Note: Descriptions are shown in the official language in which they were submitted.
CA 02435571 2003-07-22
Electrolysis Device
The present invention relates to an electrolysis device that has at least one
horizontal
electrolytic cell that has a housing and an anode with a membrane or
diaphragm, and a
cathode with a gas-diffusion electrode, as well as means for supplying and
discharging
gas into or out of the gas chamber of the cathode, and well as means for
supplying and
discharging electrolytes into or out of a first electrolytic chamber, and into
or out of a
second electrolytic chamber, the electrolytic chambers being separated from
one another
by the membrane or diaphragm.
An electrolysis device of this kind is described in EP-A-182 144. In this, the
electrolyte
is supplied and discharged by way of openings disposed on the edge between the
electrodes. Because of this, the cross sectional area of the openings is
restricted by the
dimensions of the electrodes and the distance between them. Since the spacing
between
the electrodes amounts to only a few millimeters, the cross sectional area
that is
available for supplying and discharging the electrolyte is relatively small.
For this
reason, such electrolysis devices are suitable only for electrolytic cells
that are connected
electrolytically in parallel, since only small quantities of electrolyte pass
through these.
In the case of cells that are electrolytically connected in series, as is
described, for
example, in EP-B-0 865 516, the quantity of electrolyte that passes through
them is
greater, in keeping with the number of cells, and unacceptable pressure losses
can be
caused at the openings because of high electrolyte speeds. This is
particularly so if an
electrode is coated with a porous gas-diffusion electrode. An hydraulic
pressure acts on
the gas-diffusion electrode as a function of the pressure losses at the
openings, and this
CA 02435571 2003-07-22
can result in flooding of the electrode if the gas pressure on the other side
of the
electrode is not great enough. It is true that-generally speaking-gas-
diffusion
electrodes are hydrophobic since they contain a considerable quantity of
Teflon that
binds the carbon, so that they can in part be loaded with water columns
greater than 500
mm without the water penetrating into the cells. However, practice has shown
that this
is not the case in an electrolysis device since, when current is flowing and
ions are
present, the surface will be wetted even at pressures below 40 mm of water
column. In
the same way that hydraulic pressure increases as the length of pipe line
through which
there is a flow increases, so the pressure acting on the gas-diffusion
electrode increases
with the increasing number of cells connected electrolytically in series. This
results in
the highest pressure being found in the first cell, and the lowest pressure
being found in
the last cell. In such a case, flooding of the gas-diffusion electrode can
only be
prevented by maintaining a specific gas pressure in each individual cell.
In order to permit operation of the cells in a manner that is less costly and
thus more
economical, and with only one gas pressure, a cascade flow has to be
generated, i.e., the
electrolyte passes in overflow from the outlet pipe of one cell into the inlet
pipe of the
next cell. At the overflow point, which corresponds to an adjustable-height
overflow as
described in EP-B-0 865 S 16, the hydraulic pressure falls off so that the
pressure is equal
in each cell. In the case of cells with vertical electrodes, this can be
achieved by the
lengths of the inlet and outlet pipes, which correspond to the hydraulic
pressures. 1n
contrast to this, this is not possible in the case of horizontal electrodes,
such as those
described in EP-A-0182 114 and in EP-B-0 856 S 16. In the present case, the
permissible
variation of the acceptable hydraulic pressure is determined by the installed
height of the
2
______._.,.
CA 02435571 2003-07-22
cell. As a rule, this amounts to a few centimeters in order to save both space
and
materials. For this reason, one possibility for generating only appropriately
low
hydraulic pressures lies in the structural enlargement of the inlet and outlet
openings.
This can be achieved in that the inlet and the outlet openings are not
arranged between
the electrodes, but rather adjacent to the electrodes, as proposed in EP-A-0
168 600,
EP -A-0 330 849 and EP-B-0 865 516. The cross sectional areas of the openings
is then
no longer limited by the amount of space between the electrodes, but can be
matched to
the increased quantities of electrolyte by the appropriate layout of the frame
geometry in
the case of electrolytically series connection. However, one disadvantage with
such an
arrangement of the openings is the additional requirement for a sealing frame
that joins
the membrane of the diaphragm to the frame so that it is gas-tight and liquid-
tight, in
order that the quantities in the individual chambers are prevented from
mixing. Since it
is located between the electrodes, such a frame also means that the distance
between the
electrodes and will be increased by the thickness of the frame. This causes
the voltage
drop in the electrolytes to increase and increases energy consumption.
