Note: Descriptions are shown in the official language in which they were submitted.
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
SOLVENT EXTRACTION OF LOW MOLECULAR WEIGHT
COMPONENTS FROM SOLID POLYMERS
TECHNICAL FIELD OF THE INVENTION
This invention relates generally to the removal of low molecular weight
components
from solid.polyrner resins and articles, and, more particularly, the invention
relates to improved
s.,u
.gin
methods for solvent extraction of low molecular weight components from
polymers comprised
of ethylenic backbones having pendant groups selected from alkyl acrylate
groups, cyclic
olefinic groups and/or benzylic groups.
BACKGROUND OF THE INVENTION
Reactive extrusion is a convenient technique for the post-polymerization
modification
of polymers. Typically, a polymer, reactive agents, and a catalyst are
introduced into the
reactive extruder under heating sufficient to promote a melt reaction between
reactive agents
and the molten polymer and to produce a new modified polymer. The polymer melt
can then
be extruded into a form useful for fizrther storage or processing. One polymer
that can be
produced by ' reactive extrusion is ethylene/methyl
acrylate/cyclohexenylinethyl acrylate
(EMCM).
However, in addition to the desired polymer, reactive extrusion processes
commonly
generate traces of oligomers with molecular weight less than about 2000, as
well as low
molecular weight components, e.g. residual reactive agents and reaction by-
products. The
presence of oligomers and low molecular weight components in the polymer is
generally not
preferred. For example, if the polymer is intended for a food packaging
application, traces of
the low molecular weight components may migrate into the packaged food, which
may give
rise to a malodor or an off taste or may lead to further study to meet
requirements set by
regulatory agencies, such as the U.S. Food and Drug Administration (FDA).
Although reactive extrusion processing of the polymers can frequently
substantially
eliminate malodor or off taste due to low molecular weight components (such as
by vacuum
devolatilization or nitrogen/stearn stripping), and reduce migration of the
volatile low
molecular weight components to very low levels (<50 ppb in the edible dietary
intake (EDI])
which are beneath regulatory thresholds, it is desirable in many instances to
have low cost and
effective alternative processes to reduce any non-volatile Iow molecular
weight components or
fixrther remove these low molecular weight components from EMCM and other
polymers,
particularly when the polymers are to be used for packaging food items.
Traditional cleaning
1
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
of solid polymers to remove non-volatile low molecular weight by-products has
involved, for
example, solvent extraction of finely divided resin particles, and, with
increased cost and mass
transport problems, precipitation from a dilute polymer solution from a
solvent into a non-
solvent.
Lewellen et al., U.S. Patent No. 6,010,391, teaches the polishing of soft
acrylic articles.
In one step, the articles are treated with a mineral spirits fraction,
whereafter residual mineral
spirits can be removed by ultrasonication using a solvent, such as an aqueous
solution of 2-
butoxyethanol and detergents.
Flynn et al., U.S. Patent No. 6,008,179, teaches a composition comprising a
methyl
1o ether of a perfluorobutyl compound and an organic solvent. The composition
can be used in a
method of ultrasonic cleaning.
However, there remains a need for efficient and cost-effective alternative
methods for
extraction or cleaning of solid polymer particles with large surface to volume
ratios such as
powders, pellets, fibers, strands, thin sheets and films. The present
invention provides such an
efficient and cost-effective method.
SUMMARY OF THE INVENTION
According to one aspect of the present invention, a method is provided for
treating a
solid polymer to remove low molecular weight components, e.g., components with
a molecular
2o weight less than about 2000, less than about 1000, or less than about 500,
contained therein. A
solid polymer is contacted with one or more solvents under conditions
effective for extracting
the low molecular weight components from the solid polymer. The solid polymer
treated in
accordance with the invention can be in the form of powder, pellets, fibers,
strands, thin sheets,
films, and the like, and will generally comprises an ethylenic backbone
containing alkyl
acrylate pendant groups, cyclic olefmic pendant groups and/or benzylic pendant
groups. The
solvent with which the solid polymer is contacted is typically selected from
the group
consisting of Cl-C4 alcohols, C3-C6 ketones, C3-C8 acetates, and C3-C8 ethers.
According to another aspect of the present invention, a method is provided for
treating a
solid polymer, in which a polyethylene-methyl acrylate) copolymer, a
poly(cyclohexene
3o methyl acrylate) homopolymer (CHAR), a poly(ethylene/cyclohexene-methyl
acrylate)
copolymer, a poly(ethylene/vinyl cyclohexene) copolymer (EVCH), or a
poly(ethylene/methyl
acrylate/cyclohexene-methyl acrylate) (EMCM) terpolymer is contacted with one
or more
solvents selected from the group consisting of C1-C3 alcohols and acetone
under conditions
effective for extracting low molecular weight components having molecular
weights less than
2
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
about 2000 from the solid polymer.
In a related embodiment, the solid polymer and the solvent can further be
heated to a
temperature below the melting point or the glass transition temperature of the
polymer.
