Note: Descriptions are shown in the official language in which they were submitted.
CA 02432662 2003-06-09
WO 97!29743 PCT/IJS96/13632
IL-8 RECEPTOR ANTAGONISTS
s
FIELD OF THE INVENTION
This invention relates to a novel group of phenyl urea compounds, processes
for
the preparation thereof, the use thereof in treating IL,-8, GROG, GRO~, GROy
and
NAP-2 mediated diseases and pharmaceutical compositions for use in such
therapy.
BACKGROUND OF THE INVENTION
Many different names have been applied to Interleukin-8 (IL,-8), such as
neutrophil attractant/activation protein-1 (NAP-1}, monocyte derived
neutrophil
chemotactic factor (MDNCF), neutrophil activating factor (NAF), and T-cell
lymphocyte chemotactic factor. Interleukin-8 is a chemoattractant for
neutrophils,
basophils, and a subset of T-cells. It is produced by a majority of nucleated
cells
including macrophages, fibroblasts, endothelial and epithelial cells exposed
to TNF,
iL-1 a, IL-1 b or LPS, and by neutrophils themselves when exposed to LPS or
chemotactic factors such as FMLP. M. Baggiolini et al, J. Clin. Invest. 84,
1045
( l 989); J. Schroder et al, J. Immunol. 139, 3474 ( 1987) and J. Immunol.
144, 2223
( 1990) ; Strieter, et al, Science 243, 1467 ( 1989) and J. Biol. Chem. 264,
10621 ( 1989);
Cassatella et al, J. Immunol. 148, 3216 (1992). _
GROG, GRO~i, GROy and NAP-2 also belong to the chemokine a family. Like
IL-8 these chemokines have also been referred to by different names. For
instance
GROa, ~3, y have been referred to as MGSAa, b and g respectively (Melanoma
Growth
Stimulating Activity), see Richmond et al, J. Cell Physiology 129, 375 ( 1986)
and
Chang et al, J. Immunol 148, 451 ( 1992). All of the chemokines of the a-
family which
possess the ELR motif directly preceding the CXC motif bind to the FL-8 B
receptor.
IL-8, GROa, GRO~i, GROy and NAP-2 stimulate a number of functions in
vitro. They have all been shown to have chemoattractant properties for
neutrophils,
while IL-8 and GROG have demonstrated T-lymphocytes, and basophiles
chemotactic
activity. In addition IL-8 can induce histamine release from basophils from
both
normal and atopic individuals GRO-a and IL-8 can in addition, induce Iysozomal
enzyme release and respiratory burst from neutrophils. IL-8 has also been
shown to
increase the surface expression of Mac-1 (CDIIb/CD18) on neutrophils without
de
novo protein synthesis. This may contribute to increased adhesion of the
neutrophils to
vascular endothelial cells. Many known diseases are characterized by massive
CA 02432662 2003-06-09
WO 97/29743 PGTIUS96/13632
neutrophil infiltration. As IL-8, GROa, GRO~i, GROy and NAP-2 promote the
accumulation and activation of neutrophils, these chemokines have been
implicated in a
wide range of acute and chronic inflammatory disorders including psoriasis and
rheumatoid arthritis, Baggiolini et al, FEES Lett. 307, 97 (1992); Miller et
al, rit R v.
Immunol. I2, 17 (1992); Oppenheim et al, Annu. Rev. Immunol. 9, 617 (1991);
Seitz
et al., J. CIin. Invest. 87, 463 ( 1991 ); Miller et al., Am. Rev. Re~pir.
Dis. 146, 427
( 1992); Donnely et al., Lancet 341, 643 ( 1993). In addition the ELR
chemokines
(those containing the amino acids ELR motif just prior to the CXC motif) have
also
been implicated in angiostasis. Strieter et al, Science 258, 1798 ( 1992).
to In vitro, IL-8, GROa, GRO~i, GROy and NAP-2 induce neutrophil shape
change, chemotaxis, granule release, and respiratory burst, by binding to and
activating
receptors of the seven-transmembrane, G-protein-linked family, in particular
by binding
to IL-8 receptors, most notably the B-receptor. Thomas et al., J. Biol. Chem.
266,
14839 ( 1991 ); and Holmes et al., Science 253, 1278 ( 1991 ). The development
of non-
peptide small molecule antagonists for members of this receptor family has
precedent.
For a review see R. Freidinger in: Progress in Drub Research, Vol. 40, pp. 33-
98,
Birkhauser Verlag, Basel 1993. Hence, the 1L-8 receptor represents a promising
target
for the development of novel anti-inflammatory agents.
Two high affinity human IL-8 receptors (77% homology) have been
2o characterized: TL-8Ra, which binds only IL-8 with high affinity, and IL-
8Rb, which has
high affinity for IL-8 as well as for GRO-a, GROb, GROg and NAP-2. See Holmes
et
al.. supra; Murphy et al., Science 253, 1280 (i991); Lee et al., J. Biol.
Che~n. 267,
16283 ( 1992); LaRosa et al., J. Biol. Chem. 267, 25402 ( 1992); and Gayle et
al., ~
Biol. Chem. 68, 7283 ( 1993).
There remains a need for treatment, in this field, for compounds which are
capable of binding to the IL-8 a or b receptor. Therefore, conditions
associated with an
increase in IL-8 production (which is responsible for chemotaxis of neutrophil
and T-
cells subsets into the inflammatory site) would benefit by compounds which are
inhibitors of IL-8 receptor binding.
SUMMARY OF THE I1WENTION
This invention provides for a method of treating a chemokine mediated disease,
wherein the chemokine is one which binds to an IL-8 a or b receptor and which
method
comprises administering an effective amount of a compound of Formula (n or a
pharmaceutically acceptable salt thereof. In particular the chemokine is IL-8.
-2-
CA 02432662 2003-06-09
WO 97/29743 PCTIUS96/13632
This invention also relates to a method of inhibiting the binding of IL-8 to
its
receptors in a mammal in need thereof which comprises administering to said
mammal
an effective amount of a compound of Formula (I).
Compounds of Formula (I) useful in the present invention are represented by
the
structure:
R
n~Y)~ J,.~ ~ R~)m
H H
(I)
wherein
X is oxygen or sulfur;
R is any functional moiety having an ionizable hydrogen and a pKa of 10 or
less;
R 1 is independently selected from hydrogen; halogen; vitro; cyano; C 1 _ 10
~Yl:
halosubstituted'C1_10 alkyl; C2_10 alkenyl; C1-10 alkoxy; halosubstituted
Cl-l0~koxy; azide; S(O)tR4; (CRBRg)q S(O)tR4; hydroxy; hydroxy substituted
C 1 alkyl; aryl; aryl C 1 ~ alkyl; aryl C2_ 10 alkenyl; aryloxy; aryl C 1 ~
alkyloxy;
heteroaryl; heteroarylalkyl; heteroaryl C2_ 10 alkenyl; heteroaryl C 1 ~
alkyloxy;
heterocyclic, heterocyclic Cl~alkyl; heterocyclicCl~alkyloxy;
heterocyclicC2_10
alkenyl; (CRBRg)q NR4R5; (CRBRg)q C(O)NR4R5; C2_ l0 aikenyl C(O)NR4R5;
(CRBRg)q C(O)NR4R10; S(O)~H; S(O)3Rg; (CRBRg)q C(O)R11; C2-10 ~kenyl
C(O)R11; C2_IO alkenyl C(O)ORI1; (CRBRg)q C{O)OR11; (CRBRg)q OC(O)R11;
(CRBRg)qNR4C(O)R11; (CRBRg)q C(NR4}NR4R5; (CRBRg)q NR4C(NRS)R11 ,
(CRBRg)q NHS(O)ZR13; (CRBRg)q S(O)2NR4R5, or two R1 moieties together may
form O-(CH2)s0- or a 5 to 6 membered unsaturated ring, and wherein the alkyl,
aryl,
arylalkyl, heteroaryl, heterocyclic moities may be optionally substituted;
t is 0, or an integer having a value of 1 or 2;
s is an integer having a value of i to 3;
R4 and RS are independently hydrogen, optionally substituted C1_4 alkyl,
optionally
substituted aryl, optionally substituted aryl Cl~alkyl, optionally substituted
heteroaryl, optionally substituted heteroaryl Cl~alkyl, heterocyclic,
heterocyclic
C 1 ~ alkyl, or R4 and RS together with the nitrogen to which they are
attached form
a 5 to 7 member ring which may optionally comprise an additional heteroatom
selected from O/N/S;
Y is hydrogen; halogen; vitro; cyano; halosubstituted C 1 _ 10 alkyl; C 1-10
~Yl~ C2-10
alkenyl; C 1 _ 10 alkoxy; halosubstituted C 1 _ 10 alkoxy; azide;
(CRBRg)qS(O)tR4,
(CRBRg)qOR4; hydroxy; hydroxy substituted C 1 alkyl; aryl; aryl C 1 ~ alkyl;
_3_
CA 02432662 2003-06-09
WO 97/29743 PCT/US96II3632
aryloxy; arylC 1 _q. alkyloxy; aryl C2_ I0 alkenyl; heteroaryl;
heteroarylalkyl;
heteroaryl C 1 ~ alkyioxy; heteroaryl C2_ 10 alkenyl; heterocyclic,
heterocyclic
C 1 _4alkyi; heterocyclicC2_ 10 alkenyl; (CRgRg)qN'R4R5; CZ_ 10 alkenyl
C(O)NR4R5; (CRBRg)qC(O)NR4R5; (CRBRg)q C(O)NR4R10; S(O)3Rg;
(CRBRg)qC(O)R11; C2-10 ~kenylC(O)Rl l; (CRBRg)qC(O)OR11:
C2-l0~kenylC(O)OR11; (CRBRg)qOC(O)Rli; {CRBRg)qNR4C(O)R11:
{CRBRg)qNHS(O)2Rb; (CR8R8)qS(O)ZNRøR5; (CRgRg)qC(NR4)NR4R5;
(CRBRg)q NR4C(NRS)R1 l; or two Y moieties together may form O-(CH~,)s0- or a
to 6 membered unsaturated ring; and wherein the alkyl, aryl, arylalkyl,
heteroaryl,
i0 heteroaryl alkyl, heterocyclic, heterocyclicalkyl groups may be optionally
substituted;
q is 0 or an integer having a value of 1 to 10;
m is an integer having a value of 1 to 3;
R( and R7 are independently hydrogen or a C 1..4 alkyl group, or R6 and R~
together
with the nitrogen to which they are attached form a 5 to 7 member ring which
ring
may optionally contain an additional heteroatom which heteroatom is selected
from
oxygen, nitrogen or sulfur;
Rg is hydrogen or C 1 ~ alkyl;
R 10 is C 1 _ 10 alkyl C(O)ZRg;
R 1 l is hydrogen, optionally substituted C 1 ~ alkyl, optionally substituted
aryl,
optionally substituted aryl C 1 alkyl, optionally substituted heteroaryl,
optionally
substituted heteroarylC 1 alkyl, optionally substituted heterocyclic, or
optionally
substituted heterocyclicC 1 alkyl;
R 12 is hydrogen, C 1 _ 10 alkyl, optionally substituted aryl or optionally
substituted
arylalkyl;
R 13 is suitably C l~ alkyl, aryl, aryl C 1 alkyl, heteroaryl, heteroarylC 1
alkyl,
heterocyclic, or heterocyclicCl_4alkyl;
Rb is NR6R~, alkyl, aryl, aryl C1~ alkyl, aryl C2~ alkenyl, heteroaryl,
heteroaryl
C 1 ~ alkyl, heteroarylC2~ alkenyl, heterocyclic, heterocyclic C 1 ~ alkyl,
heterocyciic C2~ alkenyl, or camphor, all of which groups may be optionally
substituted;
or a pharmaceutically acceptably salt thereof.
Another aspect of the present invention is to a method of treating a chemokine
mediated disease, wherein the chemokine is one which binds to an IL-8 a or b
receptor
and which method comprises administering an effective amount of a compound of
Formula (II) or a pharmaceutically acceptable salt thereof, as defined herein.
-4-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
This invention also relates to a method of inhibiting the binding of IL-8 to
its
receptors in a mammal in need thereof which comprises administering to said
mammal
an effective amount of a compound of Formula (II), as defined herein.
This invention also relates to the novel compounds of Formula (II), or a
pharmaceutically acceptable salt thereof, as defined herein.
Another aspect of the present invention is to a method of treating a chemokine
mediated disease, wherein the chemokine is one which binds to an IL-8 a or b
receptor
and which method comprises administering an effective amount of a compound of
Formula (III) or a pharmaceutically acceptable salt thereof, as defined
herein.
to This invention also relates to a method of inhibiting the binding of IL-8
to its
receptors in a mammal in need thereof which comprises administering to said
mamma!
an effective amount of a compound of Formula (III), as defined herein.
This invention also relates to the novel compounds of Formula (111), or a
pharmaceutically acceptable salt thereof, as defined herein.
DETAILED DESCRIPTION OF THE INVENTION
The compounds of Formula (I) may also be used in association with the
veterinary treatment of mammals, other than humans, in need of inhibition of
IL-8 or
other chemokines which bind to the II,-8 a and b receptors. Chemokine mediated
2o diseases for treatment, therapeutically or prophylactically, in animals
include disease
states such as those noted herein in the Methods of Treatment section.
In compounds of Formula (1), R is suitably any functional moiety which
provides an ionizable hydrogen having a pKa of 10 or less, preferably from
about 3 to
9, more preferably from about 3 to '7. Such functional groups include, but are
nat
limited to, hydroxy, carboxylic acid, thioi, -SR2 -OR2, -NH-C(O)Ra, -
C(O)NR(R~, a
substituted sulfonamides of the formula -NHS(O)2Rb, -S(O)2NHR~, NHC(X2)NHRb,
or a tetrazolyl; wherein X2 is oxygen or sulfur, preferably oxygen.
Preferably, the
functional group is other than a sulfonic acid, either directly or as a
substituent group on
the aryl, heteroaryl, or heterocyclic moiety ring, such as in SR2 or OR2. More
preferably R is OH, SH, or NHS(O 2Rb.
Suitably, R2 is a substituted aryl, heteroaryl, or heterocyclic moiety which
ring
has the functional moiety providing the ionizable hydrogen having a pKa of 10
or less.
Suitably, R6 and R~ are independently hydrogen or a C 1..4 alkyl group, or R(
and R~ together with the nitrogen to which they are attached form a 5 to 7
member ring
-S-
CA 02432662 2003-06-09
WO 97/Z9743 FCTlUS96/13632
which ring may optionally contain an additional heteroatom which heteroatom is
selected from oxygen, nitrogen or sulfur. This heteroring may be optionally
substituted
as defined herein.
Suitably Ra is an alkyl, aryl, arylC 1 alkyl, heteroaryl, heteroaryIC 1 alkyl,
heterocyclic, or a heterocyclic C 1 ~alkyi moiety, all of which may be
optionally
substituted, as defined herein below.
Suitably, Rb is a NR6R~, alkyl, aryl, aryl Cl~ alkyl, aryl C2~ alkenyl,
heteroaryl, heteroaryl C 1 ~ alkyl, heteroarylC2_4 alkenyl, heterocyclic,
heterocyclic
C1~ alkyl, a heterocyclic C2~ alkenyl moiety, or camphor, all of which groups
may be
optionally substituted one to three times independently by halogen; vitro;
halosubstituted C 1 ~ alkyl, such as CF3; C 1 ~ alkyl, such as methyl; C l~
alkoxy, such
as methoxy; aryl; heteroaryl; heterocyclic; NR9C(O)Ra; C(O)NR6R~, S(O)3H,
S(O)m~Ra (wherein m' is 0, 1 or 2), or C(O)OC 1 ~ alkyl. When Rb is an aryl or
arylalkyl, preferably it is an optionally substituted phenyl, benzyl, or
styryl. When Rb
is a heteroaryl preferably it is an optionally substituted thiazole,
optionally substituted
thienyl, optionally substituted quinalinyl or isoquinolyl ring, or pyridyl
ring.
R9 is hydrogen or a C 1 ~ alkyl, preferably hydrogen. Suitably, when the
substituent group on the Rb moiety is NR9C(O)Ra, then Ra is preferably an
alkyl
group, such as methyl.
Suitably Rc is hydrogen, alkyl, aryl, arylC 1 alkyl, arylC l~alkenyl,
heteroaryl,
heteroarylC 1 alkyl, heteroarylC 1 ~alkenyi, heterocyclic, or heterocyclic C 1
alkyl, or
a heterocyclic C 1 ~alkenyl moiety, all of which groups may be optionally
substituted
one to three times independently by halogen, vitro, halosubstituted C 1 ~
alkyl, C 1..4
alkyl, Cl~ alkoxy, NR9C(O)Ra, C(O)NR6R7, S(O)3H, or C(O)OCI~ alkyl, wherein
R9 is hydrogen or a C1~ alkyl. Preferably, Rc is an optionally substituted
phenyl.
When R is an ORZ or SRZ moiety it is recognized by one of skill in the art
that
the aryl ring must, therefore, contain the required ionizable hydrogen. The
aryl ring
may also be additionally substituted, independently, by one to three groups,
which
groups may also contain an additional ionizable group, and which include but
are not
limited to, halogen, vitro, halosubstituted C 1 ~ alkyl, C 1.~ alkyl, C 1 ~
alkoxy, hydroxy,
SH, -C(O)NR(R~, -NH-C(O)Ra, -NHS(O)2Rb, S(O)2NR6R~, C(O)ORg~ or a
tetrazolyl ring.
-6-
CA 02432662 2003-06-09
WO 97/29'143 PG"T/US96/13632
In compounds of Formula (I), suitably R1 is suitably an electron withdrawing
moiety. R 1 may be independently selected from hydrogen; halogen; nitro;
cyano;
halosubstituted C 1 _ 10 alkyl, such as CF3; C 1 _ 10 alkyl, such as methyl,
ethyl, isopropyl,
or n-propyl; C2_ 10 alkenyl; C 1 _ 1p alkoxy, such as methoxy, or ethoxy;
halosubstituted
CI-10 alkoxy, such as trifluoromethoxy; azide; S(O)tR~, wherein t is 0, 1 or
2;
(CRgRg)q S(O)tR4; hydroxy; hydroxy substituted Cl~alkyl, such as methanol or
ethanol; aryl, such as phenyl or naphthyl: aryl C 1 _4 alkyl, such as benzyl;
aryl C2_ I O
alkenyl ; aryloxy, such as phenoxy; aryl C 1 ~ alkyloxy, such as benzyloxy;
heteroaryl;
t0 heteroarylalkyl: heteroaryl C1~ alkyloxy; heteroaryl C2_10 alkenyi;
(CRgRg)qNR4R5;
C2-10 alkenyl-C(O)NR4R5; (CRgRg)qC(O)NR4R5; (CRgRg)qC(O)NR4R10; S(O)3H;
S(O)3Rg; (CRgRg)q C(O)R1 ~, such as trifluromethyl ketone ; C2_10 alkenyl
C(O)Rl 1,
C2-10 ~enylC{O)OR11; (CRgRg)qC(O)OR11, such as carboxy, methylcarboxyiate or
phenylbenzoate; (CRgRg)qC(O)OR12; (CRgRg)qOC(O)R11; (CRgRg)q NR4C(O)R11;
~5 (CRgRg)qNHS(O)2R13; (CRgRg)qS(O)2NR4R5; or two R1 moieties together may
form O-(CH2)s0- or a 5 to 6 membered unsaturated ring; and s is an integer
having a
value of 1 to 3. The alkyl, aryl, arylalkyl, aryialkenyl, heteroaryl,
heteroarylalkyl,
heteroarylalkenyl, heterocyclic, heterocyclicalkyl, and heterocyclicalkenyl
moieties may
all be optionally substituted as defined herein below. Preferably R1 is other
than azido
20 or S(O)3Rg. Rg is independently hydrogen or C 1~ alkyl, which may be
branched or
straight.
When RI forms a dioxybridge, s is preferably 1. When Rl forms an additional
unsaturated ring, it is preferably 6 membered resulting in a naphthylene ring
system.
25 This naphthylene ring may be substituted independently, 1 to 3 times by the
other R1
moieties as defined above.
Suitably, R4 and RS are independently hydrogen, optionally substituted C1~
alkyl, optionally substituted aryl, optionally substituted aryl Cl_4alkyl,
optionally
3o substituted heteroaryl, optionally substituted heteroaryl Cl~alkyl,
heterocyclic,
heterocyclicC 1~ alkyl, or R4 and Rg together with the nitrogen to which they
are
attached form a 5 to 7 member ring which may optionally comprise an additional
heteroatom selected from O/N/5. The optionally substituted moieties are as
defined
herein below.
R10 is suitably C1_10 alkyl C(O)2Rg, such as CH2C(O)2H or CH2C(O)2CH3.
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
R 11 is suitably hydrogen, optionally substituted C 1 _4 alkyl, optionally
substituted aryl, optionally substituted aryl C1_4 alkyl, optionally
substituted heteroaryl,
optionally substituted heteroaryl C 1..4alkyl, optionally substituted
heterocyclic, or
optionally substituted heterocyclic C 1 _q.alkyl. The optionally substituted
moieties are as
defined herein below.
R 12 is suitably hydrogen, optionally substituted C 1 _ 10 alkyl, optionally
substituted aryl or optionally substituted arylalkyl. The optionally
substituted moieties
are as defined herein below.
to
Preferably R1 is halogen, cyano, vitro, CF3, C(O)NR4R5, alkenyl C(O)NR~RS,
C(O) R4R 10, alkenyl C(O)OR 12, heteroaryl, heteroarylalkyl, heteroaryl
alkenyl, or
S(O)NR4R5, and preferably R4 and RS are both hydrogen or one is phenyl. A
preferred ring substitution for R1 is in the 4-position of the phenyl ring.
When R is OH, SH or NS02Rb than R1 is preferably substituted in the 3-
position, the 4- position or di-substituted in the 3,4- position. The
substituent group is
suitably an electron withdrawing moiety. Preferably when R is OH, SH or
NSOZRb,
than R1 is vitro, halogen, cyano, trifluoromethyl group, C(O)NR4R5.
When R is carboxylic acid, than R 1 is preferably hydrogen, or R 1 is
preferably
substituted in the 4-position, more preferably substituted by trifluoromethyl
or chloro.
In compounds of Formula (I), suitably Y is independently selected from
hydrogen; halogen; vitro; cyano; halosubstituted C 1 _ 10 alkyl; C 1 _ 10
alkyl; C2_ 10
alkenyl; C 1 _ 10 alkoxy; halosubstituted C 1 _ l p alkoxy; azido;
(CRBRg)qS(O)tR4,
wherein q is 0 or an integer having a value of 1 to I0; (CRBRg)qOR4; hydroxy;
hydroxy C 1 alkyl; aryl; aryl C 1 ~ alkyl; aryloxy; arylC 1.4 alkyloxy; aryl
C2_ 10
alkenyl; heteroaryi; heteroarylallcyl; heteroaryl C 1 ~. alkyloxy; heteroaryl
C2_ 10
3o alkenyl; heterocyclic, heterocyclic C 1 alkyl; heterocyclicC2_ 10 alkenyl;
(CRgR~qNR4Rg; C2_ 10 alkenyl C(O)NR4R5; (CRBRg)qC(O)NR4R5;
(CRBRg)qC(O)NR4R10; S(O)3R8; (~gRg)qC(O)R11; C2-IO alkenyl C(O)R11;
C2-10 ~kenyl C(O)OR11; (CRBRg)q C(O)OR12; (CRBRg)qOC(O)R11;
(CRBRg)qNR4C(O)R11; (CRBRg)q NHS(O)2Rb; (CRBRg)qS(O)2NR4R5;
3s CRBRg)qC(NR4)NR4R5; (CRBRg)q NR4C(NRS)R11, or two Y moieties together may
form O-(CH2)s0- or a 5 to 6 membered unsaturated ring. When Y forms a
dioxybridge, s is preferably 1. When Y forms an additional unsaturated ring,
it is
_g_
CA 02432662 2003-06-09
WO 97/29743 PCTlCTS96l13632
preferably 6 membered resulting in a naphthylene ring system. This naphthylene
ring
may be substituted 1 to 3 times by another Y moiety, such as defined above.
Additionally ail of the various aryl, heteroaryl and heterocyclic groups noted
above, as
well as the R4, RS and Rl 1 substituent groups, may be optionally substituted
as defined
herein in the specification below. Preferably Y is other than azido or
S(O)3Rg. Rg is
independently hydrogen or C 1 ~ alkyl.
Y is preferably a halogen, C 1 ~ alkoxy, optionally substituted aryl,
optionally
substituted aryloxy, optionally substituted aryialkoxy, optionally substituted
1o arylalkyloxy, optionally substituted heteroarylalkyloxy, methylenedioxy,
NR4R5,
thioC 1 alkyl, thioaryl, halosubstituted alkoxy, optionally substituted C 1 ~
alkyl, or
hydroxy alkyl. Y is more preferably mono-substituted halogen, disubstituted
halogen,
mono-substituted allcoxy, disubstituted allcoxy, methylenedioxy, aryl, or
alkyl, more
preferably these groups are mono or di-substituted in the 2'- position or 2'-,
3'-position.
While Y may be substituted in any of the 5 ring pdsitions, preferably when R
is
OH, SH, or NHS02Rb, Y is preferably mono-substituted in the 2'-position or 3'-
position, with the 4'- preferably being unsubstituted. If the ring is
disubstituted, when R
is OH, SH, or NHS02Rb, substituents are preferably in the 2' or 3' position of
a
2o monocyclic ring. While both R1 and Y can both be hydrogen, it is prefered
that at least
one of the rings be substituted, preferably both rings are substituted.
In compounds of Formula (I), X is suitably oxygen or sulfur, preferably
oxygen.
2s While not explicitly covered by Formula (n, (Ia-c), (II), {IIa-c), or (11n,
another
aspect of this invention are the symmetrical bis compounds which are included
for each
structure.
Compounds exemplified by this bis like structure include:
3o N-{Bis (2-hydroxy-4-vitro phenyl-N'-(dianisdine~iurea
4-Methylene bis(N-(2-chloro phenyl)-N'-(2-hydroxy 4-nitrophenyl)urea)
Exemplified compounds of Formula (I) include:
N-[2-Hydroxy-4-(methoxycarbonyl)phenyl]-N'-phenylurea;
35 N-[5-Nitro-2-hydroxyphenyl]-N'-phenyl urea
3-Hydroxy-4-{ [(phenylamino)carbonyl)amino )benzamide
N-(z-Hydroxy-4-fluorophenyl)-N'-phenyl urea
-9-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96l13632
2- { [(Phenylamino)carbonyl]amino } thiophenol
N-(2-Carboxy-4-hydroxyphenyl)-N'-phenyl urea
N-[2-Hydroxy-4-(trifluoromethyl)phenyl]-N'-phenyl urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-hydroxy-4-nitrophenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-phenyl-thiourea
N-(4-Nitro-2-(phenylsulfonylamino)phenyl)-N'-phenyl urea
N-(2-Hydroxy-5-nitrophenyl)-N'-(3-methoxy-2-thienyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-methoxy-2-thienyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-methoxyphenyl)urea
t0 N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-trifluoromethylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-trifluoromethylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-trifluoromethylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-{3-bromophenyl)urea
N-(2-Hydroxy-4-nitraphenyl)-N'-(4-bromophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-( 1-naphthyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-{2-nitrophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-fluorophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2,6-difluorophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-ethoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-{2-ethylphenyl)urea
N-(2-Hydroxy-4-nitraphenyl)-N'-(2-trifluoromethoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl) N'-(2-methylthiophenyl) urea
N-(2-Hydroxy-4-nitrophenyl) N'-(2-chloro 6-methyl phenyl) urea
N-(2-Hydroxy-4-nitrophenyl) N'-(2-sulfoxymethyl phenyl) urea
N-(4-Trifluoromethyl-2-hydroxy phenyl) N'-{2-bromo phenyl) urea
N-(4-Carbomethoxy 2-hydroxy phenyl) N'-(2-bromophenyl) urea
3o N-(4-Trifluoromethyl-2-hydroxy phenyl) N'-(2-phenyl phenyl) urea
N-(4-Carbomethoxy 2-hydroxy phenyl) N'-(2-phenyl phenyl) urea
N-(2-Hydroxy-4-nitrophenyl) N'-(2,3-dichloro phenyl) urea
N-(2-Hydroxy-4-nitraphenyl) N'-(2,4-dichloro phenyl) urea
N-(2-Hydroxy-4-nitrophenyl) N'-(2-chloro phenyl) urea
N-(2-Hydroxy-4-nitrophenyl) N'-(2,4-dibromaphenyl) urea
N-(2-Hydroxy-1-napthyl)-N'-(2-bromophenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2,3-methylenedioxyphenyl)urea
- 10-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96l13632
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-chloro-2-methoxyphenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methyiphenyl) urea
N-(4-(Benzylamino)carbonyl-2-hydroxyphenyi)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-phenoxyphenyl) urea
S N-(2-Hydroxy-4-fluoro phenyl)-N'-(2-bromophenyl) urea
N-(2-Hydroxy-3-napthyl)-N'-(2-bromophenyl) urea
N-(3,4-Difluoro-2-hydroxyphenyl)-N'-(2-bromophenyl) urea
N-(2-Hydroxy 4-phenylphenyl)-N'-(2-bromophenyl) urea
N-(2-Hydroxy-4-methylphenyl)-N'-(2-bromophenyl) urea
t0 N-(2-Hydroxy-4-nitrophenyl)-N'-(2-phenylaminophenyl) urea
N-(2-Hydroxy-3-carboxyphenyl)-N'-(2-bromophenyl) urea
N-(2-Sulfhydryl-4-bromophenyl)-N'-(2-bromophenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-iodophenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-bromophenyl) thiourea
15 N-[(2-Phenylsulfamido)-4-cyanophenyl]-N'-{2-bromophenyl) urea
N-(2-(Aminosulfonamidophenyl)phenyl)-N'-(2-bromophenyl) urea
N-(2-{Aminosulfonylstyryl) phenyl)-N'-(2-bromophenyl) urea
2-[(3,4-Di-methoxyphenylsulfonyl)amino]phenyl)-N'-(2-bromophenyl) urea
N-(2-[(4-Acetamidophenylsulfonyl)amino]phenyl)-N'-(2-bromophenyl) urea
20 N-(2-{Aminosulfonyl (2-thiophene)phenyl)-N'-(2-bromophenyl) urea
N-(2-{Aminosulfonyl (3-tolyl) phenyl)-N'-(2-bromophenyl) urea
N-(2-(Aminosulfonyl (8-quinolinyl))phenyl)-N'-(2-bromophenyl) urea
N-{2-(Aminosulfonyl benzyl) phenyl)-N'-{2-bromophenyl) urea
N-(2-Hydroxy-4-azidophenyl)-N'-(2-methoxyphenyl)urea
25 N-[2-Hydroxy-5-cyanophenyl]-N'-[2-bromophenyl]urea
N-[2-Hydroxy-3-fluorophenyl]-N'-[2-bromophenylurea
N-(2-Hydroxy-3-fluoro-5-bromophenyl]-N'-[2-bromophenyl]urea
N-[2-Hydroxy-3-chlorophenyl]-N'-[2-bromophenyl]urea
N-[2-Hydroxy-3-trifluoromethylphenyl]-N'-[2-bromophenyi]urea
30 N-[2-Hydroxy-3,4-diphenyl-phenyl]-N'-[2-bromophenyl]urea
N-[2-Hydroxy-3-glycinemethylestercarbonylphenyl]-N'-[2-bromopheny1] urea
N-[2-Hydroxy-3-glycincarbonylphenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3,5-dichlorophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-nitrophenyl]-N'-[2-bromophenyl] urea
35 N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-cyanophenyl]-N'-[2-bromophenyi] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[2-bromophenyl] urea
-11-
CA 02432662 2003-06-09
WO 97/29743 PCT/11S96/13632
N-[2-Hydroxy-4-cyanophenyl]-N'-{4-methoxyphenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[2-phenylphenyl] urea
t~I-[2-Hydroxy-4-cyanophenyl]-N'-[2-methylphenyl} urea
N-[2-Hydroxy-4-cyanophenyi]-N'-[2-trifluoromethylphenyi} urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[4-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-n-propylphenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-4-ethylphenyl]-N'-(2-bromophenyl] urea
N-(2-Hydroxy-3-phenylaminocarbonyl phenyl]-N'-(2-bromophenyl] urea
to N-(2-Hydroxy-3-cyano-4-methylphenyl]-N'-[2-bromophenyi] urea
N-[2-Hydroxy-4-carbophenyl phenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-carbophenyl phenyl]-N'-[2-bromophenyl] urea
N-[3-Benzyloxy-2-hydroxyphenyi]-N'-[2-bromophenyl] urea
(E)-N-[4-[2-(Methoxycarbonyl)ethenyl)-2-hydroxyphenyl]-N'-[2-bromophenyl]urea
t5 (E)-N-(3-[2-(Methoxycarbonyl)ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenyl}
urea-
N'-[2-bromophenyl]urea
(E)-N-(3-[2-(Aminocarbonyl)ethenyl}-2-hydroxyphenyl]-N'-(2-bromophenyl]urea-N'-
[2-bromopheayl]urea
(E)-N-[4-[2-(Aminocarbonyl)ethenyl}-2-hydroxyphenyl]-N'-[2-bromophenyl]urea-N'-
2o ' [2-bromophenyl]urea
N-[2-Hydroxy-4.-benzamide phenyl]-N'-[2-bromophenyl]urea
N-[4-Aminocarbonyl-2-hydroxyphenyl]-N'-(2-bromophenyl]urea
N-(2-Hydroxy-3,5,6-trifluorophenyi)-N'-(2-bromophenyl)urea
N-(2-Hydr~xy-3-fluoro-4-trifluoromethylphenyl)-N'-(2-bromophenyl)urea
i5 N-(2-Hydroxy-3-iodophenyl)-N'-(2-bromophenyl)urea
N-(2-[([2-(Trifluoromethyl)phenyl}sulfonyl]amino]phenyl]-N'-(2-
bromophenyl)urea
N-(2-Bromophenyl)-N'-[2-dimethylaminosulfonylamino]phenyl]urea
N-(2-(Phenethylsuifonylamino)phenyl]-N'-(2-bromophenyi)urea
N-[2-[(2-Acetamido-4-methylthiazol-5-yl)sulfonylamino]phenyl]-N'-(2-
30 bromophenyl)urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[4-phenylphenyl] urea
N-(2-Hydroxy-4-cyanophenyl]-N'-(2,3-dichlorophenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[2-methoxyphenyl] urea
N-[2-Hydroxy-4-cyanophenyl}-N'-(3-methoxyphenyi] urea
35 N-[2-Hydroxy-5-fluorophenyl]-N'-[2-bromophenyl} urea
N-[2-Hydroxy-5-trifluoromethylphenyl}-N'-[2-bromophenyl}urea
N-(2-Hydroxyphenyl]-N'-[2-bromophenyl] urea
- 12-
CA 02432662 2003-06-09
WO 97!29743 PCTILTS96/13632
N-[Trans-3-styrl-2-hydroxyphenyl]-N'-[2-bromophenyl] urea
N-(2-Hydroxy-3.4-dichlorophenyl]-N'-[2-methoxyphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[4-methoxyphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-phenylphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[4-phenylphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyI]-N'-[2,3-dichlorophenyl] urea
N-(2-Hydroxy-4-isopropylphenyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-naphthyl]-N'-[2,3-dichlorophenyl] urea
N-(2-[(2,3-Dichlorothien-5-yl)]sulfonylamino]phenyl]-N'-(2-bromophenyi)urea
N-[2-[(3,5-Bistrifluoromethylphenyl)sulfonylamino]phenyl]-N'-(2-
bromophenyl)urea
N-[2-[(2-Benzyl)sulfonylamino]-(S-trifluoromethyl)phenyl]-N'-(2-
bromophenyl)urea
N-[2-[2-(3-Nitrophenyl)sulfonylamino]phenyl]-N'-(2-bromophenyl)urea
N-[2-(2-(4-Phenoxyphenyl)sulfonylamino]phenyl]-N'-(2-bromophenyl) urea
~5 N-[[2-(1S)-10-Camphorsulfonylamino]phenyl]-N'-(2-bromophenyl)urea
N-[[2-( 1R)-10-Camphorsulfonylamino]phenyl]-N'-(2-bromophenyl)urea
N-(2-[2-(2-Nitro-(4-trifluoromethyl)phenyl)sulfonylamino]phenyl-N'-(2-
bromophenyl)urea
N-(2-Hydroxy-4-azidophenyl)-N'-(2-iodophenyl)urea
2o N-(2-Hydroxy-3-azidophenyl)-N'-(2-bromophenyl)urea
N-[2-Hydroxy-3-cyanophenyl]-N'-[2-methoxyphenyl] urea
N-[2-Hydroxy-3-cyanophenyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-cyanophenyl]-N'-[2-phenylphenyl] urea
N-[2-Hydroxy-3-cyanophenyl]-N'-(2,3-dichlorophenyl] urea
25 N-(2-Hydroxy-4-isopropylphenyl]-N'-[2,3-dichlorophenyl] urea
N-[2-Hydroxy-4-isopropylphenyl]-N'-[2-chloro-5-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-phenylphenyl]-~N'-(2,3-dichlorophenyl] urea
N-[2-Hydroxy-5-nitrophenyl]-N'-(2-methoxyphenyl] urea
N-(2-Hydroxy-5-nitrophenyl]-N'-[3-trifluoromethylphenyl] urea
30 N-[2-Hydroxy-5-nitrophenyl]-N'-[2-phenylphenyl] urea
N-(2-Hydroxy-5-nitrophenyl]-N'-[2,3-dichlorophenyl] urea
N-(2-Hydroxy-5-ethylsulfonylphenyl]-N'-(2,3-dichlorophenyl) urea
N-[2-(2-Amino-(4-trifluoromethyl) phenyl) sulfonylamino] phenyl]- N'-(2-
bromophenyl)urea
35 N-(2-(Aminosulfonyl phenyl) 3-amino phenyl] N'-(2-bromo phenyl) urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2,4 dimethoxyphenyl] urea
N-(2-Hydroxy-3,4-dichlorophenyl]-N'-[2-chloro-5-trifluoromethylphenyl] urea
- 13-
CA 02432662 2003-06-09
WO 97129743 PCT/US96113632
N-[2-Hydroxy-3-naphthyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-5-naphthalenesulfonic acid]-N'-[2-bromophenyl] urea;
N-[2-Hydroxy-4-naphthalenesulfonic acid]-N'-[2-bromophenyl] urea.
