Note: Descriptions are shown in the official language in which they were submitted.
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
1
CABLE WITH RECYCLABLE COVERING
The present invention relates to a cable with recyclable covering. In
particular, the invention relates to a cable for transporting or distributing
medium or high voltage electric energy, wherein an extruded covering layer
based on a thermoplastic polymer material in admixture with a dielectric
liquid
with superior mechanical and electrical properties is present, enabling, in
particular, the use of high operating temperatures and the transportation of
high power energy.
The requirement for products of high environmental compatibility,
composed of materials which, in addition to not being harmful to the
environment during production or utilization, can be easily recycled at the
end
of their life, is now fully accepted in the field of electrical and
telecommunications cables.
However the use of materials compatible with the environment is
conditioned by the need to limit costs while, for the more common uses,
providing a performance equal to or better than that of conventional
materials.
In the case of cables for transporting medium and high voltage energy,
the various coverings surrounding the conductor commonly consist of
polyolefin-based crosslinked polymer, in particular crosslinked polyethylene
(XLPE), or elastomeric ethylene/propylene (EPR) or ethylene/propylene/diene
(EPDM) copolymers, also crosslinked. The crosslinking, effected after the step
of extrusion of the polymeric material onto the conductor, gives the
iri.aterial
satisfactory performance even under hot conditions during continuous use and
with current overload.
It is well known however that crosslinked materials cannot be recycled,
so that manufacturing wastes and the covering material of cables which have
reached the end of their life can be disposed of only by incineration.
Electric cables are also known having their insulation consisting of a
multi-layer wrapping of a paper or paper/polypropylene laminate impregnated
with a large quantity of a dielectric liquid (commonly known as mass
impregnated cables or also oil-filled cables). By completely filling the
spaces
present in the multi-layer wrapping, the dielectric liquid prevents partial
discharges arising with consequent perforation of the electrical insulation.
As
dielectric liquids products are commonly used such as mineral oils,
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
2
polybutenes, alkylbenzenes and the like (see for example US-4,543,207, US-
4,621,302, EP-A-0987718, WO 98/32137).
It is however well known that mass impregnated cables have numerous
drawbacks compared with extruded insulation cables, so that their use is
currently restricted to specific fields of application, in particular to the
construction of high and very high voltage direct current transmission lines,
both for terrestrial and in particular for underwater installations. In this
respect, the production of mass impregnated cables is particularly complex and
costly, both for the high cost of the laminates and for the difficulties
encountered during the steps of wrapping the laminate and then of
impregnating it with the dielectric liquid. In particular, the dielectric
liquid
used must have low viscosity under cold conditions to allow rapid and uniform
impregnation, while at the same time it must have a low tendency to migrate
during installation and operation of the cable to prevent liquid loss from the
cable ends or following breakage. In addition, mass impregnated cables cannot
be recycled and their use is limited to an operating temperature of less than
90 C.
Within non-crosslinked polymeric materials, it is known to use high
density polyethylene (HDPE) for covering high voltage cables. HDPE has
however the drawback of a lower temperature resistance than XLPE, both to
current overload and during operation.
Thermoplastic low density polyethylene (LDPE) insulating coverings are
also used in medium and high voltage cables: again in this case; these
coverings are limited by too low an operating temperature (about 70 C).
WO 99/13477 describes an insulating material consisting of a
thermoplastic polymer forming a continuous phase which incorporates a liquid
or easily meltable dielectric forming a mobile interpenetrating phase within
the
solid polymer structure. The weight ratio of thermoplastic polymer to
dielectric
is between 95:5 and 25:75. The insulating material can be produced by mixing
the two components while hot either batchwise or continuously (for example by
means of an extruder). The resultant mixture is then granulated and used as
insulating material for producing a high voltage electric cable by extrusion
onto
a conductor. The material can be used either in thermoplastic or crosslinked
form. As thermoplastic polymers are indicated: polyolefins, polyacetates,
cellulose polymers, polyesters, polyketones, polyacrylates, polyamides and
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
3
polyamines. The use of polymers of low crystallinity is particularly
suggested.
