Note: Descriptions are shown in the official language in which they were submitted.
CA 02428896 2003-05-16
MICROBUBBLE CONSTRUCT IFOR SENSITIVITY ENHANCED MR
MANOMETRY
CROSS REFERENCE TO RELATED UNITF,D STATES PATENT
APPLICATIONS
This patent application relates to United States Provisional patent
application Serial No. 60f378,048 filed on May 16, 2002, entitled MICROBUBBLE
CONSTRUCT FOR SENSITIVITY ENHANCED MR MANOMETRY, and
Canadian patent application Serial No. 2,418,229 filed on Tanuary 31, 2003,
entitled MICROBUBBLE CONSTRUCT FOR SENSITIVITY ENHANCED MR
MANOMETRY, both published in English, and both patent applications
being incorporated herein by reference in their entirety.
FIELD OF THE INVENTION
The present invention relates to microbubbles for sensitivity enhanced
manometry, and more particularly the present invention relates to a magnetic
resonance
manometry method for measuring intravascular or intracardiac pressure using
microbubbles of high magnetic susceptibility.
BACKGROUND OF THE INVENTION
A quantitative intracardiac pressure measurement can provide clinicians with a
strong measure of the functional integrity of the cardiovascular system as
noted in E.
Braunwald et al. in Heart Disease, p 780-806. It is possible to infer the
pressure in
the left ventricle with a sphygmomanometer and to date, there have been
numerous
efforts made to develop a similar non-invasive means of measuring pressure in
the right
ventricle (RV) as noted in M Bergen et al. in "Quantitative assessment of
pulmonary
CA 02428896 2003-05-16
2
hypertension in patients with tricuspid regurgitation using continuous wave
Doppler ultrasound," Am J Caxdiol 1985; 6:359-365. Such efforts have been made
with
the intent of replacing widely used catheterization procedures and the
associated physical
discomfort and risk of infection in patients as noted in D Raeside et al. in
"Making
measurements in the pulmonary circulation: when and how?'°, Thorax
1997; 52:9-11.
R~1 pressure measurement using continuous wave Doppler echocardiography based
on
the peak velocity of the tricuspid jet with the modified Bernoulli's equation
is possible
only when tricuspid insufficiency exists. However, this usu,~lly does not set
in until peak
RV pressure is greater than 75 mmHg as noted in B3 Kircher et al. in
"hloninvasive
estimation of right atrial pressure from the inspiratory collapse of the
inferior vena
cave", Am J Cardiol 1990;66:493-496. However, the progression of many
congenital
heart diseases involve small continuous changes in RV pressure. Pulmonary
hypertension
is defined as an increase in RAT systolic pressure above 30 nunHg (~or 5 mmHg
above the
normal systolic pressure of the RV) as defined in Braunwald E et al. Hence,
one of the
essential requirements of a non-invasive pressure measurement technique is
exquisite
sensitivity to detect small pressure changes associated with pulmonary
hypertension. It
has long been realized that distensible micro-bubbles can serve as pressure
sensors for
non-invasive manometry. Since the 1970's many ultrasound techniques have tried
to take
advantage of this idea. However, various technical difficulties have prevented
their
advancement in vivo. A magnetic resonance (MR) based technique that has the
potential
for detecting intravascular pressure with the aid of a microbubble contrast
agent was
recently proposed by AL Alexander et al. in "Microbubbles as novel pressure-
sensitive
MR contrast agents" , Magn Reson Med 1996. 35:801-806. Their hypothesis was
based
on the observation of earlier reports of other works which showed that the
rate of
CA 02428896 2003-05-16
3
relaxation of the MR signal (R2) from a solution containing spheres (with a
susceptibility
mismatch relative to the solution) is related to the size of sphere. Since
microbubbles
respond to pressure changes via volume changes, they correctly predicted that
R2 can be
used to calibrate and serve as a pressure marker in vivo. While the early
experimental
results in vitro have shown this successfully, an in vivo use of this
technique for early
detection of pulmonary hypertension (25 mmHg above right ventricular systolic
pressure
or 50 mmHg above atmospheric pressure) is currently limited by inadequate
sensitivity.
The current limitations of microbubble based MR manometxy are as follows.
(1) Inadequate R2 measurement accuracy associated with detecting small changes
in
pressure. The measurement errors in R2 originate from cardiac motion,
breathing, flow
dephasing, and partial volume effects.
2) Suboptimal changes in R2 for a given pressure change in the presence of
microbubbles in the blood stream for a given microbubble dose. In the presence
of
microbubbles in vivo,
RG lood i R~~s -E' R~C ,"- R~ubb
where RZD'ss is the rate constant associated with the decay of the MR signal
due to
dissipative mechanisms such as dipole-dipole coupling and is ~ 4 s°1 .
R2~oand RB"bv
are the rate constants connected with decay of the MR signal due diffusion of
spins in a
field gradient set up by the red blood cells and the bubbles respectively. At
1.5T, with a
refocusing interval (zl8o) of 6 ms at oxygenation. saturation of 70% Oz in the
pulmonary
trunk is R2~o~ 1.2 s 1. Hence, under similar conditions, we anticipate that
for the
bubbles to -dominate the relaxation process, RB~b should be at least 5 s 1.
CA 02428896 2003-05-16
4
3) Even large pressure changes {100's mmHg) cannot be detected without
physiologically
toxic microbubble dose. Toxicity testing of microbubble formulations to date
has shown
that, when the dose of bubble formulations exceed 1 cclkg, clinical
complications
emerge. The volume fraction of gas used by Alexander et a:L corresponds to a
dose of
approximately 3 cc/kg (assuming 4 L of blood in a 70 kg body) that would be
toxic in
vivo. This means that a large enough R2B°bh needs to be established in
vivo with the
smallest possible microbubble dose. Preliminary experiments at 4.7 T by others
have
revealed that, when microbubbles containing air (mean radius of 3.03 ~ 0.53 m)
are used
3 ec/kg, the spin echo R2B°bb 1S 1 ~ s 1, which is large enouglx to
produce the adequate
sensitivity. However, considering the target clinical utility, the low
pressure sensitivity of
R2, toxic microbubble doses are required. In addition, at large static field
strengths (such
as 4.7T) the contribution of R2~ to R2B1°°d will also be higher.
In addition, at high
fields it is anticipated that the motion and flow artifacts will further
degrade the accuracy
of the measurement technique.
