Note: Descriptions are shown in the official language in which they were submitted.
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
CARBOXAMIDE COMPOUNDS AND THEIR USE AS ANTAGONISTS OF A HUMAN 11CBY RECEPTOR
This invention relates to a method of treatment using an antagonist of the
human 11 CBy
receptor; a new therapeutic use of a class of carboxamide compounds which are
antagonists to a human 11 CBy receptor; also to novel compounds within that
class, and to
methods for malting the compounds.
International Patent Application Publication Nmnber WO 01/21577 (Takeda
Chemical
Industries Ltd.) discloses certain bisaryl compounds as melanin concentrating
hormone
antagonists.
WO 98/00401 (Merck & Co. Int.) discloses benzamide derivatives as fibrinogen
receptor
antagonist prodrugs.
European Patent EP 0 358 118 (Boehringer Mamiheim GmbH) discloses certain
bisaryl
compounds as inhibitors of erythrocyte aggregation and useful in the treatment
of cardiac
and circulatory disease.
European Patent Application EP 0 968 999 (Mitsui Chemical Inc.) discloses
certain
anilide derivatives useful in the treatment of arrhythmia.
WO 99/01127 (SmithKline Beecham) discloses certain N [(amino allcoxy)phenyl]
benzamides that are active as CCRS receptor ligands, including the compoLUZds
N-[4-[2-[bis(1-methylethyl)amino]ethoxy]-2-fluorophenyl]-[ 1,1'-biphenyl]-4-
carboxamide and N-[4-[2-[bis(1-methylethyl)amino]-ethoxy]-phenyl]-[1,1'-
biphenyl]-4-
carboxamide. Also WO 99/06146 (SmithKline Beecham) discloses certain
substituted
anilides that are antagonists of the CCRS receptor, including the compounds:
biphenyl-4-carboxylic acid [4-(2-dimethylamino-ethoxy)-phenyl]-amide,
biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-phenyl]-amide,
N [4-(2-diisopropylamino-ethoxy)-phenyl]-4-phenoxy-benzamide,
N [4-(2-diethylawino-ethoxy)-phenyl]-4-phenoxy-benzamide,
N [4-(2-diisopropylamino-ethoxy)-phenyl]-3-phenoxy-benzamide,
N [4-(2-diethylawino-ethoxy)-phenyl]-3-phenoxy-benzamide,
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
4-cyclohexyl-N [4-(2-diisopropylamino-ethoxy)-phenyl]-benzamide,
4-cyclohexyl-N -[4-(2-diethylamino-ethoxy)-phenyl]-benzamide,
4-benzyl-N [4-(2-diisopropylamino-ethoxy)-phenyl]-benzamide,
4-benzyl-N -[4-(2-diethylamino-ethoxy)-phenyl]-benzamide,
4'-ethyl-biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-phenyl]-
amide,
and 4'-ethyl-biphenyl-4-carboxylic acid [4-(2-diethylamino-ethoxy)-phenyl]-
amide.
The present invention is based on the fording that a class of carboxamides
overlapping
with the above-mentioned benzamides and anilides, are, surprisingly,
antagonists of a
human 11 CBy receptor disclosed in Nature, 400, 261-265 (1999).
Accordingly these compounds axe believed to have a role in preventing,
ameliorating or
correcting dysfunctions or diseases, including, but not limited to, infections
such as
bacterial, fungal, protozoan and viral infections, particularly infection
caused by HIV-1 or
HIV-2; pain; cancers; diabetes; obesity; feeding and drinking abnormalities,
such as
anorexia and bulimia; asthma; Parkinson's disease; both acute and congestive
heart
failure; hypotension; hypertension; urinary retention; osteoporosis; angina
pectoris;
myocardial infarction; ulcers; allergies; benign prostatic hypertrophy;
psychotic and
neurological disorders, including anxiety, schizophrenia, manic depression,
delirium,
dementia or severe mental retardation; and dyskinesias, such as Huntington's
disease or
Gilles de la Tourette's syndrome, among others, hereinafter referred to as
"the
Disorders".
According to the present invention there is provided a method of treating the
Disorders
which comprises administering to a mammal suffering from one or more of the
Disorders
an effective amount of a compound of fomnula (I), or a pharmaceutically
acceptable salt
or solvate thereof, in which:
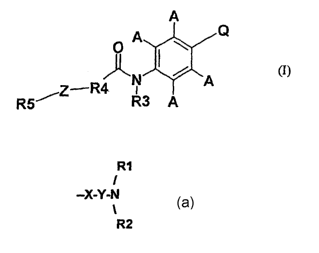
A
Q
p A
,~Z'R4 N
R3 A
R5 (I)
2
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
each A is independently hydrogen, a Cl_6 alkyl optionally substituted by
hydroxyl, C1_6
alkoxy, C1_6 allcenyl or C1_6 acyl group or a halogen atom or hydroxyl, CN or
CF3 group;
R3 is hydrogen, methyl or ethyl.
Preferably R3 is methyl.
R4 is an optionally substituted aromatic carbocyclic or heterocyclic ring.
Z is an O or S atom, or an NH or CH2 group, or a single bond, at the 3 or 4
position of R4
relative to the carbonyl group.
Preferably, Z is a bond.
More preferably, Z is a bond at the 4-position of R4 relative to the carbonyl
group.
RS is an optionally substituted aromatic carbocyclic or heterocyclic ring, or
an optionally
substituted, saturated or unsaturated, carbocyclic or heterocyclic ring.
Preferably, RS is a phenyl ring.
R1
I
and Q is -X-Y-N
1
R2
' (a) where X is an O or S atom, preferably an O atom;
Y is a linear or branched C2-q. allcylene group, preferably a C3 alkylene
group, optionally
substituted by a hydroxyl group, or is a CS_6 cycloalkylene group,
Rl and R2 are independently a linear or branched C1_6 alkyl, preferably ethyl;
phenyl C1_
6 alkyl group; or
(b) where X is an O or S atom;
Y is a linear or branched C2_q alkylene group, optionally substituted by a
hydroxyl group,
Rl and R2 are linked to form a 5, 6 or 7-membered ring, preferably a 5-
membered ring,
optionally containing one or more further heteroatoms selected from O, S or N,
where N
or C ring atoms are optionally substituted by Ra, -CO-Ra, -CO-NH-Ra, or CO-O-
Ra,
where Ra is a linear or branched C1_6 alkyl or aryl group; and the 5, 6 or 7-
membered
ring is optionally fused to an optionally substituted benzene ring, or a ring
atom of the 5,
6 or 7-membered ring is optionally linked by a single bond or methylene group
to Y; or
(c) where X is an O or S atom,
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Y is a C2_4 alkylene group, Rl is a C2_q. alkylene group linked to Y to form a
5 or 6
membered ring and R2 is a linear or branched C1_6 alkyl group; or
(d) where X is a N atom,
Y is a C2_q. allcylene group, Rl is a C2_q. alkylene group linked to X to form
a 5 or 6
membered ring and R2 is a linear or branched C1_6 alkyl group.
Alkyl groups, including alkyl groups that are part of alkoxy, acyl, etc
groups, typically
contain 1 to 6 carbon atoms, and may be linear or branched, such as methyl,
ethyl, i-
propyl and t-butyl, and optionally substituted by hydroxyl. Aryl groups are
typically
phenyl, but may include bicyclic groups such as naphthyl. Cycloalkyl groups
typically
contain from 3 to 7 carbon atoms. Heterocyclic groups may be monocylic 5 to 7
membered rings containing up to three hetero atoms, such as pyridyl or
imidazole, or
bicyclic, especially heterocyclic rings fused to benzene rings, such as
benzoxazole or
benzimidazole. Aryl, cycloalkyl and heterocyclic groups may be optionally
subsituted by
up to three substituents, which may suitably be selected from aryl, alkyl,
alkoxy, halogen,
hydroxy and cyano, or by linked substituents such as dioxymethylene.
Suitable aromatic rings for use as R4 include phenyl, pyridyl, thienyl,
furanyl and
pyrazolyl. Suitable optional substituents for R4 include halogen, CF3, C1_q.
alkyl, C1_4
alkoxy. R4 may have 2 or 3 substituents, but preferably has only 1 substituent
in addition
to Z, or more preferably is unsubstituted apart from Z. Particularly suitable
substituents
for R4 include chloro, fluoro, trifluoromethyl, methyl, methoxy.
R5 may be monocyclic, for example thienyl, furanyl, imidazolyl, oxadiazolyl,
phenyl,
pyridinyl, cyclohexyl, piperidinyl, piperazinyl, pyrazinyl, pyrimidinyl; or a
fused bacyclic
ring system, for example naphthyl, 3,4-dioxymethylene-phenyl, benzofuranyl,
indolyl; or
a bicyclic system in which a monocyclic ring has a cyclic substituent such as
oxadiazolyl,
benzyloxy. Suitable optional substituents for R5 include halogen, CF3, CF30,
CHF20,
CN, amino, mono- or di-C 1 _6 alkylamino, C 1 _g alkyl, C 1 _6 allcoxy, C 1 _6
acyl, C 1 _6 alkyl-
S-, C1_6 alkyl-S02-, Cl_6 alkenyl, phenyl-C1_6 alkyl, phenyl-C1_6 alkoxy. R5
may have
2 or 3 substituents, but preferably has only 1 substituent, especially in the
para position
relative to Z. Particularly suitable substituents for R5 include chloro,
fluoro,
4
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
trifluoromethyl, cyano, amino, methyl, ethyl, t-butyl, methoxy, acetyl,
formyl,
methylthio, methanesulphonyl, vinyl, benzyl, benzyloxy, hydrogen.
As for the ring substituents A, all A substituents may be hydrogen, but it is
advantageous
that no more than 3 are hydrogen. Suitable A substituents include halogen,
C1_6 alkyl
optionally substituted with hydroxy, C1_6 alkoxy, C1-6 acyl and Cl_6 allcenyl.
Particularly suitable A substituents include C1-2alkoxy, C1-2alkyl, C1-2 acyl.
Preferable
substituents for A include chloro, fluoro, methyl, ethyl, hydroxyethyl,
methoxy, formyl,
acetyl, vinyl and allyl. More preferable substituents for A include methoxy.
Suitably, the A substituent is adjacent to the group Q.
In the system Q, in conf guration (a) particularly suitable substituents for
Rl and R2
include methyl, ethyl, isopropyl, benzyl, phenethyl. Y may especially be -
(CH~)2-,
-(CH2)3-, -(CH2)q-, -CHI-CH(CH3)-CH2-. When Y is substituted by hydroxy, it
may be
for example -CHI-CH(OH)-CHI-.
In configuration (b) of system Q, the ring formed by linking Rl and R2 inay be
pyrrolidinyl, piperidinyl, azepanyl, or imidazolyl. Fused rings include
indolinyl,
tetrahydxoisoquinolinyl, tetrahydroquinolinyl and benzoazepinyl. When a second
heteroatom is present, suitable rings include thiazinyl, oxazinyl and
piperazinyl. A
second N atom may be substituted, for example by phenyl, methyl, ethyl,
isopropyl or
acetyl. Y is typically -(CH2)2-. The ring may be linlced back to Y to form a
quinuclidinyl
group.
In configuration (c) of system Q, the ring formed by linking Rl to Y may be a
pyrrolidinyl or piperidinyl ring. The linkage to Y may be such as to cxeate a
ring linked
by a single bond from a ring carbon atom directly to X or via a methylene or
ethylene
linl~ing group. R2 is typically methyl so that the N atom of the ring is
substituted by
methyl.
5
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
In configuration (d) of system Q, the ring formed by linking Rl to N is
suitably a 5 or 6-
membered ring such as diazinyl or piperazinyl. Y is typically -(CH2)2-. R2 is
typically
methyl so that the second N atom (other than X) of the ring is substituted by
methyl.
Within the scope of formula (I) is a class of compounds of general formula
(II)
~R1
O I ~ O~N~R2
\ H / Or
R5
(II)
where A = H and OMe, R3 = H, X = O, Y = CH2CH2 , Z = a bond, R4 = Ph, RS is
either
I O meta or para substituted on R4, and Rl, R2 and RS are as defined for
formula (I).
Also within the scope of formula (I) is a class of compounds of general
formula (III)
Z
R5/
(III)
where A = H and OMe, R3 = H, X = O, Y = CH2-CH2 , Z = O, CH2 or NH and is
either
meta or para substituted on R4, R4 = Ph, RS is Ph, and Rl and R2 are as
defined for
formula (I).
Also within the scope of formula (I) is a class of compounds of general
formula (IV)
~ ~ ~~N~
R5'~Z~R4~N / O~
H
6
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
where A = H and OMe, Rl = R2 = iPr, R3 = H, X = O, Y = CHI-CH2 , and R4 and RS
are substituted phenyl or heterocycles as defined for formula (I)
Also within the scope of formula (I) is a class of compounds of general
formula (V)
~1
R7 O~'N~R2
O
R6
R5''Z
where R3 = H, X = O, Y = CHI-CH2, Z = O, CHI, NH or a bond, R4 = Ph, RS is Ph
or
cyclohexyl (Cy), Z is either meta or para substituted on R4, and A (R6,R7) and
R1,R2 are
as defined in formula (I).
