Note: Descriptions are shown in the official language in which they were submitted.
CA 02411879 2002-12-04
- 1
DESCRIPTION
ALLOYED HOT-DIP ZINC-COATED STEEL SHEET WITH EXCELLENT
SLIDING PROPERTY
Technical Field
The present invention relates to an alloyed hot-dip
galvanized steel sheet with excellent sliding, property which
is suitable as an anti-corrosive steel sheet for use in car
bodies.
Background Art
Alloyed hot-dip galvanized steel sheets are widely used
as anti-corrosive steel sheets for use in car bodies. The "''
alloyed hot-dip galvanized steel sheets are molded into car
bodies by pressing and must therefore be excellent not only
in anti-corrosive properties but also in sliding properties.
Process steps for manufacturing an alloyed hot-dip
galvanized steel sheet are broadly divided into the process
step of immersing a material steel sheet in a plating bath
to form a hot-dip galvanized layer on the surface of the
steel sheet, and the step of subjecting the steel sheet
carrying the hot-dip galvanized layer to alloying to thereby
form an alloyed hot-dip galvanized layer.
CA 02411879 2002-12-04
- 2 -
The hot-dip galvanized layer formed on the surface of
the steel sheet in the plating bath comprises an
intermetallic compound of Zn and Fe (~, 81, r), and the
sliding property of the alloyed hot-dip galvanized layer
formed by alloying varies depending on the composition of
the intermetallic compound. Various techniques have
therefore been proposed in which the sliding property of an
alloyed hot-dip galvanized steel sheet is improved by
controlling the composition of such an intermetallic
compound of the hot-dip galvanized layer formed~prior to
alloying.
For example, Japanese Unexamined Patent Application
Publication No. 9-209106 discloses a steel sheet for use in
':
alloyed hot-dip galvanized and an alloyed hot-dip galvanized
steel sheet. This technique intends to form an alloyed hot-
dip galvanized layer with satisfactory sliding property by
controlling the composition of the base steel sheet.
However, the composition of the hot-dip galvanized layer
varies with changes in operating conditions of the plating
process step and affects the sliding property of the
resulting alloyed hot-dip galvanized layer. Accordingly,
the'technique disclosed in Japanese Unexamined Patent
Application Publication No. 9-209106 cannot significantly
yield satisfactory sliding property stably.
CA 02411879 2002-12-04
- 3 -
Japanese Unexamined Patent Application Publication No.
11-200009 discloses an alloyed hot-dip galvanized steel
sheet with excellent sliding property. This technique
intends to manufacture an alloyed hot-dip galvanized steel
sheet with high sliding property and resistance to plating
adhesion failure by depositing a crystal mainly containing
Ti and A1 on the surface of the alloyed hot-dip galvanized
layer. To deposit a crystal mainly containing Ti and Al, a
plating bath must comprise Ti. However, when the plating
bath comprises Ti, a Ti-A1 intermetallic compound (so-called
"dross") is formed and adheres to the hot-dip galvanized
layer to cause problems in the surface appearance of the
resulting galvanized steel sheet.
Disclosure of Tnvention
Accordingly, an object of the present invention is to
solve the above problems and to provide an alloyed hot-dip
galvanized steel sheet stably exhibiting excellent sliding
property.
The present inventors have made detailed investigations
on alloyed hot-dip galvanized layers with excellent sliding
property.
Specifically, the present inventors have made
investigations on electrolytic behavior of alloyed hot-dip
CA 02411879 2002-12-04
- 4 -
galvanized layers by electrolyzing them according to a
constant potential electrolysis using various alloyed hot-
dip galvanized steel sheets as an anode and an aqueous zinc
sulfate-sodium chloride solution as an electrolyte.
