Note: Descriptions are shown in the official language in which they were submitted.
CA 02415849 2003-01-08
Pharmaceutical powder cartridge, and inhaler equipped
with same
Description
The invention relates to a pharmaceutical powder
cartridge for powder inhalers for holding a
pharmaceutical depot for a large number of
pharmaceutical powder doses, having at least one
storage space and an integrated metering device, said
integrated metering device comprising at least one
metering slide which can be moved approximately
transversely in a metering slide channel at least from
a filling position to an emptying position,
approximately transversely with respect to the
direction of flow of the pharniaceutical powder out from
the at least one storage space, and an inhaler equipped
accordingly.
Background of the invention
In the field of treatment of bronchial diseases, and
also of other diseases in which medication can be given
via the airways, it is known not only to atomize
solutions or suspensions into inhalable aerosols but
also to administer powdered medicaments. Many examples
of such medicaments are described in the literature,
and of these we refer purely by way of illustration to
WO 93/11773, EP 0 416 950 Al and EP 0 416 951 Al.
A customary form of administration in this connection
is delivery via an inhalation device (inhaler).
Known inhalers for powdered pharmaceuticals include
those for administration of a single dose and also
inhalation devices which have a storage space for a
plurality of pharmaceutical doses. In connection with
the latter, it is known either to provide separate
storage spaces for each indi.vidual dose or to provide
CA 02415849 2003-01-08
- 2 -
one single receiving space for receiving a multiplicity
of doses of a medicament.
Known inhalers in which a large number of individual
doses are provided in separate storage spaces include
those in which individual areas of the inhaler are each
filled with a pharmaceutical ciose. An example of such
an inhaler is described in US 5,301,666 A. However, it
is also known to accommodate a large number of
pharmaceutical powder doses in separate areas of what
are called blister packs. An example of such a blister
pack for use with an inhaler is described in DE 44 00
083 C2. Such a blister pack, which is designed at the
same time as a disposable irihaler, is described for
example in DE 44 00 084 Al.
An inhalation device into which blister packs can be
inserted, which each have separate storage spaces for
individual doses of a powdered pharmaceutical and which
can be emptied one after another with the aid of the
inhalation device, is described, for example, in
DE 195 23 516 Cl.
Many examples of inhalers with a storage space for a
large number of pharmaceutical doses are described in
the prior art. One example with an exchangeable storage
container is described in German Patent Specification
846 770, and another in WO 95/31237.
An important problem with inhalation systems in which a
large number of doses of a medically active substance
are accommodated in a common storage space concerns the
apportioning of an individual dose for one individual
inhalation. In this connection, a great many solutions
have been proposed, for example those which are
described in US 2,587,215 A and US 4,274,403 A. Other
types of arrangements for metering an individual dose
of pharmaceutical powder from a storage space for a
large number of pharmaceutical doses are furthermore
CA 02415849 2003-01-08
- 3 --
described in WO 92/09322, WO 93/16748 and DE 35 35 561
C2 and in GB 2 165 159 A. An excharigeable cartridge
for receiving a large riurnber of doses of a
pharmaceutical powder with an integrated metering slide
is known from DE 195 22 415 Al.
Another important problem with inhalation of
pharmaceutical powders concerris the breakdown of the
galenic powder formulations into particles which can
access the lungs. The active substances administered in
this way are generally combined with vehicles in order
to achieve a reasonable dosing capacity of the
medically active substance and to set further
properties of the pharmaceutical powder, which for
example can influence the storage life.
Proposed solutions concerning the designs of powder
inhalers with which particles which can access the
lungs are intended to be made available in an air
stream for inhalation are described for example in EP 0
640 354 A2, US 5,505,196 A, US 5,320,714 A, US
5,435,301 A, US 5, 301, 666 A, DE 195 22 416 Al and WO
97/00703. Proposals are also known to use auxiliary
energy to generate the air stream, for example in ZA-A
916741.
In the use of medicaments for inhalation in powder
form, it is also quite generally known to combine
active substances by administering prepared active
substance mixtures. Corresponding proposals are found
in EP 0 416 951 Al and WO 93/11773, for example for
combination of salmeterol and fluticasone or formoterol
and budesonide.
