Note: Descriptions are shown in the official language in which they were submitted.
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
OXYGEN SCAVENGING COMPOSITIONS SUITABLE
FOR HEAT TRIGGERING
1. Field of the Invention
The present invention relates generally to the field of oxygen scavenging
materials.
More particularly, it concerns methods of initiating oxygen scavenging in
oxygen scavenging
compositions by heating the composition.
2. Description of Related Art
It is well known that limiting the exposure of oxygen-sensitive products to
oxygen
maintains and enhances the quality and shelf life of the product. For
instance, by limiting the
oxygen exposure of oxygen sensitive food products in a packaging system, the
quality of the
food product is maintained, and food spoilage is avoided. In addition, such
packaging also
keeps the product in inventory longer, thereby reducing costs incurred from
waste and
restocking. In the food packaging industry, several means for limiting oxygen
exposure have
already been developed, including modified atmosphere packaging (MAP), vacuum
packaging
and oxygen barrier film packaging.
Another means for limiting oxygen exposure involves incorporating an oxygen
scavenger into the packaging structure. Incorporation of a scavenger in the
package can
provide a uniform scavenging effect throughout the package. In addition, such
incorporation
can provide a means of intercepting and scavenging oxygen as it is passing
through the walls
of the package (herein referred to as an "active oxygen barrier"), thereby
maintaining the
lowest possible oxygen level throughout the package.
In many cases, however, the onset of oxygen scavenging in this system may not
occur for days
ZS or weeks. The delay before the onset of useful oxygen scavenging is
hereinafter referred to as
the induction period.
Much work has been done to minimize the induction period. Speer et al., United
States
Patent No. 5,211,875, and Ching et al., U.S. Patent No. 5,859,145, disclose
methods for
minimizing the induction period by initiating oxygen scavenging via exposure
to radiation.
Both teach methods that rely on radiation that comprises UV or visible light,
with wavelengths
that comprise UV radiation being preferred. Such UV initiation systems are
especially useful
for oxygen scavenging compositions that comprise non-aromatic polymers.
Although UV triggering permits control of when oxygen scavenging is initiated
, the
use of such methods that rely on UV radiation for induction of oxygen
scavenging has
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
limitations. First, oxygen-scavenging compositions can comprise materials that
are opaque to
UV radiation, thus limiting the ability of the UV radiation to activate oxygen
scavenging. For
example, oxygen scavenging compositions that comprise polymers like
polyethylene
terephthalate (PET) or polyethylene naphthalate (Pelf are difficult to trigger
using UV
initiation methods because these polymers absorb UV light. Furthermore; due to
the
geometric and physical constraints associated with UV radiation, it can be
difficult to achieve
uniform W treatment of preformed, angular oxygen scavenging packaging
articles.
Examples of such angular packaging articles are gable-top cartons,
parallelepiped cartons,
plastic bottles, and glass bottles, among other containers. Still further,
methods of initiation of
oxygen scavenging that rely on UV irradiation are most often associated with
oxygen
scavenging compositions that comprise photoinitiators. In general, such
photoinitiators are
relatively expensive. Furthermore, certain photoinitiators can actually have
undesirable traits
(e.g. cause yellowing) that must be taken into consideration when designing
compositions and
articles that incorporate them.
A need exists for the ready initiation of oxygen scavenging in oxygen
scavenging
compositions that is efficient regardless of whether UV opaque materials are
present in the
composition. It is also desirable to have methods of initiating oxygen
scavenging that are
effective with oxygen scavenging compositions that comprise aromatic polymers.
Improved
methods for uniform initiation of oxygen scavenging in angular packaging
articles would be
~0 useful. Furthermore, it would be beneficial to have oxygen scavenging
compositions and
packaging articles that do not require photoinitiators for efficient
initiation of oxygen
scavenging.
SUMMARY OF THE INVENTION
~5
The present invention is directed to methods of initiating oxygen scavenging
that rely
on heating an oxygen scavenging composition.
