Note: Descriptions are shown in the official language in which they were submitted.
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
HOUSING CAPABLE OF CONNECTING A CONTAINER
TO A MEDICAL DEVICE
BACKGROUND OF THE INVENTION
1. Field of the Invention
The invention relates to a housing that is capabie of connecting a container
and
a medical device. The medical device can comprise, for example, a syringe, an
infusion set, or the like. The container can comprise a bag, a vial, a tube,
or other
container.
2. Discussion of the Art
Many pharmaceutical products are delivered to pharmacies in sealed containers.
such as glass or plastic vials, glass or plastic bottles, and flexible bags.
Such
containers can contain a powdered or lyophilized formulation of a
pharmaceutical
product that must be reconstituted with an aqueous diluent prior to
administration to a
patient. In addition, such containers can contain a solution or suspension
formulation
of a pharmaceutical product that can be withdrawn from the container and
administered
directly to a patient, for example, by parenteral administration.
Most pharmaceutical vials are sealed by a pierceable stopper that is press-fit
into
the mouth of a vial to thereby isolate the contents of the vial from the
external
environment of the vial. In order to access the pharmaceutical product within
the vial, it
is necessary either to pierce the stopper or remove the stopper from the vial.
A
conventional syringe can be used to add a diluent to the vial and/or to
withdraw liquid
from the vial. The syringe has a hollow needle that is pushed through the
stopper and
into communication with the contents of the vial. The plunger of the syringe
can be
depressed to dispense a diluent into the vial or pulled outwardly to draw
liquid from the
vial into the syringe.
-1-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
The piercing of stoppers of vials typically has been achieved through the use
of
sharp, small-bored needles. Standard hypodermic syringe needles are
particularly
useful for this purpose because they allow the pharmaceutical product to be
aseptically
withdrawn from the vial and parenterally administered to a patient using a
single device,
thereby minimizing risk of contamination of the pharmaceutical product. In
this mode,
needles are typically connected to the syringe by means of a Luer-lock
fitting. Luer-lock
fittings are known to those skilled in the art. Such a fitting has been used
in medical
applications for joining an injection needle to a syringe, to link catheters,
to link infusion
sets, and to provide fluid communication for aqueous solutions of medicaments
in a
variety of settings. Typically, the Luer-lock fitting has a taper that matches
the taper of
the interior wall portion of the opening of the device into which it is
inserted. Force is
used to introduce the Luer-lock fitting into the opening of the device, and
the matched
tapers provide a substantially leak-proof seal.
The tapered structure of the Luer-lock fitting is subject to well-known and
understood mechanical standards. Luer-lock fittings can be made of glass or
metal, but
are typically made of thermoplastic materials, such as, for example,
polycarbonate,
polypropylene, polystyrene, polyvinyl chloride, and the like. The experience
of the
industry has been that the Luer-lock fitting has great value, but is not free
of operating
problems. Conventionally, Luer-lock fittings are made from rigid thermoplastic
materials, which provide little flexibility in the seal region and limit the
depth that the
male member of the Luer-lock fitting can be inserted into the receiving member
of the
Luer-lock fitting.
Examples of conventional tapered fittings are shown in Dennehey et al., U.S.
Patent No. 4,439,188 and Dalton, U.S. Patent No. 5,312,377. The surfaces of
the
tapered male member of the fitting and the tapered female member of the
fitting have a
matched taper in these fittings. In these conventional designs, it is required
that the
taper of the mating surfaces of the tapered male member of the fitting and the
tapered
female member of the fitting be closely matched and come in close contact to
make a
satisfactory seal. Slight errors in the manufacturing of these tapered members
typically
result in a seal that either cannot be initially achieved or subsequently
maintained.
` -2-
CA 02404444 2008-11-25
Any moment of force normal to the line of contact of the male member of
the fitting and the female member of the fitting can result in failure of the
seal.
There is a need for a device that can compensate for Luer-lock fittings that
deviate
dimensionally from current industry standards, yet still provide a
satisfactory seal.
