Note: Descriptions are shown in the official language in which they were submitted.
CA 02435050 2003-07-23
60895-1606D
- 1 -
COMPOSITIONS, SYSTEMS, AND METHODS FOR
CREATING IN SITU, CHEMICALLY CROSS-LINKED,
MECHANICAL BARRIERS OR COVERING STRUCTURES
This is a divisional application of Canadian
Patent Application Ser. No. 2,340,648 filed on
August 25, 1999. The subject matter of this divisional
application is restricted to a biocompatible and
biodegradable material to be applied to seal a vascular
puncture comprising: (A) a buffered protein solution having
a pH value of between about 7 and about 10 and comprising
recombinant or natural human serum albumin at a
concentration of about 25~ or less, and (B) a polymer
solution comprising cross-linkable polyethylene
glycol)(PEG) derivative with a functionality of at least
three, wherein, upon mixing, the buffered protein solution
and the polymer solution cross-link to form a solid matrix
that seals the vascular puncture without use of a photo-
initiator and ultraviolet light energy.
FIELD OF THE INVENTION
The invention generally relates to the formation
and application of barrier materials in a tissue region,
e.g., to close vascular puncture sites in humans and other
animals. The invention also generally relates systems and
methods for arresting or controlling the bleeding or leakage
of fluid in body tissues, e.9., diffuse organ bleeding, lung
punctures, anastomotic leakage, and the like.
However, it should be borne in mind that the
expression "this invention" or the like throughout the
specification encompasses the subject matters of both the
divisional and parent applications.
CA 02435050 2003-07-23
60895-1606D
- la -
BACKGROUND OF THE INVENTION
There are over seven million diagnostic and
therapeutic coronary interventions performed each year. By
far, the majority of these interventions are performed using
percutaneous puncture of the femoral artery to gain access
to the arterial system.
Once the intervention is concluded, the vascular
puncture site has to be sealed to prevent bleeding, while
natural healing processes close the puncture site.
Conventional management of the puncture site has typically
involved external compression using, e.g., digital pressure,
C-clamps, or sandbags, followed by immobilization and bed
rest. Proper placement of compression devices to stop
bleeding calls for trained clinical skills. Likewise,
strong nursing skills are required to monitor for
rebleeding. The patient can suffer local
CA 02435050 2003-07-23
60895-1606D
' - 2 -
discomfort, which may exceed the pain associated
with the diagnostic or therapeutic procedure
requiring vascular access in the first instance.
Complications are not uncommon, which can lead to
prolonged hospitalization, transfusion, and direct
surgical repair of the puncture site.
Various alternative methods for sealing a
vascular puncture site.have been tried. For example,
collagen plugs have been used to occlude the
puncture orifice. The collagen plugs are intended
to activate platelets and accelerate the natural
healing process. Holding the collagen seals in place
using an anchor located inside the artery has also
been tried. Still, patient immobilization is
required until clot formation stabilizes the site.
Other problems, such as distal embolization of the
collagen, rebleeding, and the need for external
pressure to achieve hemostatis, also persist.
As another example, devices that surgically
suture the puncture site percutaneously have also
been used. The devices require the practice of fine
surgical skills to place four needles at a precise
distance from the edges of the puncture orifice and
to form an. array of suture knots, which are
tightened, resulting in puncture edge apposition.
There remains a need for fast and
straightforward mechanical and chemical systems and
methods to close vascular puncture .sites and to
accelerate the patient's return to ambulatory status
without pain and prolonged immobilization.
Bleeding may also be caused by trauma, e.g.
splenic, kidney, and liver lacerations, or may be
caused during surgery, e.g. tumor removal or bone
bleeding. Hemostatic barriers are routinely called
upon to control this type of bleeding.
CA 02435050 2003-07-23
60895-1606D
' - 3 -
Bleeding is conventionally controlled by
the application of solid sheets of material, e.g.
gauze, GelfoamT"" material, or SurgicelT"' material.
These materials can be soaked with a hemostatic
agent, such~as thrombin or epinephrine, or sprayable
formulations such as fibrin glue.
Conventional treatment modalities require
the use of these hemostatic agents in conjunction
with pressure to achieve hemostasis. The various
hemostatic agents can include coagulation factors
(e. g. thrombin), platelet activators (e. g.
collagen), vasoconstrictors (epinephrine), or
fibrinolytic inhibitors.
In some instances, conventional treatments
achieve hemostasis in a clinically acceptable time.
Still, there are a number of drawbacks.
For example, many treatment modalities
consist of bovine collagen and bovine thrombin to
cause the desired clotting action. These products
have the potential for the transmission to humans of
bovine spongiform encephalopathy (also called "Mad
Cow Disease"). Regardless, the bovine thrombin
marketed today is relatively impure, and these
impurities can lead to complications in certain
patient populations. Furthermore, fibrin glue,
generally composed of purified fibrinogen and
thrombin from pooled human blood, has safety and
efficacy concerns as well. Additionally, many
products do not achieve hemostasis in a clinically
acceptable period, particularly in cases of brisk
bleeding.
In addition to hemostatic agents, surgical
sealants are also commonly used to control bleeding
or fluid leakage along anastomoses formed by suture
or staple lines, e.g., between blood vessels, bowel,
CA 02435050 2003-07-23
60895-1606D
' - 4 -
or lung tissue. In cases of blood leakage, fibrin
glue can be utilized to seal an anastomosis. Still,
fibrin glue's lack of adhesion to moist tissue,
safety concerns, and cost precludes its widespread
use as a surgical sealant for blood vessel
anastomoses.
Conventional hemostatic agents and surgical
sealants for blood vessel anastomoses achieve
hemostasis using the application of pressure and by'
activating the coagulation pathway of the blood.
Yet, many of the surgeries where hemostatic barriers
and surgical sealants are required also require the
administration of anti-coagulation therapies, such
as heparin. The hemostatic barrier or surgical
sealant, which is promoting coagulation, is hindered
by the effect of the heparin, which is preventing
coagulation.
Despite conventional treatment modalities
for hemostatic barriers and surgical sealants, there
is a need for a biomaterial that safely, quickly,
and reliably arrests or controls fluid leakage in
body tissues through the application of pressure and
without interaction with the patient's coagulation
pathways.
SUMMARY OF THE INVENTION
The invention provides biocompatible and
biodegradable matrix materials which can be applied,
e.g., to seal a vascular puncture site, to arrest
the flow of blood or fluid from body tissue, or to
arrest diffuse bleeding.
One embodiment provides a compound, which
is chemically cross-linked without use of an enzyme
to form a non-liquid mechanical barrier, which can
be used, e.g., to seal a vascular puncture site. In
one embodiment, the compound comprises a mixture of
CA 02435050 2003-07-23
60895-1606D
- 5 -
a first liquid component and a second liquid
component chemically cross-linked, without use of an
enzyme, to form the non-liquid mechanical matrix. In
one embodiment, the compound comprising a mixture of
a protein solution and a polymer solution including
a derivative of a hydrophilic polymer with a
functionality of at least three, wherein, upon
mixing, the protein solution and the polymer
solution cross-link to form a mechanical non-liquid
matrix.
Another embodiment provides a hydrogel
compound free of a hemostatic agent, which can be
applied to provide a covering structure that, e.g.,
arrests the flow of blood or fluid from body tissue
or arrests diffuse bleeding.
Another embodiment provides a biocompatible
and biodegradable material, which can be applied,
e.g., to arrest the flow of blood or to seal tissue,
comprising a mixture of a protein solution and a
polymer solution including a derivative of a
hydrophilic polymer with a functionality of at least
three, wherein, upon mixing, the protein solution
and the polymer solution cross-link to form a
mechanical non-liquid covering structure.
Another embodiment provides a system for
applying in contact with a tissue region a
biocompatible, non-liquid matrix. The system
comprises a delivery device defining a fluid
delivery channel movable into association with the
tissue region. The system also includes a first
dispenser containing a protein solution, and a
second dispenser containing a polymer solution
including a derivative of a hydrophilic polymer with
a functionality of at least three. The system
includes an introduces attachable in communication
CA 02435050 2003-07-23
60895-1606D
' - 6 -
with the fluid delivery channel and including a
holder to mutually support the first and second
dispensers while conveying the protein solution and
polymer solution from the dispensers into the fluid
delivery channel for mixing as a result of flow
through the channel, wherein, upon mixing, the
protein solution and the polymer solution cross-link
to form the non-liquid matrix.
Features and advantages of the inventions
are set forth in the following Description and
Drawings, as well as in the appended Claims.
BRIEF DESCRIPTION OF THE DRAAINGS
Fig. 1 is a plan view of a system for
creating a mechanical barrier to seal a vascular
puncture site, showing the components of the system
prepackaged in a site access kit and a barrier
component kit;
Fig. 2 is an exploded plan view of the
contents of the site access kit and barrier
component kit shown in Fig. 1, illustrating their'
assembly for use;
Fig. 3 is an enlarged view of the distal
end of the catheter tube of a catheter device
contained in the site access kit shown in Fig. 1,
showing two deformable regions in a relaxed
condition for deployment to a vascular puncture
site;
Fig. 4 is an enlarged view of the distal
end of the catheter tube shown in Fig. 3,
illustrating two def ormable regions in an enlarged
condition, ready for use at the vascular puncture
site;
Fig. 5 is a schematic perspective view of
the distal catheter end in the relaxed condition
shown in Fig. 3, when deployed at a vascular
CA 02435050 2003-07-23
60895-1606D
_ 7 _
puncture site;
Fig. 6 is a schematic perspective view of
the distal catheter end in the enlarged condition
shown in Fig. 4, when deployed at a vascular
puncture site;
Fig. 7A is an exploded, perspective view of
the site access kit shown in Fig. 1;
Fig. 7B is an exploded, perspective view of
the barrier component kit shown in Fig. l;
Figs. 8A to 8D are perspective views
showing the manipulation of syringes contained in
the barrier component kit shown in Fig. 78, to
create a liquid PEG solution for use with the
system;
Fig. 9 is a perspective view of the barrier
material introducer/mixer contained in the site
access kit shown in Fig. 1, with the syringes
containing the liquid albumin solution and the
liquid PEG solution (mixed as shown in Figs. 8A to
8D) mounted and ready for use;
Fig. 10 is a perspective view of the
.barrier material introducer/mixer shown in Fig. 9
attached for operation with the catheter device
contained in the site access kit shown in Fig. 1;
Fig. 11 is a schematic, perspective view of
the vascular puncture site shown in Fig. 6, as the
barrier material introducer/mixer is being operated
to convey a liquid mixture of albumin and PEG
solution into a tissue region outside the puncture
site;
Fig. 12 is a schematic, perspective view of
the vascular puncture site shown in Fig. 11, as the
liquid mixture of albumin and PEG solution cross
links to form a non-liquid barrier network in the
tissue region outside the puncture site;
CA 02435050 2003-07-23
60895-1606D
8
Fig. 13 is a schematic, perspective view of
the vascular puncture site shown in Fig. 12, with
the non-liquid barrier network remaining in the
tissue region outside the puncture site, to seal the
puncture site, after withdrawal of the catheter
device;
Fig. 14 is a plan view of an alternative
embodiment of a catheter device which can be used in
association with the system shown in Fig. 1, with
the deformable region on the distal end shown in a
collapsed condition;
Fig. 15 is an enlarged view of the distal
end of the catheter device shown in Fig. 14, with
the deformable region in an expanded condition;
Fig. 16 is an enlarged sectional view of
the distal end of the catheter device shown in Fig.
15;
Fig. 1? is a schematic perspective view of
the distal end of the catheter device shown in Fig.
14, when deployed in the collapsed condition at a
vascular puncture site;
Fig. 18 is a schematic perspective view of
the distal end of the catheter device shown in Fig.
17, when expanded for use at the vascular puncture
site;
Fig. 19 is a schematic perspective view of
the distal end of the catheter device shown in Fig.
18, as barrier material is dispensed in liquid form
in tissue outside the vascular puncture site;
Fig. 20 is the non-liquid barrier network
formed after the liquid barrier material cross-links
in situ in tissue to seal the vascular puncture
site;
Fig. 21 is a perspective view of the
barrier material introducer/mixer shown in Fig. 9
CA 02435050 2003-07-23
60895-1606D
_ 9 _
when used in association with a sprayer or a
cannula, to dispense barrier material without use of
a catheter device;
Fig. 22 is an enlarged sectional view
showing the interior of a mixing chamber usable in
association with the barrier material introduces
shown in Fig. 9, the interior containing an array of
baffle funnels with staggered interruptions to
establish a circular flow path through the chamber
for the purpose of accelerating mixing of the liquid
components of the barrier material;
Fig. 23 is an enlarged sectional view
showing the interior of a mixing chamber usable in
association with the barrier material introduces
shown in Fig. 9, the interior containing an array of
baffle walls with staggered interruptions to
establish a zig-tagging flow path through the
chamber for the purpose of accelerating mixing of
the liquid components of the barrier material;
Fig. 24 is an enlarged sectional view
showing the interior of a mixing chamber usable in
association with the barrier material introduces
shown in Fig. 9, the interior containing a spiral
baffle to establish a circular flow path through the
chamber for the purpose of accelerating mixing of
the liquid components of the barrier material;
Fig. 25 is an enlarged sectional view
showing the interior of a mixing chamber usable in
association with the barrier material introduces
shown in Fig. 9, the interior containing an array of
staggered baffle walls to establish a cascading flow
path through the chamber for the purpose of
accelerating mixing of the liquid components of the
barrier material;
Fig. 26 is an enlarged sectional view
CA 02435050 2003-07-23
60895-1606D
' - 10 -
showing the interior of a mixing chamber usable in
association with the barrier material introduces
shown in Fig. 9, the interior establishing
tangential f low paths within through the' chamber for
the purpose of accelerating mixing of the liquid
components of the barrier material;
Fig. 27 is an enlarged sectional view
showing the interior of a mixing chamber usable in
association with the barrier material introduces
shown in Fig. 9, the interior containing multiple,
independent inlet ports to convey liquid components
into the chamber for the purpose of accelerating
mixing of the liquid components of the barrier
material; '
Fig. 28 is a side elevation view of an
alternative embodiment of an introducer/mixer, which
can be used in association with the system shown in
Fig. 1; -
Fig. 29 is a top view of an alternative
embodiment of an introducer/mixer of the type shown
in Fig. 28, showing the presence of skirts to resist
side-to-side deflection of syringes supported by the
introducer/mixer;
Fig. 30 is a side elevation view of an
other alternative embodiment of an introducer/mixer,
which can be used in association with the system
shown in Fig. 1;
Fig. 31 is a plan view of a-system for
arresting or controlling bleeding or leakage of
fluid in body tissue, showing the components of the
system prepackaged in sterile kits;
Fig. 32 is a diagrammatic view of a
compromised tissue region, upon which a covering
structure that embodies the features of the
invention has been dispersed to arrest or control
CA 02435050 2003-07-23
60895-1606D
' - 11
bleeding;
Fig. 33 is a side view of the covering
structure shown in Fig. 32, taken generally along
line 33-33 in Fig. 32;
Fig. 34 is a side view of an
introducer/mixer, with the syringes containing a
liquid albumin solution and a liquid PEG solution
mounted and ready for use, the introduces mixer
having an attached mixing spray head to disperse the
solutions to form the covering structure shown in
Figs. 32 and 33;
Fig. 35 is a side view of an
introducer/mixer, with the syringes containing a
liquid albumin solution and a liquid PEG solution
mounted and ready for use, the introduces mixer
having an attached cannula to disperse the solutions
to form the covering structure shown in Figs. 32 and
33;
Fig. 36A is an exploded, perspective view
of the kit shown in Fig. 31 that contains the liquid
and solid components and syringe dispensers for the
covering structure;
Fig. 36B is an exploded, perspective view
of the kit shown in Fig. 31 that contains the
introducer/mixer shown in Figs. 34 and 35, which
receives the syringes shown in Fig. 36A during use;
Figs. 37A, 37B, and 37C illustrate use of
the system shown in Fig. 31 to control or arrest
diffuse organ bleeding;
Figs. 38A, 38B, and 38C demonstrate use of
the system shown in Fig. 31 to seal a puncture site
in a lung;
Figs. 39A, 39B, and 39C illustrate use of
the system shown in Fig. 31 to control or arrest
bleeding through an anastomosis; and
CA 02435050 2003-07-23
60895-1606D
- 12 -
Figs. 40A to 40D are perspective views
showing the manipulation of syringes contained in
the kit shown in Fig. 36A, to create a liquid PEG
solution for use with the system shown in Fig. 31.
