Note: Descriptions are shown in the official language in which they were submitted.
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
TITLE: DISPERSANT/VISCOSTTY 1MPROVERS FOR LUBRICATING
OlL AND FUELS
FIELD OF THE INVENTION
This invention relates to hydrocarbyl substituted carboxylic compositions
and derivatives prepared therefrom. The carboxylic compositions and
derivatives are
useful as dispersantlviscosity irnprovers for lubricating oil and fuel
compositions.
BACKGROUND OF THE INVENTION
The viscosity of lubricating oils, particularly the viscosity of mineral oil
based lubricating oils, is generally dependent upon temperature. As the
temperature
of the oil is increased, the viscosity usually decreases.
The function of a viscosity improver is to reduce the extent of the decrease
in
viscosity as the temperature is raised or to reduce the extent of the increase
in
viscosity as the temperature is lowered, or both. Thus, a viscosity improver
ameliorates the change of viscosity of an oil containing it with changes in
temperature. The fluidity characteristics of the oil are improved.
Numerous types ~ of additives are used to improve lubricating oil and fuel
compositions. Such additives include, but are not limited to dispersants and
detergents of the ashless and ash-containing variety, oxidation inhibitors,
anti-wear
additives, friction modifiers, and the like. Such materials are well known in
the art
and are described in many publications, for example, Smalheer, et al,
"Lubricant
Additives", Lezius-Hiles Co., Cleveland, OH, USA (1967); M.W. Ranney, Ed.,
"Lubricant Additives", Noyes Data Corp., Park Ridge, NJ, USA (1973); M.J.
Satriana, Ed., "Synthetic Oils and Lubricant Additives, Advances since 1977",
Noyes Data Corp., Park Ridge NJ, USA (1982), W.C. Gergel, "Lubricant Additive
Chemistry", Publication 694-320-6581 of the Lubrizol Corp., Wickliffe, OH, USA
(1994); and W.C. Gergel et al, "Lubrication Theory and Practice" Publication
794-
320-5983 of the Lubrizol Corp., -Wicldiffe, OH, USA (1994); and in numerous
United States patents, for example Chamberlin, III, US 4,326,972, Ripple et
al, US
4,904,401, and Ripple et al, US 4,981,602.
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Dispersants are well-known in the lubricating art. Dispersants are employed
in lubricants to keep impurities, particularly those formed during operation
of
mechanical devices such as internal combustion engines, automatic
transmissions,
etc. in suspension rather than allowing them to deposit as sludge or other
deposits on
the surfaces of lubricated parts.
Conventional dispersants are poor contributors to improving high
temperature, e.g., 100°C, viscosity. Mixtures of conventional
dispersants with
polymeric viscosity improvers are often used but such combinations are costly
and
may adversely affect low temperature viscometric performance.
Multifunctional additives that provide both viscosity improving properties
and dispersarit properties are likewise known in the art. Such products are
described
in numerous publications including Dieter Klamann, "Lubricants and Related
Products", Verlag Chemie Ginbh (1984), pp. 185-193; C. V. Smalheer and R. K.
Smith, "Lubricant Additives",. Lezius-Hiles Co. (196T); M. W. Ranney,
"Lubricant
Additives", Noyes Data Corp. (1973), pp. 92-145, M. W. Ranney, "Lubricant
Additives, Recent Developments", Noyes Data Corp. (1978), pp. 139-164; and M.
W. Ranney, "Synthetic Oils and Additives for Lubricants", Noyes Data Carp.
(1980), pp. 96-166. Each of these publications is hereby expressly
incorporated
herein by reference.
Dispersant-viscosity improvers are generally 'prepared by functionalizing,
i.e., adding polar groups, to a hydrocarbon polymer .
Hayashi et al, U.S. 4,670,173 relates to compositions suitable for use as
dispersant-viscosity improvers made by reacting an acylating reaction product
which
is formed by reacting a hydrogenated bloclc copolymer and an alpha,beta
olefinically
unsaturated reagent in the presence of free-radical initiators, then reacting
the
acylating product with a primary amine and optionally with a polyamine and a
mono-functional acid.
Chung et al, US 5,035,821 relates to viscosity index improver-dispersants
comprised of the reaction products of an ethylene copolymer grafted with
ethylenically unsaturated carboxylic acid moieties, a polyamine having two or
more
primary amino groups or polyol and a high functionality long chain hydrocarbyl
substituted dicarboxylic acid or anhydride.
2
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Van Zon et al, U.S. 5,049,294, relates to dispersant/VI improvers produced
by reacting an alpha,beta-unsaturated carboxylic acid with a selectively
hydrogenated star-shaped polymer then reacting the product so formed with a
Iong
chain alkane-substituted carboxylic acid and with a C1 to Cl8 amine containing
1 to 8
nitrogen atoms andlor with an alkane polyol having at least two hydroxy groups
or
with the preformed product thereof.
Bloch et al, U.S. 4,517,104, relates to oil soluble viscosity improving
ethylene copolymers reacted or grafted with ethylenically unsaturated
carboxylic
acid moieties then with polyamines having two or more primary amine groups and
a
carboxylic acid component or the preformed reaction product thereof.
Gutierrez et al, U.S. 4,632,769, describes oil-soluble viscosity improving
ethylene copolymers reacted or grafted with ethylenically unsaturated
carboxylic
acid moieties and reacted with polyamines having two or more primary amine
groups and a C22 to C28 olefin carboxylic acid component.
Lange, et al, U.S. 4,491,527 relates to ester-heterocycle compositions useful
as "lead paint" inhibitors in lubricants. The compositions comprise
derivatives of
substituted carboxylic ~ acids in which the substituent is a substantially
aliphatic,
substantially saturated hydrocarbon based radical containing at least about 30
aliphatic carbon atoms; said derivatives being the combination of: (A) at
least one
ester of said carboxylic acids in which all the alcohol moieties are derived
from at
least on mono- or polyhydroxyalleane; and (B) at least one heterocyclic
condensation
product of said substituted carboxylic acids containing at least one
heterocyclic
moiety which includes a 5- or 6-membered ring which contains at Ieast two ring
hetero atoms selected from the group consisting of oxygen, sulfur and nitrogen
separated by a single carbon atom, at least one of said hetero atoms being
nitrogen,
and at least one carboxylic.moiety; the carboxylic and heterocyclic moieties
either
being linked through an ester or amide linkage or being the same moiety in
which
said single carbon atom separating two ring hetera atoms corresponds to a
carbonyl
carbon atom of the substituted carboxylic acid.
Lange, et al, U.S: 5,512,192 teach dispersant viscosity improvers for
lubricating oil compositions comprising a vinyl substituted aromatic-aliphatic
conjugated dime block copolymer grafted with an ethylenically unsaturated
3
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
carboxylic acid reacted with at least one polyester containing at least one
condensable ~hydroxy group and at least one polyamine having at least one
condensable primary or secondary amino group, and optionally, at least one
hydrocarbyl substituted carboxylic acid or anhydride.
Lange, U.S. 5,540,851 describes dispersant viscosity improvers for
lubricating oil compositions which are the reaction product of (a) an oil
soluble
ethylene-alpha olefin copolymer wherein the alpha olefin is selected from the
group
consisting Of C3-Zg alpha olefins, said polymer having a number average
molecular
weight ranging from about 30,000 to about 300,000 grafted with an
ethylenically
unsaturated carboxylic acid or functional derivative thereof; with at least
one polyester
containing at least one condensable hydroxyl group, and at least one polyamine
having
at least one condensable primary or secondary amino group, and optionally
at.least one
hydrocarbyl substituted carboxylic acid or anhydride.
Each of these patents is hereby expressly incorporated herein by reference.
. For additional disclosures concerning multi-purpose additives and
particularly viscosity improvers and dispersants, the disclosures of the
following
United States patents are incorporated herein by reference:
2,973,344 ~ 3,488,049 3,799,877
3,278,550 3,513,095 3,842,010
3,311,558 3,563,960 3,864,098
3,312,619 3,598,738 3,864,268
3,326,804 ~ 3,615,288 3,879,304
3,403,011 3,637,610 4,033,889
3,404,091 3,652,239 4,051,048
3,445,389 3,687,849 4,234,435
Many such additives are derived from carboxylic reactants, for example,
acids, esters, anhydrides, lactones, and others. Specific examples of commonly
used
carboxylic compounds used as intermediates for preparing lubricating oil
additives
include high molecular weight hydrocarbyl group substituted carboxylic acids
such
as succinic acids and anhydrides, aromatic acids, such as salicylic acids, and
others.
Illustrative carboxylic compounds are described in Lange et al , US 5,512,192,
Lange US 5,540,851 and 5,811,378 and Hayashi et al US 4,670,173.
4
CA 02413979 2002-12-19
04-07-2002 US0119880
Such carboxylic acids are typically prepared by thermally reacting or free
radical grafting of carboxylic groups such as malefic anhydride, acrylic
compounds,
etc. with a high molecular weight hydrocarbon. Reaction rates are relatively
low.
Attempts to improve the conversion rate by increasing the reaction temperature
and/or using super-atmospheric pressure often results in degradation of
malefic
anhydride to carbon dioxide, water and tar-like solids
In industry, it is also desirable to have available a wide variety of
reactants
available to prepare compositions. Materials shortages, costs, etc. contribute
to
uncertainties in the industry. These uncertainties can be relieved when more
than a
IO limited number of types raw materials are available to a manufacturer. The
compositions of this invention are prepared employing raw materials that are
different from, and are not suggested by, traditionally used raw materials.
SUMMARY OF THE INVENTION
This invention relates to carboxylic compositions and derivatives thereof
useful as dispersant viscosity improvers for lubricating oils and fuels. The
carboxylic compositions are also useful as intermediates for preparing
derivatives
for use as dispersant viscosity improvers. Both the carboxylic compositions
and the
derivatives thereof find utility as dispersant/viscosity improvers for
lubricating oil
and fuel compositions. Hydrocarbyl group substituted carboxylic compositions
are
derived from (A) a hydrocarbon polymer having M" ranging from about 20,000 to
about 500,000 and (B) an a,(i-unsaturated carboxylic compound prepared by
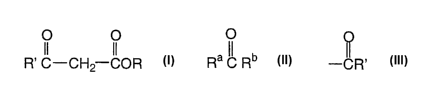
reacting (1) an active methylene compound of the formula
R' c-cH2 coR
and (2} a carbonyl compound of the general formula
Ra IC Rb
wherein R° is H or hydrocarbyl and Rb is
5
AMENDED SHEET
CA 02413979 2002-12-19
04-07-2002 US0119880
r
R
-CR' ~ v,rherein each R' is independently R or OR and each R is,
independently,
H or a hydrocarbyl group; and lower alkyl acetals, ketals, hemiacetals and
hemiketals of the carbonyl compound (2). Carboxylic derivative compositions
are
obtained by reacting the carboxylic compositions with a reactant selected from
the
group consisting of (a) amines characterized by the presence within their
structure of
at least one condensable H-N< group, (b) alcohols, {c) reactive metal or
reactive
metal compounds, and {d) a combination of two or more of any of (a} through
(c),
the components of (d) being reacted with the carboxylic composition
simultaneously
or sequentially, in any order.
DETAILED DESCRIPTION OF THE PREFERRED EMBODllVIENTS
As used herein, the terms "hydrocarbon", "hydrocarbyl" or "hydrocarbon
based" mean that the group being described has predominantly hydrocarbon
character within the context of this invention. These include groups that are
purely
hydrocarbon in nature, that is, they contain only carbon and hydrogen. They
may
also include groups containing substituents or atoms which do not alter the
predominantly hydrocarbon character of the group. Such substituents may
include
halo-, alkoxy-, nitro-, etc. These groups also may contain hetero atoms.
Suitable
hetero atoms will be apparent to those skilled in the art and include, for
example,
sulfur, nitrogen and oxygen. Therefore, while remaining predominantly
hydrocarbon in character within the context of this invention, these groups
may
contain atoms other than carbon present in a chain or ring otherwise composed
of
carbon atoms.
In general, no more than about three non-hydrocarbon substituents or hetero
atoms, and preferably no more than one, will be present for every 10 carbon
atoms
in the hydrocarbon or hydrocarbon based groups. Most preferably, the groups
are
purely hydrocarbon in nature, that is they are essentially free of atoms other
than
carbon and hydrogen.
Throughout the specification and claims the expression oil soluble or
dispersible is used. By oil soluble or dispersible is meant that an amount
needed to
provide the desired level of activity or performance can be incorporated by
being
6
AMENDED SHEET
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
dissolved, dispersed or suspended in an oil of lubricating viscosity. Usually,
this
_ means that at least about 0.001 % by weight of the material can be
incorporated in a
lubricating oil composition. For a further discussion of the terms oil soluble
and
dispersible, particularly "stably dispersible", see U.S. Patent 4,320,019
which is
expressly incorporated herein by reference for relevant teachings in this
regard. .
It must be noted that as used in this specification and appended claims, the
singular forms also include the plural unless the context clearly dictates
otherwise.
Thus the singular forms "a", "an", and "the" include the plural; for example
"an
amine" includes mixtures of amines of the same type. As another example the
singular form "amine" is intended to include both singular and plural unless
the
context clearly indicates otherwise.
Hydrocarbon Polymer
As used herein, the expression 'polymer' refers to polymers of all types,
i.e.,
homopolymers and copolymers. The term homopolymer refers to polymers derived
from essentially one monomeric species; copolymers are defined herein as being
derived from 2 or more monomeric species.
The hydrocarbon polymer is an essentially hydrocarbon based polymer,
usually one having a number average molecular weight ( M n) between about
20,000
and about 500,000, often from about 20,000 to about 300,000, frequently from
about
40,000 to about 200,000. Molecular weights of the hydrocarbon polymer are
determined using well known methods described in the literature. Examples of
procedures for determining the molecular weights are gel permeation
chromatography (GPC) (also known as size-exclusion chromatography) and vapor
phase osmometry (VPO). It is understood that these are average molecular
weights.
GPC molecular weights are typically accurate within about 5-10%. Even with
narrow polydispersity, a polymer with M n of about 20,000 rnay have some
species
as low as about 15,000. A polymer with M W about 35,000 and M n about 20,000
may have GPC peaks corresponding to polymer components as low as about 10,000
and as high as 75,000.