One aspect of the invention is directed to a method of initiating oxygen
scavenging by
an oxygen scavenging composition that comprises an oxidizable organic compound
and a
30 transition metal catalyst. The oxidizable organic compound has a polymeric
backbone with
cyclic olefmic moieties, and initiation of oxygen scavenging is accomplished
by heating the
oxygen scavenging composition. Preferably the polymeric backbone is ethylenic.
It is also
preferred that the cyclic olefinic moieties are pendant to the polymeric
backbone, though in
2
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
certain other embodiments of the present invention the polymeric backbone of
the oxidizable
organic compound can comprise at least one ring carbon of the cyclic organic
moiety.
Heating of the oxygen scavenging composition to an extent sufficient to
initiate oxygen
scavenging can take place during the process of forming the oxygen scavenging
composition
into a packaging article or film, or it can take place after the oxygen
scavenging composition
has been formed into a packaging article or film.
In addition to the oxidizable organic compound and the transition metal
catalyst, the
oxygen scavenging composition can further comprise a material selected from
antioxidants,
co-catalysts, additional polymers and pigments.
By using methods of initiating oxygen scavenging of the present invention,
initiation of
oxygen scavenging can be achieved without the use of UV irradiation. Exposing
oxygen
scavenging materials to a source of heat does not involve the same types of
physical
constraints as UV radiation, and heat can be used to initiate oxygen
scavenging in certain
oxygen scavenging compositions that can not be readily activated by UV
radiation (i.e.
compositions comprising aromatic polymers or UV opaque materials). For
example, certain
oxygen scavenging compositions that cannot be successfully triggered by UV
exposure
because they comprise UV opaque materials can be triggered by heat.
Furthermore, when the
oxygen scavenging composition is part of an angular packaging article, heat
initiation methods
can be used successfully for initiating oxygen scavenging, even though
initiation by UV
ZO exposure can be difficult in such angular packaging articles. In addition,
methods of the
present invention do not require photoinitiators or UV exposure, and in
certain embodiments
the heating step can be combined with a process of forming a packaging article
or a film from
the oxygen scavenging composition; thus methods of the present invention can
be less
expensive than UV initiation methods.
?5
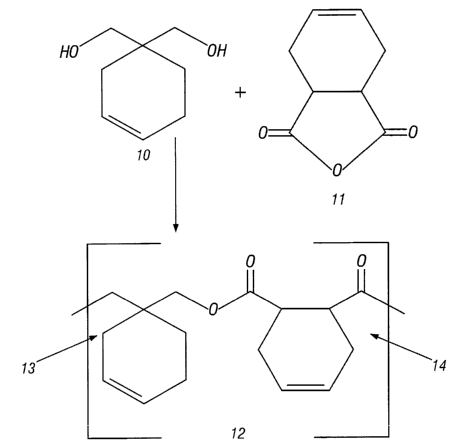
BRIEF DESCRIPTION OF DRAWING
Figure 1 is a schematic of a synthesis of an oxidizable organic compound that
can be
used in certain embodiments of the present invention.
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
One embodiment of the present invention is a method of initiating oxygen
scavenging
that comprises providing an oxygen scavenging composition that comprises an
oxidizable
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
organic compound and a transition metal catalyst, wherein the oxidizable
organic compound
comprises an polymeric backbone and at least one cyclic olefinic group; and
heating the
oxygen scavenging composition to an extent sufficient to initiate oxygen
scavenging.
Preferably the heating resulting in oxygen scavenging initiation is performed
for up to about
60 minutes, more preferably between about 0.5 minutes and 60 minutes. The
polymeric
backbone can be polyester, polyether, polythioether, polycarbonate, polyamide
or
polyethylene, or a combination of two or more thereof. Preferably the
polymeric backbone is
ethylenic. It is also preferred that the cyclic olefinic group be pendant to
the polymeric
backbone. However in other embodiments, the polymeric backbone can comprise at
least one
L 0 ring carbon of the cyclic olefinic group and thus the cyclic olefinic
group is not pendant.