Also within the scope of formula (I) is a class of compounds of general
formula (VI)
R1
R8
O I ~ O~N~R2
N / R9
R5~Z / R3
(VI)
where X = O, Y = CHI-CH2, R4 = phenyl, RS = phenyl or cyclohexyl (Cy), Z = O,
CHI
or a bond, and A (R8,R9), R3 and R1,R2 are as defined in formula (I).
Also within the scope of formula (I) is a class of compounds of general
formula (VII)
7
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
R1
i
O~N~R2
N O
H
(VII)
where A = H and OMe, X = O, R3 = H, R4 = 3-pyridyl (with respect to the
carbonyl
group), RS = phenyl, Z = a para bond , and Rl,R2 are as defined in formula
(I).
Also within the scope of formula (I) is a class of compounds of general
formula (VIII)
~1
p-.Y.-N~2
N / ~i
R5~Z / H
(VIII)
where A = H and OMe, R3 = H, X = O, R4 = phenyl, Z = O, CH2 or a bond, RS = Ph
or
cyclohexyl (Cy), Y is a chain of 3 or 4 carbon atoms optionally substituted by
an
hydroxyl group, and R1,R2 are as defined in formula (I).
Also within the scope of formula (I) is a class of compounds of general
formula (IX)
~ N'~N-R1
N ~ / Oi
H
R5
(IX)
where A = H and OMe, R3 = H, X = N, R4 = phenyl, Z = a para substituted bond,
RS =
Ph or cyclohexyl (Cy), Y and R2 form a piperazinyl ring between X and N, and
Rl is as
defined in formula (I).
8
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A preferred sub-class of compounds for use in the method of treatment of this
invention
are compounds of formula (I) in which R3 is methyl.
Within formula (I) is a novel group of compounds in which R3 is methyl or
ethyl. The
novel compounds, or a salt or solvate thereof, form a further aspect of this
invention.
A particular group of novel compounds is a class of compounds of general
formula (VI)
R5
(VI)
where R8 and R9 are as defined for A in formula (I), Rl, R2 and RS are as
defined in
formula (I), and R3 is methyl or ethyl.
Suitably RS is phenyl or cyclohexyl optionally substituted by halogen,
haloalkyl, alkyl or
alkoxy; Z is O, CH2 or a single bond; R8 and R9 are independently selected
from
hydrogen, halogen, alkyl and alkoxy; Rl and R2 are alkyl or linlced together
to form a
ring; and R3 is ethyl or methyl.
Another aspect of this invention is a class of novel compounds, or a salt or
solvate
thereof, which are the compounds of formula (I) excluding the compounds:
N-[4-[2-[bis(1-methylethyl)amino]ethoxy]-2-fluorophenyl]-[1,1'-biphenyl]-4-
carboxamide,
N-[4-[2-[bis(1-methylethyl)amino] ethoxy]phenyl]-[1,1'-biphenyl]-4-
carboxamide,
biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-phenyl]-amide,
N [4-(2-diisopropylamino-ethoxy)-phenyl]-4-phenoxy-benzamide,
N [4-(2-diethylamino-ethoxy)-phenyl]-4-phenoxy-benzamide,
N [4-(2-diisopropylamino-ethoxy)-phenyl]-3-phenoxy-benzamide,
N [4-(2-diethylamino-ethoxy)-phenyl]-3-phenoxy-benzamide,
4-cyclohexyl-N [4-(2-diisopropylamino-ethoxy)-phenyl]-benzamide,
4-cyclohexyl-N -[4-(2-diethylamino-ethoxy)-phenyl]-benzamide,
9
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
4-benzyl-N [4-(2-diisopropylamino-ethoxy)-phenyl]-benzamide,
4-benzyl-N -[4-(2-diethylamino-ethoxy)-phenyl]-benzamide,
4'-ethyl-biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-phenyl]-
amide,
and 4'-ethyl-biphenyl-4-carboxylic acid [4-(2-diethylamino-ethoxy)-phenyl]-
amide.
A further aspect of this invention is those compounds of the Examples herein
which are
novel.
The compounds of formulae (I) to (IX), or their salts or solvates, are
preferably in
pharmaceutically acceptable or substantially pure form. By pharmaceutically
acceptable
form is meant, inter alia, of a pharmaceutically acceptable level of purity
excluding
normal pharmaceutical additives such as diluents and carriers, and including
no material
considered toxic at normal dosage levels.
Suitable salts and solvates include pharmaceutically acceptable salts and
pharmaceutically acceptable solvates.
Suitable pharmaceutically acceptable salts include metal salts, such as for
example
aluminium, alkali metal salts such as lithium, sodium or potassium, alkaline
earth metal
salts such as calcium or magnesium and ammonium or substituted ammonium salts,
for
example those with lower alkylamines such as triethylamine, hydroxy
alkylamines such
as 2-hydroxyethylamine, bis-(2-hydroxyethyl)-amine or tri-(2-hydroxyethyl)-
amine,
cycloalkylamines such as bicyclohexylamine, or with procaine,
dibenzylpiperidine,
N-benzyl-(3-phenethylamine, dehydroabietylamine, N,N'-bisdehydroabietylamine,
glucamine, N-methylglucamine or bases of the pyridine type such as pyridine,
collidine,
quinine or quinoline.
Suitable pharmaceutically acceptable salts also includes pharmaceutically
acceptable acid
addition salts, such as those provided by pharmaceutically acceptable
inorganic acids or
organic acids.
Suitable pharmaceutically acceptable acid addition salts provided by
pharmaceutically
acceptable inorganic acids includes the sulphate, nitrate, phosphate, borate,
hydrochloride
and hydrobromide and hydroiodide.
Suitable pharmaceutically acceptable acid addition salts provided by
pharmaceutically
acceptable organic acids includes the acetate, tartrate, maleate, fumarate,
malonate,
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
citrate, succinate, lactate, oxalate, benzoate, ascorbate, methanesulphonate,
a-keto
glutarate and a,-glycerophosphate.
Suitable pharmaceutically acceptable solvates include hydrates.
A substantially pure form will generally contain at least 50% (excluding
normal
pharmaceutical additives), preferably 75%, more preferably 90% and still more
preferably 95% of the compound of formula (I) to (IX) or its salt or solvate.
One preferred pharmaceutically acceptable form is the crystalline form,
including such
form in a pharmaceutical composition. In the case of salts and solvates the
additional
ionic and solvent moieties must also be non-toxic.
Examples of pharmaceutically acceptable salts of a compound of formula (I) to
(IX)
include the acid addition salts with the conventional pharmaceutical acids,
for example,
malefic, hydrochloric, hydrobromic, phosphoric, acetic, fumaric, salicylic,
citric, lactic,
mandelic, tartaric, succinic, benzoic, ascorbic and methanesulphonic.
The compounds of formula (I) to (IX) may exist in more than one stereoisomeric
form,
and the invention extends to all such forms as well as to their mixtures
thereof, including
racemates.
The compounds of formula (I) to (IX), or salts or solvates thereof, may be
prepared by the
methods illustrated in the following general reaction schemes, or by
modification thereof,
using readily available starting materials, reagents and conventional
synthetic procedures.
If a particular enantiomer of a compound of the present invention is desired,
it may be
synthesised starting from the desired enantiomer of the starting material and
performing
reactions not involving racemization processes or it may be prepared by chiral
synthesis,
or by derivatisation with a chiral auxiliary, where the resulting
diastereomeric mixture is
separated and the auxiliary group cleaved to provide the pure desired
enantiomers.
.Alternatively, where the molecule contains a basic functional group, such as
amino, or an
acidic functional group, such as carboxy, diastereomeric salts are formed with
an
appropriate optically active acid or base, followed by resolution of
diastereomeric salts by
fractional crystallization and subsequent recovery of the pure enantiomers.
Compounds of formula (I) to (IX) may prepared by condensing suitably
substituted aryl
or heteroarylcarboxylic acids and suitably substituted amines, which are
commercially
11
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
available or synthesized by methods known to the art from coxmnercially
available
starting materials, using methods known to the art. For example, suitably
substituted aryl
or heteroarylcarboxylic acids are treated with an activating reagent, such as
thionyl
chloride, at a suitable temperature, such as at reflux, to afford aryl or
heteroarylcarbonyl
chlorides, and the aryl- or heteroarylcarbonyl chlorides are condensed with
suitably
substituted anilines in the presence of a suitable base, such as
diisopropylethylamine, in a
suitable solvent, such as dichloromethane, to give compounds of formula (I).
In particular, the preparation of certain carboxamides of formula (I) in which
R3 is H is
disclosed in WO 99/01127 and WO 99/06146 mentioned above, and analogous
methods
of preparation may be used in the present invention. Many additional methods
for
converting a carboxylic acid to an amide are known, and can be found in
standard
reference books such as "Compendium of Organic Synthetic Methods", Vol. I-VI
(published by Wiley-Interscience).
For example the compounds of formula (I) may be prepared by reacting a
compound of
formula (X)
R5-Z-R4-COL (X)
where L is a leaving group such as halogen, especially chlorine or bromine
with a compound of formula (XI)
A
H
Q
R3 A (xI)
where A, Z, R3, R4, RS and Q are as defined for formula (I).
In this process, groups convertible to Rl, R2, R3, R4 and RS may be present
during the
coupling, and converted to Rl, R2, R3, R4 and RS after coupling. Also it may
be
convenient to convert one Rl, R2, R3, R4 and RS to another Rl, R2, R3, R4 and
RS
group after coupling. In particular, ring formation between the groups Rl,
X,Y, R2 or
12
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
the addition of suitable cyclic groups embodying Rl, X, Y, R2, may be
performed after
coupling.
Accordingly, there is provided a process for the preparation of a compound of
formula
(I), or a salt or solvate thereof, wherein R3 is methyl or ethyl which process
comprises the
reaction of a compound of formula (X) as hereinbefore defined with a compound
of
formula (XI) wherein A and Q are as hereinbefore defined and R3 is methyl or
ethyl.
There therefore also provided a process for the preparation of a compound of
formula (I),
or a salt or solvate thereof, with the proviso that the following compounds
are excluded;
N-[4-[2-[bis( 1-methylethyl)amino]ethoxy]-2-fluorophenyl]-[1,1'-biphenyl]-4-
carboxamide,
N-[4-[2-[bis(1-methylethyl)amino]ethoxy]phenyl]-[1,1'-biphenyl]-4-carboxamide,
biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-phenyl]-amide,
N [4-(2-diisopropylamino-ethoxy)-phenyl]-4-phenoxy-benzamide,
N [4-(2-diethylamino-ethoxy)-phenyl]-4-phenoxy-benzamide,
N [4-(2-diisopropylamino-ethoxy)-phenyl]-3-phenoxy-benzamide,
N [4-(2-diethylamino-ethoxy)-phenyl]-3-phenoxy-benzamide,
4-cyclohexyl-N [4-(2-diisopropylamino-ethoxy)-phenyl]-benzamide,
4-cyclohexyl-N -[4-(2-diethylamino-ethoxy)-phenyl]-benzamide,
4-benzyl-N [4-(2-diisopropylamino-ethoxy)-phenyl]-benzamide,
4-benzyl-N -[4-(2-diethylamino-ethoxy)-phenyl]-benzamide,
4'-ethyl-biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-phenyl]-
amide,
and 4'-ethyl-biphenyl-4-carboxylic acid [4-(2-diethylamino-ethoxy)-phenyl]-
amide.
which process comprises the reaction of a compound of formula (X) as
hereinbefore
defined with a compound of formula (XI) as hereinbefore defined.
The compounds of formula (XI) may be prepared in a number of ways, for example
when
X is O or S coupling an appropriately substituted nitrobenzene compound with a
dialkyaminoalcohol or thiol, and converting the N02 group to NH2 by
hydrogenation in
13
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
the presence of palladium catalyst (or with iron/ammonium chloride) before
coupling
with an acid chloride, for example as illustrated below:
+
\ O~ NaH ~+ i H N O~
O',N \ O HZ I Pd on C z \
CI DMF
EtOH O
+ O
HOUN~ N
U
O
Et3N / ~ [ ~GI
CHaCl2 \ \
Acid chlorides of formula (X) may be prepared from the corresponding acids
which are
commercially available or described in the literature or may be prepared by
methods
analogous to those of the literature.
Alternatively the acids of formula (X) may be prepared by combining moieties
containing
respectively RS and R4 via Z.