There is also a need to reduce the amount of force or effort required by the
user to
insert the male member of a Luer-lock fitting into the opening of the female
member of the Luer-lock fitting. It is also desired that the seal effected
between
the male member of the Luer-lock fitting and the female member of the Luer-
lock
fitting provide a positive sense of engagement, or "feel" to the user, in
order to
confirm that the male member of the Luer-lock fitting and the female member of
the Luer-lock fitting are fully engaged, i.e., that the seal has been formed
in such a
manner that it will survive expected use and remain engaged even when
subjected
to a moment of force normal to the line of contact of the male member of the
Luer-lock fitting and the female member of the Luer-lock fitting.
SUMMARY OF THE INVENTION
In one aspect, this invention provides a housing that can be used to form
and maintain a fluid-tight connection between a container and a medical
device.
The housing comprises an internal receiving surface circumscribing a cavity
into
which a fitting of the medical device can be inserted. The internal receiving
surface comprises a first wall portion having a first contact annulus and a
second
wall portion having a second contact annulus. Upon insertion of the fitting
into an
opening in the cavity of the housing, the fitting forms a primary seal with
the first
contact annulus and a secondary seal with the second contact annulus.
Thus, in accordance with the invention, there is provided a housing for
establishing a fluid connection between a fitting having a tip with a tapered
exterior surface and a medical device, said housing comprising an internal
receiving surface circumscribing a cavity into which said fitting can be
inserted,
the internal receiving surface comprising a first wall portion having a first
contact
annulus that defines a first diameter and a second wall portion having a
second
contact annulus that defines a second diameter, the second contact annulus
being
spaced a distance from the first contact annulus so as to be distinct
therefrom, the
second wall portion having more flexibility than the first wall portion, the
first
-3-
CA 02404444 2008-11-25
diameter being less than the second diameter, wherein upon insertion of the
fitting
into the cavity of said housing, said first contact annulus contacts the
fitting to
form a primary seal with the tapered exterior surface of the tip of said
fitting and
said second contact annulus contacts said tapered exterior surface of the tip
of said
fitting to form a secondary seal.
In another aspect of the invention, there is provided an assembly
comprising a medical device, a container, and a housing, the assembly
comprising:
(a) a medical device having a fitting having a tapered exterior surface
having a reduced diameter at a distal end and an axial passageway,
io (b) a container, and
(c) a housing of the invention as defined hereinbefore.
In still another aspect of the invention, there is provided an assembly
comprising a medical device, a container, and a housing, the assembly
comprising:
(a) a medical device having a fitting having a tapered exterior surface
having a reduced diameter at a distal end and an axial passageway,
(b) a container comprising a mouth, which mouth is occluded by a
stopper, and
(c) a housing of the invention as defined hereinbefore, wherein the
housing further comprises a penetrator device disposed in the cavity of the
housing, the penetrator device capable of sliding in the cavity of the
housing, the
penetrator device further capable of penetrating the stopper upon movement of
the
penetrator device within the cavity of the housing.
In the invention the primary seal is formed as the surface of the fitting
contacts the first contact annulus of the internal receiving surface of the
housing.
A hoop stress caused by the contact between the fitting and the first contact
annulus produces a strain in the wall portion of the housing adjacent to the
first
contact annulus. This strain allows the diameter of the first contact annulus
to
expand. As the fitting continues to advance in the cavity, the first contact
annulus
eventually expands to a
-3a-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
sufficient diameter to allow the fitting to contact the second contact
annulus. The
secondary seal is formed as the surface of the fitting contacts the second
contact
annulus of the internal receiving surface of the housing.
The housing uses a relatively flexible receiving surface in conjunction with a
relatively rigid fitting -of a medical device to form a reliable fluid-tight
connection.
Neither the interior of the housing nor the fitting require either precisely
matched
tapered surfaces or precise machining. The housing provides retention of the
medical
device by providing frictional contact between the first contact annulus of
the internal
receiving surface of the housing and the fitting and frictional contact
between the
second contact annulus of the internal receiving surface of the housing and
the fitting.
