Note: Descriptions are shown in the official language in which they were submitted.
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
DEVICE AND METHOD FOR THE CESSATION OF SMOKING
BACKGROUND OF THE INVENTION
Field of the Invention
The field of the present invention is pharmacology. More specifically, the
invention
relates to smoking cessation methods and aids that provide a non-irritating
form of nicotine to
the user as well as the oral and tactile stimulation of smoking.
Summary of the Related Art
Cigarette smoking is the leading cause of preventable disease and death in the
United
States. Each year, over 400,000 adults die from tobacco-related diseases (A
Report of the
Surgeon General, Rockville, MD: Public Health Service (2000)). In addition to
health risks
facing smokers themselves, the annoyance and risks of second-hand smoke are
receiving
increased attention. Although airlines, workplaces, and restaurants often ban
smoking, almost
88% of non-smokers have detectable blood levels of nicotine metabolites
(Morbidity and
Mortality WeeklX, November 5, 1999). Environmental tobacco smoke remains a
preventable
health hazard for smokers' co-workers and family members (A Report of the
Surgeon General,
Rockville, MD: Public Health Service (2000)).
Unfortunately, 48 million adults in the United States, 24.7% of the
population, continue
to smoke. Despite public health initiatives, smoking prevalence among adults
has not changed
significantly throughout the 1990's (Morbidity and Mortality Weekly, November
5, 1999).
Although most smokers in the United States wish to stop smoking, and over one
third of them
attempt to give up smoking each year, only about 2.5% succeed (A Resort of the
Surgeon
General, Rockville, MD: Public Health Service (2000)).
-1-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
Various techniques have been advanced to aid smoking cessation. The five major
pharmacotherapies for treating tobacco dependence are nicotine gum (see, e.g.,
U.S. Patent
No. 3,845,217); nicotine transdermal patch (see, e.g., U.S. Patent No.
4,915,950); nicotine nasal
spray (see, e.g., AU 664 41); nicotine inhaler (see, e.g., U.S. Patent Nos.
4,920,989 and
4,953,572); and sustained-release bupropion hydrochloride (see, e.g., Jorenby
et al., N. En~l.
J. Med., 340:685-91(1999)). Other examples of smoking cessation aids include
nicotine nose
drops (see, e.g., U.S. Patent No. 4,579,858); nicotine lozenges (see, e.g.,
U.S. Patent
Nos. 4,806,35 and 5,549,906); smoke-free cigarettes (see, e.g., U.S. Patent
Nos. 4,284,089,
4,676,259, 4,736,755, 4,813,437, 5,284,163, and 6,041,789); compositions
comprising nicotine
metabolites (see, e.g. U.S. Patent No. 5,869,505); and drinkable nicotine
solutions (see, e.g.,
WO 99155371).
Studies have shown that smokers using nicotine gum, patch, nasal spray,
inhaled nicotine,
or nicotine sublingual tablets are about 1.5 to 2 times more likely to stop
smoking than smokers
using no cessation aid, and evidence suggests that bupropion may be even more
effective (see,
Silagy et al., Cochrane Database Syst. Rev., No. 2, p.CD000146 (2000)).
However, each of the
existing smoking cessation aids has drawbacks, and none has proven fully
effective.
For example, nicotine gum and lozenges can cause high localized nicotine
concentration
in the mouth, which tastes unappealing, and the gum may be difficult to chew.
In addition,
chewing gum or eating lozenges may appear unprofessional. Nicotine patches may
irritate the
skin, and some smokers are dissatisfied with the lack of rapid nicotine
absorption from the patch.
Nicotine nasal sprays may irritate the nose and throat. More importantly, none
of these smoking
cessation aids simulates the tactile sensations or hand-to-mouth behaviors
that form an integral
part of the smoker's addiction.
-2-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
Studies have shown that sensory aspects affect smoking behavior and cigarette
cravings
as much as nicotine intake does. Indeed, smokers attempting to give up
cigarettes have
complained that, aside from experiencing nicotine withdrawal, they miss the
sensations and
hand-to-mouth behaviors associated with smoking (see, Rose, Ann. Rev. Med.,
47:493-507
(1996)). In a study prepared for the American Lung Association, 41 % of
smokers reported that
their most recent attempt to stop smoking was unsuccessful because they missed
having
something to hold or to do with their hands (Smoking Cessation Study,
Yankelovich Partners,
July 27, 1998). Cigarette smoking involves a hand-to-mouth ritual that may be
repeated over
70,000 times per year. Since smoking cessation requires giving up a highly
ingrained habitual
motion as well as giving up nicotine, an effective smoking cessation aid
should address the
behavioral components of smoking as well as providing nicotine replacement
therapy. A
smoking cessation aid should give the smoker the comfort of an oral and
tactile ritual, while at
the same time supplying nicotine. However, a smoking cessation device that is
too similar to a
conventional cigarette and provides oral sensations and tactile stimuli that
too closely mimic
I5 tobacco smoking may not be ideal. A smoker using such a device might find
it too easy to
relapse into cigarette smoking (see, e.g., Schneider et al., Addiction,
91:1293-1306 (1996)).
Thus, a smoking cessation aid that provides the synergistic combination of
nicotine plus oral and
tactile stimuli, while not too closely approximating a conventional tobacco
cigarette, seems most
desirable.
