Note: Descriptions are shown in the official language in which they were submitted.
CA 02408856 2002-11-21
71916-15D
-1--
This application is a divisional application of
copending application 2,049,973, filed February 19, 1991.
Backaround of the Invention
1. Field of the Invention
This invention relates to methods for lessening
restenosis of body lumens, and to prostheses for delivering
drugs to treat said restenosis.
2. Describtion of the Related Art
Restenosis is defined as the enclosure of a
previously dilated, ablated, or lased peripheral or coronary
vessel. It occurs at a rate of 20-50-°s for each of these
procedures and is dependent on a number of variables (i.e.,
vessel location, lesion length, etc.). Restenosis may begin
immediately following an angioplasty procedure, but ceases
at the end of approximately six (6) months. There is not a
current therapeutic procedure that has been proven to
significantly reduce this restenosis rate.
A recent technology that has been developed that
assesses the problem of restenosis is intravascular stems.
Stents are metallic devices that are permanently implanted
(expanded) in coronary and peripheral vessels. The goal of
these stems is to provide a long-term "scaffolding" or
support for the diseased (stenosed) vessels. The theory
being, if you can support the vessel from the inside, the
vessel will not close down or restenose. Unfortunately,
initia2 data from clinical sten.t implants shows that these
metallic structures do not significantly reduce the amount
of restenosis.
Many pharmacologic (biochemical) attempts have
been made to reduce the amount of restenosis. All of these
CA 02408856 2002-11-21
71916-15D
-2-
attempts have dealt with the systemic delivery of drugs via
oral or intravascular introduction. Very limited success
has been achieved with this systemic approach.
For drug delivery, it has been recognized for a
!~ long period of time that pills and injections may not be the
best mode of administration. It is very difficult with
these types of administration to get constant drug delivery.
Through repeated doses, these drugs often cycle through
concentration peaks and val:Leys, resulting in time periods
of toxicity and ineffectiveness. Thus, localized drug
treatment is warranted.
The art described in this section is not intended
to constitute an admission that any patent, publication or
other information referred to herein is "prior art" with
respect to this invention, unless specifically designated as
such. In addition, this section should not be construed to
mean that a search has been made or that no other pertinent
information as defined in 37 C.F.R. ~ 1.56(a) exists.
Summary of the Invention
The parent invention may be summarized as an
intraluminal drug-eluting prosthesis assembly comprising: a
stmt, said stent being expansible to f:ix said stmt to an
interior of a vascular lumen, said stmt being constructed
and arranged such that at least a portion of the exterior
surface of said stmt is formed from a polymer into which a
drug which limits acute or chronic closure of said vascular
lumen is compounded; and a stmt delivery catheter for
delivering said stmt through a blood vessel.
In one aspect, the present application provides
use of a drug to limit acute or chronic closure of a
CA 02408856 2002-11-21
71916-15D
-2a-
vascular lumen in the manufacture of a polymeric composition
for use in a method of treating a vascular lumen with a
stmt .
In a further aspect, the present application
provides a pharmaceutical composition for drug delivery to a
vascular lumen by a stmt comprising a drug to limit acute
or chronic closure of a vascular lumen incorporated within a
polymeric material.
The invention provides prostheses which may be
inserted into a lumen of a body and fixed to the lumen wall
adjacent an area needing treatment. Mcst typically, the
lumen will be part of ~he vascular system which may
restenose. However, the methods and devices of the
invention are also suited to treatment of any body lumen,
including the vas deferens; ducts of the gallbladder,
prostate gland, trachea, bronchus and liver or any other
lumen of the body where medication cannot be applied without
a surgical procedure. The invention applies to acute and
chronic closure or reclosure of body lumens.
