Note: Descriptions are shown in the official language in which they were submitted.
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
COMBINED OX'Y~GEN ~-1,ND NOx SENSOR
CROSS REFERENCE TO RELATED APPLrCATIONS
"his application claims the benefit of U.S_ provisional Application Serial No.
601254,081, filed December 7, 2000. This application is also a continuation-in-
part o~'CJ.S.
Patent application Serial No. 09/662,773, filed September 15, 2000, which
claims the beneft of
U.S. Provisional Application Serial No. 601155,817, fled September 23, 1999.
'$ACI~GROUND OF THE fNV~NT~O1V
The pz~eserat invention, relates to a device for sensing the partial pressure
of oxygen in a
gas, and more particularly to an active nnultilayer sensor utilizing an oxygen
ion conducting
matexial. The present invention also relates to a combined sensor for
measuring oxygen content
and NOx content in a gas. ~tOx is utiaized herein to represent nitzic oxide,
zaitrogen dioxide,
nitrogen trioxide, etc_
It is vtridely recognized that one of the most important diagrxostics for
monitoring the
efficiency of any combustion process is the measuzemeut of the oxygen partzaJ
pressure in ara
exhaust gas. Thus, oxygen sensoxs have loug been used to measure the oxygen
content of
exhaust gases from such divezse combustion processes as internal combustion
engines in motor
vehicles and coal, natural gas, or oil burning power generations facilities.
2O The most widely lrnown and used oxygen sensors are based on partially
sfiabilized
zirconia (PSZ) as the ion conductor. Such sensors function by monitoring the
electxarnotive
force (EMF) developed across an ion conductor which is exposed to different
partial pressures of
oxygen. Oxygen tends to move from a gas containing a high concerxtration of
oxygen to vne of
lower concentration. Tftwo gases are separated from each other by an
electroded oxygen ion
conductor, the oxygerx molecules will dissociate on one surface of tlae
couductar and absorb
electrons to loran oxygen ions. These ions then diffuse through the ionic
conductor, leaving the
entry surface with a def cieracy of electrons (OZ + 4e = 20'2). One the exit
or lorw oxygen
concenixation side of the conductor, oxygen iozzs leaving the conductor must
give up electrons to
form molecular oxygen, thus leaving the exit surface with an excess of
electrons. This creates
the $M11 between the two surfaces of floe ion eonduetor_
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
One problem with the use of partially stabilized zirconia sensors is that they
must be
operated at temperatures in the range of about 800 C to reduce intez-rtal
resistance to a point
where a current carp be measured. Further, the raw material costs of
stabili2ed zireonia is
zelatively high, and the ~xlelting point of zizconia is quite high (2700 C) so
that formation of
sensors is expensive.
Lawless, in U.S. Patent No. 4,462,89 x, describes a passive oxygen sensor
using eeram
ion conducting materials based ors nickel niobates and Bismuth oxides. The
oxygen sensor
includes a plurality of layers of tlxe ceramic material and a porous metallic
conductor axrangec
form a body having alterxzating ceramic and metallic layers, with first
alternate ones of the
metallic layers being exposed along one side of the body and second alternate
ones of the
metallic layers being exposed along an opposite side of the body. The first
and second alterna
ones of the metallic layers are exposed to separate gases, one of the gases
being a reference ga
in order to cxeate a voltage output signal across electrodes connected to
alternate raaetallic laye:
The voltage output signal is indicative ofthe relative oxygen partial
pressures of the separate
gases. Thus, the passive oxygen sensor cannot provide an oxygen partial
pressure indication
unless the first arid second metallic layers present in the body are exposed,
respectively, to a
sample gas and a separate reference gas having a known oxygen partial
pressure, i.e., each side
of the sensoz body r~nust be exposed to a separate gas.
More recently, arnperomeiric sensors have been introduced which also use
partially
stabilized zircoz~ia but which do not require a reference gas to operate. Such
a sensor 80 is
illustrated in Fig, 1 aad comprises a cavity 100 iz~ communication with the
unknown gas ihrou~
a diffusion hole 120. The base of the cavity 100 is a PSZ electrolyte 1~.0
which is connected
through electrodes 160, 160' to a voltage source x 70. The application of a
voltage causes oxyg
tv be pumped from the cavity through diffusion into the suzround~g gas as
shown by the arrov
~f the cavity is sealed atop the base, and if the top of the cavity has the
small diffusion hole '12C
then a point is rEached on increasing the voltage where no more oxygen can be
pumped out of
the cavity than is entering through the diffusion hole. The current drawn at
this point is called
the amperometric current. The larger the oxygen partial pressure in the
surrounding gas, the
larger will be the amperometric current. Thus, a measurement of the
amperometric current
gelds the oxygen parl5al pressure. Again, however, this sensor suffers .from
some of the same
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
drawbacks in that materials and fa'6rication costs are relatively high. An
extremely small
diffusion hole is required, about Spm, and requires precise machining because
the size is cz~itical
to the operation of the sensor. Additionally, the manufacture o~ the sensor of
Fig. 1 requires five
silk screen operations and four burnout steps. Finally, these sensor. s lose
their sensitivity above
about 80% oxygen and the diffusion bole is prone to plugging.
Accordingly, there renr~a~ins a need int the art for art amperometric oxygen
sensor which is
relatively inexpensive to manufacture and provides enhanced oxygen
sensitivity. There is also a
need in the art for a sensor which is capable of providing an independent
indication of NOx
content in a gas.