l,1'-{4-Methyl-2-phenylene)bis[2-thio-3-tolylurea]
N-(2-Carboxyphenyl)-N'-phenylurea
N-(2-Hydroxy-4-nitrophenyl)-N'-phenylurea
1-{2-Carboxyphenyl)-3-(4-chlorophenyl)urea
2-(3,4-Dichlorophenylcarbonyldiimino}-5-trifluoromethylbenzoic acid
2-(4-Chlorophenylcarbonyldiimino}-5-trifluoromethylbenzoic acid
1-(p-Anisyl)-3-(2-carboxyphenyl)urea
1-(2-Carboxyphenyl)-3-(3-fluorophenyl)urea
1-(2-Carboxyphenyl)-3-(3-chlorophenyl)urea
1-(m-Anisyl)-3-(2-carboxyphneyl)urea
1-(o-Anisyl)-3-(2-carboxyphenyl)urea
1-(2-Carboxyphenyl)-3-(3,4-dichlorophenyl)urea
1-(2-Carboxyphenyl)-3-(2,4-dichlorophenyl)urea
N-(5-Chloro-2-hydroxy-4-nitrophenyl)-N'-phenylurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-nitrophenyl)urea
N-[2-[2-(4-Chloro-3-aminophenyl)sulfonylamino]phenyl)-N'-(2-bromophenyl)urea
N-[2-(3-Aminophenyl)sulfonylaminophenyl]-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(4-methoxyphenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-{3-trifluoromethyphenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(2-phenylphenyl)urea
2s N-(2-Hydroxy-3-nitrophenyl)-N'-(2,3dichlorophenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(4-phenylphenyl)urea
N-( 2-Hydroxy-3-nitrophenyl)-N'-(2;4-dimethoxyphenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(2-chloro-S-trifluoromethylphenyl)urea
N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2-methoxyphenyl)urea
N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2-phenylphenyl)urea
N-(2-Benzenesuifonylamino-4-cyanophenyl)-N'-(3-trifluoromethylphenyl)urea
N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2,3dichlorophenyl)urea
N-(2-Hydroxy-4-amidinophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3,4-dichloro phenyl) N'( phenyl) urea
N-(2-Hydroxy 4-cyano phenyl) N'( phenyl) urea
N-(2-Hydroxyphenyl 3-carboxylic acid)N'( phenyl) urea
N-(2-Hydroxy-3-nitrophenyl}-N'-phenylurea
14-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96I13632
N-(2-Hydroxy-3-cyano phenyl ) N'(phenyl) urea
N-(2-Hydroxy-3-cyano-4-chlorophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-fluorophenyl)-N'-(phenyl)urea
N-(2-Hydroxy-3,4-difluorophenyl)-N'-(phenyl)urea
N-[2-(Benzylsulfonylamino)-4-cyanophenyl]-N'-(2,3-dichlorophenyl)urea
N-[2-(Phenylsulfonylamino)-4-trifluoromethylphenyl]-N'-(2,3-
dichlorophenyl)urea
N-[2-(3-Pyridinesulfonylamino)-4-cyanophenyl]-N'-(2,3-dichlorophenyl)urea
N-[2-(5-Isoquinolinesulfonylamino)-4-cyanophenyl]-N'-(2,3-dichlorophenyl)urea
N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-{2-chlorophenyl)urea
1o N-[(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-fluoro phenyl) urea
N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-thiomethylphenyl)urea
N-[2-(Phenylsulfonylamino)-4-cyano phenyl]-N'-(2-trifluoromethoxyphenyl)urea
N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-trifluoromethylphenyl)urea
N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methylphenyl) urea
15 N-[2-(Phenylsulfonylamino)-4-cyano phenyl]-N'-(2-methoxy 3-chloro phenyl)
urea
N-[2-(4-cyanophenyl)-N'-(3-fluoro phenyl) urea
N-(2-Thiophenesulfonylamino-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-[(2-Pyrid-2-yl)thiophene-5-sulfonylamino-4-cyanophenyl]-N'-(2,3-
dichlorophenyl)urea
N-[(2-Acetamino-4-methyl-5-thiazolesulfonylamino-4-cyanophenyl]-N'-(2,3-
2o dichlorophenyl)urea
N-((2-Aminosulfonylphenyl) 4-cyano phenyl) N'-(2-methyl 3-chloro phenyl) urea
N-(2-Benzenesulfonylamino-3-cyanophenyi)-N'-(2,3dichlorophenyl)urea
N-[(Benzylsulfonylamino)-5-cyanophenyl]-N'-(2,3-dichlorophenyl)urea
N-((2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-nitrophenyl)urea
25 N-[(2-Phenylsulfonyiamino)-4-cyanophenyl]-N'-(2-methyl-3-nitrophenyl)urea
N-j(2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methyl-3-aminophenyl)urea
N-[(2-Phenylsulfonyiamino)-4-cyanophenyl)-N'-(2-aminophenyl)urea
N-(2-(2-Pyridinesulfonylamino-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Benzenesulfonylamino-3-trifluoromethylphenyl-N'-(2,3-dichlorophenyl)urea -
30 N-(4-Benzenesulphonylthiophene-2-sulphonylamino-4-cyanophenyl}-N'-(2,3-
dichlorophenyl)urea
N-(2-Trifluoromethylbezenesulfonylacnino-4-cyanophenyl)-N'-(2,3-
dichlorophenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2,3-methylenedioxyphenyl)urea
N-[2-(2-Nitrophenylthio)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
35 N-(2-Hydroxy-3-trifluoromethylphenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-3-trifluoromethylphenyl)-N'-(2-phenylphenyl}urea
N-(2-Hydroxy-4-nitrophenyl)-N'~-(2-benzylphenyl)urea
-15-
CA 02432662 2003-06-09
WO 97!29743 PCT/C1S96/13632
N-{2-Hydroxy-4-nitrophenyl)-N'-[2-(phenylthiomethyl)phenyl]urea
N-{2-Hydroxy-4-vitro phenyl)-N'-[2-(phenyloxymethyl)phenyl]urea
N-{2-Hydroxy-4-nitrophenyl)-N'-[2-(phenylethyl)phenyl]urea
N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(4-trifluorophenyl)phenyl]urea
N-( 2-Hydroxy-3-trifloromethylphenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-acetoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-(2-cyanophenylthio)phenyl]urea
N-(2-Hydroxy-3-trifluoromethylphenyl)-N'-(2-chlorophenyl)urea
N-(2-Hydroxyethyl)-N'-{2-hydroxy-4-nitrophenyi)urea
1o N-2-(Benzyoxyphenyi)-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(2-Thienylsulfonylamino)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-(2-Benzenesulfonylamino-4-nitrophenyl)-N'-(2,3-dichlorophenyi)urea
N-(2-Benzenesulfonylanuno-4-nitrophenyl)-N'-(2-bromophenyl)urea
N-(2-Benzylsulfonyiamino-4-nitrophenyl)-N'-(2-bromophenyl)urea
~5 N-(2-Benzylsulfonylamino-4-nitrophenyl)-N'-(2,3dichlorophenyl)urea
N-[2-(3-Pyridylmethoxy)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(4-Pyridylmethoxy)phenyl]-N'-(2-hydmxy-4-nitrophenyl)urea
N-[2-(Methoxycarbonylamino)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(Methylsulfonylamino)-4-nitrophenyl]-N'-(2-brornophenyl)urea
20 N-[2-(Propylsulfonylamino)-4-nitrophenyl)-N'-(2-bromophenyl)urea
N-[2-(Propylsulfonylamino)-4-nitrophenyl]-N'-(2,3-dichlorophenyl)urea
N-[[(2-acetamino-4-methyl-5-thiazolyl)sulforay!amino]-4-nitrophenyl)-N'-{2,3-
dichlorophenyl)urea
N-[2-(3-Pyridinesulforay!amino)-4-nitrophenyi]-N'-(2,3-dichlorophenyl)urea
z5 N-[2-(3-Pyridinesulforay!amino)-4-nitrophenyi]-N'-(2-bromophenyi)urea
N-[2-(Methylsulforay!amino)-4-nitrophenyl]-N'-(2,3-dichloropheny!)urea
N-(2-Hydroxyeth-1-yloxyphenyl)-N-(2-hydroxy-4-nitrophenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-benzylaminophenyl)urea
N'-[2-(2-Pyridylmethoxy)phenyl]-N'-(2-Hydroxy-4-nitrophenyl)urea
3o N-[2-(2-Methoxycarbonylbenzyloxyphenyl]-N-(2-hydroxy-4-nitmpheny!)urea
N-[2-(2-Carboxybenzyloxy)phenyl)-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(Benzoylamino)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
Additionally exemplified compounds of Formula ()~ include:
35 N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(benzyloxy)phenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-{2-(2-pyridylmethyloxy)phenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(3-pyridylmethyloxy)phenyl)urea
- 16-
CA 02432662 2003-06-09
WO 97129743 PCT/US96113632
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(4-pyridylmethyloxy)phenyl)urea
N-(2-Hydroxy-4-trifluoroacetophenone)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-trifluorosulfonylphenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-bromo-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-chloro-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-trifluoromethyl-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-cyanophenyl-3-carboxylic acid)-N'-(2-bromophenyl)urea
N-{2-Hydroxy-4-trifluoroacetophenone)-N'-(2,3-dichlorophenyl)urea
N-{2-Hydroxy-4-trifluorosulfonylphenyl)-N'-(2,3-dichlorophenyl)urea
1o N-(2-Hydroxy-3-bromo-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-3-chloro-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-3-trifluoromethyl-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-4-cyanophenyl-3-carboxylic acid)-N'-(2.3-dichlorophenyl)urea
Prefered compounds of Formula (n include:
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-bromophenyl)urea
N-{2-Hydroxy-4-nitrophenyl)-N'-{2-phenylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methylthiophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-{2,3-dichlorophenyl)urea
N-(2-hydroxy 4-nitro phenyl) N'-(2-chloro phenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2,3-methylenedioxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methoxy-3-chlorophenyl)urea
N-(2-hydroxy 4-nitro phenyl) N'-{2-phenyloxy phenyl) urea
N-(3-Chloro-2-hydroxyphenyl)-N'-(bromophenyl)urea
N-(2-Hydroxy-3-glycinemethylestercarbonylphenyl)-N'-(2-bromophenyl)urea
N-(3-Nitro-2-hydroxyphenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3,4-dichlorophenyl)-N'-{2-bromophenyl)urea
3o N-(3-Cyano-2-hydroxyphenyl)-N'-{2-bromophenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-phenylphenyl)urea
N-(2-Hydorxy-4-cyanophenyl-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-methylphenyl)urea
N-(2-Hydroxy-3-cyano-4-methylphenyl)-N'-(2-bromophenyl)urea
N-(4-Cyano-2-hydroxyphenyl)-N'-(2-trifluoromethylphenyl)urea
N-(3-Trifluoromechyl-2-hydroxyphenyl)-N'-{2-bromophenyl)urea
-17-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96I13632
N-(3-Phenylaminocarbonyl-2-hydroxyphenyl)-N'-(2-bromophenyl)urea
N-(2-hydroxy 4-vitro phenyl) N'-(~-iodo phenyl) urea
N-(2-hydroxy 4-vitro phenyl) N'(2-bromo phenyl) thiourea
N-(2-phenylsulfonamido)-4-cyanophenyl-N'(2-bromo phenyl)urea
(E)-N-[3-((2-Aminocarbonyl)ethenyi]-2-hydroxyphenyl]-N'-(2-bromophenyl)urea
N-(2-Hydroxy,3,4-dichlorophenyi)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy,3,4-dichlorophenyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-3,4-dichlorophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-5-nitrophenyl)-N'-(2,3-dichlorophenyl)urea
1o N-(2-Hydroxy-3-cyanophenyl)-N'-(2,3 dichlorophenyl)urea
As used herein, "optionally substituted" unless specifically defined shall
mean
such groups as halogen, such as cyano, vitro, fluorine, chlorine, bromine or
iodine;
hydroxy; hydroxy substituted C1-l0alkyl; C1-10 alkoxy, such as methoxy or
ethoxy;
t5 S(O)m~ C1-10 alkyl, wherein m' is 0, 1 or 2, such as methyl thio, methyl
sulfinyl or
methyl sulfonyl; amino, mono & di-substituted amino, such as in the NR4R5
group;
NHC(O)R4; C(O)NR4R5; C(~)~R11; S(0)2NR4R5; NHS(O)2R13;,C1-10 alkYh such
as methyl, ethyl, propyl, isopropyl, or t-butyl; halosubstituted C1-10 ~Yl~
such CF3; an
optionally substituted aryl, such as phenyl, or an optionally substituted
arylalkyl, such
2o as benzyl or phenethyl, optionally substituted heterocylic, optionally
substituted
heterocylicalkyl, optionally substituted heteroaryl, optionally substituted
heteroaryl
alkyl, wherein these aryl, hetroaryi, or heterocyclic moieties may themselves
be
optionally substituted one to two times by halogen; hydroxy; hydroxy
substituted alkyl;
C1-10 alkoxy; S(C)m~Cl-10 ~Yl: ono, mono & di-substituted amino, such as in
the
25 NR4R5 group; C 1-10 aIkYI, or halosubstituted C 1-10 alkyl, such as CF3.
R 13 is suitably C 1 ~ alkyl, halosubstituted C 1 ~ alkyl, aryl, aryl C 1
alkyl,
heteroaryl, heteroarylC 1-4alkyl, heterocyclic, or heterocyclicC 1 _4alkyl.
Another aspect of the present invention are the novel compounds of Formula
30 (II), or a pharmaceutically acceptable salt thereof, as described below,
which are also
useful in inhibiting the binding of IL-8 to its receptors in a mammal in need
thereof.
This invention also relates to the pharmaceutical compositions comprising a
compound
of Formula (II) and a pharmaceutically acceptable diluent or carrier.
Compounds of
Formula (II) are also useful for treating a chemokine mediated disease,
wherein the
35 chemokine is one which binds to an IL-8 a or b receptor and which method
comprises
administering an effective amount of a compound of Formula (lI)-or a
pharmaceutically
acceptable salt thereof.
- 18-
CA 02432662 2003-06-09
WO 97129743 PCTIUS96/13632
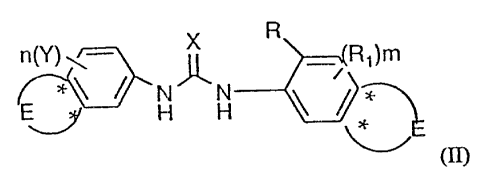
Compounds of Formula (II) are represented by the structure:
R
1
Rt)m
H
(II)
wherein
X is oxygen or sulfur;
R is any functional moiety having an ionizable hydrogen and a pKa of 10 or
less;
R I is independently selected from hydrogen; halogen; vitro; cyano; C I_ IO
~Y1:
halosubstituted C 1 _ 10 ~Yl ~ C2-10 ~kenyl; C I-10 alkoxy; halosubstituted
C1_l0alkoxy; azide; S(O)tR,~; (CRBRg)q S(O)tR~; hydroxy; hydroxy substituted
C 1 _4alkyl; aryl; aryl C 1 ~ alkyl; aryl C2_ I0 alkenyl; aryloxy; aryl C 1 ~
alkyloxy;
t0 heteroaryl; heteroarylalkyi; heteroaryl C2_10 alkenyl; heteroaryl CI~
alkyloxy;
heterocyclic, heterocyclic C 1 _q.alkyl; heterocyclicC 1 ~.alkyloxy;
heterocyclicC2_ 10
aikenyl; (CRBRg)q NR,4R5; (CRBRg)q C(O)NR4R5; C2_10 alkenyl C(O)NR4R5;
(CRBRg)q C(O)NR4RI0; S(O)3Rg; (CRBRg)q C(O)RI I; C2-10 ~enyl C(O)RI I;
C2-10 ~kenyl C(O)ORI I; (CRBRg)q C(O)OR11; (CRBRg)q OC(O)R11;
(CRBRg)qNR4C(O)RI1: (~8R8)q C(NR4)NR4R5: (CR8R8)q ~4C~5)R11.
(CRBRg)q NHS(O)2R13; (CRBRg)q S(O)2NR4R5, or two R1 moieties together may
form O-(CH2)s0- or a 5 to 6 membered unsaturated ring, and wherein the alkyl,
aryl,
arylalkyl, heteroaryl, heterocyclic moities may be optionally substituted;
t is 0, or an integer having a value of 1 or 2;
s is an integer having a value of 1 to 3;
R4 and R5 are independently hydrogen, optionally substituted C L.~ alkyl,
optionally
substituted aryl, optionally substituted aryl C I alkyl, optionally
substituted
heteroaryl, optionally substituted heteroaryl Cl~alkyl, heterocyclic,
heterocyclicC 1 _4 alkyl, or R4 and R5 together with the nitrogen to which
they are
attached form a 5 to 7 member ring which may optionally comprise an additional
heteroatom selected from O/N/S;
Y is hydrogen; halogen; vitro; cyano; halosubstituted C 1_ I0 alkyl: C I-10
~Yl: C2-10
alkenyl; C 1_ 10 alkoxy; halosubstituted C 1_ 10 alkoxy; azide;
(CR8R8)qS(O)tR4,
(CRBRg)qOR~; hydroxy; hydroxy substituted C I alkyl; aryl; aryl C 1 ~ alkyl;
aryloxy; arylC 1 ~ alkyloxy; aryl CZ_ I0 alkenyl; heteroaryl;
heteroarylallryl;
heteroaryl C 1 ~ alkyloxy; heteroaryl C2_ 10 alkenyl; heterocyclic,
heterocyclic
C 1 _4alkyl; heterocyclicC2_ l p alkenyl; (CRBRg)qNR4R5; C2_ 10 alkenyl
C(O)NR4R5; (CRgRg)qC(O)NR4Rg; (CRgRg)q C(O)NR4R10: S(O)3R8:
- 19-
CA 02432662 2003-06-09
WO 97129743 PCT/US96I13632
(CRgRg)qC(O)Rl 1; C2-10 ~kenylC(O)R11; (CRgRg)qC(O)OR11;
C2-l0~kenylC(O)OR11; (CRgRg}qOC(O) R11; (CRgRg)qNR4C(O)R11:
(CRgRg)q NHS(O)~Rb; (CRgRg)q S(O)2NR4R5; (CRgRg)qC(NR4)NR4R5;
(CRgRg)q NRq,C(NRS)R 11; or two Y moieties together may form O-(CH2)sO- or a
5 to 6 membered unsaturated ring; and wherein the alkyl, aryl, arylalkyl,
heteroaryl;
heteroaryl alkyl, heterocyclic, heterocyclicalkyl groups may be optionally
substituted;
q is 0 or an integer having a value of 1 to 10;
n is an integer having a value of 1 to 3;
m is an integer having a value of 1 to 3;
R6 and R~ are independently hydrogen or a C 1 _4 alkyl group, or R( and R~
together
with the nitrogen to which they are attached form a 5 to 7 member ring which
ring
may optionally contain an additional heteroatom which heteroatom is selected
from
oxygen, nitrogen or sulfur;
Rg is hydrogen or C 1 _4 alkyl;
Rl0 is C1_10 alkyl C(O)2Rg;
R11 is hydrogen, optionally substituted C1~ alkyl, optionally substituted
aryl,
optionally substituted aryl C 1 alkyl, optionally substituted heteroaryl,
optionally
substituted heteroarylC 1 alkyl, optionally substituted heterocyclic, or
optionally
substituted heterocyclicC 1 ~aikyl;
R 12 is hydrogen, C 1 _ 10 alkyl, optionally substituted aryl or optionally
substituted
arylalkyl;
R13 is suitably Cl~ alkyl, aryl, aryl C1_4alkyl, heteroaryl,
heteroarylCl~alkyl,
heterocyclic, or heterocyclicCl~alkyl;
Rb is NR6R~, alkyl, aryl, aryl Ci~ alkyl, aryl C2~ alkenyl, heteroaryl,
heteroaryl
C 1 _q. alkyl, heteroarylC2~ alkenyl, heterocyclic, heterocyclic C 1 ~ alkyl,
heterocyclic C2~ alkenyl, or camphor, all of which groups may be optionally
substituted;
E is optionally selected from
O
O ~
; ; R~ , or R~ ,
the asterix * denoting point of attachment of the ring, with at least one E
being present;
or a pharmaceutically acceptably salt thereof.
-20-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
Suitably, the variables for Formula (II), such as X, R, R1, R4 , RS, R6, R~,
Rg,
R9, Y, Ra, Rb, Rc, n, m, and s terms, etc. are as defined in Formula (I)
above. The E
ring denoted by its point of attachment through the asterix (*) may optionally
be
present. If if it is not present the ring is a phenyl moiety which is
substituted by the R
and R 1 terms as shown. At least one E ring is necessary. The E ring may be
substituted
by the R 1 or Y moiety in any ring, saturated or unsaturated, and is shown for
purposes
herein substituted only in the unsaturated ring(s).
to Another aspect of the present invention are the novel compounds of Formula
(IIa), (IIb) and (IIc) which are similar to those described herein for
Formulas (Ia), {Ib)
and (Ic) but which require one of the two phenyl rings to posses an E ring.
Suitably, for compounds of Formula (IIa-c), the variables are as defined
herein
for Formulas (I) and (II).
Compounds of Formula (IIa) are represented by the structure:
R
n~Yy ~ R~)m
x. ~ /
H H
(IIa)
wherein
X is oxygen or sulfur;
2o R is -NHS(O)2Rb;
Ra is an alkyl, aryl, arylCl~alkyl, heteroaryl, heteroaryl Cl~alkyl,
heterocyclic, or a
heterocyclic Cl~alkyl moiety, all of which may be optionally substituted;
Rb is a NR6R7, alkyl, aryl, arylC 1 alkyl, aryl C2~alkenyl, heteroaryl,
heteroarylCl~alkyl, heteroarylC2_4 alkenyl, heterocyclic, or heterocyclic
Cl~alkyl, or a heterocyclic C2~alkenyl moiety, camphor, all of which may be
optionally substituted one to three times independently by halogen; vitro;
halosubstituted C1~ alkyl; C1~ alkyl; C1~ alkoxy; NR9C(O)Ra; S(O)m~Ra,
C(~)N1t6R7; S(U)3H, or C(O)OC 1-4 alkyl;
R6 and R~ are independently hydrogen or a C 1 ~ alkyl group, or R( and R~
together
3o with the nitrogen to which they are attached form a 5 to 7 member ring
which ring
may optionally contain an additional heteroatom which heteroatom is selected
from
oxygen, nitrogen or sulfur, which ring may be optionally substitued;
R9 is hydrogen or a C1_4 alkyl, preferably hydrogen;
-21-
CA 02432662 2003-06-09
WO 97129743 PCTlLTS96/13632
R 1 is independently selected from hydrogen; halogen; vitro; cyano; C 1-10
alkyl;
halosubstituted C 1 _ 10 alkyl; CZ_ 10 aikenyl; C 1-10 alkoxy; halosubstituted
C1-l0alkoxy; azide; S(O)tR4; (CRgRg)q S(O)tR4; hydroxy; hydroxy substituted
C 1 _4alkyl; aryl; aryl C 1 ~ alkyl; aryl C2_ 10 alkenyl; aryloxy; aryl C 1 ~
alkyloxy;
heteroaryl; heteroarylalkyl; heteroaryl C2_10 alkenyl; heteroaryl C1~
alkyloxy;
heterocyclic, heterocyclic C 1..4alkyl: heterocyclicC 1 _4alkyloxy;
heterocyclicC2_ 10
alkenyi: (CRgRg)q NR4R5; (CRgRg)q C(O)NR4Rg; C2_ 10 alkenyl C(O)NR.4R5;
(CRgRg)q C(O)NR4R10; S(O)3Rg; (CRgRg)q C(O)R11; C2-10 alkenyl C(O)R11;
C2-10 ~kenyl C(O)OR11; (CRgRg)q C(O)OR11; (CRgRg)q OC(O)R11;
~o (CRgRg)qNR4C(O)R11; (CRgRg)q C(NRq.)NRdRS; {CRgRg)q NR4C(NRS)R11~
(CRgRg)q NHS(O)2R13; (CRgRg)q S(O)2NR4R5, or two R1 moieties together may
form O-(CH2)sO- or a 5 to 6 membered unsaturated ring, and wherein the alkyl,
aryl,
arylalkyl, heteroaryl, heterocyclic mollies may be optionally substituted;
t is 0, or an integer having a value of 1 or 2;
s is an integer having a value of 1 to 3;
R4 and RS are independently hydrogen, optionally substituted C 1 ~ alkyl,
optionally
substituted aryl, optionally substituted aryl Cl~aikyl, optionally substituted
heteroaryl, optionally substituted heteroaryl C l~alkyl, heterocyclic,
heterocyclicC 1~ alkyl, or R4 and RS together with the nitrogen to which they
are
2o attached form a 5 to 7 member ring which may optionally comprise an
additional
heteroatom selected from O/N/S;
Y is hydrogen; halogen; vitro; cyano; halosubstituted C 1 _ 1 O ~Yl ~ C 1-10
~Yl: C2-10
alkenyl; C1_10 alkoxy; halosubstituted C1-10 alkoxy; azide; (CRgRg)qS(O)tR4,
(CRgRg)qOR4; hydroxy; hydroxy substituted Cl~.alkyl; aryl; aryl C1~ alkyl;
aryloxy; arylC 1 _4 alkyloxy; aryl C2_ 10 alkenyl; heteroaryI;
heteroarylalkyl;
heteroaryl C 1 ~ alkyloxy; heteroaryl C2_ 10 alkenyl; heterocyclic,
heterocyclic
C 1 alkyl; heterocyclicC2_ 10 alkenyl; (CRgRg)qNR4R5; C2_ 10 alkenyl
C(O)NR4R5: (CRBRg)qC(O)NR4R5: (CRgRg)q C(O)NR4R10~ S(O)3R8~
(CRgRg)qC(O)R11; C2-10 ~kenylC{O)R11; (CRgRg)qC(O)ORII;
3o C2_lpalkenylC(O)OR11; (CRgRg)qOC(O) R11; (CRgRB)qNRq.C(O)R11~
(CRgRgyq NHS(O)2Rb; (CRgRg)q S(O)2NR4R5, (CRgRg)qC(NR4)NR4R5;
(CRgRg)q NR4C(NR5)R11; or two Y moieties together may form O-(CH2)s0- or a
5 to 6 membered unsaturated ring; and wherein the alkyl, aryl, aryialkyl,
heteroaryl,
heteroaryl alkyl, heterocyclic, heterocyciicalkyl groups may be optionally
substituted;
q is 0 or an integer having a value of 1 to 10;
n is an integer having a value of 1 to 3;
-22-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
m is an integer having a value of 1 to 3;
Rg is hydrogen or C 1 _~ alkyl;
R 10 is C 1-10 alkyl C(O)2Rg;
R 11 is hydrogen, optionally substituted C 1 ~ alkyl, optionally substituted
aryl,
optionally substituted aryl C1-alkyl, optionally substituted heteroaryl,
optionally
substituted heteroarylC 1-4alkyl, optionally substituted heterocyclic, or
optionally
substituted heterocyclicC 1 _4alkyl;
R 12 is hydrogen, C 1 _ 10 alkyl, optionally substituted aryl or optionally
substituted
arylalkyl:
R13 is suitably C1~ alkyl, aryl, aryl Cl~alkyl, heteroaryl,
heteroarylCl~alkyl,
heterocyclic, or heterocyclicC 1 alkyl;
E is optionally selected from
O
*
* ~ ~ ~
l
O
. ~ , R' , or Ri
the asterix * denoting point of attachment of the ring; with the proviso that
at least one
E ring being present;
or a pharmaceutically acceptably salt thereof.
Formula (IIb) compounds contain the R functionality of X1R2 wherein R2 is R2 -
is a substituted aryl, heteroaryl, or heterocyclic ring which ring has a
functional moiety
2o providing the ionizable hydrogen having a pKa of 10 or less; and the
remaining
variables as defined ahove for compounds of Formula (I) and (II).
Formula (IIc) compounds contain the R functionality X 1H, wherein X 1 is
oxygen or sulfur and the remainder of the variables are as defined in Formula
(I) and
(II) above.
Exemplified compounds of Formula (In include:
N-[2-hydroxy-S-indanone]-N'-[2-bromophenyl] urea;
N-[1-hydroxyfluorene]-N'-j2-bromophenyl] urea;
3o N-[3-hydroxy-9,10-anthraquinon-2-yl]-N'-[2-bromophenyl] urea
-23-
CA 02432662 2003-06-09
WO 97129743 PCTIUS96l13632
Another aspect of the present invention are the novel compounds of Formula
(III), or a pharmaceutically acceptable salt thereof, as described below,
which are also
useful in inhibiting the binding of IL-8 to its receptors in a mammal in need
thereof.
This invention also relates to the pharmaceutical compositions comprising a
compound
of Formula (III) and a pharmaceutically acceptable diluent or carrier.
Compounds of
Formula (III) are also useful for treating a chemokine mediated disease,
wherein the
chemokine is one which binds to an IL-8 a or b receptor and which method
comprises
administering an effective amount of a compound of Formula (III) or a
pharmaceutically acceptable salt thereof.
i0 Compounds of Formula (III:) are represented by the structure:
(Y)n
R
R m
S N~ _
H H
wherein
X is oxygen or sulfur;
R is any functional moiety having an ionizable hydrogen and a pKa of 10 or
less;
t 5 R 1 is independently selected from hydrogen; halogen; vitro; cyano; C I _
10 alkyl;
halosubstituted C 1 _ 10 alkyl; CZ_ 1p alkenyl; C 1-10 alkoxy; halosubstituted
Cl-l0~koxy; azide; S(O)tR4; (CRBRg)q S(O)tR4; hydroxy; hydroxy substituted
C 1 alkyl; aryl; aryl C 1 ~ alkyl; aryl C2_ 10 alkenyl; aryloxy; aryl C 1 ~
allcyloxy;
heteroaryl; heteroarylallcyl; heteroaryl C2_ 10 alkenyl; heteroaryl C 1 ~
allcyloxy;
20 heterocyclic, heterocyclic C 1 _4alkyi; heterocyclicC 1 ~alkyloxy;
heterocyclicC2_ I O
alkenyl; (CRBRg)q NR4R5; (CRBRg)q C(O)NR4R5; C2_10 alkenyi C(O)NR4R5;
(CRBRg)q C(O)NR4R10; S(O)3Rg; (CRBRg)q C(O)R11; C2-10 ~enyl C(O)R11;
C2-10 ~kenyl C(O)ORI I; (CRBRg)q C(O)OR11; (CRBRg)q OC(O)R11;
(CRgRg)qNR4C(O)R11; (~gRg)q C(1'IR4)NR4R5; (CRgRg)Q NR4C(NRS)R11~ .
25 (CRBRg)q NHS(O)2R13; (CRBRg)q S(O)ZNR~RS, or two R1 moieties together may
form O-(CH2)s0- or a 5 to 6 membered unsaturated ring, and wherein the alkyl,
aryl,
arylalkyl, heteroaryl, heterocyclic moities may be optionally substituted;
q is 0 or an integer having a value of 1 to I0;
t is 0, or an integer having a value of 1 or 2;
30 s is an integer having a value of 1 to 3;
R4 and RS are independently hydrogen, optionally substituted C 1 ~ alkyl,
optionally
substituted aryl, optionally substituted aryl C 1 alkyl, optionally
substituted
-24-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96113632
heteroaryl, optionally substituted heteroaryl C 1 _4alkyl, heterocyclic,
heterocyclicCl_4 alkyl, or R4 and R5 together with the nitrogen to which they
are
attached form a 5 to 7 member ring which may optionally comprise an additional
heteroatom selected from O/N/S;
Y is hydrogen: halogen; nitro; cyano; halosubstituted C1-10 ~kYl; C1-10 alkyl;
C2_10
alkenyl; C1_lp alkoxy; halosubstituted C1-10 ~koxY; azide; (CRgRg)qS(O)tR4,
(CRgRg)qOR4; hydroxy; hydroxy substituted Cl~alkyl; aryl; aryl C1~ alkyl;
aryloxy; arylC 1 _4 alkyloxy; aryl C2_ 10 alkenyl; heteroaryl;
heteroarylalkyl;
heteroaryl C 1 ~. alkyloxy; heteroaryl C2_ 10 alkenyl; heterocyclic,
heterocyclic
1 o C 1 _4alkyl; heterocyclicC2_ 10 alkenyl; (CRgRg)qNR4R5; C2_ 10 alkenyl
C(O)NR4R5; (CRgRg)qC(O)NR4R5; (CRgRg~ C(O)NR4R10; S(O)3Rg;
(CRgRg)qC(O)R 11; C2-10 ~kenylC(O)R 11; (CRgRg)qC(O)OR 11;
C2-l0~enylC(O)OR11; (CRgRg)qOC(O) R11; (CRgRg)qNR4C(O)R11:
(CRgRg)q NHS(O)2Rb; {CRgRg)q S(O)2NR4R5; (CRgRg)qC(NR4)NR~RS;
t5 (CRgRg)q NR4C(NRS)R11; or two Y moieties together may form O-(CH2)s0- or a
to 6 membered unsaturated ring; and wherein the alkyl, aryl, arylalkyl,
heteroaryl,
heteroaryl alkyl, heterocyclic, heterocyclicalkyl groups may be optionally
substituted;
n is an integer having a value of 1 to 3;
20 m is an integer having a value of 1 to 3;
R6 and R~ are independently hydrogen or a C1_r~ alkyl group, or R6 and R~
together
with the nitrogen to which they are attached form a 5 to 7 member ring which
ring
may optionally contain an additional heteroatom which heteroatom is selected
from
oxygen, nitrogen or sulfur;
25 Rg is hydrogen or C 1 _4 alkyl;
R 1 p is C 1 _ 1 p alkyl C(O)2Rg;
R 11 is hydrogen, optionally substituted C 1..4 alkyl, optionally substituted
aryl,
optionally substituted aryl C l alkyl, optionally substituted heteroaryl,
optionally
substituted heteroarylC 1 _4alkyl, optionally substituted heterocyclic, or
optionally
30 substituted heterocyclicCl~alkyl;
R12 is hydrogen, Cl-10 alkyl, optionally substituted aryl or optionally
substituted
arylalkyl;
R 13 is suitably C 1 _4 alkyl, aryl, aryl C 1 _4alkyl, heteroaryl, heteroarylC
1 alkyl,
heterocyclic, or heterocyclicC 1 alkyl;
35 Rb is NR6R~, alkyl, aryl, aryl C1~ alkyl, aryl C2~, alkenyl, heteroaryl,
heteroaryl
C 1 _4 alkyl, heteroarylC2_4 alkenyl, heterocyclic, heterocyclic C 1 ~ alkyl,
-25-
CA 02432662 2003-06-09
WO 97!29743 PCT/US96/i3632
heterocyclic C2_4 alkenyl, or camphor, all of which groups may be optionally
substituted;
E is optionally selected from
O
* \~
* *
Ri, i
/ ~/
. R~ , or R'
the asterix * denoting point of attachment of the ring;
or a pharmaceutically acceptably salt thereof.
Suitably, the variables, etc. for Formula (III) are the same as those defined
for
Formula (n above, such as for example the R, R1 and Y variables. Suitably the
E term
is the same as previously defined for Formula (II).
Exemplified compounds of Formula (III) include:
N-{2-Hydroxy-4=nitrophenyl)-N'-(3-methoxy-2-thienyl)urea; and
N-(2-hydroxy-5-nitrophenyl)-N'-{3-methoxy-2-thienyl)urea.
Another aspect of the present invention is the novel compounds of Formula
(Ia),
a subset of compounds of Formula (I) useful for treating a chemokine mediated
disease
as defined herein. This invention also relates to the pharmaceutical
compositions
comprising a compound of Formula (Ia) and a pharmaceutically acceptable
diluent or
carrier.
The compounds of Formula (Ia) are represented by the strucuture:
NHS(O)2F~
~~N~N
H H
(Ia)
wherein
X is oxygen or sulfur;
Ra is an alkyl, aryl, arylC 1 alkyl, heteroaryl, heteroaryl C 1..4allcyl,
heterocyclic, or a
heterocyclic C 1 alkyl moiety, all of which may be optionally substituted;
Rb is a NR(R~, alkyl, aryl, arylC 1 alkyl, aryl C2~alkenyl, heteroaryi,
heteroarylCl~alkyl, heteroarylC2~ alkenyl, heterocyclic, or heterocyclic
-26-
CA 02432662 2003-06-09
WO 97129743 PCTIUS96/13632
C1_4alkyl, or a heterocyclic C2_4alkenyl moiety, camphor, all of which may be
optionally substituted one to three times independently by halogen; vitro;
halosubstituted C 1 _4 alkyl; C 1 _4 alkyl; C 1 _4 alkoxy; NR9C(O)Ra;
S(O)m~Ra,
C(O)NR6R?, S(O)3H, or C(O)OC1_4 alkyl;
R6 and R7 are independently hydrogen, or a C1_4 alkyl group, or R6 and R7
together
with the nitrogen to which they are attached form a 5 to 7 member ring which
ring
may optionally contain an additional heteroatom which heteroatom is selected
from
oxygen, nitrogen or sulfur, which ring may be optionally substitued;
R9 is hydrogen or a C1_4 alkyl, preferably hydrogen;
t o R 1 is independently selected from hydrogen; halogen; vitro; cyano; C 1 _
10 alkyl;
halosubstituted C 1 _ 10 alkyl; C2_ 10 alkenyl; C 1 _ 10 alkoxy;
halosubstituted
C1-l0~koxy; azide; S(O)tR4; (CRgRg)q S(O)tR4; hydroxy; hydroxy substituted
C 1 alkyl; aryl; aryl C 1 ~ alkyl; aryl C2_ 10 alkenyi; aryloxy; aryl C 1 ~
alkyloxy;
heteroaryl; heteroarylalkyl; heteroaryl C2_ 10 alkenyl; heteroaryl C 1 ~
alkyloxy;
heterocyclic, heterocyclic C 1 _4alkyl; heterocyclicC 1 _4aikyloxy;
heterocyciicC2_ 10
alkenyl; (CRgRg)q NR4R5; (CRgRg)q C(O)NR4R5; C2_10 alkenyl C(O)NR4R5;
(CRgRg)q C(O)NR4R10; S(O)3Rg; (CRgRg)q C(O)R11; C2-10 ~enyl C(O)R11;
C2-10 ~kenyl C(O)OR11; (CRgRg)q C(O)OR11; (CRgRg)q OC(O)R11;
(CRgRg)qNR4C(O)R11; (~gRg)q C(~4)NR4R5~ (CRgRg)q NR4C(NR5)R11~
2o (CRgRg)q NHS(O)2R13; (CRgRg)q S(O)2NR4R5, ar two R1 moieties together may
form O-(CH2)s0- or a 5 to 6 membered unsaturated ring, and wherein the alkyl,
aryl,
arylalkyl, heteroaryl, heterocyclic mollies may be optionally substituted;
t is 0, or an integer having a value of 1 or 2;
s is an integer having a value of 1 to 3;
R4 and RS are independently hydrogen, optionally substituted C1~ alkyl,
optionally
substituted aryl, optionally substituted aryl C1_4alkyl, optionally
substituted
heteroaryl, optionally substituted heteroaryl C 1 alkyl, heterocyclic,
heterocyclicC 1 _4 alkyl, or R4 and RS together with the nitrogen to which
they are
attached form a 5 to ? member ring which may optionally comprise an additional
heteroatom selected from O/N/S;
Y is hydrogen; halogen; vitro; cyana; halosubstituted C1_10 ~Yl~ C1-10 ~Yl~ C2-
10
alkenyl; C 1 _ 10 alkoxy; halosubstituted C 1 _ 10 alkoxy; azide;
(CRgRg)qS(O)tR4,
(CRgRg)qOR4; hydroxy; hydroxy substituted Cl~alkyl; aryl; aryl C1~ alkyl;
aryloxy; arylC l ~. aikyloxy; aryl C2_ 10 alkenyl; heteroaryl;
heteroarylalkyl;
heteroaryl C 1 ~ alkyloxy; heteroaryl C2_ 10 alkenyl; heterocyclic,
heterocyclic
C l.~alkyl; heterocyclicC2_ 1p alkenyl; (CRgRg)qNR4R5; C2_ 10 alkenyl
C(O)NR4R5; (CRgRg)qC(O)NR4R5; (CRgRg)q C(O)NR4R10; S(O)3Rg;
- 27 _
CA 02432662 2003-06-09
WO 97IZ9743 PCT/US96/13632
(CR$Rg)qC(O)RI I; C2-10 ~kenylC(O)RI I; (CRgRg)qC(O)ORI1,
C2-10a1kenylC(O)ORI I; (CR$Rg)qOC(O) RI I; (CR$Rg)qNRq.C(O)RI I,
(CR$Rg)q NHS(O)2Rb; (CR$Rg)q S(O)2NR4R5; (CR$Rg)qC(NR4)NR4R5;
(CR$Rg)q NR4C(NRS)RI I; or two Y moieties together may form O-(CHZ)s0- or a
5 to 6 membered unsaturated ring; and wherein the alkyl, aryl, arylalkyl,
heteroaryl,
heteroaryl alkyl, heterocyclic, heterocyclicalkyl groups may be optionally
substituted;
q is 0 or an integer having a value of 1 to 10;
n is an integer having a value of 1 to 3;
m is an integer having a value of 1 to 3;
Rg is hydrogen or C I ~ alkyl;
RI0 is C I-10 alkyl C(O)ZRg;
RI I is hydrogen, optionally substituted C1~ alkyl, optionally substituted
aryl,
optionally substituted aryl C I alkyl, optionally substituted heteroaryl,
optionally
substituted heteroarylC I alkyl, optionally substituted heterocyclic, or
optionally
substituted heterocyclicCl~alkyl;
R12 is hydrogen, CI-10 alkyl, optionally substituted aryl or optionally
substituted
arylalkyi;
R I3 is suitably C I ~ alkyl, aryl, aryl C I~talkyl, heteroaryl, heteroarylC I
alkyl,
i0 heterocyclic, or heterocyclicC I-4alkyl;
or a pharmaceutically acceptably salt thereof.
Suitably, the variables for Formula (Ia) are the same as those defined for
Formula (I) above, such as for examples the R, RI, and Y variables. A
preferred ring
?5 substitution for the RI variable is monosubstituted in the 3-position, or
the 4- position,
or di-substituted in the 3,4- position. The substituent group is suitably an
electron
withdrawing moiety. Preferably RI is nitro, halogen, cyano, trifluoromethyl
group, or
C( O)NR4R5.
While Y may be substituted in any of the 5 ring positions, preferably the ring
3o with the Y moiety is mono-substituted in the 2-position or 3- position,
with the 4-
preferably being unsubstituted. If the ring is di-substituted, substituents
are preferably
in the 2'-, 3'- positions of a monocyclic ring. While both RI and Y can both
be
hydrogen, it is prefered that at least one of the rings be substituted,
preferably both rings
are at least mono-substituted, i:e. n amd m are each equal to I or more.
35 Y is more preferably a mono-substituted halogen, disubsdtuted halogen, mono-
substituted alkoxy, disubstituted alkoxy, methylenedioxy, aryl, or alkyl,
preferably these
groups are substituted in the 2'- position or 2'-,3'-position.
-2$-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
Exemplified compounds of Formula (Ia) are
N-(4-Nitro 2-(phenylsulfonylamino)phenyl)-N'-phenyl urea
N-[(2-Phenyisulfamido) 4-cyanoghenyl]- N'-(2-bromo phenyl) urea
N-(2-(Amino sulfonamido phenyl) phenyl) N'-(2-bromo phenyl) urea
N-{2-{Amino sulfonyl styryl) phenyl) N'-(2-bromo phenyl) urea
2-[(3,4 Di-methoxyphenylsulfonyl)amino] phenyl) N'-(2-bromo phenyl) urea
N-(2-[(4-Acetamidophenylsulfonyl)amino] phenyl) N'-(2-bromo phenyl) urea
N-(2-{Amino sulfonyl (2-thiophene) phenyl) N'-(2-bromo phenyl) urea
l0 N-(2-{Amino sulfonyl (3-tolyl) phenyl) N'-{2-bromo phenyl) urea
N-(2-{Amino sulfonyl (8-quinolinyl)) phenyl) N'-(2-bromo phenyl) urea
N-(2-(Amino sulfonyl benzyl} phenyl) N'-(2-bromo phenyl) urea
N-[2-[[[2-(Trifluoromethyl)phenyl]sulfonyl]amino]phenyl]-N'-(2-
bromophenyl)urea
N-(2-Bromophenyl}-N'-[2-dimethylaminosuifonylamino]phenyl]urea
15 N-[2-{Phenethylsulfonylamino)phenyl]-N'-(2-bromophenyl)urea
N-[2-[(2-Acetamido-4-methylthiazol-5-yl)sulfonylamino]phenyl]-N'-(2-
bromophenyl)urea
N-[2-[(2,3-Dichlorothien-5-yl)]sulfonylamino]phenyl]-N'-(2-bromophenyl)urea
N-[2-[(3,5-Bistrifluoromethylphenyl)sulfonylamino]phenyl]-N'-(2-
bromophenyl)urea
20 N-[2-[(2-Benzyl)sulfonylamino]-(5-trifluoromethyl)phenyl]-N'-(2-
bromophenyl)urea
N-[2-[2-{3-Nitrophenyl)sulfonylamino]phenyl]-N'-(2-bromophenyl)urea
N-[2-[2-(4-Phenoxyphenyl)sulfonylamino]phenyl]-N'-(2-bromophenyl) urea
N-[[2-( 1 S)-10-Camphorsulfonyiamino]phenyl]-N.'-(2-bromophenyl)urea
N-[[2-( 1 R)-10-Camphorsulfonylamino]phenyl]-N'-(2-bromophenyl)urea
25 N-[2-[2-(2-Nitro-(4-trifluoromethyl)phenyl)sulfonylamino]phenyl-N'-(2
bromophenyl)urea
N-[2-(2-Amino-{4-trifluoromethyl) phenyl) sulfonylamino] phenyl]- N'-(2-
bromophenyl)urea
N-[2-(aminosulfonyl phenyl)-3-aminophenyl] N'-(2-bmmo phenyl) urea
30 N-[2-[2-(4-Chloro-3-aminophenyl)sulfonylamino]phenyl]-N'-(2-
bromophenyl)urea
N-[2-{3-Aminophenyl)sulfonylaminophenyl]-N'-(2-bromophenyl)urea
N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2-methoxyphenyl)urea
N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2-phenylphenyl)urea
N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(3-trifluoromethylphenyl)urea
35 N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2,3dichlorophenyl)urea
N-[2-(Benzylsulfonylamino)-4-cyanophenyl]-N'-(2,3-dichlorophenyl}urea
N-[2-(Phenylsulfonylamino}-4-trifluoromethylphenyl]-N'-(2,3-
dichlorophenyl)urea
-29-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
N-(2-(3-Pyridinesulfonylamino)-4-cyanophenyl]-N'-(2,3-dichlorophenyl)urea
N-[z-(~-Isoquinolinesuifonylamino)-4-cyanophenyl]-N'-(2,3-dichlorophenyl}urea
N-(2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-chlorophenyl)urea
N-[(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-fluoro phenyl) urea
N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-thiomethylphenyl)urea
N-[2-(Phenylsulfonylamino)-4-cyano phenyl]-N'-(2-trifluoromethoxyphenyl)urea
N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-trifluoromethyiphenyl)urea
N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methylphenyl) urea
N-[2-(Phenylsulfonylamino)-4-cyano phenyl]-N'-(2-methoxy 3-chloro phenyl) urea
1o N-[2-(4-cyanophenyl)-N'-(3-fluoro phenyl) urea
N-(2-Thiophenesulfonylamino-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-[(2-Pyrid-2-yl)thiophene-5-sulfonylamino-4-cyanophenyl]-N'-(2,3-
dichlorophenyl)urea
N-[{2-Acetamino-4-methyl-5-thiazolesulfonylamino-4-cyanophenyl]-N'-(2,3-
dichlorophenyl)urea
15 N-((2-aminosulfonylphenyl) 4-cyano phenyl) N'-(2-methyl 3-chloro phenyl)
urea
N-(2-benzenesulfonylamino-3-cyanophenyl)-N'-{2,3dichlorophenyl)urea
N-((Benzylsulfonylamino)-5-cyanophenyl]-N'-(2,3-dichlorophenyl)urea
N-[(2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-nitrophenyl)urea
N-[(2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methyl-3-nitrophenyl)urea
zo N-[(2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methyl-3-aminophenyl)urea
N-[(2-Phenylsulfonylamino)-4.-cyanophenyl)-N'-(2-aminophenyl)urea
N-(2-(2-pyridinesulfonyiamino-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Benzenesuifonylamino-3-trifluoromethylphenyl-N'-(2,3-dichlorophenyl)urea
N-(4-Benzenesulphonylthiophene-2-sulphonylamino~l-cyanophenyl)-N'-{2,3-
25 dichlorophenyl)urea
N-(2-Trifluaromethylbezenesulfonylamino-4-cyanophenyl)-N'-(2,3-
dichloraphenyl)urea
N-[2-(2-Thieny lsulfonylamino}phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-(2-Benzenesulfonylamino-4-nitrophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Benzenesulfonylamino-4-nitrophenyl)-N'-(2-bromophenyl)urea
30 N-{2-Benzylsulfonylamino-4-nitraphenyl)-N'-(2-bromophenyl)urea
N-{2-Benzylsulfonylamino-4-nitrophenyl)-N'-(2,3dichlorophenyl)urea
Another aspect of the present invention is the novel compounds of Formula
(Ib),
a subset of compounds of Formula (I) useful for treating a chemokine mediated
disease.
