Note: Descriptions are shown in the official language in which they were submitted.
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
METHOD, SYSTEM, AND COMPUTER PROGRAM PRODUCT FOR
MEASURING HEMATOCRIT IN BLOOD VESSELS
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates generally to reflected light analysis. More
particularly, the invention relates to the use of reflected spectral imaging
to
determine a quantity of visualizable components within a fluid flowing in a
tubular system. Still more particularly, the invention relates to the use of
reflected
spectral imaging to determine a quantity of components within the blood of a
mammalian, especially human, vascular system.
2. Related Art
Widely accepted medical school doctrine teaches that the complete blood
count including the white blood cell differential (CBC+Diff) is one of the
best
. tests to assess a patient's overall health. With it, a physician can detect
or
diagnose anemia, infection, blood loss, acute and chronic diseases, allergies,
and
other conditions. CBC+Diff analyses provide comprehensive information on
constituents in blood, including the number ofred blood cells, the hematocrit,
the
hemoglobin concentration, and indices that portray the size, shape, and oxygen-
carrying characteristics of the entire red blood cell (RBC) population. The
CBC+Diff also includes the number and types of white blood cells and the
number of platelets. The CBC+Diff is one of the most frequently requested
diagnostic tests with about two billion done in the United States per year.
A conventional CBC+Diff test is done in an "invasive" manner in which
a sample ofvenous blood is drawn from apatient through aneedle, and submitted
to a laboratory for analysis. For example, a phlebotomist (an individual
specially
trained in drawing blood) collects a sample of venous blood into a tube
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
containing an anticoagulant to prevent the blood from clotting. The sample is
then
sent to a hematology laboratory to be processed, typically on automated,
multiparameter analytical instruments, such as those manufactured by Beckman
Coulter Diagnostics of Miami, Florida. The CBC+Diff test results are returned
to the requesting physician, typically on the next day.
Invasive techniques, such as for conventional CBC+Diff tests, pose
particular problems for newborns because their circulatory system is not yet
fully
developed. Blood is typically drawn using a "heel stick" procedure wherein one
or more punctures are made in the heel of the newborn, and blood is repeatedly
squeezed out into a collecting tube. This procedure is traumatic even for an
infant
in good health. More importantly, this procedure poses the risk of having to
do
a blood transfusion because of the low total blood volume of the infant. The
total
blood volume of the newborn infant is 60-70 cc/kg body weight. Thus, the total
blood volume of low birth weight infants (under 2500 grams) cared for in
newborn intensive care units ranges from 45-175 cc. Because of their low blood
volume and delay in production of red blood cells after birth, blood sampling
from preterm infants and other sick infants frequently necessitates
transfusions
for these infants. Blood bank use for transfusion of infants in neonatal
intensive
care units is second only to the usage for cardiothoracic surgery. In addition
to
newborns, invasive techniques are also particularly stressful for, and/or
difficult
to carry out on, children, elderly patients, burn patients, and patients in
special
care units.
A hierarchical relationship exists between the laboratory findings and
those obtained at the physical examination. The demarcation between the
physical
findings of the patient and the laboratory findings are, in general, the
result of
technical limitations. For instance in the diagnosis of anemia, it is
frequently
necessary to quantify the hemoglobin concentration or the hematocrit in order
to
verify the observation of pallor. Pallor is the lack of the pink color of skin
which
frequently signals the absence or reduced concentration of the heavily red
pigmented hemoglobin. However, there are some instances in which pallor may
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-3-
result from other causes, such as constriction of peripheral vessels, or being
hidden by slcin pigmentation. Because certain parts of the integument are less
affected by these factors, clinicians have found that the pallor associated
with
anemia can more accurately be detected in the mucous membrane of the mouth,
the conjunctivae, the lips, and the nail beds.
A device which is able to rapidly and non-invasively quantitatively
diagnose anemia directly from an examination of one or more of the foregoing
areas would eliminate the need to draw a venous blood sample to ascertain
anemia. Such a device would also eliminate the delay in waiting for the
laboratory results in the evaluation of the patient. Such a device also has
the
advantage of added patient comfort.