As shown by R Dharmakumar et aL, in "On the parameters affecting the
sensitivity of MR measures of pressure with microbubbles", MRM 2002. 47: 264-
273
previously there are a number of parameters that affect the sensitivity of
microbubble
based MR manometry. Results show that the MR sensitivity to pressure changes
is
strongly dependent on the bubble size at atmospheric pressure (RD), static
magnetic held
strength, magnitude of the susceptibility difference between the encapsulated
gas and
plasma (0~, and bubble volume fraction. It was also found that the optimum
bubble size
is strongly dependent on the type of nuclear magnetic resonance (I~-MR)
measurement
method and improves with increase in magnetic field strengi;h, susceptibility
difference,
and volume fraction. To reduce measurement errors associated with detecting MR
signal
CA 02428896 2003-05-16
for cardiovascular applications it has been suggested that Carr-Purcell-
Meiboom-Gill
based pulse sequence is used by GA Wright et al., in "Estimating oxygen
saturation of
blood in vivo with MR imaging at 1.5T". JMRI 1991;1:275-283. In addition,
given that
most common commercial MR scanners operate at 1.5 T and physiological
complications
based on microbubble toxicity arise when the dose exceeds 1 cc/kg of body
weight, it was
concluded that for R2Bubb t0 be larger than 5 s 1, Ro should be 2-3 ~xn and ~x
>_ 34 ppm
(SI units). Although optimum R4 is feasible, the microbubble contrast agent
with the
largest realizable Ox that is limited by the inherent low density of gases at
11 ppm (SI
units). Hence the successful clinical implementation of MR manometry relies on
improved susceptibility difference in excess of 34 ppm, physiologically
tolerable
microbubble doses, a calibration scheme to relate pressure changes to R2, and
an MR
protocol to make the requisite measurement.
SUMMARY OF THE INVENTION
The inventors show that a specialized microbubble design Can effectively
increase
the ax to desired levels through enhancing the magnetic susceptibility of
microbubble
shell. In particular they show that embedding magnetic nanc~particles of high
dipole
moment on the lipid shell of the 'typical microbubbles can increase the ~x to
desired
levels while preserving the pressure sensitivity of microbubbles in the MR
field. This is
shown by first re-deriving the governing equation of field perturbation around
a gas
containing bubble coated with a highly susceptible continuous shell. From
there it is
shown that the continuous shell case is equivalent to uniformly coating the
lipid shell
with particles of high dipole moment. It is disclosed herein tk~at the
resulting dx is a
function of particle dipole moment, size, and density on the shell. In
addition, with the
CA 02428896 2003-05-16
6
aid of Monte Carlo simulations they show that when particles of high enough
dipole
moment are coated at low volume fraction, it is feasible to elevate R2B"ab
well beyond
Ss'1. It is disclosed that microbubble dose is proportional to R2~"bb and the
present
invention establishs that by controlling the volume fraction of the particles
on the
microbubble shell it is also possible to reduce the microbubble dose within a
physiologically acceptable range. Through the theoretical work underpinning
the present
invention it is demonstrated that these specialized microbubbles are capable
of acting as
highly sensitive non-invasive pressure probes that will be instrumental in the
sensitive
detection of moderate pulmonary hypertension with magnetic resonance imaging.
The
present invention also shows how this technique may be implemented from the
fabrication of the necessary microbubbles to the MR protocol to measure
pressure.
In addition to detecting pulmonary hypertension in vivo, this technique may
also
be broadened to measure intracardiac and intravascular pressure anywhere else
in the
circulation. For instance, using this technique one should be able to measure
aortic
pressures that are not visible to sphygmomanometer, pressure changes in
atheroscelortic
regions of the vasculature, intracranial pressure, and pressure in the ocular
cavity to name
a few.
The present invention provides a microbubble for sensitivity enhanced magnetic
resonance manometry, comprising a lipid shell having a high magnetic
susceptibility.
The present invention provides a microbubble for sensitivity enhanced magnetic
resonance manometry, comprising a lipid shell including magnetic nanoparticles
having
high dipole moments embedded therein.
The present invention provides a microbubble for sensitivity enhanced magnetic
resonance manometry, comprising a lipid shell including a magnetically active
agent
CA 02428896 2003-05-16
7
attached to, or incorporated into, the surface of the bubble to give said
rnierobubble a pre-
selected magnetic susceptibility.
In another aspect of the present invention there is provided a magnetic
resonance
imaging method for measuring intravascular or intracardiac pressure in a
patient, the
method comprising the steps of;
a). intravenously administering mierobubbles to a patient, said microbubbles
comprising a lipid shell having a high magnetic susceptibility;
b). performing cardiac-gated, flow and/or motion compensated magnetic
resonance imaging to establish microbubble concentration dependent and
pressure
independent magnetic resonance (MR) signal decay in a major blood vessel or in
a
sample of blood drawn from said patient; and
c). measuring decay of the magnetic resonance signal in a region of interest
in the
patient's body, comparing a difference between pressure independent magnetic
resonance
signal decay and pressure dependent magnetic resonance aignal decay to a
calibration
curve between magnetic resonance signal decay and pressure to determine the
pressure in
the region of interest.
CA 02428896 2003-05-16
8
BRIEF DESCRIPTION OF TI3E DRAWINGS
The method of the present invention will now be described, reference being had
to
the accompanying drawings, in which:
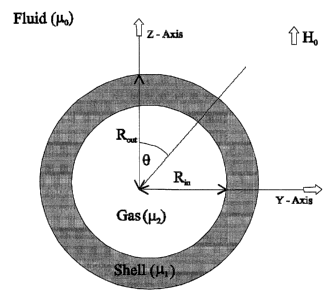
Fig. 1 is a schematic representation of a sphere with inner radius of
R;° and outer
radius R°,~ and a uniform external magnetic field of Ho directed along
the z-axis. The
magnetic permeability of the region outside the sphere is ia,~, in the shell
is p,1, and of the
gas inside the sphere is ~.2;
Fig. 2 is a plot showing the relationship between the shell thickness and
effective
magnetic susceptibility difference between the fluid and the gas containing
microbubble
with shells of non-negligible magnetic permeability;
Fig. 3 is a plot showing the relationship between the. susceptibility of
particles of
different sizes embedded on microbubble shell and the effective magnetic
susceptibility
difference between blood plasma and air containing microbubble. In all cases
microbubble radius was fixed at 2 ~,m;
Fig. 4 is a plot showing the relationship between the two ways of increasing
the
particle volume shell fraction and the effective magnetic susceptibility
difference
between blood plasma and air containing mierobubble. In both cases particle
susceptibility was fixed at 10000 ~c ppm; and rnicrobubble radius vvas fixed
at 2 ~.m;
Fig. 5 is a plot comparing the effect of pressure changes on R2 of blood
containing
free air bubble and magnetite coated microbubble. Both microbubbles are 2
E.itn in radius
and contain air in their lumen. The air bubble volume fraction was 0.1527% (or
dose of
0.87 eclkg) but the magnetite coated microbubble volume fraction 'was 0.0344%
(or a
dose of 0.2 cc/kg). Magnetite particles were 15 nm in radius and had a total
magnetic
susceptibility of 128000~t ppm. R2 was obtained via Monte Carlo simulations
with a
CA 02428896 2003-05-16
9
refocusing interval =6 ms; diffusion coefficient of water = 2.75 x 10-9 m2 ~s
1; Bo =1.ST;
and
Fig. 6(A-C) show the different microbubble constructs with magnetically active
agents that can be prepared: agents incorporated onto the surface (A), in
between the
bilayers (B), or within an oil layer of a multilamellar structured microbubble
(C).
