Note: Descriptions are shown in the official language in which they were submitted.
CA 02428339 2003-05-08
1 "PROCESS FOR PRODUCING SYNTHESIS GAS
2 BY NON-CATALYTIC PARTIAL OXIDATION OF NATURAL GAS"
3
4 FIELD OF THE INVENTION
s The invention relates to a process and apparatus for non-catalytic
6 partial oxidation of natural gas for the production of a synthesis gas.
s BACKGROUND OF THE INVENTION
9 Synthesis gas or syngas is a mixture of H2 and CO. Synthesis gas
to can be used as H2/C0 mixtures or separated into high-purity HZ and CO. As a
1 i mixture, synthesis gas is used primarily for the synthesis of methanol and
i2 oxochemicals, and as a reducing gas for iron ore. The separate products, H2
and
13 CO, have extensive uses in refinery processes, ammonia production, as a key
14 raw material for a number of chemicals, and in fuelling fuel cells.
is Production of synthesis gas is the first step in most commercial
16 technologies for converting natural gas into valuable chemicals and fuels.
The
1~ synthesis gas production step is expensive and constitutes a major portion,
often
1g 50%, of the total cost of a petrochemical plant. Conversion of natural gas
to
19 synthesis gas can be carried out through many known techniques such as
2o catalytic steam reforming or non-catalytic partial oxidation. Primarily,
the relative
2t price and availability of the hydrocarbon feedstock dictates the choice
between
22 steam reforming and partial oxidation.
23 High-temperature non-catalytic partial oxidation of natural gas is a
2a widely practiced technology for the generation of synthesis gas. To a
lesser
2s extent, compared with steam reforming, partial oxidation processes have
also
CA 02428339 2003-05-08
t been used for synthesis gas generation from gaseous feedstocks, mainly
natural
2 gas. Typically, partial oxidation processes are used in applications where
lower
3 HZ/CO ratios in the product syngas are desirable, such as for use in Fischer-
a Tropsch (FT) synthesis, carbon monoxide production, and oxoalcohol
production.
s Non-catalytic partial oxidation is a thermal process wherein
6 hydrocarbon conversion is completed by combustion. The process requires high
~ reaction temperatures, typically 1200 to 1500°C, to achieve full
conversion of the
s hydrocarbons and high-oxygen consumption. Non-catalytic partial oxidation
has
9 an advantage over catalytic processes in that other constituents, such as
sulfur
to compounds, do not need to be removed. Non-catalytic partial oxidation can
also
t t be used to handle much heavier petroleum fractions than catalytic
processes and
t2 is therefore attractive for processing diesels, logistic fuels and residual
fractions.
t3 While it is known that many others have carried out such high
to temperature partial oxidation at a large scale, Applicant has found that
is unfortunately, the reaction does not scale down well and control of the
reaction is
t6 problematic.
t~ US Patent 5,886,056 to Hershkowitz et al. teaches a partial
is oxidation process for the conversion of light hydrocarbon gases into
synthesis
t9 gas. A plurality of nozzles in a complex injector are used to inject
preheated,
2o pressurized hydrocarbon gas and oxygen containing gases into a reaction
zone
2t at a 02/C molar ratio in the range of from 0.3 up to 0.8 to 1 mole of C and
22 preferably 0.45 to 0.7 to 1.
23 US Patent 6,365,792 to Stapf et al. teaches preparation of
2a acetylene and synthesis gas using a mixture of hydrocarbon, including
natural
2s gas, and molecular oxygen at a temperature of 1200 - 1400°C,
followed by rapid
2
CA 02428339 2003-05-08
i quenching. The preferred mixture for the preparation of methanol is a ratio
of
2 methane to 02 of about 2:1. A mixture of starting material, at about
25°C, is
3 premixed in a mixing chamber and introduced to a flow-tube reactor or other
4 suitable reactor for flame-free combustion which operates at a temperature
of
s about 1300°C and atmospheric pressure. The resulting cracked gas is
passed
6 through a heat exchanger and is rapidly cooled to below 300°C. A
water-free
~ product gas containing 10.4 volume % methane, 0 volume % oxygen, 9.1 volume
s % acetylene, 25.8 volume % carbon monoxide, 50.2 volume % hydrogen and 3.4
9 volume % carbon dioxide with the remaining being nitrogen, soot and higher
to hydrocarbons, is formed.