The dielectric is preferably a synthetic or mineral oil of low or high
viscosity, in
particular a polyisobutene, naphthene, polyaromatic, a-olefin or silicone oil.
The Applicant considers as still unsolved the technical problem of
producing an electric cable with a covering made from a thermoplastic polymer
material having mechanical and electrical properties comparable to those of
cables with an insulating covering of crosslinked material. In particular, the
Applicant has considered the problem of producing a cable with a non-
crosslinked insulating covering having good flexibilty and high mechanical
strength under both hot and cold conditions, while at the same time
possessing high dielectric strength, without using products potentially
polluting during the life cycle of the cable, i.e. from its production to its
disposal.
In view of said problem, the Applicant considers that the addition of
dielectric liquids to polymer materials as proposed in the cited WO 99/13477
gives totally unsatisfactory results. In this respect, the Applicant maintains
that adding a dielectric liquid to an insulating material should both
determine
a significant increase in its electrical properties (in particular its
dielectric
strength), without changing the material characteristics (thermomechanical
properties, manageability) and without resulting in exudation of the
dielectric
liquid. In particular, the resultant cable should give substantially constant
performance with time and hence high reliability, even at high operating
temperatures (at least 90 C and beyond).
The Applicant has now found it possible to solve said technical problem
by using, as recyclable polymer base material, a thermoplastic propylene
homopolymer or copolymer mixed with a dielectric liquid as hereinafter
defined. The resultant composition possesses good flexibility even when cold,
excellent thermomechanical strength and high electrical performance, such as
to make it particularly suitable for forming at least one covering layer, and
in
particular an electrical insulating layer, of a medium or high voltage cable
of
high operating temperature, of at least 90 C and beyond. The dielectric liquid
suitable for implementing the invention has high compatibility with the base
polymer and high efficiency in the sense of improving electrical performance,
consequently allowing the use of small quantities of additive such as not to
impair the thermomechanical characteristics of the insulating layer.
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
4
High compatibility between the dielectric liquid and the base polymer
ensures homogeneous dispersion of the liquid in the polymer matrix and
improves cold behaviour of the polymer. Moreover, as the dielectric liquid
suitable for forming the cable of the invention is free of polar groups, it
absorbs
water in extremely small quantities, hence preventing formation of insulation
defects due to the presence of steam which normally forms during the process
of high temperature extrusion.
According to a first aspect, the invention therefore relates to a cable (1)
comprising at least one electrical conductor (2) and at least one extruded
covering layer (3, 4, 5) based on a thermoplastic polymer material in
admixture
with a dielectric liquid, wherein:
- said thermoplastic material comprises a propylene homopolymer or a
copolymer of propylene with at least an olefin comonomer selected from
ethylene and an a-olefin other than propylene, said homopolymer or
copolymer having a melting point greater than or equal to 140 C and a
melting_enthalpy of from 30 to 100 J/g;
- said liquid comprises at least one alkylaryl hydrocarbon having at least
two non-condensed aromatic rings and a ratio of number of aryl carbon
atoms to total number of carbon atoms greater than or equal to 0.6, and
preferably greater than or equal to 0.7.
According to a first embodiment, said extruded covering layer based on
said thermoplastic polymer material in admixture with said dielectric liquid
is
an electrically insulating layer.
According to a further embodiment, said extruded covering layer based
on said thermoplastic polymer material in admixture with said dielectic liquid
is a semiconductive layer.
Preferably, the propylene homopolymer or copolymer has a melting point
of from 145 to 170 C.
Preferably, the propylene homopolymer or copolymer has a melting
enthalpy of from 30 to 85 J/g.
Preferably, the propylene homopolymer or copolymer has a. flexural
modulus, measured in accordance with ASTM D790, at room temperature, of
from30 to 1400 MPa, and more preferably from 60 to 1000 MPa.