WO 00/74754 and many other publications over a period
of more than twenty years have described how,
particularly in powder inhalers, there is a
considerable problem with moisture. Not only can
moisture have a disadvantageous effect on the
CA 02415849 2003-01-08
- 4 -
pharmaceutically active composition of the medicament,
it can also impair in particular the interplay of
physical and chemical parameters of the combination of
active substance and auxiliaries. As a result, lumps
may form, for example, or the breakdown of the inhaled
powder into particles which can access the lungs may be
impaired. All these circumstances can lead to problems
affecting the metering and the efficacy of the
administration of a powdered medicament.
To minimize these disadvantages, various attempts have
already been made in the past to reduce the penetration
of moisture into a powder inhaler by using seals.
Attempts have also been made to reduce the
disadvantageous effects of penetrated moisture by
providing desiccants to absorb the moisture, in
particular to keep the air moisture in storage chambers
to a minimum.
Prior art
In the prior art, WO 00/74754 expressly describes how
attempts to solve this problem have generally been made
only by the use of desiccants in various forms. The
Applicant there claims to have solved this problem by
for the first time providing a seal intended to prevent
penetration of moisture into the inhaler, particularly
into the storage container of a powder inhaler with in
particular elastic sealing elements.
To this end, reference is made to sealing elements made
from "all conventionally known materials, for example
natural or synthetic rubber, a silicone or PTFE" and
like materials.
Subsequently, with reference to the "Clickhaler" powder
inhaler from Innovata Biomed, an arrangement is
described in detail which concerns a particular
arrangement of the metering mechanism of this inhaler,
CA 02415849 2003-01-08
- 5 -
which comprises a metering device in the manner of a
star feeder in the form of an inclined truncated cone.
In the embodiment described;, the sealing element
provided is a likewise frustoconical sealing sleeve
which is fitted over the truncated metering cone and is
intended to be pivotable, so that it can assume a
sealing position and a non-sealing position. Here, page
5 interestingly describes how this sealing sleeve is
preferably to be made of a syrithetic material like that
of the metering cone. It is further proposed that the
sealing sleeve be provided with the same number of
holes as the metering cone element has metering
cavities. The sealing sleeve and the metering element
are to be designed in such a way that, when both are
turned, a metering chamber formed by an opening in the
sealing sleeve first takes up the medicament from the
reservoir and then, upon further turning, deposits this
in the metering cavity in the actual metering cone and
is finally conveyed from there into an air channel.
A particularly advantageous embodimerit is described in
which the outer contour of the sealing sleeve forms a
spherical section and provides a good fit with a
corresponding curvature of the medicament storage
space. The inner contour of the sealing sleeve is
intended to be adapted to the frustum of the metering
cone.
From US 6,132,394 A, it is known to provide, in a
medicament chamber of an inhaler, a separate container
containing a desiccant. This is described as differing
from US 4,274,403 A in that a completely closed
separate container is used which is made of a material
which is as far as possible permeable to moisture and
in which the desiccant, for example silica gel, is to
be placed. An important advantage of this is stated to
be the fact that, compared to conventional dry
capsules, there ar-e no assembly or connection points
CA 02415849 2003-01-08
- 6 --
through which small amounts of the desiccant can pass
into the medicament chamber and thus contaminate the
powdered medicament. In conventional dry capsules, such
connection points are to be present in particular
between capsule body and porous membrane through which
the water vapour is to pass into the desiccant.
The separate container is accordingly intended to be
made as far as possible from a single material,
preferably one with a high degree of water vapour
permeability. Suitable materials proposed are
polycarbonate (PC) and ABS (acrylonitrile-butadiene-
styrene) . The drying behaviour over a relatively long
period of time is intended here to be adapted via the
material of the container.
WO 01/46038 discloses the use of a stopper, a foil, a
tablet or a lining of an EVA copolymer with 35-80% by
weight of a desiccant such as silica gel, clay or zinc
chloride as desiccant capsule or embedded in a storage
container in particular for packaged foods, mention
being made of deficierit mechanical stability of the
stopper etc., and the risk of mechanical decomposition
at relatively high concentrations of desiccant. Here,
the EVA types described as being suitable have quite
high proportions of vinyl acetate copolymers, so that
these materials have very high water vapour
permeability.
WO 01/21238 discloses a powder inhaler with hermetic
sealing when not in use. To this end, in the case of a
powder inhaler with a medicanlent storage space and with
an air channel running through under the storage space,
a sealing skirt is provided on each side of the storage
container and covers an air inlet opening and an
inhalation opening of the air channel in a position of
rest. When an actuating cap is actuated to move a
metering plunger through the storage space in order to
convey a dose of medicament from the reservoir into the
CA 02415849 2003-01-08
- 7 -
air channel, the two sealiiig skirts secured on the
actuating cap are moved downwards too until the
metering plunger has reached its emptying position.
