Note: Descriptions are shown in the official language in which they were submitted.
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
AUTOMATIC IDENTIFICATION OF COMPOUNDS IN A SAMPLE MIXTURE
BY MEANS OF NMR SPECTROSCOPY
BACKGROUND OF THE INVENTION
1. Field of Invention
This invention relates to qualitative and quantitative chemical analysis, and
more particularly to processes, apparatus, media and signals for automatically
identifying compounds in a sample.
2. Description of Related Art
The field of biometric identification has grown tremendously over the recent
decade both from its relevance to medical diagnostics and to its application
as
a way to uniquely identify a person or an animal, for example. As diagnostic
tools have become more sophisticated, complex liquid mixtures, such as
human blood or urine for example, can now be analyzed to identify or search
for particular compounds that can provide important diagnostic information to
a medical technician or a doctor.
Generally, the separation and characterization of mixtures is fundamental to
nearly every aspect of analytical chemistry and biochemistry. Most
approaches to identify and quantify biological compounds in liquid mixtures
require an initial compound separation (chromatographic or physical
separation) step to separate a particular compound or set of compounds from
the mixture. For example, gas chromatography, electrophoresis, and liquid
chromatography are used to separate pure chemical
components/compounds, for example, from a mixture before analysis is
performed. Initial compound separation is required because most spectral
identification processes, such as mass spectrometry or infrared, visible, and
ultraviolet spectroscopy, require relatively pure samples in order to minimize
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-2-
noise and increase the accuracy of the measuring device. Spectral
identification processes are expensive, manually intensive and require a great
deal of technical expertise to be performed properly in an accurate, timely
manner.
Nuclear magnetic resonance (NMR) has recently been shown to be an
alternative approach to identify and quantify biological compounds without
chromatographic separation. In this approach, radio frequency (RF)
electromagnetic radiation is applied to a mixture of organic compounds to
extract and measure a characteristic RF absorption spectrum of nuclei
belonging to each specific organic compound. A large number of compounds
are associated with well-defined peaks in the absorption spectrum and
knowing which peaks are associated with certain compounds makes it
possible to manually identify some of the compounds in the liquid mixture
without resorting first to chromatographic separation. However, this process
is
still quite slow and requires a great deal of a priori information that
relates
each peak to a given compound. It can take a number of years for experts in
NMR spectroscopy to acquire the knowledge required to analyze NMR
spectra to accurately identify and quantify compounds in sample mixtures.
Therefore what is desired is a process and apparatus for quickly, accurately
and automatically identifying a number of compounds which may be present
in complex liquid mixtures without involving chromatographic separation and
without requiring people who are experts in NMR techniques.
SUMMARY OF THE INVENTION
Overall Process
The embodiments of the invention disclosed herein provide for automated,
accurate analysis of a test spectrum obtained from a sample, to quantitatively
and qualitatively identify compounds present in the sample.
CA 02423260 2008-06-09
-3-
In accordance with one aspect of the invention there is provided a computer-
implemented process for automatically identifying compounds in a sample
mixture. The process involves receiving a representation of a measured
condition of the sample mixture, using the representation of a measured
condition of the sample mixture to select a set of reference spectra of
compounds suspected to be contained in the sample mixture, from a library of
reference spectra, and receiving a representation of a test spectrum having
peaks associated with compounds therein, the test spectrum being produced
from the sample mixture under the measured condition. The process also
involves combining reference spectra from the set of reference spectra to
produce a matching composite spectrum having peaks associated with at
least some of the suspected compounds, that match peaks in the test
spectrum, the compounds associated with the reference spectra that combine
to produce the matching spectrum being indicative of the compounds in the
sample mixture, and storing a representation of the matching composite
spectrum.
The process may involve identifying compounds associated with the
representative reference spectra.
Identifying compounds may involve identifying quantities of the compounds.
Identifying compounds may involve identifying concentrations of the
compounds.
The process may involve identifying a peak associated with a calibration
compound, in the test spectrum.
Identifying the peak may involve identifying a peak meeting a set of criteria
that associate the peak with the calibration compound.
CA 02423260 2008-06-09
-4-
Identifying the peak may involve producing Lorentzian line shape parameters
to represent the peak.
The process may involve receiving a measured condition value representing
the measured condition of the sample.
The process may involve producing the measured condition value.
Producing the measured condition value may involve measuring pH of the
sample.
Producing the measured condition value may involve producing the condition
value from the test spectrum.
Producing the condition value may involve identifying in the test spectrum a
peak position associated with a condition reference compound.
Identifying a peak position may involve identifying a peak meeting a set of
criteria that associate the peak with the condition reference compound.
Producing the condition value may involve producing the condition value as a
function of the peak position and parameters of a sample solvent.
Producing the measured condition value may involve determining a pH value
for the sample, from the test spectrum.
Determining a measured pH value may involve determining from the test
spectrum, the location of a peak associated with a pH reference compound, in
relation to a peak associated with a calibration reference compound.
Producing the condition value may involve producing the condition value as a
function of the peak location and parameters of a sample solvent.
CA 02423260 2008-06-09
-5-
The process may involve adjusting a set of base reference spectra according
to the measured condition value, to produce the set of reference spectra.
Adjusting the set of base reference spectra may involve adjusting parameters
of the base reference spectra according to a pH of the sample.
The process may involve producing a derived reference spectrum in response
to the measured condition value and a reference spectrum.
Producing the derived reference spectrum may involve identifying a reference
spectrum that is associated with a condition value nearest to the measured
condition value.
Producing the derived reference spectrum may involve deriving a value from
at least one reference spectrum that is associated with a condition value
nearest to the measured condition value.
Producing the derived reference spectrum may involve performing
mathematical operations on parameters of a reference spectrum to produce
new parameters for use as parameters of the derived reference spectrum.
The process may involve identifying in the test spectrum a peak associated
with a calibration compound and producing Lorentzian line shape parameters,
including a line width parameter, to represent the peak.
The derived reference spectrum may be represented by at least one set of
Lorentzian line shape parameters including a line width parameter, the
process may further involve calibrating the line width parameter associated
with the derived reference spectrum relative to the line width parameter
associated with the calibration compound.
CA 02423260 2008-06-09
-5a-
Identifying representative reference spectra may involve adjusting a
parameter of the at least one derived reference spectrum until the at least
one
derived reference spectrum best aligns with the test spectrum.
Identifying representative reference spectra may involve producing a cluster
position indicator for the derived reference spectrum, which causes the
positions of peaks in the derived reference spectrum to match corresponding
peaks of the test spectrum.
Identifying representative reference spectra may involve producing an upper
bound concentration estimate of a quantity of a compound associated with the
derived reference spectrum.
Producing an upper bound concentration estimate may involve selecting as
the upper bound concentration estimate, a lowest concentration value
selected from a plurality of concentration values computed for respective
peaks in the test spectrum.
Producing the upper bound concentration estimate may involve finding the
height of a peak in the test spectrum that corresponds to a peak in the
reference spectrum.
Producing the upper bound concentration estimate may involve determining a
concentration value for a peak as a function of the height of the peak.
Producing the upper bound concentration estimate may involve predicting
whether the height of a peak in the test spectrum is greater than a threshold
level and not determining a concentration for the peak when the height is less
than the threshold level.