Preferably, the propylene homopolymer or copolymer has a melt flow
index (MFI), measured at 230 C with a load of 21.6 N in accordance with ASTM
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
D1238/L, of from 0.05 to 10.0 dg/min, more preferably from 0.5 to 5.0 dg/min.
If a copolymer of propylene with an olefin comonomer is used, this latter
is preferably present in a quantity of less than or equal to 15 mo1%, and more
preferably of less than or equal to 10 mol%. The olefin comonomer is, in
5 particular, ethylene or an (x-olefin of formula CH2=CH-R, where R is a
linear or
branched C2-C10 alkyl, selected for example from: 1-butene, 1-pentene, 4-
methyl-l-pentene, 1-hexene, 1-octene, 1-decene, 1-dodecene and the like, or
combinations thereof. Propylene/ethylene copolymers are particularly
preferred.
Preferably, said thermoplastic material is selected from:
a) a propylene homopolymer or a copolymer of propylene with at least one
olefin comonomer selected from ethylene and an a-olefin other than
propylene, having a flexural modulus generally of from 30 to 900 MPa,
and preferably of from 50 to 400 MPa;
b) a heterophase copolymer comprising a thermoplastic phase based on
propylene and an elastomeric phase based on ethylene copolymerized
with an (x-olefin, preferably with propylene, in which the elastomeric
phase is present in a quantity of at least 45 wt% on the total weight of
the heterophase copolymer.
The homopolymers or copolymers of class a) show a single-phase
microscopic structure, i.e. substantially devoid of heterogeneous phases
dispersed as molecular domains of size greater than one micron. These
materials do not show in fact the optical phenomena typical of heterophase
polymer materials, and in particular are characterised by better transparency
and reduced whitening due to local mechanical stresses (commonly known as
"stress whitening").
Particularly preferred of said class a) is a propylene homopolymer or a
copolymer of propylene with at least one olefin comonomer selected from
ethylene and an a-olefin other than propylene, said homopolymer or copolymer
having:
- a melting point of from 140 to 165 C;
- a melting enthalpy of from 30 to 80 J/g;
- a fraction soluble in boiling diethyl ether in an amount of less than or
equal to 12 wt%, preferably from 1 to 10 wt%, having a melting enthalpy
of less than or equal to 4 J/g, preferably less than or equal to 2 J/g;
CA 02412891 2008-08-27
6
- a fraction soluble in boiling n-heptane in an amount of from 15 to 60
wt%, preferably from 20 to 50 wt%, having a melting enthalpy of from 10
to 40 J/g, preferably from 15 to 30 J/g; and
- a fraction insoluble in boiling n-heptane in an amount of from 40 to 85
wt%, preferably from 50 to 80 wt%, having a melting enthalpy of greater
than or equal to 45 J/g, preferably from 50 to 95 J/g.
Further details of these materials and their use in covering cables are
given in WO 01 /37289.
The heterophase copolymers of class b) are thermoplastic elastomers
obtained by sequential copoly:nerization of: i) propylene, possibly containing
minor quantities of at least one olefin comonomer selected from ethylene and
an a-olefin other than propylene; and then of: ii) a mixture of ethylene
~&rith an
a-olefin, in particular propylene, and possibly with minor portions of a
diene.
This class of product is also commonly known by the term "thermoplastic
reactor elastomers".
Particularly preferred of the said class b) is a heterophase copolymer in
which the elastomeric phase consists of an elastomeric copolymer of ethylene
and propylene comprising from 15 to 50 wt% of ethylene and from 50 to 85
wt% of propylene on -the weight of the elastomeric phase. Further details of
these materials and their use in covering cables are given in Canadian patent
application 2356851 filed on 21,12,1999 in the name of the Applicant.
-~ -
Products of class a) are available commercially for example under the
trademark RexflexR of the Huntsman Polymer Corporation.
Products of class b) are available commercially for example under the
trademark HifaxR of Montell.