Soft tissue, such as mucosal membranes or unpigmented skin, does not
absorb light in the visible spectra and regions in the near-infrared. Tn
particular,
soft tissue does not absorb light in the spectral region where hemoglobin
absorbs
light. This allows vascularization to be differentiated by spectral absorption
from
surrounding soft tissue background. However, the surface of soft tissue
strongly
reflects light and the soft tissue itself effectively scatters light after
penetration of
only 100-500 microns. Therefore, ih vivo visualization of the circulation is
generally impractical because of the complexities involved in either fording
suitable areas and/or compensating for multiple scattering and for specular
reflection from the surface. Studies on the visualization of cells in
microcirculation consequently have been almost exclusively invasive, using a
thin
section (less than the distance for multiple scattering) of tissue containing
the
microcirculation, such as the mesentery, that can be observed by a microscope
using light transmitted through the tissue section. Other studies have
experimented with producing images of tissues from within the multiple
scattering region by time gating (see, Yodh, A. and B. Chance, Physics Today,
March, 1995, 34-40). However, the resolution of such images is limited because
of the scattering of light, and the computations for the scattering factor are
complex.
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-4-
Some imaging studies have been done in the reflected light of the
microcirculation of the nail beds on patients with Raynauds, diabetes, and
sickle
cell disease. These studies were done to obtain experimental data regarding
capillary density, capillary shape, and blood flow velocity, and were limited
to
gross physical measurements on capillaries. No spectral measurements, or
individual cellular measurements, were made, and Doppler techniques were used
to assess velocity. The non-invasive procedure employed in these studies could
be applied to most patients, and in a comfortable manner.
One non-invasive device for ih vivo analysis is disclosed in U.S. Patent
No. 4,998,533 to Winkelman. The Winkelman device uses image analysis and
reflectance spectrophotometry to measure individual cell parameters such as
cell
size and number. Measurements are taken only within small vessels, such as
capillaries where individual cells can be visualized. Because the Winkelinan
device takes measurements only in capillaries, measurements made by the
Winkelman device will not accurately reflect measurements for larger vessels.
This inaccuracy results from the constantly changing relationship of volume of
cells to volume of blood in small capillaries resulting from the non-Newtonian
viscosity characteristic of blood. Consequently, the Winkelman device is not
capable of measuring the central or true hematocrit, or the total hemoglobin
concentration.
The Winkelman device measures the number ofwhite blood cells relative
to the number of red blood cells by counting individual cells as they flow
through
a micro-capillary. The Winkelman device depends upon accumulating a
statistically reliable number of white blood cells in order to estimate the
concentration. However, blood flowing through a micro-capillary will contain
approximately 1000 red blood cells for every white cell, making this an
impractical method. The Winkelman device does not provide any means by
which platelets can be visualized and counted. Further, the Winkelman device
does not provide any means by which the capillary plasma can be visualized, or
the constituents of the capillary plasma quantified. The Winkelman device also
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-5-
does not provide a means by which abnormal constituents of blood, such as
tumor
cells, can be detected.
Othernon-invasive devices for in vivo analysis are disclosed in commonly
assigned U.S. Patent No. 5,983,120, issued November 9, 1999 to Warren Groner
and Richard G. Nadeau, and entitled "Method and Apparatus for Reflected
Imaging Analysis" (hereinafter referred to as "the ' 120 patent"), and in
commonly
assignedU.S.PatentApplicationNo.09/401,859,filedSeptember22,1999inthe
names of Christopher Cook and Mark M. Meyers, and entitled "Method and
Apparatus for Providing High Contrast Imaging" (hereinafter referred to as
"the
'859 application"). The disclosure of the '120 patent and the '859 application
is
incorporated herein by reference as though set forth in its entirety. The
devices
of the '120 patent and the '859 application provide for complete non-invasive
in
vivo analysis of a vascular system. These devices provide for high resolution
visualization of blood cell components (red blood cells, white blood cells,
and
platelets), blood rheology, blood vessels, and vascularization throughout the
vascular system. The devices of the '120. patent and the '859 application
allow
quantitative determinations to be made for blood cells, normal and abnormal
contents of blood cells, as well as for normal and abnormal constituents of
blood
plasma.
The devices of the '120 patent and the '859 application capture a raw
reflected image of a blood sample, and normalize the image with respect to the
background to form a corrected reflected image. An analysis image is segmented
from the corrected reflected image to include a scene of interest for
analysis. The
method and apparatus disclosed in the '120 patent and the '859 application
employ Beer's law to determine such characteristics as the hemoglobin
concentration per unit volume of blood. The reflected images obtained with the
devices of the '120 patent and the '859 application can also be useful in
determining the number of white blood cells per unit volume of blood, a mean
cell volume, the number of platelets per unit volume of blood, and the ratio
of the
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-6-
cellular volume of blood to its total volume which is generally called the
hematocrit.