Increasing the spacing can be avoided if the membrane or diaphragm or-as is
proposed
in US-A 4 436 608-even the gas diffusion electrode is bent round at the sides.
How-
ever, this entails the danger that too great a shear force will act at the
corners of the
frame, so that the membrane or diaphragm will no longer be tight because it
has been
damaged.
For this reason, it is the objective of the present invention to describe an
electrolysis
device of the kind referred to in the introduction hereto, in which the above
cited
3
CA 02435571 2003-07-22
problems associated with the prior art have been resolved, and in particular
to describe
an electrolysis device of this kind which is of simple construction and can be
operated
economically.
According to the present invention, this problem has been solved, for example,
in that
the anode as well as the membrane or the diaphragm each have at least one
opening for
supplying electrolytes to the second electrolytic chamber, and at least one
additional
opening for discharging electrolytes from the second electrolytic chamber.
In this respect, it is particularly advantageous if the membrane or the
diaphragm be
clamped so as to be gas and liquid tight in the area of the electrolyte supply
opening and
the electrolyte discharge opening in a sealing frame, the thickness of which
does not
exceed the thickness of the anode, and on the sealing frames and the seals
that lie on the
anodes. Such an arrangement entails the advantage that the spacing between the
electrodes is not affected by this clamping and the shear forces acting on the
membrane
or the diaphragm are minimized.
1 S Most of today's electrolysis cells are manufactured from metal because,
providing
appropriate alloys are used, it is possible to ensure long-term resistance to
chemical and
mechanical stresses at very high temperatures. Disadvantages of metal
structures are the
materials and production costs, which are mostly very high and which, as a
rule, include
costly welding operations. This is particularly the case with cells that use
different
materials for the anodes and cathodes, for example chlorine-alkali membrane
cells, in
which the anode is of a titanium-palladium alloy that is coated with ruthenium
oxide,
and the cathode is of nickel. Such cells basically comprise an anode and a
cathode bath
4
CA 02435571 2003-07-22
with the particular electrodes. In the case of electrical series connection,
the individual
baths are welded together, e.g., through explosive plated, bipolar rails.
Ideally, the cells
are welded together by way of such rails using a laser, when the welding range
or the
temperature zone can be so arranged spatially that any mixing of the different
alloys
involved, and thus corrosion, can be prevented. It is simpler to manufacture
an
electrolytic cell if the anode and the cathode are of identical material, as
is the case, for
example, with a cell for producing hydrogen peroxide in alkaline solution
using a gas-
diffusion cathode. In this case, nickel can be used as the material. In order
to obtain a
bipolar cell, the electrodes are simply connected to one another electrically
through
connectors or the cell walls themselves. In this cell, it is important that
when a
diaphragm is used at the anode there be a gas tight partition between the
anode and the
cathode-as is described in EP-B-0 865 516-so that the gas pressure, which is
meant to
prevent flooding of the gas-diffusion cathode by the catholyte, does not act
on the
anolytes. In contrast to a membrane, a diaphragm is liquid permeable, so that
pressure
that acts on the anolytes also acts on the catholytes. Without a partition,
the pressure
differential would act on the gas-diffusion cathode and cause flooding.
However,
installation of such a partition requires a considerable outlay from the
standpoint of
manufacturing technology, since the requirement for gas-tight sealing does not
permit
the use of spot welding, so that the partition must be welded to the
connectors and the
cell walls by continuous welds. In most cases, however, this leads to warping,
since the
thinnest possible material is selected for reasons of economy, and the welding
heat is not
dissipated effectively. As is the case with chlorine-alkali membrane
electrolysis, laser
welding is recommended because the temperature zone can be determined very
5
CA 02435571 2003-07-22
precisely. However, because of costly set up, long preparation times, and the
great
demands made on its quality, laser welding is very cost intensive.
In order to avoid these production and material costs that result from using
two metal
baths, as in EP-0-A 182 114, for example, a further development of the
inventive
concept proposes that the housing of the electrolysis cell be formed from two
plastic
panels, between which the electrolysis chambers and the gas chamber are
delimited by
the use of frame-like seals.
At the same time, when a plurality of electrolytic cells are arranged one
above the other,
the middle plastic panels) forms or form the bottom of the upper electrolysis
cell and
the cover of the electrolysis cell that is located below.