I0
This may also be achieved conveniently by first coupling a compound of R4-CO-L
with
the compound of formula (XI) followed by reaction with a compound RS-Z-L (or L-
R4-
CO-L with RS-Z). For example an amine of formula (XI) may be reacted with an
appropriately substituted bromobenzoyl chloride which may be then reacted
with, for
15 example, an appropriately substituted phenyl moiety with a leaving group,
or a cyclic
amine, as in the following scheme:
14
SUBSTITUTE SHEET (RULE 26)
Pmaeuure r,a ~
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
H E t3N ~ O HzN O~
N I \ O/ MDC
\ CI + ~O
Br ~O
Br / N
B(oH N
Procedure A93 )z ~ BINAP Pd(OAc)z
EtOH PhH \ HN~ CszC03 DME
Na CO
z a / ~ Procedure A107
Pd(PPh3)4 n.
BINAP = (S)-(+)_2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
Similar reactions building up the structure of formula (I) may be carried out
starting with
the compound of formula (X) and adding the equivalent of formula (XI) in
sections, as in
the scheme below where an N protecting group on Q, here a piperazine ring, may
be
removed after coupling the components of formula (I) and replacement by a
desired
substituent:
N~ O~ HZN \ O~ O
O%N I \ O (Boc)z0 O~ I ~ H2~ I / ~ \ CI
N~ H CH C ~~ EtOH N ~O + I /
O O ~O
DIEA resin
O
CH.,CL,
\ N Oi TFA
I I\
-E-
N~NH ~ ~ OA
EtOH ~ Formaldehyde
AcOH Amberlyst BH3CN
\ N O~ Procedure Hi
I / ~ .i
N~N~
In an alternative strategy for building up the compounds of formula (XI)
before coupling,
so as to introduce a hydroxy group in Y, an appropriately substituted
nitrophenol is linked
to an epoxy compound which is then reacted with an amine forming a group Q
which is -
O-Y(OH) NR1R2, before coupling with RS-Z-R4-CO-L, as illustrated by:
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
EtZNH
~+ ~ NaH / DMF ~N+ O~ CHI OoN+ \ O'~ NEt
O.N I \ O ~ O' I \ Ti(O~Pr)4 ~ / O z
O~ ~O~
~OH i
Nos O O OH
EtOH
i) Et3SiOTf I Et3N HCI I EtaO
O ii) CHzCIz O Ha / Pd on C
N I ~ O NEtz ~ I CI HZN
\ ~O \ _/ , \ N Eta
~i
OH O
OH
Procedure G9 iii) MeOH / Na2C03
Nos = p-nitrobenzenesulphonyl
Novel compounds of formula (I) where the amide nitrogen is alkylated (R3 is
methyl or
ethyl) may be prepared by alkylating an anilide of formula (XI) before
coupling with an
acid chloride of formula (X), for example, by utilising the following
reductive amination
S procedure:
H CHs
HZN ~ O~ ~) Et0- \ OEt HN ~ O~
OEt
I TFA I ,i O
O
N ii) NaBH41 EtOH ~N~
'+
Et3N /
O, CHZC12
O
NiPr2
Procedure E1
The compounds of formula (I) may be converted into their pharmaceutically
acceptable
salts by reaction with the appropriate organic or mineral acids.
I0 Solvates of the compounds of formula (I) may be formed by crystallization
or
recrystallization from the appropriate solvent. For example, hydrates may be
formed by
crystallization or recrystallization from aqueous solutions, or solutions in
organic solvents
containing water.
16
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Also salts or solvates of the compounds of formula (I) which are not
pharmaceutically
acceptable may be useful as intermediates in the production of
pharmaceutically
acceptable salts or solvates. Accordingly such salts or solvates also form
part of this
invention.
The above-listed compounds and pharmaceutically acceptable salts thereof,
especially the
hydrochloride, and pharmaceutically acceptable solvates, especially hydrates,
form a
preferred aspect of the present invention.
By virtue of the activity of these compounds as antagonists of a human 11 CBy
receptor,
the compounds of formula (I) are believed to have a role in preventing,
ameliorating or
correcting dysfunctions of diseases, including, but not limited to, "the
Disorders"
previously mentioned.
It is also considered that the treatment of certain of the Disorders mentioned
above by an
antagonist to the human llCBy receptor are novel. Accordingly, the invention
also
provides a method for the treatment of diabetes, major depression, manic
depression,
anxiety, schizophrenia and sleep disorders, in human or non-human mammals
which
method comprises the administration of a therapeutically effective amount of
an
antagonist to the human llCBy receptor. In particular the the invention
provides a
method for the treatment of diabetes in human or non-human mammals which
method
comprises the administration of a therapeutically effective amount of an
antagonist to the
human llCBy receptor. In particular the invention provides a method for the
treatment
of major depression, in human or non-human mammals which method comprises the
administration of a therapeutically effective amount of an antagonist to the
human
llCBy receptor. In particular the invention provides a method for the
treatment of manic
depression, in human or non-human mammals which method comprises the
administration of a therapeutically effective amount of an antagonist to the
human
llCBy receptor. In particular the the invention provides a method for the
treatment of
anxiety in human or non-human mammals which method comprises the
administration of
a therapeutically effective amount of an antagonist to the human llCBy
receptor. In
particular the the invention provides a method for the treatment of
schizophrenia in
human or non-human mammals which method comprises the administration of a
therapeutically effective amount of an antagonist to the human llCBy receptor.
17
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
In particular the the invention provides a method for the treatment of sleep
disorders, in
human or non-human mammals which method comprises the administration of a
therapeutically effective amount of an antagonist to the human llCBy receptor.
The administration of such compounds to a mammal may be by way of oral
(including
sub-lingual), parenteral, nasal, rectal or transderinal administration.
An amount effective to treat the Disorders hereinbefore described depends on
the usual
factors such as the nature and severity of the disorders being treated and the
weight of the
mammal. However, a unit dose will normally contain 1 to 1000 mg, suitably 1 to
500
mg, for example an amount in the range of from 2 to 400 mg such as 2, 5, 10,
20, 30, 40,
50, 100, 200, 300 and 400 mg of the active compound. Unit doses will normally
be
administered once or more than once per day, for example l, 2, 3, 4, 5 or 6
times a day,
more usually 1 to 4 times a day, such that the total daily dose is normally in
the range, for
a 70 kg adult of 1 to 1000 mg, for example 1 to 500 mg, that is in the range
of
approximately 0.01 to 15 mg/kg/day, more usually 0.1 to 6 mg/kg/day, for
example 1 to 6
mg/lcg/day.
It is greatly preferred that compounds of formula (I) are administered in the
form of a
unit-dose composition, such as a unit dose oral (including sub-lingual),
nasal, rectal,
topical or parenteral (especially intravenous) composition.
Such compositions are prepared by admixture and are suitably adapted for oral
or
parenteral administration, and as such may be in the form of tablets,
capsules, oral liquid
preparations, powders, granules, lozenges, reconstitutable powders, injectable
and
infusable solutions or suspensions or suppositories. Orally administrable
compositions
are preferred, in particular shaped oral compositions, since they are more
convenient for
general use.
Tablets and capsules for oral administration are usually presented in a unit
dose, and
contain conventional excipients such as binding agents, fillers, diluents,
tabletting agents,
lubricants, disintegrants, colourants, flavourings, and wetting agents. The
tablets may be
coated according to well known methods iii the art.
18
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Suitable fillers for use include cellulose, mannitol, lactose and other
similar agents.
Suitable disintegrants include starch, polyvinylpyrrolidone and starch
derivatives such as
sodium starch glycollate. Suitable lubricants include, for example, magnesium
stearate.
Suitable pharmaceutically acceptable wetting agents include sodium lauryl
sulphate.
These solid oral compositions may be prepared by conventional methods of
blending,
filling, tabletting or the like. Repeated blending operations may be used to
distribute the
active agent throughout those compositions employing large quantities of
fillers. Such
operations are, of course, conventional in the art.
Oral liquid preparations may be in the form of, for example, aqueous or oily
suspensions,
solutions, emulsions, syrups, or elixirs, or may be presented as a dry product
for
reconstitution with water or other suitable vehicle before use. Such liquid
preparations
may contain conventional additives such as suspending agents, for example
sorbitol,
syrup, methyl cellulose, gelatin, hydraxyethylcellulose, carboxymethyl
cellulose,
aluminium stearate gel or hydrogenated edible fats; emulsifying agents, for
example
lecithin, sorbitan monooleate, or acacia; non-aqueous vehicles (which may
include edible
oils), for example, almond oil, fractionated coconut oil, oily esters such as
esters of
glycerine, propylene glycol, or ethyl alcohol; preservatives, for example
methyl or propyl
p-hydroxybenzoate or sorbic acid; and if desired conventional flavouring or
colouring
agents.
Oral formulations also include conventional sustained release formulations,
such as
tablets or granules having an enteric coating.
For parenteral administration, fluid unit dose forms are prepared containing
the
compound and a sterile vehicle. The compound, depending on the vehicle and the
concentration, can be either suspended or dissolved. Parenteral solutions are
normally
prepared by dissolving the compound in a vehicle and filter sterilising before
filling into a
suitable vial or ampoule and sealing. Advantageously, adjuvants such as a
local
anaesthetic, preservatives and buffering agents are also dissolved in the
vehicle. To
19
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
enhance the stability, the composition can be frozen after filling into the
vial and the
water removed under vacuum.
Parenteral suspensions are prepared in substantially the same manner except
that the
compound is suspended in the vehicle instead of being dissolved and is
sterilised by
exposure to ethylene oxide before suspending in the sterile vehicle.
Advantageously, a
surfactant or wetting agent is included in the composition to facilitate
uniform
distribution of the compound of the invention.
As is common practice, the compositions will usually be accompanied by written
or
printed directions for use in the medical treatment concerned.
Compounds of the present invention may be employed alone or in conjunction
with other
compounds, such as therapeutic compounds.
No adverse toxicological effects are expected for the compounds of the
invention, when
administered in accordance with the invention.
Accordingly, in a further aspect, the present invention provides a
pharmaceutical
composition for use in the treatment and/or prophylaxis of one or more of the
Disorders
which comprises a compound of this invention, or a pharmaceutically acceptable
salt or
solvate thereof, and a pharmaceutically acceptable carrier.
The present invention also provides a method of treatment and/or prophylaxis
of one or
more of the Disorders comprising administering to the sufferer in need thereof
an
effective or prophylactic amount of a compound of this invention, or a
pharmaceutically
acceptable salt or solvate thereof.
In a further aspect the invention provides the use of a compound of this
invention, or a
pharmaceutically acceptable salt or solvate thereof, for the manufacture of a
medicament
fox the treatment and/or prophylaxis of one or more of the Disorders.
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
In a still further aspect the invention provides the use of a novel compound
of this
invention, or a pharmaceutically acceptable salt or solvate, thereof as a
therapeutic agent,
in particular for the treatment and/or prophylaxis of one or more of the
Disorders.
Compounds for use in this invention and their preparation are illustrated in
the following
Examples and Tables.
These Examples illustrate general procedures and sources of chemicals utilised
to prepare
compounds whose structures are shown in the Tables of data which follow the
Examples.
In the case of Examples prepared as members of a coupled array, the synthetic
origin of
all starting components of the array are shown in the Examples. Rather than
detailing the
experimental procedure for each case, the method by which individual members
of the
array were prepared is indicated in a Table by reference to a related Example.
Mass
spectral characterisation of all Examples is provided in the tables of data.
Additional
characterisation is provided for selected representative Examples with full
experimental
procedures.
Example A1 [WO-00!06146]
Utilising the procedure of Example A7 with 4-biphenylcarboxylic acid [Aldrich]
in place
of 2'-methyl-4-biphenylcarboxylic acid.
Example A2
Correspondingly Example A7 with 4-(5-methyl-[1,2,4]oxadiazol-3-yl)-benzoic
acid
[J.Org.Chem. 50; 8; 1985; 1182].
Example A3
Correspondingly Example A7 with 4-pyrazol-1-yl-benzoic acid [Can.J.Chem.; 41;
1963;
1540].
Example A4
Correspondingly Example A7 with 3-biphenylcarboxylic [Med.Chem.Res.; 6; 2;
1996].
Example AS
21
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Correspondingly Example A7 with 4-(2-pyridyl)-benzoic acid [J.Chem.Soc.; 1940;
355,
356].
Example A6
Correspondingly Example A7 with 3'-acetyl-biphenyl-4-carboxylic acid [Patent
WO-
9743262].
Example A7
2-methylphenyl-4-phenylcarboxylic acid [3-methoxy-4-(2-bis-(2-
methylethyl)amino)-
ethoxy)-phenyl amide.
