Note: Descriptions are shown in the official language in which they were submitted.
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
ALKYNYL AMIDES AND THEIR THERAPEUTIC APPLICATIONS
TECHNICAL FIELD
The present invention relates to alkynyl amides, to the use of these compounds
to treat
seizures, migraine, and psychiatric disorders, and to the preparation of these
compounds.
BACKGROUND OF THE INVENTION
Epilepsy affects roughly 1% of the world's population. Among the drugs
employed for
control of epileptic seizures is valproic acid. Valproic acid (also referred
to as VPA, valproate,
di-n-propylacetic acid, DPA, 2-propylvaleric acid or 2-propylpentanoic acid)
is an effective
anticonvulsant.
Migraine is defined as a periodically occurring vascular headache
characterized by pain
in the head (usually unilateral), nausea and vomiting, photophobia,
phonophobia, vertigo and
general weakness. Migraine is the most common type of vascular headache and
affects as much
as 15% of the world's population. Of the different types of migraines,
classical migraine and
common migraine are the two most prevalent. The major difference between the
two types of
migraines is that classical migraines are preceded by the appearance of
neurological symptoms
before an attack whereas common migraines are not preceded by such symptoms.
Migraine is
caused by intermittent brain dysfunction. However, the precise
pathophysiological mechanisms
involved are not understood. The head pain is believed to involve blood vessel
dilation.
Analgesics are often used to treat infrequent and mild migraines. Analgesics
reduce the
pain of a migraine and, in the case of aspirin, also discourage platelet
aggregation. However, for
moderate to severe migraines, stronger medications such as ergotamine and
valproic acid are
often necessary. Ergotamine tartrate is a vasoconstrictor which counteracts
the painful dilation
stage of the headache. When taken during the early stages of an attack,
ergotamine tartrate helps
to relieve the classic and common migraine symptoms. Valproic acid has been
shown to be
effective in prevention of migraine, however, its mechanism of anti-migraine
action is unclear.
It is believed that valproic acid increases brain gamma-aminobutyric acid
(GABA) levels and in
doing so may activate the GABA receptor and suppresses migraine-related
events.
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
A number of psychiatric disorders may be treated with valproic acid, see:
Loscher W., ed.
Valproate, Basel Switzerland: Birkhauser Verlag, 1999; Post R.M., In Tasman
A., Goldfinger,
S.M., Kaufmann, C.A., eds. Review of psychiatry, volume 9, Washington DC:
American
Psychiatric Press, 1990, 170-202; and Jensen R., Brinck T., Olesen J.,
Neurology 44, 1994, 647.
Such psychiatric disorders include: i) Mood Disorders; ii) Anxiety Disorders;
iii) Attention-
Deficit and Disruptive Behavior Disorders; iv) Behavioral Disturbances
associated with
dementia; v) Behavioral Disturbances associated with autism; vi)
Schizophrenia; vii) Impulse
Control Disorders; viii) Personality Disorders; and ix) Substance-related
Disorders.
Mood Disorders include, but are not limited to, Depressive Disorders and
Bipolar
Disorders such as Manic episodes and Mixed episodes. Symptoms associated with
Mood
Disorders include, but are not limited to, depression, elevated, expansive or
irritable mood,
insomnia/hypersomnia, agitation and distractability or impulsivity.
Anxiety Disorders include, but are not limited to, Panic Disorder,
Posttraumatic Stress
Disorder and Generalized Anxiety Disorder. Symptoms associated with Anxiety
Disorders
include, but are not limited to, anxious mood, panic attacks, irritability,
outbursts of anger and
exaggerated startle response.
Attention-Deficit and Disruptive Behavior Disorders include, but are not
limited to,
Attention-Deficit/Hyperactivity Disorder Hyperactive-Impulsive Type, Conduct
Disorder,
Oppositional Defiant Disorder and Disruptive Behavior Disorder. Symptoms
associated with
Attention-Deficit and Disruptive Behavior Disorders include, but are not
limited to, impulsivity,
aggression, anger and loss of temper.
Symptoms associated with Behavioral Disturbances for both dementia and autism
include, but are not limited to, verbal and physical agitation and aggression.
Symptoms associated with Substance-related Disorders, include but are not
limited to,
withdrawal and dependence.
Symptoms associated with schizophrenia, include but are not limited to,
positive
symptoms, negative symptoms and agitation.
Impulse Control Disorders include, but are not limited to, Intermittent
Explosive
Disorder. Symptoms associated with Impulse Control Disorders, include but are
not limited to,
verbal or physical aggressive impulses.
2
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Personality Disorders include, but are not limited to, Borderline Personality
Disorder.
Symptoms associated with Personality Disorders, include but are not limited
to, mood lability,
irritability, agitation and aggression.
The symptoms listed for these psychiatric disorders are not an exhaustive
description of
the diagnostic category or disorder, but merely reflect some of the symptoms
that may improve
when treated with valproic acid. For Example, valproic acid may be used to
treat general
agitation or aggression not necessarily associated with any particular
psychiatric disorder.
Further, excitatory neurotransmitters such as glutamate and aspartate, as well
as a variety
of voltage-gated ion channels, are thought to play a central role in mediating
cell death after a
variety of cerebral insults including, but not limited to, ischemia, trauma,
seizure and
hypoglycemia. Many studies have shown that compounds or therapeutic strategies
that decrease
excitatory neurotransmission, for example, glutamate antagonists, ion channel
blockers,
anticonvulsants, and the like, elicit a neuroprotective effect in animal
models of cerebral insults.
Recently, VPA has been shown to increase the levels of the neuroprotective
protein B
cell lymphoma protein-2 (bcl-2) in frontal cortex, findings that may have
implications for the
long-term treatment of various neurodegenerative disorders (Chen G., et al,
Journal of
Neurochemistry, 1999, 879-882).
Neuropathic pain affects a significant number of patients suffering from
disorders of the
brain or spinal cord, such as stroke, trauma, multiple sclerosis, and
diabetes. Several known
anticonvulsant compounds are efficacious in various analgesia models relevant
to identifying
therapeutic candidates for treating neuropathic pain (Lloyd and Morselli, in
Psychopharmacology: The Third Generation of Progress, Raven Press, 1987). The
use of
anticonvulsants like valproate to treat various pain states has been
documented extensively
(Swendlow, J. Clin. Neuropharmacol., 7, 1984, 51-82).
Restlessness syndrome denotes a somatic (non-mental) restlessness
characterized by
involuntary movement of the limbs, as well as by a sense of physical (rather
than mental)
agitation, which is independent of mood and, hence, is distinguished from
restlessness per se, see
(Sachdev et al., Austral. New Zealand J. Psychiatry 30, 1996, 38-53.
The genus of restlessness syndromes, inclusive of numerous indications, can be
observed
in association with many organic and non-organic psychiatric illnesses. For
example, drug-
3
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
induced restlessness (tardive, chronic, and withdrawal akathisias), such as
drug-induced
extrapyramidal symptoms, is one of the most common side effects of
neuroleptic, drug therapy.