The electrolysis supply and discharge channels for the second electrolyte
chamber can
be incorporated in these plastic panels in a simple manner, in particular, by
being milled
into them. The same applies to the electrolyte supply and discharge channels
for the first
electrolyte chamber.
PP, PVC, and post-chlorinated PVC can be used as the plastic. These plastics
are
resistant to a number of chemicals, even at temperatures of up to
approximately 80°C.
The plastic panels can be fitted with seals so that the necessary electrolyte
and gas
chambers are left between the electrodes and a plastic panel without incurring
any major
expense. Thus, it is possible to dispense with a material-intensive version
with two
baths, and without welding a partition into place.
6
CA 02435571 2003-07-22
In the case of electrolysis operations other than that used for the
electrolysis of peroxide,
it is preferred that the plastic panels be of materials that differ from one
another since the
anolyte and the catholyte consist of different compounds. Since the anolyte
and the
catholyte are routed across the identical plastic panel, these can more
usefully consist of
two different plastics.
In the case of a plurality of electrolysis cells, the electrolyte discharge
channels of the
upper electrolysis cell can in each instance be electrically connected to the
electrolyte
supply channels of the electrolysis cell located below so as to permit a flow,
by way of
external connecting lines.
If plastic panels are used as a housing, it is not possible to supply current
to the
electrodes by way of the housing wall since these are then non-conductive. A
conventional electrical connection by way of connectors that are located in
the
electrolyte area should also be avoided, since these would have to be
additionally sealed
against the plastic panel. It would also be necessary to mill passages into
the plastic
panel, a procedure that would degrade the rigidity of the panel.
Accordingly, the present invention proposes that the anode and the cathode be
routed out
through the seals that delimit the electrolyte chambers and the gas chamber to
the
outside, and that they be fitted with their electrical connectors or
connections from the
anode to the cathode outside the chamber.
7
CA 02435571 2003-07-22
The electrical connectors or connections can also be located within the
plastic panel, and
edge recesses or openings can be provided for this purpose; they can also be
axranged
externally. The rigidity of the plastic panel will not be degraded in this
case.
The materials used for the electrical connectors and connections can be
selected as
desired since these connectors and connections are no longer exposed to the
chemical
and thermal stresses generated by the electrolytes. For this reason, it is
possible to use
highly conductive copper, for example, which is not normally used at this
location
because of its poor chemical and thermal resistance. This leads to a
favourable reduction
of the cost entailed for the number and the dimensions of the electrical
connectors and
connections, to which must be added the corresponding conductor rails to which
the
electrical connections are made.
Particularly easy assembly is ensured if the connectors and/or connections to
the anodes
and the cathodes are made by way of clamping elements. Cost-intensive welding
is then
no longer necessary.
When gas diffusion electrolysis is used, a major role is also played by the
gas
requirement. This must be many times the stoichiometric requirement for the
reactions
taking place in gas diffusion electrolysis, so that no losses of efficiency
result. In most
cases, the oxygen in a gas diffusion cathode is converted with the hydrogen
that is
generated at the cathode during the production of energy. As a rule, for
reasons of
economy, air is used in place of oxygen. Since, as is known, air contains only
21%
oxygen, correspondingly larger quantities of it must be introduced into the
electrolysis
cell. This requires supply and discharge lines that are of appropriately large
cross-
8
CA 02435571 2003-07-22
section, which means that the thickness of the cell frame must be increased;
this is
undesirable. Any reduction of the cross section with a simultaneous increase
in the
number of lines must in most instances be precluded for reasons of economy.
According to another proposal made by the present invention, for this reason a
gas
supply channel and a gas removal channel pass through the plastic panels that
define the
electrolysis cells) and optionally the anodes and the cathodes, from above to
below,
whilst sealing the electrolyte chambers, and in a flow connection with the
particular gas
chamber. The cross section of the supply and removal openings can thus be
determined
regardless of the thickness of the panel. To this end, there are openings with
identical
dimensions in the individual plastic panels and, optionally, in the
electrodes, and these
are aligned with each other in order that the gas, for example the air, is
distributed within
the cell stack with the least possible loss of pressure and in a manner that
is favorable
from the energy standpoint. The openings are so laid out that the required
cross-section
is available and so that sufficient material is left over for the flow of
current. Because the
1 S air flows downward from above, any electrolyte that passes through the gas
diffusion
electrode by way of minor leaks can be removed. A further advantage of the
possibility
for converting large quantities of gas is the increased absorption of the
evaporative heat
that is generated at the gas diffusion electrode, so that internal cooling
takes place and
this then replaces external cooling and eliminates the costs that would be
incurred for a
heat exchanger.