Through-holes are provided in the sealing skirts and
are arranged in such a way that, with the actuating cap
in the end position, they free the openings of the air
channel. As long as the actuating cap is kept
depressed, the air can be sucked through the air
channel. When the actuating cap is released and returns
to its starting position, the openings of the air
channel are closed again.
By means of an additional guide arrangement and an
elastic design of the sealing skirts, these are pressed
against the outer wall in order to increase the sealing
effect. In this arrangement, as the distance travelled
from the opening position to the closing position
increases, the sealing skirts are pressed increasingly
more strongly against the outer wall of the inhaler
transverse to the direction of movement. An elastic
seal in the form of a bellows is also provided between
actuating cap and inhaler housing in order to close the
gap between said structural parts.
Summary of the invention
The object of the inventiori is therefore to improve
known systems for administering powdered
pharmaceuticals.
According to the invention, this object is achieved by
means of a pharmaceutical powder cartridge for powder
inhalers for holding a pharmaceutical depot for a large
number of pharmaceutical powder doses, having at least
one storage space and an integrated metering device,
said integrated metering device comprising at least one
metering slide which can be moved approximately
transversely in a metering slide channel at least from
a filling position to an emptying position,
CA 02415849 2003-01-08
- 8 -
approximately transversely with respect to the
direction of flow of the pharmaceutical powder out from
the at least one storage space, the metering slide
channel with the at least one metering slide being
sealed off from the environment at least in the filling
position of the metering slide.
By means of the design according to the invention, and
for only the slightest additional outlay, an effective
protection of the pharmaceutical storage space against
moisture from the environment is obtained, in
particular during intermediate storage during the.
period of use after the patient has begun using the
storage space. This advantage applies both while the
pharmaceutical cartridge is fitted in an inhaler and
also when it is being stored outside the inhaler.
Compatibility with known powder inhalers for
exchangeable pharmaceutical powder cartridges of the
type mentioned at the outset can be maintained.
In a particularly preferred embodiment, a
pharmaceutical powder cartridge according to the
invention is characterized in that the metering slide
channel has, at one end, an opening to the environment
through which a part of the metering slide can pass,
and, around the opening, a contact surface is provided
for a seal.
A particularly reliable function can be achieved if the
metering slide has a sealing surface provided in a
plane approximately transverse to its direction of
movement from the f.illirig position to the emptying
position. In this way, it is at the same time possible
to avoid a change of frictional forces during the
movement of the metering slide which, in known
inhalers, can be caused by a movement of the seal along
the sealing surface, by powder residues or wear of the
seal.
CA 02415849 2003-01-08
- 9 -
Particularly reliable sealing is achieved if said
sealing is provided by an elastic seal.
In the case of prolonged storage prior to the
pharmaceutical powder cartridge being inserted into an
inhaler, an especially permanent and effective sealing
is guaranteed if the metering slide can further be
moved into an additional storing position and the seal
is elastically prestressed sealingly at least in the
storing position of the metering slide, especially if
the metering slide is fixed iri the storing position by
resiliently elastic means.
In a preferred embodiment, a pharmaceutical powder
cartridge according to the invention is characterized
in that the metering device comprises at least one
metering cavity for holding a predetermined quantity of
a pharmaceutical powder.
For administering active substance combinations, it can
also be advantageous if the pharmaceutical powder
cartridge has at least two storage spaces, in
particular if the pharmaceutical powder cartridge has a
metering device, said metering device having, for each
of the storage spaces, a metering cavity for
apportioning a predetermined quantity of each medically
active substance provided in the storage spaces.
Depending on the active substance combination provided,
it is also advantageous if the metering devices of the
individual pharmaceutical powder cartridges have
metering cavities of identical or different volume.
A particularly economical application, especially
suitable for expensive pharmaceutical powders with only
occasional administration, is possible if the
pharmaceutical powder cartridge further has a device
for indicating the quantity of pharmaceutical doses
which remain in the storage chambers or which have been
removed from the storage chambers.
