Note: Descriptions are shown in the official language in which they were submitted.
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
METHOD AND APPARATUS FOR OBJECTIVELY MEASURING PA1N_,
PAIN TREATMENT AND OTHER RELATED TECHNIQUES
FIELD OF THE INVENTION
This invention relates to non-invasive measurement methods and systems and
more
particularly to a method and apparatus for measuring indices of brain activity
during acute and
chlronic pain, and the ability to measure treatment effects on acute or
chroluc pain. It is also a novel
method for determining quantitative indices from neLUOimaging signals.
BACKGROUND OF THE INVENTION
As is 1110W11 111 the art, magnetic resonance imaging (MRI) (also referred to
as nuclear
magnetic resonance or NMR) and other non-invasive techniques such as
functional magnetic
resonance imaging (fMRI), magnetic resonance spectroscopy (MRS),
electroencephgraphy (EEG),
magnetoencephalography (MEG), positron emission tomography (PET), optical
imaging (OR),
single phOt011 eln1SS1011 COlnpLlter tomography (SPELT), filnctional
computerized tomography (fCT)
have been proposed to be able to directly examine a combination of brain
(cortical and subcol-tical),
brainstem and spinal cord regions in hlunans for the evaluation of acute and
cluonic pain states,
analgesic responses, therapies including pharmacological or gene products, and
placebo responses.
To date, this goal has not been accomplished. The major hurdle to this
proposed goal has
been the inability to define an objective set of indices that characterize the
pain state, its progression
over time and its alteration tluough intervention.
Pain is a complex response that has been functionally categorized into
sensory, adaptive, and
affective components. The sensory aspect includes information about stimulus
location and
intensity while the adaptive component may be considered to be the activation
of endogenous pain
modulation and motor plalming for escape responses. The affective component
appears to include
evaluation of pain Lmpleasantness and st1111L1hL1S tlueat as well as negative
emotions triggered by
memory and context of the painftil stimulus. Extensive electrophysiological
research in alumals has
defined likely neu roanatomical substrates for some of the sensory attributes
of pain, SLlch as
localization and intensity, and some of the adaptive responses, such as
descending analgesia. Other
regions activated by painful stimuli have also been identified which may be
involved in the affective
response, however the neural substrates for the motivational and emotional
response to pain remain
3 5 a topic of debate.
1
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Ronald Melzaclc and Kenneth Casey state " To consider only the ser2sory
features of pain,
and ignore its motivational and affective properties, is to look at only part
of the problem, not even
the most important part at that". In Donald Price's treatise on the Psychology
of Pain, he defines
pain as a somatic perception containing: (1) a bodily sensation with qualities
like those reported
during tissue-damaging stimulation; (2) an experienced threat associated with
this sensation and
(3) a feeling of unpleasantness or other negative emotion based on this
experienced threat.
To date, although there are clear affective, motivational and emotional
components of pain that
can be evaluated subjectively, a clear delineation of the neural circuitry
involved in the motivational
and emotional aspects of pain are only begirming to be evaluated in animal
models. A typical
current formulation of CNS systems involved in the evaluation of pain
intensity (algosity) and
unpleasantness ("classic pain circuitry") is presented in "Pain An Unpleasant
Topic," Pain 1999
Suppl. 6 ~61-69, H. L. Fields.
Despite hypotheses about what was constitutes "classic pain circuitry", the
ISSUe Of WhlCh
brain regions process sensory information vs. those that mediate affective
responses remains an area
of active discussion. Indeed, it is unclear whether unpleasantness is a
sensation or an emotion.
Another approach for determining which neuroanatomical regions mediate
emotional processes
regarding pain stimuli might focus on those regions 1a10w11 to be active for
motivational processes
which underlie emotion. When animals organize behavior in response to aversive
or rewarding
stimuli, they respond to multiple informational dimensions of these goal-
objects or events. These
informational dimensions include rate, delay, incidence, intensity and amount
and location of the
stimulus. A nLUnber of brain regions have been consistently implicated in the
organization of
?5 responses to aversive and rewarding stimuli in animals. More recently,
these regions have been
specifically implicated in reward processes in h lunans. These regions, which
include the nucleus
accwnbens (NAc), the sublenticular extended amygdala of the basal forebrain
(SLEA), the
amygdala, the ventral tegmentlun (VT) and the orbital gyrus (GOb), have been
shown to be
activated in stLldies of drug-associated reward; in general, these regions are
thought to be important
for irrforrnation processing in the service of emotional and motivational
states. Traditionally, these
regions have been considered in the domain of rewarding rather than aversive
stimuli, though, it has
been previously postulated that pain and reward are at opposite ends of the
same behavioral
spectrum.
2
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Motivational states (including aversive states such as pain) which lead to
goal-directed
behavior depend on a complex informatics system comprised of a set of
subprocesses for the
moment-by-moment modulation of behavior. The informatics subprocesses can be
grouped into
three general categories for (1) perceptual processing of goal-objects and
other putative rewards,
(2) valuation of goal-object woz-th, and (3) approximation of temporal
infolznation and conditional
probabilities about the potential reward. The amygdala appears to be a central
component of the
brain circuitry mediating the first informatics subprocess, while other
regions such as tile
sublenticutear extended amygdala (SLEA) of the basal forebrain, and the
IlLlCheLlS aCClllnbellS (NAc)
appear to be central to the second and third subprocesses respectively. In
regard to reward fiu lction,
input from the dopalninergic neurons ofthe ventral tegmentlun (VT) to the
alnygdala, SLEA, and
NAc is an impol-tant featLlre of this extended system. To date objective
indices of function in these
regions have not been directly comlected to the perception, evaluation, and
integration of painful
St1111L1h.
Recent neu roimaging studies have sought to define the principal CNS
structures involved
in the perception, evaluation and integration of painful stimuli. These
studies have contributed to
oLlr understanding of the complex natlue of the CNS response to pain but have
not clearly separated
circuitry involved in reward/aversion and emotion from circuitry involved With
sensory processing.
Direct interrogation of any brain circuitry to objectively define the pain
state has hithertofore not
been accomplished.
One lneans for evaluating the brain circuitry mediating acute and chronic
palll lIIVOIVeS
"invasive" approaches. These approaches, have been predolninalltly restricted
to animal research
and methods such as placing electrodes into the brain of an animal for
electrical recordings, or
sacrificing the animal to collect brain tissue for cell culture,
immunohistochemistry or other
molecular biological techniques.
It would, be desirable to provide a technique and system to non-invasively
interrogate the
brain of an individuate hlunal>/animal regarding acute and chronic pain. It
would be further desirable
to be able to objectively assess pain in humans or allilnals, or the effects
of therapeutic interventions
Oll aCLlte alld Cte1T0111C pa111.
SUMMARY OF THE INVENTION
In accordance with the present invention, a system includes a non-invasive
measurement
3
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
apparatus for obtaining signals of central nervous system (CNS) activity, a
localization
processor, coupled to the non-invasive measurement system, for localizing
signals to specific
anatomical and flmctional brain regions, a correlator for correlating an
experimental process to
brain activity and a processor for interpreting the result of the correlation
to a specific
application.
With this pal-ticuhar arrangement, a system for measuring indices of brain
activity dluing
motivational and emotional function is provided. It should be appreciated that
the non-invasive
measluement apparatus may be provided aS Olle WhICh CaI11I11pIeInellt fMRI,
PET, IR, SPECT, fCT,
MRS, MEG and EEG or other tecluuques to non-invasively measure indices ofbrain
activity during
motivational and emotional function. The CNS signal processor and the
correlation processor
cooperate to determine indices of brain activity during motivational axed
emotional function. Once
CNS signals are obtained, the signals are localized to examine the function in
a particular region of
the brain. The particular malmer in which such the signals are localized are
dependent upon a
variety of factors including but not limited to the technique or techniques
(111Chudlllg eqtllplnellt)
used to extract the signals. Once signals are extracted, the correlation
processor correlates
empirical data with the 122eaSLIred 51g11a1S alld interprets the results of
the correlation to a specific
application. It should be appreciated that although the CNS apparatus and
correlation processors
are described as separate and distinct pieces of equipment, in practice the
functions performed by
these pieces of equipment may be performed by a single processor or by more
than one processor.
In accordance with a fiuther aspect of the present invention, a method for
measuring
indices of brain activity dining motivational and emotional function includes
the steps of non-
invasively acquiring central nervous system (CNS) signals, statistically
analyzing and then
localizing the CNS signals to specific anatomical and functional brain
regions, evaluating the
CNS signahs with regard to patterns of activity within and between functional
brain regions, and
interpreting the results of the correlation to a specific application. With
this pal-ticular
arrangement, a technique for measuring indices of brain activity dluing
motivational and
emotional flmction is provided. In one embodiment, the CNS signals are
acquired (e.g. via an
MRI, PET or other non-invasive measurement system) while the subject lmdergoes
one or more
experimental paradigms focused on one or snore motivatioll/emotion processes.
In other
embodiments, the CNS signals are acquired while the subject is exposed to
certain stimulus (e.g.
the subject views photographs of people or food or consumer products) or while
the subject
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
performs particular tasks (e.g. presses a bar to get a particular result).
Alternatively, the subject
could perform some combination of the above tasks.
Data associated with the experimental/paradigm is correlated with patterns of
activity
and other measures.
In the step of interpreting the results of the core elation to a specific
application, the
subject's brain response to a laiown stimulus in a particular application is
measured. For
example, if a subject is being tested to determine whetller or hOW 111L1Ch
they like a particular
I0 pTOdLICt, the a111OLlllt alld/Or II1te11Slty Of aCtlvlty 111 CeI'talll
reg10115 Of tile SLlb~eCt'S bTaln 1S
compared with signals from the subject's brain (or from a database of known
brain region
responses) in response to stimuli considered to elicit from a subject
responses with a limited
variance (e.g., extreme liking vs. extreme aversion). Based upon this
information, a
determination can be made as to whether or Ilow much the subject liked the
particular product.
IS The comparison call be based on one or more of spatial, temporal,
integration-derivative
characteristics, moment analysis, Laterality, synchrony, volume, differential
power function,
power spectrum analysis and Ixlatrix values. In one embodiment for example,
brain responses in
the alnygdala region of the brain is evaluated for habituation to aversion
stimuli. If it does slot
habituate at or below a population normed average then individuals who are
being tested with
20 the diagnosis of obsessive compulsive disorder will not be referred for
behavioral therapy since
a common component of behavioral therapy is the ability to habituate or be de-
conditioned to
aversive stimuli.
BRIEF DESCRIPTION OF THE DRAWINGS
25 The foregoing features of the invention, as well as the invention itself
may be more fully
Lmderstood from the following detailed description of the drawings, in which:
Fig. 1 is a flow diagram showing a general method for measuring indices of
central
nervous system activity during motivational and emotional function and
determining indices of
30 brain activity during motivational and emotional function;
Fig. 2A is a schema of brain functional illness and its relationship to
motivatioll/emotion
fL111Ct1011;
35 Fig. 2B is a schema detailing fimctional illnesses that can be the sequelae
of chronic
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
pain;
Fig. 2C is a generalized schema which illustrates three phases of motivational
flmction;
Fig. 2D is a schema dissecting one of the three phases of motivational
filnction into its
StlbCOlllpOllellt5;
Fig. 3 is a block diagram of brain circuitry of reward and aversive flulction
alld illustrates
brain anatomy of reward and aversive function that is implicated in motivated
behavior;
Fig. 3A is a plot of signal strength from the Left nucleus acculnbens vs. time
for
morphine infusions;
Fig. 3B is a plot of signal strength from the Left 11LIC1eLIS aCCLlIllbe115
VS. time for morphine
infusions;
Fig. 3C is a plot of signal strength front the left and right nucleus
accumbens vs. time for
morphine infusions;
Fig. 3D is a plot of signal strength from the left and right nucleus accumbens
vs. time for
saline infusions;
Fig. 3E, is a statistical activation map of significant signal change in the
right nucleus
acclunbens dtllhllg a painful stimulus;
Fig. 3F is a plot of signal strength change in the rigllt nucleus accunlbens
vs. time;
Fig. 3G is a blocl~ diagram of limbic and paralimbic brain regions observed in
drug
studies;
Fig. 3H, is a series of plots showing absolute fMRI signals reflecting
expectancy
responses for six regions of interest in reward regions vs. time;
6
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 3I, is a series of plots showing absolute fMRI signals for four regions
of interest in
reward regions vs. time for three different outcomes on each spinner;
Fig. 3J is a plot of signal change vs. time for the SLEA;
Fig. 3K is a diagram of a portion of a bralll ShOW111g early phase activation
of the SLEA
brain region in response to an aversive thermal stimulus;
Fig. 3L is a diagram of a portion of a brain showing no late phase activation
of the SLEA
brain region to an aversive thermal stimulus;
Fig. 3M is a diagram of an early phase activation map of the primary
somatosensory
cortex (SI) in response to an aversive thermal stimulus;
I S Fig. 3N is a diagram of a Late phase an activation map of the primary
somatosensory
col-tex (SI) in response to a an aversive thermal stimulus;
Fig. 30 is a plot of signal change vs. time of a signal in the primary
somatosensory
cortex (SI) of a brain;
Fig. 4 is a block diagram of a noninvasive measurement apparatus and system
for
measuring indices of brain activity dLlring motivational and emotional
function;
Fig. 5A is a flow diagram illustrating the general phases of a
motivationallelnotional
mapping process (MEMP) According to the present invention;
Figs. SB-SD are a series of flow diagrams illustrating a MEMP schema for
mapping
motivational/emotional response;
Fig. 6 is a diagram illustrating a nLUnber of distract spatial scales of CNS
fzmction, and
the tec1ll11C~t1eS SLlch aS 11et1TOllllaglllg used to interrogate these
scales.
Fig. 7A is a diagram of a portion of a brain showing activation ofthe aCG
brain region in
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
response to a thermal stimulus;
Fig. 7B is a plot of signal change vs. tune of a signal in aCG brain region in
response to a
thermal stimulus;
Fig. 7C is a diagram of a portion of a brain showing activation of the aCG
brain region in
response to a painful thermal stimulus;
Fig. 7D is a plot of signal change vs. time of a signal in aCG brain region in
response to a
painful thermal stimulus;
Fig. 7E is a diagram of a portion of a brain showing activation of the NAc
brain region in
response to a thermal stimulus;
Fig. 7F is a plot of signal change vs. time of a signal in NAc brain region in
response to a
thermal stimulus;
Fig. 7G is a diagram of a portion of a brain showing activation of the NAc
brain region in
response to a painful thermal stimulus;
Fig. 7H is a plot of signal change vs. time of a signal in the NAc brain
region in response
to a painful thermal stimulus;
Fig. 7I is a plot of signal change vs. time of a signal in the Gob brain
region in response to
a painful thermal stimulus;
Fig. 7J is a plot of signal change vs. time of a signal in the VT/PAG brain
region in response
to a painful thermal 5t11nL1111S;
Fig. 8A is a diagram of a portion of a brain showing activation of the aCG
brain region in
response to a thermal stimulus and an application of capsaicin;
Fig. 8B is a plot of signal change vs. tune of a signal in aCG brain region in
response to a
thermal stimulus and an application of capsaicin;
s
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 8C is a diagram of a portion of a brain showing activation of the NAc
brain region in
response to a thermal stimulus and an application of capsaicin;
Fig. 8D is a plot of signal change vs. time of a signal in NAc brain region in
response to
a thermal St1mL1111S alld an application of capsaicin;
Fig. 9A is a diagram of a portion of a brain showing activation of the aCG and
NAc brain
regions of a subject with neuropathic pain in response to a thermal stimulus;
Fig. 9B is a plot of signal change vs. time of a signal in aCG brain region of
a subject with
nemopathic pain in response to a thermal stimulus;
Fig. 9C is a plot of signal change vs. time of a signal in NAc brain region of
a subject with
neuropathic pain in response to a thermal stimulus ;
Fig. 10A is a diagram of a portion of a brain showing activation of the NAc
brain region in
response to a painful thermal stimulus and an infusion of saline;
Fig. l OB is a plot of signal change vs. time of a signal in NAc brain region
in response to
a painful thermal stimulus and an intravenous infusion of saline;
Fig. lOC is a diagram of a portion of a brain showing activation of the NAc
brain region in
response to a painful thermal stimulus and all 111traVe110L1S 111f11S1011 of
lnOrphllle;
Fig. l OD is a plot of signal change vs. time of a signal in NAc brain region
in response to
a painful thermal stimulus and an intravenous infusion of morphine;
Fig. 10E is a diagram of a portion of a brain showing activation of the VT/PAG
brain region
in response to a thermal stimulus during the intravenous administration of
naloxone;
Fig. l OF is a plot of signal change vs. image nlunber of a time coluse of a
signal in VT/PAG
brain region in response to a thermal stimulus before and dLUing the
intravenous administration of
naloxone;
9
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 11 is a diagram of a system for determining central nervous system
activity in
reward/aversion cir cuitry;
S Figs. 1 lA-11K are a series of figures which illustrate quantities derived
from WCA
waveform based correlation;
Figs. 12A -12F, axe a series of figures which illustrate activation in tile
brainstem region
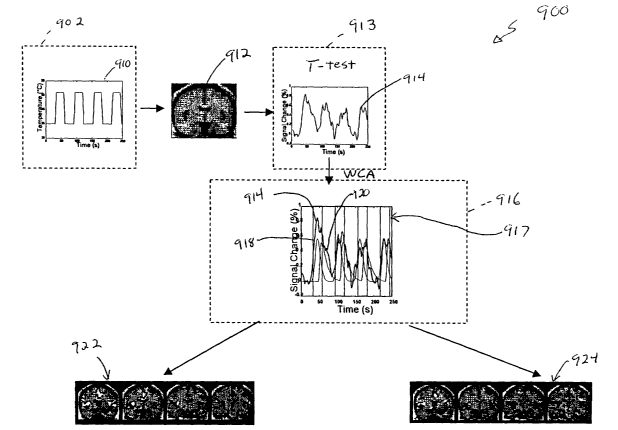
spV following noxious heat (46°C) applied to the sl{in of the face;
Figs. 13 and 13A illustrate activation in the brainstem region spV and
thalamus
following allodynia produced by a heat-capsaicin model in a healthy volunteer.
Fig. 14A is a diagram of a portion of a brain showing activation of the NAc
brain region of
subjects during brush-induced allodynia in a subject with chronic pain;
Fig. 14B is a plot of signal change vs. time of a signal in the NAc brain
region of
subjects dLlrlng brllSh-111d11Ced allodynia in a subject Wlth ChrO111C palll;
Fig. 15 is a set of statistical maps showing brain activation for men (left
column), women
in the mid-follicular stage (middle column), and Women in the mid-luteal phase
(right column)
for the perforated cortex (top row), insula (middle row), and aCG (bottom
row);
Fig. 15A is a plot of signal change vs. time for the mean he1110dyna1111C
TeSpOIISe for all
signiflcalltly activated voxels in the brain for men;
Fig. 15B is a plot of signal change vs. time for the lneall hemodynalnic
response for all
significantly activated voxels in the brain for women in the mid-follicular
stay of their menstrual
cycle;
Fig. 15C is a plot of signal change vs. time for the mean hemodynamic response
for all
significantly activated voxels in the brain for women in the mid-luteal stage
of their menstlmal stage;
Fig. 16 is a schematic diagram of a method for rapid drug evaluation in
hLUnans;
and
to
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 17 is a flow diagram of a method to image CNS regions in the brainstem
(trigeminal
nucleus).
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Before proceeding with a description of the invention, some terminology is
explained.
As used therein below, the term "central nervous system" or "CNS" as referred
to in the
descriptions below includes both the brainstem and spinal cord regions.
Reference is also made
herein to noninvasively ObtalI11I1g signals of a CNS. Such references refer to
recording CNS
signals noninvasively. It should be appreciated that in some applications it
may be desirable or
necessary to inject a substance (e.g. a dye or other substance) into a subject
prior to recording
the CNS signals. The signal responses, however, are still measLUed in a
noninvasive malmer.
Referring now to Fig. 1, a flow diagram shows the processing to determine
indices of
Central Nervous System (CNS) activity during motivational and emotional
function. Such
processing may be performed by a processing apparatus which may, for example,
be provided as
part of noel-invasive measLlrement system such as that to be described below
in conjunction with
Fig. 4.
In the flow diagram of Figs. 1 and SA - SC, the rectangular elements in the
flow
diagrams are herein denoted "processing blocks" and represent computer
software instructions
Or grOLlpS Of 111StrLlCt1011S.
Alternatively, the processing blocks represent steps performed by
fiulctionally equivalent
C1TCL11tS Such aS a digital signal processor circuit or an application
specific integrated circuit
(ASIC). It should be appreciated that some of the steps described in the flow
diagram may be
implemented via computer software while others may be implemented in a
different planner e.g.
via an empirical procedure. The flow diagrams do not depict the syntax of any
particular
programming language. Rather, the flow diagrams illustrates the fiulctional
information one of
ordinary skill in the art requires to fabricate circuits or to generate
computer software to perform
the processing required of the particular apparatus. It should be noted that
many routine
program elements, such as initialization of loops and variables alld the use
of temporary
variables are not shown. It will be appreciated by those of ordinary shill in
the art that Lmless
11
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
otherwise indicated herein, the particular sequence of steps described is
illustrative only amd can
be varied without departing from the spirit of the invention.
Turning now to Fig. 1, processing begins in step 10 in which after positioning
subjects to
be tested (e.g. persons who are Lender going a lie detection test) and
instructing the subjects to
remain as still as possible, CNS signals are acquired. A measuring apparatus
which non-
invasively obtains the GNS signals is used, hl one embodiment, the subject to
be tested is
placed in a brain ~camier of an MRI, fMRI, MEG, fCT, OI, SPECT, or PET system
of the type
to be described below in conjLmction with Fig. 4.
The CNS signals can be acquired while the subject undergoes an experimental
paradigm
focused on one or more "motivation/emotion" processes. Alternatively, the CNS
signals can be
acquired while the subject is exposed to certain stimuli (e.g. the subject
views photographs of
people or food or consumer products) or while the subject performs particular
taslcs (e.g. presses
a bar to get a particular result). Alternatively still, the subject can
perform two or more of the
above tasks while the CNS signals are obtained.
Processing then proceeds to step 11 where the non-invasively obtained CNS
signals are
statistically analyzed and then localized to specific anatomical and
flmctional brain regions. The
details of the processes for statistically analyzing the CNS signals and
localizing the signals to
specific brain regions are described below in conjunction with Figs. 3-30 and
SA-SC.
Processing next proceeds to processing step 12 where the CNS signals obtained
in step
I O are evaluated with regard to patterns of activity within and between
functional brain regions.
Data associated with the experimental paradigm is correlated with patterns of
activity and other
measLUes.
In process step 13, an interpretation of the correlation obtained in step 12
to a specific
application is then made. In this step, the subject's response to a lalov~m
response for a particular
application is made. For example, if a subject is being tested to determine
whether or how much
they lilce a particular product, the amount and/or intensity of responses in
certain regions of the
subject's brain is compared with predetermined responses from the subject's
brain (or from a
database of signals corresponding to I~lOWIl brain region responses) in
response to stimuli which
elicits a response from the subject considered to be statistically normal. By
comparing the
12
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
response generated by the subject when exposed to the particular product with
the premeasured
response, a variation from the subjects normal response can be folmd. Based
upon this
information, a determination can be made as to whether or how much the subj
ect lilted the
particular product. In another embodiment, brain responses in the alnygdala
region of the brain
are evaluated for habituation to aversion stimuli. If the amygdala region does
not habituate at or
below a population normed average then individuals who are being tested with
the diagnosis of
obsessive compulsive disorder will not be referred for behavioral therapy
since a colnlnon
component of behavioral therapy is the ability to habituate or be de-
conditioned to aversive
stimuli.
It should be appreciated that the responses are measured in particular regions
of the
subject's brain. The pal-ticular bra111 reg10I1S lIl WhlCh the responses
should be measured depend,
at least in part, t1p011 the type of determination trying to be made. For
example, if one is trying
to determine whether a subject lilies a particular object, then the response
in a first p1 ~ality of
brain regions are examined. If, on the other hand, one is trying to determine
whether a subject is
being truthful, then the response in a second plurality of brain regions are
examined.
Fig. 2A is a schema of brain functional illness and its relationship to
motivatiol~/emotion
fL111Ct1011. That is, Fig. 2A illustrates the lil~lcage of functional illness
to motivation and emotion.
Psychiatric illnesses, pain disorders, and illnesses producing
neuropsychiatric dysfiu~lction are
examples of brain functional illnesses. At the core of all psychiatric
illness, is some dysfimction
of motivatiol~/elnotion. This has been most closely evaluated for substance
abuse/addiction.
The schema of Fig. 2A shows that relationships between circuitry of motivation
20 and a
plurality of different categories of disorders designated by reference numbers
22-30 exists. Oval
shaped reference lines 32-40 indicate that relationships exist between each of
the disorder
categories 32-40 and the circuitry of motivation and emotion 20. The details
of the circuitry of
motivation and emotion 20 are described 111 CO11~t111Ct1011 Wlth Figs. 3-SC
below.
Fig. 2A illustrates the lil~lcage between psychiatric disease and dysfimction
of all or
CO111pOllellts Of the C1TCL11tTy of motivation or emotion. Thus, whatever the
cause of the
dySfL111Ct1011, this cause can be identified in the circuit 20.The circuitry
of motivation 20 is
related via a relationship 22 to anxiety disorders 24. The precise
relationship 32 is reported to
include altered function of amagdala subnuclei shown in Fig. 3, though the
full details remain a
topic of clurent research. The circuitry of motivation 20 is also related via
relationship 34 to
13
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
psychosis 24. In this case the precise relationship is reported to involve
altered function of the
ventral tegmentutn and prefrontal cortex illustrated in Fig. 3, and
potentially the thalamus.
Again, research continues to seek the details of relationship 34.
The circuitry of motivation 20 is further related via relationship 38 to
addiction 28.
Extensive research implicates the nucleus accLUnbLms, amygdala subnuclei,
SLEA, ventral
tegmentum, and orbital cortex with the development and progression of
addictive disorders.
The circuitry of motivation 20 is related via relationship 36 to mood
disorders 26.
Currently, motivation oircuitry such as the amygdala subnuclei and prefrontal
cortex have been
connected to hedonic defect syndromes.
Lastly, the circuitry of motivation 20 is related via relationship 40 to
attention deficit
disorder 30. Motivation circuitry implicated in disorders of attentional
dysfunction include the
ventral teglnentum and prefrontal cortex.
Referring now to Fig. 2B, a chart or schema 46 illustrates the relationship
between
circuitry of motivation altered by chronic pain 48 and a plLlrality of
different behavioral states 50
- 58. Reference lines 62-70 indicate that relationships exist between each of
the behavioral
states 50 - 58 and the circuitry of motivation and emotion 48. It should be
understood that pain
is not traditionally considered a psychiatric disorder. Rattler, pain is
considered to have a
number of functional sequelae. Thus, Fig. 2B is a schema detailing possible
functional sequelae
of chronic pain. Long term behavioral manifestations of pain include a
constellation of
symptoms aside from pain intensity, which closely parallel symptoms related to
disordered
motivation and emotion fimction observed with psychiatric illness. Thus, a
close similarity
exists between Figs. 2A and 2B.
Referring now to Fig. 2C a conventional schema 79 of motivational function
illustrates
that motivated behavior necessitates at least three ftuldamental operations
80, 82, 84. Operation
80 includes selection of short-term and long-term objectives focused on
attaining rewarding
outcomes while avoiding aversive outcomes, operation 82 involves processing of
perceptual
features regarding the rate, delay, incidence, intensity, (i.e., worth),
amount, and category of
these potential outcomes to plan behavior, and operation 84 includes the
actual determination of
physical plans involving IlILISCLllatLlre Or Orgall fUnCt1011 t0 ObtaIIl these
OutCOIIIeS.