Thus, according to the present invention it is possible to construct
electrolysis devices
for use in gas diffusion electrolysis, and to do so in a simple and economical
manner. No
9
CA 02435571 2003-07-22
costly welding operations are needed. The individual parts can be assembled
directly at
the intended site of operation, which means that the costs associated with
intermediate
assembly are eliminated and transportation costs can be reduced. The
combination of
different materials for the electrodes and plastics means that cells for
various electrolysis
processes can be assembled in a cost-effective, modular system
Additional objectives, features, advantages, and possible applications for the
present
invention are set out in the following description of the embodiments, on the
basis of the
drawings appended hereto. Either alone or in any combination, all of the
features that
the described or illustrated herein constitute the object of the present
invention,
regardless of their combination in the individual claims or their references.
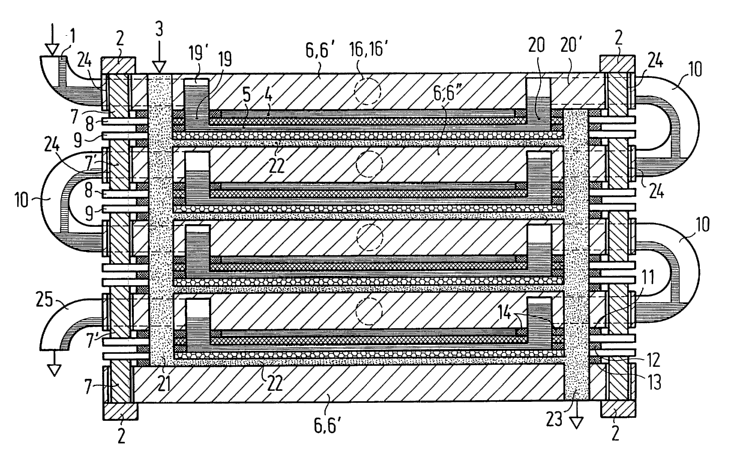
The drawing show the following:
Figure 1: A diagrammatic representation of an the electrolysis device
according to
the present invention, which is assembled from four electrolysis cells;
Figure 2: An enlarged view showing a section of a pair of electrodes in the
area of an
electrolyte supply opening or an electrolyte discharge opening;
Figure 3: a plan view of a sealing frame as is used in Figure 2.
The electrolysis device shown in Figure 1 has four horizontal electrolysis
cells that are
stacked one above the other, and a housing b that is formed from plastic
panels 6', 6", the
uppermost plastic panel 6' forming a cover and the lowest plastic panel 6"
forming a
bottom for the uppermost or lowest electrolysis cell, respectively, whereas
the middle
CA 02435571 2003-07-22
plastic panel 6" simultaneously forms the bottom of the electrolysis cell that
is located
above it and the cover for the electrolysis cell that is located beneath it.
Each electrolysis cell has an anode 8 with a membrane or a diaphragm 18, and a
cathode
9 with a gas-diffusion electrode 17, a first electrolyte chamber 4 being
formed by the
S seals 11, 12, 13 as an anode chamber and a second electrolyte chamber S
being formed
as a cathode chamber, with a gas chamber 22 being formed on the outside of the
cathode
9.
Electrolyte 1 is routed by way of an electrolysis supply channel 19' in the
upper most
plastic panel 6' to an opening 19 in the anode 8 and to the associated
membrane or to the
associated diaphragm 18 and thus to the second electrolyte chamber 5.
Electrolyte is
removed from the second electrolytic chamber 3 through an electrolyte removal
opening
into an electrolyte removal channel 20' which analogously to the electrolyte
supply
channel 19' is milled into the first uppermost plastic panel 6' and runs from
the second
electrolyte chamber S first vertically and then horizontally. Corresponding
channels and
15 openings also provided in the remaining plastic panels, and anodes and
membranes or
diaphragms. In the same way that the electrolyte 1 is routed through a side
supply
opening 26 to the outer edge of the uppermost plastic panel 6', the
electrolyte 1 flows
from the electrolyte removal channel 20' laterally to the outside and into a
connecting
line 20 to the second plastic panel 6", which defines the uppermost
electrolysis cell as
20 the bottom and then into an electrolyte feed channel which corresponds to
the supply
channel 19 ' of the uppermost plastic panel 6 ', or until the electrolyte is
discharged from
the side of the next to last plastic panel 6" through an outlet tube 2S.
