Note: Descriptions are shown in the official language in which they were submitted.
CA 02420626 2003-03-13
CATHETER HAVING COEXTRUDED TUBING
The present applir_ation is a divisional of
copending Canadian Patent Application Serial No. 2,140,506
filed January 18, 1995.
BACKGROUND OF THE INVENTION
Intravascular catheters, such as catheters for
advancement into the arterial system around the heart,
are presently in wide clinical use both for angiography
and angioplasty (PTCA). As is well known, the catheter
must be made of a flexible material which, nevertheless,
exhibits a certain stiffness so that the catheter may be
advanced through the various twists and turns of the
arterial system to bring an angioplasty balloon or other
clot opening device, for example, to the desired site of
arterial occlusion. Also, while the plastic body of the
catheter must exhibit this desired stiffness and other
particular characteristics, the catheter lumen must have
a low friction surface so that the catheter can be
advanced along a guidewire, and also a guidewire or inner
catheter can be advanced through the catheter lumen.
Conventionally, a catheter having a nylon body
or a body of polyethylene, polyurethane, polyethylene
terephtha~.ate) (known as PET) is used, with the catheter
lumen having a lubricating coating or containing a
preformed polytetrafluoroethylene (PTFE) sleeve. This
13853
CA 02420626 2003-03-13
inner PTFE sleeve provides the desired low friction to
the catheter lumen, while the balance of the catheter can
provide other desired qualities. However, it has
sometimes been found that the presence of the PTFE tubing
can make the catheter too stiff, as well as unduly
subject to kinking, when the catheter turns a sharp
corner in the arterial system of the patient in which it
is used.
PTFE is of course a stiff plastic, and this has
to be accommodated for by reducing the thickness of the
outer catheter layer by an amount that may cause other
characteristics of the catheter to be less than optimum.
Also, the PTFE of course does not bond in any significant
way to the nylon or PET outer catheter layer. This
provides an added possible disadvantage that the PTFE
tubing may shift with respect to the outer catheter layer
and slide out of the lumen to a certain extent. As is
well known, PTFE bonds only w~.th great difficulty to most
other plastic materials.
zf it were desired to replace the inner PTFE
tubing in conventional catheters with another low
friction plastic such as a high density polyethylene,
this material also is incompatible with nylon, for
example, and does not form a sign_i.ficant bond with nylon
upon coextrusion of tubing with a nylon outer layer and
a high density polyethylene inner layer. Such layers
- 2
CA 02420626 2003-03-13
also can slip in their relative position because of the
essential absence of a bond, even after coextrusion.
As another prior art expedient, the interior of
a catheter is coated with a friction reducing material
such as a silicone in liquid form, which is then dried.
Such processes are particularly difficult with small
diameter intravascular catheters. Also, silicone resins
and the like which are used for such coating lack the
best low friction characteristics to ,facilitate
advancement of such catheters along a guidewire.
In accordance with this invention, a catheter
is provided having tubing with inner and outer tubular
layers which are bonded to each other far firm retention
and ease of manufacture. The manufacturing may be simply
by coextrusion, while obtaining the desired bond and
other desired characteristics of the respective inner and
outer catheter layers to optimise overall catheter
performance.
DESCRIPTION OF THE INVENTION
By this invention, an intravascular catheter is
provided which comprises a length of flexible plastic
tubing. Such catheter is claimed in the Parent Application
No. 2,140,506. The tubing comprises an outer plastic layer
and an inner plastic layer, the plastic materials of the
- 3 -
CA 02420626 2003-03-13
outer and inner plastic layers being different, and
covalently bonded to each other.
Preferably, the plastic material of the inner
plastic layer defines a catheter lumen having the walls
which exhibit lower frictional characteristics than the
material of the outer plastic layer. Thus, the selection
of the desired outer plastic layer materials for the
catheter may be greatly broadened to optimize catheter
performance. For instance, a material may be selected
which bonds optimally to the desired balloon material of
catheters which carry such balloons for good bonding,
while the Lumen of the catheter provides desired lumen
characteristics, particularly low friction for a
guidewire or a catheter advancing through the lumen.
Preferably, the material of the outer plastic
Layer has a greater stiffness than the material of the
inner plastic layer, to provide a catheter which is
flexible, but stiff enough for optimal "pushability" for
advancement thereof, particularly into the arterial
system of a patient adjacent the heart. For example,
nylon, polyurethane, or PET may be used as the outer
plastic layer material.
Preferably, a chemical bond is used between the
outer and inner plastic layers in the catheter tubing.
The material of the inner plastic layer
typically comprises a vinylic polymer having functional
CA 02420626 2003-03-13
groups bonded to the material of the outer plastic layer.
The vinylic polymer may be a copolymer having a mayor
amount of ethylene units and a minor amount of units of
unsaturated carboxylic acid or an anhydride thereof.