14
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
A simplistic rendition of subsystems needed for pulling H (where H corresponds
to
information as conceived and defined by Shannon & Weaver which is hereby
incorporated
herein by reference in its entirety) from the environment regarding potential
rewards and
aversive outcomes might segregate a subsystem for modulation of attention to
putative goal-
object features, a subsystem for probability assessment, and a subsystem for
valuation. In
congruence with prospect theory, probability computations would be processed
in parallel with
computations assessing value to determine the reward OLItCOIne aS ShOW111I1
Fig. 2D.
Fig. 2D illustrates tluee phases: (a) an expectancy phase 86; (b) an
evaluation of worth
phase 88; and (c) an outcome phase 90. If one considers variables needed to
determine wol-th,
one fundamental variable is the "rareness" of the goal-object in the
envirolunent, while a second
is the value of the goal-object to the organism for reducing an existing
"deficit state". The
former variable of "rareness" depends on a probability assessment for its
computation, and thus
is an important input to any fimetion of worth evaluation.
The integration of perceptual features regarding the rate, delay, incidence,
intensity,
amount, and category of these potential outcomes as shown in block 82 can be
represented as
ShOWIl 111 blocl~s 92-98 of Fig. 2D. In blocl~ 86, modulation of attention to
H refers to the
increased attention a subject gives to the source of information "H." This
increased attention
leads to an evaluation of goal-object features for "valuation of H" as shown
in block 94.
Fig. 3 is a block diagram of brain circuitry 100 corresponding to brain
circuitry of reward
and aversive fllllctloll (i.e. here collectively referred to as
reward/aversion circuitry). That is,
Fig. 3 shows the route by which the brain receives external/internal
information and how that
information propagates to various regions of the brain to produce motivated
behavior. It should
thus be appreciated that circuitry 100 illustrates brain regions of
reward/aversive function that is
implicated in motivated behavior.
The brain circuitry 100 includes a prefrontal and sensory cortex 102. The
prefrontal
cortex includes medial prefrontal col-tex 102a and lateral prefrontal cortex
102b. The region 102
also includes the primary sensory and motor components 102c-102h. These
components include
the primary somatosensory cortex (S1) 102f, the secondary somatosensory cortex
(S2) 1028, the
primary motor cortices (M1) 102d, and secondary motor cortices (M2) 102e.
Motor behavior
involves regions such as M1 and M2, along with supplementary motor cortex
(SMA) 102c. The
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
fiontal eye fields (102h) modulate motor aspects of eye control relating to
directing the reception
of visual signals from the envirozunent to the brain.
Brain circuitry 100 also includes the thalamus region 104, the dorsal striatum
region 106
and the lateral and medial temporal cortex regions 108, I 10. The medial
temporal cortex region
110 includes, for example, the hippocampus 110a, the basolateral aznygdala 1 l
Ob, and the
entorhinal cortex 110. Also included as part of the brain circuitry 100 are
paralimbic regions
112, which include, for example, the insula 112a, the orbital cortex 112b, the
parahippocaznpus
112c and the anterior cingulate 112d. Current perspectives of reward circuitry
also include the
hypothalamus 114, the ventral pallidum 116 and a ph~rality of regions
collectively designated
118.
The regions collectively designated 118 comprises the nucleus accumbums (NAc)
120,
the central aznygdala 122, the sublenticular extended aznygdala of the basal
forebrain
SLEA/basal forebrain or SLEA/BF) I24, the ventral tegmentum (ventral tier) I26
and the
ventral tegmentum (dorsal tier) 126.
The regions 118 collectively represent a number of regions having significant
involvement in motivational and emotional processing. It should be appreciated
that other
components such as the basolateral amygdala 110c are also important but not
included in the
regions designated by reference wunber 118. Other regions that are fiuther
important to this
type of processing include the hypothalamus (114), the orbitofrontal cortex
(112b), the insula
(1 I2a) and the anterior cingulate cortex (112d). Further regions are also
important but listed
separately such as the ventral pallidum (116), the thalamus (104), the dorsal
striatum (106), the
hippocampus (1 10a), the medial prefrontal cortex (102a), and the lateral
prefrontal cortex
(102b). Not listed in this figure but also involved in processing sensory
information for its
emotional implications is the cerebellum.
The fimctional contribution of each of these major regions are discussed
below. It ShOLlld
be noted that what follows is a gross simplification and does not convey the
complexity nor the
diversity of the functions that these regions have been implicated with and
may in the future be
corrected to. Further note that there is currently a debate regarding the
modular vs. non-modular
function of these brain regions, i.e., can a specific fL111Ct1o11 be
attributed to each region in
isolation. Accordingly what is listed below is information which provides one
of ordinary skill
16
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
in the art with the understanding that this function may be mediated by the
connection of this
region with many other regions (i.e., the fzlnction mediated by a distributed
set of regions, of
which the identified region is a fundamental component).
As a brain region the NAc 120 has previously been implicated in tile
processing of
rewarding/addicting stimuli, and is thought to leave a number of functions
with regard to
probability assessments and reward evaluation. It has also~has been implicated
in the moment
by moment modulation of behavior (e.g., initiation of behavior). S1g11a1S
111eaSllred fr0111 the
NAc are shown and described below in conjunction with Figs. 3A-3D.
The SLEA/BF has been implicated in reward evaluation based on its likely role
in brain
stimulation reward effects. It is thought t0 be important for estimating the
intensity of a reward
value. It aild other sections of the basal forebrain appear to be important
for the processing of
emotional stimuli in general, and it has been implicated in drug addiction.
Lilce the NAc, the amygdala has been implicated in both processing of
emotional
information along with processing of pain and analgesia information. The
alnygdala has been
implicated in both the orienting to and the memory of motivationally salient
stimuli across the
entire spectrum from aversion to reward. It may be important for the
processing of signals with
social salience in real time. In this context it is often referred to with
regard to fear. A number of
its anatomical C01111eCt1021S to primary sensory cortices, suggest that it is
impol-tant for the
modulation of attention to motivationally salient stimuli.
With respect to the VT/PAG, doparmimlergic projections are present from the VT
to the
SLEA, the orbitofrontal cortex, the amygdala, and the NAc. Indeed
dopalninergic projections go
to host subcortical and prefrontal sites. In Fig. 3, the fundamental
importance of the VT/PAG
projection (124, 126) is focussed on the NAc (120), central Amygdala (122),
and SLEA/BF
(124), though it also projects to regions 110, 112, 116, 102a axed 102b. The
VT has been
implicated in reward prediction processes, motor functions and a number of
learning processes
arotuld motivational events in general. The PAG has also been implicated as a
modulator of
pain stimuli, for example, and may therefore be a region that signals early
information on
rewarding or aversive stimuli.
The GOb component of the prefrontal cortex has been implicated in a nlunber of
17
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
cognitive, memory, and plamling functions around emotional stimuli or
regarding rewarding or
aversive outcomes in animal and human studies. This section of the prefrontal
cortex has also
been implicated in modulating pain. It has afferent and efferent connections
with a nlunber of
subcortical structures (118). The GOb is involved in a nlunber of different
reward processes
including those of expectancy determination and reward valuation. Patients
with lesions in this
region tend to have impulse control problems.
The hypothalamus (114) is involved in the monitoring and maintenance of
homeostatic
systems (e.g., endocrine control, satiety, thennoregulation, thirst
monitoring, reproductive
control, and pain processing). It also has been both implicated in the
evaluation of the relevance
for rewarding and aversive stimuli in order to maintain homeostatic
equilibrium. The
hypothalamus is highly important for meeting the objectives which optimize
fitness over time
and meet the requirements necessary for survival.
The cingulate cortex (112d) has been interpreted to be involved in attention
and
plamling, the processing of pain lulpleasantness, the processing of reward
events and emotions
in general, and the evaluation of emotional conflict . The cingulate col-tex
is an extensive region
of brain cortex and appears to have emotional and cognitive subdivisions, to
name a few.
The insula (112a) has been implicated 11111Lllnber Of fL111Ct1011S
111C1t1d111g the processing of
emotional stimuli, the processing of somatosensory functions (e.g., pain), and
the processing of
visceral fimction.
The thalamus (104) is composed of a number of sub-nuclei which have been
implicated
111 a dlVerSe range Or f11I1Ct10I1S. FuIldaInelltal aI11021g these
111I1Ct1011S appears t0 be that of being
an informational relay of sensory and other information between the external
and internal
enviromnent. It has also been directly implicated in both rewarding and
aversive processes, and
damage to the structure may result in dySfLIIICtlOn StlCh as Chr0111C pain.
The hippocampus (110a) has been extensively implicated in fiulctions fOr
ellCOdnlg and
retrieval of information. Lesions to this structure lead to severe impairment
in the ability to
form new memories. Motivated behavior is heavily dependent on such memories:
for instance,
how a particular behavior in the past led to obtaining a goal object which
would reduce a
particular deficit state such as thirst.
is
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
The ventral palladium (116) is one of the primary output sources of the NAc
and bas a
number of projection sites including tile dorsomedial nucleus of the thalamus
(109). Via this
comlection, it is one of the maj or relays between the NAc and the rest of the
brain, ill particular
prefrontal cortical regions (102). It has been strongly implicated in reward
functions and is a
site thought to be important for tile development of addiction.
The medial prefrontal cortex 102a of the brain has been strongly implicated in
reward
functions and has been fond to be one of the few brain sites into which
cocaine self
administration can be initiated 111 a111111a1S.
In response to reward and aversion situations, Certain regions of the brain
clrcultry 100
play a role in processing reward/aversive information to plan behavioral
responses as discussed
above. These regions are designated reward/aversion regions of the brain. The
activation of
such reward/aversion regions can be observed dLlring positive and negative
reinforcement using
neuroimaging technology. These reward/aversion regions produce specific
fiulctional
contributions to motivated behavior. For example, contributions made by
regions such as the
include assessment ofprobability (i.e. expectancy).
Central to performing valuation, probability assessment, and other information
processing tasks needed for plalllling behavior in response to reward and
aversion situations are
a number of COre bralll reglOnS 111C1L1d1I1g the 1111C1e11S aCCLllnbellS (NAC)
120, the SllblelltlClllar
extended amygdala of the basal forebrain (SLEA/BF) 124, alnygdala (multiple
nuclei) 1 l Oc,
122, the ventral tegmentum/periaqueductal gray (VT/PAG) 124, 126, the
hypothalamus 114 and
the orbirtal gyros (GOb). The GOb is designated as the orbital cortex 112b in
Fig. 3. Also
important to reward alld aversion information processing are regions such as
the insula 112a,
anterior cingulate 112d, thalamus 104, ventral pallidum 116, medial prefrontal
cortex 102 a, arid
cerebellum (not shown in Fig. 3). The cerebelhull is associated with
integrating motor and
autonomic behavior. It appears to have specific roles in reward and emotion,
including the
detection of errors in information processing or the implementations of motor
behaviors.
As shown on Fig. 3, when a subject receives or senses an input 128, the
sensory input is
generally processed by multiple components of the brain circuitry
simultaneously. The arrows
in Fig. 3 indicate lalown afferent and/or efferent projections between those
regions. While Fig.
19
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
3 provides a simplistic overview of the connections along which information
processing occurs,
it is important to note that processing may occur simultaneously between
regions or sequentially
across brain regions...
Each of these interactions cause the regions to produce specific functional
contributions
to motivated behavior whieh is manifested as indicated at 130.
Referring now to Fig. 3, in one experiment, core brain regions implicated in
reward/aversive fimction were observed to activate in cocaine addicts after
cocaine
administration. In that experiment, the cocaine was administered after a brief
abstinence from
the drug in a randomized double-blind fashion relative to saline. Significant
signal change was
observed for the NAc 120 and SLEA 124 following cocaine with distinct time
courses that
correlated with subjective repol-ts made by the subjects. Subjective reports
of rush and craving
from cocaine were correlated with distinct sets of brain regions activated. In
particular, the NAc
I 5 120 and amygdala 1 l Oc, 122 were correlated with the motivational state
of craving, while areas
such as the SLEA/BF 118 and VT 124, 126 were correlated with the rush produced
by cocaine.
The cloves shown in Figs. 3A-3C illustrate that activation of reward regions
such as the
NAc 20 can be observed after low dose morphine in healthy volunteers (as
opposed to addicts).
Figs. 3A-3D illustrate signal chal2ges in the NAc I20 observed in individuals
over a period of time
to saline vs. morphine. Figs. 3A-3D thus demonstrate the power of neuroimaging
to interrogate
reward/aversion circuitry 111 111d1v1dL1alS eVell Wlth lnlld euphoria such as
that produced by very low
doses of morphine.
Turning now to Figs. 3A and 3B, curves 132-142 correspond to time-course data
(curves
measured from the left NAc in five subjects for both morphine and saline
infusions (Figs. 3A, 3B
respectively). Percent signal change in Figs. 3A and 3B are normalized
relative to each subjects
pre-infusion baseline, belt not detrended. The curves are plotted as percent
signal change. The
average signal change for the flue subjects is shown as lines 136, 142, and
the average infusion
interval, given cardiac-gating of the acquisition begins at 300 seconds and
ends at 780 seconds. The
time-course data Was sampled from each individual using a region of interest
from the aggregate
statistical map with each voxel localized in NAc meeting probability a
threshold of p < 0.05.
Figs. 3A, 3B show that individual signals can be readily obtained in these
shall
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
motivationally relevant regions. It also shows that there is a congruence of
positive signal for a
rewarding stimulus for this particular region.
Referring now to Figs. 3C and 3D, individual time-course data after morphine
and after
saline is averaged separately for the right (curve 146 - morphine: cwve 148 -
saline) and left
(curve 144 - morphine: 150 - saline) NAc. Error bars are included for the MRI
data acquired as
the 20' tilne-point, the 70' time-point, the 150' time-point, aald the 250'
time-point. Time is
represented in seconds using a conversion of repetition time (TR) = 6 RR
intervals = 6 seconds.
These graphs show that there were bilateral NAc changes to this particular
rewarding St1111111L1s,
WhlCh 15 llOt always the case as Noted 111 the SLllnlnary figure for mLlltlple
reward eXper1111entS.
(Table II)
Referring now to Fig. 3E, the statistical activation snap for significant
signal change in
the right nucleus NAc (152), averaged for six subjects is shown. Reference
numbers 154, 157
denote time interval during which a 46°C stimulus is applied to a hand
of a subject.
Referring now to Fig. 3F, curve 156 corresponds to the average time course
(i.e.,
signal change vs. time) of the activation shown in Fig. 3E. Note the
correlation between the
change in signal and the duration of the painful thermal stimuli (46°C)
shown as regions 154,
157. The time periods designated 154 and 157 correspond to periods in which
painful thermal
stimulus is applied to the subject. It should be noted that the signal goes
down during these
periods of time. After period 154 the signal 156 returns toward baseline
during the
inter-stimulus interval (i.e., between offset of 154 and onset of 157) and
goes negative again
during the second application of the thermal stimulus which takes place during
time period 157.
The decrease in signal during periods 154, 157 is highly significant because
it shows that all
aversive stimulus is negatively valenced (i.e. an aversive stimulus results in
a signal change
opposite to that of rewarding stimuli-e.g. cocaine, morphine monetary reward,
beauty).
Referring now to Fig. 3G, reward and aversion regions activated for cocaine in
addicts,
and morphine in healthy volunteers, are juxtaposed to demonstrate the
commonality of this
circuitry. Fig. 3G thus corresponds to a summary schematic of limbic and
paralimbic brain
regions observed with double blind cocaine infitsions in cocaine dependent
subjects, and
unblinded low-dose morphine infusions in drug-naive subjects.
21
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Regions activated to a sigrliflcant degree in the morphine and cocaine studies
and not
associated with heterogeneity of activation valence (i.e.-, positive vs.
negative signal changes),
are summarized in the brain schematic at the bottom of the image. Regions
symbolized by a
circle are sub-cortical regions traditionally associated with reward fvlnction
in animal studies,
while regions symbolized with squares are those associated in humans with
emotion fLlllCtloll 111
general. The commonality of activation across two distinct categories of
drugs, in the NAc
(120), SLEA (118), VT (124), and amygdala (110c 122) along with regions such
as the cingulate
cortex (112d) and orbital cortex - GOb (I 12b), suggests that a broad set of
brain regions may be
involved with generalized reward 11111Ct1011S. Other regions included in the
figure are the insula
(112a), the thalamus (104) which is involved in sensory and 1110tOT
111tegrat1o11 alld transmission
and the parahippocampal gyros (112c) which is involved in processing facial
and location
features. This composite figure strongly argues that there is a generalized
circuit of
reward/aversion that responds to divergently different categories of drug.
In another experiment, a game of chance (sinular to gambling) was used. In
this experiment,,
a wheel of fortune (i.e. a "spimler") having a spinning arrow on it was used.
The arrow lands to
signal the reception of a reward or "outcome" (honey). This gives an example
of the type of
experiment that can be done for almost any demographic group. In such an
experhnent, expectancy
(predicted chance of W11711111g) and outcome (actual W11n1111g or dollars
earned) processes are
segregated in time.
In the experiment, subjects have the OppOrtL1111ty t0 lose money as well aS
Wlll 111olley
since spimlers are randomly presented in this experiment. The overall sequence
of potential
wimlings and losses resembles a random walls process like that of a stoclc
index. This Follows
the psychology of prospect theory, which is the basis of behavioral finance
and decision malting
with regard to saving and spending honey. An experiment was performed to map
the
hemodynahic changes that anticipate and accompany monetary losses and gains
under varying
conditions of controlled expectation and colmterfactual comparison. The
paradigm involved
subjects viewing stimuli projected onto a mirror within the bore of the
magnet. The display
consisted of either a fixation point or one of three disks ("spilmers"). Each
spilmer was divided
into 3 equal sectors. The "good" spimler could yield $10, $2.50, or $0.0
outcomes, the "bad"
spinier could yield -$6.00, -$1.50, or $0.0 outcomes, and the "intermediate"
spimler could yield
$2.50, $0.0, or -$1.50.
22
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Details of activation in different regions in temps of expectancies
(prospects) and outcomes
(wimings or losses) are shown in Table I below. As observed in Table I,
multiple regions show
differential patterns of signal change to good, bad and intermediate
prospects. Each region of , °. ,
interest (ROI) in Table I below is defined a priori. A priori ROI's are
anatomically defined prior
to the experiment. Other regions not expected to activate can be determined to
be significant if they
meet conventional post-hoc statistical tluesholds. A focus of activation is a
group of pixels showing
significant activation compared with baseline that are fond in a region of
gray matter of the brain.
Table I sLUmnarizes the anatomic location of regions of interest (ROIs),
deviations of
BOLD signals from baseline, and ANOVA results. "Coordinates" denotes the
Talairach
coordinates using the atlas of Talairach a~ld Tournoux (1988) of the voxel
with the strongest p-
value at the center of each of the I6 ROIs..Coordinates are expressed in mm
from the anterior
commissl~re: R/L, right (+)/left (-); A/P, anterior (+)/posterior (-); S/I,
superior (+)/inferior (-
)."Change from Baseline" identifies ROIs in which the 95% confidence interval
aroLmd the
BOLD signal cleared zero. For the "Prospect" column, the spinier responsible
for the deviation
from zero is indicated by a "G", "I" or "B", for the good, intermediate and
bad spimiers,
respectively. For the Outcomes colunm, numerals refer to the trial type as
follows: l, 2, and 3
represent the $10.00, $2.50, and $0.00 outcomes, respectively, on the good
spinier. For the
intermediate spimler, 4, 5, and 6 represent the $2.50, $0.00, and -$1.50
outcomes, respectively,
and 7, 8, and 9 represent the $0.00, -$1.50, and -$6.00 outcomes,
respectively, on the bad
' spinier. "Time points Clearing Baselines" lists how many time points
reliably cleared the
baseline for prospect and for outcome data. In both the "Prospects" and the
"Outcomes"
coliunns, (+) refers to positive deviations from zero, and (-) refers to
negative deviations from
zero. The "ANOVA" colunm lists the ROIs for which significant main effects or
interactions
were fOLllld. ROIs with nonsignificant results are designated by a dash ("-").
For the expectancy
phase, ROIs with a significant main effect of spinier are indicated by "SP",
and ROIs with a
significant interaction of spiimer and time point are indicated by "SP~'TP".
Similarly, ROIs with
significant main effects of trial type dicing the outcome phase are designated
by "BI", whereas
ROIs with significant interaction of trial type and time point are indicated
by "BI*TP".
Table I
Coordinates Change from Baseline ANOVA
Anatomy RO R/L A/P S/I Prospects Outcom Prospects Outco
I # es mes
23
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Frontal
Lobe
GOb 1 -25 47 -18 B 2, 8 SP*TP BI
GOb 2 15 34 -21 G, I 1 - -
GOb 3 -12 66 -6 - - - -
GOb 4 18 19 -25 - l, 9 - BI
GOb 5 6 59 -12 G 3 - BI
GOb 6 25 59 -18 G 2, 8 - BI'''TP
GOb 7 -34 38 -18 B 2 ~ - -
GOb 8 -12 31 -21 G 6 - BI
GOb 9 28 44 -12 G, B - - -
GOb 10 -25 13 -9 B 2, 3, SP BI,
7
BI'''TP
Temporal
Lobe
Medial
A~nygdal11 -18 3 -15 B 5 SP*TP BI
a
Amygdal12 21 -3 -21 - 9 - BI
a
Subcortic
al Gray
NAc 13 12 16 -G G, I, 1-3, SP BI,
B 6, 7,
9 BI'''TP
SLEA 14 18 0 -G G, I, 1-3, SP BI
B 6-9
Hypothal15 9 -3 -6 G, I, 3, 6, SP, SPrTPBI
B 9
anus
Brainste
m
VT 16 12 -18 -12 G, I, 3 - BI
B
It has also been shovm that the clustering of regions involved in expectancy
and outcome
assessment in different hemispheres of the brain exists. As can be seen from
the prospect colmnn
and coordinates columns, it is notable that there appears to be a right
hemisphere predominance
(note positive values in the R/L column), for deep brain structL~res (e.g.,
NAc, SLEA) with regard
to positive stimuli, while there is a left hemisphere dominance for negative
stimuli in regions such
as the amygdala and GOb ROI wunbers 1, 7, 10. Data such as this show that
right or left brain
activation of reward/aversion may be important for interpreting the signal
changes
As noted above, many brain regions showing expectancy effects also show
outcome
effects.
Referring now to Fig. 3H, absolute fMRI signals are displayed for six regions
of interest
in reward/aversion regions. Signals were zeroed relative to an eight second
pre-stimulus epoch.
24
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
The time-courses for the good ( ~ ), intermediate (~), and bad ( ~ ) spimlers
are displayed. The
dashed lines segregate the expectancy and outcome phases of the experiment.
The bottom
graphs illustrate the good, intermediate, and bad spinner time-coL~rses
together, using the same
COdIllg as in tile colLU~lus of signals above them. In FIG. 3H, the first five
columns show signals
representing activity in the GOb(5) 170, NAc 172, SLEA 174, hypothalamus 176
("Hyp" in the
Fig. 3H), and VT 178, all of these regions show strong good spilmer effects
dtuing the
expectancy phase of the experiment. In the last column in FIG. 3H, the signal
from the left
amygdala 180 region shows minimal effects, during the good and intermediate
spinners, and
strong biphasic effects dl~rlllg the bad spiluer. Namely, the bad spinier
produces a signal that
I O becomes negative and then positive during the time it is spilming. For all
six regions,
differential responses to discrete expectancy CO11d1t1011S are ShOWll. The
expectancy response of
the NAc, SLEA hypothalamus, VT and GOb occurs in temporal lil~Ic to the
spinner being
initially presented, & spimzing. It reflects the assessment of contingent
probabilities around
potential gains and losses shown on the spinier. A discrete expectancy is one
of good,
I S intermediate, or bad outcomes. This is the first demonstration of
controlled expectancy effects
in humans and further shows that the waveforms in each of these regions were
significantly
different. This data provides evidence that prObablllty f1i11Ct1011S are
COIIIpLlted by dlStrlbLlted sets
of reward regions.
20 Referring now to Fig. 3I, the robust time-courses for bin effects in four
ROIs 182-190 are
illustrated. Bins (monetary outcomes, for example $10, $2.50 and $0.00 ) on
the good spimler
ar a shown in the top row of graphs, while bins for the intermediate spimler
are shown in the
middle row, and bins for the bad spimler are shown in the bottom row. A bin
effect corresponds
to the response to each spimler landing on one of tluee possible outcomes. The
eight seconds of
25 data acquired before the outcome phase of the experiment are used to zero
the data. The three
colwnns of data from the NAc 182, SLEA 184, and Hyp 186 in are grouped to
illustrate regions
that show differential effects for predominant gains as outcomes in the
context of good
expectancy. It should be noted that these tlwee ROIs 182, 184, 186 show
differential effects for
the outcomes on the good spimler and demonstrate strict ordering on the basis
of outcome
30 magnitude. That is, on the good spilu-ler, outcomes of $0.00, $2.50 and
$10.00 are possible, and
discrete ordering of the results are observed depending on the outcome.
Similar orderings are
not observed for outcomes in the context of intermediate and bad expectancies.
These orderings
are salient for supporting the notion that a distributed set of human brain
regions represents
stimulus wol-th in a parametric fashion. The GOb 190 is presented to
illustrate a very different
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
profile of outcome responses. Namely, this ROI appears to respond to extremes,
such as the
$10.00 outcome in the context of good expectancy, and the -$6.00 outcome in
the context of bad
expectancy. Differential responses to discrete monetary outcomes in a number
of reward
regions demonstrate that magnitude differences in the valuation of rewarding
stimuli can be
distinguished. This shows that reward functions are not just "on" "off
phenomena but produce a
gradation of response across the continuum of reinforcement (i.e., between
reward and
aversion). These data indicate that the brain can discriminate nuances in
value across the
continuum between reward and ptmislnnent, and between pleasure and pain. Such
observations
show that a mechanism exists for determilung what an organism values, and the
relationship of
this valuation to valuation of other objects, events, or internal states.
Figs. 3J - 30 illustrate early and late activation in different brain regions.
Referring now to Fig. 3J, curve 192 corresponds to a time course of the signal
(signal
change vs. time) for activation in the SLEA following a 46°C stimulus.
It should be noted that
there is a large initial change in the signal 192 during the first epoch 193
of the thermal stimulus
and not during subsequent thermal epochs 194, 196, 200. Curve 192 illustrates
that early and
profound activation in one area of the rewardlaversion (SLEA) compared with
late activation
illustrated by curve 211 (Fig. 30) in SI (somatosensory cortex).
Referring now to Figs. 3I~ and 3L, these figures shows activation in the SLEA
during the
early 202 phase and no activation in the region during the late phase 204 of a
46°C St1111t1h1S.
Other activations in the figure represent 1C110W11 leg1o11S 111Chldlllg the
right and left insula (112 -
in Fig 3) and the cingulate gyros (112d -- in Fig 3).
Referring now to Figs. 3M and 3N, the figures show relatively little
activation in the
primary somatosensory cortex (S 1) 206 and designated as 102f in Fig. 3 during
the early phase
of the stimulus while there is significant activation of the Sl region 208
dtuing the late phase of
the stimulus. Other areas of activation include the insula (112 - in Fig 3).
3O
Referring now to Fig. 30, curve 211 corresponding to a time course of the
signal in the
primary somatosensory cortex 208 (and designated as 102F in Fig. 3) extends
across multiple
time periods or epochs 212-215. It should be noted that activation exists
within region 208 in
each of the time periods 212_215 during which the thermal stimulus is applied.