CA 02415849 2003-01-08
- 10 -
The advantages of the invention can be used especially
in long-term use of a pharmaceutical powder cartridge
for powder inhalers for holding a pharmaceutical depot
for a large number of pharmaceutical powder doses,
having at least one storage space and an integrated
metering device, said integrated metering device being
able to assume at least a filling position and an
emptying position and being able to move from the
filling position to the emptying position, and with a
seal being provided which substantially seals off the
storage space from the envir.orlment and against entry of
moisture, at least in the filling position of the
metering device, said seal being elastically
deformable, during a movement of the metering device
from its emptying position to its filling position,
without any sliding movement of the seal relative to
the sealing surfaces.
In a preferred embodiment of the invention, the seal is
made of a silicone rubber or an elastomer, more
preferably a thermoplastic elastomer, preferably of
TPEE (thermoplastic polyester elastomer).
The improvement in the application properties afforded
by the invention, particularly through actively
reducing the effect of moisture on a pharmaceutical
powder during the period of use by a patient or a
hospital establishment, is further achieved by means of
a pharmaceutical powder cartridge for powder inhalers
or a powder inhaler having at least one storage space
for holding a pharmaceutical depot for a large number
of pharmaceutical powder doses, including a housing
body and a lid which substantially enclose the at least
one storage space, and where the housing body and/or
the lid are made predominantly of a PVDC
(polyvinylidene chloride), a pharmaceutically
compatible plasti_c coated completely or partially with
PVDC, an olefin copo]ymer with heterocyclic side groups
CA 02415849 2003-01-08
- 1.1 -
(COC or mPP), or a PCTFE (polychloro-
trifluoroethylene).
In a particularly advantageous embodiment, a
pharmaceutical powder cartridge according to the
invention is characterized in that at least one
metering slide as a component of the rnetering device is
made predominantly of a PVDC (polyvinylidene chloride),
a pharmaceutically compatible plastic coated completely
or partially with PVDC, an olefin copolymer with
heterocyclic side groups, an at least partially
oriented PP (polypropylene) or a PCTFE (polychloro-
trifluoroethylene).
To limit the effects of moisture, which has penetrated
into the cartridge or is present therein, on a
pharmaceutical powder, it is further expedient if a
pharmaceutical powder cartridge for powder inhalers or
a powder inhaler according to the invention is
characterized in that the housing body and/or lid
comprises, on at least part of the side facing the
storage space, a blend of desiccant embedded in a
thermoplastic matrix.
To avoid impairment of the pharmaceutical by desiccant
residues, it is advantageous, according to the
invention, to provide a pharrnaceutical powder cartridge
for powder inhalers or a powder inhaler having at least
one storage space for holding a pharmaceutical powder
depot for a large number of pharmaceutical doses,
containing at least one shaped body made of a blend of
a thermoplastic matrix with a desiccant embedded
therein, preferably silica gel, bentonite or molecular
sieve, particularly if' channels are formed in a matrix
of a thermoplastic of low water absorption, as are
obtainable by dissolving soluble co-extrudate
components. For rapid uptake of residual moisture in
the storage space, it can also be expedient in this
case if fibres which absorb water vapour are embedded
CA 02415849 2003-01-08
- 12 -
as filler in a matrix of a thermoplastic of low water
absorption.
It is particularly advantageous, for economic mass
production, if the blend in a matrix of a thermoplastic
of low water absorption and a desiccant embedded
therein is designed at least as part of an inner wall
of a storage space by multi-component injection-
moulding in a housing body made of a plastic
substantially impermeable to water vapour.
Within the meaning of the invention, it is further
advantageous if housing body and lid are sealed
watertight, preferably by ultrasonic welding.
For particularly economic production, the seal is co-
injected onto the housing body or the metering'slide.
The invention can be advantageously exploited in
economic terms using an inhaler for powdered
pharmaceuticals with a pharmaceutical powder cartridge
according to the invention, and with an inhaler for
powdered pharmaceuticals, in which the pharmaceutical
can be taken by a patient by way of an air stream,
characterized by a holder for a pharmaceutical powder
cartridge according to the invention.
The advantages of the invention are particularly
beneficial for patients requiring treatment with a
pharmaceutical powder cartridge according to the
invention containing a powder with one or more of the
following active substances: analgesics, anti-
allergics, antibiotics, anticholinergics, anti-
histamines, anti-inflammatory substances, antipyretics,
corticoids, steroids, antitussives, bronchodilators,
diuretics, enzymes, substances acting on the
cardiovascular system, hormones, proteins and peptides.