Among the existing smoking cessation aids, patches provide no oral or tactile
stimulus,
gum, lozenges, and drinkable solutions stimulate only the mouth, and nasal
sprays stimulate only
the airways. These aids lack the important synergistic combination of
nicotine, oral stimulation,
and hand-to-mouth behaviors that smokers desire.
-3-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
Some existing smoking cessation aids, such as nicotine inhalers and various
smoke-free
cigarettes, do provide nicotine as well as some behavioral aspects of smoking.
However, these
products have major drawbacks. For example, U.S. Pat. No. 4,953,572 (Rose et
al.) discloses a
nicotine aerosol spray designed to simulate the sensations of inhaling tobacco
smoke. The spray
may be administered through inhalation from a hand-held nebulizer. However,
the nicotine dose
of the spray, which is limited by the volatility of the inhaler's nicotine
base at room temperature,
is low compared to that of tobacco smoke, and thus may be insufficient for
many smokers.
Additionally, such a spray may irritate the oral cavity.
U.S. Patent No. 5,284,163 (I~nudsen et al.) discloses a smoke-free cigarette
substitute
comprising a nicotine granulate in a tubular sleeve. The end of the sleeve
contains a gum plug,
which is bitten off and held in the mouth as chewing gum. When a person draws
on the
cigarette, nicotine granulate is pulled into the mouth and can be chewed into
the gum, thus
dispensing nicotine into the oral cavity. This product may possess the
drawbacks of nicotine
chewing gum described above. Also, as with any smoke-free cigarette, using it
may approximate
the behavioral aspects of smoking so closely that smoking cessation becomes
more difficult.
U.S. Patent Nos. 4,284,089 and 4,813,437 (Ray) disclose non-pyrolytic devices
shaped
like ordinary cigarettes and containing porous polymer plugs holding volatile
liquid nicotine.
Drawing on the device delivers nicotine vapors to a person's lungs. However,
these devices are
unable to deliver sufficient uniform doses of nicotine. Additionally, the
vaporizable nicotine
tastes unpleasant and is unstable, such that the devices have a very short
shelf life.
U.S. Patent No. 6,041,789 (Bankert et al.) addresses some of these problems
with a non-
pyrolytic cigarette substitute that delivers a nicotine-simulating vapor
mixture with a cigarette-
like taste and aroma. Instead of vaporizable nicotine, the device contains a
volatile
-4-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
nicotinomimetic agonist and volatile palatability enhancing agents. However,
this device does
not actually deliver nicotine. Additionally, the cigarette-like structure,
taste, and aroma may
make the device so similar to a conventional cigarette that smokers using it
as a cessation aid are
likely to relapse.
Japanese Patent No. 02190178 (Akimichi et al.) discloses a smoke-free tobacco
"perfume
solution" that may be vaporized and administered through a "tubular flexible
casing." However,
the solution may present health risks due to the carcinogens and irntating
particulates contained
in tobacco. Additionally, as with other smoke-free cigarettes, using the
device may approximate
the actual tastes, smells, and motions of cigarette smoking so closely that
smoking cessation is
made more difficult.
LT.S. Patent No. 5,293,883 (Edwards) discloses a non-pyrolytic cigarette
containing two
tobacco-filled chambers, which house unburned and pre-burned tobacco, and a
mouth filter that
holds nicotine-filled ampules that release pure liquid nicotine into a
person's mouth when the
person breaks them open by biting or manually crushing the mouth filter. This
device provides
nicotine and a cigarette-like taste, but it may suffer from the drawbacks of
liquid nicotine
described above. Additionally, as discussed above, the device presents the
health hazards of
tobacco and may make smoking cessation more difficult by too closely
approximating actual
tobacco cigarettes.
Thus, a continuing need exists for new and improved smoking cessation aids to
help
reduce the ongoing public health hazards associated with cigarette smoking and
second-hand
smoke. Particularly needed is a smoking cessation aid that synergistically
provides an easily
controllable nicotine dose in a non-irritating form along with a delivery
device that supplies the
-5-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
oral and tactile stimulation that smokers crave, without too closely
approximating actual
cigarette smoking.
SUMMARY OF THE INVENTION
The present invention solves the foregoing problems by providing a smoking
cessation
aid that delivers nicotine in non-irntating solution form and offers the oral
and tactile stimulation
that smokers crave. Drawing a nicotine solution through the device and into
the mouth is similar
to the accustomed hand-to-mouth behavior of smoking. However, because the
nicotine is
delivered as a solution rather than as inhaled vapors, using the present
smoking cessation aid is
different enough from actual smoking that behavioral similarities should not
encourage smokers
to relapse. Nicotine 'delivered as a solution also does not irritate the oral
cavity as do inhaled
vapors from a nicotine spray.
Accordingly, in one aspect, the invention provides an oral nicotine delivery
device
comprising a tubular chamber, a nicotine granulate contained within the
chamber, and a retainer
in the chamber. The chamber has a first end suitable for taking in a liquid
from an external
source and a second end suitable for oral application of suction. The retainer
prevents release of
the nicotine granulate and liquid from the first end of the chamber. When oral
suction is applied
to the second end of the chamber, liquid enters the chamber from the external
source through the
first end of the chamber, and then the liquid and the nicotine are delivered
through the second
end of the chamber. As used herein, the term "comprises" means "includes, but
is not limited
to.
-6-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
In one embodiment, the tubular chamber of the device approximates the size and
shape of
a conventional cigarette. In another embodiment, the tubular chamber
approximates the size and
shape of a conventional drinking straw.
