Note: Descriptions are shown in the official language in which they were submitted.
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
PROCESS FOR PREPARATION OF HYDRATES OF OLANZAPINE AND THEIR CONVERSION INTO
CRYSTALLINE FORMS OF OLANZAPINE
The present invention relates to a method for the preparation of hydrates of 2-
methyl-4-(4-methyl-1-piperazinyl)-lOH-thieno [2,3-b] [1,5~ benzodiazepine
(hereinafter referred to as Olanzapine). The present invention also relates to
a process
for conversion of these hydrates into a pure crystalline form of olanzapine
referred to
as form-I. The present invention also relates to a method of converting
Olanzapine
Form-2 to Form-1.
This invention more particularly relates to the preparation of hydrates of
olanzapine and their conversion into crystalline form of Olanzapine Fonn-1
through
recrystallization from a solvent. Olanzapine is represented by the following
structure.
~CH3
N_ /N~
N
H g CH3
Olanzapine
Olanzapine is useful for treating psychotic patients and mild anxiety states.
Preparation of Olanzapine and its acid salts, having pharmaceutical properties
particularly in the treatment of disorders of the central nervous system has
been
discussed in U.S. Patent No. 5,229,382.
U.S. Patent No. 5,229,382 does not refer to any specific polymorphic
crystalline form of Olanzapine. European patent specification No. 733635A1
claims Form-2 of Olanzapine. The process under this patent describes
preparation of
Form-2 from ethyl acetate. This patent also designated the product obtained
according to the process described in U.S. Patent No. 5,229,382 as Form-1.
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-2
Furthermore, EP 733635A1 discloses the d values for Form-1 and Form-2
from their X-ray Diffractograms. The values are:
d value d value
Form-1 Form-2
9.94 10.26
8.55 8.57
8.24 7.47
6.88 7.12
6.37 6.14
6.24 6.07
5.58 5.48
5.30 5.21
4.98 5.12
4.83 4.98
4.72 4.76
4.62 4.71
4.53 4,47
4.46 ~ 4.33
4.29 4.22
4.23 4.14
4.08 3.98
3.82 3.72
3.74 3.56
3.69 3.53
3.58 3.38
3.50 3.25
3.33 3.12
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-3-
3.28 3.08
3.21 3.06
3.11 3.01
3.05 2.87
2.94 2.81
2.81 ~ 2.72
2.75 2.64
2.65 2.60
2.63
2.59
It is noteworthy to mention that EP 0 831 098 A2 discloses the preparation of
a
series of dehydrates of olanzapine namely Dehydrate B, Dehydrate D and
Dehydrate E.
The d values from the X-ray Diffractograms for these forms are listed in EP 0
831
098 A2.
We conducted experiments to obtain Olanzapine Form I by recrystallization of
olanzapine from acetonitrile using the process described in Example l, sub
example 4
of U.S. Patent No. 5,229,382. The process is described herein for reference:.
A
mixture of 4-amino-2-methyl-lOH-thieno-[ 2,3-b] [1,5]benzodiazepine HCl (100
g),
N-methyl piperizine (350m1), DMSO (465 ml) and toluene (465 ml) was heated to
reflux. The reaction mass was maintained at reflux for 19 hours and then
cooled to
50°C and water was added. The reaction mass was cooled to 0-10°C
and stirred at
the same temperature for 6 hours. The crude Olanzapine separated was filtered
and
dried in oven to a constant weight (76.5 g). The crude compound was added to
acetonitrile (750 ml) at boiling temperature. The mixture was boiled for
further 5
minutes. The mixture was filtered to remove the undissolved solid. The
filtrate was
treated with carbon and filtered. The filtrate was distilled to a minimum
volume,
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-4-
cooled to 0-5°C and maintained at the same temperature for 1.0 hour and
filtered.
The compound was dried to a constant weight in an oven (51.6g).
The polymoiphic form obtained from these experiments was characterized for
its X-ray Powder Diffraction on Rigaku D / Max 2200. As clearly observed, the
d
values fox this product (Fig. 1) matched with those of Olanzapine Form-2
claimed in
EP 733635A1. It is therefore inferred that the recrystallization of Olanzapine
in
acetonitrile produces Form-2 and not Form-1.