Application of the invention
CA 02415849 2003-01-08
-- 13 -
By means of the invention, i.t is possible to make
available pharmacodynamically active substances in the
form of powdered pharmaceuticals over a long period of
use, even when they are sensitive to moisture or are
under unfavourable climatic c:oriditions, and in so doing
also to obtain the advantages of re-usable inhalers
with exchangeable pharmaceutical powder cartridges.
It is also possible to make available powdered
pharmaceuticals for inhalation in different active
substance combinations under improved storage
conditions, of which individual active substances have
increased sensitivity to moisture affecting their
storage life, their stability or their dosability.
Active substances for which the invention can be used
can also be for example from the group of beta-
sympathomimetics: salbutamol, reproterol, fenoterol,
formoterol, salmeterol. Possible examples from the
group of corticosteroids are: budesonide,
beclomethasone, fluticasone, triamcinolone,
loteprednol, mometasone, flunisolide, ciclosonide.
Possible examples froni the group of anticholinergics
are: ipatropium bromide, thiotropium bromide,
glycopyrrolate.
Possible examples from the group of analgesics and
anti-migraines are: morphine, tramadol, flupirtine,
sumatryptan. The following can be used for example from
the group of peptides and proteins: cetrorelix,
insulin, calcitonin, parathyroid hormone, factor VIII
analogs, interferon alpha, interferon beta, heparin,
FSH (follicle-stimulating hormone), colistin,
tobramycin.
Use is not limited to the active substances mentioned
here. The pharmaceutical powder cartridge described is
suitable for all active substances which can be metered
in powder form and administered by inhalation. By
CA 02415849 2003-01-08
- 14 -
appropriate modification of the system and of the
metering device, the inventi_on described is also
suitable for combination of active substances which
contain liquid formulations, for example solutions or
suspensions of pharmacodynamically active substances.
Pharmaceutical powder formulations which can
expediently be used with the pharmaceutical powder
cartridge system according to the invention can contain
various active substances, such as, for example,
analgesics, anti-allergics, antibiotics,
anticholinergics, antihistamines, anti-inflammatory
substances, antipyretics, corticoids, steroids,
antitussives, bronchodilators, diuretics, enzymes,
cardiovascular agents, hormones, proteins and peptides.
Examples of analgesics are codeine, diamorphine,
dihydromorphine, ergotamine, fentanyl and morphine;
examples of anti-allergics are cromoglycinic acid and
nedocromil; examples of antibiotics are cephalosporins,
fusafungine, neomycin, penicillins, pentamidine,
streptomycin, sulphonamides and tetracyclines,
colistin, tobramycin; examples of anticholinergics are
atropine, atropine methonitrate, ipratropium bromide,
oxitropium bromide, trospium chloride and thiotropium
bromide; examples of antihistamines are azelastine,
flezelastine and methapyrilene; examples of anti-
inflammatory substances are beclomethasone, budesonide,
loteprednol, dexamethasone, flunisolide, fluticasone,
tipredane, triamcinolone, mometasone; examples of
antitussives are narcotine and noscapine; examples of
bronchodilators are bambuterol, bitolterol, carbuterol,
clenbuterol, ephedrine, epinephrine, formoterol,
fenoterol, hexoprerialine, i.buterol, isoprenaline,
isoproterenol, metaproterenol, orciprenaline,
phenylephrine, phenylpropanolamine, pirbuterol,
procaterol, reproterol, rimiterol, salbutamol,
salmeterol, sulfonteros, terbutaline and tolobuterol;
examples of diuretics are amiloride and furosemide; an
example of an enzyme is trypsin; examples of
CA 02415849 2003-01-08
-- 15 -
cardiovascular agents are diltiazem and nitroglycerin;
examples of hormones are cortisone, hydrocortisone and
prednisolone; examples of proteins and peptides are
cyclosporine, cetrorelix, glucagon and insulin. Further
active substances which can be used are adrenochrome,
coichicine, heparin, scopolamine. The active substances
listed by way of example can be used as free bases or
acids or as pharmaceutically acceptable salts.
Counterions which can be used include, for example,
physiological alkaline earth metals or alkali metals or
amines, for example acetate, benzene sulphonate,
benzoate, hydrogen carbonate, hydrogen tartrate,
bromide, chloride, iodide, carbonate, citrate,
fumarate, malate, maleate, cluconate, lactate, pamoate
and sulphate. Esters can also be used, for example
acetate, acetonide, propionate, dipropionate, valerate.
