Note: Descriptions are shown in the official language in which they were submitted.
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
Apparatus and Method for Container Filling
FIELD OF THE INVENTION
The present invention relates generally to systems for the
aseptic packaging of food products. More particularly, the
present invention relates to an aseptic packaging system for the
aseptic packaging of food products in containers such as bottles
or jars. The present invention also relates to a method and
apparatus for interior bottle sterilization. The present
invention also relates to an apparatus and method for providing
container product filling in the aseptic packaging system. The
present invention further relates to a method and apparatus for
providing container lidding and sealing in the aseptic packaging
system.
BACKGROUND OF THE INVENTION
Sterilized packaging systems in which a sterile food
product is placed and sealed in a container to preserve the
product for later use are well known in the art. Methods of
sterilizing incoming containers, filling the containers with
pasteurized product, and sealing the containers in an aseptic
sterilization tunnel are also known.
1
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
Liquid product fillers are known in the art. Generally, a
container is placed under a filler head. The filler head opens
and dispenses the liquid product. When the container is filled
to a desired level, the filler head closes and stops the flow of
liquid product into the container. Commonly, in line aseptic
fillers use completely mechanical devices for measuring and
dosing product into containers. These devices include a first
apparatus for measuring the amount of material to be dispensed,
and a second apparatus which functions as a filling nozzle.
Typically, the first apparatus includes a piston cylinder
apparatus for measuring the amount of material. The amount of
material measured by the piston cylinder apparatus is limited by
the diameter and stroke of the piston. The first and second
apparatus include complicated mechanical members which are
difficult to sterilize, clean, and maintain.
Typically, rotary fillers include multiple filling stations
and allow about 7 to 15 seconds for filling. Some of the
rotary bottle filers use electronic measuring devices for dosing
the desired amount of product into a bottle. In order to meet
FDA (Food and Drug Administration) "aseptic" standards and 3A
Sanitary Standards, all surfaces of the filler that come into
contact with the liquid product must be sterilized. Before
filling commences, a plurality of interior parts of the filler
must be removed, sterilized, and replaced. This time consuming
and expensive process is necessary in order to ensure the
2
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
complete sterilization of all surfaces that come into contact
with the liquid product.
Packaged food products can generally be categorized as high
acid products (Ph below 4.5) or low acid products (Ph of 4.5 and
above). The high acid content of a high acid product helps to
reduce bacteria growth in the product, thereby increasing the
shelf life of the product. The low acid content of a low acid
product, however, necessitates the use of more stringent
packaging techniques, and often requires refrigeration of the
product at the point of sale.
Several packaging techniques, including extended shelf life
(ESL) and aseptic packaging, have been developed to increase the
shelf life of low acid products. During ESL packaging, for
example, the packaging material is commonly sanitized and filled
with a product in a presterilized tunnel under "ultra-clean"
conditions. By using such ESL packaging techniques, the shelf
life of an ESL packaged product is commonly extended from about
10 to 15 days to about 90 days. Aseptic packaging techniques,
however, which require that the packaging take place in a
sterile environment, using presterilized containers, etc., are
capable of providing a packaged product having an even longer
shelf life of 150 days or more. In fact, with aseptic
packaging, the shelf life limitation is often determined by the
quality of the taste of the packaged product, rather than by a
limitation caused by bacterial growth.
3
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
For the aseptic packaging of food products, an aseptic
filler must, for example, use an FDA (Food and Drug
Administration) approved sterilant, meet FDA quality control
standards, use a sterile tunnel or clean room, and must
aseptically treat all packaging material. The food product must
also be processed using an "Ultra High Temperature" (UHT)
pasteurization process to meet FDA aseptic standards. The
packaging material must remain in a sterile environment during
filling, closure, and sealing operations.
Many attempts have been made, albeit unsuccessfully, to
aseptically fill containers, such as bottles or jars having
small openings, at a high output processing speed. In addition,
previous attempts for aseptically packaging a low acid product
in plastic bottles or jars (e.g., formed of polyethylene
terepthalate (PET) or high density polyethylene (HDPE)), at a
high output processing speed, have also failed. Furthermore,
the prior art has not been successful in providing a high output
aseptic filler that complies with the stringent United States
FDA standards for labeling a packaged product as "aseptic." In
the following description of the present invention, the term
"aseptic" denotes the United States FDA level of aseptic.
Si]MMARY OF THE INVENTION
In order to overcome the above deficiencies, the present
4
CA 02416094 2008-10-20
invention provides an apparatus and method for interior container
sterilization, container
product filling, and container lidding and sealing in an aseptic processing
apparatus.
In a broad aspect, the present invention relates to and apparatus comprising:
a valve
for controlling a flow of product; a first sterile region surrounding a region
where the
product exits the valve; a continuously sterilized second sterile region
positioned
proximate said first sterile region whereby said second sterile region is
continuously
sterilized during operation; a valve activation mechanism for controlling the
opening or
closing of the valve by extending a portion of the valve from the continuously
sterilized
second sterile region into the first sterile region and by retracting the
portion of the valve
from the first sterile region back into the continuously sterilized second
sterile region.
In another broad aspect, the present invention relates to and apparatus
comprising:
a tank for containing a supply of a pressurized product; a measuring device
connected to
the tank for measuring an amount of the product flowing from the tank to a
container; a
filling nozzle connected to the measuring device for directing product flow
into the
container; a valve located within the filling nozzle for controlling the flow
of product; a
first sterile region surrounding a region where the product exits the valve; a
valve stem
attached to the valve for controlling the opening or closing of the valve; a
sterilization
chamber surrounding a first portion of the valve stem; and a valve activation
mechanism
for controlling the opening or closing of the valve by extending the first
portion of the
valve stem from the sterilization chamber into the first sterile region and by
retracting the
first portion of the valve stem from the first sterile region back into the
sterilization
chamber.
In another broad aspect, the present invention relates to a method comprising
the
steps of: controlling a flow of product using a valve; surrounding a region
where the
product exits the valve with a sterile region; providing a continuously
sterilized second
sterile region positioned proximate said first sterile region whereby said
second sterile
region is continuously sterilized during operation; and controlling the
opening or closing of
the valve by extending a portion of the valve from the continuously sterilized
second sterile
5
CA 02416094 2008-10-20
region into the first sterile region and by retracting the portion of the
valve from the first
sterile region back into the continuously sterilized second sterile region.
In another broad aspect, the present invention relates to and apparatus
comprising:
a first supply source of sterile air; a supply source of sterilant; an
atomizing system
producing an atomized sterilant from the mixing of the sterile air from the
first supply
source of sterile air with the sterilant; a second supply source of a hot
sterile air for
providing the hot sterile air to the atomized sterilant; a probe for applying
the atomized
sterilant into an interior of a container; and a third supply source of a hot
sterile drying air
for activating and drying the sterilant in the interior of the container.
In another broad aspect, the present invention relates to a method comprising:
providing a first supply of sterile air; providing a supply of sterilant;
producing an
atomized sterilant by mixing the first supply of sterile air with the
sterilant; providing a
second supply of hot sterile air to the atomized sterilant; providing a probe
for applying the
atomized sterilant into an interior of a container; and supplying a third
supply of hot sterile
drying air for activating and drying the sterilant in the interior of the
container.
In another broad aspect, the present invention relates to an apparatus
comprising: a
continuous chain of lids; a bath of sterilant for covering the chain of lids
with the sterilant;
a drier for heating and drying the sterilant on the chain of lids; and a guide
for positioning
each lid over a container opening.
In another broad aspect, the present invention relates to a method for lidding
aseptically sterilized foodstuffs comprising the steps of: providing a chain
of lids;
providing a bath of sterilant for covering the chain of lids with the
sterilant; providing a
drier for heating and drying the sterilant on the chain of lids; and providing
a guide for
positioning each lid over a container opening.
In another broad aspect, the present invention relates to and apparatus
comprising:
means for providing a continuous chain of lids; means for providing a
sterilant for
covering the chain of lids with the sterilant; means for providing a drier for
heating and
5a
CA 02416094 2008-10-20
drying the sterilant on the chain of lids; and means for positioning each lid
over a container
opening.
In another broad aspect, the present invention relates to method comprising
the
steps of: controlling a flow of product using a valve; surrounding a region
where the
product exits the valve with a sterile region; providing a second sterile
region positioned
proximate said first sterile region; controlling the opening or closing of the
valve by
extending a portion of the valve from the second sterile region into the first
sterile region
and by retracting the portion of the valve from the first sterile region back
into the second
sterile region; providing a tank for containing a supply of pressurized
product flowing to
the valve; providing a measuring device for measuring the amount of
pressurized product
flowing from the tank to the valve; exposing the valve, an interior surface of
the tank, and
an interior surface of the measuring device with steam; covering an exit of
the valve; and
allowing a build-up of steam pressure inside the tank to above a temperature
of about
250 F, a steam pressure of about 50 psig, for about 30 minutes.
In another broad aspect, the present invention relates to a method comprising
the
steps of: controlling a flow of product using a valve; surrounding a region
where the
product exits the valve with a sterile region; providing a second sterile
region positioned
proximate said first sterile region; controlling the opening or closing of the
valve by
extending a portion of the valve from the second sterile region into the first
sterile region
and by retracting the portion of the valve from the first sterile region back
into the second
sterile region; providing a second apparatus wherein the container is filled
to a first level
with the product exiting from the first apparatus, and the container is filled
to a second
level with the product exiting from the second apparatus; uncovering the exit
of the valve;
and providing sterile air to reduce the temperature of the valve, the interior
surface of the
tank, and the interior surface of the measuring device to the temperature of
the product.