Certain oxidizable organic compounds that comprise a cyclic olefinic group
that is not pendant
can be produced through polymerization of a diol with an anhydride, the cyclic
olefinic group
can be introduced into polymer through either the diol or the anhydride or
both.
One synthesis scheme for an oxidizable organic compound in which the cyclic
olefinic
LS moiety is not pendant is shown in Figure 1. This scheme involves reacting 3-
cyclohexene-1,1-
dimethanol 10 with cis-1,2,3,6-tetrahydrophthalic anhydride 11. The resulting
polymer 12 has
two different cyclic olefinic groups: one that is introduced through the
anhydride with two
ring carbons belonging to the polymeric backbone 14, and another that is
introduced through
the diol with one ring carbon belonging to the polymeric backbone 13.
a0 Oxygen scavenging compositions used in this method can be provided in
several
different forms. They can be provided as manufacturing intermediates, like
polymer blends or
preforms, or alternatively the oxygen scavenging compositions can be provided
as finished
articles. Finished articles can be provided in the form of a single or
multilayer film, in the
form of a film that is part of a packaging article, or in the form of a rigid,
semi-rigid, or
?5 flexible packaging article that has a single layer or multiple layers.
Alternatively the
composition can be provided as a component or layer of an oxygen scavenging
film or
packaging article.
In certain embodiments of the present invention, the oxygen scavenging
composition
consists essentially of an oxidizable organic compound and a transition metal
catalyst.
30 Preferably the oxygen scavenging composition does not comprise a
photoinitiator, though this
is not intended to imply that oxygen scavenging compositions that comprise
photoinitiators
could not be used in the methods of the present invention. Thus the preferred
oxygen
scavenging compositions of the present invention do not require W radiation
exposure to
initiate oxygen scavenging. In other preferred embodiments, the oxygen
scavenging
4
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
composition can further comprise at least one material selected from
antioxidants, co-catalysts,
additional polymers, and pigments.
Heat applied during the process of forming a packaging article from an oxygen
scavenging composition can be sufficient to cause heat triggering, or
additional heat can be
applied to a finished article. Examples of finished articles that can be heat
triggered
subsequent to their manufacture are packaging articles and films. Heat sources
for heat
triggering can be selected from those known in the art. For example, hot air
can be blown on
the oxygen scavenging composition or infraxed radiation can be used to heat
the oxygen
scavenging composition. The heat triggering can be performed under nitrogen or
in a low
oxygen atmosphere, in which the oxygen concentration is lower than in air.
Regardless of
when the oxygen scavenging composition is heated, during or after the
formation of a
packaging article or film, preferably the oxygen scavenging composition is
heated to a
temperature between about 75°C and about 300°C. If the heat
triggering is performed during
extrusion or co-extrusion of a film or an article, it is preferred that the
temperature be between
about 170°C and about 280°C. It should be noted that mixing
temperature and time must be
carefully controlled to obtain a blend of oxidizable organic compound and
transition metal
catalyst that is not triggered until processing that occurs after mixing. The
temperature of the
heating apparatus and the duration of exposure that is sufficient for heat
triggering will vary
depending on the oxygen scavenging composition, the oxidizable organic
compound, the
ZO presence and quantity of transition metal salts, antioxidants, and other
additives in the
composition, the design of the heating apparatus, the proximity of the
packaging article to the
heat source, the nature of heat transfer (typically convection), and other
parameters apparent to
one of ordinary skill in the art.
