Note: Descriptions are shown in the official language in which they were submitted.
CA 02417483 2003-O1-27
1
A PROCESS FOR PURIF~fING ENERGETIC GASES SUCH AS BIOGAS AND
NATURAL GAS
FIELD OF THE INVENT10N
This invention concerns in general the field of processes and apparatuses for
the
s separation of a gaseous compound from a mixture of gaseous compounds. The
process is based on the use of biochemical catalysts in the accelerated
chemical
transformation of specific gaseous compounds found in a mixture of gases. More
specifically, it concerns the purification of energetic gases such as biogas
and natural
gas. Even more specifically, the invention concerns the purification of
methane
Zo containing energetic gases by removing therefrom the carbon dioxide.
BACKGROUND OF THE INVENTION
Substantial reserves of low concentration gaseous methane, that is, between 40
and
80% (vlv) exist. Impurities, i.e. the other compounds, as for example COZ,
might be
extracted from the gas in order to obtain natural gas containing over 05% of
15 methane. This natural gas can be used as a source of energy to heat, to
make
electricity, or it can be used in the composition of more complex chemical
products,
etc. However, the extraction of these impurities from the valuable energetic
gas by
way of conventional techniques is neither always profitable nor efficient. On
the other
hand, the gas mixture contains greenhouse gases and, if released in the
2 o atmosphere, will contribute to the earth's global warming.
Various technologies for the separation of C02 and methane have been
developed.
Conventional technology in the natural gas industry uses an amine in solution
that
has the characteristic of absorbing the CO2 (US6156096; CA10T8300; CA2200130;
CA950364; EP180670; GB848528; JP 08-252430), A packed column. or aspersion
25 column is usually used to increase contact between the liquid and gas
phases. This
physico-chemical method is generally suitable for large volumes of gas and, is
less
efficient in the presence of oxygen. The oxygen is present in variable
concentrations
in biogases and gases produced during the extraction of coal. A glycol
derivative that
CA 02417483 2003-O1-27
2
functions under high pressures (up to 300 psi) is also used as an adsorbent.
This,
however, tends to elevate operation costs. The recuperatian of the
hydrocarbons
composing the said gas is then obtained by cryogenic and distillation
procedures that
have the disadvantage of expending a lot of energy. A variant of this physico-
chemical conventional adsorption process consists of continuously flushing the
gas
inside hollow and porous fibres. The adsorbent in solution can be found
outside of
this fibre pattern.
The separation of gases can also be carried out using a porous polymeric
membrane
acting as a filter (0S4681605; 04681612; l,IS6128919; CA2294531; JP08-252430).
~o This membrane functions under a pressure differential and is composed of
pores
having dimensions selective to the gases present. This method provides for a
certain
separation but a pure gas is not obtained. Moreover, the temperature of the
treated
gas must be inferior to 200°C. This technique as well as the physico-
chemical
approach using an adsorbent is generally chosen when a high pressure (> 300
psi)
gas mixture is available.
Another gas separation process is referred to as PSA (Pressure Swing
Adsorption).
This technology is based ors the selective adsorption of certain gases on a
solid
matrix (0S5938819; FR2758740; GB1120483; CN1227255; JP57-130527; JP11-
050069). Raising the pressure heightens the selectivity of adsorption. When
the
2 o pressure is reduced, the tendency to adsorb a gas is lowered. These
phenomena,
exploited in cycles of pressurization I depressurization, allow the selective
adsorption
and desorption (regeneration) of a gas contained in a mixture of gases. The
solid
used has a high specific surface. The most frequently used solids include:
activated
carbon, silica gel, and zeotites, which can be very costly, Also high
operation costs
2 5 must be added since the pressures (1000 psi) and operating temperature
0700°C)
of the PSA process, which depend on the adsorbent used, are very high. A
variant
to this process is the VSA (\/acuum Swing Adsorption). This process adsorbs at
ambient pressure but regenerates the adsorbent with a negative pressure. The
PSA
and VSA processes are generally used when the pressure of the mixture of gases
to
CA 02417483 2003-O1-27
3
be treated is low (<300 psi). The presence of water vapour in the gas or a
high
gaseous temperature decreases the efficiency of the technology.
Also known in the prior art, there is a process where a mixture of gases
containing a
high concentration of C02 is liquefied by increasing the pressure and reducing
the
temperature. Examples of such process are disclosed in CA1190470; CA2361809;
and EP0207277. This process is essentially a distillation of the mixture of
gases and
requires an important quantity of energy. Furthermore, the mixture of gases
must
have been previously dried in order to avoid the formation of ice in the
equipment.