The invention may be embodied in several
forms without departing from its spirit or essential
characteristics. The scope of the invention is
defined in the appended claims, rather than in the
specific description preceding them. All embodi-
ments that fall within the meaning and range of
equivalency of the claims are therefore intended to
be embraced by the claims.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
I. SEALING VASCULAR PUNCTURE SITES
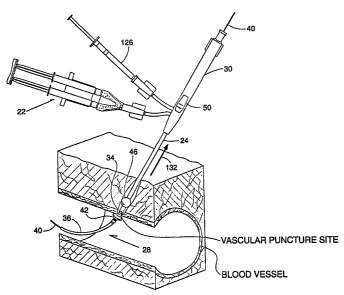
Fig. 1 shows a system 10 of functional
instruments for sealing a vascular puncture site. As
will be described in greater detail, the instruments
of the system l0 are, during use, deployed in a
purposeful manner to gain subcutaneous access to a
vascular puncture site. At the site, the instruments
of the system 10 are manipulated to introduce an
inert barrier material in liquid form outside the
blood vessel at the puncture site. The material
quickly transforms into a non-liquid structure in
situ, forming a barrier outside the vessel, which
mechanically seals the puncture. The barrier exists
long enough to prevent blood leakage while natural
healing processes close the puncture site. The
barrier is, over time, degraded by hydrolysis by in
the host body and cleared by the kidneys in the
urine.
As Fig. 1 shows, in the illustrated embodiment,
the system 10 is consolidated in two functional
kits 12 and 14.
The first kit 14 contains a vascular puncture
CA 02435050 2003-07-23
60895-1606D
- 13 -
site access assembly 16. The purpose of the access
assembly 16 is to gain subcutaneous access to the
vascular puncture site for the purpose of delivering
the fluid barrier material. .
The second kit 14 contains a barrier component
assembly 18. The purpose of the barrier component
assembly 18 is to house the components of the fluid
barrier material prior to use. As will be described
in greater detail later, these components are mixed
and delivered by the access assembly 16 to the
puncture site, forming the barrier.
The kits 12 and 14 can take various forms. In
the illustrated embodiment, each kit 12 and I4
comprises a sterile, wrapped assembly, the details
of which will be discussed in greater detail later.
The Access Assembly
As Fig. 2 shows, the access assembly 16
comprises a catheter device 20 and a barrier
material introducer/mixer 22.
1. The Catheter Device
The catheter device 20 includes a flexible
catheter tube 24 having a proximal end 26 and a
distal end 28. The catheter tube 24 can be
constructed, for example, using standard flexible,
medical grade plastic materials, like vinyl, nylon,
poly(ethylene), ionomer, poly(urethane),
poly(amide), and polyethylene terephthalate). The
distal end 28 has an outside diameter of, e.g., 4 Fr
to 16 Fr. The proximal end 26 carries a handle
30 to facilitate gripping and maneuvering the
catheter tube 24 by a physician.
As Fig. 3 shows, an interior lumen 32 extends
through the catheter tube 24. The lumen
accommodates passage of a conventional guide wire
40.
CA 02435050 2003-07-23
60895-1606D
- 14 -
As will be described in greater detail later,
the guide wire 40 typically will have been
previously introduced subcutaneously, through a wall
of the vessel, to guide passage of a desired
therapeutic or diagnostic instrument into' the
vessel, e. g. , to perf orm coronary angioplasty. After
performing the intended procedure, the instrument is
withdrawn, leaving the guide wire 40. As Fig. 5
shows, the distal end 28 of the catheter tube 24 is
passed over the same guide wire 40 into the blood
vessel. Manipulation of the distal end 28 closes
the vascular puncture site and stops bleeding.
As Figs. 3 and 4 show, the distal end 28 of the
catheter tube 24 includes a circumferentially spaced
array of nozzles 34. The barrier material is
conveyed in liquid form and. dispensed in a
circumferential manner through the nozzles 34 at the
puncture site.
As Figs. 3 and 4 also show, the distal end 28
also includes a flexible, elongated leader 36, which
extends distally beyond the nozzles 34. In use (see
Fig. 5), the leader 36 is located inside the blood
vessel immediately interior to the puncture site. In
use (see Fig. 5), the array of nozzles 34 is located
outside the blood vessel immediately exterior to the
puncture site.
Referring again to Figs. 3 and 4, the distal end
28 also includes a first deformable region 38, which
is located between the nozzles 34 and the leader 36.
The region 38 normally presents a generally
cylindrical, low profile condition (shown in Fig.
3), matching the leader 36. When in the low profile
condition, the region 38 follows the leader 36 over
the guide wire into the vessel (see Fig. 5).'
The region 38 can be deformed into a radially
CA 02435050 2003-07-23
60895-1606D
- 15 -
enlarged condition, which forms a positioner 42 (see
Fig. 4). In use (see Fig. 6), the positioner 42
resists passage of the leader 36 back through the
puncture site in response to rearward tension along
the catheter tube 24, as shown by arrow 132 in Fig.
6. Moreover, as Fig. 6 shows, rearward tension along
the catheter tube 24 seats the positioner 42 against
the interior of vessel wall at the puncture site.
The positioner 42 serves to position the nozzles 34
.10 at a proper distance outside the vessel. The
positioner 42 also serves to support the puncture
site inside the vessel while the liquid barrier
material is introduced outside the vessel through
the nozzles 34.
Referring back to Figs. 3 and 4, a second
deformable region 44 is spaced a distance proximal
to the nozzles 34. Like the nozzles 34 (see Fig. 5),
the deformable region 44 is intended, during use, to
lay outside the vessel.
The deformable region 44 presents a normally,
generally collapsed condition for deployment over
the guide wire 40 (shown in Figs. 3 and 5). The
deformable region 44 can be expanded into, e.g., an
elliptical dam 46(see Figs. 4 and 6). The dam 46
serves block proximal egress of the liquid barrier
a~ateriai conveyed through the nozzles 34.
The deformation of the regions 38 and 44 can be
accomplished in various ways. In the illustrated
embodiment, the leader 36 moves along a slide tube
48 (see Figs. 3 and 4) toward and away from the
nozzles 34. A push-pull lever 50 on the handle 30
(shown in Fig. 2) is coupled by a stylet 52 to the
leader 36 to affect axial movement of the leader 36
along the slide tube 48.
In this arrangement, the region 38 comprises a
CA 02435050 2003-07-23
60895-1606D
- 16 -
generally elastic material surrounding the slide
tube 48. The material is attached at one end to the
leader 36 and at the other end to the catheter tube
24 near the nozzles 34. Drawing the leader 36
toward the nozzles 34 pushes against and radially
deforms the material into the positioner 42.
Advancement of the leader 36 away from the nozzles
34 relaxes the material.
In the illustrated embodiment, the second region
44 comprises an expandable balloon material attached
about the catheter tube 24. The catheter tube 24
includes an interior lumen 56 (shown in Figs. 3 and
4), which communicates with the interior of the
balloon material. A fitting 54 carried by the handle
30 (see Fig. 2) communicates with the lumen 56. The
fitting 54 couples the lumen to an auxiliary
syringe 126, which injects air under pressure
through the lumen 56 into the space surrounded by
the balloon material, causing the material to expand
and form the dam 46.
2. Harrier Material Introducer/Mixer
As will be described in greater detail later,
the barrier material is formed from two liquid
components, which are mixed at the instant of use.
The two components cross-link to form the non-liquid
barrier.
Before mixing, the components are housed in
sterile dispensing syringes 60 and 62 contained in
the kit 14 (see Fig. 1). As Fig. 2 shows, the
barrier material introducer/mixer 22 receives the
two dispensing syringes 60 and 62 for use in
association with the catheter device 20. The barrier
material introducer/mixer 22 allows the physician to
uniformly express the two components in a liquid
state from the dispensing syringes 60 and 62.
CA 02435050 2003-07-23
60895-1606D
' - 17 -
The barrier material introducer/mixer 22 also
mixes the components while flowing in the liquid
state from the dispensing syringes 60 and 62. This
obviates the need for static mixing prior to
dispensing. This mixing of liquid components within
a flow channel will, in shorthand, be called
"channel-mixing.n
To accomplish these functions (see Fig. 2), the
barrier material introducer/mixer 22 includes
syringe support 64. The support 64 includes side-by
side channels 66. Each channel 66 accommodates in
a snap-friction-tit the barrel 78 of a conventional
syringe of desired size, e.g., 3 cc (as Figs. 9 and
10 also showj.
The barrier material introducer/mixer 22 also
includes a syringe clip 68. The syringe clip 68
includes spaced apart walls 7o forming an interior
race 72. As Figs. 9 and 10 show, the race 72
receives in a sliding friction fit the thumb rests
74 of the dispensing syringe pistons 76, in axial
alignment with the syringe barrels 78 carried by the
syringe support 64. The syringe clip 68 mechanically
links the syringe pistons 76 together for common
advancement inside their respective syringe barrels
78.
To facilitate handling (see Figs. 2, 9 and 10j,
the syringe support 64 includes opposed finger rests
80, and the syringe clip 68 includes a thumb rest
82. The orientation of these rests 80 and 82
parallel the orientation of the finger rests and
thumb rests of a single syringe. The physician is
thereby able to hold and operate multiple syringes'
60 and 62 in the same way as a single syringe. -
The barrier material introducer/mixer 22 also
includes a joiner 84. The joiner 84 includes side
CA 02435050 2003-07-23
60895-1606D
- 18 -
by side female luer fittings 86. The female luer
fittings 86 each receives the threaded male luer
fitting 88 at the dispensing end of the dispensing
syringes 60 and 62. The female luer fittings 86 are
axially aligned with the barrels 78 of the
dispensing syringes 60 and 62 carried in the syringe
support 64.
The physician is thereby able to quickly and
conveniently ready the dispensing syringes 60 and 62
for use by securing the dispensing syringes to the
joiner 84, snap fitting the syringe barrels 78 into
the syringe support 64, and slide fitting the
syringe thumb rests 74 into the clip 68.
The joiner 84 includes interior channels 90
coupled to the female luer fittings 86. The channels
90 merge at a Y-junction into a single outlet port
92. The joiner 84 maintains two fluids dispensed by
the syringes 60 and 62 separately until they leave
the joiner 84. This design minimizes plugging of the
joiner 84 due to a mixing reaction between the two
fluids. The syringe clip 68 ensures even application
of individual solutions through the joiner 84.
The barrier material introducer/mixer 22 further
includes a mixing chamber 94, which, in use, is
coupled to the single outlet port 92 (as Fig. 10
shows). Expressed in tandem from the dispensing
syringes 60. and 62, which are mechanically linked
together by the joiner 84, support 64, and clip 68,
the two components of the barrier material come into
contact in the liquid state in the mixing chamber
94. Channel-mixing of the two components occurs as
they flow through the mixing chamber 94 under
pressure from operation of the mechanically linked
dispensing syringes 60 and 62.
In the illustrated embodiment (see Figs. 2 and
CA 02435050 2003-07-23
60895-1606D
- 19 -
10), the mixing chamber 94 is carried at the end of
a tube 96 attached to the handle 30 of the catheter
device 20. The tube 96 communicates with interior
lumens 134 in the catheter tube 24 (shown in Fig.
3), which, in turn, are coupled to the dispensing
nozzles 34. The mixing chamber 94 includes a luer
fitting 98, which threadably connects with the
single outlet port 92 of the joiner 84.
The parts of the barrier material
IO introducer/mixer 94 are made, e.g., by molding
medical grade plastic materials, such as
polycarbonate and acrylic.
Harrier Component Assembly
The barrier component assembly 18 includes the
already described dispensing syringes 60 and 62 for
the two components of the barrier material.
According to the invention, the barrier material
comprises a compound that is chemically cross-linked
without the use of an enzyme, to form a non-liquid
mechanical matrix.
As defined in this Specification, an
"enzymatically cross-linked" barrier material is
formed by the mixture of an enzyme and a substrate.
Solutions of the substrate and enzyme can be
delivered to the application site simultaneously, or
separate solutions of the enzyme and substrate can
be mixed at the application site. The enzyme cross-
links to the substrate, transforming the solution to
a solid. Examples of these materials include fibrin
glue (in which the enzyme is thrombin and the
substrate is fibrinogen), and transglutaminase
cross-linked materials (in which the enzyme is
transglutaminase and the substrate is selected from
materials containing amino groups.