These and other procedures are described in. numerous publications
including:
7
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
P.J. Flory, "Principles of Polymer Chemistry", Cornell University Press
(1953), Chapter VII, pp. 266-316,
"Macromolecules, an Introduction to Polymer Science", F.A. Bovey and
F.H. Winslow, Editors, Academic Press (1979), pp. 296-312, and
W.W. Yau, J.J. Kirkland and D.D. Bly, "Modern Size Exclusion Liquid
Chromatography", John Wiley and Sons, New York, 1979.
Unless otherwise indicated, GPC molecular weights referred to herein are
polystyrene equivalent weights, i.e., are molecular weights determined
employing
polystyrene standards.
A measurement which is complementary to a polymer's molecular weight is
the melt index (ASTM D-1238). Polymers of high melt index generally have low
molecular weight, and vice versa. The polymers of the present invention
preferably
have a melt index of up to 20 dg/min., more preferably 0.1 to 10 dg/rnin.
These publications are hereby incorporated by reference for relevant
disclosures contained therein relating to the determination of molecular
weight.
When the molecular weight of a polymer is greater than desired, it may be
reduced by techniques known in the art. Such techniques include mechanical
shearing
of the polymer employing masticators, ball mills, roll mills, extruders and
the like.
~xidative or thermal shearing or degrading techniques are also useful and are
known.
Details of numerous procedures for shearing polymers are given in U.S.
5,348,673
which is hereby incorporated herein by reference for relevant disclosures in
this regard.
Reducing molecular weight also tends to improve the subsequent shear stability
of the
polymer.
The polymer may contain aliphatic, aromatic or cycloaliphatic components,
or mixtures thereof. When the polymer is prepared from the monomers, it may
contain substantial amounts of olefinic unsaturation, oftentimes far in excess
of that
which is desired for this invention. The polymer may be subjected to
hydrogenation
to reduce the amount of unsaturation to such an extent that the resulting
hydrogenated polymer has olefinic unsaturation, based on the total number of
carbon
to carbon bonds in the polymer, of less than 5%, frequently less than
2°7o, often no
more than 1% olefinic unsaturation.
8
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
In one embodiment, the polymer is substantially saturated. By substantially
saturated is meant that no more than 5% of the carbon to carbon bonds, often
no
more than 1% and frequently no more than 0.5% of the carbon to carbon bonds
are
olefinically unsaturated. Most often, substantially saturated means that the
polymer
is essentially free of olefinic unsaturation. In the case where the polymer is
substantially saturated, the reaction with (B) is conducted employing a free
radical
initiator. Such processes are described in U.S. Patents 5,512,192 and
5,540,851
which are incorporated herein by reference.
In another embodiment, the polymer (A) contains olefinic unsaturation and
the reaction is conducted thermally, employing the well lcnown "ene" process,
optionally in the presence of added chlorine. The use of added chlorine during
the
reaction often facilitates the reaction. Nonetheless, in order to avoid the
presence of
chlorine in the grafted product and derivatives thereof, it is preferred to
conduct the
grafting reaction thermally or in the presence of a free radical initiator.
_ The "ene" process is described in the literature, fox example in U.S. Patent
No. 3,412,111 and Ben et al, "The Ene Reaction of Malefic Anhydride With
Alkenes", J.C.S Perl~in II (1977), pp. 535-537, both of which are incorporated
herein
by reference for relevant disclosures contained therein.
Chlorine assisted grafting is described in numerous patents including
U.S. Patents 3,215,707; 3,912,764; and 4,234,435, which are incorporated
herein by
reference.
Typically, from about .90 to about 99.9%, often 100% of carbon to carbon
bonds in the polymer are saturated. As noted, the choice of grafting procedure
typically depends upon the extent of olefinic unsaturation present in the
polymer.
Free radical initiators are typically used when the polymer is substantially
saturated;
the thermal "ene" process may be used when the polymer contains significant
amounts of olefinic unsaturation.
Aromatic unsaturation is not considered olefinic unsaturation within the
context of this invention. Depending on hydrogenation conditions, up to about
20%
of aromatic groups may be hydrogenated; however, typically no more than about
5%,' usually less than 1% of aromatic bonds are hydrogenated. Most often,
substantially none of the aromatic bonds are hydrogenated.
9
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
In one typical embodiment, the polymer contains an average of from 1 to .
. , about 9,000 olefinic double bonds, more often from about 1 to about 100
olefinic
double bonds, even more often from about 1, frequently 2 to about 10, up to
about
50, olefinic double bolds per molecule based on the M n of the polymer. In
another
embodiment, the polymer contains about I olefinic double bond for about every
20,
often for about every 70 to 7000 carbon atoms. In still another embodiment,
the
hydrocarbon polymer contains about 1 olefinic double bond for every 4,000 to
20,000 on M n basis, often, about 1 olefinic double bond per 1,000 to 40,000
on M n
basis. Thus, for example, in this embodiment a polymer of M n = 80,000 would
contain from about 2 to about 80 olefinic double bonds per molecule, often
from
about 4 to about 20 double bonds per molecule. In yet another embodiment, the
hydrocarbon polymer (P) contains about 1 olefinic double bond for about every
300
to 100,000 on M n basis.
As noted hereinabove, in another embodiment, the polymer is substantially
saturated, as defined hereinabove.
The equivalent weight per mole of carbon to carbon double bonds is defined
herein as the mole-equivalent weight. For example, a polymer having M n of
100,000 and which contains an average of 4 moles of carbon to carbon double
bonds, has a mole equivalent weight of 100,000/4 = 25,000. Conversely, the
polymer has one mole of carbon to carbon double bonds per 25,000 M n.
In preferred embodiments, the hydrocarbon polymer is at least one oil
soluble or dispersible homopolymer or copolymer selected from the group
consisting of:
(1) polymers of dimes;
(2) copolymers of conjugated dimes with vinyl substituted aromatic
compounds;
(3) polymers of aliphatic olefins having from 2 to about 28 carbon atoms;
(4) olefin-dime copolymers; and
(5) star polymers.
These preferred polymers are described in greater detail hereinbelow.
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
(1) Polymers of Dienes
The hydrocarbon polymer may be a homopolymer or copolymer of one or
more dimes. The dimes may be conjugated such as isoprene, butadiene and
piperylene or non-conjugated such as 1-4 hexadiene, ethylidene norbornene,
vinyl
norbornene, 4-vinyl cyclohexene, and dicyclopentadiene. Polymers of conjugated
dimes are preferred. Such polymers are conveniently prepared via free radical
and
anionic polymerization techniques. Emulsion techniques are commonly employed
for
free radical polymerization.
As noted hereinabove, useful polymers have M n ranging from about 20,000
to about 500,000. More often, useful polymers of this type have M n ranging
from
about 50,000 to about 150,000.
These polymers rnay be and often are hydrogenated to reduce the amount of
olefinic unsaturation present in the polymer. They may or may not be
exhaustively
hydrogenated. Hydrogenation is often accomplished employing catalytic methods.
Catalytic techniques employing hydrogen under high pressure and at elevated
temperature are well-known to those skilled in the chemical art. Other methods
are
also useful and are well lrnown to those spilled in the art.
Extensive discussions of dime polymers appear in the "Encyclopedia of
Polymer Science and Engineering", Volume 2, pp. 550-586 and Volume 8, pp. 499-
532, Wiley-Interscience (1986), which are hereby expressly incorporated herein
by
reference for relevant disclosures in this regard.
The polymers include homopolymers and copolymers of conjugated dimes
including polymers of 1,3-dimes of the formula
~1' ~2 ~3 /R4
C= C- C= C
ERs
wherein each substituent denoted by R, or R with a numerical subscript, is
independently hydrogen or hydrocarbon based, wherein hydrocarbon based is as
defined hereinabove. Preferably at least one substituent is H. Normally, the
total
carbon content of the dime will not exceed 20 carbons. Preferred dimes for
preparation of the polymer are piperylene, isoprene, 2,3-dimethyl-1,3-
butadiene,
chloroprene and 1,3-butadiene.
m
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Suitable homopolymers of conjugated dimes are described, and methods for
their preparation are given in numerous U.S. patents, including the following:
3,547,821
3,835,053
3,959,161
3,965,019
4,085,055
4,116,917
As a specific example, U.S. 3,959,161 teaches the preparation of
hydrogenated polybutadiene. In another example, upon hydrogenation,
1,4-polyisoprene becomes an alternating copolymer of ethylene and propylene.
Copolymers of conjugated dimes are prepared from two or more conjugated
dimes. Useful dienes are the same as those described in the preparation of
homopolymers of conjugated dimes hereinabove. The following U.S. Patents
describe dime copolymers and methods for preparing them:
3,965,019
4,073,737
4,085,055 _
4,116,917
For example, U.S. Patent 4,073,737 describes the preparation and hydrogenation
of
butadiene-isoprene copolymers.
~2) Copolymers of Conjugated Dienes with Vinyl Substituted Aromatic Compounds
In one embodiment, the hydrocarbon polymer is a copolymer of a vinyl-
substituted aromatic compound and a conjugated dime. The vinyl substituted
aromatics generally contain from 8 to about 20 carbons, preferably from 8 to
12
carbon atoms and most preferably, 8 or 9 carbon atoms.
Examples of vinyl substituted aromatics include vinyl anthracenes, vinyl
naphthalenes and vinyl benzenes (styrenic compounds). Styrenic compounds are
preferred, examples being styrene, alpha-methystyrene, ortho-methyl styrene,
meta-
methyl styrene, para-methyl styrene, para-tertiary-butylstyrene and
chlorostyrene,
with styrene being preferred.
I2
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
The conjugated dimes generally have from 4 to about 10 carbon atoms and
preferably from 4 to 6 carbon atoms. Example of conjugated dienes include
piperylene, 2,3-dimethyl-1,3-butadiene, chloroprene, isoprene and 1,3-
butadiene,
with isoprene and 1,3-butadiene being particularly preferred. Mixtures of such
conjugated dimes are useful.
The vinyl substituted aromatic content of these copolymers is typically in the
range of about 20% to about 70% by weight, preferably about 40% to about 60%
by
weight. The aliphatic conjugated dime content of these copolymers is typically
in
the range of about 30% to about 80% by weight, preferably about 40% to about
60%
by weight.
The polymers, and in particular, styrene-dime copolymers, can be random
copolymers or block copolymers, which include regular block copolymers or
random block copolymers. Random copolymers are those in which the comonomers
are randomly, or nearly randomly, arranged in the polymer chain with no
significant
blocking of homopolymer of either monomer. Regular block copolymers are those
in which a small number of relatively long chains of homopolymer of one type
of
monomer are alternately joined to a small number of relatively long chains of
homopolymer of another type of monomer. Random block copolymers are those in
which a larger number of relatively short segments of homopolymer of one type
of
monomer alternate with relatively short segments . of homopolymer of another
monomer.
The random, regular bloclc and random block polymers used in this invention
may be linear, or they may be partially or highly branched. The relative
arrangement of homopolymer segments in a linear regular block or random block'
polymer is obvious. Differences in structure lie in the number and relative
sizes of
the homopolymer segments; the arrangement in a linear block polymer of either
type
is always alternating in homopolymer segments.
Normal or regular block copolymers usually have from 1 to about 5, often 1
to about 3, preferably only from 1 to about 2 relatively large homopolymer
blocks of
each monomer. Thus, a linear regular diblock copolymer of styrene or other
vinyl
aromatic monomer (S) and dime (D) would have a general structure represented
by
a large block of homopolymer (S) attached to a large block of homopolymer (D),
as:
' 13
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
(S)s~)d
where subscripts s and d are as described hereinbelow. Similarly, a regular
linear
tri-block copolymer of styrene or other vinyl aromatic monomer (S) and dime
monomer (D) may be represented, for example, by
(S)s~)d(S)s ~r ~)d(~)s~)d~
Techniques vary for the preparation of these "S-D-S" and "D-S-D" triblock
polymers, and are described in the literature for anionic polymerization.
A third monomer (T) may be incorporated into linear, regular block
copolymers. Several configurations are possible depending on how the
hornopolymer segments are arranged with respect to each other. For example,
linear
triblock copolymers of monomers (S), (D) and (T) can be represented by the
general
configurations:
(S)s ~)d-~)c~ (S)s (T)c-~)d~ or ~)d-(S)s (T)c~
wherein the lower case letters s, d and t represent the approximate number of
monomer units in the indicated block.
The sizes of the blocks are not necessarily the same, but may vary
considerably. The only stipulation is that any regular bloclc copolymer
comprises
relatively few, but relatively large, alternating homopolymer segments.
As an example, when (D) represents blocks derived from dime such as
isoprene or butadiene, "d" usually ranges from about 100 to about 2000,
preferably
from about 500 to about 1500; when (S) represents, for example, blocles
derived
from styrene, "s" usually ranges from about 100 to about 2000, preferably from
about 200 to about 1000; and when a third block (T) is present, "t" usually
ranges
from about 10 to about 1000, provided that the ~M n of the polymer is within
the
ranges indicated as useful for this invention.
The copolymers can be prepared by methods well known in the art. Such
copolymers usually are prepared by anionic polymerization using Group Ia
metals in
the presence of electron-acceptor aromatics, or preformed organornetallics
such as
sec-butyllithium as polymerization catalysts.
The styrene/diene block polymers are usually made by anionic
polymerization, using a variety of techniques, and altering reaction
conditions to
produce the most desirable features in the resulting polymer. In an anionic
14
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
polymerization, the initiator can be either an organometallic material such as
an
alkyl lithium, or the anion formed by electron transfer from a Group Ia metal
to an
aromatic material such as naphthalene. A preferred organometallic material is
an
alkyl lithium such as sec-butyl lithium; the polymerization is initiated by
addition of
the butyl anion to either the dime monomer or to the styrene.
When an alkyl lithium initiator is used, a homopolymer of one monomer,
e.g., styrene, can be selectively prepared, with each polymer molecule having
an
anionic terminus, and lithium gegenion. The carbanionic terminus remains an
active initiation site toward additional monomers. The resulting polymers,
when
monomer is completely depleted, will usually all be of similar molecular
weight and
composition, and the polymer product will be "monodisperse" (i.e., the ratio
of
weight average molecular weight to number average molecular weight is very
nearly 1.0). At this point, addition of 1,3-butadiene, isoprene or other
suitable
anionically polymerizable monomer .to the homopolystyrene-lithium "living"
polymer produces a second segment which grows from the terminal anion site to
produce a living di-block polymer having an anionic terminus, with lithium
gegenion.