DETAILED DESCRIPTION OF THE INVENTION
I. A Continuum Model
When a spherical microbubble is placed fn a fluid with a magnetic permeability
of
,cry in which an external uniform magnetic field Ho is present, the field
around the
microbubble is disturbed. The equation which represents this field is the
solution to the
associated 3D Laplace equation of the magnetic scalar potential, A. If we let
~.2 to
represent the magnetic permeability of gas inside the bubble, ~,1 represent
the magnetic
permeability of the shell of the micro-bubble, and H represent the magnetic
field intensity
and B represents the magnetic field (where B = ~.H) then the Maxwell's
equations
corresponding to this magnetostatic can be combined in a 3D Laplacian for
magnetic
scalar potential given by
~2A = 0, (2)
which is a well known partial differential equation which has the following
solution in
spherical coordinates (r,8, ~):
Ao = (-Hor + Io / r2)cos8, r > Ri
Al = (-Hrr + h / rz~cos8, Rl > r > R2 (3)
AZ = -H2 rcos8, r < R2,
CA 02428896 2003-05-16
1d
where Ao, Al, A2 correspond to the magnetic scalar potential outside the
bubble, in the shell, and
inside the bubble respectively and h, Hl, and H2 are to be solved from the
boundary conditions.
R~ is the radius of the bubble without the shell and Rout is the radius of the
bubble with the
shell (refer to Fig. 1).
The boundary conditions for the problem must satisfy the following criteria:
the tangential
component of H (or HB) and the radial (or normal) component of L (or Br) are
continuous across
the different boundaries [9). From here, it is possible to show that the z
component of the local
field perturbation in the vicinity of the bubble in terms of the dipole
moment, a, in the spherical
coordinate system is given by
~Bx = ~ ~ ~3 cos2 B - 1 ) , (4)
where
- 91~Wo6x2Bo~ ~ _ OXi 3
(W + 2~CO) (2W + ~2)(2f~o -1- y) - 20Xz ~ OX1. - 3 + !~1 +2~oBoRout~
out
with ~Xl = f~l - I~o; L~k2 = !~2 - W ; Bo = I~Ho~ I~ = 1 - X, where ~~ the
magnetic susceptibility.
(A). Shell-free or Lipid-shelled Gas Bubble in Plasma
When there is no shell present (Rout = Rin) or when the shell is made of lipid
bilayer
(~Cl ~~ ~2), the first term in the above equation vanishes and we get
a ' I~1~x2l~o B~R°ut ~ (b)
Moreover if the magnetic susceptibility of the fluid and the gas are very
small then ,uo N u1 ~, 1
and hence
g lBoRout~
Thus from Eq. (4), it follows that the field perturbation along the z-axis is
3
OBz = 3 ~ GlXlBo ~ ~'~out ~ . ~3 cos2 9 - 1~ . ('T)
CA 02428896 2003-05-16
~1
This is the equation originally reported by JA Glasel et al in "On the
interpretation of water
nuclear magnetic resonance relaxation times in heterogeneous systems". J Am
Chem Soc
96:970 (1974). These were the same equations that were used by R Dharmakumar
et al
with lipid-shelled microbubbles. Under these conditions the only way to change
the magnetic
susceptibility of such bubbles in the plasma is to change the susceptibility
of the encapsulated
gas.
(B). Gas Bubble avith Highly Susceptible ,Shell in Plasma
Now suppose the sphere has a shell that is highly permeable to the static
magnetic field. If
the susceptibility of this shell is much larger than the gas inside the bubble
or fluid outside
the bubble ( or X2 « Xl and Xo « Xl), the magnetic dipole moment can be
reduced to
1 ()
~ = 3 ' ~Xeff ° Bo ° Routs g
where
~x 3 ~X~ + 4 ' (1 + x~) ° ~X2 ~ ,~3
eff = [3 -I- Xz~ ~ ~ (20X1 + 3) (Xs + 3) -I- 2Xi ~ ~3~ (
and denote Rm = ~3Rout where ,(3 E (0,1), and ~1 = 1 + Xl. From Eq. (4), it
follows that
the field perturbation along the z-axis is
3
OBz = 3 . dXe ff . Bo . Bout ~ . (3 co s~ 8 - 1} (10)
The above equation is quite appealing since it is identical in form to the
shell-free equation
we used in our earlier work but now with OX = ~Xeff(a, X~, X19 X2). The
dependence of
OXe ff on shell thiclmess and shell susceptibility is shown in Fig 2. From
this figure it is clear
that increasing the shell thiclrness or increasing the magnetic susceptibility
of the shell is
equivalent to improving the susceptibility difference between the bubble and
its environment.
CA 02428896 2003-05-16
12
A Discrete Model
In practice one means of enhancing the microbubble shell. susceptibility is by
coating or embedding
magnetically active particles of high magnetic dipole moment. Using finite
element analysis, we
modeled the field perturbations around the bubble as function of particle size
(Rp), density (5Z), and
total magnetic susceptibility (xT°t). Finite element analysis was
performed with the aid of Maxwell
3D(Ansoft Corp, Pittsburgh, USA). The finite element consisted of mesh volumes
of no larger than
0.05 ;um3; at this meshing the theoretical prediction of the field surrounding
a free bubble agreed to
more than 99% with the fields computed with Maxwell 3D. One eighth of a sphere
of 2 pm radius
was placed at one of the corners of a cube of 15 E.cm length. On this sphere,
spherical particles
of radius of known size and XTot were uniformly distributed at different
~.ensities. Exploiting the
symmetry in the model and taking the inside of the bubble to contain air and
the outside to be
blood plasma, the fields surrounding the bubbles was computed on a PC with AMD
Athlon XP
1600- processor (Sunnyvale, CA). The field patterns surrounding the bubble
were then used to fit
Eq. (10~ and Oxen was computed.