Alternatively, as thermoplastic base material, a propylene homopolymer
or copolymer as hereinabove defined can be used in mechanical mixture with a
low crystallinity polymer, generally with a melting enthalpy of less than 30
J/g,
which mainly acts to increase flexibility of the material. The quantity of low
crystallinity polymer is generally less than 70 wt%, and preferably of from 20
to
60 wt%, on the total weight of the thermoplastic material.
Preferably, the low crystallinity polymer is a copolymer of ethylene with
a C3-C12 a-olefin, and possibly with a diene. The a-olefui is preferably
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
7
selected from propylene, 1-hexene and 1-octene. If a diene comonomer is
present, this is generally C4-C20, and is preferably selected from: conjugated
or non-conjugated linear diolefins, such as 1,3-butadiene, 1,4-hexadiene, 1,6-
octadiene or their mixtures and the like; monocyclic or polycyclic dienes,
such
as 1,4-cyclohexadiene, 5-ethylidene-2-norbornene, 5-methylene-2-norbornene,
5-vinyl-2-norbornene or their mixtures and the like.
Particularly preferred ethylene copolymers are:
(i) copolymers having the following monomer composition: 35-90 mol%
of ethylene; 10-65 mol% of an a-olefin, preferably propylene; 0-10 mol% of a
diene, preferably 1,4-hexadiene or 5-ethylene-2-norbornene (EPR and EPDM
rubbers are within this class);
(ii) copolymers having the following monomer composition: 75-97 mol%,
preferably 90-95 mol%, of ethylene; 3-25 mol%, preferably 5-10 mol%, of an a-
olefin; 0-5 mol%, preferably 0-2 mol%, of a diene (for example ethylene/ 1-
octene copolymers, such as the products EngageR of Dow-DuPont Elastomers).
The alkylaryl hydrocarbon of the invention preferably has a dielectric
constant, at 25 C, of less than or equal to 3.5 and preferably less than 3
(measured in accordance with IEC 247).
According to a further preferred aspect, the alkylaryl hydrocarbon of the
invention has a predetermined viscosity such as to prevent fast diffusion of
the
liquid within the insulating layer and hence its outward migration, while at
the
same time such as to enable it to be easily fed and mixed into the polymer.
Generally, the dielectric liquid of the invention has a kinematic viscosity,
at
20 C, of between 1 and 500 mm2/s, preferably between 5 and 100 mm2/s
(measured in accordance with ISO 3104).
According to a further preferred aspect, the alkylaryl hydrocarbon of the
invention has a hydrogen absorption capacity greater than or equal to 5
mm3/min, preferably greater than or equal to 50 mm3/min (measured in
accordance with IEC 628-A).
According to a preferred aspect, an epoxy resin can be added to the
dielectric liquid suitable for forming the cable of the invention, generally
in a
quantity of less than or equal to 1 wt% on the weight of the liquid, this
being
considered to mainly act to reduce the ion migration rate under an electrical
field, and hence the dielectric loss of the insulating material.
In a preferred embodiment, the dielectric liquid of the invention
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
8
comprises at least one alkylaryl hydrocarbon having at least three non-
condensed aromatic rings.
Even more preferably, the dielectric liquid of the invention comprises at
least one alkylaryl hydrocarbon having at least three non-condensed aromatic
rings in a quantity of not less than 10 wt% on the total weight of the
dielectric
liquid.
Preferably, the dielectric liquid of the invention comprises at least one
alkylaryl hydrocarbon having the structural formula:
Rl CH3 R3 R4
R2
0 CH2 CHz ~ CHZ O
nl n2
wherein:
Rl, R2, R3 and R4, equal or different, are hydrogen or methyl;
n 1 and n2, equal or different, are zero, 1 or 2, with the proviso that the
sum
nl+n2 is less than or equal to 3.
The dielectric liquid can also contain minor quantities of at least one
triphenylmethane, either unsubstituted or substituted by at least one radical
selected from methyl, benzyl and methylbenzyl. Examples of
triphenylmethanes are: ditoluylphenylmethane, dixylylphenylmethane,
xylyltoluylphenylmethane and the like, or their mixtures.
