Note: Descriptions are shown in the official language in which they were submitted.
CA 02412892 2003-O1-03
1
DENSIFYING AGENTS FOR ENHANCING FIBER DENSIFICATION
Field of the Invention
. This invention concerns organic and inorganic polymeric and non
polymeric densifying agents for fibers and the use of such agents in enhancing
the
densification of fibers. The fibers treated with such agents may be easily
densified by
external application of pressure. The binders may be applied to fibers on a
wet-Laid
fiber sheet manufacturing line, and subsequently fiberized for processing
using air lay
equipment. In particular embodiments, the invention concerns cellulosic fibers
which
may then be used, for example, to make absorbent fibers that are densified and
incorporated into absorbent products.
20
30
CA 02412892 2003-O1-03
2
10
BACKGROUND OF THE INVENTION
Superabsorbent polymers have been developed in recent years that are
capable of absorbing many times their own weight of liquid. These polymers,
which
are also known as water insoluble hydrogels, have been used to increase the
absorbency of sanitary products such as diapers and sanitary napkins:
Superabsorbent
polymers are often provided in the form of particulate powders, granules, or
fibers that
are distributed throughout absorbent cellulosic products to increase the
absorbency of
the product. Superabsorbent particles are described, for example, in U.S.
Patent No.
4,160,059; U.S. Patent No. 4,676;784; U.S. Patent No. 4,673,402; U.S. Patent
No.
5,002,814; and U.S. Patent No. 5,057,166. Products such as diapers that
incorporate
absorbent hydrogels are shown in U.S. Patent No. 3,669,103 and U.S Patent No.
3,670,731.
One problem with the use of superabsorbents is that the superabsorbent
material can be physically dislodged from the cellulosic fibers of an
absorbent product.
Separation of the superabsorbent from its substrate reduces the absorbency of
the
product and diminishes the effectiveness of the superabsorbent material. This
problem
was addressed in European Patent Application 442 18S Al, which discloses use
of a
polyaluminum chloride binder to bind an absorbent polymer to a fibrous
substrate. The
polyaluminum binder, however, suffers from the drawback of being an inorganic
product that is not readily biodegradable. Moreover, that European patent does
not
CA 02412892 2003-O1-03
3
offer any guidance far selecting binders other than polyaluminum chloride that
would
be useful in binding absorbent particles.
A method of immobilizing superabsorbents is disclosed in U.S. Patent
No. 4,410;571 in which a water swellable absorbent polymer is converted to a
non-
particulate immobilized confluent layer. Polymer particles are converted to a
coated
film by plasticizing them in a polyhydroxy organic compound such as glycerol,
ethylene/glycol; or propylene glycol. The superabsorbent assumes a non-
particulate
invnobilized form that can be foamed onto a substrate. The individual
particulate
identity of the superabsorbent polymer is lost in this process. The confluent
nature of
the superabsorbent material can also result in gel blocking, in which
absorption is
diminished as the water swollen polymers block liquid passage through the film
layer.
U.S. Patent No. 4,412;036 and U.S. Patent No. 4,467,012 disclose
absorbent laminates in which a hydrolyzed starch polyacrylonitrile graft
copolymer and
glycerol mixture is laminated between two tissue layers. The tissue layers are
laminated to each other by applying external heat and pressure. The reaction
conditions form covalent bonds between the tissue layers that firmly adhere
the tissue
layers to one another.
Numerous other patents have described methods of applying binders to
fibrous webs. Examples include U.S. Patent No. 2,757;150; U.S. Patent No.
4,584,357; and U.S. Patent No. 4,600,462. Such binders are not described as
being
useful in binding particulates, such as superabsorbent particles, to fibers.
Yet other
patents disclose crosslinking agents such as polycarboxylic acids that form
covalent
intrafiber bonds with individualized cellulose fibers, as in European Patent
Application
440 472 Al; European Patent Application 427 317 A2; European Patent
Application
427 316 A2; and European Patent Application 429 112 A2. The covalent
intrafiber
bonds are formed at elevated temperatures and increase the bulk of cellulose
fibers
treated with the crosslinker by forming intrafiber ester crosslir. ~;s.
Crosslinking must
occur under acidic conditions to prevent reversion of the ester bonds. The
covalent
bonds within the fibers produce a pulp sheet that is more difl~cult to
compress to
conventional pulp sheet densities than in an untreated sheet. Covalent
crosslink bonds
CA 02412892 2003-O1-03
4
may also form between the fibers and particles, and occupy functional groups
that
would otherwise be available for absorption, hence absorption efficiency is
decreased.
A particular disadvantage of forming covalent ester intrafiber crosslinks
is that the resulting fiber product resists densification. Energy requirements
for making
densified absorbent products are increased because very high compression
pressures
must be used to densify the absorbent product. It would be advantageous to
provide a
method of enhancing densification of crosslinked fibers by reducing energy
requirements for densification.
Many different types of particles other than superabsorbents may be
added to fibers for different end uses. Antimicrobials, zeolites and fire
retardants are
but a few examples of particles that are added to fibers. It would be
advantageous to
provide a method of attaching particles that could be accommodated to the many
different particle needs of end users. Moreover, it would be advantageous to
reduce
particulate waste in the attachment process, and simplify shipment of fiber
products
that require particulate addition. It would be further advantageous to bind
particulates
to fibers without requiring the shipment of bulk fibers with adhered
particulates
because shipping and excessive handling of these fibers subject them to
mechanical
impact which can dislodge some particles from the fibers. It would also be
advantageous to incorporate binders onto fibers during the initial pulp sheet
manufacturing process so that the fibers are ready for activation and use at a
remote
product manufacturing location.
It has previously been important that particles added to cellulose
products be insoluble in liquids such as water or liquid binders. It has been
thought
that liquid insolubility (particularly water insolubility) was an essential
characteristic for
particles bound to cellulose fibers because soluble particles would be
dissolved by a
water containing binder. Although the particle could eventually resolidify as
the binder
evaporated, dissolution of the particle in the binder would cause the particle
to diffuse
to areas of the product where it was not needed or desired. Water soluble
panicles
have therefore not been used for particles that were to be bound to fibers
using a
binder.
CA 02412892 2003-O1-03
S
SUMMARY OF THE INVENTION
The foregoing and other problems have been overcome by providing
fibers with hydrogen bonding functional sites, and binders that have a
volatility less
than water. The binder has a functional group that is capable of forming a
hydrogen
bond with the fibers, and a functional group that is also capable of forming a
hydrogen
bond or . a coordinate covalent bond with particles that have a hydrogen
bonding or
coordinate covalent bonding functionality.
The fibers of the present invention may have particles bound to the
fibers with a polymeric or non-polymeric binder. The binders comprise binder
molecules. The polymeric binder may be selected from the group consisting of
polyglycols [especially poly(propyleneglycol)], a polycarboxylic acid, a
polycarboxylate, a poly(3actone) polyol, such as diols, a polyamide, a
polyamine, a
polysulfonic acid, a polysulfonate and combinations thereof. Specific examples
of
some of these binders, without limitation, are as follows: polyglycols may
include
polypropylene glycol (PPG) and polyethylene glycol (PEG); poly(lactone) diols
include
poly(caprolactone) diol; polycarboxylic acid include polyacrylic acid (PAA);
polyamides include polyacrylamide or polypeptides; polyamines include
polyethylenimine and polyvinylpyridine; polysulfonic acids or polysulfonates
include
poly(sodium-4-styrenesulfonate) or poly(2-acrylamido-methyl-1-propanesulfonic
acid);
and copolymers thereof (for example a polypropylene glycol/polyethylene glycol
copolymer). The polymeric binder typically has repeating units. The repeating
unit
may be the backbone of a compound, such as with a polypeptide, wherein the
repeating polyamides occur in the peptide chain. The repeating unit also may
refer to
units other than backbones, for instance a repeating acrylic acid unit. In
such a case,
the repeating units may be the same or different. The binder molecules have at
least
one functional group capable of forming a hydrogen bond or a coordinate
covalent
bond with particles, and at least one functional group capable of forming a
hydrogen
bond with the fibers. At this time, the most preferred polymeric binder is
polyethylene
glycol although another especially preferred polymeric binder is an amide
binder such
as a polypeptide binder with polyglycine being a specifically preferred
example.
CA 02412892 2004-02-17
6
The non-polymeric binder has a volatility less than water. The non-
polymeric binder molecules have at least one functional group that is capable
of
forming a hydrogen bond or coordinate covalent bond with the particles, and at
least
one functional group that is capable of forming hydrogen bonds with the
cellulose
fibers. The nonpolymeric binder is an organic binder, and preferably includes
a
functional group selected from the group consisting of a carboxyl (for
example,
carboxylic acids), a carboxylate, a carbonyl (for example, aldehydes), a
sulfonic acid, a
sulfonate, a phosphoric acid, a phosphate, a hydroxyl (for example, an alcohol
or
polyol), an amide, amine, and combinations thereof (for example, amino acid or
hydroxy acid), wherein there are at least two functionalities on the molecule
selected
from this group, and the two functionalities are the same or different.
Examples of
such binders include polyols, polyamines (a non-polymeric organic binder with
more
than one amine group), polyamides (a non-polymeric organic binder with more
than
one amide goup), polycarboxylic acids (a non-polymeric organic binder with
more
than one carboxylic acid functionality), polyaldehydes (a non-polymeric
organic binder
with more than one aldehyde), amino alcohols, hydroxy acids. These binders
have
functional groups that are capable of forming the specified bonds with the
particles and
fibers.
More preferably, the organic non-polymeric binder is selected from the
group consisting of glycerin, a glycerin monoester, a glycerin diester,
glyoxal, ascorbic
acid, urea, glycine, pentaerythritol, a monosaccharide or a disaccharide,
citric acid,
tartaric acid, taurine (2-aminoethanesulfonic acid), p-aminosalicylic acid,
dipropylene
glycol, and urea derivatives, such as DMDHEU, and combinations thereof .
Suitable
saccharides include glucose, sucrose, lactose, ribose, fructose, mannose,
arabinose, and
erythrose. The preferred binders are non-polymeric molecules with a plurality
of
hydrogen bonding functionalities that permit the binder to form hydrogen bonds
to
both the fibers and particles. Particularly preferred binders include those
that can form
five or six membered rings, most preferably six membered rings, with a
functional
group on the particle surface. At present, preferred binders include glycerin,
glycerin
monoesters, including monoglycerides, a glycerin diester, including
diglycerides,
polyglycerin oligomers, a propylene glycol oligomer, urea and combinations
thereof
(such as glycerin and urea).
CA 02412892 2003-O1-03
7
As used herein, an oligomer refers to a condensation product of polyols,
wherein the
condensation product contains less than ten monomer units. A polyglycerin
oligomer
as referred to herein means a condensation product of two or more glycerin
molecules.
A propylene glycol oligorner as referred to herein means a condensation
product of
two or more propylene glycol molecules. At this time, a specifically preferred
non-
polymeric binder is glycerin.
The fibrous material may be cellulosic or synthetic fibers that are
capable of forming hydrogen bonds with the binder, while the particles are
selected to
be of the type that are capable of forming hydrogen bonds or coordinate
covalent
bonds with the binder. It has unexpectedly been found that this binder system
secures
particles to fibers exceptionally well. A superior fibrous product is
therefore produced
that has improved absorbent properties as compared to unbound or covalently
bound
particles. Formation of the noncovalent bond allows production of a fiber
product that
is easily manufactured and a web that is easily densified, and that is readily
biodegradable and disposable.
In one preferred embodiment, an absorbent product comprises a fibrous
cellulosic mat that contains superabsorbent hydrogel particles in particulate
form. The
superabsorbent particles are capable of forming hydrogen bonds or coordinate
covalent
bonds with the binder, depending upon the binder, while the binder in turn
forms
hydrogen bonds with the hydroxyl groups of the cellulose fibers. These
noncovalent,
relatively flexible bonds between the binder and particles maintain the
particles in
contact with the fibers, and resist dislodgement of the particles by
mechanical forces
applied to the mat during manufacture, storage or use. The amount of binder
present
typically depends on a number of factors, including the nature of the binder
and
particles, and whether the particles are immediately added to the fibers or
after a
period of time. Hence, one skilled in the art will realize that the amount of
binder
suitable and particularly useful for a particular application will vary.
However, the
binder may suitably be present in an amount of from about 1 to 80 percent of
the total
weight of the fibrous material. An especially suitable range of binder is 1 to
40 percent
by weight, or I to 25 percent by weight of the fibrous material. The particles
bound by
the binder of the present invention (via hydrogen/coordinate covalent bonds)
may
CA 02412892 2003-O1-03
g
suitably be present in an amount of .OS to 80 percent, preferably 1 to 80
percent or 3
to 80 percent, or more than 3 percent by weight of the total weight of the
fibrous
material and the particles. A particularly suitable range of particles is 3 to
40 percent
by weight of the fibrous material and particles. A preferred weight ratio of
particle to
binder is 8:1 to 50:1. An example of a suitable particle is a superabsorbent
polymer
such as a starch graft polyacrylate hydrogel fine or larger size particle such
as a
granule, which forms hydrogen bonds with the binder. The binder also forms
hydrogen
bonds with the hydroxyl groups of the cellulose, thereby securely attaching
the
superabsorbent particles to the fibers.
The present invention also includes a method of binding particles to
fibers wherein the particles are substantially insoluble in the binder (and
soluble in
water) and therefore retain their solid particulate form following binding.
The
particles, whether or not water soluble, preferably have functional groups
that can
form hydrogen bonds or coordinate covalent bonds with the binder, and the
binder in
turn is capable of forming hydrogen bonds to the fibers. Other particles
without the
desired functionality also may be included in the fiber product, but such
particles will
not be bound as strongly in the same manner.
In especially preferred embodiments, the fibers are cellulosic and the
particles are superabsorbent particles that are bound to the binder by
hydrogen bonds.
The fibers may also be continuous or discontinuous synthetic or natural fibers
having a
hydrogen bonding functional group that hydrogen bonds with the binder. The
binder is
suitably applied to the fibers in an amount of at least 1 percent, and
preferably no more
than 80 percent, by total weight of the fibrous material. The particles may be
bound to
the fibers at less than 150°C or without any external application of
heat at ambient
temperature (e.g., about 25°C). Particles may also be bound in the
absence of any
external application of pressure, or in the absence of external heat and
pressure.
In some embodiments the binder is associated with the fibers as a solid
(for example, a dry powder or a dried liquid), and the fibers contain at least
7 percent
water by weight when the binding step is performed. This level of moisture in
the
fibers provides sufficient mobility of reactants to allow the particles and
fibers to bind
well to each other. When a liquid binder is used (for example, glycerin or a
solution of
CA 02412892 2003-O1-03
9
glycine powder), the fibers suitably contain at least about 0.5 percent water
by weight.
A solid binder is suitably used with fibers having less than 0.5 percent water
by weight
if the binder is heated above its melting point to liquefy it. The solid can
be applied to
the fibers as a supersaturated solution or the solid binder may be heated
above its
S melting point to liquefy the binder, which is later applied to the fibers.
Upon
solidifying the binder is deactivated. A solid binder may be thermoplastic or
meltable,
such that it can be heated above its melting point/or softening point and then
cooled to
fuse fibers to each other. The thermoplastic properties of the binder can also
provide
additional mechanical adherence between the particles and fibers. In some
embodiments, a thermoplastic binder such as urea may be employed which can
adhere
particles both thermoplastically and with hydrogen bonding.
In other embodiments, the particles are soluble in water but have
reduced solubility in the binder such that the particles can be bound in solid
particulate
form to the fibers. Addition of the binder does not dissolve the particle and
cause it to
1 S diil'use away from its desired site of attachment to the fibers.
The binder attaches the particles to the fibers, and forms a bond that has
been found to be resistant to mechanical disruption. A significant advantage
of these
binders is that the binder and particle together on the fiber have been found
to reduce
the pressure required to densify the fibers. The binders can also be present
on fibers in
an inactive state for more than a week, a month, or a even a year, then later
activated
or reactivated to bind particies to the fibers.
Liquid binders (which includes neat liquids or aqueous solutions of solid
binders) can be placed on the fibers, dried, and later activated by moistening
the fibers.
Alternatively, a dry solid binder may be added to the fibers and later
activated by
addition of-a liquid. An inactive binder can also be activated by applying
kinetic energy
to the fibers after the binder and fibers reach an equilibrium moisture
content with the
atmosphere (hereinafter referred to as "air dry"). Kinetic energy can be
applied to the
binder and fibers, for example and without limitation, by applying mechanical
agitation,
pressure from an external source, or using ultrasonics. In yet other
embodiments, the
binder may be activated or reactivated by heating the fibers after applying
the binder to
the fibers.
CA 02412892 2003-O1-03
The capacity for activation or reactivation allows the binder to be
applied to the fibers, which are then shipped to distribution points with the
binder in an
inactive form. The binder is then activated at the distribution point (for
example, a
customer's facility) where particles are added to the fibers and bound
thereto. As used
5 herein, binder "activation" includes both activation of previously inactive
binders (such
as solid binders in the absence of liquid) or activation of previously active
binders (such
as a liquid binder that has been dried).
Another advantage of the present invention is that the binder can be
activated or reactivated in a pattern that corresponds to a desired
distribution of
10 particles in fibrous material. An activation fluid, such as an activation
liquid, for
example, can be applied to the areas of a diaper that will be initially
moistened by urine
during use. Examples, without limitation, of a suitable activation liquid
include water,
lower-alkyl alcohols, polyols such as the glycols, acetone; and combinations
thereof,
such as water and glycerin. When the activating fluid is a liquid such as
water, the
water may be sprayed or otherwise applied and may be provided in the form of
steam
or moisture-laden gas, such as humid air. Other liquid activation fluids may
be applied
in the same manner. Superabsorbent particles can be added to activated areas
of the
diaper and adhered almost exclusively in those areas where initial urine
absorption is
required. Targeted activation of binder allows particles to be efficiently and
economically attached . to the fibers, with reduced particle wastage.
Moreover,
targeted binder activation and particle adherence increases the absorptive
efficiency of
the product by diminishing excessive wicking of liquid within the plane of an
absorptive product.
The invention also is directed to fibrous products produced by any of
the methods described herein, and to absorbent articles comprised of such
fibrous
products. These fibrous products include fibers with inactive or activatable
binders. A
fibrous product may be individual fibers or webs made thereof.
The present invention relates to the above objects, features and
advantages individually as well as collectively. The foregoing and other
features and
advantages of the invention will become more apparent from the following
detailed
descriptions and accompanying drawings.
CA 02412892 2003-O1-03
11
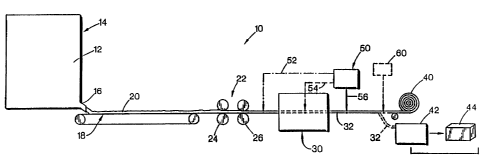
BRIEF DESCRIPTION OF THE DRAWINGS
FIG: 1 is a schematic illustration of a wet laid sheet manufacturing line
illustrating the application of binders in accordance with the present
invention during
the manufacture of a fiber sheet.
FIG. 2 is a schematic illustration of a binder activation and particulate
attachment process in accordance with the present invention.
FIG. 3 is a schematic depiction of the components of an apparatus of
the present invention that produce high bulk fibers.
FIG. 4 is a top plan view of a structure into which fibers of the present
invention are incorporated with attached particles, the fibers being in the
form of an
illustrated absorbent pad.
FIG. 5 represents a partial sectional view of the pad of FIG. 4.
FIG. 6 illustrates a plan view of a bandage incorporating fibers of the
present invention.
FIG. 7 is a sectional view of the bandage of FIG. 6, taken along line 7-7
of FIG. 6.
FIG. 8 is a plan view of a disposable diaper including a core of fibers of
the present invention.
FIG. 9 is a vertical sectional view along line 9-9 of the diaper of FIG. 8.
FIG. 10 is a view of an enlarged fiber with particles bonded to the fiber
with the binders of the present invention.
FIG. 11 is a schematic view of a cellulose mat with particles bound to
all its surfaces and throughout its depth.
FIG: 12 is a photomicrograph of particles adhered to fibers with an
ascorbic acid binder.
FIGS. 13A, 13B, 13C, and 13D are photomicrographs of particles
bound to fibers with lactose.
FIG. 14 is a photomicrograph of oxalic acid particles bound to a fiber
with a glycerin binder.
CA 02412892 2004-02-17
12
FIG. 15 is a photomicrograph of aluminum sulfate (alum) bound to a
fiber with a glycerin binder.
FIG. 16 is a photomicrograph of EDTA (ethylenediaminetetraacetic
acid) particles bound to a crossiinked fiber with a glycerin binder.
DETAILED DESCRIPTION OF THE SEVERAL PREFERRED
EMBODIIvviENTS OF THE INVENTION
I. Processing of Fibers
FIG. 1 illustrates a wet laid sheet manufacturing line such as a pulp
sheet manufacturing line 10. In this manufacturing Line, a pulp slurry 12 is
delivered
from a headbox 14 through a slice 16 and onto a Fourdrinier wire 18. The pulp
slurry
12 typically includes cellulose fibers such as wood pulp fibers and may also
include
synthetic or other non-cellulose fibers as part of the slurry. Water is drawn
from the
pulp deposited on wire 18 by a conventional vacuum system, not shown, leaving
a
deposited pulp sheet 20 which is carried through a dewatering station 22,
illustrated in
this case as two sets of calendar rolls 24, 26 each defining a respective nip
through
which the pulp sheet or mat 20 passes. From the dewatering station, the pulp
sheet 20
enters a drying section 30 of the pulp manufacturing Line. In a conventional
pulp sheet
manufacturing line, drying section 30 may include multiple canister dryers
with the
pulp mat 20 following a serpentine path around the respective canister dryers
and
emerging as a dried sheet or mat 32 from the outlet of the drying section 30.
Other
alternate drying mechanisms, alone or in addition to canister dryers, may be
included in
the drying stage 30. The dried pulp sheet 32 has a maximum moisture content
pursuant to the manufacturer's specifications. Typically, the maximum moisture
content is no more than 10% by weight of the fibers and most preferably no
more than
about 6% to 8% by weight. Otherwise, the fibers tend to be too damp. Unless
overly
damp fibers are immediately used, these fibers are subject to degradation by,
for
example, mold or the like. The dried sheet 32 is taken up on a roll 40 for
transportation to a remote location, that is, one separate from the pulp sheet
manufacturing line, such as at a user's plant for use in manufacturing
products.
CA 02412892 2003-O1-03
13
Alternatively, the dried sheet 32 is collected in a baling apparatus 42 from
which bales
of the pulp 44 are obtained for transport to a remote location.
A binder of the type explained in detail below is applied to the pulp
sheet from one or more binder applying devices, one of which is indicated at
50 in FIG.
