Note: Descriptions are shown in the official language in which they were submitted.
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
TITLE
Hybrid Sleeve Material and Structure
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a Continuation-In-Part application from US
Application No. 091668,496, filed September 22, 2000, the entire contents of
which is
hereby incorporated by reference.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH
Not Applicable
BACKGROUND OF THE INVENTION
Field of The Invention
This invention relates to medical device delivery catheters in general, and
specifically to balloon catheters for use in delivering a medical device such
as a stmt to a
desired body location, such as in a blood vessel. More specifically, this
invention relates
to socks or sleeves used in retaining the stmt in the unexpanded state which
have
reduced frictional engagement with the ends of a stmt andlor balloon cones. In
the
present invention such reduced frictional interaction is made possible by
providing the
sleeve or sleeves with an inside surface of which at least a portion is
characterized as
being harder than the outside surface.
Description Of The Related Art:
Stents and stmt delivery assemblies are utilized in a number of medical
procedures and situations, and as such their structure and function axe well
known. A
stmt is a generally cylindrical prosthesis introduced via a catheter into a
lumen of a body
vessel in a configuration having a generally reduced diameter and then
expanded to the
diameter of the vessel. In its expanded configuration, the stent supports and
reinforces
the vessel walls while maintaining the vessel in an open, unobstructed
condition.
Both self expanding and inflation expandable stents are well known and
widely available in a variety of designs and configurations. Self expanding
scents must
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
be maintained under positive external pressure in order to maintain their
reduced
diameter configuration during delivery of the stent to its deployment site.
Inflation
expandable stems may be crimped to their reduced diameter about the delivery
catheter,
maneuvered to the deployment site, and expanded to the vessel diameter by
fluid
inflation of a balloon positioned on the delivery catheter. The present
invention is
particularly concerned with delivery and deployment of inflation expandable
stems,
although it is generally applicable to self expanding stems when used with
balloon
catheters.
In advancing an inflation expandable stent through a body vessel to the
deployment site, there are a number of important considerations. The stmt must
be able
to securely maintain its axial position on the delivery catheter, without
translocating
proximally or distally, and especially without becoming separated from the
catheter. The
stmt, particularly its distal and proximal ends, must be protected to prevent
distortion of
the stmt and to prevent abrasion and/or reduce trauma of the vessel walls.
Inflation expandable stmt delivery and deployment assemblies are known
which utilize restraining means that overlie the stmt during delivery. U.S.
Patent No.
4,950,227 to Savin et al, relates to an expandable stmt delivery system in
which a sleeve
overlaps the distal or proximal margin (or both) of the stmt during delivery.
That patent
discloses a stmt delivery system in which a catheter carries, on its distal
end portion, a
stmt which is held in place around the catheter prior to and during
percutaneous delivery
by means of one and preferably two sleeves. The sleeves are positioned around
the
catheter with one end portion attached thereto and overlap an end portions) of
the stent
to hold it in place on the catheter in a contracted condition. Each sleeve is
elastomeric in
nature so as to stretch and release the stmt when it expands for implantation.
The stent is
expandable by means of the expandable balloon on the catheter. During
expansion of the
stent at the deployment site, the stmt margins are freed of the protective
sleeve(s). U.S.
Patent 5,403,341 to Solar, relates to a stent delivery and deployment assembly
which
uses retaining sheaths positioned about opposite ends of the compressed stmt.
The
retaining sheaths of Solar are adapted to tear under pressure as the stent is
radially
expanded, thus releasing the stent from engagement with the sheaths. U.S.
Patent No.
5,108,416 to Ryan et al., describes a stent introducer system which uses one
or two
2
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
flexible end caps and an annular socket surrounding the balloon to position
the stent
during introduction to the deployment site.
Copending U.S. Patent Application No. 09/407,836 which was filed on
September 28, 1999 and entitled Stent Securement Sleeves and Optional Coatings
and
Methods of Use, and which is incorporated in its entirety herein by reference,
also
provides for a stmt delivery system having sleeves. In 09/407,836 the sleeves
may be
made up of a combination of polytetrafluoroethylene (PTFE) as well as one or
more
thermoplastic elastomers. Other references exist which disclose a variety of
stent
retaining sleeves.