In another broad aspect, the present invention relates to an apparatus
comprising:
an inline bottle filing apparatus including: a valve for controlling a flow of
product; a first
sterile region surrounding a region where the product exits the valve; a
second sterile
5b
CA 02416094 2008-10-20
region positioned proximate said first sterile region; a valve activation
mechanism for
controlling the opening or closing of the valve by extending a portion of the
valve from the
second sterile region into the first sterile region and by retracting the
portion of the valve
from the first sterile region back into the second sterile region.
In another broad aspect, the present invention relates to an apparatus
comprising:
a valve for controlling a flow of product into a bottle; a first sterile
region surrounding a
region where the product exits the valve; a second sterile region positioned
proximate said
first sterile region; a valve activation mechanism for controlling the opening
or closing of
the valve by extending a portion of the valve from the second sterile region
into the first
sterile region, such that the valve does not contact the bottle, and by
retracting the portion
of the valve from the first sterile region back into the second sterile
region.
The present invention provides an apparatus and method for providing container
interior sterilization in an aseptic processing apparatus. The interior
container sterilization
is applied in an apparatus for providing aseptically processed low acid
products in a
container having a small opening, such as a glass or plastic bottle or jar, at
a high output
processing speed. The present invention includes a plurality of sterile air
supply sources.
For example, a first supply source of sterile air is used to atomize a
sterilant (e. g.,
hydrogen peroxide), within an atomizing venturi. A second supply source of
sterile air is
used to provide hot sterile air to the atomized sterilant leaving the
atomizing venturi. A
third supply source of sterile air is used to provide hot sterile air for
activating and drying
the sterilant on the interior surface of the container. The second supply
source of heated
sterile air, prevents the formation of hydrogen peroxide droplets. This
results in a design
that will meet the FDA regulations for each and every bottle that is
manufactured.
Typically, in the aseptic packaging industry, a low volume of air at a high
temperature is
applied to the packaging materials. This method works well when the container
material
can withstand relatively
5c
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
high temperatures such as when cups are made of polypropylene.
However, this often results in deformation and softening of
packaging materials formed of PET or HDPE. In order to prevent
softening and deformation of the bottles, when formed from these
types of plastic materials, the present invention applies high
volumes of air at relatively low temperatures over an extended
period of time in the activation and drying apparatus. A long
exposure time is predicated by the geometry of the bottle and
the softening temperature of the material used to form the
bottle. In the present invention, about 24 seconds are allowed
for directing hot sterile air from the third supply source of
sterile air into the interior of the bottles. In order to
achieve aseptic sterilization, the bottle is maintained at about
131 F for at least 5 seconds. Many features are incorporated
into the interior bottle sterilization apparatus in order to
meet the various FDA aseptic standards and the 3A Sanitary
Standards and Accepted Practices.
The present invention provides both a "Clean In Place"
(CIP) process for cleaning, and a "Sterilizing in Place" for
sterilizing all of the interior surfaces of the filler without
having to disassemble the filler. The filler apparatus includes
a smooth filling tube which is easy to clean and sterilize. The
filler apparatus is used in a system for providing aseptically
processed low acid products in a container having a small
opening, such as a glass or plastic bottle or jar, at a high
6
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
output processing speed. Many features are incorporated into
the filler apparatus in order to meet various FDA aseptic
standards and 3A Sanitary Standards and Accepted Practices.
The present invention provides an apparatus and method for
providing container lidding and sealing in an aseptic processing
apparatus. The lidding is applied in an apparatus for providing
aseptically processed low acid products in a container having a
small opening, such as a glass or plastic bottle or jar, at a
high output processing speed. Many features are incorporated
into the lidding and sealing apparatus in order to meet the
various FDA aseptic standards and the 3A Sanitary Standards and
Accepted Practices.
A lid sterilization and heat sealing apparatus of the
present invention sterilizes and seals container lids supplied
from a continuous chain of lids. When a narrow band of material
interconnects lids, this form of lidding material is also known
as a "daisy chain." The daisy chain eliminates the material
waste resulting from die cutting lids from a continuous roll of
material. Without the waste material generated by a die cutting
process, the daisy chain requires only a supply reel and
eliminates the need for having a take-up reel. Since the daisy
chain provides a single thickness of interconnected lids, the
possibility of two lids sticking together is eliminated. The
daisy chain passes through a sterilant bath that ensures
complete sterilization of all surfaces of the daisy chain before
7
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
entry into the sterilization tunnel.
BRIEF DESCRIPTION OF THE DRAWINGS
The features of the present invention will best be
understood from a detailed description of the invention and a
preferred embodiment, thereof selected for the purposes of
illustration, and shown in the accompanying drawings in which:
FIG. 1 is a plan view of an aseptic processing apparatus in
accordance with a preferred embodiment of the present invention;
FIG. 2 is a side view of the aseptic processing apparatus
of FIG. 1;
FIG. 3 is a partial cross-sectional side view of the
aseptic processing apparatus of FIG. 1;
FIG. 4 is a cross-sectional side view of a bottle infeed
and sterilization apparatus;
FIG. 5 illustrates a cross-sectional top view of the bottle
infeed and sterilization apparatus taken along line
5--5 of FIG. 4;
FIG. 6 is an interior sectional view of an interior wall
taken along line 6--6 of FIG. 4;
FIG. 7 is a cross-sectional view of the bottle infeed and
sterilization apparatus taken along line 7--7 of FIG. 4;
FIG. 8 is a perspective view of a conveying plate for use
in the aseptic processing apparatus of the present invention;
8
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
FIG. 9 is a perspective view of a partition in a
sterilization tunnel;
FIG. 10 is a cross-sectional side view of an interior
bottle sterilization apparatus and the partition located between
stations 8 and 9;
FIG. 11 is a cross-sectional side view of the partition
located between stations 22 and 23;
FIG. 12 is a cross-sectional side view of the partition
located between stations 35 and 36;
FIG. 13 is a cross-sectional side view of a lid
sterilization and heat sealing apparatus;
FIG. 14 is a side view of a lifting apparatus with a
gripper mechanism for lifting the bottles from the sterilization
tunnel;
FIG. 15 is a top view of the aseptic processing apparatus;
FIG. 16 is a side view of the aseptic processing apparatus
indicating the control and monitoring locations that are
interfaced with a control system;
FIG. 17 is a plan view of a daisy chain of lids;
20. FIG. 18 is a plan view of another embodiment of a daisy
chain of lids with holes for receiving pins of a drive wheel;
FIG. 19 is another embodiment of the lid sterilization and
heat sealing apparatus including a pin drive apparatus;
FIG. 20 is a perspective view of the heat sealing and
gripper apparatus;
9
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
FIG. 21 is a schematic diagram of a sterilization control
system for the interior bottle sterilization apparatus;
FIG. 22 is a side view of a main product filler apparatus;
FIG. 23 is a cross-sectional view of a valve in a closed
position in a first sterile region;
FIG. 24 is a cross-sectional view with a portion of a valve
stem displaced from a non-sterile region into the first sterile
region;
FIG. 25 is a cross-sectional view of the valve in a closed
position in a first sterile region, and with the portion of the
valve stem located in a second sterile region; and
FIG. 26 is a cross-sectional view of the valve in an open
position where the portion of the valve located in the second
sterile region has been displaced into the first sterile region.
DETAILED DESCRIPTION OF THE INVENTION
Although certain preferred embodiments of the present
invention will be shown and described in detail, it should be
understood that various changes and modifications may be made
without departing from the scope of the appended claims. The
scope of the present invention will in no way be limited to the
number of constituting components, the materials thereof, the
shapes thereof, the relative arrangement thereof, etc., and are
disclosed simply as an example of the preferred embodiment. The
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
features and advantages of the present invention are illustrated
in detail in the accompanying drawings, wherein like reference
numerals refer to like elements throughout the drawings.
Although the drawings are intended to illustrate the present
invention, the drawings are not necessarily drawn to scale.
The present invention provides an aseptic processing
apparatus 10 that will meet the stringent United States FDA
(Food and Drug Administration) requirements and 3A Sanitary
Standards and Accepted Practices required to label a food
product (foodstuffs) as "aseptic." Hereafter, "aseptic" will
refer to the FDA level of aseptic. The present invention
provides an aseptic processing apparatus 10 for producing at
least about a 12 log reduction of Clostridium botulinum in food
products. In addition, the present invention produces packaging
material with at least about a 6 log reduction of spores.
Actual testing of the aseptic processing apparatus is
accomplished with spore test organisms. These test organisms
are selected on their resistance to the media selected used to
achieve sterility. For example, when steam is the media, the
test organism is Bacillus stearothermophilus. When hydrogen
peroxide is the media, then the test organism is Bacillus
subtilis var. globigii.
The present invention processes containers such as bottles
or jars that have a small opening compared to its height and its
greatest width (e.g., the ratio of the opening diameter to the
11
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
height of the container is less than 1.0). In the preferred
embodiment, a bottle 12 (see, e.g., FIG. 8) is illustrated as
the container. The container may alternately comprise a jar.
The bottle 12 is preferably formed of a plastic such as
polyethylene terepthalate (PET) or high density polyethylene
(HDPE), although other materials such as glass may also be used.