As further defined in this Specification, a
CA 02435050 2003-07-23
60895-1606D
20 -
"chemically cross-linked" barrier material refers to
all barrier materials not formed through the use of
enzymes. Cross-linking can occur, e.g., as a result
of energy (heat or light), or cross-linking chemical
reactions (active esters, isocyanates, epoxides).
Examples of these materials includes photo-cross-
linked acrylates and nucleophilic attack of
electrophiles.
In a preferred embodiment, the barrier material
is a protein/polymer composite hydrogel. The
material is nontoxic, biodegradable, and possesses
suitable mechanical properties to seal arterial
pressure.
The barrier material is most preferably formed
from the mixture of a protein solution and a
solution of an electrophilic derivative of a
hydrophilic polymer with a functionality of at least
three. The.barrier material of this composition has
Buff icient cohesive strength, adhesive strength, and
elasticity to seal arterial pressure. The rate of
cross-linking and gelation can be controlled through
buffer selection and concentration. The rate of
degradation after cross-linking can be controlled
through the selection of a degradation control
region.
1. Barrier Material Components
a. Natural Plasma-BaseB Protein
In the illustrated embodiment (see Fig 1), the
first dispensing syringe 60 contains a concentration
of buffered protein solution 100. The protein
solution is supplemented with the appropriate
buffers, sterile filtered, aseptically filled into
the syringe 60, and the syringe 60 is capped for
storage prior to use.
Suitable proteins for incorporation into barrier
CA 02435050 2003-07-23
60895-1606D
- 21 -
material include non-immunogenic, hydrophilic
proteins. Examples include solutions of albumin,
gelatin, antibodies, serum proteins, serum
fractions, and serum. Also, water soluble
derivatives of hydrophobic proteins can also be
used. Examples include collagen, fibrinogen,
elastin, chitosan, and hyaluronic acid. The protein
can be produced from naturally occurring source or
it may be recombinantly produced.
The preferred protein solution is 25$ human
serum albumin, USP. Human serum albumin is
preferred due to its biocompatibility and its ready
availability.
Buffer selection and concentration maintains the
pH of the reactive mixture. Buffers that are well
tolerated physiologically can be used. Example8
include carbonate and phosphate buffer systems. Care
should be taken to select buffers that do not
participate in or interfere with the cross-linking
reaction. The preferred range of buffer
concentration is from about 0.01 M to about 0.3 M,
. and the preferred range of pH is from about 7.0 to
about 10Ø A preferred buffer system for vascular
puncture sealing is phosphate buffer at a
concentration of 0.05 M at a pH value of about 8 to
about 9. As will be described later, there is a
relationship between pH and the time for cross-
linking (also called °geiation").
As will be described in greater detail later,
the syringe 60 is kept before use within inner and
outer wraps, which are peripherally sealed by heat
or the like. The wraps are made, at least in part,
from a material that is permeable to ethylene oxide
sterilization gas, e.g., TYVERT"" plastic material
available from Du Pont. The outer surfaces of
CA 02435050 2003-07-23
60895-1606D
' - 22
syringe 60 can thereby be sterilized using ethylene
oxide gas.
b. Electrophilic Water Soluble
Polymer
In the illustrated embodiment (still referring
principally to Fig 1), the second dispensing syringe
62 contains an inert, electrophilic, water soluble
polymer 102. The polymer cross-links the protein to
form an inert, three dimensional mechanical network
or matrix. The matrix forms a mechanical barrier,
which, when appropriately positioned in tissue at a
vascular puncture site outside the vessel, serves to
seal the puncture site. The barrier is, over time,
resorbed.
The polymer 102 comprises a hydrophilic,
biocompatible polymer, which is electrophilically
derivatized with a functionality of at least three.
A number of polymers could be utilized, including
polyethylene glycol), polyethylene oxide),
polyvinyl alcohol), poly(vinylpyrrolidone),
poly(ethyloxazoline), and polyethylene
glycol)-co-poly(propylene glycol) block copolymers.
The polymer portion is not restricted to synthetic
polymers as polysaccharides, carbohydrates, and
proteins could also be electrophilically
derivatized.
Preferably, the polymer 102 is comprised of
polyethylene glycol) (PEG) with a molecular weight
between 1,000 and 30,000 g/mole, more preferably
between 2,000 and 15,000 g/mole, and most preferably
between 10,000 and 15,000 g/mole. PEG has been
demonstrated to be biocompatible and non-toxic in a
variety of physiological applications.
The preferred polymer can be generally expressed
as compounds of the formula:
CA 02435050 2003-07-23
60895-1606D
- 23 -
PEG-(DCR-CG)"
where:
DCR is a degradation control region.
CG in a cross-linking group.
' n i3
While the preferred polymer is a multi-armed
structure, a linear polymer with a functionality of
at least three can also be used. The desired
functionality of the PEG polymer f or forming the
barrier can be expressed in terms of (i) how quickly
the polymer cross-links the protein and transforms
to a nonfluent gel state (i.e., the mechanical
barrier material) (a preferred gelation time is
under three minutes) , and (ii) the mechanical
properties of the barrier after gelation in terms of
its liquid sealing characteristics, physical
strength, resistance to fragmentation (i.e.,
brittleness), and bioresorption. The optimization of
both attributes (i) and (ii) is desirable.
The inventors have discovered that the utility
of a given PEG polymer significantly increases when
the functionality is increased to be greater than or
equal to three. The observed incremental increase in
functionality occurs when the functionality is
increased from two to three, and again when the
functionality is increased from three to four.
Further incremental increases are minimal when the
functionality exceeds about four.
The use of PEG polymers with functionality of
greater than three provides a surprising advantage.
When cross-linked with higher functionality PEG
polymers, the concentration of albumin can be~
reduced to 25% and below. Past uses of difunctional
PEG polymers require concentrations of albumin well
above 25%, e.g. 35% to 45%. Use of lower
CA 02435050 2003-07-23
60895-1606D
- 24 -
concentrations of albumin results in superior
sealing properties with reduced brittleness,
facilitating reentry through the nonfluid barrier
material, without fragmentation. Additionally, 25%
human serum albumin, USP is commercially available
from several sources, however higher concentrations
of USP albumin are not commercially available. By
using commercially available materials, the dialysis
and ultrafiltration of the albumin solution, as
disclosed in the prior art, is eliminated,
significantly reducing the cost and complexity of
the preparation of the albumin solution.
In the illustrated embodiment, the polymer 102
is initially packaged prior to use in the second
dispensing syringe 92 in an inert atmosphere (e. g.,
argon) in a stable, powder form. In this
arrangement, the barrier component assembly 18
includes a third syringe 104, which contains sterile
water 106 for dissolution of the powder polymer 102
just before mixing with the albumin component 100.
In facilitating mixing, a stopcock valve 108 is
secured to the luer fitting 88 at the dispensing end
of the second dispensing syringe 62. The dispensing
end 110 of the water syringe 104 couples to the
stopcock valve 108, so that the water 106 can be
mixed with the polymer 102 in the dispensing syringe
72 prior to use. Further details of the preparation
of the polymer prior to use will be described later.
In the illustrated embodiment, the second and
third dispensing syringes 62 and 104 are placed in
inner and outer wraps peripherally sealed by heat.
The wraps are made, at least in part, from a
material that is transparent to electron beam
irradiation. The contents of the second and third
dispensing syringes 62 and 104 can thereby be
CA 02435050 2003-07-23
60895-1606D
- 25 -
sterilized, e.g., by exposure to electron beam
irradiation.
(1) Selection of the
Degradation Control
Region DCR
The rate of degradation is controlled by the
selection of chemical moiety in the degradation
control region DCG. If degradation is desired, a
hydrolytically or enzymatically degradable moiety
can be selected,
Examples of hydrolytically degradable moieties
include saturated di-acids, unsaturated di-acids,
poly(glycolic acid), poly(DL-lactic acid),
poly(L-lactic acid), poly ( -caprolactone),
poly(5-valerolactone), poly(y-butyrolactone),
poly(amino acids), poly(anhydrides),
poly(orthoesters), poly(orthocarbonates), and
poly(phosphoesters).
Examples of enzymatically degradable regions
include Leu-Glyc-Pro-Ala (collagenase sensitive
linkage) and Gly-Pro-Lys (plasmin sensitive
.linkage).
The preferred degradable control regions for
degradable barrier materials are ester containing
linkages, as are present when succinic acid or
glutaric acid are coupled to a PEG molecule. The
preferred degradable control regions for
nondegradable barrier materials are ether containing
linkages. The barrier material can also be created
without the introduction of a degradation control
region.
(2) . Selection of the
Cross-Linking Group CG
The cross-linking group is responsible for the
cross-linking of the albumin, as well as the binding
CA 02435050 2003-07-23
60895-1606D
- 26 -
to the tissue substrate. The cross-linking group can
be selected to selectively react with sulfhydryl
groups, selectively react with amines, or can be
selected to react with sulfhydryl, primary amino,
and secondary amino groups. Cross-linking groups
that react selectively with sulfhydryl groups
include vinyl sulfone, N-ethyl maleimide,
iodoacetamide, and orthopyridyl disulfide. Cross-
linking groups specific to amines include aldehydes.
Non-selective electrophilic cross-linking groups
include active esters, epoxides, carbonylimidazole,
nitrophenyl carbonates, tresylate, mesylate,
tosylate, and isocyanate. The preferred cross
linking group is an active ester, specifically an
ester of N-hydroxysuccinimide.
To minimize the liberation of heat during the
cross-linking reaction, the concentration of the
cross-linking groups is preferably kept less than 5%
of the total mass of the reactive solution, and more
preferably about 1% or less. The low concentration
of the cross-linking group is also beneficial so
that the amount of the leaving group is also
minimized. In a preferred embodiment, the cross-
linking group portion comprising a
N-hydroxysuccinimide ester has demonstrated ability
to participate in the cross-linking reaction with
albumin without presenting the risk of local or
systemic immune responses in humans.
( 3 ) Pref erred Multiple Arm
PEG Polymer
In a preferred embodiment, the polymer is
comprised of a 4-arm PEG with a molecular weight of
about 10,000 g/mole, the degradation control region
is comprised of glutaric acid, and the cross-linking
group is comprised of a N-hydroxysuccinimide ester.
CA 02435050 2003-07-23
60895-1606D
' - 27 -
Thus, a preferred polymer is polyethylene glycol)
tetra-succinimidyl glutarate, which is available
from Shearwater Polymers, Huntsville, AL. The
preferred polymer will, in shorthand, be called
4-PEG-SG. The polymer is dissolved in water prior
to use. Preferred concentrations of the polymer are
from 5% to 35% w/w in water.
The solution of 4-PEG-SG mixes with 25% serum
albumin to form a liquid solution that quickly
cross-links to form a non-liquid, three dimensional
network for the barrier. With these barrier material
formulations, it is possible.to intimately mix the
water soluble polymer with the albumin protein
without static mixing. Effective mixing occurs as
the multiple arm PEG polymer and albumin are jointly
passed through a confined flow path. This
beneficial phenomenon has been earlier referred to
in this specification as "channel-mixing."
As will be demonstrated later, the rate of
reaction can be controlled by the pH of the reactive
solution. An increase in temperature is not observed
during formation of the barrier network, due to the
low concentration of reactive groups, which account
for only about 1% of the total mass. In a typical
clinical application, about 50 mg of a non-toxic
leaving group is produced during the cross-linking
reaction, which is a further desired result.
The resulting nonfluent barrier material created
by mixing 25% albumin and 4-PEG-SG is approximately
80% water, 13% albumin, and 7% PEG. The barrier
material is well tolerated by the body, without
invoking a severe foreign body response. Over a
controlled period of time, the barrier material is
degraded via hydrolysis. Histological studies have
shown a foreign body response consistent with a
CA 02435050 2003-07-23
60895-1606D
- 28 -
biodegradable material, such as VICRYLTM sutures. As
the material is degraded. the tissue returns to a
quiescent state. The molecules of the degraded
barrier material are cleared from the bloodstream by
the kidneys and eliminated from the body in the
urine. In a preferred embodiment of the invention,
the barrier material loses its physical strength
during the first twenty days, and total resorption
occurs in about 4 weeks.
The following Examples demonstrate the superior
features of the barrier material of the invention.
Example 1: Preparation of Cross-Linked
Barrier Networks
Cross-linked barrier networks were formed by the
mixture of an 4-PEG-SG and albumin. A solution of 4
PEG-SG was prepared by dissolving 0.40g in 2.0 mL of
water. The albumin solution consisted 25% human
serum alburmin, USP (Plasbumin-25, Bayer
Corporation), as received.
Dispensing syringes containing 2.0 mL of the
polymer solution and 2.0 mL of albumin solution were
connected to the joiner 84, to which a spray head
was coupled. The solutions were sprayed into a
polystyrene weigh boat. A cross-linked barrier
network formed at room temperature in about 90
seconds.
Example 2: Control of the Rate of Gelatioa
The rate of formation of the cross-linked
barrier network of 4-PEG-SG and albumin (i.e.,
gelation) can be controlled by the pH of the
reactive solution. To increase the rate of cross-
linking, the pH of the solution is increased, and
conversely, to decrease the rate of cross-linking,
the pH of the solution is decreased. The pH of the
solution is controlled by both the buffer strength
CA 02435050 2003-07-23
60895-1606D
29
and buffer pH.
Table 1 shows the effect of buffer strength on
the rate of gelation of 17% w/w 4-PEG-SG in water
for injection and 25% human serum albumin, USP at
room temperature. The rate of gelation can also be
controlled by adjusting the pH of the buffer at a
constant buffer concentration. The buffer was placed
in the solution of albumin. The gelation time is the
amount of time required for the formulation to
transform from the liquid state to the cross-linked
solid state.
Table 1: Effect of Buffer Strength and Buffer pH
on Gel Formation
Buffer Buff er pii Gelation Time
Concentration
300 mM 9 < 1 sec
200 mM 9 5 sec
100 mM 9 10 sec
50 mM 9 20 sec
0 mM 7 90 sec
Example 3: Channel-Mixing
A solution of 4-PEG-SG was prepared by
dissolving 0.408 in 2.0 mL of water. The albumin
solution consists 25% human serum albumin, USP
(Plasbumin-25, Bayer Corporation), buffered to pH
9Ø
Syringes containing 2.o mL of the polymer
solution and albumin solution were connected to the
joiner 84. A cannula channel having an inside
diameter of 1 mm and a length of 20 cm was attached
to the outlet port 92 of the joiner 84. The
solutions were expressed through the cannula channel
into a polystyrene weigh boat.
