Note: Descriptions are shown in the official language in which they were submitted.
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
Determination of the Properties of a Solution or Solid Using Raman Ratios
Field of the Invention
This invention relates to an apparatus and a method for analyzing properties
of a solution or
solid using Raman spectroscopy and in particular to the application of Raman
peak intensity
ratios for analyzing and predicting the properties of a solution or solid.
Background of the Invention
In many industrial chemical processes, the amount of reactants, or input
components,
that are used is less than or more than the amount necessary to carry the
reaction to the
point of obtaining a desired characteristics) of the product stream. If too
little of the
input component is used, often the desired target value of a characteristic
from the
process is not obtained. Alternatively, if an excessive amount of an input
component is
used, the desired characteristic may be obtained, but the excess input
component is
typically released as waste in the effluent of the process. In other cases,
excessive
amounts of an input component may cause undesirable reactions to occur that
produce
unwanted characteristics. Further, the wasted input component is economically
costly
and can become an environmental pollutant if it is released into the
environment without
being removed or recycled from the effluent. The difficulty in controlling
chemical
processes, such as bleaching, in the pulp and paper manufacturing industry can
be
caused by a number of factors including qualitative and quantitative
variability of the
pulp or wood furnish, the composition of the process chemicals, and the
consistency (%
wood or pulp) of the furnish. Further, changing market requirements for paper
products
may require a paper manufacturing operation to produce a wide variety of paper
grades.
New paper processing methods, equipment, and chemicals force the paper
bleaching
operation to adapt to these technical changes while still monitoring various
characteristics of the pulp.
It is therefore desirable to be able to precisely control the input components
to obtain the
desired target characteristics) with little waste. To obtain this control, a
characteristic
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
of the effluent of an industrial process should be precisely monitored in real
time in
order to provide feedback control on the amount of input components, which
should be
added to the reactor to avoid under use or excessive use, and waste, of the
input
component.
For example, in the pulp and paper industry, hydrogen peroxide and the
hydroperoxy
anion (H02~) are important input components for the oxidation and bleaching of
wood
pulps. In a typical pulp bleaching plant situation, the control of the
bleaching chemicals
is based on the brightness of the incoming pulp, the pulp flow, and the target
brightness
and pulp physical properties that are to be achieved. The factors of incoming
pulp
brightness, pulp flow, and target brightness are then used to calculate the
amount of
bleaching chemicals required to be added to the pulp to achieve a certain
final target
brightness. In another system, the brightness of the pulp is measured after
bleaching
chemicals are added and after allowing the reaction to occur for a defined
reaction time.
The resultant brightness value of the reaction is then measured and is used
for feedback
regulation of the bleaching chemicals.
Typically with these feedback systems, the amount of hydrogen peroxide that is
used
exceeds or overshoots the amount necessary to reach a final target
characteristic, such as
pulp final target brightness, yellowness, residual peroxide, brightness
efficiency,
yellowness efficiency, and delignification efficiency. The resultant unwanted
variation
in these pulp characteristics may cause additional processing problems in the
pulp and
paper processing mill. Further, in the case of peroxide bleaching, excessive
use of
hydrogen peroxide results in waste hydrogen peroxide in the pulp effluent,
which is both
costly and environmentally harmful.
In order to solve these problems the prior art has offered various solutions.
For
example, United States Patent No. 4,878,998 teaches a method for bleaching of
mechanical, thermomechanical and chemi-mechanical pulps whereby peroxide
bleaching is controlled by the addition of a preset amount of bleaching
chemicals at a
first bleaching stage, measuring the brightness of the pulp, feed forwardly
adjusting the
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
amount of bleaching chemicals to be added at a second bleaching stage as a
function of
the measured brightness of the pulp from the first stage, and then bleaching
the pulp at
the second stage.
Canadian Patent No. 2,081,907 teaches a method and apparatus for determining
information characteristics of the concentration of each of at least three
intermixed
components in kraft liquors having the steps of: identifying detectable
characteristics
that are detectable in relation to the concentration of the components,
developing a
mathematical relationship between the component and the characteristics, such
as
regression analysis, analysing a sample of solution with a UV detector, and
then
controlling the concentration of each of the three components by using the
information
from the analysis of the sample.
While current brightness sensors are able to provide a measure of the pulp
brightness,
they are unable to measure the bleaching efficiency of the bleaching reaction
itself.
Bleaching efficiency is a change in brightness of a pulp divided by the amount
of
peroxide consumed during the bleaching reactions. Further, measurement of
yellowness
efficiency, which is defined as a change in pulp yellowness divided by the
peroxide
consumed during the bleaching reactions, also requires a method by which the
residual
peroxide in the pulp effluent can be measured. Other efficiency measures
relating the
relative improvement in pulp optical and strength properties to the
consumption of
chemicals and the generation of dissolved species derived from wood are of
interest, but
are not readily available.
United States Patent No. 5,842,150 and WO 961122183 to Renberg et al. disclose
a
method, based on UV/VIS/NIR/IR, for the qualitative and quantitative
determination of
quality parameters in pulp and paper and/or the organic content in effluents
from pulp
and paper production by applying chemometric methods. Renberg et al. provide a
review and discussion of the "state of the art" and the need for on-line
measurement of
variables related to pulp and effluent quality. A discussion is provided for
the use of
spectral analysis with standard chemometric procedures to derive calibrated
values for
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
pulp and paper strength and brightness parameters and also measures of amounts
of
organic substances, for example the Total Organic Carbon (TOC), the Chemical
Oxygen
Demand (COD), and the Biological Oxygen Demand (BOD). The abstract and the
disclosure indicate that the invention relates to UV/VIS/NIR spectroscopy,
including
Raman spectroscopy. However, the disclosure does not provide any examples or
further
disclosure with respect to the use of Raman spectroscopy. The examples
disclosed in
column 7 in United States Patent No. 5,842,150 are all examples related to UV
absorption techniques between 200 and 360 nm to some other property. It is
noted that
United States Patent No. 5,842,150 does not disclose any wavelength region
outside the
UV region. Furthermore, Raman spectroscopy is an emission technique and does
not
extend to absorption, transmittance or reflectance techniques as discussed in
United
States Patent No. 5,842,150. The reflectance technique disclosed therein is
not the same
as an emission by inelastic scattering as it occurs in Raman spectroscopy. The
prior art
does not relate the spectral parameters to organic indicators and does not
discuss the
properties related to the oxidative capacity of inorganic components that may
exist in
multiple oxidation states, the development of substances that contribute to
scale
deposition of effluent components, the physical properties of polymerizable
species,
such as the number of endgroups, the extent of network formation, and the
chain length,
or the development of bulk, yield or fiber flexibility.
The following two references, including references within, provide a general
review of
the application and interpretation of Raman spectroscopy with respect to
lignocellulosics, Chapter 9 "An Overview of Raman Spectroscopy as Applied to
Lignocellulosic Materials" by Umesh P. Agarwal from a book entitled "Advances
in
Lignocellulosics Characterization", edited by Dimitris S. Argyropoulos,
published by
Tappi Press 1999 (ISBN 0-89852-357-5), and an article by Umesh P. Agarwal and
Sally
A. Ralph in Applied Spectroscopy Volume 51, Number 11, 1997. pp 1648-1655,
entitled "FT Raman Spectroscopy of Wood: Identifying Contributions of Lignin
and
Carbohydrate Polymers in the Spectrum of Black Spruce (Picea Mariana).
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
Unfortunately, it is well known that there is a present lack of an appropriate
method or
device for the monitoring and control of pulp bleaching reaction
characteristics,
including pulp final target brightness, yellowness, residual peroxide, and
efficiency,
with respect to brightness or physical strength development, the amount of
peroxide
consumed, or the loss of lignin or carbohydrate substances. Also, it is known
that pH
measurement probes and electrochemical methods of measuring hydrogen peroxide,
such as the Kajaani Polarox sensor made by Valmet Automation, can be
unreliable
under pH and chemical concentration conditions which are typically used for
pulp
brightening reactions. BTG Spectris (Sweden) has an instrument and method of
measuring the peroxide concentration that employs the use of a catalyst to
decompose
the hydrogen peroxide to generate oxygen gas that increases the reaction
vessel pressure.
This instrument, the RPA-5000, then relates the change in the pressure of the
reaction
vessel to the concentration of peroxide. This method, while providing a badly
needed
measure of the peroxide concentration, is complicated and indirect and subject
to
variability related to sample preparation and instrument maintenance.
Measurement of the concentrations of other pulp bleaching chemicals such as
sodium
hydrosulfite (dithionite), chlorine dioxide, and hypochlorite present similar
difficulties
to those encountered to for hydrogen peroxide. In general these bleaching
compounds
are oxidative or reductive substances, normally existing as one or more
species of
inorganic oxianions in solution. A review of the current state of the art of
pulp
bleaching practices, including methods for measurement and control, has been
published
(Pulp Bleaching. Principles and Practice. Canton W. Dence, and Duglas W. Reeve
Editors. Tappi Press, Atlanta 1996).
Summary of the Invention
In accordance with the invention there is provided a method for measuring an
amount of
peroxide or peroxyl ion of a sample comprising the following steps: (a)
irradiating at
least a portion of the sample with a laser light for generating a Raman
spectrum of the
sample; (b) obtain a Raman spectrum for obtaining at least two measurements at
two
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
different wavenumbers, a first measurement related to a Raman intensity
related to an
amount of peroxide or an amount of peroxyl ion, the second measurement related
to the
other of the amount of hydrogen peroxide and the amount of peroxyl ion; and
(c)
formulating a relationship between a Raman intensity for hydrogen peroxide and
a
Raman intensity for the peroxyl ion by comparing information related to the
two
measurements for determing the amount of peroxide or peroxyl ion.
In accordance with another embodiment of the invention there is provided a
method for
determining a property of a sample comprising the steps of: (a) irradiating at
least a
portion of the sample with a laser light for generating a Raman emitted light
from the
sample; (b) obtaining at least two measurements of the Raman emitted light
between
200 cm 1 and 4000 cm 1, a first measurement at a first wavenumber and a second
measurement at a second wavenumber; and (c) determining a non-linear
relationship
between the at least two measurements and the property of the sample.