Also within the restlessness syndrome rubric are the so-called "restless leg
syndrome" and
"sleep-related periodic leg movements," pathologies that can be associated
with head and/or
spinal cord trauma and with lesions of the spinal cord. Idiopathic restless
leg syndrome follows
an autosomal dominant inheritance, with a variable clinical expression of
symptoms.
Diminished GABAergic neurotransmission is implicated in the neurochemical
basis of
restlessness syndromes. Consistent with this notion, for instance, is the
efficacy of drugs such as
baclofen, valproate, gabapentin and the benzodiazepines, in the treatment of
restless leg
syndrome, an important indication, see (O'Keefe, Arch. Intern. Med.
156,1996,243-48; Danek
et al., in Neurological Disorders: Course and Treatment, pages 819-23,
Academic Press, 1996;
and Mellick and Mellick, Neurology 45(suppl), 1995, 285-86).
A relationship has been reported between epilepsy, migraine and psychiatric
disorders
(Post and Silberstein, Neurology, 1994, 44(suppl 7), S37-S47). Although the
three disorders are
distinct, they all are paroxysmal dysregulations of the nervous system that
partially overlap in
their pharmacology. Some drugs, such as valproic acid, are effective in
treating all three
syndromes, see: (Post R.M., In Tasman A., Goldfinger, S.M., Kaufinann, C. A.,
eds. Review of
psychiatry, volume 9, Washington DC: American Psychiatric Press, 1990, 170-
202; and Jensen
R., Brinck T., Olesen J., Neurology 44, 1994, 647), suggesting the presence of
shared underlying
pathophysiology, while other drugs are effective for treating one syndrome.
For example, (3-
adrenergic adrenoreceptor blockers which are effective against migraine are
not useful for
treating the other two syndromes and may even exasperate depression.
There has been considerable effort to discover analogs of valproic acid that
are equally
effective. One study has demonstrated that the valproic acid analog 2-propyl-4-
hexynoic acid is
an effective antiepileptic with a longer duration of activity.
Alpha-fluorinated valproic acid, 2-fluoro-2-propylpentanoic acid, has also
been reported
(Ph.D. thesis of Wei Tang, University of British Columbia, 1996; W. Tang et
al., Chem. Res.
Toxicol. (1995), 8(5), 671-682; M. Jurima-Romet et al., Toxicology (1996),
112(1), 69-85; W.
Tang and F. Abbott, Drug Metab. Dispos. (1997), 25(2), 219-227). The
anticonvulsant activity
and pharmacokinetics of this compound were studied, and its pharmaceutical
potential was
4
W002/36546 CA 02427560 2009-09-16 PCT/USOI/45704
speculated upon (F. Abbott, W. Tang, 7. Palaty, J. Pharmacol. Exp. Ther.
(1997), 282, 1163-
1172). The compound was reported to be less potent than VPA, and the
hepatotoxic, sedative, or
teratogenic properties were not disclosed.
SUMMARY OF THE INVENTION
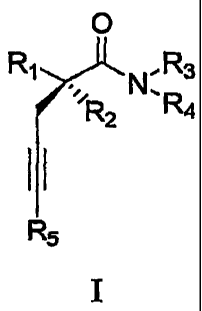
In its principle embodiment, the present invention discloses compounds of
formula (I):
0
Rr"R N,'3
erein
wh
Rl is selected from the group consisting of alkyl and haloalkyl;
R2 is selected from the group consisting of hydrogen, alkyl and fluorine;
R3 is selected from the group consisting of hydrogen, alkyl and
(NRARB)carbonylallyl
wherein RA and Fa are each independently selected from the group consisting of
hydrogen and
alkyl;
R4 is selected from the group consisting of hydrogen and alkyl; and
R5 is selected from the group consisting of hydrogen,and alkyl.
An aspect of the invention is to provide a compound of formula (1)
0
RI N:R3
IrR2 R4
lRS
x,
wherein
RI is alkyl or haloalkyl;
R2 is hydrogen, alkyl or fluorine;
R3 is (NRARB)carbonylalkyl wherein RA and RB are each independently hydrogen
or
alkyl;
R4 is hydrogen or alkyl; and
CA 02427560 2009-09-16
R5 is hydrogen or alkyl,
wherein the alkyl is C 1-C 10 alkyl, haloalkyl is C 1-C 10 haloalkyl, and
(NRARB)carbonylalkyl is C 1-C 10 (NRARB)carbonylalkyl.
The above identified compound of formula I, the compound can be (2R)-2-propyl-
4-
hexynamide.
In the above identified compound of formula I, Rl can be alkyl wherein said
alkyl is propyl;
and R5 can be alkyl wherein said alkyl is methyl. The above identified
compound can be
(2R)-N-(2-amino-2-oxoethyl)-2-propyl-4-hexynamide; (2R)-N-[(1 S)-1-
(aminocarbonyl)-2-
methylpropyl]-2-propyl-4-hexynamide; (2R)-N-[(1 S)-1-(aminocarbonyl)-3-
methylbutyl]-2-
propyl-4-hexynamide; (2R)-N-[(lS)-2-amino-l-methyl-2-oxoethyl]-2-propyl-4-
hexynamide;
or (2R)-N-[(1R)-2-amino-l-methyl-2-oxoethyl]-2-propyl-4-hexynamide. R2 can be
fluorine or
R2 can be alkyl.
Another aspect of the invention is to provide a use of a therapeutically
effective amount of a
compound of formula (I) of any one of the above identified compounds for the
following:
(a) the treatment of seizures in a mammal in need of such treatment, (b) the
treatment of
epilepsy in a mammal in need of such treatment, (c) the treatment of migraine
in a mammal
in need of such treatment, (d) the treatment of psychiatric disorders wherein
the psychiatric
disorders are Mood Disorders, Anxiety Disorders, Attention-Deficit and
Disruptive Behavior
Disorders, Behavioral Disturbances associated with dementia, Substance Abuse-
related
Disorders, Schizophrenia, Impulse Control Disorders, Personality Disorders or
Behavioral
Disturbances associated with autism in a mammal in need of such treatment, (e)
the treatment
of neuropathic pain in a mammal in need of such treatment, (f) the treatment
of restlessness
syndrome in a mammal in need of such treatment, and (g) to provide
neuroprotection to a
mammal suffering from a cerebral insult. For the above uses of the
therapeutically effective
amount of a compound, the compound can be (2R)-2-propyl-4-hexynamide. For the
above
uses of the therapeutically effective amount of a compound, the compound can
be
(2R)-N-(2-amino-2-oxoethyl)-2-propyl-4-hexynamide;
(2R)-N-[(1 S)-1-(aminocarbonyl)-2-methylpropyl]-2-propyl-4-hexynamide;
(2R)-N-[(1S)-1-(aminocarbonyl)-3-methylbutyl]-2-propyl-4-hexynamide;
5a
CA 02427560 2009-09-16
(2R)-N-[(1S)-2-amino-l-methyl-2-oxoethyl]-2-propyl-4-hexynamide; or
(2R)-N-[(1 R)-2-amino- l -methyl-2-oxoethyl]-2-propyl-4-hexynamide.
