Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
~~ TTENANT LES PAGES 1 A 245
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 245
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE:
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrokine-alpha and Neutrokine-alpha Splice Variant
Field of the Ifzve~ztiozz
[0001] The present invention relates to a novel cytokine which has been
designated
Neutrokine-alpha ("Neutrokine-alpha"). In addition, an apparent splicing
variant of
Neutrokine-alpha has been identified and designated Neutrokine-alphaSV. In
specific
embodiments, the present invention provides nucleic acid molecules encoding
Neutrokine-alpha and Neutrokine-alphaSV polypeptides. In additional
embodiments,
Neutrokine-alpha and Neutrokine-alphaSV polypeptides are also provided, as are
vectors,
host cells and recombinant methods for producing the same.
Related Af-t
[0002] Human tumor necrosis factors (TNF-alpha) and (TNF-beta, or lymphotoxin)
are related members of a broad class of polypeptide mediators, which includes
the
interferons, interleukins and growth factors, collectively called cytokines
(Beutler, B. and
Cerami, A., Afzrzu. Rev. Irramunol. 7:625-655 (1989)). Sequence analysis of
cytokine
receptors has defined several subfamilies of membrane proteins (1) the Ig
superfamily, (2)
the hematopoietin (cytolcine receptor superfamily) and (3) the tumor necrosis
factor
(TNF)/nerve growth factor (NGF) receptor superfamily (for review of TNF
superfamily
see, Gruss and Dower, Blood 85(12) :3378-3404 (1995) and Aggarwal and
Nataraaan, Eur.
Cytokine Netw., 7(2):93-124 (1996)). The TNF/NGF receptor superfamily contains
at
least 10 different proteins. Gruss and Dower, supra. Ligands for these
receptors have
been identified and belong to at least two cytokine superfamilies. Gruss and
Dower,
supra.
[0003] Tumor necrosis factor (a mixture of TNF-alpha and TNF-beta) was
originally
discovered as a result of its anti-tumor activity, however, now it is
recognized as a
pleiotropic cytokine capable of numerous biological activities including
apoptosis of some
transformed cell lines, mediation of cell activation and proliferation and
also as playing
important roles in immune regulation and inflammation.
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0004] To date, known members of the TNF-ligand superfamily include TNF-alpha,
TNF-beta (lymphotoxin-alpha), LT-beta, OX40L, Fas ligand, CD30L, CD27L, CD40L
and 4-IBBL. The ligands of the TNF ligand superfamily are acidic, TNF-like
molecules
with approximately 20% sequence homology in the extracellular domains (range,
12%-36%) and exist mainly as membrane-bound forms with the biologically active
form
being a trimeric/multimeric complex. Soluble forms of the TNF ligand
superfamily have
only been identified so far for TNF, LT-beta, and Fas ligand (for a general
review, see
Gruss, H. and Dower, S.K., Blood, 85(12) :3378-3404 (1995)), which is hereby
incorporated by reference in its entirety. These proteins are involved in
regulation of cell
proliferation, activation, and differentiation, including control of cell
survival or death by
apoptosis or cytotoxicity (Aimitage, R.J., Curr. Opih. Immufaol. 6:407 (1994)
and Smith,
C.A., Cell 75:959 (1994)).
[0005] Tumor necrosis factor-alpha (TNF-alpha; also termed cachectin;
hereinafter
"TNF") is secreted primarily by monocytes and macrophages in response to
endotoxin or
other stimuli as a soluble homotrimer of 17 kD protein subunits (Smith, R.A.
et al., J.
Biol. Chefyz. 262:6951-6954 (1987)). A membrane-bound 26 kD precursor form of
TNF
has also been described (Kriegler, M. et al., Cell 53:45-53 (1988)).
[0006] Accumulating evidence indicates that TNF is a regulatory cytokine with
pleiotropic biological activities. These activities include: inhibition of
lipoprotein lipase
synthesis ("cachectin" activity) (Beutler, B. et al., Nature 316:552 (1985)),
activation of
polymorphonuclear leukocytes (Klebanoff, S.J. et al., J. Immu~col. 136:4220
(1986);
Perussia, B., et al., J. Immunol. 138:765 (1987)), inhibition of cell growth
or stimulation
of cell growth (Vilcek, J. et al., J. Exp. Med. 163:632 (1986); Sugarman, B.
J. et al.,
Scief2ce 230:943 (1985); Lachman, L.B. et al., J. If~2mufzol. 138:2913
(1987)), cytotoxic
action on certain transformed cell types (Lachman, L.B. et al., supra;
Darzynkiewicz, Z. et
al., Canc. Res. 44:83 (1984)), antiviral activity (Kohase, M. et al., Cell
45:659 (1986);
along, G.H.W. et al., Nature 323:819 (1986)), stimulation of bone resorption
(Bertolini,
D.R. et al., Nature 319:516 (1986); Saklatvala, J., Nature 322:547 (1986)),
stimulation of
collagenase and prostaglandin E2 production (payer, J.-M. et al., J. Exp. Med.
162:2163
(1985)); and immunoregulatory actions, including activation of T cells
(Yokota, S. et al.,
J. Immunol. 140:531 (1988)), B cells (Kehrl, J.H. et alr, J. Exp. Med. 166:786
(1987)),
monocytes (Philip, R. et al., Nature 323:86 (1986)), thymocytes (Ranges, G.E.
et al., J.
2
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Exp. Med. 167:1472 (1988)), and stimulation of the cell-surface expression of
major
histocompatibility complex (MHC) class I and class II molecules (Collins, T.
et al., Pr-oc.
Natl. Acad. Sci. USA 83:446 (1986); Pujol-Borrel, R. et al., Nature 326:304
(1987)).
[0007] TNF is noted for its pro-inflammatory actions which result in tissue
injury,
such as induction of procoagulant activity on vascular endothelial cells
(Pober, J.S. et al.,
J. Ifnrnunol. 136:1680 (1986)), increased adherence of neutrophils and
lymphocytes
(Pober, J.S. et al., J. Immufzol. 138:3319 (1987)), and stimulation of the
release of platelet
activating factor from macrophages, neutrophils and vascular endothelial cells
(Camussi,
G. et al., J. Exp. Med. 166:1390 (1987)).
[0008] Recent evidence implicates TNF in the pathogenesis of many infections
(Cerami, A. et al., Imnaunol. Today 9:28 (1988)), immune disorders, neoplastic
pathology,
e.g., in cachexia accompanying some malignancies (Oliff, A. et al., Cell
50:555 (1987)),
and in autoimmune pathologies and graft-versus host pathology (Piguet, P.-F.
et al., J.
Exp. Med. 166:1280 (1987)). The association of TNF with cancer and infectious
pathologies is often related to the host's catabolic state. A major problem in
cancer
patients is weight loss, usually associated with anorexia. The extensive
wasting which
results is known as "cachexia" (Kern, K. A. et al. J. Parent. Enter. Nutr.
12:286-298
(1988)). Cachexia includes progressive weight loss, anorexia, and persistent
erosion of
body mass in response to a malignant growth. The cachectic state is thus
associated with
significant morbidity and is responsible for the majority of cancer mortality.
A number of
studies have suggested that TNF is an important mediator of the cachexia in
cancer,
infectious pathology, and in other catabolic states.
[0009] TNF is thought to play a central role in the pathophysiological
consequences of
Gram-negative sepsis and endotoxic shock (Michie, H.R. et al., Br. J. Surg.
76:670-671
(1989); Debets, J. M. H. et al., Second Vienna Shock Forunz, p.463-466 (1989);
Simpson,
S. Q. et al., Crit. Care Clin. 5:27-47 (1989)), including fever, malaise,
anorexia, and
cachexia. Endotoxin is a potent monocyte/macrophage activator which stimulates
production and secretion of TNF (Kornbluth, S.K. et al., J. Immunol. 137:2585-
2591
(1986)) and other cytokines. Because TNF could mimic many biological effects
of
endotoxin, it was concluded to be a central mediator responsible for the
clinical
manifestations of endotoxin-related illness. TNF and other monocyte-derived
cytokines
mediate the metabolic and neuxohormonal responses to endotoxin (Michie, H.R.
et ad., N.
3
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
EfZg. J. Med. 318:1481-1486 (1988)). Endotoxin administration to human
volunteers
produces acute illness with flu-like symptoms including fever, tachycardia,
increased
metabolic rate and stress hormone release (Revhaug, A. et al., Arch. Surg.
123:162-170
(1988)). Elevated levels of circulating TNF have also been found in patients
suffering
from Gram-negative sepsis (Waage, A. et al., Lancet 1:355-357 (1987);
Hammerle, A.F.
et al., Secofad Vienfza Shock Forum p. 715-718 (1989); Debets, J. M. H. et
al., Crit. Care
Med. 17:489-497 (1989); Calandra, T. et al., J. Infec. Dis. 161:982-987
(1990)).
[0010] Passive immunotherapy directed at neutralizing TNF may have a
beneficial
effect in Gram-negative sepsis and endotoxemia, based on the increased TNF
production
and elevated TNF levels in these pathology states, as discussed above.
Antibodies to a
"modulator" material which was characterized as cachectin (later found to be
identical to
TNF) were disclosed by Cerami et al. (EPO Patent Publication 0,212,489, March
4, 1987).
Such antibodies were said to be useful in diagnostic immunoassays and in
therapy of
shock in bacterial infections. Rubin et al. (EPO Patent Publication 0,218,868,
April 22,
1987) disclosed monoclonal antibodies to human TNF, the hybridomas secreting
such
antibodies, methods of producing such antibodies, and the use of such
antibodies in
immunoassay of TNF. Yone et al. (EPO Patent Publication 0,288,088, October 26,
1988)
disclosed anti-TNF antibodies, including mAbs, and their utility in
immunoassay
diagnosis of pathologies, in particular Kawasaki's pathology and bacterial
infection. The
body fluids of patients with Kawasaki's pathology (infantile acute febrile
mucocutaneous
lymph node syndrome; Kawasaki, T., Allergy 16:178 (1967); Kawasaki, T.,
Shouica
(Pediatrics) 26:935 (1985)) were said to contain elevated TNF levels which
were related
to progress of the pathology (Yone et al., supra).
[0011] Other investigators have described mAbs specific for recombinant human
TIFF
which had neutralizing activity in vitro (Liang, C-M. et al. Biochem. Biophys.
Res. Comtr2.
137:847-854 (1986); Meager, A. et al., Hybridor~aa 6:305-311 (1987); Fendly et
al.,
Hybridoma 6:359-369 (1987); Bringman, T S et al., Hybridoma 6:489-507 (1987);
Hirai,
M. et al., J. Imrraunol. Meth. 96:57-62 (1987); Moller, A. et al. (Cytokine
2:162-169
(1990)). Some of these mAbs were used to map epitopes of human TNF and develop
enzyme immunoassays (Fendly et al., supra; Hirai et al., supra; Moller et al.,
supra) and
to assist in the purification of recombinant TNF (Bringman et al., supra).
However, these
studies do not provide a basis for producing TNF neutralizing antibodies that
can be used
4
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
for ifz vivo diagnostic or therapeutic uses in humans, due to immunogenicity,
lack of
specificity and/or pharmaceutical suitability.
[0012] Neutralizing antisera or mAbs to TNF have been shown in mammals other
than
man to abrogate adverse physiological changes and prevent death after lethal
challenge in
experimental endotoxemia and bacteremia. This effect has been demonstrated,
e.g., in
rodent lethality assays and in primate pathology model systems (Mathison, J.C.
et ad., J.
Clin. Invest. 81:1925-1937 (1988); Beutler, B. et al., Science 229:869-871
(1985}; Tracey,
K. J. et al., Nature 330:662-664 (1987); Shimamoto, Y. et al., Immunol. Lett.
17:311-318
(1988); Silva, A. T. et al., J. Infect. Dis. 162:421-427 (1990); Opal, S. M.
et al., J. Infect.
Dis. 161:1148-1152 (1990); Hinshaw, L.B. et al., Circ. Shock 30:279-292
(1990)).
[0013] To date, experience with anti-TNF mAb therapy in humans has been
limited
but shows beneficial therapeutic results, e.g., in arthritis and sepsis. See,
e.g., Elliott, M. J.
et al., Baillieres Clin . Rheur~aatol. 9:633-52 (1995); Feldmann M, et al.,
Anfz. N. Y Acad.
Sci. USA 766:272-8 (1995); van der Poll, T. et al., Shock 3:1-12 (1995);
Wherry et ad.,
Crit. Care. Med. 21:S436-40 (1993); Tracey K. J., et al., Crit. Care Med.
21:S415-22
(1993).
[0014] Mammalian development is dependent on both the proliferation and
differentiation of cells as well as programmed cell death which occurs through
apoptosis
(Walker, et al., Methods Achiev. Exp. Patlzol. 13:18 (1988). Apoptosis plays a
critical role
in the destruction of immune thymocytes that recognize self antigens. Failure
of this
normal elimination process may play a role in autoimmune diseases (Gammon et
al.,
Immutzology Today 12:193 (1991)).
[0015] Itoh et al. (Cell 66:233 (1991)) described a cell surface antigen,
Fas/CD95 that
mediates apoptosis and is involved in clonal deletion of T-cells. Fas is
expressed in
activated T-cells, B-cells, neutrophils and in thymus, liver, heart and lung
and ovary in
adult mice (Watanabe-Fukunaga et al., J. Immunol. 148:1274 (1992)) in addition
to
activated T-cells, B-cells, neutrophils. In experiments where a monoclonal Ab
is
cross-linked to Fas, apoptosis is induced (Yonehara et al., J. Exp. Med.
169:1747 (1989);
Trauth et al., Science 245:301 (1989)). In addition, there is an example where
binding of a
monoclonal Ab to Fas is stimulatory to T-cells under certain conditions
(Alderson et al., J.
Exp. Med. 178:2231 (1993)).
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0016] Fas antigen is a cell surface protein of relative MW of 45 I~d. Both
human and
murine genes for Fas have been cloned by Watanabe-Fukunaga et al., (T.
Inzmunol.
148:1274 (1992)) and Itoh et al. (Cell 66:233 (1991)). The proteins encoded by
these
genes are both transmembrane proteins with structural homology to the Nerve
Growth
Factor/Tumor Necrosis Factor receptor superfamily, which includes two TNF
receptors,
the low affinity Nerve Growth Factor receptor and CD40, CD27, CD30, and OX40.
[0017] Recently the Fas ligand has been described (Suda et al., Cell 75:1169
(1993)).
The amino acid sequence indicates that Fas ligand is a type II transmembrane
protein
belonging to the TNF family. Thus, the Fas ligand polypeptide comprises three
main
domains: a short intracellular domain at the amino terminal end and a longer
extracellular
domain at the carboxy terminal end, connected by a hydrophobic transmembrane
domain.
Fas ligand is expressed in splenocytes and thymocytes, consistent with T-cell
mediated
cytotoxicity. The purified Fas ligand has a MW of 40 kD.
[0018] Recently, it has been demonstrated that FaslFas ligand interactions are
required
for apoptosis following the activation of T-cells (Ju et al., Nature 373:444
(1995); Brunner
et al., Nature 373:441 (1995)). Activation of T-cells induces both proteins on
the cell
surface. Subsequent interaction between the ligand and receptor results in
apoptosis of the
cells. This supports the possible regulatory role for apoptosis induced by
Fas/Fas ligand
interaction during normal immune responses.
[0019] Accordingly, there is a need to provide cytokines similar to TNF that
are
involved in pathological conditions. Such novel cytokines may be used to make
novel
antibodies or other antagonists that bind these TNF-like cytokines for
diagnosis and
therapy of disorders related to TNF-like cytokines.
Sumynary of the lya~ention
[0020] In accordance with one embodiment of the present invention, there is
provided
a novel extracellular domain of a Neutrokine-alpha polypeptide, and a novel
extracellular
domain of a Neutrokine-alphaSV polypeptide, as well as biologically ~ active
and
diagnostically or therapeutically useful fragments, analogs and derivatives
thereof.
[0021] In accordance with another embodiment of the present invention, there
are
provided isolated nucleic acid molecules encoding human Neutrokine-alpha or
Neutrokine-alphaSV, including mRNAs, DNAs, cDNAs, genomic DNAs as well as
6
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
analogs and biologically active and diagnostically or therapeutically useful
fragments and
derivatives thereof.
[0022] The present invention provides isolated nucleic acid molecules
comprising, or
alternatively, consisting of, a polynucleotide encoding a cytolcine and an
apparent splice
variant thereof that are structurally similar to TNF and related cytolcines
and have similar
biological effects and activities. This cytokine is named Neutrokine-alpha and
the
invention includes Neutrokine-alpha polypeptides having at least a portion of
the amino
acid sequence in Figures 1A and 1B (SEQ >D N0:2) or amino acid sequence
encoded by
the cDNA clone (HNEDU15) deposited on October 22, 1996 assigned ATCC number
97768. The nucleotide sequence determined by sequencing, the deposited
Neutrokine-alpha clone, which is shown in Figures 1A and 1B (SEQ >D NO:1),
contains
an open reading frame encoding a complete polypeptide of 285 amino acid
residues
including an N-terminal methionine, a predicted intracellular domain of about
46 amino
acid residues, a predicted transmembrane domain of about 26 amino acids, a
predicted
extracellular domain of about 213 amino acids, and a deduced molecular weight
for the
complete protein of about 31 kDa. As for other type II transmembrane proteins,
soluble
forms of Neutrokine-alpha include all or a portion of the extracellular domain
cleaved
from the transmembrane domain and a polypeptide comprising the complete
Neutrokine-alpha polypeptide lacking the transmembrane domain, i.e., the
extracellular
domain linked to the intracellular domain. The apparent splice variant of
Neutrokine-alpha
is named Neutrokine-alphaSV and the invention includes Neutrokine-alphaSV
polypeptides comprising, or alternatively, consisting of, at least a portion
of the amino
acid sequence in Figures 5A and 5B (SEQ >D N0:19) or amino acid sequence
encoded by
the cDNA clone HDPMC52 deposited on December 10, 1998 and assigned ATCC number
203518. The nucleotide sequence determined by sequencing the deposited
Neutrokine-alphaSV clone, which is shown in Figures 5A and 5B (SEQ JD N0:18),
contains an open reading frame encoding a complete polypeptide of 266 amino
acid
residues including an N-terminal methionine, a predicted intracellular domain
of about 46
amino acid residues, a predicted transmembrane domain of about 26 amino acids,
a
predicted extracellular domain of about 194 amino acids, and a deduced
molecular weight
for the complete protein of about 29 kDa. As for other type II transmembrane
proteins,
soluble forms of Neutrokine-alphaSV include all or a portion of the
extracellular domain
7
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
cleaved from the transmembrane domain and a polypeptide comprising the
complete
Neutrolcine-alphaSV polypeptide lacking the transmembrane domain, i.e., the
extracellular
domain linked to the intracellular domain.
[0023] Thus, one embodiment of the invention provides an isolated nucleic acid
molecule comprising, or alternatively consisting of, a polynucleotide having a
nucleotide
sequence selected from the group consisting of: (a) a nucleotide sequence
encoding a
full-length Neutrokine-alpha polypeptide having the complete amino acid
sequence in
Figures 1A and 1B (SEQ ID N0:2) or as encoded by the cDNA clone contained in
the
deposit having ATCC accession number 97768; (b) a nucleotide sequence encoding
the
predicted extracellular domain of the Neutrokine-alpha polypeptide having the
amino acid
sequence at positions 73 to 285 in Figures 1A and 1B (SEQ ID N0:2) or as
encoded by
the clone contained in the deposit having ATCC accession number 97768; (c) a
nucleotide
sequence encoding a fragment of the polypeptide of (b) (e.g., amino acids 134-
285) having
Neutrokine-alpha functional activity (e.g., biological acitivity); (d) a
nucleotide sequence
encoding a polypeptide comprising the Neutrokine-alpha intracellular domain
(predicted
to constitute amino acid residues from about 1 to about 46 in Figures 1A and
1B (SEQ ID
N0:2)) or as encoded by the clone contained in the deposit having ATCC
accession
number 97768; (e) a nucleotide sequence encoding a polypeptide comprising the
Neutrokine-alpha transmembrane domain (predicted to constitute amino acid
residues
from about 47 to about 72 in Figures 1A and 1B (SEQ )D N0:2) or as encoded by
the
cDNA clone contained in the deposit having ATCC accession number 97768; (f) a
nucleotide sequence encoding a soluble Neutrokine-alpha polypeptide having the
extracellular and intracellular domains but lacking the transmembrane domain;
and (g) a
nucleotide sequence complementary to any of the nucleotide sequences in (a),
(b), (c), (d),
(e) or (f) above.
[0024] Further embodiments of the invention include isolated nucleic acid
molecules
that comprise, or alternatively consist of, a polynucleotide having a
nucleotide sequence at
least 80%, 85% or 90% identical, and more preferably at least 95%, 96%, 97%,
98% or
99% identical, to any of the nucleotide sequences in (a), (b), (c), (d), (e),
(f) or (g) above,
or a polynucleotide which hybridizes under stringent hybridization conditions
to a
polynucleotide in (a), (b), (c), (d), (e), (f) or (g) above. This
polynucleotide which
8
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
hybridizes does not hybridize under stringent hybridization conditions to a
polynucleotide
having a nucleotide sequence consisting of only A residues or of only T
residues.
[0025] Another embodiment of the invention provides an isolated nucleic acid
molecule comprising, or alternatively consisting of, a polynucleotide having a
nucleotide
sequence selected from the group consisting of: (a) a nucleotide sequence
encoding a
full-length Neutrokine-alphaSV polypeptide having the complete amino acid
sequence in
Figures 5A and 5B (SEQ ID N0:19) or as encoded by the cDNA clone contained in
the
ATCC Deposit deposited on December 10, 1998 as ATCC Number 203518; (b) a
nucleotide sequence encoding the predicted extracellular domain of the
Neutrokine-alphaSV polypeptide having the amino acid sequence at positions 73
to 266 in
Figures 1A and 1B (SEQ ID N0:2) or as encoded by the cDNA clone contained in
ATCC
203518 deposited on December 10, 1998; (c) a nucleotide sequence encoding a
polypeptide comprising the Neutrokine-alphaSV intracellular domain (predicted
to
constitute amino acid residues from about 1 to about 46 in Figures 5A and 5B
(SEQ ID
N0:19)) or as encoded by the cDNA clone contained in ATCC No. 203518 deposited
on
December 10, 1998; (d) a nucleotide sequence encoding a polypeptide comprising
the
Neutrokine-alphaSV transmembrane domain (predicted to constitute amino acid
residues
from about 47 to about 72 in Figures 5A and 5B (SEQ ID N0:19) or as encoded by
the
cDNA clone contained in ATCC No. 203518 deposited on December 10, 1998; (e) a
nucleotide sequence encoding a soluble Neutrokine-alphaSV polypeptide having
the
extracellular and intracellular domains but lacking the transmembrane domain;
and (f) a
nucleotide sequence complementary to any of the nucleotide sequences in (a),
(b), (c), (d),
or (e) above.
[0026] Further embodiments of the invention include isolated nucleic acid
molecules
that comprise, or alternatively consist of, a polynucleotide having a
nucleotide sequence at
least 80%, 85% or 90% identical, and more preferably at least 95%, 96%, 97%,
98% or
99% identical, to any of the nucleotide sequences in (a), (b), (c), (d), (e)
or (f) above, or a
polynucleotide which hybridizes under stringent hybridization conditions to a
polynucleotide in (a), (b), (c), (d), (e) or (f) above. This polynucleotide
which hybridizes
does not hybridize under stringent hybridization conditions to a
polynucleotide having a
nucleotide sequence consisting of only A residues or of only T residues.
9
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0027] In one embodiment, the invention includes isolated nucleic acid
molecules that
comprise, or alternatively consist of, a polynucleotide having a nucleotide
sequence
encoding the apparent splice variant of Neutrokine-alpha comprising, or
alternatively
consisting of, at least a portion of the amino acid sequence from Gly-142 to
Leu-266 as
shown in Figures 5A and 5B (SEQ ID N0:19) or amino acid sequence encoded by
the
cDNA clone HDPMC52 deposited on December 10, 1998 and assigned ATCC Deposit
No. 203518.
[0028] In another preferred embodiment, the invention include isolated nucleic
acid
molecules that comprise, or alternatively consist of, a polynucleotide having
a nucleotide
sequence encoding the apparent splice variant of Neutrokine-alpha comprising,
or
alternatively consisting of, at least a portion of the amino acid sequence
from Ala-134 to
Leu-266 as shown in Figures 5A. and 5B (SEQ ID N0:19) or amino acid sequence
encoded by the cDNA clone HDPMC52 deposited on December 10, 1998 and assigned
ATCC Deposit No. 203518.
[0029] In additional embodiments, the nucleic acid molecules of the invention
comprise, or alternatively consist of, a polynucleotide which encodes the
amino acid
sequence of an epitope-bearing portion of a Neutrokine-alpha or Neutrokine-
alphaSV
polypeptide having an amino acid sequence in (a), (b), (c), (d), (e), (f) or
(g) above. A
further nucleic acid embodiment of the invention relates to an isolated
nucleic acid
molecule comprising, or alternatively consisting of, a polynucleotide which
encodes tl~e
amino acid sequence of a Neutrokine-alpha or Neutrokine-alphaSV polypeptide
having an
amino acid sequence which contains at least one amino acid addition,
substitution, and/or
deletion but not more than 50 amino acid additions, substitutions and/or
deletions, even
more preferably, not more than 40 amino acid additions, substitutions, and/or
deletions,
still more preferably, not more than 30 amino acid additions, substitutions,
and/or
deletions, and still even more preferably, not more than 20 amino acid
additions,
substitutions, and/or deletions. Of course, in order of ever-increasing
preference, it is
highly preferable for a polynucleotide which encodes the amino acid sequence
of a
Neutrokine-alpha or Neutrokine-alphaSV polypeptide to have an amino acid
sequence
which contains not more than 10, 9, 8, 7, 6, 5, 4, 3, 2 or 1 or 1-100, 1-50, 1-
25, 1-20, 1-15,
1-10, or 1-5 amino acid additions, substitutions and/or deletions.
Conservative
substitutions are preferable.
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0030] The present invention also relates to recombinant vectors, which
include the
isolated nucleic acid molecules of the present invention, and to host cells
containing the
recombinant vectors, as well as to methods of making such vectors and host
cells and for
using them for production of Neutrokine-alpha polypeptides by recombinant
techniques.
[0031] In accordance with a further embodiment of the present invention, there
is
provided a process for producing such polypeptides by recombinant techniques
comprising culturing recombinant prokaryotic and/or eukaryotic host cells,
containing a
Neutrokine-alpha or Neutrokine-alphaSV nucleic acid sequence of the invention,
under
conditions promoting expression of said polypeptide and subsequent recovery of
said
polypeptide.
[0032] The invention further provides an isolated Neutrokine-alpha polypeptide
comprising, or alternatively consisting of, an amino acid sequence selected
from the group
consisting of: (a) the amino acid sequence of the full-length Neutrokine-alpha
polypeptide
having the complete amino acid sequence shown in Figures 1A and 1B (i.e.,
positions
1-285 of SEQ ID N0:2) or as encoded by the cDNA plasmid contained in the
deposit
having ATCC accession number 97768; (b) the amino acid sequence of the full-
length
Neutrokine-alpha polypeptide having the complete amino acid sequence shown in
SEQ >D
N0:2 excepting the N-terminal methionine (i.e., positions 2 to 285 of SEQ ff~
N0:2); (c)
a fragment of the polypeptide of (b) having Neutrokine-alpha functional
activity (e.g.,
biological activity); (d) the amino acid sequence of the predicted
extracellular domain of
the Neutrokine-alpha polypeptide having the amino acid sequence at positions
73 to 285 in
Figures 1A and 1B (SEQ >D N0:2) or as encoded by the cDNA plasmid contained in
the
deposit having ATCC accession number 97768; (e) an amino acid sequence
encoding the
mature soluble form of Neutrokine-alpha polypeptide having the amino acid
sequence at
positions 134-285 in Figures 1A and 1B (SEQ ID N0:2); (f) the amino acid
sequence of
the Neutrokine-alpha intracellular domain (predicted to constitute amino acid
residues
from about 1 to about 46 in Figures 1A and 1B (SEQ ID N0:2)) or as encoded by
the
cDNA plasmid contained in the deposit having ATCC accession number 97768; (g)
the
amino acid sequence of the Neutrokine-alpha transmembrane domain (predicted to
constitute amino acid residues from about 47 to about 72 in Figures 1A and 1B
(SEQ >D
N0:2)) or as encoded by the cDNA plasmid contained in the deposit having ATCC
accession number 97768; (h) the amino acid sequence of the soluble Neutrokine-
alpha
11
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polypeptide having the extracellular and intracellular domains but lacking the
transmembrane domain, wherein each of these domains is defined above; and (i)
fragments of the polypeptide of (a), (b), (c), (d), (e), (f), (g) or (h). The
polypeptides of
the present invention also include polypeptides having an amino acid sequence
at least
80% identical, more preferably at least 85% or 90% identical, and still more
preferably
95%, 96%, 97%, 98% or 99% identical to those described in (a), (b), (c), (d),
(e) (f), (g),
(h) or (i) above, as well as polypeptides having an amino acid sequence with
at least 80%,
85%, or 90% similarity, and more preferably at least 95% similarity, to those
above.
Additional embodiments of the invention relates to polypeptides which
comprise, or
alternatively consist of, the amino acid sequence of an epitope-bearing
portion of a
Neutrokine-alpha polypeptide having an amino acid sequence described in (a),
(b), (c), (d),
(e), (f), (g), (h) or (i) above. Polypeptides having the amino acid sequence
of an
epitope-bearing portion of a Neutrokine-alpha polypeptide of the invention
include
portions of such polypeptides with at least 4, at least 5, at least 6, at
least 7, at least 8, and
preferably at least 9, at least 10, at least 11, at least 12, at least 13, at
least 14, at least 15,
at least 20, at least 25, at least 30, at least 40, at least 50, and more
preferably at least
about 30 amino acids to about 50 amino acids, although epitope-bearing
polypeptides of
any length up to and including the entire amino acid sequence of a polypeptide
of the
invention described above also are included in the invention.
[0033] Highly preferred embodiments of the invention are directed to nucleic
acid
molecules comprising, or alternatively consisting of a polynucleotide having a
nucleotide
sequence at least 80%, 85%, 90% identical and more preferably at least 95%,
96%, 97%,
98%, 99% or 100% identical to a polynucleotide sequence encoding the
Neutrokine-alpha
polypeptide having the amino acid sequence at positions 134-285 in Figures 1A
and 1B
(SEQ m N0:2). Preferred embodiments of the invention are directed to nucleic
acid
molecules comprising, or alternatively consisting of a polynucleotide having a
nucleotide
sequence at least 90% identical to a polynucleotide sequence encoding the
Neutrokine-
alpha polypeptide having the amino acid sequence at positions 134-285 in
Figures 1A and
1B (SEQ ID N0:2). More preferred embodiments of the invention are directed to
nucleic
acid molecules comprising, or alternatively consisting of a polynucleotide
having a
nucleotide sequence at least 95% identical to a polynucleotide sequence
encoding the
Neutrokine-alpha polypeptide having the amino acid sequence at positions 134-
285 in
12
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Figures 1A and 1B (SEQ ID N0:2). More preferred embodiments of the invention
are
directed to nucleic acid molecules comprising, or alternatively consisting of
a
polynucleotide having a nucleotide sequence at least 96% identical to a
polynucleotide
sequence encoding the Neutrolcine-alpha polypeptide having the amino acid
sequence at
positions 134-285 in Figures 1A and 1B (SEQ ID N0:2). Additionally, more
preferred
embodiments of the invention are directed to nucleic acid molecules
comprising, or
alternatively consisting of a polynucleotide having a nucleotide sequence at
least 97% to a
polynucleotide sequence encoding the Neutrokine-alpha polypeptide having the
amino
acid sequence at positions 134-285 in Figures 1A and 1B (SEQ )D N0:2).
Additionally,
more preferred embodiments of the invention are directed to nucleic acid
molecules
comprising, or alternatively consisting of a polynucleotide having a
nucleotide sequence at
least 98°7o to a polynucleotide sequence encoding the Neutrokine-alpha
polypeptide having
the amino acid sequence at positions 134-285 in Figures 1A and 1B (SEQ ID
N0:2).
Additionally, more preferred embodiments of the invention are directed to
nucleic acid
molecules comprising, or alternatively consisting of a polynucleotide having a
nucleotide
sequence at least 99°Io identical to a polynucleotide sequence encoding
the Neutrokine-
alpha polypeptide having the amino acid sequence at positions 134-285 in
Figures 1A and
1B (SEQ ID N0:2).
[0034] The present invention also encompasses the above polynucleotide
sequences
fused to a heterologous polynucleotide sequence. Polypeptides encoded by these
polynucleotides and nucleic acid molecules are also encompassed by the
invention.
[0035] The invention further provides an isolated Neutrokine-alphaSV
polypeptide
comprising, or alternatively consisting of, an amino acid sequence selected
from the group
consisting of: (a) the amino acid sequence of the full-length Neutrokine-
alphaSV
polypeptide having the complete amino acid sequence shown in Figures 5A and 5B
(i.e.,
positions 1-266 of SEQ » N0:19) or as encoded by the cDNA clone contained in
ATCC
No. 203518 deposited on December 10; 1998; (b) the amino acid sequence of the
full-length Neutrokine-alphaSV polypeptide having the complete amino acid
sequence
shown in SEQ ID N0:19 excepting the N-terminal methionine (i.e., positions 2
to 266 of
SEQ ID N0:19); (c) the amino acid sequence of the predicted extracellular
domain of the
Neutrokine-alphaSV polypeptide having the amino acid sequence at positions 73
to 266 in
Figures 5A and 5B (SEQ m N0:19) or as encoded by the cDNA clone contained in
13
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
ATCC No. 203518 deposited on December 10, 1998; (d) the amino acid sequence of
the
Neutrokine-alphaSV intracellular domain (predicted to constitute amino acid
residues
from about 1 to about 46 in Figures 5A and 5B (SEQ >D N0:19)) or as encoded by
the
cDNA clone contained in ATCC No. 203518 deposited on December 10, 1998; (e)
the
amino acid sequence of the Neutrokine-alphaSV transmembrane domain (predicted
to
constitute amino acid residues from about 47 to about 72 in Figures 5A and 5B
(SEQ ID
N0:19)) or as encoded by the cDNA clone contained in ATCC No. 203518 deposited
on
December 10, 1998; (f) the amino acid sequence of the soluble Neutrokine-
alphaSV
polypeptide having the extracellular and intracellular domains but lacking the
transmembrane domain, wherein each of these domains is defined above; and (g)
fragments of the polypeptide of (a), (b), (c), (d), (e), or (f). The
polypeptides of the
present invention also include polypeptides having an amino acid sequence at
least 80%
identical, more preferably at least 85% or 90% identical, and still more
preferably 95%,
96%, 97%, 98% or 99% identical to those described in (a), (b), (c), (d), (e)
(f), or (g)
above, as well as polypeptides having an amino acid sequence with at least
80%, 85%, or
90% similarity, and more preferably at least 95% similarity, to those above.
Additional
embodiments of the invention relates to polypeptides which comprise, or
alternatively
consist of, the amino acid sequence of an epitope-bearing portion of a
Neutrokine-alphaSV polypeptide having an amino acid sequence described in (a),
(b), (c),
(d), (e), (f), or (g) above. Peptides or polypeptides having the amino acid
sequence of an
epitope-bearing portion of a Neutrokine-alphaSV polypeptide of the invention
include
portions of such polypeptides with at least 4, at least 5, at least 6, at
least 7, at least 8, and
preferably at least 9, at least 10, at least 11, at least 12, at least 13, at
least 14, at least 15,
at least 20, at least 25, at least 30, at least 40, at least 50, and more
preferably at least
about 30 amino acids to about 50 amino acids, although epitope-hearing
polypeptides of
any length up to and including the entire amino acid sequence of a polypeptide
of the
invention described above also are included in the invention.
[0036] Certain non-exclusive' embodiments of the invention relate to a
polypeptide
which has the amino acid sequence of an epitope-bearing portion of a
Neutrokine-alpha or
Neutrokine-alphaSV polypeptide having an amino acid sequence described in (a),
(b), (c),
(d), (e), (f), (g), (h) or (i) above. In other embodiments, the invention
provides an isolated
antibody that binds specifically (i.e., uniquely) to a Neutrokine-alpha or
Neutrokine-
14
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
alphaSVpolypeptide having an amino acid sequence described in (a), (b), (c),
(d), (e), (f),
(g), (h) or (i) above.
[0037] The invention further provides methods for isolating antibodies that
bind
specifically (i.e., uniquely) to a Neutrolcine-alpha or Neutrokine-alphaSV
polypeptide
having an amino acid sequence as described herein. Such antibodies are useful
diagnostically or therapeutically as described below.
[0038] The invention also provides for pharmaceutical compositions comprising
soluble Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides, particularly
human
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides, andlor anti-
Neutrokine-alpha
antibodies and/or anti-Neutrokine-alphaSV antibodies which may be employed,
for
instance, to treat, prevent, prognose and/or diagnose tumor and tumor
metastasis,
infections by bacteria, viruses and other parasites, immunodeficiencies,
inflammatory
diseases, lymphadenopathy, autoimmune diseases, graft versus host disease,
stimulate
peripheral tolerance, destroy some transformed cell lines, mediate cell
activation, survival
and proliferation, mediate immune regulation and inflammatory responses, and
to enhance
or inhibit immune responses.
[0039] In certain embodiments, soluble Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptides of the invention, or agonists thereof, are administered, to
treat, prevent,
prognose and/or diagnose an immunodeficiency (e.g., severe combined
immunodeficiency (SLID)-X linked, SCID-autosomal, adenosine deaminase
deficiency
(ADA deficiency), X-linked agammaglobulinemia (XLA), Bruton's disease,
congenital
agammaglobulinemia, X-linked infantile agammaglobulinemia, acquired
agammaglobulinemia, adult onset agammaglobulinemia, late-onset
agammaglobulinemia,
dysgammaglobulinemia, hypogammaglobulinemia, transient hypogammaglobulinemia
of
infancy, unspecified hypogammaglobulinemia, agammaglobulinemia, common
variable
immunodeficiency (CVTD) (acquired), Wiskott-Aldrich Syndrome (WAS), X-linked
immunodeficiency with hyper IgM, non X-linked immunodeficiency with hyper IgM,
selective IgA deficiency, IgG subclass deficiency (with or without IgA
deficiency),
antibody deficiency with normal or elevated Igs, immunodeficiency with
thymoma, Ig
heavy chain deletions, kappa chain deficiency, B cell lymphoproliferative
disorder
(BLPD), selective IgM immunodeficiency, recessive agammaglobulinemia (Swiss
type),
reticular dysgenesis, neonatal neutropenia, severe congenital leukopenia,
thymic
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
alymphoplasia-aplasia or dysplasia with immunodeficiency, ataxia-
telangiectasia, short
limbed dwarfism, X-linked lymphoproliferative syndrome (XLP), Nezelof syndrome-
combined immunodeficiency with Igs, purine nucleoside phosphorylase deficiency
(PNP),
MHC Class II deficiency (Bare Lymphocyte Syndrome) and severe combined
immunodeficiency.) or conditions associated with an immunodeficiency.
[0040] In a specific embodiment, Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides or polynucleotides of the invention, or agonists thereof, is
administered to
treat, prevent, prognose and/or diagnose common variable immunodeficiency.
[0041] In a specific embodiment, Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides or polynucleotides of the invention, or agonists thereof, is
administered to
treat, prevent, prognose and/or diagnose X-linked agammaglobulinemia.
[0042] In another specific embodiment, Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptides or polynucleotides of the invention, or agonists thereof, is
administered to
treat, prevent, prognose and/or diagnose severe combined immunodeficiency
(SCID).
[0043] In another specific embodiment, Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptides or polynucleotides of the invention, or agonists thereof, is
administered to
treat, prevent, prognose and/or diagnose Wiskott-Aldrich syndrome.
[0044] In another specific embodiment, Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptides or polynucleotides of the invention, or agonists thereof, is
administered to
treat, prevent, prognose and/or diagnose X-linked Ig deficiency with hyper
IgM.
[0045] In another embodiment, Neutrokine-alpha antagonists and/or Neutrokine-
alphaSV antagonists (e.g., an anti-Neutrokine-alpha antibody), are
administered to treat,
prevent, prognose and/or diagnose an autoimmune disease (e.g., rheumatoid
arthritis,
systemic lupus erhythematosus, idiopathic thrombocytopenia purpura, autoimmune
hemolytic anemia, autoimmune neonatal thrombocytopenia, autoimmunocytopenia,
hemolytic anemia, antiphospholipid syndrome, dermatitis, allergic
encephalomyelitis,
myocarditis, relapsing polychondxitis, rheumatic heart disease,
glomerulonephritis (e.g,
IgA nephropathy), an immune-based rheumatologic disease (e.g., SLE, rheumatoid
arthritis, CREST syndrome (a variant of scleroderma characterized by
calcinosis,
Raynaud's phenomenon, esophageal motility disorders, sclerodactyly, and
telangiectasia.),
Seronegative spondyloarthropathy (SpA), Polymyositis/dermatomyositis,
Microscopic
polyangiitis, Hepatitis C-asociated arthritis, Takayasu's arteritis, and
undifferentiated
16
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
connective tissue disorder), Multiple Sclerosis, Neuritis, Uveitis Ophthalmia,
Polyendocrinopathies, Purpura (e.g., Henloch-Scoenlein puzpura), Reiter's
Disease, Stiff-
Man Syndrome, Autoimmune Pulmonary Inflammation, Guillain-Barre Syndrome,
insulin
dependent diabetes mellitis, and autoimmune inflammatory eye, autoimmune
thyroiditis,
hypothyroidism (i.e., Hashimoto's thyroiditis, Goodpasture's syndrome,
Pemphigus,
Receptor autoimmunities such as, for example, (a) Graves' Disease , (b)
Myasthenia
Gravis, and (c) insulin resistance, autoimmune hemolytic anemia, autoimmune
thrombocytopenic purpura , schleroderma with anti-collagen antibodies, mixed
connective
tissue disease, polymyositis/dermatomyositis, pernicious anemia, idiopathic
Addison's
disease, infertility, glomerulonephritis such as primary glomerulonephritis
and IgA
nephropathy, bullous pemphigoid, Sjogren's syndrome, diabetes millitus, and
adrenergic
drug resistance (including adrenergic drug resistance with asthma or cystic
fibrosis),
chronic active hepatitis, primary biliary cirrhosis, other endocrine gland
failure, vitiligo,
vasculitis, post-MI, cardiotomy syndrome, urticaria, atopic dermatitis,
asthma,
inflammatory myopathies, and other inflammatory, granulamatous, degenerative,
and
atrophic disorders) or conditions associated with an autoimmune disease. In a
specific
preferred embodiment, rheumatoid arthritis is treated, prevented, prognosed
and/or
diagnosed using anti-Neutrokine-alpha antibodies and/or anti-Neutrokine-
alphaSV
antibodies and/or other antagonist of the invention. In another specific
preferred
embodiment, systemic lupus erythemosus is treated, prevented, prognosed,
and/or
diagnosed using anti-Neutrolcine-alpha antibodies and/or anti-Neutrokine-
alphaSV and/or
other antagonist of the invention. In another specific preferred embodiment,
idiopathic
thrombocytopenia purpura is treated, prevented, prognosed, and/or diagnosed
using anti-
Neutrokine-alpha antibodies and/or anti-Neutrokine-alphaSV and/or other
antagonist of
the invention. In another specific preferred embodiment IgA nephropathy is
treated,
prevented, prognosed and/or diagnosed using anti-Neutrokine-alpha antibodies
and/or
anti-Neutrokine-alphaSV and/or other antagonist of the invention. In a
preferred
embodiment, the autoimmune diseases and disorders andlor conditions associated
with the
diseases and disorders recited above are treated, prevented, prognosed and/or
diagnosed
using anti-Neutrokine-alpha antibodies and/or anti-Neutrokine-alphaSV
antibodies.
[0046] The invention further provides compositions comprising a Neutrokine-
alpha or
Neutrokine-alphaSV polynucleotide, a Neutrokine-alpha or Neutrokine-alphaSV
17
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polypeptide, and/or an anti-Neutrokine-alpha antibody or anti-Neutrokine-
alphaSV
antibody, for administration to cells ifz vitro, to cells ex vivo, and to
cells isz vivo, or to a
multicellular organism. In preferred embodiments, the compositions of the
invention
comprise a Neutrokine-alpha and/or Neutrokine-alphaSV polynucleotide for
expression of
a Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide in a host organism
for
treatment of disease. In a most preferred embodiment, the compositions of the
invention
comprise a Neutrokine-alpha and/or Neutrokine-alphaSV polynucleotide for
expression of
a Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide in a host organism
for
treatment of an immunodeficiency and/or conditions associated with an
immunodeficiency. Particularly preferred in this regard is expression in a
human patient
for treatment of a dysfunction associated with aberrant endogenous activity of
a
Neutrokine-alpha, Neutrokine-alphaSV, Neutrokine alpha receptor, and/or
Neutrokine-alphaSV receptor gene (e.g., expression to enhance the normal B-
cell function
by expanding B-cell numbers or increasing B cell lifespan).
[0047] The present invention further encompasses methods and compositions for
preventing, treating and/or ameliorating diseases or disorders associated with
aberrant or
inappropriate Neutrokine-alpha, Neutrokine-alphaSV, Neutrokine-alpha receptor,
and/or
Neutrokine-alphaSV receptor expression or function in an animal, preferably a
mammal,
and most preferably a human, comprising, or alternatively consisting of,
administering to
an animal in which such treatment, prevention or amelioration is desired one
or more
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides (including molecules
which
comprise, or alternatively consist of, Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptide fragments or variants thereof) in an amount effective to treat
prevent or
ameliorate the disease or disorder.
[0048] The present invention further encompasses methods and compositions for
killing cells of hematopoietic origin, comprising, or alternatively consisting
of, contacting
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide with cells of
hematopoietic
origin. In preferred embodiments, the cells of hematopoietic origin are B
cells.
[0049] The present invention further encompasses methods and compositions for
killing cells of hematopoietic origin, comprising, or alternatively consisting
of,
administering to an animal in which such killing is desired, a Neutrokine-
alpha and/or
Neutrokine-alphaSV polypeptide (e.g., a radiolabelled Neutrokine-alpha and/or
18
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrokine-alphaSV polypeptide) in an amount effective to kill cells of
hematopoietic
origin. In preferred embodiments, the cells of hematopoietic origin are B
cells.
[0050] The present invention further encompasses methods and compositions for
stimulating immunoglobulin production, comprising, or alternatively consisting
of,
contacting an effective amount of Neutrokine-alpha and/or Neutrokine-alphaSV
with cells
of hematopoietic origin, wherein the effective amount of the Neutrokine-alpha
andlor
Neutrokine-alphaSV binding polypeptide stimulates Neutrokine-alpha and/or
Neutrokine-
alphaSV-mediated immunoglobulin production.
[0051] The present invention further encompasses methods and compositions for
stimulating immunoglobulin production comprising, or alternatively consisting
of,
administering to an animal in which such stimulation is desired, a Neutrokine-
alpha and/or
Neutrokine-alphaSV polypeptide in an amount effective to stimulate
immunoglobulin
production.
[0052] The present invention further encompasses methods and compositions for
stimulating proliferation of cells of hematopoietic origin, comprising, or
alternatively
consisting of, contacting an effective amount of Neutrokine-alpha and/or
Neutrokine-
alphaSV polypeptide with with cells of hematopoietic origin, wherein the
effective
amount of Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide stimulates
Neutrokine-alpha and/or Neutrokine-alphaSV-mediated cell proliferation. In
preferred
embodiments, the cells of hematopoietic origin are B cells.
[0053] The present invention further encompasses methods and compositions for
stimulating proliferation of cells of hematopoietic origin, comprising, or
alternatively
consisting of, administering to an animal in which such stimulation is
desired, a
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide in an amount effective
to
stimulate Neutrokine-alpha and/or Neutrokine-alphaSV-mediated cell
proliferation. In
preferred embodiments, the cells of hematopoietic origin are B cells.
[0054] The present invention further encompasses methods and compositions for
increasing activation of cells of hematopoietic origin, comprising, or
alternatively
consisting of, contacting an effective amount of Neutrokine-alpha andlor
Neutrokine-
alphaSV polypeptide with cells of hematopoietic origin, wherein the effective
amount of
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide increases Neutrokine-
alpha
19
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
and/or Neutrokine-alphaSV-mediated activation of cells of hematopoietic
origin. In
preferred embodiments, the cells of hematopoietic origin are B cells.
[0055] The present invention further encompasses methods and compositions for
increasing activation of cells of hematopoietic origin, comprising, or
alternatively
consisting of, administering to an animal in which such increase is desired, a
Neutrokine-
alpha and/or Neutrokine-alphaSV polypeptide in an amount effective to increase
Neutrokine-alpha and/or Neutrokine-alphaSV-mediated activation of cells of
hematopoietic origin. In preferred embodiments, the cells of hematopoietic
'origin are B
cells.
[0056] The present invention further encompasses methods and compositions for
increasing lifespan of cells of hematopoietic origin, comprising, or
alternatively consisting
of, contacting an effective amount of Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptide with cells of hematopoietic origin, wherein the effective amount
of
Neutroleine-alpha andlor Neutrokine-alphaSV binding polypeptide increases
Neutrokine-
alpha and/or Neutrokine-alphaSV-regulated lifespan of cells of hematopoietic
origin. In
preferred embodiments, the cells of hematopoietic origin are B cells.
[0057] The present invention further encompasses methods and compositions for
increasing lifespan of cells of hematopoietic origin, comprising, or
alternatively consisting
of, administering to an animal in which such increase is desired, a Neutrokine-
alpha
and/or Neutrokine-alphaSV polypeptide in an amount effective to increase
Neutrokine-
alpha and/or Neutrokine-alphaSV-regulated lifespan of cells of hematopoietic
origin. In
preferred embodiments, the cells of hematopoietic origin are B cells.
[0058] The present invention further encompasses methods and compositions for
inhibiting or reducing immunoglobulin production, comprising, or alternatively
consisting
of, contacting an effective amount of Neutrokine-alpha and/or Neutrokine-
alphaSV with
cells of hematopoietic origin, wherein the effective amount of the Neutrokine-
alpha and/or
Neutrokine-alphaSV binding polypeptide inhibits or reduces Neutrokine-alpha
and/or
Neutrokine-alphaSV-mediated immunoglobulin production. In preferred
embodiments,
the cells of hematopoietic origin are B cells.
[0059] The present invention further encompasses methods and compositions for
inhibiting or reducing immunoglobulin production comprising, or alternatively
consisting
of, administering to an animal in which such inhibition or reduction is
desired, a
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrokine-alpha andlor Neutrokine-alphaSV polypeptide in an amount effective
to
inhibit it reduce immunoglobulin production.
[0060] The present invention further encompasses methods and compositions for
inhibiting or reducing proliferation of cells of hematopoietic origin,
comprising, or
alternatively consisting of, contacting an effective amount of Neutrokine-
alpha and/or
Neutrokine-alphaSV polypeptide with cells of hematopoietic origin, wherein the
effective
amount of Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide inhibits it
reduces
Neutrokine-alpha and/or Neutrokine-alphaSV-mediated cell proliferation. In
preferred
embodiments, the cells of hematopoietic origin are B cells.
[0061] The present invention further encompasses methods and compositions for
inhibiting or reducing proliferation of cells of hematopoietic origin,
comprising, or
alternatively consisting of, administering to an animal in which such
inhibition or
reduction is desired, a Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide
in an
amount effective to inhibit or reduce Neutrokine-alpha and/or Neutrokine-
alphaSV-
mediated cell proliferation. In preferred embodiments, the cells of
hematopoietic origin
are B cells. .
[0062] The present invention further encompasses methods and compositions for
decreasing activation of cells of hematopoietic origin, comprising, or
alternatively
consisting of, contacting an effective amount of Neutrokine-alpha and/or
Neutrokine-
alphaSV polypeptide with cells of hematopoietic origin, wherein the effective
amount of
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide decreases Neutrokine-
alpha
and/or Neutrokine-alphaSV-mediated activation of cells of hematopoietic
origin. In
preferred embodiments the cells of hematopoietic origin are B cells.
[0063] The present invention further encompasses methods and compositions for
decreasing activation of cells of hematopoietic origin, comprising, or
alternatively
consisting of, administering to an animal in which such increase is desired, a
Neutrokine-
alpha and/or Neutrokine-alphaSV polypeptide in an amount effective to decrease
Neutrokine-alpha and/or Neutrokine-alphaSV-mediated activation of cells of
hematopoietic origin. In preferred embodiments the cells of hematopoietic
origin are B
cells.
[0064] The present invention further encompasses methods and compositions for
decreasing lifespan of B cells, comprising, or alternatively consisting of,
contacting an
21
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
effective amount of Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide
with cells
of hematopoietic origin, wherein the effective amount of Neutrolcine-alpha
and/or
Neutrokine-alphaSV binding polypeptide decreases Neutrokine-alpha and/or
Neutrolcine-
alphaSV-regulated lifespan of cells of hematopoietic origin. In preferred
embodiments the
cells of hematopoietic origin are B cells.
[0065] The present invention further encompasses methods and compositions for
decreasing lifespan of cells of hematopoietic origin, comprising, or
alternatively
consisting of, administering to an animal in which such reduction is desired,
a Neutrokine-
alpha and/or Neutrokine-alphaSV polypeptide in an amount effective to decrease
Neutrokine-alpha and/or Neutrokine-alphaSV-regulated lifespan of cells of
hematopoietic
origin. In preferred embodiments the cells of hematopoietic origin are B
cells.
[0066] The present invention also provides a screening method for identifying
compounds capable of enhancing or inhibiting a cellular response induced by
Neutrokine-alpha and/or Neutrokine-alphaSV which involves contacting cells
which
express Neutrokine-alpha and/or Neutrokine-alphaSV with the candidate
compound,
assaying a cellular response, and comparing the cellular response to a
standard cellular
response, the standard being assayed when contact is made in absence of the
candidate
compound; whereby, an increased cellular response over the standard indicates
that the
compound is an agonist and a decreased cellular response over the standard
indicates that
the compound is an antagonist.
[0067] In another embodiment, a method for identifying Neutrokine-alpha and/or
Neutrokine-alphaSV receptors is provided, as well as a screening assay for
agonists and
antagonists using such receptors. This assay involves determining the effect a
candidate
compound has on Neutrokine-alpha and/or Neutrokine-alphaSV binding to the
Neutrokine-alpha and/or Neutrokine-alphaSV receptor. In particular, the method
involves
contacting a Neutrokine-alpha and/or Neutrokine-alphaSV receptor with a
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide of the invention and a
candidate
compound and determining whether Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptide binding to the Neutrokine-alpha and/or Neutrokine-alphaSV receptor
is
increased or decreased due to the presence of the candidate compound. The
antagonists
may be employed to prevent septic shock, inflammation, cerebral malaria,
activation of
the HIV virus, graft-host rejection, bone resorption, rheumatoid arthritis,
cachexia
22
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
(wasting or malnutrition), immune system function, lymphoma, and autoimmune
disorders
(e.g., rheumatoid arthritis and systemic lupus erythematosus).
[0068] The present inventors have discovered that Neutroltine-alpha is
expressed not
only in cells of monocytic lineage, but also in kidney, lung, peripheral
leukocyte, bone
marrow, T cell lymphoma, B cell lymphoma, activated T cells, stomach cancer,
smooth
muscle, macrophages, and cord blood tissue. The present inventors have further
discovered that Neutrokine-alphaSV appears to be expressed highly only in
primary
dendritic cells. For a number of disorders of these tissues and cells, such as
tumor and
tumor metastasis, infection of bacteria, viruses and other parasites,
immunodeficiencies
(e.g., chronic variable immunodeficiency), septic shock, inflammation,
cerebral malaria,
activation of the HIV virus, graft-host rejection, bone resorption, rheumatoid
arthritis,
autoimmune diseases (e.g., rheumatoid arthritis and systemic lupus
erythematosus) and
cachexia (wasting or malnutrition). It is believed that significantly higher
or lower levels
of Neutrokine-alpha and/or Neutrokine-alphaSV gene expression can be detected
in
certain tissues (e.g., bone marrow) or bodily fluids (e.g., serum, plasma,
urine, synovial
fluid or spinal fluid) taken from an individual having such a disorder,
relative to a
"standard" Neutrokine-alpha and/or Neutrokine-alphaSV gene expression level,
i.e., the
Neutrokine-alpha and/or Neutrokine-alphaSV expression level in tissue or
bodily fluids
from an individual not having the disorder. Thus, the invention provides a
diagnostic
method useful during diagnosis of a disorder, which involves: (a) assaying
Neutrokine-alpha and/or Neutrokine-alphaSV gene expression level in cells or
body fluid
of an individual; (b) comparing the Neutrokine-alpha and/or Neutrokine-alphaSV
gene
expression level with a standard Neutrokine-alpha and/or Neutrokine-alphaSV
gene
expression level, whereby an increase or decrease in the assayed Neutrokine-
alpha and/or
Neutrokine-alphaSV gene expression level compared to the standard expression
level is
indicative of a disorder.
[0069] An additional embodiment of the invention is related to a method for
treating
an individual in need of an increased or constitutive level of Neutrokine-
alpha and/or
Neutrokine-alphaSV activity in the body comprising administering to such an
individual a
composition comprising a therapeutically effective amount of an isolated
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide of the invention or an
agonist
thereof.
23
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0070] A still further embodiment of the invention is related to a method for
treating
an individual in need of a decreased level of Neutrokine-alpha and/or
Neutrokine-alphaSV
activity in the body comprising, administering to such an individual a
composition
comprising a therapeutically effective amount of an Neutrokine-alpha and/or
Neutrokine-alphaSV antagonist. Preferred antagonists for use in the present
invention are
Neutrokine-alpha-specific and/or Neutrokine-alphaSV-specific antibodies.
BYief Description of the Figures
[0071] The following drawings are illustrative of embodiments of the invention
and
are not meant to limit the scope of the invention as encompassed by the
claims.
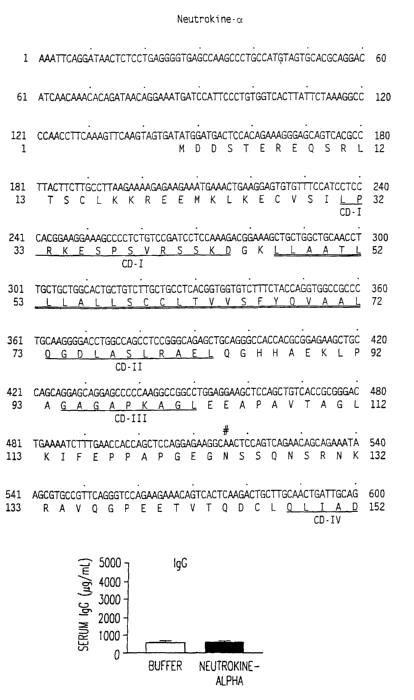
[0072] Figures 1A and 1B show the nucleotide (SEQ ID NO:1) and deduced amino
acid~(SEQ ID N0:2) sequences of Neutrokine-alpha. Amino acids 1 to 46
represent the
predicted intracellular domain, amino acids 47 to 72 the predicted
transmembrane domain
(the double-underlined sequence), and amino acids 73 to 285, the predicted
extracellular
domain (the remaining sequence). Potential asparagine-linked glycosylation
sites are
marked in Figures 1A and 1B with a bolded asparagine symbol (N) in the
Neutrokine-alpha amino acid sequence and a bolded pound sign (#) above the
first
nucleotide encoding that asparagine residue in the Neutrokine-alpha nucleotide
sequence.
Potential N-linked glycosylation sequences are found at the following
locations in the
Neutrokine-alpha amino acid sequence: N-124 through Q-127 (N-124, S-125, S-
126,
Q-127) and N-242 through C-245 (N-242, N-243, S-244, C-245).
[0073] Regions of high identity between Neutrokine-alpha, Neutrokine-alphaSV,
TNF-alpha, TNF-beta, LT-beta, and the closely related Fas Ligand (an alignment
of these
sequences is presented in Figures 2A, 2B, 2C, and 2D) are underlined in
Figures 1A and
1B. These regions are not limiting and are labeled as Conserved Domain (CD)-I,
CD-II,
CD-III, CD-IV, CD-V, CD-VI, CD-VII, CD-VIII, CD-IX, CD-X, and CD-XI in Figures
1A and 1B.
j0074] Figures 2A, 2B, 2C, and 2D show the regions of identity between the
amino
acid sequences of Neutrokine-alpha (SEQ TD N0:2) and Neutrokine-alphaSV (SEQ
ID
N0:19), and TNF-alpha ("TNFalpha" in Figures 2A, 2B, 2C, and 2D; GenBank No.
215026; SEQ ID N0:3), TNF-beta ("TNFbeta" in Figures 2A, 2B, 2C, and 2D;
GenBank
No. 215026; SEQ ID N0:4), Lymphotoxin-beta ("LTbeta" in Figures 2A, 2B, 2C,
and 2D;
24
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
GenBank No. L11016; SEQ ID .N0:5), and FAS ligand ("FASL" in Figures 2A, 2B,
2C,
and 2D; GenBank No. LT11821; SEQ ID N0:6), determined by the "MegAlign"
routine
which is part of the computer program called "DNA~~STAR." Residues that match
the
consensus are shaded.
[0075] Figure 3 shows an analysis of the Neutrol~ine-alpha amino acid
sequence.
Alpha, beta, turn and coil regions; hydrophilicity and hydrophobicity;
amphipathic
regions; flexible regions; antigenic index and surface probability are shown,
as predicted
for the amino acid sequence of SEQ ID N0:2 using the default parameters of the
recited
computer programs. In the "Antigenic Index - Jameson-Wolf" graph, the indicate
location
of the highly antigenic regions of Neutrokine-alpha i.e., regions from which
epitope-bearing peptides of the invention may be obtained. Antigenic
polypeptides
include from about Phe-115 to about Leu-147, from about Ile-150 to about Tyr-
163, from
about Ser-171 to about Phe-194, from about Glu-223 to about Tyr-246, and from
about
Ser-271 to about Phe-278, of the amino acid sequence of SEQ ID N0:2.
[0076] The data presented in Figure 3 are also represented in tabular form in
Table I.
The columns are labeled with the headings "Res", "Position", and Roman
Numerals
I-XIV. The column headings refer to the following features of the amino acid
sequence
presented in Figure 3, and Table I: "Res": amino acid residue of SEQ ID N0:2
and
Figures 1A and 1B; "Position": position of the corresponding residue within
SEQ ID N0:2
and Figures 1A and 1B; I: Alpha, Regions - Gamier-Robson; II: Alpha, Regions -
Chou-Fasman; III: Beta, Regions - Gamier-Robson; IV: Beta, Regions - Chou-
Fasman; V:
Turn, Regions - Gamier-Robson; VI: Turn, Regions - Chou-Fasman; VII: Coil,
Regions -
Garnier-Robson; VIII: Hydrophilicity Plot - I~yte-Doolittle; IX:
Hydrophobicity Plot -
Hopp-Woods; X: Alpha, Arnphipathic Regions - Eisenberg; XI: Beta, Amphipathic
Regions - Eisenberg; XII: Flexible Regions - Karplus-Schulz; XIII: Antigenic
Index -
Jameson-Wolf; and XIV: Surface Probability Plot - Emini.
[0077] Figures 4A, 4B, and 4C show the alignment of the Neutrokine-alpha
nucleotide sequence determined from the human cDNA deposited in ATCC No. 97768
with related human cDNA clones of the invention which have been designated
HSOAD55
(SEQ ID N0:7), HSLAH84 (SEQ ID N0:8) and HLTBM08 (SEQ ID N0:9).
[0078] Figures 5A and 5B shows the nucleotide (SEQ ID N0:18) and deduced amino
acid (SEQ ID N0:19) sequences of the Neutrokine-alphaSV protein. Amino acids 1
to 46
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
represent the predicted intracellular domain, amino acids 47 to 72 the
predicted
transmembrane domain (the double-underlined sequence), and amino acids 73 to
266, the
predicted extracellular domain (the remaining sequence). Potential asparagine-
linked
glycosylation sites are marlced in Figures 5A and 5B with a bolded asparagine
symbol (N)
in the Neutrokine-alphaSV amino acid sequence and a bolded pound sign (#)
above the
first nucleotide encoding that asparagine residue in the Neutrokine-alphaSV
nucleotide
sequence. Potential N-linked glycosylation sequences are found at the
following locations
in the Neutrokine-alphaSV amino acid sequence: N-124 through Q-127 (N-124, S-
125,
S-126, Q-127) and N-223 through C-226 (N-223, N-224, S-225, C-226). Antigenic
polypeptides include from about Pro-32 to about Leu-47, from about Glu-116 to
about
Ser-143, from about Phe-153 to about Tyr-173, from about Pro-218 to about Tyr-
227,
from about Ala-232 to about Gln-241; from about Ile-244 to about Ala-249; and
from
about Ser-252 to about Val-257 of the amino acid sequence of SEQ ID N0:19.
[0079] Regions of high identity between Neutrokine-alpha, Neutrokine-alphaSV,
TNF-alpha, TNF-beta, LT-beta, and the closely related Fas Ligand (an augment
of these
sequences is presented in Figure 2) are underlined in Figures 1A and 1B. These
conserved
regions (of Neutrokine-alpha and Neutrokine-alphaSV) are labeled as Conserved
Domain
(CD)-I, CD-II, CD-III, CD-V, CD-VI, CD-VII, CD-VIII, CD-IX, CD-X, and CD-XI in
Figures 5A and 5B. Neutrokine-alphaSV does not contain the sequence of CD-IV
described in the legend of Figures 1A and 1B.
[0080] An additional alignment of the Neutrokine-alpha polypeptide sequence
(SEQ
II? N0:2) with APRIL, TNF alpha, and LT alpha is presented in Figures 7A-1 and
7A-2.
In Figures 7A-1 and 7A-2, beta sheet regions are indicated as described below
in the
legend to Figures 7A-1 and 7A-2.
[0081] Figure 6 shows an analysis of the Neutrokine-alphaSV amino acid
sequence.
Alpha, beta, turn and coil regions; hydrophilicity and hydrophobicity;
amphipathic
regions; flexible regions; antigenic index and surface probability are shown,
as predicted
for the amino acid sequence of SEQ ID NO:19 using the default parameters of
the recited
computer programs. The location of the highly antigenic regions of the
Neutrokine-alpha
protein, i.e., regions from which epitope-bearing peptides of the invention
may be
obtained is indicated in the "Antigenic Index - Jameson-Wolf" graph. Antigenic
polypeptides include, but are not limited to, a polypeptide comprising amino
acid residues
26
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
from about Pro-32 to about Leu-47, from about Glu-116 to about Ser-143, from
about
Phe-153 to about Tyr-173, from about Pro-218 to about Tyr-227, from about Ser-
252 to
about Thr-258, from about Ala-232 to about Gln-241; from about Ile-244 to
about
Ala-249; and from about Ser-252 to about Val-257, of the amino acid sequence
of SEQ ID
N0:19.
[0082] The data shown in Figure 6 can be easily represented in tabular format
similar
to the data shown in Table I. Such a tablular representation of the exact data
disclosed in
Figure 6 can be generated using the MegAlign component of the DNA*STAR
computer
sequence analysis package set on default parameters. This is the identical
program that
was used to generate Figures 3 and 6 of the present application.
[0083] Figures 7A-1 and 7A-2. The amino-acid sequence of Neutrokine-alpha and
alignment of its predicted ligand-binding domain with those of APRIL, TNF-
alpha, and
LT-alpha (specifically, amino acid residues 115-250 of the human APRIL
polypeptide
(SEQ ID N0:20; GenBank Accession No. AF046888 (nucleotide) and AAC6132
(protein)), amino acid residues 88-233 of TNF alpha (SEQ ID N0:3; GenBank
Accession
No. 215026), and LT alpha ((also designated TNF-beta) amino acid residues 62-
205 of
SEQ ID N0:4; GenBank Accession No. 215026)). The predicted membrane-spanning
region of Neutrokine-alpha is indicated and the site of cleavage of Neutrokine-
alpha is
depicted with an arrow. Sequences overlaid with lines (A thru H) represent
predicted
beta-pleated sheet regions.
[0084] Figure 7B. Expression of Neutrokine-alpha mRNA. Northern hybridization
analysis was performed using the Neutrokine-alpha orf as a probe on blots of
poly (A)+
RNA (Clonetech) from a spectrum of human tissue types and a selection of
cancer cell
lines. A 2.6 kb Neutrokine-alpha mRNA was detected at high levels in placenta,
heart,
lung, fetal liver, thymus, and pancreas. The 2.6 kb Neutrokine-alpha mRNA was
also
detected in HL-60 and I~562 cell lines.
[0085] Figures 8A, 8B and 8C. Neutrokine-alpha expression increases following
activation of human monocytes by IFN-gamma. Figures 8A and 8B. Flow cytometric
analysis of Neutrokine-alpa protein expression on ifa vitro cultured
monocytes. Purified
monocytes were cultured for 3 days in presence or absence of IFN-gamma (100
U/ml).
Cells were then stained with a Neutrokine-alpha-specific mAb (2E5) (solid
lines) or an
isotype-matched control (IgGl) (dashed lines). Comparable results were
obtained with
27
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
monocytes purified from three different donors in three independent
experiments. Figure
8C. Neutrolcine-alpha-specific TaqMan primers were prepared and used to assess
the
relative Neutrolcine-alpha mRNA expression levels in unstimulated and IFN-
gamma (100
U/rnL) treated monocytes. Nucleotide sequences of the TaqMan primers are as
follows:
(a) Probe: 5'-CCA CCA GCT CCA GGA GAA GGC AAC TC-3' (SEQ ID N0:24); (b) 5'
amplification primer: 5'-ACC GCG GGA CTG AAA ATC T-3' (SEQ >D N0:25); and (c)
3' amplification primer: 5'-CAC GCT TAT TTC TGC TGT TCT GA-3' (SEQ 1D N0:26).
[0086] Figures 9A and 9B. Neutrokine-alpha is a potent B lymphocyte
stimulator.
Figure 9A. The biological activity of Neutrokine-alpha was assessed in a
standard B-
lymphocyte co-stimulation assay utilizing Staphylococcus aureus cowan 1 SAC as
the
priming agent. SAC alone yielded background counts of 1427 +/- 316. Values are
reported as mean +/- standard deviation of triplicate wells. Similar results
were obtained
using recombinant Neutrokine-alpha purified from stable CHO transfectants and
transiently transfected HEK 293T cells. Figure 9B. Proliferation of tonsillar
B cells with
Neutrokine-alpha and co-stimulation with anti-IgM. The bioassay was performed
as
described for SAC with the exception that individual wells were pre-coated
with goat anti-
human IgM antibody at 10 micrograms/mL in PBS.
[0087] Figures 10A, 10B, 10C, 10D, 10E, 10F and 10G. Neutrokine-alpha receptor
expression among normal human peripheral blood mononuclear cells and tumor
cell lines.
Figures 10A, 10B, 10C, 10D and 10E. Human peripheral blood nucleated cells
were
obtained from normal volunteers and isolated by density gradient
centrifugation. Cells
were stained with biotinylated Neutrokine-alpha followed by PE-conjugated
streptavidin
and FITC or PerCP coupled mAbs specific for CD3, CD20, CD14, CD56, and CD66b.
Cells were analyzed on a Becton Dickinson FACScan using the CellQuest
software. Data
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
were counter-stained with Mayer's hematoxylin. CD45R(B220) expressing cells
appear
brown. Figures 11B and 11C. Flow cytometric analyses of normal (left panel)
and
Neutrolcine-alpha-treated (right panel) stained with PE-CD45R(B220) and FITC-
ThB
(Ly6D). Figures 11D, 11E, and 11F. Serum IgM, IgG, and IgA levels in normal
and
Neutrol~ine-alpha treated mice.
Detailed Description
[0089] The present invention provides isolated nucleic acid molecules
comprising a
polynucleotide encoding a Neutrokine-alpha polypeptides having the amino acid
sequences shown in Figures 1A and 1B (SEQ ID N0:2), which was determined by
sequencing a cDNA clone. The nucleotide sequence shown in Figures 1A and 1B
(SEQ
ID N0:1) was obtained by sequencing the HNEDU15 clone, which was deposited on
October 22, 1996 at the American Type Culture Collection, 10801 University
Boulevard,
Manassas, Virginia 20110-2209, and assigned ATCC Accession No. 97768. The
deposited clone is contained in the pBluescript SK(-) plasmid (Stratagene, La
Jolla, CA).
The ATCC deposits were made pursuant to the terms of the Budapest Treaty on
the
international recognition of the deposit of microorganisms for the purposes of
patent
procedure.
[0090] The present invention also provides isolated nucleic acid molecules
comprising
a polynucleotide encoding Neutrokine-alphaSV polypeptides having the amino
acid
sequences shown in Figures 5A and 5B (SEQ ID N0:19), which was determined by
sequencing a cDNA clone. The nucleotide sequence shown in Figures 5A and 5B
(SEQ
ID N0:18) was obtained by sequencing the HI~PMC52 clone, which was deposited
on
December 10, 1998 at the American Type Culture Collection, and assigned ATCC
Accession No. 203518. The deposited clone is contained in the pBluescript SK(-
) plasmid
(Stratagene, La Jolla, CA). The ATCC deposits were made pursuant to the terms
of the
Budapest Treaty on the international recognition of the deposit of
microorganisms for the
purposes of patent procedure.
[0091] The Neutrokine-alpha and Neutrokine-alpha polypeptides of the present
invention share sequence homology with the translation products of the human
mRNAs
for TNF-alpha, TNF-beta, LTbeta, Fas ligand, APRIL, and LTalpha. (See, Figures
2A,
2B, 2C, 2D, 7A-1 and 7A-2). As noted above, TNF-alpha is thought to be an
important
29
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
cytokine that plays a role in cytotoxicity, necrosis, apoptosis,
costimulation, proliferation,
lymph node formation, immunoglobulin class switch, differentiation, antiviral
activity,
and regulation of adhesion molecules and other cytokines and growth factors.
Nucleic Acid Molecules
[0092] Unless otherwise indicated, all nucleotide sequences determined by
sequencing
a DNA molecule herein were determined using an automated DNA sequencer (such
as the
Model 373 from Applied Biosystems, Inc., Foster City, CA), and all amino acid
sequences
of polypeptides encoded by DNA molecules determined herein were predicted by
translation of a DNA sequence determined as above. Therefore, as is known in
the art for
any DNA sequence determined by this automated approach, any nucleotide
sequence
determined herein may contain some errors. Nucleotide sequences determined by
automation are typically at least about 90% identical, more typically at least
about 95% to
at least about 99.9% identical to the actual nucleotide sequence of the
sequenced DNA
molecule. The actual sequence can be more precisely determined by other
approaches
including manual DNA sequencing methods well known in the art. As is also
known in
the art, a single insertion or deletion in a determined nucleotide sequence
compared to the
actual sequence will cause a frame shift in translation of the nucleotide
sequence such that
the predicted amino acid sequence encoded by a determined nucleotide sequence
will be
completely different from the amino acid sequence actually encoded by the
sequenced
DNA molecule, beginning at the point of such an insertion or deletion. ,
[0093] By "nucleotide sequence" of a nucleic acid molecule or polynucleotide
is
intended, for a DNA molecule or polynucleotide, a sequence of
deoxyribonucleotides, and
for an RNA molecule or polynucleotide, the corresponding sequence of
ribonucleotides
(A, G, C and U), where each thymidine deoxyribonucleotide (T) in the specified
deoxyribonucleotide sequence is replaced by the ribonucleotide uridine (U).
[0094] Using the information provided herein, such as the nucleotide sequence
in
Figures 1A and 1B, a nucleic acid molecule of the present invention encoding a
Neutrokine-alpha polypeptide may be obtained using standard cloning and
screening
procedures, such as those for cloning cDNAs using mRNA as starting material.
Illustrative of the invention, the nucleic acid molecule described in Figures
1A and 1B
(SEQ ID NO:1) was discovered in a cDNA library derived from neutrophils.
Expressed
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
sequence tags corresponding to a portion of the Neutrolcine-alpha cDNA were
also found
in kidney, lung, peripheral leukocyte, bone marrow, T cell lymphoma, B cell
lymphoma,
activated T cells, stomach cancer, smooth muscle, macrophages, and cord blood
tissue. In
addition, using the nucleotide information provided in Figures 5A and 5B, a
nucleic acid
molecule of the present invention encoding a Neutrokine-alphaSV polypeptide
may be
obtained using standard cloning and screening procedures, such as those for
cloning
cDNAs using mRNA as starting material. Illustrative of the invention, the
nucleic acid
molecule described in Figures 5A and 5B (SEQ ID N0:18) was discovered in a
cDNA
library derived from primary dendritic cells.
[0095] The Neutrokine-alpha plasmid HNEDU15 deposited as ATCC Accession No.
97768 contains an open reading frame encoding a protein of about 285 amino
acid
residues, a predicted intracellular domain of about 46 amino acids (amino acid
residues
from about 1 to about 46 in Figures 1A and 1B (SEQ ' ID N0:2)), a predicted
transmembrane domain of about 26 amino acids (underlined amino acid residues
from
about 47 to about 72 in Figures 1A and 1B (SEQ D7 N0:2)), a predicted
extracellular
domain of about 213 amino acids (amino acid residues from about 73 to about
285 in
Figures 1A and 1B (SEQ ID N0:2)); and a deduced molecular weight of about 31
kDa.