A common problem which occurs in catheter assemblies is friction or
adhesion between various parts which periodically come into contact with one
another
during the medical procedure. For instance, friction can occur between the
guide catheter
and guide wire, between the introducer sheath and the guide catheter, or
between the
guide catheter and the balloon catheter, for instance, and may increase the
difficulty of
insertion, cause loss of catheter placement, and result in discomfort to the
patient or
damage to the vasculature. In catheters equipped with stent retaining socks or
sleeves,
friction between the balloon and sleeve, and/or the stent and sleeve may also
cause
retraction of the sleeves to be made more difficult. In stmt delivery systems
where the
stmt employs a relatively soft coating material on it surface, such as a drug
carrier, the
relatively soft coating may increase its friction to the sock or sleeve
system. An example
of which may be seen in U.S. Patent No. 5,693,085 to Buirge et al., the entire
contents of
which is incorporated herein by reference.
It is therefore desirable to reduce the friction due to the sliding between
the various parts of the catheter assemblies. Copending U.S. Application No.
09/549,286
which was filed April 14, 2000 describes a reduced columnar strength stmt
retaining
sleeve having a plurality of holes. The relatively reduced columnar and radial
strength
provided by the holes allows the sleeve to be retracted off of a stmt without
the need for
lubricant.
Lubricants however may be used in a variety of stent delivery catheters.
Many lubricants and lubricious coatings types have been used in conjunction
with
balloon catheters. Both hydrophilic and hydrophobic coatings and lubricants
are well
3
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
known in the catheter art. For example: copending U.S. Patent Application No.
09/407,836 which was filed on September 28, 1999 and entitled Stet Securement
Sleeves and Optional Coatings and Methods of Use, provides for a stmt delivery
system
having sleeves. In 09/407,836 the sleeves may be made up of a combination of
polytetrafluoroethylene (hereinafter PTFE) as well as one or more
thermoplastic
elastomers. Copending U.S. Patent Application No. 09/427,805 filed October 27,
1999,
and entitled End Sleeve Coating for Stent Delivery, describes the use of stent
retaining
sleeves having lubricious coatings applied thereto.
Copending U.S. Patent Application No. 09/273,520 filed March 22, 1999,
entitled Lubricated Sleeve Material For Stent Delivery likewise describes the
use of stmt
retaining sleeves and lubricants.
Stent delivery systems which may not require the use of lubricants have
been proposed, such as copending U.S. Application No. 09/549,286 mentioned
above.
Another example of a stent delivery system and retaining sleeve which may not
require ,
lubrication is Copending application 09/668,496 filed September 22, 2000 and
entitled
Striped Sleeve For Stent Delivery describes a two component sleeve having one
or more
substantially longitudinally oriented stripe of a hard material and a softer
material. The
striped configuration of materials in the sleeve allows the sleeve to radially
expand but
with limited or no longitudinal expansion. The unique expansion
characteristics provided
by the striped configuration helps avoid a need to use a lubricant with the
sleeve, though
a lubricant may still be utilized therewith if desired.
The entire content of all patents and applications listed within the present
patent application are incorporated herein by reference.
BRIEF SUMMARY OF THE INVENTION
The instant invention is directed to a medical device delivery system
comprising a catheter assembly having a medical device receiving region and at
least one
retaining sleeve for retaining the medical device on the receiving region
prior to delivery.
An expandable medical device, such as a stmt, is disposed about the medical
device
receiving region of the catheter assembly. At least
4
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
one retaining sleeve is disposed about an end of the expandable medical device
and at
least a portion of the catheter assembly.
'The at least one retaining sleeve further comprises an inside surface and
an outside surface. The outside surface being comprised of a first material
and at least
the portion of the inside surface which is constructed to overlay a stmt being
comprised
of a second material. The first and second materials having different
harnesses, the
second material being harder than the first. As is known, for most polymer
materials, the
hardness represents the capacity of elongation when the polymer is exposed to
an outside
acting force, this is especially true for elastomeric materials (e.g. the
lower a material's
hardness the higher the material's elasticity).