The process may involve adjusting the relative positions of peaks associated
with one of the compounds according to pre-defined criteria.
CA 02423260 2008-06-09
-5b-
Identifying representative reference spectra may involve determining scaling
factors for peaks in a plurality of reference spectra such that the sum of the
peaks scaled by the scaling factors optimally matches the test spectrum.
The process may involve determining concentrations of compounds
associated with the reference spectra as a function of the scaling factors.
The process may involve producing an indication of compounds associated
with reference spectra having peaks that when scaled by the scaling factors
have a height greater than a threshold.
The process may involve outputting a value representing at least one of the
concentrations.
The process may involve receiving the test spectrum from a spectrum
measurement device.
The process may involve doping the sample with a condition indicator.
Doping may involve doping the sample with a pH indicator.
The process may involve doping the sample with a chemical shift reference
compound.
The process may involve employing Nuclear Magnetic Resonance (NMR) to
produce free induction decay data operable to be transformed into an NMR
spectrum operable to be used as the test spectrum.
The process may involve receiving Nuclear Magnetic Resonance (NMR) free
induction decay (FID) data and processing the FID data to produce a
CA 02423260 2008-06-09
-5c-
representation of a measured spectrum having well-defined Lorentzian lines,
a flat baseline and peaks that have positive well defined areas.
The may involve roducin the test s ec rum from h
process p g p t t e measured
spectrum.
Producing the test spectrum may involve producing a conditioned spectrum.
Producing the conditioned spectrum may involve producing a baseline
corrected spectrum from the measured spectrum.
In accordance with another aspect of the invention there is provided a
computer-readable medium for providing computer readable instructions for
directing a processor circuit to identify compounds in a sample, the
instructions. The computer readable medium includes a set of codes
operable to cause the processor circuit to receive a representation of a
measured condition of the sample mixture, a set of codes operable to cause
the processor circuit to use the representation of a measured condition of the
sample mixture to select a set of reference spectra of compounds suspected
to be contained in the sample mixture, from a library of reference spectra,
and
a set of codes operable to cause the processor circuit to receive a
representation of a test spectrum, produced from the sample mixture under
the measured conditions. The computer readable medium further includes a
set of codes operable to cause the processor circuit to combine reference
spectra from the set of reference spectra to produce a matching composite
spectrum having peaks representing at least some of the suspected
compounds, that match peaks the test spectrum, the compounds associated
with the reference spectra that combine to produce the matching spectrum
being the compound in the sample mixture, and a set of codes operable to
cause the processor circuit to store a representation of the matching
composite spectrum.
CA 02423260 2008-06-09
-5d-
In accordance with another aspect of the invention there is provided an
apparatus for identifying compounds in a sample mixture. The apparatus
includes a processor circuit programmed to receive a representation of a
measured condition of the sample mixture, use the representation of a
measured condition of the sample mixture to select a set of reference spectra
of compounds suspected to be contained in the sample mixture, from a library
of reference spectra, and receive a representation of a test spectrum,
produced from the sample mixture under the measured conditions. The
processor circuit is also programmed to combine reference spectra from the
set of reference spectra to produce a matching composite spectrum having
peaks representing at least some of the suspected compounds, that match
peaks the test spectrum, the compounds associated with the reference
spectra that combine to produce the matching spectrum being the compound
in the sample mixture.
In accordance with another aspect of the invention there is provided an
apparatus for identifying compounds in a sample mixture. The apparatus
includes provisions for receiving a representation of a measured condition of
the sample mixture, provisions for using the representation of a measured
condition of the sample mixture to select a set of reference spectra of
compounds suspected to be contained in the sample mixture, from a library of
reference spectra, and provisions for receiving a representation of a test
spectrum, produced from the sample mixture under the measured conditions.
The apparatus also includes provisions for combining reference spectra from
the set of reference spectra to produce a matching composite spectrum
having peaks representing at least some of the suspected compounds, that
match peaks the test spectrum, the compounds associated with the reference
spectra that combine to produce the matching spectrum being the compound
in the sample mixture.
CA 02423260 2008-06-09
-5e-
Other aspects and features of the present invention will become apparent to
those ordinarily skilled in the art upon review of the following description
of
specific embodiments of the invention in conjunction with the accompanying
Figures.
BRIEF DESCRIPTION OF THE DRAWINGS
In drawings which illustrate embodiments of the invention,
Figure 1 is a system for determining the quantity of compounds in a test
sample, according to a first embodiment of the invention;
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-6-
Figure 2 is a flow chart illustrating an automatic process for conditioning a
measured spectrum, as implemented by a workstation shown in
Figure 1;
Figure 3 is a pictorial representation of a measured spectrum produced
by the workstation shown in Figure 1;
Figure 4 is a flow chart of a routine executed on the workstation shown in
Figure 1, for conditioning the measured spectrum to suppress a
peak caused by a solvent in a sample for which the measured
spectrum is produced;
Figure 5 is a flow chart of a process for identifying compounds executed by
a spectrum analysis apparatus shown in Figure 1;
Figure 6 is a pictorial representation of a reference spectrum associated
with lactic acid at pH of 5.10;
Figures 7A and 7B are a tabular representation of an Extensible Markup
Language (XML) file representation of the reference spectrum of
Figure 6;
Figure 8 is a flow chart of a process by which base reference spectrum
records such as shown in Figures 7A and 7B may be produced;
Figure 9 is a process executed by the spectrum analysis apparatus
shown in Figure 1 to identify a peak associated with a calibration
compound in a test spectrum;
Figures IOA and 10B are a flow chart of the process for identifying
compounds, shown in Figure 5, in greater detail;
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-7-
Figure 11 is a flow chart of a process executed by the spectrum analysis
apparatus for determining a pH value from the test spectrum;
Figure 12 is a flow chart of a process executed by the spectrum analysis
apparatus for producing a derived reference spectrum;
Figures 13A and 13B are a tabular representation of a base reference
spectrum record associated with lactic acid at a pH of 5.45;
Figures 14A and 14B are a tabular representation of a derived reference
spectrum record associated with lactic acid at a pH of 5.28;
Figures 15A and 15B are a tabular representation of a generic type of derived
reference record in which equations specify center Parts Per
Million (PPM) values for peak clusters, according to one
embodiment of the invention;
Figures 16A and 16B are a tabular representation of a derived record
comprising look-up table links to center PPM values according
to another embodiment of the invention;
Figures 17 is a flow chart of a process for determining an upper bound
concentration estimate;
Figure 18 is a flow chart of a least squares fitting routine referenced by
Figure 10B;
DETAILED DESCRIPTION
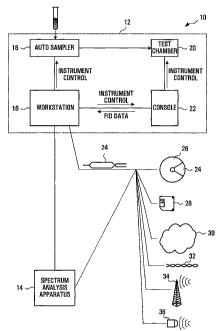
Referring to Figure 1, a system, according to a first embodiment of the
invention, for determining the quantity of compounds in a test sample is
shown generally at 10. The system includes a spectrum producing apparatus
12 and a spectrum analysis apparatus shown generally at 14. In this
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-8-
embodiment, the spectrum producing apparatus 12 is a Nuclear Magnetic
Resonance (NMR) System provided by Varian Inc. of California, U.S.A.