Another aspect of the invention is to provide a pharmaceutical composition
comprising a
therapeutically effective amount of the compounds of formula I identified
above in
combination with a pharmaceutically acceptable carrier.
Another aspect of the invention is to provide a use of the compound of formula
(I) of any
one of the compounds above, in the manufacture of a medicament for the
following: (a) the
treatment of seizures, (b) the treatment of epilepsy, (c) treatment of
migraine, (d) treatment of
psychiatric disorders wherein the psychiatric disorders are Mood Disorders,
Anxiety
Disorders, Attention-Deficit and Disruptive Behavior Disorders, Behavioral
Disturbances
associated with dementia, Substance Abuse-related Disorders, Schizophrenia,
Impulse
Control Disorders, Personality Disorders or Behavioral Disturbances associated
with autism,
(e) treatment of neuropathic pain, (f) treatment of restlessness syndrome, and
(g) to provide
neuroprotection to a mammal suffering from a cerebral insult. For the above
uses of the
compounds of formula I for the manufacture of a medicament, the compound can
be (2R)-2-
propyl-4-hexynamide. For the above uses of the compounds of formula I for the
manufacture
of a medicament, the compound can be
(2R)-N-(2-amino-2-oxoethyl)-2-propyl-4-hexynamide;
(2R)-N-[(1 S)-1-(aminocarbonyl)-2-methylpropyl]-2-propyl-4-hexynamide;
(2R)-N- [(1S)-1-(aminocarbonyl)-3 -methylbutyl]-2-propyl-4-hexynamide;
(2R)-N-[(1 S)-2-amino- l -methyl-2-oxoethyl]-2-propyl-4-hexynamide; or
(2R)-N-[(1 R)-2-amino- l -methyl-2-oxoethyl]-2-propyl-4-hexynamide.
DETAILED DESCRIPTION OF THE INVENTION
In its principle embodiment, the present invention discloses compounds of
formula (I):
O
R, rR2" N, RS
5b
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
wherein
R1 is selected from the group consisting of alkyl and haloalkyl;
R2 is selected from the group consisting of hydrogen, alkyl and fluorine;
R3 is selected from the group consisting of hydrogen, alkyl and
(NRARB)carbonylalkyl
wherein RA and RB are each independently selected from the group consisting of
hydrogen and
alkyl;
R4 is selected from the group consisting of hydrogen and alkyl; and
R5 is' selected from the group consisting of hydrogen and alkyl.
In a preferred embodiment, compounds of the present invention have formula (I)
wherein
R2 is hydrogen; R1, R3, R4 and R5 are as defined in formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R2 is alkyl; R1, R3, R4 and R5 areas defined in formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R2 is fluorine; R1, R3, R4 and R5 are as defined in formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R1 is propyl; R5 is is methyl; and R2, R3 and R4 are as defined in
formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R1 is propyl; R3 is-hydrogen; R5 is methyl; and R2 and R4 are as
defined in formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R1 is propyl; R3 is (NRARB)carbonylalkyl; R5 is methyl; and R2 and R4
are as defined in
formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R1 is propyl; R2 is hydrogen; R3 is (NRARB)carbonylalkyl; R5 is
methyl; and R4 is as
defined in formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R1 is propyl; R2 is fluorine; R3 is (NRARB)carbonylalkyl; R5 is
methyl; and R4 is as
defined in formula (I).
In another preferred embodiment, compounds of the present invention have
formula (I)
wherein R1 is propyl; R2 is alkyl; R3 is (NRARB)carbonylalkyl; R5 is methyl;
and R4 is as defined
in formula (I).
6
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Another embodiment of the present invention relates to pharmaceutical
compositions
comprising a therapeutically effective amount of a compound of formula (I) in
combination with
a pharmaceutically acceptable carrier.
Another embodiment of the present invention relates to a method of treating
epilepsy,
migraine or a psychiatric disorder selected from Mood Disorders, Anxiety
Disorders, Attention-
Deficit and Disruptive Behavior Disorders, Behavioral Disturbances associated
with dementia,
Substance Abuse-related Disorders, Schizophrenia, Impulse Control Disorders,
Personality
Disorders and Behavioral Disturbances associated with autism in a mammal
comprising
administering to a mamml in need of such treatment a therapeutically effective
amount of a
compound of formula (I) in combination with a pharmaceutically acceptable
carrier.
A preferred embodiment of the present invention relates to a method of
treating epilepsy,
migraine or a psychiatric disorder selected from Mood Disorders, Anxiety
Disorders, Attention-
Deficit and Disruptive Behavior Disorders, Behavioral Disturbances associated
with dementia,
Substance Abuse-related Disorders, Schizophrenia, Impulse Control Disorders,
Personality
Disorders and Behavioral Disturbances associated with autism in a mammal
comprising
administering to a mamml in need of such treatment a therapeutically effective
amount of (2R)-
2-propyl-4-hexynamide in combination with a pharmaceutically acceptable
carrier.
Another preferred embodiment of the present invention relates to a method of
treating
epilepsy, migraine or a psychiatric disorder selected from Mood Disorders,
Anxiety Disorders,
Attention-Deficit and Disruptive Behavior Disorders, Behavioral Disturbances
associated with
dementia, Substance Abuse-related Disorders, Schizophrenia, Impulse Control
Disorders,
Personality Disorders and Behavioral Disturbances associated with autism in a
mammal
comprising administering to a mammal in need of such treatment a
therapeutically effective
amount of a compound selected from (2R)-N-(2-amino-2-oxoethyl)-2-propyl-4-
hexynamide;
(2R)-N-[(1S)-1-(aminocarbonyl)-2-methylpropyl]-2-propyl-4-hexynamide; (2R)-N-
[(1S)-1-
(aminocarbonyl)-3 -methylbutyl]-2-propyl-4-hexynamide; (2R)-N-[(1 S)-2-amino-l
-methyl-2-
oxoethyl]-2-propyl-4-hexynamide; and (2R)-N-[(1R)-2-amino-l-methyl-2-oxoethyl]-
2-propyl-4-
hexynamide in combination with a pharmaceutically acceptable carrier.
Definition of Terms
7
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
As used throughout this specification and the appended claims, the following
terms have
the following meanings:
The term "alkyl," as used herein, refers to a straight or branched chain
hydrocarbon
containing from 1 to 10 carbon atoms. Representative examples of alkyl
include, but are not
limited to, methyl, ethyl, propyl, butyl, pentyl, hexyl, 3-methylhexyl, 2,2-
dimethylpentyl, 2,3-
dimethylpentyl, heptyl, octyl, nonyl, and decyl.
The term "halo" or "halogen," as used herein, refers to -Cl, -Br, -I or -F.
The term "haloalkyl," as used herein, refers to at least one halogen, as
defined herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of haloalkyl include, but are not limited to,
trifluoromethyl,
pentafluoroethyl, 3,3,3-trifluoropropyl, 2,2-difluoropropyl, 2,3-
difluoropropyl, 2,2,3,3,3,-
pentafluoropropyl and 2-chloro-3-fluoropentyl.
The term "carbonyl," as used herein, refers to a -C(O)- group.
