Note: Descriptions are shown in the official language in which they were submitted.
CA 02407265 2002-11-13
HIGH DENSITY ARRAY
BACKGROUND
High density arrays of immobilized natural or synthetic target substances
allow
the simultaneous screening of analytes for the presence of specific
properties. Such high
density arrays have proven useful in a variety of technological fields
including chemistry,
genetics, immunology, material sciences, medicine, molecular biology and
pharmacology.
For example, high density arrays of nucleic acids are used to ascertain gene
sequences, to
detect the presence of genetic mutations and to detect the qualitative and
quantitative
differential expression of gene products. Similarly, high density arrays of
peptides are used
to map epitopic sequences that elicit immune responses. Further, arrays of
target substances
are used to identify compounds for the development of pharmaceutical agents.
Currently, methods for the construction of high density arrays of test
substances are generally of two types. First, arrays are constructed by
individually applying
preformed natural or synthetic target substances, such as biomolecules,
directly to specific
locations on a support. Supports include membranes of nitrocellulose, nylon,
polyvinylidine
difluoride, glass, silicon or other materials, and the target substances can
be immobilized to
the support by exposing the support to ultraviolet radiation or by baking the
support, among
other techniques. One such method is disclosed in Pietu et al., "Novel Gene
Transcripts
Preferentially Expressed in Human Muscles Revealed by Quantitative
Hybridization of a
High Density cDNA Array, " Genome Research (1996) 6: 492-503. Various
devices have been devised to automate the application method.
The second method of constructing high density arrays involves synthesizing
individual target substances at specific locations in situ on a support. In
one version of this
method, photosynthetic chemistry is used to simultaneously prepare series of
different target
substances at unique locations on the support. In another version of this
method, target
substances are synthesized by physically masking or blocking selected areas on
a support and
the desired chemical synthesis reaction is carried out on the unmasked portion
of the support.
Examples of this method are disclosed in, Fodor et al., "Light-Directed,
Spatially
Addressable Parallel Chemical Syntheses," Science (1991) 251:767-777; United
States Patent
5,436,327; and Southern, E.M. et al., " Analyzing and Comparing Nucleic Acid
Sequences
CA 02407265 2002-11-13
2
by Hybridization to Arrays of Oligonucleotides; Evaluation using Experimental
Models,"
Genomics (1992) 13: 1008-1017.
Both methods of constructing high density arrays are associated with several
disadvantages. First, the methods can only produce a relatively limited number
of identical
arrays at one time. Secondly, it is difficult to check the arrays being
produced by these
methods during production to determine the integrity of the production steps.
Third, many
potential target substances cannot be applied to supports and cannot be
synthesized in situ on
supports by currently used methods. Further, arrays composed of test
substances from more
than one chemical category, such as arrays of peptides and nucleic acid test
substances, are
not described. Also, the methods are only capable of producing arrays of
target substances in
a layer having a relatively limited thickness. Additionally, each method can
produce arrays
of target substance zone dimensions having only relatively limited sizes.
Therefore, there is a need for an alternate method of producing high density
arrays which does not have the disadvantages inherent in the known methods of
high density
array production. For example, the method should preferably be able to produce
large
numbers of identical arrays simultaneously, rapidly and cost effectively. The
method should
be able to use a wide variety of target substances and supports, including
test substances and
supports that cannot be incorporated into arrays by presently used methods.
The method
should be able to produce arrays in more than two dimensions, in varying
thicknesses and
sizes, and in configurations other than a planar configuration. Additionally,
the method
should be able to produce arrays having a variety of target substance zone
dimensions,
including dissimilarly sized zones for different target substances in an
array. Also, the
method should be able to produce high density arrays of test substances from
different
categories or chemical classes, such as arrays of peptide and nucleic acid
test substances.
Further, the method should be able to use preformed target substances or to
use target
substances that are synthesized in situ, as necessary, to incorporate the
advantages of these
methods.
CA 02407265 2002-11-13
2a
SUMMARY
According to one aspect of the present invention, there is provided a high
density array of target substances produced by sectioning a bundle comprising
a plurality
of target-strands, wherein the plurality target-strands comprise target
substances, and
wherein the location of each target substance within the bundle is noted in a
database.
A method of producing such an array is also provided and comprises the
steps of sectioning a bundle of target-strands, wherein the target-strands
comprise the
target substances, and wherein the sectioning results in a high density array
of target
substances present in three Cartesian axes. The method can also comprise a
step
CA 02407265 2002-11-13
3
of stabilizing the bundle, incorporating an additional material into the
bundle or interrogating
the high density array.
A high density array may be produced by sectioning a bundle
comprising a plurality of target-strands, wherein the plurality target-strands
comprise target
substances. The high density arrays can have target substances present in one,
two or three
Cartesian aces.
A method of making a high density array may comprise the
step of rolling a membrane or stacking membranes impregnated with Iines target
substances
to produce a bundle. A method of ascertaining gene sequences may involve
detecting
the presence of genetic mutations, detecting the qualitative or quantitative
differential
expression of gene products, mapping epitopic sequences that elicit immune
responses, or
id~tifying compounds for the development of pharmaceutical agents, using the
kugh density
arrays produced ~acoording to the present invention.