Generally, the system is operable to receive a specially prepared liquid
biological test sample and produce a data file comprised of a plurality of
(x,y)
values which define a measured NMR spectrum. This measured NMR
spectrum is then supplied to the spectrum analysis apparatus 14, where a
process according to another aspect of the invention is carried out to provide
an indication of the quantities of certain compounds in the specially prepared
biological test sample.
The system 10 is suitable for use with biological samples, for example blood
or urine, in which the solvent is water, for example. Such samples may be
"prepared" by doping them with a small quantity of a condition indicator
compound, also referred to as a condition reference compound, and a
chemically inert chemical shift calibration standard compound also referred to
as a calibration compound. The condition indicator may be trimethylsilyl-l-
propanoic acid or Imidazole, where the distortion factor is pH, for example.
Alternatively, the sample itself may have a naturally occurring, inherent
condition indicator such as glycine, creatinine, urea, citrate, or
trimethylamine-
N-oxide, for example. The chemical shift calibration standard compound may
be 3-[trimethylsilyl]-1-propanesulfonic acid, also known as DSS, for example.
Alternatively, the chemical shift calibration standard may be
dimethylsulphoxide (DMSO), acetone, or tetramethylsilane (TMS), for
example.
In this embodiment, the spectrum producing apparatus 12 is comprised of a
computer workstation 16, an auto sampler 18, a test chamber 20, and a
console 22. The workstation is a Sun Workstation with a 400 MHz
UltraSPARC Ili CPU with 2 MB level 2 cache, 128 MB RAM, on-board PGX24
graphics controller, 20 GB 7200 r.p.m. EIDE hard disk 48x CD-ROM drive
1.44 MB floppy drive and 17" flat screen color monitor. The workstation runs
Varian VNMR software which includes routines for controlling the auto
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-9-
sampler 18 and the console 22 to cause the specially prepared biological
liquid sample to be received in the test chamber 20 and to cause the console
to acquire and provide to the workstation Free Induction Decay (FID) data
representing the free induction decay of electromagnetic radiation absorptions
produced by protons in the compounds of the liquid sample as a result of
changes in magnetic properties of the protons due to a nuclear magnetic
resonance process initiated in the test chamber 20 by the console 22.
Process for Producing a Measured Spectrum
The FID data is received and stored in memory at the workstation 16. Then, in
this embodiment, a process according to an embodiment of another aspect of
the invention, is carried out to cause the workstation to produce a measured
spectrum for use by the spectrum analysis apparatus 14. Instructions for
directing the workstation to automatically carry out the process for producing
the measured spectrum are embodied in computer readable codes 24. These
computer readable codes 24 may be provided to the workstation 16 in a
variety of different forms including a file or files on a computer readable
medium such as a CD-ROM 26, or floppy disk 28, for example, or as a file
received as a signal from a communications medium such as an internet 30,
extranet or intranet, electrical 32, Radio Frequency (RF) 34, or optical
medium
36 or any other medium by which a file comprised of said codes may be
provided to the workstation 16 to enable the workstation to be directed by the
codes to execute the process described herein to produce a measured
spectrum.
Autoprocessing
Generally, an automatic computer-implemented process for producing a
measured spectrum from NMR data, may involve operating on free induction
decay (FID) data produced by a spectrometer to produce a trace file
comprised of intensity and frequency values representing a measured
spectrum having a flat baseline and well defined peaks that have positive,
well-defined areas, for use in a computer-implemented spectrum analysis
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-10-
process such as the process described herein. In particular, the process may
involve performing a Fourier Transform on Free Induction Decay (FID) data to
produce an initial spectrum, filtering a selected region of the initial
spectrum to
produce a filtered spectrum and phasing the filtered spectrum to produce a
measured spectrum having a flat baseline and well defined positive peaks.
Referring to Figure 2, a flowchart depicting functional blocks implemented by
the codes to cause the workstation to execute a specific process for
producing a measured spectrum is shown generally at 50. The process
begins with a first block 52 that causes the workstation 16 to read and
perform
an initial Weighted Fourier Transform on the FID data to produce an initial
measured spectrum representing signal intensity (i) versus frequency (F).
Then block 54 causes the workstation 16 to produce parameters for use in a
later-executed Fourier Transform performed on the FID data to produce a
representation of a measured spectrum having well defined Lorentzian lines
with a flat baseline and peaks that have positive, well-defined areas. Thus,
the result of block 54 is a set of parameters that controls Fourier Transforms
later performed on the FID data to produce a representation of a measured
spectrum.
Block 56 directs the workstation to save the set of parameters in association
with the FID data. Block 58 directs the workstation 16 to perform a Fourier
Transform on the FID data, using the parameters produced by block 54 to
produce a trace file, which is a file comprised of a plurality of (x,y) values
that
represent a trace of the measured spectrum, representing intensity versus
frequency. Block 59 then causes the workstation to save the trace file for
transmission to the spectrum analysis apparatus 14 shown in Figure 1.
An example of a measured spectrum is shown generally at 41 in Figure 3.
The spectrum is a plot of intensity versus frequency. The x-axis 43 is
referenced to parts per million (ppm) and depicts a window of the overall
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-11-
spectrum, the window containing relevant information or features for
identifying compounds in the sample. The y-axis 39 is referenced to a zero
value and the spectrum has a baseline 37 representing a noise level from
which a plurality of peaks 45, 47, 49, 51, 53, 55, 57, 59, 61, 63 associated
with various compounds in the sample extend. For example peaks 45 and 47
are associated with Imidazole, peak 49 is associated with Urea and peaks 51
and 53 are associated with Creatinine. Peaks 55 and 57 form a first cluster
associated with citric acid and peaks 59 and 61 form a second cluster
associated with that compound. Peak 63 is associated with DSS, the
calibration compound.
Referring back to Figure 2, block 54 which processes the FID data, is shown
in greater detail. Block 54 includes sub-functional blocks including a Fourier
Transform block 60, a filter selected and/or solvent region block 66 and an
automatic phasing block 68, each of which is automatically executed in turn,
in the order shown. The process may include an optional spectral window
setting block 62 and an optional drift correction block 64, to further process
the spectrum, for example.
The Fourier Transform block 60 has an optional sub-block 70 that causes the
workstation to perform a weighted Fourier Transform with weights that provide
for enhancement of the initial spectrum. These weights may perform a line
broadening function to the initial spectrum, for example. To do this in this
embodiment, block 70 causes the workstation to set signal enhancement
parameters for use in a subsequently executed weighted Fourier Transform
block 72. Such signal enhancement parameters may effect line broadening,
line narrowing, or gaussian sine-bell conditioning, for example, to the
resulting
spectrum produced by the Fourier Transform block 72. In the Varian VNMR
software, this is effected by setting a line broadening variable "Ib" to a
specified value, which may be 0.5, for example. Also in the VNMR software,
the weighted Fourier Transform may be executed by calling the VNMR macro
"wft" to perform a weighted Fourier Transform on the FID data, using the lb
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-12-
parameter value set at block 70. This has the effect of broadening the lines
or
peaks of the spectrum and averaging the spectrum to produce a measured
spectrum with a better signal to noise ratio than would be produced without
averaging. It also has the effect of eliminating glitches to produce a
measured
spectrum of better quality.