More preferably, the dielectric liquid of the invention comprises at least
one alkylaryl hydrocarbon of the aforegiven formula (I) in which the sum n
1+n2
is other than zero.
Alkylaryl hydrocarbons corresponding to formula (I) in which the sum
nl+n2 is equal to zero, and usable advantageously in this invention, are for
example: benzyltoluene, benzylxylene, (methylbenzyl) toluene,
(methylbenzyl)xylene and the like, or their mixtures.
Alkylaryl hydrocarbons corresponding to formula (I) in which the sum
nl+n2 is other than zero, and usable advantageously in this invention, are for
example: dibenzyltoluene, dibenzylxylene, di(methylbenzyl) toluene,
di(methylbenzyl)xylene and the like, or their mixtures.
The alkylaryl hydrocarbons of formula (I) are generally prepared by
reacting benzylchloride, methylbenzylchloride or their mixtures, with an
aromatic hydrocarbon selected from benzene, toluene, xylene or their mixtures,
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
9
in the presence of a Friedel-Crafts catalyst (for example FeC13, SbC13, TiC14
or
A1C13). Further details regarding the preparation of alkylaryl hydrocarbons of
formula (I) are given for example in US-5,192,463, US-5,446,228, US-
5,545,355 and US-5,601,755.
The dielectric liquid suitable for implementing the invention has good
heat resistance, considerable gas absorption capacity, in particular for
hydrogen, and hence high resistance to partial discharges, so that dielectric
loss is not high even at high temperature and high electrical gradient. The
weight ratio of dielectric liquid to base polymer material of the invention is
generally between 1:99 and 25:75, preferably between 2:98 and 20:80, and
more preferably between 3:97 and 15:85.
According to a preferred aspect, the cable of the invention has at least
one extruded covering layer with electrical insulation properties formed from
the thermoplastic polymer material in admixture with the aforedescribed
dielectric liquid.
According to a further preferred embodiment, the cable of the invention
has at least one extruded covering layer with semiconductive properties formed
from the thermoplastic polymer material in admixture with the aforedescribed
dielectric liquid. To form a semiconductive layer, a conductive filler is
generally
added to the polymer material. To ensure good dispersion of the conductive
filler within the base polymer material, this latter is preferably selected
from
propylene homopolymers or copolymers comprising at least 40 wt% of
amorphous phase, on the total polymer weight.
In a preferred embodiment, the cable of the invention has at least one
electrical insulation layer and at least one semiconductive layer formed from
a
thermoplastic polymer material in admixture with a dielectric liquid as
hereinabove described. This prevents the semiconductive layers from
absorbing, with time, part of the dielectric liquid present in the insulating
layer, so reducing its quantity just at the interface between the insulating
layer
and semiconductive layer, in particular the inner semiconductive layer where
the electrical field is higher.
According to a further aspect, the invention relates to a polymer
composition comprising a thermoplastic polymer material in admixture with a
dielectric liquid, in which:
- said thermoplastic material comprises a propylene homopolymer or a
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
copolymer of propylene with at least one olefin comonomer selected from
ethylene and an a-olefin other than propylene, said homopolymer or
copolymer having a melting point of greater than or equal to 140 C and
a melting enthalpy of from 30 to 100 J/g;
5 - said liquid comprises at least one alkylaryl hydrocarbon with at least
two non-condensed aromatic rings and a ratio of number of aryl carbon
atoms to total number of carbon atoms greater than or equal to 0.6,
preferably greater than or equal to 0.7.
According to a further aspect, the invention relates to the use of a
10 polymer composition, as described hereinabove, as the base polymer material
for preparing a covering layer (4) with electrical insulation properties, or
for
preparing a covering layer (3, 5) with semiconductive properties.
In forming a covering layer for the cable of the invention, other
conventional components can be added to the aforedefined polymer
composition, such as antioxidants, processing aids, water tree retardants, and
the like.