1. Any binder applying device may be used, such as sprayers, roll coaters,
immersion
applicators or the like. Sprayers are typically easier to utilize and
incorporate into a
pulp sheet manufacturing line. As indicated by the arrows 52, 54 and 56, the
binder
may be applied at various locations or at multiple locations on the pulp sheet
manufacturing line, such as ahead of the drying stage 30 (indicated by line
52),
intermediate the drying stage 30 (as indicated by line 54), or downstream from
the
drying stage 30 (as indicated by the line 56). Water-based binders, such as
non-
polymeric urea, are typically applied at a location where sufficient drying
can still take
place in the drying stage to produce a dried binder containing fiber sheet
with no more
than the maximum desired moisture content. Consequently, to take advantage of
the
I5 drying stage 30, water-based binders are typically applied at locations 52
or 54. At
location 52, the water remaining in the sheet or mat 20 at this stage tends to
interfere
with the penetration of the binder into the sheet. Consequently, application
of the
binder after some drying has taken place, for example at location 54, is
preferable. If
water-based binders are applied at location 56 in an amount which would cause
the
moisture content of the sheet to exceed the desired maximum level, an
additional
drying stage (not shown) may be included in the pulp manufacturing line to
bring the
moisture content down to the desired level.
A non-aqueous based binder, such as glycerin, is most preferably added
downstream from the dryng stage at location 56 or during the drying stage as
indicated by location 54. However, liquid non-aqueous binders may also be
added at a
location, such as location 52, upstream of the drying stage. At this latter
location, the
water in the wet web at this point may tend to attract these binders into the
mat or
sheet as the binders tend to be hydroscopic. Since non-aqueous binders
typically do
not enhance the degradation of the product due to the addition of moisture to
the
sheet, they can be applied downstream from the drying stage without bringing
the
moisture content of the sheet above the desired maximum Level.
CA 02412892 2003-O1-03
14
The particulate materials, selected as explained below, may be added to
the sheet and adhered thereto by the binders on the pulp manufacturing line,
such as
indicated by the particulate applicator 60, which may comprise a bulk or
volumetric
metering device. These particles may be sprinkled, poured or otherwise added
to the
S sheet. To facilitate the adherence of these particulates to the sheet at
this location,
enough moisture must remain in the sheet, in the case of aqueous binders, to
enable the
bonding between the particles and fibers as explained below. For non-aqueous
binders,
the particles in this case are preferably added while the binder is still wet
or heated to
facilitate the.reaction. Particles can be added on the pulp sheet
manufacturing line in
this manner, with a subsequent drying stage being utilized to reduce the
moisture
content following particulate addition. However, if a water-based binder makes
the
fibers too wet following the addition of the particles, this is not the
preferred approach.
Although the above approach is advantageous because the particles are
strongly bound to the fibers, during transportation of rolls or bales of these
fibers it is
possible for particles to become dislodged by mechanical impact during
transport. In
addition, this approach interferes with the customization of the fiber
application at a
user's location. For example, a user may want the capability of selecting
particular
types or brands of particles for adherence to the fibers in the user's
products, without
having this selection made by a pulp sheet manufacturer who incorporates the
particles
into the pulp sheet during its manufacture. Also, certain particles rnay
degrade over
time, making it advantageous to add such particles immediately prior to
incorporation
into products. For example, superabsorbent particles are susceptible to
absorbing
moisture from the atmosphere during shipment. Particles with a relatively
short shelf
life, such as certain zeolites (e.g. Abscents with odor absorbing materials
which can
become saturated with odors over time) being one example, may also degrade
over
time. Another example is zeolites with silver salts as antimicrobiai agents
which can
photodegrade. Therefore, it is also advantageous to provide a fibrous product
in
which the end user of the product may incorporate the desired particles at the
time the
fibers are converted into products.
Therefore, in keeping with this latter preferred approach, as illustrated
in FIG. 2, the respective rolls 40 or bales 44 of binder-containing fibers,
without
CA 02412892 2003-O1-03
IS
particles, are transported to a remote location for use by a user. These rolls
or bales
(or otherwise transported fibers, e.g., bagged, containerized or otherwise in
bulk form)
are then refiberized by a fiberizing apparatus 70. Although any fiberizer may
be used,
a typical fiberizing apparatus 70 is 'a hammermili which may be used alone or
in
conjunction with other devices such as picker rolls or the like for breaking
up the sheet
32 or bales 42 into individual fibers.
A particulate material adding mechanism 72 (e.g., like mechanism 60)
delivers the desired particulate materials to the fibers at the desired
location in the
user's process. Again, the device 72 typically comprises a metering mechanism,
IO although any suitable device for adding particulates to fibrous materials
may be used.
For example, the particulates may be delivered as indicated by line 74 to the
fiberizing
apparatus 70. In the case of some binders; agitation of fibers within the
fiberizer 70, as
explained in greater detail below, activates the binders and causes the
particulates to be
adhered to the fibers by the binder. Alternatively, an activating fluid, which
may be a
I S liquid such as water, glycerin, lower-alkyl alcohols, polyols such as the
glycols,
acetone, and combinations thereof such as water and glycerin, may be sprayed
or
otherwise applied to the fibers, such as from an activation fluid tank or
source 78 by
way of a sprayer (not shown) at location 80. The particles may then be
applied, as
indicated by line 84 to the fibers downstream from the application of the
activation
20 liquid 80. Alternatively, the particles which may be added prior to or at
location 80,
are adhered to the fibers by the binder upon activation of the hinder at
location 80. As
yet another alternative, the fiberized fibers are delivered to an air-laying
device 90 and
reformed into a desired product such as a web indicated at 92. In the case of
air-laid
fibers, the activation fluid or liquid may be applied to the web at location
96 with the
25 particles then being added at location 98 as shown with the activated
binder then
adhering the particles to the fibers. The particles may be applied at a
location in the
process upstream from the application of the activating liquid at location 96.
Alternatively, the activating fluid may be added simultaneously with the
addition of
particles, so that the activation occurs simultaneously with the addition of
particles.
30 The activating fluid also may be added after the particles are added to the
fibers. In
addition, the binder may be activated at specifically defined locations on the
web 92,
CA 02412892 2003-O1-03
16
such as in target zones of an absorbent core of a product with the particles
then only
being applied to these target zones, thereby minimizing the wasting of the
particulate
material. A specific example of a target zone is the crotch region of a diaper
where
most diaper wetting would occur. The application of superabsorbent particles
to such
a zone places these particles at a location where they are most useful in
absorbing
liquid. The web 92, with or without other components of the end user's
product, is
then processed into the user's product, such as being included within a
disposable
diaper 100.
Again, with this approach, the end user of the fibers may readily select
particles to be applied to its product and may activate the binder as required
to
enhance the efficient production of the user's product. In addition, the user
has
flexibility in air laying or otherwise combining the binder containing fibers
into a
finished product with the desired particulates. The binder containing fibers,
because
the binders are all water soluble, are preferably not wet Laid because wet
laying would
remove at least some of the binder. Not only is handling and shipping of the
particulate containing products avoided by the manufacturer of the pulp sheet,
enhanced adhesion of particulates to the fibers results because the particles
are not
subjected to mechanical forces between the location of manufacture of the
fibers and
the location at which the particulate materials are added.
II. Fiber Characteristics
The present invention includes a method of binding particles to fibers,
and the product, including absorbent end-products, that are produced by such
method.
In particularly preferred embodiments, the product is a cellulosic or
synthetic fiber to
which superabsorbent hydrogel polymer particles are adhered by a binder, and
absorbent products made therefrom. Suitable fibers include wood pulp fibers,
which
can be obtained fi-orn well known chemical processes such as the kraft and
sulfite
processes. The invention also includes a combination of wood pulp and certain
binders, which for the purpose of this combination are bulk fibers in roll
form having a
basis weight of at least 350 grams per square meter (g/m2) or bale form. The
bulk
fibers can have a density of at least about 400 kg/m3. Preferred bulk fibers
are wood
CA 02412892 2003-O1-03
17
pulp fibers or softwood pulp fibers. The pulp fibers may be chemical or
thermomechanical or chernithermornechanical or combinations thereof. The
preferred
pulp fiber is chemical. In these processes, the best starting material is
prepared from
long fiber coniferous wood species, such as pine, douglas fir, spruce and
hemlock.
Wood pulp fibers can also be obtained from mechanical processes, such as
ground
wood, mechanical, thermomechanical; chemimecharucal, and chemithermomechanical
pulp processes: The fibers are preferably elongated, for example having a
length to
width ratio of about 10:1 to 5: I.
The fibers of the present invention also include fibers that are pretreated
prior to the application of a binder to the fibers. This pretreatment may
include
physical treatment, such as subjecting the fibers to steam or chemical
treatment, such
as cross-linking the fibers. Although not to be construed as a iimitatinn,
examples of
pretreating fibers include the application of fire retardants to the fibers,
such as by
spraying the fibers with fire retardant chemicals. Specific fire-retardant
chemicals
I S include, by way of example, sodium borate/boric acid, urea,
urea/phosphates, etc. In
addition, the fibers may be pretreated with surfactants or other liquids; such
as water
or solvents, which modify the surface of the fibers. Other pretreatments
include
exposure to antimicrobials or pigments.
The fibers also may be pretreated in a way which increases their
wettability. The fibers also may be pretreated with conventional cross-linking
materials
and may be twisted or crimped, as desired. Pretreating cellulose fibers with
chemicals
which result in lignin or cellulose rich fiber surfaces also may be performed
in a
conventional manner.
Bleaching processes, such as chlorine or ozoneloxygen bleaching may
also be used in pretreating the fibers: In addition, the fibers may be
pretreated, as by
slurrying the fibers in baths containing various solutions. For example,
antimicrobial
solutions (such as solutions of antimicrobial particles as set forth below),
as well as
solutions of fertilizers and pesticides, and/or fragrances and flavors, for
release over
time during the life of the fibers. Fibers pretreated with other chemicals,
such as
thermoplastic and thermoset resins also may be used. Combinations of
pretreatments
CA 02412892 2003-O1-03
I8
also may be employed with the resulting pretreated fibers then being subjected
to the
application of the binder coating as explained below.
Ground wood fibers, recycled or secondary wood-pulp fibers; and
bleached and unbleached wood-pulp fibers can be used. Details of the
production of
wood pulp fibers are well known to those skilled in the art. These fibers are
commercially available from a number of companies including Weyerhaeuser
Company, the assignee of the present invention.
The fibers also can be any of a variety of other natural or synthetic
fibers; however, all of the fibers to which particles are attached in
accordance with the
present invention include a hydrogen-bonding functionality. This does not
preclude the
blending of such fibers with fibers lacking this characteristic. However, the
fibers
lacking a hydrogen bonding functionality will not have particles bonded
thereto with
the strength and manner of the bonds that would be present if the fibers had a
hydrogen-bonding functionality.
1S A hydrogen bond is an intermolecular force that occurs between
hydrogen atoms ,that are covalently bonded to small, strongly electronegative
elements
(such as nitrogen and oxygen) and nonbonding electron pairs on other such
electronegative elements. A hydrogen bonding functionality is a functional
group that
contains an oxygen or nitrogen atom, for example hydroxyls, carboxyls,
sulfonic acids,
sulfonamides, ethers, esters, epoxides; carbonyls, amines, urethanes and
others, that is
capable of forming a hydrogen bond. The orbitals of the nonbonding electron
pairs on
the oxygen or nitrogen overlap with the relatively empty is orbital of the
hydrogen
covalently bonded to another nitrogen or oxygen atom. The is orbital of the
hydrogen
is relatively empty due to the unequal sharing of the electrons in the
covalent bond
between it and: the small electronegative atom (oxygen or nitrogen) to which
it is
bound.
Specific examples of natural fibers that contain a hydrogen bonding
functionality include chopped silk fibers, wood pulp fibers, bagasse, hemp,
jute, rice,
wheat, bamboo, corn, sisal, cotton, flax, kenaf, peat moss, and mixtures
thereof.
Suitable synthetic fibers with hydrogen bonding funetionalities include
acrylic,
polyester, cacboxylated polyoiefins; rayon and nylon. The hydrogen-bonding
CA 02412892 2003-O1-03
19
functionality is an ester in acrylic fibers and a carboxylic acid in
carboxylated polyolefin
fibers, an ester in polyester, an amide in nylon, and a hydroxyl in rayon.
Polyethylene
and polypropylene would be unsuitable fibers for use in particle to fiber
bonding in the
manner of the present invention because they include only carbons and
hydrogens
without any other atoms, such as oxygens or nitrogens, that can participate in
hydrogen bonds:
For purposes of convenience, and not to be construed as a limitation,
the following description proceeds with reference to the treatment of
individual
chemical wood-pulp fibers. The fibers are individualized, for example by
defiberization
in a hammermill: Such individualized fibers are conventionally formed into a
mat, and
are commercially available, for example as NB 416 fibers from the Weyerhaeuser
Company. Another suitable cellulosic mat would include Rayfloc JI,D from ITT
Rayonier. The cellulose fibers may be in the form of a cellulosic web or loose
cellulose
fibers.
III Particle Characteristics
In accordance with the present invention, particles are added to the
fibers to give the fibers desired properties, such as, by way of example only,
increased
absorbency, abrasiveness, or antimicrobial activity. The particle can be any
particulate
material that has the desired property and which is capable of forming
hydrogen bonds
or coordinate covalent bonds with the binder. Hydrogen bonds can be formed, as
discussed above, by particles that contain certain functional groups,
particularly those
having an oxygen or nitrogen. Coordinate covalent bonds, in contrast, are
formed by
donation of a lone pair of electrons on one atom to an empty orbital of
another atom.
Coordinate covalent bonds differ from covalent bonds in that covalent bonds
are
formed by a pair of electrons wherein one of the electrons is donated from
each of the
atoms that participate in the bond. Particles can form coordinate covalent
bonds if
they have an empty p or d or f orbital that is capable of accepting a pair of
electrons
from the binder.
A coordinate covalent bond occurs between a donor atom that has a
Lone pair of electrons to donate to the bond, and an acceptor atom that has an
empty
CA 02412892 2003-O1-03
orbital to accept the lone pair of electrons from the donor. According to the
Aufbau
and Pauli principles, electrons occupy the lobes of atomic orbitals one at a
time with a
maximum of two electrons (with opposite spins) per lobe. The most basic
orbital is the
s orbital, which .is available for bonding the elements in the first row of
the periodic
5 table. In the second row of the periodic table, electrons fill first the 2s
orbital of Li and
Be. However, metals in periods less than three do not have sui~cient afl3nity
for
electrons to participate in coordinate covalent bonding. Beginning with Group
~
(boron), the threep orbitals participate in coordinate covalent bonding and
the Lobes of
the p orbitals begin to fill. Boron has one electron in one of the 2p
orbitais, thus
10 leaving the other 2p orbitals empty and available for coordinate covalent
bonding. An
example of a coordinate covalently bonded boron containing particle is boric
acid,
which is used as an astringent, antiseptic and fire retardant. As shown below,
the
boron atom of boric acid acts as an acceptor for a lone pair of electrons
donated by an
oxygen atom of polypropylene glycol (PPG); thereby forming a coordinate
covalent
15 bond between a boric acid particle and a PPG binder. The depiction of boric
acid
shown below is not typical of the aqueous chemistry of boron, but rather is
provided to
illustrate the type of bonding that is believed to occur in a coordinate
covalent bond.
20 C~ CH3
PPG -~O ~~~ O
( O ) (i ~Ow
O )
-.. H3 ;
~ O 0~.8
boric acid W
H H
H H
The next element, carbon, usually hybridizes to have one electron in the
2s orbital and the three remaining electrons are singly placed in the three p
orbitals.
This leaves no lobes empty for coordinate covalent bonding and electron
additions
proceeding further across that row of the periodic table also leave no lobes
empty.
CA 02412892 2003-O1-03
2I
Hence, boron is the only element in the second row of the periodic table that
is capable
of forming coordinate covalent bonds.
Next the third row begins to fill, and the two 3s electrons fill first in
sodium and magnesium.
Sodium and magnesium have empty d orbitals available for
coordination. Examples of magnesium coordination compounds are common. Then
aluminum, Like boron, places one electron in one of the 3p Iobes, and the two
other 3p
Iobes are empty and available for coordinate covalent bonding. The same trends
continue across the third row, but the third row elements also have available
five 3d
lobes so the potential for coordination bonding exists even though 3p orbitals
are
occupied in the third row. Hence, AI, P, S, and CI are capable of accepting a
pair of
electrons from an electron-pair donor to form a coordinate covalent bond. An
example
of this is found in the bonding in PCIs, aluminum trihydrate; or phosphorous
pentasulfide. A phosphorous pentasulfide particle can be used to increase
flammability
of a product, while aluminum trihydrate is a fire retardant. An example of a
coordinate
covalently bonded aluminum compound is
CH3 CH3
P~ /~ .~,~.4~ p O
( O ) r
~O l
'~" H3 ,
i
O
Aluminum O ~ O 'p
trihydrate
H H
H
H
H
aluminum trihydrate, which may participate in a coordinate covalent bond with
a
polypropylene glycol (PPG) polymer. In this example, the aluminum atom of
aluminum trihydrate acts as an electron acceptor for an electron pair donated
by an
oxygen atom of the polypropylene glycol (PPG) binder. The depiction of
aluminum
trihydrate shown above is not typical of the aqueous chemistry of aluminum,
but rather
CA 02412892 2003-O1-03
22
is provided to illustrate the type of bonding that may occur in a coordinate
covalent
bond.
In the next row, the 4s orbital is filled first, then the 3d lobes begin to
fill one electron per lobe until all have added a single then a second
electron to each
lobe until all lobes are filled. However, 4p and 4f orbitals also are
available, hence
many of the transition elements are capable of forming coordinate covalent
bonds.
The elements that have empty orbitals that participate in coordinate
covalent bonding include all those except the metals (which excludes hydrogen)
in
periods one and two, and C, N, 0, F, Ne and He. These metals do not have
sufficient
aiEnity for electrons to participate in coordinate covalent bonding.
Especially
preferred particles contain boron, aluminum, iron, rhodium, osmium, platinum,
and
palladium, and most particularly boron. Examples of particles that are capable
of
coordinate covalent bonding are aluminum trihydrate, antimony oxide, arsenic
disul$de, bismuth aluminate, bismuth iodide oxide, bismuth phosphate, bismuth
subcarbonate, bismuth subgallate; cadmium salycilate, chromic carbonate,
chromic
hydroxide, chromic oxide, and chromic phosphate. All of the polymeric binders
of the
present invention [polyglycols (such as PPG), polycarboxylic acids (such as
PAA),
poly(lactone) diols (such as poly(caprolactone) diol); polyamides, polyamines,
etc. ] are
capable of donating a lone pair of electrons from an electronegative atom,
such as
oxygen or nitrogen, to form a coordinate covalent bond with a suitable
particle that
includes an atom having an empty. orbital for accepting electrons to form a
coordinate
covalent bond.
IV. Superabsorbent Particles
In one disclosed embodiment the added particles are superabsorbent
particles, which comprise polymers that swell on exposure to water and form a
hydrated geI (hydrogel) by absorbing large amounts of water. Superabsorbents
are
defined herein as materials that exhibit the ability to absorb large
quantities of liquid,
i.e., in excess of 10 to 15 parts of liquid per part thereof. These
superabsorbent
materials generally fall into three classes, namely starch graft copolymers,
crosslinked
carboxymethylcellulose derivatives and modified hydrophilic polyacrylates:
Examples
CA 02412892 2004-02-17
23
of such absorbent polymers are hydrolyzed starch-acrylonitrile graft
copolymer, a
neutralized starch-acrylic acid graft copolymer, a saponified acrylic acid
ester-vinyl
acetate copolymer, a hydrolyzed acrylonitrile copolymer or acrylamide
copolymer, a
modified cross-linked polyvinyl alcohol, a neutralized self crosslinking
polyacrylic acid,
a crosslinked polyacrylate salt, carboxylated cellulose, and a neutralized
crosslinked
isobutylene-malefic anhydride copolymer.
Superabsorbent particles are available commercially, for example starch
graft polyacrylate hydrogel fines (IMIOOOF~ from Hoechst-Celanese of
Portsmouth,
VA, or larger particles such as granules. Other superabsorbent particles are
marketed
under the trademarks SANWE'I~(supplied by Sanyo Kasei Kogyo Kabushiki Kaisha),
SUMIICA GEL# {supplied by Sumitomo Kagaku Kabushiki Kaisha and which is
emulsion polymerized and spherical as opposed to solution polymerized ground
particles), FAVOR (supplied by Stockhausen of Greensboro, North Carolina), and
NORSOCRYL (supplied by Atochem). The superabsorbent particles come in a
variety
IS of sizes and morphologies, for example IM 1000#and IMIOOOF# The 1000F is
finer
and will pass through a 200 mesh screen whereas IM 100 has some particles that
will
not pass through a 60 mesh screen. Another type of superabsorbent particle is
IM
5600 (agglomerated fines). Superabsorbent particulate hydrophilic polymers
also are
described in detail in U.S. Patent No. 4,102,340. That patent discloses
hydrocolloid
absorbent materials such as cross-linked polyacrylamides.
V. Other Particles
Many particles that form hydrogen bonds or coordinate covalent bonds
are suitable for use with the present invention. Some such particles are
listed in Table I
with an indication of the function of the listed particles. The particles
listed in Table I
are water-insoluble particles.
