Note: Descriptions are shown in the official language in which they were submitted.
CA 02424714 2003-04-01
METHOD FOR THIOSULFATE LEACHING OF
PRECIOUS METAL-CONTAINING MATERIALS
FIELD OF THE INVENTION
The present invention is directed generally to the recovery of precious metals
from
precious metal-containing material and specifically to the recovery of
precious metals from
precious metal-containing material using thiosulfate lixiviants.
BACKGROUND OF THE INVENTION
A traditional technique for recovering precious metal(s) from precious metal-
containing ore is by leaching the material with a cyanide lixiviant. As used
herein, a
"precious metal" refers to gold, silver, and the platinum group metals (e.g.,
platinum,
palladium, ruthenium, rhodium, osmium, and iridium). Many countries are
placing severe
limitations on the use of cyanide due to the deleterious effects of cyanide on
the environment.
Incidents of fish and other wildlife having been killed by the leakage of
cyanide into
waterways have been reported. The limitations being placed on cyanide use have
increased
substantially the cost of extracting precious metal(s) from ore, thereby
decreasing precious
metal reserves in many countries. Cyanide is also unable to recover precious
metals such as
CA 02424714 2003-04-01
gold from refractory ores without a pretreatment step. "Refractory ores" refer
to those ores
that do not respond well to conventional cyanide leaching. Examples of
refractory ores
include sulfidic ores (where at least some of the precious metals are locked
up in the sulfide
matrix), carbonaceous ores (where the precious metal complex dissolved in the
lixiviant
adsorbs onto carbonaceous matter in the ores), and ores that are both sulfidic
and
carbonaceous.
Thiosulfate has been actively considered as a replacement for cyanide.
Thiosulfate
is relatively inexpensive and is far less harmful to the environment than
cyanide. Thiosulfate
has also been shown to be effective in recovering precious metals from
pretreated refractory
preg-robbing carbonaceous ores and sulfidic ores. As used herein, "preg-
robbing" is any
material that interacts with (e.g., adsorbs or binds) precious metals after
dissolution by a
lixiviant, thereby interfering with precious metal extraction, and
"carbonaceous material" is
any material that includes one or more carbon-containing compounds, such as,
but not
limited to, humic acid, graphite, bitumins and asphaltic compounds.
Where gold is the precious metal, thiosulfate leaching techniques have
typically relied
on the use of copper ions to catalyze and accelerate the oxidation of gold,
ammonia to
facilitate the formation and stabilization of cupric ammine ions and/or a pH
at pH 9 or above
to maintain a region of stability where both the cupric ammine and gold
thiosulfate
complexes are stable.
It is well known in the art that the catalytic effect of copper and ammonia in
conventional thiosulfate leaching of gold is described by the following
sequence ofreactions.
Formation of the cupric ammine complex:
-2-
CA 02424714 2003-04-01
Cu" +4NH, -* Cu(NH);' (1)
Oxidation of gold by cupric ammine, gold complexation as the gold-thiosulfate
anion, and
reduction of cupric ammine to cuprous thiosulfate:
Au + Cu(NH3 )4+ + 5S203 -+ Au(S2O3 )z + Cu(S2O3 )3 + 4NH3 (2)
Oxidation of the cuprous thiosulfate back to cupric ammine with oxygen:
5 -
+4NH3 +/02 +%H2O-4Cu(NH3)4+ +35203 +OH- (3)
Cu(5203)3
Summing equations (2) and (3) yields the overall thiosulfate leach reaction
for gold:
Au+ 2S2032 + yOZ + H2O -* Au(S2O3)2 + OH- (4)
It can be seen from the above equations that copper and ammonia act as
catalysts in
that they are neither produced nor consumed in the overall leach reaction.
Cupper and ammonia can be a source ofproblems. Added copper tends to
precipitate
as cupric sulfide, which is speculated to form a passive layer on gold,
thereby inhibiting gold
leaching as well as increasing copper and thiosulfate consumption:
Cue + 5203 + 20H- -* CuS + SO4 + H2O (5)
Rapid oxidation of thiosulfate by cupric ammine also occurs, leading to
excessive
degradation and loss of thiosulfate:
2Cu(NH3)4i + 85203 -4 2Cu(S2O3)3 + S406 + 8NH3 (6)
-3-
CA 02424714 2003-04-01
Loss of ammonia by volatilization occurs readily, particularly in unsealed gas-
sparged
reactors operating at pH greater than 9.2, leading to excessive ammonia
consumption:
NH4 + OH- -* NH3(.,) + H20-4 NH3(,) + H2O (7)
Like cyanide, copper and ammonia are highly toxic to many aquatic lifeforms
and are
environmentally controlled substances.
Other problems encountered with thiosulfate leaching include difficulty in
recovering
gold out of solution as a result of the formation of polythionates, such as
tetrathionate and
trithionate, which adsorb competitively with gold onto adsorbents, such as
resins. The
formation of polythionates further increases thiosulfate consumption per unit
mass of
processed ore.
SUMMARY OF THE INVENTION
These and other needs have been addressed by the methodologies and systems of
the
present invention. The methodologies can recover precious metals, such as gold
and silver,
from a variety of materials, including refractory carbonaceous or sulfidic
ores, double
refractoryores (e.g., ores containing both sulfide-locked gold and
carbonaceous preg-robbing
matter), oxide ores, nonrefractory sulfidic ores, and ores also containing
copper minerals and
other materials derived from such ores (e.g., concentrates, tailings, etc.).
In one embodiment, a thiosulfate leaching process is provided that includes
one or
more of the following operating parameters:
(a) an oxygen partial pressure that is preferably superatmospheric and more
preferably ranges from about 4 to about 500 psia;
-4-
CA 02424714 2003-04-01
(b) a leach slurry pH that is preferably less than pH 9;
(c) a leach slurry that is preferably at least substantially free of (added)
ammonia
and more preferably contains less than 0.05M (added) ammonia such that the
leach slurry has
a maximum total concentration of ammonia of preferably less than 0.05M and
more
preferably no more than about 0.025M;
(d) a leach slurry that is preferably at least substantially free of (added)
copper
ion and more preferably contains no more than about 15 ppm (added) copper
ions;
(e) an (added) sulfite concentration that is preferably no more than about.
0.01M
such that the slurry has a maximum total concentration of sulfite of
preferably no more than
about 0.02M and more preferably no more than about 0.01M; and/or
(f) a leach slurry temperature preferably ranging from about 20 to about 100 C
and more preferably from about 20 to about 80 C.
The foregoing parameters can yield a high level of precious metal extraction
from the
precious metal-containing material, which can be at least about 70% and
sometimes at least
about 80%.
The thiosulfate lixiviant can be derived from any suitable form(s) of
thiosulfate, such
as sodium thiosulfate, calcium thiosulfate, potassium thiosulfate and/or
ammonium
thiosulfate. Sodium and/or calcium thiosulfate are preferred.
The leaching process can be conducted by any suitable technique. For example,
the
leaching can be conducted in situ, in a heap or in an open or sealed vessel.
It is particularly
preferred that the leaching be conducted in an agitated, multi-compartment
reactor such as
an autoclave.
-5-
CA 02424714 2003-04-01
The precious metal can be recovered from the pregnant leach solution by any
suitable
technique. By way of example, the precious metal can be recovered by resin
adsorbtion
methods such as resin-in-pulp, resin-in-solution, and resin-in-leach or by
solvent extraction,
cementation, electrolysis, precipitation, and/or combinations of two or more
of these
techniques.
Reducing or eliminating the need to have copper ions and/or ammonia present in
the
leach as practiced in the present invention can provide significant multiple
benefits. First, the
cost of having to add copper and ammonia reagents to the process can be
reduced
significantly or eliminated. Second, environmental concerns relating to the
presence of
potentially harmful amounts of copper and ammonia in the tailings or other
waste streams
generated by the process can be mitigated. Third, the near-absence or complete
absence of
copper and ammonia in the leach can provide for a much more reliable and
robust leaching
process, yielding more stable leachates, able to operate over a wider pH and
oxidation-
reduction potential (ORP) range than is possible with conventional thiosulfate
leaching. The
latter process must operate in the relatively narrow window of pH and ORP
where both the
cupric ammine complex and the gold thiosulfate complex co-exist. With the
process of the
present invention, the pH of the thiosutfate lixiviant solution in the
leaching step can be less
than pH 9 and the ORP less than 200 mV (referenced to the standard hydrogen
electrode).
Fourth, minimizing the amount of copper in the system can lead to increased
loading of gold
onto resins due to reduced competitive adsorption of copper ions. Resin
elutions are also
simplified as little, if any copper, is on the resin. Finally, the near-
absence or complete
absence of copper and ammonia in the leach can reduce or eliminate entirely a
host of
-6-
CA 02424714 2003-04-01
deleterious side reactions that consume thiosulfate and are otherwise
difficult or impossible
to prevent.
The elimination or near elimination of sulfite from the thiosulfate leach also
can have
advantages. Sulfite can depress the rate of dissolution of precious metal from
the precious
metal-containing material by reducing significantly the oxidation reduction
potential (ORP)
of the leach solution or lixiviant. As will be appreciated, the rate of
oxidation of the gold
(and therefore the rate of dissolution of the gold) is directly dependent on
the ORP.