According to another aspect of the invention, an ultrasound-assisted solvent
extraction
method is provided in which a solid polymer is contacted with one or more
solvents to form a
polymer/solvent mixture. The polymer solvent mixture is subj ected to an
ultrasonication
treatment under conditions and for a duration effective for extracting the
desired low molecular
weight components from the polymer. The solid polymer generally comprises an
ethylenic
backbone containing alkyl acrylate pendant groups, cyclic olefinic pendant
groups, benzylic
l0 pendant groups, or a combination thereof. The solvent with which the solid
polymer is
contacted is selected from the group consisting of C1-C4 alcohols, C3-C6
ketones, C3-C8
acetates, and C3-C8 ethers.
Preferably, in this aspect of the invention, the polymer is selected from a
poly(ethylene
methyl acrylate) copolymer, a poly(cyclohexene-methyl acrylate) homopolymer
(CHAA), a
poly(ethylene/cyclohexene-methyl acrylate) copolymer, a poly(ethylene/vinyl
cyclohexene)
copolymer (EVCH), or a poly(ethylene/methyl acrylate/cyclohexene-methyl
acrylate)
terpolymer; and the one or more solvents are selected from Cl-C3 alcohols and
acetone.
In yet another embodiment of this invention, an ultrasound- and heat-assisted
solvent
extraction method is provided in which a solid polymer is contacted with one
or more solvents
2o to form a polymer/solvent mixture. The polymer solvent mixture is subjected
to an
ultrasonication treatment under conditions and for a duration effective for
extracting the desired
low molecular weight components from the polymer, wherein the conditions
comprise heating
the mixture to a temperature below the melting point or the glass transition
temperature of the
polymer.
By practice of the present invention, greater than about 75%, preferably
greater than
about 85%, more preferably greater than about 95% of components having
molecular weights
less than about 2000 are extracted from the solid polymer. Moreover, the
methods of the
invention provide good extraction selectivity such that removal of low
molecular weight
components is maximized while extraction of higher molecular weight polymeric
components
is minimized. Thus, greater than about 90%, preferably greater than about 98%
of the
extractives removed according to the present invention will have molecular
weights less than
about 2000, and preferably less than about 1000.
3
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
BRIEF DESCRIPTION OF THE DRAWINGS
The invention may be understood by reference to the following description
taken in
conjunction with the accompanying drawings, in which like reference numerals
identify like
elements, and in which:
Figures 1 illustrates gel permeation chromatography traces of EMAC and low
molecular
weight components extracted from the EMAC with various solvents;
Figure 2 illustrates gel permeation chromatography traces of repelletized EMAC
and
low molecular weight components extracted from the repelletized EMAC with
various
solvents;
l0 Figure 3 illustrates normalized gel permeation chromatography traces of
repelletized
EMAC and low molecular weight components extracted from the repelletized EMAC
with
various solvents;
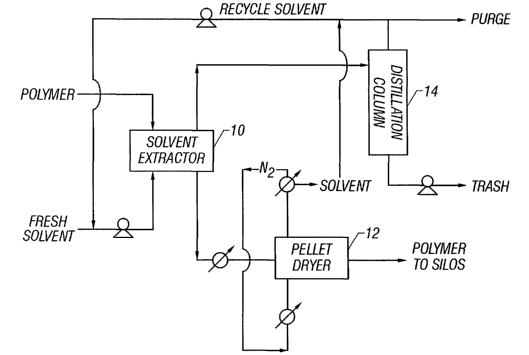
Figure 4 is a representation of one illustrative implementation of a solvent
extraction
procedure according to the present invention; and
Figure 5 is a representation of one illustrative implementation of an
ultrasound-assisted
solvent extraction procedure according to the present invention.
While the invention is susceptible to various modifications and alternative
forms,
specific embodiments thereof have been shown by way of example in the drawings
and are
herein described in detail. It should be understood, however, that the
description herein of
2o specific embodiments is not intended to limit the invention to the
particular forms disclosed,
but on the contrary, the intention is to cover all modifications, equivalents,
and alternatives
falling within the spirit and scope of the invention as defined by the
appended claims.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
Illustrative embodiments of the invention are described below. In the interest
of clarity,
not all features of an actual implementation are described in this
specification. It will of course
be appreciated that in the development of any such actual embodiment, numerous
implementation-specific decisions must be made to achieve the developers'
specific goals, such
as compliance with system-, enviromnental-, and business-related constraints,
which will vary
from one implementation to another. Moreover, it will be appreciated that such
a development
effort might be complex and time-consuming, but would nevertheless be a
routine undertaking
for those of ordinary skill in the art having the benefit of tlus disclosure.
The present invention provides methods for the removal of Iow molecular weight
components, such as Iow molecular weight monomers, oligomers, additives,
odorous and other
4
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
by-products, and the like, from a solid polymer. The solid polymer from which
the low
molecular weight components are removed may be in any of a variety of forms
when treated
according to this invention. By way of example, the polymer may be in the form
of pellets,
powders, fibers, strands, thin sheets, films, etc.
The solid polymer will generally have an ethylenic backbone and will further
comprise
pendant groups linked to the ethylenic backbone, the pendant groups being
selected from alkyl
acrylate pendant groups, cyclic olefinic pendant groups, benzylic pendant
groups, or a
combination thereof.