To a solution of the acid (2'-methyl-biphenyl-4-carboxylic acid) [Patent WO-
9901127]
(SSmg, 0.26mmo1) in dimethylformamide were added (1-(3-dimethylaminopropyl)-3-
ethyl carbodiimide hydrochloride [Aldrich] (SOmg, 0.26mmo1) and 1-hydroxy-7-
azabenzotriazole [Aldrich] (35mg, 0.26mmo1) followed by diisopropylethylamine
(0.04rn1, 0.2Smmo1) and the aniline (4-(2-diisopropylamino-ethoxy)-3-methoxy-
phenylamine) (69mg, 0.22mmo1), [prepared using the method used to form 3-
methoxy-4-
(2-pyrrolidin.-1-yl-ethoxy)-phenylamine in Example A51 but with 2-
diisopropylamino-
ethanol in place of 1-(2-hydroxyethyl)-pyrrolidine]. The reaction mixture was
stirred at
room temperature for 16 hours. The solvent was evaporated, and the residue re-
dissolved
in dichloromethane (1 Oml), filtered through an SAX [Varian] column (2g), and
the filtrate
was then stirred with PS-isocyanate resin [Argonaut Technologies] (100mg,
0.38mmo1)
for 16 hours. The mixture was filtered, evaporated, and the residue purified
by flash
chromatography on silica gel using dichloromethane - aq. ammonia - methanol as
eluent,
to afford the title compound as an oil.
1H NMR (CDCIg): 8 1.04 (12H, d), 2.28 (3H, s), 2.90 (2H, t), 3.05 (2H, m),
3.91 (3H, s),
3.95 (2H, t), 6.88 (1H, d), 7.03 (1H, dd), 7.27-7.32 (4H, m), 7.44, (2H, d),
7.53 (1H, d),
7.94 (2H, d) and 8.01 (1H, bs); MS (AP+ve): m/z 461 [M+H]+.
Example A8
Utilising the procedure of Example A7 with cyclohexyl-4-benzoic acid
[Aldrich], in
place of (2'-methyl-biphenyl-4-carboxylic acid).
Example A9
22
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Correspondingly Example A7 with 4-(2-thienyl)-benzoic acid [J.Chem.Soc.Perkin
Trasis.l; 17; 1992; 2203].
Example A10
Correspondingly Example A7 with 4-(1-methyl-1H pyrazol-4-yl)-benzoic acid
[Patent: WO-9906409].
Example AlI
Correspondingly Example A7 with 4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-
carboxylic acid [Patent:WO -9743262].
Example A12
Correspondingly Example A7 with 4-benzyl-carboxylic acid [Apin].
Example A13
Correspondingly Example A7 with 3'-cyano-biphenyl-3-carboxylic acid
[J.Chem.Soc.Perlcin Trans.2; 1; 1984; 35-38].
Example A14
Correspondingly Example A7 with 3'-methanesulfonyl-biphenyl-4-carboxylic acid
[Izv.Sib.Otd.Akad.Nauk SSSR Ser.Khim.Nauk; 11; 1966; 62].
Example A15
Correspondingly Example A7 with 3-thiophen-2-yl-benzoic acid [Tetrahedron
Lett.; 39;
24; 1998; 4175].
Example A16
Correspondingly Example A7 with 3-thiophen-3-yl-benzoic acid [J.Chem.Soc.B;
1970;
1595].
Example A17
Correspondingly Example A7 with 4-acetyl-4-biphenylcarboxylic acid [Aldrich].
23
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example A18
Correspondingly Example A7 with 4'-cyano-3'-methylbiphenyl-4-carboxylic acid
[WO-
9850358].
Example A19
Correspondingly Example A7 with 4'-(5-methyl-[1,3,4]oxadiazol-2-yl)-biphenyl-4-
carboxylic acid [Patent:WO-9743262].
Example A20
Correspondingly Example A7 with 4-thiophen-3-yl-benzoic acid [J.Chem.Soc.B;
1970;
1595].
Example A21
Correspondingly Example A7 with 4-pyrazin-2-yl-benzoic acid [Patent WO-
9854164].
Example A22
Utilising the procedures of Example A93 with 2-methoxyphenylboronic acid
[Aldrich] in
place of 4-methylphenylboronic acid, and Example A51 with 2-(diisopropylamino)-
ethanol in place of 1-(2-hydroxyethyl)-pyrrolidine .
Example A23
Utilising the procedure of Example A22 with 4-trifluoromethylphenylboronic
acid
[Aldrich], in place of 2-methoxyphenylboronic acid [Aldrich]
Example A24
Correspondingly Example A23 with 3-aminophenylboronic acid [Aldrich].
Example A25
Correspondingly Example A23 with 4-benzyloxyphenylboronic acid [Lancaster].
Example A26
Correspondingly Example A23 with 2-naphthylboronic acid [Lancaster].
24
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example A27
Correspondingly Example A23 with 3-naphthylboronic acid [Lancaster].
Example A28
Correspondingly Example A23 with 4-methylphenylboronic acid [Lancaster].
Example A29
Correspondingly Example A23 with 4-methylthiophenylboronic acid [Lancaster].
Example A30
Correspondingly Example A23 with 3-trifluoromethylphenylboronic acid
[Lancaster].
Example A31
Correspondingly Example A23 with 4-carbonylphenylboronic acid [Aldrich].
Example A32
Correspondingly Example A23 with 3,4-(methylenedioxy)phenylboronic acid
[Aldrich].
Example A33
Correspondingly Example A23 with 4-vinylphenylboronic acid [Aldrich].
Example A34
Correspondingly Example A23 with 3-methoxyphenylboronic acid [Lancaster].
Example A35
Utilising the procedure of Example A51 with 1-(2-hydroxyethyl)morpholine
[Aldrich] in
place of 1-(2-hydroxyethyl)pyrrolidine.
Example A36
Utilising the procedure of Example A35 with 4-cyclohexylbenzoic acid
[Aldrich]. in
place of 4-biphenylcarboxylic acid.
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example A37
Utilising the procedure of Example A51 with 2-dimethylaminoethanol [Aldrich],
in place
of 1-(2-hydroxyethyl)pyrrolidine.
Example A39
Correspondingly Example A51 with (R)-(+)-1-methyl-2-pyrrolidinemethanol
(Patent
WO-9932480).
Example A41
Correspondingly Example A51 With 3-hydroxy-1-methylpiperidine [Aldrich].
Example A43
Correspondingly Example A51 with 2-dimethylamino-1-propanol [ICN-RF].
Example A45
Correspondingly Example A51 with 2-(diethylamino)-ethanol [Aldrich].
Example A47
Correspondingly Example A51 with (S)-(-)-1-methyl-2-pyrrolidinemethanol
[Aldrich].
Example A49
Correspondingly Example A51 with N-benzyl-N methylethanolamine [Aldrich].
Example A51
Biphenyl-4-carboxylic acid (3-methoxy-4-(2-pyrrolidin-I-yl-ethoxy)-phenyl
amide.
To a solution of the hydroxy amine, (1-(2-hydroxyethyl)-pyrrolidine)
[Aldrich], (1.87m1,
l6mmol) in dimethylforman ude was added portionwise sodium hydride [60%
dispersion
in oil, (544mg, l6rmnol). After stirring at room temperature for 10 minutes a
solution of
the halonitrobenzene, (1-chloro-2-methoxy-4-vitro-benzene) [Avocado] (3g,
l6mmol) in
dimethylformamide (1 Oml) was added dropwise. The reaction mixture was left
stirring at
room temperature for l6hrs then concentrated. The residue was dissolved in
ethyl acetate
26
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
(200m1) and washed with water (3 x SOmI). The organic phase was dried with
magnesium
sulphate, evaporated and the residue purified by flash chromatography on
silica gel using
dichloromethane - aq. ammonia - methanol as eluent to afford 1-[2-(2-methoxy-4-
nitro-
phenoxy)-ethyl]-pyrrolidine as a brown oil.
1H NMR (CDC13): 81.82 (4H, m), 2.65 (4H, m), 3.01 (2H, t), 3.94 (3H, s), 4.24
(2H, t),
6.92 (1H, d), 7.74 (1H, d), and 7.89(IH, dd); MS (AP +ve): m/z 267 [M+H]+.
To a solution of the amine, 1-[2-(2-methoxy-4-nitro-phenoxy)-ethyl]-
pyrrolidine (2.3g,
8.6mtnol) in ethanol (100m1) was added 10% Pd/C (SOmg). The mixture was
stirred at
room temperature under an atmosphere of hydrogen at atmospheric pressure for
16h, then
filtered through celite and the filtrate concentrated to give the
corresponding aiuline; 3-
methoxy-4-(2-pyrrolidin-1-yl-ethoxy)-phenylasnine, as a brown solid.
1H NMR (CDC13): b 1.80 (4H, m), 2.62 (4H, m), 2.89 (2H, t), 3.80 (3H, s), 4.06
(2h, t),
6.20 (1H, dd), 6.29 (1H, d) and 6.75 (1H, d); MS (AP +ve): srrlz 237 [M+H]+.
To the caxboxylic acid, (4-biphenyl carboxylic acid) [Aldrich] (47.Smg,
0.24mmo1)
suspended in dichloromethane (1m1) was added oxalyl chloride [Aldrich]
(0.06m1,
0.72nunol) followed by one drop of dimethylformamide. The reaction mixture was
stirred
at room temperature for 1 hour, concentrated, then co-evaporated three times
with
dichloromethane to give 4-phenylbenzoyl chloride. This was dissolved in
dichloromethane (1m1) and added to a solution containing the amine, (3-methoxy-
4-(2-
pyrrolidin-1-yl-ethoxy)-phenylamine), (47 mg, 0.2mmo1), triethylamine (0.14m1,
Immol)
and dichloromethane (1m1). The reaction mixture was stiiTed for 16 hours at
room
temperature, concentrated, re-dissolved in dichloromethane (lOml), filtered
through an
SAX column [Varian] (2g) and stirred with PS-isocyanate resin [Argonaut
Technologies]
(100mg, 0.38mmo1) for 16 hours. The mixture was filtered, evaporated then
purified by
flash chromatography on silica gel using dichloromethane - aq. ammonia -
methanol as
eluent to afford the title compound as an oil.
1H NMR (CDCl3): S I.88 (4H, m), 2.90 (4H, m), 3.08 (2H, t), 3.84 (3H, s), 4.2I
(2H, t),
6.83 (1H, d), 7.03 (1H, dd), 7.27-7.70 (8H, m) and 8.01 (2H, d); MS (AP+ve):
m/z 417
[M+H]+.
Example A54
27
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Utilising the procedure of Example A51 with 1-dimethylamino-2-propanol
[Aldrich] in
place of 1-(2-hydroxyethyl)-pyrrolidine.
Example A56
Correspondingly Example A51 with 1-(2-hydroxyethyl)-piperidine [Aldrich].
Example A58
Correspondingly Example A51 with 2-(hexamethyleneamino)-ethanol [Lancaster].
Example A60
Utilising the procedures of Example A93 with 3-aminophenylboronic acid in
place of 2-
methoxyphenylboronic acid and Example 51 with 2-dimethylaminoethanol in place
of 1-
(2-hydroxyethyl)pyrrolidine.
Example A63
Utilising the procedure of Example A60 with 4-carboxyphenylboronic acid
[Aldrich] in
place of 3-aminophenylboronic acid.
Example A70
Correspondingly Example A63 with (3,4-methylenedioxyphenyl)boronic acid
[Aldrich].
Example A72
Utilising the procedure of Example 51 with N (2-phenyl)-ethyl-N methyl-
ethanolamine
[J. Org. Chem. 1985, 50(22), 4359] in place of 1-(2-hydroxyethyl)-pyrrolidine.
Example A74
Correspondingly Example 51 with 2-dimethylaminocyclohexanol [J. Chem. Soc. C
(1969), (2), 248-52].
Example A76
Correspondingly Example 51 with 2-(1,2,4,5-tetrahydro-benzo[d]azepin-3-yl)-
ethanol
[Patent US-394682]
28
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example A78
Correspondingly Example 51 with 2-(3,4-dihydro-1H-isoquinolin-2-yl)-ethanol
[Patent
WO-9719926].
Example A80
Correspondingly Example 51 with 2-(4-phenyl-piperazin-1-yl)-ethanol
[J. Med. Chem. 1994, 37(13), 1964].
Example A82
Correspondingly Example 51 with 1-methyl-3-pyrrolidinol
[Aldrich].
Example A84
Utilising the procedures of Example A93 with 4-methoxy-phenylboronic acid
[Aldrich]
in place of 2-methoxyphenylboronic acid and Example A51 with 2-
diethylaminoethanol
in place of 1-(2-hydroxyethyl)pyrrolidine.
Example A88
Utilising the procedures of Example A84 with 4-methoxy-3-pyridylboronic acid
[Patent
WO-9924440] in place of 4-methoxy-phenylboronic acid.
Example A89
Correspondingly Example A88 with 2-methoxy-3-pyridylboronic acid [Patent WO-
9910331].
Example A90
Correspondingly Example A88 with benzo-[b]-furan-2-boronic acid [Aldrich].
Example A91
Correspondingly Example A88 with thiophene-3-boronic acid [Aldrich].
29
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example A92
Correspondingly Example A88 with indole-5-boronic acid [Frontier].