Using Beer's law to quantitatively measure components of a blood vessel
in a spectral image requires the components to be uniformly distributed
throughout the vessel. For instance, Beer's law can be used to determine the
hemoglobin concentration from in vivo measurements of optical density at an
isobestic wavelength of the hemoglobin absorption spectrum. However, this
technique presupposes the blood vessel is uniformly filled with red blood
cells.
Since the measurements are taken from a spectral image, it is paramount that
this
image contains a representative sample of blood components, i.e. red blood
cells.
Should the blood vessel contain a non-uniform distribution of red blood cells,
the
spectral image would most likely not contain a representative sample of blood
components. Moreover, the optical densitymeasurements would fluctuate widely
over time and individual measurements would not accurately reflect the subj
ect's
true hemoglobin concentration.
The size of the vessel diameter directly influences the distribution of
blood components. In large vessels where the vessel diameter is many times the
diameter of the blood cells, it has been shown that red blood cells, and hence
the
hemoglobin they contain, are uniformly distributed along and within the
vessel.
Therefore, a spectral image of a large blood vessel is prone to contain a
representative sample of blood components, i.e. an average number of red blood
cells. Beer's law, in this instance, can be used to produce an accurate
measurement of hemoglobin concentration.
However, in smaller vessels, the variation in the number ofred blood cells
is more prominent. As the vessel diameter becomes smaller, a lesser number of
red blood cells are able to pass side by side through the vessel. In the
smallest
vessels, only a single stream of red blood cells is able to pass. In smaller
vessels,
the number of red blood cells in a spectral image is likely to vary
significantly
over both time and length along the vessel. As a result, it is difficult to
get a
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
representative image of the subject's blood vessel. Therefore, the optical
density
and hemoglobin measurements from Beer's law are limited by imprecision.
In smaller vessels, the red blood cell count can be measured as an
alternative to calculating the hemoglobin concentration. This can be
accomplished by counting the number of red blood cells per unit length in
ablood
vessel. This technique is effective in the smallest vessels where only a
single
stream of red blood cells is able to pass.
In medium sized vessels, whichhave avessel diameter considerably larger
than the diameter of a single red blood cell yet are not large enough to be
filled
uniformly with red blood cells, neither the cell counting technique or the
Beer's
law method can be used without a substantial variation in results. However, it
is
this range of vessel diameters that are most easily accessible for
visualization and
measurement when one uses the methods of the '120 patent and the '859
application.
Thus, there is a need in the art for a method and system for quantitatively
analyzing select images of a fluid stream having a non-uniformly distributed
concentration of cellular components by using non-invasive in vivo techniques.
SUMMARY OF THE INVENTION
The present invention is directed to analyzing reflected spectral images
of a microcirculatory system to measure the volume and concentration of a
blood
vessel, including arteries, veins and capillaries. The method and system of
the
present invention quantitatively analyzes a fluid stream having a non-uniform
distribution of cellular components. The present invention can be used to
evaluate
the cellular concentration in an unfilled blood vessel. Basically, the method
and
system of the present invention measures the vessel diameter and optical
density
at various locations along the axis of the vessel. The coefficient of
variation in the
diameter and/or optical density measurements are used to estimate blood
characteristics, such as the hematocrit.
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
_g_
The method is used to perform in vivo analyses of blood in vessels from
a spectral image. The method of the present invention can also be used to
perform
in vitro analyses by imaging blood in, for example, a narrow tube or flow
cell.
The method of the present invention can also be used to analyze other
types of fluids containing visible suspended particles. The spectral imaging
system canbe used to analyze fluids forparticulate impurities. It is
onlynecessary
that the walls of the fluid path be sufficiently transparent to permit light
to pass
through to image the fluid and any impurities flowing in the path.
Features and Advantages
A feature of the present invention is that it can be used to determine
characteristics, such as the hematocrit through the use of reflected spectral
imaging.
Another feature of the present invention is that it can be used to determine
blood characteristics in vessels having a non-uniform distribution of blood
components.
An advantage of the present invention is that it provides a means for the
rapid, non-invasive measurement of clinically significant parameters of the
CBC+Diff test. It advantageously provides immediate results. As such, it can
be
used for point-of care testing and diagnosis.
A further advantage of the present invention is that it eliminates the
invasive technique of drawing blood. This eliminates the pain and difficulty
of
drawing blood from. newborns, children, elderly patients, burn patients, and
patients in special care units. The present invention is also advantageous in
that
it mitigates the risk of exposure to AIDS, hepatitis, and other.blood-borne
diseases.