Oxygen scavenging compositions of the present invention comprise an oxidizable
JS organic compound that comprises a polymeric backbone and at least one
cyclic olefinic
pendant group. Preferably, the polymeric backbone is ethylenic and the cyclic
olefinic
pendant group has the structure (I):
q3
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
wherein q1, qa, q3, q4, and r are independently selected from hydrogen,
methyl, or ethyl; m is
-(CHZ)n , wherein n is an integer from 0 to 4, inclusive; and, when r is
hydrogen, at least one
of q1, qa, q3, and q4 is also hydrogen. One preferred oxidizable organic
compound is ethylene-
vinyl cyclohexene copolymer (EVCH). The oxidizable organic compound can
further
comprise a linking group linking the polymeric backbone and the cyclic
olefinic pendant
group, wherein the linking group is selected from:
-O-(CHR)ri ; -(C=O)-O-(CHR)ri ; -NH-(CHR)p ; -O-(C=O)-(CHR)ri ;
-(C=O)-NH-(CHR)n ; or -(C=O)-O-CHOH-CHa-O-;
wherein R is hydrogen, methyl, ethyl, propyl, or butyl; and n is an integer
from 1 to 12,
inclusive. Preferred oxidizable organic compounds that have a linking group
between their
cyclic olefinic pendant groups and their backbones are ethylene/methyl
acrylate/cyclohexenyl
methyl acrylate terpolymer (EMCM) and cyclohexenylmethyl acrylate (CHAA)
homopolymer.
The oxygen scavenging composition comprises a transition metal catalyst. The
transition metal catalyst accelerates the rate of oxygen scavenging. Though
not to be bound by
theory, useful catalysts include those which can readily interconvert between
at least two
oxidation states. See Sheldon, R. A.; I~ochi, J. I~.; "Metal-Catalyzed
Oxidations of Organic
Compounds" Academic Press, New York 1981.
Preferably, the catalyst is in the form of a salt, with the transition metal
selected from
the first, second or third transition series of the Periodic Table. Suitable
metals and their
oxidation states include, but are not limited to, manganese II or III, iron II
or III, cobalt II or
III, nickel II or III, copper I or II, rhodium II, III or IV, and ruthenium.
The oxidation state of
the metal when introduced need not necessarily be that of the active form. The
metal is
preferably iron, nickel, manganese, cobalt or copper; more preferably
manganese or cobalt;
and most preferably cobalt. Suitable counterions for the metal include, but
are not limited to,
chloride, acetate, stearate, palmitate, 2-ethylhexanoate, neodecanoate or
naphthenate.
Preferably, the salt, the transition metal, and the counterion are either on
the U.S. Food and
Drug Administration GRAS (generally regarded as safe) list, or exhibit
substantially no
migration to the product from the oxygen scavenging composition when it is
part of a
packaging article (i.e. less than 50 ppb in edible dietary intake (EDI)).
Particularly preferable
salts include cobalt oleate, cobalt stearate, and cobalt neodecanoate. The
metal salt can also be
6
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
an ionomer, in which case a polymeric counterion is employed. Such ionomers
are well
known in the art.
Typically, the amount of transition metal catalyst can range from 0.001 to 1%
(10 to
10,000 ppm) the oxidizable organic compound, based on the metal content only
(excluding
ligands, counterions, etc.). Preferably the transition metal catalyst is
blended directly with the
oxidizable organic compound. The transition metal catalyst can be a component
of a layer that
comprises the oxidizable organic compound (e.g. an oxygen scavenging layer)
or, less
preferably, it can be a component of a layer adjacent to such an oxygen
scavenging layer. In
the event the amount of transition metal catalyst is less than 1 %, it follows
that the oxidizable
organic compound, and any additional polymer or additives, will comprise
substantially all of
the scavenging composition or article, i.e. more than 99%.
Antioxidants can be used with oxygen scavenging compositions to control
scavenging
initiation. An antioxidant as defined herein is a material which inhibits
oxidative degradation
or cross-linking of polymers. Typically, antioxidants are added to facilitate
the processing of
polymeric materials or prolong their useful lifetime. In relation to this
invention, such
additives prolong the induction period for oxygen scavenging in the absence of
heat that
triggers oxygen scavenging. When it is desired to accelerate the commencement
of oxygen
scavenging by an oxygen scavenging composition, the composition is exposed to
heat that is
suited to triggering oxygen scavenging in that particular composition.