Conventional antioxidants suitable for the purpose are for example
distearyl-thiopropionate and pentaerithryl-tetrakis [3-(3,5-di-tertbutyl-4-
hydroxyphenyl)propionate} and the like, or their mixtures.
Processing aids which can be added to the polymer base include, for
example, calcium stearate, zinc stearate, stearic acid, paraffin wax and the
like,
or mixtures thereof.
With particular reference to medium and high voltage cables, the
polymer materials as defined hereinabove can be advantageously used to form
an insulating layer. As stated above, these polymer materials show indeed good
mechanical characteristics both at ambient temperature and under hot
conditions, and also show improved electrical properties. In particular they
enable high operating temperature to be employed, comparable with or even
exceeding that of cables with coverings consisting of crosslinked polymer base
materials.
If a semiconductive layer is to be formed, a conductive filler, in
particular carbon black, is generally dispersed within the polymer material in
a
quantity such as to provide the material with semiconductive characteristics
(i.e. such as to obtain a resistivity of less than 5 Ohm.m at ambient
temperature). This quantity is generally between 5 and 80 wt%, and preferably
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
11
between 10 and 50 wt%, of the total weight of the mixture.
The possibility to use the same type of polymer composition for both the
insulating layer and the semiconductive layers is particularly advantageous in
producing cables for medium or high voltage, in that it ensures excellent
adhesion between adjacent layers and hence better electrical behaviour,
particularly at the interface between the insulating layer and the inner
semiconductive layer, where the electrical field and hence the risk of partial
discharges are higher.
The compositions of the invention can be prepared by mixing together
the base polymer material, the dielectric liquid and any other additives
possibly
present by methods known in the art. Mixing can be carried out for example by
an internal mixer of the type with tangential rotors (Banbury) or with
interpenetrating rotors, or, preferably, in a continuous mixer of Ko-Kneader
(Buss) type, or of co- or counter-rotating double-screw type.
Alternatively, the dielectric liquid of the invention can be added to the
polymer material during the extrusion step by direct injection into the
extruder
cylinder.
According to the present invention, the use of the aforedefined polymer
composition in covering cables for medium or high voltage enables recyclable,
flexible coverings to be obtained with excellent mechanical and electrical
properties.
Greater compatibility has also been found between the dielectric liquid
and thermoplastic base polymer of the invention than in the case of similar
mixtures of the same polymer material with other dielectric liquids known in
the art. This greater compatibility leads, inter alia, to less exudation of
the
dielectric liquid and hence a reduction of the already discussed migration
phenomena. Because of their high operating temperature and their low
dielectric loss, the cables of the invention can carry, for the same voltage,
a
power at least equal to or even greater than that transportable by a
traditional
cable with XLPE covering.
For the purposes of the invention the term "medium voltage" generally
means a voltage of between 1 and 35 kV, whereas "high voltage" means
voltages higher than 35 W.
Although this description is mainly focused on the production of cables
for transporting or distributing medium or high voltage energy, the polymer
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
12
composition of the invention can be used for covering electrical devices in
general and in particular cables of different type, for example low voltage
cables, telecommunications cables or combined energy/telecommunications
cables, or accessories used in constructing electrical lines, such as
terminals
or connectors.
Further characteristics will be apparent from the detailed description
given hereinafter with reference to the accompanying drawing, in which:
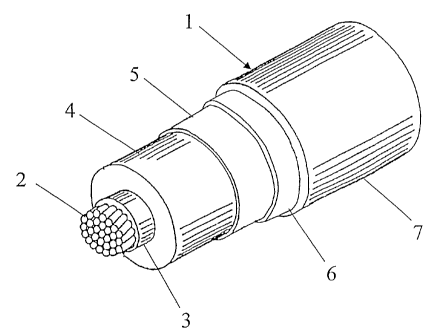
- Figure 1 is a perspective view of an electric cable, particularly suitable
for
medium or high voltage, according to the invention.