The Neutrokine-alpha polypeptide shown in Figures 1A and 1B (SEQ ID NO:2) is
about
20% similar and about 10 % identical to human TNF-alpha, which can be accessed
on
GenBank as Accession No. 339764.
[0096] The Neutrokine-alphaSV plasmid HDPMC52, deposited as ATCC Accession
No. 203518, contains a predicted open reading frame encoding a protein of
about 266
amino acid residues, a predicted intracellular domain of about 46 amino acids
(amino acid
residues from about 1 to about 46 in Figures 5A and 5B (SEQ ID N0:19)), a
predicted
transmembrane domain of about 26 amino acids (underlined amino acid residues
from
about 47 to about 72 in Figures 5A and 5B (SEQ ID N0:19)), a predicted
extracellular
domain of about 194 amino acids (amino acid residues from about 73 to about
266 in
Figures 5A and 5B (SEQ ID N0:19)); and a deduced molecular weight of about 29
kDa.
The Neutrokine-alphaSV polypeptide shown in Figures 5A and 5B (SEQ ID N0:19)
is
about 33.9% similar and about 22.0% identical to human TNF-alpha which can be
accessed on GenBank as Accession No. 339764. As one of ordinary skill would
appreciate, due to the possibilities of sequencing errors discussed above, the
actual
31
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
complete Neutrokine-alpha and/or Neutrolcine-alphaSV polypeptides encoded by
the
deposited cDNAs, which comprise about 285 and 266 amino acids, respectively,
may be
somewhat shorter. In particular, the determined Neutrokine-alpha and
Neutroltine-alphaSV coding sequences contain a common second methionine codon
which
may serve as an alternative start codon for translation of the open reading
frame, at
nucleotide positions 210-212 in Figures 1A and 1B (SEQ ID N0:1) and at
nucleotide
positions 64-66 in Figures 5A and 5B (SEQ ID NO:18). More generally, the
actual open
reading frame may be anywhere in the range of ~20 amino acids, more likely in
the range
of ~10 amino acids, of that predicted from either the first or second
methionine codon
from the N-terminus shown in Figures 1A and 1B (SEQ LD NO:1) and in Figures 5A
and
5B (SEQ ID N0:18). It will further be appreciated that, the polypeptide
domains
described herein have been predicted by computer analysis, and accordingly,
that
depending on the analytical criteria used for identifying various functional
domains, the
exact "address" of the extracellular, intracellular and transmembrane domains
of the
Neutrokine-alpha and Neutrokine-alphaSV polypeptides may differ slightly. For
example,
the exact location of the Neutrokine-alpha and Neutrokine-alphaSV
extracellular domains
in Figures 1A and 1B (SEQ ~ N0:2) and Figures 5A and 5B (SEQ TD N0:19) may
vary
slightly (e.g., the address may "shift" by about 1 to about 20 residues, more
likely about 1
to about 5 residues) depending on the criteria used to define the domain. In
this case, the
ends of the transmembrane domains and the beginning of the extracellular
domains were
predicted on the basis of the identification of the hydrophobic amino acid
sequence in the
above indicated positions, as shown in Figures 3 and 6 and in Table I. In any
event, as
discussed further below, the invention further provides polypeptides having
various
residues deleted from the N-terminus and/or C-terminus of the complete
polypeptides,
including polypeptides lacking one or more amino acids from the N-termini of
the
extracellular domains described herein, which constitute soluble forms of the
extracellular
domains of the Neutrokine-alpha and Neutrokine-alphaSV polypeptides.
[0097] As indicated, nucleic acid molecules and polynucleotides of the present
invention may be in the form of RNA, such as mRNA, or in the form of DNA,
including,
for instance, cDNA and genomic DNA obtained by cloning or produced
synthetically.
The DNA may be double-stranded or single-stranded. Single-stranded DNA or RNA
may
32
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
be the coding strand, also known as the sense strand, or it may be the non-
coding strand,
also referred to as the anti-sense strand.
[0098] By "isolated" nucleic acid molecules) is intended a nucleic acid
molecule
(DNA or RNA), which has been removed from its native environment. For example,
recombinant DNA molecules contained in a vector are considered isolated for
the
purposes of the present invention. Further examples of isolated DNA molecules
include
recombinant DNA molecules maintained in heterologous host cells or purified
(partially or
substantially) DNA molecules in solution. Isolated RNA molecules include if2
vivo or in
vitro RNA transcripts of the DNA molecules of the present invention. However,
a nucleic
acid contained in a clone that is a member of a library (e.g., a genomic or
cDNA library)
that has not been isolated from other members of the library (e.g., in the
form of a
homogeneous solution containing the clone and other members of the library) or
a
chromosome isolated or removed from a cell or a cell lysate (e.g., a
"chromosome spread",
as in a karyotype), is not "isolated" for the purposes of this invention. As
discussed
further herein, isolated nucleic acid molecules according to the present
invention may be
produced naturally, recombinantly, or synthetically.
[0099] Isolated nucleic acid molecules of the present invention include DNA
molecules comprising, or alternatively consisting of, an open reading frame
(ORF) with an
initiation codon at positions 147-149 of the nucleotide sequence shown in
Figures 1A and
1B (SEQ ID NO:1). In addition, isolated nucleic acid molecules of the
invention include
DNA molecules which comprise, or alternatively consist of, a sequence
substantially
different from those described above, but which due to the degeneracy of the
genetic code,
still encodes the Neutrokine-alpha protein. Of course, the genetic code is
well known in
the art. Thus, it would be routine for one skilled in the art to generate the
degenerate
variants described above. In another embodiment, the invention provides
isolated nucleic
acid molecules comprising, or alternatively consisting of, a sequence encoding
the
Neutrokine-alpha polypeptide having an amino acid sequence encoded by the cDNA
contained in the plasmid having ATCC accession number 97768. Preferably, this
nucleic
acid molecule comprises, or alternatively consists of a sequence encoding the
extracellular
domain the mature or soluble polypeptide sequence of the polypeptide encoded
by the
cDNA contained in the plasmid having ATCC accession number 97768.
33
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0100] Isolated nucleic acid molecules of the present invention also include
DNA
molecules comprising an open reading frame (ORF) with an initiation codon at
positions
1-3 of the nucleotide sequence shown in Figures 5A and 5B (SEQ ID N0:18). In
addition, isolated nucleic acid molecules of the invention include DNA
molecules which
comprise, or alternatively consist of, a sequence substantially different from
those
described above, but which due to the degeneracy of the genetic code, still
encodes the
Neutrokine-alphaSV polypeptide. Of course, the genetic code is well known in
the art.
Thus, it would be routine for one skilled in the art to generate the
degenerate variants
described above. In another embodiment, the invention provides isolated
nucleic acid
molecules comprising, or alternatively consisting of, a sequence encoding the
Neutrokine-alphaSV polypeptide having an amino acid encoded by the cDNA
contained
in the plasmid having ATCC accession number 203518. Preferably, this nucleic
acid
molecule comprises, or alternatively consists of, a sequence encoding the
extracellular
domain or the mature soluble polypeptide sequence of the polypeptide encoded
by the
cDNA contained in the plasmid having ATCC accession number 203518.
[0101] The invention further provides an isolated nucleic acid molecule
comprising, or
alternatively consisting of, the nucleotide sequence shown in Figures 1A and
1B (SEQ ID
NO:1) or the nucleotide sequence of the Neutrokine-alpha cDNA contained in the
plasmid
having ATCC accession number 97768, or a nucleic acid molecule having a
sequence
complementary to one of the above sequences. In addition, the invention
provides an
isolated nucleic acid molecule comprising, or alternatively, consisting of,
the nucleotide
sequence shown in Figures 5A and 5B (SEQ ID N0:18) or the nucleotide sequence
of the
Neutrokine-alpha SV cDNA contained in the plasmid having ATCC accession,
number
203518, or a nucleic acid molecule having a sequence complementary to one of
the above
sequences. Such isolated molecules, particularly DNA molecules, have uses
which
include, but are not limited to, as probes for gene mapping by ifZ situ
hybridization with
chromosomes, and for detecting expression of the Neutrokine-alpha and
Neutrokine-alphaSV in human tissue, for instance, by Northern or Western blot
analysis.
[0102] In one embodiment, the polynucleotides of the invention comprise, or
alternatively consist of, the sequence shown in SEQ ID N0:22. The sequence
provided as
SEQ )D N0:22 was constructed from several overlapping mouse EST sequences
obtained
from GenBank (AI182472, AA422749, AA254047, and AI122485). The EST sequences
34
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
were aligned to generate the Neutrolune-alpha-like polynucleotide sequence
provided as
SEQ ID N0:22. The amino acid sequence resulting from the translation of SEQ ID
N0:22 is provided as SEQ ff~ N0:23. Fragments, variants, and derivatives of
the
sequences provided as SEQ ID N0:22 and SEQ ID N0:23 are also encompassed by
the
invention.
[0103] In another embodiment, the polynucleotides of the invention comprise,
or
alternatively consist of, the sequence shown in SEQ ID NO:27, and/or a
sequence
encoding the amino acid sequence disclosed in SEQ ID N0:28, fragments,
variants, and
derivatives thereof. These polynucleotides are also encompassed by the
invention. For
example, certain embodiments of the invention are directed to polynucleotides
comprising, or alternatively consisting of, a sequence encoding a polypeptide
sequence
that is at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98%, or 99% identical to
amino
acids 68-219 of SEQ ID N0:28. The amino acid sequence resulting from the
translation
of SEQ ID N0:27 is provided as SEQ ID N0:28. Polypeptides comprising, or
alternatively consisting of, the amino acid sequence of SEQ D7 N0:28, and
fragments,
variants, and derivatives of the sequence provided as SEQ ID N0:28 are also
encompassed by the invention. For example, certain embodiments of the
invention are
directed to polypeptides comprising, or alternatively consisting of, a
polypeptide sequence
that is at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98%, or 99% identical to
amino
acids 68-219 of SEQ )D N0:28. A nucleic acid molecule having the sequence
provided as
SEQ ID NO:27 was obtained by RT-PCR from cyanomologous monkey (i.e., Macaca
irus) PBMC using two degenerate primers. Briefly, total RNA was prepared from
cyanornologous monkey PBMC by using Trizol (available from Life Technologies,
Inc.,
Rockville, MD) according to the manufacturer's protocol. Then a single
stranded cDNA
was synthesized from the cyanomologous monkey PBMC preparation using standard
methods with an oligo-dT primer. Neutrokine-alpha-specific primers were
designed based
on the conserved region between the mouse and human Neutrokine-alpha molecules
(SEQ
ID NOs:22 and 1, respectively). A cyanomologous monkey Neutrokine-alpha
nucleic acid
molecule was then generated by PCR using the cDNA template in combination with
the
following two degenerate oligonucleotide primers. 5' primer: 5'-TAC CAG ITG
GCI
GCC ITG CAA G-3' (SEQ ID NO:35) and 3' primer: 5'-GTI ACA GCA GTT TIA IIG
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
CAC C-3' (SEQ ID N0:36). In the sequence of the degenerate primers (SEQ ID
NOs:35
and 36), "I" represents deoxyinosine or dideoxyinosine.
[0104] In another embodiment, the polynucleotides of the invention comprise,
or
alternatively consist of, the sequence shown in SEQ ID N0:29, and/or a
sequence
encoding the amino acid sequence disclosed in SEQ ID N0:30, fragments,
variants, and
derivatives thereof. These polynucleotides are also encompassed by the
invention. For
example, certain embodiments of the invention are directed to polynucleotides
comprising, or alternatively consisting of, a sequence encoding a polypeptide
sequence
that is at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98%, or 99% identical to
amino
acids 68-219 of SEQ ID N0:30. The amino acid sequence resulting from the
translation
of SEQ ID N0:29 is provided as SEQ ID N0:30. Polypeptides comprising, or
alternatively consisting of, the amino acid sequence of SEQ ID N0:30, and
fragments,
variants, and derivatives of the sequences provided as SEQ ID N0:29 and SEQ ID
N0:30
are also encompassed by the invention. For example, certain embodiments of the
invention are directed to polypeptides comprising, or alternatively consisting
of, a
polypeptide sequence that is at least 80%, 85%, 9Q%, 92%, 95%, 96%, 97%, 98%,
or 99%
identical to amino acids 68-219 of SEQ Ip N0:30. A nucleic acid molecule
having the
sequence provided as SEQ ID N0:29 was obtained by RT-PCR from rhesus monkey
PBMC using two degenerate primers. Briefly, total RNA was prepared from rhesus
monkey PBMC by using Trizol (available from Life Technologies, Inc.,
Rockville, MD)
according to the manufacturer's protocol. Then a single stranded cDNA was
synthesized
from the rhesus monkey PBMC preparation using standard methods with an oligo-
dT
primer. Neutrokine-alpha-specific primers were designed based on the conserved
region
between the mouse and human Neutrokine-alpha molecules (SEQ ID NOs:22 and 1,
respectively). A rhesus monkey Neutrokine-alpha nucleic acid molecule was then
generated by PCR using the cDNA template in combination with the following two
degenerate oligonucleotide primers. 5' primer: 5'-TAC CAG ITG GCI GCC ITG CAA
G-
3' (SEQ ID N0:35) and 3' primer: 5'-GTI ACA GCA GTT TIA IIG CAC C-3' (SEQ ID
N0:36). In the sequence of the degenerate primers (SEQ ID NOs:35 and 36), "I"
represents deoxyinosine or dideoxyinosine.
[0105] The invention also provides nucleic acid molecules having nucleotide
sequences related to extensive portions of SEQ ID NO:1 and SEQ ID N0:18 which
have
36
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
been determined from the following related cDNA clones: HSOAD55 (SEQ ID N0:7),
HSLAH84 (SEQ ID N0:8), and HLTBM08 (SEQ ID N0:9).
[0106] The present invention is further directed to nucleic acid molecules
encoding
portions of the nucleotide sequences described herein, as well as to fragments
of the
isolated nucleic acid molecules described herein. In one embodiment, the
invention
provides a polynucleotide having a nucleotide sequence representing the
portion of SEQ
ID NO:1 which consists of the nucleotides at positions 1-1001 of SEQ ff~ NO:1.
In
another embodiment, the invention provides a polynucleotide having a
nucleotide
sequence representing the portion of SEQ ID N0:18 which consists of positions
1-798 of
SEQ )D NO:18.
[0107] The present invention is further directed to fragments of the nucleic
acid
molecules (i.e. polynucleotides) described herein. By a fragment of a nucleic
acid
molecule having, for example, the nucleotide sequence of the cDNA contained in
the
plasmid having ATCC accession number 97768, a nucleotide sequence encoding the
polypeptide sequence encoded by the cDNA contained in the plasmid having ATCC
accession number 97768, the nucleotide sequence of SEQ ID NO:1, a nucleotide
sequence
encoding the polypeptide sequence of SEQ ID N0:2, the nucleotide sequence of
the
cDNA contained in the plasmid having ATCC accession number 203518, a
nucleotide
sequence encoding the polypeptide sequence encoded by the cDNA contained in
the
plasmid having ATCC accession number 203518, the nucleotide sequence of SEQ ID
NO:18, a nucleotide sequence encoding the polypeptide sequence of SEQ ID
N0:19, or
the complementary strand thereto, is intended fragments at least 15 nt, and
more
preferably at least 20 nt or at least 25 nt, still more preferably at least 30
nt, and even more
preferably, at least 40, 50, 100, 150, 200, 250, 300, 325, 350, 375, 400, 450,
or 500 nt in
length. These fragments have numerous uses which include, but are not limited
to,
diagnostic probes and primers as discussed herein. Of course, larger
fragments, such as
those of 501-1500 nt in length are also useful according to the present
invention as are
fragments corresponding to most, if not all, of the nucleotide sequences of
the cDNA
contained in the plasmid having ATCC accession number 97768, the nucleotide
sequence
of SEQ ID NO:l, the nucleotide sequences of the cDNA contained in the plasmid
having
ATCC accession number 203518, and the nucleotide sequence of SEQ ID N0:18.
Preferred nucleic acid fragments of the present invention include nucleic acid
molecules
37
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
encoding polypeptides comprising, or alternatively, consisting of, epitope-
bearing portions
of the Neutrolcine-alpha andlor Neutrokine-alphaSV polypeptide as identified
in Figures
1A and 1B (SEQ ID N0:2) and in Figures 5A and 5B (SEQ ID N0:19), respectively,
and
described in more detail below. Polypeptides encoded by these polynucleotide
fragments
are also encompassed by the invention.
[0108] Also by a fragment of a nucleic acid molecule having, for example, the
nucleotide sequence of SEQ 117 N0:21, the nucleotide sequence of SEQ ID N0:22,
the
nucleotide sequence of SEQ ID N0:27, the nucleotide sequence of SEQ ID N0:29,
the
nucleotide sequence of SEQ ID N0:37, a nucleotide sequence encoding the
polypeptide
sequence of SEQ ID N0:23, a nucleotide sequence encoding the polypeptide
sequence of
SEQ ID N0:28, a nucleotide sequence encoding the polypeptide sequence of SEQ
ID
N0:30, a nucleotide sequence encoding the polypeptide sequence of SEQ 117
N0:38, a
nucleotide sequence encoding the polypeptide sequence of SEQ ID N0:39, a
nucleotide
sequence encoding the polypeptide sequence of SEQ ID N0:40, a nucleotide
sequence
encoding the polypeptide sequence of SEQ ID N0:41, a nucleotide sequence
encoding the
polypeptide sequence of SEQ ID N0:42, a nucleotide sequence encoding the
polypeptide
sequence of SEQ ID N0:43, a nucleotide sequence encoding the polypeptide
sequence of
SEQ ID NO:44, or the complementary strands thereof, is intended fragments at
least 15 nt,
and more preferably at least 20 nt or at least 25 nt, still more preferably at
least 30 nt, and
even more preferably, at least 40, 50, 100, 150, 200, 250, 300, 325, 350, 375,
400, 450, or
500 nt in length. These fragments have numerous uses which include, but are
not limited
to, diagnostic probes and primers as discussed herein. Of course, larger
fragments, such as
those of 501-1500 nt in length are also useful according to the present
invention as are
fragments corresponding to most, if not all, of the nucleotide sequence of SEQ
ID N0:21,
the nucleotide sequence of SEQ ID N0:22, the nucleotide sequence of SEQ ID
N0:27, the
nucleotide sequence of SEQ ID N0:29, the nucleotide sequence of SEQ ID NO:37,
a
nucleotide sequence encoding the polypeptide sequence of SEQ ID NO:23, a
nucleotide
sequence encoding the polypeptide sequence of SEQ ID N0:28, a nucleotide
sequence
encoding the polypeptide sequence of SEQ ID N0:30 a nucleotide sequence
encoding the
polypeptide sequence of SEQ ID N0:38, a nucleotide sequence encoding the
polypeptide
sequence of SEQ ID N0:39, a nucleotide sequence encoding the polypeptide
sequence of
SEQ ID N0:40, a nucleotide sequence encoding the polypeptide sequence of SEQ
ID
38
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
N0:41, a nucleotide sequence encoding the polypeptide sequence of SEQ ID
N0:42, a
nucleotide sequence encoding the polypeptide sequence of SEQ ID N0:43, a
nucleotide
sequence encoding the polypeptide sequence of SEQ )D N0:44, or the
complementary
strands thereof. Polypeptides encoded by these polynucleotide fragments are
also
encompassed by the invention.
[0109] Representative examples of Neutrokine-alpha polynucleotide fragments of
the
invention include, for example, fragments that comprise, or alternatively,
consist of, a
sequence from about nucleotide 1 to 50, 51 to 100, 101 to 146, 147 to 200, 201
to 250,
251 to 300, 301 to 350, 351 to 400, 401 to 450, 451 to 500, 501 to 550, 551 to
600, 600 to
650, 651 to 700, 701 to 750, 751 to 800, 800 to 850, 851 to 900, 901 to 950,
951 to 1000,
1001 to 1050, and/or 1051 to 1082, of SEQ ID NO:1, or the complementary strand
thereto,
or the cDNA contained in the plasmid having ATCC accession number 97768. In
this
context "about" includes the particularly recited ranges, and ranges that are
larger or
smaller by several (5, 4, 3, 2, or 1) nucleotides, at either terminus or at
both termini.
[0110] Representative examples of Neutrokine-alphaSV polynucleotide fragments
of
the invention include, for example, fragments that comprise, or alternatively,
consist of, a
sequence from about nucleotide 1 to 50, 51 to 100, 101 to 150, 151 to 200, 201
to 250,
251 to 300, 301 to 350, 351 to 400, 401 to 450, 451 to 500, 501 to 550, 551 to
600, 600 to
650, 651 to 700, 701 to 750, 751 to 800, 800 to 850, and/or 851 to 900 of SEQ
>D NO:18,
or the complementary strand thereto, or the cDNA contained in the plasmid
having ATCC
accession number 203518. In this context "about" includes the particularly
recited ranges,
and ranges that are larger or smaller by several (5, 4, 3, 2, or 1)
nucleotides, at either
terminus or at both termini.
[0111] In certain preferred embodiments, polynucleotide of the invention
comprise, or
alternatively, consist of, nucleotide residues 571-627, 580-627, 590-627, 600-
627,
610-627, 571-620, 580-620, 590-620, 600-620, 571-610, 580-610, 590-610, 571-
600,
580-600, and/or 571-590 of SEQ ID NO:1.
[0112] In certain other preferred embodiments, polynucleotides of the
invention
comprise, or alternatively, consist of nucleotide residues 1-879, 25-879, 50-
879, 75-879,
100-879, 125-879, 150-879, 175-879, 200-879, 225-879, 250-879, 275-879, 300-
879,
325-879, 350-879, 375-879, 400-879, 425-879, 450-879, 475-879, 500-8?9, 525-
879,
550-879, 575-879, 600-879, 625-879, 650-879, 675-879, 700-879, 725-879, 750-
879,
39
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
775-879, 800-879, 825-879, 850-879, 1-850, 25-850, 50-850, 75-850,'100-850,
125-850,
150-850, 175-850, 200-850, 225-850, 250-850, 275-850, 300-850, 325-850, 350-
850,
375-850, 400-850, 425-850, 450-850, 475-850, 500-850, 525-850, 550-850, 575-
850,
600-850, 625-850, 650-850, 675-850, 700-850, 725-850, 750-850, 775-850, 800-
850,
825-850, 1-825, 25-825, 50-825, 75-825, 100-825, 125-825, 150-825, 175-825,
200-825,
225-825, 250-825, 275-825, 300-825, 325-825, 350-825, 375-825, 400-825, 425-
825,
450-825, 475-825, 500-825, 525-825, 550-825, 575-825, 600-825, 625-825, 650-
825,
675-825, 700-825, 725-825, 750-825, 775-825, 800-825, 1-800, 25-800, 50-800,
75-800,
100-800, 125-800, 150-800, 175-800, 200-800, 225-800, 250-800, 275-800, 300-
800,
325-800, 350-800, 375-800, 400-800, 425-800, 450-800, 475-800, 500-800, 525-
800,
550-800, 575-800, 600-800, 625-800, 650-800, 675-800, 700-800, 725-800, 750-
800,
775-800, 1-775, 25-775, 50-775, 75-775, 100-775, 125-775, 150-775, 175-775,
200-775,
225-775, 250-775, 275-775, 300-775, 325-775, 350-775, 375-775, 400-775, 425-
775,
450-775, 475-775, 500-775, 525-775, 550-775, 575-775, 600-775, 625-775, 650-
775,
675-775, 700-775, 725-775, 750-775, 1-750, 25-750, 50-750, 75-750, 100-750,
125-750,
150-750, 175-750, 200-750, 225-750, 250-750, 275-750, 300-750, 325-750, 350-
750,
375-750, 400-750, 425-750, 450-750, 475-750, 500-750, 525-750, 550-750, 575-
750,
600-750, 625-750, 650-750, 675-750, 700-750, 725-750, 1-725, 25-725, 50-725,
75-725,
100-725, 125-725, 150-725, 175-725, 200-725, 225-725, 250-725, 275-725, 300-
725,
325-725, 350-725, 375-725, 400-725, 425-725, 450-725, 475-725, 500-725, 525-
725,
550-725, 575-725, 600-725, 625-725, 650-725, 675-725, 700-725, 1-700, 25-700,
50-700,
75-700, 100-700, 125-700, 150-700, 175-700, 200-700, 225-700, 250-700, 275-
700,
300-700, 325-700, 350-700, 375-700, 400-700, 425-700, 450-700, 475-700, 500-
700,
525-700, 550-700, 575-700, 600-700, 625-700, 650-700, 675-700, 1-675, 25-675,
50-675,
75-675, 100-675, 125-675, 150-675, 175-675, 200-675, 225-675, 250-675, 275-
675,
300-675, 325-675, 350-675, 375-675, 400-675, 425-675, 450-675, 475-675, 500-
675,
525-675, 550-675, 575-675, 600-675, 625-675, 650-675, 1-650, 25-650, 50-650,
75-650,
100-650, 125-650, 150-650, 175-650, 200-650, 225-650, 250-650, 275-650, 300-
650,
325-650, 350-650, 375-650, 400-650, 425-650, 450-650, 475-650, 500-650, 525-
650,
550-650, 575-650, 600-650, 625-650, 1-625, 25-625, 50-625, 75-625, 100-625,
125-625,
150-625, 175-625, 200-625, 225-625, 250-625, 275-625, 300-625, 325-625, 350-
625,
375-625, 400-625, 425-625, 450-625, 475-625, 500-625, 525-625, 550-625, 575-
625,
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
600-625, 1-600, 25-600, 50-600, 75-600, 100-600, 125-600, 150-600, 175-600,
200-600,
225-600, 250-600, 275-600, 300-600, 325-600, 350-600, 375-600, 400-600, 425-
600,
450-600, 475-600, 500-600, 525-600, 550-600, 575-600, 1-575, 25-575, 50-575,
75-575,
100-575, 125-575, 150-575, 175-575, 200-575, 225-575, 250-575, 275-575, 300-
575,
325-575, 350-575, 375-575, 400-575, 425-575, 450-575, 475-575, 500-575, 525-
575,
550-575, 1-550, 25-550, 50-550, 75-550, 100-550, 125-550, 150-550, 175-550,
200-550,
225-550, 250-550, 275-550, 300-550, 325-550, 350-550, 375-550, 400-550, 425-
550,
450-550, 475-550, 500-550, 525-550, 1-525, 25-525, 50-525, 75-525, 100-525,
125-525,
150-525, 175-525, 200-525, 225-525, 250-525, 275-525, 300-525, 325-525, 350-
525,
375-525, 400-525, 425-525, 450-525, 475-525, 500-525, 1-500, 25-500, 50-500,
75-500,
100-500, 125-500, 150-500, 175-500, 200-500, 225-500, 250-500, 275-500, 300-
500,
325-500, 350-500, 375-500, 400-500, 425-500, 450-500, 475-500, 1-475, 25-475,
50-475,
75-475, 100-475, 125-475, 150-475, 175-475, 200-4?5, 225-475, 250-475, 275-
475,
300-475, 325-475, 350-475, 375-475, 400-475, 425-475, 450-475, 1-450, 25-450,
50-450,
75-450, 100-450, 125-450, 150-450, 175-450, 200-450, 225-450, 250-450, 275-
450,
300-450, 325-450, 350-450, 375-450, 400-450, 425-450, 1-425, 25-425, 50-425,
75-425,
100-425, 125-425, 150-425, 175-425, 200-425, 225-425, 250-425, 275-425, 300-
425,
325-425, 350-425, 375-425, 400-425, 1-400, 25-400, 50-400, 75-400, 100-400,
125-400,
150-400, 175-400, 200-400, 225-400, 250-400, 275-400, 300-400, 325-400, 350-
400,
375-400, 1-375, 25-375, 50-375, 75-375, 100-375, 125-375, 150-375, 175-375,
200-375,
225-375, 250-375, 275-375, 300-375, 325-375, 350-375, 1-350, 25-350, 50-350,
75-350,
100-350, 125-350, 150-350, 175-350, 200-350, 225-350, 250-350, 275-350, 300-
350,
325-350, 1-325, 25-325, 50-325, 75-325, 100-325, 125-325, 150-325, 175-325,
200-325,
225-325, 250-325, 275-325, 300-325, 1-300, 25-300, 50-300, 75-300, 100-300,
125-300,
150-300, 175-300, 200-300, 225-300, 250-300, 275-300, 1-275, 25-275, 50-275,
75-275,
100-275, 125-275, 150-275, 175-275, 200-275, 225-275, 250-275, 1-250, 25-250,
50-250,
75-250, 100-250, 125-250, 150-250, 175-250, 200-250, 225-250, 1-225, 25-225,
50-225,
75-225, 100=225, 125-225, 150-225, 175-225, 200-225, 1-200, 25-200, 50-200, 75-
200,
100-200, 125-200, 150-200, 175-200, 1-175, 25-175, 50-175, 75-175, 100-175,
125-175,
150-175, 1-150, 25-150, 50-150, 75-150, 100-150, 125-150, 1-125, 25-125, 50-
125,
75-125, 100-125, 1-100, 25-100, 50-100, 75-100, 1-75, 25-75, 50-75, 1-50, 25-
50, and/or
1-25 of SEQ m N0:18.
41
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0113] In certain additional preferred embodiments, polynucleotides of the
invention
comprise, or alternatively, consist of nucleotide residues 400-627, 425-627,
450-627,
475-627, 500-627, 525-627, 550-627, 575-627, 600-627, 400-600, 425-600, 450-
600,
475-600, 500-600, 525-600, 550-600, 575-600, 400-575, 425-575, 450-575, 475-
575,
500-575, 525-575, 550-575, 400-550, 425-550, 450-550, 475-550, 500-550, 525-
550,
400-500, 425-500, 450-500, 475-500, 400-475, 425-475, 450-475, 400-450, 425-
450,
571-800, 600-800, 625-800, 650-800, 675-800, 700-800, 725-800, 750-800, 775-
800,
571-775, 600-775, 625-775, 650-775, 675-775, 700-775, 725-775, 750-775, 571-
750,
600-750, 625-750, 650-750, 675-750, 700-750, 725-750, 571-725, 600-725, 625-
725,
650-725, 675-725, 700-725, 571-700, 600-700, 625-700, 650-700, 675-700, 571-
675,
600-675, 625-675, 650-675, 571-650, 600-650, 625-650, 571-625, 600-625, and/or
571-600 of SEQ ID NO:1.
[0114] In additional preferred embodiments, polynucleotides of the invention
comprise, or alternatively, consist of nucleotide residues 147-500, 147-450,
147-400, 147-
350, 200-500, 200-450, 200-400, 200-350, 250-500, 250-450, 250-400, 250-350,
300-500,
300-450, 300-400, 300-350, 350-750, 350-700, 350-650, 350-600, 350-550, 400-
750,
400-700, 400-650, 400-600, 400-550, 425-750, 425-700, 425-650, 425-600, 425-
550,
450-1020, 450-1001, 450-950, 450-900, 450-850, 450-800, 450-775, 500-1001, 500-
950,
500-900, 500-850, 500-800, 500-775, 550-1001, 550-950, 550-900, 550-850, 550-
800,
550-775, 600-1001, 600-950, 600-900, 600-850, 600-800, 600-775, 650-1001, 650-
950,
650-900, 650-850, 650-800, 650-775, 700-1001, 700-950, 700-900, 700-850, 700-
800,
700-775, 825-1082, 850-1082, 875-1082, 900-1082, 925-1082, 950-1082, 975-1082,
1000-1082, 1025-1082, and/or 1050-1082 of SEQ ID NO:l.
[0115] Preferably, the polynucleotide fragments of the invention encode a
polypeptide
which demonstrates a Neutrokine-alpha and/or Neutrokine-alphaSV functional
activity.
By a polypeptide demonstrating "functional activity" is meant, a polypeptide
capable of
displaying one or more known functional activities associated with a full-
length and/or
secreted Neutrokine-alpha polypeptide and/or Neutrokine-alphaSV polypeptide.
Such
functional activities include, but are not limited to, biological activity
(e.g., ability to
stimulate B cell proliferation, survival, differentiation, and/or activation),
antigenicity
(ability to bind or compete with a Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptide for binding to an anti-Neutrokine-alpha and/or anti-Neutrokine-
alphaSV
42
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
antibody], immunogenicity (ability to generate antibody which binds to a
Neutrokine-
alpha and/or Neutrokine-alphaSV polypeptide), ability to form multimers (as
described
below in the "Neutrolcine-alpha Polypeptides" section) with Neutrokine-alpha
and/or
Neutrokine-alphaSV polypeptides of the invention, ability to form
heteromultimers (as
described below in the "Neutroltine-alpha Polypeptides" section) with APRIL
polypeptides (e.g., SEQ ID N0:20 or SEQ ID N0:47; PCT International
Publication
Number W097/33902; GenBank Accession No. AF046888 (nucleotide) and AAC6132
(protein); J. Exp. Med. 188(6):1185-1190), ability to bind to a receptor or
ligand (e.g.,
transmembrane activator and CAML interactor (TACI, GenBank accesion number
AAC51790), and B-cell maturation antigen (BCMA, GenBank accession number
NP 001183)) for a Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide, and
ability
to stimulate a Neutrokine-alpha and/or Neutrokine-alphaSV receptor signalling
cascade
(e.g., to activate calcium-modulator and cyclophilin ligand ("CAML"),
calcineurin,
nuclear factor of activated T cells transcription factor ("NF-AT"), nuclear
factor-kappa B
("NF-kappa B"), activator protein-1 (AP-1), SRF, extracellular-signal
regulated kinase 1
(ERK-1), polo like kinases (PLK), ELF-l, high mobility group I (HMG-I), and/or
high
mobility group Y (HMG-Y)).
[0116] In additional specific embodiments, the polynucleotide fragments of the
invention encode a polypeptide comprising, or alternatively, consisting of the
predicted
intracellular domain (amino acids 1 to 46 of SEQ ID N0:2), the predicted
transmembrane
domain (amino acids 47 to 72 of SEQ ID N0:2), the predicted extracellular
domain
(amino acids 73 to 285 of SEQ ID N0:2), or the predicted TNF conserved domain
(amino
acids 191 to 284 of SEQ ID N0:2) of Neutrokine-alpha. In additional
embodiments, the
polynucleotide fragments of the invention encode a polypeptide comprising, or
alternatively, consisting of any combination of l, 2, 3, or all 4 of the above
recited
domains. Polypeptides encoded by these polynucleotides are also encompassed by
the
invention.
[0117] In additional specific embodiments, the polynucleotide fragments of the
invention encode a polypeptide comprising, or alternatively, consisting of the
predicted
intracellular domain (amino acids 1 to 46 of SEQ 1D N0:19), the predicted
transmembrane domain (amino acids 47 to 72 of SEQ ID N0:19), the predicted
extracellular domain (amino acids 73 to 266 of SEQ m N0:19), or the predicted
TNF
43
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
conserved domain (amino acids 172 to 265 of SEQ ID N0:19) of Neutrokine-
alphaSV. In
additional embodiments, the polynucleotide fragments of the invention encode a
polypeptide comprising, or alternatively, consisting of any combination of 1,
2, 3, or all 4
of the above recited domains. Polypeptides encoded by these polynucleotides
are also
encompassed by the invention.
[0118] In another embodiment, polynucleotide fragments of the invention
comprise, or
alternatively consist of, polynucleotides which encode an amino acid sequence
selected
from residues Met-1 to Lys-113, Leu-114 to Thr-141, Ile-142 to Lys-160, Gly-
161 to Gln-
198, Val-199 to Ala-248, and Gly-250 to Leu-285 of SEQ ID N0:2. Moreover,
polynucleotides that encode any combination of two, three, four, five or moxe
of these
amino acid sequences are also encompassed by the invention. Polypeptides
encoded by
these polynucleotides are also encompassed by the invention.
[0119] In another embodiment, polynucleotide fragments of the invention
comprise, or
alternatively consist of, polynucleotides which encode an amino acid sequence
selected
from residues Met-1 to Lys 113, Leu-114 to Thr-141, Gly-142 to Gln-179, Val-
180 to
Ala-229, and Gly-230 to Leu-266 of SEQ ID N0:19. Moreover, polynucleotides
that
encode any combination of two, three, four, five or more of these amino acid
sequences
are also encompassed by the invention. Polypeptides encoded by these
polynucleotides
are also encompassed by the invention.
[0120] In another embodiment, polynucleotide fragments of the invention
comprise, or
alternatively consist of, polynucleotides which encode an amino acid sequence
selected
from residues Met-1 to Lys-106, Leu-107 to Thr-134, Glu-135 to Asn-165, Ile-
167 to Lys-
184, Gly-185 to Gln-224, Val-225 to Ala-272, and Gly-273 to Leu-309 of SEQ ID
N0:39.
Moreover, polynucleotides that encode any combination of two, three, four,
five or more
of these amino acid sequences are also encompassed by the invention.
Polypeptides
encoded by these polynucleotides are also encompassed by the invention.
[0121] In another embodiment, polynucleotide fragments of the invention
comprise, or
alternatively consist of, polynucleotides which encode an amino acid sequence
selected
from residues Tyr-1 to Lys-47, Leu-48 to Thr-75, Ile-76 to Lys-94, Gly-95 to
Gln-132,
Val-133 to Ala-182, and Gly-183 to Ala-219 of SEQ ID N0:28. Moreover,
polynucleotides that encode any combination of two, three, four, five or more
of these
44
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
amino acid sequences are also encompassed by the invention. Polypeptides
encoded by
these polynucleotides are also encompassed by the invention.
[0122] In another embodiment, polynucleotide fragments of the invention
comprise, or
alternatively consist of, polynucleotides which encode an amino acid sequence
selected
from residues Tyr-1 to Lys-47, Leu-48 to Thr-75, Ile-76 to Lys-94, Gly-95 to
Gln-132,
Val-133 to Ala-182, and Gly-183 to Ala-219 of SEQ ID N0:30. Moreover,
polynucleotides that encode any combination of two, three, four, five or more
of these
amino acid sequences are also encompassed by the invention. Polypeptides
encoded by
these polynucleotides are also encompassed by the invention.
[0123] In another embodiment, the polynucleotides of the invention comprise,
or
alternatively consist of, the sequence shown in SEQ ID N0:21. The sequence
shown as
SEQ ID N0:21 encodes a polypeptide consisting of an initiating methionine
residue
linked to residues Ala-134 through Leu-285 of the Neutrokine-alpha polypeptide
sequence
shown as SEQ ID N0:2. Polypeptides encoded by these polynucleotides are also
encompassed by the invention.
[0124] In certain additional preferred embodiments, polynucleotides of the
invention
comprise, or alternatively, consist of nucleotide residues 1-459, 15-459, 30-
459, 45-459,
60-459, 75-459, 90-459, 105-459, 120-459, 135-459, 150-459, 165-459, 180-459,
195-459, 210-459, 225-459, 240-459, 255-459, 270-459, 285-459, 300-459, 315-
459,
330-459, 345-459, 360-459, 375-459, 390-459, 405-459, 420-459, 435-459, 450-
459,
1-450, 15-450, 30-450, 45-450, 60-450, 75-450, 90-450, 105-450, 120-450, 135-
450,
150-450, 165-450, 180-450, 195-450, 210-450, 225-450, 240-450, 255-450, 270-
450,
285-450, 300-450, 315-450, 330-450, 345-450, 360-450, 375-450, 390-450, 405-
450,
420-450, 435-450, 1-435, 15-435, 30-435, 45-435, 60-435, 75-435, 90-435, 105-
435,
120-435, 135-435, 150-435, 165-435, 180-435, 195-435, 210-435, 225-435, 240-
435,
255-435, 270-435, 285-435, 300-435, 315-435, 330-435, 345-435, 360-435, 375-
435,
390-435, 405-435, 420-435, 1-420, 15-420, 30-420, 45-420, 60-420, 75-420, 90-
420,
105-420, 120-420, 135-420, 150-420, 165-420, 180-420, 195-420, 210-420, 225-
420,
240-420, 255-420, 270-420, 285-420, 300-420, 315-420, 330-420, 345-420, 360-
420,
375-420, 390-420, 405-420, 1-405, 15-405, 30-405, 45-405, 60-405, 75-405, 90-
405,
105-405, 120-405, 135-405, 150-405, 165-405, 180-405, 195-405, 210-405, 225-
405,
240-405, 255-405, 270-405, 285-405, 300-405, 315-405, 330-405, 345-405, 360-
405,
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
375-405,390-405, 1-390, 15-390, 30-390, 45-390, 60-390, 105-390,
75-390, 90-390,
120-390,135-390, 150-390, 165-390, 180-390, 195-390, 240-390,
210-390, 225-390,
255-390,270-390, 285-390, 300-390, 315-390, 330-390, 375-390,
345-390, 360-390,
1-375,5-375, 30-375, 45-375, 60-375, 75-375, 90-375, 135-375,
1 105-375, 120-375,
150-375,165-375, 180-375, 195-375, 210-375, 225-375, 270-375,
240-375, 255-375,
285-375,300-375, 315-375, 330-375, 345-375, 360-375, , 45-360,
1-360, 15-360, 30-360
60-360,75-360, 90-360, 105-360, 120-360, 135-360, 150-360,180-360,
165-360,
195-360,210-360, 225-360, 240-360, 255-360, 270-360, 315-360,
285-360, 300-360,
330-360,345-360, 1-345, 15-345, 30-345, 45-345, 60-345, 105-345,
75-345, 90-345,
120-345,135-345, 150-345, 165-345, 180-345, 195-345, 240-345,
210-345, 225-345,
255-345,270-345, 285-345, 300-345, 315-345, 330-345, , 45-330,
1-330, 15-330, 30-330
60-330,75-330, 90-330, 105-330, 120-330, 135-330, 150-330,180-330,
165-330,
195-330,210-330, 225-330, 240-330, 255-330, 270-330, 315-330,
285-330, 300-330,
1-315, 135-315,
15-315,
30-315,
45-315,
60-315;
75-315,
90-315,
105-315,
120-315,
150-315,165-315, 180-315, 195-315, 210-315, 225-315, 270-315,
240-315, 255-315,
285-315,300-315, 1-300, 15-300, 30-300, 45-300, 60-300, 105-300,
75-300, 90-300,
120-300,135-300, 150-300, 165-300, 180-300, 195-300, 240-300,
210-300, 225-300,
255-300,270-300, 285-300, 1-285, 15-285, 30-285, 45-285,90-285,
60-285, 75-285,
105-285,120-285, 135-285, 150-285, 165-285, 180-285, 225-285,
195-285, 210-285,
240-285,255-285, 270-285, 1-270, 15-270, 30-270, 45-270,90-270,
60-270, 75-270,
105-270,120-270, 135-270, 150-270, 165-270, 180-270, 225-270,
195-270, 210-270,
240-270,255-270, 1-255, 15-255, 30-255, 45-255, 60-255, 105-255,
75-255, 90-255,
120-255,135-255, 150-255, 165-255, 180-255, 195-255, 240-255,
210-255, 225-255,
1-240, 135-240,
15-240,
30-240,
45-240,
60-240,
75-240,
90-240,
105-240,
120-240,
150-240,165-240, 180-240, 195-240, 210-240, 225-240, , 45-225,
1-225, 15-225, 30-225
60-225,75-225, 90-225, 105-225, 120-225, 135-225, 150-225,180-225,
165-225,
195-225,210-225, 1-210, 15-210, 30-210, 45-210, 60-210, 105-210,
75-210, 90-210,
120-210,135-210, 150-210, 165-210, 180-210; 195-210, , 45-195,
1-195, 15-195, 30-195
60-195, 5,
75-195, 1-180,
90-195,
105-195,
120-195,
135-195,
150-195,
165-195,
180-19
15-180, 150-180,.
30-180,
45-180,
60-180,
75-180,
90-180,
105-180,
120-180,
135-180,
165-180,1-165, 15-165, 30-165, 45-165, 60-165, 75-165, 120-165,
90-165, 105-165,
135-165,150-165, 1-150, 15-150, 30-150, 45-150, 60-150, 105-150,
75-150, 90-150,
46
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
120-150, 135-150, 1-135, 15-135, 30-135, 45-135, 60-135, 75-135, 90-135, 105-
135,
120-135, 1-120, 15-120, 30-120, 45-120, 60-120, 75-120, 90-120, 105-120, 1-
105,
15-105, 30-105, 45-105, 60-105, 75-105, 90-105, 1-90, 15-90, 30-90, 45-90, 60-
90, 75-90,
1-75, 15-75, 30-75, 45-75, 60-75, 1-60, 15-60, 30-60, 45-60, 1-45, 15-45, 30-
45, 1-30,
and/or 15-30 of SEQ ID N0:21. Polypeptides encoded by these polynucleotides
are also
encompassed by the invention.
[0125] Accordingly, specific embodiments of the invention are directed to
polynucleotides encoding polypeptides which comprise, or alternatively consist
of, the
amino acid sequence of beta pleated sheet region A, A', B, B', C, D, E, F, G,
or H
disclosed in Figures 7A-1 and 7A-2 and described in Example 6. Additional
embodiments
of the invention are directed to polynucleotides encoding Neutrokine-alpha
polypeptides
which comprise, or alternatively consist of, any combination of 1, 2, 3,~ 4,
5, 6, 7, 8, 9 or
all 10 of beta pleated sheet regions A-H disclosed in Figures 7A-1 and 7A-2
and described
in Example 6. Additional preferred embodiments of the invention are directed
to
polypeptides which comprise, or alternatively consist of, the Neutrokine-alpha
amino acid
sequence of beta pleated sheet region A, A', B, B', C, D, E, F, G, or H
disclosed in Figures
7A-1 and 7A-2 and described in Example 6. Additional embodiments of the
invention are
directed Neutrokine-alpha polypeptides which comprise, or alternatively
consist of, any
combination of l, 2, 3, 4, 5, 6, 7, 8, 9 or all 10 of beta pleated sheet
regions A through H
disclosed in Figures 7A-1 and 7A-2 and described in Example 6.
[0126] In certain other preferred embodiments, polynucleotides of the
invention
comprise, or alternatively consist of, nucleotide residues 34-57, 118-123, 133-
141,
151-159, 175-216, 232-255, 280-315, 328-357, 370-393, and/or 430-456 of SEQ ID
N0:21. Polypeptides encoded by these polynucleotides are also encompassed by
the
invention. These polynucleotide and polypeptide fragments correspond to the
predicted
beta-pleated sheet regions shown in Figures 7A-1 and 7A-2. In certain
embodiments,
polynucleotides of the invention comprise, or alternatively consist of, a
polynucleotide
sequence at least 90%, 95%, 96%, 97%, 98% or 99% identical to the
polynucleotide
sequence encoding one, two, three, four, five, six, seven, eight, nine or ten
of the beta-
pleated sheet regions described above. The present invention also encompasses
the above
polynucleotide sequences fused to a heterologous polynucleotide sequence.
Polypeptides
encoded by these polynucleotide sequences are also encompassed by the
invention. In
47
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
another embodiment, the invention provides an isolated nucleic acid molecule
comprising
a polynucleotide which hybridizes under stringent hybridization conditions to
one, two,
three, four, five, six, seven, eight, nine or ten of the beta-pleated sheet
polynucleotides of
the invention described above. The meaning of the phrase "stringent
conditions" as used
herein is described infra.
[0127] In further preferred embodiments, polynucleotides of the invention
comprise,
or alternatively consist of, nucleotide residues 576-599, 660-665, 675-683,
693-701,
717-758, 774-803, 822-857, 870-899, 912-935, and/or 972-998 of SEQ ID NO:l.
Polypeptides encoded by these polynucleotide fragments are also encompassed by
the
invention. These polynucleotide and polypeptide fragments correspond to the
predicted
beta-pleated sheet regions shown in Figures 7A-1 and 7A-2.
[0128] In additional preferred embodiments, polynucleotides of the invention
comprise, or alternatively consist of, nucleotide residues 457-462, 472-480,
490-498,
514-555, 571-600, 619-654, 667-696, 699-732, and/or 769-795 of SEQ ID N0:18.
Polypeptides encoded by these polynucleotide fragments are also encompassed by
the
invention. These polynucleotide and polypeptide fragments correspond to the
predicted
beta-pleated sheet regions shown in Figures 7A-1 and 7A-2.
[0129] In yet further preferred embodiments, polynucleotides of the invention
comprise, or alternatively consist of, nucleotide residues 124-129, 139-147,
157-165,
181-222, 238-267, 286-321, 334-363, 376-399, and/or 436-462 of SEQ ID NO:22.
Polypeptides encoded by these polynucleotide fragments are also encompassed by
the
invention. These polynucleotide and polypeptide fragments correspond to the
predicted
beta-pleated sheet regions shown in Figures 7A-1 and 7A-2. Polypeptides
comprising, or
alternatively, consisting of the amino acid sequence of any combination of
one, two, three,
four, five, six, seven, eight, nine, ten, or all of these regions are
encompassed by the
invention.
[0130] The relative positions of several intron/exon boundaries were
determined for
the mouse Neutrokine-alpha (SEQ ID N0:39) based on sequence analysis of mouse
genomic DNA. The apparent second axon from the 5' end of the mouse Neutrokine-
alpha
genomic clone (preliminarily designated "Exon 2") consists of Tyr-187 to Gln-
222 of the
sequence shown in SEQ ID N0:39. The apparent third axon from the 5' end of the
mouse
48
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrokine-alpha genomic clone (preliminarily designated "Exon 3") comprises
Val-223
to Gly-273 of the sequence shown in SEQ m N0:39.
[0131] Thus, in one embodiment, the invention provides polynucleotides
encoding
polypeptides comprising, or alternatively consisting of, the amino acid
sequence of
residues Tyr-187 to Gln-222 of SEQ )D N0:39. The present invention is also
directed to
nucleic acid molecules comprising, or alternatively, consisting of, a
polynucleotide
sequence at least 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% identical to the
polynucleotide sequence encoding the mouse Neutrokine-alpha polypeptides
described
above. The present invention also encompasses the above polynucleotide
sequences fused
to a heterologous polynucleotide sequence. Polypeptides encoded by these
nucleic acids
and/or polynucleotide sequences are also encompassed by the invention.
[0132] In another embodiment, the invention provides polynucleotides encoding
polypeptides comprising, or alternatively consisting of, the amino acid
sequence of
residues Val-223 to Gly-273 of SEQ )D N0:39. The present invention is also
directed to
nucleic acid molecules comprising, or alternatively, consisting of, a
polynucleotide
sequence at least 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% identical to the
polynucleotide sequence encoding the mouse Neutrokine-alpha polypeptides
described
above. The present invention also encompasses the above polynucleotide
sequences fused
to a heterologous polynucleotide sequence. Polypeptides encoded by these
nucleic acids
and/or polynucleotide sequences are also encompassed by the invention.
[0133] Moreover, the relative positions of the corresponding intron/exon
boundaries
were determined for human Neutrokine-alpha (SEQ ID NO:1 and SEQ ID NO:2) based
on
an alignment of the sequences of mouse and human Neutrokine-alpha
polypeptides. The
apparent second exon from the 5' end of human Neutrokine-alpha (also
preliminarily
designated "Exon 2") consists of, Tyr-163 to Gln-198 of the sequence shown in
SEQ ID
N0:2. The apparent third exon from the 5' end of human Neutrokine-alpha (also
preliminarily designated "Exon 3") consists of, Val-199 to Gly-249 of the
sequence shown
in SEQ ID N0:2.
[0134] Thus, in one embodiment, the invention provides polynucleotides
encoding
polypeptides comprising, or alternatively consisting of, the amino acid
sequence of
residues Tyr-163 to Gln-198 of SEQ ID N0:2. The present invention is also
directed to
nucleic acid molecules comprising, or alternatively, consisting of, a
polynucleotide
49
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
sequence at least 80%, 85%; 90%, 95%, 96%, 97%, 98% or 99% identical to the
polynucleotide sequence encoding the Neutrolcine-alpha polypeptides described
above.
The present invention also encompasses the above polynucleotide sequences
fused to a
heterologous polynucleotide sequence. Polypeptides encoded by these nucleic
acids
and/or polynucleotide sequences are also encompassed by the invention.
[0135] In another embodiment, the invention provides polynucleotides encoding
polypeptides comprising, or alternatively consisting of, the amino acid
sequence of
residues Val-199 to Gly-249 of SEQ ID N0:2. The present invention is also
directed to
nucleic acid molecules comprising, or alternatively, consisting of, a
polynucleotide
sequence at least 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% identical to the
polynucleotide sequence encoding the Neutrokine-alpha polypeptides described
above.
The present invention also encompasses the above polynucleotide sequences
fused to a
heterologous polynucleotide sequence. Polypeptides encoded by these nucleic
acids
and/or polynucleotide sequences are also ' encompassed by the invention.The
functional
activity of Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides, and
fragments,
variants derivatives, and analogs thereof, can be assayed by various methods
as described
herein and as are well known in the art.
[0136] For example, in one embodiment where one is assaying for the ability to
bind
or compete with full-length Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptide for
binding to anti-Neutrokine-alpha and/or anti-Neutrokine-alphaSV antibody or
binding to
Neutrokine-alpha receptors) and/or Neutrokine-alphaSV receptors) on B cells,
various
immunoassays known in the art can be used, including but not limited to,
competitive and
non-competitive assay systems using techniques such as radioimmunoassays,
ELISA
(enzyme linked immunosorbent assay), "sandwich" immunoassays,
immunoradiometric
assays, gel diffusion precipitation reactions, immunodiffusion assays, iT~
situ
immunoassays (using colloidal gold, enzyme or radioisotope labels, for
example), western
blots, precipitation reactions, agglutination assays (e.g., gel agglutination
assays,
hemagglutination assays), complement fixation assays, immunofluorescence
assays,
protein A assays, and immunoelectrophoresis assays, etc. In one embodiment,
antibody
binding is detected by detecting a label on the primary antibody. In another
embodiment,
the primary antibody is detected by detecting binding of a secondary antibody
or reagent
to the primary antibody. In a further embodiment, the secondary antibody is
labeled.
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Many means are known in the art for detecting binding in an immunoassay and
are within
the scope of the present invention.
[0137] In another embodiment, where a Neutrokine-alpha andlor Neutrolcine-
alphaSV
ligand is identified, or the ability of a polypeptide fragment, variant or
derivative of the
invention to multimerize is being evaluated, binding can be assayed, e.g., by
means well-
known in the art, such as, for example, reducing and non-reducing gel
chromatography,
protein affinity chromatography, and affinity blotting. See generally,
Phizicky, E., et al.,
1995, Microbiol. Rev. 59:94-123. In another embodiment, physiological
correlates of
Neutrokine-alpha and/or Neutrokine-alphaSV binding to its substrates (signal
transduction) can be assayed.
[0138] In addition, assays described herein (see e,g., Examples 6 and 7) and
otherwise
known in the axt may routinely be applied to measure the ability of Neutrokine-
alpha
and/or Neutrokine-alphaSV polypeptides and fragments, variants derivatives and
analogs
thereof to elicit Neutrokine-alpha andlor Neutrokine-alphaSV related
biological activity
(e.g., to stimulate, or alternatively to inhibit (in the case of Neutrokine-
alpha and/or
Neutrokine-alphaSV antagonists) signalling mediated by Neutrokine-alpha and/or
Neutrokine-alphaSV; to stimulate, or alternatively to inhibit B cell
proliferation,
differentiation andlor activation; and/or to increase or decrease B cell
survival in vitro or
ira vivo).
[0139] Other methods will be known to the skilled artisan and are within the
scope of
the invention.
[0140] In additional embodiments, the polynucleotides of the invention encode
polypeptides comprising, or alternatively consisting of, functional attributes
of
Neutrokine-alpha and Neutrokine-alphaSV. Preferred embodiments of the
invention in
this regard include fragments that comprise, or alternatively consist of,
alpha-helix and
alpha-helix forming regions ("alpha-regions"), beta-sheet and beta-sheet
forming regions
("beta-regions"), turn and turn-forming regions ("turn-regions"), coil and
coil-forming
regions ("coil-regions"), hydrophilic regions, hydrophobic regions, alpha
amphipathic
regions, beta amphipathic regions, flexible regions, surface-forming regions
and high
antigenic index regions of Neutrokine-alpha and Neutrokine-alphaSV
polypeptides.
51
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0141] It is believed one or more of the beta pleated sheet regions of
Neutrolcine-alpha
disclosed in Figures 7A-1 and 7A-2 is important for dimerization and also for
interactions
between Neutrolcine-alpha and its ligands.
[0142] Certain preferred regions in this regard are set out in Figure 3 (Table
I). The
data presented in Figure 3 and that presented in Table I, merely present a
different format
of the same results obtained when the amino acid sequence of SEQ TD N0:2 is
analyzed
using the default parameters of the DNA~STAR computer algorithm.
[0143] The above-mentioned preferred regions set out in Figure 3 and in Table
I
include, but are not limited to, regions of the aforementioned types
identified by analysis
of the amino acid sequence set out in Figures 1A and 1B. As set out in Figure
3 and in
Table I, such preferred regions include Gamier-Robson alpha-regions, beta-
regions,
turn-regions, and coil-regions, Chou-Fasman alpha-regions, beta-regions, and
coil-regions,
Kyte-Doolittle hydrophilic regions and hydrophobic regions, Eisenberg alpha-
and
beta-amphipathic regions, Karplus-Schulz flexible regions, Emini surface-
forming regions
and Jameson-Wolf regions of high antigenic index. Among highly preferred
polynucleotides in this regard are those that encode polypeptides comprising,
or
alternatively consisting of, regions of Neutrokine-alpha andlor Neutrokine-
alphaSV that
combine several structural features, such as several (e.g., l, 2, 3 or 4) of
the features set
out above. Polypeptides encoded by the polynucleotides are also encompassed by
the
invention.
[0144] Additionally, the data presented in columns VIII, IX, XIII, and XIV of
Table I
can routinely be used to determine regions of Neutrokine-alpha which exhibit a
high
degree of potential for antigenicity (column VIII of Table I represents
hydrophilicity
according to Kyte-Doolittle; column IX of Table I represents hydrophobicity
according to
Hopp-Woods; column XIII of Table I represents antigenic index according to
Jameson-
Wolf; and column XIV of Table I represents surface probability according to
Emini).
Regions of high antigenicity are determined from the data presented in columns
VIII, IX,
XIII, andlor IV by choosing values which represent regions of the polypeptide
which are
likely to be exposed on the surface of the polypeptide in an environment in
which antigen
recognition may occur in the process of initiation of an immune response. The
data
presented in Figure 6 can also routinely be presented in a similar tabular
format by simply
examining the amino acid sequence disclosed in Figure 6 (SEQ ID N0:19) using
the
52
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
modules and algorithms of the DNA*STAR set on default parameters. As above,
the
amino acid sequence presented in Figure 6 can also be used to determine
regions of
Neutrokine-alpha which exhibit a high degree of potential for antigenicity
whether
presented as a Figure (as in Figure 6) or a table (as in Table I).
53
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I
Res I II III V VI VII VIIIIX X XI XIIXIIIXIV
Position IV
Met1 A . . . . . . 0.73-0.71. . . 0.951.39
Asp2 A . . . . T . 1.12-O.G6* . . 1.151.56
Asp3 A . . . . T . 1.62-1.09* . . 1.152.12
Ser4 A . . . . T . 2.01-1.51. . . 1.154.19
Thr5 A . . . . T . 2.40-2.13. . F 1.304.35
Glu6 A A . . . . . 2.70-1.73* * F 0.904.51
Arg7 A A . . . . . 2.81-1.34* * F 0.904.51
Glu8 A A . . . . . 2.00-1.73* * F 0.906.12
Gln9 A A . . . . . 1.99-1.53* * F 0.902.91
Ser10 A . . B . . . 2.00-1.04* * F 0.902.15
Arg11 A . . B . . . 1.33-0.66* ~ F 0.901.66
Leu12 A . . B . . . 0.41-0.09* * F 0.450.51
Thr13 A . . B . . . 0.460.20 * * F -0.150.32
Ser14 A A . . . . . 0.50-0.19* * . 0.300.32
Cys15 A A . . . . . 0.91-0.19* * . 0.300.78
Leu~ 16 A A . . . . . 0.80-0.87* * F 0.901.06
Lys17 A A . . . . . 1.61-1.36. * F 0.901.37
Lys18 A A . . . . . 132 -1.74. * F 0.904.44
Arg19 A A . . . . . 1.67-1.70. * F 0.905.33
Glu20 A A . . . . . 1.52-2.39. * F 0.905.33
Glu21 A A . . . . . 2.38-1.70. * F 0.902.20
Met22 A A . . . . . 2.33-1.70. * F 0.902.24
Lys23 A A . . . . . 1.62-1.70* * F 0.902.24
Leu24 A A . . . . . 0.66-1.13* * F 0.750.69
Lys25 A A . . . . . 0.36-0.49. * F 0.450.52
Glu26 A A . B . . . -0.53-0.71* * . 0.600.35
Cys27 A A . B . . . -0.74-0.03* * . 0.300.30
Val28 A A . B . . . -1.00-0.03* * . 0.300.12
Ser29 A A . B . . . -0.080.40 * * . -0.300.11
Ile30 A . . B . . . -0.080.40 * * . -0.300.40
Leu31 A . . B . . . -0.08-0.17* . . 0.451.08
Pro32 . . . B . . C 0.29-0.81* . F 1.101.39
Arg33 . . . . T . . 0.93-0.81. * F 1.502.66
,
Lys34 . . . . T . . 0.93-1.07. . F 1.844.98
Glu35 . . . . . . C 0.97-1.37* * F 1.984.32
Ser36 . . . , . T C 1.89-1.16* * F 2.521.64
Pro37 . . . . T C 1.80-1.16* * F 2.861.60
Ser38 . . . . T T . 1.39-0.77* . F 3.401.24
Val39 A . . . . T . 1.39-0.39. * F 2.361.24
Arg40 A . . . . . . 1.39-0.77* * F 2.461.60
54
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I (continued)
Res I II III IV V VI VII VIII IX X XI XII XIV
Position XIII
Ser41 A . . . . . . 1.34 -1.20* * F 2.46~
2.00
Ser42 . . . . T T . 1.60 -1.16. * F 3.062.67
Lys43 . . . . T T . 1.09 -1.80. * F 3.062.72
Asp44 . . . . T T . 1.13 -1.11* * F 3.401.67
Gly45 A . . . . T . 0.43 -0.81* * F 2.661.03
Lys46 . A . . . . . 0.14 -0.70. . F 1.770.52
A
Leu47 A A . . . . . 0.13 -0.20* . . 0.980.31
Leu48 A A . . . . . -0.72 0.29* . . 0.040.46
Ala49 A A . . . . . -1.53 0.54. * . -0.600.19
Ala50 A A . . . . . -2.00 1.23. . . -0.600.19
Thr51 A A . . . . . -2.63 1.23. . . -0.600.19
Leu52 A A . . . . . -2.63 1.04. . . -0.600.19
Leu53 A A . . . . . -2.63 1.23. . . -0.600.15
Leu54 A A . . . . . -2.34 1.41. . . -0.600.09
Ala55 A A . . . . . -2.42 1.31. . . -0.600.14
Leu56 A A . . . . . -2.78 1.20. . . -0.600.09
Leu57 A . . . . T . -2.78 1.09. . . -0.200.06
Ser58 A . . . . T . -2.28 1.09. . . -0.200.05
Cys59 A . . . . T . -2.32 1.07. . . -0.200.09
Cys60 A . . . . T . -2.59 1.03. . . -0.200.08
Leu61 . . B B . . . -2.08 0.99. . . -0.600.04
Thr62 . . B B . . . -1.97 0.99. . . -0.600.11
Val63 . . B B . . . -1.91 1.20. . .. -0.600.17
Val64 . . B B . . . -1.24 1.39. . . -0.600.33
Ser65 . . B B . . . -1.43 1.10. . . -0.600.40
Phe66 A . . B . . . -1.21 1.26. . . -0.600.40
Tyr67 A . . B . . . -1.49 1.11. . . -0.600.54
Gln68 A . . B . . . -1.44 0.97. . . -0.600.41
Val69 A . . B . . . -0.59 1.27. . . -0.600.39
Ala70 A . . B . . . -0.63 0.89. . . -0.600.43
~
Ala71 A . . B . . . 0.07 0.56. * . -0.600.25
Leu72 A . . . . T . -0.50 0.16. * . 0.100.55
Gln73 A . . . . T . -1.09 0.20. . F 0.250.45
Gly74 A . . . . T . -0.53 0.20. . F 0.250.45
Asp~ A . . . . T . -0.76 0.09. * F 0.250.73
75
Leu76 A A . . . . . -0.06 0.09. * F -0.150.35
Ala77 A A . . . . . 0.17 -0.31. * . 0.300.69
Ser78 A A . . . . . 0.17 -0.24. * . 0.300.42
Leu79 A A . . . . . -0.30 -0.24. * . 0.300.88
Arg80 A A . . . . . -0.30 -0.24. * . 0.300.72
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I (continued)
Res I II III IV V VI VII VIII IX X XI XII XIV
Position XIII
Ala81 A A . . . . . 0.17 -0.34. * . 0.300.93
Glu82 A A . . , . . 0.72 -0.30. * . 0.451.11
Leu83 A A . . . . . 0.99 -0.49. * . 0.300.77
Gln84 A A . . . . . 1.21 0.01. * . -0.151.04
Gly85 A A . . . . . 1.10 0.01* * . -0.300.61
His86 A A . . . . . 1.73 0.01* * . -0.151.27
His87 A A . . . . . 0.92 -0.67. * . 0.751.47
Ala88 A A . . . . . 1.52 -0.39. * . 0.451.22
Glu89 A A . . . . . 0.93 -0.39. . . 0.451.39
~
Lys90 A A . . . . . 0.93 -0.39* . F 0.601.03
Leu91 A . . . . T . 0.38 -0.46* . . 0.851.01
Pro92 A . . . . T . 0.07 -0.46. . . 0.700.59
Ala93 A . . . . T . 0.07 -0.03. . . 0.700.29
Gly94 A . . . . T . -0.140.47. . . -0.200.36
Ala95 A . . . . . . -0.140.21. * . -0.100.36
Gly96 A . . . . . . 0.08 -0.21. . F 0.650.71
Ala97 A . . . . . . -0.06-0.21. . F 0.650.72
Pro98 A . . . . . . -0.28-0.21. * F 0.650.71
Lys99 A A . . . . . 0.07 -0.03. . F 0.450.59
Ala100 A A . . . . . 0.66 -0.46. . F 0.601.01
Gly101 A A . . . . . 0.41 -0.96. . F 0.901.13
Leu102 A A . . . . . 0.79 -0.89. . F 0.750.57
Glu103 A A . . . . . 0.41 -0.46* . F 0.450.88
Glu104 A A . . . . . -0.49-0.46* . F 0.450.89
Ala105 A A . . . . . -0.21-0.24. . . 0.300.81
Pro106 A A . . . . . -0.46-0.44. . . 0.300.67
Ala107 A A . . . . . 0.01 0.06. . . -0.300.39
Val108 A A . . . . . -0.800.49. * . -0.600.38
Thr109 A A . . . . . -0.760.67. * . -0.600.20
Ala110 A A . . . , . -1.060.24'~'* . -0.300.40
Gly111 A A . . . . . -1.540.43.* * . -0.600.38
Leu.112 A A . . . . . -0.960.57* * . -0.600.23
Lys113 . A B . . . . -0.310.09* * . -0.300.39
Ile114 . A B . . . . -0.210.01* . . -0.300.61
Phe115 . A B . . . . -0.210.01* . . 0.151.15
Glu116 . A . . . . C -0.08-0.17* . F 1.250.58
Pro' . A . . . . C 0.39 0.26* ~' F 1.101.28
117
Pro118 . . . . , . C 0.34 -0.00. . F 2.201.47
Ala119 . . . . . T C 0.89 -0.79. * F 3.001.47
Pro120 . . . . . T C 1.59 -0.36. * F 2.250.94
56
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I (continued)
Res I II III V VI VII VIIIIX X XI XII XIV
Position IV XIII
Gly 121 . . . . T T . 1.29-0.39. * F 2.15 0.98
Glu 122 . . . . T T . 1.20-0.43. . F 2.00 1.30
~
Gly 123 . . . . . . C 1.41-0.54. . F 1.60 1.12
Asn 124 . . . . . T C 2.00-0.57. . F 1.50 1.97
Ser 125 . . . . . T C 1.91-0.60. * F 1.50 1.82
Ser 126 . . . . . T C 2.37-0.21. * F 1.54 2.47
Gln 127 . . . . . T C 2.37-0.64. * F 2.18 3.01
Asn 128 . . . . . . C 2.76-0.64. . F 2.32 3.61
Ser 129 . . . . . T C 2.87-1.03. . F 2.86 5.39
Arg 130 . . . . T T . 2.58-1.41* . F 3.40 6.09
Asn 131 . . . T T . 2.02-1.31* . F 3.06 3.83
Lys 132 . . . . T T . 2.02-1.07* . F 2.72 2.12
Arg 133 . . . . T . . 1.68-1.06* . F 2.18 1.88
Ala 134 . . . . . . C 1.77-0.63* . F 1.64 1.15
Val 135 . . . . . . C 1.66-0.60* . F 1.49 0.89
Gln 136 . . . . . . C 1.66-0.60* . F 1.83 0.79
Gly 137 . . . . . T C 1.30-0.60* . F 2.52 1.35
Pro 138 . . . . . T C 0.33-0.61* . F 2.86 2.63
Glu 139 . . . . T T . 0.61-0.61* . F 3.40 1.13
Glu 140 A . . . . T . 1.47-0.53* . F 2.66 1.64
Thr 141 A . . . . . . 1.47-0.56. . F 2.12 1.84
Val 142 A . . . . . . 1.14-0.99. . F 1.78 1.77
Thr 143 A . . . . T . 0.54-0.41. . F 1.19 0.55
Gln 144 A . . . . T . 0.540.27 * . F 0.25 0.31
Asp 145 A . . . . T . -0.270.19 * . F 0.25 0.73
Cys 146 A . . . . T . -0.840.23 * . . 0.10 0.42
Leu 147 A A . . . . . -0.580.43 * . . -0.600.17
Gln 148 A A . . . . . -0.270.53 * . . -0.600.10
Leu 149 A A . . . . . -0.570.53 * * - -0.300.32
.
Ile 150 A A . . . . . -0.570.34 * . . 0.30 0.52
Ala 151 . A . . . . C -0.21-0.34. * . 1.40 0.52
Asp 152 . . . . T T . 0.39-0.26. * F 2.45 0.91
Ser 153 . . . . . T C 0.08-0.51. . F 3.00 2.00
Glu 154 . . . . . T C -0.00-0.71. . F 2.70 2.86
Thr 155 . . . . . T C 0.89-0.53* . F 2.40 1.20
Pro 156 . . . B . . C 1.52-0.13* . F 1.56 1.55
Thr 157 . . . B T . . 1.18-0.51* . F 1.92 1.79
Ile 158 A . . B . . . 1.18-0.09. . F 1.08 1.23
Gln 159 . . . . T T . 0.93-0.19. . F 2.04 1.07
Lys 160 . . . . T T . 0.930.14 * . F 1.60 1.16
57
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I (continued)
Res I II IIIIV V VI VII VIIIIX X XI XII XIV
Position XIII
Gly161 . . . . T T . 0.44Ø14 * . F 1.442.38
Ser162 . . . . T T . -0.100.24 * . F 1.281.19
Tyr163 . . . B T . . 0.580.49 * . . 0.120.44
Thr164 . . B B . . . 0.290.91 * . . -0.440.69
Phe165 . . B B . . . -0.571.40 * . . -0.600.54
Val166 . . B B . . . -1.031.70 . . . -0.600.29
Pro167 . . B B . . . -1.031.63 . . . -0.600.16
Trp168 A . . B . . . -1.491.53 . * . -0.600.25
Leu169 A . . B . . . -1.131.53 * . . -0.600.29
Leu170 A . . B . . . -0.320.89 * . . -0.300.38
Ter171 A . . . . . . 0.190.46 * . . 0.200.71
Phe172 . . . .. T . . 0.10-0.03* . . 1.800.85
Lys173 . . . . T T . -0.20-0.33* . F 2.601.38
Arg174 . . . . . T C -0.20-0.51. . F 3.001.04
Gly175 . . . . . T C 0.61-0.21. . F 2.250.99
Ser176 A . . . . T . 0.91-1.00* . F 2.050.86
Ala177 A A . . . . . 1.66-1.00* . F 1.350.76
Leu178 A A . . . . . 1.61-1.00. . F 1.201.54
Glu179 A A . . . . . 1.50-1.43. . F 0.901.98
Glu180 A A . . . . . 1.89-1.41* . F 0.903.16
Lys181 A A . . . . . 1.30-1.91* . F 0.907.66
Glu182 A A . . . . . 1.08-1.91. . F 0.903.10
Asn183 A A . . . . . 1.03-1.23* * F 0.901.48
Lys184 A A . . . . . 1.08-0.59* . F 0.750.55
Ile185 A A . . . . . 1.08-0.59* * . 0.600.63
Leu186 A A . . . . . 0.72-0.59* * . 0.600.68
Val187 A A . . . . . 0.38-0.50. * . 0.300.49
Lys188 A A . . . . . 0.13-0.07* * F 0.450.69
Glu189 A . . . . T . -0.610.00 "' * F 0.401.32
Thr190 . . . . T T . -0.420.10 . * F 0.801.54
Gly191 . . . . T T . -0.500.24 * . F 0.650.67
Tyr192 . . . . T T . 0.110.93 * * . 0.200.27
Phe193 . . B B . . . -0.281.69 . . . -0.600.29
Phe194 . . B B . . . -0.281.63 . * . -0.600.29
Ile195 . . B B . . . -0.821.60 . . . -0.600.32
Tyr196 . . B B . . . -1.291.49 . . . -0.600.28
Gly197 . . . B T . . -1.291.39 . . . -0.200.26
Gln198 . . . B T . . -0.901.36 . . . -0.200.59
V 199 . . . B . . C -0.201.16 . . . -0.400.54
al
Leu200 . . . B . . C 0.730.40 . . . -0.100.92
58
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I (continued)
Res I II III IV V VI VII VIII IX X XI XII XIV
Position XIII
Tyr201 . . . . T T . 0.67 -0.03. . . 1.251.06
Thr202 . . . . T T . 0.77 0.06. . F 0.802.06
Asp203 . . . . T T . 0.18 0.17. . F 0.803.91
Lys204 A . . . . T . 0.43 -0.01. . F 1.002.52
Thr205 A A . . . . , 0.90 -0.16. . F 0.601.73
Tyr206 A A . . . . . 1.11 -0.21. . . 0.451.03
Ala207 A A . . . . . 0.61 0.29. . . -0.300.70
Met208 A A . . . . . -0.280.97. . . -0.600.40
Gly209 A A . B . . . -0.321.17* . . -0.600.18
His210 A A . B . . . 0.10 0.81* . . -0.600.31
Leu211 A A . B . . . 0.39 0.31. . . -0.300.61
Ile212 A A . B . . . 1.02 -0.30. . . 0.451.22
Gln213 A A . ~B . . . 0.77 -0.73. * . 0.751.80
Arg214 A A . B . . . 1.08 -0.59. * F 0.901.62
Lys215 A A . B . . . 0.26 -0.77* * F 0.903.14
Lys216 A A . B . . . 0.37 -0.81. * F 0.901.35
Val217 . A B B . . . 0.91 -0.43* * . 0.300.60
His218 . A B B . . . 0.91 -0.00. * . 0.300.29
Val219 . A B B . . . 0.80 -0.00* * . 0.300.25
Phe220 . . B B . . . -0.06-0.00* . . 0.300.57
Gly221 A . . B . . . -0.400.04. * . -0.300.35
Asp222 A . . . . . . -0.36-0.07* . . 0.500.63
Glu223 A . . . . . . -1.18-0.03* . . 0.500.60
Leu224 A . . B . . . -0.63-0.17. . . 0.300.45
Ser225 A . . B . . . -0.74-0.11. . . 0.300.39
Leu226 A . . B . . . -1.100.57. * . -0.600.18
Val227 A . . B . . . -0.991.36. * . -0.600.19
Thr228 A . . B . . . -1.660.67* * . -0.600.28
Leu229 A . . B . . . -1.730.86* . . -0.600.18
Phe230 A . . B . . . -1.430.86* . . -0.600.17
Arg231 A . . B . . . -0.620.61* . . -0.600.21
Cys232 . . . B T . . -0.370.53* . . -0.200.41
Ile233 . . . B T . . -0.270.46* . . -0.200.46
Gln234 . . . B T . . 0.54 0.10* . . 0.100.37
Asn235 . . . B . . C 0.93 0.10* . . 0.051.19
Met236 . . . B . . C 0.01 0.01* . F 0.202.44
'
Pro237 . . . B . . C 0.47 0.01* . F 0.441.16
Glu238 . . . . T . . 1.36 0.04* . F 1.081.12
Thr239 . . . . . . C 1.36 0.04* . F 1.121.82
Leu240 . . . . , . C 1.06 -0.17* . F 1.961.89
59
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I (continued)
Res I II III IV V VI VII VIIIIX X XI XII XIV
Position XIII
Pro241 . . . . T . . 0.99 -0.21. . F 2.40 1.46
Asn242 . . . . T . . 0.96 0.36 . . F 1.41 0.54
Asn243 . . . . T T . 0.66 0.63 . . F 1.22 1.03
Ser244 . . . . T T . 0.38 0.33 . . F 1.13 0.89
Cys245 . . . . T T . 0.84 0.40 . . . 0.74 0.56
Tyr246 . . . . T T . 0.17 0.43 . . . 0.20 0.35
Ser247 A . . . . . . -0.42 0.71 . . . -0.400.18
Ala248 A A . . . . . -0.38 0.83 . . . -0.600.34
Gly249 A A . . . . . -0.89 0.26 . . . -0.300.43
Ile250 A A . . . . . -0.22 0.19 * . . -0.300.27
Ala251 A A . . . . . 0.02 -0.20~' . . 0.30 0.46
Lys252 A A . . . . . -0.02 -0.70. . . 0.60 0.80
Leu253 A A . . . . . 0.57 -0.70. . F 0.90 1.13
Glu254 A A . . . . . 0.91 -1.39. . F 0.90 1.87
Glu255 A A . . . . . 0.99 -1.89. . F 0.90 1.62
Gly256 A A . . . . . 1.58 -1.20. ~- F 0.90 1.62
Asp257 A A . . . . . 0.72 -1.49. * F 0.90 1.62
Glu258 A A . . . . . 0.94 -0.80* * F 0.75 0.77
Leu259 A A . . . . . 0.06 -0.30* * . 0.30 0.79
Gln260 A A . . , . . -0.16 -0.04* . . 0.30 0.33
Leu261 A A . . . . . 0.30 0.39 * . . -0.300.30
Ala262 A A . . . . . 0.30 0.39 * . . -0.300.70
Ile263 A A . . . . . 0.30 -0.30. * . 0.30 0.70
Pro264 A . . . . T . 0.52 -0.30. * F 1.00 1.37
Arg265 A . . . . T . 0.52 -0.49. * F 1.00 1.37
Glu266 A . . . . T . 0.44 -0.59* * F 1.30 3.38
Asn267 A . . . . T . 0.73 -0.59* * F 1.30 1.53
Ala268 A . . . . . . 0.81 -0.63* * . 0.95 1.05
Gln269 A . . . . . . 1.02 0.06 * * . -0.100.50
Ile270 A . . . . . . 0.57 0.06 . * . 0.15 0.52
Ser271 . . . . . . C 0.57 0.09 . * . 0.60 0.51
Leu272 . . . . . . C -0.29 -0.41. * F 1.60 0.49
Asp273 . . . . T T . -0.01 -0.17. * F 2.25 0.52
Gly274 . . . . T T . -0.71 -0.37. * F 2.50 0.56
Asp275 . . . . T T . -0.52 0.03 . * F 1.65 0.59
Val276 A . . . . T . -0.57 0.13 . * F 1.00 0.30
Thr277 A . . B . . . -0.34 0.56 . * . -0.100.30
Phe278 A . . B . . . -1.16 0.63 . * . -0.350.18
Phe279 A . . B . . . -0.77 1.31 . * . -0.600.20
Gly280 A A . , . . . -1.58 0.67 . * . -0.600.28
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Table I (continued)
Res Position I II III IV V VI VII VIII IX X XI XTI XIII XIV
Ala281 A A . . . . . -1.53 0.87 . * . -0.600.27
Leu282 A A . . . . . -1.61 0.77 * . . -0.600.26
Lys283 A A . . . . . -1.30 0.41 * . . -0.600.33
Leu284 A A . . . . . -0.99 0.41 . . . -0.600.42
Leu285 A A . . . . . -1.03 0.34 * . . -0.300.65
61
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0145] Additional preferred nucleic acid fragments of the present invention
include
nucleic acid molecules comprising, or alternatively, consisting of a sequence
encoding one
or more epitope-bearing portions of Neutrokine-alpha. In particular, such
nucleic acid
fragments of the present invention include nucleic acid molecules comprising,
or
alternatively consisting of, a sequence encoding a polypeptide selected from:
from about
Phe-115 to about Leu-147, from about Ile-150 to about Tyr-163, from about Ser-
171 to
about Phe-194, from about Glu-223 to about Tyr-246, and from about Ser-271 to
about
Phe-278, of the amino acid sequence of SEQ ID N0:2. In this context, "about"
means the
particularly recited ranges and ranges larger or smaller by several, a few, 5,
4, 3, 2 or 1
amino acid residues at either or both the amino- and carboxy-termini.