In another embodiment, an extraction agent is preferably contacted with a
pregnant
(precious metal-containing) thiosulfate leach solution at a temperature of
less than about
70 C and more preferably less than about 60 C in the substantial absence of
dissolved
molecular oxygen to isolate the precious metal and to prevent conversion of
thiosulfate in
the pregnant leach solution into polythionate. In one configuration, the
extraction agent is
an adsorbent, such as a resin, which loads the precious metal onto the
adsorbent. As used
herein, an "adsorbent" is a substance which has the ability to hold molecules
or atoms of
other substances on its surface. Examples of suitable resin adsorbents include
weak and
strong base resins such as "DOWEX 21K", manufactured by Dow Chemical. In
another
configuration, the extraction agent is a solvent extraction reagent that
extracts the precious
metals into an organic phase, from which the precious metals can be later
recovered. As will
be appreciated, the thiosulfate decomposition to polythionates is
significantly reduced in the
substantial absence of dissolved molecular oxygen.
In yet another embodiment, the pregnant leach solution from a thiosulfate
leaching
step is contacted, after the leaching step, with a reagent to convert at least
about 50% and
-7-
CA 02424714 2003-04-01
typically at least most of polythionates (particularly trithionate and
tetrathionate) into
thiosulfate. The reagent or reductant can be any suitable reactant to convert
polythionates
into thiosulfate, with any sulfide, and/or polysulfide (i.e., a compound
containing one or a
mixture of polymeric ion(s) S..2 where x = 2-6, such as disulfide, trisulfide,
tetrasulfide,
pentasulfide and hexasulfide) being particularly preferred. A sulfite reagent
can also be used
but is generally effective only in converting polythionates of the form SxO62-
, where x = 4 to
6, to thiosulfate. The sulfite, sulfide, and/or polysulfide can be compounded
with any cation,
with Group IA and IIA elements of the Periodic Table, ammonium, and hydrogen
being
preferred.
In yet another embodiment, a precious metal solubilized in a solution, such as
a
pregnant leach solution or eluate, is electrowon in the presence of sulfite.
In the presence of
sulfite, the precious metal is reduced to the elemental state at the cathode
while the sulfite
is oxidized to sulfate at the anode. Sulfite is also believed to improve the
precious metal
loading capacity of the resin by converting loaded tetrathionate to
trithionate and thiosulfate.
In yet another embodiment, the formation of polythionates is controlled by
maintaining a (pregnant or barren) thiosulfate leach solution in a
nonoxidizing (or at least
substantially nonoxidizing) atmosphere and/or sparging a nonoxidizing (or at
least
substantially nonoxidizing) gas through the leach solution. As will be
appreciated, the
atmosphere or gas may contain one or more reductants, such as hydrogen sulfide
and/or
sulfur dioxide. The molecular oxygen concentration in the atmosphere and/or
sparge gas is
preferably insufficient to cause a dissolved molecular oxygen concentration in
the leach
solution of more than about I ppm and preferably of more than about 0.2 ppm.
Preferably,
-8-
CA 02424714 2003-04-01
the inert atmosphere (or sparge gas) is at least substantially free of
molecular oxygen and
includes at least about 85 vol. % of any inert gas such as molecular nitrogen
and/or argon.
By controlling the amount of oxidant(s) (other than thiosulfate and
polythionates) in the
atmosphere and/or (pregnant or barren) leach solution the rate or degree of
oxidation of
thiosulfates to form polythionates can be controlled.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. I is a flow schematic of a first embodiment of the present invention;
Fig. 2 is a flow schematic of second embodiment of the present invention;
Fig. 3 is a flow schematic of a third embodiment of the present invention;
Fig. 4 is a flow schematic of a fourth embodiment of the present invention;
Fig. 5 is a plot of gold extraction in percent (vertical axis) versus leach
time in hours
(horizontal axis);
Fig. 6 is another plot of gold extraction in percent (vertical axis) versus
leach time
in hours (horizontal axis);
Fig. 7 is another plot of gold extraction in percent (vertical axis) versus
leach time
in hours (horizontal axis);
Fig. 8 is another plot of gold extraction in percent (vertical axis) versus
leach time
in hours (horizontal axis);
Fig. 9 is a plot of gold extraction in percent (left vertical axis) and
thiosulfate
remaining in percent (right vertical axis) versus leach time in hours
(horizontal axis); and
-9-
CA 02424714 2003-04-01
Fig. 10 is a plot of gold extraction in percent (vertical axis) and time
(horizontal axis)
for aerated and unaerated columns.
DETAILED DESCRIPTION
The present invention provides an improved thiosulfate leaching process for
the
recovery ofprecious metals from precious metal-bearing material. The precious
metal(s) can
be associated with nonprecious metals, such as base metals, e.g., copper,
nickel, and cobalt.
The precious metal-bearing material includes ore, concentrates, tailings,
recycled industrial
matter, spoil, or waste and mixtures thereof. The invention is particularly
effective for
recovering precious metals, particularly gold, from refractory carbonaceous
material.
Figure 1 is a flow chart according to a first embodiment of the present
invention. The
process of the flow chart is particularly effective in recovering gold from
carbonaceous
material and oxide material and mixtures thereof.
Referring to Figure 1, a precious metal-bearing material 100 is subjected to
the steps
of wet and/or dry crushing 104 and wet and/or dry grinding 108 to reduce the
particle size
of the material sufficiently to enable the solids to be suspended in an
agitated vessel and to
allow for the efficient leaching of the precious metals. Preferably, wet
grinding is employed
with the recycled thiosulfate leach solution and water being used as the
liquid component in
the slurry. In that event, the slurry 112 containing the comminuted material
typically
contains from about 0.05 to about 0.1 M thiosulfates and from about 0.0005 to
about 0.025
m polythionates. The fully comminuted material particle size is preferably at
least smaller
than 80% passing about 48 mesh (300 microns), more preferably 80% passing
about 100
-10-
CA 02424714 2003-04-01
mesh (150 microns), and most preferably 80% passing about 200 mesh (75
microns). The
typical solids content of the slurry 112 ranges from about 20 to about 30
wt.%. As will be
appreciated, other techniques can be used to comminute the material to the
desired particle
size(s). By way of illustration, blasting can be used alone with or without
crushing and
grinding and crushing and grinding can be used alone with or without another
comminution
technique.
The ground slurry 112 is then thickened 116 to adjust the pulp density to a
value
suitable for leaching. The ideal leach pulp density will vary according to the
type of material
being leached. Typically, the pulp density ranges from about 20 to about 50%
solids by
weight, but could be as low as about 1% or as high as about 60%. Thickening
116 will
generally not be required if the desired pulp density (after wet comminution
or formation of
the comminuted material into a slurry) is less than about 20%.
The thickener overflow solution 120 is recycled back to grinding 108 in the
event that
wet grinding is employed. Otherwise, the overflow solution 120 is returned to
the optional
slurry formation step (not shown).
Fresh makeup thiosulfate is added, as necessary, at any suitable location(s),
such as
to the slurried material during comminution 108 and/or in the thickener 116,
to the underflow
or overflow solution 124, 120, to leaching 132 and/or to the regenerated
thiosulfate solution
128 (discussed below). In any event, the optimum solution thiosulfate
concentration to
maintain during leaching 132 will depend on the nature of the material being
leached, but
will preferably range from about 0.005 to about 2 molar (M), more preferably
about 0.02 to
about 0.5 M, and even more preferably from about 0.05 to about 0.2 M. The
source of
-11-
CA 02424714 2003-04-01
makeup thiosulfate can be any available thiosulfate-containing compound, such
as sodium
thiosulfate, potassium thiosulfate, calcium thiosulfate, or any other
thiosulfate-containing
material or thiosulfate precursor. Ammonium thiosulfate can also be used but
its use is less
preferred for environmental reasons. Alternatively, thiosulfate can be
generated in situ or in
a separate step by reaction of elemental sulfur with a source of hydroxyl
ions, in accordance
with the following reaction:
2(x + 1)S + 60H- -+ S2O3 + 2S2X + 3H20 (8)
where x = 3-6, or by reaction of bisulfide with bisulfate:
2HS- + 4HSO3 -+ 3S2O3 + 3H20 (9)
or by reaction of elemental sulfur with sulfite:
S + S03 -* S2 03 (10)
If the desirable temperature is above ambient, it may be desirable to recover
waste
heat for recycle to leaching. In that event, the underflow slurry 124 is
directed through an
indirect heat exchanger 136, preferably a shell and tube heat exchanger system
in which the
hot slurry from resin-in-pulp pretreatment 140 (discussed below) is passed
through the inner
tubes and the cold feed (or underflow) slurry 140 is passed through the
annular space
between the tubes (or vice versa). In this way waste heat is transferred from
the leached
slurry 144 to the feed (or underflow) slurry 124, reducing the amount of new
heat that must
be added in leaching 132 to maintain the desired leach temperature. Typically,
the approach
temperature of the incoming feed slurry 148 is from about 2 to about 5 C below
the leach
-12-
CA 02424714 2003-04-01
temperature (discussed below) and heat is added to the leach vessel by
suitable techniques
to makeup the difference.
The heated slurry 148 is subjected to leaching 132 in the presence of oxygen
and
thiosulfate. Leaching is conducted in the presence of an oxygen-enriched
atmosphere at
atmospheric pressure, or at a pressure above atmospheric pressure using an
oxygen-
containing gas to reduce or eliminate the need for the presence of copper
and/or ammonia
in the leach. Using gold as an example, the thiosulfate leaching of precious
metal-bearing
material in the absence or substantial absence of copper and ammonia under
elevated oxygen
partial pressure can be illustrated by the following reaction:
Au+ 2S20; + 4 02 + %H2O-4 Au(S203)2 + OH- (11)
The increased oxygen partial pressure in the leach increases the rate of the
above
reaction in the absence or near absence of copper and ammonia. To accomplish
this goal,
the oxygen-containing gas may include atmospheric air, or it may include
relatively pure
(95%+) oxygen such as that produced from any commercially available oxygen
plant, or it
may include any other available source of oxygen. The desired oxygen partial
pressure (PO2)
maintained during leaching will depend on the material being leached, but it
will be at least
higher than that provided under normal ambient conditions by air at the
elevation the process
is applied. Thus, if the process is practiced at sea level for example the
oxygen partial
pressure will be in excess of about 3 pounds per square inch absolute pressure
(psia) to as
high as about 500 psia, preferably from about 10 to about 115 psia, and most
preferably from
about 15 to about 65 psia. The total operating pressure is the sum of the
molecular oxygen
-13-
CA 02424714 2003-04-01
partial pressure and the water vapor pressure at the temperature employed in
the leaching
step 132, or preferably ranges from about 15 to about 600 psia and more
preferably from
about 15 to about 130 psia.
