Note: Descriptions are shown in the official language in which they were submitted.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
CARBOHYDRATE MEDICAL SOLUTION AND SULPHITE STABILISATOR IN A
MULTIPLE COMPARTMENT CONTAINER AND USE THEREOF
TECHNICAL FIELD
The present invention relates to multiple compartment containers including
sterile medical solutions, in which at least one solution contains
carbohydrate
compounds. The invention further relates to stabilising carbohydrates in a
sterile
medical solution.
BACKGROUND
Sterilisation of medical solutions such as, for example, peritoneal
dialyses (PD) solutions, is commonly performed through the addition of energy,
either in the form of radiation or heat. WO-A-9705852 discloses a multiple
compartment container including sterile peritoneal dialyses solutions, which
is heat-
sterilised in an autoclave.
In recent years scientists have become aware of the toxicity of decomposition
compounds of carbohydrates in PD solutions. Wieslander et al., reported that
all
major brands of commercial PD solutions were toxic in contrast to PD solutions
sterilised by filtration (Wieslander et al., 1991, Kidney Int, 40:77-79). The
PD
solutions were tested after dilution with cell growth media on cultured
fibroblasts.
Furthermore, Wieslander et al. have reported that the glucose degradation
products
also affect the functional responses involved in host defence (Wieslander et
al.,
1995, Peritoneal Dialysis Int, 15 (supply.
A patient on peritoneal dialysis (PD) uses between 8 and 20 litres of dialysis
solution every day, depending on the treatment. This results in the
consumption of
3-7 tons of solution with 1.5-4% glucose (50-175 kg pure glucose) every year.
(Wieslander, 1996, Nephrol Dial Transplant 11:958-959), which if the glucose
undergoes decomposition also means a non-negligible amount of decomposition
compounds. Furthermore, it is well lmown that some patients experience pain
during
inflow of the dialysis fluid. It has been speculated that the pain could be
the result of
glucose degradation (Henderson et al., 1985 Frontiers in peritoneal dialysis,
ed.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
2
Winchester, New York: Field, Rich, 261-264) and that these degradation
products
mediate basal cytotoxicity (Barile FA, 1994, Introduction to ih vitro
cytotoxicity.
Florida:CRC Press, 27-35). This means that they act upon fundamental life
processes, which involve structures and functions common to all living cells
such as
membrane integrity, mitochondria) activity, or synthesis of proteins and DNA.
These basal cell functions support organ specific cell functions. Thus,
glucose
degradation products capable of affecting basal cell activities are likely to
interfere
with specialised cell functions such as IL-1[3 release from mononuclear cells.
Glucose, an osmotic agent commonly used in PD solutions is known to
degrade into carbonyls such as formaldehyde, acetaldehyde, metylglyoxal, 3-
deoxyglucosone and glyoxal.
Sulphite compounds have commonly been used as antioxidant in parenteral
emergency drugs to prevent oxidation. The mechanism of decomposition of
carbohydrates in PD solutions has appears however to have less to do with
oxidation
and sulphite is not intended to be used as an antioxidant ifa vitro in the
present
invention. Further, the anti-microbial or antioxidant corripounds in
parenteral
emergency drugs are typically used in concentration which deliver 0.5 to 2 mg
of
sulphite per ml of undiluted drug injection (Smolinske S, 1992, Clinical
toxicology
30:597-606). Such concentrations for preventing oxidation could not be used in
PD
solutions since they would administer too much sulphite to the patient
resulting in
adverse toxic effects.
SUMMARY OF THE INVENTION
On the above background it is an object of the present invention to provide a
multiple compartment container for sterile medical solutions of the kind
referred to
above in which decomposition of carbohydrates and/or the negative effects of
the
decomposition products are reduced. The multiple compartment container
comprises
at least one sulphite compound in one or more of the compartments to stabilise
decomposition of carbohydrates or to scavenge decomposition products formed
during sterilisation and/or storage.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
3
The invention further relates to a medical solution wherein the solution
contains at least one carbohydrate compound and at least one sulphite compound
to
stabilise decomposition of the carbohydrates or to scavenge decomposition
products
formed from the carbohydrates during sterilisation or storage of the medical
solutions.