In this embodiment optional block 62 causes the workstation 16 to define a
window on the initial spectrum and this may involve scaling the initial
spectrum. It is desirable to set the spectral window to a preset size, i.e. a
pre-defined range of frequency, to enable the acquisition of repeatable data
and for all useful data to be in a pre-defined window and to scale the
spectrum such that the height of its maximum peak is a percentage of the
height of the window. In this embodiment, this is effected through the VNMR
software by executing three sub-functional blocks 74, 76 and 78 that cause
the workstation 16 to call the VNMR macros "f', "full', and the VNMR
command "vsadj", respectively, in the order shown. The `f' macro sets display
parameters "sp" and "wp" for a full display of a 1 D spectrum, the `full'
macro
sets display limits for a full screen so that the spectrum can be seen as wide
as possible in the window, and the `vsadj' command sets up automatically the
vertical scale "vs" in the absolute intensity mode "ai", so that the largest
peak
is of the required height. Effectively this provides for scaling of the
spectrum
so that the highest peak is 90% of the total window height.
Optional block 64 causes the workstation to produce parameters that perform
drift correction on the spectrum to correct the measured spectrum for drift
effects, effectively setting the two extremes of the baseline of the spectrum,
i.e. the left and right sides of the spectrum to have zero slope. In this
embodiment, using the Varian VNMR software, this is achieved by block 80
which causes the workstation 16 to call the "dc" macro of the VNMR software.
Effectively the "dc" macro calculates a linear baseline correction. The
beginning and end of a straight line to be used for baseline correction are
determined from the display parameters "sp" and "wp". The "dc" command
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-13-
applies this correction to the spectrum and stores the definition of the
straight
line in the parameters "9vl" (level) and "tlt" (tilt) of the VNMR software.
(cdc
resets the parameters "Ivl" and "tlt" to zero.)
Block 66 causes the workstation to filter a selected region of the spectrum to
adjust the intensity of the spectrum in that region. Filtering may involve
applying a notch filter to a selected or solvent region, for example, to
suppress
a peak associated with a contaminant or solvent in the contaminant or solvent
region. This ensures that the solvent region or contaminant region of the
spectrum is correctly phased with the rest of the spectrum so that the entire
spectrum can be properly phased later. In order to permit the entire spectrum
to be phased, the solvent or contaminant residual must be in phase with the
rest of the spectrum, ideally reducing the solvent or contaminant region to
zero. The solvent region is the region of the spectrum in which solvent
compounds in the sample may be found. For example the solvent may be
water, in which case the region around the peak in the measured spectrum
associated with the compound H20 is considered to be the solvent region.
The contaminant region is a region of the spectrum where peaks associated
with contaminants are present.
Referring to Figure 4, a routine for filtering the selected region is shown
generally at 66 and involves a first block 92 that causes the workstation 16
to
apply a notch filter to the selected region to suppress a peak in that region.
A
set of initial notch filter parameters specifying the attenuation, width and
position of the notch filter is used.
Applying a notch filter may further involve producing an adjusted set of notch
filter parameters and applying a notch filter employing the adjusted set of
notch filter parameters to the selected region. The set of notch filter
parameters may be adjusted to produce an adjusted set of notch filter
parameters that may be applied to the notch filter to filter the selected
region
until a sum of the absolute values of areas defined by peaks above and below
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-14-
a baseline of the initial spectrum is minimized. In this embodiment this is
done by block 94 which causes the workstation to adjust the set of initial
notch
filter parameters and re-apply the notch filter until the sum of the absolute
values of the areas of the spectrum in the selected region, is minimized. One
quick way of doing this and minimizing the number of iterations of application
of the notch filter is to employ numerical methods to successive values
produced. For example, in this embodiment, using the Varian VNMR
software, the parameter "sslsfrq" specifies a notch filter value that affects
the
minimization of the sum of the areas above and below the baseline. Brent's
method, as described in Brent, R.P. 1973, Algorithms for Minimization without
Derivatives (Englewood Cliffs, NJ: Prentice-Hall), Chapter 5, [1], for example
may be used to find an optimum value for "sslsfrq".
Referring back to Figure 2, after filtering the selected region block 68 is
invoked to automatically phase the entire spectrum and make the peaks as
symmetrical as possible. This may be done iteratively, for example, by
adjusting the real and imaginary components of the transformed FID data until
the resulting spectrum has positive, well defined peaks. In this embodiment,
employing the Varian VNMR software, this is achieved by invoking block 84
which calls the "aphO" command of the VNMR software. Some versions of
the VNMR software may require more than one successive execution of the
aphO command.
After automatic phasing parameters of the spectrum have been produced,
optionally, a baseline correction block 69 may be executed to flatten out the
baseline of the spectrum. Alternatively, baseline correction may be performed
later. Baseline correction may be done by analysing the spectrum to
determine areas with peaks and areas devoid of peaks and setting areas
devoid of peaks to have a common intensity value such as zero, for example.
An example of baseline correction available at
www.acdlabs.com/publish/nmr-ar.htmi published by Advanced Chemistry
Development Inc. of Toronto, Ontario, Canada.
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-15-
Block 56 then causes the workstation 16 to save parameters produced by the
various sub-processes of block 54 in association with the FID data and text,
if
desired. With the Varian VNMR software this may be achieved using the
`svf($savefid)' command.
Block 58 then directs the workstation 16 to produce a trace file comprised of
(x,y) values representing intensity versus frequency, by performing a Fourier
Transform on the FID data, using the parameters produced as described
above and associated with FID data. The trace file is then transferred or
transmitted to the spectrum analysis apparatus 14 or is stored for later
transfer to that apparatus.
Spectrum Analysis Apparatus
In the embodiment shown, the spectrum analysis apparatus (SAA) 14 is a
separate component and includes a Linux workstation configured to receive
the trace file representing the measured spectrum, from the spectrum
producing apparatus 12. The spectrum analysis apparatus 14 is configured to
receive and execute instructions embodied in computer readable codes to
carry out a process for identifying compounds in a sample according to an
embodiment of another aspect of the invention. The codes may be provided
to the spectrum analysis apparatus through any of the media described above
including the CD-ROM 26, Floppy disk 28, internet 30, extranet, intranet,
electrical 32, RF 34, and optical 36 media and/or any other media capable of
providing codes to the spectrum analysis apparatus.
It will be appreciated that the workstation 16 may alternatively be configured
with both the codes to effect the process for producing a measured spectrum
shown in Figure 2 and the codes to effect the process for identifying
compounds, or either of these. It is desirable however, to execute the
process for identifying compounds at a computer other than the workstation
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-16-
16, to enable the process for identifying compounds to be executed while
another sample is being subjected to the NMR process, for example.
Process for Identifying Compounds
Referring to Figure 5, generally, the process for identifying compounds
involves identifying representative reference spectra from a set of reference
spectra associated with detectable compounds and selected according to a
condition of the sample, which collectively define a composite reference
spectrum having features matching a set of features in a test spectrum
produced from the sample. Once the representative reference spectra have
been identified, compounds with which they are associated may be identified.