Furthermore, in accordance with yet another embodiment of the present
invention there
is provided a method for determining a potential of an oxidative reductive
process
comprising the following steps: (a) irradiating at least a portion of the
sample with a
laser light for generating a Raman emitted light from the sample; (b)
obtaining at least
two measurements of the Raman emitted light between 200 cm 1 and 4000 cm 1, a
first
measurement at a first wavenumber, and a second measurement at a second
wavenumber; and (c) determining a relationship between the two measurements
and the
potential of the oxidative reductive process. The term peak refers herein
after to a
maximum intensity value or a region about the maximum intensity, near or about
the
peak.
In accordance with the invention there is further provided a method for
measuring an
amount of at least one of hydrogen peroxide and peroxyl ion (H00-) in a
solution,
comprising the steps of: irradiating at least a portion of the solution with
light of a
suitable wavelength and intensity to obtain information relating to a Raman
spectrum
thereof, said information containing data related to at least one of an
intensity peak
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
corresponding to peroxide and an intensity peak corresponding to peroxyl ion;
and,
processing the information to determine indicia of a concentration of at least
one of
hydrogen peroxide and peroxyl ion, the processing including an analysis of at
least one
of data related to the intensity peak corresponding to peroxide, data related
to the
intensity peak corresponding to peroxyl ion, a sum of data related to the
intensity peaks
of the peroxide and peroxyl ion, a product of data related to the intensity
peaks of the
peroxide and peroxyl ion, and a ratio of data related to the intensity peaks
of the
peroxide and peroxyl ion.
In accordance with a further embodiment of the present invention there is
provided an
apparatus for determining a property of a sample comprising: a laser light
source for
irradiating at least a portion of the sample for generating a Raman emitted
light from the
sample; a detector for detecting the Raman emitted light from the sample, said
detector
for obtaining at least two measurements of the Raman emitted light, a first
measurement
at a first wavenumber and a second measurement at a second wavenumber; and a
processor for receiving and processing data from the detector for determining
a non-
linear relationship between the at least two measurements and the property of
the
sample.
In accordance with another aspect of the invention there is provided a system
for
determining a property of a sample comprising: means for determining a non-
linear
relationship between at least two measurements and the property of the sample,
the at
least two measurements corresponding to Raman emitted light between 200 cm 1
and
4000 cm 1, and the at least two measurements comprising a first measurement at
a first
wavenumber and a second measurement at a second wavenumber.
Furthermore, in accordance with the invention there is provided a system for
determining a property of a sample comprising: means for comparing at least
two
measurements including a first measurement at a first wavenumber and a second
measurement at a second wavenumber, the at least two measurements
corresponding to
Raman emitted light between 200 cm 1 and 4000 cm 1 when the sample is
irradiated with
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
a laser; means for determining a non-linear relationship between the at least
two
measurements and the property of the sample; and, means for determining the
property
of the sample in dependence upon the non-linear relationship.
In accordance with a further aspect of the invention there is provided a
system for
determining an amount of at least one of hydrogen peroxide and HOO- in a
solution,
comprising: means for receiving information containing data related to at
least one of a
Raman intensity peak corresponding to peroxide and a Raman intensity peak
corresponding to peroxyl ion; and, means for processing the information to
determine
indicia of a concentration of at least one of peroxide and peroxyl ion, the
processing
including an analysis of at least one of data related to the intensity peak
corresponding to
peroxide, data related the intensity peak corresponding to peroxyl ion, a sum
of data
related to the intensity peaks of the peroxide and peroxyl ion, a product of
data related to
the intensity peaks of the peroxide and peroxyl ion, and a ratio of data
related to the
intensity peaks of the peroxide and peroxyl ion.
Brief Description of the Drawings
Exemplary embodiments of the invention will now be described in accordance
with the
drawings in which:
Figure 1 presents a schematic diagram of a pulp bleaching process in
accordance with an
embodiment of the present invention;
Figure 2 shows a matrix plot for an example of a bleaching process and shows
five
dependent properties and six corresponding UV absorbance values;
Figure 3 shows a similar matrix plot, which was formed using the same
characteristic
properties and a representative set of ratios from the absorbance values;
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
Figure 4 presents an example from pulp and paper process waters and shows a
plurality
of absorbance spectra obtained at different temperatures between 20 and 80
degrees
Celsius;
Figure 5 shows a matrix plot with one dependent property, viz. the
temperature, and 6
corresponding UV-visible absorbance values;
Figure 6 shows a matrix plot with one dependent property, viz. the
temperature, and a
representative set of ratios from the absorbance values;
Figure 7 shows a plot presenting Raman spectra from a dispersive system with a
514.5
nm laser and a FT system with a 1064 nm laser;
Figure ~ shows a Fourier Transform (FT) scan of a Raman scattering signal from
a pulp
bleaching mixture of hydrogen peroxide, silicate, and sulfate;
Figure 9 shows a series of Raman spectra of pressates from peroxide bleaching
of pulp;
Figure 10 shows a matrix plot for variables related to Aspen TMP pulp
bleaching with
hydrogen peroxide, Raman intensities, Raman intensity ratios and the pulp and
bleaching
pressate properties;
Figure 11 presents Raman Spectra of pressates from hydrogen peroxide bleaching
of aspen
TMP pulp at different pH values;
Figure 12 shows a graph for the prediction of pulp brightness from a model
based on a
combination of Raman peak intensities and Raman peak intensity ratios;
Figure 13 presents the Raman spectra showing HOO- and HOOH peaks at 850 cm 1
and 877
cm 1, respectively;
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
Figure 14 shows a matrix plot of Raman intensities and Raman ratios as a
function of pH;
Figures 15 and 16 show Raman spectra of different sulfur oxianion in a dibasic
form;
5 Figure 17 presents spectra showing Raman intensities of a solution of sodium
hydrosulfite
(Na2S204) oxidizing to sulfate and sulfite ions;
Figure 18 shows the S-O stretching region of the Raman spectrum during the
oxidation of
hydrosulfite to sulfate;
Figure 19 presents a matrix plot showing Raman intensities, and Raman
intensity ratios
with time and oxidation reduction potential (ORP);
Figure 20 presents a series of silicate Raman spectra as a function of varying
hydroxide
concentration as taken from Prabir K. Dutta and Dah-Chung Shieh, published in
Applied
Spectroscopy, Vol. 39, No. 2, pp. 343-346 (1985);
Figure 21 shows the intensities of the different peaks as derived from the
spectra presented
in Figure 20;
Figure 22 showing a matrix plot of Raman intensity ratios as a function of the
HO-/Si ratio;
Figure 23 shows Raman spectra of white water samples from ANC and MWP; and
Figure 24 shows Raman spectra of acetic acid and acetic acetate buffer
solution. Top line:
0.05M acetic acetate buffer solution. Bottom line: 5% acetic acid solution
(scaling X 0.2).
Detailed Description of the Invention
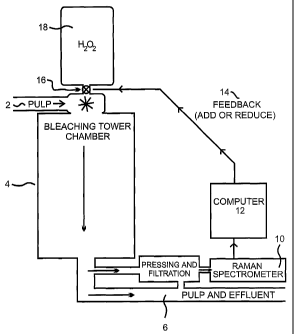
Figure 1 presents a schematic diagram of a pulp bleaching process in
accordance with an
embodiment of the present invention. As shown in Figure 1, wood pulp 2 is
bleached in
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
11
a bleaching chamber 4, by bleaching agent hydrogen peroxide (H202), as input
component. H202 flows from reservoir 18 through control valve 16 into the
bleaching
chamber 4. The output of the bleaching process is a process effluent 6, which
includes
both the bleached pulp as well as the bleaching liquor.
A sample of the effluent 6 is diverted to a Raman spectrometer 10 for the
purpose of
obtaining at least two Raman measurements of the effluent 6 and then
calculating the
ratio of the two measurements. The ratio of the Raman emitted light intensity
measurements have been found to correlate to various characteristics of the
pulp
effluent. For example, characteristics such as pulp brightness, pH, and pulp
yellowness,
and residual peroxide can be determined through the use of different Raman
wavenumbers in the ratio.
The bleaching process causes structural changes in the lignin or extractive
components
of the pulp including ionization of the phenolic groups of the lignin
molecule. Lignin
degradation typically results in an increase in the number of phenolic groups
that can be
ionized by changes in pH. As a result, the relative amount of ionized phenolic
groups
usually depends on the extent of lignin degradation and pH.
In accordance with an embodiment of the present invention, the effluent 6 is
filtered by
a 0.05 ~m cross-flow membrane filter (Koch Filtration #5-HMF-451 PNE-PP)
filter to
remove colloidal material from the pulp effluent. If the colloidal material is
not
removed from the effluent, the resultant turbidity may interfere and offset
the Raman
intensities and hence affect the Raman ratio of the present invention.
Computer 12 calculates a mathematical relationship between two or more Raman
measurements, which is a ratio or combination of ratios in this embodiment of
the
present invention. Moreover, computer 12 stores in its memory a predetermined
value
for a characteristic of the effluent.
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
12
Appropriate software provides means for receiving information pertaining to
the Raman
measurements and means for processing this information. The processing of the
information includes the comparison of measurements, the determination of non-
linear
relationships, and the determination of a property or properties of the
sample.
Computer 12 acts as a comparing means for comparing the measured H202 value
with a
predetermined H202 value. This comparison step determines the actual empirical
value
of the residual weight percentage of H202, a characteristic of the effluent
being
monitored.
According to this embodiment, computer 12 determines whether to send a
feedback
signal 14 to control valve 16 to adjust the amount of H202 being fed into the
bleaching
process taking place in bleaching chamber 4. Thus, computer 12 together with
control
valve 16 acts as a means for adjusting the amount of input component, such as
H202,
according to the value of the characteristic of the effluent 6, H202
concentration, which
was determined by the ratio of the Raman emitted light measurements.