~0 Antioxidants such as 2,6-di(t-butyl)-4-methylphenol(BHT), 2,2'-methylene-
bis(6-t-
butyl-p-cresol), triphenylphosphite, tris-(nonylphenyl)phosphite,
dilaurylthiodipropionate,
vitamin E, and tetra [methylene (3,5-di-tert-butyl-4-
hydroxyhydrocinnamate)]methane are
suitable for use with this invention.
' The amount of an antioxidant, when present, can also have an effect on
oxygen
~5 scavenging. As mentioned earlier, such materials are usually present with
oxidizable organic
compounds or additional polymers to prevent oxidation or gelation of the
polymers.
Typically, they are present in about 0.005 to 0.05% by weight of the
oxidizable organic
compound. However, additional amounts of antioxidant can also be added if it
is desired to
tailor the induction period.
30 Oxygen scavenging compositions of the present invention can comprise one or
more
additional polymers. Such additional polymers can be structural polymers that
are
thermoplastic and render the oxygen scavenging composition more adaptable for
use in a
packaging article. Suitable structural polymers include, but are not limited
to, polyethylene,
low density polyethylene, very low density polyethylene, ultra-low density
polyethylene, high
7
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
density polyethylene, polyethylene terephthalate (PET), polyvinyl chloride,
and ethylene
copolymers such as ethylene-vinyl acetate, ethylene-alkyl (meth)acrylates,
ethylene-
(meth)acrylic acid, and ethylene-(meth)acrylic acid ionomers. In rigid
articles, such as
beverage containers, PET is often used. Blends of different structural
polymers can also be
used. However, the selection of the structural polymer largely depends on the
article to be
manufactured and the end use thereof. Such selection factors are well known in
the art. For
instance, the clarity, cleanliness, effectiveness as an oxygen scavenger,
burner properties,
mechanical properties, or texture of the article can be adversely affected by
a blend containing
a structural polymer that is incompatible with the oxidizable organic
compound.
LO Oxygen scavenging compositions can flxrther comprise at least one co-
catalyst to speed
heat triggering. A co-catalyst can be an amine or an amide. Preferred co-
catalysts are low
molecular weight polyethers having at least one amine terminal group,
polyamides, and
nylons, among others .
An oxygen scavenging composition can be provided in the form of a film or a
LS packaging article, including a component (integral or non-integral) of a
packaging article.
When provided in the form of a film, the film can be autonomous or can be an
integral or non-
integral paxt of a packaging article. Packaging articles suitable for
comprising oxygen
scavenging compositions can be flexible, rigid, semi-rigid or some combination
thereof.
Examples of oxygen scavenging packaging articles that can be used in the
present invention,
?0 include gable-top cartons, parallelepiped cartons, trays, cups, bags and
bottles among other
containers. Materials that can be used in making such containers include
paper, cardboard,
fiberboard, glass or plastic. Such containers can be used as juice cartons,
soft drink containers,
tofu containers, and beer bottles, among other uses. Rigid packaging articles
typically have
wall thicknesses in the range of 100 to 1000 micrometers. Typical flexible
packages that can
?5 be used in the present invention include those used to package food items
such as meats,
cheeses, fresh pastas, snack foods, or coffees, among others, and they
typically have
thicknesses of 5 to 250 micrometers. Furthermore, the oxygen scavenging
composition can be
provided in a npn-integral oxygen scavenging component or a layer of a
package, e.g., it can
be in the form of a coating, a bottle cap liner, an adhesive or a non-adhesive
sheet insert, a
30 gasket, a sealant, or a fibrous mat insert, among others. Oxygen scavenging
components can
also consist of a single layer or multiple layers. Generally, packaging
articles (flexible, rigid,
semi-rigid, or combinations of these) and packaging components comprising
oxygen
scavenging compositions can be used in packaging any product for which it is
desirable to
8
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
inhibit oxygen damage during storage, e.g. foods, beverages, cosmetics,
pharmaceuticals,
medical products, corrodible metals, or electronic devices, among others.