The compounds associated with respective reference spectra of the identified
set are the compounds that may be expected to be present in the sample.
Quantities of the compounds may be determined from the intensities of
certain representative peaks in the test spectrum which are associated with
the compounds, relative to the intensity of a peak associated with the
chemical shift calibration standard compound which is unaffected by the
condition of the sample. A condition may be the pH of the sample, for
example, and an accurate measurement of pH can be obtained from the test
spectrum. Thus, given a test spectrum of a sample and given a set of
reference spectra, the process can identify and quantify compounds present
in the sample. Alternatively, the condition may be temperature, osmality, salt
concentration, chemical composition, or solvent, for example.
Reference Spectra
Before the process for identifying compounds can be carried out, a set of
reference spectra for compounds to be detected in the sample must be made
available to the SAA 14. This can be done by storing data relating to
reference spectra associated with respective compounds and allowing the
SAA 14 access to the data. An exemplary reference spectrum for a given
compound may initially be represented in the form of intensity versus
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-17-
frequency (x,y) values, which may be represented graphically. A reference
spectrum for lactic acid is shown in Figure 6, for example. It will be
appreciated that such a spectrum may have a plurality of peaks and/or
clusters of peaks 150, 152, 154, 156, 158, 160, 162, 164, 166, 168, 170
superimposed upon a featureless background, such as noise 172. The
resolution along the x axis is dependent upon the frequency of the Magnet
used in the Nuclear Magnetic Resonance Process employed to acquire the
sample. The peaks that are associated with lactic acid are found in first and
second clusters 166 and 154. These clusters are centered at 1.322 ppm and
4.119 ppm respectively. The first cluster is comprised of two peaks and the
second cluster 154 is comprised of four peaks.
A reference spectrum of the type shown in Figure 6 can be represented in
various formats including mathematical representations such as Lorentzian
equations which may specify peaks associated with the compound the
spectrum is intended to represent. Such equations have the form:
f(x) = a w2
w2 + 4(x-c)2
where: a represents amplitude of the peak
w represents width of the peak; and
c represents the center of the peak
Thus, for example, the two peaks associated with the cluster centered on
1.322 ppm may be specified by two sets of Lorentzian line shape parameters
a, w and c.
The Lorentzian line shape parameters for each peak associated with a given
compound may be stored in a base reference spectrum record embodied in
an XML file as shown in Figures 7A and 7B, for example. Such a file may
have fields 200, 202 and 204, for example, for storing compound information,
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-18-
experiment information and cluster/peak information respectively. The
compound information field may include sub-fields for storing the name of the
compound with which the record is associated, and the molecular weight of
the compound, for example. The experiment field may have sub-fields for
storing information about the experiment, such as conditions under which the
peak information about the compound was collected. This may include the
pH of the solution that was analyzed, the temperature of the solution, the
calibration reference compound ratio, the concentration of the compound in
the solution, a timestamp, a sourcefile name, the frequency of the magnet
used in the NMR process, and the spectral width of the entire spectrum, for
example. The cluster/peak information fields may include separate fields 206
and 208 for each cluster (166 and 154 in Figure 6).
Each cluster field 206 and 208 may include sub fields 210, 212, 214, 216 and
218 for representing information relating to the proton number of the cluster,
the quantification of the cluster, the Lorentzian line width adjustment of the
cluster and first and second peak subfields respectively. The first and
second peak subfields may include fields 220, 222, and 224 for representing
offset center information, height information and proton ratio information
relating to a respective peak in the cluster, respectively.
Effectively, the Lorentzian line shape parameters (a) and (c) for each peak
may be stored in the height and offset center fields 222 and 220 respectively
and each peak in a given cluster is considered to have the same width (w)
which is specified by the contents of the Lorentzian line width adjust field
214
associated with the cluster.
Referring to Figure 8, a process by which base reference spectrum records
may be produced is shown generally at 230. The process begins with block
232 representing the preparation of a liquid solution containing a reference
compound such as lactic acid, a calibration compound such as DSS and a
condition indicator compound such as Imidazole. The liquid solution is
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-19-
prepared to a carefully calibrated concentration of the calibration compound
at
a carefully controlled temperature and pH. This step is carried out in a
laboratory, by a human or by a mechanized process, for example.
Once the liquid solution containing the reference compound has been
produced, as shown in block 234, it is subjected to the NMR process carried
out by the apparatus 12 shown in Figure 1 to produce FID data.
At block 236, the apparatus 12 subjects the FID data produced by the NMR
process to the process shown in Figure 2, to produce a measured reference
spectrum.
Having obtained a measured reference spectrum, a process as shown in
block 238 is initiated to identify the calibration compound and obtain
calibration parameters. This process is shown in greater detail at 238 in
Figure 9. Referring to Figure 9, the codes direct the SAA 14 to derive from
the measured reference spectrum a characterization of the calibration
compound contained in the sample. This involves identifying a position of a
peak of the measured reference spectrum that meets a set of criteria that
associate the peak with the calibration compound and further involves
producing parameters for a mathematical model of the peak, that best
represents the peak. Thus, in this embodiment the characterization is a list
of
Lorentzian line shape parameters (w, c and a) representing width, peak
position and center amplitude respectively of a Lorentzian curve that best
describes a feature, that is, a peak, of the measured reference spectrum, that
is associated with the calibration compound. It will be appreciated that other
characterizations could be used, such as those produced by peak picking,
linear least squares fitting, the Levenberg-Marquardt method, or a
combination of these methods.
To find a peak associated with the calibration compound and to produce a list
of Lorentzian line shape parameters that characterize it, the SAA 14 is
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-20-
programmed with codes that include a first block 250 that directs the SAA 14
to determine a noise level at a pre-defined area of the measured reference
spectrum. In this embodiment, it is known that an area on the x-axis
(frequency) corresponding to positions 64,000 and 65,000 for example can be
expected to be void of peaks and contain only noise. The standard deviation
of the y-value (signal intensity) over this region of the measured reference
spectrum is representative of the noise level of the entire spectrum and
provides a measure of the noise level.
Next block 252 directs the SAA 14 to scan the measured reference spectrum
in the negative x-direction beginning at the higher order end of the spectrum,
to find a y-value that meets a certain criterion. For example, the criterion
may
be that the y-value must exceed the noise level by a pre-determined amount,
such as a factor of 10, at the top of a peak. A y-value meeting this criterion
is
assumed to be associated with an x-value that represents the position of a
peak associated with the calibration compound.
Block 254 then directs the SAA 14 to employ the x-value representing the
approximate position of the calibration peak in the test spectrum in a fitting
algorithm that fits a curve to the calibration peak and specifies width,
height
and position values. For example a Lorentzian line shape-fitting algorithm
may be employed to produce Lorentzian line shape parameters (a, w and c)
that define a Lorentzian line shape that best matches the calibration peak.
Referring back to Figure 8, having calculated Lorentzian line shape
parameters that identify and characterize the calibration compound, block 240
is carried out to associate other input data with the measured reference
spectrum. Other input data may include information associated with the name
and experiment fields 200 and 202 and information such as the number of
protons (proton number in XML file) for each cluster and the proton ratio for
each peak, for example.