Polypeptides
encoded by these nucleic acid molecules are also encompassed by the invention.
Polypeptide fragments which bear antigenic epitopes of the Neutrokine-alpha
may be
easily determined by one of skill in the art using the above-described
analysis of the
Jameson-Wolf antigenic index, as shown in Figure 3. Methods for determining
other such
epitope-bearing portions of Neutrokine-alpha are described in detail below.
[0146] Additional preferred nucleic acid fragments of the present invention
include
nucleic acid molecules comprising, or alternatively consisting of a sequene
encoding one
or more epitope-bearing portions of Neutrokine-alphaSV. In particular, such
nucleic acid
fragments of the present invention include nucleic acid molecules comprising,
or
alternatively consisting of a sequence encoding a polypeptide selected from
about Pro-32
to about Leu-47, from about Glu-116 to about Ser-143, from about Phe-153 to
about
Tyr-173, from about Pro-218 to about Tyr-227, from about Ser-252 to about Thr-
258,
from about Ala-232 to about Gln-241; from about Ile-244 to about Ala-249; and
from
about Ser-252 to about Val-257, of the amino acid sequence of SEQ )D N0:19. In
this
context, "about" means the particularly recited ranges and ranges larger or
smaller by
several, a few, 5, 4, 3, 2 or 1 amino acid residues at either or both the
amino- and carboxy-
termini. Polypeptides encoded by these nucleic acid molecules are also
encompassed by
the invention. Polypeptide fragments which bear antigenic epitopes of the
Neutrokine-
alpha may be easily determined by one of skill in the art using the above-
described
analysis of the Jameson-Wolf antigenic index. Methods for determining other
such
epitope-bearing portions of Neutrokine-alphaSV are described in detail below.
62
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0147] In specific embodiments, the polynucleotides of the invention are less
than
100,000 kb, 50,000 lcb, 10,000 kb, 1,000 kb, 500 lcb, 400 kb, 350 kb, 300 lcb,
250 kb, 200
kb, 175 kb, 150 lcb, 125 kb, 100 kb, 75 kb, 50 lcb, 40 kb, 30 kb, 25 lcb, 20
kb, 15 kb, 10 kb,
7.5 kb, or 5 kb in length.
[0148] In further embodiments, polynucleotides of the invention comprise at
least 15,
at least 30, at least 50, at least 100, or at least 250, at least 500, or at
least 1000 contiguous
nucleotides of Neutrokine-alpha coding sequence, but consist of less than or
equal to 1000
kb, 500 kb, 250 kb, 200 kb, 150 kb, 100 kb, 75 lcb, 50 kb, 30 kb, 25 kb, 20
kb, 15 kb, 10
kb, or 5 kb of genomic DNA that flanks the 5' or 3' coding nucleotide set
forth in Figures
1A and 1B (SEQ ID NO:l) or Figures 5A and 5B (SEQ ID N0:18). In further
embodiments, polynucleotides of the invention comprise at least 15, at least
30, at least
50, at least 100, or at least 250, at least 500, or at least 1000 contiguous
nucleotides of
Neutrokine-alpha coding sequence, but do not comprise all or a portion of any
Neutrokine-alpha intron. In another embodiment, the nucleic acid comprising
Neutrokine-alpha coding sequence does not contain coding sequences of a
genomic
flanking gene (i.e., 5' or 3' to the Neutrokine-alpha gene in the genome). In
other
embodiments, the polynucleotides of the invention do not contain the coding
sequence of
more than 1000, 500, 250, 100, 50, 25, 20, 15, 10, 5, 4, 3, 2, or 1 genomic
flanking
gene(s).
[0149] In another embodiment, the invention provides an isolated nucleic acid
molecule comprising a polynucleotide which hybridizes under stringent
hybridization
conditions to a portion of the polynucleotide in a nucleic acid molecule of
the invention
described above, for instance, the sequence complementary to the coding and/or
noncoding sequence depicted in Figures 1A and 1B (SEQ ID NO:l), the sequence
of the
cDNA clone contained in the deposit having ATCC accession no. 97768, the
sequence
complementary to the coding sequence andlor noncoding sequence depicted in
Figures 5A
and 5B (SEQ ID N0:18), the sequence of the cDNA clone contained in the deposit
having
ATCC accession no. 203518, the sequence complementary to the coding sequence
and/or
noncoding sequence (i.e., transcribed, untranslated) depicted in SEQ ID N0:21,
the
sequence complementary to the coding sequence and/or noncoding sequence
depicted in
SEQ ID N0:22, the sequence complementary to the coding sequence and/or
noncoding
sequence depicted in SEQ ID N0:27, the sequence complementary to the coding
sequence
63
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
and/or noncoding sequence depicted in SEQ ID N0:29, the sequence complementary
to
the coding sequence and/or noncoding sequence depicted in SEQ ID N0:37, or
fragments
(such as, for example, the open reading frame or a fragment thereof) of these
sequences, as
described herein. By "stringent hybridization conditions" is intended
overnight incubation
at 42°C in a solution comprising: 50% formamide, 5x SSC (750 mM NaCl,
75 mM
trisodium citrate), 50 mM sodium phosphate (pH 7.6), 5x Denhardt's solution,
10°70
dextran sulfate, and 20 ~,g/ml denatured, sheared salmon sperm DNA, followed
by
washing the filters in O.lx SSC at about 65°C.
[0150] By a polynucleotide which hybridizes to a "portion" of a polynucleotide
is
intended a polynucleotide (either DNA or RNA) hybridizing to at least about 15
nucleotides (nt), and more preferably at least about 20 nt, still more
preferably at least
about 30 nt, and even more preferably about 30-70 (e.g., 40, 50, or 60)
nucleotides, and
even more preferably about any integer in the range of 30-70 or 80-150
nucleotides, or the
entire length of the reference polynucleotide. These have uses, which include,
but are not
limited to, diagnostic probes and primers as discussed above and in more
detail below. By
a portion of a polynucleotide of "at least about 20 nt in length," for
example, is intended to
include the particularly recited ranges, larger or smaller by several (i.e. 5,
4, 3, 2, 1, or 0)
amino acids, at either extreme or at both extremes of the nucleotide sequence
of the
reference polynucleotide (e.g., the sequence of one or both of the deposited
cDNAs, the
complementary strand of the nucleotide sequence shown in Figures 1A and 1B
(SEQ m
NO:1), the complementary strand of the nucleotide sequence shown in Figures 5A
and 5B
(SEQ ID N0:18), the complementary strand of the nucleotide sequence shown in
SEQ m
N0:21, the complementary strand of the nucleotide sequence shown in SEQ ID
NO:22,
the complementary strand of the nucleotide sequence shown in SEQ ID N0:27, the
complementary strand of the nucleotide sequence shown in SEQ ID N0:29, and/or
the
complementary strand of the nucleotide sequence shown in SEQ ID N0:37). Of
course, a
polynucleotide which hybridizes only to a poly A sequence (such as the 3'
terminal poly
(A) tract of the Neutrokine-alpha cDNA shown in Figures 1A and 1B (SEQ m
NO:1), the
3' terminal poly(A) tract of the Neutrokine-alphaSV cDNA shown in Figures 5A
and 5B
(SEQ ID N0:18) or the 3' terminal poly(A) tract of the Neutrokine-alphaSV cDNA
shown
in SEQ ll~ N0:22), or to a complementary stretch of T (or U) residues, would
not be
included in a polynucleotide of the invention used to hybridize to a portion
of a nucleic
64
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
acid of the invention, since such a polynucleotide would hybridize to any
nucleic acid
molecule containing a poly (A) stretch or the complement thereof (e.g.,
practically any
double-stranded cDNA clone generated using oligo dT as a primer).
[0151] As indicated, nucleic acid molecules of the present invention which
encode a
Neutrokine-alpha polypeptide or a Neutrokine-alphaSV polypeptide may include,
but are
not limited to, polynucleotides encoding the amino acid sequence of the
respective
extracellular domains of the polypeptides, by themselves; and the coding
sequence for the
extracellular domains of the respective polypeptides and additional sequences,
such as
those encoding the intracellular and transmembrane domain sequences, or a pre-
, or pro-
or prepro- protein sequence; the coding sequence of the respective
extracellular domains
of the polypeptides, with or without the aforementioned additional coding
sequences.
[0152] Also encoded by nucleic acids of the invention are the above protein
sequences
together with additional, non-coding sequences, including for example, but not
limited to,
introns and non-coding 5' and 3' sequences, such as the transcribed, non-
translated
sequences that play a role in transcription, mRNA processing, including
splicing and
polyadenylation signals, for example, ribosome binding and stability of mRNA;
an
additional coding sequence which codes for additional amino acids, such as
those which
provide additional functionalities.
[0153] Thus, the sequence encoding the polypeptide may be fused to a marker
sequence, such as a sequence encoding a peptide which facilitates purification
of the fused
polypeptide. In certain preferred embodiments of this embodiment of the
invention, the
marker amino acid sequence is a hexa-histidine peptide, such as the tag
provided in a pQE
vector (QIAGEN, Inc., 9259 Eton Avenue, Chatsworth, CA, 91311), among others,
many
of which are commercially available. As described in Gentz et al., Proc. Natl.
Acad. Sci.
USA 86:821-824 (1989), for instance, hexa-histidine provides for convenient
purification
of the fusion protein. The "HA" tag is another peptide useful for purification
which
corresponds to an epitope derived from the influenza hemagglutinin protein,
which has
been described by Wilson et al., Cell 37: 767 (1984). As discussed below,
other such
fusion proteins include the Neutrokine-alpha or the Neutrokine-alphaSV
polypeptides
fused to Fc at the N- or C-terminus. '
[0154] The present invention further relates to variants of the nucleic acid
molecules
of the present invention, which encode portions, analogs or derivatives of the
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrokine-alpha or Neutrokine-alphaSV polypeptides of SEQ )D N0:2. Variants
may
occur naturally, such as a natural allelic variant. By an "allelic variant" is
intended one of
several alternate forms of a gene occupying a ' given locus on a chromosome of
an
organism. Genes 11, Lewin, B., ed., John Wiley & Sons, New York (1985). Non-
naturally
occurring variants may be produced using art-known mutagenesis techniques,
which
include, but are not limited to oligonucleotide mediated mutagenesis, alanine
scanning,
PCR mutagenesis, site directed mutagenesis (see e.g., Carter et al., Nucl.
Acids Res.
13:4331 (1986); and Zoller et al., Nucl. Acids Res. 10:6487 (1982)), cassette
mutagenesis
(see e.g., Wells et al., GeTae 34:315 (1985)), restriction selection
mutagenesis (see e.g.,
Wells er al., Philos. Trans. R. Soc. London SerA 317:415 (1986)).
[0155] Such variants include those produced by nucleotide substitutions,
deletions or
additions. The substitutions, deletions or additions may involve one or more
nucleotides.
The variants may be altered in coding regions, non-coding regions, or both.
Alterations in
the coding regions may produce conservative or non-conservative amino acid
substitutions, deletions or additions. Especially preferred among these are
silent
substitutions, additions and deletions, which do not alter~the properties and
activities of the
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides or portions thereof.
Also
especially preferred in this regard are conservative substitutions.
[0156] Additional embodiments of the invention are directed to isolated
nucleic acid
molecules comprising a polynucleotide which encodes the amino acid sequence of
a
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide (e.g., a Neutrokine-
alpha and/or
Neutrokine-alphaSV polypeptide fragment described herein) having an amino acid
sequence which contains at least one conservative amino acid substitution, but
not more
than 50 conservative amino acid substitutions, even more preferably, not more
than 40
conservative amino acid substitutions, still more preferably, not more than 30
conservative
amino acid substitutions, and still even more preferably, not more than 20
conservative
amino acid substitutions, 10-20 conservative amino acid substitutions, 5-10
conservative
amino acid substitutions, 1-5 conservative amino acid substitutions, 3-5
conservative
amino acid substitutions, or 1-3 conservative amino acid substitutions. Of
course, in order
of ever-increasing preference, it is highly preferable for a polynucleotide
which encodes
the amino acid sequence of a Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptide to
66
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
have an amino acid sequence which contains not more than 10, 9, 8, 7, 6, 5, 4,
3, 2 or 1
conservative amino acid substitutions.
[0157] Further embodiments include an isolated nucleic acid molecule
comprising, or
alternatively consisting of, a polynucleotide having a nucleotide sequence at
least 80%,
85%, or 90% identical, and more preferably at least 95%, 96%, 97%, 98% or 99%
identical to a polynucleotide selected from the group consisting of: (a) a
nucleotide
sequence encoding the Neutrokine-alpha polypeptide having the complete amino
acid
sequence in Figures 1A and 1B (i.e., positions 1 to 285 of SEQ )D N0:2); (b) a
nucleotide
sequence encoding the Neutrokine-alpha polypeptide having the complete amino
acid
sequence in SEQ ID N0:2 excepting the N-terminal methionine (i.e., positions 2
to 285 of
SEQ >D NO:2); (c) a fragment of the polypeptide of (b) having Neutrokine-alpha
functional activity (e.g., antigenic or biological activity); (d) a nucleotide
sequence
encoding the predicted extracellular domain of the Neutrokine-alpha
polypeptide having
the amino acid sequence at positions 73-285 in Figures 1A and 1B (SEQ ID
NO:2); (e) a
nucleotide sequence encoding the Neutrokine-alpha polypeptide having the amino
acid
sequence at positions 134-285 in Figures 1A and 1B (SEQ D7 N0:2); (f) a
nucleotide
sequence encoding the Neutrokine-alpha polypeptide having the complete amino
acid
sequence encoded by the cDNA clone contained in the deposit having ATCC
accession
number 97768; (g) a nucleotide sequence encoding the extracellular domain of
the
Neutrokine-alpha polypeptide having the amino acid sequence encoded by the
cDNA
contained in the deposit having ATCC accession number 97768; and (h) a
nucleotide
sequence complementary to any of the nucleotide sequences in (a), (b), (c),
(d), (e), (f),
(g), or (h) above.The present invention also encompasses the above
polynucleotide
sequences fused to a heterologous polynucleotide sequence. Polypeptides
encoded by
these polynucleotides and nucleic acid molecules are also encompassed by the
invention.
[0158] Highly preferred embodiments of the invention are directed to nucleic
acid
molecules comprising, or alternatively consisting of a polynucleotide having a
nucleotide
sequence at least 80%, 85%, 90% identical and more preferably at least 95%,
96%, 97%,
98%, 99% or 100% identical to a polynucleotide sequence encoding the
Neutrokine-alpha
polypeptide having the amino acid sequence at positions 134-285 in Figures 1A
and 1B
(SEQ ID N0:2). Preferred embodiments of the invention are directed to nucleic
acid
molecules comprising, or alternatively consisting of a polynucleotide having a
nucleotide
67
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
sequence at least 90% identical to a polynucleotide sequence encoding the
Neutrolune-
alpha polypeptide having the amino acid sequence at positions 134-285 in
Figures 1A and
1B (SEQ ID N0:2). More preferred embodiments of the invention are directed to
nucleic
acid molecules comprising, or alternatively consisting of a polynucleotide
having a
nucleotide sequence at least 95% identical to a polynucleotide sequence
encoding the
Neutrokine-alpha polypeptide having the amino acid sequence at positions 134-
285 in
Figures 1A and 1B (SEQ ID N0:2). More preferred embodiments of the invention
are
directed to nucleic acid molecules comprising, or alternatively consisting of
a
polynucleotide having a nucleotide sequence at least 96% identical to a
polynucleotide
sequence encoding the Neutrokine-alpha polypeptide having the amino acid
sequence at
positions 134-285 in Figures 1A and 1B (SEQ ID N0:2).
[0159] Additionally, more preferred embodiments of the invention are directed
to
nucleic acid molecules comprising, or alternatively consisting of a
polynucleotide having
a nucleotide sequence at least 97% to a polynucleotide sequence encoding the
Neutrokine-
alpha polypeptide having the amino acid sequence at positions .134-285 in
Figures 1A and
1B (SEQ ID N0:2). Additionally, more preferred embodiments of the invention
are
directed to nucleic acid molecules comprising, or alternatively consisting of
a
polynucleotide having a nucleotide sequence at least 98% to a polynucleotide
sequence
encoding the Neutrokine-alpha polypeptide having the amino acid sequence at
positions
134-285 in Figures 1A and 1B (SEQ ID N0:2). Additionally, more preferred
embodiments of the invention are directed to nucleic acid molecules
comprising, or
alternatively consisting of a polynucleotide having a nucleotide sequence at
least 99%
identical to a polynucleotide sequence encoding the Neutrokine-alpha
polypeptide having
the amino acid sequence at positions 134-285 in Figures 1A and 1B (SEQ ID
N0:2).
[0160] A further embodiment of the invention relates to an isolated nucleic
acid
molecule comprising a polynucleotide which encodes the amino acid sequence of
a
Neutrokine-alphaSV polypeptide (e.g., a Neutrokine-alphaSV polypeptide
fragment
described herein) having an amino acid sequence which contains at least one
conservative
amino acid substitution, but not more than 50 conservative amino acid
substitutions, even
more preferably, not more than 40 conservative amino acid substitutions, still
more
preferably not more than 30 conservative amino acid substitutions, and still
even more
preferably not more than 20 conservative amino acid substitutions. Of course,
in order of
68
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
ever-increasing preference, it is highly preferable for a polynucleotide which
encodes the
amino acid sequence of a Neutrokine-alpha polypeptide to have an amino acid
sequence
which contains not more than 7-10, 5-10, 3-7, 3-5, 2-5, 1-5, 1-3, 10, 9, 8, 7,
6, 5, 4, 3, 2 or
1 conservative amino acid substitutions.
[0161] Further embodiments include an isolated nucleic acid molecule
comprising, or
alternatively, consisting of a polynucleotide having a nucleotide sequence at
least 80%,
85% or 90% identical, and more preferably at least 95%, 96%, 97%, 98% or 99%
identical
to a polynucleotide selected from the group consisting of: (a) a nucleotide
sequence
encoding the Neutrokine-alphaSV polypeptide having the complete amino acid
sequence
in Figures 5A and 5B (i.e., positions 1 to 266 of SEQ >D NO:19); (b) a
nucleotide
sequence encoding the Neutrokine-alphaSV polypeptide having the complete amino
acid
sequence in SEQ )I~ N0:19 excepting the N-terminal methionine (i.e., positions
2 to 266
of SEQ ID N0:2); (c) a nucleotide sequence encoding the predicted
extracellular domain
of the Neutrokine-alphaSV polypeptide having the amino acid sequence at
positions
73-266 in Figures 5A and 5B (SEQ >L~ N0:19); (d) a nucleotide sequence
encoding the
Neutrokine-alphaSV polypeptide having the complete amino acid sequence encoded
by
the cDNA clone contained in the deposit having ATCC accession number 203518;
(e) a
nucleotide sequence encoding the extracellular domain of the Neutrokine-
alphaSV
polypeptide having the amino acid sequence encoded by the cDNA clone contained
in the
deposit having ATCC accession number 203518; and (f) a nucleotide sequence
complementary to any of the nucleotide sequences in (a), (b), (c), (d) or (e),
above.
[0162] Further, the invention includes a polynucleotide comprising, or
alternatively,
consisting of, a sequence at least 90%, or at least 95%, identical to any
portion of at least
about 10 contiguous nucleotides, about 20 contiguous nucleotides, about 25
contiguous
nucleotides, or about 30 contiguous nucleotides, preferably at least about 40
nucleotides,
or at least about 50 nucleotides, of the sequence from nucleotide 1 to
nucleotide 1082 in
Figures 1A and 1B (SEQ )D N0:1), preferably excluding the nucleotide sequences
determined from the above-listed 4 cDNA clones and the nucleotide sequences
from
nucleotide 797 to 1082, 810 to 1082, and 346 to 542. The invention also
includes a
polynucleotide comprising, or alternatively consisting of, a sequence at least
90%, or at
least 95%, identical to any portion of at least about 10 contiguous
nucleotides, about 20
contiguous nucleotides, about 25 contiguous nucleotides, or about 30
contiguous
69
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
nucleotides, preferably at least about 40 nucleotides, or at least about 50
nucleotides, of
the sequence in Figures 5A and 5B (SEQ ID N0:18), preferably excluding the
nucleotide
sequences determined from the above-listed 4 cDNA clones. The invention also
includes
a polynucleotide comprising, or alternatively consisting of a sequence at
least 90%, or at
least 95%, identical to any portion of at least about 10 contiguous
nucleotides, about 20
A
contiguous nucleotides, about 25 contiguous nucleotides, or about 30
contiguous
nucleotides, preferably at least about 40 nucleotides, or at least about 50
nucleotides, of
the sequence in SEQ ID N0:21, preferably excluding the nucleotide sequences
determined
from the above-listed 4 cDNA clones. The invention also includes a
polynucleotide
comprising a sequence at least 90%, or at least 95%, identical to any portion
of at least
about 10 contiguous nucleotides, about 20 contiguous nucleotides, about 25
contiguous
nucleotides, or about 30 contiguous nucleotides, preferably at least about 40
nucleotides,
or at least about 50 nucleotides, of the sequence in SEQ ID N0:22, preferably
excluding
the nucleotide sequences determined from the above-listed 4 cDNA clones. The
invention
also includes a polynucleotide comprising a sequence at least 90%, or at least
95%,
identical to any portion of at least about 10 contiguous nucleotides, about 20
contiguous
nucleotides, about 25 contiguous nucleotides, or about 30 contiguous
nucleotides,
preferably at least about 40 nucleotides, or at least about 50 nucleotides, of
the sequence in
SEQ ID N0:27, preferably excluding the nucleotide sequences determined from
the
above-listed 4 cDNA clones. The invention also includes a polynucleotide
comprising a
sequence at least 90%, or at least 95%, identical to any portion of at least
about 10
contiguous nucleotides, about 20 contiguous nucleotides, about 25 contiguous
nucleotides,
or about 30 contiguous nucleotides, preferably at least about 40 nucleotides,
or at least
about 50 nucleotides, of the sequence in SEQ ID N0:29, preferably excluding
the
nucleotide sequences determined from the above-listed 4 cDNA clones. The
invention also
includes a polynucleotide comprising a sequence at least 90%, or at least 95%,
identical to
any portion of at least about 10 contiguous nucleotides, about 20 contiguous
nucleotides,
about 25 contiguous nucleotides, or about 30 contiguous nucleotides,
preferably at least
about 40 nucleotides, or at least about 50 nucleotides, of the sequence in SEQ
ID N0:37,
preferably excluding the nucleotide sequences determined from the above-listed
4 cDNA
clones. In this context "about" includes the particularly recited ranges,
larger or smaller
by several (i.e. 5, 4, 3, 2 or 1) amino acids, at either extreme or at both
extremes.
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0163] By a polynucleotide having a nucleotide sequence at least, fox example,
95%
"identical" to a reference nucleotide sequence encoding a Neutrolcine-alpha
and/or
Neutrokine-alphaSV polypeptide is intended that the nucleotide sequence of the
polynucleotide is identical to the reference sequence except that the
polynucleotide
sequence may include up to five mismatches per each 100 nucleotides of the
reference
nucleotide sequence encoding the Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptide. In other words, to obtain a polynucleotide having a nucleotide
sequence at
least 95% identical to a reference nucleotide sequence, up to 5% of the
nucleotides in the
reference sequence may be deleted or substituted with another nucleotide, or a
number of
nucleotides up to 5%, of the total nucleotides in the reference sequence may
be inserted
into the reference sequence. These mutations of the reference sequence may
occur at the
5' or 3' terminal positions of the reference nucleotide sequence or anywhere
between those
terminal positions, interspersed either individually among nucleotides in the
reference
sequence or in one or more contiguous groups within the reference sequence.
The
reference (query) sequence may be the entire nucleotide sequence encoding
Neutrokine-
alpha or Neutrokine-alphaSV, as shown in Figures 1A and 1B (SEQ ID NO:1) and
Figures
5A and 5B (SEQ ID NO:18),-respectively, or any Neutrokine-alpha such as, for
example,
the Neutrokine-alpha polynucleotides shown as SEQ ID NOs:2l, 22, 27, 29, or
37, or any
Neutrokine-alpha or Neutrokine-alphaSV polynucleotide fragment as described
herein.
[0164] As a practical matter, whether any particular nucleic acid molecule is
at least
80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% identical to, for instance, the
nucleotide
sequences shown in Figures 1A and 1B, or the nucleotide sequences shown in
Figures 5A
and 5B, or to the nucleotides sequence of the deposited cDNA clones, or to any
Neutrokine-alpha polynucleotide such as, for example, the Neutrokine-alpha
polynucleotides shown as SEQ >D NOs:2l, 22, 27, 29, or 37, or fragments
thereof, can be
determined conventionally using known computer programs such as the Bestfit
program
(Wisconsin Sequence Analysis Package, Version 8 for Unix, Genetics Computer
Group,
University Research Park, 575 Science Drive, Madison, WI 53711). Bestfit uses
the local
homology algorithm of Smith and Waterman to find the best segment of homology
between two sequences (Advances irz Applied Mathefnatics 2:482-489 (1981)).
When
using Bestfit or any other sequence alignment program to determine whether a
particular
sequence is, for instance, 95% identical to a reference sequence according to
the present
71
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
invention, the parameters are set, of course, such that the percentage of
identity is
calculated over the full length of the reference nucleotide sequence and that
gaps in
homology of up to 5°7o of the total number of nucleotides in the
reference sequence are
allowed.
[01651 In a specific embodiment, the identity between a reference (query)
sequence (a
sequence of the present invention) and a subject sequence, also referred to as
a global
sequence alignment, is determined using the FASTDB computer program based on
the
algorithm of Brutlag and colleagues (Comp. App. Biosei. 6:237-245 (1990)). In
a
sequence alignment the query and subject sequences are both DNA sequences. An
RNA
sequence can be compared by converting U's to T's. The result of said global
sequence
alignment is in percent identity. Preferred parameters used in a FASTDB
alignment of
DNA sequences to calculate percent identity are: Matrix=Unitary, k-tuple=4,
Mismatch
Penalty=l, Joining Penalty=30, Randomization Group Length=0, Cutoff Score=1,
Gap
Penalty=5, Gap Size Penalty 0.05, Window Size=500 or the length of the subject
nucleotide sequence, whichever is shorter. According to this embodiment, if
the subject
sequence is shorter than the query sequence because of 5' or 3' deletions, not
because of
internal deletions, a manual correction is made to the results to take into
consideration the
fact that the FASTDB program does not account for 5' and 3' truncations of the
subject
sequence when calculating percent identity. For subject sequences truncated at
the 5' or 3'
ends, relative to the query sequence, the percent identity is corrected by
calculating the
number of bases of the query sequence that are 5' and 3' of the subject
sequence, which are
not matched/aligned, as a percent of the total bases of the query sequence. A
determination of whether a nucleotide is matchedlaligned is determined by
results of the
FASTDB sequence alignment. This percentage is then subtracted from the percent
identity, calculated by the above FASTDB program using the specified
parameters, to
arrive at a final percent identity score. This corrected score is what is used
for the
purposes of this embodiment. Only bases outside the 5' and 3' bases of the
subject
sequence, as displayed by the FASTDB alignment, which are not matched/aligned
with
the query sequence, are calculated for the purposes of manually adjusting the
percent
identity score. For example, a 90 base subject sequence is aligned to a 100
base query
sequence to determine percent identity. The deletions occur at the 5' end of
the subject
sequence and therefore, the FASTDB alignment does not show a matched/alignment
of
72
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
the first 10 bases at 5' end. The 10 unpaired bases represent 10% of the
sequence (number
of bases at the 5' and 3' ends not matched/total number of bases in the query
sequence) so
10% is subtracted from the percent identity score calculated by the FASTDB
program. If
the remaining 90 bases were perfectly matched the final percent identity would
be 90%.
In another example, a 90 base subject sequence is compared with a 100 base
query
sequence. This time the deletions are internal deletions so that there are no
bases on the 5'
or 3' of the subject sequence which are not matched/aligned with the query. In
this case
the percent identity calculated by FASTDB is not manually corrected. Once
again, only
bases 5' and 3' of the subject sequence which are not matched/aligned with the
query
sequence are manually corrected for. No other manual corrections are made for
the
purposes of this embodiment.
[0166] The present application is directed to nucleic acid molecules at least
80%, 85%,
90%, 92%, 95%, 96%, 97%, 98% or 99% identical to the nucleic acid sequences
(i.e.,
polynucleotides) disclosed herein (e.g., those disclosed in Figures 1A and 1B
(SEQ ID
NO:1) or to the nucleic acid sequence of the deposited cDNAs), irrespective of
whether
they encode a polypeptide having Neutrokine-alpha andlor Neutrokine-alphaSV
functional
activity (e.g., biological activity). In addition, the present application is
also directed to
nucleic acid molecules at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99%
identical to the nucleic acid sequence shown in Figures 5A and 5B (SEQ ID
NO:18) or to
the nucleic acid sequence of the deposited cDNA, irrespective of whether they
encode a
polypeptide having Neutrokine-alphaSV activity. Moreover, the present
application is
also directed to nucleic acid molecules at least 80%, 85%, 90%, 92%, 95%, 96%,
97%,
98%, 99% identical to the nucleic acid sequence shown in SEQ ID NOs:2l, 22,
27, 29, or
37, irrespective of whether they encode a polypeptide having Neutrokine-alpha
activity.
This is because even where a particular nucleic acid molecule does not encode
a
polypeptide having Neutrokine-alpha andlor Neutrokine-alphaSV activity, one of
skill in
the art would still know how to use the nucleic acid molecule, for instance,
as a
hybridization probe or a polymerise chain reaction (PCR) primer. Uses of the
nucleic
acid molecules of the present invention that do not encode a polypeptide
having
Neutrokine-alpha and/or Neutrokine-alphaSV activity include, inter alia, (1)
isolating the
Neutrokine-alpha and/or Neutrokine-alphaSV gene or allelic variants thereof in
a cDNA
library; (2) in situ hybridization (e.g., "FISH") to metaphase chromosomal
spreads to
73
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
provide precise chromosomal location of the Neutrokine-alpha and/or
Neutrolcine-alphaSV gene, as described in Verma et al., Hun2arz Clar-
ornosonies: A Manual
of Basic Techniques, Pergamon Press, New York (1988); and Northern Blot
analysis for
detecting Neutrokine-alpha and/or Neutrokine-alphaSV mRNA expression in
specific
tissues.
[0167] Preferred, however, are nucleic acid molecules having sequences at
least 80%,
85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to the nucleic
acid~sequences
disclosed herein (e.g., the nucleotide sequence shown in Figures 1A and 1B
(SEQ ID
NO:1) and the nucleic acid sequence of the deposited cDNAs, or fragments
thereof),
which do, in fact, encode a polypeptide having Neutrokine-alpha and/or
Neutrokine-alphaSV polypeptide functional activity (e.g., biological
activity). Also
preferred are nucleic acid molecules having sequences at least 80%, 85%, 90%,
92%,
95%, 96%, 97%, 98% or 99% identical to the nucleic acid sequence shown in
Figures 5A
and 5B (SEQ ID N0:18) or to the nucleic acid sequence of the deposited cDNA
which do,
in fact, encode a polypeptide having Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptide functional activity (e.g., biological activity). Also preferred
are nucleic acid
molecules having sequences at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or
99%
identical to the nucleic acid sequence shown SEQ )D NOs:2l, 22, 27, 29, or 37,
which do,
in fact, encode a polypeptide having Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptide functional activity (e.g., biological activity).
[0168] By "a polypeptide having Neutrokine-alpha polypeptide functional
activity"
(e.g., biological activity) and "a polypeptide having Neutrokine-alphaSV
polypeptide
functional activity" (e.g., biological activity) are intended polypeptides
exhibiting activity
similar, but not necessarily identical, to an activity of the extracellular
domain or the
full-length Neutrokine-alpha or Neutrokine-alphaSV polypeptides of the
invention, as
measured in a particular functional assay (e.g., immunological or biological
assay). For
example, Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide functional
activity can
be measured by the ability of a polypeptide sequence described herein to form
multimers
(e.g., homodimers and homotrimers) with the complete Neutrokine-alpha and/or
Neutrokine-alphaSV or extracellular domain of Neutrokine-alpha andlor
Neutrokine-
alphaSV, and to bind a Neutrokine-alpha and/or Neutrokine-alphaSV ligand.
Additionally, Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide
functional
74
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
activity can be measured by the ability of a polypeptide sequence described
herein to form
heteromultimers with APRIL (e.g., SEQ ID N0:20 and SEQ ID N0:47) or APRIL
fragments or variants, especially the extracellular soluble domain of APRIL
(e.g., amino
acids 105-250 of SEQ ID N0:47). Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptide functional activity can be also be measured by determining the
ability of a
polypeptide of the invention to induce lymphocyte (e.g., B cell)
proliferation,
differentiation or activation and/or to extend B cell survival. These
functional assays can
be routinely performed using techniques described herein (e.g., see Example 6)
and
otherwise known in the art. Additionally, Neutrokine-alpha or Neutrokine-
alphaSV
polypeptides of the present invention modulate cell proliferation,
cytotoxicity, cell
survival and cell death. An i~z vitro cell proliferation, cytotoxicity, cell
survival, and cell
death assay for measuring the effect of a protein on certain cells can be
performed by
using reagents well known and commonly available in the art for detecting cell
replication
and/or death. For instance, numerous such assays for TNF-related protein
activities are
described in the various references in this disclosure. Briefly, an example of
such an assay
involves collecting human or animal (e.g., mouse) cells and mixing with (1)
transfected
host cell-supernatant containing Neutrokine-alpha protein (or a candidate
polypeptide) or
(2) nontransfected host cell-supernatant control, and measuring the effect on
cell numbers
or viability after incubation of certain period of time. Such cell
proliferation andlor
survival modulation activities as can be measured in this type of assay are
useful for
treating tumor, tumor metastasis, infections, autoimmune diseases,
inflammation and other
immune-related diseases.
[0169] Neutrokine-alpha modulates cell proliferation and differentiation in a
dose-dependent manner in the above-described assay. Accordingly, it is
preferred that "a
polypeptide having Neutrokine-alpha polypeptide functional activity" (e.g.,
biological
activity) includes polypeptides that also exhibit any of the same cell
modulatory
(particularly immunomodulatory) activities in the above-described assays , in
a
dose-dependent manner. Although the degree of dose-dependent activity need not
be
identical to that of the Neutrokine-alpha polypeptides, preferably, "a
polypeptide having
Neutrokine-alpha polypeptide functional activity" will exhibit substantially
similar
dose-dependence in a given activity as compared to the Neutrokine-alpha
polypeptides
(i.e., the candidate polypeptide will exhibit greater activity or not more
than about 25-fold
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
less and, preferably, not more than about tenfold less activity relative to
the reference
Neutrokine-alpha polypeptides).
[0170] In certain preferred embodiments, "a polypeptide having Neutrokine-
alpha
polypeptide functional activity" (e.g., biological activity) and "a
polypeptide having
Neutrokine-alphaSV polypeptide functional activity" (e.g., biological
activity) includes
polypeptides that also exhibit any of the same B cell (or other cell type)
modulatory
(particularly immunomodulatory) activities described in Figures 8A, 8B, 8C,
9A, 9B, 10A,
IOB, 10C, 10D, 10E, 10F, 11A, 11B, 11C, 11D, 11E, and 11F and in Example 6.
[0171] Like other members of TNF family, Neutrokine-alpha exhibits activity on
leukocytes including, for example, monocytes, lymphocytes (e.g., B cells) and
neutrophils.
For this reason Neutrokine-alpha is active in directing the proliferation,
differentiation and
migration of these cell types. Such activity is useful for immune enhancement
or
suppression, myeloprotection, stem cell mobilization, acute and chronic
inflammatory
control and treatment of leukemia. Assays for measuring such activity are
known in the
art. For example, see Peters et al., Immun. Today 17:273 (1996); Young et al.,
J. Exp.
Med. 182:1111 (1995); Caux et al., Nature 390:258 (1992); and Santiago-Schwarz
et al.,
Adv. Exp..Med. Biol. 378:7 (1995). '
[0172] Of course, due to the degeneracy of the genetic code, one of ordinary
skill in
the art will immediately recognize that a large number of the nucleic acid
molecules
having a sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98%, or 99%
identical
to the nucleic acid sequence contained in cDNA clone deposited in ATCC
accession no.
97768, or the nucleic acid sequence shown in Figures 1A and 1B (SEQ ID NO:1),
or
fragments thereof, will encode a polypeptide "having Neutrokine-alpha
polypeptide
functional activity" (e.g., biological activity). One of ordinary skill in the
art will also
immediately recognize that a large number of the nucleic acid molecules having
a
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98%, or 99% identical to
the
nucleic acid sequence contained in cDNA clone deposited in ATCC accession no.
203518
or the nucleic acid sequence shown in Figures 5A and 5B (SEQ ff~ N0:18) will
encode a
polypeptide "having Neutrokine-alphaSV polypeptide functional activity''
(e.g., biological
activity). In fact, since degenerate variants of these nucleotide sequences
all encode the
same polypeptide, this will be clear to the skilled artisan even without
performing the
above described comparison assay. It will be further recognized in the art
that, for such
76
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
nucleic acid molecules that are not degenerate variants, a reasonable number
will also
encode a polypeptide having Neutrolcine-alpha and/or Neutrohine-alphaSV
activity. This
is because the skilled artisan is fully aware of amino acid substitutions that
are either less
likely or not likely to significantly effect protein function (e.g., replacing
one aliphatic
amino acid with a second aliphatic amino acid), as further described below.
[0173] Similarly, polynucleotides encoding polypeptides which contain all or
some
portion of the region V-142 through K-160 of SEQ ID N0:2 are likely to be
valuable
diagnostic and therapeutic polynucleotides with regard to detecting and/or
altering
expression of either Neutrokine-alpha or Neutrokine-alphaSV polynucleotides.
In
addition, polynucleotides which span the junction of amino acid residues T-141
and G-142
of the Neutrokine-alphaSV polypeptide shown in SEQ ID N0:19 (in between which
the
V-142 through K-160 amino acid sequence of Neutrokine-alpha is apparently
inserted),
are also likely to be useful both diagnostically and therapeutically. Such T-
141/G-I42
spanning polynucleotides will exhibit a much higher likelihood of
hybridization with
Neutrokine-alphaSV polynucleotides than with Neutrokine-alpha polynucleotides.
A
partial, non-limiting, non-exclusive list of such Neutrokine-alphaSV
polypeptides which
are encoded by polynucleotides of the invention includes polypeptides
comprising, or
alternatively consisting of, an amino acid sequence selected from the
following: G-121
through E-163; E-122 through E-163; G-123 through E-163; N-124 through E-163;
S-125
through E-163; S-126 through E-163; Q-127 through E-163; N-128 through E-163;
S-129
through E-163; R-130 through E-163; N-131 through E-163; K-132 through E-163;
R-133
through E-163; A-134 through E-163; V-135 through E-163; Q-136 through E-163;
G-137
through E-163; P-138 through E-163; E-139 through E-163; E-140 through E-163;
T-141
through E-163; G-142 through E-163; S-143 through E-163; Y-144 through E-163;
T-145
through E-163; F-146 through E-163; V-147 through E-163; P-148 through E-163;
W-149
through E-163; L-150 through E-163; L-151 through E-163; S-152 through E-163;
F-153
through E-163; K-154 through E-163; R-155 through E-163; G-156 through E-163;
S-157
through E-163; A-158 through E-163; L-159 through E-163; E-160 through E-163;
E-161
through E-163; K-162 through E-163; G-121 through K-162; G-121 through E-161;
G-121 through E-160; G-121 through L-159; G-121 through A-158; G-121 through S-
157;
G-121 through G-156; G-121 through R-155; G-121 through K-154; G-121 through
F-153; G-12I through S-152; G-121 through L-151; G-121 through L-150; G-121
through
77
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
W-149; G-121 through P-148; G-121 through through F-146;
V-147; G-121 G-121
through T-145; G-121 through Y-144; G-121 G-121 through
through S-143; G-142;
G-121 through T-141; G-121 through E-140;
G-121 through E-139; G-121 through P-138;
G-121 through G-137; G-121 through Q-136; V-135; G-121
G-121 through through
A-134; G-121 through R-133; G-121 through through N-131;
K-132; G-121 G-121
through R-130; G-121 through S-129; G-121 G-121 through
through N-128; Q-127;
G-121 through S-126; G-121 through S-125; N-124; G-121
G-121 through through
G-123; and G-121 through E-122 of SEQ ID
N0:19. Polypeptides encoded by these
polynucleotides are also encompassed by the
invention.
Vectors and Host Cells
[0174] The present invention also relates to vectors which include the
isolated DNA
molecules of the present invention, host cells which are genetically
engineered with the
recombinant vectors, or which are otherwise engineered to produce the
polypeptides of the
invention, and the production of Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides, or fragments thereof, by recombinant or synthetic techniques.
[0175] In one embodiment, the polynucleotides of the invention are joined to a
vector
(e.g., a cloning or expression vector). The vector may be, for example, a
phage, plasmid,
viral or retroviral vector. Retroviral vectors may be replication competent or
replication
defective. In the latter case, viral propagation generally will occur only in
complementing
host cells. The polynucleotides may be joined to a vector containing a
selectable marker
for propagation in a host. Introduction of the vector construct into the host
cell can be
effected by techniques known in the art which include, but are not limited to,
calcium
phosphate transfection, DEAF-dextran mediated transfection, cationic lipid-
mediated
transfection, electroporation, transduction, infection or other methods. Such
methods are
described in many standard laboratory manuals, such as Davis et al., Basic
Methods In
Molecular Biology (1986),
[0176] Generally, recombinant expression vectors will include origins of
replication
and selectable markers permitting transformation of the host cell, e.g., the
ampicillin
resistance gene of E. coli and S. cerevisiae TRP1 gene, and a promoter derived
from a
highly-expressed gene to direct transcription of a downstream structural
sequence. Such
promoters can be derived from operons encoding glycolytic enzymes such as
78
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
3-phosphoglycerate kinase (PGI~), a-factor, acid phosphatase, or heat shock
proteins,
among others. The heterologous structural sequence is assembled in appropriate
phase
with translation initiation and termination sequences, and preferably, a
leader sequence
capable of directing secretion of translated protein into the periplasmic
space or
extracellular medium. Optionally, the heterologous sequence can encode a
fusion protein
including an N-terminal identification peptide imparting desired
characteristics, for
example, stabilization or simplified purification of expressed recombinant
product.
[0177] In one embodiment, the DNA of the invention is operatively associated
with an
appropriate heterologous regulatory element (e.g., promoter or enhancer), such
as, the
phage lambda PL promoter, the E. coli lac, trp, phoA, and tac promoters, the
SV40 early
and late promoters and promoters of retroviral LTRs, to name a few. Other
suitable
promoters will be known to the skilled artisan.
[0178] As indicated, the expression vectors will preferably include at least
one
selectable marker. Such markers include dihydrofolate reductase, 6418 or
neomycin
resistance for eukaryotic cell culture and tetracycline, kanamycin or
ampicillin resistance
genes for culturing in E. coli and other bacteria. Representative examples of
appropriate
hosts include, but are not limited to, bacterial cells, such as E. coli,
Streptomyces and
Salmonella typlzimurium cells; fungal cells, such as yeast cells (e.g.,
Saccharomyces
cer-evisiae or Pichia pastoris (ATCC Accession No. 201178)); insect cells such
as
Drosophila S2 and Spodoptera Sf9 cells; animal cells such as CHO, COS, 293 and
Bowes
melanoma cells; and plant cells. Appropriate culture mediums and conditions
for the
above-described host cells are known in the art.
[0179] The host cell can be a higher eukaryotic cell, such as a mammalian cell
(e.g., a
human derived cell), or a lower eukaryotic cell, such as a yeast cell, or the
host cell can be
a prokaryotic cell, such as a bacterial cell. The host strain may be chosen
which
modulates the expression of the inserted gene sequences, or modifies and
processes the
gene product in the specific fashion desired. Expression from certain
promoters can be
elevated in the presence of certain inducers; thus expression of the
genetically engineered
polypeptide may be controlled. Furthermore, different host cells have
characteristics and
specific mechanisms for the translational and post-translational processing
and
modification (e.g., phosphorylation, cleavage) of proteins. Appropriate cell
lines can be
chosen to ensure the desired modifications and processing of the foreign
protein
79
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
expressed. Selection of appropriate vectors and promoters for expression in a
host cell is a
well-known procedure and the requisite techniques for expression vector
construction,
introduction of the vector into the host and expression in the host are
routine skills in the
art.
[0180] Useful expression vectors for bacterial use are constructed by
inserting a
structural DNA sequence encoding a desired protein together with suitable
translation
initiation and termination signals in operable reading phase with a functional
promoter.
The vector will comprise one or more phenotypic selectable markers and an
origin of
replication to ensure maintenance of the vector and to, if desirable, provide
amplification
within the host. Suitable prokaryotic hosts for transformation include E.
coli, Bacillus
subtilis, Salmonella typhirrauriurn, and various species within the genera
Pseudorraonas,
Streptornyces, and Staphylococcus, although others may also be employed as a
matter of
choice. As a representative, but nonlimiting example, useful expression
vectors for
bacterial use can comprise a selectable marker and bacterial origin of
replication derived
from commercially available plasmids comprising genetic elements of the well-
known
cloning vector pBR322 (ATCC 37017). Such commercial vectors include, for
example,
pKK223-3 (Pharmacia Fine Chemicals, Uppsala, Sweden) and GEMl (Promega Biotec,
Madison, WI, USA). These pBR322 "backbone" sections are combined with an
appropriate promoter and the structural sequence to be expressed. Among
vectors
preferred for use in bacteria include pHE4-5 (ATCC Accession No. 209311; and
variations thereof), pQE70, pQE60 and pQE-9, available from QIAGEN, Inc.,
supra; pBS
vectors, Phagescript vectors, Bluescript vectors, pNHBA, pNHl6a, pNHl8A,
pNH46A,
available from Stratagene; and ptrc99a, pKK223-3, pKK233-3, pDR540, pRITS
available
from Pharmacia. Preferred expression vectors for use in yeast systems include,
but are not
limited to, pYES2, pYDl, pTEFl/Zeo, pYES2lGS, pPICZ, pGAPZ, pGAPZalpha, pPIC9,
pPIC3.5, PHIL-D2, pHIL-S1, pPIC3.5K, pPIC9K, and PA0815 (all available from
Invitrogen, Caxlsb~ad, CA). Among preferred eukaryotic vectors are pWLNEO,
pSV2CAT, pOG44, pXT1 and pSG available from Stratagene; and pSVK3, pBPV, pMSG
and pSVL (available from Pha.rmacia). Other suitable vectors will be readily
apparent to
the skilled artisan.
[0181] Following transformation of a suitable host strain and growth of the
host strain
to an appropriate cell density, the selected promoter is induced by
appropriate means (e.g.,
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
temperature shift or chemical induction) and cells are cultured for an
additional period.
Cells are typically harvested by centrifugation, disrupted by physical or
chemical means,
and the resulting crude extract retained for further purification.
[0182] Microbial cells employed in expression of proteins can be disrupted by
any
convenient method, including freeze-thaw cycling, sonication, mechanical
disruption, or
use of cell lysing agents, such methods are well know to those skilled in the
art.
[0183] In one embodiment, the yeast Pichia pastoris is used to express
Neutrokine-
alpha protein in a eukaryotic system. Pichia pastoris is a methylotrophic
yeast which can
metabolize methanol as its sole carbon source. A main step in the methanol
metabolization pathway is the oxidation of methanol to formaldehyde using 02.
This
reaction is catalyzed by the enzyme alcohol oxidase. In order to metabolize
methanol as
its sole carbon source, Pichia pastoris must generate high levels of alcohol
oxidase due, in
part, to the relatively low affinity of alcohol oxidase for OZ. Consequently,
in a growth
medium depending on methanol as a main carbon source, the promoter region of
one of
the two alcohol oxidase genes (AOXI ) is highly active. In the presence of
methanol,
alcohol oxidase produced from the AOXI gene comprises up to approximately
30°10 of the
total soluble protein in.Pichia pastoris. See, Ellis, S.B., et al., Mol. Cell.
Biol. 5:1111-21
(1985); Koutz, P.J, et al., Yeast 5:167-77 (1989); Tschopp, J.F., et al.,
Nucl. Acids Res.
15:3859-76 (1987). Thus, a heterologous coding sequence, such as, for example,
a
Neutrokine-alpha or Neutrokine-alphaSV polynucleotide of the present
invention, under
the transcriptional regulation of all or part of the AOXl regulatory sequence
is expressed at
exceptionally high levels in Pichia yeast grown in the presence of methanol.
[0184] In one example, the plasmid vector pPIC9K is used to express DNA
encoding
a Neutrokine-alpha or Neutrokine-alphaSV polypeptide of the invention, as set
forth
herein, in a Picl2ea yeast system essentially as described in "Pichia
Protocols: Methods in
Molecular Biology," D.R. Higgins and J. Cregg, eds. The Humana Press, Totowa,
NJ,
1998. This expression vector allows expression and secretion of a Neutrokine-
alpha or
Neutrokine-alphaSV protein of the invention by virtue of the strong AOXl
promoter
linked to the Pichia pastoris alkaline phosphatase (PHO) secretory signal
peptide (i.e.,
leader) located upstream of a multiple cloning site.
81
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0185] Many other yeast vectors could be used in place of pPIC9K, such as,
pYES2,
pYDl, pTEFl/Zeo, pYES2/GS, pPICZ, pGAPZ, pGAPZalpha, pPIC9, pPIC3.5, pHIL-
D2, pHIL-S1, pPIC3.5K, and PA0815, as one skilled in the art would readily
appreciate,
as long as the proposed expression construct provides appropriately located
signals for
transcription, translation, secretion (if desired), and the like, including an
in-frame AUG as
required.
[0186] In one embodiment, high-level expression of a hetcrologous coding
sequence,
such as, for example, a Neutrokine-alpha or Neutrokine-alphaSV polynucleotide
of the
present invention, may be achieved by cloning the heterologous polynucleotide
of the
invention into an expression vector such as, for example, pGAPZ or pGAPZalpha,
and
growing the yeast culture in the absence of methanol.
[0187] Transcription of the DNA encoding the polypeptides of the present
invention
by higher eukaryotes is increased by inserting an enhancer sequence into the
vector.
Enhancers are cis-acting elements of DNA, usually about from 10 to 300 by that
act on a
promoter to increase its transcription. Examples including the SV40 enhancer
on the late
side of the replication origin by 100 to 270, a cytomegalovirus early promoter
enhancer,
the polyoma enhancer on the late side of the replication origin, and
adenovirus enhancers.
[0188] Various mammalian cell culture systems can also be employed to express
recombinant protein. Examples of mammalian expression systems include the COS-
7
lines of monkey kidney fibroblasts, described by Gluzman (Cell 23:175 (1981)),
and other
cell lines capable of expressing a compatible vector, for example, the 0127,
3T3, CHO,
HeLa and BHK cell lines. Mammalian expression vectors will comprise an origin
of
replication, a suitable promoter and enhancer, and also any necessary ribosome
binding
sites, polyadenylation site, splice donor and acceptor sites, transcriptional
termination
sequences, and 5' flanking nontranscribed sequences. DNA sequences derived
from the
SV40 splice, and polyadenylation sites may be used to provide the required
nontranscribed
genetic elements.
[0189] In a specific embodiment, constructs designed to express a portion of
the
extracellular domain of the Neutrokine-alpha (e.g., amino acid residues Ala-
134 through
Leu-285) are preferred. One of skill in the art would be able to use the
polynucleotide and
polypeptide sequences provided as SEQ ID NO:1 and SEQ DJ N0:2, respectively,
or SEQ
82
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
ID N0:18 and SEQ ID N0:19, respectively, to design polynucleotide primers to
generate
such an expression construct.
[0190] In another embodiment, constructs designed to express the entire
predicted
extracellular domain of the Neutrokine-alpha (i.e., amino acid residues Gln-73
through
Leu-285) are preferred. One of skill in the art would be able to use the
polynucleotide and
polypeptide sequences provided as SEQ ID NO:1 and SEQ ID N0:2, respectively,
or SEQ
ID N0:18 and SEQ ID N0:19, respectively, to design polynucleotide primers to
generate
such an expression construct.
[0191] In addition to encompassing host cells containing the vector constructs
discussed herein, the invention also encompasses primary, secondary, and
immortalized
host cells of vertebrate origin, particularly mammalian origin, that have been
engineered to
delete or replace endogenous genetic material (e.g., Neutrokine-alpha coding
sequence),
andlor to include genetic material (e.g., heterologous polynucleotide
sequences) that is
operably associated with Neutrokine-alpha polynucleotides of the invention,
and which
activates, alters, and/or amplifies endogenous Neutrokine-alpha
polynucleotides. For
example, techniques known in the art may be used to operably associate
heterologous
control regions (e.g., promoter and/or enhancer) and endogenous Neutrokine-
alpha
polynucleotide sequences via homologous recombination (see, e.g., U.S. Patent
No.
5,641,670, issued June 24, 1997; International Publication No. WO 96129411,
published
September 26, 1996; International Publication No. WO 94112650, published
August 4,
1994; Roller et al., Proc. Natl. Acad. Sci. USA 86:8932-8935 (1989); and
Zijlstra et al.,
Nature 342:435-438 (1989), the disclosures of each of which are incorporated
by reference
in their entireties).
[0192] The host cells described infra can be used in a conventional manner to
produce
the gene product encoded by the recombinant sequence. Alternatively, cell-free
translation systems can also be employed to produce the polypeptides of the
invention
using RNAs derived from the DNA constructs of the present invention.
[0193] The polypeptide of the invention may be expressed or synthesized in a
modified form, such as a fusion protein (comprising the polypeptide joined via
a peptide
bond to a heterologous protein sequence (of a different protein)), and may
include not only
secretion signals, but also additional heterologous functional regions. Such a
fusion
protein can be made by ligating polynucleotides of the invention and the
desired nucleic
83
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
acid sequence encoding the desired amino acid sequence to each other, by
methods known
in the art, in the proper reading frame, and expressing the fusion protein
product by
methods known in the art. Alternatively, such a fusion protein can be made by
protein
synthetic techniques, e.g., by use of a peptide synthesizer. Thus, for
instance, a region of
additional amino acids, particularly charged amino acids, may be added to the
N-terminus
of the polypeptide to improve stability and persistence in the host cell,
during purification,
or during subsequent handling and storage. Also, peptide moieties may be added
to the
polypeptide to facilitate purification. Such regions may be removed prior to
final
preparation of the polypeptide. The addition of peptide moieties to
polypeptides to
engender secretion or excretion, to improve stability and to facilitate
purification, among
others, are familiar and routine techniques in the art.
[0194] In one embodiment, polynucleotides encoding Neutrokine-alpha and/or
Neutrokine-alphaSV polypeptides of the invention may be fused to signal
sequences
which will direct the localization of a protein of the invention to particular
compartments
of a prokaryotic or eukaryotic cell andlor direct the secretion of a protein
of the invention
from a prokaryotic or eukaryotic cell. For example, in E. coli, one may wish
to direct the
expression of the protein to the peripIasmic space. Examples of signal
sequences or
proteins (or fragments thereof) to which the polypeptides of the invention may
be fused in
order to direct the expression of the polypeptide to the periplasmic space of
bacteria
include, but are not limited to, the pelB signal sequence, the maltose binding
protein
(MBP) signal sequence, MBP, the ompA signal sequence, the signal sequence of
the
periplasmic E. coli heat-labile enterotoxin B-subunit, and the signal sequence
of alkaline
phosphatase. Several vectors are commercially available for the construction
of fusion
proteins Which will direct the localization of a protein, such as the pMAL
series of vectors
(particularly the pMAL-p series) available from New England Biolabs. In a
specific
embodiment, polynucleotides encoding Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptides of the invention may be fused to the pelB pectate lyase signal
sequence to
increase the efficiency of expression and purification of such polypeptides in
Gram-
negative bacteria. See, U.S. Patent Nos. 5,576,195 and 5,846,818, the contents
of which
are herein incorporated by reference in their entireties.
[0195] Examples of signal peptides that may be fused to a polypeptide of the
invention
in order to direct its secretion in mammalian cells include, but are not
limited to, the
84
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
MP1F-1 signal sequence (amino acids 1-21 of GenBank Accession number
AAB51134),
the stanniocalcin signal sequence (MLQNSAVLLLLVISASA, SEQ >D N0:45), and a
consensus signal sequence (MPTWAWWLFLVLLLALWAPARG, SEQ ID N0:46). A
suitable signal sequence that may be used in conjunction with baculoviral
expression
systems is the gp67 signal sequence, (amino acids 1-19 of GenBank Accession
Number
AAA72759). ,
[0196] A preferred fusion protein comprises a heterologous region from
immunoglobulin that is useful to stabilize and purify proteins. For example,
EP-A-O 464
533 (Canadian counterpart 2045869) discloses fusion proteins comprising
various portions
of constant region of immunoglobulin molecules together with another human
protein or
part thereof. In many cases, the Fc part in a fusion protein is thoroughly
advantageous for
use in therapy and diagnosis and thus results, for example, in improved
pharmacolunetic
properties (EP-A 0232 262). On the other hand, for some uses it would be
desirable to be
able to delete the Fc part after the fusion protein has been expressed,
detected and purified
in the advantageous manner described. This is the case when Fc portion proves
to be a
hindrance to use in therapy and diagnosis, for example when the fusion protein
is to be
used as antigen for immunizations. In drug discovery, for example, human
proteins, such
as hIL-5 has been fused with Fc portions for the purpose of high-throughput
screening
assays to identify antagonists of hIL-5. See, D. Bennett et al., J. Molecular
Recog~aition
8:52-58 (1995) and I~. Johanson et al., J. Biot. Chenz. 270:9459-9471 (1995).
[0197] Polypeptides of the present invention include naturally purified
products,
products of chemical synthetic procedures, and products produced by
recombinant
techniques from a prokaryotic or eukaryotic host, including, for example,
bacterial, yeast,
higher plant, insect and mammalian cells. Depending upon the host employed in
a
recombinant production procedure, the polypeptides of the present invention
may be
glycosylated or may be non-glycosylated. In addition, polypeptides of the
invention may
also include an initial modified methionine residue, in some cases as a result
of
host-mediated processes.
[0198] Polypeptides of the invention can be chemically synthesized using
techniques
known in the art (e.g., see Creighton, 1983, Proteins: Structures and
Molecular Principles,
W.H. Freeman & Co., N.Y., and Hunkapiller, M., et al., 1984, Nature 310:105-
111). For
example, a peptide corresponding to a fragment of the complete Neutrokine-
alpha or
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrolcine-alphaSV polypeptides of the invention can be synthesized by use of
a peptide
synthesizer. Furthermore, if desired, nonclassical amino acids or chemical
amino acid
analogs can be introduced as a substitution or addition into the Neutrolune-
alpha or
Neutrokine-alphaSV polynucleotide sequence. Non-classical amino acids include,
but are
not limited to, to the D-isomers of the common amino acids, 2,4-diaminobutyric
acid, a-
amino isobutyric acid, 4-aminobutyric acid, Abu, 2-amino butyric acid, g-Abu,
e-Ahx,
6-amino hexanoic acid, Aib, 2-amino isobutyric acid, 3-amino propionic acid,
ornithine,
norleucine, norvaline, hydroxyproline, sarcosine, citrulline, homocitrulline,
cysteic acid, t-
butylglycine, t-butylalanine, phenylglycine, cyclohexylalanine, b-alanine,
fluoro-amino
acids, designer amino acids such as b-methyl amino acids, Ca-methyl amino
acids, Na-
methyl amino acids, and amino acid analogs in general. Furthermore, the amino
acid can
be D (dextrorotary) or L (levorotary).
[0199] The invention encompasses Neutrokine-alpha or Neutrokine-alphaSV
polypeptides which are differentially modified during or after translation,
e.g., by
glycosylation, acetylation, phosphorylation, amidation, derivatization by
known
protecting/blocking groups, proteolytic cleavage, linkage to an antibody
molecule or other
cellular ligand, etc. Any of numerous chemical modifications may be carried
out by
known techniques, including but not limited, to specific chemical cleavage by
cyanogen
bromide, trypsin, chymotrypsin, papain, V8 protease, NaBH4, acetylation,
formylation,
oxidation, reduction, metabolic synthesis in the presence of tunicamycin, etc.
[0200] Additional post-translational modifications encompassed by the
invention
include, for example, e.g., N-linked or O-linked carbohydrate chains,
processing of
N-terminal or C-terminal ends), attachment of chemical moieties to the amino
acid
backbone, chemical modifications of N-linked or O-linked carbohydrate chains,
and
addition or deletion of an N-terminal methionine residue as a result of
procaryotic host cell
expression. The polypeptides may also be modified with a detectable label,
such as an
enzymatic, fluorescent, radioisotopic or affinity label to allow for detection
and isolation
of the protein. .
[0201] Examples of suitable enzymes include horseradish peroxidase, alkaline
phosphatase, beta-galactosidase, glucose oxidase or acetylcholinesterase;
examples of
suitable prosthetic group complexes include streptavidin/biotin and
avidin/biotin;
examples of suitable fluorescent materials include biotin, umbelliferone,
fluorescein,
86
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
fluorescein isothiocyanate, rhodamine, dichlorotriazinylamine fluoxescein,
dansyl chloride
or phycoerythrin; an example of a luminescent material includes luminol;
examples of
bioluminescent materials include luciferase, luciferin, and aequorin; and
examples of
suitable radioactive material include a radioactive metal ion, e.g., alpha-
emitters such as,
for example, 213Bi, or other radioisotopes such as, for example, iodine (1311,
l2sh lz3h lall)~
carbon (14C), sulfur (35S), tritium (3H), indium (llsmln~ llsmln~ llaln~
111In), and technetium
(~~Tc, ~9'T'Tc), thallium (2olTi), gallium (GBGa, G7Ga), palladium (lo3Pd),
molybdenum
(~~Mo), xenon (ls3Xe), fluorine (1sF)~ ls3Sm~ 177Lu~ ls9Gda 149Pm~ l4oLa~
17s~~ lG6Ho~ ~oY,
475~~ lsGRe~ IBSRe~ 142Pr~ losRh~ 97Ru~ GsGe~ 57C~~ GSzn~ 85Sr~ 32P~ lssGd~
1G9~,b~ SlCr~ s4Mn,
7sSe, 113Sn, and 117Tin.
[0202] In specific embodiments, Neutrokine-alpha andlor Neutrokine-alphaSV
polypeptides of the invention are attached to macrocyclic chelators useful for
conjugating
radiometal ions, including but not limited to, 111In, 177Lu, 9°y,
IGGHo, and lssSm, to
polypeptides. In a preferred embodiment, the radiometal ion associated with
the
macrocyclic chelators attached to Neutrokine-alpha andlor Neutrokine-alphaSV
polypeptides of the invention is 111In. In another preferred embodiment, the
radiometal
ion associated with the macrocyclic chelator attached to Neutrokine-alpha
and/or
Neutrokine-alphaSV polypeptides of the invention is 9°Y. In specific
embodiments, the
macrocyclic chelator is 1,4,7,10-tetraazacyclododecane-N,N',N",N"'-tetraacetic
acid
(DOTA). In other specific embodiments, the DOTA is attached to the Neutrokine-
alpha
andlor Neutrokine-alphaSV polypeptide of the invention via a linker molecule.
Examples
of linker molecules useful for conjugating DOTA to a polypeptide are commonly
known
in the art - see, for example, DeNardo et al., Clin Cancer Res. 4(10):2483-90,
1998;
Peterson et al., Bioconjug. Chem. 10(4):553-7, 1999; and Zimmerman et al,
Nucl. Med.
Biol. 26(8):943-50, 1999 which are hereby incorporated by reference in their
entirety. In
addition, U.S. Patents 5,652,361 and 5,756,065, which disclose chelating
agents that may
be conjugated to antibodies, and methods for making and using them, are hereby
incorporated by reference in their entireties. Though U.S. Patents 5,652,361
and
5,756,065 focus on conjugating chelating agents to antibodies, one skilled in
the art could
readily adapt the method disclosed therein in order to conjugate chelating
agents to other
polypeptides.
87
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0203] In one embodiment, Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides of the invention may be labeled with biotin. In other related
embodiments,
biotinylated Neutrolcine-alpha and/or Neutrokine-alphaSV polypeptides of the
invention
may be used, for example, as an imaging agent or as a means of identifying one
or more
Neutrokine-alpha and/or Neutrokine-alphaSV receptors) or other coxeceptor or
coligand
molecules.
[0204] Also provided by the invention are chemically modified derivatives of
Neutrokine-alpha or Neutrokine-alphaSV which may provide additional advantages
such
as increased solubility, stability and in vivo ox in vitro circulating time of
the polypeptide,
or decreased imrnunogenicity (see U. S. Patent No. 4,179,337). The chemical
moieties for
derivitization may be selected from water soluble polymers such as
polyethylene glycol,
ethylene glycol/propylene glycol copolymers, carboxymethylcellulose, dextran,
polyvinyl
alcohol and the like. The polypeptides may be modified at random positions
within the
molecule, or at predetermined positions within the molecule and may include
one, two,
three or more attached chemical moieties.
[0205] The polymer may be of any molecular weight, and may be branched or
unbranched. For polyethylene glycol, the preferred molecular weight is between
about 1
kDa and about 100 kDa (the term "about" indicating that in preparations of
polyethylene
glycol, some molecules will weigh more, some less, than the stated molecular
weight) for
ease in handling and manufacturing. Other sizes may be used, depending on the
desired
therapeutic profile (e.g., the duration of sustained release desired, the
effects, if any on
biological activity, the ease in handling, the degree or Lack of antigenicity
and other known
effects of the polyethylene glycol to a therapeutic protein or analog). For
example, the
polyethylene glycol may have an average molecular weight of about 200, 500,
1000, 1500,
2000, 2500, 3000, 3500, 4000, 4500, 5000, 5500, 6000, 6500, 7000, 7500, 8000,
8500,
9000, 9500, 10,000, 10,500, 11,000, 11,500, 12,000, 12,500, 13,000, 13,500,
14,000,
14,500, 15,000, 15,500, 16,000, 16,500, 17,000, 17,500, 18,000, 18,500,
19,000, 19,500,
20,000, 25,000, 30,000, 35,000, 40,000, 50,000, 55,000, 60,000, 65,000,
70,000, 75,000,
80,000, 85,000, 90,000, 95,000, or 100,000 kDa.
[0206] As noted above, the polyethylene glycol rnay have a branched structure.
Branched polyethylene glycols are described, for example, in U.S. Patent No.
5,643,575;
Morpurgo et al., Appl. Biochem. Biotechfzol. 56:59-72 (1996); Vorobjev et al.,
88
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Nucleosides Nucleotides 18:2745-2750 (1999); and Caliceti et al., Bioconjug.
Claeni.
10:638-646 (1999), the disclosures of each of which are incorporated herein by
reference.
[0207] The polyethylene glycol molecules (or other chemical moieties) should
be
attached to the protein with consideration of effects on functional or
antigenic domains of
the protein. There are a number of attachment methods available to those
skilled in the
art, e.g., EP 0 401 384, herein incorporated by reference (coupling PEG to G-
CSF), see
also Malik et al., Exp. Hematol. 20:1028-1035 (1992) (reporting pegylation of
GM-CSF
using tresyl chloride). For example, polyethylene glycol may be covalently
bound
through amino acid residues via a reactive group, such as, a free amino or
carboxyl group.
Reactive groups are those to which an activated polyethylene glycol molecule
may be
bound. The amino acid residues having a free amino group may include, for
example,
lysine residues and the N-terminal amino acid residues; those having a free
carboxyl group
may include aspartic acid residues, glutamic acid residues, and the C-terminal
amino acid
residue. Sulfhydryl groups may also be used as a reactive group for attaching
the
polyethylene glycol molecules. Preferred for therapeutic purposes is
attachment at an
amino group, such as attachment at the N-terminus or lysine group.
[0208] As suggested above, polyethylene glycol may be attached to proteins via
linkage to any of a number of amino acid residues. For example, polyethylene
glycol can
be linked to a proteins via covalent bonds to lysine, histidine, aspartic
acid, glutamic acid,
or cysteine' residues. One or more reaction chemistries may be employed to
attach
polyethylene glycol to specific amino acid residues (e.g., lysine, histidine,
aspartic acid,
glutamic acid, or cysteine) of the protein or to more than one type of amino
acid residue
(e.g., lysine, histidine, aspartic acid, glutamic acid, cysteine and
combinations thereof) of
the protein.
[0209] One may specifically desire proteins chemically modified at the N-
terminus.
Using polyethylene glycol as an illustration, one may select from a variety of
polyethylene
glycol molecules (by molecular weight, branching, etc.), the proportion of
polyethylene
glycol molecules to protein (or peptide) molecules in the reaction mix, the
type of
pegylation reaction to be performed, and the method of obtaining the selected
N-terminally pegylated protein. The method of obtaining the N-terminally
pegylated
preparation (i.e., separating this moiety from other monopegylated moieties if
necessary)
may be by purification of the N-terminally pegylated material from a
population of
89
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
pegylated protein molecules. Selective proteins chemically modified at the N-
terminus
modification may be accomplished by reductive alleylation which exploits
differential
reactivity of different types of primary amino groups (lysine versus the N-
terminal)
available for derivatization in a particular protein. Under the appropriate
reaction
conditions, substantially selective derivatization of the protein at the N-
terminus with a
carbonyl group containing polymer is achieved.
[0210] As indicated above, pegylation of the proteins of the invention may be
accomplished by any number of means. For example, polyethylene glycol may be
attached to the protein either directly or by an intervening linker.
Linkerless systems for
attaching polyethylene glycol to proteins are described in Delgado et al.,
Crit. Rev. Thera.
Drug Caf-rier Sys. 9:249-304 (1992); Francis et al., hZtena. J. of Hematol.
68:1-18 (1998);
U.S. Patent No. 4,002,531; U.S. Patent No. 5,349,052; WO 95/06058; and WO
98132466,
the disclosures of each of which are incorporated herein by reference.
[0211] One system for attaching polyethylene glycol directly to amino acid
residues of
proteins without an intervening linker employs tresylated MPEG, which is
produced by
the modification of monmethoxy polyethylene glycol (MPEG) using tresylchloride
(C1S02CH2CF3). Upon reaction of protein with tresylated MPEG, polyethylene
glycol is
directly attached to amine groups of the protein. Thus, the invention includes
protein-
polyethylene glycol conjugates produced by reacting proteins of the invention
with a
polyethylene glycol molecule having a 2,2,2-trifluoreothane sulphonyl group.
[0212] Polyethylene glycol can also be attached to proteins using a number of
different intervening linkers. For example, U.S. Patent No. 5,612,460, the
entire
disclosure of which is incorporated herein by reference, discloses urethane
linkers for
connecting polyethylene glycol to proteins. Protein-polyethylene glycol
conjugates
wherein the polyethylene glycol is attached to the protein by a linker can
also be produced
by reaction of proteins with compounds such as MPEG-succinimidylsuccinate,
MPEG
activated with 1,1'-carbonyldiimidazole, MPEG-2,4,5-trichloropenylcarbonate,
MPEG-p-
nitrophenolcarbonate, and various MPEG-succinate derivatives. A number
additional
polyethylene glycol derivatives and reaction chemistries for attaching
polyethylene glycol
to proteins are described in WO 98/32466, the entire disclosure of which is
incorporated
herein by reference. Pegylated protein products produced using the reaction
chemistries
set out herein are included within the scope of the invention.
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0213] The number of polyethylene glycol moieties attached to each protein of
the
invention (i.e., the degree of substitution) may also vary. For example, the
pegylated
proteins of the invention may be linked, on average, to 1, 2, 3, 4, 5, 6, 7,
8, 9, 10, 12, 15,
17, 20, or more polyethylene glycol molecules. Similarly, the average degree
of
substitution within ranges such as 1-3, 2-4, 3-5, 4-6, 5-7, 6-8, 7-9, 8-10, 9-
11, 10-12, 11-
13, 12-14, 13-15, 14-16, 15-17, 16-18, 17-19, or 18-20 polyethylene glycol
moieties per
protein molecule. Methods for determining the degree of substitution are
discussed, for
example, in Delgado et al., Crit. Rev. Thera. Drug Carrier Sys. 9:249-304
(1992).
[0214] The Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides can be
recovered and purified by known methods which include, but are not limited to,
ammonium sulfate or ethanol precipitation, acid extraction, anion or cation
exchange
chromatography, phosphocellulose chromatography, hydrophobic interaction
chromatography, affinity chromatography, hydroxylapatite chromatography and
lectin
chromatography. Most preferably, high performance liquid chromatography
("HPLC") is
employed for purification.
Neutrokiue-alpha Polypeptides
[0215] The Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides of the
invention may be in monomers or multimers (i.e., dimers, trimers, tetramers
and higher
multimers). Accordingly, the present invention relates to monomers and
multimers of the
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides of the invention,
their
preparation, and compositions (preferably, pharmaceutical compositions)
containing them.
In specific embodiments, the polypeptides of the invention are monomers,
dimers, trimers
or tetramers. In additional embodiments, the multimers of the invention are at
least
dimers, at least trimers, or at least tetramers.
[0216] Multimers encompassed by the invention may be homomers or heteromers.
As
used herein, the term homomer, refers to a multimer containing only Neutrokine-
alpha
and/or Neutrokine-alphaSV polypeptides of the invention (including Neutrokine-
alpha
and/or Neutrokine-alphaSV fragments, variants, and fusion proteins, as
described herein).
These homomers may contain Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides
having identical or different amino acid sequences. In a specific embodiment,
a homomer
of the invention is ~ a multimer containing only Neutrokine-alpha and/or
91
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrokine-alphaSV polypeptides having an identical amino acid sequence. In
another
specific embodiment, a homomer of the invention is a multimer containing
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides having different amino
acid
sequences. In specific embodiments, the multimer of the invention is a
homodimer (e.g.,
containing Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides having
identical or
different amino acid sequences) or a homotrimer (e.g., containing Neutrokine-
alpha and/or
Neutrokine-alphaSV polypeptides having identical or different amino acid
sequences). In
a preferred embodiment, the multimer of the invention is a homotrimer. In
additional
embodiments, the homomeric multimer of the invention is at least a homodimer,
at least a
homotrimer, or at least a homotetramer.