The leaching temperature will be dictated by the type of material being
leached. The
temperature will vary typically from about 5 C to about 150 C, preferably from
about 20
to about 100 C, and most preferably from about 40 to about 80 C. Higher
temperatures
accelerate the leaching of precious metals but also accelerate the degradation
of thiosulfate.
If required, a source of makeup heat such as steam is added to the leach
reactors to maintain
the desired temperature.
The leaching retention time is dependent on the material being leached and the
temperature, and will range from about 1 hour to 96 hours, preferably from
about 2 to about
16 hours, and most preferably from about 4 to about 8 hours.
The absence or substantial absence of copper and/or ammonia in the leach
greatly
simplifies the process. Elimination or near-elimination of ammonia and copper
from the
leach provides the advantage of allowing for a consistently high and
reproducible precious
metal extraction over a broader pH range than was previously possible with the
other
thiosulfate leaching processes. Preferably, the (added and/or total solution)
copper
concentration is no more than about 20 ppm, more preferably no more than about
15 ppm,
and even more preferably no more than about 10 ppm while the (added and/or
total solution)
ammonia concentration is no more than about 0.05 M, more preferably no more
than about
0.03 M, and even more preferably no more than about 0.01 M. In the present
invention
leaching can be operated at about pH 7-12, preferably about pH 8-11, more
preferably about
-14-
CA 02424714 2003-04-01
pH 8-10, and even more preferably at a pH less than pH 9. The oxidation-
reduction potential
(ORP) preferably ranges from about 100 to about 350 mV and more preferably
from about
150 to about 300 mV (vs. the standard hydrogen electrode (SHE)).
Oxidative degradation of thiosulfate ultimately to sulfate can also occur,
possibly by
the following sequence of reactions that involve the formation of intermediate
polythionates
(polythionates can be represented by Sõ 06 , where n = 2-6):
Tetrathionate formation: 2S203 + , 02 + H2O -+ 5406 + 20H- (12)
+ 2 H+ (13)
Trithionate formation: 354 06 +-1502 + H20-+4S306
Sulfite formation: 5306 + / 02 + 2H2 0 --4 3S03 + 4H+ (14)
Sulfate formation: 2503 + 02 -+ 2SO0 (15)
Overall: S203 + 202 + H2O -* 2S04 + 2H+ (16)
Oxidative degradation of thiosulfate to polythionates and sulfates is
accelerated
markedly in the presence of copper ions and/or ammonia. The oxidative
degradation
reactions are slowed considerably at elevated oxygen partial pressure in the
absence or near-
absence of copper and ammonia.
The leaching step 132 may be conducted in a batch or continuous basis but
continuous operation is preferred. Continuous leaching is carried out in a
multiple series of
-15-
CA 02424714 2009-07-24
one or more reactors that are agitated sufficiently to maintain the solids in
suspension.
Agitation may be accomplished by mechanical, pneumatic or other means. In a
preferred
configuration, gassing impellers are employed, such as those disclosed in U.S.
Patent No.
6,183,706 and copending U.S. Patent 6,368,381, filed April 27, 2000. Such
impellers can
significantly enhance the amount of dissolved molecular oxygen in the leach
solution.
Leaching may also be carried out in a multi-compartment autoclave containing
one or more
compartments, (with 4 to 6 compartments being preferred) similar in design to
the
autoclaves used to pressure oxidize sulfide-bearing ores or concentrates.
However, owing to
the non-acidic, moderate temperature, relatively mild conditions employed in
the present
invention, the autoclave materials of construction are much less expensive
than those found
to be necessary when oxidizing sulfide minerals. The latter autoclaves are
normally
constructed of a steel shell fitted with a lead liner and refractory brick
liner and containing
metallic components constructed of titanium or other expensive corrosion-
resistant alloys.
The leach reactors and contained metallic components employed by the present
invention
can be simply constructed of stainless steel and do not require lead or brick
liners.
The extraction of precious metals in the leaching step 132 is relatively high,
particularly for carbonaceous ores. Typically, at least about 50%, more
typically at least
about 70%, and even more typically at least about 80% of the precious metal in
the precious
metal-containing material is extracted or solubilized into the pregnant leach
solution 144.
The concentration of the dissolved precious metal in the pregnant leach
solution typically
ranges from about 0.05 to about 100 ppm and more typically from about 1 to
about 50 ppm.
16
CA 02424714 2003-04-01
The pregnant leach slurry 144 containing the precious metal-bearing leach
solution
and gold-depleted solid residue may optionally be directed to RIP pretreatment
140 to reduce
the concentration ofpolythionates in solution. As will be appreciated, the
molecular oxygen
sparged through the leach slurry in the leaching step 132 will oxidize a minor
portion of the
thiosulfate into polythionates. Polythionates have the undesired effect of
reducing the
loading of precious metals on to resin by competitive adsorption. Lowering the
polythionate
concentration will have the beneficial effect of increasing the loading of
precious metals on
to resin, thereby improving the efficiency of resin recovery of precious
metals.
The RIP pretreatment step 140 can be performed using any one or more of a
number
of techniques for converting polythionates to other compounds that do not
compete with the
precious metal for collection by the extraction agent.
In one embodiment, a polythionate reductant is added to the slurry 144 to
reduce
polythionates to thiosulfates. Any of a number of reductants are suitable for
performing the
conversion.
By way of example, a sulfide-containing reagent can reduce the polythionates
back
to thiosulfate, as shown by the following reactions:
25406 + S2- + / H2O-* -* %8203 + 3H+ (17)
S306'- +S2- S2 -+ 2S203'- (18)
Any reagent that releases sulfide ions on dissolution will suffice, such as
sodium
bisulfide, NaHS, sodium sulfide, Na2S, hydrogen sulfide gas, H2S, or a
polysulfide. The use
-17-
CA 02424714 2003-04-01
of a sulfide reagent has the advantages of rapidly and efficiently reducing
polythionates to
thiosulfate at ambient or moderately elevated temperature. The treatment can
be carried out
in an agitated reactor in batch mode or in a series of 1-4 reactors operating
in continuous
mode, or in a multi-compartment autoclave. Alternatively the treatment can be
carried out
in a pipe reactor or simply by injecting sulfide ions in the piping system
directing the leach
slurry to gold recovery, or the first stage of RIP. The treatment is carried
out at a controlled
pH of about pH 5.5 to about pH 10.5, preferably about pH 7 to about pH 10,
most preferably
less than about pH 9. The temperature employed can range from about 20 C to
about 150 C,
preferably from about 40 to about 100 C, more preferably from about 40 to
about 80 C, and
even more preferably from about 60 to about 80 C. The retention time can range
from as
low as 5 minutes, preferably greater than 30 minutes, most preferably from
about I to about
3 hours.
Alternatively, a sulfite-containing reagent can also reduce polythionates to
thiosulfates as shown by the following reaction:
(19)
S4 06 + SO 3' -~ S2 03 + S3O 6'
Sulfite treatment is effective in reducing tetrathionate quickly, but a
disadvantage is it is
ineffective in reducing trithionate. The sulfite can be added in any form
and/or can be
formed in situ. For example, sulfite can be added in the form of sodium
metabisulfite or
sulfur dioxide.
When using sulfite, the temperature of the leach slurry in the RIP
pretreatment 140
is preferably less than 60 C to inhibit, at least substantially, the
precipitation of precious
-18-
CA 02424714 2003-04-01
metal(s) from the leach slurry 144. More preferably, the RIP pretreatment 140
with sulfite
is performed at a temperature in the range of from about 10 to about 50 C and
even more
preferably at ambient temperature.
When using sulfite, the residence time of the leach slurry 144 in the
regeneration step
140 is preferably at least about I second, more preferably greater than about
5 minutes, and
even more preferably greater than about 10 minutes and no more than about 1
hour, with
about 15-30 minutes being most preferable.
The pH of the leach slurry during sulfite treatment typically ranges from
about pH 5.5
to about pH 10.5 and more typically from about pH 7 to about pH 10.
Other suitable polythionate reductants include hydrogen, fine, reactive
elemental
sulfur, carbon monoxide, and mixtures thereof.
In another embodiment, the pretreatment step 140 is performed by maintaining
the
temperature of the leach slurry at a sufficiently high value in the absence of
oxygen to effect
the following hydrolytic disproportionation reactions:
4S4O6 + SH2O -> 7S203 + 2SO4 + IOH+ (20)
S3O6 + H2O -4 S2O1 + SO4 + 2H+ (21)
Hydrolytic treatment can be carried out in an agitated reactor in batch mode
or in a
series of 1-4 reactors operating in continuous mode, or a multi-compartment
autoclave. The
temperature is preferably maintained in the range of from about 60 to about
150 C,
preferably of from about 70 to about 100 C, and most preferably of from about
80 to about
-19-
CA 02424714 2003-04-01
90 C by adding a source of heat, such as steam. The retention time typically
ranges from
about 15 minutes to 8 hours, preferably from about I to about 6 hours, and
most preferably
from about 2 to about 4 hours. Hydrolytic treatment is generally less
preferable than sulfide
treatment because the former method results in irreversible loss of some of
the polythionate
to sulfate.