A still further advantage of the present invention is that it provides for
overall cost savings by eliminating sample transportation, handling, and
disposal
costs associated with conventional invasive techniques.
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-9-
A still further advantage of the present invention is that it provides for the
measurement of the hematocrit without having to individually count the red
blood
cells. As such, the present invention can precisely determine the red blood
cell
count even if the individual red blood cells cannot be clearly imaged.
BRIEF DESCRIPTION OF THE FIGURES
The present invention is described with reference to the accompanying
drawings. In the drawings, like reference numbers indicate identical or
functionally similar elements. Additionally, the left-most digits) of a
reference
number identifies the drawing in which the reference number first appears.
FIG.1 a shows a cross-sectional view of a medium blood vessel containing
red blood cells;
FIG. 1b shows a cross-sectional view of a small blood vessel containing
red blood cells;
FIG. lc shows a cross-sectional view of a large blood vessel containing
red blood cells;
FIG. 2 illustrates vessel diameter measurements being taken by an
embodiment of the present invention;
FIG. 3 illustrates intensity measurements being taken by an embodiment
of the present invention;
FIG. 4 shows a flow chart representing the general operational flow for
measuring the hematocrit from the variation in diameter measurements according
to an embodiment of the present invention;
FIG. 5 shows a flow chart representing the general operational flow for .
measuring the hematocrit from variations in optical density measurements
according to an embodiment of the present invention;
FIG. 6 compares hematocrit measurements according to the present
invention versus in vitro hematocrit measurements; and
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-10-
FIG. 7 shows a block diagram of an example computer system useful for
implementing the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is directed to a method and system for performing
quantitative analyses, particularly non-invasive, in vivo analyses of a
subject's
vascular system. The in vivo measurements discussed herein can also be
performed ivy vitro by imaging blood in, for example, a tube or flow cell, as
would
be apparent to a person skilled in the relevant art(s). The images can be
obtained
from a spectral imaging apparatus preferably, but not necessarily, of the type
described in the '120 patent or the '859 application. Nonetheless, the image
can
be obtained from any type of imaging apparatus designed to produce a contrast
image of a suspension of particles in a vascular system in either transmitted
or
reflected light. As disclosed inthe '120 patent orthe '859 application, the
spectral
imaging apparatus. includes a light source that is used to illuminate the
portion of
the subj ect's vascular system to be imaged. The reflected light is captured
by an
image capturing means. Suitable image capturing means include, but are not
limited to, a camera, a fi1W medium, a photocell, a photodiode, or a charge
coupled device camera, An image correcting and analyzing means, such as a
computer, is coupled to ~ the image capturing means for carrying out image
correction, scene segmentation, and blood characteristic analysis.
The in vivo method of the present invention is carried out by imaging a
portion of the subj ect's vascular system. For example, the image can be
created
from a sub-surface region of a subj ect's tissues or organs. The tissue
covering the
imaged portion is thus preferably transparent to light, and relatively thin,
such as
the mucosal membrane on the inside of the lip or the sclera of the eyeball of
a
human subject. As used herein, "light" refers generally to electromagnetic
radiation of any wavelength, including the infrared, visible, and ultraviolet
portions of the spectrum. A particularly preferred portion of the spectrum is
that
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-11-
portion where there is relative transparency of tissue due to absences of
water
abortion, such as in visible and near-infrared wavelengths. It is to be
understood
that for the present invention, light can be coherent light or incoherent
light, and
illumination can be steady or in pulses of light.
Human blood is made up of formed elements and plasma. There are three
basic types of formed blood cell components: red blood cells (erythrocytes);
white blood cells (leukocytes); and platelets. As noted above, red blood cells
contain hemoglobin that carries oxygen from the lungs to the tissues of the
body.
White blood cells are of approximately the same size as red blood cells, but
do
not contain hemoglobin. A normal healthy individual will have approximately
5,000,000 red blood cells per cubic millimeter ofblood, and approximately
7,500
white blood cells per cubic millimeter of blood. Therefore, a normal healthy
individual will have approximately one white blood cell for every 670 red
blood
cells circulating in the vascular system.