26
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
It should be appreciated that Figs. 3J - 30 show why regions such as the SLEA,
which
has been heavily implicated in goal-object valuation, (i.e., how rewarding or
aversive is a
stimulus) respond to an aversive stimulus ahead of systems involved with
primary
somatosensory perception. The SLEA time coluse is orthogonal to typical time-
courses of
subjective ratings of pain. Namely, it's signal returns to baseline at the
time subjects are rating
maximal pain intensity from a pulsalite thermal stimulus. . The SLEA response
this occurs before
subjects malce conscious ratings that they are feeling pain. This is an
example of how
neuroimaging can be used to potentially differentiate conscious from non-
conscious processes
with relevance to motivation.
It should also be appreciated that distinct patterns of reward/aversive
circuitry flmction cars
be observed after presentation of different valences of stimuli (particularly
with regard to the left
amygdala) (i.e., fearful vs. happy or neutral faces) to different subjects. It
is important to note, for
IS example, that both happy and fearful signal habituates rapidly over the
course of an experiment.
This indicates that the brain adapts to novel emotional information quickly
and that the techniques
of the present invention can be used to observe this function.
It has also been observed that right alnygdala activation occurs after a
different category of
aversive stimulus (i.e., sad faces). Thus, it should be appreciated that
C0111pO11el1tS Of the
reward/aversion may respond in different degrees to various motivational and
emotional stimuli.
It should also be appreciated that demographic differences in subjects can
lead to different
activation in different groups of subjects (e.g. male us. female) to the salve
stilnuhus. For example,
NAc and amygdala activation to fearful faces are different in groups of men
and women.
Demographic differences in subjects can lead to different activation in
different groups of
subjects (e.g. male us. female) to the salve stimulus. For example, distinct
differences in activation
of reward/aversion regions between men and women, particularly for the mid-
huteah phase of the
menstrual cycle have been found.
Also, drug expectancy effects can be observed prior to tile 111fL1S1011 Of
cocaine us. saline.
For example, NAc activation can be observed prior to and shortly after cocaine
infilsions, but
before the onset of airy pharmacological effects. These effects result from
probability assessments
regarding the potential of receiving a drug reward (i.e. a previously
experienced reward). This
27
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
demonstrates that subsystems of motivational circuitry function can be
interrogated in isolation of
other subsystems. In addition, subjects did not intend to signal their
expectancy of drug, yet the
neuroimaging technology recorded it.
Table II provides a sLUmnary of activation across multiple studies using
different categories
of reward/aversion. Table II shows that a colnlnon circuitry processes reward
information,
regardless of the category of the reward stimulus, whether drug, money or
social stnnlxlus (e.g.
cocaine, morphine, monetary reward beautiful faces). Regions designated x in
the Table II are
activated. The observation that this is a generalized circuitry means that any
type of object can be
assessed regarding its rewarding/aversive properties to see how it falls along
the continuum of
reward aazd aversion (see Figs. 3H, 3I regarding evaluating how it falls along
the continuum of
reward). Of further importance, the areas of brain activation that are common
across these
categories of reward were also observed to be activated~during the perception
of an aversive
stimulus (see Figs. 3E, 3F, and 3H, 3I). This commonality does not imply that
all these regions
work in the salve way for rewarding and aversive stimuli (i.e. not all regions
axe activated at the
same time- they are aII activated differentially). For example, negatively
valenced signal is
observed in the NAc to a painfill stimulus, while positively valenced signal
is observed in the NAc
for a drug reward such as morphine. Other regions may provide different levels
of activation or
different timing with respect to activation depending on the valence of the
St1111L1ILlS along the
reward-aversion continulun.
Table II is divided into two main sections, one on expectancy, and one
regarding
reward/aversion outcomes. The left section on expectancy shows that across two
studies with
monetary reward and cocaine reward, expectancy effects lead to activation in a
nlunber of colxllnon
areas, namely the GOb and bilateral NAc. These effects are different than the
outcome effects in
terms of signal intensity and waveform. Across a number of experiments - two
experiments with
cocaine infusions, one experiment with morphine, one experiment with monetary
reward, and one
experiment with a social reward (beautifixl faces)-colnlnon foci of activation
were observed in the
right GOb, NAc, SLEA, and potentially the VT. The X's in the cohunns of Table
II axe
superscripted to indicate more than one foci of activation in that region
(i.e., X2 = 2 foci of
activation, X3 - 3 foci of activation). Brackets around an X indicate that the
statistical sigluflcance
of the findings were just subthueshold for the experiment in question. It
should be noted that there
are two columns for the cocaine experiments, representing two completely
separate cocaine
experiments. The two columns for the beauty study represent positive vs.
aversive outcomes. hl thllS
28
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
strldy, it was folmd that yolmg men looking at beautiful male faces, devalued
the images, indicating
they were non-rewarding, while valuing the beautiful female faces, indicating
that they, in contrast,
were rewarding. It should be noted that the beauty experiment is not the only
one with aversive and
rewarding outcomes. For example a monetary reward experiment discussed below
also had very
explicit rewards vs. losses. The strongest results regarding aversive
outcomes, though, are the pain
studies, which show activation in the same right GOb, NAc, and SLEA regions
that are common
across category of rewal-d.
Table II
)rYpectancy MonetaryCocaine Outcomes Monetary
Region Reward >JYpectancyRegion CocaineMorphineReward Beauty
(1) (~) (-)
(Z)
Gob R X- X Gob R X X (X) X' (X
)
L X X L X X X'
NAC R X X NAc R X X x X X (X)
~
L X L X X X
SL>;A R SL)JA R (X) X- X (X)
X
L L X X
AmygdalaR AmygdalaR (X) X
X
L X L X X (X)
vT R VT R X X X
L L X X X ~ (X) I
I
Referring now to Fig. 4, a noninvasive measluement apparatus and system for
measuring
indices of brain activity during motivational and emotional function is shown.
In this particular
example a magnetic resonance imaging (MRI) system 216 that may be programmed
to non-
invasively aid in the determination of indices of brain activity dluing
motivational and emotional
function in accordance with the present invention is shown. Its should be
appreciated however that
other teC1n11qL1eS 111Cltldlllg bLlt not limited to fMRI, PET, OI, SPECT, CT,
fCT, MRS, MEG and
EEG lnay also be used to non-invasively measure indices of brain activity
Bluing motivational and
eI210t10Ilal llll1Ct10I1.
MRI system 215 includes a magnet 216 having gradient coils 216a and RF coils
216b
disposed thereabout in a particular mamler to provide a magnet system 217. In
response to
control signals provided from a controller processor 218, a transmitter 219
provides a signal to
the RF coil 216b tluough an RF power amplifier 220. A gradient amplifier 221
provides a
current to the gradient coils 216a also in response to signals provided by the
control processor
218.
For generating a Lmiform, steady magnetic field required for MRI, the magnet
system 217
may be provided having a resistance or superconducting coils and which are
driven by a generator.
29
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
The magnetic fields are generated in an examination or scanning space or
region 222 in which the
object to be examiiZed is disposed. For example, if the object is a person or
patient to be examined,
the person or portion of the person to be examined is disposed in the region
222.
The transmitter / amplifier combination 219, 220 drives the coil 216b. After
activation
of the transmitter coil 216b, spin resonance signals are generated in the
object situated in the
examination space 222, which signals are detected and are collected by a
receiver 223.
Depending upon the measuring technique to be executed, the same coil can be
used as the
transmitter coil and the receiver coil or use can be made of separate coils
for transmission and
reception. The detected resonance signals are sampled, digitized in a
Digitzer/Aray proceser
224. Digitizer/Array processor 224 converts the analog signals to a stream of
digital bits which
represent the measured data and provides the bit stream to the control
processor 218.
A display 226 coupled to the control processor 218 is provided for the display
of the
reconstructed image. The display 226 may be provided for example as a monitor,
a terminal,
such as a CRT or flat panel display.
A user provides scan and display operation coimnands and parameters to the
control
processor 218 tluough a scan interface 228 and a display operation interface
230 each of which
provide means for a user to interface with and control the operating
parameters of the MRI
system 215 in a meaner well lalown to those of ordinary skill in the art.
The control processor 218 also has coupled thereto a CNS signal processor 232,
a
correlation processor 234 and a data store 236. It should be appreciated that
each of the
components depicted in Fig. 4, except for the CNS signal processor 232 and~the
correlation
processor 234 are standard equipment in commercially available magnetic
resonance imaging
systems.
It should also be appreciated that the MRI system must be capable of acquiring
the data
which can be used by CNS signal processor 232 and the correlation processor
234. In some
embodiments, the CNS signal processor 232 and the correlation processor 234
may be provided
as a general propose processors or computers programmed in accordance with the
techniques
described herein to determine indices of brain activity during motivational
and emotional
function. For example, in some applications it may be desirable to provide a
single processor or
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
computer which is appropriately programmed to perform the functions of control
processor 216,
the CNS signal processor 232 and the correlation processor 234. In other
embodiments, the
CNS signal processor 232 and the correlation processor 234 lnay be provided as
specially
designed processors (e.g. digital signal processors) or other specially
designed circuits. In any
event the CNS signal processor 232 and the correlation processor 234 are
unique in that they are
programmed or otherwise designed to determine indices of brain activity during
motivational
and emotional function in accordance with the present invention as described
herein.
The CNS signal processor 232 and the correlation processor 234 cooperate to
determine
indices of brain activity during motivational and emotional function. One pal-
ticular technique
For determining indices of brain activity during motivational and emotional
function is described
below in conjlmction with Figs. SA-SC. Suffice it here to say that once CNS
signals are
obtained (e.g. via a non-invasive technique including but not limited to M1RI,
fMl2I, PET, etc...),
the signals are localized to examine the function in a particular region of
the brain. The
particular manner in which such the signals are localized are dependent upon a
variety of factors
including but not limited to the technique or teclmiques (111Chldlllg
equipment) used to extract
the signals.
Once signals are extracted, the correlation processor 234 correlates empirical
data with
the measured signals. The correlation processor 234 then interprets the
results of the correlation
to a specific application. The CNS signal processor 232 and the correlation
processor 234
perform many of tile functions described in phases 502-509 below in
conjlmction with Figs.
SA-SC which describe the Motivational/Emotional Mapping Process (MEMP)
classification.
It should be appreciated that although processors 232, 234 are here shown as
separate
and distinct processors, in practice the functions described herein may
involve the use of both
processors 232, 234. Moreover, in practice all functions described herein can
be performed by
different processors (e.g. processors 218, 232, 234) or may be performed by a
single processor
or by more than thuee processors. Thus, processors 232, 234 may cooperate as
inter-digitated
processors. Processor 232 may be involved in performing all or portions of
steps 502-507 (Fig.
5A) while processor 234 may be involved in performing all or portions of steps
502, 503, 508a,
508b.
The remaining components of Fig. 4 perform tl-le functions described in phase
501 of
31
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 5A arld step 518 of Fig. 5B.
Referring now to Fig. 5A, general phases used in a Motivational/Emotion
Mapping
Process (MEMP) are illustrated. This process can be partially implemented
using a CNS
measluement system, such as system 215 described above in conjunction with
Fig. 4. In a setup
phase 500, the experimental paradigm is developed, subjects are screened and
selected, and
neLlroimaging parameters are optimized. The experimental paradigms are
developed by
considering a variety of factors including but not limited to part
experiments, lmowledge of a
particular characteristic of participants, 1nowhedge of what region in being
interrogated.
In phase 501, brain imaging data is collected along with physiological and
psychophysical data. Preferably a lion-invasive measurement system such as the
MRI system
215 of Fig. 4 is used to image the brain. It should be appreciated, however,
that there are several
other techniques 1C11oW11 111 the art to obtain brain imaging with sufficient
resolution
(approximately 5 x 5 x 5 mm) for the MEMP.
In a signal processing and statistical mapping of imaging data phase 502,
signal
processing involves the normalization of data across subjects and experimental
conditions, and
transformation of data into a uniform space for averaging, or anatomically
precise sampling of
signals. Standard signal processing techniques of fMRI include, but are not
limited to motion
correction, signal intensity scaling, detrending, spatial fihtering, temporal
filtering, and morphing
of the functional imaging data into a uniform space such as that of Tahair ach
and Tournoux.
Statistical mapping involves evaluating fMRI 3D data across time for
significant changes
relating to experimental conditions or any other variables such as subject
physiology or
psychophysical responses. Although here four dimensions (f MRI 3D and time)
are used, those
of ordinary skill in the art will appreciate that N dimensions can also be
used. Statistical
evaluation involves some degree of location and scale estimation along with
techniques for
computing general effects and pairwise differences between experimental
conditions. The type
and sequence of signal processing and statistical mapping of imaging data may
vary across the
technique of imaging used (including but not limited to MRI, fMRI, PET, EEG,
MEG, etc.).
In an anatomic localization phase 503, anatomic templates for precise
localization of
fMRI signal changes are prepared. Anatomic scans, acquired either at the time
of f~.mctional
neuroimaging with the experiments or at another time, are transformed into the
same uniform
32
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
space as the functional brain data. For example, this may involve a Talairach
transformation
(i.e., brain anatomy from individuals is normalized into a standardized 3D
reference system) or
cortical flattening. Alternatively, the anatomic and functional data may be
registered into the
same coordinate system so that they have an aligned set of 3D axis and the
anatomic data can be
segmented and parcellated into precise anatomic locations for later
superposition on the
fwctional data. Segmentation and parcelhation is a reproducible method using a
standard
format for locating and defining the boundaries of brain regions. The
quantified vohune of each
brain region is one output of the process. Anatomic and functional data are
ultimately co-
registered so that fMRI flmctionah data can be evaluated for each individual
on their native
anatomy. Such techniques lnay be the primary means of anatomic localization of
significant
signal changes, or be a supplement to use, of lmiform anatomic spaces such as
that of Talairach
and Tournoux for primary anatomic analysis.
In a hypothesis testing and determination of significant activity phase 504,
targeted
anatomic regions having significant signal changes relating to experimental
conditions,
physiology, and psychophysical measures are evaluated. Experimental
CO11d1t1o11S 111Cltlde
variables built into the experimental paradigm, variables built around the
group or groups of
subjects being scalmed and potentially compared, variables involving any
administered drugs or
compounds, and variables involving repeated administration of the paradigm, or
comparison of
this paradigm to another paradigm. Hypothesis testing involves correction for
the multiple
comparisons between experimental cO11d1t1o11S being made. Deter111111at1011 Of
S1g111f1Ca11t activity
tluoughout the entire brain, or throughout the entire set of acquired
functional data, will also be
performed using a correction for this larger set of comparisons. Hypothesis
testing and
determination of significant change will also be performed for comparisons
generated by the
physiology and psychophysics data.
In a signal evaluation phase 506, signal features relative to the experiment
are evaluated.
Evaluation of signal features involves qL1a11t1f1Cat1011 Of 111d1CeS
111C1L1d111g btlt not limited to Talairach
coordinates and subregions or subnuculei, T'p and , rate of signal change,
first, second and third
moments, right side activation (i.e. measure of activation of a structure in
the right hemisphere-
denoted R), left side activation (i.e. measure of activation of a structlue in
the left hemisphere -
denoted L), fractional laterality (i.e. an index of how lateral an index is
computed as (R-L/R+L),
correlation factor (R), volume, exponent of power f1111Ct1o11, a111p11tL1deS
Of har111o111CS alld
subharlnolucs, aanplitude changes between plateaus (computed via integration
of an fMRI signal of
33
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
a region) and maxilnuln rate of change and time to achieve the maximum rate of
change (computed
by tal{ing a derivative of an fMRI signal in a region).
This evaluation of signal feaW res is important for understanding how a signal
in a specified
anatomic region may be significantly different between experimental
conditions, or across
physiological changes or changes in psychophysics responses. The evaluation of
signal features is
not limited to the four categories mentioned above. These four categories in
particular, are mentioned
because they allow one to evaluate patterns of signal within specified
anatomic regions. These
patterns witlin one anatomic region can also be compared to patterns within
other anatomic regions.
Sets of regions with similar signal features can then be "clustered" together
for discussing the
dynamics of activation across multiple brain regions.
In a signal quantification phase 507, a calculation of specific indices which
can be
compared across experimental conditions across brain regions, and sometimes
across separable
experimental paradigms is made. Quantities which are included in the
computation of the
indices will be dlSCLlSSed below 111 C011~Lillctloll Wlth Figs. 11-11J. The
primary 115e Of qllalltlfled
indices of an fMRI signal is that sets of these indices become very precise
descriptors of signal
events in anatomic regions. These sets of indices (e.g., characteristics of
the wavefonn such as
the time-to-peals measure) can be used to categorize large numbers of brain
regions by
experimental condition. These categorizations of multiple regions quantify a
"pattern" of
activation which can be evaluated across multiple experimental conditions, or
can be used to
compare experimental condition effects to physiological effects or to
psychophysics-relevant
effects. These patterns can also be used to compare individual subjects, or
follow them over
time. Quantified signal indices compliment but do not replace the signal
features described in
step 506 above.
In a comparison of experimental vs. physiological effects phase 508a, patterns
of
significant signal change in hypothesized brain regions and elsewhere in the
brain are compared
and contrasted between experimental conditions and effects related to
physiology. Similarly,
signah features and quantified signal indices are compared and contrasted
between experimental
conditions and physiology. This is done to determine what experimental effects
are truly
independent of mainly global effects produced by body physiological changes.
In a comparison of experimental vs. psychophysical effects phase 508b,
patterns of
34
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
significant change, signal features and quantified signal indices in
hypothesized brain regions,
and elsewhere in the brain axe compared and contrasted between experimental
conditions and
effects associated with the psychophysical responses. This is done to
determine which
experimental condition effects and psychophysical response effects are
(dependently) lil~l~ed,
and which are independent.
In an interpretation of experimental results phase 509, experimental condition
effects and
psychophysical response effects which are independent and dependent on each
other are
evaluated with regard to lalown functions of the targeted (hypothesized) brain
regions and other
brain regions. Interpretation of experimental paradigm results in individual
subjects or groups
of subjects is performed against a background of established brain response
features and
quantified indices for particular paradigm conditions ial -~ a"~, which
reflect (or were designed
to interrogate) specific motivational or emotional functions. Thus, components
of motivation
fL111ct1o11 from blocks 80 , 82 , or 84 (in Figure 2C), such as expectancy
phase 86 through
outcome phase 96; which reflect subfimctions of bloclc 82, are colmected to
experimental
paradigm conditions or psychophysical responses. This connection of
experimental paradigm
and psychophysics results to motivation and emotion functions is then used to
answer the query
leading to the initial formulation of the experiment.
Referring now to Figs. SB-SD, the steps in the Motivational/Emotion Mapping
Process
(MEMP) are illustrated. The process described in conjunction with Figs. SB-SD
corresponds to
both the process used to determine the circuitry as well as the process used
to arrive ai a
CO11C11151011 (e.g. "the subject lilces the product" or "the subject is
lying"). The process begins as
shown in step 510 in which an experimental paradigm is developed targeting
~a
motivational/emotional function from one of the three general processes needed
for motivated
behavior. These processes are (1) determination of objectives for survival and
optimization of
fitness, (2) extracting information from the envirolnnent regarding potential
goal objects, events
or internal states, of relevance to motivational function and meeting the
above objectives; and
(3) definition of behavior to obtain the goal objects and thus meet the
objectives for survival.
The experimental paradigm involves a number of discrete conditions which are
to be
independently measured or compared and are referred to herein below as
conditions {a, -~ a" J=.
It is important to note that experimental conditions include variables built
around the group or
groups of subjects being scamled and potentially compared, variables involving
any
administered drugs or compounds, and variables involving repeated
admilustration of one
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
paradigm or comparison of this paradigm to another paradigm. The experimental
paradigm may
be integrated with parallel physiological measures (e.g., heart rate (HR),
blood pressure (BP),
temperature , skin galvanic response SGR, etc.) and/or with parallel
psychophysics measures
(e.g., analog rating scales of pain or pleasure, response times etc.)
The types of experiments which can be developed in step 510, can be quite
diverse.
Examples of experiments wllich can ve split into conditions ~a~ ~~ a"I are
provided by a
representative cocaine vs. saline infusions study, a monetary reward study and
a beauty bar-press
procedure
IO
For example, the cocaine vs. saline infusions experiments were split into pre-
vs. post-
lllfLlS1011 COIldIt1011S. Namely, condition a~ = pre-cocaine infusion,
condition a2 = post-cocaine
lllfllSIOIl, COIIdItI0I1 a3 = pre-SalIIle II2flISIOn, and COIIdItI0I1 aq =
pOSt-SalIIle IIIfL1S1O11.
Fox the monetary experiment, there were Mine experimental conditions depending
on the
combination of expectancy and outcome conditions for a wheel of fortwle.
Namely, given three
possible outcomes on each spilmer, and three spinners, there were three total
expectancy/outcome combinations.
In the be,~uty bar-press pr~cedLU~e, subjects bar-press to lceep a pictLUe up
longer, bar-
press to get rid of a picture quiclcer, or do nothing. The time interval
before each of these 3
conditions represents al, a~, and a3. These experiments result in a set of
experimental conditions
{al ~ a"} which are separable either in time, or by correlation with
physiological or
psychophysical measLUes.
Experiments developed in step 510 incorporate principles from neurobiology,
clinical
pharmacology, cognitive netuoscience, decision theory, neurocomputation and
medicine including
psychiatry and neurology. The experiments are hypothesis driven. Regions can
be specified a priori ~
on the basis ofthe cLlrrent neuroscience and medical literature at the time.
Experiments incorporate
a number of conditions whose comparison make it possible to attribute function
to targeted brain
regions. Examples of such experiments can be seen in double-blind cocaine
infusions, thermal
stimulation experiments to evaluate pain processing and monetary reward
experiments (described
below in more detail). Step 510 includes the development any off line testing
if required.
In step 512, subjects are selected and screened for study. The subjects may be
hlunan, or
36
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
animal, depending on the experimental question behind the experiment developed
in step 510.
In step 514, neuroimaging parameters are optimized and tested. The optimized
parameters are integrated into the experimental paradigm {al -~ a"~. The
integration of any
potential 111fL1S1011 Wlth radioligand, nucleotide, or contrast material into
the sequence of scans
plamled for experimental conditions {a1 -~ a" J occurs in step 514.
A number of regions can be targeted, for example the subcortical gray matter
structures.
An attempt is made to reduce potential artifacts affecting signal from deep
gray matter structLUes
by optimizing machine parameters. For example, to see the nucleus accumbens or
amygdala,
One llllght acquire signals using nearly isotropic voxel dimensions and
reduced echo times. In
addition, shimming methods 1a10w11111 the art can be used to e1W ance the
homogeneity of the
mean magnetic field via use of second or higher order shims.
In step 516, paradigm conditions {a~ -~ a"} are administered in temporal
lil~lcage with
step 518.
In step 518, brain imaging results in signal acquisition in time and space
using optimized
machine parameters (including potential infusion with radioligand or contrast
agent).
In step 520, physiological and psychophysics parameters are measured in
lil~cage with
brain imaging from step 518. Non-invasive physiological parameters (measured
outside or
inside the functional brain imaging unlt) include anylall measLlre/s of
physiological function
such as heart rate (HR), blood pressure (BP) including systolic, diastolic and
mean using a cuff,
skin galvanic response (SGR), skin blood flow as measured by laser Doppler,
respiratory rate
(RR), electrocardiogram (EKG), pupilometry, electroencephalography (EEG) etc.
Invasive physiologic parameters can include blood presswre (via arterial
line), blood
oxygenation levels or any similar pulmonary measure using blood sampling,
hormonal levels as
measured by repeated blood sampling and subsequent assays, drug levels or
levels of any injected
compound which may be part of the experiment, etc.
Psychophysical parameters include any subjective response (which may be
recorded by
voice )or a device (such as a mouse) used in the magnet by the subject to
respond to questions
presented to them inside or outside the magnet. Examples include visual
analogue scores, hedonic
measures, reaction times, experiment~guided responses (e.g., true/false), or
other means of
37
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
communicating internal states etc.
Note, most of the physiological parameters can be measwed in animals and
humans.
However, psychological parameters are mostly specific to humans.
In step 522, an examination of the imaging signal in 3-D relative to the
experimental paradigm
is made. As an example of the many signal processing and statistical mapping
tecluuques available
for fMRI data, two basic approaches to fMRI data analysis will be described.
In the first approach,
the system targets a set of anatomically defined regions of interest (i.e.,
NAc, amygdala, SLEA,
VT/PAG for a reward/aversion stzzdy), and evaluates signals from these regions
using two statistical
mapping techniques. A second approach evaluates signals tluoughout the entire
brain, including the
extended set of regions implicated in reward/aversion functions, such as the
GOb, medial prefrontal
cortex, aCG, and insula. This post-hoc analysis evaluates averaged data with a
similar set of statistical
methods as for targeted reward regions, but could also be focussed on
individual data. The
examination of the imaging signals, occurs in 3-D, relative to experimental
paradigm. It should be
appreciated that some of the MEMP steps could become automated or semi-
automated.
Prior to statistical mapping, initial signal processing involves motion
correction which uses
the automated image registration or some similar type of motion correction
(AIR) algorithm or similar
programs which are applied to individual data sets. After motion correction,
all individual images are
evaluated for residual motion artifacts. Functional MRI data may be intensity
scaled and linearly
detxended. Spatial filtering may be performed using a Hamling filter with a
1.5 voxel radius, and then
mean signal intensity is removed on a voxel by voxel basis.
During analysis of the targeted reward regions, all individual structural and
functional data
sets can be transformed into a uniform anatomic space such as Talairach space
or a group specific .
anatomic space to allow statistically significant findings to be aggregated
across subjects. In contrast,
for voxel-by-voxel analysis, whole brain stTUCtzzral and functional data are
transformed into a zmiform
anatomic space such as Talairaeh space or a group specific anatomic space
prior to averaging across
subjects. The averaged fimctional data is then statistically evaluated as
described below in
conjunction with steps 522 through 566.
In parallel to the analysis of functional data using parasnetric statistical
mapping (and
multiple correlation mapping described below), as shown in Phases 502, 503 the
structural scans
for each individual have the targeted brain regions segmented (e.g., NAc,
SLEA, amygdala, and
38
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
VT). These segmentation volumes can then be transformed into a universal
anatomic space such
as Talairach space, or a group specific space. Each activation cluster
identified on the group
average data is evaluated to determine its localization in these segmentation
volumes. Each
cluster, which is localized in a particular segmentation volume for 80% or
more of the
individuals comprising the average, is kept for subsequent analysis.
For the statistical parametic maps, these selected clusters in the targeted
regions (e.g.,
NAc, SLEA, amygdala, and VT/PAG) can be used to sample the individual
Talairach-
transformed functional data (or functional data transformed into another
Lmiversal or group
specific anatomic space). This individual data can be submitted for robust
location a~zd scale
estimation usiilg the Tulcey bisquare method to evaluate experimental
conditions and determine
differences between them. Differences across experimental conditions may
emerge
quantitatively when conditions are sampled together (i.e., morphine vs. saline
effects on thermal
pain stimuli), or qualitatively in the form of differences in patterns of
activation in each of the a
priori structures when the conditions are sampled separately. For each
analysis across
conditions, clusters which have a significant result by robust analysis of
variance (ANOVA) will
then undergo pairwise contrasts.
In step 524, am anatomic framework or map in 3-D is generated which can
localize fMRI
signals.
In step 526, examination of imaging signal, in 3-D, relative to physiology,
and,
separately relative to psychophysical function, can be performed to produce
location and scale
estimates for statistical evaluation of physiology, & psychophysical effects
on brain fLUlctloll.
As part of step 526, individual fMRI data are also evaluated for correlational
mapping of
subjective effects (as from hedonic analog scales), and correlational mapping
of physiological
measLUes correlational analysis will involve multiple correlation of
subjective ratings'and/or
physiological measures with the fMRI data set dLUing which they were collected
in each subject.