The term "-NRARB," as used herein, refers to two groups, RA and RB, which are
appended
to the parent molecular moiety through a nitrogen atom. RA and RB are each
independently
selected from hydrogen and alkyl.
The term "(NRARB)carbonyl," as used herein, refers to a -NRARB group, as
defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined herein.
The term "(NRARB)carbonylalkyl," as used herein, refers to a (NRARB)carbonyl
group, as
defined herein, appended to the parent molecular moiety through an alkyl
group, as defined
herein. Representative examples of (NRARB)carbonylalkyl include, but are not
limited to, 2-
amino-2-oxoethyl, (1 S)-2-amino-1 -methyl-2-oxoethyl, (1R)-2-amino-l-methyl-2-
oxoethyl, (1 S)-
1 -(aminocarbonyl)-2-methylpropyl, (1 R)-1-(aminocarbonyl)-2-methylpropyl, (1
S)-1-
(aminocarbonyl)-3 -methylbutyl, (1R)-1-(aminocarbonyl)-3-methylbutyl, and
(1S,2R)-4-amino-2-
ethyl- l-isobutyl-4-oxobutyl.
Preferred compounds of formula (I) include,
(2R)-N-(2-amino-2-oxoethyl)-2-propyl-4-hexynamide;
(2R)-N-[(1 S)-1-(aminocarbonyl)-2-methylpropyl]-2-propyl-4-hexynamide;
(2R)-N-[(1 S)-1-(aminocarbonyl)-3-methylbutyl]-2-propyl-4-hexynamide;
(2R)-N-[(1S)-2-amino-l-methyl-2-oxoethyl]-2-propyl-4-hexynamide; and
8
CA 02427560 2008-11-05
WO 02/36546 PCT/US01/45704
(2R)-N-[ (1 R)-2-amino-l-methyl-2-oxoethyl]-2-propyl-4-hexynamide.
A most preferred compound is (2R)-2-propyl-4-hexynamide.
Abbreviations
Abbreviations which have been used in the descriptions of the schemes and the
examples
that follow are: DMF for N,N-dimethylformamide; DMSO for dimethylsulfoxide;
HRMS for
High Resolution Mass Spectroscopy; and LDA for lithium diisopropylamine.
Preparation of Compounds of The Invention
The compounds and processes of the present invention will be better understood
in
connection with the following synthetic schemes and methods which illustrate a
means by which
the compounds of the invention can be prepared.
The compounds of this invention can be prepared by a variety of synthetic
routes.
Representative procedures are shown in Schemes 1-3.
Scheme I
1) catalytic DMF
0 CI(CO)2CI 0
R, OH CH2CI2 R~ NR
3R4
2) NHR3R4
RS (1) (2) R5 (3)
Alkynyl amides of general formula (3), wherein R1, R3, R4 and R5 are as
defined in
formula (I), may be prepared as described in Scheme 1. Alkynyl acids of
general formula (1),
prepared according to US patent No. 5786380, may be
treated with oxalyl chloride and catalytic DMF in a solvent such as methylene
chloride to
provide crude acid chlorides. The crude acid chlorides may then be treated
with an amine of
general formula (2) in a solvent such as methylene chloride to provide alkynyl
amides of general
formula of general formula (3).
9
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Scheme 2
O HCI/CH3OH O LDA 0
R, OH fRj OCH3 R2X R, OCH3
R2=alkyl Rz
X=CI, Br or I
R5 (1) R5 (5) R5 (6)
MOH 0 chiral O O
M=Na, K, or Li R1 OH auxiliary R1 OH R1y 0H
6
R R2 + (8) R2
() R2
water/ethanol
(7) 5 R5
1) catalytic DMF 0
CI(CO)2CI
CH2CI2 R, NR3R4
(8) 2) NR3R4 R2
(2) R5 (9)
Alkynyl amides of general formula (9), wherein R1, R3, R4 and R5 are as
defined in
formula (I) and R2 is alkyl, may be prepared as described in Scheme 2. Alkynyl
acids of general
5 formula (1) may be treated with HCl in methanol to provide alkynyl esters of
general formula
(5). Alkynyl esters of general formula (5) may be treated with lithium
diisopropylamine and an
alkyl halide to provide esters of general formula (6). Esters of general
formula (6) may be
treated with aqueous base such as potassium hydroxide in a solvent such as
water/ethanol to
provide acids of general formula (7). Acids of general formula (7) may be
separated into
individual enantiomers using chiral auxiliaries which is well known in the art
such as the use of
chiral oxazolidinones (Evans et al. J. Am. Chem. soc., (1982) 104 1737-1739)
to provide chiral
acids of general formula (8). An example of separation of similar acids is
described in US Patent
No. 5786380. Chiral acids of general formula (8) may be processed as described
in Scheme 1 to
provide alkynyl amides of general formula (9).
CA 02427560 2008-11-05
WO 02/36546 PCT/USOI/45704
Scheme 3
0 LDA O
Ri OCH3 (CF3SO2)2NF R1 F OCI-{3
j
R5 (5) R5 (11)
0
Scheme 2 R, NR3R4
(11)
F
R5 (12)
Alkynyl amides of general formula (12), wherein R1, R3, R4 and R5 are as
defined in
formula (I), may be prepared as described in Scheme 3. Esters of general
formula (5) may be
treated with lithium diisopropylamide and an electrophilic source of fluorine
such as N-fluoro-N-
[(trifluoromethyl)sulfonyl]trifluoromethanesulfonamide, other reagents known
to be electrophilic
sources of fluorine may be purchased commercially or prepared as described in
(Chem. Rev.,
(1996) vol. 96 No.5; and Clark, J.H., Wails, D., Bastock, T.W., Aromatic
Fluorination, CRC
Press, 1996), to provide fluorinated ester of general formula (11).
Fluorinated esters of general
formula (11) may be processed as described in Scheme 2 to provide alkynyl
amides of general
.formula (12).
The compounds and processes of the present invention will be better understood
by
reference to the following examples, which are intended as an illustration of
and not a limitation
upon the scope of the invention:
Example 1
(2R)-N-(2-amino-2-oxoethyl)-2-propyl-4-hexynamide
(2R)-2-Propyl-4-hexynoic acid (2.0 g, 13 mmol, prepared according to US patent
No.
5786380) in dichloromethane (20 mL) was treated with 2 drops of N,N-
dimethylformamide and
freshly distilled oxalyl chloride (1.25 equivalents) at 0 C. The mixture was
allowed to warm to
ambient temperature. After stirring for 3 hours at ambient temperature, the
solvents were
removed under reduced pressure to provide crude acid chloride. The crude acid
chloride was
dissolved in dichloromethane (15 mL) and added dropwise to a solution of 2-
aminoacetamide
11
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
hydrochloride (14 mmol) and triethylamine (3.5 mL) in water at 0 C. The
mixture was allowed
to warm to ambient temperature. After stirring for 3 hours at ambient
temperature, the mixture
was cooled to about 5 C and treated with IN HCl until pH=2. The resulting
white solid was
collected by filtration, dried and recrystallized from ethyl acetate to
provide the title compound
(70% yield).