#Trademark
CA 02412892 2003-O1-03
24
Table I
Water-Insoluble Particulates For Bindine
Name Function
Aluminum Trihydrate Fire retardant, astringent
Acediasulfone Antibacterial
Agaricic acid Antiperspirant
Alclometastone Topical anti-inflammatory
Calcium alginate Topical hemostatic
Amidomycin Fungicide
Antimony oxide Fire retardant
Apigenin Yellow dye, mordant
I Arsenic disulfide Red Pigment
S
Aspirin Anti-inflammatory; antipyretic
Azanidazole Antiprotozoal (Trichomonas)
Azelaic acid Antiacne
Baicalein Astringent
Bendazac Anti-inflammatory
Benomyl Fungicide; ascaricide
Benzestrol Estrogen
Benzylpenicillinic acid Antibacterial
Benzylsulfamide Antibacterial
Bergaptene Antipsoriatic
Betasine Iodine source
Bezitramide Narcotic analgesic
Bibrocathol , Topical antiseptic
Bietanautine Antihistaminic
Bifenox Herbicide
Bifonazole Antifungal
Binapacryl Fungicide, miticide
Bis(p-chlorophenoxy) methaneMiticide
Bismuth aluminate Antacid
Bismuth iodide oxide Anti-infective
Bismuth phosphate Antacid; protectant
Bismuth subcarbonate Topical protectant
Bismuth subgallate Astringent, antacid; protectant
Bisphenol A Fungicide
Bitertanol Agricultural fungicide
Bithionol Topical anti-infective
BromaciI Herbicide
Bromadiolone Rodenticide
Bromcresol green Indicator
Bromcresol purple Indicator
Bromethalinlin Rodenticide
CA 02412892 2003-O1-03
(Table I cont'd)
Name Function
5 p-Bromoacetanilide Analgesic; antipyretic
3 Bromo-d-camphor Topical counterirntant
Bromophos Insecticide
Bromopropylate Acaricide
5-Bromosalicyl-hydroxamic antibacterial (tuberculostatic)
acid
10 5-BromosaIycilic acid acetateAnalgesic
Bromosatigenin ~ Anti-inflammatory
Bromthymol blue Indicator
Broxyquinoline Antiseptic; disinfectant
Bucetin Analgesic
15 Bumadizon Analgesic; anti-inflammatory;
antipyretic
Bupirimate Fungicide
Busulfan carcinogen, insect sterilant,
antineoplastic
Butamben Topical anesthetic
Butrylin Insecticide
20 Butylated hydroxy-anisole Antioxidant (BHA)
Butyl paraben Pharmaceutic aid; food preservative
4-tert-Butylphenyl salicylateLight absorber
Cacotheline Indicator
Cactinomycin Antineoplastic
25 Cadmium saiycilate Antiseptic
Calamine Skin protectant
Calcium carbonate Antacid
Calcium saccharate Pharmaceutic aid
Calcium tartrate Preservative; deodorant; antacid
Cambendazole Anthelminthic
Candicidin Topical antifungal
Candidin Topical antifizngal
Capsaicin Topical analgesic
Captan Fungicide; bacteriostat
Carbadox Antimicrobial
Carbamazepine Anticonvulsant; analgesic
Carbarsone Antiamebic
Carbaryl Contact insecticide
Carbazochrome salycilate Antihemorrhagic
Carbendazim Fungicide
CarbochIoral Hypnotic
Carbophenothion Miticide; insecticide
Carboquone Antineoplastic
Carisoprodol Skeletal muscle relaxant
Carthamin Dye
Carvacrol Disinfectant
CA 02412892 2003-O1-03
26
(Table I cont'd)
Name Function
S Cephalin Local hemostatic
Ghalcomycin Antibiotic
Chartreusin Antibiotic
C~~ Vulnerary
Chloramben Herbicide
Chloramphenacol palmitateAntimicrobial
Chloranil Fungicide
Chlotbetamide Antiamebic
Chlordimeform Insecticide
Chlorfenac Herbicide
Chlorfenethol Acaricide
Chlorhexidine Topical antibacterial
Chioroazodin Antibacterial; topical anesthetic
Chlorophacinone Anticoagulant rodenticide
p-Chlorophenol Antiseptic
Chlorothricin Antibiotic
Chlorotrianisene Estrogen
Chloroxylenol Antiseptic; germicide
Chlorphenesin Topical antifu' regal
Chlorphenesin carbamate Relaxant (skeletal muscle)
Chlorphenoxamide Antiamebic
Chlorpropamide Antidiabetic
Chlorpyrifos Insecticide
Chlorquinaldol Topical antibacterial
Chlorsulfwon Herbicide
Chlorothion Insecticide
Chlozoxazone Relaxant
Cholesterol Pharmaceutic aid
Chromic carbonate Pigment
Chromic hydroxide Pigment
Chromic oxide Abrasive
Chromic phosphate Green pigment
Chrysamminic acid Explosive
Chrysarobin Antipsoriatic
Cilastazol Antithrombotic
Cinoxate Sunscreen agent
Other suitable water-insoluble particles include proteins, vitamins,
zeolites and silica, each of which contains electronegative atoms, such as
oxygen or
nitrogen groups, or both. An example of a suitable zeolite is Abscents odor
absorber
CA 02412892 2003-O1-03
27
available from UOP of Tarrytown, New York. An example of a suitable
antimicrobial
particle is chlorhexidine (N,N"-Bis(4-chlorophenyl~3,12-diimino-2,4, i 1,13-
tetraazatetradecanediimidamide). The List in Table I is by no means exhaustive
as it
can be readily determined for each type of particle whether it is capable of
forming a
hydrogen bond or a coordinate covalent bond. Many of the particles are non-
absorbent, or not superabsorbent polymers.
The particles fisted in Table I have chemical properties that make them
suitable for binding to fibers with the binders of the present invention. The
listed
particles are organic or inorganic compounds that have little or no water
solubility, yet
IO have the capacity to hydrogen bond: Water solubility is preferably low, for
example,
less than-10 g dissolves completely in 300 ml of water at 25°C, more
preferably less
than about I g in 300 ml at 25°C. This low solubility allows the
particles to remain
solid, and the hydrogen-bonding capacity allows them to adhere to the fibers
even in
cases when an aqueous binder is used. Once bound, the particles substantially
retain a
discrete particulate form instead of dissolving or fusing. Hence, once bound
more of
the particles are discrete than fused:
Many water-soluble particles that are capable of forming hydrogen
bonds or coordinate covalent bonds are suitable for use with the binders of
the present
invention. Some such water-soluble particles are listed in Table II with an
indication of
the function of the listed particles.
Table II
Particulates For B~;ndina
Name Function
Ethylenediaminetetraacetic acid Odor absorbent
(EDTA)
disodium salt of EDTA Chelator
Sodium bicarbonate Odor absorbent/pH
modifier
Acarbose Antidiabetic
Acefylline Piperazine Bronchodilator
Acenocoumarol, sodium salt Anticoagulant
Acephate Insecticide
Acetaminophen Analgesic
Acetylleucine Monoethanolamine Antivertigo agent
CA 02412892 2003-O1-03
28
Table II (cont'd)
Name Function
Acid Violet 7B Dye/Stain
Acitretin Antipsoriatic
Acranil Antiprotozoal (Giardia)
Acriflavine Anti-infective
Actaplanins Growth stimulant ,
Algestone Acetophenide Antiacne
Algin Hemostatic
Almagate Antacid
(-~Ambroxide Fragrance
Ambucaine hydrochloride Local anesthetic
Amodiaquin Antimalarial
Anabasine hydrochloride Insecticide
o-Anisaldehyde Fragrance
Anisomycin hydrochloride Topical antitrichomonal
Araikonium chloride Antiseptic, germicide
Asiaticoside Dermatide, wounds, burns
Aspartame Non-nutritive sweetener
Azidoamphenicol Antimicrobial in eye infections
Bebeerine Antimalarial
Potassium benzoate Preservative, antifungal
Benzoyl peroxide Dermatide, antiacne
Benzylidene acetone Fragrance
Bidrin Insecticide
Biphenamine hydrochloride Antiseborrheic
Bishydroxycoumarin Anticoagulant
Bismuth tribromophenate Topical antiseptic
Blasticidin S hydrochlorideAntimicrobial
Bromocresyl green Indicator
Bromophenol blue Indicator
3 Butathamine hydrochloride Anesthetic
S
Caffeine hydrochloride CNS Stimulant
Calcium ascorbate Vitamin C/Calcium source
Calcium bisulfite Germicide
Calcium thioglycollate Depilatory
Carbachol Ophthalmic parasympathomimetic
Carbowax Ointment base
Cetalkonium chloride Antibacterial
Cethoxonium bromide Antiseptic
Chartreusin Antimycobacterial
Chloramine-T Topical antiseptic
Cinnamic acid Fragrance
CA 02412892 2003-O1-03
29
Table II (cont'd)
Name Function
Cotarnine chloride . Hemostatic
Demercarium bromide Topical antiglaucoma
D-2-deoxyribose DNA synthesis
Dequalinium chloride Antiseptic
Dermostatin Anti fungal
Dexamethasone Glucocorticoid
Diacetone acrylamide Mfr coatings, adhesives
2,4-Diamino-6-hydroxypyrimidineIndicator of nitrates/nitrites
2,4-DiaminophenoT dihydrochloridePhotographic developer
Diamthazole dihydrochloride Antifungal
Diatrizoate sodium Diagnostic aid
Dibekacin sulfate Antibacterial
Disodium 4',5'-dibromofluoresceinFDA approved dye
3, 5-Dibromo-4-hydroxybenzenesulfonic
acid, sodium salt Topical disinfectant
Dibromopropamidine Cosmetic preservative
Diflorasone Topical anti-inflammatory
Dihydroxyacetone Artificial tanning
agent
Diisobutyl sodium suifosuccinateWetting agent/detergent
DikeguIac Plant growth regulator
Dimethisoquin Topical anesthetic
Diphenicillin sodium Antibacterial
Diphetarsone Antiamebic
Dipyrone Analgesic,. antipyretic
Diquat dibromide Herbicide, defoliant
Dodine Fungicide
Domiphen bromide Topical anti-infective
Dulcin Non-nutritive sweetener
Dymixal~ Topical burn treatment
Ecognidine Topical anesthetic
3 Edetic acid Antioxidant
5
Edoxudine Antiviral
Ellagic acid Hemostatic
Endothal Herbicide, defoliant
Eosine I bluish Dye
Eosine yellowish Cosmetic dye
Erythrosine Food dye
Esculin Skin protectant
Ethacridine Antiseptic
Ethambutol hydrochloride Antibacterial (tuberculostatic)
Ethamsylate Hemostatic
Ethylidene dicoumarol Anticoagulant
CA 02412892 2003-O1-03
Table II (cont'd)
Name Function
5 Ethylstibamine . Antiprotozoal
Euprocin dihydrochIoride Topical anesthetic
Fast green FCF Food coloring
Fenticonazole nitrate Topical antifungal
Ferric albuminate Hematinic
10 Ferric chloride hexahydrate Astringent, styptic
Ferric formate Silage pFeservative
Ferrulic acid, sodium salt Food preservative
Fluorescein, disodium salt Diagnostic aid
Fluoridamid Plant growth retardant
15 Forminitrazol Antiprotozoal (Trichomonas)
Fortimicin(s) Antibacterial
Foscarnet sodium Antiviral (HIV-1)
Fosetyl A1 Systemic fungicide
Fungichromin Topical antifungal
20 Gallic acid Astringent, styptic
Gentian violet Topical anti-infective
Gluconolactone Cleaner
Gossypol Rubber antioxidant
Heparin Anticoagulant
25 Hexamethylolmelamine Fireproofing agent
Mexamidine Antiseptic, anti-acne
Homatropine Anticholinergic (opthtalmic)
Hydrastinine hydrochloride Uterine hemostatic
Hydrocortisone phosphate,
30 disodium salt Glucocorticoid
Hydroquinine hydrochloride hemihydrateDepigmentor
Hydroxyamphetamine hydrobromideAndregenic (opthtalmic)
Hydroxybutyranilide Antioxidant
3-Hydroxycamphor Topical antipruritic
1-(Hydroxymethyl)-5,5-dimethylhydantionCosmetic preservative
8-Hydroxyquinoline sulfate Antiperspirant; deodorant
Iodic acid Astringent
Itraconazole Antifungal
Kanamycin(s) Antibacterial
Kermesic acid Dye
Kojic acid Flavor enhancer
Laccaic acid Crimson dye
Lactic acid Acidulant
Litmus Indicator
L-Lysine L-glutamate Flavor additive
Lyxoflavine Feedstui~, growth-promoter
CA 02412892 2003-O1-03
31
Table II (cont'd)
Name Function
Maclurin Dye
Malachite green Dye
Maltol Flavor enhancer
Maneb Agricultural fungicide
Manganese acetate Mordant
Meralein sodium Topical and-infective
Plus a host of others, including a wide range of inorganic salts.
The list in Table II is by no means exhaustive as it can be readily
determined for each type of particle whether it is capable of farming a
hydrogen bond
or a coordinate covalent bond. All or most of the particles are nonabsorbent,
or not
superabsorbent polymers. Solubility of the particle in water and the binder
can be
easily ascertained, for example in standard chemical reference materials.
The particles listed in Table II have chemical properties that make them
suitable for binding to fibers with the binders of the present invention. The
listed
particles are organic or inorganic compounds that are water soluble, yet have
the
capacity to hydrogen bond. Water solubility is preferably high. By water
soluble it is
meant that more than about '10 g of the particles will dissolve in 300 ml of
water at
25°C. The range of solubilities can extend, for example, from a lower
limit of I0 g in
300 ml of water at 25°C, to an upper limit in which the particle is
miscible in all
proportions with water at 25°C. This high solubility allows the
particles to dissolve
when exposed, to aqueous liquids such as urine, but the hydrogen bonding
capacity
allows them to adhere to the fibers in the presence of binder but in the
absence of
aqueous Liquid during use by an end user after the manufacturing process is
completed.
While bound, the particles substantially retain a discrete particulate form
instead of
dissolving ar fusing, at least until -they are exposed to an aqueous liquid.
Mare of the
particles are discrete rather than agglomerated while bound in the absence of
an
aqueous liquid. If the particles are exposed to fibers with binder in liquid
form, far the
particles to retain their particulate form, a binder is preferably selected so
that the
particles are sparingly soluble in the binder. By sparingly soluble it is
meant that no
more than about 5 g of particles dissolve in 300 mI of the binder at
25°C. Particles
CA 02412892 2003-O1-03
32
may be soluble in the binder as long as a sufficiently small amount of binder
is used so
that an effective portion of the particles remain in particulate form.
The amount of particles added to the fibers can vary widely, far
example from .OS to 80 percent of the total weight of the fibrous material and
particles.
Antimicrobials, such as chlorhexidine or other nonabsorbent particles, are
effective in
very .low amounts, such as .OS to 10 percent. Superabsorbent particles are
preferably
added in an amount of 3-?0 percent, especially 20.40 percent by weight of the
fibrous
materials and particles: The particles may be combined to include more than
one type
of particle, .far example superabsorbent and nonsuperabsorbent particles, or
two types
of superabsorbent particles. When two types of particles are used, the total
weight of
the particles will not exceed 80 percent of the total weight of the fibrous
material and
particles.
VI: Polymeric Binder Characteristics
1 S The particles may be bound to the fibers by a polymeric binder, which
may be water soluble, selected from a predetermined group of polymeric
binders. The
polymeric binders comprise binder molecules, wherein the binder molecules have
at
least one hydrogen bonding functionality or coordinate covalent band forming
functionality. The polymeric binder may comprise repeating units, wherein each
repeating unit of the polymer preferably, but not necessarily, includes at
least one
hydrogen banding functionality or coordinate covalent bond forming
functionality. In
accordance with the present invention, the predetermined groups of polymeric
binders
include the group of binders consisting of palyglycols [especially
poly(propyleneglycol)]; a poiycarboxyic acid, a poIycarboxylate, a
poly(iactone)
polyol, such as diols, a polyamide, a polyarnine, a polysulfonic acid, a
polysulfonate,
and combinations Thereof. Specific examples of some of these binders, without
limitation, are as follows: polyglycols may include polypropylene glycol
(PPG);
poly(lactone) diols include poly(caprolactone) diol; polycarboxylic acid
include
polyacrylic acid (PAA); polyamides include polyacrylamide or polypeptides;
polyamines include polyethylenimine and polyvinylpyridine; polysulfonic acids
or
polysulfonates include poly(sodium-4-styrenesulfonate) or poly(2-acrylamido-
methyl-
CA 02412892 2003-O1-03
33
I-propanesulfonic acid; and copolymers thereof (for example a polypropylene
glycol/polyethylene glycol copolymer). The polymeric binder typically has
repeating
units. The repeating unit may be the backbone of a compound, such as with a
polypeptide, wherein the repeating polyamides occur in the peptide chain. The
repeating unit also may refer to units other than backbones, for instance
repeating
acrylic-acid units. In such a case, the repeating units may be the same or
different.
The binder molecule has a functional group capable of forming a hydrogen bond
or a
coordinate covalent bond with particles, and a functional group capable of
forming a
hydrogen bond with the fibers.
As used herein, a polymer is a macromolecule formed by chemical
union of 5 or more identical or different combining units (monomers). A
polyamine is
a polymer that contains amine functional groups and a polyamide is a polymer
that
contains amide functional groups. Each of the binders has a hydrogen bonding
or a
coordinate covalent bonding functionality. The functionality may be a
hydroxyl, a
carboxyl, a carboxylaLe, a sulfonic acid, a sulfonate, an amide, an ether, an
amine or
combinations thereof. These binders are capable of forming hydrogen bonds
because
they have a functional group that contains an electronegative element, such as
oxygen
or a nitrogen.
The polyglycol has repeating ether units with hydroxyl groups at the
terminal ends of the molecule. The polycarboxylic acid, such as polyacrylic
acid, has a
repeating carboxyl group in which a hydrogen is bound to .an electronegative
oxygen,
creating a dipole that leaves the hydrogen partially positively charged. The
polyamide
(such as a polypeptide) or polyamine has a repeating NR group in which a
hydrogen
may be bound to an electronegative nitrogen that also leaves the hydrogen
partially
positively charged. The hydrogen in both cases can then interact with an
electronegative atom, particularly oxygen or nitrogen, on the particle or
fiber to form a
hydrogen bond that adheres the binder to the particle and fiber. The
electronegative
oxygen or nitrogen of the binder also can form a hydrogen bond with hydrogen
atoms
in the particle or fiber that have positive dipoles induced by electronegative
atoms,
such as oxygens or nitrogens, to which the hydrogen is attached: The polyamide
also
has a carbonyl group with an electronegative oxygen that can interact with
hydrogen
CA 02412892 2003-O1-03
34
atoms in the particles or fibers. Thus, the polymeric binders can enhance the
hydrogen
bonding (a) between the fibers and binder; and (b) in the case of particles
with
hydrogen bonding functionalities; between the binder and the particles.
Alternatively, the polymeric binder may form a coordinate covalent
bond with the particles and a hydrogen bond to the fibers. For example, the
oxygen or
nitrogen on the binder has an unbound pair of electrons that can be donated to
an
empty orbital in the particle to form a coordinate covalent bond. For example,
one
free pair of electrons on the oxygen or nitrogen can be donated to the empty p
orbital
of a boron-containing particle to form a coordinate covalent bond that adheres
the
particle to the binder. The fibers themselves contain functional groups that
can form
hydrogen bonds with the binder, and allow the binder to adhere to the fiber.
Ceilulosic
and synthetic fibers, for example, may contain hydroxyl, carboxyl, carbonyl,
amine,
amide, ether and ester groups that will hydrogen bond with the hydroxyl,
carboxylic
acid, amide or amine groups of the binder. Hence, the polymeric binder will
adhere the
IS particle with a coordinate covalent bond and the fiber will adhere with a
hydrogen
bond.
In some preferred embodiments, the polymeric binder is bound to both
the fibers and the particle by hydrogen bonds. A polypropylene glycol binder,
for
example, can be used to bind water-insoluble polyacrylate hydrogel particles
to
cellulosic fibers. The hydroxyl and ether groups on the glycol binder
participate in
hydrogen-bonding interactions with the hydroxyl groups on the cellulose fibers
and the
carboxyl groups on the polyacrylate hydrogel, as shown below:
CA 02412892 2003-O1-03
3s
)n
s acrylic SAP
° 00 0o ao °
io - H H H H
CH3
4w o~ i)
polypropylene O~ O
9 fY~i
is ~~-H Cf-i~3 n~H
O
2o cellulose
)n
H
2s
Alternatively, a polypropylene glycol (PPG) binder, for example, can be used
to bind a
water-soluble particle to cellulosic fibers. The hydroxyl and ether groups on
the glycol
30 binder participate in hydrogen bonding interactions with the hydroxyl
groups on the
cellulose fibers and appropriate functionalities on the water-soluble
particle, as shown
below:
CA 02412892 2003-O1-03
36
$ EDTA HOOC~ ~H
N ~'''~/N O
HOOC---~
O
H
PPG O ~,~.0
% CH
H 3 H
O ~N
CE1J..ULOSE
Hence, the binder will adhere both the particle and fiber with hydrogen bonds.
The
presence of a hydrogen-bonding functionality on each repeating unit of the
polymeric
binder has been found to increase the number of hydrogen bonding interactions
per-
unit-mass of polymer, which provides superior binding efficiency and
diminishes
separation of particles from the fibers. The repeating ether functionality on
the glycol
binder provides this efi~ciency in the examples diagranuned above. A repeating
carboxyl group is the repeating functionality on poiyacrylic acid, while
repeating
carbonyls and NR, groups (wherein R is either an H or alkyl, preferably lower
alkyl i.e.,
less than five carbon atoms, in a normal or iso configuration) of the amide
linkages are
the repeating fimctionalities on polyamides such as polypeptides. A repeating
amine
group is present on polyamines.
CA 02412892 2003-O1-03
37
The polymeric organic binders of the present invention have been found
to increase in binding efficiency as the length of the polymer increases, at
least within
the ranges of molecular weights that are reported in the examples below. This
increase
in binding ef~tciency is attributable to the increased number of hydrogen
bonding or
S coordinate covalent bonding groups on the polymer with increasing molecular
length.
Each of the polymeric binders has a hydrogen bonding or coordinate covalent
bonding
functionality. If each repeating unit of the polymer has repeating
functianalities, longer
polymers provide more hydrogen bonding groups or coordinate covalent bonding
groups that can participate in hydrogen bonding interactions or in coordinate
covalent
bonds.
Although the invention is not limited to polymeric binders of particular
molecular weights, polymeric binders having a molecular weight greater than
500
grams/mole are preferred because they provide attractive physical properties,
and the
solid is less volatile as compared to low-molecular-weight polymeric binders.
Polymeric binders with molecular weights greater than 4000 grams/mole are
especially
preferred because they have minimal volatility and are less likely to
evaporate from the
fibers. Low-molecular weight materials typically are more mobile than are the
higher-
molecutar weight materials. Low-molecular weight materials can more easily
move to
the fiber-particle interface, and are more easily absorbed by the fiber where
they are
less available to bond the particles to the fibers. The higher molecular
weight materials
are less apt to be absorbed by the fibers, and are less volatile than the low-
molecular
weight materials. As a result, higher molecular weight polymeric binders, to a
greater
extent, remain on the surface of the particles where they are more available
to bond
particles to fibers. In some particular embodiments, polymers with molecular
weights
between 4000 and 8000 gramslmole have been used. Polymers with molecular
weights
above 8000 may be used, but such exceedingly high molecular weight polymers
may
decrease binding efficiency because of processing di~cuities.
Certain polymeric binders have greater binding ef~cieney because their
repeating functionality is a more efficient hydrogen bonding group. It has
been found
that repeating amide groups are more efficient than repeating carboxyl
functionalities,
which are more efficient than repeating hydroxyl functionalities, which in
turn are more
CA 02412892 2003-O1-03
38
efficient than amine or ether functionalities. Hence, polymeric binders may be
preferred that have repeating amine or ether functionalities, more preferably
repeating
hydroxyl functionalities, and even more preferably repeating carbonyl or
carboxyl
functionaiities, and most preferably repeating amide funciionalities. Binding
may occur
at any pH, but is suitably performed at a neutral pH of 5-8, preferably 6-8,
to diminish
acid hydrolysis of the resulting fibrous product. Suitable binders may be
selected from
the group consisting of polyglycols such as polyethylene glycol or
polypropylene
glycol, polycarboxylic acids such as polyacrylic acid, polyamides; polyamines,
poly(lactone) diols, such as poly(caprolactone) diol and combinations or
copolymers
IO thereof.
The group consisting of polycarboxylic acids (such as acrylic acid),
polyamides and polyamines has been found to have a especially good binding
efficiency. Among polyamides, polypeptides are especially preferred.