BRTIJF SUMMAT~Y OF THE INVENTION'
These needs are met by the present invention wherein a combined oxygen and NOx
1 S sensor is provided. Generally, the combined sensor employs a sensor body
that iuncludes two
different types of electrodes - oxygen-porous electrode layers and
dissociative oxygen-porous
electrode layers.
In accordance with one embodiment of the present invention, a combined sensor
for
measuring oxygen content and NOx content in a gas is provided. The sensor
comprises a sensor
body, an oxygen content electrical sigual output, and a N'Ox content
elecixical signal output. The
sensor body is disposed in the gas and comprises (t) a plurality of oxygen
porous electrode
layers, (ii) a plurality of dissoczative oxygen porous electrode layers,
wherein the dissociative
oxygen porous electrode layers comprise a material selected to catalyze
dissociation of~l'Ox into
nitrogen arid oxygen, and (iii) a plurality of oxygen ion conductive ceramic
layers interposed
between respective ones of the oxygen-porous electrode layers and respective
ones of the
dissociative oxygen-porous electrode layers. The oxygen content electrical
signal output is
coupled to the plurality o~oxygen-porous electrode layers. Similarly, the NOx
content electrical
signal output is coupled to the plurality of dissociative oxygen porous
electrode layers. The NOx
content electrical signal output is electrically isolated from the oxygen
content electrical signal
output.
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
In accvzdance with another embodiment of the present invention, a combined
sensor for
measuring oxygen content and NOx content in a gas is provided where the
dissociative oxygen-
porous electrode layers comprise sufficient Rh to catalyze dissociation of NOX
ixtto nitrogen and
oxygen.
Tn accordance with yet another embodiment of tlxe present invention, a
cozobined sensor
for measuring oxygen content and NOx content in a gas is provided. The sensor
comprises a
partial enclosure defining a gas passage, a sensoz body, and a diffusion
baxz~ier_ The diffusion
barrier defines a diffusion-limited portion of the gas passage and the sensor
body is disposed in
the diffusion-limited portion ofthe gas passage.
In accordance with yet another embodiment of the present invention, a sensor
body is
provided comprising a plurality of oxygen porous electrode layers, a plurality
of dissociative
oxygen-porous electrode layers, and a plurality of oxygen ion conductive
cerazxuic layers. The
dissociative oxygen-porous electrode layers comprise a material selected to
catalyze dissociation
of NOx into nitrogen and oxygen. The plurality o~ oxygen ion conductive
ceramic layers are
1 ~ intezposed betweEn respective ones of the oxygen-porous electrode layers
and zespective ones of
the dissociative oxygen-porous electrode layers.
Accordingly, it is an object of the present invention to provide an improved
oxygen and
NOx sensing device. Other objects of the pzesent invention will be apparent
art light of the
description of the invention embodied hezein.
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
BRIEF DESCRIPTxON OF ~E SEVERAh V'rE'hfS OF'1~ DRA'WIN'GS
The following detailed desc~.ption of the preferred embodixx~ents of the
present invention
can be best understood when read ix~ conjunction with the following drawings,
where like
stricture is indicated with like reference numerals and in which:
Fig. 1 is a schematic representation of a pz~or art oxygen sensor;
Fig. 2 is a schemai~ic representation of an oxygen sensor in accordance with
the present
invention;
Figs. 3-5 are illustrations of an alternative heating circuit arrangement
according to the
IO present invention;
Figs. C~~, and 6B are ilhtsirations of a packaging scheme according to one
embodiment of.
the present invention;
Fig. 7 is an illustration of a sensor body for use in a combined sensor for
measuring
oxygen content and'~1'Ox content in a gas; and
15 Figs. 8.A.-8C illustzate a combined sensor for measuring oxygen content and
NC7X content
m a gas.
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
DETAILEA DESCI~fTTOZV
The present invention is described herein with initial reference to an
amperometric
oxygen sensor and with subsequent reference to a combined oxygen and I~Ox
sensor that utilizes
an oxygen sensor and additional structure similar to the basic oxygen sensor
structure.
~eror~netric Ox yen Sensor
A, seherrxatie representation of an amperometric oxygen sensor constructed
according to
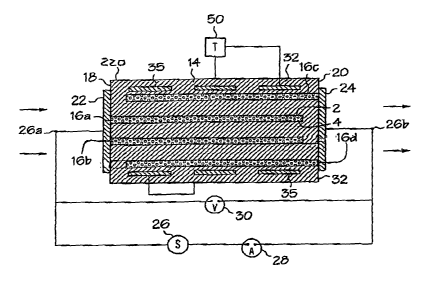
the present invention is shown an Fig. 2. As seen in Fig. 2, oxygen sensor 10
includes a sensor
body I2 having alternating layers of an oxygen ion conducting xnatcrial 14 and
an oxygen-porous
electrically conductive material 16a, 16b, I 6c, 16d. A Erst set of oxygen
porous conductive
layers 16a and 16b have end portions that are exposed along a first edge 18 of
the seztsor body
12. For the purpose of describing and defining the present invention, an
oxygen ion conductor is
any material capable of achieving electrical conductivity due to displacement
of oxygen ions
within its crystal lattice.
Electrical connections are made to the conductive layers I6a and 16b by firing
electrically conductive oxygen-porous terminations.22 onto the ends of the
conductive layers
1 Gay 16b to form a plurality of cathode layers. A second set of oxygen porous
conductive layers
16c and 16d have end portions that are exposed along a second edge 20 of the
sensor body 12.