The invention also allows the doctor to very accurately
adapt the dose to the patient over a long period of
time, without the need to dispose of partially emptied
cartridges, which would have a disadvantageous effect
on treatment costs, and without compromising
compatibility with other cartridges with different
metering devices, e.g. with the cartridge known from WO
97/00703.
Brief description of the drawings
Figure 1 shows a pharmaceutical powder cartridge
according to the invention, in a perspective view;
Figure 2 shows a plan view of a seal of a
pharmaceutical powder cartridge according to the
invention;
Figure 3 shows a view of a carrier mechanism of a
metering slide in a pharmaceutical powder cartridge
according to the invention;
CA 02415849 2003-01-08
- 16 -
Figure 4A shows a view of a metering slide body in a
pharmaceutical powder cartridge according to the
invention;
Figure 4B shows a longitudinal section through the
metering slide body of a pharmaceutical powder
cartridge according to the invention from Figure 4A;
Figure 5 shows a longitudinal section through a
pharmaceutical powder cartridge according to the
invention, in an inhaler with the metering slide in the
emptying position; and
Figure 6 shows a longitudinal section through a
pharmaceutical powder cartridge according to the
invention, in an inhaler with the metering slide in the
filling position.
Description of preferred illustrative embodiments
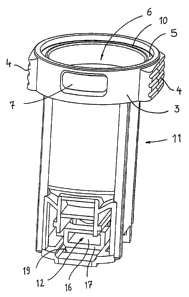
Fig. 1 shows a perspective view of a housing body 11 of
a pharmaceutical powder cartridge 1 according to the
invention for exchangeable insertion into a powder
inhaler 2. The pharmaceutical powder cartridge 1 shown
here has, at the upper area of its housing body 11, an
edge 3 which includes two grip areas 4 in order to
permit easy insertion of the pharmaceutical powder
cartridge 1 into a powder inhaler 2. In the
illustrative embodiment shown, a device for indicating
the amount of pharmaceutical doses which remain in the
storage space 6 or have been removed from the storage
space 6 is provided at the same time in the edge 3, in
an annular channel 5 formed therein (the device is not
shown in detail however), for example in the form of
foil strips with corresponding markings as is described
in detail in WO 97/00703. The user can then read off
the markings through the viewing window 7 in the edge
3.
CA 02415849 2006-09-15
- 17 -
The edge 3 also serves to receive a lid 8 with which
the storage space 6-forming the main part of the
pharmaceutical powder cartridge 1 can be sealed off.
Such a lid 8 is expediently sealed in a watertight
manner to a collar 10 formed within the edge 3, for
example by ultrasonic welding.
Arranged below the storage space 6 there is a metering
slide channel 12 in which a metering slide acting as
metering device is movably arranged, which metering
slide, in the illustrative embodiment described here,-
is made up of three parts, namely a carrier 13, the
actual metering slide body 1.4, and a seal 15 (Figures
2, 3, 4A and 4B). The metering slide is configured in
such a way that 'the seal 15 shown in Figure 2 is
inserted into the carrier 13 shown in Figure 3 and the
carrier 13 is clipped with seal 15 onto the metering
slide body 14.
As can be clearly seen from Figure 1, the metering
slide channel 12 at one end has an opening 16 and-,
formed around the opening 16, there is a contact-
surface 17 for the seal 15 of the metering slide. The
contact surface 17 is at the same time provided as a
sealing surface and extends in a plane approximately
perpendicular to the direction of movement of the
metering slide from a filling position, as is shown in
Figure 6, to an emptying position, as is shown in Figure 5.
The metering slide body 14 shown in Figures 4A and 4B
comprises a metering cavity 18 whose holding volume
represents the dose quantity to be made available for
an inhalation. The seal 15 can for example be co-
injected in multi-component injection-moulding and for
this purpose can be made, for example, of a
thermoplastic elastomer.
Correspondingly, a sealing surface can be provided on
CA 02415849 2003-01-08
- 18 -
the metering slide and the elastic seal 15 can be
mounted or better still injection-moulded in the area
of the opening 16 of the metering slide channel 12.
The housing body 11 and/o.r the lid 8 and/or the
metering slide body 14 can advantageously be made of a
COC by injection-moulding. A suitable material with the
name TOPASO 8007 is commercially available as a trial
product from the company Ticona in Germany.