Typically, known resins manufactured and sold by the
Quantum Chemical Company under the trademark Plexar may
be used for the inner plastic layer.. These materials are
vinylic (for example polyethylene caf varying densities,
polypropylene, or polyethylene vinyl acetate) which are
copolymerized with a small amount o~f malefic acid. These
materials have been used chiefly as "tie layers" for
multilayer plastic sheeting, the Plexar material being an
inner layer which bonds together dissimilar outer plastic
layers. Other unsaturated carboxylic acids such as
fumaric acid, cinnamic acid, crotonic acid, linoleic
acid, or the like may also be used as a substitute for
Malefic acid.
In accordance with this invention, a plastic
material similar to Plexar or the like may be used as
typically the inner layer of a multiple layer catheter
tubing, taking advantage of the good chemical bonding
power of the Plexar or other similar plastic, preferably
without using the functional plastic material to bond two
dissimilar layers together, but making use of the
material 'in .its own right for its desired
characteristics. For example, high density polyethylenes
- 5 -
CA 02420626 2003-03-13
which have been copolymeri~ed with a minor amount of
functional groups such as an unsaturated carboxylic acid
or an anhydride thereof may be used to provide a firm
bond with an outer layer of nylon, PET, or polyurethane
for example, while providing a low friction surface to
the catheter lumen.
Specifically, the inner plastic layer may
comprise a vinylic polymer which comprises from one to
five mole percent of malefic anhydride polymer units,
copolymerized preferably with ethylene to provide a high
density polyethylene having low friction characteristics.
The flexible plastic tubing of~ this invention
is easily coextruded, to provide a catheter tubing which
requires no separate PTFE or other low friction sleeve,
or coating for a reduction in overall. cost of manufacture
of the catheter tubing of this invention.
The catheter tubing of this invention may be
simply coextruded as a multiple tubular layer catheter,
with the reactive polymer used in this invention being
typically the innermost Layer, with that innermost layer
becoming chemically bonded during the coextrusion to an
outer plastic layer which is made of a different
material. Thus, normally incompatible plastic materials
may be bonded together in tt~e catheter tubing of this
invention to provide both a firm bond and the desired
characteristics of the respective materials selected.
CA 02420626 2003-03-13
For example, nylon and high density
polyethylene are normally quite sealing incompatible
with each other. By this invention, a high density
polyethylene copolymer serving as an inner catheter
tubing layer may be firmly, covalently bonded to a nylon
outer catheter layer, so that the nylon can provide
desired stiffness to the catheter while still permitting
flexibility, and the high density polyethylene inner
layer can provide low friction to a guidewire or inner
catheter. The chemical bonding between the two catheter
layers can take place during the extrusion process, or,
if desired, subsequent heat treating or the like may be
provided.
Accordingly, in one aspect of the present
invention, there is provided a method which comprises
coextruding a length of flexible plastic tubing by
bringing a tubular, molten outer plastic layer into
contact with a tubular, molten inner plastic layer to
form a unitary, multilayer plastic tubing, the plastic
materials of said outer and inner plastic layers being
different, whereby banding takes place between said
plastic layers during the coextrusion process.
DESCRIPTION OF THE DRAWINGS
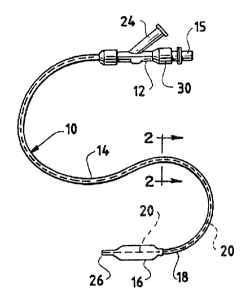
Fig. 1 is a perspective view of a PTCA balloon
catheter in accordance with this invention;
Fig. 2 is an enlarged sectional view taken
along line 2-2 of Fig. l;
CA 02420626 2003-03-13
Fig. 3 is a schematic view of the extrusion of
catheter tubing in accardance with this invention;
- 7a -
CA 02420626 2003-03-13
Fig. 4 is a longitudinal sectional view of
another embodiment of a PTCA balloon catheter in
accordance with this inventions and
Fig. 5 is an enlarged transverse sectional view
taken along line 5-5 of Fig. 4.
DESCRIPTION OF SPECIFIC EMBODIMENTS
Referring to Figs . 1-3 , an angioplasty catheter
is disclosed, which may be of conventional design and
use for PTCA procedures except as otherwise indicated
herein.
Catheter 10 defines a proximal end having a
conventional hub 12.
Catheter 10 comprises an outer tubular body 14
which connects with the Y-connector at the proximal end
15, and also connects with a balloon 1G at its distal end
18. Outer catheter tube 14 terminates at its point of
connection 18 with the proximal end of balloon 16.