Alternatively, any or all of the above-techniques for converting
polythionate(s) into
thiosulfate can be combined in the same process configuration.
In a preferred embodiment, the reductant used to convert polythionates into
thiosulfates is the sulfide reagent. Sulfide addition is preferred because one
sulfide reacts
with one tri- or two tetrathionates to form multiple thiosulfates without any
sulfur-containing
byproducts. Sulfite addition only reduces tetrathionate and is not capable of
reducing
trithionate at common operating temperatures and pH's. Heating of the leach
solution is
energy intensive and produces byproducts. Trithionate and tetrathionate are
each converted
into thiosulfate, sulfate, and hydrogen ions, thus the thiosulfate yield is
not as high as with
sulfide addition.
RIP pretreatment 140 can be performed in any suitable vessel(s), preferably
agitated.
Preferably, RIP pretreatment is performed in a series of tanks or in a
multistaged vessel.
The addition of a sulfide such as NaHS is preferred. Preferably, the amount of
the
reductant generally and, sulfide reagent specifically added to the slurry 144
is sufficient to
convert at least most of the polythionates into thiosulfate. The amount of
sulfide contacted
with the slurry 144 preferably is at least about 100 to about 150% of the
stoichiometric
amount required to convert at least substantially all of the polythionates in
the slurry into
-20-
CA 02424714 2009-07-24
thiosulfates. Typically, at least about 50%, more typically at least most, and
even more
typically from about 80 to about 95 % of the polythionates are converted into
thiosulfates in
RIP pretreatment 140.
The temperature of the slurry 144 preferably is at least about 60 C and the
ORP of
the exiting slurry 152 is at least below about 100 mV (SHE) and more
preferably ranges
from about -100 to about 100 mV (SHE) to substantially minimize precious metal
precipitation.
The exiting RIP pretreated slurry 152 is passed through heat exchanger 136 and
conditioned in a conditioner 156 to resolubilize any precious metal
precipitated during RIP
pretreatment 140 and/or heat exchange 136. Conditioning 156 is performed in an
agitated
single- or multi-compartment vessel which has an oxidizing atmosphere, such as
air, to
cause solubilization of the precious metal precipitates. Although
polythionates will form in
the presence of an oxidant, such as molecular oxygen, the rate of conversion
of thiosulfate to
polythionates is much slower than the rate of precious metal solubilization.
Preferably, the
residence time (at ambient temperature and pressure) is selected such that at
least about 95 %
of the precious metal precipitates are solubilized while no more than about 5
% of the
thiosulfate is converted into polythionates. Preferably, the slurry residence
time in
conditioning 156 is no more than about 12 hrs and more preferably ranges from
about 1 to
about 6 hrs.
The conditioned slurry 160 is next subjected to resin-in-pulp treatment 164 to
extract
the precious metal from the conditioned slurry 160. The resin-in-pulp step 164
can be
performed by any suitable technique with any suitable ion exchange resin.
Examples of
suitable techniques include that discussed in
21
CA 02424714 2009-07-24
U.S. Patent 6,251,163, filed March 4, 1998, entitled "Method for Recovering
Gold from
Refractory Carbonaceous Ores" and U.S. Patents 5,536,297 and 5,785,736.
Preferred resins
include anion exchange resins, preferably a strong base resin including a
quaternary amine
attached to a polymer backbone. A strong base resin is preferred over a weak
base resin.
The precious metal loading capacity of a strong base resin is typically
greater than that of a
weak base resin, such that a lower volume of resin is required. Gel resins and
macroporous
resins are suitable. Suitable resins include all commercial strong-base resins
of either Type I
(triethylamine functional groups) or Type II (triethyl ethanolamine functional
groups).
Specific strong-base ion exchange resins include "A500", manufactured by
Purolite,
"A600" manufactured by Purolite, "21K" manufactured by Dow Chemical,
"AMBERLITE IRA 410" manufactured by Rohm and Haas, " AMBERLITE IRA 900"
manufactured by Rohm and Haas, and "VITROKELETM 911" supplied by Signet.
Because
the RIP pretreatment and resin-in-pulp steps 140 and 164 are preferably
performed in the
same vessel (though they may be performed in different vessels), the
temperature, leach
slurry pH, and residence time typically depend on which of the above
techniques are used to
reduce the polythionate concentration.
Resin-in-pulp treatment can be performed in any suitable vessel. A preferred
vessel
is a Pachuca tank, which is an air-agitated, conical bottomed vessel, with air
being injected
at the bottom of the cone. An advantage of the Pachuca system is reduced resin
bead
breakage and improved dispersion of the resin beads in the slurry as compared
to
22
CA 02424714 2003-04-01
mechanically agitated systems. The RIP recovery is preferably carried out in
four or more
tanks connected in series, more preferably between four and eight such Pachuca
tanks.
During resin-in-pulp 164, the resin will become "loaded" with the dissolved
precious
metals. Typically, at least about 99% and more typically at least about 99.8%
of the precious
metal(s) in the leach slurry will be "loaded" or adsorbed onto the resin.
To inhibit the formation ofpolythionates and the consequent precious metal
recovery
problems and increased reagent consumption, the leach slurry can be maintained
in an inert
(or an at least substantially nonoxidizing) atmosphere and/or an inert (or an
at least
substantially nonoxidizing) gas can be sparged through the leach slurry. The
atmosphere is
preferably maintained (and/or gas sparging used) during RIP pretreatment 140
and resin-in-
pulp 164. As used herein, "inert" refers to any gas which is at least
substantially free of
oxidants, such as molecular oxygen, that can cause thiosulfate to be converted
into a
polythionate. For example, an "inert" gas would include a reducing gas.
Typically, the inert
atmosphere will include at least about 85 vol % of an inert gas, preferably
nitrogen gas, and
no more than about 5 vol % oxidants, such as oxygen gas, that can cause
thiosulfate
conversion into a polythionate. The molecular nitrogen can be a byproduct of
the oxygen
plant that is employed in the leaching step to provide superatmospheric
partial pressures of
oxygen gas. As will be appreciated, the leach slurry 144 during transportation
between the
leaching and RIP pretreatment steps 132 and 140 and if applicable from the RIP
pretreatment
and resin-in-pulp steps 140 and 164 (except during conditioning 156) is
typically in a conduit
that is not open to the surrounding atmosphere. If the leach slurry is open to
an atmosphere
-23-
CA 02424714 2003-04-01
during transportation in either or both of these stages, the leach slurry
should be maintained
in the presence of the inert atmosphere during any such transportation.
While not wishing to be bound, it is believed that sparging is more effective
than an
inert atmosphere without sparging in controlling polythionate production.
Sparging appears
to inhibit molecular oxygen ingress into the solution, even where the reactor
is open to the
ambient atmosphere, because of the outflow of inert gas from the surface of
the solution.
The barren leach slurry 168 (which will typically contain no more than about
0.01
ppm precious metals or 1% of the precious metal(s) in the leach solution 144)
is subjected
to one or more stages of counter current decantation ("CCD") 172. In CCD 172,
the solids
are separated in the underflow 176 from the barren leach (or overflow)
solution 180 and
sent to the tailings pond. The barren leach solution 180 is separated in the
overflow from
the solids and forwarded to regeneration step 184 to convert polythionates to
thiosulfate. As
will be appreciated, CCD performs liquid/solid separation, provides water
balancing in the
circuit, and prevents build up of impurities in the leach circuit by removing
a portion of the
leach solution with the solids.
Regeneration 184 can be performed in one or more vessel(s) and/or by in line
sulfide
(and/or sulfite) addition to a conduit carrying the stripped lixiviant
solution. If a number of
the techniques are employed, they can be performed simultaneously (in the same
reactors)
or sequentially (in different reactors), as desired.
The regenerated lixiviant solution 128 is recycled to the grinding step 108
along with
the thickener overflow 120 and ultimately to the leaching step 132.
-24-
CA 02424714 2009-07-24
The loaded resin 188 is screened 190 and washed with water to remove any leach
slurry (liquid and/or leached material) from the resin beads.
The recovered beads 192 are contacted with an eluant to strip or elute 194
adsorbed
precious metal into the eluate and form a pregnant solution 196 containing
typically at least
most (and more typically at least about 95%) of the precious metal on the
resin and a
stripped resin 197.
The eluant can be any suitable eluant that can displace the adsorbed precious
metal
from the loaded resin beads. The eluant could include salts, such as one or
more types of
polythionate ions and a nitrate, a thiocyanate, a sulfite, a thiourea, a
perchlorate and
mixtures thereof.
Typically, the concentration of the eluant in the pregnant solution 196 ranges
from
about 0.25 to about 3 M; the temperature of elution 194 from about 5 to about
70 C, and the
pH of elution 194 from about pH 5 to about pH 12. Under the conditions, at
least about 90 %
and more typically from about 95 to about 99% of the precious metal adsorbed
on the resin
is displaced by the eluant into the pregnant solution 196.