35 This invention also relates to the pharmaceutical compositions comprising a
compound
of Formula (Ib) and a pharmaceutically acceptable diluent or carrier.
The compounds of Formula (Ib) are represented by the structure:
-30-
CA 02432662 2003-06-09
WO 97129743 PCT/I1S96/13632
X1 R2
n(Y)~ ~ R~)m
H
wherein
X is oxygen or sulfur;
X 1 is oxygen or sulfur;
R 1 is independently selected from hydrogen; halogen; vitro; cyano; C 1 _ 10
~Yl:
halosubstituted C 1 _ 10 alkyl; C2_ 10 alkenyl; C 1 _ 1p alkoxy;
halosubstituted
C1_l0alkoxy; azide; S(O)tR~; (CRBRg)q S(O)tRq,; hydroxy; hydroxy substituted
C 1 alkyl; aryl; aryl C 1 ~ alkyl; aryl C2-10 alkenyl; aryloxy; aryl C 1 ~
alkyloxy;
to ~ heteroaryl; heteroaryialkyl; heteroaryl C2_ 10 alkenyl; heteroaryl C 1 ~
alkyloxy;
heterocyclic, heterocyclic C1_4alkyl; heterocyclicCl_4alkyloxy;
heterocyclicC2_10
alkenyl; (CRBRg)q NR4R5; (CRBRg)q C(O)NR4R5; C2_ 10 allcenyl C(O)NR4R5;
(CRgRg)q C(O)NR4R10; S(O)3R8; (~gRg)q C(O)R11; C2-10 ~enyl C(O)R11;
C2-10 ~kenyl C(O)OR11; (CRBRg)q C(O)OR11; (CRBRg)q OC(O)R11;
15 (~8R8)q~4C(O)R11; (~8R8)q C(~4)~4R5: (CRgR8)q ~4C(NRS)R 11
(CRBRg)q NHS(O)2R13; (CR8R8)q S(O)2NR4R5; or two R1 moieties together
may form O-(CH2)s0- or a 5 to 6 membered unsaturated ring, and wherein the
alkyl, aryl, arylalkyl, heteroaryl, heterocyclic mollies may be optionally
substituted;
t is 0, or an integer having a value of 1 or 2;
2a s is an integer having a value of 1 to 3;
R2 is a substituted aryl, heteroaryl, or heterocyclic ring which ring has a
functional
moiety providing the ionizable hydrogen having a pKa of 10 or less;
R4 and RS are independently hydrogen, optionally substituted Cl~ alkyl,
optionally
substituted aryl, optionally substituted aryl C 1 _aalkyl, optionally
substituted
25 heteroaryl, optionally substituted heteroaryl C1_4allcyl, heterocyclic,
heterocyclicCl~ alkyl, or R4 and RS together with the nitrogen to which they
are
attached form a 5 to 7 member ring which may optionally comprise an additional
heteroatom selected from O/N/S;
Y is hydrogen; halogen; vitro; cyano; halosubstituted C1-10 ~yl~ Cl-10 a.~Yl;
C2-10
30 alkenyl; C1-10 alkoxy; halosubstituted C1-10 alkoxy; azide;
(CRBRg)qS(O)tR4,
(CRBRg)qOR4; hydroxy; hydroxy substituted Cl..~alkyl; aryl; aryl C1~ alkyl;
aryloxy; arylC 1 ~ alkyloxy; aryl C2_ 10 alkenyl; heteroaryl; heteroarylalkyl;
heteroaryl C 1 ~ alkyloxy; heteroary! C2-1 p alkenyl; heterocyclic,
heterocyclic
C 1 _4alkyl; heterocyclicC2_ 10 alkenyl; (CRBRg)qNR4R5; C2_ 10 alkenyl
-31-
CA 02432662 2003-06-09
WO 97/29743 PCT/US9b/13632
C(O)NR4R5; (CRgRg)qC(O)NR4R5; (CRgRg)q C(O)NR4Rlp; S(O)3Rg;
(CRgRg)qC(O)R I I ; C2- I O ~kenylC{O)R I 1; (CRgRg)qC(O)OR I 1;
C~_lpalkenylC(O)OR1I; (CRgRg)qOC(O) RI I; (CRgRg)qNR4C(O)RI1;
(CRgRg)q NHS{O)2Rb; (CRgRg)q S(O)2NR4R5; (CRgRg)qC(NR4)NR4R5;
(CRgRg)q NR4C(NRS)R1 I; or two Y moieties together may form O-(CH2)s0- or a
to 6 membered unsaturated ring; and wherein the alkyl, aryl, arylalkyl,
heteroaryl,
heteroaryl alkyl, heterocyclic, heterocyclicalkyl groups may be optionally
substituted;
q is 0 or an integer having a value of 1 to 10;
to n is an integer having a value of I to 3;
m is an integer having a value of I to 3;
R6 and R~ are independently hydrogen or a C I~ alkyl group, or R( and R7
together
with the nitrogen to which they are attached form a 5 to 7 member ring which
ring
may optionally contain an additional heteroatom which heteroatom is selected
from
oxygen, nitrogen or sulfur;
Rg is hydrogen or C 1 _4 alkyl;
R 1 p is C I _ I p alkyl C(O)2Rg;
R I I is hydrogen, optionally substituted C I~ alkyl, optionally substituted
aryl,
optionally substituted aryl C 1 alkyl, optionally substituted heteroaryl,
optionally
2o substituted heteroarylCl~alkyl, optionally substituted heterocyclic, or
optionally
substituted heterocyclicC 1 _~alkyl;
R12 is hydrogen, CI_10 alkyl, optionally substituted aryl or optionally
substituted
arylalkyl;
R I3 is suitably C 1 ~ alkyl, aryl, aryl C I alkyl, heteroaryl, heteroarylC I
alkyl,
heterocyclic, or heterocyclicC l..~alkyi;
Rb is NR6R7, alkyl, aryl, aryl CIA alkyl, aryl C2~ alkenyl, heteroaryl,
heteroaryl
C 1 ~ alkyl, heteroarylC2~ alkenyl, heterocyclic, heterocyclic C I ~ alkyl,
heterocyclic C2~ alkenyl, or camphor, all of which groups may be optionally
substituted;
or a pharmaceutically acceptable salt thereof.
Suitably, the variable, etc. for Formula (Ib) are the same as those defined
for
Formula (I) above, such as for example the functional moieties on the R2 group
having
an ionizable hydrogen with a pKa of 10 or less. Suitably such functional
groups
include, but are not limited to, hydroxy, carboxylic acid, thiol, -NH-C(O)Ra, -
C(O)NR6R7, substituted sulfonamides of the formula -NHS(O)2Rb, -S(O)2NHRc,
NHC(X2)NHRb, or tetrazoyl (as defined for Formula (I).
-32-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
Suitably for compounds of Formula (Ib), a preferred ring substitution for R1
is
in the 3-position, the 4- position or is preferably di substituted in the 3,4-
position. The
substituent group is suitably an electron withdrawing moiety. Preferably R 1
is vitro,
halogen, cyano, trifluoromethyl group, or C(O)NR4R5.
While Y may be substituted in any of the 5 ring positions, preferably the ring
with the Y moiety is mono-substituted in the 2-position or 3- position, with
the 4-
preferably being unsubstituted. If the ring is disubstituted, substituents are
preferably in
the 2' or 3' position of a monocyclic ring. While both R1 and Y can both be
hydrogen,
0 it is prefered that at least one of the rings be substituted, preferably
both rings are at
least mono-substituted, i.e. n amd m are each equal to 1 or more.
Suitably for compounds of Formula (/b), Y is more preferably disubstituted
halogen, mono-substituted halogen, disubstituted alkoxy, mono-substituted
alkoxy,
methylenedioxy, aryl, or alkyl, preferably in the 2'position or 2',3'-
position.
IS
Another aspect of the present invention is the novel compounds of Formula
(Ic),
a subset of compounds of Formula (n useful for treating a chemokine mediated
disease.
This invention also relates to the pharmaceutical compositions comprising a
compound
of Formula (Ic) and a pharmaceutically acceptable diluent or carrier. The
compounds
20 of Formula (Ic) are represented by the strucuture:
X~H
n (Y) y ~ \~ -.~( R, )m
~ N N--J
H H
(Ic)
wherein
X is oxygen or sulfur;
X 1 is oxygen or sulfur;
25 R1 is independently selected from hydrogen; halogen; vitro; cyano; C1-10
~Yl;
halosubstituted C 1-10 ~Yl; C2-10 ~enyl; C 1-10 foxy; halosubstituted
C 1-l0~oxy; azide; S(O)tR4; (CRgRg)q S(O)tR4; hydroxy; hydroxy substituted
C 1 alkyl; aryl; aryl C 1 ~ alkyl; aryl CZ_ 10 alkenyl; aryloxy; aryl C 1 ~
alkyloxy;
heteroaryl; heteroaryialkyl; heteroaryl C2-10 alkenyl; heteroaryl C1~
alkyloxy;
3o heterocyclic, heterocyclic C 1 _4alkyl; heterocyclicC 1 ~alkyioxy;
heterocyclicC2-10
alkenyl; (CRgRg)q NR4R5; (CRgRg)q C(O)NR4R5; C2_ 10 ~enyl C(O)NR4R5;
(CRgRg)q C(O)NR4Rlp; S(O)3Rg; (CRgRg)q C(O)R11; C2-10 ~enyl C(O)R11;
C2-10 ~kenyl C(O)OR11; (CRgRg)q C(O)OR11; (CRgRg)q OC(O)R11;
(CRgRg)qNR4C(O)R11; (CRgRg)q C(NR4)NR4R5; (CRgRg)q NR4C(NRS)R11,
-33-
CA 02432662 2003-06-09
WO 97!29743 PCT/I1S96/13632
(CRBRg)q NHS(O)2R13; (CRBRg)q S(O)2NR4R5, or two RI moieties together may
form O-(CH2)s0- or a 5 to 6 rnembered unsaturated ring, and wherein the alkyl,
aryl,
arylalkyl, heteroaryl, heterocyclic rnoities may be optionally substituted;
t is 0, or an integer having a value of 1 or 2;
s is an integer having a value of 1 to 3;
R~ and RS are independently hydrogen, optionally substituted C 1 ~ alkyl,
optionally
substituted aryl, optionally substituted aryl C 1 _4alkyl, optionally
substituted
heteroaryl, optionally substituted heteroaryl C 1 ~ alkyl, heterocyclic,
heterocyclic
CI_4 alkyl, or R4 and RS together with the nitrogen to which they are attached
form
a 5 to 7 member ring which may optionally comprise an additional heteroatom
selected from O!N/S;
Y is hydrogen; halogen; vitro; cyano; halosubstituted CI_10 ~Yl: C1-10 ~Yl~ C2-
10
alkenyl; CI_ !p alkoxy: halosubstituted C 1-10 alkoxy; azide;
(CRgRg)qS(O)tR,~,
(CRBRg)qOR4; hydroxy; hydroxy substituted Cl~alkyl; aryl; aryl C1~ alkyl;
t5 aryloxy; arylCl_4 alkyloxy; aryl C2_IO alkenyl; heteroaryl;
heteroarylalkyl;
heteroaryl C 1 ~ alkyloxy; heteroaryl CZ_ 10 alkenyl; heterocyclic,
heterocyclic
Cl~alkyl; heterocyclicC2_i0 alkenyl; (CRBRg)qNR4R5; C2_10 alkenyl
C{O)N~S: (CRgRg)qC(O)NR.4R5: (CRBRg)9 C{O)NR4R10; S(O)3R8:
(CRBRg)qC(O)R11; C2-IO ~enylC(O)RI1; (CR8R8)qC(O)OR11:
2o C2-l0~kenylC{O)OR11; (CRgRg}qOC{O) RI l; (CRgRg)qNR4C(O)R119
(CRBRg)q NHS(O)2Rb; (CR8R8)q S(O)2NR4R5{CRSR8)qC(NR4}NR4R5:
(CRBRg)q NR4C(NRg)R11; or two Y moieties together may form O-(CH2)s0- or a
5 to 6 membered unsaturated ring; and wherein the alkyl, aryl, arylalkyl,
heteroaryl,
heteroaryl alkyl, heterocyclic, heterocyclicalkyl groups may be optionally
25 substituted;
q is 0 or an integer having a value of 1 to 10;
n is an integer having a value of 1 to 3;
m is an integer having a value of 1. to 3;
R6 and R~ are independently hydrogen or a C 1 ~ alkyl group, or R6 and R~
together
30 with the nitrogen to which they are attached form a 5 to ~ member ring
which ring
may optionally contain an additional heteroatom which heteroatom is selected
from
oxygen, nitrogen or sulfur;
Rg is hydrogen or CIA alkyl;
R I O is C 1 _ 1 p alkyl C(O)2Rg;
35 R 11 is hydrogen, optionally substituted C 1 ~ alkyl, optionally
substituted aryl,
optionally substituted aryl C 1 ~talkyl, optionally substituted heteroaryl,
optionally
-34-
CA 02432662 2003-06-09
WO 97129743 PCT/ITS96/13632
substituted heteroarylC 1-4alkyl, optionally substituted heterocycIic, or
optionally
substituted heterocyclicC 1 _rlalkyl:
R 12 is hydrogen, C 1-10 ~kYl, optionally substituted aryl or optionally
substituted
arylalkyl;
R13 is suitably Cl~ alkyl, aryl, aryl C1-4alkyl, heteroaryl,
heteroarylCl~alkyl,
heterocyclic, or heterocyclicCl-4alkyl;
Rb is NR6R~, alkyl, aryl, aryl CI_4 alkyl, aryl C2_q. alkenyl, heteroaryl,
heteroaryl
Cl_4 alkyl, heteroarylC2-q, alkenyl, heterocyclic, heterocyclic C1~ alkyl,
heterocyclic CZ_4 alkenyl, or camphor, all of which groups may be optionally
t o substituted; provided that
when n =1 than Y is substituted in the 2- or 3- position;
when n =2 than Y is di-substituted in the 2'- 3'- position, the 2'-5'-
position, the
2'-6' position, the 3'-5' or the 3'-6' position;
when n = 3 than Y is trisubstituted in the 2'-3'-5' or the 2'-3'-6'-
positions;
further provided that
when X1 is O, m=2, R1 is 2-t-butyl, 4-methyl, and n=3 than Y is not 2'-OH,3'-t-
butyl, 5'-methyl;
when XI is O, m=1, R1 is 4-methyl, and n=2 than Y is not 2'-OH, 5'-methyl;
when Xl is O, m=l, R1 is hydrogen, and n=2 than Y is not 2'-6'-diethyl;
2o when X1 is O, m=l, RI is 6-OH, and n=2 than Y is not 2'-5'-methyl;
when Xl is S, m=1, RI is 4-ethyl, and n=1 than Y is vat 2-methoxy;
or a pharmaceutically acceptably salt thereof.
Suitably, the variables, etc. for Formula (Ic) are the same as those defined
for
Formula (I) above unless indicated.
Suitably for compounds of Formula (Ic), a preferred ring substitution for Rl
is
in the 3-position, the 4- position or dl substituted in the 3,4- position.
Preferably RI is
other than hydrogen. The substituent group is suitably an electron withdrawing
moiety.
Preferably R I is vitro, halogen, cyano, trifluoromethyl group, or C(O)NR4R5.
While Y may be substituted in any of the 5 ring positions, preferably the ring
with the Y moiety is mono-substituted in the 2-position or 3- position, with
the 4-
greferably being unsubstituted. If the ring is disubstituted, substituents are
preferably in
the 2' or 3' position of a monocyclic ring. While both Rl and Y can both be
hydrogen,
it is prefered that at least one of the rings be substituted, preferably both
rings are at
least mono-substituted, i.e. n amd m are each equal to 1 or more.
-35-
CA 02432662 2003-06-09
WO 97129743 PCT/L1S96/13632
Suitably for compounds of Formula (Ic), Y is more preferably a mono-
substituted halogen, disubstituted halogen, mono-substituted alkoxy,
disubstituted
alkoxy, methylenedioxy, aryl, or alkyl, preferably with these groups in the
2'position or
2,3-position.
Exemplified compounds of Formula (Ic) are:
N-[2-Hydroxy-4-(methoxycarbonyl)phenyl]-N'-phenylurea;
N-[2-Hydroxy-5-vitro-phenyl]-N'-phenyl urea
N-(2-Hydroxy-4-fluorophenyl)-N'-phenyl urea
to N-[2-Hydroxy-4-(trifluoromethyl)phenyl]-N'-phenyl urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-hydroxy-4-nitrophenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-phenyl-thiourea
N-(2-Hydroxy-5-nitrophenyl)-N'-(3-methoxy-2-thienyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-methoxy-2-thienyl)urea
15 N-{2-Hydroxy-4-nitrophenyl)-N'-(3-methoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-trifluoromethylphenyl)urea
N-{2-Hydroxy-4-nitrophenyl)-N'-(2-trifluoromethylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-trifluoromethylphenyl)urea
2o N-(2-Hydroxy-4-nitrophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(;3-bromophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-bromophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-phenylphenyl)urea
N-{2-Hydroxy-4-nitrophenyl)-N'-{2-nitrophenyl)urea
25 N-(2-Hydroxy-4-nitrophenyl)-N'-(2-fluorophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2,6-difluorophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-ethoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-ethylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-trifluoromethoxyphenyl)urea
3o N-{2-Hydroxy-4-nitrophenyl) N'-(2-methylthiophenyl) urea
N-(2-Hydroxy-4-nitro-phenyl) N'-(2-chloro 6-methyl phenyl) urea
N-(2-Hydroxy-4-vitro-phenyl) N'-(2-sulfoxymethyl phenyl) urea
N-(2-Hydroxy-4-trifluoromethyl phenyl)-N-(2-bromo phenyl) urea
N-(2-Hydroxy-4-trifluoromethyl phenyl)-N'-(2-phenyl phenyl) urea
35 N-(2-Hydroxy-4-carbomethoxy phenyl)-N'-(2-phenyl phenyl) urea
N-{2-Hydroxy-4-nitrophenyl)-N'-(2,3-dichloro phenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2,4-dichloro phenyl) urea
- 36 -
CA 02432662 2003-06-09
WO 97/29743 PCT/CTS96i13632
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-chloro phenyl) urea
N-{2-Hydroxy-4-nitrophenyl)-N'-(2,4-dibromo phenyl) urea
N-{2-Hydroxy-1-napthyl)-N'-(2-bromo phenyl) urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2,3-methylenedioxyphenyl}urea
N-(2-Hydroxy-4-nitrophenyl) N'-(3-chloro 2-methoxy phenyl) urea
N-[2-Hydroxy-4-(Benzylamino)carbonyl phenyl]-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-vitro phenyl)-N'-(2-phenoxy phenyl) urea
N-{2-Hydroxy-4-fluoro phenyl)-N'-(2-bromo phenyl) urea
N-{2-Hydroxy-3,4-difluoro phenyl)-N'-(2-bromo phenyl) urea
to N-(2-Hydroxy 4-phenyl phenyl) N'-(;2-bromo phenyl) urea
N-(2-Hydroxy 4-methyl phenyl)-N'-(2-bromo phenyl) urea
N-(2-Hydroxy-4-vitro phenyl)-N'-(2-phenylamino phenyl) urea
N-(2-Hydroxy 3-carboxyphenyl)-N'-(2-bromo ghenyl) urea
N-(2-Sulfhydryl-4-bromo phenyl)-N'-(2-bromo phenyl) urea
t 5 N-(2-Hydroxy 4-vitro phenyl)-N'-(2-iodo phenyl) urea
N-(2-Hydroxy 4-vitro phenyl)-N'-(2-bromo phenyl) thiourea
N-{2-Hydroxy-4-azidophenyl)-N'-(2-methoxyphenyl)urea
N-[2-Hydroxy-5-cyanophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-fluorophenyl]-N'-[~-bromophenyl] urea
2o N-(2-Hydroxy-3-fluoro-5-bromophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-chlorophenyl]-N'-(2-bromophenyl] urea
N-[2-Hydroxy-3-trifluoromethylphenyl]-N'-[2-bromophenyl] urea
N-[2-hydroxy-3,4-diphenyl phenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-glycinemethylestercarbonylphenyl]-N'-[2-bromopheny1] urea
25 N-(2-Hydroxy-3-glycincarbonylphenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3,5-dichlorophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-nitrophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-cyanophenyl]-N'-[2-bromophenyl] urea
3o N-[2-Hydroxy-4-cyanophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[4-methoxyphenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[2-phenylphenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[2=methylphenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[2-trifluoromethylphenyl] urea
35 N-[2-Hydroxy-4-cyanophenyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[4-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-n-propylphenyl]-N'-[2-bromophenyl] urea
-37-
CA 02432662 2003-06-09
WO 97/19743 PCTlUS96/13632
N-[2-Hydroxy-4-ethylphenyl]-N'-[2-bromophenylJ urea
N-[2-Hydroxy-3-phenylaminocarbonyl phenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-cyano-4-methyiphenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-4-carbophenyl phenyl]-N'-[2-bromophenyl] urea
N-(2-Hydroxy-3-carbophenyl phenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-benzyloxy phenyl]-N'-[2-bromophenyl] urea
{E)-N-[4-[2-{Methoxycarbonyl) ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenyl]
urea
(E)-N-[3-[2-(Methoxycarbonyl)ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenyl]urea-
N'-
[2-bromophenyl] urea
{E)-N-[3-[2-(Aminocarbonyl~thenyl]-2-hydroxyphenyl]-N'-[2-bromophenyl]urea-N'-
[2-bromophenyl] urea
{E)-N-[4-[2-{Aminocarbonyl)ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenyl]urea-N'-
[2-bromophenyl] urea
N-[2-Hydroxy-4-benzamide phenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-4-aminocarbonyl phenyl]-N'-[2-bromophenyl] urea
N-(2-Hydroxy-3,5,6-trifluorophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-fluoro-4-trifluoromethylphenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-iodophenyl)-N'-(2-bromophenyl)urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[4-phenylphenyl] urea
2o N-[2-Hydroxy-4-cyanophenyl]-N'-[2,3-dichlorophenyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-(2-methoxyphenylJ urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[3-methoxyphenyl] urea
N-(2-Hydroxy-5-fluorophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-5-trifluoromethylphenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxyphenyl]-N'-[2-bromophenyl] urea
N-[Trans-3-styrl-2-hydroxyphenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-methoxyphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[4-methoxyphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[3-trifluoromethylphenyl] urea
3o N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-phenylphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[4-phenylphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2,3-dichlorophenyl] urea
N-[2-Hydroxy-4-isopropylphenyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-naphthyl]-N'-[2,3-dichlorophenyl] urea
N-(2-Hydroxy-4-azidophenyl)-N'-(2-iodophenyl)urea
N-{2-Hydroxy-3-azidophenyl)-N'-{2-bromophenyl)urea
N-[2-Hydroxy-3-cyanophenyl]-N'-(2-methoxyphenyl] urea
-38-
CA 02432662 2003-06-09
WO 97129743 PCTlUS96113632
N-[Z-Hydroxy-3-cyanophenyl]-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-cyanophenyl]-N'-[2-phenylphenyi) urea
N-[2-Hydroxy-3-cyanophenyl]-N'-[2,3-dichlorophenyll urea
N-[2-Hydroxy-4-isopropylphenyl]-N'-[2,3-dichlorophenyl] urea
N-[2-Hydroxy-4-isopropylphenyl]-N'-[2-chloro-5-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-phenylphenyl)-N'-[2,3-dichlorophenyl] urea
N-[2-Hydroxy-5-nitrophenyl]-N'-{2-methoxyphenyi] urea
N-[2-Hydroxy-5-nitrophenyl]-N'-{3-trifluoromethylphenyl) urea
N-[2-Hydroxy-5-nitrophenyl)-N'-[2-phenylphenyl] urea
N-[2-Hydroxy-5-nitrophenyl]-N'-(2,3-dichlorophenyi) urea
N-[2-Hydroxy-5-ethylsulfonylphenyl]-N'-(2,3-dichlorophenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl)-N'-(2,4-dimethoxyphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-chloro-5-trifluvromethylphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-{benzyl] urea
~5 N-[2-Hydroxy-4-isopropylphenyl]-N'-[3-trifluoromethylphenyl] urea
N-{2-Hydroxy-3-(phenylaminocarbonyl) phenyl]-N'-[benzoyl] urea
N-{2-Hydroxy-3-trifluoromethylphenyl]-N'-{benzoyl] urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[benzoyl] urea
N-[2-Hydroxy-3-naphthyl)-N'-[3-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-naphthyl]-N'-(2,3-dichlorophenyl] urea
N-[2-Hydroxy-3-naphthyl]-N'-[benzyl) urea
N-[2-Hydroxy-5-naphthalenesulfonic acid)-N'-{2-bromophenyl] urea;
N-[2-Hydroxy-4-naphthalenesulfonic acid)-N'-(2-bromophenyl) urea;
N-(2-Hydroxy 3-napthyl} N'-(2-bromo phenyl} urea;
N-(2-Hydroxy-I-napthyl)-N'-(2-bromo phenyl) urea;
N-(2-Hydroxy-4-nitrophenyl)-N'-( 1-naphthyi)urea;
N-(2-Hydroxy-3-nitrophenyl}-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(4-methoxyphenyl)urea
N-(2-Hydroxy-3-nitropheny!)-N'-(3-trifluoromethyphenyl}urea
3o N-(2-Hydroxy-3-nitrophenyl)-N'-(2-phenylphenyl}urea
N-(2-Hydroxy-3-nitrophenyl~N'-(2,3dichlorophenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(4-phenylphenyl)urea
N-(2-Hydroxy-3-nitrophenyl}-N'-(2,4-dimethoxyghenyl)urea
N-(2-Hydroxy-3-nitrophenyl)-N'-(2-chloro-5-trifluoromethylphenyl)urea
N-(2-Hydroxy-4-amidinopheny!)-N'-{2-bromophenyl)urea
N-(2-Hydroxy-3,4-dichloro phenyl} N'( phenyl} urea
N-(2-Hydroxy-4-cyano phenyl) N'( phenyl) urea
-39-
CA 02432662 2003-06-09
W4 97!29743 PCT1US96/13632
N-{2-Hydroxyphenyl-3-carboxylic acid)N'( phenyl) urea
N-(2-Hydroxy-3-nitrophenyl)-N'-phenylurea
N-(2-Hydroxy-3-cyanophenyl ) N'(phenyl) urea
N-(2-Hydroxy-3-cyano-4-chlorophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-fluorophenyl)-N'-(phenyl)urea
N-(2-Hydroxy-3,4-difluorophenyl)~-N'-(phenyl)urea
N-{2-Hydroxy-4.-cyanophenyl)-N'-f.2,3-methylenedioxyphenyl)urea
N-[2-(2-nitrophenylthio)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-{2-hydroxy-3-trifluoromethylphenyl)-N'-(2,3-dichiorophenyl)urea
N-{2-hydroxy-3-trifluoromethylphenyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-benrylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(phenylthiomethyl)phenyl]urea
N-(2-Hydroxy-4-nitro phenyl)-N'-[2-(phenyloxymethyl)phenyl]urea
N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(phenylethyl)phenyl]urea
~5 N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(4-trifluorophenyl)phenyl]urea
N-(2-Hydroxy-3-trifloromethylphenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-acetoxyphenyl)urea
N-{2-Hydroxy-4-nitrophenyl)-N'-[2-(2-cyanophenylthio)phenyl]urea
N-(2-hydroxy-3-trifluoromethy!phenyl)-N'-(2-chlorophenyl)urea
N-(2-Hydroxyethyl)-N'-(2-hydroxy-4-nitrophenyl)urea
N-2-{Benzyoxyphenyl)-N'-(2-hydroxy-4-nitrophenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-benzylaminophenyl)urea
N-[2-(2-Pyridylmethoxy)phenyl]-N'-(2-hydroxy-4-niuophenyl)urea
N-[2-(2-Methoxycarbonylbenzyloxy)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
i5 N-[2-(2-Carboxybenzyloxy)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(Benzoylamino)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(3-Pyridylmethoxy)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(4-Pyridylmethoxy)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
N-[2-(Methoxycarbonylamino)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
3o N-(2-Hydroxyeth-1-yloxyphenyl)-N'-(2-hydroxy-4-nitrophenyl)urea
N-(2-Hydroxy-4-cyanophenyl~N'-(2-benzylaminophenyl)urea
N'-[2-(2-Pyridylmethoxy)phenylJ-N'-(2-Hydroxy-4-nitrophenyl)urea
N-[2-(2-Methoxycarbonylbenzyloxyphenyl]-N-{2-hydroxy-4-nitrophenyl)urea
N-[2-(2-Carboxybenzyloxy)phenyl)-N'-(2-hydroxy-4-nitrophenyl)urea
35 N-[2-(Benzoylamino)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
Additionally exemplified compounds of Formula (Ic) include:
-40-
CA 02432662 2003-06-09
WO 9?!29743 PCT/US96/13632
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(benzyloxy)phenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(2-pyridylmethyloxy}phenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(3-pyridylmethyloxy)phenyl)urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(4-pyridylmethyloxy)phenyl)urea
N-(2-Hydroxy-4-trifluoroacetophenone)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-trifluorosulfony!phenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-bromo-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-chloro-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-trifluoromethyl-4-cyanophenyl)-N'-(2-bromophenyl)urea
t0 N-(2-Hydroxy-4-cyanophenyl-3-carboxylic acid)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-trifluoroacetophenone)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-4-trifluorosulfonylphenyl)-N'-(2,3-dichlorophenyl)nrea
N-(2-Hydroxy-3-bromo-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-3-chloro-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-3-trifluoromethyl-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-4-cyanophenyl-3-carboxylic acid)-N'-(2,3-dichlorophenyl)urea
Suitable pharmaceutically acceptable salts are well known to those skilled in
the
art and include basic salts of inorganic and organic acids, such as
hydrochloric acid,
hydrobromic acid, sulphuric acid, phosphoric acid, methane sulphonic acid,
ethane
sulphonic acid, acetic acid, malic acid, tartaric acid, citric acid, lactic
acid, oxalic acid,
succinic acid, fumaric acid, malefic acid, benzoic acid, salicylic acid,
phenylacetic acid
and mandelic acid. In addition, pharmaceutically acceptable salts of compounds
of
Formula (I) may also be formed with a pharmaceutically acceptable cation, for
instance,
if a substituent group comprises a carboxy moiety. Suitable pharmaceutically
acceptable cations are well known to those skilled in the art and include
alkaline,
alkaline earth, ammonium and quaternary ammonium cations.
The following terms, as used herein, refer to:
~ "halo" - all halogens, that is chloro, fluoro, bromo and iodo.
~ "C1_lpalkyl" or "alkyl" - both straight and branched chain radicals of 1 to
10
carbon atoms, unless the chain length is otherwise limited, including, but not
limited to,
methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, iso-butyl, ten butyl,
n-pentyl and
the like.
~ The term "cycloalkyl" is used herein to mean cyclic radicals, preferably of
3 to
8 carbons, including but not limited to cyclopropyl, cyclopentyl, cyclohexyl,
and the
like.
-41 -
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
~ The term "alkenyl" is used herein at all occurrences to mean straight or
branched chain radical of 2-10 carbon atoms, unless the chain length is
limited thereto,
including, but not limited to ethenyl, 1-propenyl, 2-propenyl, 2-methyl-1-
progenyl, 1-
butenyl, 2-butenyl and the like.
~ "aryl" - phenyl and naphthyl;
~ "heteroaryl" (on its own or in any combination, such as "heteroaryloxy", or
"heteroaryl alkyl") - a 5-10 membered aromatic ring system in which one or
more rings
contain one or more heteroatoms selected from the group consisting of N, O or
S, such
as, but not limited, to pyrrole, pyrazale, furan, thiophene, quinoline,
isoquinoline,
1o quinazolinyl, pyridine, pyrimidine, oxazole, thiazole, thiadiazole,
triazole, imidazole, or
benzimidazole.
~ "heterocyclic" (on its own or in any combination, such as
"heterocyclicalkyl")
- a saturated or partially unsaturated 4-10 membered ring system in which one
or more
rings contain one or more heteroatorns selected from the group consisting of
N, O, or S;
t 5 such as, but not limited to, pyrrolidine, piperidine, piperazine,
morpholine,
tetrahydropyran, or imidazolidine.
~ The term "arylalkyl" or "heteroarylalkyl" or "heterocyclicalkyl" is used
herein
to mean C 1 _ 10 alkyl, as defined above, attached to an aryl, heteroaryl or
heterocyclic
moiety, as also defined herein, unless otherwise indicated.
2o ~ "sulfinyl" - the oxide S (O) of the corresponding sulfide, the term
"thio"
refers to the sulfide, and the term "sulfonyl" refers to the fully oxidized
S(O)2 moiety.
The term "wherein two R1 moieties (or two Y moieties) may together form a
or 6 membered unsaturated ring" is used herein to mean tile formation of a
napthylene
ring system or a phenyl moiety having attached a 6 membered partially
unsaturated ring
25 such as a C6 cycloalkenyl, i.e hexene, or a CS cyloalkenyl moiety,
cyclopentene.
The compounds of Formula (1), (Ia), (Ib), (Ic), (II, (IIa ), (IIb), (IIc), and
(III)
may be obtained by applying synthetic procedures, some of which are
illustrated in the
Schemes below. The synthesis provided for in these Schemes is applicable for
the
3o producing compounds of Formula (1), (Ia), (Ib), (Ic), (II, (IIa ), (IIb),
(IIc), and (III)
having a variety of different R, Rl, and Ar groups which are reacted,
employing
optional substituents which are suitably protected, to achieve compatibility
with the
reactions outlined herein. Subsequent deprotection, in those cases, then
affords
compounds of the nature generally disclosed. Once the urea nucleus has been
35 established, further compounds of these formulas may be prepared by
applying standard
techniques for functional group interconversion, well known in the art. While
the
-42-
CA 02432662 2003-06-09
WO 97!29743 PCT/US96I13632
schemes are shown with compounds only of Formula {17 this is merely for
illustration
purposes only.
Scheme t
R a ~ \ R O
s
NH2 / N' _ N
1 2
R=NH2, OH, COZH, SH a)PhNCO
NHS02R
Ortho substituted phenyl ureas shown in 2-scheme 1 may be prepared by
standard conditions involving the condensation of commercially available ortho
substituted aniline(Aldrich Chemical Co., Milwaukee, Wi) with the commercially
available optionally substituted aryl isocyanate (Aldrich Chemical Co.,
Milwaukee, Wi)
to in an aprotic solvent (DMF, toluene). When the 1-{RS02NH)2-{NH2)Ph is not
commercially available it can be made by treating the commercially available
RS02CI
with the cooresponding 2-phenylene diamine in the presence of an base like
triethyl
amine or NaH in an aprotic solvent (like methylene chloride or DMF).
S~;heme 2
R» R. R.
\ ~ ~ \ -b
/
/ N02 / NH2
4
R'=OH, NH2, NHS02R a)HNO3, 23 °C b)SnC4~, EtOH
If the desired 2-substituted aniline 5-scheme 2. is not commercially available
the corresponding vitro compound can be prepared from 3-scheme 2, under
standard
nitration conditions (using HN03 or BF4N03) at 23 °C. The vitro
compound is then
2o reduced to the corresponding aniline using SnCl2 in EtOH(or alternately
H2JPd or
LiAlH4).
- 43 -
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
ch m
N SH
> ~ / %~' NHp ~
NH2 a S b NH2
6_ 7 $
a)NH4SCN, Br2
b)NaOH EtOH
If the desired 2-amino benzenethiol 8-scheme 3 is not commercially available
it
can be synthesized by reaction of the phenyl aniline with the thiocyanate
anion in the
presence of an oxidant(like bromine) to produce the 2-amino benzthiazole 7-
scheme 3.
This thiazole can then be hydrolyzed to the desired 2-amino benzeneihiol 8-
scheme 3
with a suong base like NaOH in a erotic solvent (i.e., EtOH).
Scheme 4
OzN ~ OH O~ OTBS
a,b \ X
NH2 . ~ / N It
1Q
c 021'1 ~ \ OH X /
N~ N
X=S, O
a)TBSCI, imid, DMF b)i)CICXCI, NaHCog, ii)PhNH2 c)Et3N~HF, CH3CN
In the case where the thioisocyanate or phenyl isocyanate is not commercially
available, the thiourea or urea 11-scheme 4 may be prepared from the
commercially
lo available ortho substituted aniline. This compound is first protected with
a protecting
group (tert-butyl dimethyl silyl or benzyl ) by conditions well known in the
art(see
Greene, T Protecting Gmups in Organic Synthesis. Wiley&Sons, New York, 1981).
This protected aniline is then reacted, in the presence of a base(like
triethyl amine or
sodium bicarbonate), with either thiophosgene or a solution of phosgene in an
aprotic
solvent (ie. DMF, toluene), followed by aniline to produce the protected
thiourea or
urea respectively. The corresponding urea or thiourea is then deprotected,
using
conditions standard in the art, to form the desired thiourea or urea 11-scheme
4.
CA 02432662 2003-06-09
WO 97129743 PCT/LTS96I13632
hem 5
a. b /~ ~ /
S C02H S N N
12 1~ X
a)(Ph0)2PON3,Et3N b)PhXNH2
X=OH, NHS02R. SH
Alternately the urea can be formed using a Curtius rearrangement from the
corresponding aromatic or thiophene carboxylic acid 12-scheme 5. The
carboxylic acid
is submitted to standard Curtius conditions ((Ph0)2PON3, Et3N or CICOCOCI
followed by NaN3) and the intermediate isocyanate is trapped by an
appropriately
substituted aniline.
Pharmaceutically acceptable salts of compounds of Formula (I) may be obtained
in known manner, for example by treatment thereof with an appropriate amount
of acid
or base in the presence of a suitable solvent.
Another aspect of the present invention is the novel synthesis of cyano
nitrophenol intermediates. Numerous conversions of aryl halides to aryl cyano
derivatives with copper (I) cyanide have been published. However, no examples
of an
t5 aryl ring with a hydroxy group present were mentioned. Several attempts to
obtain a
cyano phenol moiety with published results failed. Using known conditions of
elevated
temperatures, greater than 170°C, such as from 180 to 210° did
not yield displacment of
the halogen to a cyano moiety. Standard bases, such as DMF and pyridine
further
provided no desired product. Intermediates such as 2-amino-5-fluorophenol, 2-
vitro-5-
fluorophenol, 2-vitro-5-methyl-6-bromophenol were tried with a change of
halogens,
from fluorine to chlorine to bromine, and with use of copper (n cyanide. The
use of a
bmmine derivative, such as 2-vitro-5-methyl-6-bromophenol, with
dimethylformamide~
and using triethylamine with a catalytic amount of dimethylamino pyridine and
copper
(I) cyanide at reduced temperatures,. i.e. <100° C, preferably 60 to
about 80° C for
reduced times from strandarized procedures, i.e., < 18 hours, preferably about
4 to 6
hours yielded the desired products.
Therefore one aspect of the invention is to a process for producing a cyano
phenol derivative of the formula:
- 45 -
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/I3632
H
W, c-N
J
wherein R 1 is as defined for Formula (I) above, which
method comprises reacting a compound of the fotznula:
H
X
~J
R' wherein X is halogen with copper (1) cyanide,
dimethylformamide, triethylamine and a catalytic amount of dimethylamino
pyridine.
Preferably, the process is run at reduced temperatures of about 60 to about
80° C.
Preferably X is bromine.
In the Examples, ail temperatures are in degrees Centigrade (°C). Mass
spectra
were performed upon a VG Zab mass spectrometer using fast atom bombardment,
1o unless otherwise indicated. 1H-NMR (hereinafter "NMR") spectra were
recorded at 250
MHz or 400MHz using a Bruker AM 250 or Am 400 spectrometer, respectively.
Multiplicities indicated are: s=singlet, d=doublet, t=triplet, q=quartet,
m=multiplet and
br indicates a broad signal. Sat. indicates a saturated solution, equiv.
indicates the
proportion of a molar equivalent of reagent relative to the principal
reactant_
15 Flash chromatography is run over Merck Silica gel 60 (230 - 400 mesh).
SYNTHETIC EXAMPLES
The invention will now be described by reference to the following examples
which are merely illustrative and are not to be construed as a limitation of
the scope of
2o the present invention. All temperatures are given in degrees centigrade,
all solvents
used herein are of the highest available purity and all reactions are run
under anhydrous
conditions in an argon atmosphere unless otherwise indicated.
General Method A: Synthesis of N, N'- phenyl urea To a solution of substituted
25 phenyl isocyanate { 1.0 equiv.) in toluene (5 miliLiters (hereinafter
"mL")) the
corresponding aniline {1.0 equiv.) was added. The reaction mixture was stirred
at about
80°C until complete (24-48 hours (hereinafter "hrs" or "h")), then
cooled to room
temperature. The purifications, yields and spectral characteristics for each
individual
compound are listed below.
-46-
CA 02432662 2003-06-09
WO 97!29743 PCTIUS96/13632
General Method B: Synthesis of N, N'~ phenyl urea To a solution of phenyl
isocyanate ( 1.0 equiv. ) in dimethyl .formamide ( 1 mL) the corresponding
aniline ( 1.0
equiv.) was added. The reaction mixture was stirred at about 80 C until
complete (24-
48 hours), then the solvent was removed under vacuum. The purifications,
yields and
spectral characteristics for each individual compound are listed below.