[0217] As used herein, the term heteromer refers to a multimer containing
heterologous polypeptides (i.e., polypeptides of a different protein) in
addition to the
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides of the invention. In a
specific
embodiment, the multimer of the invention is a heterodimer, a heterotrimer, or
a
heterotetramer. In additional embodiments, the heteromeric multimer of the
invention is
at least a heterodimer, at least a heterotrimer, or at least a heterotetramer.
In highly
preferred embodiments, the heteromeric multimer of the invention is a
heterotrimer
comprising both Neutrokine alpha-polypeptides and APRIL polypeptides (e.g.,
SEQ ID
NO:20 or SEQ ID N0:47; PCT International Publication Number W097/33902;
GenBank
Accession No. AF046888 (nucleotide) and AAC6132 (protein); J. Exp. Med.
188(6):1185-1190). In other highly preferred embodiments, the heteromeric
multimer of
the invention is a heterotrimer consisting of one Neutrokine alpha-polypeptide
and two
APRIL polypeptides. In other highly preferred embodiments, the heteromeric
multimer of
the invention is a heterotrimer consisting of two Neutrokine alpha-
polypeptides and one
APRIL polypeptide. In a further nonexclusive embodiment, the heteromers of the
invention contain CD40 ligand polypeptide sequence(s), or biologically active
fragments)
or variants) thereof.
[0218] Multimers of the invention may be the result of hydrophobic,
hydrophilic, ionic
and/or covalent associations and/or may be indirectly linked, by for example,
liposome
formation. Thus, in one embodiment, multimers of the invention, such as, for
example,
homodimers or homotrimers, are formed when polypeptides of the invention
contact one
another in solution. In another embodiment, heteromultimers of the invention,
such as, for
92
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
example, heterotrimers or heterotetramers, are formed when polypeptides of the
invention
contact antibodies to the polypeptides of the invention (including antibodies
to the
heterologous polypeptide sequence in a fusion protein of the invention) in
solution. In
other embodiments, multimers of the invention are formed by covalent
associations with
and/or between the Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides of
the
invention. Such covalent associations may involve one or more amino acid
residues
contained in the polypeptide sequence (e.g., that recited in SEQ ID N0:2 or
SEQ ID
N0:19, or contained in the polypeptide encoded by the clones deposited in
connection
with this application). In one instance, the covalent associations are cross-
linking between
cysteine residues located within the polypeptide sequences which interact in
the native
(i.e., naturally occurring) polypeptide. In another instance, the covalent
associations are
the consequence of chemical or recombinant manipulation. Alternatively, such
covalent
associations may involve one or more amino acid residues contained in the
heterologous
polypeptide sequence in a Neutrokine-alpha and/or Neutrokine-alphaSV fusion
protein. In
one example, covalent associations are between the heterologous sequence
contained in a
fusion protein of the invention (see, e.g., US Patent Number 5,478,925). In a
specific
example, the covalent associations are between the heterologous sequence
contained in a
Neutrokine-alpha-Fc and/or Neutrokine-alphaSV-Fc fusion protein of the
invention (as
described herein). In another specific example, covalent associations of
fusion proteins of
the invention are 'between heterologous polypeptide sequence from another TNF
family
ligand/receptor member that is capable of forming covalently associated
multimers, such
as for example, oseteoprotegerin (see, e.g., International Publication No. WO
98/49305,
the contents of which are herein incorporated by reference in its entirety).
In another
specific example, covalent associations of fusion proteins of the invention
are between
heterologous polypeptide sequence from CD40L, or a soluble fragment thereof.
In
another embodiment, two or more Neutrokine-alpha and/or Neutrokine-alpha
polypeptides
of the invention are joined through synthetic linkers (e.g., peptide,
carbohydrate or soluble
polymer linkers). Examples include those peptide linkers described in U.S.
Pat. No.
5,073,627 (hereby incorporated by reference). Proteins comprising multiple
Neutrokine-
alpha and/or Neutrokine-alphaSV polypeptides separated by peptide linkers may
be
produced using conventional recombinant DNA technology.
93
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0219] Another method for preparing multimer Neutrokine-alpha and/or
Neutrolcine-
alphaSV polypeptides of the invention involves use of Neutrokine-alpha and/or
Neutrolcine-alphaSV polypeptides fused to a leucine zipper or isoleucine
zipper
polypeptide sequence. Leucine zipper or isoleucine zipper domains are
polypeptides that
promote multimerization of the proteins in which they are found. Leucine
zippers were
originally identified in several DNA-binding proteins (Landschulz et al.,
Science
240:1759, (1988)), and have since been found in a variety of different
proteins. Among
the known leucine zippers or isoleucine zippers are naturally occurring
peptides and
derivatives thereof that dimerize or trimerize. Examples of leucine zipper
domains
suitable for producing soluble multimeric Neutrokine-alpha and/or Neutrokine-
alphaSV
proteins are those described in PCT application WO 94/10308, hereby
incorporated by
reference. Recombinant fusion proteins comprising a soluble Neutrokine-alpha
and/or
Neutrokine-alphaSV polypeptide fused to a peptide that dimerizes or trimerizes
in solution
are expressed in suitable host cells, and the resulting soluble multimeric
Neutrokine-alpha
and/or Neutrokine-alphaSV is recovered from the culture supernatant using
techniques
known in the art.
[0220] Certain members of the TNF family of proteins are believed to exist in
trimeric
form (Beutler and Huffel, Sciesace 264:667, 1994; Banner et al., Cell 73:431,
1993). Thus,
trimeric Neutrokine-alpha andlor Neutrokine-alphaSV may offer the advantage of
enhanced biological activity. Preferred leucine zipper moieties are those that
preferentially form trimers. One example is a leucine zipper derived from lung
surfactant
protein D (SPD), as described in Hoppe et al. (FEBS Letters 344:191, (1994))
and in U.S.
patent application Ser. No. 08/446,922, hereby incorporated by reference.
Other peptides
derived from naturally occurring trimeric proteins may be employed in
preparing trimeric
Neutrokine-alpha and/or Neutrokine-alphaSV.
[0221] In another example, proteins of the invention are associated by
interactions
between the Flag~ polypeptide sequence contained in Flag~-Neutrokine alpha or
Flag~-
Neutrokine-alphaSV fusion proteins of the invention. In a further embodiment,
proteins
of the invention are associated by interactions between the heterologous
polypeptide
sequence contained in Flag-Neutrokine-alpha or Flag-Neutrokine-alphaSV fusion
proteins of the invention and anti-Flag~ antibody.
94
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0222] The multimers of the invention may be generated using chemical
techniques
known in the art. For example, polypeptides desired to be contained in the
multimers of
the invention may be chemically cross-linked using linker molecules and linker
molecule
length optimization techniques known in the art (see, e.g., US Patent Number
5,478,925,
which is herein incorporated by reference in its entirety). Additionally,
multimers of the
invention may be generated using techniques known in the art to form one or
more inter-
molecule cross-links between the cysteine residues located within the sequence
of the
polypeptides desired to be contained in the multimer (see, e.g., US Patent
Number
5,478,925, which is herein incorporated by reference in its entirety).
Further, polypeptides
of the invention may be routinely modified by the addition of cysteine or
biotin to the C
temninus or N-terminus of the polypeptide and techniques known in the art may
be applied
to generate multimers containing one or more of these modified polypeptides
(see, e.g.,
US Patent Number 5,478,925, which is herein incorporated by reference in its
entirety).
Additionally, techniques known in the art may be applied to generate liposomes
containing the polypeptide components desired to be contained in the multimer
of the
invention (see, e.g., US Patent Number 5,478,925, which is herein incorporated
by
reference in its entirety).
[0223] Alternatively, multimers of the invention may be generated using
genetic
engineering techniques known in the art. In one embodiment, polypeptides
contained in
multimers of the invention are produced recombinantly using fusion protein
technology
described herein or otherwise known in the art (see, e.g., US Patent Number
5,478,925,
which is herein incorporated by reference in its entirety). In a specific
embodiment,
polynucleotides coding for a homodimer of the invention are generated by
ligating a
polynucleotide sequence encoding a polypeptide of the invention to a sequence
encoding a
linker polypeptide and then further to a synthetic polynucleotide encoding the
translated
product of the polypeptide in the reverse orientation from the original C-
terminus to the
N-terminus (lacking the leader sequence) (see, e.g., US Patent Number
5,478,925, which
is herein incorporated by reference in its entirety). In another embodiment,
recombinant
techniques described herein or otherwise known in the art are applied to
generate
recombinant polypeptides of the invention which contain a transmembrane domain
and
which can be incorporated by membrane reconstitution techniques into liposomes
(see,
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
e.g., US Patent Number 5,478,925, which is herein incorporated by reference in
its
entirety).
[0224] In one embodiment, the invention provides an isolated Neutrolcine-alpha
polypeptide having the amino acid sequence encoded by the cDNA clone contained
in
ATCC No. 97768, or the amino acid sequence in Figures 1A and 1B (SEQ ID N0:2),
or a
polypeptide comprising a portion (i.e., a fragment) of the above polypeptides.
In another
embodiment, the invention provides an isolated Neutrokine-alphaSV polypeptide
having
the amino acid encoded by the cDNA clone contained in ATCC No. 203518, or the
amino
acid sequence in Figures 5A and 5B (SEQ ID N0:19), or a polypeptide comprising
a
portion (i.e, fragment) of the above polypeptides.
[0225] Polypeptide fragments of the present invention include polypeptides
comprising or alternatively, consisting of, an amino acid sequence contained
in SEQ ID
N0:2, encoded by the cDNA contained in the plasmid having ATCC accession
number
97768, or encoded by nucleic acids which hybridize (e.g., under stringent
hybridization
conditions) to the nucleotide sequence contained in the deposited clone, or
the
complementary strand of the nucleotide sequence shown in Figures lA-B (SEQ ID
NO:1.
[0226] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID N0:19, encoded by the cDNA contained in the plasmid having ATCC
accession number 203518, or encoded by nucleic acids which hybridize (e.g.,
under
stringent hybridization conditions) to the nucleotide sequence contained in
the deposited
clone, or the complementary strand of the nucleotide sequence shown in Figures
5A-B
(SEQ ID N0:18).
[0227] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence encoded
by nucleic acids which hybridize (e.g., under hybridization conditions
described herein) to
the complementary strand of the nucleotide sequence shown in SEQ ID N0:21.
[0228] Polypeptide fragments of the present invention also include
polypeptides
comprising or alternatively, consisting of, an amino acid sequence contained
in SEQ ID
N0:23, or encoded by nucleic acids which hybridize (e.g., under hybridization
conditions
described herein) to the complementary strand of the nucleotide sequence shown
in SEQ
ID N0:22.
96
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0229] In addition, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID N0:28, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of the nucleotide
sequence
shown in SEQ ID N0:27.
[0230] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID N0:30, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of the nucleotide
sequence
shown in SEQ ID N0:29.
[0231] ~ Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID N0:38, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of the nucleotide
sequence
shown in SEQ ID N0:37.
[0232] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID NO:39, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of a nucleotide
sequence
encoding the polypeptide of SEQ ID N0:39.
[0233] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID NO:40, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of a nucleotide
sequence
encoding the polypeptide of SEQ ID N0:40.
[0234] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID N0:41, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) ~ to the complementary strand of a nucleotide
sequence
encoding the polypeptide of SEQ ID N0:41.
[0235] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
97
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
in SEQ ID N0:42, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of a nucleotide
sequence
encoding the polypeptide of SEQ ID N0:42.
[0236] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID N0:43, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of a nucleotide
sequence
encoding the polypeptide of SEQ ID N0:43.
[0237] Additionally, polypeptide fragments of the present invention include
polypeptides comprising or alternatively, consisting of, an amino acid
sequence contained
in SEQ ID N0:44, or encoded by nucleic acids which hybridize (e.g., under
hybridization
conditions described herein) to the complementary strand of a nucleotide
sequence
encoding the polypeptide of SEQ ID N0:44.
[0238] Polypeptide fragments of the present invention include polypeptides
comprising or alternatively, consisting of, an amino acid sequence contained
in SEQ ID
N0:2, encoded by the cDNA contained in the deposited clone, or encoded by
nucleic acids
which hybridize (e.g., under stringent hybridization conditions) to the
nucleotide
sequence contained in the deposited clone, or shown in Figures 1A and 1B (SEQ
ID
NO:l) or the complementary strand thereto. Protein fragments may be "free-
standing," or
comprised within a larger polypeptide of which the fragment forms a part or
region, most
preferably as a single continuous region. Representative examples of
polypeptide
fragments of the invention, include, for example, fragments that comprise or
alternatively,
consist of from about amino acid residues: 1 to 50, 51 to 100, 101 to 150, 151
to 200, 201
to 250, and/or 251 to 285 of SEQ ID N0:2. Moreover, polypeptide fragments can
be at
least 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 175 or
200 amino
acids in length.
[0239] In specific embodiments, polypeptide fragments of the invention
comprise, or
alternatively consist of, amino acid residues: 1-46, 31-44, 47-72, 73-285, 73-
83, 94-102,
148-152, 166-181, 185-209, 210-221, 226-237, 244-249, 253-265, and/or 277-284,
as
depicted in Figures 1A and 1B (SEQ m NO:2). Polynucleotides encoding these
polypeptides are also encompassed by the invention.
98
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0240] It will be recognized by one of ordinary skill in the art that
mutations targeted
to regions of a Neutrokine-alpha polypeptide of the invention which encompass
the
nineteen amino acid residue insertion which is not found in the Neutrolcine-
alphaSV
polypeptide sequence (i.e., amino acid residues Val-142 through Lys-160 of the
sequence
presented in Figures 1A and 1B and in SEQ ID N0:2) may affect the observed
biological
activities of the Neutrokine-alpha polypeptide. More specifically, a partial,
non-limiting
and non-exclusive list of such residues of the Neutrokine-alpha polypeptide
sequence
which may be targeted for mutation includes the following amino acid residues
of the
Neutrokine-alpha polypeptide sequence as shown in SEQ ID N0:2: V-142; T-143;
Q-144; D-145; C-146; L-147; Q-148; L-149; I-150; A-151; D-152; S-153; E-154; T-
155;
P-156; T-157; I-158; Q-159; and I~-160. Polynucleotides encoding Neutrokine-
alpha
polypeptides which have one or more mutations in the region from V-142 through
K-160
of SEQ 117 N0:2 are contemplated. Polypeptides encoded by these
polynucleotides are
also encompassed by the invention.
[0241] Polypeptide fragments may be "free-standing," or comprised within a
larger
polypeptide of which t$e fragment forms a part or region, most preferably as a
single
continuous region. Representative examples of polypeptide fragments of the
invention,
include, for example, fragments that comprise or alternatively, consist of
from about
amino acid residues: 1 to 15, 16-30, 31-46, 47-55, 56-72, 73-104, 105-163, 163-
188, 186-
210 and 210-284 of the amino acid sequence disclosed in SEQ ID N0:2.
Additional
representative examples of polypeptide fragments of the invention, include,
for example,
fragments that comprise or alternatively, consist of from about amino acid
residues: 1 to
143, 1-150; 47-143, 47-150, 73-143, 73-150, 100-150, 140-145, 142-148, 140-
150, 140-
200, 140-225, and 140-266 of the amino acid sequence disclosed in SEQ ID
N0:19.
Moreover, polypeptide fragments can be at least 10, 20, 30, 40, 50, 60, 70,
80, 90, 100,
110, 120, 130, 140, 150, 175 or 200 amino acids in length. In this context,
"about" means
the particularly recited ranges and ranges larger or smaller by several, a
few, 5, 4, 3, 2 or 1
amino acid residues at either or both the amino- and carboxy-termini.
Polynucleotides
encoding these polypeptide fragments are also encompassed by the invention.
[0242] Additional preferred embodiments encompass polypeptide fragments
comprising, or alternatively consisting of, the predicted intracellular domain
of
Neutrokine-alpha (amino acid residues 1-46 of SEQ ID N0:2), the predicted
99
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
transmembrane domain of Neutrokine-alpha (amino acid residues 47-72 of SEQ ID
N0:2), the predicted extracellular domain of Neutroltine-alpha (amino acid
residues
73-285 of SEQ ID N0:2), the predicted TNF conserved domain of Neutrokine-alpha
(amino acids 191 to 284 of SEQ ID N0:2), and a polypeptide comprising, or
alternatively,
consisting of the predicted intracellular domain fused to the predicted
extracellular domain
of Neutrokine-alpha (amino acid residues 1-46 fused to amino acid residues 73-
285 of
SEQ ID N0:2). Polynucleotides encoding these polypeptides are also encompassed
by the
invention.
[0243] Further additional preferred embodiments encompass polypeptide
fragments
comprising, or alternatively consisting of, the predicted intracellular domain
of
Neutrokine-alphaSV (amino acid residues 1-46 of SEQ ID N0:19), the predicted
transmembrane domain of Neutrokine-alphaSV (amino acid residues 47-72 of SEQ
>D
N0:19), the predicted extracellular domain of Neutrokine-alphaSV (amino acid
residues
73-266 of SEQ ID N0:19), the predicted TNF conserved domain of Neutrokine-
alphaSV
(amino acids 172 to 265 of SEQ ID N0:19), and a polypeptide comprising, or
alternatively, consisting of the predicted intracellular domain fused to the
predicted
extracellular domain of Neutrokine-alphaSV (amino acid residues 1-46 fused to
amino
acid residues 73-266 of SEQ ID NO:19). Polynucleotides encoding these
polypeptides are
also encompassed by the invention.
[0244] Certain additional embodiments of the invention encompass polypeptide
fragments comprising, or alternatively consisting of, the predicted beta-
pleated sheet
regions identified in Figures 7A-1 and 7A-2. These polypeptide fragments of
the
invention comprise, or alternatively consist of, amino acid residues Gln-144
to Ala-151,
Phe-172 to Lys-173, Ala-177 to Glu-179, Asn-183 to Ile-185, Gly-191 to Lys-
204, His-
210 to Val-219, Leu-226 to Pro-237, Asn-242 to Ala-251, Gly-256 to Ile-263
and/or Val-
276 to Leu-284 of SEQ )D N0:2. In another, nonexclusive embodiment, these
polypeptide fragments of the invention also comprise, or alternatively consist
of, amino
acid residues Phe-153 to Lys-154, Ala-158 to Glu-160, Asn-164 to Ile-166, Gly-
172 to
Lys-185, His-191 to Val-200, Leu-207 to Pro-218, Asn-223 to Ala-232, Gly-237
to Ile-
244 and/or Val-257 to Leu-265 of SEQ )D N0:19; and amino acid residues Phe-42
to Lys-
43, Ala-47 to Glu-49, Asn-53 to Ile-55, Gly-61 to Pro-74, His-80 to Val-89,
Leu-96 to
Pro-107, Asn-112 to Ala-121, Gly-126 to Ile-133 and/or Asp-146 to Leu-154 of
SEQ ID
loo
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
N0:23. In further nonexclusive embodiments, these polypeptide fragments of the
invention also comprise, or alternatively consist of, amino acid residues Gln-
78 to Ala-85;
Phe-106 to Lys-107, Ala-111 to Glu-113, Asn-117 to Ile-119, Gly-125 to Lys-
138, His-
144 to Val-153, Leu-160 to Pro-171, Asn-176 to Ala-185, Gly-190 to Ile-197
and/or Val-
210 to Leu-218 of SEQ ID N0:28; and amino acid residues Gln-78 to Ala-85; Phe-
106 to
Lys-107, Ala-111 to Glu-113, Asn-117 to Ile-119, Gly-125 to Lys-138, His-144
to Val-
153, Leu-160 to Pro-171, Asn-176 to Ala-185, Gly-190 to Ile-197 and/or Val-210
to Leu-
218 of SEQ ID N0:30. Polynucleotides encoding these polypeptide fragments are
also
provided.
[0245] , A partial, non-limiting, and exemplary list of polypeptides of the
invention
which comprise, or alternatively consist of, combinations of amino acid
sequences of the
invention includes, for example, [Met-1 to Lys-113] fused to [Leu-114 to Thr-
141] fused
to [Val-142 to Lys-160] fused to [Gly-161 to Gln-198] fused to [Val-199 to Ala-
248]
fused to [Gly-249 to Leu-285] of SEQ » N0:2; or [Met-1 to Lys-113] fused to
[Val-142
to Lys-160] fused to [Gly-161 to Gln-198] fused to [Val-199 to Ala-248] fused
to [Gly-.
249 to Leu-285] of SEQ ID N0:2; or [Met-1 to Lys-113] fused to [Leu-114 to Thr-
141]
fused to [Val-142 to Lys-160] fused to [Gly-161 to Gln-198] fused to [Gly-249
to Leu-
285] of SEQ ID N0:2. Other combinations may include the polypeptide fragments
in an
order other than that recited above (e.g., [Leu-114 to Thr-141] fused to [Val-
199 to Ala-
248] fused to [Gly-249 to Leu-285] fused to [Val-142 to Lys-160] of SEQ ID
N0:2).
Other combinations may also include heterologous polypeptide fragments as
described
herein andlor other polypeptides or polypeptide fragments of the present
invention (e.g.,
[Met-1 to Lys-113] fused to [Leu-114 to Thr-141] fused to [Val-142 to Lys-160]
fused to
[Gly-161 to Gln-198] fused to [Gly-249 to Leu-285] of SEQ ID N0:2 fused to a
FLAG
tag; or [Met-1 to Lys-113] of SEQ lD N0:2 fused to [Leu-114 to Thr-141] of SEQ
ID
N0:2 fused to [Glu-135 to Asn-165] of SEQ ID N0:39 fused to [Val-142 to Lys-
160] of
SEQ ID N0:2 fused to [Gly-161 to Gln-198] of SEQ ID N0:2 fused to [Val-199 to
Ala-
248] of SEQ ID N0:2 fused to [Gly-249 to Leu-285] of SEQ ID NO:2).
Polynucleotides
encoding any of these polypeptides are encompassed by the invention.
[0246] An additional partial, non-limiting, and exemplary list of polypeptides
of the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Met-1 to Lys-113] fused to [Leu-114 to Thr-
141] fused
101
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
to [Gly-142 to Gln-179] fused to [Val-180 to Ala-229] fused to [Gly-230 to Leu-
266] of
SEQ ID N0:19; [Met-1 to Lys-113J fused to [Gly-142 to Gln-179J fused to [Val-
180 to
Ala-229] fused to [Gly-230 to Leu-266] of SEQ ID N0:19; or [Met-1 to Lys-113]
fused to
[Leu-114 to Thr-141J fused to [Gly-142 to Gln-179] fused to [Gly-230 to Leu-
266] of
SEQ ID N0:19. Other combinations may include the polypeptide fragments in an
order
other than that recited above (e.g., [Leu-114 to Thr-141] fused to [Val-180 to
Ala-229J
fused to [Gly-230 to Leu-266] fused to [Gly-142 to Gln-179J of SEQ ID N0:19).
Other
combinations may also include heterologous polypeptide fragments as described
herein
and/or other polypeptides or polypeptide fragments of the present invention
(e.g., [Met-1
to Lys-113] fused to [Leu-114 to Thr-141] fused to [Gly-142 to Gln-179] fused
to [Gly-
230 to Leu-266] of SEQ ID NO:19 fused to a FLAG tag or , [Met-1 to Lys-113] of
SEQ
ID N0:19 fused to [Leu-114 to Thr-141] of SEQ ID N0:19 fused to [Glu-135 to
Asn-165J
of SEQ ID N0:39 fused to [Gly-142 to Gln-179] of SEQ ID N0:19 fused to [Val-
180 to
Ala-229] of SEQ ID N0:19 fused to [Gly-230 to Leu-266] of SEQ ID N0:19).
Polynucleotides encoding any of these polypeptides are encompassed by the
invention.
[0247] A further partial, non-limiting, and exemplary list of polypeptides of
the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Met-1 to Lys-106] fused to [Leu-107 to Thr-
134] fused
to [Ile-166 to Lys-184J fused to [Gly-185 to Gln-222] fused to [Val-223 to Ala-
272J fused
to [Gly-273 to Leu-309] of SEQ ID NO:39; [Met-1 to Lys-106] fused to [Glu-135
to Asn-
165] fused to [Ile-166 to Lys-184] fused to [Gly-185 to Gln-222] fused to [Val-
223 to Ala-
272] fused to [Gly-273 to Leu-309] of SEQ ID N0:39; or [Met-1 to Lys-106]
fused to
[Leu-107 to Thr-134] fused to [Glu-135 to Asn-165] fused to [Ile-166 to Lys-
184] fused to
[Gly-185 to Gln-222] fused to [Gly-273 to Leu-309] of SEQ ID N0:39. Other
combinations may include the polypeptide fragments in an order other than that
recited
above (e.g., [Met-1 to Lys-106] fused to [Gly-185 to Gln-222] fused to [Ile-
166 to Lys-
184] fused to [Val-223 to Ala-272] fused to [Leu-107 to Thr-134J fused to [Gly-
273 to
Leu-309J of SEQ lD N0:39). Other combinations may also include heterologous
polypeptide fragments as described herein and/or other polypeptides or
polypeptide
fragments of the present invention (e.g., [Met-1 to Lys-106] fused to [Glu-135
to Asn-165]
fused to [Ile-166 to Lys-184J fused to [Gly-185 to Gln-222] fused to [Val-223
to Ala-272]
102
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
fused to [Gly-273 to Leu-309] of SEQ ID N0:39 fused to a FLAG tag).
Polynucleotides
encoding any of these polypeptides are encompassed by the invention.
[0248] A further partial, non-limiting, and exemplary list of polypeptides of
the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes,.for example, [Met-1 to Lys-106] fused to [Leu-107 to Thr-
134] fused
to [Glu-135 to Asn-165] fused to [Ile-166 to Pro-180] fused to [Ala-181 to Gln-
202] fused
to [Val-203 to Ala-252] fused to [Gly-253 to Leu-289] of SEQ ID N0:38; [Met-1
to Lys-
106] fused to [Leu-107 to Thr-134] fused to [Ile-166 to Pr0-180] fused to [Ala-
181 to Gln-
202] fused to [Val-203 to Ala-252] fused to [Gly-253 to Leu-289] of SEQ ID
N0:38;
[Met-1 to Lys-106] fused to [Leu-107 to Thr-134] fused to [Glu-135 to Asn-165]
fused
[Ala-181 to Gln-202] fused to [Val-203 to Ala-252] fused to [Gly-253 to Leu-
289] of SEQ
)D N0:38; [Met-1 to Lys-106] fused to [Leu-107 to Thr-134] fused to [Ala-181
to Gln-
202] fused to [Val-203 to Ala-252] fused to [Gly-253 to Leu-289] of SEQ ID
N0:38;
Other combinations may include the polypeptide fragments in an order other
than that
recited above (e.g., [Met-1 to Lys-106] fused to [Ala-181 to Gln-202] fused to
[Ile-166 to
Pro-180] fused to [Val-203 to Ala-252] fused to [Leu-107 to Thr-134] fused to
[Gly-253
to Leu-289] of SEQ ID N0:38). Other combinations may also include heterologous
polypeptide fragments as described herein andlor other polypeptides or
polypeptide
fragments of the present invention (e.g., [Met-1 to Lys-106] fused to [Glu-135
to Asn-165]
fused to [Ile-166 to Pro-180] fused to [Ala-181 to Gln-202] fused to [Val-203
to Ala-252]
fused to [Gly-253 to Leu-289] of SEQ ID NO:38 fused to a FLAG tag).
Polynucleotides
encoding any of these polypeptides are encompassed by the invention.
[0249] A further partial, non-limiting, and exemplary list of polypeptides of
the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Met-1 to Lys-106] fused to [Leu-107 to Thr-
134] fused
to [Glu-135 to Asn-165] fused to [Arg-166 to Gln-203] fused to [Val-204 to Ala-
253]
fused to [Gly-254 to Leu-290] of SEQ ID N0:40; [Met-1 to Lys-i06] fused [Glu-
135 to
Asn-165] fused to [Arg-166 to Gln-203] fused to [Val-204 to Ala-253] fused to
[Gly-254
to Leu-290] of SEQ >D N0:40; [Met-1 to Lys-106] fused to [Leu-107 to Thr-134]
fused to
[Arg-166 to Gln-203] fused to [Val-204 to Ala-253] fused to [Gly-254 to Leu-
290] of
SEQ ID NO:40; or [Met-1 to Lys-106] fused to [Leu-107 to Thr-134] fused to
[Glu-135 to
Asn-165] fused to [Arg-166 to Gln-203] fused to [Gly-254 to Leu-290] of SEQ ID
N0:40.
103
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Other combinations may include the polypeptide fragments in an order other
than that
recited above (e.g., [Met-1 to Lys-106] fused to [Arg-166 to Gln-203] fused to
[Val-204 to
Ala-253] fused to [Leu-107 to Thr-134] fused to [Gly-254 to Leu-290] of SEQ >D
N0:40).
Other combinations may also include heterologous polypeptide fragments as
described
herein andlor other polypeptides or polypeptide fragments of the present
invention (e.g.,
[Met-1 to Lys-106] fused to [Glu-135 to Asn-165] fused to [Arg-166 to to Gln-
202] fused
to [Val-204 to Ala-253] fused to [Gly-254 to Leu-290] of SEQ ID N0:38 fused to
a FLAG
tag). Polynucleotides encoding any of these polypeptides are encompassed by
the
invention.
[0250] A further partial, non-limiting, and exemplary list of polypeptides of
the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Tyr-1 to Lys-47] fused to [Leu-48 to Thr-75]
fused to
[Val-76 to Lys-94] fused to [Gly-95 to Gln-132] fused to [Val-133 to Ala-182]
fused to
[Gly-183 to Leu-219] of SEQ ID N0:28;. [Tyr-1 to Lys-47] fused to [Leu-48 to
Thr-75]
fused to [Val-76 to Lys-94] fused to [Val-133 to Ala-182] of SEQ m N0:28; or
[Tyr-1 to
Lys-47] fused to [Val-76 to Lys-94] fused to [Val-133 to Ala-182] fused to
[Gly-183 to
Leu-219] of SEQ ID N0:28. Other combinations may include the polypeptide
fragments
in an order other than that recited above (e.g., [Tyr-1 to Lys-47] fused to
[Gly-183 to Leu-
219] fused to [Val-133 to Ala-182] fused to [Leu-48 to Thr-75] of SEQ ID
N0:28). Other
combinations may also include heterologous polypeptide fragments as described
herein
andlor other polypeptides or polypeptide fragments of the present invention
(e.g., [Leu-48
to Thr-75] fused to [Val-76 to Lys-94] fused to [Gly-95 to Gln-132] fused to
[Val-133 to
Ala-182] of SEQ ID N0:28 fused to an Fc receptor tag). Polynucleotides
encoding any of
these polypeptides are encompassed by the invention.
[0251] A further partial, non-limiting, and exemplary list of polypeptides of
the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Tyr-1 to Lys-47] fused to [Leu-48 to Thr-75]
fused to
[Val-76 to Lys-94] fused to [Gly-95 to Gln-132] fused to [Val-133 to Ala-182]
fused to
[Gly-183 to Leu-219] of SEQ ID N0:30; [Tyr-1 to Lys-47] fused to [Leu-48 to
Thr-75]
fused to [Val-76 to Lys-94] fused to [Val-133 to Ala-182] of SEQ )D N0:30; or
[Tyr-1 to
Lys-47] fused to [Val-76 to Lys-94] fused to [Val-133 to Ala-182] fused to
[Gly-183 to
Leu-219] of SEQ ID N0:30. Other combinations may include the polypeptide
fragments
104
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
in an order other than that recited above (e.g., [Tyr-1 to Lys-47] fused to
[Gly-183 to Leu-
219] fused to [Val-133 to Ala-182] fused to [Leu-48 to Thr-75] of SEQ ID
N0:30). Other
combinations may also include heterologous polypeptide fragments as described
herein
and/or other polypeptides or polypeptide fragments of the present invention
(e.g., [L,eu-48
to Thr-75] fused to [Val-76 to Lys-94] fused to [Gly-95 to Gln-132] fused to
[Val-133 to
Ala-182] of SEQ ID N0:30 fused to an Fc receptor tag). Polynucleotides
encoding any of
these polypeptides are encompassed by the invention.
[0252] A further partial, non-limiting, and exemplary list . of polypeptides .
of the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Ala-1 to Thr-8] fused to [Val-9 to Lys-27]
fused to
[Gly-28 to Gln-65] fused to [Val-66 to Ala-115] fused to [Gly-116 to Leu-152]
of SEQ ID
N0:41; [Ala-1 to Thr-8] fused to [Gly-28 to Gln-65] fused to [Val-66 to Ala-
115] fused to
[Gly-116 to Leu-152] of SEQ ID N0:41; [Ala-1 to Thr-8] fused to [Val-9 to Lys-
27] fused
to [Gly-28 to Gln-65] fused to [Gly-116 to Leu-152] of SEQ ID NO:41; Other
combinations may include the polypeptide fragments in an order other than that
recited
above (e.g[Ala-1 to Thr-8] fused to [Gly-116 to Leu-152] fused to [Val-66 to
Ala-115]
fused to [Val-9 to Lys-27] of SEQ DJ NO:41). Other combinations may also
include
heterologous polypeptide fragments as described herein and/or other
polypeptides or
polypeptide fragments of the present invention (e.g., [Ala-1 to Thr-8] fused
to [Val-9 to
Lys-27] fused to [Gly-28 to Gln-65] fused to [Val-66 to Ala-115] fused to [Gly-
116 to
Leu-152] of SEQ ID N0:41 fused to an Fc receptor tag). Polynucleotides
encoding any of
these polypeptides are encompassed by the invention.
(0253] A further partial, non-limiting, and exemplary list of polypeptides of
the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Ala-1 to Thr-8] fused to [Glu-9 to Thr-40]
fused to
[Arg-41 to Gln-78] fused to [Val-79 to Ala-128] fused to [Gly-129 to Leu-165]
of SEQ ID
N0:42; [Ala-1 to Thr-8] fused to [Arg-41 to Gln-78] fused to [Val-79 to Ala-
128] fused to
[Gly-129 to Leu-165] of SEQ ID N0:42; [Ala-1 to Thr-8] fused to [Glu-9 to Thr-
40] fused
to [Arg-41 to Gln-78] fused to [Gly-129 to Leu-165] of SEQ ID N0:4. Other
combinations may include the polypeptide fragments in an order other than that
recited
above (e.g[Ala-1 to Thr-8] fused to [Gly-129 to Leu-165] fused to [Val-79 to
Ala-128]
fused to [Arg-41 to Gln-78] fused to [Glu-9 to Thr-40] of SEQ ID N0:42). Other
105
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
combinations may also include heterologous polypeptide fragments as described
herein
and/or other polypeptides or polypeptide fragments of the present invention
(e.g., [Ala-1
to Thr-8] fused to [Glu-9 to Thr-40] fused to [Arg-41 to Gln-78] fused to [Val-
79 to Ala-
128] fused to [GIy-129 to Leu-165] of SEQ ID N0:42 fused to an Fc receptor
tag).
Polynucleotides encoding any of these polypeptides are encompassed by the
invention.
[0254] A further partial, non-limiting, and exemplary list of polypeptides of
the
invention which comprise, or alternatively consist of, combinations of amino
acid
sequences includes, for example, [Ala-1 to Thr-8] fused to [GIu-9 to Thr-40]
fused to [Ile-
41 to Lys-59] fused to [Gly-60 to Gln-97] fused to [Val-98 to Ala-147] fused
to [Gly-148
to Leu-184] of SEQ ID N0:43; [Ala-1 to Thr-8] fused [Gly-60 to Gln-97] fused
to [Gly-
148 to Leu-184] of SEQ ID N0:43; [Ala-1 to Thr-8] fused to [Glu-9 to Thr-40]
fused to
[GIy-60 to Gln-97] fused to [Val-98 to Ala-147] fused to [Gly-148 to Leu-184]
of SEQ ll~
N0:43; [Ala-1 to Thr-8] fused to [Ile-41 to Lys-59] fused to [Gly-60 to Gln-
97] fused to
[Val-98 to Ala-147] fused to [Gly-148 to Leu-184] of SEQ ID N0:43; or [Ala-1
to Thr-8]
fused to [Glu-9 to Thr-40] fused to [IIe-4I to Lys-59] fused to [Gly-60 to GIn-
97] fused to
[Gly-148 to Leu-184] of SEQ ID N0:43; Other combinations may include the
polypeptide
fragments in an order other than that recited above . (e.g., [Ala-1 to Thr-8]
fused to [Gly-
148 to Leu-184] fused to [Val-98 to Ala-147] fused to [Ile-41 to Lys-59] fused
to [Glu-9
to Thr-40] fused to [Gly-60 to Gln-97] of SEQ ID N0:43). Other combinations
may also
include heterologous polypeptide fragments as described herein and/or other
polypeptides
or polypeptide fragments of the present invention (e.g., [Ala-1 to Thr-8]
fused to [Glu-9 to
Thr-40] fused to [Ile-41 to Lys-59] fused to [Val-98 to Ala-147] fused to [Gly-
148 to Leu-
184] of SEQ ID N0:43 fused to an Fc receptor tag). Polynucleotides encoding
any of
these polypeptides are encompassed by the invention.
[0255] Additional embodiments of the invention encompass Neutrokine-alpha
and/or
Neutrokine-alphaSV polypeptide fragments comprising, or alternatively
consisting of,
functional regions of polypeptides of the invention, such as the Gamier-Robson
alpha-regions, beta-regions, turn-regions, and coil-regions, Chou-Fasman alpha-
regions,
beta-regions, and coil-regions, Kyte-Doolittle hydrophilic regions and
hydrophobic
regions, Eisenberg alpha- and beta-amphipathic regions, Karplus-Schulz
flexible regions,
Emini surface-forming regions and Jameson-Wolf regions of high antigenic index
set out
in Figures 3 and 6 and in Table I and as described herein. In a preferred
embodiment, the
106
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polypeptide fragments of the invention are antigenic. The data presented in
columns VIII,
IX, XIII, and XIV of Table I can be used to routinely determine regions of
Neutrokine-alpha which exhibit a high degree of potential for antigenicity.
Regions of
high antigenicity are determined from the data presented in columns VIII, IX,
XIII, .and/or
IV by choosing values which represent regions of the polypeptide which are
likely to be
exposed on the surface of the polypeptide in an environment in which antigen
recognition
may occur in the process of initiation of an immune response. Among highly
preferred
fragments of the invention are those that comprise regions of Neutrokine-alpha
and/or
Neutrokine-alphaSV that combine several structural features, such as several
(e.g., 1, 2, 3
or 4) of the features set out above. Polynucleotides encoding these
polypeptides are also
encompassed by the invention.
[0256] In another embodiment, the invention provides a polypeptide comprising,
or
alternatively consisting of, an epitope-bearing portion of a polypeptide of
the invention.
Polynucleotides encoding these polypeptides are also encompassed by the
invention. The
epitope of this polypeptide portion is an immunogenic or antigenic epitope of
a
polypeptide of the invention. An "immunogenic epitope" is defined as a part of
a protein
that elicits an antibody response when the whole protein is the immunogen. On
the other
hand, a region of a protein molecule to which an antibody can bind is defined
as an
"antigenic epitope." The number of immunogenic epitopes of a protein generally
is less
than the number of antigenic epitopes. See, for instance, Geysen et al., Proc.
Natl. Acad.
Sci. USA 51:3998- 4002 (1983).
[0257] As to the selection of polypeptides bearing an antigenic epitope (i.e.,
that
contain a region of a protein molecule to which an antibody can bind), it is
well known in
that art that relatively short synthetic peptides that mimic part of a protein
sequence are
routinely capable of eliciting an antiserum that reacts with the partially
mimicked protein.
See, for instance, Sutcliffe, J. G., Shinnick, T. M., Green, N. and Learner,
R. A. (1983)
"Antibodies that react with predetermined sites on proteins", Science, 219:660-
666.
Peptides capable of eliciting protein-reactive sera are frequently represented
in the primary
sequence of a protein, can be characterized by a set of simple chemical rules,
and are
confined neither to immunodominant regions of intact proteins (i.e.,
immunogenic
epitopes) nor to the amino or carboxyl terminals. Antigenic epitope-bearing
peptides and
polypeptides of the invention are therefore useful to raise antibodies,
including
107
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
monoclonal antibodies, that bind specifically to a polypeptide of the
invention. See, for
instance, Wilson et al., Cell 37:767-778 (1984) at 777.
[0258] Antigenic epitope-bearing peptides and polypeptides of the invention
preferably contain a sequence of at least 4, at least 5, at least 6, at least
7, more preferably
at least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at
least 14, at least 15, at
least 20, at least 25, at least 30, at least 40, at least 50, and, most
preferably, between about
15 to about 30 amino acids contained within the amino acid sequence of a
polypeptide of
the invention. Preferred polypeptides comprising immunogenic or antigenic
epitopes are
at least 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90,
95, or 100 amino
acid residues in length. Additional non-exclusive preferred antigenic epitopes
include the
antigenic epitopes disclosed herein, as well as portions thereof.
[0259] Non-limiting examples of antigenic polypeptides or peptides that can be
used
to generate Neutrokine-alpha- and/or Neutrokine-alphaSV-specific antibodies
include: a
polypeptide comprising, or alternatively consisting of, amino acid residues
from about
Phe-115 to about Leu-147 in Figures 1A and 1B (SEQ W N0:2); a polypeptide
comprising, or alternatively consisting of, amino acid residues from about Ile-
150 to
about Tyr-163 in Figures 1A and 1B (SEQ ID N0:2); a polypeptide comprising, or
alternatively consisting of, amino acid residues from about Ser-171 to about
Phe-194 in
Figures 1A and 1B (SEQ ID N0:2); a polypeptide comprising, or alternatively
consisting
of, amino acid residues from about Glu-223 to about Tyr-246 in Figures 1A and
1B (SEQ
ID N0:2); and a polypeptide comprising, or alternatively consisting of, amino
acid
residues from about Ser-271 to about Phe-278 in Figures 1A and 1B (SEQ ID
N0:2). In
this context, "about" means the particularly recited ranges and ranges larger
or smaller by
several, a few, 5, 4, 3, 2 or 1 amino acid residues at either or both the
amino- and carbaxy-
termini. These polypeptide fragments have been determined to bear antigenic
epitopes of
the Neutrokine-alpha polypeptide by the analysis of the 3ameson-Wolf antigenic
index, as
shown in Figure 3 and Table I, above.
[0260] Non-limiting examples of antigenic polypeptides or peptides that can be
used
to generate Neutrokine-alpha- and/or Neutrokine-alphaSV-specific antibodies
include: a
polypeptide comprising, or alternatively consisting of, amino acid residues
from about
Pro-32 to about Leu-47 in Figures 5A and 5B (SEQ ID N0:19); a polypeptide
comprising,
or alternatively consisting of, amino acid residues from about Glu-116 to
about Ser-143
1o8
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
in Figures 5A and 5B (SEQ ID N0:19); a polypeptide comprising, or
alternatively
consisting of, amino acid residues from about Phe-153 to about Tyr-173 in
Figures 5A
and 5B (SEQ ID N0:19); a polypeptide comprising, or alternatively consisting
of, amino
acid residues from about Pro-218 to about Tyr-227 in Figures 5A and 5B (SEQ ID
N0:19); a polypeptide comprising, or alternatively consisting of, amino acid
residues
from about Ala-232 to about Gln-241 in Figures 5A and 5B (SEQ ID N0:19); a
polypeptide comprising, or alternatively consisting of, amino acid residues
from about
Ile-244 to about Ala-249 in Figures 5A and 5B (SEQ ID N0:19); and a
polypeptide
comprising, or alternatively consisting of, amino acid residues from about Ser-
252 to
about Val-257 in Figures 5A and 5B (SEQ ID N0:19). In this context, "about"
means
the particularly recited ranges and ranges larger or smaller by several, a
few, 5, 4, 3, 2 or 1
amino acid residues at either or both the amino- and carboxy-termini.
Polynucleotides
encoding these polypeptides are also encompassed by the invention. These
polypeptide
fragments have been determined to bear antigenic epitopes of the Neutrokine-
alphaSV
polypeptide by the analysis of the Jameson-Wolf antigenic index, as shown in
Figure 6
and a tabular representation of the data presented in Figure 6 generated by
the Protean
component of the DNA*STAR computer program (as set forth above).
[0261] The epitope-bearing peptides and polypeptides of the invention may be
produced by any conventional means. See, e.g., Houghten, R. A. (1985) General
method
for the rapid solid-phase synthesis of large numbers of peptides: specificity
of
antigen-antibody interaction at the level of individual amino acids. Proc.
Natl. Acad. Sci.
USA 82:5131-5135; this "Simultaneous Multiple Peptide Synthesis (SMPS)"
process is
further described in U. S. Patent No. 4,631,211 to Houghten et al. (1986).
[0262] Epitope-bearing peptides and polypeptides of the invention have uses
that
include, but are not limited to, to induce antibodies according to methods
well known in
the art. See, for instance, Sutcliffe et al., supra; Wilson et al., supra;
Chow, M. et al., Proc.
Natl. Acad. Sci. USA 82:910-914; and Bittle, F. J. et al., J. Gezz. Virol.
66:2347-2354
(1985). Immunogenic epitope-bearing peptides of the invention, i.e., those
parts of a
protein that elicit an antibody response when the whole protein is the
immunogen, are
identified according to methods known in the art. See, for instance, Geysen et
al., supra.
Further still, U.S. Patent No. 5,194,392 to Geysen (1990) describes a general
method of
detecting or determining the sequence of monomers (amino acids or other
compounds)
109
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
which is a topological equivalent of the epitope (i.e., a "mimotope") which is
complementary to a particular paratope (antigen binding site) of an antibody
of interest.
More generally, U.S. Patent No. 4,433,092 to Geysen (1989) describes a method
of
detecting or determining a sequence of monomers which is a topographical
equivalent of a
ligand which is complementary to the ligand binding site of a particular
receptor of
interest. Similarly, U.S. Patent No. 5,480,971 to Houghten, R. A. et aI.
(1996) on
Peralkylated Oligopeptide Mixtures discloses linear C1-C7-alkyl peralkylated
oligopeptides and sets and libraries of such peptides, as well as methods for
using such
oligopeptide sets and libraries for determining the sequence of a peralkylated
oligopeptide
that preferentially binds to an acceptor molecule of interest. Thus, non-
peptide analogs of
the epitope-bearing peptides of the invention also can be made routinely by
these methods.
[0263] The present invention encompasses polypeptides comprising, or
alternatively
consisting of, an epitope of the polypeptide having an amino acid sequence of
SEQ ID
N0:2, or an epitope of the polypeptide sequence encoded by a polynucleotide
sequence
contained in ATCC deposit No. 97768, or encoded by a polynucleotide that
hybridizes to
the complement of the sequence of SEQ ID NO:1 or the cDNA sequence contained
in
ATCC deposit No. 97768 (e.g., under hybridization conditions described
herein). The
present invention further encompasses polynucleotide sequences comprising, or
alternatively consisting of, a sequence encoding an epitope of a polypeptide
sequence of
the invention (such as, for example, the sequence disclosed in SEQ ID NO:l),
polynucleotide sequences of the complementary strand of a polynucleotide
sequence
encoding an epitope of the invention, and polynucleotide sequences which
hybridize to the
complementary strand (e.g., under hybridization conditions described herein).
[0264] The present invention also encompasses polypeptides comprising, or.
alternatively consisting of, an epitope of the polypeptide having an amino
acid sequence of
SEQ ID N0:19, or an epitope of the polypeptide sequence encoded by a
polynucleotide
sequence contained in ATCC deposit No. 203518, or encoded by a polynucleotide
that
hybridizes to the complement of the sequence of SEQ )D N0:18 or the cDNA
sequence
contained in ATCC deposit No. 203518 (e.g., under hybridization conditions
described
herein). The present invention further encompasses polynucleotide sequences
comprising,
or alternatively consisting of, a sequence encoding an epitope of a
polypeptide sequence of
the invention (such as, for example, the sequence disclosed in SEQ m N0:18),
1I0
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polynucleotide sequences of the complementary strand of a polynucleotide
sequence
encoding an epitope of the invention, and polynucleotide sequences which
hybridize to the
complementary strand (e.g., under hybridization conditions described herein).
[0265] The term "epitopes," as used herein, refers to portions of a
polypeptide having
antigenic or immunogenic activity in an animal, preferably a mammal, and most
preferably in a human. In a preferred embodiment, the present invention
encompasses a
polypeptide comprising an epitope, as well as the polynucleotide encoding this
polypeptide. An "immunogenic epitope," as used herein, is defined as a portion
of a
protein that elicits an antibody response in an animal, as determined by any
method known
in the art, for example, by the methods for generating antibodies described
infra. (See, for
example, Geysen et al., Proc. Natl. Acad. Sci. USA 81:3998- 4002 (1983)). The
term
"antigenic epitope," as used herein, is defined as a portion of a protein to
which an
antibody can immunospecifically bind its antigen as determined by any method
well
known in the art, for example, by the immunoassays described herein.
Immunospecific
binding excludes non-specific binding but does not necessarily exclude cross-
reactivity
with other antigens. Antigenic epitopes need not necessarily be immunogenic.
[0266] Fragments which function as epitopes may be produced by any
conventional
means. (See, e.g., Houghten, Proc. Natl. Acad. Sc~. USA 82:5131-5135 (19.85),
further
described in U.S. Patent No. 4,631,211).
[0267] In the present invention, antigenic epitopes preferably contain a
sequence of at
least 4, at least 5, at least 6, at least 7, more preferably at least 8, at
least 9, at least 10, at
least 11, at least 12, at least 13, at least 14, at least 15, at least 20, at
least 25, at least 30, at
least 40, at least 50, and, most preferably, between about 15 to about 30
amino acids.
Preferred polypeptides comprising immunogenic or antigenic epitopes are at
least 10, 15,
20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, or 100 amino
acid residues in
length. Additional non-exclusive preferred antigenic epitopes include the
antigenic
epitopes disclosed herein, as well as portions thereof. Antigenic epitopes are
useful, for
example, to raise antibodies, including monoclonal antibodies, that
specifically bind the
epitope. Preferred antigenic epitopes include the antigenic epitopes disclosed
herein, as
well as any combination of two, three, four, five or more of these antigenic
epitopes.
Antigenic epitopes can be used as the target molecules in immunoassays. (See,
for
111
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
instance, Wilson et al., Cell 37:767-778 (1984); Sutcliffe et al., Science
219:660-666
(1983)).
[0268] Similarly, immunogenic epitopes can be used, for example, to induce
antibodies according to methods well known in the art. (See, for instance,
Sutcliffe et al.,
supra; Wilson et al., supra; Chow et al., Proc. Natl. Acad. Sci. USA 82:910-
914; and
Bittle et al., J. Gen. Virol. 66:2347-2354 (1985). Preferred immunogenic
epitopes include
the immunogenic epitopes disclosed herein, as well as any combination of two,
three, four,
five or more of these immunogenic epitopes. The polypeptides comprising one or
more
immunogenic epitopes may be presented for eliciting an antibody response
together with a
carrier protein, such as an albumin, to an animal system (such as rabbit or
mouse), or, if
the polypeptide is of sufficient length (at least about 25 amino acids), the
polypeptide may
be presented without a carrier. However, immunogenic epitopes comprising as
few as 8 to
amino acids have been shown to be sufficient to raise antibodies capable of
binding to,
at the very least, linear epitopes in a denatured polypeptide (e.g., in
Western blotting).
[0269] Epitope-bearing polypeptides of the present invention may be used to
induce
antibodies according to methods well known in the art including, but not
limited to, i~c vivo
immunization, in vitro immunization, and phage display methods. See, e.g.,
Sutcliffe et
al., supra; Wilson et al., supra, and Bittle et al., J. Gen. Virol., 66:2347-
2354 (1985). If
in vivo immunization is used, animals may be immunized with free peptide;
however, anti-
peptide antibody titer may be boosted by coupling the peptide to a
macromolecular carrier,
such as keyhole limpet hemacyanin (KLH) or tetanus toxoid. For instance,
peptides
containing cysteine residues may be coupled to a carrier using a linker such
as
maleimidobenzoyl-N-hydroxysuccinimide ester (MBS), while 'other peptides may
be
coupled to carriers using a more general linking agent such as glutaraldehyde.
Animals
such as rabbits, rats and mice are immunized with either free or carrier-
coupled peptides,
for instance, by intraperitoneal and/or intradermal injection of emulsions
containing about
100 micrograms of peptide or carrier protein and Freund's adjuvant or any
other adjuvant
known for stimulating an immune response. Several booster injections may be
needed, for
instance, at intervals of about two weeks, to provide a useful titer of anti-
peptide antibody
which can be detected, for example, by ELISA assay using free peptide adsorbed
to a solid
surface. The titer of anti-peptide antibodies in serum from an immunized
animal may be
increased by selection of anti-peptide antibodies, for instance, by adsorption
to the peptide
112
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
on a solid support and elution of the selected antibodies according to methods
well known
in the art.
[0270] As one of skill in the art will appreciate, and as discussed above, the
polypeptides of the present invention comprising an immunogenic or antigenic
epitope can
be fused to other polypeptide sequences. For example, the polypeptides of the
present
invention may be fused with the constant domain of immunoglobulins (IgA, IgE,
IgG,
IgM), or portions thereof (CH1, CH2, CH3, or any combination thereof and
portions
thereof), or albumin (including but not limited to recombinant human albumin
or
fragments or variants thereof (see, e.g., U.S. Patent No. 5,876,969, issued
March 2, 1999,
EP Patent 0 413 622, and U.S. Patent No. 5,766,883, issued June 16, 1998,
herein
incorporated by reference in their entirety)), resulting in chimeric
polypeptides. Such
fusion proteins may facilitate purification and may increase half life ifz
vivo. This has
been shown for chimeric proteins consisting of the first two domains of the
human CD4-
polypeptide and various domains of the constant regions of the heavy or light
chains of
mammalian immunoglobulins. See, e.g., EP 394,827; Traunecker et al., Nature,
331:84-
86 (1988). Enhanced delivery of an antigen across the epithelial barner to the
immune
system has been demonstrated for antigens (e.g., insulin) conjugated to an
FcRn binding
partner such as IgG or Fc fragments (see, e.g., PCT Publications WO 96/22024
and WO
99/04813). IgG Fusion proteins that have a disulfide-linked dimeric structure
due to the
IgG portion desulfide bonds have also been found to be more efficient in
binding and
neutralizing other molecules than monomeric polypeptides or fragments thereof
alone.
See, e.g., Fountoulakis et al., J. Biochem., 270:3958-3964 (1995). Nucleic
acids
encoding the above epitopes can also be recombined with a gene of interest as
an epitope
tag (e.g., the hemagglutinin ("HA") tag or flag tag) to aid in detection and
purification of
the expressed polypeptide. For example, a system described by Janknecht et al.
allows for
the ready purification of non-denatured fusion proteins expressed in human
cell lines
(Janknecht et al., 1991, Proc. Natl. Acad. Sci. USA 88:8972- 897). In this
system, the
gene of interest is subcloned into a vaccinia recombination plasmid such that
the open
reading frame of the gene is translationally fused to an amino-terminal tag
consisting of
six histidine residues. The tag serves as a matrix-binding domain for the
fusion protein.
Extracts from cells infected with the recombinant vaccinia virus are loaded
onto Ni~+
113
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
nitriloacetic acid-agarose column and histidine-tagged proteins can be
selectively eluted
with imidazole-containing buffers.
[0271] In another embodiment, the Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides of the present invention and the epitope-bearing fragments
thereof are fused
with a heterologous antigen (e.g., polypeptide, carbohydrate, phospholipid, or
nucleic
acid). In specific embodiments, the heterologous antigen is an immunogen.
[0272] In a more specific embodiment, the heterologous antigen is the gp120
protein
of HIV, or a fragment thereof. Polynucleotides encoding these polypeptides are
also
encompassed by the invention.
[0273] In another embodiment, the Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides of the present invention and the epitope-bearing fragments
thereof are fused
with polypeptide sequences of another TNF ligand family member (or
biologically active
fragments or variants thereof). In a specific embodiment, the Neutrokine-alpha
and/or
Neutrokine-alphaSV polypeptides of the present invention are fused with a
CD40L
polypeptide sequence. In a preferred embodiment, the CD40L polypeptide
sequence is
soluble.
[0274] The techniques of gene-shuffling, motif-shuffling, exon-shuffling,
andlor
codon-shuffling (collectively referred to as "DNA shuffling") may be employed
to
modulate the activities of Neutrokine-alpha andlor Neutrokine-alphaSV thereby
effectively generating agonists and antagonists of Neutrokine-alpha and/or
Neutrokine-
alphaSV. See generally, U.S. Patent Nos. 5,605,793, 5,811,238, 5,830,721,
5,834,252,
and 5,837,458, and Patten, P. A., et al., Curr. Opinio~z Biotechfzol. 8:724-33
(1997);
Harayama, S. Trends Biotechs2ol. 16(2):76-82 (1998); Hansson, L. O., et al.,
T. Mol. Biol.
287:265-76 (1999); and Lorenzo, M. M. and Blasco, R. Biotechfziques 24(2):308-
13
(1998) (each of these patents and publications are hereby incorporated by
reference). In
one embodiment, alteration of Neutrokine-alpha and/or Neutrokine-alphaSV
polynucleotides and corresponding polypeptides may be achieved by DNA
shuffling.
DNA shuffling involves the assembly of two or more DNA segments into a desired
Neutrokine-alpha and/or Neutrokine-alphaSV molecule by homologous, or site-
specific,
recombination. In another embodiment, Neutrokine-alpha andlor Neutrokine-
alphaSV
polynucleotides and corresponding polypeptides may be altered by being
subjected to
random mutagenesis by error-prone PCR, random nucleotide insertion or other
methods
114
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
prior to recombination. In another embodiment, one or more components, motifs,
sections, parts, domains, fragments, etc., of Neutrokine-alpha and/or
Neutrokine-alphaSV
may be recombined with one or more components, motifs, sections, parts,
domains,
fragments, etc. of one or more heterologous molecules. In preferred
embodiments, the
heterologous molecules are, for example, TNF-alpha, lymphotoxin-alpha (LT-
alpha, also
known as TNF-beta), LT-beta (found in complex heterotrimer LT-alpha2-beta),
OPGL,
Fast, CD27L, CD30L, CD40L, 4-1BBL, DcR3, OX40L, TNF-gamma (International
Publication No. WO 96/14328), AIM-I (International Publication No. WO
97/33899),
AIM-II (International Publication No. WO 97/34911), APRIL (J. Exp. Med.
188(6):1185-
1190), endokine-alpha (International Publication No. WO 98/07880), OPG, OX40,
and
nerve growth factor (NGF), and soluble forms of Fas, CD30, CD27, CD40 and 4-
IBB,
TR2 (International Publication No. WO 96/34095), DR3 (International
Publication No.
WO 97/33904), DR4 (International Publication No. WO 98/32856), TR5
(International
Publication No. WO 98/30693), TR6 (International Publication No. WO 98/30694),
TR7
(International Publication No. WO 98/41629), TRANK, TR9 (International
Publication
No. WO 98/56892), TR10 (International Publication No. WO 98/54202),312C2
(International Publication No. WO 98/06842), TR12, CAD, and v-FLIP. In further
embodiments, the heterologous molecules are any member of the TNF family.
[0275] In a preferred embodiment, Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides of the invention (inlcuding biologically active fragments or
variants thereof),
are fusedwith soluble CD40L polypeptides, or biologically acitve fragments or
variants
thereof.
[0276] In another preferred embodiment, Neutrokine-alpha and/or Neutrokine-
alphaSV polypeptides of the invention (inlcuding biologically active fragments
or variants
thereof), are fused with soluble APRIL polypeptides (e.g., SEQ ID NO:20 or SEQ
ID
N0:47), or biologically acitve fragments or variants thereof.
[0277] To improve or alter the characteristics of Neutrokine-alpha and/or
Neutrokine-alphaSV polypeptides, protein engineering may be employed.
Recombinant
DNA technology known to those skilled in the art can be used to create novel
mutant
proteins or "muteins including single or multiple amino acid substitutions,
deletions,
additions or fusion proteins. Such modified polypeptides can show, e.g.,
enhanced
activity or increased stability. In addition, they may be purified in higher
yields and show
115
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
better solubility than the corresponding natural polypeptide, at least under
certain
purification and storage conditions. For instance, for many proteins,
including the
extracellular domain or the mature forms) of a secreted protein, it is known
in the art that
one or more amino acids may be deleted from the N-terminus or C-terminus
without
substantial loss of biological function. For instance, Ron et al., J. Biol.
Chem.,
268:2984-2988 (1993) reported modified KGF proteins that had heparin binding
activity
even if 3, 8, or 27 amino-terminal amino acid residues were missing.
[0278] In the present case, since the protein of the invention is a member of
the TNF
polypeptide family, deletions of N-terminal amino acids up to the Gly (G)
residue at
position 191 in Figures 1A and 1B (SEQ )D N0:2) may retain some biological
activity
such as, for example, the ability to stimulate lymphocyte (e.g., B cell)
proliferation,
differentiation, and/or activation, and cytotoxicity to appropriate target
cells. Polypeptides
having further N-terminal deletions including the Gly (G) residue would not be
expected
to retain biological activities because it is known that this residue in TNF-
related
polypeptides is in the beginning of the conserved domain required for
biological activities.
However, even if deletion of one or more amino acids from the N-terminus of a
protein
results in modification or loss of one or more biological functions of the
protein, other
functional activities may still be retained. Thus, the ability of the
shortened protein to
induce and/or bind to antibodies which recognize the complete or extracellular
domain of
the protein generally will be retained when less than the majority of the
residues of the
complete or extracellular domain of the protein are removed from the N-
terminus.
Whether a particular polypeptide lacking N-terminal residues of a complete
protein retains
such immunologic activities can readily be determined by routine methods
described
herein and otherwise known in the art.
[0279] Accordingly, the present invention further provides polypeptides having
one or
more residues deleted from the amino terminus of the amino acid sequence of
the
Neutrokine-alpha shown in Figures 1A and 1B (SEQ ID N0:2), up to the glycine
residue
at position 191 (Gly-191 residue from the amino terminus), and polynucleotides
encoding
such polypeptides. In particular, the present invention provides polypeptides
comprising,
or alternatively consisting of, the amino acid sequence of residues n1-285 of
SEQ ID
N0:2, where n1 is an integer in the range of the amino acid position of amino
acid residues
2-190 of the amino acid sequence in SEQ )D N0:2. Polynucleotides encoding
these
116
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polypeptides are also encompassed by the invention. More in particular, the
invention
provides polynucleotides encoding polypeptides comprising, or alternatively
consisting of,
an amino acid sequence selected from the group consisting of residues 2-285, 3-
285,
4-285, 5-285, 6-285, 7-285, 8-285, 9-285, 10-285, 11-285, 12-285, 13-285, 14-
285,
15-285, 16-285, 17-285, 18-285, 19-285, 20-285, 21-285, 22-285, 23-285, 24-
285,
25-285, 26-285, 27-285, 28-285, 29-285, 30-285, 31-285, 32-285, 33-285, 34-
285,
35-285, 36-285, 37-285, 38-285, 39-285, 40-285, 41-285, 42-285, 43-285, 44-
285,
45-285, 46-285, 47-285, 48-285, 49-285, 50-285, 51-285, 52-285, 53-285, 54-
285,
55-285, 56-285, 57-285, 58-285, 59-285, 60-285, 61-285, 62-285, 63-285, 64-
285,
65-285, 66-285, 67-285, 68-285, 69-285, 70-285, 71-285, 72-285, 73-285, 74-
285,
75-285, 76-285, 77-285, 78-285, 79-285, 80-285, 81-285, 82-285, 83-285, 84-
285,
85-285, 86-285, 87-285, 88-285, 89-285, 90-285, 91-285, 92-285, 93-285, 94-
285,
95-285, 96-285, 97-285, 98-285, 99-285, 100-285, 101-285, 102-285, 103-285,
104-285,
105-285, 106-285, 107-285, 108-285, 109-285, 110-285, 111-285, 112-285, 113-
285,
114-285, 115-285, 116-285, 117-285, 118-285, 119-285, 120-285, 121-285, 122-
285,
123-285, 124-285, 125-285, 126-285, 127-285, 128-285, 129-285, 130-285, 131-
285,
132-285, 133-285, 134-285, 135-285, 136-285, 137-285, 138-285, 139-285, 140-
285,
141-285, 142-285, 143-285, 144-285, 145-285, 146-285, 147-285, 148-285, 149-
285,
150-285, 151-285, 152-285, 153-285, 154-285, 155-285, 156-285, 157-285, 158-
285,
159-285, 160-285, 161-285, 162-285, 163-285, 164-285, 165-285, 166-285, 167-
285,
168-285, 169-285, 170-285, 171-285, 172-285, 173-285, 174-285, 175-285, 176-
285,
177-285, 178-285, 179-285, 180-285, 181-285, 182-285, 183-285, 184-285, 185-
285,
186-285, 187-285, 188-285, 189-285, and 190-285 of SEQ ID N0:2. Polypeptides
encoded by these polynucleotides are also encompassed by the invention. The
present
invention is also directed to nucleic acid molecules comprising, or
alternatively, consisting
of, a polynucleotide sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98%
or
99% identical to the polynucleotide sequence encoding the Neutrokine-alpha
and/or
Neutrokine-alphaSV polypeptides described above. The present invention also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising, or
alternatively
consisting of, an amino acid sequence at least 80%, 85%, 90%, 92%, 95%, 96%,
97%,
117
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
98°Io or 99% identical to the amino acid sequence described above, and
polynucleotides
that encode such polypeptides.
[0280] Furthermore, since the predicted extracellular domain of the Neutrolune-
alpha
polypeptides of the invention may itself elicit biological activity, deletions
of N- and
C-terminal amino acid residues from the predicted extracellular region of the
polypeptide
(spanning positions Gln-73 to Leu-285 of SEQ ID N0:2) may retain some
biological
activity such as, for example, ligand binding, stimulation of lymphocyte
(e.g., B cell)
proliferation, differentiation, and/or activation, and modulation of cell
replication or
modulation of target cell activities. However, even if deletion of one or more
amino acids
from the N-terminus of the predicted extracellular domain of a Neutrokine-
alpha
polypeptide results in modification or loss of one or more biological
functions of the
polypeptide, other functional activities may still be retained. Thus, the
ability of the
shortened polypeptides to induce and/or bind to antibodies which recognize the
complete
or mature or extracellular domains of the polypeptides generally will be
retained when less
than the majority of the residues of the complete or mature or extracellular
domains of the
polypeptides are removed from the N-terminus. Whether a particular polypeptide
lacking
N-terminal residues of a complete polypeptide retains such immunologic
activities can
readily be determined by routine methods described herein and otherwise known
in the art.
[0281] Accordingly, the present invention further provides polypeptides having
one or
more residues deleted from the amino terminus of the amino acid sequence of
Neutrokine-alpha shown in SEQ )D N0:2, up to the glycine residue at position
number
280, and polynucleotides encoding such polypeptides. In particular, the
present invention
provides polypeptides comprising, or alternatively consisting of, the amino
acid sequence
of residues n2-285 of SEQ ~ N0:2, where n2 is an integer in the range of the
amino acid
position of amino acid residues 73-280 in SEQ ID N0:2, and 73 is the position
of the
first residue from the N-terminus of the predicted extracellular domain of the
Neutrokine-alpha polypeptide (disclosed in SEQ ID N0:2). Polynucleotides
encoding
these polypeptides are also encompassed by the invention. More in particular,
in certain
embodiments, the invention provides polynucleotides encoding polypeptides
comprising,
or alternatively consisting of, an amino acid sequence selected from the group
consisting
of residues of Q-73 to L-285; G-74 to L-285; D-75 to L-285; L-76 to L-285; A-
77 to
L-285; S-78 to L-285; L-79 to L-285; R-80 to L-285; A-81 to L-285; E-82 to L-
285; L-83
118
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
to L-285; Q-84 to L-285; G-85 to L-285; H-86 to L-285; H-87 to L-285; A-88 to
L-285;
E-89 to L-285; K-90 to L-285; L-91 to L-285; P-92 to L-285; A-93 to L-285; G-
94 to
L-285; A-95 to L-285; G-96 to L-285; A-97 to L-285; P-98 to L-285; K-99 to L-
285;
A-100 to L-285; G-101 to L-285; L-I02 to L-285; E-103 to L-285; E-I04 to L-
285; A-105
to L-285; P-106 to L-285; A-107 to L-285; V-108 to L-285; T-109 to L-285; A-
110 to
L-285; G-111 to L-285; L-112 to L-285; K-113 to L-285; I-114 to L-285; F-115
to L-285;
E-116 to L-285; P-117 to L-285; P-118 to L-285; A-119 to L-285; P-120 to L-
285; G-121
to L-285; E-122 to L-285; G-123 to L-285; N-124 to L-285; S-125 to L-285; S-
126 to
L-285; Q-127 to L-285; N-128 to L-285; S-129 to L-285; R-130 to L-285; N-131
to
L-285; K-132 to L-285; R-I33 to L-285; A-I34 to L-285; V-I35 to L-285; Q-I36
to
L-285; G-137 to L-285; P-138 to L-285; E-139 to L-285; E-140 to L-285; T-141
to L-285;
V-142 to L-285; T-143 to L-285; Q-144 to L-285; D-145 to L-285; C-146 to L-
285; L-147
to L-285; Q-148 to L-285; L-149 to L-285; I-150 to L-285; A-151 to L-285; D-
152 to
L-285; S-153 to L-285; E-154 to L-285; T-155 to L-285; P-156 to L-285; T-157
to L-285;
I-I58 to L-285; Q-159 to L-285; K-160 to L-285; G-261 to L-285; S-I62 to L-
285; Y-163
to L-285; T-164 to L-285; F-165 to L-285; V-166 to L-285; P-167 to L-285; W-
168 to
L-285; L-169 to L-285; L-170 to L-285; S-17I to L-285; F-172 to L-285; K-173
to L-285;
R-174 to L-285; G-175 to L-285; S-176 to L-285; A-177 to L-285; L-I78 to L-
285; E-I79
to L-285; E-180 to L-285; K-181 to L-285; E-182 to L-285; N-183 to L-285; K-
184 to
L-285; I-I85 to L-285; L-I86 to L-285; V-187 to L-285; K-188 to L-285; E-I89
to L-285;
T-190 to L-285; G-191 to L-285; Y-I92 to L-285; F-193 to L-285; F-194 to L-
285; I-I95
to L-285; Y-196 to L-285; G-197 to L-285; Q-198 to L-285; V-199 to L-285; L-
200 to
L-285; Y-201 to L-285; T-202 to L-285; D-203 to L-285; K-204 to L-285; T-205
to
L-285; Y-206 to L-285; A-207 to L-285; M-208 to L-285; G-209 to L-285; H-210
to
L-285; L-211 to L-285; I-212 to L-285; Q-213 to L-285; R-214 to L-285; K-215
to L-285;
K-216 to L-285; V-217 to L-285; H-218 to L-285; V-219 to L-285; F-220 to L-
285;
G-221 to L-285; D-222 to L-285; E-223 to L-285; L-224 to L-285; S-225 to L-
285; L-226
to L-285; V-227 to L-285; T-228 to L-285; L-229 to L-285; F-230 to L-285; R-
231 to
L-285; C-232 to L-285; I-233 to L-285; Q-234 to L-285; N-235 to L-285; M-236
to
L-285; P-237 to L-285; E-238 to L-285; T-239 to L-285; L-240 to L-285; P-241
to L-285;
N-242 to L-285; N-243 to L-285; S-244 to L-285; C-245 to L-285; Y-246 to L-
285; S-247
to L-285; A-248 to L-285; G-249 to L-285; I-250 to L-285; A-251 to L-285; K-
252 to
119
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
L-285; L-253 to L-285; E-254 to L-285; E-255 to L-285; G-256 to L-285; D-257
to
L-285; E-258 to L-285; L-259 to L-285; Q-260 to L-285; L-261 to L-285; A-262
to
L-285; I-263 to L-285; P-264 to L-285; R-265 to L-285; E-266 to L-285; N-267
to L-285;
A-268 to L-285; Q-269 to L-285; I-270 to L-285; S-271 to L-285; L-272 to L-
285; D-273
to L-285; G-274 to L-285; D-275 to L-285; V-276 to L-285; T-277 to L-285; F-
278 to
L-285; F-279 to L-285; and G-280 to L-285 of SEQ ID N0:2. Polypeptides encoded
by
these polynucleotides are also encompassed by the invention. The present
invention is
also directed to nucleic acid molecules comprising, or alternatively,
consisting of, a
polynucleotide sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99%
identical to the polynucleotide sequence encoding the Neutrokine-alpha and/or
Neutrokine-alphaSV polypeptides described above. The present invention also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids andlor polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising, or
alternatively
consisting of, an amino acid sequence at least 80%, 85%, 90%, 92%, 95%, 96%,
97%,
98% or 99% identical to the amino acid sequence described above, and
polynucleotides
that encode such polypeptides.
[0282] Highly preferred embodiments of the invention are directed to nucleic
acid
molecules comprising, or alternatively consisting of a polynucleotide having a
nucleotide
sequence at least 80%, 85%, 90% identical and more preferably at least 95%,
96%, 97%,
98%, 99% or 100% identical to a polynucleotide sequence encoding the
Neutrokine-alpha
polypeptide having the amino acid sequence at positions 134-285 in Figures 1A
and 1B
(SEQ ID N0:2). Preferred embodiments of the invention are directed to nucleic
acid
molecules comprising, or alternatively consisting of a polynucleotide having a
nucleotide
sequence at least 90% identical to a polynucleotide sequence encoding the
Neutrokine-
alpha polypeptide having the amino acid sequence at positions 134-285 in
Figures 1A and
1B (SEQ ID N0:2). More preferred embodiments of the invention are directed to
nucleic
acid molecules comprising, or alternatively consisting of a polynucleotide
having a
nucleotide sequence at least 95% identical to a polynucleotide sequence
encoding the
Neutrokine-alpha polypeptide having the amino acid sequence at positions 134-
285 in
Figures 1A and 1B (SEQ ID N0:2). More preferred embodiments of the invention
are
directed to nucleic acid molecules comprising, or alternatively consisting of
a
120
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polynucleotide having a nucleotide sequence at least 96°70 identical to
a polynucleotide
sequence encoding the Neutrokine-alpha polypeptide having the amino acid
sequence at
positions 134-285 in Figures 1A and ~1B (SEQ ID N0:2).
[0283] Additionally, more preferred embodiments of the invention are directed
to
nucleic acid molecules comprising, or alternatively consisting of a
polynucleotide having
a nucleotide sequence at least 97°1o to a polynucleotide sequence
encoding the Neutrokine-
alpha polypeptide having the amino acid sequence at positions 134-285 in
Figures 1A and
1B (SEQ )D N0:2). Additionally, more preferred embodiments of the invention
are
directed to nucleic acid molecules comprising, or alternatively consisting of
a
polynucleotide having a nucleotide sequence at least 98% to a polynucleotide
sequence
encoding the Neutrokine-alpha polypeptide having the amino acid sequence at
positions
134-285 in Figures 1A and 1B (SEQ ID N0:2). Additionally, more preferred
embodiments of the invention are directed to nucleic acid molecules
comprising, or
alternatively consisting of a polynucleotide having a nucleotide sequence at
least 99%
identical to a polynucleotide sequence encoding the Neutrokine-alpha
polypeptide having
the amino acid sequence at positions 134-285 in Figures 1A and 1B (SEQ ID
N0:2).
[0284] In specific embodiments, a polypeptide comprising, or alternatively
consisting
of, one of the following N-terminally deleted polypeptide fragments of
Neutrokine-alpha
and/or Neutrokine-alphaSV are preferred: amino acid residues Ala-71 through
Leu-285,
amino acid residues Ala-81 through Leu-285, amino acid residues Leu-112
through
Leu-285, amino acid residues Ala-134 through Leu-285, amino acid residues Leu-
147
through Leu-285, and amino acid residues Gly-161 through Leu-285 of SEQ ID
N0:2.
Polynucleotides encoding these polypeptides are also encompassed by the
invention.
[0285] Similarly, many examples of biologically functional C-terminal deletion
muteins are known. For instance, Interferon gamma shows up to ten times higher
activities by deleting 8-10 amino acid residues from the carboxy terminus of
the protein
(Dobeli et al., J. BiotechfZOlogy 7:199-216 (1988). Since the present protein
is a member
of the TNF polypeptide family, deletions of C-terminal amino acids up to the
leucine
residue at position 284 are expected to retain most if not all biological
activity such as, for
example, ligand binding, the ability to stimulate lymphocyte (e.g., B cell)
proliferation,
differentiation, and/or activation, and modulation of cell replication.
Polypeptides having
deletions of up to about 10 additional C-terminal residues (i.e., up to the
glycine residue at
121
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
position 274) also may retain some activity such as receptor binding, although
such
polypeptides would lack a portion of the conserved TNF domain which extends to
about
Leu-284 of SEQ ID N0:2. However, even if deletion of one or more amino acids
from
the C-terminus of a protein results in modification or loss of one or more
biological
functions of the protein, other functional activities rnay still be retained.
Thus, the ability
of the shortened protein to induce and/or bind to antibodies which recognize
the complete
or mature protein generally will be retained when less than the majority of
the residues of
the complete or mature protein are removed from the C-terminus. Whether a
particular
polypeptide lacking C-terminal residues of a complete protein retains such
immunologic
activities can readily be determined by routine methods described herein and
otherwise
known in the art.