Subsequent introduction of additional styrene can produce a new poly
S-block-poly D-block-poly S, or S-D-S triblock polymer; higher orders of block
polymers can be made by consecutive stepwise additions of different monomers
in
different sequences.
Alternatively, a living dibloclc polymer can be coupled by exposure to an
agent such as a dialkyl dichlorosilane. When the carbanionic "heads" of two S-
D
dibloclc living polymers are coupled using such an agent, precipitation of
LiCl
occurs to give an S-D-S triblock polymer.
Block copolymers made by consecutive addition of styrene to give a
relatively large homopolymer segment (S), followed by a dime to give a
relatively
large homopolymer segment (D), are referred to as poly-S-block-poly-D
copolymers, or S-D diblock polymers.
When metal naphthalide is employed as initiator, the dianion formed by
electron transfer from metal, e.g., Na, atoms to the naphthalene ring can
generate
dianions which may initiate polymerization, e.g. of monomer S, in two
directions
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
simultaneously, producing essentially a homopolymer of S having anionic
termini at
both ends. '
Subsequent exposure of the poly (S) dianion to a second monomer (D)
results in formation of a poly D-block-poly S-block-poly D, or a D-S-D
triblocle
polymeric dianion, which may continue to interact with additional anionically-
polymerizable monomers of the same, or different chemical type, in the
formation
of higher order -block polymers. Ordinary block copolymers are generally
considered to have up to about 5 such bloclcs.
Usually, one monomer or another in a mixture will polymerize faster,
leading to a segment that is richer in that monomer, interrupted by occasional
incorporation of the other monomer. This can be used to build a type of
polymer
referred to as a "random block polymer", or "tapered block polymer". When a
mixture of two different monomers is anionically polymerized in a non-polar
paraffinic solvent, one will initiate selectively, and usually polymerize to
produce a
relatively short segment of homopolymer. Incorporation of the second monomer
is
inevitable, and this produces a short segment of different structure.
Incorporation of
the first monomer type then produces another short segment of that
homopolymer,
and the process continues, to give a "random" alternating distribution of
relatively
short segments of homopolymers, of different lengths. Random blocle polymers
are
generally considered to be those comprising more than 5 such blocks. At some
point, one monomer will become depleted, favoring incorporation of the other,
leading to ever longer blocks of homopolymer, resulting in a "tapered block
copolymer."
An alternative way of preparing random or tapered block copolymers
involves initiation of styrene, and interrupting with periodic, or step,
additions of
dime monomer. The additions are programmed according to the relative
reactivity
ratios and rate constants of the styrene and particular dime monomer.
"Promoters" are electron-rich molecules that facilitate anionic initiation and
polymerization rates while lessening the relative differences in rates between
various monomers. Promoters also influence the way in which dime monomers are
incorporated into the block polymer, favoring 1,2-polymerization of dimes over
the
normal 1,4-cis- addition.
16
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
These polymers may. have considerable olefinic unsaturation, which may be
reduced, if desired. Hydrogenation to reduce the extent of olefinic
unsaturation may
be carried out to reduce approximately 90-99.1 % of the olefinic. unsaturation
of the
initial polymer, such that from about 90 to about 99.9°70 of the carbon
to carbon
bonds of the polymer are saturated.. In. general, it is preferred that these
copolymers contain no more than about 10%, preferably no more than 5% and
often no more than about 0.5% residual olefinic unsaturation on the basis of
the
total amount of olefinic double bonds present in the polymer prior to
hydrogenation. Unsaturation can be measured by a number of means well
known to those of shill in the art, including infrared, nuclear magnetic
resonance spectroscopy, bromine 'number; iodine number, and other means.
Aromatic unsaturation is not considered to be olefinic unsaturation within the
context of this invention.
Hydrogenation techniques are well known to those of skill in the art. One
common method is to contact the copolymers with, hydrogen, often at
superatmospheric pressure in the presence of a metal catalyst such as
colloidal
nickel, palladium supported on charcoal, etc. Hydrogenation may be carried out
as
part of the overall production process, using finely divided, or supported,
nickel
catalyst. Other transition metals may also be used to effect the
transformation.
Other techniques are known in the art.
Other polymerization techniques such as emulsion polymerization can be
used.
Often the arrangement of the various homopolymer blocles is dictated by the
reaction conditions such as catalyst and polymerization characteristics of the
monomers employed. Conditions for modifying arrangement of polymer blocks are
well known to those of skill in the polymer art. Literature references
relating to
polymerization techniques and methods for preparing certain types of block
polymers include:
1) "Encyclopedia of Polymer Science and Engineering", i~liley-
Interscience Publishing, New York, (1986);
2) A: Noshay and J.E. McCrrath, "Block Copolymers", Academic Press,
New York, (1977);
17
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
3) R.J. Ceresa, ed., "Blocl~ and Graft Copolymerization", John Wiley
and Sons, New York, .(1976); and
4) D.J. Meier, ed., (Block Copolymers", MMI Press, Harwood
Academic Publishers, New Yorlc, (1979).
Each of these is hereby incorporated herein by reference for relevant
disclosures relating to block copolymers.
Examples of suitable commercially available regular linear diblocle
copolymers as set forth above include SHELLVIS°-40, and SHELLVIS "-50,
both
hydrogenated styrene-isoprene block copolymers, manufactured by Shell
Chemical.
Examples of commercially available random block and tapered block
copolymers include the various GLISSOVISCAL° styrene-butadiene
copolymers
manufactured by BASF. A previously available random block copolymer was
PH1L-AD° viscosity improver, manufactured by Phillips Petroleum.
The copolymers preferably have M n in the range of about 20,000 to about
500,000, more preferably from about 30,000 to about 150,000. The weight
average
molecular weight ( M W) for these copolymers is generally in the range of
about 50,000
to about 500,000, preferably from about 50,000 to about 300,000.
Copolymers of conjugated dimes with olefins containing aromatic groups,
e.g., styrene, methyl styrene, etc. are described in numerous patents
including the
following:
3,554,911 4,082,680
3,992,310 4,085,055
3,994,815 4,116,917
4,031,020 4,136,048
4,073,738 4,145,298
4,077,
893
For example, U.S. Patent 3,554,911 describes a random butadiene-
styrene copolymer, its preparation and hydrogenation.
(3) Polymers of Aliphatic Olefins
Another useful hydrocarbon polymer is one which in its main chain is
composed essentially of aliphatic olefin, especially alpha olefin, monomers.
The
18
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
polyolefins of this embodiment thus exclude polymers which have a large
component
of other types of monomers copolymerized ~in the main polymer , such as ester
monomers, acid monomers, and the like. The polyolefin may contain impurity
amounts of such materials, e.g., less than 5% by weight, more often less than
1% by
weight, preferably, less than 0.1% by weight of other monomers. Useful
polymers
include oil soluble or dispersible polymers of alpha-olefins.
The olefin copolymer preferably has a number average molecular weight ( M n)
determined by gel-permeation chromatography employing polystyrene standards,
ranging from about 20,000 to about 500,000, often from about 30,000 to about
300,000, often to about 200,000, more often from about 50,000 to about
150,000, even
more often from about 80,000 to about 150,000. Exemplary polydispersity values
( M W/ M ") range from about 1.5 to about 3.5, often to about 3.0, preferably,
from about
1.7, often from about 2.0, to about 2.5.
These polymers may be homopolymers .or copolymers and are preferably
polymers of alpha-olefins having from 2 to about 28 carbon atoms. Preferably
they are
copolymers, more preferably copolymers of ethylene and at least one other cc-
olefin
having from 3 to about 28 carbon atoms, i.e., one of the formula CH2 = CHRl
wherein
Rl is straight chain or branched chain allcyl radical comprising 1 to 26
carbon atoms.
Preferably Rl is alkyl of from 1 to 8 carbon atoms, and more preferably is
alkyl of from
1 to 2 carbon atoms. Examples include homopolymers from monoolefins such as
propylene, 1-butene, isobutene, 1-pentene, 1-hexene, 4-methyl-1-pentene, 1-
heptene,
1-octene, 1-nonene, 1-decene, etc and copolymers, preferably of ethylene with
one
or more of these monomers. Preferably, the polymer of olefins is an ethylene-
propylene copolymer.
The ethylene content is preferably in the range of 20 to 80 percent by weight,
and more preferably 30 to 70 percent by weight. When propylene and/or 1-butene
are
employed as comonomer(s) with ethylene, the ethylene content of such
copolymers
most preferably is 45 to 65 percent, although higher or lower ethylene
contents may be
present. Most preferably, these polymers are substantially free of ethylene
homopolymer, although they may exhibit a degree of crystallinity due to the
presence
of small crystalline polyethylene segments within their microstructure.
19
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
In one particular embodiment, the polymer is a homopolymer derived from a
butene, particularly, isobutylene. Especially preferred is where the polymer
comprises
terminal vinylidene olefinic double bonds.
The polymers employed in this embodiment may generally be prepared
substantially in accordance with procedures which are well known in the art.
Catalysts employed in the production of the reactant polymers are likewise
well known. One broad class of catalysts particularly suitable for
polymerization of
a-olefins, comprises coordination catalysts such as Ziegler or Ziegler-Natta
catalysts
comprising a transition metal atom. Ziegler-Natta catalysts are composed of a
combination of a transition metal atom with an organo aluminum halide and may
be
used with additional complexing agents..
Other useful polymerization catalysts are the metallocene compounds. These
are organometallic coordination compounds obtained as cyclopentadienyl
derivatives of a transition metal or metal halide. The metal is bonded to the
cyclopentadienyl ring by electrons moving in orbitals extending above and
below
the plane of the ring (~ bond). The use of such materials as catalysts for the
preparation of ethylene-alpha olefin copolymers is described .in U.S. Patent
5,446,221. The procedure described therein provides ethylene-alpha olefin
copolymers having at least 30% of terminal ethenylidene unsaturation. This
patent
is hereby incorporated herein by reference for relevant disclosures.
Polymerization using coordination catalysis is generally conducted at
temperatures ranging between 20° and 300° C, preferably between
30° and 200°C.
Reaction time is not critical and may vary from several hours or more to
several
minutes or less, depending upon factors such as reaction temperature, the
monomers
to be. copolymerized, and the like. One of ordinary skill in the art may
readily obtain
the, optimum reaction time for a given set of reaction parameters by routine
experimentation. Preferably, the polymerization will generally be completed at
a
pressure of 1 to 40 MPa (l0.to 400 bar).
The polymerization may be conducted employing liquid monomer, such as
liquid propylene, or mixtures of liquid monomers (such as mixtures of liquid
propylene and 1-butene), as the reaction medium. Alternatively, polymerization
may be accomplished in.the presence of a hydrocarbon inert to the
polymerization
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
such as butane, pentane, isopentane, hexane, isooctane, decane, toluene,
xylene, and
the like.
When carrying out the polymerization in a batch-type fashion, the reaction
diluent (if any) and the alpha-olefin comonomer(s) are charged at appropriate
ratios to
a suitable reactor. Care should be taken that all ingredients are dry, with
the reactants
typically being passed through molecular sieves or other drying means prior to
their
introduction into the reactor. Subsequently, components) of the catalyst are
introduced while agitating the reaction mixture, thereby causing
polymerization to
commence. Alternatively, components) of the catalyst may be premixed in a
solvent
and then fed to the reactor. As polymer is being formed, additional monomers
may be
added to the reactor. Upon completion of the reaction, unreacted monomer and
solvent
are either flashed or distilled off, if necessary by vacuum, and the copolymer
withdrawn from the reactor.
The polymerization may be conducted in a continuous manner by
simultaneously feeding the reaction diluent (if employed), monomers,
components) of
the catalyst to a reactor .and withdrawing solvent, unreacted monomer and
polymer
from the reactor so as to allow a residence time of ingredients long enough
for forming
polymer of the desired molecular weight; and separating the polymer from the
reaction
mixture.
In those situations wherein the molecular weight of the polymer product that
would be produced at.a given set of operating conditions is higher than
desired, any
of the techniques known in the prior art for control of molecular weight, such
as
polymerization temperature control, may be used.
The polymers are preferably formed in the substantial absence of added H2
gas, that is, H2 gas added in amounts effective to substantially reduce the
polymer
molecular weight.
The polymers can be random copolymers, block copolymers, and random
block copolymers. Ethylene propylene copolymers are usually random copolymers.
Block copolymers may be obtained by conducting the reaction in a tubular
reactor.
Such a procedure is described in U.S. 4,804,794 which is hereby incorporated
by
reference for relevant disclosures in this regard.
21
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Numerous United States patents, including the following, describe the
preparation of copolymers of alpha olefins.
3,513,096 4,068,057
3,551,336 4,081,391
3,562,160 4,089,794
3,607,749 4,098,710
3,634,249 4,113,636
3,637,503 4,132,661
3,992,310 4,137,185
4,031,020 4,138,370
4,068,056 4,144,181
Copolymers of ethylene with higher alpha olefins are the most common
copolymers of aliphatic olefins. Ethylene-propylene copolymers are the most
common ethylene-alpha-olefin copolymers and are preferred for use in this
invention. A description of an ethylene-propylene copolymer appears in
U.S. 4,137,185 which is hereby incorporated herein by reference.
Useful ethylene-alpha olefin, usually ethylene-propylene, copolymers are
commercially available from numerous sources including the Exxon, Texaco and
Lubrizol Corporations.
(4) ~lefin-Diene Copol, mers
Another useful hydrocarbon polymer is one derived from olefins, especially
lower olefins, and dimes. Preferred olefins are alpha olefins. Dimes may be
non
conjugated or conjugated, usually non-conjugated. Useful olefins and dienes
are the
same as those described hereinabove and hereinafter in discussions of other
polymer
types.
In one embodiment, the copolymer is an ethylene-lower olefin-dime
copolymer. As used herein, the term lower refers to groups or compounds
containing
no more than 7 carbon atoms. Preferably, the dime is non-conjugated.
Especially
preferred are ethylene-propylene-diene copolymers.