By "ethylenic backbone," it is meant that the backbone comprises a chain
structure or
to backbone of saturated carbon atoms which, generally, is created during a
polymerization
process. For example, homopolymerization of ethylene provides an ethylenic
backbone.
Copolymerization of ethylene and acrylic acid, methacrylic acid, alkyl
acrylate, or alkyl
methacrylate also results in an ethylenic backbone with pendant acid or ester
moieties. In
general, the number of carbon atoms in the backbone is a number between 2 and
about 30,000.
In general, the polymer will contain between about one and about 100 mole
percent of
the pendant benzylic, alkyl acrylate and/or cyclic olefinic moieties.
Preferably, the composition
contains between about 5 and 50 mole percent of the pendant moieties, more
preferably
between about 10 and 25 mole percent. However, the skilled individual will
appreciate that
such levels are illustrative only and can be varied to suit the needs of a
particular application of
interest.
In one illustrative embodiment, the polymer treated in accordance with tlus
invention
will comprise an ethylenic backbone having alkyl acrylate pendant groups
linked to the
ethylenic backbone. Such polymers are also commonly referred to as ethylene-
alkyl acrylate
copolymers, and are well known in the art to include copolymers of ethylene
and acrylic or
methacrylic esters of linear, branched or cyclic alkanols having, for example,
1-2~ carbon
atoms. One illustrative ethylene-alkyl acrylate copolymer of particular
interest comprises an
ethylene-methyl acrylate copolymer. The specific ethylene-alkyl acrylate
employed in the
process of the present invention is not critical and can include copolymers
containing high
weight percentages of alkyl acrylate or high weight percentages of ethylene.
Such polymers are
3o commercially available. For example, a suitable copolymer containing about
76 wt.% ethylene
and 24 wt.% methyl acrylate is available as EMAC 2260 from Chevron Chemical
Company
(San Francisco, Ca.)
Methods for the preparation of these polymers are well known. One example of
the
preparation of ethylene-methyl acrylate copolymers is disclosed in U.S. Pat.
No. 3,350,372
5
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
which is incorporated herein by reference in its entirety. Additional
preparation methods are
disclosed, for example, in U.S. Pat. Nos. 5,631,325 and 5,543,477, the
disclosures of which are
incorporated herein by reference in their entireties.
In another embodiment of this invention, the solid polymer comprises an
ethylenic
backbone having cyclic olefinic pendant groups, such as those described in
International
Application No. W0/99/48963, from Chevron Chemical Company, and pending U.S.
Patent
Application Serial No. 09/127,316, the disclosure of which is incorporated
herein by reference
in its entirety. Many illustrative cyclic olefinic pendant groups will conform
with the structure
below:
l0
where q1, q2, q3, q4, and r are selected from the group consisting of -H, -
CHI, and -C2H5;
and where m is -(CH2)n with n being an integer in the range from 0 to 4; and
wherein, when r
is H, at least one of q1, qa, q3 and q4 is H.
Illustratively, but not by way of limitation, the cyclic olefinic pendant
groups can be
selected from the group consisting of cyclohexene-4-methylene radical, 1-
methyl cyclohexene
4-methylene radical, 2-methyl cyclohexene-4-methylene radical, 5-methyl
cyclohexene-4
methylene radical, 1,2-dimethyl cyclohexene-4-methylene radical, 1,5-dimethyl
cyclohexene-4-
methylene radical, 2,5-dimethyl cyclohexene-4-methylene radical, 1,2,5-
trimethyl cyclohexene-
4-methylene radical, cyclohexene-4-ethylene radical, 1-methyl cyclohexene-4-
ethylene radical,
2-methyl cyclohexene-4-ethylene radical, 5-methyl cyclohexene-4-ethylene
radical,
1,2-dimethyl cyclohexene-4-ethylene radical, 1,5-dimethyl cyclohexene-4-
ethylene radical,
2,5-dimethyl cyclohexene-4-ethylene radical, 1,2,5-trimethyl cyclohexene-4-
ethylene radical,
cyclohexene-4-propylene radical, 1-methyl cyclohexene-4-propylene radical, 2-
methyl
cyclohexene-4-propylene radical, 5-methyl cyclohexene-4-propylene radical, 1,2-
dimethyl
cyclohexene-4-propylene radical, 1,5-dimethyl cyclohexene-4-propylene radical,
2,5-dimethyl
3o cyclohexene-4-propylene radical, 1,2,5-trimethyl cyclohexene-4-propylene
radical,
cyclopentene-4-methylene radical, 1-methyl cyclopentene-4-methylene radical, 3-
methyl
cyclopentene-4-methylene radical, 1,2-dimethyl cyclopentene-4-methylene
radical,
3,5-dimethyl cyclopentene-4-methylene radical, 1,3-dimethyl cyclopentene-4-
methylene
radical, 2,3-dimethyl cyclopentene-4-methylene radical, 1,2,3-trimethyl
cyclopentene-4-
6
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
methylene radical, 1,2,3,5-tetramethyl cyclopentene-4-methylene radical,
.cyclopentene-4-
ethylene radical, 1-methyl cyclopentene-4-ethylene radical, 3-methyl
cyclopentene-4-ethylene
radical, 1,2-dimethyl cyclopentene-4-ethylene radical, 3,5-dimethyl
cyclopentene-4-ethylene
radical, 1,3-dimethyl cyclopentene-4-ethylene radical, 2,3-dimethyl
cyclopentene-4-ethylene
radical, 1,2,3-trimethyl cyclopentene-4-ethylene radical, 1,2,3,5-tetramethyl
cyclopentene-4-
ethylene radical, cyclopentene-4-propylene radical, 1-methyl cyclopentene-4-
propylene radical,
3-methyl cyclopentene-4-propylene radical, 1,2-dimethyl cyclopentene-4-
propylene radical,
3,5-dimethyl cyclopentene-4-propylene radical, 1,3-dimethyl cyclopentene-4-
propylene radical,
2,3-dimethyl cyclopentene-4-propylene radical, 1,2,3-trimethyl cyclopentene-4-
propylene
l0 radical, and 1,2,3,5-tetramethyl cyclopentene-4-propylene radical.