Also, catheter 10 comprises an inner catheter
tubing 2o which extends through the lumen of outer
catheter tubing 14, defining a generally cylindrical
space 22 between outer eatY~eter tubing 14 and inner
catheter tubing 20. Connector arm 24 of Y-connector 12
communicates in conventional manner with generally
g _
CA 02420626 2003-03-13
cylindrical space 22 at the proximal end thereof.
cylindrical space 22 extends along the catheter between
catheter tubings 14 and 20, to terminate in communication
with the interior of balloon 1G. Inner catheter tubing
20, however, extends through balloon 16 as shown in Fig.
l, being sealed to balloon 16 at its distal end 26 in
such a manner that the lumen 28 of inner catheter tubing
20 is open at its distal end. Second arm 30 of Y-
connector 12 communicates with lumen 28 of inner catheter
tubing 20, so that fluid communication is possible
throughout the entire length of the catheter from
connector arm 30 through the open distal end 26 of inner
catheter tube 20, extending through balloon 16.
The above structure per se is used in
commercially available prior art catheter designs.
2n accordance with. this invention, catheter 10
comprises a length of flexible plastic tubing which, in
turn, comprises an outer plastic layer 32 and an inner
plastic layer 34, the plastic materials of the outer and
inner layers being different and chemically bonded to
each other. While that specific plastic tubing is inner
catheter tubing 20 in this specific embodiment, it could
be used for the outer catheter tubing 14 as well. As an
alternative, a bonding plastic layer could be placed both
inside and outside of a catYieter having a nylon middle
layer, for example.
- 9 -
CA 02420626 2003-03-13
Outer tubular plastic layer 32 of catheter tube
20 may preferably be selected from the group consisting
of nylon, polyurethane, and polyester, with such
materials preferably being of greater stiffness than the
material of inner tubular plastic layer 34.
Outer plastic layer 32 may typically comprise
about GO to 90 percent of the overall wall thickness of
tube 20, providing a desired amount of stiffness to the
tube while tube 20 retains a thin wall.
The material of inner tubular plastic layer 34
may be made of a material which exhibit lower frictional
characteristics than the material of outer plastic layer
32, to facilitate the advancement of a guidewire or a
separate, smaller catheter, for example; through the
catheter of this invention, while at the same time
enjoying the benefit of the physical properties provided
to the catheter by the presence of and the physical
properties of outer tubular layer 32.
Accordingly, for an angioplasty or angiography
catheter, the overall catheter may exhibit a desired
level of stiffness while still remaining flexible, due to
the combined properties of inner and outer catheter tubes
20, 14. At the same time the frictional characteristics
of the walls of lumen 2~ may remain low irrespective of
the frictional characteristics of layer 32. Furthermore,
this may be accomplished without the separate addition of
- 10 -
CA 02420626 2003-03-13
a PTFE sleeve, a coating, or the like in the catheter
lumen, which requires a complexity in the manufacturing
process. The layers of inner catheter tube 20 can be
simply coextruded. ."
As previously stated, the material of the inner
plastic layer 34 is preferably no more than half the
overall thickness of the wall of inner catheter tubing
20, and it may be as low as about 5 or 10 percent of the
overall thickness if desired. The material of inner
plastic layer 34 is preferably a copolymer of a major
amount of a vinylic polymer such as ethylene and a minor
amount of an unsaturated carboxylic acid or an anhydride
thereof, for example, a polyethylene which contains about
1 to 5 mole percent of malefic anhydride polymer units
present in the molecule (on a mole percent basis).
More specifically, the material of inner
plastic layer 34 may be a high density polyethylene,
modified for example with the presence of about 1 or 2
mole percent of copolymerized malefic and anhydride units.
As previously stated, such modified polyethylene resins
are commercially available from the Quantum Chemical
Corporation under the trademark Plexar, being used
conventionally as tie layer resins for the bonding of
dissimilar plastics together in coextruded films.
However, nonreactive materials may be used for the inner
layer 34 when coextrusion provides a physical bond of
- 21 -
CA 02420626 2003-03-13
adequate strength with layer 32. Specific extrusion
conditions for the best bonding depend on the product
used.
In this invention, however, such materials as
Plexar resins may be used in their own right as the
second layer of this invention, to coat a layer of a
catheter on the inside thereof to achieve the advantages
previously described herein.
The functional groups which are found on the
vinylic polymers used in this invention to promote a
chemical bond between the outer and inner plastic layers
may include copolymerized units such as an unsaturated
carboxylic acid, or an anhydride thereof, or functional
groups which are substituents of vinyl-containing
molecules, polymerized or co-polymerized to form the
vinylic polymer, for example hydroxyl in the case of
polyvinyl alcohol, or other pendant reactive groups as
may be desired to permit the formation of a chemical bond
between two plastic materials.