A major hurdle to the massive use of low concentrated biogases or gaseous
to hydrocarbons as an energy source is the high cost of extracting gas
contaminants.
Furthermore, a system, which works without concentration, temperature or
humidity
limits, would increase the acceptance of this large-scale process. One also
needs a
fast contaminant-specific purification process that doers not use compounds
toxic for
the environment.
Another alternative is the use of an enzyme to accelerate the solubilization
of COZ in
water. Carbonic anhydrase is easily available and has a strong tendency to
react.
The enzyme has, for these reasons, already been used in its immobilized form
for
the purification by affinity column, for the transportation through membranes
and
recently, for the reduction of carbon dioxide emissions in enzymatic reactors.
belated
2 o to this, Trachtenberg (US6143556) describes a systern for the gas phase
treatment
of gas effluents with an enzyme, i.e. carbonic anhydrase. EP0991462;
W09855210;
CA2291785 in the name of the applicant also proposes a process for the use of
the
enzyme in the treatment of a C02-containing gas. Although these processes have
proved to be effective to remove the C02 contained in a mixture of gases, they
are
not adapted or suitable for the purification of energetic gases such as biogas
or
natural gas on a large scale.
Although there has been a lot of development made irr the field of gas
separation or
gas purification, there is still a need for a process that would allow a large-
scale
CA 02417483 2003-O1-27
4
production of energetic gases such as methane contained in the biogas and the
natural gas at a relatively low cost.
SUMMARY OF THE INVENTION
An object of the present invention is to provide a process that satisfies the
above-
s mentioned need and that overcomes several of the above-mentioned drawbacks
concerning the prior process for the purification of energetic gases such as
biogas
and natural gas.
An auxiliary object, which is obtained with a preferred embodiment of the
invention, is
to reduce greenhouse gases.
l o In accordance with the present invention, that object is achieved with a
process for
purifying a gas stream containing a contaminant gas and an energetic gas. The
process comprises the steps of:
a) providing a bioreactor comprising:
-a reaction chamber containing a solvent and a biocatalyst capable of
15 catalyzing a transformation reaction of the contaminant gas dissolved in
the
solvent into ions;
b) extracting the contaminant gas from the gas stream, comprising the steps
of:
-feeding the gas stream in the reaction chamber and thereby allowing
2 o the contaminant gas to dissolve and transform into ions, yielding the
energetic
gas free of the contaminant gas and leaving a spent solvent containing the
ions in solution;
c) separately releasing the energetic gas and the spent solvent obtained in
step b) from the reaction chamber;
25 d) removing the ions from the spent solvent to recycle the solvent; and
e) feeding the recycled solvent of step d) in the reaction chamber.
CA 02417483 2003-O1-27
In step a) above, the solvent is preferably exempt of the contaminant gas and
saturated with the energetic gas to be cleaned.
As can be appreciated, and thanks to the fact that the spent solvent is
regenerated
and recycled back into the reaction chamber, the process of the invention
makes
5 possible the production on a large scale of energetic Bases. Indeed, since
the spent
solvent, which is essential to dissolve the gaseous contaminant, is recycled
back into
the process, the process i s operable without the need of an outside source of
solvent. Without the recycling of the spent solvent, enormous quantity of
fresh
solvent from an outside source would have to be supplied to the bioreactorto
enable
to the purification of energetic gases on a large scale.
Also, since a certain amount of the energetic gas might as well have dissolved
in the
solvent, the spent solvent, which is recycled back into the reaction chamber,
is
saturated of energetic gas.
Another advantage of the invention in comparison to other available
technologies is
that the mixture of gas requires no pre-treatment (dehydration, preliminary
extraction)
before arrival in the transfer system.
Still another advantage of this invention is that everything takes place at
ambient
temperature and pressure conditions. The operating costs are therefore
decreased
with regard to other technologies.
2 o In accordance with a preferred aspect, the process is used to clean a
biogas or a
natural gas, which contain methane and carbon dioxide. In that particular
case, the
energetic gas is methane, the contaminant gas is carbon dioxide, the
biocatalyst is
carbonic anhydrase or an analog thereof and the solvent contains water.
The process can also be used to purify the other gases found in the natural
gas,
namely ethane, propane, butane, iso-butane, pentane, iso-pentane, hexane,
nitrogen, hydrogen, oxygen, argon, helium, and neon.
CA 02417483 2003-O1-27
6
Also preferably, step e) of removing the ions from the spent solvent is
performed by
means of an ion exchange resin and the process further comprises a step of
regenerating the ion exchange resin.