CA 02435050 2003-07-23
60895-1606D
The barrier network formed at room temperature
in about 20 seconds. Qualitatively, the mechanical
properties of the barrier network when sprayed (as
in Example 1) and the barrier network when expressed
5 through the cannula channel were equivalent.
This demonstrates that the barrier network can
be formed by channel-mixing the liquid components,
without static mixing, by delivery through a small
diameter channel.
10 Puncture Site Closure Using the System
1. The Kits
As Figs. 7A and 7B show, in the illustrated
embodiment, each kit 12 and 14 includes an interior
tray 112 made, e.g., from die cut cardboard, plastic
15 sheet, or thermo-formed plastic material.
The catheter device 20 and barrier material
introducer/mixer 22 are carried by the tray 112 in
the first kit 12. The first, second, and third
syringes 60, 62, and 114 and stopcock valve 108 are
20 carried by the tray 112 in the second kit 14.
Each kit 12 and 14 presents its contents ~ in a
user-friendly orientation on the tray 112, to
facilitate quick preparation of the barrier material
using straightforward, intuitive steps, and the
25 subsequent attachment of the dispensing syringes 60
and 62 to the catheter device 20.
As shown in Fig. 7A, the kit 12 includes an
inner wrap 114, which is peripherally sealed by heat
or the like, to enclose the tray 112 from contact
30 with the outside environment. One end of the inner
wrap 114 includes a conventional peel away seal 116.
The seal 116 provides quick access to the tray 1l2
at the instant of use, which preferably occurs in a
suitable environment, such as within a
catheterization lab.
CA 02435050 2003-07-23
60895-1606D
' ' 31 -
The kit 12 is further wrapped in an outer wrap
118, which is also peripherally sealed by heat or
the like, to enclose the interior tray 112. One end
of the inner wrap 118 includes a conventional peel
away seal 120, to provide quick access to the
interior tray 112 and its contents.
The outer wrap 118 and the inner wrap 114 are
made, at least in part, from a material that is
permeable to ethylene oxide sterilization gas, e.g.,
TYVEKT"" plastic material (available from DuPont) . Kit
12 is sterilized utilizing ethylene oxide gas or
electron beam irradiation.
As shown in Fig. 78, kit 14 includes a polymer
package 138 (which contains the prefilled powder
polymer syringe 62 and water syringe. 104) and an
albumin package 140 (which contains the prefilled
albumin syringe 64). Each polymer package 138 and
albumin package 140 includes an individual wrap 142,
' which is peripherally sealed by heat or the like, to
enclose package 138 and 140 from contact with the
outside environment. One end of the individual wrap
142 includes a conventional peel away seal 144, to
provide quick access to the contents of the packages
138 and 140 at the instant of use, such as within a
catheterization lab.
Polymer package 138 and albumin package 140 are
further wrapped in an outer wrap 118, which is also
peripherally sealed by heat or the like. One end of
the outer wrap 118 includes a conventional peel away
seal 148, to provide quick access to the packages
138 and 140. After sterilization treatment, the
packages 138 and 140 and the tray 112 are further
wrapped in container 146 for the user's convenience.
The wraps 142 and 118 are made, at least in
part,~from a material that is permeable to ethylene
CA 02435050 2003-07-23
60895-1606D
- 32 -
oxide sterilization gas, e.g., TYVEK~"'' plastic
material (available from DuPont). The albumin
package 140 is prepared, sterilized utilizing
ethylene oxide gas, and placed into kit 14. The
polymer package 138 is prepared, sterilized
utilizing electron beam irradiation, and place into
kit 14.
In the illustrated embodiment, each kit 12 and
14 also preferably includes directions 122 for using
the contents of the kit to carry out a desired
procedure. Exemplary directions 122 will be
described later.
2: Use of the Kits to Access and Seal a
Vascular Puncture Site
The directions 122 can, of course vary,
according to the particularities of the desired
procedure. Furthermore, the directions 122 need not
be physically present in the kits 12 and 14. The
directions 122 can be embodied in separate
instruction manuals, or in video or audio tapes.
In the illustrated embodiment, exemplary
directions 122 are described, which instruct the
physician how to use of the system l0 to close a
vascular puncture site following percutaneous
transliminal coronary angioplasty. It should be
appreciated that the specific contents of the
directions 122 are merely exemplary. The objectives
set forth in the exemplary directions 122 can be
accomplished in different ways, using different
devices, and different sequences of steps.
It should also be appreciated that the use of
the system 10 is not limited to angioplasty
procedures. The system 10 can be used with other
diverse procedures, which provide vascular access
through a puncture site.
CA 02435050 2003-07-23
60895-1606D
- 33 -
In the illustrated embodiment, at the time the
system 10 is readied for use, the guide wire 40 has
already been deployed through a conventional
introduces through a vascular puncture site into,
e.g., the femoral artery. An angioplasty balloon
has been deployed over the guide wire 40 through the
puncture site and into the artery. The angioplasty
balloon has been advanced over the guide wire 40 to
the occluded treatment site. The balloon has been
expanded and manipulated to open the occluded site.
The balloon has been withdrawn over the guide wire
40.
When use of the system 10 is desired, the outer
wrap 118 of the kits 12 and 14 are removed. The
trays 112, still contained in the inner wraps 118,
are placed in the sterile operating field.
The physician opens the inner wrap 118 of the
second kit l4 to gain access the first, second, and
third syringes 60, 62, and 104.
In the illustrated embodiment, the directions
122 for use instruct the physician to remove from
the second kit tray 112 the second dispensing
syringe 62, which contains, in sterile powder form,
a predetermined amount of the polymer 102 (e. g.,
about 0.3 to 0.5 g). The directions 122 also
instruct the physician to remove from the second kit
14 the third syringe 104, which contains sterile
water 106 (e.g., about 2 cc). Both are contained in
the polymer package 138.
As Fig. 8A shows, the directions 122 instruct
the physician to couple the dispensing end of the
water syringe 104 to the stopcock valve 108 on the
second dispensing syringe 62. The stopcock valve 108
is closed at this point. As instructed by the
directions 122, the physician opens the stopcock
CA 02435050 2003-07-23
60895-1606D
34
valve 108 (see Fig. 88) and transfers water from the
water syringe 104 into the powder 100 in the second
dispensing syringe 62 (see Fig. 8C). The physician
is instructed.to repeatedly transfer the water and
powder mixture between the two syringes 62 and 104,
to syringe-mix the powder and water until all solids
are dissolved. The syringe-mixing places the water
soluble, polymer material into solution. The
syringe-mixing process generally takes about two
minutes.
After syringe mixing, the physician, following
the directions 122, transfers the PEG solution 136
(about 2 cc) into one of the syringes (which, in the
illustrated embodiment, is the second syringe 62).
The physician waits for bubbles to dissipate, which
generally takes about an additional two minutes.
According to the directions 122, the physician
now closes the stopcock valve 108 (as Fig. 8D
shows). The physician removes the stopcock valve
108 by unscrewing it from the luer fitting on the
dispensing end of the second syringe 62. The PEG
solution 136 is ready for use. Mixing of the PEG
solution 136 should take place generally within one
hour of use. If the PEG solution 136 remains unused
over one hour after mixing, it should be discarded.
The directions 122 instruct the physician to
remove from the second kit tray 112 the dispensing
syringe 60 containing the albumin 100. As before
described, the albumin 100 has been premixed in a
buffered form to the desired concentration (e. g.,
25%), then sterile filtered, and aseptically filled
into the syringe 60. A closure cap normally closes
the dispensing end inside the tray 112.
The physician now, or at a previous time, opens
the outer wrap 118 of the first kit 12 to gain
CA 02435050 2003-07-23
60895-1606D
' - 35
access to the catheter device 20 and barrier
material introducer/mixer 22. Using an auxiliary
syringe (not shown), the physician is instructed to
instructed to flush the interior lumen leading to
the nozzles 34 with sterile saline. The physician
is also directed to flush the interior guidewire
lumen 32 with sterile saline. The physician
attaches another auxiliary syringe 126 filled with
about 1 cc of air to the fitting 54 for inflating
the deformable region 44 to confirm its
functionality, and then returns the deformable
region 44 to the collapsed state.
As illustrated in Fig. 9, the directions 122
instruct the physician to remove the closure cap and
screw the dispensing end of the first syringe 60 to
the luer fitting 86 on the joiner 84. The physician
is also instructed to screw the dispensing end of
the second syringe 62 (now containing the mixed PEG
solution 136) to the other luer fitting 86 on the
joiner 84.
Following the directions 122 (as Fig. 9 also
shows), the physician snaps the barrels 78 of the
syringes 60 and 62 to the holder channels 66. The
physician captures the thumb rests 74 of the two
syringes 60 and 62 inside the race 72 of the syringe
clip 68. The directions 122 instruct the physician
to attach the joiner 84 to the mixing channel 94 (as
Fig. 10 shows).
The physician is now ready to deploy the
catheter tube 24. As Fig. 5 shows, the physician iS
instructed to pass the distal end 28 of the catheter
tube 24 over the guide wire 40 through the puncture
site. The physician advances the distal end 28 to
situate the first deformable region 38 inside the
vessel, while the nozzles 34 are deployed outside
CA 02435050 2003-07-23
60895-1606D
- 36 -
the vessel. The physician can monitor the
advancement tactilely, without using fluoroscopy.
However, the physician can use fluoroscopy or an
other form of visualization, if desired.
According to the directions 122 (as Fig. 6
shows), the physician pulls the lever 50 rearward,
causing the first deformable region 38 to expand
radially into the positioner 42. The physician is
instructed to place slight rearward tension on the
catheter tube 24 (shown by arrow 132 in Fig. 6), to
bring the positioner 42 into contact with the
interior of the vessel. The physician will, by
tactile feedback, know that the positioner 42 has
contacted the vessel interior. Due to the. slight
Z5 rearward tension, the positioner 42 seats against
and supports the puncture site. The guide wire
lumen 32 of the catheter tube 24 can be used to
inject suitable contrast media to aid in the
visualization of the puncture site region.
While maintaining slight rearward tension on the
catheter tube Z4, the physician is instructed to
manipulate the syringe 126 to inject air (e. g. about
0.7 cc to 0.8 cc) into the second deformable region
44. The second deformable region 44 expands (as Fig.
6 shows), forming the dam 46 outside the vessel.
The physician is instructed to continue to apply
a slight rearward tension on the catheter tube 24,
sufficient to keep the positioner 42 against the
interior of the vessel, without pulling it through
the vessel wall.
The physician is instructed to grasp the finger
rests 80 and thumb rest 82 of the barrier material'
introducer/mixer 22, as if grasping an ordinary
syringe. The physician expresses the albumin 100
from the first dispensing syringe 60 while
CA 02435050 2003-07-23
60895-1606D
' - 37 -
simultaneously also expressing the PEG solution 136
from the second dispensing syringe 62.
The albumin and PEG solutions come into contact
in the mixing chamber 94 and, from there, proceed
through the catheter tube 24 to the nozzles 34. The
albumin 200 and PEG solution 136 intimately channel-
mix in transit.
As Fig. 11 shows, the mixture of albumin 100 and
PEG solution 136 flows in liquid form through the
nozzles 34. Conveyed circumferentially about the
catheter tube 24 by the nozzles 34, the liquid
mixture 130 of albumin 100 and PEG solution 136
enters and fills the tissue region surrounding the
puncture site.
As Fig. 12 shows, according to the directions
122, the physician waits the requisite gelation
period, during which the liquid mixture 130 of
albumin 100 and PEG material 136 transform into a
non-fluid barrier network 128 outside the puncture
site. Using 4-PEG-SG and albumin, the gelation
period is about 15 to 60 seconds.
During the gelation period, the physician is
instructed to continue to apply a slight rearward
tension on the catheter tube 24 to seat the
positioner 42 against the interior vessel wall.
This, in effect, suspends the vessel on the distal
end of the catheter tube 24, while the solid barrier
network 128 forms outside the vessel to seal the
puncture site. The positioner 42 and the catheter
tube 24 resist seepage of the liquid mixture 130
into the vessel during the gelation period.
After the requisite gelation period, the
physician is instructed to push the lever 50 forward
to relax the positioner 42. The physician also
relieves air pressure from the dam 46. The physician
CA 02435050 2003-07-23
60895-1606D
_ gg
withdraws the guide wire 40 and the distal end 28 of
the catheter tube 24 from the vessel. As shown by
Fig. 13, during withdrawal, the distal end 28 and
the guide wire 40 pass through the barrier network
128 that has, by now, formed over the puncture site.
If desired, the guidewire 40 may be left in place
for removal at a future time.
After withdrawing the catheter tube 24, the
physician is instructed to apply manual pressure to
the skin over the blood vessel, e.g., for about
three minutes, to aid in the sealing process. This
time allows the barrier material to fully cross
link. The physician then confirms that the puncture
site has been sealed by observing the lack of blood
seepage about the guide wire 40 access.
The puncture site of the, vessel naturally closes
and heals. As Fig. 13 shows, the presence of the
barrier network 128 outside the puncture site
prevents blood leakage while natural healing takes
ZO place. The barrier network 128 obviates the need for
the patient to forgo ambulation and normal
activities while this natural healing process takes
place. The body resorbs the barrier network 128 over
time, e.g., within 3o days.
Example 4: Femoral Puncture Site Closure
A solution of 4-arm PEG succinimidyl gl~ttarate,
MW 10,000 (Shearwater Polymers, Huntsville, AL) was
prepared by dissolving 0.40g in 2.0 mL of water for
injection. The albumin solution consists 25% human
serum ~ albumin, USP (Plasbumin-25, Bayer
Corporation), buffered to pH 9Ø
Syringes containing 2.0 mL of the polymer
solution and 2.0 mL of albumin solution were
connected to the joiner coupled to the catheter
device having an 8 French catheter tube 24.
CA 02435050 2003-07-23
60895-1606D
' - 39
Aseptically, the distal end of the catheter tube
24 was inserted into the femoral artery of a sedated
sheep. The first and second deformable regions were
enlarged inside and outside the artery. The material
in the dispensing syringes were simultaneously
injected through the mixing chamber into the
catheter tube 24, and dispensed through the nozzles
34 at the tissue site.