a
3
CA 02428339 2003-05-08
t SUMMARY OF THE INVENTION
2 A non-catalytic partial oxidation process and apparatus are
3 provided which produce a synthesis gas yield of between 1.2 to 1.6 with
reduced
4 undesirable C02 formation of between 4 to 6 mole%; improved CO selectivity
of
s between 85 to 90%; and a natural gas conversion of between 60 to 80%.
6 In accordance with a broad aspect of the invention, the process is
~ directed to an non-catalytic partial oxidation process for the production of
g gaseous mixtures comprising H2 and CO, and other gaseous materials from
9 hydrocarbon feed gas comprising: providing a stream of oxygen (02)
containing
to gas preheated to a temperature in the range of 20 to 200°C;
providing a stream
t t of hydrocarbon feed gas preheated to a temperature of 20 to 300°C;
controlling
i2 the relative flow rates of the preheated hydrocarbon feed gas and 02
containing
t3 gas streams; intimately mixing the preheated hydrocarbon feed gas and 02
to containing gas streams together into a gaseous mixture substantially
Is simultaneously on discharge into a partial oxidation reaction zone, the
gaseous
tb mixture having a fuel equivalence ratio in the range of about 2 to about 4;
t~ reacting the gaseous mixture in the partial oxidation reaction zone at a
reaction
tg temperature in the range of about 800°C to about 1400°C at
atmospheric
t9 pressure so as to produce an effluent gas stream comprising at least Hz and
CO;
2o and cooling the effluent gas stream for producing a product gas.
2t Preferably, a fuel equivalence ratio [(Fuel/Oxidant
22 a~t~ay/(Fuel/Oxidant stoichiomeuic)~ ~p is maintained at about 3.5, the
feed gas and
23 oxygen streams are heated to about 200°C and the reaction is carried
out in a
24 refractory lined combustion chamber according to another broad aspect of
the
2s invention as discussed below. The oxygen (Oa) containing gas is selected
from
4
CA 02428339 2003-05-08
1 the group consisting of air or oxygen enriched air and preferably oxygen
enriched
2 air having an oxygen content of greater than 95%.
3 More preferably, the separately heated gas streams are introduced
4 into the partial oxidation reaction zone or combustion chamber using a
swirling jet
s burner which acts to mix the streams substantially simultaneously upon
discharge
6 into the combustion chamber. The swirling jet burner, which may protrude
directly
~ into the combustion chamber, is preferably cooled using a water jacket to
prevent
s damage as a result of the reaction temperatures in the coupled combustion
9 chamber.
to Flow control means, typically flowmeters are used to adjust the flow
11 rates of the gas streams to ensure that the fuel equivalence ratio cp is
maintained
Iz at a ratio of about 2 to about 4 and preferably at about 3.5. Further, a
space
t3 velocity of 50-100 hr' is maintained.
la In another broad aspect of the invention, apparatus for the non-
is catalytic partial oxidation process comprises: a heater for preheating a
stream of
tb hydrocarbon feed gas; a heater for preheating a stream of oxygen containing
t~ gas; a temperature controlled refractory-lined combustion chamber capable
of
ig providing a reaction temperature in the range of about 800°C to
about 1400°C at
i9 atmospheric pressure so as to produce an effluent gas stream comprising at
least
2o H2 and CO; a temperature controlled swirling jet burner coupled to and
2t discharging into the refractory lined combustion chamber for intimately
mixing the
22 preheated hydrocarbon feed gas and 02 containing gas streams together into
a
23 gaseous mixture substantially simultaneously on discharge into the
refractory-
2a lined combustion chamber; flow control means for adjusting the flow of the
2s hydrocarbon feed gas and oxygen containing gas streams for maintaining a
fuel
CA 02428339 2003-05-08
i equivalence ratio in the range of about 2 to about 4 in the gaseous mixture;
and a
2 quenching chamber for cooling the effluent gas stream for producing a
product
3 gas.