In some embodiments, the retainer is fixed proximal to the first end of the
chamber. In
other embodiments, the retainer is transportable toward the second end of the
chamber with the
nicotine granulate and the liquid when suction is applied to the second end of
the chamber.
In some embodiments, the nicotine granulate comprises coated particles of
powdered
nicotine. In particular embodiments, the nicotine particles are coated to
enhance palatability. In
some embodiments, the nicotine granulate comprises particles of powdered
nicotine that are
incorporated in spheres comprising at least one material selected from the
group consisting of
sugar, starch, acacia, sodium alginate, carbomer, cellulose, dextrotes, ethyl
cellulose, methyl
cellulose, and povidone.
In another embodiment, the tubular chamber contains from about 1 milligram to
about 40
milligrams of nicotine. In yet another embodiment, the tubular chamber
contains from about 4
milligrams to about 12 milligrams of nicotine. The nicotine is preferably
selected from the group
consisting of levo nicotine, dextro nicotine, racemic mixtures thereof, and
pharmaceutically
acceptable salt forms thereof.
In some embodiments, a solution of nicotine is formed when the liquid enters
the
chamber and contacts the nicotine. In particular embodiments, the solution is
a suspension.
In another aspect, the invention provides a method for reducing the incidence
of tobacco
smoking by a person. The method comprises orally administering nicotine to the
person using
the device according to the previous aspect of the invention. The person
applies oral suction to
the second end of the chamber, such that the liquid enters the chamber from
the external source
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
through the first end of the chamber. The liquid and the nicotine are then
delivered through the
second end of the chamber into the mouth of the person.
In one embodiment of the method, a single dose of nicotine administered to the
person is
from about 1 milligram to about 40 milligrams of nicotine. In a particular
embodiment, the dose
administered is from about 4 milligrams to about 12 milligrams of nicotine. In
a preferred
embodiment, the total daily dose of nicotine administered to the person is
from about 4
milligrams to about 144 milligrams of nicotine. In some embodiments, the blood
level of
nicotine in the person after administration of the nicotine is at least about
5 nanograms of
nicotine per 1 milliliter of blood. In some preferred embodiments, the blood
level of nicotine in
the person after administration of the nicotine is from about 10 nanograms to
about 50
nanograms of nicotine per 1 milliliter of blood.
In some embodiments of the method, a solution of nicotine is formed when the
liquid
enters the chamber and contacts the nicotine. In particular embodiments, the
solution has an
acidic pH.
In another aspect, the invention provides an, oral nicotine delivery device
comprising a
tubular chamber, a nicotine solution contained within the chamber, and a
retainer. The chamber
has a first end suitable for taking in a liquid or a gas from an external
source and a second end
suitable for oral application of suction. The retainer is for preventing
release of the nicotine
solution from the first end of the chamber. The nicotine solution is delivered
through the second
end of the chamber when oral suction is applied to the second end of the
chamber.
In some embodiments of the device of the invention, a liquid enters the
chamber from an
external source through the first end of the chamber when oral suction is
applied to the second
_g_
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
end of the chamber. The liquid and the nicotine solution are then delivered
from the device
through the second end of the chamber.
In other embodiments, a gas enters the chamber from an external source through
the first
end of the chamber when oral suction is applied to the second end of the
chamber. The nicotine
solution is then delivered from the device through the second end of the
chamber.
In some embodiments, the tubular chamber approximates the size and shape of a
conventional cigarette. In alternative embodiments, the tubular chamber
approximates the size
and shape of a conventional drinking straw.
In some preferred embodiments, the retainer is transportable toward the second
end of the
chamber with the nicotine solution when suction is applied to the second end
of the chamber.
In a particular embodiment, the nicotine solution is a nicotine suspension. In
a preferred
embodiment, the nicotine suspension comprises a nicotine granulate. In some
embodiments, the
nicotine granulate comprises coated particles of powdered nicotine, and in
some preferred
embodiments, the nicotine particles are coated to enhance palatability. In
some embodiments,
the nicotine granulate comprises particles of powdered nicotine that are
incorporated in spheres
comprising at least one material selected from the group consisting of sugar,
starch, acacia,
sodium alginate, carbomer, cellulose, dextrotes, ethyl cellulose, methyl
cellulose, and povidone.
In a preferred embodiment, the nicotine solution contains from about 1
milligram to
about 40 milligrams of nicotine. In a particular embodiment, the nicotine
solution contains from
about 4 milligrams to about 12 milligrams of nicotine. In a particular
embodiment, the chamber
contains from about 1 milliliter to about 5 milliliters of the nicotine
solution. The nicotine in the
nicotine solution is preferably selected from the group consisting of levo
nicotine, dextro
nicotine, racemic mixtures thereof, and pharmaceutically acceptable salts
thereof. In some
-9_
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
embodiments, the nicotine solution has an acidic pH. In some embodiments, the
nicotine
solution further comprises a flavoring.
In yet another aspect, the invention provides a method for reducing the
incidence of
tobacco smoking by a person. The method comprises orally administering
nicotine to the person
using the device according to the previous aspect of the invention. The person
applies oral
suction to the second end of the chamber, such that the nicotine solution is
delivered from the
chamber through the second end of the chamber into the mouth of the person.