The cyclic olefinic pendant groups described above are generally linked with
the
ethylenic backbone of the polymer by means of one or more linking groups. Such
linking
groups are known within the art, and, most typically, will be selected from
the following:
-O- CHR n:
-(C=O)-O-(CHR)ri
-NH-(CHR)n
-O-(C=O)-(CHR)ri
-(C=O)-NH-(-CHR)ri and
-(C=O)-O-CHOH-CH2-O-
wherein R is hydrogen or an alkyl group selected from the group consisting of
methyl, ethyl,
propyl and butyl groups and where n is an integer in the range from about 1 to
12.
However, it should be noted that a linking group is not required.
Preferred polymers include a polyethylene-methyl acrylate) copolymer, a
poly(cyclohexene-methyl acrylate) homopolymer (CHAA), a
poly(ethylene/cyclohexene-
methyl acrylate) copolymer, a poly(ethylene/vinyl cyclohexene) copolymer
(EVCH), or a
poly(ethylene/methyl acrylate/cyclohexene-methyl acrylate) (EMCM) terpolymer.
In another illustrative embodiment, the polymer treated in accordance with the
present
invention comprises an ethylenic backbone having a combination of alkyl
acrylate pendant
groups and cyclic olefinic pendant groups. Examples of such polymers can be
found, for
example, in International Application No. W0/99/48963, from Chevron Chemical
Company,
and pending U.S. Patent Application Serial No. 09/127,316, the disclosure of
which is
incorporated herein by reference in its entirety. One particularly preferred
polymer according to
this aspect of the invention is exemplified by the terpolymer, ethylene-alkyl
acrylate-
cyclohexene methyl acrylate, also referred to herein as EMCM.
7
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
In another illustrative embodiment of the invention, the.solid polymer used in
the
disclosed process comprises an ethylenic backbone having benzylic pendant
groups, such as
those described in U.S. Patent No. 5,859,145, assigned to Chevron Chemical
Company, the
disclosure of which is incorporated herein by reference in its entirety. Thus,
the solid polymer
can comprise an ethylenic backbone and moieties which contain a benzyl radical
and which are
pendant or terminal to the ethylenic backbone. A pendant moiety which contains
a benzyl
radical, as that term is used herein, is any group which is a side-chain or
branch or is terminal to
the ethylenic backbone and which contains a benzyl radical.
The benzyl radical can comprise a phenyl radical directly bonded to a
methylene
to radical. The methylene radical may be joined to other alkyl or alkylene,
alkenyl, alkynyl, aryl,
or heteroatom-containing substituents that, together with the benzyl radical,
form the
unsubstituted moiety that is pendant to the ethylenic backbone. These radicals
may be
substituted with a hydrocarbyl radical or a heteroatom or heteroatom-
containing radical or may
be unsubstituted. A substituted phenyl radical has at least one radical
substituted in place of at
least one hydrogen atom of the phenyl radical. An unsubstituted methylene
radical generally
consists of one carbon atom and two or three hydrogen atoms. A substituted
methylene radical
generally consists of one carbon atom, one hydrogen atom, and at least one
radical substituted
in place of one of the hydrogen atoms. A benzyl radical may be bonded to the
remainder of its
pendant moiety through its phenyl radical. In this case, its methylene radical
may be a methyl
2o radical or a substituted methyl radical.
Preferably, the benzyl radical is a component of a cyclic benzylic side chain,
by which
is meant a side chain in which at least two of the carbons of the benzyl group
are members of a
cyclic allcyl, alkenyl, or alkynyl group that is not coextensive with the
benzyl group. Preferred
cyclic benzyl radicals include cyclic benzyl ether groups, cyclic benzyl amine
groups, or cyclic
benzyl amide groups.
A heteroatom-containing radical is any radical which contains an element other
than
carbon and hydrogen. The heteroatom-containing radical generally improves the
S oxygen-
scavenging abilities of the composition. A heteroatom having pi bonds to
adjacent carbon
atoms is preferred. When present, the heteroatom-containing radical is
preferably bonded
3o directly to the benzyl radical with no moieties present between the
heteroatom-containing
radical and the benzyl radical. The heteroatom-containing radical may be
bonded to the benzyl
radical in any combination of three possible ways. For example, the heteroatom-
containing
radical may be bonded to the methylene radical. It may also be substituted
onto the methylene
radical in place of one of the hydrogen atoms, in which case the methylene
radical is attached
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
directly to the backbone or the moiety attached to the backbone or to another
heteroatom-
containing moiety. Or, the heteroatom-containing radical may be substituted in
place of one of
the hydrogen atoms of the phenyl radical. Examples of heteroatom-containing
radicals include
amine, ether, sulfide, and ketone radicals, and preferred radicals are esters
and amides.