The application of only a relatively low force is needed to form the primary
and the
secondary seals, and the seals thus formed will provide to the user a positive
sense of
engagement that indicates to the user that a durable, fluid-tight connection
has been
formed.
In another aspect, the housing of the invention can be used to establish a
fluid-
tight connection between a fitting, e. g., a Luer-lock fitting, of a medical
device, e. g., a
syringe, and a stopper disposed in a container, e. g., a vial. The connection
results
from the interaction between the fitting and the housing, which is disposed
over the
stopper in the container. The housing has an internal receiving surface
surrounding a
cavity into which the fitting is inserted. The internal receiving surface of
the housing
comprises a first wall portion having a first contact annulus and a second
wall portion
having a second contact annulus, which annuli provide two seals, a primary
seal at the
first contact annulus and a secondary seal at the second contact annulus. The
seals
are formed in the same manner as described previously. The housing can be used
in
conjunction with a penetrator device that is disposed within the cavity of the
housing,
which is disposed over the stopper. As the fitting is introduced into the
opening in the
cavity of the housing, the fitting can cause the penetrator device to move
towards the
stopper and pierce the stopper by means of a pointed end on the penetrator
device,
thereby forming an opening in the stopper. Once the stopper is penetrated,
then fluid
-4-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
can be withdrawn from the container by means of the medical device, such as,
for
example, a syringe.
The housing of this invention provides a reliable fluid-tight connection
between a
container and a medical device. The fluid-tight connection results from the
primary seal
that is formed by the contact between the exterior surface of the fitting and
the first
contact annulus of the internal receiving surface of the housing and the
secondary seal
that is formed by the contact between the exterior surface of the fitting and
the second
contact annulus of the internal receiving surface of the housing. As a result
of the force
generated in placing the fitting into the housing, the primary and secondary
seals are
formed quickly, creating a reliable seal with minimal insertion force.
The fitting can be inserted into the cavity of the housing with relatively low
force
to provide high frictional retention forces. Such an insertion preferably
requires less
than a full turn of the fitting to provide a satisfactory fluid-tight seal.
The invention facilitates rapid and safe access to the contents stored within
a
sealed container. The invention is especially suitable for use with a
container such as a
glass or plastic vial containing a pharmaceutical product or medicament.
However, it
will be appreciated that other applications of the present invention are
feasibie,
including, but not limited, applications in connection with parenteral tube
sets. The
pharmaceutical product may be in liquid form, e. g., a solution or suspension
of the
product, or in a solid form, e.g., a powdered or lyophilized form of the
product. The
invention is especially useful with a conventional vial, which is normally
sealed with a
rubber stopper, which, in turn, is to be pierced by the hollow needle of a
hypodermic
syringe. When the stopper is pierced, the contents of the vial can be diluted
or
reconstituted with the contents of the syringe. Alternatively, the contents of
the vial can
be withdrawn into the syringe for subsequent discharge into another container
system
or for administration to a patient.
Numerous other advantages and features of the present invention will become
readily apparent from the following detailed description of the invention,
from the
claims, and from the accompanying drawings and legends.
-5-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
BRIEF DESCRIPTION OF THE DRAWINGS
In the accompanying drawings, which form part of the specification, and in
which
like numerals are employed to designate like parts throughout the same:
FIG. 1 is a side view in elevation, broken away, of a housing configured for
use
with a conventional glass or plastic vial;
FIG. 2 is a cross-sectional view of the housing shown in FIG. 1;
FIG. 3 is a partial cross-sectional view of the internal receiving surface of
the
housing shown in FIG. 1;
FIG. 4 is a partial cross-sectional view of a conventional Luer-lock fitting
situated
in a position preparatory to being inserted into the housing of this invention
to form the
primary and the secondary seals;
FIG. 5 is a cross-sectional view of the fitting of FIG. 4 inserted in the
housing;
FIG. 6 is a perspective view of a conventional syringe employing a Luer-lock
fiffing;
FIG. 7 is a cross-sectional view showing the syringe of FIG. 6 attached to the
housing, with the housing installed on a vial. This view shows the penetrator
device in
the fully extended lowered position penetrating the stopper in the mouth of
the vial.