Furthermore, the present inventors have made investigations
on the relationship between the quantity of electricity
required by the constant potential electrolysis and the
sliding property. As a result, they have found that alloyed
hot-dip galvanized steel sheets exhibiting a potential of
less than or equal to a specific level, when they are
immersed in the electrolyte, have satisfactory sliding
property and that alloyed hot-dip galvanized steel sheets, ,
in which the total quantity of electricity consumed until ,,
the completion of electrolysis is less than or equal to a "°~
specific level, have satisfactory sliding property.
The present invention has been accomplished based on
these findings and further investigations.
Specifically, the invention provides an alloyed hot-dip
galvanized steel sheet with excellent processability and
particularly with excellent sliding property, exhibiting a
potential to a saturated calomel electrode of less than or
equal to -850 mV when it is immersed in a zinc sulfate-
sodium chloride electrolyte. The invention also provides an
alloyed hot-dip galvanized steel sheet with excellent
CA 02411879 2002-12-04
- 5 -
processability and particularly with excellent sliding
property in which, when the alloyed hot-dip galvanized steel
sheet is electrolyzed according to a constant potential
electrolysis process in a zinc sulfate-sodium chloride
electrolyte at a potential to a saturated calomel electrode
of from -940 mV to -920 mV, the quantity of electricity
consumed is less than or equal to 0.5 C/cm2.
When the quantity of the electricity is less than or
equal to 0.3 C/cm2, the resulting alloyed hot-dip galvanized
steel sheet exhibits preferable sliding property.
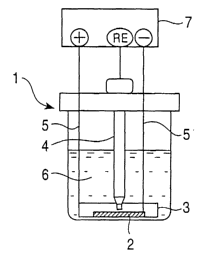
Brief Description of the Drawings
Figs. 1 and 2 are a vertical sectional view and a
':
perspective view, respectively, schematically illustrating
an example of constant-potential electrolysis devices.
Best Mode for Carrying Out the Invention
The alloyed hot-dip galvanized steel sheet of the
present invention satisfies the following requirement.
When the.alloyed hot-dip galvanized steel sheet is
electrolyzed according to the constant potential
electrolysis process in a zinc sulfate-sodium chloride
electrolyte at a potential to a saturated calomel electrode
in a range of from -940 mV to -920 mV, the quantity of
CA 02411879 2002-12-04
- 6 -
electricity consumed is less than or equal to 0.5 C/cmZ.
Alternatively, when the alloyed hot-dip galvanized steel
sheet is immersed in the electrolyte, it exhibits a
potential to a saturated calomel electrode of less than or
equal to -850 mV. Base steel sheets having some textures or
surface dimensions do not always satisfy the both
requirements, but the objects can be achieved only if either
of the two requirements is satisfied.
When the quantity of electricity consumed during
constant potential electrolysis is less than or~equal to 0.5
C/cm2, the alloyed hot-dip galvanized steel sheet exhibits
satisfactory properties in various tests for determining
sliding property. The end point of constant potential
electrolysis is set at the time when an electrolysis current
density decreases and reaches 5 N.A/cm2. An example of such
tests for determining sliding property is a cylindrical
flat-bottom cup drawing test. The constant potential
electrolysis is performed in a zinc sulfate-sodium chloride
electrolytic solution using an alloyed hot-dip galvanized
steel sheet as an anode at a potential to a saturated
calomel electrode in a range of from -940 mV to -920 mV. To
electrolyze portions of the alloyed hot-dip galvanized layer
which significantly affect the sliding property selectively,
the potential is set at -940 mV to -920 mV. The
CA 02411879 2002-12-04
electrolysis is performed in a zinc sulfate-sodium chloride
electrolyte, because this type of electrolyte hardly
dissolves the alloyed hot-dip galvanized layer chemically
and is hardly.affected by an oxide film formed on the
surface of the alloyed hot-dip galvanized layer. When the
type of the electrolyte is changed, the potential at which
portions of the alloyed hot-dip galvanized layer which
significantly affect the sliding property are selectively
electrolyzed changes, and the change of the potential must
be verified by a preliminary test.