These copolymers most often will have M n ranging from about 20,000 to
about 500,000, preferably from about 50,000 to about 200,000. In another
22
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
embodiment, the M n ranges from about 70,000 to about 350,000. These polymers
often have a relatively 'narrow range of molecular weight as represented by
the
poIydispersity value M W/Ma. Typically, the polydispersity values are less
than 10,
more often less than 6, and preferably less than 4, often between 2 and 3.
'There are numerous commercial sources for lower olefin-diene copolymers.
For example, ORTHOLEUM~ 2052 (a product marlceted by the DuPont Company)
which is a terpolymer having an ethylene:propylene weight ratio of about 57:43
and
containing 4-5 weight % of groups derived from 1,4-hexadiene monomer. Other
commercially available olefin-dime copolymers including ethylene-propylene
copolymers with ethylidene norbornene, with dicyclopentadiene, with vinyl
norbornene, with piperylene (1,3-pentadiene), with 4-vinyl cyclohexene, and
numerous other such materials are readily available. Olefin-dime copolymers
and
methods for their preparation are described in numerous patents including the
following U.S. Patents:
3,291,780
3,300,459
3,598,738
4,026,809
4,032,700
4,156,061
3,320,019
4,357,250
U.S. Patent 3,598,738, which describes the preparation of ethylene-propylene-
1,4-
hexadiene terpolymers, is illustrative. This patent also Iists numerous
references
describing the use of various polymerization catalysts.
Another useful polymer is an olefin-conjugated dime copolymer. An
example of such a polymer is butyl rubber, an isobutylene-isoprene copolymer.
Details of various types of polymers, reaction conditions, physical
properties,
and the like are provided in the above patents and in numerous booles,
including:
"Riegel's Handbook of Industrial Chemistry", 7th edition, James A. Kent
Ed., Van Nostrand Reinhold Co., New York (1974), Chapters 9 and 10,
23
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
P.J. Flory, "Principles of Polymer Chemistry", Cornell University Press,
Ithaca, N.Y. (1953),
"Kirk-Othmer Encyclopedia of Chemical Technology", 3rd edition, Vol. 8
(Elastomers, Synthetic, and various subheadings thereunder), John Wiley and
Sons,
New York (1979).
Each of the above-mentioned books and patents is hereby expressly
incorporated herein by reference for relevant disclosures contained therein.
Polymerization can also be effected using free radical initiators in a well-
known process, generally employing higher pressures than used with
coordination
catalysts. These polymers may be and frequently are hydrogenated to bring
unsaturation to desired levels. As noted, hydrogenation may talce place before
or
after reaction with the carboxylic reactant.
~,5) Star Pol"
Star polymers are polymers comprising a nucleus and polymeric arms.
Common nuclei include polyalkenyl compounds, usually compounds having at least
two non-conjugated alkenyl groups, usually groups attached to electron
withdrawing
groups, e.g., aromatic nuclei. The polymeric arms are often homopolymers and
copolymers of dimes, preferably conjugated dimes, vinyl substituted aromatic
compounds such as monoalkenyl arenes, homopolymers of olefins such as butenes,
especially isobutene, and mixtures thereof.
Molecular weights (GPC peak) of useful star polymers range from about
20,000 often from about 50,000 to about 500,000. They frequently have M"
ranging from about 100,000 to about 250,000.
. The polymers thus comprise a poly(polyalkenyl coupling agent) nucleus with
polymeric arms extending outward therefrom. The star polymers are usually
hydrogenated such that at least 80°l0 of the olefinic carbon-carbon
bonds are
saturated, more often at least 90°70 and even more preferably, at least
95°7o are
saturated. As noted herein, the polymers contain olefinic unsaturation;
accordingly,
they are not exhaustively saturated before reaction with the carboxylic
reactant.
The polyvinyl compounds making up the nucleus are illustrated by
polyallcenyl arenes, e.g., divinyl benzene and poly vinyl aliphatic compounds.
24
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Dimes malting up the polymeric arms are illustrated by butadiene, isoprene
and the like. Monoalkenyl compounds include, for example, styrene and
alkylated
derivatives thereof. In one embodiment, the arms are derived from dienes. In
'another
embodiment, the arms are derived from dimes and vinyl substituted aromatic
compounds. In yet another embodiment, the anus comprise polyisobutylene
groups,
often, isobutylene-conjugated diene copolymers. Arms derived from dimes or
from
dimes and vinyl substituted aromatic compounds are frequently substantially
hydrogenated. .
Star polymers are well known in the arf. Such material and methods for
preparing same are described in numerous publications and patents, including
the
following United States patents which are hereby incorporated herein by
reference
for relevant disclosures contained therein:
4,116,917,
4,141,847,
4,346,193,
4,358,565,
and 4,409,120.
Star polymers are commercially available, for example as Shellvis 200 sold
by Shell Chemical Co.
Mixtures of two or more hydrocarbon polymers may be used.
a,(3-Unsaturated Carboxylic Compound
The a,(3-unsaturated carboxylic compound used in the preparation of the
hydrocarbyl substituted carboxylic compositions of this invention are
themselves
prepared by reacting (1) an active methylene compound and (2) a carbonyl
compound as described in detail herein. They are preferably polycarboxylic
compounds of the general formula
H~ ~COOR
C=C .
R~ ~ COOK
CA 02413979 2002-12-19
04-07-2002 ~ US0119880
O
wherein R~ is -C "OR or --CHO;
and each R is, independently, H or hydrocarbyl.
With the reaction of dimethyl malonate and the methyl hemiacetal of methyl
glyoxylate, a minor amount (ca. 5% yield) of a product having the formula
H3C02 COzCH3
H3C~2C COZCH3
has been obtained.
Several compounds of this type are described in Hall et al, Polymer Bulletin
I6, 405-9 (1986); Evans et al, J. Org. Chem. 54 2$49 (1989); Hall et al,
Macromolecules 8 22, (1975); Stetter et al, Synthesis 626 (1981); Wilk,
Tetrahedron 53, 7097 (1997); Hawlcins et al, US 4,049,698 and Roblin et al
U.S.
2,293,309.
The reacting of (1) an active methylene compound and (2) a carbonyl
compound take place with ar without solvent and with or without catalyst.
Generally, the reaction takes place at temperatures between about 120°C
and 170°
for 4 to 8 hours with liberated water being removed during reaction. The
Knoevenagel reaction wherein a,ø-unsaturated compounds can be prepared by
reaction of active methylene compounds with aldehydes is illustrative. Such
reactions take place with or without solvent and with or without catalyst.
Generally,
the reaction takes place at temperatures between about 120°C and
170° for 4 to 8
hours with liberated water being removed during reaction. The reaction
products are
often fractionally distilled to obtained the desired a,ø-unsaturated compound.
The reaction products are often fractionally distilled to obtained the desired
a, J3-unsaturated compound.
26
AMENDED SHEET
04-07-2002 CA 02413979 2002-12-19 US0119880
Active MethYlene Compound
Active methylene compounds (1) used to prepare (B) the a,[3-unsaturated
carboxylic compound have the general formula
O O
R' C-CH2 COR
wherein each R' is independently R or OR and each R is, independently, H or a
hydrocarbyl group. Useful active methylene compounds include malonic acid and
esters thereof, especially di-lower alkyl malonate esters, and acetoacetic
acid esters,
particularly, lower alkyl, such as methyl, ethyl and propyl acetoacetates.
Especially preferred di-lower alkyl malonate esters are dimethyl malonate,
diethyl malonate and methyl ethyl malonate. Especially preferred lower alkyl
acetaacetates include methyl- or ethyl- acetoacetate.
Carbonvl Compound
Carbonyl compounds used to prepare (B) the a,~i-unsaturated carboxylic
compound have the general formula
O
Ra ~C Rb
wherein Ra is H or hydrocarbyl, especially H or lower alkyl, and Rb is "'CR',
wherein each R' is independently R or OR and each R is, independently, H or a
hydrocarbyl group; and lower alkyl acetals, ketals, hemiacetals and hemiketals
of the
carbonyl compound
In a preferred embodiment the carbonyl compound is a compound having the
general formula
~7
AMENDED SHEET
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
~~-~r~~
wherein each R' is independently R or OR and each R is, independently, H or a
hydrocarbyl group; or a lower allcyl hemiacetal .thereof. Preferably, R' is a
group of
the formula OR wherein R is independently H or lower alkyl.
Preferred carbonyl compounds are glyoxylic acids and reactive equivalents
thereof. In one preferred embodiment, the carbonyl compound is glyoxylic acid
or
the hydrate thereof. Particularly preferred are lower allcyl esters of
glyoxylic -acid.
Especially preferred is a lower alkyl hemiacetal of a lower alkyl glyoxylate,
most
preferably, the methyl hemiacetal of methyl glyoxylate.
The following examples illustrate several a,~i-unsaturated carboxylic
compounds used in the preparation of the hydrocarbyl substituted carboxylic
compositions of this invention. In these and in examples that follow, unless
indicated otherwise, all parts are parts by weight, temperatures are in
degrees
Celsius, and pressures are atmospheric. The relationship between parts by
weight
and parts by volume is as grams to milliliters. Filtrations are conducted
employing a
diatomaceous earth filter aid.
Example B)-1
A reactor is charged with 30 parts dimethyl malonate and 27.2 parts
glyoxylic acid methyl ester methyl hemiacetal (hereinafter GMHA). While these
are
being mixed, 23.17 parts acetic anhydride are added from an addition funnel at
ambient temperature. Heating is begun and after 0.7 hour the temperature is
105°C.
Heating is continued while distillate is collected in a Dean-Stark trap.
Heating is
continued for 4.7 hours while the temperature is increased to 130°C. At
this point 8
parts by volume distillate has been collected in the Dean-Stark trap. The
temperature
is increased to 160°C and is maintained for 7.5 hours while collecting
8.2 parts by
volume additional distillate. Heating at 160°C is continued for 7 hours
followed by .
heating to 200°C .and vacuum distillation at 10 mm Hg pressure. Two
fractions are
obtained. Yield of desired product is 11.84 parts (25.8%). '
28
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Example (B)-2
A reactor is charged with 30 parts dimet~yl malonate and 27.2 parts GMHA.
The materials are heated, under N2 to 140 °C over 1 hour then
temperature is
maintained for 1.5 hours while collecting 6 parts by volume distillate in Dean-
Stark
trap The temperature is increased to 160°C and is maintained, for 13
hours. The
temperature is increased to 170°C and the materials are vacuum stripped
at 5.2 mm
Hg pressure. Solids and clear colorless liquid distill over and 6.48 parts
white solid
is isolated from the liquid by filtration through filter paper. The solid is
the product
at 14.13% yield.
Example (.~)-3
A reactor is charged with 253.73 parts dimethyl malonate and 230.65 parts
GMHA. The materials are heated, under NZ to 117°C then to 125°C
'over 5 hours
while collecting 50 parts by volume distillate in a Dean-Stark trap. The
temperature
is increased to 130°C then to 170°C over 6.5 hours while
collecting an additional
~ 36.2 parts distillate. The temperature is increased to a maximum of
188°C at 4.5 mm
Hg pressure while collecting 199.65 parts distillate (51% yield). The
distillate is the
product.
Example (B)-4
A reactor is charged with,132.12 parts dimethyl malonate and 120.1 parts
GMHA. To the stirring mixture are added 1.79 parts dibutylamine. The materials
are
heated under N2, to 130°C over 8.25 hours while collecting a total of
36.5 parts by
weight distillate in a Dean-Starlc trap. The materials are cooled to
110°C and vacuum
distilled. The fraction collected at 6-10 mm Hg pressure and head temperature
134-
152°C (93.9 parts, 46.4% yield) is the product.
Example (B)-5
A reactor is charged with 132.12 parts dimethyl malonate and 120.1 parts
GMHA. The materials are heated, under N2, over 7 hours while collecting a
total of
273 parts by volume (235 parts by weight) distillate in a Dean-Stark trap. The
temperature is increased to 170°C and the materials are vacuum
distilled. The
fraction collected at 156-171°C pot temperature (21-5 mm Hg pressure,
139-I70°C
head temperature) (398.95 parts, 39.5 % yield) is the product.
29
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Example (B)-6
A reactor is charged with 264.24 parts dimethyl malonate, '240.2 parts GMHA
and
5.49 parts 70% aqueous methane sulfonic acid. The materials are heated to
140°C
over 6.25 hours while collecting a total of 63.8 parts distillate in a Dean-
Stark trap.
The temperature is .increased to 160°C and is maintained for 2.5
hours while
collecting an additional 29 parts by volume distillate. The materials are
vacuum
distilled collecting 230.68 parts, (57.07% yield) at pot temperature 154-
162°C, head
temperature 130-140°C and 5.6-30 mm Hg pressure as the product.
Example B)-7 ,
A reactor is charged with 132.12 parts dimethyl malonate, 120.01 parts
GMHA and 0.89 parts (3-alanine. The materials are heated, under NZ, to
130°C while
collecting a total of 13.58 parts distillate in a Dean-Stark trap. The
temperature is
increased to 160°G over 4.5 hours and is maintained for 2 hours while
collecting an
additional 11,65 parts distillate. The materials are vacuum distilled
collecting 93.73
parts (46.37% yield) at pottemperature~of 134-178°C, head temperature
108-120°C
at 4.4-7.5 mm Hg pressure as the product.
Example B )-8
A reactor is charged with 132.12 parts dimethyl malonate, 120:01 parts
GMHA and 1.77 parts 30% aqueous NH40H. The materials are heated, under N2, to
151°C over 3 hours while collecting a total of 30.5 parts distillate in
a Dean-Starlc
trap. The materials are vacuum distilled collecting 54.07 parts (26.74% yield)
at pot
temperature 145-157°C, head temperature I00-134°C at 6-7.8 mrn
Hg pressure as
the product.
Example (B)-9
A reactor is charged with 532.3 parts GMHA, 585.6 parts dimethyl rnalonate
and 6.08 parts 70% aqueous methanesulfonic acid. The materials are heated,
under
N2, to 130°C over 5.5 hours while collecting 43.79 parts distillate in
a Dean-Starlc
trap. The temperature is increased to 140°C and is maintained for 1
hour while
collecting an additional 91.82 parts distillate. The temperature is increased
to 150°C
~ over 1 hour and is maintained for 5.5 hours while collecting an additional
84.3 parts
distillate. To the residue are added 4.62 parts Na2C03, the materials are
filtered then
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
vacuum distilled to 150°C and 10 mm Hg pressure. The fraction
distilling at 100-
130°C (246.01 parts, 27.5 % yield) is collected as the product.