Additionally the invention relates to a method of stabilising a carbohydrate
containing solution wherein the solution contains at least one sulphite
compound to
stabilise decomposition of carbohydrates or to scavenge decomposition products
formed during sterilisation and/or storage.
Furthermore the invention relates to the use of a carbohydrate containing
solution for the preparation of a multiple compartment container.
Finally, the invention relates to use of a carbohydrate containing solution
for
the preparation of a multiple compartment container for the treatment of a
patient in
need thereof.
BRIEF DESCRIPTION OF THE DRAWING
In the following detailed portion of the present description, the invention
will
be explained in more detail with reference to an exemplary embodiment shown in
the drawings, in which Figure 1 is a frontal view on a multiple compartment
container according to an embodiment of the invention.
DETAILED DESCRIPTION OF THE INVENTION
The invention is intended for use in treatments of diseases such as uremic
disorder or kidney malfunctions, including for example treatments of diseases
using
peritoneal dialysis.
Definitions
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
4
The term "multiple compartment container for a medical solution" is intended
to mean any container comprising more than one compartment, particularly two
or
three but not limited to three, compartments. One example is a multiple
compartment container used for peritoneal dialysis containing medical
solutions,
which are sold under the brand Physioneal° and Gambrosol° trio.
The term "medical solution" is intended to mean any solution useful for
medicinal purposes in which, a carbohydrate may be present and in which the
carbohydrate undergoes decomposition during either the sterilisation procedure
or
storage resulting in disadvantageous decomposition products unfavourable for
living
cells. Decomposition products contemplated are for example products such as
mono
and dicarbonyl compounds, formaldehyde, acetaldehyde, methylglyoxal, 3-
deoxyglucosone and glyoxal or the like. The storage conditions could be any
conventional storage condition, such as room temperature for 2 years. One
example
of a medical solution is a solution, present in one or more of the
compartments, used
for peritoneal dialysis.
The term "final solution" is intended to mean a solution obtained by mixing
one or more of the medical solutions in the container.
The term "peritoneal dialysis solution" is intended to mean a solution
comprising an electrolyte, a buffer and an osmotically active compound,
wherein the
electrolyte comprises ions, such as sodium, potassium, calcium and magnesium;
the
buffer comprises components, such as acetate, lactate and bicarbonate; and the
osmotic compound is a carbohydrate as defined hereinafter. Examples of medical
solutions for use as peritoneal dialysis solutions may be found in Wieslander
et al.,
1991, Kidney Int 40:77-79. The peritoneal dialysis solution could, prior to
dialysis,
be present in one or more compal-tments. In the case of multiple compartments
the
solutions are mixed prior to peritoneal dialysis.
The term "carbohydrate compound" is intended to mean sugars or sugars
acids such as glucose, fructose, mannose, aldonic, alduronic, aldaric acids
and their
esters with saccharides or a polymer of glucose, fructose, mannose, aldonic,
alduronic, aldaric acids and their esters with saccharides or a synthetic form
of
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
glucose, fructose, mannose, aldonic, alduronic, aldaric acids and their esters
with
saccharides or derivatives and mixtures thereof.
The term "sulphite compound" is intended to mean a sulphite containing
compound with the properties to reduce the content of decomposition products,
by
for example stabilising the solution, including preventing the generation of
decomposition products, or scavenging already formed decomposition products,
produced during sterilisation and/or storage of medical solutions containing
carbohydrate compounds, as defined above.
Furthermore, the sulphite compound could be used as an antioxidant or to
scavenge toxic or allergenic compounds ira vivo, such as in the body fluids.
Examples of such toxic or allergenic compounds are metylglyoxal, 3-
deoxyglucosone and glyoxal. The effect of the sulphite compound can be
measured
according to the method "A~Zalysis of glucose degradation products" mentioned
under "Material and Methods" hereinafter. Examples of such sulphite compounds
are any sulphite, having a positive counter ion, such as sodium, potassium,
calcium,
magnesium and ammonium, for example HS03-, Sa052- and 5032-. Examples of
sulphite compounds to be used are NaHS03, Na2S205 and Na2S03 or any other of
sulphite compound or derivative thereof, natural or synthetic, or mixtures
thereof.