DETAILED DESCRIPTION
While this invention is susceptible of embodiment in many different forms,
this
specification and the accompanying drawings disclose only some specific forms
as
examples of the invention. However, the invention is not intended to be
limited to the
embodiments so described. The scope of the invention is pointed out in the
appended
claims.
For ease of description, the components of this invention are described in the
positions depicted in the accompanying drawings, and terms such as upper,
lower,
-6-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
horizontal, etc., are used with reference to this position. It will be
understood, however,
that the components of this invention may be manufactured, stored,
transported, used,
and sold in an orientation other than the position described.
Figures illustrating the components show some mechanical elements that are
known and that will be recognized by one skilled in the art. The detailed
descriptions of
such known elements are not necessary to an understanding of the invention,
and
accordingly, are herein presented only to the degree necessary to facilitate
an
understanding of the novel features of the invention.
This invention can be used with certain other conventional instruments and/or
components, the details of which, although not fully illustrated or described,
will be
apparent to those having skill in the art and an understanding of the
necessary
functions of such components.
In one aspect, the invention provides a housing for establishing a fluid-tight
connection between a fitting, such as, for example, a male Luer tapered
fitting having
an axial passageway, and a container, such as, for example, a vial of fluid.
The
housing of this invention is especially suitable for use with a syringe
employing a Luer-
lock fitting. However, it will be appreciated that other applications of the
present
invention are feasible including, but not limited to, applications involving
parenteral tube
sets. The invention is especially useful for providing a reliable connection
with a fitting
that may have dimensional variations. The reliable connection exhibits
increased
retention forces, thereby increasing the resistance to disconnection of the
fitting from
the housing.
As used herein, the term "housing" means an element that covers, protects, or
supports. The term "wall" means an element serving to enclose an area. The
term
"shoulder" means a shoulderlike slope or projection. The expression "contact
annulus"
means a ringlike figure, part, structure, or marking of the housing that
touches a fitting.
The term "seal" means a fluidtight closure. The term "receiving surface" means
a
surface of an element that takes in or holds a different element. The term
"diameter"
means a straight line segment passing through the center of a figure, such as,
for
example, a circle, and terminating at the periphery. In this invention, the
annulus is
-7-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
substantially circular in shape; however, on account of the difficulty
encountered in
forming annuli that are perfectly circular in shape, the annuli may not be
perfectly
circular in shape, in which case, the term "diameter" refers to a measured
value that
approximates the diameter of a circle.
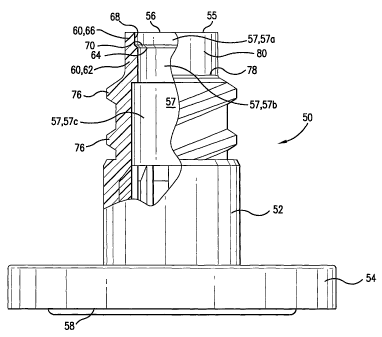
FIGS. 1 and 2 illustrate a housing 50 configured for use with a conventional
glass or plastic vial. However, it will be appreciated that the housing 50 can
be adapted
to a wide variety of containers and devices for storage of solids and fluids
and transport
of fluids. The depiction herein of the housing 50 is not intended to be
limiting but
instead represents one useful application of the present invention. For the
purpose of
this disclosure, all references to the terms "container" and "vial" are
intended to include
vials, bottles, flexible containers, parenteral or enteral tube sets, and
equivalents
thereof.
The housing 50 has a cylindrical neck 52 terminating in an annular flange 54,
which facilitates attachment of the housing 50 to a container or vial. The
housing 50
has an upper end 55 having an opening 56, which opens to an internal cavity
57, which
extends through the housing 50. The cavity 57 comprises an upper cavity
portion 57a,
an intermediate cavity portion 57b, and a lower cavity portion 57c. The
housing also
has a lower end 58 having an opening 59, which opens to the internal cavity
57. The
internal cavity 57 is surrounded by a wall 60, which comprises a first wall
portion 62,
which includes a first contact annulus 64, and a second wall portion 66, which
includes
a second contact annulus 68. The ratio of the thickness of the first wall
portion 62 to
the thickness of the second wall portion 66 is greater than unity, i. e., 1.