General Method C:Synthesis of sulfonamide The ortho substituted aniline (1
equiv.),
triethyl amine ( 1 equiv.) and the desired sulfonyl chloride ( I equiv.) were
combined in
methylene chloride and allowed to stir at about 23 °C until complete (
12-36 h). The
t0 reaction mixture was partitioned between water and methylene chloride. The
organic
layer was separated and dried over magnesium sulfate, filtered and
concentrated in
vacuo. The purifications of each compound are listed below.
xam !e 1
Prevaration of ~-I2-Hvdroxv-4-lmethoxvcarbonvl)v~envll N'-nhenvl urea
N-{2-Hydroxy-4-(methoxycarbonyi)phenyl]-N'-phenyl urea was prepared from
methyl-4-amino-3-hydroxybenzoate (200 mg, 1.19 mmol) and phenyl isocyanate (
1.19
mmol) according to the procedure noted above in General Method A. The product
was
purified by precipitation from toluene, and filtering, to afford the titled
compound (309
mg, 90%). mp: 188.4-188.8°C; 1H NMR (CD30D/CDC13): d 8.15 (d, 1H, J =
8.25
Hz), 7.70 (s, 1H), 7.51 (d, 1H, J = 8.25 Hz), ?.43 (d, 2H, J = 8.25 Hz), 7.30
(t, 2H, 3 =
8.25 Hz), 7.01 (t, 1H, J = 8.25 Hz), 3.87 (s, 3H); BI-MS m/z 286 (M+H)+; Anal.
(C 15H 14N204) C, H, N.
am Ie 2
Prevaration of N-L5-vitro-2-hvdroxyphen !v 1-N'-phenyl urea
The N-{5-vitro-2-hydroxyphenyl]-N'-phenyl urea was prepared from the S-vitro
2-hydroxy aniline and phenyl isocyanate according to the procedure in General
Method
A. The product was purified by precipitation from toluene and filtering to
afford the
titled compound (100 mg, 30%). 1H NMR (CD30D): d 9.48 (s, IH, NH), 9.07 (d,1=
1.56 Hz, NH), 8.55 (s, 1H), 7.80 (dd, 1H, J = 6.25 Hz and J = 1.56 Hz), 7.50
(d, 2H, J =
6.25. Hz), 7.30 (t, 2H, J = 6.25 Hz), 7.01 (m, 2H). EI-MS mlz 273 (M+H)+.
x 3
Preparation of 3-hydroxy-4-{ flphenvlaminolcarbonvllaminolbenzamide
a)Pteparation
of 0.67 Molar (hereinafter "M") Stock Solutions of Aluminum Amide Reagents
- 47 -
CA 02432662 2003-06-09
WO 97/29743 PCTlUS96113632
To a suspension of the appropriate hydrochloride (0.02 mole (hereinafter
"mol")) in dry toluene (20 mL) at about 0°C, was slowly added a
solution of (2M, 10
mL) of trimethyl aluminum in toluene. After the addition was complete, the
reaction
mixture was allowed to warm to room temperature and was stirred for about I-2
hours
until gas evolution has ceased.
b)Preparation of 3-hydroxy-4-{[(phenylamino)carbonyl]amino}benzamide
To a solution of the N-[2-hydroxy-4-(methoxycarbonyl)phenyl]-N'-phenyl urea
(60 miligram (hereinafter "mg"), 0.2 mmol) in toluene (2 mL) was added
aluminum
amide reagent (0.9 mL, 0.67M). The reaction mixture was stirred at reflux for
about 12
1o hours. The reaction mixture was cooled to room temperature and was
carefully
quenched with 5% HCI. The organic layer was separated and the aqueous layer
was
extracted three times with ethyl acetate. The organic extracts were combined,
dried over
MgS04, filtered and concentrated under reduced pressure. Chromatography of the
resulting solid on silica gel (ethyl acetate) gave the desired amide (28 mg,
49%). mp:
106.8-107.1°C; 1H NMR (CD30DICDC13): d 7.98 (d, 1H, J = 8.25 Hz), ?.35
(d, 2H, J
= 8.25 Hz), 7.30 (d, 2H, J = 8.25 Hz), 7.17 (t, 2H, J = 8.25 Hz), 6.91 (t, 1H,
J = 8.25
Hz); EI-MS tn/z 271 (M+H)+; Anal. (C I4H 13N303) C, H, N.
Example 4
'reparation of N-l2-hydroxy-4-fluorophen 1v 1-1V_-,phenyl urea
a)Preparation of 2-amino-5-fluoro phenol
A mixture of 5-fluoro-2-nitrophenol (500 mg, 3.18 nzmol) and tin (II) chloride
( 1.76 g, 9.2 mmol) in ethanol ( 10 mL) was heated at 80°C under argon.
After 30 min,
the starting material had disappeared and the solution was showed to cool down
and
then poured into ice. The pH was made slightly basic (pH 7-8), by addition of
5%
aqueous sodium bicarbonate, before being extracted with ethyl acetate.-The
organic
phase was washed with brine, dried over MgS04 and filtered. Evaporation of the
solvent gave the title compound(335 mg, 83%). /H NMR (CD30D/CDCl3): d 6.6 (m,
1H), 6.38 (dd, IH. J = 8.3 Hz and J = 2.8 Hz), 6.29 (m, IH).
b)Preparation of N-(2-hydroxy-4-fluorophenyl)-N'-phenyl urea
N-(2-Hydroxy-4-fluorophenyl)-N'-phenyl urea was prepared from 2-amino-5-
fluoro phenol (200 mg, 1.57 mmol) and phenyl isocyanate according to the
procedure in
General Method A. The product was purified by precipitation from toluene and
filtering
to afford the titled compound (352 mg, 91 %). mp: 195.5-195.7°C; 1H NMR
(CD30D/CDC13): d 7.70 (m, 1H), 7.3 (d, 2H, J = 8.25 Hz), 7.15 (t, 2H, J = 8.25
Hz),
6.89 (t, 1H, J = 8.25 Hz), 6.50 - 6.38 (m, 2H); EI-MS mlz 246 (M+H)+; Anal.
(C 13H11N202 F) C, H, N.
-48-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
Example S
Preparation of 2-( ffohenylaminolcarbonyllamino~hiophenol
2-}[(Phenylamino)carbonyl)amino}thiophenol was prepared from2-
aminothiophenol (200 mg, 1.6 mmol) and phenyl isocyanate according to the
procedure
in General Method A. The product was purified by precipitation from toluene
and
filtering to afford the titled compound (330 mg, 85 %). mp: 194.5°C; 1H
NMR
(CD30D/CDC13): d 7.48 - 7.26 (m, 4H), 7.25 - 7.10 (m, 3H), 7.04 - 6.79 (m,
2H); EI-
MS mJz 244 (M+H)+; Anal. (C13H12N2OS) C, H, N.
to
ExamRle 6
Preparation of N-l2-Carboxy"-4-hydroxw-phenyl)-N'=phenvl~ea
N-{2-Carboxy-4-hydroxyphenyl)-N'-phenyl urea was prepared from 2-amino-S-
hydroxy benzoic acid ( 1 g, 6.53 mmol) according to the procedure in General
Method
B. The reaction mixture was partitianed between ethyl acetate and water. The
organic
phase was washed with brine, dried aver MgS04 and filtered. Removal of solvent
under
reduced pressure and chromatography of the resulting solid on silica gel
(hexane : ethyl
acetate, 1:1 to 100% ethyl acetate) gave the titled compound ( 1.5 g, 84%). 1
H NMR
(CD30D/CDC13): d 8.36 (d, 1H, I = 8.25 Hz), 7.63 (m, 4H), 7.48 (t, 2H, J =
8.25 Hz),
7.20 (m, 1 H); EI-MS m/z 272 (M+H)+; Anal. (C 14H 12N204) C, H, N.
am 1e 7
Preparation of N - f2 - h~droxy,- 4- ftr'r,~'Iuorometh~~Rheny~ - N' - ~het~ 1v
urea
a)Preparation of 2-vitro-5-trifluoromethylphenol
2-Nitro-S-trifluoromethylphenol was prepared by adding concentrated HN03 (6
mL) drop-wise to a,a,a-trifluoro-m-cresol (5g, 30.8 mmol) at room temperature.
After
the addition was complete the reaction was quenched with saturated ammonium
acetate
and extracted with EtOAc. The organic was separated, dried over sodium sulfate
and
filtered. Concentration of the solution in vacuo afforded an oil which was
purified by
column chromatography (gradient 100% hexane to 50% EtOAc/hexanes) to afford
the
titled compound as an oil(1.7 g, 27%). 1H NMR (CDCI3): 10.6 (s, 1H, OH),
8.26(d,
1H, J = 7.8 Hz), 7.45(s, 1H, arom), 7.26(d, 1H, J= 7.8 Hz)
b)Preparation of 2-amino-S-trifluoromethylphenol
2-Amino-S-trifluoromethylphenol was prepared by treating 2-nitro-5-
trifluoromethylphenol (500 mg, 2.41 mmol) with a solution of 5nC12(3.Sg, mmol)
in
EtOH at 23 °C for 12h. The mixture was concentrated to 50 mL and
adjusted to pH 7
using saturated sodium bicarbonate. The reaction mixture was partitioned
between H20
-49-
CA 02432662 2003-06-09
WO 97/29?43 PCT/US96/13632
and EtOAc. The aqueous layer was separated and extracted with EtOAc. The
combined organic extracts were dried over sodium sulfate, filtered and
concentrated in
vacuo. The resulting colorless oil(370 mg, 87%) was used without further
purification.
1H NMR (CDCl3}: 7.6 (s, 1H), 7.39(d, 1H, J = 8.5 Hz), 7.08(d, 1H, J= 8.5 Hz)
c)Preparation of N - [2 - hydroxy - 4- (trifluoromethyl) phenyl] - N' - phenyl
urea
N - [2 - Hydroxy - 4- (trifluaromethyl) phenyl] - N' - phenyl urea was
prepared
from 2-amino-5-trifiuoromethylphenol ( 150 mg, 1.09 mmol) and phenyl
isocyanate( 1.09 mmol) according to the procedure in General method A. The
product
was purified by precipitation from methylene chloride and filtering to afford
the titled
1o compound ( 230 mg, 87% ), mp: °C; 1H NMR (DMSO-d6): d 9.45 (s, 1H,
NH), 8.50
(s, 1 H, NH), 8.31 (d, 1 H, J = I0.0 Hz), 7.45 (d, 2H, J = 10.0 Hz), 7.29 (t,
2H, J = 6.67
Hz), 7.10 (m, 2H), 6.99 (t, IH, J = 6.67 Hz). EI-MS m/z 296 (M+). Anal.
(C 14H 11 N202F3)C, H, N.
is Example 88
Preparation of N-l2-hvdro_xy-4-nitro~enyl)-N'-(2-hyd~xy-4-nitrovhenvll urea
a}Preparation of 2-(tert-butyldimethylsilyloxy)-4-nitroaniline
To a solution of 2-amino-5-nitrophenol ( I g, 6.49 mmol) and imidazole (0.88
g,
20 12.3 mmol) in DMF ( 15 mL), tert -butyldimethylsilyl chloride ( 11.2 mL,
64.9 mmol)
was added. The resulting mixture was allowed to stir at 23°C for 48
hours. The reaction
mixture was partitioned between 0.1 % HCl and ethyl acetate. The combined
organic _
phase was washed with brine, dried over MgS04 and filtered. Removal of solvent
at
reduced pressure and chromatography of the resulting oil on silica gel (hexane
: ethyl
25 acetate; 5:1) gave the titled compound (1.7 g, 98 %). 1H NMR (CDC13): d
7.78 (dd,
1 H, 3 = 6.7 Hz and J = 2.7 Hz), 7.61 (d, 1 H, J = 2.7 Hz), 6.7 (d, 1 H, J =
8.8 Hz), 1.0 (s,
9H), 0.28 (s, 6H).
b)Preparation of N-[(2-tert-butyldimethylsilyloxy)-4-nitrophenyl]-N-[(2-tert-
butyldimethylsiloxy)-4- nitrophenyl] urea
3o To a solution of 2-(ten-butyldimethylsilyloxy)-4-nitroaniline(200 mg, 0.75
mmol) in toluene (10 mL) triethylamine (0.I3 mL, 1.64 mmol) and triphosgene
(88.4
mg, 0.3 mmol) were added. The reaction mixture was stirred at 70°C for
2 hours, then
cooled to room temperature. Then more 2-(tent -butyldimethylsilyloxy)-4-
nitroaniline
(200 mg, 0.75 mmol) was added. The resulting mixture was allowed to stir at
70°C for
35 48 hours then cooled to room temperature. The reaction mixture was
partitioned
between water and ethyl acetate. The combined organic phase was washed with
brine,
dried over MgS04 and filtered. Removal of solvent at reduced pressure and
- 50 -
CA 02432662 2003-06-09
WO 97129743 PG"T/US96/I3632
chromatography of the resulting oil on silica gel (hexane : ethyl acetate,
10:1 ) gave the
titled compound( 130 mg, 31%). 1H NMR (CDCl3): d 8.36 (d, 2H, J = 8.3 Hz),
7.90
(dd. 2H, J = 8.3 Hz and J = 2.8 Hz), 7.71 (d, 2H, J = 2.8 Hz), 7.22 (s, 2H),
1.02 (s,
18H), 0.35 (s, 12H).
c)Preparation of N-(2-Hydroxy-4-nitrophenyl)-N'-(2-hydroxy-4-nitrophenyl) urea
To a solution of N-[(2-ten-butyldimethylsilyloxy)-4-nitrophenyl]-N'-[(2-tert-
butyldimethylsilyloxy)-4- nitrophenyl] urea(50 mg, 0.089 mmol) in THF (2 mL),
tetrabutylammonium fluoride ( 1 M, 0.09 mL, 0.089 mmol) was added at
0°C. The
reaction mixture was stirred at 23°C. After 1 hour, the starting
material had
to disappeared. The reaction mixture was partitioned between water and ethyl
acetate. The
combined organic phase was dried over MgS04 and filtered. Removal of solvent
at
reduced pressure and chromatography of the resulting oil on silica gel (hexane
: ethyl
acetate; 1:1 to 100% ethyl acetate) gave the titled compound(24 mg, 81%). 1H
NMR
(CD30D/CDCl3):, d 8.32 (d, 2H, J = 8.25 Hz), 7.80 (dd, 2H, J = 8.25 Hz and J =
2.06
t5 Hz), 7.7 (d, 2H, J = 2.06 Hz). EI-MS m/z 334 (M+H)+. Anal. (C 13H 10N407)
C, H, N.
Exa
Preparation of N-l2-hydroxv-4-nitro~henyl)-N'-Rhen"yl-thiourea
a)Preparation of N-(2-tert-butyldimethysilyloxy-4-nitrophenyl)-N'-phenyl-
thiourea
2o N-(2-tert-Butyldimethysilyloxy-4-nitrophenyl)-N'-phenyl-thiourea was
prepared
by treating a biphasic solution of 2-tent-butyldimethysilyloxy-4-
nitroaniline(80 mg,
0.308 mmol) and NaHC03 in CHC13:H20(2.5:1, 7mL) with thiophosgene at
0°C. The
solution was allowed to warm to 23°C and the reaction was continued
overnight. The
CHC13 layer was separated and dried over sodium sulfate. The solution was
25 concentrated in vacuo and the residue was dissolved in toluene and treated
with aniline
( 100 uL) at 23 °C for 12 h. The reaction mixture was concentrated and
the residue was
purified by flash chromatography ( 10% EtOAc/hexanes) to afford the titled
compound
as a yellow solid ( 120.8 mg, 98%) mp: 144-145°C;1H NMR (CD30D/CDCl3):
d 8.65
(d, 1H, J = 10.0 Hz), 7.58 (d, 1H, J = 10.0 Hz), 7.47 (d, 1H, J = 1.25 Hz),
7.26 (m, 4H),
30 7.10 (m, 1 H).
b)Preparation of N-(2-hydroxy-4-nitrophenyl}-N'-phenyl-thiourea
N-(2-Hydroxy-4-nitrophenyl)-N'-phenyl-2-thiourea was prepared by treating a
solution of N-(2-tent-butyldimethysilyloxy-4-nitrophenyl)-N'-phenyl-thiourea (
100 mg,
0.248 mmoi) in CH3CN ( 1 mL) with Et3N~HF ( 100uL, 0.62 mmol) in acetonitrile
for
35 10 minutes at 23°C. The solution was concentrated and flushed
through a silica plug
with EtOAc to afford the desired compound as an orange solid (55 mg, 77%).
-51-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
mp: 144-145°C;1H NMR (CD30DlCDCI3): d 8.65 (d, 1H, J = 10.0 Hz), 7.58
(d, 1H, J
= 10.0 Hz), 7.47 (d, 1H, J = 1.25 Hz), 7.26 (m, 4H), 7.10 (m, 1H).
Example 10
Preparation of N-l4- nitro 2-lphenylsulfonvlamino)phen~il-N'-phen~,rl urea
a) Preparation of 4-vitro 2-(phenylsulfonylamino) aniline
A solution of 4-vitro 1,2-phenylene diamine(I.53 g, 10.0 mmol) in DMF was
treated with phenyl sulfonyl chloride( 1.76 g, 10.0 mmol) and triethyl amine(
1.01 g) in
DMF for 12 h at 23 °C. The reaction mixture was partitioned between
saturated NH4C1
and methylene chloride. The organic layer was dried over sodium sulfate,
filtered and
concentrated in vacuo. The resulting solid was recrysiallized (EtOH) to afford
desired
(0.2?5 g, 9%). 1H NMR(DMSO) 9.5(s, 1H, br), 7.83 (dd, 1H, J=10 Hz, 2 Hz),
7.74{d,
2H, J=8 Hz), 7.76(t, 1H, J=8 Hz), 7.56(t, 2H, J=8 Hz), 7.55(d,lH, J=2Hz), 6.79
(d, 1H,
J=8Hz), 6.5(s, 2H, br)
t5 b)Preparation of N-(4- vitro 2-(phenylsulfonylamino)phenyl)-N'-phenyl urea
N-(4-Nitro 2-(phenylsulfonylamino)phenyl)-N'-phenyl urea was prepared from
4- vitro 2-(phenylsulfonyiamino) aniline(82 mg) and phenyl isocyanate(33 mg)
by
method A. The reaction was cooled and then partitioned between saturated
ammonium
chloride and 9:1 methylene chloride and methanol. The organic phase was dried
over
2o magnesium sulfate, filtered and concentrated in vacuo. The residue was
purified by
column chromatography (ethyl acetate/hexanes) to afford desired(30.8 mg, 26%).
EI-
MS mlz 413(M+H)+
Exam 1
25 Preparation of N-f2-hvdroxy-5-nitro~henyll-N'-l3-methoxy-2-thien 1)y urea
a)Preparation of 3-methoxy-2-thienylcarboxlic acid
To a solution of 3-methoxythiophene (4.81 g, 42.1 mmol) in ether (20 mL) at -
78°C, butyllithium ( 17 mL, 47.6 mmol) was added. The reaction mixture
was stirred at
-78°C for 1 hour, then it was warmed to 0 °C for 3 hours. After
to recooling -78°C
30 the reaction mixture was poured into a beaker filled with crushed dry ice (
14.5 g) and
allowed to stand until the excess dry ice had completely sublimed. Then the
reaction
mixture was poured into a mixture of ice ( 10 g) to which conc. HCI (24 mL)
had been
added. The product was purified by precipitation from ether and filtering
(6.42 g, 96
%). EI-MS mlz 169 (M+H)+.
35 b)Preparation of N-{2-hydroxy-5-nitrophenyl)-N'-{3-methoxy-2-thienyl)urea
-52-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
To a solution of 3-methoxy-2-thiophene carboxylic acid (200 mg, 1.27 mmol) in
benzene, (Ph0)2PON3 (0.33 mL), 2-amino-4-nitrophenol ( 195.7 mg, 1.27 mmol)
and
triethylamine ( 1.1 equiv., 0.25 mL) were added. The reaction mixture was
stirred at
reflux overnight. The reaction nuxture was partitioned between Solo citric
acid and ethyl
acetate. The organic layer was separated and-the aqueous layer was extracted
three
times with ethyl acetate. The organic extracts were combined, dried over
MgS04,
filtered and concentrated under reduced pressure. Chromatography of the
resulting
solid on silica gel (hexane:ethyl acetate; l : l ) gave a solid product ( 160
mg, 41 %). mp:
172.6-173.0°C; 1H NMR (CD30D/CDC13): d 8.96 (d, 1H, J = 2.5 Hz), 7.74
(dd, 1H, J
t0 =5.0 Hz and J = 1.25 Hz), 6.82 (d, 1H, J =7.5 Hz), 6.76 (s, 2H), 3.80 (s,
3H); EI-MS
m/z 309 (M+H)+; Anal. (C 12H 11N305S) C, H, N.
Example 12
Prgparation of N-(2-hxdroxy-4-nitrophenyI)-N'-(3-methoxy-2-thien~l)urea
t5 To a solution of 3-methoxy-2-thiophene carboxylic acid (example l la, 200
mg,
1.27 mmol) in toluene, (Ph0)2PON3 (0.33 mL) and triethylamine ( 1.1 equiv.,
0.25 mL)
were added. The reaction mixture was stirred at 70°C for 2 hours and
cooled down to
room temperature then 2-amino-S-nitrophenol was added. The reaction mixture
was
stirred at 70°C overnight. The reaction mixture was partitioned between
5% citric acid
20 and ethyl acetate. The organic layer was separated and the aqueous layer
was extracted
three times with ethyl acetate. The organic extracts were combined, dried over
MgS04,
filtered and concentrated under reduced pressure. Chromatography of the
resulting
solid on silica gel (hexane:ethyl acetate; l : l ) gave the product ( 190 mg,
48%). 1 H NMR
(CD30D/CDCI3): d 8.38 (d, 1H, J = 5.0 Hz), 7.85 (dd, 1H, J = 5.0 Hz and J =
1.25 Hz),
25 7.76 (d, 1H, J = 2.5 Hz), 6.9 (s, 2H), 3.95 (s, 3H); EI-MS mlz 309 (M+H}+;
Anal.
(C12H11N305S} C, H, N.
Example 13
Preparation of N-l2-hvdroxv-4-nitrophenvl)-N'-l3-methoxyphenyDurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-methoxyphenyl)urea was prepared from 2=
30 hydroxy 4-vitro aniline ( 154 mg, 1.0 mmol) and 3-methoxy phenyl
isocyanate( 1.0
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound ( 140 mg, 46%). EI-MS m/z 302(M-H) -
35 Example 14
P~paration of N-f2-hydroxv-4-nitrophen~rll-N'-(2-methoxy~he_nvllurea
-53-
CA 02432662 2003-06-09
WO 97129743 PGTlUS96/13632
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methoxyphenyl)urea was prepared from 2-
hydroxy 4-vitro aniline ( 154 mg, l _0 mmol) and 2-methoxy phenyl isocyanate(
1
mmol.) according to the procedure in General Method B. The product was
purified by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound (82 mg, 27%). EI-MS m/z 302(M-H)-
~xamnle 1 S
Preparation of N 12-h~o_xy-4-nitrophen~l-N'-(3-trifluoromethYl_phenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-methoxyphenyl)urea was prepared from 2-
to hydroxy 4-vitro aniline ( 154 mg, 1.0 mmol) and 3-trifluoromethyl phenyl
isocyanate ( 1
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound ( 180 mg, 52%). EI-MS m/z 342(M+H) +
l~xamp",~e 16
15 Preparatior~of N-(2-hy~roxv-4-nitro~h_envll-N'-l2-trifluorometh; lphen
llurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-trifluoromethylphenyl)urea was prepared
from 2-hydraxy 4-vitro aniline ( 154 mg, 1.0 mmol) and 2-trifluoromethyl
phenyl
isocyanate ( 1.0 mmol) according to the procedure in General Method B. The
product
was purified by dilution with methylene chloride and precipitation with
hexanes.
20 - Filtering afforded the title compound (180 mg, 52%). EI-MS m/z 342(M+H) +
Example 1?
Preparation of N-l2-h~~v-4-nitrophenyll-N'-l4-trifluorometh~nhenyllurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-trifluoromethylphenyl)urea was prepared
25 from 2-hydroxy 4-vitro aniline ( 154 mg, 1.0 mmol) and 4-trifluoromethyl
phenyl
isocyanate ( 1.0 mmol) according to the procedure in General Method B. The
product
was purified by dilution with methylene chloride and precipitation with
hexanes.
Filtering afforded the title compound ( 111 mg, 32%). EI-MS m/z 340(M-H)-
Example 18
30 Preparation of N-t2-hydroxy-4-nitro~henyl)-N'-f2-bromoph~yl_lurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-bromophenyl)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 2-bromophenyl isocyanate (3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
35 title compound(530 mg, 47%). EI-MS m/z 350(M-H) '
Example 19
-54-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
Preparation of N-E2-hvdroxv-4-nitrophen~rll-N'-f 3-bromophenvl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(3-bromo phenyl)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 3-bromo phenyl isocyanate
(3.24
mmol)according to the procedure in General Method B. The product was purified
by
s dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound(0.96g, 87%). EI-MS m/z 350(M-H) -
Example 20
Preparation of N-(2-hydroxy-4-nitrophenyt)-N'-f4-bromo hen 1Y lurga
1o N-(2-Hydroxy-4-nitrophenyl)-N'-(4-bromo pheny!)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 4-bromo phenyl isocyanate
(3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound(0.41 g, 37%). EI-MS m/z 352(M+H) +
Example 21
l~renaration of N-l2-hvdroxy-4-nitrophenyl)-N'-(2-phenvl~hen 1)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-phenylphenyl)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 2-phenyl phenyl isocyanate
(3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound(0.22 g, 19%). EI-MS m/z 350(M+H) +
Example 22
Pre~araticZn of N-l~.-ll,~rdroxv-4-nitrophenyl)-N'-t1-naphth 1)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-( 1-naphthyl)urea was prepared from 2-hydroxy
4-vitro aniline (500 mg, 3.24 mmol;) and 1-naphthyl isocyanate (3.24 mmol)
according
to the procedure in General Methad B. The product precipitated from methylene
chloride and filtered. The resulting solid was titruated with 1:3 triethyl '
amine:methylene chloride. The filterate was concentrated in vacuo. The
resulting
residue was dissolved in methylene chloride and treated with 1N HCI in water.
The
desired product precipitated from solution and was collected by filtration(0.1
1g, 10%).
EI-MS m/z 324(M+H) +
E ample 23
Preparation of N-(2-t~ydrox~-4-nitro~henyl)-N'-(2-nitro~henvl)urea
-55-
CA 02432662 2003-06-09
WO 97J29743 PCT/US96113632
N-(2-Hydroxy-4-nitrophenyl)-N°-(2-vitro phenyl)urea was prepared
from 2-
hydroxy 4-vitro aniline {500 mg, 3.24 mmol) and 2-vitro phenyl isocyanate
{3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound(0.44 g, 44%). EI-MS m/z 319(M+H) +
Example 24
Preparation of N-l2-hydroxy-4-nitrophen~,~)-N'-l2-fluorophenyllurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-fluorophenyl)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 2-fluoro phenyl isocyanate
(3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methyiene chloride and precipitation with hexanes. Filtering
afforded the
title compound(0.59 g, 31%). EI-MS m/z 292(M+H) +
Examp1~25
Preparau~gn of N-(2-hydrox~!-4-nitrophenyl)-N'-(2.6-difluorophenvl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-{2,6-difluorophenyl)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 2,6-difluoro phenyl
isocyanate(3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound(0.91 g, 91 %). EI-MS m/z 308(M-H) °
Example 26
Preparation of N-(2-hxdrox~-4-nitrophenvl)-1V'-(2-ethoxYphenyllurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-ethoxyphenyl)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 2-ethoxy phenyl isocyanate
(3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with rnethylene chloride and precipitation with hexanes. Filtering
afforded the
title compound{0.84 g, 81%). EI-MS m/z 318(M+H) +
Exam 1~ a 27
Preparation of N- 2-hydroxy-4-nitro~henyll-N'-l2-ethylphenyl urea
3o N-(2-Hydroxy-4-nitrophenyl)-N'-(2-ethylphenyl)urea was prepared from 2-
hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and 2-ethyl phenyl isocyanate
(3.24
mmol) according to the procedure in General Method B. The product was purified
by
dilution with methylene chloride and precipitation with hexanes. Filtering
afforded the
title compound(0.44 g, 43%). EI-MS m/z 302(M+H) +
Example 28
Preparation of N-l2-hydroxv-4-vitro phenyll-N'-(2-t 'fluoromethoxyphetl, llv
urea
-56-
CA 02432662 2003-06-09
WO 97129743 PCT/L1S96113632
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-trifluoromethyloxyphenyl)urea was
prepared from 2-hydroxy 4-vitro aniline (500 tng, 3_24 mmol) and 2-
trifluoromethoxy
phenyl isocyanate (3.24 mmol) according to the procedure in General Method B.
The
product was purified by dilution with methylene chloride and precipitation
with
hexanes. Filtering afforded the title compound(0.69 g, 60%). EI-MS m/z
358(M+H) +
Example 29
Synthesis of N-l2-h~drox~-4-vitro phenyl) N'-(2-methvlthio~hen l~rea
The urea was prepared from 2-hydroxy 4-vitro aniline (500 mg , 3.24 mmol)
t0 and 2-methylthio phenyl isocyanate(3.24 nnmol) by general Method B. The
product
was purified by dilution with methylene chloride and precipitation with
hexanes.
Filtering afforded the title compound(0.63 g, 61 %). EI-MS m/z 320(M+H) +
Exam a 30
15 Synthesis of N-(2-hvdro~y-4-vitro phenyl) N'-(2-chloro 6-met_iiyl phenyl)
urea
The urea was prepared from 2-hydroxy 4-vitro aniline (500 mg, 3.24 mmol) and
2-chloro 6-methyl phenyl isocyanate by general Method B. It was purified by
dilution
with methylene chloride and precipitation with hexane. Filtering afforded the
desired
compound(0.31 g, 29%). El-MS m/z 322(M+H) +
Exam )p a 31
Synthesis of N-(2-h~~-4-vitro phenyl) N'-(2- meth3rl sulfoxYphenyl) urea
The urea was synthesized by treatment of N-( 2-hydroxy 4-vitro phenyl) N'-(2-
methyl thio phenyl) urea(example 28, 100 mg) with sodium periodate( 100 mg) in
t-
butanollwater for 12 hours at 23 °C. The product precipitated from the
reaction
mixture(30 mg, 29%). EI-MS m/z 336(M+H) +
Exam"Rle 32
Synthesis of N-(2-hydroxy 4-trifluorometh~~henyll N'-(2-byomo nhen,1~ urea The
urea was prepared from 2-hydroxy 4-trifluoromethyl aailine(example 7a, 0.171g,
1
mmol) and 2-bromo phenyl isocyanate(1 mmol) by general Method B. It was
purified
by dilution with methylene chloride and precipitation with hexane. Filtering
afforded
the desired compound(0.25 g, 54%). EI-MS m/z 375(M+H) +
xa 33
Synthesis of N-(2-hydroxy 4-carbomethoxy phenyl) N'-(2-bromo enyl) urea
-57-
CA 02432662 2003-06-09
WO 97/29743 PCT/L1S96113632
The urea was prepared from 2-hydroxy 4-carbomethoxy aniline(0.167 g, 1
mmol} and 2-bromo phenyl isocyanate(1 mmol) by general Method B. It was
purified
by dilution with methylene chloride and precipitation with hexane. Filtering
afforded
the desired compound(0.12 g, 33%). EI-MS m/z 363(M-H) -
Example 34
Synthesis of N-f2-h~droxv 4-trifluQromethyl phenyl) N'-l2-phgnxl phenyl) urea
The urea was prepared from 2-hydroxy 4-trifluoromethyl aniline(example 7a,
0.171 g, 1 mmol)) and 2-phenyl phenyl isocyanate by general Method B. It was
t0 purified by dilution with methylene chloride and precipitation with hexane.
Filtering
afforded the desired compound(0.24 g, 64%). EI-MS m/z 373(M+H)+
Example 35
Synthesis ofN-(2-hydroxy 4-carbomethoxy phen~rl, N'-l2~henyl nhenyrl) urea
The urea was prepared from 2-hydroxy 4-carbomethoxy aniline(0.167 g, 1
mmol) and 2-phenyl phenyl isocyanate( l mmol) by general Method B. It was
purified
by dilution with methylene chloride and precipitation with hexane. Filtering
afforded
the desired compound(0.185 g, 50%). EI-MS m/z 363(M-H)
Example 36
Synthesis of N-l2-hvdroxv 4-nitro~henvll N'-(2.3-dic oro hen~)u_r~a The urea
was
prepared from 2-hydroxy 4-vitro aniline(308 mg, 2 mmol) and 2,3-dichloro
phenyl
isocyanate(2 mmoI) by general Method B. It was purified by dilution with
methylene
chloride and precipitation with hexane. Filtering afforded the title
compound(0.5 g,
73%). EI-MS m/z 342(M+H) +
Example 37
Synthesis of N-f2-h~droxv 4-vitro phenyll N'-(2.4-dichloro ~yll urea
The urea was prepared from 2-hydroxy 4-vitro aniline(308 mg, 2 mmol) and
2,4-dichloro phenyl isocyanate(2 mmol) by general Method B. It was purified by
dilution with methylene chloride and precipitation with hexane. Filtering
afforded the
title compound(0.26 g, 38%). EI-MS m/z 342 (M+H) +
Example 38
Synthesis ol~N-l2-hy, roxy-4-nitro,phenyl) N'-(2-chloro Rhe~Srl) urea
_5g_
CA 02432662 2003-06-09
WO 97129743 PCT/I1S96/13632
The urea was prepared from 4-vitro 2-hydroxy aniline(308 mg, 2 mmol) and 2-
chloro phenyl isocyanate(2 mmol) by general Method B. It was purified by
dilution
with methylene chloride and precipitation with hexane. Filtering afforded the
title
compound(0.29 g, 47%). EI-MS m/z 308(M+H) +
Example 39
Synthesis of N-12-hvdroxv-4-nitroahenvl) N'-(2.4-dibromo Qhen~l~ urea
The urea was prepared from 4-vitro 2-hydroxy aniline(308 mg, 2 mmoi) and
2,4-dibromo phenyl isocyanate(2 mmol) by general Method B. It was purified by
dilution with methylene chloride and precipitation with hexane. Filtering
afforded the
title compound(0.34 g, 39%). EI-MS m/z 430(M+H) +
Exam 1~ 40
S~thesis of 1-~(2-hydroxynapthyl~ N'-(2-bromo phenXll urea
The urea was prepared from 1-amino 2-hydroxy napthalene( 195 mg, 1 mmol)
and 2-bromo phenyl isocyanate( 1 mmol) by general Method B. It was purified by
dilution with methylene chloride and precipitation with hexane. Filtering
afforded the
title compound(0.030 g, 8%). EI-MS m/z 357(M+H) +
Example 41
Synthesis of N-(2-h~roxy-4-nitrophenyl)-N'-(2.3-methylenedioxy~heny~,lurea
a)Preparation of 2,3-methylenedioxyphenylcarboxylic acid
A solution of 1,3-benzodioxole (3.09 g, 32 mmol) in dry ether (50 mL) was
treated dropwise at -10°C with 2.5 M n-butyllithium ( 15 mL, 35 mmol)
in hexane.
When the addition was complete, the mixture was stirred under reflux fvr one
hour.
After cooling to room temperature, it was added to crushed solid carbon
dioxide, and
after 24 hours, the residue was treated with 10 % aq. NaHC03 and ether. The
alkali
layer was separated, washed with ether, then acidified with cold concentrated
HCl, and
extracted with chloroform. The combined organic layers were dried over MgS04,
filtered and concentrated under reduced pressure (1.1 g, 20 %). EI-MS mJz 167
(M+H)+
b)Preparation of N-(2-hydroxy-4-nitrophenyl)-N'-(2,3-methylenedioxyphenyl)urea
To a solution of the 2,3-methylenedioxyphenylcarboxylic acid in toluene,
triethylamine (0.27 mL, 1.95 mmol) and diphenylphosphoryl azide (DPPA) (0.32
mL,
1.5 mmol) were added. The reaction mixture was stirred at 60°C for 2
hours, then 2-
amino-5-nitrophenol (250 mg, 1.5 mmol) was added. The reaction mixture was
stirred
-59-
CA 02432662 2003-06-09
WO 97/29743 PCT/i1S96/13632
at 100°C for 18 hours. After the reaction mixture was cooled to room
temperature, it
was partitioned between 5 % citric acid and ethyl acetate. The organic layer
was
separated and the aqueous layer was extracted three times with ethyl acetate.
The
organic extracts were combined, dried over MgS04, filtered and concentrated
under
reduced pressure. Chromatography of the resulting solid on silica gel (hexane
: ethyl
acetate; 5:1 ) gave product (200 mg, 42 %). EI-MS m/z 318 (M+H)+
Example 42
~nthesis of N-(2-hydro~ 4-vitro phenyl) N'-(2-method 3-chloro phenyl? urea
The urea was prepared from 2-hydroxy 4-vitro aniline(308 mg, 2 mmol) and 2-
chloro 3-methoxy phenyl isocyanate(2 mmol) by general Method B. It was
purified by
dilution with methylene chloride and precipitation with hexane. Filtering
afforded the
title compound(0.48 g, 63%). EI-MS mlz 338(M+H) +
Example 43
~nthesis of N-l2-hydroxx4-vitro phenyll N'-(2-methyl ~enyl urea
The urea was prepared from 2-hydroxy 4-vitro aniline(308 mg, 2 mmol) and 2-
methyl phenyl isocyanate(2 mmol) by general Method B. It was purified by
dilution
with methylene chloride and precipitation with hexane. Filtering afforded the
title
2o compound(0.38 g, 53%). EI-MS m/z 288(M+H) +
Example 44
Synthesis of N(bis (2-hydroxv 4-vitro phenyl) N'-(dianisdine diurea
The urea was prepared from 2-hydroxy 4-vitro aniline(616 mg, 4 mmol) and
dianidisdine diisocyanate(2 mmol) by general Method B(except 2 equiv. of 4-
vitro 2-
hydroxy aniline was used instead of lequiv.). The product was purified by
dilution
with methylene chloride and precipitation with hexane. Filtering afforded the
title
compound( 0.08 g, 6%).EI-MS m/z 605(M+H) +
Example 45
~nthesis of 4-methylene bis(N-(2-chloro phenvI~ N'-(2-h3rdroxy 4-nitr~henyll
urea)
The urea was prepared from 2-hydroxy 4-vitro aniline(616 mg, 4 mmol) and 4-
methylene bis(N-(2-chloro phenyl) diisocyanate(2 mmol) by general Method
B(except 2
equiv. of 4-vitro 2-hydroxy aniline was used instead of lequiv.). The product
was
purified by dilution with methylene chloride and precipitation with hexane.
Filtering
afforded the title compound(0.10 g, 8%). EI-MS mlz 627(M+H) +
_60_
CA 02432662 2003-06-09
WO 97/29743 PCTIUS96/13632
Example 46
Synthesis of N-f 2-hydroxy 4-(benzylaminolcarbonyl phenyll-N'-(2-bromophenyl
urea
a)Synthesis of N-(2-hydroxy 4-carboxylate phenyl) N'-(2-bromo phenyl) area
The urea was prepared from 3-hydroxy 4-amino benzoic acid (3.69 g, 24 mmol)
and 2-bromo phenyl isocyanate(24 mmol) by general Method B. It was purified by
dilution of the DMF solution with methylene chloride and precipitation with
hexane(4.0
g, 48%). EI-MS m/z 351(M+H) +
t0 b)Preparation of N-(4-(benzyiamino)carbonyl-2-hydroxyphenylJ-N'-(2-
bromophenyI)urea To a solution of the N-(2-hydroxy 4-carboxylate phenyl) N'-(2-
bromo phenyl) urea (200 mg, 0.58 mmol) in DMF ( 15 mL), EDC ( 121.9 mg, 0.58
mmol), HOBT ( 156.6 mg, 11.6 mmol) were added . The reaction mixture was
stirred at
room temperature for 16 hours. Then the benzyl amine ( 123 mg, 11.6 mmol) was
t 5 added. The reaction mixture was stirred at same temperature for 24 hours.
Then the
reaction mixture was partitioned between water and ethyl acetate. The organic
layer was
separated and the aqueous layer was extracted three times with ethyl acetate.
The
organic extracts were combined, dried over MgS04, filtered and concentrated
under
reduced pressure. Chromatography of the resulting solid on silica gel (hexane
: ethyl
2o acetate; 1:1 ) gave benzylamino product (500 mg, 65 %). EI-MS m/z 441
(M+H)+
Example 47
Synthesis of N-(2-hvdrox~4-n~r_o ~henvl) N'-(2-nhenoxv nhenvl) urea The urea
was
synthesized by the treatment of 2-phenoxyphenyl carboxylic acid(2 mmol,) with
25 diphenyl phosphoryl azide(0.475 mL) and triethyl amine(.14 mL) in DMF at 80
°C after
24 hours the 2-amino 5-vitro phenol ( 1 equiv.) was added. The reaction was
heated for
24 hours at 80°C. The reaction product was oiled out with hexane. The
residue was
dissolved in methanol and the solid was precipitated out with water.( 180 mg,
24%) EI-
MS m/z 364{M-H)
example 4~
S~rnthesis of N-(2-hydrox~-4-fluoro ~enyll N'-(2-bromo phen, I~u_re_a
a)Synthesis of 2-hydroxy 4-fluoro aniline
3-fluoro 6-vitro phenol (2 g, I 1 mmol} was treated with I0%PdlC(1 g) at 23
°C.
The reaction mixture was flushed with hydrogen gas and the reaction was
allowed to
-61-
CA 02432662 2003-06-09
WO 97/29743 PCTlI1S96/13632
stir 12 h before it was filtered through celite. The filtrate was concentrated
in vacuo to
afford the title compound {1.4 g, 77%). EI-MS mlz 169(M+H) +
b)Synthesis of N-(2-hydroxy-4-fluoro phenyl) N'-(2-bromo phenyl) urea
The urea was prepared from 2-hydroxy 4-fluoro aniline(254 mg, 2 mmol) and
2-bromo phenyl isocyanate by general Method B. It was purified by dilution
with
methylene chloride and precipitation with hexane( 173 mg, 26%). EI-MS m/z 325
(M+H) +
Example 49
S~rnthesis of N-( 2-h~rdroxy 3.4-difluoro phen l~'-,~2-bromo_~henyl) urea
a)Synthesis of 2-hydroxy 3,4-difluoro aniline
2,3 difluoro 6-vitro phenol (2 g, 11 mmol) was treated with 10%Pd/C( 1 g) at
23
°C. The reaction mixture was flushed with hydrogen gas and the reaction
was allowed
to stir 12 h before it was filtered through celite. The filtrate was
concentrated in vacuo
to afforded the title compound ( 1.6 g, 97%). EI-MS m/z I46(M+H)+
b)Synthesis of N-(2-hydroxy 3,4-difluoro phenyl) N'-(2-bromo phenyl) urea
The urea was prepared from 2-hydroxy 3,4-difluoro aniline(0.290 g, 2 mmol)
and 2-bromo phenyl isocyanate(0.4 g) by general Method B. it was purified by
dilution
with methylene chloride and precipitation with hexane(0.254 g, 37%). EI-MS mlz
' 343(M+H) +
Example 50
~nthesis of N-(2-by oxy 3-napthyll N'-l2-bromo phenyll urea
The urea was prepared from 3-amino 2-hydroxy napthaiene(0.320 g, 2 mmol)
and 2-bromo phenyl isocyanate(.40 g) by general Method B. It was purified by
dilution
of the with methylene chloride and precipitation with hexane(0.339, 47%).EI-MS
nn/z
357(M+H)+
Exam l
~;~,nthesis of N-(2-hydroxv 4-den r~l phenyl) N'-(2-bromo ~envl) urea
a)Synthesis of 2-vitro 5-phenyl phenol
A solution of 3-phenyl phenol(2 g, 11 mmol) in acetic acid was treated with
concentrated nitric acid drop-wise until all starting material was consumed.