The term "stabilising" is intended to mean preventing the generation of
decomposition products, or scavenging already formed decomposition products,
produced during sterilisation and/or storage of medical solutions containing
carbohydrate compounds
The term "carbohydrate decomposition products" is intended to mean
products produced in a carbohydrate solution during any kind of sterilisation
and/or
during storage, which are products obtained from decomposition of
carbohydrates,
such as glucose and toxic to eucaryotic and procaryotic cells. Specifically
contemplated are mono and dicarbonyl compounds, such as formaldehyde,
acetaldehyde, methylglyoxal, 3-deoxyglucosone and glyoxal or the like. The
toxicity
can be measured according to the method "in vitro assay for cytotoxity"
mentioned
in "Materials and Methods hereinafter.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
6
The term "sterilisation" is intended to mean any kind of sterilisation, such
as
radiation, pressure, heat, LTV-radiation, radioactive radiation, sterile
filtration,
radiation using micro waves or other sterilisation methods. Furthermore the
sterilisation can be performed using different approaches such as short
sterilisation
time at a high temperature, sterilisation at low pH, sterilisation with high
glucose
concentration after removal of catalytic substances.
Multiple compartment containers employing a medical solution.
The invention relates to multiple compartment containers for sterile medical
solutions, particularly solutions for peritoneal dialysis (PD), wherein the
medical
solutions are present in one or more compartments. One or more of the compart-
ments comprises a carbohydrate and at least one sulphite compound in order to
reduce the amount of the carbohydrate decomposition products produced during
sterilisation and/or storage. Furthermore one or more of the compartments may
include an electrolyte, a buffer and any other pharmaceutically acceptable
additive
or other component.
Additionally, the container comprises at least two compartments, preferably
three or more compartments, most preferably three. In at least one of the
compart-
ments there is provided a carbohydrate compound in solution and in at least
one of
the compartments there is provided a sulphite compound to reduce the formation
or
scavenge already produced decomposition products formed from carbohydrate.
Furthermore the "sulphite compound" could be used as an antioxidant or to
scavenge toxic or allergenic compounds in vivo, such as in the body fluids.
Commonly used medical solutions either in single or multiple compartment
containers) for peritoneal dialysis preferably contain glucose in the final
solution
in a concentration in the range of 1.5 to 4,0 % preferably substantially 1.5,
2.5 or 4
by weight (based on the final solution).
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
7
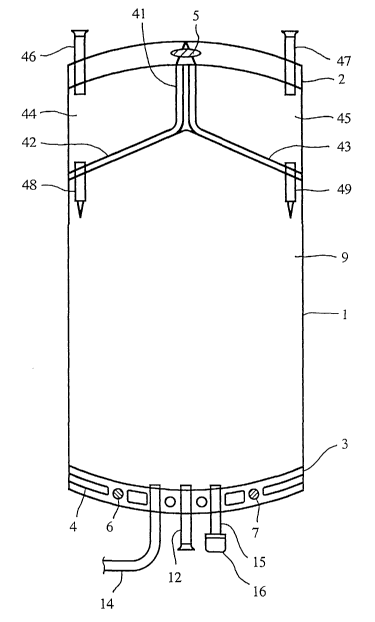
Fig. 1 shows a preferred embodiment of the container, in this case a three-
compartment bag . The bag 1 is made from a continuous tube of a plastics
material,
which is sealed at both ends by sealing borders 2,3.
As shown in FIG. 1 each sealing border comprises several
embossments 4 and apertures 5,6,7. The embossments 4 enhance the stability of
the
border 2. The aperture 5 in the upper border 2 is intended for hanging the bag
during
use and the apertures 6,7 in the lower border 3 are for fixation of the bag
during the
manufacturing operation.
The lower border 3 is also provided with an outlet tube 14, which
connects compartment 9 with the consumer, for example a catheter ending in the
abdominal cavity of a patient for peritoneal dialysis. Often tube 14
terminates in a
luer connector (not shown in FIG. 1).
Furthermore, border 3 is provided with a filling tube 12, a medicament tube 15
including a removable cap 16. When cap 16 is removed, tube 15 forms an
entrance
site for introducing any type of beneficial agent or medicament into
compartment 9
as desired, such as antibiotics.