In some preferred embodiments of this aspect of the invention, a single dose
of nicotine
administered to the person is from about 1 milligram to about 40 milligrams of
nicotine. In a
particular embodiment, the single dose administered is from about 4 milligrams
to about 12
milligrams of nicotine. In some preferred embodiments, the total daily dose of
nicotine
administered to the person is from about 4 milligrams to about 144 milligrams
of nicotine. In
some embodiments, the blood level of nicotine in the person after
administration of the nicotine
solution is at least about 5 nanograms of nicotine per 1 milliliter of blood.
In some preferred
embodiments, the blood level of nicotine in the person after administration of
the nicotine
solution is from about 10 nanograms to about 50 nanograms of nicotine per 1
milliliter of blood.
In a preferred embodiment, the nicotine solution is a nicotine suspension.
-10-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
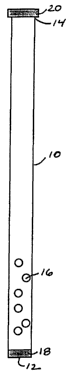
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a diagrammatic representation of a frontal view of a nicotine
delivery device of
the invention that approximates the shape and size of a conventional drinking
straw.
FIG. 2 is a diagrammatic representation of a frontal view of a nicotine
delivery device of
the invention that approximates the shape and size of a conventional
cigarette.
-11-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
DETAILED DESCRIPTION
The issued U.S. patents, published patent applications, and references that
are cited
herein are hereby incorporated by reference to the same extent as if each were
specifically and
individually indicated to be incorporated by reference. Any inconsistency
between these
publications and the present disclosure shall be resolved in favor of the
present disclosure.
The present invention provides smoking cessation methods and smoking cessation
aids
that deliver a nicotine solution through a tubular device to a user. The
advantage of delivering
nicotine through a tubular device is that the oral and tactile sensations
experienced by the user
approximate the ingrained hand-to-mouth behaviors associated with smoking.
Thus, the device
of the present invention offers the smoker oral and manual focus, which helps
to alleviate
cigarette cravings by providing an object for the smoker to manipulate and
chew.
As shown in FIG. 1, the device of the invention may approximate a conventional
drinking
straw in size and shape. Alternatively, as shown in FIG. 2, the device may
approximate a
conventional cigarette in size and shape. The term "approximate" means that
the device
comprises an elongated tubular chamber 10, with a first end 12 adapted for
drawing in air or
liquid from an external source and a second end 14 adapted for the application
of oral suction,
and that the dimensions of the device are similar to the dimensions of a
conventional drinking
straw or a conventional cigarette. In some preferred embodiments, as shown in
FIG. l, the
device approximates a conventional drinking straw in shape and size. The
chamber 10 is
preferably from about 13 to about 20 centimeters long, with a diameter of from
about 4 to about
S millimeters. More preferably, the chamber 10 is from about 15 to about 17
centimeters long,
with a diameter of from about 6 to about 7 millimeters. Alternatively, as
shown in FIG. 2, the
-12-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
device approximates a conventional cigarette in shape and size. In these
embodiments, the
chamber 10 is preferably from about 7 to about 11 centimeters long, with a
diameter of from
about 7 to about 10 millimeters. More preferably, the chamber 10 is from about
8 to about 10
centimeters long, with a diameter of about 8 millimeters. Examples of the
tubular delivery
devices of the present invention include, without limitation, devices such as
those disclosed in
U.S. Patent No. 5,718,681 (Manning) and U.S. Patent Nos. 5,780,058, 5,985,324,
5,989,590,
6,024,721, and 6,106,845 (along et al.).
Materials for making the tubular chamber 10 of the present invention may
include,
without limitation, paper, plastic such as propyleneistyrene copolymers,
polypropylene, high
density polyethylene, low density polyethylene, ethylene vinyl acetate
copolymer, and the like.
The tubular chamber 10 of the device of the present invention contains
nicotine 16 that
will be delivered to the user. The nicotine 16 may be in any useful form, such
as levo nicotine,
dextro nicotine, or a racemic mixture thereof. Pharmaceutically acceptable
salt forms of nicotine
are also suitable for use in the present invention. Nonlimiting examples of
such suitable salt
forms of nicotine include the dihydrochloride, sulfate, bitartrate,
salicylate, hydrogen tartrate,
and hemisulfate salts. Nicotine and its salt forms are commercially available,
for example, from
Sigma-Aldrich, St. Louis, MO.
Preferably, the nicotine 16 is a granulate. The term "granulate" means that
the nicotine is
in the form of a particulate such as grains or beads. The size of the grains
or beads preferably is
small enough not to be felt as separate particles in the mouth, preferably
less than about 200
micrometers in diameter.
In some preferred embodiments, the nicotine granulate comprises particles of
powdered
nicotine which have been coated according to standard techniques known in the
art (see, e.g.,
-13-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
Deasy, Crit Rev. Ther. Dru~Carrier S std, 8:39-89 (1991)). The term "powdered"
means
composed of fine particles. Glatt Air Technique (Ramsay, NJ) and Particle and
Coating
Technologies, Inc. (St. Louis, MO) provide particle coating services. Suitable
coatings include,
without limitation, hydroxy propyl cellulose, sugar, starch, polymer, resin,
gum, wax, and fat.
The diameter of the nicotine particles before coating is preferably less than
about 200
micrometers, more preferably from about 20 to about 100 micrometers, and still
more preferably
from about 40 to about 60 micrometers.
In some preferred embodiments, the nicotine granulate comprises particles of
powdered
nicotine that are incorporated into microspheres, which may be coated as
described above.