The first
contact annufus 64 has a diameter that is less than the diameter of the second
contact
annulus 68. The first contact annulus 64 has a diameter that is less than the
diameter
of the upper cavity portion 57a. Examples of dimensions of the housing range
from a
height of %-inch to 3/-inch measured from the upper end 55 to the lower end 58
of the
housing 50. An example of the diameter of the first contact annulus 64 is
0.166 inch.
An example of the diameter of the second contact annulus 68 is 0.179 inch. An
example of the thickness of the first wall portion 62 is 0.022 inch. The
difference in
annular diameter between the first contact annulus 64 and the second contact
68
-8-
CA 02404444 2008-11-25
WO 01/74292 PCT/USOI/10431
preferably ranges from about 0.005 to about 0.02 inch. An annular shoulder 70
is
located adjacent the first contact annulus 64.
Referring now to FIGS. 3, 4, and 5, the wall porti~on 62 containing the first
contact annulus 64 has a greater thickness than the wall portion 66 containing
the
second contact annulus 68. Thus, the first contact annulus 64 is associated
with a
relatively more rigid wall structure than is the seoond contact annulus 68.
The wall
portion 66 has relatively more flexibility than does the wall portion 62. The
difference in
flexibiiity.in the wall portions in the embodiment shown is obtained by
employing a wall
portion 66 that is thinner than the wall portion 62. However, in aitematlve
embodiments, the difference in flexibility can be obtained by providing a
plurality of
slots, preferably elongated, paraaliy or completely through the wall pofion
66. In this
alternative embodiment, the wall pordon 66 can be of equal thkkness or of
greater
thiclmess than the wall portion 62. The distance between the first contact
annulus 64
and the second contact annulus 68 must be sufficient so that at least two
distinct seals,
a-primary seal 72 and a secondary seal 74, are fomW. The primary seal 72 and
the
secondary seal 74. are shown in F{G. 5. The distance between the first contact
annulus
64 and the, second contact annulus 68 cannot be so great that the formation of
at least
two distinct seals is prohibifed. An example of the nominal distance between
the first
contact annufus 64 and the second contact annulus 68 Is 0.03 inch. As will be
explained with respect to a Luer-iock fitting, these structural features
provide an
excellent fluid-tight connection and Increased contact stress (higher force
per unit area
at the first and the second contact annuli), thereby increasing the resistance
to
disconnecdon between the Luer-lock fitting and the housing 50. Luer
connectors,
induding Luer-lock fitdngs, ane.described in detail in U. S. Patent No.
5,312,377.
Although only two contact annuli are shown in the embodiments of this
inven6on,
it is within the soope of this invention to add a third contact annulus, or
even more
contact annuli, to the housing 50. Each additionat contact annulus, if
employed, would
resuit in an addibonal seal. Each additional seal would be placed at a higher
level on
the housing 50 than the previously numbered seal, e. g., the tertiary seal
would bq
-9-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
placed at a higher level than the secondary seal, and each additional seal
would have
the same relationship to the previously numbered seal that the secondary seal
74 has
to the primary seal 72.
A portion of the housing 50 includes a laterally projecting formation 76, such
as a
conventional Luer-lock, double lead, helical thread, or the like. This
laterally projecting
formation 76 projects from the exterior surface 80 of the housing 50. The
laterally
projecting formation 76 is designed for engaging a mating thread system on an
annular
skirt of a medical device having a Luer connector, such as, for example, a
syringe
employing a Luer-lock fitting (as described in detail hereinafter). The
laterally projecting
formation 76 begins at a point 78 on the exterior surface 80 of the housing
50. It is
preferred that the point 78 be placed at such a distance from the upper end 55
of the
housing 50 that the axial length of the laterally projecting formation 76 is
rendered
sufficiently short that less than a full turn of the medical device is
required to engage
the Luer-lock fitting. It is preferred that the laterally projecting formation
76 extends
along the exterior surface 80 of the housing 50 a distance sufficient to
engage the Luer-
lock fitting approximately one-half turn. However, it is within the scope of
this invention
that the laterally projecting formation 76 can be longer and can even extend
to the,
upper end 55 of the housing 50.