The bag 1 is divided into three compartments 9,44,45 by welding seal lines
41,42,43. The upper compartments 44,45 divided by welding seal line 11 are of
equal size and separated form the lower compartment ~ by two sloping welding
lines 42,43. Thus there is formed a first upper compartment 44 and a second
upper
compartment 45, each being accessed via introduction tubes 46,47. The large
lower
compartment 9 comprises the electrolytes necessary for the solution to be
formed
(final solution), such as NaCI, MgCh , lactate etc., dissolved in water in a
manner
known per se.
The first compartment 44 comprises glucose solution having a concentration
of about 30% and the second compartment 45 comprises a glucose solution having
a
concentration of about 50%.
When breaking the breakable portion of connection tube 4~, the contents of
the first compartment 44 is mixed with the contents of the lower compartment 9
to
form a peritoneal dialysis solution having a concentration of 1.5% of glucose.
If the
breakable portion of connection tube 49 is broken, the contents of compartment
45
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
8
is mixed with the contents of both compartment 9 thereby forming a dialysis
solution having a concentration of about 2.5% of glucose. If both breakable
portions
of connection tubes 48,49 are broken, the contents of both compartments 44 and
45
are mixed with the contents of compartment 9 thereby to form a dialysis
solution
having a concentration of about 4% of glucose. The above dialysis solutions
formed
by mixing at least one of the glucose containing compartments.
If the bag should be used as a nutritional solution, the large compartment 9
may comprise only NaCl or any other suitable composition as used today but
excluding glucose.
It is mentioned that the glucose can be exchanged with a glucose like
component, such as glucose polymers, as an osmotic agent.
Furthermore, the sterile medical solutions comprising a carbohydrate, may
include an electrolyte, a buffer such as lactate and any other
pharmaceutically
acceptable additive.
According to one embodiment of the invention the carbohydrate compound is
separately provided in one or more compartments, the rest of the peritoneal
dialysis
solution compounds being provided in one or more the other compartments. The
sulphite compounds) may be present in one or more of said compartments or
separately presented in one or more compartments. The sulphite compounds) may
be introduced in any of the carbohydrate or electrolyte solution compartments
before
or after sterilisation. However, some or all components of the medical
solutions)
and the sulphite compound are mixed prior to peritoneal dialysis to obtain a
final
solution.
If a monosulphite compound such a bisulphate is used, it is preferably added
to the carbohydrate and/or the electrolyte compartment in an amount to give a
final
solution within the range of 0.01 - 10 mM, preferably 0.05 - 1 mM, most
preferably
0.05 - 0.5 mM. If a disulphite compound is used it is preferably added to the
carbohydrate compartment in an amount to give a final solution within the
range of
0.005 - 5 mM, preferably 0.025 - 0.5 mM, most preferably about 0.025 - 0.25
mM.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
9
If a disulphite compound is used it is preferably added in an amount to give a
final solution which is half of the concentration used for the corresponding
monosulphite compound.
The pH of the solution in the carbohydrate compartment is preferably
between pH 2.0-7.5, more preferably pH 2-5.5, even more preferably pH 3-4 and
most preferably about pH 3.2. The pH of the final solution is preferably
between
5.0 - 8.0, more preferably between 6.5 - 8.0, most preferably between 7.0 -
7.5 or
absolutely most preferably 7.4.
Additionally, in a preferred embodiment, the multiple compartment container
containing the medical solution is sterilised. Any conventional methods and
apparatus for sterilisation may be used, such as those mentioned under the
definition
of the term "sterilisation". Preferably the sterilisation is performed by heat
treatment
most preferable at about 121° C for 20 minutes (Ph. Eur. (current)).
Solution
The invention further relates to a medical solution comprising at least one
sulphite compound to be included for the ability to reduce the concentration
of
decomposition products formed from a carbohydrate by stabilisation or
scavenging
already formed decomposition products, obtained during sterilisation and/or
storage
of solutions containing carbohydrate compounds, as defined above. Furthermore
the
"sulphite compound" could be used as an antioxidant or to scavenge toxic or
allergenic compounds iyz vivo, such as in the body fluids.
The invention further relates to a medical solution such as a solution used
for
peritoneal dialysis either in a single or a multiple compartment container,
comprising at least one sulphite compound to be included for the ability to
reduce
decomposition of a carbohydrate present in the solution exposed to
sterilisation.