In Figure 1, the cable 1 comprises a conductor 2, an inner layer with
semiconductive properties 3, an intermediate layer with insulating properties
4, an outer layer with semiconductive properties 5, a metal screen 6, and an
outer sheath 7.
The conductor 2 generally consists of metal wires, preferably of copper
or aluminium, stranded together by conventional methods. At least one
covering layer selected from the insulating layer 4 and the semiconductive
layers 3 and 5 comprises the composition of the invention as heretofore
defined. Around the outer semiconductive layer 5 there is usually positioned a
screen 6, generally of electrically conducting wires or strips wound
helically.
This screen is then covered by a sheath 7 of a thermoplastic material, for
example non-crosslinked polyethylene (PE) or preferably a propylene
homopolymer or copolymer as heretofore defined.
The cable can also be provided with an outer protective structure (not
shown in Figure 1) the main purpose of which is to mechanically protect the
cable against impact or compression. This protective structure can be, for
example, a metal reinforcement or a layer of expanded polymer as described in
WO 93 j 52197.
Figure 1 shows only one possible embodiment of a cable according to
the invention. Suitable modifications known in the art can evidently be made
to this embodiment, but without departing from the scope of the invention.
The cable of the invention can be constructed in accordance with known
methods for depositing layers of thermoplastic material, for example by
extrusion. The extrusion is advantageously carried out in a single pass, for
example by the tandem method in which individual extruders are arranged in
series, or by co-extrusion with a multiple extrusion head.
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
13
The following examples illustrate the invention, but without limiting it.
EXAMPLES
Table 1 shows the characteristics of the dielectric liquids used in the
following
examples.
TABLE 1
-------------------------------------------------------------------------------
-------
Dielectric Dielectric Total carbon Ratio
liquid constant (*) atoms C(aryl)/C(total)
-------------------------------------------------------------------------------
-------
JarylecR 2.8 MXX = 16 0.75
Exp 4 DXX = 24
-------------------------------------------------------------------------------
--------
JarylecR 2.7 21 0.86
Exp 3
-------------------------------------------------------------------------------
--------
BaysiloneR 2.6 - -
-------------------------------------------------------------------------------
--------
(*) at 25 C in accordance with IEC 247
The dielectric liquids according to the invention were:
JarylecRExp4 (commercial product of Elf Atochem):
a mixture containing 85 wt% of monoxylylxylene (MXX)
CH3
H3C
CHZ
H3C
and 15 wt% of dixylylxylene (DXX)
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
14
CH3
H 3 C
CH2
CHz
H3C
CH3
JarylecRExp3 (commercial product of Elf Atochem):
dibenzyltoluene (DBT)
H3C
CHz
CHZ
The comparison dielectric liquids were:
BaysiloneRPDS (commercial product of General Electric - Bayer):
polyphenylmethylsiloxane (PPMS), polyaromatic dielectric oil as
described in IEEE Transactions on Electrical Insulation Vol. 26, No.4,
1991, having a viscosity of 4 mm2 / sec at 25 C;
FlexonR641 (commercial product of Esso) :
naphthene-based aromatic oil having a viscosity of 22 mm2/sec at 40 C,
consisting of 40 wt% aromatic hydrocarbons, 57 wt% saturated
hydrocarbons and 3 wt% polar compounds.
The following polymer materials were used:
- a flexible propylene homopolymer with melting point 160 C, melting
enthalpy 56.7 J/g, MFI 1.8 dg/min and flexural modulus 290 MPa
(RexflexRWL105 - commercial product of Huntsman Polymer Corp.)
(Examples 1-6)
- a propylene heterophase copolymer with an ethylene/propylene
elastomeric phase content of about 65 wt% (propylene 72 wt% in the
elastomeric phase), melting enthalpy 32 J/g, melting point 163 C, MFI
0.8 dg/min and flexural modulus of about 70 MPa (Hifa.xRKSO81 -
commercial product of Montell).
Composition preparation
CA 02412891 2006-04-27
The polymer in granular form was preheated to 80 C in a turbomixer.