Example B)-IO
A reactor is charged with 720.6 parts GMHA and 660 parts dimethyl
malonate. The materials are heated, under N2, to 120°C over 6 hours,
collecting
81.22 parts distillate in a Dean-Stark trap. The temperature is increased to
150°C
over 6 hours; collecting an additional 121.93 parts distillate. The
temperature is
maintained for 6. hours, collecting an additional 47.52 parts distillate. The
materials
are vacuum distilled collecting 401.69 parts (39.7% yield) at 150-160°C
pot
temperature, 100-127°C head temperature at 5 mm Hg pressure as the
product.
Example B)-I1
A reactor is charged with 160.17 parts dimethyl malonate and 120.1 parts
GMHA. The materials are heated under N2 to 150°C over 8 hours,
collecting a total
of 43 parts by volume distillate in a Dean-Stark trap. The temperature is
maintained
for 4 hours, collecting an additional 37.8 parts distillate. The materials are
vacuum
distilled. The fraction distilling 100-120°C head temperature at 3.3 mm
Hg pressure
(121.87 parts, 52.9% yield) is collected as product.
Example (B)-I2
A reactor is charged with 30 parts dimethyl malonate, 27.2 parts GMHA and
0.62 parts 70% aqueous methanesulfonic acid. The materials are heated to
160°C
over 5 hours then the temperature is maintained for 3 hours. The materials are
vacuum stripped to 130°C and 4.9 mm Hg. The solid-liquid mixture is
obtained. The
mixture is filtered through paper and 12,85 parts white solids (28% yield) is
collected as the product.
Example (B)-13
A reactor is charged with 240.2 parts GMHA; 264.24 parts dimethyl
rnalonate and 25 parts of sulfonated polystyrene-co-divinylbenzene) resin
(AMBERLYST° 35, Rohm and Haas)). The materials are heated, under N2, to
120°C over 7 hours, then maintained at temperature for 13.5 hours. The
materials are
filtered to remove Amberlyst 35, and the liquid filtrate is vacuum distilled.
The
fraction distilling at 150°C pot temperature, 95-125°C head
temperature at 8.3 mm
Hg pressure (138.1 parts, 34.1% yield, is collected as the product.
31
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Example (B)-14
A reactor is charged with 65.07 parts ethyl acetoacetate, 61.02 parts GMHA,
5.0 parts 3-aminopropyl-functionalized silica gel and I00 parts by volume
toluene.
The materials are heated, under N2, to 70°C over 0,5 hour, then
temperature is
maintained for hours. The temperature is increased to 80°C over 3.25
.hour then to
90°C over 2 hours. The temperature is maintained at 90°C for 7
hours. The materials
are vacuum distilled. The fraction distilling at 130°C pot temperature,
115°C head
temperature at 5.4 mm Hg pressure (55.3 parts, 54.7% yield, .is collected as
the
product. The product is 93.3% triethyl ethylenetricarboxylate as determined by
gas
chromatography/MS.
Example B)-15
A reactor is charged with 300 parts dimethyl rnalonate and 272.7 parts
GMHA. The materials are heated, under N2, to 123°C at which time a
strong reflux
is observed. The materials are heated to 170°C over 6.5 hours while
107.1 parts
distillate are collected. The residue, 400.9 parts, 87.3% yield is the
product.
Example B)-16
The crude liquid product (225 parts) of Example (B)-15 is vacuum distilled
at maximum pot temperature of 200°G and 4 mm Hg pressure. The
distillate, a white
solid in a clear liquid (total 148.87 parts) is collected and is the product.
Example (B)-17
A reactor is charged with 1322.2 parts dimethyl malonate and I201.8 parts
GMHA. The materials are heated, under N2 to 150°C over 5 hours then
temperature
is maintained for 5 hours. The temperature is increased to 145°C, is
maintained for 1
hour, then is increased to 150°C and is maintained at temperature for 4
hours. A total
of 427.38 parts distillate is collected. The matez~als are vacuum distilled,
collecting
the fraction distilling at 130-150°C/5 mm Hg pxessure (477.3 parts,
23.6 % yield).
Example B)-18
A reactor is charged with 260.28 parts ethyl acetoacetate, 240.2 parts
GMHA, 20 parts 3-aminopropyl-functionalized silica gel and 400 parts by volume
toluene. The materials are heated, under N2, to 90°C over 1 hour then
temperature is
maintained at 90°C for 7.5 hours while removing distillate. The
materials are
filtered through filter paper which is subsequently washed with 100 parts by
volume
32
04-07-2002 CA 02413979 2002-12-19 US0119880
toluene. The filtrate and washings are vacuum stripped to 110°C pot
temperature
(80°C head temperature) at 3 mm Hg pressure, yielding 367.51 parts
(91.78% yield)
as the major product.
Example (B)-19
A portion of the product of Example (B)-18 (280 parts) is vacuum distilled to
135°C and 4 mm Hg pressure yielding 233.07 parts distillate as product.
HYdrocarbyl Group Substituted Carboxylic Compositions
This invention is also directed to hydrocarbyl group substituted carboxylic
compositions and a process for preparing said carboxylic compositions
comprising
reacting
(A) a hydrocarbon polymer having M" ranging from about 20,000 to
about 500,000, and
(B) an a,~3-unsaturated carboxylic compound prepared by reacting
(1) an active methylene compound of the formula
O O
is R' C-CH2-COR
and
(2) a carbonyl compound of the general formula
O'
Ra IC Rb
wherein R~ is H or hydrocarbyl and Rb is -'CR~, wherein each R' is
independently
R or OR and each R is, independently, H or a hydrocarbyl group; and lower
alkyl
acetals, ketals, hemiacetals and hemiketals of the carbonyl compound (2).
Preferred
reactants for use in the process are the same as those described hereinabove.
Reactants (A) and (B) are generally reacted in amounts ranging from about
0.95 to about 4 moles (B) per equivalent of (A), wherein an equivalent of (A)
is
defined as the molecular weight of (A) divided by the number of olefinic
groups
therein. For example, the equivalent weight of a EPDM co-polymer having
AMENDED SHEET
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
molecular weight of 20,000 and containing 4 olefinic groups is 5,000. In one
preferred embodiment, about 3 moles (B) are reacted per equivalent of (A),
while in
another preferred embodiment, about 1 mole (B) is reacted with one equivalent
of
(A)~
The process may be conducted at ambient pressure, under superatmospheric
pressure or under reduced pressure. Usually, except when volatile by-products
are
being removed from the reaction mixture under reduced pressure, there is
usually no
advantage to conduct the reaction under other than ambient pressure.
When the hydrocarbon polymer is substantially saturated, the process is
. conducted employing free radical conditions.
Radical, grafting is preferably carried out using free radical initiators such
as
peroxides, hydroperoxides, and azo compounds which decompose thermally within
the grafting temperature range to provide said free radicals.
Free radical generating reagents are well know to those spilled in the art.
Examples include benzoyl peroxide, t-butyl perbenzoate, t-butyl
metachloroperbenzoate, t-butyl ~ peroxide, sec-butylperoxydicarbonate,
azobisisobutyronitrile, and the like. Numerous examples of free radical-
generating
reagents, also known as free-radical initiators, are mentioned in the above
referenced tests by Flory and by Bovey and Winslow. An extensive listing of
free
radical initiators appears in J. Brandrup and E. H. Tmmergut, Editor, "Polymer
Handbook", 2nd edition, John Wiley and Sons, New York (1975), pages II-1 to II-
40. Preferred free radical-generating reagents include t-butyl peroxide, t-
butylhydroperoxide, t-butyl perbenzoate, t-amyl peroxide, cumyl peroxide,
t-butyl peroctoate, t-butyl-m-chloroperbenzoate and azobisisovaleronitrile.
The free-radical initiators are generally used in an amount from 0.01 to about
10 percent by weight based on the total weight of ~ the reactants. Preferably,
the
initiators are used at about 0.05 to about 1 percent by weight.
The reaction is usually conducted at temperatures ranging between about
80°C to about 200°C, . preferably between about 130°C to
about 170°C.
Considerations for determining reaction temperatures include reactivity of the
system and the half-life of the initiator at a particular temperature.
34
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
The choice of free radical generating reagent can be an important
consideration. For example, when a polymer undergoing grafting with a monomer
is diluted with a solvent such as a hydrocarbon oil, grafting of, the monomer
onto the
oil diluent may occur. It has been observed that the choice of initiator
affects the
extent of grafting of the monomer onto the oil diluent. Reducing the amount of
monomer grafted onto the diluent usually results in an increased amount of
monomer grafted onto the polymer. Improved efficiency of monomer grafting onto
substantially saturated copolymer resins has been described by Lange et al. in
U.S.
5,298,565 which is hereby incorporated herein by reference for relevant
disclosures
in this regard. .
When the hydrocarbon polymer is olefinically unsaturated, the process may
be conducted thermally at temperatures ranging from ambient, usually from at
least
about 20°C up to about 250°C, more often from about 80°C
to about 220°C.
In one embodiment, the process is conducted wherein said reacting of (A) the
hydrocarbon polymer and (B) the a,(3-unsaturated carboxylic compound is
conducted with the addition of from about 0.1 to about 2.5 moles Cla per mole
of (B)
polycarboxylic compound. In another embodiment, the reacting is conducted with
the addition of from about 0.1 to about 2.2 moles Cl2 per equivalent of
olefinically
unsaturated hydrocarbon. The process with added chlorine is also generally
conducted at an elevated temperature, typically from about 130°C up to
about
200°C.
The following examples illustrate hydrocarbyl group substituted carboxylic
compositions of this invention. Temperatures, pressures, and amounts are
expressed
in terms as set forth hereinabove. Filtrations are conducted employing a
diatomaceous earth filter aid.
Example 1
A reactor is charged with 2000 parts of a 14 weight % in mineral oil solution
of an ethylene/propylene/dicyclopentadiene copolymer (weight ratio of
63/36/1.5)
having M W 150,000 and an average of about 12 C=C per molecule (Uniroyal
Chemical). The solution is heated with stirring to 100°C under N2, 10
parts of the
product of Example (B)-5 are added then the temperature is increased to
130°C. A
solution of 5 parts t-butyl peroxybenzoate and 5 parts toluene is added
dropwise
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
over 0.75 hour. The temperature is maintained for' 3 hours then the materials
are
cooled whereupon streaks of solid material appeared on reactor walls. Toluene,
200
parts by volume, is added and 'the materials are heated to 130°C
whereupon a
solution of 2.5 parts t-butyl peroxybenzoate and 2.5 parts toluene added over
0.25
hour. The reaction is continued at I30°C for 3 hours. Upon cooling, a
significantly
reduced amount (estimated 1-2 parts) of solids adhere to reactor wall. The
materials
are vacuum stripped for 0.5 hour at 150°C and 20 mm Hg pressure. The
residue is
mixed with 800 parts mineral oil and the oil solution is collected as the
product..
The hydrocarbyl group substituted carboxylic compositions of this invention
are useful as additives for lubricating oil compositions and may be
incorporated in a
minor amount into a major amount of an oil of lubricating viscosity. They also
serve
as intermediates to undergo further reaction with amines, alcohols and metal
containing compounds to prepare derivative compositions which are useful as
dispersant viscosity improvers for lubricants and fuels. The carboxylic
derivative
compositions are also incorporated in a minor amount into a major amount of an
oil
of lubricating viscosity. A major amount is defined herein as any amount
greater
than 50% by weight and a minor amount is any amount less than 50% by weight
provided the total of all components is 100%.
Hvdrocarbvl Group Substituted Carboxylic Derivative Compositions
The instant invention is also directed to derivatives of the hydrocarbyl
substituted carboxylic compositions. These derivatives are hydrocarbyl group
substituted carboxylic derivative compositions prepared by reacting at least
one
hydrocarbyl group substituted carboxylic composition of this invention with a
reactant selected from the group consisting of (a) amines characterized by the
presence within their structure of at least one condensable H-N< group, (b)
alcohols,
(c) reactive metal or reactive metal compounds, and (d) a combination of two
or
more of any of (a) through (c), the components of (d) being reacted with the
carboxylic composition simultaneously or sequentially, in any order.
The hydrocarbyl group substituted carboxylic compositions are described in
detail hereinabove.
36
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Amines
The amines may be monoamines or polyamines, typically
polyamines, preferably ethylene amines, amine bottoms or amine condensates.
The
amines can be aliphatic, cycloaliphatic, aromatic, or heterocyclic, including
aliphatic-substituted cycloaliphatic, aliphatic-substituted aromatic,
aliphatic-
substituted heterocyclic, cycloaliphatic-substituted aliphatic, cycloaliphatic-
substituted heterocyclic, aromatic-substituted aliphatic, aromatic-substituted
cycloaliphatic, aromatic-substituted heterocyclic, heterocyclic-substituted
aliphatic,
heterocyclic-substituted alicyclic, and heterocyclic-substituted aromatic
amines and
~ may be saturated or unsaturated.
Monoamines useful in this invention generally contain from 1 to
about 24 carbon atoms, preferably 1 to about 12, and more preferably 1 to
about 6.
Examples of primary monoamines useful in the present invention include
methylamine, propylamine, butylamine, cyclopentylanune, dodecylamine,
allylamine, cocoamine and stearylamine. Examples of secondary monoamines
include dimethylamine, dipropylamine, dicyclopentylamine, methylbutylamine,
etc.
The monoamine may be an alkanol amine represented by at least one of the
formulae:
H2N -R'- OH,
and
H
~N-R'-OH,
R~
wherein each R4 is independently a hydrocarbyl group of one to about 22 carbon
atoms or hydroxyhydrocarbyl group of two to about 22 carbon atoms, preferably
one
to about four, and R' is a divalent hydrocarbyl group of about two to about 18
carbon
atoms, preferably two to about four. The group -R'-OH in such formulae
represents
the hydroxyhydrocarbyl group. R' can be an acyclic, alicyclic or aromatic
group.