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-21-
Next at block 242, the measured reference spectrum is characterized by
employing the well-known Conjugate Gradient method to determine
Lorentzian line shape parameters (a, w and c) for each peak or to determine
sets of such parameters that define a mathematical model or models of peaks
that best fits the important peaks of the measured reference spectrum.
At block 244, a base reference spectrum record of the type shown in Figures
7A and 7B is produced from the other input data and the characterization of
the spectrum. At block 246, the base reference spectrum record is stored in a
reference record library, which effectively includes a plurality of reference
records for various different reference compounds. For example, the
reference record library may include base reference spectrum records for: L-
phenylaianine, L-Threonine , Glucose, Citric Acid, Creatinine, Dimethylamine,
Glycine, Hippuric acid, L-alanine, L-Histidine, L-Lactic Acid, L-Lysine, L-
Serine, Taurine, Trimethylamine, Trimethylamine-N-Oxide, Urea, L-Valine,
and Acetone.
Reference records may include base reference records or derived reference
records. Base reference spectrum records may be produced by empirical
processes as described above. New records known as derived reference
records may be produced by operating on data from base reference records,
and represent derived reference spectra. Operating on data may include
interpolation and/or performing mathematical operations, and/or using a
lookup table, for example. Thus, for example, a limited set of base reference
spectrum records can be produced, including a record representing the
spectrum for lactic acid at a pH of 5.1, and a record for lactic acid at a pH
of
5.45, for example. A derived reference record representing the spectrum of
lactic acid in a solution having a pH of 5.28, for example, can then be
produced by performing mathematical operations on the Lorentzian line shape
parameters specified by the base records associated with solutions at pH 5.1
and pH 5.45 to interpolate values for a solution at a pH of 5.28. Thus, a
derived set of reference records can be produced for solutions of any pH,
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-22-
within a reasonable range, when required, thereby avoiding a priori production
of base reference records for every pH condition. As will be appreciated
below, this feature may be exploited by determining the pH value of a sample
under test and using the determined pH value to produce a set of derived
reference records for use in identifying compounds present in the sample. In
other words, reference records for use in the process for identifying and
quantifying compounds are selected from existing base reference records or
are "selected" by producing derived reference records, according to a
condition of the sample. In this embodiment, the condition is pH.
Process for Identifying Compounds
After having produced a reference library of base reference spectrum records,
the process of identifying and quantifying compounds in a test sample can be
carried out.
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-23-
Process for Identifying and Qualifying Compounds
The process is shown generally at 300 in Figure IOA and 10B and begins
with an optional first block of codes 302 that cause the SAA to perform a
spectrum conditioning step.
Spectrum Conditioning
If the measured NMR spectrum of the test sample is of sufficient quality, it
can
be used directly in subsequent operations of the process disclosed herein.
However, usually, the measured spectrum will not be of sufficient quality and
will require further processing to condition it for later use. This further
conditioning may involve baseline correction as described earlier, for
example, to produce a conditioned spectrum.
Thus the following description will refer to a test spectrum, which may be the
measured spectrum described above, if such measured spectrum is of
sufficient quality or it may be a conditioned spectrum. A measured spectrum
having a corrected baseline, for example, would be an example of a
measured spectrum that would not need to be subjected to further processing
to condition it. Usually however the process will involve producing a test
spectrum from the measured spectrum.
Calibration Determination
After being provided with, or after producing, a test spectrum of the type
described, the process involves block 304 to produce a characterization of a
calibration compound in the sample or block 306 to determine a
representation of a condition of the sample. These two functions can be done
independently or the determination of the condition of the sample can be
determined after first characterizing the calibration compound.
The process of characterizing the calibration compound generally involves
identifying a peak associated with a calibration compound, in the test
spectrum. This may involve identifying a peak meeting a set of criteria that
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-24-
associate the peak with the calibration compound. The peak associated with
the calibration compound may be characterized by producing Lorentzian line
shape parameters to represent the peak.
Block 304 relating to characterizing the calibration compound involves a call
to the process shown in Figure 9 to cause the SAA 14 to produce a set of
Lorentzian values (a, w and c) which best represent the peak associated with
the calibration compound in the test spectrum.
Condition Factor Determination
Optionally, as shown by block 308, a separate measuring device may be used
to measure the selected condition of the test sample. In this embodiment, the
measured condition is pH which may be measured by a separate pH meter to
produce a pH condition value that may be supplied to the SAA as indicated at
"C" in Figure 10, for use in later functions of the process.
If the condition value has not already been obtained desirably the condition
value can be derived from the test spectrum itself as shown at block 306.
This is possible where the measured condition is pH because the
identification of a peak associated with a pH indicator compound in a sample
can be readily determined from the test spectrum and the Lorentzian line
shape values that characterize the representation of the calibration compound
in the test spectrum.
Referring to Figure 11, a process for determining a pH condition value from
the test spectrum is shown generally at 310. Basically, the process involves
identifying a position, height and width of a peak associated with a condition
reference compound in the test spectrum and this may involve identifying a
peak meeting a set of criteria that associate the peak with the condition
reference compound. Once the peak is identified the measured condition
value may be produced as a function of the peak position and parameters of
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-25-
the sample medium, the parameters being the parameters that define the
calibration compound.
To achieve this, in this embodiment, the codes include a block 312 which
directs the SAA 14 to employ the Lorentzian line shape parameter (c)
associated with the calibration compound to locate a window in the test
spectrum, where a peak associated with the pH indicator compound is
expected to be. The window is then scanned along the x-axis (frequency)
from left to right, for example, for a y-value (intensity) that is greater
than the
amplitude value specified by the Lorentzian line shape parameter (a).
When a y-value meeting the above criteria is found, block 314 causes the
SAA 14 to execute a characterization algorithm to produce at least a center
value (c) representing the center of the peak associated with the pH reference
compound. For example a Lorentzian curve algorithm may be used to
produce Lorentzian parameters a, w and c defining the peak associated with
the pH reference compound.
Block 316 then directs the SAA 14 to execute a modified pH titration Equation
as shown below, on the center value c and to use certain parameters of the
sample solvent, in the equation, to produce a condition value representing pH
of the sample:
pH = pKa - logL &bs CSA
&HA - & bs
where: Sabs is the observed chemical shift (center c);
8A is the chemical shift of the conjugate base;
8HA is the chemical shift of the conjugate acid; and
PKA is an association constant for the conjugate base.
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-26-
Assume that no matter what method of determining pH is used, a pH value of
5.28 is obtained for the sample. Referring Back to Figure 10B block 320
directs the SAA to receive the condition value either produced externally,
such
as by measurement or produced internally such as by using the test spectrum
as described above, to produce a derived reference record representing a
derived reference spectrum for use in later functions of the process. Separate
derived reference records may be produced from corresponding base
reference spectrum records associated with corresponding compounds
expected to be in the sample. Thus, in effect a representation of a set of
derived reference spectra may be produced from a set of reference spectra
and the measured condition value. In general, a process for producing a
representation of a spectrum for a hypothetical solution containing a
compound, for use in determining the composition of a test sample, involves
producing a position value for at least one peak of a reference spectrum as a
function of the measured condition of the test sample and a property of the at
least one peak in a base reference spectrum. The property may be a position
of a peak, amplitude of the peak or width of the peak for example. In this
embodiment, a derived reference record is used to represent a representation
of a spectrum for the hypothetical solution.