VII. Non-Polymeric Binder Characteristics
The particles may be bound to the fibers by a nonpoIymeric organic
binder selected from a predetermined group of binders that each have a
volatility less
than water. The vapor pressure of the binder may, for example, be less than I
O mm
Hg at 25°C, and more preferably less than I mm Hg at 25°C. The
non-polymeric
binders comprise non-polymeric binder molecules wherein the molecules have at
least
one functional group that forms hydrogen bonds or coordinate covalent bonds
with the
particles. In accordance with the present invention, the predetermined group
of non-
polymeric binders may include a functional group selected from the group
consisting of
a carboxyl, a carboxylate, a carbonyl; a sulfonic acid, a sulfonate, a
phosphate, a
phosphoric acid, a hydroxyl; an amide, an amine, and combinations thereof
(such as an
amino acid or hydroxy acid) wherein each binder includes at least two such
functionalities, and the two functionalities are the same or different. A
requirement for
the non-polymeric binder is .that it have a plurality of functional groups
that are capable
of hydrogen bonding, or at least one group that can hydrogen bond and at least
one
group that can form coordinate covalent bonds. As used herein, the term "non-
polymeric" refers to a monomer, dimer, trimer, tetramer, and oIigomers,
although
CA 02412892 2003-O1-03
39
some particular non-polymeric binders are monomeric and dimeric, preferably
monomeric.
Particularly preferred non-polymeric organic binders are capable of
forming five or six membered rings with a functional group on the surface of
the
particle. An example of such a binder is an amine or amino acid (for example,
a
primary amine or an amino acid such as glycine) which forms six-membered rings
by
forming hydrogen bonds:
IO
SAP ~'~ey-~4~~~
' H
: , n
~R
or
. saP
o-~ (c-~
r r
H
amino "~,: n
add H'
A six-membered ring also is formed by the hydroxyl groups of
carboxylic acids; alcohols, and amino acids, for example:
R~~O-- __
w0--H-
A five membered ring can be formed by the binder and the functionality on the
surface
of the particle, for example:
CA 02412892 2003-O1-03
5 . OiR
I
H'
H-O' ~ ' O-H
CH-CH
10 Ri
wherein the particle is a water-insoluble particle such as SAP and the binder
is an
alcohol, such as a polyoi with hydroxyl groups on adjacent carbons, for
example 2,3-
15 butanediol. A binder that forms a five-membered ring can also be used with
a water
soluble particle, for example wherein the particle is EDTA and the binder is
an alcohol,
such as a polyol with hydroxyl groups on adjacent carbons, for example 2,3-
butanediol.
Other alcohols that do not form a five-membered ring also can be used,
20 for example alcohols that do not have hydroxyl groups on adjacent carbons.
Examples
of suitable. alcohols include primary, secondary or tertiary alcohols.
Amino alcohol binders are alcohols that contain an amino group (-NR2),
and include binders such as ethanolamine (2-aminoethanol), and diglycolamine
(2-(2-aminoethoxy) ethanol)). Non-polymeric polycarboxylic acids contain more
than
25 one carboxylic acid functional group, and include such binders as citric
acid, propane
tricarboxylic acid, malefic acid, butanetetracarboxylic acid,
cyclopentanetetracarboxylic
acid, benzene tetracarboxylic acid and tartaric acid. A polyol is an alcohol
that
contains a plurality of hydroxyl groups, and includes diols such as the
glycols (dihydric
alcohols), ethylene glycol, propylene glycol and trimethylene glycol; triols
such as
30 glycerin (1,2,3-propanetriol). Esters of hydroxyl-containing binders also
may be used
with mono- and diesters of glycerin, such as monoglycerides and diglycerides,
being
especially preferred. In the case of the diglycerides, at least one of the
esterifying acid
moieties must also include a functional group that is capable of forming at
least one
hydrogen bond with the fibers, or at least one functional group capable of
forming a
35 hydrogen bond or a coordinate covalent bond with the particles. Examples of
CA 02412892 2003-O1-03
4I
polyhydroxy or polycarboxylic acid compounds include tartaric acid or ascorbic
acid
(vitamin C):
H~ your
2
H~C~CH~C~C~C
i 1
C C
CHr OOH
Yrtam~t C
Hydroxy acid binders are acids that contain a hydroxyl group, and
include hydroxyacetic acid (CHZOHCOOH) and lactic, tartaric, ascorbic, citric,
and
salicylic acid. Amino acid binders include any amino acid, such as glycine,
alanine,
valine, serine, threonine, cysteine, glutamic acid, lysine, or ~i alanine.
Sulfonic acid binders and sulfonates are compounds that contain a
sulfonic acid group (-S03H) or a sulfonate (-S03 ). Amino-sulfonic acids also
can be
used. One example of an amino-sulfonic acid binder suitable for the present
invention
is taurine, which is 2-aminoethanesulfonic acid. Non-polymeric polyamide
binders are
small molecules (for example, monomers or dimers) that have more than one
amide
group, such as oxamide, urea and biuret. Similarly, a non-polymeric polyamine
binder
is a non-polymeric molecule that has more than one amine group, such as
ethylene
- diamine, EDTA or the amino acids asparagine and glutamine.
Although other non-polymeric organic binders are suitable in
accordance with the discussion above, the nonpolymeric organic binder is
preferably
selected from the group consisting of glycerin, a glycerin monoester, a
glycerin diester,
glyoxal, ascorbic acid, urea, glycine, pentaerythritol, a monosaccharide, a
disaccharide,
citric acid, taurine, tartaric acid, dipropyleneglycol, urea derivatives,
phosphate,
phosphoric acid, a hydroxy acid; and combinations thereof. The non-polymeric
binder
also is most preferably selected from the group consisting of glycerin, a
glycerin
monoester, a glycerin diester, polyglycerin oligomers, urea and combinations
thereof.
The non-polymeric binders also preferably include functionalities selected
from the
CA 02412892 2003-O1-03
42
group consisting of a carboxyl, a carboxylate, a carbonyl, a sulfonic acid, a
sulfonate, a
phosphate, a phosphoric acid, a hydroxyl, an amine, an amide, and combinations
thereof (such as an amino acid or hydroxy acid). The non-polymeric binders
must have
at least two functianaIities from such group, and the groups may be the same
or
different.
Each of the non-polymeric binders disclosed above is capable of
forming hydrogen bonds because it has a functional group that contains
electronegative
atoms, particularly oxygens or nitrogens, or has electronegative groups,
particularly
groups containing oxygens or nitrogens, and that also include a hydrogen. The
amino
alcohol, amino acid, carboxylic acid; alcohol and hydroxy acid all have a
hydroxyl
group in which a hydrogen is bound to an electronegative oxygen, creating a
dipole
that leaves the hydrogen partially positively charged. The amino alcohol,
amino acid,
amide and amine all have an NR group in which a hydrogen may be bound to an
electronegative nitrogen that also leaves the hydrogen partially positively
charged. The
partially positively charged hydrogen in both cases then can interact with an
electronegative element, such as oxygen ar nitrogen, on the particle or fiber
to help
adhere the binder to the particle and fiber. The polycarboxylic acid, hydroxy
acid,
amino acid and amide also. have a carboxyl group with an electronegative
oxygen that
can interact with hydrogen atoms in the particles and fibers, or in
intermediate
molecules between the binder and particles or fibers. similarly,
electronegative atoms
(such as oxygen or nitrogen) on the fiber or particle can interact with
hydrogen atoms
on the binder that have positive dipoles, and partially positive hydrogen
atoms on the
fiber or particle can interact with electronegative atoms on the binder.
Several proposed hydrogen bonding interactions of two of the binders
(glycine and 1,3-propanediol) with cellulose are shown below:
CA 02412892 2003-O1-03
43
H OH d~i~H H OH
p H H p H
CECWLaSE H H H~ , H ti t v H~ H~ ~ ~ Ht v
p ~ ~ H H ~ p 1~N H'O
H L~~_~_H
',
,'
H'~
H ,
grit=--C:--C--O H
lO H ~ H H H H
(~t,~P~OPMI~IOL
15 The hydrogen bonding interactions are shown as dotted lines. One such
interaction is shown between the nitrogen of glycine and a hydrogen of an -OH
on
cellulose. A hydrogen bond with glycine is also shown between an oxygen of the
-OH
on giycine and the hydroxy hydrogen of an alcohoi sidechain on cellulose.
Hydrogen
bonding interactions of the 1,3-propanediol are shown in dotted lines between
an
20 oxygen on an -OH group of the binder and a hydrogen of an -OH group on the
cellulose molecule. Another hydrogen bond is also shown between a hydrogen on
an
-OH group of the glycol binder and an oxygen in an alcohol sidechain of the
cellulose.
It also is possible for water or other hydrogen bonding molecules to be
interposed between the fiber and binder, such that the fiber and binder are
both
25 hydrogen bonded to the water molecule.
Alternatively, an atom on the binder may have an unbound pair of
electrons, such as a lone pair of electrons from an oxygen or nitrogen atom,
that can be
donated to an empty orbital of an acceptor atom in the particle to form a
coordinate
covalent bond. The free pair of electrons on the oxygen or nitrogen can be
donated to
30 the empty p, d or f orbital of a particle (for example a boron-containing
particle) to
form a coordinate covalent bond that adheres the particle to the binder. The
fibers
themselves do not normally contain functional groups that can act as electron
acceptors in the formation of coordinate covalent bonds with the binders, but
hydrogen
bonding interactions allow the binder to adhere to the fiber. Cellulosic and
synthetic
35 fibers, for example, contain hydroxyl, carboxyl and ester groups that will
hydrogen
CA 02412892 2003-O1-03
44
bond with the hydroxyl, carboxylic acid, amide, amine or other groups of the
binder.
Non-cellulosic or non-synthetic fibers that have these functionalities also
can be used,
for example silk, which has an amide linkage. Hence the binder wilt adhere the
particle
with a coordinate covalent bond and the fiber with a hydrogen bond.
S In some preferred embodiments, the binder is bound to both the fibers
and the pardcIe by hydrogen bonds. A polyol binder, for example, can be used
to bind
polyacrylate hydrogel particles to cellulasic fibers. The hydroxyl groups on
the polyot
binder participate in hydrogen-bonding interactions with the hydroxyl groups
on the
cellulose fibers and the carboxyl groups on the polyacrylate hydrogel. Hence,
the
IO binder will adhere to both the particle and fiber with hydrogen bonds.
These hydrogen
bonds provide excellent binding efficiency and diminish separation of bound
particles
from the fibers.
A structural drawing is shown below in which citric acid, vitamin C and
urea adhere water-insoluble SAP particles to cellulose with hydrogen bonds, or
water-
15 soluble EDTA particles. Some of the possible hydrogen bonding interactions
are
shown as dashed lines. It is possible that other molecules (such as water
molecules)
also may participate in some of these bonds, for example, as an intermediary
between
the binder and particle or fiber.
CH~7H H OH CH~OH H QH
20 0 0
H H H H
CELWtQSE ~ H ~ ~ N ~ ~ ~ H i v ~ H t
O t It H O ~H H O
H ~ ~~ H
..
. ,
. .
;' , .
25 ' '
. ;' . .
,
a o . . ;
.. OH OH \C Oli~. ~ ; H
H ,~l H-Hi .
HOCHt C ~~O\~ ~~ CEO
O OOH acW O~ ~OH ~ H
.
.,
POLYTE , \~C OH O\~OH O\C~OH O\C OH O'C~OH O\C OH
.__ .~~ ~..~ ~-C -CHt-C --Ctii ( __.
H H H H H !i
n
CA 02412892 2003-O1-03
4$
CHipH H OH G i=p~.( H OH
O a
CELLaILpSE H ~ H , ~ ' H~ H ~ H'
$ O ~H H~~~ O ~'H H H~~~O
f_.
:~ :1~
. : ~- .
.' . .
. .
. .
.
;~
.
O
sa~ER ~ o ~ off pc ~', oN' . o
~.lis = HQ~[: C-C~ ~C~O ~Cs.~3
p~pH ~ H ~~ ~ urea
.
.
~
~ .
.
' . ' p ~~ -
Eor~ ~~ ~.~,
0
0
H~
Particularly efficient hydrogen bonding binders include those with
carboxyl groups, such as ascorbic acid, or amide groups; such as urea.
Hydroxyl
groups are also very efficient binders. Amine and ether funetionalities are
less efficient
binders.
Binders have functional groups that may be, selected independently or in
combination from the group consisting of a carboxyl, a carboxylate, a
carbonyl, a
hydroxyl, a sulfonic acid, a sulfonate, a phosphoric acid, a phosphate, an
amide, an
amine, and combinations thereof. These functional groups might be provided by
the
following exemplary chemical compounds: a carboxyl group could be provided by
carboxylic acids, such as ascorbic acid; a carboxylate, which is an ionized
carboxylic
acid, could be provided by a material such as ascorbate; a carbonyl group can
be
provided by an aldehyde, such as ketone; a hydroxyl, such as an alcohol or a
polyol,
such as glycerol, or a mono- or diglyceride, which are esters of glycerol; an
amide,
such as a peptide; and an amine, which may be provided by an alkyl amine, such
as
ethylenimine wherein the binder has at least two of these functional groups,
and each
of the functional groups can be the same (for example, a polyol, polyaldehyde,
CA 02412892 2003-O1-03
46
polycarboxylic acid, polyamine or polyamide) or different (for example, an
amino
alcohol; hydroxyamide, carboxyamide, or amino acid). Functional groups also
may be
selected independently or in combination from the group consisting of
carboxyl, an
alcohol, an amide and an amine. An aldehyde may optionally be a member of each
of
these groups, particularly if it is oxidized to a carboxylic acid.
Combinations of the polymeric and non-polymeric binders, as well as
with other binders, also may be used, providing that they are non-reactive.
That is,
providing that the binders do not react in a manner which prevents the binders
from
possessing the functional groups required to be present for binding in
accordance with
the present invention.
VIII. Process Advantages
The binders of the present invention also provide numerous process
advantages. Binding of particles to the fibers can occur, for example, without
external
application of heat. Hence, if desired, particle binding may occur at ambient
temperature. The present invention therefore is distinct from prior-art
crosslinking
processes in which elevated temperatures are required to covalently crosslink
cellulose
groups to one another. Moreover, the binders of the present invention have the
advantage of being activatable by addition of a fluid, such as a liquid
solvent
(sometimes referred to herein as a activation liquid, one example of which is
water).
Hence, a liquid binder (which would include a solution of a solid or liquid
binder, or a
binder that has a melting point or softening point below room temperature) can
be
applied to a cellulose mat in the absence of the particles to be bound and the
binder
allowed to dry, for example until the fiber product reaches an equilibrium
moisture
content with the moisture in the ambient air. The binders then may be
activated to
bind the particles in place. Some of the binders (especially the liquid
binders) diil'use
throughout the fibers to reach an equilibrium distribution of the binder.
Alternatively,
the binder can be applied as a solid, for example as particles or a powder. At
a later
stage of processing, water or another activating fluid or liquid may be added
to those
portions of the mat where particulate binding is desired. The particles then
may be
added to the mat and adhered to those portions of the mat that have been
moistened.
CA 02412892 2003-O1-03
47
Alternatively, the particles may be added to the mat prior to or
simultaneously with
activation of the binder.
The binders may be liquids at room temperature (such as glycerin), or
liquid solutions of binders that are solids at room temperature {for example,
an
aqueous solution of glycine), or liquid hot melts of solid binders. Solid
binders can be
applied to the fibers as a supersaturated solution or the solid binder may be
heated
above its melting point and applied to the fibers. Upon solidifying the binder
is
deactivated. Solid binders may be added to fibers in particulate form, for
example, by
sprinkling binder particles on the fibers, provided they are fixed by the
subsequent
application of heat or liquid.
The binding reaction of the present invention can occur across a broad
range of pH without requiring a catalyst. A suitable pH range without a
catalyst is
1-14, but preferred ranges are 5-8 or 6-8 because such neutral pH ranges will
produce
fibrous products (such as cellulose products) that are less prone to damage by
acid
hydrolysis. A non-acidic pH (7 or greater) will provide an environment that
inhibits
formation of ester bonds, and promotes formation of the hydrogen bonds or
coordinate
covalent bonds that adhere the particles of the present invention to the
fibers with the
binder.
When water-insoluble particles are used, the moisture content of the
fibers during the binding reaction is 0.5-50%, suitably 5-40%, or preferably 5-
20%
water by weight of the fibers, binder and particle. A moisture content greater
than
20%, preferably 30%, or in the range 20-50%, or 30-50%, can be used even
though
such high moisture contents interfere with intermediate anhydride formation
and
inhibits formation of covalent bonds in the production of high-bulk
crosslinked fibers.
When water-soluble particles are used, the moisture content of the fibers
during the
binding reaction is 0.5-30%, suitably 5-25%, preferably 12-20%. Particles may
be
added to the fibers with the particles distributed throughout a fibrous
product without
being confined to a surface of the product. The particles can be distributed
throughout
the depth of a fiber product such as a mat or web.
The binder suitably is present in the treated product in an amount of at
least 1 percent, and no more than 80 percent, by weight of the fibrous
material
CA 02412892 2003-O1-03
48
("percent by weight"). In especially preferred embodiments, the binder is
present in an
amount of 1 - 80, or more preferably, 1 to 40 or 1 to 25 percent by weight of
the
fibrous material. Below about I percent, when placed on the fiber, an
insufficient
amount of binder is present to achieve adequate binding. Using excessive
amounts of
binder can introduce unnecessary expense into the binding process. High
percentages
of binder can also cause processing problems because the binder material
transfers to
equipment surfaces. Therefore, it is often preferred to use no more binder
than is
required to bind the particles and fibers.
Thermoplastic binders also may be used to help bind fibers to each
other and particles to fibers. The binder that has the hydrogen bonding or
coordinate
covalent bonding functionalities itself may be thermoplastic. The polymeric
binders
and some non-polymeric binders of the present invention have the advantage of
being
thermoplastic solids. Hence, fibers treated in accordance with the present
invention
can be thermobonded by elevating the fiber temperature above the softening
temperature of the binder to soften the thermoplastic binder and
thermoplastically bind
the fibers to each other and the fibers to the particles. Alternatively, an
auxiliary or
second binder can be applied to the fibers as a solid at room temperature, and
the
temperature of the second binder elevated above its softening point to
thermobond-the
fibers and particles. The auxiliary binder may be applied to the fibers either
before or
after the primary binder is applied, but before thermobonding.
The binders of the present invention may be used with fibers that have
substantial intrafiber covalent crosslinks (such as HBA available from
Weyerhaeuser)
or fibers which are substantially free of intrafiber covalent crosslinking.
Examples of
individualized intrafiber crosslinked fibers are seen in European Patent
Applications
440 472 AI and 427 3I7 A2, which produce products that those publications
describe
as being substantially free of interfiber bonds. The fibers of the present
invention do
not need to be processed as in those European applications to eliminate
interfiber
bonds. Binders of the present invention can therefore be used with natural
fibers that
have substantial interfiber bonding, which are defined as fibers that have not
been
processed as in European Applications 440 472 AI and 427 317 A2 to
substantially
CA 02412892 2003-O1-03
49
eliminate interfiber bonds. Cellulose fibers that have not been so processed
are
substantially free of intrafiber bonds.
The fibrous product of the present method (with or without intrafiber
crosslinking) may further be densi~ed by external application of pressure. The
densified product is compact and easily transported. And, when the particles
are
superabsorbent particles, the resulting fibrous product has superior
properties as
compared to nondensified products: The inventors have found that the binders
of the
present invention produce a product that can be easily densified. Easy
densification is
associated with the hydrogen bonds and coordinate covalent bonds formed
between
the binder and the particles and fibers. The fibers are particularly easily
densified when
at least f% by weight of the fibers, particles and binder, more preferably
10%, are SAP
particles adhered to the fibers.
In accordance with this invention, the binders may be applied to fibers
before, subsequent, or simultaneously with addition of the particles.
Simultaneous
addition can be accomplished by two separate streams of particles and binder
that are
simultaneously directed at a fibrous substrate, or alternatively merged
immediately or
some time prior to impacting against the substrate. Without limiting the
invention, it
appears that the addition of small amounts of moisture to the particles may
help bind
superabsorbent particles and perhaps other types of particles to the fibers.
For
example, exposing the particles to air at 65 percent humidity as they are
delivered to
binder containing fibers has been found to enhance the particle bonding.
Binding may be performed under conditions that favor formation of
hydrogen bonds or coordinate covalent bonds, and discourage formation of
covalent
bonds. Conditions that favor covalent bonds are those disclosed in U. S.
Patent No.
4,412,036 and U.S. Patent No. 4,467,012 wherein particle and binder would be
laminated between tissue layers under high temperature and pressure to form
laminated
adherent tissue layers. That patent teaches that minimal adhesion occurs at
200 pli
(pounds per linear inch, as in a calendar press) if no external heat is
supplied, but
adhesion improves as the reaction temperature increases. Improved adhesion of
the
tissue layers occurs because of enhanced covalent bonding as the temperature
increases.
CA 02412892 2003-O1-03
Conditions that favor covalent bond formation are also shown in
European Patent Applications 440 472 Al; 427 317 A2; 427 316 A2; and 429 112
A2.
These European publications use polycarboxylic acid crosslinkers, and require
elevated
temperatures (for example above 145°C) and -acidic conditions (pH less
than 7) to
5 promote formation of intrafiber covalent ester bonds and inhibit reversion
of the ester
bonds. The present invention, in contrast, can form hydrogen or coordinate
covalent
bonds below 145°C, below 100°C; and even at room temperature:
The binders of the
present invention also can bind particles to fibers under neutral or alkaline
conditions,
i.e., at a pH above 7, but preferably at a pH of 5-8 or 7-8. Fibers that have
high bulk
10 from intrafiber covalent crosslinks are prepared by individualizing the
fibers (for
example, in a fiberizer) and curing them at an elevated temperature (above I
SO°C).
Initial application of the binder on such high-bulk fibers preferably occurs
after the
curing step, particularly if the binder is capable of functioning as a
crosslinking
material. The specific types of binders disclosed herein that also can
crosslink are
15 polyols, polyaldehydes, polycarboxylic acids, and polyamines (polymeric or
nonpolymeric binders with more than one amine group). If such binders are
present
during curing, the binder will be consumed during the curing step to form
covalently
crosslinked bonds. When this occurs, the binder is no longer available for
hydrogen
bonding or coordinate covalent bonding, and particle binding to fibers is
ineffective.
20 The intrafiber covalent bond forming processes described in the above
European publications requ'cre formation of an anhydride that then reacts with
a
hydroxy group on cellulose to form a covalent ester bond. The presence of more
than
about 20% water by weight in the fibers is believed to interfere with the
formation of
the anhydride and inhibits covalent bond formation. Hence, in processes that
use
25 polycarboxylic acids, polyols and polyamines (which includes both polymeric
and
nonpolymeric amines having more than one amine group) as binders in the
present
invention, the fibers should contain at least 2Q% water (or 20-50% water) by
weight if
the particles and binder are present in the fibers when curing occurs. The
water
inhibits covalent bond formation, and prevents all of the binder from being
used to
30 form covalent intrafiber crosslinks. Hence, some of the binder remains
available to
CA 02412892 2004-02-17
51
form the non-covalent bonds with the particles and produce ease of
densification in
fiber products made by the process of the present invention.