Thus, as shown in the embodiment of the invention, shown in Figure l, the
disclosed
invention permits a real time feedback control of a pulp bleaching process.
The
feedback system provides sufficient H2O2 to the pulp bleaching process without
producing excessive waste residual H202 or pollution.
If desired, other input components, such as NaOH, MgS04, or a chelating agent,
such as
DTPA (diethylenetriamine pentaacetic acid), are added to the bleaching
reaction in
reduced or increased amounts in accordance this feedback system.
Richardson et al. disclose in U.S. Patent No. 5,242, 602 a method for
simultaneously
analyzing the concentration of performance indicators in aqueous systems by
determining an absorbance or emission spectrum in a wavelength range from 200
to
2500 nm and applying chemometric algorithms to the absorbance or emission
spectrum.
The concentrations of the performance indicators determined by the chemometric
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
13
algorithms are compared to predefined ranges for the respective performance
indicators.
Thus, this technique teaches the application of cher~ometrical methods for
multicomponent analysis using multiwavelength spectroscopy. Richardson et al.
describe the following four basic algorithms to extract and analyze features
in the
overall absorption or emission spectra that are specific to the qualitative
and quantitative
contributions from the specific performance indicators:
1. Quantification. Measurement of the absorption or emission spectra on a
series of samples with known concentrations of the performance indicators.
2. Processing. The processing of raw data to reduce noise and optimize the
ability of the chemometric techniques to compare known spectra with unknown
spectra or to act on specific features for the spectra of a multi-components
solution to permit analysis of mufti-components solutions or to adjust for
noise
or drift. The following preprocessing steps were explicitly identified: a)
noise
reduction or smoothing; b) Fourier or Walsh transformations; c) first or
second
derivatives; and d) correction for drift.
3. Analysis. Analysis of the spectra using chemometric techniques. The
following statistical methods were specifically identified: a) principal
component
analysis; b) regression analysis including multiple regression methods; and c)
discriminant analysis.
4. Comparison. Comparison of results from the analysis to actual values.
This step may involve the use of multiple linear regressions on 2-4 Principal
components to obtain a calibration for different performance indicators.
In the method disclosed by Richardson et al. the chemometric algorithms are
applied
directly to the absorbance or emission spectrum. However, the Analysis methods
described in U.S. Patent No. 5,242,602 are ineffective if there is a small
variation or a
high degree of correlation in the data matrix. The present invention discloses
the use of
a ratio or multiple ratios from the at least two measurements for computing an
empirical
value of the characteristic. This is a significant improvement from the method
described
by Richardson et al. in that the present invention can extract the variation
even if the
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
14
obtained absorbance or emission spectra are similar, i.e. they show little
variation. The
method disclosed by Richardson et al. does not provide any mention in the
processing
step for the use of ratios as a means to improve the analysis step.
Furthermore, there is
no specific mention of using ratios as a means of reducing the redundancy of
the data
that may lead to analysis problems. The invention disclosed by Richardson et
al. does
not address problems related to the reliability of the estimation of a
property/characteristic that has a small variation relative to the total
variation in the data.
The present invention discloses the use of at least one ratio and a value
formulated from
at least two Raman measurements, each at different wavenumber, to determine a
characteristic of a process effluent. Combinations of the disclosed ratios may
be further
used to monitor and control characteristics of the process effluent. Multiple
regression
analysis, using a forward stepwise multiple regression, was conducted using
these
Raman ratios to determine the best combination of these ratios and
coefficients, (i.e. a
predetermined relationship) which best predicted the final property of the
pulp (i.e.
optical properties and other descriptors of bleaching including
delignification efficiency
and residual peroxide). As shown in the graphs, the present invention can use
this
predetermined relationship to determine a pulp property (final brightness,
delignification
efficiency, or residual peroxide) using a minimum number of Raman ratio
measurements.
A more detailed description of the problems associated with the extraction of
multiple
properties from a series of similar ultraviolet-visible spectra is given
below. The use of
ratios formulated from Raman measurements provides a means for accentuating
the
difference between the obtained spectra. A description of the problems with
the
extraction of multiple properties from a series of similar Raman spectra. The
method
disclosed by Richardson et al. could not be used to separate the variables or
parameters
since they often cause the same kind of changes in the spectra or the absolute
changes
are relatively small. The present invention makes use of relative changes to
control the
number of parameters.
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
The analysis methods, such as Principal Component Analysis, Regression
Analysis, and
Discriminant Analysis, described by Richardson et al. relate linear
combinations of
measured variables with the observable characteristic. The instant invention
relates at
least one ratio and a value to a characteristic of pulp or the process
effluent and
5 implicitly accommodates nonlinear relationships between the measured values
and the
determined characteristic. Strictly speaking a chemical concentration should
be linear
with the absorbance or emission at different wavelengths. One object of the
present
invention is to obtain a relationship between a set of emission values and a
characteristic
that is a complicated nonlinear function of a plurality of different chemical
and physical
10 factors, such as the delignification efficiency. However, there are many
problems with
applying statistical analysis directly to a series of similar Raman spectra
that are
described below. Ultimately, the best result is only as good as the inherent
variance in
the data. Analysis using a set of absorbance or emission ratios alone or in
combination
with Raman emission or scattering values amounts to generating a new or
expanded data
15 set that has a greater variance than the original data set. A new data set
will generate
more accurate and stable solutions if it contains arrays that relate more
closely to the
characteristic to be fit than the original data. Analysis using a set of
absorbance or
emission ratios amounts to generating a new data set, based on functions from
the initial
data set, that is used with the chemometric techniques.
The statistical methods described by Richardson et al. require that the
variation of the
measured absorbance at different wavelengths changes substantially as a
function of the
different properties that are mathematically related to the absorbance values.
Thus,
small variation in the data matrix will result in computational difficulties
in multiple-
linear regression analysis. Further, a small variation in the relationships
between
variables, as expressed in the correlation matrix, will result in additional
computational
difficulties in the principle component analysis. In this case, small relative
variations
may be easily masked by large absolute variations. The presence of small
variations
contributing to the response for different variables in the presence of large
variations
that are similar in the different variables results in an ill-conditioned or
singular matrix
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
16
of the absorbance values that is not amenable to the statistical analysis
described by
Richardson et al. as discussed below in more detail using an exemplary data
matrix.
The use of ratios obtained from ultraviolet-visible measurements can condition
the data
matrix to emphasize the variation of emission (or conversely absorbance) at
one
wavelength to that of another. The analysis is then performed on the relative
variation
rather than on the absolute variation. If in the process to be monitored or
controlled one
substance is transformed into another, or if a small amount of one substance
can have a
substantial effect on the concentration of another, then the method of
observing the
relative changes is much more powerful than looking at absolute changes. The
present
invention as defined in the claims uses at least one ratio and hence provides
a means to
selectively weigh the contributions from the at least two measurements, each
at a
different wavelengths.
The outstanding problems associated with the analysis of similar and
featureless spectra
are well known and a discussion of those problems is described by O. Thomas
and S.
Gallot. in Fresenius Journal for Analytical Chemistry, 1990, Volume 33~, pages
234-
237; and by B. Karlberg in the following PCT publication W095/01560. The prior
art
does not provide any references to solve this problem by using a ratio.
Despite
numerous attempts to directly address the computational difficulties related
to
regression and chemometrics with colinear data, there are no reports directly
addressing
the computational problem by using ratios.
In the case of the quantification or analysis methods described by Richardson
et al., the
data matrix would consist of a series of absorption or emission measurements
on i2
different samples (indexed by i) and p different wavelengths (indexed by j).
For
example: '
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
17
A1,1, ... Al,~ ... A1 P
A = Ai,l ... Ai,~ ... Ai,p [1~
Ar2,1 . . . AjZ,~ ... A~, p
In the statistical techniques described by Richardson et al., the sample
correlation (r~~)
between variables is measured as a function of the variance (s~~) and
covariance (s~k) of
the data where j and k are two different indexes for the wavelength for the
emission (or
conversely absorbance) measurement and i is the index for n different samples.
s .k
rlk = ~ [2l
S~~ Skk ,
sii - si - 1 ~ ~''~t,; - A~ ~ [
h-1 ;-1
Sik - ~ 1 1 ~ ~~,k - Ak ~A;,; - A; ~ [4]
From these calculations the sample covariance matrix S and correlation matrix
R may
be calculated.
SllS12 ~..
Slp
x'21S22 . . [5~
g2n
Sp1Sp2 ...
SPP
1 Y12 ...
Ylp
R = Y211 . . L
.
Y2
p
Y~1Yp2 ...
1
Multiple regression calculations require the calculation of the determinant of
the data
matrix A. The determinant of an n x n matrix is the sum of the products of the
elements
of a row of the matrix and their cofactors. A cofactor is a determinant of a
matrix
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
18
obtained by eliminating the row and column of the element. Thus each cofactor
may be
obtained by calculating the products of elements of a row and the determinant
of a
matrix obtained by eliminating the row and column of each element. The
calculation of
the determinant has several important consequences:
1. Least squares multiple regression methods require calculation of the
determinant
of the data matrix to obtain a linear combination of the different variables.
Colinear
data, or a high degree of covariance, similarity or redundancy in the data
matrix will
lead to an ill-conditioned matrix that produces unstable results.
a) If two rows or columns are nearly identical then the determinant will be
nearly zero.
b) Solutions to a set of linear equations, for example as addressed when
using multiple regression to obtain correlated physical properties from
Raman spectra, depend on the division using the determinant of the
matrix. Colinear variables are problematic in these types of calculations.
Therefore, the division by a very small number leads to instability in the
calculation in cases where the determinant is near zero.
2. In Principal Component Analysis (PCA) the colinearity problem is resolved
by
forming a new set of orthogonal variables, the principal components. The
principal
components are linear combinations of variables that express the maximum
variance.