Correlation maps are composed of correlation factors for each pixel.
Correlation factors are
transformed into probability values using a Fisher transformation. Correlation
maps for each
individual are anatomically morphed into the Talairach domain or another
universal or group
anatomic space. These p-value maps are evaluated across each experimental
group using a
C011~L111Ct1011 allalySlS to quantify the commonality of activations across
experimental C011d1t10115.
The conjLmction maps representing the association of subjective effects with
fMRI data in
individuals axe evaluated by identifying clusters of activation in the NAc,
SLEA, amygdala, and
39
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
VT (or other a priori reward/aversion regions).
Evaluation of brain data from regions not included in the initial set of
targeted regions can
involve use of whole brain data averaged or aggregated across subjects.
Alternately, it could also be
done in individuals given a sufficiently large cohort for statistical power
reasons. A number of
statistical mapping procedures are currently available for post-hoc analysis.
In one embodiment, a
statistical mapping procedure is performed on a voxeh-by-voxeh basis, using
both a warefonn based
correlation (WCA) analysis, and a multiple correlation analysis.
Analysis of fMRI data can be broadly grouped as model-flee or model-based
methods, and time-
preserving or non-time preserving methods. Most data analysis methods use
distribution statistics,
SLICK as Student's T-test or Kolmogorov-Smimov statistics. In these designs a
constant hemodynamic
response dluing stimulation is assluned. These techniques are not time-
preserving since they compare
distribution of activated time points versus resting time points regardless of
their time order. Model-
based, time-preserving techniques, such as colTehation analysis and in some
cases, event-related fMRT,
maintain the temporal information by mcludmg in their analysis the particular
time evolution of the
model for the fMRI response. These techniques may have some limitations in
detecting CNS
activation if more than one hemodynamic response is present. The use of an a
priori helnodynamic
model may maslc structures whose responses differ from the chosen model.
In step 524, anatomical localization is performed. SLICK localization can be
accomplished
LlSlllg a niunber of different techniques. Preferably, anatomic localization
is performed using
Luliversal anatomic coordinate systems (e.g., Talairach c~ Tollrrlollx),
111dlvldllah anatomy (e.g.,
as with segmented brain volumes), and/or anatomically morphed anatomy (e.g.,
inflated
flattened cortical surfaces).
Preferably, anatomically segmented and parcellated brain regions are used for
anatomical
localization of signal changes. It should be appreciated that alternate
embodiments may be
developed in the filtLUe for more sophisticated and detailed anatomical
localization of signal
changes observed with functional imaging.
The segmentation methodology, founded upon intensity contour and differential
intensity
contour concepts is used in step 524. The cortical parcelhation technique is
based upon the concept
of limiting sulci aald planes and tales advantage of the observed
relationships between cortical surface
features and the location of fimctionah cortical areas. An example set of
operational definitions is
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
presented in Caviness et al., 1996; Malcris et al., 199 which is hereby
incorporated herein by reference
in its entirety. A critical advantage of this method is that definitions are
Lulambiguously definable in
a standardized fashion from the information visible in high resolution MRI.
As is lalown in the art, targeted regions (e.g., the NAc, SLEA, alnygdala,
VT/PAG) will have
specific anatomic definitions. For instance, for the NAc, SLEA, anygdala, and
VTIPAG, the
following definitions can be used. The NAc is identified at the inferior
jLmction between the head of
caudate and the putamen. The NAc is delimited superiorly by a line colnlecting
the inferior corner
of the lateral ventricle and the inferior most point of the internal capsule
abutting the NAc and
laterally by a vertical line passing from the latter point. The VT/PAG and
amygdala is directly
visualized, and the posterior extent of amygdala is located at the identical
coronal plane as the anterior
tip of the anterior hippocalnpus. The PAG is contained in parcellation tulits
that include the n lidbrain
tegmentLUn. The SLEA region is identified anterioposteriorly from the
midsection of the NAc
extending back to the first substration nigra (SN) coronal section. It is
identified medially by the
~ hypothalamus (whicll extends anteroposteriorly from anterior commisure to
include p0steriorly the
mallunily body (MB), having a vertical line at the level of the optic tract or
the lateralmost extent of
the optic clliasm of the internal capsule as its lateral border and the
interhemispheric midline as its
medial border).
It should be appreciated that the signal processing and statistical analysis
is described in terms
of the cLlrrent state of the art for fMRI data. It is recognized that data
collection teclnliques will lil~ely
change over the coming years. The statistical procedlues may vary somewhat
between neLlroimaging
teC1ll11queS, bLlt ShOLlld all 111VO1Ve lOCat1011 alld scale estimation,
aI011g Wlth techniques f01 COmpLltlllg
general effects and pairwise differences between experimental conditions. The
inventive method is
compatible with other imaging teclllliques and future imaging techniques which
produce location and
scale measurements having equivalent resolution characteristics to current
fMRI imagers (i.e. at 3
Tesla and 7 Tesla).
As discussed above, in step 522, an examination of imaging signal, in 3-D,
relative to
experimental conditions dal ~ a";, produces location and scale estimates for
statistical
evaluation of paradigm effects. It should be appreciated, however, that the
exact sequence of
steps between step 522 and step 566, regarding statistical evaluation and
anatomic localization
may vary, as may the specific method for statistical evaluation or anatomic
localization.
In step 528, images from step 522 with those in 524 are merged to allow
localization of
41
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
brain imaging signal for experimental co11d1t1011S {al -j a"f .
In step 530, brain imaging signals associated with physiology and
psychophysics
lrleasLUes are localized.
During the hypothesis testing and determination of significant activity phase
504, brain
impulse signal from targeted regions is identified on the basis of previous
for reward/pain
relevant regions, other imaging studies, or animal data.
The hypothesis testing alld determination of significant activity ShOWl1 111
phase 504,
includes steps 532-SGG.
In step 532, an operator or an automated process splits localized results for
experimental
C011d1t1o11S {al ~ a" J 111t0 reg10115 Wh1C11 are 1 priori (i.e., targeted)
and those which are not.
In step 534, an operator or an automated process splits localized results for
physiology
and psychophysical conditions to regions which are a priori (i.e., targeted)
and those which are
110t.
Hypothesis testing continues in steps 544 - 550. h step 544, statistical
threshold testing
based on step 510 is performed on the targeted regions within the motivational
and e1210t1o11a1
circuits y.
In steps 544, 54G, 548, 550, thresholds of significance are computed for the
statistical tests to
allow for multiple statistical comparisons. This is done in a different
fashion depending on the
type of statistical analysis being performed. One method involves Using a
region of interest
analysis to sample maxima of signal Change Wlth111 targeted regions. The
signal from these
targeted regions in individuals is then submitted to an ANOVA analysis where
the p value
threshold is corrected for the number of regions being sampled. In contrast to
this, a voxel by
voxel technique of analysis might incorporate another format of threshold
correction. One
means of doing this is to measure the vohune of tissue sampled in
targeted/hypothesized regions,
to determine how many voxels cover this tissue, and to divide the p < 0.05/x,
where x = tile
number of voxels, to maintain an overall alpha level of less tllan 0.05. Tlle
volume of tissue for
the entire brain is also then sampled and used in a similar fashion to produce
a correction similar
to a Bonferroni correction. After computing thresholds of significance for
targeted and
42
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
non-targeted regions, imaging data from targeted regions is evaluated to
determine which data
meet a priori and post-hoc tllresholds.
In step 544, targeted brain regions are evaluated to determine if they have
significant general
effects and significant effects between experimental conditions.
In step 546, evaluation of whole brain data (i.e., this may be on a voxel by
voxel basis for
every voxel acquired during tile experiment in the brain), is performed to
determine if tllere are
significant general effects and effects between conditions. In step 548, the
same procedLLre is
followed regarding the evaluation of physiologic and psychophysical effects in
the fMRI data. In
step 550, the salve procedLlre used in step 546 is followed, to evaluate
physiological and
psychophysical effects. The output of the process in step 544 is noted as
steps 552 and 554, the
output of step 546 is noted as steps 556 alld 558, the output of step 548 is
noted as steps 560 and
562, and tile output of step 550 is noted as steps 564 and 566. The rationale
for segregating these
OL1tp11tS 111 this fashion, is that only steps 552 and 556 contribute the
input to the processing which
takes place in step 568. Similarly, o111y the output of step 560 and step 564
contribute the input to
the processing of step 570.
In step 552, significant activity in targeted regions from threshold
assessment in step 544
15 deteT111111ed. I11 Step 554, SL1bt111eS1101d aCtIVlty Ill targeted TegI011S
fr0111 threshold assessment
in step 544 is determined. In step 556, significant activity in non-targeted
regions from
threshold assessment in step 546 is determined. In step 558, subtllreshold
activity in non-
targeted regions from threshold assessment in step 546 is determined. In step
560 signiflcallt
activity in taxgeted regions from threshold assessment in step 548 is
determined. In step 562,
subtllreshold activity in targeted regions from threshold assessment in step
548 is determined. In
step 564, significant activity in noel-targeted regions from threshold
assessment in step 550 is
determined. In step 566, subtllreshold activity in noel-targeted regions from
threshold
assessment in step 560 is determined.
In step 568, the system evaluates signal features relative to the experiment
(e.g. signal valence,
graded intensity information, intensity over time and adaptation dynamics).
Two examples of
evaluating signal features with biological significance are described below.
In particular, the use of
valence information (from pain and facial expression stimuli), and graded
intensity information (from
monetary reward stimuli) are described.
43
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
In step 568, during fMRI of rewarding or aversive stimuli in hlunans, positive
activation
(signal change) in the NAc following rewarding stimuli (including monetary
reward, beauty, and drug
reward) and negative activation (decreased signal change) following noxious
thermal stimuli is
observed. These findings directly show that painful stimuli are assessed
distinctly from rewarding
stimuli, as reflected by all altered valence of NAc signal change. In step
570, the system evaluates
of signal features relative to subj ective ratings (intensity over time).
One example of the steps included in phase 506 would be a comparison of
cocaine infusion
maps generated by the comparison of the pre-infusion interval with the post-
infusion interval with
the statistical maps generated by correlation of subjective ratings with the
brain signal. Thus,
activations produced by the cross-correlation of rush and/or craving ratings
with brain signal can
be overlaid with the activations which represent the response to cocaine in
general. Some
activations from the general cocaine map will correspond with the activations
that correlate to nlsh,
others will correspond with the activations that correlate to craving, while a
third set may
correspond to both, and a fourth set may not correlate to either craving or
rush.
In steps 572 and 574, the signals are quantified and compared between
experimental
conditions. In step 572, the signal features within the same anatomic foci and
between different
anatomic foci are quantified (i.e., to produce for instance, time to peals and
dispersion measures) and
compared to experimental conditions dal -~ a" J . Also in steps 572 and 574,
tile use of quantified
signal indices can describe signal events in anatomic regions. These anatomic
regions can then be
categorized by these descriptions to show a pattern of signal response across
many regions. For
example, thermal pain data can be evaluated to produce time-to-peals measlues
(T~,) and dispersion
' measures (~) (i.e. the width of the signal change in response to a painful
stimulus from the point of
inflection of the signal to its return to baseline). These T~, aald d measures
can then be evaluated
across all regions showing significant signal change (both targeted/
hypothesized regions, along with
all other brain areas) and divided on the basis of being above or below the
mean Tp and mean ~. This
division was legitimized since there were two peaks of T~, and D across the
set of regions with
significant change. The categorization of regions into a matrix with (a) Tp <
T~, mean and D < 0
~ 0 llleall, (b) Tp < Tp llleall alld O > ~ lneall, (C) T~, > ~ 111ea11 alld ~
< ~ mean, alld (d) T~, > T~, mean and
4 > ~ mean, categorizes the entire set of anatomic regions activated by the
experimental condition
of applying an aversive (painful) thermal stimulus. This pattern of activated
regions can be directly
compared to the patterns from other experimental conditions to determine
differences between
conditions in terms of anatomic regions involved in the different conditions.
The categorization of
44
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Tp and D above was compared to that from a non-aversive/non-painful thermal
stimulus to show the
differences in brain regions processing these two categories of stimulus.
There are many potentially
quantifiable signal indices. Dependilig on the number of indices used, an N-
dimensional matrix can
be used to categorize the regional activations so by with the N indices.
In step 574, the signal features within the same anatomic foci and between
different
anatomic foci are quaaltified and compared to physiological and psychophysical
measurements.
In step 576, the overlap between experimental condition and physiological
effects, and the
overlap between experimental conditions and psychophysical effects is
evaluated. For example,
autonomic (e.g. GSR) responses, physiological measures (e.g. EKG, BP, RR) or
psychological
measures (e.g. pain intensity, pain unpleasantness) can be correlated with the
brain signal. In
this way one can correlate the specificity of the responses with specific
regions of the brain that
may mediate these physiological/psychological responses.
Time coluse verification of statistical maps occurs in Phases 506 and 507.
Foci of
apparent significant change in hypothesized regions, and elsewhere in the
brain, are further
evaluated by examining the corresponding signal intensity vs. time curves,
both for time course
data taken from ROI constrained activation clusters (in individuals), and for
post-hoc voxel-by-
voxel focused activation maps. This also provides a means of determining an
estimate of mean
signal change and confirming that regional activation coincides with the
timing of stimulus
presentation.
In step 578, experimental conditions which camlot be segregated from
physiological
conditions are identified. These regions do not receive any more processing.
In step 580,
experimental cOl1d1t1o11S Whlch Call be segregated from physiological
conditions in the same
anatomic foci, and between different ones are identified. In step 582,
experimental Col1d1t1o11S
which calmot be segregated from psychophysical effects in the same anatomic
foci, or between
different ones are identified. In step 584, experimental conditions which can
be segregated from
psychophysical effects in the same anatomic foci, or between different ones
are identified. In
step 582 or step 584 the subject can be either conscious or non-conscious.
In step 586 offline studies (done outside neuroimaging system) or
questiolinaires can
optionally be used to modulate interpretation of imaging data. Performance on
offline studies or
scores from offline questionnaires can be correlated with quantitative signal
measures from the
functional imaging process. It must be stressed here that the primary data is
the neuroimaging data,
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
and that data from offline studies are merely used to fine-tLme the
interpretation of the neuroimaging
results.
In step 588, the system interprets the results from the experiment in terms of
motivational and emotional function, or changes therein. Signal features in
specific anatomic
regions or between different anatomic regions convey a specific picture or
script of
motivatiorzlemotion fUllctloll. The biological signals define the motivational
and emotional
fiulction effected by the experimental paradigm.
It should be appreciated that in phases 502-504 statistical analysis is
performed on
hypothesized/targeted regions (e.g., such as the NAc, SLEA, VT/PAG, aznygdaha)
and post-
hoc/non-targeted regions. Parametric statistical mapping of experimental
effects in individual
fMRI data may begin with an aggregation process, i.e., all experimental rllzls
for an individual
are concatenated. Individual data for the aggregated experiments may then be
transformed into a
zmiversal anatomic space such as the Talairach domain. Data common to each
experiment is
then averaged or aggregated across all individuals. This averaged or
aggregated functional data
then undergoes a statistical comparison of its baseline condition vs. all
categorically common
experimental conditions, to produce the masks used to collect signal intensity
data from
individual subjects. Thus, for each experimental condition, a test is
performed between a
coznznon baseline and all time-points for all experimental conditions which
may be subsequently
compared. From these statistical comparisons, clusters of activation are
identified using a
cluster-growing algoritlun. To maintain an overall alpha < 0.05, this
algoritlun will localize
activation meeting a corrected threshold of p < 0.05/x, (i.e., P for the max
vox) where x could be
the number of hypothesized brain regions interrogated. The cluster growing
algoritlnn will select
voxels with p<0.05/x in a set radius (e.g., 7 mm) of a voxel with a minimum p-
value (i.e., max
vox). Max vox peala are within a cluster of a standardized nLUnber of voxels
(e.g., tluee voxels),
each of which meets the statistical threshold. Max vox peaks will also be
separated by a
standardized distance (such as 4mm) from any other putative peal.
As an alternative to the statistical analysis technique described above in
phases 502-504, an
WCA approach can be used. The WCA approach determines statistical significance
using cross
correlation of each pixel with a mean hemodynaznic response (MHR). The MHR is
obtained for a
subset of active pixels found active by using a T-test. The WCA approach has
been used for a
noxious heat experiment, and has been fOtlzld to yiehd more information than
standard approaches,
including more robust levels of significance for signah changes, increased
numbers of brain regions
46
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
that are observed to be activated, and temporal differences in signal time
courses for proximate
activations (e.g. early activation in some reward/aversion regions and late
activation in others).
Also in phases 502-504, ll1 COIl~uTlctloll Wlth the WCA analysis, a multiple
correlation analysis
of the averaged whole brain data using averaged subjective ratings is
performed. For both the WCA
and multiple correlation analysis, significance is determined by applying a
correction for multiple
comparisons. Correction levels are determined as follows:
(1) for a priori regions the corrected p value is 0.05 divided by lhpr;ori (a
priori = nlunber of
pixels sampled in the a priori regions)
(2) for post hoc regions, the p value is 0.05 divided by n post hoc (posthoc =
number of pixels in
whole brain gray matter region sampled, and approximates a Bonferroni life
correction).
Phases 502-506 allow one to detennilie that the effects of aversive stimuli
are distinct from
rewarding 5t1m1111 011 the basis of the pattern of reward/aversion activation.
This is shown by distinct
patterns of reward/aversion region activity seen dluing studies of the visual
processing of negative
vs. positive facial expressions. In these studies with facial expressions
(i.e., studies with facial
expressions which are responses to aversive stimuli, or responses to rewarding
stimuli), positive left
and right amygdala activation is observed dluing the visual processing of
fearftil faces, positive right
amygdala activation is observed following presentation of sad faces, and
positive left alnygdala
activation is observed with happy faces.
Experiments can be explicitly designed to dissect the sub-functions of the
informational
system for motivated behavior. For instance, in one experiment, monetary
reward in a game of
chance resembling gambling at a slot machine is used to dissect out activity
in reward regions related
to the evaluation of probability information (i.e., expectancy), and valuation
infolznation (in this case
under the general outcome phase of the systemJ. This monetary reward
experiment represents the
first demonstration that circuitry involved in hlllnall 1110t1Vatloll Call be
dissected 111t0 Sllb-COlnp011el1t
11111ct1O11S. All llnpOrtallt feature of the ability to dissect sub-ftmctions
of the informational system for
motivated behavior is ordered activation in sets of targeted reward regions
which reflect the relative
magnitude of the reward. Observing the NAc, SLEA, hypothalamus, and amygdala,
can determine
how rewarding stimuli are relative to each other.
Referring now to Fig. 6, a chart shows the relationship of distinct scales of
brain fimction
47
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
and the research teclmiques used to investigate these scales. Oval shaped
reference lines 610-
618 indicate that relationships exist between each of the measurement
categories cognitive
neuroscience (behavior) 600, hlunan neuroimaging (distributed neural
ensembles) 604, animal
neluoimaging 606, ehectrophysiology (cells, neural ensembles) 608 and
molecular biology and
genetics at the molecular and gene level 602. Fig. 6 is a diagram illustrating
an association
between filnctional nellroimaging in humans and animals. The importance of
functional
neltroimaging in humans and animals is apparent when considering that it is
the primary means
by which gene and molecular function can be lil~l~ed to their behavioral
effects.
Fig. 6 describes a working format for the interaction of a number of basic
neuroscience
techniques that measLlre braill/neural signals fiom various spatial scales.
Thus for example,
molecular biology and genetic studies predominantly work with animals to
define the contribution
of specific genes, modification of these genes or gene products (e.g.,
receptors) and the effects of
molecules (e.g., neurotransmitters) on neuronal fimction. This evaluation is
performed at a
cellular/molecular level. However, such techniques may use neuronal markers of
activity (e.g., c-
fos) to determine the fzmction of groups of neurons tllTOLlghOtlt the
11et1raX15. However, this measure
is made in-vitro (i.e., special staining methods of brain tissue harvested
from animals).
I;hectrophysiology on the other hand may measlue the response of a single or
multiple neurons to
specific activatioll/perturbation (which may be sensory, mechanical or
chemical). Groups of
llellrOlls Wlthlll the CNS may therefore show patterns of response indicative
of a particular function
of a neuron, group of neurons or brain regions. Neuroimaging, animal or human,
allows for the
evaluation of signals from neuronal circuits in the living condition. Lastly,
cognitive neuroscience
and other experimental psychological disciplines allow a description of
behavior that can be
quantified and interdigitated with neuroimaging (e.g., monetary reward
paradigm, using techniques
from prospect theory).
Several experiments specific to motivation and emotion fimction have been
performed
using the teclmiques described above. These experiments have produced 5peC1flC
111fOTlllat1011
regarding motivation/ emotion functions. For instance, these experiments have
involved graded
responses to monetary reward in a game of chance, bar press experiments
indicating a
preference to various stimuli, and experiments evolving direct
aversive/rewarding sensations.
In one experiment, the principles of prospect theory (as that term is
understood in
experimental psychology and behavioral finance) were incorporated into a game
of chance with
48
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
money to evaluate normative reward/aversion function during fvmctional
magnetic resonance
imaging (fMRI) at 3 Tesla. The paradigm involved a sequence of single trials
with spinners that
shared a subset of outcomes, and segregated expectancy from monetary loss or
gain.
In one experiment involving monetary loss and gain, twenty right-handed male
subjects
were recruited of which eight subsequently were shown after the experiment to
have
uncorrectable motion or spiking artifact, leading to twelve usable data sets.
All subjects were
medically, neurologically, and psychologically normal by self report and
review of systems.
This experiment was performed to map the hemodynamic changes that anticipate
and
accompany monetary losses and gains under varying conditions of controlled
expectation and
counterfactual comparison. The paradigm developed in step 510 involved
subjects viewing
stimuli projected onto a mirror within the bore of the magnet, while
maintaining a stable head
position by means of an individually molded bite bar. The display consisted of
either a fixation
point or one of 3 dislcs ("spimers"). Each spimler was divided into 3 equal
sectors. The "good"
spimler could yield either a large gain (+$10), a small gain (+$2.50), or no
gain ($0), the "bad"
spinner could yield a large loss (-$6), a smaller loss (-$1.50), or no loss
($0), and the
"intermediate" spinner could yield a small gain (+$2.50), a small loss (-
$1.50), or neither a loss
nor a gain ($0). Providing larger gains than losses was implemented to
compensate for the
tendency of subjects to assign greater weight to a loss than to a gain of
equal magnitude (per
prospect theory).
Before the game began, subjects were shown each spimler 3 times so as to learn
its
composition. Each trial consisted of (1) a "prospect phase," when a spinner
was presented and
an arrow spvm aroLmd it, and (2) an "outcome" phase, when the arrow landed on
one sector and
the corresponding amount was added to or subtracted from the subject's
wimlings. During the
prospect phase, the image of one of the tluee spiimers was projected for six
seconds and the
subject pressed one of three buttons to identify the displayed spiimer, thus
providing a measure
of vigila~lce. The display was static for the first one-half second, and then
a superimposed arrow
would begin to rotate. The arrow would come to a halt at six seconds, marking
the end of the
prospect phase. During the first five and one-half seconds of the ensuing
outcome phase, the
sector where the arrow had come to rest would flash,.indicating the outcome. A
black disk was
then projected as a visual mask during the last one-half second of the twelve
second trial. On
fixation-point trials, an asterisk would appear in the center of the display
for fifteen and one-half
49
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
seconds, followed by the 0.5-sec mask.
The pseudo-random trial sequence was fully counter-balanced to the first order
so that
trials of a given type (spinner + outcome) were both preceded and followed
once by all nine
spimer/outcome combinations and three times by fixation-point trials. Thus,
the average 1-trial
"history" arid "future" was the same for trials of every type. Eight runs with
nineteen trials
apiece were presented to subjects. Only results of the last eighteen trials
were scored for each
run, since the initial trial was inserted into the run sequence pL~rely to
maintain counter-
balancing. Runs were separated by two to four minute rest periods. The same
trial sequence was
used for all subjects, generating wimlings of $142.50, to which was added a
$50 endov~nnent. At
the end of the scanning session, subjects completed a questiomaire rating
their subjective
experience of each spinier and outcome using an eleven point opponent scale.
The timing of stimulus events in this experiment, and the rationale for the
data analysis,
are based on two fundamental assumptions. A first assmnption is that the
hemodynamic control
system is approximately linear in the brain regions targeted by this
experiment, on the basis of
results frOlll CO11d1t1011S tested to date. A second assumption is that, given
appropriate
comterbalancing, the compound response can be "peeled apart" by means of
selective averaging
axed comparison of impulse-like hemodynamic responses.
Subject instructions were developed and administered (see e.g. steps 510, 516
in Fig.
5B). Using a set text, subjects were informed that they would be participating
in a series of
games of chance. At the start of these games, they would receive an endowment
of $50 to cover
possible losses, and informed of the maximum they could win over the course of
the experiment.
In the unlilcely event that they lost more then their endovv~nent, they would
receive no money,
but would receive a picture of their brain in action and have a clinical scan
on record, worth
approximately $1600. For each game of chance they would see a wheel of chance
with tluee
sectors. The wheel would move for some time, and the spimier would eventually
land on one of
the sectors, determining how much they received for that particular game.
There would be tluee
wheels of chance, which differ in their general level of outcomes, and would
be temned the bad,
medium, and good spimlers. Subjects were informed they would see each of these
spimers in a
short video to acquaint them with the game. They were fimther informed to
identify each
spinier shown for each game as rapidly as possible using a button box,. and to
refrain from
speech during the scan. After reading the instruction text, subjects'
questions were answered,
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
and they then observed a brief set of 10 trials (including the fixation trial)
to familiarize them
with the stimuli.
Physiological and psychophysical measures of behavior were monitored (in
accordance
with step 520 discussed above). Subjects made behavioral responses tluoughout
the study,
consisting of identification of each spinier as it was presented. Subjects
identified spilmers
using a button box, with the first l~ey on the left (index finger) being used
to identify the bad
spinner, the second l~ey on the left (middle finger) being used to identify
the lnedi~.un spinner,
and the third lcey on the left (ring finger) being used to identify the good
spilmer.
Subjects were scalmed (in accordance with step 518 discussed above) on an
instascan
device (3 T Geileral Electric Signa; modified by Advanced NMR Systems,
Wilmington, MA)
using a GE head coil. II11ag1llg fOI' all experiments started with a sagittal
localizes scan
(conventional T1-weighted spoiled gradient refocused gradient echo (SPGR)
sequence; tluough-
I 5 plane resolution = 2.8 mm; 60 slices) to orient, fox subsequent scans, the
slices to be acquired for
functional scanning. This scan was also used as the structural scan for
Talairach transformation.
Next, an automated shimming teclmique was used to optimize BO homogeneity.
Radio-
frequency full-width half maximlun (FWHM) line-width after shimming of primary
and
secondary shims produced a measlue of 32.4 ~ 2.2 Hertz (Hz) for the I2
SLlb~eCtS Wlth 1120t1on-
correctable functional data. After shimming, experimental slices were
prescribed, with 18 slices
parallel to the AC-PC line and covering the NAc, amygdala, SLEA/BF, and VT. In
this
orientation, an SPGR T1-weighted flow-compensated scan was obtained
(resolution = 1.6 nun x
1.6: mm x 3 lnln), primarily to aid Talairach transformation during data
analysis. The fourth scam
was a T1-weighted echo planar inversion recovery sequence (TI = 1200 cosec, in-
plane
resolution = 1.57 mm) for high-resolution structural images to be used in
preliminary statistical
maps (but not with Talairach transformed or averaged maps). Finally,
filnctional scans involved
a T2'''-weighted gradient echo sequence (TR=2s, TE=35ms; Flip=70°; in
plane resolution =
3.125 x 3.125 mm, through-plane resolution = 3lxlln, FOV = 40 x 20 cm; 18
contiguous slices,
images per slice = I08 per slur). The shortened TE and nearly isotropic voxel
dimensions had
been optimized previously in step 514 t0 lllll'111111Ze llllaglllg artifacts
in the regions of interest.