The present invention, in contrast, produces a product under conditions
. that favor formation of hydrogen or coordinate covalent bonds. Hence, the
particles
can be bound to the fibers in the absence of the external application of heat
or
pressure. Particles also may be bound and the resulting fiber product
densified, for
example at less than 200 pli (about 8000 psi) with SAP, or less than 100 pli
(about
4000 psi) with SAP, in the absence of external application of heat to produce
a product
in which a substantial portion of the particles are bound by non-covalent
bonds
(hydrogen or coordinate covalent bonds). A substantial portion of particles
bound by
non-covalent bonds means at least half of the bonds binding particles to
fibers are other
than covalent bonds, for example, hydrogen or coordinate covalent bonds.
In yet other examples, particles may be bound in the absence of external
application of pressure, but at elevated temperatures.
In particularly preferred embodiments, the particles are substantially
entirely bound to the fibers non-covalently.
IX. Binding Examples for Pol~nneric Binders and Water Insoluble Particles
Several examples are provided below to illustrate using the polymeric
binders within the present invention to attach superabsorbent particles to
southern
bleached kraft pulp.
EXAMPLE 1
A 321 gram amount of NB-41 ~ southern bleached kraft fluff obtained
from Weyerhaeuser Company may be air-entrained in a blender-like mixing device
and
100 grams of poly(caprolactone) diol (average molecular weight 2000, supplied
by
Aldrich Chemical Company of Milwaukee, Wisconsin) dissolved in 100 ml of
deionized water may be sprayed onto the fluff as a binder. Then 435 grams of
starch
graft polyacrylate hydrogel fines (IM 1000F ~ supplied by Hoechst-Celanese of
Portsmouth, Virginia) may be added and mixed. The product may then be removed
from the blender, and spread out in a fume hood to dry overnight. The
resulting
#Trademark
CA 02412892 2004-02-17
52
product may then be airlaid on a small airlay line, from M & J Machines (of
Horsens,
Denmark) and thermobonded at 140°C for one minute to produce a web
containing
40% superabsorbent particles (SAP) attached to the individualized fibers. This
binder
has a low melting point, hence raising the temperature to 140°C melted
the binder and
allows it to flow over the fibers and particles to enhance hydrogen bonding
interactions, thereby further binding the fibers and particles. This is an
example of
activating a solid binder by heating it, without liquid addition. A
polypropylene
glycoUpolyethylene glycol copolymer binder would also behave in this manner.
EXAMPLE 2
A 321 gram amount of southern kraft fluff was air-entrained in a
blender-Like mixing device and 154 grams of a 65% solution of polyacrylic acid
(average molecular weight = 2,000; supplied by Aldrich Chemical Company of
Milwaukee, Wisconsin) diluted with 100 ml of deionized water was sprayed onto
the
1 S fluff. Then 435 gams of polyacrylate hydrogel (FAVOR 800 supplied by
Stockhausen
of Greensboro, North Carolina) was added into the mixing device and mixed with
the
fluff and polyacrylic acid binder. The product was removed and spread out to
dry and
then fed to a hammermill with a three-eighths inch round hole screen and
shunted to a
small airlay line to produce a web containing 40% SAP attached to the
individualized
fibers.
EXAMPLE 3
A 321 gram amount of southern bleached kraft fluff is air-entrained in a
blender-like mixing device and 100 grams of polyglycine (molecular weight =
5,000-
15,000; supplied as a dry powder by Sigma Chemical Company of St. Louis,
Missouri)
diluted with 100 ml of deionized water is sprayed onto the fluff. Then 435
grams of
starch graft polyacrylate hydrogel fines (IM1000P~ supplied by Hoechst-
Celanese of
Portsmouth, Virginia) is added and mixed. The product is removed and spread
out in
a fume hood to dry overnight. The resulting product is fed into a Fitz
hammermill with
a three-eighths inch round hole screen and shunted to a small M & J airlay
line to
produce a web containing 40% SAP attached to the fibers.
#Trademark
CA 02412892 2003-O1-03
S3
EXAMPLE 4
A 321 gram amount of southern bleached kraft fluff is air-entrained in a
blender-like mixing device and 200 .grams of a SO% solution of
polyethyleneimine
S (molecular weight = 50,000-100,000; supplied by ICN Biomedicals, Inc. of
Costa
Mesa, California), or polyvinyl pyridine is sprayed on the fluff. Then 43S
grams of
starch graft polyacrylate hydrogel fines (IM1OOOF; supplied by Hoechst-
Celanese of
Portsmouth, Virginia) is added and mixed. The product is removed and spread
out in
a fume hood to dry overnight. The resulting product is fed into a Fitz
hammermill with
a three-eighths inch round hole screen and shunted to a small M & J airlay
line to
produce a web containing 40% SAP attached to the fibers.
The classes of polymeric binders that encompass those described in
Examples 1-4 are especially preferred over other multiple hydrogen bonding
functionality polymers for a number of reasons. One important reason is that
their
1 S functionalities produce very strong, effective hydrogen bonding. Other
important
reasons include their relative lack of activity (as compared with
polyaldehydes or
polyisocyanates) and their Iow toxicity (again, as compared with polyaldehydes
or
polyisocyanates).
EXAMPLE S
As previously described, repetition of a hydrogen bonding group on
each repeating unit of a polymer has been found to produce a binder that
provides
superior binding of particles to fibers, as compared to polymeric binders in
which the
hydrogen bonding functionality is not present on all the repeating units. This
example
2S shows the difference in binding efficiency between a 2U% carboxylated
polymer and a
100% carboxylated polymer. A bound sample was prepared as in Example 1 using a
20% carboxylated ethylene acrylic acid copolymer and a 100% carboxylated PAA.
A
sample of each was subjected to the same mechanical agitation (to simulate
machine
processing required to make a web); screened through a descending series of
sieves to
remove unattached SAP, and subjected to an absorbent capacity test (less
attached
SAP would result in a lower absorbent capacity). The result of the test was
measured
CA 02412892 2003-O1-03
54
by weighing the unabsorbed liquid (0.9% saline) from a standardized result. A
lower
number indicates more liquid absorbed, which corresponds to a higher absorbent
capacity.
A sample of the 20% carboxylated polymer (15% of the total mix) gave
a beaker test result of 19.5 grams. A similar sample of polypropylene glycol
would
give a result of about 20.0 grams. However, the hydrogen bonding functionality
of
PPG is not as efficient as the carboxyl functionality of PAA. A similar sample
of
polyacryIic acid (I00% carboxyl functionality of PAA) gave a result of 11.3
grams. A
comparison of the 20% and 100% carboxylated polymers shows a substantial
increase
in SAP binding efficiency, as measured by an increase in absorbency of the
product.
X. Non-Polymeric Binding Examples
Several examples are provided below to illustrate the use of several
non-polymeric organic binders of the present invention to attach
superabsorbent
particles to southern bleached kraft pulp. Several examples of binder
activation and
activation also are provided.
EXAMPLE 6
A 3171 gram amount of southern bleached kraft fluff was air-entrained
in a blender-like mixing device and 1000 grams of glycerin (96%, USP; supplied
by
Dow Chemical Co. of Midland, MI) diluted with 300 mI of deionized water was
sprayed onto the fluff. Then 4348 grams of starch graft polyacrylate hydrogel
fines
(ll~i1000F; supplied by Hoechst-Celanese of Portsmouth, VA) were added to the
mixing device and mixed with the fluff' and binder. The material was then
shunted into
a flash tube dryer at 142°F, blown into a cyclone and fed into a Danweb
airlay machine
to form a web containing bound 40% IM1000F that is substantially immobile in
the
web because the particles are bound to the fibers instead of mechanically
entrapped by
the matrix. Glycerin is advantageous because it tends to penetrate the fibers
and soften
them in addition to binding the particles to the fibers. However, over time
less glycerin
is available at the surface of the fibers for use in binding particles in the
event the
glycerin/fiber material is stored for long periods prior to use in adhering
particles (e:g.
CA 02412892 2003-O1-03
if activation is delayed for several weeks or more). This can be compensated
for in
part by using higher percentages of glycerin on the fibers. Also,
monoglyceride and
diglyceride binders do not penetrate as readily into the fibers and therefore
can be
stored longer before activation to adhere particles.
EXAMPLE 7
A 900 gram amount of southern bleached kraf3 fluff pulp sheet was
sprayed with a 50% solution of glycine (supplied as a dry powder by Aldrich of
Milwaukee, WI) so that the moisture content was 17-21 % as the sheet was fed
into a
Fitz hammermill fitted with a three-eighths inch hole screen. Starch graft
polyacrylate
hydrogel fines (nvIl000F; supplied by Hoechst-Celanese of Portsmouth, VA) were
simultaneously added to the mill by a screw feed device, mixed with the fluflc
shunted
to an M & J airlay forming machine and airlaid to foirn a web. The web that
resulted
contained 20% SAP attached to the fibers substantially uniformly throughout
the web
without being confined to a surface of the web:
EXAMPLE 8
A 900 gram amount of southern bleached kraft fluff pulp sheet was
sprayed with'a 50% solution of pentaerythritol (supplied by Aldrich of
Milwaukee, WI)
so that the moisture content was 17-21 % as the sheet was fed into a Fitz
hammermill
fitted with a three-eighths-inch hole screen. Starch graft polyacrylate
hydrogel fines
(IM 1000F; supplied by Hoechst-Celanese of Portsmouth, VA) were simultaneously
added to the mill by a screw feed device, mixed with the fluff, shunted to an
M & J
airlay forming machine and airlaid to form a web. The web that resulted
contained
20% SAP attached to the fibers.
EXAMPLE 9
A 900-gram amount of southern bleached kraft fluff pulp sheet was fed
into a Fitz hammermill fitted with a three-eighths-inch hole screen. The sheet
was
defiberized, shunted to an M c~c J airlay line, and airlaid into a web. As the
web
emerged, target zones of the web were misted with a 50% solution of lactose to
raise
CA 02412892 2003-O1-03
the moisture content to 17-21 %. Five gram aliquots of starch graft
polyacrylate
hydrogel fines (IM1000F; supplied by Hoechst-Celanese of Portsmouth, VA) were
subsequently sifted onto the target zones: The web that resulted contained
target
zones with 5 grams of SAP attached to the fibers of each target zone. Portions
of the
web that were not targeted for lactose application did not adhere the
particles welt.
This is an example of applying the binder to a target zone so that SAP
primarily
adheres to the target areas where the binder was applied. Target-zone
application of
SAP can be advantageous because it reduces the cost of the product to provide
SAP
only in areas of a product where the SAP is needed, for example, the crotch
area of a
diaper. Placement of SAP in the area where a liquid insult is expected also
decreases
the necessity for wicking liquid to a SAP impregnated region. This is an
advantage
because the requirement for wicking can increase liquid leakage iri an
absorbent
product such as a diaper.
EXAMPLE 10
A 321 gram amount of southern bleached kraft fluff was air-entrained in
a blender-like mixing device and 100 grams of glycerin (96%, USP; supplied by
Daw
of Midland, MI) diluted with; 30 ml of deionized water were sprayed onto the
fluff. 71
grams of Aliscents (an odor absorbing zeolite supplied by UOP of Tarrytown,
NY)
was then added and mixed in the mixing device with the fibers and glycerin for
15
seconds until a homogenous mixture was achieved. The material was then spread
out
in a fume hood overnight to dry, airlaid into a web and tested for particulate
retention
by an ash test. The pad so produced contained 7% particulate. The original
addition
amount should have produced 15%, hence 50% particle retention was observed.
This
compares favorably to particulate retention with latex binders under similar
conditions
in which only about 3% of particles are retained.
XI. Binding.Exampies for Water-Soluble Particles
Several examples are provided below to illustrate using binders of the
34 present invention to attach water-soluble particles to southern bleached
kraft pulp.
CA 02412892 2003-O1-03
57
EXAMPLE 11
A 321 gram amount of NB-416 southern bleached kraft fluff obtained
from Weyerhaeuser Company (Tacoma, Washington) was air-entrained in a biender-
like mixing device and 50 grams of glycerin (supplied by Dow Chemicals of
Midland,
lVflchigan) were sprayed onto the fluff Then 288 grams of disodium
ethylenediamine
tetraacetic acid (EDTA) (supplied by Mallinkrodt Chemical Works of St. Louis,
l~ssouri) were added and mixed in the device. The blender was stopped, the
product
was vacuumed out, and spread out in a fume hood to dry overnight. The
resulting
product was examined by scanning electron microscope and revealed disodium
EDTA
particles attached to fibers.
EXAMPLE 12
A 321 gram amount of HBA pulp (a crosslinked high bulk fiber
I S available from Weyerhaeuser Company, Tacoma; Washington) was air-entrained
in a
blender-like mixing device and 50 grams of glycerin (supplied by , Dow
chemical of
Midland, Michigan) were sprayed onto the fluff. Then 288 grams of sodium
bicarbonate (supplied by J.T. Baker Chemical Co. of Phillipsburg, New Jersey)
were
added and mixed: in the device. The blender was stopped, the product was
vacuumed
out, and spread out in a fume hood to dry overnight. The resulting product was
examined by scanning electron microscope (SEM) and revealed fibers with
attached
sodium bicarbonate particles.
EXAMPLE 13
An NB 416 pulp sheet (southern bleached kraft available from
Weyerhaeuser Company of Tacoma, Washington) was treated with glycerin on a
roll
coater so that the product contained 10% glycerin by weight. That pulp sheet
was fed
into a hammermiii and ground while simultaneously adding a polyacrylate
hydrogel
(IM 3900, supplied by Hoechst Celanese of Portsmouth, Virginia) and
ethylenediamine
tetraacetic acid to the mill at rates such that the product contained 54%
treated fiber,
42% IM 3900, and 4% EDTA. That mixture was shunted to an airlay device from M
CA 02412892 2003-O1-03
5g
8t J Machines (of Horsens, Denmark) and airlaid into a continuous web. The
resulting
product was examined by scanning electron microscope and revealed fibers with
attached polyacrylate hydrogel and EDTA particles.
EXAMPLE 14
A procedure similar to the one described in Example 13 was performed
using KittyHawk pulp (a thermobondable blend of southern bleached kraft and
polyethylene fibers available from Weyerhaeuser Company of Tacoma,
Washington).
The resulting product was thermobonded by passing the web through a through-
air
oven at 140°C for 0.5 minutes. The resulting product was examined by
scanning
electron microscope, and revealed fibers with attached polyacrylate hydrogel
and
EDTA particles.
EXAMPLE 15
In this example, oxalic acid is bound to the fibers by the binders of the
present invention. A pulp sheet with 10% binder was prepared as in Example 13.
The
pulp sheet was conditioned at 90% relative humidity for 4 hours, then the
sheet was
fiberized in a Waring blender. Particles of oxalic acid were then added to the
blender
and blending continued. The product was dried and an SEM obtained, which is
shown
in FIG. 14. The feathery particle of oxalic acid is shown near the center of
the
photograph bound to the cellulose fiber by the glycerin.
EXAMPLE 16
Fibers were prepared as in Example 15, except aluminum sulfate (alum)
was substituted for oxalic acid. The SEM of alum bound to the fibers is shown
in FIG.
15.
EXAMPLE 17
A mixture of binders also may be used to bind particles to the fibers.
Fibers may be supplied as in Example I1, but the 50 grams of glycerin would be
substituted with a mixture of urea and glycerin. A 40/60 mixture (by weight)
of urea
CA 02412892 2003-O1-03
59
and glycerin is mixed by dissolving urea in the glycerin, and heating the
solution to 70-
80°C. The heated binder mixture is then applied to bind the particles
to the fibers as in
Example 11. The urea/glycerin mixture provides several advantages over the use
of
glycerin alone. Urea lowers the cost of the binder, while glycerin softens the
fibers.
The mixture also provides manufacturing advantages.
In other embodiments urea alone as well as the other binders of the type
specified in the foregoing detailed description of the invention and
combinations
thereof may be used as the binder.
XII. Product Characteristics
The following examples illustrate how SAP retention, pad integrity,
wettability, bulk and liquid retention are affected by the glycerin binder of
the present
invention.
EXAMPLE 18
Superabsorbent particles were bound to cellulose fibers with a glycerin
binder, as described in Example 6 above. For purposes of comparison,
superabsorbent
particles were bound to a separate sample of cellulose fibers using a
polyvinyl acetate
(PVAc) binder that was about 3% carboxylated, that is only about 3% of the PVA
monomers were carboxylated. Binding was performed as in Example 6, but PVAc
was
substituted for glycerin. A 100-gram sample of the glycerin and PVAc treated
fluff
with attached SAP was fed into a fan that was connected by a hose to. a small
cyclone
mounted on top of a material containment box. This was done in an effort to
simulate
forces of mechanical agitation the fluff would encounter during the airlay
process.
After collection in the material containment device, fiber with attached SAP
was
removed and weighed: A five gram sample of the fiber with attached SAP was
then
placed in a column of sieves with decreasing mesh sizes and subjected to a
shaking and
thumping action for ten minutes in order to further dislodge any poorly
attached SAP.
Unattached or poorly attached SAP sifted through screens having a range of 5-
60
mesh, while the fiber with well attached SAP remained on the 5 mesh screen.
CA 02412892 2003-O1-03
A 2.00 gam sample of the fibers that remained near the top of the sieve
column was then placed in a 75 ml sample of 0.9% saline for exactly one
minute. After
that minute, the liquid that was not absorbed was poured ofd' into a separate,
tared
beaker and weighed. The relative amounts of liquid absorbed is indicative of
the
S amounts of SAP bound to the fiber. Fiber retaining higher amounts of SAP
tend to
absorb more liquid and give a smaller amount of liquid not absorbed. These
results are
shown in Table III:
TABLE III
10 Glycerin Binder
Comparing SAP Retention with Glycerin and PVAc Binders
Binder Beaker result
40-504
VAc 22.8
3666H
VAc 22:0
GI cerin 5:5
Table III illustrates that the glycerin binder provides a product that has
an absorbency increase of 400% compared to the PVAc binder. A substantial
portion
15 of-this improvement is believed to be due to better adhesion between the
fibers and
SAP, such that the particles are not dislodged from the fibers.
EXAMPLE 19
Pad integrity was compared in fibrous products that used no binder and
20 a glycerin binder at 7% and l l% by weight. Each of these binders was used
to bind
SAP to fibers as in Example 6, and properties of the pad were measured and are
shown
in Table IV:
CA 02412892 2004-02-17
61
TABLE IV
Tensile Results
Pad integrity (low density):
Sam le Basis Wei ht Densi Tensile Index
NB-416 464 gsm 0.12 g/cc 0.257 Nm/g
control
NB-416/T/o 437.6 gsm 0.126 g/cc 0.288 Nm/g
Gl cerin
NB-416/11 % 402.5 gsm 0.13 5 g/cc 0. 53 8 Nm/g
Gl cerin
Pad Integrity (high density):
Sam le Basis Wei ht Densit Tensile Index
NB-416 482.1 gsm 0.218 g/cc 0.475 Nm/g i
control
NB-416/7% 460.7 gsm 0.219 g/cc 0.882 Nm/g
Gl cerin
NB-416/11% 421.6 gsm 0.248 g/cc 1.536 Nm/g
Gl cerin
The glycerin binder in this example produced a product that had a
higher tensile index than an untreated product. The increased tensile strength
was
especially enhanced in the densi8ed (high density) product, and particularly
when at
least 11% of the binder was used.
EXAMPLE 20
The effect of binders on the wettability and bulk of fibers was tested
using the following fibers: NB-318'(a standard southern bleached kraft pulp
with no
1 S binder) ; GNB#as used herein is an NB pulp (a standard southern bleached
kraft pulp)
with 25% glycerin (entrained and sprayed); HBA#pulp (a high bulk intra-fiber
crosslinked fiber available from the Weyerhaeuser Company that contains
intrafiber
covalent crosslinks); and GHB~ as used herein is I3BA fibers treated with a
glycerin
binder in amounts of 12.5% and 25% by weight. Results are given in Tables V
and VI.
FAQ time was determined by airlaying a specific quantity (4.00 grams)
of the fluff to be tested into a clear plastic tube that was fitted with a
screen at one end.
The fluff and tube were then placed into a well in the test device and a metal
plunger
#Trademark
CA 02412892 2003-O1-03
62
was lowered onto the fluff and the pad's bulk calculated. Water then flowed
from
underneath the pad, passed through the screen and wicked up through the pad.
Absorbency time was measured from when the liquid makes contact with the
bottom
screen until the water completes an electrical circuit by contacting the foot
of the
plunger resting on top of the pad. Lower absorbency times indicate better
absorbency.
Since the absorption of the liquid by the pad was accompanied with some
collapse of
the pad's structure, the bulk of the wet pad was then recalculated. The amount
of
liquid absorbed was then measured and a gram-per-gram capacity for the
material was
calculated.
ZO Table V gives FAQ time as a measure of wettability. A lower FAQ
time indicates a product that is more absorbent and wicks faster. Table VI
gives wet
bulk of fibers and the adjusted bulk of the fibers. The adjusted bulk is a
calculated
number obtained by dividing the bulk by the actual percent of pulp in the
sample.
TABLE V
Wettability
Fiber FA time
NB-3I6 3.0 sec
GNB 25% 3.2 sec
HBA 13.5 sec
GHBA I2:5% 4.5 sec
GHBA 25% 0.4 sec
CA 02412892 2003-O1-03
63
TABLE VI
Bulk
S
Fiber Wet Bulk Ad'usted Bulk
NB-316 12.7 cc/ 12.7 cc/
GNB 2S% 10:9 ccl 14.5 cc/
HBA 19.4 cc/ 19.4 cc/
GHBA 12.5% 16.1 cc/ 18.4 cc/
GHBA 2S% 14.9 ccJg 19.9 cc/g
The low FAQ times (Table V) in the glycerin-treated fibers (GNB,
GHBA) show that wettability is as good as the untreated fiber (NB-316). The
GHBA
25% had significantly better wettability than untreated HBA pulp. Bulk of
glycerin
treated fibers (Table VI) was not significantly decreased or changed at all
levels of
glycerin binder on a fiber to fiber comparison basis.
EXAMPLE 21
Liquid retention of bound fibers was determined and compared to fibers
in which no binder was added. NB-316 is a pulp sheet available from
Weyerhaeuser
1 S Company in which no binder is used. I-iBA pulp is described in Example 20.
HBA/GIy SAP was an HBA pulp fiber that was bound with glycerin (12% binder,
48%
fiber) and which contained 40% SAP particles. NB-316/Gly SAP is NB-316 fibers
to
which glycerin and SAP fibers were added.