If a monosulphite compound such a bisulphite is used, it is preferably added
to the carbohydrate and/or the electrolyte compartment in an amount to give a
final
solution with a concentration within the range of 0.01 - 10 mM, preferably
0.05 - 1
mM, most preferably about 0.05 - 0.5 mM. If a disulphite compound is used it
is
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
preferably added to the carbohydrate compartment in an amount to give a final
solution with a concentration within the range of 0.005 - 5 mM, preferably
0.025 -
0.5 mM, most preferably 0.025 - 0.25 mM.
If a disulphite compound is used it is preferably added in an amount to give a
5 final solution which is half of the concentration used for the corresponding
monosulphite compound.
The pH of the carbohydrate solution is not critical and could be in any range,
suitable the pH is between pH 2.0-7.5, more preferably pH 2-5.5, even more
preferably pH 3-4 and even more preferably about pH 3.2. The pH of the final
10 solution is preferably between 5.0 - 8.0 and more preferably 6.5 - 8.0 and
most
preferably 7.0 - 7.5.
Preferably the medical solution is a sterile medical solution.
Additionally, the sulphite compound may be provided to the carbohydrate
solution prior or after sterilisation.
Furthermore, the sterilisation is performed using any conventional
sterilisation method as defined above under the term "sterilisation".
Preferably the
sterilisation is performed by heat treatment within the range of 100-
150° C, for 1-
130 minutes, more preferably at 121° C for 20 minutes (Ph. Eur.
(current)).
The solution may be any medical solution which comprises a carbohydrate
with or without other components. Preferably the solution is a medical
solution such
as a solution used for peritoneal dialysis, preferably medical solutions) for
single or
multiple compartment containers) for peritoneal dialysis, more preferably two
or
three compartment containers, even more preferably a three compartment
container.
Medical solutions used for peritoneal dialysis preferably contain glucose in
an
amount to give a glucose concentration in the range of 1.5 to 4%, preferably
about
1.5, 2.5 or 4 % by weight in the final solution (based on the total final
solution).
Additionally the medical solution is a solution used to scavenge toxic or
allergenic compounds in vivo, preferably a medical solution used to scavenge
toxic
or allergenic compounds in body fluids.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
11
Method
The invention further relates to a method for stabilising or scavenging the
decomposition of carbohydrate components produced in a medical solution during
sterilisation and/or storage, comprising providing a sulphite compound to the
solution prior or after the sterilisation process in order to reduce
decomposition of
the carbohydrate components in the medical solution.
Preferably the method is used for preparation of medical solutions) used for
peritoneal dialysis.
Even more preferably the medical solution (s) is/are used in a multiple
compartment container for peritoneal dialysis, such as a three compartment
container.
Preferably, the method is used for preparation of a multiple compartment
container used for peritoneal dialysis, wherein the sulphite compound may be
added
either to the carbohydrate compartment or to the electrolyte compartment of a
multiple compartment.
If a monosulphite compound is used it is preferably added to the carbohydrate
solution in an amount to give a concentration in the final solution within the
range
of 0.01 - 10 mM, preferably 0.05 - 1 mM, most preferably 0.05 - 0.5 mM.
If a disulphite compound is used it is preferably added to the carbohydrate
solution in an amount to give a concentration in the final solution within the
range
of 0.005 - 5 mM, preferably 0.025 - 0.5 mM, most preferably 0.025 - 0.25 mM.
When a disulphite compound is used it is also preferably added in an amount
to give a concentration in the final solution which is half of the
concentration used
for a corresponding monosulphite compound.
The pH of the carbohydrate compartment is preferably between pH 2.0-7.5,
more preferably pH 2-5.5, even more preferably pH 3-4 and most preferably
about
pH 3.2.
More preferably the method is used for the preparation of a sterile multiple
compartment.
Additionally, the sulphite compound is provided to the carbohydrate solution
prior or after sterilisation.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
12
Furthermore, the sterilisation is performed using any conventional
sterilisation method as defined above under the term "sterilisation".
Preferably the
sterilisation is performed by heat treatment within the range of 100-
150° C, for 1-
130 minutes, more preferably at 121° C for 20 minutes (Ph. Eur.