'Typically, R° is an acyclic straight or branched alkylene group such
as an ethylene,
1,2-propylene, 1,2-butylene, 1,2-octadecylene, etc. group. When two R4 groups
are
present in the same molecule they can be joined by a direct carbon-to-carbon
bond
or, through a heteroatom (e.g., oxygen, nitrogen or sulfur) to form a 5-, 6-,
7- or 8-
membered ring structure. Examples of such heterocyclic amines include
37
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
N-(hydroxyl . lower alkyl)-morpholines, -thiomorpholines, -piperidines, - -
oxazolidines, -thiazolidines and the like. Typically, however, . each R4 is
independently a methyl, ethyl, propyl, butyl, pentyl or hexyl group.
Examples of allcanolamines include mono- and di- ethanolamine,
ethylethanolamine, monomethylethanolamine, etc.
The hydroxyamines can also be ether N-(hydroxyhydrocarbyl) amines.
These are hydroxy poly(hydrocarbyloxy) analogs of the above-described hydroxy
amines (these analogs also include hydroxyl-substituted oxyalkylene analogs).
Such
N-(hydroxyhydrocarbyl) amines can be conveniently prepared, for example, by
reaction of epoxides with aforedescribed amines and can be represented by the
formulae:
H2N - (R'O)X - H, and
H
jN-(R' O)x H,
Ra
wherein x is a number from about 2 to about 15 and R4 and R' are as described
above. R4 may also be a hydroxypoly (hydrocarbyloxy) group.
Other useful amines include ether amines of the general formula
R60R1NHR7
wherein R6 is~a hydrocarbyl group, preferably an aliphatic group, more
preferably an
alkyl group, containing from 1 to about 24 carbon atoms, R1 is a divalent
hydrocarbyl group, preferably an alkylene group, containing from two to about
18
carbon atoms, more preferably two to about 4 carbon atoms and R7 is H or
hydrocarbyl, preferably H or aliphatic, more preferably H or alkyl, more
preferably
H. When R7 is not H, then it preferably is alkyl containing from one to about
24
carbon atoms. Especially preferred ether amines are those available under the
name
' SURFAMo produced and marketed by Sea Land Chemical Co., Westlake, Ohio.
The amine may also be a polyamine. The polyamine may be aliphatic,
cycloaliphatic, heterocyclic or aromatic. Examples of useful polyamines
include
alkylene polyamines, hydroxy containing polyamines, polyoxyallcylene
polyamines,
arylpolyamines, and heterocyclic polyamines.
Alkylene polyamines are represented by the formula
38
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
H2N--~Alkylene- N~-nRg
RS
wherein n has an average value between about 1 and about 10, preferably about
2 to
about 7, more preferably about 2 to about 5, and the "Alkylene" group has from
1 to
about 10 carbon atoms, .preferably about 2 to about 6, more preferably about 2
to
about 4. RS is independently hydrogen, an aliphatic group or a hydroxy-
substituted
or amino-substituted aliphatic group of up to about 30 carbon atoms.
Preferably RS
is H or lower alkyl, most preferably, H.
Alkylene polyanunes include methylene-, ethylene-, butylene-, propylene-,
pentylene- and other polyamines. Higher homologs and related heterocyclic
amines
such as piperazines and N-amino alkyl-substituted piperazines are also
included.
Specific examples of such polyamines are ethylene diamine, diethylene
triamine,
triethylene tetramine, tris-(2-amin0ethyl)amine, propylene diamine, N,N-
dimethylaminopropylamine, trirnethylene diamine, tripropylene tetramine,
tetraethylene pentamine, hexaethylene heptamine, pentaethylenehexamine,
aminoethyl piperazine, etc.
Higher homologs obtained by condensing two or more of the above-noted
alkylene amines are similarly useful as are mixtures of two or more of the
aforedescribed polyamines.
Ethylene polyamines, such as some of those mentioned above, are preferred.
They are described in detail under the heading "Diamines and Higher Amines" in
Kirk Othrner's . "Encyclopedia of Chemical Technology",, 4th Edition, Vol. 8,
pages
74-108, John Wiley and Sons, New York (1993) and in Meinhardt, et al, U.S.
4,234,435, both of which are hereby incorporated herein by reference for
disclosure
of useful polyamines. Such polyamines are most conveniently prepared by the
reaction of ethylene dichloride with ammonia or by reaction of an ethylene
imine
with a ring opening reagent such as water, ammonia, etc. These reactions
result in
the production of a complex mixture of polyalkylene polyamines including
cyclic
condensation products such as the aforedescribed piperazines. Ethylene
polyamine
mixtures are useful.
39
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Other useful types of polyamine mixtures are those resulting from stripping
of the above-described polyamine mixtures to leave as residue what is often
termed
"polyamine bottoms". In general, alkylene polyamine bottoms can be
characterized
as having less than two, usually less tham 1% (by weight) material boiling
below
about 200°C. A typical sample of such ethylene polyamine bottoms
obtained from
the Dow Chemical Company of Freeport, Texas, designated "E-100" has a specific
gravity at 15.6°C of 1.0168, % nitrogen of 33.15 and a viscosity at
40°C of 121
centistokes. Gas chromatography analysis shows such a sample contains about
0.93% "Light Ends" (most probably diethylenetriamine), 0.72%
triethylenetetramine, 21.74% tetraethylenepentamine and 76.6I% pentaethylene
hexamine and higher (by weight). These alkylene polyaxnine bottoms include
cyclic
condensation products such as piperazine and higher analogs of
diethylenetriamine,
triethylenetetramine and the like.
Another useful polyamine is a condensation product obtained by reaction of
_ at least one hydroxy allcyl compound with at least one polyamine reactant
containing
at least one primary or secondary amino group. The hydroxy compounds are
preferably polyhydric alcohols and amines. Preferably the hydroxy compounds
are
polyhydric amines. Polyhydric amines include any of the above-described
monoamines reacted with an alkylene oxide (e.g.~ ethylene oxide, propylene
oxide,
butylene oxide, etc.) having two to about 20 carbon atoms, preferably two to
about
four. Examples of polyhydric amines include tri-(hydroxypropyl)amine, tris-
(hydroxymethyl)ami.no methane, 2-amino-2-methyl-1,3-propanediol, N,N,N',N'-
tetrakis(2-hydroxypropyl) ethylenediamine, and N,N,N',N'-tetrakis(2-
hydroxyethyl)
ethylenediamine. .
Polyamine reactants, which react with the polyhydric alcohol or amine to
form the condensation products or condensed amines, are described above.
Preferred polyamine reactants include triethylenetetramine (TETA),
tetraethylenepentamine (TEPA), pentaethylenehexamine (PEHA), and mixtures of
polyarnines such as the above-described "amine bottoms".
The condensation reaction of the polyamine reactant with the hydroxy
compound is conducted at an elevated temperature, usually about 60°C to
about
265°C in the presence of an acid catalyst.
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
The amine condensates and methods of making the same are described in
Steckel (IJS Patent 5,053,152) which is incorporated by reference for its
disclosure
to the condensates and methods. of making 'amine condensates.
The polyamines may be hydroxy-containing polyamines. These include
hydroxy-containing polyamine analogs of hydroxy monoamines, particularly
alkoxylated allcylenepolyamines. Such polyamines can be made by reacting the
above-described alkylene amines with one or more of the above-described
alkylene
oxides. .
Specific examples of alkoxylated alkylenepolyamines include N-(2-
hydroxyethyl) ethylenediamine, N,N-di-(2-hydroxyethyl)-ethylenediamine, 1-(2-
hydroxyethyl) piperazine, mono-(hydroxypropyl)-substituted
tetraethylenepentamine, N-(3-hydroxybutyl)-tetramethylene diamine, etc. Higher
homologs obtained by condensation of the above illustrated hydroxy-containing
polyamines through amino groups or through hydroxy groups are likewise useful.
Condensation through amino groups results in a higher amine accompanied by
removal of ammonia while condensation through the hydroxy groups results in
products containing ether linlcages accompanied by removal of water. Mixtures
of
two or more of any of the aforesaid polyamines are also useful.
The polyamines may be polyoxyalkylene polyarnines, including
polyoxyethylene and polyoxypropylene diamines and the polyoxypropylene
triamines having average molecular weights ranging from about 200 to about
2000.
Polyoxyalkylene polyamines, including polyoxyethylene-polyoxypropylene
polyamines, are commercially available, for example under the tradename
JEFFAMII~ES" from Texaco Chemical Co. U.S. Patent numbers 3,804,763 and
3.948,800 contain disclosures of polyoxyallcylene polyamines and are
incorporated
herein by reference for their disclosure of such materials.
In another embodiment, the polyamine may be a heterocyclic polyamine.
The heterocyclic polyamines include aziridines, azetidines, azolidines, tetra-
and
dihydropyridines, pyrroles, indoles, piperidines, inudazoles, di- and
tetrahydroimidazoles, piperazines, isoindoles, purines, N-aminoalkyl-
thiomorpholines, N-aminoalkylmorpholines, N-aminoalkyl-piperazines, N,N'-
bisaminoalkyl piperazines, azepines, azocines, azonines, azecines and tetra-,
di- and
41
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
perhydro derivatives of each of the above and mixtures of two or more of these
heterocyclic amines. Preferred heterocyclic .amines are the saturated 5- and ~-
membered heterocyclic amines containing only nitrogen, or nitrogen with oxygen
and/or sulfur in the hetero ring, especially the piperidines, piperazines,
thiomorpholines, morpholines, pyrrolidines, and the like. Piperidine,
aminoallcylsubstituted piperidines, piperazine, aminoallcylsubstituted
piperazines,
morpholine, aminoalkylsubstituted morpholines, pyrrolidine, ~ and aminoallcyl-
substituted pyrrolidines, are especially preferred. Usually the aminoallcyl
substituents are substituted on a nitrogen atom forming part of the hetero
ring.
Specific! examples of such heterocyclic amines include N-
amirlopropylmorpholine,
N-amino-ethylpiperazine, and N,N'-diaminoethyl-piperazine. Hydroxy alkyl
substituted heterocyclic polyamines are also useful. Examples include
N-hydroxyethylpiperazine and the like.
Another useful amine is the condensation product of a hydrocarbyl,
preferably aliphatic, containing from about 30 to about 200 carbon atoms,
substituted mono- or polycarboxylic acid with at least one of the
aforementioned
polyamines in relative amounts such that the resulting condensation product
contains
at least one condensable N ~I group. The condensation product may be pre-
formed
condensation or formed in situ. The pre-formed~condensation product is
preferred.
Examples include polyisobutenyl ( M n~ 1000) substituted succinic anhydride-
ethylene polyamine and polypropylene ( M n~800) substituted propionic acid-
ethylene polyamine reaction products wherein each contains at least one
condensable N-H group.
Hydrazine and substituted-hydrazine can also be used to form nitrogen
containing carboxylic dispersants. At -least one of the nitrogens in the
hydrazine
must contain a hydrogen directly bonded thereto. Preferably there are at least
two
hydrogens bonded directly to hydrazine nitrogen and, more preferably, both
hydrogens are on the same nitrogen. The substituents which may be present on
the
hydrazine include alkyl, alkenyl, aryl, aralkyl, alkaryl, and the like.
Usually, the
substituents are alkyl, especially lower alleyl, phenyl, and substituted
phenyl such as
lower alkoxy-substituted phenyl or lower alkyl-substituted phenyl. Specific
examples of substituted hydrazines are methylhydrazine, N,N-dimethyl-
hydrazine,
42
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
N,N'-dimethylhydrazine, phenylhydrazine, N-phenyl-N'-ethylhydrazine, N-(para-
tolyl)-N =(n-butyl)-hydrazine, N-(para-nitrophenyl)-hydrazine, N-(para-
nitrophenyl)-
N-methyl-hydrazine, N,N'-di(para-chlorophenol)-hydrazine, N-phenyl-N'-
cyclohexy~hydrazine, amino guanidine bicarbonate, and the like.
The carboxylic derivative compositions produced by reacting the
hydrocarbyl group substituted carboxylic composition of the invention and the
amines described above are acylated amines which include amine salts, amides,
imides and imidazolines as well as mixtures thereof. To prepare the carboxylic
derivative compositions from the amines, one or more of the hydrocarbyl group
substituted carboxylic composition and one or more amines are heated,
optionally in
the presence .of a normally liquid, substantially inert organic liquid
solvent/diluent,
at temperatures in the range of from about 80°C up to the decomposition
point of
any of the reactants or the product, but normally at temperatures in the range
of from
about 100°C up to about 300°C, provided 300°C does not
exceed the decomposition
point. Temperatures of about 125°C to about 250°C are normally
used. The
carboxylic composition and the amine are reacted in an amount sufficient to
provide
from about one-half equivalent up to two moles of amine per equivalent of the
carboxylic composition. In another embodiment, the carboxylic composition is
reacted with from about one-half equivalent up to one mole of amine per
equivalent
of the carboxylic composition. For the purpose of this invention, an
equivalent of
amine is that amount of amine corresponding to the total weight of amine
divided by
the total number of nitrogens present having at least one H-N< group. Thus,
octyl
amine has an equivalent weight equal to its molecular weight; ethylenediamine
has
an equivalent weight equal to one-half its molecular weight, and
aminoethylpipera-
zine, with 3 nitrogen atoms but only two having at least one H-N< group, has
an
equivalent weight equal to one-half of its molecular weight.
Alcohols
The carboxylic compositions may be reacted with (b) alcohols. Alcohols
useful as (b) in preparing carboxylic derivative compositions of this
invention from
the hydrocarbyl group substituted carboxylic composition previously described
include those compounds of the general formula
Rs-(~~m
43
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
wherein R3 is a monovalent or polyvalent organic radical joined to the -OH
groups
through carbon-to-oxygen bonds (that is,
-C-OH
wherein the carbon is not part of a carbonyl group) and rn is an integer of
from 1 to
about 10, usually 2 to about 6. As with the amine reactant (a), the alcohols
can be
aliphatic, cycloaliphatic, aromatic, and heterocyclic, including aliphatic-
substituted
cycloaliphatic alcohols, aliphatic-substituted aromatic alcohols, aliphatic-
substituted
heterocyclic alcohols, cycloaliphatic-substituted aliphatic alcohols,
cycloaliphatic-
substituted aromatic alcohols, cycloaliphatic-substituted heterocyclic
alcohols,
heterocyclic-substituted aliphatic alcohols, heterocyclic-substituted
cycloaliphatic
alcoho~s, and heterocyclic-substituted aromatic alcohols. Except for
polyoxyalkylene alcohols, the mono- and polyhydric alcohols corresponding to
the
above formula 'will usually contain not more than about 40 carbon atoms and
generally not more than about 20 carbon atoms. The alcohols may contain non-
' 15 hydrocarbon substituents of the same type mentioned with respect to the
amines
above, that is, non-hydrocarbon substituents which do not interfere with the
reaction
of the alcohols with the acylating reagents of this invention. In general,
polyhydric
alcohols are preferred.