Referring to FIG. 2, the lower cavity portion 57c communicates with the.
intermediate cavity portion 57b. The lower cavity portion 57c is open at the
lower end
58 of the housing 50.
FIG. 4 shows a partial view in cross-section of a tip 90 of a medical device
(not
shown), such as, for example, a syringe, having a conventional Luer-lock
fitting. The tip
90 has a wall 92 surrounding a bore 94, which is in communication with the
body of the
medical device (not shown). The exterior surface of the tip 90 is tapered,
whereby the
exterior diameter of the tip 90 is reduced to its minimum dimension at the
distal end 96
of the tip 90. As used herein, the distal end 96 of the tip 90 is the end of
the tip 90 that
first enters the opening 56 of the housing 50. FIG. 4 illustrates the tip 90
in position to
be inserted into the housing 50, a partial cross-sectional view of which is
shown.
-10-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
FIG. 5 shows the tip 90 fully inserted in the housing 50. Upon insertion of
the tip
90 in the internal cavity 57 of the housing 50, the wall 92 of the tip 90
initially contacts
the first contact annulus 64. A primary seal 72 is formed as the surface of
the tip 90
contacts the first contact annulus 64. This contact causes a hoop stress to be
exerted
on the first contact annulus 64. This hoop stress creates a strain in the wall
portion
adjacent to the first contact annulus 64. This strain causes lateral expansion
of the first
contact annulus 64. As the tip 90 continues to advance in the cavity 57, the
first
contact annulus 64 eventually expands to a sufficient diameter to allow the
tip 90 to
contact the second contact annulus 68. The secondary seal is formed as the
exterior
surface of the tip 90 contacts the second contact annulus 68 of the housing
50.
Contact of the tip 90 with the second contact annulus 68 is facilitated by the
tapered
shape of the tip 90.
When the tip 90 is fully inserted into the housing 50, a primary seal 72 is
formed
between the wall 92 of the tip 90 and the first contact annulus 64 and a
secondary seal
74 is formed between the wall 92 of the tip 90 and the second contact annulus
68.
After the primary seal 72 and the secondary seal 74 are established, the first
contacfi
annulus 64 and the second contact annulus 68 are displaced axially. If a
lateral force
sufficient to create a moment is exerted anywhere along the major axis of a
medical
device attached to the fitting held by the primary seal 72 and the secondary
seal 74, the
primary seal 72 and the secondary seal 74 will resist being broken by the
force.
Moreover, the primary seal 72 and the secondary seal 74 will resist the
disengagement
of the medical device from the housing.
Any thermoplastic material can be used in the manufacture of the housing 50
containing the contact annuli 64, 68. Conventional thermoplastic materials
include
homopolymers and copolymers containing olefinic monomer groups. Representative
examples of such homopolymers and copolymers include, but are not limited to,
polyethylene, polypropylene, copolymers comprising ethylene and propylene
monomeric groups, copolymers containing ethylene and hexalene monomeric
groups,
ethylenediene copolymers, and other polyolefinic materials. Additionally,
other
engineering plastics, such as, for example, polyamides, such as nylon,
polyvinyl
- 11 -
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
chlorides, polycarbonates, etc., can be used. The materials used to form the
components used in this invention are selected on the basis of structural
properties,
ease of manufacture, and cost. Preferred materials for this invention comprise
homopolymers and copoiymers containing oiefinic monomeric groups.
The housing 50 is preferably molded as a unitary structure from a
thermoplastic
material, such as polypropylene, nylon, polyethylene, ethylenecopropylene
copolymers,
ethylenecohexylene copolymers, and polyvinyl chloride. Polypropylene is an
especially
preferred material.