'H NMR (300 MHz, DMSO-d6) 6 8.1 (t, J=5.7 Hz, 1H), 7.0 (s, 2H), 3.6 (m, 2H),
2.4-2.1 (m,
3H), 1.7 (t, 2 Hz, 3H), 1.4 (m, 2H), 1.2 (m, 2H) 0.8 (t, J=7.5 Hz, 3H); MS
(ESI+) m/z 211
(M+H)+; HRMS: calculated 211.1447, found 211.1442.
Example 2
(2R)-N-[(1 S)- I -(aminocarbonyl-2-methylpropyl]-2-propyl-4-hexynamide
(2R)-2-Propyl-4-hexynoic acid and (2S)-2-amino-3-methylbutanamide were
processed as
described in Example 1.
'H NMR (300 MHz, DMSO-d6) 6 7.7 (d, J=9 Hz, 1H), 7.2 (s, 1H), 6.9 (s, 1H), 4.1
(m, 1H), 3.0
(m, I H), 2.5 (m, I H), 2.1 (m, 2H), 1.9 (m, I H), 1.6 (s, 3H), 1.3 (m, 2H),
1.2 (m, 2H), 0.8 (m,
9H); MS (CI) m/z 253 (M+H)*.
Example 3
(2R [(1 S)-1 -(aminocarbonyl-3-methylbutyll-2-propel-4-hexynamide
(2R)-2-Propyl-4-hexynoic acid and (2S)-2-amino-4-methylpentanamide were
processed
as described in Example 1.
'H NMR (300 MHz, DMS O-d6) 6 7.9 (d, J=9 Hz, I H), 7.1 (s, I H), 6.9 (s, I H),
4.2 (m, 1), 3.0 (m,
1H), 2.4 (m, 1H), 2.1 (m, 2 H), 1.6 (s, 3H), 1.6-1.1 (m, 6H), 0.8 (m, 9H); MS
(Cl:) m/z 253
(M+H)+.
Example 4
(2R)-N-[(1 S)-2-amino-l-methyl-2-oxoethyl]-2ropyl-4-hexynamide
(2R)-2-Propyl-4-hexynoic acid and (2S)-2-aminopropanamide were processed as
described in Example 1.
12
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
'H NMR (300 MHz, DMSO-d6) S 7.9 (d, J=8 Hz, 1H), 7.3 (s, 1H), 6.9 (s, 1H), 4.2
(m, 1H), 3.0
(m, I H), 2.4 (m, 1H), 2.2 (m, 2H), 1.7 (m, I H), 1.4 (s, 3H), 1.2 (m, 2H),
0.8 (t, J=7 Hz, 3H); MS
(Cl) m/z 225 (M+H)+.
Example 5
(2R)[(1R)-2-amino-l-methyl-2-oxoeth, l-2-propyl-4-hexvnamide
(2R)-2-Propyl-4-hexynoic acid and (2R)-2-aminopropanamide were processed as
described in Example 1.
1H NMR (300 MHz, DMSO-d6) S 8.0 (d, J=8 Hz, IH), 7.0 (s, 2H), 4.2 (m, 1H), 3.1
(q, J=6 Hz,
1H), 2.4 (m, 1H), 2.2 (m, 2H), 1.7 (m, 1H), 1.4 (s, 3H), 1.2 (m, 2H), 0.8 (t,
J=7 Hz, 3H); MS (CI)
m/z 225 (M+H)+.
Example 6
(2R)-2-propyl-4-hexvnamide
(2R)-2-Propyl-4-hexynoic acid (1.5 g 10 mmol) in dichloromethane (15 mL) was
treated
with 2 drops of N,N-dimethylformamide and freshly distilled oxalyl chloride
(1.25 equivalents)
at 0 C. The mixture was allowed to warm to ambient temperature. After
stirring for 3 hours at
ambient temperature, the solvents were removed under reduced pressure to
provide the crude
acid. The acid chloride was dissolved in dichloromethane (15 mL) and ammonia
gas was
bubbled through the mixture at 0 C for 30 minutes. The mixture was allowed to
warm to
ambient temperature and ammonia gas was bubbled through the mixture an
additional 20
minutes. The mixture was treated with water (20 mL) and the resulting white
solid collected by
filtration, dried and recrystallized from ethyl acetate to provide the title
compound (75% yield).
'H NMR (300 MHz, DMSO-d6) S 7.3 (s, IH), 6.8 (s, 1H), 2.2 (m, 2H), 2.1 (m, 1
H), 1.7 (s, 3H),
1.4(m, 2H), 1.2(m, 2H), 0.8 (t, J=7 Hz, 3H); MS (CI) m/z 154 (M+H)+.
Determination of anticonvulsant effect
The anticonvulsant effect of a representative number of compounds of the
present
invention were determined using the procedure described hereinafter.
13
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Male CD1 mice (Charles River, Portage, MI) weighing 25-35 g were used and
during the
experiment the mice were housed individually in clear polycarbonate cages for
observation. For
each experiment, ten mice were each pretreated intra-peritoneally with a
compound of the
present invention (1400 gmol/kg) prior to pentylenetetrazole (PTZ) (Sigma, St.
Louis, MO)
injection. Seizures were induced by the subcutaneous injection of PTZ (85
mg/kg) just below
the nape of the neck. The animals were observed for 15 minutes and time to
seizure was noted.
The data in Table 1 represents the average time for PTZ induced seizure. Also
noted were the
number of mice out of ten that did not seize within the 15 minute period
following PTZ injection.
This data is shown in Table 2. Control animals were not pretreated with a
compound of the
present invention but were injected with PTZ (85 mg/kg) subcutaneously.
Table 1
Latency to PTZ Induced Seizures in Mice
Time to Seizure
Example Number (seconds)
control 240
1 675
2 305
3 575
4 610
5 525
6 No Seizures
Table 2
Mice Protected Against PTZ Induced Seizures
Number of Mice
Example Number Protected
control 0
1 5
2 1
14
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
3 4
4 6
2
6 10
The data in Tables 1 and 2 illustrate the anticonvulsant effect of compounds
of the
present invention in mammals,
Maximum Electrical Shock method (IVIES)
Male CD1 mice (Charles River) weighing from 25-35 g were used. Mice were
pretreated
5 orally with compounds of the present invention prior to electrical
stimulation. Mice were
challenge by pulsed electrical stimulation (50 mA, 0.4 s duration, pulse width
0.5 ms, 60
pulses/sec) via corneal electrodes to induce seizure. The stimulation was
delivered with an ECT
Unit (Ugo Basile #7801). The electrodes of the unit were coated with
electrocardiogram
electrolyte (Signa Creme, Parker Labs #1708) to ensure good contact with the
corneas. Mice
were observed post-stimulation for the onset of seizures and death. Mice were
considered to
have had a seizure only if there was an extended extension (>90 from plane of
body) of the hind
legs. Mice were assigned scores of either "positive" or "negative." A positive
score indicates
that seizure occurred; a negative score indicated that seizure did not occur.
Animals which gave
a positive seizure response were observed an additional 30 seconds for death.