The present invention uses an aseptic sterilant such as hydrogen
peroxide (H202) or oxonia (hydrogen peroxide and peroxyacetic
acid) to sterilize the bottles 12. In the preferred embodiment
of the present invention, hydrogen peroxide is used as the
sterilant. The present invention uses hydrogen peroxide with a
concentration of less than about 35% and ensures that the
bottles 12 have less than about .5ppm of residual hydrogen
peroxide after each bottle 12 is sterilized.
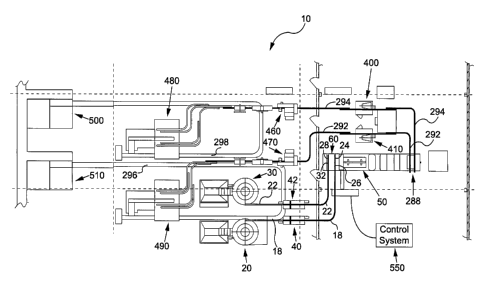
FIGS. 1-3 illustrate several views of an aseptic processing
apparatus 10 in accordance with a preferred embodiment of the
present invention. As shown, the aseptic processing apparatus
10 includes a first bottle unscrambler 20, a second bottle
unscrambler 30, and a bottle lifter 40 for providing a supply of
properly oriented empty bottles. The empty bottles are
delivered to a filler apparatus 50 after passing through a
bottle infeed and sterilization apparatus 60 for aseptic
sterilization. The filled bottles are sealed at a first capping
apparatus 400 or a second capping apparatus 410. A control
system 550 monitors and controls the operation of the aseptic
12
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
processing apparatus 10. The filled and sealed bottles are
packed and palletized using a first case packing apparatus 480,
a second case packing apparatus 490, a first palletizer 500, and
a second palletizer 510.
The bottles 12 arrive at a first bottle unscrambler 20 with
a random orientation, such that an opening 16 (see FIG. 8) of
each bottle 12 can be oriented in any direction. The first
bottle unscrambler 20 manipulates the bottles 12 until the
opening 16 of each bottle 12 is in a top vertical position. The
bottles 12 leave the first bottle unscrambler 20 in a series
formation with the opening 16 of each bottle 12 oriented
vertically. The bottles 12 travel in single file in a first
lane 18 to a first bottle lifter 40. The first bottle lifter 40
lifts and transports the bottles 12 to a bottle infeed and
sterilization apparatus 60. A second bottle unscrambler 30 may
also used to provide a supply of vertically oriented bottles 12.
The bottles 12 output from the second bottle unscrambler 30
travel in single file in a second lane 22 to a second bottle
lifter 42, which lifts and transports the bottles 12 to the
bottle infeed and sterilization apparatus 60.
FIG. 3 illustrates the bottle infeed, sterilization, and
conveying apparatus 60 attached to the filler apparatus 50.
FIG. 4 illustrates a cross-sectional side view of the bottle
infeed, sterilization, and conveying apparatus 60. FIG. 5
illustrates a cross-sectional top view of the bottle infeed,
13
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
sterilization, and conveying apparatus 60 taken along line 5--5
of FIG. 4. The bottle infeed and sterilization apparatus 60
preferably inputs six bottles 12 in a horizontal direction from
the first lane 18 and six bottles in a horizontal direction from
the second lane 22 (FIG. 5). A gate 76 in the first lane 18
selectively groups six bottles 12 at a time in first horizontal
row 24. A gate 78 in the second lane 22 selectively groups six
bottles 12 at a time in a second horizontal row 28. An infeed
apparatus 80 includes a pushing element 84 for pushing the
bottles 12 in the first horizontal row 24 into a first vertical
lane 26. A corresponding infeed apparatus 80 includes a pushing
element 86 for pushing.the bottles 12 in the second horizontal
row 28 into a second vertical lane 32. The six bottles 12 in
the first vertical lane 26 and the six bottles 12 in the second
vertical lane 32 are directed downward into the bottle infeed
and sterilization apparatus 60.
Referring to FIG. 4, as the bottles 12 move downward in the
first vertical lane 26 and the second vertical lane 32, a
sterilant 14, such as heated hydrogen peroxide, oxonia, or other
aseptic sterilant, is applied to an outside surface 34 of each
bottle 12 by a sterilant application apparatus 36. The outside
surface 34 of a bottle 12 is illustrated in greater detail in
FIG. S. The bottles 12 may move downward in the first vertical
lane 26 and the second vertical lane 32 by the force of gravity.
Alternatively, controlled downward movement of the bottles 12
14
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
can be created by the use of a conveying device such as a moving
conveying chain. A plurality of pins are attached to the
conveying chain. Each bottle 12 rests on one of the pins
attached to the conveying chain. Therefore, the motion of each
bottle is controlled by the speed of the moving conveying chain.
A sterilant such as hydrogen peroxide may be provided to
the sterilant application apparatus 36 in many ways. For
example, liquid hydrogen peroxide may be provided in a reservoir
at a level maintained by a pump and overflow pipe. A plurality
of measuring cups (e.g., approximately 0.5m1 each) connected by
an air cylinder are submerged into the reservoir and are lifted
above the liquid level. Thus, a measured volume of liquid
hydrogen peroxide is contained in each measuring cup.
Each measuring cup may include a conductivity probe that is
configured to send a signal to the control system 550 indicating
that the measuring cup is full. A tube (e.g., having a diameter
of about 1/16") is positioned in the center of the measuring
cup. A first end of the tube is positioned near the bottom of
the measuring cup. A second end of the tube is connected'to the
sterilant application apparatus 36. The sterilant application
apparatus 36 includes a venturi and a heated double tube heat
exchanger. When the measuring cup is full, and a signal is
received from the control system 550, a valve is opened allowing
pressurized sterile air to enter the venturi. The pressurized
air flow causes a vacuum to be generated in second end of the
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
tube causing liquid hydrogen peroxide to be pulled out of the
measuring cup. The liquid hydrogen peroxide is sprayed into a
sterile air stream which atomizes the hydrogen peroxide into a
spray. The atomized hydrogen peroxide enters the double tube
heat exchanger in order to heat the atomized hydrogen peroxide
above its vaporization phase. The double tube heat exchanger is
heated with steam and the temperature is monitored and
controlled by the control system 550. In FIG. 4, the
application of the sterilant 14 by the sterilant application
apparatus 36 is accomplished through the use of spray nozzles 64
that produce a sterilant fog which is directed to the entire
outside surface 34 of each bottle 12.
Alternatively, a direct spray of heated hydrogen peroxide
may be continuously applied to the outside surface 34 of each
bottle 12. For producing the direct spray, a metering pump
regulates the amount of hydrogen peroxide, a flow meter
continuously measures and records the quantity of hydrogen
peroxide being dispensed, a spray nozzle produces a fine mist,
and a heat exchanger heats the hydrogen peroxide above the
vaporization point.
FIGS. 3 and 4 illustrate the sterilization chamber 38 for
activation and drying of bottles 12 which is included in the
bottle infeed, sterilization, and conveying apparatus 60. The
sterilization chamber 38 sterilizes the outside surface 34 of
each bottle 12. The sterilization chamber 38 encloses a conduit
16
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
39. Sterile heated air, which is generated by a sterile air
supply system 146 (FIG. 3), enters the conduit 39 of the
sterilization chamber 38 through ports 67 and 68 located at the
bottom of the sterilization chamber 38. The sterile heated air
also enters through a bottom opening 62 of the bottle infeed and
sterilization apparatus 60. The sterile heated air travels up
through the conduit 39 of the sterilization chamber 38, and
exits the top of the sterilization chamber 38 through an exhaust
conduit 70. The sterile heated air continuously flows in an
upward direction through the sterilization chamber 38, thus
preventing any contaminants from entering the bottle infeed and
sterilization apparatus 60. To create the sterile heated air,
the air is first passed through a filtering system (e.g., a
group of double sterile air filters to sterilize the air. The
air is then heated in a heating system (e.g., an electric
heater) to about 230 F. The air temperature is regulated by the
control system 550. Other techniques for providing the sterile
heated air may also be used. The control system 550 monitors
the air pressure and flow rate of the sterile heated air to
ensure that an adequate flow of the hot sterile air is
maintained in the bottle sterilization chamber 38 of the bottle
infeed and sterilization apparatus 60.
As illustrated in FIGS. 4, 6, and 7, the sterilization
chamber 38 includes two opposing, interior, perforated walls
72A, 72B. The perforated walls 72A and 72B guide the bottles 12
17
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
downward in the first vertical lane 26 and the second vertical
lane 32, respectively. The perforated walls 72A, 72B also allow
the complete circulation of hot sterile air around the outside
surface 34 of each bottle 12 in the sterilization chamber 38.
The sterilization chamber 38 supplies hot sterile air to the
outside surface 34 of each bottle 12 between the sterilant
application apparatus 36 and the bottom opening 62 of the bottle
infeed and sterilization apparatus 60. This sterilant may be
hydrogen peroxide or oxonia (hydrogen peroxide and peroxyacetic
acid).
In accordance with the preferred embodiment of the present
invention, twelve drying positions are provided in the
sterilization chamber 38. Each bottle 12 is exposed to the hot
sterile air in the sterilization chamber 38 for about at least
24 seconds. This provides time sufficient time for the hydrogen
peroxide sterilant to break down into water and oxygen, to kill
any bacteria on the bottles 12, and to evaporate from the
outside surface 34 of the bottles 12.
An exhaust fan 73 is located at a top of the exhaust
conduit 70 to provide an outlet from the sterilization tunnel
90, and to control the sterile air flow rate through the
sterilization chamber 38. The exhaust fan 73 is controlled by
the control system 550. The control system 550 controls the
sterile air temperature preferably to about 230 F, and controls
the sterile air flow rate through the sterilization chamber 38.