The procedure for determining liquid retention was to weigh triplicate
small portions (near 0.2 grams) of samples to the nearest 0.0001 gram and then
heat-
seal the small portions inside an envelope of a heat-sealable, nonwoven tea
bag. The
samples were then immersed in an excess of 0.9% saline for thirty minutes,
then
drained by suspending them from a clip for fifteen minutes. The samples were
weighed
to determine the amount of liquid absorbed. The grams of liquid absorbed per
gram of
2S sample were calculated and the samples were spun in a centrifuge for one
minute. The
sampies were then reweighed and a percent-liquid-retention was calculated.
Results are shown in the following Table VII:
CA 02412892 2004-02-17
64
TABLE VII
Liquid Retention (after centrifueel
FiberBinder % Retention
NB-316/none less than 1
HBA/none - less than 1
HBA/Gl SAP 23%
NB-316/Gly 31.5%
SAP
The results in Table VII illustrate that fibers that have SAP bound to
them retain liquid well, while fibers without SAP retain liquid poorly. The
glycerin
binders provided excellent adherence of SAP to the fibers.
XIII. Auxiliary Binder
As previously described, an auxiliary binder or additional binder or
binders can be used in addition to the non-polymeric or polymeric binders or
combinations thereof in accordance with the present invention. However, the
additional binders) is selected to not react with the binder or binder
combination of
the present invention in a manner which prevents this latter binder from
having the
required functionality. Thus, the preferred auxiliary binders are non-reactive
in this
way. In addition, polymeric and non-polymeric binders of the invention may be
combined with one another and with other binders as long as they do not react
to block
the desired functionality.
EXAMPLE 22
A 321 gram amount of a southern bleached kraft fiber (NB-41(i;
supplied by Weyerhaeuser) was air entrained in a blenderlike mixing device and
sprayed with 212.8 grams of a polyvinyIacetate latex (PN-3666H, supplied by
H.B.
Fuller of Minneapolis, Minnesota). While still mixing, 438 grams of a water
swellable
polyacrylate hydrogel (Favorsab 800; supplied by Stockhausen of Greensboro,
NC)
ZS was added and the resulting nuxture was then sprayed with 100 grams of a
50%
solution of glycerin (supplied by Dow of Midland, Michigan). The blender was
then
stopped and the mixture was vacuumed from the blender and placed in a fume
hood to
air dry overnight. The dried product was then airlaid into a 6" diameter pad
in a
xTrademark
CA 02412892 2003-O1-03
laboratory padformer, pressed to a density of approximately 0.077 g/cc, and
thermobonded at 140°C for thirty seconds. The resulting pads had 40%
bound SAP
and improved tensile strength as compared to untreated fluff with SAP and as
also
compared to binder treated fluffwith $AP without the auxiliary binder.
5 Tensile strength was highest with polyvinylacetate atone, followed by a
combination of polyvinylacetate and glycerin, then glycerin alone. , Lowest
tensile
strength was seen with no binder at all.
EXAMPLE 23
10 Binders of the present invention may be used to bind particles to pulp
fibers that contain synthetic thermobonding fibers. In this example, Kittyhawk
pulp
(available from Weyerhaeuser Company) is a mixture of NB316 southern bleached
kraft and 22% polyethylene thermoplastic binder fibers. The Kittyhawk pulp is
used to
produce a pulp web, with SAP bound to the fibers as described in Example 3.
The web
15 with adhered SAP is then passed through a thermobonder to soften the
polyethylene
fibers and fuse the fibers of the web to each other to increase web strength.
XIV: Spectroscopic Evaluations
Spectroscopic measurements were made of the fiber products made
20 according to the present invention. The results of the NMR and IR studies
are
presented below.
A. NMR Analysis
EXAMPLE 24
25 Solid sample 13C NMR spectra were obtained on cellulose fibers treated
with ascorbic acid to bind SAP to the fibers. An NMR spectra also was obtained
on L-
ascorbic acid. In both cases; separate spectra were acquired using recovery
delays of 1
sec and 5 sec between acquisitions.
The peaks in the treated-fiber spectrum were assigned readily to the
30 components: SAP polyacrylate carboxyl (185 ppm) and backbone (50-30 ppm)
carbons; cellulose ( 106, 90; 84, 76, 73 and 66 ppm); and ascorbic acid ring
carbons C-
CA 02412892 2003-O1-03
66
1, C-2 and C-3 (175, 119 and 156/153 ppm, respectively); the other ascorbic
acid
carbons are in the cellulose region, two of them being resolved at 69 and 61
ppm. The
ascorbic acid carbon chemical shifts is this ternary mixture were essentially
identical
0.2 ppm) to their values in pure ascorbic acid. This indicated that the
ascorbic acid
in the treated fibers had undergone no gross structural changes, such as total
neutralization, oxidation or ring opening.
The signal-accumulation rates observed at the two different recovery
delay times showed that the proton spins in pure ascorbic acid relaxed after
excitation
much more slowly than they did in the ternary mixture. As shown in the
following
IO table, slow relaxation yields higher signal strength at the long recovery
delay relative to
the short one. The fast proton spin-lattice relaxation in the coated fibers
indicated that
the ascorbic acid in this system is held more tightly in place (i.e., is less
mobile) than in
the bulk acid. The ascorbic acid apparently-is held tightly by one or both of
the other
two components, cellulose and SAP, and not by other ascorbic acid molecules.
I S If the bonding were purely ionic, involving ascorbate ion and an acrylic
acid unit in the SAP, then the NMR of the treated fibers would show the
ascorbic acid
in the salt form. NMR reference spectra were found of the acid and its salt in
aqueous
solution, and C-3 is seen to shift dramatically on ionization of its OH group:
156 ppm
in the acid to 176 ppm in the salt. Thus, since the 1VMR spectrum of the
ternary
20 mixture contains the peaks at around 156 ppm, the ascorbic acid in this
system is not
ionized.
Looking at acidities, ascorbic and polyacrylic acids have nearly identical
pK, values (4.2 vs 5, respectively). They are both typical strong organic
acids with
weak conjugate bases. Thus, there is no compelling reason for one of these
acids to be
25 neutralized (ionized) by the conjugate base of the other acid. Rather,
there should be a
strong tendency for an ascorbic acid and an acrylate ion to share a hydrogen
ion
between them, resulting in a long hydrogen bond between partially ionic
ascorbic and
acrylic acid units. This sharing of hydrogen ions would certainly be reflected
in the IR
spectrum, yet satisfies the NMR data by not invoking full ionization of
ascorbic acid.
30 The spectroscopic data are fully consistent with a hydrogen bonding
mechanism between ascorbic acid and an acrylate unit in the superabsorber.
CA 02412892 2003-O1-03
67
Acrylic Acid NMR Amplitude Ratios at Different
Recovery Delay Times.
Signal Ratio. 5 sec/1 sec
Peak Frea.. onm Treated Fibers Pure Acid
176 1.99 5.21
156 1.92 --
153 1.80 5.35
119 2. l U 4.26
B: Infrared Analysis
EXAMPLE 25
Fibers With Superabsorber
And Ascorbic Acid
Infrared transmission spectra of the untreated NB316 pulp, the treated
NB316 pulp, ascorbic acid, and the IM 1OOF superabsorber were prepared. Then,
a
subtraction spectrum representing the treated pulp minus the untreated control
was
obtained.
Examination of that subtraction spectrum indicated several infrared
bands that obviously were associated with the ascorbic acid. They were evident
at
1755, 1690 (shifted slightly from 1660-1670), 868, 821, and 756 wave numbers
(crri 1).
However, several other bands that were prominent in the ascorbic acid spectrum
were
absent in that subtraction spectrum. They included the following: 3525, 3410,
3318,
1319, 1119, and 1026 crri'.
° The higher frequency bands (3300-3600 crri') in ascorbic acid are
indicative of bonded OH groups. The infrared bands at 1319, 1119, and 1026 cm'
may also be associated with OH vibrations. Consequently, the IR suggested that
the
subtraction spectrum reflected primarily a loss of the OH groups that were
attached
directly to the ring. A likely possibility is that the OH groups were replaced
by
sodium: The only other major band in the subtraction spectrum was located at
1589
cm 1. This was probably due to the superabsorber C=O which had shifted to a
slightly
higher frequency (from 1562 cm 1).
CA 02412892 2003-O1-03
68
The infrared spectra, point to substantial disruption in the structure of
the ring OH groups, comparing pure ascorbic acid with the treated fibers, with
the
ascorbic acid in the mixture resembling ascorbate salts in having some of the
OH
stretching bands missing.
XV. Activation
The binders of the present invention have the advantage of being
activatable from an inactive state on the f hers by addition of liquid,
heating, or by
kinetic energy such as may be supplied by mechanical agitation, pressure, or
ultrasonics. Hence, a liquid binder can be applied to cellulose fibers, loose
or in
another form, such as a cellulose mat, in the absence of the particles to be
bound. The
binder is then dried or allowed to dry, for example until the binder and fiber
reach an
equilibrium moisture content with ambient air. Alternatively, the binder can
be applied
as a solid, for example, particles sprinkled onto a fiber mat. At a later
stage of
processing, a liquid such as water is added to the fibers resulting in an
activation of the
binder. The particulates may then be added, and the binder secures the
particulates to
the fibers. This subsequent processing of the fibers to attach the particles
can occur,
for example, at a separate location from the location where the. binder was
applied to
the fibers. Therefore, manufacturers of products can add particulates of
interest (e.g.,
superabsorbent particles or fibers; antimicrobial particles, etc.) at the
place of
manufacture of the end products that incorporate the treated fibers. Also,
more than
one type of particulate material (including water soluble and water insoluble
particles)
may be added, if desired. Particles without the required functionality would
not be
bound in the same manner.
It also has been found that some of the binders of the present invention
can be activated by mechanical agitation (the application of kinetic energy).
For
example, glycerin binder may be applied to fibrous cellulose. The glycerin
binder may
be allowed to dry, and the fibers then mechanically agitated in the presence
of
superabsorbent particles and/or other particles to activate the glycerin
binder and bind
the particles to the fibers. Mechanical agitation may take place, for example,
in a
defiberizer where a sheet or mat of glycerin treated cellulose fibers are
defiberized
CA 02412892 2003-O1-03
69
while being intimately mixed with SAP that is bound to the fibers by the
mechanical
agitation.
XVI. Binder Activation Examples
Binder activation in the present invention allows binder to be added to
fibers either before or after particles are added to the fibers. The binder is
subsequentIyactivated by addition of liquid; heat, orby kinetic energy such as
resulting
from agitation, and particles are bound to the fibers. The particles may be
added to the
fibers either before binder activation, after binder activation, or
simultaneous with
activation. If SAP and/or other particles are to be added to cellulose fibers,
for
example, the binder may be applied to a pulp sheet which is subsequently
fiberized. A
liquid such as water may be added to the pulp before or after fiberization,
and SAP
may be added before or after water addition, or simultaneously with the water.
If SAP
is added after water addition, the SAP should be applied to the fibers prior
to complete
evaporation of the added water from the fibers. Water also can be added in
other
ways, such as by very humid air; a fog or mist, or as steam.
Activafton can be of all the fibers, or only portions of the fibers, such as
target zones or portions of the mat where particulate binding is desired. The
particles
may then be added to the mat and adhered to the target zones of the mat which
have
been activated. In some embodiments, the binder is applied as a solid and
heated
during a later processing stage to activate the binder by softening it such
that it binds
the particles to the fibers. The particles may be added in a pattern
corresponding to a
desired distribution (for example a non-homogeneous distribution) of particles
in the
fibrous material. Most commonly, however, activation is accomplished by using
a
binder solvent to moisten a targeted area of the product into which an
inactive (dry or
dried) binder has already been introduced.
In yet other embodiments, the binder is applied to the fibers and then
activated by applying kinetic energy to the fibers. Neat polypropylene glycol
(MW
2000) binder, for example, may be sprayed on fibers and allowed to dry.
Desired
particles are then added to the fibers as the fibers are mechanically agitated
in a blender
or defiberizer to kinetically activate the binder and bind the particles to
the fibers. For
CA 02412892 2003-O1-03
kinetic activation, the binder may be added as a liquid or a solid to the
fibers. In the
case of liquid addition, the liquid is allowed to dry, and then activated by
mechanically
agitating the fibers and binder. In the case of solid binder addition, the
binder is
applied as a solid, and then moistened (for example, to a total fiber moisture
content of
about 7%) and then mechanically agitated.
Activation of the binder may be performed prior to adding the particles,
subsequent to adding the particles, or simultaneously with addition of the
particles.
Once the binder is activated, it adheres a substantial portion of the
particles to the
fibers, wherein "a substantial portion" refers to about half of the particles
added, at
least where the particles are not added in excess. Of the particles that are
adhered, at
least half of them (and more ypically substantially all of them, e.g., over
80%) are
adhered to the fibers.
In embodiments in which the binder is applied to the fibers as a solid,
the activating step can comprise applying a liquid to the fibers. after the
binder has been
applied to the fibers, shortly before the binder is applied to the fibers, or
simultaneously
with application of the binder to the fibers.
The activating step may be performed after the curing step is complete,
if a curing step is to be performed.
The following example will illustrate several specific applications of the
activation process, and are not intended to limit the invention to the
disclosed methods.
EXAMPLE 26
The method of Example 1 above could be modified such that the SAP is
not added until after the web is heated to 140°C. A solid polyethylene
glycol/poly-
propylene glycol copolymer could be substituted for the binder of Example 1,
and it
would melt well below 140°C, and in its liquid form bind the SAP to the
fibers. The
SAP could be applied randomly across the heated product; or applied
specifically to a
targeted zone of the product where enhanced absorbency is specifically
desired.
CA 02412892 2003-O1-03
71
EXAMPLE 27
A southern kraft pulp sheet would be immersed or sprayed with 154
grams of a 55% solution of polyacrylic acid diluted with 100 ml of deionized
water.
The sheet is then allowed to dry overnight, heated in an oven at 80°C
for thirty minutes
S and conditioned in a 50% relative humidity chamber overnight. The sheet is
then
misted with water to raise its moisture content to 17-20% as it is fed into a
Fitz
hammermill filled with a three-eighths inch hole screen. Polyacrylate hydrogel
particles
of FAVOR 800 supplied by Stockhausen would simultaneously be added to the mill
by
a screw feed device, mixed with the flub shunted to an M & J airlay forming
machine
and airIaid to form a web containing bound SAP throughout the web, i.e:,
without
being confined to a surface of the web. IVfixing SAP throughout the fluff'
helps
produce a product in which SAP is homogeneously or randomly distributed, which
diminishes problems of get blocking.
EXAMPLE 28
900 grams of KittyHawk pulp sheet {from the Weyerhaeuser Co.,
containing 22% synthetic fiber) is immersed in a 10% by weight solution of
polyglycine
for thirty minutes. The 5 inch wide sheet was then uncoiled on a lab bench to
dry
overnight, heated in an oven at 80°C for thirty minutes and conditioned
in a 50%
relative humidity chamber overnight. The sheet is fed into a Fitz hammermill
fitted
with a three-eighths inch hold screen, defiberized, shunted to an M & J airlay
line, and
airlaid into a web. As the web emerges, circular target zones of the web are
misted
with water from a spray bottle to raise the moisture content to 17-Z I % in
the target
zone. Five gram aliquots of starch graft polyacrylate hydrogel fines (IM1000F;
supplied by Hoechst-Celanese of Portsmouth, VA) are subsequently sifted onto
the
target zones to yield a web with SAP bound in target zones. The SAP does not
form a
confluent layer; but is instead present in particulate form on and below the
surface of
the web.
CA 02412892 2003-O1-03
72
EXAMPLE 29
A 900 gram amount of a southern bleached kraft pulp sheet was
immersed in a 2% by mass solution of ascorbic acid (supplied as a dry powder
by
Aldrich Chemical Co. of Milwaukee, WI) for thirty minutes. The S inch wide
sheet
was then uncoiled on a lab bench to dry overnight, heated in an oven at
80°C for thirty
minutes and conditioned in a 50% relative humidity chamber overnight. The
sheet was
then gravimetrically determined to be about 7% by weight ascorbic acid. The
sheet
was misted with water to raise its moisture content to 17-20% as it was fed
into a Fitz
hammermill fitted with a three-eighths inch hole screen. Misting with water
activated
the binder prior to addition of superabsorbent particles (SAP). Starch graft
polyacrylate hydrogel fines (Ilvil000F supplied by Hoechst-Celanese of
Portsmouth,
VA) were added as SAP to the hammermill by a screw feed device, mixed with the
fluff, shunted to an M & J airlay forming machine (from Horsens, Denmark) and
airlaid
to form a web. The web that resulted contained 20% SAP attached to the fibers
by the
binder.
EXAMPLE 30
A 900 gram amount of KittyHawk pulp sheet (from the Weyerhaeuser
Co., containing 22% synthetic fibers) was immersed in a 10% by weight solution
of
urea (supplied by Aldrich of Milwaukee, WI) for thirty minutes. The 5-inch-
wide sheet
was then uncoiled on a lab bench to dry overnight, heated in an oven at
80°C for thirty
minutes and conditioned in a SO% relative humidity chamber overnight. The
sheet was
then gravimetricalIy determined to be about 30% by weight urea. The sheet was
fed
into a Fitz hammermill fitted with a three-eighths-inch hole screen,
defiberized, shunted
to an M & J airlay line, and airlaid into a web. As the web emerged, the
binder in the
dried web was activated by misting arget zones of the web with deionized water
in a
circular pattern from a spray bottle to raise the moisture content of the web
or the
target zones to 17-21%. Five gram aliquots of polyacrylate hydrogel (FAVOR 800
supplied by Stockhausen of Greensboro, North Carolina) were subsequently
sifted
onto each activated target zone. The web that resulted contained target zones
with 5
grams of SAP attached to the fibers in each target zone. Alternative spray
patterns
CA 02412892 2003-O1-03
73
could be provided by selecting spray heads or different control devices that
mist
different patterns.
XVII. Thermoplastic Binders
An auxiliary binder also may be used to help bind fibers to each other
above the melting point of the auxiliary binder. The auxiliary binder may be a
solid
thermoplastic material that is applied to the fibers and softened by elevating
the
temperature during the binding step to above the softening temperature of the
auxiliary
binder. The auxiliary binder is thereby temporarily softened, rendered more
fluid
(which for purposes of convenience may be referred to as auxiliary binder
melting) and
subsequently resolidified as the temperature cools, which thermoplastically
binds the
fibers to each other, and the particles to the fibers. The auxiliary binder
may also
contain a hydrogen bonding functionality that hydrogen bonds the particles to
the fiber.
Examples of auxiliary binders that are thermoplastic and also contain hydrogen
bonding groups include ethylene vinyl alcohol, polyvinyl acetate, acrylates,
polycarbonates; polyesters and polyamides. Further information about the use
of such
auxiliary binders can be found in LT.S. Patent No. 5,057,166.
The auxiliary or second binder can be added to the fibers, either before
or after a first binder, to help bind the fibers to each other and provide
additional
binding between the fibers and particles. A suitable . second binder would be
a
thermoplastic or thermosetting binder. In the case of thermoplastic polymers,
the
polymers may be a material which remains permanently thermoplastic.
Alternatively,
such polymers may be a material which is partially or fully crosslinkable,
with or
without an external catalyst, into a thermosetting type polymer. As a few
specific
examples, suitable thermoplastic binders can be made of the following
materials:
ethylene vinyl alcohol; polyvinyl acetate; acrylic, polyvinyl acetate
acryiate, acrylates,
polyvinyl dichloride, ethylene vinyl acetate, ethylene vinyl chloride,
polyvinyl chloride,
styrene, styrene acrylate, styrene/butadiene, styrene/acrylonitrile,
butadiene/
acrylonitrile, acrylonitrile/butadiene/styrene, ethylene acrylic acid,
polyethylene,
urethanes, polycarbonate, oxide polypropylene, polyesters, and polyimides.
CA 02412892 2004-02-17
74
In addition, a few specific examples of thermoset binders include those
made of the following materials: epoxy, phenolic, bismaleimide, polyimide,
melamine/
formaldehyde, polyester, urethanes, urea, and urea/formaldehyde.
More than one of these materials may be used to treat the fibers. For
example, a first coating or sheath of a thermoset material may be used
followed by a
second coating of a thermoplastic material. The superabsorbent particles or
other
particles are then typically adhered to the outer binder material. During
subsequent
use of the fibers to make products, the thermoplastic material may be heated
to its
softening or tack temperature without raising the thermoset material to its
curing
temperature. The remaining thermoset material permits subsequent heating of
the
fibers to cure the thermoset material during further processing.
Alternatively, the
thermoset material may be cured at the same time the thermoplastic material is
heated
by heating the fibers to the curing temperature of the thermoset with the
thermoplastic
material also being heated to its tack temperature.
Certain types of binders enhance the fire resistance of the treated fibers,
and thereby products made from these fibers. For example, polyvinyl chloride,
polyvinyl dichloride, ethylene vinyl chloride and phenolic are fire retardant.
Surfactants may also be included in the liquid binder as desired. Other
materials may also be mixed with the liquid binder to impart desired
characteristics to
the treated fibers. For example, particulate material, such as pigments, may
also be
included in the binder for application to the fibers.
EXAMPLE 31
As previously described, an auxiliary binder can be used in addition to
the polymeric binders of the present invention. A 3210 gram amount of southern
bleached kraft binder (NB-416# supplied by Weyerhaeuser Company) is air
entrained in
a blenderlike mixing device and sprayed with 2128 grams of a polyvinyl acetate
latex
(PN-3666H,~supplied by H.B. Fuller of Minneapolis, Minnesota). While still
mixing,
4073 grams of a water swellable polyacrylate hydrogel (IM 1000-60# supplied by
Hoechst-Celanese of Portsmouth, Virginia) is added and the resulting mixture
is then
sprayed with 1160 grams of a 50% solution of polypropylene glycol (supplied by
#~rademark
CA 02412892 2003-O1-03
Union Carbide of Danbury; Connecticut). The blender is not stopped and the
mixture
is shunted into a flash tube dryer. The dried product is then airlaid as a 16
inch wide
web on a Danweb airlay machine, pressed to a density of approximately 0.15
g/cc, and
thermobanded at 140°C for thirty seconds. The resulting web would have
40% bound
5 SAP and improved tensile strength (as compared to untreated fluff with SAP).
Alternatively; 189 grams of EDTA can be substituted for the 4073
grams of polyacrylate hydrogel.
XVIII. Application of Binder
10 The binders of the present invention can be added to the fibers in any
convenient manner. One such procedure is to spray the binder or binders on a
web of
the fibers that is conveyed past a sprayer on a' conveyor belt. Alternatively,
loose
fibers may be allowed to fall past a sprayer, or loose fibers may be moved on
a
conveyor belt past a sprayer. The loose fibers may also be slurned with or
immersed in
15 binder. It is also preferable to roll coat the binders on the web,
particularly if the
binder is viscous. For solid binders, blending of the fiber and binder may be
accomplished or the binder may simply be sprinkled onto or otherwise
commingled
with the fibers, followed by a fixation step such as addition of heat or
liquid. The
fibers may also be sprayed or immersed in the binder, or binder particles may
be
20 applied thereto. These fibers can, while still wet in the case of a liquid
binder or
following activation of a liquid or solid, be combined with the particles.