Example A93
4'-Methyl-biphenyl-4-carboxylic acid [3-methoxy-4-(2-pyrrolidin-1-yl-ethoxy)-
phenyl]-amide
A mixture of 3-methoxy-4-(2-pyrrolidin-1-yI-ethoxy)-phenylamine [Example A51]
(4.7mM I.Ig) and triethylamine (I4mmol) was treated with 4-brornobenzoyl
chloride
[Aldrich] in dichloromethane (20m1) and kept at room temperature for I6 hours.
The solvent was evaporated and the crude product purified by chromatography on
silica gel using dichloromethane - methanol - aq. ammonia to afford 4-bromo
-N [3-methoxy-4(2-pyrrolidin-1-yl-ethoxy)-phenyl]-benzamide as a white solid
in 72%
yield.
1H NMR (DMSO-d6): 8 7.91 (2H, dd), 7.73 (2H, dd), 7.50 (1H, d), 7.30 (1H, dd),
6.94 (1H, d), 4.02 (2H, t), 3.76 (3H, s), 2.77 (2H, t), 2.51 (4H, m under DMSO-
d-5
signal) and 1.67 (4H, m); MS: (ES+ve) m/z 419, 421 [M+H]+
The amide, 4-bromo-N [3-methoxy-4(2-pyrrolidin-1-yl-ethoxy)-phenyl]-benzamide
(O.lmM 42mg), and 4-methyl-benzene boronic acid [Aldrich] (O.lmM l4mg) were
refluxed for 16 hours in a mixture of benzene (8m1), ethanol (2m1) and 2M
aqueous
sodium carbonate (2m1) in the presence of tetrakis-(triphenylphosphine)-
palladium[0]
(5mg) under an argon atmosphere. The mixture was cooled, the upper Iayer
decanted,
and this solution purified by chromatography on silica gel using
dichloromethane:
methanol (10:1) followed by acetonitrile: satd. aqueous ammona (25:1) to
afford the
title compound as a white solid.
1H NMR (CDC13): 8 7.92 (2H, dd), 7.68 (2H, dd), 7.50 (2H, dd), 7.26 (3H,
dddd),
6.96 ( 1 H, dd), 6. 8 8 ( 1 H, d), 4.13 ( 1 H, t), 3 .87 (3 H, s), 2.92 (2H,
t), 2.60 (4H, m),
2.41 (3H, s) and 1.80 (4H, m); MS: (AP-ve) nz/z 429 [M-H]-; (AP+ve) m/z 431
[M+H]+,
Example A100
Utilising the procedure of Example A93 with 4-(2,6-dimethoxypyrimidinyl)-
boronic acid
[Frontier] in place of 4-methyl-benzene boronic acid.
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example A103
Correspondingly Example A93 with furan-3-boronic acid [Frontier].
Example A144
Correspondingly Example A93 with mesityl-boronic acid [Frontier].
Example AlOS
Utilising the procedure of Example A51 except employing chloroform in place of
dichloromethane as a solvent and eluent and utilising 3-quinuclidinol
[Aldrich] in place
of 1-(2-hydroxyethylpyrrolidine)
Example A107
Utilising the procedure of Example B37 except using piperidine in place of
aniline.
Example B1
Utilising the procedure of Example A7 with 3-phenoxybenzoic acid [Aldrich] in
place of
2'-methyl-biphenyl-4-carboxylic acid.
Example B2
Correspondingly Example B1 using 4-benzylbenzoic acid [Apin].
Example B34
Correspondingly Example Bl using 3-benzylbenzoic acid [Patent WO-9828268].
Example B35
Correspondingly Example B1 using 4-phenoxybenzoic acid [Aldrich].
Example B37
N [-[3-Methoxy-4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-4-phenylamino-benzamide
Dry cesium carbonate (O.lSmM, 49mg), (S)-B1NAP [Aldrich] (0.015 mM, 9mg) and
palladium acetate (0.0075rnM, 2mg) were sonicated in anhydrous ethyleneglycol
31
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
dimethyl ether (15 ml) for 40 minutes under an argon atmosphere. This
suspension was
treated with 4-bromo-N [3-methoxy-4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-
benzamide
[Example A93] (0.lmM, 42mg) and aniline (0.1 lmM, lOmg) then refluxed for 40
hours.
The suspension was filtered through a hydrophobic membrane, concentrated, then
purified on C 18 R.P. silica using acetonitrile:water to afford the title
compound as a white
solid.
1H NMR (MeOH-d4): 8 7.96 (2H, dd) 7.92 (1H, d), 3.1 (2H, dd), 7.20 (1H, dd),
7.04
(1H, d), 4.28 (2H, t), 3.92 (3H, s), 3.78 (2H, m), 3.60 (2H, t), 3.58-3.13
(6H, m) and 2.26-
1.47 (10H, m); MS: (ES+ve) m/z 424 [M+H]+
Example C1
Utilising the procedure of Example A7 with 2-methylbiphenyl-4-carboxylic acid
[Patent
WO-9606079] in place of 2'-methyl-biphenyl-4-carboxylic acid.
Example C2
Correspondingly Example CI using 3-methoxybiphenyl-4-carboxylic acid [Patent
WO-
9534540].
Example C3
Correspondingly Example C1 using 3-methylbiphenyl-4-carboxylic acid [Patent WO-
9534540].
Example C4
Correspondingly Example C1 using 4-phenylthiophene-2-carboxylic acid [Specs].
Example CS
Correspondingly Example C1 using 4-(3,5-dichlorophenoxy)-furan-2-carboxylic
acid
[Maybridge].
Example C6
Correspondingly Example C1 using 5-methyl-1-phenylpyrazole-4-carboxylic acid
[Maybridge] .
32
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example C7
Correspondingly Example C1 using 6-phenyl-nicotinic acid [WO-0006085].
Example C8
Correspondingly Example C1 using 3-chloro-biphenyl-4-carboxylic acid [Patent
JP-
09221476] .
Example C9
Correspondingly Example Cl using 5-(4-chlorophenyl)-2-trifluoromethyl-furan-3-
carboxylic acid [Maybridge].
Example C10
Correspondingly Example C1 using 2-(4-chlorophenyl)-3-(trifluoromethyl)-
pyrazole-4-
carboxylic acid [Maybridge].
Example C11
Correspondingly Example Cl using 5-(2-pyridyl)-thiophene-2-carboxylic acid
[Maybridge] .
Example C12
Correspondingly Example C1 using 5-(methyl-trifluoromethyl-2-H-pyrazol-3-yl)-
thiophene-2-carboxylic acid [Maybridge].
Example Dl
Utilising the procedure of Example DS with 3,4-dichloronitrobenzene [Aldrich]
in place
of 2,4-dichloronitrobenzene.
Example DS
Biphenyl-4-carboxylic acid [2-chloro-4-(2-diisopropylamino-ethoxy)-phenyl]-
amide.
To a three-neck flask (fitted with condenser, dropping funnel and themnometer)
containing iron powder (938mg, 16.8mmo1) mixed with a solution of ammonium
chloride
33
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
(28mmol) in water (28m1), was added the amine [2-(3-chloro-4-nitro-phenoxy)-
ethyl]-
diisopropyl-amine [prepared by the method used to form 1-[2-(2-methoxy-4-nitro-
phenoxy)-ethyl]-pyrrolidine in Example A51 but with 2-4-dichloronitrobenzene
[Aldrich] in place of 4-chloro-3-methoxynitrobenzene and 2-
diisopropylaminoethanol in
place of 1-(2-hydroxylethyl)-pyz~olidine], dropwise over 10 minutes. The
reaction
mixture was gently xefluxed until t.l.c. analysis showed no starting material.
The mixture
was filtered while hot and the inorganic residues washed with methanol. The
combined
filtrates wexe partitioned between water (5m1) and ethyl acetate(3 x 1 Oml),
the organic
phase dried (MgS04), filtered, and evaporated. The aqueous phase was treated
with satd.
aq. sodium bicarbonate (lOml), extracted with ethyl acetate (3 x 10m1), dried
(MgS04),
and evaporated. Residues from both extractions were combined and purified by
flash
chromatography on silica gel using dichloromethane - methanol - aq. ammonia as
eluent
to affoxd 2-chloro-4-(2-diisopropylamino-ethoxy)-phenylamine as a brown oil.
1H NMR (CDCIg): 8 1.02(12H, d), 2.77(2H, t), 3.03(2H, sept.), 3.72(2H, bs),
3.80(2H, t),
6.68(2H, m) and 6.85(1H, m); MS (AP+ve): m/z 271, 273 [M+H]+.
This material was used in place of 3-methoxy-4-(2-pyrrolidin-1-yl-ethoxy)-
phenylamine
in the procedure of Example A51 to afford the title compound as clear oil.
1H NMR (CDC13): b 1.26 (12H, d), 3.07 (2H, m), 3.35 (2H, m), 4.22 (2H, m),
6.89 (1H,
dd), 7.01 (1H, m), 7.44 (3H, q), 7.62 (2H, d), 7.71 (2H, d), 7.97 (2H, d) and
8.34 (1H, d);
MS (AP+ve): m/z 452, 454 [M+H]+.
Example D9
Utilising the procedure of Example A51 with 2,4-difluoronitrobenzene [Aldrich]
in place
of 4-chloro-3-methoxynitrobenzene.
Example D12 [WO-00/06146]
Utilising the procedure of Example A51 with 3,4-difluoronutrobenzene [Aldxich]
in place
of 4-chloro-3-methoxynitrobenzene.
Example D16
34
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Utilising the procedure of Example A51 with 2-methyl-4-fluoronitrobenzene
[Aldrich] in
place of 4-chloro-3-methoxynitrobenzene
Example D20
Utilising the procedure of Example A51 with 3-methyl-4-fluoronitrobenzene
[Aldrich] in
place of 4-chloro-3-methoxynitrobenzene
Example D24
Utilising the procedure of Example A51 with 3-acetyl-4-fluoronitrobenzene
[Aldrich] in
place of 4-chloro-3-methoxynitrobenzene
Example DZS
Biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-2-formyl-5-methoxy-
phenyl]-amide
Biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-3-methoxy-phenyl]-
amide
[Patent WO-9901127] (223 mg, 0.5 mmol) was treated with glyoxylic acid
trihydrate
(1m1), dichloromethane (5 ml) and methanesulphonic acid (0.5 ml). The mixture
was
stirred vigorously for 24 hours then treated with satd. aq. sodium bicarbonate
(30m1) and
extracted with dichloromethane (3 x 20m1). The combined organic phases were
dried
(MgS04), filtered and evaporated, then subjected to flash chromatography on
silica gel
[chloroform - methanol - aqueous acetic acid] to obtain the title compound as
the acetate
salt, a white solid.
1H NMR (CDCl3): ~ 1.13 (12H, d), 2.04 (3H, s), 3.02 (2H, t), 3.20 (2H, kept.),
4.05 (3H,
s), 4.10 (2H, t), 5.0 (1H, bs), 7.22 (1H, s), 7.40 (1H, t), 7.48 (2H, d), 7.65
(2H, d), 7.76
(2H, d), 8.14 (2H, d), 8.72 (1H, s) and 9.34 (1H, s); MS (AP+ve): m/z 475
[M+H+].
Example D26
Biphenyl-4-carboxylic acid [4-(2-diethylamino-ethoxy)-3-(1-hydroxy-ethyl)-
phenyl]-
amide
To biphenyl-4-carboxylic acid [3-acetyl-4-(2-diethylamino-ethoxy)-phenyl]-
amide
[Example D24] (20mg, O.OSmmol) dissolved in a 1:1 mixture of tetrahydrofuran l
ethanol (3m1), was added sodium borohydride [Aldrich] (6mg, O.lSmmol). The
reaction
mixture was stirred at ambient temperature for 16 hours. The solvent was
evaporated and
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
the residue purified by flash chromatography on silica gel using
dichloromethane - aq.
ammonia - methanol as eluents, to afford the title compound as a white solid.
1H NMR (CDC13): 8 1.09 (6H, t), 1.49 (3H, d), 2.75 (4H, q), 2.95 (2H, t),
4.15(2H, t),
5.01 (1H, q), 6.84 (1H, d), 7.45-7.67 (9H, m) and 7.95 (2H, d)
MS (AP+ve): n~/z 433 [M+H+]
Example D27
Biphenyl-4-carboxylic acid (4-(2-diethylamino-ethoxy)-3-ethyl-phenyl]-amide
To biphenyl-4-carboxylic acid [3-acetyl-4-(2-diethylamino-ethoxy)-phenyl]-
amide
[Example D24] (25mg, 0.06mmol) dissolved in dichloromethane (1.5m1), was added
triethylsilane (O.SmI) and trifluoroacetic acid (0.25m1). The resulting yellow
solution was
stirred at room temperature for 120h. The solvents were evaporated and the
residue
purified by flash chromatography on silica gel using dichloromethane - aq.
ammonia -
methanol as eluents to afford the title compound as white solid.