(A). Effect of RP and xTot on ~Xe,~
To study the effect of particle size on ~xe~, particle size wa,s vaxied from 5
nm to 30 nm
in interval of 5 nm while keeping the particle density fixed at a solid angle
of approximately
5°: To ensure Maxwell's program correctness criteria, all overlapping
particles on the surface
of the sphere weie eliminated. To study the effect of XTot on ~xe~; while
keeping all other
parameters the same, xTot was changed incrementally by the following sequence
400n ppm,
2000~r ppm, 4000~r ppm, 6000~r pprn, 8000~c ppm, 10000n ppm.
(B). E,~ect of S2 on axe,
To study the effect of particle density on the effective magnetic
susceptibility, Rp and X~t
were fixed at 15 nm and 10000~r ppm respectively and S2 was varied from
3.75° to 15° with
CA 02428896 2003-05-16
13
Ta.hlP 1: Magnetic nronerties of naturallv available minerals
Mineral RsD (rcrn)X~ot ( X 103 Density (g/cm3
pPm) )
Iron (Fe) 4-13 1400 7.874
Magnetite (Fe304)12-30 400 5.197
Maghemite (y-Fe304)5-30 320 5.074
Hematite (~-Fe304)13-7500 2.1 ~ 5.271
(a) XT°t is the total magnetic susceptibility at 1.5T
SZ E (3.75°, 5°, 7.5°, 9°,15°).
From the theoretical results so far inventors foresee that any magnetic
particle of any size
that can positively enhance the ~xe~ can be attached to the microbubble would
enhance the
sensitivity of magnetic resonance imaging based manometry. In nature there are
many such
particles and in Table 1 inventors list a few such particles with their
physical and magnetic
properties as reported by DJ Dunlop et al in Rock magnetism: fundamenta,d and
frontiers.
Cambridge University Press, 1997., p.51 and 131.
(C). Effect of Cladngang Pressure on R2 zaith Cotated Microbubblcs
To study the effect of changing pressure with microbubbles coated with
magnetically active
particles, inventors simulated the experiment with microbubbles (Ro = 2~Crn)
coated with
single domain magnetite particles at SZ = 5° and XT°t = 0.12,8.
Assuming the isothermal
pressure volume relationship holds, the bubble size at pressure (Po -+ OP) was
computed
according to
go y3
R \Po + OP~ ° Ro
where Po is the atmospheric pressure and DP is the incremental change in
pressure above
Po. By keeping S2 = 5° and X~pt = 0.128x fixed and varying the bubble
size by changing
L1,P in increments of 50 mmHg from 0 mmHg up to 150 mmHg, ~Xe~ was computed
via
CA 02428896 2003-05-16
14
finite element analysis assuming th.e inside of the bubble is composed of air
and outside of the
bubble was blood plasma. This data were used in conjunction with a Monte Carlo
simulation
as performed earlier and the relation between pressure and R~(CPMG) was
computed. The
parameters for the Monte Carlo simulations were (1)Diffusie~n coefficient of
water in blood
plasma = 2.?5 x10-9m2 ~ s-1;(2)bubble volume fraction = 0.0344 % corresponding
to a dose
j 0.2 cc/kg; (3) Tl8o = 6 ms; (4) time step of protons = 10 ~,s; (5) number of
protons = 104;
(6) Bo = 1.5T.
(D). Simulation Results
Increasing the magnetic susceptibility of the spherical inclusions on the
microbubble shell or
their size, monotonically increases the overall effective magnetic
susceptibility between the
bubble and its environment. This is shown in Fig. 3. It is also evident from
this figure that
the dependence between particle size and ~~e~ is nonlinear arid d~Xe~/dRp is
much smaller
at small particle sizes such as 5 nm compared to larger particle sizes such
a,s 20 nm. This
implies that for a given number of particles distributed at a kruown surface
density, GXe~ can
be more effectively increased with larger particles than smaller ones. In
addition, increasing
the particle density also increases the ~Xe~. The effect of increasing
particle density is related
to increasing particle size as they both contribute to increasin;~ the
rr~icrobubble shell volume
fraction of the particles where shell volume fraction (ilFshe:jl) is defined
as the composite
volume of all the particles on the microbubble shell divided by the volume of
the microbubble
shell or
2
VFshell - ~ ° [ Rp~ ~ (11)
where n is the number of particles on microbubble surface a,nd R is the size
of the bubble
(refer to Appendix B). This implies that changing the densii;y changes n and
changing the
particle size changes Rp. Using this common parameter we show the effect of
changing volume
fraction on ~Xe~ by changing particle size and particle density in Fig. 4. tom
this figure
CA 02428896 2003-05-16
it is evident that, at low VFshell> ~Xeff could be improved I>y increasing
density. However,
beyond a critical point, improvement in ~Xe~ is best achieved by increasing
particle size.
Finally, the effect of changing pressure in the presence of coai;ed
microbubbles and shell-free
bubbles containing air on mufti-echo R2 is shown in Fig. 5.
It is clear that pressure sensitivity is improved with coated microbubbles in
comparison with air
bubbles. However, unlike susceptibility enhancing contrast agents as suggested
in U.B. Pat Nos.
5215680, 5088499, 6416740, and the world pat No. W009851284 these must be
fabricated so that
their ability to report pressure and hence their compressibility in relation
to conventional magnetic
agent-free microbubbles is not adversely altered. In the manufacturing process
the compressibility
of the shells can be studied with the ultrasound scattering properties ca,n be
used to study the
compressibility changes in the shell due to the loading of magnetically active
agents.
Fabrication of Pressure Sensitive and Shell Susceptibility lEnhanced
Medical-grade Microbubbles
Magnetically active agents not limited to paramagnetic, superparamagnetic, or
ferromagnetic origin
or their variations may be incorporated on the surface, as transmebrane
structures, or embedded
in a compartment of a multilamellar lipid-shelled microbubble construct/ in a
number of ways.
The methods listed below can serve as a means of preparing the desired
construct for sensitivity
enhanced MR manometry.
(A). lhlonolayered Lipid-shelled Nlaermbubbles Externally Canted urith
Lanthanide ~'riva-
lent Metalic Complexes
1-~s disclosed in U.S. Pat. No. 5,215,680 which is incorporated herein by
reference, the prior
art fabrication is performed in a two step process where the medical grade
lipid-shelled mi-
crobubbles are produced and then are subject to subsequent paramagnetic
labeling.