Twenty seconds was allowed for gelation. The
deformable regions were relaxed, and the catheter
tube 24 was withdrawn from the artery.
Direct pressure was applied to the artery for an
additional 3 minutes to allow the barrier material
to fully harden. When the pressure was relieved,
blood loss through the tissue track or hematoma
formation was not observed. Doppler analysis
confirmed blood flow distally from the arteriotomy.
The time between application of liquid barrier
material to the formation of a non-liquid barrier to
affect complete sealing was 3.5 minutes.
The treated sheep was upright and bearing weight
evenly on its legs within 45 minutes after
deployment of the barrier material. After about one
hour from the completion of the procedure, hay was
25~ placed in the pen. The sheep immediately began
eating. Approximately 2 hours after the procedure,
the animal was bright, alert, and responsive without
a hematoma. The animal did not exhibit any adverse
effects from the treatment and was indistinguishable
from non-treated sheep.
Thirty days post-operative, the animal was
sacrificed and the femoral artery was removed en
bloc, placed in formalin, and evaluated using
standard histological techniques. Approximately 10%
of the implanted material was still remaining at
CA 02435050 2003-07-23
60895-1606D
' - 40 -
thirty days. The evaluating pathologist noted a
foreign body response to the material that was
consistent with a biodegrading material. Additional
studies have shown that, after the material has
entirely degraded, the tissue returns to a quiescent
state.
Example 5: Additional Femoral Puncture Site
Closure Procedures in Sheep
A number of additional procedures have been
performed using the barrier material in various
sizes of puncture sizes using heparinized sheep. The
following Table summarizes the results:
Table 2: Femoral Sealing Results (Heparinizeti
Sheep
Barrier Catheter Number $leeding Total Procedure
cube of
Material24 DiameterProceduresStopped in Time Less
less than
than 3 minutes10 Minutes
.
(Measured (Measured
Bctween Between
Material Insertion
of
Application Catheter Tube
and
When Bleedingand Stoppage
of
Stopped) Bleeding After
Removal of
Cathetcr Tube)
4-atixt 6 Fr 1 1 of 1 Not Applicable
PEG
/Albumin
.
4-arm 8 Fr 3 2 of 3 3 of 3
PEG
/Albumin
4-arm 8 Fr 3 2 of 3 3 of 3
PEG
/Albumin
+ Heparin
CA 02435050 2003-07-23
60895-1606D
' - 41 -
Example 6: Additional Femoral Puncture Site
Closure Procedures in Pigs
A number of additional procedures have been
performed using the barrier material in various
sizes of puncture sizes in pigs. The procedure used
in the porcine experiments is identical to that used
in the ovine experiments. ,
The following Table summarizes the results.
Table 3: Femoral Sealing Results (Pigs)
Barrier Catheter Number Bleeding Total Procedure
of Stopped
Materialtube 24 Proceduresin Iess thanTime Less
3.5 than
Diameter minutes 10 Minute:
(Measured (Measured
Between MaterialBetween
Application Insertion
and of
When BleedingCatheter Tube
Stopped) and Stoppage
of
Bleeding After
Removal of
Catheter Tubej
4-arm 8 Fr 4 3 of 4 4 of 4
PEG
/Albumin
4-arm 7 Fr 1 7 of 1 Not Applicable
PEG
/Albumin
Alternative Embodiments
1. Catheter Device
Fig. 14 shows an alternative embodiment of a
catheter device 220 that the system 10 can
incorporate instead of the catheter device 20.~
Like the catheter device 20, the catheter device
220 includes a flexible catheter tube 224 having a
proximal end 226 and a distal end 228. The catheter
CA 02435050 2003-07-23
60895-1606D
42
tube 224 can be constructed from the same medical
grade plastic materials as the catheter tube 24,
already described. As with the catheter tube 24, the
distal end 228 has an outside diameter of, e.g., 4
Fr to 16 Fr. Unlike the distal end 28, the distal
end 228 has a uniform diameter along its entire
length, which also matches the outside diameter of
the entire catheter tube 24.
The proximal end 226 carries a handle 230 to
facilitate gripping and maneuvering the catheter
tube 224 by a physician. As shown in Fig. 14, the
handle 230 is of reduced size, compared to the
handle 30. The reduced size of the handle 230
facilitates holding the handle 330 between the
forefinger and thumb, for better fine control and
tactile feedback.
As Fig. 16 shows, an interior lumen 232 extends
through the catheter tube 224. The lumen
accommodates passage of a conventional guide wire
40, as already described.
Like the catheter device 20, the catheter device
220 includes, at its distal end 228, a
circumferentially spaced array of. nozzles 234 (see
Fig. 15). The barrier material is conveyed in
liquid form and dispensed in a circumferential
manner through the nozzles 234 at the puncture site.
As Fig. 15 shows, the distal end 228 includes a
single deformable region 238, which is located a
short distance from the nozzles 234. Unlike the
catheter device 20, the distal end 228 of the
catheter device 220 does not includes a leader,
extending distally from the deformable region 238.
The distal end 228 terminates a short distance from
the deformable region 238.
The deformable region 238 normally presents a
CA 02435050 2003-07-23
60895-1606D
' - 43 -
generally cylindrical, low profile condition (shown
in Fig. 14), presenting an outside diameter that is
generally the same as the distal end 238 itself.
When the low profile condition, the region 238
passes over the guide wire into the vessel (as Fig.
17 shows).
The region 238 can be deformed into a radially
enlarged condition, which forms a positioner 242
(see Fig. 15). In use (see Fig. 18), the positioner
242 resists passage through the puncture site in
response to rearward tension along the catheter tube
224, as shown by arrow 132 in Fig. 18. The
positioner 242 serves to position the nozzles 234 at
a proper distance outside the vessel, while the
liquid barrier material is introduced outside the
vessel through the nozzles 34.
Unlike the catheter device 20, the catheter
device 220 does not include a second deformable
region spaced proximal to the nozzles 34. It has
been found that the gelation of the liquid barrier
material, as described above, occurs quickly enough
to obviate the need for a proximal dam.
The deformation of the region 238 can be
accomplished in various ways. In the illustrated
embodiment, the region 238 comprises an expandable
balloon material attached about the catheter tube
224. The catheter tube 224 includes an interior
lumen 256 (shown in Fig. 16), which communicates
through an aperture 258 with the interior of the
balloon material. A fitting 254 carried by the
handle 230 (see Fig. 14) communicates with the lumen
256. The fitting 254 couples the lumen to an
auxiliary syringe 126, which injects air under
pressure through the lumen 256 into the space
surrounded by the balloon material, causing the
CA 02435050 2003-07-23
60895-1606D
- 44 -
material to expand and form the positioner 242.
As Fig. 14 shows, a mixing chamber 294 is
carried at the end of a tube 296 attached to the
handle 230 of the catheter device 220. The tube 296
communicates with interior lumens 334 in the
catheter tube 224 (shown in Fig. 16), which, in
turn, are coupled to the dispensing nozzles 234. The
mixing chamber 294 includes a luer fitting 298,
which threadably connects with the single outlet
port 92 of the joiner 84 (see Fig. 17).
In use, the barrier material introducer/mixer 22
expresses the albumin 100 and polymer solution 136
in tandem from the dispensing syringes 60 and 62,
which are mechanically linked together by the joiner
84, support 64, and clip 68, in the. manner already
described. The two components of the barrier
material come into contact in the liquid state in
the mixing chamber 294. Channel-mixing of the two
components occurs as they flow through the mixing
chamber 294 to the nozzles 234.
Prior to deploying the catheter device 220 for
use, the physician prepares the PEG solution 136,
and couples the syringes 60 and 62 to the barrier
introducer/mixer 22, in the manners previously
described.
As Fig. 17 shows, according to appropriate
instructions 122, the physician is instructed to
pass the distal end 228 of the catheter tube 224
over the guide wire 40 through the puncture site.
The physician advances the distal end 228 to situate
the deformable region 238 inside the vessel, while
the nozzles 234 are deployed outside the vessel. The
physician can monitor the advancement tactilely. The
presence of the uniform diameter distal end 228
seals the puncture site.
CA 02435050 2003-07-23
60895-1606D
- 45 -
According to the directions 122 (as Fig. 18
shows), the physician is instructed to attach an
auxiliary syringe 126 filled with about 1 cc of air
to the fitting 254. The physician~injects the air
to inflate the region 238, which expands radially
into the positioner 242. The physician is then
instructed to place slight rearward tension on the
catheter tube 224 (shown by arrow 132 in Fig. 18) ,
to bring the positioner 242 into contact with the
interior of the vessel. Due to the slight rearward
tension, the positioner 242 seats against and
supports the puncture site. The physician will, by
tactile feedback, know that the positioner 42 has
contacted the vessel interior. The guidewire lumen
32 of the catheter tube 24 can be used to inject
suitable contrast media to aid in the visualization
of the puncture site region.
The physician is instructed to continue to apply
a slight rearward tension on the catheter tube 224,
sufficient to keep the positioner 242 against the
interior of the vessel, without pulling it through
the vessel wall.
The physician is instructed to grasp the finger
rests 80 and thumb rest 82 of the barrier material
introducer/mixer 22, as if grasping an ordinary
syringe. The physician expresses the albumin 100
from the first dispensing syringe 60 while
simultaneously also expressing the PEG solution 136
from the second dispensing syringe 62.
The albumin and PEG solutions come into contact
in the mixing chamber 294 and, from there, proceed
through the catheter tube 224 to the nozzles 234.
The albumin 100 and PEG solution 136 intimately
channel-mix in transit. w
As Fig. 19 shows, the mixture of albumin 100 and
CA 02435050 2003-07-23
60895-1606D
- 46 -
PEG solution 136 flows in liquid form through the
nozzles 234. The liquid mixture 130 of albumin 100
and PEG solution 13~ enters and fills the tissue
region surrounding the puncture site.
As Fig. 19 shows, according to the directions
122, the physician waits the requisite gelation
period, during which the liquid mixture 130 of
albumin 100 and PEG material 136 transform into a
non-fluid barrier network 128 outside the puncture
site. During the gelation period, the physician is
instructed to continue to apply a slight rearward
tension on the catheter tube 224 to seat the
positioner 242 against the interior vessel wall, as
the solid barrier network 128 forms outside the
vessel to seal the puncture site. The catheter tube
224 resists seepage of the liquid mixture 130 into
the vessel during the gelation period.
After the requisite gelation period, the
physician is instructed to operate the syringe 126
to remove air pressure and collapse the positioner
242. The physician withdraws the guide Wire 40 and
the distal end 228 of the catheter tube 24 from the
vessel. As shown by Fig. 20, during withdrawal, the
distal end 28 and the guide wire 40 pass through the
barrier network 128 that has, by now, formed over
the puncture site.
After withdrawing the catheter tube 24, the
physician is instructed to apply manual pressure to
the skin over the blood vessel, e.g., for about
three minutes, to aid in the sealing process. This
time allows the barrier material to fully cross-
link. The physician then confirms that the puncture
site has been sealed by observing the lack of blood
seepage about the guide wire access.
The puncture site of the vessel naturally closes
CA 02435050 2003-07-23
60895-1606D
- 47 -
and heals. As Fig. 20 shows, the presence of the
barrier network 128 outside the puncture site
prevents blood leakage while natural healing takes
place. The body resorbs the barrier network 128 over
time, e.g., within 30 days.
2. Mixing Chambers
There are various alternative constructions for
a mixing chamber 94 usable in association with the
barrier material introducer/mixer 22. The
construction selected depends upon the particular
geometry and size of a given mixing chamber, as well
as how readily the components of the barrier
material intimately mix to initiate the cross-
linking reaction.
In the illustrated embodiment, the enhanced
functionality of the preferred 4-PEG-SG material
allows channel mixing to take place, as the
components of the barrier are conveyed in tandem to
the targeted puncture site. In this arrangement, the
mixing chamber 94 serves the function of rapidly
guiding the polymer solution 136 and the protein
solution 100 into intimate flow contact as they
leave the port 92.
The mixing chamber 94 can, if desired, include
other structure to. mechanically enhance and
accelerate the mixing effect.
For example, as shown in Fig. 22, a mixing
chamber 94 can include an array of interior funnel
walls 156. The funnel walls 156 include
interruptions 158, which are arranged in a
alternative pattern along the flow center and along
the f low perimeter of the chamber 154. Polymer
solution 136 and protein solution 100 are directed'
through the interruptions 158 in a circumferential
and.circular flow path through the chamber 154. The
CA 02435050 2003-07-23
60895-1606D
- - 48 -
circumferential and circular flow of the polymer
solution 136 and protein solution 100 accelerates
the channel-mixing process.
Alternatively (as Fig. 23 shows), baffle walls
166 can be arranged perpendicular to.the flow path
through the mixing chamber 94. The baffle walls 166
include staggered interruptions 168. The
interruptions 168 cause the polymer solution 136 and
protein solution 100 to advance through the chamber
94 in a zig-tagging path, from one side of the
chamber 94 to the other. The zig-tagging path is
particularly advantageous if the polymer solution
136 and protein solution 100 are introduced into the
chamber 94 through separate inlet ports 170 and
17 2 ) .
Alternatively, baffles 160 can be arranged about
a hub 162 in a spiral pattern (as Fig. 24 shows) or
in a non-spiral pattern (as Fig. 25 shows). The
baffles 160 establish a cascading flow Within the
chamber 94 to accelerate mixing of the polymer
solution 136 and protein solution 100. The hub 162
can include an interior lumen 164 to accommodate
passage of, e.g., the guide wire 40 or the air
conveyed to expand a deformable region on the distal
end of the catheter tube 24 or 224.
As Fig. 26 shows, the polymer solution 136 and
the protein solution 100 can be introduced into the
chamber 94 through separate tangential ports 174 and
1?6, which are diagonally spaced apart. The chamber
94 includes a center outlet port 178. Solutions 100
and 136 entering the ports 174 and 176 flow in a
swirling pattern about the periphery of the chamber'
94, before exiting the center outlet port 178. The
swirling flow pattern accelerates intimate mixing.