The components of the invention can be manufactured using any thermoplastic
manufacturing technique that will provide the desired result. The components
are
preferably made by injection molding of thermoplastic materials into precision
molds
under pressure using conventional techniques. The dimensions of the first
contact
annulus 64 and the second contact annulus 68 can reasonably be maintained
using
conventional thermoplastic injection molding techniques.
FIGS. 6 and 7 illustrate the use of the housing of the present invention to
couple
a syringe 100 employing a Luer-lock fitting with a vial 130 containing a
liquid. It will be
appreciated that this description is for exemplary purposes only and that use
of the
present invention is not limited to a syringe employing a Luer-lock fitting or
a vial
containing a liquid.
The syringe 100 includes a barrel 102 and a telescopically received plunger
104.
The distal end of the plunger 104 includes a piston 106 that engages with the
interior
cylindrical surface of the barrel 102 to form a seal between the piston 106
and the
interior cylindrical surface of the barrel 102.
The distal end of the syringe 100 has an annular skirt 108, which is
internally
threaded with a conventional Luer-lock, double lead, helical thread system
110. A tip
112 projects from the distal end of the barrel 102 within the annular skirt
108. The
exterior surface of the tip 112 is tapered, whereby the exterior diameter of
the tip 112 is
reduced to its minimum dimension at the distal end of the tip 112. The tip 112
has
formed therein a bore 114, which is in communication with the interior chamber
115 of
the barrel 102, which chamber 115 is located below the piston 106.
-12-
CA 02404444 2008-11-25
WO 01/74292 PCT/USOl/10431
As shown in FIG. 7, the syringe 100 can be coupled with the vial 130. The
thread system 110 engages the thread system 76 of the housing 50. As relative
rotation is effected between the syringe 100 and the vial.130, the tip 112 of
the syringe
100 moves downwardly against the upper end of a penetrator device. 120. This
movement causes the penetrator device 120 to move downwardly through the
intemal
cavity 57 of the housing 50.
The vial 130 has a cylindrical neck 132 terminating in an annular flange 134,
which defines an opening 136 of the vial 130.
The mouth of the vial 130- contains a stopper 140. The stopper 140 is
typically
made from rubber or other suitable elastomeric material. The stopper 140
includes a
central generally annular plug portion 142 and a head portion 144. The
diameter of the
head portion 144 is greater than the diameter of the plug portion 142. The
head poraon
144 functions as a supporting flange and is normally seated on the top end-
surface 145
of the flange 134 of the vial 130. The annular plug portion 142 of the stopper
140
defines an intemal recess 146, which opens downwardly toward the contents of
the vial
130. The stopper 140 prevents the removal of the contents of vial 130 unless
and untii
the stopper 140 is either removed. or penetrated. Typically, the annular
plug'portion
142 of the stopper 140 is received in the opening 136 of the vial 130 in a
radially
inwardly compressed condition and i$ retained within the opening 136 in the
vial 130 by -
frictional engagement, which is established by the outward fonoe of the
annular plug
pordon 142 on the neck 132 of the vial 130.
The housing 50 is posfioned on the head por6on 144 of the stopper 140 and is
held in place by ferrule 150, which retains the radially extending lower end
of the
housing 50. Ferrules are discussed in U. S. Patent 6,610,041, issued
August 26, 2003. . A ring 152 engages the head portion 144 of the
stopper 140, thereby effecting a seal between the ring 152 and the head
porflon 144. A
bottom peripheral porGon of the ferrule 150 is crimped about the lower edge of
the
flange 134 of the vial 130. A penetrator device 120 is disposed in the lower
cavity portion 57c of the
housing 50 and is axially a)igned therein. The penetrator device 120 is
capable of
-13-
CA 02404444 2008-11-25
WO 01/74292 PCT/[JS01/10431
sliding in the lower cavity portion 57c of the housing 50. The penetrator
device 120,
which is adapted for being received in the housing 50, Is a unitary structure
made from
plastic or metal material. The penetrator device 120 has a shank 122 having a
point
defining a pointed distal end 124. The penetrator device 120 has a hub 126 at
the end
of the shank 122 opposite the pointed distal end 124. The hub 126 defines the
upper
end of the penetrator device 120. The lower por6on of the hub 126 indudes an
annular
bead 128 having a diameter that establishes the diameter of the hub 126.