18
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
The flow rate is preferably about 1800 scfm through the
sterilization chamber 38. The bottles 12 leave the
sterilization chamber 38 with a hydrogen peroxide concentration
of less than 0.5PPM.
As shown in FIGS. 3.and 4, a plurality of proximity sensors
71 located along the sides of the vertical lanes 26, 32 detect
any bottle 12 jams that occur within the sterilization chamber
38. The proximity sensors 71 transmit an alarm signal to the
control system 550. The bottles 12 leave the bottle infeed and
sterilization apparatus 60 through the bottom opening 62, and
enter a sterilization tunnel 90 of the filler apparatus 50.
In the preferred embodiment of the present invention, the
filler apparatus 50 includes forty-one (41) index stations 92,
hereafter referred to as "stations." Various index stations 92
are illustrated in FIGS. 3, 4, and 11-15. The conveying motion
of the bottles 12 to the various stations 92 through the filler
apparatus 50 is based on an indexing motion. The filler
apparatus 50 is designed to convey the bottles 12 through the
various operations of the filler 50 in a two by six matrix. The
twelve bottles 12 in the two by six matrix are positioned in,
and displaced by, a conveying plate 94 as illustrated in FIG. 8.
Therefore, twelve bottles 12 are exposed to a particular station
92 at the same time. A conveying apparatus 100 moves the set of
twelve bottles 12 in each conveying plate 94 sequentially
through each station 92.
19
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
Referring to FIGS. 3 and 4,. the bottles 12 are supplied
from an infeed chamber 102 to station 2 of the filler apparatus
50 through the bottom opening 62 of the bottle infeed and
sterilization apparatus 60. The infeed chamber 102 is enclosed
to direct heated hydrogen peroxide laden air completely around
the outer surface 34 of the bottles 12. A mechanical scissors
mechanism and a vacuum "pick and place" apparatus 104 position
twelve bottles 12 at a time (in a two by six matrix, FIG. 8)
into one of the conveying plates 94.
A plurality of conveying plates 94 are attached to a main
conveyor 106. The main conveyor 106 forms a continuous element
around conveyor pulleys 108 and 110 as illustrated in FIG. 3. A
bottle support plate 107 supports a bottom 120 of each bottle 12
as the bottles 12 are conveyed from station to station through
the filler apparatus 50. Each conveying plate 94 passes through
stations 1 through 41, around pulley 108, and returns around
pulley 110 to repeat the process. The main conveyor 106,
conveying plates 94, and pulleys 108 and 110 are enclosed in the
sterilization tunnel 90.
At station 4, the bottles 12 in the conveying plate 94
enter a bottle detection apparatus 112. The bottle detection
apparatus 112 determines whether all twelve bottles 12 are
actually present and correctly positioned in the conveying plate
94. Proximity sensors 114 detect the presence and the alignment
of each bottle 12. In the present invention, a bottle 12 with
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
correct alignment is in an upright position with the opening 16
of the bottle 12 located in an upward position. Information
regarding the location of any misaligned or missing bottles 12
is relayed to the control system 550. The control system 550
uses this location information to ensure that, at future
stations 92, bottle filling or sealing will not occur at the
locations corresponding to the misaligned or missing bottles 12.
At station 7, as illustrated in FIGS. 3 and 10, the bottles
12 in the conveying plate 94 enter an interior bottle
sterilization apparatus 116. A sterilant, such as hydrogen
peroxide, oxonia, or any other suitable aseptic sterilant is
applied as a heated vapor fog into the interior 118 of each
bottle 12. Preferably, hydrogen peroxide is used as the
sterilant in the present invention. The application of
sterilant is accomplished with the use of a plurality of
sterilant measuring devices 121 and a plurality of probes 123.
Each probe 123 includes any practical means for transferring the
sterilant from the probe 123 to the interior surface 119 of the
bottle 12. For example, an opening or a plurality of openings
may be used for ejecting the sterilant onto the interior surface
119. Preferably, in the present invention, an applicator spray
nozzle 122 is included in each probe 123. The applicator spray
nozzle 122 provides uniform sterilant application without
droplet formation on the interior surface 119 of the bottle 12.
A separate measuring device 121 and the probe 123 are used for
21
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
each of the twelve bottle 12 locations in the conveying plate
94. Each sterilant measuring device 121 may include a spoon
dipper 304 (e.g., approximately 0.5m1 each) as illustrated in
FIG. 21. Each bottle 12 is supplied with the same measured
quantity of sterilant, preferably in the form of a hot vapor
fog. A pump 306 provides a sterilant (e.g., hydrogen peroxide)
from a sterilant supply tank 310 to a reservoir 124. An
overflow pipe 308 maintains the sterilant liquid level in the
reservoir 124 by returning excess sterilant to the sterilant
supply tank 310. The spoon dipper 304 connected to an air
cylinder 316 is submerged into the reservoir 124 and is lifted
above the liquid level. Thus, a measured volume of liquid
hydrogen peroxide (e.g., approximately 0.5m1) is contained in
each spoon dipper 304.
Each spoon dipper 304 may include a conductivity probe that
is configured to send a signal to the control system 550
indicating that the spoon dipper 304 is full. A tube 312 (e.g.,
having a diameter of about 1/16") is positioned in the center of
the spoon dipper 304. A first end of the tube 312 is positioned
near the bottom of the spoon dipper 304. A second end of the
tube 312 is connected to an atomizing venturi 314.
A pressurized air source 318 is connected by a conduit 320
to a flow adjust valve 322. A conduit 324 connects the flow
adjust valve 322 to a regulator valve 326. A conduit 328
connects the regulator valve 326 with a solenoid actuated valve
22
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
330. A conduit 332 connects the solenoid actuated valve 330
with the air cylinder 316. The control system 550 controls the
solenoid actuated valve 330 which controls the compressed air
supplied to the air cylinder 316. Compressed air supplied to the
air cylinder 316 lowers or lifts the spoon dipper 304 into or
out of the liquid sterilant.
A conduit 334 connects the flow adjust valve 322 with the
regulator valve 336. A conduit 338 connects the regulator valve
336 with a sterile air filter 340. A conduit 342 connects the
sterile air filter 340 with a solenoid actuated valve 344. A
conduit 346 connects the solenoid actuated valve 344 with the
atomizing venturi 314. When the spoon dipper 304 is full, and a
signal is received from the control system 550, the solenoid
actuated valve 344 is opened allowing pressurized sterile air to
enter the atomizing venturi 314 through the conduit 346. The
pressurized air flow causes a vacuum to be generated in the
second end of the tube 312 causing liquid hydrogen peroxide to
be pulled out of the spoon dipper 304.
A first supply of sterile air is supplied through conduit
346. The pressurized air supplied through conduit 346 is used
to atomize the hydrogen peroxide sterilant in the atomizing
venturi 314. Atomization of the liquid hydrogen peroxide may be
provided by other means such as by using ultrasonic frequencies
to atomize the liquid hydrogen peroxide.
A conduit 348 connects with the atomizing venturi 314,
23
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
passes through a heat exchanger 350 (e.g., double tube heat
exchanger), and connects with a probe 123 including the
applicator spray nozzle 122. A conduit 352 connects a steam
supply 354 with a valve 356. A conduit 358 connects the valve
356 with a regulator valve 360. A conduit 382 connects the
regulator valve 360 with the heat exchanger 350.
A second supply of hot sterile air is supplied to the
atomized sterilant through a conduit 378. A humidity control
apparatus 362 maintains the humidity level of the air entering a
blower 364. A conduit 366 connects the blower 364 with a heater
368. A conduit 370 connects the heater 368 with a sterile
filter 372. A conduit 374 connects the sterile filter 372 with
a flow adjust valve 376. The conduit 378 connects the flow
adjust valve 376 with the conduit 348. A conduit 380 connects
the sterile filter 372 with a bypass valve 382. The blower 364
operates continuously supplying humidity controlled air to the
heater 368. The flow of heated sterile air is controlled with
the flow adjust valve 376 and travels through conduit 378.
Exiting conduit 378, the second supply of hot sterile air
enters the conduit 348 to mix with the atomized hydrogen
peroxide from the atomizing venturi 314. Excess flow of heated
sterile air travels through conduit 380 and passes through the
bypass valve 382. The second supply of hot sterile air assists
in obtaining a uniform concentration of hydrogen peroxide in the
air stream in conduit 348 and provides enough momentum to ensure
24
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
that all portions of the bottle 12 interior 118 are contacted by
hydrogen peroxide. Furthermore, the second supply of hot
sterile air is continuously blowing, whereas the first supply of
sterile air and hydrogen peroxide in conduit 346 is intermittent
corresponding to the movement of the bottles'12. Since the
second supply of hot sterile air is continuous, hydrogen
peroxide does not have the ability to fall out of the air stream
and deposit in the delivery conduit 348 in the form of drops.
This ensures that the delivery of hydrogen peroxide is
consistent from one bottle 12 application to the next and does
not allow a drop to be directed into the bottle 12 interior 118.