Radicals which may be substituted or joined onto the benzyl radical include
alkyl
radicals containing from 1 to 18 carbon atoms, alkoxy radicals having from 1
to 16 carbon
atoms, alkenyl or alk3myl radicals containing from 2 to 18 carbon atoms,
alkenoxy or alkynoxy
radicals having from 2 to 18 carbon atoms, amine radicals having from 1 to 6
carbon atoms,
aryl radicals or substituted aryl radicals having 6 to 24 carbon atoms, aryl
ether radicals or
l0 substituted aryl ether radicals having from 6 to 24 carbon atoms, and ester
and amide radicals
of acids having from 1 to 16 carbon atoms. Aryl and aryl ether radicals can be
substituted in the
same manner as the methylene and the phenyl radicals, subj ect to the
limitation that the aryl
and aryl ether radicals, after substitution, have 6 to 24 carbon atoms total.
Preferably, the
radicals which are substituted onto the benzyl radical are selected from the
group consisting of
15 alkyl radicals containing from 1 to 6 carbon atoms, alkoxy radicals having
from 1 to 6 carbon
atoms, amine radicals having from 1 to 6 carbon atoms, aryl radicals and
substituted aryl
radicals having 6 to 15 carbon atoms, aryl ether radicals and substituted aryl
ether radicals
having from 6 to 15 carbon atoms, and ester and amide radicals of acids having
from 1 to 6
carbon atoms. Preferred radicals which provide higher oxygen scavenging rates
are alkyl,
20 alkoxy, and amine radicals.
Preferably, the benzyl moieties which are pendant to the ethylenic backbone
comprise
benzyl thioester, more preferably benzyl amide, and most preferably benzyl
ester moieties.
Preferably, the amide or ester is bonded directly to the ethylenic or
polyethylenic backbone.
Other preferable pendant moieties contain benzyl ether groups, benzyl amine
groups, and -CH2
25 -aryl containing groups where the aryl group includes more than one ring,
such as 1,3-
dihydroisoindole, anthracene, phenanthrene, naphthalene and the like.
In one preferred embodiment, a polymeric composition of the present invention
contains between about one and 20 mole percent benzyl radicals. More
preferably, the
composition contains between about two and 15 percent, and more preferably
still, between
3o about 5 and 12 mole percent benzyl radicals. Preferably, the benzyl
radicals are bonded directly
to a heteroatom-containing group. The exact amount of benzyl radicals and
heteroatom-
containing radicals as well as the amount of transition-metal salt are
normally determined by
the application in which the composition is going to be employed.
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
According to the present invention, one or more of the solid polymers
described above
is contacted with a solvent in order to extract undesirable low molecular
weight components
from the polymer. By low molecular weight components, it is meant the
components present in
the polymer after it is produced that have molecular weights less than about
2000, less than
about 1000, or less than about 500, depending on the particular polymer being
produced and/or
the particular components that are desired to be removed.
The solvent with which the solid polymer is contacted is selected from Cl-C4
alcohols,
C3-C6 ketones, C3-C8 acetates, and C3-C8 ethers. or a mixture thereof. In one
illustrative
embodiment of the invention, the solvent is selected from ethanol,
isopropanol, acetone, ethyl
l0 acetate, or a mixture thereof. The solvents of the invention have been
found to advantageously
extract the low molecular weight components from the solid polymer matrix,
while typically
not extracting higher molecular weight polymeric material that is desired to
remain in the solid
polymer following extraction. This selectivity of the solvents identified
herein is particularly
advantageous for extracting components from the solid polymer having molecular
weights less
than about 2000, preferably less than about 1000, while causing only minimal
extraction of
polymeric material having higher molecular weights.
One or more of the solid polymers described above are contacted with the
solvent or
solvent mixture under conditions effective for extracting these low molecular
weight
components from the polymer. This will typically involve contacting the solid
polymer with
the solvent for a duration in the range of about 0.5 hr. to about 2 days. Most
typically, the
contact time between the polymer and the solvent will range from about 2 to 20
hrs. The
duration of contact between the polymer and the solvent can, of course, be
varied depending on
a number of factors, e.g., the temperature at which the extraction is
performed, the particular
solvent used, the solid polymer being treated, the nature and quantity of the
low molecular
weight components to be removed from the polymer, the particle size of the
polymer, polymer-
solvent interactions (such as swelling), the intensity of ultrasonic energy
that may also applied
to the mixture, etc. The temperature of the extraction solvent can be varied
as a matter of
operational convenience provided the temperature does not exceed the melting
temperature or
the glass transition temperature of the polymer being treated. Generally, the
extraction
3o temperature will be in the range of about 15 °C to about 65
°C. Most typically, the extraction
temperature will be between about 35 °C and about 55 °C. In
addition, physical agitation or
circulation can be used to maximize the efficiency to remove oligomer and
odorous
compounds.
to
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
The skilled individual in the art will recognize that the solvent extraction
approach
described herein can be employed in any of a variety of settings and can be
readily adapted for
use in a large scale polymer production facility if desired. For example,
Figure 4 illustrates one
possible approach for the implementation of the disclosed process. The pellet
extraction
system shown in Figure 4 centers around the solvent extractor 10. Operating
temperatures and
flow rates for the solvent extractor 10 and other pieces of equipment depend
on the nature of
. the polymer and the extraction solvent.