The conductive layers 16c and I6d are electrically connected to one another by
an electrically
conductxvc oxygen-porous termination 24, to forxxi a plurality of anode
layers. Silver or oxygen-
porous platinum are suitable nnaterials for use as the electrically conductive
oxygen porous
termit~ations 22, 24. The tezxninations 22, 24 are used to electrically
connect the ceramic layers
in pan~allel to reduce the electrical resistance of the sensor and allow
increased amperometri,e
current.
Each of the conductive layers 16a 16d includes two major surfaces. For
example,
cortduetive layer 16a includes major surfaces 2 and 4_ Each oxygen ion
coz~duetor layer 14 is
disposed between major surfaces of opposing conductive layers. Further, both
major surfaces of
each conductive layer are unexposed, i.e., enclosed by the sensor body 12. It
is contemplated by
the present invention that any number of oxygen-porous conductive layers and
ion conductor
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
layers may be used to construct the sensor body 12. The number of layers shown
in Fig. 2 is
merely pzesented for illustrative pwcposes.
A voltage source 26 is electrically connected to the t~rminatxvr~s 22 and 24.
Tn this
manner, a fiucst pole 2Ga of the voltage source 26 is electrically eonuected
to tlae cathode layers
forr~aed by conductive layers I 6a and 1 Gb and a second pole 26b of the
voltage source 26 is '
electzically connected to the anode layers formed by conductive layers 16e and
I6d. An
amperoanetric current meter 28 is connected betwveen the voltage source 2G and
the termination
2A. A voltage meter 30 is connected across the voltage source 2G.
The oxygen-porous electrically conductive material fornvng conductive layers
16a-d
I O preferably comprises oxygen-porous platinum, although any suitable
electxically conductive
material which is porous to oxygen and catalyzes oxygen molecules to ions at
the cathode layers
and catalyzes ions to oxygen molecules at the anode layers nn.ay be used.
Platinum electrodes can be made porous to oxygen by well-known methods. For
example, the use of coarse Pt panicles in elecitodzng ink results in porous
electrodes. Otk~er
additions to the electrvding ink, such as zireonia particles, further increase
the porosity. A
platinum electrode having 530% of its volume occupied by pores is oz~e
preferred example. As
another example, 85 parts, by weight, of a coarse Pt powder available as
platinum gorwder
number 5432\0101 froxxj Uemetron, GMBH, Hanau, Germany, xnay be combined with
1S parts,
by weight, o~ a d.00 mesh zirconia powder in a suitable silk screening slurry.
:Cn one ezobodxxn~ent o~ the present invention, the width of the sensor body
12; i.e., the
dimEnsion of the sensor body ~rom the first edge I 8~ to the second edge 20,
is about 0.20" (0.5
can), the short ends of the conductive layers lGa,16b, 16c, 16d terminate
about 0.030" (0.075
em) from respective side edges, leaving a 0.14" (0.3b cm) conductive layer
overlap. The lez~gtb
of the sensor body 12 is about 0.18" (0.46 cm). The thi,cltc~ess of the sensor
body 12 is defined
by the number and thickness of the oxygen ion conductor layers 19., the
conductive layers 1 fia,
lGb, 16c, 16d, and any layers dedicated to a heating circuit (desczibed
below). In one
embodiment ofthe present invention, eleven oxygen ion conductor layers 14 are
positioned
between alternate ones of twelve conductive layers 16a,16b, 16e, 16d. The
oxygen ion
conductor layers 14 may comprise 0.0030" (0.076 mm) thick yttria-stabilized
zireonia layers.
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
The conductive layers comprise 0.0001" (0.0025 mm) thick porous platinum. The
result is an
oxygen sensor that is relatively compact in size and relatively inexpensive to
produce.
A number of eerarnie oxygen ion conductor materials may be used in accordance
witty the
present Invention. Indeed, the present invention's advantages of simplicity
ofconstruction and
reduced electrical resistance due to sensor geometry are applicable to any of
a wide vaxiety of
ceramic materials used. Preferably, the oxygen ion conductor of tlae present
invention is a
ceramic eXectrolyte and more specifically, comprises yttria-stabilized
zirconia (ZrOz stabilized
with Y203) but may also comprise stabilized bismuth oxide, stabilized ceria,
etc. The zirconia
ceramic may be stabilized with materials other than Yz43-
Fine gzain sized powders of ZrQz:'Y2C~ can be sintered to high density at 1150-
1300 C,
making it possible to manufacture multi-layer seffsor bodies from 'this oxygen
ion conductor.
Because of the convenient sintering temperatures of the ceramic materials of
the present
invention, the ceramics can be "tape cast" into a monolithic body. As is well
lrnown in the
ceramic art, tape casting is a process for making a multilayered body (for
example, a cerarrxic
capacitor) wherein appropriate metal electrodes arE interdispersed between the
ceramic layers. A,
tape casting technique may be employed such as that described in U.S. Patent
No. 4,462,891,
incorporated herein by reference. The cet~amic layers are quite thin, having a
thickness of from
about 25-100 ~.xr~. Further, this tape casting method requires only a single
sills screening
operation and a single burnout step.
Higher porosity levels in the conductive layers are more suitable for sensing
very low
levels of oxygen in a gas, e.8., as low as 1 ppm oxygen partial pressure.