4 Preferably, the gas streams are heated using electrical heaters.
s Water jackets are used to control the temperature of the combustion chamber,
6 the burner and the quenching chamber.
The quenching chamber is further equipped with access for
s cleaning and a water sump which maintains a constant water level.
6
CA 02428339 2003-05-08
t BRIEF DESCRIPTION OF THE DRAWINGS
2 Figure 1 is a schematic of an apparatus of the preferred
3 embodiment of the invention used for commercial production of synthesis
product
a gas according to the process of the present invention;
s Figure 2 is a partial sectional view of a swirling jet burner according
6 to Fig. 1;
Figure 2a is a plan view of the swirling jet burner at section A-A
s illustrating tangential inlet ports for the feed gas and oxidant gas
according to Fig.
9 2,
to Figure 3 is a partial section view of a refractory lined combustion
t t chamber according to Fig. 1;
t2 Figure 4 is a partial sectional view of a quenching chamber
t3 according to Fig. 1;
to Figure 5 is a graphical representation of the H2/CO ratio in a
is product gas stream at varying fuel equivalence ratios, a constant natural
gas flow
tb rate of 12 I/min and a constant temperature of 20°C for both a
natural gas feed
t~ gas stream and an oxygen containing gas stream;
tg Figure 6 is a graphical representation of the CO selectivity using the
t9 conditions according to Fig. 5;
2o Figure 7 is a graphical representation of the CH4 conversion using
21 the conditions according to Fig. 5;
22 Figure 8 is a graphical representation of the synthesis gas yield
2s using the conditions according to Fig. 5;
7
CA 02428339 2003-05-08
t Figure 9 is a graphical representation of the H2/CO ratio at varying
2 temperatures and fuel equivalence ratios cp and with a constant natural gas
flow
3 rate of 12 I/min.;
a Figure 10 is a graphical representation of the CO selectivity using
s the conditions according to Fig. 9;
6 Figure 11 is a graphical representation of the CH4 conversion using
~ the conditions according to Fig. 9;
s Figure 12 is a graphical representation of the synthesis gas yield
9 using the conditions according to Fig. 9;
to Figure 13 is a graphical representation of the H2/C0 ratio at varying
t t natural gas flow rates and fuel equivalence ratios cp and at a constant
t2 temperature and a constant temperature of 20°C for both a natural
gas feed gas
t3 stream and an oxygen containing gas stream;
t4 Figure 14 is a graphical representation of the CO selectivity using
is the conditions according to Fig. 13;
tb Figure 15 is a graphical representation of the CH4 conversion using
t~ the conditions according to Fig. 13; and
is Figure 16 is a graphical representation of the synthesis gas yield
19 using the conditions according to Fig. 13.
8
CA 02428339 2003-05-08
t DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
2 The present invention pertains to both an improved process for
3 operating a non-catalytic partial oxidation gas generator for the production
of a
a synthesis gas, reducing gas, or fuel gas and to the apparatus utilized in
the
s process.
6 Having reference to Fig. 1, apparatus 1 for implementing a
~ preferred embodiment of the process of the invention is shown. The apparatus
1
a is divided into several main sections, particularly:
9 an electric preheating section 2 for preheating gaseous reactants,
to particularly a feed gas and an oxygen containing gas,
t t a water-jacketed swirling jet burner 3 for receiving the preheated
t2 gaseous reactants, and for mixing the heated reactants at an exit nozzle,
t3 a partial oxidation reaction zone comprising a water jacketed
t4 refractory-lined combustion chamber 4 into which the preheated and mixed
is gaseous reactants are discharged and from which an effluent gas stream is
t6 produced; and
t~ a water-jacketed spray quenching chamber 5 for cooling the
to partially oxidized effluent gas stream for producing a product gas.