Accordingly, the present invention provides a novel method for preparation of
hydrates of olanzapine, which are different from those reported in the
literature.
These hydrates are named Olanzapine monohydrate-I and Olanzapine dehydrate-I
fox
convenience.
Accordingly, the present invention also provides a novel method for
preparation of Olanzapine Form-1 by recrystallization of olanzapine or its
hydrates in
dichloromethane.
The present invention also provides a novel method for converting Olanzapine
Form-2 to Olanzapine Form-1
According to the present invention the process for the preparation of
olanzapine monohydrate-I comprises:
a) refluxing a mixture of 4-amino-2-methyl-lOH-thieno-[2,3-b]
[1,5]benzodiazepine hydrochloride, N-methyl piperazine, dimethyl sulfoxide
(DMSO)
and toluene for 5 to 20 hours;
b) cooling the mixture to 20 to 90°C;
c) adding water;
d) cooling the mixture to -5 to 2S°C and stinging for 2-10 hours;
e) filtering the mixture and washing with water; and
f) drying at 30 to 50°C to a constant weight.
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-5-
According to the present invention the process for the preparation of
olanzapine dehydrate -I comprises:
a) refluxing a mixture of 4-amino-2-methyl-l OH-thieno-[2,3-b]
' [1,5]benzodiazepine hydrochloride, N-methyl piperazine, dimethyl sulfoxide
(DMSO)
and toluene for 5 to 20 hours;
b) cooling the mixture to 20 to 90°C;
c) adding water;
d) cooling the mixture to -5 to 25°C and stirnng for 2-10 hours;
e) filtering the mixture and washing with water; and
f) drying at ambient temperature to a constant weight.
The preferred ratio of 4-amino-2-methyl-lOH-thieno-[ 2,3-b] [1,5]
benzodiazepine HCI, N-methyl piperizine, DMSO and toluene that can be used for
preparation.of the monohydrate and dehydrate are:
N-methyl piperizine (2.0 - 8.4 moles with respect to 1.0 mole of 4-Amino-2-
methyl-lOH-thieno-[ 2,3-b] [1,5]benzodiazepine HCl).
DMSO (2 - 8 times by volume with respect to 1.0 mole of 4-Amino-2-methyl-
lOH-thieno-[ 2,3-b] [1,5]benzodiazepine HCl).
Toluene (3 - 8 times by volume with respect to 1.0 mole of 4-Amino-2-methyl-
lOH-thieno-[ 2,3-b] [1,5]benzodiazepine HCl).
According to this invention, Olanzapine Form -I is prepared by heating to
reflux a suspension of olanzapine or its hydrates in dichloromethane wherein
the
amount of dichloro-methane used is 4.5 to 13 volume/weight of Olanzapine to
obtain
a clear solution. The resultant solution is then treated with carbon followed
by
filtration. Upon completion of this step the filtrate is cooled to 0 to
5°C and stirred at
the same temperature for 60-90 minutes. The separated solid was filtered and
washed
with dichloromethane. The product obtained on drying in an oven at 60-
70°C to a
constant weight is Form-1 of Olanzapine.
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-6-
The process described in U.S. 5,229,382 was used to prepare olanzapine crude
and the process described in EP 733 635 A1 was used to prepare olanzapine Form-
2
for our studies. However, other methods may be used to prepare olanzapine
crude
and olanzapine Form-2 and any other methods that can be used to prepare
olanzapine
crude and olanzapine Form 2 can be used in the processes of this invention.
The following examples are provided for purposes of illustration and are not
to
be construed as limiting the scope of the invention.