Unlike the 09/668,496 application, from which the present application
depends, and which provides for a sleeve having reduced longitudinal
elongation, the
present invention improves sleeve retractability by providing at least the
portion of the
inside surface of the sleeve which may overlay a stent with a material which
has a greater
hardness than the majority of the sleeve material. Such a relatively hard
material
preferably provides the sleeve with a surface having lower frictional
engagement to the
stent.
In an embodiment of the invention the first material and second material
are co-extruded polymers.
In an embodiment of the invention the second material is a coating on the
first material.
In an embodiment of the invention the inside surface is comprised entirely
of the second material.
In an embodiment of the invention only the portion of the inside surface
which is constructed and arranged to overlay a stent is comprised of the
harder material.
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
A detailed description of the invention is hereafter described with specific
reference being made to the drawings in which:
FIG. 1 is a side view of a first embodiment of the invention;
FIG. 2 is a side view of a second embodiment of the invention;
FIG. 3 is a side view of a third embodiment of the invention; and
FIG. 4 is a side view of a forth embodiment of the invention.
DETAILED DESCRIPTION OF THE INVENTION
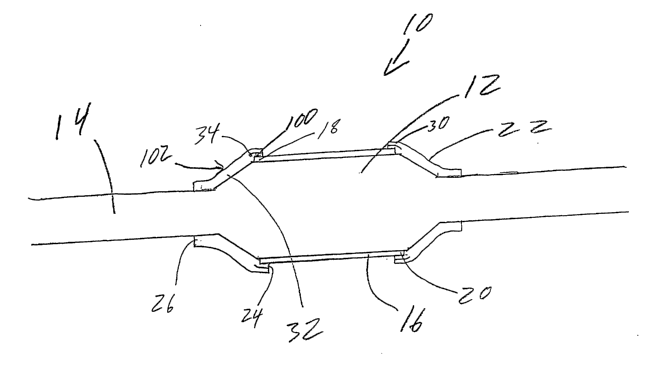
As may be seen in FIG. l, the present invention may be embodied in a
stent delivery catheter, indicated generally at 10. Catheter 10, includes a
stent mounting
region 12, the stent mounting region 12 may be an inflatable portion of the
catheter or
may be a separate balloon mounted to the catheter shaft 14. The balloon 12 may
have an
unexpanded state and an expanded state. A stmt 16, disposed about the stent
mounting
region 12 may ~be delivered when the balloon 12 is expanded to the expanded
state.
The stent 16 includes a proximal end 18 and a distal end 20. In the
embodiment shown a stmt retaining sleeve 22 overlies at least a portion of
each end 18
and 20. As is known in the art, when the balloon 12 and stmt 16 are expanded
to their
expanded state, the ends of the stent retaining sleeves 22 are configured to
retract off of
the stmt ends 18 and 20. In the present invention, the sleeves 22 have a
unique
construction which provides the first portion 24 of the sleeve which overlies
the stmt 16,
with a reduced frictional engagement with the stmt 16 by providing the inside
surface
100 of the first portion 24 with a material 34 which is harder than that of
the outer
surface 102 of the sleeve 22.
The second portion 26 of the sleeve 22 is disposed about and is engaged
to a portion of the catheter shaft 14 adjacent to the balloon 12.
As stmt 16 is expanded, the stmt ends 18 and 20 will eventually be drawn
from underneath the stent retaining sleeves 22. By providing a sleeve 22 which
has a
reduced frictional engagement with the stmt ends 18 and 20 the present
invention
ensures that the stmt is delivered with improved sleeve retractability.
As previously indicated, the sleeves 22 are constructed from at least two
6
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
materials having different hardness characteristics. The first material 30 is
formed into a
generally tubular body 32 which provides the sleeve with its shape as well as
its outer
surface 102. At least a portion of the first end 24 of the inside surface 100
is composed
of the second material 34.
The first material 30 may be any elastic material known which has a
hardness as measured by a Shore durometer of less than SSD. Preferably the
durometer
hardness of the first material is between 40A and 100A. The second material 34
may be
any material having a durometer hardness greater than about SSD: In at least
one
embodiment of the invention the first material 30 has a hardness of 35D and
the second
material 34 has a hardness of 70D.