The stripped resin 197 is recycled to the resin-in-pulp step 164. Optionally,
the
stripped resin 197 can be regenerated (not shown) by known techniques prior to
reuse of the
resin. As will be appreciated, the resin can be regenerated by acid washing
the resin with an
acid such as nitric acid or hydrochloric acid. The acid wash removes adsorbed
eluant and/or
impurities from the resin and frees up the functional sites on the resin
surface (previously
occupied by the eluant) to adsorb additional precious metal. In the case of a
polythionate
eluant, the resin can be regenerated by contacting the resin with sulfide
and/or sulfite to
CA 02424714 2009-07-24
reduce the polythionate ions to thiosulfate ions and sulfate ions. After
regeneration, the
resin and regeneration product solution are separated by screening and
washing.
The pregnant solution 196, which includes the eluant and typically no more
than
about 100 ppm and more typically from about 10 to about 500 ppm solubilized
precious
s metals, is subjected to electrowinning 198 to recover the solubilized
precious metals and
form a barren solution 199. Problems in electrowinning of precious metals out
of a medium
containing polythionates and/or thiosulfate have been encountered. When the
eluant is a
polythionate, the polythionate and thiosulfate tend to be co-reduced with the
precious metal
at the cathode to produce elemental sulfur, which interferes with the
efficient continued
operation of the electrowinning circuit while the polythionate and thiosulfate
are also
wastefully oxidized to sulfate ions at the anode.
These problems are overcome by the present invention through the use of
sulfite in
the pregnant solution. Sulfite is added to the eluant and/or to the pregnant
solution 196 prior
to, during, or after electrowinning. Preferably, sulfite is added to the
eluant prior to the
elution step 194. In the presence of sulfite, the precious metal is reduced at
the cathode
while the sulfite is oxidized to sulfate at the anode. This has the benefit of
lowering the cell
voltage required. Preferably, the concentration of sulfite in the pregnant
solution 196 (in the
elution and electrowinning steps 194, 198) is at least about 0.01M and more
preferably
ranges from about 0.1 to about 2 M. The sulfite is preferably in the pregnant
solution with
another eluant, such as any of the eluants noted above.
26
CA 02424714 2003-04-01
The stripped or barren solution 199 is removed from the electrowinning cell(s)
and
returned to the elution step 194. A bleedstream (not shown) of the barren
solution 199 can
be used to control buildup of impurities such as sulfate.
The recovered precious metal 195, which contains the precious metal recovered
in
electrowinning and impurities, is subjected to retorting 193 by known
techniques to remove
the impurities and form precious metal sludge. The sludge, which contains at
least most of
the precious metal in the recovered precious metal 195, is refined to produce
a precious metal
product of high purity.
Fig. 2 depicts another embodiment of a process for thiosulfate leaching of a
refractory
precious metal-containing material. Fig. 2 shows an alternative to resin-in-
pulp for precious
metal recovery. Following leaching 132, the precious metal bearing solution
144 is separated
200 from the solids by any suitable means, such as by counter-current
decantation washing
and/or filtration. Preferably, at least about 95% and more preferably at least
about 99% of
the precious metal is separated from the solids with the latter going to
tailings impoundment.
The separated precious metal bearing solution 204 is directed to the precious
metal
precipitation - thiosulfate regeneration step 208. This process can be carried
out in any
suitably agitated reactor or plurality of agitated reactors. The pH of the
precious metal
bearing solution 204 is adjusted if necessary to about pH 5.5-12, more
preferably about pH
7-11, even more preferably about pH 9-11 using a suitable basic reagent such
as sodium
hydroxide and the solution is contacted with a reductant, preferably a sulfide
and/or bisulfide
and/or polysulfide reagent to precipitate at least about 99% of the precious
metal and convert
at least about 90% of the polythionates to thiosulfate, effectively
regenerating the thiosulfate
-27-
CA 02424714 2003-04-01
lixiviant. The effectiveness of the conversion causes significantly less
thiosulfate reagent to
be consumed during the process than for conventional thiosulfate leaching
processes. The
use of a sulfide and/or bisulfide and/or polysulfide has the added benefit of
reducing
impurities such as copper or mercury or manganese from solution thereby
reducing the rate
of thiosulfate degradation. While not wishing to be bound by any theory, it is
believed that
the most likely composition of the precipitate is the metallic precious metal
and/or a precious
metal sulfide, such as Au2S. Maximum precipitation of gold and regeneration of
thiosulfate
is accomplished by adding at least a stoichiometric amount of reductant
(relative to the
dissolved precious metal and polythionate concentrations) to reduce the
solution ORP to at
least about -150 mV (SHE). The temperature is preferably maintained in the
range of about
5 to 40 C, and more preferably at ambient temperature, about 20 C. The
retention time is
about 5 minutes to about 2 hours, more preferably about 15 minutes to about 1
hour. The
process is conducted under oxygen-depleted conditions, with the solution
preferably
containing no more than about 1 ppm dissolved molecular oxygen and more
preferably less
than about 0.2 ppm dissolved molecular oxygen concentration, by bubbling an
oxygen-
deficient gas such as nitrogen into the slurry and/or maintaining a blanket of
nitrogen in the
atmosphere over the slurry as noted above.
The precious metal bearing precipitate is separated from the regenerated
solution 212
by any suitable method such as filtration, CCD, and the like and the separated
precious metal
216 is recovered by refining in furnaces.
The regenerated solution 220 is directed to the conditioning step 224, which
can be
conducted in any suitably agitated reactor or plurality of reactors. The
solution pH is adjusted
-28-
CA 02424714 2003-04-01
to a value suitable for recycling the solution back to grinding 108 and/or for
precious metal
scavenging 228. Preferably, the pH ranges from about pH 7 to about pH 12, more
preferably
about pH 8 to pH 10. The solution 220 is agitated in the presence of an oxygen-
containing
atmosphere, such as air, to oxidize any remaining reductant (such as sulfide
or bisulfide or
polysulfide) carried over from the precious metal precipitation - thiosulfate
regeneration step
208. The duration ofthe conditioning step 224 is preferably not sufficient to
cause more than
about 5% of the thiosulfate to form polythionates, or to yield a polythionate
concentration
of more than about 0.003M. The majority (typically at least about 80 vol%) of
the
conditioned solution 232 is then recycled in recycle solution 236. A minor
portion (e.g.,
from about 2 to about 20 vol%) of the conditioned solution or bleed stream 240
may have
to be bled to tailings to control the buildup of impurities, such as soluble
sulfate and metallic
impurities. Prior to discharge to tailings the bleed portion 240 of the
conditioned solution 232
is directed to the precious metal scavenging step 228 to recover any precious
metals
remaining in solution that were not recovered in the precious metal
precipitation - thiosulfate
regeneration step 208. Precious metal scavenging can be accomplished, by any
suitable gold
recovery technique such as by passing the bleed solution 240 through a column
containing
a strong base resin to adsorb the precious metal. While not wishing to be
bound by any
theory, precipitated precious metal can be redissolved due to trace amount of
molecular
oxygen in the solution and incomplete reduction of polythionates in the
solution. Because
the amount of polythionates in the bleed is negligible, a resin-in-column
recovery technique
will have an excellent ability to load any remaining dissolved precious metal.
-29-
CA 02424714 2003-04-01
In an alternative configuration (not shown), the precious metal precipitates
are
redissolved in a suitable solvent, such as nitric/hydrochloric acid, cyanide,
thiosulfate,
thiourea chloride/chlorine and bromide/bromine to provide a precious metal-
containing
solution. The precious metal can then be recovered by electrolysis as noted
above in
connection with step 198 of Fig. 1.
This process is preferred in certain applications over the process of Fig. 1.
For certain
precious metal-containing materials, it is difficult to obtain high rates of
precious metal
adsorption onto resins while maintaining the precious metal in solution. The
use of an RIP
pretreatment step, though beneficial, can be difficult to use without
experiencing some
precious metal precipitation. Conditioning 156 may not be completely effective
in
redissolving gold precipitates, which would be discarded with the barren
solids to tailings.
The process of Fig. 2 can also be more robust, simpler, and therefore easier
to design and
operate than the process of Fig. 1.
Fig. 3 shows an alternative to Fig. 2 in which thiosulfate leaching is
conducted in two
stages to achieve more effective recovery of the precious metal content.
Leaching is first
conducted at atmospheric pressure and ambient temperature in the presence of
an oxygen-
containing gas such as air or industrially available oxygen (step 300) to
dissolve from about
30 to 95% of the leachable precious metal content. The leachable precious
metal content is
defined as that portion of the precious metal content that is physically
accessible to the
thiosulfate lixiviant and is not encapsulated within constituents contained in
the host
material. The precious metal bearing solution 304 is separated from the solids
308 (step 200),
the solids 308 are repulped with a portion 310 of the recycle solution 236,
and the resulting
-30-
CA 02424714 2003-04-01
slurry 308 is then directed to pressure leaching (step 312) to dissolve the
majority, ie. about
5-70%, of the remaining leachable precious metal content that was not
recovered in
atmospheric leaching 300. In pressure leaching the solids are leached under
superatmospheric
conditions such as the conditions described previously (step 132 of Fig. 1).
The molecular
oxygen partial pressure in leach 300 preferably ranges from the molecular
oxygen partial
pressure at ambient conditions (e.g., more than about 3 psia at sea level) to
about 15 Asia and
the molecular oxygen partial pressure in leach 312 preferably ranges from more
than 15 psia
to about 500 psia. The slurry 316 exiting pressure leaching 312 is then
processed in
essentially the same manner as the slurry exiting leaching 300 in Fig. 2. That
is, the slurry
316 is subjected to solid/liquid separation 320 in the presence of wash water
to separate the
barren solid material 324 from the (second) pregnant leach solution 328. The
first and
second pregnant leach solutions 304, 328 are subjected to precious metal
precipitation -
thiosulfate regeneration 208, further solid/liquid separation 212,
conditioning 224 and
precious metal scavenging 228 as noted above in connection with Fig. 2.