PREPARATION OF OLANZAPINE MONOHYDRA.TE-1
EXAMPLE 1
A mixture of 4-amino-2-methyl-lOH-thieno-[2,3-b][1,5]benzodiazepine
hydrochloride (20 Kg), N-methyl piperazine (42 lit), dimethyl sulfoxide (40
lit) and
toluene (95 lit) was heated to reflux. The reaction mass was maintained at
reflux for
17 hours and 15 minutes and then cooled to 40-50°C. Water (95 lit) was
added
slowly at 40-50°C. The reaction mass was cooled to -0.6 to 1.2°C
and stirred at the
same temperature for six hours. The Olanzapine crude that separated was
filtered and
washed with water (10 lit). The product was dried at 30.5 to 31.8°C for
10 hrs and 50
minutes. Yield: 20 Kg. A 20 gm sample from the above material after prolonged
heating for an additional 72 hours gave the product with a moisture content of
5.22%.
PREPARATION OF OLANZAPINE DIHYDR.ATE-I
EXAMPLE 2
A mixture of 4-amino-2-methyl-lOH-thieno-[2,3-b][1,5]benzodiazepine
hydrochloride (200 g), N-methyl piperazine (420 ml), dimethyl sulfoxide (200
ml)
and toluene (940 ml) was heated to reflux. The reaction mass was maintained at
reflux
for I2 hours and then cooled to 40°C. Water (940 ml) was added slowly
at 40-44°C.
The reaction mass was cooled to 0-5°C and stirred at the same
temperature for five
hours. The Olanzapine crude that separated was filtered and washed with water
(100
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
ml). The solid obtained was dried atmospherically (25-35°C) for 24
hours (Yield
241 g).
PREPARATION OF FORM-1
EXAMPLE 3
Crude 2-methyl-4-(4-methyl-1-piperazinyl)-lOH-thieno-[2,3-b][1,5]
benzodiazepine (35.0 g) was suspended in dichloromethane (160.0 ml). The
suspension was heated to reflux to obtain a clear solution. The resultant
solution was
then treated with carbon (3.5 g) followed by filtration. Upon completion of
this step
the filtrate was cooled to 0 to 5°C and stirred at the same temperature
for one hour.
The separated solid was filtered and washed with chilled dichloromethane
(10.0m1).
The product obtained on drying in oven at 65 to 70°C to a constant
weight gave
Form-1 of Olanzapine (Yield 22.0 g).
CONVERSION OF FORM-2 TO FORM-1
EXAMPLE 4
The stirred suspension of pure form-2 of 2-methyl-4-(4-methyl-1-piperazinyl)-
lOH-thieno-[2,3-b][1,5]benzodiazepine (20.0 g) in dichloromethane (90.0 ml)
was
heated to reflux to obtain a clear solution. The clear solution was filtered
and the
filtrate was then cooled to 3 to 5°C and stirred at same temperature
for one hour. The
crystalline solid separated was filtered and washed with dichloromethane (4.0
ml).
Subsequent drying at 60 to 70°C to a constant weight yielded Olanzapine
Form-1.
(Yield: 12.7 g).
PREPARATION OF FORM-1 FROM MONOHYDRATE-I OF OLANZAPINE
EXAMPLE 5
Monohydrate-I of 2-methyl-4-(4-methyl-1-piperazinyl)-lOH-thieno-[2,3-
b][1,5] benzo- diazepine (25.0 g) prepared as per Example-1 was suspended in
dichloromethane (325.0 ml). The suspension was heated to reflux to obtain a
clear
solution. The resultant solution was then treated with carbon (2.5 g) followed
by
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-g_
filtration. Upon completion of this step the filtrate was distilled to a
minimum
volume and then cooled to 2 to 4°C and stirred at the same temperature
for 90
minutes. The product separated was filtered arid washed with chilled
dichloromethane (10 ml). The product obtained on drying in oven at 60 to
70°C to a
constant weight gave Form-1 of Olanzapine (Yield 16.5 g)
PREPARATION OF FORM-1 FROM DIHYDRATE-I OF OLANZAPINE
EXAMPLE 6
Dehydrate-I of 2-methyl-4-(4-methyl-1-piperazinyl)-lOH-thieno-[2,3-b][1,5]
benzodiazepine (40.0 g) prepared as per Example-2 was suspended in
dichloromethane (520.0 ml). The suspension was heated to reflux to obtain a
clear
solution. The resultant solution was then treated with carbon (4.0 g) followed
by
filtration. Upon completion of this step the filtrate was distilled to a
minimum
volume and the left over reaction mass was cooled to 0 to 2°C and
stirred at the same
temperature for one hour. The separated solid was filtered and washed with
dichloromethane (10.0m1). The product obtained on drying in oven at 65 to
70°C to a
constant weight renders Form-1 of Olanzapine (Yield 26.0 g).