Conversely, lower
porosity levels in the conductive layers are more suitable far sensing
applications over a broad
range of oxygen paatial pressure up to a maximum of l Os ppm. According to one
eznbodino.ent of
the present invention, the amperometrac oxygen sensoz 10 is pxoduced by
sintering the entire
sensoz body 12, i.e., the oxygen ion canductvr layers 14, the conductive
layers 16a, I 6b,16c,
16d, and any layers dedicated to the heating circuit 12, at a sintering
temperature selected to
yield a predetermined oxygen porosity in the conductive layers 16a, 16b,16c,
16d. Sintering at
relatively high temperatures for relatively large amounts of time decreases
the porosity in the
electrode layers because the density of the sensor body increases. Conversely,
sintering at
relatively low temperatures fez' relatively short amounts of time does not
lead to equally
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
significant decreases in porosity in the electrode layers because the density
of the sensor body
does not izacrease as much as is the case for higher temperature arid longer
duration sintering.
Accordixtgly, a~~ amperametric oxygen sensor according to the present
invention may be
produced by providing an unsintered sensor. body, selecting a target porosity
for the oxygen-
porous electrode layers, and selecting a corresponding sintering temperature
for the sensor body,
'fhe sintering temperature is selected to correspond to the target porosity
anal may be determined
~khrough experimentation. Tlae sensor body is sintered at the selected
sintering temperature to
yield a siu~tered sensor body including oxygen porous electrode layers having
a target porosity.
For example, where the conductive layers are sintered at about 1200°C,
for a duration of about 2
hours, the sintered sensor body is suitable for oxygen sensing in gases having
art o~tygen~ content
ranging from a value typically found in air to values as low as 1 ppm or
lower. Xf the sensor
'body is sintered at a higher temperature, e.g.,1275°C, for the same
duration, a less porous layer
is formed and the sintered sensor body is more suitable for oxygen sensing of
gases having
higlaer oxygen concentrations, e.g_, up to I00% oxygez~_
There may be some increase in resistance in the oxygerx porous electrode
layers over time
as a result of sintering of platinum particles in the electrodes at the
operating temperature of the
sensor. The long term stability of sensors according to the present invention
may be improved it
some irxstanees by stabilizing the oxygen porous electrode layers against
sintering. If should be
appreciated by those practicing the present invention that a variety of
methods are available for
stabilizing platinum electrodes agai~ast sintering.
Tn operation, the oxygen sensor I O is immersed in a gas whose oxygen partial
pressure is
to be determined_ If there is not already oxygezz present in the porous
conductive layers 16a-d,
oxygen froru the gas passes through the porous terminations 22 and 24 and
enters the porous
electrodes 16a-d through diffusion. A. voltage from voltage source 26 is
applied across the
terxninations 22 and 24. The resulting voltage difference between the
conductive layers 16a and
16b, also referred to herein as the cathode layers, and the conductive layers
16c and 16d, also
referred to herein as the anode layers, will cause oxygen to be pumped through
the Layers of
oxygen ion conducting material 14. Since the porous electrode layers lda d
catalyze oxygen
molecules to ions at the cathode layers 16a, 16b and catalyze ia~ns to oxygen
zx~olecules at the
anode layers I6e, 16d, oxygen enters at the cathode layers 16a, 16b, is pumped
througia the
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
layers of ion conductor material 14, grad exits through the anode layers
16c,16d. The resulting
electrical current is measured by the amperor~aetric meter 28 and is
indicative of the oxygen
partial pressure of the gas.
Sensors based on stabilized zircouia tend to have operating temperatures above
700°C_
The applied voltage is monitored by the voltage meter 30_ It has been found
that applied do
voltages at and above 0.2 volts often lead to instabilities in the sensor and
that an applied voltage
of 0.05 volts has been found to yield unstable current signals at Iarge oxygen
partial pressures.
~1n applied voltage of 0.1 volts is the preferred bias voltage. The voltage
source rnay be a do
voltage source or oat ac voltage source operating at about 3 Hz. The preferred
ac frequency is
less than 50Hz sine, as the ac .frequency increases, the sensor response to
oxygen decreases_
Because the oxygen sensor of the present invention operates at an elevated
temperature, it is
preferable to provide a heater and thermometer fox the sensor body.
Resistive heating electrodes 35 are provided in the mamaer ihustrated in Figs.
2~5. As is
illustrated in Figs. 2-5, cover plate heating electrodes 35 in the form of
platinum tracks are
embedded in the ion conductor zoaterial 14 of the sensor body 12, more
specifically in the top
and bottom cover plates 32. Referring specifically to Figs. 3-5, tlae sensor
body 12 is provided
with a top heater track 2 and a bottom heater track 4. 'fhe rear face S of the
sensor body 12 is
provided wiih a conductive tez~xaxnation arranged to couple conductively the
top heater track 2 to
the bottom heater track 4. In addition, the front face 7 of the sensor body 12
is provided with a
pair of conductive tenninations 6 coupled conduetively to respective ones of
the top heater track
2 and the bottom heater track 4. Tin this manner, a complete circuit is formed
by coupling a
heating voltage source (incorporated in heating circuit controller 50) grad
terminals 8 to
respectiwe~ ones of the conductive tezminations 6.
The measured resistance in the embedded platinum heater track 35 typically
varies from
about 2.3 to about 6.5 ohms between 2S° C and 800° C,
respectively. The measured beater
power required to maintain the sensor body 12 ranges up to about 2 watts at
800° C, a pz~eferred
sensor operating temperature_ A heating voltage is applied across the heating
circuit by
connecting a heating voltage source across the heating electrodes 35. ~'he
resistivity of the
heating circuit generates heat when a voltage is applied_ The resistance of
the heating electrodes
35 varies as a function of temperature. This temperature/resistance relation
provides a means for
to
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
measuring the tennperaturc of the sensor body 12. l.'referably, the heating
electrodes 35 are
coupled to a beating circuit controller 50 programmed to control the
resistance of the heating
electrodes 35 by applying a constant current to the beating electrodes 35 and
controlling the
voltage applied thereto.