In general, the lower is the potential of the alloyed
hot-dip galvanized steel sheet when it is immersed in the
electrolyte, the lower is the quantity of electricity ,
consumed during constant potential electrolysis. Figs. 1
and 2 illustrate an example of a constant potential
electrolytic apparatus 1. The electrolytic apparatus 1 uses
an alloyed hot-dip galvanized steel sheet (a test sample) 2
as an anode and a platinum ring or platinum sheet, for
example, as a counter electrode (a cathode) 3. Each of
these components is connected to a device 7 for setting the
potential via a platinum wire 5. The potential is
preferably set using a potentiostat with a reference
electrode (RE) 4 such as a saturated calomel electrode br a
silver-silver chloride electrode.
CA 02411879 2002-12-04
8 -
As an electrolyte 6, an aqueous zinc sulfate-sodium
chloride solution is used. This type of electrolytes hardly
dissolves the alloyed hot-dip galvanized layer chemically
and is hardly affected by an oxide film formed on the
surface of the alloyed hot-dip galvanized layer. The
concentrations of zinc sulfate and of sodium chloride are
preferably controlled to within ranges from 1 to 50 mass ~
and 1 to 30 mass ~, respectively. The alloyed,hot-dip
galvanized steel sheet of the present invention is not
specified by its manufacturing process, but can~be
manufactured, for example, by controlling alloying
conditions according to procedures disclosed in Japanese .
Unexamined Patent Application Publications No. 7-41925 and
':
No. 10-130802 and by further exactly controlling plating and
alloying conditions. Particularly, the alloying operation
should preferably be performed at temperatures higher than
those in ordinary cases, by controlling the A1 content in
the zinc-coated layer at a high level.
To form an alloyed hot-dip galvanized layer having
characteristics of the alloyed hot-dip galvanized steel
sheet of the present invention, the following conditions are
preferred: quantity of plating on a single side: 40 to 60
g/m2, Fe content in the zinc-coated layer: 9 to 13 mass o,
A1 content: 0.20 to 0.30 mass ~, Pb content: 0.002 to 0.2
CA 02411879 2002-12-04
_ g -
mass $, Mn content: 0.001 to 0.1 mass o, Si content:
0.0001 to 0.01 mass o, and P content: 0.0001 to 0.01 mass o.
Mn, Si and P are not necessarily incorporated concurrently.
The steel is not specifically limited in its grade, but
is preferably a ultra low carbon steel (e.g., C 0.0020-Si
0.01-Mn 0.10-P 0.01-A1 0.030-Ti 0.025-Nb 0.010 mass o). In
particular, by satisfying either or both requirements of B
content of 0.0002 to 0.015 mass o and Sb content of 0.002 to
0.015 mass ~, a steel sheet having a significantly highly
slidable galvanized layer can be manufactured.
<Examples>
A test piece of a ultra low carbon steel having the
composition shown in Table 1 was processed into an ingot in ,,
a converter, was then continuously cast and thereby yielded
a slab. The slab was subjected to hot-rolling process at a
slab heating temperature of 1150°C to 1250°C and a finished
temperature in hot-rolling process of 920°C, was rolled at
550°C and thereby yielded a hot-rolled sheet coil 3.2 mm
thick. The coil was subjected to acid pickling to remove
mill scale, was subjected to cold rolling and thereby
yielded a cold rolled steel sheet 0.8 mm thick.
TABLE 1
C Si Mn P A1 Ti Nb Sb B
(mass (mass (mass (mass (mass (mass (mass (mass (mass
~) ~) ~) $) $) ~) $) ~) $)
0.0020 0.01 0.10 0.01 0.030 0.025 0.010 0.007 0.0005
CA 02411879 2002-12-04
- 10 -
The cold rolled steel sheet was subjected to a
continuous hot-dip galvanized line at an annealing
temperature of 790°C to 830°C, a temperature of incoming
sheet into the plating bath of 460°C to 470°C, a bath
temperature of the plating bath of 460°C to 470°C, and an
alloying temperature of 490°C to 530°C and thereby yielded an
alloyed hot-dip galvanized steel sheet. The quantity of
plating on a single side was set at 40 to 50 g/mz, and the
quantity of plating a single on both sides were controlled
to be equal to each other.