11
CA 02435571 2003-07-22
Gas, for example, oxygen or air, is routed downwards into a gas supply channel
21 that
passes through all of the plastic panel 6', 6" of the housing 6 that is sealed
off from the
electrolyte chambers 4, 5 so as to be both gas and liquid tight, although
there is a flow
connection to the corresponding gas chamber 22 of the particular electrolysis
cell.
Below, the gas supply channel 21 opens out in the lowest gas chamber. On the
opposite
side of the stack of electrolysis cells, a vertical gas removal channel 23
extends from the
first gas chamber 22 as far as a lower outlet opening in the lowest plastic
panel 6'.
In the middle of the plastic panel 6', 6" there is in each instance an
electrolyte supply or
electrolyte discharge opening 16 of the first electrolysis chamber 4 (anode
chamber) and
the associated electrolyte supply and electrolyte discharge channels 16'. The
corres-
ponding channels I6' can also be milled into the plastic panel 6', 6" in the
same way as
the channels 19', 20', as well as the gas passage openings which are aligned
with each
other and located in the edge area of the particular plastic panel 6', 6" and
form the
vertical gas channels 21, 23.
Figure 1 also shows that the electrodes 8, 9 are routed out at the side
through the seals
that define the electrolyte chambers 4, 5 and the gas chamber 22 and are in
this way
traversed by the vertical gas channels 21, 23.
In the outermost edge area, the plastic panels 6', 6" are provided with edge
recesses 24
that are aligned with each other. Within these, on both sides, both above and
below,
there are electrical connectors 8 (above) and the cathode 9 (below) that are
connected to
the contact rails 2; in the middle plastic panel 6" there are electrical
connections 7'
between the cathode 9 and the anode 8 of the electrolysis cells that follow
one another.
12
CA 02435571 2003-07-22
The contact rails 2 as well as the connectors 7 and the connections 7' can be
of a
material, such as copper, that possesses good current-conducting properties.
The
connectors 7 and the connections T can also be secured to the anode 8 and the
cathode 9
through clamping elements (not shown herein).
Figure 2 shows how sealing is effected in the area of and in electrolyte
supply opening
19 and in an electrolyte discharge opening 20. Whereas the gas diffusion
electrode
coating 17 on the cathode is continuous as far as the edge area of the cathode
9, where it
is covered by a sealing element 12; in the area of the openings 19, 20 the
membrane or
the diaphragm 18 is angled upward so as to lie on a sealing frame 15, which is
no thicker
than the anode 8. The sealing frame 15 is accommodated in a large cutout 27 in
the
anode 8, and internally it defines the openings 19, 20. Above the angled area
of the
membrane or of the diaphragm 18 there is a sealing element 14 above the anode
8. In
the vicinity of the openings 19, 20 the membrane or diaphragm 18 is clamped by
the
edge that faces the openings 19,20 between sealing frames 15 and sealing
element 14 so
as to be gas tight and liquid tight.
Figure 3 shows that the sealing frame 50, which is shown in Figure 2 in
vertical cross-
section on the line II-II is narrow and its short sides are curved and thus
enclose the
openings 19, 20.
13
CA 02435571 2003-07-22
Reference Numbers
1 Electrolyte
2 Contact rails (of copper)
3 Gas, e.g., OZ or air
4 First electrolyte chamber (anode)
Second electrolyte chamber (cathode)
6 Housing
6', 6" Plastic panels
7', 7" Electrical connectors or connections
8 Anode, cross hatched area covered by membrane or diaphragm
9 Cathode, cross hatched area covered by gas-diffusion electrode
Connecting line
11, 12 Sealing element
13, 14 Sealing element
Sealing frame
16 Electrolyte supply and discharge opening of first electrolyte chamber
(anode
chamber)
16' Electrolyte supply and discharge channel of first electrolyte chamber
(anode
chamber)
17 Gas-diffusion electrode
18 Membrane or diaphragm
19 Electrolyte supply opening of the second electrolyte chamber (cathode
chamber)
19' Electrolyte supply channel of the second electrolyte chamber (cathode
chamber)
Electrolyte discharge opening of the second electrolyte chamber (cathode
chamber)
20' Electrolyte discharge channel of the second electrolyte chamber (cathode
chamber)
21 Gas supply channel
22 Gas chamber
23 Gas discharge channel
24 Edge cutout
Outlet pipe
14
Image