[0286] Accordingly, the present invention further provides polypeptides having
one or
more residues deleted from the carboxy terminus of the amino acid sequence of
the
Neutrokine-alpha polypeptide shown in Figures 1A and 1B (SEQ JD N0:2), up to
the
glycine residue at position 274 (Gly-274) and polynucleotides encoding such
polypeptides. In particular, the present invention provides polypeptides
comprising, or
alternatively consisting of, the amino acid sequence of residues 1-m1 of the
amino acid
sequence in SEQ ID N0:2, where ml is any integer in the range of the amino
acid position
of amino acid residues 274-284 in SEQ ID N0:2. Polynucleotides encoding these
polypeptides are also encompassed by the invention. More in particular, the
invention
provides polynucleotides encoding polypeptides comprising, or alternatively
consisting of,
an amino acid sequence selected from the group consisting of residues 1-274, l-
275,
1-276, 1-277, 1-278, 1-279, 1-280, 1-281, 1-282, 1-283 and 1-284 of SEQ 1D
N0:2.
Polypeptides encoded by these polynucleotides are also encompassed by the
invention.
The present invention is also directed to nucleic acid molecules comprising,
or
alternatively, consisting of, a polynucleotide sequence at least 80%, 85%,
90%, 92%,
95%, 96%, 97%, 98% or 99% identical to the polynucleotide sequence encoding
the
Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides described above. The
present
invention also encompasses the above polynucleotide sequences fused to a
heterologous
polynucleotide sequence. Polypeptides encoded by these nucleic acids and/or
polynucleotide sequences are also encompassed by the invention, as are
polypeptides
comprising, or alternatively consisting of, an amino acid sequence at least
80%, 85%,
122
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
90%, 92%, 95%, 96%, 97%, 98% or 99% identical to the amino acid sequence
described
above, and polynucleotides that encode such polypeptides.
[0287] Also provided are polypeptides comprising, or alternatively consisting
of, one
or more amino acids deleted from both the amino and the carboxyl termini,
which may be
described generally as having residues nl-ml of SEQ )17 N0:2, where n1 and m1
are
integers as defined above. Also included are a nucleotide sequence encoding a
polypeptide comprising, or alternatively consisting of, a portion of the
complete
Neutrokine-alpha amino acid sequence encoded by the deposited cDNA clone
contained
in ATCC Accession No. 97768 where this portion excludes from 1 to 190 amino
acids
from the amino terminus or from 1 to 11 amino acids from the C-terminus of the
complete
amino acid sequence (or any combination of these N-terminal and C-terminal
deletions)
encoded by the cDNA clone in the deposited plasmid. Polynucleotides encoding
all of the
above deletion polypeptides are encompassed by the invention.
[0288) Similarly, deletions of C-terminal amino acid residues of the predicted
extracellular domain of Neutrokine-alpha up to the leucine residue at position
79 of SEQ
m N0:2 may retain some biological activity, such as, for example, ligand
binding,
stimulation of lymphocyte (e.g., B cell) proliferation, differentiation,
and/or activation,
and modulation of cell replication or modulation of target cell activities.
Polypeptides
having further C-terminal deletions including Leu-79 of SEQ >D NO:2 would not
be
expected to retain biological activities.
[0289] However, even if deletion of one or more amino acids from the C-
terminus of a
polypeptide results in modification or loss of one or more biological
functions of the
polypeptide, other functional activities may still be retained. Thus, the
ability of the
shortened polypeptide to induce and/or bind to antibodies which recognize the
complete,
mature or extracellular forms of the polypeptide generally will be retained
when less than
the majority of the residues of the complete, mature or extracellular forms of
the
polypeptide are removed from the C-terminus. Whether a particular polypeptide
lacking
C-terminal residues of the predicted extracellular domain retains such
immunologic
activities can readily be determined by routine methods described herein and
otherwise
known in the art. '
[0290] Accordingly, the present invention further provides polypeptides having
one or
more residues deleted from the carboxy terminus of the amino acid sequence of
the
123
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
predicted extracellular domain of Neutrokine-alpha polypeptide shown in SEQ ID
N0:2,
up to the leucine residue at position 79 of SEQ ID N0:2, and polynucleotides
encoding
such polypeptides. In particular, the present invention provides polypeptides
comprising,
or alternatively consisting of, the amino acid sequence of residues 73-m2 of
the amino acid
sequence in SEQ ID N0:2, where m~' is any integer in the range of the amino
acid position
of amino acid residues 79 to 285 in the amino acid sequence in SEQ ID N0:2,
and residue
78 is the position of the first residue at the C- terminus of the predicted
extracellular
domain of the Neutrokine-alpha polypeptide (disclosed in SEQ ID N0:2).
PoIypeptides
encoded by these polynucleotides are also encompassed by the invention. More
in
particular, in certain embodiments, the invention provides polynucleotides
encoding
polypeptides comprising, or alternatively consisting of, an amino acid
sequence selected
from the group consisting of residues Q-73 to Leu-285; Q-73 to L-284; Q-73 to
K-283;
Q-73 to L-282; Q-73 to A-281; Q-73 to G-280; Q-73 to F-279; Q-73 to F-278; Q-
73 to
T-277; Q-73 to V-276; Q-73 to D-275; Q-73 to G-274; Q-73 to D-273; Q-73 to L-
272;
Q-73 to S-271; Q-73 to I-270; Q-73 to Q-269; Q-73 to A-268; Q-73 to N-267; Q-
73 to
E-266; Q-73 to R-265; Q-73 to P-264; Q-73 to I-263; Q-73 to A-262; Q-73 to L-
261;
Q-73 to Q-260; Q-73 to L-259; Q-73 to E-258; Q-73 to D-257; Q-73 to G-256; Q-
73 to
E-255; Q-73 to E-254; Q-73 to L-253; Q-73 to K-252; Q-73 to A-251; Q-73 to I-
250;
Q-73 to G-249; Q-73 to A-248; Q-73 to S-247; Q-73 to Y-246; Q-73 to C-245; Q-
73 to
S-244; Q-73 to N-243; Q-73 to N-242; Q-73 to P-241; Q-73 to L-240; Q-73 to T-
239;
Q-73 to E-238; Q-73 to P-237; Q-73 to M-236; Q-73 to N-235; Q-73 to Q-234; Q-
73 to
I-233; Q-73 to C-232; Q-73 to R-231; Q-73 to F-230; Q-73 to L-229; Q-73 to T-
228; Q-73
to V-227; Q-73 to L-226; Q-73 to S-225; Q-73 to L-224; Q-73 to E-223; Q-73 to
D-222;
Q-73 to G-221; Q-73 to F-220; Q-73 to V-219; Q-73 to H-218; Q-73 to V-217; Q-
73 to
K-216; Q-73 to K-215; Q-73 to R-214; Q-73 to Q-213; Q-73 to I-212; Q-73 to L-
211;
Q-73 to H-210; Q-73 to G-209; Q-73 to M-208; Q-73 to A-207; Q-73 to Y-206; Q-
73 to
T-205; Q-73 to K-204; Q-73 to D-203; Q-73 to T-202; Q-73 to Y-201; Q-73 to L-
200;
Q-73 to V-199; Q-73 to Q-198; Q-73 to G-197; Q-73 to Y-196; Q-73 to I-195; Q-
73 to
F-194; Q-73 to F-193; Q-73 to Y-192; Q-73 to G-191; Q-73 to T-190; Q-73 to E-
189;
Q-73 to K-188; Q-73 to V-187; Q-73 to L-186; Q-73 to I-185; Q-73 to K-184; Q-
73 to
N-183; Q-73 to E-182; Q-73 to K-181; Q-73 to E-180; Q-73 to E-179; Q-73 to L-
178;
Q-73 to A-177; Q-73 to S-176; Q-73 to G-175; Q-73 to R-174; Q-73 to K-I73; Q-
73 to
124
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
F-172; Q-73 to S-171; Q-73 to L-170; Q-73 to L-169; Q-73 to W-168; Q-73 to P-
167;
Q-73 to V-166; Q-73 to F-165; Q-73 to T-164; Q-73 to Y-163; Q-73 to S-162; Q-
73 to
G-161; Q-73 to K-160; Q-73 to Q-159; Q-73 to I-158; Q-73 to T-157; Q-73 to P-
156;
Q-73 to T-155; Q-73 to E-154; Q-73 to S-153; Q-73 to D-152; Q-73 to A-151; Q-
73 to
I-150; Q-73 to L-149; Q-73 to Q-148; Q-73 to L-147; Q-73 to C-146; Q-73 to D-
145;
Q-73 to Q-144; Q-73 to T-143; Q-73 to V-142; Q-73 to T-141; Q-73 to E-140; Q-
73 to
E-139; Q-73 to P-138; Q-73 to G-137; Q-73 to Q-136; Q-73 to V-135; Q-73 to A-
134;
Q-73 to R-133; Q-73 to K-132; Q-73 to N-131; Q-73 to R-130; Q-73 to S-129; Q-
73 to
N-128; Q-73 to Q-127; Q-73 to S-126; Q-73 to S-125; Q-73 to N-124; Q-73 to G-
123;
Q-73 to E-122; Q-73 to G-121; Q-73 to P-120; Q-73 to A-119; Q-73 to P-118; Q-
73 to
P-117; Q-73 to E-116; Q-73 to F-115; Q-73 to I-114; Q-73 to K-113; Q-73 to L-
112; Q-73
to G-111; Q-73 to A-110; Q-73 to T-109; Q-73 to V-108; Q-73 to A-107; Q-73 to
P-106;
Q-73 to A-105; Q-73 to E-104; Q-73 to E-103; Q-73 to L-102; Q-73 to G-101; Q-
73 to
A-100; Q-73 to K-99; Q-73 to P-98; Q-73 to A-97; Q-73 to G-96; Q-73 to A-95; Q-
73 to
G-94; Q-73 to A-93; Q-73 to P-92; Q-73 to L-91; Q-73 to K-90; Q-73 to E-89; Q-
73 to
A-88; Q-73 to H-87; Q-73 to H-86; Q-73 to G-85; Q-73 to Q-84; Q-73 to L-83; Q-
73 to
E-82; Q-73 to ~A-81; Q-73 to R-80; and Q-73 to L-79 of SEQ ID NO:2.
Polypeptides
encoded by these polynucleotides are also encompassed by the invention. The
present
invention is also directed to nucleic acid molecules comprising, or
alternatively, consisting
of, a polynucleotide sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98%
or
99% identical to the polynucleotide sequence encoding the Neutrokine-alpha
andlor
Neutrokine-alphaSV polypeptides described above. The present invention also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising, or
alternatively
consisting of, an amino acid sequence at least 80%, 85%, 90%, 92%, 95%, 96%,
97%,
98% or 99% identical to the amino acid sequence described above, and
polynucleotides
that encode such polypeptides.
[0291] The invention also provides polypeptides having one or more amino acids
deleted from both the amino and the carboxyl termini of the predicted
extracellular
domain of Neutrokine-alpha, which may be described generally as having
residues n~-m2
of SEQ ID N0:2 where n2 and m2 are integers as defined above.
125
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0292] In another embodiment, a nucleotide sequence encoding a polypeptide
consisting of a portion of the extracellular domain of the Neutrokine-alpha
amino acid
sequence encoded by the cDNA plasmid contained in the deposit having ATCC
accession
no. 97768, where this portion excludes from 1 to about 206 amino acids from
the amino
terminus of the extracellular domain of the amino acid sequence encoded by the
cDNA
plasmid contained in the deposit having ATCC accession no. 97768, or from 1 to
about
206 amino acids from the carboxy terminus of the extracellular domain of the
amino acid
sequence encoded by the cDNA plasmid contained in the deposit having ATCC
accession
no. 97768, or any combination of the above amino terminal and carboxy terminal
deletions, of the entire extracellular domain of the amino acid sequence
encoded by the
cDNA plasmid contained in the deposit having ATCC accession no. 97768.
[0293] As mentioned above, even if deletion of one or more amino acids from
the
N-terminus of a polypeptide results in modification or loss of one or more
functional
activities (e.g., biological activity) of the polypeptide, other functions or
biological
activities may still be retained. Thus, the ability of a shortened Neutrokine-
alpha mutein
to induce and/or bind to antibodies which recognize the full-length or mature
forms or the
extracellular domain of the polypeptide generally will be retained when less
than the
majority of the residues of the full-length or mature or extracellular domain
of the
polypeptide are removed from the N-terminus. Whether a particular polypeptide
lacking
N-terminal residues of a complete polypeptide retains such immunologic
activities can
readily be determined by routine methods described herein and otherwise known
in the art.
It is not unlikely that a Neutrokine-alpha mutein with a large number of
deleted
N-terminal amino acid residues may retain some functional (e.g., biological or
immunogenic) activities. In fact, peptides composed of as few as six
Neutrokine-alpha
amino acid residues may often evoke an immune response.
[0294] Accordingly, the present invention further provides polypeptides having
one or
more residues deleted from the amino terminus of the predicted full-length
amino acid
sequence of the Neutrokine-alpha shown in SEQ ID N0:2, up to the glycine
residue at
position number 280 of the sequence shown SEQ ID N0:2 and polynucleotides
encoding
such polypeptides. In particular, the present invention provides polypeptides
comprising
the amino acid sequence of residues n3-285 of the sequence shown in SEQ ID
N0:2,
I26
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
where n3 is an integer in the range of the amino acid position of amino acid
residues 1 to
280 of the amino acid sequence in SEQ ID N0:2.
[0295] More in particular, the invention provides polynucleotides encoding
polypeptides comprising, or alternatively consisting of, an amino acid
sequence selected
from the group consisting of residues of D-2 to L-285; D-3 to L-285; S-4 to L-
285; T-5 to
L-285; E-6 to L-285; R-7 to L-285; E-8 to L-285; Q-9 to L-285; S-10 to L-285;
R-11 to
L-285; L-12 to L-285; T-13 to L-285; S-14 to L-285; C-15 to L-285; L-16 to L-
285; K-17
to L-285; K-18 to L-285; R-19 to L-285; E-20 to L-285; E-21 to L-285; M-22 to
L-285;
K-23 to L-285; L-24 to L-285; K-25 to L-285; E-26 to L-285; C-27 to L-285; V-
28 to
L-285; S-29 to L-285; I-30 to L-285; L-31 to L-285; P-32 to L-285; R-33 to L-
285; K-34
to L-285; E-35 to L-285; S-36 to L-285; P-37 to L-285; S-38 to L-285; V-39 to
L-285;
R-40 to L-285; S-41 to L-285; S-42 to L-285; K-43 to L-285; D-44 to L-285; G-
45 to
L-285; K-46 to L-285; L-47 to L-285; L-48 to L-285; A-49 to L-285; A-50 to L-
285; T-51
to L-285; L-52 to L-285; L-53 to L-285; L-54 to L-285; A-55 to L-285; L-56 to
L-285;
L-57 to L-285; S-58 to L-285; C-59 to L-285; C-60 to L-285; L-61 to L-285; T-
62 to
L-285; V-63 to L-285; V-64 to L-285; S-65 to L-285; F-66 to L-285; Y-67 to L-
285; Q-68
to L-285; V-69 to L-285; A-70 to L-285; A-71 to L-285; L-72 to L-285; Q-73 to
L-285;
G-74 to L-285; D-75 to L-285; L-76 to L-285; A-77 to L-285; S-78 to L-285; L-
79 to
L-285; R-80 to L-285; A-81 to L-285; E-82 to L-285; L-83 to L-285; Q-84 to L-
285; G-85
to L-285; H-86 to L-285; H-87 to L-285; A-88 to L-285; E-89 to L-285; K-90 to
L-285;
L-91 to L-285; P-92 to L-285; A-93 to L-285; G-94 to L-285; A-95 to L-285; G-
96 to
L-285; A-97 to L-285; P-98 to L-285; K-99 to L-285; A-100 to L-285; G-101 to L-
285;
L-102 to L-285; E-103 to L-285; E-104 to L-285; A-105 to L-285; P-106 to L-
285; A-107
to L-285; V-108 to L-285; T-109 to L-285; A-110 to L-285; G-111 to L-285; L-
112 to
L-285; K-113 to L-285; I-114 to L-285; F-115 to L-285; E-116 to L-285; P-117
to L-285;
P-118 to L-285; A-119 to L-285; P-120 to L-285; G-121 to L-285; E-122 to L-
285; G-123
to L-285; N-124 to L-285; S-125 to L-285; S-126 to L-285; Q-127 to L-285; N-
128 to
L-285; S-129 to L-285; R-130 to L-285; N-131 to L-285; K-132 to L-285; R-133
to
L-285; A-134 to L-285; V-135 to L-285; Q-136 to L-285; G-137 to L-285; P-138
to
L-285; E-139 to L-285; E-140 to L-285; T-141 to L-285; V-142 to L-285; T-143
to L-285;
Q-144 to L-285; D-145 to L-285; C-146 to L-285; L-147 to L-285; Q-148 to L-
285; L-149
to L-285; I-150 to L-285; A-151 to L-285; D-152 to L-285; S-153 to L-285; E-
154 to
127
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
L-285; T-155 to L-285; P-156 to L-285; T-157 to L-285; I-158 to L-285; Q-159
to L-285;
K-160 to L-285; G-161 to L-285; S-162 to L-285; Y-163 to L-285; T-164 to L-
285; F-165
to L-285; V-166 to L-285; P-167 to L-285; W-I68 to L-285; L-169 to L-285; L-
170 to
L-285; S-171 to L-285; F-172 to L-285; K-173 to L-285; R-174 to L-285; G-175
to L-285;
S-176 to L-285; A-177 to L-285; L-178 to L-285; E-I79 to L-285; E-180 to L-
285; K-18I
to L-285; E-182 to L-285; N-183 to L-285; K-184 to L-285; I-185 to L-285; L-
186 to
L-285; V-187 to L-285; K-188 to L-285; E-189 to L-285; T-I90 to L-285; G-191
to
L-285; Y-192 to L-285; F-193 to L-285; F-194 to L-285; I-195 to L-285; Y-196
to L-285;
G-197 to L-285; Q-198 to L-285; V-199 to L-285; L-200 to L-285; Y-201 to L-
285; T-202
to L-285; D-203 to L-285; K-204 to L-285; T-205 to L-285; Y-206 to L-285; A-
207 to
L-285; M-208 to L-285; G-209 to L-285; H-210 to L-285; L-211 to L-285; I-212
to
L-285; Q-213 to L-285; R-214 to L-285; K-215 to L-285; K-216 to L-285; V-217
to
L-285; H-218 to L-285; V-219 to L-285; F-220 to L-285; G-221 to L-285; D-222
to
L-285; E-223 to L-285; L-224 to L-285; S-225 to L-285; L-226 to L-285; V-227
to L-285;
T-228 to L-285; L-229 to L-285; F-230 to L-285; R-231 to L-285; C-232 to L-
285; I-233
to L-285; Q-234 to L-285; N-235 to L-285; M-236 to L-285; P-237 to L-285; E-
238 to
L-285; T-239 to L-285; L-240 to L-285; P-241 to L-285; N-242 to L-285; N-243
to L-285;
S-244 to L-285; C-245 to L-285; Y-246 to L-285; S-247 to L-285; A-248 to L-
285; G-249
to L-285; I-250 to L-285; A-251 to L-285; K-252 to L-285; L-253 to L-285; E-
254 to
L-285; E-255 to L-285; G-256 to L-285; D-257 to L-285; E-258 to L-285; L-259
to
L-285; Q-260 to L-285; L-261 to L-285; A-262 to L-285; I-263 to L-285; P-264
to L-285;
R-265 to L-285; E-266 to L-285; N-267 to L-285; A-268 to L-285; Q-269 to L-
285; I-270
to L-285; S-271 to L-285; L-272 to L-285; D-273 to L-285; G-274 to L-285; D-
275 to
L-285; V-276 to L-285; T-277 to L-285; F-278 to L-285; F-279 to L-285; and G-
280 to
L-285 of SEQ ID N0:2. The present application is also directed to nucleic acid
molecules
comprising, or alternatively, consisting of, a polynucleotide sequence at
least 80%, 85%,
90%, 92%, 95%, 96%, 97%, 98% or 99% identical to the polynucleotide sequence
encoding the Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides described
above.
The present invention also encompasses the above polynucleotide sequences
fused to a
heterologous polynucleotide sequence. Polypeptides encoded by these nucleic
acids
and/or polynucleotide sequences are also encompassed by the invention, as are
polypeptides comprising an amino acid sequence at least 80%, 85%, 90%, 92%,
95%,
128
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
96%, 97%, 98% or 99% identical to the amino acid sequence described above, and
polynucleotides that encode such polypeptides.
[0296] Also as mentioned above, even if deletion of one or more amino acids
from the
C-terminus of a protein results in modification or loss of one or more
functional activities
(e.g., biological activity) of the protein, other functional activities may
still be retained.
Thus, the ability of a shortened Neutrokine-alpha mutein to induce and/or bind
to
antibodies which recognize the complete or mature form or the extracellular
domain of the
polypeptide generally will be retained when less than the majority of the
residues of the
complete or mature form or the extracellular domain of the polypeptide are
removed from
the C-terminus. Whether a particular polypeptide lacking C-terminal residues
of a
complete polypeptide retains such immunologic activities can readily be
determined by
routine methods described herein and otherwise known in the art. It is not
unlikely that a
Neutrokine-alpha mutein with a large number of deleted C-terminal amino acid
residues
rnay retain some functional (e.g., biological or immunogenic) activities. In
fact, peptides
composed of as few as six Neutrokine-alpha amino acid residues may often evoke
an
immune response.
[0297] Accordingly, the present invention further provides in another
embodiment,
polypeptides having one or more residues deleted from the carboxy terminus of
the amino
acid sequence of the Neutrokine-alpha shown in SEQ ID N0:2, up to the glutamic
acid
residue at position number 6, and polynucleotides encoding such polypepfides.
In
particular, the present invention provides polypeptides comprising the amino
acid
sequence of residues 1-m3 of SEQ TD N0:2, where m3 is an integer in the range
of the
amino acid position of amino acid residues 6-284 of the amino acid sequence in
SEQ ID
NQ:2.
[0298] More in particular, the invention provides polynucleotides encoding
polypeptides comprising, or alternatively consisting of, an amino acid
sequence selected
from the group consisting of residues M-1 to L-284; M-1 to K-283; M-1 to L-
282; M-1 to
A-281; M-1 to G-280; M-1 to F-279; M-1 to F-278; M-1 to T-277; M-1 to V-276; M-
1 to
D-275; M-1 to G-274; M-1 to D-273; M-1 to L-272; M-1 to S-271; M-1 to I-270; M-
1 to
Q-269; M-1 to A-268; M-1 to N-267; M-1 to E-266; M-1 to R-265; M-1 to P-264; M-
1 to
I-263; M-1 to A-262; M-1 to L-261; M-1 to Q-260; M-1 to L-259; M-1 to E-258; M-
1 to
D-257; M-1 to G-256; M-1 to E-255; M-1 to E-254; M-1 to L-253; M-1 to K-252; M-
1 to
129
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
A-251; M-1 to I-250; M-1 to G-249; M-I to A-248; M-1 to S-247; M-1 to Y-246; M-
1 to
m
C-245; M-1 to S-244; M-1 to N-243; M-1 to N-242; M-1 to P-241; M-1 to L-240; M-
1 to
T-239; M-1 to E-238; M-1 to P-237; M-1 to M-236; M-1 to N-235; M-1 to Q-234; M-
1 to
I-233; M-1 to C-232; M-1 to R-231; M-1 to F-230; M-1 to L-229; M-1 to T-228; M-
1 to
V-227; M-I to L-226; M-1 to S-225; M-1 to L-224; M-1 to E-223; M-1 to D-222; M-
1 to
G-221; M-I to F-220; M-1 to V-219; M-I to H-218; M-1 to V-217; M-1 to K-216; M-
1 to
K-215; M-I to R-214; M-I to Q-213; M-1 to I-212; M-I to L-2I1; M-I to H-210; M-
1 to
G-209; M-1 to M-208; M-1 to A-207; M-I to Y-206; M-1 to T-205; M-1 to K-204; M-
1 to
D-203; M-I to T-202; M-1 to Y-201; M-1 to L-200; M-I to V-199; M-1 to Q-198; M-
1 to
G-197; M-1 to Y-196; M-I to I-195; M-1 to F-194; M-I to F-193; M-1 to Y-192; M-
1 to
G-191; M-1 to T-I90; M-1 to E-189; M-1 to K-I88; M-1 to V-I87; M-1 to L-I86; M-
1 to
I-185; M-I to K-184; M-1 to N-183; M-1 to E-182; M-I to K-181; M-1 to E-I80; M-
1 to
E-179; M-1 to L-I78; M-1 to A-177; M-1 to S-I76; M-1 to G-175; M-1 to R-174; M-
1 to
K-173; M-1 to F-172; M-1 to S-171; M-1 to L-170; M-I to L-169; M-1 to W-168; M-
1 to
P-167; M-1 to V-166; M-1 to F-I65; M-1 to T-164; M-1 to Y-I63; M-1 to S-I62; M-
1 to
G-161; M-1 to K-160; M-1 to Q-I59; M-I to I-I58; M-1 to T-157; M-1 to P-156; M-
I to
T-I55; M-1 to E-I54; M-1 to S-153; M-I to D-152; M-1 to A-151; M-I to I-150; M-
I to
L-149; M-1 to Q-148; M-1 to L-147; M-1 to C-146; M-1 to D-145; M-1 to Q-144; M-
1 to
T-143; M-I to V-142; M-1 to T-141; M-I to E-140; M-1 to E-I39; M-1 to P-I38; M-
I to
G-137; M-1 to Q-136; M-1 to V-135; M-1 to A-134;.M-1 to R-133; M-1 to K-132; M-
I to
N-131; M-I to R-130; M-1 to S-129; M-1 to N-128; M-1 to Q-127; M-1 to S-126; M-
1 to
S-125; M-1 to N-124; M-1 to G-123; M-I to E-122; M-1 to G-121; M-1 to P-120; M-
1 to
A-I19; M-1 to P-118; M-1 to P-117; M-1 to E-116; M-1 to F-I15; M-I to I-114; M-
1 to
K-113; M-1 to L-112; M-1 to G-111; M-1 to A-110; M-1 to T-I09; M-1 to V-108; M-
1 to
A-107; M-1 to P-106; M-1 to A-105; M-1 to E-104; M-1 to E-103; M-1 to L-102; M-
1 to
G-101; M-1 to A-100; M-1 to K-99; M-1 to P-98; M-1 to A-97; M-1 to G-96; M-I
to
A-95; M-1 to G-94; M-1 to A-93; M-I to P-92; M-1 to L-91; M-1 to K-90; M-1 to
E-89;
M-1 to A-88; M-1 to H-87; M-I to H-86; M-1 to G-85; M-1 to Q-84; M-I to L-83;
M-1 to
E-82; M-1 to A-81; M-I to R-80; M-1 to L-79; M-I to S-78; M-1 to A-77; M-1 to
L-76;
M-1 to D-75; M-1 to G-74; M-1 to Q-73; M-1 to L-72; M-1 to A-7I; M-1 to A-70;
M-1 to
V-69; M-1 to Q-68; M-1 to Y-67; M-1 to F-66; M-1 to S-65; M-1 to V-64; M-1 to
V-63;
M-1 to T-62; M-1 to L-6I; M-1 to C-60; M-1 to C-59; M-1 to S-58; M-1 to L-57;
M-1 to
130
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
L-56; M-1 to A-55; M-1 to L-54; M-1 to L-53; M-1 to L-52; M-1 to T-51; M-1 to
A-50;
M-1 to A-49; M-I to L-48; M-1 to L-47; M-1 to K-46; M-1 to G-45; M-I to D-44;
M-1 to
K-43; M-1 to S-42; M-1 to S-41; M-1 to R-40; M-1 to V-39; M-1 to S-38; M-1 to
P-37;
M-1 to S-36; M-I to E-35; M-1 to K-34; M-I to R-33; M-I to P-32; M-1 to L-3I;
M-1 to
I-30; M-I to S-29; M-I to V-28; M-1 to C-27; M-1 to E-26; M-1 to K-25; M-1 to
L-24;
M-1 to K-23; M-I to M-22; M-1 to E-21; M-1 to E-20; M-1 to R-19; M-1 to K-18;
M-1 to
K-17; M-1 to L-16; M-1 to C-15; M-1 to S-14; M-1 to T-13; M-1 to L-12; M-1 to
R-11;
M-1 to S-10; M-1 to Q-9; M-1 to E-8; M-1 to R-7; and M-1 to E-6 of SEQ 1D
N0:2. The
present application is also directed to nucleic acid molecules comprising, or
alternatively,
consisting of, a polynucleotide sequence at least 80%, 85°70, 90%, 92%,
95%, 96%, 97%,
98% or 99% identical to the polynucleotide sequence encoding the Neutrokine-
alpha
and/or Neutrokine-alphaSV polypeptides described above. The present invention
also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising an amino
acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
the
amino acid sequence described above, and polynucleotides that encode such
polypeptides.
[0299] The invention also provides polypeptides having one or more amino acids
deleted from both the amino and the carboxyl termini of a Neutrokine-alpha
polypeptide,
which may be described generally as having residues n3-m3 of SEQ ID N0:2,
where n3
and m3 are integers as defined above.
[0300] Furthermore, since the predicted extracellular domain of the
Neutrokine-alphaSV polypeptides of the invention may itself elicit functional
activity
(e.g., biological activity), deletions of N- and C-terminal amino acid
residues from the
predicted extracellular region of the polypeptide at positions Gln-73 to Leu-
266 of SEQ
>D N0:19 may retain some functional activity, such as, for example, ligand
binding, to
stimulation of lymphocyte (e.g., B cell) proliferation, differentiation,
and/or activation,
modulation of cell replication, modulation of target cell activities and/or
immunogenicity.
However, even if deletion of one or more amino acids from the N-terminus of
the
predicted extracellular domain of a Neutrokine-alphaSV polypeptide results in
modification or loss of one or more functional activities of the polypeptide,
other
functional activities may still be retained. Thus, the ability of the
shortened polypeptides
131
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
to induce and/or bind to antibodies which recognize the complete or mature or
extracellular domains of the polypeptides generally will be retained when less
than the
majority of the residues of the complete or mature or extracellular domains of
the
polypeptides are removed from the N-terminus. Whether a particular polypeptide
lacking
N-terminal residues of a complete polypeptide retains such immunologic
activities can
readily be determined by routine methods described herein and otherwise known
in the art.
[0301) Accordingly, the present invention further provides polypeptides having
one or
more residues deleted from the amino terminus of the amino acid sequence of
Neutrokine-alphaSV shown in SEQ ID N0:19, up to the glycine residue at
position
number 261, and polynucleotides encoding such polypeptides. In particular, the
present
invention provides polypeptides comprising the amino acid sequence of residues
n4-266 of
SEQ ID N0:19, where n4 is an integer in the range of the amino acid position
of amino
acid residues 73-261 of the amino acid sequence in SEQ ID NO:I9, and 261 is
the
position of the first residue from the N-terminus of the predicted
extracellular domain
Neutrokine-alphaSV polypeptide (shown in SEQ ID N0:19).
[0302] More in particular, in certain embodiments, the invention provides
polynucleotides encoding polypeptides comprising, or alternatively consisting
of, an
amino acid sequence selected from the group consisting of residues of Q-73 to
L-266;
G-74 to L-266; D-75 to L-266; L-76 to L-266; A-77 to L-266; S-78 to L-266; L-
79 to
L-266; R-80 to L-266; A-81 to L-266; E-82 to L-266; L-83 to L-266; Q-84 to L-
266; G-85
to L-266; H-86 to L-266; H-87 to L-266; A-88 to L-266; E-89 to L-266; K-90 to
L-266;
L-91 to L-266; P-92 to L-266; A-93 to L-266; G-94 to L-266; A-95 to L-266; G-
96 to
L-266; A-97 to L-266; P-98 to L-266; K-99 to L-266; A-I00 to L-266; G-10I to L-
266;
L-102 to L-266; E-103 to L-266; E-104 to L-266; A-105 to L-266; P-106 to L-
266; A-207
to L-266; V-108 to L-266; T-109 to L-266; A-110 to L-266; G-111 to L-266; L-
lI2 to
L-266; K-113 to L-266; I-1I4 to L-266; F-115 to L-266; E-116 to L-266; P-117
to L-266;
P-118 to L-266; A-119 to L-266; P-120 to L-266; G-121 to L-266; E-122 to L-
266; G-123
to L-266; N-124 to L-266; S-125 to L-266; S-126 to L-266; Q-127 to L-266; N-
128 to
L-266; S-I29 to L-266; R-130 to L-266; N-131 to L-266; K-132 to L-266; R=133
to
L-266; A-134 to L-266; V-135 to L-266; Q-136 to L-266; G-137 to L-266; P-138
to
L-266; E-139 to L-266; E-140 to L-266; T-141 to L-266; G-142 to L-266; S-143
to.L-266;
Y-144 to L-266; T-145 to L-266; F-I46 to L-266; V-147 to L-266; P-148 to L-
266; W-I49
132
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
to L-266; L-150 to L-266; L-151 to L-266; S-152 to L-266; F-153 to L-266; K-
154 to
L-266; R-155 to L-266; G-156 to L-266; S-157 to L-266; A-158 to L-266; L-159
to
L-266; E-160 to L-266; E-161 to L-266; K-162 to L-266; E-163 to L-266; N-164
to
L-266; K-165 to L-266; I-166 to L-266; L-167 to L-266; V-168 to L-266; K-169
to L-266;
E-170 to L-266; T-171 to L-266; G-172 to L-266; Y-173 to L-266; F-174 to L-
266; F-175
to L-266; I-176 to L-266; Y-177 to L-266; G-178 to L-266; Q-179 to L-266; V-
180 to
L-266; L-181 to L-266; Y-182 to L-266; T-183 to L-266; D-184 to L-266; K-185
to
L-266; T-186 to L-266; Y-187 to L-266; A-188 to L-266; M-189 to L-266; G-190
to
L-266; H-191 to L-266; L-192 to L-266; I-193 to L-266; Q-194 to L-266; R-195
to L-266;
K-196 to L-266; K-197 to L-266; V-198 to L-266; H-199 to L-266; V-200 to L-
266;
F-201 to L-266; G-202 to L-266; D-203 to L-266; E-204 to L-266; L-205 to L-
266; S-206
to L-266; L-207 to L-266; V-208 to L-266; T-209 to L-266; L-210 to L-266; F-
211 to
L-266; R-212 to L-266; C-213 to L-266; I-214 to L-266; Q-215 to L-266; N-216
to L-266;
M-217 to L-266; P-218 to L-266; E-219 to L-266; T-220 to L-266; L-221 to L-
266; P-222
to L-266; N-223 to L-266; N-224 to L-266; S-225 to L-266; C-226 to L-266; Y-
227 to
L-266; S-228 to L-266; A-229 to L-266; G-230 to L-266; I-231 to L-266; A-232
to L-266;
K-233 to L-266; L-234 to L-266; E-235 to L-266; E-236 to L-266; G-237 to L-
266; D-238
to L-266; E-239 to L-266; L-240 to L-266; Q-241 to L-266; L-242 to L-266; A-
243 to
L-266; I-244 to L-266; P-245 to L-266; R-246 to L-266; E-247 to L-266; N-248
to L-266;
A-249 to L-266; Q-250 to L-266; I-251 to L-266; S-252 to L-266; L-253 to L-
266; D-254
to L-266; G-255 to L-266; D-256 to L-266; V-257 to L-266; T-258 to L-266; F-
259 to
L-266; F-260 to L-266; and G-261 to L-266 of SEQ ID N0:19. The present
application is
also directed to nucleic acid molecules comprising, or alternatively,
consisting of, a
polynucleotide sequence at least 80%, $5%, 90%, 92%, 95%, 96%, 97%, 98% or 99%
identical to the polynucleotide sequence encoding the Neutrokine-alpha and/or
Neutrokine-alphaSV polypeptides described above. The present invention also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising an amino
acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
the
amino acid sequence described above, and polynucleotides that encode such
polypeptides.
133
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0303] Similarly, deletions of C-terminal amino acid residues of the predicted
extracellular domain of Neutrokine-alphaSV up to the leucine residue at
position 79 of
SEQ ID N0:19 may retain some functional activity, such as, for example, ligand
binding,
the ability to stimulate lymphocyte (e.g., B cell) proliferation,
differentiation, and/or
activation, modulation of cell replication, modulation of target cell
activities and/or
immunogenicity. Polypeptides having further C-terminal deletions including Leu-
79 of
SEQ ID N0:19 would not be expected to retain biological activities.
[0304] However, even if deletion of one or more amino acids from the C-
terminus of a
polypeptide results in modification or loss of one or more functional
activities (e.g.,
biological activity) of the polypeptide, other functional activities may still
be retained.
Thus, the ability of the shortened polypeptide to induce and/or bind to
antibodies which
recognize the complete, mature or extracellular forms of the polypeptide
generally will be
retained when less than the majority of the residues of the complete, mature
or
extracellular forms of the polypeptide are removed from the C-terminus.
Whether, a
particular polypeptide lacking C-terminal residues of the predicted
extracellular domain
retains such immunologic activities can readily be determined by routine
methods
described herein and otherwise known in the art.
[0305] Accordingly, the present invention further. provides polypeptides
having one or
more residues from the carboxy terminus of the amino acid sequence of the
predicted
extracellular domain of Neutrokine-alphaSV shown in SEQ ID N0:19, up to the
leucine
residue at position 79 of SEQ ID NO:I9, and polynucleotides encoding such
polypeptides.
In particular, the present invention provides polypeptides having the amino
acid sequence
of residues 73-m4 of the amino acid sequence in SEQ ID N0:19, where m4 is any
integer
in the range of the amino acid position of amino acid residues 79-265 of the
amino acid
sequence in SEQ ID N0:19.
[0306] More in particular, in certain embodiments, the invention provides
polynucleotides encoding polypeptides comprising, or alternatively consisting
of, an
amino acid sequence selected from the group consisting of residues Q-73 to L-
265; Q-73
to K-264; Q-73 to L-263; Q-73 to A-262; Q-73 to G-261; Q-73 to F-260; Q-73 to
F-259;
Q-73 to T-258; Q-73 to V-257; Q-73 to D-256; Q-73 to G-255; Q-73 to D-254; Q-
73 to
L-253; Q-73 to S-252; Q-73 to I-251; Q-73 to Q-250; Q-73 to A-249; Q-73 to N-
248;
Q-73 to E-247; Q-73 to R-246; Q-73 to P-245; Q-73 to I-244; Q-73 to A-243; Q-
73 to
134
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
L-242; Q-73 to Q-241; Q-73 to L-240; Q-73 to E-239; Q-73 to D-238; Q-73 to G-
237;
Q-73 to E-236; Q-73 to E-235; Q-73 to L-234; Q-73 to K-233; Q-73 to A-232; Q-
73 to
I-231; Q-73 to G-230; Q-73 to A-229; Q-73 to S-228; Q-73 to Y-227; Q-73 to C-
226;
Q-73 to S-225; Q-73 to N-224; Q-73 to N-223; Q-73 to P-222; Q-73 to L-221; Q-
73 to
T-220; Q-73 to E-219; Q-73 to P-218; Q-73 to M-217; Q-73 to N-216; Q-73 to Q-
215;
Q-73 to I-214; Q-73 to C-213; Q-73 to R-212; Q-73 to F-211; Q-73 to L-210; Q-
73 to
T-209; Q-73 to V-208; Q-73 to L-207; Q-73 to S-206; Q-73 to L-205; Q-73 to E-
204;
Q-73 to D-203; Q-73 to G-202; Q-73 to F-201; Q-73 to V-200; Q-73 to H-199; Q-
73 to
V-198; Q-73 to K-197; Q-73 to K-196; Q-73 to R-195; Q-73 to Q-194; Q-73 to I-
193;
Q-73 to L-192; Q-73 to H-191; Q-73 to G-190; Q-73 to Q-7389; Q-73 to A-188; Q-
73 to
Y-187; Q-73 to T-186; Q-73 to K-185; Q-73 to D-184; Q-73 to T-183; Q-73 to Y-
182;
Q-73 to L-181; Q-73 to V-180; Q-73 to Q-179; Q-73 to G-178; Q-73 to Y-177; Q-
73 to
I-176; Q-73 to F-175; Q-73 to F-174; Q-73 to Y-173; Q-73 to G-172; Q-73 to T-
171;
Q-73 to E-170; Q-73 to K-169; Q-73 to V-168; Q-73 to L-167; Q-73 to I-166; Q-
73 to
K-165; Q-73 to N-164; Q-73 to E-163; Q-73 to K-162; Q-73 to E-161; Q-73 to E-
160;
Q-73 to L-159; Q-73 to A-158; Q-73 to S-157; Q-73 to G-156; Q-73 to R-155; Q-
73 to
K-154; Q-73 to F-153; Q-73 to S-152; Q-73 to L-151; Q-73 to L-150; Q-73 to W-
149;
Q-73 to P-148; Q-73 to V-147; Q-73 to F-146; Q-73 to T-145; Q-73 to Y-144; Q-
73 to
S-143; Q-73 to G-142; Q-73 to T-141; Q-73 to E-140; Q-73 to E-139; Q-73 to P-
138;
Q-73 to G-137; Q-73 to Q-136; Q-73 to V-135; Q-73 to A-134; Q-73 to R-133; Q-
73 to
K-132; Q-73 to N-131; Q-73 to R-130; Q-73 to S-129; Q-73 to N-128; Q-73 to Q-
127;
Q-73 to S-126; Q-73 to S-125; Q-73 to N-124; Q-73 to G-123; Q-73 to E-122; Q-
73 to
G-121; Q-73 to P-120; Q-73 to A-119; Q-73 to P-118; Q-73 to P-117; Q-73 to E-
116;
Q-73 to F-115; Q-73 to I-114; Q-73 to K-113; Q-73 to L-112; Q-73 to G-111; Q-
73 to
A-110; Q-73 to T-109; Q-73 to V-108; Q-73 to A-107; Q-73 to P-106; Q-73 to A-
105;
Q-73 to E-104; Q-73 to E-103; Q-73 to L-102; Q-73 to G-101; Q-73 to A-100; Q-
73 to
K-99; Q-73 to P-98; Q-73 to A-97; Q-73 to G-96; Q-73 to A-95; Q-73 to G-94; Q-
73 to
A-93; Q-73 to P-92; Q-73 to L-91; Q-73 to K-90; Q-73 to E-89; Q-73 to A-88; Q-
73 to
H-87; Q-73 to H-86; Q-73 to G-85; Q-73 to Q-84; Q-73 to L-83; Q-73 to E-82; Q-
73 to
A-81; Q-73 to R-80; Q-73 to L-79; and Q-73 to S-78 of SEQ ID N0:19. The
present
application is also directed to nucleic acid molecules comprising, or
alternatively,
consisting of, a polynucleotide sequence at least 80%, 85%, 90%, 92%, 95%,
96%, 97%,
135
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
98% or 99% identical to the polynucleotide sequence encoding the Neutrokine-
alpha
and/or Neutrolcine-alphaSV polypeptides described above. The present invention
also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising an amino
acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
the
amino acid sequence described above, and polynucleotides that encode such
polypeptides.
[0307] The invention also provides polypeptides having one or more amino acids
deleted from both the amino and the carboxyl termini of the predicted
extracellular
domain of Neutrokine-alphaSV, which may be described generally as having
residues
n4-m4 of SEQ ID N0:19 where n4 and m4 are integers as defined above.
[0308] In another embodiment, a nucleotide sequence encoding a polypeptide
consisting of a portion of the extracellular domain of the Neutrokine-alphaSV
amino acid
sequence encoded by the cDNA clone contained in the deposit having ATCC
Accession
No. 203518, where this portion excludes from 1 to about 260 amino acids from
the amino
terminus of the extracellular domain of the amino acid sequence encoded by
cDNA clone
contained in the deposit having ATCC Accession No. 203518, or from 1 to about
187
amino acids from the carboxy terminus of the extracellular domain of the amino
acid
sequence encoded by cDNA clone contained in the deposit having ATCC Accession
No.
203518, or any combination of the above amino terminal and carboxy terminal
deletions,
of the entire extracellular domain of the amino acid sequence encoded by the
cDNA clone
contained in the deposit having ATCC Accession No. 203518.
[0309] As mentioned above, even if deletion of one or more amino acids from
the
N-terminus of a polypeptide results in modification or loss of one or more
functional
activities (e.g., biological activity) of the polypeptide, other functional
activities may still
be retained. Thus, the ability of a shortened Neutrokine-alphaSV mutein to
induce.and/or
bind to antibodies which recognize the full-length or mature forms or the
extraeellular
domain of the polypeptide generally will be retained when less than the
majority of the
residues of the full-length or mature or extracellular domain of the
polypeptide are
removed from the N-terminus. Whether a particular polypeptide lacking N-
terminal
residues of a complete polypeptide retains such' immunologic activities can
readily be
determined by routine methods described herein and otherwise known in the art.
It is not
136
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
unlikely that a Neutrolcine-alphaSV mutein with a large number of deleted N-
terminal
amino acid residues may retain functional (e.g., immunogenic) activities. In
fact, peptides
composed of as few as six Neutrolcine-alphaSV amino acid residues may often
evoke an
immune response.
[0310] Accordingly, the present invention further provides polypeptides having
one or
more residues deleted from the amino terminus of the predicted full-length
amino acid
sequence of the Neutrokine-alphaSV shown in SEQ ID N0:19, up to the glycine
residue at
position number 261 of the sequence shown SEQ ID N0:19 and polynucleotides
encoding
such polypeptides. In particular, the present invention provides polypeptides
comprising
the amino acid sequence of residues n5-266 of the sequence shown in SEQ ID
N0:19,
where n5 is an integer in the range of the amino acid position of amino acid
residues 1 to
261 of the amino acid sequence in SEQ ID N0:19.
[0311] More in particular, the invention provides polynucleotides encoding
polypeptides comprising, or alternatively consisting of, an amino acid
sequence selected
from the group consisting of residues of D-2 to L-266; D-3 to L-266; S-4 to L-
266; T-S to
L-266; E-6 to L-266; R-7 to L-266; E-8 to L-266; Q-9 to L-266; S-10 to L-266;
R-11 to
L-266; L-12 to L-266; T-13 to L-266; S-14 to L-266; C-15 to L-266; L-16 to L-
266; K-17
to L-266; K-18 to L-266; R-19 to L-266; E-20 to L-266; E-21 to L-266; M-22 to
L-266;
K-23 to L-266; L-24 to L-266; K-25 to L-266; E-26 to L-266; C-27 to L-266; V-
28 to
L-266; S-29 to L-266; I-30 to L-266; L-31 to L-266; P-32 to L-266; R-33 to L-
266; K-34
to L-266; E-35 to L-266; S-36 to L-266; P-37 to L-266; S-38 to L-266; V-39 to
L-266;
R-40 to L-266; S-41 to L-266; S-42 to L-266; K-43 to L-266; D-44 to L-266; G-
4S to
L-266; K-46 to L-266; L-47 to L-266; L-48 to L-266; A-49 to L-266; A-50 to L-
266; T-51
to L-266; L-52 to L-266; L-53 to L-266; L-54 to L-266; A-55 to L-266; L-56 to
L-266;
L-57 to L-266; S-58 to L-266; C-59 to L-266; C-60 to L-266; L-61 to L-266; T-
62 to
L-266; V-63 to L-266; V-64 to L-266; S-65 to L-266; F-66 to L-266; Y-67 to L-
266; Q-68
to L-266; V-69 to L-266; A-70 to L-266; A-71 to L-266; L-72 to L-266; Q-73 to
L-266;
G-74 to L-266; D-75 to L-266; L-76 to L-266; A-77 to L-266; S-78 to L-266; L-
79 to
L-266; R-80 to L-266; A-81 to L-266; E-82 to L-266; L-83 to L-266; Q-84 to L-
266; G-85
to L-266; H-86 to L-266; H-87 to L-266; A-88 to L-266; E-89 to L-266; K-90 to
L-266;
L-91 to L-266; P-92 to L-266; A-93 to L-266; G-94 to L-266; A-95 to L-266; G-
96 to
L-266; A-97 to L-266; P-98 to L-266; K-99 to L-266; A-100 to L-266; G-101 to L-
266;
137
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
L-102 to L-266; E-103 to L-266; E-104 to L-266; A-105 to L-266; P-106 to L-
266; A-107
to L-266; V-108 to L-266; T-109 to L-266; A-110 to L-266; G-111 to L-266; L-
112 to
L-266; K-113 to L-266; I-114 to L-266; F-115 to L-266; E-116 to L-266; P-117
to L-266;
P-118 to L-266; A-119 to L-266; P-120 to L-266; G-121 to L-266; E-122 to L-
266; G-123
to L-266; N-124 to L-266; S-125 to L-266; S-126 to L-266; Q-127 to L-266; N-
128 to
L-266; S-129 to L-266; R-130 to L-266; N-131 to L-266; K-132 to L-266; R-133
to
L-266; A-134 to L-266; V-135 to L-266; Q-136 to L-266; G-137 to L-266; P-138
to
L-266; E-139 to L-266; E-140 to L-266; T-141 to L-266; G-142 to L-266; S-143
to L-266;
Y-144 to L-266; T-145 to L-266; F-146 to L-266; V-147 to L-266; P-148 to L-
266; W-149
to L-266; L-150 to L-266; L-151 to L-266; S-152 to L-266; F-153 to L-266; K-
154 to
L-266; R-155 to L-266; G-156 to L-266; S-157 to L-266; A-158 to L-266; L-159
to
L-266; E-160 to L-266; E-161 to L-266; K-162 to L-266; E-163 to L-266; N-164
to
L-266; K-165 to L-266; I-166 to L-266; L-167 to L-266; V-168 to L-266; K-169
to L-266;
E-170 to L-266; T-171 to L-266; G-172 to L-266; Y-173 to L-266; F-174 to L-
266; F-175
to L-266; I-176 to L-266; Y-177 to L-266; G-178 to L-266; Q-179 to L-266; V-
180 to
L-266; L-181 to L-266; Y-182 to L-266; T-183 to L-266; D-184 to L-266; K-185
to
L-266; T-186 to L-266; Y-187 to L-266; A-188 to L-266; M-189 to L-266; G-190
to
L-266; H-191 to L-266; L-192 to L-266; I-193 to L-266; Q-194 to L-266; R-195
to L-266;
K-196 to L-266; K-197 to L-266; V-198 to L-266; H-199 to L-266; V-200 to L-
266;
F-201 to L-266; G-202 to L-266; D-203 to L-266; E-204 to L-266; L-205 to L-
266; S-206
to L-266; L-207 to L-266; V-208 to L-266; T-209 to L-266; L-210 to L-266; F-
211 to
L-266; R-212 to L-266; C-213 to L-266; I-214 to L-266; Q-215 to L-266; N-216
to L-266;
M-217 to L-266; P-218 to L-266; E-219 to L-266; T-220 to L-266; L-221 to L-
266; P-222
to L-266; N-223 to L-266; N-224 to L-266; S-225 to L-266; C-226 to L-266; Y-
227 to
L-266; S-228 to L-266; A-229 to L-266; G-230 to L-266; I-231 to L-266; A-232
to L-266;
K-233 to L-266; L-234 to L-266; E-235 to L-266; E-236 to L-266; G-237 to L-
266; D-238
to L-266; E-239 to L-266; L-240 to L-266; Q-241 to L-266; L-242 to L-266; A-
243 to
L-266; I-244 to L-266; P-245 to L-266; R-246 to L-266; E-247 to L-266; N-248
to L-266;
A-249 to L-266; Q-250 to L-266; I-251 to L-266; S-252 to L-266; L-253 to L-
266; D-254
to L-266; G-255 to L-266; D-256 to L-266; V-257 to L-266; T-258 to L-266; F-
259 to
L-266; F-260 to L-266; and G-261 to L-266 of SEQ ID N0:19. The present
application is
also directed to nucleic acid molecules comprising, or alternatively,
consisting of, a
138
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polynucleotide sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99%
identical to the polynucleotide sequence encoding the Neutrokine-alpha and/or
Neutrokine-alphaSV polypeptides described above. The present invention also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising an amino
acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
the
amino acid sequence described above, and polynucleotides that encode such
polypeptides.
[0312] Also as mentioned above, even if deletion of one or more amino acids
from the
C-terminus of a protein results in modification or loss of one or more
functional activities
(e.g., biological activities) of the protein, other functional activities may
still be retained.
Thus, the ability of a shortened Neutrokine-alphaSV mutein to induce and/or
bind to
antibodies which recognize the complete or mature form or the extracellular
domain of the
polypeptide generally will be retained when less than the majority of the
residues of the
complete or mature form or the extracellular domain of the polypeptide are
removed from
the C-terminus. Whether a particular polypeptide lacking C-terminal residues
of a
complete polypeptide retains such immunologic activities can readily be
determined by
routine methods described herein and otherwise known in the art. It is not
unlikely that a
Neutrokine-alphaSV mutein with a large number of deleted C-terminal amino acid
residues may retain some functional (e.g., immunogenic) activities. In fact,
peptides
composed of as few as six Neutrokine-alphaSV amino acid residues may often
evoke an
immune response.
[0313] Accordingly, the present invention further provides in another
embodiment,
polypeptides having one or more residues deleted from the carboxy terminus of
the amino
acid sequence of the Neutrokine-alphaSV shown in SEQ ID N0:19, up to the
glutamic
acid residue at position number 6, and polynucleotides encoding such
polypeptides. In
particular, the present invention provides polypeptides comprising the amino
acid
sequence of residues 1-m5 of SEQ )D N0:19, where m5 is an integer in the range
of the
amino acid position of amino acid residues 6 to 265 in the amino acid sequence
of SEQ ID
N0:19.
[0314] More in particular, the invention provides polynucleotides encoding
polypeptides comprising, or alternatively consisting of, an amino acid
sequence selected
139
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
from the group consisting of residues M-1 to L-265; M-1 to K-264; M-1 to L-
263; M-1 to
A-262; M-1 to G-261; M-1 to F-260; M-1 to F-259; M-1 to T-258; M-1 to V-257; M-
1 to
D-256; M-1 to G-255; M-1 to D-254; M-1 to L-253; M-1 to S-252; M-1 to I-251; M-
1 to
Q-250; M-1 to A-249; M-1 to N-248; M-1 to E-247; M-1 to R-246; M-1 to P-245; M-
1 to
I-244; M-1 to A-243; M-1 to L-242; M-1 to Q-241; M-1 to L-240; M-1 to E-239; M-
1 to
D-238; M-1 to G-237; M-1 to E-236; M-1 to E-235; M-1 to L-234; M-1 to K-233; M-
1 to
A-232; M-1 to I-231; M-1 to G-230; M-1 to A-229; M-1 to S-228; M-1 to Y-227; M-
1 to
C-226; M-1 to S-225; M-1 to N-224; M-1 to N-223; M-1 to P-222; M-1 to L-221; M-
1 to
T-220; M-1 to E-219; M-1 to P-218; M-1 to M-217; M-1 to N-216; M-1 to Q-215; M-
1 to
I-214; M-1 to C-213; M-1 to R-212; M-1 to F-211; M-1 to L-210; M-1 to T-209; M-
1 to
V-208; M-1 to L-207; M-1 to S-206; M-1 to L-205; M-1 to E-204; M-1 to D-203; M-
1 to
G-202; M-1 to F-201; M-1 to V-200; M-1 to H-199; M-1 to V-198; M-1 to K-197; M-
1 to
K-196; M-1 to R-195; M-1 to Q-194; M-1 to I-193; M-1 to L-192; M-1 to H-191; M-
1 to
G-190; M-1 to M-189; M-1 to A-188; M-1 to Y-187; M-1 to T-186; M-1 to K-185; M-
1 to
D-184; M-1 to T-183; M-1 to Y-182; M-1 to L-181; M-1 to V-180; M-1 to Q-179; M-
1 to
G-178; M-1 to Y-177; M-1 to I-176; M-1 to F-175; M-1 to F-174; M-1 to Y-173; M-
1 to
G-172; M-1 to T-171; M-1 to E-170; M-1 to K-169; M-1 to V-168; M-1 to L-167; M-
1 to
I-166; M-1 to K-165; M-1 to N-164; M-1 to E-163; M-1 to K-162; M-1 to E-161; M-
1 to
E-160; M-1 to L-159; M-1 to A-158; M-1 to S-157; M-1 to G-156; M-1 to R-155; M-
1 to
K-154; M-l .to F-153; M-1 to S-152; M-1 to L-151; M-1 to L-150; M-1 to W-
149;.M-1 to
P-148; M-1 to V-147; ~VI-1 to F-146; M-1 to T-145; M-1 to Y-144; M-1 to S-143;
M-1 to
G-142; M-1 to T-141; M-1 to E-140; M-1 to E-139; M-1 to P-138; M-1 to G-137; M-
1 to
Q-136; M-1 to V-135; M-1 to A-134; M-1 to R-133; M-1 to K-132; M-1 to N-131; M-
1 to
R-130; M-1 to S-129; M-1 to N-128; M-1 to Q-127; M-1 to S-126; M-1 to S-125; M-
1 to
N-124; M-1 to G-123; M-1 to E-122; M-1 to G-121; M-1 to P-120; M-1 to A-119; M-
1 to
P-118; M-1 to P-117; M-1 to E-116; M-1 to F-115; M-1 to I-114; M-1 to K-113; M-
1 to
L-112; M-1 to G-111; M-1 to A-110; M-1 to T-109; M-1 to V-108; M-1 to A-107; M-
1 to
P-106; M-1 to A-105; M-1 to E-104; M-1 to E-103; M-1 to L-102; M-1 to G-101; M-
1 to
A-100; M-1 to K-99; M-1 to P-98; M-1 to A-97; M-1 to G-96; M-1 to A-95; M-1 to
G-94;
M-1 to A-93; M-1 to P-92; M-1 to L-91; M-1 to K-90; M-1 to E-89; M-1 to A-88;
M-1 to
H-87; M-1 to H-86; M-1 to G-85; M-1 to Q-84; M-1 to L-83; M-,1 to E-82; M-1 to
A-81;
M-1 to R-80; M-1 to L-79; M-1 to S-78; M-1 to A-77; M-1 to L-76; M-1 to D-75;
M-1 to
140
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
G-74; M-1 to Q-73; M-1 to L-72; M-1 to A-71; M-1 to A-70; M-1 to V-69; M-1 to
Q-68;
M-1 to Y-67; M-1 to F-66; M-1 to S-65; M-1 to V-64; M-1 to V-63; M-1 to T-62;
M-1 to
L-61; M-1 to C-60; M-1 to C-59; M-1 to S-58; M-1 to L-57; M-1 to L-56; M-1 to
A-55;
M-1 to L-54; M-1 to L-53; M-1 to L-52; M-1 to T-51; M-1 to A-50; M-1 to A-49;
M-1 to
L-48; M-1 to L-47; M-1 to K-46; M-1 to G-45; M-1 to D-44; M-1 to K-43; M-1 to
S-42;
M-1 to S-41; M-1 to R-40; M-1 to V-39; M-1 to S-38; M-1 to P-37; M-1 to S-36;
M-1 to
E-35; M-1 to K-34; M-1 to R-33; M-1 to P-32; M-1 to L-31; M-1 to I-30; M-1 to
S-29;
M-1 to V-28; M-1 to C-27; M-1 to E-26; M-1 to K-25; M-1 to L-24; M-1 to K-23;
M-1 to
M-22; M-1 to E-21; M-1 to E-20; M-1 to R-19; M-1 to K-18; M-1 to K-17; M-1 to
L-16; .
M-1 to C-15; M-1 to S-14; M-1 to T-13; M-1 to L-12; M-1 to R-11; M-1 to S-10;
M-1 to
Q-9; M-1 to E-8; M-1 to R-7; and M-1 to E-6 of SEQ ID N0:19. The present
application
is also directed to nucleic acid molecules comprising, or alternatively,
consisting of, a
polynucleotide sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99%
identical to the polynucleotide sequence encoding the Neutrokine-alpha andlor
Neutrokine-alphaSV polypeptides described above. The present invention also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising an amino
acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
the
amino acid sequence described above, and polynucleotides that encode such
polypeptides.
[0315] The invention also provides polypeptides having one or more amino acids
deleted from both the amino and the carboxyl termini of a Neutrokine-alphaSV
polypeptide, which may be described generally as having residues ns-m5 of SEQ
ID
NO:19, where n5 and ms are integers as defined above.
[0316] In additional embodiments, the present invention provides polypeptides
comprising the amino acid sequence of residues 134-m~ of SEQ ff~ N0:2, where
rri6 is an
integer from 140 to 285, corresponding to the position of the amino acid
residue in SEQ
ll~ N0:2. For example, the invention provides polynucleotides encoding
polypeptides
comprising, or alternatively consisting of, an amino acid sequence selected
from the group
consisting of residues A-134 to Leu-285; A-134 to L-284; A-134 to K-283; A-134
to
L-282; A-134 to A-281; A-134 to G-280; A-134 to F-279; A-134 to F-278; A-134
to
T-277; A-134 to V-276; A-134 to D-275; A-134 to G-274; A-134 to D-273; A-134
to
141
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
L-272; A-134 to S-271; A-134 to I-270; A-134 to Q-269; A-134 to A-268; A-134
to
N-267; A-134 to E-266; A-134 to R-265; A-134 to P-264; A-134 to I-263; A-134
to
A-262; A-134 to L-261; A-134 to Q-260; A-134 to L-259; A-134 to E-258; A-
134.,to
D-257; A-134 to G-256; A-134 to E-255; A-134 to E-254; A-134 to L-253; A-134
to
K-252; A-134 to A-251; A-134 to I-250; A-134 to G-249; A-134 to A-248; A-134
to
S-247; A-134 to Y-246; A-134 to C-245; A-134 to S-244; A-134 to N-243; A-134
to
N-242; A-134 to P-241; A-134 to L-240; A-134 to T-239; A-134 to E-238; A-134
to
P-237; A-134 to M-236; A-134 to N-235; A-134 to Q-234; A-134 to I-233; A-134
to
C-232; A-134 to R-231; A-134 to F-230; A-134 to L-229; A-134 to T-228; A-134
to
V-227; A-134 to L-226; A-134 to S-225; A-134 to L-224; A-134 to E-223; A-134
to
D-222; A-134 to G-221; A-134 to F-220; A-134 to V-219; A-134 to H-218; A-134
to
V-217; A-134 to K-216; A-134 to K-215; A-134 to R-214; A-134 to Q-213; A-134
to
I-212; A-134 to L-211; A-134 to H-210; A-134 to G-209; A-134 to M-208; A-134
to
A-207; A-134 to Y-206; A-134 to T-205; A-134 to K-204; A-134 to D-203; A-134
to
T-202; A-134 to Y-201; A-134 to L-200; A-134 to V-199; A-134 to Q-198; A-134
to
G-197; A-134 to'Y-196; A-134 to I-195; A-134 to F-194; A-134 to F-193; A-134
to
Y-192; A-134 to G-191; A-134 to T-190; A-134 to E-189; A-134 to K-188; A-134
to
V-187; A-134 to L-186; A-134 to I-185; A-134 to K-184; A-134 to N-183; A-134
to
E-182; A-134 to K-181; A-134 to E-180; A-134 to E-179; A-134 to L-178; A-134
to
A-177; A-134 to S-176; A-134 to G-175; A-134 to R-174; A-134 to K-173; A-134
to
F-172; A-134 to S-171; A-134 to L-170; A-134 to L-169; A-134 to W-168; A-134
to
P-167; A-134 to V-166; A-134 to F-165; A-134 to T-164; A-134 to Y=163; A-134
to
S-162; A-134 to G-161; A-134 to K-160; A-134 to Q-159; A-134 to I-158; A-134
to
T-157; A-134 to P-156; A-134 to T-155; A-134 to E-154; A-134 to S-153; A-134
to
D-152; A-134 to A-151; A-134 to I-150; A-134 to L-149; A-134 to Q-148; A-134
to
L-147; A-134 to C-146; A-134 to D-145; A-134 to Q-144; A-134 to T-143; A-134
to
V-142; A-134 to T-141; and A-134 to E-140 of SEQ ID N0:2. The present
application is
also directed to nucleic acid molecules comprising, or alternatively,
consisting of, a
polynucleotide sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99%
identical to the polynucleotide sequence encoding the Neutrokine-alpha andlor
Neutrokine-alphaSV polypeptides described above. The present invention also
encompasses the above polynucleotide sequences fused to a heterologous
polynucleotide
142
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
sequence. Polypeptides encoded by these nucleic acids and/or polynucleotide
sequences
are also encompassed by the invention, as are polypeptides comprising an amino
acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
the
amino acid sequence described above, and polynucleotides that encode such
polypeptides.
[0317] Additional preferred polypeptide fragments of the invention comprise,
or
alternatively consist of, an amino acid sequence selected from the group
consisting of
residues: M-1 to C-15; D-2 to L-16; D-3 to K-17; S-4 to K-18; T-5 to R-19; E-6
to E-20;
R-7 to E-21; E-8 to M-22; Q-9 to K-23; S-10 to L-24; R-11 to K-25; L-12 to E-
26; T-13 to
C-27; S-14 to V-28; C-15 to S-29; L-16 to I-30; K-17 to L-31; K-18 to P-32; R-
19 to R-
33; E-20 to K-34; E-21 to E-35; M-22 to S-36; K-23 to P-37; L-24 to S-38; K-25
to V-39;
E-26 to R-40; C-27 to S-41; V-28 to S-42; S-29 to K-43; I-30 to D-44; L-31 to
G-45; P-32
to K-46; R-33 to L-47; K-34 to L-48; E-35 to A-49; S-36 to A-50; P-37 to T-51;
S-38 to
L-52; V-39 to L-53; R-40 to L-54; S-41 to A-55; S-42 to L-56; K-43 to L-57; D-
44 to S-
58; G-45 to C-59; K-46 to C-60; L-47 to L-61; L-48 to T-62; A-49 to V-63; A-50
to V-64;
T-51 to S-65; L-52 to F-66; L-53 to Y-67; L-54 to Q-68; A-55 to V-69; L-56 to
A-70; L-
57 to A-71; S-58 to L-72; C-59 to Q-73; C-60 to G-74; L-61 to D-75; T-62 to L-
76; V-63
to A-77; V-64 to S-78; S-65 to L-79; F-66 to R-80; Y-67 to A-81; Q-68 to E-82;
V-69 to
L-83; A-70 to Q-84; A-71 to G-85; L-72 to H-86; Q-73 to H-87; G-74 to A-88; D-
75 to E-
89; L-76 to K-90; A-77 to L-91; S-78 to P-92; L-79 to A-93; R-80 to G-94; A-81
to A-95;
E-82 to G-96; L-83 to A-97; Q-84 to P-98; G-85 to K-99; H-86 to A-100; H-87 to
G-101;
A-88 to L-102; E-89 to E-103; K-90 to E-104; L-91 to A-105; P-92 to P-106; A-
93 to A-
107; G-94 to V-108; A-95 to T-109; G-96 to A-110; A-97 to G-111; P-98 to L-
112; K-99
to K-113; A-100 to I-114; G-101 to F-115; L-102 to E-116; E-103 to P-117; E-
104 to P-
118; A-105 to A-119; P-106 to P-120; A-107 to G-121; V-108 to E-122; T-109 to
G-123;
A-110 to N-124; G-111 to S-125; L-112 to S-126; K-113 to Q-127; I-114 to N-
128; F-115
to S-129; E-116 to R-130; P-117 to N-131; P-118 to K-132; A-119 to R-133; P-
120 to A-
134; G-121 to V-135; E-122 to Q-136; G-123 to G-137; N-124 to P-138; S-125 to
E-139;
S-126 to E-140; Q-127 to T-141; N-128 to V-142; S-129 to T-143; R-130 to Q-
144; N-
131 to D-145; K-132 to C-146; R-133 to L-147; A-134 to Q-148; V-135 to L-149;
Q-136
to I-150; G-137 to A-151; P-138 to D-152; E-139 to S-153; E-140 to E-154; T-
141 to T-
155; V-142 to P-156; T-143 to T-157; Q-144 to I-158; D-145 to Q-159; C-146 to
K-160;
L-147 to G-161; Q-148 to S-162; L-149 to Y-163; I-150 to T-164; A-151 to F-
165; D-152
143
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
to V-166; S-153 to P-167; E-154 to W-168; T-155 to L-169; P-156 to L-170; T-
157 to S-
171; I-158 to F-172; Q-159 to K-173; K-160 to R-174; G-161 to G-175; S-162 to
S-176;
Y-163 to A-177; T-164 to L-178; F-165 to E-179; V-166 to E-180; P-167 to K-
181; W-
168 to E-182; L-169 to N-183; L-170 to K-184; S-171 to I-185; F-172 to L-186;
K-173 to
V-187; R-174 to K-188; G-175 to E-189; S-176 to T-190; A-177 to G-191; L-178
to Y-
192; E-179 to F-193; E-180 to F-194; K-181 to I-195; E-182 to Y-196; N-183 to
G-197;
K-184 to Q-198; I-185 to V-199; L-186 to L-200; V-187 to Y-201; K-188 to T-
202; E-189
to D-203; T-190 to K-204; G-191 to T-205; Y-192 to Y-206; F-193 to A-207; F-
194 to M-
208; I-195 to G-209; Y-196 to H-210; G-197 to L-211; Q-198 to I-212; V-199 to
Q-213;
L-200 to R-214; Y-201 to K-215; T-202 to K-216; D-203 to V-217; K-204 to H-
218; T-
205 to V-219; Y-206 to F-220; A-207 to G-221; M-208 to D-222; G-209 to E-223;
H-210
to L-224; L-211 to S-225; I-212 to L-226; Q-213 to V-227; R-214 to T-228; K-
215 to L-
229; K-216 to F-230; V-217 to R-231; H-218 to C-232; V-219 to I-233; F-220 to
Q-234;
G-221 to N-235; D-222 to M-236; E-223 to P-237; L-224 to E-238; S-225 to T-
239; L-
226 to L-240; V-227 to P-241; T-228 to N-242; L-229 to N-243; F-230 to S-244;
R-231 to
C-245; C-232 to Y-246; I-233 to S-247; Q-234 to A-248; N-235 to G-249; M-236
to I-
250; P-237 to A-251; E-238 to K-252; T-239 to L-253; L-240 to E-254; P-241 to
E-255;
N-242 to G-256; N-243 to D-257; S-244 to E-258; C-245 to L-259; Y-246 to Q-
260; 5-
247 to L-261; A-248 to A-262; G-249 to I-263; I-250 to P-264; A-251 to R-265;
K-252 to
E-266; L-253 to N-267; E-254 to A-268; E-255 to Q-269; G-256 to I-270; D-257
to S-
271; E-258 to L-272; L-259 to D-273; Q-260 to G-274; L-261 to D-275; A-262 to
V-276;
I-263 to T-277; P-264 to F-278; R-265 to F-279; E-266 to G-280; N-267 to A-
281; A-268
to L-282; Q-269 to K-283; I-270 to L-284; and S-271 to L-285 of SEQ ID N0:2.
Preferably, these polypeptide fragments hive one or more functional activities
(e.g.,
biological activity, antigenicity, and immunogenicity) of Neutrokine-alpha
andlor
Neutrokine-alpha SV polypeptides of the invention and may be used, for
example, to
generate or screen for antibodies, as described further below. The present
invention is also
directed to polypeptides comprising, or alternatively, consisting of, an amino
acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
an
amino acid sequence described above. The present invention also encompasses
the above
amino acid sequences fused to a heterologous amino acid sequence as described
herein.
Polynucleotides encoding these polypeptides are also encompassed by the
invention.
144
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0318] Additional preferred polypeptide fragments of the invention comprise,
or
alternatively consist of, an amino acid sequence selected from the group
consisting of
residues: M-1 to C-15; D-2 to L-16; D-3 to K-17; S-4 to K-18; T-5 to R-19; E-6
to E-20;
R-7 to E-21; E-8 to M-22; Q-9 to K-23; S-10 to L-24; R-11 to K-25; L-12 to E-
26; T-13 to
C-27; S-14 to V-28; C-15 to S-29; L-16 to I-30; K-17 to L-31; K-18 to P-32; R-
19 to R-
33; E-20 to K-34; E-21 to E-35; M-22 to S-36; K-23 to P-37; L-24 to S-38; K-25
to V-39;
E-26 to R-40; C-27 to S-41; V-28 to S-42; S-29 to K-43; I-30 to D-44; L-31 to
G-45; P-32
to K-46; R-33 to L-47; K-34 to L-48; E-35 to A-49; S-36 to A-50; P-37 to T-51;
S-38 to
L-52; V-39 to L-53; R-40 to L-54; S-41 to A-55; S-42 to L-56; K-43 to L-57; D-
44 to S-
58; G-45 to C-59; K-46 to C-60; L-47 to L-61; L-48 to T-62; A-49 to V-63; A-50
to V-64;
T-51 to S-65; L-52 to F-66; L-53 to Y-67; L-54 to Q-68; A-55 to V-69; L-56 to
A-70; L-
57 to A-71; S-58 to L-72; C-59 to Q-73; C-60 to G-74; L-61 to D-75; T-62 to L-
76; V-63
to A-77; V-64 to S-78; S-65 to L-79; F-66 to R-80; Y-67 to A-8I; Q-68 to E-82;
V-69 to
L-83; A-70 to Q-84; A-71 to G-85; L-72 to H-86; Q-73 to H-87; G-74 to A-88; D-
75 to E-
89; L-76 to K-90; A-77 to L-91; S-78 to P-92; L-79 to A-93; R-80 to G-94; ~-81
to A-95;
E-82 to G-96; L-83 to A-97; Q-84 to P-98; G-85 to K-99; H-86 to A-100; H-87 to
G-101;
A-88 to L-102; E-89 to E-103; K-90 to E-I04; L-91 to A-105; P-92 to P-106; A-
93 to A-
107; G-94 to V-108; A-95 to T-109; G-96 to A-110; A-97 to G-111; P-98 to L-
112; K-99
to K-113; A-100 to I-114; G-101 to F-115; L-102 to E-116; E-103 to P-117; E-
104 to P-
118; A-105 to A-119; P-106 to P-120; A-107 to G-121; V-108 to E-122; T-109 to
G-123;
A-110 to N-124; G-111 to S-125; L-112 to S-126; K-l I3 to Q-127; I-114 to N-
128; F-115
to S-129; E-116 to R-130; P-117 to N-131; P-118 to K-132; A-119 to R-133; P-
120 to A-
134; G-121 to V-135; E-122 to Q-136; G-123 to G-137; N-124 to P-138; S-125 to
E-139;
S-I26 to E-140; Q-I27 to T-I41; N-128 to G-142; S-I29 to S-143; R-I30 to Y-
I44; N-131
to T-145; K-132 to F-146; R-133 to V-147; A-134 to P-148; V-135 to W-149; Q-
136 to L-
150; G-I37 to L-151; P-I38 to S-152; E-139 to F-I53; E-140 to K-I54; T-14I to
R-155;
G-142 to G-156; S-143 to S-157; Y-I44 to A-158; T-145 to L-I59; F-146 to E-
160; V-147
to E-161; P-148 to K-162; W-149 to E-163; L-150 to N-164; L-151 to K-165; S-
152 to I-
166; F-153 to L-167; K-154 to V-168; R-155 to K-169; G-156 to E-170; S-157 to
T-171;
A-158 to G-172; L-159 to Y-173; E-160 to F-174; E-161 to F-175; K-162 to I-
176; E-163
to Y-177; N-164 to G-178; K-165 to Q-179; I-166 to V-180; L-167 to L-181; V-
168 to Y-
I82; K-169 to T-183; E-170 to D-184; T-171 to K-185; G-172 to T-186; Y-173 to
Y-187;
145
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
F-174 to A-188; F-175 to M-189; I-176 to G-190; Y-177 to H-191; G-178 to L-
192; Q-
179 to I-193; V-180 to Q-194; L-181 to R-195; Y-182 to K-196; T-183 to K-197;
D-184
to V-198; K-185 to H-199; T-186 to V-200; Y-187 to F-201; A-188 to G-202; M-
189 to
D-203; G-190 to E-204; H-191 to L-205; L-192 to S-206; I-193 to L-207; Q-194
to V-
208; R-195 to T-209; K-196 to L-210; K-197 to F-211; V-198 to R-212; H-199 to
C-213;
V-200 to I-214; F-201 to Q-215; G-202 to N-216; D-203 to M-217; E-204 to P-
218; L-205
to E-219; S-206 to T-220; L-207 to L-221; V-208 to P-222; T-209 to N-223; L-
210 to N-
224; F-211 to S-225; R-212 to C-226; C-213 to Y-227; I-214 to S-228; Q-215 to
A-229;
N-216 to G-230; M-217 to I-231; P-218 to A-232; E-219 to K-233; T-220 to L-
234; L-221
to E-235; P-222 to E-236; N-223 to G-237; N-224 to D-238; S-225 to E-239; C-
226 to L-
240; Y-227 to Q-241; S-228 to L-242; A-229 to A-243; G-230 to I-244; I-231 to
P-245;
A-232 to R-246; K-233 to E-247; L-234 to N-248; E-235 to A-249; E-236 to Q-
250; 6-
237 to I-251; D-238 to S-252; E-239 to L-253; L-240 to D-254; Q-241 to G-255;
L-242 to
D-256; A-243 to V-257; I-244 to T-258; P-245 to F-259; R-246 to F-260; E-247
to G-261;
N-248 to A-262; A-249 to L-263; Q-250 to K-264; I-251 to L-265; and S-252 to L-
266 of
SEQ ID N0:19. Preferably, these polypeptide fragments have one or more
functional
activities (e.g., biological activity, antigenicity, and imrimnogenicity) of
Neutrokine-alpha
and/or Neutrokine-alpha SV polypeptides of the invention and may be used, for
example,
to generate or screen for antibodies, as described further below. The present
invention is
also directed to polypeptides comprising, or alternatively, consisting of, an
amino 'acid
sequence at least 80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to
an
amino acid sequence described above. The present invention also encompasses
the above
amino acid sequences fused to a heterologous amino acid sequence as described
herein.