Those that did not
seize were considered to be protected. The percent protection from seizures
was calculated by
dividing the number of protected mice by the total number of mice for the
group. The
percentage of mice protected from electrical shock induced seizures at doses
of 600, 1200, 1800
and 2400 mole/kg is illustrated in Table 3 for Example 1 and Example 6. The
corresponding
acid, (2R)-2-propyl-4-hexynoic acid, was also tested for comparison.
Table 3
Percentage of Mice Protected Against
Electrical Shock Induced Seizures
Dose ( mole/kg)
600 1200 1800 2400
Example 1 0% 70% 90% 100%
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Example 6 0% 20% 90% NT
(2R)-2-propyl-4-hexynoic acid 0% 0% 0% 20%
NT in Table 3 means Not Tested.
The data in Table 3 illustrates the ability of alkynyl amides of the present
invention to
protect mice from electrical shock induced seizures. Alkynyl amides of the
present invention
unexpectedly protect a significantly higher percentage of mice when compared
to the
corresponding acid, (2R)-2-propyl-4-hexynoic acid.
The compounds of the present invention, including but not limited to those
specified in
the examples, possess an anticonvulsant effect as demonstrated by the data
illustrated in Tables
1-3. As anticonvulsants, the compounds of the present invention may be useful
for the treatment
of a variety of disorder such as seizures, epilepsy, migraine, psychiatric
disorders, neuropathic
pain and restlessness syndrome.
Additionally, compounds of the present invention, including but not limited to
those
specified in the examples, may be useful for providing neuroprotection.
The ability of the compounds of the invention, including but not limited to
those
specified in the examples, to treat epilepsy may be demonstrated according to
the methods
described in (Loscher W., ed. Valproate, Basel Switzerland: Birkhauser Verlag,
1999; and
Cereghino et al., "Introduction," in Antiepileptic Drugs, 4th ed., pages 1-11,
Raven Press, 1995).
The ability of the compounds of the invention, including but not limited to
those
specified in the examples, to treat different types of migraine such as
classical migraine and
common migraine may be demonstrated according to the methods described in
(Loscher W., ed.
Valproate, Basel Switzerland: Birkhauser Verlag, 1999; Hering and Kuritzky,
Cephalalgia, 12,
1992, 81-84; Hering and Kuritzky, Cephalalgia, 9, 1989, 195-198; and Mathew
and Sabiha,
Headache, 31, 1991, 71-74).
The ability of the compounds of the invention, including but not limited to
those
specified in the examples, to treat psychiatric disorders such as Mood
Disorders, Anxiety
Disorders, Attention-Deficit and Disruptive Behavior Disorders, Behavioral
Disturbances
associated with dementia, Substance Abuse-related Disorders, Schizophrenia,
Impulse Control
Disorders, Personality Disorders and Behavioral Disturbances associated with
autism may be
demonstrated according to the methods described in (Loscher W., ed. Valproate,
Basel
16
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Switzerland: Birkhauser Verlag (1999); Post R.M., In Tasman A., Goldfinger,
S.M., Kaufmann,
C.A., eds. Review of psychiatry, volume 9, Washington DC: American Psychiatric
Press, (1990)
170-202; Jensen R., Brinck T., Olesen J., Neurology 44 (1994) 647; Bemasconi
et al., in
Anticonvulsants In Affective Disorders, pages 14-32, Excerpta Medica, 1984;
Lloyd and
Morselli, in Psychopharmacology: The Third Generation of Progress, Raven
Press, 1987).
The ability of the compounds of the invention, including but not limited to
those
specified in the examples, to provide neuroprotection may be demonstrated
according to the
methods described in (Chen G., et al, Journal of Neurochemistry, 1999, 879-
882; and Lyden,
Chapter 10 in "Neuroprotective Agents and Cerebral Ischaemia", IRN 40,
Academic Press
Limited, 1997.
The ability of the compounds of the invention, including but not limited to
those
specified in the examples, to treat neuropathic pain may be demonstrated
according to the
methods described in (Lloyd and Morselli, in Psychopharmacology: The Third
Generation of
Progress, Raven Press, 1987; and Swendlow, J. Clin. Neuropharmacol., 7, 1984,
51-82).
The ability of the compounds of the invention, including but not limited to
those
specified in the examples, to treat Restlessness Syndrome may be demonstrated
according to the
methods described in (O'Keefe, Arch. Intern. Med. 156, 1996, 243-48; Danek et
al., in
Neurological Disorders: Course and Treatment, pages 819-23, Academic Press,
1996; and
Mellick and Mellick, Neurology 45(suppl), 1995, 285-86.
Compounds of the present invention may exist as stereoisomers wherein,
asymmetric or
chiral centers are present. These stereoisomers are "R" or "S" depending on
the configuration of
substituents around the chiral carbon atom. The terms "R" and "S" used herein
are
configurations as defined in IUPAC 1974 Recommendations for Section E,
Fundamental
Stereochemistry, Pure Appl. Chem., 1976, 45: 13-30. In particular, the
stereochemistry at the 2-
position of the 4-hexynamides, as shown in formula (I), may be only (R) when
R2 is hydrogen or
alkyl and may be only (S) when R2 is fluorine. Additionally, when R3 is
(NRARB)carbonylalkyl,
the alkyl portion may contain one or two chiral centers. The one or two chiral
centers contained
within the alkyl portion of (NRARB)carbonylalkyl may be independently either
(R) or (S), unless
specifically noted otherwise. However, the present invention does contemplate
various
stereoisomers and mixtures thereof and are specifically included within the
scope of this
17
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
invention. Stereoisomers include enantiomers and diastereomers, and mixtures
of enantiomers
or diastereomers. Individual stereoisomers of compounds of the present
invention may be
prepared synthetically from commercially available starting materials which
contain asymmetric
or chiral centers or by preparation of racemic mixtures followed by resolution
well-known to
those of ordinary skill in the art. These methods of resolution are
exemplified by (1) attachment
of a mixture of enantiomers to a chiral auxiliary, separation of the resulting
mixture of
diastereomers by recrystallization or chromatography and liberation of the
optically pure product
from the auxiliary or (2) direct separation of the mixture of optical
enantiomers on chiral
chromatographic columns or (3) formation of a salt with a chiral amine and
separating the
enantiomers via fractional crystallization.
The present invention contemplates prodrugs that are transformed by in vivo
biotransformation into compounds of formula (I).
The term "prodrug,"as used herein, represents those prodrugs of the compounds
of the
present invention which are, within the scope of sound medical judgement,
suitable for use in
contact with the tissues of humans and lower animals without undue toxicity,
irritation, allergic
response, and the like, commensurate with a reasonable benefit/risk ratio, and
effective for their
intended use. Prodrugs of the present invention may be rapidly transformed in
vivo to
compounds of formula (I), for example, by hydrolysis in blood. A thorough
discussion is
provided in T. Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, V.
14 of the A.C.S.
Symposium Series; and in Edward B. Roche, ed., Bioreversible Carriers in Drug
Design,
American Pharmaceutical Association and Pergamon Press, 1987.