Post-paradigm subjective reports were collected. After finishing the paradigm,
subjects
completed a questiolmaire regarding cmnulative gains, and their experience of
the prospect and
outcome phases of the experimental trials as a means of determining whether
they experienced
51
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
the monetary taslc in the mamer predicted by prospect theory. The
questiomiaire specifically
queried subjects' ability to follow cumulative gains/losses during the
experiment, estimates of
total winnings, and their subj ective experience of spirmer presentation, plus
outcome from each
spinier. To male these ratings of each spimler, and each outcome on the tluee
spizmers, subjects
marked their response on an 11-point opponent scale ranging from very bad (-5)
to very good
(+5). Subjects were subsequently informed of their total gains from the
experiment. In this
particular study, no further offline or neuropsychological measures unrelated
to the paradigm
itself were performed (as in step 586 Fig. SD).
Data analysis on behavioral data collected during the paradigm was performed
in step
526. The integer output for each behavioral rating was checked against the
trial sequence, and
performance was listed for each individual. The mean ~ standard error of the
mean (SIJM) were
computed across the twelve subjects with motion-correctable functional data
for each of the
eight rLU~.s to ascertain that response errors were less than fve percent per
subject.
Data analysis on post-paradigm data was performed in step 526. The real-number
responses of subjects with motion-correctable functional data were tabulated
and evaluated
using robust methods paralleling those detailed for the fMRI data (see steps,
522-566 Fig. 5B).
Specifically, for the subjective ratings of spinner a statistical expert
system performed an
analysis of raw residuals and recommended against use of variance-adjusted
weights and the
TLdcey bisquare estimator. The efficiency of the robust (bisquare) analysis
was only eighty-five
percent as great as the efficiency of the traditional least-squares approach,
so the
recommendation of the expert system was accepted, and a least-squares
components ANOVA
(one-way) performed with subsequent pairwise comparisons.
For the subjective ratings of outcomes, boxplots of the residuals indicated a
number of
potential outliers, the presence of which were confirmed with am analysis of
raw residuals form
the robust fit. The efficiency of the robust (bisquare) analysis was greater
than the efficiency of
the least squares approach as confirmed with a normal probability plot of
residuals, a~zd hence
the expert system recommended use of variance-adjusted means and the Tulcey
bisquare
estimator. This recommendation was accepted, and a bisquare components ANOVA
(two way -
bins nested in spinier) performed with subsequent pairwise contrasts.
The fMRI data was then processed (as in phases 502, 504 in Fig. 5A) and signal
52
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
processing of fMRI blood oxygen level dependency (BOLD) data before
statistical mapping was
performed (in accordance with step 522 of Fig. 5B). To reduce head motion,
each subject was
positioned using a bitebar, and BOLD data was motion corrected using a motion
correction
algoritlun. After motion correction, time-series data were inspected to assure
that no data set
evidenced residual motion in the form of cortical rim or ventricular artifacts
> 1 voxel. From
this analysis, eight of the twenty subjects were fOlllld to have uncorrectable
motion or spiking
autifact, leaving a final cohort of twelve subjects for further evaluation.
Motion correction
(mean ~ SEM) of the BOLD data revealed m average maximal displacement for each
of eight
runs of 0.43 + 0.097 mm; 0.67 ~ 0.16 mm, 0.72 ~ 0.18 min, 0.71 ~ 0.15 nnn,
0.80 ~ 0.19 mm,
1.16 ~ 0.30 mm, 1.33 ~ 0.39 rmn, 1.47 ~ 0.43 111111. Motion displacement led
to corrections for
movement, in terms of the mean correction per time point for each of these
rLms, of 0.22 + 0.04
mm, 0.49 ~ 0.13 mm, 0.56 ~ 0.15 mm, 0.55 ~ 0.11 mm, 0.65 ~ 0.16 mm, 1.00 ~
0.29 mm, 1.19
~ 0.37 mm, 1.29 ~ 0.41 mm.
For all eight runs, fMRI data in the Talairach domain was normalized by
intensity
scaling on a voxel-by-voxel basis to a standard value of 1000, so that all
mean baseline raw
magnetic resonance signals were equal (corresponding to step 522 in Fig. 5B).
This data was
then detrended to remove any linear drift over the course of scan. Spatial
filtering was performed
using a Hamling filter with 1.5 voxel radius (this approximates a 0.7 voxel
gaussian filter).
Lastly, mean signal intensity was removed on a voxel-by-voxel basis.
In this experiment, the trials were selectively averaged. In total, there were
ten trial types
(spinier + outcome), including the fixation baseline. Prospect and outcome
phases of the trials
each lasted six seconds. Given the standard delay of two seconds for the onset
of the
hemodynamic response to neural activity, at least fourteen seconds of BOLD
response needed to
be sampled for selective averaging across trial type. Six seconds of pre-
stimulus sampling were
incorporated for use in subsequent data analysis as a baseline to zero the
onset of each trial.
This is a cormnon practice in evoked response experimentation.
Counterbalancing was
performed to the first order, so that the six seconds before the onset of each
trial, when averaged
across all iterations of that trial, would represent a common baseline against
which to
normalized the onset of each trial. Accordingly, selective averaging was
performed for twenty
second epochs.
Each individual's set of infusion images, along with the associated
conventional
53
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
structural scans, were transformed into Talairach space and resliced in the
coronal orientation
with isotropic voxel dimensions (x,y,z = 3.125 lnln) (steps 522, S24 in Fig.
5B). Optimized fit
between functional data and structural scans was then obtained via translation
of exterior
contours.
Talairach-transformed structural and functional data (i.e., the selectively
averaged trials,
N=10) were averaged across the twelve subjects with interpretable BOLD data
(steps 522, 524
Figure SB).
Statistical mapping, ROI-based analysis and statistical mapping of main
effects as ROI's
was then performed (as discussed in phases 502-504 above). All time-points
collected during
the prospect phase of the experiment, and all time-points collected during the
outcome phase of
the experiment were statistically evaluated by correlation analysis with a
theoretical impulse
function. The impulse flmction for the predicted hemodynamic response was
generated using a
gamma fLl11Ct1011. To eliminate cross-trial hemodynalnic overlap, the
correlation maps were
generated with the difference between all prospect data and fixation epoch
data, and with the
difference between all outcome data and fixation epoch data using time-point
by time-point
comparison. Subsequently, clusters of activation were identified using a
cluster-growing
algoritlun. In order to maintain an overall a < 0.05, this algoritlnn
specifically localized
activation which met a corrected p-value threshold of p<0.007 for the number
of hypothesized
brain regions being interrogated. Regions of interest (ROIs) were delineated
by the voxels with
p<0.007 in a 7mm radius of the voxel with the minimum p-value (i.e., max vox).
Max voxel
pealts had to be within a cluster of at least 3 voxels, malting the
statistical threshold, and
separated by at least 4 mm from any other putative max vox peals. ROIs
identified in this
mamler were then used to sample the individual prospect data (N=10 ROIs) and
OL1tC0111e data
(N=6 ROIs).
During the anatomic localization phase 503, statistical, maps of group
averaged data were
superimposed over high-resolution conventional Tl-weighted images which had
been
transformed into the Talairach domain and averaged. Primary anatomic
localization of activation
foci was performed by Talairach coordinates of the maximLUn voxel from each
activation cluster
with secondary confirmation of this via inspection of the juxtaposition of
statistical maps with
these coronally resliced Tl-weighted structural scans. Confirmation of
subcortical localization
of activations followed the region of interest conventions described
previously for the NAc
54
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
SLEA, amygdala, and VT. The GOb ROI conventions were not previously described,
and are
here delineated. Namely, the GOb (BA 11/47) was identified anteriorly behind
the ventral
surface of the frontal pole (BA10). It began with the orbital gyri (anterior,
lateral, and medial)
which are visible by the begimzing of the orbital sulci, and extended
posteriorly to the begilming
of the SLEA of the basal forebrain which is visible by the extinguishing of
the orbital SLllc1
(transverse orbital sulcus). Laterally, the GOb extended to the anterior
horizontal ramus of the
Sylvian fissure, and medially, it extended to the olfactory sulcus.
A5 S110W11 111 Phases 502-504 priori regions evaluated for activation clusters
included the
NAc, amygdala, and VT (for prospects), and the SLEA, amygdala, hypothalamus,
and GOb (for
outcomes). Regions hypothesized for one condition (i.e., prospects or
outcomes), were also
evaluated for the other. In total, ten clusters of signal change were noted
fox these a priori
regions during the prospect phase ofthe experiment. Six other clusters of
signal change were
noted in a priori regions during the outcome phase of the experiment .
Signal time-course analysis of ROI's was performed in phases 502-504. The
normalized fMRI signal was averaged, at each time point, within each
activation cluster falling
within an ROI. As described above, the averaged data were assembled into time
coluses, 20 sec
in duration, which included a 6-sec epoch prior to trial onset.
An exploratory analysis of the time courses was performed in order to examine
the
across-subject distribution of the averaged fMRI signal in each cluster.
rirst, the signals for each
subject were transformed into deviations from the across-subject mean at each
time point within
each trial type. The deviation scores for the period begimiing 2 sec following
trial onset and
ending 2 sec following the end of the trial were selected for exploratory
analysis; this segment
was used because it contained the data that were later used for hypothesis
testing concerning
expectancy and outcome responses. The deviation scores within the selected
time period were
combined across time points and trial types and displayed as a normal
probability (t'quantile-
quantile") plot. If the scores of the subjects were distributed normally, such
a plot would be a
straight line passing tluough the origin, with a slope equal to the standard
deviation.
Normal probability plots of data from some clusters did not deviate strongly
from
linearity, suggesting that the signals recorded from the different subjects
were distributed in an
approximately normal fashion. In contrast, substantial deviations from
linearity, consistent with
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
the properties of contaminated normal distributions, were noted in the case of
several clusters.
Thus, it was decided to employ robust statistical methods to describe the time
courses. Such
statistics are less subject than conventional parametric statistics to the
influence of extreme
values ("outliers") and provide more efficient estimates of the central
tendency ("location") and
dispersion ("scale") of contaminated normal distributions. As described below,
a formal test of
the relative efficiency of the conventional arid robust measures was carried
out in order to
determine whether robust or conventional least-square statistics were the most
appropriate for
hypothesis testing.
The robust estimates of location and scale are based on the Tukey bisquare
estimator
(phases 502-504). This estimator weights scores as a fvuzction of their
deviation from the sample
median. The weights decline smoothly to zero in a bell-shaped fashion as the
deviation from the
median grows. To compute the location estimate, each score is first expressed
as a scaled
deviation from the sample median:
x; - M
u, _
c x MAD
Where x; = fMRI signal for subject i at a given time point
M= median of the fMRI signals for all subjects at that time point
c = a tuning constant and
MAD = the median of the absolute deviations from the median
The weighting function is
w;_~1-u; ~iflu;l_<1;~~;=0iflu;l)1,
the robust estimate of location (Tb;) is
~~x~ - M~'~ ~'%
T,,;=M+ ,
1N;
a~ld the robust estimate of scale (sG;) is
n2 x ~~x;-M~~ x~l-u,2)4>'
s~» = o
~w;~ ~e~l-Su;-~
where a = the number of subjects
The turning constant, c, determines the point at which the weighting function
reaches
56
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
zero. As the value of this constant grows, progressively fewer data points
receive zero weight,
and the location estimate approaches the mean; as the value of this constant
sluinhs,
progressively fewer data points are rejected, and the location estimate
approaches the median. A
tuning constant of 6 was employed to compute the location and scale estimates
used to graph the
signal time courses and their confidence intervals. Given normally distributed
data, such a
tLming constant would result in assignment of a zero weight to all
observations falling more than
4 standard deviations from the median. In the case of the observed
distributions, the median
percentage of data points assigned a weight of zero was 1.24%. The range for
15 of the 16
clusters was 0.47 - 2.16%, whereas the percentage of data points rejected in
the case of the
remaining cluster was 5.86%.
A baseline adjustment was made. The robust estimates of location and scale
were
computed first from untransformed data. A within-subject subtraction procedure
was then used
to align the signal time cotuses for each trial type with a common baseline.
As shown in Fig. 3H
in the case of the data to be used for analysis of expectancy responses, the
subtrahend consisted
of the median fMRI signal dl~ring the six seconds prior to trial onset plus
the first two seconds of
the trial. (Due to the delay in the hemodynamic response, the signal during
the first two seconds
of the trial should reflect neural activation prior to trial onset.) This
median value was then
subtracted from the fMRI signals obtained during the subsequent twelve
seconds. In the case of
the data to be used for analysis of outcome responses, the subtrahend
consisted of the median
fMRI signal during the first six seconds of the trial (the prospect phase)
plus the first two
seconds followiilg presentation of the outcome. Thus, in both cases, the
median of the signals
recorded during the preceding epoch was subtracted from the signals from a
given trial phase.
Following the application of the subtraction procedure, new robust estimates
of location and
scale were computed.
The robust estimates of location and scale were used to compute the 95%
confidence
intervals. Due to the fact that the average weight is less than one, the
degrees of freedom must
be corrected accordingly. The nLUnber of degrees of freedom were multiplied by
0.7 in
constructing confidence intervals about the robust estimates of location. The
expression for the
confidence interval is
5,~;1
T~j ~ ~t(°.7x(n-,» X ,~- J
In the hypothesis testing and determination of significant activity phase 504,
tests for
57
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
differences between time courses were carried out using a statistical expert
system such as
RS/Explore. It should be appreciated that there are several methods and expert
systems which
call perform the statistical analysis. Separate analyses of the transformed
data for the
expectancy and outcome phases were conducted.
The multiple-regression module of RS/Explore was employed to carry OLIt an
analysis of
variance (ANOVA) as part of steps 544 - 550. In the cases of 12 of the 1 G
clusters, the data
selected for this analysis consisted of the transformed fMRI signals during
the period begimling
2 sec following trial onset and ending 8 sec following trial onset. This
period lags the timing of
the expectancy phase of the trial by 2 sec, consistent with other reports of
helnodynamic delay
post experimental stimulation. Examination of the time courses for these 12
clusters confirmed
that signals whose confidence intervals cleared zero did indeed lag the onset
of the trial by 2 sec.
However, in the case of the remaining clusters, the lag was longer. For
example, the peals signal
in cluster GOb(6) occurred at 6 sec, and that the signal was still elevated at
8 sec. In the four
cases such as this one, signal epochs selected for statistical analysis
matched the time interval
during which the peals signal was attained, and the Inaxilnum signal under the
curve was
observed. Thus, for cluster GOb(6), a 4 second lag allowed selection of the
time interval with
both the peals signal and maximum signal order the curve.
The data segment selected for analysis of expectancy responses in the case of
the 3 other
ROIs also consisted of the points at 4, 6, and 8 seconds. Regardless of the
hemodynalnic lag, the
duration of the sampled period was 6 seconds.
The dependent variable in the expectancy ANOVA was the transformed BOLD
signal,
and the predictors were the spimler and time point. Both spinner and time
point were defined as
categorical (110n-C011t111LlOLlS) variables, thLlS fOTClllg the analysis
software to carry out an
ANOVA in lieu of fitting a regression surface. By defining the independent
variables in this
fa5h10I1, It WaS possible t0 aVOld malting asslunptions about the form of the
tune courses.
At the outset of the analysis, the statistical expect system compared the
relative
efficiencies of the Tulcey bisquare estimator and conventional least-square
statistics. In the cases
of 15 of the 16 clusters, the Tulcey bisquare estimator was found to be more
efficient and thus, a
robust ANOVA Was carried out; graphical Confirmation of the need for a robust
estimator was
provided by normal probability plots. In the remaining case, the least-squares
estimator Was
5s
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
found to be slightly (~l%) more efficient and thus, as reconunended by the
expert system,
conventional least-square methods were employed.
A second test carried out prior to the ANOVA compared the within-cell
variances. In 15
of 16 clusters, these were found to be sufficiently similar that the use of
variance-adjusted
weights was not recommended. However, in the remaining cluster, the
differences between the
witlun-cell variances were sufficiently large as to cause the expert system to
recommend the use
of variance-adjusted weights.
The results of primary interest in the expectancy ANOVA were the main effect
of
spinier and the spinier x time point interaction. A main effect of spinier
indicates a difference
in the magnitude of the fMRI signals corresponding to the presentation of the
three spimers; a
spinner x time point interaction indicates the form of the signal time courses
differed across
spimiers: Given that ANOVAs were carried out on the signals from 16 different
clusters, a more
stringent alpha level (0.003) was used than the conventional 0.05 value as the
threshold fox a
significant effect.
In cases that met the criterion alpha level, the pair-wise across-spinier
contrasts were
computed at each of the three time points. Regardless of whether the main
effect of spinier or
the spinner x time point interaction met the significance criterion, the
confidence band
swrounding the location estimate was compared to zero. Given that multiple
comparisons were
carried out, simultaneous confidence intervals reflecting the variance at all
time points during
the expectancy phase were used in this comparison.
The outcome-phase ANOVA was largely analogous to the expectancy-phase ANOVA.
In all cases, the data employed fell within a 6-sec period beginning 2 sec
after the onset of the
outcome phase. The BOLD signal served as the dependent variable, and spimler,
trial type, and
time point served as the predictors; trial type, a categorical variable, was
nested within spinner.
(A $10 win following the presentation of the good spinner constitutes one
trial type, whereas a
$2.50 win constitutes another.)
Prior to the ANOVA, the expert system was used to determine whether robust or
least-
square statistics were more efficient and whether the use of variance-adjusted
weights was
recommended. A robust ANOVA was carried out in the case of 13 clusters, and a
conventional
59
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
least-square analysis was carried out in the remaining 3 clusters. Variance-
adjusted weights were
used in 7 of the 16 clusters. In aII cases, the recommendations of the
statistical expert system
were accepted.
The results of primary interest in the outcome ANOVA were the main effect of
trial type
and the trial type x time point interaction. A main effect of trial type
indicates a difference in the
magnitude of the fMRI signals corresponding to the presentation of the
different Wlth111-spllnler
outcomes; a trial type x time point interaction indicates that the form of the
signal time course
varied across trial type. As in the case of the expectancy-phase ANOVAs, the
criterion alpha
level was set to 0.003.
In cases that met the criterion alpha level, pair-wise contrasts were computed
between
the three trial types within each spinner, at each ofthe tluee time points.
Regardless of whether
the main effect of trial type or the trial type ~e time point interaction met
the significance
criterion, the confidence band surrounding the location estimate was compared
to zero. As in
the case of the data from the expectancy phase, simultaneous confidence
intervals were used in
this comparison.
In steps 522 and 524 as part of the Statistical Mapping of Imaging Data phase
502 data
was produced for the post-hoc voxel-by-voxel correlational analysis in steps
546 and 550. This
analysis sought to determine if regions not included in the hypotheses were
potentially active
dLlTlllg either the prospect/expectancy phase of the experiment, or the
outcome phase. Toward
this end, statistical correlational leaps were generated against a theoretical
impulse (i.e., gannna)
function. Specific paired comparisons for the prospect and outcome data were
the same as the
post-hoc comparisons after the ANOVA analysis. These paired comparisons were
all performed
against the medium prospect or the intermediate outcome with one exception,
namely all
comparisons between the good and bad spinners, or the high and low outcomes,
were deemed to
be redlmdant since their main comparison was already contained in the dyadic
comparisons of
good to intermediate, and bad to intermediate spilmers.
Clusters of positive and negative signal change were identified for each
paired
C0111par1S011 LlSlllg the automated cluster growing algoritlun described
above. In order to
maintain an overall oc < 0.05, this algoritlnn specifically localized
activation which lnet a
corrected p-value threshold for the volume of tissue sampled in the a priori
regions (i.e., p<4.96
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
x I0-5 for both prospects and outcomes). All other regions had to meet a
corrected (Bonferroni)
threshold for sigluficance of p<7. I x 10-~ for the estimated volume of brain
tissue per subject
sampled in this experiment. As previously, mar vox peaks identified by the
cluster growing
algoritlun had to be within a cluster of at Ieast three voxels, of which the
two voxels which were
not the peak had to meet the statistical tlueshold of p<0.07 and be within a
7lrlln radius of the
mar vox.
All activations were further checked against the functional image data to
ascel-tain that
they did IlOt overlap areas of susceptibility artifact. Such overlap was
determined by whether or
not a voxel's signal intensity during the baseline was less than the average
voxel in its slice by
50% of the difference between the average voxel signal intensity in the slice
and the average
voxel signal intensity outside of the slice.
In Phase 506 S1g111f1Ca11t differential responses to monetary outcomes were
recorded from
the NAc, SLEA, and hypothalamus to the three outcomes on the good spiln-ler
($10.00, $2.50,
$0.00). For these ROIs, the time courses diverged similarly, with signal
declines during the
$0.00 outcome, and less marked declines in the case of the $2.50 outcome. The
highest signal
levels were recorded in response to the highest value ($10.00) outcome, and in
the NAc and
SLEA, the outcome phase response to this outcome rises towards the end of the
trial. In these
ROIs, the value of the normalized BOLD signal during the outcome phase tracks
the subjects'
wimlings.
The outcome-phase time coluses were aligned to a common baseline by
subtracting the
median of the normalized BOLD signals recorded during the prospect phase.
Thus, even in the
absence of a hemodynamic response to the outcome, the recorded signal may have
decreased
during the outcome phase simply due to the waning of the prospect response.
The lcey to
d1St111gt115hlng b011a fide responses to the outcomes from the decaying phase
of preceding
prospect responses is the differential nahlre of the outcome-phase responses.
As shown by the
significant effect of outcome or the outcome by time point interaction in the
ANOVAs carried
out in 12 of the 16 ROIs, differential outcome-phase responses were indeed
observed,
distinguishing these outcome results from those of the preceding prospect
phase. Nonetheless,
the decay of prospect-phase responses may have contributed to driving the
outcome-phase
signals below zero, which was the case at 37 of the 49 time points at which
the outcome-phase
signals differed reliably fiom the baseline. Thirty of these 37 time points
moving below zero
61
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
belong to the NAc, SLEA, and hypothalamus alone. In contrast to these
subcortical signals, 11
of the I2 time points that move reliably above the baseline belong to GOb
ROIs.
The dominant pattern in the most sustained outcome-phase responses (those that
cleared
the baseline reliably at the greatest munber of time points) is typified by
the signals recorded
from the NAc, SLEA, and hypothalamus. For these three ROIs, relative to the
median of the
prospect-phase responses, the signal at the end of the outcome phase is lowest
in response to the
worst outcome on the good spimler ($0.00), somewhat higher in response to the
small gain
($2.50), and highest in response to the large gain ($10.00).
A strilcingly different pattern is observed in the case of cluster GOb(4). In
that case, the
responses to the two most extreme outcomes ($10.00, -$6.00) are higher than
the responses to
the other outcomes on the respective spinners. Thus, the responses in this ROI
provide
information about the magnitude of the outcome but not about its sign. Only
one other time
course, the response to the worst outcome on the bad spinier (-$6.00) in the
right amygdala,
deviates reliably from the baseline at more than one outcome-phase time point.
Again, it is the
response to an extreme outcome that stands out.
In phase 507, a nLUnber of prospect responses demonstrated signals with
distinct time to
peals measures. Signals from subcortical and brainstem structures with robust
simultaneous 95%
confidence bands that cleared the baseline, peaked at 4 seconds in 10 of 13
cases. Several ofthe
signals that peaked later were recorded in GOb ROIs, for instance,
differential lags are apparent
during responses to the good spinier in the SLEA and in GOb(6). It is
important to note, for the
SLEA and GOb(6), that slice acquisition occ~.ured in interleaved fashion in
the axial domain,
parallel to the AC-PC line, with a through-plane resolution of 3 mm. The
functional data from
activations in the SLEA (Talairach coordinates: 18, 0, -6) and GOb(6)
(Talairach coordinates:
25, 59, -18) were acquired only one slice apart. Thus, at each time point, at
most 100 cosec
separated acquisition of signal in the SLEA and GOb(6). In contrast, the peals
of SLEA signal
leads the peak of the GOb(6) signal by 2 seconds, and the GOb(6) response
remains near its
peals value for an additional 2 seconds during which time, the SLEA signal
declines. The
temporal separation of these acquisitions camiot be accounted for by teclmical
or averaging
constr amts.
Phase 508, was not applicable to this experiment.
62
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Research on the psychology of monetary gains and losses shows that the
subjective
response to an outcome depends on the alternative outcomes available and on
prior expectation.
In Phase 509, the interpretation of the results suggest that this was also the
case in the BOLD
signals recorded in the NAc, SLEA, and hypothalamus in response to the $0
outcomes. On the
good spilmer, $0 is the worst of the three outcomes available. The responses
to this outcome fall
throughout the outcome phase, dropping below the other time courses. In
contrast, the NAc and
SLEA responses to the $0 outcome on the bad spilmer are rising at the end of
the outcome
phase, around the time when a hemodynalnic response to an outcome might be
expected to peals;
these signals climb above the responses to the $0 outcome on the good spimler,
as does the bad-
spinner response in the hypothalamus. The $0 outcome on the bad spiln~er is
the best available
on that spinner. Indeed, the form of the BOLD time courses recorded during the
outcome phase
of bad-spinner trials on which the outcome was $0 resembles the form of the
responses in the
NAc and SLEA to the best outcome ($10.00) on good-spinner trials. Finally, the
psychological
I S research predicts that the $0 outcome on the intermediate spilmer, which
falls between the two
other values, will be experienced as near-neutral. The normalized BOLD time
courses
corresponding to presentation of this outcome fluctuate near the zero
baseline.
The design of this experiment takes into account several principles that have
emerged
from the psychological study of judgment and decision. Paramount among these
is the view that
the emotional impact of an outcomes depends strongly on the context within
which they are
experienced. Thus, the experiment was designed so as to control and manipulate
prior
expectations as well as post-hoc comparisons with the alternative
("counterfactual") outcomes
available. Both the psychological and neurobiological literature suggest that
different processes
are brought to bear when anticipating and experiencing outcomes. Thus, the
trials were
structured so as to separate over time the responses of the subjects to
prospects and outcomes.
Psychological research shows that losses with respect to a neutral point tend
to loom larger than
gains of the same magnitude. Larger gains than losses were employed in an
attempt to offset
this tendency. Five different monetary amolmts were used, enabling us to
determine how the
BOLD signal varied as a function of the magnitude and sign of the outcomes. By
111ClLldlllg one
common outcome on all three spilmer s, the influence of expectation and
counterfactual
comparison could be assessed. The asset position (C111n111at1Ve W11ll1121gS)
of the subject was not
displayed, thus increasing the likelihood that performance on each trial would
be referenced to a
colnlnon baseline. Modeling of the design of the present study on principles
well established in
63
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
prior psychological research on judgment and decision may have been crucial to
the clarity and
orderliness of the BOLD signals as well as to their tight linl~age to trial
events.
The importance of functional neuroimaging in humans and animals is apparent
when
considering that it is the primary means by which gene and molecular function
can be lined to
their behavioral effects.
The description below considers three categories of pain: (acute) experimental
pain
(sometimes referred to herein as "pain 1") (acute) sensitized (e.g.,
hyperalgesia) or inflammatory
pain, (sometimes referred to herein as "pain 2") and chronic pain nociceptive
or neLUOpathic,
(sometimes referred to herein as "pain 3")
As used herein, the term "reward/aversion" circuitry refers to those regions
referred to in
the art as "classic pain regions" and "reward regions." In accordance with the
present invention,
it has been discovered that a formerly unalown relationship uWnown exist,
between these
regions and thus those regions are referred to herein as "reward/aversion"
regions or
"reward/aversion circuitry."