Referring to Figure 12, producing a derived reference record may involve
accessing a pre-defined record specifying peaks in a reference spectrum and
adjusting a position value in the record, the position value being the
position
value of the at least one peak. This may be done by block 322 which causes
the SAA to identify a base reference spectrum record that is associated with a
condition nearest to the measured condition of the sample and to use such
reference spectrum as the derived reference spectrum.
Producing a position value for a peak may involve interpolating a position
value from position values associated with base reference spectra associated
with condition values above and below the measured condition value
associated with the sample. For example, block 324 may be employed to
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-27-
cause the SAA 14 to produce a position value by calculating the position
value as a function of pH of the sample and to effectively produce or
interpolate a derived reference spectrum.
To interpolate a derived reference spectrum, assume that at block 322 a base
reference record for lactic acid at a pH of 5.10 is located as being the base
reference spectrum record for lactic acid that is nearest to the pH of the
sample, 5.28. Such a record is shown in Figures 7A and 7B. Referring back
to Figure 12, block 324 may direct the SAA 14 to find another base reference
spectrum record for lactic acid that is associated with a pH value greater
than
the pH of the sample. Assume that it locates a base reference spectrum
record associated lactic acid at a pH of 5.45. A record of this type is shown
in
Figures 13A and 13B. On locating this second base reference spectrum
record, block 324 directs the SAA 14 to create a new derived reference
spectrum record for lactic acid at a pH of 5.28. To do this the SAA 14 is
directed to make a copy of the base reference spectrum record associated
with a pH of 5.45 and then to replace the frequency values for the center
position of each cluster shown in that record, with interpolated values. A
simple linear interpolation is used to find the value 1.3202 for the first
cluster
and the value 4.1149 for the second cluster. Figures 14A and 14B show the
resulting derived reference spectrum record for a pH of 5.28, for lactic acid,
produced using this method. Similarly, derived reference spectrum records
are produced for each compound in the reference library to produce derived
reference records for a pH of 5.28 for each compound represented in the
library.
Alternatively; adjusting the position of a peak may involve locating a
measured condition value dependent function in a base reference record, or
pre-defined record, producing the position value from the function and
associating the position value with the pre-defined record. Associating may
involve storing the position value in the pre-defined record, for example. To
effect this method of adjusting the position of a peak, a generic type of
derived
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-28-
record may be kept, in which equations, effectively specifying the centerPPM
values for the two clusters as a function of pH may be provided in the field
associated with the centerPPM value for each cluster, as shown in Figures
15A and 15B. Then, whenever a pH value is found from a sample, a copy of
the record can be made and the pH value may be used in the equations in the
copied record to produce centerPPM values. These center PPM values can
then be substituted for the respective equations that produced them, in the
copied record, thereby producing a new derived record for use in later
calculations.
Alternatively, producing a position value may involve producing the position
value by addressing a lookup table of position values with the measured
condition value of the sample. For example the position value of a peak may
be adjusted by locating, in a pre-defined record, a link to a lookup table
specifying peak positions for various condition values, retrieving the
position
value from the lookup table and associating the position value with the pre-
defined record. To do this a second generic type of derived record may be
kept, in which lookup table links, effectively specifying links to lookup
tables
(not shown) that return centerPPM values for input pH values may be
provided in the field associated with the centerPPM value for each cluster, as
shown in Figures 16A and 16B. Then, whenever a pH value is found from a
sample, a copy of the record can be made and the pH value may be used to
address the lookup tables associated with the links specified in the record to
produce centerPPM values. These center PPM values can then be
substituted for the respective links that produced them, in the copied record,
thereby producing a new derived record for use in later calculations.
Referring back to Figure 10B, after having produced a derived reference
spectrum for each compound that is likely to be in the sample, block 326
causes the SAA 14 to calibrate the Lorentzian line width values for the
derived
reference spectrum relative to the test spectrum to provide for a better fit
to
the test spectrum. To do this, block 326 may direct the SAA 14 to calibrate to
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-29-
the (a, c and w) values associated with the calibration compound in the
sample, the spectral linewidths of peaks associated with each of the reference
compounds. In this embodiment block 326 may direct the SAA 14 to employ
the contents of the Lorentzian width adjust field 214 of each derived
reference
spectrum record to produce respective absolute values representing actual
linewidths relative to the calibration compound Iinewidth. These modified
spectral line widths may be associated with respective peaks in the same
cluster of each reference compound, by storing these modified spectral line
widths in an internal data structure (not shown) that associates modified
spectral information with derived reference records.
Still referring to Figure 10B, optionally, compound specific adjustments as
shown by block 328 may be made to the contents of the fields of the derived
reference records, where it is known, for example that certain effects occur
when certain reference compounds are present in the test sample. For
example, the shift of peaks associated with citrate is affected by the
presence
or absence of certain divalent cations and therefore the process may include
a compound-specific adjustment to compensate for shifts known to occur
when the presence of such divalent cations is known. Other compound-
specific adjustments may be made to compensate for shifts due to
temperature, chemical interactions, dilution effect and other ligand effects.
Cluster Centering
Still referring to Figure 10B the process may further involve a cluster
centering
step as shown at 330 for shifting the derived reference spectrum in frequency
(x-direction) to better align it with the test spectrum. This may involve
producing a cluster position indicator for a derived reference spectrum, which
causes the positions of peaks in the derived reference spectrum to match
corresponding peaks in the test spectrum. A cluster position indicator already
associated with the derived reference spectrum may be used or a cluster
position indicator that produces a match of the derived reference spectrum to
the test spectrum to a defined degree may be derived from the cluster position
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-30-
indicator already associated with the derived reference spectrum. In the
embodiment shown, producing a cluster center indicator is achieved by
attempting to fit the cluster to the test spectrum. To do this, cluster center
values around the cluster center value already associated with the derived
reference spectrum are assigned to the derived reference spectrum and used
to effectively shift the derived reference spectrum to the left and right of
the
current cluster center value. For example, cluster center values +/- 0.001
ppm points are successively assigned to the derived reference spectrum to
successively shift the center of the derived reference spectrum at successive
points in a window extending -0.003 ppm to +0.003ppm from the currently
assigned cluster center. At each point, the derived reference spectrum is
used in a Levenberg-Marquardt (LM) fitting algorithm that determines a
correlation value for each position of the center of the derived reference
spectrum in the window. The center position that causes the LM fitting
algorithm to produce the best correlation value is then associated with the
derived reference spectrum correlation value and is used in later
calculations.
Thus in effect, the derived reference spectrum is "wiggled" into alignment
with
the test spectrum. This wiggling is done independently for each cluster of
peaks in the derived reference spectrum.