The
solution was partitioned between water and methylene chloride. The organic
phase was
separated and the aqueous phase was extracted once more with methylene
chloride. The
combined organic phases were dried over sodium sulfate, filtered and
concentrated in
-62-
CA 02432662 2003-06-09
WO 97129743 PCT/US96113632
vacuo. The residue was purified by silica gel chromatography(ethyl
acetate/hexanes) to
afford desired ( 1.2 g, 50%).1H NMR (CDC13): d 10.65(s, 1H), 8.18 (d, 1H, J =
10.0
Hz), 7.65 (d, 2H, J = 6.0 Hz), 7.49 (m, 3H), 7.34 (s, 1H), 7.10 (d, 1H,
J=10.0Hz).
b)Synthesis of 2-amino 5-phenyl phenol
A solution of 2-vitro 5-phenyl phenol( 1.2 g, 5.5 mmol) in methanol was
treated
with 10% Pd/C( 1.28). The reaction mixture was flushed with hydrogen and
allowed to
stir overnight. The reaction mixture was filtered through celite and the
filtrate was
concentrated in vacuo to afford desired ( 1.01 g, 98%).EI-MS m/z 186(M+H)+
c)Synthesis of N-(2-hydroxy 4-phenyl phenyl) N'-(2-bromo phenyl) urea
The urea was prepared from 2-hydroxy 4-phenyl aniline(0.185 g, 1 mmol) and
2-bromo phenyl isocyanate(0.198 g} by general Method B. It was purified by
dilution
of the DMF solution with methylene chloride and precipitation with hexane(215
mg,
56%).EI-MS m/z 383(M+H) +
Example 52
Synthesis of N-(2-hvdrox~ethvl nhenyl) N'-(2-bromo ahenvl) urea
The urea was prepared from 2-hydroxy 4-methyl aniline(.274g, 2 mmol) and 2-
bromo phenyl isocyanate(0.40 g, 2 mmol) by general Method B. It was purified
by
dilution of the DMF solution with methylene chloride and precipitation with
2o hexane(249 mg, 39%). EI-MS m/z 319(M-H)
Example 53
Synthesis of N(2-hydroxy 4-vitro ~yl) N'-(2-phenvlamino phen~ll ureaThe urea
was
synthesized by the treatment of 2-tertbutyldimethylsilyloxy 4-vitro phenyl
isocyanate(example 9a, 0.4198, 1.5 equiv.) with 2-anilino aniline(0.184 g, 1
equiv.) in
THF overnight at 40 °C. The desired product precipitated out of the
reaction
mixture(30 mg, 8%). EI-MS m/z 365(M+H) +
Example 54
Synthesis of N-l2-h~droxy 3-carbox 1v ate phenyl) N'-(2-bromo phenyll urea
The urea was prepared from 2-hydroxy 3-amino benzoic acid(300 mg, 2 mmol)
and 2-bromo phenyl isocyanate by general Method B. It was purified by dilation
of the
DMF solution with methylene chloride and precipitation with hexane(.287 g, 41
%). EI-
MS m/z 351(M+H) +
Exam 1p a 55
-63-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
Synthesis of Nf2-sulfh~rvl 4-bromo phenyl) N'-l2-bromo.phenyll ureaa)Synthesis
of
2-amino 6-bromo thiazole
4-Bromo aniline(4.3 g, 25 mmol, 1 equiv.) and ammonium th.iocyanate(5.7 g,
3equiv.) was dissolved in acetic acid and treated with bromine(4 g, lequiv.)
at room
temperature. After complete disappearance of starting material the reaction
mixture
was poured into water and the solid was collected. The solid was used in the
next step
without any purification(3.6 g, 46%). EI-MS m/z 229(M+H) +
b)Synthesis of bis (3-bromo 6-amino phenyl) disulfide
The 2-amino 6-bromo thiazole hydrobromide (500 mg, 1.6 mmol) in
1o water(SmL) was treated with KOH (2.5 g) was heated at reflux for 8 h at
reflux. The
reaction mixture was then acidified to ph 4 with acetic acid and extracted
with
methylene chloride. The methylene chloride mixture was concentrated in vacuo.
The
residue was dissolved in DMSO and treated with I2. After stirring overnight at
room
temperature the reaction mixture was partitioned between methylene chloride
and
t5 saturated sodium bicarbonate. The methylene chloride layer was dried with
magnesium
sulfate and concentrated in vacuo. The resulting solid was purified by flash
chromatography(ethyl acetatelhexane) to afford the title compound (230 mg,
34%). EI-
MS m/z 405(M+H) +
c)Synthesis of N(2-sulfhydryl 4-bromo phenyl) N'-{2-bromo phenyl) urea
Zo A solution of (3-bromo b-amino phenyl) disulfide(201 mg, .5 mmol) in DMF
was treated with 2-bromo phenyl isocyanate( 1 mmol) at 80 °C overnight.
The reaction
mixture was diluted with methylene chloride and a solid was precipitated out
with
hexanes. The solution was dissolved in MeOH and treated with NaBH4. After gas
evolution ceased the reaction mixture was carefully acidified with 1N HCI and
the
25 resulting solid was filtered(52 mg, 13%). EI-MS m/z 399 (M-H)
E_~Dle 56
Synthesis of N-l2-h day 4-vitro phenyl) N'-(2-iodo~henyll urea
The urea was synthesized by the treatment of 2-iodo benzoic acid(5 g, 20 mmoi)
3o with diphenyl phosphoryI azide( 1 equiv.) and triethyl amine ( 1 equiv.) in
DMF at 80
°C after gas evolution ceased the 5-vitro 2-amino phenol (3 g, I
equiv.) was added. 'The
reaction was heated overnight at 80°C. The reaction mixture was
purified by filtering
through a plug of silica with methylene chloride. The desired product was then
precipitated out with hexane. Filtering afforded the desired compound(1.08 g,
13%).
35 EI-MS m/z 398(M-H)
CA 02432662 2003-06-09
WO 97129743 PCT/US96113632
Example 57
Synthesis of N-(2-hvdroxy 4-nitro phenyl) N'-(2-bromo phenyl) thiourea
The thiourea was synthesized by treatment of the 2-tert-butyldimethylsilyloxy
4-
nitro phenyl thioisocyanate(see example 9a , 3.73 mmol) with 2-bromo aniline
in
toluene at 88°C over 36 h. The solution was concentrated and the
residue was purified
by flash chromatography(EtOAc/Hexanes). The fraction slightly lower rf than
starting
material contained the desired compound. This fraction was concentrated and
then
treated with triethyl amine hydrofluoride in acetonitrile for 15 minutes at 23
°C. The
reaction mixture was then concentrated in vacuo and the residue was purified
by flash
1o chromatography(ethyl actate/hexanes) to give N-(2-hydroxy 4-nitro phenyl)
N'-(2-
bromo phenyl) thiourea(52 mg, 4%;1. EI-MS m/z 369(M+H) +
Example 58
~nthesis of N-(2-vhenvlsulfamido) 4-cyanop~en_3r1 N'-(2-bromo phenyll urea
t 5 a)Synthesis of 3-(phenylsulfamido) benzonitrile
The of 3-(phenylsulfamido) benzonitrile was synthesized from the 3-cyano
aniline (23.9 g, .2 mol) by Method C. It was purified by recrystalization from
EtOH(15.8 g, 31%).1H NMR (CDC13): d 7.95(s, 1H), 7.84 (d, 2H, J = 8.0 Hz),
7.59 (t,
1H, J = 8.0 Hz), 7.45 (m, 2H), 7.35 (m, 4H).
2o b)Synthesis of 3-(phenylsulfamido) 4-nitro benzonitrile
The 3-(phenylsulfamido) benzonitrile(10 g, 39 mmol) was dissolved in acetic
anhydride and treated with concentrated nitric acid dropwise at room
temperature until
all the starting material had been consumed. The reaction mixture was then
quenched by
carefully pouring it into sodium bicarbonate and left to sit until all gas
evolution had
25 subsided. It was then partitioned between methylene chloride and water. The
organic
layer was dried over sodium sulfate and filtered. The reaction mixture was
concentrated in vacuo, absorbed onto silica gel and purified by column
chromatography(methylene chloride/hexane) to afford the title compound ( 1.7g,
15%).
EI-MS mlz 302(M+H) +
30 c)Synthesis of 3-(phenylsulfamido) 4-amino benzonitrile
The 3-(phenylsulfamido) 4-nitro benzonitrile(1.5 g, 4.9 rnmol) was treated
with
tin chloride dihydrate in EtOH at 80 °C for 12h. It was then
concentrated and flushed
through a plug of silica gel with 5% methanol/methylene chloride. The
filterate was
absorbed onto silica gel and purified by flash chromatography(ethyl
acetatelhexane) to
35 afford the title compound (0.9 g, 60%). EI-MS m/z 274 (M+H) +
d)Synthesis of N-(2-phenylsulfamido) 4-cyanophenyl N'-(2-bromo phenyl) urea
-65-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96113632
The urea was synthesized from 2-(phenylsulfamido) 4-amino benzonitrile(77
mg, 0.28 mmol) and 2-bromo phenyl isocyanate by general Method C. It was
purified
by column chromatography(ethyl acetate/hexane) to afford the title compound
(30 mg,
22%). EI-MS m/z 469(M-H)
Example 59
Synthesis of N-(2-(phenyl sulfamidol phenvll N'-(2-bromophenvl) urea
a)Synthesis of 2-( phenyl sulfamido) aniline
The sulfonamide was synthesized from phenyl sulfonyl chloride(0.01 mmol) and
to o-phenylene diamine( 1.08 g, 0.01 mmol) by general Method C. it was
purified by
recrystallization from EtOH(1.0 g, 40%).EI-MS m/z 249(M+H) +
b)Synthesis of N-(2-(phenyl suifamido) phenyl) N'-(2-bromo phenyl) urea
The urea was synthesized Z-(phenyl sulfamido) aniline( 1 mmol)
and 2-bromo phenyl isocyanate by general Method B. It was purified by dilution
with
15 methylene chloride and precipitation with hexane. Filtering afforded the
desired
compound(0.234 g, 52%).EI-MS m/z 446(M+H) +
Example 60
Synthesis of N-(2-( styrvi sulfamido) phenvll N'-(2-bromo phenyll urea
20 a)Synthesis of 2-( styryl sulfamido) aniline
The sulfonamide was synthesized from styryl sulfonyl chloride(0.01 mol) and o-
phenylene diamine(0.01 mol) by general Method C. It was purified by
recrystallization
from EtOH( 1.2 g, 60%)EI-MS m/z 199(M+H) +
b)Synthesis of N-(2-(styryl sulfamido) phenyl) N'-(2-bromo phenyl) urea
25 The urea was synthesized from 2-(styryl sulfamido) aniline( 1 mmol) and 2-
bromo phenyl isocyanate( 1 mmol) by general Method B. It was purified by
dilution
with methylene chloride and precipitation with hexane. Filtering afforded the
desired
compound(0.309 g, 65%). EI-MS m/z 472(M+H} +
30 Example 61
Synthesis of 2-ff3.4 dimethoxyphenvl)sulfonyl arninol nhenvll N'-(2-bromo
phen~
urea
a)Synthesis of 2-[(3,4-dimethoxyphenyl)sulfonyl amino]phenyl aniline
The sulfonamide was synthesized from 3,4-dimethoxy phenyl sulfonyl
35 chloride(0.01 mol) and o-phenylene diamine by general Method C. It was
purifed by
recrystallization from EtOH(0.65 g, 21%). EI-MS m/z 309(M+H) +
-66-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
b)Synthesis of 2-((3,4-dimethoxyphenyl)sulfonylamino] phenyl) N'-(2-bromo
phenyl)
urea
The urea was synthesized from 2-[(3,4-dimethoxyphenyl)sulfonyl amino]phenyl
aniline( 1 mmol) and 2-bromo phenyl isocyanate by general Method B. It was
purified
by dilution with methylene chloride and precipitation with hexane. Filtering
afforded
the desired compound(0.062 g, 12%).EI-MS m/z 504(M-H) -
Exam~e 62
~nthesis of N-f2-f(4-acetamidophenvllsulfonvlam'no7 phenyl) N'-(2-bromo
phe~rl)
urea
a)Synthesis of 2-[(4-acetamidophenyl)sulfonylamino]phenyl aniline
The sulfonamide was synthesized from 4-acetamidophenyl sulfonyl
chloride(0.01 mol) and o-phenylene diamine(0.01 mol) by general Method C. It
was
purified by recrystallization from EtOH( 1.27 g,40%)EI-MS m/z 304(M-H) .
b)Synthesis of N-(2-[(4-acetamidophenyisulfonyl)amino] phenyl) N'-(2-bromo
phenyl)
urea
The urea was synthesized from 2-[(4-acetamidophenyl)sulfonylanuno]phenyl
aniline( 1 mmol) and 2-bromo phenyl isocyanate(1 mmol) by general Method B. It
was
purified by dilution with methylene chloride and precipitation with hexane.
Filtering
afforded the desired compound(0.12 g, 24%). EI-MS m/z 501 (M-H) -
Example 63
S~rnthesis of N-f2-(2-thio~hene sulfamido phenvll N'-(2-bromo phenyll urea
a)Synthesis of 2-(2-thiophene sulfamido) aniline
The sulfonamide was synthesized from 2-thiophene sulfonyi chloride(0.01 mol)
and o-phenylene diamine(0.01 mol) by general Method C. It was purified by
recrystallization from EtOH(0.77 g, 30%). EI-MS m/z 255 (M+H)+
b)Synthesis of N-{2-(2-thiophene sulfonyl amino phenyl) N'-(2-bromo phenyl)
urea
The urea was synthesized from 2-( 2-thiophene sulfonyl amino) aniline( 1
mmol) and 2-bromo phenyl isocyanate( 1 mmol) by general Method B. It was
purified
by dilution with methylene chloride and precipitation with hexane. Filtering
afforded
the desired compound(0.29 g, 64%). EI-MS m/z 450(M-H)
Exam,~le 64
Synthesis of N-(2-(3-told sulfon~amino ~henyll N'-l2-bromo phenyll urea
a)Synthesis of 2-( 3-tolyl sulfonyl amino) aniline
-67-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13532
The sulfonamide was synthesized from 3-tolyl suifonyl chloride(O.OI mol) and
o-phenylene diamine(0.01 mol) by general Method C. It was purified by
recrystallization from EtOH(0.73g, 28%}.EI-MS m/z 263 (M+H)+
b)Synthesis of N-{2-((3-tolyl sulfonyl amino) phenyl) N'-(2-bromo phenyl) urea
The urea was synthesized from 2-{3-tolyl sulfonyl amino) aniline( 1 mmol) and
2-bromo phenyl isocyanate( 1 mmol) by general Method B. It was purified by
dilution
with methylene chloride and precipitation with hexanes. It was recrysallized
two times
with EtOH(25 mg, 5%). EI-MS m/z 458(M-H)
1o Example 65
S~rnthesis of N-l2-(8-auinolinyl sulfonXl aminol phenyl) N'-(2-brQ,mo hen )
urea
a)Synthesis of 2-(8-quinoiinyl sulfonyl amino) aniline
The sulfonamide was synthesized from 8-quinolinyl sulfonyl chloride(0.01 mol)
and o-phenylene diamine(0.01 mol;1 by general Method C. It was purified by
recrystallization from EtOH(0.82 g, 27%).EI-MS mlz 300 (M+H) +
b)Synthesis of N-(2-( (8-quinolinyl) sulfonyI amino) phenyl) N'-{2-bromo
phenyl) urea
The urea was synthesized from 2-((8-quinolinyl) sulfonyl amino) aniline( 1
mmol) and 2-bromo phenyl isocyanate( 1 mmol) by general Method B. It was
purified
by dilution with methylene chloride and precipitation with hexane. Filtering
afforded
2o the desired compound(0.23 g, 46%).EI-MS m/z 495(M-H)
Example 66
S, nY these of N-(2-( benzyl sulfon'rl aminol phen Il N'- 2-bromo phenyll urea
a)Synthesis of 2-(benzyl sulfonyl amino) aniline
The sulfonamide was synthesized from benzyl sulfonyl chloride(0.01 moi) and
o-phenylene diamine(0.01 mol) by general Method C. It was purified by
recrystallization from EtOH(0.87g, 33%). EI-MS m/z 263(M+H)+.
b)Synthesis of N-(2-( benzyl sulfonyl amino) phenyl) N'-(2-bromo phenyl) urea
The urea was synthesized from 2-{ benzyl sulfonyl amino} aniline( 1 mmol)
3o and 2-bromo phenyl isocyanate(lmmol) by general Method B. It was purified
by
dilution with methylene chloride and precipitation with hexane. Filtering
afforded the
desired compound(0.11 g, 23%). EI-MS m!z 460 (M+H)+
Example 67
Svnthes~s o~jV-(2-h~droxx-4-azidoghenxll-N'-~2-methoxyphen_vllurea
a)Synthesis of N-(2-hydroxy-4-aminophenyl)-N'-(2-methoxyphenyl)urea
-68-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
To a solution of N-(2-hydroxy-4-nitro phenyl)-N'-(2-methoxyphenyl)urea( 1.0 g,
example IS) in methanol, palladium (on activated carbon, 10%) (100 mg) was
added.
Then the reaction mixture was hydrogenated under a hydrogen balloon for 18
hours.
The solid was filtered off by celite and washed three times by methanol. The
filtrate was
concentrated under reduced pressure to give amine compound (0.8 g, 89%). EI-MS
m/z
2?4 (M+H)+
b)Synthesis of N-(2-hydroxy-4-azidophenyl)-N'-(2-methoxyphenyl)urea
The N-{2-hydroxy-4-aminophenyl)-N'-(2-methoxyphenyl)urea (300 mg, 1. I7
mmol) was added to HCI/H20 ( 1.17 mLJ2.34 mL), cooled to 0°C. Sodium
nitrite (80.7
mg, 1.17 mmol) was added to the reaction mixture. The reaction mixture was
stirred at
0°C for 30 minutes. The sodium azide (76 mg, 1.17 mmol) was added to
reaction
mixture and it was warmed to room temperature. The reaction mixture was
stirred at
room temperature for 18 hours. Then it was extracted with three times by ethyl
acetate.
The organic extracts were combined, dried over MgS04, filtered and
concentrated
under reduced pressure and chromatography of the resulting solid on silica gei
(hexane
ethyl acetate; 5:1) gave product (125 mg, 38%). EI-MS m/z 300 (M+H)+
Example 68
Preparation of N-f2-hvdroxy-5-c~anophen 1y_ 1-N'-'~2-bromophen 11 urea
a)Preparation of 2-amino-4-cyanophenol
To a solution of 2-nitro-4-cyanophenol(lOg, 61mmo1) in methanol(250mL) was
added 10% PdIC ( l g). The mixture was flushed with argon, then hydrogen was
bubbled through the solution for 10 min. and a hydrogen atmosphere was
maintained at
balloon pressure overnight. The mixture was filtered through celite and the
celite was
washed with methanol. The solvent was evaporated and chromatography of the
resulting solid on silica gel (5%MeOH/ CH2C12) gave the desired product(8.0 g,
97%).
1H NMR (CD30D): d 6.96 (d, 1H), 6.90 (dd, 1H), 6.77 (d, 1H).
b)Preparation of N-[2-hydroxy-5-cyanophenyl]-N'-[2-bromophenyl] urea .
N-[2-hydroxy-5-cyanophenyl]-N'-[2-bromophenyl] urea was prepared from 2-
amino-4-cyanophenol(268mg, 2.00 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
hexane( 1/20) and filtering. (540mg,81%). 1H NMR (CD30D): d 8.10 (d, 1H), 7.87
(d,
1H}, 7.43 (d, 1H), 7.20 (t, 1H), 7.09 (d, 1H), 6.86 (t, 1H), 6.77 (d, 1H).
Example 69
Pre,~aration of N-f 2-hydroxy-3-fluoro~Yll-N'-~?-bromophen 11 urea
-69-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
a)Preparation of 2-amino-3-fluorophenol
To a solution of 2-nitro-3-fluorophenol( 1 g, 6.4mmol) in methanol(250mL) was
added 10% Pd/C (1g). The mixture was flushed with argon, then hydrogen was
bubbled through the solution for 10 min. and a hydrogen atmosphere was
maintained at
balloon pressure overnight. The mixture was filtered through celite and the
celite was
washed with methanol. The solvent was evaporated and chromatography of the
resulting solid on silica gel (5%MeOH! CH2C12) gave the desired product{650
mg, 80.2
%). IH NMR (CD30D): d 6.41-6.1? (m, 3H).
b)Preparation of N-(2-hydroxy-3-fluorophenyl]-N°-(2-bromophenyl] urea
to N-[2-Hydroxy-3-fluorophenyl]-N'-[2-bromo phenyl] urea was prepared from 2-
amino-3-fluorophenol (254mg, 2.00 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
hexane( 1l20) and filtering. {500 mg, 77%). 1H NMR (CD30D): d 8.05 (d, 1H),
7.50
(d, 1H), 7.26 (t, 1H), 7.18 (d, 1H), 6.92 (t, 1H), 6.86-6.68 (m, 2H).
Exam~nle 7-0
Preparation of N-2-f I-hydroxyfluorenel-N'-f2-bromophen 1Y-1 urea
a)Preparation of 2-amino-1-hydroxyfluorene
To a solution of I-hydroxy-2-nitrofluorene(250 mg, 1.23mmol) in
2o methanol(250mL) was added 10% Pd/C ( l g). The mixture was flushed with
argon,
then hydrogen was bubbled through the solution for 10 min. and a hydrogen
atmosphere
was maintained at balloon pressure overnight. The mixture was filtered through
celite
and the celite was washed with methanol. The solvent was evaporated and
chromatography of the resulting solid on silica gel (5%MeOH/ CH2C12) gave the
desired product(171 mg, 81.2 96). 1H NIviR (CD30D): d 7.60 (d, 1H), 7.47 (d,
1H),
7.28 (t, 1H), 7.18 (m, 2H), 6.82 (d, 1H), 3.76 (s, 2H).
b)Preparation of N-2-[1-hydroxyfluorene]-N'-[2-bromophenyl] urea
N-2-[1-hydroxyfluorene]-N'-[2-bromo phenyl] urea was prepared from 2- .
amino-1- hydroxyfluorene ( 170mg, 0.86 mmol) according to the procedure in
General
3o Method B. The product was purified by chromatography of the resulting solid
on silica
gel (30%EtOAc/ Hexane) to give the desired product (300mg, 84.5%). 1H NMR
(CD3C1): d 8.04 (d, 1H), 7.66 (d, 1H), 7.49 (t, 2H), 7.35-7.20 (m, 4H), 7.09
(d, 1H),
6.90 (t, 1H).
Example 71
-70-
CA 02432662 2003-06-09
R'O 97!29743 PCTIUS96/13632
Preparation of N-3-f2-hydroxy-9.10-anthraquinonvll-N'-f2-bromophenyl] urea N-3-
[2-Hydroxy-9.10-anihraquinonyl]-N'-[2-bromophenyl] urea was prepared from 2-
hydroxy-3-aminoanthraquinone(480mg, 2.00 mmol) according to the procedure in
General Method B. The product was purified by precipitation from methylene
chloride!
hexane(1120) and filtering. (610mg, 70%). 1H NMR (CD30D): d 8.93 (s,lH), 8.12
(m, 2H), 8.02 (d> 1H), 7.77 (m, ZH), 7.61 (d, 1H), 7.52 (s, 1H), 7.38 (t, 1H),
7.05 (t,
1 H).
Example 72
t0 Preparation of N-f2-hydroxv-3-fluoro-5-bromophenyll-N'-f2-bromophenyl_]
urea
a)Preparation of 2-amino-6-fluoro-4-bromophenol
A mixture of 4-bromo-2-fluoro 6-nitrophenol( 1 g, 4.2mmo1) and tin (II)
chloride
(4.78 g, 21.2mmol) in ethanol(SOmL) was heated at 80°C under argon.
After 2 hours,
the starting material had disappeared and the solution was allowed to cool
down and
then poured into ice. The pH was made slightly basic (pH7-8), by addition of
solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired
product(710
mg, 82 %). 1H NMR (CD30D}: d f.51-6.40 (m, 2H).
2o b)Preparation of N-[2-hydroxy-3-fluoro-5-bromophenyl]-N'-[2-bromophenyl]
urea
N-[2-hydroxy-3-fluoro-5-bromophenyl]-N'-[2-bromophenyl] urea was prepared
from 2-amino-6-fluoro-4-bromophenol (254mg, 2.00 mmol) according to the
procedure
in General Method B. The product was purified by precipitation from methylene
chloride/ hexane(1120} and filtering. (500 mg, 77%). 1H NMR (CD30D): d 7.98
(s,
1H), 7.91 (d, 1H), 7.60 (d, 1H), 7.:33 (t, 1H), 7.00 (t, 1H), 6.94 (d, 1H).
Example 73
PreRa~ation of N-f2-h~droxv-3-chlorophenvll-N'-f2-bromophen !v_! urea
a)Preparation of 2-amino-3-chlorophenol
A mixture of 3-chloro-2-nitrophenol(250 mg, l.4mmoi) and tin (I17 chloride
( 1.2 g, 5.3mmol) in ethanol(SOmL) was heated at 80~C under argon. After 2
hours, the
starting material has disappeared and the solution was allowed to cool down
and then
poured into ice. The pH was made slightly basic (pH7-8), by addition of solid
NaOH,
before being extracted with ethyl acetate. The organic phase was washed with
brine,
dried over MgS04 and filtered. The solvent was evaporated and chromatography
of the
-?1
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
resulting solid on silica gel (4%MeOHI CH2CI2) gave the desired product(143
mg, 69
%). IH NMR (CD30D): d 6.75 (t"IH), 6.70 (d, 1H), 6.65 (d, 1H).
b)Preparation of N-[2-hydroxy-3-chlorophenyl]-N'-[2-bromophenyl] urea
N-[2-hydroxy-3-chlorophenyl]-N'-[2-bromophenyl] urea was prepared from 2-
amino-3-chlorophenol ( 143mg, 1.00 mmol) according to the procedure in General
Method B. The product was purified by chromatography of the resulting solid on
silica
gel (30%EtOAc/ Hexane) to give the desired product(195mg, 57%). 1H NMR
(CD30D): d 7.81 (d, 1H), 7.68 (d, 1H), 7.47 (d, 1H), 7.20 (t, IH), 6.90 (m,
2H), 6.70
(t. 1H).
lo
Example 74
Preparation of N-t2-h~~3-trifluoromethyiphenyll-N'-f2-bromo henxll
ureaa)Preparation of 2-vitro-6-trifluoromethylphenol
2-trifluoromethylphenol (3.40g, lB.Smmo1) was dissolved in methylene
15 chloride(40mL) followed by the addition of sodium nitrate ( I .73g,
20.4mmol). The
addition of sulfuric acid (23 mIJ 3M) was then made, followed by addition of a
catalytic amount of sodium nitrite. The mixture was allowed to stir. After 24
hours, the
reaction mixture was diluted with methylene chloride and extracted with water.
The
organic layer was dried over MgS04 and filtered. The solvent was evaporated
and
20 chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave
the
desired product( 1.84 g, 47 %). IH NMR (CD3COCD3): d 8.35 (d,lH), 7.95 (d,
IH),
7.13 (t, IH).
b)Preparation of 2-amino-6- trifluoromethylphenol
A mixture of 6-trifluoromethyl-2-nitrophenol( 1.84 g, 8.67mmol) and tin (In
25 chloride (6.0 g, 26.2 mmol} in ethanol( 1 SOmL) was heated at 80°C
under argon. After
2 hours, the starting material has disappeared and the solution was allowed to
cool down
and then poured into ice. The pH was made slightly basic (pH7-8), by addition
of solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
30 of the resulting solid on silica gel (4~vMeOH/ CH2Cl2) gave the desired
product( 1.35 g,
88 %). 1H NMR (CD30D): d 6.93 (d, 1H), 6.82 (t, 1H), 6.78 (d, 1H).
c)Preparation of N-[2-hydroxy-3- trifluoromethylphenyl]-N'-[2-bromophenyl]
urea
N-[2-hydroxy-3-trifluoromethylphenyl]-N'-[2-bromophenyl] urea was prepared
from 2-amino-6-trifluoromethylphenol (280mg, 1.60 mmol) according to the
procedure
35 in General Method B. The product was purified by precipitation from
methylene
-72-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
chloride/ hexane( I/20) and filtering. (390mg, 65%). /H NMR {CD30D): d 7.99
(d,
1H), 7.60 (d, 1H), 7.58 (d, 1H), 7.34 (t, 1H), 7.30 (d, 1H), 7.00 (t, 1H),
6.96 (d, 1H).
Example 75
Preparation of N-f3.4 diphenyl-2-h'rdroxyphenyll-N'-[2-bromophen~~llurea
N-[3,4 diphenyi-2-hydroxyphenyl]-N'-[2-bromophenyl] urea was prepared from
2-amino-5,6 diphenylphenol (50mg, 0.19 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane(II20) and filtering (6lmg, 69%). /H NMR (CD30D): d 7.97 (d, 1H), 7.66
(d,
1H), 7.58 (d, 1H), 7.31 (t, 1H), 7.25-7.00 (m, 11H), 6.91 (d, 1H).
Exam , Ip a 76
Preparation of N-f2-hvdroxv-3-~Ivcinemethvlestercarbon~~yll-N' j2-bromonhenvll
urea
N-[2-hydroxy-3-glycinemethylestercarbonylphenyl]-N'-[2-bromopheny1] urea
was prepared from 6-glycinemethylestercarbonyl-2-aminophenol (50mg, 0.22
mmol),
purchased from the University of New Hampshire, according to the procedure in
General Method B. The product was purified by precipitation from methylene
chloride/
hexane(1/20) and filtering (b5mg, 69%). 1H NMR (CD30D): d 8.14 (d, 1H), 7.96
(d,
2o IH). 7.49 (d, 1H), 7.24 (t, 2H), 6.89 (dd, 1H), 6.81 (t, 1H), 4.10 (s,2H),
3.74 (s,3H).
Exam 1p a ?7
Preparation of N-f2-hydroxv-3-glvcinecarbon r~iphen~rl]-N'l2-bromopheny[Lurea
N-[2-
Hydroxy-3-glycinecarbonylphenyi]-N'-[2-bromophenyl] urea was prepared from N-
[2-
hydroxy-3-glycinemethylestercarbonylphenyl]-N'-[2-bromophenyl] urea(SOmg, 0.12
mmol) by stirring in a 3/1 ratio of methanol/water ( 10 mL). Addition of 1
equiv. of
lithium hydroxide was added and stirring continued until the starting material
had
disappeared. (45mg, 92%). The product was purified by chromatography of the
resulting solid on silica gel (9/1/0.1 CH2C12/ MeOH/ AcOH) to give the desired
product(195mg, 57%). 1H NMR (CD30D): d 8.14 (d, IH), 7.92 (d, 1H), 7.60 (d,
1H),
7.46 (d, 1H), 7.34 (t, 1H), 7.04 (t, 1H), 6.82 (t, IH), 3.96 {2H).
Example 78
Preparation of N-f2-hydroxv-3.5-dichlorophen~]-N'-(2-bromopheny, urea
a)Preparation of 2-amino-4,6-dichlorophenol
-73-
CA 02432662 2003-06-09
WO 97/29743 PCTILTS96I13632
A mixture of 4,6-dichloro-2-nitrophenol( 1 g, 4.8mmo1) and tin (II) chloride
(3.2
g, 14.4mmol) in ethanol(SOmL) was heated at 80° C under argon. After 2
hours, the
starting material had disappeared and the solution was allowed to cool down
and then
poured into ice. The pH was made slightly basic (pH7-8), by addition of solid
NaOH.
before being extracted with ethyl acetate. The organic phase was washed with
brine,
dried over MgS04 and filtered. The solvent was evaporated and chromatography
of the
resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the desired product(685
mg, 80
%). 1H NMR (CD30D): d 6.75 (s,lH), 6.61 (s, 1H).
b)Preparation of N-[2-hydroxy-3,5-dichlorophenyl]-N'-[2-bromophenyl] urea
to N-[2-Hydroxy-3,5-dichlorophenyl]-N'-[2-bromophenyl] urea was prepared from
2-amino-4,6-dichlorophenol (143mg, 1.00 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane( 1/20) and filtering. (660mg, 88%). 1H NMR (CD30D): d 7.96 (s, 1H),
7.89
(d, 1H), 7.60 (d, 1H), 7.35 (t, 1H), 7.00 (t, 1H), 6.95 (dd, 1H).
I5
Example 79
Prep anon of N-f2-hvdroxv-3-nitro~henjrll-N'-f2-bromonhen 1~1 ureaN-[2-Hydroxy-
3-
nitrophenyl]-N'-[2-bromophenyl] urea was prepared from 2-hydroxy-3-
nitroaniline
{ 1.25g, 8.1 mmol) according to the procedure in General Method B. The product
was
20 purified by precipitation from methylene chloride/ hexane( 1120) and
filtering. (2.4g,
84%). 1H NMR (CD30D): d 8.45 (d, 1H), 7.94 (d, 1H), 7.78 (d, 1H), 7.60 (d,
1H),
7.35 (t, 1H), 7.01 (m, 2H).
Example 80
25 Preparation of N-f 2-h r~droxv-4-nanhthalenesulfonic acidl-N'-f 2-
bromonheavll urea
N-[2-hydroxy-4-naphthalenesulfonic acid]-N'-[2-bromophenyl] urea was
prepared from 1-amino-2-hydroxy-4-naphthalensulfonic acid (0.48g, 2.0 mmol)
according to the procedure in General Method B and the addition of 1mL of
triethylamine The product was purified by precipitation from methylene
chloride/
30 hexane( 1/20) and filtering. (690 mg, 79%). 1 H NMR (CD30D): d 8.14 (s,
1H), 8.04
(d, 1H), 7.98 (m, 2H), 7.61-7.55 (m, 3H), 7.43 (t, 1H), 6.98 (t, 1H).
Exa~n~le 81
Preparation of N-f2-hvdroxy 5-na~hthalenesulfonic acidl-N'-f2-brQmophenyll
urea
35 N-3-[2-hydroxy-5-naphthalensulfonic acid]-N'-[2-bromophenyl] urea was
prepared from 2-amino-3-hydroxy-6-naphthalensulfonic acid (0.48g, 2.0 mmol)
-74-
CA 02432662 2003-06-09
WO 97!29743 PCTIUS96/i3632
according to the procedure in General Method B and the addition of 1mL of
triethylamine The product was purified by precipitation from methylene
chloride/
hexane(1/20) and filtering. (715 mg, 82%). 1H NMR (CD30D): d 8.09 (s, 1H),
7.96
(d, 1H), 7.65-7.48 (m, 3H), 7.36 (t, 1H), 7.25 (s, 1H), 7.04 (m, 2H).
Example 82
Preparation of N-f 2-hvdroxy-3.4-diehlorophen~]-N'-f 2-bromophe X11 urea
a)Preparation of 2-vitro-5,6 dichlorophenol
2,3-dichlorophenol (3.26g, ZOmmoi) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate ( 1.88g, 22mmol).
The
addition of sulfuric acid (20mL/ 3M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with methylene chloride and extracted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the
desired product(1.8 g, 44 %). 1H NMR (CD3COCD3): d 8.04 (d,lH), 7.15 (d, 1H).
b)Preparation of 2-amino-5,6 dichlarophenol
A mixture of 5,6-dichloro-2-nitrophenol( 1.8 g, 8.7mmol) and tin (II) chloride
(5.8 g, 26.1mmol) in ethanol(50mL) was heated at 80_C under argon. After 2
hours,
the starting material had disappeared and the solution was allowed to cool
down and
then poured into ice. The pH was made slightly basic (pH7-8), by addition of
solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the desired
product( 1.4
mg, 90 %). 1H NMR (CD30D): d 6.71 {d, 1H), 6.45 (d, 1H).
c)Preparation of N-[2-hydroxy-3,4-dichlorophenyi]-N'-[2-bromophenyi] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-bromophenyl] urea was prepared from
2-amino-5,6-dichlorophenol (350mg, 2.00 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1/20) and filtering. (670mg, 89%). 1H NMR {CD30D): d 7.90 (d, 1H), 7.85
(d, 1H), 7.59 (d, 1H), 7.3I (t, 1H), 6.99 (t, 1H), 6.96 (d, (1H).
Example 83
Preparation of N12-h d~--rox_y-3-c~anopheny_1]-N'-f2-bromophen 11 urea
a)Preparation of 2-vitro-6-cyanophenol
_75_
CA 02432662 2003-06-09
WO 97/29743 PCT/I1S96/13632
2-cyanophenol (2.38g, 20mmol) was dissolved in methylene chloride(40mL)
followed by the addition of sodium nitrate ( 1.88g, 22mmol}. The addition of
sulfuric
acid (20mL/ 3M) was then made, followed by addition of a catalytic amount of
sodium
nitrite. The mixture was allowed to stir. After 24 hours, the reaction mixture
was
diluted with methylene chloride and extracted with water. The organic layer
was dried
over MgS04 and filtered. The solvent was evaporated and chromatography of the
resulting solid on silica gel (4%MeOHI CH2Cl2) gave the desired product( 1.4
g, 42 %).
1H NMR (CD3COCD3): d 8.47 (d,lH), 8.15 (d, 1H), 7.30 {t, 1H).
b)Preparation of 2-amino-6-cyanophenol
1o A mixture of 6-cyano-2-nitrophenol(600 mg, l.Ommo1) and tin (II) chloride
(3.2
g, 14.4mmol) in acetic acid(SOmL) was heated at 80_C under argon. After 2
hours, the
starting material has disappeared and the solution was allowed to cool down
and then
poured into ice. The pH was made slightly basic (pH7-8), by addition of solid
NaOH,
before being extracted with ethyl acetate. The organic phase was washed with
brine,
dried over MgS04 and filtered. The solvent was evaporated and chromatography
of the
resulting solid on silica gel (4%MeOHI CH2C12) gave the desired product(365
mg, 75
%). 1H NMR (CD30D): d 6.92 (d, IH}, 6.85-6.69 (m,2H).
c)Preparation of N-[2-hydroxy-3-cyanophenyl]-N'-[2-bromophenylj urea
N-[2-Hydroxy-3-cyanophenyi]-N'-[2-bromophenyl] urea was prepared from 2-
2o amino-6-cyanophenol ( I34mg, 1.00 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1J20) and filtering. (260mg, 78%). IH NMR (CD30D): d 7.98 (d, 1H), 7.74
(d, 1H), 7.57 (d, IH), 7.30 (t, 1H), 7.22 (d, 1H), 6.98 (t, 1H), 6.94 (t,
(1H).
Exam 1p a 84_
Preparation of N-f2-h dy roxy-4-cyanophenyrll-N'-f2-bromophenyl] urea
a)Preparation of 2-nitro-5-cyanophenol
3-cyanophenol (2.388, 20mmo1) was dissolved in methylene chloride(40mL)
followed by the addition of sodium nitrate ( 1.88g, 22mmo1). The addition of
sulfuric
acid (20mL 3M) was then made, followed by addition of a catalytic amount of
sodium
nitrite. The mixture was allowed to stir. After 24 hours, the reaction mixture
was
diluted with methyiene chloride and extracted with water. The organic layer
was dried
over MgS04 and filtered. The solvent was evaporated and chromatography of the
resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired product(910
mg, 28
%}. 1H NMR (CD3COCD3): d 8.30 (d,lH), 7.67 (s,lH), 7.49 (d, IH).
b)Preparation of 2-amino-5-cyanophenol
_76_
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
A mixture of 5-cyano-2-nitrophenol(250 mg, l.Smmo1) and tin (II) chloride {3.2
g, 14.4mmol) in ethanol(SOmL) was heated at 80_C under argon. After 2 hours,
the
starting material has disappeared and the solution was allowed to cool down
and then
poured into ice. The pH was made slightly basic (pH7-8), by addition of solid
NaOH,
before being extracted with ethyl acetate. The organic phase was washed with
brine,
dried over MgS04 and filtered. The solvent was evaporated and chromatography
of the
resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired product( 175
mg, 86
%). 1H NMR (CD30D): d 7.00 (d, 1H), 6.88 (s,lH), 6.69 (d, 1H).
c)Preparation of N-[2-hydroxy-4-cyanophenyl]-N'-[2-bromophenyl] urea
t0 N-[2-Hydroxy-4-cyanophenyl]-N'-[2-bromophenylJ urea was prepared from 2-
amino-5-cyanophenol ( 170mg, 1.27 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1/20) and filtering (310mg, 74%). 1H NMR (CD30D): d 8.25 (d, 1H), 7.91
(d,
1H), 7.59 (d, 1H), 7.33 (t, 1H), 7.17 (d, 1H), 7.07 (s, 1H), 7.01 (t, (1H).
Example 85
Preparation of N-(2-h~droxy-4-c~phenyll N'-L4-methoxyPhen, 1i urea
N-[2-Hydroxy-4-cyanophenyl]-N'-(4-methoxyphenyl] urea was prepared from
2-amino-5-cyanophenol (60mg, 0.45 mmol) according to the procedure in General
2o Method B. The product was purified by precipitation from methyiene
chloride/
hexane(1/20) and filtering. ( 110mg,86%). 1H NMR (CD30D): d 8.23 (d, 1H), 7.61-
7.51 (m, 2H), 7.32 (d, 1H), 7.20 (d, 1H), 7.15 (d, 1H), 7.03 (s, 1H).
Example 86
Preparation of N-f2-hydroxy-4-c~phen~]-N'-f2-phen l~nhenyll urea N-[2-
Hydroxy-4-cyanophenyl]-N'-(2-phenylphenyl] urea was prepared from 2-amino-5-
cyanophenol ( 170 mg, 1.27 mmol) according to the procedure in General Method
B.
The product was purified by precipitation from methylene chloridel
hexane(1/20) and
filtering. (150mg, 85%). 1H NMR (CD3OD): d 8.20 (d, 1H), 7.73 (d, 1H), 7.51-
7.20
(m, 8H), 7.13 (d, 1 H), 7.01 (s, ( 1 H).
xa 7
Preparation of N-f2-hvdroxv-4-cvanoohenvll-N'-f2-methvlt~l envll urea
N-[2-Hydroxy-4-cyanophenyl]-N'-(2-methylphenyl] urea was prepared from 2-
amino-5-cyanophenol (60mg, 0.45 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
_77_
CA 02432662 2003-06-09
WO 97/29743 PCT/US96113632
hexane( 1/20) and filtering. (90mg, 75%). 1H NMR (CD30D): d 8.25 (d, IH), 7.59
(d,
1H). 7.26-7.00 (m, SH), 2.30 (s, 3H).