The prostheses of the invention include at least
one drug which will release from the device at a controlled
rate to supply the drug where needed without the overkill of
systemic delivery. The prostheses include means for fixing
the device in the lumen where desired. The prostheses may
be completely biodegradable or may be bioabsorbable in whole
or in part such that the prostheses wil_1 be completely
incorporated into the lumen wall as a result of tissue
growth, i.e. endothelialization. Alternatively, the
prostheses may be biostable in which case the drug is
diffused out from the biostable materials in which it is
incorporated.
CA 02408856 2002-11-21
71916-15D
-2b-
The prosthesis comprises a generally flexible
tubular body which is fixed against the lumen walls by a
mechanical action. The device should not cause an
appreciable reduction in the lumen cross-section where
inserted. Conventional stmt designs which provide an
CA 02408856 2002-11-21
-3-
expansion of the vessel are suitable, though not required.
In all cases, the prostheses of the invention require the
presence of an elutable drug compounded to the prosthesis
itself. With conventional metal stems, the invention
requires a drug-carrying coating overlying at least a portion
of the metal.
The drugs in the prosthesis may be of any type
which would be useful in treating the lumen. In order to
prevent restenosis in blood vessels, migration and subsequent
l0 proliferation of smooth muscle cells must be checked.
Platelet aggregation and adhesion can be controlled with
antiplatelets and anticoagulants. Growth factor and receptor
blockers and antagonists may be used to limit the normal
repair response.
The current invention contemplates the usage of any
prosthesis which elutes drugs locally to treat a lumen in
need of repair. Controlled release, via a bioabsorbable
polymer, offers to maintain the drug level within the desired
therapeutic range for the duration of the treatment. When
"stmt" is referred to herein, it may include the classical
definition of stems as they are used in intravascular
applications. "Stent" used herein also includes any
prosthesis which may be inserted and held where desired in a
lumen. It includes, but is not limited to, structures such
as those shown and described in U.S. Patent 4,886,062 to
Wiktor.
Brief Description of the Drawings
A detailed description of the invention is
hereafter described with specific reference being made to the
drawings in which:
FIG. 1 is a greatly enlarged side view of an
intralumenal drug-eluting prosthesis of the invention;
FIG. 2 is a greatly enlarged side view of an
alternative embodiment to the prosthesis of Fig. 1:
FIG. 3A is a greatly enlarged fragment of the
embodiment of Fig.l
FIG. 3B is a greatly enlarged fragment of the
embodiment of Fig. 1 in which two layers of polymer are
CA 02408856 2002-11-21
71916-15
-4-
present, each having a different drug;
FIG. 4 is a greatly enlarged fragment of the
embodiment of Fig. 2;
FIG. 5 is a greatly enlarged microscopic
fragmentary detail of drug shown eluting from the porous
structure of a filament or filament coating in a prosthesis
into tissue or the vessel lumen;
FIG. 6 is a greatly enlarged cross-section of a
blood vessel showing plaque profile immediately post-balloon
catheter dilation procedure;
FIG. 7 is a greatly enlarged cross-section of the
subject of Fig. 6 at a later date showing restenosis;
FIG. 8 is a greatly enlarged cross-section of a
blood vessel showing plaque-prosthesis profile immediately
post-prosthesis implant procedure;
FIG. 9 is a greatly enlarged cross-section of the
subject of Fig. 8 after ingrowth has occurred;
FIG. 10 is a greatly enlarged fragmentary
perspective view of a blood vessel wall and prosthesis
filament of Figs. 1 and 3 immediately after implantation;
FIG. 11 is a greatly enlarged fragmentary
perspective view of the subject of Fig. 10 after about one
month;
FIG. 12 is a greatly enlarged fragment of a loose
weave of prosthesis filaments;
FIG. 13 is a greatly enlarged fragment of a coated
metal filament in a loose weave;
CA 02408856 2002-11-21
71916-15
-5-
FIG. 14 is a greatly enlarged fragment of a melted
junction weave of prosthesis filaments in a loose weave;
FIG. 15 is a greatly enlarged fragment of a kinked
junction weave of prosthesis filaments;
FIG. 16 is a greatly enlarged fragment of multi
strand weave of prosthesis filaments; and
FIG. 17 is an alternative embodiment to Fig. 16,
in which the strands are not woven.