The monohydric and polyhydric alcohols useful as (b) include monohydroxy
and polyhydroxy aromatic compounds. Monohydric and polyhydric phenols and
naphthols are preferred hydroxyaromatic compounds. These hydroxy-substituted
aromatic compounds may contain other substituents in addition to the hydroxy
substituents such as halo, alkyl, alkenyl, allcoxy, alkyl-mercapto, nitro and
the Iike.
Usually, the hydroxy aromatic compound will contain 1 to 4 hydroxy groups. The
aromatic hydroxy compounds are illustrated by the following specific examples:
phenol, beta-naphthol, cresols, resorcinol, catechol, carvacrol, thymol,
eugenol, p,p'-
dihydroxybiphenyl, hydroquinone, pyxogallol, phloroglucinol, orcin, guaicol,
2,4-
dibutylphenol, propenetetramer-substituted phenol, didodecylphenol, 4,4'-
methylene-bis-methylene-bis-phenol, alpha-decyl-beta-naphthol, polyisobutenyl-
(molecular weight of about 1000)-substituted phenol, the condensation product
of
heptylphenol with 0.5 mole of formaldehyde, the condensation product of
octylphenol with acetone, di(hydroxyphenyl)oxide, di(hydroxyphenyl)sulfide,
44
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
di(hydroxyphenyl)disulfide, and 4-cyclohexylphenol. Phenol itself and
aliphatic
hydrocarbon-substituted phenols, e.g., alkylated phenols having up to 3
aliphatic
hydrocarbon substituents are especially preferred. Each of the aliphatic
hydrocarbon
substituents may contain 100 or more carbon atoms but usually will have from 1
to
20 carbon atoms. Alkyl and alkenyl groups are the preferred aliphatic
hydrocarbon
substituents. '
Further specific examples of monohydric alcohols which can be used as (b)
include monohydric alcohols such as methanol, ethanol, isooctanol,
cyclohexanol,
behenyl alcohol, neopentyl alcohol, isobutyl alcohol, benzyl alcohol, beta-
phenethyl
alcohol, 2,-methylcyclohexanol, monomethyl ether of ethylene glycol, monobutyl
ether of ethylene glycol, monopropyl ether of diethylene glycol, monododecyl
ether
of triethylene glycol, monooleate of ethylene glycol, monostearate of
diethylene
glycol, sec-pentyl alcohol, tert-butyl alcohol, and dioleate of glycerol.
Alcohols
within (b) may be unsaturated alcohols such as allyl alcohol, cinnamyl
alcohol, 1
cyclohexene-3-of and oleyl alcohol.
Other specific examples of alcohols useful as (b) are the ether alcohols and
amino alcohols including, for example, the oxyalkylene, oxy-arylene-, amino-
alkylene-, and aminoarylene-substituted alcohols having one or more
oxyallcylene,
aminoalkylene or amino-aryleneoxy-arylene groups. They are exemplified by
CELLOSOLVE", CARBITOL°, phenoxyethanol, heptylphenyl-
(oxypropylene)~-
OH, octyl-(oxyethylene)3a-OH phenyl-(oxyoctylene)2-OH, mono-(heptylphenyl-
oxypropylene)-substituted glycerol, polystyrene oxide), aminoethanol, 3-amino-
ethylpentanol, di(hydroxyethyl)amine, p-aminophenol, tri(hydroxypropyl)amine,
N-
hydroxyethyl ethylenediamine, N,N,N',N'-tetrahydroxy-trimethylenediamine, and
the like. The polyhydric alcohols preferably contain from 2 to about 10
hydroxy
groups. They are illustrated, for example, by the alkylene glycols and
polyoxyalkylene glycols mentioned above such as ethylene , glycol, triethylene
glycol, tetraethylene glycol, dipropylene glycol, dibutylene glycol, and other
alkylene glycols and polyoxyalkylene glycols in which the alkylene groups
contain 2
to about 8 carbon atoms.
Other useful polyhydric alcohols include glycerol, monooleate of glycerol,
monostearate of glycerol, monomethyl ether of glycerol, pentaerythritol, n-
butyl
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
ester of 9,10-dihydroxy stearic acid, methyl ester of 9,10-dihydroxy stearic
acid, 1,2-
butanediol, 2,3-hexanediol, 2,4-hexanediol, pinacol, erythritol, arabitol,
sorbitol,
mannitol, 1,2-cyclohexanediol, and xylene glycol. Carbohydrates such as
sugars,
starches, celluloses, and so forth likewise can be cased as (b). I The
carbohydrates
may be exemplified by glucose, fructose, sucrose, rhamnose, mannose,
glyceraldehyde, and galactose.
Polyhydric alcohols having at least 3 hydroxyl groups, some, but not all of
which have been esterified with an aliphatic monocarboxylic acid having from
about
8 to about 30 carbon atoms such as octanoic acid, oleic acid, stearic acid,
linoleic
acid, dodecanoic acid or tall oil acid are useful as (b). Further specific
examples of
such partially esterified polyhydric alcohols are the monooleate of sorbitol,
distearate of sorbitol, monooleate of glycerol, monostearate of glycerol, di-
dodecanoate of erythritol, and the like.
A preferred class of alcohols suitable as (b) are those polyhydric alcohols
containing up to about 12 carbon atoms, and especially those containing 3 to
10
carbon atoms. This class of alcohols includes glycerol, erythritol,
pentaerythritol,
dipentaerythritol, gluconic acid, glyceraldehyde, glucose, arabinose,
heptanediols,
hexanetriols, butanetriols, guinic acid, 2,2,6,6-tetrakis-
(hydroxymethyl)cyclohexanol, 1,10-decanediol, digitalose, and the like.
Aliphatic
alcohols containing at least three hydroxyl groups and up to 10 carbon atoms
are
particularly preferred.
An especially preferred class of polyhydric alcohols for use as (b) are the
polyhydric alleanols containing 3 to 10 carbon atoms and particularly, those
containing 3 to 6 carbon atoms and having at least three hydroxyl groups. Such
alcohols are exemplified by glycerol, erythritol, pentaerythritol, mannitol,
sorbitol,
2-hydroxymethyl-2-methyl-1,3-propanediol(trimethylolethane), 2-hydroxy-methyl-
2-ethyl-1,3-propanediol(trimethylpropane), 1,2,4-hexanetriol, and the like.
From what has been stated above, it is , seen that (a) may contain alcoholic
hydroxy substituents and (b) can contain primary, secondary, or tertiary amino
substituents. Thus, amino alcohols can fall into both (a) and (b) provided
they
contain at least one primary or secondary amino group. If only tertiary amino
groups are present, the amino alcohol belong only in (b).
46
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Reactive Metals
Reactive metals' or reactive metal compounds useful as (c) are those which
will form carboxylic acid metal salts with the hydrocarbyl group substituted
carboxylic composition of this invention and those which will form metal-
containing
complexes with the carboxylic derivative compositions produced by reacting the
hydrocarbyl group substituted carboxylic composition with amines and/or
alcohols
as discussed above.
Reactive metal compounds useful for preparing metal salts of hydrocarbyl
group substituted carboxylic composition of this invention include those salts
containing metals selected from the group consisting of Group I metals, Group
II
metals, Al, Pb, Sn, Co and Ni. Examples of compounds include the oxides,
hydroxides, alcoholates, and carbonates of Li, Na, K, Ca, Ba, Pb, Al, Sn, Ni
and
others. While reactive metals may also be employed, it is generally more
convenient,
and often more economical to employ the metal salts as reactants. An extensive
listing of reactive metal compounds useful for preparing the metal salts of
the
hydrocarbyl group substituted carboxylic composition is provided in U.S.
3,271,310
(LeSuer) which is expressly incorporated herein by reference.
Reactive metal compounds useful as (c) for the formation of complexes with
the reaction products of the acylating reagents of this invention and amines
are
disclosed in U.S. Patent 3,306,908. Complex-forming metal reactants useful as
(c)
include the nitrates, nitrites, halides, carboxylates, phosphates, phosphates,
sulfates,
sulfites, carbonates, borates, and oxides of cadmium as well as metals having
atomic
numbers from 24 to 30 (including chromium, manganese, iron, cobalt, nickel,
copper and zinc). These metals are the so-called transition or coordination
metals,
i.e., they are capable of forming complexes by means of their secondary or
coordina-
tion valence. Specific examples of the complex-forming metal compounds useful
as
the reactant in this invention are cobalt, cobaltous oxide, cobaltous
chloride, cobaltic
chloride, chromous acetate, chromic acetate, chromic sulfate, chromic
hexanoate,
manganous acetate, manganous benzoate, manganous nitrate, ferrous acetate,
ferric
benzoate, ferrous bromide, nickel nitrate, nickel dioleate, nickel stearate,
copper (I)
acetate, cupric benzoate, cupric formate, cupric nitrite; zinc benzoate, zinc
borate,
zinc chromate, cadmium benzoate, cadmium carbonate, cadmium butyrate.
47
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Hydrates of the above compounds are especially convenient for use in the
process of
this invention.
U.S. Patent 3,306,908 is expressly incorporated herein by reference for its
discussion of reactive metal compounds suitable for forming such complexes and
its
disclosure of processes for preparing the complexes. Basically, those
processes are
applicable to the carboxylic derivative compositions of the acylating reagents
of this
invention with the amines as described above by substituting, or on an
equivalent
basis, the acylating reagents of this invention with the high molecular weight
carboxylic acid acylating . agents disclosed in U.S. Patent 3,306,908. The
ratio of
equivalents of the acylated amine thus produced and 'the complex-forming metal
reactant remains the same as disclosed in U.S. Patent 3,306,908.
The following examples illustrate carboxylic derivative compositions of this
invention. Temperatures, pressures, and amounts are as set forth hereinabove.
Exam l~
A reactor is charged with 2637 parts of the product of Example 1 (equivalent
weights 65,000/C=C, 21,667/C=0). The materials are heated to 150°C
under NZ
whereupon 9.7 parts polyamine bottoms (E-100, Dow) are added dropwise over 0.5
hour. The materials are reacted for 2 hours at 150°C then vacuum
stripped to 150°C
at 20 mm Hg pressure. The residue is the product containing 0.134% N, total
base
number = 2.82 and total acid number = 0.35.
Exam 1p a $
A reactor is charged with 2200 parts of a 14 weigh % in mineral oil solution
of an ethylene/propylene/dicyclopentadiene copolymer {weight ratio 63/36/1.5
%)
having M W 150,000 and an average of about 12 C=C per molecule (Uniroyal
Chemical) and 220 parts toluene. While stirring, 11 parts of the product of
Example
(B)-5 are added followed by heating to 130°C under N2. A solution of
8.8 parts each
t-butyl peroxybenzoate and toluene is added over 0.75 hour. The materials are
heated for 2 hours, then vacuum stripped to 150°C at 20 mrn Hg
pressure. A portion
of the residue is removed from the reactor leaving 2550 parts remaining. To
this
material are added 450 parts of a 50% in oil solution of the reaction product
of
polyisobutylene (M" 1600) substituted succinic anhydride and polyethylene
polyamine bottoms having total base number about 27, total acid number about 2
48
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
and containing about 1.16% N. The materials are heated to I50°C under
N2, reacted
at 150°C for 3 hours then vacuum stripped to 150°C at 20 mm Hg
pressure. The
residue is the product containing 0.169% N and having total acid number =
1.34.
The Oil of Lubricating ViscositX
The lubricating compositions of this invention employ an oil of lubricating
viscosity, including natural or synthetic lubricating oils and mixtures
thereof.
Natural oils include animal oils and vegetable oils (e.g. castor oil, lard
oiI) as
well as mineral lubricating oils such as liquid petroleum oils.and solvent-
treated or
acid treated mineral lubricating oils of the paraffinic, naphthenic or mixed
paraffinic-naphthenic types. Oils of lubricating viscosity derived from coal
or shale
are also useful. Synthetic lubricating oils include hydrocarbon oils and
halosubstituted hydrocarbon oils such as polymerized and interpolymerized
olefins,
etc. and mixtures thereof, alkylbenzenes, polyphenyl, (e.g., biphenyls,
terphenyls,
allcylated polyphenyls, etc.), alkylated diphenyl ethers and alkylated
diphenyl
sulfides and the derivatives, analogs and homologues thereof and the like.
Alkylene oxide polymers and interpolymers and derivatives thereof where
their terminal hydroxyl groups have been modified by esterification,
etherification,
etc., constitute another useful class of lcnown synthetic lubricating oils.
Another suitable class of synthetic lubricating oils that can be used
comprises
the esters of di- and polycarboxylic acids and those made from C$ to C2o
monocarboxylic acids and polyols and polyolethers.
Other synthetic lubricating oils include liquid esters of phosphorus-
containing acids, polymeric tetrahydrofurans and the like, silicon-based oils
such as
the polyalkyl-, polyaryl-, polyalkoxy-, or polyaryloxy-siloxane oils and
silicate
oils.
Unrefined, refined and rerefined oils, either natural or synthetic (as well as
mixtures of two or more of any of these) of the type disclosed hereinabove can
be
used in the compositions of the present invention. Unrefined oils are those
obtained
directly from natural or synthetic sources without further purification
treatment.
' Refined oils are similar to the unrefined oils except they have been further
treated in
one or more purification steps to improve one or more properties. Refined oils
49
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
include solvent refined oils, hydrorefined oils, hydrofinished oils,
hydrotreated oils,
and oils obtained by hydrocracking and hydroisomerization techniques.
Rerefined oils are obtained by processes similar to those used to obtain
refined oils applied to refined oils which have been already used in service.
Such
rerefined oils often are additionally processed by techniques directed to
removal of
spent additives and oil breakdown products.