The present invention also concerns a process for purifying a gas stream
containing
methane as an energetic gas and carbon dioxide as a contaminant gas, the
process
comprising the steps of:
a) providing a bioreactor comprising:
-a reaction chamber filled with an aqueous solvent containing a biocatalyst
capable
of catalyzing the chemical conversion of dissolved cGirbon dioxide into an
aqueous
1 o solution;
b) extracting the carbon dioxide from the gas stream, comprising the steps of:
-feeding the gas stream in the reaction chamber and thereby allowing the
carbon
dioxide to dissolve and transform infio hydrogen ions and bicarbonate ions,
yielding
the energetic gas free of carbon dioxide and leaving a spent solvent
containing the
5 hydrogen ions and bicarbonate ions in solution;
c) releasing the energetic g.as and the spent solvent obtained in step b) from
the
reaction chamber;
d) removing the hydrogen ions and bicarbonate ions from the spent solvent to
recycle the solvent; and
2 o e) feeding the recycled solvent of step d) in the reaction chamber.
The bicarbonate ions are then preferably precipitated as a solid or re-
transformed
into pure C02. The application of this invention can allow the recuperation of
large
quantities of potentially energetic gases while avoiding the emission of
greenhouse
gases and allowing the geological sequestration of CO2.
2s BRIEF DESCRIPTfON OF THE DRAWINGS
Other objects and advantages of the invention will become apparent upon
reading
the detailed description and upon referring to the drawings in which:
CA 02417483 2003-O1-27
7
Figure 1 is a schematic process diagram of a first preferred embodiment of the
process according to the present invention.
Figure 2 is a schematic process diagram of a second preferred embodiment of
the
process according to the present invention
s While the invention will be described in conjunction with example
embodiments, it will
be understood that it is not intended to Limit the scope of the invention to
such
embodiments. On the contrary, it is intended to cover all alternatives,
modifications
and equivalents as may be included as defined by the appended claims.
DESCRIPTION OF PREFERRED EMBODIMENTS
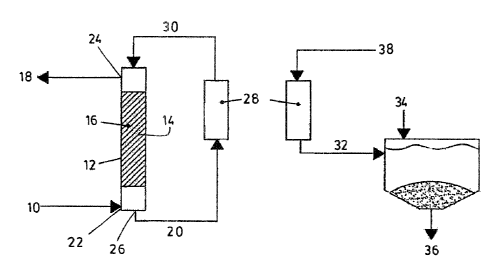
1 o Referring to figure 1, and broadly described, a process according to the
invention is
for purifying a gas stream (10) containing a contaminant gas, such as for
example
carbon dioxide, and an energetic gas, biogas or natural gas. The process is
performed in a bioreactor (12) comprising a reaction chamber (14) filled or
flushed
with a solvent. The reaction chamber (14) contains a biocatalyst (16) capable
of
i5 catalyzing a transformation reaction of the contaminant gas dissolved in
the solvent
into ions. In the case where carbon dioxide has to be extracted from the gas
stream
(10), the biocatalyst used is preferably the enzyme carbonic anhydrase or an
analog
thereof and the solvent contains water.
Since the bioreactor (12) is used in part for dissolving the contaminant gas,
it might
2 o also be referred to hereinbelow as the dissolution module or the gas-
liquid transfer
system.
The bioreactor (12) represented in figure 1 is in the form of a packed tower,
such as
the one described in the abave-mentioned prior applications CA 2291785 and WO
9855210 in the name of the applicant. It is however worth mentioning that the
2 s invention is not limited to this particular type of bioreactor and that
other bioreactors
already known in the prior art may advantageously bE: used.
CA 02417483 2003-O1-27
This system allows for the transformation of gaseous COZ into bicarbonate and
hydrogen ions. The transformation of C02 into bicarbonate ions, usually a slow
naturally occurring process, is catalyzed by an enzyme, which is in an
immobilized or
free state inside the reaction chamber (14} of the bi~oreactor (12). The
equilibrium
reaction must undergo an intermediate hydration that slows the transformation
of
COZ into bicarbonate ions. The enzymatic system catalyses this hydration of
dissolved carbon dioxide. The following equations describe the relevant
processes:
without enzyme: dissolved C02 ~ H2C03 r~ H+ + HCO3 (fV)
with enzyme: dissolved CO2 ~ H* + HC03' (V)
1 o The process thus comprises the steps a) of providing such a bioreactor
(12) and then
b) extracting the C02 contaminant gas from the gas stream (10). In order to
extract
the contaminant gas, the gas stream (10} is fed in the reaction chamber (14)
via an
appropriate gas inlet (22) thereby allowing the coni:aminant gas to dissolve
and
transform into hydrogen and bicarbonate ions within the reaction chamber (14),
yielding the methane energetic gas (18) free of COz contaminant gas and
leaving a
spent solvent (20) containing the ions in solution, and a certain amount of
dissolved
energetic gas in an equilibrium concentration.