S As is illustrated in Figs. 2-S, top and bottom dielectric cover plates 32
preferably
comprise a 0_02" (0.05 em) thick dielectric material added above and below the
uppermost and
lowermost electroded layers of the sensor body 12 for electrical insulation
and structural
integrity. The sensor body 12 may be incorporated into a four pin package, two
connections fox
the heating circuit, a cathode connection, and an anode connection, surrounded
by thermal
insula~on, and enclosed by a Teflon parhiculate filter.
Co~ad~uctive Au or 1't leads may be coupled to the various sensor electrodes
by attaching
the leads to the exposed electrode portions on the sensor body 12 wifih an Au
or Pt paste.
Aleernatavely, sensor packaging can be simplified by er~nbedding the
conductive leads in the
sensor body 12. Specificahy, small holes (~0.6 mm) may be drilled in the
sensor body 12 prior
to sintering and Pt or Au vvizes may be inserted, with a suitable conductive
paste, into the holes.
.~ preferxed heating contxol scheme involves applying tl~e constant current to
the heater
electrodes 35 in square-wave pulses and using the voltage signal to control
the pulse width of the
current pulses (pulse-width modulation)_ Undex feedback conixol the pulse
width is modulated td
maintain the voltage constant, thereby maintaining the resistance of the
heating electrodes 35
constant, as desired. Stated differently, modulating the pulse width of the
current controls the
heating power applied to the heating electrodes 35 to maintain the se~asor
temperature constant.
The voltage can easily be read using a x b bit A/l~ converter to an accuracy
of ~ 0.0015°fo.
Conventional Eurrent control schemes allow maintenance of a constant current
within about
O.OX°.lo. Therefore, the temperature of the integrated sensor body can
be controlled within
acceptable ranges.
A preferred microprocessor-based heating circuit cor~traller 50 consists of a
temperature's
control section and a sensor-output section. The latter section would supply a
constant voltage to
the heating electrodes 3S and read the amperometric current in the heating
electrodes 35. The
c~urent signal may be converted to a readout of the oxygen partial pressure
and may be converted
to an output suitable for controlling a combustion process_
11
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
The sensor I O xnay be calibrated and used by first identifying the resistance
of the heating
electrodes 35 in the desired operating temperature range_ This resistance
value, e.g. 9-10 ohms
at 600 C, is known and typically is well defined within a given temperature
range.
Corresponding current and voltage parameters, e.g., 0.47 A and 4.1 volts, arc
prograaxuned into
the heating circuit controller 50~ and the controller 50 is programmed to
maintain these values.
The actual operating tempezature of any individual sensor is held constant
within the sensor's
operating range.
As an illustrative example, where 1 mil = 0.001 inches = 0.0254 mm, a
preferred sensor
body is 166 znil x 124 mil x 53 rxail (4.22 mm x 3.15~mm x 1.35 mm) and weighs
144 mg, rn the
embodiment of tlae present invention where cover plate heating electrodes 35
are exnployed, the
total electrode overlap area per layer is pzeferably about 12_7 znu2 and the
total area to thickness
ratio of the oxygen sensor body 12 is about 199 em. The exposed edge of each
eleci~ode is 50
rail (1.27 mna) wide, and each electrode extends 1 S3 mil (3.89 mm) into the
body. 1'he resistive
heating electrodes are preferably porous Pt tracks approximately x 66 mil
(4.22 mrx~) in length
and 22 mil (0.559 zrnn) in width, whereby a heater current of 223 rxaA is
typical for a control
temperature of about 600° C.
Referring now to Figs. 6A and 6E, a packaging scberrae according to orte
embodiment of
the present invention is illustrated. In the illustrated embodiment, the
sensor body 12 is enclosed
in a stainless steel tube 60. The thicla~,ess of the tube 60 is preferably
selected to be machinablE
for threads for mounting the package into a bulkhead or exhaust flue. The
sensor bod~r 12 is
stabilized and thermally insulated within the tube 60 by means of suitable gas
permeable thermal.
insulation 62 (e.g., N'extel 312 thezznal insulation). A back end 64 of the
tube 60 is sealed with a
ceramic 66_ Electrical connections 68 to the sensor body 12 ate potted in the
ceramic 66 and
routed through the insulation b2. Preferably, the electrical connections
cozxiprise 20 gauge
copper leads coupled to the few sensor leads. A front ez~d 65 of the tube 60
is provided with a
stainless steel screen 69 to permit gas to reach the sensoz body 12.
VPlzile cextaiz~ representative embodiments and details have been shown far
purposes of
illustrating the invention, it will be apparent to those skilled in the alt
that various changes in the
methods and apparatus disclosed herein may be made without departing from the
scope of the
invention, which is de .fined in the appended claims. For example, although
the sensor 10 of the
12
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
present invention is well suited for measuring excess oxygen partial pressure
because the
o~cygen-porous terminatzons 22, 24 present a catalysis area for the combustion
of CO and other
combustibles, it is noted that the present invention may be arranged for
measuring actual oxygen
partial pressurE rather than excess oxygen partial pressure. Specil5eally, the
cathode electrodes
16a, 16b exposed on the first edge i8 ofthe sensor body 12 are very thin and
present a very
small catalysis area for the combustion of CO and other combustibles.