The principal components may be used in a multiple regression technique to
derive
relationships with properties related to the variables. The regression on
principal
component solves problems related to matrix singularity leading to problems
with
inversion. However, the number of principal components may be less than the
number
of variables and some valuable information may be lost in principal components
that are
considered statistically insignificant.
a) When the regression matrix R or the covariance matrix S is singular or
ill-conditioned problems may occur in extracting principal components.
b) If the relevant underlying effects are small in comparison with some
irrelevant ones, then they may not appear among the first few principal
components. The resulting component selection problem is difficult as
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
19
an arbitrary inclusion of the first n principal components may serve to
degrade the performance of the model.
3. Partial Least Squares (PLS) regression is a multivariate data analysis
technique
that can be used to extracts components (now called factors) that relate
several response
(Y) variables to several explanatory (X) variables. The method aims to
identify the
underlying factors, or linear combination of the X variables, which best model
the Y
dependent variables. PLS can deal efficiently with data sets where there are
very many
variables that are highly correlated and involving substantial random noise.
a) Richardson et al. in U.S. Patent No. 5,242,602 does not describe the use
of PLS although other descriptions of chemometrics describe the power
of this technique in chemometric analysis.
b) However, the partial least squares analysis is no better than the different
linear functions that may be described from the data. If the
property/characteristic that is to be predicted cannot be described as a
linear function of the variables x then the regression will introduce
systematic error into the regression model.
The analysis method described in accordance with the present invention amounts
to
generating a new data matrix that has the general appearance shown below.
Using a set of ratios derived from the original data, the data matrix A is
transformed into
a new matrix that may be called the ratio matrix Q. This matrix is based upon
a,
predetermined set of wavenumbers for the numerators and denominators of the
ratios.
The i index is the index for the sample and the j index is the index for the
wavelength of
the numerators with a total of jp numerators that define the set of quotients
used, the k
index is the index for the set of wavelengths corresponding to the set of
denominator s
that match the j indexed emission (or conversely absorbance) values for each
sample.
There are a total of kp index values for the denominators for that define the
set of
quotients used. The ratio matrix Q may be generally written as:
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
Ai,i~Ahi A'ep
. . . .
Al'kiAl k A
Aa,i,... A'e ... A'''PU)
Aa,k,Ar,k A~.kP
. . . ~ .
An,klAn,k An,kp
The wavelengths used for the numerators A;,/ and the denominators A;,k will be
selected from those absorbance or emission wavelengths that yield ratios that
5 correspond well to the component concentration, process or physical property
that is to
modeled or optimized.
The method disclosed by Richardson et al. in U.S. Patent No. 5,242,602
comprises the
direct determination of an emission or absorbance spectrum. However, the
method
10 disclosed by Richardson et al. would not work with the present invention.
For example,
in order to control pulp bleaching processes it is necessary to separate the
variables, i.e.
it is necessary to control a plurality of parameters. In peroxide bleaching
processes, the
pH and the amount of peroxide are the most important parameters to control.
Nevertheless, to control those parameters it is not sufficient to look only at
the levels of
15 those two parameters. It is important to look at how the reaction has
progressed which
means it is necessary to extract a measure of how much peroxide is present, to
extract a
measure of the pH, to extract a measure of how~bright the pulp is, and to
extract a
measure of how yellow the pulp is. Using the method taught by Richardson et
al. it
would not be possible to extract these parameters because often these
parameters cause
20 generally the same kind of changes in the measured spectra or alternatively
the absolute
changes in some important areas of the spectrum are fairly small. In using the
relative
changes, i.e. ratios, it is possible to extract the variables. For example, in
peroxide
bleaching processes there are two main components to consider. One component
is
peroxide and the other component is lignin. For the lignin component three
aspects
have to be considered: i) How much lignin has been released from the pulp; ii)
What is
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
21
the intensity of the color of the removed lignin components; and iii) what is
the extent of
lignin ionization? Thus to optimize this reaction the peroxide and the amount
of lignin
removed is maximized under the constraint of minimizing the color of the
removed
lignin. Pulp consists of about 30% lignin which causes its yellow color and
hence
removing the lignin from the pulp makes the pulp brighter. Lignin has a
relatively broad
diffuse spectrum and could be monitored at almost any wavelength in the UV
region.
Since the bleaching process involves a plurality of parameters this process
cannot
simply be optimized by maximizing the amount of peroxide. The problem is that
the
competition between productive bleaching reactions involving the nucleophilic
peroxyl
anion compete with destructive reactions initiated by a (second order)
bimolecular
decomposition involving hydrogen peroxide and the peroxyl anion. Variation in
pH or
peroxide concentrations yields a point where the peroxide decomposition
dominates
over the productive bleaching reactions. Before that point the improvements in
the
bleaching process are basically proportional to the pH or the peroxide
concentration
until a maximum is reached in both. At the maximum the peroxide decomposition
leads
to darkening reactions that neutralize the productive bleaching reactions.
Thus at this
pH and this peroxide concentration the amount of bleaching that has been done
is no
longer related to the amount of peroxide consumed and also it is no longer
related to the
amount of lignin released. In fact, the trend is opposite and has a non-linear
equation.
2,0 In the beginning, the amount of bleaching is linear for a certain region
when observing
the bleaching versus the amount of peroxide consumed or the bleaching versus
the
amount of lignin released. The bleaching is linear in some regions but in fact
it is a
parabolic function and one observes a maximum. Using the method disclosed by
Richardson et al., i.e. a linear function, it appears that the bleaching
process is
progressing but in reality this is not happening. Thus, it is necessary to
identify when an
unproductive loss in hydrogen peroxide and an unproductive generation of the
lignin
occurs. This becomes important when the peroxide starts to decompose and
superoxide
and hydroxyl free radicals are formed. These free radicals start reacting with
the lignin
and large amounts of lignin are removed. However, the pulp whiteness decreases
and
increased amounts of peroxide are used without reaching optimum bleaching
conditions
or improving the brightness of the pulp. Therefore, it is important that this
point is
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
22
characterized. The present invention discloses that when this point is
approached, the
color of the lignin changes with a resulting increase in the absorbance value
at 350 nm,
for example. The relative amount of color in the lignin increases
substantially at that
critical point. The method taught by Richardson et al. would detect this
increase in
absorbance as a result of lignin removal. Lignin has intrinsic or natural
color and when
more lignin is removed more color is observed. This means, that in the initial
bleaching
stages lignin is removed with the intrinsic color. However, when the peroxide
starts to
break down, then a new kind of reaction occurs and more lignin is removed that
is
highly colored. Problems arise because at the same time these highly colored
lignin
species are generated on the pulp. The method by Richardson et al. would
measure
changes in the amount of lignin removed but because this method does not make
use of
relative amounts it cannot distinguish between the desired removal of natural
lignin and
the undesired case where removal of highly colored lignin occurs as a result
of a
competing reaction when the peroxide breaks down. Thus without making use of
absorbance or emission ratios it is not possible to obtain sufficient
information to
distinguish between one reaction happening and another reaction happening. The
method taught in U.S. Patent No. 5,242,602 can not manage this non-linear
relationship.
The following example demonstrates the advantage of the present invention and
how it
addresses the critical point of extracting the variation by using ratios
instead of applying
analysis techniques directly on the spectra or on the processed spectra as it
was taught in
the method disclosed by Richardson et al. The presented example utilizes
ratios
obtained from UV absorbance measurements. This example is an example of a
bleaching process and shows five dependent properties and six corresponding UV
absorbance values. The absorbance values are representative, but are chosen to
provide
a large variance. Figure 2, shows a matrix plot for an example of a bleaching
process
and shows five dependent properties and six corresponding UV absorbance
values.
Each miniature plot in Figure 2 shows a bivariate plot of the corresponding
diagonal
elements. The variable on any given plot axis is determined by vertical and
horizontal
tracing to the variable at the plot diagonal, much like a mileage chart on a
map. This
matrix plot demonstrates that the absorbance functions are highly colinear
with each
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
23
other. There are five different non-linear relationships between the
absorbance values
and the different properties: pH, residual H202, brightness, yellowness
(indicated as
BSTAR) and brightness efficiency. Furthermore, it is seen that there is
scatter in the
direct relationships between some of the characteristic properties and the
absorbance
values.
Figure 3 shows a similar matrix plot, which was formed using the same
characteristic
properties and a representative set of ratios from the absorbance values. Note
that the
colinearity between the ratio variables is much smaller than the colinearity
between the
absorbance values. Furthermore it is seen that the different UV-visible ratios
vary
linearly with different properties. There is a high correlation coefficient
between
different single ratios and each of the characteristic properties. These
correlation
coefficients are given in Table 1 below.
Table 1
Ratio Wavelength Single Linear
Pro ert UV ratio CorrelationUV Correlation
absorbance
pH A230/A250 -0.98 A250 0.96
H~02 ResidualA230/A280 0.96 A280 -0.93
Pulp BrightnessA350/A250 -0.83 A400 -0.80
Yellowness A350/A280 0.95 A400 0.95
(B~)
Brightness A230/A400 0.52 A450 -0.42
Efficienc
Principle Component Analysis:
Using 9 UV values, only one factor may be extracted. This factor explains
97.6% of the
data variation. There is one other factor with an eigenvalue between 1.0 and
0.1.
However, using 9 UV ratios, two factors may be extracted. One factor explains
67.6%
of the data variation and the other explains 22.2% of the data variation.
There are three
other factors with eigenvalues between 0.1 and 1Ø
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
24
The ability to extract more factors using principle component analysis
indicates that the
data variation may be broken down into more independent factors using the
ratio
method.
Figure 4 presents an example from pulp and paper process waters. Figure 4
shows a
plurality of absorbance spectra obtained at different temperatures between 20
and 80
degrees Celsius. The small variation in the obtained spectra shows that there
is a
chemical change as a function of the temperature. These formless spectra with
their
small variations as a function of the process variable are typically
encountered in the
pulp and paper industry as well as in many other industries consuming a large
amount of
process water. Figures 5 and 6 present the matrix plots for this example. The
set of
spectra for this example shows small variations in the absorbance values.