Description of the Preferred Embodiments
Restenosis
In the summary, a very simple definition of
restenosis was given. As a complement to this definition,
there are several more clinical definitions. Several of
these definitions are listed below:
1. Loss of at least 50s of the initial gain achieved
in angioplasty.
2. Decrease of at least 30o in the lumen diameter
compared to post-angioplasty result.
3. A return to within l00 of the pre-angioplasty
diameter stenosis.
4. An immediate post-angioplasty diameter stenosis of
less than 50o that increases to 70% or greater at follow-up.
6. Deterioration of 0.72 mm in minimal luminal
diameter or greater from post-angioplasty to follow-up.
7. As for 6, but with a deterioration of 0.5 mm.
CA 02408856 2002-11-21
71916-15
-5a-
These definitions are used by cardiologists to
clinically (angiographically) define restenosis.
Several hypotheses exist on why and how restenosis
occurs. The current, most widely accepted explanation is
that restenosis is a natural healing process in response to
the arterial injury that occurs during all types of
angioplasty procedures. This very complex healing process
results in intimal hyperplasia, more specifically migration
and proliferation of medial smooth muscle cells (SMC). The
problem associated with this arterial healing process is
that in some instances, it does not shut off. The artery
continues to "heal" until it becomes occluded. It should be
noted that restenosis is not a re-deposition of the plaque-
like cholesterol material that originally occluded the
artery.
The following is a possible scenario for
restenosis according to the vessel healing hypothesis.
Successful angioplasty of stenotic lesions produces cracking
of the plaque, dissection into the media, denudation and
destruction of endothelial cells, exposure of thrombogenic
collagens, released tissue thromboplastin, and an increased
loss of
CA 02408856 2002-11-21
-6-
protacyclin production. All of these lead to the aggregation
of active platelets.
Figs. 6 and 7 show a typical vessel 30 in cross-
section after angioplasty procedures showing the interior 32
of the lumen. In Fig. 6 the interior of the lumen is rough
and includes intimal flaps 34. Damage causes healing with
deposition of platelets, fibrin formation and proliferation
of neointima 37 which as shown i.n Fig. 7 significantly
reduces the interior of the lumen.
Activated platelets release several mitogens
including platelet derived growth factor (PDGF), epidermal
growth factor, and transforming growth factor. PDGF has both
mitogenic and chemotactic properties and thus, may induce
both migration of SMC from the medial layer to the intimal
layer as well as proliferation (intimal hyperplasia). PDGF
causes SMC proliferation by binding to specific PDGF
receptors. Once the PDGF is bound to the receptors,
deoxyribose nucleic acid (DNA) synthesis occurs and new cells
are replicated. Minor endothelial injury may cause platelet
adhesion and activation with the resultant release of PDGF.
Thus, even the deposition of a monolayer of platelets may be
sufficient to induce SMC proliferation.
Deeper arterial injury which is sometimes
associated with complex stenotic lesions leads to more
extensive platelet deposition and activation which may cause
an even greater availability of the mitogenic factors. Thus,
increased SMC proliferation and intimal hyperplasia.
Arterial injury from angioplasty may result in release of
PDGF-like compounds from not only platelets but also
macrophages, monocytes, endothelial cells, or SMC's
themselves.
Activated SMC from human atheroma or following
experimental arterial injury secrete PDGF-like molecules
which appears to lead to self perpetuation of SMC
proliferation by the release of their own PDGF-like
substances. Thus, any or all of the cells which can secrete
PDGF related substances (platelets, macrophages, monocytes,
endothelia, and smooth muscle cells) may contribute to the
CA 02408856 2002-11-21
-7_
cascading effect of restenosis after angioplasty.