The fibers also can be allowed to dry for later activation with an
activation fluid, such as an activation liquid, and combined with the
particles at that
time. An example of when it may be desirable to apply the binder to the fiber
and
25 thereafter activate the binder in the presence of particles is when the
particles are
added at a remote site: For instance, the binder may be activated from an
inactive state
at a second location that is remote from a first location where the binder is
applied to
the fibers. The second location may be, for example, a location where a
manufacturer
combines fibers and particles into articles, such as absorbent articles.
Particles may be
30 added from conventional volumetric feeders in a hammermill or from
injectors on a
paper making line.
CA 02412892 2003-O1-03
76
One method for uniformly coating the fibers with a binder and adding
the particles is shown in U.S. Patent No. 5,064,689. However, the invention is
not
limited to any specific mechanism for combining the fiber, binder and
particles.
XIX. Production of High Bulk Fibers
Production of high bulk fibers with intrafiber crosslinks is known in the
art. Processes for making such fibers are described in EP 440 472 Al; EP 427
317 A2;
EP 427 316 A2; and EP 429 112 A2, as well as U.S. Patent No. 5,324,391. These
high bulk fibers may be used in the present invention, with particles bound to
them by
the binders disclosed herein. Since methods of making high bulk fibers are
known,
only a brief description of one such process is given below.
A. Overall S~rstem
The apparatus 110 (FIG. 3) of the present invention comprises a
conveying device 112 for transporting a mat 114 of cellulose fibers or other
fibers
through a fiber treatment zone 116; an applicator 118 for applying a treatment
substance such as a crosslinking substance from a source 119 thereof to the
mat 114 at
the fiber treatment zone 116; a fiberizer 120 for completely separating the
individual
cellulose fibers comprising the mat 114 to form a fiber output comprised of
substantially unbroken cellulose fibers substantially without nits or knots;
and a dryer
122 coupled to the fiberizer for flash evaporating residual moisture from the
fiber
output and for curing the crosslinking substance, thereby forming dried and
cured
cellulose fibers.
The mat 114 of cellulose fibers is preferably in an extended sheet form
stored in the form of a roll 124 until use. It is normally not necessary that
the cellulose
fibers comprising the mat 114 be completely dry. Since cellulose is a
hydrophilic
substance, molecules thereof will typically have a certain level of residual
moisture,
even after drying. The level of residual moisture is generally 10% wt/wt or
less, which
is not detectable as "wetness." FIG. 3 also shows that more than one supply,
such as
CA 02412892 2003-O1-03
77
multiple rolls 124, of the mat 114 of cellulosic fibers can be simultaneously
processed
using the present invention.
At the fiber treatment zone 116, sprayers or other applicators 118 apply
chemicals such as crosslinking agents to the mat. Typically chemicals are
applied
uniformly to both sides of the mat. The wetted mat passes between a pair of
rollers
128 which assist in distributing the chemicals uniformly through the mat.
Other
applicators may also, of course, be used.
The crosslinking substance is a liquid solution of any of a variety of
crosslinking solutes known in the art: If required, the crosslinking substance
can
include a catalyst to accelerate the bonding reactions between molecules of
the
crossIinking substance and cellulose molecules. However, many if not most
crosslinking substances do not require a catalyst.
Preferred types of crosslinking substances are selected from a group
consisting of urea derivatives such as methylolated urea, methylolated cyclic
areas,
methylolated lower alkyl substituted cyclic areas, methylolated dihydroxy
cyclic areas,
and mixtures thereof. A specifically preferred crosslinking substance would be
dimethyloldihydroxyethylene urea (DMDHEU). In addition, crosslinking
substances
can be polycarboxylic acids, such as citric acid. Crosslinking materials are
known in
the art, such as described in the previously mentioned Chung patent, U. S.
Patent No.
4,935,022 to Lash, et al., U:S. Patent No. 4,889,595 to Herron, et al., U.S.
Patent No.
3,819,470 to Shaw, et al.; U.S. Patent No. 3,658,613 to Steijer, et al., U.S.
Patent No.
4,822,453 to Dean, et al., and U.S. Patent No. 4,853,086 to Graef, et al.
Suitable catalysts include acidic salts which can be usefi~l when urea-
based crosslinking substances are used. Such salts include ammonium chloride,
ammonium sulfate, aluminum chloride, magnesium chloride, or mixtures of these
or
other similar compounds. Alkali metal salts of phosphorus-containing acids may
also
be used.
In FIG. 3, the crosslinking substance applied to the mat 114 is obtained
from a supply 119 thereof; such as a tank or analogous vessel.
Crosslinked cellulose fibers are individual fibers each comprised of
multiple cellulose molecules where at least a portion of the hydroxyl groups
on the
CA 02412892 2003-O1-03
78
cellulose molecules have been covalently bonded to hydroxyl groups an
neighboring
cellulose molecules in the same fiber via crosslinking reactions with
extraneously added
chemical reagents termed "crosslinking substances" or "crosslinking agents."
Suitable
crosslinking agents are generally of the bifunctional type which create
covalently ,
bonded "bridges" between said neighboring hydroxyl groups.
B. ConveXing Device
Referring further to FIG. 3, each mat 114 of cellulosic fibers is
conveyed by a conveying device 112, which carries the mats through the fiber
treatment zone 116. FIG. 3 also shows a further portion of one type of
conveying
device comprised of a first pair of rollers 126 and a second pair of rollers
128 for each
mat 114. The first and second pair of rollers 126, 128 are particularly
effective for
urging the corresponding mat at a substantially constant and controlled rate
of speed.
C. Fiber Treatment Zone
Each mat 114 is urged by the first and second pair of rollers 126, 128
through the fiber treatment zone 116 where the mat 114 is impregnated with a
liquid
crosslinking substance. The crosslinking substance is preferably applied to
one or both
surfaces of the mat using any of a variety of methods known in the art useful
for such a
purpose, such as spraying, rolling, dipping, or analogous method. Combinations
of
spray and roller applicators can also be employed.
The crosslinking substance is typically applied in an amount ranging
from about 2 kg to about 200 kg chemical per ton of cellulose fiber and
preferably
about 20 kg to about 100 kg chemical per ton of cellulose fiber.
D. Fiberizer
The next subsystem following the fiber treatment zone is a fiberizer 120
which serves to comminute one or more mats 130 impregnated with the
crosslinking
substance into individual substantially unbroken cellulose fibers comprising a
fiber
output.
CA 02412892 2003-O1-03
79
Referring further to FIG. 3, a first conveyer fan 260 of conventional
design can be utilized for propelling the fibers from the outlet 162 of the
attrition
device 132 through a conduit 262.
An optional component of the fiberizer 120 is a first cyclone 264 or
similar apparatus known in the art, utilized in a conventional manner to
concentrate the
fibers passing out of the outlet 162 of the attrition device 132. The first
cyclone 264
receives the fibers through the conduit 262 coupled thereto.
Excess air can be recovered at the top 266 of the first cyclone 264 and
recycled as required through a conduit 268 to a location upstream of the first
conveyer
fan 260 (if used). Such additional air can be beneficial for easing the
transfer of the
fibers through the first conveyor fan 260.
A disk refiner 268 is another optional component of the fiberizer 120
which can be employed to effect additional separation of fibers (removal of
knots) if
required. The disk refiner 268 is of a type known in the art and comprises a
disk
refiner inlet 270 and a disk refiner outlet 272. A representative disk refiner
268 is type
DM36 manufactured by Sprout-Bauer, Incorporated of Muncie, Pennsylvania. If
the
disk refiner 268 is used, the inlet 270 thereof is coupled via a conduit 274
to an outlet
276 of the first cyclone 264.
A second conveyor fan 278 may optionally be utilized to urge
propagation of the fibers through a conduit 180 downstream of the disk refiner
268.
Excess air can be recovered from the top 266 of the first cyclone 264 and
routed via a
conduit 281 to a tee 282 just upstream of the second conveyor fan 278.
Another optional component of the fiberizer 120 is a fluff generator 290
which receives the fibers from the optional second conveyor fan 278 through a
conduit
2g4. The fluff generator is described in detail below and in U.S. Patent No.
5,277,371.
E. Drier
Referring further to FIG. 3, a preferred embodiment of the present
apparatus 110 includes a dryer I22 which is utilized to perform two sequential
functions: remove residual moisture from the fibers and cure the crossIinking
agent.
CA 02412892 2003-O1-03
Preferably, the dryer 122 comprises a drying zone 373 for receiving fibers,
e.g. from
fluff generator outlet 304 and for removing residual moisture from the fibers
via a
"flash drying" method and a second drying zone 360, 362 for curing the
crosslinking
agent. In FIG. 3, the curing starts in zone 360 and continues through zone
362.
5 The FIG. 1 embodiment shows that zone 373 is coupled to the fluff
generator outlet by a conduit 372 and to a source 374 of heated air, typically
produced
by combustion of a supply of natural gas 376 and fresh air 378. The
temperature of
heated air is regulated to maintain the temperature of the drying zone 373
within a
range of about 200°C to about 315°C. As the fiber output passes
into the drying zone
10 373, the wet fibers comprising the fiber output are substantially
instantaneously
exposed to the high temperature in this zone. Such rapid exposure to high
temperature
imparts a "flash drying" erect to the fibers, thereby causing rapid and
thorough drying
and separation of the fibers. The passage time through the drying zone 373 is
preferably less than one second.
15 The FIG. 3 embodiment shows that the first zone 360 is comprised of a
first tower 364 comprised of a body portion 366, an inlet 368, and a first
tower outlet
370. The dryer zone 373 is coupled via a conduit 372 to the outlet of the
fluff
generator 290.
In FIG. 3, the first tower 364 is shown preferably coupled via a conduit
20 380 to a down tube 382, which is coupled via a conduit 384 to a third
conveyor fan
386 located at an inlet 388 of a econd tower 390. The third conveyor fan 386
transports the fibers through the dryer which thereby pass into the second
tower 390.
As the fibers are lofted through the second tower 390, they are still exposed
to a
curing temperature within a range of about 140°C to about 180°C,
which is sufficient
25 to effect curing of the crosslinking agent without scorching the dry
fibers. The lofting
keeps the fibers separated until the crosslinking reaction is complete. The
curing
temperature depends upon the type of crosslinking material used to treat the
fibers and
also is set at a level so as to not scorch the fibers during curing. It should
be noted
that single stage dryers may also be used.
30 The dried and cured fibers exiting the dryer outlet of tower 390 have an
extremely low level of nits and virtually no knots. Further, they are not
discolored
CA 02412892 2003-O1-03
81
from scorching and the like, and have a median fiber length substantially
unchanged
from the median length of the fibers comprising the mat 14.
FIG. 3 also shows a second cyclone 400 of conventional design coupled
via a conduit 402 to the outlet of tower 390, serving to concentrate the
fibers passing
therethrough in preparation for collection. The resulting concentrated fibers
can be
collected using any of a number of collection devices 408 known in the art,
such as
fiber bagging devices.
E~ArvIPLE 32
3n this example, non-woven fibrous mats were impregnated with a
crosslinking agent, fiberized, dried, and cured using the apparatus as
diagrammed
schematically in FIG. 3.
Two 52-inch-wide mats of southern pine kraft wood pulp fibers (type
NB3I6 from Weyerhaeuser Company) and having a basis weight of 680 g/M2 were
fed
I 5 to the apparatus. The mats were impregnated using
dimethyloldihydroxyethylene urea
at a concentration of about 5%, applied over both sides of each mat using a
combination of spray nozzles and impregnation rollers. The loading level of
crosslinking agent was about 4.5% w/w.
The treated fiber mats were fed at the rate of 8 meters/min to the
attrition device 32. The specific attrition device used in this example was
equipped
with six mat inlets and a rotor having 16 rows of hammers as described above
around
the circumference of the rotor. The rotor had a diameter of 30 inches and was
rotated
at an angular velocity of 1200 rpm by an electric motor. Other rpm rates have
also
been tested and have proven satisfactory, including extremely high rpm rates.
Random samples of fibers were obtained from the output attrition
device and observed for nits. These samples were 2.6 grams and were
consistently
observed to have fewer than three nits on the average with most samples having
no
nits. The attrition device was flushed with water once every sixteen hours for
cleaning
purposes.
A disk refiner was employed downstream of the attrition device. This
specific disk refiner was a DM36 refiner as previously mentioned. A fluff
generator as
CA 02412892 2003-O1-03
82
described in FIGS. 7-9 was also employed in this downstream of the disk
refiner. The
temperature at the dryer input in this example was within the range of
200°C to 315°C.
The temperature at the second tower outlet was within the range of
140°C to 180°C.
Crosslinked fiber at the output of the dryer was produced at a rate of about
5000 ,
pounds per hour. The particle binders and particles of the present invention
can be
added before, after, or simultaneously with curing. The term "curing in the
presence of
the binder" means that the binder is added before or simultaneously with
curing.
Curing in the presence of the binder is not usually a problem because the
binder cannot
always participate in the intrafiber ctosslinking reaction, and the binder is
not affected
by the curing step. In certain situations, however, the binder can also form
covalent
intrafiber crosslinks. Polycarboxylic acids (such as citric acid), polyots
(such as
dipropylene glycol) and' polyamines (such as ethylene diamine) can function as
crosslinking agents, and are consumed during the curing step in the formation
of
covalent crosslinks. Hence in the limited case in which the crosslinking agent
is also a
binder material, steps should be taken to prevent the binder from being
consumed as a
crosslinker in the curing step.
Formation of the intrafiber covalent ester bond requires an anhydride
intermediate. Formation of the anhydride intermediate can be inhibited by the
presence
of water. The present inventors have found that about 20% water (more
preferably at
least f0% water) by weight in the fibers will sufficiently retard curing so
that adequate
binder functional groups will remain available in the fibers to bind the
particles to the
fibers. Hence when curing the crosslinking material. in the presence of a
binder that is
also a crosslinking material, the fibers should contain at least about 20%
water by
weight of the fibers when curing begins. When curing the crosslinking material
in the
presence of a binder that is not also a crosslinking material; steps to
prevent covalent
bond formation are not usually necessary. When the crosslinking material is
not cured
in the presence of the binder, that is when the binder is applied after
curing, no steps
need be taken ro inhibit covalent bond formation.
CA 02412892 2003-O1-03
83
XX. Composite Absorbent Product
In accordance with the present invention; absorbent structures or
articles may be made from the fibers; with binder and adhered particulates.
These
articles may be composite structures (e.g., made of plural materials). For
example, the
articles may have a core of plural types of fibers, or fiber layers, with or
without
covering materials. These products are capable of absorbing significant
quantities of
water and other fluids, such as urine and other body fluids. Such products
include, but
are not limited to, disposable diapers, sanitary napkins; incontinent gads,
towels and
the Iike.
FIGS. 4-5 illustrate an absorbent pad structure which may be formed
from fibers of the present invention, whether or not they are blended with
other fibers.
FIGS. 4 and S represent an absorbent pad 410 having a heat embossed screen
pattern
4I2. Pads having no pattern may also be used. A pad having a cover sheet 414
and a
backing sheet 416 may be formed, for example, by placing a square fiber piece
cut
from the sheet onto a corresponding precut backing sheet. A corresponding
precut
cover sheet is placed over the top of the fiber 418 on the backing sheet. This
assembly
may then be adhesively bonded around a continuous margin 420.
With reference to FIGS. 6-7, an absorbent structure in the form of a
bandage is shown. A bandage 430 for application to a wound to absorb blood and
other bodily fluids is shown. An absorbent pad 440 is securely mounted to an
exterior
or pad mounting surface 434 of a backing strip 436: Fibers 441 are contained
in pad
440, and particles are attached to the fibers 441 in accordance with the
present
invention. Any suitable mounting or securing means may be used to affix pad
440 to
the surface 434 of he strip 436. However, it is preferable for surface 434 to
be coated
with an adhesive so that the pad 440 may be adhesively mounted in place. An
exemplary adhesive is ethylene vinyl acetate adhesive. It is also desirable
for the
overall surface 438 of backing strip 436 to be coated with a conventional
adhesive.
Surface 438 is the surface which is affixed to the area of the skin
surrounding the
wound. Conventional "peel-back" tabs may be used to protect the adhesive
coating
and pad 440 until the bandage is to be applied. This type of backing strip is
well
known in the art.
CA 02412892 2003-O1-03
84
The backing strip 436 may be of any known flexible material suitable
for application to the skin. It is preferable for the strip 416 to be of a
material which is
impermeable to the passage of liquid so that fluid from a wound is contained
by the
bandage. However, the strip 436 may be apertured or otherwise breathable to
permit
air to reach the wound to promote the healing process. A specific example of a
suitable backing' strip 436 is a polyethylene film.
As in the other structures described, a variety of combinations of
antimicrobials and other particles may be used in the fibers 441 of such a
bandage.
Again, however, the particles are adhered securely in place when the particles
have a
hydrogen bonding or a coordinate covalent bonding functionality, the fibers to
which
these particles are bound have a hydrogen bonding functionality, and wherein
the
binder is selected from the group consisting of a polypropylene glycol, a
polypropylene
glycoUpolyethylene glycol copolymer, a polycarboxylic acid, such as
polyacrylic acid, a
poly(lactone) diol, such as poly(caprolactone) diol, a polyamide, a polyamine,
a
polysulfonic acid, a polysulfonate, polycarboxylate and combinations thereof.
The
polymeric binder has a hydrogen bonding or a coordinate covalent bond forming
functionality: Nonpolymeric binders would include organic binders such as
glycerin,
monoglycerides; diglycerides, ascorbic acid, urea, glycine, pentaerythritol, a
monosaccharide or a disaccharide, citric acid, tartaric acid, taurine,
dipropylene glycol,
and urea derivatives such as DMDHEU. Suitable saccharides include glucose,
sucrose, lactose, ribose, fiuctose, mannose, arabinose, and erythrose. Two
different
particles, such as different antimicrobials in particulate form, may be
adhered to the
same fiber. In the alternative, each different type of antimicrobial particle
or other
particle may be adhered separately to different fibers. Also, blends of fibers
may be
included in absorbent structures such as pad 366. For example, these blends
may
include fibers with adhered antimicrobial (one or more antimicrobials)
particles and
adhered superabsorbent particles; fibers with one or more antimicrobial
particles
without superabsorbent particles blended with fibers having adhered
superabsorbent
particles with or without antimicrobial particles; and combinations of such
fibers with
untreated fibers and/or binder coated fibers without superabsorbent particles
or
CA 02412892 2003-O1-03
antimicrobial particles. In addition, other particles, such as anticoagulants
or
hemostatics may be attached to the fibers.
The absorbent pad of bandage 430 may also include a cover sheet that
is typically made of any suitable material which will readily permit the
passage of liquid
S through the cover sheet to the fibers 441, such as nonwoven fiber webs of
fibers such
as, for example, rayon, nylon, polyester; propylene and blends ' thereof. One
specifically preferred cover sheet material is a 70 percent rayon/30 percent
polyester
blend having a basis weight of 18 g/m2 from Scott Paper Company.
FIGS. 8 and 9 illustrate a conventional disposable diaper 550 with a
10 core 552 which is comprised of fibers of the present invention with adhered
superabsorbent particulate materials. These particulate materials may be
confined to a
target zone (for example, the front or crotch portion of a diaper indicated at
556) or of
a heavier concentration in the target zone. This can be accomplished by
airlaying fibers
of the present invention in such a zone. Also, the core may be activated by
melting the
15 binder or moistening the target zone with water. The superabsorbent
particles may be
sprinkled on or otherwise applied to this wetted zone. As the zone dries, the
particles
are adhered in place.
~. Densification
20 The products such as described above, as well as webs of the fibers of
the present invention, can also be densified by external application of
pressure to the
web. The web could be densified by passing it through a set of calendar rolls
set at 60
and 94 pli (pounds per linear inch, as in a calendar press) respectively to
yield sheets
with increased densities. Densification may alternatively be provided by
compaction
25 rolls or presses. The inventors have found that densification is
facilitated in SAP-
containing products treated with binders of the present invention, when the
densification occurs with the binder in an active state. Products that are
treated with
these binders require less heat and pressure than untreated fibers to densify
to a given
density. Densification is preferably performed to produce a product that has a
density
30 of about 0.05 to 0.7 g/cc; more preferably O. l to 0.3 gicc.
CA 02412892 2003-O1-03
86
An example of densification using some of the binders of the present
invention is given below:
EXAMPLE 33
The products of the present invention can be formed into 550
gratn/square meter sheets, six inches in diameter, in a laboratory padformer.
Those
pads are then passed through a set of calendar rolls set at 60 and 90 pli,
respectively to
yield sheets with densities of 0.3 and 0.5 g/cc.
IO EXAMPLE 34
A 50 gram amount of polypropylene glycol is -dilufed with 50 gams
deionized water. The resulting solution is sprayed on 321 grams of an
intrafiber
crosslinked cellulose fluff (HBA pulp from Weyerhaeuser Company of Tacoma, WA)
that was air entrained in a blender like mixing device. While the HBA fiber is
still
damp; 438 grams of IM1000F (supplied by Hoechst-Celanese, of Portsmouth,
Virginia) is added to ~ the mixture. The resultant mixture is then vacuumed
from the
blender and spread on a counter to dry overnight. Then 550 gram/square meter
handsheets, six inches in diameter, are made in a laboratory padformer. Those
pads
are then pressed at 2000 and 3000 psi (or 60 and 90 pli in a calendar roll);
respectively,
to yield sheets with densities of 0.3 and 0.5 g/cc. Alternatively, pads of
untreated
HBA pulp blended with 45% IMI000F would require heating to 100°C and
pressures
between 8,000 and 11,000 psi to produce pads of similar densities.
EXAMPLE 35
HBA pulp with 40% IM 1000F and HBA pulp with 12% glycerin and
40% IM1000F were formed into six-inch pads in the padformer then pressed at
about
6500 psi for 15 seconds. HBA pulp without glycerin binder reached a density of
0.4
g/cc and HBA pulp with glycerin bound particles reached a density of 0.57
g/cc. This
example illustrates that fibers treated with the method of the present
invention achieve
a greater density than untreated fibers at the same compression pressure.
CA 02412892 2003-O1-03
87
XXII. Water Addition
In some embodiments of the invention, a crosslinking material is added
to the fibers and cured to form intrafiber covalent bonds that produce high
bulk fibers.
If the crosslinking material and binder are the same (for example, a
polycarboxylic
acid), or are both different but capable of intrafiber crosslinking, and the
binder is
added before curing occurs, substantially all of the crosslinking
material/binder will be
used in the covalent crosslinking reaction, such that none will be available
for
subsequent binding of the particles to the fibers with hydrogen bonds and
coordinate
covalent bonds. In this particular instance (where the crosslinking material
and binder
are both capable of crosslinking, and are added before curing) water may be
added to
the fibers before curing to retard initiation of the curing step and ensure
that a portion
of the binder's functionality is not consumed in the crosslinking reaction. At
least 20%
water by weight in the fibers sufficiently retards intrafiber covalent bond
formation to
allow residual polycarboxylic acid on the fibers to bind the particles to the
fibers. The
following example illustrates this process.