Suitable materials for the microspheres include, without limitation, sugar,
starch, acacia, sodium
alginate, carbomer, cellulose, dextrotes, ethyl cellulose, methyl cellulose,
povidone, and mixtures
thereof. For example, the microspheres may comprise a degradable composition.
The
microspheres have a diameter of between about 300 micrometers and about 1000
micrometers,
preferably between about 500 micrometers and about 600 micrometers. U.S.
Patent No.
5,939,100 (Albrechtson et al.) discloses further examples of nicotine
microspheres.
The coating on the nicotine particles or microspheres preferably is designed
to mask the
taste of the nicotine, and thus enhance palatability. Most preferably, the
coating is formulated
such that most, but not all, of the nicotine taste is masked. The advantage of
such a formulation
is that enough of the nicotine taste is masked that the nicotine particles are
palatable, but enough
nicotine taste remains that the user is aware that nicotine is being ingested.
Thus, in some
preferred embodiments, the formulation may comprise both coated and uncoated
particles. The
coating on the particles or microspheres also may be designed to achieve other
functions,
including, but not limited to, preventing clumping and preventing water
absorption.
-14-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
Preferably, a single dose of nicotine 16 from the device of the present
invention contains
a therapeutically effective amount of nicotine. The term "single dose"
means~the quantity of
nicotine 16 provided in the chamber 10 and delivered to the user upon
application of oral suction
to the second end 14 of the chamber 10.' The term "therapeutically effective
amount" means an
amount sufficient to reduce a smoker's need for nicotine from burnt tobacco.
This amount can
be determined by the user's physician. An advantage of the smoking cessation
devices of the
invention is their ability to deliver a large dose of nicotine when necessary,
or a smaller dose of
nicotine when appropriate. Preferably, from about 1 milligram to about 40
milligrams of
nicotine are provided by the device. Useful amounts include from about 1
milligram to about 5
milligrams, from about 4 milligrams to about 12 milligrams, from about 4
milligrams to about 20
milligrams, from about 5 milligrams to about 15 milligrams, from about 5
milligrams to about 20
milligrams, and from about 20 milligrams to about 40 milligrams of nicotine
provided by the
device in a single dose. A smoker attempting to stop smoking may use the
device of the present
invention from about 1 to about 12 times per day, preferably from about 5 to
about 12 times per
day, such that a total daily dose of from about 1 milligram to about 480
milligrams, preferably
from about 5 milligrams to about 480 milligrams, of nicotine is delivered
depending on
perceived need. Useful total daily doses include from about 1 milligram to
about 60 milligrams,
from about 4 milligrams to about 144 milligrams, from about 4 milligrams to
about 240
milligrams, from about 5 milligrams to about 180 milligrams, from about 5
milligrams to about
240 milligrams, and from about 240 milligrams to about 480 milligrams of
nicotine. The term
"total daily dose" means the quantity of nicotine delivered to the user during
a 24-hour period.
Such a dosing regimen preferably provides a blood level of nicotine of at
least about 5
nanograms of nicotine per milliliter of blood, with a maximum peak of about 60
nanograms of
-15-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
nicotine per milliliter of blood. Preferably, the blood level of nicotine in
the user of the device is
from about 10 nanograms to about 50 nanograms of nicotine per milliliter of
blood. More
preferably, the user's blood level of nicotine is about 20 nanograms of
nicotine per milliliter of
blood.
Blood levels of nicotine preferably may be measured by gas chromatography with
nitrogen phosphorous detection as described, for example, by Jacob et al., J.
Chromatography,
222: 61-70 (1981). The dosing frequency may be adjusted so as to achieve a
steady state
concentration of nicotine in the blood. The steady state blood levels of
nicotine preferably fall
within the preferred blood level ranges described above. The quantities of
nicotine described
above are sufficient to deliver a therapeutically effective amount of nicotine
into the user's
metabolism, even after first-pass absorption by the liver. It will be
understood by those skilled in
the art that the preferred doses and blood levels of nicotine will vary
according to the
preferences, metabolism, and former smoking habits of the individual user of
the smoking
cessation aid of the present invention.
In one device encompassed by the present invention, the nicotine granulate is
delivered to
the user as a nicotine solution. The term "solution," as used herein, refers
to a liquid into which
the nicotine is dissolved, or a suspension or emulsion of nicotine in a
liquid. Delivery of nicotine
as a solution provides several advantages. Swallowing the solution provides
the oral stimulation
that smokers crave. However, the solution does not irritate the oral cavity as
does nicotine gum.
Additionally, nicotine in solution form is absorbed in the body more slowly
than nicotine from a
nasal spray. Such slow absorption allows for less frequent dosing and provides
less potential for
abuse. Finally, delivery of liquid along with the nicotine prevents high local
concentrations of
nicotine, which may result in cramping, for example, when a nicotine capsule
is swallowed.
-16-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
In some devices, the first end 12 of the chamber 10 is placed in contact with
an external
source of liquid and the user applies oral suction to the second end 14 of the
chamber 10.
According to one method of the invention, a liquid is drawn into the first end
12 of the chamber
from the external source. The liquid then forms a suspension of the nicotine
16 granulate as it
5 is drawn through the chamber 10, and the suspension is delivered through the
second end 14 of
the chamber 10 into the user's mouth. Some or all of the nicotine 16 granulate
also may dissolve
in the liquid, such that the nicotine 16 is delivered as a solution. WO
99/55371 (Westman et al.)
discloses non-limiting nicotine solutions whose properties may be desirable
for use with the
present invention.