The aforementioned crystalline forms in examples 1 to 6 have been examined
for their structural and analytical data viz., Powder X-Ray Diffraction,
Differential
Scanning Calorimetry, and Infrared Absorption Spectroscopy. The results
obtained
are discussed and the respective drawings attached (Fig. 2 -19).
The X-Ray Diffraction Pattern set out herein for examples 1 to 6 were obtained
using Rigaku D / Max-2200 X-Ray Powder Diffractometer having a copper K
radiation source of wavelength ~,=1.54 A°. The samples were scanned
between 3-45
degrees 2A.
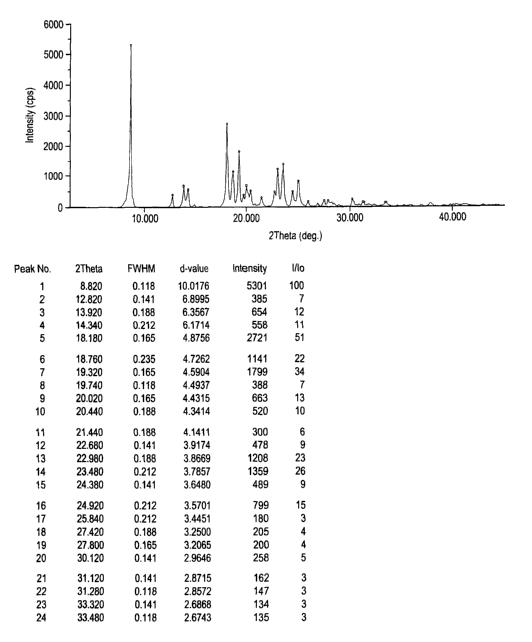
The d values for the monohydrate-1 in Example-I are herewith enclosed (Fig.
2).
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-9-
d value I/Io
10.0176 100
6.8995 7
' 6.3567 12
6.1714 11
4.8756 51
4.7262 22
4.5904 34
4.4937 7
4.4315 13
4.3414 10
4.1411 6
3.9174 9
3.8669 23
3.7857 26
3.6480 9
3.5701 15
3.4451 3
3.2500 4
3.2065 4
2.9646 5
2.8715 3
2.8572 2
The d values for the
dihydrate-1 in Example-2
are herewith given
(Fig. 5).
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-10-
d value I/Io
9.9949 100
9.6887 7
7.0418 2
' 6.4117 2
6.2495 7
6.1205 6
5.4534 6
5.2358 2
4.8230 33
4.7162 9
4.5717 15
4.4847 6
4.3924 8
4.3080 4
4.2070 3
4.0735 3
3.9974 3
3.9242 9
3.8438 12
3.7699 9
3.7386 13
3.6837 3
3.6509 4
3.6072 5
3.5256 11
3 .4242 2
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-11-
3.1773 2
3.1207 2
2.9917 2
2.9569 3
2.8733 2
2.8483 2
The X-Ray Diffiaction Pattern obtained for the products from examples 3 to 6
is identical with those reported in EP 733 635 Al.
BRIEF DESCRIPTION OF DRAWINGS
Fig.l is a characteristic X-ray powder diffraction pattern of Form-2 obtained
on recrystallization with acetonitrile (Vertical axis: Intensity (CPS);
Horizontal axis:
Two Theta (degrees)).
Fig. 2 is a characteristic X-ray powder diffraction pattern of Olanzapine
monohydrate-I (Vertical axis: Intensity:(CPS); Horizontal axis: Two Theta
(degrees)).
Fig. 3 is a characteristic infrared absorption spectrum in potassium bromide
of
Olanzapine monohydrate-I (Vertical axis, Tramission (%); Horizontal axis: Wave
number (cm 1)). .