(current)).
The method according to the invention is intended to be used for medical
solutions, in which the medical solution needs to be sterile, by a method as
defined
under the term "sterilisation", and preferably the method will be used for the
preparation of medical solutions used for peritoneal dialysis or the like. By
way of
adding a sulphite compound in small amounts to the solution either prior or
after
sterilisation, decomposition of the carbohydrate components into toxic
compounds
in the solution is prevented or the toxic compounds are scavenged. Preferably,
medical solutions to be used for peritoneal dialysis, preferably contain
glucose in an
amount to give a glucose concentration range between 1.5 to 4%, preferably
about
1.5, 2.5 or 4 % by weight in the final solution.
Furthermore the sulphite compound could be used as an antioxidant or to
scavenge toxic or allergenic compounds in vivo, such as in the body fluids.
Additionally the invention provides the use of a carbohydrate containing
solution for the preparation of a multiple compartment container, preferably a
three
compartment container, suitable for peritoneal dialysis.
Specifically the invention provides the use of a carbohydrate containing
solution for the preparation of a multiple compartment container for the
treatment of
an animal in need thereof.
MATERIALS AND METHODS
Determination of glucose degradation products:
Chemicals: Acetonitrile (Lab Scan, Ireland) and methanol (Lab Scan,
Ireland) were of HPLC grade. 2,3- diaminonaphtalene was supplied by ICN, USA.
3-deoxyglucosone 56 % (weight/weight) was synthesised by T. Henle Technische
Univeritat Dresden. Sodiumphosphate p.a. and Glyoxal 30 % (weight/volume)
supplied by Merck (Germany), methylglyoxal 40 % (weight/volume), 2,4-di-
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
13
nitrophenylhydrazine (2,4-DNPH) and 1,2-phenylenediamine were supplied by
Sigma Chemical (USA). Acetaldehyde p.a. was supplied by Fluka (Germany).
Equipment: Two HPLC systems were used for the determination of glucose
degradation products (GDP). One HPLC consisted of an Hewlett Packard liquid
chromatograph serie 1050 equipped with an UV-detector and an autosampler. The
second HPLC system consisted of an Hewlett Packard liquid chromatograph serie
1100 equipped with an autosampler and Waters Refractive Index detector model
410. Hewlett Packard Chem Station software rev. A.06.03, NT 4.0 was used for
the
data handling.
Determination of 3-deoxyglucosone (3-DG): 3-DG was determined using
2,3-diaminonaphtalene as derivative reagent. The samples were diluted 50 times
to a
total volume of 1 ml prior to analysis. The standards were prepared in the
range 1-6
~M. Standards and samples were prepared by adding 100,10.1% (2,3-
diaminonaphtalene to 1 ml sample and incubated for 16 hours in room
temperature
in dark. The analytical column was a Water Symmetry C18 column (5 ~,m, 25 cm
x4, 6 mm). The elution of the substance was performed at constant flow rate of
1.0
ml/min by using a gradient of acetonitrile/water. The percentage of
acetonitrile/water (volume/volume) was initially 25175, and 12 minutes later
25/75,
at 15 minutes 60/40 and at the gradient stop 30 minutes 60/40. The wavelength
was
set at 268 nm and the injected volume was 20 ~.1. The limit of quantification
was
1 ~,M.
Determination of acetaldehyde and formaldehyde: The samples for the
determination of acetaldehyde were diluted 20 times to a final volume of 4 ml,
prior
to analysis. Acetaldehyde was prepared as hydrazone derivatives using 2,4-DNPH
as derivative reagent. The standards were prepared in range 1.1 - 11.4 ~,M
acetaldehyde, and 1.7-16.7 ~M formaldehyde. Standards and samples were
prepared
by adding 2 ml 0.08 % (weight/volume) 2,4-DNPH to 4 ml of each sample. The
sample were concentrated on a solid phase extraction C18 column (Bond Elut LRC
200 mg/3 ml) and after rinsing with water, eluted with 1.6 ml acetonitrile.