Accordingly, by omitting
the oxygen-porous tcrminations 22, 24, the sensor 10 of tl~e present invention
may be arranged
for measuring actual oxygen paaiial pressure rather than excess oxygen partial
pressure.
huxther, it is contemplated by tlae present invention that a pair of sensors
could be
packaged to yield both actual and excess oxygen measurerner~is simply by
providing the o~cygen-
porous terminaiions 22, 24 on one sensor body only. Finally, it is noted that
an alteznate method
of measuring actual and excess oxygen using two sensors would be to maintain
one sensor below
the ignition te~.nperature of CO (600-650°C) and the second sensor
above this temperature, also
in a single package.
Combined Oxy~and NOx Sensor
Referring now to Figs. 7 and 8A-8C, a combined sensor 200 for measuring oxygen
content and 1\10X content in a gas is described. 'fhe sensor 200 comprises a
partial enclosure
210, a sensor body 220 disposed in the partial enclosure 210, a diffusion
barrier 230, and axr
oxygen sensor 240. As will be described in further detail below, the sensor
body 220 is
conFgured to provide an indication of the N'Ox content of tl~e gas and the
oxygen sensor 240 is
configured to provide an indication of the oxygen content of the gas. The
sensor 200 includes
many components identical or similar in structure to those described in detail
above with
reference to Fig. 2. Like reference numerals are utilized in Figs. 2 and 7
corresponding to the
life elements and reference is made to the discussion of Fig_ 2 for a
description of these
elements_
The partial enclosure 210 defines a gas passage 2I2 and as referred to herein
as "partial's
because it encloses a defined space but also defines the gas passage 212, an
inlet portion 2I4,
and an outlet portion 216. ~'he partial enclosure 210 typically comprises au
o~tygen-ion
conductive ceramic tube. It is noted that, although the enclosure is
illustrated with a rectangula.e
13
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
cross-section, an enclosure with a circuaar cross seci~on is likely to be more
efFective and easier
to mariufacture_
The diffusion barrier 230 extends across the gas passage 212 and defines a
diffusion-
limited portion 2I8 of the gas passage 2I2 between the inlet portion 214 and
the outlet portion
216. The enclosure 210, the diffusion barzaer 230, and the sensor body 220 are
configured such
that the diffusion-limited portion 218 of the gas passage 212 comprises a
hermetically sealed
Zone including a diffusion inlet defined by the diffusion barrier 230 and a
sensor outlet defined
by the sensor body 220. An oxygen pumping portion 250, described in detail
below, is also
provided in the hermetically sealed zone.
The diffusion barrier 230 is porous to oxygen and NOx and may comprise, for
example, a
substantially uniform zirconia partition. Typically, the diffusion barrier is
configured to pass an
amount of gas that varies as a function of oxygen partial pressure of gas
within an inlet portion of
the gas passage. It is contemplated that the diffusion barrier may define a
variety of
con~~gurativns including, for example, a perforated plate, a plate including a
single restricted
x5 aperture, etc.
The sensor body 220 extends across the outlet porkion 216 of the gas passage
212 and is
disposed in the diffusion-limited portion 218 of the gas passage 212. The
sensor body 220
differs from the sEnsor body 12 illustrated in Fig. 2 in that selected ones of
the oxygen porous
conductive layers are formed from a material that catalyzes the dissociation
of NOx into N2 and
02. In this manner, dissociated 02 may be measured as an amperometric current
sad the
amperometric current may be related to NOx contex~t_ 'fhe conductive layexs
that do not catalyze
the dissociation of NOx into N2 and 02, i.e., the non-dissociative electrode
layers, are utilized to
pxovide an indication of oxygen content, as will be described in further
detail herein.
Specifically, the sensor body 220 comprises a plurality of oxygen-porous
electrode layers
ZS 16a, 16c and a pluo:ality of dissociative oxygen-porous electrode layers
16b, 16d. As is described
above with reference to the oxygen sensor of Fig. 2, the oxygen-porous
electrode layers 16a, 16c
catalyze cause oxygen to be pumped through the layers of oxygen ion conducting
rx~ate~al 14 by
catalysing oxygen molecules to ions at the cathode layers and catalyzing ions
to oxygen
molecules at the anode layers. The resulting electrical current is measured by
the arnperometric
meter 28 and is indicative of the oxygen partial pressure o~the gas_ The
dissociative oxygen-
14
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
porous eIecixode layers 16b, 16d pump oxygen through this process as well but
additionally
pump oxygen dissociated from NOx in the gas by catalyzing the dissociation of
NOx into N2 and
02 at the cathode layers. As a result, the resulting electrical current at the
dissoeiative oxygen-
porous electrode layers 16b, 16d provides an indication of NOx present in the
gas.
As is the case with the embodiment ofFig_ 2, a plurality of oxygen ion
conductive
cerarr~ic layers are interposed between respective ones of the oxygen=porous
electrode layers 16a,
16c and respective ones of the dissociative oxygen-porous electrode layers
16b,16d. As will be
appreciated by those practicing the present invention, an oxygen content
electrical signal output
is provided in the form of electrical leads coupled to the plurality of oxygen
porous electrode
layers 16a,16c. Similarly, a NOx content electrical signal output is provided
in the fozxu of
electrical leads coupled to the plurality of dissoeiative oxygen-porous
electrode layers 16b, 16d.