FIGURES
The features, aspects and advantages of the present invention will become
better understood with regard to the following description, appended claims
and
accompanying figures where:
Figures I through 3 depict the production of high density arrays using a
bundle
of fibers which comprise target substances according to the present invention;
Figures 4 through 5 depict the production of high density arrays using a
bundle
comprising a membrane having lines of target substances applied on the
membrane according
to the present invention;
Figums 6 through 8 depict the production of high density arrays using a bundle
comprising a plurality of membranes having lines of known target substances
applied on the
membrane according to the present invention;
Figures 9 through 11 depict the production of high density arrays using a
bundle comprising a rolled membrane having lines of lmown target substances
applied on the
membrane according to the preset invention;
Figures 12 through 14 depict the production of high density arrays using a
bundle comprising tubes filled with target substances according to the present
invention;
Z figure 15 is a photograph of an autoradiograph showing the result of a
hybridization study pGrfforaned on au array producxd acxording to the present
invention; and
CA 02407265 2002-11-13
4
Figure 16 is a photograph of an autoradiograph showing the result of a
hybridization study performed on another array produced according to the
present invention.
DF5CR1~ON
According to one embodiment of the present invention, there is provided a
method of malting a high density array of target substances for deterznining
the identity or
properties of analytes or for determining the identity or properties of the
target substances.
According to another embodiment of the present invention, there is provided a
high density
array of target substances for determining the identity or properties of
analyzes or for
determining the identity or properties of the target substances.
As used herein, the term "target substance" refers to the component of the
high density array that potentially interacts with one or more analytes of
interest. Target
substances can be atoms, molecules, complex chemicals, organelles, viruses,
cells or
materials, or can be combinations of these entities, or can be other entities
as will be
understood by those with skill in the art with reference to the disclosure
herein. For
example, the target substances of a high density array according to the
present invention can
be selected from one or more of the group of atoms such as zinc, sulfur, and
gold;
biomolocules such as polynucleotides, DNA, RNA, peptides, proteins,
glycoproteins,
lipoproteins, carbohydrates, lipids, immunoglobuIins, and their synthetic
analogs and
variants; viruses; sub-cellular components such as microdisected chromosomes
and
mitochondria; cells including prokaryotic cells, archaebacteria, and
eukaryotic cells; and
materials such as metallic alloys, ceramics, glasses, semiconductors,
superconductors,
plastics, polymeric materials, wood, fabric and concrete.
As used herein, the term "analyte" refers to an entity whose identity or
properties are to be determined by interaction with the target substances on a
high density
array according to the present invaition. Alternately or simultaneously, the
analyte can be
used to determine the identity or properties of the target substances by
interaction with the
target substances on a high density array according to the present invention.
Analytes can be
selected from the same group as target substances, such as proteins or nucleic
acids, or can
be a physical or environmental condition such as one or more condition
selected from the
group a~nsisting of temperature, pH, or salt concentration.
As used heron, the term "target-strand" refers to a strip of target substance.
These strips can consist eatirely of one or more target substances, or can
comprise one or
CA 02407265 2002-11-13
S
more target substances with a support or a container. The target substances
can be absorbed
to, adsorbed to, attached to, embedded in, or coated on the support, or
contained within the
container. For example, the target-strands can include cast rods of target
substances such as
metal alloys, concrete or plastic, or can include target substances absorbed
onto glass fibers
or silk threads, attached to polymer fibers, embedded in a porous rod, coated
on a metal
wire, or contained within a matrix of gelatin. Further, target-strands can
include lines of
target substances which are written, drawn, printed or embossed on a glass
slide or on a
membrane such as a thin planar sheet of polymeric substance, or on an
equivalent support.
Additionally, target-strands can include target substances attached to the
inside of tubes.
As used herein, the term "matrix" refers to a matezial in which target
substances can be embedded or to which target substances can be attached to
supply
additional structural support, to serve as a spacer, to display the target
substance to the
analyte, or to influence the interaction between the target substance and the
analyte such as
by electrically insulating target substances from each other. Matrices can be
polymeric
materials such as one or more substances selected from the group consisting of
aerogel,
agarose, albumin, gelatin, hydro-gel and polyacrylamide.
As used herein, the term "bundle" refers to an ordered arrangement or
assembly of target-strands. For example, a bundle can include a stack of
target-strands
where each target-strand comprises a tube filled with a target substance, or
where each
target-strand comprises lines of target substances drawn on a membrane, or
where each
target-strand comprises a wire of a target substance.
Method of Producing High Density Arrays
The method of producing high density arrays according to the present
invention comprises the steps of (a) assembling a bundle of target-strands,
and (b) sectioning
the bundle to produce an array. Additionally, the method can include a step of
stabilizing the
target-strands or bundles. Further, the method can include a step of
incorporating one or
more additional materials into the high density arrays. Also, the method can
include a step
of interrogating the high density array.
_ Ambling a Bnndle of Target-Strands
Anaysbundle of target-strands can be produced by a number of methods. Por
example, a bundle of targets-strand can be produced by first filling tubes
with target
substances or with target substanoGS in combination with a mattrio. The target
substance can
CA 02407265 2002-11-13
6
be enclosed within the matrix without being chemically bound to the matrix or
can be
attached to the matrix by covalent forces, by ionic forces, by hydrogen
bonding or by other
forms of attachment. The tubes are then arranged and secured substantially
parallel to their
long axes to produce the bundle of target-strands.