The previous restenosis scenario resulted from
normal angioplasty procedures. During balloon angioplasty if
the balloon is undersized or not totally inflated and the
plaque cracking and extensive endothelial denudation does not
occur the lesion would restenose. Rheologic factors
contribute as well to the interaction between platelets and
the arterial wall. Residual stenosis, resulting from
inadequate balloon expansion, produces a high local shear
rate and enhances platelet deposition and activation. These
stenoses may be important as a stimulus for some
proliferation through enhanced platelet deposition and
secretion of growth factors. This hypothesis correlates with
the increased incidence of restenosis in patients with high-
grade residual stenoses or transtenotic gradients.
Prevention of Restenosis
In order to prevent restenosis, one must stop the
proliferation of smooth muscle cells. As stated earlier,
this is a biochemical process which cannot be treated
mechanically. Several hypotheses exist on how to
biochemically stop restenosis. Some of which are:
1. Reduce the adhesion and aggregation of the platelets at
. the arterial injury site.
2. Block the expression of the growth factors and their
receptors.
3. Develop competitive antagonists of the above growth
factors.
4. Interfere with the receptor signaling in the responsive
cell.
5. Find a "natural" inhibitor of smooth muscle
proliferation.
Item #1 is directly related to the formation of
thrombus, a major problem with all types of angioplasty
procedures. Items #2, #3, and #4 are closely related. They
deal with blocking restenosis during the massive cell
CA 02408856 2002-11-21
-8-
migration and replication cycle. Unlike item #1, these items
address the growth factors that are produced from sources
other than platelets. Item n5 is listed to address the
question, why don't the 50-80% of the people who don't
restenose, restenose. There may be some type of natural
inhibitor that these people produce that stops the
proliferation of smooth muscle cells.
There are at least two (2) different ways to
l0 prevent the adhesion and aggregation of platelets. One
method is to use an antiplatelet and another is to use an
anticoagulant.
Antiplatelet drugs include drugs such as aspirin
and dipyridamole. Aspirin is classified as an analgesic,
antipyretic, anti-inflammatory, antiplatelet drug. It has
been clinically tested and proven to reduce the risk of
sudden death and/or non-fatal reinfarction in post myocardial
infarction (heart attack) patients. The proposed mechanism
of how aspirin works, relates directly to the platelets. It
ZO somehow blocks the platelets, restricting coagulation. This
prevents the cascading platelet aggregation found in thrombus
and restenosis. Aspirin is therefore a possible restenosis
inhibitor. Dipyridamole is a drug similar to aspirin, in
that is has anti-platelet characteristics. Dypridimole is
also classified as a coronary vasodilator. It increases
coronary blood flow by primary selective dilatation of the
coronary arteries without altering systemic blood pressure or
blood flow in peripheral arteries. These vasodilation
characteristics are thought to be possibly beneficial for
restenosis prevention.
Anticoagulant drugs include Heparin, Coumadin,
Protamine, and Hirudin. Heparin is the most common
anticoagulant used today. Heparin, in one form or another,
is used in virtually every angioplasty procedure performed.
All four (4) of these drugs function as an anticoagulant by
'preventing the production of thrombin, a binding agent which
causes blood to clot. This too, may reduce the cascading
effect of platelet aggregation at the lesion site, thus
CA 02408856 2002-11-21
_g_
possibly reducing restenosis. The use of Protamine in the
presence of Heparin causes the Protamine to function as a
Heparin antagonist, blocking the effect of the Heparin.
Protamine, however, used alone, acts as an anticoagulant.
Hirudin is singled out because it is not normally found in
the human body. Hirudin is a drug that is found in the
salivary glands of leeches. It is a very concentrated
anticoagulant that functions in the same manner as Heparin,
Coumadin, and Protamine.
There are several types of drugs that interrupt
cell replication. Antimitotics (cytotoxic agents) work
directly to prevent cell mitosis (replication), whereas
antimetabolites prevent deoxyribose nucleic acid (DNA)
synthesis, thus preventing replication. The action of the
antimitotics and antimetabolites are so similar, they will be
grouped into one category. This category will be known as
the anti- replicate drugs.