The
analytical column was a Supelco C18 column (5 Vim, 15 cm x 4,6 mm). The
elution
of the substances was performed at constant flow of 1.7 ml/min by using a
linear
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
14
gradient of acetonitrile/water. The percentage acetonitrile/water
(volume/volume)
was initially 35/65 and at the gradient stop 12 minutes later 80/20. The
wavelenght
was set at 240 nm and the injected volume was 20 ~.1. The limit of
quantification
was for acetaldehyde. 1.1 ~M. And for formaldehyde 1.7 ~M.
Determination of glyoxal and methylglyoxal: Glyoxal and methylglyoxal
were determined as quinoxalines using 1,2-phenylenediamine. The standards were
prepared in the range 3.5-51.7 ~,M methylglyoxal. Standards and samples were
prepared by adding 0.6 m10.4 % (volume/volume) 1,2-phenylenediamine to 1 ml of
each sample. The analytical column was Supelco C18 column (5 ~,m, 25 cm x 4,6
mm). The elution of the substances was performed at constant flow of 1.0 ml /
min
using a mobile phase of initial 25 % (volume/volume) acetonitrile and 75
(weight/volume) 0.05 M sodiumphosphate. At the gradient at 6 minutes the
mobile
phase was 30 % acetonitt-ile and 70 % millipore water and at gradient stop 9
minutes
the percentages were 25/75. The wavelength was set at 312 nm and the injected
volume was 20 ~,1. The limit of quantification for glyoxal was 3.5 ~,M and for
methylglyoxal 2.8 ~,M.
In vitro assa.~. otoxicitx
Medical solutions used for peritoneal dialysis were mixed with one part cell
growth medium and 10% (volume/volume) fetal calf serum was added (Wieslander
et al., 1991, Kidney Int. 40:77-79). Basal cytotoxicity of medical solution
used for
peritoneal dialysis were determined on mouse fibroblasts cells L-929 (CCL-1;
ATTC, Rockville, MD, USA) as described earlier (Wieslander et al. 1993,
Advances in Peritoneal Dialys, 9:31-35) and expressed as inhibition of
cellgrowth
(ICG).
EXAMPLE 1.
Three compartment container with sulphite in the glucose compartment
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
A multiple compartment container as shown in FIG. l, containing following
medical solutions in the three compartments 44, 45 and 9.
Compartment 44 containing 100 ml of the comaosition:
5 glucose 30%
calcium 20 mM
magnesium 5 mM
sodium 132 mM
bisulphate 1 mM
10 pH 3.2
Comaartment 45 contains 100 ml of the combosition:
glucose 50%
calcium 33 mM
15 magnesium ~ mM
sodium 132 mM
bisulphate 1 mM
pH 3.2
Compartment 9 contains 1900 m1 with the composition:
bicarbonate 40 mM
sodium 132 mM
pH 6.7
By mixing the contents of compartment 44 and compartment 9, a final
solution suited for peritoneal dialysis is obtained with the following
concentrations:
glucose 1.5%
calcium 1.0 mM
bicarbonate 3~ mM
sodium 132 mM
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
16
magnesium 0.25 mM
bisulphite 0.05 mM
By mixing the contents of compartment 45 and compartment 9, a final
solution suited for peritoneal dialysis is obtained with the following
concentrations:
glucose 2.5%
calcium 1.65 mM
bicarbonate 38 mM
sodium 132 mM
magnesium 0.4 mM
bisulphite 0.05 mM
By mixing the contents of both compartments 44 and 45 with the contents of
compartment 9, a final solution suited for peritoneal dialysis is obtained
with the
following concentrations:
glucose 4.0%
calcium 2.5 mM
bicarbonate 36 mM
sodium 132 mM
magnesium 0.6 mM
bisulphite 0.1 mM
EXAMPLE 2.
Three compartment container with sulphite in electrolyte compartment
A multiple compartment container as shown in FIG. 1, containing following
medical solutions in the three compartments 44, 45 and 9.