Example 88
Preparation of N-f2-hYdroxy-4-c~anophenyll-N'-f2-trifluoromethyl~henvll urea
N-{2-Hydroxy-4-cyanophenylJ-N'-[2-trifluoromethylphenyl] urea was prepared
from 2-amino-5-cyanophenol (60mg, 0.45 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane( 1/20) and filtering. ( 1 lOmg, 76%). 1 H NMR (CD30D): d 8.25 (d, 1H),
7.81
to (d, 1H), 7.68 (d, 1H), 7.6I (t, 1H), 7.32 (t, 1H), 7.15 (dd, 1H), 7.09 (s,
(1H).
Exam~1~89
Preparation of N-f2-hvdroxv-4-cvanonhenvll-N'-f3-trifluoromethvlQhenvll urea
N-[2-hydroxy-4-cyanophenyl)-N'-[3-trifluoromethyiphenyl] urea was prepared
is from 2-amino-5-cyanophenol (60mg, 0.45 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1/20) and filtering. (114mg, 79%). 1H NMR (CD30D): d 8.30 (d, 1H), 7.92
(s, 1H), 7.60 (d, 1H), 7.47 (t, 1H), 7.29 (d, IH), 7.18 (dd, IH), 7.06 (s,
1H).
2o Example 90
Preparation of N-f2-hydroxy-4-c,~phenvll-N'-f4-trifluoromethyl~nyll urea
N-[2-Hydroxy-4-cyanophenylJ-N'-[4-trifluoromethylphenylJ urea was prepared
from 2-amino-5-cyanophenol (60mg, 0.45 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
25 hexane(1/20) and filtering. (108mg, 75%). 1H NMR (CD30D): d 8.31 (d, 1H),
7.68
(d, 2H), 7.59 (d, 2H), 7.20 (dd, 1H), 7.07 (s, 1H).
Example 91
P~,eparation of N-f 2-hydroxk 3-n-propylphenyll-N'-f2-bromo hr ~~envll urea
3o a)Preparation of 2-nitro-6-n-propylphenol
2-n-propylphenol (5.00g, 36.8mmol) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate (3.438, 40.Smmo1).
The
addition of sulfuric acid (45mL13M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
35 mixture was diluted with methylene chloride and extracted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
_78_
CA 02432662 2003-06-09
WO 97/29743 PCT/US96113632
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product(3.2 mg, 48 %). 1H NMR (CD3COCD3): d 7.99 (d,lH), 7.46 dd, 1H),
6.90 (t, 1H), 2.70 (t, 2H), 1.70 (m, 2H), 1.00 (t, 3H).
b)Preparation of 2-amino-6-n-propylphenol
To a solution of 2-vitro-6-n-propylphenol(2g, 11.Ommo1) in methanol( 100mL)
was added 10% Pd/C (200 mg). The mixture was flushed with argon, then hydrogen
was bubbled through the solution for 10 min. and a hydrogen atmosphere was
maintained at balloon pressure overnight. The mixture was filtered through
celite and
the celite was washed with methanol. The solvent was evaporated and
chromatography
to of the resulting solid on silica gel (5%MeOH/ CH2C12) gave the desired
product( I.50 g,
80.2 %). 1H NMR (CD30D): d 6.65 (m, 2H), 6.55 (t, 1H), 2.58 (t, 2H), 1.61 (m,
2H),
0.96 (t, 3H).
c)Preparation of N-[2-hydroxy-3-n-propyiphenyi]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-3-n-propyl phenyl]-N'-[2-bromo phenyl] urea was prepared from
2- amino-6-n-propyl phenol (302mg, 2.00 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1/20) and filtering. (640mg,92%). 1H NMR (CD30D): d 8.00 (d, 1H), 7.58
(d,
1H), 7.32 (t, 1H), 7.26 (t, 1H), 6.96 (dd, 1H), 6.89 (t, IH), 6.78 (d, 1H).
2o Example 92
Preparation of N-f2-hydroxY-4-ethvlphenyll-N'-f2-bromophe,~,~ll urea
a)Preparation of 2-vitro-5-ethylphenol
3-ethylphenol (S.OOg, 41 mmol) was dissolved in methylene chloride(40 mL)
followed by the addition of sodium nitrate (3.83g, 45 mmol). The addition of
sulfuric
acid (SOmL/ 3M) was then made, followed by addition of a catalytic amount of
sodium
nitrite. The mixture was allowed to stir. After 24 hours, the reaction mixture
was diluted
with methylene chloride and extracted with water. The organic layer was dried
over
MgS04 and filtered. The solvent was evaporated and chromatography of the
resulting
solid on silica gel (4%MeOH/ CH2Cl2) gave the desired product( I .7 g, 25 %).
1 H NMR
(CD3COCD3): d 8.02 (d,lH), 6.99 (s,lH), 6.85 (d, 1H), 2.69 (q, 2H), 1.30 (t,
3H).
b)Preparation of 2-amino-5-ethylphenol
To a solution of 2-vitro-5-ethylphenol(lg, 6.4mmo1) in methanol(250mL) was
added 10% PdIC ( I00 mg). The mixture was flushed with argon, then hydrogen
was
bubbled through the solution for 10 min. and a hydrogen atmosphere was
maintained at
balloon pressure overnight. The mixture was filtered through celite and the
celite was
washed with methanol. The solvent was evaporated and chromatography of the
- 79
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
resulting solid on silica gel (5%MeOH/ CH2C12) gave the desired product(750
mg, 91
%). 1 H NMR (CD30D): d 6.41-6.17 (m, 3H).
c)Preparation of N-[2-hydroxy-4-ethylphenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-4-ethyIphenyl3-N'-[2-bromo phenyl] urea was prepared from 2-
amino-5-ethylphenol (274mg, 2.00 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1/20) and filtering. (520 mg, 77%}. 1H NMR (CD30D): d 7.96 (d, 1H),
7.62
(s, 1H}, 7.56 (d, 1H), 7.30 (t, 1H}, 6.96 (t, 1H), 6.82 (d, 1H), 6.76 (d, 1H).
Example 93
Preparation of N-f2-hvdroxv 3-phenvlaminocarbonYl_phenyll-N'-f2-bromophenvll
urea
a)Preparation of 2-vitro-6-phenylaminocarbonylphenol
2-Phenylaminocarbonylphenol (S.OOg, 23 mmol) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate (2.208, 25.5 mmol).
The
addition of sulfuric acid (30mL/ 3M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with methylene chloride and extracted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the
desired product(2.50 g, 42 %). 1H NMR (CD3COCD3): d 8.15 (d,lH), 8.09 (d,lH),
7.51 (d, 1H), 7.30 (d, 1H), 7.10 (t, 1H), 7.01 (t, 1H).
b)Preparation of 2-amino-6-phenylaminocarbonylphenol
To a solution of 2-vitro-6-phenylaminocarbonylphenol (1g, 4.0 mmol) in
methanol(250mL) was added 10% PdIC ( 100 mg). The mixture was flushed with
argon, then hydrogen was bubbled through the solution for 10 min. and a
hydrogen
atmosphere was maintained at balloon pressure overnight. The mixture was
filtered
through celite and the celite was washed with methanol. The solvent was
evaporated
and chromatography of the resulting solid on silica gel (5%MeOH/ CH2C12) gave
the
desired product(800 mg, 91 %). 1H NMR (CD30D): d 7.73-7.57 (m, 2H), 7.43-7.27
(m, 3H), 7.25-7.10 (m, 1H), 6.94 (t, 1H), 6.74 (t, 1H).
c)Preparation of N-[2-hydroxy 3-phenylaminocarbonyl phenyl]-N'-[2-bromophenyl]
urea
N-[2-hydroxy 3-Phenylaminocarbonyl phenyl]-N'-[2-bromo phenyl] urea was
prepared from 2-amino-6-phenylaminocarbonylphenol (456mg, 2.00 mmol) according
to the procedure in General Method B. The product was purified by
precipitation from
methylene chloride/ hexane(1/20) and filtering. (800mg,94%). 1H NMR (CD30D):
80 -
CA 02432662 2003-06-09
WO 97!29743 PCT/US96/13632
1H NMR (CD30D): d 25 (d, 1H), 7.94 (d, 1H), 7.75-7.57 (m, 4H), 7.48-7.30 (m,
3H},
7.21 (t, 1H), 7.02 (dd, 1H), 6.92 (t, 1H).
Example 94
Preparation of N-f2-hydroxv-3-cvano-4-methvl~ !y 1-N'-(2-bromophe~"vll ureaa)
Preparation of the 2-nitro 5-methyl 6-bromo phenol
A solution of t-butyl amine(6.88 mL, 4.79 g, 2 equiv.) in methylene chloride
was treated with bromine ( 1.67 mL, 5.2 g, 1 equiv.) at -20 °C. The
flask was then
cooled to -78 °C and the the 2-nitro 5-methyl 6-bromo phenol (5 g, 1
equiv., in
to methylene chloride) was added drop-wise with vigrous stirring. The reaction
mixture
was slowly warmed to -30 °C for I h, then to -10 °C for 2 hours.
The reaction mixture
was then partitioned between methylene chloride and 5% aqueous acetic acid.
The
organic layer was dried over magnesium sulfate, filtered and concentrated in
vacuo.
The reaction mixture was purified by flash chromatography(Ethyl acetate/
hexanes) to
remove dibrominated species. The 2-nitro 4-bromo 5-methyl phenol was then
selectively crystallized out of methylene chloride. A final silica gel
column(5%ethyl
acetate/ hexanes) yielded desired isomer in 90% purity.(1.05 g, 14%). 1H NMR
(CDC13): d 7.95 (d, IH, 3 = 10.0 Hz), 6.91 (d, 1H, 3 = 10.0 Hz), 2.52 (s, 3H).
b) Preparation of 2-nitro-5-methyl-6-cyanophenol
2-Nitro-5-methyl-6-bromophenol ( 100 mg, 0.433 mmol) was dissolved in
dimethyl formamide (2mL) followed by the addition of triethylamine (0.175g,
1.73
mmol). The addition of a catalytic amount dimethylamino pyridine was then
made,
followed by addition of copper (I) cyanide (155mg, 1.73mmol). The mixture was
allowed to stir at 80_C for 4 hours. The solvent was evaporated and
chromatography of
the resulting solid on silica gel (2%MeOH/ CH2C12) gave the desired product
(70 mg,
9I %). 1H NMR (CD3COCD3): d 8.30 (d,lH), 7.15 (d,lH), 2.6I (s, 3H).
c) Preparation of 2-amino-5-methyl 6-cyanophenol
A mixture of 5-cyano-2-nitrophenol(70 mg, 0.39mmo1) and tin (II) chloride .
(265 mg, 1.18mmo1) in ethanol(20mL) was heated at 80_C under argon. After 2
hours,
3o the starting material has disappeared and the solution was allowed to cool
down and
then poured into ice. The pH was made slightly basic (pH7-8), by addition of
solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired
product( 175
mg, 86 %). 1H NMR (CD30D): d 6.87 (d, 1H), 6.75 (d,lH), 6.32 (s, 3H).
d) Preparation of N-[2-hydroxy 3-cyano 4-methyl phenyl]-N'-[2-bromophenyl]
urea
-81-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
N-[2-hydroxy 3-cyano 4-methyl phenyl)-N'-(2-bromophenyl] urea was prepared
from 2-amino-5-methyl-b-cyano phenol (50mg, 0.34 mmol) according to the
procedure
in General Method B. The product was purified by precipitation from methylene
chloride/ hexane( 1/20) and filtering. (70mg, 60%). 1H NMR (CD30D): d 7.92 (d,
1H), 7.68 (d, 1H), 7.59 (d, 1H), 7.31 (t, 1H), 7.00 (t, 1H), 6.62 (t, 1H),
2.49 (s, (3H).
Example 95
preparation of N-f2-hydroxv 4-Carboxyphenvl phenyli-N'-f2-bromophe~yll
ureaa)Preparation of 4-vitro-3-hydroxybenzophenone
l0 3-Hydroxybenzophenone (3.00g, 15. Immo1) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate ( 1.42g, 16.7mmo1).
The
addition of sulfuric acid (25mL/ 3M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with methylene chloride and exuacted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the
desired product( 1.10 g, 30 %). 1H NMR (CD3COCD3): d 8.25 (d,1H), 7.86 (d,lH),
?.71 (m, 1H), 7.59 (d, 1H}, 7.48 (s, 1H), 7.39 (dd, 1H).
b)Preparation of 4-amino-3-hydroxybenzophenone
2o A mixture of 4-vitro-3-hydroxybenzophenone (900 mg, 3.7mmol) and tin (I~
chloride (2.5 g, l l.lmmol) in ethanol(50mL) was heated at 80°C under
argon. After 2
hours, the starting material has disappeared and the solution was allowed to
cool down
and then poured into ice. The pH was made slightly basic (pH7-8), by addition
of solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the desired
product(685
mg, 87 %). 1H NMR (CD30D): d 7.65 (d, 2H}, 7.55 (d,lH), 7.49 (t, 2H), 7.26 (s,
1H),
7.16 (dd, 1H), 6.68 {d, 1H).
c)Preparation of N-[4-Carboxyphenyl-2-hydroxyphenyl]-N'-[2-bromophenyl] urea
3o N-[4-Carboxyphenyl-2-hydroxyphenyl]-N'-[2-bromophenyl] urea
was prepared from 4-amino-3-hydroxybenzophenone (330mg, 1.5 mmol) according to
the procedure in General Method B. The product was purified by precipitation
from
methylene chloride/ hexane(1/20) and filtering. (490mg, 79%). 1H NMR (CD30D):
d
8.40 (d, 1H), 8.09 (d, 1H), 7.83 (d, 2H), 7.65-7.60 (m, 4H), 7.48 (s, 1H),
7.43 (d, 1H),
7.35 (d, ( 1 H), 7.10 (t, i H).
-82-
CA 02432662 2003-06-09
WO 97129743 PCTIUS96113632
Example 96
Preparation of N-f2-hydroxy 3-carbox~vhen~ phenyll-N'12-bromophe~ll
ureaa}Preparation of 3-vitro-2-hydroxybenzophenone
2-Hydroxybenzophenone (3.00g, lS.Immo1) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate ( 1.42g, 16.7mmol).
The
addition of sulfuric acid (25mL/ 3M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with methylene chloride and extracted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
1o chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave
the
desired product( I.60 g, 44 %}. IH NMR (CD3COCD3): d 8.30 (d,lH), 7.86 (m,3H),
7.71 (m, 1H), 7.78 (d, 1H), 7.56 (dd 2H), 7.24 (t, 1H).
b)Preparation of 3-amino-2-hydroxybenzophenone
A mixture of 3-vitro-2-hydroxybenzophenone (600 mg, 2.Smmo1) and tin (In
chloride ( 1.7 g, 7.Smmol) in ethanol(SOmL) was heated at 80°C under
argon. After 2
hours, the starting material had disappeared and the solution was allowed to
cool down
and then poured into ice. The pH was made slightly basic (pH7-8), by addition
of solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired
product(490
mg, 92 %). 1H NMR (CD30D): d 7.65-7.40 (m, SH), 6.98 (d,lH), 6.86 (d, 1H),
6.67
(t, 1H).
c)Preparation of N-[2-hydroxy 3-carboxyphenyl phenyl]-N'-[2-bromophenyl] urea
N-(2-hydroxy 3-carboxyphenyl phenyl]-N'-(2-bromophenyl] urea was prepared
from 3-amino-2-hydroxybenzophenone (250mg, 1.20 mmol) according to the
procedure
in General Method B. The product was purified by precipitation from methylene
chloridel hexane(U20) and filtering. (200mg, 78%). 1H NMR (CD30D): d 8.35 (d,
1H), 7.96 (d, 1H), 7.72 (d, 2H), 7.65-7.50 (m, 4H), 7.35 (d, 1H), 7.30 (d,
1H), 7.01 (dd,
(1H), 6.92 (t, 1H).
I xample 97
Preparation of N-f2-hydroxv 3-benzvloxy phenvll-N'-f2-bromophenyll urea
a)Preparation of 2-vitro-6-benzyloxy phenol
2-Benzyloxyphenol (S.OOg, 25.Ommo1) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate (2.30g, 27.Smmol).
The
addition of sulfuric acid (3lmL/ 3M) was then made, followed by addition of a
catalytic
-83-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96I13632
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with methylene chloride and extracted with water. The
organic
layer was dried aver MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product(2.6 g, 43 %). 1H NMR (CD3COCD3): d 7.70 (d,IH), 7.50-7.28 (m,
5H), 7.14 (d, 1H), 6.92 (t, 1H), 5.21 (s, 2H).
b)Preparation of 2-amino-6-benzyloxy phenol
A mixture of 2-vitro-6-benzyloxy phenol ( 1.00 g, 4. l0mmol) and tin (II)
chloride (2.75 g, I2.2 mmol) in ethanol( 150mL) was heated at 80°C
under argon. After
2 hours, the starting material had disappeared and the solution was allowed to
cool
down and then poured into ice. The pH was made slightly basic (pH7-8), by
addition of
solid NaOH, before being extracted with ethyl acetate. The organic phase was
washed
with brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
t5 desired product(1.35 g, 88 %). 1H NMR (CD30D): d7.46 (d, 2H), 7.40-7.35 (m,
SH),
6.55 (d, 1H), 6.40 (d, 1H), 5.10 (s, 2H).
b)Preparation of N-[2-hydroxy3-benzyloxy phenyl]-N'-[2-bromophenyl] urea
N-[3-benzyloxy-2-hydroxyphenyl]-N'-[2-bromophenyl] urea was prepared from
2-vitro-6-benzyloxy phenol (430mg, 2.00 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane( 1/20) and filtering. (630mg, 76%). 1H NMR (CD30D): d 7.93 (d, 1H),
7.58
{d, 1H), 7.54-7.42 (m, 3H), 7.40-7.25 (m, 4H), 7.00 (t, 1H), 6.69 (d, 2H),
5.16 (s, 2H).
Example 98
Preparation of N-3-f2-hydmxy-5-indanonel-N'-t2-bromophenyll urea
a)Preparation of 2-hydroxy-3-vitro-5-indanone
2-Hydroxy-5-indanone(3.OOg, 20.Ommol) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate ( 1.95g, 21.Ommo1).
The .
addition of sulfuric acid (25mLJ 3M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with methylene chloride and exuacted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the
desired product(1.5 g, 39 %). 1H NMR (CD3COCD3): d 7.70 (d,lH), 7.04 (d, 1H),
3.04 (d, 2H), 2.74 (d, 2H).
b)Preparation of 3-amino-2-hydroxy-5-indanone
-gq._
CA 02432662 2003-06-09
WO 97/29743 PCTIUS96/13632
A mixture of 2-hydroxy-3-nitro-5-indanone ( 1.50 g, 7.80mmo1) and tin (II)
chloride (5.25 g, 23.3 mmol) in ethanol( 150mL) was heated at 80° C
under argon.
After 2 hours, the starting material had disappeared and the solution was
allowed to cool
down and then poured into ice. The pH was made slightly basic (pH7-8), by
addition of
solid NaOH, before being extracted with ethyl acetate. The organic phase was
washed
with brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product( 1.00 g, 79 %). 1 H NMR (CD30D): d 6.85 (d, l H), 6.45 (d, 1
H), 2.95
(d, 2H). 2.60 (d, 2H).
to c) Preparation N-3-[2-hydroxy-5-indanone]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-5-indanone]-N'-[2-bromophenylJ urea was prepared from 3-
amino-2-hydroxy-5-indanone (326mg, 2.00 mmol) according to the procedure in
General Method B. The product was purified by precipitation from methylene
chloride/
hexane{1/20) and filtering. .(610mg, 85%). 1H NMR (CD30D): d 7.92 (d, 1H),
7.65
(m, 2H), 7.45 (t, 1H), 7.09 (t, 1H), 7.00 (d, 1H), 2.90 (d, 2H), 2.66 (d, 2H).
Example 99
Preparation of (E)-N-[4-f2-lMethoxycarbonyll ethenyl]-2-hydroxy~hen,~l-N=,L2-
bromoohen, i1 urea
a) Preparation of 4-nitro-3-hydroxycinnamic acid
3-Hydroxycinnamic acid (3.00g, 18.3 mmol) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate ( 1.70 g, 26.1
mmol). The
addition of sulfuric acid (25 mLJ 3M) was then made, followed by addition of a
catalytic amount of sodium nitrite. The mixture was allowed to stir. After 24
hours, the
reaction mixture was diluted with methylene chloride and extracted with water.
The
organic layer was dried over MgS04 and filtered. The solvent was evaporated
and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product(1.0 g, 26 %). 1H NMR (CD3C4CD3): d 8.07 (d, 1H), 7.69 (d, 1H),
7.51 (s, 1H), 7.46 (d, 2H), 6.75 (d,lH).
b) Preparation of 4-nitro-3-hydroxymethylcinnamate
4-Nitro-3-hydroxycinnamic acid was stirred in excess methanol with a catalytic
amount of sulfuric acid. The solvent was evaporated and chromatography of the
resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired product( 1.0
g, 94 %}.
1H NMR (CD3COCD3): d 8.17 (d, 1H), 7.69 (d, 1H), 7.52 (s, 1H), 7.45 (d, 2H),
6.75
(d,1H), 3.80 (s, 3H).
c)Preparation of 4-amino-3-hydroxymethylcinnamate
-85-
CA 02432662 2003-06-09
WO 97129743 PCT/US96Ii3632
A mixture of 4-vitro-3-hydroxymethylcinnamate ( 1.0 g, 4.50mmo1) and tin (II)
chloride (3.0 g, 13.4 mmol) in ethanol(50mL) was heated at 80_C under argon.
After 2
hours, the starting material had disappeared and the solution was allowed to
cool down
and then poured into ice. The pH was made slightly basic (pH7-8), by addition
of solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the desired product
(650
mg, 75 %). 1H NMR (CD30D): d7.50 (d,lH), 6.94 (s, 1H), 6.89 (d, 1H), 6.68 (d,
IH),
6.18 (d, LH), 3.74 (s, 3H).
d)Preparation (E)-N-[4-[2-(Methoxycarbonyl) ethenyl]-2-hydroxyphenylj-N'-[2-
bromophenyl] urea
(E)-N-[4-[2-(Methoxycarbonyl) ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenyi]
urea was prepared from 4-amino-3-hydroxymethylcinnamate (250mg, 1.3 mmol)
according to the procedure in General Method B. The product was purified by
precipitation from methyiene chloride/ hexane(I/20) and filtering. (300mg,
59%). 1H
NMR (CD30D): d 8.24 (d,lH), 8.05 (d, 1H), 7.69 (d, 1H), 7.65 (d, 1H), 7.42 (t,
1H),
7.21 (s, 1H), 7.19 (d, IH), 7.10 (t, 1H) 6,45 (d,lH) 3.81 (s, 3H).
Example 100
2o Preparation of (E)-N-f3-f2-(Methoxycarbonyi) ethenyll-2-hydrox3r~henXl,]-N'-
f2-
~omophe~yl] urea N'-f2-bromo~n_yil urea
a)Preparation of 3-vitro-2-hydroxycinnamic acid
2-Hydroxycinnamic acid (3.00g, 18.3 mmol) was dissolved in methylene
chloride(4~mL) followed by the addition of sodium nitrate (2.21 g, 26.1mmo1).
The
addition of sulfuric acid (30 mL 3M) was then made, followed by addition of a
catalytic amount of sodium nitrite. The mixture was allowed to stir. After 24
hours, the
reaction mixture was diluted with methylene chloride and extracted with water.
The
organic layer was dried over MgSO4 and filtered. The solvent was evaporated
and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2Ci2) gave the
desired product(2.0 g, 52 %). 1H NMR (CD3COCD3): d 8.21 (d, iH), 8.16 (d, 1H),
8.05 (d, 1H), 7.19 (t, 1H), 6.72 (d, 1H)
b) Preparation of 3-vitro-Z-hydroxymethylcinnamate
3-vitro-2-hydroxycinnamic acid was stinted in excess methanol with a catalytic
amount of sulfuric acid. The solvent was evaporated and chromatography of the
resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the desired product( 1.0
g, 94 %).
- 86 -
CA 02432662 2003-06-09
WO 97/29743 PCTIUS96/13632
1 H NMR (CD3COCD3 ): d 8.25 (d, 1 H), 7.8.15 (d, I H), 8.06 (s, 1 H), 7.20 (t,
2H), 6.76
(d.IH), 3.80 (s, 3H).
c)Preparation of 3-amino-2-hydroxymethylcinnamate
A mixture of 3-nitro-2-hydroxymethylcinnamate ( I .0 g, 4.5 mmol) and tin (II)
chloride (3.0 g. 13.4 mmol) in ethanol(SOmL) was heated at 80_C under argon.
After 2
hours, the starting material had disappeared and the solution was allowed to
cool down
and then poured into ice. The pH was made slightly basic (pH7-8), by addition
of solid
NaOH, before being extracted with ethyl acetate. The organic phase was washed
with
brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired product
(700
mg, 81 %). 1H NMR (CD30D): d 8.04 (d, 1H), 6.93 {d, 1H),6.79 (d, 1H), 6.71 (t,
1H),
6.43 (d, IH), 3.72 (s, 3H).
d)Preparation(E)-N-[3-[2-(Methoxycarbonyl) ethenyt]-2-hydroxyphenyl]-N'-[2-
bromophenyl] urea
i5 (E)-N-[3-[2-(Methoxycarbonyl) ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenyl]
urea was prepared from 3-amino-2-hydroxymethylcinnamate ( 100 mg, 0.52 mmol)
according to the procedure in General Method B. The product was purified by
precipitation from methylene chloride/ hexane( 1120) and filtering. ( 150mg,
74%).IH
NMR (CD30D): d 8.I0 (d,lH), 8.00 (d, 1H), 7.69 (d, IH), 7.65 (d, 1H), 7.42 (t,
1H),
7.38 (t, 1H), 7.32 (d, IH), 7.05 (t, 1H) 6.55 (d,lH) 3.8I (s, 3H).
Example IOI
Preparation of iEl-N-f3-(2-(Aminocarbon~) ethenvil-2-h' dr roxvnhenvll-N'-I2-
bromophen~rll urea N'-[2-bromophenvll urea
a)Preparation of 2-hydroxycinnamide
2-Hydroxycinnamic acid (2.00g, 12.3 mmol) was dissolved in dimethyl
formamide( IOmL) followed liy the addition of benzotriazol-1-yloxy-
tris(dimethylamino)phosphonium hexafluorophosphate (5.4g, 12.3 mmoi) and
triethylamine ( I.7mL, 12.3mmol). Ammonia gas was bubbled into the reaction
3o mixture for 30 minutes. The mixture was allowed to stir for 24 hours, the
reaction
mixture was diluted with methylene chloride and extracted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4% MeOH/ CH2C12) gave the
desired product( 1.5 g, 75 %).
b)Preparation of 3-nitro-2-hydroxycinnamide
-87_
CA 02432662 2003-06-09
WO 97129743 PCTIUS96/13632
2-Hydroxycinnamide (750 mg, 4.6 mmol) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate (430 mg, S.lmmol).
The
addition of sulfuric acid (7 mL/ 3M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with meLhylene chloride and extracted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product(350 mg, 36 %}. 1H NMR (CD3COCD3): d 8.19 (d, 1H), 8.02 (d,
1H},
7.88 (d, 1 H), 7.15 (t, 1 H), 6.84 (d, 1 H}
to c)Preparation of 3-amino-2-hydroxycinnamide
A mixture of 3-vitro-2-hydroxymethylcinnamate (350 mg, 1.7 mmol) and tin (II)
chloride (3.0 g, 13.4 mmol) in ethanol(SOmL) was heated at 80° C under
argon. After 2
hours, the starting material had disappeared and the solution was allowed to
cool down and
then poured into ice. The pH was made slightly basic (pH7-8), by addition of
solid NaOH,
is before being extracted with ethyl acetate. The organic phase was washed
with brine, dried
over MgS04 and filtered. The solvent was evaporated and chromatography of the
resulting solid on silica gel (4%MeOH/ CH2C12) gave the desired product(244
mg, 80%).
d)Preparation of (E)-N-[3-[2-(Aminocarbonyl) ethenyl]-2-hydmxyphenyl]-N'-[2-
bromophenyl] urea
20 (E)-N-[3-[2-(Aminocarbonyl) ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenyl]
urea was prepared from 3-amino-2-hydroxycinnamide ( 100 mg, 0.56 mmol)
according
to the procedure in General Method B. The product was purified by
precipitation from -
methylene chloride! hexane( 1/20) and filtering. ( 110 mg, 52%).1 H NMR
(CD30D): d
8.00 (d,lH), 7.90 (d, 1H), 7.63 {d, 1H), 7.55 (d, 1H), 7.35 (m, 2H), 7.05 (t,
IH), 6.95
25 {t, 1H), 6.70 (d,lH) .
Example 102
Preparation of lE)-N-f4-f2-lAminocarbonvil etheny~]-2-hydrox3rphenv_l]-N'-(~-
~romqrhenyll urea N'-f2-bmmophenyll urea
30 a)Preparation of 3-hydroxycinnamide
3-Hydroxycinnamic acid (2.00 g, I2.3 mmol) was dissolved in dimethyl
formamide( 10 mL) followed by the addition of benzotriazol-1-yloxy-
tris(dimethylamino)phosphonium hexafluorophosphate (5.4g, 12.3 mmol) and
triethylamine ( 1.7 mL, 12.3mmo1). Ammonia gas was bubbled into the reaction
35 mixture for 30 minutes. The mixture was allowed to stir for 24 hours, the
reaction
mixture was diluted with methylene chloride and extracted with water. The
organic
_88_
CA 02432662 2003-06-09
WO 97J29743 PCT/US96113632
layer was dried over MgSO4 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product( 1.3 g, 65 %).
b)Preparation of 4-vitro-3-hydroxycinnamide
3-Hydroxycinnamide (750 mg, 4.6 mmol) was dissolved in methylene
chloride(40 mL) followed by the addition of sodium nitrate (430 mg, S.lmrnol).
The
addition of sulfuric acid (7 mI,/ 3M) was then made, followed by addition of a
catalytic
amount of sodium nitrite. The mixture was allowed to stir. After 24 hours, the
reaction
mixture was diluted with methylene chloride and extracted with water. The
organic
layer was dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the
desired product(240 mg, 25 %). 1H NMR (CD3COCD3): d 8.09 (d, 1H), 7.49 (d,
1H),
7.26 (s, 1 H), 7.16 (d, 1 H), 6.71 (d, 1H)
c)Preparation of 4-amino-2-hydroxycinnamide
A mixture of 4-vitro-3-hydroxymethylcinnamate (300 mg, 1.40 mmol) and tin
{II) chloride (980 mg, 4.30 mmol) in ethanol(50 mL) was heated at 80 C under
argon.
After 2 hours, the starting material had disappeared and the solution was
allowed to cool
down and then poured into ice. The pH was made slightly basic (pH 7-8), by
addition
of solid NaOH, before being extracted with ethyl acetate. The organic phase
was
?0 washed with brine, dried over MgS04 and filtered. The solvent was
evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the
desired product (200 mg, 74 %).
d)Preparation(E)-N-[3-[2-(Aminocarbonyl) ethenyl]-2-hydroxyphenyl]-N'-[2-
bromopheiiyl] urea
(E)-N-[3-(2-(Aminocarbonyl) ethenyl]-2-hydroxyphenyl]-N'-[2-bromophenylj
urea was prepared from 4-amino-2-hydroxycinnamide ( 100mg, 0.56 mmol)
according
to the procedure in General Method B. The product was purified by
precipitation from
methylene chloride/ hexane( 1/20) and filtering. {125mg, 54%).1H NMR (CD30D):
d
8.05 (d,lH), 7.92 (d, 1H), 7.60 (d, 1H), 7.4 5 (d, 1H), 7.35 (t, 1H), 7.05 (m,
2H), 6.50
(d,lH) .
Example 103
Preparation of N-f2-hydroxx4-(phenyl amino carbox~phenyll-N'-f2-bromophenvll
urea
N-[2-hydroxy 4-(phenyl amino carboxy) phenyl]-N'-[2-bromophenylj urea was
prepared from 5-(phenyl amino carboxy) 2-amino phenol (0.50 mmol) according to
the
_ 89 -
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
procedure in General Method B. The product was purified by precipitation from
methylene chloride/ hexane( 1/20) and filtering. ( 150 mg, 70%). 1 H NMR
(CD30D): d
8.25 (d, 1H), 8.00 (d, 1H), 7.75 (d, 2H), 7.64 (d, 1H), 7.50 (d, 2H), 7.41 (m,
3H), 7.16
(t, 1H), 7.05 (t, 1H).
Example 104
Preparation of N-f4-aminocarbonyl-2-hydroxvphenyll-N'-f2-bromophenvll urea N-
[4-
Aminocarbonyl -2-hydroxyphenyl]-N'-[2-bromophenyl] urea was prepared from 5-
aminocarbonyl-2-amino phenol (304 mg, 0.50 mmol) according to the procedure in
General Method B. The product was purified by precipitation from methylene
chloride/
hexane( 1/20) and filtering. (440 mg, 62%). 1H NMR (CD30D): d 8.09 (d, 1H),
7.91
(d, 1H), 7.60 (d, 1H), 7.45 (m, 3H). 7.00 (d, 1H).
Example 105
Preparation of N-[2-HYdroxy-3.5.6-trifluorophenyl)-N'-(2-bromophen 1)urea N-(2-
Hydroxy-3,5,6-trifluorophenyl)-N'-(2-bromophenyl)urea was prepared from 3,5,6-
trifluoro-2-hydroxyaniline (83 mg, 0.51 mmol) and 2-(bromophenyl)isocyanate (
100
mg, 0.53 mmol) according to the procedure in General Method B. The product was
purified by preparation thin layer chromatography. EI-MS mlz 359 (M-H) .
Example 106
Preparation of N-(2-Hydroxy-3-fluoro-4-trifluoromethylnhenyll-N'-l2-
bromophenxl_lurea
N-(2-Hydroxy-3-fluoro-4-trifluoromethylphenyl)-N'-(2-bromophenyl)urea was
prepared from 4-trifluoromethyI-3-fluoro-2-hydroxyaniline (239 mg, 1.2 mmol)
and 2-
(bromophenyl)isocyanate (243 mg, 1.2 mmol) according to the procedure in
Genera!
Method B. Removal of solvent under reduced pressure and chromatography of the
resulting solid on silica gel (hexane:ethyl acetate) gave the title compound
(20 mg, 4%).
EI-MS m/z 391 (M-H) .
Example 107
Preparation of N-(2-Hydroxy-3-iodophenyl)-N'-f2-bromophenyl)urea
N-(2-Hydroxy-3-iodophenyl)-N'-(2-bromophenyl)urea was prepared from 3-
iodo-2-hydroxyaniline (200 mg, 0.85 mmol) and 2-(bromophenyl)isocyanate ( 169
mg,
0.85 mmol) according to the procedure in General Method B. Removal of solvent
under reduced pressure and chromatography of the resulting solid on silica gel
-90-
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
(hexane:ether) gave the title compound E40 mg, 11%). 1H NMR (DMSO): d 9.45 (s,
1H), 9.15 (s, IH), 8.8 (s, IH}, 7.9~ (d, 1H), 7.8 (d, 1H),7.65 (d, 1H), 7.4
(d, 1H), 7.3 (t,
1H), 7.0 (t, 1H), 6.65(t, 1H).
Example 108
Preparation of N-f2-fff2-ltrifluorometh r~l phenvllsulfonyllaminolphen, 1~~-
bromophen I)y urea
a)Preparation of [2-[2-(tritluoromethyl)phenyl](sulfonamido)aniline]
The title compound was prepared according to General Method C using 2-
(trifluoromethyl)benzenesulfonyl chloride (1 equiv.). The product was purified
by
chromatography on silica gel (methylene chloride:methanol) ( 1.04 g, 33%). EI-
MS m/z
317 (M+H)+.
b)Preparation of N-[2-[[[2-(trifluoromethyl)phenyl]sulfonyl]amino]phenyl]-N'-
(2-
bromophenyl)urea
The title compound was prepared using[2-[2(trifluoromethyl)phenyl]
(sulfonamido)aniline ( 1.04 g, 3.2 mmol) and 2-(bromophenyl)isocyanate (652
mg, 3.2
mmol) according to General Method B. The solvent was evaporated to give the
desired
urea ( 1.03 g, 61 %). EI-MS m/z 514 (M+H)+.
2o Example 109
Preparation of N-l2-Bromophenyl)-N'-f2-dimethylaminosulfonvlamino~~envllurea
a) Preparation of [2-[1,1-(dimethylamino)]sulfonamidoaniline]
The title compound was prepared according to General Method C using
dimethylsulfamoyi chloride ( 1 equiv.). The product was purified by
chromatography on
silica gel (methylene chloride:methanol). ES-MS m/z 216 (M+H)+
b)Preparation of N-(2-Bromophenyl)-N'-[2-
(dimethylaminosulfonylamino]phenyl]urea
The title compound was prepared from [2-[ 1,1-(dimethlyamino)sulfonamido-
aniline ( 137 mg, 0.6 mmol) and 2-(bromophenyl)isocyanate ( 126 mg, 0.6 mmol)
according to General Method B. The solvent was evaporated and chromatography
on
silica gel (ethyl acetate:hexane) gave the desired urea. EI-MS m/z 413 (M+H)+
Example 110
Preparation of N-[2-fPhenethylsulfonvlaminol phenyl-N'-f2-bromophe~ llurea
[2-(Phenethylsulfonamido) aniline] (example 60, 300mg, 1.09 mmol) was
placed in a Parr shaker bottle containing palladium (180 mg) under an argon
stream.
Methanol ( 150 mL) was added and the container placed on a Parr shaker (55
psi) for
-91-
CA 02432662 2003-06-09
WO 97129743 PCT/LTS96/13632
several hours. The reaction mixture was filtered through Celite and the
filtrate was
evaporated to give the desired aniline (269 mg, 90%). EI-MS m/z 277 (M+H)+.
b)Preparation of N-[2-(Phenethylsulfonylamino)phenyl]-N'-{2-bromophenyl)urea
The title compound was prepared from [2-(phenethyIsulfonamido) aniline] (269
mg, 0.97 mmol) and 2-(bromophenyl)isocyanate ( 193 mg, 0.97 mmol) according to
General Method B. The desired urea was precipitated out of toluene/hexane (384
mg,
78%). EI-MS m/z 472 (M-H) .
Exam 1~~
to Preparation of N-f2-jj2-acetamido-4-methylthiazol-5-vl
sulfonvlaminolphenyll-N'-f2-
~romqphenyl)urea
a)Preparation of [2-[(2-acetamido-4-methyl-5-thiazole)sulfonamido]aniline]
The title compound was prepared using 2-acetamido-4-methyl-5-
thiazolesuifonyl chloride (1 equiv.) according to General Method C. A solid
i5 precipatated from the reaction mixture and was filtered to give the desired
aniline (1.68
g, 52%). ES-MS m/z 327 (M+H)+..
b)Preparation of N-[2-[(2-acetamido-4-methylthiazol-5-yl)sulfonylamino)phenyl]-
N'-
(2-bromophenyl)urea
The title compound was prepared from [2-[(2-acetamido-4-methyl-5-
2o thiazole)sulfonamido]aniiine] ( 1.68 g,5.14 mmol) and 2-
(bromophenyl)isocyanate
( 1.02 g, 5.14 mmol) according to General Method B. The product was
precipitated
from ethyl acetate/hexane (220 mg, 8%). EI-MS m/z 524 (M+H)+.
Jrxam~ 1e 112
25 Preparation of N-j2-hydrox~~a_nophenvll-N'-f4- h~enyl~h_enyll urea N-[2-
Hydroxy-4-cyanophenyl]-N'-[4-phenylphenyl] urea was prepared from 2-amino-5-
cyanophenol (60mg, 0.45 mmol) according to the procedure in General Method B.
The
product was purified by precipitation from methylene chloride/ hexane( 1/20)
and
filtering. (135 mg, 75%). 1H NMR (CD30D): d 8.33 (d, 1H), 7.71-7.29 (m, 9H),
7.25
30 (d, 1H), 7.12 (s, 1H).
Example 113
PreR,aration of,lT j2-~,vdroxv" ~-cyanophenyll-N'-f2.3-dichloroohenvll urea
N-{2-Hydroxy-4-cyanophenyl]-N'-[2,3 dichlorophenyl] urea was prepared from
35 2-amino-5-cyanophenol (60mg, 0.45 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
-92-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
hexane(1/20) and filtering. (125mg, 86%). 1H NMR (CD30D): d 8.27 (d, 1H), 8.15
(m, 1H), 7.39-7.20 (m, 2H), 7.16 (d, 1H), 7.06 (s, 1H).
Example 114
Preparation of N-f2-hydroxv-4-cvanonhenvll-N'-f2-methoxvohenvll urea
N-[2-Hydroxy-4-cyanophenyl]-N'-[2-methoxyphenyl] urea was prepared from
2-amino-5-cyanophenol (60mg, 0.45 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1/20) and filtering (105mg, 83%). 1H NMR (CD30D): d 8.26 (d, 1H), 8.02
(d,
to 1H), 7.14 (d, 1H), 7.05 (s, 1H), 7.00-6.83 (m, 3H}, 3,84 (s, 3H).
Example 115
Preparation of N-f2-hvdroxv-4-c~phenyll-N'-[3-methoxxnhenYlj urea N-[2-
Hydroxy-4.-cyanophenyl]-N'-[3-methoxyphenyl] urea was prepared from 2-amino-5-
t5 cyanophenol (60mg, 0.45 mmol) according to the procedure in General Method
B. The
product was purified by precipitation from methylene chloride/ hexane( 1120)
and
filtering. ( 102mg, 80%). 1 H NMR (CD30D}: d 8.25 (d, 1H), 7.25-7.08 (m, 3H),
7.04
(s, 1H), 6.90 (t, 1H), 6.58 (d, 1H).
20 Exam 116
Preparation of N-f2-hydroxy-5-fluorophenyll-N'-f2-bromophenvl]' urea
a)Preparation of 2-amino-4-fluorophenol
A mixture of 4-fluoro-2-nitrophenol( 1 g, 4.b4mmol) and tin (II) chloride (5.4
g,
24.2mmol) in ethanol(SOmL) was heated at 80°C under argon. After 2
hours, the
z5 starting material had disappeared and the solution was allowed to cool down
and then
poured into ice. The pH is made slightly basic (pH7-8), by addition of solid
NaOH,
before being extracted with ethyl acetate. The organic phase was washed with
brine,
dried over MgS04 and filtered. The solvent was evaporated and chromatography
of the
resulting solid on silica gel (4%MeOH/ CH2Cl2) gave the desired product(622
mg, 85
30 %). 1H NMR (CD30D): d 6.51 (dd, 1H), 6.32 (dd, 1H), 6.17 (ddd, 1H).
b)Preparation of N-[2-hydroxy-5-tluorophenyl]-N'-[2-bromophenyl] urea
N-[2-Hydroxy-5-fluorophenyl]-N'-[2-bromophenyl] urea was prepared from 2-
amino-6-fluoro phenol (254mg, 2.00 mrnol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
35 hexane(1/20) and filtering. (520mg,80%). 1H NMR (CD30D): d 7.88 (d, 1H),
7.79
(dd, 1H), 7.57 (d, 1H), 7.31 (t, 1H), 7.00 (t, 1H), 6.76 (dd, 1H), 6.57
(ddd,lH).