A bundle of target-strands can also be produced by first coating or
impregnating a support, such as a membrane, fiber, tube, or rod, with a target
substance, or
by applying solutions of target substance onto a support with a fountain pen
nib such as an
artist's crow's- quill pen nib or an air-brush, or by ink jet printing,
embossing or thermally
transferring solutions of target substances onto a support. Next, these
supports are stacked,
rolled or folded to produce the bundle of target-strands. The resultant bundle
contains rows
of target substances that are aligned relatively parallel to the long axis of
target substance
application.
Sectioning the Bundles to Produce the Arrays
After assembling, the bundles are sectioned to produce the arrays. The bundles
can be-sectioned with a microtome, laser, saw, hot wire or other cutting
device or method as
will be understood by those with skill in the art with reference to the
disclosure herein. The
sectioning can result in a high density array with target substances having
any of a wide
variety of thicknesses. For example, the array can have target substances with
a thickness of
between about 0.1 ~m to about 1 mm or thicker. Further, unlike prior known
methods of
producing arrays, the method disclosed herein can readily produce arrays
having target
substances with a thiclrness of greater than 50 Vim. This is advantageous as
it can increase
the signal generated by the target substance as compared to signals generated
by target
substances on thinner arrays.
In a preferred embodiment, the assembled bundle has target-strands which
have long axes substantially parallel to each other and the bundle is
sectioned substantially
perpendicular to the long axes of the target-strands to produce the high
density arrays. The
sectioning can also be performed at an angle other than substantially
perpendicular to the
long axes of the target-strands, such as to produce oval arrays from a
cylindrical bundle.
Depending on the form of the bundle and the direction of sectioning, the
sectioning step can produce high density arrays with one, two or three
analytical axes, that is,
high density arrays having target substances in one, two or three Cartesian
axes. For
example, arrays with one analytical axis can result from cxoss-sectioning a
bundle having
CA 02407265 2002-11-13
7
target substances lying in a single plane. Arrays with two analytical axes can
result from
cross-sectioning a bundle having target substances lying in a plurality of
planes. Arrays with
two analytical axes can also be produced by combining multiple, single
analytical axis arrays.
Arrays with three analytical axes can be produced by combining multiple,
single analytical
S axis arrays, by combining a single analytical axis array with an array with
two analytical
axes, or by combining a plurality of arrays with two analytical axes.
For example, a high density array with one analytical axis can be produced by
sectioning a bundle formed from target-strands made by depositing target
substances in
parallel lines on a flat membrane, where sectioning is performed in a plane
perpendicular to
the plane formed by the lines. Similarly, a high density array with two
analytical axes can be
produced by sectioning a bundle formed from target-strands comprising a stack
of
membranes, where each membrane has target substances deposited in parallel
lines, and
where sectioning is performed in a plane perpuidicular to the long axes of the
target
substance lines. Further, a high density array with three analytical axis can
be produced by
stacking a plurality of high density array with two analytical axes produced
by this
sectioning.
Stabilizing the Bundle of Target-Strands
The method of producing high density arrays according to the present
invention can also include a step of stabilizing the bundle of target-strands.
Stabilization can
improve the form or the function of the bundle or array, such as making the
bundle easier to
section, or isolating target substances from each other in the array. The
stabilizing step can
be performed at any time during or after the assembly of the bundle of target-
strands, as is
appropriate to the type of stabilization. For example, stabilization can be
accomplished by
embedding the bundle of target-strands in a matrix, such as epoxy,
polypropylene or
polystyrene.
Incorporating Additional Materials Into the High Density Arrays
The method of producing high density arrays according to the present
invention can also include a step of incorporating one or more additional
materials into high
density arrays during or after assembly of the bundle of target-strands,
including after the
sectioning step. These materials can improve the form or the function of the
high density
array. For example, the incorporation step can include adding antioxidants or
microbial
inhibitors or other subset's to maintain the integrity of the high density
array over time.
CA 02407265 2002-11-13
Further, the incorporation step can include adding substances to the matrix
which reduce background noise, such as a nonfluoresoent counterstain, or which
increase the
detection signal. Similarly, the incorporation step can include adding a
scintillant to the
matrix to facilitate the detection of radioactive analyzes. Also, the
incorporation step can
include adding cofactors necessary for lain modes of detection to the matrix,
such as
secondary enzymes which are necessary for enzymatic color development, or an
energy
transfer dye which can enhance the detection of a fluorescent label.
Additionally, a surface
of a high density array produced by the method disclosed herein can be coated
with silver or
another reflective material to enhance the amount of light available for
detection.
Interrogating the High Density Arrays
The method of producing high density arrays according to the present
invention can also include a step of interrogating the high density arrays. In
a preferred
embodiment, the interrogating step is selected from the group of visual
inspection with or
without magnification, chemical deposition, electrical probing, mechanical
sensing and
magnetic sensing. In another embodiment, the step of interrogating comprises
placing the
array in close proximity to a collection of interdigitated electrodes and
measuring capacitance
changes resulting from interactions between the target substances on the high
density array
and the interdigitated electrodes.
Production of High Density Arrays from Bundles Comprising F'~rs
In one embodiment, high density arrays are produced from bundles of target-
strands comprising fibers or threads. The fibers or threads can comprise
natural or synthetic
material selected from the group consisting of cotton, silk, nylon, and
polyester, or can be
other materials as will be understood by those with skill in the art with
reference to the
disclosure herein.