The copolymerized, unsaturated carboxylic acid
or anhydride thereof thus can serve as a functional group
which bonds a vinylic polymer of the inner layer to the
plastic of the outer layer. For example, particularly
when the first layer is a polyamide such as nylon or
another nitrogen-containing polymer such as polyurethane,
this can be effectively accomplished. Similarly, such
- 12 -
CA 02420626 2003-03-13
vinylic polymers having acid functional groups may react
with hydroxyl-containing polymers of the outer layer,
under proper reaction conditions. Such reactions may
take place during co-extrusion of the two layers, to form
a covalent bond between the outer and inner plastic
layers of the catheter tubing as it is formed by the
extrusion.
Preferably, inner plastic layer 34 is about
0.0005 to 0.003 inch thick while outer plastic layer 32
is about 0.003 to 0.00G inch thick, preferably giving a
total wall thickness for the inner catheter tube 20 of
0.005 to 0.008 inch. The overall diameter of inner
catheter tubing 20 is typically about 0.02 to 0.035 inch.
Outer catheter tubing 14 has a typical wall thickness of
about 0.003 to 0.005 inch, and a typical diameter of 0.04
to 0.05 inch, to provide a generally cylindrical space
22, although it is understood that inner tubing 20 is
unconstrained and will not remain in exactly coaxial
relationship with outer catheter tubing 14.
Inner catheter tubing 20 may be coextruded in
generally conventional manner as schematically
illustrated in Fig. 3. Extruder dies 36,37 bring
cylindrical streams of molten plastic which are to form
the outer layer 32 and inner layer 34 into coaxial,
physical contact around maxrdrel 33, to pass through
extruder die 35 while molten, to form catheter tubing 20.
- 13 -
CA 02420626 2003-03-13
During this process, the reactive moieties of the plastic
formulation which comprises inner layer 34 forms chemical
bonds with the plastic of outer layer 32. Specifically,
the nylon plastic of outer layer 32 is believed to react
by forming amide-like linkages with the malefic anhydride
units of the high density, copolymeri~ed polyethylene
plastic of layer 34 to form a strong bond between the
layers. Then, the manufactured tubing 20 may be
assembled in conventional manner to form the catheter of
this invention, as disclosed herein.
High density polyethylene is generally
understood by those skilled in the art to comprise a
density of at least about 0.94 g./cc.. For purposes of
this invention a polyethylene containing reactive groups
and being of this density or greater is defined to be
"high density polyethyleneB'.
Referring to Figs. 4 and S, another embodiment
of an intravascular balloon catheter in accordance with
this invention is disclosed.
A catheter which comprises the conventional hub
12a similar to hub 12 is connected to the proximal end of
double lumen tubing 40, which may be manufactured by an
extrusion process in accordance with Fontirroche U.S.
Patent No. 5, 063, 018, modified to coextrude the tubing 40
disclosed herein. Tubing 40 comprises an outer layer or
body 42, plus an inner layer 44 which may be added by
- 14 -
CA 02420626 2003-03-13
coextrusion, typically in lumen 46, through which the
guidewire extends.
The side arm 24a of hub 12a communicates with
the other lumen 48 of tubing 40. The distal end of
tubing 40 is sealed to a second length of flexible
plastic tubing 50 which may be made of nylon or the like
to have sufficient stiffness characteristics. Inner
tubular layer 44 may be made of a material similar to
inner tubular layer 34 of the previous embodiment for
similar purposes. The outer catheter layer 42 may be
similar to outer catheter layer 32 of the previous
embodiment although possibly not of a material of greater
stiffness than the material of layer 44 since the mass of
material 42 in cross section is relatively greater.
Basically the inventive principles described above still
hold. The material of layer 42 is relied upon to provide
desired stiffness to the catheter. The material of layer
44 provides desired low frictional characteristics to
lumen 46, both of these being provided in a single
catheter by a coextrusion process.
Tube 50 connects at its distal end to a
catheter balloon 52. Lumen 48 communicates with the
interior of balloon 52 through tube 50, communicating at
the proximal end of lumen 4 8 with side arm 24a of the
hub.
- 15 -
CA 02420626 2003-03-13
An inner catheter tube 54 is sealed to the
distal end of lumen 4G and extends proximally through
balloon 52 to be also sealed to the balloon at the
proximal catheter end 56. Tubing 54 may be similar in
structure to tubing 20 of the previous embodiment, having
an inner layer and an outer layer analogous to layers 32,
34 to perform the desired functions thereof as described
above.
Thus, two different embodiments of,a catheter
are disclosed which may be of a desired stiffness and
having a desired sealing compatibility to a catheter
balloon 16 or 52, for example. At the same time, a
catheter lumen similar to lumen 28 or 4G may have a low
friction wall made of a different material bonded, and
preferably covalently bonded, to the plastic of tube 32
or 42.
The above has been offered for illustrative
purposes only, and is not intended to limit the scope of
the invention of this application, which is as defined in
the claims below.
- 1G -