CA 02428896 2003-05-16
16
(i) Formation of Medical grade lipid-shelled Microbubbles
A surfactant mixture with a preferred composition of Glyerol 11!Ionolaurate,
Cholesterol
Benzoate, Cholesterol, Cholesterol Acetate, and Glycerol Tripalmitate is
formed by ad-
mixing the agents in a weight ratio of 3:1:1:1:1. A saturated lipid emulsion
is obtained
when this surfactant mixture is mixed in saline solution (0.02 to 0.4g of
sufactant mix-
ture: 100 cc of saline). The resulting mixture is shaken vigorously for 10
seconds in air
or other gaseous material at room temperature. After 5 min, shaking is
repeated 2 or
3 more times. Following the shaking, the solution is allowed to stand for N 30
min so
that the undissolved material settles out of solution. The resulting solution
is filtered
through a polysulfone membrane filter (Gelman Sciences, Ann Arbor, MIJ with
average
pore diameter of 0.45 ~cm. Particle sizing can be performed with
electroimpedance-sensed
volumetric sizing with Coulter Multisizer with Coulter's Accucomp data
handling soft-
ware. The expected characteristics is as follows: maximum bubble diameter of 6
~cm
(mean diameter of 2~,m, 99% below 4.5 E.cm in diameter) at a paxticle density
of 540,000
~ 15% per mL.
(ii) Labeling the Monolayered Microbubbles with a desired Paramagnetic Label
The surface active paramagnetic label is obtained as a lyophilized powder.
Dissolve 15
g of polyalanine (a moderately hydrophobic, neutral amino acid copolymer of
1000-5000
daltons in molecular weight) in 2500 mL of 1.0 M phosphate bufl:er and filter
through 0.5
~,m pore-diameter filter. Add 20 fold molar excess of solid DTPA (Sigma
Chemical, St.
Louis, MO~ to protein solution and adjust pH to 8 by adding sodium phosphate
buffer.
Stir for 30 min and lower the pH to 5.6 by adding glacial acetic acid (or
concentrated
HCl). Add 30 fold molar excess of GDC13 (Aldrich Chemical Co, Milwaukee, WI~to
protein. Perform dialysis with the solution against 0.1.5 M saline at 5 ' for
96 hours
using 1000-Dalton cut-off dialysis tubing. Lyophilize the resulting 1.8-2.OL
solution over
CA 02428896 2003-05-16
17
several days to give white, solid derivative (N 20g). In order to incorporate
Gd-DTPA
derivative into lipid-coated rn.icrobubbles the powdered derivative needs to
be combined
with the lipid-sufactant mixture used to form the microbubbles at 5-10% w/w.
When
this mixture is shaken in isotonic saline, paramagnetic labelled surface
active derivative is
incorporated into microbubble's surrounding lipid monoleyer with Gd-DTPA
remaining
exposed to aqueous exterior. Refer to Fig. 6 A
(B). Lipid-shelled Iron O~ide .Ea-zcraPsa~lccted i~ oligoLarnelldr~
Mic~obubbles
As disclosed in U.S. Pat. No. 5,088,499 which is incorporated herein b;y
reference, the prior art
fabrication is performed by incorporating iron oxide particula~tes
internalized by pre-formed
liposomes via base catalysis.
(i) A. Liposome Construction
The lipids used may be of either natural or synthetic origin. Such materials
include, but
are not limited to, lipids such as cholesterol, phosphatidylcholine,
phosphatidylethanolamine,
phosphatidylserine, phosphatidylglycerol, phosphatidicaciid,
phosphatidylinositol, lysolipids,
fatty acids, sphingomyelin, glycosphingolipids, glucolipids, glycolipids,
sulphatides, lipids
with ether and ester-linked fatty acids, polymerizable lipids, and
combinations thereof.
The liposomes may be PG,17 synthesized in the absence or presence of
incorporated
glycolipid, complex carbohydrate, protein or synthetic polymer, using
conventional pro-
cedures. The surface of a liposome may also be modified with a polymer, such
as, for
example, with polyethylene glycol (PEG), using procedures readily apparent to
those
skilled in the art. Any species of lipid may be used, with the sole proviso
that the lipid
or combination of lipids and associated materials incorporated within the
lipid matrix
should form a bilayer phase under physiologically relevant conditions. The
composition
of the liposomes may be altered to modulate the biodistr ibution and clearance
proper-
ties of the resulting liposomes. To incorporate ionophores into t:he liposome
membrane,
CA 02428896 2003-05-16
I8
the ionophores, which are lipophilic, are simply added to the lipid mixture,
and the
liposomes are prepared in the usual fashion. In addition, the size of the
vesicles can
be adjusted by a variety of procedures including filtration, sonication,
homogenization
and similar methods to modulate liposomal biodistribution and clearance. To
increase
internal aqueous trap volume, the vesicles can be subjected to repeated cycles
of freezing
and thawing. The liposomes of the invention may be of varying sizes, but
preferably
have a mean outer diameter between about :30 nm and about 10 ~,m.
(ii) Internalizing Iron O~ide Magnetite
~ne method of entrapping a particulate solid contrast enhancing agent such as
magnetite,
within an existing liposome is via base catalysis. In this method, a mixture
of ferrous
and ferric salts is entrapped within the aqueous core of the liposome
containing a gaseous
precursor. An ionophore such as valinomycin is incorporated within the matrix
of the
liposome in order to increase the rate of proton flux across the: membrane.
Prior to or
during use, the pH on the exterior of the vesicle is then increased by the
addition of the
appropriate alkali resulting in an increase in the pH in the interior of the
liposome. The
increase in pH in turn promotes base catalysis which results vin the in sit~x
formation
of highly susceptible magnetite within the liposome. It is equally possible to
entrap
preformed solid contrast enhancing agents such a,s preforrned magnetite in the
liposomes.
The magnetite containing microbubbles with gaseous p~°ecurso:r can then
be converted
to gas containing microbubbles via change in pH in the internal enviornment,
exposure
to UV light, or increased temperature. In this forumulation, the iron oxide
magnetite is
incorporated into the walls of the lipid-based microbubbles. Refer to ~'ig. 6B
(C). Lipid-shelled Magnetically Active agents Internalized; in the Oil Lezyer
of r~
lVfulitlammelar Microbubble
As disclosed in World Pat. No. W00985I284 and U.S. Pat. hTo. 6,416,740 which
are in-
CA 02428896 2003-05-16
19
corporated herein by reference, the prior art fabrication is performed by
incorporating any
magnetically active agent in the oil layer of the multilamellar microbubble
construct: Such a
microbubble contrast system will comprise of an oil, surfactant, a
magnetically active agent
complexed with a lipophillic agent, and a gas. Magnetically active elements
such as Gd(III),
Mn(II), Cu(II), Cr(II), Fe(II), Fe(III), Co(II), Er(II), Ni(II), Eu(II), and
Dy(II) are incorpo-
rated through covalent or non-covalent association, to complex:ing agents,
including lipophffic
derivatives, or to proteinaceous marcomolecules. Other magnetically active
agents such as
paramagnetic, superparamagnetic, ferrormagnetic agnets, or the variations of
them that en-
hance magnetic susceptibility may also be incorporated within the oil layer
when alkylated
or combined with other derivatives. The magnetically active agents are then
dissolved in the
oil or wax with the partition coefficient greater than 10. This composition is
then added or
dispersed into an aqueous. phase containing one or more surfactant and
stabilizing media.