Specific examples of the above-described oils of lubricating viscosity are
given in Chamberlin, llI, U.S. 4,326,972, European Patent Publication 107,282,
and
A. Sequeria, Jr., Lubricant Base Oil and Wax Processing, Chapter 6, Marcel
Decker,
Inc., New York (1994), each of which is hereby incorporated by reference for
relevant disclosures contained therein.
A basic, brief description of lubricant base oils appears in an article by
D.V.
Broclc, "Lubrication Engineering", Volume 43, pages 184-5, March, 1987, which
article is expressly incorporated herein by reference for relevant disclosures
contained therein.
The Normally Liquid Fuels
As indicated hereinabove, the products of this invention may also be used
as additives for normally liquid fuels.
The fuels used in the fuel compositions of this invention are well known to
those skilled in the art and usually contain a major portion of a normally
liquid fuel
such as hydrocarbonaceous petroleum distillate fuel (e.g., motor gasoline as
defined
by ASTM Specifications D-439-89 and D-4814-91 and diesel fuel or fuel oil as
defined in ASTM Specifications D-396-90 and D-975-91). Fuels containing non-
hydrocarbonaceous materials such as alcohols, ether, organo-nitro compounds
and
the like, are also within the scope of this invention as are liquid fuels
derived from
vegetable or mineral sources. A range of alcohol and ether type compounds are
described as oxygenates. Oxygenate-containing fuels are described in ASTM
D-4814-91. Mixtures of any of the above-described fuels are useful.
Particularly preferred fuels are gasoline, that is, a mixture of hydrocarbons
having an ASTM boiling point of 60°C at the 10% distillation point to
about 205°C
at the 90% distillation point, oxygenates, and gasoline-oxygenate blends, all
as
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
defined in the aforementioned ASTM Specifications for automotive gasolines.
Most
preferred is gasoline.
. The fuel compositions typically contain from about 0.001 % to about 2% by
weight, more often up to about 0.5%, even more often up to about 0.2% by
weight
of the additives of this invention.
The fuel compositions of the present invention may contain other additives
which are well known to those skilled in the art. These can include anti-knock
agents such as tetra-alkyl lead compounds, lead scavengers such as halo-
alkanes,
dyes, antioxidants such as hindered phenols, rust inhibitors such as alkylated
succinic acids and anhydrides and derivatives thereof, bacteriostatic agents,
auxiliary
dispersants and detergents, gum inhibitors, fluidizers, metal deactivators,
demulsifiers, anti-icing agents and the like. The fuel compositions of this
invention
may be lead-containing or lead-free fuels. Preferred are lead-free fuels.
Other Additives
As mentioned, lubricating oil compositions of this invention may contain
other components. The use of such additives is optional and the presence
thereof in
the compositions of this invention will depend on the particular use and level
of
performance required. Thus the other additive may be included or excluded. ~
The
compositions may comprise a zinc salt of a dithiophosphoric acid. Zinc salts
of
dithiophosphoric acids are often referred to as zinc dithiophosphates, zinc
O,O'-
dihydrocarbyl dithiophosphates, and other commonly used names. They are
sometimes referred to by the abbreviation ZDP. One or more zinc salts of
dithiophosphoric acids may be present in a minor amount to provide additional
extreme pressure, anti-wear and anti-oxidancy performance.
, In addition to zinc salts of dithiophosphoric acids discussed hereinabove,
other additives that may optionally be used in the lubricating oils of this
invention
include, for example, auxiliary detergents and dispersants, viscosity
improvers,
oxidation inhibiting agents, pour point depressing agents, extreme pressure
agents,
anti-wear agents, color stabilizers and anti-foam agents. The above-mentioned
dispersants and viscosity improvers may be used in addition. to the
compositions of
this invention.
51 '
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
Auxiliary extreme pressure agents and corrosion and oxidation inhibiting
agents which may be included in the compositions of the invention are
exemplified
by chlorinated aliphatic hydrocarbons, organic sulfides and polysulfides,
phosphorus
esters including dihydrocarbon and trihydrocarbon phosphites, molybdenum
compounds, and the like.
Auxiliary viscosity improvers (also sometimes referred to as viscosity index
improvers or viscosity modifiers) may be included in the compositions of this
invention. Viscosity improvers are usually polymers, including polyisobutenes,
polymethacrylic acid esters, dime polymers, polyalkyl styrenes, esterified
styrene-
malefic anhydride copolymers, alkenylarene-conjugated dime copolymers and
polyolefins. Multifunctional viscosity improvers, other than those of the
present
invention, which also have dispersant and/or antioxidancy properties are known
and
may optionally be used in addition to the products of this invention. Such
products
are described in numerous publications including those mentioned in the
I5 Background of the Invention. Each of these publications is hereby expressly
incorporated by reference.
Pour point depressants are often included in the lubricating oils described
herein. See for example, page 8 of 'Lubricant Additives" by C.V. Smalheer and
R.
Kennedy Smith (Lezius-Riles Company Publisher, Cleveland, Ohio, 1967). Pour
point depressants, techniques for their preparation and their use are
described in U.
S. Patent numbers 2,387,501; 2,015,748; 2,655,479; 1,815,022; 2,191,498;
2,666,748; 2,721,877; 2,721,878; and 3,250,715 which are expressly
incorporated
by reference for their relevant disclosures.
Anti-foam agents used to reduce or prevent the formation of stable foam
include silicones or organic polymers. Examples of these and additional anti-
foam
compositions are described in "Foam Control Agents", by Henry T. Kerner (Noyes
Data Corporation, 1976), pages 125-162.
Detergents and dispersants may be of the ash-producing or ashless type. The
ash-producing detergents are exemplified by oil soluble neutral and basic
salts of
alkali or alkaline earth metals with sulfonic acids, carboxylic acids, phenols
or
organic phosphorus acids characterized by a least one direct carbon-to-
phosphorus
linkage.
52
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
The term "basic salt" is used to designate metal salts wherein the .metal is
present in stoichiometrically larger amounts than the organic acid radical.
Basic
salts and techniques for preparing and using them are well known to those
skilled in
the art and need not be discussed in detail here.
Ashless detergents and dispersants are so-called despite the fact that,
depending on its constitution, the detergent or dispersant may upon combustion
yield a nonvolatile residue such as boric oxide or phosphorus pentoxide;
however, it
does not ordinarily contain metal and therefore does not yield a metal-
containing ash
on combustion. Many types are known in the art, and are suitable for use in
the
lubricants of this invention. The following are illustrative:
(1) Reaction products of carboxylic acids (or derivatives thereof) containing
at least about 34 and preferably at least about 54 . carbon atoms with
nitrogen
containing compounds such as amine, organic hydroxy. compounds such as phenols
and alcohols, and/or basic inorganic materials. Examples of these "carboxylic
dispersants" are described in British Patent number 1,306,529 and in many U.S.
patents including the following:
3,163,603 3,38.1,022 . 3,542,680
3,184,474 3,399,141 3,567,637
3,215,707 3,415,750 3,574,101
3,219,666 3,433,744 3,576,743
3,271,310 3,444,170 3,630,904
3,272,746 3,448,048 3,632,510
3,281,357 3,448,049 3,632,511
3,306,908 3,451,933 3,697,428
3,311,558 3,454,607 3,725,441
~
3,316,177 3,467,668 4,194,886
3,340,281 3,501,405 .4,234,435
3,341,542 3,522,179 4,491,527
3,346,493 3,541,012 5,696;060
3,351,552 3,541,678 5,696,067
RE 26,433
(2) Reaction products of relatively high molecular weight aliphatic or
alicyclic halides with amines, preferably polyalkylene polyamines. These may
be
characterized as "amine dispersants" ,and examples thereof are described for
example, in the following U.S. patents:
53
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
3,275,554 3,454,555
3,438,757 3,565,804
(3) Reaction products of alkyl phenols in which the allcyl groups contains
at least about 30 carbon atoms with aldehydes (especially formaldehyde) and
amines
(especially polyalkylene polyamines), which may be characterized as "Mannich
dispersants". The materials described in the following U. S. patents are
illustrative:
3,413,347 3,725,480
3,697,574 3,726,882
3,725,277
(4) Products obtained by post-treating the carboxylic amine or Mannich
dispersants with such reagents as urea, thiourea, carbon disulfide, aldehydes,
ketones, carboxylic acids, hydrocarbon-substituted succinic anhydrides,
nitriles,
epoxides, boron compounds, phosphorus compounds or the like' Exemplary
materials of this kind are described in the following U.S. patents:
3,036,003 3,282,955 3,493,520 3,639,242
3,087,936 3,312,619 3,502,677 3,649,229
3,200,107 3,366,569 3,513,093 3,649,659
3,216,936 3,367,943 3,533,945 3,658,836
' 3,254,025 3,373,111 3,539,633 3,697,574
3,256,185 3,403,102 3,573,010 3,702,757
3,278,550 3,442,808 3,579,450 3,703,536
3,280,234 3,455,831 3,591,598 3,704,308
3,281;428 3,455,832 3,600,372 3,708,522
4,234,435
~ (5) Polymers and copolymers of oil-solubilizing monomers such as decyl
methacrylate, vinyl decyl ether and high molecular weight olefins with
monomers
containing polar substituents, e.g., aminoallcyl acrylates or methacrylates,
acrylamides and poly-(oxyethylene)-substituted acrylates. These may be
characterized as "polymeric dispersants" and examples thereof are disclosed in
the
following U.S. patents:
3,329,658 3,666,730
3,449,250 3,687,849
3,519,565 3,702,300
54
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
The above-noted patents are incorporated by reference herein for their
disclosures of
ashless dispersants.
The above-illustrated additives may each be present in lubricating
compositions at a concentration of as little as 0:001% by weight, usually
ranging
from about 0.01% to about 20% by weight. In most instances, they each
contribute
from about 0.1% to about 10% by weight, more often up to about 5% by weight.
Additive Concentrates
The various corripositions and other additives described herein can be added
directly to the lubricant. Preferably, however, they are diluted with a
substantially
inert, normally liquid organic diluent such as mineral oil, naphtha, benzene,
toluene
or xylene, to form an additive concentrate. Preferred additive concentrates
contain
the diluents referred to hereinabove. These concentrates usually comprise
about 0.1
to about 80% by weight of the compositions of this invention and may contain,
in
addition, one or more other additives known in the art or described
hereinabove.
Concentrations such as 15%, 20%, 30% or 50% or higher may be employed.
Lubricating Oil Compositions
The instant invention also relates to lubricating oil compositions containing
the carboxylic compositions of the invention. As noted hereinabove,'the
carboxylic
compositions of this invention may be blended directly into an oil or
lubricating
viscosity or, more often, are incorporated into an additive concentrate
containing one
or more other additives which in turn is blended into the oil.
Lubricant Examples AA-BB
SAE 15W-40 lubricating oil compositions are prepared by blending 0.1 part
of a 40% in oil solution of a styrene-maleate copolymer neutralized with
aminopropylmorpholine; 6.5 parts of an additive concentrate prepared by
combining
55.385 parts of a 50% in oil solution of a polyisobutylene ( M n 1600)
substituted
succinic anhydride-ethylene polyaxnine reaction product, 8.05 parts of Zn
mixed
isopropyl-methyl amyl phosphorodithioate, 3.G5 parts sulfurized butadiene-
butyl
acrylate Diels-Alder adduct, Ø23 parts 2,5-bis(t-nonyldithio)-1,3,4-
thiadiazole
(Amoco 158 Amoco), 6.58 parts Ca overbased (MR 2.3) sulfurized alkyl phenol,
5.35 parts calcium overbased (M12 11) alkyl benzene sulfonic acid, 3.67 parts -
calcium overbased (M12 2.8) alkyl benzene sulfonic acid, , 007 parts of a
kerosene
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
solution of a commercial silicone antifoam and sufficient mineral oil to bring
the
total weight of the additive concentrate to 100 parts; and the indicated
amounts of
the product of the listed Example, in sufficient mineral oil (Exxon stoclcs)
to prepare
100 parts of lubricant:
Lubricant
AA BB
Product of Example: A (8.0) B ~ (9.4)
(pbw)
Lubricant Examples CC and DD
SAE 15W-40 lubricating oil compositions are prepared by blending 0.1 part
of a 40°~o in oil solution of a styrene-maleate copolymer neutralized
with
aminopropylmorpholine; 13 parts of the additive concentrate described in
Example
AA and BB; and the indicated amounts of the product of the listed Example, in
sufficient mineral oil (Exxon stocks) to prepare 100 parts of lubricant:
Lubricant
CC DD
Product of Example: A (8.0) B (9.4)
(pbw)
It is known that some of the materials described above may interact in the
final formulation, so that the components of the final formulation may be
different
from those that are initially added. For instance, metal ions (of, e.g., a
detergent)
can migrate to other acidic sites of other molecules. The products formed
thereby,
including the products formed upon employing the composition of the present
invention in its intended use, may not susceptible of easy description.
Nevertheless,
all such modifications and reaction products are included within the scope of
the
present invention; the present invention encompasses the composition prepared
by
admixing the components described above.
Each of the documents referred to above is incorporated herein by reference.
Except in the examples, or where otherwise explicitly indicated, all numerical
quantities in this description specifying amounts of materials, reaction
conditions,
molecular weights, number of carbon atoms, and the like, are to be understood
as
modified by the word "about". Unless otherwise indicated, each chemical or
56
CA 02413979 2002-12-19
WO 01/98440 PCT/USO1/19880
composition referred to herein should be interpreted as being a commercial
grade
material which may contain the isomers, by-products, derivatives, and other
such
materials which are normally understood to be present in the commercial grade.
However, the amount of each chemical component is presented exclusive of any
solvent or diluent oil which may be customarily present in the commercial
material,
unless otherwise indicated. It is to be understood that the upper and lower
amount,
range, and ratio limits set forth herein may be independently combined. As
used
herein, the expression "consisting essentially of" permits the inclusion of
substances
which do not materially affect the basic and novel characteristics of the
composition
under consideration.
While the invention has been explained in relation to its preferred
embodiments, it is to be understood that various modifications thereof will
become
apparent to those skilled in the art upon reading the specification.
Therefore, it is to
be understood that the invention disclosed herein is intended to cover such
modifications that fall within the scope of the appended claims.
57