Referring to Figs. 7A - 7J, in which like elements are provided having life
reference
designations throughout the several figures, central nervous system (CNS)
activity in
reward/aversion circuitry is shown in response to application of thermal
stimuli to a subject over
varying ranges of temperature. The response may be measured, for example, by
using a system
such as that to be described below in conjLmction with Fig. 11.
Referring now to Fig. 7A, an image of the anterior cingulate gyros (aCG)
having an
activation 702 in response to a 41 °C thermal stimulus is shown. The
thermal stimulus is
delivered to a subject using a Pettier based thermode (manufactured by Medoc,
Haifa Israel).
The size of the activation shown in Fig. 7A indicates the relative extent
within each region. The
size of the region corresponds to the amount of activation vohune in the aCG.
Thus, a relatively
small size corresponds to a relatively low activation volume in the aCG while
a relatively large
size corresponds to a relatively large activation volume in the aCG.
The aCG is lalov~m to activate in pain studies bilaterally (i.e. in both the
left and right
brain hemispheres.) A similar pattern is observed in the insula and the
thalamus regions of the
64
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
CNS. As is known, a conventional two sample Student's T-test will detect a
bilateral activation,
but will not indicate a temporal sequence of activation in these structures.
Fig. 7B shows a curve 704 representing a thermal stimulus delivered in the
form of a
series of blocks or thermal pulses 704a-704d. Each of the thermal pulses 704a-
704d are
provided having a pulse duration typically of about twenty-five seconds
followed by a resting
period 705 having a dw-ation typically of about thirty seconds and during
which time a neutral
temperature is applied to the subject. The curve 704 in Fig. 7B, indicates
that the thermal
St11I1L11uS 704 changes from a first temperature dwmg time periods 705 to a
second temperature
during time periods 704a - 704d. In one application, the first temperatLlre
corresponds to a
neutral temperature (i.e. a temperature which does not cause pain to a
subject) and the second
temperature corresponds to a temperature which is different from the neutral
temperature but
which also does slot cause significant pain to a subject (referred to as a non-
painful temperature).
In one experiment, the first temperature (i.e. the neutral temperature)
corresponds to a
temperature typically of about 35°C and the second temperature (i.e.
the non-painful
temperature) corresponds to a temperature typically of about 41 °C.
Thus, pulses 704a-7044 in
Fig. 7B vary from a temperature typically of about 35° C to a
temperature typically of about 41°
C.
Also shown in the plot of Fig. 7B is a curve 708 which corresponds to a zero
baseline
signal and a second curve 706 which corresponds to a plot of signal change (in
percent) vs. time
(in seconds) of a signal in the aCG brain region generated in response to a
thermal stimulus (e.g.
the thermal pulses 704a-704d) being applied to the subject. The x-axis
represents time in
seconds over the length of the experiment and the y-axis represents a
percentage signal change
with reference to the baseline value which is calculated by averaging
dimensiox~less pixel signal
values when the St1111111L1S 1S llot present using a teclmique which is
generally lalown in the art.
It should be appreciated that for each thermal pulse 704a - 704d, there is a
corresponding
positive percentage change in the temporal response as evidenced by regions
706a - 706d of curve
706 in the aCG. That is, each time one of the thermal pulses (e.g. one of
pulses 704a - 704d) is
applied to the subject, an increase is measured in the response of the aCG to
the thermal pulse as
shown by regions 706a - 706d in cwve 706 in Fig. 7B. As is lalown, the aCG is
part of the
reward/aversion circuitry in the brain and since application of one of the
thermal pulses 704a-704d
elicits a corresponding increase 706a-706d (as measured by percentage signal
change) in the aCG
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
response, the aCG is said to be positively valenced with respect to pain. It
should be noted that the
shape of pulse 706b is an artifact of noise rather than a measure of a
biologically relevant feature.
Fig. 7C shows activation in the aCG 710 iiz the CNS reward/aversion region
being activated
in response to a painfiil thermal stimulus. The size and color coding used for
the activation in Fig.
7A to convey certain information are similarly used to represent information
in Fig. 7C.
Importantly, it should be noted, that it is not possible to determine, fiom
the images shovv~m
in Figs. 7A and 7C, an objective marker of pain experience nor to determine
which activation map
corresponds to the more painful stimulus. That is, while the images in both
Figs. 7A and 7C convey
that the subject has activation in a reward/aversion region of the brain (i.e.
the aCG), one stimulation
was a thermal sensation and the other was a painfiil one. Yet they both
activate the same structure,
albeit with different volumes of activation (i.e., there is no differentiation
of "warm" non painful
from "painful" heat).
Referring now to Fig. 7D a curve 730 represents a thermal stimulus delivered
in the form
of a series of blocks of thermal pulses 730a-730d, each of the thermal pulses
730a-7304 having a
pulse dLUation typically of about twenty-five seconds during which time a
relatively high
temperature is applied to the subject followed by a resting period 731 having
a dwation typically
of about thirty seconds and during which time a neutral temperature is applied
to the subject.
The curve 730 in Fig. 7D, indicates that the thermal stimulus 730 changes from
a first
temperature to a second temperature. In one application, tile first
temperature corresponds to a
neutral temperature (e.g. a temperature typically about 35°C) and the
second application
corresponds to a temperature which is different from the neutral temperature
(e.g. a temperature
typically about 46°C which corresponds to a relatively painful
temperature). Thus, pulses 714a-
714d in Fig. 7D change from a temperature typically of about 35° C to a
temperature typically of
about 4G° C.
Also shown in the plot of Fig. 7D is a first curve 716 which corresponds to a
zero
baseline signal and a second curve 714 which corresponds to a plot of signal
change (in percent)
vs. time (in seconds) of a signal in aCG brain region generated in response to
the thermal
stimulus (e.g. the thermal pulses 730a-730d) being applied to the subject. The
x-axis represents
time in seconds over the length of the experiment and the y-axis represents a
percentage signal
change with reference to the baseline value which is calculated by averaging
dimensionless pixel
66
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
signal values when the stimulus is not present using a technique which is
generally known in the
art.
It should be appreciated that for each therznah pulse 730a - 730d, there is a
corresponding
positive percentage change in the temporal response 714a - 7144 in the aCG.
That is, each time one
of the thermal pulses (e.g. one of pulses 730a - 730d) is applied to the
subject, a corresponding
increase is measured in the response of the aCG to the thermal pulse as shown
by regions 714a -
7146d in curve 714 in Fig. 7D. As is lalown, the aCG is part of the
reward/aversion circuitzy in the
brain and since application of one of the thermal pulses 730a-730d elicits a
corresponding increase
7I4a-714d (as measwed by percentage signal change) in the aCG, the aCG is said
to be positively
vahenced with respect to pain. The decreasing size of the pulse 714c, 714d
indicate habituation to
repetitive 46 C stimuli for the subject and thus is in agreement with prior
art measurements of
subjective responses to similar experiments
As in the case of Figs. 7A and 7C, it should be noted that it is not possible
to objectively
determine which waveform 706 or 714 corresponds to the pairzfzil stimulus.
Additionally it is not
possible to objectively detect a level of pain caused by non-painful thermal
stimulus 704 and painfzzl
thermal stiznulus 712 by evaluating waveforms 706 and 714 which are measzzred
in the classic pain
center regions. That is, while the curves in both Figs. 7B and 7D convey that
the subject has
activation in a reward/aversion region of the brain (i.e. the ACG), it is not
possible from the czuves
of Figs. 7B and 7D to determine whether the subject felt pain in either case.
Referring now to Fig. 7E, an image of a NAc region 7I 8 of the in response to
a 41 °C
thermal stimulus is shown. There are no color coded regions in Fig. 7E
indicating no response in
this rewardlaversion region to the non-painful stimulus.
Referring now to Fig. 7F, when the thermal pulses 704a - 704d (shown as shaded
regions in Fig. 7F) described abOVe 211 CO11J1111Ct1o11 Wlth Fig. 7B are
applied to the subject, a
measure of the response in the NAc brain region 718 (Fig. 7E) is plotted as
cLZrve 724. As can
be seen from Fig. 7F, czuve 724 produces no net change from its baseline, and
thus can be said
to resemble the zero baseline 719. Curve 719 thus indicates that there is no
response in the
reward/aversion region to the thermal pulse train described 704. Thus, a non-
painful stimulus
such as the thermal pulse train 704 does not produce any response in the NAc
while such a pulse
train does produce a response in the aCG.
67
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 7G shows the NAc in the CNS reward region 726 being activated in response
to a
painful (i.e. 46°C) thermal stimulus. The size and color coding ofthe
activation areas are similar
to the coding described above in conjunction with Fig. 7A. The red colored
response depicted in
Fig. 7G indicates a highly significant statistical activation in the NAc.
Referring now to Fig. 7H, when the thermal pulses 730a - 7304 (sllowll as
shaded
regions in Fig. 7H) described abOVe 111 cOll~llllct1011 Wlth Fig. 7D are
applied to the subject, a
measLlre of the response in the NAc brain region 726 (Fig. 7G) is plotted as
cLlrve 734. As can
be seen fron l Fig. 7H, clove 734 fluctuates substantially below a zero
baseline 732.
It should be appreciated that for each thermal pulse 730a - 7304, tllere is a
corresponding
negative percentage change in the temporal response 734 in the NAc. That is,
each time one of the
thermal pulses (e.g. one of pulses 730a- 730d) is applied to the subject, a
corresponding decrease
is measLlred in the response of the NAc to the thermal pulse as shown by
regions 734a - 7344 in
curve 734 in Fig. 7H. As described herehl above in accordance with the present
invention, the NAc
is part of the reward/aversion circuitry and since application of one of the
thel~nal pulses 730a-730d
elicits a corresponding decrease 734a-7344 (as measured by percentage signal
change) in the NAc,
the NAc is said to be negatively valenced with respect to pain.
Thus, while it is not possible to distinguish a painful thermal stimulus from
a poll-painful
thermal stimulus by simply using measurements from a reward/aversion region
such as the aCG,
It 1S possible t0 distinguish a painful thermal St1111L11L1s fr0111 a 11011-
palllflll thermal St1111L1h1S by
examining the response from two reward/aversion regions such as the aCG and
the NAc.
Specifically, the aCG responses 702, 706 (Figs. 7A, 7B respectively) and 710,
714 (Figs. 7C
and 7D respectively) do not contain enough information to allow one to
distinguish a painful
stimulus from a non-painful stimulus by examination (i.e. it does not provide
an objective
determination that a subject is actually experiencing pain). However, by
examining the
reSpOIISeS frOln 170tH the aCG alld the NAc, It is possible t0 dlStlllglllSh
the painful StII2ILlILIS flOln
the non-painful stimulus due to the different responses 719, 734 (Figs 7F, 7H,
respectively)
which appear ill the NAc.
For each pulse 730a - 730d representing an increase in temperatLUe to
46°C in the thermal
stimulus, there is a corresponding negative percentage change in the temporal
response.734a - 7344
68
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
in the NAc. 734c may reflect an adaptation of the BOLD response. As shown
above, activation
irLforlnation fiom only the rewardlaversion region, signal 714, does not
provide enough data to male
an objective characterization of the brain activity. However, combining
information derived from
the correlation of the negative response wavefonn 734 representing the NAc
with signal 714, allows
an objective determination that the subject is actually experiencing pain
produced by the high
temperature (46°C) thermal stimulus.
Referring now to Fig. 7I, a cL~rve 736 of the GOb region of the brain
representing the
response to a series of thermal pulses 730a, 7304 followed by periods of
neutral temperat~.me is
ShOWll. As described above, the GOb is another brain region nnphcated in
reward/aversion. Curve
739 represents a zero baseline signal. Clove 736 is plotted as percent signal
change vs. time
(seconds). Vertical time lines 738 indicate the peals of early activation
phase for thermal stimuli
pulses 730a- 730d and vertical time lines 740 indicate the peals of late
activation phase for thermal
stimuli pulses 730a - 730d.
For each pulse 730a - 730d representing an increase in temperature to
46°C in the thermal
stimulus, there is a corresponding positive percentage change in the temporal
response 736a- 7364
in the GOb
Referring now to Fig.7J, a curve 742 of the VT/PAG region of the brain
representing the
response to a series of thermal pulses 730a - 7304 followed by periods of
neutral temperature is
ShoWll. As described above, the VT/PAG regI0I1 IS allOther brain region
implicated in
reward/aversion fL111Ct1o11. CLIrve 743 represents a zero baseline signal.
Curve 742 is plotted as
percent signal change vs. time (seconds). Vertical time lines 744 indicate the
peals of early
activation phase for thermal stimuli pulses 730a- 730d and vertical time lines
746 indicate the peals
of late activation phase for thermal stimuli pulses 730a - 730d.
For each pulse 730a - 730d representing an increase in temperature to
46°C in the
thermal stimulus, there is a corresponding positive percentage change in the
temporal response
742a - 7424 in the VT/PAG region. As shown above, activation information from
only the
classic pain regions, (e.g. signals 706, 714 in Figs. 7B, 7D respectively),
does not provide an
objective determination that a subject is actually experiencing pain from the
above-described
experiment. However as will be described in further detail below, by combining
information
derived from pain and other regions which are part of the reward/aversion
circuitry an objective
69
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
determination that the subject is actually experiencing pain produced by the
high temperature
(46°C) thermal stimulus can be made.
It should be noted that there is a large initial change in the signal 742
during the first
epoch 730a of the thermal stimulus and not during subsequent thermal epochs
730b-730d.
Habituation reflects adaptation of the reward/aversion system to repeated
axzd/ox controlling
aversive stimulation. The decreasing size of pulses 742a-742d indicates an
adaptation of the
brain response.
Referring to Figs. 8A - 8D, in which lilfe elements are provided having like
reference
designations throughout the several figures, central nervous system (CNS)
activity in is shown
in response to application of heat as a 41°C stimulus to a subject
sensitized to heat by capsaicin
to produce a model of sensitization/hyperalgesia (Pain 2). The response may be
measured, for
example, by using a system such as that to be described below in conjLmction
with Fig. 11.
Referring now to Fig. 8A, an image of an anterior cingulate gyros (aCG) having
an
activation 750 in response to a 41 °C thermal stimulus is shown. The
thermal stimulus is
delivered to a subject using a Peltier based thermode. It should be
appreciated of course that any
thermal mechanical, chemical device can be used to produce pain. The size and
color of the
aversion shown in Fig, 8A indicate the relative extent and statistical
significance respectively
within each region. The size of the region corresponds to the amount of
activation in a volume
in the aCG. Thus, a relatively small size corresponds to a relatively low
activation vohune in the
aCG while a relatively large size corresponds to a relatively large activation
volume in the aCG .
Also, a region having a blue color indicates a less significant activation
while a region having a
red or yellow color indicates a more significant activation. Other models of
sensitization
produced thermal, mechanical, chemical stimuli could be used, for example
prolonged hot
thermal stimulus or mustard oil or any stimulus well laloml to those of
ordinary skill in the art
into the subject to produced by hyperalgesia could be used
The aCG is 1C110Wn to activate in pain studies bilaterally. A similar pattern
is observed in
the insula and the thalamus regions of the CNS. As is lalown, a conventional
two sample
Student's T-test will detect a bilateral activation, but will not indicate a
temporal correlation
with other regions.
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 8B shows a series of unshaded regions 752 and shaded regions 754
representing a
resting period and a thermal stimulus respectively delivered in the form of a
series of blocl~s or
thermal pulses. The thermal pulses 754a-754b are provided having a pulse
duration typically of
about thirty seconds followed by a resting period 752b and 752c having a
duration typically of
about thirty seconds and during which time a neutral temperatwe is applied to
the subject. In
one application, the resting temperature corresponds to a neutral temperature
(i.e. a temperature
which does not cause pain to a subject) and the second application corresponds
to a temperature
which is different from the neutral temperature but which also does not cause
significant pain to
a subject (referred to as a non-painful temperature). In one experiment, the
first temperature (i.e.
the neutral temperatLUe) corresponds to a temperature typically of about
35°C and the second
temperature (i.e. the non-painful temperature) corresponds to a temperature
typically of about
41°C. Thus, pulses 752 and 754 in Fig. 8B vary from a temperature
typically of about 35° C to a
temperature typically of about 41 ° C.
Also shown in the plot of Fig. 8B is a curve 756 which corresponds to a zero
baseline
signal and a second curve 758 which corresponds to a plot of signal change (in
percent) vs. time
(in seconds) of a signal in the aCG brain region generated in response to a
thermal St1111L1htlS (e.g.
the thermal pulses 752 and 754) being applied to the subject. The x-axis
represents time in
seconds over the length of the experiment and the y-axis represents a
percentage signal change
with reference to the baseline value which is calculated by averaging
dimensionless pixel signal
values when the stimulus is not present using a technique which is generally
lalown in the art.
It should be appreciated that for each thermal pulse 752 and 754, there is a
corresponding
positive percentage change in the temporal response as evidenced by regions
758a - 758b of curve
758 in the aCG. That is, each time one of the thermal pulses (e.g. one of
pulses 752 and 754) is
applied to the subject, an increase is measured in the response of the aCG to
the thermal pulse as
shown by regions 758a - 758b in curve 758 in Fig. 8B. As is lmown, the aCG is
part of the
reward/aversion circuitry in the brain and since application of one of the
thermal pulses 752 and 754
elicits a corresponding increase 758a - 758b (as measlued by percentage signal
change) in the aCG
response, the aCG is said to be positively valenced with respect to pain.
Referring now to Fig. 8C, an image of aNAc region760 in response to a
41°C thermal
stimulus is shown. The thermal stimulus is delivered to a subject using a
thermal stimuli. The
size and color of the activations showlz in Fig, 8C indicate the relative
activation and statistical
71
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
significance respectively within each region. The size of the region
corresponds to .the amount
of activation volume in the NAc. Thus, a relatively small size corresponds to
a relatively low
activation in a volume in the NAc while a relatively large size corresponds to
a relatively large
activation volume in the NAc.
Fig. 8D shows a series of unshaded regions 762 and shaded regions 764
representing a
resting period and a thermal stimulus respectively delivered in the form of a
series of blocks or
thermal pulses. Each of the thermal pulses 764a-764b are provided having a
pulse duration
typically of about twenty five seconds followed by a resting period 762b and
762c having a
duration typically of about thirty seconds and during which time a neutral
temperature is applied
to the subject. In one application, the resting temperature corresponds to a
neutral temperattu-e
(i.e. a temperattue which does not cause pain to a subject) and the second
application
corresponds to a temperature which is different from the neutral temperature
but which also does
not cause significant pain to a subject (referred to as a non-painful
temperature). In one
experiment, the first temperature (i.e. the neutral temperatwe) corresponds to
a temperature
typically of about 35°C and the second temperature (i.e. the non-
painful temperature)
corresponds to a temperature typically of about 41°C. Thus, pulses 762
and 764 in Fig. 8D vary
from a temperature typically of about 35°C to a temperature typically
of about 41°C.
Also shov~m in the plot of Fig. 8D is a curve 766 which corresponds to a zero
baseline
signal and a second curve 768 which corresponds to a plot of signal change (in
percent) vs. time
(in seconds) of a signal in aCG brain region generated in response to a
thermal stimulus (e.g. the
thermal pulses 762 and 764) being applied to the subject. The x-axis
represents time in seconds
over the length of the experiment and the y-axis represents a percentage
signal change with
reference to the baseline value which is calculated by averaging dimensionless
pixel signal
values when the stimulus is not present using a technique which is generally
lazovm in the art.
It should be appreciated that for each thermal pulse 762 and 764, there is a
corresponding
negative percentage change in the temporal response as evidenced by regions
768a - 768b of curve
768 in the NAc. That is, each time one of the thermal pulses (e.g. one of
pulses 762 and 764) is
applied to the subject, a decrease is measured in the response of the NAc to
the thermal pulse as
shown by regions 768a - 768b in curve 768 in Fig. 8D. As is lazown, the NAc is
part of the
reward/aversion circuitry in the brain and since application of one of the
thermal pulses 762 ayd 764
elicits a corresponding decrease 768a - 768b (as measl~red by percentage
signal chaalge) in the NAc
72
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
response, the NAc is said to be negatively valenced with respect to pain. It
should be appreciated
that cold temperatures (in addition to hot temperatures) can be used to induce
pain.
RefelTing to Figs. 9A - 9C, in which life elements are provided having life
reference
designations tlwoughout the several figures, central nervous system (CNS)
activity in
reward/aversion circuitry is shown in response to application of a mechanical
stimulus ( brush)
by hand at a rate of about 2 strolces per second stimulus to an area in which
a subject has
neuropathic pain to produce a model of chronic pain (Pain 3). It should be
appreciated that othex
mechanical stimulus may also be used, and static or dynamic mechanical stimuli
may be used.
The response may be measured, for example, by using a system such as that to
be described
below in conjLmction with Fig. 1 1A.
Referring now to Fig. 9A, an image of an anterior cingulate gyros (aCG) having
an
activation 770 ill response to the brush StllnLllLlS is ShOWIl. The size of
the activation shown in
Fig, 9A indicate the relative extent within each region. The size of the
region corresponds to the
alnOl111t Of aCtlVat1011 111 a V01111ne 111 tl2e aCG. Thus, a relatively small
size corresponds to a
relatively low activation volume in the aCG while a relatively large size
corresponds to a
relatively large activation vohune in the aCG.
The aCG is lmown to activate in pain studies bilaterally. A similar pattern is
observed in
the insula and the thalaanus regions ofthe CNS. As is lazown, a conventional
two sample
Student's T test will detect a bilateral activation, but will not indicate a
temporal sequence of
activation in these regions.
Fig. 9B shows a series of unshaded regions 772 and shaded regions 774
representing a
resting period and a brush stimulus respectively delivered in the form of a
series of blocla. Each
of the bTLlSh pulses 774x-774b are provided having a pulse duration typically
of about twenty
five seconds followed by a resting period 772b and 772c having a duration
typically of about
thirty seconds and during which time no brush stimulus is applied to the
subject. Also shown in
the plot of Fig. 9B is a curve 776 which corresponds to a zero baseline signal
and a second curve
778 which corresponds to a plot of signal change (in percent) vs. time (in
seconds) of a signal in
the aCG brain region generated in response to a brLISh 5tIZ11111t1S being
applied to the subject.
The x-axis represents time in seconds over the length of the experiment and
the y-axis represents
a percentage signal change with reference to the baseline value which is
calculated by averaging
73
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
dimensionless pixel signal values when the stimulus is not present using a
technique which is
generally lmown in the art.
It should be appreciated that for each brush pulses 772 and 774, there is a
corresponding
positive percentage change in the temporal response as evidenced by regions
778a - 778d of curve
778 in the aCG. That is, each time one of the brush pulses (e.g. one of pulses
772 and 774) is
applied to the subject, an increase is measured in the response of the aCG to
the brush pulse as
shown by regions 778a - 778d in curve 778 in Fig. 9B. As is lazown, the aCG is
part of the
reward/aversion in the brain and since application of one of the brush pulses
772 and 774 elicits a
corresponding increase 778a - 778d (as measured by percentage signal change)
in the aCG response,
the aCG is said to be positively valenced with respect to pain.
Referring again to Fig. 9A, an image of a NAc region 780 in response to a 41
°C
mechanical stimulus is shown. The mechanical stimulus is delivered to a
subject using a
mechanical stimulus (e.g., applied by hand or a specialized delivery unit).
The size and color of
the activations shown in Fig, 9A indicate the relative extent and statistical
significance
respectively within each region. The size of the region corresponds to the
amount of activation
volume in the NAc. Thus, a relatively smalh size corresponds to a relatively
low activation
vohune in the NAc while a relatively large size corresponds to a relatively
large activation
vohune in the NAc.
Fig. 9C shows a series of unshaded regions 782 and shaded regions 784
representing a
resting period and a brush stimulus respectively delivered in the form of a
series of blocks. Each
of the brush pulses 784a-784b are provided having a pulse duration typically
of about twenty
Five seconds followed by a resting periods 782b-a having a duration typicalhy
of about thirty
seconds and during which time no brush stimulus is applied to the subject.
Ahso shown in the plot of Fig. 9C is a curve 786 which corresponds to a zero
baseline
signal and a second curve 788 which corresponds to a plot of signal change (in
percent) vs. time
(in seconds) of a signal in aCG brain region generated in response to a brush
St1111L11t1S being
applied to tile subject. The x-axis represents time in seconds over the length
of the experiment
and the y-axis represents a percentage signal change with reference to the
baseline value which
is calculated by averaging dimensionless pixel signal values when the stimulus
is not present
tlSlllg a technique which is generally known in the art.
74
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
It should be appreciated that for each mechanical stimulus 784, there is a
corresponding
positive percentage change in the temporal response as evidenced by regions
788a - 788d of curve
788 in the NAc. That is, each time one of the brush pulses (e.g. one of pulses
782 and 784) is
applied to the subject, an increase is measured in the response of the NAc to
the thermal pulse as
shown by regions 788a - 788d in curve 788 in Fig. 9C. As is lmown, the NAc is
part of the
reward/aversion circuitry in the brain and since application of one of the
thermal pulses 782 and 784
elicits a corresponding increase 788a - 788d (as measured by percentage signal
change) in the NAc
response, the NAc is said to be positively valenced with respect to pain of
the category of pain 3.
Note that both the NAc and aCG are activated positively, in this pain 3
study,unlilce tile
pattern of NAc and aCG activation for pain 1 studies illustrated in Figs. 7
and 8.
Referring to Figs. 10A - l OD, in which life elements are provided having like
reference
designations throughout the several figures, central nervous system (CNS)
activity in
xeward/aversions is shown in response to application of heat as a 46°C
stimulus and either saline
or morphine is administered to a subject to measure an analgesic effect. The
response may be
measured, for example, by using a system such as that to be described below in
conjunction with
Fig. 11. Ill the x-axis of Figs. l OB, l OD, the nnage llLllnber corresponds
to four cardiac pulses.
Referring now to Fig. 10A, an image of the NAc having an activation 790 in
response to
a 46°C thermal stimulus in a subject who has been administered
intravenous saline is shown .
The saline is administered using conventional intravenous techniques. The
thermal stimulus is
delivered to a subject using a Pehtier based thernode. The size of the
activations shown in Fig.
10A indicate the relative extent within each region. The size of the region
corresponds to the
amount of activation in a volume in the NAc. Thus, a relatively small size
corresponds to a
relatively low activation volume in the NAc while a relatively large size
corresponds to a
relatively large activation volume in the NAc.
Fig. lOB shows a series of unshaded regions 792a and 792b and a shaded region
794
representing a resting period and a thermal stimulus respectively delivered in
the form of a series
of bloclcs or thermal pulses. The thermal pulse 794 is provided having a pulse
duration typically
of about thirty seconds followed by a resting period 792b having a duration
typically of about
thirty seconds and during which time a neutral temperature is applied to the
subject. In one
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
application, the resting temperature corresponds to a neutral temperature
(i.e. a temperatL~re
which does not cause significant pain to a subject) and the second application
corresponds to a
temperature which is different froze the neutral temperatLUe and causes pain
to a subject
(referred to as a painful temperature). In one experiment, the first
temperatLUe (i.e. the neutral
temperature) corresponds to a temperature typically of about 35°C and
the second temperature
(i.e. the painful temperatL~re) corresponds to a temperatvzre typically of
about 46°C. Thus, pulses
792 and 794 in Fig. lOB vary from a temperature typically of about 35°C
to a temperature
typically of about 46°C.