The term "pharmaceutically acceptable carrier," as used herein, means a non-
toxic, inert
solid, semi-solid or liquid filler, diluent, encapsulating material or
formulation auxiliary of any
type. Some examples of materials which can serve as pharmaceutically
acceptable carriers are
sugars such as lactose, glucose and sucrose; starches such as corn starch and
potato starch;
cellulose and its derivatives such as sodium carboxymethyl cellulose, ethyl
cellulose and
cellulose acetate; powdered tragacanth; malt; gelatin; talc; excipients such
as cocoa butter and
suppository waxes; oils such as peanut oil, cottonseed oil, safflower oil,
sesame oil, olive oil,
corn oil and soybean oil; glycols; such a propylene glycol; esters such as
ethyl oleate and ethyl
laurate; agar; buffering agents such as magnesium hydroxide and aluminum
hydroxide; alginic
18
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
acid; pyrogen-free water; isotonic saline; Ringer's solution; ethyl alcohol,
and phosphate buffer
solutions, as well as other non-toxic compatible lubricants such as sodium
lauryl sulfate and
magnesium stearate, as well as coloring agents, releasing agents, coating
agents, sweetening,
flavoring and perfuming agents, preservatives and antioxidants can also be
present in the
composition, according to the judgment of the formulator.
The present invention provides pharmaceutical compositions which comprise
compounds
of the present invention formulated together with one or more non-toxic
pharmaceutically
acceptable carriers. The pharmaceutical compositions can be formulated for
oral administration
in solid or liquid form, for parenteral injection or for rectal
administration.
Further included within the scope of the present invention are pharmaceutical
compositions comprising one or more of the compounds of formula (I) prepared
and formulated
in combination with one or more non-toxic pharmaceutically acceptable
compositions. The
pharmaceutical compositions can be formulated for oral administration in solid
or liquid form,
for parenteral injection or for rectal administration.
The pharmaceutical compositions of this invention can be administered to
humans and
other mammals orally, rectally, parenterally, intracisternally,
intravaginally, intraperitoneally,
topically (as by powders, ointments or drops), bucally or as an oral or nasal
spray. The term
"parenterally," as used herein, refers to modes of administration which
include intravenous,
intramuscular, intraperitoneal, intrastemal,'subcutaneous, intraarticular
injection and infusion.
Pharmaceutical compositions of this invention for parenteral injection
comprise
pharmaceutically acceptable sterile aqueous or nonaqueous solutions,
dispersions, suspensions or
emulsions and sterile powders for reconstitution into sterile injectable
solutions or dispersions.
Examples of suitable aqueous and nonaqueous carriers, diluents, solvents or
vehicles include
water, ethanol, polyols (propylene glycol, polyethylene glycol, glycerol, and
the like), suitable
mixtures thereof, vegetable oils (such as olive oil) and injectable organic
esters such as ethyl
oleate. Proper fluidity may be maintained, for example, by the use of a
coating such as lecithin,
by the maintenance of the required particle size in the case of dispersions,
and by the use of
surfactants.
These compositions may also contain adjuvants such as preservative agents,
wetting
agents, emulsifying agents, and dispersing agents. Prevention of the action of
microorganisms
19
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
may be ensured by various antibacterial and antifungal agents, for example,
parabens,
chlorobutanol, phenol, sorbic acid, and the like. It may also be desirable to
include isotonic
agents, for example, sugars, sodium chloride and the like. Prolonged
absorption of the injectable
pharmaceutical form may be brought about by the use of agents delaying
absorption, for
example, aluminum monostearate and gelatin.
In some cases, in order to prolong the effect of a drug, it is often desirable
to slow the
absorption of the drug from subcutaneous or intramuscular injection. This may
be accomplished
by the use of a liquid suspension of crystalline or amorphous material with
poor water solubility.
The rate of absorption of the drug then depends upon its rate of dissolution
which, in turn, may
depend upon crystal size and crystalline form. Alternatively, delayed
absorption of a
parenterally administered drug form is accomplished by dissolving or
suspending the drug in an
oil vehicle.
Suspensions, in addition to the active compounds, may contain suspending
agents, as, for
example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan esters,
microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar,
tragacanth, and
mixtures thereof.
If desired, and for more effective distribution, the compounds of the present
invention
can be incorporated into slow-release or targeted-delivery systems such as
polymer matrices,
liposomes, and microspheres. They may be sterilized, for example, by
filtration through a
bacteria-retaining filter or by incorporation of sterilizing agents in the
form of sterile solid
compositions, which may be dissolved in sterile water or some other sterile
injectable medium
immediately before use.
The active compounds can also be in micro-encapsulated form, if appropriate,
with one
or more excipients as noted above. The solid dosage forms of tablets, dragees,
capsules, pills,
and granules can be prepared with coatings and shells such as enteric
coatings, release
controlling coatings and other coatings well known in the pharmaceutical
formulating art. In
such solid dosage forms the active compound can be admixed with at least one
inert diluent such
as sucrose, lactose, or starch. Such dosage forms may also comprise, as is
normal practice,
additional substances other than inert diluents, e.g., tableting lubricants
and other tableting aids
such a magnesium stearate and microcrystalline cellulose. In the case of
capsules, tablets and
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
pills, the dosage forms may also comprise buffering agents. They may
optionally contain
opacifying agents and can also be of'such composition that they release the
active ingredient(s)
only, or preferentially, in a certain part of the intestinal tract in a
delayed manner. Examples of
embedding compositions which can be used include polymeric substances and
waxes.
Injectable depot forms are made by forming microencapsulated matrices of the
drug in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of drug to
polymer and the nature of the particular polymer employed, the rate of drug
release can be
controlled. Examples of other biodegradable polymers include poly(orthoesters)
and
poly(anhydrides) Depot injectable formulations are also prepared by entrapping
the drug in
liposomes or microemulsions which are compatible with body tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium just prior to use.
Injectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions
may be formulated according to the known art using suitable dispersing or
wetting agents and
suspending agents. The sterile injectable preparation may also be a sterile
injectable solution,
suspension or emulsion in a nontoxic, parenterally acceptable diluent or
solvent such as a
solution in 1,3-butanediol. Among the acceptable vehicles and solvents that
may be employed
are water, Ringer's solution, U.S.P. and isotonic sodium chloride solution. In
addition, sterile,
fixed oils are conventionally employed as a solvent or suspending medium. For
this purpose any
bland fixed oil can be employed including synthetic mono- or diglycerides. In
addition, fatty
acids such as oleic acid are used in the preparation of injectables.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders, and
granules. In such solid dosage forms, the active compound is mixed with at
least one inert,
pharmaceutically acceptable excipient or carrier such as sodium citrate or
dicalcium phosphate
and/or a) fillers or extenders such as starches, lactose, sucrose, glucose,
mannitol, and silicic
acid; b) binders such as carboxymethylcellulose, alginates, gelatin,
polyvinylpyrrolidinone,
sucrose, and acacia; c) humectants such as glycerol; d) disintegrating agents
such as agar-agar,
calcium carbonate, potato or tapioca starch, alginic acid, certain silicates,
and sodium carbonate;
21
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
e) solution retarding agents such as paraffin); f) absorption accelerators
such as quaternary
ammonium compounds; g) wetting agents such as cetyl alcohol and glycerol
monostearate;)
absorbents such as kaolin and bentonite clay; and i) lubricants such as talc,
calcium stearate,
magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate, and
mixtures thereof. In
the case of capsules, tablets and pills, the dosage form may also comprise
buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high molecular
weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be prepared
with coatings and shells such as enteric coatings and other coatings well
known in the
pharmaceutical formulating art. They may optionally contain opacifying agents
and can also be
of a composition that they release the active ingredient(s) only, or
preferentially, in a certain part
of the intestinal tract in a delayed manner. Examples of embedding
compositions which can be
used include polymeric substances and waxes.