The process of Fig. 3 typically performs the bulk of the leaching, or precious
metal
dissolution, under ambient conditions, which is much cheaper than leaching
under
superatmospheric conditions. The more-difficult-to-dissolve precious metals
and weakly
preg-robbed precious metals are then dissolved in a higher pressure leach.
Because less
precious metal remains to be dissolved, the high pressure leach can have a
shorter residence
time and therefore lower capacity than would be possible in the absence of the
ambient
pressure leach.
-31-
CA 02424714 2003-04-01
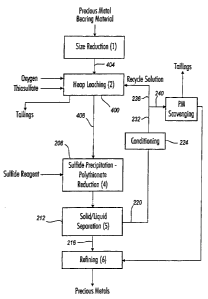
Fig. 4 depicts another embodiment of the present invention. The process is
similar
to those discussed above except that thiosulfate leaching is performed by heap
leaching 400
techniques.
Before heap leaching can commence, the heap is formed on a leach pad. To
provide
desired levels of heap porosity and permeability, the precious metal-
containing material is
comminuted to a desired size range, typically by wet or dry crushing or may be
placed as
mined ore. Typically, the material is comminuted to a P80 size of from about 2
inches to
about 1/4 inch. Prior to placement on the leach pad, fine particles (particles
preferably
having a size of less than about 150 pm and more preferably of less than about
500 m) are
removed from the comminuted material by suitable screens, filters, and the
like.
Alternatively or additionally, the comminuted precious metal-containing
material can be
formed into agglomerates or particulates by known techniques. Thiosulfate
lixiviant can be
contacted with the comminuted material immediately before or during
agglomeration to
assist in agglomerate formation and "jump start" the leaching process and/or a
suitable binder
can be added to provide more robust agglomerates. Possible binders include
NALCO 9704,
water, barren thiosulfate-containing solution, and leachate. In any event, it
is preferred that
the P80 size of the particles/agglomerates formed into the heap is at least
about 150 m, more
preferably at least about 500 m, and even more preferably at least about
1,000 m.
Thiosulfate lixiviant can be contacted with the particles of material and/or
agglomerates as
the particles/agglomerates are being placed on the heap. In the process
configuration of Fig.
4, the comminuted precious metal-containing material 404 is directly formed
into a heap
without prior agglomeration.
-32-
CA 02424714 2003-04-01
The pad typically includes a liquid impervious liner, which is placed beneath
the
heap, to collect the pregnant leach solution and prevent the pregnant leach
solution from
being lost to the surrounding environment. The height of each lift of the heap
is typically
from about 4 to about 8 m and of the heap itself can be up to 100 m. Rather
than moving the
heap after thiosulfate leaching is completed (which is done in a dynamic heap
configuration),
further heaps or lifts can be built on top of exhausted heap(s) (which is done
in a static heap
configuration).
During heap construction, a network of aerating pipes can be located in a
lower
portion of the heap to force an oxygen-containing gas through the heap during
thiosulfate
leaching. The pipes can be perforated so as to provide a substantially uniform
dispersion of
the gas throughout the heap. The oxygen-containing gas is typically forced
through the pipes
using a single- or multi-stage compressor, blower, fan, or other mechanical
device. When
the oxygen-containing gas is pressurized and forced through the pipes, it
typically has a
pressure greater than the ambient atmospheric pressure, more typically of at
least about 1
inch H2O, and even more typically of at least about 30 inches H2O greater than
the ambient
atmspheric pressure. Preferably, at least a stoichiometric amount of molecular
oxygen
(relative to the amount of precious metal in the precious metal-containing
material) is
deliberately introduced into the heap during leaching. More preferably, at
least about 0.5 kg
of molecular oxygen and even more preferably from about 1 to about 10 kg of
molecular
oxygen is introduced into the heap during leaching for each ton of material to
be leached in
the heap. Preferably, at least about 2 and more preferably from about 4 to
about 40 m3 of
-33-
CA 02424714 2003-04-01
oxygen-containing gas is introduced into the heap for each liter of lixiviant
applied to the
heap.
To control evolution ofammonia gas during forced air introduction into the
heap, the
pH of the thiosulfate lixiviant is controlled. Preferably, the pH of the
thiosulfate lixiviant is
maintained (when introduced into the heap and during passage through the heap)
at a pH of
no more than about pH 9, more preferably of no more than about pH 8.75, and
even more
preferably of from about pH 6.5 to about pH 8.75. Alternatively, the
concentration of free
ammonia can be maintained below levels sufficient to result in evolution of
significant
amounts of ammonia gas. In some configurations, the concentration of free
ammonia in the
thiosulfate lixiviant applied to the top of the heap is maintained at a level
of no more than
about 2000 ppm, more preferably no more than about 1000 ppm, and even more
preferably
no more than about 500 ppm. This can be realized, for example, by using sodium
thiosulfate
alone as the lixiviant or using a mixture of ammonium and sodium thiosulfate.
The pH can be controlled by using suitable (acid or base) buffering agents to
produce
the desired change in pH. In one embodiment, carbonaceous compounds, such as
calcium
carbonates, are added to the lixiviant before or after application to the heap
and/or to the heap
itself. Surprisingly, the carbon component in the buffering agent has been
found under
suitable conditions to perform, at most, only a minimal degree preg robbing.
Typical
consumption of carbonates is in the range of about 0.5 to about 101b/ton of
material in the
heap.
To apply the thiosulfate lixiviant to the heap, a number of techniques can be
employed. For example, spray systems (such as spray nozzles), drip and/or
trickle systems
-34-
CA 02424714 2003-04-01
(such as drip emitters and perforated pipes), injection holes in the heap, and
irrigation ditches
on top of the heap can be used to apply the lixiviant. The preferred lixiviant
distribution
system preferably applies the lixiviant at least substantially uniformly
throughout the heap.
In a preferred configuration, the applied lixiviant flows countercurrently
through the heap
relative to the flow of the oxygen-containing gas. Typically, the lixiviant
flows from the top
to the bottom of the heap while the gas flows from the bottom to the top of
the heap. In a
preferred configuration, at least about 0.5 and even more preferably from
about 1 to about
l Ot solution It ore of lixiviant is applied to the top of the heap from start-
to-finish of heap
leaching. In this configuration the lixiviant is applied for at least about
0.5 and even more
preferably from about I to 48 months from the start to finish of heal
leaching.
In one configuration, the lixiviant is sparged with the oxygen-containing gas
before
application to the heap or the gas is otherwise contacted with the lixiviant
before application
to the heap (such as by in-line mixing) to cause the lixiviant to have a
heightened dissolved
molecular oxygen content. Additional gas may be deliberately introduced into
the heap
separately from the lixiviant, if desired. The dissolved molecular oxygen
content of the
lixiviant preferably is at least about I mg/L and even more preferably ranges
from about 5
to about 40 mg/L.
After construction of the heap, the thiosulfate lixiviant (which commonly
includes
a recycled thiosulfate lixiviant 236 mixed with a makeup (fresh) thiosulfate
solution(not
shown)) is applied to the top of the heap while the oxygen-containing gas is
introduced to
the bottom of the heap. The pregnant leach solution 408 is collected from the
base of the
heap. The pregnant leach solution is recycled to the top of the heap. The
recycle rate is
-35-
CA 02424714 2003-04-01
sufficient to provide an application rate of the lixiviant to the top of the
heap of from about
2 and more preferably from about 4 to about 24 L/h/m2 of top surface area for
the heap.
During recycle, at least a portion (typically at least about 5 vol.% and more
typically
from about 50 to about 100 vol.%) of the pregnant leach solution is removed
and subjected
to precious metal recovery, such as by any of the techniques referred to
above. A particularly
preferred technique is to recover the dissolved precious metal by sulfide
precipitation. As
noted, sulfide precipitation not only precipitates the precious metal but also
regenerates the
thiosulfate by converting polythionates into thiosulfate. Sulfide
precipitation is typically
conducted under anerobic conditions, as noted above. Such conditions can be
realized and
maintained by de-aerating the pregnant leach solution with a vacuum, inert gas
bubbling,
and/or allowing the pregnant leach solution to stand dormant for a sufficient
period of time
for the dissolved oxygen level to decrease to desired levels. Sufficient
sulfide is added to the
pregnant leach solution to precipitate at least most of the dissolved precious
metal(s) as
precious metal sulfides and to convert at least most of the polythionates to
thiosulfate. The
barren thiosulfate lixiviant, after precious metal recovery, can be recombined
with the
remaining pregnant leach solution and returned to the top of the heap.
While not wishing to be bound by any theory, it is believed that the precious
metal
thiosulfate complex may be unstable under certain conditions, including those
set forth
above, and that the precious metal can be stripped from the thiosulfate-
containing solution
by a number of substances commonly encountered in precious metal-containing
materials.
The substances or preg robbing materials typically absorb, adsorb or
precipitate the precious
metal. Such preg-robbing materials include carbonaceous materials, pyrite-
containing
-36-
CA 02424714 2003-04-01
materials, chacopyrite and iron oxides. Surprisingly and unexpectedly,
blinding agents may
be used in the thiosulfate lixiviant to prevent or inhibit preg robbing of the
precious metal
by the preg robbing material. The blinding agent itself absorbs or adsorbs (in
preference to
the precious metal) or otherwise neutralizes (such as by chemical reaction)
the preg robbing
sites on the material. Suitable blinding agents include one or more of
hydrocarbon-
containing substances, such as petroleum products (e.g., kerosene, diesel
fuel, and gasoline),
alcohols, esters, or aldehydes; surfactants such as detergents, sodium lauryl
sulfonate, or
organic phosphates; and reactive metal salts such as lead, mercury, cadmium,
tin, and silver
salts. In such situations, the thiosulfate lixiviant, in any of the leaching
processes discussed
above, typically includes at least about 0.1 mg/L, more typically at least
about 1 mg/L, and
even more typically from about 2 to about 200 mg/L of the blinding agent.