The mixture of heated sterile air and atomized hydrogen
peroxide in conduit 348 passes through the double tube heat
exchanger 350. The double tube heat exchanger 350 adds
additional heat to the atomized hydrogen peroxide. Heat is
supplied to the double tube heat exchanger 350 from the steam
supply 354 controlled by the regulator valve 360. Generally,
hydrogen peroxide has chemical stabilizers in it that may cause
a white powder precipitate to form on the inner surfaces of the
double tube heat exchanger 350. This occurs when the
temperature differential between the supplied steam heat and the
gas to be heated is large. In the present invention, the
temperature of the atomized hydrogen peroxide is typically about
the same as the supplied steam heat so that a minimal amount of
precipitate occurs. Another embodiment of the invention
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
eliminates the need for the double tube heat exchanger 350
because the temperature of the atomized hydrogen peroxide is
already at the desired temperature.
The temperature of the atomized gas entering the interior
118 of the bottle 12 is in the range of about 100 C to 120 C.
This temperature is limited to prevent the plastic bottles 12
from melting. The droplet size occurring on the interior
surface 119 of the bottles 12 is in the range of about 300 to
500 micrometers. The initial concentration level of hydrogen
peroxide on the interior surface 119 of the bottle 12 is about
35%.
As illustrated in FIG. 21, the control system 550 monitors
the temperatures at locations denoted as "T" in the interior
bottle sterilization apparatus 116. The temperatures "T" are
measured in the conduit 348, in the heater 368, and in the
conduit 370. Additionally, the control system 550 monitors the
pressures at locations denoted as "P" as illustrated in FIG. 21.
The pressures "P" are measured in the conduit 328, conduit 338,
and in the conduit 382.
The control system 550 monitors and controls a spray
apparatus 126 that includes the probe 123 including the
applicator spray nozzles 122 FIG 10. Each applicator spray
nozzle 122 sprays the sterilant into the interior 118 of a
corresponding bottle 12 as a hot vapor fog. The probe 123
including applicator spray nozzles 122 are designed to extend
26
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
through the bottle openings 16. The probe 123 including
applicator spray nozzles 122 descends into the interior 118 and
toward the bottom of the bottles 12. This ensures the complete
application of sterilant to the entire interior 118 and interior
surface 119 of each bottle 12. Alternately, the probe 123
including the applicator spray nozzles 122 may be positioned
immediately above the bottle openings 16 prior to the
application of sterilant.
FIG. 9 illustrates a perspective view of a partition 130
that provides control of sterile air flow within the
sterilization tunnel 90 of the filler apparatus 50. The
partition 130 includes a top baffle plate 132, a middle baffle
plate 134, and a bottom baffle plate 136. The top baffle plate
132 and the middle baffle plate 134 are provided with cut-outs
133 which correspond to the outer shape of each bottle 12 and to
the outer shape of the conveyor plate 94. The cut-outs 133
allow each bottle 12 and each conveyor plate 94 to pass through
the partition 130. A space 138 between the middle baffle plate
134 and the bottom baffle plate 136 allows each empty conveyor
plate 94 to pass through the partition 130 as it travels on its
return trip from the pulley 108 toward the pulley 110.
As illustrated in FIG. 3, partitions 130A, 130B, and 130C,
are located within the sterilization tunnel 90. FIG. 10
illustrates a cross-sectional view of partition 130A including
baffle plates 132A, 134A, and 136A. The partition 130A is
27
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
located between stations 8 and 9. FIG. 11 illustrates a cross-
sectional view of partition 130B including baffle plates 132B,
134B, and 136B. The partition 130B is located between stations
22 and 23. FIG. 12 illustrates a cross-sectional view of
partition 130C including baffles 132C, 134C, and 136C. The
partition 130C is located between stations 35 and 36. As
illustrated in FIG. 3, sterile air is introduced through sterile
air supply sources (e.g., conduits 140, 142, and 144) into the
sterilization tunnel 90. The sterile air conduit 140 is located
at station 23 (FIG. 11), the sterile air conduit 142 is located
at station 27 (FIG. 3), and the sterile air conduit 144 is
located at station 35 (FIG. 12).
The partition 130A separates an activation and drying
apparatus 152 from the interior bottle sterilization apparatus
116. The partition 130B separates the activation and drying
apparatus 152 from a main product filler apparatus 160 and a lid
sterilization and heat sealing apparatus 162. Thus, a first
sterilization zone 164 is created that includes the activation
and drying apparatus 152. Partition 130C separates the main
product filler apparatus 160 and the lid sterilization and heat
sealing apparatus 162 from a bottle discharge apparatus 280.
Thus, partitions 130B and 130C create a second sterilization
zone 166 that includes the main product filler apparatus 160 and
the lid sterilization and heat sealing apparatus 162. A third
sterilization zone 172 includes the bottle discharge apparatus
28
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
280. A fourth sterilization zone 165 includes the interior
bottle sterilization apparatus 116. The second sterilization
zone 166 provides a highly sterile area where the bottles 12 are
filled with a product and sealed. The second sterilization zone
166 is at a higher pressure than the first sterilization zone
164 and the third sterilization zone 172. Therefore, any gas
flow leakage is in the direction from the second sterilization
zone 166 out to the first sterilization zone 164 and the third
sterilization zone 172. The first sterilization zone 164 is at
a higher pressure than the fourth sterilization zone 165.
Therefore, gas flow is in the direction from the first
sterilization zone 164 to the fourth sterilization zone 165.
The partitions 130A, 130B, and 130C create sterilization
zones 164, 165, 166, and 172 with different concentration levels
of gas laden sterilant (e.g., hydrogen peroxide in air). The
highest concentration level of sterilant is in the fourth
sterilization zone 165. For example, with the sterilant
hydrogen peroxide, the concentration level of hydrogen peroxide
is about 1000 ppm (parts per million) in the fourth
sterilization zone 165. The hydrogen peroxide sterilant level
is about 3 ppm in the first sterilization zone 164. The lowest
concentration level of sterilant is in the second sterilization
zone 166. In the second sterilization zone 166, the hydrogen
peroxide sterilant concentration level is less than .5ppm and
typically about .lppm. Advantageously, this helps to maintain
29
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
the main product filler apparatus 160 and the lid sterilization
and heat sealing apparatus 162 at a low sterilant concentration
level. This prevents unwanted high levels of sterilant to enter
the food product during the filling and lidding process. The
hydrogen peroxide sterilant concentration level is about .1 ppm
in the third sterilization zone 172.
As illustrated in FIG. 3, a gas such as hot sterile air
enters the first sterilization zone 164 at a rate of about 2400
cfm (cubic feet per minute). The temperature of the hot sterile
air is about 230 F. The hot sterile air enters the first
sterilization zone 164 through conduit 148. Additional hot
sterile air enters the second sterile zone through sterile air
conduits 140, 142, and 144 at a total rate of about 1000 cfm
(FIG. 3). Also, hot sterile air enters at a rate of about 1800
cfm through ports 67 and 68 leading into the infeed and
sterilization apparatus 60. A portion of the hot sterile air
exits the sterilization tunnel 90 at a rate of about 1500 cfm
through a plurality of exhaust ports 153 located in the first
sterilization zone 164 (FIG. 15). A portion of the hot sterile
air exits the sterilization tunnel 90 at a rate about 100 cfm
through an opening 282 (FIG. 14). The bottles 12 exit the
sterilization tunnel 90 through the opening 282. The continuous
flow of sterile air flow out through the opening 282 prevents
contaminants from entering the sterilization tunnel 90.
As illustrated in FIG. 3, the hot sterile air is drawn out
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
of the fourth sterilization zone 165 of the sterilization tunnel
90 through the bottom opening 62 in the bottle infeed and
sterilization apparatus 60. Next, the hot sterile air from the
infeed and sterilization apparatus together with the fourth
sterilization zone 165 exits out of the exhaust conduit 70 of
the infeed and sterilization apparatus at a rate of about 3600
cfm. This outflow of hot sterile air from the bottle infeed and
sterilization apparatus 60 prevents contaminants from entering
the bottle infeed sterilization apparatus 60 and the
sterilization tunnel 90.
Stations 10 through 21 include twelve stations for
directing hot sterile air into each bottle 12 for the activation
and removal of the sterilant from the interior of the bottle 12.
In these twelve stations, a third supply of hot sterile air is
provided through the sterile air supply system 146. The sterile
air supply system 146 supplies hot sterile air to a plurality of
nozzles 150 in the activation and drying apparatus 152. The hot
sterile air flow in each bottle 12 is about 40 SCFM. Hot
sterile air is supplied to the sterile air supply system 146
through conduit 148. The air is first passed through a
filtration system to sterilize the air. The air is then heated
in a heating system to about 230 F. The air temperature is
regulated by the control system 550. Also, the control system
550 monitors the air pressure and flow rate to ensure that an
adequate flow of hot sterile air is maintained in the
31
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
sterilization tunnel 90 of the application and drying apparatus
152.
As shown in FIG. 8, each bottle 12 generally has a small
opening 16 compared to its height "H." A ratio of a diameter
"D" of the bottle 12 to the height "H" of the bottle 12 is
generally less than 1Ø The small bottle opening 16 combined
with a larger height "H" restricts the flow of hot gas into the
interior 118 of the bottle 12. Also, PET and HDPE bottle
materials have low heat resistance temperatures. These
temperatures commonly are about 55 C for PET and about 121 C for
HDPE. Typically, in the aseptic packaging industry, a low
volume of air at a high temperature is applied to the packaging
materials. This often results in deformation and softening of
packaging materials formed of PET and HDPE. In order to prevent
softening and deformation of the bottles 12, when formed from
these types of materials, the present invention applies high
volumes of air at relatively low temperatures over an extended
period of time in the activation and drying apparatus 152. The
plurality of nozzles 150 of the activation and drying apparatus
152 direct hot sterile air into the interior 118 of each bottle
12 (FIG. 11). A long exposure time is predicated by the
geometry of the bottle 12 and the softening temperature of the
material used to form the bottle 12. In the present invention,
about 24 seconds are allowed for directing hot sterile air from
the plurality of nozzles 150 into each bottle for the activation
32
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
and removal of sterilant from the interior surface 119 of the
bottle 12. To achieve aseptic sterilization, a minimum bottle
temperature of about 131 F should be held for at least 5 seconds.