In a prefe~cred embodiment, the bundles of target-strands are produced by
directly impregnating fibers with an aqueous solution of the target substance.
A series of
such fibers are impregnated with different target substances and the identity
of each the target
substance eaair fiber contains is recorded in a database. The fibers are
washed to elute
unbound target substances and are treated with a non-interfering substance to
block
nonspecific binding sites on the fibers and the immobilized target substances.
The fibers are
then dried to fix the bloclang agent to the fiber and to the immobilized
target substances.
The fiber are then assembled into bundles with the location of each fiber and
CA 02407265 2002-11-13
9
its associated immobilized target substance noted in the database. The bundle
of fibers is
preferably stabilized by embedding or otherwise impregnating the bundle in a
matrix to
provide structural support to the bundle.
The bundle is then sectioned substantially perpendicular to the long axis of
the
fibers using suitable instrumentation to provide a plurality of high density
arrays. Preferably,
the sectioning results in a plurality of identical high density arrays. The
identity and location
of the target substances on each array are tzacked through the information in
the database.
These arrays can be utilized to simultaneously screen analyzes for the
presence of specific
properties, or can be utilized for other purposes as will be understood by
those with skill in
the art with reference to the disclosure herein.
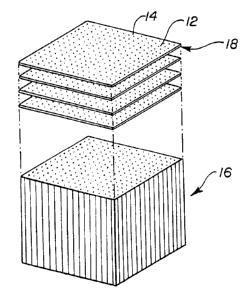
Referring now to Figures 1 to 3, there are shown respectively, target-strands
10 comprising a series of coated fibers 12 impregnated with known target
substances; the
target-strands 10 embedded in a matri~c 14 and assembled into a bundle 16; and
the bundle 16
being sectioned to produce a plurality of identical high density arrays 18,
where each array
has target substances in two analytical axes.
Production of High Density Arrays from Bundles Comprising Membra~s
In one embodiment, high density arrays are produced from bundles comprising
membranes. The membranes can comprise thin planar sheets of a polymeric
substance, or
can comprise other materials as will be understQOd by those with skill in the
art with
referencx to the disclosure herein.
In a preferred embodiment, the bundles are produced by applying lines of a
composition containing the target substances on the membranes by writing,
drawing, printing
or embossing. The identity and location of each target substance is recorded
in a database.
The membranes are then , if necessary, to fix the target substances to the
membrane.
One membrane producxd in this manner can be sectioned to produce a
plurality of high density arrays, each array having target substances arranged
in one
analytical axis. Referring now to Figures 4 and 5, there are shown
respectively, bundle 20
comprising a membrane 22 having lines of known target substances 24 applied on
the
membrane 22; and the bundle 20 being sectioned to produce a plurality of high
density arrays
26, where each array has target substances arranged in one analytical axis.
Alternately, a plurality of membranes produced in this manner can be
assembled into bundles with the identity and location of each immobilized
target substance
CA 02407265 2002-11-13
noted in the database. Assembly can comprise rolling or folding the membrane,
or can
comprise stacking a plurality of target substance impregnated membranes. If
necessary, the
bundle is stabilized such as by embedding or otherwise impregnating the bundle
in a matrix
to provide structural support to the bundle.
5 The bundle is then sectioned substantially perpendicular to the long axis of
the
target substance lines on the membranes using suitable instrumentation to
provide a plurality
of high density arrays, where each array has target substances arranged in two
analytical
axes. Preferably, the sectioning results in a plurality of identical high
density arrays. The
location and identity of the target substances are tracked through the
information in the
10 database. These arrays can be utilized to simultaneously screen analytes
for the presence of
specific properties, or can be utilized for other purposes as will be
understood by those with
skill in the art with reference to the disclosure herein.
Referring now to Figures 6 to 8, there are shown respectively, a plurality of
membranes 28 having lines of target substances 30 applied on each membranes
28; the
membranes 28 stacked and stabilized to form the bundle 32; and the bundle 32
being
sectioned to produce a plurality of high density arrays 34, where each array
has target
substances 28 arranged in two analytical axes.
Referring now to Figures 9 to 11, there are shown respectively, a membrane
36 having lines of lmown target substances 38 applied on membrane 36; the
membrane 36
being rolled and stabilized to form a bundle 40; and the bundle 40 being
sectioned to produce
a plurality of high density arrays 42, where each array has target substances
38 arranged in
two analytical axis.
Production of High Density Arrays from Bundles Comprising T~bes
In one cmbodiment, high density arrays are produced from target-strands
comprising tubes. The tubes can comprise polyimide, nylon, polypropylene,
polyurethane,
silicone, ethyl vinyl acetate, stainless steel, copper, glass, or fused
silica, or can be other
materials as will be understood by those with skill in the art with reference
to the disclosure
herein.
In a preferred embodiment, target strands are produccd by cc~aating the inside
of the tubes with an aqueous solution of the target substance such that the
target substance is
absorbed, adsorbed or covalently bound to the interior surface of the tubes.
Alternately, the
tubes can be filled with the target substances with or without embedding the
target substances
CA 02407265 2002-11-13
11
in a matrix. A series of such tubes are produced by coating or filling the
tubes with different
target substances and the identity of each target-strand and the target
substance it contains is
recorded in a database.