A complete blood count (CBC) without white blood cell differential
reports eightparameters: (1) hemoglobin (Hb); (2) hematocrit (Hct); (3)
redblood
cell count (RBC); (4) mean cell volume (MCV); (5) mean cell hemoglobin
(MCH); (6) mean cell hemoglobin concentration (MCHC); (7) white blood cell
count (WBC); and (8) platelet count (Plt). The first six parameters are
referred to
herein as RBC parameters. Concentration measurements (measurements per unit
volume of blood) are necessary for producing values for Hb, Hct, RBC, WBC,
and Plt. Hb is the hemoglobin concentration per unit volume of blood. Hct is
the
volume of cells per unit volume of blood. Hct can be expressed as a
percentage,
i.e.,:
(red blood cell volume = volume of blood) X 100% (Eqn. 1)
RBC is the number of red blood cells per unit volume of blood. WBC is the
number of white blood cells per unit volume of blood. Plt is the number of
platelets per unit volume of blood.
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-12-
Red blood cell indices (MCV, MCH, and MCHC) are cellular parameters
that depict the volume, hemoglobin content, and hemoglobin concentration,
respectively, of the average red blood cell. The red blood cell indices can be
determined by making measurements on individual cells, and averaging the
individual cell measurements. Red blood cells do not change volume or lose
hemoglobin as they move through the vascular system. Therefore, red blood cell
indices are constant throughout the circulation, and can be reliably measured
in
small vessels. The three red blood cell indices are related by the equation:
MCHC = MCH = MCV (Eqn. 2)
Thus, only two red blood cell indices are independent variables.
To determine values for the six RBC parameters listed above, the
following two criteria must be met. First, three of the parameters must be
independently measured or determined. That is, three of the parameters must be
measured or determined without reference to any other of the six parameters.
Second, at least one of the three independently measured or determined
parameters must be a concentration parameter (per unit volume of blood).
Therefore, values for the six key parameters can be determined by making three
independent measurements, at least one of which is a concentration measurement
which cannot be made in a small vessel.
As disclosed in the '120 patent or the '859 application, Hb and Hct can
be directly measured by reflected spectral imaging of large vessels, while MCV
and MCHC can be directly measured by reflected spectral imaging of small
vessels. In this manner, three parameters are independently measured, and two
of
the parameters (Hb and Hct) are concentration parameters measured per unit
volume of blood. As such, the six RBC parameters listed above can be
determined in the following manner:
Hb Directly measured
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-13-
Hct Directly measured
RBC Hct = MCV
MCV Directly measured
MCH MCV x (Hb-Hct)
MCHC Hb = Hct
Alternatively, as disclosed in the '120 patent or the '859 application, Hb
can be directly measured by reflected spectral imaging of large vessels, and
MCV
and MCHC can be directly measured by reflected spectral imaging of small
vessels. In this manner, three parameters are independently measured, and one
of
the parameters (Hb) is a concentration parameter measured per unit volume of
blood. As such, the six RBC parameters listed above can be determined in the
following manner:
Hb Directly measured
Hct Hb = MCHC
RBC Hb = (MCV x MCHC)
MCV Directly measured
MCH MCV x MCHC
MCHC Directly measured
Hemoglobin is the main component of red blood cells. Hemoglobin is a
protein that serves as a vehicle for the transportation of oxygen and carbon
dioxide throughout the vascular system. Hemoglobin absorbs light at particular
absorbing wavelengths, such as 550 nm, and does not absorb light at other non-
absorbing wavelengths, such as 650 nm. Under Beer's law, the negative
logarithm of the measured transmitted light intensity is linearly related to
concentration. As explained more fully in the '120 patent or the '859
application,
a spectral imaging apparatus can be configured so that reflected light
intensity
follows Beer's law. Assuming Beer's law applies, the concentration of
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-14-
hemoglobin in a particular sample of blood is linearly related to the negative
logarithm of reflected light absorbed by hemoglobin. The more 550 nm light
absorbed by a blood sample, the lower the reflected light intensity at 550 nm,
and
the higher the concentration of hemoglobin in that blood sample. The
concentration of hemoglobin can be computed by taking the negative logaritlun
of the measured reflected light intensity at an absorbing wavelength such as
550
nm. Therefore, if the reflected light intensity from a particular sample of
blood
is measured, the concentration in the blood of such components as hemoglobin
can be directly determined.
The method and system of the present invention can be used to directly
measure the hematocrit and can be used to quantitatively analyze a vascular
system even if the measured components are not uniformly distributed. For
example, the present invention can be used to measure the hematocrit of a
blood
vessel that does not have a uniform distribution of red blood cells.