As stated above, the oxygen scavenging composition can be provided as an
article that
has a single layer or multiple layers. An oxygen scavenging layer comprises
the oxidizable
organic compound. When a packaging article or film comprises an oxygen
scavenging layer,
it can further comprises one or more additional layers, one or more of the
additional layers can
comprise an oxygen barrier layer, i.e. a layer having an oxygen transmission
rate equal to or
less than 100 cubic centimeters per square meter (cc/m2) per day per
atmosphere at room
temperature (about 25°C). Typical oxygen barriers comprise polyethylene
vinylalcohol),
polyacrylonitrile, polyvinyl chloride, poly(vinylidene dichloride),
polyethylene terephthalate
(PET), oriented PET, silica, foil, polyamides, or mixtures thereof.
Other additional layers of the oxygen scavenging packaging article can include
one or
more layers which are permeable to oxygen. For example, one embodiment of the
present
invention, a packaging article, can be comprised of the following layers, in
order starting from
the outside of the packaging article to the innermost layer (forming the
hollow interior) of the
packaging article, (i) a structural layer, (ii) an oxygen barrier layer, (iii)
an oxygen scavenging
layer comprising an oxidizable organic compound and a transition metal
catalyst, and
optionally, (iv) an oxygen-permeable seal or food-contact layer. Control of
the oxygen barrier
property of (ii) allows regulation of the scavenging life of the package by
limiting oxygen
ingress from the atmosphere to the scavenging layer (iii), and thus slows the
consumption of
oxygen scavenging capacity by atmospheric oxygen. Layer (iv) can improve the
heat-
sealability, clarity, or resistance to blocking of the multi-layer packaging
article. Also, control
of the oxygen permeability of layer (iv) allows alteration of the rate of
oxygen scavenging for
the overall structure independent of the composition of the scavenging
component (iii). Layer
,5 (iv) can permit oxygen from the headspace inside the package to pass to the
oxygen
scavenging layer (iii), while acting as a barrier to migration of the
components of the
scavenging layer, or by products of scavenging, into the package interior.
Further additional layers, such as adhesive layers, can also be used in a
multi-layer
packaging article or film. Compositions typically used for adhesive layers
include anhydride
functional polyolefins and other well-known adhesive layers.
Oxygen scavenging layers and oxygen scavenging packaging articles of the
present
invention can be made by a number of different methods known in the art. For
example, to
prepare oxygen scavenging layers, films and articles, the desired components
thereof can be
melt-blended at a temperature between about 150°C and about
300°C. Preferably the oxygen
9
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
scavenging composition is heat triggered after melt-blending, and this should
be considered in
choosing the melt-blend temperature and duration, along with other factors
known to those of
skill in the art. Alternatives to melt-blending, such as the use of a solvent
followed by
evaporation, can also be employed in preparing a polymer blend. The blending
can
immediately precede the formation of the finished article, film or preform or
precede the
formation of a feedstock or masterbatch for later use in the production of
finished packaging
articles or films. When the blended composition is used to make an oxygen
scavenging layer,
film or a packaging article, (co-)extrusion, solvent casting, inj ection
molding, stretch blow
molding, orientation, thermoforming, extrusion coating, coating and curing,
lamination, or
combinations thereof would typically follow the blending. Heat triggering can
be used during
these processes or after they have been implemented, and the temperature of
the oxygen
scavenging composition and apparatuses used during these processes should be
adjusted
accordingly.
The following examples are included to demonstrate preferred embodiments of
the
invention. It should be appreciated by those of skill in the art that the
techniques disclosed in
the examples which follow represent techniques discovered by the inventor to
function well in
the practice of the invention, and thus may be considered to constitute
preferred modes for its
practice. However, those of skill in the art should, in light of the present
disclosure, appreciate
that many changes may be made in the specific embodiments which are disclosed
and still
~0 obtain a like or similar result without departing from the spirit and scope
of the invention.