Anti-replicate drugs include among others:
Methotrexate, Azathioprine, Vincristine, VinBlastine,
Fluorouracil, Adriamycin, and Mutamycin. The target systemic
molarity desired with methotrexate is on the order of 106 M
with a range of between 103 to 108 Molar. Locally, the
molarity of the drug may be highly variable, which is one of
the great disadvantages in systemic administration of the
drug. When drugs are delivered locally via.the prosthesis of
the invention, they may be at therapeutic levels at the
diseased site while at the lower limits of detectability in
the bloodstream. So little drug is required for effective
local treatment of a lumen that the drug may not be
detectable in blood samples.
If the restenosis process ranges from immediately
after injury to about 4 months later, then the generalized
elution rates contemplated are that the drug should start to
be released immediately after the prosthesis is secured to
the lumen wall to lessen cell proliferation. The drug
should then continue to elute for about four months in total.
Complex systems of drugs may be carried by the
CA 02408856 2002-11-21
-10'_
prosthesis. An anticoagulant or antiplatelet may be included
on the outermost surface of the device in order to elute off
very quickly for the first several weeks. Antireplicates can
be formulated into the device to continue to elute later,
when in contact with non-blood cells after neointima
overgrowth has surrounded the device. This usually occurs in
about two weeks. The drug elution rate does not need to be
uniform, and may be tailored to fit the need of the patient.
Prosthesis ~Stent~ DesicLn
The current invention contemplates the usage of any
prosthesis which elutes drugs locally to treat a lumen in
need of repair. When "stent" is referred to herein, it may
include the classical definition of stents as they are used
in intravascular applications. "Stent" used herein also
includes any prosthesis which may be inserted and held where
desired in a lumen.
Figs. 1 through 17 show features of some of the
prostheses which may be used to carry and elute restenosis
limiting-drugs.
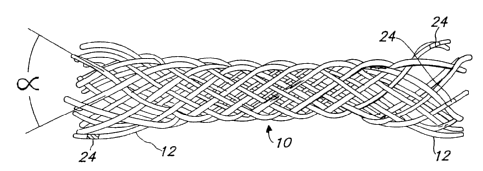
The current preferred stent 10 configuration
consists of a single filar, monofilament braided mesh design
as shown in Fig. 1. There are sixteen (16) filaments 12,
eight (8) of which are wound in one helical direction, and
the remaining eight (8) which are wound in the opposite
direction. The stent l0 is self-expanding to a predetermined
diameter. The profile (diameter) of the stent 10 can be
easily reduced by pulling the stent l0 longitudinally. In
this reduced profile configuration, the stent 10 can be
loaded into a catheter for delivery into the vessel.
The stent 20 shown in Figures 2 and 4 is a metallic
malleable design which may be forced against a lumen wall by
a balloon catheter which fixes it into position. The
exterior surface of the metal filaments 22 would include a
coating 14 with a drug-eluting polymer described previously.
The polymer may be biostable or bioabsorbable. If biostable,
the drug would diffuse out of the polymer.
The variations of design shown in the embodiments
CA 02408856 2002-11-21
--11-
of Figs. 1 and 2 show that the prosthesis of the invention
must be secured against a lumen wall and must carry a drug-
eluting polymer.
There are many variables in the design of stmt 10.
The angle (a) of the filaments 12 is a major variable. The
angle a can vary from 0 degrees to 180 degrees. The design in
the Figures is based on an angle in the 60 degree to 90
degree range.
There are many options for fabricating the drug
eluting stents. One option is to have all sixteen (16)
filaments be drug eluting. Or, you could have any number of
filaments up to sixteen (16) degrade and elute drugs.