Upper Bound Concentration Estimates
Still referring to Figure 10B, in this embodiment, the process for identifying
and quantifying further involves block 332 which causes the SAA 14 to
produce an upper bound estimate of a quantity of a compound associated
with a derived reference spectrum, for use in a least squares algorithm later
in
the process. In general, producing an upper bound concentration estimate
comprises selecting as the upper bound concentration estimate, a lowest
concentration value selected from a plurality of concentration values
calculated from respective peaks in the test spectrum. This may involve
finding the height of a peak in the test spectrum that corresponds to a peak
in
the reference spectrum and determining a concentration value for the peak as
a function of its height. Prior to determining a concentration estimate for a
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-31-
peak, the process may involve predicting whether the height of a peak in the
test spectrum is greater than a threshold level and deciding not to determine
a
concentration for the peak when the height is less than the threshold level.
Referring to Figure 17 a process implemented by program codes operating on
the SAA 14 of Figure 1, for producing an upper bound concentration estimate
is shown generally at 340. A first block 342 causes the SAA 14 to select a
reference record. Next block 344 causes the SAA 14 to sort by height those
peaks in the reference record that have a quantification value equal to 1.
This
causes the process to consider only those peaks that provide reliable
concentration estimates. Next, block 346 directs the SAA to address the
(next) highest peak of those that have just been sorted at block 344.
Reference is made to the "next" high peak because the peaks are considered
in succession. On the first pass through the process however, the highest
peak found in the sort is the first peak addressed.
Next block 348 causes the SAA 14 to use the position of the currently
addressed peak in the reference spectrum to locate a corresponding peak in
the test spectrum. This may involve looking for a peak in a window positioned
at a corresponding position in the test spectrum. On finding such a peak, the
maximum intensity value (max(y)) associated with that peak is found.
At block 350, the SAA 14 is directed to calculate a concentration value as a
function of the max (y) value, using the following equation:
Ct = adjustedwidth * max(y) * dssconcentration * dssprotonratio (17)
Dssheight * peakprotonratio
Where: Ct is the concentration value for the peak
adjustedwidth is the width of the peak as determined from
the variable w calculated as shown in
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-32-
Figure 9 and the Lorentzian width adjust
value stored in the reference record
max(y) is the maximum y-value associated with the
corresponding peak in the test spectrum
dssconcentration is the concentration of DSS in the sample
0.5mM, for example
dssprotonratio is the DSS proton ratio (9, for example)
Dssheight is the DSS height value a, calculated as
shown in Figure 9
Peakprotonratio is the proton ratio of the peak, as indicated
in the reference record.
At block 352 SAA 14 is directed to determine whether the currently calculated
concentration value is less than the previously calculated value. If so, then
block 354 causes the SAA 14 to set a preliminary upper bound concentration
value to the current concentration value. If at block 352, the currently
calculated concentration value is not less than the previously calculated
value,
the preliminary upper bound concentration estimate value remains at its
former value. The effect of blocks 352 and 354 is to cause the preliminary
upper bound concentration estimate to be set to the lowest concentration
value calculated for any of the peaks.
Once the preliminary value has been determined from the current pass, block
356 directs the SAA 14 to determine whether all peaks with quantification
values of 1 have been considered. If so, the SAA 14 is directed to optional
block 357 in Figure 17. If not, the SAA 14 is directed to block 358 which
causes the SAA to calculate the expected height of the next peak associated
with the compound, in the test spectrum. To do this equation 17 above is
solved for max(y) using the current preliminary concentration estimate, and
the Lorentzian width adjust value, and the peak proton ratio of the next
highest peak from the list of sorted peaks. Then, block 359 in Figure 17
causes the SAA 14 to determine whether the max(y) value so found is less
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-33-
than the noise level of the spectrum. (noise level was calculated at block 250
in Figure 9). If not, then the next peak is worth considering and the SAA 14
is
directed to resume processing at block 346 to address the next highest peak
in the sorted list.
If the estimated height of the next highest peak found at block 358 is less
than
the noise level of the spectrum, the SAA 14 is directed to an optional block
357 which increases the amplitude of the preliminary concentration estimate
value by the amplitude of the noise in the test spectrum to produce a true
estimate of the upper bound concentration limit for the compound. This is
useful where concentration values are very low.
Then, finally, block 355 directs the SAA 14 to associate the true upper bound
concentration estimate with the reference record, such as by storing the upper
bound concentration estimate value in a field (not shown) of the record, or in
a
field of a data structure maintained in the SAA 14 to create such
associations.
Least squares Fitting
Referring back to Figure 10B, the process for identifying and quantifying
compounds involves a block 334 which causes the SAA 14 to perform a least
squares fitting algorithm using all of the derived reference records and the
test
spectrum to produce scaling values for each peak in each reference spectrum
such that when all peaks from all reference spectra are summed they produce
a composite spectrum that best matches the test spectrum.
Referring to Figure 18, the least squares fitting routine includes a first
block
360 which causes the SAA 14 to produce "signature" spectra comprised of
(x,y) pairs that define a composite spectrum representative of the sum of all
Lorentzians in a given derived reference record. A separate signature
spectrum is produced for each derived reference record. Thus a separate
(x,y) array is produced for each derived reference record.
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-34-
Block 362 then provides each signature spectrum, upper bound
concentrations and the (x,y) array representing the test spectrum to a Linear
Least Squares fitting routine, which in this embodiment is LS SOL licensed
from Stanford University of California, USA. This routine returns scaling
factors for each peak in each applicable reference record, such that when the
scaled Lorentzian models specified in all applicable reference records are
summed together to make a composite spectrum, the composite spectrum
has features matching features in the test spectrum produced from the
sample. These scaling factors thus identify representative reference spectra
from a set of reference spectra associated with detectable compounds and
selected according to the measured condition of the sample.
In this embodiment, an indication of compounds associated with reference
spectra having peaks that when scaled by the scaling factors have a height
greater than a threshold may be produced. This may involve producing a list
of compounds, for example. Thus, scaled peaks having a height less than
the threshold may indicate that the presence of the associated compound in
the sample is questionable and therefore such compound should not be listed
as being present in the sample.
Block 364 then causes the SAA 14 to employ these scaling factors in the
following equation to quantify each compound by producing concentration
values for each compound represented by a reference record:
Conc. = (DSSRatio * scalingFactor * cdb) / pxDSS
Where: Conc.: concentration of the given compound in the
sample
DSSRatio: the DSSRatio entry for the given compound
(see field 202 in Figure 7A)
scalingFactor: the scaling factor of the highest peak in the
given compound (from least squares fitting)
CA 02423260 2003-03-24
WO 02/056048 PCT/CA01/01544
-35-
cdb: the concentration of the given database
entry (see field 202 in Figure 7A)
pxDSS: the pixel height of DSS in the spectrum (the
value a as determined by the process
shown in Figure 9)
Block 366 then causes the SAA 14 to associate these concentration values
with the compounds associated with the derived reference records.
Block 368 then causes the SAA 14 to produce a list or indication of
compounds in the sample, along with their associated concentration values.
This list may be printed and/or displayed on a monitor, for example.
Concentration values may be expressed in moles, mmol/L, g/L or moles/mole,
for example and absolute quantities may be obtained by a simple equation
converting concentration to absolute quantity values, in moles, for example.
While specific embodiments of the invention have been described and
illustrated, such embodiments should be considered illustrative of the
invention only and not as limiting the invention as construed in accordance
with the accompanying claims.