Figure 5 shows one dependent property, viz. the temperature, and 6
corresponding UV-
visible absorbance values. In this case, one principle component accounts for
97% of
the data variation. The general trend is for the absorbance values to decrease
with
increasing temperature. The principle components method can only resolve this
effect,
even though it is clear that more is happening. A second principle component
may be
found if eigenvalues between 0.1 and 1.0 are allowed. This second eigenvalue
accounts
for only 2% of the variation.
Figure 6 shows one dependent property, viz. the temperature, and a
representative set of
ratios from the absorbance values. In this case, two principle components
account for
61.2% of the data variation and 27.6% of the data variation. A third principle
component may be found if eigenvalues between 0.1 and 1.0 are allowed. Once
rotated,
the three eigenvalues may account for 40%, 34%, and 25% of the data variation.
In accordance with an embodiment of the present invention it is taught that an
efficiency
and ultimate extent of a mechanical pulp bleaching operation using hydrogen
peroxide
is monitored and controlled by analysis of the UV-visible spectrum or Raman
spectrum
of the pressate from the bleaching process. It was not previously known that
the
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
reactions on the components in the pressate were representative of the pulp
bleaching
process. Bleaching control has always been done using pulp brightness and
without
observing the color of the pressate. Measurements of pulp brightness suffer
from
variability related to pulp consistency, pulp surface area or scattering and
insensitivity.
5 The present invention teaches that the amount of lignin in the pressate and
the color of
the pressate, as determined by UV ratios, reflected the bleaching progress
during a
mechanical pulp bleaching process. Previously, these bleaching processes have
been
described only in terms of the reactions on the pulp and the reactions on the
components
in the pressate were thought to be trivial and different from the bleaching
reactions. The
10 method of using a series of ratios of absorbance or alternatively emission
values from
pressates has a further advantage because pressates are easier to analyze
optically than
the pulp. At the point of maximum pulp brightness the response of pulp
brightness is a
flat function of pulp bleaching variables such as pH and peroxide
concentration. For
this reason, pulp brightness is a very poor control parameter. Another
unexpected and
15 advantageous result is that a combination of UV ratios or Raman ratios
provides
sensitive measures at this point where traditional bleaching control sensors
that monitor
a pulp brightness fail. The analysis disclosed in the patent describes that
hydrogen
peroxide bleaching of mechanical pulps can be controlled by monitoring a
combination
of the amount of hydrogen peroxide anion, the amount of lignin removed, and
the
20 relative amount of color removed during the bleaching process. Three points
may be
made concerning these parameters:
1. The removal of lignin was identified as an important process in the
bleaching of
pulp. In the prior art this was not thought to be the case for mechanical
pulps.
25 However, excessive lignin removal can occur during inefficient bleaching
process that
leads to a parallel increase in colored components. The best bleaching
occurred when
the most lignin could be removed while generating the smallest amount of
colored
components.
2. The bleaching process was most efficient when the relative amount of
hydrogen
peroxide anion was maintained at high levels. Underlying this simple statement
are the
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
26
realizations that the principle cause of inefficient bleaching is the
degradation of
peroxide at high concentrations and high pH values and an important reason for
incomplete bleaching is low pH values that do not fully activate the hydrogen
peroxide
to the hydrogen peroxide anion. Furthermore, peroxide degradation leads to
pulp
yellowing and darkening processes. The optimum bleaching process then is one
at
which the bleaching occurs at the highest levels of peroxide anion
concentration that are
consistent with the beneficial effects of bleaching but which exclude the
negative effects
of peroxide degradation.
3. A strategy of maximizing the relative concentration of the peroxide (H00-)
bleaching agent and the beneficial effects of the bleaching agent of color
removal are
taught in accordance with an embodiment of the present invention. This method
does
not require a multiple regression with respect to a concentration in the
effluent. A
learning step with respect to the final pulp properties is advantageous but
not necessary.
As of yet, reliable means for determining hydrogen peroxide concentration are
not
available. The value of hydrogen peroxide consumed at a paper mill is
typically
between two and ten million dollars a year. Much of this peroxide is wasted
and the
development of a reliable means for monitoring hydrogen peroxide in the pulp
and
paper industry is important in making the bleaching process more economical.
The
advantages of using a set of UV absorbance or alternatively Raman scattering
ratios
obtained from a filtrate to determine the peroxide concentration have not been
previously recognized. A simple measurement of the hydrogen peroxide ion
concentration as a function of UV absorbance or Raman emission would be
obvious.
However, the measurement of the hydrogen peroxide concentration in a
background of
strongly UV absorbing substances, such as lignin, presents substantial
deconvolution
problems. Furthermore, the measurement of the peroxide concentration under pH
conditions where the relative amounts of hydrogen peroxide and the hydrogen
peroxide
concentrations are varying is a difficult problem. The present invention
obviates this
problem, in that by using ratios, i.e. relative values, obtained from
ultraviolet-visible
measurements the contributions from lignin and the variation due to pH are
factored out.
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
27
A plurality of different methods for measuring peroxides is known. Among
those,
polarigraphic measurements are thought to be unreliable and commercial methods
using
catalytic peroxide decomposition were not yet available. Presently, a hydrogen
peroxide
measurement is available from BTG a Division of Spectris Technologies, but the
reliability of this measurement is still in question and it has not been
widely adapted.
In accordance with the present invention ratios of Raman peak intensities are
used to
predict the properties of a solution or a solid such as pulp that is processed
with the
solution. The method and apparatus of the present invention extend the
multiple ratio
strategy to Raman scattering intensities observed at visible or near IR
wavelengths. In
this case the intensity of the Raman shifted data is used to create the ratio.
These
intensities are related to the concentration of species dissolved in the
liquid. A preferred
measurement is using a transmissive Raman scattering measurement using a
Nd:YAG
(1064 nm) laser to minimize sample fluorescence. If desired, baseline
corrected spectra
. and a scattering from a water reference is subtracted before extraction of
intensities for
ratios. The Raman scattering intensities provide a good measure of the
concentration of
small oxygenated molecules. Relevant small molecules and complex ions in the
pulp
and paper industry include, but are not exhaustive, SO42-, SO32-, H202, C102,
HC103,
silicates, acetic acid, Chloric Acid HC103, Chlorate C103(-), Chlorous Acid
HC102,
Chlorite C102(-), Hypochlorous Acid HC10. Hypochlorite Cl0(-), phosphate,
nitrate,
nitrites. If desired, the present invention is used to measure large molecules
such as
hemicellulose, extractives and pectic substances. The use of Raman spectra to
measure
small, oxygenated molecules is well documented and extensive lists exist of
Raman
shifts for molecules and functional groups, which are found for example in
"Irzfrared
and Ramarz Spectra of Inorganic and Coordirzatiorz Corrzpourzds Part A:
Tlzeory arzd
Applications in Inorgazzic Chemistry" Kazuo Nakamoto, 1997, John Wiley and
Sons,
Inc, New York. The value of using Raman emissions to measure and control
species of
oxidative and reductive oxygenated inorganic species has not been recognized
before
this invention. Furthermore, the importance of measurement of the relative
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
28
concentrations of different related species, for example: HOOH and HOO- or
C102,
C102 , HC10 and C103 or SZO42-, SO32-, 502-, has not been recognized or
adapted.
Figure 7 shows an example of dispersive and Fourier Transform (FT) Raman
spectra to
demonstrate an interference using a laser at shorter wavelengths. The plot
presents
Raman data from a dispersive system with a 514.5 nm laser and a FT system with
a
1064 nm laser. The data from the dispersive system shows interference that
probably is
due to fluorescence, at 800-1000 cm 1. With advances in Raman spectroscopy
lasers at a
longer wavelength than 514.5 nm are used to obviate a fluorescence problem.
Thus a
preferred configuration in accordance with an embodiment of the present
invention is a
laser operating at a higher wavelength than 514.5 nm.
Figure 8 shows a Fourier Transform (FT) scan of a Raman scattering signal from
a pulp
bleaching mixture of hydrogen peroxide, silicate, and sulfate. Steps in the
processing of
the raw data include a fast Fourier transform, a baseline correction and then
subtraction
of the water signal. The peak at 400 cm 1 and below is an experimental
artifact due to a
silicate cell and the detection geometry. An optimized system would have a
different
geometry, such as 90 degree detection, or cell material, such as sapphire, to
minimize
the interference of the cell material when measuring silicate solutions and
colloids.
Figure 9 shows a series of Raman spectra of pressates from peroxide bleaching
of pulp.
These spectra were obtained using a 1064 nm laser with FT signal processing,
baseline
correction and subtraction of water spectra. The samples were quantitatively
diluted to
pH 7 before their measurement. The series progresses from high bleaching pH at
the top
to low bleaching pH at the bottom. Raman peaks are observed at approximately
530
cm 1 for silicate, at approximately 877 cm 1 for hydrogen peroxide (H202 , at
approximately 990 cm 1 for sulfate, and at approximately 1077 cm 1 for
carbonate. The
Raman peaks for sulfate shown in Figure 8 appear due to the addition of
sulfate. Sulfate
was added in order to lower the pH value. The Raman peaks for carbonate are
observed
because C02 is absorbed from the surrounding atmosphere to form carbonate
ions.
Alternatively, this method is used for predicting scale formation since it is
capable of
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
29
detecting sulfate ions and carbonate ions. In this case, care is taken that
the pressates are
not exposed to carbon dioxide.
Table 2 shows a relationship between pulp yield and Raman intensity ratios.
Table 2
Regression Summary for Dependent Variable: YIELD
R= .99912365 RZ= .99824807 Adjusted RZ= .99562018
F(3,2)=379.87 p<.00263 Std.Error of estimate: .27325
BETA St. Err. B St. Err. t(2) p-level
of of B
BETA
Interce 37.37 4.841 7.72089 .016364
t
RH202UV2 .992604.080541 8322.67 675.313 12.32417.006520
H202 .958102.091885 32733.36 3139.227 10.42720.009072
RSILH202 .942172.122940 38.20 4.985 7.66366 .016604
Table 3 shows a relationship between residual hydrogen peroxide and Raman
ratios.