Fig. 4 is a characteristic of differential scanning calorimetry thermogram of
Olanzapine monohydrate-I. (Vertical axis: mW; Horizontal axis: Temperature
(°C)).
Fig. 5 is a characteristic X-ray powder diffraction pattern of Olanzapine
dehydrate-T (Vertical axis: Tntensity (CPS); Horizontal axis: Two Theta
(degrees)).
Fig. 6 is a characteristic infrared absorption spectrum in potassium bromide
of
Olanzapine dehydrate-I. (Vertical axis, Tramission (%); Horizontal axis: Wave
number (cm 1)).
Fig. 7 is a characteristic of differential scanning calorimetry thermogram of
Olanzapine dehydrate-I. (Vertical axis: mW; Horizontal axis: Temperature
(°C)).
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-12-
Fig. 8 is a characteristic X-ray powder diffraction pattern of Form-1 produced
by recrystallizing crude Olanzapine in dichloromethane. (Vertical axis:
Intensity
(CPS); Horizontal axis: Two Theta (degrees)).
Fig.9 is a characteristic infrared absorption spectrum in potassium bromide of
Form-1 produced by recrystallizing crude Olanzapine in dichloromethane.
(Vertical
axis, Tramission (%); Horizontal axis: Wave number (cm 1)).
Fig.lO is a characteristic of differential scanning calorimetry thermogram of
Form-1 produced by recrystallizing crude Olanzapine in dichloromethane.
[Vertical
axis: mW; Horizontal axis: Temperature (°C))].
Fig.11 is a characteristic X-ray powder diffraction pattern of Form-I obtained
on conversion of Form-2 to Form-1 Olanzapine in dichloromethane (Vertical
axis:
Intensity (CPS); Horizontal axis: Two Theta (degrees)).
Fig.12 is a characteristic infrared absorption spectrum in potassium bromide
of
Form-1 obtained on conversion of Form-2 to Form-1 Olanzapine in
dichloromethane
(Vertical axis, Tramission (%); Horizontal axis: Wave number
(cm I))]'
Fig.l3 is a characteristic of differential scanning calorimetry thermogram of
Form-1 obtained on conversion of Form-2 to Form-1 Olanzapine in
dichloromethane
(Vertical axis: mW; Horizontal axis: Temperature (°C))].
Fig.l4 is a characteristic X-ray powder diffraction pattern of Form-1 obtained
on conversion of olanzapine monohydrate-I to Form-1 Olanzapine in
dichloromethane
(Vertical axis: Intensity (CPS); Horizontal axis: Two Theta (degrees)).
Fig.lS is a characteristic infrared absorption spectrum in potassium bromide
of
Form-1 obtained on conversion of olanzapine monohydrate-I to Form-1 Olanzapine
in
dichloromethane (Vertical axis, Tramission (%); Horizontal axis: Wave number
(cm m)).
CA 02420987 2003-02-28
WO 02/18390 PCT/USO1/07258
-13-
Fig.l6 is a characteristic of differential scanning calorimetry thermogram of
Form-I obtained on conversion of olanzapine monohydrate-I to Form-I
Olanzapine in dichloromethane. (Vertical axis: mW; Horizontal axis:
Temperature (°C)).
Fig.17 is a characteristic X-ray powder diffraction pattern of Fonn-1 obtained
on conversion of olanzapine dehydrate-I to Form-1 Olanzapine in
dichloromethane
(Vertical axis: Intensity (CPS); Horizontal axis: Two Theta (degrees)).
Fig.l 8 is a characteristic infrared absorption spectrum in potassium bromide
of
Fouln-1 obtained on conversion of olanzapine dehydrate-I to Fol~n-1 Olanzapine
in
dichloromethane. ([Vertical axis, Tramission (%); Horizontal axis: Wave number
Fig.l9 is a characteristic of differential scanning calorimetry thermogram of
Form-1 obtained on conversion of olanzapine dehydrate-I to Form-1 Olanzapine
in
dichloromethane. (Vertical axis: mW; Horizontal axis: Temperature
(°C)).