The first material 30 may be selected from one or more of the following
substances: soft grade polyester/polyether elastomers such as ArnitelTM
available from
DSM Engineering, polyurethane-polyether polymers, such as Tecothane~M 1074A
available from Thermedics, Inc.; polyester-polyurethanes, such as PellethaneTM
2102-
75A sold by Dow Chemical; polyester-polyurethanes, such as EstaneT~ 5703P sold
by
BF Goodrich; polyether block amides, such as PebaxTM 2533 available from Elf
Atochem; and styrene-butadien-styrene triblock copolymers such as KratonTM
D1101
sold by Shell Chemical company. Other materials which may also be used in the
production of the first material 30 include, but are not limited to styrenic
block
copolymers, polyurethanes, silicone rubber, natural rubber, copolyesters,
polyamides,
EPDM rubber/polyolefm, nitril rubber/PVC, fluoroelastomers, butyl rubber,
epichlorohydrin, soft block copolymers, and any combinations thereof.
The second material 34 may be selected from one or more of the
following substances: polyethyleneterephthalate (PET), polybutylene
terephthalate
(PBT), polytrimethylene terephthalate (PTT),NylonTM, engineering thermoplastic
polyurethanes, fluoropolymers, polyester/polyether elastomers such as
ArnitelTM
available from DSM Engineering, polyurethane-polyether polymers, such as
TecothaneTM l OSSD or 1075D both of which are available from Thermedics, Inc.;
polyester-polyurethanes, such as EstaneTM 58170 sold by BF Goodrich; polyether
block
amides, such as PebaxTi''i 7233 or 6333 both of which are available from Elf
Atochem.
Other materials which may also be used in the production of the second
material 34
7
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
include, but are not limited to: polyolefins, polystyrene, polyvinyl chloride,
acrylonitrile-
butadiene-styrene polymers, polyacrylonitrile, polyacrylate, vinyl acetate
polymer,
cellulose plastics, polyurethanes, polyethylene terephthalate, polyacetal,
polyethers,
polycarbonates, polyamides, polyphenylene sulfide, polyarylethersulfones,
polyaryletherketones, polytetrafluoroethylene, and any combinations thereof.
The above examples of the first and second materials 30 and 34 are in no
way exhaustive of the potential substances or combinations of substances which
may be
used. The present invention is directed to a sleeve composed of any materials
which
have the hardness qualities previously described for the respective materials
30 and 34.
As may be seen in the various figures, the present invention may be
embodied in a variety of manners. For instance, in the embodiment shown in
FIG. 1 the
catheter 10 is seen with a pair of sleeves 22 each of which have a first
portion 24 with an
inner surface 100 which is composed of a second material 34, such as is
described above.
Second material 34 may be a coating of hardened material applied to the inside
surface
100 of the sleeve 22. Alternatively, the material 34 may be bonded or welded
to the
sleeve 22, or first material 30 and second material 34 may have been co-
extruded
together in the form of sleeve 22 shown. Other methods for joining the
materials 30 and
34, such as selective coating by printing, may also be utilized.
As may be seen in FIG. 2, the entire inside surface 100 of the sleeves) 22
may be composed of the second material 34.
FIGs. 3 and 4 show the sleeve configurations respectfully described in
relation to FIGs. l and 2 as they may be embodied on a sleeve 22 exclusive of
the stent
delivery catheter 10.
In alternative embodiments, notably those utilized pecifically for delivery
of a self expanding stmt, a retractable sheath (not shown) such as axe known
in the art,
may be employed to overlay the stmt. In such embodiments a single sleeve or
two
sleeves such have been shown and described may be employed to retain the self
expanding stent in place. When the sheath is retracted the stent will expand
causing the
sleeves) to retract.
In addition to being directed to the embodiments described above and
claimed below, the present invention is further directed to embodiments having
different
8
CA 02424061 2003-03-27
WO 02/39929 PCT/USO1/50707
combinations of the features described above and claimed below. As such, the
invention
is also directed to other embodiments having any other possible combination of
the
dependent features claimed below.
The above examples and disclosure are intended to be illustrative and not
exhaustive. These examples and description will suggest many variations and
alternatives to one of ordinary skill in this art. All these alternatives and
variations are
intended to be included within the scope of the attached claims. Those
familiar with the
art may recognize other equivalents to the specific embodiments described
herein which
equivalents are also intended to be encompassed by the claims attached hereto.
9