The tubes are then assembled into bundles with the location of each tube and
its associated target substance noted in the database. The bundle of tubes is
preferably
stabilized by embedding the bundle in a matrix to provide structural support
to the bundle.
The bundle is then sectioned substantially perpendicular to the long axis of
the
tubes using suitable instrumentation to provide a plurality of high density
arrays. Preferably,
the sectioning results in a plurality of identical high density arrays. The
identity and location
of the target substances are tracked thmugh the information in the database.
These arrays
can be utilized to simultaneously screen analytes for the presence of specific
properties, or
can be utilized for other purposes as will be understood by those with skill
in the art with
reference to the disclosure herein.
>teferring now to Figures 12 to 14, there are shown respectively, target-
strands
44 comprising a series of tubes 46 filled with known target substances 48; the
target-strands
44 embedded in a matrix 50 and assembled into a bundle 52; and the bundle 52
being
sectioned to produce high density arrays 54, where each array has target
substances 48
arranged in two analytical axis.
F;XANll'LE I
Production and Use of High Density Arrays Comprising DNA Coated Threads
The method of producing high density arrays from a bundle comprising fibers
or threads according to the present invention is used to produce high density
arrays of DNA
target substances as follows. Cotton thread is evaluaxed for wetability by an
aqueous solution
by dipping the thread in water. Water beading on the surface of the thread
indicates that the
thread could have binders, oils or other materials on its surface that can
negatively affect the
wetability of the thread for producing target-strands. If beading occurs
during the wetability
test, the threads should be washed in methanol, ethanol or another suitable
solvent miscible
with water to remove the undesirable materials. The threads are then placed in
water and the
water exchanged several times until each thread is fully wetted.
Next, the threads are transfen~ed into an aqueous solution of a polymeric
cationic substance such as poly L-lysine and allowed to equilibrate with the
poly L lysine
solution for a few hours. The threads are removed from the poly L-lysine
solution and dried
CA 02407265 2002-11-13
12
to fix the poly L-lysine to the surface of the threads. After fixation, the
threads are washed in
buffered solution and the buffer is exchanged several times. The threads are
removed from
the buffer and allowed to dry.
The threads are then cut into lengths, varying from a centimeter to a few
meters, as appropriate to the dimensions of the bundle being constructed. Each
thread
destined for the bundle is preferably cut to the same length.
Next, each cut thread is placed in contact with a solution of DNA having a
specific known sequence that is to be the immobilized target substance. The
DNA sequence
is preferably different for each thread. The DNA used should preferably be
single stranded if
IO it is to be utilized for nucleic acid hybridization studies, but can
otherwise be left in double
stranded farm. The DNA can be from natural sources such as plasmid
preparations, yeast
artificial chromosomes, BAC libraries, YAC libraries or other DNA libraries
such as
expressed sequence tags, or can be synthetically produced by the polymerase
chain reaction
or other synthetic processes. The thread and the DNA solution are incubated
for a period
ranging from a few minutes to a few hours, as is needed to fully saturate the
available binding
sites on the thread with DNA.
The DNA coated threads are then dried in an oven at approximately
60°C for a
period sufficient to a~x the DNA to the threads. Alternatively, the DNA can be
fixed to the
threads by wetting the dried DNA coated thread with 100 % ethanol or methanol
for a few
minutes and allowing the threads to dry. The identity of each thread and its
sequence of
immobilized DNA target substance is recorded in a database. Next, the threads
are
individually washed in a buffer such as lx TE (10 mM tris, 1 mM EDTA, pH 7.6)
to remove
unbound DNA from the thread. The DNA coated threads are again dried.
A bundle of DNA coated threads is then assembled by placing the threads
parallel and adjacent to one another with the location of each thread in the
bundle and its
associated DNA recorded in the database. The bundle of threads is stabilized
by embedding
it in a matrix such as palymethacrylate, epoxy resins, polyethylene glycol,
paraffin waxes,
gums, poly acrylamide and other similar materials which can, preferably, be
handled in liquid
form at elevated temperature or in unpolymerized form suitable for embedding
the threads.
The embedded threads are allowed to harden or to crosslink to impart a rigid
structure to the
bundle.
In a preferred embodiment, the threads are prevented from becoming fully
CA 02407265 2002-11-13
13
impregnated with embedding matrix and sequestering the immobilized DNA by
coating the
threads with a substance such as gelatin, sucrose or polyvinyl alcohol, to
which the matrix is
impermeant. This is accomplished by wetting the threads bearing the fixed,
immobilized
DNA in a solution containing from about 0.019b to about 10 X by weight of the
substance
and allowing the threads to dry before being embedded in the matrix.
The stabilized bundle is then sectioned perpendicular to the long axis of the
threads using a microtome or similar device to create a plurality of high
density arrays
preferably having a thiclrness of between about 0.1 and 100 microns. Each
resultant high
density array has the same pattern of DNA sequences in specific spatial
regions or zones of
the array with the target substances arranged in two analytical axis.
One use for these DNA arrays is to detect labeled DNA sequ~ces in an
sample which are complimentary to single stranded DNA target substances in the
array by
incubating the sample and array under hybridizing conditions for a sufficient
period of time
for hybridization to occur. Unhybridized DNA is removed by washing. The labels
are then
detected and the zones providing signal are determined. These zones are
compared to the
database containing the identity of the DNA target substances on the array to
establish the
identity of the labeled DNA in the sample.