The alloyed hot-dip galvanized steel sheet was stamped
into a disc shape 15 mm in diameter and was subjected to
constant potential electrolysis at a potential to a ,,
saturated calomel electrode of -930 mV using 20 mass o zinc \~~'~
sulfate-10 mass ~ sodium chloride aqueous solution as an
electrolyte. The electrolysis was performed until a current
density became 5 N.A/cm2 or below, and the quantity of
electricity consumed from the beginning of electrolysis was
determined. It took about 10 to 20 minutes for electrolysis.
The end point of constant potential electrolysis was set at
the time when an electrolysis current density decreased to 5
~A/cm2. However, sice the current is low in the vicinity of
the end point, even if a current density level somewhat
lower than the above specified current density would be
CA 02411879 2002-12-04
- 11 -
employed, since there is not influence upon determination of
quantity of electricity,, and accordingly accurate estimation
can be made.
Separately, using the steel sheet stamped into the disc
shape as a test piece, the immersing potential of the test
piece to a saturated calomel electrode in the aforementioned
electrolyte was determined.
To estimate sliding property for comparison, a
conventional rust preventive oil was applied to the alloyed
hot-dip galvanized steel sheet in an amount of ~1.5 g/m2, the
alloyed hot-dip galvanized steel sheet was then subjected to
a drawing test using a cylindrical flat-bottom cup 33 mm in.
diameter to determine a limiting drawing ratio. The lower ,,
the rating of the limiting drawing ratio is, the higher the
sliding property is. The limiting drawing ratio was rated
as follows: the limiting drawing ratio of equal to or more
than 2.0%: Rating 1, from 1.9% to 2.0%: Rating 2, from
1.8% to 1.9%: Rating 3, from 1.7% to 1.8%: Rating 4, and
less than or equal to 1.7%: Rating 5. The results are
shown in Table 2.
CA 02411879 2002-12-04
- 12 -
TABLE 2
Quantity Content PotentialQuantity of Sliding
in
of plating upon electricity property
layer
Plating Zn Fe immersingduring constantrating
on single(mass (mass (mV vs potential
~) $)
side SCE) electrolysis
( /m2) (C/cmZ)
Inventive90 90.9 9.6 -900 0.13 1
Example
1
Inventive97 88.3 11.6 -880 0.16 1
Example
2
Inventive40 88.5 11.5 -886 0.21 1
Examgle
3
Inventive95 89.6 10.4 -870 0.30 1
Example
4
Inventive93 90.1 9.9 -852 0.97 3
Example
Inventive45 90.8 9.2 -845 0.47 3
Example '
6
Inventive97 91.0 9.0 -859 0.52 3
Example
7
Comp. 42 90.0 10.0 -825 0.55 5
Ex.
The zinc-coated steel sheet according to Comparative
Example in which the quantity of electricity exceeds 0.5
C/cm2 exhibits deteriorated sliding property of "Rating 5".
In contrast, the galvanized steel sheets in which the
quantity of electricity is less than or equal to 0.5 C/cmz
exhibit satisfactory sliding property of "Rating 3" or below.
Particularly, all the galvanized steel sheets in which the
quantity of electricity is less than or equal to 0.3 C/cm2
exhibit significantly satisfactory sliding property of
"Rating 1".
CA 02411879 2002-12-04
- 13 -
In addition, all the steel sheets exhibiting an
immersing potential of less than or equal to -850 mV exhibit
satisfactory sliding property of "Rating 3" or below.
Industrial Applicability
The present invention can provide an alloyed hot-dip
galvanized steel sheet stably exhibiting excellent sliding
property.