Compositions for rectal or vaginal administration are preferably suppositories
which can
be prepared by mixing the compounds of this invention with suitable non-
irritating excipients or
carriers such as cocoa butter, polyethylene glycol or a suppository wax which,
are solid at
ambient temperature but liquid at body temperature and therefore melt in the
rectum or vaginal
cavity and release the active compound.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, microemulsions, solutions, suspensions, syrups and elixirs. In
addition to the active
compounds, the liquid dosage forms may contain inert diluents commonly used in
the art such
as, for example, water or other solvents, solubilizing agents and emulsifiers
such as ethyl
alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol,
benzyl benzoate,
propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular,
cottonseed,
groundnut, corn, germ, olive, castor, and sesame oils), glycerol,
tetrahydrofurfuryl alcohol,
polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof.
Besides inert
diluents, the oral compositions can also include adjuvants such as wetting
agents, emulsifying
and suspending agents, sweetening, flavoring, and perfuming agents.
22
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Compounds of the present invention may also be administered in the form of
liposomes.
As is known in the art, liposomes are generally derived from phospholipids or
other lipid
substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid
crystals that are
dispersed in an aqueous medium. Any non-toxic, physiologically acceptable and
metabolizable
lipid capable of forming liposomes may be used. The present compositions in
liposome form
may contain, in addition to the compounds of the present invention,
stabilizers, preservatives,
excipients, and the like. The preferred lipids are the natural and synthetic
phospholipids and
phosphatidylcholines (lecithins) used separately or together.
Methods to form liposomes are known in the art. See, for example, Prescott,
Ed.,
Methods in Cell Biology, Volume XIV, Academic Press, New York, N. Y., (1976),
p 33 et seq.
The term "pharmaceutically acceptable cation," as used herein, refers to a
positively-
charged inorganic or organic ion that is generally considered suitable for
human consumption.
Examples of pharmaceutically acceptable cations are hydrogen, alkali metal
(lithium, sodium
and potassium), magnesium, calcium, ferrous, ferric, ammonium, alkylammonium,
dialkylammonium, trialkylammonium, tetraalkylammonium, diethanolammmonium, and
choline. Cations may be interchanged by methods known in the art, such as ion
exchange.
The term "pharmaceutically acceptable salt," as used herein, refers to salts
that are well
known in the art. For example, S. M Berge et al. describe pharmaceutically
acceptable salts in
detail in Q. Pharmaceutical Sciences, 66:1-19 (1977)). Examples of
pharmaceutically
acceptable, nontoxic acid addition salts are salts of an amino group formed
with inorganic acids
such as hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid
and perchloric acid
or with organic acids such as acetic acid, oxalic acid, maleic acid, tartaric
acid, citric acid,
succinic acid, or malonic acid or by using other methods used in the art such
as ion exchange.
Other pharmaceutically acceptable salts include nitrate, bisulfate, borate,
formate, butyrate,
valerate, 3-phenylpropionate, camphorate, adipate, benzoate, oleate,
palmitate, stearate, laurate,
lactate, fumarate, ascorbate, aspartate, nicotinate, p-toluenesulfonate,
camphorsulfonate,
methanesulfonate, 2-hydroxyethanesulfonate, gluconate, glucoheptonate,
lactobionate,
glycerophosphate, pectinate, lauryl sulfate, and the like, metal salts such as
sodium, potassium,
magnesium or calcium salts or amino salts such as ammonium, triethylamine
salts, and the like,
all of which may be prepared according to conventional methods.
23
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants. The active compound is mixed under
sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives, buffers or
propellants which can be required. Opthalmic formulations, eye ointments,
powders and
solutions are also contemplated as being within the scope of this invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of this
invention can be varied so as to obtain an amount of the active compound(s)
which is effective to
achieve the desired therapeutic response for a particular patient,
compositions and mode of
administration. The selected dosage level will depend upon the activity of the
particular
compound, the route of administration, the severity of the condition being
treated and the
condition and prior medical history of the patient being treated. However, it
is within the skill of
the art to start doses of the compound at levels lower than required for to
achieve the desired
therapeutic effect and to gradually increase the dosage until the desired
effect is achieved.
Aqueous liquid compositions of the present invention may be particularly
useful for the
treatment and prevention of seizures, in particular, seizures associated with
epilepsy.
When used in the above or other treatments, a therapeutically effective amount
of one of
the compounds of the present invention can be employed in pure form or, where
such forms
exist, in pharmaceutically acceptable salt form. Alternatively, the compound
can be
administered as a pharmaceutical composition containing the compound of
interest in
combination with one or more pharmaceutically acceptable excipients. The
phrase
"therapeutically effective amount" of the compound of the invention means a
sufficient amount
of the compound to treat disorders, at a reasonable benefit/risk ratio
applicable to any medical
treatment. It will be understood, however, that the total daily usage of the
compounds and
compositions of the present invention will be decided by the attending
physician within the scope
of sound medical judgement. The specific therapeutically effective dose level
for any particular
patient will depend upon a variety of factors including the disorder being
treated and the severity
of the disorder; activity of the specific compound employed; the specific
composition employed;
the age, body weight, general health, sex and diet of the patient; the time of
administration, route
of administration, and rate of excretion of the specific compound employed;
the duration of the
treatment; drugs used in combination or coincidental with the specific
compound employed; and
24
CA 02427560 2003-05-01
WO 02/36546 PCT/US01/45704
like factors well known in the medical arts. For example, it is well within
the skill of the art to
start doses of the compound at levels lower than required to achieve the
desired therapeutic
effect and to gradually increase the dosage until the desired effect is
achieved.
The total daily dose of the compounds of this invention administered to a
human or lower
animal may range from about 0.003 to about 30 mg/kg/day. For purposes of oral
administration,
more preferable doses can be in the range of from about 0.01 to about 10
mg/kg/day. If desired,
the effective daily dose can be divided into multiple doses for purposes of
administration;
consequently, single dose compositions may contain such amounts or
submultiples thereof to
make up the daily dose.
It is understood that the foregoing detailed description and accompanying
examples are
merely illustrative and are not to be taken as limitations upon the scope of
the invention, which is
defined solely by the appended claims and their equivalents. Various changes
and modifications
to the disclosed embodiments will be apparent to those skilled in the art.
Such changes and
modifications, including without limitation those relating to the chemical
structures, substituents,
derivatives, intermediates, syntheses, formulations and/or methods of use of
the invention, may
be made without departing from the spirit and scope thereof.