ERAMPLE B
Production and Use of High Density Arrays Comprising Peptide Coated Threads
The method of producing high density arrays from a bundle comprising fibers
or threads according to the present invention is used to produce high density
arrays of peptide
target substances as follows. Cotton thread is evaluated for wetability by an
aqueous solution
by dipping the thread in water. Water beading on the surface of the thread
indicates that the
thread could have binders, oils or other materials on its surface that can
negatively affect the
wetability of the thread for producing target-strands. If beading occurs
during the wetability
test, the threads should be washod in methanol, ethanol or another suitable
solvent miscible
with water to remove the undesirable materials. The tht~eads are then placed
in water and the
water eacchanged several times until each thread is fully wetted.
Next, the thzeads are transferred into an aqueous solution of a polymeric
cationic substancx such as poly Irlysine and allowed to equilibrate with the
poly L-lysine
solution for a few hours. The threads are removed from the poly L-lysine
solution and dried
to fix the poly L-lysine to the surfacx of the threads. After fitcation, the
threads are washed
CA 02407265 2002-11-13
14
buffered solution and the buffer is exchanged several times. The threads are
removed from
the buffer and allowed to dry.
The threads are then cut into lengths, varying from a centimeter to a few
meters, as appropriate to the dimensions of the bundle being constructed. Each
thread
destined for the bundle is preferably cut to the same length. Cotton thread is
evaluated for
wetability by an aqueous solution, transferred into an aqueous solution of a
polymeric
cationic substance such as poly L-lysine, and allowed to equilibrate with the
poly L-lysine
solution for a few hours. The threads are removed from the poly L-lysine
solution and dried
to fix the poly L-lysine to the surface of the threads. After fixation, the
threads are washed in
buffered solution and the buffer is exchanged several times. The threads are
removed from
the buffer and allowed to dry.
Next, each cut thread is placed in contact with a dimethylsulfoxide (DMSO)
solution of peptide having a specific known sequence which is to be the
immobilized target
substance. The peptide sequence is preferably different for each thread.
Individual peptides
for use as target substances are obtained commercially or are made by
Merifield synthesis,
(such as discussed in Bodanszky, M. and Troust, B. Eds. Principles of Peptide
Synthesis, 2nd
ed., Springer-Verlag, New York, 1993, as will be
understood by those with skill in the art with reference to the disclosure
herein. Each thread
and peptide solution are incubated for a period ranging from a few minutes to
a few hours, as
is needed to fully saturate the available binding sites on the thread with
peptide.
The peptide coated threads are blotted free of excess DMSO solution and then
incubated with mixed pentanes or an equivalent substance to precipitate the
peptides onto the
surface of the threads. The peptide coated threads are dried at room
temperature or between
about 60°C and 70°C, with or without a vacuum. The identity of
each thread and its
sequence of immobilized peptide target substance is recorded in a database.
The peptide
coated threads are then washed in aqueous buffer such as 0.01 to 1.0 M tris pH
7.0 or
phosphate buffered saline pH 7.0, such as 120 mM sodium chloride, 2.7 mM
potassium
chloride and 10 mM phosphate (available from Sigma Chemical Co., St. Louis,
MO, USA)
to remove unbound peptides from the threads and dried again at room
temperature or between
about 60°C and 70°C, with or without a vacuum.
A bundle of peptide coated threads is then assembled by placing the threads
parallel and adjacent to one another with the location of each thread in the
bundle and its
CA 02407265 2002-11-13
associated peptide recorded in the database. The bundle of threads is
stabilized by
embedding it in a matrix such as polymethacrylate, epoxy resins, polyethylene
glycol,
paraffin waxes, gums, poly acrylamide and other similar materials which can,
preferably, be
handled in liquid form at elevated temperature or in unpolymerized form
suitable for
5 embedding the threads. The embedded threads are allowed to harden or to
cmsslink to
impart a rigid structure to the bundle.
In a preferred embodiment, the threads are prevented from becoming fully
impregnated with embedding matrix and sequestering the immobilized peptide by
coating the
threads with a substance such as gelatin, sucrose or polyvinyl alcohol, to
which the matrix is
10 impermeant. This is accomplished by wetting the threads bearing the fixed,
immobilized
DNA in a solution containing from about 0.01 to about 109 by weight of the
substance and
allowing the threads to dry before being embedded in the matrix.
The stabilized bundle is then sectioned perpendicular to the long axis of the
threads using a microtome or similar device to create a plurality of high
density arrays
15 preferably having a thickness of between about 0.1 and 100 microns. Each
resultant high
density array has the same pattern of peptide sequences in specific spatial
regions or zones of
the array.
One use for these peptide arrays is to detect the presence of antibody analyte
in
a sample, where the antibody is capable of binding to at least one peptide
target substance on
the array. The presence of the antibody analytes is determined by incubating
the sample and
array under suitable conditions for a sufficient period of time for binding
between the
antibody analyte to occur. Unbound sample is removed by washing. The bound
antibody is
then detected using biotinylated secondary antibodies and labeled streptavidin
detection such
as alkaline phosphatase, fluorescein or gold labeled streptavidin, according
to techniques
known to those with skill in the art, and the identity of the peptide target
substances on the
zones displaying binding are established by reference to the database. Binding
indicates the
presence of antibody having an epitopic domain for the peptide in the zone.