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
17
Compartment 44 contains 100 ml of the comaosition:
glucose 30%
calcium 20 mM
magnesium 5 mM
sodium 132 mM
pH 3.2
Combartment 45 contains 100 ml of the composition:
glucose 50%
calcium 33 mM
magnesium 8 mM
sodium 132 mM
pH 3.2
Comaartment 9 contains 1900 ml with the composition:
bicarbonate 40 mM
sodium 132 mM
bisulphate 0.1 mM
pH 6.7
By mixing the
contents of compar~nent
44 and compartment
9, a final
solution suited peritoneal dialysis is obtained with the following
for concentration:
glucose 1.5%
calcium 1.0 mM
bicarbonate 38 mM
sodium 132 mM
magnesium 0.25 mM
bisulphate 0.095 mM
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
18
By mixing the contents of compartment 45 and compartment 9, a final
solution suite for peritoneal dialysis is obtained with the following
concentration:
glucose 2.5%
calcium 1.65 mM
bicarbonate 38 mM
sodium 132 mM
magnesium 0.4 mM
bisulphite 0.095 mM
By mixing the contents of both comparhnents 44 and 45 with the contents of
compartment 9, a final solution suited for peritoneal dialysis is obtained
with the
following concentration:
glucose 4.0%
calcium 2.5 mM
bicarbonate 36 mM
sodium 132 mM
magnesium 0.6 mM
bisulphite 0.090 mM
EXAMPLE 3
Two compartment container with sulphite in glucose compartment or three
compartment container with both glucose compartments mixed.
The solutions used in example 3 and 4 were as described in example 1 and 2
except for that 50 % glucose was used in all glucose compartments giving
slightly
different volumes for the compartments.
Two different sets of solutions were prepared, one electrolyte compartment
with the volume 1.875 L and one glucose containing compartment with different
amounts of bisulphite added, this volume was 125 ml. The solutions were
sterilised
at 121 ° C for 1 hour and mixed post sterilisation. The concentrations
post mixing of
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
19
electrolytes were 132 mM Na+, 1.35 mM Ca+, 0.25 mM Mg2~, 95.2 mM Cl- and 40
mM lactate.
The concentration of glucose were 4 % (w/v) and the concentration of
sulphite were 0. 0.01, 0.05, 0.1, 0.2 and 0.5 mM in the final solution.
The sterilised solutions were analysed in the Ih vats°o assay of
cytotoxicity
mentioned under Materials & Methods. The results from the assay showed that an
increase of the sodium bisulphate resulted in a decrease of the ICG value,
which
means that there is a decrease in the content of the toxic decomposition
products
after addition of a sodium bisulphate.
EXAMPLE 4
Two com~aartment container with sulphite in electrolyte compartment or three
compartment container with both glucose compartments mixed
As described in example 3 except for that sulphite was added to the
electrolyte compartment. The concentrations of electrolytes, glucose and
sulphite in
the final solution were as in example 3. The sterilised solutions were
analysed as in
example 3. The results from the assay showed that an increase of the sodium
bisulphate resulted in a decrease of the ICG value, which means that there is
a
decrease in the content of the toxic decomposition products.
EXAMPLE 5
Analysis of a solution with or without sulphite for the presence of
formaldehyde
acetaldehyde and ICG.
Three solutions containing 132 mM Nay, 1.35 mM Ca2+, 0.25 mM Mgr'+, 95.2
mM Cl-, 40 mM lactate and 1.5 % glucose was heat sterilised at 121° C
for 1 hour.
The sterilised solutions were mixed with sodium bisulphate to three different
concentrations of sodium bisulphate (0, 0.5, 1 mM).
Three solutions containing 132 mM Na+, 1.35 mM Ca2+, 0.25 mM Mg2+, 95.2 mM
Cl-, 40 mM lactate and 1.5 % glucose were mixed with sodium bisulfate to
different
CA 02403574 2002-09-18
WO 01/89478 PCT/SE01/01125
concentration of sodium bisulphate (0, 0.5, 1 mM) and was heat sterilised at
121° C
for 1 hour.
The sterilised six solutions were analysed in the Iya vitro assay of
cytotoxicity
and determination of acetaldehyde aid foy°maldehyde mentioned under
Materials &
5 Methods. The results from the Ih vats°o assay of cytotoxicity showed
that an increase
of the sodium bisulphate resulted in a decrease of the ICG value, which means
that
there is a decrease in the content of the toxic decomposition products. The
results
from the assay " determination of acetalde7~yde aszd formaldehyde" showed de-
creased levels of both acetaldehyde and formaldehyde in the solutions
containing
10 sodium bisulphate, even down to levels under detection limit.