FIG. 1 a illustrates a typical segment of a blood vessel 100 of length L and
diameter D. Vessel I00 has a discrete number (N) of red blood cells. The
hematocrit (Hct) of vessel 100 can be expressed as NVB = (~/4)DzL, where VB is
the mean volume of a red blood cell. Hence, the hematocrit for any region of
vessel 100 can be expressed by the following probability function:
F(1~ = NVB = (~/4)DZL (Eqn. 3)
where N is a parameter that varies along the vessel length L at any given
time,
and also varies in time, at any given point along the vessel length L. The
distribution of the variable N is best described bythe poisson distribution
wherein
the variance is proportional to the square root of the variable. For instance,
at any
given time, a section of blood vessel 100 would have an average number of red
blood cells measured by:
N = (Hct)(~/4)DzL = VB (Eqn. 4)
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-15-
The standard deviation of the mean N is proportional to the square root of N ,
and the coefficient ofvariation (C.V.) canbe calculated as the standard
deviation
over the mean, or:
1
C. V. _ ~ (Eqn. 5)
Combining the equations 4 and 5, the measure of the fluctuation or variation
in
the number of red blood cells can be shown by: '
C.V.(I~ = 1 4~B (Eqn. 6)
D (Hct)~zL
Thus, the coefficient of the variation of N is a function of the Hct and the
vessel
diameter. This variation will be manifested as a variation in the optical
density
or the diameter along an image formed of the vessel. Alternatively, it will
manifest as a variation in either the diameter or the optical density in a
time series
of images of any one point in the vessel.
As discussed above, the use of reflected spectral imaging to measure Hb
is based on the assumption that the blood vessels are uniformly filled. High
variations in the number of red blood cells measured along the length of a
vessel
suggest that the vessel is not uniformly filled. Refernng to equation 6, it
can be
seen that the coefficient of variation is inversely related to the vessel's
diameter.
Larger blood vessels, having diameters exceeding 50 microns, have low
coefficient of variations which suggest they have a uniform distribution of
Hb.
FIG. 1b illustrates a typical small blood vessel of diameter D. Small blood
vessels are those having a diameter less than 6 microns. The dimensions of the
vessels in this range are comparable to the dimensions of the red blood cells
(shown as diameter d). Therefore, typically only a single stream of red blood
cells
is permitted to flow through the vessels. In this instance, one can use
spectrophotometry (i.e., Beer's law) to calculate the MCHC but not to measure
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-16-
the Hct and Hb. Red blood cell concentration can only be determined by
counting
red blood cells along the vessel.
For medium sized vessels (such as vessel 100 shown in FIG.1 a), between
the range of 6 to 60 microns, the high variation (e.g., 10-20%) indicates a
non-
uniform distribution of Hb and complicates the use of Beer's law to estimate
the
Hb or the MCHC. The vessel size is large enough to permit multiple streams of
red blood cells which impair the use of spectrophotometry to determine precise
measures of RBC by counting individual cells. In fact, vessels in this range
can
be as large as 2 to 15 red blood cells in diameter. For unfilled vessels, the
method
and system of the present invention can be used to estimate the Hct from the
coefficient of variation and vessel diameter.
FIG. 1c illustrates a typical large vessel of diameter D. In large vessels,
discrete measurements ofreflective properties, and hence spectrophotometry
(i.e.,
Beer's law), can be used to estimate the Hb. For smaller blood vessels, the
high
variation in the number of red .blood cells imposes a profound effect on
estimating Hb as a function of the optical density measured from a reflected
spectral image.
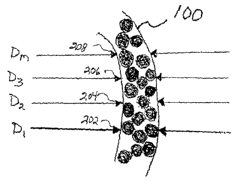
FIG. 2 illustrates a vessel's diameter being measured. As shown, "m"
diameter measurements (202, 204, 206 and 208) are made along the axis of
vessel
100.
FIG. 3 illustrates a vessel's intensity being measured. As shown, "m"
intensity measurements are made along the axis of vessel 100. The original
light
(302, 304, 306 and 308) is depicted as Io", and the attenuated light (312,
314, 316
and 318) is depicted as I".
Referring to FIG. 4, flowchart 400 represents the general operational flow
of the present invention. More specifically, flowchart 400 shows an example of
a control flow for measuring the hematocrit of a blood vessel.
FIG. 4 begins at step 401. At step 405, an image is retrieved from a
memory source or image directory. The images can be obtained from an input
file
stored in a temporary or permanent memory location on a haxd disk drive or
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-17-
removable storage device, such as a floppy diskette, magnetic tape, optical
disks,
or the like, as would be apparent to a person skilled in the relevant art(s).
The
input file also includes the subject number or other data used to identify the
subject. Alternatively, the images can be obtained in real time from an
imaging
S apparatus preferably, but not necessarily, of the type described in the '120
patent
or the '8S9 application.