Table 3
Regression Summary for Dependent Variable: H202 RESIDUAL
R= .99822460 R2= .99645235 Adjusted RZ= .99113086
F(3,2)=187.25 p<.00532 Std.Error of estimate: .11298
BETA St. Err. B St. Err.t(2) p-level
of of B
BETA
Interce -12.085 1.7914 -6.74606.021275
t
RH202UV2 1.045293.122605 2546.622 298.70048.52567 .013480
RH202SIL 1.376609.171798 1.718 .2144 8.01298 _.015220
RSILH202 1.395214.236563 16.437 2.7869 5.89786 .027565
~ ~ ~
Figure IO shows a matrix plot for Aspen TMP pulp bleaching with hydrogen
peroxide
and the pulp and bleaching pressate properties. This matrix plot shows
variables
describing the peroxide bleaching process and Raman peak intensity and
intensity ratios
from bleaching pressates obtained by bleaching with hydrogen peroxide under
varied pH
conditions. The samples were diluted to a constant pH before measurement so
that the
information in them relates to changes developed during the bleaching process.
The
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
pulp variables include an ultimate brightness, bulk, which is a measure of the
specific
volume of the pulp, and yield, which is the % mass of the bleached pulp
relative to the
unbleached pulp. The bleaching pressate values are characterized by the
process
variable pH, the residual peroxide as a % on pulp when 4% by weight of
peroxide and
5 pulp that was initially put on the pulp, and the total dissolved solids
(TDS). Raman
intensity ratios provided relate to the peak intensity for silicates at 530 cm
1 (silicate,
530 cm 1), and hydrogen peroxide at 877 cni 1 (H202, 877 cm 1). The Raman
intensity
ratio of RH202SIL presented in Figure 10 is a ratio of (H202 (877 cm 1)
intensity!
intensity of silicate 0530 cm 1) and intensity of Raman intensity to UV
absorbance is
10 given by the following ratio RH202UV2 as expressed by (H202 (877crri 1)/UV
absorbance at 280 nm). For each subplot the X-axis relates to the relative
concentration
of the variable above and the Y-axis relates to the relative concentration of
the variable
to the right.
15 The ratio formulated between the Raman peak intensity for the peroxide and
the silicate
is of importance for indicating an optimal stabilizing effect through the
silicate. Silicate
is added to the bleaching solution to stabilize the peroxide. Thus the amount
of silicate
with respect to hydrogen peroxide is maximized such that it provides best
stabilizing
effects for the amount of hydrogen peroxide.
Table 4 shows data for peroxide bleaching at different pH values. The Raman
intensity
ratios are compared to pulp and pressate properties.
Table 4
StartPulp Pressate Raman intensities
and Properties of
pH
adjusted
pressates
pH BleachHa02 TDS BrightnessPulp SilicateH202 SOa C03
Yield residual ISO % Bulk 530crri877cW 980cW 1077crri
1 1 1 1
9.5 97.2 3.74 1.1773.84 3.27 0.25 1.81 1.28 0.9G
9.7 96.1 2.38 1.1674.52 3.17 0.28 1.05 1.27 0.92
10.0 96.0 1.7677.97 3.12 0.32 1.08 1.29 1.04
10.3 93.6 2.82 1.9578.93 2.80 0.21 1.06 1.24 0.97
10.5 92.8 1.90 2.2178.99 2.92 0.20 1.05 1.24 0.96
10.7 93.0 1.89 2.2079.68 2.38 0.32 0.98 1.34 0.91
11.1 90.2 1.26 2.6981.82 2.60 0.30 0.98 1.28 0.94
11.5 88.9 0.91 3.1583.45 2.00 0.40 0.86 1.46 1.01
12.1 86.7 0.04 5.2680.33 1.69 0.41 0.89 1.38 0.97
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
31
Figure 11 presents Raman Spectra of pressates from hydrogen peroxide bleaching
of
aspen TMP pulp at different pH values. The spectra are obtained directly, i.e.
without
dilution or pH adjustment. The Raman peaks for hydrogen peroxide (H202) at 877
cm l,
for carboxylic acid (COO-) at 925 cm 1, for a C-H bending mode at 1350 crri 1,
and for a
C-H bending mode at 1415 cm 1 are shown. The relative decrease of the hydrogen
peroxide peak and increase in the peaks representing different bleaching by-
products can
be related to pulp properties developed during bleaching. During the pulp
bleaching
process with hydrogen peroxide competing chemical processes result in the
destruction
of colored species, the cleavage and removal of colored species, and the
formation of
colored species during non-productive cleavage of lignin substances. Peroxide
concentrations decrease during both productive and non-productive reactions
through
bimolecular degradation of the peroxide or reaction with the wood substances.
The
spectra in figure 11 and the associated tables 5 and 6 below show that the
productive
reactions leading to high brightness with minimal loss of yield and bulk
generate
carboxylic groups with Raman intensities at 925 cm 1. Smaller amounts of
brightness
gain and greater yield or bulk loss correlate well with the increase in the
intensity of
Raman emission peaks at 1350 cm 1 and 1415 cm 1. Hence the greatest
improvement in
brightness gain with the least loss of yield or bulk occurs when the amplitude
of the
intensity ratio of the peak at 925 cm 1 to the intensity at 1350 cm 1 or 1415
crri 1 is
maximized. The peak intensity for the hydrogen peroxide at 877 cm -~ should be
maximized to preserve the hydrogen peroxide and to prevent unnecessary radical
reactions. These results provide a basis for improved relationships between
the Raman
intensities and the pulp properties shown in Tables 7-12. In Table 7 the
brightness
development is optimized when the relative consumption of the hydrogen
peroxide to
the development of the C-H peak at 1350 cm 1 is minimized. Table 9 relates the
bulk to
the product of the peaks at 1350 cm 1 and 1415 cm l, as formation of compounds
contributing to both of these peaks contributes to brightness loss.
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
32
Table 5
H ISO % H202 % on
% in
initialfinal BrightnessFreenessBulk pressatepul TOC Yield
9.3 6.4 72.17 164.5 2.931 0.577 2.942 1580 98.102
9.6 6.5 72.56 149.0 2.967 0.548 2.825 1550 98.138
10.0 7.1 74.49 148.0 2.901 0.359 1.850 3030 96.361
10.2 7.3 75.17 143.0 2.774 0.311 1.604 3610 95.664
10.5 7.4 76.05 147.0 2.750 0.277 1.428 4070 95.112
10.7 7.6 76.03 152.0 2.815 0.245 1.262 4570 94.511
11.0 8.0 77.58 142.0 2.620 0.147 0.760 6550 92.133
11.4 8.4 78.32 132.0 2.453 0.088 0.451 6400 92.313
11.8 9.0 78.25 129.0 1.954 0.075 0.388 ~ 84.266
13000
Table 6
H UV Abs Raman
Intensities
initial final 280 nm Ig~~ 1925 11350 hdis
9.3 6.4 0.588 1.960 0.532 0.364 0.496
9.6 6.5 0.578 1.585 0.434 0.263 0.409
10.0 7.1 0.860 1.207 0.799 0.344 0.666
10.2 7.3 1.013 0.996 0.893 0.441 0.653
10.5 7.4 1.183 0.888 0.964 0.469 0.797
10.7 7.6 1.269 0.797 0.971 0.471 0.795
11.0 8.0 2.164 0.656 1.559 0.731 1.181
11.4 8.4 3.193 0.324 1.647 0.877 1.334
11.8 9.0 4.371 0.587 1.782 ~ 1.216 1.750
Figure 12 shows a graph for the prediction of pulp brightness from a model
based on a
combination of Raman peak intensities and Raman peak intensity ratios. The
observed
values are plotted against the predicted values with the ISO brightness being
a
dependent variable and the independent variables being the Raman intensities
and
intensity ratios.
Tables 7 to 12 below demonstrate the prediction of pulp properties from Raman
ratios
and Raman intensities. The pulp properties presented are brightness in Table
7, bulk in
Tables 8 and 9, residual hydrogen peroxide on the pulp in Table 10, and total
organic
carbon (TOC) in Tables 11 and 12.