Polymer, in pellet form, passes along with fresh and recycled extraction
solvent through
the solvent extractor 10, a device that allows for intimate contacting of
polymer pellets and
to solvent. Residence time in this extractor can be on the order of minutes to
several hours,
depending on the polymer-solvent system requirements and the equipment used.
The
extraction rate in this equipment can be enhanced by the use of ultra-sound,
thereby reducing
the required residence time, as described elsewhere in this document.
Pellets leaving the solvent extractor undergo separation of the solvent from
the polymer
by filtration, and then the pellets are passed to a pellet dryer 12. The
purpose of this step is not
only to remove solvent from the surface of the polymer, but also to allow for
removal of
solvent absorbed by the polymer. Evaporating solvent from the pellets without
first extracting
a large proportion of the solvent by filtration or similar technique may lead
to the retention of
non-volatile oligomers in the pellet. Warm, dry nitrogen gas, or other dry
inert gas, is passed
2o through the pellets. The nitrogen stream leaving the dryer 12 is rich in
solvent vapor. This
stream passes to a condenser where the solvent vapor is condensed and recycled
back to the
extractor. The nitrogen is recycled back to the dryers.
Spent solvent leaving the solvent extractor 10 is sent to one or more
distillation columns
14 for removal of dissolved impurities (i.e. lower molecular weight compounds
and
comonomers) from the solvent stream. Recovered solvent is recycled back to the
solvent
extractor 10 and impurities are removed.
In another aspect of the present invention, a method for ultrasound-assisted
solvent
extraction is provided. It has been found that ultrasonication of the solid
polymer while in
contact with the disclosed solvents significantly facilitates the removal
efficiency of the low
molecular weight components from the solid polymer, possibly by increasing the
mobility of
the low molecular weight components. Illustratively, six hours of solvent
extraction used in
conjunction with ultrasonication removed a greater quantity of low molecular
weight
components than were removed after 24 hours of extraction with the same
solvent without
ultrasonication.
11
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
Therefore, according to this aspect of the present invention, a method is
provided in
which solid polymer is contacted with solvent to form a polymer/solvent
mixture and ultrasonic
energy is applied to the polymer/solvent mixture under conditions for
improving the extraction
efficiency of low molecular components from the polymer. This use of
ultrasonication
effectively reduces the contact time between the polymer and the extraction
solvent that is
necessary to extract a given quantity of low molecular weight components. For
example, the
extraction efficiency, as measured by extractives removed per unit time, can
be increased by
greater than about 2 to 10 times, preferably greater than about 5 times, using
ultrasound-
assisted solvent extraction compared with extraction with the same solvents)
in the absence of
l0 ultrasonication.
The ultrasonic energy may be applied by any of a variety of known techniques.
Illustratively, the ultrasound can be applied from outside an extraction
vessel, or, alternatively,
from inside an extraction vessel, for example using a probe design. The latter
may be a
preferred approach for use in a large scale operation. For certain
applications, the vessel in
which extraction is performed can contain a fluidized bed or a current
counterflow of solid
versus solvent to achieve optimal extraction efficiency.
The ultrasonic energy level that is applied to the solid polymer while in
contact with
solvent can be varied to best suit the needs of a given implementation of this
invention. The
energy level employed may vary, for example, depending on the design of the
system, e.g.,
2o external versus internal design. Typically, but not by way of limitation,
the energy level of the
ultrasound applied to a polymer/solvent mixture will be in the range of about
50-10000 watts.
For lab scale applications, the energy level will be in the range of about 50-
500 watts.
The conditions under which the ultrasonic energy is applied, e.g., the energy
level,
contact time, etc., are selected so as to effectively remove the desired
quantity of low molecular
weight components from the solid polymer of interest. In one illustrative
embodiment, the
treatment conditions are selected such that the levels of low molecular weight
components in
the solid polymer are reduced by at least about ~5%, preferably by at least
about 90%,
following one or more solvent extractions described herein. In another
illustrative
embodiment, the levels of the low molecular weight components are reduced to
below about
100ppm, preferably below about 30ppm, for each low molecular weight component
present in
the solid polymer.
The process of this invention can be readily adapted for use on essentially
any scale
from small, laboratory scale solvent extractions to large, commercial scale
operations. Figure 5
illustrates one possible implementation of this aspect of the invention in
which, fresh resin to be
12
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
cleaned is to be fed continuously from a feeder 20. The resin pellets travel
through a tubelpipe
22 with assistance from screw pressure (not shown), while the fresh solvent is
injected into the
system near the outlet of the cleaned pellets at solvent inlet 24. The resin
pellets are cleaned as
they travel through the tube/pipe 22 equipped with at least one ultrasonicator
26. The tube/pipe
22 may be heated to increase the cleaning efficiency. The dirty solvent is
taken out at waste
outlet 28 near the inlet of the fresh resin and sent to a solvent recycling
unit 30, where the
solvent is purified by distillation. The purified solvent is fed back to the
system through the
solvent inlet 24. The cleaned pellets are sent out to a dryer (not shown),
which removes further
solvent left in the pellets.