As shown in Fig. 27, the chamber 94 can include
CA 02435050 2003-07-23
60895-1606D
- 49 -
multiple spaced apart inlet ports 180, 182, 184, 186
arranged about a common center outlet port 188. The
ports 180, 182, 184, 186, and 188 are arranged
parallel .to the intended flow path through the
chamber 94. Polymer solution 136 is introduced
through opposed ports 180 and 184, while protein
solution is introduced through the opposed ports 182
and 186. The multiple spaced-apart inlet paths
feeding a common center outlet port 188 enhance the
desired mixing effect of the chamber 94.
3. other Uses for the Barrier Material
Introducer/Mixer
The barrier material introducer/mixer 22 can be
used to dispense barrier material without
association with the catheter device 20 or 220. As
Fig. 21, the outlet port 92 can be coupled to
various dispensing devices, such as a sprayer 150 or
a cannula or needle 152.
The physician can select the sprayer 150 and
operate the material introducer/mixer 22 in the
manner previously described, to locally dispense the
barrier material (or an other tissue adhesive or
sealing material) at an exposed puncture or suture
site, e.g., during an open surgical procedure or on
the skin. Atomization through the sprayer 150 will
mix the liquid components of the barrier or adhesive
material sufficiently to initiate the cross-linking
reaction.
Alternatively, the physician can select the
cannula 152 and operate the material
introducer/mixer 22 to inject the barrier material
(or other selected material) at a targeted
subcutaneous puncture site. Passage of the liquid
components of the barrier or other material through
the cannula 152 will channel-mix the materials
CA 02435050 2003-07-23
60895-1606D
' - 50
sufficiently to initiate the cross-linking reaction.
It should thus be appreciated that the barrier
material introducer/mixer 22 can be used in diverse
ways throughout the body for dispensing any material
formed by intimate mixing of two liquid components
conveyed in tandem to a targeted treatment site. The
barrier material introducer/mixer 22 can be used for
exterior or interior introduction and application of
any such material, with or without catheter access.
4. Introducer/Mixer
Fig. 28 shows an alternative embodiment of an
introducer/mixer 300. In this embodiment, a molded
joiner 320 includes side-by-side female luer
fittings 304. Each fitting 304 receives the threaded
male luer fittings 306 of the dispensing syringes 60
and 62. A syringe clip 308 also preferably links the
syringe pistons 76 for simultaneous advancement when
dispensing materials from the syringes 60 and 62..
In this alternative embodiment, the
introducer/mixer 300 does not include a separate
channeled syringe support member (as shown by
reference numeral 34 in Fig. 2). The molded strength
of the female luer fittings 304 on the joiner,302,
can, when threaded to the male fittings 306, itself
be sufficient to hold the syringes 60 and 62 during
dispensement of their liquid contents, as~ already
described. This reduces the number of parts required
for the introducer/mixer 300.
As Fig. 29 shows, the joiner 302 can include
opposing skirts 310 molded to peripherally surround
the fittings 304. The skirts 310 resist side-to
side deflection of the syringes 60 and 62, when held'
by the joiner 302.
As Fig. 28 shows, the joiner 302 includes
interior channels 312 and 314, which are coupled to
CA 02435050 2003-07-23
60895-1606D
' - 51 -
the luer fittings 304. The interior channels 312
and 314 criss-cross within the joiner 302, without
fluid communication. The criss-crossing channels
312 and 314 keep the liquid contents of the syringes
60 and 62 free of mixing. The channels 312 terminate
with separate outlet ports 316 and 318.
As Fig. 28 also shows, in use, the joiner 302 is
coupled to a mixing chamber 320, which is of the
type shown in Fig. 27. The liquid contents of the'
syringes 60 and 62 are transported through the
outlet ports 316 and 318 from the joiner 302 into
separate, spaced-apart ports 322 in the mixing
chamber 320. The ports 322 lead to a common center
outlet port 324. As before explained, the flow of
the liquid contents through separate spaced-apart
inlet ports 322 into a common outlet port 324
enhances the mixing effects of the chamber 320.
Fig. 30 shows yet another alternative embodiment
of an introducer/mixer 326. In this embodiment, a
molded joiner 328 includes female leer fittings
330, to receive the threaded male luer fittings 306
of the dispensing syringes 60 and 62. In this
embodiment, the fittings 330 extend in a generally
v-shape, at an angle and not parallel with respect
to each. This allows the main body of the joiner
328 to be reduced in size. A syringe clip (not
shown) can be used to link the syringe pistons
coupled to the joiner 328 for simultaneous
advancement.
In this alternative embodiment, the
introducer/mixer 326 also does not include a
separate channeled syringe support member (as shown
by reference numeral 34 in Fig. 2). The molded
strength of the female huer fittings 330 itself can
be .sufficient to support the syringes 60 and 62
CA 02435050 2003-07-23
60895-1606D
- 52 -
during use. As Fig. 30 shows, an intermediate wall
332 can be provided between the fittings 330 to
resist inward deflection of the syringes 60 and 62
during use.
As Fig. .30 shows, the joiner 328 includes criss
crossing interior channels 334 and 336, like those
shown in Fig. 28. The channels 334 and 336 terminate
with separate outlet ports 338 and-340, which, in
use, are coupled to a mixing chamber 342 of the type
shown in Fig. 28 and previously described.
Of course, the joiners 302 and 328 can be
coupled to other types of mixing chambers.
II. ARRESTING OR CONTROLLING LOSS OF BLOOD OR OTHER
FLUIDS
Fig. 31 shows a system 410 of functional
instruments for arresting or controlling the loss of
blood or other fluids in body tissue:
During use, the instruments of the system 410
are brought to a compromised tissue region (shown as
an incision INC in Figs. 32 and 33), where bleeding
or loss of another body fluid is occurring, e.g.,
due to diffuse bleeding or anastomosis. The parts of
the system 410 are manipulated by a physician or
medical support personnel to create a liquid
material, which is immediately dispersed as a spray
directly onto the surface of the compromised tissue
region. The liquid material transforms as it is
being dispersed as a result of cross-linking into an
in situ-formed non-liquid covering structure. The
covering structure intimately adheres and conforms
to the surface the compromised tissue region, as
Fig.~3 best shows.
Due to the physical characteristics of the
covering structure and the speed at which it forms
CA 02435050 2003-07-23
60895-1606D
' - 53 -
in situ, the presence of the covering structure
mechanically arrests or blocks further blood or
fluid loss from the compromised tissue region,
without'need for a hemostatic agent. The covering
structure exists long enough to prevent blood or
fluid leakage while the compromised tissue region
heals by natural processes. The covering structure
is, over time, degraded by hydrolysis by in the host
body and cleared by the kidneys from the blood
stream and removed in the urine.
In the illustrated embodiment (see Fig. 31) , the
system 41o is consolidated in two functional kits
412 and 414.
The kit 412 houses the component assembly 418,
which contains the formative components from which
the covering structure is created. The kit 412 holds
the components in an unmixed condition until the
instant of use.
The kit 414 contains a dispersing assembly,416.
The dispersing assembly 416 brings the components in
the assembly 418, while in liquid form, into
intimate mixing contact. At the same time, the
assembly 416 disperses the liquid mixture onto the
surface of the compromised tissue region, to
ultimately form the in situ covering structure.
The Covering Structure
The covering structure comprises a material that
is chemically cross-linked, to form a non-liquid
mechanical matrix or barrier.
In a preferred embodiment, the material of the
covering structure is a protein/polymer composite
hydrogel. The material is most preferably formed
from the mixture of a protein solution and a
solution of an electrophilic derivative of a
hydrophilic polymer with a functionality of at least
CA 02435050 2003-07-23
60895-1606D
' - 54 -
three. The material is nontoxic, biodegradable, and
possesses mechanical properties such as cohesive
strength, adhesive strength, and elasticity
sufficient to block or arrest diffuse organ
bleeding, or to block or arrest seepage as a result
of anastomosis, or to seal lung punctures.
The material also permits the rate of cross-
linking and gelation to be controlled through buffer
selection and concentration. The rate of
degradation after cross-linking can be controlled
through the selection of a degradation control
region.
1. Material components
In the illustrated embodiment {see Fig 31), the
component assembly 418 includes first and second
dispensing syringes 460 and 462, in which the
formative components of the covering structure are
stored prior to use.
a. Natural Plasma-Based Protein
The first dispensing syringe 460 contains a
concentration of buffered protein solution 500. The
protein solution is supplemented with the
appropriate buffers, sterile filtered, aseptically
filled into the syringe 460, and the syringe 460 is
capped for storage prior to use.
Suitable proteins for incorporation into
material include non-immunogenic, hydrophilic
proteins. Examples include solutions of albumin,
gelatin, antibodies, serum proteins, serum
fractions, and serum. Also, water soluble
derivatives of hydrophobic proteins can also be
used. Examples include collagen, fibrinogen;
elastin, chitosan, and hyaluronic acid. The protein
can be produced from naturally occurring source or
it may be recombinantly produced. ,
CA 02435050 2003-07-23
60895-1606D
' ~ - 55 -
The preferred protein solution is 25% human
serum albumin, USP. Human serum albumin is
preferred due to its biocompatibility and its ready
availability.
Buffer selection and concentration maintains the
pH of the reactive mixture. Buffers that are well
tolerated physiologically can be used. Examples
include carbonate and phosphate buffer systems. Care
should be taken to select buffers that do not
participate in or interfere with the cross-linking
reaction. The preferred range of buffer
concentration is from about 0.03 M to about 0.4 M,
and the preferred range of pH is from about 7.0 to
about 10Ø A pref erred buffer system for the
covering structure is carbonate buffer at a
concentration of 0.315 M at a pH value of about 9 to
about 10. As will be described later, there is a
relationship between~pH and the time for cross-
linking (also called "gelation").
b. Electrophilic Water soluble
Polymer
In the illustrated embodiment (still referring
principally to Fig 31), the second dispensing
syringe 462 contains an inert, electrophilic, water
soluble polymer 502. The polymer cross-links the
protein to form an inert, three dimensional
mechanical network or matrix. The matrix forms the
mechanical covering structure. The covering
structure adheres and conforms to the surface of the
tissue region on which ,it is dispensed. The covering
structure is, over time, resorbed.
The polymer 502 comprises a hydrophilic,
biocompatible polymer, which is electrophilically
derivatized with a functionality of at least three.
A number of polymers .could. be utilized, including
CA 02435050 2003-07-23
60895-1606D
' - 56 -
polyethylene glycol), polyethylene oxide),
polyvinyl alcohol), poly(vinylpyrrolidone),
poly(ethyloxazoline), and polyethylene
glycol)-co-poly(propylene glycol) block copolymers.
The. polymer portion is not restricted to synthetic
polymers as polysaccharides, carbohydrates, and
proteins could also be electrophilically
derivatized.
Preferably, the polymer 502 is comprised of
polyethylene glycol) (PEG) with a molecular weight
between 1,000 and 30,000 g/mole, more preferably
between 2,000 and 15,000 g/mole, and most preferably
between 10,000~and 15,000 g/mole. PEG has been
demonstrated to be biocompatible and non-toxic in a
variety of physiological applications.
The preferred polymer can be generally expressed
as compounds of the formula:
P~G-(DCR-CG)"
where:
DCR is a degradation control region.
CG in a cross-linking group.
n i3
While the preferred polymer is a multi-armed
structure, a linear polymer with a functionality of
at least three can also 'be used. The desired
functionality of the PEG polymer for forming the
covering structure can be expressed in terms of (f)
how quickly the polymer cross-links the protein and
. transforms to a nonfluent gel state (i.e., the
mechanical material) (a preferred gelation time is
under three seconds) , and (ii) the mechanical
properties of the covering structure after gelation
in terms of its liquid sealing characteristics,
physical strength, resistance to fragmentation
(i.e., brittleness), and bioresorption. The
CA 02435050 2003-07-23
60895-1606D
_ g~
optimization of both attributes (i) and (ii) is
desirable.
The inventors have discovered that the utility
of a given PEG polymer significantly increases when
the functionality is increased to be greater than or
equal to three. The observed incremental increase in
functionality occurs when the functionality is
increased from two to three, and again when the
functionality is~ increased from three to four.
Further incremental increases are minimal when the
functionality exceeds about four.
The use of PEG polymers with functionality of
greater than three provides a surprising advantage.
When cross-linked with higher functionality PEG
polymers, the concentration of albumin can be
reduced to 25% and below. Past uses of difunctional
PEG polymers require concentrations of albumin well
above 25%, e.g. 35% to 45%. Use of lower
concentrations of albumin results in superior
sealing properties with reduced brittleness,
facilitating reentry through the nonfluid material,
without fragmentation. Additionally, 25% human
serum albumin, USP is commercially available from
several sources, however higher concentrations of
USP albumin are not commercially available. By using
commercially available materials, the dialysis and
ultrafiltration of the albumin solution, as
disclosed in the prior art, is eliminated,
significantly reducing the cost and complexity of
the preparation of the albumin solution.
In the illustrated embodiment, the polymer 502
is initially packaged prior to use in the second
dispensing syringe 462 in an inert atmosphere (e. g.,
argon) in a stable, powder form. In this
arrangement, the component assembly 418 includes a
CA 02435050 2003-07-23
60895-1606D
_ 58
third syringe 504, which contains sterile water 506
for dissolution of the powder polymer 502 just
before mixing with the albumin component 500.
In facilitating mixing, a stopcock valve 508 is
secured to the luer fitting 488 at the dispensing
end of the second dispensing syringe 462. The
dispensing end 110 of the water syringe 504 couples
to the stopcock valve 508, so that the water 106 can
be mixed with the polymer 502 in the dispensing
syringe 562 prior to use.
The rate of degradation is controlled by the
selection of chemical moiety in the degradation
control region DCG. If degradation is desired, a
hydrolytically or enzymatically degradable moiety
can be selected. The degradation control region DCR
can be selected for the covering structure using the
criteria previously described with respect to the
polymer that, when cross-linked, forms the
mechanical barrier f or sealing a vascular puncture
site. The preferred degradable control regions for
degradable materials are ester containing linkages,
as are present when succinic acid or glutaric~acid
are coupled to a PEG molecule. The preferred
degradable control regions for nondegradable
materials are ether containing linkages. The
material can also be created without the
introduction of a degradation control region.
The cross-linking group CG is responsible.for
the cross-linking of the albumin, as well as the
3~0 binding to the tissue substrate. The cross-linking
group CG for the covering structure can be selected
using the criteria previously described with respect '
to the polymer that, when cross-linked, forms the
mechanical barrier for sealing a vascular puncture
site. The preferred cross-linking group is an active
CA 02435050 2003-07-23
60895-1606D
- - 59 - '
ester, specifically an ester of
N-hydroxysuccinimide.