10 The external source of liquid to be drawn into the tubular chamber 10 of a
device of the
invention is preferably a beverage. Most preferably, the beverage contains a
flavoring, which
helps to make the nicotine solution palatable. This is less crucial where the
nicotine taste has
already been masked by coating the particles of the nicotine granulate. The
term "palatable"
means that the taste of the solution is tolerable to the user. The beverage
may comprise, but is
not limited to, coffee, soda, sugar, fruit juice, carbonation, or ethyl
alcohol, such as wine, beer, or
hard liquor. Preferably, the beverage does not contain solids such as pulp. To
be palatable, the
nicotine solution preferably has an acidic pH. The term "acidic pH" means a pH
of less than
about 6.9. More preferably, the nicotine solution has a pH of less than about
5.5. Most
preferably, the nicotine solution has a pH from about 2.0 to about 4Ø
Flavorings such as coffee,
alcohol, and fruit juice may be used to regulate the pH of the nicotine
solution. For example,
lime juice, cranberry juice, grapefruit juice, orange juice, tonic water,
soda, and wine have pH's
from about 2.0 to about 4.0, and beer, seltzer water, coffee, and have pH's
less than about 6.9.
-17-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
An advantage of the smoking cessation devices of the present invention is
their
compatibility with beverages that are acidic, as the majority of popular
beverages are. Existing
smoking cessation devices, such as nicotine gum and nicotine inhalers, may not
be used in
combination with acidic beverages because an alkaline environment is required
for buccal
absorption of the nicotine delivered by such devices. Acidic beverages must be
avoided for a
period of about one hour surrounding each use of a smoking cessation device
relying on buccal
absorption of nicotine. Thus, smokers attempting to use such devices to give
up smoking must
also avoid consuming a majority of beverages much of the time. In contrast,
smokers using
nicotine delivery devices of the invention, through which nicotine is orally
ingested, may
consume acidic beverages whenever they choose.
The tubular chamber 10 of the device of the present invention may comprise two
or more
lumens. The multiple lumens provide a plurality of smaller cross-sectional
flow paths, which
help to optimize the flow velocity and flow volume of the liquid from the
external source, thus
assuring rapid, uniform, and complete delivery of the nicotine 16 contained
within the chamber
10. The nicotine 16 may be contained in one of the lumens, or in multiple
lumens. The multiple
lumens may be of identical size or of different sizes.
Alternatively, the chamber 10 of the device of the present invention contains
a pre-
formulated solution of nicotine 16 such as, for example, a nicotine
suspension. About 1 milliliter
to about 40 milliliters of nicotine solution may be provided in the chamber.
Preferably, from
about 1 milliliter to about 10 milliliters, most preferably from about 1
milliliter to about 5
milliliters, of solution are provided. The preferred single doses of nicotine,
total daily doses of
nicotine, and nicotine blood levels are as described above for the nicotine
granulate.
-18-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
In another method of the invention, the user applies oral suction to the
second end 14 of
the chamber 10 of the device, such that a gas such as air is drawn through the
first end 12 of the
chamber 10 into the device. As the air is drawn into the chamber 10, the
nicotine 16 solution
contained in the chamber 10 is delivered through the second end 14 of the
chamber 10 into the
user's mouth.
Alternatively, the user places the first end 12 of the chamber 10 in contact
with an
external source of a liquid and applies oral suction to the second end 14 of
the chamber 10, such
that the liquid is drawn into the first end 12 of the chamber 10 from the
external source. As the
liquid is drawn through the chamber 10, it is mixed with the solution of
nicotine 16 contained in
the chamber 10, and the mixture is delivered through the second end 14 of the
chamber 10 into
the user's mouth. The external source of liquid is preferably a beverage, as
described above for
the device in which the chamber 10 contains a nicotine 16 granulate. Most
preferably, the
beverage comprises flavorings and pH ranges as described above.
The nicotine 16 solution contained within the chamber 10 may itself contain
the
flavorings described above: Preferably, the solution is pre-formulated to fall
within the
aforementioned pH levels through use of an acid such as, for example, carbonic
acid, citric acid,
acetic acid, tartaric acid, malefic acid, ascorbic acid, adipic acid, or
combinations thereof. Such
pre-formulation to optimize flavoring and pH is especially desirable if the
user will be drawing
air, rather than liquid, through the first end of the chamber, such that the
nicotine 16 solution
contained in the chamber 10 is delivered without any liquid from an external
source that might
enhance palatability.
The chamber 10 of the device of the present invention further contains a
retainer 18 for
preventing release of nicotine 16 from the first end 12 of the chamber 10. The
term "retainer"
-19-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
refers to a disc, float, plug, or other restriction that blocks the diameter
of the chamber 10 such
that the nicotine 16 contained within the chamber 10 cannot be released from
the first end 12 of
the chamber 10. The retainer 18 allows gas or liquid from an external source
to enter the first
end 12 of the chamber 10, but prevents release of nicotine 16 from the first
end 12 of the
chamber 10. The retainer 18 also discourages release from the first end 12 of
the chamber 10 of
liquid drawn into the chamber 10 from an external source or contained within
the chamber 10 as
part of a pre-formulated solution of nicotine 16.
The retainer 18 may be fixed proximal to the first end 12 of the chamber 10.