I O Also shown in the plot of Fig. l OB is a curve 796 which corresponds to a
maximzun
percentage signal change and a second curve 798 which corresponds to a plot of
signal change
(in percent) vs. time (in seconds) of a signal in NAc brain region generated
in response to a
thermal stimulus (e.g. the thermal pulses 792 and 794) being applied to a
subject infused with
saline. The x-axis represents an image number instead of time (because data is
cardiac gated)
15 over the length of the experiment and the y-axis represents a percentage
signal change with
reference to a zero value which is calculated by averaging dimensionless pixel
signal values
when the stimulus is not present using a technique which is generally known in
the art.
It should be appreciated that for the thermal pulse 792 and 794, there is a
corresponding
20 negative percentage change in the temporal response as evidenced by region
798a of curve 798
in the NAc. That is, when the thermal pulse 792 and 794 is applied to the
subject, a percentage
decrease is measured in the response of the NAc to the thermal pulse as shown
by regions 798a
in curve 798 in Fig. l OB. As is known, the NAc is part of the reward/aversion
reward/aversion
in the brain and since application the thermal pulse 792 and 794 elicits a
corresponding decrease
25 798a (as measured by percentage signal change) in the NAc response, the NAc
is said to be
negatively valenced with respect to pain. When compared to the results in Fig.
7H, prior saline
infusion has no effect on the negatively valenced signal in the NAc following
the 46°C
5t11nL1111S. Note the similar pattern of decreased activation after noxious
heat alone as show in
Fig. 7H. By comparing curve 798 to curve 808 it can be observed that injection
of morphine
30 attenuates the response thus the curves 798, 808 correspond to an objective
measure of the drug
on pain
Referring now to Fig. l OC, an image of an NAc region 800 in response to a
46°C thermal
stimulus being applied to a subject infused with morphine is shown. The
thermal stimulus is
76
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
delivered to a subject using a Peltier based thennode. The morphine dose was
4mg/701cg. The
size and color of the activations shown in Fig, l OC indicate the relative
extent and statistical
significance respectively within each region. The size of the colored region
corresponds to the
amount of activation volume in the NAc. Thus, a relatively small size
corresponds to a
relatively low activation volume in the NAc while a relatively large size
corresponds to a
relatively high activation vohune in the NAc. Also, a region having a blue
color indicates a less
S1gI11f1CaIlt activation while a region having a red or yellow color indicates
a more significant
activation.
Fig. I OD shows a series of unshaded regions 802 and a shaded region 804
representing a
resting period and a thermal stimulus respectively delivered in the form of a
series of blocks of
thermal pulses. The thermal pulses 804 is provided having a pulse duration
typically of about
twenty five seconds followed by a resting period 802b having a duration
typically of about thirty
seconds and during which time a neutral temperature is applied to the subject.
In one
application, the resting temperatLUe corresponds to a neutral temperature
(i.e. a temperature
which does not cause pain to a subject) and the second application corresponds
to a temperature
which is different from the neutral temperature and causes pain to a subject
(referred to as a
painful temperature). In one experiment, the first temperatlue (i.e. the
neutral temperatLlre)
corresponds to a temperature typically of about 35°C and the second
temperature (i.e. the painfzll
temperature) corresponds to a temperature typically of about 46°C.
Thus, pulses 802 and 804 in
Fig. l OD vary from a temperature typically of about 35°C to a
temperature typically of about 46°
C.
Also shown in the plot of Fig. l OD is a curve which corresponds to the
maximum
percentage signal change 796 (Fig. l OB) and a second curve 808 which
corresponds to a plot of
signal change (in percent) vs. time (in seconds) of a signal in NAc brain
region generated in
response to a thermal stimulus (e.g. the thermal pulses 802 and 804) being
applied to the subject.
Curve 796 is provided as a means to compare signals 808 and 798 (Fig. l OB).
The x-axis
represents an image nLUnber instead of time (because data is cardiac gated)
over the length of the
experiment and the y-axis represents a percentage signal change with reference
to a zero value
which is calculated by averaging dimensionless pixel signal values when the
stimulus is not
present using a technique which is generally known in the art.
It should be appreciated that for the thermal pulse 802 and 804, there is a
greatly reduced
77
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
negative percentage change in the temporal response as evidenced by region
808a of cLlrve 808
in the NAc. That is, each time one of the thermal pulses (e.g. one of pulses
804) is applied to the
subject, a decrease is measured in the response of the NAc to the thermal
pulse as shown by
regions 808a-b in curve 808 in Fig. 10D. Note that the magnitude of signal
decrease (~l%) is
much less than the decrease produced by heat plus saline (2%, as indicated by
curve 798). As is
lmown, the NAc is part of the reward/aversion in the brain and since
application of one of the
thermal pulses 802 and 804 elicits a corresponding decrease 808a (as measLlred
by percentage
signal change) in the NAc response, the NAc is said to be negatively valenced
with respect to
pain. By examining the responses from the NAC and the results from the Pain 1
experiments
(Figs. 7A - 7H), it is possible to objectively determine the effect of the
saline and the morphine
on a painful stimulus. It can be objectively determined that morphine at an
example dose of
4lng/701cg attenuates pain by measuring decrease in activation produced by
noxious heat (46°C)
in the NAc in the subj ect.
This alteration of the signal by an analgesic drug morphine, bLlt not by a
placebo control,
indicates this method may be used to evaluate drugs with potential analgesic
effects, or more
general drugs with effects on reward/aversion circuitry that may be used to
treat pain, vs. long-
term sequelae of pain. Similar techniques may also be used to evaluate drug
effects in
functional illnesses mediated by altered functions in these rewardlaversion
brain regions.
Referring to Figs. 10E and 10F, central nervous system (CNS) activity in
reward/aversive regions is shown in response to an infusion of naloxone (in a
dose of 4mg/701cg)
in a subject. The response may be measured, for example, by using a system
such as that to be
described below in conjunction with Fig. 11.
Referring now to Fig. 10E, the VT/PAG (combined left and right components in
region
820) having an activation 820 in response t0 all 111fL1S1011 Of llalOXOlle 111
a SLIbJeCt. The size and
shade of the region 420a indicates the extent of activation and statistical
significance
respectively within the region. Thus, a relatively shall size corresponds to a
relatively low
activation in a volume in the VT/PAG while a relatively large size corresponds
to a relatively
larger volume in the VT/PAG. In region 820, a dancer shade of gray indicates a
less significant
activation while a region indicated by a lighter shade of gray indicates a
more significant
activation.
7s
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. l OF shows an unshaded region and a shaded region representing a
preinfusion
period 822a o~naloxone and a period during which naloxone is being infused
822b. The period
822a has a dL~ration typically of about five minutes followed by the infusion
period 822b having
a dLUation typically of about five minutes.
The response is represented by curve 828 showing preinfusion (white
baclcgromd) acid
post-infvision (stippled background) time points. The x-axis represents time
over the length of
the experiment and the y-axis represents a percentage signal change with
reference to a zero
value which is calculated by averaging dimensionless pixel signal values
before the infusion
using a technique which is generally known in the art.
It should be appreciated that for the infusion period 822b, there is a
corresponding
negative percent change in the temporal response as evidenced by curve 828 in
the VT/PAG. As
is lQloWIl, the VT/PAG is part of the reward/aversion circuitry in the brain
and since infusion of
naloxone elicits a corresponding decrease (as measured by percentage signal
change) in the
VT/PAG response, the VT/PAG is said to be negatively valenced with respect to
a drug that
affects pain function, and analgesic responses to pain.
Now referring to Fig. 1 l, a system 900 for determining central nervous system
(CNS)
activity in the reward/aversion circuitry over time in response to varying
temperature ranges of
noxious thermal stimuli includes means for delivering a noxious thermal
stimulus 902 to a
subject (not shov~m) having a central nervous system (CNS) 912.
A measurement system 913 is disposed about the subject to non-invasively
measure one
or more signals produced by the CNS 912 in response to the thermal stimulus.
The system 913
also produces a statistical activation map by any number of methods including
but IlOt limited to
applying a so-called Student's T-test and using the results of the T-test to
obtain a mean
hemodynamic response (MHR), represented as cL~rve 914 for a subset of active
pixels found
LlSlng the T-test. The x axis represents time in seconds over the length of
the experiment. The y
axis represent a percentage signal change with reference to a baseline value
which is calculated
be averaging dimensionless pixel signal values when the stimulus is not
present. The curve 914
corresponds to the sum of all responses in the brain detected by the T-test.
A waveform-based correlation analysis WCA processor 916 is coupled to receive
the
79
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
MHR values from the system 913. The WCA processor 916 processes the MHR values
914 to
decompose the values which form curve 914 to provide a pair of temporal
components 918 and
920 of the MHR values. It will be appreciated by those of ordinary skill in
the art that the MHR
can be decomposed into multiple phases. In general the nmnber of components is
a
characteristic of the brain response to the motivational salient stimulus. For
example, the
experiment described in conjLmction with Fig. 7 produces activity in
reward/aversion circuitry,
and the MHR can be decomposed into two components. WCA processor thus
decomposes the
MHR values into an early phase 918 and a late phase 920. . The early phase 918
generally
represents the reward and motivationemotional response, and the late phase 920
generally
represents the pain and sensory responses. Once the early and late phase
components 918, 920
are provided, the system 900 correlates the brain response in selected regions
with the early and
late phase components 918, 920 on a pixel by pixel basis to produce activation
maps in the
motivationemotional/reward circuitry 922 and sensory/pain circuitry 924.
However, if more
regions were implicated in the response, then it may be desirable to decompose
the signal into
still more components.
In one application, means 902 provides the noxious thermal stimulus to a
subject (not
shovm) in a predetermined pattern selected to elicit a predetermined response
from the subject
exposed to the stimulus. In this particular example, the noxious thermal
stimulus is delivered in
a block design oftwenty-five seconds of a relatively high temperature (i.e.
thermal stimulus
"on") followed by thirty seconds of neutral temperatwe (i.e. thermal
StlInLllLlS "off') as
represented by cwve 910. The block waveform indicates the noxious thermal
stimulus 910
changing from a first temperature to a second higher temperature. In one
specific embodiment,
the first temperatt~re corresponds to a lower neutral temperature (e.g. a
temperature of about
35°C) and the second temperature corresponds to a higher noxious
temperature (e.g. a
temperature of about 46°C). The thermal stimulus produces activity
measured by system 913
as a neuroimaging signal in the CNS 912. An analysis applied to fMRI images
produces data
that are motion corrected, intensity normalized, and talairach transformed.
The T-test produces
a statistical activation map. Conventionally, after the activation regions
were identified by T-
test analysis, analysis of the imaged signals was concluded.
The analysis of the imaged signals is continued in the present invention by
obtaiung the mean
hemodynamic response (MHR) curve 914 for a subset of active pixels found using
the T-test.
Waveform analysis is then evaluated and gamma curves fitted to these signals.
so
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Several functions have been proposed to model hemodynamic response (in this
case the
MHR). In one embodiment, gamma functions as expressed in Equation (1) below
call be used
with an added delay to account for different thermal stimulus delivery times.
S Equation (1) Y= cz+b*(t-c)d*e-~t-~~~e
In which:
a is an offset correction parameter;
b is a measure of the amplitude of the hemodynalnic response;
c is a time delay; and
d aald a determine the time to peals and width of the helnodynalnic response.
It should be appreciated, however, that other fimctions such as gaussian and
poison can
be used to do the fit.
1S In the thermal study described above in conjunction with Figs. 7A-7J, four
thermal
stimuli are delivered for each experiment and it was assumed that the galnlna
functions across
the fOllr St1111L111 W0111d have the same width and amplitude, but start at
different times. It should
be noted, however, that an analysis in which the amplitude is also variable
and adjusted for each
stimulus can be performed. Hence the values b, d, and a were optimized for the
four responses,
while the parameter c was adjusted for each response. A least-squares approach
was used to fit
the gamma fLl11Ct1o11S. It should appreciated, however, that the values b, d,
and c can be adjusted
for each response.
Two sets of gamma fiulctions which were used to model the MHR were obtained
and
statistical maps for each set, representing the early and late phases
respectively, were generated
in a similar fashion as the WCA method, i.e., using each set of fitted gallnna
functions (labeled
as early and late phases) as the MHR to calculate Pearson moment correlation
coefficients on a
voxel-by-voxel 111a1111eT. In order to improve statistics and to reduce bias
in the calculation due
to the simultaneous presence of both phases in certain structures, the time
courses of all pixels
were selectively blocked. Thus, in analyzing the early phase, for example,
time points
corresponding to the late phase were not included, and vice versa. Time points
were blocked
between the time of intersection of both hemodynamic models to time points in
which the
undesired hemodynalnic model dropped to amplitudes less than 10% of the
maximum
amplitude. Final ad~LiStInelltS 111 the 211t1llber Of tune pOlllts Were made
50 that each phase had the
s1
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
wane munber of residual time points.
It should be appreciated, however, that other methods can be used to account
for the
overlap of phases, such as subtraction methods.
The WCA processor 913 analysis provides time cowse data from the MHR signal
data 914
by decomposing the MHR signal data 914 into two temporal signal components
represented by curves
918, 920. Plot 917 shows curves 918 and 920 temporally aligned and
superimposed with the MHR
curve 914. For the thermal pain experiment curve 918 represents an early phase
activation signal
correlated with activations in some reward/aversion regions and not others. In
contrast to cLUVe 918,
these regions all respond before subjects report strong subjective effects of
the aversive stimulus.
Cwve 920 represents the late phase activation signal correlated with
activations in distinct
reward/aversion regions along with sensory regions that produce signal changes
temporally correlated
with the subject ratings of pain. WCA analysis thus allows the dissection of
early information
processing systems from conscious sensory processing systems because of the
temporal aligznnent
of the early and late phase activation signals 918, 920. This pattern of
regionally localized signal
changes for 918 and 920 characterizes a pain response to the 46° C
stimulus in reward/aversion
circuitry that is objectively distinct (Figs. 7C,7D, 7G, 7H) from responses to
the non-aversive thermal
stimulus of 41°C (Figs. 7A, 7B, 7E, 7F)
It has, in accordance with the present invention, been recognized that the
above-described
WGA processing is more sensitive than processing which utilizes only T-test
processing. Thus, the
more sensitive WCA analysis can detect regions activated in the
rewardlaversion not recognized with
prior art techniques, because the T-test alone is not sensitive enough to
detect significant activity in
some regions.
The WCA approach determines statistical significance using cross correlation
of each
pixel in a region of interest with the MHR derived from a BOLD signal. WCA
analysis looks at
pixel by pixel activation on a time of activation basis, but instead of
performing pair correlation
calculations among all pixels, each pixel is itself correlated with the MHR.
Activation maps for the regions shov~m in 922 and sensory/pain region 924 are
generated
conventionally by fusing anatomical images with statistical information
indicating a range of
validity values. Activation maps allow highly significant areas to be located
and correlated to
specific CNS structures.
82
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
It should be noted that different CNS regions are activated at different
times. The early
and late phase activation signals 918, 920 are used to derive images 922, 924
which indicate
CNS regions generally activated (image 922) fox the reward/aversion regions
vs. others(image
924) regions respectively. The derivation process includes detecting any
temporally correlated
activity for a CNS structure of interest (i.e. compare the values in curves
918, 920 and with the
CNS regions active during those times).
The early phase activation signal 918 and late phase activation signal 920 can
thus be
used repeatedly to detect any temporally correlated activity for any CNS
structure of interest.
The WCA can be applied either on a voxel by voxel basis or by regions of
interest such as the
NAc.
Further techniques can be used to quantify the activations after the WCA
analysis. These
techniques can also be applied to the MHR waveform. The methods include but
are not limited
to spatial comparison; a temporal comparison, a comparison of slope, moment
analysis,
laterality, synchrony, vohLUne, differential power function, power spectrum
a~ialysis, and region
matrix aalahysis. For example, an activation in the NAc can be quantified in
time as occurring
five seconds before an activation in the aCG.
Figs. 11A- 11I illustrate quantitative indices and qualification describes
derived from
WCA analysis of brain responses to a~z aversive stimulus (i.e., 46°C)
that were not observed for
the 41°C stimulus. Observations in this set include but are not limited
to: (a) categorical signal
differences for some reward/aversion regions; (b) increased vohune of temporal
lobe signal; (c)
signal habituation; (d) biphasic distribution of signal dispersion (4); (e)
differential pattern of
activation organized by time to peals (Tp) and dispersion (0) measures; (fj
alterations in the
MHR waveforms; and (g) synchrony of activation among reward/aversion regions
that respond
early vs. those that respond late. All these measures provide for the
objective dissection of the
CNS response to pain. Psychophysical measures provide subjective but not
objective
assessments of the intensity, unpleasantness or presence of pain. By assessing
quantitative
descriptors and quantitative indices of function in reward/aversion circuitry,
brain imaging can
provide an objective measlue of the pain experience.
Referring now to Fig. 1 1A, the results of a spatial comparison technique are
illustrated.
83
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Images 930-934 correspond to slices taken in differing spatial locations
through the thalamus
region. It is possible-to spatially differentiate activation after the WCA
analysis and detect
different nuclei activated with pain by referring to an anatomical atlas of
the thalamus.
The lower left side acronyms in images 930 - 934 identify the different
thalamic nuclei.
Thus image 930 corresponds to the anterior nucleus (na), (v1) image 932
corresponds to the
ventroposteriorlaetral (vpl) and image 934 corresponds to the ventroposterior
medial/
dorsomedial (vpm/dm) The upper right numbers in each of the images 930-934
correspond to
anterior posterior coordinate from the Talairach atlas.
Such spatial differentiation is useful because each nuclei shown in images 930-
934 has
been implicated in different functions. When some clinical conditions are
added which alter the
functions of the thalamus, such alterations can be observed using the
techniques of the present
invention described above in conjunction with Fig. 11. Prior alt techniques
where unable to
trace such changes for pain. The thalamus has a number of nuclei each of which
serves different
functions (e.g. some at sensory vs. limbic/affective functions) Fig. 11A shows
different
activations in different nuclei which subserve different functions.
Referring now to Fig. 11B, a technique for quantifying a signal response (e.g.
a signal
response as measLlred in Figs. 7I, 7J) includes integrating an MHR signal over
time and
measuring the change between any resulting plateau regions produced by the
integration.
Such integrating and measuring steps were performed for the 46°C
experiment (as
described 111 C011~1lllctloll Wlth Figs. 7 and 11 to produce a curve 960.)
Similarly, integrating the
MHR over time for the 41 °C experiment produces a curve 966. The
relative slope of each curve
corresponds to an index for the total response as detected by WCA to the
stimuli.
Cwve 960 has plateau regions 961 a-9614 and rise regions 962a-962d. The
distances
between consecutive plateau regions 961 a-961 d are designated 964a-964c.
Thus, distance 964a
represents the vertical distance between plateau region 961a and plateau
region 961b. Similarly
distances 964b represents the vertical distance between plateau regions 961b
and 961 c and
distance 964c represents the vertical distance between plateau regions 961c
and 961d. For
example illustrated in Fig. 11B, distance 964a corresponds to 12 nits,
distance 964b
corresponds to 8 units and distance 964c corresponds to 6 units. CLlrve 960
was generated by
84
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
applying four stimuli to a subject (i.e. thermal probe applied to a subject)
and measuring the
response in various brain regions as described abOVe 111 C011~1111Ct1011 Wlth
Figs. 7-11. To
generate curve 960, a 46°C thermal probe was applied to the subject
during the 30-60, 80-120,
150-190 and 220-250 time intervals as measured on the x-axis of the plot in
Fig. 1 IB. It should
be appreciated that tile 46°C thermal probe requires 5 seconds to reach
a temperature of 46°C
when starting from a temperatilre of 35°C.
Since the distances 964a-964c vary, this is a sign of adaptation of activation
in the region
being measured. In a similar mamier to curve 960, curve 966 was generated by
applying four
stimuli to a subject (i.e. a thermal probe applied to a subject) and measuring
the response in
Var10L1S brain regions as described above in conjunction with Figs. 7-11. To
generate cLUVe 966,
a 41°C thermal probe-was applied to the subject during the same time
intervals described above
for the 46°C probe. It should be appreciated that the 41°C
thermal probe requires 2 seconds to
reach 41°C from 35°C
Curve 966 has plateau regions 967a-967d separated by vertical distances 968a-
968c.
Each of the distances 968a-968c are approximately 4.5 units. Since the
distances 968x-968c do
not vary, this indicates that there is no sign of adaptation of activation in
the region in response
to the 41 °C thermal probe.
The 46°C thermal probe is considered a painfill stimulus (VAS score
greater than 5 out
of 10) while the 41 °C thermal probe in considered a non-painful
stimulus (VAS score greater
between 0 and 3). Thus, curves 960,966 can be used to generate quantitive
indices such as
measures of signal adaptatiol~/habitation which are used to provide an
objective measure of pain,
between stimuli such as the 46°C and 41 ° C inputs.
Referring now to Fig. 11 C, a plot of the first derivatives with respect to
time of the
curves 960, 966 of Fig. 11B are shown. Specifically curve 969 in Fig. 11C
corresponds to the
first derivative with respect to time of the MHR signal 914 in Fig. 11 for the
46°C thermal probe
experiment and curve 970 in Fig. 11C corresponds to the first derivative of
the MHR signal fiom
the 41°C thermal probe experiment in Fig. 11
Cloves 969 and 970 correspond to the first derivative of the MHR's for the 46
and 41 °C
experiments respectively. Curve 969 is obtained by differentiating MHR signal
914 (Fig. 11)
for the 46°C experiment. Arrows 972 marls points of inflexion that can
be used as indices for
s5
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
the onset of activation. The peals-to-peals times can further be used to
quantitate the duration of
activation, and ful-ther differentiating in a quantitative fashion, the
effects of the 46C and 41 C
stimuli.
Now referring to Fig. 1 ID, a means of quantifying the MHR curve 914 (Fig. 11)
using moment
analysis is illustrated. Histograms 980-986a represent the time-to-peals (Tp)
and the width or dispersion
(~) in CNS regions in response to thermal stimuli. The first moment, Tp, is an
index of the onset-time
for the response and the second moment, 4, is an index for the duration of the
response. The y axis
reflects the count of regions of activation and the x-axis represents time in
seconds. The histograms are
generated in a thermal stimulus experiment (as described above in conjunction
with Figs. 7 and 11).
Histograms 980 and 982 depict the distribution of the value of Tp and ~
respectively for
activated areas during the early phase of the MHR (Fig. 11) using a
46°C stimulus. Histograms
980a and 982a depict the distribution of the value of Tp and D respectively
for activated areas
IS dLlrmg the late phase of the MHR (Fig. 11) L1S111g a 46°C
stimulus.
Histograms 984 and 986 depict the distribution of the value of Tp and 0
respectively for
activated areas during the eaxly phase of the MHR (Fig. I1) using a
41°C stimulus. Histograms
984a and 986a depict the distribution of the value of Tp and D respectively
for activated areas
during the late phase of the MHR (Fig. 11 ) using a 41 °C stimulus.
The distributions allow one to objectively differentiate between the
46°C (pain) and the
4I°C )(non-pain) stimuli.
As mentioned above, the time-to-peals (Tp) and dispersion (4) measures can be
used to
segregate activations into a swnmary matrix as described below in conjunction
with Fig. 11J.
The response to the first stimulus of each activated area in both the early
and late phase
responses was fitted to a galnlrla fimction. The resulting fitting parameters
can be used to
calculate the time-to-peals (Tp) and the dispersion (0) according to the
following formulas and
the parameters of equation (2):
(2) Tp = c+ d~~e- 30
(3) ~-2~~~e
86
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
in which:
Tp is defined as the time at which the first derivative of the gamma fixnction
becomes
zer o;
4 is defined as the time span between the two inflection points in the gamma
function
which could be obtained from the roots of the second order derivative of the
gamma fLlllct1011.
In equation (2) 30 seconds were subtracted from Tp to shift the zero time, to
the onset of
the fir st stimulus.
Referring now to Fig. 11E, a means of quantifying the MHR curve 914 (Fig.
11) for laterality differentiation is illustrated. Curve 990 is the fMRI
response as detected by
WCA in the right aCG when the left hand is stimulated with 46°C
stimulus. Curve 992 is the
response observed in the contralateral (left) aCG when the left hand is
stimulated with 46°C
stimulus. The shaded area 994 represents the time when the stimulus is
applied. Curve 996
represents a zero baseline signal. The x-axis represents tune in seconds over
the length of the
experiment. The y-axis represents a percentage signal change with reference to
the baseline
value which is calculated by averaging dimensionless pixel signal values when
the stimulus is
not present.
Using WCA, bilateral signal changes can be deconvolved into early or late
phase
activations, and potentially localized in opposite hemispheres. In this way,
one can quantify
the temporal ordering of the brain activation. It should be noted that curves
990, 992 illustrate
that both the left and right aCG activate, but that the activations have
different times-to-peak
and durations of activation, even though the same stimulus was applied. Fig.
11E thus
illustrates that one can measure a sequence of events for subcomponents of the
same structure.
There may be some components that, although dominant, require that another
component be
involved to achieve an integrated response.
Fig. 11F shows the synchrony of activation within two sets of reward/aversion
regions 950 and 952 following a noxious thermal stimulus. This synchrony
pattern separates the
pain response from the non-pain response. In the past, regions shown in region
950 were termed
reward regions while regions shown in region 952 were thought to be classic
pain regions.
Establishing a temporal sequence of activation is one method to qllalltlfy the
lllapp111g results of
the WCA analysis. The structL~res in the region 950 that shows significant
correlation consist of
the SLEA, VT/PAG, orbital gyrus and anterior cingulate cortex. Analysis of the
correlation
87
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
among structures in the region 952 indicated significant correlation of the
insula, the thalamus,
the SI, and the aCG.
The lines intercomlecting each pair of regions represent a temporal
correlation between
the two regions. The thicker the line the greater the correlation. For
example, the correlation
coefficient between the aCG and the SLEA 956a is between .3-.4. In 952, the
correlation
coefficient between the aCG and the INS 956h is greater than 0.9. It should be
appreciated that
no single part of the brain defines the response to chronic, acute or arty
other pain process.
Generally the activation's illustrated in 950 occur in a early phase that
occurs before the
activations illustrated in 952.
Activation's for the 46°C stimulus that are temporally correlated can
be identified via ~a
Pearson's correlation analysis. Significant correlation's (p < 0.0025) can be
observed for activatioii in
the early phase of some reward/aversion. A strong correlation exists between
the SLEA and VT/PAG
along with the GOb and the aCG . In contrast, the NAc , which displayed a
negative signal, does not
correlate with the form or phase of signal froze these regions. It should be
appreciated that although
only positive correlation's are shown in Fig. 11F, negative correlation's can
be calculated.
Highly significant positive correlation is observed between structzzres such
as SI somatosensory
cortex, insula, and thalamus, that also occur in the late phase. The results
indicate that a number of
regions classically identified with pain function show correlated activation
dzuing the late phase.
Referring now to Fig. 11 G, an example of the quantification of the volume of
activation
as detected using both the WCA technique described above in conjunction with
Fig. 11 and the
standard T-test is shown both for 41°Caand 46°C thermal probe
experiments. By comparing the
relative volzunes via bars 1002a-1002f from the results of the T-test to the
relative volume
expressed as bars 1004a-1004f from the results of the WCA analysis, it is seen
that the WCA
analysis is more sensitive than the T-test (i.e. the WCA analysis is able to
measure a greater
volume of signal change with a greater sensitivity than the T-test approach).
The volwnes are
measwed as the total number of voxels activated above a statistical threshold
in a particular
region (the tlireshold is defined using a priori or post hoc criteria def ned
previously). Fig. 11 G
illustrates measured volumes for each technique in the frontal lobes, the
parietal lobes, temporal
lobes, medial paralimbic regions, subcoz~tical gray matter, and the brainstem
and cerebellum.
ss
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
For parietal, temporal, paralimbic particular subcortical, and brainstem
regions,
activation fox the 46°C experiment, and for most of the regions for the
41 °C experiment, WCA
detects more volume than the standard Student T-test analysis. These distinct
volumes for the
46C and 41 C conditions, as detected by WCA, filrther distinguish the pain
response from tlse
non-pain response.