In this manner, the o~tygen-porous electrode layers 16a, 16e are coupled to an
electrical signal
output indicative of an oxygen content of gas within the diffusion-lizxaited
portion 218 of the gas
passage 212 and the dissociative oxygen-porous electrode layers 16b, x6d are
coupled to an
electrical signal output indicative of an NOX content of gas within the
diffusion-limited portion
218 of the gas passage 212.
The NOx content electrical signal output is electrically isolated frorr~ the
oxygen content
electrical signal output to ensure proper device performance. To further
enhance device
performance, flee power source 30 and the electrode layers 16a, 16b, 16c,16d
are arranged such
that the oxygen-porous electrode layer 16a and the dissociative oxygen porous
electrode layer
16b define the sole adjacent pair of different-type electrode layers and have
matching polarity.
The electrode layers 16a, 16b are also at substantially equivalent electrical
potential (e_g_,
0.1VDC). In this manner, pumping of oxygen between the oxygen-porous electrode
layer 16a
and the dissociative oxygen-porous electrode layer 1Gb is .inhibited. In
contrast, the sensor
arrangement illustrated in Fig 2 nncludes electrode layers of alternating
polarity.
At elevated temperatures, e.g., above about 600°C, Rh catalyzes the
dissociation of NOx
into NZ and Oz. Accordingly, the dissociative oxygen-porous electrode layers
ldb, 16d may
comprise Rh_ The non-dissoeiative electrode layers 16a, 16c may comprise
oxygen porous
platinum, as described above, and may additionally include Au in an amount
sufficient to
discourage catalysis of the dissociation of NOx. As is noted above with
reference to the oxygen
is
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
sensor of Fig. 2, a heater or heating electrode is preferably configured to
elevate the operating
temperature of the combined sensor well above room temperature, typically an
the vicinity of an
operating temperature of about 800°C. The sensor is temperature
independent in this range. The
heater may, for example, be providEd in the form of a heating electrode formed
about the
enclosure 210_
The paz-tial enclosure 2I0 also defines an oxygen pumping portion 250 that is
configured
to maintain a favorable NOx to oxygen ratio in the diffusion limited portion
218 of the gas
passage 212. Depending upon the operation. constraints of the equipment used
with the present
invention, accurate measurement of l~Ox content may be problematic if the
amount of oxygen in
the diffusion limited portion relative to the amount of NOx is too high. The
oxygen pumping
portion 250 comprises an oxygen-porous cathode electrode 252, an oxygen-porous
anode
electrode 254, and axz oxygen-ion conductive ceramic material 256. The oxygen-
porous cathode
electrode 252 is positioned over an interior surface of the partial enclosure
210 within the
diffusion-limited portion 218 of the gas passage 212. The oxygen porous anode
electrode Z54 is
1 S positioned over azi exterior surface of the partial enclosure 210 outside
of the diffusion-limited
portion 218 of the gas passage 212. The oxygen-ion conductive ceramic material
2SG is typically
formed by the body of the enclosure 210 and, as such, is interposed between
the cathode
electrode 252 and the anode electrode 254. 'f he oxygen-porous anode electrode
254 may
eonoprise platinum and the oxygen-porous cathode electrode 252 may also
comprise platinum
with an amount of gold additive su:~cient to discourage dissociation of NOx.
Preferably, the NOx to oxygen ratio in the diffusion lix~aited portion 218 is
below about 5
parts oxygen to 1 part NOx but x~c.~ay be higher if the equipment used to
measure amperometric
current and control the voltages at the electrodes is optimized to accowlt for
higher oxygen
levels. Accurate measurement of NO~c content is problematic if the amount of
oxygen in the
diffiusion limited portion relative to the amount o~NOx is too high. For
example, there is a
logarithmically linear relafiionship between amperometric current and oxygen
partial pressure
below about 1000 ppm but accurate measurement is problexx~atic above this
level. A feedback
loop xnay be coupled between the sensor body 220 and the oxygen pumpxz~g
portion 250. The
feedback loop may be configured to control the oxygen pumping porhion 250 in
response to the
amount of oxygen sensed by the sensor body 220. Specifically, using the oxygen
measurement
16
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
from the sensor body 220, the rate of pumping oxygen out of the diffusion
limited portion 218
can be continuously adjusted so that no more oxygen is pumped out ofthe tube
interior than is
needed to provide an accurate measurement of t'he NOx content (e.g., tv keep
the ratio ofNOx-
released oxygen to background oxygen at, say, 1:5). The feedback loop may also
be configured
to switch the pumping funetioxx on and offin response to the amount of sensed
oxygen. Tn this
mannez~, operation of the oxygen pumping portion 250 rxxay be operated to
minimize power
eonsurnption of the combined sensor 200.
The oxygen sensor 240 is positioned in the inlet portion 214 of the gas
passage 212 and
provides a signal indicative of the oxygen partial pressure of the gas ixx the
inlet portion 214.
'Thus, the combined sensor 200 is 'configured to provide independent
indicatioxas of oxygen
partial pressure and NOX content.
Turning now to the manner in which the NOX content is determined, NOx present
in the
gas v~rithin tile diffusion limited portion 218 dissociates on the
dissociative oxygen porous
electrode layers 16b, 16d and the released oxygen creates an ampexometric
current at the NOx
content electrical signal output. Oxygen in the surrou~oding gas also
contributes to the NOx
content electrical signal output, increasing the amperometric current because
tlae dissociative
electrodes lbb, 16d pump the oxygen in the gas and the oxygen dissociated $rom
the NOx
present in the gas. This "background" oxygen axad the inczeased amperometric
current can be
accounted for using the oxygen content electrical signal output from the
electrodes 16a,16c
ZO because the corresponding amperometric current at the non-dissociative
olect~rodes 1,6a,16c
provides an izxdependent xr~easure of the background oxygen.