At step 410, multiple measurements are taken along the axis of the vessel
to calculate the diameter at different segments. Referring back to FIG. 2, "m"
diameter measurements (202, 204, 206 and 208) are made along the axis of
vessel
~ 100. As would be apparent to a person skilled in the relevant art(s), the
number
of measurements or segments should be sufficient to obtain an accurate
measurement of the diameter. At step 41 S, the diameter measurements are
analyzed to determine the coefficient of variation.
At step 420, the fractional volume of a cellular component (e.g., red blood
1 S cell) is determined for the vessel. Two methods can be used to determine
the
fractional volume. Under the first method, the fractional volume of the
cellular
component is determined at each segment by taking the reciprocal of the
product
of the coefficient of variation and the diameter measurements for each
segment.
Under the second method, the fractional volume of the cellular component can
be determined by taking the reciprocal of the product of the coefficient of
variation and the average of the diameter measurements.
At step 425, the Hct is calculated from the mean fractional volume. If the
first method is used to determine the fractional volume, the average of all
the
fractional volume measurements is calculated and used to estimate the Hct. If,
2S however, the second method is used, the mean fractional volume calculated
at
step 420 would be used to estimate the Hct. After the Hct is calculated, the
control flow of flowchart 400 ends as indicated by step 495.
Referring to FIG. S, flowchart S 00 represents the general operational flow
of another embodiment ofthe present invention. More specifically, flowchart
S00
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
_18_
shows an example of a control flow for using optical density to measure the
hematocrit of a blood vessel.
FIG. 5 begins at step 501. The control flow proceeds to step 405 through
step 410 as discussed above with reference to FIG. 4. At step 510, the vessel
is
illuminated at multiple sections along its axis to measure the original and
attenuated light intensities. Refernng back to FIG. 3, "m" intensity
measurements
are made along the axis of vessel 100. An intensity profile is created by
computing the negative logarithm of the ratio of the measured attenuated light
intensity and the original light intensity to produce the optical density. As
would
be apparent to a person skilled in the relevant art(s), the number of
measurements
or segments should be sufficient to obtain an accurate measurement of the
optical
density.
At step 515, the optical density measurements are analyzed to determine
the coefficient of variation. At step 520, the fractional volume of the
cellular
component is determined at each segment by taking the reciprocal of the
product
of the coefficient of variation and the diameter measurements from each
segment.
As discussed in reference to FIG. 4, the fractional volume of the cellular
component can also be determined at each segment by taking the reciprocal of
the
product of the coefficient of variation and the average of the diameter
measurements.
At step 525, the average of all the fractional volume measurements is
calculated to estimate the Hct. If, however, the second method is used to
determine the fractional volume, the mean fractional volume determined at step
520 would be used to estimate the Hct. The control flow of flowchart 500 ends
as indicated by step 595.
Hence, it can be demonstrated that the hematocrit of a subject can be
determined from the coefficient of variation in optical density or the
diameter,
either along an image formed of a blood vessel, or in a time series of images
of
any one point in the vessel. FIG. 6 shows the results of a comparative study
that
implements the methods and systems of the present invention. During the study,
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-19-
in vitro measurements of blood samples were taken from nine subj ects and used
to determine their hematocrit. Using the methods of the present invention, ira
vivo
images from the eye (i. e., sclera) of the nine subj ects were obtained by
orthogonal
polarization spectroscopy, as described in the '120 patent or the '~59
application.
For each subject, five blood segments were selected to determine diameter
and optical density measurements. The variation in the optical density along
the
respective blood vessel was also measured. Next, the reciprocal of the product
of
the variation and the diameter for each segment was computed. Then, the
average
of this quantity for each of the nine subj ects was calculated to combine the
results
from the in vivo measurements into a single hematocrit estimate.
The results from the in vivo and in vitro measurements are shown in
FIG. 6. The ordinate axis represents the hematocrit determined from the in
vitro
measurements, and the abscissa represents the hematocrit from the in vivo
measurements. The slope for the hematocrit calculation is 0.91, which suggests
that the in vivo measurements are close approximations for the in vitro
measurements.
As is apparent from the foregoing description, the present invention was
developed primarily to analyze blood components in a non-invasive manner.
However, it will be clear to persons skilled in the relevant arts) that the
analysis
techniques of this invention have utility beyond the medical applications
described above. The invention has application outside the medical area and
can
be used generally to quantitatively analyze visualizable components in a fluid
flowing in any vascular system, such as a tube, the walls of which are
transparent
to transmitted and reflected light. The present invention is most effective
for
analyzing vessels having diameters between 6 to 60 microns, which represents
the
most likely sized vessel to be detected with spectrophotometry. A preferred
range
is 15 to 50 microns where the coefficient of variation averages 10 to 20%.