EXAMPLES
The following examples are provided to demonstrate certain illustrative
embodiments of
this invention. It should be appreciated by those skilled in the art that the
techniques disclosed
in the examples which follow represent those found by the inventors to
function in the practice
of the invention and thus can be considered to constitute examples of
illustrative modes for its
practice. However, those skilled in the art should, in light of the present
disclosure, appreciate
that many changes can be made in the specific embodiments which are disclosed
and still
obtain a like or similar result without departing from the spirit and scope of
the invention.
EXAMPLE I
250 grams of EMAC or EMCM pellets and 400-450 grams of solvent were added to a
one-litter bottle. The pellets and solvent were agitated by tumbling the
bottle on a roller for 22-
24 hours at room temperature. The pellets and extract were filtered with #2
filter paper and
rinsed with 3x25 mL solvent. The polymer pellets were dried in a vacuum oven
at 50-60 °C for
24-48 hours. The solvent was removed from the polymer extractives using a
rotary evaporator
at 50-70 °C. The weight percentage of low molecular weight component
was calculated based
on the weights of the total extractives and the starting polymer.
Various solvents were tested for their efficiency in removing low molecular
weight
components from EMAC and EMCM. These components, because of their low
molecular
weights 0200-2000 MW), can migrate from inside of the polymer pellets to the
solvents. The
3o results of these experiments are shown below in Table 1.
13
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
Table 1. Effect of Solvent on Oligomer Extraction from EMAC
Exp. No. EMAC Solvent Extractives (wt.%)
02380-02-D Repelletized Ethyl Acetate 1.8
02380-02-E EMAC-2260 THF/Acetone 1.7
02380-02-A (165 pellets/g Acetone , 0.6
vs.
02380-02-B 33 pellets/g IpA 0.5
for
02380-02-C starting EMAC- Ethanol 0.2
2260)
The EMAC/solvent mixtures were agitated by tumbling on a roller at room
temperature for 22-24 hrs. In 02380-E, 200 g of THF was used to swell the EMAC
for 2 hrs before acetone (260 g) was added.
Figure 1 illustrates the GPC traces of EMAC-2260 and low molecular weight
components extracted from it with various solvents. The low molecular weight
components
extracted from various solvents have different molecular weight distributions
which vary
to depending on the particular solvent used. It can be seen that solvents like
THF and ethyl
acetate effectively extract lower molecular weight components from the solid
polymer.
However, some higher molecular weight polymeric material was extracted as well
by these
solvents.
Consequently, solvents having high solubility with the high and low molecular
weight
components of the solid polymer may not be the best choice for oligomer
extraction and EMAC
clean up where it is desired to optimize the extraction of low molecular
weight components,
e.g., less than about 2000 MW, while minimizing the extraction of higher
molecular weight
polymer. For example, if it is desired to remove components with molecular
weights less than
about 1000, acetone, isopropyl alcohol and ethanol remove almost as much as
THF and ethyl
2o acetate, but remove very little of the higher molecular weight polymer. In
addition, strong
solvents may cause excessive swelling of EMAC (especially at elevated
temperatures). This
helps to remove the oligomers, but may cause problems in large production
scale. These
problems include reduced effective capacity, and difficulty in transferring
polymer and in
removing the solvent after extraction. In view of the above, the tested
disclosed solvents are
effective for removing the low molecular weight components from EMAC, but for
many
applications, acetone, ethanol and/or isopropyl alcohol will be preferred.
14
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
ExAMPLE 2
250 grams of EMAC or EMCM pellets and 400-450 grams solvent were added to a
one-
litter bottle. The bottle was ultrasonicated in an ultrasound bath having an
average sonic power
of 45-150 w for 45 minutes to 20 hours. The polymer was filtered with #2
filter paper and
rinsed with 3x25 mL solvent. The ultrasonication, filtration and rinsing were
repeated one or
several times. In some instances, the polymer/solvent mixtures were agitated
by tumbling on a
roller before filtration and rinsing. The polymer was dried in a vacuum oven
at 50-60 °C for
24-48 hours. The solvent was removed from the polymer extractives using a
rotary evaporator
l0 at 50-70 °C.
Ultrasonication dramatically increased oligomer mobility and improved the
efficiency
of the solvent extraction process. As shown in Table 2, solvent extraction
performed for 1 hour
in combination with ultrasound at 50 °C removed more than one-third of
the oligomer removed
at room temperature during a 24 hour extraction without ultrasound. Six hours
of extraction
performed in combination with ultrasound removed more oligomers than was
removed after 24
hours of extraction without ultrasound.