As is nol~ed above, to accurately measure tk~e NOx content, it is also
z~eecssaacy to reduce
the background oxygen in the diffusion limited portion to a level commensurate
with the NOx
released oxygen (e.g., to a ratio of about 5:1 (oxygen to NO~.
As is noted above, the sensor body 220 has two separated sets of porous
electrodes, one
of which catalyzes the dissociation of NOx to nitrogen and o~cygen. For
convenience of
illustration, Fig_ 7 merely illustrates a pair of electa-ode layers in eack~
set. However, it is
contemplated that a large number of electrode layers could be provided in each
set. Preferably,
an equal number of electrode layers are provided in each set. However, it is
contemplated by the
17
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
present izzvention that more electrode layers could be providEd in one set,
relative to the other, as
long as the difference in nurxaber is accounted for in the subsequent NOx
content calculation.
The sensor 200 may be mounted directly iz~ an exhaust or sample gas. There is
no need
for a reference gas supply. Particulate filters or ether types of. filters may
be provided to present
damage to the sensor and extend sensor life.
The sensor 200 is preferably manufactured in a manner similar to that
discussed above
with reFerence to the oxygen sensor of Fig. 2. Although a variety
ofznanu~acturir~g techniques
are available, multi-layering manufaeturiz~g processes have the flexibility of
producing layers
electroded with PtlAu and separate layers electroded with Rh in tb,e same
sensor body. Sensor
leads are preferably embedded in the sensor body by drillixrg small holes
(~0.5 xnm) in the sensor
body 220 in the green state. The sensor body 220 is thezt sintered and Pt
wires are feed in the
holes with a Pt paste. The stiffness of the ft wires has the advantage
ofprovidiz~g mechanical
support. Leads for the oxygen sensor 240 are similarly errabedded.
The length of the actual coxxibined sensor nnay be about one inch (2.5 cm) and
the major
outside diameter may be about %x 111C11 (1.25 cm). The enclosure 210 may
comprise a zircania
tube made by slip casting. The tube i.s ypically milled in the green state to
pmvide passageways
for electrical leads and is subsequently sintered. The PtJAu and Pt electrodes
252, 254 are then
fired on the intezior and exterior of the tube, respectively. Finally, using a
commercial glass for
sealing zirconia parts together, the sensor body 220, the diffusion battier
230, anal the oxygen
sensor 240 are sealed in the zirconia tube in a single firing_ The first two
components are sealed
hermetically. .A. Pt lead for the internal Pt/Au electrode passes through the
wall of the tube and is
also sealed hermetically. A slot in the zirconia tube at the large open end
provides the
passagerWay fez two oxyge~a sensoz leads, and opposing slots in the snrxall
closed end provide
passageways for four du.a.1-sensor leads. The sensor body or dual sensor 220
arid its four leads
are hermetically sealed in the xirconia tube rwith a commerciahy available
glass.
Generally, the operation of the combined sensor 200 is as follows: The device
Xs heated
to, and maintained at, the operafiing temperature (e.g., 800 °C), axed
the oxygen sensor 240
measures the oxygen partial pressure of the exhaust or sample gas. The gas
diffuses through the
diffusion bawier 230 into the interior diffusion limited portion 218 of the
enclosure or tube 210.
A voltage applied across tlae cathode 252 and the anode 254 causes the oxygen
in the interior to
18
CA 02431018 2003-06-06
WO 03/008957 PCT/USO1/47465
be pumped to a sufficiently low Ievel. The sensor body 220 measures this low
oxygen level with
the non-dissociative layers 16a, 16e. The dissociative electrode layers 16b,
16d measure both the
low oxygen level and the oxygen released from the NOx dissociation. ~laese
amperometrie
currents from both sets of electrodes are then used to determine the NOx
content.
The 2irconia diffusion barrier 230 diffusion-limits the amount of exhaust gas
entering the
interior of the tube and thereby ensures that a low level of oxygen can be
reached in the interior
by the pumping process (i.e., without this plug the interior would be
constantly flooded with the
exhaust gas). The NOx diffuses through this plug as molecular NOx.
Tlae heater (not shown in Fig. 2) has a temperature-dependent resistance and
thereby
provides a aneans for measuring and controlling the operating temperature. ,A.
tradeoff is
involved with the operating temperature, however: On the one hand, the higher
the temperature,
the more power is consmned by the lieater in maintaining this temperature. On
the other hand,
the temperatuze slZVUld be high enough to reduce the resistance of the
zirconia tube to a low
value to avoid consuming large amounts of powex in pumping the oxygen out of
the tube
interior.
For tlae purposes of describing and defining the present invention it is noted
that the term
"substantially" is utilized herein to represent the inherent degree of
uncertainty that may be
attributed to any quantitative comparison, value, measurement, or other
representation. The terra
"substantially" is also utilized herein to represent the degree by which a
quantitative
representation may vary from a stated reference without resulting in a change
in the basic
function of the subject matter at issue.
.'l3aving described tl~e invention in~ detail and by reference to preferred
erxxbodiments
thereof, it will be apparent that modifZCations and variations are possible
without departing from
the scope ofthe invention defined iu~ the appended claims. More specifically,
although some
aspects of the present invention are identified herein as preferred oz-
particularly advantageous, it
is contemplated that the present invezttian is not necessarily limited to
these preferred aspects of
the invez~tion_
What is claimed is:
19