This composition is placed in a vial and is sealed with a head space of a pre-
selected gas. The
vial is then shaken for 45 seconds on an Espe CAPAMIX dental amalgamator at
4500 RPM.
This process results in liospheres containing magnetically active agents in
the oil layer with
a central gas bubble. Refer to Fig. 6C.
Microbubble Specifications: Size, Gas Type, and Biodistribution
The ideal microbubble contrast system should be made with a gas that has a
solubility that is
less than that of nitrogen so as to remain stable in circulation within the
imaging time. Gaseous
precursors that change state from liquid to gas due to shaking or that axe
temperature sensitive such
as those composed of perfluorocarbon or those gas containing microbubbles in
native state in room
teiriperature axe ideal choices. Microbubbles should be formed in a manner
that biodistribution
be narrowly distributed between 4-10 ~,m with a mean diameter of approximately
ø6 ~cm. The
aforementioned prior art describe in detail the specific composition of lipid,
volume of gas in the
CA 02428896 2003-05-16
headspace of the vial, ideal surfactant, the duration and the vigor of
shaking, and the filtration
techniques that axe necessary to make the desired formulations.
Microbubble and Nanoparticle Toxicity
When considering the toxicity associated with the proposed contrast agent
system one needs to
consider two different sources of toxicity: microbubble toxicity and the
toxicity of the magnetic
agents that get chelated/embedded onto the surface of the microbubbles.
(A). Lipid-Shelled Micr~bubble T~:racity
The consensus among experts on high doses of microbubble;s (in excess of
lcc/kg of body
mass) is quite varied. Alexander et al note that since LD50 o~f these contrast
agents in mice
are above l5cc/kg and they expeca lcc/kg would not cause any physiological
complications
in humans. Another group has found physiological complicavtions start to
emerge after an
administration of 0.3 cc/kg with the primary complication being reduced
systolic and dias-
tolic pressure levels as reported by NC Nanda et al in "Echo-enhancing Agents:
safety ". In:
N Nanda et al eds. Advances in echo imaging using contra;~t enhancement,
Dubai:Kluwer
academic publishers;1997. p 115-131. In other studies involving lipid coated
microbubbles,
Phase I clinical studies have shown that 0.15 cc/kg was safe and well
tolerated by all subjects
as reported by TA Fritz et al in ":Phase I clinical trials of MR.X-115: A new
ultrasound con-
tract agent, Inves Radiol 1997; 32:735-740. With the contrast agents the
inventors propose
for pressure measurements it shouad be possible to produce mi.crobubble
contrast agents that
can be sensitive even when the doses are below 0.30 cc/kg.
(B). IVano~~rtacle Toxicity
We have identified a number of different superparamagnetic agents that in
theory can be
CA 02428896 2003-05-16
21
chelated/embedded onto the lipid shells of the microbubbles. However, we
choose to use
Magnetite (Fe304) or the fully oxidized form of magnetite - maghemite (7-
Fe203) as they
have already seen clinical use in MRI. In an earlier work, for sensitive
detection of pressure,
we showed that be in excess of 34 ppm in SI units at imaging the field
strength of 1.5T with
microbubble dose of 0.87 cc/kg is required. Our calculations to date show that
of 50 ppm
(SI) at a microbubble dose of 0.17 cc/kg can be obtained when
superparamagnetic magnetite
particles of radius 15 nm are dispersed in lipid shell at a sb.ell volume
fraction (defined as
the ratio of total volume of the particles to volume of shell) of 1.02%. This
is tantamount to
uniformly dispersing approximately 2350 magnetite particles on each of the
nearly 8.2 billion
lipid shelled medical .grade bubbles of 2 ~cm radius. This coating is
equivalent to a total
iron dose of 1.8 mg that is well below the dose (in excess of :280 mg) at
which physiological
complications emerge as reported by M Taylor et al in "Safety and preliminary
findings with
the intravascular contrast agent NC100150 injection for M~u, coronary
angiography, JMRI
1999; 9:220-227.
Dose dependence on Measurement Accuracy in R2 for MIf~ Manometry
As pointed out earlier, the measured R2Bto°d in the presence of
microbubble will be a combination of
R2 due to dipole-dipole coupling and diffusion through local field
inl~omoge:neities that is dependent
on the oxygen state of the blood and the presence of micobubbles. If we can
detect the changes in
~Bubb perfectly, to detect a pressure change of ~1' with 95~ confidence
subject to an error of Q in
R2 of blood without bubbles (R2I ), it can be shown that
~Bubb > 2 ' ~ ' ~2 (12)
k~~P '
where k is the relative change in I~Bubb due to change in pressure. From our
calculations, we
observed k . 0.066 mmHg 1. Hence, to detect a pressure change o~f 50 mmHg
above atmospheric
pressure the minimum necessary RzBubb will be related to the measurement
accuracy of R2I. Table
CA 02428896 2003-05-16
22
Table 2: Dependence of measurement accuracy of R2 on
RBnbb for sensitive detection of moderate pulmonary hypertension
Percent accuracy in the measurementminimum aRBubh
of R2 (o-)
14
4 11
3 8.6
2 5.7
1. 2.9
(i). as predicted by Eq.(12)
2 lists the minimum RZBubb values needed to detect 50 mmHg pressure change to
the atmospheric
pressure when 0.01 < Q < 0.05. As 6 decreases, R2Bubb ado decreases indicating
that as the
measurement accuracy of R2r increases, the microbubble dose necessary to make
the measurement
can be decreased further.