Another option is to have a multi-filar stent. Instead of a
single filament braided into the stent, it is possible to
have two (2), three (3), or even four (4) strands 16 braided
to form a filament 12 as shown in Fig. 16. This would create
a stent with much greater expansile force, but also have much
more material in the surface area. This is a common trade-
off in stent design. Similar to the single-filar design, the
multi-filar form shown in Fig. 16 could have varying numbers
of strands 16 that are drug eluting. Figs. 16 and 17 show
that the mufti-filar design may be braided or upbraided. One
(1), two (2), three (3), or four (4) of the filaments could
be impregnated with a drug and biodegradably elute.
Alternatively, the polymer may be biostable which allows for
diffusion of the drug without degradation.
The stent 10 of Fig. 1 consists of- a wound braided
mesh which is self-expanding to a predetermined diameter and
whose profile diameter can be greatly reduced for catheter
introduction. The radial expansile force increases with
diameter to the point of the self- expanded diameter limit,
at which point the angle between the filaments and the
longitudinal axis is a maximum. Figures 12 through 15 show
alternative construction techniques to alter the radial
expansive force. Figure 12 shows the filaments 12 being
woven without any connection. Figure 13 is similar except
the filament 22 is formed with a metal core 16 and a coating
14. In Fig. 14 the individual filaments 12 are shown with a
bonded juncture 18. The bonding at the junctures 18 prevents
CA 02408856 2002-11-21
-12-
the individual filaments 12 from sliding relative to each
other, which improves the radial strength. The mechanically
kinked junction 19 shown in Fig. 15 also limits the sliding
of the filaments to change the radial strength. A heated
platen press may be pressed against the wound stmt while
still on the forming mandrel to form the kinks. Higher
temperatures may be used to form the melted junctures 18.
The devices may be made more visible under
fluoroscopy and x-ray by incorporating radiopaque materials
into marker bands 24 to the individual filaments 12 at the
ends of the stent 10 as shown in Fig. 1. Such bands could
help to locate the stmt and assure proper placement and
patency.
Bioabsorbable Prosthesis (Stmt) Materials
Controlled release, via a bioabsorbable polymer,
offers to maintain the drug level within the desired
therapeutic range for the duration of the treatment. In the
case of stents, the prosthesis materials will maintain vessel
support for at least two weeks or until incorporated into the
vessel wall even with bioabsorbable, biodegradable polymer
constructions.
Several polymeric compounds that are known to be
bioabsorbable and hypothetically have the ability to be drug
impregnated may be useful in prosthesis formation herein:
These compounds include: poly-1-lactic acid/polyglycolic
acid, polyanhydride, and polyphosphate ester. A brief
description of each is given be~iow.
Poly-1-lactic acid/polyglycolic acid has been used
for many years in the area of bioabsorbable sutures. It is
currently available in many forms, i.e., crystals, fibers,
blocks, plates, etc. These compounds degrade into non-toxic
lactic and glycolic acids. There are, however, several
problems with this compound. Ths degradation artifacts
(lactic acid and glycolic acid) are slightly acidic. The
acidicity causes minor inflammation in the tissues as the
polymer degrades. This same inflammation could be very
detrimental in coronary and peripheral arteries, i.e., vessel
occlusion. Another problem associated with this polymer is
CA 02408856 2002-11-21
-13-
the ability to control and predict the degradation behavior.
It is not possible for the biochemist to safely predict
degradation time. This would be very detrimental for a drug
delivery device.
Another compound which could be used are the
polyanhydrides. They are currently being used with several
chemotherapy drugs for the treatment of cancerous tumors.
These drugs are compounded in the polymer which is molded
into a cube-like structure and surgically implanted at the
to tumor site.
Polyanhydrides have weaknesses in their mechanical
properties, due to low molecular weights. This drawback
makes them difficult to process into a filament form. Also,
polyanhydrides have poor solubility, making characterization
and fabrication difficult.