Now referring to Fig. 11H, a quantification of the differential power flmction
as a
flmction of temperatwe is shown. Curve 1020 corresponds to the percentage
change of signal
amplitude of activation as detected by WCA for the insula. Curve 1022
corresponds to the
percentage change of signal amplitude of activation as detected by WCA for the
SLEA. Both
curves 1020 and 1022 display differential power law dependence on temperature.
Such
differences are used as indices for quantlfymg reward/aversion circuitry
responses to painful vs.
non-painfill stimulation.
It should be appreciated that different structures might have a power flmctlon
relationship t0 temperature which is different from each other. A wunber of
reward/aversion
structures may have a response similar to that ShOwl1 for the insula, Whlle
Others play have
responses similar to that shown for the SLEA.
An exponent of power function for each for each brain regions is computed as:
(T-3 5)''
Structtue X
SLEA 4.3
INS 2.1
Each of the responses of these brain regions can then be characterized by
these indices.
Now referring to Fig. 1 1I, fourier-transforms of MHR signals are shown for
four
temperatwe stimulus experiments. CLUVe 1024 represents a spectrum for a
temperature
experiment performed using male subjects. Curve 1026 represents a spectrlun
for an experiment
performed using female subjects during the follicular phase of the menstrual
cycle.
Curve 1028 represents a spectrum for an experiment performed using female
subjects
dining the luteal phase of the menstrual cycle. Curve 1030 corresponds to the
power spectrLUn
of the actual temperature cwve of the probe.
89
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
The inset graph is a continuation of the x-axis but at a different scale (as
shown on the y-
axis of the inset).
Each curve includes different contributions of other harmonics, tal~en
together these
harmonics uniquely characterize each curve. For example, signals having
relatively high
haT1110111CS 111 the frequency range of .02Hz to .OSHz tend to have a
relatively rapid onset and a
relatively rapid return to baseline (curve 1028). Signals having responses in
the frequency range
of 0 to about .0125 HZ tend to have a relatively long lasting response. AS
ShOwll by cLlrVes (1026
and 1028) this power spectrwn analysis reveals relatively large differences
for brain activation
in female subjects at different points in their menstrual cycle. Thus power
spectrmn analyses
provide another technique to quantify the response of a signal. Thus if it is
desired to quantify
the response to pain in three different groups, then power spectrum analysis
can be used to
segregate and identify the different groups.
Referring now to Fig. 11 J, a matrix 1040 can be generated for classifying
various regions
on the basis of response time (rapid pear or delayed peals), dispersion time
(fast or slow), and
location (left or right). It should be appreciated that matrix 1040
corresponds to a pattern
recognition matrix having a pattern recognition format for a brain function.
In this particular
example, matrix 1040 provides a matrix pattern fOT reCOgllltloll Of nOXIOLIS
heat. It should be
appreciated, however, that other matrix patterns will be used for other
stimuli (e.g. drug effects,
etc...). Matrix 1040 includes four quadrants 1042-1048. Each of the quadrants
1042 -1048
include a left cohunn 1042a-1048a and a right column 1042b-1048b.
Quadrants 1042, 1044 have listed therein brain regions having a dispersion
time of
greater than 14.9 seconds and which are thus characterized as having a
relatively slow dispersion
characteristic. Quadrants 1046, 1048 have listed therein brain regions having
a dispersion time
of less than 14.9 seconds and thus which are characterized as having a
relatively fast dispersion
characteristic.
Columns 1042a, 1042b, 1046a, 1046b have listed therein left and right brain
regions
having a peals response time of less than 19.6 seconds respectively and thus
which are
characterized as having a relatively rapid peals response time. Columns 1044a,
1044b, 1048a,
1048b are the left and right brain regions having a peals response time of
greater than 19.6
seconds respectively and thus which are characterized as having a delayed
peals response time.
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Many of the brain regions also are listed with parenthetical reference numbers
which
correspond to Brodmann areas. It should be appreciated that regions 1049a,
1049b, 1049c and
1049d correspond to portions of the reward/aversion circuitry that in the past
have had formally
been considered to mediate reward and 210t pain functions. The work profiled
here shows that
these traditional reward regions are part of a generalized reward/aversion
circuitry.
Activations can be classified as having a "rapid response" where Tp < Tp",e1"
or having a
"fast dispersion" where 4< 0",e,", (Tp",ean(46°C) ' 19.6 ~ 7.5 s;
~",e~,n(46°C) = 14.9 ~ 6.8 S (111ea11 ~
SD)). Structures with a Tp or a 4 larger than the average can be described as
having a "slow
response" or a "slow dispersion." Examples of regions with a rapid response
and rapid
dispersion to the 46°C stimulus include the GOb while an example of a
region with a slow
response and slow dispersion is observed with SI somatosensory cortex .
It should be appreciated that matrix 1040 defines a pattern of indices for a
particular pain
or analgesic state (i.e. pain 1-a 46°C thermal stimulus).
It is recognized, however, that for a different pain or analgesic state the
pattern of indices
will differ from that shown in Fig. 11J. For example, in response to an
analgesic or non-noxious
stimulus, the NAc, SLEA will not activate and thus no corresponding index will
appear in the
matrix 1040. As a~iother example, the computation of the indices includes the
valence
characteristic of the regions. In a pain-2 state it is l~nov~m that thalamus
will change valence.
Thus, the value of the index associated with the thalamus in the matrix will
change from the
value which is computed in the pain-1 case.
For Pain 3 both the NAc and the thalamus change valence and thus the values of
these
indices will change from that computed in the pain-1 case.
Also, the position of the indices within the matrix 1040 may change. That is
NAc index
may move from quadrant to another quadrant in response to some stimuli.
Fig. I 1K illustrates how WGA analysis enables evaluation of foci of
activation in a
structure of interest such as the aCG. As described above, a focus of
activation is a group of
pixels showing significaalt activation compared with baseline that are found
in a the gray matter
91
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
of the brain. Typically one considers a focus of activation within a single
structure, for instance,
it is possible to differentiate activation within a structure (e.g. such as
the aCG that occurs early
or late after a aversive thermal stimulus).
hnages 1060 -1068 represent 3.1251nm MRI sagittal slices across the brain
midline.
Vertical lines indicate the location of the anterior commissaure (thiclc
vertical line) 1059, and
head of the corpus callosum 1058 (thin vertical line).
The top row, images 1060, 1061 and 1062, depicts activation in the aCG
detected by
WCA of the MHR. The middle row, images 1063, 1064 and 1065, depicts activation
detected in
the early phase, and the bottom row, images 1066, 1067 and 1068, depicts
activation detected in
the Late phase. The center slice, images 1061, 1064, and 1067, runs through
the middle of the
brain, the others are located 3 mm to the right (left column) and 3 lnm to the
left (right column).
By deconvolving the WCA analysis of the MHR into the early and late phase (as
described abOVe 111 CO11J1111Ct1011 Wlth Fig. 11), images 1063-1068 divide
some activations into
sets of activatians with distinct temporal behaviors. hnage 1061 has an
activation region 1061 a
which can be deconvolved into early and late activation regions as shown in
images 1064, 1067.
For example, the pattern of activation in the aCG could be divided into number
of foci, some
within the putative "cognitive division", and the other within the putative
"affective division" of
the aCG.
The activation localized in the putative "cognitive division" could be
dissected
using WCA analysis into 4 foci in the early phase 1063a, 1064a, 1064b, 1065a
and one focus in
the late phase 1068a (images 1063-1068). No focus within the "affective
division" of the aCG
appeared during the early phase images 1063-1065 , though at least two foci
1067a, 1067b
activated in the late phase images 1066-1068.
Activation in the aCG that occurs early or late may represent activation in
ftmctionally
different CO1np011e11tS Of the StrllCtllre.
This functional partition of the structure on the basis of its temporal
response to an
aversive stimulus distinguishes this response from the structures response
dlulng non-aversive
stimulation, and can be used to identify the pain response as such merely from
the functional
92
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
imaging data.
To distinguish subtypes of pain, such as acute thermal pain (pain 1), from a
surrogate
model of neuropathic pain (e.g., capsaicin induced hyperalgesia, pain 2), or
between acute pain
(pain 1) and actual neuropathic pain (pain 3), other patterns of
rewardlaversion circuitry
activation can be evaluated. For instance, to first focus on the distinction
of acute thermal pain
(pain 1) from an acute model of neuopathic pain (pain 2) by the patterns of
brain activation,
brain regions such as the brainstem spV region and the thalamus can be
interrogated.
Referring now to Figs. 12A-12F in which like elements are provided having like
reference designations throughout the several views, the images reveal an
activation of the spV
(Sty' cranial nerve nuclei in the brainstem) following application of noxious
heat (46° C) in the
mamer described above in conjunction with Figs. 7-11 to the skin of a healthy
volunteers.
Activation measured using a non-invasive measurement technique is shown in a
coronal plane
1202 (Fig. 12) at an activation point 1204, in a horizontal plane 1206 (Fig.
12A) at an activation
point 1208 and a sagittal plane 1210 (Fig. 12B) at an activation point 1212.
The statistical
tlueshold for activation wasp < 0.01.
The activation sites shown in Figs. 12 -12B can be compared with an anatomic
map
1214 shown in Fig. 12C. A region 1216 in the anatomic map 1214 corresponds to
an activation
in the spV. Reference designators 1218 (Figs. 12C, 12E) show the approximate
location of
activation in sagittal and horizontal anatomical sections. Reference
designators 1220 (Figs.
12D, 12F) show the location of the spV in caudal pons and caudal medulla. The
designators
"R," "L," "D," and "V" indicates right, left, dorsal, and ventral regions
respectively.
Referring now to Fig. 13, surrogate models of sensifiization are explained.
Activation in the spV and thalamus following allodynia produced by a heat-
capsaicin model in a
healthy volunteer are, together, different than in acute pain.
In one experiment, the following paradigm was used. First allodynia was
induced by
application of heat in the form of a heat probe as described above to portions
of the face of a
subject. The heat was applied at a temperature of 44° C fox time period
of 5 minutes. Next, a
0.075% capsaicin cream was applied for 20 minutes in the same facial area
where the heat probe
had been. The capsaicin cream was modified following the method described in
"A new hmnan
93
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
experimental pain model: the heat/capsaicin sensitization model," Petersen and
Rowbotham,
Neuroreport. 1999; 10(7):I511-6. Allodynia was produced by the application of
normally non-
110X1011s brush and 41 °C stimuli to the right (R) and left (L) V2
division of the trigeminal node.
For the 5 minute thermal application, the capsaicin application and the pain
induced by
normally non-noxious mechanical and thermal stimuli to the right or left V2
region, the subjects
rated the intensity of the pain they experienced using a conventional on-Iine
VAS rating scheme
(i.e. an 11 point visual analogue scale 0-10; where 0 = no pain and 10 =
maximwn pain). The
subjects rated the 5 minute thermal application and the capsaicin application
on the V2R as
approximately 5 and 2.5, respectively, on the VAS rating scale. The
application of the brush to
the V2R region was rated as a 2.5 on the VAS scale and the brush to the V2L
region (i.e. the
wtreated V2 region) produced no pain. Also, application of the 41° C
probe to the V2R region
was rated as approximately 9 on the VAS scale while application of the 41
° C pr obe to the V2L
region was rated as approximately 1 on the VAS scale.
Following the application of the 41 ° C thermal probe to the V2 area of
the skin treated
with capsaicin, activation in the ipsilateral spV was observed using a
noninvasive measurement
tech~lique (e.g. fMRI) while no activation was observed in the
contralateralhmtreated V2 side
using the same noninvasive measurement teclmique. This indicates that the
measured
activations in the ipsilateral spV correspond to the ratings provided by the
subjects on the VAS
scale, and are the same during a surrogate model of neuropathic pain (pain 2)
and during acute
pain (pain 1)
As shown in Fig. 13, a curve 1300 of the spV region representing the response
to a series
of non-noxious thermal pulses 1302x, 1302b (as administered via 41° C
thermal probe pulses)
followed by periods of neutral temperature 1304x, 1304b is shown. Curve 1306
represents a zero
baseline signal. Curve 1300 is plotted as percent signal change vs. time
(seconds).
For each thermal pulse period 1302x, 1302b representing an increase in
temperature to 41 °
C in the thermal stimulus, there is a corresponding positive percentage change
in the temporal
response 1308x, 1308b in the ipsilateral spV. Thus the ipsilateral spV is
positively valenced with
respect to thermal pain indices by experimental allodynia (pain 2). This
response can be used in
conjLmction with responses from other reward/aversion circuitry (e.g. GOb,
NAc) to allow an
objective determination of whether a subject is actually experiencing pain to
be made.
94
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
To distinguish between pain 1 and pails 2, differential responses in the
thalamus may be
used. In acute pain, the thalamus produces positive signal change (Fig. 1F,
952).
Referring now to Fig. 13A, a curve 1310 representing the response of the
thalamus to a
series of brush strobes of an example of pain 2 is shown. Curve 1312
represents a zero baseline
signal. The brush strobes are applied during time periods designated 1314a,
1314b. Curve 1310
shows a decreased signal change in the thalamus in the selisitized state as
evidenced by regions
131 Ga, 1316b.
That is, there is a decrease in signal in the contralateral thalamus following
brush
induced allodynia compared with no signal induced by brush on the
contralateral mirror side
alone (p c 0.01, t-test).
I S Differences in the sign of signal change in reward/aversion regions such
as the thalamus
may be used to d1St111gL11Sh StlbtypeS Of palls SLlch aS pain 1 and pain 2.
Referring to Figs. 14A and 14B, a means for objectively differentiating acute
physiological or acute pain (pain I) from chronic pain (pain 3) is shown.
Central nervous
system (CNS) activity in the NAc is shovcm in response to application of
capsaicin and a brush
stimulus. A camel hair brush is applied to the skin to produce a painful
response in a cluonic
pain subject with damaged nerves (allodynia). The response may be measured,
for example, by
using a system such as that to be described below in conjunction with Fig. I I
.
Referring now to Fig. 14A, an image of a NAc having al activation 1400 in
response to a
brush stimulus is shown. The brush stimulus is delivered to a subject using a
camel hair brush.
The size and color of the activation shown in Fig. 14A indicate the relative
extent and statistical
significance respectively within each region. The size of the colored region
corresponds to the
amount of activation volume in the NAc. Thus, a relatively small size
corresponds to a
relatively low activation volume in the NAc while a relatively large size
corresponds to a
relatively large activation vohune in the NAc . Also, a region having a blue
color indicates a
less significant activation while a region having a red or yellow color
indicates a snore
significant activation.
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Fig. 14B shows a series of unshaded regions 1402 and shaded regions 1404
representing
a resting period and a brush stimulus respectively delivered in the form of a
series of brush
strobes. Each of the' brush stimuli 1404a-1404b are provided having a pulse
duration typically
of about twenty-five seconds followed by a resting period 1402b and 1402c
having a duration
typically of about thirty seconds and during which time no stimulus is applied
to the subject.
Also shown in the plot of Fig. 14B is a curve 1406 which corresponds to a zero
baseline signal and a second curve 1408 which corresponds to a plot of signal
change (in
percent) vs. time (in seconds) of a signal in NAc generated in response to the
stimulus (e.g. the
series of brush strokes 1404a and 1404b) being applied to the subject. The x-
axis represents
time in seconds over the length of the experiment and the y-axis represents a
percentage signal
change with reference to the baseline value which is calculated by averaging
dilnensionless
pixel signal values when the stimulus is not present using a technique which
is generally
known in the art.
It should be appreciated that for each the series of brush strokes 1404a and
1404b, there
is a corresponding positive percentage change in the temporal response as
evidenced by regions
1408a - 1408b of curve 1408 in the NAc. That is, each time one of the series
of brush strokes
1404a and 1404b is applied to the subject, an increase is measured in tile
response of the NAc to
the series of brush strokes as shown by regions 1408a - 1408b in curve 1408 in
Fig. 14B. As is
lalown, the NAc is part of the reward/aversion in the brain and since
application of one of the
series of brush strokes 1404a and 1404b elicits a corresponding increase 1408a
- 1408b (as
measured by percentage signal change) in the NAc response, the NAc is said to
be positively
valenced with respect to pain. Typically heat pain produces negative/decreased
signal in the
NAc (i.e., pain activation in the normal and sensitized state in a normal
nervous system produces
decreased signal in the NAc). However, pain produced by brush in a chronic
pain patient with
damaged nerve (allodynia) results in a positive signal in the NAc. By
recognizing this type of
response using WCA, a means for objectively differentiating acute
physiological or acute pain
(pain 1) from chronic pain (pain 3) is provided.
Referring now to Figs. 15 - 15C, activations for a thermal stimulus experiment
(as
described 111 CO11~L111Ct1o11 Wlth Figs. 7 and 11) in three struct~.ires for
men, for women during the
follicular phase of the menstrual cycle, and for women during the lateral
phase of the menstrual
cycle are shown.
96
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
In Fig. 15, images 1502, 1503, and 1504 depict activation in the frontal
lobes, while
images 1505, 1506, and 1508 depict activation in the insula and images 1510,
1511, and 1512
depict activation in the aCG.
Figs. 15A-15C show curves 1514, 1516, and 1518 which correspond to measured
MHR's for men, women during the mid-follicular and during the mid-lateral
phases
respectively. Curves 1514, 151 G, 1518 correspond to average MHR signals for
the entire brain.
It should be noted that the responses as evidenced by curves 1514, 151 G, 1518
are different for
each ofthe different groups of subjects. That is, the MHR curve 1514 for men
differs from the
MHR curves 1516, 1518 for women daring the follicular and during the luteal
phases
respectively. Thus an objective lneasllre of gender differences is provided.
Similarly, curve 1 S 1 G for women during the follicular phase differs from
curve 1518
during the luteal phase. Thus an objective measure of differences between
women at different
points in their menstrual cycle is provided.
Such results can be incorporated in a pattern matrix such as the pattern
matrix described
above in conjunction with Fig. 11J. Furthermore, in addition to measuring
differences in gender
and differences in women at different phases of their menstrual cycle, the
measurements can
also be used in selecting subjects for a drug study. For example, if one is
performing a drug
study using men and women, it is desirable to have the subjects as closely
correlated as possible.
Thus, it may be desirable to use the above objective measure to select, for
example, women in
the follicular rather than luteal phases of their menstrual cycle if they are
to be compared to a
group of men.
Referring low to Fig. 1 G, a drug evaluation technique for rapidly eVahlatlllg
dltlgS 111
subjects, 111Ch1d111g hlllnall SLlb~ectS, begins with step 1602 111 Whlch
Ca11d1dateS are SeheCted fOT
Ch1I11Cah teStlllg. The step Of selecting candidates includes selecting a
group Of SLlb~eCts and
performing conventionah molecular discovery and pre-clinical evaluation to
select candidates for
the clinical testing. The selection step may include, for example, the
selection of an eluiched
group (e.g. a group in which the subjects have a response to a particular
drug/test which
indicates that the subjects are mechanistically similar or a group in which
there is a pain
response after withdrawing medication). The selection step may alternatively
seek a random
97
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
group of subjects meeting study inclusion criteria. Other methods, well known
to those of
ordinary skill in the art for selecting relatively small groups for drug
testing may also be used.
The tecluique then proceeds to step 1604 in which each of the selected
candidates are
randomly selected to be included in one of the first and second subsets (i.e.
the candidates are
divided into two groups). Next, as shown in step 1606, each of the candidates
in the first subset
has a drug administered to them and each of the candidates in the second
subset has a placebo
administered to them. The dosage of the drug or placebo administered to each
of the candidates
corresponds to an amount equal to a therapeutic or sub-therapeutic dose of the
drug to be tested
or the placebo.
Before describing steps 1608-1616 it should be noted that these steps are
preferably
performed simultaaleously. However, it may be possible to practice some of
steps1608-1616 at a
different time than other of steps 1606-1608.
In step 1608, the first neuroimaging study is then performed on both the first
and second
groups of candidates to non-invasively measure signals from the their central
nervous systems
(CNS), specifically focused on reward/aversion circuitry, or output/input
regions to them. In one
example, fMRI measurements from a central nervous system (CNS) are then
processed using the
WCA method described above in conjunction with Figs. 7 and 1 l, to evaluate
signals for various
CNS regions in each candidate in response to the effects of the drugs and
placebo. Thus, in
steps 1606 and 1608, a drug being investigated is provided to the first subset
of candidates while
a placebo is given to the second subset of candidates and a noninvasive
measurement of a
response in a brain region is made.
The teclmique then continues by administering a placebo to each of the
candidates in the
first subset and a drug to each of the candidates in the second subset as
shown in step 1610 and
then performing a second neuroimaging scan on both the first and second subset
of candidates as
shov~m in step 1612. Thus, in steps 1610, 1612, a drug being investigated is
provided to the
second subset of candidates while a placebo is given to the first subset of
candidates and a
noninvasive measurement of a response in a brain region is made.
The process continues in steps 1614, 1616 in which the psychophysical
responses a~ld
physiological responses axe collected for each of the subjects in response to
the effects of the
98
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
drugs and placebo. The physiological data may be collected, for example,
during a series of
experiments in which stimuli are provided to the subject. Such psychophysicah
and
physiological responses are described above 111 CO11~L1nCtloll Wlth the MEMP
processing
described in Fig. 5.
The fMRI data (showing differential activation), on-line psychophysical (e.g.,
paid
ratings and other hedonics) and physiological data (e.g. heart rate (HR),
electrocardiogram
(ECG), ETC02, GSR or laser-Doppler measures of skin-blood flow) are recorded
for
correlation analysis.
In step 1618 the fMRI data, psychophysics data and physiological data are
correlated.
Such correlation maybe performed, for example, as described above in
conjunction with Fig. 5.
The obj ective measures provided by the fMRI teclmique allows fewer test subj
ects to be used
than in prior art techniques. By computing fMRI data for each of the
candidates in the first and
second groups and correlating the fMRI data with the psychophysics data and
the physiological
data, the effect of the drug on the candidate can be rapidly evaluated.
In one embodiment, the technique utilizes an N of 1 design method with a
double-blind
cross-over design (e.g. neuroimaging I and II). This may then be repeated on a
third trial for
either a placebo or a drug. The candidates receive three scans with a drug or
a placebo in a
double-blind, randomized, cross-over (Neuroimaging I and II or III) design.
This procedure can
optionally be repeated in a third trial for either the placebo or the drug.
The
physiological/psychophysical and fMRI data sets are all collected during the
experiments. By
correlating the fMRI measurement with physiological arid psychophysical
measures, one is able
to dissect the fMRI brain data into its functional subcomponents as discussed
above. It is
desirable to correlate fMRI data with physiological and psychophysicah since a
positive
correlation between the fMRI and physiological and psychophysical
measurements, one can
objectively define the relationship~between structure and function. It also
allows verification
that the data is not tainted by physiohogical artifacts.
JO
The data can be further correlated to results from testing a similar drug or a
drug which
has desirable properties. The results can be used to look at analgesic effects
of drugs by
objectively examining the time correlated effects in the reward/aversion
regions with the
psychophysical and the psychophysical measurements.
99
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
The technique of the present invention can thus be used to evaluate drugs more
rapidly
than conventional methods because it uses physiological and psychophysical
data which is
correlated With activations in CNS regions (i.e. an absolutely objective
measure provided via the
fMRI process) which are implicated in the effects of the drug compound.
Conventional techlniques fail to provide an obj ective test for measuring the
effect of a
drug on chronic pain. Animal models may not adequately define the human
condition during
cluonic pain, and thus are frequently not helpful for early determination of
potential clinical
I O efficiency. The qualitative description and quantitative indices
characterizing the pain respoilse
(for Pain l, 2, or 3), in reward/aversion circuitry as accessed by
neuroimaging will further allow
investigators to discover where a particular drug acts on the CNS to produce
its effects
Clinical trials using the technique of the present invention can provide an
accLUate
15 assessment of a drug by evaluating a low number of subjects (i.e., 20
subjects) instead of the
large cohort typically needed by other empirical techniques. Furthermore, the
current invention
gives an absolute objective measure of pain.
The experiments and stimuli provided to the subjects can be developed using
empirical
20 techniques. In the above examples, thermal probes and mechanical brushes
were used. It should
be appreciated, however, that other thermal, mechanical, chemical or other
stimuli can also be
used.
It will be appreciated by those of ordinary skill in the art that the
technique of the present
25 invention can be used to evaluate various compounds, drugs, and
biopharmaceuticals both 111
therapeutic and sub-therapeutic dosages. The technique of the present
invention can also be
used to discover new drugs, gene products , and therapy (for example
acupunctLUe).
Coupled with specifically designed experiments, this method can augment or
replace
30 clinical experts and pal2els using techniques such as the Diagnostic
Statistical Malmals (such as
DSM-NR) for psychiatric classification of disease. This method would
specifically evaluate
reward/aversion regions implicated in the presentation of psychiatry and
psychological
dysfimctions, to objectively determine the presence of such psychiatric or
psychological
problems in clients and patients. This method would thus produce a set of
radiological tools and
35 techniques to replace the current use ofpatient signs and symptoms as used
in current DSM-NR
100
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
or other diagnostic formulations to diagnose psychiatric and psychological
dysfunction, to
predict treatment response, to monitor treatment progress, and ultimately to
determine successful
treatment. It is important to note, that this method would also be applicable
to evaluating and
diagnosing functional sequelae of pain syndromes.
This technique reduces the number of subjects typically required for an
evaluation to a
substantially smaller cohort site (for example, N=10 subjects).
Referring now to Fig. 17, a technique for imaging the trigeminal nucleus is
shown. It is
desirable to image the SpV since the SpV is the first synapse from the
periphery and thLlS It
provides information regarding pain input to reward/aversion circuitry (i.e.
it is the "gateway" to
the central nervous system). Conventionally, CNS regions in the spinal cord
1702 have not been
imaged to detect pain because the region is difficult to image with MRI and
not accessible with
PET. The degradation of the MRI signal is due to the noise induced by cardiac-
induced effects.
The cardiac-induced signal fluctuations overwhelm or partially mask the signal
of interest,
mahcing it difficult to process. The artifact in the images occurs because the
standard imaging
plane is orthogonal to the spinal cord. The conventional method and imaging
axis tend to be
optimized for imaging other areas of the brain and not the brain stem. A non-
standard plane is
used in the technique of the present invention to minimize cardiac-induced
signah fluctuations on
the signals of interest.
The selection of planes (called "slice prescription") was discovered by
observing slices
capturing the brain stem. It was noticed that the brain stem was coming in and
out of the image
with each cardiac pulse. Those slices were prescribed per standard methodology
(i.e. a
methodology in which alignment is done with brain landmarks such as the
anterior commissar-
posterior commissar axis). In the present technique, slices axe prescribed
that are parallel to the
brain stem. In one embodiment, the teclmique includes prescribing 3-4 slices
out of 30 behind
the brain-stem with each slice being 3 nnn thick. It is thus not necessary to
measure angles, as
with any sta~ldard prescription of slices. In one embodiment, slices can be
aligned with certain
landmarks. In one particular example, the fifth slice is placed at the
posterior edge of the brain
stem and runs as parallel as possible along it. Cardiac gating can also be
used with the above
techuque to further improve the measurement results.
All references cited herein are hereby incorporated herein by reference in
their entirety.
101
CA 02403974 2002-09-25
WO 01/74240 PCT/USO1/10377
Having described preferred embodiments of the invention, it will now become
apparent
to one of ordinary shill in the art that other embodiments incorporating their
concepts may be
used. It is felt herefore that these embodiments should not be limited to
disclosed embodiments,
but rather should be limited only by the spirit and scope of the appended
claims.
What is claimed is:
102