Microbubble Based in vivo MR Manometry
Microbubble based MR Manometry relies on the intravenous delivery of
microbubble contrast
media, a calibration curve for a given contrast agent at a given dose between
pressure and R2Blood~
a flow independent and motion compensated MRI protocol to measure R2Blood in a
region where
the gauge pressure is approximately zero and in the region of interest where
the pressure is to be
measured. Calibration curve between the the ambient pressure and R2Blood can
be established for
all physiological doses of interest with the aid of a catheter for a given
contrast agent. Following
this, a physiologically tolerable dose of sensitivity enhanced microbubble
contrast media is delivered
intravenously either as a bolus or as a~ continuous infusion.. The passage
towards steady state
microbubble concentration can be monitored by measuring the MR signal changes
in a large vein
CA 02428896 2003-05-16
23
such as the brachiocephalic vein where the gauge pressures are nearly zero.
Following this a similar
MR, pulse sequence as outlined in the U.S. 1'at No. 6,094,591 which is
incorporated herein by
reference in its entirety, be used to measure intracardiac or vascular R2Biopd
in the presence of the
microbubble contrast media.
The prior art pulse sequence begins with a 90x excitation pulse followed by a
train of 180y
refocusing pulses, which are equally separated by a refocusing interval termed
Tlso. Spatial local-
ization is performed using a final slice-selective pulse followed by an
imaging gradient. To measure
R2BUod a series of T2-weighted images is acquired with this pulse sequence in
which the duration
of the refocusing train is set to different values by changing the number of
refocusing pulses used.
With these images, R2Btood can be estimated by extracting the signal amplitude
within the blood
vessel and fitting the data points as a monoexponential decay using a weighted
least squares fit.
To minimize flow sensitivity when using this pulse sequence, the excitation
pulse and refocus-
ing train are non-selective. Thus, there are no gradients applied and no
moments to be hulled. In
addition, the regular refocusing achieved by the train of 180y pulses. lessens
the amount of dephas-
ing due to flow through susceptibility gradients. Assuming ideal R,F
homogeneity, phase accrued
by spins moving at a constant velocity through local Bo inhomogeneity can be
modeled as a linear
gradient. The validity of such a model improves as Tlso decreases because
spins travel a shorter
distance between pulses.
In this implementation of a T2-weighted magnetization preparation the T2-
weighted magne-
tization produced by the train of 180y refocusing pulses is returned to the
longitudinal axis at the
echo of the final refocusing pulse. Manipulation of T2 contrast from the
transverse plane back to
the longitudinal axis is achieved using a 90_~ tip-up pulse. At this time, a
spoiler gradient is also
applied along the slice-select axis to dephase any residual transverse
magnetization.
The principal advantage of temporary longitudinal storage of T2 contrast using
the present
invention is the flexibility it allows in the choice of imaging pulse
sequences. For example, in one
CA 02428896 2003-05-16
24
embodiment the T2 preparation segment is followed by an imaging pulse sequence
in which a series
of tip-up angle RF excitations follow the tip-up RF pulse at the completion of
the T2 preparation
segment. Different slices or different part of k-space may be acquired after
each small tip angle RF
excitation pulse. In the preferred embodiment described below, a single slice
imaging pulse sequence
is used in which a spectrally and spatially selective RF excitation pulse and
spiral interleaf readout
is employed. Because the spectral-spatial RF pulse selectively excites water
while isolating the slice
of interest, this sequence rejects lipids. The spiral acquisition is well-
suited for vascular imaging
due to its excellent flow properties.
In addition to tipping the T2-weighted magnetization back into the
longitudinal axis, the T2
preparation segment addresses a number of important issues. The effect s of RF
and static field
inhomogeneities during the refocusing train are handled using trains of
relatively simple composite
refocusing pulses with good RF cycling patterns. It is preferred that a MLEV
pattern of 90x -
180y - 90~ composite refocusing pulses is used and all pulses are rectangular
and non-selective with
yBl/2~r c 1 kliz. When using composite refocusing pulses, methods are used to
compensate for T1
signal decay effects during each refocusing pulse. Solutions include
decreasing the pulse duration,
increasing the refocusing interval, and using post-processing methods. It is
that one uses a simple
shift of echo times to account for T1 signal decay effects without
constraining the pulse duration
or the refocusing interval.
The effects of RF field offsets on the 90~/90_~ excitation/tip-up pulse pair
is addressed by
using phase-cycling methods which subtract out the T1 bias or by using
composite 90° excitation
and tip-up pulses which ensure an efficient manipulation of magnetization
between the transverse
plane and the longitudinal axis. It is preferred one uses a 360x - 274x - 90y
pulse for excitation
and a 45-x - 90-y - 90-x - 45~ pulse for tip-up. This pulse combination
provides dual RF and
static field insensitivity without increasing the imaging time.
Following the preparation interval, T2 contrast is stored temporarily along
the longitudinal
CA 02428896 2003-05-16
axis. During this time, the T2-weighting will degrade gradually by Tl
relaxation effects. Methods
which remove the additive Tl recovery -term will preserve the prepared T2
contrast. The preferred
embodiment cycles the longitudinally-stored T2 contrast between the ~z axes by
applying a robust
inversion pulse immediately following the tip-up pulse on subsequent
excitation. The additive term
is removed upon subtraction of the acquired data. When using a series of small-
tip angle excitations,
the sensitivity to subtraction errors can be reduced by applying ari inversion
pulse following each
small-tip angle excitation.
Due to the strong dependence of the contrast agent effect on ri$o, careful
selection of this
parameter is an important aspect of the oximetry protocol. Hence an optimal
TlBO that sufficiently
balances the contrast that can be developed within this time yet one that
reduces the flow artifacts
for a given microbubble dose and ~x is necessary. It is preferred that Tl.BO
of 6 ms or smaller is
used to optimize the pressure contrast based on R281oo~.
A signal-to-noise ratio per pixel greater than 10 at the time of the longest
T2 preparation
interval is essential to avoid noise bias in the R2Blood measurement. In the
large vessels, which
are closer to the body surface, this SNR is achieved easily using a
conventional 5 inch surface
coil. Due to the rapid drop-off of sensitivity with depth when using such a
coil, the SNR/pixel
may be prohibitively low for measurements in small and centrally located
vessels, such as those
in and around the heart. To overcome this problem it is desirable to use an
array of local coils
to receive the MR signal. Good visualization of a vessel for Manornetry
requires adequate spatial
resolution and an imaging slice which is perpendicular to the vessel wall.
This is straightforward
for measurements in large vessels with little motion. Measurements within
smaller vessels which
move considerably, such as those in and around the heart, pose a greater
challenge for reliable
visualization. Spatial resolution can be increased by sampling higher spatial
frequencies during
the data acquisition. The preferred method is to place at least 6 pixels
across the vessel diameter.