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
33
Table 7
Regression Summary for Dependent Variable: Brightness
R= .99774854 R~= .99550215 Adjusted RZ= .99280344
F(3,5)=368.88 p<.00000 Std.Error of estimate: .19239
Raman BETA St. Err. B St. Err.t(5) p-level
Intensity of of B
BETA
Interce 77.16 .4319 178.6687.000000
t
I876/I1350-.318581.111927 -.35 .1226 -2.8463 .035979
I1415 .279821 .054338 750.02 145.64425.1497 .003616
I876 -.444773.102212 -1016.37 233.5693-4.3515 .007349
Table 8
Regression Summary for Dependent Variable: BULK
R= .98249292 RZ= .96529234 Adjusted R2= .96033411
F(1,7)=194.68 p<.00000 Std.Error of estimate: .06333
BETA St. Err. B St. Err. t(7) p-level
of of
BETA B
Interce 3.264 .04657 70.0903 .000000
t
I1350 -.982493.070415 -521.78337.39594 -13.9529.000002
Table 9
The Ratio Predicts bulk better
Regression Summary for Dependent Variable: BULK
R= .99113735 RZ= .98235324 Adjusted R2= .97983227
F(1,7)=389.67 p<.00000 Std.Error of estimate: .04516
BETA St. Err. B St. Err.t(7) p-level
of
BETA of B
Interce 3. .022 138.9712.000000
t
P13501415-.991137 .050209 -127757.6471.954-19.7401.000000
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
34
Table 10
Regression Summary for Dependent Variable: H2O2 residual on Pulp
R= .99806738 RZ= .99613850 Adjusted R2= .99382159
F(3,5)=429.94 p<.00000 Std.Error of estimate: .07356
BETA St. Err. B St. Err.t(5) p-level
of of B
BETA
Interce .799 .16514 4.84114 .004710
t
88761350 .360850.103708 .163 .04689 3.47948 .017669
I1415 -.252501.050347 -279.28955.68855-5.01519 .004052
I876 .427770.094706 403.38489.307624.51680 .006301
Table 11
Regression Summary for Dependent Variable: TOC
R= .96431621 Ra= .92990576 Adjusted RZ= .91989230
F(1,7)=92.866 p<.00003 Std.Error of estimate: 1002.7
BETA St. Err. B St. Err. t(7) p-level
of
BETA of B
Interce -1394. 737.4 -1.89022.100643
t
I1350 .964316 .100067 5705570.592068.1 9.63668 .000027
Table 12
Regression Summary for Dependent Variable: TOC
R= .99132396 R2= .98272319 Adjusted RZ= .97235711
F(3,5)=94.802 p<.00008 Std.Error of estimate: 589.00
BETA St. Err.B St. Err. t(5) p-level
of of B
BETA
Interce -1989. 1509. -1.31791.244683
t
Product 2.031475.447598 29173073E2642774566.4.53862 .006177
I1350I1415
Ratio .208308 .072085 2406. 832. 2.88976 .034197
I924/I1350
UV280 Abs -,993688.435777 -2691. 1180. -2.28027.071514
Figure 13 presents the Raman spectra showing HOO- and HOOH peaks at 850 cm 1
and
877 cm 1, respectively. The spectra presented in Figure 13 are shown after
subtraction
of a reference spectrum of water and baseline correction. When looking at the
Raman
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
spectrum of hydrogen peroxide two peaks are observed for peroxides. The
intensity of
the two peaks varies with the pH value of the solution, i.e. at a lower pH
value more
hydrogen peroxide is observed and at a higher pH value more peroxyl anion (H00-
) is
observed as a result of ionization of the hydrogen peroxide. In bleaching
processes it is
5 important to know what percentage of the hydrogen peroxide is ionized at a
particular
pH value. This is determined by looking at the two Raman peaks at 850 cm 1 and
877
cm 1 and comparing them with each other. The active species in bleaching
processes are
the ionized species and thus the amount of peroxyl anion (H00-) needs to be
maximized
since too much peroxyl anion (H00-) would cause a degradation of the hydrogen
10 peroxide. In accordance with an embodiment of the present invention Raman
spectroscopy conveniently provides a means for determining an extent of
ionization of
hydrogen peroxide by comparing the Raman peaks for the peroxyl anion and
hydrogen
peroxide.
15 Figure 14A to 14J show a matrix plot of Raman intensities and Raman ratios
as a
function of pH. The considered Raman intensities are the Raman intensities for
hydrogen peroxide HOOH (I877) and peroxyl ions HOO- (I850) as a function of
pH.
Two ratios of Raman peak intensities are also shown in the matrix plot. The
Raman
intensity ratio of 8850877 is the intensity of the peak at 850 cm 1 to the
intensity of the
20 peak at 877 cm 1, the Raman intensity ratio R877T is the ratio of the 877
cm 1 peak to
the sum of the two peak intensities at 850 cm 1 and 877 cm 1. These intensity
r atios give
new non-linear variables that relate to the peroxide solutions properties. The
matrix plot
shows the relationship between the solution pH and the Raman peak intensities
of the
HOO- and HOOH peroxide species. The use of these peak intensities for the
25 measurement and control of hydrogen peroxide concentrations in pulp and
paper and
water treatment has not been described. Thus in accordance with an embodiment
of the
present invention the Raman peak intensities of the Raman peaks at 850 cm 1
and 877
cm 1 are used to control the hydrogen peroxide concentration. The ratio of the
peak
intensities provides a new relationship with respect to the solution
properties (pH) as
30 shown in plot 14C. The ratio is different than the direct peak intensities
as shown in
Figures 14F and 14H. Raman peak intensity ratios or products provide
alternative
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
36
means by which the properties can be modeled. A second ratio may be defined by
the
intensity of one peak to the sum of the intensity of both Raman peaks. This
ratio is
expected to follow relationships relevant to the pica of the peroxide. Figure
14F and
14H show nonlinear relationships between the ratio and the direct peak
intensity thus
confirm the matrix plot presented in Figure 14 does not show a product of the
intensities
of the two Raman peak intensities at 850 cm 1 and 877 cm 1. However, the
product of
these two Raman peak intensities provides similar results as the ratio between
two
Raman peak intensities.
The Measurement of the Redox Properties and Relative Concentrations of Related
Oxidative and Reductive Species
Figures 15 and 16 show Raman spectra of different sulfur oxianion in a dibasic
form.
Figure 17 presents spectra showing Raman intensities of a solution of sodium
hydrosulfite (Na2S204) oxidizing to sulfate and sulfite ions. The management
of
oxidizing and reducing substances in industrial applications is problematic in
part
because measures such as an oxidation-reduction potential (ORP) are very
sensitive to
pH, ionic strength, temperature and the influence of interfering substances.
The use of
Raman peak intensities provides a means to directly measure the concentrations
and
relative concentrations of the different species contributing to the oxidation
potential of
the solution.
Figure 18 shows the S-O stretching region of the Raman spectrum during the
oxidation
of hydrosulfite to sulfate. The isobestic point is the point at which the
total
concentration is always a same function of intensity no matter what proportion
of the
two different species, i.e. hydrosulfite and sulfate, are present.
Figure 19 presents a matrix plot showing Raman intensities, and Raman
intensity ratios
with time and oxidation-reduction potential (ORP). Regression lines marked in
the
matrix plot of Figure 19 demonstrate that the Raman intensity ratios correlate
better with
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
37
the ORP than their components. Plots P and R within Figure 19 demonstrate that
a
Raman intensity ratio provides the best direct measure of the ORP of the
sulfur species
in solution since these plots are almost linear.
Table 13 provides a regression summary for the oxidation-reduction potential
and Table
14 shows the correlations for hydrosulfite oxidation.
Table 13
Regression Summary for Dependent Variable: ORP
R= .99514836 R2= .99032025 Adjusted RZ= .98709366
F(1,3)=306.93 p<.00041 Std..Error of estimate: 6.0576
BETA St. Err. of B St. Err. t(3) p-level
BETA of B
Interce -206.4679.147723 -22.5703 .000190
t
Ratio -.995148.056803 -75.0624.284508 -17.5193 .000405
I460/I980
ORP=-206.467-75.062*I460/I980
I5 Table 14
Correlations Hydrosuliite Oxidation
Marked correlations are significant at p < .05000
Ratio
58319801 460/ 980/
Time ORP I228 ~I460I583 I980I998 I1022980 998 980 1022
TIME 1.00 .94 -1.00-.99-1.00.94 -1.0 -.79 -.94.90 -.94 .90
ORP .94 1.00 -.98 -.99-.95 .97 -.88 -.96 -.99.93 -1.00.9G
I228 -1.00-.98 1.00 1.001.00 -.94.99 .79 .94 -.91 .94 -.90
I460 -.99 -.99 1.00 1.001.00 -.94.99 .79 .96 -.89 .96 -.89
I583 -1.00-.95 1.00 1.001.00 -.941.0 .79 .96 -.89 .95 -.89
I980 .94 .97 -.94 -.94-.94 1.00-.95 -.93 -.87.98 -.87 .99
I998 -1.00-.88 .99 .99 1.00 -.951.0 .81 .95 -.91 .94 -.91
I1022 -.79 -.96 .79 .79 .79 -.93.81 1.00 .75 -.91 .76 -.96
Ratio -.94 -.99 .94 .96 .96 -.87.95 .75 1.00-.77 1.00 -.81
583/
980
Ratio .90 .93 -.91 -.89-.89 .98 -.91 -.91 -.771.00 -.77 .99
980/
998
Ratio -.94 -1.00.94 .96 .95 -.87.94 .76 1.00-.77 1.00 -.80
460/
980
Ratio .90 .96 -.90 -.89-.89 .99 -.91 -.96 -.81.99 -.80 1.00
980/
1022
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
38
The Oxidation/Reduction Potential (ORP) is a measurement of the potential for
a
reaction to occur. Oxidation-reduction represents electron concentration and
activity
level. An ORP in the plus range indicates oxidation, i.e. the absence of
energy, and an
inability to perform additional chemical reactions. An ORP in the negative
range
indicates chemical reduction, i.e. the presence of electrons, potential
energy, and the
ability to generate additional chemical reactions. ORP is therefore a measure
of energy
potential. The more negative the ORP, the more electrons present (in relation
to the
number of protons), and the more energy available. Biological redox reactions
are a
result of hydrogen being the essential electron donor, and oxygen being the
essential
electron acceptor.
Oxidation-reduction potential, ORP, measurements are used to determine the
oxidizing
or reducing properties of a solution. Typical applications include the
neutralization of
waste water containing sulfides, cyanides, chromates, nitrites or organic
waste and
controlling the addition of oxidants to drinking water, swimming pools or
cooling
towers. ORP can also be used to determine the ion activity of metals in
solution and
determine the endpoint of titrations.
The standard method for determining the ORP is given in "Standaxd Methods for
the
Examination of Water and Waste Water", 18'h Edition, 1992, method 2580 B.
Discussion of relevant background information is included in this reference.
The term potential of an oxidative reductive process within this specification
is defined
as the oxidation-reduction potential and also as an oxidation-reduction
indicator. The
potential of an oxidative reductive process is a measure of a composite state
of the
oxidation-reduction capacity of a sample and not of the oxidative-reductive
capacity of
the individual species.'
ORP is commonly measured as pE using a metal electrode, a reference electrode
and a
high input impedance millivolt meter such as a pH meter. However,
electrochemical
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
39
methods for measuring the ORP cannot discriminate between different
oxidative/reductive species. In the environment or in industrial applications
usually
many different influences on the ORP from other oxidative or reductive species
are
present. In accordance with an embodiment of the present invention Raman
spectroscopy is employed to determine the contribution to ORP of certain
species more
accurately by monitoring the oxidative/reductive properties and relative
concentrations
of the species of interest. Alternatively, additional oxidativelreductive
species
contributing to the ORP are monitored if their molecular vibrations are Raman
active.
The Nernst equation relates the oxidative and reductive properties of a
certain regime.