t9 The gaseous reactants comprise a feed gas stream, typically
2o natural gas (NG) as fuel for the partial oxidation process and an oxygen
(02)
2t containing gas stream as an oxidant. The Oz containing gas can be air or
oxygen
2z enriched air. The oxygen enriched air is preferably at least 95% 02. The
gaseous
2s reactants NG, Oz are supplied independently from cylinders 6 having
appropriate
24 pressure regulators 7, each equipped with a solenoid-controlled valve 8, a
needle
9
CA 02428339 2003-05-08
t valve 9 to control the flow rates and a calibrated flow meter 10 to monitor
flow
2 rates.
3 The gaseous reactants NG, 02 are preferably preheated. One
4 suitable form of heater is an electric heater 11. The streams of natural gas
NG
s and 02 are preheated separately using individual electric heaters 11 wherein
the
6 temperature of the gaseous reactants NG, OZ is increased by passage through
a
~ heat transfer system with an electrically heated, high temperature coiled
tube or
s heating coil (not shown) and the temperatures are controlled using an on/off
9 temperature indicator controller 12. Once preheated, the gaseous reactants
NG,
to 02 are fed to the burner 3 under the control of the flowmeters 10, which
permits
t t control of a fuel equivalence ratio cp [(Fuel/Oxidant
a~,"al)/(Fuel/Oxidant S~;~,~ome~~)]-
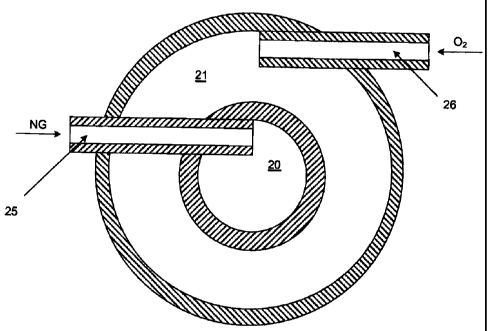
12 Having reference to Figs. 2, 2a and 3, the burner 3 comprises a
t3 separate fuel gas passage 20 and a separate annular oxygen passage 21 which
to intersect at a discharge or swirling jet, mixing head 22. At the mixing
head 22, the
is oxygen passage 21 is angled radially inward to interset with the fuel
passage 20.
t6 The burner 3 is a rapid flow-through burner which permits rapid discharge
of the
t~ gaseous reactants NG, OZ at the mixing head 22. The burner 3 is suspended
into
is an inlet 30 at the top of the combustion chamber 4 and is preferably tit
with a
t9 water jacket 23 which surrounds at least an intruding portion of the burner
3 and
2o acts to maintain the temperature of the burner 3 below the reaction
temperature
2t in the combustion chamber 4. Flange 24, extending outward from the burner
3,
22 engages a cooperating flange 31 at the combustion chambers inlet 30
permitting
23 coupling of the burner 3 to the combustion chamber 4.
CA 02428339 2003-05-08
1 As shown in Fig. 2a, inlet ports 25, 26 through which the gaseous
z reactants NG, 02 enter the burner 3, enter at a tangent to passages 20, 21,
3 respectively, which acts to impart rotation to the gases.
a Preferably, the burner 3 has a viewing glass 25 extending through a
s cap 26 at a top 27 of the burner 3
6 Having reference again to Fig. 1, the structural integrity of the
~ burner 3 is protected from high combustion temperatures using the water-
cooled
s jacket 23 positioned circumferentially about the burner 3. Water, used for
cooling
9 the burner 3, is circulated through a heat exchanger 40 using a water pump
41.
io Heat, absorbed from the burner 3, is released to the heat exchanger 40 and
the
a resulting cooled water is recirculated through the burner jacket 23 for
further heat
12 absorption. Preferably, the cooling water used is distilled water to
prevent fouling
13 of the water lines.
is Having reference to Fig. 3, the gaseous reactants, NG, 02, are
is mixed by the burner 3 substantially simultaneously upon discharge into the
ib combustion chamber 4. The combustion chamber 4 is a refractory-lined down-
m flow non-catalytic reactor and is an open-volume vessel which is free from
any
ig internal packing material. Partial oxidation of the natural gas NG in the
presence
19 of 02 takes place by combustion in the combustion chamber 4 at reaction
2o temperatures of about 800°C to about 1400°C for forming an
effluent stream of
2~ product gases.