1H NMR (CDC13): b 1.17 (6H, m), 2.64 (2H, q), 2.8 (4H, c~, 3.06 (2H, t), 4.15
(2H, t),
6.82 (1H, d), 7.35-7.71 (9H, m) and 7.96 (2H, d)
MS (AP+ve): m/z 417 (M+H]+
Example D28 [W09901127]
Utilising the procedure of Example A51 with 4-fluoronitrobenzene [Aldrich] in
place of
4-chloro-3-methoxynitrobenzene, and 2-diisopropylaminoethanol in place of 1-(2-
hydroxyethyl)-pyrrolidine
Example D30 [W09901127]
Utilising the procedure of Example D28 with 2-dimethylaminoethanol [Aldrich]
in place
of 2-diisopropylaminoethanol.
Example D32 (W09901127)
Utilising the procedure of Example D28 with 2-diethylaminoethanol [Aldrich] in
place
of 2-diisopropylaminoethanol
Example D38 [W09901127]
36
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Utilising the procedure of Example A22 with 4-fluoronitrobenzene [Aldrich] in
place of
4-chloro-3-methoxynitrobenzene, and 4-ethylphenylboronic acid in place of 4-
methoxyphenylboroiuc acid
Example D39 [W09901I27)
Utilising the procedure of Example A84 with 4-fluoronitrobenzene [Aldrich] in
place of
4-chloro-3-methoxynitrobenzene, and 4-ethylphenylboronic acid in place of 4-
methoxyphenylboronic acid.
Example E1
Biphenyl-4-carboxylic acid [4-(2-diisopropylamino-ethoxy)-3-methoxy-phenyl]-
methyl-amide.
To 4-(2-diisopropylamino-ethoxy)-3-methoxy-phenylamine (lmmol) [Example A7]
were added triethylorthoformate (8m1) and trifluoroacetic acid (0.15m1). The
resulting
solution was heated to 90°C for 4hr. The solution was evaporated then
redissolved in
ethanol and cooled to approximately -10°C. Sodium borohydride (190mg,
Smmol) was
introduced portionwise over 10 minutes then the mixture allowed to warm to
room
temperature. The solution was stirred at room temperature for 16h, then
acidified to pH 1
with 2M hydrochloric acid. The mixture was concentrated to approximately l
OmI, then
partitioned between ethyl acetate and water. The aqueous phase was adjusted to
pH 14
using 2M aq sodium hydroxide solution, and extracted with dichloromethane
(x3), dried
(MgS04), filtered and evaporated. The residue was purified by flash
chromatography on
silica gel using dichloromethane - aq. ammonia - methanol as eluent to afford
[4-(2-
diisopropylamino-ethoxy)-3-rnethoxy-phenyl]-methyl-amine as an oil.
~H NMR (CDC13): 8 1.03 (12H, d), 2.80 (3H, s), 2.85 (2H, t), 3.02 (2H, q),
3.80 (3H, s),
3.86 (2H, t), 6.13 (1H, dd), 6.23 (1H, d) and 6.80 (1H, d); MS (AP+ve): yrrlz
281[M+H]+.
To 4-phenylbenzoic acid (0.2mmo1) suspended in dichloromethane was added
oxalyl
chloride (0.6mmo1) followed by dimethylformamide (1 drop). The reaction
mixture was
stirred for 1h, evaporated, co-evaporated (x3) with dichloromethane then
redissolved in
dichloromethane(lml). A solution containing the amine [4-(2-diisopropylamino-
ethoxy)-
3-methoxy-phenyl]-methyl-amine (0.2mrno1) and triethylamine (140mg, lrnmol)
dissolved in dichloromethane (1m1) was added. This solution was stirred at
ambient
37
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
temperature for 14 hours, evaporated, dissolved in dichloromethane (1m1) and
treated
with PS-isocyanate resin [Argonaut Technologies] (150mg). After a further 18h
shaking
at ambient temperature, the mixture was filtered, passed through an SAX column
[Varian] (1g), evaporated, and the residue purified by chromatography on
silica gel using
dichloromethane - aq. ammonia - methanol as eluent to afford the title
compound as an
oil.
1H NMR (CDC13): b 1.21 (12H, bd), 2.88-3.24 (4H, m), 3.32 (3H, s), 3.87 (3H,
s), 4.11
(2H, m), 6.82-6.91 (3H, m) and 7.26-7.56 (9H, m); MS (AP+ve): fnlz 476 [M+H]+.
Example ES
Utilising the procedure of Example EI with triethyl orthoacetate [Aldrich] in
place of
triethyl orthoformate.
Example E12
1 S Biphenyl-4-carboxylic acid [2-chloro-4-(2-diisopropylamino-ethoxy)-5-
methoxy-
phenyl]-amide
Biphenyl-4-carboxylic acid [4-(2-diethylamino-ethoxy)-3-methoxy-phenyl]-methyl-
amide [Example E9] (45mg, 0.1 mmol), was dissolved in chloroform (1m1) and
treated
with benzotriazole [Aldrich] (12 mg, 0.1 mmol) and N chlorosuccinimide (13 mg,
0.11mmo1). The mixture was stirred at ambient temperature for 16 hours then
evaporated
and subjected to flash chromatography on silica gel (dichloromethane -
methanol -
aqueous ammonia) to afford the title compound as an oil.
1H NMR (CDCl3): 8 1.06 (6H, t), 2.63 (4H, q), 2.90 (2H, t), 3.39 (3H, s), 3.67
(3H, s),
4.03 (2H, t), 6.57 (1H, s), 6.84 (1H, s) and 7.31-7.53 (9H, m); MS (AP+ve):
mlz 467, 469
[M+H]+.
Example E13
Utilising the procedures of Example A93 with [4-(2-diethylamino-ethoxy)-3-
rnethoxy-
phenyl]-methyl-amine [Example E9] in place of 4-(2-diethylamino-ethoxy)-3-
methoxy-
phenylamine and 2-fluoromethylphenylboronic acid [Aldrich] in place of 4-
methoxyphenylboronic acid and of Example 51 with (N-diethyl)ethanolamine in
place of
1-(2-hydroxyethyl)pyrrolidine.
38
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example E14
Utilising the procedure of Example E13 with 2-methylphenylboronic [Aldrich] in
place
of of 4-chlorophenylboronic acid.
Example E26
Correspondingly Example E14 with 2-chloromethylphenylboronic acid [Aldrich].
Example E17
Correspondingly Example E14 with 4-fluoromethylphenylboronic acid [Aldrich].
Example E21
Correspondingly Example E14 with 4-chloromethylphenylboronic acid [Aldrich].
Example E22
Correspondingly Example E14 with 4-ethylphenylboronic acid [Aldrich].
Example E23
Correspondingly Example E14 with 4-tef°tbutylphenylboronic acid
[Aldrich].
Example E24
4-Biphenylcarboxylic acid [4-(2-diethylamino-ethoxy)-3-methoxy-phenyl]-methyl-
amide
[Example E9] (45mg, 0.lmmol), was dissolved in acetonitrile (1m1) and treated
with N
fluoro-N'-chloromethyl-triethylenediamine-bis(tetrafluoroborate) (43mg,
0.12mmo1) and
heated to ~OoC for 6 hours. The solvent was evaporated and the residue
subjected to
flash chromatography on silica gel (dichloromethane - methanol - aqueous
ammonia) to
afford the title compound as an oil.
MS (AP+ve): m/z 451 [M+H]+.
Example E25
TJtilising the procedure of Example El with 4-(2-diisopropylamino-ethoxy)-3-
methyl-
phenylamine [Example D20] in place of 4-(2-diisopropylamino-ethoxy)-3-methoxy-
phenylamine and triethyl orthoacetate in place of triethyl orthoformate.
39
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example F1
Utilising the procedure of Example A7 with 6-phenyl-nicotinic acid (Patent WO-
0006085) in place of 2'-methyl-4-biphenylcarboxylic acid and N-
dimethylethanolamine
in place of 2-(diisopropylamino)ethanol.
Example G1
Biphenyl-4-carboxylic acid [4-((R)-diethylamino-hydroxy-propoxy)-3-methoxy-
phenyl]-amide
4-Nitro-2-methoxyphenol [Aldrich] (845mg, 5mmo1) was dissolved in DMF (25 ml)
and
treated with sodium hydride (60% oil dispersion, 200mg). When the
effervescence
ceased, the mixture was treated with (R)-p-nitrophenylsulphonyl glycidol
[Aldrich] and
warmed to 50°C with stirring. After 16 hours, the mixture was cooled,
evaporated,
partitioned between water (20m1) and dichloromethane (3 x 25m1), dried
(MgS04),
filtered and evaporated. The residue was purified by flash chromatography on
silica gel
(hexane - ether) to give (R)-2-(2-methoxy-4-vitro-phenoxymethyl)-oxirane as a
pale
brown solid in 80% yield.
1H NMR (CDCl3): ~ 2.79 (1H, dd), 2.95 (1H, dd), 3.41 (1H, dddd), 3.96 (3H, s),
4.06
(1H, dd), 4.43 (1H, dd), 6.98 (1H, d), 7.75 (1H, d) and 7.87 (1H, dd).
(R)-2-(2-Methoxy-4-vitro-phenoxymethyl)-oxirane (0.5mmol, 113mg), in
dichloromethane (3 mI) was treated with the amine (diethylamine) [Aldrich]
(1.5 mmol,
110 mg) and titanium tetraisopropoxide [Aldrich] (50u1). The solution was
stirred at
ambient temperature for 24 h, treated with water (1m1) and shaken vigorously
for 10
minutes. The resulting suspension was passed through a hydromatnx cartridge
[Varian
ChemElut] (5m1) eluting with dichloromethane (lOml) to give (R)-diethylamino-
(2-
methoxy-4-vitro-phenoxy)-propan-2-of as a yellow oil
1H NMR (CDC13): 8 1.07 (6H, t), 2.55-2.72 (7H, m), 3.94 (3H, s), 4.09-4.13
(3H, m),
6.97 (1H, d), 7.74 (1H, d) and 7.89 (1H, dd); MS (AP+ve): m/z 299 [M+H~].
This material was dissolved in ethanol (5m1) and treated with hydrogen
chloride (2M in
diethyl ether) 0.1 nnl then 10% palladium on charcoal (20 mg) and hydrogenated
at
atmospheric pressure for 24 hours. The solution was purged with argon then
filtered
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
through celite and evaporated to give (R)-(4-amino-2-methoxy-phenoxy)-
diethylamino-
propan-2-of hydrochloride as a white crystalline solid.
1H NMR (CD30D): 8 1.19 (6H, t), 3.36-3.45 (6H, m), 3.88 (s, 3H), 4.02-4.11
(2H, m),
4.03 (1H, m), 6.95-7.03 (2H, m) and 7.13 (1H, d).
A solution of this material in dichloromethane (2m1) was treated with
triethylamine (2
mmol, 280u1) and triethylsilyl trifluoromethanesulphonate (lmmol, 264mg).
After 30
minutes, 4-biphenylcarboxylic acid chloride [Example 1] (lmmol, 217mg) was
introduced and the mixture stirred for 12 hours. The solvent was evaporated
and the
residue dissolved in methanol (100m1) and treated with potassium carbonate
(2g). After
stirring for six hours, the suspension was evaporated, formed into a slurry
with
dichloromethane (20m1), filtered, the filtrate evaporated, and the residue
purified by flash
chromatography (dichloromethane - methanol - aq. ammonia) to give the title
compound
as a white solid.
1H NMR (CDC13): 8 1.11 (6H, t), 2.61-2.78 (6H, m,), 3.88 (3H, s), 3.5-4.5 (1H,
vbs),
3.99-4.13 (3H, m), 6.92 (1H, d), 6.99 (1H, dd), 7.41-7.49 (3H, m), 7.56 (1H,
d), 7.63 (2H,
d), 7.69 (2H, d) and 7.97 (3H, d); MS (AP+ve): m/z 449 [M+H+].
Example GS
Utilising the procedure for the preparation of (R)-diethylamino-(2-methoxy-4-
nitro-
phenoxy)-propan-2-of [Example Gl] but replacing dichloromethane with 1,2-
dichloroethane and diethylamine with diisopropylamine. In addition, the
mixture of
amine and epoxide was heated at 80oC for 12h rather than being kept at ambient
temperature for 24 hours.
Example G8
Utilising the procedure of Example G1 but using (S)-p-nitrophenylsulphonyl-
glycidol in
place of (R)-p-nitrophenylsulphonyl-glycidol, and pyrrolidine in place of
diethylamine.
Example G22
Utilising the procedure of Example A51 but using 4-dimethylamino-1-butanol
[ICN-RF]
in place of 1-(2-hydroxyethyl)-pyrrolidine.
41
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Example Hl
4-Cyclohexyl-N-(3-methoxy-4-(4-methyl-piperazin-1-yl)-phenyl]-benzamide
A solution of 1-(2-methoxy-4-vitro-phenyl)-piperazine (Patent WO-9906382) (1
Ommol,
2.37g) in dichloromethane (50m1) was treated with diter~tbutyl dicarbonate
(lOmmol,
2.18g) with stirring. Vigorous evolution of gas occurred which ceased after 1
hour. The
solution was then evaporated to a yellow solid 4-(2-methoxy-4-vitro-phenyl)-
piperazine-
1-carboxylic acid tertbutyl ester.