-93-
CA 02432662 2003-06-09
WO 97/29743 PCT/U596113632
Example 117
Preparation of N-f2-hvdroxv-5-trifluoromethvln...~-hen lY,l-N'-[2-bromophenvll
urea
a)Preparation of 2-amino-4- trifluoromethylphenol
A mixture of 4-trifluoromethyl-2-nitrophenol{ 1.0 g, 4.8mmol) and tin (II)
chloride (5.4 g, 24.2 mmol) in ethanol( 150mL) was heated at 80°C under
argon. After
2 hours, the starting material had disappeared and the solution was allowed to
cool
down and then poured into ice. The pH was made slightly basic (pH7-8), by
addition of
solid NaOH, before being extracted with ethyl acetate. The organic phase was
washed
with brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product(708 mg, 83 %). 1H NMR (CD30D): d 6.87 (s, 1H), 6.80 (d, 1H),
6.69
(d, 1 H).
b)Preparation of N-[2-hydroxy-5-trifluoromethylphenyl]-N'-[2-bromophenyl] urea
t5 N-[2-hydroxy-5-irifluoromethylphenyl]-N'-[2-bromophenyl) urea was prepared
from 2-amino-4-trifluoromethylphenol (354mg, 2.00 mmol) according to the
procedure in
General Method B. The product was purified by precipitation from methylene
chloride/
hexane(lequiv./20equiv.) and filtering. (490mg, 65%). 1H NMR (CD30D): d 8.40
(s,
1H), 7.94 (d, 1H), 7.60 (d, 1H), 7.35 (t, 1H), 7.18 {d, 1H), 7.03 (t, 1H),
6.95 (d, 1H).
Example 118
Preparation of N-f2-hydroxyn, hen~rl]-N'-f2-bromophenyll urea
N-[2-hydroxyphenyl]-N'-[2-bromo phenyl] urea was prepared from 2- amino-
phenol ( 141mg, 1.30 mmol) according to the procedure in General Method B. The
product was purified by precipitation from methylene chloride/ hexane( 1120)
and
filtering. (300mg,75%). 1H NMR (CD30D): d 8.05 (d, 1H), 7.49 (d, 1H), 7.25 (t,
2H), 6.96 (t, 1H), 6.90 (t, 2H), 6.68 (t, 1H).
Examples 19
Prepara ~n of N-ftrans-3-s;yrl 2-h~drox~ph~ll-N'-f -bromonhenvll
ureaa)Preparation of traps-6-styrl-2~-nitrophenol
Traps-2-styrlphenol (500 mg, 2.55 mmol) was dissolved in methylene
chloride(40mL) followed by the addition of sodium nitrate (240 mg, 2.81mmo1).
The
addition of sulfuric acid (3 mL of 3M) was then made, followed by addition of
a
catalytic amount of sodium nitrite. The mixture was allowed to stir. After 24
hours, the
reaction mixture was diluted with methylene chloride and extracted with water.
The
-94-
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
organic layer was dried over MgS04 and filtered. The solvent was evaporated
and
chromatography of the resulting solid on silica gel (4%MeOH/ CH2C12) gave the
desired product (200 mg, 36 %). 1H NMR (CD3COCD3): d 8.05 (d, 1H), 7.90 (d,
2H),7.65-7.20 (m,7H),7.00 (t,lH).
b)Preparation of traps-6-styrl-2-aminophenol
A mixture of traps-6-styrl-2-nitrophenol (200 mg, 0.83 mmol) and tin (II)
chloride (560 mg, 2.60 mmol) in ethanol(50mL) was heated at 80° C under
argon.
After 2 hours, the starting material has disappeared and the solution was
allowed to cool
down and then poured into ice. The pH is made slightly basic (pH7-8), by
addition of
14 solid NaOH, before being extracted with ethyl acetate. The organic phase
was washed
with brine, dried over MgS04 and filtered. The solvent was evaporated and
chromatography of the resulting solid on silica geI (4%MeOH/ CH2C12) gave the
desired product (50 mg, 29 %a). 1H NMR (CD30D): d 7.51 (m, 3H), 7.29 (m,
3H),7.11
(t, 1 H), 7.00 (m, 2~-i), 6.69 (m, 2H).
i5 c)Preparation of N-[traps-3-styrl-2-hydroxyphenyl]-N'-[2-bromophenyl] urea
N-[traps-3-styrl-2-hydroxyphenyl]-N'-[2-bromophenyl] urea was prepared from
traps-6-styrl-2-aminophenol (35mg, 0.17 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane(1/20) and filtering. (36mg, 53%). 1H NMR (CD3OD): d7.97 (d, 1H), 7.62-
20 7.48 (m, 4H), 7.45-7.26 (m, 5H), 7.25 (t, 1H), 7.15 (d, 1H), ?.O1 (t, 1H),
6.88 (t 2H).
Example 120
Preparation of N-f2-hydroxv-3.4-dichlorophen-yll-N'-f2-methoxy~henyl] urea
N-[2-hydroxy-3,4-dichiorophenyl]-N'-[2-methoxyphenyl] urea was prepared
25 from 2-amino-5,6-dichlorophenol (80mg, 0.50 mmol, example 82b) according to
the
procedure in General Method B. The product was purified by precipitation from
methylene chloride/ hexane( 1/20) and filtering. ( 125mg,77%). 1 H NMR
{CD30D): d
8.02 (d, 1 H), 7.79 (d, 1 H), 7.05-6.86 (m, 4H), 3.92 (s, 3H).
30 Exam 1p a 12l
Preparation of N-f2-hvdroxY-3.4-dichloronhenvll-N'-f4-methoxv~henvll urea
N-[2-hydroxy-3,4-dichlorophenyl]-N'-[4-methoxyphenyl] urea was prepared
from 2-amino-5,6-dichlorophenol (80mg, 0.50 mmol, example 82b) according to
the
procedure in General Method B. The product was purified by precipitation from
35 methylene chloride/ hexane(lequiv./20equiv.) and filtering. (120mg, 74%).
1H NMR
(CD30D): d 7.89 (d, 1H), 7.35 (d, 2H), 6.99 (d, 1H), 6.90 (dd, 2H), 3.80 (s,
3H).
-95-
CA 02432662 2003-06-09
WO 97/29743 PCTIUS96/i3632
Example 122
Preparation of N-L2-h, d,~r roxy-3.4-dichlorophenvll-N'-j3-
trifluoromethvlphenyll urea
N-[2-hydroxy-3,4-dichlorophenyl]-N'-[3-trifluoromethylphenyl] urea was
prepared from 2-amino-5,6-dichiorophenol (80mg, 0.50 mmol, example 82b)
according
to the procedure in General Method B. The product was purified by
precipitation from
methyiene chloride/ hexane(lequiv./20equiv.) and filtering. (130mg, 71%). 1H
NMR
(CD30D): d 7.96 (d, 2H), 7.60 (d, 1H), 7.48 (t, 1H), 7.30 (d, IH), 7.00 (d,
1H).
~ca~~le 1~3
Prenaration~N-f2-hvdroxv-3.4-dishloronhenvll-N'-f2-nhenylnhenvll urea
N-[2-hydroxy-3,4-dichlorophenyl]-N'-[2-phenylphenyl] urea was prepared from
2-amino-5,6-dichlorophenol (80mg, 0.50 mmoi, example 82b) according to the
procedure in General Method B. The product was purified by precipitation from
methylene chloride/ hexane(lequiv.J20equiv.) and filtering. (1 IOmg, 59%). 1H
NMR
(CD30D): d 7.77 (d, 1H), 7.73 (d, 1H), 7.53-7.14 (m, 8H), 6.95 (d, 1H).
ExamRle 124
Preparation of N-~,2-hydr~c X 3 4-dichlorophe~rl]-N'-~2 3-dichloro~he~ 1~1
urea
N-[2-Hydroxy-3,4-dichlorophenyi]-N'-[2,3-dichlorophenyl] urea was prepared
from 2-amino-5,6-dichlorophenol (80mg, 0.50 mmol, example 82b) according to
the
procedure in General Method B. The product was purified by precipitation from
methylene chloride! hexane(lequiv./20equiv.) and filtering. (130mg, 71%). 1H
NMR
(CD30D): d 8.06 (dd, IH), 7.91 (d, 1H), 7.25 (m, 2H), 7.00 (d, 1H).
Example 125
Preparation Qf N-12-hvdroxv-4-isonronvhhenvll-N'-C3-trifluoromethvlnhen !y 1
urea
a)Preparation of 2-nitro-5-isopropylphenol
3-isopropylphenoi (3.00g, 22 mmol) was dissolved in methylene chloride(40m1)
3o followed by the addition of sodium nitrate (2.06g, 24mmol). The addition of
sulfuric
acid (25mL 3M) is then made, followed by addition of a catalytic amount of
sodium
nitrite. The mixture was allowed to stir. After 24 h, the reaction mixture is
diluted with
methylene chloride and extracted with v~ater. The organic layer is dried over
MgS04
and filtered. The solvent was evaporated and chromatography of the resulting
solid on
silica gel (4%MeOHI CH2Cl2) gave the desired product(1.09g, 27 %). 1H NMR
(CD3COCD3): d 7.95 (d,IH), 7.62 (d,lH), 7.11 (d, IH), 2.95 (m, 1H), I.24 (d,
6H).
-96-
CA 02432662 2003-06-09
WO 97129743 PCT/US96I13632
b) Preparation of 2-amino-5-isopropylphenol
To a solution of 2-vitro-5-isopropyiphenol(lg, 6.4 mmol) in methanol(50 mL)
was added 10% Pd/C ( 100 mg). The mixture was flushed with argon, then
hydrogen
was bubbled through the solution for 10 min. and a hydrogen atmosphere was
maintained at balloon pressure overnight. The mixture was filtered through
ceiite and
the celite was washed with methanol. The solvent was evaporated and
chromatography
of the resulting solid on silica gel (S%MeOH/ CH2Cl2) gave the desired
product(775
mg, 93 %). 1H NMR (CD30D): d 6.71-6.44 (m, 3H), 2.73 (m, 1H), 1.20 (d, 6H).
c) Preparation of N-[2-hydroxy-4-isopropylphenyl]-N'-[3-trifluoromethylphenyi]
urea
N-[2-hydroxy-4-isopropylphenyl]-N'-[3-trifluoromethylphenyl] urea was
prepared from 2-amino-5-isopropylphenol (75mg, 0.50 mmol) according to the
procedure in General Method B. The product was purified by precipitation from
methylene chloride/ hexane(lequiv./20equiv.) and filtering. (140mg, 83%). 1H
NMR
(CD30D): d 7.91 (d, 2H), 7.62 (d, 1H), 7.47 (t, 1H), 7.39 (d, IH), 6.75 (s,
1H), 6.72 (d,
IH), 2.80 (m, 1H), 1.21 (d, 6H).
Example 126
Preparation of N-f2-hvdroxv-3-nanhthvll-N'-f2.3-dichloronhenvll urea
N-[2-hydroxy-3-naphthyl]-N'-[2,3-dichlorophenyl] urea was prepared from 3
2o amino 2-naphthol ( 160mg, 1.00 mmol) according to the procedure in General
Method
B. The product was purified by precipitation from methylene chloride/
hexane(lequiv./20equiv.) and filtering. (285mg, 82%). 1H NMR (CD30D): d 8.48
(s,
1H), 8.10 (d, IH), 7.68 (d, 1H), 7.57 (d, 1H), 7.40-7.23 (m, 4H), 7.18 (d,
1H).
Example 127
Preparation of N-f2-fj2.3-Dichlorothien-5-y111su1fonYlaminoiphenvll-N'-(2-
bromophet~vllurea
a)Preparation of [2-[(2,3-Dichlorothien-5-yl)]sulfonylaminoaniline)
The title compound was prepared according to General Method C using 2,3-
dichlorothiophene-5-sulfonyl chloride (( 1 eq). The product was purified by
flash
chromatography on silica gel (ethyl acetate/hexane 20/80-methylene
chloride:methanol
90!10) ( 1.25 g, 39 %). EI-MS m/z 321 (M-H)-
b)Preparation of N-[2-[(2,3-Dichlorothien-5-yl))sulfonyiamino]phenyl]-N'-(2-
bromophenyl)urea
The title compound was prepared from [2-[(2,3-dichlorothien-5-
yl))sulfonylaminoaniline (1.25 g,3.9 mmol) and 2-(bromophenyl)isocyanate (768
mg,
-97-
CA 02432662 2003-06-09
WO 97129743 PCT/US96113632
3.9 mmol) according to General Method B. The product was purified by flash
chromatography on silica gel (ethyl acetate:hexane 30/70) (272 mg, 13 %) EI
~~IS m/z
520 (M-H)
g Example 128
Preparation of N-f2-[(3.5-Bistrifluoromethylphenyllsulfgnylamino phenyjl-N'-(2-
bromophenyllurea
a)Preparation of [2-(3,5-Bistrifluoromethylphenyl)sulfonylaminoaniline]
The title compound was prepared according to General Method C using 3,5-
(bistrifluoromethyl)phenylsulfonyl chloride ( 1.28 g, 4.1 mmol) and o-
phenylenediamine
(441 mg, 4.1 mmol). The product was purified by flash chromatography on silica
gel
(methylene chIoride:meihanol 95/5) (61 I mg, 39 %). EI-MS m/z 383 {M-H)-
b)Preparation of N-[2-[(3,5-Bistrifluoromethylphenyl)sulfonylamino]phenyl]-N'-
(2-
bromophenyl)urea
The title compound was prepared from [2-(3,5-bistrifluoromethylphenyl)
sulfonylaminoaniline (591 mg, 1.5 mmol) and 2-bromophenylisocyanate (305 mg,
1.5
mmol) according to General Method B. The product was purified by flash chroma-
tography on silica gel (ethyl acetate:hexane 30/70) ( 10 mg, I %).EI-MS m/z
580 (M-H)-
2o Exam 1p a 129
Preparation of N-f2-f(2-Benzvllsulfonylami~ol 15-trifluorometh rill h~en~rll-
N'-(2-
bromophenyllurea
a)Preparation of [(4-Benzylsulfonylamino)-{3 -nitro)-benzotrifluoride]
4-Amino-3-nitro-benzotrifluoride ( I.0 g, 4.85 mmol) was mined in DMF and
the reaction mixture was cooled to 0°C. Sodium hydride ( 175 mg, 7.28
mmol) was
added to the cold mixture and allowed to mix for ten minutes ( a deep red
color was
noted). Toluenesulfonyl chloride (925 mg, 4.85 mmol) was added ( reaction
color
changed to yellow) and the reaction was mixed for sixteen hours at room
temperature.
The reaction was quenched in NH4C1 and extracted with ethyl acetate:hexane {
1:I).
3o The groduct was purified by flash chromatography on silica gel ( ethyl
acetate:hexane
30170) (878 mg, 52 %) EI-MS m/z 359 (M-Hj .
b)Preparation of [(4-Benzylsuifonylamino)-(3-amino)-benzotrifluoride]
[(4-Benzylsuifonylamino)-(3-nitro)-benzotrifluoride (230 mg, 0.64 mmol) was
mixed in methanol and poured into a Parr bottle. Palladium on carbon ( 15 mg)
was
3s added under an argon stream. The reaction mixture was placed on a Parr
shaker ( 55
- 98 _
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
psi, H2) for several hours. The reaction mixture was filtered through Celite
to give the
title compound. (210 mg, 99%) EI-MS m/z 329 (M-H) .
c)Preparation of N-[2-[{2-Benzyl)sulfonylamino]-(5-trifluoromethyl)phenyl]-N'-
(2-
bromophenyl)urea
The title compound was prepared from [(4-benzylsulfonylamino)-(3-amino)-
benzotrifluoride (210 mg, 0.64 mmol) and 2-bromophenylisocyanate ( 126 mg,
0.64
mmol) according to the procedure in General Method B. The product was purified
by
flash chromatography on silica gel {ethyl acetate:hexane 30/70) {70 mg, 21%)
EI-MS
mlz 526 (M-H)-
to
Example 130
Preparation of N-f2-f2-l3-Nitrophenyl)sulfonvlaminolphen,~rll-N'-(2-
bromonhenvl)urea
a)Preparation of [2-((3-Nitrophenyl)sulfonylamino)aniline]
The title compound .was prepared according to General Method C using 3-
t5 nitrobenzenesulfonyl chloride (1 eq). The product was purified by flash
chromatogrphy
on silica gel (methylene chloride:methanol 96/4).( 1.07 g, 37 %) EI-MS m/z 294
(M+H)~
b)Preparation of N-[2-[(3-Nitrophenyl)sulfonylamino]phenyl]-N'-(2-
bromophenyl)urea
The title compound was prepared from [2-(3-nitrophenyl)sulfonylaminoaniline]
(590 mg, 2.0 mmol) and 2-(bromophenyl)isocyanate (398 mg, 2.0 mmol) according
to
20 the procedure in General Method B. The product was purified by flash
chromatography
on silica gel (ethyl acetate:hexane 30/70) (400 mg, 40%). EI-MS m/z 489 (M-Hj
Example 131
Preparation of N-f2-f2-(4-Phenoxy~hen_yllsulfonvlaminolphen~rll-N'-l2-
bromg~heny1)
25 urea
a)Preparation of [2-((4-Phenoxyphenyl)sulfonylamino)aniline]
The title compound was prepared according to General Method C using 4-
phenoxyphenylsulfonyl chloride (969 mg, 3.6 mmol) and o-phenylenediamine (300
mg,
2.77 mmol). The reaction mixture was partitioned between water (200 ml) and
30 toluene:methylene chloride ( 1:3). 'The organic phase collected and the
methyleae
chloride evaporated leaving the toluene. Hexane added and the product
precipatated
from solution. (317 mg, 34 %) EI-MS m/z 341 (M+H)+
b)Preparation of N-[2-[(4-Phenoxyphenyl)sulfonylamino]phenyl]-N'-(2-
bromophenyl)urea
35 The title compound was prepared from [2-(4-phenoxyphenyl)suifonyl
aminoaniline (276 mg, 0.8 mmol) and 2-(bromophenyl)isocyanate ( 161 mg; 0.8
mmol)
-99-
CA 02432662 2003-06-09
WO 97!29743 PCT/US96/13632
according to the procedured in General Method B. The product was purified by
flash
chromatography on silica gel (ethyl acetate:hexane 30/70) (240 mg, 55 %) EI-MS
m/z
536 (M-H)
g Exam lie 132
Preparation of N-([2-llSl-10-Cam orsulfon laminojphenyll-N'-(2-
bromophen3rllurea
a)Preparation of 2-((1S)-10-Camphorsulfonylamino)aniline
The title compound was prepared according to General Method C using ( 1 S)(+)
t0 10-Camphorsulfonyl chloride ( 1.16 g, 4.6 mmol) and o-phenylenediamine (500
mg, 4.6
mmoI). The reaction mixture was partitioned between water (200 ml) and
toluene:methylene chloride ( 1:3). The organic phase was separated and the
methylene
chloride evaporated leaving the toluene. Hexane was added and solid
precipitated from
solution. ( I 30 mg; 9%) EI-MS m/z 323 (M+H)+
i5 b)Preparation of N-[[2-(1S)-10-Camphorsulfonylamino]phenyl]-N'-(2-
bromophenyl)urea
The title compound was prepared from [2-( 1 S)-10-
camphorsulfonylamino]aniline ( 130 mg, 0.4 mmol) and 2-(bromophenyl)isocyanate
(80
mg, 0.4 mmol) according to the procedure in General Method B. The solvent was
20 evaporated and product was precipitated from methylene chloride:hexane.
(200 mg, 95
%). EI-MS m/z SI8 (M-H)-
Example 133
Preparation of N-112-f 1R)-10-Car~phorsulfonylamino henY1_LN'-(2-
b~omophenyl)urea
a)Preparation of 2-((1R)-10-Camphorsulfonylamino)aniline
The title compound was prepared according to General Method C using (1R)(-)-
10-camphorsulfony! chloride ( 1.16 g, 4.6 mmol) and o-phenylenediamine (500
mg, 4.6
mmol). The reaction mixture was partitioned between water (200 mL) and
3o toluene:methylene chloride( 1:3). The organic phase was separated and the
methylene
chloride evaporated leaving the toluene. Hexane was added and the product
precipitated from solution. (563 mg, 38%). EI-MS m1z 323 (M+H)+
b)Preparation of N-[[2-(1R)-10-Camphorsulfonylamino]phenyl)-N'-(2-
bromophenyl)urea
The title compound was prepared from [I-{IR)-IO-
camphorsulfonylaminoaniline] (563 mg, 1.75 mmol) and 2-(bromophenyl)isocyanate
100 -
CA 02432662 2003-06-09
WO 97129743 PCT/LTS96113632
(346 mg, 1.75 mmo!) according to the procedure in General Method B. The
product
was purified by flash chromatography on silica gel (ethyl acetate:hexane
30170) (263
mg, 29 %) EI-MS m/z 518 (M-H)-
Example 134
Preparation of N j2-L2-(2-Nitro-(4-trifluoromethylyhenvllsulfon3rlaminolphenxl-
N'-~2-
bromophenxl)urea
a)Preparation of [2-[(2-Nitro)-(4-trifluoromethyl)phenyl]sulfonylamino]aniline
The title compound was prepared according to General Method C using 2-nitro-
to 4-(trifluoromethyl)benzenesulfonyl chloride ( 1 eq). The product was
purified by flash
chromatography on silica gel ( methylene chloride:methanol 96/4) (875 mg, 25
%) EI-
MS m/z 362 (M+H)+
b)_Preparation of N-[2-[2-{2-Nitro-(4-
trifluoromethyl)phenyl)sulfonylaminoJphenyl-N'-
(2-bromophenyl)urea
t5 The title compound was prepared from [2-[(2-nitro)-(4-trifluoromethyl)
phenylJsulfonylamino]aniline (740 mg, 2.1 mmol) and 2-(bromophenyl)isocyanate
(406 mg, 2.1 mmol) according to General Method B. The product was purified by
flash
chromatography on silica gel (ethyl acetate:hexane 30/70). The product was
further
purified by recrystallization in ethyl acetate:hexane. (320 mg, 28 %) EI-MS
m/z 557
20 (M-H)
Exam ip a 135
Preparation of N-l2-hydroxy-4-azidophenyl)-N'-f2-iodophenyllurea
a)Preparation of N-(2-hydroxy-4-aminophenyl)-N'-(2-iodophenyl)urea
To a solution of N-(2-hydroxy-4-nitrophenyl)-N'-(2-iodophenyl)urea (220 mg,
25 0.55 mmol) in ethanol (15 mL), Tin chloride (522 mg, 2.75 mmol) was added.
The
reaction mixture was stirred at reflux for 16 hours then cooled to room
temperature. The
reaction mixture was basified to pH 8 with aq. NaHC03 then extracted with
ethyl
acetate (3x). The organic extracts were combined, dried over MgS04, filtered
and
concentrated under reduced pressure to give product ( 180 mg, 89%). EI-MS mlz
370
3a (M+H)+
b)Preparation of N-(2-hydroxy-4-azidophenyl)-N'-(2-iodophenyi)urea
The N-(2-hydroxy-4-aminophenyl)-N'-(2-iodophenyl)urea(77 mg, 0.21 mmol)
was added to HC1/H20 (0.21 mi./0.42 mL), and cooled to 0°C. Sodium
nitrate ( 14.5
mg, 0.21 mmol) was added to the reaction mixture. The reaction mixture was
stirred at
35 0°C for 30 minutes. Sodium azide ( 14 mg, 0.21 mmol) was added to
reaction mixture
and it was warmed to room temperature. The reaction mixture was stirred at
room
101 -
CA 02432662 2003-06-09
WO 97/29')43 PCT/US96113632
temperature for 18 hours. Then it was extracted with three times by ethyl
acetate. The
organic extracts were combined, dried over MgS04, filtered and concentrated
under
reduced pressure and chromatography of the resulting solid on silica gel
(hexane : ethyl
acetate; 5:1 ) gave product (20 mg, 24%). EI-MS m/z 396 (M+H)+.
s
Example 136
Preparation of N-(2-hydroxy-3-azidophenYl-7-N'-l2-bromo henyl)urea
a) Preparation of N-(2-hydroxy-3-aminophenyl)-N'-(2-bromophenyl)urea
To a solution of N-(2-hydroxy-3-nitrophenyl)-N'-(2-bromophenyl)urea (300 mg,
0.85 mmol) in ethanol (20 mL), Tin chloride (958 mg, 4.25 mmol) was added. The
reaction mixture was stirred at reflux for 16 hours then cooled to room
temperature. The
reaction mixture was basified to pH 8 with aq. NaHC03 then extracted with
ethyl
acetate (3x). The organic extracts were combined, dried over MgS04, filtered
and
concentrated under reduced pressure to give product (274 mg, 99%). EI-MS m/z
323
(M+H)+.
b) Preparation of N(2-hydroxy-3-azidoghenyl)-N'-(2-bramophenyl)urea
The N-(2-hydroxy-3-aminophenyl)-N'-(2-bromophenyl)urea(274 mg, 0.85
mmol) was added to HCl/H20 (0.85 mIJl.7 mL), cooled to 0°C. Sodium
nitrate (58.6
mg, 0.85 mmol) was added to the reaction mixture. The reaction mixture was
stirred at
0°C for 30 minutes. Sodium azide (55 mg, 0.85 mmol) was added to
reaction mixture
and it was warmed to room temperature. The reaction mixture was stirred at
room
temperature for 18 hours then it was extracted with three times with ethyl
acetate. The
organic extracts were combined, dried over MgS04, filtered and concentrated
under
reduced pressure and chromatography of the resulting solid on silica gel
(hexane : ethyl
acetate: 5:1) gave product (210 mg, 71°!0). EI-MS m/z 349 (M+H)+.
Exam l~ a 137
Preparation of N-f2-hvd~ox~3-c~ano~henvll-N'-f2-methoxytlhenvll urea
N-[2-hydroxy-3-cyanophenyl]-N'-[2-methoxyphenyl] urea was prepared from 2-
3o amino-6-cyanophenol ( 134mg, 1.00 rrimol) according to the procedure in
General
Method B. The product was purified by precipitation from tnethylene chloride)
hexane(lequiv./20equiv.) and filtering. (230 mg, 81%). 1H NMR (CD30D): d 8.06
(d,
1H), 7.79 {d, 1H), 7.49-7.35 (m, 2H), 7.05-6,87 (m, 3H), 3.95 (s, 3H).
- 102 -
CA 02432662 2003-06-09
WO 97129743 PCT/L1S96113632
Example 138
Preparation of N-f2-h~rdroxy-3-cyanophenyl]-N'~(3-trifluoromethyl~henyIl urea
N-[2-hydraxy-3-cyanophenyl)-N'-[3-trifluoromethylphenyl) urea was prepared
from 2-amino-6-cyanophenol ( 134mg, 1.00 mmoi, example 83a) according to the
procedure in General Method B. The product was purified by precipitation from
methylene chloride/ hexane(lequiv./20equiv,) and filtering. (280mg, 87%). 1H
NMR
(CD30D): d 8.10 (d, 1H), 7.96 (s, 1H), 7.54 (d, 1H), 7.55-7.25 (m, 3H), 7.01
(t, 1H).
Exam L
Preparation of N-f2-hvdroxv-3-cvanonhenylLN'-f2-nhenvLDhenyll a
N-[2-hydroxy-3-cyanophenyl]-N'-[2-phenylphenyl] urea was prepared from 2-
amino-6-cyanophenol ( 134mg, 1.00 mmol, example 83a) according to the
procedure in
General Method B. The product was purified by precipitation from methylene
chloride/
hexane(lequiv./20equiv.) and filtering. (270mg, 82%). 1H NMR (CD30D): d 7.81
(d,
~5 1H), 7.75 (d, 1H), 7.56-7.15 (m, 9H), 6.91 (t, 1H).
Example 140
P~paration of N-12-hydroxx-3-cXanophenylLN~2,3-dichlorophen~rll urea N-[2-
hydroxy-3-cyanophenyl]-N'-[2,3 dichlorophenyl] urea was prepared from 2-amino-
6-
cyanophenol ( 134mg, 1.00 mmol, example 83a) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride!
hexane( lequiv./20equiv.) and filtering. (300mg, 93%). 1H NMR (CD30D): d 8.11
(d,
1H), 8.01 (d, 1H), 7.33-7.25 (m, 3H), 7.00 (t, 1H).
Example 141
Preaaraeion of N-f2-h~rdroxv-4-isonronvlnhenvll-N'-I2.3-dichloronhenvll urea
N-[2-hydroxy-4-isopropylphenyl]-N'-[2,3-dichlorophenyl) urea was prepared from
2-
amino-5-isopropyIphenol ( 150 mg, 1.00 mmol, example 128x) according to the .
procedure in General Method B. The product was purified by precipitation from
3o methylene chloridel hexane(lequiv.l20equiv.) and filtering (285mg, 84%). 1H
NMR
(CD30D): d 8.05 (d, 2H), ?.77 (s, 1H), 7.26 (m, 2H), 6.88 (m, 2H), 2.82 (m,
1H), 1.25
(d, 6H).
- 103 -
CA 02432662 2003-06-09
WO 97129743 PCT/US96113632
Example 142
Pre aration of N- 2-h dro v-4-i o r 1 hen 1 -N'- 2-chior - -t 'flu ro eth I
hen 1
urea
N-[2-hydroxy-4-isopropylphenyl]-N'-[2-chloro-5-trifluoromethylphenyl] urea
was prepared from 2-amino-5-isopropylphenol ( 150mg, I .00 mmol, example 128x)
according to the procedure in General Method B. The .product was purified by
precipitation from methylene chloride/ hexane(lequiv./20equiv.) and filtering.
(275mg,
82%). 1H NMR (CD30D): d 8.50 (s, 1H), 7.70 (s, 1H), 7.51 (d, 1H), 7.22 (d,
1H),
6.70 (m, 2H), 6.62 (dd, IH), 2.76 (m, (1H), 1.16 (d, 6H).
E,~ample 143
Preparation of N-f2-hydroxv-3-phenylphenyll-N'-f2.3-dichlorophenyl_] urea
a)Preparation of 2-nitro-6-phenylphenol
2-phenylphenol (3.00g, 17.6mmo1) was dissolved in methylene chloride(40m1)
followed by the addition of sodium nitrate ( 1.65g, 19.4mmol). The addition of
sulfuric
acid (25m1/ 3M) was then made, followed by addition of a catalytic amount of
sodium
nitrite. The mixture was allowed to stir. After 24 hrs, the reaction mixture
was diluted
with methylene chloride and extracted with water. The organic layer was dried
over
MgS04 and filtered. The solvent was evaporated and chromatography of the
resulting
solid on silica gel (4%MeOH/ CH2C12) gave the desired product(900 mg, 24 %). I
H
NMR (CD3COCD3): d 8.19 (d,lH), 7.79 (d,lH), 7.64 (d, 2H), 7.50 (t, 2H), 7.45
(t,
1H), 7.22 (t, 1H).
b)Preparation of 2-amino-6-phenyiphenol
To.a solution of 2-nitro-6-phenylphenol(900 mg, 4.2mmo1) in methanol(SOmI)
was added 10% Pd/C ( 100 mg). The mixture was flushed with argon, then
hydrogen
was bubbled through the solution for IO min. and a hydrogen atmosphere was
maintained at balloon pressure overnight. The mixture was filtered through
celite and
the ceiite was washed with methanol. The solvent was evaporated and
chromatography
of the resulting solid on silica get (5%MeOH/ CH2Cl2) gave the desired
product(700
mg, 90 %). 1H NMR (CD30D): d 7.55-7.27 (m, SH), 6.77-6.6I (m, 3H)
c)Preparation of N-[2-hydroxy-3-phenylphenyl]-N'-[2,3-dichlorophenyl] urea
N-[2-hydroxy-3-phenylphenyl]-N'-j2,3-dichlorophenyl] urea was prepared from
2- amino-6-phenylphenol (92.Smg, 0.50 mmol) according to the procedure in
General
Method B. The product was purified by precipitation from methylene chloride/
hexane(lequiv./20equiv.) and filtering. (150mg,81%). IH NMR (CD30D): d 8.06
(d,
1H),7.65 (d, 1H), 7.54 (d, 2H),7.40 (t, 2H), 7.32 (d, 1H) 7.22 (m, 2H), 7.04-
6.88
- 104 -
CA 02432662 2003-06-09
WO 97129743 PCTIUS96/13632
Preparation of N-[2-hydroxy-3-phenylphenyl]-N'-[2,3-dichlorophenyl] urea
b)N-[2-hydroxy-3-phenylphenyl]-N'-[2,3-dichlorophenyl] urea was prepared from
2-
amino-6-phenylphenol (92.Smg, 0.50 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
hexane(lequiv./20equiv.) and filtering. (150 mg, 81%). 1H NMR (CD30D): d 8.06
(d,
IH),7.65 (d, 1H), 7.54 (d, 2H),7.40 (t, 2H), 7.32 (d, 1H) 7.22 (m, 2H), 7.04-
6.88 (m, 2H).
Example 144
Preparation of N-12-h~drox_v-5-nitrophenyll-N'-[2-methoxyphenXll urea
N-[2-hydroxy-5-nitrophenyl]-N'-[2-methoxyphenyl] urea was prepared from 2-
amino-4-nitrophenol ( 154 mg, 1.00 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
hexane(
lequiv./20equiv.) and filtering. (270 mg, 89%). 1H NMR (CD30D): d 9.10 (s,
1H),
8.10 (d, 1 H), 7.85 '(d, 1 H), 7.08-b"88 (m, 4H), 3.96 (s, 3H).
Example 145
Preparation of N I2-hvdroxy-S-nitronhenvll-N'-f3-trifluoromethvlnhenvll urea
N-[2-hydroxy-5-nitrophenyl]-N'-[3-trifluoromethylphenyl] urea was prepared
from 2-amino-4-nitrophenol { 154 rng, 1.00 mmol) according to the procedure in
2o General Method B. The product was purified by precipitation from methylene
chloride/
hexane(lequiv.l20equiv.) and filtering. (290 mg, 85%). 1H NMR (CD30D): d 9.12
(s,
IH), 7.89 (d, IH), 7.68 (d, 1H), 7.55 (m, 2H), 7.45 (d, 1H), 7.00 (d, 1H}.
Example 146
Preparation of N-E2-hxdrox, -5-nitro~henyll-N'-I[2-phenyjnhenvll urea N-[2-
hydroxy-5-nitrophenyl]-N'-[2-phenylphenyl] urea was prepared from 2-amino-4-
nitrophenol ( 154 mg, 1.00 mmol) according to the procedure in General Method
B. The
product was purified by precipitation from methylene chloridel
hexane(lequiv./20equiv.) and filtering. (285 mg, 81%). 1H NMR (CD30D): d 8.09
(s,
3o IH}, 7.86 (d, iH), 7.58-7.20 (m, 9H), 6.95 (d, 1H).
Example 147
Preparation of N-L2-hydroxv-5-nitrophenvll-N'-12.3-dichlorophenyll urea
N-[2-hydroxy-5-nitrophenyl]-N'-[2,3-dichlorophenyl] urea was prepared from 2-
amino-4-nitrophenol ( 154 mg, 1.00 mmol) according to the procedure in General
Method B. The product was purified by precipitation from methylene chloride/
- 105 -
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
hexane{ lequiv./20equiv,) and filtering. (290 mg, 85%). 1H NMR (CD30D): d 9.11
(s,
1H), 8.17 (d, 1H), 7.89 (d, 1H), 7.34 (m, 2H), 6.95 (d, 1H).
Example 148
Pr~aration of N-=j2-h dv roxy-5-ethxlsulfonvIphenyll-N'-f2.3-dichlorophenyl],
urea
N-[2-hydroxy-5-ethylsulfonylphenyl)-N'-[2,3-dichlorophenyl] urea was
prepared from 2-amino-4-{ethylsulfonyl)phenol ( 185 mg, 1.00 mmol) according
to the
procedure in General Method B. The product was purified by precipitation from
methylene chloride/ hexane( lequivJ20equiv.) and filtering. (310 mg, 84%). 1H
NMR
t0 (CD30D): b 8.65 (s, IH), 8.18 (d, IH), 7.45 (d, 1H), 7.26 (m, 2H), 7.00 (d,
1H), 3.33
(q, 2H), 1.24 (t, 3H).
The following compounds of Formula (1) have been prepared in accordance with
the examples and schemes as described above:
Example 149 : N-[2-(2-Amino-{4-trifluoromethyl) phenyl) sulfonylamino] phenyl]-
N'-
(2-bromophenyl)ureaEI-MS m/z 527 (M-H)-.
Example 150 : N-[2-(aminosulfonyl phenyl) 3-amino phenyl] N'-(2-bromo phenyl)
ureaEI-MS m/z 426 (M+H)+;
Example 15I : N-[2-[2-(4-Chloro-3-aminophenyl)sulfonylamino]phenyl)-N'-(2-
2o bromophenyl)urea
Example 152 : N-[2-(3-Aminoghenyl)sulfonylaminophenyl]-N'-(2-bromophenyl)urea
Example 153 : N-(2-Hydroxy-3-nitrophenyl)-N'-(2-methoxyphenyi)urea EI-MS m/z
302.3
(M-H)-.
Example 154 : N-(2-Hydroxy-3-nitrophenyl)-N'-(4-methoxyphenyl)urea urea EI-MS
m/z
302.3 (M-H)-.
Example 155 : N-(2-Hydroxy-3-nitrophenyl)-N'-(3-trifluoromethyphenyl)urea urea
EI-MS
mlz 340.3 (M-H)-
Example 156 : N-(2-Hydroxy-3-nitrophenyi)-N'-(2-phenylphenyl)urea 1H NMR
(DMSO),
8.83(lH,s) 8.63(lH,s), 8.41 (lH,d) 7.79 (lH,d), 7.56 (lH,d) 7.51-7.32 (6H,m)
7.23 (lH,ds)
7.18 ( lH,d) 6.97 ( lH,t)
Example 157 : N-(2-Hydroxy-3-nitrophenyl)-N'-(2,3dichlorophenyl) EI-MS m/z
340.3 (M-
H)-
Example 158 : N-(2-Hydroxy-3-nitrophenyl)-N'-(4-phenylphenyl) EI-MS mlz 348,3
(M-
H)-
Example 159 : N-(2-Hydroxy-3-nitrophenyl)-N'-(2,4-d.imethoxyphenyl)urea EI-MS
mlz
333.4. (M+H)+;
_ 106
CA 02432662 2003-06-09
WO 97129743 PCTJUS96113632
Example 160 : N-(2-Hydroxy-3-nitrophenyl)-N'-(2-chloro-5-
trifluoromethyiphenyl}urea
EI-MS m/z 374.2 (M-H)-
Example 161 : N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2-
methoxyphenyl)urea EI-
MS mlz 421.3 (M-H)-
Example 162 : N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(2-phenylphenyl)urea
EI-
MS mJz 467.3 (M-H)-
Example 163 : N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-(3-
trifluoromethyiphenyl)urea EI-MS m1z 459.3 (M-H)-
Example 164 : N-(2-Benzenesulfonylamino-4-cyanophenyl)-N'-
(2,3dichlorophenyl)urea
t o EI-MS m/z 461. I (M+H)+;
Exampie 165 : N-(2-Hydroxy-4-amidinophenyl)-N'-(2-bromophenyl)urea 1H NMR
(CD30D): b 8.10( 1 H,8) 7.92( 1 H,8) 7.58 ( i H,b) 7.40-7.25 (3H,m) 7.02 ( 1
H, t); EI-MS mlz
348.0 (M-H)-
Example 166 : N-(2-Hydroxy-3,4-dichioro phenyl) N'( phenyl) urea EI-MS m/z
297.0
(M+H)+
Example 167 : N-(2-Hydroxy 4-cyano phenyl) N'( phenyl) urea EI-MS m!z 284.0
(M+H)+
Example 168 : N-(2-Hydroxyphenyl 3-carboxylic acid)N'( phenyl) urea EI-MS m/z
273.0
(M+H)+
Example 169 : N-(2-Hydroxy-3-nitrophenyi)-N'-phenylurea EI-MS m/z 274.0 (M+H)+
z0 Example 170 : N-(2-hydroxy-3-cyano phenyl ) N'(phenyl) urea EI-MS m/z 254.0
(M+H)+
Example 171 : N-{2-Hydroxy-3-cyano-4-chlorophenyl)-N'-{2-bromophenyl)urea EI-
MS
m/z 264.2 (M-H)-
Example 172 : N-(2-Hydroxy-3-fluorophenyl)-N'-(phenyl)urea EI-MS m/z 247.0
(M+H)+
Example 173 : N-(2-Hydroxy-3,4-difluorophenyl)-N'-(phenyl)urea EI-MS m/z 265.0
(M+H)+
Example 174 : N-[2-(Benzylsulfonylamino)-4-cyanophenyl]-N'-(2,3-
dichlorophenyl)urea
EI-MS m/z 473.0 (M-H)-
Example 175 : N-[2-(Phenylsulfonylamino)-4-trifluoromethylphenyl]-N'-(2,3-
dichlorophenyl)urea EI-MS rnlz 502.0 (M-H)-
3o Example 176 : N-[2-(3-Pyridinesulfonylamino)-4-cyanophenyl]-N'-(2,3-
dichlorophenyl)urea 1H NMR (CD30D): 8 8.76(lH,s) 8.70(iH,d), 8.19 (lH,d) 8.00
( 1 H, dd) 7.92 ( 1 H,dd) 7.54 ( 1 H. dd) 7.54 ( 1 H, dd) 7.45 ( 1 H,dd) 7.19
( 1 H,d) 7.17 ( 1 H, s)
6.86 (lH,d)
Example 178 : N-[2-(5-Isoquinolinesulfonylamino)-4-cyanophenyl]-N'-(2,3-
dichlorophenyl)urea 1H NMR (CD30D): 8 9.37 (lH,s) 8.51-8.39 (3H,m} 8.29 (lH,d)
8.00
(lH,dd) 7.93 (lH,d) 7.67 (1H, t) 7..50 (lH,dd) 7.25 (lH,d) 7.24 (lH,s) 6.91
(lH,d)
107 -
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
Example I79 : N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-
chlorophenyl)urea EI-
MS mlz 427.0 (M+H)+
Example 180 : N-[(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-fluoro phenyl)
urea EI-MS
m/z 411.0 (M+H)+
Example l 81 : N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-
thiomethylphenyl)urea
EI-MS m/z 439.0 (M+H)+
Example 182 : N-[2-(Phenylsulfonylamino)-4-cyano phenyl]-N'-(2-
trifluoromethoxyphenyl)urea EI-MS m/z 477.0 (M+H)+
Example 183 : N-[2-(Phenylsulfonylamino)-4-cyanophenyl)-N'-(2-
trifluoromethylphenyl)urea EI-MS m/z 461.0 (M+H)+
Example 184 : N-[2-(Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methylphenyl)
urea EI-
MS m/z 407.0 (M+H)+
Example 185 : N-[2-(Phenylsulfonylamino)-4-cyano phenyl]-N'-(2-methoxy 3-
chloro
phenyl) urea EI-MS m/z 457.0 (M+H)+
Example 186 : N-[2-(4-cyanophenyl)-N'-(3-fluoro phenyl) urea EI-MS ~a~/z 409.0
(M-H)-
Example 187 : N-(2-Thiophenesulfonylamino-4-cyanophenyl)-N'-(2,3-
dichlomphenyl)urea
m.p. : 138.5 - 139.2
Example 188 : N-[(2-Pyrid-2-yl)thiophene-5-sulfonylamino-4-cyanophenyl]-N'-
(2,3-
dichlorophenyl)urea ; m.p. : 147.5 - 148.3
2o Example 189 : N-[(2-Acetamino-4-methyl-5-thiazolesulfonylamino-4-
cyanophenyl]-N°-
(2,3-dichlorophenyl)urea EI-MS m/z 540.4 (M+H)+
Example 190 : N-{(2-aminosulfonyiphenyl) 4-cyano phenyl) N'-(2-methyl-3-chloro
phenyl)
urea EI-MS nn/z 439.0 (M-H)-
Example 191 : N-(2-benzenesulfonylamino-3-cyanophenyl)-N'-(2,3-
dichlorophenyi}urea
1H NMR (DMSO): S 10.00 (IH,s) 9.05 (lH,s) 8.93 (lH,s) 8.19 (lH,dd) 8.00
(lH,dd) 7~.72-
7.42 (7H,m) 7.35 ( lH,d) 7.32 ( lH,s)
Example I92 : N-[(Benzylsulfonyiamino)-5-cyanophenyl]-N'-{2,3-
dichlorophenyl)urea EI-
MS cn/z 474.0 (M-H)-
Example 193 : N-[(2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-nitrophenyl)urea
EI-MS
mlz 438.0 (M-H)-
Example 194 : N-[(2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methyl-3-
nitrophenyl)urea EI-MS m/z 450.0 (M-H)-
Example 195 : N-[(2-Phenylsulfonylamino)-4-cyanophenyl]-N'-(2-methyl-3-
aminophenyl)urea EI-MS m/z 422.0 (M+H)+
Example 196 : N-[(2-Phenylsulfonylamino)-4-cyanophenyl)-N-(2-aminophenyl)urea
EI-
MS m/z 408.0 (M+H)+
- 108 -
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
Example 197 ; N-{2-(2-pyridinesulfonylamino-4-cyanophenyl)-N'-(2,3-
dichlorophenyl)urea
1H NMR (CD30D): 8 8.90 ( lH,d) 8.33 (1H>d) 8.14(2H,m) 7.99 (lH,d) 7.78 (lH,dd)
7.67
( 1 H,dd) 7.40 ( 1 H,d) 7.39 ( 1 H,s) 7.18 ( I H,s)
Example 198 : N-(2-Benzenesulfonylamino-3-trifluoromethylphenyl-N'-(2,3-
dichlorophenyl)urea 1H NMR (CD30D): 8 8.0$ (lH,.dd) 7.90 (lH,dd) 7.78 (2H,m)
7.50
(2H,d) 7.41 (3H,m) 7.27 (2H,d)
Example 199 . N-(4-benzenesulphonylthiophene-2-sulphonylamino-4-cyanophenyl}-
N'-
(2,3-dichlorophenyl)urea EI-MS m/z 609.0 (M+H)+
Example 200 : N-(2-trifluoromethylbezenesulfonylamino-4-cyanophenyl)-N'-(2,3-
to dichlorophenyl)urea EI-MS m/z 527.1 (M+H)+
Example 201 : N-(2-Hydroxy-4-cyanophenyl)-N'-(2, 3-methylenedioxyphenyl)urea IH
NMR (CD30D): 8 8.22 (lH,d) 7.49 (lH,d) 7.18 (lH.d) 7.08 (lH,s) 6.82 (lH,t)
6.61(IH,d)
6.00 (2H,s)
Example 202 : N-[2-(2-nitrophenylthio)phenyl]-N'-(2-hydroxy-4-
nitrophenyl)urea; m.p.