While not wishing to be bound by any theory, other agents may also be suitable
as
blinding agents. Preferably, the agents do not destabilize thiosulfate in
solution. Agents
which act as oxidation catalysts, can destabilize thiosulfate in solution. By
way of example,
copper salts under certain conditions are not preferred as a blinding agent as
copper salts
under these conditions can catalyze thiosulfate decomposition.
To facilitate extraction of gold from sulfidic and/or carbonaceous materials,
the
thiosulfate leach step in any of the above processes can be preceded by one or
more
pretreatment steps to destroy or neutralize the carbon-containing and/or
sulfidic minerals.
By way of example, the intermediate steps can include one or more of
biooxidation or
chemical oxidation to oxidize sulfides, ultrafine grinding to liberate
occluded precious
-37-
CA 02424714 2003-04-01
metals, conventional roasting to destroy carbon- and/or sulfide-containing
minerals, and/or
microwave roasting.
EXAMPLE 1
A gold ore from Nevada, designated Sample A, was subjected to thiosulfate
leaching
under oxygen pressure at varying temperatures. The ore assayed 24.1 g/t gold,
2.59% iron,
0.31% total sulfur, 0.28% sulfide sulfur, 3.40% total carbon, 1.33% organic
carbon and
0.02% graphitic carbon. From a diagnostic leaching analysis of the ore it was
determined that
a maximum of 83% of the contained gold was capable of being solubilized while
the
remaining gold was inaccessible to a lixiviant because it was encapsulated
within pyrite
and/or other minerals contained in the ore.
The ore was ground to 80% passing 200 mesh (75 m). Samples of the ore were
slurried with water to a pulp density of 33% solids in a mechanically agitated
laboratory
autoclave. The natural pH of the slurry at ambient temperature was 8.3. The pH
of the slurry
was adjusted to 9 with sodium hydroxide and a quantity of sodium thiosulfate
reagent was
added to adjust the initial leach solution thiosulfate concentration to 0.1
molar (M). The
autoclave was sealed and pressurized to 100 psig oxygen with pure (95% plus)
oxygen gas
and the slurry was heated to the desired temperature (if required). Leaching
was maintained
for 6 hours, during which pulp samples were taken at 2 and 4 hours in order to
monitor gold
extraction with time. Upon termination of leaching, the slurry was filtered
and the residue
solids were washed with a dilute thiosulfate solution. The residue solids and
leach solution
were assayed for gold to determine the final gold extraction.
The results were as follows:
-38-
CA 02424714 2003-04-01
Leach Temp. Leach Time Calc'd Head Residue Au Ext'n
( C) (hours) Au (g/t) Au (g/t) (%)
20 2 33.3
4 41.9
6 22.8 9.44 58.5
40 2 51.2
4 55.1
6 26.4 9.25 64.9
60 2 63.7
4 68.5
6 22.8 4.26 81.3
60 (repeat) 2 65.2
4 73.0
6 80.9
The results indicate that the rate and extent of gold extraction was improved
with
increasing temperature and leach time in the temperature range 20-60 C. The
best results
were obtained at 60 C, with about 81 % gold extraction obtained after 6 hours
leaching, this
representing about 98% of the leachable gold content of the ore.
EXAMPLE 2
A second gold ore from Nevada, designated Sample B, was subjected to
thiosulfate
leaching under oxygen pressure at varying initial pH's. The ore assayed 9.45
g/t gold, 2.50%
iron, 0.39% total sulfur, 0.36% sulfide sulfur, 4.20% total carbon, 1.46%
organic carbon and
0.05% graphitic carbon. From a diagnostic leaching analysis of the ore it was
determined that
82% of the contained gold was capable of being solubilized. Samples of the ore
were
prepared and leached as described in Example 1, except the temperature was 60
C in each
test, the autoclave was pressurized with 50 psig oxygen, the initial pH was
adjusted to either
9, 11 or 12, and the leach retention time was extended to 8 hours for the pH
11 and 12 tests.
-39-
CA 02424714 2003-04-01
The results were as follows:
Initial Leach Time Calc'd Head Residue Au Ext'n
pH (hours) Au (g/t) Au (g/t) (%)
9 1 50.2
2 62.4
4 72.0
6 8.49 2.10 75.3
11 1 41.3
2 63.0
4 69.3
8 8.61 2.00 76.8
12 1 6.4
2 1.0
4 13.6
8 8.61 3.34 61.2
The results indicate that there was not much difference in gold leaching
behaviour
over the initial pH range of 9-11 (it should be noted that the pH tended to
decline during
leaching). However, gold leaching was suppressed during the first 4 hours of
leaching at pH
12, but then started to recover.
EXAMPLE 3
A third gold ore sample from Nevada, Sample C, was subjected to thiosulfate
leaching under oxygen pressure at varying temperatures. The head analysis of
the ore was as
follows:
Gold Ore Sample C
Au, g/t 9.50 C (t), % 4.45
Fe, % 2.52 C (CO3)1% 3.12
Cu, ppm 40 C (org), % 1.38
As, ppm 647 S(2-),% 0.35
Hg, ppm 14 S (t), % 0.27
-40-
CA 02424714 2003-04-01
Ca, % 9.0 Mg, % 1.5
From a diagnostic leaching analysis of the ore it was determined that 83% of
the
contained gold was capable of being solubilized.
The ore was ground to 80% passing 200 mesh (75 m). Samples of the ore were
slurried with water to a pulp density of 33% solids in a mechanically agitated
laboratory
autoclave. The initial pH of the slurry was adjusted to approximately 11 with
sodium
hydroxide, after which the autoclave was sealed and pressurized to 100 psig
oxygen with
pure (95% plus) oxygen gas and the slurry was heated to the desired
temperature. To initiate
leaching, a quantity of sodium thiosulfate stock solution was injected to
adjust the leach
solution thiosulfate concentration to 0.1 M. Leaching was continued for 6 to
10 hours, during
which no additional reagents were added. Pulp samples were taken at set
intervals during
leaching in order to monitor gold extraction with time. Upon termination of
leaching, the
slurry was filtered and the residue solids were washed with a dilute
thiosulfate solution. The
residue solids and leach solution were assayed for gold to determine the final
gold extraction.
Fig. 5 depicts graphically the effect of leach temperature, in the range 40-80
C, on
the rate of gold extraction from Sample C. It can be seen that the gold
leached quickly at
60 C and 80 C, there being little difference in the extraction rate at the two
temperatures. The
gold extraction peaked at approximately 83%, the maximum extractable, after 6
hours
leaching. Gold leaching was slowed if the temperature was lowered to 40 C, but
80% gold
extraction was still obtained after 10 hours leaching at 40 C.
An overall summary of the results is provided below:
-41-
CA 02424714 2003-04-01
Parameter Test #6 Test #25 Test #15
80 C 60 C 40 C
Leach time, hours 8 6 10
Final pH 7.0 8.7 9.3
Final ORP, mV (SHE) 307 242 225
Calc'd Head Au, g/t 9.48 9.43 9.27
Residue Au, g/t 1.59 1.63 1.81
Au Ext'n, % 83.2 82.7 80.5
EXAMPLE 4
The gold ore designated Sample C was subjected to thiosulfate leaching at
varying
oxygen pressures. Samples of the ore were prepared and leached as described in
Example 3
except the temperature was maintained at 60 C in each test and the oxygen
partial pressure
was varied.
Fig. 6 portrays the effect of oxygen partial pressure, in the range 0-200
psig, on the
rate of gold extraction from Sample C (in the 0 psig 02 test, the autoclave
was not
pressurized but the head space was maintained with pure oxygen at atmospheric
pressure).
It can be seen that the rate of gold leaching was somewhat sensitive to oxygen
pressure, in
that the rate increased with increasing pressure, particularly during the
first two hours of
leaching. After 6 hours leaching, gold extraction varied from a low of 78% at
0 psig 02 to
a high of 83% at 200 psig 02.
An overall summary of the results is provided below:
Parameter Test #7 Test #25 Test #22 Test #28 Test #31
200 psig 02 100 psig 02 50 psig 02 10 psig 02 0 psig 02
Leach time, hours 8 6 6 6 6
Final pH NA 8.7 9.0 9.3 9.4
Final ORP, mV (SHE) NA 242 223 216 232
Calc'd Head Au, g/t 9.78 9.43 9.40 8.95 9.08
Residue Au, g/t 1.68 1.63 1.77 1.72 2.00
-42-
CA 02424714 2003-04-01
Au Ext'n, % 82.8 82.7 81.1 80.8 78.0
NA = not analyzed
EXAMPLE 5
The gold ore designated Sample C was subjected to thiosulfate leaching under
oxygen
pressure at varying initial sodium thiosulfate concentrations. Samples of the
ore were
prepared and leached as described in Example 3 except the temperature was
maintained at
60 C in each test and the initial sodium thiosulfate concentration was varied.
Fig. 7 portrays the effect of initial sodium thiosulfate concentration, in the
range 0.05-
0.2 M, on the rate of gold extraction from Sample C. It can be seen that the
rate of gold
leaching was insensitive to initial thiosulfate concentration in the 0.1-0.2 M
range. At 0.05
M thiosulfate, the rate was reduced significantly, particularly during the
first two hours of
leaching. After 6 hours leaching gold extraction was 78% at 0.05 M thiosulfate
compared to
82% achieved at both 0.1 M and 0.2 M thiosulfate concentration.