1H NMR (CDCl3): cS 1.50 (9H, s), 3.16 (4H, t), 3.61 (4H, t), 3.96 (3H, s),
6.88 (1H, d),
7.72 (1H, d) and 7.86 (1H, dd).
This material was dissolved in ethanol (50m1) and treated with 10% Pd on
carbon
(100mg). The suspension was hydrogenated at 1 atmosphere for 2 hours, then
filtered
through celite and evaporated to give 4-(4-amino-2-methoxy-phenyl)-piperazine-
I-
carboxylic acid tertbutyl ester as a brown oil.
1H NMR (CDCl3): S 1.48 (9H, s), 2.86-2.91 (4H, t), 3.52-3.60 (4H, t), 3.81
(3H, s), 6.22-
6.27 (2H, m) and 6.73 (1H, d).
This aniline (0.2rnmol, 6I mg) was dissolved in dichloromethane (1m1) and
treated
successively with DIEA resin [Argonaut Technologies] (0.5 g) and 4-
cyclohexylbenzoyl
chloride [Example A36]. The mixture was shaken gently for 12 hours then
filtered,
evaporated and the residue purified by flash chromatography on silica gel
(dichloromethane - methanol - aq. ammonia) to afford 4-(4-{ [1-(4-cyclohexyl-
phenyl)-
methanoyl]-amino)-2-methoxy-phenyl)-piperazine-1-carboxylic acid tertbutyl
ester as a
white crystalline solid
1H NMR (CDC13): ~ 1.25-1.47 (5H, m), 1.54 (9H, s), 1.75-1.88 (5H, m), 2.56
(1H, m),
2.98 (4H, t), 3.61 (4H, t), 3.91 (3H, s), 6.87 (1H, d), 6.93 (1H, dd), 7.32
(2H, d), 7.54
(1H, s), 7.77, (1H, s) and 7.78 (2H, d); MS (AP+ve): m/z 493 [M+H+].
This material was dissolved in dichloromethane (5m1) and treated with anisole
(1m1) and
trifluoroacetic acid (5m1). After 2 hours the solution was evaporated, then co-
evaporated
twice from toluene. The residue was dissolved in dichloromethane (lOml),
washed with
satd. sodium bicarbonate (2m1), the organic phase dried (MgS04), fzltered and
evaporated
to a brown oil, 4-cyclohexyl-N (3-methoxy-4-piperazin-1-yl-phenyl)-benzamide.
42
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
1H NMR (CDCl3): 8 1.22-I.87 (10, m), 2.57 (l, m), 3.04-3.12 (8H, m), 3.9I (3H,
s), 6.95
(2H, bs), 7.32 (2H, d), 7.54 (1H, m), 7.77 (1H, s) and 7.78 (2H, d); MS
(AP+ve): n~/z 394
[M+H+].
This amine (O.lmmol, 39mg) was dissolved in ethanol (3m1) and treated with
metafolmaldehyde (100mg), Amberlyst cyanoborohydride resin [Novabiochem]
(100mg), and acetic acid (50u1). The mixture was stirred at ambient
temperature for three
hours then filtered, evaporated and the residue purified by flash
chromatography on silica
gel (dichloromethane - methanol - aq. ammonia) to afford the title compound as
a pale
brown oil. Tlus was evaporated from dilute acetic acid to give the monoacetate
salt
hydrate.
1H NMR (CDC13): ~ 1.22-1.45 (5H, m), 1.76-1.87 (5H, m), 2.02 (6H, 2xs), 2.56
(1H, m),
3.22-3.23 (4H, t), 3.29-3.30 (4H, t), 3.88 (3H, s), 6.86 (1H, d), 6.94 (1H,
dd), 7.30 (1H,
d), 7.59 (1H, d), 7.79 (2H, d), 7.98 (1H, s) and 8.54 (4H, bs);
MS (AP+ve): m/z 408 [M+H~].
The following tables give Examples which illustrate but do not limit the
invention in any
way.
43
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table A
Encompassing compounds of general formula (II), a subset of formula (I) where
A = H
and OMe, R3 = H, X = O, Y = CH2CH~ , Z = a bond; R4 = Ph and RS is either meta
or
para substituted on R4.
eR1
O I ~ O~N~R2
\ H / Oi
R5
(II)
Example RS ~1 meta / [M+H]+ Procedure
No. N ara
.. O~ wR2 p
A1 Ph ~- p 447 A7
.O~N~
A2 ~~N p 453 A7
/ \N
A3 ~,N~ ~ p 437 A7
~N___ ;.off--N
A4 Ph ~- m 447 A7
.O~N~
AS N ~ p 448 A7
'I O~N
A6 ,, o ~ p 489 A7
/ \ ; .o~-N
A7 ~ p 461 A7
__ _ ; .0~..~.N~
A8 ~ p 453 A7
. 'O~N~
44
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A9 p 453 A7
~ \ ___ _ --o
. '~--1'
A10 \N \ ____ ~ p 451 A7
N_ . .o~'N~
All ,N _ ~ p 529 A7
~ ~ _ ..o~~--N
~N
A12 -~ ~ p 461 A7
-O~'N
A13 / I ~- m 472 A7
- .. \ vN : .O~'N~
A14 ~~ , ~- p 52S A7
,, ~ \ so - .o~/--N
A15 - _ / ~ ~ m 453 A7
O~N
S
A16 S ~ ~ m 453 A7
\ . : .O~N~
A17 O ~ ~ ____ ~- p 489 A7
\~ - -O~N
A18 New ~- p 486 A7
/ I :-O~N
A19 N-N ~ ~ _ ~ p 529 A7
/'O -:ofN
A20 S ~ p 453 A7
____ ;-O~--N
A21 N ~ p 449 A7
i \ ____ - o~~'
'=-N
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A22 ~ ~ ~ ~ p 477 A22
:.O~N~
A23 F - ~ _ ~ p 515 A22
.O~_"'N~
FF
A24 H2N / ~ p 462 A22
\ ..0~"-N I
A25 ~ \ ~- p 553 A22
,.0~_"N~
_-
\ ___
A26 / \ ~. ~- p 497 A22
O~N
A27 . ~ p 497 A22
\ ~ _._ .: ~~N~
A28 -- _ ~- p 461 A22
_ : O~N
A29 --- _ ~ p 493 A22
.O~N~
A30 F ~ p 515 A22
'~ ; .o~--N~
FF I
46
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A31 ~ -- ~ p 475 A22
_
:.O~N~
A32 JO ~ p 491 A22
.-.- "'_N
O ..,0~
~ ~
A33 -- ~- p 473 A22
__ ..O~N~
_
A34 ~ ,' ~ p 477 A22
O / ~ , o~N~
A35 Ph ~~ p 433 A51
.O~N~
A36 ~0 p 439 A51
___ ,~~N~
A37 Ph ~ p 397 A51
,,.0~~"'N~
A38 ~ p 391 A51
___
A39 [ p 423 ASI
___
A40 Ph [ p 417 A51
A41 Ph p 417 A51
Nw
A42 p 423 A51
___ ''O N\
47
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A43 Ph ~ p 405 A51
..O~Nw
A44 N p 411 A51
___ _,0
A45 Ph ~ p 419 A51
._O-'~N~
A46 ~ p 425 A51
_ _ _ ,_o~N~/
A47 Ph ~\~/~~ p 417 A51
---O
A48 ~\~/~~ p 423 A51
___ ___O
A49 Ph ~-Ph p 467 A51
.: o~N~
A50 ~-Ph p 473 A51
___ ,,,o~N~
AS I Ph p 417 A51
,,.0~'-"N
A52 p 423 A51
___ ,,,o\~---N
A53 ~ 0~ ~ p 421 A22
.. . ...0~'_'N~
A54 Ph , ,O N/ p 405 A51
A55 _-_ , ,O N/ p 411 A51
48
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A56 Ph ~ p 431 A51
,,.0~"N
A57 ~ p 437 A51
___ ,, O~"-N
A58 Ph P 445 A51
. .p~-N
A59 P 451 A51
_ _ _ , -O~--N
A60 HZN / 406 A60
p
,,,o~/--N\
A61 Ph~~ ~ p 497 A63
,,.O~N\
A62 CFs ~ 459 A63
,,,0~~"N p
A63 ~-'' N p 419 A63
Hoc ~ ''off \
A64 ~ N p 417 A63
-o
A65 ~~ i ~ p 421 A63
,,,o~/--N
\
A66 ~ p 441 A63
w v ~ , .O~N\
A67 /. /- ~ p 441 A63
..off--N\
\ \
A68 / ~ p 404 A63
,,.off--N~
A69 / ~ p 437 A63
~ ~ ~! , ,o~N~
s
A70 /O / I --- - ~ p 434 A63
.O~N~
49
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A71 ~--' N p 459 A63
F3C ~ I W ~ v
A72 Ph ~ 481 A51
~~' ~N\ I / p
A73 ~--' 'w ~ ~ p 487 A51
Nw I ~
A74 Ph -~-~ p 445 A51
A75 ---~ p 451 A51
N
A76 Ph o~N I ~ p 493 A51
i
A77 ~-' 1,~N ~ ~ p 499 A51
Q i
A78 Ph o N I ~ p 479 A51
i
A79 ~--' o N I ~ p 485 A51
i
A80 Ph o N~ p 508 A51
~N~Ph
A81 ~-' O ~ p 514 A51
N.Ph
A82 Ph a p~,~ p 403 A51
~~N
A83 ~--' - ,p~ p 409 A51
~N~\
A84 i /~ p 449 A84
W I ... . .O~N~
O
A85 i /~ p 445 A88
- .O~/~'N~
A86 ~ ~ p 487 A88
I .. . 'o~N~/
F3C
A87 ~ ___- /~ p 425 A88
- .O~"N~
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A88 i /~ p 450 A88
.O~N~./
O N
A89 \ I -''- / ' /O N p 450 A88
N O
A90 \ ~ ~ ___ ' .OWN p 459 A88
o ~/
A91 j ___ '- o~N p 425 A88
A92 N \ ~'' ' 'OWN p 458 A88
H
A93 ' \ I -'' ' Io N~ p 447 A93
O
A94 \ \ I -'' , 'o N~ p 443 A93
A95 \ I -' r Io N~ P 485 A93
F3C
A96 \ ~ ___- r '0 N~ p 423 A93
S
A97 \ I -'' y IO N~ p 431 A93
A98 \ \ I -' - o N~ p 448 A93
O N
A99 \ I -'' - '0 N~ p 431 A93
A100 N~ - ~ p 479 A93
,.0~"N
~0~~0' '
A101 \ I 1 ___ ' 'o~N~ p 457 A93
0
A 102 ~ _ p 423 A93
S~ __ ; .O~N~
A103 ~ _ p 407 A93
O~ __ - O~N
A104 ~ -y0 N~ p 459 A93
A105 Ph /~~ p 429 A105
51
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
A106 GN'' ' /~ p 426 A107
..O~N~
A107 GN'' ' ~ p 424 A107
,,0~--N
A108 ~N'' ~ p 454 A107
,: O~N
52
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table B
Encompassing compounds of general formula (III), a subset of formula (1 )
where A = H
and OMe, Rl = R2 = Me2, R3 = H, X = O, Y = CH2-CH2 , Z = O, CH2 or NH; R4 =
Ph,
RS is Ph and Z is either meta or para substituted on R4.
R1
O I ~ O~N~R2
\ H / Oi
Z
R5/
(III)
Example Z meta ~ ~ [M+H]+ Procedure
/
No. para ,,O
~N~R2
Bl O m ~ 463 Bl
,,.0~'N~
B2 CH2 p ~ 461 B1
,,.O~N~
B3 m ~O 229 A51
O ,: O
B4 CH2 p ~O 4447 A51
,,.0~~"'N~
BS O m ~ 407 A51
,,.0~''N~
B6 CH2 p ~ 405 A51
,,.0~"N~
B7 O m 433 A51
.O
B8 CH2 p 431 ASI
.O
53
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
B9 O m ~ 433 A51
.O N
B 10 CH2 p ~ 431 A51
N
,,.0~,~~
B 11 O m ~ 421 A51
,,.0 N \
B 12 CH2 p ~ 419 A51
,,.0 N\
B13 O m /- 435 A51
,,,0~'N~
B14 CH2 p /~ 433 A51
,,.O~N~
B 15 O m 433 A51
... ~,,,,
'' N~
B 16 OH2 p 431 A51
.0~, ,, Nw
,,
B17 O m ~Ph 483 A51
. .O~N\
B18 CH2 p ~'Ph 481 A51
. .O~N\
B19 O m 433 A51
.p~-N
.