For pharmaceutical combinations in which the powders
cannot be stored, or cannot adequately be stored, as a
mixture, it can also be expedient to provide two
storage chambers instead of the one storage space 6.
Figures 5 and 6 show a longitudinal section through the
pharmaceutical powder cartridge 1 inserted into an
inhaler 2. As can be seen from the figures, the
metering slide, designated overall by 9, can move in
the metering slide channel 12 at least from the filling
position shown in Figure 6 to the emptying position
shown in Figure 5.
In the filling position shown in Figure 6,
pharmaceutical powder can fall. from the storage space 6
into the metering cavity 18. When the metering cavity
18 has been filled, as desired, with a pharmaceutical
powder, the metering slide 9 can be moved to the
emptying position shown in E'igure 5 with the aid of
engagement means in a powder inhaler which are only
indicated schematically here, for example such as those
described in US 5,840,279 A, and which cooperate with
the carrier 13.
The emptying position is reached when the metering
cavity 18 is situated over an emptyirig opening 19. When
the metering slide 9 has reached this position, the
pharmaceutical powder can fall from the metering cavity
18 through the emptying opening 19, for example into a
CA 02415849 2003-01-08
- 19 -
powder channel 20 of an inhaler 2.
The filling position of the metering slide 9 can be
seen clearly in Figure 6, with the metering cavity 18
under a hole 21 on the underside of the storage space
6. To reach the empting position, the metering slide 9
is pushed to the left in Figure 6 until this metering
cavity 18 covers the emptying opening 19 and the
pharmaceutical powder can fall down from it.
It can also be clearly seen in Figure 6 that the seal
of the metering slide 9 lies on the contact surface
17 of the metering slide channel 12 and ensures a good
sealing, preferably with slight elastic deformation.
15 This can be done by prestressing with resiliently
elastic means, in particular via an actuating device in
the inhaler for the metering slide 9, which expediently
also effects an immediate reverse movement of the
metering slide 9 from the emptying position, as shown
in Figure 5, to its sealed filling position, as shown
in Figure 6, as soon as a pharmaceutical dose has been
removed.
For a higher contact pressure of the seal 15 of the
metering slide 9 on the contact surface 17 of the
metering slide channel 12, and thus for a particularly
reliable sealing during storage of a pharmaceutical
powder cartridge according to the invention, especially
prior to its first use in a powder inhaler, it is
advantageous to provide, slightly further to the right
in the view in Figure 6, an additional storing position
for the metering slide 9 of a filled pharmaceutical
powder cartridge 1 in which the metering slide 9 can be
fixed by the releasable snap connection 22 shown. In
this storing position, the seal 15 of the metering
slide 9 on the contact surface 17 of the metering slide
channel 12 is subject to an increased prestressing
force.
CA 02415849 2003-01-08
- 20 -
In the upper area of the storage space there is also
advantageously a shaped body 23 which is preferably
secured in its position via corresponding shaped edges
24, in order to avoid mechanical loading of the
pharmaceutical powder. The shaped body 23 is
expediently produced by injection-moulding from a blend
of a thermoplastic matrix and a desiccant. The
desiccant is intended in particular to absorb moisture
which is situated in the storage space 6 or which has
penetrated through the metering cavity 18. The use of
such a shaped body 23 ensures that no crumbs of the
desiccant, typically silica gel, can pass into the
pharmaceutical powder and thus into the airways of a
patient. Such a shaped body can be made of a PP matrix
which itself does not take up water and which is
injected mixed with a water-soluble compound and the
desiccant, for example polyethylene glycol, and the
water-soluble compound is then washed out. This results
in a sponge-like structure with channels which, after
the shaped body has dried, permit rapid water
absorption of the (not water-soluble) silica gel
through large surfaces using capillary condensation.
In order to obtain a rapid water absorption in the
whole shaped body 23, it may also be expedient to embed
suitable fibres as filler in the blend of desiccant and
thermoplastic matrix, which fibres, by means of their
capillary action, ensure rapid transport of the air
moisture and of the water to the desiccant.
The shaped body 23 can also be designed in the form of
a wall lining in the manner of an itisert, as is shown
purely by way of example iri Figure 5, or, by multi-
component injection-mouldirig in the production of the
pharmaceutical powder cartridge, can form all or part
of an inner wal.l of the storage space 6.