"Proximal
to" the first end 12 of the chamber 10 means toward or at the first end 12 of
the chamber 10.
Alternatively, the retainer 18 may be transportable toward the second end 14
of the chamber 10.
For example, the retainer 18 is transported toward the second end 14 of the
chamber 10 as the
user applies oral suction to the second end 14 of the chamber 10. Gas or
liquid from an external
source is drawn through the first end 12 of the chamber 10 into the device,
and the nicotine 16
solution in the chamber 10 is delivered into the user's mouth. As it is drawn
toward the second
end 14 of the chamber 10 behind the nicotine 16 solution, the transportable
retainer 18 assures
that all of the nicotine 16 in the chamber 10 is cleanly delivered through the
second end 14 of the
chamber 10 into the user's mouth. The second end 14 of the chamber 10 is
constructed such that
the retainer 18 itself is blocked from entering the user's mouth. For example,
the diameter at the
second end 14 of the chamber 10 maybe smaller than the diameter elsewhere in
the chamber 10
and smaller than the diameter of the retainer 18, such that the retainer 18
may pass from the first
end 12 of the chamber 10 to the second end 14 of the chamber 10, but cannot
pass through the
second end 14 of the chamber 10 into the user's mouth. The diameter at the
second end 14 of the
chamber 10 may be made smaller than the diameter elsewhere in the chamber 10
by a crimping
-20-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
of the chamber 10, a series of dimples in the circumference of the chamber 10,
or a continuous
indentation in the circumference of the chamber 10 at the second end 14 of the
chamber 10. A
transportable retainer 18 is particularly useful when the device is prefilled
with liquid, i.e.,
contains a pre-formulated solution of nicotine 16.
The retainer 18 may comprise a restriction and a plug. The term "restriction"
means that
the diameter near the first end 12 of the chamber 10 is smaller than the
diameter elsewhere in the
chamber 10 and smaller than the diameter of the plug, such that the plug is
contained within the
chamber 10 and prevents release of nicotine 16 through the first end 12 of the
chamber 10. The
restriction may result from, for example, a crimping of the chamber 10, a
series of dimples in the
circumference of the chamber 10, or a continuous indentation in the
circumference of the
chamber 10 near the first end 12 of the chamber 10.
The retainer 18 may alternatively comprise a particle barrier with apertures
or slits that
allow liquid to pass through the barrier when oral suction is applied to the
second end 14 of the
chamber 10. A cap may be placed over the first end 12 of the chamber 10 prior
to use to avoid
any possible loss of nicotine 16 or entry of contaminants through the particle
barrier apertures or
slits. The apertures must be small enough that nicotine 16 cannot pass through
them and be
released from the first end 12 of the chamber 10. The slits are easier to
manufacture and seal the
chamber 10 more completely prior to use than the apertures do. Alternatively,
the barrier is
made from a material such as fine mesh or porous paper that will retain the
nicotine 16 in the
chamber 10 but requires no barrier apertures or slits to allow liquid to be
drawn into the chamber
10. For ease in construction, the barrier is preferably cone-shaped and
located all the way at the
first end 12 of the chamber 10.
-21-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
Alternatively, the retainer 18 comprises a one-way plug or valve. The plug or
valve seals
the first end 12 of the chamber 10 at atmospheric pressure. However, when
suction is applied to
the second end 14 of the chamber 10, the plug is deformed and permits liquid
from an external
source to flow around the plug and into the chamber 10 through the first end
12 of the chamber
10. The plug preferably has a density of less than one, such that it is drawn
toward the second
end 14 of the chamber 10 when suction is applied to the second end 14 of the
chamber 10 and the
nicotine 16 contained within the chamber 10 is delivered into the mouth of the
user. When
suction is removed from the second end 14 of the chamber 10, the plug relaxes,
seals the
chamber 10, and remains stationary. Transportation of the plug all the way to
the second end 14
of the chamber 10 indicates that the entire dose of nicotine 16 contained
within the chamber 10
has been delivered.
The retainer 18 alternatively comprises a cylindrical body with at least one
protrusion on
its exterior surface. Liquid from an external source is drawn through or
around the retainer 18
and into the chamber 10, but the protrusions prevent nicotine 16 solution from
leaking through or
around the sides of the retainer 18 and out of the first end 12 of the chamber
10. The protrusions
are fins, ridges, or rings which act as a seal for the chamber 10 and also
create friction or drag
between the retainer 18 and the chamber 10, which allows time for the liquid
from the external
source to mix with the nicotine 16 after passing through or around the
retainer 18.
The retainer 18 of the present invention may be made from, for example,
thermoplastic
materials or low or high density foam materials such as, without limitation,
ethylene vinyl
acetate copolymers, polyolefins such as polyethylene, polypropylene, and the
like, or closed cell
foam. Alternatively, the retainer 18 may comprise, for example, a compressible
plug of bonded
fibers. The fibers may be polymeric fibers, such as polyolefin fibers with or
without a polyester
-22-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
core, polyester, cellulose acetate, nylon, felt, or cotton. The uncompressed
fiber plug preferably
has a diameter slightly larger than the diameter of the chamber 10. Thus,
after insertion in the
chamber 10, the plug seals and prevents the release of nicotine 16 from the
chamber 10, but still
allows liquid to be drawn into the chamber 10 when oral suction is applied to
the second end 14
of the chamber 10.
In some embodiments, the chamber 10 of the device of the present invention
preferably
also contains a removable end cap 20 or seal located at the second end 14 of
the chamber 10.