The gaseous phase of the eraergetic gas (18) and the ;>pent solvent (20) are
released
from the reaction chamber (14) via a respective gas outlet (24) and a liquid
outlet
2 0 (26). Then the ions are removed from the spent solvent (20) to recycle the
same
within the reaction chamber (14). More specifically, the spent solvent (20)
passes
through an ion exchange resin (28) where contaminants transformed into ions in
solution are removed. The recycled solvent (30), vvhich now only contains the
energetic gas in solution in an equilibrium concentration, is fed back into
the reaction
2 s chamber (14) ready to extract contaminants. When the resin (28) no longer
contains
active sites capable of adsorbing ions, it is regenerated with a chemical
regenerator
(38). The obtained solution (32) will be concentrated in ions. The ions can,
by the
addition of additional ions (34), be precipitated as a solid (36).
CA 02417483 2003-O1-27
9
Referring to Figure 2 a second preferred version of the process according to
the
invention is represented. This embodiment provides a method for obtaining a
gas of
superior purity. This method can also be used for extracting several different
contaminants. Figure 2 shows two dissolving bioreactors (12a, 12b) organized
in
series where the exit of purified gas (11 ) from the first bioreactor (12a)
returns to the
second bioreactor, which contains the same enzyme as the first or a different
enzyme (16). The rest of the treatment sequence follows the same steps as
figure 1.
As can be appreciated, the invention is directed to the use of enzymes, so as
to
extract one or several compounds from a potentially energetic gaseous mixture.
The
to gas or gases to be extracted are previously dissolved in a liquid phase
called a
solvent to be then transformed by one or several ionized enzymes. This enzyme
can
be immobilized on a support or in suspension in the solution. This way,
contaminants
can be removed from them to become a concentrated or purified gas mixture. One
or
several separated gases transformed into aqueous ions can be converted into
inert
z.5 solids or reconverted into pure gas.
Every gaseous compound has a solubility equilibrium with a given solvent and
the
maximum concentration of the dissolved compound is dependent on temperature
and partial pressure conditions of the gas. The transfer of the compound
between
the two phases is interrupted when this maximum concentration is reached.
Table 1
2 0 lists a number of compounds originating from landfill sites, which can be
found in a
biogas.
It is worth noting that, if one wants to remove the COZ from this gas mixture
and to
transfer it to a liquid phase, that the water solubility of C02 is 24 times
that of
methane. Therefore, the C02 will preferentially be adsorbed in water as
opposed to
25 methane. If the solubility equilibrium is reached for methane but not for
the carbon
dioxide, only the gas phase COZ will continue to diffuse in the liquid phase.
Since a
physico-chemical mechanism allows for the removal of C02 as it is formed, the
equilibrium is never achieved and the gaseous COZ continues to be dissolved in
the
aqueous phase.
CA 02417483 2003-O1-27
In the process according to the invention, the gas mixture (10) enters into
contact
with a solvent inside conventional gas I liquid transfer systems (12) such as
a
packed column, aspersion tower, triphasic column or any other system (without
limiting itself to it). The liquid phase, that is to say the v,olvent,
preferably contains the
5 to-be-purified compound in solution in an equilibrium concentration, thus
saturated,
with the gas phase. Furthermore, this solvent is free of the to-be-extracted
compound. An enzyme (16) specially selected to catalyze the transformation of
the
to-be-extracted compound is found inside the gas ! liquid transfer system.
This
transformation generates ions in solution.
1 o The solvent can then flush through the ion exchange resin (28) where only
selected
ionic compounds are trapped. The compounds that are not ionized in solution,
i.e.
those that have strong covalent bonds, stay in solution. A regeneration of the
resin
(28) is necessary once all of the resin's active sites are occupied. A second
solution
strongly charged in ions is then obtained.
5 The ions in solution can be precipitated as an inert solid (34) by the
addition of
cations or additional anions. The produced ions can also be re-transformed as
a gas
by means of temperature andlor pressure change. In both cases, the solid and
the
gas generated are preferred.