The dielectric liquid was added, in the quantities specified for the
formulations
given in Table 2, to the polymer preheated in the turbomixer under agitation
at
80 C over 15 min. After the addition agitation was continued for a further
5 hour at 80 C until the liquid was completely absorbed in the polymer
granules.
After this first stage, the resultant material was kneaded in a laboratory
double-screw Brabender PlasticorderTM PL2000 at a temperature of 185 C to
complete homogenization. The material left the double-screw mixer in the form
of granules.
10 Measurement of dielectric strength (DS)
The dielectric strength of the polymer compositions obtained was
evaluated on test-pieces of insulating material having the geometry proposed
by the EFI (Norwegian Electric Power Research Institute) in the pubiication
"The EFI Test Method for Accelerated Growth of Water Trees" (IEEE
15 International Symposium on Electrical insulation, Toronto, Canada, June 3-6
1990). In this method, the cable is simulated with glass-shaped test pieces of
insulating material having their base coated on both sides with a
semiconductive material coating.
The glass-shaped test-pieces were formed by moulding discs of
insulating material at 160-170 C from a plate of thickness 10 mm obtained by
compressing granules at about 190 C.
The inner and outer surfaces of the base, which had a thickness of
about 0.40-0.45 mm, were coated with a semiconductive coating. The DS
measurement was made by applying to these specimens, immersed in silicone
oil at 20 C, an aiternating current at 50 Hz starting with a voltage of 25 kV
and
increasing in steps of 5 kV every 30 minutes until perforation of the test-
piece
occurred. Each measurement was repeated on 10 test-pieces. The values
given in Table 2 are the arithmetic meanof the individual measured values.
TABLE 2
----------------------------------------- -------------------------------------
-------- -------
Ex. Polymer Dielectric % dielectric DS
liquid liquid by weight (mean)
-------------------------------------------------------------------------------
--------------
92
1* RexflexR -- --
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
16
WL 105
-------------------------------------------------------------------------------
--------------
2* RexflexR BaysiloneR 5 90
WL 105 PD5
-------------------------------------------------------------------------------
--------------
3* RexflexR FlexonR641 5 94
WL 105
-------------------------------------------------------------------------------
-------------
4 RexflexR JarylecR 6 128
WL 105 Exp4
-------------------------------------------------------------------------------
-------------
5 RexflexR JarylecR 15 150
WL 105 EXP4
-------------------------------------------------------------------------------
-------------
6 RexflexR JarylecR 4 143
WL 105 Exp3
-------------------------------------------------------------------------------
--------------
7* HifaxR -- -- 90
KS081
-------------------------------------------------------------------------------
--------------
8 HifaxR JarylecR 15 140
KS081 Exp4
-------------------------------------------------------------------------------
----------=--
* comparison
The dielectric strength values given in Table 2 highlight the
improvement in electrical performance deriving from the dielectric liquids of
the invention, compared to that of the base polymer as such or when mixed
with the comparison dielectric liquids.
Migration tests
Using the polymer/dielectric liquid compositions prepared in Examples
5 and 6 moulded into 5 mm plates at 190 C, the loss of dielectric liquid
(expressed as percentage by weight on the initial quantity) was measured
against time at 20 C in air in order to verify the diffusivity of the
dielectric
CA 02412891 2002-12-20
WO 02/03398 PCT/EP01/06820
17
liquids in the pQlymer and hence their stability with time in these
compositions.
TABLE 3
-------------------------------------------------------------------------------
Days Composition Composition
Example 6 Example 3
-------------------------------------------------------------------------------
0 100.00 100.00
1 100.00 99.84
4 99.97 99.32
5 99.97 99.14
6 99.97 99.14
8 99.75 98.6
12 99.45 97.91
18 99.34 96.69
28 99.24 94.92
39 99.14 93.54
------------------------------------------------------------------------------
The data of Figure 3 show the high compatibility of the dielectric liquids
with the described base polymer material and consequently the low tendency of
these liquids to migrate to the outside of the polymer material.