Example 1. 988.4 Parts by weight of polyethylene/ methyl acrylate/ cyclohexene-
methyl acrylate) (EMCM, manufactured by Chevron) was mixed at 250-270
°C with 23.2
~5 parts by weight of cobalt oleate toluene solution containing 50 wt.% cobalt
oleate on a twin
screw extruder (Werner & Pfleiderer ZSK-30). 150 ppm of Irgonox 1010
antioxidant were
also added to the blend. The extruder was equipped with two vacuum vents to
remove the
toluene during the mixing. The product was tumble dried at 40-50 °C for
4 hrs and then
vacuum packaged in aluminum bags.
Example 2. Commercial PET resin was dried at 170 °C for 4 hrs. A three-
layer film
having PET as outlayers (4-8 mil thickness) and thermal triggerable oxygen
scavenging
polymer (OSP) made in Example 1 as a core layer (2-4 mil thickness) was made
on a
Randcastle film extruder. The temperature for the feedblock, die and various
zones was set to
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
270-280 °C. The thick, three-layer film, after cooling, was reheated to
100 °C and then
biaxially stretched 2.5-3.0 times. The film was stored at room temperature for
2-3 weeks and
then tested on a Mocon OX-TRAM 2/20 for oxygen transmission rate at room
temperature and
20-30% relative humidity. The oxygen transmission test showed that no oxygen
permeated
through the film. As a control, an analogous test was performed on a single
layer PET film
prepared in the same manner as the three-layer film described above without an
OSP core
layer, and this test showed that the control film has an oxygen transmission
rate of 15-60 cc
OZ/m2/day.
Example 3. 105.5 Parts by weight of neat cobalt oleate was mixed with 894.5
parts by
weight of polyethylene/ methyl acrylate) (EMAC, manufactured by Chevron) at
220-260 °C
on a twin screw extruder (Werner & Pfleiderer ZSK-30). The cobalt containing
masterbatch
product was tumble dried at 40-50 °C for 4 hrs and then vacuum packaged
in aluminum bags.
Example 4. Commercial PET resin was dried at 170 °C for 4 hrs. A three-
layer film
having PET as outer layers (4-8 mil thickness) and a thermal triggerable
oxygen scavenging
polymer core layer (2-4 mil thickness) was made on a Randcastle film extruder.
The thermal
triggerable layer contained a blend from 90 parts by weight of polyethylene/
methyl acrylate/
cyclohexene-methyl acrylate) (EMCM, manufactured by Chevron) and 10 parts by
weight of
cobalt masterbatch made in Example 3. The temperature for the feedblock, die
and various
zones was set to 270-280 °C. The thick, three-layer film, after
cooling, was reheated to 100 °C
and then biaxially stretched 2.5-3.0 times. The film was stored at room
temperature for 2-3
weeks and then tested on a Mocon OX-TRAM 2/20 for oxygen transmission rate at
room
temperature and 20-30% relative humidity. The oxygen transmission test showed
that no
oxygen permeated through the film. As a control, an analogous test was
performed on a single
layer PET film prepared in the same manner as the three-layer film described
above without
an OSP core layer, and this test showed that the control film has an oxygen
transmission rate
of 15-60 cc 02/m2/day.
All of the compositions and methods disclosed and claimed herein can be made
and
executed without undue experimentation in light of the present disclosure.
While the
compositions and methods of this invention have been described in terms of
preferred
embodiments, it will be apparent to those of skill in the art that variations
can be applied to the
compositions and methods and in the steps or in the sequence of steps of the
method described
11
CA 02425579 2003-04-08
WO 02/33024 PCT/USO1/31434
herein without departing from the concept, spirit and scope of the invention.
More
specifically, it will be apparent that certain agents which are both
chemically related may be
substituted for the agents described herein while the same or similar results
would be
achieved. All such similar substitutes and modifications apparent to those
skilled in the art are
deemed to be within the spirit, scope and concept of the invention as defined
by the appended
claims.
12