To achieve this bottle temperature and time requirements,
including the time required to heat the bottle, the sterilant is
applied for about 1 second and the hot sterile air is introduced
for about 24 seconds. The hot sterile air leaves the nozzles
150 at about 230 F and cools to about 131 F when it enters the
bottle 12. The hot sterile air is delivered at a high volume so
that the bottle 12 is maintained at about 131 F for at least 5
seconds. The about 24 seconds provides adequate time for the
bottle 12 to heat up to about 131 F and to maintain this
temperature for at least 5 seconds. After bottle 12 has dried,
the residual hydrogen peroxide remaining on the bottle 12
surface is less than 0.5 PPM.
A foodstuff product is first sterilized to eliminate
bacteria in the product. An "Ultra High Temperature" (UHT)
pasteurization process is required to meet the aseptic FDA
standard. The time and temperature required to meet the aseptic
FDA standard depends on the type of foodstuff. For example,
milk must be heated to 282 F for not less than 2 seconds in order
to meet the aseptic standards.
After UHT pasteurization, the product is delivered to a
main product filler apparatus 160. The main product filler
apparatus is illustrated in FIGS. 3, 13, and 22. The main
33
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
product filler 160 can be sterilized and cleaned in place to
maintain aseptic FDA and 3A standards. A pressurized reservoir
apparatus 180 that can be steam sterilized is included in the
main product filler apparatus 160. As illustrated in FIG. 22,
the pressurized reservoir apparatus 180 includes an enclosed
product tank 182 with a large capacity (e.g., 15 gallons). The
product tank 182 is able to withstand elevated pressures of
about 60 psig or more. The pressurized reservoir apparatus 180
also includes a level sensor 184, a pressure sensor 186, at
least one volumetric measuring device 188 (two are shown as
188A, 188B), and at least one filling nozzle 190 (two are shown
as 190A, 190B). The product tank 182 includes a single product
inlet 250 with a valve cluster (not shown) including a sterile
barrier to separate the product supply system (not shown) from
the main product filler apparatus 160. The product tank 182 has
an outlet with twelve connections. At each connections is a
volumetric measuring device 188 such as a mass or volumetric
flow meter. Pressurized steam or sterile air is supplied into
the product tank 182 through the inlet 252. The product level
254 in the product tank 182 is measured by the level sensor 184.
The control system 550 maintains the product level and pressure
in the product tank 182. This supplies each filling nozzle 190
(e.g. 190A, 190B) with a constant pressure that ensures proper
product delivery to the bottles 12.
Filling nozzles 190A, 1903 are provided at stations 23, 25,
34
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
respectively. Additionally, there are a plurality of
corresponding volumetric measuring devices 188A and 188B to
measure the volume of product entering each bottle 12 at
stations 23 and 25, respectively. In accordance with the
present invention, the volumetric measuring devices 188A and
188B are preferably electronic measuring devices such as a
magnetic flow meter which measures the volume of product flow,
or a mass flow meter which measures the weight of product flow.
The electronic measuring devices provide filling accuracies of
about 0.5%. The control system 550 calculates the desired
volume of product to be inserted into each bottle 12, and
controls the product volume by opening or closing a plurality of
valves 194A and 194B included in the filling nozzles 190A and
190B, respectively. The amount of product delivered to the
bottles 12 is controlled by the duration of time that.the
plurality of valves 194A and 194B are open. The control system
550 controls the duration of time. Thus, any desired quantity
of product may be selected by controlling the duration of time
that the valves 194A and 194B are open.
The activation mechanisms for valves 194A and 194B include
valve stems 256A and 256B attached to actuators 258A and 258B,
respectively. Each actuator 258A, 258B may include any suitable
actuating apparatus (e.g. hydraulic, pneumatic, electrical,
etc.). Preferably, in the present invention, the actuators 258A
and 258B include air cylinders controlled by the control system
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
550. The actuators 258A and 258B are attached to the valve
stems 256A and 256B, respectively. The actuators 258A and 258B
displace the valve stems 256A and 256B in an upward and downward
direction.
FIG. 23 illustrates the valve stem 256A attached to the
valve 194A. A first sterile region 260 surrounds the nozzle
196A through which product 262A exits. The first sterile region
260 is connected to, and is at the same sterilization level as,
the second sterilization zone 166 (FIG. 3) of the sterile tunnel
90. The valve 194A is in a closed position against nozzle 196A
blocking the flow of product 262A into a bottle 12 (not shown)
located in the first sterile region 260. A first portion 264A
of the valve stem 256A is surrounded by a non-sterile region
268, for example, the area located outside of the sterile tunnel
90. Thus, the first portion 264A of the valve stem 256A is
exposed with contaminants.
As illustrated in FIG. 24, the actuator 258A has displaced
the valve stem 256A in a downward direction. The valve 194A is
removed from the nozzle 196A allowing product 262A to flow into
a bottle 12 (not shown). The first portion 264A of the valve
stem 256A has entered the first sterile region 260. This may
create a problem because the first portion 264A of the valve
stem 256A may carry contaminants from the non-sterile region 268
into the first sterile region 260. In order to overcome this
difficulty, the present invention has introduced a second
36
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
sterile region 270 as illustrated in FIG. 25.
The second sterile region 270A is enclosed by a housing 272
and by a wall 274. The wall 274 separates the second sterile
region 270A from the first sterile region 260. The first
sterile region 260 is connected to, and is at the same
sterilization level, as the second sterilization zone 166 of the
sterile tunnel 90. A sterilizing media 424 is supplied to the
second sterile region 270A through the inlet conduit 420A. An
outlet conduit 422A may be added to allow the sterilizing media
424 to leave the second sterile region 270A. The sterilizing
media 424 may include any suitable sterilant (e.g. steam,
hydrogen peroxide, oxonia, etc.). The non-sterile region 268
lies outside of the housing 272. A second portion 266A of the
valve stem lies in the non-sterile region 268. As illustrated
in FIG. 25, the valve 194A is in a closed position against the
nozzle 196A blocking the flow of product 262A into a bottle 12
(not shown) in the first sterile region 260. The first portion
264A of the valve stem 256A is surrounded by the second sterile
region 270A. Thus, the first portion 266A of the valve stem
256A is maintained in a sterile condition.
As illustrated in FIG. 26, the actuator 258A has displaced
the valve stem 256A in a downward direction. The valve 194A is
removed from the nozzle 196A allowing product 262A to flow into
a bottle 12 (not shown). The first portion 264A of the valve
stem 256A has entered the first sterile region 260. In the
37
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
present invention, the first portion 264A of the valve stem 256A
has not introduced contaminants into the first sterile region
260 because the first portion 264A of the valve stem 256A was
pre-sterilized in the second sterile region 270A before entering
the first sterile region 260. The second portion 266A of the
valve stem 256A has entered the second sterile region 270A from
the non-sterile region 268. The second portion 266A of the
valve stem 256A is sterilized in the second sterile region 270A
removing any contaminants. Therefore, the second sterile region
270A removes any contaminants from the valve stem 256A before
any portion of the valve stem 256A enters the first sterile
region 260. Thus, contaminants are prevented from entering the
sterile tunnel 90 through the filling nozzles 190A and 190B, and
the valves 194A and 194B, respectively.
The plurality of valves 194A control the volume of product
flowing through a corresponding plurality of nozzles 196A into
the bottles 12 at station 23. The plurality of valves 194B
control the volume of product flowing through a corresponding
plurality of nozzles 196B into the bottles 12 at station 25.
The control system 550 uses previously stored information
provided by the bottle detection apparatus 112 to only allow
filling to occur at the locations where bottles 12 are actually
present and correctly aligned.
The initial sterilization process for the pressurized
reservoir apparatus 180 includes the step of exposing all of the
38
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
surfaces of the pressurized reservoir apparatus 180 that come in
contact with the product to steam at temperatures above about
250 F for a minimum of about 30 minutes. Elements such as cups
198A and 198B (FIG. 22) are used to block off nozzle outlets
196A and 196B, respectively, to allow a build-up of steam
pressure to about 50 psig inside the pressurized reservoir
apparatus 180. Condensate generated as the steam heats the
interior surfaces of the pressurized reservoir apparatus 180 is
collected in the cups 198A and 198B. This condensate is
released when the cups 198A and 198B are removed from the nozzle
outlets 196A and 196B. Once the interior surfaces of the
pressurized reservoir apparatus 180 are sterilized, the steam is
shut off, and sterile air is used to replace the steam. The
sterile air reduces the interior temperature of the pressurized
reservoir apparatus 180 to the temperature of the product before
the product is allowed to enter the enclosed product tank 182.
As shown in FIG. 13, sterile air is directed through sterile air
conduits 142 and 144 into the second sterilization zone 166 at a
volume rate of about 800 scfm. The sterile air flow entering
the second sterilization zone 166 provides sterile air to the
main product filler apparatus 160 and to the lid sterilization
and heat sealing apparatus 162.