B20 CH2 p 431 A51
,p~--N
B21 CH2 p ~,~ ~ 419 ASI
'~N
\
B22 O m 447 A51
' ,p~--N
54
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
B23 CH2 p 445 A51
I ,p~--N
B24 O m / 497 A51
..off U h
B25 CH2 p ' ~N Ph 495 A51
.O
B26 O m Q,~N ' ~ 509 A51
i
B27 CH2 p Q,,~N I ~ 507. A51
i
B28 O m , -p~N \ ~ 495 A51
B29 CH2 p ,-p ~ 493 A51
,
B30 O m ,--o~N~ 524 A51
~N~Ph
B31 CH2 p - -o~N~ 522 A51
~N~Ph
B32 O m , ,p~~N~ 419 A51
B33 CH2 p , .O.~~N~ 417 A51
B34 CH2 m ~ 461 Bl
B35 O p ~ 463 Bl
B36 NH p ~ 462 B37
-.0~~
B37 NH p 432 B37
.: O~N
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table C
Encompassing compounds of general formula (IV) a subset of formula (I) where A
= H
and OMe, Rl = R2 = Me2, R3 = H, X = O, Y = CH2-CH2 ; R4, RS = substituted
phenyl
or heterocycle,
O . I ~ O
R5~Z~R4~N ~ O~
H
(IV)
Example Z 3l4 ~ RS [M+H]+ Method
No. substitution
w.r.t. C=O R4
C1 bond 4 ~ Ph 461 C1
C2 bond 4 ~ Ph 477 C1
I
C3 bond 4 ~ Ph 461 C1
i
C4 bond 3 ~ Ph 453 C1
S
CS O 3 ~ c~ ~ ~'' 521, C1
523, 525
O ci
C6 bond 3 N r l o Ph 451 C1
N
C7 bond 4 ~ Ph 448 C1
NJ
56
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
C8 bond 4 CI ~ Ph 481, Cl
483
C9 bond 3 F3C I ~ '' 539, C1
~ ~ 541
c1
C10 bond 3 ~i ~ I ~ '' 539 Cl
CF3 CI
C 11 bond 3 ~ I W = 453 Cl
~N
S
C12 bond 3 ~ ,N 525 C1
I w
/
__
~ CF~
S
57
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table D
Encompassing compounds of general formula (V) a subset of formula (I) where R3
= H,
X = O, Y = CH2-CH2, Z = O, CH2, NH or a bond; R4 = Ph, RS is Ph or cyclohexyl
(Cy)
and Z is either meta or para substituted on R4.
~1
R /7
O ~ \ O~N~R2
R6
R5~Z
ExampleZ R6 R7 RS meta ~~ [M+H] Method
No. / p N +
~ ~R2
para
D1 bond Cl H Ph p ~ 452, D1
~~N 454
D2 O Cl H Ph m ~ 468, Dl
470
D3 CH2 C1 H Ph p ~ 466, Dl
~~,N 468
D4 bond Cl H Cy p ~ 458, Dl
~~N 460
D5 bond H Cl Ph p ~ 452, DS
~~,N 454
D6 O H CI Ph m ~ 468, D5
~~N 470
58
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
D7 CH2 H Cl Ph p ~ 466, DS
/ ~O~N 468
D8 bond H Cl Cy p ~ 458, DS
' y~~N 460
D9 bond F H Ph p ~ 435 D9
. .O~N~
D10 CH2 F H Ph p ~ 449 D9
..O~N~
D11 bond F H Ph p ~ 441 D9
..O~N~
D12 bond H F Ph p ~ 435 D12
,,.O~N~
D13 O H F Ph m ~ 451 D12
..O~N~
D14 CH2 H F Ph p ~ 449 D12
..O~N~
D 15 bond H F Cy p ~ 441 D 12
.0~~"'N~
D16 bond Me H Ph p ~ 431 D16
. .O~N~
D17 O Me H Ph m ~ 447 D16
. .O~N~
59
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
D18 CH2 Me H Ph p ~ 445 D16
..O~N~
D19 bond Me H Cy p ~ 437 D16
,.O~N~
D20 bond H Me Ph p ~ 431 D20
. .O~N~
D21 O H Me Ph m ~ 447 D20
,O~A""N~
D22 CH2 H Me Ph p ~ 445 D20
..O~N~
D23 bond H Me Cy p ~ 437 D20
.,O~N~
D24 bond COCH3 H Ph p ~ 431 D24
..O~N~
D25 bond OMe CHO Ph p ~ 475 D25
..O~N~
D26 bond CH(OI~CH3H Ph p ~ 433 D26
. .O~N~
D27 bond Et H Ph p ~ 417 D27
,O~"'-N~
D28 bond H H Ph p ~ 417 D28
,C?~'_'N~
D29 O H H Ph m ~ 433 D28
.O~.N~
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
D30 bond H H Ph p / 361 D30
,,O~Nw
,
D31 O H H Ph p 433 D28
,,.0~_"N~
D32 O H H Ph p ~' 405 D32
.,O~N~
D33 O H H Ph m ~' 405 D32
. .O~N~
D34 bond H H Cy p ~ 423 D28
. .O~N~
D35 bond H H Cy p ~ 395 D32
. .O~N~
D36 CH2 H H Ph p ~ 431 D28
. .O~N~
D37 CH2 H H Ph p ~ 403 D32
. .O~N~
D38 bond H H p- p ~ 445 D38
EtPh I ,~~N
D39 bond H H p- p ~ 417 D39
EtPh , ,p~--N~
61
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table E
Encompassing compounds of general formula (VI) a subset of formula (1) where A
= H,
Cl, F and OMe, X = O, Y = CH2-CH2 ; R4 = phenyl, RS = phenyl or cyclohexyl
(Cy), Z
= O, CH2 or a bond
R1
R8
O I ~ O~N~R2
N ~ R9
R5~Z / R3
(VI)
ExampleZ o/pR3 R8 R9 RS /~~ [M+H] Method
No. . -O~N~2 +
E1 bond p Me H Me0 Ph >-- 461 El
y .off--N
E2 O m Me H Me0 Ph >-- 477 El
. .O~N~
E4 CH2 p Me H Me0 Ph >-- 475 El
' O~N
ES bond p Me H Me0 Cy ~ 467 El
.O~N~
E6 bond p Et H Me0 Ph ~ 447 El
. .O~N~
E7 bond p Et H MeO Ph ~ 445 El
' ,p~-,--N
E8 bond p Me H Me0 Ph ~ 431 El
. .O~N
E9 bond p Me H Me0 Ph ~ 433 El
. .O~N~
E10 bond p Et H Me0 Cy ~ 453 E1
. .O~N~
E11 bond p Et H MeO Cy ~ 451 El
,.0~'N
E12 bond p Me C1 MeO Ph ~ 468, E12
-~~'N~/ 470
62
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
E13 bond P Me H Me0 2-F-Ph~ 451 E13
:.O~N~
E14 bond p Me H Me0 2-Me- ~ 447 E14
Ph ,: ~~/-'N~/
E15 bond p Me H Me0 2- ~ 463 E14
Me0- ,:0~/-'N~/
' Ph
E16 bond p Me H Me0 2-C1-Ph~ 468, E14
.: o~N~/ 470
El7 bond p Me H Me0 4-F-Ph~ 451 E14
.O~'N~
E18 bond p Me H Me0 4-F3C-~ 501 E14
Ph :O~N~
E19 bond p Me H Me0 4-Me- ~ 447 E14
Ph .= O~'N~/
E20 bond p Me H Me0 4- ~ 463 E14
Me0- ,:0~/"N~/
Ph
E21 bond p Me H Me0 4-C1-Ph~' 468, E14
,:0~/"N~/ 470
E2.2 bond p Me H Me0 4-Et-Ph/' 461 E14
.: O~'Nw/
E23 bond p Me H Me0 4tBu- j' 489 E14
Ph ,..0~'_'N~/'
E24 bond p Me F Me0 Ph ~ 451 E24
:.O~N~
E25 bond p Et H Me Ph ~'' 459 E25
:.O~N~
E26 bond p Et H Me Cy ~'' 465 E25
.: O~N
E27 CH2 P Et H Me Ph ~'' 473 E25
:.O~N~
63
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table F
Encompassing compounds of general formula (VII) a subset of fonmula (1) where
A = H
and OMe, X = O, R4 = 3-pyridyl, R5 = phenyl, Z = a para bond
R'1
O~N~R2
i
N O
H
(VII)
Example R' [M+H]+ Method
No. --''~~N'R2
F1 Rl = R2 = Me 392 F1
F2 418 Fl
, \~
:O N~
F3 418 F 1
', o~~
F4 ~ 448 F1
.O~N~
64
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table G
Encompassing compounds of general formula (VIII) a subset of formula (I) where
A = H
and OMe, R3 = H, X = O; R4 = phenyl, Z = O, CH2 or a bond and RS = Ph or
cyclohexyl (Cy), Y is a chain of 3 or 4 carbon atoms optionally substituted by
an
hydroxyl group.
~1
O ~ ~ O--Y~-N~R2
N ~ O'~
R5--Z / H
(VIII)
Example Z rn/ RS XYN ~1 [M+H]+ Method
No.
p ~~~N~R2
G1 bond p Ph o~N ~ 449 G1
off ''~N~
G2 bond p Ph O~N ~ 461 Gl
-'
OH N
'
G3 bond p Ph O~N ~N~ 476 G1
r /
,N
OH
G4 bond p Ph O~N ~ .476 G1
- '
OH
GS bond p Ph o~N 'I,~~ 465 GS
f
O ~
H
G6 bond p Ph O~/~N ~ 475 G1
/''
OH N
G7 bond p Ph o-~/~N '~ 475 Gl
OH '' N
G8 bond p Cy o~N ~ 453 G8
O
OH -
.- N
G9 bond p Ph o~N ~ 447 G8
off '
.. N
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
G10 bond p Cy o~N ~' 455 G8
''-N~
off
G11 bond p Ph o~N ~' 449 G8
off
G12 bond p Cy o~N ~ 483 GS,G8
OH '--N
G13 bond p Ph o~N ~ 477 GS,G8
OH .--N
G14 bond p Cy o~N - ~-' 482 G8
N
'
-
OH
G15 bond p Ph o~N ' ~~ 476 G8
N
'
-
OH
G16 bond p Cy o~N ~ 481 G8
r-
OH N
-
G17 bond p Cy O~N '~ 481 G8
OH N
G18 bond p Ph o~N '~ 475 G8
N
OH '
G19 bond p Ph O~N ~ 475 G8
.y
OH N
-
G20 bond p Ph O~N ~ 444 G8,G5
,.-N /
OH
G21 bond p Ph o~N ~ 461 G8
OH --
' N
G22 bond P Ph o%'~/~N NMe2 419 G22
G23 O m Ph o%'~/~N NMe2 435 G22
G24 CH2 p Ph o%'~/~N NMe2 433 G22
G25 bond p Cy o%'~/~/N NMe2 425 G22
66
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
Table H
Encompassing compounds of general formula (IX) a subset of formula (I) where A
= H
and OMe, R3 = H, X = N; R4 = phenyl, Z = a para substituted bond and RS = Ph
or
cyclohexyl (Cy), Y and R2 four a piperazinyl ring between X and N.
O ~ V -R1
N I / Oi
/ H
R5
(IX)
Io
Example RS R1 [M+H]+ Method
No.
H1 Cy Me 408 H1
H2 Cy Et 436 Hl
H3 Cy ~ iPr 422 H1
I S The activity of the compounds used in this invention may be assessed by
competitive
binding assays to llCBy receptors, as follows:
RadioIigand Binding Studies
20 Radioligand binding assays were carried out on well washed membranes from
HEK293
cells stably expressing IlCBy receptors. Membranes (5-15 mg protein) were
incubated
with [125I]-Melann Concentrating Hormone (0.22 nM)(obtained from NEN) in the
presence and absence of competing test compounds for 45 min at 37°C in
a buffer
(pH7.4), containing SOmM Tris and 0.2% BSA. Non-specific binding was defined
using
25 0.1 mM Melanin Concentrating Hormone (obtained from Bachem). The test
compounds
were added at concentrations between l OM and lOpM in 10 concentration steps.
Following incubation, the reaction was stopped by filtration through GF/B
filters and
67
SUBSTITUTE SHEET (RULE 26)
CA 02417638 2003-O1-29
WO 02/10146 PCT/EPO1/08637
washed with 4 x lml of ice-cold SOmM Tris buffer. Microscint 20 (Paclcard) was
added to
the filters and the radioactivity measured using a Paclcard TopCount.
Bound cpm in the presence of test compound was expressed as a fraction of the
bound
cpm in the absence of test compound and plotted against the concentration of
compound.
Frorn tlus an IC50 was determined from which the pKi was calculated.
The most potent compounds of the present invention have pKi values in the
range of 7.1
to 7.8 For example:
Example pKi range
A48 7.5-7.8
B2 7.1-7.4
C8 7.1-7.4
D 15 7.5-7.8
E9 7.5-7.8
F4 7.1-7.4
Gl 7.1-7.4
H1 7.1-7.4
68
SUBSTITUTE SHEET (RULE 26)