This binding can
be evidence of exposure to or infection by an organism, if the sample was
derived from a
patient's serum.
F~ III
Production and Use of High Density Arrays Comprising DNA Impregnated on a
Membrane
CA 02407265 2002-11-13
16
The method of producing high density arrays of target substances according to
the present invention was used to create arrays from DNA impregnated on a
membrane as
follows. The Saccharomyces Genome Database at Stanford University, Palo Alto,
California, USA was used as a source for identifying naturally existing
genomic sequences.
Using this information, 16 oligonucleotides having similar melting
temperatures were
randomly selected from the yeast genome as target substances. Each sequence
was between
28 and 35 nucleotides and was synthesized by standard
cyanoethylphosphoramidite chemistry
according to the method disclosed in Gait, M.J., Ed., Oligonucleotide
Synthesis: A Practical
Approach, IIZL Press, Oxford, 1984. Each target substance sequence had 100
thymidine
residues at the 3' end to facilitate binding of the oligonucleotide to the
membrane. See, for
example, Erlich, Henry A. and Bugawan, Teodorica L., HLA Class II Gene
Polymorphism:
DNA Typing, Evolution, and Relationship to Disease Susceptibility in PCR
Technology:
Principles and Applications for DNA Amplification. Stockton Press, New York,
pp. 193-208,
1989. The 16 target substances, labeled #1
through #1.6, were individually dissolved in diethylpyrocarconate treated
water to a final
concentration of 10 ug/~.1:
The target substances were applied using an application nib having a reservoir
with a capacity of 11 ~1 connected to the tip by a small capillary channel.
The nib was used
to draw lines of target substances approximately 1 mm to 3 mm apart on 20 cm x
20 cm
membranes of Hybond~' N+charged nylon membranes (Amersham, Arlington. Heights,
IL,
USA). The nib reservoir was filled with 10.51 of a solution of the first of
the 16 target
substances using an Eppendorf~ 2-10 ~1 pipetor.
The first membrane, membrane #1, was placed on a clean, flat tabletop with
the sheet of a waxed paper larger than the membrane that was used as a
separator in the
manufacture's packaging placed between the membrane and the tabletop. The nib
was
aligned such that both sides of the capillary channel touched the waxed paper
about 1 cm
from the edge of the membrane and the nib was smoothly drawn across the waxed
paper and
membrane manually using a ruler as a guide to draw a straight line of target
substance
parallel to one edge of the membrane. The solution of target substance was
drawn out of the
nib and exhausted after drawing a line approximately 12-16 cm long. This cycle
was
repeated for each solution of target substance on the first membrane until
membrane #1
comprised 16 parallel lines of different DNA target substances approximately 1
mm to 3 mm
CA 02407265 2002-11-13
17
apart from each other.
This procedure was repeated to produce two additional membranes,
membranes #2 and #3, except that each solution of DNA target substance was
applied three
times consecutively resulting in a total of 48 parallel lines of target
substances on membrane
#2 and #3. Each line of target substance was labeled for identification
purposes on all of the
membranes.
The membranes comprising the lines of DNA target substances were allowed
to air dry for about 2 hours and were then crosslinked by application of 1200
~cjoules of UV
electromagnetic radiation for 35 seconds using a Stratagene 2400 Stratalinkerm
(Stratagene,
La Jolla, CA, USA). Starting with the edge of the membrane containing the
leading edge of
the target substance lines, one strip about 2 cm in width by 20 cm in length
was cut from
each of the three membranes so that the lines of target substances were
parallel to the 2 cm
edge of the strips.
Radioactively lavbeled DNA probes which were complimentary to the sequence
of target substances #1 and #7 were prepared using standard techniques.
Hybridization was
attempted between the radioactively labeled probes and an array produced from
membrane #1
using standard techniques. In summary, the DNA oligonucleotides was labeled
using the
Ready to Go Kinase~'" ldt {Pharrnacia, Piscataway, NJ, USA) using gamma-~P-ATP
(ICN
Radiochemicals, Irvine, CA, USA) according to the manufact<uer's instructions.
The labeled
probes were purified using Nick'TM columns (Pharmacia) according to the
manufacturer's
instructions, and diluted to approximately 1x106 cpm/ml.
Prehybridization and hybridization was performed using 10 ml HyperHybt'r'
buffer (Research Genetics, Inc. Huntsville, AL, USA) according to the
manufacturer's
instructions in a Mini-6"~' hybridization oven (Hybaid, Ltd., Middlesex, UK)
at 42°C for one
hour each. Post-hybridization washes were performed using three 10 ml washes
for 15
minutes each in IxSSC, 0.01 sodium dodecyl sulfate (SDS) at 42°C. A
final wash was
performed in 100 ml of the lxSSC (0.15 M NaCI, 0.015 M sodium citrate, pH 7.2)
(Research Genetics), 0.0196SDS buffer (Sigma) at 42°C for 15 minutes. A
final rinse was
performed in 10 ml lzSSC buffer. The membranes were then air dried for 1-2
hours at room
tempetahue.