The main product filler apparatus 160 includes a separate
filling position for each bottle. A bottle 12 moves into
position under a nozzle 196. The bottle stops and the valve 194
39
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
opens allowing product 262 to enter the bottle 12. The
volumetric measuring device 188 measure the amount of product
entering the bottle 12. Next, when the desired bottle 12 fill
level is achieved, the valve 194 is closed. The control system
550 controls the valve opening and closing. Additionally, the
control system 550 does not allow product 262 to flow if a
bottle 12 is not present. The bottle 12 filling operation is
completed for six bottles at station 23 and for six bottles at
station 25. The filling cycle is repeated for each cycle of the
aseptic processing apparatus 10. In the present invention the
bottle filling time is about 1.5 seconds. Another embodiment of
the present invention adds a second main product filler
apparatus 160B located at, for example, stations 27 and 29 (FIG.
22). In this embodiment, the bottles 12 are partially filled by
the first main product filler apparatus 160 at stations 23 and
25. Next, the bottles are moved to the second main product
filler apparatus 160B where the filling of each bottle is
completed at stations 27 and 29. For example, in filling each
16 fluid ounce bottle 12, the first main product filler
apparatus 160 would fill the first 8 ounces in about 1.5
seconds. Next, the second main product filler apparatus 160
would fill the remaining 8 ounces in each bottle 12 in another
about 1.5 seconds. The second main product filler 160B allows
the operation to be kept to about 1.5 seconds at each main
product filler apparatus 160, 16013. This allows the conveying
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
apparatus 100 to move the bottles through the aseptic processing
apparatus 10 at speeds greater than about 350 bottles 12 per
minute.
FIGS. 3, 13, 16 and 19 illustrate the lid sterilization and
heat sealing apparatus 162. A lid 200 is applied to each of the
twelve bottles 12 at station 33. For a fully aseptic bottle
filler, complete lid 200 sterilization is necessary, and
therefore a sterilant such as hydrogen peroxide is typically
used. In the present invention, the lids are formed of a
material such as foil or plastic. The lids 200 are joined
together by a small interconnecting band 203 that holds them
together to form a long continuous chain of lids 200,
hereinafter referred to as a "daisy chain" 202. The daisy chain
202 of lids is illustrated in FIGS. 17. A daisy chain 202 of
lids 200 is placed on each of a plurality of reels 210. For the
twelve bottle configuration of the present invention, six of the
reels 210, each holding a daisy chain 202 of lids 200, are
located on each side of a heat sealing apparatus 214. Each
daisy chain 202 of lids 200 winds off of a corresponding reel
210 and is sterilized, preferably using a hydrogen peroxide bath
204. The concentration of hydrogen peroxide can range from
about 30 to 40%, however, preferably the concentration is about
35%. Each lid 200 remains in the hydrogen peroxide bath 204 for
at least about 6 seconds. A plurality of hot sterile air knives
208, which are formed by jets of hot sterile air, activate the
41
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
hydrogen peroxide to sterilize the lids 200 on the daisy chain
202. The hot sterile air temperature is about 135 C. The hot
air knives 208 also remove excess hydrogen peroxide from the
lids 200. A plurality of heated platens 205 further dry the
lids 200 so that the residual concentration of hydrogen peroxide
is less than .5 PPM. The hydrogen peroxide bath 204 prevents
any contaminants from entering the sterilization tunnel 90 via
the lidding operation.
Once sterilized, the lids 200 enter the sterilization
tunnel 90 where they are separated from the daisy chain 202 and
placed on a bottle 12. Each lid is slightly larger in diameter
then that of the opening 16 of a bottle 12. During the
placement of the lid 200 on the bottle 12, a slight mechanical
crimp of the lid 200 is formed to locate and hold the lid 200 on
the bottle 12. The crimp holds the lid 200 in place on the
bottle 12 until the bottle 12 reaches a station 33 for sealing.
Sealing may also be accomplished without having to provide the
mechanical crimp on the lid 200.
Another embodiment of a lid sterilization and heat sealing
apparatus 552 is illustrated in FIG. 19. As illustrated in FIG.
18, the daisy chain 215 of lids 200 includes a hole 207 located
in each interconnecting band 203. Each hole 207 receives a pin
209 of a drive sprocket 211.
The daisy chain 215A, 215B of lids 200 is placed on each of
a plurality of reels 210 (e.g. 210A and 210B). For the twelve
42
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
bottle configuration of the present invention, six of the reels
210, each holding a daisy chain 215A, 215B of lids 200, are
located on each side of a heat sealing apparatus 214. Each
daisy chain 215A, 215B of lids 200 winds off of a corresponding
reel 210 and is sterilized preferably using a hydrogen peroxide
bath 204. The concentration of hydrogen peroxide can range from
about 30 to 40%, however, preferably the concentration is about
35%. The lids 200 remain in the hydrogen peroxide bath 204 for
at least about 6 seconds. A plurality of hot sterile air knives
208, which are formed by jets of hot sterile air, activate the
hydrogen peroxide to sterilize the lids 200 on the daisy chain
215A, 215B. The hot sterile air temperature is about 135 C.
The hot air knives 208 also remove excess hydrogen peroxide form
the lids 200. A plurality of heated platens 205 further dry the
lids 200 so that the residual concentration of hydrogen peroxide
is less than .5 PPM. The hydrogen peroxide bath 204 prevents
any contaminants from entering the sterilization tunnel 90 via
the lidding operation. The drive sprocket 211A includes a
plurality of pins 209 that engage with the holes 207 of the
daisy chain 215A. The drive sprocket 211A rotates in a
counterclockwise direction and indexes and directs the daisy
chain 215A, through a plurality of guides 217A. The guides 217A
may include a plurality of rollers 221A to further guide and
direct an end 219A of the daisy chain 215A over the bottle 12A.
The drive sprocket 211B includes a plurality of pins 209 that
43
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
engage with the holes 207 of the daisy chain 215B. The drive
sprocket 211B rotates in a clockwise direction and indexes and
directs the daisy chain 215B through a plurality of guides 217B.
The guides 217B may include a plurality of rollers 2212 to
further guide and direct an end 219B of the daisy chain 21513
over the bottle 12B.
Once sterilized, the lids 200 enter the sterilization
tunnel 90 where they are separated from the daisy chain 215A,
217B and placed on the bottle 12A, 12B. At station 33, the lids
200 are applied to the bottles 12. As illustrated in FIGS. 13
and 20, the heat sealing apparatus 214 includes a heated platen
216 that applies heat and pressure against each lid 200 for a
predetermined length of time, to form a seal between the lid 200
and the bottle 12A, 12B. Although lidding for a bottle has been
described, it should be appreciated that lidding of other
containers (e.g. jars) can be provided by the present invention.
FIG. 20 illustrates a perspective view of the heat sealing
apparatus 214, the daisy chain 215A, the gripper apparatus 554,
the bottle 12A, and the conveying plate 94. The lid 200 is
located above the bottle opening 16. The gripper apparatus 554
includes a grip 223 for capturing the bottle 12A by a bottle lip
225. The gripper apparatus 554 lifts the bottle 12A in an upward
direction so that the lid 200 is pressed between a bottle top
lip 227 and the heated platen 216. The interconnecting band 203
severs and separates the lid 200 on the bottle 12 from the next
44
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
lid on the daisy chain 215A. The heated platen 216 is in a two
by six configuration to seal twelve of the bottles 12 at a time.
There is a separate gripper apparatus 554 for each of the twelve
bottles 12. After each bottle 12 is sealed, its gripper
apparatus 554 lowers and releases the bottle 12 and each bottle
12 continues to station 37.
At station 37, the lid 200 seal and bottle 12 integrity are
checked in a known manner by a seal integrity apparatus (not
shown) comprising, for example, a bottle squeezing mechanism and
a proximity sensor. Each bottle 12 is squeezed by the bottle
squeezing mechanism which causes the lid 200 on the bottle 12 to
extend upward. The proximity sensor detects if the lid 200 has
extended upward, which indicates an acceptable seal, or whether
the seal remains flat, which indicates a leaking seal or bottle
12. The location of the defective bottles 12 are recorded by
the control system 550 so that the defective bottles will not be
packed.
Bottle discharge from the sterilization tunnel 90 of the
filler apparatus 50 occurs at stations 38 and 40 as illustrated
in FIGS. 3, 13 and 14. A bottle discharge apparatus 280 is
located at stations 38 and 40. At this point in the filler
apparatus 50, the filled and sealed bottles 12 are forced in an
upward direction such that a top portion 284 of each bottle 12
protrudes through the opening 282 in the sterilization tunnel 90
(FIG. 14). A rotating cam 290 or other suitable means (e.g., an
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
inflatable diaphragm, etc.) may be used to apply a force against
the bottom 120 of each bottle 12 to force the bottle 12 in an
upward direction.
As illustrated in FIG. 14, the bottle discharge apparatus
280 comprises a lifting apparatus 286 that includes a gripper
288 that grasps the top portion 284 of each bottle 12 and lifts
the bottle 12 out through the opening 282 in the sterilization
tunnel 90. In order to ensure that contaminated air cannot
enter the sterilization tunnel 90, the sterile air in the
sterilization tunnel 90 is maintained at a higher pressure than
the air outside the sterilization tunnel 90. Thus, sterile air
is always flowing out of the sterilization tunnel 90 through the
opening 282. In addition, the gripper 288 never enters the
sterilization tunnel 90, because the top portion 284 of the
bottle 12 is first lifted out of the sterilization tunnel 90 by
the action of the rotating cam 290 before being grabbed by the
gripper 288.