To minimize the liberation of heat during the
cross-linking reaction, the concentration of the
5' cross-linking groups' is preferably kept less than 5%
of the total mass of the reactive solution, and more,
preferably about 1% or less. The low concentration
of the cross-linking group is also beneficial so
that the amount of the leaving group is also
minimized. In a preferred embodiment, the cross
linking group portion comprising a
N-hydroxysuccinimide ester has demonstrated ability
to participate in the cross-linking reaction with
albumin without presenting the risk of local or
systemic immune responses in humans.
c. Preferred Multiple Arm PEG
Polymer
In a preferred embodiment, the polymer is
comprised of a 4-arm PEG with a molecular weight of
about 10,000 g/mole, the degradation control region
is comprised of glutaric acid, and the cross-linking
group is comprised of a N-hydroxysuccinimide ester.
Thus, a preferred polymer is polyethylene glycol)
tetra-succinimidyl glutarate, which is available
from Shearwater Polymers, Huntsville, AL. The
preferred polymer will, in shorthand, be called
4-PEG-SG. The polymer is dissolved in water prior
to use. Preferred concentrations of the polymer are
from 5% to 35% w/w in water.
The solution of 4-PEG-SG mixes with 25% serum
albumin to form a liquid solution that quickly
cross-links to form a non-liquid, three dimensional
network for the covering structure. With these
material formulations, it is possible to intimately
mix the water soluble polymer with the albumin
CA 02435050 2003-07-23
60895-1606D
' - 60
protein using, e.g., atomization, or static mixing,
or in-line channel mixing.
As will be demonstrated later, the rate of
reaction can be controlled by.the pH of the reactive
solution. An increase in temperature is not observed
during formation of the covering structure network,
due to the low concentration of reactive groups,
which account for only about 1% of the total mass.
In a typical clinical application, about 50 mg of s
non-toxic leaving group is produced 'during the
cross-linking reaction, which is a further desired
result.
The resulting nonfluent material created by
mixing 25% albumin and 4-PEG-SG is approximately 80%
water, 13% albumin, and 7% PEG. The material is well
tolerated by the body, without invoking a severe
foreign body response. Over a controlled period of
time, the material is degraded via hydrolysis.
Histological studies have shown a foreign body
response consistent with a biodegradable material,
such as VICRYL~'" sutures. As the material is
degraded. the tissue returns to a quiescent state.
'The molecules of the degraded material are cleared
from the bloodstream by the kidneys and eliminated
from the body in the urine. In a preferred
embodiment of the invention, the material loses its
physical strength during the first twenty days, and
total resorption occurs, in about 4 weeks.
The following Examples demonstrate the superior
features of the material of the invention.
Example 1: Preparation of Cross-Linked
Networks
Cross-linked covering structure networks were
formed by the mixture of an 4-PEG-SG and albumin. A
solution of 4-PEG-SG was prepared by dissolving
CA 02435050 2003-07-23
60895-1606D
' - 61 -
0.408 in 2.0 mL of water. The albumin solution
consisted 25% human serum alburmin, USP (Plasbumin-
25, Bayer Corporation), as received.
Dispensing syringes containing 2.0 mL of the
polymer solution and 2.0 mL of albumin solution were
connected to the joiner 84, to which a spray head
was coupled. The solutions were sprayed into a
polystyrene weigh boat. A cross-linked covering
structure network formed at room temperature in
about 90 seconds.
Example 2: Control of the Rate of Gelation
The rate of formation of the cross-linked
covering structure network of 4-PEG-SG and albumin
(i.e., gelation) can be controlled by the pH of the
reactive solution. To increase the rate of cross
linking, the pH of the solution is increased, and
conversely, to decrease the rate of cross-linking,
the pH of the solution is decreased. The pH of the
solution is controlled by both the buffer strength
and buffer pH.
Table 1 shows the effect of buffer strength on
the rate of gelation of 17% w/w 4-PEG-SG in water
for injection and 25% human serum albumin, USP at
room temperature. The rate of gelation can also be
controlled by adjusting the pH of the buffer at a
constant buffer concentration. The buffer was placed
in the solution of albumin. The gelation time is the
amount of time required for the formulation to
transform from the liquid state to the cross-linked
solid state.
Table 1: Effect of Buffer Strength and Buffer
pH on Gel Formation
CA 02435050 2003-07-23
60895-1606D
- 62 -
Buffer Buffer pH Gelation Time
Concentration
300 mM 9 < 1 sec
200 mM 9 5 sec
100 mM 9 10 sec
50 mM 9 20 sec
0 mM -- 90 sec
The Dispersing assembly
As Fig. 34 shows, the dispersing assembly 416
comprises a material introducer/mixer 422. The
material introducer/mixer 422 receives the two
dispensing syringes 460 and 462. The material
introducer/mixer 422 allows the physician to
uniformly dispense the two. components in a liquid
state from the dispensing syringes 460 and 462.
The material introducer/mixer 422 also mixes the
components while flowing in the liquid state from
the dispensing syringes 460 and 462.
To accomplish these functions (see Fig. 34) , the
material introducer/mixer 422 includes syringe
support 464. The support 464 includes side-by-side
channels 466 (see Fig. 31, too). The channel 466
accommodates in a snap-friction-fit the barrels of
the syringes 460 and 462.
The material introducer/mixer 422 also includes
a syringe clip 468. The syringe clip 468 includes
spaced apart walls 470 forming an interior race 472.
The race 472 receives in a sliding friction fit the
thumb rests 474 of the pistons 476 of the dispensing
syringes 460 and 462, in axial alignment with the
syringe barrels carried by the syringe support 464.
The syringe clip 468 mechanically links the syringe
pistons 476 together for common advancement inside
CA 02435050 2003-07-23
60895-1606D
- 63 -
their respective syringe barrels.
To facilitate handling, the syringe support 464
includes opposed finger rests 480, and the syringe
clip 468 includes a thumb rest 482. The orientation
~ of these rests 480 and 482 parallel the orientation
of the finger rests and thumb rests of a single
syringe. The physician is thereby able to hold and
operate multiple syringes 460 end 462 in the same
way. as a single syringe.
The material introducer/mixer 422 also includes
a joiner 484. The joiner 484 includes side by side
female luer fittings 486. The female luer fittings
486 each receives the threaded male luer fitting 488
at the dispensing end of the dispensing syringes 460
and 462. The female luer fittings 486 are axially
aligned with the barrels 478 of the dispensing
syringes 46o and 462 carried in the syringe support
64.
The physician is thereby able to quickly and
conveniently ready the dispensing syringes 460 and
462 for use by securing the dispensing syringes to
the joiner 484, snap fitting the syringe barrels 478
into the syringe support 464, and slide fitting the
syringe thumb rests 474 into the clip 468.
The joiner 484 includes interior channels 490
coupled to the female luer fittings 486. The
channels 490 merge at a Y-junction into a single
outlet port 492. The joiner 484 maintains-two
fluids dispensed by the syringes 460 and 462
separately until they leave the joiner 484. This
design minimizes plugging of the joiner 484 due to
a mixing reaction between the two fluids. The
syringe clip 468 ensures even application of
individual solutions through the joiner 484.
The material introducer/mixer 422 further
CA 02435050 2003-07-23
60895-1606D
64
includes a mixing spray head 494, which, in use, is
coupled to the single outlet port 492. In Fig. 31,
the kit 414 contains several interchangeable mixing
spray heads 494, in case one mixing spray head 494
becomes clogged during use. '
The mixing spray head 494 may be variously
constructed. It may, for example, comprise a spray
head manufactured and sold by Nemaedics.
Alternatively, the material introducer/mixer 422
can include a cannula 552, which, in use, can be
coupled~to the outlet port 492 instead of the mixing
spray head (see Fig. 35).
Expressed in tandem from the dispensing syringes
460 and 462, which are mechanically linked together
by the joiner 484, support 464, and clip 468, the
two components of the barrier material come into
contact in the liquid state either in the mixing
spray head 494 or the cannula 552. Atomization of
the two components occurs as they .are dispersed
through the mixing spray head 494 under pressure
from operation of the mechanically linked dispensing
syringes 460 and 462. Passage of the liquid
components through the cannula 552 will channel-mix
the materials. Either by atomization or channel
mixing, the liquid components are sufficiently mixed
to immediately initiate the cross-linking reaction.
The parts of the introducer/mixer 422 are made,
e.g., by molding medical grade plastic materials,
such as polycarbonate and acrylic.
The. Kits
As Figs. 36A and 36B show, in the illustrated
embodiment, each kit 412 and 414 includes an
interior tray 512 made, e.g., from die cut
cardboard, plastic sheet, or thermo-formed plastic
material.
CA 02435050 2003-07-23
60895-1606D
- 65 -
The component assembly 418 is carried by the
tray 512 in the kit 412 (see Fig. 36A). The
dispersing assembly 416 is carried by the tray 512
in the kit 414 (see Fig. 36B).
As shown in Fig. 368, the kit 414 includes an
inner wrap 514, which is peripherally sealed by heat
or the like, to enclose the tray 512 from contact
with the outside environment. One end of the inner
wrap 114 includes a conventional peel away seal 516.
The seal 116 provides quick access to the tray 512
at the instant of use, which preferably occurs in a
suitable sterile environment.
The kit 414 is further wrapped in an outer wrap
518, which is also peripherally sealed by heat or
the like, to enclose the interior tray 512. One end
of the inner wrap 518 includes a conventional peel
away seal 520, to provide quick access to the
interior tray 512 and its contents.
The outer wrap 518 and the inner wrap 514 are
made, at least in part, from a material that is
permeable to ethylene oxide sterilization gas, e.g.,
TYVEKT"' plastic material (available from DuPont) . Kit
12 is sterilized utilizing ethylene oxide gas or
electron beam irradiation.
As shown in Fig. 36A, kit 412 includes a polymer
package 538 (which contains the prefilled powder
polymer syringe 462 and water syringe 504) and an
albumin package 540 (which contains the prefilled
albumin syringe 464). Each polymer package 138 and
albumin package 540 includes an individual wrap 542,
which is peripherally sealed by heat or the like, to
enclose package 538 and 540 from contact with the
outside environment. One end of the individual wrap
542 includes a conventional peel away seal 544, to
provide quick access to the contents of the packages
CA 02435050 2003-07-23
60895-1606D
- 66 -
538 and 540 at the instant of use.
Polymer package 538 and albumin package 540 are
further wrapped in an outer wrap 518, which is also
peripherally sealed by heat or the like. One end of
the outer wrap 518 includes a conventional peel away
seal 548, to provide quick access to the packages
538 and 540. After sterilization treatment, the
packages 538 and 540 and the tray 512 are further
wrapped in container 546 for the user's convenience.
The wraps 542 and 518 are made, at least in
part, from a material that is permeable to ethylene
oxide sterilization gas, e.g., TYVEKT"" plastic
material (available from DuPont). The albumin
package 540 is prepared, sterilized utilizing
ethylene oxide gas, and placed into kit 414. The
polymer package 538 is prepared, sterilized
utilizing electron beam irradiation, and place into
kit 414.
In the illustrated embodiment, each kit 412 and
414 also preferably includes directions 522 for
using the contents of the kit to carry out a desired
procedure. The directions 522 can, of course~vary,
according to the particularities of the desired
procedure. Furthermore, the directions 522 need not
be physically present in the kits 412 and 414. The
directions 122 can be embodied in separate
instruction manuals, or in video or audio tapes.
Using the System
1. Controlling or Arresting Diffuse Organ
Bleeding
In this embodiment, exemplary directions 522 are
described, which instruct the physician how to use
of the system 410 to arrest diffuse bleeding of an
injured or compromised body organ. In the
illustrated embodiment (see Fig. 37A), diffuse
CA 02435050 2003-07-23
60895-1606D
- 67 -
bleeding is shown to occur diagrammatically through
an incision in the organ.
The system 410 is applicable for use to control
or arrest diffuse bleeding in diverse types of
organs, e.g., the liver, spleen, kidney, or bone.
The cause of diffuse bleeding that the system 10
controls or arrests can also vary. The diffuse
bleeding can occur as a result of trauma or
accidental injury. The diffuse bleeding can also
occur~during normal surgical intervention, e.g., by
organ resection, or tumor excision, or (in the case
of bone) by sternotomy, orthopedic procedure, or
craniotomy. The diffuse bleeding can also occur
through needle tracks formed during tissue biopsy,
or by capillary bed bleeding, as a result of
saphenous vein harvesting, adhesiolysis, or tumor
removal. It should be appreciated that the
ef f ectiveness of the system 410 does not depend upon
where the diffuse bleeding is occurring or its
underlying cause.
When use of the system 410 is desired, the outer
wrap 518 of the kits 412 and 414 are removed. The
trays 512, still contained in the inner wraps 518,
are placed in the sterile operating ffield. The
physician opens the inner wrap 518 of the kit 412 to
gain access the first, second, and third syringes
460, 462, and 504.
The directions 522 for use instruct the
physician to remove from the kit tray 512 the second
dispensing syringe 462, which contains, in sterile
powder form, a predetermined amount of the polymer
502 (e.g., about 0.3 to 0.5 g). The directions 522
also instruct the physician to remove from the kit
412 the third syringe 504, which contains sterile
water 506 (e.g., about 2 cc). Both are contained in
CA 02435050 2003-07-23
60895-1606D
- 68 -
the polymer package 538.
As Fig. 40A shows, the directions 522 instruct
the physician to couple the dispensing end of the
water syringe 504 to the stopcock valve 508 on the
second dispensing syringe 462. The stopcock valve
508 is closed at this point. As instructed by the
directions .522, the physician opens the stopcock
valve 508 (see Fig. 40H) and transfers water from
the water syringe 504 into the powder 500 in the
second dispensing syringe 462 (see Fig. 40C). The
physician is instructed to repeatedly transfer the
water and powder mixture between the two syringes
462 and 504, to syringe-mix the powder and water
until all solids are dissolved. The syringe-mixing
places the water soluble, polymer material into
solution. The syringe-mixing process generally takes
about two minutes.
After syringe mixing, the physician, following
the directions 522, transfers the PEG solution 536
(about 2 cc) into one of the syringes (which, in the
illustrated embodiment, is the second syringe 462).
The physician waits for bubbles to dissipate, which
generally takes about an additional two minutes.