The compound which is preferred is a polyphosphate
ester. Polyphosphate ester is a proprietary compound which
is currently being developed by Dr. Kam Leong at John Hopkins
University (JHU). Similar to the polyanhydrides,
polyphosphate ester is being researched for the sole purpose
of drug delivery. Unlike the polyanhydrides, the
polyphosphate esters have high molecular weights (600,000
average), yielding attractive mechanical properties. This
high molecular weight leads to 'transparency, and film and
fiber properties. It has also been observed that the
phosphorous-carbon-oxygen plasticizing effect, which lowers
the glass transition temperature, makes the.poly~ner desirable
for fabrication.
The basic structure of polyphosphate ester monomer is shown
below.
O
--(-P-O-R1-O-)--
OR
where P corresponds to Phosphorous,
CA 02408856 2002-11-21
-14-
O corresponds to Oxygen,
and R and R1 are functional groups.
Reaction with water leads to the breakdown of this compound
into monomeric phosphates (phosphoric acid) and diols (see
below) .
O
-- (-P-O-Rl-O-) -- + Hz0 - - H3P04 + ROH + HO - R1 - OH
OR
It is the hydrolytic instability of the phosphorous ester
bond which makes this polymer attractive for controlled drug
release applications. A wide range of controllable
degradation rates can be obtained by adjusting the
hydrophobicities of the backbones of the polymers and yet
assure biodegradability.
The functional side groups allow for the chemical
linkage of drug molecules to the polymer. This is shown
below.
O
__(_p_O_R1_O_)__
O
C (O)
drug
The drug may also be incorporated into the backbone
of the polymer.
CA 02408856 2002-11-21
-15-
O
--(-P-O-drug-O-)__
OR
In summary, the highly hydrolytically reactive
phosphorous ester bond, the favorable physical properties,
and the versatile chemical structure make the polyphosphate
esters a superior drug delivery system for a prosthesis.
Figs. 3A and 3B show that the filaments 12 may be
made from one or several layers of polymer. In Fig. 3A only
a single polymer is present to carry the drugs, In Fig. 3B a
second layer of polymer 15 is shown. That layer 15 may be a
simple barrier which limits diffusion of drugs in the polymer
14. In that event, the smaller molecules could elute out
immediately, while larger compounds would not elute until
later when the layer 15 has biodegraded. Alternatively,
layer 15 may include a different drug incorporated therein
from that found in layer 14. The barrier coating 15 could be
as simple as a silicone or polyurethane.
Operation
The prosthesis is inserted into the lumen wherever
needed as per the usual procedure for stents. The device is
fixed into place either by radial expansion in devices such
as shown in Fig. 1 or are deformed by a balloon catheter in
the case of devices in accordance with Fig. .2.
Figures 8 through 11 show the placement and effects
of the drug-eluting prosthesis of the invention. The
prosthesis tacks up any intimal flaps and tears caused by any
prior ballooning. The initial deposition of platelets and
subsequent thrombus formation 38 is controlled and minimized
by the stent design and the elution of drug which limits
platelet aggregation and other immediate repair responses
described previously. Localized thrombus formations in the
areas of cracked and roughened plaques and newly exposed
underlying collagen and .fibro-muscular tissues is also
decreased. This results in limited but quick neointima
formation 40 and intimal proliferation over individual stmt
CA 02408856 2002-11-21
-16-
filaments progressing to mature endothelial lining. Long
term restenosis is therefore limited. Elution of the anti-
replicates alone or in conjunction with the initial elution
of anti-coagulants can also limit the restenosis which occurs
in the natural healing process.
While this invention may be embodied in many
different forms, there are shown in the drawings and
described in detail herein specific preferred embodiments of
the invention. The present disclosure is an exemplification
of the principles of the invention and is not intended to
limit the invention to the particular embodiments
illustrated.
This completes the description of the preferred and
alternate embodiments of the invention. Those skilled in the
art may recognize other equivalents to the specific
embodiment described herein which equivalents are intended to
be encompassed by the claims attached hereto.