In addition, the SNR limitation also require that microbubble dose do not
exceed the limits of
CA 02428896 2003-05-16
26
detection so as to create pockets of "black-blood" in the images. Flence, it
is imperative pressure
sensitive MR contrast is developed in a manner that limits dose both due to
toxicity limitations
and due to measurement limitations.
R2~lood measurements in and around the heart are inherently sensitive to
respiratory motion
due to the relatively long data acquisition times. if not compensated for,
blurring and motion
artifacts will degrade the quality of each T2-weighted image. A number of
respiratory compen-
sation methods exist which can improve image quality. Schemes which rely on
breath-holding,
rapid imaging, or motion monitoring and re-acquisition methods attempt to
reduce the number of
respiratory phases in the acquired data. Other methods rely on the periodicity
of the respiratory
cycle to implement post-processing corrections.
It is preferred that one uses a respiratory bellows and the signal processing
unit of the MR
imager to monitor and record the respiratory phase at the time of each data
acquisition. Following
the collection of a full data set, a histogram of the respiratory phases is
constructed. Overscanning
and the well-known Diminishing Variance Algorithm are then applied to "freeze"
the respiratory
motion. In addition, if not compensated, cardiac motion can introduce
considerable artifacts and
blurring into a T2-weighted image. Methods to "freeze" heart motion rely on
prospective gating
using a plethysmograph placed on a finger for an ECG trigger. Due to the
considerable delay
between the R wave and the triggering of the plethysmograph, the preferred
embodiment uses the
R wave of the ECG signal for triggering the pulse sequence.
Because the acquisition of T2-weighted images in and around the heart requires
multiple data
acquisitions, a steady-state longitudinal magnetization is desirable at the
time of each excitation.
For vascular T2 measurements, a steady-state magnetization is difficult to
achieve due to variability
in the heart rate. The simplest method to reduce the effects of heart rate
variability on the
R2Blooa measurement is to allow more than one heart beat for T1 recovery.
Other methods control
the duration of T1 recovery by nulling the longitudinal magnetization at a set
time before each
CA 02428896 2003-05-16
27
excitation pulse. To overcome this problem it is desirable to ;acquire data
following every
other heart beat.
By collecting the R2Bl~~~ at pressure independent region such as the
brachiocephalic or jugular vein and the region of interest where the pressure
is to be
measured, and computing the differences between the respective R~Bi~~d s and
using the
aforementioned calibration curve, pressure in a region of interest is mapped.
As used herein, the terms "comprises", "comprising", "inclt~.ding" and
"includes"
are to be construed as being inclusive and open ended, and not exclusive.
Specifically,
when used in this specification including claims, the terms "compri ses",
"comprising",
"including" and "includes" and variations thereof mean the specified features,
steps or
components are included. These terms are not to be interpreted to exclude the
presence of
other features, steps or components.
The foregoing description of the preferred embodiments of the invention has
been
presented to illustrate the principles of the invention and not to limit the
invention to the
particular embodiment illustrated. It is intended that the scope of the
invention be defined
by all of the embodiments encompassed within the following claims and their
equivalents.
CA 02428896 2003-05-16
2~
OTHER PUBLICATIONS
Rich S, Braunwald E, Grossman W. Pulmonary hypertension. In: )3raunwald E,
editor.
Heart Disease, 5th edition. Philadelphia: W. B. Saunders Compna~r; 1998. p 780-
806.
Berger M, Haimowitz A, Tosh AV. (quantitative assessment of puhr.~onary
:hypertension in patients
with tricuspid regurgitation using continuous wave Dopplerultrasound. Arn J
Cardiol 1985; 6:359-
365.
Bouchard A, Higgins CB, Byrd, BF. Magnetic resonance imaging in pulmonary
hypertension. Am
J Cardiol 1985;56: 938-942
Urchuk SN, Plewes DB. MR measurement of. tune-dependent blood pressure
variations. J Magn
Reson Imag 1995;5:621-627
Raeside D, Peacock, A. Making measurements in the pulmonary circulation: when
and how?.
Thorax 1997; 52:9-11.
Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation o:E right atria,l
pressure from the
inspiratory collapse of the inferior vane cave. Am J Caxdiol 1990;6n:493-496
Fairbank WM, Scully M. A new noninvasive technique for cardiac pressure
measurement: resonant
scattering of ultrasound from bubbles, IEEE Trans Biomed Eng 1997; BME- 24:107-
.110
Tickner EG. Precision Micro-bubbles for right side intracaxdiac pressure and
flow measurements. In:
Meltzer RS, Roelandt JTCR, ads. Contrast Echocardiography. Vo1.15.London:
Martinus Nijho.,
CA 02428896 2003-05-16
29
1982:313-324.
Bouakaz A, Finking PJA, Bom N. Noninvasive measurement of the hydrostatic
pressure in fluid-
filled cavity based on the disappearance time of micrometer-size free gas
bubbles. Ultrasound Med
Bio 1999. 25:1407-1415.
Alexander AL, McCreery TT, Barrette TR, Gmitro AF, Unger E. Microbubbles as
novel pressure-
sensitive MR contrast agents. Magn Reson Med 1996. 35:801-806
Wright GA, Hu M, Macovski A. Estimating oxygen saturation of blood in vivo
with MR imaging
at 1.5T. J Magn Reson Imag 1991;1:275-283
Nanda NC, Cartensen EL. Echo-enhancing Agents: safety. In.: Nanda N, Schlief
R, Goldberg BB,
eds. Advances in echo imaging using contrast enhancement, 2nd edition.
Dubai:Kluwer academic
publishers;1997, p 115-131
Dharmakumar R, Plewes D, Wright GA. On the parameters affecting the
sensitivityof MR measures
of pressure with microbubbles. Magn Reson Med 2002. 47: 264-273.
Glasel JA, Lee KH. On the interpretation of water nuclear magnetic resonance
relaxation times in
heterogeneous systems. J Am Chem Soc 96:970 (1974).
Dunlop DJ, Ozdemir O. Rock magnetism: fundamental and frontiers. Cambridge
University Press,
1997. p.51 and 131.
Fritz TA, Unger EC, Sutherland G, Sahn D. Phase I clinical trials of MRX-:L15:
A new ultrasound
contrast.agent. Inves Radiol 1997; 32:735-740
Taylor M, Panting JR, Keegan J, Gatehous PD, Jhooti P, Yang GZ, MeGill S,
Barman ED, Francis
JM, Firmin DN, Pennell DJ. Safety and preliminary findings with the
intravascular contrast agent'
NC100150 injection for MR coronary angiography. J Magn Reson Iniag 1999; 9:220-
227.