The present invention can be implemented using hardware, software or
a combination thereof and can be implemented in one or more computer systems
or other processing systems. In fact, in an embodiment, the invention is
directed
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-20-
toward one or more computer systems capable of carrying out the functionality
described herein.
Referring to FIG. 7, an example computer system 700 useful in
implementing the present invention is shown. Computer system 700 includes one
or more processors, such as processor 704. The processor 704 is connected to a
communication infrastructure 706 (e.g., a communications bus, cross-over bar,
or network). Various software embodiments are described in terms of this
exemplary computer system. After reading this description, it will become
apparent to a person skilled in the relevant arts) how to implement the
invention
using other computer systems andlor computer architectures.
Computer system 700 can include a display interface 702 that forwards
graphics, text, and other data from the communication infrastructure 706 (or
from
a frame buffer not shown) fox display on the display unit 730.
Computer system 700 also includes a main memory 708, preferably
random access memory (R.AM), and can also include a secondary memory 710.
The secondary memory 710 can include, for example, a hard disk drive 712
and/or a removable storage drive 714, representing a floppy disk drive, a
magnetic tape drive, an optical disk drive, etc. The removable storage drive
714
reads from and/or writes to a removable storage unit 718 in a well-known
manner. Removable storage unit 718, represents a floppy disk, magnetic tape,
optical disk, etc. which is read by and written to removable storage drive
714. As
will be appreciated, the removable storage unit 718 includes a computer usable
storage medium having stored therein computer software and/or data.
In alternative embodiments, secondary memory 710 can include other
similar means for allowing computer programs or other instructions to be
loaded
into computer system 700. Such means can include, for example, a removable
storage unit 722 and an interface 720. Examples of such can include a program
cartridge and cartridge interface (such as that found in video game devices),
a
removable memory chip (such as an EPROM, or PROM) and associated socket,
and other removable storage units 722 and interfaces 720 which allow software
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-21-
and data to be transferred from the removable storage unit 722 to computer
system 700.
Computer system 700 can also include a communications interface 724.
Communications interface 724 allows software and data to be transferred
between
computer system 700 and external devices. Examples of communications
interface 724 can include a modem, a network interface (such as an Ethernet
card), a communications port, a PCMCIA slot and card, etc. Software and data
transferred via communications interface 724 are in the form of signals 728
which
can be electronic, electromagnetic, optical or other signals capable of being
received by communications interface 724. These signals 728 are provided to
communications interface 724 via a communications path (i.e., channel) 726.
This channel 726 carries signals 728 and can be implemented using wire or
cable,
fiber optics, a phone line, a cellular phone link, an RF link and other
communications channels.
In this document, the terms "computer program medium" and "computer
usable medium" are used to generally refer to media such as removable storage
drive 714, a hard disk installed in hard disk drive' 712, and signals 728.
These
computer program products are means for providing software to computer system
700. The invention is directed to such computer program products.
Computer programs (also called computer control logic) are stored in
main memory 708 and/or secondary memory 710. Computer programs can also
be received via communications interface 724. Such computer programs, when
executed, enable the computer system 700 to perform the features of the
present
invention as discussed herein. In particular, the computer programs, when
executed, enable the processor 704 to perform the features of the present
invention. Accordingly, such computer programs represent controllers of the
computer system 700.
In an embodiment where the invention is implemented using software, the
software can be stored in a computer program product and loaded into computer
system 700 using removable storage drive 714, hard drive 712 or communications
CA 02428866 2003-05-14
WO 02/43561 PCT/USO1/43097
-22-
interface 724. The control logic (software), when executed by the processor
704,
causes the processor 704 to perform the functions of the invention as
described
herein.
In another embodiment, the invention is implemented primarily in
hardware using, for example, hardware components such as application specific
integrated circuits (ASICs). Implementation of the hardware state machine so
as
to perforn the functions described herein will be apparent to persons skilled
in
the relevant art(s).
In yet another embodiment, the invention is implemented using a
combination of both hardware and software.
While various embodiments of the present invention have been described
above, it should be understood that they have been presented by way of
example,
and not limitation. It will be apparent to persons skilled in the relevant
arts) that
various changes in form and detail can be made therein without departing from
the spirit and scope of the invention. Thus, the present invention should not
be
limited by any of the above described exemplary embodiments.