Polynucleotides encoding these polypeptides are also encompassed by the
invention.
[0319] Additional preferred polypeptide fragments of the invention comprise,
or
alternatively consist of, an amino acid sequence selected from the group
consisting of
residues: M-1 to F-15; D-2 to C-16; E-3 to S-17; S-4 to E-18; A-5 to K-19; K-6
to G-20;
T-7 to E-21; L-8 to D-22; P-9 to M-23; P-10 to K-24; P-11 to V-25; C-12 to G-
26; L-13 to
Y-27; C-14 to D-28; F-15 to P-29; C-16 to I-30; S-17 to T-31; E-18 to P-32; K-
19 to Q-
33; G-20 to K-34; E-21 to E-35; D-22 to E-36; M-23 to G-37; K-24 to A-38; V-25
to W-
39; G-26 to F-40; Y-27 to G-41; D-28 to I-42; P-29 to C-43; I-30 to R-44; T-31
to D-45;
P-32 to G-46; Q-33 to R-47; K-34 to L-48; E-35 to L-49; E-36 to A-50; G-37 to
A-51; A-
146
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
38 to T-52; W-39 to L-53; F-40 to L-54; G-41 to L-55; I-42 to A-56; C-43 to L-
57; R-44
to L-58; D-45 to S-59; G-46 to S-60; R-47 to S-61; L-48 to F-62; L-49 to T-63;
A-50 to
A-64; A-51 to M-65; T-52 to S-66; L-53 to L-67; L-54 to Y-68; L-55 to Q-69; A-
56 to L-
70; L-57 to A-71; L-58 to A-72; S-59 to L-73; S-60 to Q-74; S-61 to A-7S; F-62
to D-76;
T-63 to L-77; A-64 to M-78; M-65 to N-79; S-66 to L-80; L-67 to R-81; Y-68 to
M-82;
Q-69 to E-83; L-70 to L-84; A-71 to Q-85; A-72 to S-86; L-73 to Y-87; Q-74 to
R-88; A-
75 to G-89; D-76 to S-90; L-77 to A-91; M-78 to T-92; N-79 to P-93; L-80 to A-
94; R-81
to A-95; M-82 to A-96; E-83 to G-97; L-84 to A-98; Q-85 to P-99; S-86 to E-
100; Y-87 to
L-101; R-88 to T-102; G-89 to A-103; S-90 to G-104; A-91 to V-105; T-92 to K-
106; P-
93 to L-107; A-94 to L-108; A-95 to T-109; A-96 to P-110; G-97 to A-111; A-98
to A-
112; P-99 to P-113; E-100 to R-114; L-101 to P-115; T-102 to H-116; A-103 to N-
117; 6-
104 to S-118; V-105 to S-119; K-106 to R-120; L-107 to G-121; L-108 to H-122;
T-109
to R-123; P-110 to N-124; A-111 to R-125; A-112 to R-126; P-113 to A-127; R-
114 to F-
128; P-115 to Q-129; H-116 to G-130; N-117 to P-131; S-118 to E-132; S-119 to
E-133;
R-120 to T-134; G-121 to E-135; H-122 to Q-136; R-123 to D-137; N-124 to V-
138; 8-
125 to D-139; R-126 to L-140; A-127 to S-141; F-128 to A-142; Q-129 to P-143;
G-130
to P-144; P-131 to A-145; E-132 to P-146; E-133 to C-147; T-134 to L-148; E-
135 to P-
149; Q-136 to G-150; D-137 to C-151; V-138 to R-152; D-139 to H-153; L-140 to
S-154;
S-141 to Q-155; A-142 to H-156; P-143 to D-157; P-144 to D-158; A-145 to N-
159; P-
146 to G-160; C-147 to M-161; L-148 to N-162; P-149 to L-163; G-150 to R-164;
C-151
to N-165; R-152 to I-166; H-153 to I-167; S-154 to Q-168; Q-155 to D-169; H-
156 to C-
170; D-157 to L-171; D-158 to Q-172; N-159 to L-173; G-160 to I-174; M-161 to
A-175;
N-162 to D-176; L-163 to S-177; R-164 to D-178; N-165 to T-179; I-166 to P-
180; I-167
to A-181; Q-168 to L-182; D-169 to E-183; C-170 to E-184; L-171 to K-185; Q-
172 to E-
186; L-173 to N-187; I-174 to K-188; A-175 to I-189; D-176 to V-190; S-177 to
V-191;
D-178 to R-192; T-179 to Q-193; P-180 to T-194; A-181 to G-195; L-182 to Y-
196; E-
183 to F-197; E-184 to F-198; K-185 to I-199; E-186 to Y-200; N-187 to S-201;
K-188 to
Q-202; I-189 to V-203; V-190 to L-204; V-191 to Y-205; R-192 to T-206; Q-193
to D-
207; T-194 to P-208; G-195 to I-209; Y-196 to F-210; F-197 to A-211; F-198 to
M-212; I-
199 to G-213; Y-200 to H-214; S-201 to V-215; Q-202 to I-216; V-203 to Q-217;
L-204
to R-218; Y-205 to K-219; T-206 to K-220; D-207 to V-221; P-208 to H-222; I-
209 to V-
223; F-210 to F-224; A-211 to G-225; M-212 to D-226; G-213 to E-227; H-214 to
L-228;
147
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
V-215 to S-229; I-216 to L-230; Q-217 to V-231; R-218 to T-232; K-219 to L-
233; K-220
to F-234; V-221 to R-235; H-222 to C-236; V-223 to I-237; F-224 to Q-238; G-
225 to N-
239; D-226 to M-240; E-227 to P-241; L-228 to K-242; S-229 to T-243; L-230 to
L-244;
V-231 to P-245; T-232 to N-246; L-233 to N-247; F-234 to S-248; R-235 to C-
249; C-236
to Y-250; I-237 to S-251; Q-238 to A-252; N-239 to G-253; M-240 to I-254; P-
241 to A-
255; K-242 to R-256; T-243 to L-257; L-244 to E-258; P-245 to E-259; N-246 to
G-260;
N-247 to D-261; S-248 to E-262; C-249 to I-263; Y-250 to Q-264; S-251 to L-
265; A-252
to A-266; G-253 to I-267; I-254 to P-268; A-255 to R-269; R-256 to E-270; L-
257 to N-
271; E-258 to A-272; E-259 to Q-273; G-260 to I-274; D-261 to S-275; E-262 to
R-276; I-
263 to N-277; Q-264 to G-278; L-265 to D-279; A-266 to D-280; I-267 to T-281;
P-268 to
F-282; R-269 to F-283; E-270 to G-284; N-271 to A-285; A-272 to L-286; Q-273
to K-
287; I-274 to L-288; and S-275 to L-289 of SEQ ID N0:38. Preferably, these
polypeptide fragments have one or more functional activities (e.g., biological
activity,
antigenicity, and immunogenicity) of Neutrokine-alpha and/or Neutrokine-alpha
SV
polypeptides of the invention and may be used, for example, to generate or
screen for
antibodies, as described further below. The present invention is also directed
to
polypeptides comprising, or alternatively, consisting of, an amino acid
sequence at least
80%, 85%, 90%, 92%, 95%, 96%, 97%, 98% or 99% identical to an amino acid
sequence
described above. The present invention also encompasses the above amino acid
sequences
fused to a heterologous amino acid sequence as described herein.
Polynucleotides
encoding these polypeptides are also encompassed by the invention.
[0320] It will be recognized by one of Qrdinaiy skill in the art that some
amino acid
sequences of the Neutrolcine-alpha and Neutrokine-alphaSV polypeptides can be
varied
without significant effect of the structure or function of the polypeptide. If
such
differences in sequence are contemplated, it should be remembered that there
will be
critical areas on the polypeptide which determine activity.
[0321] Thus, the invention further includes variations of the Neutrokine-alpha
polypeptide which show Neutrokine-alpha polypeptide functional activity (e.g.,
biological
activity) or which include regions of Neutrokine-alpha polypeptide such as the
polypeptide fragments described herein. The invention also includes variations
of the
Neutrokine-alphaSV polypeptide which show Neutrokine-alphaSV polypeptide
functional
activity (e.g., biological activity) or which include regions of Neutrokine-
alphaSV
148
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polypeptide such as the polypeptide fragments described herein. Such mutants
include
deletions, insertions, inversions, repeats, and type substitutions selected
according to
general rules known in the art so as have little effect on activity. For
example, guidance
concerning how to make phenotypically silent amino acid substitutions is
provided in
Bowie, J. U. et al., "Deciphering the Message in Protein Sequences: Tolerance
to Amino
Acid Substitutions," Science 247:1306-1310 (1990), wherein the authors
indicate that
there are two main approaches for studying the tolerance of an amino acid
sequence to
change. The first method relies on the process of evolution, in which
mutations are either
accepted or rejected by natural selection. The second approach uses genetic
engineering
to introduce amino acid changes at specific positions of a cloned gene and
selections or
screens to identify sequences that maintain functionality.
[0322] As the authors state, these studies have revealed that proteins are
surprisingly
tolerant of amino acid substitutions. The authors further indicate which amino
acid
changes are likely to be permissive at a certain position of the protein. For
example, most
buried amino acid residues require nonpolar side chains, whereas few features
of surface
side chains are generally conserved. Other such phenotypically silent
substitutions are
described in Bowie, J. U. et al., supra, and the references cited therein.
Typically seen as
conservative substitutions are the replacements, one for another, among the
aliphatic
amino acids Ala, Val, Leu and Ile; interchange of the hydroxyl residues Ser
and Thr,
exchange of the acidic residues Asp and Glu, substitution between the amide
residues Asn
and Gln, exchange of the basic residues Lys and Arg and replacements among the
aromatic residues Phe, Tyr.
[0323] Thus, the fragment, derivative or analog of the polypeptide of Figures
1A and
1B (SEQ ID N0:2), or that encoded by the deposited cDNA plasmid, may be (i)
one in
which one or more of the amino acid residues are substituted with a conserved
or
non-conserved amino acid residue (preferably a conserved amino acid residue)
and such
substituted amino acid residue may or may not be one encoded by the genetic
code, or (ii)
one in which one or more of the amino acid residues includes a substituent
group, or (iii)
one in which the extracellular domain of the polypeptide is fused with another
compound,
such as a compound to increase the half-life of the polypeptide (for example,
polyethylene
glycol), or (iv) one in which the additional amino acids are fused to the
extracellular
domain of the polypeptide, such as an IgG Fc fusion region peptide or leader
or secretory
149
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
sequence or a sequence which is employed for purification of the extracellular
domain of
the polypeptide or a proprotein sequence. Such fragments, derivatives and
analogs are
deemed to be within the scope of those slcilled in the art from the teachings
herein.
[0324] Furthermore, the fragment, derivative or analog of the polypeptide of
Figures
5A and 5B (SEQ ID N0:19), or that encoded by the deposited cDNA plasmid, may
be (i)
one in which one or more of the amino acid residues are substituted with a
conserved or
non-conserved amino acid residue (preferably a conserved amino acid residue)
and such
substituted amino acid residue may or may not be one encoded by the genetic
code, or (ii)
one in which one or more of the amino acid residues includes a substituent
group, or (iii)
one in which the extracellular domain of the polypeptide is fused with another
compound,
such as a compound to increase the half-life of the polypeptide (for example,
polyethylene
glycol), or (iv) one in which the additional amino acids are fused to the
extracellular
domain of the polypeptide, such as, a soluble biologically active fragment of
another TNF
ligand family member (e.g., CD40 Ligand), an IgG Fc fusion region peptide or
leader or
secretory sequence or a sequence which is employed for purification of the
extracellular
domain of the polypeptide or a proprotein sequence. Such fragments,
derivatives and
analogs are deemed to be within the scope of those skilled in the art from the
teachings
herein.
[0325] Thus, the Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides of
the
present invention may include one or more amino acid substitutions, deletions
or
additions, either from natural mutations or human manipulation. As indicated,
changes
are preferably of a mitnor nature, such as conservative amino acid
substitutions that do not
significantly affect the folding or activity of the protein (see Table II).
TABLE II. Conservative Amino Acid Substitutions.
150
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
B asic Arginine
Lysine
Histidine
Acidic ~ Aspartic Acid
Glutamic Acid
Small Alanine
Serine
Threonine
Methionine
Glycine
[0326] In one embodiment of the invention, polypeptide comprises, or
alternatively
consists of, the amino acid sequence of a Neutrokine-alpha or Neutrokine-
alphaSV
polypeptide having an amino acid sequence which contains at least one
conservative
amino acid substitution, but not more than 50 conservative amino acid
substitutions, even
more preferably, not more than 40 conservative amino acid substitutions, still
more
preferably, not more than 30 conservative amino acid substitutions, and still
even more
preferably, not more than 20 conservative amino acid substitutions. Of course,
in order of
ever-increasing preference, it is highly preferable for a peptide or
polypeptide to have an
amino acid sequence which comprises the amino acid sequence of a Neutrokine-
alpha
polypeptide, which contains at least one, but not more than 10, 9, 8, 7, 6, 5,
4, 3, 2 or 1
conservative amino acid substitutions.
[0327] For example, site directed changes at the amino acid level of
Neutrokine-alpha
can be made by replacing a particular amino acid with a conservative
substitution.
Preferred conservative substitution mutations of the Neutrokine-alpha amino
acid
sequence provided in SEQ >D N0:2 include: Ml replaced with A, G, I, L, S, T,
or V; D2
replaced with E; D3 replaced with E; S4 replaced with A, G, I, L, T, M, or V;
T5 replaced
with A, G, I, L, S, M, or V; E6 replaced with D; R7 replaced with H, or K; E8
replaced
with D; Q9 replaced with N; S10 replaced with A, G, I, L, T, M, or V; R11
replaced with
H, or K; L12 replaced with A, G, I, S, T, M, or V; T13 replaced with A, G, I,
L, S, M, or
V; S14 replaced with A, G, I, L, T, M, or V; L16 replaced with A, G, I, S, T,
M, or V; K17
replaced with H, or R; K18 replaced with H, or R; R19 replaced with H, or K;
E20
replaced with D; E21 replaced with D; M22 replaced with A, G, I, L, S, T, or
V; K23
replaced with H, or R; L24 replaced with A, G, I, S, T, M, or V; K25 replaced
with H, or
151
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
R; E26 replaced with D; V28 replaced with A, G, I, L, S, T, or M; S29 replaced
with A, G,
I, L, T, M, or V; I30 replaced with A, G, L, S, T, M, or V; L31 replaced with
A, G, I, S,, T,
M, or V; R33 replaced with H, or K; K34 replaced with H, or R; E35 replaced
with D; S36
replaced with A, G, I, L, T, M, or V; S38 replaced with A, G, I, L, T, M, or
V; V39
replaced with A, G, I, L, S, T, or M; R40 replaced with H, or K; S41 replaced
with A, G, I,
L, T, M, or V; S42 replaced with A, G, I, L, T, M, or V; K43 replaced with H,
or R; D44
replaced with E; G45 replaced with A, I, L, S, T, M, or V; K46 replaced with
H, or R; L47
replaced with A, G, I, S, T, M, or V; L48 replaced with A, G, I, S, T, M, or
V; A49
replaced with G, I, L, S, T, M, or V; A50 replaced with G, I, L, S, T, M, or
V; T51
replaced with A, G, I, L, S, M, or V; L52 replaced with A, G, I, S, T, M, or
V; L53
replaced with A" G, I, S, T, M, or V; L54 replaced with A, G, I, S, T, M, or
V; A55
replaced with G, I, L, S, T, M, or V; L56 replaced with A, G, I, S, T, M, or
V; L57
replaced with A, G, I, S, T, M, or V; S58 replaced with A, G, I, L, T, M, or
V; L61
replaced with A, G, I, S, T, M, or V; T62 replaced with A, G, I, L, S, M, or
V; V63
replaced with A, G, I, L, S, T, or M; V64 replaced with A, G, I, L, S, T, or
M; S65
replaced with A, G, I, L, T, M, or V; F66 replaced with W, or Y; Y67 replaced
with F, or
W; Q68 replaced with N; V69 replaced with A, G, I, L, S, T, or M; A70 replaced
with G,
I, L, S, T, M, or V; A71 replaced with G, I, L, S, T, M, or V; L72 replaced
with A, G, I, S,
T, M, or V; Q73 replaced with N; G74 replaced with A, I, L, S, T, M, or V; D75
replaced
with E; L76 replaced with A, G, I, S, T, M, or V; A77 replaced with G, I, L,
S, T, M, or V;
S78 replaced with A, G, I, L, T, M, or V; L79 replaced with A, G, I, S, T, M,
or V; R80
replaced with H, or K; A81 replaced with G, I, L, S, T, M, or V; E82 replaced
with D; L83
replaced with A, G, I, S, T, M, or V; Q84 replaced with N; G85 replaced with
A, I, L, S,
T, M, or V; H86 replaced with K, or R; H87 replaced with K, or R; A88 replaced
with G,
I, L, S, T, M, or V; E89 replaced with D; K90 replaced with H, or R; L91
replaced with A,
G, I, S, T, M, or V; A93 replaced with G, I, L, S, T, M, or V; G94 replaced
with A, I, L, S,
T, M, or V; A95 replaced with G, I, L, S, T, M, or V; G96 replaced with A, I,
L, S, T, M,
or V; A97 replaced with G, I, L, S, T, M, or V; K99 replaced with H, or R;
A100 replaced
with G, I, L, S, T, M, or V; 6101 replaced with A, I, L, S, T, M, or V; L102
replaced with
A, G, I, S, T, M, or V; E103 replaced with D; E104 replaced with D; A105
replaced with
G, I, L, S, T, M, or V; A107 replaced with G, I, L, S, T, M, or V; V108
replaced with A,
G, I, L, S, T, or M; T109 replaced with A, G, I, L, S, M, or V; A110 replaced
with G, I, L,
152
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
S, T, M, or V; 6111 replaced with A, I, L, S, T, M, or V; L112 replaced with
A, G, I, S, T,
M, or V; K113 replaced with H, or R; I114 replaced with A, G, L, S, T, M, or
V; F115
replaced with W, or Y; E116 replaced with D; A119 replaced with G, I, L, S, T,
M, or V;
6121 replaced with A, I, L, S, T, M, or V; E122 replaced with D; 6123 replaced
with A,
I, L, S, T, M, or V; N124 replaced with Q; 5125 replaced with A, G, I, L, T,
M, or V;
5126 replaced with A, G, I, L, T, M, or V; Q127 replaced with N; N128 replaced
with Q;
S 129 replaced with A, G, I, L, T, M, or V; 8130 replaced with H, or K; NI31
replaced
with Q; K132 replaced with H, or R; 8133 replaced with H, or K; A134 replaced
with G,
I, L, S, T, M, or V; V135 replaced with A, G, I, L, S, T, or M; Q136 replaced
with N;
6137 replaced with A, I, L, S, T, M, or V; E139 replaced with D; E140 replaced
with D;
T141 replaced with A, G, I, L, S, M, or V; V142 replaced with A, G, I, L, S,
T, or M;
T143 replaced with A, G, I, L, S, M, or V; Q144 replaced with N; D145 replaced
with E;
L147 replaced with A, G, I, S, T, M, or V; Q148 replaced with N; L149 replaced
with A,
G, I, S, T, M, or V; I150 replaced with A, G, L, S, T, M, or V; A151 replaced
with G, I, L,
S, T, M, or V; D152 replaced with E; 5153 replaced with A, G, I, L, T, M, or
V; E154
replaced with D; T155 replaced with A, G, I, L, S, M, or V; T157 replaced with
A, G, I, L,
S, M, or V; I158 replaced with A, G, L, S, T, M, or V; Q159 replaced with N;
K160
replaced with H, or R; 6161 replaced with A, I, L, S, T, M, or V; S 162
replaced with A,
G, I, L, T, M, or V; Y163 replaced with F, or W; T164 replaced with A, G, I,
L, S, M, or
V; F165 replaced with W, or Y; V166 replaced with A, G, I, L, S, T, or M; W168
replaced
with F, or Y; L169 replaced with A, G, I, S, T, M, or V; L170 replaced with A,
G, I, S, T,
M, or V; 5171 replaced with A, G, I, L, T, M, or V; F172 replaced with W, or
Y; .K173
replaced with H, or R; 8174 replaced with H, or K; 6175 replaced with A, I, L,
S, T, M,
or V; S176 replaced with A, G, I, L, T, M, or V; A177 replaced with G, I, L,
S, T, M, or
V; L178 replaced with A, G, I, S, T, M, or V; E179 replaced with D; E180
replaced with
D; K181 replaced with H, or R; E182 replaced with D; N183 replaced with Q;
K184
replaced with H, or R; I185 replaced with A, G, L, S, T, M, or V; L186
replaced with A,
G, I, S, T, M, or V; V 187 replaced with A, G, I, L, S, T, or M; K188 replaced
with H, or
R; E189 replaced with D; T190 replaced with A, G, I, L, S, M, or V; 6191
replaced with
A, I, L, S, T, M, or V; Y192 replaced with F, or W; F193 replaced with W, or
Y; F194
replaced with W, or Y; I195 replaced with A, G, L, S, T, M, or V; Y196
replaced with F,
or W; 6197 replaced with A, I, L, S, T, M, or V; Q198 replaced with N; V199
replaced
153
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
with A, G, I, L, S, T, or M; L200 replaced with A, G, I, S, T, M, or V; Y201
replaced with
F, or W; T202 replaced with A, G, I, L, S, M, or V; D203 replaced with E; K204
replaced
with H, or R; T205 replaced with A, G, I, L, S, M, or V; Y206 replaced with F,
or W;
A207 replaced with G, I, L, S, T, M, or V; M208 replaced with A, G, I, L, S,
T, or V;
6209 replaced with A, I, L, S, T, M, or V; H210 replaced with K, or R; L211
replaced
with A, G, I, S, T, M, or V; I212 replaced with A, G, L, S, T, M, or V; Q213
replaced with
N; 8214 replaced with H, or K; K215 replaced with H, or R; K2I6 replaced with
H, or R;
V217 replaced with A, G, I, L, S, T, or M; H218 replaced with K, or R; V219
replaced
with A, G, I, L, S, T, or M; F220 replaced with W, or Y; 6221 replaced with A,
I, L, S, T,
M, or V; D222 replaced with E; E223 replaced with D; L224 replaced with A, G,
I, S, T,
M, or V; S225 replaced with A, G, I, L, T, M, or V; L226 replaced with A, G,
I, S, T, M,
or V; V227 replaced with A, G, I, L, S, T, or M; T228 replaced with A, G, I,
L, S, M, or
V; L229 replaced with A, G, I, S, T, M, or V; F230 replaced with W, or Y; 8231
replaced
with H, or K; I233 replaced with A, G, L, S, T, M, or V; Q234 replaced with N;
N235
replaced with Q; M236 replaced with A, G, I, L, S, T, or V; E238 replaced with
D; T239
replaced with A, G, I, L, S, M, or V; L240 replaced with A, G, I, S, T, M, or
V; N242
replaced with Q; N243 replaced with Q; 5244 replaced with A, G, I, L, T, M, or
V; Y246
replaced with F, or W; S247 replaced with A, G, I, L, T, M, or V; A248
replaced with G,
I, L, S, T, M, or V; 6249 replaced with A, I, L, S, T, M, or V; I250 replaced
with A, G, L,
S, T, M, or V; A251 replaced with G, I, L, S, T, M, or V; K252 replaced with
H, or R;
L253 replaced with A, G, I, S, T, M, or V; E254 replaced with D; E255 replaced
with D;
6256 replaced with A, I, L, S, T, M, or V; D257 replaced with E; E258 replaced
with D;
L259 replaced with A, G, I, S, T, M, or V; Q260 replaced with N; L261 replaced
with A,
G, I, S, T, M, or V; A262 replaced with G, I, L, S, T, M, or V; I263 replaced
with A, G, L,
S, T, M, or V; 8265 replaced with H, or K; E266 replaced with D; N267 replaced
with Q;
A268 replaced with G, I, L, S, T, M, or V; Q269 replaced with N; I270 replaced
with A,
G, L, S, T, M, or V; S271 replaced with A, G, I, L, T, M, or V; L272 replaced
with A, G,
I, S, T, M, or V; D273 replaced with E; 6274 replaced with A, I, L, S, T, M,
or V; D275
replaced with E; V276 replaced with A, G, I, L, S, T, or M; T277 replaced with
A, G, I, L,
S, M, or V; F278 replaced with W, or Y; F279 replaced with W, or Y; 6280
replaced with
A, I, L, S, T, M, or V; A281 replaced with G, I, L, S, T, M, or V; L282
replaced with A,
G, I, S, T, M, or V; K283 replaced with H, or R; L284 replaced with A, G, I,
S, T, M, or
154
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
V; and/or L285 replaced with A, G, I, S, T, M, or V. Polynucleotides encoding
these
polypeptides are also encompassed by the invention. The resulting Neutrolune-
alpha
proteins of the invention may be routinely screened for Neutrokine-alpha
and/or
Neutrokine-alphaSV functional activity and/or physical properties (such as,
for example,
enhanced or reduced stability and/or solubility). Preferably, the resulting
proteins of the
invention have an increased and/or a decreased Neutrokine-alpha and/or
Neutrol~ine-
alphaSV functional activity. More preferably, the resulting Neutrokine-alpha
and/or
Neutrolune-alphaSV proteins of the invention have more than one increased
and/or
decreased Neutrokine-alpha andlor Neutrolune-alpha SV functional activity
and/or
physical property.
[0328] In another embodiment, site directed changes at the amino acid level of
Neutrokine-alphaSV can be made by replacing a particular amino acid with a
conservative
substitution. Preferred conservative substitution mutations of the Neutrokine-
alphaSV
amino acid sequence provided in SEQ ID,N0:19 include: M1 replaced with A, G,
I, L, S,
T, or V; D2 replaced with E; D3 replaced with E; S4 replaced with A~ G, I, L,
T, M, or V;
T5 replaced with A, G, I, L, S, M, or V; E6 replaced with D; R7 replaced with
H, or K; E8
replaced with D; Q9 replaced with N; S10 replaced with A, G, I, L, T, M, or V;
R11
replaced with H, or K; L12 replaced with A, G, I, S, T, M, or V; T13 replaced
with A, G,
I, L, S, M, or V; S14 replaced with A, G, I, L, T, M, or V; L16 replaced with
A, G, I, S, T,
M, or V; K17 replaced with H, or R; K18 replaced with H, or R; R19 replaced
with H, or
K; E20 replaced with D; E21 replaced with D; M22 replaced with A, G, I, L, S,
T, or V;
K23 replaced with H, or R; L24 replaced with A, G, I, S, T, M, or V; K25
replaced with
H, or R; E26 replaced with D; V28 replaced with A, G, I, L, S, T, or M; S29
replaced with
A, G, I, L, T~ M, or V; I30 replaced with A, G, L, S, T, M, or V; L31 replaced
with A, G,
I, S, T, M, or V; R33 replaced with H, or K; K34 replaced with H; or R; E35
replaced with
D; S36 replaced with A, G, I, L, T, M, or V; S38 replaced with A, G, I, L, T,
M, or V; V39
replaced with A, G, I, L, S, T, or M; R40 replaced with H, or K; S41 replaced
with A, G, I,
L, T, M, or V; S42 replaced with A, G, I, L, T, M, or V; K43 replaced with H,
or R; D44
replaced with E; G45 replaced with A, I, L, S, T, M, or V; K46 replaced with
H, or R; L47
replaced with A, G, I, S, T, M, or V; L48 replaced with A, G, I, S, T, M, or
V; A49
replaced with G, I, L, S, T, M, or V; A50 replaced with G, I, L, S, T, M, or
V; T51
replaced with A, G, I, L, S, M, or V; L52 replaced with A, G, I, S, T, M, or
V; L53
155
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with A, G, I, S, T, M, or V; L54 replaced with A, G, I, S, T, M, or
V; A55
replaced with G, I, L, S, T, M, or V; L56 replaced with A, G, I, S, T, M, or
V; L57
replaced with A, G, I, S, T, M, or V; S58 replaced with A, G, I, L, T, M, or
V; L61
replaced with A, G, I, S, T, M, or V; T62 replaced with A, G, I, L, S, M, or
V; V63
replaced with A, G, I, L, S, T, or M; V64 replaced with A, G, I, L, S, T, or
M; S65
replaced with A, G, I, L, T, M, or V; F66 replaced with W, or Y; Y67 replaced
with F, or
W; Q68 replaced with N; V69 replaced with A, G, I, L, S, T, or M; A70 replaced
with G,
I, L, S, T, M, or V; A71 replaced with G, I, L, S, T, M, or V; L72 replaced
with A, G, I, S,
T, M, or V; Q73 replaced with N; G74 replaced with A, I, L, S, T, M, or V; D75
replaced
with E; L76 replaced with A, G, I, S, T, M, or V; A77 replaced with G, I, L,
S, T, M, or V;
S78 replaced with A, G, I, L, T, M, or V; L79 replaced with A, G, I, S, T, M,
or V; R80
replaced with H, or K; A81 replaced with G, I, L, S, T, M, or V; E82 replaced
with D; L83
replaced with A, G, I, S, T, M, or V; Q84 replaced with N; G85 replaced with
A, I, L, S,
T, M, or V; H86 replaced with K, or R; H87 replaced with K, or R; A88 replaced
with G,
I, L, S, T, M, or V; E89 replaced with D; K90 replaced with H, or R; L91
replaced with A,
G, I, S, T, M, or V; A93 replaced with G, I, L, S, T, M, or V; G94 replaced
with A, I, L, S,
T, M, or V; A95 replaced with G, I, L, S, T, M, or V; G96 replaced with A, I,
L, S, T, M,
or V; A97 replaced with G, I, L, S, T, M, or V; K99 replaced with H, or R;
A100 replaced
with G, I, L, S, T, M, or V; 6101 replaced with A, I, L, S, T, M, or V; L102
replaced with
A, G, I, S, T, M, or V; E103 replaced with D; E104 replaced with D; A105
replaced with
G, I, L, S, T, M, or V; A107 replaced with G, I, L, S, T, M, or V; V108
replaced with A,
G, I, L, S, T, or M; T109 replaced with A, G, I, L, S, M, or V; A110 replaced
with G, I, L,
S, T, M, or V; 6111 replaced with A, I, L, S, T, M, or V; L112 replaced with
A, G, I, S, T,
M, or V; K113 replaced with H, or R; I114 replaced with A, G, L, S, T, M, or
V; F115
replaced with W, or Y; E116 replaced with D; A119 replaced with G, I, L, S, T,
M, or V;
6121 replaced with A, I, L, S, T, M, or V; E122 replaced with D; 6123 replaced
with A,
I, L, S, T, M, or V; N124 replaced with Q; 5125 replaced with A, G, I, L, T,
M, or V;
S126 replaced with A, G, I, L, T, M, or V; Q127 replaced with N; N128 replaced
with Q;
5129 replaced with A, G, I, L, T, M, or V; 8130 replaced with H, or K; N131
replaced
with Q; K132 replaced with H, or R; 8133 replaced with H, or K; A134 replaced
with G,
I, L, S, T, M, or V; V135 replaced with A, G, I, L, S, T, or M; Q136 replaced
with N;
6137 replaced with A, I, L, S, T, M, or V; E139 replaced with D; E140 replaced
with D;
156
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
T141 replaced with A, G, I, L, S, M, or V; 6142 replaced with A, I, L, S, T,
M, or V;
S 143 replaced with A, G, I, L, T, M, or V; Y144 replaced with F, or W; T145
replaced
with A, G, I, L, S, M, or V; F146 replaced with W, or Y; V147 replaced with A,
G, I, L, S,
T, or M; W149 replaced with F, or Y; L150 replaced with A, G, I, S, T, M, or
V; L151
replaced with A, G, I, S, T, M, or V; 5152 replaced with A, G, I, L, T, M, or
V; F153
replaced with W, or Y; K154 replaced with H, or R; 8155 replaced with H, or K;
6156
replaced with A, I, L, S, T, M, or V; 5157 replaced with A, G, I, L, T, M, or
V; A158
replaced with G, I, L, S, T, M, or V; L159 replaced with A, G, I, S, T, M, or
V; E160
replaced with D; E161 replaced with D; K162 replaced with H, or R; E163
replaced with
D; N164 replaced with Q; K165 replaced with H, or R; I166 replaced with A, G,
L, S, T,
M, or V; L167 replaced with A, G, I, S, T, M, or V; V168 replaced with A, G,
I, L, S, T,
or M; K169 replaced with H, or R; E170 replaced with D; T171 replaced with A,
G, I, L,
S, M, or V; 6172 replaced with A, I, L, S, T, M, or V; Y173 replaced with F,
or W; F174
replaced with W, or Y; F175 replaced with W, or Y; I176 replaced with A, G, L,
S, T, M,
or V; Y177 replaced with F, or W; 6178 replaced with A, I, L, S, T, M, or V;
Q179
replaced with N; V 180 replaced with A, G, I, L, S, T, or M; L181 replaced
with A, G, I, S,
T, M, or V; Y182 replaced with F, or W; T183 replaced with A, G, I, L, S, M,
or V; D184
replaced with E; K185 replaced with H, or R; T186 replaced with A, G, I, L, S,
M, or V;
Y187 replaced with F, or W; A188 replaced with G, I, L, S, T, M, or V; M189
replaced
with A, G, h L, S, T, or V; 6190 replaced with A, I, L, S, T, M, or V; H191
replaced with
K, or R; L192 replaced with A, G, I, S, T, M, or V; I193 replaced with A, G,
L, S, T, M,
or V; Q194 replaced with N; 8195 replaced with H, or K; K196 replaced with H,
or R;
K197 replaced with H, or R; V198 replaced with A, G, I, L, S, T, or M; H199
replaced
with K, or R; V200 replaced with A, G, I, L, S, T, or M; F201 replaced with W,
or Y;
6202 replaced with A, I, L, S, T, M, or V; D203 replaced with E; E204 replaced
with D;
L205 replaced with A, G, I, S, T, M, or V; 5206 replaced with A, G, I, L, T,
M, or V;
L207 replaced with A, G, I, S, T, M, or V; V208 replaced with A, G, I, L, S,
T, or M;
T209 replaced with A, G, I, L, S, M, or V; L210 replaced with A, G, I, S, T,
M, or V;
F211 replaced with W, or Y; 8212 replaced with H, or K; I214 replaced with A,
G, L, S,
T, M, or V; Q215 replaced with N; N216 replaced with Q; M217 replaced with A,
G, I, L,
S, T, or V; E219 replaced with D; T220 replaced with A, G, I, L, S, M, or V;
L221
replaced with A, G, I, S, T, M, or V; N223 replaced with Q; N224 replaced with
Q; 5225
157
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with A, G, I, L, T, M, or V; Y227 replaced with F, or W; S228
replaced with A,
G, I, L, T, M; or V; A229 replaced with G, I, L, S, T, M, or V; 6230 replaced
with A, I, L,
S, T, M, or V; I231 replaced with A, G, L, S, T, M, or V; A232 replaced with
G, I, L, S, T,
M, or V; K233 replaced with H, or R; L234 replaced with A, G, I, S, T, M, or
V; E235
replaced with D; E236 replaced with D; 6237 replaced with A, I, L, S, T, M, or
V; D238
replaced with E; E239 replaced with D; L240 replaced with A, G, I, S, T, M, or
V; Q241
replaced with N; L242 replaced with A, G, I, S, T, M, or V; A243 replaced with
G, I, L, S,
T, M, or V; I244 replaced with A, G, L, S, T, M, or V; 8246 replaced with H,
or K; E247
replaced with D; N248 replaced with Q; A249 replaced with G, I, L, S, T, M, or
V; Q250
replaced with N; I251 replaced with A, G, L, S, T, M, or V; 5252 replaced with
A, G, I, L,
T, M, or V; L253 replaced with A, G, I, S, T, M, or V; D254 replaced with E;
6255
replaced with A, I, L, S, T, M, or V; D256 replaced with E; V257 replaced with
A, G, I, L,
S, T, or M; T258 replaced with A, G, I, L, S, M, or V; F259 replaced with W,
or Y; F260
replaced with W, or Y; 6261 replaced with A, I, L, S, T, M, or V; A262
replaced with G,
I, L, S, T, M, or V; L263 replaced with A, G, I, S, T, M, or V; K264 replaced
with H, or
R; L265 replaced with A, G, I, S, T, M, or V; and/or L266 replaced with A, G,
I, S, T, M,
or V. Polynucleotides encoding these polypeptides are also encompassed by the
invention. The resulting Neutrokine-alpha proteins of the invention may be
routinely
screened for Neutrokine-alpha and/or Neutrokine-alphaSV functional activity
and/or
physical properties (such as, for example, enhanced or reduced stability
and/or solubility).
Preferably, the resulting proteins of-the invention have an increased and/or a
decreased
Neutrokine-alpha and/or Neutrokine-alphaSV functional activity. More
preferably, the
resulting Neutrokine-alpha and/or Neutrokine-alphaSV proteins of the invention
have
more than one increased and/or decreased Neutrokine-alpha and/or Neutrokine-
alpha SV
functional activity and/or physical property.
[0329] In another embodiment, site directed changes at the amino acid level of
Neutrokine-alpha can be made by replacing a particular amino acid with a
conservative
substitution. Preferred conservative substitution mutations of the Neutrokine-
alpha amino
acid sequence provided in SEQ ID N0:23 include: R1 replaced with H, or K; V2
replaced
with A, G, I, L, S, T, or M; V3 replaced with A, G, I, L, S, T, or M; D4
replaced with E;
L5 replaced with A, G, I, S, T, M, or V; S6 replaced with A, G, I, L, T, M, or
V; A7
replaced with G, I, L, S, T, M, or V; A10 replaced with G, I, L, S, T, M, or
V; L13
158
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with A, G, I, S, T, M, or V; G15 replaced with A, I, L, S, T, M, or
V; R17
replaced with H, or K; H18 replaced with K, or R; S19 replaced with A, G, I,
L, T, M, or
V; Q20 replaced with N; H21 replaced with K, or R; D22 replaced with E; D23
replaced
with E; N24 replaced with Q; G25 replaced with A, I, L, S, T, M, or V; M26
replaced with
A, G, I, L, S, T, or V; N27 replaced with Q; L28 replaced with A, G, I, S, T,
M, or V; R29
replaced with H, or K; N30 replaced with Q; R31 replaced with H, or K; T32
replaced
with A, G, I, L, S, M, or V; Y33 replaced with F, or W; T34 replaced with A,
G, I, L, S,
M, or V; F35 replaced with W, or Y; V36 replaced with A, G, I, L, S, T, or M;
W38
replaced with F, or Y; L39 replaced with A, G, I, S, T, M, or V; L40 replaced
with A, G, I,
S, T, M, or V; S41 replaced with A, G, I, L, T, M, or V; F42 replaced with W,
or Y; K43
replaced with H, or R; R44 replaced with H, or K; G45 replaced with A, I, L,
S, T, M, or
V; N46 replaced with Q; A47 replaced with G, I, L, S, T, M, or V; L48 replaced
with A,
G, I, S, T, M, or V; E49 replaced with D; E50 replaced with D; K51 replaced
with H, or R;
E52 replaced with D; N53 replaced with Q; K54 replaced with H, or R; I55
replaced with
A, G, L, S, T, M, or V; V56 replaced with A, G, I, L, S, T, or M; V57 replaced
with A, G,
I, L, S, T, or M; R58 replaced with H, or K; Q59 replaced with N; T60 replaced
with A, G,
I, L, S, M, or V; G61 replaced with A, I, L, S, T, M, or V; Y62 replaced with
F, or W; F63
replaced with W, or Y; F64 replaced with W, or Y; I65 replaced with A, G, L,
S, T, M, or
V; Y66 replaced with F, or W; S67 replaced with A, G, I, L, T, M, or V; Q68
replaced
with N; V69 replaced with A, G, I, L, S, T, or M; L70 replaced with A, G, I,
S, T, M, or
V; Y71 replaced with F, or W; T72 replaced with A, G, I, L, S, M, or V; D73
replaced
with E; I75 replaced with A, G, L, S, T, M, or V; F76 replaced with W, or Y;
A77
replaced with G, I, L, S, T, M, or V; M78 replaced with A, G, I, L, S, T, or
V; G79
replaced with A, I, L, S, T, M, or V; H80 replaced with K, or R; V81 replaced
with A, G,
I, L, S, T, or M; I82 replaced with A, G, L, S, T, M, or V; Q83 replaced with
N; R84
replaced with H, or K; K85 replaced with H, or R; K86 replaced with H, or R;
V87
replaced with A, G, I, L, S, T, or M; H88 replaced with K, or R; V89 replaced
with A, G,
I, L, S, T, or M; F90 replaced with W, or Y; G91 replaced with A, I, L, S, T,
M, or V; D92
replaced with E; E93 replaced with D; L94 replaced with A, G, I, S, T, M, or
V; S95
replaced with A, G, I, L, T, M, or V; L96 replaced with A, G, I, S, T, M, or
V; V97
replaced with A, G, I, L, S, T, or M; T98 replaced with A, G, I, L, S, M, or
V; L99
replaced with A, G, I, S, T, M, or V; F100 replaced with W, or Y; 8101
replaced with H,
159
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
or K; I103 replaced with A, G, L, S, T, M, or V; Q104 replaced with N; N105
replaced
with Q; M106 replaced with A, G, I, L, S, T, or V; K108 replaced with H, or R;
T109
replaced with A, G, I, L, S, M, or V; L110 replaced with A, G, I, S, T, M, or
V; N112
replaced with Q; N113 replaced with Q; S114 replaced with A, G, I, L, T, M, or
V; Y116
replaced with F, or W; 5117 replaced with A, G, I, L, T, M, or V; A118
replaced with G,
I, L, S, T, M, or V; 6119 replaced with A, I, L, S, T, M, or V; I120 replaced
with A, G, L,
S, T, M, or V; A121 replaced with G, I, L, S, T, M, or V; 8122 replaced with
H, or K;
L123 replaced with A, G, I, S, T, M, or V; E124 replaced with D; E125 replaced
with D;
6126 replaced with A, I, L, S, T, M, or V; D127 replaced with E; E128 replaced
with D;
I129 replaced with A, G, L, S, T, M, or V; Q130 replaced with N; L131 replaced
with A,
G, I, S, T, M, or V; A132 replaced with G, I, L, S, T, M, or V; I133 replaced
with A, G, L,
S, T, M, or V; 8135 replaced with H, or K; E136 replaced with D; N137 replaced
with Q;
A138 replaced with G, I, L, S, T, M, or V; Q139 replaced with N; I140 replaced
with A,
G, L, S, T, M, or V; 5141 replaced with A, G, I, L, T, M, or V; 8142 replaced
with H, or
K; N143 replaced with Q; 6144 replaced with A, I, L, S, T, M, or V; D145
replaced with
E; D146 replaced with E; T147 replaced with A, G, I, L, S, M, or V; F148
replaced with
W, or Y; F149 replaced with W, or Y; 6150 replaced with A, I, L, S, T, M, or
V; A151
replaced with G, I, L, S, T, M, or V; L152 replaced with A, G, I, S, T, M, or
V; K153
replaced with H, or R; L154 replaced with A, G, I, S, T, M, or V; and/or L155
replaced
with A, G, I, S, T, M, or V. Polynucleotides encoding these polypeptides are
also
encompassed by the invention. The resulting Neutrokine-alpha proteins of the
invention
may be routinely screened for Neutrokine-alpha andlor Neutrokine-alphaSV
functional
activity and/or physical properties (such as, for example, enhanced or reduced
stability
andlor solubility). Preferably, the resulting proteins of the invention have
an increased
and/or a decreased Neutrokine-alpha and/or Neutrokine-alphaSV functional
activity.
More preferably, the resulting Neutrokine-alpha and/or Neutrokine-alphaSV
proteins of
the invention have more than one increased and/or decreased Neutrokine-alpha
and/or
Neutrokine-alpha SV functional activity and/or physical property.
[0330] In another embodiment, site directed changes at the amino acid level of
Neutrokine-alpha can be made by replacing a particular amino acid with a
conservative
substitution. Preferred conservative substitution mutations of the Neutrokine-
.alpha amino
acid sequence provided in SEQ ID N0:38 include: Ml replaced with A, G, I, L,
S, T, or
160
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
V; D2 replaced with E; E3 replaced with D; S4 replaced with A, G, I, L, T, M,
or V; A5
replaced with G, I, L, S, T, M, or V; K6 replaced with H, or R; T7 replaced
with A, G, I,
L, S, M, or V; L8 replaced with A, G, I, S, T, M, or V; L13 replaced with A,
G, I, S, T, M,
or V; F15 replaced with W, or Y; S17 replaced with A, G, I, L, T, M, or V; E18
replaced
with D; Kl9 replaced with H, or R; G20 replaced with A, I, L, S, T, M, or V;
E21 replaced
with D; D22 replaced with E; M23 replaced with A, G, I, L, S, T, or V; K24
replaced with
H, or R; V25 replaced with A, G, I, L, S, T, or M; G26 replaced with A, I, L,
S, T, M, or
V; Y27 replaced with F, or W; D28 replaced with E; I30 replaced with A, G, L,
S, T, M,
or V; T31 replaced with A, G, I, L, S, M, or V; Q33 replaced with N; K34
replaced with
H, or R; E35 replaced with D; E36 replaced with D; G37 replaced with A, I, L,
S, T, M, or
V; A38 replaced with G, I, L, S, T, M, or V; W39 replaced with F, or Y; F40
replaced
with W, or Y; G41 replaced with A, I, L, S, T, M, or V; I42 replaced with A,
G, L, S, T,
M, or V; R44 replaced with H, or K; D45 replaced with E; G46 replaced with A,
I, L, S, T,
M, or V; R47 replaced with H, or K; L48 replaced with A, G, I, S, T, M, or V;
L49
replaced with A, G, I, S, T, M, or V; A50 replaced with G, I, L, S, T, M, or
V; A51
replaced with G, I, L, S, T, M, or V; T52 replaced with A, G, I, L, S, M, or
V; L53
replaced with A, G, I, S, T, M, or V; L54 replaced with A, G, I, S, T, M, or
V; L55
replaced with A, G, I, S, T, M, or V; A56 replaced with G, I, L, S, T, M, or
V; L57
replaced with A, G, I, S, T, M, or V; L58 replaced with A, G, .I, S, T, M, or
V; S59
replaced with A, G, I, L, T, M, or V; S60 replaced with A, G, I, L, T, M, or
V; S61
replaced with A, G, I, L, T, M, or V; F62 replaced with W, or Y; T63 replaced
with A, G,
I, L, S, M, or V; A64 replaced with G, I, L, S, T, M, or V; M65 replaced with
A, G, I, L,
S, T, or V; S66 replaced with A, G, I, L, T, M, or V; L67 replaced with A, G,
I, S, T, M,
or V; Y68 replaced with F, or W; Q69 replaced with N; L70 replaced with A, G,
I, S, T,
M, or V; A71 replaced with G, I, L, S, T, M, or V; A72 replaced with G, I, L,
S, T, M, or
V; L73 replaced with A, G, I, S, T, M, or V; Q74 replaced with N; A75 replaced
with G, I,
L, S, T, M, or V; D76 replaced with E; L77 replaced with A, G, I, S, T, M, or
V; M78
replaced with A, G, I, L, S, T, or V; N79 replaced with Q; L80 replaced with
A, G, I, S, T,
M, or V; R81 replaced with H, or K; M82 replaced with A, G, I, L, S, T, or V;
E83
replaced with D; L84 replaced with A, G, I, S, T, M, or V; Q85 replaced with
N; S86
replaced with A, G, I, L, T, M, or V; Y87 replaced with F, or W; R88 replaced
with H, or
K; G89 replaced with A, I, L, S, T, M, or V; S90 replaced with A, G, I, L, T,
M, or V; A91
161
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with G, I, L, S, T, M, or V; T92 replaced with A, G, I, L, S, M, or
V; A94
replaced with G, I, L, S, T, M, or V; A95 replaced with G, I, L, S, T, M, or
V; A96
replaced with G, I, L, S, T, M, or V; G97 replaced with A, I, L, S, T, M, or
V; A98
replaced with G, I, L, S, T, M, or V; E100 replaced with D; L101 replaced with
A, G, I, S,
T, M, or V; T102 replaced with A, G, I, L, S, M, or V; A103 replaced with G,
I, L, S, T,
M, or V; 6104 replaced with A, I, L, S, T, M, or V; V 105 replaced with A, G,
I, L, S, T,
or M; K106 replaced with H, or R; L107 replaced with A, G, I, S, T, M, or V;
L108
replaced with A, G, I, S, T, M, or V; T109 replaced with A, G, I, L, S, M, or
V; A111
replaced with G, I, L, S, T, M, or V; A112 replaced with G, I, L, S, T, M, or
V; 8114
replaced with H, or K; H116 replaced with K, or R; N117 replaced with Q; 5118
replaced
with A, G, I, L, T, M, or V; 5119 replaced with A, G, I, L, T, M, or V; 8120
replaced with
H, or K; 6121 replaced with A, I, L, S, T, M, or V; H122 replaced with K, or
R; 8123
replaced with H, or K; N124 replaced with Q; 8125 replaced with H, or K; 8126
replaced
with H, or K; A127 replaced with G, I, L, S, T, M, or V; F128 replaced with W,
or Y;
Q129 replaced with N; 6130 replaced with A, I, L, S, T, M, or V; E132 replaced
with D;
E133 replaced with D; T134 replaced with A, G, I, L, S, M, or V; E135 replaced
with D;
Q136 replaced with N; D137 replaced with E; V138 replaced with A, G, I, L, S,
T; or M;
D139 replaced with E; L140 replaced with A, G, I, S, T, M, or V; 5141 replaced
with A,
G, I, L, T, M, or V; A142 replaced with G, I, L, S, T, M, or V; A145 replaced
with G, I, L,
S, T, M, or V; L148 replaced with A, G, I, S, T, M, or V; 6150 replaced with
A, I, L, S, T,
M, or V; 8152 replaced with H, or K; H153 replaced with K, or R; 5154 replaced
with A,
G, I, L, T, M, or V; Q155 replaced with N; H156 replaced with K, or R; D157
replaced
with E; D158 replaced with E; N159 replaced with Q; 6160 replaced with A, I,
L, S, T,
M, or V; M161 replaced with A, G, I, L, S, T, or V; N162 replaced with Q; L163
replaced
with A, G, I, S, T, M, or V; 8164 replaced with H, or K; N165 replaced with Q;
I166
replaced with A, G, L, S, T, M, or V; I167 replaced with A, G, L, S, T, M, or
V; Q168
replaced with N; D169 replaced with E; L171 replaced with A, G, I, S, T, M, or
V; Q172
replaced with N; L173 replaced with A, G, I, S, T, M, or V; I174 replaced with
A, G, L, S,
T, M, or V; A175 replaced with G, I, L, S, T, M, or V; D176 replaced with E;
S177
replaced with A, G, I, L, T, M, or V; D178 replaced with E; T179 replaced with
A, G, I, L,
S, M, or V; A181 replaced with G, I, L, S, T, M, or V; L182 replaced with A,
G, I, S, T,
M, or V; E183 replaced with D; E184 replaced with D; K185 replaced with H, or
R; E186
162
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with D; N187 replaced with Q; K188 replaced with H, or R; I189
replaced with
A, G, L, S, T, M, or V; V 190 replaced with A, G, I, L, S, T, or M; V 191
replaced with A,
G, I, L, S, T, or M; 8192 replaced with H, or K; Q193 replaced with N; T194
replaced
with A, G, I, L, S, M, or V; 6195 replaced with A, I, L, S, T, M, or V; Y196
replaced with
F, or W; F197 replaced with W, or Y; F198 replaced with W, or Y; I199 replaced
with A,
G, L, S, T, M, or V; Y200 replaced with F, or W; S201 replaced with A, G, I,
L, T, M, or
V; Q202 replaced with N; V203 replaced with A, G, I, L, S, T, or M; L204
replaced with
A, G, I, S, T, M, or V; Y205 replaced with F, or W; T206 replaced with A, G,
I, L, S, M,
or V; D207 replaced with E; I209 replaced with A, G, L, S, T, M, or V; F210
replaced
with W, or Y; A211 replaced with G, I, L, S, T, M, or V; M212 replaced with A,
G, I, L,
S, T, or V; 6213 replaced with A, I, L, S, T, M, or V; H214 replaced with K,
or R; V215
replaced with A, G, I, L, S, T, or M; I216 replaced,with A, G, L, S, T, M, or
V; Q217
replaced with N; 8218 replaced with H, or K; K219 replaced with H, or R; K220
replaced
with H, or R; V221 replaced with A, G, I, L, S, T, or M; H222 replaced with K,
or R;
V223 replaced with A, G, I, L, S, T, or M; F224 replaced with W, or Y; 6225
replaced
with A, I, L, S, T, M, or V; D226 replaced with E; E227 replaced with D; L228
replaced
with A, G, I, S, T, M, or V; S229 replaced with A, G, I, L, T, M, or V; L230
replaced with
A, G, I, S, T, M, or V; V231 replaced with A, G, I, L, S, T, or M; T232
replaced with A,
G, I, L, S, M, or V; L233 replaced with A, G, I, S, T, M, or V; F234 replaced
with W, or
Y; 8235 replaced with H, or K; I237 replaced with A, G, L, S, T, M, or V; Q238
replaced
with N; N239 replaced with Q; M240 replaced with A, G, I, L, S, T, or V; K242
replaced
with H, or R; T243 replaced with A, G, I, L, S, M, or V; L244 replaced with A,
G, I, S, T,
M, or V; N246 replaced with Q; N247 replaced with Q; 5248 replaced with A, G,
I, L, T,
M, or V; Y250 replaced with F, or W; 5251 replaced with A, G, I, L, T, M, or
V; A252
replaced with G, I, L, S, T, M, or V; 6253 replaced with A, I, L, S, T, M, or
V; I254
replaced with A, G, L, S, T, M, or V; A255 replaced with G, I, L, S, T, M, or
V; 8256
replaced with H, or K; L257 replaced with A, G, I, S, T, M, or V; E258
replaced with D;
E259 replaced with D; 6260 replaced with A, I, L, S, T, M, or V; D261 replaced
with E;
E262 replaced with D; I263 replaced with A, G, L, S, T, M, or V; Q264 replaced
with N;
L265 replaced with A, G, I, S, T, M, or V; A266 replaced with G, I, L, S, T,
M, or V; I267
replaced with A, G, L, S, T, M, or V; 8269 replaced with H, or K; E270
replaced with D;
N271 replaced with Q; A272 replaced with G, I, L, S, T, M, or V; Q273 replaced
with N;
163
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
I274 replaced with A, G, L, S, T, M, or V; S275 replaced with A, G, I, L, T,
M, or V;
8276 replaced with H, or K; N277 replaced with Q; 6278 replaced with A, I, L,
S, T, M,
or V; D279 replaced with E; D280 replaced with E; T281 replaced with A, G, I,
L, S, M,
or V; F282 replaced with W, or Y; F283 replaced with W, or Y; 6284 replaced
with A, I,
L, S, T, M, or V; A285 replaced with G, I, L, S, T, M, or V; L286 replaced
with A, G, I, S,
T, M, or V; K287 replaced with H, or R; L288 replaced with A, G, I, S, T, M,
or V; and/or
L289 replaced with A, G, I, S, T, M, or V. Polynucleotides encoding these
polypeptides
are also encompassed by the invention. The resulting Neutrokine-alpha proteins
of the
invention may be routinely screened for Neutxokine-alpha andlor Neutrokine-
alphaSV
functional activity and/or physical properties (such as, for example, enhanced
or reduced
stability and/or solubility). Preferably, the resulting proteins of the
invention have an
increased and/or a decreased Neutrokine-alpha and/or Neutrokine-alphaSV
functional
activity. More preferably, the resulting Neutrokine-alpha andlor Neutrokine-
alphaSV
proteins of the invention have more than one increased and/or decreased
Neutrokine-alpha
andlor Neutrokine-alpha SV functional activity and/or physical property.
[0331] Amino acids in the Neutrokine-alpha and/or Neutrokine-alphaSV
polypeptides
of the present invention that are essential for function can be identified by
methods known
in the art, such as site-directed mutagenesis or alanine-scanning mutagenesis
(Cunningham and Wells, Science 244:1081-1085 (1989)). The latter procedure
introduces
single alanine mutations at every residue in the molecule. The resulting
mutant molecules
are then tested for functional activity, such ligand binding and the ability
to stimulate
lymphocyte (e.g., B cell) as, for example, proliferation, differentiation,
and/or activation.
[0332] Of special interest are substitutions of charged amino acids with other
charged
or neutral amino acids which may produce proteins with highly desirable
improved
characteristics, such as less aggregation. Aggregation may not only reduce
activity but
also be problematic when preparing pharmaceutical formulations, because
aggregates can
be immunogenic (Pinckard et al., Clizz. E~p. Immunol. 2:331-340 (1967);
Robbins et al.,
Diabetes 36: 838-845 (1987); Cleland et al., Crit. Rev. Therapeutic Drug
Carrier Systems
10:307-377 (1993).
[0333] In another embodiment, the invention provides for polypeptides having
amino
acid sequences containing non-conservative substitutions of the amino acid
sequence
provided in SEQ ID N0:2. For example, non-conservative substitutions of the
164
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Neutrokine-alpha protein sequence provided in SEQ ID N0:2 include: Ml replaced
with
D, E, H, K, R, N, Q, F, W, Y, P, or C; D2 replaced with H, K, R, A, G, I, L,
S, T, M, V, N,
Q, F, W, Y, P, or C; D3 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P, or
C; S4 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T5 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; E6 replaced with H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y, P,
or C; R7 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
E8 replaced
with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; Q9 replaced with
D, E, H, K,
R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; S 10 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; R11 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; L12
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T13 replaced with D; E,
H, K, R, N,
Q, F, W, Y, P, or C; S14 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
C15
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P; L16
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; K17 replaced with D, E, A, G, I,
L, S, T, M, V,
N, Q, F, W, Y, P, or C; K18 replaced with D, E, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
or C; R19 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
E20 replaced
with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E21 replaced
with H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; M22 replaced with D, E, H, K,
R, N, Q, F,
W, Y, P, or C; K23 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y,
P, or C; L24
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; K25 replaced with D, E,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; E26 replaced with H, K, R, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; C27 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
or P; V28 replaced with D, E, H; K, R, N, Q, F, W, Y, P, or C; S29 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; I30 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C; L31
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P32 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; R33 replaced with D, E, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, P, or C; K34 replaced with D, E, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
or C; E35 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; S36
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P37 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; S38 replaced with D, E, H, K, R, N,
Q, F, W, Y,
P, or C; V39 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; R40 replaced
with D, E,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; S41 replaced with D, E, H, K,
R, N, Q, F,
W, Y, P, or C; S42 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; K43
replaced with
165
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; D44 replaced with H, K,
R, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; G45 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; K46 replaced with D~ E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
L47 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; L48 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; A49 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A50
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; T51 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; L52 replaced with D, E, H, K, R, N, Q, F,.W, Y, P, or C; L53 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; L54 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; A55
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L56 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; L57 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
S58
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; C59 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or P; C60 replaced with D, E, H, K, R, A,
G, I, L, S, T,
M, V, N, Q, F, W, Y, or P; L61 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; T62
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V63 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; V64 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
S65
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F66 replaced with D, E,
H, K, R, N,
Q, A, G, I, L, S, T, M, V, P, or C; Y67 replaced with D, E, H, K, R, N, Q, A,
G, I, L, S, T,
M, V, P, or C; Q68 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W,
Y, P, or C;
V69 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A70 replaced with D,
E, H, K,
R, N, Q, F, W, Y, P, or C; A71 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; L72
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q73 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, F, W, Y, P, or C; G74 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; D75 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; L76
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A77 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; S78 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
L79
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; R80 replaced with D, E,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; A81 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
E82 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L83
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q84 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, F, W, Y, P, or C; G85 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; H86
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; H87
replaced with D,
E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; A88 replaced with D, E, H,
K, R, N, Q,
166
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
F, W, Y, P, or C; E89 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; K90 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L91
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; P92 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, or C; A93 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
G94 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A95 replaced with D,
E, H, K,
R, N, Q, F, W, Y, P, or C; G96 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; A97
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P98 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; K.99 replaced with D, E, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, P, or C; A100 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; 6101
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L102 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; E103 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
or C; E104 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; A105
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P106 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; A107 replaced with D, E, H, K, R, N,
Q, F, W,
Y, P, or C; V108 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T109
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; A110 replaced with D, E, H, K, R, N, Q,
F, W, Y,
P, or C; 6111 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L112
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; K113 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; I114 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
F115 replaced
with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; E116 replaced with
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; P117 replaced with D, E, H, K, R,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, or C; P118 replaced with D, E, H, K, R, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, or C; A119 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
P120
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; 6121
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; E122 replaced with H, K, R, A, G,
I, L, S, T,
M, V, N, Q, F, W, Y, P, or C; 6123 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
N124 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
5125 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; S 126 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; Q127 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y,
P, or C;
N128 replaced With D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
5129 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; 8130 replaced with D, E, A, G, I,
L, S, T, M,
V, N, Q, F, W, Y, P, or C; N131 replaced with D, E, H, K, R, A, G, I, L, S, T,
M, V, F, W,
167
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Y, P, or C; K132 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; 8133
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; A134
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; V135 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; Q136 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
6137
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P138 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; E139 replaced with H, K, R, A, G, I,
L, S, T, M,
V, N, Q, F, W, Y, P, or C; E140 replaced with H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, P, or C; T141 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V142
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; T143 replaced with D, E, H, K, R, N, Q,
F, W, Y,
P, or C; Q144 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P,
or C; D145
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; C146
replaced with
D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P; L147 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; Q148 replaced with D, E, H, K, R, A, G, I, L, S, T,
M, V, F, W,
Y, P, or C; L149 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; I150
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; A151 replaced with D, E, H, K, R, N, Q,
F, W; Y,
P, or C; D152 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; 5153
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; E154 replaced with H, K,
R, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; T155 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; P156 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y,
or C; T157
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;1158 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; Q159 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
F, W, Y, P,
or C; K160 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
6161
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; S 162 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; Y163 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T,
M, V, P, or
C; T164 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F165 replaced
with D, E, H,
K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; V166 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; P167 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, or C;
W168 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; L169
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; L170 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; S171 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F172
replaced with
D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; K173 replaced with D, E,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, P, or C; 8174 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
168
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
F, W, Y, P, or C; 6175 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; S
176 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; AI77 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; LI78 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; E179
replaced with
H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; EI80 replaced with H,
K, R, A, G,
I, L, S, T, M, V, N, Q, F, W, Y, P, or C; KI81 replaced with D, E, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, P, or C; E282 replaced with H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; N183 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P,
or C; K184
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; I185
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; L186 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; V187 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; K188 replaced
with D, E, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E189 replaced with H, K, R, A, G,
I, L, S, T,
M, V, N, Q, F, W, Y, P, or C; T190 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
GI9I replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Y192 replaced with
D, E~ H, K,
R, N, Q, A, G, I, L, S, T, M, V, P, or C; F193 replaced with D, E, H, K, R, N,
Q, A, G, I,
L, S, T, M, V, P, or C; F194 replaced with D, E, H, K, R, N, Q, A, G, I, L, S,
T, M, V, P,
or C; I195 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; YI96 replaced
with D, E,
H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; 6197 replaced with D, E, H, K,
R, N; Q, F,
W, Y, P, or C; Q198 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W,
Y, P, or C;
V199 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L2,00 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, ar C; Y201 replaced with D, E, H, K, R, N, Q, A, G, I, L,
S, T, M, V,
P, or C; T202 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D203
replaced with H,
K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K204 replaced with D, E,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, P, or C; T205 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; Y206 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C;
A207 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; M208 replaced with D, E, H, K, R,
N, Q, F,
W, Y, P, or C; G~09 replaced with D, E, H, K; R, N, Q, F, W, Y, P, or C; H2I0
replaced
with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L21I replaced with
D, E; H, K,
R, N, Q> F, W, Y, P, ox C; I21~ replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; Q213
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; 8214
replaced with
D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K2I5 replaced with D, E,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, P, or C; K~16 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; V217 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
HZI8
is~
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with D, E, A, G, I, L,. ,S; T, M, V, N, Q, F, W, Y, P, or C; V219
replaced with D,
E, H, K, R, ~T, Q, F, W, Y, P, or C; F220 replaced with D, E, H, K, R, N, Q,
A, G, I, L, S,
T, M, V, P, or C; 6221 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
D222
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E223
replaced with
H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L224 replaced with D,
E, H; K, R,
N, Q, F, W, Y, P, or C; S225 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; L226
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V227 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; T228 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
L229
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F230 replaced with D, E,
H, K, R, N,
Q, A, G, I, L, S, T, M, V, P, or C; 8231 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; C232 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
or P; I233 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q234 replaced
with D, E,
H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; N235 replaced with D, E, H,
K, R, A, G,
I, L, S, T, M, V, F, W, Y, P, or C; M236 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; P237 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or
C; E238
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; T239
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; L240 replaced with D, E, H, K, R, N, Q,
F, W, Y,
P, or C; P241 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W,
Y, or C;
N242 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
N243 replaced
with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; 5244 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; C245 replaced with D, E, H, K, R, A, G, I, L, S,
T, M, V, N,
Q, F, W, Y, or P; Y246 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M,
V, P, or C;
S247 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A248 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; 6249 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; I250
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A251 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; K252 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; L253 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; E254 replaced
with H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E255 replaced with H, K, R, A,
G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; 6256 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or
C; D257 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
E258
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L259
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; Q260 replaced with D, E, H, K, R, A, G,
I, L, S, T,
170
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
M, V, F, W, Y, P, or C; L261 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; A262
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; I263 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; P264 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, or C; 8265 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; E266
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; N267
replaced with
D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; A268 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; Q269 replaced with D, E, H, K, R, A, G, I, L, S, T, M,
V, F, W, Y,
P, or C;.I270 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 5271
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; L272 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; D273 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
6274
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D275 replaced with H, K,
R, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; V276 replaced with D, E, H, K, R, N, Q,
F, W, Y,
P, or C; T277 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F278
replaced with D,
E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; F279 replaced with D, E, H,
K, R, N, Q,
A, G, I, L, S, T, M, V, P, or C; 6280 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
A281 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L282 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; K283 replaced with D, E, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; L284 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; and/or L285
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C. Polynucleotides encoding these
polypeptides
are also encompassed by the invention. The resulting Neutrokine-alpha proteins
of the
invention may be routinely screened for Neutrokine-alpha andlor Neutrokine-
alphaSV
functional activities andlor physical properties (such as, for example,
enhanced or reduced
stability and/or solubility) described throughout the specification and known
in the art.
Preferably, the resulting proteins of the invention have an increased and/or a
decreased
Neutrokine-alpha and/or Neutrokine-alphaSV functional activity. More
preferably, the
resulting Neutrokine-alpha and/or Neutrokine-alphaSV proteins of the invention
have
more than one increased and/or decreased Neutrokine-alpha and/or Neutrokine-
alphaSV
functional activity and/or physical property.
[0334] In an additional embodiment, Neutrokine-alpha polypeptides of the
invention
comprise, or alternatively consist of, more than one amino acid (e.g., 2, 3,
4, 5, 6, 7, 8, 9,
10, 15, 20, 30 and 50) replaced with the substituted amino acids as described
above (either
conservative or nonconservative).
171
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0335] In another embodiment of the invention, non-conservative substitutions
of the
Neutrolcine-alphaSV protein sequence provided in SEQ ID N0:19 include: Ml
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; D2 replaced with H, K, R, A, G, I,
L, S, T, M,
V, N, Q, F, W, Y, P, or C; D3 replaced with H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, P, or C; S4 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T5
replaced with D, E,
H, K, R, N, Q, F, W, Y, P, or C; E6 replaced with H, K, R, A, G, I, L, S, T,
M, V, N, Q, F,
W, Y, P, or C; R7 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y,
P, or C; E8
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; Q9
replaced with D,
E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; S 10 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; R11 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; L12 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T13 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; S 14 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; C 15
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P; L16
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; K17 replaced with D, E, A, G, I,
L, S, T, M, V,
N, Q, F, W, Y, P, or C; K18 replaced with D, E, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
or C; R19 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
E20 replaced
with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E21 replaced
with H, K, R,
A~ G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; M22 replaced with D, E, H, K,
R, N, Q, F,
W, Y, P, or C; K23 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y,
P, or C; L24
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; K25 replaced with D, E,
A, 'G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; E26 replaced with H, K, R, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; C27 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
or P; V28 replaced with D, E, H, .K, R, N, Q, F, W, Y, P, or C; S29 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; I30 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C; L31
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P32 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; R33 replaced with D, E, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, P, or C; K34 replaced with D, E, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
or C; E35 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; S36
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P37 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; S38 replaced with D, E, H, K, R, N,
Q, F, W, Y,
P, or C; V39 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; R40 replaced
with D, E,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; S41 replaced with D, E, H, K,
R, N, Q, F,
172
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
W, Y, P, or C; S42 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; K43
replaced with
D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; D44 replaced with H, K,
R, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; G45 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; K46 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
L47 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; L48 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; A49 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A50
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; T5I replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; L52 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L53 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; L54 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; A55
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L56 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; L57 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
S58
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; C59 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or P; C60 replaced with D, E, H, K, R, A,
G, I, L, S, T,
M, V, N, Q, F, W, Y, or P; L61 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; T62
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V63 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; V64 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
S65
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F66 replaced with D, E,
H, K, R, N,
Q, A, G, I, L, S, T, M, V, P, or C; Y67 replaced with D, E, H, K, R, N, Q, A,
G, I, L, S, T,
M, V, P, or C; Q68 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W,
Y, P, or C;
V69 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A70 replaced with D,
E, H, K,
R, N, Q, F, W, Y, P, or C; A71 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; L72
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q73 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, F, W, Y, P, or C; G74 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; D75 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; L76
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A77 replaced with D, E,
H, K, R~ N,
Q, F, W, Y, P, or C; S78 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
L79
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; R80 replaced with D, E,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; A81 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
E82 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L83
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q84 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, F, W, Y, P, or C; G85 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; H86
replaced with D, E, A, G, I, L, S, T,.M, V, N, Q, F, W, Y, P, or C; H87
replaced with D,
173
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; A88 replaced with D, E, H,
K, R, N, Q,
F, W, Y, P, or C; E89 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; K90 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L91
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; P92 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, or C; A93 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
G94 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A95 replaced with D,
E, H, K,
R, N, Q, F, W, Y, P, or C; G96 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; A97
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P98 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; K99 replaced with D, E, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, P, or C; A100 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; 6101
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L102 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; E103 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
o . or C; E104 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y,
P, or C; A105
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P106 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; A107 replaced with D, E, H, K, R, N,
Q, F, W,
Y, P, or C; V108 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T109
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; A110 replaced with D, E, H, K, R, N, Q,
F, W, Y,
P, or C; 6111 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L112
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; K113 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; I114 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
F115 replaced
with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; E116 replaced with
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; P117 replaced with D, E, H, K, R,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, or C; P118 replaced with D, E, H, K, R, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, or C; A119 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
CP120
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; 6121
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; E122 replaced with H, K, R, A, G,
I, L, S, T,
M, V, N, Q, F, W, Y, P, or C; 6123 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
N124 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
S125 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; S 126 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; Q127 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y,
P, or C;
N128 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
5129 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; 8130 replaced with D, E, A, G, I,
L, S, T, M,
174
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
V, N, Q, F, W, Y, P, or C; N131 replaced with D, E, H, K, R, A, G, I, L, S, T,
M, V, F, W,
Y, P, or C; K132 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; 8133
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; A134
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; V 135 replaced with D, E, H, K, R, N, Q,
F, W, Y, P, or
C; Q136 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
6137
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P138 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; E139 replaced with H, K, R, A, G, I,
L, S, T, M,
V, N, Q, F, W, Y, P, or C; E140 replaced with H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, P, or C; T141 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 6142
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; S 143 replaced with D, E, H, K, R, N,
Q, F, W, Y, P,
or C; Y144 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C;
T145
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F146 replaced with D, E,
H, K, R, N,
Q, A, G, I, L, S, T, M, V, P, or C; V 147 replaced with D, E, H, K, R, N, Q,
F, W, Y, P, or
C; P148 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or
C; W149
replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; L150
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; L151 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; 5152 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F153 replaced
with D, E, H,
K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; K154 replaced with D, E, A, G, I,
L, S, T, M,
V, N, Q, F, W, Y, P, or C; 8155 replaced with D, E, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; 6156 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 5157
replaced with D,
E, H, K, R., N, Q, F, W, Y, P, or C; A158 replaced With D, E, H, K, R, N, Q,
F, W, Y, P, or
C; L159 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; E160 replaced
with H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E161 replaced with H, K, R, A,
G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; K162 replaced with D, E, A, G, I, L, S, T, M,
V, N, Q, F,
W, Y, P, or C; E163 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W,
Y, P, or C;
N164 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
K165 replaced
with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; I166 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; L167 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; V168
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; K169 replaced with D, E,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, P, or C; E170 replaced with H, K, R, A, G, I, L, S,
T, M, V, N,
Q, F, W, Y, P, or C; T171 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
6172
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Y173 replaced with D, E,
H, K, R, N,
175
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Q, A, G, I, L, S, T, M, V, P, or C; F174 replaced with D, E, H, K, R, N, Q, A,
G, I, L, S,
T, M, V, P, or C; F175 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M,
V, P, or C;
I176 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Y177 replaced with
D, E, H, K,
R, N, Q, A, G, I, L, S, T, M, V, P, or C; 6178 replaced with D, E, H, K, R, N,
Q, F, W, Y,
P, or C; Q179 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P,
or C; V180
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L181 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; Y182 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T,
M, V, P, or
C; T183 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D184 replaced
with H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K185 replaced with D, E, A, G,
I, L, S, T,
M, V, N, Q, F, W, Y, P, or C; T186 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
Y187 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; A188
replaced
0
with D, E, H, K, R, N, Q, F, W, Y, P, or C; M189 replaced with D, E, H, K, R,
N, Q, F,
W, Y, P, or C; 6190 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; H191
replaced
with D, E, A~ G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L192 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; I193 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; Q194
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; 8195
replaced with
D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K196 replaced with D, E,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, P, or C; K197 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, .W, Y, P, or C; V198 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
H199
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; V200
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; F201 replaced with D, E, H, K, R, N, Q, A,
G, I, L, S,
T, M, V, P, or C; 6202 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
D203
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E204
replaced with
H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L205 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; 5206 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; L207
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V208 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; T209 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
L210
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F211 replaced with D, E,
H, K, R, N,
Q, A, G, I, L, S, T, M, V, P, or C; 8212 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; C213 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
or P; I214 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q215 replaced
with D, E,
H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; N216 replaced with D, E, H,
K, R, A, G,
176
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
I, L, S, T, M, V, F, W, Y, P, or C; M217 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; P218 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or
C; E219
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; T220
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; L221 replaced with D, E, H, K, R, N, Q,
F, W, Y,
P, or C; P222 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W,
Y, or C;
N223 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
N224 replaced
with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; 5225 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; 0226 replaced with D, E, H, K, R, A, G, I, L, S,
T, M, V, N,
Q, F, W, Y, or P; Y227 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M,
V, P, or C;
5228 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A229 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; 6230 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; I231
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A232 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; K233 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; L234 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; E235 replaced
with H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E236 replaced with H, K, R, A,
G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; 6237 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or
C; D238 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
E239
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L240
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; Q241 replaced with D, E, H, K, R, A, G,
I, L, S, T,
M, V, F, W, Y, P, or C; L242 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; A243
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; I244 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; P245 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, or C; 8246 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; E247
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; N248
replaced with
D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; A249 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; Q250 replaced with D, E, H, K, R, A, G, I, L, S, T, M,
V, F, W, Y,
P, or C; I251 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; S252
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; L253 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; D254 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
6255
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D256 replaced with H, K,
R, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; V257 replaced with D, E, H, K, R, N, Q,
F, W, Y,
P, or C; T258 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F259
replaced with D,
177
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; F260 replaced with D, E, H,
K, R, N, Q,
A, G, I, L, S, T, M, V, P, or C; 6261 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
A262 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L263 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; K264 replaced with D, E, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; L265 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; and/or L266
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C. Polynucleotides encoding these
polypeptides
are also encompassed by the invention. The resulting Neutrokine-alpha proteins
of the
invention may be routinely screened for Neutrokine-alpha and/or Neutrolcine-
alphaSV
functional activities and/or physical properties (such as, for example,
enhanced or reduced
stability and/or solubility) described throughout the specification and known
in the art.
Preferably, the resulting proteins of the invention have an increased andlor a
decreased
Neutrokine-alpha and/or Neutrokine-alphaSV functional activity. More
preferably, the
resulting Neutrokine-alpha and/or Neutrokine-alphaSV proteins of the invention
have
more than one increased and/or decreased Neutrokine-alpha and/or Neutrokine-
alphaSV
functional activity andlor physical property.
[0336] In an additional embodiment, Neutrokine-alpha polypeptides of the
invention
comprise, or alternatively consist of, more than one amino acid (e.g., 2, 3,
4, 5, 6, 7, 8, 9,
10, 15, 20, 30 and 50) replaced with the substituted amino acids as described
above (either
conservative or nonconservative).