FIG. 15 illustrates a top view of the filler apparatus 50
including the bottle infeed and sterilization apparatus 60, the
interior bottle sterilization apparatus 116, and the activation
and drying apparatus 152. FIG. 15 additionally illustrates the
main filler apparatus 160, the lid steril'ization and heat
sealing apparatus 162, and the bottle discharge apparatus 280.
Referring again to FIGS. 1 and 14, the lifting apparatus
286 lifts the bottles 12 at station 38 and places the bottles 12
46
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
in a first lane 292 that transports the bottles 12 to a first
capping apparatus 410. In addition, the lifting apparatus 286
lifts the bottles 12 at station 40 and places the bottles 12 in
a second lane 294 that transports the bottles 12 to a second
capping apparatus 400.
The first capping apparatus 410 secures a cap (not shown)
on the top of each bottle 12 in the first lane 292. The second
capping apparatus 400 secures a cap on the top of each bottle 12
in the second lane 294. The caps are secured to the bottles 12
in a manner known in the art. It should be noted that the
capping process may be performed outside of the sterilization
tunnel 90 because each of the bottles 12 have previously been
sealed within the sterilization tunnel 90 by the lid
sterilization and heat sealing apparatus 162 using a sterile lid
200. '
After capping, the bottles 12 are transported via the first
and second lanes 292,,294 to labelers 460 and 470. The first
labeling apparatus 470 applies a label to each bottle 12 in the
first lane 292. The second labeling apparatus 460 applies a
label to each bottle 12 in the second lane 294.
From the first labeling apparatus 470, the bottles 12 are
transported along a first set of multiple lanes (e.g., 4) to a
first case packing apparatus 490. From the second labeling
apparatus 460, the bottles 12 are transported along a second set
of multiple lanes to a second case packing apparatus 480. Each
47
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
case packing apparatus 480, 490 gathers and packs a plurality of
the bottles 12 (e.g., twelve) in each case in a suitable (e.g.,
three by four) matrix.
A first conveyor 296 transports the cases output by the
first case packer 490 to a first palletizer 510. A second
conveyor 298 transports the cases output by the second case
packer 480 to a second palletizer 500. A vehicle, such as a
fork lift truck, then transports the pallets loaded with the
cases of bottles 12 to a storage warehouse.
Referring again to FIG. 3, the main conveyor 106 and each
conveying plate 94 are cleaned and sanitized once during each
revolution of the main conveyor 106. Specifically, after each
empty conveying plate 94 passes around the pulley 108, the
conveying plate 94 is passed through a liquid sanitizing
apparatus 300 and a drying apparatus 302. The liquid sanitizing
apparatus 300 sprays a mixture of a sterilizing agent (e.g.,
oxonia, (hydrogen peroxide and peroxyacetic acid)) over the
entire surface of each conveying plate 94 and associated
components of the main conveyor 106. In the drying apparatus
302, heated air with is used to dry the main conveyor 106 and
conveying plates 94.
Stations 1 through 40 are enclosed in the sterilization
tunnel 90. The sterilization tunnel 90 is supplied with air
that is pressurized and sterilized. The interior of the
sterilization tunnel 90 is maintained at.a pressure higher than
48
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
the outside environment in order to eliminate contamination
during the bottle processing. In addition, to further ensure a
sterile environment within the sterilization tunnel 90, the
sterile air supply provides a predetermined number of air
changes (e.g., 2.5 changes of air per minute) in the
sterilization tunnel 90.
Before bottle production is initiated, the bottle infeed
and sterilization apparatus 60 and the filler apparatus 50 are
preferably sterilized with an aseptic sterilant. For example, a
sterilant such as a hot hydrogen peroxide mist may be applied to
all interior surfaces of the bottle infeed and sterilization
apparatus 60 and the filler apparatus 50. Then, hot sterile air
is supplied to activate and remove the hydrogen peroxide, and to
dry the interior surfaces of the bottle infeed and sterilization
apparatus 60 and the filler apparatus 50.
FIG. 16 is a side view of the aseptic processing apparatus
10 of the present invention indicating the location of the
control and monitoring devices that are interfaced with the
control system 550. The control system 550 gathers information
and controls process functions in the aseptic processing
apparatus 10. A preferred arrangement of the control and
monitoring devices are indicated by encircled letters in FIG.
16. A functional description of each of the control and
monitoring devices is listed below. It should be noted that
these control and monitoring devices are only representative of
49
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
the types of devices that may be used in the aseptic processing
apparatus 10 of the present invention. Other types and
combinations of control and monitoring devices may be used
without departing from the intended scope of the present
invention. Further, control system 550 may respond in different
ways to the outputs of the control and monitoring devices. For
example, the control system 550 may automatically adjust the
operational parameters of the various components of the aseptic
processing apparatus 10, may generate and/or log error messages,
or may even shut down the entire aseptic processing apparatus
10. In the preferred embodiment of the present invention, the
control and monitoring devices include:
A. A bottle counter to ensure that a predetermined number
of the bottles 12 (e.g., six bottles) on each upper horizontal
row 24, 28 enter the loading area of the bottle infeed and
sterilization apparatus 60.
B. A proximity sensor to ensure that the first group of
bottles 12 has dropped into the first bottle position in the
bottle infeed and sterilization apparatus 60.
Cl. A conductivity sensor to ensure that the measuring
cup used by the sterilant application apparatus 36 is full.
C2. A conductivity sensor to ensure that the measuring
cup used by the sterilant application apparatus 36 is emptied in
a predetermined time.
C3. A pressure sensor to ensure that the pressure of the
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
air used by the sterilant application apparatus 36 is within
predetermined atomization requirements.
C4. A temperature sensor to ensure that each heat
heating element used by the sterilant application apparatus 36
is heated to the correct temperature.
D. A proximity sensor (e.g., proximity sensor 71,
FIG. 3) to ensure that a bottle jam has not occurred within the
bottle infeed and sterilization apparatus 60.
E. A temperature sensor to ensure that the
temperature of the heated sterile air entering the bottle infeed
and sterilization apparatus 60 is correct.
F. A proximity sensor that to ensure that each
conveying plate 94 is fully loaded with bottles 12.
Gl. A conductivity sensor to ensure that the measuring
cup used by the interior bottle sterilization apparatus 116 is
full.
G2. A conductivity sensor to ensure that the measuring
cup used by the interior bottle sterilization apparatus 116 is
emptied in a predetermined time.
G3. A pressure sensor to ensure that the pressure of the
air used by the interior bottle sterilization apparatus 116 is
within predetermined atomization requirements.
G4. A temperature sensor to ensure that each heat
heating element used by the interior bottle sterilization
apparatus 116 is heated to the correct temperature.
51
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
H. A temperature sensor to ensure that the air drying
temperature within the activation and drying apparatus 152 is
correct.
I. A plurality of flow sensors to ensure that the airflow
rate of the sterile air entering the sterilization tunnel 90 is
correct.
J. A pressure sensor to ensure that the pressure of the
sterile air entering the activation and drying apparatus 152 is
correct.
K. A measuring device (e.g., volumetric measuring device
188, FIG. 3) to ensure that each bottle 12 is filled to a
predetermined level.
L. A pressure sensor to ensure that the pressure in the
product tank 182 is above a predetermined level.
M. A level sensor to ensure that the level of product in
the product tank 182 is maintained at a predetermined level.
N. Proximity sensors to ensure that the daisy chains 202
of lids 200 are present in the lid sterilization and heat
sealing apparatus 162
O. A level sensor to ensure that the hydrogen peroxide
level in the hydrogen peroxide bath 204 in the lid sterilization
and heat sealing apparatus 162 is above a predetermined level.
P. A temperature sensor to ensure that the temperature of
the hot sterile air knives 208 of the lid sterilization and heat
sealing apparatus 162 is correct.
52
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
Q. A temperature sensor to ensure that the heat sealing
apparatus 214 is operating at the correct temperature.
R. Proximity sensors to ensure that the bottles 12 are
discharged from the filler.
S. A speed sensor to measure the speed of the conveying
apparatus 100.
T. A concentration sensor to ensure that the
concentration of oxonia is maintained at a predetermined level
in the sanitizing apparatus 300.
U. A pressure sensor to ensure that the pressure of the
oxonia is maintained above a predetermined level in the
sanitizing apparatus 300.
V. A temperature sensor to ensure that the drying
temperature of the drying apparatus 302 is correct.
The following steps are performed during the "Clean In Place"
(CIP) process in the filler apparatus 50;
23. Conductivity sensor to verify caustic and acid
concentrations. _
24. Temperature sensor to verify "Clean In Place" solution
temperatures.
25. Flow meter to verify "Clean In Place" flow rates.
26. Time is monitored to ensure that adequate cleaning
time is maintained.
The follow steps are performed during sterilization of the
bottle filler apparatus 50;
53
CA 02416094 2003-01-14
WO 01/05658 PCT/US00/19188
27. Temperature sensors for measuring steam temperatures.
28. Proximity sensors to ensure filler nozzle
cleaning/sterilization cups are in position.
29. Temperature sensors for air heating and cooling.
30. Flow meter for hydrogen peroxide injection.
31. Time is monitored to ensure the minimum time periods
are met (steam, hydrogen peroxide application and
activation/drying).
The foregoing description of the present invention has been
presented for purposes of illustration and description. It is
not intended to be exhaustive or to limit the invention to the
precise form disclosed, and many modifications and variations
are possible in light of the above teaching. Such modifications
and variations that may be apparent to a person skilled in the
art are intended to be included within the scope of this
invention.
54