An overall summary of the results is provided below:
Parameter Test #4 Test #25 Test #8
0.2 M 0.1 M 0.05 M
Leach time, hours 8 6 6
Final pH 8.7 8.7 8.5
Final ORP, mV (SHE) NA 242 262
Calc'd Head Au, g/t 8.85 9.43 9.40
Residue Au, g/t 1.50 1.63 1.87
Au Ext'n, % 83.0 82.7 80.1
NA = not analyzed
EXAMPLE 6
The gold ore designated Sample C was subjected to thiosulfate leaching under
oxygen
pressure at two different pulp densities. Samples of the ore were prepared and
leached as
-43-
CA 02424714 2003-04-01
described in Example 3, except the temperature was maintained at 60 C in each
test and the
leach pulp density was either 33% or 45% solids by weight.
Fig. 8 portrays the effect of 33% vs. 45% pulp density on the rate of gold
extraction
from Sample C. The rate of gold leaching was found to be essentially
insensitive to pulp
density in this range.
An overall summary of the results is provided below:
Parameter Test #26 Test #25
45% pulp 33% pulp
density density
Leach time, hours 6 6
Final pH 8.5 8.7
Final ORP, mV (SHE) 286 242
Calc'd Head Au, g/t 9.29 9.43
Residue Au, g/t 1.68 1.63
Au Ext'n, % 81.9 82.7
EXAMPLE 7
A fourth gold ore sample from Nevada, Sample D, was subjected to thiosulfate
leaching at 60 C and 10 psig oxygen pressure at the natural pH of the slurry,
for 8 hours. The
head analysis of the ore was as follows:
Gold Ore Sample D
Au, g/t 12.15 C (t), % 4.31
Fe, % 2.09 C (CO3), % 3.02
Cu, ppm 39 C (org), % 1.30
As, ppm 692 S(2-),% 0.12
Hg, ppm 27 S (t), % 0.22
Ca, % 8.9 Mg, % 1.3
From a diagnostic leaching analysis of the ore it was determined that 80% of
the
contained gold was capable of being solubilized.
-44-
CA 02424714 2003-04-01
The ore was ground to 80% passing 200 mesh (75 pm). A sample of the ore was
slurried with water to a pulp density of 40% solids in a mechanically agitated
laboratory
autoclave. The autoclave was sealed and pressurized to 100 psig oxygen with
pure (95%
plus) oxygen gas and the slurry was heated to 60 C. To initiate leaching, a
quantity of sodium
thiosulfate stock solution was injected to adjust the leach solution
thiosulfate concentration
to 0.1 M. Leaching was continued for 8 hours, during which no additional
reagents were
added. Pulp samples were taken at set intervals during leaching in order to
monitor gold
extraction and remaining thiosulfate with time. Upon termination of leaching,
the slurry was
filtered and the residue solids were washed with a dilute thiosulfate
solution. The residue
solids and leach solution were assayed for gold to determine the final gold
extraction.
Fig. 9 depicts percent gold extraction and percent remaining thiosulfate with
time.
Gold extraction reached 79.3% after 8 hours, or 99% of the leachable gold
content.
Thiosulfate consumption was low, with 86.7% of the thiosulfate remaining after
8 hours and
available for reuse.
An overall summary of the results is provided below:
Parameter Test #37-01
Leach time, hours 8
Initial pH 7.9
Final pH 9.0
Initial ORP, mV (SHE) 411
Final ORP, mV (SHE) 397
Calc'd head Au, g/t 11.50
Residue Au, g/t 2.38
Gold extraction, % 79.3
Amount of thiosulfate remaining, % 86.7
-45-
CA 02424714 2003-04-01
EXAMPLE 8
A thiosulfate leach discharge slurry was heated to 60 C in an agitated reactor
in
preparation for RIP pre-treatment, the objective being to reduce the
polythionate content
without precipitating gold. The slurry was kept under a nitrogen atmosphere to
ensure the
dissolved oxygen content was maintained below 0.2 mg/L. A single dose ofa 0.26
M sodium
bisulfide (NaHS) solution, adjusted to pH 9, was added and the pretreatment
was allowed to
proceed at 60 C and ambient pressure for 2 hours. The amount of sulfide added
was 150%
of stoichiometric based on the amount required to convert the polythionates
back to
thiosulfate in accordance with the following reactions:
2S406 + S2_ + / H2O -* %S203 + 3H+
5306 + s2 -* 2S2 O3
A summary of the results is provided below:
Time Au S2032 S4062" S3062 ORP pH
(min) (mg/L) (8/L) (P~L) (g/L) (mV)
0 4.36 8,38 0.51 0.59 240 6.9
120 4.36 11.0 0.06 0.10 5 6.7
The tetrathionate and trithionate concentrations were reduced by 88% and 83%
respectively while all of the gold remained in solution.
EXAMPLE 9
A pregnant thiosulfate leach solution was adjusted to pH 10 with sodium
hydroxide
in an agitated reactor in preparation for sulfide treatment, the objective
being to regenerate
thiosulfate and precipitate the gold. The solution was kept under a nitrogen
atmosphere to
-46-
CA 02424714 2003-04-01
ensure the dissolved oxygen content was maintained below 0.2 mg/L. A single
dose ofa 0.26
M sodium sulfide (Na2S) solution was added and the treatment was allowed to
proceed for
2 hours at ambient temperature (22 C) and pressure. The amount of sulfide
added was 100%
of stoichiometric based on the amount required to convert the polythionates
back to
thiosulfate in accordance with the following reactions:
25406 +S2-+/H20-) %5203 +3H+
S306 + S2 -* 25203
A summary of the results is provided below:
Time Au 52032- 54062. 53062- ORP pH
(min) (mg/L) (g/L) (g/L) (9/L) (mV)
0 4.12 7.8 0.84 1.47 200 10.0
10 0.05 9.9 0.01 0.01 -210 11.0
0.02 9.9 0.01 0.01 -220 10.4
0.01 9.9 0.01 0.01 -230 10.2
15 60 0.01 9.8 0.01 0.01 -260 10.3
90 0.01 10.1 0.01 0.01 -260 10.3
120 0.01 10.2 0.01 0.01 -260 10.3
The rate of conversion of polythionates to thiosulfate was extremely fast
under
20 ambient conditions, with essentially complete conversion achieved after
about 10 minutes.
Similarly, gold precipitation was also fast and essentially complete after
about 30 minutes.
EXAMPLE 10
A gold ore from Nevada, designated sample X, was subjected to thiosulfate
leaching
in a column. The ore assayed 2.48 g/t gold, 2.2 wt.% iron, 0.2 wt.% total
sulfur, 0.13 wt.%
25 sulfide sulfur, 6.1 wt.% total carbon and 1.1 wt.% organic carbon. The ore
was crushed to
-47-
CA 02424714 2003-04-01
100% passing 1 inch and then loaded into a 10 inch diameter, 8 foot tall
column constructed
from PVC fitted with a retaining plate to keep the ore in the column.
A solution containing 15 g/L ammonium thiosulfate at a pH of 9.0 was applied
continuously to the top of the column and was collected at the bottom. The
solution was not
recycled. Solution was applied at a rate of 12 LJh/m2. To the aerated column,
air was forced
through the bottom of the column at a rate of 130 L/h/m2. No air was forced
through the
unaerated column. The temperature of both tests averaged 22 C.
Upon termination of leaching, the residue solids were washed with water and
the
residue solids and collected leach solution were assayed to determine the
final gold
extraction.
The results are as follows:
Leach Aerated Column Unaerated Column
Time Au Extraction Au Extraction
(days) (mg/L) (mg/L)
0.00 0.00 0% 0.00 0%
0.89 1.80 0% 0.00 0%
1.93 5.20 9% 2.38 2%
2.99 3.70 27% 1.86 11%
4.01 2.20 36% 1.40 17%
5.09 1.30 43% 1.02 22%
8.00 0.62 52% 0.64 32%
11.99 0.34 59% 0.38 39%
14.91 0.24 62% 0.26 43%
18.91 0.20 64% 0.16 46%
21.81 0.16 66% 0.12 48%
25.96 0.16 68% 0.10 50%
28.78 0.10 70% 0.08 51%
33.89 0.08 71% 0.06 52%
-48-
CA 02424714 2003-04-01
The results indicate that the rate and extent of gold extraction was improved
with the
addition of forced aeration.
While this invention has been described in conjunction with the specific
embodiments thereof, it is evident that many alternatives, modifications, and
variations will
be apparent to those skilled in the art. Accordingly, preferred embodiments of
the invention
as set forth herein are intended to be illustrative, not limiting. By way of
example, any
source of sulfur species with an oxidation state less than +2 may be used in
any of the above
process steps to convert polythionates to thiosulfate. The regeneration step
184 in Fig. 1 can
be performed in a variety of locations. For example, regeneration 184 can be
performed in
the recycle loop after CCD 172 and before grinding 108, between grinding 108
and
thickening 116, in the thickener 116 immediately before or during, leaching
132 and/or
between resin in pulp 164 and CCD 172. Fresh thiosulfate can also be added in
a number
of locations. For example, fresh thiosulfate can be added in any of the
locations referred to
previously for the regeneration step 184 and can be added after or during
regeneration 184
as noted above or in a separate tank or location. In Fig. 3, a lixiviant other
than thiosulfate,
such as cyanide, can be used in the atmospheric leach 300 with thiosulfate
being used in the
pressure leach 312. These and other changes may be made without departing from
the spirit
and scope of the present invention.
-49-