204.1 - 205.3
Example 203 : N-{2-hydroxy-3-trifluoromethyiphenyl)-N'-(2,3-
dichlorophenyl)urea; m.p.
204.3 - 205.2
Example 204 : N-(2-hydroxy-3-trifluoromethylphenyl}-N'-(2-phenylphenyl)urea
m.p. 136.7
- 137.3
2o Example 205 : N-(2-Hydroxy-4-nitrophenyl)-N'-(2-benzylphenyl)urea EI-MS m/z
364.0
(M+H)+
Example 206 : N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(phenylthiomethyl)phenyl]urea
EI-MS
m/z 394.0 (M-H)-
Example 207 : N-(2-Hydroxy-4-vitro phenyl~N'-[2-(phenyloxymethyl)phenyl]urea
EI-MS
mlz 378.0 (M-H)-
Example 208 : N-(2-Hydroxy-4-nitrvphenyl)-N'-[2-(phenylethyl}phenyl]urea EI-MS
m/z
376.0 (M-H)-
Example 209 : N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(4-trifluorophenyl)phenyl]urea
EI-MS
mlz 416.0 (M-H)-
Example 210 : N-(2-Hydroxy-3-trifloromethylphenyl~N'-(2-methoxyphenyl)urea EI-
MS
mIz 327.3 (M+H)+
Example 211 : N-(2-Hydroxy-4-nitrophenyl)-N'-(2-acetoxyphenyl)urea EI-MS m/z
332.0
(M+H)+
Example 212 : N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(2-cyanophenylthio)phenyl]urea
EI-MS
m/z 407.0 (M+H)+
- 109 -
CA 02432662 2003-06-09
WO 97129743 PCT/US96113632
Example 213 : N-(2-hydroxy-3-trifluoromethylphenyl)-N'-(2-chlorophenyl)urea
m.p.
179.3°C
Example 214 : N-(2-Hydroxyethyl)-N'-(2-hydroxy-4.-nitrophenyl)urea m.p. 168.2 -
168.8°C
Example 215 : N-2-(benzyoxyphenyl)-N'-(2-hydroxy-4-nitrophenyl)urea m.p. 179.0
-
179.6°C
Example 216 : N-[2-(2-thienylsulfonylamino)phenyl]-N°-(2-hydroxy-4-
nitrophenyl)urea
m.p. 149.0 - 149.6°C
Example 217 : N-(2-Benzenesulfonylamino-4-nitrophenyl)-N'-(2,3-
dichlorophenyi)urea
IH NMR (CD30D): 89.92 (IH,s) 9.68 (iH,s) 9.58 (lH,s) 8.40 (lH,d) 8.14 {IH,dd)
8.00
( I H,d) 7.76-7.57 (6H,m) 7.38 ( 1 H,dO 7.23 ( 1 H,d)
Example 218 : N-(2-Benzenesulfonylamino-4-nitrophenyl)-N'-(2-bromophenyl)urea
IH
NMR (CD30D): 89.89 (IH,s) 9.51{lH,s) 9.35 (lH,s) 8.41(lH,d) 8.13(lH,dd) 7.87
(lH,d)
7.69-7.57 (6H,m) 7.40 ( 1 H,t) 7.22 ( 1 H,dd) 7.10 ( 1 H,t)
Example 219 : N-(2-Benzylsulfonylamino-4-nitrophenyl)-N'-(2-bromophenyl)urea
IH
35 NMR (CD30D): 8 9.58 (IH,s) 9.30 (IH,s) 9.14 (lH,s) 8.33 (lH,d) 8.13-8.05
(2H,m) 7.88
( I H,d) 7.69 ( 1 H,d) 7.50 -7.30 (6H,m) 7.08 ( 1 H,t) 4.61 {2H,s)
Example 220 : N-(2-Benzylsulfonylamino-4-nitrophenyl)-N'-
(2,3dichlorophenyl}urea 1H
NMR (CD30D): 8 9.60 ( 1 H,s) 9.42( 1 H,s) 9.40 ( 1 H,s) 8.32 ( I H,d) 8.15( 1
H,dd) 7.45-7.25
(7H,m} 4.62 (2H,s)
2o Example 221 : N-[2-(3-Pyridylmethoxy)phenylJ-N'-(2-hydroxy-4-
nitrophenyl)urea m.p.
185.4 - 186.2
Example 222 : N-[2-(4-Pyridylmethoxy)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea
m.p.
189.3 - 189.7
Example 223 : N-[2-{Methoxycarbonylamino)phenyl]-N'-{2-hydroxy-4-
nitrophenyl)urea
25 m.p. 199.3 - 199.6
Example 224 : N-[2-(Methylsulfonylamino)-4-nitrophenylJ-N'-(2-bromophenyl)urea
Example 225 : N-[2-(Propylsulfonylamino)-4-nitrophenyl)-N'-(2-bromophenyl)urea
Example 226 : N-[2-(Propylsulfonylamino)-4-nitrophenyl)-N'-{2,3-
dichlorophenyl)urea
30 Example 227 : N-[[(2-acetamino-4-methyl-5-thiazolyl)sulfonylamino]-4-
nitrophenyl]-N'-(2,3dichlorophenyl)urea
Example 228 : N-[2-(3-Pyridinesulfonylamino)-4-nitrophenyl]-N'-(2,3-
dichlorophenyl)urea
Example 229 : N-[2-(3-Pyridinesulfonylamino)-4-nitrophenyl}-N'-(2-
35 bromophenyl)urea
- 110 -
CA 02432662 2003-06-09
WO 97129743 PCTIUS96113632
Example 230 : N-[2-(Methylsulfonylamino)-4-nitrophenyl]-N'-(2,3-
dichlorophenyl)urea
Example 231 : N-(2-Hydroxyeth-1-yloxyphenyl)-N'-(2-hydroxy-4-nitrophenyl)urea
Example 232 : N-(2-Hydroxy-4-cyanophenyl)-N'-(2-benzylaminophenyl)urea m.p.:
108.8-
109.4
Example 233 : N'-[2-(2-Pyridylmethoxy)phenyl]-N'-(2-Hydroxy-4-nitrophenyl)urea
m.p.
193.5-194.0
Example 234 : N-[2-(2-Methoxycarbonylbenzyloxyphenyl]-N-(2-hydroxy-4-
nitrophenyl)urea m.p. 177.2 - 178.0
1o Example 235 : N-[2-(2-Carboxybenzyloxy)phenyl)-N'-(2-hydroxy-4-
nitrophenyl)urea m.p.
164.1
Example 236 : N-[2-{Benzoylamino)phenyl]-N'-(2-hydroxy-4-nitrophenyl)urea m.p.
188.7- 189.3 ° C
t s The following compounds of Formula (I) may be prepared in accordance with
the examples and schemes as described above:
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(benzyloxy)phenyi)urea
N-{2-Hydroxy-4-cyanophenyl)-N'-(2-(2-pyridylmethyloxy)phenyl}urea
N-(2-Hydroxy-4-cyanophenyl)-N'-(2-(3-pyridylmethyloxy)phenyl)urea
2o N-{2-Hydroxy-4-cyanophenyl}-N'-{2-{4-pyridylmethyloxy)phenyl)urea
N-(2-Hydroxy-4-trifluoroacetophenone)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-trifluorosulfonylphenyi)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-bromo-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-chloro-4-cyanophenyl)-N'-(2-bromophenyl)urea
?5 N-(2-Hydroxy-3-triflaoromethyl-4-cyanophenyl)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4.-cyanophenyl-3-carboxylic acid)-N'-(2-bromophenyl)urea
N-(2-Hydroxy-4-trifluoroacetophenone)-N'-(2,3-dichlorophenyl)urea
N-{2-Hydroxy-4-trifluorosulfonylphenyi)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-3-bromo-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
3o N-{2-Hydroxy-3-chloro-4-cyanophenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-3-trifluoromethyl-4-cyanophenyl)-N'-(2,3-dichiorophenyl)urea
N-(2-Hydroxy-4-cyanophenyl-3-carboxylic acid)-N'-{2,3-dichlorophenyl)urea
The following compounds of Formula (I) may be prepared in accordance with
35 the examples and schemes as described above, or may also be purchased
commercially
from well recognized sources. For instance, from Elldrich Chemical Company:
N-(2-Hydroxy-4-nitrophenyl)-N'-phenylurea
111 -
CA 02432662 2003-06-09
WO 97129743 PCT/I1S96/13632
For instance, from the Alfred Bader Collection of Aldrich Chemical:
1-(2-Carboxyphenyl)-3-(3-fluorophenyl)urea
1-( 2-Carboxyphenyl)-3-( 3-chlorophenyl)urea
Available from Gallard Schlesinger Company andlor the Sigma Aldrich Library
of Rare Compounds:
1-(2-Carboxyphenyl)-3-(4-chlorophenyl)urea
!-(p-Anisyl)-3-(2-carboxyphenyl)urea Available from Gallard Schlisinger
Company
l0 2-{3,4-Dichlorophenylcarbonyldiimino)-5-trifluoromethylbenzoic acid
2-(4-Chlorophenylcarbonyldiimino)-5-trifluoromethylbenzoic acid
N-Phenyl-N'-(2-carboxyphenyl)urea
From Maybridge Chemical Company, Cambridge England:
15 I,1'-(4-Methyl-2-phenylene)bis[3-tolyl)]thiourea
N-(5-Chloro-2-hydroxy-4-nitrophenyl)-N'-phenylurea
The following compounds of Formula {I) may be prepared in accordance with
the examples and schemes as described above, or as indicated by their
respective
2o citations in Chemical Abstracts:
1-(m-Anisyi)-3-{2-carboxyphneyl)urea
I -(o-Anisyl)-3-(2-carboxyphenyl)urea
I -(2-Carboxyphenyl)-3-(3,4-dichlorophenyl)urea
1-(2-Carboxyphenyl)-3-(2,4-dichlorophenyl)urea
METHOD OF TREATMENT
The compounds of Formula (I), (la), (Ib), {Ic), (II), (IIa), (IIb), (IIc), and
(III),
or a pharmaceutically acceptable salt thereof can be used in the manufacture
of a
medicament for the prophylactic or therapeutic treatment of any disease state
in a
3o human, or other mammal, which is exacerbated or caused by excessive or
unregulated
IL-8 cytokine production by such mammal's cell, such as but not limited to
monocytes
andlor macrophages, or other chemolcines which bind to the 1L-8 a or b
receptor, also
referred to as the type I or type II receptor.
For purposes herein, the compounds of Formula (I), {Ia), (Ib), (Ic), (II, (Lia
),
(IIb), (IIc), and (III) all have the same dosages, and dosage formulations as
that of
Formula (I) and are used interchangeably.
- II2-
CA 02432662 2003-06-09
WO 97!29743 PCT/US96113632
Accordingly, the present invention provides a method of treating a chemokine
mediated disease, wherein the chemokine is one which binds to an IL,-8 a or b
receptor
and which method comprises administering an effective amount of a compound of
Formula (I) or a pharmaceutically acceptable salt thereof. In particular, the
chemokines ars IL-8, GROG, GR0~3, GROy or NAP-2.
The compounds of Formula (I) are administered in an amount sufficient to
inhibit cytokine function, in particular IL-8, GROa, GROG, GROy or NAP-2 ,
such that
they are biologically regulated down to normal levels of physiological
function, or in
t0 some case to subnormal levels, so as to ameliorate the disease state.
Abnormal levels of
IL-8, GROa, GRO~i, GROy or NAP-2 for instance in the context of the present
invention, constitute: (t) levels of free IL-8 greater than or equal to 1
picogram per mL;
(ii) any cell associated IL-8, GROa, GR0~3, GRO~y or NAP-2 above normal
physiological levels: or (iii)the presence of IL-8, GROG, GRO~, GRO~y or NAP-2
15 above basal levels in cells or tissues in which IL-8, GROG, GRO~i, GROy or
NAP-
2respectively, is produced.
There are many disease states in which excessive or unregulated IL-8
production
is implicated in exacerbating and/or causing the disease. Chemokine mediated
diseases
20 include psoriasis, atopic dermatitis, arthritis, asthma, chronic
obstructive pulmonary
disease, adult respiratory distress syndrome, inflammatory bowel disease,
Crohn's
disease, ulcerative colitis, stroke, septic shock, endotoxic shock, gram
negative sepsis,
toxic shock syndrome, cardiac and-renaFreperfusion~ 'r~rtrry,-
glomerulonephritis,
thrombosis, graft vs. host reaction, alzheimers disease, allograft rejections,
malaria,
25 restinosis, angiogenesis or undesired hematopoietic stem cells release.
These diseases are primarily characterized by massive neutrophil infiltration,
T-
cell infiltration, or neovascular growth, and are associated with increased IL-
8 GROa,
GRO~3, GROY or NAP-2 productian which is responsible for the chemotaxis of
30 neutrophils into the inflammatory site or the directional growth of
endothelial cells. In
contrast to other inflammatory cytokines (IL-1, TNF, and IL-b), IL-8 GROa,
GRO~i,
GROy or NAP-2 has the unique property of promoting neutrophil chemotaxis,
enzyme
release including but not limited to elastase release as well as superoxide
production and
activation. The a-chemokines but particularly, GROG, GRO~, GROy or NAP-2,
35 working through the IL-8 type I or II receptor can promote the
neovascularization of
tumors by promoting the directional growth of endothelial cells. Therefore,
the
113 -
CA 02432662 2003-06-09
WO 97!29743 PCTIUS96/13632
inhibition of IL-8 induced chemotaxis or activation would lead to a direct
reduction in
the neutrophil infiltration.
The compounds of Formula (I) are administered in an amount sufficient to
inhibit IL-8, binding to the IL-8 alpha or beta receptors, from binding to
these receptors,
such as evidenced by a reduction in neutrophil chemotaxis and activation. The
discovery that the compounds of Farmula (I) are inhibitors of IL-8 binding is
based
upon the effects of the compounds of Formulas (I) in the in vitro receptor
binding
assays which are described herein. The compounds of Formula (I) have been
shown to
be dual inhibitors of both recombinant type I and type II IL-8 receptors.
Preferably the
compounds are inhibitors of only one receptor, preferably Type II.
As used herein, the term "IL-8 mediated disease or disease state" refers to
any
and all disease states in which IL-8, GROa, GRO~i, GROy or NAP-2 plays a role,
either
by production of IL-8, GROa, GRO~, GROy or NAP-2 themselves, or by IL-8 GROa,
GRO~i, GROy or NAP-2 causing another monokine to be released, such as but not
limited to IL-1, IL-6 or TNF. A disease state in which, for instance, IL-1 is
a major
component, and whose production or action, is exacerbated or secreted in
response to
IL-8, would therefore be considered a disease stated mediated by IL-8.
As used herein, the term ''chemokine mediated disease or disease state" refers
to
any and all disease states in which a chemokine which binds to an IL-8 a or b
receptor
plays a role, such as but not limited to IL-8, GROG, GRO~, GROy or NAP-2. This
would include a disease state in which, IL-8 plays a role, either by
production of IL-8
itself, or by IL-8 causing another monokine to be released, such as but not
limited to IL-
l, IL-6 or TNF. A disease state in which, for instance, IL-1 is a major
component, and
whose production or action, is exacerbated or secreted in response to IL-8,
would
therefore be considered a disease stated mediated by IL-8.
As used herein, the term "cytokine" refers to any secreted polypeptide that
affects the functions of cells and is a molecule which modulates interactions
between
cells in the immune, inflammatory or hematopoietic response. A cytokine
includes, but
is not limited to, monokines and Iymphokines, regardless of which cells
produce them.
For instance, a monokine is generally referred to as being produced and
secreted by a
mononuclear cell, such as a macrophage and/or monocyte. Many other cells
however
also produce monokines, such as natural killer cells, fibroblasts, basophils,
neutrophils,
endothelial cells, brain astrocytes, bone marrow stromal cells, epideral
keratinocytes
114
CA 02432662 2003-06-09
WO 97IZ9743 P(;TIL1S96113632
and B-lymphocytes. Lymphokines are generally referred to as being produced by
lymphocyte cells. Examples of cytokines include, but are not limited to,
Interleukin-1
(IL-1), Interleukin-6 (IL-6), Interleukin-8 (IL-8), Tumor Necrosis Factor-
alpha (TNF-
a) and Tumor Necrosis Factor beta (TNF-B).
As used herein, the term "chemokine" refers to any secreted polypeptide that
affects the functions of cells and is a molecule which modulates interactions
between
cells in the immune, inflammatory or hematopoietic response, similar to the
term
"cytokine" above. A chemokine is primarily secreted through cell
transmembranes and
causes chemotaxis and activation of specific white blood cells and leukocytes,
neutrophils, monocytes, macrophages, T-cells, B-cells, endothelial cells and
smooth
muscle cells. Examples of chemokines include, but are not limited to, IL-8
GROG,
GRO~i, GROy, NAP-2, IP-10, MIP-la, MIP-b, PF4, and MCP l, 2, and 3.
In order to use a compound of Formula (I) or a pharmaceutically acceptable
salt
thereof in therapy, it will normally be formulated into a pharmaceutical
composition in
accordance with standard pharmaceutical practice. This invention, therefore,
also
relates to a pharmaceutical composition comprising an effective, non-toxic
amount of a
compound of Formula (I) and a pharmaceutically acceptable carrier or diluent.
Compounds of Formula (I), pharmaceutically acceptable salts thereof and
pharmaceutical compositions incorporating such may conveniently be
administered by
any of the routes conventionally used for drug administration, for instance,
orally,
topically, parenterally or by inhalation. The compounds of Formula (n may be
administered in conventional dosage forms prepared by combining a compound of
Formula (I) with standard pharmaceutical carriers according to conventional
procedures. The compounds of Formula (I) may also be administered in
conventional
dosages in combination with a known, second therapeutically active compound.
These
procedures may involve mixing, granulating and compressing or dissolving the
34 ingredients as appropriate to the desired preparation. It will be
appreciated that the
form and character of the pharmaceutically acceptable character or diluent is
dictated by
the amount of active ingredient with which it is to be combined, the route of
administration and other well-known variables. The carriers) must be
"acceptable" in
the sense of being compatible with the other ingredients of the formulation
and not
deleterious to the recipient thereof.
- 115 -
CA 02432662 2003-06-09
WO 97/29743 PCT/US96113632
The pharmaceutical carrier employed may be, for example, either a solid or
liquid. Exemplary of solid carriers are lactose, terra alba, sucrose, talc,
gelatin, agar,
pectin, acacia, magnesium stearate, stearic acid and the like. Exemplary of
liquid
carriers are syrup, peanut oil, olive oil, water and the like. Similarly, the
carrier or
diluent may include time delay material well known to the art, such as
glyceryi mono-
stearate or glyceryl distearate alone, or with a wax.
A wide variety of pharmaceutical forms can be employed. Thus, if a solid
carrier is used, the preparation can be tableted, placed in a hard gelatin
capsule in
powder or pellet form or in the form of a troche or lozenge. The amount of
solid carrier
will vary widely but preferably will be from about 25mg. to about 1g. When a
liquid
carrier is used, the preparation will be in the form of a syrup, emulsion,
soft gelatin
capsule, sterile injectable liquid such as an ampule or nonaqueous liquid
suspension.
Compounds of Formula (I) may be administered topically, that is by non-
systemic administration. This includes the application of a compound of
Formula (n
externally to the epidermis or the buccal cavity and the instillation of such
a compound
into the ear, eye and nose, such that the compound does not significantly
enter the blood
stream. In contrast, systemic administration refers to oral, intravenous,
intraperitoneal
and intramuscular administration.
Formulations suitable for topical administration include liquid or semi-liquid
preparations suitable for penetration through the skin to the site of
inflammation such as
liniments, lotions, creams, ointments or pastes, and drops suitable for
administration to
the eye, ear or nose. The active ingredient may comprise, for topical
administration,
from 0.001 % to 10% w/w, for instance from 1 % to 2% by weight of the
Formulation.
It may however comprise as much as 10% w/w but preferably will comprise less
than
5% w/w, more preferably from 0.1% to 1% w/w of the Formulation.
Lotions according to the present invention include those suitable for
application
to the skin or eye. An eye lotion may comprise a sterile aqueous solution
optionally
containing a bactericide and may be prepared by methods similar to those for
the
preparation of drops. Lotions or liniments for application to the skin may
also include
an agent to hasten drying and to cool the skin, such as an alcohol or acetone,
and/or a
moisturizer such as glycerol or an oil such as castor oil or arachis oil.
- 116 -
CA 02432662 2003-06-09
WO 97!29743 PCT/US96/I3632
Creams, ointments or pastes according to the present invention are semi-solid
formulations of the active ingredient for external application. They may be
made by
mixing the active ingredient in finely-divided or powdered form, alone or in
solution or
suspension in an aqueous or non-aqueous fluid, with the aid of suitable
machinery, with
a greasy or non-greasy base. The base may comprise hydrocarbons such as hard,
soft or
liquid paraffin, glycerol, beeswax, a metallic soap; a mucilage; an oil of
natural origin
such as almond, corn, arachis, castor or olive oil; wool fat or its
derivatives or a fatty
acid such as steric or oleic acid together with an alcohol such as propylene
glycol or a
macrogel. The formulation may incorporate any suitable surface active agent
such as an
to anionic, cationic or non-ionic surfactant such as a sorbitan ester or a
polyoxyethylene
derivative thereof. Suspending agents such as natural gums, cellulose
derivatives or
inorganic materials such as silicaceous silicas, and other ingredients such as
lanolin,
may also be included.
Drops according to the present invention may comprise sterile aqueous or oily
solutions or suspensions and may be prepared by dissolving the active
ingredient in a
suitable aqueous solution of a bactericidal and/or fungicidal agent and/or any
other
suitable preservative, and preferably including a surface active agent. The
resulting
solution may then be clarified by filtration, transferred to a suitable
container which is
2o then sealed and sterilized by autoclaving or maintaining at 98-100
°C. for half an hour.
Alternatively, the solution may be sterilized by filtration and transferred to
the container
by an aseptic technique. Examples of bactericidal and fungicidal agents
suitable for
inclusion in the drops are phenylmercuric nitrate or acetate (0.002%),
benzallconium
chloride (0.01 %) and chlorhexidine acetate (0.01 %). Suitable solvents for
the
preparation of an oily solution include glycerol, diluted alcohol and
propylene glycol.
Compounds of formula (I) may be administered parenterally, that is by
intravenous, intramuscular, subcutaneous intranasal, intrarectal, intravaginal
or
intraperitoneal administration. The subcutaneous and intramuscular forms of
parenteral
administration are generally preferred. Appropriate dosage forms for such
adminisuation may be prepared by conventional techniques. Compounds of Formula
(I) may also be administered by inhalation, that is by intranasal and oral
inhalation
administration. Appropriate dosage forms for such administration, such as an
aerosol
formulation or a metered dose inhaler, may be prepared by conventional
techniques.
For all methods of use disclosed herein for the compounds of Formula (1], the
daily oral dosage regimen will preferably be from about 0.01 to about 80 mg/kg
of total
117 -
CA 02432662 2003-06-09
WO 97/29743 PCT/US96/13632
body weight. The daily parenteral dosage regimen about 0.001 to about 80 mg/kg
of
total body weight. The daily topical dosage regimen will preferably be from
0.1 mg to
1 SO mg, administered one to four, preferably two or three times daily. The
daily
inhalation dosage regimen will preferably be from about 0.01 mg/kg to about 1
mg/kg
per day. It will also be recognized by one of skill in the art that the
optimal quantity
and spacing of individual dosages of a compound of Formula (I) or a
pharmaceutically
acceptable salt thereof will be determined by the nature and extent of the
condition
being treated, the form, route and site of adminisuation, and the particular
patient being
treated, and that such optimums can be determined by conventional techniques.
It will
also be appreciated by one of skill in the art that the optimal course of
treatment, i.e.,
the number of doses of a compound of Formula (I) or a pharmaceutically
acceptable salt
thereof given per day for a defined number of days, can be ascertained by
those skilled
in the art using conventional course of treatment determination tests.
The invention will now be described by reference to the following biological
examples which are merely illustrative and are not to be construed as a
limitation of the
scope of the present invention.
BIOLOGICAL EXAMPLES
The IL-8, and Gro-a chemokine inhibitiory effects of compounds of the present
invention were determined by the following in vitro assaye
Receptor Binding Assays:
~ 125I~ R,_g (human recombinant) was obtained from Amersham Corp.,
Arlington Heights, 1L, with specific activity 2000 Ci/mmol. Gro-a was obtained
from
NEN- New England Nuclear. All other chemicals were of analytical grade. High
levels
of recombinant human IL-8 type a and b receptors were individually expressed
in
Chinese hamster ovary cells as described previously (Holmes, et al.,
Science,1991, 253,
1278). The Chinese hamster ovary membranes were homogenized according to a
previously described protocol (Haour, et al., J Biol Chem., 249 pp 2195-2205
(1974)).
Except that the homogenization buffer was changed to lOmM Tris-HCL. 1mM MgS04,
0.5mM EDTA (ethylene-diaminetetra-acetic acid), ImMPMSF (a-toluenesulphonyl
fluoride), 0.5 mglL Leupeptin, pH 7.5. Membrane protein concentration was
determined using Pierce Co. micro-assay kit using bovine serum albumin as a
standard.
All assays were performed in a 96-well micro plate format. Each reaction
mixture
contained 1251 ~-g (0_25 nM) or 1251 Gro_a and 0.5 pg/mL of IL-8Ra or 1.0
pg/mL of
IL-8Rb membranes in 20 mM Bis-Trispropane and 0.4 mM Tris HCl buffers, pH 8.0,
containing 1.2 mM MgS04, 0.1 mM EDTA, 25 mM NaC1 and 0.03° CHAPS. In
- 118 -
CA 02432662 2003-06-09
WO 97!29743 PCT/US96/13632
addition, drug or compound of interest was added which had been pre-dissolved
in
DMSO so as to reach a final concentration of between O.OlnM and 100 uM. The
assay
was initiated by addition of 125I-IL_g. After 1 hour at room temperature the
plate was
harvested using a Tomtec 96-well harvester onto a glass fiber filtermat
blocked with 1
polyethylenimine/0.5% BSA and washed 3 times with 25 mM NaCI> 10 mM TrisHCl, 1
mM MgS04, 0.5 rnM EDTA, 0.03 % CHAPS, gH 7.4. The filter was then dried and
counted on the Betaplate liquid scintillation counter. The recombinant IL-8
Ra, or Type
I, receptor is also referred to herein as the non-permissive receptor and the
recombinant
IL,-8 Rb, or Type II, receptor is referred to as the permissive receptor.
All of the exemplified compounds of Formulas (I) to (!in noted herein in the
Synthetic Chemistry Section, of Examples 1 to 222 plus the additional
purchased
compounds demonstrated an IC50 from about 45 to about <1 ~tg/mL in the
permissive
models for II,-8 receptor inhibition. All of these compounds were also found
to be
inhibitors of Gro-a binding at about the same level. The compound 1-(2-
Carboxyphenyl)-3-(4-chloro-2-methylphenyl)urea was found to be active at about
75
pg/mL.
The following compounds, generally tested at levels of up to 45 ltg/mL were
2o found to not demonstrate levels of IL-8 receptor antagonism within the
criteria set forth
above at the dosage levels tested. These compounds are:
1-(4-Chloro-alpha,alpha,alpha-trifluoro-3-tolyl)-3-{2-(4-chlorophenyl)thio]-5-
chlorophenylurea
1-(6-Chlorv-alpha,alpha,alpha-trifluoro-3-tolyl)-3-[2-(4-chlorophenoxy)-5-
chlorophenyl]urea
1-(2-Mercaptophenyl)-3-phenyl-2-thiourea
1-( 2-Hydroxyphenyl)-3-phenyl-2-thiourea
3,3'-(Carbonothioyldiimino)bis[4-hydroxybenzoic acid]
m,m'-( 1,3-thioureylene)di(4-hydroxybenzoic acid)
1-(2-Tolyl}-3-(3-chloro-6-hydroxyphenyl)-2-thiourea
1-{(2-Hydroxy-4-aminophenyl)]-(3-phenyl)-urea
N-(2-Carboxy-4-trifluromethylphenyl)-N'-(3-chlorophenyl)urea
N-(2-Carboxyphenyl)-N'-(2,5-dichlorophenyl)urea
1-(2-Carboxyphenyl)-3-(2-ChToro-~-trifluoromethylphenyl)urea
2-[2-[3-(4-Bromophenyl)ureido]-4-trifluoromethylphenoxy]benzoic acid;
2-{2-[3-(4-Chlorophenyl)ureido]phenoxyJbenozic acid
2-{2-[3-(4-Chloro3-(trifluromethyl)phenyl)ureido]phenoxy]benozic acid
- 119
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
N- (2-Hydroxyphenyl) -N'-phenyl urea N-[2-Hydroxy-5-(methoxycarbonyl)phenyl]-
N'-
phenylurea
N-[4-Carboxy-2-hydroxyphenyl]-n'-phenylurea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-nitrophenyl)urea;
1-(2-Carboxyphenyl)-3-(2,6-xylyl)urea
1-( 6-Carboxy-2,4-dichlorophenyl)-3-(2,4,6-trichlorophenyl)urea
1-(2-Carboxyphenyl)-3-(2,5-dimethoxyphenyl)urea
1-{2-Carboxyphenyl)-3-(2-methylphenyl)urea
1-[(2-Hydroxyphenyl)-3-(2-methyl)-5-nitrophenyl]urea
1-(2,5-Dichlorophenyl)-3-(2-hydroxy-4-nitrophenyl)urea
1-(2-Carboxyphenyl)-3-(4-chloro-2-methylphenyl)urea
N-(2-phenylsulfonylaminophenyl-N'-phenylurea
N-(2-Hydroxy-4.-nitrophenyl)-N'-(4-ethoxycarbonylphenyl)urea
N-{2-Hydroxy-4-nitrophenyl)-N'-(2-ethoxycarbonylphenyl)urea
N-(2-Hydroxy-4-nitropheny!)-N'-(3-ethoxycarbonylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-phenylphenyl)urea
N-{2-Hydroxy-4-nitrophenyl)-N'-(4-phenoxyphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(4-propylphenyl)urea
N-(4-Trifluromethyl-2-(4-nitrobenzenesulfonyl)amino}-N'-phenylurea
2o N-{3-Carboxyphenyl)-N'-2-hydroxy-4-nitrophenyl)urea
N-{4-Trifluromechyl-2-(methylsulfonyl)amino]-N'-phenylurea
N-(2-Hydroxy-4-nitrophenyl)-N'-[2-(isopropyl)phenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2,6-dimethylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-fluoro-5-nitrophenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-chloro-5-trifluromethylphenyl)urea
N-(2-Hydroxy-4-nitrophenyl)-N'-(2-methoxy-4-nitrophenyl)urea
N-(2-Hydroxy-1-napthyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-5-ethylsulfonylphenyl)-N'-(2-bromophenyl)urea
N-(2-hydroxy 3,4 dichlorophenyl )-N'-(4-phenylphenyl)urea
3o N-{2-hydroxy-3-naphthyl)-N'-(2-methoxyphenyl)urea
N-(2-hydroxy-3-naphthyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-3-naphthyl)-N'-(4-methoxyphbnyl)urea
N-(2-Hydroxy-3-naphthyl)-N'-{3-trifluoromethylphenyl)urea
N-(2-Hydroxy-3-naphthyl)-N'-(4-phenylphenyl)urea
N-[2-(2-Carboxyphenylsulfonylamino)phenyl]-N'-(2-bromophenyl)urea
N-(2-Hydroxy-3-phenylphenyl)-N'-(2-methoxyphenyi)urea
N-(2-Hydroxy-3-phenylphenyl)-N'-(4-methoxyphenyl)urea
120 -
CA 02432662 2003-06-09
WO 97!29743 PCTlUS96113632
N-(2-Hydroxy-3-phenylphenyl)-N'-(3-triflouromethylphenyi)urea
N-(2-Hydroxy-3-phenylphenyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-3-phenylphenyl)-N'-{4-phenylphenyl)urea
N-[2-[(2,5-Dichlorothien3-yl)sulfonylamino]phenyl]-N'-(2-bromophenyl)urea
N-(2-Hydroxy,3,4-dichiorophenyl~N'-(2,4 dimethoxyphenyi)urea
N-(2-Hydroxy,3,4-dichlorophenyl)-N'-(2-chloro-5-trifloromethylphenyl)urea
N-(2-Hydroxy-3-naphthyl)-N'-(2,4 dimethoxyphenyl)urea
N-(2-Hydroxy-3-naphthyl)-N'-(2-chloro-5-trifluoromethylphenyl)urea
N-(2-Hydroxy-3 phenyiphenyl)-N'-{2,4-dimethoxyphenyl)urea
to N-{2-Hydroxy-4-isopropylphenyl)-N'-(2,4-dimethoxyphenyl)urea
N-(2-Hydroxy-3-phenylphenyl}-N'-(2-chloro-5-trifluoromethylpheny!)urea
N-(2-Hydroxy-5-nitrophenyl)-N'-(2,4-dimethoxyphenyl)urea
N-(2-Hydroxy-5-nitrophenyl)-N'-(2-chloro-5-trifluoromethylphenyl)urea
N-(2-Hydroxy-3-cyanophenyl}-N'-(4-methoxyphenyl)urea
t5 N-(2-Hydroxy-3-cyanophenyl)-N'-(4-phenylphenyl)urea
N-(2-Hydroxy-3-cyanophenyl)-N'-(2,4 dimethoxyphenyl)urea
N-(2-Hydroxy-3-cyanophenyl)-N'-(2-chloro-5-trifluoromethylphenyl)urea
N-(2-Hydroxy- 5-phenylphenyl)-N'-(2-methoxyphenyl)urea
N-(2-Hydroxy- 5-phenylphenyl)-N'-(4-methoxyphenyl)urea
z0 N-(2-Hydroxy- 5-phenylphenyl)-N'-(3-trifluoromethylphenyl)urea
N-{2-Hydroxy- 5-phenylphenyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-5-phenylphenyl)-N'-(4-phenylphenyl)urea
N-(2-Hydroxy-5-phenylphenyl)-N'-(2,3-dichlorophenyl)urea
N-(2-Hydroxy-5-phenylphenyl)-N'-(2,4-dimethoxypheny!)urea
25 N-(2-Hydroxy-5-phenylphenyl)-N'-(2-chloro-5-trifluoromethylpheny!}urea
N-(2-Hydroxy-5-ethylsulfonylphenyl}-N'-(4-methoxyphenyl)urea .
N-(2-Hydroxy-5-ethylsulfonylphenyl)-N'-(3-trifluoromethylphenyl)urea
N-(2-Hydroxy-5-ethylsulfonylphenyl)-N'-(2-phenylphenyl)urea
N-(2-Hydroxy-5-ethylsulfonylphenyl}-N'-(4-phenylphenyl)urea
3o N-(2-Hydroxy-5-ethylsulfonylphenyl)-N'-(2,4-dimethoxyphenyl}urea
N-(2-Hydroxy-5-ethylsulfonylphenyl)-N'-(2-chloro-5-trifluoromethylphenyl)urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2,4 dimethoxyphenyl] urea
N-[2-Hydroxy-3,4-dichlorophenyl]-N'-[2-chloro-5-trifluoromethylphenyl] urea
N-[2-Hydroxy-3-naphthyl]-N'-[3-trifluoromethylphenyl] urea
Chemotaxis Assav
- I21 -
CA 02432662 2003-06-09
WO 97129743 PCTIUS96/13632
The in vitro inhibitory properties of these compounds were determined in the
neutrophil chemotaxis assay as described in Current Protocols in Immunology,
vol I,
Suppl 1, Unit 6.12.3., whose disclosure is incorporated herein by reference in
its
entirety. Neutrophils where isolated from human blood as described in Current
Protocols in Immunology Vol I, Suppl 1 Unit 7.23.1, whose disclosure is
incorporated
herein by reference in its entirety. The chemoattractants IL-8, GRO-a, GRO-b,
GRO-g
and NAP-2 where placed in the bottom chamber of a 48 multiwell chamber (Neuro
Probe, Cabin John, MD) at a concentration between 0.1 and 100 nM. The two
chambers where separated by a Sum polycarbonate filter. When compounds of this
invention were tested, they where mixed with the cells (0.001 - 1000 nM) just
prior to
the addition of the cells to the upper chamber. Incubation was allowed to
proceed for
between about 45 and 90 min at about 37oC in a humidified incubator with 5%
C02.
At the end of the incubation period, the polycarbonate membrane was removed
and the
top side washed, the membrane was then stained using the Diff Quick staining
protocol
t5 (Baxter Products, McGaw Park, IL, USA). Cell which had chemotaxed to the
chemokine were visually counted 'using a microscope. Generally, four fields
where
counted for each sample, these number where averaged to give the average
number of
cells which had migrated. Each sample was tested in triplicate and each
compound
repeated at least four times. To certain cells (positive control cells) no
compound was
added, these cells represent the maximum chemotactic response of the cells. In
the
case where a negative control (unstimulated) was desired, no chemokine was
added to
the bottom chamber. The difference between the positive control and the
negative
control represents the chemotactic activity of the cells.
Elastase Release Assav:
The compounds of this invention where tested for their ability to prevent
Elastase release from human neutrophils. Neutrophils where isolated from human
blood
as described in Current Protocols in Immunology Vol I, Suppl 1 Unit 7.23.1.
PMNs
0.88 x 106 cells suspended in Ringer's Solution (NaCI 118, KCl 4.56, NaHC03
25,
3o KH2P04 1.03, Glucose 11.1, HEPES 5 mM, pH 7.4) where placed in each well of
a 96
well plate in a volume of 50 u1. To this plate was added the test compound
(0.001 -
1000 nM) in a volume of 50 u1, Cytochalasin B in a volume of 50 u1 (20ug/ml)
and
Ringers buffer in a volume of 50 u!. These cells where allowed to warm (37 oC,
5%
C02, 95% RH) for 5 min before IL-8, GROa, GROb, GROg or NAP-2 at a f nal
concentration of 0.01 - 1000 nM was added. The reaction was allowed to proceed
for
min before the 96 well piate was centrifuged (800 xg 5 min) and 100 u1 of the
supernatant removed. This suppernatant was added to a second 96 well plate
followed
- 122 -
CA 02432662 2003-06-09
WO 97129743 PCT/US96/13632
by an artificial elastase substrate (MeOSuc-Ala-Ala-Pro-Val-AMC, Nova
Biochern, La
Jolla, CA) to a final concentration of 6 ug/ml dissolved in phosphate buffered
saline.
Immediately, the plate was placed in a fluorescent 96 well plate reader
(Cytofluor 2350,
Millipore, Bedford, MA) and data collected at 3 min intervals according to the
method
of Nakajima et al 3. Biol Chem 254 4027 ( 1979}. The amount of Elastase
released from
the PMNs was calculated by measuring the rate of MeOSuc-Ala-Ala-Pro-VaI-AMC
degradation.
The above description fully discloses the invention including preferred
to embodiments thereof. Modifications and improvements of the embodiments
specifically disclosed herein are within the scope of the following claims.
Without
further elaboration, it is believed that one skilled in the are can, using the
preceding
description, utilize the present invention to its fullest extent. Therefore
the~Examples
herein are to be construed as merely illustrative and not a limitation of the
scope of the
present invention in any way. The embodiments of the invention in which an
exclusive
property or privilege is claimed are defined as follows.
- 123 -