22 The combustion chamber 4 comprises a vessel 32 surrounded by a
z3 circumferential water-cooled, jacket 33 having a first inner layer of
refractory
2a material 34, such as alumina, capable of resisting the elevated reaction
2s temperature. Further, the vessel has a second outer layer of insulating
ceramic
11
CA 02428339 2003-05-08
1 fiber 35, such as KAOWOOLTM positioned between the refractory layer 34 and a
2 vessel wall 36. The two layer lining 34,35 is capable of resisting
temperatures to
3 at least 1800°C. Internal reactor temperature are monitored using a
refractory-
a protected thermocouple 37 of platinum or platinum-rhodium, typically ceramic
s coated.
6 Having reference to Fig. 4, after partial oxidation has occurred, an
~ effluent gas stream, typically containing at least H2 and CO, is directed to
the
s quenching chamber 5 for cooling into a product gas. The quenching chamber 5
Is
9 coupled directly to the combustion chamber 4. The quenching chamber 5
io comprises a vessel 50 having a conical lower portion 51 extending to a
water
i i sump 52 which maintains a minimum water level sustained using a U tube 53.
I2 Two hemispherical towers 54, each fit with a plurality of water injection
nozzles
13 55 for direct spray quenching of the product gases are positioned within
the
14 vessel 50, adjacent an inlet 56. The nozzles 55 are typically sized about 1
mm.
is Preferably, the water used for injection through the nozzles 55 is fresh.
In order
tb to maintain a minimum water level in the sump 51, excess water is drained
to an
i~ open drain and to a sewer 57 through the U tube 53. A sight glass 58
enables
s visual monitoring of the water level. The quench chamber 5 is equipped
19 thermocouples 59 for monitoring of the temperature and a hand hole 60 for
2o cleaning purposes. The quenching chamber 5 further comprises a water jacket
zl 49 for assisting in the cooling the product gases.
2z Finally, as shown in Fig. 1, the quenched product gases exit the
23 system 1 for recovery of the H2 and CO and use in downstream systems.
2a Determination of the composition of the product gas is carried out by
sampling of
12
CA 02428339 2003-05-08
t the exit gas followed by analysis using a refinery gas analyzer 61,
typically a gas
2 chromatograph.
3 Preferably, an injection line 70 and solenoid valve 71 are used to
a inject an inert gas, typically N2, into the combustion chamber 4 after each
run for
s safety purposes, to flush the system of any remaining combustible material.
6
~ IN USE
s The flow rates of the gaseous reactants NG, 02 into the burner are
9 individually adjusted so as to obtain a desired raw product gas while
maintaining
to a substantially constant free oxygen 02 to carbon C atomic ratio in a
reaction
1 t zone in the combustion chamber. The molar ratio of free oxygen 02 to
carbon C
t2 in the gaseous reactants NG, 02 is adjusted by adjusting the individual
flow rates
t3 using the flowmeters 10.
to Applicant has determined that the apparatus as previously
is disclosed can be used in combination with a variety of unique process
variables
16 to produce a product gas having reduced undesirable C02 formation and
m improved CO selectivity, natural gas conversion and synthesis gas yield.