The Nernst equation can be expressed in pE notations as:
pE = pE + ~I loglo {{A ed} [
pE is a notation related to the oxidation form and the reduction form of the
species in a
regime. Presented below is the example for a water regime.
The limit of pE in water can be determined using the Nernst equation. The
stability
regime of water is determined by the reduction and oxidation of water. The
reduction of
water defines the lower limit to pE
H+ + e- -~ ~ H20 [9]
H+
pE = pE- +loglo { 1 } [10]
2
fH2
As pE-is zero by definition and the boundary condition commonly used is a
hydrogen
fugacity, fH2 of unity, this gives
pE = pH [ 11 ]
The upper boundary for the stability of water is given by the oxidation
reaction:
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
402+H++e -+ ~HZO [12]
pE=pE-+loglo f~{H+} [13]
5
The boundary condition used is an oxygen fugacity of unity. The upper pE limit
becomes:
pE = 20.75 - pH [ 14]
The pE for natural waters can be calculated using equation [6]. The unknowns,
which
must be measured, are pH and the 02 partial pressure (concentration). For
example, the
pE for surface water in equilibrium with the atmosphere (pot = 0.21 atm) and
have a pH
of 8 would be:
pE = pE- + loglo f ~ f H+ ~ [ 13]
pE = pE- - pH + loglo f ~ [ 15]
pE = 20.75 - 8 + log to (0.21) ~ =12.58 [ 16]
The stability boundaries for water were calculated earlier. The upper and
lower limits,
respectively, are:
pE = 20.75 - pH ~ [ 14]
pE = -pH [ 11 ]
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
41
Measurements of Properties of Species Forming Complexes, Polymerized and
Networked Structures, and Multiple degrees of Ionization.
Figure 20 presents a series of silicate Raman spectra as a function of varying
hydroxide
concentration as taken from Prabir K. Dutta and Dah-Chung Shieh, published in
Applied Spectroscopy, Vol. 39, No. 2, pp. 343-346 (1985). These spectra yield
the
intensities and ratios presented in Figures 21 and 22. Silicates can take
different
structural forms through ionization and polymerization in dependence upon a pH
value.
Silicates can be used in a plurality of industrial applications. For example,
silicates are
added to a bleaching solution in order to stabilize peroxides.
The set of spectra shows how silicate types vary with solution conditions.
Figure 21 shows the intensities of the different peaks as derived from the
spectra
presented in Figure 20. Figure 21 shows a matrix plot of Raman intensities as
a
function of the NaOH-/Si ratio as derived from Prabir K. Dutta and Dah-Chung
Shieh;
Applied Spectroscopy, Vol. 39, No. 2, 343-346 (1985). Peaks from silicate
relate to
different vibrational modes that depend on the ionization and degree of
polymerization
of the silicate. Vibrations from silicate monomers yield peaks at 925 cm 1 (Si-
O- stretch,
monomer ionized) 772 cm 1 (Si-O-H stretch, monomer not ionized), 482 cxri 1
(Si-OH
stretch) and 446 cm 1 (Si02(OH)z2- symmetric bend). Dimer groups yield peaks
at 597
cm 1 (Si-O-Si stretch, dimer bridge) and 1014 cm 1 (Si03 stretch, dimer
endgroup).
Cyclic trimers have a breathing vibration at 531 cm 1. The peak at 1014 cm 1
(SiO~
stretch, dimer endgroup) shifts to 1030 cm 1 with cyclic trimers.
The prior art has looked only at the Raman intensities. In accordance with the
present
invention ratios are used as they are capable of presenting different non-
linear
relationships with respect to solution properties than the intensities do.
Ratios present the physical properties of the silicates better than
intensities alone do.
This is demonstrated in Figure 22 showing a matrix plot of Raman intensity
ratios as a
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
42
function of the NaOH/Si ratio from Prabir K. Dutta and Dah-Chung Shieh,
Applied
Spectroscopy, Vol. 39, No. 2, 343-346 (1985). It is noted that this article
does not teach
the use of ratios. The intensities given in this article were used to derive
Raman
intensity ratios in order to demonstrate that the use of Raman intensity
ratios is much
better in predicting the physical properties of silicates. The ratios relate
the relative
concentrations of different functional groups characterizing the silicate
speciation.
These ratios provide new variables that logically relate to the properties of
the silicate
solution. The ratio 8597/1014 is related to the degree of polmerization or the
chain
length because individual intensities represent the amount of bridging/the
amount of end
groups, i.e. ratio of the peak intensity at 597 cm 1 to the intensity at 1014
cm 1. The
degree of polymerization decreases with increasing alkali concentration. The r
atio
8925/772 is related to the degree of amount of ionization of the silicate
monomers (ratio
of the peak intensity at 925 cm 1 to the intensity at 772 cm 1). The ratio
8531/597 is
related to the ratio of cyclic trimer groups to dimer groups (ratio of the
peak intensity at
531 cm 1 to the intensity at 597 cm 1). The ratio 8531/772 is related to the
ratio of
cyclic trimer groups to protonated monomer groups (ratio of the peak intensity
at 531
crri 1 to the intensity at 772 cm 1). The ratio 8531/925 is related to the
ratio of cyclic
trimer groups to ionized monomer groups (ratio of the peak intensity at 531
crri 1 to the
intensity at 925 cm 1). The ratio 81014/925 is related to the ratio of ionized
dimer
groups to ionized monomer groups (ratio of the peak intensity at 1014 cm ~ to
the
intensity at 925 cm 1)
FT-Raman spectra were recorded using a Bruker IFS-88 Fourier Transform
Infrared
(FT-IR) spectrometer equipped with FRA 106 Raman accessory. Excitation was
provided by an Nd:YAG laser (~, = 1.064mm, v =9394 cm 1). Solid state samples
were
measured using an aluminum sample holder and solutions were contained in a 0.5
cm
cuvettes. Sepctra were recorded at a resolution of 4 cm 1, utilizing a laser
power typical
50mw for a solid sample and 280mw for a solution sample.
The spectrum in solid-state is different from the spectrum from the solution.
In most
cases an aqueous solution was measured. In general, Raman spectroscopy has an
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
43
advantage over IR spectroscopy with respect to working on an aqueous phase.
The
detection limit can be pushed down to 0.05% at current experimental conditions
(Laser
power 280mw and single path at 180° sample collection configurations).
In order to
increase the detection limit, higher laser power andlor multi-path sample
collection
configuration are needed in FT-Raman spectroscopy.
Example of The Use of Raman Spectra and Ratios for Measurement and Control
of Pulp Bleaching.
There are 10 different samples (PH = 9.5, 9.7, 10.0, 10.3, 10.5, 10.7 11.5,
11.7, 12.1 and
12.7). Since each sample adjusts the final pH by using buffer solution (pH =
6.9) and
1M NaOH, these 10 samples have been divided into two groups. There is one
group
with three peaks at 877, 980 and 1077 cm 1, and another group having two broad
bands
at 1100 and 1630 cm 1. These two broad bands are due to water and the Raman
sample
cell. As described above, water was subtracted from the obtained Raman spectra
before
processing the data.
According to the reference Raman spectra of H202, the peak at 877 cm 1 in
bleaching
water is due to the H2O2. The amount of the H202 is approximately 0.1 %. The
peak at
980 cm 1 is due to S04 stretching band of the sulfate (reference see previous
work). The
amount of H202 decreases with the pH increase, while the amount of the sulfate
increases with the pH increase. The detection limit for H202 and S04 can be
lowered
down to 0.1 % at current instrument condition. Interfering peaks due to
carboxylic acid
vibrations occur at 890 cm 1 for acetic acid and 925 cm 1 for the acetate ion.
The third peak in bleaching water sample is at 1077 cm 1. The intensity of
this peak does
not change with pH increase.
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
44
Other Examples of Raman Spectra of Ultrafiltered Pulp Processing Waters
Raman spectra of two white water samples from Millar Western Pulp (MWP) and
one
from Alberta Newsprint (ANC) are collected and shown in Figure 23. These
samples
are filtered through a 0.45, filter before data is collected. Only one band at
980 cm j
appears in ANC water. It is the contribution of the S04 (sulfate ion) band
from the
sulfate. There are also some sulfates in two kinds of Millar Western Pulp
water. The
amount of sulfate is different according to the band intensities. The peak at
877 crri l in
both Millar Western Pulp water samples also indicates the existence of H202.
Another
band at 925 cni 1 in two MWP water samples is the contribution of carboxylate
groups
in the water. Figure 24 gives the Raman spectra of acetic acid and acetic
acetate buffer
solution (0.05M). The peak at 925 cni 1 is due to vC-O of the carboxylic group
(basic
form), the peak at 890 cm 1 is due to the same vibration mode but from the
acid form.
Other relevant small molecules and complex ions in the pulp and paper industry
may be
detected. These species include SO42-, SZO32-, 5032-, H202, C102, HC103,
silicates,
acetic acid, Chloric Acid HC103, Chlorate C1O3(-), Chlorous Acid HC102,
Chlorite
C102(-), Hypochlorous Acid HC10. Hypochlorite C10(-), phosphate, nitrate,
nitrites and
carbonate.
The invention provides an additional set of process parameters that are
closely tied to the
process chemistry. Using these new variables with functions that predict,
model and
control the state and outcome of a multi-dimensional process provides a
significant
advantage over standard chemometric and linear regression methods. Advanced
control
systems including those utilizing fuzzy logic, time correlation analysis,
neural networks,
adaptive control, principle component analysis and partial least squares
provides a means of
developing software programming and logic solutions to problems with a high
dimensionality. It is an object of this invention to provide new variables
that may be used
to better control input and output parameters in a complicated process with
many
interrelated variables.
SUBSTITUTE SHEET (RULE 26)
CA 02403008 2002-08-O1
WO 01/59437 PCT/CA01/00123
The a above-described embodiments of the invention are intended to be examples
of the
present invention and alterations and modifications may be affected thereto,
by those of
skill in the art, without departing from the scope of the invention which is
defined solely
by the claims appended hereto.
5
SUBSTITUTE SHEET (RULE 26)