One of the advantages c~f the invention by comparison to other available
2 o technologies, is that the mixture of gas requires no pre-treatment
(dehydration,
preliminary extraction) before its arrival in the transfer system. A cooling
system may
be necessary for cases where the gas temperature increases that of the liquid
to
temperatures superior to what the enzyme can tolerate. Using an enzyme that
tolerates high temperature, pH and pressure can be also envisaged.
2 s Another advantage of this invention is that the process can take place at
normal
temperature and pressure conditions. The operating costs are therefore
decreased
with regard to other technologies.
CA 02417483 2003-O1-27
If several gases must be extracted from the initial mixture, several types of
enzymes
may be present in the dissolving module. It could also be advantageous to use
several modules in series which will have for an individual task the
extraction of a
single compound at a time.
A first flushing of the gas mixture will not necessarily produce a gas free of
contaminants. However, to increase the purity of the treated gas, the user can
re-
flush the gas in the dissolving module (12) until the required concentration
is
obtained.
Table l:Solubility in water of certain gases contained in biogas found found
in
landfills.
Pure Gas Solubility (Mol xrtotal Moi'
ethane (C2H4) 3,40 x 10-
Methane (CH4) ! 2,55 x 1~
Oxygen (02) 2,29 x 10-~
_ i
Hydrogen (H2) 1,41 x 10-
Nitrogen (N2) 1,18 x 10-
Hexane (C6H~4) 1,1 x 10'
Propane (C3H$) 2,73 x 10
Isobutane {C4Hlp) 1,66 x 10~
Carbon dioxide (C~z) 6,15 x 10~
Hydrogen sulphide {H2S) 1,85 x 10-
'~ normal pressure and temperature
Examples
The biogas found in landfill sites is formed from the anaei°obic
decomposition of
buried biodegradable matter. This gas mainly consists of nitrogen (N2), carbon
i 5 dioxide {C02) and methane (CH4). Volatile hydrocarbons as well as volatile
sulphured
compounds are found in weaker concentrations. The release into the atmosphere
of
CA 02417483 2003-O1-27
12
C02 and CH4, both recognised as principal greenhouse gases, aggravates the
global
warming problem.
In a sustainable development context, the gas resulting from the
biomethanation of
organic matter is a renewable source of energy in the same way as the energy
s exploitation of the biomass. The capture of biogases and their burning can
sometimes provide the recovery of energy which can be used to produce, among
others, some electricity or vapour. To achieve this, the methane concentration
of the
biogas must be sufficiently high, and one must be using equipment adapted to
this
type of gas mixture. Furthermore, the use of a gas with a low concentration of
to methane may require the use of a catalyst or an adequate dose of oxygen and
favours a high production of NOx due to a high flame temperature. 1n most
cases,
the heat or energy generators cannot work directly with biogases and a
preliminary
separation of the C02 is necessary.
For an application of this technology in the treatment of the biogas escaping
from a
15 landfill site, the biogas (10) is put in contact with a solvent containing
some methane
in equilibrium balance with the gas phase. The enzyme (16) used in thE: gases
dissolving module (12) is the carbonic anhydrase, which has the capacity to
catalyze
the transformation of aqueous C02 in ionic bicarbonate.
The bicarbonate is removed from the solution by adsorption on an anionic resin
and,
2 o subsequently, concentrated in resin's regeneration solution. The
bicarbonate can be
coupled with a cation such as calcium (34) to form solid calcium carbonate
(36). This
inert precipitate can be used at the landfill site as a recovering material.
The purified methane, found in concentrations superior to 95%, can be used as
fuel.
No greenhouse gases are therefore emitted into the atmosphere.
2 s Purification of the natural gas
The natural gas must be purified of its water vapour, carbon dioxide and other
contaminants content before its liquefaction. This raw natural gas may come
from the
CA 02417483 2003-O1-27
Z3
extraction of coal or petroleum. Once the gas is liquefied, it can be
transported by
pipeline. If the purification steps are too expensive and result in an
unprofitable
operation, the gas will be burned, thereby producing greenhouse gases and
sacrificing a potential source of energy.
s The process described in the present invention is used to extract the COz
and other
contaminants contained in a mixture of natural gas. The only step necessary
before
the liquefaction of the gas is a final dehydration. The C02 so separated is
preferably
combined with cations to form an insoluble precipitate. This precipitate can
be
exploited on the market or still accumulated in convenient areas such as a
pit, and
1 o hence, establish an effective, secure and non-polluting form of geologic
sequestration of C02.
Although preferred embodiments of the present invention have been described in
detail herein and illustrated in the accompanying drawings, it is to be
understood that
the invenfiion is not limited to these precise embodiments and that various
changes
15 and modifications may be effected therein without departing from the scope
or spirit
of the present invention.