Such
tg process variables include:
t9 ~ preheating of the gaseous reactants NG, 02 prior to the partial
20 oxidation reaction; for the natural gas NG in a range from ambient to about
2t 300°C; and for the oxygen containing gas 02 in a range from ambient
to about
22 200°C. Preheating of the gaseous reactants NG, 02 aids in
maintaining higher
23 flame temperatures in the combustion chamber 4, which results in higher
24 conversion of the natural gas NG, higher selectivity and also higher yield
and
13
CA 02428339 2003-05-08
1 advantageously gives rise to lower soot formation, minimizing the need to
clean
2 the combustion chamber frequently;
3 ~ the fuel equivalence ratio cp being the methane to oxygen ratio
a relative to the corresponding stoichiometric value, in a range of about 2 to
about
s 4; and
6 ~ space velocities which are determined by the volumetric flow
~ rate entering the reactor (I/hr) and the reactor volume (L), which is
typically a .
s constant, and are typically maintained at a range of about 50 to about 100
hr-1.
9
io The major components of the product gas are HZ and CO, with no
i i measurable amount of 02 remaining. Natural gas is typically used as fuel
for the
12 partial oxidation process. The composition of the feed gas mixture of
natural gas
13 NG typically contains about 80 to 98 volume% of methane CH4. The oxidizer
is
la typically free-oxygen containing gas. Air or oxygen-enriched air of more
than 21
is mole% oxygen may be used. Oxygen-enriched air can also include
substantially
I6 pure oxygen such as that having at least 95 mole% oxygen.
m
14
CA 02428339 2003-05-08
t Examples
2 Analysis of the effect of variation of process variables:
3 In order to discuss the experimental results shown in Figs 5-16, four
4 evaluation parameters are defined as follows:
HZ/CO ratio = y"2'°nd
YCO,llut
...............................................................................
............ ( 1 )
Yco X 100
...............................................................................
........ (2)
XClly ~%~ - FClly.in - FCll4,uut X 100
FCH4,in
........................,................................................ J
s and
F", + Fco
Yc~r,
Fcua.rn W'cna,~ur
,..............................................................................
.......... (4)
t o where:
t t Sco is the CO selectivity,
t2 X~Ha is the natural gas conversion,
t3 YcHa is the yield with respect to converted natural
gas,
t4 F; is molar flow rate of component i,
is y; is the mole fraction of component i,
tb N. is the number of C atoms per molecule of i and
"prods"
t~ refers to all product species.
tg
t9 As shown in Figs. 5 to 8, evaluation parameters at different fuel
zo equivalence ratios cp are shown, respectively Hz/C0 ratio, CO selectivity,
natural
CA 02428339 2003-05-08
i gas conversion and synthesis gas yield. The flow rate of NG is maintained at
a
2 constant flow of 12 I/min and temperature of the gaseous reactants NG, 02
are
3 maintained at 20°C.
a As shown in Fig. 5, a maximum value for H2/CO ratio is achieved at
s a fuel equivalence ratio cp of 3.5. Fig. 6 illustrates that the CO
selectivity varies
6 between 80 to 90 % and thus the effect of the variation in the fuel
equivalence
~ ratio is not remarkable. Fig. 7 illustrates a reduction in methane CH4
conversion
a with increased fuel equivalence ratios cp, while Fig. 8 illustrates that the
synthesis
9 gas yield is increased with increased fuel equivalence ratios cp.
to Figs. 9 - 12 illustrate evaluation parameters at different
t r temperatures and different fuel equivalence ratios cp, respectively H2/CO
ratio, CO
i2 selectivity, natural gas conversion and synthesis gas yield. The flow rate
of NG
13 was maintained at a constant flow of 12 I/min. As shown, an increase in the
i4 temperature of the gaseous reactants NG, 02 results in an overall
improvement in
is the evaluation parameters.
ib As shown in Figs. 13 to 16, evaluation parameters at different NG
i~ flow rates and fuel equivalence ratios cp are shown, respectively H2/C0
ratio, CO
is selectivity, natural gas conversion and synthesis gas yield. The
temperatures of
19 the gaseous reactants NG, 02 were maintained at 20°C.