Table 2. Oligomer Extraction from EMAC, Roller vs. Ultrasonic Bath
Exp. No. EMAC Extraction Method Extractives
(Wt. %)
C2380-03-C 24 hr on roller, RT 1.5
Commercial
C2380-03-B 1 hr ultrasonic bath, 0.6
50 C
2260
C2380-07-A 2x3 hr ultrasonic bath, 2.6
50 C
Solvent: ethyl acetate.
EXAMPLE 3
We also tested the efficacy of using solvent mixtures for removal of the low
molecular
weight components from EMAC. 250 grams of EMAC pellets were swelled with 250-
350
grams of chloroform or THF in a 1-liter bottle. Weak solvent (300-450 grams of
isopropyl
alcohol, acetone, etc.) was added to the bottle. The mixture was agitated by
tumbling on a
roller for 4-10 hours at room temperature. The mixture was washed with
solvents one or more
times to extract the oligomers.
When a solvent mixture was used, a small amount of relatively strong solvent,
such as
THF and chloroform, was first mixed EMAC to swell the polymer. We typically
added equal
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
weights of the strong solvent and polymer. Excess amount of solvent dissolved
the polymer
and made the polymer pellets stick together. After all the solvent was
absorbed by the pellets, a
weaker solvent, such as isopropyl alcohol or ethanol was used for the
extraction of low
molecular weight components. Using a strong solvent such as chloroform
extracts more higher
molecular weight oligomer from the EMAC pellets, but it does not help
significantly in
removing lower molecular weight components having molecular weights below
about 1000.
EXAMPLE 4
Multiple solvent washes also improve the efficiency of oligomer removal as
shown in
l0 Table 3. In large production scale, a continuous process with fresh solvent
and fresh polymer
feeding in opposite directions is expected to further improve the process.
Table 3. Oligomer Removal from EMAC via Solvent Extraction
Single Wash vs. Triple Wash, Commercial vs. Repelletized
Exp. No. EMAC 2260 Extraction Method Weight
C2380-03-A Repelletized Single wash, 1 hr 0.9
C2380-07-B (165/g) Triple wash, 6 hr 3.7
C2380-03-B Commercial Single wash, 1 hr 0.6
C2380-07-A (33/g) Triple wash, 6 hr 2.6
Extraction was done with ethyl acetate in an ultrasonic bath at about 50
°C.
Repelletized EMAC was produced as small spheres with pellets per gram being
about
165, and having diameters about 1/5 the size of commercial EMAC pellets (which
are
somewhat elliptical or disk shaped with pellets per gram of about 33). As
shown in Table 3,
about 30% more oligomers were removed from repelletized EMAC than from the
commercial
EMAC under the same extraction condition. However, the extractives from
repelletized
EMAC contained more polymers than extracted from the commercial pellets, but
similar
quantities of oligomers with molecular weight of less than 500.
EXAMPLE 5
We also tested the effectiveness of the solvent wash process using 10 day, 40
°C, 95%
ethanol extraction conditions and using cleaned EMAC monolayer film. Monolayer
film (1.5-2
mils thick) was made from cleaned EMAC or EMCM using a Randcastle extruder.
About 100
grams of the monolayer film were soaked in 1 gallon of 95% ethanol in a one-
gallon bottle.
16
CA 02421666 2003-03-06
WO 02/28916 PCT/USO1/28890
The film/ethanol mixture was put in a 40°C oven. The film was removed
after 10 days at 40°C.
The ethanol was removed from the ethanol extract using a rotary evaporator at
about 50°C.
When the solution volume was reduced to about 30mL, the concentrated mixture
was
transferred to a 50mL flask. Ethanol was then completely removed by further
rotary
evaporation before weighing the film extractives.
Using a typical commercial EMAC without solvent extraction clean up, about
0.5% low
molecular weight components were extracted from the monolayer film. As shown
in Table 5,
less than 0.04% low molecular weight components were extracted from film made
from triple
solvent washed EMAC. This represents about a 93% reduction in low molecular
weight
to components from the EMAC.
Table 5.10 Day 40 °C Ethanol Extraction on Solvent Extracted
EMAC Monolayer Film
Exp. No. EMAC 2260 Clean Up Method Residual (wt.%)
02380-OS-A Commercial 1 ethyl acetate wash0.114
02380-OS-B Repelletized 1 acetone wash 0.157
02380-05-C Repelletized 1 IPA wash 0.233
02380-07-A Cormnercial 3 ethyl acetate washes0.039 .
02380-07-B Repelletized 3 ethyl acetate washes0.035
Film extraction was done by soaking the monolayer EMAC film in 1 gallon of
95% ethanol at 40 °C for 10 days. In 02380-05, the actual time and
temperature
were 75 hrs at 50 °C and 165 hrs at 40 °C).
The particular embodiments disclosed above are illustrative only, as the
invention may
be modified and practiced in different but equivalent manners apparent to
those skilled in the
2o art having the benefit of the teachings herein. More specifically, it will
be apparent that certain
agents which are chemically related may be substituted for the agents
described herein while
the same or similar results would be achieved. Furthermore, no limitations are
intended to the
details of construction or design herein shown, other than as described in the
claims below. It
is therefore evident that the particular embodiments disclosed above may be
altered or modified
and all such variations are considered within the scope and spirit of the
invention. Accordingly,
the protection sought herein is as set forth in the claims below.
17