[0337] For example, preferred non-conservative substitutions of the Neutrokine-
alpha
protein sequence provided in SEQ ID N0:23 include: R1 replaced with D, E, A,
G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; V2 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
V3 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D4~replaced with H, K,
R, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; L5 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; S6 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A7 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; P8 replaced with D, E, H, K, R, A, G, I, L, S, T,
M, V, N, Q, F,
W, Y, or C; P9 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, or C;
AlO replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P11 replaced with D,
E, H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; C12 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, or P; L13 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
P14 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C;
G15
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; C16 replaced with D, E,
H, K, R, A,
178
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
G, I, L, S, T, M, V, N, Q, F, W, Y, or P; R17 replaced with D, E, A, G, I, L,
S, T, M, V, N,
Q, F, W, Y, P, or C; H18 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; S 19 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q20 replaced with
D, E, H, K,
R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; H21 replaced with D, E, A, G, I,
L, S, T, M, V,
N, Q, F, W, Y, P, or C; D22 replaced with H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; D23 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; N24
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; G25
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; M26 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; N27 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or
C; L28
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; R29 replaced with D, E,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; N30 replaced with D, E, H, K, R, A, G, I, L,
S, T, M, V,
F, W, Y, P, or C; R31 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W,
Y, P, or C;
T32 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Y33 replaced with D,
E, H, K, R,
N, Q, A, G, h L, S, T, M, V, P, or C; T34 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; F35 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C;
V36 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; P37 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, or C; W38 replaced with D, E, H, K, R, N, Q, A, G, I,
L, S, T, M,
V, P, or C; L39 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L40
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; S41 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; F42 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; K43
replaced
with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; R44 replaced with
D, E, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; G45 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; N46 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or
C; A47
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L48 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; E49 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
or C; E50 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; K51
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E52
replaced with H,
K, R, A, G, I, L, S, T, M, V, N, Q, F, W; Y, P, or C; N53 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, F, W, Y, P, or C; K54 replaced with D, E, A, G, I, L, S,
T, M, V, N, Q,
F, W, Y, P, or C; I55 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V56
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; V57 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; R58 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; Q59
179
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; T60
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; G61 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; Y62 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; F63
replaced
with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; F64 replaced with
D, E, H, K, R,
N, Q, A, G, I, L, S, T, M, V, P, or C; I65 replaced with D, E, H, K, R, N, Q,
F, W, Y, P, or
C; Y66 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; S67
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q68 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, F, W, Y, P, or C; V69 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; L70
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Y71 replaced with D, E,
H, K, R, N,
Q, A, G, I, L, S, T, M, V, P, or C; T72 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; D73 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
P74 replaced
with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; I75 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; F76 replaced with D, E, H, K, R, N, Q, A, G, I,
L, S, T, M,
V, P, or C; A77 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; M78
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; G79 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; H80 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
V81 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; I82 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; Q83 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y,
P, or C; R84
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K85
replaced with D,
E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K86 replaced with D, E, A,
G, I, L, S, T,
M, V, N, Q, F, W, Y, P, or C; V87 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
H88 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; V89
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; F90 replaced with D, E, H, K, R, N, Q,
A, G, I, L,
S, T, M, V, P, or C; G91 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
D92
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E93
replaced with
H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L94 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; S95 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; L96
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V97 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; T98 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
L99
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F100 replaced with D, E,
H, K, R, N,
Q, A, G, I, L, S, T, M, V, P, or C; 8101 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; C 102 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W, Y,
180
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
or P; I103 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q104 replaced
with D, E,
H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; N105 replaced with D, E, H,
K, R, A, G,
I, L, S, T, M, V, F, W, Y, P, or C; M106 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; P107 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or
C; K108
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; T109
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; L110 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; P111 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or
C; N112
replaced with D, E, H, K, R, A, G, T, L, S, T, M, V, F, W, Y, P, or C; N113
replaced with
D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; S 114 replaced with
D, E, H, K, R,
N, Q, F, W, Y, P, or C; C115 replaced with D, E, H, K, R, A, G, I, L, S, T, M,
V, N, Q, F,
W, Y, or P; Y116 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P,
or C; 5117
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A118 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; 6119 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
I120
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; AI21 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; 8122 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; L123 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; E124 replaced
with H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E125 replaced with H, K, R, A,
G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; 6126 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or
C; D127 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
E128
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; I129
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; Q130 replaced with D, E, H, K, R, A, G,
I, L, S, T,
M, V, F, W, Y, P, or C; L131 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; A132
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; I133 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; P134 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, or C; 8135 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; E136
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; N137
replaced with
D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; A138 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; Q139 replaced with D, E, H, K, R, A, G, I, L, S, T, M,
V, F, W, Y,
P, or C; I140 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 5141
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; 8142 replaced with D, E, A, G, I, L, S, T,
M, V, N, Q,
F, W, Y, P, or C; N143 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F,
W, Y, P, or
C; 6144 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D145 replaced
with H, K, R,
181
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; D146 replaced with H, K, R, A,
G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; T147 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or
C; F148 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C;
F149 replaced
with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; 6150 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; A151 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; L152
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; K153 replaced with D, E,
A, G, I, L,
S, T~ M, V, N, Q, F, W, Y, P, or C; L154 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; andlor L155 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C.
Polynucleotides
encoding these polypeptides are also encompassed by the invention. The
resulting
Neutrokine-alpha proteins of the invention may be routinely screened for
Neutrokine-
alpha and/or Neutrokine-alphaSV functional activities and/or physical
properties (such as,
for example, enhanced or reduced stability andlor solubility) described
throughout the
specification and known in the art. Preferably, the resulting proteins of the
invention have
an increased and/or a decreased Neutrokine-alpha and/or Neutrokine-alphaSV
functional
activity. More preferably, the resulting Neutrokine-alpha and/or Neutrokine-
alphaSV
proteins of the invention have more than one increased andlor decreased
Neutrokine-alpha
and/or Neutrokine-alphaSV functional activity and/or physical property.
[0338] In an additional embodiment, Neutrokine-alpha polypeptides of the
invention
comprise, or alternatively consist of, more than one amino acid (e.g., 2, 3,
4, 5, 6, 7, 8, 9,
10, 15, 20, 30 and 50) replaced with the substituted amino acids as described
above (either
conservative or nonconservative).
[0339] For example, preferred non-conservative substitutions of the Neutrokine-
alpha
protein sequence provided in SEQ ID N0:38 include: M1 replaced with D, E, H,
K, R, N,
Q, F, W, Y, P, or C; D2 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P, or
C; E3 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
S4 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; A5 replaced with D, E, H, K, R, N,
Q, F, W,
Y, P, or C; K6 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; T7
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L8 replaced with D, E, H,
K, R, N, Q,
F, W, Y, P, or C; P9 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y, or
C; P10 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or
C; P11
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; C12
replaced
with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P; L13 replaced
with D, E, H,
1s2
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
K, R, N, Q, F, W, Y, P, or C; C14 replaced with D, E, H, K, R, A, G, I, L, S,
T, M, V, N,
Q, F, W, Y, or P; F15 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M,
V, P, or C;
C16 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P;
S17 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; E18 replaced with H, K, R, A, G,
I, L, S, T, M,
V, N, Q, F, W, Y, P, or C; K19 replaced with D, E, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; G20 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; E21 replaced
with H, K,
R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; D22 replaced with H, K, R,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; M23 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
K24 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; V25
replaced with
D; E, H, K, R, N, Q, F, W, Y, P, or C; G26 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; Y27 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C;
D28 replaced
with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; P29 replaced
with D, E, H, K,
R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; I30 replaced with D, E, H, K,
R, N, Q, F,
W, Y, P, or C; T31 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P32
replaced with
D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; Q33 replaced with
D, E, H, K,
R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; K34 replaced with D, E, A, G, I,
L, S, T, M, V,
N, Q, F, W, Y, P, or C; E35 replaced with H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; E36 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; G37
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A38 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; W39 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T,
M, V, P, or
C; F40 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; G41
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; I42 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; C43 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, or P;
R44 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; D45
replaced with
H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; G46 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; R47 replaced with D, E, A, G, I, L, S, T, M, V, N, Q,
F, W, Y, P,
or C; L48 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L49 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; A50 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
A51 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T52 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; L53 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; L54
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L55 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; A56 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
L57
183
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L58 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; S59 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
S60
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 561 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; F62 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T,
M, V, P, or
C; T63 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A64 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; M65 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; S66
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L67 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; Y68 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T,
M, V, P, or
C; Q69 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
L70 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; A71 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; A72 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L73
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; Q74 replaced with D, E, H, K, R, A, G, I,
L, S, T, M,
V, F, W, Y, P, or C; A75 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
D76
replaced with H, K, R, A, G, I, L, 5, T, M, V, N, Q, F, W, Y, P, or C; L77
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; M78 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; N79 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or
C; L80
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; R81 replaced with D, E,
A, G, I, L, S,
T, M, V, N, Q, F, W, Y, P, or C; M82 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
E83 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L84
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q85 replaced with D, E, H, K, R,
A, G, I, L, S,
T, M, V, F, W, Y, P, or C; S86 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; Y87
replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; R88
replaced with D, E,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; G89 replaced with D, E, H, K,
R, N, Q, F,
W, Y, P, or C; S90 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A91
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; T92 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; P93 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y,
or C; A94
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A95 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; A96 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
G97
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A98 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; P99 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, or C; E100 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; L101
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T102 replaced with D, E,
H, K, R, N,
184
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Q, F, W, Y, P, or C; A103 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
6104
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V 105 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; K106 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; L107 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L108 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; T109 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
P110 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C;
A111
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A112 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; P113 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, or C; 8114 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; P115
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; H116
replaced
with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; N117 replaced with
D, E, H, K,
R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; S 118 replaced with D, E, H, K,
R, N, Q, F, W,
Y, P, or C; S 119 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 8120
replaced with
D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; 6121 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; H122 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; 8123 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
N124 replaced
with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; 8125 replaced
with D, E, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; 8126 replaced with D, E, A, G, I,
L, S, T, M,
V, N, Q, F, W, Y, P, or C; A127 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; F128
replaced with D, E, H, K, R, N, Q,~A, G, I, L, S, T, M, V, P, or C; Q129
replaced with D,
E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; 6130 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; P131 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
Y, or C; E132 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; E133
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; T134
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; E135 replaced with H, K, R, A, G, I,~L,
S, T, M, V,
N, Q, F, W, Y, P, or C; Q136 replaced with D, E, H, K, R, A, G, I, L, S, T, M,
V, F, W, Y,
P, or C; D137 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; V138
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D139 replaced with H, K,
R, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; L140 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; S141 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A142 replaced
with D, E,
H, K, R, N, Q, F, W, Y, P, or C; P143 replaced with D, E, H, K, R, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, or C; P144 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V,
N, Q, F, W,
185
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Y, or C; A145 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P146
replaced with D,
E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; C147 replaced with D,
E, H, K, R,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P; L148 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; P149 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, or C;
6150 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; C151 replaced with
D, E, H, K,
R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P; 8152 replaced with D, E, A, G,
I, L, S, T,
M, V, N, Q, F, W, Y, P, or C; H153 replaced with D, E, A, G, I, L, S, T, M, V,
N, Q, F,
W, Y, P, or C; S 154 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q155
replaced
with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; H156 replaced
with D, E, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; D 157 replaced with H, K, R, A,
G, I, L, S, T,
M, V, N, Q, F, W, Y, P, or C; D158 replaced with H, K, R, A, G, I, L, S, T, M,
V, N, Q, F,
W, Y, P, or C; N159 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W,
Y, P, or C;
6160 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; M161 replaced with
D, E, H,
K, R, N, Q, F, W, Y, P, or C; N162 replaced with D, E, H, K, R, A, G, I, L, S,
T, M, V, F,
W, Y, P, or C; L163 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 8164
replaced
with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; N165 replaced with
D, E, H, K,
R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; I166 replaced with D, E, H, K, R,
N, Q, F, W,
Y~ P, or C; I167 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q168
replaced with
D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; D 169 replaced with
H, K, R, A, G,
I, L, S, T, M, V, N, Q, F, W, Y, P, or C; C170 replaced with D, E, H, K, R, A,
G, I, L, S,
T, M, V, N, Q, F; W, Y, or P; L171 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
Q172 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C;
L173 replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; I174 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C; A175 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; D176
replaced with
H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; S 177 replaced with
D, E, H, K, R,
N, Q; F, W, Y, P, or C; D178 replaced with H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; T179 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P180
replaced with D,
E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; A181 replaced with D,
E, H, K, R,
N, Q, F, W, Y, P, or C; L182 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or
C; E183
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; E184
replaced with
H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K185 replaced with D,
E, A, G, I,
L, S, T, M, V, N, Q, F, W, Y, P, or C; E186 replaced with H, K, R, A, G, I, L,
S, T, M, V,
186
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
N, Q, F, W, Y, P, or C; N187 replaced with D, E, H, K, R, A, G, I, L, S, T, M,
V, F, W, Y,
P, or C; K188 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or
C; I189
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; V 190 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; V 191 replaced with D, E, H, . K, R, N, Q, F, W, Y, P, or
C; 8192
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; Q193
replaced with D,
E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; T194 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; 6195 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
Y196
replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; F197
replaced with D,
E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; F198 replaced with D, E, H,
K, R, N, Q,
A, G, I, L, S, T, M, V, P, or C; I199 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
Y200 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; S201
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q202 replaced with D, E, H, K, R,
A, G, I, L,
S, T, M, V, F, W, Y, P, or C; V203 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
L204 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Y205 replaced with
D, E, H, K,
R, N, Q, A, G, I, L, S, T, M, V, P, or C; T206 replaced with D, E, H, K, R, N,
Q, F, W, Y,
P, or C; D207 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P,
or C; P208
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or C; I209
replaced
with D, E, H, K, R, N, Q, F, W, Y, P, or C; F210 replaced with D, E, H, K, R,
N, Q, A, G,
I, L, S, T, M, V, P, or C; A211 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; M212
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 6213 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; H214 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; V215 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; I216 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; Q217 replaced with D, E, H, K, R, A, G, I, L, S,
T, M, V, F,
W, Y, P, or C; 8218 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y,
P, or C;
K219 replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; K220
replaced
with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; V221 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; H222 replaced with D, E, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; V223 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; F224
replaced with D,
E, H, K, R, N, Q, A, G, I, L, 5, T, M, V, P, or C; 6225 replaced with D, E, H,
K, R, N, Q,
F, W, Y, P, or C; D226 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F,
W, Y, P, or
C; E227 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
L228
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 5229 replaced with D, E,
H, K, R, N,
1s7
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Q, F, W, Y, P, or C; L230 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
V231
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; T232 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; L233 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
F234
replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P, or C; 8235
replaced with D,
E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; C236 replaced with D, E, H,
K, R, A, G,
I, L, S, T, M, V, N, Q, F, W, Y, or P; I237 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; Q238 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or
C; N239
replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; M240
replaced with
D, E; H, K, R, N, Q, F, W, Y, P, or C; P241 replaced with D, E, H, K, R, A, G,
I, L, S, T,
M, V, N, Q, F, W, Y, or C; K242 replaced with D, E, A, G, I, L, S, T, M, V, N,
Q, F, W,
Y, P, or C; T243 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; L244
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; P245 replaced with D, E, H, K, R, A, G,
I, L, S, T,
M, V, N, Q, F, W, Y, or C; N246 replaced with D, E, H, K, R, A, G, I, L, S, T,
M, V, F,
W, Y, P, or C; N247 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W,
Y, P, or C;
5248 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or~C; C249 replaced with
D, E, H, K,
R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, or P; Y250 replaced with D, E, H, K,
R, N, Q, A,
G, I, L, S, T, M, V, P, or C; S251 replaced with D, E, H, K, R, N, Q, F, W, Y,
P, or C;
A252 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 6253 replaced with
D, E, H, K,
R, N, Q, F, W, Y, P, or C; I254 replaced with D, E, H, K, R, N, Q, F, W, Y, P,
or C; A255
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 8256 replaced with D, E,
A, G, I, L,
S, T, M, V, N, Q, F, W, Y, P, or C; L257 replaced with D, E, H, K, R, N, Q, F,
W, Y, P, or
C; E258 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
E259
replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; 6260
replaced with
D, E, H, K, R, N, Q, F, W, Y, P, or C; D261 replaced with H, K, R, A, G, I, L,
S, T, M, V,'
N, Q, F, W, Y, P, or C; E262 replaced with H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; I263 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q264
replaced with D,
E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P, or C; L265 replaced with D, E,
H, K, R, N,
Q, F; W, Y, P, or C; A266 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
I267
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; P268 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, N, Q, F, W, Y, or C; 8269 replaced with D, E, A, G, I, L,
S, T, M, V,
N, Q, F, W, Y, P, or C; E270 replaced with H, K, R, A, G, I, L, S, T, M, V, N,
Q, F, W, Y,
P, or C; N271 replaced with D, E, H, K, R, A, G, I, L, S, T, M, V, F, W, Y, P,
or C; A272
188
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; Q273 replaced with D, E,
H, K, R, A,
G, I, L, S, T, M, V, F, W, Y, P, or C; I274 replaced with D, E, H, K, R, N, Q,
F, W, Y, P,
or C; S275 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; 8276 replaced
with D, E,
A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; N277 replaced with D, E, H, K,
R, A, G, I,
L, S, T, M, V, F, W, Y, P, or C; 6278 replaced with D, E, H, K, R, N, Q, F, W,
Y, P, or C;
D279 replaced with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C;
D280 replaced
with H, K, R, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; T281 replaced
with D, E, H,
K, R, N, Q, F, W, Y, P, or C; F282 replaced with D, E, H, K, R, N, Q, A, G, I,
L, S, T, M,
V, P, or C; F283 replaced with D, E, H, K, R, N, Q, A, G, I, L, S, T, M, V, P,
or C; 6284
replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C; A285 replaced with D, E,
H, K, R, N,
Q, F, W, Y, P, or C; L286 replaced with D, E, H, K, R, N, Q, F, W, Y, P, or C;
K287
replaced with D, E, A, G, I, L, S, T, M, V, N, Q, F, W, Y, P, or C; L288
replaced with D,
E, H, K, R, N, Q, F, W, Y, P, or C; and/or L289 replaced with D, E, H, K, R,
N, Q, F, W,
Y, P, or C. Polynucleotides encoding these polypeptides are also encompassed
by the
invention. The resulting Neutrokine-alpha proteins of the invention may be
routinely
screened for Neutrokine-alpha and/or Neutrokine-alphaSV functional activities
and/or
physical properties (such as, for example, enhanced or reduced stability
andlor solubility)
described throughout the specification and known in the art. Preferably, the
resulting
proteins of the invention have an increased and/or a decreased Neutrokine-
alpha and/or
Neutrokine-alphaSV functional activity. More preferably, the resulting
Neutrokine-alpha
and/or Neutrokine-alphaSV proteins of the invention have more than one
increased andlor
decreased Neutrokine-alpha and/or Neutrokine-alphaSV functional activity
and/or
physical property.
[0340] In an additional embodiment, Neutrokine-alpha polypeptides of the
invention
comprise, or alternatively consist of, more than one amino acid (e.g., 2, 3,
4, 5, 6, 7, 8, 9,
10, 15, 20, 30 and 50) replaced with the substituted amino acids as described
above (either
conservative or nonconservative).
[0341] Replacement of amino acids can also change the selectivity of the
binding of a
ligand to cell surface receptors. For example, Ostade et al., Nature 361:266-
268 (1993)
describes certain mutations resulting in selective binding of TNF-alpha to
only one of the
two known types of TNF receptors. Since Neutrokine-alpha and Neutrokine-
alphaSV are
189
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
members of the TNF polypeptide family, mutations similar to those in TNF-alpha
are
likely to have similar effects in Neutrokine-alpha and/or Neutrolcine-alphaSV.
[0342] Sites that are critical for ligand-receptor binding can also be
determined by
structural analysis such as crystallization, nuclear magnetic resonance or
photoaffinity
labeling (Smith et al., J. Mol. Biol. 224:899-904 (1992) and de Vos et al.
ScieTZCe
255:306-312 (1992)).
[0343] Since Neutrokine-alpha is a member of the TNF-related protein family,
to
modulate rather than completely eliminate functional activities (e.g.,
biological activities)
of Neutrokine-alpha, mutations may be made in sequences encoding amino acids
in the
TNF conserved domain, i.e., in positions Gly-191 through Leu-284 of Figures 1A
and 1B
(SEQ ID N0:2), more preferably in residues within this region which are not
conserved in
all, most or several members of the TNF family (e.g., TNF-alpha, TNF-beta, LT-
beta, and
Fas Ligand) (see e.g., Figures 2A, 2B, 2C, and 2D). By making a specific
mutation in
Neutrokine-alpha in the position where such a conserved amino acid is
typically found in
related TNFs, the Neutrokine-alpha mutein will act as an antagonist, thus
possessing
activity for example, which inhibits lymphocyte (e.g., B cell) proliferation,
differentiation,
and/or activation. Accordingly, polypeptides of the present invention include
Neutrokine-alpha mutants. Such Neutrokine-alpha mutants comprise, or
alternatively
consist of, fragments, variants or derivatives of the full-length or
preferably the
extracellular domain of the Neutrokine-alpha amino acid sequence shown in
Figures 1A
and 1B (SEQ 1D N0:2). Polynucleotides encoding the above Neutrokine-alpha
mutants
are also encompassed by the invention.
[0344] Since Neutrokine-alphaSV is a member of the TNF-related protein family,
to
modulate rather than completely eliminate functional activities (e.g.,
biological activities)
of Neutrokine-alphaSV, mutations may be made in sequences encoding amino acids
in the
TNF conserved domain, i.e., in positions Gly-172 through Leu-265 of Figures 5A
and 5B
(SEQ m N0:19), more preferably in residues within this region which are not
conserved
in all, most or several members of the TNF family (e.g., TNF-alpha, TNF-beta,
LT-beta,
and Fas Ligand) (see e.g., Figures 2A 2B, 2C and 2D). By making a specific
mutation in
Neutrokine-alphaSV in the position where such a conserved amino acid is
typically found
in related TNFs, the Neutrokine-alphaSV mutein will act as an antagonist, thus
possessing
activity for example, which inhibits lymphocyte (e.g., B cell) proliferation,
differentiation,
190
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
and/or activation. Accordingly, polypeptides of the present invention include
Neutrokine-alphaSV mutants. Such Neutrolcine-alphaSV mutants comprise, or
alternatively consist of, fragments, variants or derivatives of the full-
length or preferably
the extracellular domain of the Neutrokine-alphaSV amino acid sequence shown
in
Figures 5A and 5B (SEQ ID N0:19 Polynucleotides encoding the above
Neutrokine-alpha SV mutants are also encompassed by the invention.
[0345] In addition, it will be recognized by one of ordinary skill in the art
that
mutations targeted to regions of a Neutrokine-alpha polypeptide of the
invention which
encompass the nineteen amino acid residue insertion which is not found in the
Neutrokine-alphaSV polypeptide sequence (i.e., amino acid residues Val-142
through
Lys-160 of the sequence presented in Figures 1A and 1B and in SEQ ID N0:2) may
affect
the observed functional activities (e.g., biological activity) of the
Neutrokine-alpha
polypeptide. More specifically, a partial, non-limiting and non-exclusive list
of such
residues of the Neutrokine-alpha polypeptide sequence which may be targeted
for
mutation includes the following amino acid residues of the Neutrokine-alpha
polypeptide
sequence as shown in SEQ ID NO:2: V-142; T-143; Q-144; D-145; C-146; L-147;
Q-148; L-149; I-150; A-151; D-152; S-153; E-154; T-155; P-156; T-157; I-158; Q-
159;
and K-160.
[0346] Recombinant DNA technology known to those skilled in the art (see, for
instance, DNA shuffling supra) can be used to create novel mutant proteins or
muteins
including single or multiple amino acid substitutions, deletions, additions or
fusion
proteins. Such modified polypeptides can show, e.g., enhanced activity or
increased
stability. In addition, they may be purified in higher yields and show better
solubility than
the corresponding natural polypeptide, at least under certain purification and
storage
conditions.
[0347] Thus, the invention also encompasses Neutrokine-alpha and/or
Neutrokine-alphaSV derivatives and analogs that have one or more amino acid
residues
deleted, added, or substituted to generate Neutrokine-alpha and/or Neutrokine-
alphaSV
polypeptides that are better suited for expression, scale up, etc., in the
host cells chosen.
For example, cysteine residues can be deleted or substituted with another
amino acid
residue in order to eliminate disulfide bridges; N-linked glycosylation sites
can be altered
or eliminated to achieve, for example, expression of a homogeneous product
that is more
191
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
easily recovered and purified from yeast hosts which are known to
hyperglycosylate N-
linked sites. To this end, a variety of amino acid substitutions at one or
both of the first or
third' amino acid positions on any one or more of the glycosylation
recognitions sequences
in the Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides of the
invention, andlor
an amino acid deletion at the second position of any one or more such
recognition
sequences will prevent glycosylation of the Neutrokine-alpha andlor Neutrokine-
alphaSV
at the modified tripeptide sequence (see, e.g., Miyajimo et al., EMBO J
5(6):1193-1197).
By way of non-limiting example, mutation of the serine at position 244 to
alanine either
singly or in combination with mutation of the asparagine at position 242 to
glutamine
abolishes glycosylation of the mature soluble form of Neutrokine-alpha (amino
acids 134-
285) of SEQ ID N0:2) when expressed in the yeast Pichea pastoris. A mutant
Neutrokine-alpha polypeptide in which only the asparagine at position 242 is
mutated to
glutamine, is still gycosylated when expressed in Pichea pastoris. In this
mutant, the
glycosylation event may be due to the activation or unmasking ' of an O-linked
glyscosylation site at serine 244. Similar mutations affecting glycosylation
could also be
made in Neutrokine alpha-SV polypeptide, i.e., aspargine-223 to glutamine
and/or serine-
224 to alanine of SEQ ID NO:19.
[0348] Additionally, one or more of the amino acid residues of the
polypeptides of the
invention (e.g., arginine and lysine residues) may be deleted or substituted
with another
residue to elminate undesired processing by proteases such as, for example,
furins or
kexins. One possible result of such a mutation is that Neutrokine-alpha
polypeptide of the
invention is not cleaved and released from the cell surface.
[0349] In a specific embodiment, Lys-132 and/or Arg-133 of the Neutrokine-
alpha
sequence shown in SEQ ID N0:2 is mutated to another amino acid residue, or
deleted
altogether, to prevent or diminish release of the soluble form of Neutrokine-
alpha from
cells expressing Neutrokine-alpha. In a more specific embodiment, Lys-132 of
the
Neutrokine-alpha sequence shown in SEQ ID N0:2 is mutated to Ala-132. In
another,
nonexclusive specific embodiment, Arg-133 of the Neutrokine-alpha sequence
shown in
SEQ ID N0:2 is mutated to Ala-133. These mutatied proteins, and/or
polynucleotides
encoding these proteins have uses such as, for example, in ex vivo therapy or
gene
therapy, to engineer cells expressing a Neutrokine-alpha polypepitde that is
retained on the
surface of the engineered cells.
192
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0350] In a specific embodiment, Cys-146 of the Neutrolcine-alpha sequence
shown in
SEQ ID N0:2 is mutated to another amino acid residue, or deleted altogether,
for
example, to aid preventing or diminishing oligomerization of the mutant
Neutrokine-alpha
polypeptide when expressed in an expression system (essentially as described
in Example
1). In a specific embodiment, Cys-146 is replaced with a serine amino acid
residue.
Polynucleotides encoding these polypeptides are also encompassed by the
invention.
[0351] In another specific embodiment, Cys-232 of the Neutrokine-alpha
sequence
shown in SEQ ID N0:2 is mutated to another amino acid residue, or deleted
altogether,
for example, to aid preventing or diminishing oligomerization of the mutant
Neutrokine-
alpha polypeptide when expressed in an expression system (essentially as
described in
Example 1). In a specific embodiment, Cys-232 is replaced with a serine amino
acid
residue. Polynucleotides encoding these polypeptides are also encompassed by
the
invention.
[0352] In yet another specific embodiment, Cys-245 of the Neutrokine-alpha
sequence
shown in SEQ ID N0:2 is mutated to another amino acid residue, or deleted
altogether,
for example, to aid preventing or diminishing oligomerization of the mutant
Neutrokine-
alpha polypeptide when expressed in an expression system (essentially as
described in
Example 1). In a specific embodiment, Cys-245 is replaced with a serine amino
acid
residue. Polynucleotides encoding these polypepti,des are also encompassed by
the
invention.
[0353] The polypeptides of the present invention are preferably provided in an
isolated
form, and preferably are substantially purified. A recombinantly produced
version of the
Neutrokine-alpha andlor Neutrokine-alphaSV polypeptides can be substantially
purified
by the one-step method described in Smith and Johnson, Gene 67:31-40 (1988).
[0354] The polypeptides of the present invention include the complete
polypeptide
encoded by the deposited cDNA (ATCC Deposit No. 97768) including the
intracellular,
transmembrane and extracellular domains of the polypeptide encoded by the
deposited
cDNA, the mature soluble polypeptide encoded by the deposited cDNA, the
extracellular
domain minus the intracellular and transmembrane domains of the protein, the
complete
polypeptide of Figures 1A and 1B (amino acid residues 1-285 of SEQ ID N0:2),
the
mature soluble polypeptide of Figures 1A and 1B (amino acids 134-285 of SEQ ID
N0:2),
the extracellular domain of Figures 1A and 1B (amino acid residues 73-285 of
SEQ ID
193
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
N0:2) minus the intracellular and transmembrane domains, as well as
polypeptides which
have at least 80%, 85%, 90% similarity, more preferably at least 95%
similarity, and still
more preferably at least 96%, 97%, 98% or 99% similarity to those described
above.
Polynucleotides encoding these polypeptides are also encompassed by the
invention.
[0355] The polypeptides of the present invention also include the complete
polypeptide encoded by the deposited cDNA including the intracellular,
transmembrane
and extracellular domains of the polypeptide encoded by the deposited cDNA
(ATCC
Deposit No. 203518), the mature soluble polypeptide encoded by the deposited
cDNA, the
extracellular domain minus the intracellular and transmembrane domains of the
protein,
the complete polypeptide of Figures 5A and 5B (amino acid residues 1-266 of
SEQ ID
N0:19), the mature soluble polypeptide of Figures 5A and 5B (amino acid
residues
134-266 of SEQ >I~ N0:19), the extracellular domain of Figures 5A and 5B
(amino acid
residues 73-266 of SEQ ID N0:19) minus the intracellular and transmembrane
domains,
as well as polypeptides which have at least 80%, 85%, 90% similarity, more
preferably at
least 95% similarity, and still more preferably at least 96%, 97%, 98% or 99%
similarity
to those described above. Polynucleotides encoding these polypeptides are also
encompassed by the invention.
[0356] Further polypeptides of the present invention include polypeptides at
least
80%, or at least 85% identical, more preferably at least 90% or 95% identical,
still more
preferably at least 96%, 97%, 98% or 99% identical to the polypeptide encoded
by the
deposited cDNA (ATCC Deposit No. 97768) or to the polypeptide of Figures 1A
and 1B
(SEQ ID N0:2), and also include portions of such polypeptides with at least 30
aiilino
acids and more preferably at least 50 amino acids. Polynucleotides encoding
these
polypeptides are also encompassed by the invention.
[0357] Further polypeptides of the present invention include polypeptides at
least
80%, or at least 85% identical, more preferably at least 90% or 95% identical,
still more
preferably at least 96%, 97%, 98% or 99% identical to the polypeptide encoded
by the
deposited eDNA (ATCC Deposit No. 203518) or to the polypeptide of Figures 5A
and 5B
(SEQ ID N0:19), and also include portions of such polypeptides with at least
30 amino
acids and more preferably at least 50 amino acids. Polynucleotides encoding
these
polypeptides are also encompassed by the invention.
194
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0358] By "% similarity" for two polypeptides is intended a similarity score
produced
by comparing the amino acid sequences of the two polypeptides using the
Bestfit program
(Wisconsin Sequence Analysis Package, Version 8 for Unix, Genetics Computer
Group,
University Research Park, 575 Science Drive, Madison, WI 53711) and the
default
settings for determining similarity. Bestfit uses the local homology algorithm
of Smith
and Waterman (Advances in Applied Mathematics 2:482-489, 1981) to find the
best
segment of similarity between two sequences.
[0359] By a polypeptide having an amino acid sequence at least, for example,
95%
"identical" to a reference amino acid sequence of a Neutrokine-alpha and/or
Neutrokine-alphaSV polypeptide is intended that the amino acid sequence of the
polypeptide is identical to the reference sequence except that the polypeptide
sequence
may include up to five amino acid alterations per each 100 amino acids of the
reference
amino acid of the Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide. In
other
words, to obtain a polypeptide having an amino acid sequence at least 95%
identical to a
reference amino acid sequence, up to 5% of the amino acid residues in the
reference
sequence may be deleted or substituted with another amino acid, or a number of
amino
acids up to 5% of the total amino acid residues in the reference sequence may
be inserted
into the reference sequence. These alterations of the reference sequence may
occur at the
amino or carboxy terminal positions of the reference amino acid sequence or
anywhere
between those terminal positions, interspersed either individually among
residues in the
reference sequence or in one or more contiguous groups within the reference
sequence.
[0360] As a practical matter, whether any particular polypeptide is at least
80%, 85%,
90%, 95%, 96%, 97%, 98% or 99% identical to, for instance, the amino acid
sequence
shown in Figures 1A and 1B (SEQ ID N0:2), the amino acid sequence encoded by
the
deposited cDNA clone HNEDU15 (ATCC Accession No. 97768), or fragments thereof,
or, for instance, to the amino acid sequence shown in Figures 5A and 5B (SEQ
ID
N0:19), the amino acid sequence encoded by the deposited cDNA clone HDPMC52
(ATCC Accession No. 203518), or fragments thereof, can be determined
conventionally
using known computer programs such the Bestfit program (Wisconsin Sequence
Analysis
Package, Version 8 for Unix, Genetics Computer Group, University Research
Park, 575
Science Drive, Madison, WI 53711). When using Bestfit or any other sequence
alignment
program to determine whether a particular sequence is, for instance, 95%
identical to a
195
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
reference sequence according to the present invention, the parameters are set,
of course,
such that the percentage of identity is calculated over the full length of the
reference
amino acid sequence and that gaps in homology of up to 5°70 of the
total number of amino
acid residues in the reference sequence are allowed.
[0361] In a specific embodiment, the identity between a reference (query)
sequence (a
sequence of the present invention) and a subject sequence, also referred to as
a global
sequence alignment, is determined using the FASTDB computer program based on
the
algorithm of Brutlag et al. (Comp. App. Biosci. 6:237-245 (1990)). Preferred
parameters
used in a FASTDB amino acid alignment are: Matrix=PAM 0, k-tuple=2, Mismatch
Penalty=1, Joining Penalty=20, Randomization Group Length=0, Cutoff Score=1,
Window Size=sequence length, Gap Penalty=5, Gap Size Penalty=0.05, Window
Size=500 or the length of the subject amino acid sequence, whichever is
shorter.
According to this embodiment, if the subject sequence is shorter than the
query sequence
due to N- or C-terminal deletions, not because of internal deletions, a manual
correction is
made to the results to take into consideration the fact that the FASTDB
program does not
account for N- and C-terminal truncations of the subject sequence when
calculating global
percent identity. For subject sequences truncated at the N- and C-termini,
relative to the
query sequence, the percent identity is corrected by calculating the number of
residues of
the query sequence that are N- and C-terminal of the subject sequence, which
are not
matched/aligned with a corresponding subject residue, as a percent of the
total bases of the
query sequence. A determination of whether a residue is matched/aligned is
determined
by results of the FASTDB sequence alignment. This percentage is then
subtracted from
the percent identity, calculated by the above FASTDB program using the
specified
parameters, to arrive at a final percent identity score. This final percent
identity score is
what is used for the purposes of this embodiment. Only residues to the N- and
C-termini
of the subject sequence, which are not matched/aligned with the query
sequence, are
considered for the purposes of manually adjusting the percent identity score.
That is, only
query residue positions outside the farthest N- and C-terminal residues of the
subject
sequence. For example, a 90 amino acid residue subject sequence is aligned
with a 100
residue query sequence to determine percent identity. The deletion occurs at
the
N-terminus of the subject sequence and therefore, the FASTDB alignment does
not show a
matching/alignment of the first 10 residues at the N-terminus. The 10 unpaired
residues
196
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
represent 10% of the sequence (number of residues at the N- and C-termini not
matched/total number of residues in the query sequence) so 10% is subtracted
from the
percent identity score calculated by the FASTDB program. If the remaining 90
residues
were perfectly matched the final percent identity would be 90%. In another
example, a 90
residue subject sequence is compared with a 100 residue query sequence. This
time the
deletions are internal deletions so there are no residues at the N- or C-
termini of the
subject sequence which are not matched/aligned with the query. In this case
the percent
identity calculated by FASTDB is not manually corrected. Once again, only
residue
positions outside the N- and C-terminal ends of the subject sequence, as
displayed in the
FASTDB alignment, which are not matched/aligned with the query sequence are
manually
corrected for. No other manual corrections are made for the purposes of this
embodiment.
[0362] The polypeptides of the present invention have uses that include, but
are not
limited to, as a molecular weight marker on SDS-PAGE gels or on molecular
sieve gel
filtration columns using methods well know n to those skilled in the art.
Additionally, as
described in detail below, the polypeptides of the present invention have uses
that include,
but are not limited to, raising polyclonal and monoclonal antibodies, which
are useful in
assays for detecting Neutrokine-alpha and/or Neutrokine-alphaSV polypeptide
expression
as described below or as agonists and antagonists capable of enhancing or
inhibiting
Neutrokine-alpha andlor Neutrokine-alphaSV function. The polypeptides of the
invention
also have therapeutic uses as described herein. Further, such polypeptides can
be used in
the yeast two-hybrid system to "capture" Neutrokine-alpha and/or Neutrokine-
alphaSV
binding proteins which are also candidate agonists and antagonists according
to the
present invention. The yeast two hybrid system is described in Fields and
Song, Nature
340:245-246 (1989).
Tz-ansgenics azzd "kzzock-outs"
[0363] The polypeptides of the invention can also be expressed in transgenic
animals.
Animals of any species, including, but not limited to, mice, rats, rabbits,
hamsters, guinea
pigs, pigs, micro-pigs, goats, sheep, cows and non-human primates, e.g.,
baboons,
monkeys, and chimpanzees may be used to generate transgenic animals. In a
specific
embodiment, techniques described herein or otherwise known in the art, are
used to
express polypeptides of the invention in humans, as part of a gene therapy
protocol.
197
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0364] Any technique known in the art may be used to introduce the transgene
(i.e.,
polynucleotides of the invention) into animals to produce the founder lines of
transgenic
animals. Such techniques include, but are not limited to, pronuclear
microinjection
(Paterson, et al., Appl. Microbiol. .Biotechf2ol. 40:691-698 (1994); Carver et
al.,
Biotechnology (NY) 11:1263-1270 (1993); Wright et al., Biotechnology (NY)
9:830-834
(1991); and Hoppe et al., U.S. Pat. No. 4,873,191 (1989)); retrovirus mediated
gene
transfer into germ lines (Van der Putten et al., Proc. Natl. Acad. Sci., USA
82:6148-6152
(1985)), blastocysts or embryos; gene targeting in embryonic stem cells
(Thompson et al.,
Cell 56:313-321 (1989)); electroporation of cells or embryos (Lo, 1983, Mol
Cell. Biol.
3:1803-1814 (1983)); introduction of the polynucleotides of the invention
using a gene
gun (see, e.g., Ulmer et al., Science 259:1745 (1993); introducing nucleic
acid constructs
into embryonic pleuripotent stem cells and transferring the stem cells back
into the
blastocyst; and sperm-mediated gene transfer (Lavitrano et al., Cell 57:717-
723 (1989);
etc. For a review of such techniques, see Gordon, "Transgenic Animals," Intl.
Rev. Cytol.
115:171-229 (1989), which is incorporated by reference herein in its entirety.
See also,
U.S. Patent No. 5,464,764 (Capecchi, et al., Positive-Negative Selection
Methods and
Vectors); U.S. Patent No. 5,631,153 (Capecchi, et al., Cells and Non-Human
Organisms
Containing Predetermined Genomic Modifications and Positive-Negative Selection
Methods and Vectors for Making Same); U.S. Patent No. 4,736,866 (Leder, et
al.,
Transgenic Non-Human Animals); and U.S. Patent No. 4,873,191 (Wagner, et al.,
Genetic
Transformation of Zygotes); each of which is hereby incorporated by reference
in its
entirety.
[0365] Any technique known in the art may be used to produce transgenic clones
containing polynucleotides of the invention, for example, nuclear transfer
into enucleated
oocytes of nuclei from cultured embryonic, fetal, or adult cells induced to
quiescence
(Campell et al., Nature 380:64-66 (1996); Wilmut et al., Nature 385:810-813
(1997)).
[0366] The present invention provides for transgenic animals that carry the
transgene
in all their cells, as well as animals which carry the transgene in some, but
not all their
cells, i.e., mosaic or chimeric animals. The transgene may be integrated as a
single
transgene or as multiple copies such as in concatamers, e.g., head-to-head
tandems or
head-to-tail tandems. The transgene may also be selectively introduced into
and activated
in a particular cell type by following, for example, the teaching of Lasko et
al. (Lasko et
198
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
al., Proc. Natl. Acad. Sci. USA 89:6232-6236 (1992)). The regulatory sequences
required
for such a cell-type specific activation will depend upon the particular cell
type of interest,
arid will be apparent to those of skill in the art. When it is desired that
the polynucleotide
transgene be integrated into the chromosomal site of the endogenous gene, gene
targeting
is preferred. Briefly, when such a technique is to be utilized, vectors
containing some
nucleotide sequences homologous to the endogenous gene are designed for the
purpose of
integrating, via homologous recombination with chromosomal sequences, into and
disrupting the function of the nucleotide sequence of the endogenous gene. The
transgene
may also be selectively introduced into a particular cell type, thus
inactivating the
endogenous gene in only that cell type, by following, for example, the
teaching of Gu et
al. (Gu et al., Science 265:103-106 (1994)). The regulatory sequences required
for such a
cell-type specific inactivation will depend upon the particular cell type of
interest, and will
be apparent to those of skill in the art. In addition to expressing the
polypeptide of the
present invention in a ubiquitous or tissue specific manner in transgenic
animals, it would
also be routine for one skilled in the art to generate constructs which
regulate expression
of the polypeptide by a variety of other means (for example, developmentally
or
chemically regulated expression).
[0367] Once transgenic animals have been generated, the expression of the
recombinant gene may be assayed utilizing standard techniques. Initial
screening may be
accomplished.by Southern blot analysis or PCR techniques to analyze animal
tissues to
verify that integration of the transgene has taken place. The level of mRNA
expression of
the transgene in the tissues of the transgenic animals may also be assessed
using
techniques which include, but are not limited to, Northern blot analysis of
tissue samples
obtained from the animal, in situ hybridization analysis, reverse
transcriptase-PCR (rt-
PCR); and TaqMan PCR. Samples of transgenic gene-expressing tissue may also be
evaluated immunocytochemically or immunohistochemically using antibodies
specific for
the transgene product.
[0368] Once the founder animals are produced, they may be bred, inbred,
outbred, or
crossbred to produce colonies of the particular animal. Examples of such
breeding
strategies include, but are not limited to: outbreeding of founder animals
with more than
one integration site in order to establish separate lines; inbreeding of
separate lines in
order to produce compound transgenics that express the transgene at higher
levels because
199
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
of the effects of additive expression of each transgene; crossing of
heterozygous
transgenic animals to produce animals homozygous for a given integration site
in order to
both augment expression and eliminate the need for screening of animals by DNA
analysis; crossing of separate homozygous lines to produce compound
heterozygous or
homozygous lines; breeding to place the transgene on a distinct background
that is
appropriate for an experimental model of interest; and breeding of transgenic
animals to
other animals bearing a distinct transgene or knockout mutation.
[0369] Transgenic and "knock-out" animals of the invention have uses which
include,
but are not limited to, animal model systems useful in elaborating the
biological function
of Neutrokine-alpha and/or Neutrokine-alphaSV polypeptides, studying
conditions and/or
disorders associated with aberrant Neutrokine-alpha and/or Neutrokine-alphaSV
expression, and in screening for compounds effective in ameliorating such
conditions
and/or disorders.
[0370] In further embodiments of the invention, cells that are genetically
engineered to
express the polypeptides of the invention, or alternatively, that are
genetically engineered
not to express the polypeptides of the invention (e.g., knockouts) are
administered to a
patient in vivo. Such cells may be obtained from the patient (i.e., animal,
including
human) or an MHC compatible donor and can include, but are not limited to
fibroblasts,
bone marrow cells, blood cells (e.g., lymphocytes), adipocytes, muscle cells,
endothelial
cells etc. The cells are genetically engineered in vatro using recombinant DNA
techniques
to introduce the coding sequence of polypeptides of the invention into the
cells, or
alternatively, to disrupt the coding sequence and/or endogenous regulatory
sequence
associated with the polypeptides of the invention, e.g., by, transduction
(using viral
vectors, and preferably vectors that integrate the transgene into the cell
genome) or
transfection procedures, including, but not limited to, the use of plasmids,
cosmids, YACs,
naked DNA, electroporation, liposomes, etc. The coding sequence of the
polypeptides of
the invention can be placed under the control of a strong constitutive or
inducible
promoter or promoter/enhancer to achieve expression, and preferably secretion,
of the
polypeptides of the invention. The engineered cells which express and
preferably secrete
the polypeptides of the invention can be introduced into the patient
systemically, e.g., in
the circulation, or intraperitoneally.
200
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0371] Alternatively, the cells can be incorporated into a matrix and
implanted in the
body, e.g., genetically engineered fibroblasts can be implanted as part of a
sltin graft;
genetically engineered endothelial cells can be implanted as part of a
lymphatic or
vascular graft. (See, for example, Anderson et al. U.S. Patent No. 5,399,349;
and
Mulligan & Wilson, U.S. Patent No. 5,460,959 each of which is incorporated by
reference
herein in its entirety).
[0372] When the cells to be administered are non-autologous or non-MHC
compatible
cells, they can be administered using well known techniques which prevent the
development of a host immune response against the introduced cells. For
example, the
cells may be introduced in an encapsulated form which, while allowing for an
exchange of
components with the immediate extracellular environment, does not allow the
introduced
cells to be recognized by the host immune system.
Antibodies
[0373] Further polypeptides of the invention relate to antibodies and T-cell
antigen
receptors (TCR) which immunospecifically bind a polypeptide, polypeptide
fragment, or
variant of SEQ ID N0:2 and/or SEQ 117 N0:19, and/or an epitope, of the present
invention (as determined by immunoassays well known in the art for assaying
specific
antibody-antigen binding). In specific embodiments, antibodies of the
invention bind
homomeric, especially homotrimeric, Neutrokine-alpha polypeptides. In other
specific
embodiments, antibodies of the invention bind heteromeric, especially
heterotrimeric,
Neutrokine-alpha polypeptides such as a heterotrimer containing two Neutrokine-
alpha
polypeptides and one APRIL polypeptide (e.g., SEQ ~ N0:20 or SEQ ID N0:47) or
a
heterotrimer containing one Neutrokine-alpha polypeptide and two APRIL,
polypeptides.
[0374] In particularly preferred embodiments, the antibodies of the invention
bind
homomeric, especially homotrimeric, Neutrokine-alpha polypeptides, wherein the
individual protein components of the multimers consist of the mature form of
Neutrokine
alpha (e.g., amino acids residues 134-285 of SEQ ID N0:2, or amino acids
residuess 134-
266 of SEQ ID N0:19.) In other specific embodiments, antibodies of the
invention bind
heteromeric, especially heterotrimeric, Neutrokine-alpha polypeptides such as
a
heterotrimer containing two Neutrokine-alpha polypeptides and one APRIL
polypeptide or
a heterotrimer containing one Neutrokine-alpha polypeptide and two APRIL
polypeptides,
201
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
and wherein the individulal protein components of the Neutrokine-alpha
heteromer consist
of the mature extracellular soluble portion of either Neutrokine-alpha or
(e.g., amino acids
residues 134-285 of SEQ ID N0:2, or amino acids residues 134-266 of SEQ ID
N0:19) or
the mature extracellular soluble portion APRIL (e.g., amino acid residues 105-
250 of SEQ
ID N0:47).
[0375] In specific embodiments, the antibodies of the invention bind
conformational
epitopes of a Neutrokine-alpha and/or Neutrokine-alphaSV monomeric protein. In
specific
embodiments, the antibodies of the invention bind conformational epitopes of a
Neutrolune-alpha and/or Neutrokine-alphaSV multimeric, especially trimeric,
protein. In
other embodiments, antibodies of the invention bind conformational epitopes
that arise
from the juxtaposition of Neutrokine-alpha and/or Neutrokine alpha SV with a
heterologous polypeptide, such as might be present when Neutrokine-alpha or
Neutrokine-
alpha SV forms heterotrimers (e.g., with APRIL polypeptides (e.g., SEQ ID
N0:20 or
SEQ ID N0:47)), or in fusion proteins between Neutrokine alpha and a
heterologous
polypeptide.
[0376] Antibodies of the invention include, but are not limited to,
polyclonal,
monoclonal, multispecxfic, human, humanized or chimeric antibodies, single
chain
antibodies, Fab fragments, F(ab~ fragments, fragments produced by a Fab
expression
library, anti-idiotypic (anti-Id) antibodies (including, e.g., anti-id
antibodies to antibodies
of the invention), and epitope-binding fragments of any of the above. The term
"antibody," as used herein, refers to immunoglobulin molecules and
immunologically
active portions of immunoglobulin molecules, i.e., molecules that contain an
antigen
binding site that immunospecifically binds an antigen. The immunoglobulin
molecules of
the invention can be of any type (e.g., IgG, IgE, IgM, IgD, IgA and IgY),
class (e.g., IgGI,
IgG2, IgG3, IgG4, IgAl and IgA2) or subclass of immunoglobulin molecule. . In
preferred embodiments, the immunoglobulin is an IgGl or an IgG4 isotype.
Immunoglobulins may have both a heavy and light chain. An array of IgG, IgE,
IgM,
IgD, IgA, and IgY heavy chains may be paired with a light chain of the kappa
or lambda
forms.
[0377] Most preferably the antibodies are human antigen-binding antibody
fragments
of the present invention and include, but are not limited to, Fab, Fab' and
F(ab~2, Fd,
single-chain Fvs (scFv), single-chain antibodies, disulfide-linked Fvs (sdFv)
and
202
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
fragments comprising either a VL or VH domain. Antigen-binding antibody
fragments,
including single-chain antibodies, may comprise the variable regions) alone or
in
combination with the entirety or a portion of the following: hinge region,
CHl, CH2, and
CH3 domains. Also included in the invention are antigen-binding fragments also
comprising any combination of variable regions) with a hinge region, CHl, CH2,
and
CH3 domains. The antibodies of the invention may be from any animal origin
including
birds and mammals. Preferably, the antibodies are human, murine (e.g., mouse
and rat),
donkey, ship rabbit, goat, guinea pig, camel, horse, or chicken. As used
herein, "human"
antibodies include antibodies having the amino acid sequence of a human
immunoglobulin
and include antibodies isolated from human immunoglobulin libraries or from
animals
transgenic for one or more human immunoglobulin and that do not express
endogenous
immunoglobulins, as described infra and, for example in, U.S. Patent No.
5,939,598 by
Kucherlapati et al.
[0378] The antibodies of the present invention may be monospecific,
bispecific,
trispecific or of greater multispecificity. Multispecific antibodies may be
specific for
different epitopes of a polypeptide of the present invention or may be
specific for both a
polypeptide of the present invention as well as for a heterologous epitope,
such as a
heterologous polypeptide or solid support material. See, e.g., PCT
publications WO
93/17715; WO 92/08802; W091100360; WO 92/05793; Tutt, et al., J. Immunol.
147:60-
69 (1991); U.S. Patent Nos. 4,474,893; 4,714,681; 4,925,648; 5,573,920;
5,601,819;
Kostelny et al., J. Immunol. 148:1547-1553 (1992).
[0379] Antibodies of the present invention may be described or specified in
terms of
the epitope(s) or portions) of a polypeptide of the present invention which
they recognize
or specifically bind. The epitope(s) or polypeptide portions) may be specified
as
described herein, e.g., by N-terminal and C-terminal positions, by size in
contiguous
amino acid residues, or listed in the Tables and Figures. Antibodies which
specifically
bind any epitope or polypeptide of the present invention may also be excluded.
Therefore,
the present invention includes antibodies that specifically bind polypeptides
of the present
invention, and allows for the exclusion of the same.
[0380] In specific embodiments, antibodies of the invention bind to
polypeptides
comprising Phe-115 to Leu-147, Ile-150 to Tyr-163, Ser-171 to Phe-194, Glu-223
to
Tyr-246, and Ser-271 to Phe-278 of the amino acid sequence of SEQ ID N0:2. In
another
203
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
specific embodiment, antibodies of the invention bind to polypeptides
consisting of
Phe-115 to Leu-147, Ile-150 to Tyr-163, Ser-171 to Phe-194, Glu-223 to Tyr-
246, and
Sex-271 to Phe-278 of the amino acid sequence of SEQ ID N0:2. In a preferred
embodiment, antibodies of the invention bind to a polypeptide comprising Glu-
223 to Tyr-
246 of SEQ ID N0:2. In another preferred embodiment, antibodies of the
invention bind
to a polypeptide consisting of Glu-223 to Tyr-246 of SEQ >D N0:2. In a more
preferred
embodiment, antibodies of the invention bind to a polypeptide consisting of
Phe-230 to
Asn-242 of SEQ ID N0:2. In further preferred, nonexclusive embodiments, the
antibodies of the invention inhibit one or more biological activities of
Neutrokine-alpha
and/or Neutrokine-alphaSV polypeptides of the invention through specific
binding. In
more preferred embodiments, the antibody of the invention inhibits Neutrokine-
alpha-
and/or Neutrokine-alphaSV-mediated B cell proliferation.
[0381] Antibodies of the present invention may also be described or specified
in terms
of their cross-reactivity. Antibodies that do not bind any other analog,
ortholog, or
homolog of a polypeptide of the present invention are included. Antibodies
that bind
polypeptides with at least 95%, at least 90%, at least 85%, at least 80%, at
least 75%, at
least 70%, at least 65%, at least 60%, at least 55%, and at least 50% identity
(as calculated
using methods known in the art and described herein) to a polypeptide of the
present
invention are also included in the present invention. In a specific
embodiment, antibodies
of the present invention cross react with APRIL (e.g., SEQ ID NO:20 or SEQ ID
N0:47;
PCT International Publication Number W097/33902; GenBank Accession No.
AF046888
(nucleotide) and AAC6132 (protein); J. Exp. Med. 188(6):1185-1190). In
specific
embodiments, antibodies of the present invention cross-react with marine, rat
and/or rabbit
homologs of human proteins and the corresponding epitopes thereof. Antibodies
that do
not bind polypeptides with less than 95%, less than 90%, less than 85%, less
than 80%,
less than 75%, less than 70%, less than 65%, less than 60%, less than 55%, and
less than
50% identity (as calculated using methods known in the art and described
herein) to a
polypeptide of the present invention are also included in the present
invention. In a
specific embodiment, the above-described cross-reactivity is with respect to
any single
specific antigenic or immunogenic polypeptide, or combinations) of 2, 3, 4, 5,
or more of
the specific antigenic and/or immunogenic polypeptides disclosed herein.
Further
included in the present invention are antibodies which bind polypeptides
encoded by
204
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polynucleotides which hybridize to a polynucleotide of the present invention
under
hybridization conditions (as described herein). Antibodies of the present
invention may
also be described or specified in terms of their binding affinity to a
polypeptide of the
invention. In specific embodiments, antibodies of the invention bind
Neutrokine-alpha
and/or Neutokine-alphaSV polypeptides, or fragments or variants thereof, with
a
dissociation constant or KD of less than or equal to 5 X 10-2 M, 10-2 M, 5 X
10-3 M,10-3 M,
X 10-4 M, 10-4 M, 5 X 10-5 M, or 10-5 M. More preferably, antibodies of the
invention
bind Neutrokine-alpha andlor Neutokine-alphaSV polypeptides or fragments or
variants
thereof with a dissociation constant or KD less than or equal to 5 X 10-6 M,
10-6 M, 5 X 10-
~ M, 10-' M, 5 X 10-8 M, or 10-8 M. Even more preferably, antibodies of the
invention
bind Neutrokine-alpha and/or Neutokine-alphaSV polypeptides or fragments or
variants
thereof with a dissociation constant or KD less than or equal to 5 X 10-~ M,10-
~ M, 5 X 10-
1° M, 10-1° IVI, 5 X 10-11 M, 10-11 M, 5 X 10-is M, 10-12 M, 5 X
-13 M,10-13 M, 5 X 10-1~ M,
10-14 M, 5 X 10-15 M, or 10-15 M. The invention encompasses antibodies that
bind
Neutrokine-alpha and/or Neutokine-alphaSV polypeptides with a dissociation
constant or
KD that is within any one of the ranges that are between each of the
individual recited
values.
[0382] The invention also provides antibodies that competitively inhibit
binding of an
antibody to an epitope of the invention as determined by any method known in
the art for
determining competitive binding, for example, the immunoassays described
herein. In
preferred embodiments, the antibody competitively inhibits binding to the
epitope by at
least 95%, at least 90%, at least 85 %, at least 80%, at least 75%, at least
70%, at least
60%, or at least 50%.
[0383] Antibodies of the present invention may act as agonists or antagonists
of the
polypeptides of the present invention. For example, the present invention
includes
antibodies which disrupt the receptorlligand interactions with the
polypeptides of the
invention either partially or fully. Preferrably, antibodies of the present
invention bind an
antigenic epitope disclosed herein, or a portion thereof. The invention
features both
receptor-specific antibodies and ligand-specific antibodies. The invention
also features
receptor-specific antibodies which do not prevent ligand binding but prevent
receptor
activation. Receptor activation (i.e., signaling) may be determined by
techniques
described herein or otherwise known in the art. For example, receptor
activation can be
205
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
determined by detecting the phosphorylation (e.g., tyrosine or
serine/threonine) of the
receptor or its substrate by immunoprecipitation followed by western blot
analysis (for
example, as described supra). In specific embodiments, antibodies are provided
that
inhibit ligand activity or receptor activity by at least 95%, at least 90%, at
least 85%, at
least 80%, at least 75%, at least 70%, at least 60%, or at least 50% of the
activity in
absence of the antibody.
[0384] The invention also features receptor-specific antibodies which both
prevent
ligand binding and receptor activation as well as antibodies that recognize
the receptor-
ligand complex, and, preferably, do not specifically recognize the unbound
receptor or the
unbound ligand. Likewise, included in the invention are neutralizing
antibodies which
bind the ligand and prevent binding of the ligand to the receptor, as well as
antibodies
which bind the ligand, thereby preventing receptor activation, but do not
prevent the
ligand from binding the receptor. Further included in the invention are
antibodies which
activate the receptor. These antibodies may act as receptor agonists, i.e.,
potentiate or
activate either all or a subset of the biological activities of the ligand-
mediated receptor
activation, for example, by inducing dimerization of the receptor. The
antibodies may be
specified as agonists, antagonists or inverse agonists for biological
activities comprising
the specific biological activities of the peptides of the invention disclosed
herein. The
above antibody agonists can be made using~methods known in the art. See, e.g.,
PCT
publication WO 96140281; U.S. Patent No. 5,811,097; Deng et al., Blood
92(6):1981-1988
(1998); Chen et al., Cancer Res. 58(16):3668-3678 (1998); Harrop et al., J.
Immunol.
161(4):1786-1794 (1998); Zhu et al., Cancer Res. 58(15):3209-3214 (1998); Yoon
et al.,
J. Immunol. 160(7):3170-3179 (1998); Prat et al., J. Cell. Sci. 111(Pt2):237-
247 (1998);
Pitard et al., J. Immunol. Methods 205(2):177-190 (1997); Liautard et al.,
Cytokine
9(4):233-241 (1997); Carlson et al., J. Biol. Chem. 272(17):11295-11301
(1997); Taryman
et al., Neuron 14(4):755-762 (1995); Muller et al., Structure 6(9):1153-1167
(1998);
Bartunek et al., Cytokine 8(1):14-20 (1996) (which are all incorporated by
reference
herein in their entireties).
[0385] Antibodies of the present invention may be used, for example, but not
limited
to, to purify, detect, and target the polypeptides of the present invention,
including both in
vitro and in vivo diagnostic and therapeutic methods. For example, the
antibodies have
use in immunoassays for qualitatively and quantitatively measuring levels of
the
206
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polypeptides of the present invention in biological samples. See, e.g., Harlow
et al.,
Antibodies: A Laboratory Manual, (Cold Spring Harbor Laboratory Press, 2nd ed.
1988)
(incorporated by reference herein in its entirety).
[0386] As discussed in more detail below, the antibodies of the present
invention may
be used either alone or in combination with other compositions. The antibodies
may
further be recombinantly fused to a heterologous~ polypeptide at the N- or C-
terminus or
chemically conjugated (including covalently and non-covalently conjugations)
to
polypeptides or other compositions. For example, antibodies of the present
invention may
be recombinantly fused or conjugated to molecules useful as labels in
detection assays and
effector molecules such as heterologous polypeptides, drugs, radionuclides, or
toxins.
See, e.g., PCT publications WO 92/08495; WO 91/14438; WO 89/12624; U.S. Patent
No.
5,314,995; and EP 396,387.
[0387] The antibodies of the invention include derivatives that are modified,
i.e, by the
covalent attachment of any type of molecule to the antibody such that covalent
attachment
does not prevent the antibody from generating an anti-idiotypic response. For
example,
but not by way of limitation, the antibody derivatives include antibodies that
have been
modified, e.g., by glycosylation, acetylation, pegylation, phosphylation,
amidation,
derivatization by known protecting/blocking groups, proteolytic cleavage,
linkage to a
cellular ligand or other protein, etc. Any of numerous chemical modifications
may be
carried out by known techniques, including, but not limited to specific
chemical cleavage,
acetylation, formylation, metabolic synthesis of tunicamycin, etc.
Additionally, the
derivative may contain one or more non-classical amino acids.
[0388] The antibodies of the present invention may be generated by any
suitable
method known in the art. Polyclonal antibodies to an antigen-of interest can
be produced
by various procedures well known in the art. For example, a polypeptide of the
invention
can be administered to various host animals including, but not limited to,
rabbits, mice,
rats, etc. to induce the production of sera containing polyclonal antibodies
specific for the
antigen. Various adjuvants may be used to increase the immunological response,
depending on the host species, and include but are not limited to, Freund's
(complete and
incomplete), mineral gels such as alurrunum hydroxide, surface active
substances such as
lysolecithin, pluronic polyols, polyanions, peptides, oil emulsions, keyhole
limpet
hemocyanins, dinitrophenol, and potentially useful human adjuvants such as BCG
(bacille
207
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Calmette-Guerin) and corynebacterium parvum. Such adjuvants are also well
known in
the art.
[0389] Monoclonal antibodies can be prepared using a wide variety of
techniques
known in the art including the use of hybridoma, recombinant, and phage
display
technologies, or a combination thereof. For example, monoclonal antibodies can
be
produced using hybridoma techniques including those known in the art and
taught, for
example, in Harlow et al., Antibodies: A Laboratory Manual, (Cold Spring
Harbor
Laboratory Press, 2nd ed. 1988); Hammerling, et al., in: Monoclonal Antibodies
and T-
Cell Hybridomas 563-681 (Elsevier, N.Y., 1981) (said references incorporated
by
reference in their entireties). The term "monoclonal antibody" as used herein
is not
limited to antibodies produced through hybridoma technology. The term
"monoclonal
antibody" refers to an antibody that is derived from a single clone, including
any
eukaryotic, prokaryotic, or phage clone, and not the method by which it is
produced.
[0390] A "monoclonal antibody" may comprise, or alternatively consist of, two
proteins, i.e., a heavy and a light chain.
[0391] Methods for producing and screening for specific antibodies using
hybridoma
technology are routine and well known in the art and are discussed in detail
in the
Examples (e.g., Example 9). In a non-limiting example, mice can be immunized
with a
polypeptide of the invention or a cell expressing such peptide. Once an immune
response
is detected, e.g., antibodies specific for the antigen are detected in the
mouse serum, the
mouse spleen is harvested and splenocytes isolated. The splenocytes are then
fused by
well-known techniques to any suitable myeloma cells, for example cells from
cell line
SP20 available from the ATCC. Hybridomas are selected and cloned by limited
dilution.
The hybridoma clones are then assayed by methods known in the art for cells
that secrete
antibodies capable of binding a polypeptide of the invention. Ascites fluid,
which
generally contains high levels of antibodies, can be generated by immunizing
mice with
positive hybridoma clones.
[0392] Accordingly, the present invention provides methods of generating
monoclonal
antibodies as well as antibodies produced by the method comprising culturing a
hybridoma cell secreting an antibody of the invention wherein, preferably, the
hybridoma
is generated by fusing splenocytes isolated from a mouse immunized with an
antigen of
the invention with myeloma cells and then screening the hybridomas resulting
from the
208
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
fusion for hybridoma clones that secrete an antibody able to bind a
polypeptide of the
invention.
[0393] Antibody fragments which recognize specific epitopes may be generated
by
known techniques. For example, Fab and F(ab~2 fragments of the invention may
be
produced by proteolytic cleavage of immunoglobulin molecules, using enzymes
such as
papain (to produce Fab fragments) or pepsin (to produce F(ab~2 fragments).
F(ab~2
fragments contain the variable region, the light chain constant region and the
CH1 domain
of the heavy chain.
[0394] For example, the antibodies of the present invention can also be
generated
using various phage display methods known in the art. In phage display
methods,
functional antibody domains are displayed on the surface of phage particles
which carry
the polynucleotide sequences encoding them. In a particular embodiment, such
phage can
be utilized to display antigen-binding domains expressed from a repertoire or
combinatorial antibody library (e.g., human or murine). Phage expressing an
antigen
binding domain that binds the antigen of interest can be selected or
identified with antigen,
e.g., using labeled antigen or antigen bound or captured to a solid surface or
bead. Phage
used in these methods are typically filamentous phage including fd and M13
binding
domains expressed from phage with Fab, Fv or disulfide stabilized Fv antibody
domains
recombinantly fused to either the phage gene III or gene VIII protein.
Examples of phage
display methods that can be used to make the antibodies of the present
invention include
those disclosed in Brinkman et al., J. Immunol. Methods 182:41-50 (1995); Ames
et al., J.
Immunol. Methods 184:177-186 (1995); Kettleborough et al., Eur. J. Immunol.
24:952-
958 (1994); Persic et al., Gene 187 9-18 (1997); Burton et al., Advances in
Immunology
57:191-280 (1994); PCT application No. PCTlGB91/01134; PCT publications WO
90/02809; WO 91/10737; WO 92/01047; WO 92/18619; WO 93/11236; WO 95115982;
WO 95/20401; and U.S. Patent Nos. 5,698,426; 5,223,409; 5,403,484; 5,580,717;
5,427,908; 5,750,753; 5,821,047; 5,571,698; 5,427,908; 5,516,637; 5,780,225;
5,658,727;
5,733,743 and 5,969,108; each of which is incorporated herein by reference in
its entirety.
[0395] As described in the above references, after phage selection, the
antibody
coding regions from the phage can be isolated and used to generate whole
antibodies,
including human antibodies, or any other desired antigen binding fragment, and
expressed
in any desired host, including mammalian cells, insect cells, plant cells,
yeast, and
209
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
bacteria, e.g., as described in detail below. For example, techniques to
recombinantly
produce Fab, Fab' and F(ab~2 fragments can,also be employed using methods
known in
the art such as those disclosed in PCT publication WO 92/22324; Mullinax et
al.,
BioTechniques 12(6):864-869 (1992); and Sawai et al., AJRI 34:26-34 (1995);
and Better
et al., Science 240:1041-1043 (1988) (said references incorporated by
reference in their
entireties).
[0396] Examples of techniques which can be used to produce single-chain Fvs
and
antibodies include those described in U.S. Patents 4,946,778 and 5,258,498;
Huston et al.,
Methods in Enzymology 203:46-88 (1991); Shu et al., PNAS 90:7995-7999 (1993);
and
Skerra et al., Science 240:1038-1040 (1988). For some uses, including in vivo
use of
antibodies in humans and in vitro detection assays, it may be preferable to
use chimeric,
humanized, or human antibodies. A chimeric antibody is a molecule in which
different
portions of the antibody are derived from different animal species, such as
antibodies
having a variable region derived from a murine monoclonal antibody and a human
immunoglobulin constant region. Methods for producing chimeric antibodies are
known
in the art. See e.g., Morrison, Science 229:1202 (1985); Oi et al.,
BioTechniques 4:214
(1986); Gillies et al., (1989) J. Immunol. Methods 125:191-202; U.S. Patent
Nos.
5,807,715; 4,816,567; and 4,816397, which are incorporated herein by reference
in their
entirety. Humanized antibodies are antibody molecules from non-human species
antibody
that binds the desired antigen having one or more complementarity determining
regions
(CDRs) from the non-human species and a framework region from a human
immunoglobulin molecule. Often, framework residues in the human framework
regions
will be substituted with the corresponding residue from the CDR donor antibody
to alter,
preferably improve, antigen binding. These framework substitutions are
identified by
methods well known in the art, e.g., by modeling of the interactions of the
CDR and
framework residues to identify framework residues important for antigen
binding and
sequence comparison to identify unusual framework residues at particular
positions. (See,
e.g., Queen et al., U.S. Patent No. 5,585,089; Riechmann et al., Nature
332:323 (1988),
which are incorporated herein by reference in their entireties.) Antibodies
can be
humanized using a variety of techniques known in the art including, for
example, CDR-
grafting (EP 239,400; PCT publication WO 91/09967; U.S. Patent Nos. 5,225,539;
5,530,101; and 5,585,089), veneering or resurfacing (EP 592,106; EP 519,596;
Padlan,
210
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Molecular Immunology 28(4/5):489-498 (1991); Studnicka et al., Protein
Engineering
7(6):805-814 (1994); Roguska. et al., PNAS 91:969-973 (1994)), and chain
shuffling
(U.S. Patent No. 5,565,332).
[0397] Completely human antibodies are particularly desirable for therapeutic
treatment of human patients. Human antibodies can be made by a variety of
methods
known in the art including phage display methods described above using
antibody
libraries derived from human immunoglobulin sequences. See also, U.S. Patent
Nos.
4,444,887 and 4,716,111; and PCT publications WO 98/46645, WO 98/50433, WO
98/24893, WO 98/16654, WO 96/34096, WO 96/33735, and WO 91/10741; each of
which is incorporated herein by reference in its entirety.
[0398] Hurnan antibodies can also be produced using transgenic mice which are
incapable of expressing functional endogenous imrnunoglobulins, but which can
express
human immunoglobulin genes. For example, the human heavy and light chain
immunoglobulin gene complexes may be introduced randomly or by homologous
recombination into mouse embryonic stem cells. Alternatively, the human
variable
region, constant region, and diversity region may be introduced into mouse
embryonic
stem cells in addition to the human heavy and light chain genes. The mouse
heavy and
light chain immunoglobulin genes may be rendered non-functional separately or
simultaneously with the introduction of human immunoglobulin loci by
homologous
recombination. In particular, homozygous deletion of the JH region prevents
endogenous
antibody production. The modified embryonic stem cells are expanded and
microinjected
into blastocysts to produce chimeric mice. The chimeric mice are then bred to
produce
homozygous offspring which express human antibodies. The transgenic mice are
immunized in the normal fashion with a selected antigen, e.g., all or a
portion of a
polypeptide of the invention. Monoclonal antibodies directed against the
antigen can be
obtained from the immunized, transgenic mice using conventional hybridoma
technology.
The human immunoglobulin transgenes harbored by the transgenic mice rearrange
during
B cell differentiation, and subsequently undergo class switching and somatic
mutation.
Thus, using such a technique, it is possible to produce therapeutically useful
IgG, IgA,
IgM and IgE antibodies. For an overview of this technology for producing human
antibodies, see Lonberg and Huszar, Int. Rev. Immunol. 13:65-93 (1995). For a
detailed
discussion of this technology for producing human antibodies and human
monoclonal
211
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
antibodies and protocols for producing such antibodies, see, e.g., PCT
publications WO
98/24893; WO 92/01047; WO 96/34096; WO 96/33735; European Patent No. 0 598
877;
U.S. Patent Nos. 5,413,923; 5,625,126; 5,633,425; 5,569,825; 5,661,016;
5,545,806;
5,814,318; 5,885,793; 5,916,771; and 5,939,598, which are incorporated by
reference
herein in their entirety. In addition, companies such as Abgenix, Inc.
(Freemont, CA) and
Genpharm (San Jose, CA) can be engaged to provide human antibodies directed
against a
selected antigen using technology similar to that described above.
[0399] Completely human antibodies which recognize a selected epitope can be
generated using a technique referred to as "guided selection." In this
approach a selectede
non-human monoclonal antibody, e.g., a mouse antibody, is used to guide the
selection of
a completely human antibody recognizing the same epitope. (Jespers et al.,
Biotechnology 12:899-903 (1988)).
[0400] Further, antibodies to the polypeptides of the invention can, in turn,
be utilized
to generate anti-idiotype antibodies that "mimic" polypeptides of the
invention using
techniques well known to those skilled in the art. (See, e.g., Greenspan &
Bona, FASEB
J. 7(5):437-444; (1989) and Nissinoff, J. Immunol. 147(8):2429-2438 (1991)).
For
example, antibodies which bind to and competitively inhibit polypeptide
multimerization
and/or binding of a polypeptide of the invention to a ligand can be used to
generate anti-
idiotypes that "mimic" the polypeptide multimerization andlor binding domain
and, as a
consequence, bind to and neutralize polypeptide and/or its ligand. Such
neutralizing anti-
idiotypes or Fab fragments of such anti-idiotypes can be used in therapeutic
regimens to
neutralize polypeptide ligand. For example, such anti-idiotypic antibodies can
be used to
bind a polypeptide of the invention and/or to bind its ligands/receptors, and
thereby block
its biological activity.
Polynucleotides Encoding Antibodies
[0401] The invention further provides polynucleotides comprising a nucleotide
sequence encoding an antibody of the invention and fragments thereof. The
invention also
encompasses polynucleotides that hybridize under stringent or lower stringency
hybridization conditions, e.g., as defined supra, to polynucleotides that
encode an
antibody, preferably, that specifically binds to a polypeptide of the
invention, preferably,
an antibody that binds to a polypeptide having the amino acid sequence of SEQ
ID N0:2.
212
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
In another preferred embodiment, the antibody binds specifically to a
polypeptide having
the amino acid sequence of SEQ ID N0:19. In another preferred embodiment, the
antibody binds specifically to a polypeptide having the amino acid sequence of
SEQ ID
N0:23. In another preferred embodiment, the antibody binds specifically to a
polypeptide
having the amino acid sequence of SEQ ID N0:28. In another preferred
embodiment, the
antibody binds specifically to a polypeptide having the amino acid sequence of
SEQ >D
N0:30. In another preferred embodiment, the antibody binds specifically to a
polypeptide
having the amino acid sequence of SEQ ID N0:39. In another preferred
embodiment, the
antibody binds specifically to a polypeptide having the amino acid sequence of
SEQ ID
N0:40. In another embodiment, the antibody binds specifically to a polypeptide
having
the amino acid sequence of SEQ ID N0:41. In another embodiment, the antibody
binds
specifically to a polypeptide having the amino acid sequence of SEQ ID N0:42.
In
another embodiment, the antibody binds specifically to a polypeptide having
the amino
acid sequence of SEQ ID N0:43. In another embodiment, the antibody binds
specifically
to a polypeptide having the amino acid sequence of SEQ )D N0:44.
[0402] The polynucleotides may be obtained, and the nucleotide sequence of the
polynucleotides determined, by any method known in the art. For example, if
the
nucleotide sequence of the antibody is known, a polynucleotide encoding the
antibody
may be assembled from chemically synthesized oligonucleotides (e.g., as
described in
Kutmeier et al., BioTechniques 17:242 (1994)), which, briefly, involves the
synthesis of
overlapping oligonucleotides containing portions of the sequence encoding the
antibody,
annealing and ligating of those oligonucleotides, and then amplification of
the ligated
oligonucleotides by PCR.
[0403] Alternatively, a polynucleotide encoding an antibody may be generated
from
nucleic acid from a suitable source. If a clone containing a nucleic acid
encoding a
particular antibody is not available, but the sequence of the antibody
molecule is known, a
nucleic acid encoding the immunoglobulin may be chemically synthesized or
obtained
from a suitable source (e.g., an antibody cDNA library, or a cDNA library
generated from,
or nucleic acid, preferably poly A+ RNA, isolated from, any tissue or cells
expressing the
antibody, such as hybridoma cells selected to express an antibody of the
invention) by
PCR amplification using synthetic primers hybridizable to the 3' and 5' ends
of the
sequence or by cloning using an oligonucleotide probe specific for the
particular gene
213
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
sequence to identify, e.g., a cDNA clone from a cDNA library that encodes the
antibody.
Amplified nucleic acids generated by PCR may then be cloned into replicable
cloning
vectors using any method well known in the art.
[0404] Once the nucleotide sequence and corresponding amino acid sequence of
the
antibody is determined, the nucleotide sequence of the antibody may be
manipulated using
methods well known in the art for the manipulation of nucleotide sequences,
e.g.,
recombinant DNA techniques, site directed mutagenesis, PCR, etc. (see, for
example, the
techniques described in Sambrook et a1.,.1990, Molecular Cloning, A Laboratory
Manual,
2d Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY and Ausubel et
al., eds.,
1998, Current Protocols in Molecular Biology, John Wiley & Sons, NY, which are
both
incorporated by reference herein in their entireties ), to generate antibodies
having a
different amino acid sequence, for example to create amino acid substitutions,
deletions,
and/or insertions.