According to the directions 522, the physician
now closes the stopcock valve 508 (as Fig. 40D
shows). The physician removes the stopcock valve
108 by unscrewing it from the luer fitting on the
dispensing end of the second syringe 462. The PEG
solution 536 is ready for use. Mixing of the PEG
solution 536 should take place generally within one
hour of use. If the PEG solution 536 remains unused
over two hours after mixing, it should be discarded.
The directions 522 instruct the physician to
remove from the second kit tray 512 the dispensing
syringe 460 containing the albumin 500. As before
CA 02435050 2003-07-23
60895-1606D
- 69 -
described, the albumin 500 has been premixed in a
buffered form to the desired concentration (e. g.,
25%), then sterile filtered, and aseptically filled
into the syringe 460. A closure cap normally closes
the dispensing end inside the tray 512.
The physician now, or at a previous time, opens
the outer wrap 518 of the kit 414 to gain access to
the material introducer/mixer 422. The directions
522 instruct the physician to remove the closure cap
and screw the dispensing end of the first syringe
460 to the luer fitting 486 on the joiner 484. The
physician is also instructed to screw the dispensing
end of the second syringe 462 (now containing the
mixed PEG solution 536) to the other luer fitting
486 on the joiner 484.
Following the directions 522, the physician
snaps the barrels 478 of the syringes 460 and 462 to
the holder channels 466. The physician captures the'
thumb rests 474 of the two syringes 460 and 462
inside the race 472 of the syringe clip 468: The
directions 522 instruct the physician to attach the
joiner 484 to the mixing spray head 494.
As Fig. 37B shows, the physician is instructed
to position the mixing spray head 494 in a close
relationship with the exposed site of diffuse
bleeding on the organ. The physician applies manual
pressure to the dispensing syringes 460 and 462:
Albumin 500 from the first dispensing syringe 460
contacts the PEG solution 536 from the second
dispensing syringe 462 in the mixing spray head 494.
Atomization of the liquid components occurs through
the mixing spray head 494 under pressure from
operation of the mechanically linked dispensing
syringes 460 and 462. The mixed liquids initiate
the cross-linking reaction as they are dispersed
CA 02435050 2003-07-23
60895-1606D
- 70 -
onto the organ surface. Within seconds (as
determined by the gel time), the liquid material
transforms by in situ cross-linking into a non-
liquid structure covering the diffuse bleeding site.
. As Fig. 37C shows, the covering structure adheres
and conforms to the organ surface, including entry
into any incision, blunt penetration, or other
surface irregularity from which the diffuse bleeding
emanates. Due to speed of cross-linking and the
physical properties of the covering structure,
diffuse bleeding does not wash away or dilute the
liquid material as it transforms into the covering
structure.
As cross linking rapidly occurs at the surface
of the organ, the covering structure entraps
diffused blood. Diffuse bleeding just as rapidly
stops as the structure forms in situ, without need
of any hemostatic agent. The covering' structure
forms an in situ barrier against further bleeding on
the surface of the organ. The covering structure
exists long enough to prevent further blood or fluid
leakage while the compromised organ heals by natural
processes.
Example 3: control of Bleeding from a
Kidney Incision
A solution of 4-arm PEG succinimidyl glutarate,
MW 10,000 (Shearwater Polymers, Huntsville, AL) was
prepared by dissolving 0.40g in 2.0 mL of water for
injection. The albumin solution consisted of 25%
human serum albumin, USP (Plasbumin-25, Bayer
Corporation), buffered with 195 mM sodium carbonate
and 120 mM sodium bicarbonate. Syringes containing
2.0 mL of the polymer solution and 2.0 mL of the
albumin solution were connected to a joiner and
sprayhead (DuoFlow, Hemaedics, Brentwood, CA).
CA 02435050 2003-07-23
60895-1606D
- 71 -
The kidney of a sedated pig was exposed. An
incision approximately an inch long and a quarter
inch deep was made on the surface of the kidney.
The continual flow of blood Was temporarily
collected with gauze. The gauze was then removed
and the sprayable hemostatic solution, consisting of
the polymer and albumin syringes, was applied using
digital pressure.
As the two solutions were mixed in the
sprayhead, the crosslinking reaction began. As the
atomized, mixed fluid landed on the surface of the
bleeding kidney, the gelation of the solution
occurred. The hydrogel adhered tenaciously to the
surface of the kidney,' preventing blood from
flowing. The hydrogel also had sufficient cohesive
strength to prevent rupture. Without the use of a.
hemostatic agent, hemostasis occurred
instantaneously using the mechanical barrier of the
hydrogel.
Example 4: Control of Bleeding from a
spleen Incision
A solution of 4-arm PEG succinimidyl glutarate,
MW 10,000 (Shearwater Polymers, Huntsville, AL) was
prepared by dissolving 0.408 in 2.0 mL of water for
injection. The albumin solution consisted of 25%
human serum albumin, USP (Plasbumin-25, Bayer
Corporation), buffered with 195 mM sodium carbonate
and 120 mM sodium bicarbonate. Syringes containing
2.0 mL of the polymer solution and 2.0 mL of the
albumin solution were connected to a joiner and
sprayhead (DuoFlow, Hemaedics, Brentwo.od,.CA).
The spleen of a sedated pig was exposed. An
incision approximately an inch long and a: quarter
inch deep was made on the surface of the spleen.
The continual flow of blood was temporarily
CA 02435050 2003-07-23
60895-1606D
' - 72 -
collected with gauze. The gauze was then removed
and the sprayable hemostatic solution, consisting of
the polymer and albumin syringes, was applied using
digital pressure.
As the two solutions , were mixed in the
sprayhead, the crosslinking reaction began. As the
atomized, mixed fluid landed on the surface of the
bleeding spleen, the gelation of the solution
occurred. The hydrogel adhered tenaciously to the
surface of the spleen, preventing blood from
flowing. The hydrogel also had sufficient cohesive
strength to prevent rupture. Without the use of a
hemostatic agent, hemostasis occurred
instantaneously using the mechanical barrier of the
hydrogel.
Example 5: control of Bleeding from a Liver
Incision
A solution of 4-arm P~G succinimidyl glutarate,
MW 10,000 (Shearwater Polymers, Huntsville, AL) was
prepared by dissolving 0.40g in 2.0 mL of water for
injection. The albumin solution consisted of 25%
human serum albumin, USP (Plasbumin-25, Bayer
Corporation), buffered with 195 mM sodium carbonate
and 120 mM sodium bicarbonate. Syringes containing
2.0 mL of the polymer solution and 2.0 mL of the
albumin solution were connected to a joiner and
sprayhead (DuoFlow, Hemaedics, Brentwood, CA).
The liver of a sedated pig was exposed. An
incision approximately an inch long and a quarter
inch deep was made on the surface of the liver. The
continual flow of blood was temporarily collected
with gauze. The gauze was then removed and the'
sprayable hemostatic solution, consisting of the
polymer and albumin syringes, was applied using
digital pressure.
CA 02435050 2003-07-23
60895-1606D
' - 73 -
As the two solutions were mixed in the
sprayhead, the crosslinking reaction began. As the
atomized, mixed fluid landed on the surface of the
bleeding liver, the gelation of the solution
occurred. The hydrogel adhered tenaciously to the
surface of the liver, preventing blood from flowing.
The hydrogel also had sufficient cohesive strength
to prevent rupture. Without the use of a hemostatic
agent, hemostasis occurred instantaneously using the
mechanical barrier of the hydrogel.
2. Controlling or Arresting Air Leaks
From a Lung Incision
The exemplary directions 522 just described can
be modified to instruct the physician how to use of
the system 410 to control or arrest the leakage of
air through a perforation or puncture in the lung
caused, e.g., by trauma (see Fig. 38A).
In this embodiment, the instructions 522
instruct the physician to prepare the dispensing
syringes 460 and 462 and coupled them to the joiner
84 in the manner previously set forth. The
physician is instructed to attach the mixing~spray
head 484 and position the mixing spray head 494 in
a close relationship with lung puncture site. The
lung is deflated (see Fig. 38B).
In the manner previously described, the
physician applies manual pressure to the dispensing
syringes 460 and 462 (as Fig. 38B shows). Albumin
500 from the first dispensing syringe 460 contacts
the PEG solution 536 from the second dispensing
syringe 462 in the mixing spray head 494.'
Atomization of the liquid components also occurs
through the mixing spray head 494 under pressure
from operation of the mechanically linked dispensing
syringes 460 and 462. The mixed liquids initiate
CA 02435050 2003-07-23
60895-1606D
- 74 -
the cross-linking reaction as they are dispersed
into contact with tissue surrounding the lung
puncture site. Within seconds, the liquid material
transforms by in situ cross-linking into a non-
liquid structure covering the puncture site (see
Fig. 38C). Air leaks through the puncture site stop
as the structure forms in situ. The covering
structure exists long enough to prevent further air
leaks, while the lung tissue heals by natural
processes.
E~cample 5: Control of Air Leaks from a Lung
Incision
A solution of 4-arm PEG succinimidyl glutarate,
MW 10,000 (Shearwater Polymers, Huntsville, AL) was
prepared by dissolving 0.40g in 2.0 mL of water for
injection. The albumin solution consisted of 25%
human serum albumin, USP (Plasbumin-25, Bayer
Corporation), buffered with 195 mM sodium carbonate
and 120 mM sodium bicarbonate. Syringes containing
2.0 mL of the polymer solution and 2.0 mL of the
albumin solution were connected to a joiner and
sprayhead (DuoFlow, Hemaedics, Brentwood, CA).
The lung of a euthanized, intubated pig was
exposed. An incision approximately an inch long and
a quarter inch deep was made on the surface of the
lung. An air leak was confirmed by manually
inflated the lung and listening for the hissing
sound of air leaks. The lung was deflated and the
surgical sealant, consisting of the polymer and
albumin syringes, was applied using digital
pressure.
As the two solutions were mixed in the
sprayhead, the crosslinking reaction began. As the
atomized, mixed fluid landed on the surface of the
lung, the gelation of the solution occurred. The
CA 02435050 2003-07-23
60895-1606D
' - 75 -
hydrogel was firmly adherent to the surface of the
lung. After about 10 seconds, the lungs were
manually inflated and examined for the presence of
air leaks. The hydrogel remained firmly attached to
the lung tissue, even during and after the expansion
of the lungs. Air leaks were not present after the
application of the hydrogel surgical sealant. The
hydrogel showed sufficient adhesion, cohesion, and
elasticity to seal air leaks of lung tissue.
3. Sealing Anastomosis
The exemplary directions 522 just described can
be modified to instruct the physician how to use of
the system 410 as a surgical sealant along suture
lines or about surgical staples, forming an
anastomosis (see Fig. 39A). The sutures or staples
can be used, e.g., to join blood vessels, bowels,
ureter, or bladder. The sutures or staples can also
be used in the course of neurosurgery or ear-nose-
throat surgery.
In this embodiment, the instructions 522
instruct the physician to prepare the dispensing
syringes 460 and 462 and coupled them to the joiner
484 in the manner previously set forth. The
physician is instructed to attach the mixing spray
head 484 and position the mixing spray head 494 in
a close relationship with the anastomosis (as Fig.
398 shows).
In the manner previously described, the
physician applies manual pressure to the dispensing
syringes 460 and 462. Albumin 500 from the first
dispensing syringe 460 contacts the PEG solution 536
from the second dispensing syringe 462 in the
mixing spray head 494. Atomization of the liquid
components also occurs through the mixing spray head
494 under pressure from operation of the
CA 02435050 2003-07-23
60895-1606D
' - 76 -
mechanically linked dispensing syringes 460 and 462.
The mixed liquids initiate the cross-linking
reaction as they are dispersed into contact with
tissue along the anastomosis (see Fig. 39B). Within
seconds, the liquid material transforms by in situ
cross-linking into a non-liquid structure covering
the anastomosis (see Fig. 39C). Blood or fluid
seepage through the anastomosis stop as the
structure forms in situ. The covering structure
exists long enough to prevent further blood or fluid
leaks, while tissue along the anastomsis heals by
natural processes.
It should be appreciated that the compositions,
systems, and methods described are applicable for
use to control or arrest bleeding or fluid leaks in
tissue throughout the body, including by way of
example, the following surgical sites and
indications:
(i) In general surgery, such as in the liver
(resection, tumor excision or trauma) ; in the spleen
(trauma or iatrogenic capsular avulsion; oncology in
general (excision of tumors); or laporoscopic
cholecystectomy (Lapchole) (to control bleeding from
the gall bladder bed);
(ii) In vascular surgery, such as peripheral
vascular procedures; anastomosis sites (carotid,
femoral and popliteal arteries); or aneurysms;
(iii) In the head, such as craniotomy (to
control bone bleeding from cut bone edges or
bleeding from soft tissue); or superior sagittal
sinus (to control bleeding from damage to thin wall
sinus and access to sinus ; '
(iv) To treat arteriovenous malformation (AVM)
(to control blood vessel bleeding from smaller
vessels);
CA 02435050 2003-07-23
60895-1606D
' - 77 -
(v) To treat tumor complications, such as tumor
bed bleeding or damage to soft tissue due to
excisions;
(vi) To treat hematomas, such as in the
control of bleeding in soft tissues~and adjacent to
vessels;
(vii) In orthopedic applications, such as
laminectomy or discectomy, to control bone bleeding
from the vertebrae; or spinal reconstruction and
fusion, to control epidural vessels and vertebral
bleeders; or in hip and knee replacements, to
control of bleeding in smooth muscle tissue, soft
tissue;
(viii) In cardiovascular and thoracic surgery,
such as control of anastomosis sites in coronary
artery bypass graft (C.A.B.G.); aorta.reconstruction
and repair, to control bleeding in surrounding
tissue; or chest cavity access through the sternum,
to control bone bleeding or soft tissue bleeding;
(ix) In urology, such as retropubic
prostatectomy, to control bleeding in soft tissue;
or partial nephrectomy, to control parenchymal
bleeding; in bladder substitution, uretero
intestinal anastomosis; urethral surgery; open
urethral surgery; or vasovasostomy;
(x) In ear-neck-throat surgery, such as during
clearing of the frontal, thmoid, sphenoid and
maxillary sinuses; or in polyp removal;
(xi) In plastic and reconstructive surgery, such
as face lifts, rhinoplasty, blepharplasty, or breast
surgery;
(xii) In emergency procedures involving trauma,
tissue fracture, or abrasions.
The features of the invention are set forth in
the following claims.