zo In order to alter the fuel equivalence ratio cp, the flow rate of 02 was
2i adjusted relative to the flow rate of NG. Space velocities for Figs. 13 and
14
22 increased with increased flow rates of NG and Oz. At a fuel equivalence
ratio cp of
23 3, the space velocities were 40 h~' for a NG flow rate of 6 I/min and 80
hr' for an
24 NG flow rate of 12 I/min. At a fuel equivalence ratio cp of 3.5, the space
velocities
16
CA 02428339 2003-05-08
t were 38 hr' for a NG flow rate of 6 Ilmin and 75 hr' for an NG flow rate of
12
2 Ilmin.
3 Space velocities calculated for Figs. 15 and 16 responded similarly.
4 At a fuel equivalence ratio cp of 2, the space velocities were 64 hr' for a
NG flow
s rate of 8 I/min and 96 hr'' for an NG flow rate of 12 I/min. At a fuel
equivalence
6 ratio cp of 2.4, the space velocities were 59 hr' for a NG filow rate of 6
I/min and
~ 88 h~' for an NG flow rate of 12 I/min. At a fuel equivalence ratio cp of
3.5, the
a space velocities were 50 hr' for a NG flow rate of 6 Ilmin and 75 hr' for an
NG
9 flow rate of 12 I/min.
to Overall, as illustrated, an increase in the natural gas NG flow rate
t t and the space velocity resulted in an improvement in the evaluation
parameters,
t2 particularly, an increase in the product gas yield (Y~Ha). The range of
each of the
t3 evaluation parameters for the illustrated experiments were as follows:
t4 H2/CO ratio 0.6 to 1.0
is CO selectivity (S~o) 80 to 90
tb natural gas conversion (X~Ha) 60 to 80%
synthesis gas yield (YcHa) 1.2 to 1.6
tg
17
CA 02428339 2003-05-08
1 EXAMPLE
A
z Natural gas was used as a fuel for the partial
oxidation process
3 according
the present
invention.
The NG
composition
was as
shown in
Table 1
4
s Table
1
Com~onent ~le %
M
___ ..................._.._._......._.._....
..._ ..
CH4 _..._...
97.534
C2H6 0.801
C3H8 0.149
i-CaH,o 0.039
n-CaH, 0 0. 074
i-C5H~2 0.042
n-C5H ~ 0.053
2
C6H~4+ 0.355
COZ 0.161
HZS 2.5 ppm
N2 0.792
6
The properties of the natural gas used were as follows:
g Molecular weight: 16.686
Specific gravity: 0.5774
to Global heating value: 9163.05 kcal/m3
> > Net heating value: 8256.5 kcal/m3.
i2 Industrial oxygen with a minimum 95% purity was used as the
i3 oxygen containing gas or oxidant.
t4 The flow rate of the natural gas stream was adjusted to 720 I/hr,
is measured at 20°C and 1 bar and the flow rate of the industrial
oxygen stream was
16 adjusted to 420 I/hr, measured at 20°C and 1 bar, resulting in a
fuel equivalence
m ratio cp of 3.5. The reactor volume was 15L resulting in a space velocity of
76 hr'.
is The gaseous reactants NG, 02 were preheated to a temperature of about
200°C
i9 using electrical preheaters. The preheating was applied using a 10m long
2o aluminum heating coil designed to have an approximate heat addition rate of
18
CA 02428339 2003-05-08
1 SkW. The preheated natural gas and oxygen were then passed through the
2 swirling jet burner 3 for mixing at discharge into the combustion chamber 4.
3 Raw product gas as effluent dry synthesis gas at a rate of 1044 I/hr
4 measured at 20°C and 1 bar was produced. The product gas was sampled
and
s analyzed using the gas chromatograph 61 and was subsequently discharged
6 from a stack 62, as shown in Fig. 1.
The composition of the dry synthesis gas produced is shown in
s Table 2. The evaluated parameters of the process are shown in Table 3.
9 Table 2
Component mole
Carbon monoxide 34.0
Hydrogen 27.0
Carbon dioxide 5.3
Methane 28.6
Nitrogen 5.3
to
tt
12
13 Table 3
Evaluation parameterValue
....
___......__._......_....__.________...______._............._____.._._..........
..__
H2/CO ratio 0.8
Sco, (%) 87
XCH4, (%) 60
YcHa 1.53
14
19