EXAMPLE 36
A 100 gram pulp sheet was sprayed with 44.5% intrafiber crosslinking
material, and the pulp sheet was then delaminated and fed in small bits into a
padformer while adding superabsorbent particles to delaminated pulp sheet at
the same
time. The material was run a second time through the padformer to fluil' it
up, and the
material was then subsequently cured for 20 minutes in an oven at
150°C.
In a first run, the crosslinking material was a modified ethylene urea and
citric acid, while the particulate material was IM1000F. To the 100 g pulp
sheet was
added 63.4 grams of the ethylene urea, 16.76 grams citric acid, and 70 grams
of
IM1000F, for a final crosslinker content of 35.2% ethylene urea and 9.3%
citric acid,
by weight. No water was added in this run.
In a second run; the 100 gram pulp sheet was crosslinked with 30.83
grams of a polyaldehyde (glyoxal), 5:03 grams of a glycol, 0.2 grams alum, 0.2
grams
CA 02412892 2003-O1-03
gg
citric acid, and 15 grams distilled water. Curing was performed after 70 grams
of
IMIOOOF SAP was added to the pad.
Attachment of the particles to the pad was poor in both of these runs.
Each of these runs was then repeated, except 50 grams of distilled
water was added before curing. Fence there was 50 g of water in the first run
and 65
g of water in the second run. Particle attachment to the fibers was greatly
improved.
Electron microscopic examination of the fibers from these runs showed
that particle bonding did not occur in the absence of the 50 g water addition.
In the
presence of 50 gams distilled water, however, electromicroscopic data showed
actual
bonding of the particles to the fibers.
XXIII. Particulate Binding
FIG. 10 shows an isolated, enlarged cellulose fiber 600 with SAP
particles 602 bound to it by a binder of the present invention. This drawing
illustrates
an example of the SAP retaining its discrete particulate form following
binding to the
fibers. Some particle to particle fusion may occur in accordance with this
invention,
but maintenance of a discrete particulate form excludes formation of a
completely
confluent film in which the particles lose their particulate identity. Such a
confluent
film produces gel blocking that interferes with efficient liquid absorption
into the fibers.
The shown fiber 600 is elongated, and has an aspect ratio (ratio of
length to width) of about 10:1 to S:1, preferably about 10:1.
FIG. 11 shows the particles 602 distributed substantially uniformly
throughout the depth 604 of a pad 606. The particles are also shown adhering
to all
the surfaces of the pad. Particles may be distributed in any desired pattern
throughout
the pad in accordance with this invention, and need not necessarily adhere to
all
surfaces or be distributed throughout the volume of the mat, or distributed
uniformly.
As can be seen from FIGS. IO-I I (and FIGS. I2-15 discussed below),
the particles are not encapsulated by the binders. The particles and fibers of
the
present invention are not encapsulated with the binder. Ibloreover, the binder
does not
agglomerate the fibers together, and in many embodiments does not bind fibers
to each
CA 02412892 2003-O1-03
89
other. Discrete individual particles retain their identity on the surface of
the fibers,
instead of being subsumed in a thermoplastic encasement around the fiber and
particle.
XXIV. Electron Photomicrog,.raphs
An electron photomicrograph of superabsorbent particles (SAP) bound
to cellulose fibers with an ascorbic acid binder is shown in FIG. 12. The SAP
is at the
left margin of the photograph, and is bound to the fiber which occupies the
central
portion of the photomicrograph. The particle is seen to be bound to the fiber,
and the
fiber has undergone some shear damage that resulted in a fracture of the
fiber. It is
significant that the fiber has experienced shear damage while the particle has
remained
bound to the fiber, because this indicates that the particle-fiber bond formed
by the
ascorbic acid is very strong and resilient, resisting mechanical disruption.
FIGS. 13A, 13B, 13C and 13D show several electron photo-
micrographs that illustrate individual particles bound to fibers with a
lactose binder.
FIG. 13C, for example, shows that SAP retains its individual particulate form
when
adhered to the fiber with a lactose binder. The particles do not form a fused
confluent
mass without particulate identity.
EXAMPLE 3 7
An electron photomicrograph of oxalic acid particles bound to cellulose
fibers with a glycerin binder is shown in FIG. 14. The bound oxalic acid is
near the
center of the photograph, and is seen bound to the fiber without mechanical
encapsulation of the fiber and particle by an encapsulating binder.
FIG 15 is an SEM illustrating a particle of aluminum sulfate (alum)
bound to a cellulose fiber with a glycerin binder. The alum particle is seen
at the center
of the photograph, and the particle retains its individual particulate form
when adhered
to the fiber. The particles do not form a confluent mass lacking particulate
identity.
Moreover, the particles are not encapsulated by a material that mechanically
holds the
particle in contact with the fiber.
CA 02412892 2003-O1-03
XXV. Fiber Mixtures
The fibers of the present invention, such as fiber 600, can be mixed with
other types of fibers; such as that disclosed in U.S. Patent No. 5,057,166.
The latex
coated fibers of that patent can be mixed with the fibers of the present
invention to
5 produce an absorbent product that has characteristics of both types of
fibers.
XXVI. Additional Binder Characteristics
U.S. Patent No. 3;903,889 discloses a process for adhering absorbent
particles to pulp fibers using syrup, honey, and other polysaccharides such as
dextrins.
10 An essential requirement of these adhesive agents is that they must possess
the
property of being permanently pliable, and not rigidifying into a brittle
film. The
binders of the present invention, in contrast, are capable of functioning as a
binder after
solidifying into a rigid crystalline material. Even the binders of the present
invention
that do not rigidify into a solid (such as glycerin and PPG) are very
hygroscopic, and
15 can be present on fibers having a total water content of no more than 15%,
or even
12%. This is in contrast to the adhesives such as honey and corn syrup
disclosed in
U.S. Patent 3,903,889 that are not hygroscopic. Polysaccharides (such as corn
syrup,
honey and dextrins) are excluded as binders from some embodiments of the
invention
because they remain tacky upon drying. Tacky binders make processing the
binder-
20 coated fibers difficult. The polysaccharide polymers are also excluded from
non-
polymeric embodiments of the binder of the present invention. Moreover, the
non-
polymeric saccharides such as monosaccharides and disaccharides, lack the high
viscosity and tacky adhesive physical properties of polysaccharides such as
corn syrup
and honey. The non-polymeric saccharides of the present invention may be
solids,
25 which avoid the viscosity and handling problems associated with polymers.
As used in this application, a particle that is soluble in water will
completely dissolve at least 10 grams of the particle in 300 ml. water at
25°C. A
particle that is sparingly soluble in the binder will completely dissolve no
more than
about 5 grams of the particle in 300 ml. of the binder at 25°C.
CA 02412892 2003-O1-03
91
Some of the binders of the present invention are also water soluble. A
binder that is water will completely dissolve at least 10 grams of the binder
in 300 ml.
water at 25°C.
XXVII. Fiber Densification Without Particles
In accordance with another aspect of this invention, the present
inventors have observed that when densifying agents such as those described
below are
applied to fibers for example those described above which have hydrogen
bonding
functionality, the fibers density to a greater degree than fibers that have
not been
treated with a densiFying agent. Densifying agents that can be used to improve
the
densifiability of the fibers generally include, but are not limited to organic
and
inorganic materials that have a density greater than the dry density of the
fibers. Such
densifying agents can be applied to the fibers in the same manner that the
binders
described above are applied to fibers. In addition to the methods for
densifying fibers
described above, the advantages of his aspect of the present invention also
apply to
densification of the fibers in a bale former.
Organic densifying agents that are useful include the organic polymeric
and non-polymeric binders described above. When such binders are employed as
densifying agents, it is not necessary that the molecules contain
functionality to bind
particles. Hence, organic molecules containing only one hydrogen bonding
functionality may be useful as a densifying agent. Although the densifying
agents are
not required to bond to the fibers, in preferred embodiments they do bond to
the fibers.
In addition to the binder functionalities expressly described above, binders
that are
useful as densifying agents may also include sulfonamide or phosphoramide
fixnctionalities. At this time, preferred non-polymeric organic densifying
agents include
sorbitol, glycerin, propylene glycol; and mixtures thereof. In accordance with
this
aspect of the present invention, the organic densifying agents can be applied
to the
fibers in an amount ranging from about 0.1% to about 30% by weight based in
the
combined weight of the fibers and densifying agent.
In addition to improving the densification properties of the fibers, it has
also observed that the preferred densifying agents sorbitol and glycerin
improve the
CA 02412892 2003-O1-03
92
softness of the fibers and articles containing the fibers before and after
densification_
The organic acid, lactic acid, while not improving the densifiabifity of
fibers when
applied thereto has been observed to soften fibers treated therewith. Softness
relates
to stiffness of the fibers or articles containing the fibers, the drape or
hand of articles
including the fibers. Another aspect of softness relates to abrasiveness or
lack thereof
of a fiber or article containing the fiber: Compressibility is another aspect
of softness.
Various tests exist to evaluate some of these aspects of softness, including
Gurley
stiffness, Tuber stiffness, measurements of coefficients of friction,
handlometers, and
the like. It should be understood that none of the tests fisted above
completely
quantifies or evaluates softness or fibers or articles containing fibers;
therefore
subjective testing is also done to assess the softness of a material.
In addition to the polymeric and non-polymeric organic densif3ring
agents described above; the present inventors have also observed that some
inorganic
materials also improve the densifiability of fibers to which the agents are
applied. As
with the organic densifying agents, the inorganic densifying agents generally
include,
but are not limited to those characterized by a density greater than the
density of the
dry fibers. 'In addition, the inorganic densifying agents when added to the
fibers will
increase the mass of the fibers without appreciably affecting the volume of
the fibers:
The inorganic densifying agents may increase the mass without appreciably
affecting
the volume of the fibers by occupying the preexisting spaces within the
fibers, such as
the lumen, pores, and the like. Preferably, any increase in volume of the
fibers caused
by the addition of the densifying agent will be less than the increase in mass
of the
fibers, such that the density of the fibers in increased.
Examples of inorganic densifying agents which may have a density
greater than the fibers and that will increase the mass of the fibers without
appreciably
affecting the volume of the fibers include inorganic acid salts in which the
cotton is
monovalent, such as alkali metal, ammonium, or substituted ammonium (such as
mono-, di-, tri-, or tetra-alkyl substituted ammonium, with the alkyl groups
having
from one to six carbon atoms, for example, triethyl or trimethyl ammonium,
tetramethyl or tetraethyl ammonium). Suitable densifying agents include alkali
metal,
ammonium, or substituted ammonium salts of pyrophosphates, hexametaphosphates,
CA 02412892 2003-O1-03
93
tripolyphosphates; hypophosphates, polyphosphoric acid,
ethylenediaminetetraacetic
acid, hydroxyethylidenediphosphonate; and aminotri-(methylenephosphonate).
Specific densi fy'mg agents include sodium hypophosphate tetrapotassium
pyrophosphate, and tetrasodium pyrophosphate. Tetrasodium pyrophosphate and
tetrapotassium pyrophosphate are particularly useful. Other specific
densifying agents
include sodium hexametaphosphate, sodium salt of ethylenediaminetetraacedc
acid;
sodium polyphosphate, sodium salt of diethylenetriaminepentaacetic acid,
sodium salt
of hydroxyethylethylenedianiinetriacetic acid, and sodium salt of
dihydroxyethylglycine. Still other densifying agents include alkali metal,
ammonium or
substituted ammonium salts of oxalates, phosphates, and tungstates. Sodium
oxalate,
dibasic sodium phosphate and sodium tungstate are particularly useful.
lVfixtures of the
foregoing densifying agents are also useful. In accordance with this aspect of
the
present invention the inorganic densifying agents can be applied in an amount
from
about 1.0% to about 50% by weight based on the fibers and densifying agent.
Without intending to limit the scope of the present invention, one
possible explanation for the effect the densifying agents have on the
densifiability and
softness of the fibers can be explained by considering the hydrogen bonding
that occurs
between cellulose molecules within a cellulose fiber and within a specific
cellulose
molecule. The densifymg agents when applied to the fibers may disrupt or
promote the
disruption of existing hydrogen bonding between cellulose molecules or within
a given
cellulose molecule. In other words, the densifying agent may occupy sites on
the
cellulose molecule that would otherwise form hydrogen bonds within the
molecule or
with other cellulose molecules.
With respect to the inorganic densifying agents, such as TKPP, another
possible explanation for the effect the inorganic densifying agent has on the
densifiability and softness of the fibers or articles containing the fibers
relates to
TKPP's hygroscopic property wherein it attracts moisture from the surrounding
environment into the fibers which have been treated with TKPP. This moisture
may
disrupt or participate in the disruption of hydrogen bonding between cellulose
molecules or within a cellulose molecule as explained above. In addition to
increasing
the densifiability of fibers, the present inventors have also observed that
TKPP softens
CA 02412892 2003-O1-03
94
the fibers when applied thereto. Other inorganic densifying agents that are
hygroscopic and may also soften the fibers include many alkali metal,
ammonium, or
substituted ammonium salts of pyrophosphates, hexametaphosphates,
tripolyphosphates, hypophosphates, potyphosphoric acid,
ethylenedianlinetetraacetic
acid, hydroxyethylidenediphosphonate; and aminotri-(methylenephosphonate).
Specific hygroscopic densifying agents include sodium hypophosphate,
tetrapotassium
pyrophosphate, and disodium phosphate: Tetrapotassium pyrophosphate is
particularly
useful. Other specific densifying agents that are hygroscopic include sodium
hexametaphosphate and potassium salt of ethylenediaminetetraacetic acid.
Mixtures of
hygroscopic and non-hygroscopic densifying agents are also useful.
The advantages of this aspect of the present invention extend to
products in composition that include fibers treated with a densifying agent as
described
above where no particulate material, such as super absorbent is present or
where a
particulate material, such as super absorbent is present but not bound to the
fibers.
The following examples illustrate how the application of a densifying
agent to pulp fibers yields a product that is easier to density than the same
untreated
pulp fibers.
EXAMPLE 38
A sample of NB 416 pulp sheet (available from Weyerhaeuser
Company, Tacoma, Washington) was prayed with different levels of glycerin,
while
passing through a slitterfrewind apparatus, to produce several rolls of
material that
comprise different levels of pulp and glycerin. The pulp sheets were then fed
into a
commercial sized hammermill connected to a pocket former and an adult
incontinent
product manufacturing line. During the final stage of manufacture, the
products made
from these pulps treated with various levels of glycerin were passed through a
set of
debulking rolls set to a constant pressure for the entire range of materials.
The finished
products were then opened up and the densities of ~ the core material was
tested.
Results are as follows: -
CA 02412892 2003-O1-03
Sample Basis Weight (glm2)Density (glcm3)
0% Glycerin 673 0.173
5% Glycerin 730 0.192
6% Glycerin 701 0.162
5 7% Glycerin 767 0.199
8% Glycerin 673 0.228
9% Glycerin 721 0.237
9% Glycerin (aged) 653 0.282
The percentages given above are weight percent glycerin based on the
10 dry weight of pulp Sbers. The 9% aged glycerin sample had glycerin applied
to the
pulp sheet approximately three months prior to feeding to the commercial sized
hammermill. These results demonstrate that increasing levels of glycerin on
pulp fibers
yield products with increasing densities for a given set of densifying
conditions: Based
on the results for the aged sample, it is believed that the additional time
allowed for
15 additional hydrogen bond disruption within the cellulose fibers.
EXAMPLE 39
This example illustrates how adding other densifying agents, along with
glycerin produces a product that is easier to density than untreated pulp
fibers.
20 A 'sample of NB 41b pulp sheet (available from Weyerhaeuser
Company, Tacoma, Washington) was sprayed with a solution of 33% lactose and
66%
glycerin so that the resulting product was comprised of 91% O.D. (i.e. oven
dried)
pulp and 9% additive. A second sample of pulp sheet was sprayed with a
solution of
50% glucose and 50% glycerin to obtain a product with similar add-on levels.
These
25 products were then; in turn; processed in a manner similar to that
described in Example
38. Measurement of the core densities of the products made from these
materials
yielded the following results:
CA 02412892 2003-O1-03
96
Sample Basis Weight (g/m=) Density (g/cm3)
0% Glycerin 673 0.173
3% Lactose/6% Glycerin 689 0.263
4.5% Glucose/4.5% Glycerin 751 0.228 ,
These results demonstrate that densifying agents in addition to glycerin
on pulp fibers yield products with increased densities for a given set of
densification
conditions.
EXAMPLE 40
This example illustrates how adding other densifying agents, by
themselves, also produces a product that is easier to density than an
untreated pulp.
Samples of NB 416 pulp sheet (available from Weyerhaeuser Company;
Tacoma, Washington) were treated, by passing through a roll coating device,
with
solutions of 70% sorbitol; 64% sorbitol and 6.4% lactic acid; and 51.2%
sorbitol,
12.8% tetrapotassium pyrophosphate, and 6.4% lactic acid respectively to
produce
sheets with 8%-9% add-on levels of densifying agent per sample. The sheets
were
then air dried over night and the treated pulp sheets were then fed into a
Fitz
hammermill fitted with a 1 in2 square holed screen, shunted to an air lay
machine (an
M & J air lay rtiachine from the M & J Company, Horsens, Denmark) and air laid
into
a web. Similar samples were made from an untreated pulp sheet and one treated
with
9% glycerin alone. All three of the sample webs and the two control webs were
then
passed through a set of debulking rolls set to a pressure of 25 psi and the
densities of
the resulting materials was determined with the following results:
Sample Basis Weight (g/m2) Density (glcm3)
0% Glycerin 176 0.1270
9% Glycerin 168 0.2242
9% Sorbitol 167 0.1904
8% SorbitoUl% Lactic acid 178 O.I396
6% SorbitoU2% Potassium Pyrophosphate/ 172 0.1719
CA 02412892 2003-O1-03
9?
1 % Lactic acid
These results clearly demonstrate that other densifying agents on pulp
also yield products with increased densities for a given set of processing
conditions.
EXAMPLE 41
This example illustrates how the method of adding glycerin to some
crosslinked pulps yields a product that is easier to density than a
corresponding
untreated crosslinked pulp.
300 gram samples of HBA and HBAFF (crosslinked pulps produced by
Weyerhaeuser Company, Tacoma; Washington) were each air entrained in a
blenderlike mixing device and 33 grams of glycerin dissolved in SO milliliters
of
deionized water was sprayed onto the pulp. In each case, the mixer was
stopped, the
product was vacuumed out and allowed to dry overnight. Ten grain samples of
the
treated pulps and ten gram samples of the corresponding untreated pulps were
air laid
into 6 inch diameter pads in a laboratory pad former then pressed at ?00 psi
for one
minute. The thickness of the resulting pads was determined and densities were
calculated with the following results:
Saiuple Basis Weight (g/~r=) Density (g/cm3)
HBAFF 542 0.206
HBAFF/10% Glycerin 520 0.285
HBA 529 0.128
I-iBA/10% Glycerin 521 0.0942
These results show that in the case of HBAFF, addition of the glycerin
gives a product that is more easily densified for a given set of densifying
conditions.
The following example demonstraxes how the application of certain
densifying agents in accordance with the present, invention on pulp yield
products with
improved softness for a given set of processing conditions.
CA 02412892 2004-02-17
98
EXAMPLE 42
This example illustrates how certain densifying agents affect the
stiffness which is one aspect of the softness of an air laid web of fibers
treated with a
densifying agent.
A sample of NB -416# pulp sheet (available from Weyerhaeuser
Company, Tacoma, Washington) was treated, by passing through a roll coating
device
to coat the pulp sheet to 5.8% weight (of a mixture of equal amounts of
sorbitol and
lactic acid) based on the mass of the fibers and added densifying agents.
Another
sample of NB-41 ~ was sprayed with a solution of 50% TKPP to a level of S%
TKPP
based on the mass of the fibers and TKPP. The sheets were then air dried over
night
and the treated pulp sheets and an untreated control were then fed into a Fitz
hammermill fitted with a 1 in2 square holed screen, while simultaneously
adding
IM 390~(a superabsorbent available from Hoechst Celanese, of Portsmouth, VA)
at a
rate to produce a material that was 60% treated fiber and 40% IM 3900. This
mixture
was shunted to an air lay machine (an M & J air lay machine from the M & J
Company,
Horsens, Denmark) and air laid into webs. Samples of each of the webs were
placed in
a flat press and pressed to obtain an equivalent density. Press conditions
were adjusted
so that all the samples were densified to approximately equal densities. The
Gurley
Stiffness test was then performed on each of the pressed samples with the
following
results:
Sample Density (g/cm3) Gurley Stiffness
NB-416~ 0.141 131.7
5.8% SorbitoULactic acid 0.145 96.2
5% TKPP 0.138 125.1
The results demonstrate how densified air laid webs of pulp fibers
treated with certain densifying agents are less stiff than corresponding
densified air laid
webs of untreated pulp fibers. This stiffness date when evaluated in
combination with
other qualities of the web such as compressibility, pressure required to
achieve the
given density, and drape, give some indication that the treated fibers are
softer than
untreated fibers.
XTrademark
CA 02412892 2003-O1-03
99
EXAMPLE 43
This example illustrates how a densifying agent added to pulp fibers
produces material with increased mass without an appreciable increase in
volume.
A sample of NB' 416 pulp sheet (available from Weyerhaeuser
Company, Tacoma; Washington) was treated, by passing through a roll coating
device
to coat the pulp sheet to 9% glycerin based on the mass of the fibers and
glycerin.
Another sample of NB 416 was sprayed with a solution of 50% TKPP to a level of
5%
TKPP based on the mass of the fibers and TKPP. The sheets were then air dried
over
night and the treated pulp sheets and an untreated control were then fed into
a Fitz
hammermill fitted with a 1 in2 square holed screen, while simultaneously
adding IM
3900 (a superabsorbent available from Hoechst Celanese, of Portsmouth, "VA) at
a rate
to produce a material that was 60% treated fiber and 40% IM 3900. This nuxture
was
shunted to an air lay machine (an M & I air lay machine from. the M & J
Company,
Horsens, Denmark) and air laid into webs. Each of the webs had a basis weight
of
approximately 375 grams/meterz. The undensified webs were then calipered and
densities were calculated.
-Sample Caliper (mm) Density (g/cm3)
0% Glycerin 4.I 0.091
9% Glycerin 3.6 0.104
5% TKPP 2.8 0.134
The results demonstrate shows how air Iaid webs made from fibers
treated with certain densifying agents exhibit a decreased caliper for the
same mass of
fibers.
Having illustrated and described the principles of the invention in many
preferred embodiments, it should be apparent to those skilled in the art that
the
invention can be modified in arrangement and detail without departing from
such
principles. We claim all modifications coming within the spirit and scope of
the
following claims.