Autoradiography was performed by placing the arrays in contact with
Biomax'"r' MS or MR x-ray film (Eastman Kodak, Rochester, NY, USA) at room
CA 02407265 2002-11-13
18
temperature for between about 'k hour to 4 hours until the desired image
intensity was
obtained. All probes hybridized with the appropriate target substance on the
array
demonstrating that the DNA target substances were attached to the membrane and
available
for probing, and that such probing gave specific, non-ambiguous hybridization
results.
Next, the remaining 20 cm by 18 cm portion of membranes #1, 2 and 3 were
used to produce arrays as follows. The membranes were immersed in 3 y6
teleostean gelatin
(Sigma) in deionized water and were incubated overnight at room temperature to
block the
membranes. The membranes were then washed three times in 600 ml of deionized
water to
remove unbound gelatin. The membranes were blotted free of excess moisture
between two
sheets of 903 blotting paper (Schleicher and Schuell, Keene, NH, USA ) and
allowed to air
dry at room temperature overnight.
Next, a 2 cm by 20 cm strip was cut from each of the three membranes #1,
#Z, #3 perpendicular to the lines of target substances with the 2 cm edge
parallel to the lines
of target substances. Each of the strips was tightly rolled about an axis
parallel to the lines of
target substances to produce a cylinder with the portion of the membrane which
did not have
target substances applied to it being the innermost part of the cylinder.
Clear nail polish was
used to seal the free 3 mm edge of the strips to prevent the cylinder from
unwinding. Each
cylinder was immersed into a plastic bulb 1.25 cm by 7.5 cm filled with
unpolymerized Llt
White''" soft embedding media (Sigma) prepared according to the manufacturer's
instructions
until the cylinder became fully impregnated by the media. Each cylinder was
then placed at
the base of the media filled bulb, centered and allowed to polymerize
overnight at 60°C.
Each bulb containing an embedded cylinder was removed and placed at ambient
temperature
and polymerization was observed to be complete.
A plurality of arrays approximately 10 microns thick was then produced by
repratod sectioning each embedded cylinder perpendicular to its long axis,
that is
perpendicular to the long axis of each line of target substance. The
sectioning was
accomplished using a hand microtome, model DK-10 (Edmund Scientific,
Barrington, NJ,
USA).
Radioactively labeled DNA probes which were complimentary to the sequence
of target substances #1 and #7 were prepared using standard techniques.
Hybridization was
attempted bawean the radioactively labeled probes and an array produced from
membrane #1
using standard techniques. In summary, the DNA oligonucleotides were labeled
using the
CA 02407265 2002-11-13
19
Ready to Go KinaseT''" Idt (Pharmacia, Piscataway, NJ, USA) using gamma-32P-
ATP (ICN
Radiochemicals, Irvine, CA, USA) according to the manufacturer's instructions.
The labeled
probes were purified using Nick""' columns (Pharmacia) according to the
manufacturer's
instructions, and diluted to 1x106 cpm/ml.
Prehybridization and hybridization was performed using 10 ml HyperHybT'"'
buffer (Research Genetics, Inc. Huntsville, AL, USA) according to the
manufacturer's
instructions in 1.5 ml screw-cap microcentrifuge tubes at 42 C for one hour in
a Mini-6
hybridization oven (Hybaid, I,td., Middlesex, UK) at 42°C. Post-
hybridization washes were
performed using three 1.5 ml washes for 15 minutes each in IxSSC, 0.01 %
sodium dodecyl
sulfate (SDS) at 42°C. A final wash was performed in 100 ml of the
lxSSC (0.15 M NaCI,
0.015 M sodium citrate, pH 7.2) (Research Genetics), 0.01 ~ SDS buffer {Sigma)
at 42 ° C for
minutes. a final rinse was performed in 10 ml IxSSC buffer. The arrays were
then air
dried for approximately 15-30 minutes. Autoradiography was performed by
placing the
arrays in contact with BiomaxT"" MS or MR x-ray film (Eastman Kodak,
Rochester, NY,
15 USA) at room temperature for between about 'fi hour to 4 hours until the
desired image
intensity was obtained. Photographs of the developed autoradiographies were
then made.
Hybridization was attempted between the radioactively labeled probes and an
array produced from membrane #1 using standard techniques. All probes
hybridized with
the appropriate target substance on the array demonstrating that the DNA
target substances
were attached to the membrane and available for probing, and that such probing
gave
specific, non- ambiguous hybridization results.
The arrays were tested for functionality as follows. A radioactively labeled
DNA probe complimentary to target substance #1 was used to probe an array
produced from
membrane #2. Referring now to Figure 15, there can be seen a photograph of the
autoradiograph of the result. As can be seen, hybridization between the probe
and three
zones on the array containing target substance #1 occurred, with minimal cross
hybridization
for the other 45 zones representing the remaining 15 DNA target substances.
Hence, the
array demonstrated both functionality for hybridization studies as well as
specificity.
Next, an array produced from membrane #3 was probed with radioactively
labeled DNA complimentary to target substances #1 and #7. Referring now to
Figure 16,
there can be seen an autoradiograph of the result. As can be seen,
hybridization between the
probes and six zones on the array occurred, with minimal cross hybridization
for the other 42
CA 02407265 2002-11-13
zones representing the remaining 14 DNA target substances.
Although the present invention has been discussed in considerable detail with
reference to certain preferred embodiments, other embodiments are possible.
Therefore, the
scope of the appeaded claims should not be limited to the description of
preferred
5 embodiments contained herein,