The removable end cap 20 or seal prevents the nicotine 16 granulate or pre-
formulated nicotine
16 solution contained within the chamber 10 from being released through the
second end 14 of
the chamber 10 during shipping and storage of the device. Before using the
device, the user
removes the end cap 20 or seal from the second end 14 of the chamber 10 so
that the nicotine 16
granulate or pre-formulated nicotine 16 solution contained within the chamber
10 may be
delivered through the second end 14 of the chamber 10 into the user's mouth.
Materials for
making the end cap 20 or seal of the present invention may include, without
limitation, paper,
foil, plastic such as propylene/styrene copolymers, polypropylene, high
density polyethylene,
low density polyethylene, ethylene vinyl acetate copolymer, and the like.
In some instances, the device, itself, may comprise a flavoring, such that it
tastes pleasant
for chewing as, before, or after the nicotine dose has been delivered. The
flavoring may be, for
example, sugar, cinnamon, spearmint, peppermint, wintergreen, bubble gum,
fruit, chocolate,
anise, nut, coffee, tobacco, or combinations thereof. Preferably, the
flavoring is selected from
the group consisting of spearmint, peppermint, wintergreen, and cinnamon.
The device as described in detail above provides the novel synergistic
combination of
oral and tactile stimulation along with a controlled dose of nicotine in non-
irritating solution
-23-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
form. Smokers attempting to stop smoking may use the device as an aid that can
satisfy nicotine
needs and at the same time prevent cigarette cravings by providing a
substitute for the hand-to-
mouth behavioral component of smoking. .The synergistic combination of the
present invention
provides an important new method for reducing the incidence of tobacco smoking
in smokers
who appreciate the health risks of smoking and wish to stop.
The following nonlimiting examples further illustrate certain preferred
embodiments
of the present invention:
EXAMPLE 1: Safety and Pharmacokinetics Study
1. Device
Nicotine~is administered orally using a straw-like smoking cessation device of
the
invention as shown in FIG. 1. The chamber 10 is a plastic drinking straw. The
retainer 18 is a
filter at the first end 12 of the straw. The user places the first end 12 of
the device in a glass of
apple juice and applies oral suction to the second end 14 of the device, such
that the juice and
nicotine 16 are delivered into the user's mouth.
The nicotine 16 in the device is in the form of coated sugar spheres of
nicotine
bitartrate having a diameter of approximately 500 micrometers. The nicotine
spheres are
prepared by spraying nicotine bitartrate dihydrate with a binder onto sugar
spheres and applying
a film coating. Using a Wurster processing unit, particles are suspended in
air using a controlled
airflow system and a coating suspension is added at a controlled rate via a
pneumatically
2,0 atomized nozzle. As atomized droplets of the coating solution contact the
particles, they spread
and coalesce on the particle surface. Excess moisture from the applied liquid
evaporates in the
apparatus, leaving a coated dry substance. Mixing of the coating suspension
occurs throughout
the manufacturing process to ensure uniformity of the suspension.
-24-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
2. Administration
On day one, following overnight abstinence from smoking, healthy adult smokers
who
wish to give up smoking receive a single dose of nicotine or placebo. Smokers
are assigned to
each dosage group in a double blind, randomized manner, as is well known in
the art. The
following data are collected before dosing, every thirty minutes for the first
two hours after
dosing, and every hour for the following six hours: plasma levels of nicotine
and cotinine, vital
signs, adverse events, and patient's assessment of cigarette cravings.
Baseline and endpoint
clinical chemistry, hematology, urinalysis, and ECG are also monitored.
On day eight, again following overnight abstinence from smoking, the same
subjects
receive the same dose of nicotine or placebo. The dose is repeated
periodically throughout the
day. The following data are collected as described above before the first
dosing and one hour
after each dose is administered: plasma levels of nicotine and cotinine, vital
signs, adverse
events, and patient's assessment of cigarette cravings. Baseline and endpoint
clinical chemistry,
hematology, urinalysis, and ECG are also monitored. The data are used to
evaluate the safety
and pharmacokinetics of nicotine delivery by the smoking cessation device.
EXAMPLE 2: Efficacy Study
In a double blind study, three hundred healthy adult smokers who wish to give
up
smoking use the smoking cessation device as described in Example 1 to deliver
nicotine or
placebo over a ten week period. Each device contains 5-10 mg of nicotine or
placebo. Subjects
are instructed to use the device as needed for smoking urges, but not to
exceed 12 doses per day.
Patients are concurrently enrolled in a counseling program to provide
behavioral support for
smoking cessation. Smoking abstinence data are collected during the last eight
weeks of
treatment, serving as the primary endpoint. Abstinence is defined as four
weeks of continuous
-25-
CA 02427283 2003-04-28
WO 02/38208 PCT/USO1/51239
abstinence from smoking during the s,ludy period. Subjects have weekly clinic
visits for
monitoring plasma levels of nicotine and cotinine, vital signs, adverse
events, and patient's
assessment of cigarette cravings. Baseline and endpoint clinical chemistry,
hematology,
urinalysis, and ECG are also monitored.
EQUIVALENTS
While the foregoing invention has been described in some detail for purposes
of clarity
and understanding, it will be appreciated by one skilled in the art from a
reading of this
disclosure that various changes in form and detail can be made without
departing from the scope
of the invention and the attached claims.
-26-