The housing 50 and the penetrator device 120 are preferably constructed in.
such a manner that they are held together by friWonal forces therebetween. The
penetrator_device 120 Is initially posidoned at an uppennost elevation (not
shown)
within the lower cavity portion 57c of the housing 50. The annular bead 128
disposed
on the shank 122 of the penetrator device 120 establishes a slight
interference fit with
the interior surface of the lower cavity porUon 57c. The lower cavity porbon
57c may
include longitudinal guide elements (not shown) for the penetrator device 120,
such as
the rib structure disciosed in U.S. Patent No. 5,954,104.
The housing 50, with the penetrator devioe 120 inserted therein, is
positioned with respect to the vial 130 in such a manner that the pointed
distal end 124,
of the penetrator device 120 Is capable of piercing the intemal recess 146 of
the
stopper 140 when the penetrator device 120 Is actuated by coupling the Luer
lock
system 110 to the housing 50.
-14-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
OPERATION
Operation of the present invention will now be described in conjunction with
FIG.
7. It will be appreciated that this description is for exemplary purposes only
and that
the present invention is not limited to the example. As shown in FIG. 7, the
syringe 100
is coupled with the housing 50. To this end, the syringe 100 is threadingly
engaged
with the thread system 76 on the housing 50. A relative rotation of
approximately one-
half turn is sufficient to effectively engage the syringe 100 and the vial
130. The tip 112
of the syringe 100 moves downwardly until it contacts the first contact
annulus 64,
thereby resulting in a hoop stress. The hoop stress resulting from this
contact produces
a strain in the wall portion of the housing adjacent to the first contact
annulus 64. This
strain allows the diameter of the first contact annulus 64 to expand. As the
tip 112 of
the syringe 100 continues to advance in the cavity, the first contact annulus
64
eventually expands to a sufficient diameter to allow the tip 112 of the
syringe 100 to
contact the second contact,annulus 68. The secondary seal 74 is formed as the
second contact annulus 68 of the housing 50 contacts the tip 112 of the
syringe 100.
Contact of the tip 112 with the second contact annulus 68 is facilitated by
the tapered
shape of the tip 112. See FIG 7.
When the tip 112 of the syringe 100 is fully inserted in the housing 50, the
primary seal 72 is formed by contact of the first contact annulus 64 with the
exterior
surface of the tip 112 of the syringe 100, and the secondary seal 74 is formed
by
contact of the second contact annulus 68 with the exterior surface of the tip
112 of the
syringe 100.
Further, the tip 112 also engages the upper end of the penetrator device 120.
This engagement pushes the penetrator device 120 downwardly along the lower
cavity
portion 57c of the housing 50. As the penetrator device 120 moves downwardly
within
the lower cavity portion 57c of the housing 50, the pointed distal end 124 of
the
penetrator device 120 pierces the stopper 140 and enters the internal recess
146 of the
stopper 140, thereby establishing fluid communication between the vial 130 and
syringe
100.
-15-
CA 02404444 2002-09-26
WO 01/74292 PCT/US01/10431
The housing of this invention can be used to form a fluid-tight seal between a
container, such as a vial, and a medical device, such as a syringe, or other
device
capable of transferring fluids. The seal can be formed easily with minimal
level of
insertion force. The seal can be made without carefully aligning the container
and the
medical device.
The foregoing detailed description of the invention and the illustrations
thereof
demonstrate that numerous variations and modifications in apparatus and
methods that
embody the invention may be effected without departing from the true spirit
and scope
of the novel concepts or principles of this invention. The invention resides
in the claims
hereinafter appended.
-16-