[0405] In a specific embodiment, the amino acid sequence of the heavy and/or
light
chain variable domains may be inspected to identify the sequences of the
complementarity
determining regions (CDRs) by methods that are well known in the art, e.g., by
comparison to known amino acid sequences of other heavy and light chain
variable
regions to determine the regions of sequence hypervariability. Using routine
recombinant
DNA techniques, one or more of the CDRs may be inserted within framework
regions,
e.g., into human framework regions to humanize a non-human antibody, as
described
supra. The framework regions may be naturally occurring or consensus framework
regions, and preferably human framework regions (see, e.g., Chothia et al., J.
Mol. Biol.
278: 457-479 (1998) for a listing of human framework regions). Preferably, the
polynucleotide generated by the combination of the framework regions and CDRs
encodes
an antibody that specifically binds a polypeptide of the invention.
Preferably, as discussed
supra, one or more amino acid substitutions may be made within the framework
regions,
and, preferably, the amino acid substitutions improve binding of the antibody
to its
antigen. Additionally, such methods may be used to make amino acid
substitutions or
deletions of one or more variable region cysteine residues participating in an
intrachain
disulfide bond to generate antibody molecules lacking one or more intrachain
disulfide
bonds. Other alterations to the polynucleotide are encompassed by the present
invention
and within the skill of the art.
214
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0406] In addition, techniques developed for the production of "chimeric
antibodies"
(Morrison et al., Proc. Natl. Acad. Sci. 81:851-855 (1984); Neuberger et al.,
Nature
312:604-608 (1984); Takeda et al., Nature 314:452-454 (1985)) by splicing
genes from a
mouse antibody molecule of appropriate antigen specificity together with genes
from a
human antibody molecule of appropriate biological activity can be used. As
described
supra, a chimeric antibody is a molecule in which different portions are
derived from
different animal species, such as those having a variable region derived from
a murine
mAb and a human immunoglobulin constant region, e.g., humanized antibodies.
[0407] Alternatively, techniques described for the production of single chain
antibodies (U.S. Patent No. 4,946,778; Bird, Science 242:423- 42 (1988);
Huston et al.,
Proc. Natl. Acad. Sci. USA 85:5879-5883 (1988); and Ward et al., Nature
334:544-54
(1989)) can be adapted to produce single chain antibodies. Single chain
antibodies are
formed by linking the heavy and light chain fragments of the Fv region via an
amino acid
a
bridge, resulting in a single chain polypeptide. Techniques for the assembly
of functional
Fv fragments in E. coli may also be used (Skerra et al., Science 242:1038-
1041 (1988)).
Methods of Producing Antibodies
[0408] The antibodies of the invention can be produced by any method known in
the
art for the synthesis of antibodies, in particular, by chemical synthesis or
preferably, by
recombinant expression techniques.
[0409] Recombinant expression of an antibody of the invention, or fragment,
derivative or analog thereof, (e.g., a heavy or light chain of an antibody of
the invention or
a single chain antibody of the invention), requires construction of an
expression vector
containing a polynucleotide that encodes the antibody. Once a polynucleotide
encoding
an antibody molecule or a heavy or light chain of an antibody, or portion
thereof
(preferably containing the heavy or light chain variable domain), of the
invention has been
obtained, the vector for the production of the antibody molecule may be
produced by
recombinant DNA technology using techniques well known in the art. Thus,
methods for
preparing a protein by expressing a polynucleotide containing an antibody
encoding
nucleotide sequence are described herein. Methods which are well known to
those skilled
in the art can be used to construct expression vectors containing antibody
coding
sequences and appropriate transcriptional and translational control signals.
These methods
215
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
include, for example, in vitro recombinant DNA techniques, synthetic
techniques, and ira
vivo genetic recombination. The invention, thus, provides replicable vectors
comprising a
nucleotide sequence encoding an antibody molecule of the invention, or a heavy
or light
chain thereof, or a heavy or light chain variable domain, operably linked to a
promoter.
Such vectors may include the nucleotide sequence encoding the constant region
of the
antibody molecule (see, e.g., PCT Publication WO 86/05807; PCT Publication WO
89/01036; and U.S. Patent No. 5,122,464) and the variable domain of the
antibody may be
cloned into such a vector for expression of the entire heavy or light chain.
[0410] The expression vector is transferred to a host cell by conventional
techniques
and the transfected cells are then cultured by conventional techniques to
produce an
antibody of the invention. Thus, the invention includes host cells containing
a
polynucleotide encoding an antibody of the invention, or a heavy or light
chain thereof, or
a single chain antibody of the invention, operably linked to a heterologous
promoter. In
preferred embodiments for the expression of double-chained antibodies, vectors
encoding
both the heavy and light chains may be co-expressed in the host cell for
expression of the
entire immunoglobulin molecule, as detailed below.
[0411] A variety of host-expression vector systems may be utilized to express
the
antibody molecules of the invention. Such host-expression systems represent
vehicles by
which the coding sequences of interest may be produced and subsequently
purified, but
also represent cells which may, when transformed or transfected with the
appropriate
nucleotide coding sequences, express an antibody molecule of the invention in
situ. These
include but are not limited to microorganisms such as bacteria (e.g., E. coli,
B. subtilis)
transformed with recombinant bacteriophage DNA, plasmid DNA or cosmid DNA
expression vectors containing antibody coding sequences; yeast (e.g.,
Saccharomyces,
Pichia) transformed with recombinant yeast expression vectors containing
antibody
coding sequences; insect cell systems infected with recombinant virus
expression vectors
(e.g., baculovirus) containing antibody coding sequences; plant cell systems
infected with
recombinant virus expression vectors (e.g., cauliflower mosaic virus, CaMV;
tobacco
mosaic virus, TMV) or transformed with recombinant plasmid expression vectors
(e.g., Ti
plasmid) containing antibody coding sequences; or mammalian cell systems
(e.g., COS,
CHO, BHK, 293, 3T3 cells) harboring recombinant expression constructs
containing
promoters derived from the genome of mammalian cells (e.g., metallothionein
promoter)
216
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
or from mammalian viruses (e.g., the adenovirus late promoter; the vaccinia
virus 7.5K
promoter). Preferably, bacterial cells such as Escherichia coli, and more
preferably,
eukaryotic cells, especially for the expression of whole recombinant antibody
molecule,
are used for the expression of a recombinant antibody molecule. For example,
mammalian cells such as Chinese hamster ovary cells (CHO), in conjunction with
a vector
such as the major intermediate early gene promoter element fzom human
cytomegalovirus
is an effective expression system for antibodies (Foecking et al., Gene 45:101
(1986);
Cockett et al., Bio/Technology 8:2 (1990)).
[0412] In bacterial systems, a number of expression vectors may be
advantageously
selected depending upon the use intended for the antibody molecule being
expressed. For
example, when a large quantity of such a protein is to be produced, for the
generation of
pharmaceutical compositions of an antibody molecule, vectors which direct the
expression
of high levels of fusion protein products that are readily purified may be
desirable. Such
vectors include, but are not limited, to the E. coli expression vector pUR278
(Ruther et al.,
EMBO J. 2:1791 (1983)), in which the antibody coding sequence may be ligated
individually into the vector in frame with the lac Z coding region so that a
fusion protein is
produced; pIN vectors (Inouye & Inouye, Nucleic Acids Res. 13:3101-3109
(1985); Van
Heeke & Schuster, J. Biol. Chem. 24:5503-5509 (1989)); and the like. pGEX
vectors may
also be used to express foreign polypeptides as fusion proteins with
glutathione S-
transferase (GST). In general, such fusion proteins are soluble and can easily
be purified
from lysed cells by adsorption and binding to matrix glutathione-agarose beads
followed
by elution in the presence of free glutathione. The pGEX vectors are designed
to include
thrombin or factor Xa protease cleavage sites so that the cloned target gene
product can be
released from the GST moiety.
[0413] In an insect system, Autographs califon~ica nuclear polyhedrosis virus
(AcNPV) is used as a vector to express foreign genes. The virus grows in
Spodoptera
frugiperda cells. The antibody coding sequence may be cloned individually into
non-
essential regions (for example the polyhedrin gene) of the virus and placed
under control
of an AcNPV promoter (for example the polyhedrin promoter).
[0414] In mammalian host cells, a number of viral-based expression systems may
be
utilized. In cases where an adenovirus is used as an expression vector, the
antibody
coding sequence of interest may be ligated to an adenovirus
transcription/translation
217
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
control complex, e.g., the late promoter and tripartite leader sequence. This
chimeric gene
may then be inserted in the adenovirus genome by in vitro or in vivo
recombination.
Insertion in a non- essential region of the viral genome (e.g., region E1 or
E3) will result
in a recombinant virus that is viable and capable of expressing the antibody
molecule in
infected hosts. (E.g., see Logan & Shenk, Proc. Natl. Acad. Sci. USA 81:355-
359
(1984)). Specific initiation signals may also be required for efficient
translation of
inserted antibody coding sequences. These signals include the ATG initiation
codon and
adjacent sequences. Furthermore, the initiation codon must be in phase with
the reading
frame of the desired coding sequence to ensure translation of the entire
insert. These
exogenous translational control signals and initiation codons can be of a
variety of origins,
both natural and synthetic. The efficiency of expression may be enhanced by
the inclusion
of appropriate transcription enhancer elements, transcription terminators,
etc. (see Bittner
et al., Methods in Enzymol. 153:51-544 (1987)).
[0415] In addition, a host cell strain may be chosen which modulates the
expression of
the inserted sequences, or modifies and processes the gene product in the
specific fashion
desired. Such modifications (e.g., glycosylati.on) and processing (e.g.,
cleavage) of protein
products may be important for the function of the protein. Different host
cells have
characteristic and specific mechanisms for the post-translational processing
and
modification of proteins and gene products. Appropriate cell lines or host
systems can be
chosen to ensure the correct modification and processing of the foreign
protein expressed.
To this end, eukaryotic host cells which possess the cellular machinery for
proper
processing of the primary transcript, glycosylation, and phosphorylation of
the gene
product may be used. Such mammalian host cells include but are not limited to
CHO, .
VERY, BHK, Hela, COS, MDCK, 293, 3T3, WI38, and in particular, breast cancer
cell
lines such as, for example, BT483, Hs578T, HTB2, BT20 and T47D, and normal
mammary gland cell line such as, for example, CRL7030 and Hs578Bst.
[0416] For long-term, high-yield production of recombinant proteins, stable
expression is preferred. For example, cell lines which stably express the
antibody
molecule may be engineered. Rather than using expression vectors which contain
viral
origins of replication, host cells can be transformed with DNA controlled by
appropriate
expression control elements (e.g., promoter, enhancer, sequences,
transcription
terminators, polyadenylation sites, etc.), and a selectable marker. Following
the
218
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
introduction of the foreign DNA, engineered cells may be allowed to grow for 1-
2 days in
an enriched media, and then are switched to a selective media. The selectable
marker in
the recombinant plasmid confers resistance to the selection and allows cells
to stably
integrate the plasmid into their chromosomes and grow to form foci which in
turn can be
cloned and expanded into cell lines. This method may advantageously be used to
engineer
cell lines which express the antibody molecule. Such engineered cell lines may
be
particularly useful in screening and evaluation of compounds that interact
directly or
indirectly with the antibody molecule.
[0417] A number of selection systems may be used, including but not limited to
the
herpes simplex virus thymidine kinase (Wigler et al., Cell 11:223 (1977)),
hypoxanthine-
guanine phosphoribosyltransferase (Szybalska & Szybalski, Proc. Natl. Acad.
Sci. USA
48:202 (1992)), and adenine phosphoribosyltransferase (Lowy et al., Cell
22:817 (1980))
genes can be employed in tk-, hgprt- or aprt- cells, respectively. Also,
antimetabolite
resistance can .be used as the basis of selection for the following genes:
dhfr, which
confers resistance to methotrexate (Wigler et al., Natl. Acad. Sci. USA 77:357
(1980);
OTIare et al., Proc. Natl. Acad. Sci. USA 78:1527 (1981)); gpt, which confers
resistance
to mycophenolic acid (Mulligan & Berg, Proc. Natl. Acad. Sci. USA 78:2072
(1981));
neo, which confers resistance to the aminoglycoside G-418 Clinical Pharmacy
12:488-
505; Wu and Wu, Biotherapy 3:87-95 (1991); Tolstoshev, Ann. Rev. Pharmacol.
Toxicol.
32:573-596 (1993); Mulligan, Science 260:926-932 (1993); and Morgan and
Anderson,
Ann. Rev. Biochem. 62:191-217 (1993); May, 1993, TIB TECH 11(5):155-215); and
hygro, which confers resistance to hygromycin (Santerre et al., Gene 30:147
(1984)).
Methods commonly known in the art of recombinant DNA technology may be
routinely
applied to select the desired recombinant clone, and such methods are
described, for
example, in Ausubel et al. (eds.), Current Protocols in Molecular Biology,
John Wiley &
Sons, NY (1993); Kriegler, Gene Transfer and Expression, A Laboratory Manual,
Stockton Press, NY (1990); and in Chapters 12 and 13, Dracopoli et al. (eds),
Current
Protocols in Human Genetics, John Wiley & Sons, NY (1994); Colberre-Garapin et
al., J.
Mol. Biol. 150:1 (1981), which are incorporated by reference herein in their
entireties.
[0418] The expression levels of an antibody molecule can be increased by
vector
amplification (for a review, see Bebbington and Hentschel, The use of vectors
based on
gene amplification for the expression of cloned genes in mammalian cells in
DNA
219
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
cloning, Vol.3. (Academic Press, New York, 1987)). When a marker in the vector
system
expressing antibody is amplifiable, increase in the level of inhibitor present
in culture of
r
host cell will increase the number of copies of the marker gene. Since the
amplified
region is associated with the antibody gene, production of the antibody will
also increase
(Grouse et al., Mol. Cell. Biol. 3:257 (1983)).
[0419] The host cell may be co-transfected with two expression vectors of the
invention, the first vector encoding a heavy chain derived polypeptide and the
second
vector encoding a light chain derived polypeptide. The two vectors may contain
identical
selectable markers which enable equal expression of heavy and light chain
polypeptides.
Alternatively, a single vector may be used which encodes, and is capable of
expressing,
both heavy and light chain polypeptides. In such situations, the light chain
should be
placed before the heavy chain to avoid an excess of toxic free heavy chain
(Proudfoot,
Nature 322:52 (1986); Kohler, Proc. Natl. Acad. Sci. USA 77:2197 (1980)). The
coding
sequences for the heavy and light chains may comprise cDNA or genomic DNA.
[0420] Once an antibody molecule of the invention has been produced by an
animal,
chemically synthesized, or recombinantly expressed, it may be purified by any
method
known in the art for purification of an immunoglobulin molecule, for example,
by
chromatography (e.g., ion exchange, affinity, particularly by affinity for the
specific
antigen after Protein A, and sizing column chromatography), centrifugation,
differential
solubility, or by any other standard technique for the purification of
proteins. In addition,
the antibodies of the present invention or fragments thereof can be fused to
heterologous
polypeptide sequences described herein or otherwise known in the art, to
facilitate
purification.
[0421] The present invention encompasses antibodies recombinantly fused or
chemically conjugated (including both covalent and non-covalent conjugations)
to a
polypeptide (or portion thereof, preferably at least 10, 20, 30, 40, 50, 60,
70, 80, 90 or 100
amino acids of the polypeptide) of the present invention to generate fusion
proteins. The
fusion does not necessarily need to be direct, but may occur through linker
sequences.
The antibodies may be specific for antigens other than polypeptides (or
portion thereof,
preferably at least 10, 20, 30, 40, 50, 60, 70, 80, 90 or 100 amino acids of
the polypeptide)
of the present invention. For example, antibodies may be used to target the
polypeptides
of the present invention to particular cell types, either in vitro or in vivo,
by fusing or
220
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
conjugating the polypeptides of the present invention to antibodies specific
for particular
cell surface receptors. Antibodies fused or conjugated to the polypeptides of
the present
invention may also be used in ira vitro immunoassays and purification methods
using
methods lcnown in the art. See e.g., Harbor et al., supra, and PCT
publication. WO
93121232; EP 439,095; Naramura et al., Immunol. Lett. 39:91-99 (1994); U.S.
Patent
5,474,981; Gillies et al., PNAS 89:1428-1432 (1992); Fell et al., J. Imrnunol.
146:2446-
2452(1991), which are incorporated by reference in their entireties.
[0422] The present invention further includes compositions comprising the
polypeptides of the present invention fused or conjugated to antibody domains
other than
the variable regions. For example, the polypeptides of the present invention
may be fused
or conjugated to an antibody Fc region, or portion thereof. The antibody
portion fused to
a polypeptide of the present invention may comprise the constant region, hinge
region,
CHl domain, CH2 domain, and CH3 domain or any combination of whole domains or
portions thereof. The polypeptides may also be fused or conjugated to the
above antibody
portions to form multimers. For example, Fc portions fused to the polypeptides
of the
present invention can form dimers through disulfide bonding between the Fc
portions.
Higher multimeric forms can be made by fusing the polypeptides to portions of
IgA and
IgM. Methods for fusing or conjugating the polypeptides of the present
invention to
antibody portions are known in the art. See, e.g., U.S. Patent Nos. 5,336,603;
5,622,929;
5,359,046; 5,349,053; 5,447,851; 5,112,946; EP 307,434; EP 367,166; PCT
publications
WO 96/04388; WO 91/06570; Ashkenazi et al., Proc. Natl. Acad. Sci. USA
88:10535-
10539 (1991); Zheng et al., J. Immunol. 154:5590-5600 (1995); and Vil et al.,
Proc. Natl.
Acad. Sci. USA 89:11337- 11341(1992) (said references incorporated by
reference in their
entireties).
[0423] As discussed, supra, the polypeptides corresponding to a polypeptide,
polypeptide .fragment, or a variant of SEQ ID N0:2 may be fused or conjugated
to the
above antibody portions to increase the in vivo half life of the polypeptides
or for use in
immunoassays using methods known in the art. Further, the polypeptides
corresponding
to SEQ ID N0:2 may be fused or conjugated to the above antibody portions to
facilitate
purification. Also as discussed, supra, the polypeptides corresponding to a
polypeptide,
polypeptide fragment, or a variant of SEQ ID NO:19 may be fused or conjugated
to the
above antibody portions to increase the in vivo half life of the polypeptides
or for use in
221
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
immunoassays using methods known in the art. Moreover, the polypeptides
corresponding to SEQ TD N0:19 may be fused or conjugated to the above antibody
portions to facilitate purification. One reported example describes chimeric
proteins
consisting of the first two domains of the human CD4-polypeptide and various
domains of
the constant regions of the heavy or light chains of mammalian
imrnunoglobulins. (EP
394,827; Traunecker et al., Nature 331:84-86 (1988). The polypeptides of the
present
invention fused or conjugated to an antibody having disulfide- linked dimeric
structures
(due to the IgG) may also be more efficient in binding and neutralizing other
molecules,
than the monomeric secreted protein or protein fragment alone. (Fountoulakis
et al., J.
~. Biochem. 270:3958-3964 (1995)). In many cases, the Fc part in a fusion
protein is
beneficial in therapy and diagnosis, and thus can result in, for example,
improved
pharmacokinetic properties. (EP A 232,262). Alternatively, deleting the Fc
part after the
fusion protein has been expressed, detected, and purified, would be desired.
For example,
the Fc portion may hinder therapy and diagnosis if the fusion protein is used
as an antigen
for immunizations. In drug discovery, for example, human proteins, such as hIL-
5, have
been fused with Fc portions for the purpose of high-throughput screening
assays to
identify antagonists of hIL-5. (See, Bennett et al., J. Molecular Recognition
8:52-58
(1995); Johanson et al., J. Biol. Chem. 270:9459-9471 (1995).
[0424] Moreover, the antibodies or fragments thereof of the present invention
can be
fused to marker sequences, such as a peptide to facilitate purification. In
preferred
embodiments, the marker amino acid sequence is a hexa-histidine peptide, such
as the tag
provided in a pQE vector (QIAGEN, Inc., 9259 Eton Avenue, Chatsworth, CA,
91311),
among others, many of which are commercially available. As described in Gentz
et al.,
Proc. Natl. Acad. Sci. USA 86:821-824 (1989), for instance, hexa-histidine
provides for
convenient purification of the fusion protein. Other peptide tags useful for
purification
include, but are not limited to, the "HA" tag, which corresponds to an epitope
derived
from the influenza hemagglutinin protein (Wilson et al., CeII 37:767 (1984))
and the
~~Bag" tag.
[0425] The present invention further encompasses antibodies or fragments
thereof
conjugated to a diagnostic or therapeutic agent. The antibodies can be used
diagnostically
to, for example, monitor the development or progression of a tumor as part of
a clinical
testing procedure to, e.g., determine the efficacy of a given treatment
regimen. Detection
222
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
can be facilitated by coupling the antibody to a detectable substance.
Examples of
detectable substances include various enzymes, prosthetic groups, fluorescent
materials,
luminescent materials, bioluminescent materials, radioactive materials,
positron emitting
metals using various positron emission tomographies, and nonradioactive
paramagnetic
metal ions. The detectable substance may be coupled or conjugated either
directly to the
antibody (or fragment thereof) or indirectly, through an intermediate (such
as, for -
example, a linker known in the art) using techniques known in the art. See,
for example,
U.S. Patent No. 4,741,900 for metal ions which can be conjugated to antibodies
for use as
diagnostics according to the present invention. Examples of suitable enzymes
include
horseradish peroxidase, alkaline phosphatase, beta-galactosidase, or
acetylcholinesterase;
examples of suitable prosthetic group complexes include streptavidinlbiotin
and
avidin/biotin; examples of suitable fluorescent materials include
umbelliferone,
fluorescein, fluorescein isothiocyanate, rhodamine, dichlorotriazinylamine
fluorescein,
dansyl chloride or phycoerythrin; an example of a luminescent material
includes luminol;
examples of bioluminescent materials include luciferase, luciferin, and
aequorin; and
examples of suitable radioactive material include iodine (131h izsh iz3h
lzll), carbon (14C),
sulfur (3sS), tritium (3I~, indium (115mIn, nsmln, mzln, niln), and technetium
(~~Tc, ~9'T''fC),
thallium (z°1Ti), gallium (~sGa, 6~Ga), palladium (losPd), molybdenum
(~~Mo), xenon
(i3sXe)~ ~uorine (isF)~ is3Sm~ m~Lu~ is~Gd~ ia~Pm~ i4oLa~ mslb~ lG6Ho~ 901,
~~Sc~ is6Re~
issRe~ iazPr~ iosRh~ ~~Ru~ 6sGe~ s~Co~ 6szn~ ssSr~ 32p~ is3Gd~ i6~Yb~ syr~
s4Mn, ~sSe~ n3Sn~
and ll~Tin.
[0426] Further, an antibody or fragment thereof may be conjugated to a
therapeutic
moiety such as a cytotoxin, e.g., a cytostatic or cytocidal agent, a
therapeutic agent or a
radioactive metal ion, e.g., alpha-emitters such as, for example, zisBi. In
specific
embodiments, antibodies of the invention are attached to macrocyclic chelators
useful for
conjugating radiometal ions, including but not limited to, lln, l~7Lu,
~°Y, i6sl3o, and
Is3Sm, to polypeptides. In preferred embodiments, the radiometal ion
associated with the
macrocyclic chelators attached to antibodies of the invention is lln. In
preferred
embodiments, the oradiornetal ion associated with the macrocyclic chelators
attached to
antibodies of the invention is ~°Y. In specific embodiments, the
macrocyclic chelator is
1,4,7,10-tetraazacyclododecane-N,N',N",N"'-tetraacetic acid (DOTA). In other
specific
embodiments, the DOTA is attached to the Neutrokine-alpha and/or Neutrokine-
alphaSV
223
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
polypeptide of the invention via a linker molecule. Examples of linker
molecules useful
for conjugating DOTA to a polypeptide are commonly known in the art - see, for
example,
DeNardo et al., Clin Cancer Res. 4(10):2483-90 (1998); Peterson et al.,
Bioconjug. Chem.
10(4):553-7 (1999); and Zimmerman et al, Nucl. Med. Biol. 26(8):943-50 (1999)
which
are hereby incorporated by reference in their entirety. In addition, U.S.
Patents 5,652,361
and 5,756,065, which disclose chelating agents that may be conjugated to
antibodies, and
methods for making and using them, are hereby incorporated by reference in
their
entireties.
[0427] A cytotoxin or cytotoxic agent includes any agent that is detrimental
to cells
and includes such molecules as small molecule toxins and enzymatically active
toxins of
bacterial, fungal, plant, or animal origin, or fragments thereof. Examples
include
paclitaxol, cytochalasin B, gramicidin D, ethidium bromide, emetine,
mitomycin,
etoposide (VP-16), tenoposide, vincristine, vinblastine, colchicin,
doxorubicin,
daunorubicin, dihydroxy anthracin dione, mitoxantrone, mithramycin,
actinomycin D, 1-
dehydrotestosterone, glucocorticoids, procaine, tetracaine, lidocaine,
propranolol, and
puromycin and analogs or homologs thereof. Therapeutic agents include, but are
not
limited to, antimetabolites (e.g., methotrexate, 6-mercaptopurine, 6-
thioguanine,
cytarabine, 5-fluorouracil decarbazine), alkylating agents (e.g.,
mechlorethamine, thioepa
chlorambucil, melphalan, carmustine (BSNU) and lomustine (CCNU),
cyclophosphamide,
busulfan, dibromomannitol, streptozotocin, mitomycin C, and cis-
dichlorodiamine
platinum (II) (DDP) cisplatin), anthracyclines (e.g., daunorubicin (formerly
daunomycin)
and doxorubicin), antibiotics (e.g., dactinomycin (formerly actinomycin),
bleomycin,
mithramycin, and anthramycin (AMC)), and anti-mitotic agents (e.g.,
vincristine and
vinblastine), improsulfan, piposulfan, benzodopa, carboquone, meturedopa,
uredopa,
altretamine, triethylenemelamine, trietylenephosphoramide,
triethylenethiophosphaoramide trimethylolomelamine, chlornaphazine,
cholophosphamide, estramustine, ifosfamide, novembichin, phenesterine,
prednimustine,
trofosfamide, uracil mustard, chlorozotocin, fotemustine, nimustine,
ranimustine,
aclacinomysins, azaserine, cactinomycin, calichearnicin, carabicin,
carminomycin,
carzinophilin, chromomycins, detorubicin, 6-diazo-5-oxo-L-norleucine,
epirubicin,
esorubicin, idarubicin, marcellomycin, mycophenolic acid, nogalamycin,
olivomycins,
peplomycin, potfiromycin, quelamycin, rodorubicin, streptonigrin, tubercidin,
ubenimex,
224
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
zinostatin, zorubicin, denopterin, pteropterin, trimetrexate, fludarabine,
thiamiprine,
ancitabine, azacitidine, 6-azauridine, carmofur, dideoxyuridine,
doxifluridine, enocitabine,
floxuridine, 5-FU, calusterone, dromostanolone propionate, epitiostanol,
mepitiostane,
testolactone, aminoglutethimide, mitotane, trilostane, frolinic acid,
aceglatone,
aldophosphamide glycoside, aminolevulinic acid, amsacrine, bestrabucil,
bisantrene,
edatraxate, defofamine, dernecolcine, diaziquone, elfornithine, elliptiniurn
acetate,
etoglucid, gallium nitrate, hydroxyurea, lentinan, lonidamine, mitoguazone,
mopidamol,
nitracrine, pentostatin, phenamet, pirarubicin, podophyllinic acid, 2-
ethylhydrazide,
procarbazine, PSKO, razoxane, sizofiran, spirogermanium, tenuazonic acid,
triaziquone, 2,
2',2"-trichlorotriethylamine, urethan, vindesine, dacarbazine, mannomustine,
mitobronitol,
mitolactol, pipobroman, gacytosine, arabinoside ("Ara-C"), taxoids, e.g.
paclitaxel
(TAXOL", Bristol-Myers Squibb Oncology, Princeton, NJ) doxetaxel (TAXOTERE",
Rh6ne-Poulenc Rorer, Antony, France), gemcitabine, ifosfamide, vinorelbine,
navelbine,
novantrone, teniposide, aminopterin, xeloda, ibandronate, CPT-I 1,
topoisomerase
inhibitor RFS 2000, difluoromethylornithine (I?MFO), retinoic acid,
esperamicins,
capecitabine, and pharmaceutically acceptable salts, acids or derivatives of
any of the
above. Also included in this definition are anti-hormonal agents that act to
regulate or
inhibit hormone action on tumors such as anti-estrogens including for example
tamoxifen,
raloxifene, aromatase inhibiting 4(5)-imidazoles, 4 hydroxytamoxifen,
trioxifene,
keoxifene, LY 117018, onapristone, toremifene (Fareston), and anti-androgens
such as
flutamide, nilutamide, bicalutamide, leuprolide, and goserelin, and
pharmaceutically
acceptable salts, acids or derivatives of any of the above.
[0428] The conjugates of the invention can be used for modifying a given
biological
response, the therapeutic agent or dnig moiety is not to be construed as
limited to classical
chemical therapeutic agents. For example, the drug moiety may be a protein or
polypeptide possessing a desired biological activity. Such proteins may
include, for
example, a toxin such as abrin, ricin A, pseudomonas exotoxin, or diphtheria
toxin; a
protein such as tumor necrosis factor, alpha-interferon, beta-interferon,
nerve growth
factor, platelet derived growth factor, tissue plasminogen activator, an
apoptotic agent,
e.g., TNF-alpha, TNF-beta, AIM I (See, International Publication No. WO
97/33899),
AIM II (See, International Publication No. WO 97/34911), Fas Ligand (Takahashi
et al.,
Int. Imrnunol., 6:1567-15?4 (1994)), VEGI (See, International Publication No.
WO
225
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
99/23105), CD40 Ligand, a thrombotic agent or an anti- angiogenic agent, e.g.,
angiostatin
or endostatin; or, biological response modifiers such as, for example,
lymphokines,
interleulcin-1 ("IL-1"), interleukin-2 ("IL-2"), interleukin-6 ("IL-6"),
granulocyte
macrophage colony stimulating factor ("GM-CSF"), granulocyte colony
stimulating factor
("G-CSF"), or other growth factors.
[0429] Antibodies may also be attached to solid supports, which are
particularly useful
for immunoassays or purification of the target antigen. Such solid supports
include, but
are not limited to, glass, cellulose, polyacrylamide, nylon, polystyrene,
polyvinyl chloride
or polypropylene.
[0430] Techniques for conjugating such therapeutic moiety to antibodies are
well
known, see, e.g., Arnon et al., "Monoclonal Antibodies For Immunotargeting Of
Drugs In
Cancer Therapy", in Monoclonal Antibodies And Cancer Therapy, Reisfeld et al.
(eds.),
pp. 243-56 (Alan R. Liss, Inc. 1985); Hellstrom et al., "Antibodies For Drug
Delivery", in
Controlled Drug Delivery (2nd Ed.), Robinson et al. (eds.), pp. 623-53 (Marcel
Dekker,
Inc. 1987); Thorpe, "Antibody Carriers Of Cytotoxic Agents In Cancer Therapy:
A
Review", in Monoclonal Antibodies '84: Biological And Clinical Applications,
Pinchera et
al. (eds.), pp. 475-506 (1985); "Analysis, Results, And Future Prospective Of
The
Therapeutic Use Of Radiolabeled Antibody in Cancer Therapy", in Monoclonal
Antibodies For Cancer Detection And Therapy, Baldwin et al. (eds.), pp. 303-16
(Academic Press 1985), and Thorpe et al., "The Preparation And Cytotoxic
Properties Of
Antibody-Toxin Conjugates", Immunol. Rev. 62:119-58 (1982).
[0431] Alternatively, an antibody can be conjugated to a second antibody to
form an
antibody heteroconjugate as described by Segal in U.S. Patent No. 4,676,980,
which is
incorporated herein by reference in its entirety.
[0432] An antibody, with or without a therapeutic moiety conjugated to it,
administered alone or in combination with cytotoxic factors) and/or
cytokine(s) can be
used as a therapeutic.
Immunophenotyping
[0433] The antibodies of the invention may be utilized for immunophenotyping
of cell
lines and biological samples. The translation product of the gene of the
present invention
may be useful as a cell specific marker, or more specifically as a cellular
marker that is
226
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
differentially expressed at various stages of differentiation and/or
maturation of particular
cell types. Monoclonal antibodies directed against a specific epitope, or
combination of
epitopes, will allow for the screening of cellular populations expressing the
marker.
Various techniques can be utilized using monoclonal antibodies to screen for
cellular
populations expressing the marker(s), and include magnetic separation using
antibody-
coated magnetic beads, "panning" with antibody attached to a solid matrix
(i.e., plate), and
flow cytometry (See, e.g., U.S. Patent 5,95,660; and Mornson et al., Cell,
96:737-49
(1999)).
[0434] These techniques allow for the screening of particular populations of
cells,
such as might be found with hematological malignancies (i.e. minimal residual
disease
(MRD) in acute leukemic patients) and "non-self" cells in transplantations to
prevent
Graft-versus-Host Disease (GVHD). Alternatively, these techniques allow for
the
screening of hematopoietic stem and progenitor cells capable of undergoing
proliferation
and/or differentiation, as might be found in human umbilical cord blood.
Assays For Antibody Binding
[0435] The antibodies of the invention may be assayed for immunospecific
binding by
any method known in the art. The immunoassays which can be used, include but
are not,
limited to, competitive and non-competitive assay systems using techniques
such as
western blots, radioimmunoassays, ELISA (enzyme linked immunosorbent assay),
"sandwich" immunoassays, immunoprecipitation assays, precipitin reactions, gel
diffusion
precipitin reactions, immunodiffusion assays, agglutination assays, complement-
fixation
assays, immunoradiometric assays, fluorescent immunoassays, protein A
immunoassays,
to name but a few. Such assays are routine and well known in the art (see,
e.g., Ausubel et
al, eds, 1994, Current Protocols in Molecular Biology, Vol. 1, John Wiley &
Sons, Inc.,
New York, which is incorporated by reference herein in its entirety).
Exemplary
immunoassays are described briefly below (but are not intended by way of
limitation).
[0436] Immunoprecipitation protocols generally comprise lysing a population of
cells
in a lysis buffer such as RIPA buffer (1% NP-40 or Triton X-100, 1% sodium
deoxycholate, 0.1% SDS, 0.15 M NaCI, 0.01 M sodium phosphate at pH 7.2, 1%
Trasylol)
supplemented with protein phosphatase andlor protease inhibitors (e.g., EDTA,
PMSF,
aprotinin, sodium vanadate), adding the antibody of interest to the cell
lysate, incubating
227
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
for a period of time (e.g., 1-4 hours) at 4°C, adding protein A and/or
protein G sepharose
beads to the cell lysate, incubating for about an hour or more at 4° C,
washing the beads in
lysis buffer and resuspending the beads in SDS/sample buffer. The ability of
the antibody
of interest to immunoprecipitate a particular antigen can be assessed by,
e'.g., western blot
analysis. One of shill in the art would be knowledgeable as to the parameters
that can be
modified to increase the binding of the antibody to an antigen and decrease
the
background (e.g., pre-clearing the cell lysate with sepharose beads). For
further
discussion regarding immunoprecipitation protocols see, e.g., Ausubel et al,
eds, 1994,
Current Protocols in Molecular Biology, Vol. l, John Wiley & Sons, Inc., New
York at
10.16.1.
[0437] Western blot analysis generally comprises preparing protein samples,
electrophoresis of the protein samples in a polyacrylamide gel (e.g., 8%- 20%
SDS-PAGE
depending on the molecular weight of the antigen), transferring the protein
sample from
the polyacrylamide gel to a membrane such as nitrocellulose, PVDF or nylon,
blocking the
membrane in blocking solution (e.g., PBS with 3% BSA or non-fat milk), washing
the
membrane in washing buffer (e.g., PBS-Tween 20), blocking the membrane with
primary
antibody (the antibody of interest) diluted in blocking buffer, washing the
membrane in
washing buffer, blocking the membrane with a secondary antibody (which
recognizes the
primary antibody, e.g., an anti-human antibody) conjugated to an enzymatic
substrate
(e.g., horseradish peroxidase or alkaline phosphatase) or radioactive molecule
(e.g., 32P or
i2$I) diluted in blocking buffer, washing the membrane in wash buffer, and
detecting the
presence of the antigen. One of skill in the art would be knowledgeable as to
the
parameters that can be modified to increase the signal detected and to reduce
the
background noise. For further discussion regarding western blot protocols see,
e.g.,
Ausubel et al, eds, 1994, Current Protocols in Molecular Biology, Vol. 1, John
Wiley &
Sons, Inc., New York at 10.8.1.
[0438] ELISAs comprise preparing antigen, coating the well of a 96 well
microtiter
plate with the antigen, adding the antibody of interest conjugated to a
detectable
compound such as an enzymatic substrate (e.g., horseradish peroxidase or
alkaline
phosphatase) to the well and incubating for a period of time, and detecting
the presence of
the antigen. In ELISAs the antibody of interest does not have to be conjugated
to a
detectable compound; instead, a second antibody (which recognizes the antibody
of
228
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
interest) conjugated to a detectable compound may be added to the well.
Further, instead
of coating the well with the antigen, the antibody may be coated to the well.
In this case, a
second antibody conjugated to a detectable compound may be added following the
addition of the antigen of interest to the coated well. One of skill in the
art would be
knowledgeable as to the parameters that can be modified to increase the signal
detected as
well as other variations of ELISAs known in the art. For further discussion
regarding
ELISAs see, e.g., Ausubel et al, eds, 1994, Current Protocols in Molecular
Biology, Vol.
l, John Wiley & Sons, Inc., New York at 11.2.1.
[0439] The binding affinity of an antibody to an antigen and the off rate of
an
antibody-antigen interaction can be determined by competitive binding assays.
One
example of a competitive binding assay is a radioimmunoassay comprising the
incubation
of labeled antigen (e.g., 3H or lzsl) with the antibody of interest in the
presence of
increasing amounts of unlabeled antigen, and the detection of the antibody
bound to the
labeled antigen. The affinity of the antibody of interest for a particular
antigen and the
binding off-rates can be determined from the data by scatchard plot analysis.
Competition
with a second antibody can also be determined using radioimmunoassays. In this
case, the
antigen is incubated with antibody of interest conjugated to a labeled
compound (e.g., 3H
or lzsl) in the presence of increasing amounts of an unlabeled second
antibody.
Therapeutic Uses
[0440] The present invention is further directed to antibody-based therapies
which
involve administering antibodies of the invention to an animal, preferably a
mammal, and
most preferably a human, patient for treating one or more of the disclosed
diseases,
disorders, or conditions. Therapeutic compounds of the invention include, but
are not
limited to, antibodies of the invention (including fragments, analogs and
derivatives
thereof as described herein) and nucleic acids encoding antibodies of the
invention
(including fragments, analogs and derivatives thereof and anti-idiotypic
antibodies as
described herein). The antibodies of the invention can be used to treat,
inhibit or prevent
diseases, disorders or conditions associated with aberrant expression and/or
activity of a
polypeptide of the invention and/or a receptor for the polypeptide of the
invention (e.g.,
transmembrane activator and CAML interactor (TACI, GenBank accesion number
AAC~1790), and B-cell maturation antigen (BCMA, GenBank accession number
229
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
NP_001183)), including, but not limited to, any one or more of the diseases,
disorders, or
conditions described herein (e.g., autoimmune diseases, disorders, or
conditions associated
with such diseases or disorders, including, but not limited to, autoimmune
hemolytic
anemia (including but not limited to cryoglobinemia or Coombs positive
anemia),
autoimmune neonatal thrombocytopenia, idiopathic thrombocytopenia purpura,
autoimmunocytopenia, autoimmune neutropenia, hemolytic anemia,
antiphospholipid
syndrome, dermatitis (e.g., atopic dermatitis), allergic encephalomyelitis,
myocarditis,
relapsing polychondritis, rheumatic heart disease, Multiple Sclerosis,
Neuritis, Uveitis
Ophthalmic, Polyendocrinopathies, Purpura (e.g., Henloch-Scoenlein purpura),
Reiter's
Disease, Stiff-Man Syndrome, Autoimmune Pulmonary Inflammation, Guillain-Barre
Syndrome, diabetes mellitus (e.g., Type I diabetes mellitus or insulin
dependent diabetes
mellitis), juvenile onset diabetes,and autoimmune inflammatory eye, autoimmune
thyroiditis, hypothyroidism (i.e., Hashimoto's thyroiditis, systemic lupus
erhythematosus,
discoid lupus, Goodpasture's syndrome, Pemphigus, Receptor autoimmunities such
as, for
example, (a) Graves' Disease , (b) Myasthenia Gravis, and (c) insulin
resistance,
autoimmune hemolytic anemia, autoimmune thrombocytopenic purpura , rheumatoid
arthritis, schleroderma with anti-collagen antibodies, mixed connective tissue
disease,
polymyositis/dermatomyositis, pernicious anemia (Addison's disease),
idiopathic
Addison's disease, infertility, glomerulonephritis such as primary
glomerulonephritis, IgA
glomerulonephritis, and IgA nephropathy, bullous pemphigoid, Sjogren's
syndrome,
diabetes millitus, and adrenergic drug resistance (including adrenergic drug
resistance
with asthma or cystic fibrosis), gluten sensitive enteropathy, dense deposit
disease,
chronic active hepatitis, primary biliary cirrhosis, other endocrine gland
failure, vitiligo,
vasculitis, post-MI, cardiotomy syndrome, urticaria, atopic dermatitis,
asthma,
inflammatory myopathies, and other inflammatory, granulamatous, degenerative,
and
.atrophic disorders) and other disorders such as inflammatory skin diseases
including
psoriasis and sclerosis, responses associated with inflammatory bowel disease
(such as
Crohn's disease and ulcerative colitis), respiratory distress syndrome
(including adult
respiratory distress syndrome, ARDS), meningitis, encephalitis, colitis,
allergic conditions
such as eczema and other conditions involving infiltration of T cells and
chronic
inflammatory responses, atherosclerosis, leukocyte adhesion deficiency,
Reynaud's
syndrome, and immune responses associated with acute and delayed
hypersensitivity
230
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
mediated by cytokines and T-lymphocytes typically found in tuberculosis,
sarcoidosis,
granulomatosis and diseases involving leukocyte diapedesis, central nervous
system
(CNS) inflammatory disorder, multiple organ injury syndrome, antigen-antibody
complex
mediated diseases, anti-glomerular basement membrane disease, Lambert-Eaton
myasthenic syndrome, Beheet disease, giant cell arteritis, immune complex
nephritis, IgA
nephropathy, IgM polyneuropathies or autoimmune thrombocytopenia etc.
[0441] In a specific embodiment, antibodies of the invention are used to
treat, inhibit,
prognose, diagnose or prevent rheumatoid arthritis. In a specific embodiment,
antibodies
of the invention are used to treat, inhibit, prognose, diagnose or prevent
advanced
rheumatoid arthritis.
[0442] In another specific embodiment, antibodies of the invention are used to
treat,
inhibit, prognose, diagnose or prevent systemic lupus erythematosis.
[0443] For example, an antibody, or antibodies, of the present invention are
used to
treat patients with clinical diagnosis of rheumatoid arthritis (RA). The
patient treated will
not have a B cell malignancy. Moreover, the patient is optionally further
treated with any
one or more agents employed for treating RA such as salicylate; nonsteroidal
anti-inflammatory drugs such as indomethacin, phenylbutazone, phenylacetic
acid
derivatives (e.g. ibuprofen and fenoprofen), naphthalene acetic acids
(naproxen),
pyrrolealkanoic acid (tometin), indoleacetic acids (sulindac), halogenated
anthranilic acid
(meclofenamate sodium), piroxicam, zomepirac and diflunisal; antimalarials
such as
chloroquine; gold salts; penicillamine; or immunosuppressive agents such as
methotrexate
or corticosteroids in dosages known for such drugs or reduced dosages.
Preferably
however the patient is only treated with an antibody, or antibodies, of the
present
invention. Antibodies of the present invention are administered to the RA
patient
according to a dosing schedule as described infra, which may be readily
determined by
one of,ordinary skill in the art. The primary response is determined by the
Paulus index
(Paulus et al. Athritis Rheum. 33:477-4~4 (1990)), i.e. improvement in morning
stiffness,
number of painful and inflamed joints, erythrocyte sedimentation (ESR), and at
least a
2-point improvement on a 5-point scale of disease severity assessed by patient
and by
physician. Administration of an antibody, or antibodies, of the present
invention will
alleviate one or more of the symptoms of RA in the patient treated as
described above.
231
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0444] In a further specific embodiment, antibodies of the invention are used
to treat,
inhibit, prognose, diagnose or prevent hemolytic anemia. For example, patients
diagnosed
with autoimmune hemolytic anemia (AIHA), e.g., cryoglobinemia or Coombs
positive
anemia, are treated with an antibody, or antibodies, of the present invention.
AIHA is an
acquired hemolytic anemia due to auto-antibodies that react with the patient's
red blood
cells. The patient treated will not have a B cell malignancy. Further adjunct
therapies
(such as glucocorticoids, prednisone, azathioprine, cyclophosphamide, vinca-
laden
platelets or Danazol) may be combined with the antibody therapy, but
preferably the
patient is treated with an antibody, or antibodies, of the present invention
as a single-agent
throughout the course of therapy. Antibodies of the present invention are
administered to
the hemolytic anemia patient according to a dosing schedule as described
infra, which
may be readily determined by one of ordinary skill in the art. Overall
response rate is
determined based upon an improvement in blood counts, decreased requirement
for
transfusions, improved hemoglobin levels and/or a decrease in the evidence of
hemolysis
as determined by standard chemical parameters. Administration of an antibody,
or
antibodies of the present invention will improve any one or more of the
symptoms of
hemolytic anemia in the patient treated as described above. For example, the
patient
treated as described above will show an increase in hemoglobin and an
improvement in
chemical parameters of hemolysis or return to normal as measured by serum
lactic
dehydrogenase and/or bilirubin.
[0445] In another specific embodiment, antibodies of the invention are used to
treat,
inhibit, prognose, diagnose or prevent adult immune thrombocytopenic purpura.
Adult
immune thrambocytopenic purpura (ITP) is a relatively rare hematologic
disorder that
constitutes the most common of the immune-mediated cytopenias. The disease
typically
presents with severe thrombocytopenia that may be associated with acute
hemorrhage in
the presence of normal to increased megakaryacytes in the bone marrow. Most
patients
with ITP have an IgG antibody directed against target antigens on the outer
surface of the
platelet membrane, resulting in platelet sequestration in the spleen and
accelerated
reticuloendothelial destruction of platelets (Bussell, J.B. Hematol. Oncol.
Clin. North Am.
(4):179 (1990)). A number of therapeutic interventions have been shown to be
effective in
the treatment of ITP. Steroids are generally considered first-line therapy,
after which most
patients are candidates for intravenous immunoglobulin (IVIG), splenectomy, or
other
232
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
medical therapies including vincristine or immunosuppressive/cytotoxic agents.
Up to
80% of patients with ITP initially respond to a course of steroids, but far
fewer have
complete and lasting remissions. Splenectomy has been recommended as standard
second-line therapy for steroid failures, and leads to prolonged remission in
nearly 60% of
cases yet may result in reduced immunity to infection. Splenectomy is a major
surgical
procedure that may be associated with substantial morbidity (15%) and
mortality (2%).
IVIG has also been used as second line medical therapy, although only a small
proportion
of adult patients with ITP achieve remission. Therapeutic options that would
interfere
with the production of autoantibodies by activated B cells without the
associated
morbidities that occur with corticosteroids andJor splenectomy would provide
an
important treatment approach for a proportion of patients with ITP. Patients
with clinical
diagnosis of ITP are treated with an antibody, or antibodies of the present
invention,
optionally in combination with steroid therapy. The patient treated will not
have a B cell
malignancy. Antibodies of the present invention are administered to the RA
patient
according to a dosing schedule as described infra, which may be readily
determined by
one of ordinary skill in the art. Overall patient response rate is determined
based upon a
platelet count determined on two consecutive occasions two weeks apart
following
treatments as described above. See, George et al. "Idiopathic Thrombocytopenic
Purpura:
A Practice Guideline Developed by Explicit Methods for The American Society of
Hematology", Blood 88:3-40 (1996), expressly incorporated herein by reference.
[0446] In other embodiments, antibody agonists of the invention are be used to
treat,
inhibit or prevent immunodeficiencies, and/or disorders, or conditions
associated with
immunodeficiencies. Such immunodeficiencies include, but are not limited to,
severe
combined immunodeficiency (SCID)-X linked, SCID-autosomal, adenosine deaminase
deficiency (ADA deficiency), X-linked agammaglobulinemia (XLA), Bruton's
disease,
congenital agammaglobulinemia, X-linked infantile agammaglobulinemia, acquired
agammaglobulinemia, adult onset agamrnaglobulinemia, late-onset
agammaglobulinemia,
dysgammaglobulinemia, hypogammaglobulinemia, transient hypogammaglobulinemia
of
infancy, unspecified hypogammaglobulinemia, agammaglobulinemia, common
variable
immunodeficiency (CVID) (acquired), Wiskott-Aldrich Syndrome (WAS), X-linked
immunodeficiency with hyper IgM, non X-linked immunodeficiency with hyper IgM,
selective IgA deficiency, IgG subclass deficiency (with or without IgA
deficiency),
233
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
antibody deficiency with normal or elevated Igs, immunodeficiency with
thymoma, Ig
heavy chain deletions, kappa chain deficiency, B cell lymphoproliferative
disorder
(BLPD), selective IgM immunodeficiency, recessive agammaglobulinemia (Swiss
type),
reticular dysgenesis, neonatal neutropenia, severe congenital leulcopenia,
thymic
alymphoplasia-aplasia or dysplasia with immunodeficiency, ataxia-
telangiectasia, short
limbed dwarfism, X-linked lymphoproliferative syndrome (XLP), Nezelof syndrome-
combined immunodeficiency with Igs, purine nucleoside phosphorylase deficiency
(PNP),
MHC Class II deficiency (Bare Lymphocyte Syndrome) and severe combined
immunodeficiency.
[0447] In another specific embodiment, antibodies of the invention are used to
treat,
inhibit, prognose, diagnose or prevent CVID, or a subgroup of individuals
having CVID.
[0448] In another specific embodiment, antibody agonists of the invention are
used as
an adjuvant to stimulate B cell proliferation, immunoglobulin production,
and/or to
enhance B cell survival.
[0449] The treatment and/or prevention of diseases, disorders, or conditions
associated
with aberrant expression and/or activity of a polypeptide of the invention
andlor a receptor
for the polypeptide of the invention (e.g., TACI, BCMA) includes, but is not
limited to,
alleviating symptoms associated with those diseases, disorders or conditions.
The
antibodies of the invention may also be used to target and kill cells
expressing Neutrokine-
alpha on their surface and/or cells having Neutrokine-alpha bound to their
surface.
Antibodies of the invention may be provided in pharmaceutically acceptable
compositions
as known in the art or as described herein.
[0450] A summary of the ways in which the antibodies of the present invention
may
be used therapeutically includes binding polynucleotides or polypeptides of
the present
invention locally or systemically in the body or by direct cytotoxicity of the
antibody, e.g.
as mediated by complement (CDC) or by effector cells (ADCC). Some of these
approaches are described in more detail below. Armed with the teachings
provided
herein, one of ordinary skill in the art will know how to use the antibodies
of the present
invention for diagnostic, monitoring or therapeutic purposes without undue
experimentation.
[0451] The antibodies of this invention may be advantageously utilized in
combination with other monoclonal or chimeric antibodies, or with lymphokines
or
234
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
hematopoietic growth factors (such as, e.g., IL-2, IL-3 and IL-7), for
example, which serve
to increase the number or activity of effector cells which interact with the
antibodies.
[0452] The antibodies of the invention may be administered alone or in
combination
with other types of treatments (e.g., radiation therapy, chemotherapy,
hormonal therapy,
immunotherapy, anti-tumor agents, antibiotics, and immunoglobulin). Generally,
administration of products of a species origin or species reactivity (in the
case of
antibodies) that is the same species as that of the patient is preferred.
Thus, in a preferred
embodiment, human antibodies, fragments derivatives, analogs, or nucleic
acids, are
administered to a human patient for therapy or prophylaxis.
[0453] It is preferred to use high affinity and/or potent in vivo inhibiting
and/or
neutralizing antibodies against polypeptides or polynucleotides of the present
invention,
fragments or regions thereof, for both immunoassays directed to and therapy of
disorders
related to polynucleotides' or polypeptides, including fragments thereof, of
the present
invention. Such antibodies, fragments, or regions, will preferably have an
affinity for
polynucleotides or polypeptides of the invention, including fragments thereof.
Preferred
binding affinities include those with a dissociation constant or Kd less than
5 X 10-5 M,
10-5 M, 5 X 10-~ M, 10-~ M, 5 X 10-~ M, 10-' M, 5 X 10-$ M, 10-$ M, 5 X 10-9
M, 10-~ M, 5
X 10-1 o M, 10-1 o M, 5 X 10-11 M, 10-11 M, 5 X 10-1 z M, 10-1 z M, 5 X 10-1 s
M, 10-13 M, 5 X
10-14 M, 10-14 M, 5 X 10-15 M, and 10-15 M.
Gene Therapy
[0454] In a specific embodiment, nucleic acids comprising sequences encoding
antibodies or functional derivatives thereof, are administered to treat,
inhibit or prevent a
disease or disorder associated with aberrant expression and/or activity of a
polypeptide of
the invention, by way of gene therapy. Gene therapy refers to therapy
performed by the
administration to a subject of an expressed or expressible nucleic acid. In
this
embodiment of the invention, the nucleic acids produce their encoded protein
that
mediates a therapeutic effect.
[0455] Any of the methods for gene therapy available in the art can be used
according
to the present invention. Exemplary methods are described below.
[0456] For general reviews of the methods of gene therapy, see Goldspiel et
al.,
Clinical Pharmacy 12:488-505 (1993); Wu and Wu, Biotherapy 3:87-95 (1991);
235
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
Tolstoshev, Ann. Rev. Pharmacol. Toxicol. 32:573-596 (1993); Mulligan, Science
260:926-932 (1993); and Morgan and Anderson, Ann. Rev. Biochem. 62:191-217
(1993);
May, TIBTECH 11(5):155-215 (1993). Methods commonly known in the art of
recombinant DNA technology which can be used are described in Ausubel et al.
(eds.),
Current Protocols in Molecular Biology, John Wiley & Sons, NY (1993); and
Kriegler,
Gene Transfer and Expression, A Laboratory Manual, Stockton Press, NY (1990).
[0457] In a preferred embodiment, the compound comprises nucleic acid
sequences
encoding an antibody, said nucleic acid sequences being part of expression
vectors that
express the antibody or fragments or chimeric proteins or heavy or light
chains thereof in a
suitable host. In particular, such nucleic acid sequences have promoters
operably linked to
the antibody coding region, said promoter being inducible or constitutive,
and, optionally,
tissue-specific. In another particular embodiment, nucleic acid molecules are
used in
which the antibody coding sequences and any other desired sequences are
flanked by
regions that promote homologous recombination at a desired site in the genome,
thus
providing for intrachromosomal expression of the antibody encoding nucleic
acids (Koller
and Smithies, Proc. Natl. Acad. Sci. USA 86:8932-8935 (1989); . Zijlstra et
al., Nature
342:435-438 (1989). In specific embodiments, the expressed antibody molecule
is a
single chain antibody; alternatively, the nucleic acid sequences include
sequences
encoding both the heavy and light chains, or fragments thereof, of the
antibody.
[0458] Delivery of the nucleic acids into a patient may be either direct, in
which case
the patient is directly exposed to the nucleic acid or nucleic acid-carrying
vectors, or
indirect, in which case, cells are first transformed with the nucleic acids in
vitro, then
transplanted into the patient. These two approaches are known, respectively,
as in vivo or
ex vivo gene therapy.
[0459] In a specific embodiment, the nucleic acid sequences are directly
administered
in vivo, where it is expressed to produce the encoded product. This can be
accomplished
by any of numerous methods known in the art, e.g., by constructing them as
part of an
appropriate nucleic acid expression vector and administering it so that they
become
intracellular, e.g., by infection using defective or attenuated retrovirals or
other viral
vectors (see U.S. Patent No. 4,980,286), or by direct injection of naked DNA,
or by use of
microparticle bombardment (e.g., a gene gun; Biolistic, Dupont), or coating
with lipids or
cell-surface receptors or transfecting agents, encapsulation in liposomes,
microparticles, or
236
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
microcapsules, or by administering them in linkage to a peptide which is known
to enter
the nucleus, by administering it in linkage to a ligand subject to receptor-
mediated
endocytosis (see, e.g., Wu and Wu, J. Biol. Chem. 262:4429-4432 (1987)) (which
can be
used to target cell types specifically expressing the receptors), etc. In
another
embodiment, nucleic ~ acid-ligand complexes can be formed in which the ligand
comprises
a fusogenic viral peptide to disrupt endosomes, allowing the nucleic acid to
avoid
lysosomal degradation. In yet another embodiment, the nucleic acid can be
targeted ira
vivo for cell specific uptake and expression, by targeting a specific receptor
(see, e.g., PCT
Publications WO 92/06180; WO 92/22635; W092/20316; W093/14188, WO 93/20221).
Alternatively, the nucleic acid can be introduced intracellularly and
incorporated within
host cell DNA for expression, by homologous recombination (Koller and
Smithies, Proc.
Natl. Acad. Sci. USA 86:8932-8935 (1989); Zijlstra et al., Nature 342:435-438
(1989)).
[0460] In a specific embodiment, viral vectors that contain nucleic acid
sequences
encoding an antibody of the invention are used. For example, a retroviral
vector can be
used (see Miller et al., Meth. Enzymol. 217:581-599 (1993)). These retroviral
vectors
contain the components necessary for the correct packaging of the viral genome
and
integration into the host cell DNA. The nucleic acid sequences encoding the
antibody to
be used in gene therapy are cloned into one or more vectors, which facilitates
delivery of
the gene into a patient. More detail about retroviral vectors can be found in
Boesen et al.,
Biotherapy 6:291-302 (1994), which describes the use of a retroviral vector to
deliver the
mdrl gene to hematopoietic stem cells in order to make the stem cells more
resistant to
chemotherapy. Other references illustrating the use of retroviral vectors in
gene therapy
are: Clowes et al., J. Clin. Invest. 93:644-651 (1994); Kiem et al., Blood
83:1467-1473
(1994); Salmons and Gunzberg, Human Gene Therapy 4:129-141 (1993); and
Grossman
and Wilson, Curr. Opin. in Genetics and Devel. 3:110-114 (1993).
[0461] Adenoviruses are other viral vectors that can be used in gene therapy.
Adenoviruses are especially attractive vehicles for delivering genes to
respiratory
epithelia. Adenoviruses naturally infect respiratory epithelia where they
cause a mild
disease. Other targets for adenovirus-based delivery systems are liver, the
central nervous
system, endothelial cells, and muscle. Adenoviruses have the advantage of
being capable
of infecting non-dividing cells. Kozarsky and Wilson, Current Opinion in
Genetics and
Development 3:499-503 (1993) present a review of adenovirus-based gene
therapy. Bout
237
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
et al., Human Gene Therapy 5:3-10 (1994) demonstrated the use of adenovirus
vectors to
transfer genes to the respiratory epithelia of rhesus monkeys. Other instances
of the use of
adenoviruses in gene therapy can be found in Rosenfeld et al., Science 252:431-
434
(1991); Rosenfeld et al., Cell 68:143- 155 (1992); Mastrangeli et al., J.
Clin. Invest.
91:225-234 (1993); PCT Publication W094/12649; and Wang, et al., Gene Therapy
2:775-783 (1995). In a preferred embodiment, adenovirus vectors are used.
[0462] Adeno-associated virus (AAV) has also been proposed for use in gene
therapy
(Walsh et al., Proc. Soc. Exp. Biol. Med. 204:289-300 (1993); U.S. Patent No.
5,436,146).
[0463] Another approach to gene therapy involves transferring a gene to cells
in tissue
culture by such methods as electroporation, lipofection, calcium phosphate
mediated
transfection, or viral infection. Usually, the method of transfer includes the
transfer of a
selectable marker to the cells. The cells are then placed under selection to
isolate those
cells that have taken up and are expressing the transferred gene. Those cells
are then
delivered to a patient.
[0464] In this embodiment, the nucleic acid is introduced into a cell prior to
administration in vivo of the resulting recombinant cell. Such introduction
can be carried
out by any method known in the art, including but not limited to transfection,
electroporation, microinjection, infection with a viral or bacteriophage
vector containing
the nucleic acid sequences, cell fusion, chromosome-mediated gene transfer,
microcell-
mediated gene transfer, spheroplast fusion, etc. Numerous techniques are known
in the art
for the introduction of foreign genes into cells (see, e.g., Loeffler and
Behr, Meth.
Enzymol. 217:599-618 (1993); Cohen et al., Meth. Enzymol. 217:618-644 (1993);
Clin.,
Pharmac. Ther. 29:69-92m (1985) and may be used in accordance with the present
invention, provided that the necessary developmental and physiological
functions of the
recipient cells are not disrupted. The technique should provide for the stable
transfer, of
the nucleic acid to the cell, so that the nucleic acid is expressible by the
cell and preferably
heritable and expressible by its cell progeny.
[0465] The resulting recombinant cells can be delivered to a patient by
various
methods known in the art. Recombinant blood cells (e.g., hematopoietic stem or
progenitor cells) are preferably administered intravenously. The amount of
cells
envisioned for use depends on the desired effect, patient state, etc., and can
be determined
by one skilled in the art.
238
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0466] Cells into which a nucleic acid can be introduced for purposes of gene
therapy
encompass any desired, available cell type, and include, but are not limited
to, epithelial
cells, endothelial cells, keratinocytes, fibroblasts, muscle cells,
hepatocytes; blood cells
such as T lymphocytes, B lymphocytes, monocytes, macrophages, neutrophils,
eosinophils, megakaryocytes, granulocytes; various stem or progenitor cells,
in particular
hematopoietic stem or progenitor cells, e.g., as obtained from bone marrow,
umbilical
cord blood, peripheral blood, fetal liver, etc.
[0467] In a preferred embodiment, the cell used for gene therapy is autologous
to the
patient.
[0468] In an embodiment in which recombinant cells are used in gene therapy,
nucleic
acid sequences encoding an antibody are introduced into the cells such that
they are
expressible by the cells or their progeny, and the recombinant cells are then
administered
ifa vivo for therapeutic effect. In a specific embodiment, stem or progenitor
cells are used.
Any stem and/or progenitor cells which can be isolated and maintained in vitro
can
potentially be used in accordance with this embodiment of the present
invention (see e.g.
PCT Publication WO 94/08598; Stemple and Anderson, Cell 71:973-985 (1992);
Rheinwald, Meth. Cell Bio. 21A:229 (1980); and Pittelkow and Scott, Mayo
Clinic Proc.
61:771 (1986)).
[0469] In a specific embodiment, the nucleic acid to be introduced for
purposes of
gene therapy comprises an inducible promoter operably linked to the coding
region, such
that expression of the nucleic acid is controllable by controlling the
presence or absence of
the appropriate inducer of transcription.
Demonstration of Therapeutic or Prophylactic Activity
[0470] The compounds or pharmaceutical compositions of the invention are
preferably
tested in vitro, and then ifa vivo for the desired therapeutic or prophylactic
activity, prior to
use in humans. For example, in vitro assays to demonstrate the therapeutic or
prophylactic utility of a compound or pharmaceutical composition include, the
effect of a
compound on a cell line or a patient tissue sample. The effect of the compound
or
composition on the cell line and/or tissue sample can be determined utilizing
techniques
known to those of skill in the art including, but not limited to, rosette
formation assays and
cell lysis assays. In accordance with the invention, ifa vitro assays which
can be used to
239
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
determine whether administration of a specific compound is indicated, include
in vitro cell
culture assays in which a patient tissue sample is grown in culture, and
exposed to or
otherwise administered a compound, and the effect of such compound upon the
tissue
sample is observed.
Therapeutic and/or Prophylactic Administration and Composition
[0471] The invention provides methods of treatment, inhibition and prophylaxis
by
administration to a subject of an effective amount of a compound or
pharmaceutical
composition of the invention, preferably an antibody of the invention. In a
preferred
embodiment, the compound is substantially purified (e.g., substantially free
from
substances that limit its effect or produce undesired side effects). The
subject is preferably
an animal, including but not limited to animals such as cows, pigs, horses,
chickens, cats,
dogs, etc., and is preferably a mammal, and most preferably human.
[0472] Formulations and methods of administration that can be employed when
the
compound comprises a nucleic acid or an immunoglobulin are described above;
additional
appropriate formulations and routes of administration can be selected from
among those
described herein below.
[0473] Various delivery systems are known and can be used to administer a
compound
of the invention, e.g., encapsulation in liposomes, microparticles,
microcapsules,
recombinant cells capable of expressing the compound, receptor-mediated
endocytosis
(see, e.g., Wu and Wu, J. Biol. Chem. 262:4429-4432 (1987)), construction of a
nucleic
acid as part of a retroviral or other vector, etc. Methods of introduction
include but are not
limited to intradermal, intramuscular, intraperitoneal, intravenous,
subcutaneous,
intranasal, epidural, and oral routes. The compounds or compositions may be
administered by any convenient route, for example by infusion or bolus
injection, by
absorption through epithelial or mucocutaneous linings (e.g., oral mucosa,
rectal and
intestinal mucosa, etc.) and may be administered together with other
biologically active
agents. Administration can be systemic or local. In addition, it may be
desirable to
introduce the pharmaceutical compounds or compositions of the invention into
the central
nervous system by any suitable route, including intraventricular and
intrathecal injection;
intraventricular injection may be facilitated by an intraventricular catheter,
for example,
attached to a reservoir, such as an Ommaya reservoir. Pulmonary administration
can also
240
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
be employed, e.g., by use of an inhaler or nebulizer, and formulation with an
aerosolizing
agent.
[0474] In a specific embodiment, it may be desirable to administer the
pharmaceutical
compounds or compositions of the invention locally to the area in need of
treatment; this
may be achieved by, for example, and not by way of limitation, local infusion
during
surgery, topical application, e.g., in conjunction with a wound dressing after
surgery, by
injection, by means of a catheter, by means of a suppository, or by means of
an implant,
said implant being of a porous, non-porous, or gelatinous material, including
membranes,
such as sialastic membranes, or fibers. Preferably, when administering a
protein,
including an antibody, of the invention, care must be taken to use materials
to which the
protein does not absorb.
[0475] In another embodiment, the compound or composition can be delivered in
a
vesicle, in particular a liposome (see Langer, Science 249:1527-1533 (1990);
Treat et al.,
in Liposomes in the Therapy of Infectious Disease and Cancer, Lopez-Berestein
and
Fidler (eds.), Liss, New York, pp. 353- 365 (1989); Lopez-Berestein, ibid.,
pp. 317-327;
see generally ibid.)
[0476] In yet another embodiment, the compound or composition can be delivered
in a
controlled release system. In one embodiment, a pump may be used (see Langer,
supra;
Sefton, CRC Crit. Ref. Biomed. Eng. 14:201 (1987); Buchwald et al., Surgery
88:507
(1980); Saudek et al., N. Engl. J. Med. 321:574 (1989)). In another
embodiment,
polymeric materials can be used (see Medical Applications of Controlled
Release, Langer
and Wise (eds.), CRC Press, Boca Raton, Florida (1974); Controlled Drug
Bioavailability,
Drug Product Design and Performance, Smolen and Ball (eds.), Wiley, New York
(1984);
Ranger and Peppas, J., Macromol. Sci. Rev. Macromol. Chem. 23:61 (1983); see
also
Levy et al., Science 228:190 (1985); During et al., Ann. Neurol. 25:351
(1989); Howard et
al., J.Neurosurg. 71:105 (1989)). In yet another embodiment, a controlled
release system
can be placed in proximity of the therapeutic target, i.e., the brain, thus
requiring only a
fraction of the systemic dose (see, e.g., Goodson, in Medical Applications of
Controlled
Release, supra, vol. 2, pp. 115-138 (1984)).
[0477] Other controlled release systems are discussed in the review by Langer
(Science 249:1527-1533 (1990)).
241
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
[0478] In a specific embodiment where the compound of the invention is a
nucleic
acid encoding a protein, the nucleic acid can be administered in vivo to
promote
expression of its encoded protein, by constructing it as part of an
appropriate nucleic acid
expression vector and administering it so that it becomes intracellular, e.g.,
by use of a
retroviral vector (see U.S. Patent No. 4,980,286), or by direct injection, or
by use of
microparticle bombardment (e.g., a gene gun; Biolistic, Dupont), or coating
with lipids or
cell-surface receptors or transfecting agents, or by administering it in
linkage to a
homeobox- like peptide which is known to enter the nucleus (see e.g., Joliot
et al., Proc.
Natl. Acad. Sci. USA 88:1864-1868 (1991)), etc. Alternatively, a nucleic acid
can be
introduced intracellularly and incorporated within host cell DNA for
expression, by
homologous recombination.
[0479] The present invention also provides pharmaceutical compositions. Such
compositions comprise a therapeutically effective amount of a compound, and a
pharmaceutically acceptable carrier. In a specific embodiment, the term
"pharmaceutically acceptable" means approved by a regulatory agency of the
Federal or a
state government or listed in the U.S. Pharmacopeia or other generally
recognized
pharmacopeia for use in animals, and more particularly in humans. The term
"carrier"
refers to a diluent, adjuvant, excipient, or vehicle with which the
therapeutic is
administered. Such pharmaceutical carriers can be sterile liquids, such as
water and oils,
including those of petroleum, animal, vegetable or synthetic origin, such as
peanut oil,
soybean oil, mineral oil, sesame oil and the like. Water is a preferred
carrier when the
pharmaceutical composition is administered intravenously. Saline solutions and
aqueous
dextrose and glycerol solutions can also be employed as liquid carriers,
particularly for
injectable solutions. Suitable pharmaceutical excipients include starch,
glucose, lactose,
sucrose, gelatin, malt, rice, flour, chalk, silica gel, sodium stearate,
glycerol monostearate,
talc, sodium chloride, dried skim milk, glycerol, propylene, glycol, water,
ethanol and the
like. The composition, if desired, can also contain minor amounts of wetting
or
emulsifying agents, or pH buffering agents. These compositions can take the
form of
solutions, suspensions, emulsion, tablets, pills, capsules, powders, sustained-
release
formulations and the like. The composition can be formulated as a suppository,
with
traditional binders and carriers such as triglycerides. Oral formulation can
include
standard Garners such as pharmaceutical grades of mannitol, lactose, starch,
magnesium
242
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
stearate, sodium saccharine, cellulose, magnesium carbonate, etc. Examples of
suitable
pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences"
by E.W.
Martin. Such compositions will contain a therapeutically effective amount of
the
compound, preferably in purified form, together with a suitable amount of
carrier so as to
provide the form for proper administration to the patient. The formulation
should suit the
mode of administration.
[0480] In a preferred embodiment, the composition is formulated in accordance
with
routine procedures as a pharmaceutical composition adapted for intravenous
administration to human beings. Typically, compositions for intravenous
administration
are solutions in sterile isotonic aqueous buffer. Where necessary, the
composition may
also include a solubilizing agent and a local anesthetic such as lignocaine to
ease pain at
the site of the injection. Generally, the ingredients are supplied either
separately or mixed
together in unit dosage form, for example, as a dry lyophilized powder or
water free
concentrate in a hermetically sealed container such as an ampoule or sachette
indicating
the quantity of active agent. Where the composition is to be administered by
infusion, it
can be dispensed with an infusion bottle containing sterile pharmaceutical
grade water or
saline. Where the composition is administered by injection, an ampoule of
sterile water
for injection or saline can be provided so that the ingredients may be mixed
prior to
administration.
[0481] The compounds of the invention can be formulated as neutral or salt
forms.
Pharmaceutically acceptable salts include those formed with anions such as
those derived
from hydrochloric, phosphoric, acetic, oxalic, tartaric acids, etc., and those
formed with
rations such as those derived from sodium, potassium, ammonium, calcium,
ferric
hydroxides, isopropylamine, triethylamine, 2-ethylamino ethanol, histidine,
procaine, etc.
[0482] The amount of the compound of the invention which will be effective in
the
treatment, inhibition and prevention of a disease or disorder associated with
aberrant
expression and/or activity of a polypeptide of the invention can be determined
by standard
clinical techniques. In addition, ifz vitro assays may optionally be employed
to help
identify optimal dosage ranges. The precise dose to be employed in the
formulation will
also depend on the route of administration, and the seriousness of the disease
or disorder,
and should be decided according to the judgment of the practitioner and each
patient's
243
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
circumstances. Effective doses may be extrapolated from dose-response curves
derived
from in vitro or animal model test systems.
[0483] For antibodies, the dosage administered to a patient is typically 0.1
mg/lcg to
100 mg/lcg of the patient's body weight. Preferably, the dosage administered
to a patient
is between 0.1 mg/kg and 20 mg/lcg of the patient's body weight, more
preferably 1 mg/kg
to 10 mg/kg of the patient's body weight. Generally, human antibodies have a
longer half-
life within the human body than antibodies from other species due to the
immune response
to the foreign polypeptides. Thus, lower dosages of human antibodies and less
frequent
administration is often possible. Further, the dosage and frequency of
administration of
antibodies of the invention may be reduced by enhancing uptake and tissue
penetration
(e.g., into the brain) of the antibodies by modifications such as, for
example, lipidation.
[0484] The invention also provides a pharmaceutical pack or kit comprising one
or
more containers filled with one or more of the ingredients of the
pharmaceutical
compositions of the invention. Optionally associated with such containers) can
be a
notice in the form prescribed by a governmental agency regulating the
manufacture, use or
sale of pharmaceuticals or biological products, which notice reflects approval
by the
agency of manufacture, use or sale for human administration.
Diagnosis and Imaging
[0485] Labeled antibodies, and derivatives and analogs thereof, which
specifically
bind to a polypeptide of interest can be used for diagnostic purposes to
detect, diagnose, or
monitor diseases and/or disorders associated with the aberrant expression
and/or activity
of a polypeptide of the invention. The invention provides for the detection of
aberrant
expression of a polypeptide of interest, comprising (a) assaying the
expression of the
polypeptide of interest in cells or body fluid of an individual using one or
more antibodies
specific to the polypeptide interest and (b) comparing the level of gene
expression with a
standard gene expression level, whereby an increase or decrease in the assayed
polypeptide gene expression level compared to the standard expression level is
indicative
of aberrant expression.
[0486] The invention provides a diagnostic assay for diagnosing a disorder,
comprising (a) assaying the expression of the polypeptide of interest in cells
or body fluid
of an individual using one or more antibodies specific to the polypeptide
interest and (b)
244
CA 02419661 2003-02-13
WO 02/18620 PCT/USO1/25549
comparing the level of gene expression with a standard gene expression level,
whereby an
increase or decrease in the assayed polypeptide gene expression level compared
to the
standard expression level is indicative of a particular disorder. With respect
to cancer, the
presence of a relatively high amount of transcript in biopsied tissue from an
individual
may indicate a predisposition for the development of the disease, or may
provide a means
for detecting the disease prior to the appearance of actual clinical symptoms.
A more
definitive diagnosis of this type may allow health professionals to employ
preventative
measures or aggressive treatment earlier thereby preventing the development or
further
progression of the cancer.
[0487] Antibodies of the invention can be used to assay protein levels in a
biological
sample using classical immunohistological methods known to those of skill in
the art
(e.g., see Jalkanen, et al., J. Cell. Biol. 101:976-985 (1985); Jalkanen, et
al., J. Cell. Biol.
105:3087-3096 (1987)). Other antibody-based methods useful for detecting
protein gene
expression include immunoassays, such as the enzyme linked immunosorbent assay
(ELISA) and the radioimmunoassay (RIA). Suitable antibody assay labels are
known in
the art and include enzyme labels, such as, glucose oxidase; radioisotopes,
such as iodine
(i3ih izsh iz3h lzil)~ c~.bon (14C), sulfur (35S), tritium (3H), indium
(llsmIn, tl3mIn, l2In,
111In), and technetium (~~Tc, ~~n'T~), thallium (z°1Ti), gallium (~BGa,
6~Ga), palladium
(io3Pd), molybdenum (9~Mo), xenon (133Xe), fluorine (1sF), ls3Sm, l~~Lu,
IS~Gd, l4~Pm,
iaoLa~ ns~,b~ l6GHo~ 9oY~ aaSc~ is6Re, lssRe, l4zpr, iosRh, ~~Ru; luminescent
labels, such as
luminol; and fluorescent labels, such as fluorescein and rhodamine, and
biotin.
[0488] In specific embodiments, antibodies of the invention are attached to
macrocyclic chelators useful for conjugating radiometal ions, including but
not limited to,
I~~Lu, ~o~,, 166H0, and IssSm, to polypeptides. In a preferred embodiment, the
radiometal
ion associated with the macrocyclic chelator attached to antibodies of the
invention is
mln. In another preferred embodiments, the radiometal ion associated with the
macrocyclic chelator attached to antibodies of the invention is ~°Y. In
specific
embodiments, the macrocyclic chelator is 1,4,7,10-tetraazacyclododecane-
N,N',N",N"'-
tetraacetic acid (DOTA). In other specific embodiments, the DOTA is attached
to the
Neutrokine-alpha andlor Neutrokine-alphaSV polypeptide of the invention via a
linker
molecule. Examples of linker molecules useful for conjugating DOTA to a
polypeptide
are commonly known in the art - see, for example, DeNardo et al., Clin Cancer
Res.
245
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
~~ TTENANT LES PAGES 1 A 245
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 245
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE: