Note: Descriptions are shown in the official language in which they were submitted.
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
DESCRIPTION
DIHYDROPYRAZOLOPYRIDINE COMPOUNDS AND PHARMACEUTICAL USE
THEREOF
Technical field
The present invention relates to new compounds for
medicaments, which have a glycogen synthase kinase-3 beta
(GSK-3(3)-inhibitory activity, and use thereof.
Background art
It has been reported that glycogen synthase kinase-3
to beta (GSK-3(3), a protein kinase, is involved in the causes of
various diseases as noted in the following.
Type-II diabetes is a disease in which the insulin
reactivity of pancreatic (3 cells becomes low and glucose in
blood increases. As a result, complications such as diabetic
nephropathy, retinosis, heart disease and the like are induced.
GSK-3(3 acts for inhibiting glycogen accumulation in peripheral
tissues, lowering insulin response and increasing glucose in
blood by phosphorylating glycogen synthase. Lithium having a
GSK-3[3-inhibitory activity actually lowers glucose in blood by
2o a GSK-3(3-inhibitory activity (Pros. Nat. Acad. Sci, 93, 8455
(1996)). Therefore, medicaments having a GSK-3(3-inhibitory
activity are considered to be a pharmaceutical agent effective
for the improvement of Type II diabetes and complications
thereof .
The developmental mechanism of Alzheimer's dementia has
not yet been elucidated. However, it is considered that
amyloid aggregation and neurofibril changes are closely
related to.the cause of the development. GSK-3(3 is involved in
both the amyloid aggregation and the neurofibril changes as
3o follows. (1) It binds with variant presenilin and increase
production of insoluble amyloid (Proc. Nat. Acad. Sci., 95,
9637 (1998)). (2) It causes phosphorylation of the Tau protein,
which causes neurofibril changes, and weakens the backbones of
1
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
neurons to induce neuronal death (Neurosci. Lett., 128, 195
(1991)). In addition to the above, (3) the direct involvement
of GSK-3(3 in nuronal death through inactivation of pyruvate
dehydrogenase by phosphorylation to decrease the production
s amount of acetylcholine necessary for maintaining cell
activity (Proc. Nat. Acad. Sci., 93, 2719 (1996)) has been
reported.
In addition, the effectiveness for AIDS encephalopathia
as a neurodegenerative disease other than Alzheimer has been
to suggested. Tat, which is a protein produced by HIV virus that
causes AIDS, enhances GSK-3(3 activity in neurons to induce
neuronal death (J. Neurochem., 73, 578 (1999)). From the above,
GSK-3(3 inhibitors are considered to be medicaments effective
for improving neurodegenerative diseases including Alzheimer's
15 dementia.
Lithium and valproic acid, which have anti-manic-
depressive activity, have a GSK-3(3 inhibitory activity (J.
Neurochem., 72, 1327 (1999)). The relationship between anti-
manic-depressive activity and GSK-3(3 inhibitory activity is
2o unclear, but a suppressive activity on glutamic acid toxicity
is considered to be partly responsible for maintaining
neuronal activity (Proc. Nat. Acad. Sci., 95, 2642 (1998)).
Based on the foregoing, GSK-3(3 inhibitors are considered to be
medicaments effective for improving manic-depressive psychosis.
z5 NF-AT, a transcription factor, is dephosphorylated by
calcineurin to increase immunological responses (Science, 275,
1930 (1997)). GSK-3(3 acts for suppressing immunological
function by conversely phosphorylating NF-AT. Therefore, GSK-
3(3 inhibitors are considered to be medicaments effective for
3o immunopotentiation .
Incidentally, JP-A-3-272189 (invention drawn to an
improved synthesis method of mevalolacton intermediates), JP-
A-2-275878 (therapeutic agents for hyperlipoproteinemia and
2
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
atherosclerosis) and JP-A-1-272584 (therapeutic agents for
hyperlipoproteinemia) disclose pyrazolo[3,4-b]pyridine
compounds wherein the 6-position is either methyl, isopropyl
or cyclopropyl. These publications do not disclose or suggest
s any action of these compounds on GSK-3(3 or the central nervous
system.
The specifications of JP-A-59-65089, JP-A-59-118786, JP-
A-60-56979, JP-A-60-197685 and the like disclose 6-methyl-4-
substituted phenyl-4,7-dihydropyrazolo[3,4-b]pyridine-5-
io carboxylate compounds used for the treatment of cardiovascular
diseases, and they are produced by similar methods. The
present inventors reproduced the following reaction A
according to the method described in JP-A-59-65089, but failed
to obtain the compound of Example 14 (formula (IV) in the
.t5 following) described therein. They confirmed that only the
pyrazolo[1,5-a]pyrimidine derivative represented by the
formula (V) could be produced. They measured IR, NMR and the
melting point of the compound of the formula (V) and found
them to be identical with IR, NMR and the melting point
ao described in the specification of this publication. It is
therefore concluded that an erroneous structural formula has
been disclosed in these publications. In other words, 6-
methyl-4-substituted phenyl-4,7-dihydropyrazolo[3,4-
b]pyridine-5-carboxylate cannot be synthesized according to
25 the methods described in these publications.
A o o / ~ °2N I ~
O~
H2N N~N I ~I 'N
H H N
H
o ° s\ °~NI..
o... o '
I
~ .N ~ ~O N'N - t v >
I ' HZN H I N "~ ~ I
H
The compound of the above formula (IV) can be
3
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
synthesized according to the method described in J. Chem. Soc.,
Perkin Trans. l, 947 (1996), and this publication discloses
methyl 4-(2-chlorophenyl)-6-methyl-4,7-dihydro-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate and the like.
s Disclosure of the invention
An object of the present invention is to provide novel
compounds having a selective and strong inhibitory activity
against glycogen synthase kinase-3 beta (GSK-3(3), and further,
medicaments comprising them.
to The present inventors have intensively studied to
achieve the above object, and have found that 4,7-
dihydropyrazolo[3,4-b]pyridine derivatives have a selective
and strong inhibitory activity on GSK-3(3, which resulted in the
completion of the present invention. That is, the present
.ts invention relates to medicaments comprising, as an active
ingredient, dihydropyrazolopyridine compounds represented by
the following formula (I), which have a GSK-3(3-inhibitory
activity and can be used as medicaments, optical isomers
thereof, pharmaceutically acceptable salts thereof, or
zo hydrates thereof.
The present invention provides the following.
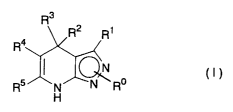
1. A dihydropyrazolopyridine compound of the formula (I):
R3
R2 R1
R4
o (I)
R5 N N R
H
25 wherein
R° is hydrogen, alkyl, acyl, cycloalkyl, formyl,
haloalkyl, aminoalkyl, alkoxyalkyl, phenoxyalkyl,
hydroxyalkyl, aminocarbonyl, alkylthiocarbonyl,
carboxyalkyl, cycloalkoxyalkyl, alkylsulfinyl,
4
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
alkylsulfonyl, phenylsulfonyl, mercaptoalkyl,
alkylthioalkyl, acyloxyacetyl, acyloxyalkyl, phenyl
optionally having substituent(s), aromatic
heterocyclic group optionally having substituent(s),
s phenylalkyl optionally having substituent(s), or a
group of the formula: -COORS (wherein R$ is hydrogen,
alkyl, aryl optionally having substituent(s) or
aralkyl optionally having substituent(s));
R1 and RZ are the same or different and each is hydrogen, alkyl,
Io acyl, cycloalkyl, hydroxy, thiol, halogen, amino,
formyl, carboxy, cyano, nitro, alkylthio, haloalkyl,
aminoalkyl, acylamino, alkoxy, cycloalkoxy, phenoxy,
phenylalkoxy, aminoalkoxy, alkoxyalkyl, phenoxyalkyl,
hydroxyalkyl, alkoxycarbonyl, aminocarbonyl,
i5 alkylthiocarbonyl, carboxyalkyl, cycloalkoxyalkyl,
phenylthio, alkylsulfinyl, alkylsulfonyl,
phenylsulfonyl, mercaptoalkyl, alkylthioalkyl, phenyl
optionally having substituent(s), aromatic
heterocyclic group or phenylalkyl;
2o R3 1s
(1) alkyl or haloalkyl,
(2) cycloalkyl,
(3) phenyl optionally having substituent(s),
(4) aromatic heterocyclic group,
as (5) a group derived from a benzene ring fused with a
saturated or unsaturated 5 or 6 membered carbocyclic
ring,
(6) a group derived from a benzene ring fused with a
saturated or unsaturated 5 to 7 membered carbocyclic
3o ring containing 1 to 3 heteroatom(s), or
(7) a group derived from a 5 to 7 membered saturated
or unsaturated carbocyclic ring containing 1 to 3
heteroatom(s), which is fused with a benzene ring,
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
wherein the groups of (2) to (7) may have one or more
substituent(s), or
a group selected from the groups represented by the
following formulas (II) and (III),
Rs R'
( II ) ( III )
wherein R6 and R' are each phenyl optionally having
substituent(s) or an aromatic heterocyclic group,
or RZ and R3 in conjunction form a ring optionally containing
heteroatom(s), wherein the ring may be fused with a
io benzene ring optionally having substituent(s);
R4 is alkoxycarbonyl, aminocarbonyl, hydrazinocarbonyl,
alkylthiocarbonyl, formyl, carbamoyl, alkylthio,
phenylthio, alkylsulfinyl, phenylsulfinyl,
alkylsulfonyl, phenylsulfonyl, dialkylphosphinyl,
15 dialkylphosphonyl, cyano or nitro; and
R5 is hydrogen, cyano, formyl, alkyl, cycloalkyl,
alkoxyalkyl, phenoxyalkyl, dialkoxyalkyl,
hydroxyalkyl, haloalkyl, carboxyalkyl,
cycloalkoxyalkyl, phenylthio, alkylsulfinyl,
ao alkylsulfonyl, phenylsulfonyl, mercaptoalkyl,
alkylthioalkyl, alkoxycarbonylalkyl,
alkoxycarbonylethenyl, aryl optionally having
substituent(s) (particularly phenyl), an aromatic
heterocyclic group or phenylalkyl, or a group derived
z5 from a 5 to 7 membered saturated or unsaturated
carbocyclic ring containing 1 to 3 heteroatom(s),
which is fused with a benzene ring,
or R4 and RS in conjunction may form a 5 or 6 membered ring
optionally containing heteroatom(s),
6
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
provided that when R°, Rl and RZ are each hydrogen, R4 is
methoxycarbonyl and RS is methyl, then R3 should not
be phenyl, 2-chloropheny, 3-nitrophenyl, 4-
carboxyphenyl or 4-methoxycarbonylphenyl,
or an optically active form thereof,. a pharmaceutically
acceptable salt thereof or a hydrate thereof.
2. The dihydropyrazolopyridine compound of the above-described
1, wherein R5 is alkyl having 2 to 8 carbon atoms, cycloalkyl,
,,
alkoxyalkyl, phenoxyalkyl, hydroxyalkyl, phenyl optionally
Io having substituent(s), an aromatic heterocyclic group or
phenylalkyl, or an optically active form thereof, a
pharmaceutically acceptable salt thereof or a hydrate thereof.
3. The dihydropyrazolopyridine compound of the above-described
1, wherein R1 is hydrogen, alkyl, phenyl optionally having
.t5 substituent(s), an aromatic heterocyclic group or phenylalkyl,
or an optically active form thereof, a pharmaceutically
acceptable salt thereof or a hydrate thereof.
4. The dihydropyrazolopyridine compound of the above-described
1, wherein RZ is hydrogen or alkyl, or an optically active form
2o thereof, a pharmaceutically acceptable salt thereof or a
hydrate thereof.
5. The dihydropyrazolopyridine compound of the above-described
1, wherein R3 is phenyl optionally having 1 to 3 substituent(s),
naphthyl, 2,1,3-benzoxadiazol-4-yl or 3,4-dihydro-2H-
z5 benzopyran-8-yl, or an optically active form thereof, a
pharmaceutically acceptable salt thereof or a hydrate thereof.
6. The dihydropyrazolopyridine compound of the above-described
1, wherein R4 is alkoxycarbonyl having 2 to 5 carbon atoms,
cyano or nitro, or an optically active dorm thereof, a
3o pharmaceutically acceptable salt thereof or a hydrate thereof.
7. The dihydropyrazolopyridine compound of the above-described
1, wherein R5 is alkyl having 2 to 4 carbon atoms, cyclopropyl,
phenyl, thienyl or hydroxyalkyl, or an optically active form
7
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
thereof, a pharmaceutically acceptable salt thereof or a
hydrate thereof.
8. The dihydropyrazolopyridine compound of the above-described
1, wherein RZ and R3 in conjunction form a ring containing
sulfur atom and the ring is condensed with a benzene ring
optionally having substituent(s), or an optically active form
thereof, a pharmaceutically acceptable salt thereof or a
hydrate thereof.
9. The dihydropyrazolopyridine compound of the above-described
l0 1, wherein R° is hydrogen or a group of the formula: -COORg
(wherein Re is alkyl, aryl optionally having substituent(s) or
aralkyl optionally having substituent(s)), or an optically
active form thereof, a pharmaceutically acceptable salt
thereof or a hydrate thereof.
10. The dihydropyrazolopyridine compound of the above-
described 1, which is selected from
(32) ethyl 4,7-dihydro-4-(2-methoxyphenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate,
(47) ethyl 4-(2-chloro-3-trifluoromethylphenyl)-4,7-dihydro-6-
zo propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate,
(66) ethyl 4,7-dihydro-4-(naphthalen-1-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate,
(73) ethyl 4-(3,4-dihydro-2H-benzopyran-8-yl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate,
z5 (87) ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-(thiophen-2-yl)-
2H-pyrazolo[3,4-b]pyridine-5-carboxylate,
(116) ethyl 4-(2,1,3-benzoxadiazol-4-yl)-4,7-dihydro-6-propyl-
2H-pyrazolo[3,4-b]pyridine-5-carboxylate,
(122) 4-(2,3-dichlorophenyl)-4,7-dihydro-5-nitro-6-propyl-2H-
3o pyrazolo[3,4-b]pyridine,
(140) 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine,
(147) 4-(2-bromo-3-cyanophenyl)-5-cyano-4,7-dihydro-6-phenyl-
8
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
2H-pyrazolo[3,4-b]pyridine,
(158) 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
phenyl-2H-pyrazolo[3,4-b]pyridine,
(171) 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
(thiophen-2-yl)-2H-pyrazolo[3,4-b]pyridine,
(182) ethyl 4-(2-bromo-3-nitrophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate,
(183) ethyl 4-(2-bromo-3-cyanophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate,
.to (189) 4-(2-bromo-3-nitrophenyl)-5-cyano-4,7-dihydro-6-propyl-
2H-pyrazolo[3,4-b]pyridine,
(205) ethyl 2-tert-butoxycarbonyl-4-(2-chlorophenyl)-4,7-
dihydro-6-propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate,
(240) ethyl 4-(2,1,3-benzoxadiazol-4-yl)-6-ethyl-4,7-dihydro-
.t5 2H-pyrazolo[3,4-b]pyridine-5-carboxylate,
(257) 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
hydroxymethyl-2H-pyrazolo[3,4-b]pyridine,
(260) 4-(2-bromo-3-cyanophenyl)-5-cyano-4,7-dihydro-6-
isopropyl-2H-pyrazolo[3,4-b]pyridine,
ao (264) 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
isopropyl-2H-pyrazolo[3,4-b]pyridine, and
(268) 4-(2-bromo-3-cyanophenyl)-5-cyano-6-cyclopropyl-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine,
a tautomer, an optically active form thereof, a
25 pharmaceutically acceptable salt thereof or a hydrate thereof.
11. A medicament comprising a dihydropyrazolopyridine compound
of the above-described 1, an optically active form thereof, a
pharmaceutically acceptable salt thereof or a hydrate thereof.
12. A pharmaceutical composition comprising a
3o dihydropyrazolopyridine compound of the above-described l, an
optically active form thereof, a pharmaceutically acceptable
salt thereof or a hydrate thereof, and a pharmaceutically
acceptable additive.
9
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
13. A glycogen synthase kinase-3 beta inhibitor comprising a
compound selected from the group consisting of a
dihydropyrazolopyridine compound of the above-described 1, an
optically active form thereof, a pharmaceutically acceptable
s salt thereof and a hydrate thereof.
14. The medicament of the above-described 11, which is used
for prevention and/or treatment of a disease caused by
glycogen synthase kinase-3 beta hyperactivity.
15. The medicament of the above-described 11, which is used
.to for prevention and/or treatment of a neurodegenerative disease.
16. The medicament of the above-described 15, wherein the
disease is selected from the group consisting of Alzheimer's
disease, ischemic cerebrovascular disorder, Down's syndrome,
cerebral ischemia due to cerebral amyloid angiopathy,
is progressive supranuclear paralysis, subacute sclerosing
panencephalitic Parkinsonism, postencephalitic Parkinsonism,
boxer's encephalopathy, Parkinson dementia complex of Guam,
Lewy body disease, Pick's disease, corticobasal degeneration,
frontotemporal dementia, AIDS encephalopathy, Huntington's
zo disease and manic-depressive psychosis.
17. The medicament of the above-described 11, which is used
for prevention and/or treatment of diabetes and diabetic
complications.
18. The medicament of the above-described 11, which is used as
a5 an immunopotentiator.
Brief Description of The Drawings
Fig. 1 shows the GSK-3~3-inhibitory activity of the
compounds of Example 47 and Example 137.
Fig. 2 shows the effect of the compound of Example 66 on
3o amyloid (3-induced cytotoxicity.
Fig. 3 shows the GSK-3(3-inhibitory effect of the
compound of Example 27 in a gerbil brain ischemia model.
Detailed Description of The Invention
l0
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The formula (I) indicates the presence of tautomers
represented by the following formulas (I-a) and (I-b), based
on the positions of hydrogen atoms of the pyrazole ring. The
present invention encompasses each isomer.of formulas (I-a)
and (I-b), and a mixture of these isomers.
Rs Ra
R2 R~ R2 R1
R4 R4
\ ~~ o
s ( ~ /N --~ s I ~ N N-R
R H No R H
R
~I_a) ~t_b)
The compounds represented by the formula (I) in the
present specification are described in detail in the following.
"Alkyl" means a linear or branched carbon chain of 1 to
.to 8 carbon atom(s), and includes methyl, ethyl, propyl, butyl,
pentyl(amyl), hexyl, or a structural isomer thereof, such as
isopropyl, isobutyl, sec-butyl, tert-butyl, isopentyl,
neopentyl, tert-pentyl and the like, with a preference for
alkyl having 1 to 4 carbon atom(s). The alkyl of RS is
preferably alkyl having 2 to 8 carbon atoms. The "alkyl having
2 to 8 carbon atoms" concretely includes ethyl, propyl, butyl,
pentyl(amyl), hexyl, heptyl and octyl, or a structural isomer
thereof, such as isopropyl, isobutyl, sec-butyl, tert-butyl,
isopentyl, neopentyl, tert-butyl and the like. Alkyl having 2
ao to 4 carbon atoms is more preferable, and propyl is
particularly preferable.
"Acyl" includes alkylcarbonyl having 2 to 8 carbon atoms,
such as acetyl, propionyl, butyryl, isobutyryl, valeryl,
pivaloyl, hexanoyl, heptanoyl and the like, and aromatic acyl
zs having 7 to 12 carbon atoms, such as benzoyl, naphthoyl,
cinnamoyl, benzylcarbonyl and the like. The benzene and
naphthalene rings may have 1 to 5 substituent(s).
11
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
"Cycloalkyl" means a cyclic carbon chain of 3 to 8
carbon atoms. Cycloalkyl concretely includes, for example,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl
and the like, with a preference for cycloalkyl having 3 to 6
s carbon atoms.
"Halogen" represents fluorine, chlorine, bromine or
iodine.
"Amino" is primary amino, or secondary or tertiary amino
having the above alkyl, and includes, for example, amino,
to methylamino, dimethylamino, ethylamino, diethylamino,
propylamino, dipropylamino, butylamino, dibutylamino and the
like, with a preference for tertiary amino. containing alkyl
having 1 to 4 carbon atom(s).
"Alkylthio" is a linear or branched alkylthio having 1
z5 to 6 carbon atom(s), and includes, for example, methylthio,
ethylthio, propylthio, butylthio, pentylthio(amylthio),
hexylthio and structural isomers thereof, such as
isopropylthio, isobutylthio, sec-butylthio, tert-butylthio,
isopentylthio, neopentylthio, tert-pentylthio and the like,
2o with a preference for alkylthio having 1 to 3 carbon atom(s).
"Haloalkyl" is the above alkyl substituted by 1 to 5
halogen(s), and represents fluoromethyl, chloromethyl,
difluoromethyl, trifluoromethyl, 2,2,2-trifluoroethyl,
2,2,3,3,3-pentafluoropropyl and the like.
2s "Aminoalkyl" is the above-mentioned alkyl having the
above amino, and includes, for example, aminomethyl,
methylaminomethyl, dimethylaminomethyl, 2-aminoethyl, 2-
methylaminoethyl, 2-dimethylaminoethyl, 2-ethylaminoethyl, 2-
diethylaminoethyl and the like, with a preference for
3o aminoalkyl containing alkyl having 1 to 4 carbon atoms)
having tertiary amino.
"Acylamino" is acylamino having the above acyl, and
represents, for example, acetylamino, propionylamino,
12
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
butyrylamino, valerylamino, pivaloylamino, benzoylamino,
phenylacetylamino, phenylpropionylamino, phenylbutyrylamino
and the like.
"Alkoxy" is alkoxy having the above alkyl, and includes,
for example, methoxy, ethoxy, propoxy, butoxy,
pentyloxy(amyloxy), hexyloxy and structural isomers thereof,
such as isopropoxy, isobutoxy, sec-butoxy, tert-butoxy,
isopentyloxy, neopentyloxy, tert-pentyloxy and the like, with
a preference for alkoxy having 1 to 4 carbon atom(s).
to "Cycloalkoxy" is alkoxy having the above cycloalkyl, and
includes, for example, cyclopropoxy, cyclobutoxy,
cyclopentyloxy, cyclohexyloxy and the like, with a preference
for cycloalkoxy having cycloalkyl having 3 to 6 carbon atoms.
"Phenylalkoxy" is phenylalkoxy having the above alkoxy,
and includes, for example, benzyloxy, 1-phenylethoxy, 2-
phenylethoxy, 3-phenylpropoxy, 4-phenylbutoxy, 1-methyl-1-
phenylethoxy, 1-methyl-2-phenylethoxy, 1-phenylpropoxy, 2-
pheylpropoxy, 1-methyl-1-phenylpropoxy, 1-methyl-2-
phenylpropoxy, 1-methyl-3-phenylpropoxy and the like, with a
zo preference for phenylalkoxy containing alkoxy having 1 to 4
carbon atom(s).
"Aminoalkoxy" is aminoalkoxy consisting of the above
alkoxy and amino, and includes, for example, aminomethoxy,
methylaminomethoxy, dimethylaminomethoxy, 2-
z5 dimethylaminoethoxy, 3-dimethylaminopropoxy, 4-
dimethylaminobutoxy and the like, with a preference for
aminoalkoxy consisting of tertiary amino containing alkyl
having 1 to 4 carbon atom(s), and alkoxy having 1 to 4 carbon
atom(s).
30 "Alkoxyalkyl" is alkoxyalkyl consisting of the above
alkoxy and alkyl, and includes, for example, methoxymethyl,
ethoxymethyl, 2-methoxyethyl, propoxymethyl, isopropoxymethyl
and the like, with a preference for alkoxyalkyl consisting of
13
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
alkoxy having 1 to 4 carbon atoms) and alkyl having 1 to 4
carbon atom(s).
"Phenoxyalkyl" is phenoxyalkyl containing the above
alkyl, and includes, for example, phenoxymethyl, 2-
phenoxyethyl, 3-phenoxypropyl and the like, with a preference
for phenoxyalkyl containing alkyl having 1 to 4 carbon atom(s).
"Dialkoxyalkyl" is dialkoxyalkyl consisting of the above
alkyl and alkoxy, and includes, for example, dimethoxymethyl,
diethoxymethyl, 2,2-dimethoxyethyl, 2,2-diethoxyethyl and the
zo like, with a preference for dialkoxyalkyl consisting of alkoxy
having 1 to 4 carbon atoms) and alkyl having 1 to 4 carbon
atom(s).
"Hydroxyalkyl" is hydroxyalkyl having the above alkyl,
and includes, for example, hydroxymethyl, 2-hydroxyethyl, 3-
.t5 hydroxypropyl and the like, with a preference for
hydroxylalkyl containing alkyl having 1 to 4 carbon atom(s).
"Alkoxycarbonyl" is alkoxycarbonyl having the above
alkoxy, and includes, for example, methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, butoxycarbonyl,
2o pentyloxycarbonyl, hexyloxycarbonyl and structural isomers
thereof, such as isopropoxycarbonyl, isobutoxycarbonyl, sec-
butoxycarbonyl, tent-butoxycarbonyl, isopentyloxycarbonyl,
neopentyloxycarbonyl, tart-pentyloxycarbonyl and the like,
with a preference for alkoxycarbonyl, in which the alkoxy
2s moiety has 1 to 4 carbon atom(s). However, R4 is preferably
alkoxycarbonyl having 2 to 5 carbon atoms.
"Aminocarbonyl" is aminocarbonyl having the above amino,
and includes, for example, aminocarbonyl (carbamoyl),
methylaminocarbonyl, dimethylaminocarbonyl, ethylaminocarbonyl,
3o diethylaminocarbonyl, propylaminocarbonyl,
dipropylaminocarbonyl, phenylcarbamoyl, benzylcarbamoyl and
the like, with a preference for tertiary-aminocarbonyl
containing alkyl having 1 to 4 carbon atom(s).
14
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
"Alkylthiocarbonyl" is alkylthiocarbonyl having the
above alkylthio, and includes, for example, methylthiocarbonyl,
ethylthiocarbonyl, propylthiocarbonyl, butylthiocarbonyl and
structural isomers thereof, such as isopropylthiocarbonyl,
isobutylthiocarbonyl, sec-butylthiocarbonyl, tert-
butylthiocarbonyl and the like, with a preference for
alkylthiocarbonyl, in which the alkyl moiety has 1 to 3 carbon
atoms.
"Carboxyalkyl" is carboxyalkyl having the above alkyl,
io and includes, for example, carboxymethyl, carboxyethyl,
carboxypropyl and the like, with a preference for carboxyalkyl
containing alkyl having 1 to 4 carbon atom(s).
"Cycloalkoxyalkyl" is cycloalkoxyalkyl having the above
cycloalkoxy and alkyl, and includes, for example,
cyclopropoxymethyl, cyclopropoxyethyl, cyclobutoxymethyl,
cyclopentyloxymethyl, cyclohexyloxymethyl and the like, with a
preference for cycloalkoxyalkyl consisting of cycloalkoxy
having 3 to 6 carbon atoms and alkyl having 1 to 4 carbon
atom(s).
"Alkylsulfinyl" is alkylsulfinyl having the above alkyl,
and,includes, for example, methylsulfinyl, ethylsulfinyl,
propylsulfinyl, isopropylsulfinyl and the like, with a
preference for alkylsulfinyl containing alkyl having 1 to 4
carbon atom(s).
zs "Alkylsulfonyl" is alkylsulfonyl having the above alkyl,
and includes, for example, methylsulfonyl, ethylsulfonyl,
propylsulfonyl, isopropylsulfonyl and the like, with a
preference for alkylsulfonyl containing alkyl having 1 to 4
carbon atom(s).
"Mercaptoalkyl" is mercaptoalkyl having the above alkyl,
and includes, for example, mercaptomethyl, mercaptoethyl,
mercaptopropyl and the like, with a preference for
mercaptoalkyl containing alkyl having 1 to 4 carbon atom(s).
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
"Alkylthioalkyl" is alkylthioalkyl having the above
alkylthio and alkyl, and includes, for example,
methylthiomethyl, methylthioethyl, methylthiopropyl,
ethylthiomethyl, ethylthioethyl, ethylthiopropyl and the like,
with a preference for alkylthioalkyl consisting of alkylthio
having 1 to 3 carbon atoms) and alkyl having 1 to 4 carbon
atom(s).
"Aryl" is aryl having 6 to 14 carbon atoms, and includes,
for example, phenyl, 1-naphthyl, 2-naphthyl, 1-anthryl, 2-
.to anthryl and the like. They may have 1 to 5 substituent(s) and
substitution sites are not particularly limited.
"Aralkyl" is aralkyl wherein the above alkyl is
substituted by the above aryl, and includes benzyl, 2-
phenylethyl, 3-phenylpropyl, 1-naphthylmethyl, 2-
.t5 naphthylmethyl and the like. These may have 1 to 5
substituent(s) on the aryl moiety.
"Acyloxyacetyl" is acyloxyacetyl having the above acyl,
and includes, for example, acetyloxyacetyl, propionyloxyacetyl,
butyryloxyacetyl, benzoyloxyacetyl and the like.
ao "Acyloxyalkyl" is acyloxyalkyl having the above acyl and
alkyl, and includes, for example, acetyloxymethyl,
propionyloxymethyl, butyryloxymethyl, benzoyloxymethyl, 2-
acetyloxyethyl, 2-propionyloxyethyl, 2-butyryloxyethyl, 2-
benzoyloxyethyl and the like.
The substituent of the "phenyl optionally having
substituent(s)" is exemplified by those mentioned for the
"substituent" below, wherein the number of the substituent is
generally 1 to 5, preferably 3. Phenyl having 1 or 2
substituent(s) is particularly preferable.
30 "Aromatic heterocyclic group" is a 5- or 6-membered
aromatic heterocyclic ring optionally containing 1 to 3
heteroatom(s) of nitrogen atom, oxygen atom and sulfur atom,
and includes, for example, thiophene, furan, pyrrole,
16
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
imidazole, pyrazole, thiazole, isothiazole, oxazole, isoxazole,
pyridine, pyridazine, pyrimidine, pyrazine and the like.
The substituent of the "aromatic heterocyclic ring
optionally having substituent(s)" is exemplified by those
s mentioned for the "substituent"'below, wherein the number of
the substituent is generally 1 to 5, preferably 3.
"Phenylalkyl" is phenylalkyl having the above alkyl, and
includes, for example, benzyl, 2-phenylethyl, 3-phenylpropyl,
4-phenylbutyl, 1-phenylethyl, 1-methyl-2-phenylethyl, 1-
.to phenylpropyl, 2-phenylpropyl, 1-methyl-1-phenylpropyl, 1-
methyl-2-phenylpropyl, 1-methyl-3-phenylpropyl and the like,
with a preference for phenylalkyl consisting of phenyl and
alkyl having 1 to 4 carbon atom(s).
The kind and the number of the substituent of the
Is "phenylalkyl optionally having substituent(s)" are the same as
those for the above-mentioned "aromatic heterocyclic ring".
"Alkoxycarbonylalkyl" is alkoxycarbonylalkyl having the
above alkoxycarbonyl and alkyl, and includes, for example,
methoxycarbonylmethyl, ethoxycarbonylmethyl,
2o ethoxycarbonylmethyl, 2-ethoxycarbonylethyl, 3-
ethoxycarbonylpropyl and the like.
"Alkoxycarbonylethenyl" is alkoxycarbonylethenyl having
the above alkoxycarbonyl, and includes, for example, 2-
methoxycarbonylethenyl, 2-ethoxycarbonylethenyl, 2-
zs butoxycarbonylethenyl, 2-tert-butoxycarbonylethenyl and the
like.
"Dialkylphosphinyl" is dialkylphosphinyl having the
above alkyl, and includes, for example, dimethylphosphinyl,
diethylphosphinyl, dipropylphosphinyl and the like, with a
3o preference for dialkylphosphinyl containing alkyl having 1 to
4 carbon atom(s).
"Dialkylphosphonyl" is dialkylphosphonyl having the
above alkyl, and includes, for example, dimethylphosphonyl,
17
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
diethylphosphonyl, dipropylphosphonyl and the like, with a
preference for dialkylphosphonyl containing alkyl having 1 to
4 carbon atom(s).
In the present specification, "substituent" includes
alkyl, acyl, cycloalkyl, phenyl, aromatic heterocyclic ring,
phenylalkyl, hydroxy, thiol, halogen, amino, formyl, carbamoyl,
cyano, nitro, alkylthio, haloalkyl, aminoalkyl, acylamino,
alkoxy, cycloalkoxy, phenoxy, phenylalkoxy, aminoalkoxy,
alkoxyalkyl, phenoxyalkyl, hydroxyalkyl, alkoxycarbonyl,
zo alkylsulfinyl, aminocarbonyl, alkylthiocarbonyl and the like.
"Ring optionally containing heteroatom(s)" is a 5 or 6
membered carbocyclic ring optionally containing 1 to 3
heteroatom(s) consisting of nitrogen atom, oxygen atom and
sulfur atom, with particular preference given to a ring
z5 containing sulfur atom. The ring may be substituted by one or
more of the above substituents or oxo groups. The substitution
site is not particularly limited. This ring is formed by R2
and R3 in the formula (I) together with the attached carbon
atom. By forming this ring, a spiro ring is formed in the
2o compound of the formula (I). The above ring can be fused with
a benzene ring optionally having substituent(s). Such a ring
includes, for example, 2,3-dihydrobenzo[b]thiophene, 2,3-
dihydrobenzo[b]thiophen-1-oxide and the like.
"A group derived from a benzene ring, which is fused
z.s with a saturated or unsaturated 5 or 6 membered carbocyclic
ring" represents a group derived from naphthalene, 1,2-
dihydronaphthalene, 1,2,3,4-tetrahydronaphthalene, indan and
the like, with preference given to naphthalene (namely
naphthyl) and particular preference given to 1-naphthyl.
30 "A group derived from a benzene ring fused with a
saturated or unsaturated 5 to 7 membered carbocyclic ring
containing 1 to 3 heteroatom(s)" includes the following groups
and the like.
18
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
o ~ o ~ s ~ o
~ o
/
/ of Q ~'~ o
0
,.N ~ / .~N. /
~N~S N
H ~(Ha
~ N /
/ CH3 ~ / GH3 ~ O
~~3
Of these, 2,1,3-benzoxadiazole and 3,4-dihydro-2H-
benzopyrane are preferable, and 2,1,3-benzoxadiazol-4-yl and
3,4-dihydro-2H-benzopyran-8-yl are particularly preferable.
"A group derived from a 5 to 7 membered saturated or
unsaturated carbocyclic ring containing 1 to 3 heteroatom(s),
which is fused with a benzene ring" includes the following
groups and the like.
/ I s / I o /
za The "5 or 6-membered ring optionally containing
heteroatom(s)" is a 5 or 6 membered carbocyclic ring
optionally containing 1 to 3 heteroatom(s) consisting of
nitrogen atom, oxygen atom and sulfur atom. Examples thereof
include furan, thiophene, pyrrole, oxazole, isoxazole,
z5 thiazole, isothiazole, imidazole, pyrazole, furazan, pyran,
pyridine, pyridazine, pyrimidine, pyrazine, pyrroline,
pyrrolidine, imidazoline and imidazolidine. Of these, furan,
19
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
thiophene, pyrrole, oxazole, isoxazole, thiazole, isothiazole,
furazan and pyridine are preferable.
The compounds, represented by the formula (I) of the
present invention can be converted to acid addition salts with
pharmaceutically acceptable acids and such acid addition salts
are also encompassed in the present invention. Such acid
addition salts include, for example, salts with inorganic
acids such as hydrochloric acid, hydrobromic acid, hydroiodic
acid, sulfuric acid, nitric acid, phosphoric acid and the like,
.to and salts with organic acids such as formic acid, acetic acid,
propionic acid, oxalic acid, malonic acid, succinic acid,
fumaric acid, malefic acid, lactic acid, malic acid, citric
acid, tartaric acid, methanesulfonic acid, benzenesulfonic
acid, p-toluenesulfonic acid, glutamic acid and the like.
i5 Furthermore, the compounds of the present invention can form
hydrates, solvates with ethanol and the like, and crystal
polymorphs. When an asymmetric carbon atom exists, optical
isomers and racemates thereof can be present, and all of these
are encompassed in the present invention.
2o Of the compounds (I) of the present invention, a
compound wherein R° is hydrogen can be synthesized as shown in
the following according to the method described in J. Chem.
Soc., Perkin Trans. 1, 947 (1996) and the like.
(First Production Method)
R3
O O R4 I R2
O O + ~ + R5~R4 R5 N- 'O
R3 R2
vii ) ( viii
(ix)
wherein Ra, R3, R4 and R5 are as defined above.
Meldrum's acid of the formula (VI) and a carbonyl
derivative of the formula (VII) are reacted with a carbonyl
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
derivative of the formula (VIII) to give an amide derivative
of the formula (IX). The reaction is carried out in the
presence of a carboxylic acid solvent inert to the reaction.
As the solvent, formic acid, acetic acid, propionic acid,
s butyric acid, valeric acid and the like are generally used.
The reaction is carried out at any temperature, for example,
from 0°C to 200°C, preferably from 60°C to 100°C.
R3 R3
R4 R2 R4 R2CH0
R5 N- 'O R5 N CI
H H
(
wherein R~, R3, R4 and RS are as defined above.
to The obtained amide derivative of the formula (IX) is
reacted in the presence of dimethylformamide and phosphorus
oxychloride to give a formyl derivative of the formula (X).
The reaction is carried out in the presence of a solvent inert
to the reaction. As the solvent, ether, tetrahydrofuran,
is dioxane, ethyl acetate, acetonitrile, benzene, toluene,
chloroform, dichloromethane, dimethylformamide, dimethyl
sulfoxide and the like are generally used. The reaction is
carried out at any temperature, for example, from 0°C to 200°C,
preferably from 60°C to 100°C.
R3 R3
R2 R2 Rt
R4 CHO Ra
l ~ -- I ~C~ c
Rs N C~ Rs N N H
H H
(X)
wherein R1 represents hydrogen, and Rz, R3, R4 and R5 are as
defined above.
The compound (I) of the present invention can be
produced by reacting the obtained formyl derivative of the
21
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
formula (X) in the presence of hydrazine. The reaction is
carried out in the presence of a solvent inert to the reaction.
As the solvent, ether, tetrahydrofuran, dioxane, ethyl acetate,
acetonitrile, benzene, toluene, chloroform, dichloromethane,
s dimethylformamide, dimethyl sulfoxide, pyridine, alcohol and
the like are generally used. The reaction is carried out at
any temperature, for example, from 0°C to 200°C, preferably
from 60°C to 100°C.
The carbonyl derivative of the formula (VII), which is a
.to starting material, can be synthesized according to the methods
described in J. Org. Chem., 46, 783 (1981), Eur. J. Med. Chem.,
31, 3 (1996) and Tetrahedron Lett., 24, 5023 (1983). The
carbonyl derivative of the formula (VIII) can be synthesized
according to the method described in Synthesis, 290 (1993).
15 (Second Production Method)
R3 2
O O R4 R R'
~~~ 4
+ Rs~R2 + Rs~R
H2N H R5 N N H
H
(XI) (VII) (VIII)
(I)
wherein R1, R2, R3, R4 and RS are as defined above.
The compounds (I) of the present invention can be
produced by reacting aminopyrazole of the formula (XI) and a
2o carbonyl derivative of the formula (VII) with a carbonyl
derivative of the formula (VIII). The reaction is carried out
in the presence of a solvent inert to the reaction. As the
solvent, ether, tetrahydrofuran, dioxane, ethyl acetate,
acetonitrile, benzene, toluene, chloroform, dichloromethane,
as dimethylformamide, dimethyl sulfoxide, alcohol and the like
are generally used. The reaction is carried out at any
temperature, for example, from 0°C to 200°C, preferably from
60°C to 100°C.
22
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Of the compounds (I) of the present invention, a
compound wherein R° is a substituent other than hydrogen can be
synthesized as follows.
(Third Production Method)
1
~ + R°-X --
~r,nN H
(XI) (X11) (I)
wherein R°, Rl, R2, R3, R4 and RS are as defined above, and X
represents halogen, provided that R° is not hydrogen.
The compounds (I) of the present invention can be
produced by reacting a dihydropyrazolopyridine derivative of
zo the formula (XI) with halide of the formula (XII) in the
presence of a base. Suitable base includes, for example,
triethylamine, diisopropylethylamine, 4-dimethylaminopyridine
and the like. The reaction is carried out in the presence of a
solvent inert to the reaction. As the solvent, one without
l5 hydroxy group is generally used, such as tetrahydrofuran,
ethyl acetate, benzene, toluene, chloroform, dichloromethane,
dimethylformamide, dimethylimidazolidinone and the like. The
reaction is carried out at any temperature, for example, from
-10°C to 200°C, preferably from 0°C to 100°C.
20 (Fourth Production Method)
R4
R2 R1
Ro-O
+ ~ o
I N H R ~ o
N R
(X~) (X111) (I)
wherein R°, Rl, R2, R3, R4 and RS are as defined above, provided
23
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
that R° is not hydrogen.
The compounds (I) of the present invention can be
produced by reacting a dihydropyrazolopyridine derivative of
the formula (XI) with anhydride of the formula (XIII) in the
s presence of a base. Suitable base includes, for example,
triethylamine, pyridine, 4-dimethylaminopyridine and the like.
The reaction is carried out in the presence of a solvent inert
to the reaction. As the solvent, one without hydroxy group is
generally used, such as tetrahydrofuran, ethyl acetate,
.~o benzene, toluene, chloroform, dichloromethane,
dimethylformamide, dimethylimidazolidinone, pyridine and the
like. The reaction is carried out at any temperature, for
example, from -10°C to 200°C, preferably from 0°C to
100°C.
The compound (I) of the present invention thus produced
Is can be isolated and purified as a free compound or a salt
thereof. Isolation and purification is carried out by a
conventional chemical process such as extraction,
concentration, evaporation, crystallization, filtration,
recrystallization, various kinds of chromatography and the
2o like. When the purified product obtained is a racemate, a
desired optically active compound can be separated by, for
example, fractional recrystallization with optically active
acid, or passing through a column packed with optically active
carrier. The present invention also encompasses optically
2s active compounds.
The compounds of the present invention obtained by the
above methods have a weak inhibitory activity on kinases other
than GSK-3(3 such as CaM kinase II, MAP kinase, Casein kinase,
PKA, PKC and ROCK, but have a strong inhibitory activity on
3o GSK-3(3. Therefore, the compounds of the present invention have
a GSK-3(3-selective inhibitory activity and can be medicaments
with small side-effect for diabetes, diabetic complications
and neurodegenerative diseases (Alzheimer's disease, ischemic
24
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
cerebrovascular disorders, Down's syndrome, cerebral ischemia
due to cerebral amyloid angiopathy, progressive supranuclear
paralysis, subacute sclerosing panencephalitic Parkinsonism,
postencephalitic Parkinsonism, boxer's encephalopathy,
s Parkinsonism dementia complex of Guam, Zewy body disease,
Pick's disease, corticobasal degeneration, frontotemporal
dementia, AIDS encephalopathy, Huntington's disease, manic-
depressive psychosis and the like). In addition, the compounds
of the present invention are useful as immunopotentiators.
.to Formulations comprising the compounds of the present
invention or salts thereof as an active ingredient are
prepared using carriers, excipients and other additives
conventionally used for formulation. The carrier and
excipient for formulation may be a solid or liquid, 'and
Is include, for example, lactose, magnesium stearate, starch,
talc, gelatin, agar, pectin, gum Arabic, olive oil, sesame oil,
cacao butter, ethylene glycol and other conventionally used
substances. Administration may be oral administration of
tablet, pill, capsule, granule, powder, solution and the like,
ao or parenteral administration of injection (intravenous
injection, intramuscular injection and the like), suppository,
transdermal agent and the like. While the dose is
appropriately determined on each case in consideration of
symptom, age and sex of the administration subject, and the
zs like, it is generally 1 - 1,000 mg, preferably 50 - 200 mg per
day for an adult person, which is orally administered once to
several times a day, or 1 - 500 mg per day for an adult person,
which is intravenously administered once to several times a
day, or continuously administered intravenously for 1 to 24
3o hours a day.
As solid compositions for oral administration according
to the present invention, tablet, powder, granule and the like
are used. In such a solid composition, one or more active
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
substances are mixed with at least one inert diluent, such as
lactose, mannitol, glucose, hydroxypropylcellulose,
microcrystalline cellulose, starch, polyvinylpyrrolidone,
metasilicic acid and magnesium aluminate. The composition may
contain, according to a conventional method, inert additives
other than diluent, for example, a lubricant such as magnesium
stearate, a disintegrator such as cellulose and calcium
glycolate, a stabilizer such as lactose and a solubilizer~such
as glutamic acid and aspartic acid. Tablet and pill may be
Io coated with a gastric or enteric coating film of, for example,
sucrose, gelatin, hydroxypropylcellulose and the like. Liquid
compositions for oral administration include pharmaceutically
acceptable emulsion, solution, suspension, syrup, elixir and
the like, and contain an inert diluent generally used, such as
purified water and ethanol. This composition may contain an
adjuvant such as wetting agent and suspending agent, a
sweetener, a flavor, an aromatic and an antiseptic, in
addition to the inert diluent. Injections for parenteral
administration contain sterile aqueous or non-aqueous solution,
~o suspension and emulsion. The aqueous solution and suspension
include, for example, propylene glycol, polyethylene glycol,
vegetable oil such as olive oil, alcohols such as ethanol,
polysorbate 80 and the like. Such a composition may contain
adjuvants such as antiseptic, wetting agent, emulsifier,
zs dispersant, stabilizer and solubilizer. These are sterilized
by, for example, filtration through a bacteria-retaining
filter, addition of an antimicrobial agent, irradiation of
ultraviolet ray and the like. Alternatively, a sterile solid
composition may be prepared and used upon dissolution in
3o sterile water or sterile solvent for injection prior to use.
Examples
The present invention is described in detail in the
following, based on Examples, Formulation Examples and
26
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Experimental Examples. The scope of the present invention is
not limited to these examples.
Example Z
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
A solution of 2-chlorobenzaldehyde (1.7 g), 3-
aminopyrazole (1.0 g) and ethyl acetoacetate (1.6 g) in
acetonitrile (20 mZ) was heated under reflux overnight. The
reaction mixture was cooled to room temperature and the
so solvent was evaporated under reduced pressure to give an oil.
The oil was purified by silica gel column chromatography
(eluent: hexane- ethyl acetate (8:2)) to give the title
compound (850 mg) as colorless crystals.
Melting Point (MP) :217-221°C.
Anal. Calcd. for:C16H1sN302C1: C,60.47;H,5.08;N,13.22.
Found:C,60.15;H,5.07;N,13.53.
MS (EI) :317 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm) : 1.00 (3H,t,J=6.8Hz) , 2.25 (3H,s) ,
3.72-3. 82 (2H,m) , 5.57 (lH,s) , 7.07-7.12 (2H,m) ,
7.18(lH,d,J=7.3Hz), 7.26(lH,s), 7.34(lH,d,J=7.9Hz),
9. 53 (lH,br. s) , 11. 98 (lH,br. s) .
IR(KBr):v=3393,3267,1670,1589,1518,1278,1217crri1.
Example 2
Ethyl 4,7-dihydro-4-(2-methoxyphenyl)-6-methyl-2H-
~s pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
methoxybenzaldehyde, 3-aminopyrazole and ethyl acetoacetate in
the same manner as in Example 1.
MP:196-200°C.
so Anal . Calcd. for: C1~H19N30 1/5 H20: C, 64 . 42;H, 6.17 ;N,13. 26 .
Found:C,64.08;H,6.05;N,13.68.
MS (EI) :313 (M+) .
zH-NMR (400MHz,DMSO-ds)8(ppm) : 1.00 (3H,t,J=6.8Hz) , 2.81 (3H,s) ,
27
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.72 (3H,s) , 3.87 (2H,q,J=6.8Hz) , 5.54 (lH,s) , 6.80 (lH,dd,J=7.3Hz
and 7.4Hz), 6.90(lH,d,J=7.8Hz), 7.04(lH,d,J=7.4Hz), 7.13-
7. 15 (2H,m) , 9. 99 (lH,br. s) , 11. 98 (lH,br. s) .
IR(KBr):v=3362,3267,3204,3090,1662,1589,1516,1275,1097ciri1.
Example 3
Ethyl 4,7-dihydro-6-methyl-4-(2-trifluoromethylphenyl)-2H-
pyrazolo[3,4-b]pyridine-5-Carboxylate
The title compound was prepared from 2-
trifluoromethylbenzaldehyde, 3-aminopyrazole and ethyl
so acetoacetate in the same manner as in Example 1.
MP:259-262°C.
Anal. Calcd. for:Cl7H~6F3N3Oz 1/5 H20:C,57.53;H,4.66;N,11.84.
Found:C,57.56;H,4.68;N,11.86.
MS (EI) :352 (M++1) .
1H-NMR (400MHz,DMSO-d6)8(ppm) : 0.74 (3H,t,J=6.9Hz) , 2.40 (3H,s) ,
3.68-3.81(2H,m), 5.42(lH,s), 7.00(lH,s),7.28(lH,dd,J=7.3Hz and
7.4Hz), 7.33(lH,d,J=7.2Hz), 7.51(lH,dd,J=7.3Hz and 7.4Hz),
7. 60 (lH,d,.J=7. 8Hz) , 9. 58 (lH,br. s) , 12. 00 (lH,br. s) .
IR(KBr):v=3277,3209,3094,1668,1593,1514,1313,1213,1153,1097,
765crn i.
Example 4
Methyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-
pyrazolo[3,4-b]pyridine-5-Carboxylate
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and methyl acetoacetate in
the same manner as in Example 1.
MP : 235°C.
Anal. Calcd.~ for:C15H14C1N302 2/5 H20:C,57.94;H,4.80;N,13.51.
Found:C,58.03;H,4.55;N,13.43.
3o MS (EI) :303 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm) : 2.40 (3H,s) , 3.34 (3H,s) ,
5.55(lH,s), 7.09-7.11(2H,m), 7.18(lH,dd,J=7.3Hz and 7.4Hz),
7. 29 (1H, s) , 7. 34 (lH,d,J=7.3Hz) , 9. 57 (lH,br. s) , 12. 00 (lH,br. s) .
28
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 5
t-Butyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
~chlorobenzaldehyde, 3-aminopyrazole and t-butyl acetoacetate
in the same manner as in Example 1.
MP:207°C.
Anal. Calcd. for:ClBHZOC1N302:C,62.52;H,5.83;N,12.15.
Found:C,62.51;H,5.79;N,12.17.
1o MS(EI) :345 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.07(9H,s), 2.36(3H,s),
5.50(lH,s), 7.11-7.15(2H,m), 7.20(lH,d,J=7.3Hz), 7.25(lH,s),
7.37(lH,d,J=7.3Hz), 9.35(lH,br.s), 11.93(lH,br.s).
Example 6
Is Isopropyl 4-(2-fluorophenyl)-4,7-dihydro-6-methyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
fluorobenzaldehyde, 3-aminopyrazole and isopropyl acetoacetate
in the same manner as in Example 1.
2o MP:218-220°C.
1H-NMR (400MHz,DMSO-d6)~(ppm): 0.66(3H,d,J=6.3Hz),
1.02(3H,d,J=6.3Hz), 2.37(3H,s), 4.66(lH,q,J=6.3Hz), 5.40(lH,s),
7.01-7.14(4H,m), 7.19(lH,s), 9.46(lH,br.s), 11.97(lH,br.s).
Example 7
25 Benzyl 4-(2-chlorophenyl)-4,7-dihydro-6-methyl-2H-
~yrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and benzyl acetoacetate in
the same manner as in Example 1.
3o MP : 234°C .
Anal. Calcd. for:CZ~H18C1N3Oa :C, 66 . 40;H, 4 .78;N,11.06 .
Found:C,66.16;H,4.86;N,10.92.
MS(EI) :379(M+) .
29
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
1H-NMR (400MHz,DMSO-d6)b(ppm): 2.43(3H,s), 4.81(lH,d,J=12.6Hz),
4.92(lH,d,J=12.6Hz), 5.62(lH,s), 6.86-6.88(2H,m), 7.13-
7.18(6H,m), 7.31-7.34(2H,m), 9.65(lH,br.s), 12.01(lH,br.s).
Example 8
4-(2-Chloro~henyl)-5-dimethylaminocarbonyl-4,7-dihydro-6-
methyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and N,N-dimethylacetamide
in the same manner as in Example 1.
1o MP:229°C.
Anal. Calcd. for:C16H1~C1N40 1/2 HzO:C,58.99;H,5.57;N,17.20.
Found:C,58.90;H,5.46;N,16.84.
MS(EI):316(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.77(3H,s), 2.72(6H,s),
15 5.39(lH,s), 7.10-7.22(4H,m), 7.30(lH,d,J=7.3Hz), 8.40(lH,br.s),
11.83(lH,br.s).
Example 9
4-(2-Chlorophenyl)-5-hydrazinocarbonyl-4,7-dihydro-6-methyl-
2H-pyrazolo[3,4-b]pyridine
ao To a solution of 4-(2-chlorophenyl)-4,7-dihydro-5-
dimethylaminocarbonyl-6-methyl-2H-pyrazolo[3,4-b]pyridine (200
mg) in acetonitrile (200 mL) was added hydrazine (200 mg) and
the mixture was heated under reflux overnight. The reaction
mixture was cooled to room temperature, and the precipitated
z5 crystals were collected by filtration and washed with ethyl
acetate to give the title compound as colorless crystals (150
mg).
MP:220°C.
Anal. Calcd. for:C14H14C1N50 3/10 HZO:C,54.39;H,4.76;N,22.65.
3o Found:C,54.36;H,4.56;N,22.65.
MS(EI):303(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.85(3H,s), 3.20-3.80(3H,br. s),
5.15(lH,s), 6.81(lH,s), 7.16-7.028(3H,m), 7.34(lH,d,J=7.3Hz),
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
10.05-11.07(2H,brs).
Example 10
4-(2-Fluorophenyl)-4,7-dihydro-6-methyl-5-
isopropylthiocarbonyl-2H-pyrazolo[3,4-b]pyridine
s The title compound was prepared from 2-
fluorobenzaldehyde, 3-aminopyrazole and acetoacetic acid
isopropyl thioester in the same manner as in Example 1.
MP:192-194°C.
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.03(3H,d,J=6.9Hz),
io 1.15(3H,d,J=6.9Hz), 2.43(3H,s), 3.35(lH,q,J=6.9Hz), 5.55(lH,s),
7.04-7.15(4H,m), 7.33(lH,s), 9.81(lH,br.s), 12.11(lH,br.s).
Example 11
4,7-Dihydro-6-methyl-5-nitro-4-(2-trifluoromethylphenyl)-2H-
pyrazolo[3,4-b]pyridine
15 The title compound was prepared from 2-
trifluoromethylbenzaldehyde, 3-aminopyrazole and 1-
nitropropan-2-one in the same manner as in Example 1.
MP:257-258°C.
1H-NMR (400MHz,DMSO-d6)b(ppm): 2.65(3H,s), 5.75(lH,s),
ao 7.19(lH,s), 7.30-7.35(2H,m), 7.51(lH,dd,J=7.3Hz and 7.8Hz),
7.66(lH,d,J=7.8Hz), 10.87(lH,br.s), 12.45(lH,br.s).
Example 12
Ethyl 4,7-dihydro-4-phenyl-6-trifluoromethyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
25 The title compound was prepared from benzaldehyde, 3-
aminopyrazole and ethyl trifluoroacetoacetate in the same
manner as in Example 1.
MP:110-115°C.
Anal. Calcd. for:C16H14N30aF3 1/2 H20:C,55.49;H,4.37;N,12.13.
3o Found:C,55.84;H,4.70;N,11.89.
MS(EI) :337 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.84(3H,t,J=6.9Hz),
3.90(2H,q,J=6.8Hz), 5.54(lH,s), 7.13-7.17(3H,m), 7.24-
31
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.28(3H,m), 9.78(lH,br.s), 12.20(lH,br.s).
IR(KBr):v=3375,3175,3067,1707,1606,1533,1278,1206,1197,1167cm 1.
'Example 13
Ethyl 4-(2-fluorophenyl)-4,7-dihydro-6-trifluoromethyl-2H-
s pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
fluorobenzaldehyde, 3-aminopyrazole and ethyl
trifluoroacetoacetate in the same manner as in Example 1.
MP:119-120°C.
so Anal. Calcd. for:C16H13F4N3OZ:C,54.09;H,3.69;N,11.84.
Found:C,53.84;H,3.57;N,11.79.
MS(EI):356(M++1).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94 (3H,t,J=6.8Hz),
3.89(2H,q,J=6.8Hz), 5.46(lH,s), 7.11-7.20(4H,m), 7.28-
I5 7.30(lH,m), 9.92(lH,br.s), 12.27(lH,br.s).
IR(KBr):v=3290,3178,3069,1703,1608,1537,1280,1232,1174,1138,
756cm 1.
Example 14
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-trifluoromethyl-2H-
2o pyrazolo[3,4-b]pyridine-5-carboxylate maleate
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and ethyl
trifluoroacetoacetate in the same manner as in Example 1.
MP:171-172°C.
25 MS (EI ) :371 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91 (3H,t,J=7.3Hz),
3.50(3H,br.s), 3.87 (2H,q,J=6.8Hz), 5.66(lH,s), 6.26(2H,s),
7.15-7.18(2H,m), 7.27(lH,d,J=7.8Hz), 7.30(lH,s),
7.40(lH,d,J=7.8Hz), 9.65(lH,br.s).
3o IR(KBr):v=3297,2935,1730,1624,1550,1479,1186cm 1.
Example 15
Ethyl 4,7-dihydro-4-(2-methoxyphenyl)-6-trifluoromethyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
32
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from 2-
methoxybenzaldehyde, 3-aminopyrazole and ethyl
trifluoroacetoacetate in the same manner as in Example 1.
MP:144-146°C.
s Anal. Calcd. for:C~7H16F3N3O3:C,55.59;H,4.39;N,11.44.
Found:C,55.55;H,4.38;N,11.43.
MS (EI ) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=6.8Hz), 3.83(3H,s),
3.89(2H,q,J=6.8Hz),5.51(lH,s), 6.84(lH,dd,J=7.3Hz and 7.4Hz),
l0 6.94-6.97(2H,m), 7.13(lH,dd,J=7.3Hz and 7.4Hz), 7.20(lH,s),
9.70(lH,br.s), 12.13(lH,br.s).
IR(KBr):v=3431,3173,3067,2993,2924,1689,1610,1527,1286,1226,
1145cm 1.
Example 16
15 Ethyl 4,7-dihydro-6-trifluoromethyl-4-(2-
trifluoromethylphenyl)-2H-pyrazolo[3,4-b]pyridine-5-
carboxylate
The title compound was prepared from 2-
trifluoromethylbenzaldehyde, 3-aminopyrazole and ethyl
2o trifluoroacetoacetate in the same manner as in Example 1.
MP:182-186°C.
Anal. Calcd. for:C17H13N3OzF6:C,50.38;H,3.23;N,10.37.
Found:C,50.21;H,3.15;N,10.39.
MS(FAB):406(M*+1).
25 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.83 (3H,t,J=6.8Hz),
3.83(2H,q,J=6.8Hz), 5.49(lH,s), 7.08(lH,s), 7.35-7.39(2H,m),
7.62(lH,dd,J=7.3Hz and 7.4Hz), 7.66(lH,d,J=7.8Hz),
9.97(lH,br.s), 12.30(lH,br.s).
IR(KBr):v=3339,3177,3067,1711,1608,1537,1313,1280,1182,1141cnW .
3o Example 17
Ethyl 4-(3-chlorophenyl)-4,7-dihydro-6-trifluoromethyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 3-
33
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
chlorobenzaldehyde, 3-aminopyrazole and ethyl
trifluoroacetoacetate in the same manner as in Example 1.
MP :144-145°C .
Anal. Calcd. for:C1gH13N3~2r3C1:C,51.69;H,3.52;N,11.30.
s Found:C,51.33;H,3.74;N,11.10.
MS(EI):371(M+).
1H-NMR (400MH2,DMS0-d6)8(ppm): 0.98 (3H,t,J=6.8Hz),
3.92(2H,q,J=6.8Hz), 5.21(lH,s), 7.11(lH,d,J=7.8Hz), 7.17(lH,s),
7.23(lH,d,J=8.7Hz), 7.29-7.33(2H,m), 9.92(lH,br.s),
io 12.30(lH,br.s).
IR(KBr):v=3321,3178,3070,1703,1610,1535,1278,1224,1184,1145cm 1.
Example 18
Ethyl 4-(4-chlorophenyl)-4,7-dihydro-6-trifluoromethyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
z5 The title compound was prepared from 4-
chlorobenzaldehyde, 3-aminopyrazole and ethyl
trifluoroacetoacetate in the same manner as in Example 1.
MP:176-178°C.
Anal. Calcd. for:C16H13F3N3~2C~-:C,51.69;H,3.52;N,11.30.
2o Found:C,51.91;H,3.77;N,11.08.
MS(EI) :371(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.98 (3H,t,J=6.8Hz),
3.90(2H,q,J=7.3Hz), 5.92(lH,s), 7.16(2H,d,J=8.2Hz), 7.27(lH,s),
7.31(2H,d,J=8.2Hz), 9.87(lH,br.s), 12.27(lH,br.s).
a5 IR(KBr):v=3476,3368,3178,3078,1714,1695,1606,1537,1278,1172,
1134cm 1.
Example 19
Ethyl 4,7-dihydro-4-(4-methoxyphenyl)-6-trifluoromethyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
3o The title compound was prepared from 4-
methoxybenzaldehyde, 3-aminopyrazole and ethyl
trifluoroacetoacetate in the same manner as in Example 1.
MP :159-161°C .
34
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C17H16FN303:C,55.59;H,4.39;N,11.44.
Found:C,55.49;H,4.54;N,11.33.
MS(EI) :367(Mk) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.99 (3H,t,J=7.3Hz), 3.68(3H,s),
s 3.89(2H,q,J=7.3Hz), 5.12(lH,s), 6.82(2H,d,J=8.7Hz),
7.03(2H,d,J=8.7Hz), 7.22-7.24(lH,m), 9.71(lH,br.s),
12.19(lH,br.s).
IR(KBr):v=3323,3231,3173,3067,1699,1610,1535,1510,1302,1248,118
4,1145cm ~.
to Example 20
Ethyl 4-(4-ethoxycarbonylphenyl)-4,7-dihydro-6- '
trifluoromethyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 4-
ethoxycarbonylbenzaldehyde, 3-aminopyrazole and ethyl
.t5 trifluoroacetoacetate in the same manner as in Example 1.
MP:157-160°C.
Anal. Calcd. for:C19H18F3N3O4:C,55.75;H,4.43;N,10.26.
Found:C,55.68;H,4.39;N,10.43.
MS(FAB):410(M++1).
20 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.96 (3H,t,J=6.9Hz), 1.28
(3H,t,J=7.3Hz), 3.89(2H,q,J=6.8Hz), 4.27(2H,q,J=7.3Hz),
5.28(lH,s), 7'.27(lH,s), 7.29(2H,d,J=8.3Hz), 7.87(2H,d,J=8.2Hz),
9.92(lH,br.s), 12.28(lH,br.s).
IR(KBr):v=3393,3188,3082,1692,1612,1539,1284ciri1.
25 Example 21
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-3-methyl-6-
trifluoromethyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared.from 2-
chlorobenzaldehyde, 3-amino-5-methylpyrazole and ethyl
3o trifluoroacetoacetate in the same manner as in Example 1.
MP:165-168°C.
MS(EI) :385 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94 (3H,t,J=7.3Hz), 1.81 (3H,s),
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.85(2H,q,J=6.8Hz), 5.54(lH,s), 7.17-7.20(2H,m),
7.27(lH,dd,J=7.3Hz and 7.4Hz), 7.36(lH,d,J=8.3Hz),
9.79(lH,br.s), 11.96(lH,br.s).
IR(KBr):v=3263,3194,3080,1668,1591,1520,1286,1232,1149,1095,
s 1062cm 1.
Example 22
Ethyl 4,7-dihydro-4-(thiophen-2-yl)-6-trifluoromethyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from thiophene-2-
Io aldehyde, 3-aminopyrazole and ethyl trifluoroacetoacetate in
the same manner as in Example 1.
MP:157-161°C.
Anal. Calcd. for:C14H1zF3N3OzS:C,49.27;H,2.95;N,12.31.
Found:C,49.10;H,3.28;N,12.13.
15 MS(EI) :343(M~) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.08 (3H,t,J=7.4Hz), 4.00
(2H,q,J=7.4Hz), 5.52(lH,s), 6.76(lH,d,J=2.9Hz),
6.87(lH,dd,J=2.9Hz and 5.4Hz), 7.30(lH,d,J=5.4Hz), 7.43(lH,s),
9.96(lH,br.s), 12.35(lH,br.s).
ao IR(KBr):v=3350,3240,3180,1693,1612,1535,1396,1371,1304,1153,109
3,1057, 694ciri x .
Example 23
Ethyl 4,7-dihydro-4-(thiophen-3-yl)-6-trifluoromethyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
25 The title compound was prepared from thiophene-3-
aldehyde, 3-aminopyrazole and ethyl trifluoroacetoacetate in
the same manner as in Example 1.
MP:140-145°C.
Anal. Calcd. for:C14H1aF3N30zS:C,49.27;H,2.95;N,12.31.
3o Found:C,49.65;H,2.64;N,12.19.
MS(EI) :343 (M+) .
~H-NMR (400MHz,DMSO-d6)8(ppm): 1.03 (3H,t,J=7.3Hz), 3.96
(2H,q,J=7.3Hz), 5.30(lH,s), 6.87(lH,d,J=4.8Hz), 7.05(lH,s),
36
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.35(lH,s), 7.39(lH,dd,J=2.9Hz and 4.8Hz), 9.76(lH,br.s),
12.25(lH,br.s).
IR(KBr):v=3356,3182,2982,2932,1689,1614,1537,1304,1224,1153cn11.
Example 24
Ethyl 4,7-dihydro-4-(1-naphthyl)-6-trif luoromethyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from naphthalene-1-
aldehyde, 3-aminopyrazole and ethyl trifluoroacetoacetate in
the same manner as in Example 1.
so MP:119-120°C.
Anal. Calcd. for:CzoH16F3N30a 1/2 HZO:C,60.45;H,4.57;N,10.57.
Found:C,60.20;H,4.77;N,10.39.
MS(FAB):388(M++1).
~H-NMR (400MHz,DMSO-d6)b(ppm): 0.69 (3H,t,J=6.8Hz),
3.73(2H,q,J=6.8Hz), 6.04(lH,s), 7.09(lH,s), 7.26(lH,d,J=6.8Hz),
7.41(lH,dd,J=7.3Hz and 7.4Hz), 7.52-7.58(2H,m),
7.75(lH,d,J=8.3Hz), 7.92(lH,dd,J=7.3Hz and 7.4Hz), 8.33(lH,s),
9.87(lH,br.s), 12.14(lH,br.s).
IR(KBr):v=3173,1670,1606,1138,1095cm:1.
2o Example 25
Ethyl 4,7-dihydro-4-phenyl-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate maleate
A solution of benzaldehyde (1.6 g), 3-aminopyrazole (1.0
g) and ethyl 3-ketohexanoate (1.9 g) in acetonitrile (20 mL)
was heated under reflux overnight. The reaction mixture was
cooled to room temperature, and the solvent was evaporated
under reduced pressure to give an oil. The oil was purified by
silica gel column chromatography (eluent: hexane-ethyl acetate
(8:2)) to give the title compound (720 mg) as colorless
3o crystals.
MP:139-141°C.
Anal. Calcd. for:C18H21N3OZC4H4O4 1/2 H~O:C, 60.54;H, 6.00;N, 9 .63.
Found: C,60.16;H,5.60;N,10.01.
37
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :311 (M+) .
~H-NMR (400MHz,DMSO-d6)b(ppm): 0.94-0.95 (6H,m), 1.62
(2H,q,J=7.8Hz), 2.66-2.77(2H,m), 3.50(3H,br.s),
3.83(2H,q,J=6.8Hz), 5.10(lH,s), 6.25(2H,s), 7.05-7.20(6H,m),
9.37(lH,br.s).
IR(KBr):v=3337,3042,1699,1593,1467,1539,1361,1203cm 1
Example 26
Ethyl 4-(2-fluorophenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
io The title compound was prepared from 2-
fluorobenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:192-194°C.
Anal. Calcd. for:ClBHzoFN302 1/2 H~O:C, 63.89;H, 6.26;N,12.42 .
i5 Found:C,63.85;H,6.Ol;N,12.36.
MS(EI) :329 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.93 (3H,t,J=7.3Hz), 0.97
(3H,t,J=7.3Hz), 1.62-1.68(2H,m), 2.71-2.83(2H,m),
3.82(2H,q,J=7.3Hz), 5.43(lH,s), 7.05-7.11(4H,m), 7.21(lH,s),
ao 9.48(lH,br.s), 11.97(lH,br.s).
IR(KBr):v=3265,3198,2964,1591,1514,1224,1209,1093cm 1.
Example 27
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
z5 The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:202-205°C.
Anal. Calcd. for:ClBHZaC1N30a:C,62.52;H,5.83;N,12.15.
3o Found:C,62.28;H,5.76;N,12.37.
M5(FAB):346(M++1).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85 (3H,t,J=6.8Hz), 0.95
(3H,t,J=7.3Hz), 1.62-1.68(2H,m), 2.67-2.87(2H,m),
38
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.78(2H,q,J=6.8Hz), 5.58(lH,s), 7.07-7.11(2H,m),
7.18(lH,dd,J=7.3Hz and 7.4Hz), 7.25(lH,s), 7.34(lH,d,J=7.8Hz),
9.49(lH,br.s), 11.97(lH,br.s).
IR(KBr):v=3263,3209,3194,3080,1668,1591,1520,1286,1232,1149,106
2, 750cni 1.
Example 28
Methyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
to chlorobenzaldehyde, 3-aminopyrazole and methyl 3-ketohexanoate
in the same manner as in Example 25.
MP:203-207°C.
Anal. Calcd. for:C17H18C1N3oZ 1/5 HZO:C,60.88;H,5.53;N,12.53.
Found:C,60.73;H,5.36;N,12.14.
MS(EI) :331(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.97 (3H,t,J=7.3Hz), 1.64-
1.66(2H,m), 2.72-2.83(2H,m), 3.31(3H,s), 5.57(lH,s),
7.10{lH,d,J=7.3Hz), 7.09-7.11(lH,m), 7.17-7.18{lH,m),
7.27(lH,s), 7.34(lH,d,J=7.8Hz), 9.54(lH,br.s), 11.97(lH,br.s).
ao IR(KBr):v=3260,3190,1672,1591,1516,1232c~ 1.
Example 29
Ethyl 4-(2-bromophenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-bromobenzaldehyde,
z5 3-aminopyrazole and ethyl 3-ketohexanoate in the same manner
as in Example 25.
MP:223°C.
Anal. Calcd. for:ClBHzoBrN30z:C,55.40;H,5.17;N,10.77.
Found:C,55.08;H,5.14;N,10.85.
3o M5(EI) :390(M+) .
1H-NMR (400MHz,DMSO-d6)&(ppm): 0.86(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.63-1.69(2H,m), 2.71-2.74(lH,m), 2.80-
2.83(lH,m), 3.77(2H,q,J=7.3Hz), 5.67(lH,s), 7.00(lH,dd,J=7.3Hz
39
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
and 7.4Hz), 7.10(lH,d,J=7.3Hz), 7.22(lH,dd,J=7.3Hz and 7.4Hz),
7:28(lH,s), 7.51(lH,d,J=7.3Hz), 9.50(lH,br.s), 11.97(lH,br.s).
Example 30
Ethyl 4,7-dihydro-4-(2-methylphenyl)-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
methylbenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:178°C.
so Anal. Calcd. for:Ci9H23N3OZ:C,70.13;H,7.12;N,12.91.
Found:C,70.12;H,7.35;N,12.99.
MS(EI) :325(M'-) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.83(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz), 1.62-1.66(2H,m), 2.44(3H,s), 2.64-
2.66(lH,m), 2.76-2.79(lH,m), 3.77(2H,q,J=7.3Hz), 5.31(lH,s),
6.93(lH,d,J=7.3Hz), 6.99-7.05(3H,m),7.18(lH, s), 9.34(lH,br.s),
11.87(lH,br.s).
Example 31
Ethyl 4,7-dihydro-6-propyl-4-(2-trifluoromethylphenyl)-2H-
2o pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
trifluoromethylbenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:198-202°C.
Anal. Caled. for:Cl9HzoF3N302 1/2.H20: C,58.76;H,5.45;N,10.81.
Found:C,58.82;H,5.92;N,10.62.
MS (EI ) : 379 (M+) .
~H-NMR (400NiHz,DMSO-d6)8(ppm): 0.76 (3H,t,J=7.3Hz),
0.98(3H,t,J=7.3Hz), 1.64-1.68(2H,m), 2.76-2.79(2H,m),
3.80(2H,q,J=7.3Hz), 5.44(lH,s), 7.00(lH,s),7.27-7.30(lH,m),
7.33(lH,d,J=7.8Hz), 7.53(lH,dd,J=7.3Hz and 7.4Hz),
7.61(lH,d,J=7.3Hz), 9.54(lH,br.s), 11.99(lH,br.s).
TR(KBr):v=3265,3198,2964,1591,1514,1224,1209,1093cm 1.
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 32
Ethyl 4,7-dihydro-4-(2-methoxyphenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
methoxybenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:169°C.
Anal. Calcd. for:C19Hz3N303:C,66.84;H,6.79;N,12.31.
Found:C,66.58;H,6.50;N,12.34.
to MS(EI) :341(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.85(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.66-1.68(2H,m), 2.66-2.70(lH,m), 2.81-
2.88(lH,m), 3.80(2H,q,J=7.3Hz), 3.85(3H,s), 5.47(lH,s),
6.76(lH,dd,J=7.3Hz and 7.4Hz), 6.89-6.94(2H,m),
7.04(lH,dd,J=7.3Hz and 7.4Hz), 7.14(lH,s), 9.29(lH,br.s),
11.82(lH,br.s).
Example 33
Ethyl 4-(2-ethoxyphenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
zo The title compound was prepared from 2-
ethoxybenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:203°C.
Anal. Calcd. for:CzoHa5N303:C,67.58;H,7.09;N,11.82.
Found:C,67.48;H,7.06;N,11.81.
MS(EI) :355 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.41(3H,t,J=7.3Hz), 1.64-1.67(2H,m), 2.68-
2.71(lH,m), 2.78-2.81(lH,m), 3.79(2H,q,J=7.3Hz), 4.03-
4.05(lH,m), 4.10-4.12(lH,m), 5.48(lH,s), 6.74(lH,dd,J=7.3Hz
and 7.4Hz), 6.87(lH,d,J=7.3Hz), 6.94(lH,d,J=7.3Hz),
7.01(lH,dd,J=7.3Hz and 7.4Hz), 7.14(lH,s), 9.28(lH,br.s),
11.79(lH,br.s).
41
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 34
Ethyl 4,7-dihydro-4-(2-propoxyphenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
propoxybenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:205°C.
Anal. Calcd. for:CaIHZ7N303:C,68.27;H,7.37;N,11.37.
Found:C,68.05;H,7.39;N,11.35.
1o MS (EI) :369 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.84(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.05(3H,t,J=7.3Hz), 1.64-1.67(2H,m), 1.81-
1.84(2H,m), 2.70-2.73(lH,m), 2.78-2.82(lH,m),
3.77(2H,q,J=7.3Hz), 3.92(lH,q,J=7.3Hz), 4.07(lH,q,J=7.3Hz),
5.52(lH,s), 6.75(lH,dd,J=7.3Hz and 7.4Hz), 6.88(lH,d,J=7.3Hz),
6.94(lH,d,J=7.3Hz), 7.01(lH,dd,J=7.3Hz and 7.4Hz), 7.11(lH,s),
9.28(lH,br.s), 11.79(lH,br.s).
Example 35
Ethyl 4,7-dihydro-4-(2-isopropyloxyphenyl)-6-propyl-2H-
2o pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
isopropyloxybenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:210°C.
Anal. Caled. for:Ca1H27N3O3:C,68.27;H,7.37;N,11.37.
Found:C,67.93;H,7.39;N,11.32.
MS (EI ) : 369 (M+) .
1H-NMR (400MHz,DMSO-ds)8(ppm): 0.84(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.25(3H,d,J=6.8Hz), 1.39(3H,d,J=6.8Hz),
1.64-1.69(2H,m), 2.68-2.72(lH,m), 2.78-2.82(lH,m),
3.77(2H,q,J=7.3Hz), 4.64-4.67(lH,m), 5.45(lH,s),
6.73(lH,dd,J=7.3Hz and 7.4Hz), 6.89-6.90(3H,m), 7.15(lH,s),
9.27(lH,br.s), 11.77(lH,br.s),.
42
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 36
Ethyl 4-(2-butoxyphenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
butoxybenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP :171°C.
Anal. Calcd. for:CZZHa9N3O3:C,68.90;H,7.62;N,10.96.
Found:C,68.66;H,7.63;N,10.89.
to MS(EI) :383(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.84(3H,t,J=7.3Hz), 0.95-
0.99(6H,m), 1.52-1.80(6H,m), 2.69-2.71(lH,m), 1.76-1.80(lH,m),
3.77(2H,q,J=7.3Hz), 3.95-3.98(lH,m),4.07-4.10(lH,m),
5.51(lH,s), 6.74(lH,dd,J=7.3Hz and 7.4Hz), 6.88-6.94(2H,m),
7.01(lH,dd,J=7.3Hz and 7.4Hz), 7.10(lH,s), 9.28(lH,br.s),
11.79(lH,br.s).
Example 37
Ethyl 4-(2-cyclopentyloxyphenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
ao The title compound was prepared from 2-
cyclopentyloxybenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:195°C.
Anal. Calcd. for:C23Ha9N3O3:C,69.85;H,7.39;N,10.62.
Found:C,69.63;H,7.28;N,10.61.
MS(EI) :395(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.83(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.65-1.98(8H,m), 2.66-2.78(2H,m),
3.76(2H,q,J=7.3Hz), 4.89-4.93(lH,m), 5.43(lH,s),
6.72(lH,dd,J=7.3Hz and 7.4Hz), 6.88-6.93(2H,m),
7.00(lH,dd,J=7.3Hz and 7.4Hz), 7.10(lH,s), 9.28(lH,br.s),
11.77(lH,br.s).
Example 38
43
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Ethyl 4-(2-benzyloxyphenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
benzyloxybenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:128°C.
Anal. Calcd. for:CZ5H27N303:C,71.92;H,6.52;N,10.06.
Found:C,71.66;H,6.73;N,9.85.
MS(EI) :417 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.84(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.64-1.67(2H,m), 2.70-2.73(lH,m}, 2.80-
2.83(lH,m), 3.80(2H,q,J=7.3Hz), 5.20(2H,d,J=30Hz), 5.60(lH,s},
6.78(lH,dd,J=7.3Hz and 7.4Hz), 6.96-7.03(3H,m), 7.08(lH,s),
7.35(lH,dd,J=7.3Hz and 7.4Hz}, 7.40-7.43(2H,m}, 7.52-
.ts 7.55(2H,m), 9.30(lH,br.s}, 11.79(lH,br.s).
Example 39
Ethyl 4,7-dihydro-4-(2-methylthiophenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
A solution of 2-methylthiobenzaldehyde (20 g), Meldrum's
2o acid (19 g), ethyl 3-ketohexanoate (21 g) and ammonium acetate
(11 g) in acetic acid (130 mL) was heated under reflux
overnight. The reaction mixture was cooled to room temperature,
and the solvent was evaporated under reduced pressure to give
colorless crystals (9.7 g). To a solution of dimethylformamide
~s (1.3 g) in chloroform (5 mL) were added phosphorus oxychloride
(1.7 mL) and a solution of the obtained colorless crystals
(1.5 g) in chloroforom (10 mL) under ice-cooling, and the
mixture was stirred overnight. Under ice-cooling, an aqueous
sodium acetate (18.5 g) solution was added and the mixture was
3o stirred for one hour. The reaction mixture was extracted with
chloroform and the solvent was evaporated under reduced
pressure to give an oil. The obtained oil was purified by
silica gel column chromatography (eluent: hexane-ethyl acetate
44
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
(8:2)) to give colorless crystals (0.9 g). To a solution of
the obtained colorless crystals (0.9 g) in pyridine (10 mL)
was. added hydrazine (0.27 g) and the mixture was stirred with
heating for 3 hours. The reaction mixture was cooled to room
s temperature, and the solvent was evaporated under reduced
pressure to give an oil. The oil was purified by silica gel
column chromatography (eluent: hexane-ethyl acetate (1:1)) to
give the title compound (230 mg) as colorless crystals.
MP:198°C.
1o Anal. Calcd. for:C19HZ3N30zS:C,63.84;H,6.49;N,11.75.
Found:C,63.56;H,6.45;N,11.64.
MS(EI) :357 (M+) .
~H-NMR (400MHz,DMSO-d6)8(ppm): 0.82(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz), 1.62-1.68(2H,m), 2.48(3H,s), 2.67-
15 2.71(lH,m), 2.79-2.83(lH,m), 3.74(2H,q,J=7.3Hz), 5.54(lH,s),
6.99-7.06(3H,m), 7.22-7.25(2H,m), 9.38(lH,br.s),
11.86(lH,br.s).
Example 40
Ethyl 4,7-dihydro-4-(2-methylsulfinylphenyl)-6-propyl-2H-
zo pyrazolo[3,4-b]pyridine-5-carboxylate
To a solution of ethyl 4,7-dihydro-4-(2-methylthio)-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate (100 mg) in
tetrahydrofuran (3.0 mL) was added metachloroperbenzoic acid
(60 mg) and the mixture was stirred at -78°C for 30 minutes.
a5 An aqueous sodium thiosulfate solution was added, and the
mixture was extracted with chloroform. The solvent was
evaporated under reduced pressure to give colorless crystals.
By recrystallization from ethyl acetate, the title compound
(50 mg) was obtained as colorless crystals.
3o MP:216°C.
Anal. Calcd. for:C19H23N3O3S:C,61.10;H,6.21;N,11.25.
Found:C,61.32;H,6.18;N,10.99.
MS(EI) :373 (M+) .
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.91(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.64-1.68(2H,m), 2.69-2.72(lH,m),
2.72(3H,s), 2.76-2.79(lH,m), 3.90(2H,q,J=7.3Hz), 5.36(lH,s),
7.15(lH,dd,J=7.3Hz and 7.4Hz), 7.20(lH,s), 7.37-7.39(2H,m),
s 7.85(lH,dd,J=7.3Hz and 7.4Hz), 9.59(lH,br.s), 12.04(lH,br.s).
Example 41
Ethyl 4,7-dihydro-4-(2-nitrophenyl)-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-nitrobenzaldehyde
to and ethyl 3-ketohexanoate in the same manner as in Example 39.
MP:218°C.
Anal. Calcd. for:C18Hz0N404:C, 60.66;H,5.66;N,15.72.
Found:C,60.25;H,5.65;N,15.44.
MS (EI ) : 356 (M+) .
15 ~H-NMR (400MHz,DMSO-d6)b(ppm): 0.80(3H,t,J=7.3Hz),
0.95(3H,t,J=7.3Hz), 1.59-1.64(2H,m), 2.69-2.73(lH,m), 2.77-
2.80(lH,m), 3.72(2H,q,J=7.3Hz), 5.45(lH,s),7.28-7.33(3H,m),
7.56(lH,dd,J=7.3Hz and 7.4Hz), 7.76(lH,d,J=7.3Hz),
9.64(lH,br.s), 10.07(lH,br.s).
zo Example 42
Ethyl 4-(2-cyanophenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-cyanobenzaldehyde
and ethyl 3-ketohexanoate in the same manner as in Example 39.
2s MP:211°C.
Anal. Calcd. for:ClgH2oN4O2:C,67.84;H,5.99;N,16.66.
Found:C,67.49;H,6.14;N,16.23.
MS(EI) :336 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89(3H,t,J=7.3Hz),
30 0.94(3H,t,J=7.3Hz), 1.61-1.67(2H,m), 2.71-2.73(lH,m), 2.79-
2.82(lH,m), 3.80(2~H,q,J=7.3Hz), 5.48(lH,s),7.21-7.29(2H,m),
7.28(lH,dd,J=7.3Hz and 7.4Hz), 7.55(lH,dd,J=7.3Hz and 7.4Hz),
7.70(lH,d,J=7.3Hz), 9.63(lH,br.s), 12.07(lH,br.s).
46
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 43
Ethyl 4-(2,3-difluorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,3-
difluorobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:207°C.
Anal. Calcd. for:C18H19FZNsOz 1/5 HZO:C,61.60;H,5.57;N,11.97.
Found:C,61.41;H,5.56;N,11.59.
to MS(EI) :347 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.90-0.97(6H,m), 1.60-1.66(2H,m),
2.68-2.71(lH,m),2.79-2.82(lH,m), 3.83(2H,q,J=7.3Hz),
5.45(lH,s), 6.87(lH,dd,J=7.3Hz and 7.4Hz), 7.03-7.13(2H,m),
7.76(lH,s), 9.55(lH,br.s), 12.03(lH,br.s).
~s Example 44
Ethyl 4-(2,3-dichlorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,3-
dichlorobenzaldehyde, 3-aminopyrazole and ethyl 3
2o ketohexanoate in the same manner as in Example 25.
MP:220°C.
Anal. Calcd. for:C18H19C1zN30a:C,56.85;H,5.04;N,11.05.
Found:C,56.35;H,5.00;N,11.01.
MS(EI) :380 (M+) .
25 sH-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.3Hz),
0.99(3H,t,J=7.3Hz), 1.66-1.69(2H,m), 2.74-2.77(lH,m), 2.82-
2.86(lH,m), 3.81(2H,q,J=7.3Hz), 5.66(lH,s), 7.10(lH,d,J=7.3Hz),
7.24(lH,dd,J=7.3Hz and 7.4Hz), 7.31(lH,s), 7.38(lH,d,J=7.3Hz),
9.59(lH,br.s), 12.04(lH,br.s).
3o Example 45
Ethyl 4-(3-fluoro-2-methylphenyl)-4,7-dihydro-6-propyl-2H-,
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 3-fluoro-2-
47
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
methylbenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:159-162°C.
Anal. Calcd. for:C19HZ2FN303 3/10 HZO:C,65.42;H,6.53;N,12.05.
s Found:C,65.56;H,6.29;N,12.40.
MS(EI) :343 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89 (3H,t,J=7.3Hz), 0.97
(3H,t,J=7.3Hz), 1.64(2H,m), 2.36(3H,s), 2.67-2.84(2H,m),
3.80(2H,q,J=7.3Hz), 5.35(lH,s), 6.86(2H,d,J=8.8Hz),
zo 7.07(lH,dd,J=7.3Hz and 7.4Hz), 7.23(lH,s), 9.42(lH,br.s),
11.94(lH,br.s).
IR(KBr):v=3265,3193,2966,2934,1668,1591,1520,1466,1240cm 1.
Example 46
Ethyl 4-(2,3-dimethoxyphenyl)-4,7-dihydro-6-propyl-2H-
ls pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,3-
dimethoxyben,zaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:205-206°C.
ao Anal. Calcd. for:C2oHZ5N304:C, 64.67; H, 6.78;N,11.31.
Found:C,64.76;H,6.81;N,11.15.
MS(EI):371(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90(3H,t,J=7.4Hz),
0.98(3H,t,J=7:3Hz), 1.66-1.68(2H,m), 2.68-2.70(lH,m), 2.80-
as 2.83(lH,m), 3.77(3H,s), 3.80(3H,s), 3.80-3.85(2H,m),~
5.44(lH,s), 6.58(lH,d,J=7.3Hz), 6.76(lH,d,J=6.8Hz),
6.88(lH,dd,J=7.3Hz and 7.4Hz),7.11(lH,s), 9.32(lH,br.s),
11.83(lH,br.s).
Example 47
3o Ethyl 4-(2-chloro-3-trifluoromethylphenyl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-chloro-3-
trifluoromethylbenzaldehyde, 3-aminopyrazole and ethyl 3-
48
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
ketohexanoate in the same manner as in Example 25.
MP:236-238°C.
Anal. Calcd. for:C19H19C1F3N3OZ:C,55.15;H,4.63;N,10.15.
Found: C,55.07;H,4.55;N,10.13.
s MS(EI) :413(M'~) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.82(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.65(2H,m), 2.70-2.90(2H,m), 3.65-
3.85(2H,m), 5.72(lH,s), 7.29(lH,s), 7.41-7.42(2H,m), 7.59-
7.61(lH,m), 9.62(lH,br.s), 12.05(lH,br.s).
.t o Example 48
Ethyl 4-(2-chloro-4-fluorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-chloro-4-
fluorobenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
Is in the same manner as in Example 25.
MS(EI) :363(M~) .
1H-NMF. (400MHz,DMSO-ds)8(ppm): 0.88(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz), 1.62-1.67(2H,m), 2.&6-2.80(2H,m),
3.77(2H,q,J=7.3Hz), 5.54(lH,s),7.08-7.13(2H,m), 7.25(lH,s),
ao 7.32(lH,dd,J=2.5Hz and 8.8Hz), 9.53(lH,br.s), 11.99(lH,br.s).
Example 49
Ethyl 4-(2,5-difluorophenyl)-4,7-dihydro-6-propyl=2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,5-
25 difluorobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:168-169°C.
MS(EI) :347 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.92-0.99(6H,m), 1.62-1.68(2H,m),
30 2.67-2.71(lH,m), 2.85-2.88(lH,m), 3.80-3.91(2H,m),
4.03(lH,q,J=6.8Hz), 5.40(lH,s), 6.77-6.80(lH,m), 6.98-
7.00(lH,m), 7.12-7.16(lH,m), 7.26(lH,s), 9.59(lH,br.s),
12.06(lH,br.s).
49
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 50
Ethyl 4-(2,5-dichlorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,5-
dichlorobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:162°C.
Anal. Calcd. for:C~8H1gC1zN~02 1/2 HzO:C,55.54;H,5.18;N,10.79.
Found:C,55.50;H,5.50;N,11~17.
1o MS(EI):380(M~).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89(3H,t,J=7.3Hz),
0.98(3H,t,J=7.3Hz), 1.62-1.66(2H,m), 2.64-2.67(lH,m), 2.86-
2.90(lH,m), 3.81(2H,q,J=7.3Hz), 5.55(lH,s), 7.04(lH,s),
7.18(lH,d,J=7.3Hz), 7.28(lH,s), 7.41(lH,d,J=7.3Hz),
s5 9.61(lH,br.s), 12.06(lH,br.s).
Example 51
Ethyl 4-(5-fluoro-2-methoxyphenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 5-fluoro-2-
2o methoxybenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:164-167°C.
Anal. Calcd. for:C1gH22FN3O3: C, 63.50;H, 6.17;N,11.69.
Found:C,63.24;H,6.09;N,11.70.
2~ MS(EI):359(Mk).
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.87(3H,t,J=7.3Hz),
0.98(3H,t,J=7.3Hz), 1.64-1.69(2H,m), 2.62-2.91(2H,m),
3.79(2H,q,J=7.3Hz), 3.85(3H,s), 5.44(lH,s), 6.33(lH,dd,J=3.OHz
and 7.8Hz), 6.83-6.91(2H,m), 7.17(lH,s), 9.41(lH,br.s),
30 11.89(lH,br.s).
IR(KBr):v=3252,2955,1657,1510,1232,1074ciri1.
Example 52
Ethyl 4-(2-chloro-5-methoxyphenyl)-4,7-dihydro-6-propyl-2H-
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-chloro-5-
methoxybenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:182°C.
Anal. Calcd. for:Cl9HZZC1N303:C,60.72;H,5.90;N,11.18.
Found:C,60.58;H,5.88;N,11.07.
MS(EI) :375 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.3Hz),
l0 0.99(3H,t,J=7.3Hz), 1.64-1.69(2H,m), 2.64-2.67(lH,m), 2.87-
2.90(lH,m), 3.79(2H,q,J=7.3Hz), 3.86(3H,s), 5.44(lH,s),
6.85(lH,d,J=7.3Hz), 6.94(lH,d,J=7.3Hz), 7.10(lH,dd,J=2.9Hz and
7.3Hz), 7.17(lH,s), 9.43(lH,br.s), 11.91(lH,br.s).
Example 53
.ts Ethyl 4-(2,5-dimethoxyphenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,5-
dimethoxybenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
zo MP:169-170°C.
MS (EI ) : 371 (M~) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.3Hz),
0.98(3H,t,J=7.3Hz), 1.68-1.70(2H,m), 2.49-2.54(lH,m), 2.94-
2.97(lH,m), 3.57(3H,s), 3.79-3.83(2H,m), 3.80(3H,s),
25 4.02(lH,q,J=7.3Hz), 5.43(lH,s), 6.49(lH,d,J=2.9Hz),
6.59(lH,dd,J=2.9Hz and 8.8Hz), 6.82(lH,d,J=8.8Hz), 7.14(lH,s),
9.32(lH,br.s), 11.83(lH,br.s).
Example 54
Ethyl 4-(2,6-difluorophenyl)-4,7-dihydro-6-propyl-2H-
3o pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,6-
difluorobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
51
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:185°C.
Anal. Calcd. for:C18H19FzN3OZ 1/2 HzO:C, 60.67;H, 5.66;N, 11.79.
Found:C,60.68;H,5.46;N,11.61.
MS(EI) :347 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90-0.97(6H,m), 1.54-1.58(2H,m),
2.51-2.54(lH,m), 2.76-2.81(lH,m), 3.82(2H,q,J=7.3Hz),
5.53(lH,s), 6.90(2H,dd,J=7.3Hz and 7.3Hz), 7.16(lH,d,J=7.3Hz),
7.20(lH,s), 9.50(lH,br.s), 11.96(lH,br.s).
Example 55
to Ethyl 4-(2,6-dichlorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,6-
dichlorobenzaldehyde and ethyl 3-ketohexanoate in the same
manner as in Example 39.
.ts MP:202°C.
Anal. Calcd. for:C18H1gC1zN302 3/10 HZO:C,56.06;H,5.12;N,10.90.
Found:C,56.28;H,5.46;N,10.78.
MS(EI) :380 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.83(3H,t,J=7.3Hz),
20 0.92(3H,t,J=7.3Hz), 1.57-1.62(2H,m), 2.47-2.51(lH,m), 2.77-
2.80(lH,m), 3.74(2H,q,J=7.3Hz), 6.03(lH,s), 7.05(lH,s),
7.13(lH,dd,J=7.3Hz and 7.4Hz), 7.22(lH,d,J=7.3Hz),
7.39(lH,d,J=7.3Hz), 9.53(lH,br.s), 11.93(lH,br.s).
Example 5f
25 Ethyl 4-(2-chloro-6-fluorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-chloro-6-
fluorobenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
3o MP:180-183°C.
MS(EI) :363(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92(3H,t,J=6.9Hz),
0.94(3H,t,J=7.3Hz), 1.56-1.61(2H,m), 2.50-2.85(2H,m),
52
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.80(2H,q,J=7.3Hz), 5.75(lH,s), 7.01-7.17(4H,m), 9.52(lH,br.s),
11.97(lH,br.s).
IR(KBr):v=3265,1591,1518,1456,1228,1097cm 1.
Example 57
s Ethyl 4,7-dihydro-6-propyl-4-(pyridin-3-yl)-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate dihydrocloride
The title compound was prepared from pyridine-3-aldehyde
and ethyl 3-ketohexanoate in the same manner as in Example 39.
MP:251°C.
to Anal. Calcd. for:Cl7HzoN40z~2HC1:C,52.99;H,5.76;N,14.54.
Found:C,52.99;H,5.67;N,14.44.
MS(EI):312(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.91(3H,t,J=7.3Hz),
1.09(3H,t,J=7.3Hz), 1.52-1.61(2H,m), 2.66-2.71(2H,m), 3.93-
.15 4.00(2H,m), 5.24(lH,s), 7.90(lH,dd,J=7.3Hz and 7.4Hz), 8.31-
8.35(2H,m), 8.66-8.69(2H,m), 10.35(lH,br.s).
Example 58
Ethyl 4,7-dihydro-6-propyl-4-(pyridin-4-yl)-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate dihydrocloride
ao The title compound was prepared from pyridine-4-aldehyde
and ethyl 3-ketohexanoate in the same manner as in Example 39.
MP:266°C.
Anal. Calcd. for:C17H2oN402 2HCl:C,52.99;H,5.76;N,14.54.
Found:C,52.63;H,5.65;N,14.69.
2s MS(EI) :312 (M+)
1H-NMR (400MHz,DMSO-d6)S(ppm): 0.91(3H,t,J=7.3Hz),
1.12(3H,t,J=7.3Hz), 1.52-1.59(2H,m), 2.64-2.72(2H,m),
4.01(2H,q,J=7.3Hz), 5.30(lH,s), 7.76(2H,d,J=6.4Hz), 8.66(lH,s),
8.72(2H,d,J=6.4Hz), 10.39(lH,br.s).
3o Example 59
Ethyl 4-(furan-2-yl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate maleate
The title compound was prepared from furan-2-aldehyde,
53
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3-aminopyrazole and ethyl 3-ketohexanoate in the same manner
as in Example 25.
MP :108-111°C .
MS(EI) :301 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92(3H,t,J=7.3Hz),
1.05(3H,t,J=6.8Hz), 1.58(2H,q,J=7.3Hz), 2.66-2.72(2H,m),
3.50(3H,br.s), 3.94(2H,q,J=6.8Hz),5.21(lH,s),5.78(lH,d,
J=2.9Hz),6.23(lH,s), 6.24(2H,s), 7.75(lH,s), 7.38(lH,s),
9.42(lH,br.s).
.to IR(KBr):v=3207,2962,1703,1479,1348,1205,1076,866ciri1.
Example 60
Ethyl 4-(furan-3-yl)-4,7-dihydro-4-(furan-3-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate maleate
The title compound was prepared from furan-3-aldehyde,
3-aminopyrazole and ethyl 3-ketohexanoate in the same manner
as in Example 25.
MP:121-123°C.
Anal. Calcd. for:C16H19N3~3C4H4~4~C~57.54;H,5.55;N,10.07.
Found:C,57.14;H,5.55;N,10.37.
2o MS(EI) :301 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90(3H,t,J=7.3Hz),
1.08(3H,t,J=7.4Hz), 1.55-1.57(2H,m), 2.62-2.70(2H,m),
3.36(lH,br.s), 3.50(2H,br.s), 3.97(2H,q,J=7.3Hz), 5.06(lH,s),
6.16(lH,s), 6.24(2H,s), 7.13(lH,s), 7.35(lH,s), 7.40(lH,s),
z5 9.31(lH,br.s).
IR(KBr):v=3350,2972,1591,1467,1361,1203,1089cm 1.
Example 61
Ethyl 4,7-dihydro-4-(2-methylfuran-3-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
3o The title compound was prepared from 2-methylfuran-3-
aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
MP:123-125°C.
54
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C17Hz1N3O3 2/5 HaO:C, 63.30;H, 6 .81;N,13 .03.
Found: C,63.51;H,6.64;N,12.96.
MS(ET) :315 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.93(3H,t,J=7.3Hz),
s 1.08(3H,t,J=7.3Hz), 1.58-1.60(2H,m), 2.20(3H,s), 2.55-
2.75(2H,m), 3.92(2H,q,J=7.3Hz), 4.99(lH,s), 5.96(lH,s),
7.21(2H,s), 9.26(lH,br.s), 11.91(lH,br.s).
IR(KBr):v=3265,3198,2964,1591,1514,1224,1209,1093cm~'1.
Example 62
Io Ethyl 4,7-dihydro-6-propyl-4-(thiophen-2-yl)-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate maleate
The title compound was prepared from thiophene-2-
aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
15 MP:129-131°C.
Anal. Calcd. for:C16H1gN3OaSC4H4O4 1/4 HZO: C, 54.85; H, 5 . 41;N, 9 .59 .
Found:C,54.59;H,5.22;N,9.97.
MS (EI ) : 317 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92(3H,t,J=7.4Hz),
2o 1.06(3H,t,J=7.3Hz), 1.58-1.60(2H,m), 2.72-2.74(2H,m),
3.50(3H,br.s), 3.94(2H,q,J=7.4Hz), 5.44(lH,s), 6.25(2H,s),
6.69(lH,s), 6.81(lH,d), 7.15(lH,d), 7.37(lH,s), 9.50(lH,br.s).
Example 63
Ethyl 4,7-dihydro-4-(3-methylthiophen-2-yl)-6-propyl-2H-
as pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 3-methylthiophene-
2-aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
MP:125°C.
3o Anal. Calcd. for:C17Hz1N30zS Hao: C,58.43;H,6.63;N,12.02.
Found: C,58.59;H,6.33;N,12.12.
MS(EI) :331(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.96(3H,t,J=7.4Hz),
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
0.98(3H,t,J=7.3Hz), 1.60-1.63(2H,m), 2.22(3H,s), 2.83-
2.90(2H,m), 3.88(2H,q,J=7.3Hz), 5.42(lH,s), 6.68(lH,d,J=4.9Hz),
7.02(lH,d,J=5.4Hz), 7.29(lH,s), 9.45(lH,br.s), 11.98(lH,br.s).
IR(KBr):v=3267,3196,2968,1664,1510,1267,1201,1091cm1.
s Example 64
Ethyl 4-(5-chlorothiophen-2-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate maleate
The title compound was prepared from 5-chlorothiophene-
2-aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
zo same manner as in Example 25.
MP:129-131°C.
Anal. Calcd. for:C16H18N302SC4H4O4:C, 51.33;H, 4.74;N, 8.98 .
Found:C,51.34;H,4.54;N,9.03.
MS(EI) :351(M+) .
15 ~H-NMR (400MHz,DMSO-d6)b(ppm): 0.92(3H,t,J=7.3Hz),
1.10(3H,t,J=6.9Hz), 1.59-1.61(2H,m), 2.57-2.82(2H,m),
3.50(2H,br.s), 3.38(lH,s), 3.98(2H,q,J=6.9Hz), 5.36(lH,s),
6.25(2H,s), 6.53(lH,d,J=3.9Hz), 6.80(lH,d,J=3.4Hz), 7.42(lH,s),
9.60(lH,br.s).
20 IR(KBr):v=3205,2964,2629,1618,1471,1363,1205,1080,889,652ciri1.
Example 65
Ethyl 4,7-dihydro-6-propyl-4-(thiophen-3-yl)-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate maleate
The title compound was prepared from thiophene-3-
z5 aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
MP:141-143°C.
Anal. Calcd. for:C16H19N3OZSC4H4O4:C,54.42;H,5.35;N,9.69.
Found:C,54.17;H,5.23;N,9.66.
3o MS(EI):317(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.92(3H,t,J=7.3Hz),
1.03(3H,t,J=6.8Hz), 1.59-1.61(2H,m), 2.60-2.78(2H,m),
3.50(2H,br.s), 3.91(2H,q,J=6.8Hz), 5.22(2H,s), 6.26(2H,s),
56
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
6.84-6.88(2H,m), 7.29(lH,dd,J=3.OHz and 4.9Hz), 7.33(lH,s),
12.0(lH,br.s).
IR(KBr):v=3346,2980,2611,1697,1467,1361,1205,1087cm 1.
Example 66
Ethyl 4,7-dihydro-4-(naphthalen-1-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from naphthalene-1-
aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
so MP:182°C.
Anal. Calcd. for:Ca2Ha3N302:C, 73.11;H,6.41;N,11.63.
Found:C,72.95;H,6.47;N,11.40.
MS(EI) :361(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.62(3H,t,J=7.3Hz),
z5 1.00(3H,t,J=7.3Hz), 1.69-1.73(2H,m), 2.73-2.76(lH,m), 2.84-
2.87(lH,m), 3.67(2H,q,J=7.3Hz), 5.95(lH,s), 7.03(lH,s),
7.23(lH,d,J=7.3Hz), 7.36(lH,dd,J=7.3Hz and 7.4Hz),
7.49(lH,dd,J=7.3Hz and 7.4Hz), 7.57(lH,dd,J=7.3Hz and 7.4Hz),
7.65(lH,d,J=7.3Hz), 7.88(lH,d,J=7.3Hz), 8.40(lH,d,J=7.3Hz),
~0 9.45(lH,br.s), 11.82(lH,br.s).
Example 67
Ethyl 4,7-dihydro-4-(naphthalen-2-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate maleate
The title compound was prepared from naphthalene-2-
25 aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
MP:136-138°C.
Anal. Calcd. for:Cz2Hz3N3O2C4H4O4 1/4 HzO:C,64.79;H,5.75;N,8.72.
Found:C,64.86;H,5.57;N,8.99.
3o MS(EI):361(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92-0.98(6H,m), 1.64-1.68(2H,m),
2.72-2.80(2H,m), 3.50(2H,br.s), 3.80(2H,q,J=7.3Hz), 5.27(lH,s),
6.25(2H,s),7.23(lH,s), 7.31(lH,d,J=8.3Hz), 7.41-7.43(2H,m),
57
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.57(lH,s), 7.73-7.77(2H,m), 9.47(lH,br.s).
IR(KBr):v=3202,2962,1701,1464,1359,1222cm 1.
Example 68
Ethyl 4,7-dihydro-4-(2-methoxynaphthalen-1-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
methoxynaphthalene-1-aldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:188-191°C.
io Anal. Calcd. for:Cz3H25N303 2/5 HzO:C, 69.29;H, 6.52;N,10.54.
Found:C,69.35;H,6.62;N,10.21.
MS(EI) :391(M+) .
1H-NMR (400MHz,DMSO-ds)b(ppm): 0.71(3H,t,J=7.3Hz),
0.95(3H,t,J=7.3Hz), 1.62-1.63(2H,m), 2.49-2.86(2H,m),
3.61(2H,q,J=7.3Hz), 3.97(3H,s), 6.27(lH,s), 6.89(lH,s), 7.16-
7.51(3H,m), 7.71-7.77(2H,m), 7.98(lH,s), 9.43(lH,br.s),
11.77(lH,br.s).
IR(KBr):v=3258,1655,1593,1082cm 1.
Example 69
2o Ethyl 4-(2,3-dihydobenzo[b]furan-7-yl)-4,7-dihydro-6-propyl-
2H- pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,3-
dihydobenzo[b]furan-7-aldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
2s MP:194-196°C.
Anal. Calcd. for:CZOHa3N303:C, 67.97;H, 6.56;N, 11.89.
Found:C,67.97;H,6.68;N,11.77.
MS(EI) :353 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.92(3H,t,J=7.4Hz),
30 0.97(3H,t,J=7.3Hz), 1.66(2H,m), 2.67-2.70(lH,m), 2.82-
2.84(lH,m), 3.15(2H,t,J=8.8Hz), 3.83-3.86(2H,m), 4.55-
4.58(2H,m), 5.29(lH,s), 6.64(lH,dd,J=7.3Hz and 7.4Hz),
6.72(lH,d,J=6.9Hz), 6.93(lH,dd,J=7.3Hz and 7.4Hz), 7.20(lH,s),
58
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
9.32(lH,br.s), 11.86(lH,br.s).
Example 70
Ethyl 4-(5-bromo-2,3-dihydobenzo[b]furan-7-yl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 5-bromo-2,3-
dihydobenzo[b]furan-7-aldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP : 200-210°C .
Anal. Calcd. for:C2oHzzBrN303: C,55.57;H,5.13;N,9.72.
To Found:C,55.23;H,5.09;N,9.89.
MS(EI) :432(M~) .
~H-NMR (400MHz,DMSO-d6)8(ppm): 0.93-0.98(6H,m),
1.64(2H,q,J=7.3Hz), 2.63(lH,m), 2.88-2.90(lH,m},
3.16(2H,t,J=8.3Hz), 3.85-3.87(2H,m), 4.57-4.60(2H,m),
is 5.23(lH,s), 6.78(lH,s), 7.11(lH,s), 7.22(lH,s), 9.44(lH,br.s),
11.94(lH,br.s).
Example 71
Ethyl 4-(5-chloro-2,3-dihydo-2-methylbenzo[b]furan-7-yl)-4,7-
dihydro-6-propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
zo maleate
The title compound was prepared from 5-chloro-2,3-
dihydo-2-methylbenzo[b]furan-7-aldehyde, 3-aminopyrazole and
ethyl 3-ketohexanoate in the same manner as in Example 25.
MP:155-158°C.
2s Anal. Calcd. for:CzlHz4N3O3C4H4O4:C,57.95;H,5.45;N,8.11.
Found:C,57.57;H,5.28;N,8.47.
MS(EI) :401 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(6H,t,J=6.8Hz),
1.03(3H,d,J=6.3Hz), 1.65(2H,m), 2.40-2.73(2H,m), 2.87(lH,m),
30 3.29(lH,m), 3.50(3H,br.s), 3.84(2H,q,J=6.8Hz), 5.05(lH,m),
5.23(lH,s), 6.25(2H,s), 6.64(lH,s), 6.95(lH,s),
7.20(lH,d,J=4.4Hz}, 9.43(lH,br.s}.
IR(KBr):v=3207,2976,1589,1462,1201,1082cni1.
59
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 72
Ethyl 4-(2H-1-benzopyran-8-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2H-1-benzopyran-8-
aldehyde and ethyl 3-ketohexanoate in the same manner as in
Example 39.
MP:194°C.
Anal. Calcd. for:Cz1H23N3~3:C,69.02;H,6.34;N,11.50.
Found:C,68.60;H,6.43;N,11.25.
1o MS(EI) :194 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz), 1.64-1.68(2H,m), 2.62-2.66(lH,m), 2.80-
2.84(lH,m), 3.81(2H,q,J=7.3Hz), 4.85(2H,dd,J=2.OHz and 9.8Hz),
5.39(lH,s), 5.89(lH,d,J=9.8Hz), 6.46(lH,d,J=9.8Hz), 6.67-
6.80(3H,m), 7.18(lH,s), 9.31(lH,br.s), 11.86(lH,br.s).
Example 73
Ethyl 4-(3,4-dihydro-2H-benzopyran-8-yl)-4,7-dihydro-6-propyl-
2H- pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 3,4-dihydro-2H-
2o benzopyran-8-aldehyde and ethyl 3-ketohexanoate in the same
manner as in Example 39.
MP:208°C.
Anal. Calcd. for:C21H25N3~3 1/2 HzO:C,67.O1;H,6.96;N,11.16.
Found:C,67.41;H,6.84;N,10.93.
MS(EI) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.63-1.68(2H,m), 1.92-1.96(2H,m), 2.67-
2.82(4H,m), 3.80(2H,q,J=7.3Hz), 4.22-4.26(2H,m), 5.41(lH,s),
6.61(lH,dd,J=7.3Hz and 7.4Hz), 6.71-6.75(2H,m), 7.17(lH,s),
~0 9.25(lH,br.s), 11.80(lH,br.s).
Example 74
Ethyl 4,7-dihydro-6- ropyl-4-(quinolin-4-y1)-2H- yrazolo[3,4-
b]pyridine-5-carboxylate
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from quinoline-4-
aldehyde and ethyl 3-ketohexanoate in the same manner as in
Example 39.
MP:198°C.
Anal. Calcd. for:C21H2aN40z 2/5 HzO:C,68.24;H,6.22;N,15.16.
Found:C,68.39;H,6.04;N,14.83.
MS(EI) :362 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.61(3H,t,J=7.3Hz),
1.02(3H,t,J=7.3Hz), 1.68-1.72(2H,m), 2.76-2.78(lH,m), 2.86-
io 2.89(lH,m), 3.66-3.68(2H,m), 5.97(lH,s), 7.07(lH,s),
7.17(lH,d,J=4.4Hz), 7.65(lH,dd,J=7.3Hz and 7.4Hz),
7.74(lH,dd,J=7.3Hz and 7.4Hz), 7.99(lH,d,J=7.3Hz),
8.48(lH,d,J=7.8Hz), 8.73(lH,d,J=4.4Hz), 9.61(lH,br.s),
11.94(lH,br.s).
I5 Example 75
Ethyl 4-(benzo[b]thiophen-3-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from benzo[b]thiophene-
3-aldehyde and ethyl 3-ketohexanoate in the same manner as in
ao Example 39.
MP:222°C.
Anal. Calcd. for:CzoHa1N302S:C,65.37;H,5.76;N,11.44.
Found:C,65.11;H,5.31;N,11.83.
MS(EI):238(M~).
25 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.64(3H,t,J=7.3Hz),
1.20(3H,t,J=7.3Hz), 1.56-1.58(2H,m), 2.66-2.78(2H,m),
4.11(2H,q,J=7.3Hz), 4.89(lH,s), 7.42-7.50(2H,m), 7.55(lH,s),
7.61(lH,s), 7.96-8.01(2H,m), 10.32(lH,br.s), 12.13(lH,br.s).
Example 76
3o Ethyl 4-(2,1,3-benzoxadiazol-4-yl)-4,7-dihydro-6-propyl-2H-
~yrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,1,3-
benzoxadiazole-4-aldehyde, 3-aminopyrazole and ethyl 3-
61
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
ketohexanoate in the same manner as in Example 39.
MP:207°C.
Anal. Calcd. for:C18H19N503:C,61.18;H,5.42;N,19.82.
Found:C,61.06;H,5.50;N,19.66.
MS(EI) :353 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.77(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 2.72-2.77(lH,m), 2.82-2.86(lH,m),
3.79(2H,q,J=7.3Hz), 5.68(lH,s), 7.11(lH,d,J=7.3Hz), 7.22(lH,s),
7.51(lH,dd,J=7.3Hz and 7.4Hz)~, 7.78(lH,d,J=7.3Hz),
l0 9.66(lH,br.s), 12.01(lH,br.s).
Example 77
Ethyl 4-(1,3-benzdioxazol-4-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 1,3-benzdioxazole-
.t5 4-aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
MP:203-207°C.
Anal. Calcd. for:C19HZ1Ns04 1/10 HaO:C,63.89;H,5.98;N,11.76.
Found:C,63.72;H,5.86;N,12.01.
2o MS(EI) :355(Mk) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.94(3H,t,J=7.3Hz),
0.96(3H,t,J=7.4Hz), 1.61.1.67(2H,m), 2.64-2.82(2H,m), 3.80-
3.88(2H,m), 5.28(lH,s), 5.99(lH,s), 6.00(lH,d,J=9.7Hz),
6.50(lH,d,J=5.9Hz), 6.65(lH,s), 6.65-6.69(lH,m), 7.25(lH,s),
25 9.40(lH,br.s), 11.94(lH,br.s).
IR(KBr):v=3265,3188,2962,1662,1587,1514,1462,1253,1215,1066cm 1.
Example 78
Ethyl 4-(6-chloro-3,4-dihydro-2,2-dimethyl-2H-1,4-benzoxazin-
8-yl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-b]pyridine-5-
3o carboxylate maleate
The title compound was prepared from 6-chloro-3,4-
dihydro-2,2-dimethyl-2H-1,4-benzoxazine-8-aldehyde, 3-
aminopyrazole and ethyl 3-ketohexanoate in the same manner as
62
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
in Example 25.
MS(EI) :430(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92(3H,t,J=7.4Hz),
0.96(3H,t,J=7.3Hz), 1.18(3H,s), 1.32(3H,s), 1.62-1.64(2H,m),
s 2.66-2.82(2H,m), 2.99(2H,s), 3.80(2H,t,J=7.3Hz), 5.32(lH,s),
6.01(2H,s), 6.14(lH,s), 6.32(lH,s), 7.14(lH,s), 9.31(lH,br.s),
11.82(lH,br.s).
IR(KBr):v=3281,2974,1672,1599,1520,1207,1155,1091cm 1.
Example 79
to Ethyl 4-(6-chloro-3,4-dihydro-2,2,4-trimethyl-1,4-benzoxazin-
8-yl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-b]pyridine-5-
carboxylate maleate
The title compound was prepared from 6-chloro-3,4-
dihydro-2,2,4-trimethyl-2H-1,4-benzoxazine-8-aldehyde, 3-
15 aminopyrazole and ethyl 3-ketohexanoate in the same manner as
in Example 25.
MS(EI) :444 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.91(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz), 1.20(6H,s), 1.35(3H,s), 1.63-1.65(2H,m),
20 2.83(2H,s), 3.00(2H,q,J=7.3Hz), 5.34(lH,s), 6.26(2H,s),
6.43(lH,d,J=2.5Hz), 7.13(lH,s), 9.33(lH,s), 11.82(lH,br.s).
IR(KBr):v=3273,2974,1666,1597,1518,1458,1259,1211cni1.
Example 80
Ethyl 4-(2,3-dihydro-1,4-benzodioxin-6-yl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate maleate
The title compound was prepared from 2,3-dihydro-1,4-
benzodioxin-6-aldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:147-149°C.
3o Anal. Calcd. for:C2QH23N3O4C4H4O4:C,59.37;H,5.60;N,8.66.
Found:C,59.12;H,5.63;N,8.57.
MS(EI) :369 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.93(3H,t,J=7.3Hz),
63
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
1.02(3H,t,J=6.8Hz), 1.60(2H,q,J=7.3Hz), 2.64-2.68(2H,m),
3.50(2H,br.s), 3.86(2H,q,J=7.3Hz), 4.14(4H,s), 4.99(lH,s),
6.26(2H,s), 6.54(lH,s), 6.57(lH,d,J=7.8Hz), 6.65(lH,d,J=7.8Hz),
7.21(lH,s), 11.97(lH,br.s).
IR(KBr):v=3211,2694,2878,2658,1697,1506,1466,1363,1302,1082cm ~.
Example 81
Ethyl 4-(benzo[b]furan-2-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate maleate
The title compound was prepared from benzo[b]furan-2-
.to aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
MP:123-125°C.
Anal. Calcd. for:C2oHZ1N3O3C4H4O4 1/2 HZO:C, 61.19;H, 5.43;N, 8.92.
Found:C,61.02;H,5.41;N,9.27.
15 MS(EI):351(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.96(3H,t,J=7.3Hz),
1.02(3H,t,J=6.8Hz), 1.63(2H,q,J=7.3Hz), 2.73-2.76(2H,m),
3.50(3H,br.s), 3.93(2H,q,J=7.3Hz), 5.36(lH,s), 6.24(2H,s),
6.43(lH,s), 7.10-7.21(2H,m), 7.41-7.48(3H,m), 9.51(lH,br.s).
2o IR(KBr):v=3190,3080,2962,1705,1581,1454,1359,1195,883cm~1.
Example 82
Ethyl 4-(2-chlorophenyl)-6-ethyl-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
25 chlorobenzaldehyde, 3-aminopyrazole and ethyl 3-ketopentanoate
in the same manner as in Example 1.
MP:213°C.
Anal. Calcd. for:C1~H18C1N3OZ:C,61.54;H,5.47;N,12.66.
Found:C,61.54;H,5.46;N,12.68.
3o MS(EI) :331(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85(3H,t,J=7.3Hz),
1.21(3H,t,J=7.3Hz), 2.78-2.84(2H,m), 3.78(2H,q,J=7.3Hz),
5.58(lH,s), 7.07-7.12(2H,m), 7.18(lH,dd,J=7.3Hz and
64
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.4Hz),7.25(lH,s), 7.34(lH,d,J=7.3Hz), 9.52(lH,br.s),
11.97(lH,br.s).
Example 83
Ethyl 6-butyl-4-(2-chlorophenyl)-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and ethyl 3-ketoheptanoate
in the same manner as in Example 1.
MP:209°C.
so Anal. Calcd. for:C19HZ2C1N302 1/5 HZO:C, 62.79;H, 6.21;N,11.56.
Found:C,62.78;H,6.11;N,11.45.
MS(EI) :359(M~") .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85(3H,t,J=7.3Hz),
0.92(3H,t,J=7.3Hz), 1.36-1.42(2H,m), 1.60-1.64(2H,m), 2.72-
ss 2.76(lH,m), 2.83-2.86(lH,m), 3.78(2H,q,J=7.3Hz), 5.58(lH,s),
7.07-7.11(2H,m), 7.18(lH,dd,J=7.3Hz and 7.4Hz), 7.24(lH,s),
7.34(lH,d,J=7.3Hz), 9.49(lH,br.s), 11.96(lH,br.s).
Example 84
Methyl 4-(2-chlorophenyl)-4,7-dihydro-6-methoxymethyl-2H-
ao pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and methyl 4-
methoxyacetoacetate in the same manner as in Example 1.
MP:160°C.
25 Anal. Calcd. for:C16H~6C1N3O3:C,57.33;H,4.83;N,12.59.
Found:C,57.53;H,4.86;N,12.58.
MS(EI) :333 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 3.36(3H,s), 3.38(3H,s),
4.67(2H,s), 5.58(lH,s), 7.08-7.13(2H,m), 7.19(lH,dd,J=7.3Hz
3o and 7.4Hz), 7.32-7.36(2H,m), 9.14(lH,br.s), 12.08(lH,br.s).
Example 85
Ethyl 4-(2-chloro henyl)-4,7-dihydro-6-phenyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and ethyl
benzoylacetoacetate in the same manner as in Example 1.
MP:214°C.
Anal. Calcd. for:C21H18C1N30z 3/10 HZO:C,65.47;H,4.87;N,10.91.
Found:C,65.29;H,4.73;N,10.93.
MS (EI ) : 379 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.57(3H,t,J=7.3Hz),
3.52(2H,q,J=7.3Hz), 5.65(lH,s), 7.14(lH,dd,J=7.3Hz and 7.4Hz),
.to 7.27(lH,dd,J=7.3Hz and 7.4Hz), 7.37-7.40(8H,m), 9.53(lH,br.s),
12.04(lH,br.s).
Example 86
Ethyl 4--(2-chlorophenyl)-4,7-dihydro-6-(4-methoxyphenyl)-2H-
pyrazolo[3,4-b~pyridine-5-carboxylate
.t5 The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and ethyl (4-
methoxybenzoyl)acetate in the same manner as in Example 1.
MP:211°C.
Anal. Calcd. for:C~2HzoC1N303:C,64.47;H,4.92;N,10.25.
ao Found:C,64.30;H,5.00;N,10.24.
MS(EI) :409(M~) .
1H-NMR (400MHz,DMSO-d6)~(ppm): 0.64(3H,t,J=7.3Hz),
3.56(2H,q,J=7.3Hz), 3.79(3H,s),5.63(lH,s), 6.95(2H,d,J=7.3Hz),
7.13(lH,dd,J=7.3Hz and 7.4Hz), 7.24-7.38(6H,m), 9.45(lH,br.s),
as 12.03(lH,br.s).
Example 87
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-(thiophen-2-yl)-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
3o chlorobenzaldehyde, 3-aminopyrazole and ethyl (thiophen-2-
carbonyl)acetate in the same manner as in Example 1.
MP:200°C.
Anal. Calcd. for:C19H16C1N30aS:C,59.14;H,4.18;N,10.89.
66
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Found:C,59.04;H,4.31;N,11.14.
MS(EI) :385 (M+) .
iH-NMR (400MHz,DMSO-d6)8(ppm): 1.02(3H,t,J=7.3Hz),
4.04(2H,q,J=7.3Hz), 5.16(lH,s), 6.58(lH,d,J=7.3Hz), 7.18-
7.70(7H,m), 9.60(lH,br.s), 12.74(lH,br.s).
Example 88
Ethyl 6-benzyl-4-(2-chlorophenyl)-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
Io chlorobenzaldehyde, 3-aminopyrazole and ethyl 4-
phenylacetoacetate in the same manner as in Example 1.
MP:247°C.
Anal. Calcd. for:CZZH2oC1N3020 1/5 HZO:C, 66 .48; H, 5 .17;N, 10 .57 .
Found:C,66.30;H,5.17;N,10.37.
15 MS(EI):393(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.81(3H,t,J=7.3Hz),
3.76(2H,q,J=7.3Hz), 4.25(2H,s), 5.65(lH,s), 7.06-7.41(lOH,m),
9.68(lH,br.s), 12.01(lH,br.s).
Example 89
2o Ethyl 6-ethyl-4,7-dihydro-4-(2-methoxyphenyl)-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
methoxybenzaldehyde, 3-aminopyrazole and ethyl 3-
ketopentanoate in the same manner as in Example 1.
2s MP:169°C.
Anal. Calcd. for:C18HZ1N303 3/10 HaO:C,64.97;H,6.54;N,12.63.
Found:C,64.86;H,6.84;N,12.33.
MS (EI ) : 327 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85(3H,t,J=7.3Hz),
30 1.18(3H,t,J=7.3Hz), 2.73-2.76(lH,m),2.81-2.85(lH,m),
3.74(2H,q,J=7.3Hz), 3.85(3H,s), 5.46(lH,s), 6.76(lH,dd,J=7.3Hz
and 7.4Hz), 6.89-6.94(2H,m), 7.04(lH,dd,J=7.3Hz and 7.4Hz),
7.14(lH,s), 9.32(lH,br.s), 11.82(lH,br.s).
67
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 90
Ethyl 6-butyl-4,7-dihydro-4-(2-methoxyphenyl)-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
methoxybenzaldehyde, 3-aminopyrazole and ethyl 3-
ketoheptanoate in the same manner as in Example 1.
MP:190°C.
Anal. Calcd. for:CzoHZ5N3O3 1/2 HzO:C, 65.91;H, 7 .19;N,11.53.
Found:C,65.92;H,7.07;N,11.88.
1o MS(EI) :355 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.85(3H,t,J=7.3Hz),
0.93(3H,t,J=7.3Hz), 1.38-1.44(2H,m), 1.59-1.64(2H,m), 2.64-
2.68(lH,m), 2.85-2.90(lH,m), 3.81(2H,q,J=7.3Hz), 3.85(3H,s),
5.47(lH,s), 6.76(lH,dd,J=7.3Hz and 7.4Hz), 6.89-6.94(2H,m),
7.04(lH,dd,J=7.3Hz and 7.4Hz), 7.14(lH,s), 9.29(lH,br.s),
11.82(lH,br.s).
Example 91
Methyl 4,7-dihydro-6-methoxymethyl-4-(2-methoxyphenyl)-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
~o The title compound was prepared from 2-
methoxybenzaldehyde, 3-aminopyrazole and methyl 4-
methoxyacetoacetate in the same manner as in Example 1.
MP:186°C.
Anal. Calcd. for:C17H19N3O4 1/5 HZO:C,61.32;H,5.87;N,12.62.
z5 Found:C,61.34;H,5.84;N,12.52.
MS(EI) :329 (M+) .
1H-NMR (400MHz,DMSO-ds)8(ppm): 3.36(3H,s), 3.38(3H,s),
3.86(3H,s), 4.68(2H,s), 5.46(lH,s), 6.77(lH,dd,J=7.3Hz and
7.4Hz), 6.90-6.94(2H,m), 7.06(lH,dd,J=7.3Hz and 7.4Hz),
30 7.22(lH,s), 8.94(lH,br.s), 11.94(lH,br.s).
Example 92
Ethyl 4,7-dihydro-4-(2-methoxy~henyl)-6-phenyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
68
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from 2-
methoxybenzaldehyde, 3-aminopyrazole and ethyl benzoylacetate
in the same manner as in Example 1.
MP:195°C.
s Anal. Calcd. for:C22Hz1N3O3:C, 70.38;H,5.64;N,11.19.
Found:C,70.41;H,5.71;N,11.27.
MS(EI) :375 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.55(3H,t,J=7.3Hz),
3.53(2H,q,J=7.3Hz), 3.88(3H,s), 5.52(lH,s), 6.84(lH,dd,J=7.3Hz
to and 7.4Hz), 6.94(lH,d,J=7.3Hz), 7.09(lH,dd,J=7.3Hz and 7.4Hz),
7.18(lH,d,J=7.3Hz), 7.23(lH,s), 7.37-7.40(5H,m), 9.33(lH,br.s),
11.90(lH,br.s).
Example 93
4-(2-Chlorophenyl)-4,7-dihydro-5-vitro-6-propyl-2H-
Is pyrazolo[3,4-b]pyridine
To an aqueous solution (50 mL) of nitromethane (50 g)
was added an aqueous solution (50 mL) of n-butylaldehyde (59
g), and the mixture was stirred with heating at 60°C for 6
hours. The reaction mixture was allowed to cool to ambient
ao temperature, and extracted with ethyl acetate. The solvent was
evaporated under reduced pressure to give a brown oil (58 g).
To a mixed solution of the obtained oil (50 g) in water (50
mL) and acetone (50 mL) was added sodium chromate (70 g).
Under ice-cooling, concentrated sulfuric acid (46 mL) was
zs added dropwise and the mixture was stirred for 5 hours. Ice-
water {200 mL) was added and the mixture was extracted with
ethyl acetate. The solvent was evaporated under reduced
pressure and the residue was purified by silica gel column
chromatography (eluent: hexane-ethyl acetate (10:1)) to give
30 1-nitropentan-2-one (40 g) as a brown oil. A solution of 2-
chlorobenzaldehyde (1.8 g), 3-aminopyrazole (1.0 g) and 1-
nitropentan-2-one (1.4 g) in acetonitrile (20 mL) was heated
under reflux overnight. The reaction mixture was cooled to
69
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
room temperature, and the solvent was evaporated under reduced
pressure to give an oil. The obtained oil was purified by
silica gel column chromatography (eluent: hexane-ethyl acetate
(8:2)) to give the title compound (680 mg) as yellow crystals.
MP:228°C.
Anal. Calcd. for:C~5H15C1N40z:C,56.52;H,4.74;N,17.58.
Found:C,56.26;H,4.91;N,17.64.
MS(EI):318(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.02(3H,t,J=7.3Hz),1.70-
.to 1.73(2H,m), 2.89-2.91(lH,m), 2.99-3.02(lH,m), 5.90(lH,s),
7.09-7.21(3H,m), 7.39(lH,d,J=7.3Hz), 7.44(lH,s),
10.84(lH,br.s), 12.43(lH,br.s).
Example 94
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
To a solution of acetonitrile (4.8 g) in THF (150 mL)
was added n-BuLi (67 mmol) at -78°C. Further, methyl butanoate
(10 g) was added and the mixture was stirred for one hour.
After acidification with hydrochloric acid, the mixture was
~o extracted with ethyl acetate. The solvent was evaporated under
reduced pressure and the residue was purified by silica gel
column chromatography (eluent: hexane-ethyl acetate (10:1)) to
give 1-cyanopentan-2-one (5.5 g) as a colorless oil. A
solution of 2-chlorobenzaldehyde (1.9 g), 3-aminopyrazole (1.0
a5 g) and 1-cyanopentan-2-one (1.6 g) in acetonitrile (20 mL) was
heated under reflux overnight. The reaction mixture was cooled
to room temperature, and the precipitated crystals were
collected by filtration to give the title compound (1.3 g) as
colorless crystals.
3o MP:248°C.
Anal. Calcd. for:Cl6HisC1N4:C,64.32;H,5.06;N,18.75.
Found:C,64.49;H,5.18;N,18.81.
MS (EI ) :298 (M+) .
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.95(3H,t,J=7.3Hz), 1.64-
1.70(2H,m), 2.38-2.42(2H,m), 5.36(lH,s), 7.23-7.26(3H,m),
7.32(lH,dd,J=7.3Hz and 7.4Hz), 7.42(lH,d,J=7.3Hz),
9.83(lH,br.s), 12.15(lH,br.s).
Example 95
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-(4-methoxyphenyl)-2H-
pyrazolo[3,4-b]pyridine
To a solution of acetonitrile (76 g) in DMSO (100 mL)
was added methyl p-anisate (100 g) and the mixture was stirred
.to with heating at 60°C for one hour. The reaction mixture was
allowed to cool, and cold water (500 mL) was added dropwise.
The mixture was acidified with hydrochloric acid and the
precipitated crystals were collected by filtration. The
obtained crystals were extracted with ethyl acetate and the
.t5 solvent was evaporated under reduced pressure. The residue was
recrystallized from ethyl acetate to give benzoylacetonitrile
(60 g) as colorless crystals. A solution of 2-
chlorobenzaldehyde (1.7 g), 3-aminopyrazole (1.0 g) and
benzoylacetonitrile (1.8 g) in acetonitrile (20 mL) was heated
ao under reflux overnight. The reaction mixture was cooled to
room temperature, and the precipitated crystals were collected
by filtration to give the title compound (2.63 g) as colorless
crystals.
MP:124°C.
25 Anal. Calcd. for:Ca0H15C1N40 8/5 HZO:C,61.34;H,4.68;N,14.31.
Found:C,61.32;H,4.88;N,14.31.
MS(EI) :3&2(M~) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.81(3H,s), 5.48(lH,s),
7.04(2H,d,J=7.3Hz), 7.26(lH,dd,J=7.3Hz and 7.4Hz), 7.32(lH,s),
30 7.35-7.39(4H,m), 7.45(lH,d,J=7.3Hz), 9.99(lH,br.s),
12.22(lH,br.s).
Example 96
4-(2-Chlorophenyl)-2,4,7,8-tetrahydrofurano[3,4-
71
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
b)pyrazolo[4,3-a]pyridin-5-one
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and ethyl 4-
chloroacetoacetate in the same manner as in Example 1.
MP:>270°C.
Anal. Calcd. for:C14H10C1N3O2 2/5 HZO:C,57.02;H,3.69;N,14.25.
Found:C,57.13;H,3.39;N,14.38.
MS(FAB):288(M++1).
1H-NMR (400MHz,DMSO-d6)8(ppm): 4.91(2H,dd,J=5.6Hz and 26.6Hz),
Io 5.34(lH,s), 7.15-7.24(3H,m}, 7.34(lH,s}, 7.41(lH,d,J=6.8Hz),
10.31(lH,br.s}, 12.20(lH,br.s}.
IR(KBr):v=3167,2966,1722,1637,1608,1510,1026ciri1
Example 97
5'-Ethoxycarbonyl-4',7'-dihydro-6'-propyl-
z5 spiro[benzo[b]thiophene-3(2H),4'-2'H-pyrazolo[3,4-b]pyridine]-
5-oxide
A solution of 2-methylthiobenzaldehyde (62 g), Meldrum's
acid (58.7 g), ethyl 3-ketohexanoate (64.4 g) and ammonium
acetate (40 g) in acetic acid (400 mL) was heated under reflux
zo overnight. After the solution was cooled to room temperature,
the solvent was evaporated under reduced pressure to give
colorless crystals (40.2 g). To a solution of
dimethylformamide (26.3 g) in chloroform (100 mL) were added,
under ice-cooling, phosphorus oxychloride (33.6 mL) and a
as solution of the obtained colorless crystals (30 g) in
chloroform (200 mL), and the mixture was stirred overnight.
Under ice-cooling, an aqueous sodium acetate (370 g) solution
was added and the mixture was stirred for one hour. The
reaction mixture was extracted with chloroform and the solvent
3o was evaporated under reduced pressure to give an oil. The oil
was purified by silica gel column chromatography (eluent:
hexane-ethyl acetate (8:2)) to give colorless crystals. To a
solution of the obtained crystals in acetone (500 mL) was
72
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
added diammonium cerium nitrate (42 g) and the mixture was
stirred for 30 minutes. The solvent was evaporated under
reduced pressure and the residue was extracted with ethyl
acetate. The solvent was evaporated under reduced pressure to
give colorless crystals. To a solution of the obtained
colorless crystals in tetrahydrofuran (500 mL) was added
metachloroperbenzoic acid (12 g) at -78°C and the mixture was
stirred for 30 minutes. An aqueous sodium thiosulfate solution
was added, and the mixture was extracted with chloroform. The
io solvent was evaporated under reduced pressure to give
colorless crystals. By recrystallization from ethyl acetate,
colorless crystals (15 g) were obtained. To a solution of the
obtained colorless crystals in tetrahydrofuran {100 mL) was
added lithium diisopropylamide (2.5 eq.) at -78°C. Immediately
thereafter, methanol and an aqueous ammonium chloride solution
were added. The mixture was extracted with chloroform and the
solvent was evaporated under reduced pressure to give an oil.
To a solution of the obtained oil in pyridine (50 mL) was
added hydrazine (4.2 g) and the mixture was stirred with
ao heating for 2 hours. The reaction mixture was cooled to room
temperature, and the solvent was evaporated under reduced
pressure to give an oil. The oil was purified by silica gel
column chromatography (eluent: hexane-ethyl acetate (1:1)) to
give title compound (0.8 g) as colorless crystals.
MP:246°C.
.Anal. Calcd. for:C19H2,,N3O3S :C, 61.44;H, 5 .70;N,11.31.
Found:C,61.58;H,5.81;N,11.16.
MS(EI):371(M+).
1H-NMR {400MHz,DMSO-d6)8(ppm): 0.70(3H,t,J=7.3Hz),
so 0.96(3H,t,J=7.3Hz), 1.63-1.68(2H,m), 2.67-2.76(2H,m),
3.07(lH,d,J=14.9Hz), 3.64(2H,q,J=7.3Hz), 4.00(lH,d,J=14.9Hz),
7.05-7.09(2H,m), 7.40(lH,dd,J=7.3Hz and 7.4Hz),
7.50(lH,dd,J=7.3Hz and 7.4Hz), 7.81(lH,d,J=7.3Hz),
73
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
9.83(lH,br.s), 12.11(lH,br.s).
Example 98
Ether 4,7-dihydro-4-(2-hydroxyphenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
s A solution of 2-methoxybenzaldehyde (15 g), Meldrum's
acid (16 g), ethyl 3-ketohexanoate (17.4 g) and ammonium
acetate (9.4 g) in acetic acid (110 mL) was heated under
reflux overnight. The reaction mixture was cooled to room
temperature, and the solvent was evaporated under reduced
to pressure to give colorless crystals (8.0 g). To a solution of
the obtained colorless crystals (5.2 g) in dichloromethane
(150 mL) were added ethanedithiol (20 mL) and aluminum
chloride (32 g), and the mixture was stirred for 2 hours.
After neutralization with 1N aqueous sodium hydroxide solution,
15 the mixture was extracted with chloroform. The solvent was
evaporated under reduced pressure to give an oil. The obtained
oil was purified by silica gel column chromatography (eluent:
hexane-ethyl acetate (8:2)) to give colorless crystals (2.0 g).
To a solution of dimethylformamide (1.9 g) in chloroform (10
2o mL) were added phosphorus oxychloride (2.5 mL) and a solution
of the obtained crystals in chloroform (20 mL) under ice-
cooling, and the mixture was stirred overnight. Under ice-
cooling, an aqueous sodium acetate (27 g) solution was added
and the mixture was stirred for one hour. The mixture was
25 extracted with chloroform and the solvent was evaporated under
reduced pressure to give an oil. The obtained oil was purified
by silica gel column chromatography (eluent: hexane-ethyl
acetate (8:2)) to give a colorless oil (1.4 g). To a solution
of the obtained oil in pyridine (10 mL) was added hydrazine
30 (0.7 g), and the mixture was stirred with heating for 2 hours.
The reaction mixture was cooled to room temperature, and the
solvent was evaporated under reduced pressure to give an oil.
The obtained oil was purified by silica gel column
74
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
chromatography (eluent: hexane-ethyl acetate (1:1)) to give
the title compound (0.2 g) as colorless crystals.
MP :177°C
Anal. Calcd. for:C1gH21N3~3:C.66.04;H,6.47;N,12.84.
s Found:C,65.96;H,6.21;N,12.66.
MS (EI ) :327 (M~) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.80(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz), 1.56-1.59(2H,m), 2.70-2.80(2H,m),
3.76(2H,q,J=7.3Hz), 5.50(lH,s), 7.28-7.33(3H,m),
l0 7.63(lH,dd,J=7.3Hz and 7.4Hz), 7.7~(lH,d,J=7.3Hz),
9.64(lH,br.s), 9.68(lH,br.s), 10.12(lH,br.s).
Example 99
Ethyl 4-(2-aminophenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
15 To a solution of ethyl 4,7-dihydro-4-(2-nitrophenyl)-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate (1.68 g) in
methanol (30 mL) was added 5~ palladium-carbon (500 mg), and
the mixture was stirred under 10 atm for 3 hours. After
removing palladium-carbon by Celite filtration, the solvent
2o was evaporated under reduced pressure to give an oil. The
obtained oil was purified by silica gel column chromatography
{eluent: hexane-ethyl acetate (1:1)) to give the title
compound (120 mg) as colorless crystals.
MP:179°C.
25 Anal. Calcd. for:ClBHzzN40z:C,66.24;H,6.79;N,17.17.
Found:C,65.96;H,6.62;N,17.16.
MS(EI) :326 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.82(3H,t,J=7.3Hz),
0.98(3H,t,J=7.3Hz), 1.58-1.64(2H,m), 2.72-2.78(2H,m),
30 3.78(2H,q,J=7.3Hz), 5.52(lH,s), 6.35-6.38(2H,br. s), 7.28-
7.36(3H,m), 7.58(lH,dd,J=7.3Hz and 7.4Hz), 7.78(lH,d,J=7.3Hz),
9.58(lH,br.s), 11.48(lH,br.s).
Example 100
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Ethyl 4-(2-ethylphenyl)-4,7-dihydro-6-propyl-1H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-ethylbenzaldehyde,
3-aminopyrazole and ethyl 3-ketohexanoate in the same manner
as in Example 25.
MP:186°C.
Anal. Calcd. for:CzoHzsN3Oz 1/5 HZO:C,70.03;H,7.46;N,12.25.
Found:C,69.91;H,7.53;N,11.98.
MS(EI):339(M~).
.to 1H-NMR (400MHz,DMSO-d6)b(ppm): 0.86(3H,t,J=7.3Hz),
0.94(3H,t,J=7.3Hz), 1.24(3H,t,J=7.3Hz), 1.64(2H,q,J=7.3Hz),
2.64-2.68(lH,m), 2.77-2.86(3H,m), 3.78(2H,q,J=7.3Hz),
5.34(lH,s), 6.98-7.01(3H,m), 7.07-7.10(2H,m), 9.34(lH,s),
11.89(lH,s).
Example 101
Ethyl 4,7-dihydro-6-propyl-4-(2-propylphenyl)-1H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-
propylbenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
~o in the same manner as in Example 25.
MP:197°C.
Anal. Calcd. for:CzlHz7N30z:C,71.36;H,7.70;N,11.89.
Found:C,71.07;H,7.73;N,11.84.
MS(EI) :353(M+) .
z5 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.3Hz), 0.94-
1.00(6H,m), 1.64(2H,q,J=7.3Hz), 2.68-2.80(4H,m),
3.79(2H,q,J=7.3Hz), 5.33(lH,s), 6.98-7.06(5H,m), 9.34(lH,s),
11.88(lH,s).
Example 102
3o Ethyl 4-(2-butylphenyl)-4,7-dihydro-6-propyl-1H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from 2-butylbenzaldehyde,
3-aminopyrazole and ethyl 3-ketohexanoate in the same manner
76
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
as in Example 25.
MP:175°C.
Anal. Calcd. for:CZ2Hz9N302:C,71.90;H,7.95;N,11.43.
Found:C,71.50;H,7.94;N,11.36.
MS(EI) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.3Hz), 0.92-
0.97(6H,m), 1.40(2H,q,J=7.3Hz), 1.60-1.66(4H,m), 2.70-
2.82(4H,m), 3.80(2H,q,J=7.3Hz), 5.33(lH,s), 6.97-7.06(5H,m),
9.34(lH,s), 11.88(lH,s).
.to Example 103
Ethyl 4,7-dihydro-4-(indan-4-yl)-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was prepared from indan-4-aldehyde,
3-aminopyrazole and ethyl 3-ketohexanoate in the same manner
Is as in Example 1.
MP :181-183°C .
Anal. Calcd. for:CZiHZ5N302:C,71.77;H,7.17;N,11.96.
Found:C,71.66;H,7.14;N,11.88.
MS(EI) :351(M~) .
20 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90(3H,t,J=7.3HZ),
0.90(3H,t,J=7.3Hz), 1.62(2H,m), 1.80-2.10(2H,m), 2.52-
3.10(6H,m), 3.77(2H,q,J=7.3Hz), 5.17(lH,s),
6.81(lH,d,J=6.8Hz),6.91-6.96(2H,m), 7.14(lH,s), 9.33(lH,br.s),
11.87(lH,br.s).
2s Example 104
Ethyl 4,7-dihydro-6-propyl-4-(1,2,3,4-tetrahydronaphthalen-5-
yl)-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Example 105
Ethyl 4-(benzo[b]furan-7-y1)-4,7-dihydro-6-propyl-2H-
30 pyrazolo[3,4-b]pyridine-5-carboxylate
Example 106
Ethyl 4-(benzo[b]thiophen-7-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
77
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from benzo[b]thiophene-
7-aldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate in the
same manner as in Example 25.
MP:166°C.
Anal. Calcd. for:CaoH21N3O2S 2HzO:C,59.53;H,6.25;N,10.41.
Found:C,59.77;H,6.46;N,9.95.
MS(EI) :367 (M+) .
~H-NMR (400MHz,DMSO-d6)8(ppm): 0.74(3,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.65-1.69(2H,m), 2.70-2.80(2H,m),
io 3.71(2H,q,J=7.3Hz), 5.48(lH,s),7.11-7.13(2H,m),
7.26(lH,dd,J=7.4Hz and 7.5Hz), 7.39(lH,d,J=5.4Hz),
7.63(lH,d,J=7.3Hz), 7.68(lH,d,J=5.4Hz), 9.57(lH,s),
11.91(lH,s).
Example 107
5'-Ethoxycarbonyl-4',7'-dihydro-6'-propyl-
spiro[benzo[b]thiophene-3(2H),4'-2'H-pyrazolo[3,4-b]pyridine]
To a solution of 5'-ethoxycarbonyl-4',7'-dihydro-6'-
propyl-spiro[benzo[b]thiophene-3(2H), 4'-2'H-pyrazolo[3,4-
b]pyridine]-1-oxide (100 mg) in carbon tetrachloride (20 mL)
~o was added trimethylsilane iodide (0.1 g), and the mixture was
stirred with heating for 30 minutes. The reaction mixture was
allowed to cool to ambient temperature, and the mixture was
extracted with chloroform. The solvent was evaporated under
reduced pressure to give an oil. The obtained oil was purified
as by silica gel column chromatography (eluent: hexane-ethyl
acetate (8:2)) to give the title compound (20 mg) as colorless
crystals.
MP:147°C.
Anal. Calcd. for:C19HZ1N302S:C,64.20;H,5.95;N,11.82.
3o Found:C,64.18;H,6.14;N,11.56.
MS (EI ) : 355 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.73(3H,t,J=7.3Hz),
0.95(3H,t,J=7.3Hz), 1.64-1.67(2H,m), 2.56-2.64(2H,m),
78
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.03(lH,d,J=10.2Hz), 3.72(2H,q,J=7.3Hz),4.03(lH,d,J=10.2Hz)~
6.69(lH,d,J=7.3Hz), 6.91(lH,dd,J=7.3Hz and 7.4Hz),
7.03(lH,dd,J=7.3Hz and 7.4Hz), 7.08(lH,s), 7.15(lH,d,J=7.3Hz),
9.65(lH,br.s), 11.96(lH,br.s).
s Example 108
Ethyl 4,7-dihydro-4-methyl-4-phenyl-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
To a solution of 5'-ethoxycarbonyl-4',7'-dihydro-6'-
propyl-spiro[benzo[b]thiophene-3(2H), 4'-2'H-pyrazolo[3,4-
.to b]pyridin]-1-oxide (100 mg) in tetrahydrofuran (10 mL) were
added disodium hydrogenphosphate (1:2 g) and methanol (5 mL)
under ice-cooling, and loo sodium amalgam (3.0 g) was added.
The mixture was stirred for 5 hours, filtered through Celite
and extracted with chloroform. The solvent was evaporated
under reduced pressure to give an oil. The obtained oil was
purified by silica gel column chromatography (eluent: hexane-
ethyl acetate (8:2)) to give the title compound (80 mg) as
colorless crystals.
MP:207°C.
ao Anal. Calcd. for:C19HZ3N3Oz:C,70.13;H,7.12;N,12.91.
Found:C,69.89;H,7.18;N,12.99.
MS(EI) :325 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.71(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz), 1.64-1.68(2H,m), 2.28(3H,s), 2.48-
zs 2.56(2H,m), 3.71(2H,q,J=7.3Hz), 6.73-7.01(5H,m), 7.10(lH,s),
9.71(lH,br.s), 11.87(lH,br.s).
Example 109
Ethyl 4,7-dihydro-6-propyl-4-(2,3,5-trichlorophenyl)-pyrazolo
[3,4-b]pyridine-5-carboxylate
so The title compound was prepared from 2,3,5-
trichlorobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:218-220°C (decomposition).
79
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C18H1gC13N3O~:C,52.13;H,4.37;N,10.13.
Found:C,51.76;H,4.37;N,10.07.
MS(EI) :414(M~) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89(3H,t,J=6.9Hz),
0.97(3H,t,J=7.3Hz), 1.62-1.67(2H,m), 2.65-2.71(lH,m), 2.85-
2.92(lH,m), 3.76-3.88(2H,m), 5.62(lH,s), 7.03(lH,d,J=l.6Hz),
7.33(lH,s), 7.59(lH,d,J=2.4Hz), 9.69(lH,s), 12.12(lH,s).
Example 110
Ethyl 4,7-dihydro-6-propyl-4-(2,3,4,5-
.to tetrahydrobenzo[b]oxepin-9-yl)-2H-pyrazolo[3,4-b]pyridine-5-
carboxylate
Example 111
Ethyl 4-(3-chloro-2-methylphenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
zs The title compound was prepared from 3-chloro-2-
methylbenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 1.
MP:185°C.
Anal. Calcd. for:Cl9HZZC1N302:C,63.42;H,6.16;N,11.68.
2o Found:C,63.37;H,6.12;N,11.65.
MS(EI):359(M+).
'H-NMR (400MHz,DMSO-d6)~(ppm): 0.87(3H,t,J=7.3Hz),
0.95(3H,t,J=7.3Hz), 1.60-1.66(2H,m), 2.67-2.69(lH,m), 2.74-
2.78(lH,m), 3.78(2H,q,J=7.3Hz), 5.39(lH,s), 6.95(lH,d,J=7.3Hz),
2~ 7.04(lH,dd,J=7.3Hz arid 7.4Hz), 7.12(lH,d,J=7.3Hz), 7.24(lH,s),
9.44(lH,br.s), 11.94(lH,br.s).
Example 112
Ethyl 4-(2,1,3-benzothiadiazol-4-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
ao The title compound was prepared from 2,1,3-
benzothiadiazole-4-aldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:180°C.
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C18H19N5OZS:C,58.52;H,5.18;N,18.96.
Found:C,58.51;H,5.19;N,18.81.
MS (EI) :369 (M+) .
1H-NMR (400MHz,DMSO-d6)~(ppm): 0.62(3H,t,J=7.3Hz),
s 1.00(3H,t,J=7.3Hz), 1.68-1.72(2H,m), 2.76-2.89(2H,m),
3.72(2H,q,J=7.3z), 6.02(lH,s), 7.16(lH,s), 7.20(lH,d,J=7.3Hz),
7.60(lH,dd,J=7.3Hz and 7.4Hz), 7.83(lH,d,J=7.3Hz), 9.55(lH,s),
11.89(lH,s).
Example 113
to Ethyl 4-(2,1,3-benzoxadiazol-4-yl)-4,7-dihydro-6-methyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,1,3-
benzoxadiazole-4-aldehyde, 3-aminopyrazole and ethyl
acetoacetate in the same manner as in Example 25.
.ts MP:228°C.
Anal. Calcd. for:Cl6HisNs03:C,59.07;H,4.65;N,21.53.
Found:C,58.85;H,4.75;N,21.17.
MS (EI ) :325 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.75(3H,t,J=7.3Hz), 2.42(3H,s),
20 3.79(2H,q,J=7.3Hz), 5.67(lH,s), 7.14(lH,d,J=6.6Hz),
7.23(lH,s),7.49(lH,dd,J=9.OHz and 6.6Hz), 7.78(lH,d,J=9.OHz),
9.69(lH,s), 12.02(lH,s).
Example 114
Ethyl 4-(2,1,3-benzoxadiazol-4-yl)-4,7-dihydro-6-phenyl-2H-
a5 pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,1,3-
benzoxadiazole-4-aldehyde, 3-aminopyrazole and ethyl
benzoylacetate in the same manner as in Example 1.
MP:190°C.
3o Anal. Calcd. for:CalH,,7N5o3:C, 65.11;H,4.42;N,18.08.
Found:C,64.99;H,4.59;N,18.06.
MS(EI) :387 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.54(3H,t,J=7.3Hz),
81
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.56(2H,q,J=7.3Hz), 5.68(lH,s), 7.24(lH,s), 7.26-7.42(6H,m),
7.72(lH,dd,J=7.3Hz and 7.2Hz), 7.94(lH,d,J=7.3Hz),
9.71(lH,s),12.08(lH,s).
Example 115
s Ethyl 4-(2,3-dichlorophenyl.)-4,7-dihydro-6-phenyl-2H-
p~yrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2,3-
dichlorobenzaldehyde, 3-aminopyrazole and ethyl benzoylacetate
in the same manner as in Example 1.
io MP:214°C.
Anal. Calcd. for:Cz1H17N503:C, 65.11;H, 4.42;N, 18.08.
Found:C,64.85;H,4.48;N,17.92.
MS(EI) :387(M'-) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.57(3H,t,J=7.3Hz),
.t5 3.52(2H,q,J=7.3Hz), 5.70(lH,s), 7.30-7.40(9H,m), 9.61(lH,s),
12.12(lH,s).
Example 116
(+)Ethyl 4-(2,1,3-benzoxadiazol-4-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
2o The compound described in Example 76 was separated using
a semi-preparative column for optical resolution (CHIRALPAK AS,
1.0 cmx25 cm, eluent n-hexane/2-
propanol/diethylamine=90/10/0.1, flow rate 2.0 mL/min, UV 254
nm, retention time 40 minutes, DAICEL CHEMICAL INDUSTRIES,
2s LTD.) to give the title compound as colorless crystals.
MP: 159°C.
MS(EI) : 353(M+) .
Specific rotation: [a]D=+260°(EtOH,c=0.5).
Example 117
30 (-)Ethyl-4-(2,1,3-benzoxadiazol-4-yl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The compound described in Example 76 was separated using
a semi-preparative column for optical resolution (CHIRALPAK AS,
82
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
1.0 cmx25 cm, eluent n-hexane/2-
propanol/diethylamine=90/10/0.1, flow rate 2.0 mL/min, UV 254
nm, retention time 55 minutes, DAICEL CHEMICAL INDUSTRIES,
LTD.) to give the title compound as colorless crystals.
s MP: 160°C.
MS(EI) : 353 (M+) .
Specific rotation: [a]D=-277°(EtOH,c=0.5).
Example 118
4-(2-Bromophenyl)-4,7-dihydro-5-nitro-6-propyl-2H-
io pyrazolo[3,4-b]pyridine
The title compound was prepared from n-butylaldehyde, 2-
bromobenzaldehyde and 3-aminopyrazole in the same manner as in
Example 93.
MP:226°C.
z5 Anal. Calcd. for:C15H1sBrN402:C,49.60;H,4.16;N,15.43.
Found:C,49.57;H,4.28;N,14.96.
MS(EI) :363 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.02(3H,t,J=7.3Hz), 1.72-
1.76(2H,m), 2.85-3.05(2H,m), 5.89(lH,s), 7.07-7.1(2H,m),
2o 7.25(lH,dd,J=7.5Hz and 7.4Hz), 7.47(lH,s), 7.56(lH,d,J=7.3Hz),
10.84(lH,s), 12.43(lH,s).
Example 119
4,7-Dihydro-4-(2-methoxyphenyl)-5-nitro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
z5 The title compound was prepared from n-butylaldehyde, 2-
methoxybenzaldehyde and 3-aminopyrazole in the same manner as
in Example 93.
MP:223°C.
Anal. Calcd. for:C16H18N4O3:C, 61.13;H, 5.77;N,17.82.
3o Found:C,61.01;H,5.87;N,17.92.
MS(EI) :314(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.03(3H,t,J=7.3Hz), 1.72-
1.78(2H,m), 2.82-3.04(2H,m), 3.86(3H,s), 5.76(lH,s),
83
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
6.78(lH,dd,J=7.5Hz and 7.4Hz), 6.90(lH,d,J=7.3Hz),
6.95(lH,d,J=7.3Hz), 7.10(lH,dd,J=7.5Hz and 7.4Hz), 7.33(lH,s),
10.68(lH,s), 12.29(lH,s).
Example 120
4,7-Dihydro-4-(2-methylthiophenyl)-5-nitro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from n-butylaldehyde, 2-
methylthiobenzaldehyde and 3-aminopyrazole in the same manner
as in Example 93.
io MP:211°C.
Anal. Calcd. for:C~6H1gN4O2S:C,58.16;H,5.49;N,16.96.
Found:C,57.94;H,5.47;N,16.53.
MS (EI ) : 330 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.02(3H,t,J=7.3z), 1.71-
1.76(2H,m), 2.8-3.00(2H,m), 5.89(lH,s), 6.98(lH,d,J=7.3Hz),
7.03(lH,dd,J=7.5Hz and 7.4Hz), 7.13(lH,dd,J=7.5Hz and 7.4Hz),
7.28(lH,d,J=7.3Hz), 7.41(lH,s), 10.74(lH,s), 12.34(lH,s).
Example 121
4,7-Dihydro-5-nitro-4-(2-nitrophenyl)-6-propyl-2H-
zo pyrazolo[3,4-b]pyridine
The title compound was prepared from n-butylaldehyde, 2-
nitrobenzaldehyde and 3-aminopyrazole in the same manner as in
Example 93.
MP : 204°C .
2s Anal. Calcd. for:C15H1sNs04:C,54.71;H,4.59;N,21.27.
Found:C,54.50;H,4.77;N,21.32.
MS(EI) :329 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.01(3H,t,J=7.3Hz), 1.69-
1.74(2H,m), 2.85-2.99(2H,m), 5.67(lH,s), 6.94(lH,d,J=7.3Hz),
30 6.98-7.03(2H,m), 7.09(lH,d,J=7.3Hz), 7.38(lH,s), 10.69(lH,s),
12.34(lH,s).
Example 122
4-(2,3-Dichlorophenyl)-4,7-dihydro-5-nitro-6-propyl-2H-
84
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine
The title compound was prepared from n-butylaldehyde,
2,3-dichlorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 93.
s MP:239°C.
.Anal. Calcd. for:C15H14C1zN40z:C,51.O1;H,4.OO;N,15.86.
Found:C,50.70;H,4.06;N,15.60.
MS(EI) :353(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.02(3H,t,J=7.3Hz), 1.70-
io 1.74(2H,m), 2.89-2.92(lH,m), 2.96-3.02(lH,m), 5.96(lH,s),
7.09(lH,d,J=7.3Hz), 7.24(lH,dd,J=7.5Hz and 7.4Hz),
7.43(lH,d,J=7.3Hz), 7.49(lH,s), 10.98(lH,s), 12.49(lH,s).
Example 123
4,7-Dihydro-4-(naphthalen-1-yl)-5-nitro-6-propyl-2H-
i5 pyrazolo[3,4-b]pyridine
The title compound was prepared from n-butylaldehyde,
naphthalen-1-aldehyde and 3-aminopyrazole in the~same manner
as in Example 93.
MP:226°C.
zo Anal. Calcd. for:C19H18N4~2:C.68.25;H,5.43;N,16.76.
Found:C,68.29;H,5.20;N,16.67.
MS(EI) :334(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.06(3H,t,J=7.3Hz), 1.76-
1.82(2H,m), 2.95-3.06(2H,m), 6.33(lH,s), 7.18-7.22(2H,m),
25 7.36(lH,dd,J=7.5Hz and 7.4Hz), 7.54(lH,dd,J=7.5Hz and 7.4Hz),
7.60(lH,dd,J=7.5Hz and 7.4Hz), 7.71(lH,d,J=7.3Hz),
7.92(lH,d,J=7.3Hz), 8.46(lH,d,J=7.3Hz), 10.80(lH,s),
12.29(lH,s).
Example 124
30 4,7-Dihydro-4-(3,4-dihydro-2H-benzopyran-8-yl)-5-nitro-6-
propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from n-butylaldehyde,
3,4-dihydro-2H-benzopyran-8-aldehyde and 3-aminopyrazole in
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
the same manner as in Example 93.
MP:234°C.
Anal. Calcd. for:C18H20N4~3~C,63.52;H,5.92;N,16.46.
Found:C,63.22;H,5.94;N,16.44.
MS(EI) :340(M+) .
1H-NMR (400MHz,DMSO-ds)b(ppm): 1.02(3H,t,J=7.3Hz), 1.71-
1.77(2H,m), 1.92-1.95(2H,m), 2.69-2.73(2H,m), 2.85-3.02(2H,m),
4.23-4.28(2, m), 5.71(lH,s), 6.61-6.67(2H,m),
6.80(lH,d,J=7.3Hz), 7.37(lH,s), 10.64(lH,s), 12.28(lH,s).
~.o Example 125
4-(2,3-Dichlorophenyl)-4,7-dihydro-6-methyl-5-nitro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from acetaldehyde, 2,3-
dichlorobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 93.
MP:>270°C.
Anal . Calcd . for : C13H1oC1zN402 : C, 48 . 02; H, 3 . 10; N, 17 . 23 .
Found:C,48.05;H,3.12;N,17.24.
MS(EI) :325 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 2.66(3H,s), 5:94(lH,s),
7.13(lH,d,J=7.2Hz), 7.22(lH,dd,J=7.3Hz and 7.2Hz),
7.42(lH,d,J=7.3Hz), 7.50(lH,s), 10.94(lH,s), 12.49(lH,s).
Example 126
4-(2,3-Dichlorophenyl)-6-ethyl-4,7-dihydro-5-nitro-2H-
z5 pyrazolo[3,4-b]pyridine
The title compound was prepared from propionaldehyde,
2,3-dichlorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 93.
MP:250°C.
3o Anal. Calcd. for : C14H1aClaN40z :C, 49 . 58; H, 3 . 57;N,16 . 52 .
Found:C,49.54;H,3.62;N,16.73.
MS(EI) :339 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.29(3H,t,J=7.3Hz), 2.98-
86
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.01(2H,m), 5.94(lH,s), 7.10(lH,d,J=7.3Hz), 7.24(lH,dd,J=7.3Hz
and 7.2Hz), 7.42(lH,d,J=7.2Hz), 7.49(lH,s), 10.93(lH,s),
12.49(lH,s).
Example 127
s 6-Butyl-4-(2,3-dichlorophenyl)-4,7-dihydro-5-nitro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from pentylaldehyde,
2,3-dichlorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 93.
1o MP:220°C.
Anal. Calcd. for:Cl6HisC1zN40z:C,52.33;H,4.39;N,15.26.
Found:C,52.64;H,4.61;N,14.51.
MS(EI) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz),. 1.41-
.15 1.46(2H,m), 1.63-1.68(2H,m), 2.94-3.04(2H,m), 5.95(lH,s),
7.08(lH,d,J=7.2Hz), 7.23(lH,dd,J=7.3Hz and 7.2Hz),
7.42(lH,d,J=7.2Hz), 7.48(lH,s), 10.97(lH,s), 12.28(lH,s).
Example 128
4-(2-Bromophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
2o pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-bromobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:237°C.
25 Anal. Calcd. for:C16H1sBrN4:C,55.99;H,4.41;N,16.32.
Found:C,55.97;H,4.45;N',16.40.
MS(EI) :343(M+) .
~H-NMR. (400MHz,DMSO-d6)b(ppm): 0.95(3H,t,J=7.3Hz),1.64-
1.70(2,m), 2.40-2.44(2H,m), 5.35(lH,s), 7.15(lH,dd,J=7.5Hz and
30 7.4Hz), 7.22(lH,d,J=7.3Hz), 7.27(lH,s), 7.36(lH,dd,J=7.5Hz and
7.4Hz), 7.59(lH,d,J=7.3Hz), 9.84(lH,s), 12.16(lH,s).
Example 129
5-Cyano-4,7-dihydro-4-(2-methoxy henyl)-6-propyl-2H-
87
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-methoxybenzaldehyde and 3-aminopyrazole in the same manner
as in Example 94.
MP:203°C.
Anal. Calcd. for:C17H18N40:C,69.37;H,6.16;N,19.03.
Found:C,69.34;H,6.25;N,19.01.
MS(EI) :294(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.96(3H,t,J=7.3Hz), 1.65-
1.70(2H,m), 2.38-2.43(2H,m), 3.83(3H,s), 5.22(lH,s),
6.89(lH,dd,J=7.5Hz and 7.4Hz), 6.99(lH,d,J=7.3Hz),
7.05(lH,d,J=7.3Hz), 7.15-7.18(2H,m), 9.65(lH,s), 12.02(lH,s).
Example 130
5-Cyano-4,7-dihydro-4-(2-methylthiophenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-methylthiobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:216°C.
2o Anal. Calcd. for:C1~H18N4S:C,65.78;H,5.84;N,18.05.
Found:C,65.68;H,5.81;N,17.83.
MS(EI) :310 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.96(3H,t,J=7.3Hz), 1.65-
1.70(2H,m), 2.40-2.46(2H,m), 2.48(3H,s), 5.34(lH,s), 7.13-
7.21(4H,m), 7.30(lH,d,J=7.3Hz), 9.75(lH,s), 12.07(lH,s).
Example 131
5-Cyano-4,7-dihydro-4-(2-methylphenyl)-6-propyl-2H-pyrazolo
[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-methylbenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:230°C.
Anal. Calcd. for:C17H18N4:C,73.35;H,6.52;N,20.13.
88
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Found:C,73.44;H,6.61;N,20.13.
MS(EI) :278 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.93(3H,t,J=7.3Hz), 1.65-
1.67(2H,m), 2.32(3H,s), 2.35-2.41(2H,m), 5.13(lH,s), 7.06-
7.16(5H,m), 9.69(lH,s), 12.07(lH,s).
Example 132
5-Cyano-4,7-dihydro-4-(2-nitrophenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
l0 2-nitrobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:216°C.
Anal. Calcd. for:C16H1sNsOz:C,62.13;H,4.89;N,22.64.
Found:C,62.16;H,4.93;N,22.57.
MS(EI) :309(M~) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz), 1.64-
1.69(2H,m), 2.36-2.42(2H,m), 5.38(lH,s), 7.27(lH,s), 7.42-
7.49(2H,m), 7.70(lH,dd,J=7.5Hz and 7.4Hz), 7.89(lH,d,J=7.3Hz),
9.91(lH,s), 12.21(lH,s).
2o Example 133
5-Cyano-4-(2-cyanophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-cyanobenzaldehyde and 3-aminopyrazole in the same manner as
as in Example 94.
MP:218°C.
Anal. Calcd. for:Cl~HlsNs:C,70.57;H,5.23;N,24.21.
Found:C,70.54;H,5.30;N,24.07.
MS(EI) :289(M~) .
30 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz), 1.63-
1.68(2H,m), 2.36-2.40(2H,m), 5.23(lH,s), 7.26(lH,s),
7.38(lH,d,J=7.3Hz), 7.43(lH,dd,J=7.5Hz and 7.4Hz),
7.69(lH,dd,J=7.5Hz and 7.4Hz), 7.80(lH,d,J=7.3Hz), 9.94(1, s),
89
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
12.22(lH,s).
Example 134
5-Cyano-4-(2,3-dichlorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
s The title compound was prepared from methyl butanoate,
2,3-dichlorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:242°C.
Anal. Calcd. for:C16H14C~-2N4 1/5 HZO:C,57.05;H,4.31;N,16.63.
to Found:C,57.23;H,4.49;N,16.25.
MS(EI) :333 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz), 1.62-
1.68(2H,m), 2.40-2.46(2H,m), 5.44(lH,s), 7.22(lH,d,J=7.3Hz),
7.30(lH,s), 7.35(lH,dd,J=7.5Hz and 7.4Hz), 7.51(lH,d,J=7.3Hz),
.t5 9.89(lH,s), 12.19(lH,s).
Example 135
5-Cyano-4,7-dihydro-4-(naphthalen-1-y1)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
ao naphthalene-1-aldehyde and 3-aminopyrazole in the same manner
as in Example 94.
MP:263°C.
Anal. Calcd. for:CZOH18N4:C,76.41;H,5.77;N,17.82.
Found:C,76.05;H,5.85;N,17.73.
25 MS(EI) :314 (M+) .
1H-NMR (400MHz,DMSO-d6)S(ppm): 0.97(3H,t,J=7.3Hz), 1.68-
1.73(2H,m), 2.44-2.48(2H,m), 5.71(lH,s),7.04(lH,s), 7.39-
7.46(4H,m), 7.81(lH,d,J=7.3Hz), 7.94(lH,d,J=7.3Hz), 9.83(lH,s),
12.02(lH,s).
3o Example 136
5-Cyano-4-(3,4-dihydro-2H-benzopyran-8-yl)-4,7-dihydro-6-
propyl-2H-pyrazolo(3,4-b]pyridine
The title compound was prepared from methyl butanoate,
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3,4-dihydro-2H-benzopyran-8-aldehyde and 3-aminopyrazole in
the same manner as in Example 94.
MP:230°C.
Anal. Calcd. for:C19H20N40:C,71.23;H,6.29;N,17.49.
s Found:C,71.20;H,6.48;N,17.55.
MS(EI) :320(Mk) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.95(3H,t,J=7.3Hz), 1.64-
1.70(2H,m), 1.92-1.95(2H,m), 2.38-2.43(2H,m), 2.72-2.76(2H,m),
4.16-4.27(2H,m), 5.16(lH,s), 6.74(lH,dd,J=7.5Hz and 7.4Hz),
.to 6.83-6.88(2H,m), 7.20(lH,s), 9.62(lH,s), 12.01(lH,s).
Example 137
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3;4-b]pyridine
The title compound was prepared from methyl butanoate,
.t5 2,1,3-benzothiadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 94.
MP:194°C.
Anal. Calcd. for:C16Hz4N6O:C,62.73;H,4.61;N,27.44.
Found:C,62.52;H,4.78;N,27.19.
2o MS(EI) :306 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92(3H,t,J=7.3Hz), 1.63-
1.68(2H,m), 2.38-2.43(2H,m), 5.40(lH,s), 7.25(lH,s),
7.40(lH,d,J=7.3Hz), 7.58(lH,dd,J=7.5Hz and 7.4Hz),
7.92(lH,d,J=7.3z), 9.93(lH,s), 12.13(lH,s).
25 Example 138
4-(2,1,3-Benzothiadiazol-4-yl)-5-c~ano-4,7-dihydro-6-propyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2,1,3-benzothiadiazol-4-aldehyde and 3-aminopyrazole in the
3o same manner as in Example 94.
MP:195°C.
Anal. Calcd. for:C16Hi4NsS:C,59.61;H,4.38;N,26.07.
Found:C,59.33;H,4.48;N,25.76.
91
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :322 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.98(3H,t,J=7.3Hz), 1.68-
1.74(2H,m), 2.45-2.50(2H,m), 5.72(lH,s), 7.19(lH,s),
7.43(lH,d,J=7.3Hz), 7.72(lH,dd,J=7.5Hz and 7.4Hz),
7.97(lH,d,J=7.3Hz), 9.87(lH,s), 12.06(lH,s).
Example 139
5-Cyano-4,7-dihydro-4-(2-methylbenzoxazol-4-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
io 2-methylbenzoxazole-4-aldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:208°C.
Anal. Calcd. for:C18H17N50 1/5 H~O:C,66.94;H,5.43;N,21.68.
Found:C,66.85;H,5.52;N,22.09.
MS(EI) :319 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.97(3H,t,J=7.3Hz), 1.67-
1.72(2H,m), 2.40-2.45(2H,m), 2.63(3H,s), 5.51(lH,s),
7.06(lH,d,J=7.3Hz), 7.16(lH,s), 7.29(lH,dd,J=7.3Hz and 7.2Hz),
7.47(lH,d,J=7.3Hz), 9.77(lH,s),. 12.06(lH,s).
2o Example 140
R(-) 4-(2,1,3-Benzoxadiazol-4-y1)-5-cyano-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine
To a solution of the compound described in Example 137
(64.5 g) in THF (1000 mL) was added (-)camphorsulfonic acid
z.s (49 g) at room temperature and the mixture was stirred for 1
hour. The solvent was evaporated under reduced pressure to
give an oil. The obtained oil was recrystallized from
acetonitrile twice to give colorless crystals (11 g). To a
solution of the obtained colorless crystals in methanol (50
3o mL) was added water (50 mZ). The mixture was neutralized with
a saturated aqueous sodium hydrogencarbonate solution and
extracted with ethyl acetate. The solvent was evaporated under
reduced pressure. The residual methanol solution was added
92
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
dropwise to water (1000 mL) and the crystals were collected by
filtration to give the title compound (11 g) as pale-yellow
crystals.
(CHIRALPAK AS, 0.25 cm~25 cm, eluent n-hexane/2-
propanol/diethylamine =80/20/0.1, flow rate 1.5 mL/min, UV 254
nm, retention time 10 minutes, DAICEL CHEMICAL INDUSTRIES,
LTD.)
MP: 170°C.
MS(EI) : 306 (M+) .
.to Spec if is rotation : [ a ] D=-8 0° ( EtOH, c=1. 0 ) .
Example 141
S(+) 4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine
To a solution of the compound (54 g), which was
z.~ recovered from the mother liquor obtained in Example 140, in
THF (600 mL) was added (+)camphorsulfonic acid (41 g) at room
temperature and the mixture was stirred for 1 hour. The
solvent was evaporated under reduced pressure to give an oil.
The obtained oil was recrystallized from acetonitrile twice to
~o give colorless crystals (12 g). To a solution of the obtained
colorless crystals in methanol (50 mL) was added water (50 mL).
The mixture was neutralized with a saturated aqueous sodium
hydrogencarbonate solution and extracted with ethyl acetate.
The solvent was evaporated under reduced pressure and the
a5 residual methanol solution was added dropwise to water (1000
mL). The crystals were collected by filtration to give the
title compound (11 g) as pale-yellow crystals.
(CHIRALPAK AS, 0.25 cmx25 cm, eluent n-hexane/2-
propanol/diethylamine =80/20/0.1, flow rate 1.5 mL/min, UV 254
3o nm, retention time 13 minutes, DAICEL CHEMICAL TNDUSTRIES,
LTD.)
MP: 170°C.
MS(EI) : 306 (M+) .
93
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Specific rotation: [a]D=+82°(EtOH,c=1.0).
Example 142
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
s The title compound was prepared from methyl benzoate, 2-
chlorobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 95.
MP:158°C.
Anal. Calcd. for:C19H13C1N4 HzO:C, 65.05;H, 4.31;N, 15.97 .
to Found:C,65.35;H,4.19;N,16.21.
MS(EI) :332(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.51(lH,s), 7.25-7.51(8H,m),
7.59-7.61(2H,m), 10.07(lH,s), 12.24(lH,s).
Example 143
Is 5-Cyano-4,7-dihydro-4-(2-methylthiophenyl)-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl benzoate, 2-
methylthiobenzaldehyde and 3-aminopyrazole in the same manner
as in Example 95.
2o MP:146°C.
Anal. Calcd. for:C2oH16N4S 4~5 HzO:C, 66.94; H, 4.94;N,15. 61.
Found:C,66.85;H,4.81;N,15.65.
MS(EI) :344(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 2.48(3H,s), 5.48(lH,s), 7.19-
2s 7.33(5H,m), 7.48-7.50(3H,m), 7.59-7.61(2H,m), 9.99(lH,s),
12.16(lH,s).
Example 144
5-Cyano-4-(2-cyanophenyl)-4,7-dihydro-.6-phenyl-2H-
pyrazolo[3,4-b]pyridine
3o The title compound was prepared from methyl benzoate, 2-
cyanobenzaldehyde and 3-aminopyrazole in the same manner as in
Example 95.
MP:148°C.
94
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:CaoH13N5 3/5 HaO:C,71.89;H,4.28;N,20.96.
Found:C,71.89;H,4.33;N,20.91.
MS(EI) :323 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.38(lH,s), 7.31(lH,s), 7.44-
7.59(7H,m), 7.70(lH,dd,J=7.3Hz and 7.2Hz), 7.83(lH,d,J=7.3Hz),
10.21(lH,s), 12.31(lH,s).
Example 145
5-Cyano-4-(2,3-dichlorophenyl)-4,7-dihydro-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
to The title compound was prepared from methyl benzoate,
2,3-dichlorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 95.
MP:162°C.
Anal. Calcd. for:C19H1zC1zN4:C,62.14;H,3.29;N,15.26.
Found:C,61.57;H,3.93;N,17.19.
MS(EI) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.59(lH,s), 7.37-7.42(3H,m),
7.48-7.55(4H,m), 7.59-7.62(2H,m), 10.14(lH,s), 12.28(lH,s).
Example 146
ao 5-Cyano-4,7-dihydro-4-(naphthalen-1-yl)-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl benzoate,
naphthalene-1-benzaldehyde and 3-aminopyrazole in the same
manner as in Example 95.
z5 MP:174°C.
Anal. Calcd. for:CagH16N4:C,79.29;H,4.63;N,16.08.
Found:C,79.50;H,4.85;N,16.58.
MS(EI) :348 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.87(lH,s),7.12(lH,s),7.50-
.~0 7.63(9H,m), 7.82(lH,d,J=7.3Hz),7.96(lH,d,J=7.3Hz),
8.34(lH,d,J=7.3Hz), 10.09(lH,s),12.12(lH,s).
Example 147
4-(2-Bromo-3-cyanophenyl)-5-cyano-4,7-dihydro-6-phenyl-2H-
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl benzoate, 2-
bromo-3-cyanobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 95.
s MP:>270°C.
Anal. Calcd. for:CZOHI2BrN5:C,59.72;H,3.01;N,17.41.
Found:C,59.53;H,3.17;N,17.30.
MS(EI) :402 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.63(lH,s), 7.39(lH,s), 7.49-
zo 7.51(3H,m), 7.60-7.63(3H,m),7.75(lH,d,J=7.3Hz),
7.85(lH,d,J=7.3Hz), 10.21(lH,s), 12.33(lH,s).
Example 148
5-Cyano-4-(3,4-dihydro-2H-benzopyran-8-y1)-4,7-dihydro-6-
phenyl-2H-pyrazolo[3,4-b]pyridine
z5 The title compound was prepared from methyl benzoate,
3,4-dihydro-2H-benzopyran-8-benzaldehyde and 3-aminopyrazole
in the same manner as in Example 95.
MP:255°C.
Anal. Calcd. for:C2zH18N40:C,74.56;H,5.12;N,15.81.
ao Found:C,74.27;H,5.11;N,15.82.
MS(EI) :354(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.94-1.97(2H,m), 2.75-2.78(2H,m),
4.20-4.30(2H,m), 5.30(lH,s), 6.80(lH,dd,J=7.3Hz and 7.2Hz),
6.91(lH,d,J=7.3Hz), 7.02(lH,d,J=7.3Hz), 7.28(lH,s), 7.49-
2s 7.51(3H,m), 7.60-7.63(2H,m), 9.88(lH,s), 12.11(lH,s).
Example 149
5-Cyano-4-(2,3-dif luorophenyl)-4,7-dihydro-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl benzoate,
30 2,3-difluorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 95.
MP:165°C.
Anal. Calcd. for:C19H1zFZN4 3/5 H20:C, 66.12;H, 3.86;N, 16 .23 .
96
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Found:C,65.87;H,3.81;N,16.46.
MS(EI) :334 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.40(lH,s), 7.16-7.38(4H,m),
7.48-7.50(3H,m), 7.57-7.59(2H,m), 10.11(lH,s), 12.30(lH,s).
Example 150
5-Cyano-4,7-dihydro-4-(2-methoxyphenyl)-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl benzoate, 2-
methoxybenzaldehyde and 3-aminopyrazole in the same manner as
so in Example 95.
MP:206°C.
Anal. Calcd. for:CzoH16N40:C, 73.15;H, 4.91;N,17.06 .
Found:C,73.23;H,5.14;N,17.19.
MS(EI) :328(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.86(3H,s), 5.36(lH,s),
6.94(lH,dd,J=7.3Hz and 7.2Hz), 7.02(lH,d,J=7.3Hz), 7.19-
7.25(3H,m), 7.48-7.51(3H,m), 7.60-7.63(2H,m), 9.91(lH,s),
12.12(lH,s).
Example 151
zo 5-Cyano-4,7-dihydro-4,6-bis(2-methoxyphenyl)-2H-pyrazolo[3,4-
b]pyridine
The title compound was prepared from methyl o-anisate,
2-methoxybenzaldehyde and 3-aminopyrazole in the same manner
as in Example 95.
MP:220°C.
Anal. Calcd. for:C21H1gN4Oz:C,70.38;H,5.06;N,15.63.
Found:C,69.97;H,5.13;N,16.15.
MS(EI) :358(Mk) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 3.86(3H,s), 3.88(3H,s),
5.32(lH,s), 6.95-7.06(3H,m), 7.14-7.25(3H,m),
7.37(lH,d,J=7.3Hz), 7.45(lH,dd,J=7.3Hz and 7.2Hz), 9.74(lH,s),
12.05(lH,s).
Example 152
97
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
5-Cyano-4,7-dihydro-4-(2-methoxyphenyl)-6-(3-methoxyphenyl)-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl m-anisate,
2-methoxybenzaldehyde and 3-aminopyrazole in the same manner
as in Example 95.
MP:192°C.
Anal. Calcd. for:Cz1H18N402:C, 70.38;H,5.06;N, 15.63.
Found:C,69.97;H,5.09;N,15.54.
MS(EI) :358 (M+) .
xo 1H-NMR (400MHz,DMSO-d6)b(ppm): 3.81(3H,s), 3.86(3H,s),
5.35(lH,s), 6.95(lH,dd,J=7.3Hz and 7.2Hz), 7.01(lH,d,J=7.3Hz),
7.07(lH,d,J=7.3Hz), 7.14(lH,s),7.18-7.23(5H,m),
7.41(lH,dd,J=7.3Hz and 7.2Hz), 9.88(lH,s), 12.12(lH,s).
Example 153
Zs 5-Cyano-4,7-dihydro-4-(2-methoxyphenyl)-6-(4-methoxyphenyl)-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl p-anisate,
2-methoxybenzaldehyde and 3-aminopyrazole in the same manner
as in Example 95.
ao MP:149°C.
Anal. Calcd. for:CZ1H18N402 1/2 HZO:C, 68 .65;H, 5.21;N, 15 .25.
Found:C,68.67;H,4.99;N,15.35.
MS(EI) :358 (M~) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 3.81(3H,s), 3.86(3H,s),
z5 5.33(lH,s), 6.94(lH,dd,J=7.3Hz and 7.2Hz), 7.01-7.05(3H,m),
7.18-7.24(3H,m), 7.56(2H,d,J=7.2Hz), 9.82(lH,s), 12.10(lH,s).
Example 154
5-Cyano-4,7-dihydro-4-(2-nitrophenyl)-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
3o The title compound was prepared from methyl benzoate, 2-
nitrobenzaldehyde and 3-aminopyrazole in the same manner as in
Example 95.
MP:221°C.
98
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C~9H13Ns0z:C,66.47;H,3.82;N,20.40.
Found:C,66.48;H,4.08;N,20.41.
MS(EI) :343(M~) .
1H-NMR (400MHz,DMSO-d6)~(ppm): 5.54(lH,s), 7.34(lH,s), 7.49
7.52(4H,m), 7.59-7.64(3H,m), 7.74(lH,dd,J=7.3Hz and 7.2Hz),
7.91(lH,d,J=7.3Hz), 10.16(lH,s), 12.30(lH,s).
Example 155
5-Cyano-4,7-dihydro-6-(2-methoxyphenyl)-4-(2-nitrophenyl)-2H-
pyrazolo[3,4-b]pyridine
io The title compound was prepared from methyl o-anisate,
2-nitrobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 95.
MP207°C.
Anal. Calcd. for:C2oH15N5o3:C, 64.34;H, 4.05;N,18.76 .
Found:C,64.03;H,4.21;N,18.68.
MS(EI) :373 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.85(3H,s),5.50(lH,s),
7.03(lH,dd,J=7.3Hz and 7.2Hz), 7.14(lH,d,J=7.3Hz), 7.33(lH,s),
7.37(lH,d,J=7.3Hz), 7.44-7.52(2H,m), 7.74-7.80(2H,m),
7.92(lH,d,J=7.3Hz), 10.02(lH,s), 12.25(lH,s).
Example 156
5-Cyano-4,7-dihydro-6-(3-methoxyphenyl)-4-(2-nitrophenyl)-2H-
~ razolo[3,4-b]pyridine
The title compound was prepared from methyl m-anisate,
2-nitrobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 95.
MP:220°C.
Anal. Calcd. for:CzoH15N503:C, 64.34;H, 4.05;N,18.76 .
Found:C,63.92;H,4.14;N,18.74.
3o MS(EI) :373(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 381(3H,s), 5.53(lH,s),
7.07(lH,d,J=7.3Hz), 7.14-7.18(2H,m), 7.33(lH,s),
7.40(lH,dd,J=7.3Hz and 7.2Hz), 7.50(lH,dd,J=7.3Hz and 7.2Hz),
99
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.63(lH,d,J=7.3Hz), 7.74(lH,dd,J=7.3Hz 7.2Hz),
7.91(lH,d,J=7.3Hz), 10.13(lH,s), 12.30(lH,s).
Example 157
5-Cyano-4,7-dihydro-6-(4-methoxyphenyl)-4-(2-nitrophenyl)-2H-
s pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl p-anisate,
2-nitrobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 95.
MP:215°C.
to Anal. Calcd. for:CZOH15N503:C, 64.34;H, 4.05;N,18 .76.
Found:C,64.13;H,,4.12;N,18.69.
MS (EI ) : 373 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.81(3H,s), 5.51(lH,s),
7.03(2H,d,J=7.3Hz), 7.33(lH,s), 7.47-7.55(3H,m), 7.61(lH,d),
.ts 7.74(lH,dd,J=7.3Hz and 7.2Hz), 7.91(lH,d,J=7.3Hz), 10.07(lH,s),
12.2$(lH,s).
Example 158
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
zo The title compound was prepared from methyl benzoate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:231°C.
Anal. Calcd. for:C19H1aN60:C,67.05;H,3.55;N,24.69.
25 Found:C,66.76;H,3.90;N,24.71.
MS(EI) :340 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.55(lH,s), 7.33(lH,s), 7.50-
7.64(7H,m), 7.95(lH,d,J=7.3Hz), 10.20(lH,s), 12.23(lH,s).
Example 159
30 4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(2-
methoxyphenyl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl o-anisate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
loo
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
same manner as in Example 95.
MP:180°C.
Anal. Calcd. for:C2pH14N6~2:C,64.86;H,3.81;N,22.69.
Found:C,64.11;H,3.98;N,22.34.
MS(EI):370(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.84(3H,s), 5.56(lH,s),
7.03(lH,dd,J=7.3Hz and 7.2Hz), 7.14(lH,d,J=6.8Hz), 7.33-
7.35(2H,m), 7.45(lH,dd,J=7.3Hz and 7.2Hz), 7.54(lH,d,J=7.3Hz),
7.65(lH,dd,J=8.8Hz and 6.8Hz), 7.94(lH,d,J=8.8Hz), 10.04(lH,s),
Io 12.18(lH,s).
Example 160
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(3-
methoxyphenyl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl m-anisate,
s5 2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:198°C.
Anal. Calcd. for:CaoH14Ns0~ 4/5 HaO:C,62.43;H,4.09;N,21.84.
Found:C,62.60;H,3.99;N,22.15.
2o MS(EI):370(M~).
1H-NMR (400MHz,DMSO-d6)b(ppm): 3.80(3H,s), 5.55(lH,s), 7.06-
7.17(3H,m), 7.33(lH,s), 7.40(lH,dd,J=7.3Hz),
7.52(lH,d,J=6.6Hz), 7.62(lH,dd,J=8.8Hz and 6.8Hz),
7.95(lH,d,J=6.8Hz), 10.18(lH,s), 12.24(lH,s).
25 Example 161
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(4-
methoxyphenyl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl p-anisate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
3o same manner as in Example 95.
MP:244°C.
Anal. Caled. for:CZOH14N602:C,64.86;H,3.81;N,22.69.
Found:C,64.77;H,3.91;N,22.49.
101
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :370(Mk) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.80(3H,s), 5.53(lH,s),
7.02(2H,d,J=7.3Hz), 7.32(lH,s), 7.50-7.53(3H,m),
7.61(lH,dd,J=8.8Hz and 6.8Hz), 7.94(lH,d,J=8.8Hz), 10.11(lH,s),
s 12.21(lH,s).
Example 162
4-(2,1,3-Benzothiadiazol-4-yl)-5-cyano-4,7-dihydro-6-phenyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl benzoate,
.to 2,1,3-benzothiadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:258°C.
MS(EI) :356 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.85(lH,s), 7.27(lH,s), 7.51
ls 7.52(3H,m), 7.61-7.67(3H,m), 7.76(lH,dd,J=8.8Hz and 6.8Hz.),
8.00(lH,d,J=8.8Hz); 10.13(lH,s), 12.16(lH,s).
Example 163
4-(2,1,3-Benzothiadiazol-4-yl)-5-cyano-4,7-dihydro-6-(2-
methoxyphenyl)-2H-pyrazolo[3,4-b]pyridine
2o The title compound was prepared from methyl o-anisate,
2,1,3-benzothiadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:231°C. 1
Anal. Calcd. for:C2oH14N6oS 3/10 H20:C, 61.30;H, 3.76;N,21.45.
2s Found:C,61.24;H,3.74;N,22.09.
MS(EI) :386 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.89(3H,s),5.85(lH,s),
706(lH,dd,J=7.6Hz and 7.3Hz), 7.17(lH,d,J=8.3Hz), 7.28(lH,s),
7.43-7.49(2H,m), 7.69(lH,d,J=6.8Hz), 7.80(lH,dd,J=8.8Hz and
30 6.8Hz), 7.99(lH,d,J=8.8Hz), 9.97(lH,s), 12.11(lH,s).
Example 164
4-(2,1,3-Benzothiadiazol-4-yl)-5-cyano-4,7-dihydro-6-(3-
methoxyphenyl)-2H-pyrazolo[3,4-b]pyridine
l02
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from methyl m-anisate,
2,1,3-benzothiadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:220°C.
s Anal. Calcd. for:CzoH14N60S:C,62.16;H,3.65;N,21.75.
Found:C,61.98;H,3.70;N,21.66.
MS (EI ) : 386 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 3.82(3H,s), 5.85(lH,s),
7.08(lH,d,J=8.3Hz), 7.19(lH,s), 7.23-7.27(2H,m),
.to 7.42(lH,dd,J=7.8Hz and 7.2Hz), 7.61(lH,d,J=6.6Hz),
7.75(lH,dd,J=8.8Hz and 6.8Hz), 7.99(lH,d,J=8.lHz), 10.10(lH,s),
12.16(lH,s).
Example 165
4-(2,1,3-Benzothiadiazol-4-yl)-5-cyano-4,7-dihydro-6-(4-
Is methoxyphenyl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl p-anisate,
2,1,3-benzothiadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:238°C.
2o MS(EI) :386 (M+) .
~H-NMR (400MHz,DMSO-d6)8(ppm): 3.81(3H,s), 5.83(lH,s),
7.04(2H,d,J=8.8Hz), 7.26(lH,s), 7.73-7.77(3H,m),
7.75(lH,dd,J=8.8Hz and 6.8Hz), 7.99{lH,d,J=8.8Hz), 10.04{lH,s),
12.14(lH,s).
2s Example 166
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(pyridin-4-
yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
isonicotinate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
3o aminopyrazole in the same manner as in Example 95.
MP:236°C.
MS(EI) :341 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.58(lH,s),7.35(lH,s), 7.54-
103
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.64(4H,m), 7.96(lH,d,J=8.lHz), 8.72(2H,d,J=5.9Hz),
10.40(lH,s), 12.29(lH,s).
Example 167
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(4-pyridin-
3-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl nicotinate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:216°C.
to Anal. Calcd. for:C18H11N~0 115 HZO:C,62.68;H,3.33;N,28.43.
Found:C,62.73;H,3.43;N,28.30.
MS(EI) :341 (M+) .
1H-NMR (400MHz,DMSO-d6)~(ppm): 5.59(lH,s), 7.35(lH,s), 7.52
7.63(3H,m), 7.95-8.00(2H,m), 8.69(lH,d,J=4.9Hz), 8.76(lH,s),
Is 10.39(lH,s), 12.28(lH,s).
Example 168
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(4- yridin-
2-yl)-2H-pyrazolo 3,4-b]pyridine
The title compound was prepared from methyl picolinate,
20 2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:188°C.
MS(EI) :341 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.59(lH,s), 7.34(lH,s), 7.51-
25 7.53(2H,m), 7.63(lH,dd,J=9.OHz and 6.6H2), 7.75(lH,d,J=6.6Hz),
7.95-7.97(2H,m), 8.69(lH,d,J=5.4Hz), 10.20(lH,s), 12.26(lH,s).
Example 169
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
(naphthalen-1-yl)-2H-pyrazolo[3,4-b]pyridine
3o The title compound was prepared from methyl 1-naphthoate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:213°C.
104
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:Cz3H14NsO:C, 70.76;H, 3.61;N, 21.53.
Found:C,70.33;H,3.74;N,21.23.
MS(EI) :390(M~) .
1H-NMR (400MHz,DMSO-d~)8(ppm): 5.65(lH,s), 7.35-7.66(7H,m),
7.96-8.21(4H,m), 10.35(lH,s), 12.23(lH,s).
Example 170
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-(furan-2-yl)-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl furan-2-
~o carboxylate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
aminopyrazole in the same manner as in Example 95.
MP:241°C.
Anal. Calcd. for:Cl~H1oN60a:C,61.82;H,3.05;N,25.44.
Found:C,61.72;H,3.19;N,25.34.
.ts MS(EI) :330(M~) .
iH-NMR (400MHz,DMSO-d6)b(ppm): 5.54(lH,s), 6.69(lH,s),
7.22(lH,d,J=3.4Hz), 7.32(lH,s), 7.48(lH,d,J=6.3Hz),
7.61(lH,dd,J=9.OHz and 6.3Hz), 7.89(lH,s), 7.94(lH,d,J=9.OHz),
10.17(lH,s), 12.26(lH,s).
2o Example 171
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(thiophen-
2-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
as aminopyrazole in the same manner as in Example 95.
MP:230°C.
Anal. Calcd, for:C17H1oN60S:C,58.95;H,2.91;N,24.26.
Found:C,58.71;H,3.08;N,24.03.
MS(EI) :346(M~) .
30 1H-NMR (400MHz,DMSO-d6)b(ppm): 5.54(lH,s), 7.17(lH,dd,J=4.9Hz
and 4.8Hz), 7.33(lH,s), 7.49(lH,d,J=6.6Hz), 7.58-7.64(2H,m),
7.77(lH,d,J=4.9Hz), 7.95(lH,d,J=9.OHz), 10.21(lH,s),
12.27(lH,s).
105
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 172
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
(naphthalen-2-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 2-naphthoate,
s 2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:228°C.
Anal. Calcd. for:CZgH14N6O:C,70.76;H,3.61;N,21.53.
Found:C,70.66;H,3.81;N,20.94.
1o MS(EI) :390(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.48(lH,s), 7.24(lH,s), 7.44-
7.55(5H,m), 7.85-7.92(4H,m), 8.05(lH,s), 10.21(lH,s),
12.14(lH,s).
Example 173
15 4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-(furan-2-yl)-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl furan-3-
carboxylate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
aminopyrazole in the same manner as in Example 95.
zo MP:237°C.
Anal. Calcd. for:Cl~H1oN60z:C,61.82;H,3.05;N,25.44.
Found:C,61.59;H,3.27;N,25.01.
MS(EI) :330 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.52(lH,s), 6.93(lH,d,J=l.OHz),
2s 7.31(lH,s), 7.48(lH,d,J=6.6Hz), 7.60(lH,dd,J=9.OHz and 6.6Hz),
7.80(lH,dd,J=l.OHz), 7.94(lH,d,J=9.OHz), 8.24(lH,s),
10.07(lH,s), 12.25(lH,s).
Example 174
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(thiophen-
30 3-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-3-
carboxylate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
aminopyrazole in the same manner as in Example 95.
106
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:242°C.
Anal. Calcd. for:C17H1oN60S:C,58.95;H,2.91;N,24.26.
Found:C,58.52;H,3.15;N,23.92.
MS (EI ) : 346 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.54(lH,s), 7.32(lH,s),
7.42(lH,d,J=5.lHz), 7.50(lH,d,J=6.6Hz), 7.61-7.66(2H,m),
7.94(lH,d,J=9.OHz), 8.00(lH,s), 10.13(lH,s), 12.24(lH,s).
Example 175
6-(Benzo[b]furan-2-yl)-4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-
.to 4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
benzo[b]furan-2-carboxylate, 2,1,3-benzoxadiazole-4-aldehyde
and 3-aminopyrazole in the same manner as in Example 95.
MP:>270°C.
i5 Anal. Calcd. for:CZlHiaNsOz:C,66.31;H,3.18;N,22.09.
Found:C,66.26;H,3.34;N,21.53.
MS(EI) :380(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.62(lH,s), 7.31-7.36(2H,m),
7.45(lH,dd,J=9.OHz and 6.7Hz), 7.53(lH,d,J=6.7Hz), 7.61-
20 7.65(3H,m), 7.77(lH,d,J=7.3Hz), 7.96(lH,d,J=9.OHz),
10.44(lH,s), 12.33(lH,s).
Example 176
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-methyl-2H-
pyrazolo[3,4-b]pyridine
2s The title compound was prepared from ethyl acetate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 94.
MP:212°C.
Anal. Calcd. for:C14H1oN60 3/5 HaO:C, 58 .17; H, 3 .91;N, 29 .07 .
3o Found:C,58.45;H,4.08;N,28.61.
MS(EI) :278 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 2.14(3H,s), 5.40(lH,s),
7.25(lH,s), 7.40(lH,d,J=6.6Hz), 7.59(lH,dd,J=9.OHz 6.6Hz),
107
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.92(lH,d,J=9.OHz), 9.98(lH,s), 12.13(lH,s).
Example 177
4-(2,1,3-Benzoxadiazol-4-yl)-6-butyl-5-cyano-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl pentanoate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 94.
MP:200°C.
Anal. Calcd. for:C17H16N60:C,63.74;H,5.03;N,26.23.
to Found:C,63.85;H,5.01;N,26.26.
MS(EI) :320 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.3Hz), 1.30-
1.39(2H,m), 1.57-1.65(2H,m), 2.06-2.40(2H,m), 5.39(lH,s),
7.25(lH,s), 7.39(lH,d,J=6.6Hz), 7.59(lH,dd,J=9.OHz and 6.6Hz),
.t5 7.91(lH,d,J=9.0Hz), 9.94(lH,s), 12.13(lH,s).
Example 178
Ethyl 4-(2-chloro-3-methylphenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
A suspension of 2-chloro-m-xylene (15 ml), N-
ao bromosuccinimide (23.3 g) and benzoyl peroxide (200 mg) in
carbon tetrachloride (150 ml) was heated under reflux for 6
hours. The insoluble material was filtered off and the
filtrate was concentrated under reduced pressure. The obtained
residue was purified by silica gel column chromatography
as (eluent: hexane) to give 2-bromomethyl-1-chloro-6-
methylbenzene (16.0 g) as a colorless oil. 2-Bromomethyl-1-
chloro-6-methylbenzene (25.4 g) and hexamethylenetetramine
(32.4 g) were dissolved in acetic acid-water (1:1, 10 ml) and
the mixture was heated under reflux for 5 hours. To the
3o reaction mixture was added concentrated hydrochloric acid (40
ml) and the mixture was heated under reflux for 1 hour. The
reaction mixture was extracted with ethyl acetate. The extract
was washed with an aqueous sodium hydrogencarbonate solution
108
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
and a saturated aqueous sodium chloride solution, and dried
over anhydrous magnesium sulfate. The solvent was evaporated
- to give 2-chloro-3-methylbenzaldehyde (19.4 g) as a yellow oil.
Subsequently, the title compound was prepared from 2-chloro-3-
s methylbenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 25.
MP:198-200°C.
Anal. Calcd. for:Cl9HaZC1N30z:C,63.42;H,6.16;N,11.68.
Found:C,63.19;H,6.14;N,11.71.
to MS(EI) :359 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.85(3H,t,J=7.3Hz),
0.97(3H,t,J=7.3Hz), 1.65(2H,m), 2.33(3H,s), 2.68-2.71(lH,m),
2.79-2.84(lH,m), 3.72-3.82(2H,m), 5.63(lH,s), 6.93-6.96(lH,m),
7.05-7.07(2H,m), 7.24(lH,s), 9.46(lH,s), 11.94(lH,s).
i5 Example 179
Ethyl 4-(2-chloro-3-nitrophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
To a solution of 2-chloro-3-nitrobenzoic acid (5.0 g) in
THF (50 ml) was added borane-tetrahydrofuran complex (1M THF
zo solution, 30 ml) under ice-cooling and the mixture was stirred
at room temperature for 24 hours. To the reaction mixture was
added a saturated aqueous sodium hydrogencarbonate solution
and the mixture was extracted with ethyl acetate. The extract
was washed with water and a saturated aqueous sodium chloride
z5 solution, and dried over anhydrous magnesium sulfate. The
solvent was evaporated to give a pale-yellow solid (3.7 g).
The obtained pale-yellow solid (1.6 g) and manganese dioxide
(1.7 g) were heated under reflux in toluene for 4.5 hours. The
insoluble material was filtered off and the filtrate was
3o concentrated under reduced pressure. The obtained residue was
purified by silica gel column chromatography (eluent: hexane-
ethyl acetate (4:1)) to give 2-chloro-3-nitrobenzaldehyde (1.3
g) as a pale-yellow solid. Subsequently, the title compound
109
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
was prepared from 2-chloro-3-nitrobenzaldehyde, 3-
aminopyrazole and ethyl 3-ketohexanoate in the same manner as
in Example 25.
MS(EI) :390(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85(3H,t,J=6.8Hz),
0.97(3H,t,J=7.3Hz), 1.64-1.68(2H,m), 2.70-2.85(2H,m), 3.73-
386(2H,m), 5.67(lH,s), 7.31(lH,s), 7.39-7.47(2H,m),
7.73(lH,dd,J=1.5,7.8Hz), 9.67(lH,s), 12.10(lH,s).
Example 180
.to Ethyl 4-(2-chloro-3-cyanophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
To a solution of 2-chloro-3-methylbenzaldehyde (19.4 g)
in ethanol (45 ml) was added an aqueous hydroxylamine
hydrochloride (9.7 g) solution (12 ml), and an aqueous sodium
hydroxide (6.9 g) solution (10 m1) was added. The mixture was
stirred at room temperature for 1.5 hours. Water (500 ml) was
added and the precipitated crystals were collected by
filtration. The obtained white crystals (16.1 g) were
dissolved in acetic anhydride (50 ml) and the mixture was
ao heated under reflux for 2.5 hours. The reaction mixture was
concentrated under reduced pressure and the obtained residue
was purified by silica gel column chromatography (eluent:
hexane-ethyl acetate (5:1)) to give 2-cyano-6-
methylchlorobenzene (10.9 g) as a white solid. A suspension of
2s 2-cyano-6-methylchlorobenzene (10.9 g), N-bromosuccinimide
(12.8 g) and benzoyl peroxide (523 mg) in carbon tetrachloride
(100 ml) was heated under reflux for 3.5 hours. The insoluble
material was filtered off and the filtrate was concentrated
under reduced pressure. The obtained residue was purified by
3o silica gel column chromatography (eluent: hexane-ethyl acetate
(20:1)) to give 2-chloro-3-cyanobenzaldehyde (12.8 g) as a
colorless oil. Subsequently, the title compound was prepared
from 2-chloro-3-cyanobenzaldehyde, 3-aminopyrazole and ethyl
110
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3-ketohexanoate in the same manner as in Example 25.
MP : 213-215°C .
Anal. Calcd. for:C19H19C1N4Oz:C,61.54;H,5.16;N,15.11.
Found:C,61.25;H,5.36;N,14.71.
s MS(EI) :370 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.86(3H,t,J=6.9Hz),
0.96(3H,t,J=7.3Hz), 1.65(2H,m), 2.70-2.80(2H,m), 3.73-
3.81(2H,m), 5.63(lH,s), 7.31(lH,s), 7.42-7.44(2H,m),
7.72(lH,dd,J=3.0,6.4Hz), 9.65(lH,s), 12.08(lH,s).
zo IR(KBr):v=3344,3292,2985,2954,2242,1652cm 1.
Example 181
Ethyl 4-(2,3-dibromophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
A suspension of 2-bromo-3-nitrotoluene (5.0 g), iron
15 (3.9 g) and ammonium chloride (3.7 g) in ethanol (50 ml)-water
(17 ml) was heated under reflux for 2 hours. The insoluble
matter was filtered off. To the filtrate was added ethyl
acetate (100 ml) and the mixture was washed with water and a
saturated aqueous sodium chloride solution, and dried over
zo anhydrous magnesium sulfate. The solvent was evaporated and
the obtained residue was purified by silica gel column
chromatography (eluent: hexane-ethyl acetate (5:1)) to give a
pale-yellow oil (4.8 g). The obtained pale-yellow oil (4.8 g)
was dissolved in 47o hydrobromic acid (50 ml). Under ice-
25 cooling, an aqueous sodium nitrite (1.6 g) solution (18 ml)
was added and the mixture was stirred under ice-cooling for 30
minutes. The reaction mixture was added dropwise to a solution
of cuprous bromide (2.0 g) in 47o hydrobromic acid (20 ml)
over 30 minutes and the mixture was stirred at 60°C for 4.5
3o hours. To the reaction mixture was added water (100 ml) and
the mixture was extracted with ethyl acetate. The extract was
washed with water and a saturated aqueous sodium
hydrogencarbonate solution, and dried over anhydrous magnesium
111
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
sulfate. The solvent was evaporated and the obtained residue
was purified by silica gel column chromatography (eluent:
hexane-ethyl acetate (9:1)) to give 2,3-dibromotoluene (2.6 g)
as a brown oil. A suspension of 2,3-dibromotoluene (2.6 g), N-
s bromosuccinimide (1.85 g) and benzoyl peroxide (50 mg) in
carbon tetrachloride (30 ml) was heated under reflux for 2
hours. The insoluble material was filtered off and the
filtrate was concentrated under reduced pressure. The obtained
residue was purified by silica gel column chromatography
to (eluent: hexane) to give a colorless oil (1.1 g). To a
solution of the obtained colorless oil (1.1 g) in dimethyl
sulfoxide (8.6 ml) - methylene chloride (2 ml) was added
trimethylamine-N-oxide (1.0 g) under ice-cooling and the
mixture was stirred at room temperature for 1 hour. The
15 reaction mixture was poured into water (50 ml) and the mixture
was extracted with ethyl acetate. The extract was washed with
5% hydrochloric acid, a saturated aqueous sodium
hydrogencarbonate solution and a saturated aqueous sodium
chloride solution, and dried over anhydrous magnesium sulfate.
ao The solvent was evaporated to give 2,3-dibromobenzaldehyde
(0.5 g) as a brown oil. Then the title compound was prepared
from 2,3-dibromobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP:180-183°C (decomposition}.
2s MS (EI ) : 469 (M+) .
1H-NMR. (400MHz,DMSO-d6)8(ppm): 0.86(3H,t,J=7.3Hz),
0.96(3H,t,J=7.3Hz),1.65(2H,m}, 2.70-2.80(2H,m), 3.72-
3.83(2H,m), 5.67(lH,s), 7.07(lH,d,J=5.8Hz),
7.18(lH,dd,J=5.8,7.8Hz), 7.48(lH,d,J=7.8Hz), 9.57(lH,s),
30 12.02(lH,s).
IR(KBr):v=3344,3292,2985,2954,2242,1652cm~'1.
Example 182
Ethyl 4-(2-bromo-3-nitrophenyl)-4,7-dihydro-6-propyl-2H-
112
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine-5-carboxylate 1/2 HZO
A suspension of 2-bromo-3-nitrotoluene (5.1 g), N-
bromosuccinimide (4.2 g) and benzoyl peroxide (229 mg) in
carbon tetrachloride (50 ml) was heated under reflux for 3
s hours. The insoluble material was filtered off and the
filtrate was concentrated under reduced pressure. The obtained
residue was purified by silica gel column chromatography
(eluent: hexane-ethyl acetate (10:1)) to give a yellow solid
(5.4 g). The obtained yellow solid (5.4 g) and
so hexamethylenetetramine (5.1 g) were dissolved in acetic acid-
water (1:1, 16 ml) and the mixture was heated under reflux for
2 hours. To the reaction mixture was added concentrated
hydrochloric acid (6 ml) and the mixture was heated under
reflux for 15 minutes. The reaction mixture was extracted with
s5 ethyl acetate. The extract was washed with water, an aqueous
sodium hydrogencarbonate solution and a saturated aqueous
sodium chloride solution and dried over anhydrous magnesium
sulfate. The solvent was evaporated, and the obtained residue
was purified by silica gel column chromatography (eluent:
zo hexane-ethyl acetate (5:1)) and crystallized (hexane-ethyl
acetate (5:1)) to give 2-bromo-3-nitrobenzaldehyde (1.2 g) as
yellow crystals. Subsequently, the title compound was prepared
from 2-bromo-3-nitrobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
2s MP : 213-215°C .
Anal. Calcd. for:C~$Hl9BrN404 1/2 HZO:C,48.66; H,4.54;N,12.61.
Faund:C,48.34;H,4.20;N,13.04.
MS(EI) :435 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.87(3H,t,J=7.3Hz),
30 0.97(3H,t,J=7.3Hz), 1.63-1.68(2H,m), 2.77-2.81(2H,m), 3.72-
3.85(2H,m), 5.68(lH,s), 7.33-7.36(2H,m),
7.47(lH,dd,J=7.8,7.8Hz), 7.66(lH,d,J=7.8Hz), 9.67(lH,s),
12.09(lH,s).
113
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 183
Ethyl 4-(2-bromo-3-cyanophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-bromo-m-xylene,
3-aminopyrazole and ethyl 3-ketohexanoate in the same manner
as in Example 180.
MP:210-212°C (decomposition).
Anal. Calcd. for:C19H19BrN402:C,54.95;H,4.61;N,13.49.
Found:C,54.98;H,4.94;N,13.11.
to MS(EI) :415 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.85(3H,t,J=6.8Hz),
0.97(3H,t,J=7.3Hz), 1.62-1.68(2H,m), 2.75-2.80(2H,m), 3.72-
3.83(2H,m), 5.63(lH,s), 7.32(lH,s), 7.39-7.48(2H,m),~
7.68(lH,dd,J=1.9,7.3Hz), 9.65(lH,s), 12.07(lH,s).
.t5 Example 184
4-(2-Chloro-3-cyanophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-chloro-3-cyanobenzaldehyde and 3-aminopyrazole in the same
ao manner as in Example 94.
MP:>250°C.
Anal. Calcd. for:C1~H14C13N5:C,63.06;H,4.36; N,21.63.
Found:C,63.10;H,4.42;N,21.61.
MS(EI) :323 (M+) .
z5 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz), 1.61-
1.71(2H,m), 2.35-2.49(2H,m), 5.47(lH,s), 7.32(lH,s), 7.52-
7.59(2H,m), 7.87(lH,dd,J=2.0,7.3Hz), 9.95(lH,s), 12.24(lH,s).
Example 185
4-(2-Chloro-3-nitrophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
3o pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-chloro-3-nitrobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
114
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:234-235°C.
Anal. Calcd. for:C16H14C1NsOZ: C,55.90;H,4.10;N,20.37.
Found:C,55.93;H,4.34;N,20.72.
MS(EI) :343 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz), 1.64-
1.69(2H,m), 2.37-2.45(2H,m), 5.52(lH,s), 7.34(lH,s), 7.54-
7.60(2H,m), 7.89(lH,dd,J=2.0,6.9Hz), 9.97(lH,s), 12.25(lH,s).
Example 186
4-(2-Bromo-3-cyanophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
Zo pyrazolo[3,4-b]pyridine 1/5 H2o
The title compound was prepared from methyl butanoate,
2-bromo-3-cyanobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:275-279°C (decomposition).
Anal. Calcd. for:C17H14BrN5 1/5 HaO:C,55.05;H,3.89;N,18.88.
Found:C,54.98;H,3.91;N,18.81.
MS(EI):368(M+)~
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz), 1.64-
1.69(2H,m), 2.38-2.43(2H,m), 5.47(lH,s), 7.33(lH,s), 7.54-
~0 7.60(2H,m), 7.83(lH,dd,J=2.0,7.4Hz), 9.95(lH,s), 12.24(lH,s).
Example 187
(+)Ethyl 4-(3,4-dihydro-2H-benzopyran-8-yl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
To a solution of the compound (1.94 g) described in
Example 73 in acetonitrile (15 ml) was added (-)-10-
camphorsulfonic acid (1.23 g) at 50°C and the mixture was
stirred under ice-cooling for 30 minutes. The precipitated
crystals were collected by filtration and recrystallized
(ethanol-ethyl acetate (2:1), 30 ml) to give white crystals
(0.81 g). The obtained white crystals were suspended in water
and a saturated aqueous sodium hydrogencarbonate solution was
added. The mixture was extracted with ethyl acetate and the
extract was washed with water and a saturated aqueous sodium
115
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
chloride solution, and dried over anhydrous magnesium sulfate.
The solvent was evaporated to give a colorless transparent oil.
The obtained colorless transparent oil was crystallized from
ethyl acetate to give the title compound (470 mg) as white
s crystals.
MP:159-161°C.
Anal. Calcd. for:Cz1H25N303:C, 68.64;H, 6.86;N,11.44.
Found:C,68.37;H,6.86;N,11.26.
Spec if is rotation : [ a. ] D=+2 0 0 ° ( EtOH, c=0 . 5 ) .
to MS (EI ) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.91(3H,t,J=6.8Hz),
0.98(3H,t,J=7.3Hz), 1.60-1.70(2H,m), 1.90-2.00(2H,m), 2.67-
2.82(4H,m), 3.81(2H,m), 4.25(2H,m), 5.42(lH,s),
6.62(lH,dd,J=7.4,7.8Hz), 6.72-6.76(2H,m), 7.18(lH,s),
is 9.26(lH,s), 11.81(lH,s).
Example 188
(-)Ethyl 4-(3,4-dihydro-2H-benzopyran-8-yl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
The filtrate obtained by filtering off the (-)-10-
2o camphorsulfonate salt in Example 187 was concentrated under
reduced pressure and suspended in water. To the suspension was
added a saturated aqueous sodium hydrogencarbonate solution
and the mixture was extracted with ethyl acetate. The extract
was washed with a saturated aqueous sodium chloride solution
z5 and dried over anhydrous magnesium sulfate. The solvent was
evaporated and the obtained residue was crystallized from
ethyl acetate to give white crystals (780 mg). By the same
process as in Example 187 using the obtained white crystals
and (+)-10-camphorsulfonic acid, the title compound (150 mg)
3o was obtained as white crystals.
MP:160-161°C.
Anal. Calcd. for:CalH2sN3~3:C, 68.64;H, 6.86; N,11.44.
Found:C,68.49;H,6.81;N,11.42.
116
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Specific rotation:[a]D=-202°(EtOH,c=0.5)
MS(EI) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.91(3H,t,J=6.8Hz),
0.98(3H,t,J=7.3Hz), 1.60-1.70(2H,m), 1.90-2.00(2H,m), 2.67-
2.82(4H,m), 3.81(2H,m), 4.25(2H,m), 5.42(lH,s), 6.62(lH,dd,
J=7.4,7.8Hz), 6.72-6.76(2H,m), 7.18(lH,s), 9.26(lH,s),
11.81(lH,s).
Example 189
4-(2-Bromo-3-nitrophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
.to pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-bromo-3-nitrobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:250-255°C (decomposition).
Anal. Calcd. for:C16H14BrN50z:C,49.50;H,3.63;N,18.04.
Found:C,49.37;H,3.76;N,18.02.
MS(EI) :388(Mk) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.95(3H,t,J=7.6Hz), 1.64-
1.70(2H,m), 2.39-2.44(2H,m), 5.53(lH,s), 7.34(lH,s),
ao 7.49(lH,d,J=7.8Hz), 7.60(lH,dd,J=7.8,8.OHz),
7.82(lH,d,J=8.OHz), 9.97(lH,s), 12.25(lH,s).
Example 190
Ethyl 4,7-dihydro-4-(2-methoxy-3-methylphenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
as A suspension of 2,6-dimethylphenol (19.5 g), iodomethane
(31 ml) and potassium carbonate (33.2 g) in dimethylformamide
(200 ml) was stirred at 60°C for 10 hours. The reaction
mixture was poured into water (300 m1) and the mixture was
extracted with ethyl acetate. The extract was washed with a
3o saturated aqueous sodium chloride solution and dried over
anhydrous magnesium sulfate. The solvent was evaporated and
the obtained residue was purified by silica gel column
chromatography (eluent: hexane) to give 2-methoxy-m-xylene (12
117
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
g) as a colorless oil. A suspension of 2-methoxy-m-xylene (5.1
g), N-bromosuccinimide (4.2 g) and bezoyl peroxide (229 mg) in
carbon tetrachloride (50 ml) was heated under reflux for 3
hours. The insoluble material was filtered off and the
filtrate was concentrated under reduced pressure. The obtained
residue was purified by silica gel column chromatography
(eluent: hexane-ethyl acetate (10:1)) to give a yellow solid
(5.4 g). The obtained yellow solid (5.4 g) and
hexamethylenetetramine (5.1 g) were dissolved in acetic acid-
.to water (1:1,16 ml) and the mixture was heated under reflux for
2 hours. To the reaction mixture was added concentrated
hydrochloric acid (6 ml) and the mixture was heated under
reflux for 15 minutes. The reaction mixture was extracted with
ethyl acetate. The extract was washed with water, an aqueous
i5 sodium hydrogencarbonate solution and a saturated aqueous
sodium chloride solution, and dried over anhydrous magnesium
sulfate. The solvent was evaporated and the obtained residue
was purified by silica gel column chromatography (eluent:
hexane-ethyl acetate (5:1)) and crystallized (hexane-ethyl
ao acetate (5:1)) to give 2-methoxy-3-methylbenzaldehyde (1.2 g)
as yellow crystals. Subsequently, the title compound was
prepared from 2-methoxy-3-methylbenzaldehyde, 3-aminopyrazole
and ethyl 3-ketohexanoate in the same manner as in Example 25.
MP : 220-222°C .
as Anal. Calcd. for:CaoHa5N303:C, 67.58;H, 7.09; N,11.82.
Found:C,67.47;H,7.02;N,11.91.
MS(EI) :355 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89(3H,t,J=7.OHz),
0.97(3H,t,J=7.3Hz), 1.60-1.70(2H,m), 2.23(3H,s), 2.66-
30 2.85(2H,m), 3.81(3H,s), 3.81-3.85(2H,m), 5.43(lH,s), 6.82-
6.91(3H,m), 7.13(lH,s), 9.31(lH,s), 11.82(lH,s).
Example 191
Ethyl 4-(3-cyano-2-methoxyphenyl)-4,7-dihydro-6-propyl-2H-
118
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-methoxy-3-
methylbenzaldehyde, 3-aminopyrazole and ethyl 3-ketohexanoate
in the same manner as in Example 180.
MP:220-222°C.
Anal. Calcd. for:C2oHz2N4O3:C, 65.56;H, 6.05; N,15.29.
Found:C,65.20;H,6.10;N,15.23.
MS(EI) :366(M'~) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89(3H,t,J=7.lHz),
ao 0.96(3H,t,J=7.3Hz), 1.60-1.70(2H,m), 2.70-2.80(2H,m), 3.75-
3.90(2H,m), 4.02(3H,s), 5.46(lH,s), 7.14-7.19(2H,m),
7.32(lH,d,J=6.lHz), 7.53(lH,d,J=7.8Hz), 9.51(lH,s),
11.97{lH,s).
Example 192
z5 5-Cyano-6-ethyl-4,7-dihydro-4-(2-nitrophenyl)-2H- ~razolo[3,4-
b]pyridine
The title compound was prepared from methyl propionate,
2-nitrobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
2o MP:228-230°C (decomposition).
Anal. Calcd. for:C~5H13NsOa :C, 61. 01; H, 4 . 44; N, 23 . 72 .
Found:C,60.72;H,4.51;N,23.78.
MS(ET):295(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.21(3H,t,J=7.4Hz), 2.42-
25 2.49(2H,m), 5.37(lH,s), 7.27(lH,s), 7.43-7.49(2H,m),
7.70(lH,dd,J=7.6,8.OHz), 7.89(lH,d,J=8.OHz), 9.94(lH,s),
12.21(lH,s).
Example 193
5-Cyano-4-(2,3-dichlorophenyl)-6-ethyl-4,7-dihydro-2H-
.~o ~ razolo[3,4-b]pyridine
The title compound was prepared from methyl propionate,
2,3-dichlorobenzaldehyde, 3-aminopyrazole and 1-cyanobutan-2-
one in the same manner as in Example 94.
119
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:>300°C.
Anal. Calcd. for:C15H12C1aN4:C,56.44;H,3.79;N,17.55.
Found:C,56.33;H,3.86;N,17.67.
MS(EI) :319 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.21(3H,t,J=7.6Hz), 2.38-
2.49(2H,m), 5.43(lH,s), 7.23(lH,d,J=6.8Hz), 7.31-7.37(2H,m),
7.51(lH,dd,J=1.7,8.1Hz), 9.92(lH,s), 12.19(lH,s).
Example 194
5-Cyano-6-ethyl-4,7-dihydro-4-(2-methoxyphenyl)-2H-
io pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl propionate,
2-methoxybenzaldehyde and 3-aminopyrazole in the same manner
as in Example 94.
MP:230-232°C.
Anal. Calcd. for:C~6H16N4O:C,68.55;H,5.75;N,19.99.
Found:C,68.16;H,5.97;N,20.39.
MS (EI) :280 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.22(3H,t,J=7.6Hz), 2.42-
2.49(2H,m), 3.84(3H,s),5.21(lH,s),6.86-6.91(lH,m),
ao 6.99(lH,d,J=8.3Hz), 7.05(lH,d,J=7.6Hz),7.15-7.19(2H,m),
9.68(lH,s), 12.02(lH,s).
Example 195
4-(2-Chloro-3-cyanophenyl)-5-cyano-6-ethyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl propionate,
2-chloro-3-cyanobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:>300°C.
Anal. Calcd. for:C16H12C1N~:C,62.04;H,3.90;N,22.61.
3o Found:C,61.74;H,4.14;N,22.93.
MS(EI):309(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.21(3H,t,J=7.6Hz), 2.42-
2.49(2H,m), 5.45(lH,s), 7.33(lH,s), 7.52-7.60(2H,m), 7.87
120
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
(lH,dd,J=2.0,7.3Hz), 9.97(lH,s), 12.23(lH,s).
Example 196
4-(2,1,3-Benzoxazol-4-yl)-5-cyano-6-ethyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
s The title compound was prepared from methyl propionate,
2,1,3-benzoxazole-4-aldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:206-208°C (decomposition).
Anal. Calcd. for:C15H12N60:C,61.64;H,4.14;N,28.75.
to Found:C,61.43;H,4.41;N,28.85.
MS(EI) :292 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.20(3H,t,J=7.6Hz), 2.40-
2.50(2H,m), 5.40(lH,s), 7.26(lH,s), 7.40(lH,d,J=6.6Hz),
7.58(lH,dd,J=6.6,9.OHz), 7.92(lH,d,J=9.OHz), 9.97(lH,s),
15 12.14(lH,s).
Example 197
4-(2-Chlorophenyl)-5-cyano-6-ethyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl propionate,
zo 2-chlorobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:>300°C.
Anal. Calcd. for:C15H13C1N4:C,63.27;H,4.60; N,19.68.
Found:C,63.14;H,4.69;N,19.67.
25 MS(EI) :284(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.21(3H,t,J=7.6Hz), 2.42-
2.49(2H,m), 5.35(lH,s), 7.22-7.26(3H,m), 7.30-7.34(lH,m),
7.42(lH,d,J=7.8Hz), 9.85(lH,s), 12.15(lH,s).
Example 198
30 4-(2-Bromo-3-cyanophenyl)-5-cyano-6-ethyl-4,7-dihydro-2H-
p~razolo[3,4-b]pyridine
The title compound was prepared from methyl propionate,
2-bromo-3-cyanobenzaldehyde and 3-aminopyrazole in the same
121
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
manner as in Example 94.
MP:>300°C.
Anal. Calcd. for:C16H12BrN5:C,54.25;H,3.41; N,19.77.
Found:C,54.13;H,3.56;N,19.98.
MS{EI) :354(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.21(3H,t,J=7.6Hz), 2.43(2H,m),
5.46(lH,s), 7.33(lH,s), 7.56-7.60(2H,m), 7.82-7.84(lH,m),
9.98(lH,s), 12.24(lH,s).
Example 199
.to 4-(2-Bromophenyl)-5-cyano-6-ethyl-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine
The title compound was prepared from methyl propionate,
2-bromobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
i5 MP:250-253°C (decomposition).
Anal. Calcd. for:C15H13BrN4:C, 54.73; H, 3.98; N,17 .02.
Found:C,54.28;H,3.96;N,16.94.
MS(EI) :329 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.21(3H,t,J=7.6Hz),2.42-
20 2.45(2H,m), 5.34(lH,s), 7.16(lH,dd,J=7.5,7.6Hz),
7.22(lH,d,J=6.6Hz), 7.27(lH,s), 7.36(lH,dd,J=6.3,7.3Hz),
7.59(lH,d,J=6.8Hz), 9.86(lH,s), 12.15(lH,s).
Example 200
Ethyl 4-(2-chlorophenyl)-6-cyano-4,7-dihydro-2H-pyrazolo[3,4-
a5 b]pyridine-5-carboxylate 1/4 hydrate
A solution of 1,1'-carbonylbis-1H-imidazole (22.5 g),
ethanol (8.1 ml) and toluene (100 ml) was stirred at room
temperature for 1.5 hours. To the reaction mixture was added
ice-water (100 m1) and the mixture was extracted with ethyl
3o acetate. The extract was washed with a saturated aqueous
sodium chloride solution and dried over anhydrous magnesium
sulfate. The solvent was evaporated and the obtained residue
was purified by silica gel column chromatography (eluent:
122
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
hexane-ethyl acetate (1:1)) to give a colorless oil (19.3 g).
A solution of the obtained residue (19.3 g) and pyruvic
aldehyde dimethyl acetal (11.1 ml) in toluene (50 ml) was
added dropwise to a suspension of sodium hydride (8.44 g) in
s toluene (250 ml) under reflux with heating, over 15 minutes,
and the mixture was heated under reflux for 1.5 hours. To the
reaction mixture was added a 10% aqueous citric acid solution
(610 ml) and the mixture was extracted with ethyl acetate. The
extract was washed with a saturated aqueous sodium chloride
to solution and dried over anhydrous magnesium sulfate. The
solvent was evaporated and the obtained residue was purified
by silica gel column chromatography (eluent: hexane-ethyl
acetate (5:1)) to give ethyl 4,4-dimethoxy-3-oxobutanoate
(15.1 g) as a colorless oil. Subsequently, ethyl 4-(2-
Zs chlorophenyl)-6-dimethoxymethyl-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate was obtained as a yellow solid from
2-chlorobenzaldehyde, 3-aminopyrazole and ethyl 4,4-dimethoxy-
2-oxobutanoate in the same manner as in Example 1. To a
solution of ethyl 4-(2-chlorophenyl)-6-dimethoxymethyl-4,7-
~o dihydro-2H-pyrazolo[3,4-b]pyridine-5-carboxylate (463 mg) in
tetrahydrofuran (5 ml) was added 1N hydrochloric acid (10 ml)
and the mixture was stirred at room temperature for 6 hours.
To the reaction mixture was added a saturated aqueous sodium
hydrogencarbonate solution and the mixture was extracted with
2s ethyl acetate. The extract was washed with a saturated aqueous
sodium chloride solution and dried over anhydrous magnesium
sulfate. The solvent was evaporated and the obtained residue
was purified by silica gel column chromatography (eluent:
hexane-ethyl acetate (1:1)) to give ethyl 4-(2-chlorophenyl)-
30 6-formyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
(290 mg) as a yellow solid. A solution of ethyl 4-(2-
chlorophenyl)-6-formyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine-
5-carboxylate (290 mg) and hydroxylamine-O-sulfonic acid
123
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
(128.5 mg) in water (10 ml)-ethanol (10 ml) was stirred at 80°C
for 2 hours. To the reaction mixture was added a saturated
aqueous sodium hydrogencarbonate solution and the mixture was
extracted with ethyl acetate. The extract was washed with a
saturated aqueous sodium chloride solution and dried over
anhydrous magnesium sulfate. The solvent was evaporated and
the obtained residue was purified by silica gel column
chromatography (eluent: hexane-ethyl acetate (1:1)) and
crystallized from ethanol-ethyl acetate to give the title
to compound (53 mg) as yellow crystals.
MP:275-278°C (decomposition).
Anal. Calcd. for:C16H13C1N4Oz 1/4 HZO:C,57.66;H,4.08;N,16.81.
Found:C,57.54;H,4.06;N,16.66.
MS(EI):328(M~).
Z5 1H-NMR (400MHz,DMSO-d6)b(ppm): 0.93(3H,t,J=7.lHz), 3.91(2H,m),
5.67(lH,s), 7.15-7.19(2H,m), 7.25(lH,dd,J=7.3,8.3Hz),
7.33(lH,s), 7.39(lH,d,J=8.3Hz), 10.81(lH,s), 12.34(lH,s).
Example 201
4-(2-Chloro-3-trifluoromethylphenyl)-5-cyano-4,7-dihydro-6-
2o propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate,
2-chloro-3-trifluoromethylbenzaldehyde and 3-aminopyrazole in
the same manner as in Example 94.
MP:>250°C.
2s Anal. Calcd. for:C17H14C1F3N4:C,55.67;H,3.85;N,15.28.
Found:C,55.81;H,3.97;N,15.44.
MS(EI) :366 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.95(3H,t,J=7.3Hz), 1.64-
1.70(2H,m), 2.40-2.43(2H,m), 5.55(lH,s), 7.31(lH,s), 7.54-
30 7.56(2H,m), 7.74(lH,dd,J=3.6,5.6Hz), 9.93(lH,s), 12.22(lH,s).
Example 202
4-(2-Chloro-3-trifluoromethylphenyl)-5-cyano-4,7-dihydro-6-
phenyl-2H-pyrazolo[3,4-b]pyridine
124
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from benzoic acid, 2-
chloro-3-trifluoromethylbenzaldehyde and 3-aminopyrazole in
the same manner as in Example 95.
MP:>250°C.
s Anal. Calcd. for:CZOHIZC1F3N4:C,59.94;H,3.02;N,13.98.
Found:C,59.74;H,3.18;N,13.95.
MS(EI) :400 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 570(lH,s), 7.39(lH,s), 7.49
7.51(3H,m), 7.57-7.62(3H,m), 7.75-7.79(2H,m), 10.18(lH,s),
l0 12.31(lH,s).
Example 203
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-isopropyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl isobutyrate,
15 2-chlorobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:>250°C.
Anal. Calcd. for:C16H15C1N4:C,64.32;H,5.06;N,18.75.
Found:C,64.18;H,5.12;N,18.84.
2o MS(EI) :298 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.23(3H,d,J=6.8Hz),
1.27(3H,d,J=6.8Hz), 3.06(lH,m), 5.34(lH,s), 7.22-7.26(3H,m),
7.30-7.34(lH,m), 7.42(lH,d,J=7.lHz), 9.63(lH,s), 12.16(lH,s).
Example 204
zs Ethyl 1-tert-butoxycarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
To a solution of the compound (1.2 g) described in
Example 27 and dimethylaminopyridine (128 mg) in THF (40 ml)
was added di-tert-butyldicarbonate (830 mg) and the mixture
3o was stirred at room temperature for one day. The solvent was
evaporated under reduced pressure and the title compound (102
mg) was obtained as colorless crystals by silica gel column
chromatography (eluent: hexane-ethyl acetate (3:1)).
125
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:112-116°C.
Anal. Calcd. for:CZ3H2gC1N3O4:C,61.95;H,6.33;N,9.42.
Found:C,61.84;H,6.33;N,9.34.
MS(EI) :445 (M+) .
s 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=6.9Hz),
0.98(3H,t,J=7.3Hz), 1.56(9H,s), 1.62-1.72(2H,m), 2.80-
2.92(2H,m), 3.85(2H,q,J=6.9Hz), 5.56(lH,s), 7.14-7.17(2H,m),
7.23(lH,dd,J=7.3 and 7.8Hz), 7.30(lH,s), 7.39(lH,d,J=7.4Hz),
8.75(lH,s).
io Example 205
Ethyl 2-tert-butoxycarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
used in Example 204 was further flowed hexane-ethyl acetate
15 (3:1) as an eluent, the title compound (300 mg) was obtained
as colorless crystals.
MP:144-147°C.
Anal. Calcd. for:Cz3Hz$C1N3O4:C,61.95;H,6.33;N,9.42.
Found:C,61.93;H,6.35;N,9.40.
2o MS(EI) :445 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.85(3H,t,J=6.9Hz),
0.97(3H,t,J=7.3Hz), 1.49(9H,s), 1.63-1.69(2H,m), 2.66-
2.85(2H,m), 3.80(2H,q,J=6.9Hz), 5.57(lH,s), 7.10-7.15(lH,m),
7.17(lH,ddd,J=1.5, 7.3 and 7.8Hz), 7.23(lH,dd,J=6.4 and 7.3Hz),
z5 7.41(lH,d,J=7.2Hz), 7.67(lH,s), 10.01(lH,s).
Example 206
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-1-methoxycarbonyl-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
.~o solid from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and methyl chloroformate in the same manner as in Example 204.
MS(EI) :403 (M+) .
126
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
IR(KBr):v=3422,1736,1699,1531,1450,1232,1086 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.lHz),
0.97(3H,t,J=7.3Hz), 1.60-1.66(2H,m), 2.86-2.89(2H,m),
3.83(2H,q,J=7.lHz), 3.94(3H,s), 5.55(lH,s),7.13-7.38(4H,m),
7.35(lH,s), 8.67(lH,s).
Example 207
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-2-methoxycarbonyl-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
to used in Example 206 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as
colorless crystals.
MP:141-143°C.
MS(EI) :403 (M+) .
.ts IR(KBr):v=3290,1774,1695,1633,1597,1523,1444,1364,1307,1209
cm 1.
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.86(3H,t,J=7.lHz),
0.95(3H,t,J=7.3Hz), 1.64-1.70,(2H,m), 2.71-2.85(2H,m),
3.78(2H,q,J=7.lHz), 3.85(lH,s), 5.57(lH,s),7.10-7.24(3H,m),
zo 7.42(lH,d,J=l.4Hz), 7.72(lH,s), 9.94(lH,s).
Example 208
Ethyl 1-benzyloxycarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
25 from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and benzyl chloroformate in the same manner as in Example 204.
MP:80°C.
Anal. Calcd. for:Cz6Hz6C1N3O4:C, 65.07;H, 5.46;N, 8 .75.
3o Found:C,65.24;H,5.71;N,8.50.
MS(EI) :479 (M+) .
IR(KBr):v=3344,1745,1701,1527,1451,1226,1084,1060 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.OHz),
127
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
0.94(3H,t,J=7.3Hz), 1.56-1.62(2H,m), 2.81-2.88(2H,m),
3.82(2H,q,J=7.OHz), 5.41(2H,s), 5.55(lH,s), 7.13-7.24(3H,m),
7.36(lH,s), 7.37(6H,m), 8.62(lH,s).
Example 209
Ethyl 2-benzyloxycarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Further elution using the column of silica gel column
chromatography in Example 208 and hexane-ethyl acetate (3:1)
as an eluent, the title compound was obtained as a colorless
to amorphous solid.
MS(EI) :479 (M+) .
IR(KBr):v=3294,1759,1697,1601,1383,1363,1300,1201 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85(3H,t,J=7.OHz),
0.95(3H,t,J=7.3Hz), 1.61-1.67(2H,m), 2.72-2.82(2H,m),
3.79(2H,q,J=7.OHz), 5.30(2H,s), 5.56(lH,s), 7.09-7.41(9H,m),
7.73(lH,s), 9.95(lH,s).
Example 210
Ethyl 1-benzoyl-4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate
2o The title compound was obtained as colorless crystals
from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and benzoyl chloride in the same manner as in Example 204.
MP:115°C.
2s Anal. Calcd. for:CZSHz4C1N3O3:C,66.74;H,5.38;N,9.34.
Found:C,66.58;H,5.41;N,9.28.
MS(EI):449(M+).
IR(KBr):v=3414,1680,1641,1516,1095 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90(3H,t,J=6.3Hz),
30 1.00(3H,t,J=7.3Hz), 1.65-1.71(2H,m), 2.90-2.93(2H,m),
3.85(2H,q,J=7.3Hz), 5.63(lH,s), 7.16-7.22(2H,m),
7.29(lH,d,J=7.3Hz), 7.40(lH,d,J=7.8Hz), 7.46(lH,s), 7.50-
7.54(2H,m), 7.65(lH,dd,J=6.3 and 7.8Hz), 7.98(lH,d,J=6.3Hz),
128
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
9.10(lH,s).
Example 211
Ethyl 2-benzoyl-4-(2-chlorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
s Through the column of silica gel column chromatography
used in Example 210 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as a
colorless amorphous solid.
MP:119-121°C.
to Anal. Calcd. for:Cz5H24C1N3O3:C,66.74;H,5.38;N,9.34.
Found:C,66.58;H,5.43;N,9.30.
MS (EI ) : 479 (M+) .
IR(KBr):v=3406,1670,1628,1601,1481,1348,1084 cm 1.
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.87(3H,t,J=6.8Hz),
.ts 0.97(3H,t,J=7.3Hz), 1.65-1.67(2H,m), 2.74-2.83(2H,m),
3.82(2H,q,J=7.3Hz), 5.65(lH,~s), 7.13-7.26(3H,m),
7.44(lH,d,J=7.8Hz), 7.47-7.51(2H,m), 7.60(lH,dd,J=7.3 and
7.3Hz), 7.91(2H,d,J=7.8), 8.00(lH,s), 10.06(lH,s).
Example 212
2o Ethyl 1-benzylcarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
solid from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
zs and phenylacetyl chloride in the same manner as in Example 204.
1H-NMR (400MHz,DMSO-d6)b(ppm):~0.89(3H,t,J=6.8Hz),
0.94(3H,t,J=7.3Hz), 1.60-1.61(2H,m), 2.84-2.86(2H,m),
3.82(2H,q,J=6.8Hz), 4.47(2H,s), 5.59(lH,s), 7.20-9.44(lOH,m),
8.90(lH,s).
~o Example 213
Ethyl 2-benz_ylcarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
129
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
used in Example 212 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as a
colorless amorphous solid.
MS(EI) :463(M+) .
IR(KBr):v=3308,1699,1628,1630,1599,1523 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=6.8Hz),
0.98(3H,t,J=7.3Hz), 1.65-1.71(2H,m), 2.77-2.84(2H,m),
3.83(2H,q,J=6.8Hz), 4.25(2H,s), 5.60(lH,s), 7.11-7.31(8H,m),
7.41(lH,d,J=7.8Hz), 7.84(lH,s), 10.30(lH,s).
to Example 214
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-1-phenylcarbamoyl-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
solid from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
is pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and phenyl isocyanate in the same manner as in Example 204.
MS(EI) :464(M+) .
IR(KBr):v=3310,1699,1597,1518,1448,1369,1228,1194,1093 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.98(3H,t,J=7.lHz),
zo 0.97(3H,t,J=7.3Hz), 1.96(2H,m), 2.87(2H,m), 3.83(2H,q,J=7.lHz),
5.61(lH,s), 7.11-7.69(BH,m), 7.67(2H,d,J=7.8Hz), 8.86(lH,s),
10.31(lH,s).
Example 215
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-2-phenylcarbamoyl-6-
25 propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
used in Example 214 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as
colorless crystals.
3o MP:145-147°C.
Anal. Calcd. for:C25HzsC1N403:C, 64.58;H, 5.42;N,12.05.
Found:C,64.10;H,5.41;N,12.30.
MS(EI) :464(M+) .
130
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
IR(KBr):v=3341,1697,1653',1630,1597,1520,1367,1197,1093 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.OHz),
0.97(3H,t,J=7.3Hz),1.68(2H,m), 2.80-2.92(2H,m),
3.82(2H,q,J=7.OHz), 5.62(lH,s),7.10-7.20(3H,m),
s 7.22(lH,dd,J=7.1 and 7.lHz), 7.31-7.33(2H,m),
7.41(lH,d,J=7.lHz),7.58-7.60(2H,m), 7.85(lH,s), 9.67(lH,s),
9.83(lH,s).
Example 216
Ethyl 1-benzylcarbamoyl-4-(2-chlorophenyl)-4,7-dihydro-6-
Io propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
solid from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and benzyl isocyanate in the same manner as in Example 204.
zs MS(EI) :478 (M+) .
IR(KBr):v=3402,1699,1637,1525,1226,1091 cm 1:
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.88(3H,t,J=7.OHz),
0.96(3H,t,J=7.3Hz), 1.61-1.63(2H,m), 2.83(2H,m),
3.82(2H,q,J=7.0Hz), 4.37(2H,d), 5.58(lH,s), 7.11-7.31(9H,m),
20 7.38(lH,d,J=7.8Hz), 8.74(lH,s), 9.01(lH,s).
Example 217
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-1-phenoxycarbonyl-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
zs solid from ethyl 4 -(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and phenyl chloroformate in the same manner as in Example 204.
MS(EI) :465 (M+) .
IR(KBr):v=3339,1728,1633,1525,1371,1302,1224,1091 cm 1.
30 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.83(3H,t,J=7.lHz),
0.96(3H,t,J=7.3Hz), 1.70(2H,m), 2.94(2H,m), 3.82(2H,q,J=7.lHz),
5.62(lH,s), 7.12-7.53(9H,m), 8.26(lH,s), 9.30(lH,s).
Example 218
131
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-2-phenoxycarbonyl-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
used in Example 217 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as
colorless crystals.
MP:156-157°C.
Anal. Calcd. for:CZSHz4C1N3O4:C,64.44;H,5.19;N,9.02.
Found:C,64.42;H,5.31;N,9.04.
1o MS(EI):465(M+).
IR(KBr):v=3325,1765,1685,1597,1525,1373,.1205,1099 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.lHz),
1.00(3H,t,J=7.3Hz), 1.67-1.69(2H,m), 2.76-2.85(2H,m),
3.82(2H,q,J=7.lHz), 5.61(lH,s), 7.15(lH,dd,J=1.7 and 6.8Hz),
7.15(lH,dd,J=1.7 and 6.8Hz), 7.17(IH,dd,J=2.0 and 7.6Hz),
7.24(lH,dd,J=1.3 and 7.4Hz), 7.27-7.31(3H,m), 7.41-7.45(3H,m),
7.89(lH,s), 10.01(lH,s).
Example 219
Ethyl 4-(2-chlorophenyl)-1-ethoxycarbonyl-4,7-dihydro-6-
2o propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and ethyl chloroformate in the same manner as in Example 204.
2s MP:88-89°C.
Anal. Calcd. for:CzlHz4C1N3O4:C,60.36;H,5.79;N,10.06.
Found:C,60.24;H,5.72;N,10.05.
MS(EI):417(M+).
IR(KBr):v=3422,1734,1705,1647,1591,1531,1228,1086,1062 cm 1.
30 1H-NMR (400MHz,DMSO-d6)b(ppm): 0.87(3H,t,J=7.lHz),
0.96(3H,t,J=7.3Hz), 1.31(3H, t,J=7.lHz), 1.61-1.66(2H,m),2.83-
2.92(2H,m), 3.83(2H,q,J=7.lHz), 4.41(2H,q,J=7.lHz), 5.55(lH,s),
7.13-7.16(2H,m), 7.25(lH,dd,J=7.0 and 7.6Hz), 7.34(lH,s),
132
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.38(lH,d,J=7.6Hz), 8.65(lH,s).
Example 220
Ethyl 4-(2-chlorophenyl)-2-ethoxycarbonyl-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
used in Example 219 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as a
colorless amorphous solid.
MS(EI):417(Mk).
.to IR(KBr):v=3325,1765,1685,1631,1597,1525,1373,1205,1099 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.lHz),
0.97(3H,t,J=7.3Hz), 1.26(3H,t,J=7.OHz), 1.63-1.69(2H,m), 2.74-
2.81(2H,m), 3.81(2H,q,J=7.lHz), 4.29(2H,q,J=7.OHz), 5.57(lH,s),
7.12(lH,dd,J=6.3 and 7.5Hz), 7.17(lH,d,J=7.8Hz),
Is 7.23(lH,dd,J=6.3 and 7.4Hz), 7.40(lH,d,J=7.8Hz), 7.71(lH,s),
9.96(lH,s).
Example 221
Eth~l 4-(2-chlorophenyl)-4,7-dihydro-1-propoxycarbonyl-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
2o The title compound was obtained as colorless crystals
from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and propyl chloroformate in the same manner as in Example 204.
MP:66-68°C.
25 MS(EI) :431(M~) .
IR(KBr):v=3356,1738,1695,1527,1282,1084 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.OHz),
0.92(3H,t,J=7.3Hz), 0.97(3H,t,J=7.3Hz), 1.62-1.67(2H,m), 1.70-
1.75(2H,m), 2.85-2.92(2H,m),3.83(2H,q,J=7.OHz),
30 4.32(2H,t,J=6.5Hz), 5.57(lH,s), 7.14-7.18(2H,m),
7.26(lH,dd,J=6.3 and 7.6Hz), 7.35(lH,s), 7.39(lH,d,J=7.8Hz)
9.10(lH,s).
Example 222
133
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-2-propoxycarbonyl-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
used in Example 221 was further flowed hexane-ethyl acetate
s (3:1) as an eluent, the title compound was obtained as
colorless crystals.
MP:59°C.
Anal. Calcd. for:CzzHa6C1N3O4:C, 61.18;H, 6 .07;N, 9.73.
Found:C,60.81;H,5.98;N,9.74.
zo MS(EI) :431(M+) .
IR(KBr):v=3296,1761,1697,1633,1599,1523,1365,1218,1089 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.lHz),
0.89(3H,t,J=7.5Hz), 0.97(3H,t,J=7.3Hz), 1.63-1.69(4H,m), 2.74-
2.81(2H,m), 3.81(2H,q,J=7.lHz), 4.21(2H,t,J=6.6Hz), 5.58(lH,s),
1s 7.12(lH,dd,J=1.8 and 7.6Hz), 7.17(lH,ddd,J=1.9, 7.3 and 7.6Hz),
7.22(lH,ddd,J=1.2, 7.3 and 7.6Hz), 7.41(lH,dd,J=1.2 and 7.8Hz),
7.72(lH,s), 9.99(lH,s).
Example 223
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-2-isobutylyl-6-propyl-2H-
zo pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
solid from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and isobutyryl chloride in the same manner as in Example 204.
2s 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.84(3H,t,J=7.OHz),
0.96(3H,t,J=7.3Hz), 1.10(3H,d,J=6.9Hz), 1.14(3H,d,J=6.8Hz),
1.64-1.70(2H,m), 2.75-2.83(2H,m), 3.53(lH,q,J=7.OHz),
3.83(2H,t,J=6.9Hz), 5.59(lH,s), 7.12(lH,s), 7.16(lH,dd,J=5.8
and 7.8Hz), 7.24(lH,dd,J=6.3 and 7.5Hz), 7.41(lH,s),
30 7.81(lH,s), 10.05(lH,s).
Example 224
Ethyl 1-acetyl-4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate
134
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was obtained as colorless crystals
from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and acetyl chloride in the same manner as in Example 204.
s MP:75-76°C.
Anal. Calcd. for:CaoHzzC1N303:C, 61.93;H, 5.72;N,10.83.
Found:C,61.77;H,5.78;N,10.90.
MS(EI) :387 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89(3H,t,J=7.3Hz),
l0 0.97(3H,t,J=7.3Hz), 1.60-1.66(2H,m), 2.66(3H,s),2.85-
2.90(2H,m), 3.81(2H,q,J=7.3Hz), 5.57(lH,s),7.14-7.18(2H,m),
7.26(lH,dd,J=7.3 and 7.6Hz), 7.38(lH,s), 7.39(lH,d,J=8.lHz),
8.90(lH,s).
Example 225
.t5 Ethyl 2-acetyl-4-(2-chlorophenyl)-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
used in Example 224 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as a
2o colorless amorphous solid.
MS(EI) :387(M~) .
IR(KBr):v=3306,1699,1633,1601,1523,1371,1197,1086 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.OHz),
0.96(3H,t,J=7.3Hz), 1.66(2H,m), 2.44(3H,s), 2.65-2.85(2H,m),
z5 3.80(2H,q,J=7.OHz), 5.58(lH,s), 7.09-7.22(3H,m),
7.40(lH,d,J=7.9Hz), 7.80(lH,s), 10.0(lH,s).
Example 226
Ethyl 1-butoxycarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
3o The title compound was obtained as a colorless amorphous
solid from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and butyl chloroformate in the same manner as in Example 204.
135
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :445 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.87(3H,t,J=7.3Hz),
0.88(3H,t,J=7.lHz), 1.33-1.38(2H,m), 1.60-1.69(4H,m), 2.85-
2.87(2H,m), 3.82(2H,q,J=7.3Hz), 4.36(2H,t,J=6.5Hz), 5.55(lH,s),
7.13-7.17(2H,m), 7.25(lH,dd,J=6.4 and 6.5Hz), 7.34(lH,s),
7.37(lH,d,J=7.5Hz), 8.61(lH,s).
Example 227
Ethyl 2-butoxycarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine-5-carboxylate
to Through the column of silica gel column chromatography
used in Example 226 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as a
colorless amorphous solid.
MS(EI) :445 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.86(3H,t,J=7.3Hz),
0.87(3H,t,J=7.3Hz), 0.96(3H,t,J=7.3Hz), 1.31-1.32(2H,m),
1.61(4H,m), 2.73-2.80(2H,m),3.80(2H,q,J=7.3Hz),
4.24(2H,t,J=6.5Hz), 5.57(lH,s), 7.09-7.22(3H,m),
7.39(lH,d,J=7.8Hz), 7.70(lH,s), 9.98(lH,s).
2o Example 228
Ethyl 4-(2-chlorophenyl)-1-cinnamoyl-4,7-dihydro-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
solid from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-1H-
z5 pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and cinnamoyl chloride in the same manner as in Example 204.
MP :131-134°C .
Anal. Calcd. for:Cz7Hz6C1N3O3:C,68.13;H,5.51;N,8.83.
Found:C,68.04;H,5.58;N,8.75.
3o MS(EI) :475(M+) .
IR(KBr):v=3396,1687,1624,1521,1394,1207,1087 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89(3H,t,J=7.OHz),
0.98(3H,t,J=7.lHz), 1.62-1.68(2H,m), 2.89-2.91(2H,m),
136
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
3.84(2H,q,J=7.OHz), 5.60(lH,s), 7.16(lH,dd,J=7.4 and 7.8Hz),
7.18(lH,d,J=6.3Hz), 7.26(lH,dd,J=6.3 and 7.4Hz),
7.39(lH,d,J=7.8Hz), 7.45(lH,s), 7.46(3H,m), 7.67(lH,d,J=6.lHz),
7.69-7.76(2H,m), 7.91(lH,d,J=7.4Hz), 9.01(lH,s).
Example 229
Ethyl 4-(2-chlorophenyl)-1-cinnamoyl-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
Through the column of silica gel column chromatography
used in Example 228 was further flowed hexane-ethyl acetate
so (3:1) as an eluent, the title compound was obtained as
colorless crystals.
MS(EI) :475(M+) .
IR(KBr):v=3304,1695,1674,1601,1521,1365,1168,1095 Cm 1~.
1H-NMR (400MHz,DMSO-d6)S(ppm): 0.86(3H,t,J=7.OHz),
.t5 0.98(3H,t,J=7.3Hz), 1.65-1.70(2H,m), 2.76-2.87(2H,m),
3.82(2H,q,J=7.OHz), 5.62(lH,s), 7.12-7.18(2H,m),
7.24(lH,dd,J=7.3 and 7.3Hz), 7.42(lH,d,J=7.8Hz), 7.45-
7.46(3H,m), 7.60(lH,d,J=6.lHz), 7.62-7.70(2H,m),
7.86(lH,d,J=6.lHz), 7.85(lH,s), 10.09(lH,s).
2o Example 230
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-3-methyl-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from 2-ethylbenzaldehyde, 3-amino-5-methylpyrazol and ethyl 3-
a5 ketohexanoate in the same manner as in Example 25.
MP:164-165°C.
MS(EI) :359 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.OHz),
1.02(3H,t,J=7.3Hz), 1.61(2H,m), 1.89(3H,s), 2.60-2.85(2H,m),
30 3.80(2H,q,J=7.OHz), 5.44(lH,s), 7.00-7.30(4H,m), 9.39(lH,s),
11.66(lH,s).
Example 231
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-2-methyl-6-propyl-2H-
137
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine-5-carboxylate
A solution of ethyl 3-ketohexanoate (7.5 g), 2-
chlorobenzaldehyde (6.6 g), piperidine (1.2 g) and acetic acid
(2.25 g) in benzene (50 ml) was heated under ref lux for 5
s hours, and the reaction mixture was dehydrated using a Dean-
Stark condenser. The solvent was evaporated under reduced
pressure, and the residue was purified by silica gel column
chromatography using an eluent (hexane-ethyl acetate (3:1)) to
give ethyl 2-(2-chlorophenyl)methylen-3-oxohexanoate
((E)/(Z)=1:1 mixture) as a yellow oil. A solution of ethyl 2-
(2-chlorophenyl)methylene-3-oxohexanoate ((E)/(Z)=1:1 mixture,
2.8 g), 3-amino-1-methylpyrazole (0.25 g) and p-
toluenesulfonic acid (25 mg) in toluene (5 mL) and
dimethylsulfoxide (0.5 mL) was heated under reflex for one day.
The solvent was evaporated under reduced pressure, and the
mixture was extracted with ethyl acetate (10 mL) and washed
with a saturated aqueous sodium chloride solution. The organic
layer was dried over anhydrous sodium sulfate and the solvent
was evaporated under reduced pressure to give an oil. The
obtained oil was purified by silica gel column chromatography
(eluent (ethyl acetate-methanol (10:1))) to give the title
compound as colorless crystals.
MP:150-151°C.
MS(EI) :359 (M+) .
2s 1H-NMR (400MHz,DMSO-d6)b(ppm): 0.83(3H,t,J=7.OHz),
0.96(3H,t,J=6.5Hz), 1.65(2H,m), 2.67-2.85(2H,m), 3.58(3H,s),
3.77(2H,q,J=7.OHz), 5.55(lH,s), 7.07-7.11(2H,m),
7.19(lH,dd,J=7.4 and 7.8Hz), 7.24(lH,d,J=8.3Hz), 9.45(lH,s).
Example 232
3o Ethyl 4-(2-chlorophenyl)-4,7-dihydro-1-methyl-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as a colorless amorphous
solid from ethyl 2-(2-chlorophenyl)methylen-3-oxohexanoate
138
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
((E)/(Z)=1:1 mixture), 3-amino-2-methylpyrazole and p-
toluenesulfonic acid.
MS(EI) :359 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.84(3H,t,J=7.OHz),
1.00(3H,t,J=7.lHz), 1.67-1.69(2H,m), 2.70-2.88(2H,m),
3.65(3H,s), 3.80(2H,q,J=7.OHz),5.55(lH,s), 6.96(lH,s), 7.08-
7.12(2H,m), 7.20(lH,dd,J=6.8 and 7.8Hz), 7.35(lH,d,J=7.8Hz),
9.31(lH,s).
Example 233
to Ethyl 4,7-dihydro-1-methyl-4-(naphthalen-1-yl)-6-propyl-1H-
pyrazolo[3,4-b]pyridine-5-carboxylate
A solution of ethyl 3-ketohexanoate (6.6 g), 1-
naphthaldehyde (7.34 g), piperidine (1.2 g) and acetic acid
(2.25 g) in benzene (50 mL) was heated under reflux for 3
i5 hours and the reaction mixture was dehydrated using a Dean-
Stark condenser. The solvent was evaporated and the residue
was purified by silica gel column chromatography using an
eluent (hexane-ethyl acetate (3:1)) to give ethyl 2-
(naphthalen-1-yl)methylene-3-oxohexanoate ((E)/(Z)=1:1
ao mixture) as a yellow oil. The title compound was obtained as a
colorless amorphous solid from ethyl 2-(naphthalen-1-
yl)methylene-3-oxohexanoate ((E)/(Z)=1:1 mixture), 3-amino-2-
methylpyrazole and p-toluenesulfonic acid.
MS(EI):375(M+).
z5 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.60(3H,t,J=6.9Hz),
1.03(3H,t,J=6.9Hz),1.74(2H,m), 2.78-2.85(2H,m), 3.65(3H,s),
3.68(2H,q,J=6.9Hz),5.94(lH,s), 6.76(lH,s), 7.20(lH,d,J=7.3Hz),
7.37(lH,dd,J=7.4 and 7.8Hz), 7.50(lH,dd,J=6.9 and 7.8Hz),
7.58(lH,m), 7.67(lH,d,J=8.3Hz), 7.88(lH,d,J=8.3Hz),
30 8.42(lH,d,J=8.8Hz), 9.26(lH,s).
Example 234
Ethyl 4-(3-chlorophenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
139
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was obtained as colorless crystals
from 3-chlorobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP :140-143°C .
Anal. Calcd. for:ClgHZOC1N30Z 2/5 HZO:C,61.24;H,5.94;N,11.90.
Found:C,61.50;H,5.94;N,11.99.
MS(EI) :345 (M+) .
IR(KBr):v=3263,1736,1666,1591,1514,1275,1222,1207,1087 cm 1.
1H-NMR (400MHz,DMSO-d6)~(ppm): 0.95(3H,t,J=7.OHz),
.to 1.04(3H,t,J=7.lHz), 1.58-1.63(2H,m), 2.63-2.81(2H,m),
3.86(2H,q,J=7.OHz), 5.11(lH,s), 7.08(lH,d,J=7.8Hz), 7.12(2H,m),
7.21(lH,d,J=8.3Hz), 7.26(lH,s), 9.84(lH,s), 11.99(lH,s).
Example 235
Ethyl 4-(4-chlorophenyl)-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from 4-chlorobenzaldehyde, 3-aminopyrazole and ethyl 3-
ketohexanoate in the same manner as in Example 25.
MP :159-161°C .
zo Anal. Calcd. for:ClBHzoCIN3Oz 1/5 HZO:C,61.87;H,5.88;N,12.03.
Found:C,61.92;H,6.23;N,11.95.
MS(EI) :345 (M+) .
IR(KBr):v=3263,1730,1662,1593,1516,1207,1091 cm 1.
1H-NMR (400MHz,DMSO-d6)~(ppm): 0.92(3H,t,J=7.OHz),
0.95(3H,t,J=7.3Hz), 1.60(2H,m), 2.64-2.80(2H,m),
3.84(2H,q,J=7.OHz), 5.10(lH,s), 7.13(2H,d,J=7.3Hz), 7.22(lH,s),
7.25(2H,d,J=7.3Hz), 9.45(lH,s), 11.96(lH,s).
Example 236
Ethyl 4,7-dihydro-4-(4-methyl-1H-imidazol-5-yl)-6-propyl-2H-
3o pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from 4-methyl-5-imidazolecarboxaldehyde, 3-aminopyrazole and
ethyl 3-ketohexanoate in the same manner as in Example 25.
140
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:219-220°C.
Anal. Calcd. for:C16Hz1NsOz 1/2 HzO:C,59.61;H,6.25;N,21.72.
Found:C,59.34;H,6.48;N,22.06.
MS(EI) :315(M+) .
s IR(KBr):v=3113,2980,1687,1620,1568,1244,1159 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz),
1.08(3H,t,J=7.OHz), 1.58-1.59(2H,m), 2.21(3H,s), 2.58-
2.79(2H,m), 3.97(2H,q,J=7.3Hz), 5.50(lH,s), 6.14(lH,s),
7.14(lH,s), 7.19(lH,s), 9.78(lH,s), 11.53(lH,s).
.to Example 237
Ethyl 4,7-dihydro-4-(1-methyl-1H-imidazol-2-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from 1-methyl-2-imidazolecarboxaldehyde, 3-aminopyrazole and
15 ethyl 3-ketohexanoate in the same manner as in Example 25.
MP:209°C.
Anal. Calcd. for:Cl6HziNsOz 3/5 HzO:C,59.28;H,6.28;N,21.60.
Found:C,59.00;H,6.52;N,21.55.
MS(EI) :315 (M+) .
ao IR(KBr):v=3254,3184,3080,1685,1593,1518,1278,1207,1078 cm 1.
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91(3H,t,J=6.8Hz),
0.93(3H,t,J=7.3Hz), 1.55-1.61(2H,m), 2.57-2.80(2H,m),
3.44(3H,s), 3.87(2H,q,J=6.8Hz), 5.29(lH,s), 6.56(lH,s),
6.84(lH,s), 7.27(lH,s), 9.38(lH,s), 11.97(lH,s).
25 Example 238
Ethyl 4,7-dihydro-4-(1H-imidazol-5-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from 3-imidazolecarboxyaldehyde, 3-aminopyrazole and ethyl 3-
3o ketohexanoate in the same manner as in Example 25.
MP:200°C.
Anal. Calcd. for:Cls~H1~N50z 1/2 HzO:C,58.43;H,5.88;N,22.71.
Found:C,58.53;H,6.25;N,22.93.
141
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :301 (M+) .
IR(KBr):v=3217,1655,1585,1506,1226,1205,1084 cm 1.
1H-NMR (400MHz,DMSO-ds)8(ppm): 0.92(3H,t,J=7.3Hz),
1.01(3H,t,J=7.3Hz), 1.57-1.59(2H,m), 2.59-2.74(2H,m),
s 3.90(2H,q,J=7.3Hz), 5.12(lH,s), 6.35(lH,s), 7.35(lH,s),
7.38(lH,s), 9.21(lH,s), 11.91(lH,s).
Example 239
Ethyl 4-(2,1,3-benzoxadiazol-4-yl)-6-butyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
.zo The title compound was prepared from 2,1,3-
benzoxadiazole-4-aldehyde, 3-aminopyrazole and ethyl 3-
ketoheptanoate in the same manner as in Example 1.
MP:213°C.
Anal. Calcd. for:C19H21N5O3:C, 62.11;H,5.76;N,19.06.
Is Found:C,62.08;H,5.75;N,18.95.
MS(EI) :367 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.77(3H,t,J=7.3Hz),
0.92(3H,t,J=7.3Hz), 1.32-1.40(2H,m), 1.60-1.64(2H,m), 2.76-
2.86(2H,m), 3.76-3.82(2H,m), 5.68(lH,s), 7.11(lH,d,J=6.6Hz),
20 7.22(lH,s), 7.51(lH,dd,J=9.OHz and 6.6Hz), 7.77(lH,d,J=9.OHz),
9.65(lH,s), 12.00(lH,s).
Example 240
Ethyl 4-(2,1,3-benzoxadiazol-4-yl)-6-ethyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
2s The title compound was prepared from 2,1,3-
benzoxadiazole-4-aldehyde, 3-aminopyrazole and ethyl 3-
ketopentanoate in the same manner as in Example 1.
MP:196°C.
Anal. Calcd. for:Cl~H1~N503:C,60.17;H,5.05;N,20.64.
3o Found:C,60.09;H,5.15;N,20.41.
MS(EI) :339 (M+) .
1H-NMR (400MHz,DMSO-d6)S(ppm): 0.75 (3H,t, J=7.3Hz), 1.21 (3H,
t, J=7.3Hz), 2.83(2H,q,J=7.3Hz), 3.73-3.84(2H,m), 5.68(lH,s),
142
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.12(lH,d,J=6.6Hz), 7.22(lH,s), 7.50(lH,dd,J=9.OHz and 6.6Hz),
7.77(lH,d,J=9.OHz), 9.68(lH,s), 12.01(lH,s).
Example 241
4(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-(thiophen-2-yl)-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, 2-chlorobenzaldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:174°C.
to Anal. Calcd. for:Cl~Hl~,CIN4S 1/10 HaO:C,59.94;H,3.31;N,16.45.
Found:C,59.82;H,3.48;N,16.93.
MS(EI) :338 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.50(lH,s), 7.18(lH,dd,J=7.3Hz
and 7.2Hz), 7.24-7.35(4H,m), 7.45(lH,d,J=7.8Hz),
z5 7.60(lH,d,J=3.6Hz), 7.77(lH,d,J=3.9Hz), 10.08(lH,s),
12.29(lH,s).
Example 242
5-Cyano-4,7-dihydro-4-(2-methoxyphenyl)-6-(thiophen-2-yl)-2H-
pyrazolo[3,4-b]pyridine
ao The title compound was prepared from methyl thiophene-2-
carboxylate, 2-methoxybenzaldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:215°C.
Anal. Calcd. for:C18H14N40S:C, 64.65;H, 4.22;N,16.75.
25 Found:C,64.66;H,4.32;N,17.02.
MS(EI) :334(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.85(3H,s), 5.34(lH,s),
6.93(lH,dd,J=7.3Hz and 7.2Hz), 7.01(lH,d,J=7.3Hz), 7.14-
7.25(4H,m), 7.60(lH,d,J=3.6Hz), 7.77(lH,d,J=5.lHz), 9.91(lH,s),
30 12.17(lH,s).
Example 243
5-Cyano-4,7-dihydro-4-(2-methylthiophenyl)-6-(thiophen-2- 1)-
2H-pyrazolo[3,4-b]pyridine
143
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from methyl thiophene-2-
carboxylate, 2-methylthiobenzaldehyde and 3-aminopyrazole in
the same manner as in Example 95.
MP:222°C.
s Anal. Calcd. for:C18H14N4Sz 2/5 HZO:C,60.44;H,4.17;N,15.66.
Found:C,60.58;H,4.44;N,15.35.
MS(EI) :350 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 2.49(3H,s), 5.48(lH,s), 7.17-
7.28(SH,m), 7.33(lH,d,J=7.3Hz), 7.60(lH,d,J=3.7Hz),
.~0 7.77(lH,d,J=3.9Hz), 10.01(lH,s), 12.22(lH,s).
Example 244
5-Cyano-4,7-dihydro-4-(2-nitrophenyl)-6-(thiophen-2-yl)-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
15 carboxylate, 2-nitrobenzaldehyde and 3-aminopyrazole in the
same manner as in Example 95.
MP:165°C.
Anal. Calcd. for:C17H11NSOzS:C,58.44;H,3.17;N,20.05.
Found:C,58.15;H,3.42;N,20.38.
2o MS(EI) :349 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.54(lH,s), 7.18(lH,dd,J=7.3Hz
and 7.2Hz), 7.34(lH,s), 7.48-7.55(2H,m),
7.60(lH,d,J=3.7Hz),7.72-7.79(2H,m), 7.92(lH,d,J=8.lHz),
10.16(lH,s), 12.35(lH,s).
z5 Example 245
4-(2,1,3-Benzothiadiazol-4-yl)-5-cyano-4,7-dihydro-6-
(thiophen-2-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, 2,1,3-benzothiadiazol-4-aldehyde and 3-
3o aminopyrazole in the same manner as in Example 95.
MP:254°C.
Anal. Calcd. for:C17H1oN6Sz:C,56.34;H,2.78;N,23.19.
Found:C,56.O1;H,2.91;N,23.19.
144
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS (EI ) :362 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.84(lH,s), 7.19(lH,dd,J=4.4Hz
and 4.3Hz), 7.28(lH,s), 7.55(lH,d,J=6.8Hz), 7.65(lH,d,J=3.7Hz),
7.72-7.79(2H,m), 7.99(lH,d,J=8.8Hz), 10.14(lH,s), 12.21(lH,s).
Example 246
5-Cyano-4,7-dihydro-4-(naphthalen-1-yl)-6-(thiophen-2-yl)-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, naphthalene-1-aldehyde and 3-aminopyrazole in the
.to same manner as in Example 95.
MP:214°C.
Anal. Calcd. for:CZIHi4N4S:C,71.16;H,3.98;N,15.81.
Found:C,,70.75;H,3.96;N,15.85.
MS(EI) :354 (M+) .
1H-NMR (400MHz,DMSO-d6)b(.ppm): 5.87(lH,s),7.13(lH,s),
7.18(lH,dd,J=4.6Hz and 3.9Hz), 7.45-7.54(4H,m),
7.62(lH,d,J=3.9Hz), 7.78(lH,d,J=4.9Hz), 7.83(lH,d,J=8.lHz),
7.95(lH,d,J=9.3Hz), 8.31(lH,d,J=7.3Hz), 10.09(lH,s),
12.17(lH,s).
2o Example 247
5-Cyano-4-(2,3-dichlorophenyl)-4,7-dihydro-6-(thiophen-2-yl)-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, 2,3-dichlorobenzaldehyde and 3-aminopyrazole in
as the same manner as in Example 95.
MP:232°C.
Anal. Calcd. for:C17H1oC1ZN4S 110 HZO:C,54.44;H,2.74;N,14.94.
Found:C,54.08;H,2.90;N,15.29.
MS(EI) :373 (M+) .
30 1H-NMR (400MHz,DMSO-d6)b(ppm): 5.58(lH,s), 7.18(lH,dd,J=7.3Hz
and 7.2Hz), 7.32-7.41(3H,m), 7.54(lH,dd,J=7.3Hz and l.SHz),
7.60(lH,d,J=3.7Hz), 7.78(lH,d,J=4.9Hz), 10.14(lH,s),
12.32(lH,s).
145
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 248
5-Cyano-4,7-dihydro-4-(2-methylphenyl)-6-phenyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl benzoate, 2-
methylbenzaldehyde and 3-aminopyrazole in the same manner as
in Example 95.
MP:246°C.
Anal. Calcd. for:CZOHisN4 1.0 HZO:C,72.71;H,5.49;N,16.96.
Found:C,72.50;H,5.26;N,17.20.
to MS(EI) :312 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 2.38(3H,s), 5.29(lH,s), 7.11-
7.23(5H,m), 7.47-7.49(3H,m), 7.55-7.58(2H,m), 9.94(lH,s),
12.17(lH,s).
Example 249
.ts 5-Cyano-4,7-dihydro-4-(2-methylphenyl)-6-(thiophen-2-yl)-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, 2-methylbenzaldehyde and 3-aminopyrazole in the
same manner as in Example 95.
ao MP:276°C.
Anal. Calcd. for:C18H14N4S:C,67.90;H,4.43;N,17.60.
Found:C,67.93;H,4.54;N,17.64.
MS(EI):318(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 2.36(3H,s), 5.28(lH,s), 7.11-
25 7.18(5H,m), 7.24(lH,s), 7.55(lH,dd,J=3.7Hz and l.OHz),
7.74(lH,dd,J=5.9Hz and l.OHz), 9.95(lH,s), 12.22(lH,s).
Example 250
4-(2-Chlorophenyl)-5-cyano-6-dimethoxymethyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
3o The title compound was prepared from methyl
dimethoxyacetate, 2-chlorobenzaldehyde and 3-aminopyrazole in
the same manner as in Example 94.
1H-NMR (400MHz,DMSO-d6)b(ppm): 3.39(6H,s), 5.18(lH,s),
146
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
5.43(lH,s), 7.23-7.27(3H,m), 7.32-7.35(lH,m),
7.44(lH,d,J=7.8Hz), 9.65(lH,s), 12.21(lH,s).
Example 251
4-(2-Chlorophenyl)-5-cyano-6-formyl-4,7-dihydro-2H-
.s pyrazolo[3,4-b]pyridine
4-(2-Chlorophenyl)-5-cyano-6-dimethoxymethyl-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine (4.4 g) was added to
trifluoroacetic acid (20 ml) under ice-cooling and the mixture
was stirred at room temperature for 2.5 hours. The reaction
to mixture was concentrated under reduced pressure and
crystallized from ethyl acetate (50 ml) to give the title
compound (1.9 g) as yellow crystals.
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.63(lH,s), 7.27-7.46(SH,m),
7.48(lH,d,J=7.lHz), 9.73(lH,s), 10.17(lH,s), 12.34(lH,s).
15 Example 252
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-hydroxymethyl-2H-
pyrazolo[3,4-b]pyridine
To a suspension of 4-(2-chlorophenyl)-5-cyano-6-formyl-
4,7-dihydro-2H-pyrazolo[3,4-b]pyridine (400 mg) in methanol
20 (10 ml) was added sodium borohydride (53 mg) under ice-cooling
and the mixture was stirred at the same temperature for 30
minutes. loo Hydrochloric acid was added to the reaction
mixture, and a saturated sodium hydrogencarbonate solution was
added. The precipitated crystals were collected by filtration
2s and washed with ethanol to give the title compound (295 mg) as
yellow crystals.
MP:205-210°C (decomposition).
Anal. Calcd. for:C14H11C1N40 1/4 HZO:C,57.74;H,3.98;N,19.24.
Found:C,57.38;H,3.93;N,18.94.
3o MS(EI):286(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 4.29(2H,d,J=5.6Hz), 5.38(lH,s),
5.49(lH,t,J=5.6Hz), 7.22-7.34(4H,m), 7.43(lH,d,J=8.OHz),
9.60(lH,s), 12.17(lH,s).
147
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 253
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-(trans-2-
ethoxycarbonylethenyl)-2H-pyrazolo[3,4-b]pyridine
To a suspension of sodium hydride (94 mg) in
s dimethoxyethane (10 ml) was added ethyl
diethylphosphonoacetate (528 mg) and the mixture was stirred
at room temperature for 15 minutes. Under ice-cooling, 4-(2-
chlorophenyl)-5-cyano-6-formyl-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine (670 mg) was added to the mixture, and the mixture
io was stirred at the same temperature for 1 hour. Water was
added to the reaction mixture and the mixture was extracted
with ethyl acetate. The extract was washed with a saturated
aqueous sodium chloride solution and dried over anhydrous
magnesium sulfate. The solvent was evaporated and the obtained
15 residue was purified by silica gel column chromatography
(eluent: hexane-ethyl acetate (1:1)) to give the title
compound (560 mg) as yellow crystals.
MP:240-243°C (decomposition).
Anal. Calcd. for:C18H15C1N40z 1/2 HzO:C, 59 .43;H, 4.43;N, 15.40.
2o Found:C,59.53;H,4.26;N,15.31.
MS(EI) :354(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.25(3H,d,J=7.lHz),
4.21(2H,q,J=7.lHz), 5.52(lH,s), 6.93(lH,d,J=15.9Hz), 7.27-
7.46(6H,m), 10.09(lH,s), 12.31(lH,s).
2s Example 254
4-(2-Chlorophenyl)-5-cyano-6-(2-ethoxycarbonylethyl)-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine
A suspension of 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-
6-(trans-2-ethoxycarbonylethenyl)-2H-pyrazolo[3,4-b]pyridine
30 (260 mg) and 5o palladium on carbon (110 mg) in ethanol was
subjected to catalytic hydrogenation at room temperature for 5
hours. The reaction mixture was filtered through Celite and
the filtrate was concentrated under reduced pressure. The
148
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
obtained residue was purified by silica gel column
chromatography (eluent: hexane-ethyl acetate (1:1)) to give a
yellow solid. The yellow solid was crystallized from ethyl
acetate-diisopropyl ether to give the title compound (160 mg)
s as pale-yellow crystals.
MP:172-174°C.
MS(EI):356(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.18(3H,t,J=7.3Hz),2.60-
2.80(4H,m), 4.08(2H,q,J=7.3Hz); 5.35(lH,s), 7.20-7.31(4H,m),
Io 7.42(lH,d,J=8.OHz), 9.84(lH,s), 12.16(lH,s).
Example 255
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-dimethoxymethyl-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
i5 dimethoxyacetate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
aminopyrazole in the same manner as in Example 94.
1H-NMR (400MHz,DMSO-d6)b(ppm): 3.35(3H,s), 3.38(3H,s),
5.16(lH,s), 5.47(lH,s), 7.26(lH,s), 7.42(lH,d,J=6.6Hz),
7.60(lH,dd,J=6.6,8.5Hz), 7.94(lH,d,J=8.5Hz), 9.77(lH,s),
2o 12.19(lH,s).
Example 256
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-formyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from 4-(2,1,3-
z5 benzoxadiazol-4-yl)-5-cyano-6-dimethoxymethyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine in the same manner as in Example 251.
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.71(lH,s), 7.33(lH,s),
7.56(lH,d,J=6.6Hz), 7.62(lH,dd,J=6.6,8.8Hz),
7.98(lH,d,J=8.8Hz), 9.73(lH,s),10.32(lH,s), 12.32(lH,s).
3o Example 257
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
hydroxymethyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from 4-(2,1,3-
149
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
benzoxadiazol-4-yl)-5-cyano-6-formyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine in the same manner as in Example 252.
MP:215-220°C (decomposition).
Anal. Calcd. for:C14H1oNsOz 1/2 HZO:C,55.44;H,3.66;N,27.71.
Found:C,55.32;H,3.68;N,27.31.
MS(EI) :294(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 4.30(2H,s), 5.45(lH,s),
5.52(lH,brs), 7.27(lH,s), 7.42(lH,d,J=6.6Hz),
7.59(lH,dd,J=6.6,9.OHz), 7.93(lH,d,J=9.OHz), 9.71(lH,s),
l0 12.16(lH,s).
Example 258
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(trans-2-
ethoxycarbonylethenyl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from 4-(2,1,3-
benzoxadiazol-4-yl)-5-cyano-6-formyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine in the same manner as in Example 253.
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.24(3H,d,J=7.lHz),
4.21(2H,q,J=7.lHz), 5.59(lH,s), 6.96(lH,d,J=16.1Hz),
7.32(lH,s), 7.39(lH,d,J=16.1Hz), 7.50(lH,m), 7.59(lH,m),
ao 7.96(lH,d,J=8.3Hz), 10.21(lH,s), 12.29(lH,s).
Example 259
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-(2-
ethoxycarbonylethyl)-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from 4-(2,1,3-
zs benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(trans-2-
ethoxycarbonylethenyl)-2H-pyrazolo[3,4-b]pyridine in the same
manner as in Example 254.
MS(EI):364(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.18(3H,t,J=7.lHz), 2.66-
30 2.80(4H,m), 4.08(2H,q,J=7.lHz), 5.40(lH,s), 7.26(lH,s),
7.42(lH,d,J=6.6Hz), 7.58(lH,dd,J=6.6,9.OHz),
7.92(lH,d,J=9.OHz), 9.96(lH,s), 12.16(lH,s).
Example 260
150
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
4-(2-Bromo-3-cyanophenyl)-5-cyano-4,7-dihydro-6-isopropyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl isobutyrate,
2-bromo-3-cyanobenzaldehyde and 3-aminopyrazole in the same
s manner as in Example 94.
MP : >25 0°C .
Anal. Calcd. for:C~7H14BrN5:C, 55.45;H, 3.83;N,19.02.
Found:C,55.30;H,3.91;N,18.98.
MS(EI) :368 (M+) .
.10 1H-NMR (400MHz,DMSO-d6)8(ppm): 1.23(3H,d,J=6.8Hz),
1.27(3H,d,J=6.8Hz), 3.03(lH,m),5.45(lH,s), 7.33(lH,s), 7.55-
7.82(2H,m), 7.83(lH,dd,J=2.0,7.1Hz), 9.76(lH,s), 12.25(lH,s).
Example 261
4-(2-Bromophenyl)-5-cyano-4,7-dihydro-6-isopropyl-2H-
.t5 pyrazolo[3,4-b]pyridine acetonitrile
The title compound was prepared from methyl isobutyrate,
2-bromobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:>250°C.
2o Anal. Calcd. for:C16H1sBrN4CZH3N:C,56.26;H,4.72;N,18.22.
Found:C,56.05;H,4.56;N,17.09.
MS(EI) :343 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.24(3H,d,J=7.lHz),
1.27(3H,d,J=7.lHz), 2.06(3H,s), 3.06(lH,m), 5.23(lH,s), 7.13-
2s 7.18(lH,m), 7.22(lH,d,J=7.6Hz), 7.27(lH,s),
7.36(lH,dd,J=1.2,7.6Hz), 7.59(lH,dd,J=1.2,8.OHz), 9.64(lH,s),
12.17(lH,s).
Example 262
5-Cyano-4,7-dihydro-6-isopropyl-4-(2-nitrophenyl)-2H-
3o pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl isobutyrate,
2-nitrobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
151
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP : 224°C .
MS(EI) :309 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.23(3H,d,J=7.lHz),
1.28(3H,d,J=7.lHz), 3.03(lH,m), 5.36(lH,s), 7.27(lH,s), 7.43-
s 7.49(2H,m), 7.70(lH,dd,J=1.2,8.8Hz), 7.89(lH,dd,J=1.2,8.3Hz),
9.71(lH,s), 12.23(lH,s).
Example 263
5-Cyano-4-(2,3-dichlorophenyl)-4,7-dihydro-6-isopropyl-2H-
pyrazolo[3,4-b]pyridine
to The title compound was prepared from methyl isobutyrate,
2,3-dichlorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:>250°C.
Anal. Calcd. for:C16H14C12N4:C,57.67;H,4.23;N,16.89.
15 Found:C,57.74;H,4.27;N,16.89.
MS(EI) :333 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.23(3H,d,J=7.lHz),
1.27(3H,d,J=7.lHz), 3.04(lH,m), 5.42(lH,s), 7.23(lH,d,J=7.6Hz),
7.31(lH,s), 7.35(lH,dd,J=7.6,7.8Hz), 7.51(lH,dd,J=1.5,7.8Hz),
ao 9.70(lH,s), 12.21(lH,s).
Example 264
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-isopropyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl isobutyrate,
zs 2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 94.
MP:222-223°C (decomposition).
Anal. Calcd. for:C16H14N60:C,62.71;H,4.61;N,27.44.
Found:C,62.71;H,4.65;N,27.45.
.~o MS(EI) :306 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.24(3H,d,J=7.lHz),
1.25(3H,d,J=7.lHz), 3.03(lH,m), 5.39(lH,s), 7.26(lH,s),
7.40(lH,d,J=6.6Hz), 7.58(lH,dd,J=6.6,8.8Hz),
i52
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.92(lH,d,J=8.8Hz), 9.74(lH,s), 12.15(lH,s).
Example 265
5-Cyano-4,7-dihydro-6-isopropyl-4-(2-methoxyphenyl)-2H-
pyrazolo[3,4-b]pyridine
s The title compound was prepared from methyl isobutyrate,
2-methoxybenzaldehyde and 3-aminopyrazole in the same manner
as in Example 94.
MP : >250°C .
Anal. Calcd. for:C17H18N40:C,69.37;H,6.16;N,19.03.
to Found:C,69.13;H,6.21;N,19.54.
MS(EI) :294 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.23(3H,d,J=7.lHz),
1.28(3H,d,J=7.lHz), 3.09(lH,m), 3.83(3H,s), 5.19(lH,s),
6.90(lH,dd,J=7.4,7.6Hz), 6.99(lH,d,J=7.6Hz),
15 7.05(lH,dd,J=1.7,7.4Hz), 7.15-7.19(2H,m), 9.47(lH,s),
12.04(lH,s).
Example 266
4-(2-Chlorophenyl)-5-cyano-6-cyclopropyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
2o The title compound was prepared from methyl
cyclopropanecalboxylate, 2-chlorobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:>250°C.
Anal. Calcd. for:C16H13C1N4:C,64.76;H,4.42;N,18.88.
as Found:C,64.71;H,4.50;N,19.05.
MS(EI) :296 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89-0.93(2H,m), 1.00-1.15(2H,m),
2.01(lH,m),5.35(lH,s), 7.22-7.26(3H,m), 7.31-7.34(lH,m),
7.42(lH,d,J=7.8Hz), 9.14(lH,s), 12.16(lH,s).
3o Example 267
4-(2-Bromophenyl)-5-cyano-6-cyclopropyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
153
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
cyclopropanecalboxylate, 2-bromobenzaldehyde and 3
aminopyrazole in the same manner as in Example 94.
MP:>250°C.
Anal. Calcd. for:C16H13BrN4:C,56.32;H,3.84;N,16.42.
s Found:C,56.18;H,3.90;N,16.48.
MS(EI) :341(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.90-0.93(2H,m), 1.00-1.15(2H,m),
2.01(lH,m), 5.34(lH,s), 7.13-7.22(2H,m),7.27(lH, s), 7.34-
7.38(lH,m), 7.59(lH,d,J=6.8Hz), 9.15(lH,s), 12.16(lH,s).
to Example 268
4-(2-Bromo-3-cyanophenyl)-5-cyano-6-cyclopropyl-4,7-dihydro-
2H-pyrazolo[3,4-b]pyridine 1/4 acetonitrile
The title compound was prepared from methyl
cyclopropanecalboxylate, 2-bromo-3-cyanobenzaldehyde and 3-
Is aminopyrazole in the same manner as in Example 94.
MP:>250°C.
Anal. Calcd. for:C17H1aBrN5H20 1/4 CH3CN:C,53.28;H,3.77;N,18.64.
Found.: C,53.28;H,3.72;N,18.81.
MS(EI) :366 (M+) .
zo 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90-0.93(2H,m), 1.03-1.08(2H,m),
1.96-2.00(lH,m), 5.45(lH,s), 7.32(lH,s), 7.54-7.60(2H,m),
7.83(lH,dd,J=1.7,7.1Hz), 9.27(lH,s), 12.25(lH,s).
Example 269
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-cyclopropyl-4,7-
25 dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclopropanecalboxylate, 2,1,3-benzoxadiazole-4-aldehyde and
3-aminopyrazole in the same manner as in Example 94.
MP:200-201°C (decomposition).
3o Anal. Calcd. for:Cl6HizNsO HzO:C,59.62;H,4.38;N,26.07.
Found:C,59.93;H,4.05;N,26.19.
MS(EI) :304(M+) .
'-H-NMR (400MHz,DMSO-d6)~(ppm): 0.88-0.93(2H,m), 1.01-1.12(2H,m),
154
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
1.99(lH,m), 5.39(lH,s), 7.25(lH,s), 7.40(lH,d,J=6.6Hz),
7.59(lH,dd,J=6.6,9.OHz), 7.92(lH,d,J=9.OHz), 9.26(lH,s),
12.15(lH,s).
Example 270
s 4-(2-Methoxyphenyl)-5-cyano-6-cyclopropyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine 1/4 acetonitrile
The title compound was prepared from methyl
cyclopropanecalboxlate, 2-methoxybenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
1o MP:241-243°C.
Anal. Calcd. for:C17H16N4O 1/4 CH3CN:C, 69.46;H, 5.58;N, 19.67.
Found:C,69.35;H,5.56;N,19.64.
MS(EI) :292 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90-0.92(2H,m), 0.99-1.10(2H,m),
i5 2.01-2.06(lH,m), 3.84(3H,s), 5.21(lH,s),
6.90(lH,dd,J=7.3,7.6Hz), 6.98-7.05(2H,m), 7.15-7.19(2H,m),
8.97(lH,s), 12.04(lH,s).
Example 271
5-Cyano-6-cyclopropyl-4-(2,3-dichlorophenyl)-4,7-dihydro-2H-
2o pyrazolo[3,4-b]pyridine 1/4 acetonitrile
The title compound was prepared from methyl
cyclopropanecalboxylate, 2,3-dichlorobenzaldehyde and 3-
aminopyrazole in the same manner as in Example,94.
MP:>250°C.
25 Anal. Calcd. for:C16H1zC1zN4 1/4 CH3CN:C, 58 .04;H, 3.76;N, 17.43.
Found:C,57.87;H,3.79;N,17.44.
MS (EI ) : 331 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.90-0.93(2H,m), 1.03-1.08(2H,m),
1.98-2.03(lH,m), 5.43(lH,s), 7.22(lH,d,J=7.8Hz), 7.31(lH,s),
30 7.35(lH,t,J=7.8Hz), 7.51(lH,dd,J=1.5,7.8Hz), 9.21(lH,s),
12.20(lH,s).
Example 272
5-Cyano-6-cyclopropyl-4,7-dihydro-4-(2-nitrophenyl)-2H-
155
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclopropanecalboxylate, 2-nitrobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:236-238°C (decomposition).
Anal. Calcd. for:C16H13N5~2:C.62.53;H,4.26;N,22.79.
Found:C,62.54;H,4.29;N,22.85.
MS(EI) :307(M~) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.90-0.93(2H,m), 1.01-1.09(2H,m),
l0 1.99(lH,m), 5.37(lH,s), 7.27(lH,s), 7.42-7.49(2H,m),
7.70(lH,dd,J=7.5,7.6Hz), 7.90(lH,d,J=8.lHz), 9.23(lH,s),
12.22(lH,s).
Example 273
Ethyl 4-(2-chlorophenyl)-6-dimethoxymethyl-4,7-dihydro-2H-
i5 pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-
chlorobenzaldehyde, 3-aminopyrazole and ethyl 4,4-dimethoxy-3-
oxobutanoate in the same manner as in Example 1.
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.87(3H,t,J=7.lHz), 3.35(3H,s),
ao 3.46(3H,s),3.82(2H,m), 5.64(lH,s), 6.11(lH,s),7.10-7.14(2H,m),
7.20-7.24(lH,m),7.27(lH, s), 7.36(lH,d,J=8.3Hz), 8.94(lH,s),
12.05(lH,s).
Example 274
Ethyl 4-(2-chlorophenyl)-6-formyl-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine-5-carboxylate
To a solution of ethyl 4-(2-chlorophenyl)-6-
dimethoxymethyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine-5-
carboxylate (463 mg) in tetrahydrofuran (5 ml) was added 1N
hydrochloric acid (10 ml) and the mixture was stirred at room
3o temperature for 6 hours. To the reaction mixture was added a
saturated aqueous sodium hydrogencarbonate solution and the
mixture was extracted with ethyl acetate. The extract was
washed with a saturated aqueous sodium chloride solution and
156
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
dried over anhydrous magnesium sulfate. The solvent was
evaporated and the obtained residue was purified by silica gel
column chromatography (eluent: hexane-ethyl acetate (1:1)) to
give the title compound (290 mg) as a yellow solid.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89(3H,t,J=7.3Hz), 3.91(2H,m),
5.70(lH,s), 7.14-7.24(3H,m), 7.31(lH,s), 7.40(lH,d,J=7.8Hz),
9.64(lH,s), 10.23(lH,s), 12.19(lH,s).
Example 275
Ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-isopropyl-2H-
lo pyrazolo[3,4-b]pyridine-5-carboxylate
2-Chlorobenzaldehyde (1.41 g), 3-aminopyrazole (0.83 g)
and ethyl isobutyrylacetate (1.58 g) were stirred in acetic
acid (10 ml) at 80°C for 2 hours. Under ice-cooling, a
saturated aqueous sodium hydrogencarbonate solution was added
i5 to the reaction mixture. The insoluble material was filtered
off, and the mixture was extracted with ethyl acetate. The
extract was washed with a saturated aqueous sodium chloride
solution and dried over anhydrous magnesium sulfate. The
extract was concentrated under reduced pressure and the
zo obtained residue was purified by silica gel column
chromatography (eluent: hexane-ethyl acetate (1:1)). The
purified product was crystallized from hexane-ethyl acetate to
give the title compound (115 mg) as white crystals.
MP : 211-213°C .
2.s 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.85(3H,t,J=7.lHz), 1,16(3H,m),
1.28(3H,d,J=7.lHz), 3.76(2H,m), 4.35(lH,m), 5.59(lH,s), 7.07-
7.13(2H,m), 7.18-7.22(lH,m),7.24(lH, s),
7.35(lH,dd,J=1.2,8.1Hz), 9.14(lH,s), 11.97(lH,s).
Example 276
3o Ethyl 4-(2-bromophenyl)-4,7-dihydro-6-isopropyl-2H-
~yrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-bromobenzaldehyde,
3-aminopyrazole and ethyl isobutyrylacetate in the same manner
157
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
as in Example 275.
MP : 214-215°C .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.84(3H,t,J=6.8Hz), 1.16(3H,m),
1.28(3H,d,J=6.8Hz), 3.76(2H,m), 4.35(lH,m), 5.56(lH,s), 7.07
7.13(2H,m), 7.02(lH,dd,J=7.3,7.8Hz), 7.11(lH,d,J=6.4Hz),
7.24(lH,dd,J=7.4,7.8Hz), 7.28(lH,s), 7.52(lH,d,J=7.8Hz),
9.15(lH,s), 11.98(lH,s).
Example 277
Ethyl 4-(2-chlorophenyl)-6-cyclopropyl-4,7-dihydro-2H-
.to pyrazolo[3,4-b]pyridine-5-carboxylate
To a solution of 2-oxazolydone (20.8 g) in
tetrahydrofuran (750 ml) was added n-butyllithium (1.56 M
hexane solution, 153 ml) at -78°C and the mixture was stirred
at the same temperature for 30 minutes. To the reaction
mixture was added a solution of cyclopropanecarbonyl chloride
(25 g) in tetrahydrofuran (50 ml) at -78°C over 30 minutes.
The mixture was stirred for 14 hours while gradually raising
the temperature to room temperature. The reaction mixture was
poured into ice-water and the mixture was extracted with ethyl
ao acetate. The extract was washed with a saturated aqueous
sodium chloride solution and dried over anhydrous magnesium
sulfate. The extract was concentrated under reduced pressure
and the obtained residue was purified by silica gel column
chromatography (eluent: hexane-ethyl acetate (1:1)) to give
z5 white crystals (26 g). A mixture of the obtained white
crystals (10 g), ethyl bromoacetate (21.5 ml) and zinc powder
(25.3 g) in tetrahydrofuran (300 ml) was ultrasonicated for 2
hours and heated under reflux for 2 hours. To the reaction
mixture was added 10o hydrochloric acid and the insoluble
3o material was filtered off through Celite. The filtrate was
extracted with ethyl acetate, and the extract was washed with
a saturated aqueous sodium chloride solution and dried over
anhydrous magnesium sulfate. The extract was concentrated
158
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
under reduced pressure and the obtained residue was purified
by silica gel column chromatography (eluent: hexane-ethyl
acetate (1:1)) to give ethyl 3-cyclopropyl-3-oxopropionate
(5.7 g) as a yellow oil. Subsequently, the title compound was
prepared from 2-chlorobenzaldehyde, 3-aminopyrazole and ethyl
3-cyclopropyl-3-oxopropionate in the same manner as in Example
275.
MP:190-192°C.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.83-0.93(4H,m), 1.10(lH,m),
l0 3.14(lH,m), 3.80(2H,m), 5.60(lH,s), 7.08-7.12(2H,m), 7.18-
7.22(lH,m), 7.25(lH,s), 7.34(lH,d,J=8.3Hz), 8.62(lH,s),
11.99(lH,s).
Example 278
4-(2-Bromo-3-cyanophenyl)-5-cyano-4,7-dihydro-6-(thiophen-2-
Ls yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, 2-bromo-3-cyanobenzaldehyde and 3-aminopyrazole
in the same manner as in Example 95.
MP:>280°C.
2o Anal. Calcd. for:C18H1oBrN5S:C,52.95;H,2.47;N,17.15.
Found:C,52.72;H,2.69;N,17.21.
MS(EI) :408 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 5.62(lH,s), 7.18(lH,dd,J=5.lHz
and 3.7Hz), 7.40(lH,s), 7.59-7.67(3H,m), 7.79(lH,d,J=3.9Hz),
zs 7.86(lH,dd,J=7.6Hz and 2.OHz), 10.20(lH,s), 12.37(lH,s).
Example 279
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-methyl-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate 2-
3o chlorobenzaldehyde and 3-amino-5-methylpyrazole in the same
manner as in Example 94.
MP:260°C.
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91(3H,t,J=7.3Hz), 1.60-
159
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
1.65(2H,m), 1.71(3H,s), 2.33(2H,q,J=7.3Hz), 5.27(lH,s), 7.20-
7.24(2H,m), 7.31(lH,dd,J=7.3Hz and 7.2Hz), 7.39(lH,d,J=7.3Hz),
9.68(lH,s), 11.83(lH,s).
Example 280
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-phenyl-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butanoate 2-
chlorobenzaldehyde and 3-amino-5-phenylpyrazole in the same
manner as in Example 94.
1o MP:262°C.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89(3H,t,J=7.3Hz), 1.61-
1.63(2H,m), 2.36(2H,q,J=7.3Hz), 5.61(lH,s), 7.09-7.34(9H,m),
9.89(lH,s), 12.62(lH,s).
Example 281
1-tert-Butoxycarbonyl-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-
6-propyl-1H-pyrazolo[3,4-b]pyridine
The title compound was obtained as a colorless amorphous
solid from 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine, dimethylaminopyridine and di-tert-
2o butyl dicarbonate in the same manner as in Example 204.
MP:98-102°C.
MS(EI) :398 (M+) .
IR(KBr):v=3391,2199,1723,1643,1529,1394,1149 cm 1.
1H-NMR (400MHz,DMSO-d6)S(ppm): 0.95(3H,t,J=7.3Hz), 1.57(3H,s),
z5 1.60-1.67(2H,m), 2.53-2.61(2H,m), 5.38(lH,s), 7.25-7.31(3H,m),
7.35(lH,ddd,J=1.4,7.3 and 7.8Hz), 7.45(lH,d,J=8.lHz),
9.20(lH,s).
Example 282
2-tert-Butoxycarbonyl-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-
30 6-propyl-2H-pyrazolo[3,4-b]pyridine
Through the column of silica gel column chromatography
used in Example 281 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as
160
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
colorless crystals.
MP:175°C (decomposition).
Anal. Calcd. for:CZIHz3C1N4O2:C,63.23;H,5.81;N,14.05.
Found:C,62.91;H,5.80;N,13.82.
MS(EI) :398 (M+) .
IR(KBr):v=3329,2197,1747,1612,1523,1369,1311,1151,949 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.93(3H,t,J=7.4Hz),
1.65(2H,q,J=7.3Hz), 2.40-2.44(2H,m), 2.48(9H,s), 5.32(lH,s),
7.27-7.36(3H,m), 7.45(lH,d,J=7.8Hz), 7.68(lH,s), 10.32(lH,s).
to Example 283
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-1-phenylcarbamoyl-6-
propyl-1H-pyrazolo[3,4-b]pyridine
The title compound was obtained as a colorless amorphous
solid from 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine, dimethylaminopyridine and phenyl
isocyanate in the same manner as in Example 204.
MP:138-140°C.
Anal. Calcd. for:Ca3H2oC1N50 1/4 HzO:C,654;H,4.89;N,16.58.
Found:C,65.20;H,5.05;N,16.17
2o MS(EI):417(M+).
IR(KBr):v=3387,3294,2202,1712,1537 cm 1.
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.95(3H,t,J=7.3Hz),
1.63(2H,q,J=7.4Hz), 2.58(2H,m), 5.43(lH,s), 7.13(lH,dd,J=7.4
and 7.5Hz), 7.24-7.36(6H,m), 7.46(lH,d,J=7.8Hz),
2s 7.69(2H,d,J=7.8Hz), 9.46(lH,s), 10.38(lH,s).
Example 284
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-2-phenylcarbamoyl-6-
propyl-2H-pyrazolo[3,4-b]pyridine
Through the column of silica gel column chromatography
3o used in Example 283 was further flowed hexane-ethyl acetate
(3:1) as an eluent, the title compound was obtained as a
colorless oil.
MP:167-171°C.
161
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :417 (M+) .
IR(KBr):v=3215,2204,1732,1631,1523,1375 cm 1.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.97(3H,t,J=7.4Hz),
1.65(2H,q,J=7.3Hz), 2.48(2H,m), 5.39(lH,s), 6.95(lH,dd,J=7.3
and 7.3Hz), 7.11(2H,dd,J=7.3 and 7.6Hz), 7.24-7.49(4H,m),
7.61(2H,d,J=7.8Hz), 7.88(lH,s), 8.63(lH,s), 9.77(lH,s),
10.17(lH,s).
Example 285
2-Acetoxyacetyl-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-
Io propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was obtained as colorless crystals
from 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine, dimethylaminopyridine and
acetoxyacetyl chloride in the same manner as in Example 204.
MP:149-150°C.
Anal. Calcd. for:CZOH19C1N4O3:C,60.23;H,4.80;N,14.05.
Found:C,60.17;H,4.83;N,13.90.
MS(EI) :398 (M+) .
IR(KBr):v=3281,3238,2197,1745,1630,1608,1523,1385,1344,1236,
1172 cm ~.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.95(3H,t,J=7.3Hz), I.64-
I.70(2H,m), 2.44(2H,q,J=7.3Hz), 3.33(3H,s), 5.26(2H,s),
5.37(lH,s), 7.29-7.35(3H,m),7.46(lH,d,J=7.8Hz), 7.86(lH,s),
10.45(lH,s).
z5 Example 286
Ethyl 1-acetoxyacetyl-4-(2-chlorophenyl)-4,7-dihydro-6-propyl-
1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-2H-
3o pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and acetoxyacetyl chloride in the same manner as in Example
204.
MP:130-131°C.
162
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:CZZHz401N305:C,59.26;H,5.43;N,9.42.
Found:C,59.17;H,5.39;N,9.31.
MS(EI) :445(M+) .
IR(KBr):v=3337,1732,1529,1390,124.6,1086 cm 1.
s 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.OHz),
0.97(3H,t,J=7.3Hz), 1.64-1.70(2H,m), 2.76-2.82(2H,m),
3.31(3H,s), 3.85(2H,q,J=7.OHz), 5.27(2H,dd,J=3.0 and 9.8Hz),
5.60(lH,s), 7.10-7.25(3H,m), 7.41(lH,dd,J=1.4 and 8.OHz),
7.82(lH,s), 10.1(lH,s).
1o Example 287
Ethyl 1-benzylcarbonyl-4-(2-chlorophenyl)-4,7-dihydro-6-
propyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was obtained as colorless crystals
from ethyl 4-(2-chlorophenyl)-4,7-dihydro-6-propyl-2H-
15 pyrazolo[3,4-b]pyridine-5-carboxylate, dimethylaminopyridine
and phenylacetyl chloride in the same manner as in Example 204.
MS(EI) :463(M+) .
IR(KBr):v=3418,1701,1521,1392,1228cn11.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.OHz),
20 0.93(3H,t,J=7.3Hz), 1.57-1.62(2H,m), 2.80-2.87(2H,m),
3.82(2H,q,J=7.OHz), 4.33(2H,s), 5.57(lH,s), 7.15(lH,dd,J=7.4
and 7.8Hz), 7.18-7.31(7H,m), 7.39(lH,d,J=7.8Hz), 7.44(lH,s),
8.94(lH,s).
Example 288
z5 4-(2,1,3-Benzoxadiazol-4-yl)-2-tert-butoxycarbonyl-5-cyano-
4,7-dihydro-6-propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was obtained as colorless crystals
from 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
propyl-1H-pyrazolo[3,4-b]pyridine, dimethylaminopyridine and
3o tert-butyldicarbonate in the same manner as in Example 204.
MP:168-170°C.
Anal. Calcd. for:CalHaaN6O3:0,62.06;H,5.46;N,20.68.
Found:C,61.92;H,5.44;N,20.52.
163
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :406 (M+) .
1H-NMR (400MHz,DMSO-d6)S(ppm): 0.89(3H,t,J=7.3Hz), 1.47(9H,s),
1.65(2H,m), 2.40(2H,m), 5.39(lH,s), 7.49(lH,d,J=6.3Hz),
7.60(lH,dd,J=6.6 and 9.OHz), 7.79(lH,s), 7.96(lH,d,J=6.6Hz),
.s 10.43(lH,s).
Example 289
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-1
phenylcarbamoyl-6-propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was obtained as colorless crystals
.to from 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine, dimethylaminopyridine and
phenyl isocyanate in the same manner as in Example 204.
MP:138-140°C.
Anal. Calcd. for:Cz3H1gN7Oz:C,64.93;H,4.50; N,23.05.
15 Found:C,65.07;H,5.05;N,21.24.
MS(EI) :425 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91(3H,t,J=7.3Hz), 1.62(2H,m),
2.58(2H,m), 5.47(lH,s), 7.13(lH,dd,J=6.3 and 6.6Hz), 7.32-
7.39(3H,m), 7.49(lH,d,J=6.5Hz), 7.61-7.91(3H,m),
2o 7.98(lH,d,J=9.lHz), 9.54(lH,s), 10.34(lH,s).
The compounds of the above-described Examples are as
follows.
164
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
o I / c1 o I ' o' o I / cF3 o I / c1 o I / c1
° I ~ NH ~° I / NH ~° I ~ NH ° I I NH ~° I
~ NH
NON NON NON NJ~N NfiNi
H H H H H
Example 1 Example 2 Example 3 Example 4 Example 5
o I ~ F o I ~ ci o I / c1 o I ' c1 o I / F
p ~ W p , wN ~ HZN.N , S /
I NH I / I H ~ I ,NH H I NH I NH
N ~N ~ N N ~N N ~Nj N ~N
H H H H H
Example 6 Example 1 Example 8 Example 9 Example 10
I
I ' CF O I ' ° F O I / CI O ~ °i
3
02N I / NH ~p I / N CF I ~N NH ~O I ~ NH I NNH
N CF3 H 'N 3 H ' CF3 H N. CF3 H
Example i1 Example 12 Example 13 Example 14 Example 15
CI ~ COOEt
~ CI
o I / cF3 o I / o I / o I / o I /
I _~ NH~o ' ~o ~' ~o ' ~° '~ I
CF3 N N~ ~ ~NNH /u~~NH I ~N,NH CFa I N ~N
H CFa H CFa '~H~ CFa H H
Example 16 Example 17 Example 18 Example 19 Example 20
- ~s
i ~ s i / ~ /
o ~cl 0 0 0 ~ o
I ~ NH ° I ~ NH ° I NH ° I NH ° I ~ NH
CF3 H ~N CFa H N CF3 H N CF3 H ~N
Example 21 Example 22 Example 23 Example 24 Example 25
I ~ I ~ c1 o I ' c1 o I ' ar
-~o ~ ~'o ~ ~o - ~o ~ ~o
I ,NH I NH I NH I NH I NH
N ~N N ~N N ~N N ~N N ~N
H H H H H
Example 26 Example 27 Example 28 Example 29 Example 30
165
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
w w w w w
cF~ o I. ' o' o I ' o~ o I ~ o~ o I l o
I ~_ NH O I ~ NH~O I ~ NH O I ~_ NH ~O I ~ NH
N N N ~N N ~N N N N ~N
H H H H H
Example 31 Example 32 Example 33 Example 34 Example 35
oI' o~ol~o oI~ o I~ oI~ s' oI~ s'
~O , ~O , ~O , ~O ~ NH~O ~ NH
I I_NNH I ~NNH I ~ I N I
N~ N N N N
H H H H H
Example 36 Example 37 Example 36 Example 39 Example 40
~ F ~ CI ~ F
O I ~ NOz ~ O I ~ CN O I ~ F O I ~ CI O I
I ~ NH~o I ~ NH ~ I NH ~ I NHS I ~ NH
N ~N N N N ~N N ,N N ,N
H H H H H
Example 41 Example 42 Example 43 Example 44 Example 45
O~ ~ CF3 F ~ CI
o I ~ o' o I ~ c1 o I ~ F o I ~ c1
~ ~ ~
O I _ NH O I \ NH O I ~ NH O I _ NH
N N N N N ~N N N
H H H H
Example 46 Example 47 Example 46 Example 49 Example 50
I I
F I ~ I I ~ F I ~ CI I ~
O ~ O O ~ CI O ~ O~ O '~ F O ~ CI
I \ NH ~o I NH ~ I NH ~o I NHS I \ NH
N N N ~N N ,N. N ,N N N
H H H H H
Example 51 Example 52 Example 53 Example 54 Example 55
~N N - O
co I ~ F o I ~ o I ~ o ~ ° o I~
~o ~ ~o ~ ~o ~ ~o ~ ~o
I NH I NH I NH I ~ NH I ~ NH
N ~N~ N ~N N ~N N N N N
H H H H H
Example 56 Example 51 Example 58 Example 59 Example 60
166
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
CI
o \\ o \ S o \ s o \ s o ~i
I ~ NH~O I ~ NH ~O I ~ NH ~O I \ ,NH ~O I ~ NH
N ~N N N N ~N N N N N
H H H H H
Example 61 Example 62 Example 63 Example 64 Example 65
I
I \ \ I ~ o I \ \ I \
O ~ O / i O / ~ O ~ O
O ~ _
I ~ NH ~O I ~ NH ~ I ~ NH ~O I ~ NH ~O I ~ NH
N N N N ~N N N
N H H H H
Example 66 Example 67 Example 68 Example 69 Example 10
c1 I ~ I \ \ I ~ IN ~ S \ /
O / ~ O / OJ O / OJ O / / O \
I ~ NH~O I ~ NH ~O I ~ NH~O I ~ NH ~O I ~ NH
N ~N N ~N N ~N N N N
H H H H H
Example 71 Example 12 Example 73 Example 14 Example 75
I
~N, I ~ CI I ~ N CI I ~ N I ~ O
O
O 'N O O O O~ O O~ O
I ~_ NH ~O I ~ ,NH ~O I ~ ,NH ~O I ~ NH ~O I ~ NH
N N N ~N N ~N N N N N
H H H H H
Example 76 Example 77 Example 78 Example 79 Example 80
/ \
- \ \ \ I
o ~ c1
0 0 -c1 o -c1 o -cl _
_ ~ _
I ~ NH~O I ~ NH ~O I ~ NH ~ I ~ NH ~ \ I N NNH
N ~N N N N ~N i N N I / H
H H H H
Example 81 Example 82 Example B3 Example 84 Example 85
I \ \
I
o ~ ct o I , o, o I , o~
o ~ c1
~o
NH /~O ~~1. I ~ ~NH _
\ I N I_ ,NH H ~N O I ~ NH O I ~ NH
~N~ ~ N
\ H / I H
S
Example 86 Example 87 ~ Example 89 Example 90
Example 88
167
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
I ~ o I i o' I ~ I
o ~ o' _ ~ c1 ~ c1
NH ~ \ I N N NH OzN I ~ NH NC I ,. .NH
~~N I , H ~~N ~~N
Exampltei 91 Example 92 Exampllie 93 Exampllei 94 \ Example 95
I ~ (~ I
O ~ CI O ~ S'O O OH O NHZ O
O I ~ NH ~O I ~ NH ~O I .~ NH~O I ~ NH~O I ~ NH
H '~ H _N N N ~ ~N ~N
Example 96 Example 97 Exampllei 98 Example 99 Example 100
I~ I~ I~ I
o ~ o ~ 0 0
~O I ' NH ~o I ~ NH ~o I ~ NH ~o I ~ NH
N ~N
H N H N H H
Example 101 Example 102 ' Example 103 Example 104
I ~ \ I ~ \ ~ ~ o I,
o ~ o o 's o ~s
o ~ /~o , o
~ NH I NH I NH I ~N NH
N ~N N ~N N
H H H H
Example 105 Example 106 Example 107 Example 108
C1 ~ C1 ~ ~ C1
o I i Cl o I i 0~ o I i
O I ~ NH ~O ( ~,NH ~O I ~ ,NH
N ~N N ~N N
I-I H H
Example 109 Example 110 Example 111
168
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
,N, / ,N, / ,N, ~ CI / ~N,
S O O I O
O \ ~N O ~ \N O ~ \N O / CI O ~ ~N
I ~ NH~O I ~ NH ~O I ~ NH ~O I ~ NH ~O
I ~ N I N I NH
H ~N t+)
Example 112 Example 113 ~ /
Example 114 Example 115 Example 116
/ ~N,
O N I ~ Br I / O I / S~ I / NO
2
/~O , OzN i O2N i OZN i OZN i
I NH I ,NH I NH I NH I NH
H ~N C_) ~ ~N ~ ~N ~ ~N ~ ~N
Example 117 Example 118 Example 119 Example 120 Example 121
a ~ ~ ~ ~ c1 ~ c1
I / a I / / I / pJ I / c1 I / c1
ON ON ON ON ON
I ~ NH 2 I ~ NH 2 I ~ NH Z I ~ NH Z I ~ NH
~N ~ ~N ~ ~N ~ ~N ~ ~N
Example 122 Example~123 Example 124 Example 125 Example 126
I ~ a I ~ I ~ I ~ I
/ CI / Br / O~ ~ S~ /
OzN NC NC NC NC
I ~ NH I ~ NH I ~ NH I ~ NH I ~ NH
~N ~ ~N ~ ~N ~ ~N ~ ~N~
Example 127 Example 128 Example 129 Example 130 Example 131
a Iw w Iw
/ NOZ / CN / CI / / / OJ
NC NC NC NC NC
I J NH I ~_ NH I ~ NH I r NH I ~ NH
N ~N N N N ~N N ~N N N
H H H H H
Example 132 Example 133 Example 134 Example 135 Example 136
,.N, / ~N, ~ O / ~N
O .S I ~~--- \ ,p \ p
N ~ ~N / N ~N ~N
NC _ NC NC NC ~ NC
NH I ~ NH I ~ NH I NH I NH
N N N ~N N ~N ~ ~N C_) ~ ~N C+)
H H H
Example 137 Example 138 Example 139 Example 140 Example 141
169
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
\ ~ I \ c~ \ \
I / ci I / S' / cN I / ~i I / /
NC NC _ Nc , NC NC _
I ~ ~NH I ~~ ~NH I NH I ~~ ~NH I ~~ ~NH
I \ H N I \ H N I \ ~ N I \ H N I \ H N
/ /
Example 142 Example 143 Example 144 Example 145 Example 146
\ CN ~ \ F ~ I / i
I / Br I / OJ I / F I / O~ NC ~O
NC ~ NC ~ NC ~ NC ~ I ~~ ~NH
I ~ ~NH I z 'NH I ~ ~NH I z ~NH I \ H N
H N I \ H N I \ H N I \ H N /
/ / / /
Example 147 Example 14B Example 149 Example 150 Example 151
I\ I\ I/ I\
-N02
/ O / NOZ NC ~ / NOZ
NC NC ( ~ ~NH NC
1 I w I ~~NH ~ N ~N I I ~~~NH
O I \ H' 'N I \ H' 'N I / H O I \ H N
Example 152 Example 153 Example 154 Example 155 Example 156
/ ,N
\ / i~ ~ ~ / iK / iN,
I / NOz ~ ~NO NC 'N ~ ~NO ~ ~NO
NC ~ NC I ~~ ~NH NC ~_ NC _
\ I N N NH I ~ NH \ N N 1 I ~NH I ~~ 'NH
I H I ~ I / H O I ~ H N I ~ H N
O / / ~O /
Example 157 Example 158 Example 159 Example 160 Example 161
/ ,N
~N, ~ S / ~N, / ~N~ / ~N,
~N S
\NS NC \ \NS \ \N \ 'NO
Yw
NC I I_ NH NC _ NC , NC
I ~ ~NH I \ H N ~ I ~~ 'NH \ I N NH ( z ~NH
I N I H N I H ~ \ H N.
/ I / ~o / N /
Example 162 Example 163 Example 164 Example 165 Example 166
/ ~K
0
~N, / ~N, ~ N / ~N, / ~N,
O ,O
\ ~N NC I ~ ~ ,NO \ ~NO
NH
NC I '_ NH NC I ~_ NH I \ p N NC I ' NH NC I ~ NH
I \ ~~N I \ HEN I \ \ N \ N
N ~N / ~ O ~ ~ S
Example 167 Example 168 Example 169 Example 170 Example 171
170
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
/ ~~l / ~~l ,N,
o ~ o / o
,N~ ,N
NC _ NC _
I ~~ ~NH I ~~ ~NH NC
I ~N NH
O S
Example 172 Example 173 Example 114 Example 175 Example 116
N Me NOZ ~ CN ~ Br
/ ~ ;O I I I I
'N O / CI O / CI O / CI O / Br
NC ~ /'~O ~ ~O ~ ~O ~ ~O
I NH I NH I NH I NH I NH
N ,N N ~N. N ~N. N ,N. N ,N
H H H H Example 181
Example 117 Example 118 Example 119 Example 180
NOZ ~ CN ~ CN ~ NO2 ~ CN
O I / Br O I / Br I / CI I / CI I / Br
O , NC ~ NC ~ NC
I ' NH I ~ NH I NH I NH I ~ ,NH
N N N N N ~N N ~N N N
H H H H H
Example 182 Example 183 Example 1B4 Example 1B5 Example 186
NO2 ~ Me ~ GN
O / OJ O / O I / Br O I / OMe O I / OMe
/\O ~ NC O
I NH I NH I ~ NH ~ I NH I NH
H ~N (+> H -N (-) H ,N, ,N ,N.
Example 187 Example 188 Example 189 Example 190 Example 191
~N,
O
I / NOz I / CI I / OMe I ~ CI ~ N
NC NC NC NC NC
I ~ NH I ~ NH I ~ NH I ~ NH I ~ NH
N ~N~ N ~N~ N ~N N ~N N ~N
H H H H H
Example 192 ' Example 193 Example 194 Example 195 Example 196
/ / GN / ~ /
CI \ Br \ I Br O I / CI ~ CI
NC I , N NC I ~ NH ~ I ~ NH ~O I ~ NH NC I ~ NH
N ~N N ~N~ N ~N NC N ~N N ~N
H H H H H
Example 197 Example 198 Example 199 Example 200 Example 201
171
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
CFa / ~ W
CI \ CI O I ~ CI O I ~ CI
O
NC I , NH NC I ~ NH ~O I I \N ~O I ~ ,N- '/~~
N~N Me N~N' N N N N OT
I l H Me H H ~O \H
Example 203 0 ~ Example 205
Example 202 Example 204
o ( ' c1 o I ~ c1 ' o I ~ ci o I ~ c1
I I N ~o I N ~ I I N ~o I
N N N ~N O- N N N N O
H ~ \ H H ~O H
_ b
Example 206 Example 207 Example 208 Example 209
I
o ~ c1 o ~ c1
I I N ~o I , N o
N H 'N
_ -O
Example 210 Example 211 txample ziz Example 213
I I
o ~ c1 o ~ c1
I I ~N / / ~o I I ~N
H ~O H ~O
N O
Example 214 Example 215 Example cio Example 217
I I I I
o ~ c1 o ~ c1 o ~ c1 o ~ c1
I / t'N~O ~O I I \N ~O I / N-~ ~O I I \N
N O ~ TO ~ N . ~ ~ ~O
O ~ O
Example 218 Example 219 Example 220 Example 221
172
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
o I '~ ci o I ~ ci o I ~ ci o I ~ ci
I ~ N-~O ~o I ~ N o ~o I I N ~ I N
N N O N ~N ~ N N N ~N
H ~ H H ~O H
Example 222 Example 223 Example 225
Example 224
Example 226 txampie LLI Example 229
Example 228
I I I I
o ~ c o ~ ci o ~ ci o
O I ~_ NH ~O I ~ N- ~O I I ~N ~O I I \N
H N H 'N H \ H
Example 230 Example 231 Example 232 Example 233
ci
rN
I , I , OHN / O N ~ N
I ~ NH ~O I ~ NH ~O I ~ NH ~O I ~ NH
N ,N N ~N ,N ~N.
H H H H
Example 234 Example 235 Example 236 Example 237
N
O ~ NH
~ NH
N ~N
H
Example 238
173
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
~N, / ~N,
~N.
O ~ O I / CI I ~ O~ I / Si
NC _ NC _ NC _
I ~ NH ~O I ~ NH I ~ ~NH I ~ NH I ~' ~NH
w N N w N N w N N
H N H ~N \ S H \ S H \ S H
Example 239 Example 240 Example 241 Example 242 Example 243
w ~ ~N.S w W w CI I w
NO ~ N I ~ ~ I ~ CI
2
NC NC NC ~ NC NC I i
I I I I ~~NH NH
NH z ,NH ~ N
w N w N N w N w N
\ S ~ \ S H \ S p \ S p I ~ b
Example 244 Example 245 Example 246 Example 247 Example 248
w
I ~ a I ~ I ~ I
~CI ~cl 'CI
NC
NC , I ~NH NC NC NC
I I' NNH ~O N N I ~ NH I ~ NH I ~ NH
H OHC N ~N~ HO N ~N EtOOC ~ N \N
\ S ~ i0 H H H
Example 249 Example 250 Example 251 Example 252 Example 253
/ ,N,
O / ~N~ / ,N / ~N,
I / ~ ~N \ _ O \ ,O \ O
CI NC N ~N ~N
NC I ~~ ~NH NC NC NC
I ~ NH i0 N N I ,NH HO I IJH I ~ NH
EtOOC N 'N O H OHC N 'N N ~N EtOOC ~ N \N
H ~ H H H
Example 254 Example 255 Example 256 Example 257 Example 258
~N ~ N ~ ~ ~ CI
,O
~N I ~ Br I ~ Br I ~ NO2 I ~ CI
NC NC ~ NC ~ NC ~ NC
I ~ NH I ~ NH I ~ NH I ~ NH I ~ ~NH
EtOOC H N H N H N H N H N
Example 259 Example 260 Example 261 Example 262 Example 263
,NO I w I ~ I ~ I ~ CN
N O ~ CI ~ Br ~ Br
NC _ NC ~- NC ~ NC ~ NC
I H~NNH I H I_NNH I ~~NNH I ~~NNH I ~~NNH
Example 264 Example 265 Examliple 266 Example 267 Exampllie 268
174
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
i ~N,o I ~ I ~ a I
O ' CI
' O ' CI ' NO2
NC ~ NC ~ NC ~ NC ~ f O ~ NH
I _ NH I _ NH I _ NH I _ NH i0 I ~ ~N
HEN ~N ~N ~N
O
Example 269 Example 270 Example 271 Example 272 Example 273
I ~ I ~ cN
O I i CI I ~ CI I ~ Br ' CI ' Br
~ EtOOC ~ / EtOOC ' NH NC I NH
EtOOC I
OHC I ~NNH Me I N ~N NH Me I N 'N NH ~~ \\ H~N.
Me H Me 'H' S
Example 274 Example 275 Example 276 Example 277 Example 278
I I I I
' c1 ' c1 ' I ' c1 ' c1
NC I ~ NH NC I ~ NH NC I I \N NC I ~'N~O
N ,N ~ ~N, ~ ~O ~ ~N J~O
H
Example 279 Example 280 Example 281 ~ Example 282
I I I I
' a ' c1 ' c1 ' c1
NC NC O NC
I I N I N I N I I ~N
H ~O H ~N N ~ ~ H N O H N
N Example 284 Example 285 ~ ~ c
Example 286
Example 283
,N
~O
~N
NC _
I N NN
H
Example 288
Example 287 Example 289
175
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 290
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-(4-methoxyphenyl)-6-
propyl-2H-pyrazolo[3,4-b]pyridine
To a solution of acetonitrile (15 g) in DMSO (25 mL) was
added methyl p-anisate (25 g) and the mixture was stirred with
heating at 60°C for 1 hour. The reaction mixture was allowed
to cool and cold water (100 mL) was added dropwise. The
mixture was acidified with hydrochloric acid and the
precipitated crystals were collected by filtration. The
to obtained crystals were extracted with ethyl acetate and the
solvent was evaporated under reduced pressure. The residue was
recrystallized from ethyl acetate to give 4-
methoxybenzoylacetonitrile (21 g) as colorless crystals. To a
solution of the obtained crystals in toluene was added
l5 hydrazine monohydrate (13 g) and the mixture was heated under
reflux for 3 hours. The mixture was cooled and the
precipitated crystals were collected by filtration to give 5-
amino-3-(4-methoxyphenyl)pyrazole (22 g). Subsequently, the
title compound was prepared from methyl butyrate, 2-
zo chlorobenzaldehyde and 5-amino-3-(4-methoxyphenyl)pyrazole in
the same manner as in Example 94.
MP:284°C.
Anal. Calcd. for:Cz3H21C1N4O:C,68.23;H,5.23;N,13.84.
Found:C,68.17;H,5.29;N,13.86.
25 MS(EI) :404 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89(3H,t,J=7.3Hz), 1.58-
1.63(2H,m), 2.32-2.38(2H,m),3.70(3H, s), 5.56(lH,s),
6.81(2H,d,J=7.2Hz), 7.09-7.12(2H,m), 7.17(lH,dd,J=7.3Hz and
7.2Hz), 7.24-7.30(3H,m), 9.85(lH,brs), 12.46(lH,brs).
3o Example 291
4-(2,1,3-Benzoxadiazol-4-yl)-6-(2-bromothiophen-5-yl)-5-cyano-
4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 5-
176
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
bromothiophene-2 -carboxylate, 2,1,3-benzoxadiazole-4-aldehyde
and 3-aminopyrazole in the same manner as in Example 95.
MP:208°C.
Anal. Calcd. for:C17H9BrN60S:C,48.O1;H,2.13;N,19.76.
s Found:C,47.94;H,2.36;N,19.78.
MS(EI) :425 (M+) .
1H-NMR (400MHz,DMSO-ds)b(ppm): 5.54(lH,s), 7.32-7.34(2H,m),
7.42(lH,d,J=3.9Hz), 7.50(lH,d,J=6.6Hz), 7.61(lH,dd,J=9.OHz),
7.95(lH,d,J=9.OHz), 10.32(lH,brs), 12.32(lH,brs).
so Example 292
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(3-
methylthiophen-2-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 3-
methylthiophene-2-carboxylate, 2,1,3-benzoxadiazole-4-aldehyde
15 and 3-aminopyrazole in the same manner as in Example 95.
MP:202°C.
Anal. Calcd. for:C1gH12N60S:C,59.99;H,3.36;N,23.32.
Found:C,59.89;H,3.53;N,23.06.
MS(EI) :360(M+) .
zo 1H-NMR (400MHz,DMSO-d6)b(ppm): 2.17(3H,s), 5.54(lH,s),
6.96(lH,d,J=5.lHz), 7.32(lH,s), 7.49(lH,d,J=6.6Hz), 7.60-
7.64(2H,m), 7.96(lH,d,J=9.OHz), 10.19(lH,brs), 12.25(lH,brs).
Example 293
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(1-
25 methoxymethylindol-3-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 1-
methoxymethylindol-3-carboxylate, 2,1,3-benzoxadiazole-4-
aldehyde and 3-aminopyrazole in the same manner as in Example
95.
3o MP:200°C.
MS(EI) :423(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 3.19(3H,s), 5.55-5.63(3H,m),
7.15(lH,dd,J=7.3Hz and 7.2Hz), 7.25(lH,dd,J=7.3Hz and 7.2Hz),
177
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.34(lH,s), 7.54(lH,d,J=7.3Hz), 7.60-7.66(3H,m), 7.93-
7.97(2H,m), 10.12(lH,brs), 12.22(lH,brs).
Example 294
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-3-(thiophen-2 -
s yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl thiophene-2-
carboxylate, methyl butyrate and 2-chlorobenzaldehyde in the
same manner as in Example 290.
MP:256°C.
io Anal. Calcd. for:CaoH17C1N4S:C,63.07;H,4.50;N,14.71.
Found:C,62.98;H,4.52;N,14.68.
MS (EI ) :380 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.86(3H,t,J=7.3Hz), 1.56-
1.62(2H,m), 2.31-2.36(2H,m), 5.46(lH,s), 7.00-7.24(5H,mj,
i5 7.36(lH,d,J=7.3Hz), 7.50(lH,d,J=4.9Hz), 9.95(lH,brs),
12.74(lH,brs).
Example 295
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-(furan-2-yl)-6-
propyl-2H-pyrazolo[3,4-b]pyridine
ao The title compound was prepared from methyl furan-2-
carboxylate, methyl butyrate and 2-chlorobenzaldehyde in the
same manner as in Example 290.
MP:253°C.
Anal. Calcd. for:CZOH17C1N40:C,65.84;H,4.70;N,15.36.
2s Found:C,65.81;H,4.84;N,15.49.
MS(EI) :364(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.3Hz), 1.58-
1.63(2H,m), 2.32-2.36(2H,m), 5.48(lH,s), 6.31(lH,d,J=3.2Hz),
6.45(lH,d,J=l.SHz), 7.14-7.23(3H,m), 7.36(lH,d,J=7.3Hz),
30 7.59(lH,s), 9.93(lH,brs), 12.76(lH,brs).
Example 296
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-(2-methoxyphenyl)-6-
propyl-2H-pyrazolo[3,4-b]pyridine
178
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from methyl 2-
methoxybezoate, methyl butyrate and 2-chlorobenzaldehyde in
the same manner as in Example 290.
MP:>270°C.
s Anal. Calcd. for:C23Hz1C1N40:C, 68 .23;H, 5.23;N,13.84.
Found:C,68.23;H,5.31;N,13,87.
MS(EI) :404(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.90(3H,t,J=7.3Hz), 1.60-
1.65(2H,m), 2.32-2.36(2H,m), 3.70(3H,s), 5.41(lH,s),
l0 6.76(lH,dd,J=7.3Hz and 7.2Hz), 6.90-6.94(2H,m), 6.98-
7.04(2H,m), 7.08-7.15(2H,m), 7.22(lH,dd,J=7.3Hz and 7.2Hz),
9.83(lH,brs), 12.21(lH,brs).
Example 297
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-(3-methoxyphenyl)-6-
15 propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 3-
methoxybenzoate, methyl butyrate and 2-chlorobenzaldehyde in
the same manner as in Example 290.
MP:239°C.
2o Anal. Calcd. for:C23Hz1C1N4O:C,68.23;H,5.23;N,13.84.
Found:C,68.16;H,5.31;N,13.80.
MS(EI) :404(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.88(3H,t,J=7.3Hz), 1.58-
1.63(2H,m), 2.31-2.36(2H,m), 3.68(3H,s), 6.78(lH,d,J=7.3Hz),
25 6.87-6.89(2H,m), 7.11-7.20(4H,m), 7.29(lH,d,J=7.3Hz),
9.92(lH,brs), 12.64(lH,brs).
Example 298
4-(2,1,3-Benzoxadiazol-4-yl)-&-(2-chlorothiophen-5-yl)-5-
cyano-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
3o The title compound was prepared from methyl 5-
chlorothiophene-2-carboxylate, 2,1,3-benzoxadiazole-4-aldehyde
and 3-aminopyrazole in the same manner as in Example 95.
MP:210°C.
179
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C17H9C1N60S:C,53.62;H,2.38;N,22.07.
Found:C,53.51;H,2.67;N,22.13.
MS(EI) :380 (M+) .
1H-NMR (400MHz,DMSO-d6)~(ppm): 5.54(lH,s), 7.23(lH,d,J=3.9Hz),
7.33(lH,s), 7.46(lH,d,J=3.9Hz), 7.50(lH,d,J=6.6Hz),
7.60(lH,dd,J=9.OHz and 6.6Hz), 7.95(lH,d,J=9.OHz),
10.31(lH,brs), 12.30(lH,brs).
Example 299
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(2-
Io methylthiophen-5-yl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 5-
methylthiophene-2-carboxylate, 2,1,3-benzoxadiazol-4-aldehyde
and 3-aminopyrazole in the same manner as in Example 95.
MP:192°C.
MS(EI) :360 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 2.50(3H,s), 5.52(lH,s),
6.87(lH,d,J=3.6Hz), 7.32(lH,s), 7.40(lH,d,J=3.7Hz),
7.48(lH,d,J=6.6Hz), 7.61(lH,dd,J=9.OHz and 6.6Hz),
7.95(lH,d,J=9.OHz), 10.12(lH,brs), 12.26(lH,brs).
zo Example 300
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-(naphthalen-1-yl)-6-
propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl naphthalene-
1-carboxylate, methyl butyrate and 2-chlorobenzaldehyde in the
a5 same manner as in Example 290.
MP:254°C.
Anal. Calcd. for:CZ6H21C1N4:C,73.49;H,4.98;N,13.19.
Found:C,73.81;H,5.05;N,13.08.
MS(EI) :424(M+) .
30 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.95(3H,t,J=7.3Hz), 1.64-
1.70(2H,m), 2.46-2.49(2H,m), 5.25(lH,s), 6.88-7.02(SH,m),
7.31(lH,dd,J=7.3Hz and 7.2Hz), 7.42-7.47(3H,m), 7.83-
7.88(2H,m), 9.95(lH,brs), 12.46(lH,brs).
180
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 301
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-(naphthalen-2-yl)-6-
propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl naphthalene-
2-carboxylate, methyl butyrate and 2-chlorobenzaldehyde in the
same manner as in Example 290.
MP:>270°C.
Anal. Calcd. for:Cz6Hz1C1N4:C,73.49;H,4.98;N,13.19.
Found:C,73.23;H,5.01;N,13.26.
1o MS(EI) :424(M~) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.91(3H,t,J=7.3Hz), 1.61-
1.66(2H,m), 2.31-2.41(2H,m), 5.76(lH,s), 7.05(lH,dd,J=7.3Hz
and 7.2Hz), 7.12-7.16(2H,m), 7.28(lH,d,J=7.3Hz), 7.45-
7.52(2H,m), 7.57(lH,d,J=7.3Hz), 7.77-7.84(4H,m), 9.94(lH,brs),
z5 12.79(lH,brs).
Example 302
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3,6-dipropyl-2H-
pyrazolo[3,4-b]pyridine
To a solution of acetonitrile (4.8 g) in THF (150 mL)
ao was added n-BuLi (67 mmol) at -78°C. Methyl butyrate (10 g)
was added and the mixture was stirred for 1 hour. The reaction
mixture was acidified with hydrochloric acid and extracted
with ethyl acetate. The solvent was evaporated under reduced
pressure and the residue was purified by silica gel column
25 chromatography (eluent: hexane-ethyl acetate (10:1)) to give
1-cyanopentan-2-one (5.5 g) as a colorless oil. To a solution
of the obtained colorless oil in toluene was added hydrazine
monohydrate (5.0 g) and the mixture was heated under reflux
for 3 hours. The mixture was cooled and the solvent was
3o evaporated under reduced pressure. The reaction mixture was
purified by silica gel column chromatography (eluent:
chloroform-methanol (10:1)) to give 5-amino-3-propylpyrazole
(5.0 g). A solution of 2-chloroaldehyde (1.7 g), 5-amino-3-
181
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
propylpyrazole (1.5 g) and 1-cyanopentan-2-one (1.6 g) in
acetonitrile (20 mL) was heated under reflux overnight. The
mixture was cooled to room temperature and the precipitated
crystals were collected by filtration to give the title
s compound (2.1 g) as colorless crystals.
MP:237°C.
Anal. Calcd. for:C19HZ1C1N4:C, 66.95;H, 6.21;N,16 .44.
Found:C,66.98;H,6.26;N,16.41.
MS(EI):340(M+).
.to 1H-NMR (400MHz,DMSO-d6)8(ppm): 0.57(3H,t,J=7.3Hz),
0.91(3H,t,J=7.3Hz), 1.02-1.07(2H,m), 1.59-1.65(2H,m),2.01-
2.12(2H,m), 2.30-2.38(2H,m), 5.28(lH,s), 7.20-7.23(2H,m),
7.30(lH,dd,J=7.3Hz and 7.2Hz), 7.38(lH,d,J=7.3Hz),
9.70(lH,brs), 11.85(lH,brs).
l5 Example 303
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-hydroxy-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butyrate, 2-
chlorobenzaldehyde and 3-amino-5-hydroxypyrazole in the same
ao manner as in Example 94.
MS(EI) :314(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89(3H,t,J=7.3Hz), 1.56-
1.60(2H,m), 2.26-2.38(2H,m), 5.11(lH,s), 7.14-7.21(3H,m),
7.27(lH,dd,J=7.3Hz and 7.2Hz), 7.34(lH,d,J=7.3Hz),
2s 9.64(lH,brs), 10.45(lH,brs).
Example 304
3-Butyl-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl pentanoate,
3o methyl butyrate and 2-chlorobenzaldehyde in the same manner as
in-Example 302.
MP:212°C.
Anal. Calcd. for:CaoH23C1N4:C,67.69;H,6.53;N,15.79.
182
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Found:C,67.58;H,6.46;N,15.75.
MS(ET) :354 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.64(3H,t,J=7.3Hz), 0.89-
0.98(6H,m), 1.10-1.14(lH,m), 1.59-1.64(2H,m),2.05-2.16(2H,m),
2.31-2.35(2H,m), 5.28(lH,s), 7.20-7.24(2H,m),
7.29(lH,dd,J=7.3Hz and 7.2Hz), 7.38(lH,d,J=7.3Hz),
9.70(lH,brs), 11.85(lH,brs).
Example 305
4-(2,1,3-Benzoxadiazol-4-yl)-6-(benzothiophen-2-yl)-5-cyano-
.to 4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
benzothiophene-2-carboxylate, 2,1,3-benzoxadiazole-4-aldehyde
and 3-aminopyrazole in the same manner as in Example 95.
MP:220°C.
z5 Anal. Calcd. for:Cz1H12N60S:C,63.62;H,3.05;N,21.20.
Found:C,63.58;H,3.29;N,21.09.
MS(EI) :396 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.60(lH,s), 7.36(lH,s), 7.44-
7.46(2H,m), 7.54(lH,d,J=6.3Hz), 7.64(lH,dd,J=9.OHz and 6.6Hz),
20 7.88(lH,s), 7.94-7.98(2H,m), 8.05(lH,d,J=9.OHz), 10.40(lH,brs),
12.31(lH,brs).
Example 306
4-(2-Chlorophenyl)-5-cyano-6-cyclohexyl-4,7-dihydro-2H-
~yrazolo[3,4-b]pyridine
25 The title compound was prepared from methyl
cyclohexanecarboxylate, 2-chlorobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:163°C.
Anal. Calcd. for:CzgHIgCIN4 1/2 HzO:C,65.61;H,5.80;N,16.11.
3o Found:C,65.40;H,5.77;N,15.86.
MS(EI) :338(M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.16-1.30(3H,m), 1.66-1.85(7H,m),
2.66-2.72(lH,m), 5.33(lH,s), 7.21-7.25(3H,m),
183
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.32(lH,dd,J=7.3Hz and 7.2Hz), 7.41(lH,d,J=7.3Hz),
9.60(lH,brs), 12.15(lH,brs).
Example 307
6-t-Butyl-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-2H-pyrazolo
[3,4-b]pyridine
The title compound was prepared from methyl pivalate, 2-
chlorobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:198°C.
to Anal. Calcd. for:C17H17C1N4:C,65.28;H,5.48;N,17.91.
Found:C,64.98;H,5.47;N,17.78.
MS(EI) :312 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.41(9H,s), 5.33(lH,s), 7.21-
7.33(4H,m), 7.41(lH,d,J=7.3Hz), 8.88(lH,brs), 12.20(lH,brs).
Example 308
4-(2-Chlorophenyl)-5-cyano-3-cyclopropyl-4,7-dihydro-6-propyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclopropanecarboxylate, methyl butyrate and 2-
2o chlorobenzaldehyde in the same manner as in Example 302.
MP:270°C.
Anal. Calcd. for:C19H19C1N4:C,67.35;H,5.65;N,16.54.
Found:C,67.34;H,5.66;N,16.62.
MS(EI) :338 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.22-0.25(lH,m), 0.41-0.44(lH,m),
0.50-0.54(lH,m), 0.62-0.66(lH,m), 0.90(3H,t,J=7.3Hz), 1.25-
1.29(lH,m), 1.58-1.63(2H,m),2.31-2.36(2H,m), 5.33(lH,s), 7.18-
7.23(2H,m), 7.30(lH,dd,J=7.3Hz and 7.2Hz), 7.38(lH,d,J=7.3Hz),
9.69(lH,brs), 11.73(lH,brs).
3o Example 309
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-ethyl-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl propionate,
184
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
methyl butyrate and 2-chlorobenzaldehyde in the same manner as
in Example 302.
MP:269°C.
Anal. Calcd. for:C18H19C1N4:C,66.15;H,5.86;N,17.14.
Found:C,66.27;H,5.86;N,17.25.
MS(EI):326(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.72(3H,t,J=7.3Hz),
0.91(3H,t,J=7.3Hz), 1.59-1.64(2H,m), 2.09-2.11(2H,m), 2.31-
2.40(2H,m), 5.29(lH,s), 7.20-7.24(2H,m), 7,30(lH,dd,J=7.3Hz
zo and 7.2Hz), 7.38(lH,d,J=7.3Hz), 9.70(lH,brs), 11.86(lH,brs).
Example 310
3-t-Butyl-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl pivalate,
.t5 methyl butyrate and 2-chlorobenzaldehyde in the same manner as
in Example 302.
MP:>270°C.
Anal. Calcd. for:CaoHz3ClN,~:C, 67 .69;H, 6.53;N,15.79.
Found:C,67.55;H,6.56;N,15.66:
2o MS(EI):354(M+).
1H-NMR (400MHz,DMSO-d6)S(ppm): 0.84(3H,t,J=7.3Hz), 0.95(9H,s),
1.53-1.59(2H,m), 2.26-2.30(2H,m), 5.39(lH,s),
6.97(lH,d,J=7.3Hz), 7.20(lH,dd,J=7.3Hz and 7.2Hz),
7.27(lH,dd,J=7.3Hz and 7.2Hz), 7.38(lH,d,J=7.3Hz),
25 9.73(lH,brs), 11.87(lH,brs).
Example 311
4-(2-Chlorophenyl)-5-cyano-3-cyclohexyl-4,7-dihydro-6-propyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
3o cyclohexanecarboxylate, methyl butyrate and 2-
chlorobenzaldehyde in the same manner as in Example 302.
MP : >27 0°C .
Anal. Calcd. for:CZ2HzsC1N4:C, 69 .37;H, 6 .62;N,14.71.
185
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Found:C,69.17;H,6.62;N,14.91.
MS(EI):380(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.89-1.17(9H,m), 1.47-1.64(6H,m),
2.06-2.08(lH,m), 2.31-2.38(2H,m), 5.30(lH,s), 7.19-7.23(2H,m),
7.29(lH,dd,J=7.3Hz and 7.2Hz), 7.38(lH,d,J=7.3Hz),
9.71(lH,brs), 11.83(lH,brs).
Example 312
4-(2-Chlorophenyl)-5-cyano-6-cycloheptyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
io The title compound was prepared from methyl
cycloheptanecarboxylate, 2-chlorobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:146°C.
MS(EI):352(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.42-1.98(l2H,m), 2.78-
2.81(lH,m), 5.33(lH,s), 7.21-7.24(3H,m), 7.32(lH,dd,J=7.3Hz
and 7.2Hz), 7.41(lH,d,J=7.3Hz), 9.61(lH,brs), 12.18(lH,brs).
Example 313
4-(2-Chlorophenyl)-5-cyano-6-cyclobutyl-4,7-dihydro-2H-
ao pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclobutanecarboxylate, 2-chlorobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:188°C.
a5 Anal. Calcd. for:C17H15C1N4:C,65.70;H,4.86;N,18.03.
Found:C,65.51;H,5.21;N,18.27.
MS(EI):310(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.72-1.77(lH,m), 1.93-1.97(lH,m),
2.09-2.12(2H,m), 2.38-2.43(2H,m), 2.58-2.61(lH,m), 5.33(lH,s),
30 7.20-7.32(4H,m), 7.41(lH,d,J=7.3Hz), 9.72(lH,brs),
12.18(lH,brs).
Example 314
4-(2-Chlorophenyl)-5-cyano-3-cyclopentyl-4,7-dihydro-6-propyl-
186
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclopentanecarboxylate, methyl butyrate and 2-
chlorobenzaldehyde in the same manner as in Example 302.
MP:>270°C.
Anal. Calcd. for:C21Hz3C1N4:C,68.75;H,6.32;N,15.27.
Found:C,68.56;H,6.36;N,15.22.
MS(EI) :366 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.89(3H,t,J=7.3Hz), 1.31-
Zo 1.74(lOH,m), 2.30-2.37(2H,m), 2.52-2.54(lH,m),
5.30(lH,s),7.17-7.22(2H,m), 7.28(lH,dd,J=7.3Hz and 7.2Hz),
7.37(lH,d,J=7.3Hz), 9.71(lH,brs), 11.86(lH,brs).
Example 315
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-3-isopropyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 2-
methylpropionate, methyl butyrate and 2-chlorobenzaldehyde in
the same manner as in Example 302.
MP:>270°C.
2o Anal. Calcd. for:C19Hz1C1N4:C,66.95;H,6.21;N,16.44.
Found:C,66.90;H,6.27;N,16.44.
MS(EI) :340(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.67(3H,d,J=7.2Hz),
0.90(3H,t,J=7.3Hz), 0.95(3H,d,J=7.3Hz), 1.57-1.63(2H,m), 2.30-
2.35(2H,m), 5.30(lH,s), 7.19-7.23(2H,m), 7.29(lH,dd,J=7.3Hz
and 7.2Hz), 7.38(lH,d,J=7.3Hz), 9.71(lH,brs), 11.88(lH,brs).
Example 316
4-(2-Chlorophenyl)-5-cyano-6-cyclopentyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
3o The title compound was prepared from methyl
cyclopentanecarboxylate, 2-chlorobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:225°C.
187
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C18H17C1N4 1/5 HaO:C, 65.83;H, 5.34;N,17 .06.
Found:C,66.02;H,5.51;N,16.62.
MS(EI):324(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.56-1.60(2H,m), 1.78-1.87(6H,m),
3.06-3.10(lH,m), 5.34(lH,s), 7.22 -7.26(3H,m),
7.32(lH,dd,J=7.3Hz and 7.2Hz), 7.42(lH,d,J=7.3Hz),
9.61(lH,brs), 12.16(lH,brs).
Example 317
4-(2-Bromo-3-cyanophenyl)-5-cyano-6-cyclopentyl-4,7-dihydro-
l0 2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclopentanecarboxylate, 2-bromo-3-cyanobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:247°C.
MS(EI):394(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.52-1.58(2H,m), 1.75-1.82(6H,m),
3.01-3.06(lH,m), 5.46(lH,s), 7.33(lH,s), 7.54-7.58(2H,m),
7.84(lH,d,J=7.3Hz), 9.73(lH,brs), 12.25(lH,brs).
Example 318
zo 4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-cyclopentyl-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclopentanecarboxylate, 2,1,3-benzoxadiazole-4-aldehyde and
3-aminopyrazole in the same manner as in Example 94.
z5 MP:193°C.
Anal. Calcd. for:C1gH16N6O:C,65.05;H,4.85;N,25.29.
Found:C,64.72;H,4.98;N,24.86.
MS(EI) :332(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.55-1.58(2H,m), 1.80-1.86(6H,m),
30 3.06-3.09(lH,m), 5.39(lH,s), 7.26(lH,s), 7.38(lH,d,J=6.6Hz),
7.60(lH,dd,J=9.OHz and 6.6Hz), 7.91(lH,d,J=9.OHz),
9.72(lH,brs), 12.15(lH,brs).
188
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Example 319
4-(2-Bromo-3-cyanophenyl)-6-t-butyl-5-cyano-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl pivalate, 2-
bromo-3-cyanobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:251°C.
Anal. Calcd. for:C18H1sBrN5 1/2 HZO:C,55.25;H,4.38;N,17.90.
Found:C,55.55;H,4.30;N,18.14.
to MS(EI) :382 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.41(9H,s), 5.46(lH,s),
7.33(lH,s), 7.54-7.60(2H,m), 7.82(lH,d,J=7.3Hz), 9.00(lH,brs),
12 . 29 ( 1H, brs ) '.
Example 320
4-(2,1,3-Benzoxadiazol-4-yl)-6-t-butyl-5-cyano-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl pivalate,
2,1,3-benzoxadiazole-4-aldehyde and 3-aminopyrazole in the
same manner as in Example 94.
2o MP:204°C.
Anal. Calcd. for:C~~H16N60 1/2 HZO:C,63.03;H,5.10;N,25.94.
Found:C,63.08;H,5.08;N,26.00.
MS(EI) :320(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.40(9H,s), 5.37(lH,s),
7.26(lH,s), 7.38(lH,d,J=6.6Hz),7.59(lH,dd,J=9.OHz and 6.6Hz),
7.91(lH,d,J=9.OHz), 9.02(lH,brs), 12.20(lH,brs).
Example 321
4-(2-Bromo-3-eyanophenyl)-5-cyano-6-cyclobutyl-4,7-dihydro-2H-
~yrazolo[3,4-b]pyridine
3o The title compound was prepared from methyl
cyclobutanecarboxylate, 2-bromo-3-cyanobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:278°C.
189
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Anal. Calcd. for:C18H14BrN5:C, 56 .86;H, 3.71;N,18 .42 .
Found:C,56.57;H,3.79;N,18.48.
MS(EI) :380 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.71(lH,m), 1.88-1.95(lH,m),
2.06-2.13(2H,m), 2.38-2.47(2H,m), 3.56-3.60(lH,m), 5.45(lH,s),
7.33(lH,s), 7.57-7.59(2H,m), 7.82(lH,d,J=7.3Hz), 9.84(lH,brs),
12.27(lH,brs).
Example 322
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-cyclobutyl-4,7-dihydro-
.to 2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclobutanecarboxylate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:194°C.
Anal. Calcd. for:C17H14N6O:C,64.14;H,4.43;N,26.40.
Found:C,64.08;H,4.51;N,26.26.
MS(EI) :318 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.72-1.76(lH,m), 1.90-1.97(lH,m),
2.10-2.14(2H,m), 2.39-2.46(2H,m), 3.56-3.60(lH,m), 5.38(lH,s),
7.26(lH,s), 7.37(lH,d,J=6.6Hz), 7.58(lH,dd,J=9.OHz and 6.6Hz),
7.91(lH,d,J=9.OHz), 9.82(lH,brs), 12.17(lH,brs).
Example 323
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-cyclohexyl-4,7-dihydro-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cyclohexanecarboxylate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:210°C.
Anal. Calcd. for:Cl9HzaNs0:C,65.88;H,5.24;N,24.26.
3o Found:C,65.88;H,5.25;N,24.19.
MS (EI ) :346 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.21-1.26(3H,m), 1.62-1.80(7H,m),
2.66-2.70(lH,m), 5.38(lH,s), 7.25(lH,s), 7.38(lH,d,J=6.6Hz),
190
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.59(lH,dd,J=9.OHz and 6.6Hz), 7.91(lH,d,J=9.OHz),
9.72(lH,brs), 12.15(lH,brs).
Example 324
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-cycloheptyl-4,7-
dih~dro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
cycloheptanecarboxylate, 2,1,3-benzoxadiazole-4-aldehyde and
3-aminopyrazole in the same manner as in Example 94.
MP:228°C.
to Anal. Calcd. for:C~oHz0N60:C,66.65;H,5.59;N,23.32.
Found:C,66.45;H,5.70;N,22.97.
MS(EI):360(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.38-1.98(l2H,m), 2.76-
2.79(lH,m), 5.37(lH,s), 7.24(lH,s), 7.38(lH,d,J=6.6Hz),
15 7.58(lH,dd,J=9.OHz and 6.6Hz), 7.91(lH,d,=9.OHz), 9.72(lH,brs),
12.13(lH,brs).
Example 325
4-(2-Bromo-3-cyanophenyl)-5-cyano-6-cyclohexyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
2o The title compound was prepared from methyl
cyclohexanecarboxylate, 2-bromo-3-cyanobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:193°C.
Anal. Calcd. for:CzoHI8BrN5 1/2 HzO:C,57.56;H,4.59;N,16.78.
a5 Found:C,57.25;H,4.37;N,16.56.
MS(EI) :408 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.21-1.26(3H,m), 1.66-1.80(7H,m),
2.66-2.69(lH,m), 5.45(lH,s), 7.33(lH,s), 7.55-7.60(2H,m),
7.82(lH,d,J=7.3Hz), 9.73(lH,brs), 12.24(lH,brs).
3o Example 326
4-(2-Bromo-3-cyanophenyl)-5-cyano-6-cycloheptyl-4,7-dihydro-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
191
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
cycloheptanecarboxylate, 2-bromo-3-cyanobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:252°C.
Anal. Calcd. for:C2lHaoBrNs 1l2 HzO:C,58.48;H,4.91;N,16.24.
s Found:C,58.53;H,4.73;N,16.I9.
MS(EI) :422 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.44-1.58(l2H,m), 2.76-
2.79(lH,m), 5.44(lH,s), 7.31(lH,s), 7.54-7.60(2H,m),
7.83(lH,d,J=7.3Hz), 9.73(lH,brs), 12.23(lH,brs).
.to Example 327
5-Cyano-4,7-dihydro-6-propyl-4-(pyridin-3-yl)-2H-pyrazolo[3,4-
b]pyridine
The title compound was prepared from methyl butyrate,
pyridine-3-aldehyde and 3-aminopyrazole in the same manner as
15 in Example 94.
MP:201°C.
.Anal. Calcd. for:ClsHlsNs:C,67.90;H,5.70;N,26.40.
Found:C,67.42;H,5.74;N,26.72.
MS(EI) :265 (M+) .
2o 1H-NMR (400MHz,DMSO-d6)b(ppm): 0.92(3H,t,J=7.3Hz),1.62-
1.67(2H,m), 2.36-2.39(2H,m), 4.98(lH,s), 7.27(lH,s),
7.35(lH,dd,J=7.3Hz and 2.9Hz), 7.54(lH,d,J=7.3Hz),8.41-
8.44(2H,m), 9.81(lH,brs), 12.18(lH,brs).
Example 328
a5 3-t-Butoxycarbonyloxy-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-
6-propyl-1H-pyrazolo[3,4-b]pyridine
To a solution of 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-
3-hydroxy-6-propyl-2H-pyrazolo[3,4-b]pyridine (12.5 g) in THF
(400 mL) was added triethylamine (4.5 g),
3o dimethylaminopyridine (0.5 g) and di-t-butylcarbonate (9.6 g)
and the mixture was stirred for 3 hours. The mixture was
extracted with ethyl acetate and the solvent was evaporated
under reduced pressure. The residue was recrystallized from
192
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
ethyl acetate to give the title compound (12 g) as colorless
crystals.
MP:182°C.
Anal. Calcd. for:CZlHasC1N403:C,60.79;H,5.59;N,13.50.
s Found:C,60.60;H,5.50;N,13.44.
M5(EI) :414 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91(3H,t,J=7.3Hz), 1.54(9H,s),
2.49-2.51(2H,m), 5.18(lH,s), 7.23-7.27(2H,m),
7.32(lH,dd,J=7.3Hz and 7.2Hz), 7.38(lH,d,J=7.3Hz),
so 9.15(lH,brs), 10.99(lH,brs).
Example 329
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-(2,2-dimethoxyethyl)-
4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 3,3-
.15 dimethoxypropionate, 2,1,3-benzoxadiazole-4-aldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:115°C.
Anal. Calcd. for:C17H16N6O3 1.0 HaO:C,55.13;H,4.90;N,22.69.
Found:C,55.30;H,4.51;N,22.99.
2o MS(EI) :352 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 2.71(2.75(2H,m), 3.28(3H,s),
3.31(3H,s), 4.74(lH,t,J=5.9Hz), 5.43(lH,s), 7.28(lH,s),
7.40(lH,d,J=6.6Hz), 7.61(lH,dd,J=9.OHz and 6.6Hz),
7.92(lH,d,J=9.OHz), 9.99(lH,brs), 12.18(lH,brs).
25 Example 330
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-(2,2-dimethoxyethyl)-
6-propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl 3,3-
dimethoxypropionate, methyl butyrate and 2-chlorobenzaldehyde
3o in the same manner as in Example 302.
MP:180°C.
Anal. Calcd. for:CZpH23C1N402:C,62.09;H,5.99;N,14.48.
Found:C,62.35;H,6.02;N,14.50.
193
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI) :386 (M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91(3H,t,J=7.3Hz), 1.59-
1.64(2H,m), 2.28-2.35(4H,m), 3.00(3H,s), 3.02(3H,s),
3.81(lH,t,J=7.3Hz), 5.31(lH,s), 7.24-7.31(3H,m),
7.40(lH,d,J=7.3Hz), 9.75(lH,brs), 11.92(lH,brs).
Example 331
4-(2,1-Benzoisoxazol-4-yl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butyrate,
Io 2,1-benzoisoxazole-4.aldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:239°C.
MS(EI):305(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.91(3H,t,J=7.3Hz), 1.64-
1.67(2H,m), 2.40-2.43(2H,m), 5.23(lH,s), 6.91(lH,d,J=6.6Hz),
7.28(lH,s), 7.36(lH,dd,J=9.3Hz and 6.6Hz), 7.52(lH,d,J=9.3Hz),
9.37(lH,s), 9.96(lH,brs), 12.21(lH,brs).
Example 332
4-(2,1-Benzoisoxazol-4-yl)-5-cyano-4,7-dihydro-6-isopropyl-2H-
2o pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl isobutyrate,
2,1-benzoisoxazole-4.aldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:245°C.
MS(EI) :305(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 1.23-1.26(6H,m),
3.03(lH,t,J=5.9Hz), 5.21(lH,s), 6.92(lH,d,J=6.6Hz), 7.30(lH,s),
7.37(lH,dd,J=9.3Hz and 6.6Hz), 7.53(lH,d,J=9.3Hz), 9.34(lH,s),
9.78(lH,brs), 7.2.23(lH,brs).
3o Example 333
4-(2,1-Benzoisoxazol-4-yl)-5-cyano-6-cyclopropyl-4,7-dihydro-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
194
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
cyclopropanecarboxylate, 2,1-benzoisoxazole-4.aldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:248°C.
MS(EI~) :303(M+) .
~H-NMR (400MHz,DMSO-d6)8(ppm): 0.89-0.91(2H,m), 1.05-1.08(2H,m),
1.94-1.98(2H,m), 5.20(lH,sj, 6.9I(lH,d,J=6.6Hz),7.28(lH,sj,
7.36(lH,dd,J=9.3Hz and 6.6Hz), 7.52(lH,d,J=9.3Hz), 9.26(lH,s),
9.36(IH,brs),12.22(lH,brs).
Example 334
Zo 4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-6-(1-t-
butoxycarbonylindol-3-yl)-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine
The title compound was prepared from methyl 1-t-
butoxycarbonylindole-3-carboxylate, 2,1,3-benzoxadiazole-4-
1.s aldehyde and 3-aminopyrazole in the same manner as in Example
95.
MP:202°C.
MS(EI) :479 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.65(9H,s), 5.60(lH,s), 7.27-
20 7.41(3H,m), 7.54-7.58(2H,m), 7.64(lH,dd,J=7.3Hz and 7.2Hz),
7.97(lH,d,J=7.3Hz), 8.03(lH,s), 8.10(lH,d,J=7.3Hz),
10.23(lH,brs), 12.26(lH,brs).
Example 335
4-(2,1,3-Benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-(indol-3-
25 yl)-2H-pyrazolo[3,4-b]pyridine
(1-t-Butoxycarbonylindol-3-yl)-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine (0.6 g) was added to trifluoroacetic
acid (15 mL) under ice-cooling and the mixture was stirred for
3 hours. The solvent was evaporated under reduced pressure,
3o and ethyl acetate and a saturated aqueous sodium
hydrogencarbonate solution were added to neutralize the
mixture. The mixture was extracted with ethyl acetate and the
solvent was evaporated under reduced pressure. The residue was
195
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
recrystallized from ethyl acetate to give the title compound
(0.4 g) as colorless crystals.
MP:238°C.
Anal. Calcd. for:C21H13N~0 3/5 HZO:C,64.64;H,3.67;N,25.13.
Found:C,64.77;H,4.05;N,25.59.
MS(EI) :379 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 5.56(lH,s), 7.08(lH,dd,J=7.3Hz
and 7.2Hz), 7.15(lH,dd,J=7.3Hz and 7.2Hz), 7.44(lH,s), 7.44-
7.54(3H,m), 7.65(lH,dd,J=7.3Hz and 7.2Hz), 7.76(lH,s),
l0 7.95(lH,d,J=7.3Hz), 9.98(lH,brs), 11.63(lH,brs), 12.20(lH,brs).
Example 336
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-3-dimethoxymethyl-6-
propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
.t5 dimethylacetate, methyl butyrate and 2-chlorobenzaldehyde in
the same manner as in Example 302.
MP:212°C.
Anal. Calcd. for:C19H21C1N40a:C,61.21;H,5.68;N,15.03.
Found:C,61.25;H,5.69;N,15.17.
2o MS(EI) :372 (M+) .
1H-NMR (400MHz,DMSO-d6)~(ppm): 0.88(3H,t,J=7.3Hz), 1.57-
1.63(2H,m), 2.28-2.35(2H,m), 2.93(6H,s),4.93(lH,sj, 5.30(lH,s),
7.10(lH,d,J=7.3Hz), 7.19(lH,dd,J=7.3Hz and 7.2Hz),
7.25(lH,dd,J=7.3Hz and 7.2Hz), 7.35(lH,d,J=7.3Hz),
25 9.80(lH,brs), 12.29(lH,brs).
Example 337
5-Cyano-4,7-dihydro-6-propyl-4-(pyridin-4-yl)-2H-pyrazolo[3,4-
b]pyridine
The title compound was prepared from methyl butyrate,
3o pyridine-4-aldehyde and 3-aminopyrazole in the same manner as
in Example 94.
MP:224°C.
Anal. Calcd. for:C1~H15N5:C,67.90;H,5.70;N,26.40.
196
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Found:C,67.90;H,5.79;N,26.31.
MS(EI) :265 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92(3H,t,J=7.3Hz), 1.62-
1.67(2H,m), 2.35-2.43(2H,m), 4.95(lH,s), 7.20(2H,dd,J=4.6Hz
and l.5Hz), 7.29(lH,s), 8.50(2H,dd,J=4.6Hz and l.5Hz),
9.84(lH,brs), 12.20(lH,brs).
Example 338
5-Cyano-4,7-dihydro-4-(3-methyl-2-nitrophenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
to The title compound was prepared from methyl butyrate, 3
methyl-2-nitrobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:250°C.
Anal. Calcd. for:C17H17N50z:C,63.15;H,5.30;N,21.66.
Found:C,62.89;H,5.51;N,22.11.
MS(EI):323(M+).
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.94(3H,t,J=7.3Hz), 1.63-
1.68(2H,m), 2.26(3H,s), 2.36-2.42(2H,m), 4.83(lH,s),
7.17(lH,s), 7.20(lH,d,J=7.3H), 7.32(lH,d,J=7.3Hz),
ao 7.48(lH,dd,J=7.3Hz and 7.2Hz), 9.91(lH,brs), 12.22(lH,brs).
Example 339
5-Cyano-4,7-dihydro-4-(3-methyl-2-nitrophenyl)-6-isopropyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl isobutyrate,
a5 3-methyl-2-nitrobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:261°C.
Anal. Calcd. for:Cl~H1~N50z 1/2 HzO:C, 61.43;H, 5.46;N, 21.07 .
Found:C,61.82;H,5.32;N,21.31.
3o MS(EI) :323 (M+) .
1H-NMR (400MHz,DMSO-d6)~(ppm): 1.21(3H,d,J=7.2Hz),
1.26(3H,d,J=7.2Hz), 2.25(3H,s), 3.01(lH,t,J=7.2Hz), 4.84(lH,s),
7.17(lH,s), 7.22(lH,d,J=7.3Hz), 7.32(lH,d,J=7.3Hz),
197
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
7.48(lH,dd,J=7.3Hz and 7.2Hz), 9.71(lH,brs), 12.24(lH,brs).
Example 340
5-Cyano-6-cyclopropyl-4,7-dihydro-4-(3-methyl-2-nitrophenyl)-
2H-pyrazolo[3,4-b]pyridine
s The title compound was prepared from methyl
cyclopropanecarboxylate, 3-methyl-2-nitrobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:265°C.
Anal. Calcd. for:C17H15N502:C,63.54;H,4.71;N,21.79.
1o Found:C,63.44;H,4.85;N,22.04.
MS(EI):321(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.90-1.07(4H,m), 1.96-
1.99(lH,m),2.26(3H,s), 4.81(lH,s), 7.17(lH,s),
7.20(lH,d,J=7.3Hz), 7.32(lH,d,J=7.3Hz), 7.48(lH,dd,J=7.3Hz and
z5 7.2Hz), 9.23(lH,brs), 12.23(lH,brs).
Example 341
Ethyl 4-(2,1,3-benzoxazol-4-yl)-4,7-dihydro-6-(1-methylethyl)-
2H-pyrazolo[3,4-b]pyridine-5-carboxylate 1/2 ethyl acetate
The title compound was prepared from 2,1,3-benzoxazole-
20 4-aldehyde, 3-aminopyrazole and ethyl isobutyrylacetate in the
same manner as in Example 275.
MP:190-193°C (decomposition)
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.7.3(3H,t,J=7.lHz),
1.19(3H,d,J=7.lHz), 1.29(3H,d,J=7.lHz), 3.77(2H,m), 4.37(lH,m),
z5 5.69(lH,s), 7.12(lH,d,J=6.6Hz), 7.22(lH,s),
7.51(lH,dd,J=6.6,9.OHz), 7.78(lH,d,J=8.8Hz), 9.31(lH,brs),
12.02(lH,brs).
Example 342
Ethyl 4-(2-nitrophenyl)-4,7-dihydro-6-(1-methylethyl)-2H-
3o pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from 2-nitrobenzaldehyde,
3-aminopyrazole and ethyl isobutyrylacetate in the same manner
as in Example 275.
198
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:205-206°C.
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.78(3H,t,J=6.8Hz),
1.15(3H,d,J=7.lHz), 1.26(3H,d,J=7.lHz), 3.71(2H,m), 4.33(lH,m),
5.44(lH,s), 7.29-7.34(3H,m), 7.58(lH,m), 7.78(lH,d,J=8.OHz),
9.33(lH,brs), 12.11(lH,brs).
Example 343
Ethyl 4-(2-methoxyphenyl)-4,7-dihydro-6-(1-methylethyl)-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate 1/2 ethyl acetate
The title compound was prepared from 2-
.to methoxybenzaldehyde, 3-aminopyrazole and ethyl
isobutyrylacetate in the same manner as in Example 275.
MP:179-180°C.
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.81(3H,t,J=7.lHz),
1.17(3H,d,J=7.lHzj, 1.27(3H,d,J=7.lHz), 3.76(2H,m), 3.85(3H,sj,
4.31(lH,m), 5.46(lH,s), 6.77(lH,m), 6.89(lH,d,J=8.OHz),
6.94(lH,d,J=7.6Hz), 7.04(lH,m), 7.14(lH,s), 8.98(lH,brs),
11.86(lH,brs).
Example 344
Ethyl 4-(2-bromophenyl)-4,7-dihydro-6-cyclopropyl-2H-
ao pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from
cyclopropanecarbonyl chloride, 2-bromobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 277.
MP:168-170°C
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.86(3H,t,J=7.lHz), 0.87
0.90(2H,m), 1.10-1.14(2H,m), 3.16(lH,m~), 3.78(2H,m),
5.57(lH,s), 7.01(lH,dd,J=5.8,7.6Hz), 7.09(lH,d,J=7.8Hz),
7.24(lH,m), 7.29(lH,s), 7.51(lH,d,J=6.8Hz), 8.65(lH,brs),
12.01(lH,brs).
3o Example 345
Ethyl 4-(2-bromo3-cyanophenyl)-4,7-dihydro-6-cyclopropyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from
199
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
cyclopropanecarbonyl chloride, 2-bromo-3-cyanobenzaldehyde and
3-aminopyrazole in the same manner as' in Example 277.
MP :168-170°C
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.86(3H,t,J=7.1H2), 0.88-
1.00(2H,m), 1.10-1.18(2H,m), 3.14(lH,m), 3.80(2H,m),
5.64(lH,s), 7.33(lH,s), 7.34-7.49(2H,m), 7.68(lH,m),
8.77(lH,brs), 12.10(lH,brs).
Example 346
4-(2-Chlorophenyl)-5-cyano-7-methyl-6-propyl-4,7-dihydro-2H-
to pyrazolo[3,4-b]pyridine
A solution of 3-aminopyrazole (3.0 g), di-t-butyl
dicarbonate (17.3 g) and dimethylaminopyridine (1.3 g) in
tetrahydrofuran (360 ml) was stirred at room temperature. The
reaction mixture was concentrated under reduced pressure. The
obtained residue was purified by silica gel column
chromatography (eluent: hexane-ethyl acetate (10:1)) to give a
mixture (7.9 g) of 1-(t-butoxycarbonyl)-3-(t-
butoxycarbonylamino)pyrazole and 2-(t-butoxycarbonyl)-3-(t-
butoxycarbonylamino)pyrazole as a white amorphous solid. To a
2o suspension of the obtained white amorphous solid (7.9 g) and
sodium hydride (I.1 g) in DMF (80 ml) was added methyl iodide
(4.0 g) under ice-cooling and the mixture was stirred at room
temperature for 1 hour. To the reaction mixture was added
water under iee-cooling and the resulting mixture was
2s extracted with ethyl acetate. The extract was washed with a
saturated aqueous sodium chloride solution, dried over
anhydrous magnesium sulfate, and concentrated under reduced
pressure. The obtained residue was purified by silica gel
column chromatography (eluent: hexane-ethyl acetate (10:1)) to
3o give a white solid (5.3 g). The obtained white solid (5.3 g)
was dissolved in methylene chloride (50 ml), and
trifluoroacetic acid (7 ml) was added. The resulting mixture
was stirred at room temperature for 20 hours. The reaction
200
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
mixture was concentrated under reduced pressure and the
obtained residue was purified by silica gel column
chromatography (eluent: chloroform-methanol (10:1)) to give 3-
methylaminopyrazole (1.54 g) as a colorless transparent oil.
Subsequently, the title compound was prepared from methyl
butyrate, 2-chlorobenzaldehyde and 3-methylaminopyrazole in
the same manner as in Example 94.
MP:170-171°C
Anal. Calcd. for:C1~H17N4C1: C, 65 .28; H, 5 .48;N,17 .91.
.to Found:C,65.14;H,5.52;N,17.72.
MS(EI) :312 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.00(3H,t,J=7.3Hz), 1.68(2H,m),
2.62(2H,m), 3.36(3H,s), 5.36(lH,s), 7.22 -7.26(2H,m), 7.30-
7.32(2H,m), 7.42(lH,d,J=8.lHz), 12.31(lH,brs).
l5 Example 347
4-(2,1,3-Benzoxazol-4-yl)-5-cyano-7-methyl-6-propyl-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butyrate,
2,1,3-benzoxazole-4-aldehyde and 3-methylaminopyrazole in the
2o same manner as in Example 346.
MP:198-200°C
Anal. Calcd. for:C17H16N6O: C,63.74;H,5.03;N,26.23.
Found:C,63.78;H,5.12;N,26.47.
MS(EI) :320 (M+) .
25 1H-NMR (400MHz,DMSO-d6)b(ppm): 0.97(3H,t,J=7.4Hz), 1.61(2H,m),
2.63(2H,m), 3.41(3H,s), 5.40(lH,s),7.32(lH,s),
7.40(lH,d,J=6.6Hz), 7.59(lH,dd,J=6.5,6.6Hz),
7.92(lH,d,J=9.3Hz), 12.30(lH,brs).
Example 348
30 4-(2-Bromo-3-cyanophenyl)-5-cyano-7-methyl-6-propyl-4,7-
dihydro-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butyrate, 2 -
bromo-3-cyanobenzaldehyde and 3-methylaminopyrazole in the
201
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
same manner as in Example 346.
MP:218-220°C
Anal. Calcd. for:C18H1sNsBr: C,56.56;H,4.22;N,18.32.
Found:C,56.60;H,4.41;N,18.18.
MS(EI) :382 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppmj: 1.00(3H,t,J=7.3Hz), 1.63(2H,m),
2.62(2H,m), 3.37(3H,s), 5.47(lH,s), 7.39(lH,s), 7.56-
7.58(2H,m), 7.83(lH,m), 12.41(lH,brs).
Example 349
.to 4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-methyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from ethyl acetate, 2-
chlorobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94.
1H-NMR (400MHz,DMSO-d6)8(ppm): 2.14(3H,s), 5.35(lH,s), 7.21-
7.33(4H,m), 7.42(lH,d,J=8.lHz), 9.87(lH,brs), 12.15(lH,brs).
Example 350
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-(morpholin-4-
yl)methyl-2H-pyrazolo[3,4-b]pyridine dihydrochloride
zo A solution of 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-
methyl-2H-pyrazolo[3,4-b]pyridine (22.9 g), di-t-butyl
dicarbonate (19.4 g) and dimethylaminopyridine (0.5 g) in
tetrahydrofuran (200 ml) was stirred at room temperature for
30 minutes. The reaction mixture was ice-cooled and the
precipitated crystals were collected by filtration to give 2-
(t-butoxycarbonyl)-4-(2-chlorophenylj-5-cyano-4,7-dihydro-6-
methyl-2H-pyrazolo[3,4-b]pyridine (21.8 g) as white crystals.
2-(t-Butoxycarbonyl)-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-
methyl-2H-pyrazolo[3,4-b]pyridine (5.0 g), N-bromosuccinimide
(2.5 g) and azobisisobutyronitrile (66 mg) were suspended in
benzene (50 ml) and the suspension was stirred for 1 hour. The
reaction mixture was concentrated under reduced pressure, and
the obtained residue was purified by silica gel column
202
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
chromatography (eluent: hexane-ethyl acetate (2:1)) and
crystallized from ethyl acetate to give 6-bromomethyl-2-(t-
butoxycarbonyl)-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine as white crystals. To a suspension of
sodium hydride (32 mg) in DMF (10 ml) was added morpholine (70
~,l) and the mixture was stirred at room temperature for 30
minutes. To the reaction mixture was added 6-bromomethyl-2-(t-
butoxycarbonyl)-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine (0.36 g) and the mixture was stirred
so under ice-cooling for 1 hour. To the reaction mixture was
added water, and the precipitated crystals were collected by
filtration and washed with hexane to give 2-(t-
butoxycarbonyl)-4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-
(morpholin-4-yl)methyl-2H-pyrazolo[3,4-b]pyridine (450 mg) as
.t5 white crystals. A solution of 2-(t-butoxycarbonyl)-4-(2-
chlorophenyl)-5-cyano-4,7-dihydro-6-(morpholin-4-yl)methyl-2H-
pyrazolo[3,4-b]pyridine (440 mg) in trifluoroacetic acid (5
ml) was stirred at room temperature for 30 minutes. The
reaction mixture was concentrated under reduced pressure and
2o 4M hydrochloric acid-dioxane solution was added. The
precipitated crystals were collected by filtration and washed
with ethyl acetate to give the title compound (250 mg) as
pale-yellow crystals.
MP:210-214°C (decomposition-).
z5 1H-NMR (400MHz,DMSO-d6)8(ppm): 3.20-3.40(3H,m), 3.84-4.00(3H,m),
4.17-4.40(4H,m), 5.49(lH,s), 7.26-7.37(4H,s),
7.45(lH,d,J=7.8Hz), 10.22(lH,brs), 11.05(lH,brs),
12.33(lH,brs).
Example 351
30 6-Benzyloxymethyl-4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from ethyl
benzyloxyacetate, 2-chlorobenzaldehyde and 3-aminopyrazole in
2 03
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
the same manner as in Example 94.
MP:165-166°C
1H-NMR (400MHz,DMSO-d6)8(ppm): 4.35(2H,d,J=2.9Hz), 4.57(2H,s),
5.42(lH,s), 7.24-7.45(lOH,m), 10.03(lH,brs), 12.22(lH,brs).
s Example 352
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-(methylpiperazin-1-
yl)methyl-2H-pyrazolo[3,4-b]pyridine dihydrochloride
4-(2-Chlorophenyl)-5-cyano-6-(t-butyldimethylsilyloxy)-
methyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine was prepared
Io from ethyl t-butyldimethylsilyloxyacetate, 2-
chlorobenzaldehyde and 3-aminopyrazole in the same manner as
in Example 94. To a solution of 4-(2-chlorophenyl)-5-cyano-6-
(t-butyldimethylsilyloxy)methyl-4,7-dihydro-2H-pyrazolo[3,4-
b]pyridine (10 g) in tetrahydrofuran (100 ml) was added a THF
15 solution (24.9 ml) of 1.0 M tetrabutylammonium fluoride and
the mixture was stirred at room temperature for 1 hour. To the
reaction mixture was added ethyl acetate (200 ml), and the
resulting mixture was washed with a saturated aqueous sodium
chloride solution and dried over anhydrous magnesium sulfate.
2o The solvent was evaporated and the obtained residue was
crystallized from ethyl acetate to give 4-(2-chlorophenyl)-5-
cyano-6-hydroxymethyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
(5.46 g) as a white solid. To a solution of 4-(2-
chlorophenyl)-5-cyano-6-hydroxymethyl-4,7-dihydro-2H-
25 pyrazolo[3,4-b]pyridine (1.0 g) and carbon tetrabromide (1.27
g) in methylene chloride (35 ml) was added triphenylphosphine
(1.0 g) under ice-cooling and the mixture was stirred under
ice-cooling for 4 hours. The reaction mixture was concentrated
under reduced pressure and the obtained residue was purified
3o by silica gel column chromatography (eluent: hexane-ethyl
acetate (1:1)) to give 4-(2-chlorophenyl)-5-cyano-6-
bromomethyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine (0.45 g) as
a pale-yellow solid. To a suspension of sodium hydride (25 mg)
204
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
in DMF (3 ml) was added 1-methylpiperazine (69 ~,l) and the
mixture was stirred at room temperature for 30 minutes. To
this reaction mixture was added a solution of 4-(2-
chlorophenyl)-5-cyano-6-bromomethyl-4,7-dihydro-2H-
pyrazolo[3,4-b]pyridine (200 mg) in DMF (3 ml) under ice-
cooling and the mixture was stirred under ice-cooling for 1
hour. To the reaction mixture was added water and the mixture
was extracted with ethyl acetate. The extract was washed with
a saturated aqueous sodium chloride solution and dried over
.to anhydrous magnesium sulfate. The solvent was evaporated and
the obtained residue was purified by silica gel column
chromatography (eluent: ethyl acetate-methanol (4:1)). The
obtained oil was treated with hydrogen chloride-methanol to
give the title compound (87 mg) as white crystals.
MP:222-225°C (decomposition)
1H-NMR (400MHz,DMSO-d6)&(ppm): 2.66-2.75(2H,m), 2.75(3H,s),
3.00-3.10(4H,m), 3.41-3.55(4H,m), 5.42(lH,s), 7.24-7.36(4H,m),
7.43(lH,d,J=8.OHz), 9.77(lH,brs), 12.17(lH,brs).
Example 353
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-6-(piperidin-1
yl)methyl-2H-pyrazolo[3,4-b]pyridine hydrochloride
The title compound was prepared from 4-(2-chlorophenyl)-
5-cyano-6-bromomethyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine
and piperidine in the same manner as in Example 352.
2s 1H-NMR (400MHz,DMSO-d6)b(ppm): 1.43(lH,m), 1.67-1.82(5H,m),
3.05-3.25(2H,m), 3.48(2H,m), 4.10(2H,m), 5.49(lH,s), 7.26-
7.35(4H,m), 7.45(lH,d,J=8.OHz), 10.28(lH,brs), 10.59(lH,brs).
Example 354
Ethyl 4-(2-nitrophenyl)-4,7-dihydro-6-cyclopropyl-2H-
3o pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from
cyclopropanecarbonyl chloride, 2-nitrobenzaldehyde and 3-
aminopyrazole in the same manner as in Example 277.
205
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MP:162-164°C (decomposition]
1H-NMR (400MHz,DMSO-d6)$(ppm): 0.81(3H,t,J=7.4Hz), 0.85-
0.95(2H,m), 1.10-1.18(2H,m), 3.12(lH,m), 3.72(2H,m),
5.46(lH,s), 7.27-7.34(3H,m), 7.58(lH,m), 7.78(lH,d,J=B.OHz),
8.78(lH,brs), 12.12(lH,brs).
Example 355
Ethyl 4-(2,1,3-benzoxazol-4-yl)-4,7-dihydro-6-cyclopropyl-2H-
pyrazolo[3,4-b]pyridine-5-carboxylate
The title compound was prepared from
1o cyclopropanecarbonyl chloride, 2,1,3-benzoxazole-4-aldehyde
and 3-aminopyrazole in the same manner as in Example 277.
MP:109-111°C (decomposition).
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.76(3H,t,J=6.8Hz), 0.85-
0.86(2H,m), 1.14-1.18(2H,m), 3.12(lH,m), 3.80(2H,m),
Is 5.69(lH,s), 7.13(lH,d,J=6.6Hz), 7.23(lH,s), 7.51(lH,m),
7.79(lH,d,J=9.OHz), 8.83(lH,brs), 12.05(lH,brs).
Example 356
4-(2,1,3-Benzoxazol-4-yl)-5-cyano-4,7-dihydro-2-
(phenylcarbamoyl)-6-propyl-2H-pyrazolo[3,4-b]pyridine
zo The title compound was obtained as colorless crystals
from 4-(2,1,3-benzoxadiazol-4-yl)-5-cyano-4,7-dihydro-6-
propyl-2H-pyrazolo[3,4-b]pyridine, dimethylaminopyridine and
phenylisocyanate in the same manner as in Example 204.
MS(EI) :425 (M+) .
25 1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91(3H,t,J=7.3Hz), 1.64(2H,m),
2.58(2H,m), 5.44(lH,s), 7.10(lH,dd,J=6.3 and 7.6Hz), 7.31
7.34(2H,m), 7.52(lH,d,J=6.6Hzj, 7.59-7.64(3H,m)~, 7.95(lH,s),
7.97(lH,d,J=9.OHz), 9.83(lH,brs), 10.30(lH,brs).
Example 357
30 4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-1-(4-pentenoyl)-6-
propyl-1H-pyrazolo[3,4-b]pyridine
The title compound was obtained as colorless crystals
from 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
206
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
pyrazolo[3,4-b]pyridine, dimethylaminopyridine and 4-pentenoyl
chloride in the same manner as in Example 204.
MP:140°C.
.Anal. Calcd. for:CzzHzlClNg0:C,66.22;H,5.62;N,14.71.
Found:C,66.20;H,5.60;N,14.65.
MS(EI) :380(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.95(3H,t,J=7.3Hz), 1.&2(2H,m),
2.39-2.58(4H,m), 3.11(2H,t,J=7.6Hz), 4.98(lH,d,J=7.lHz),
5.06(lH,d,J=10.3Hz), 5.40(lH,s), 5.85(lH,m), 7.27-7.37(4H,m),
zo 7.46(lH,d,J=7.0Hz), 9.58(lH,brs).
Example 358
4-(2-Chlorophenyl)-5-cyano-4,7-dihydro-2-(4-pentenoyl)-6-
propyl-2H-pyrazolo[3,4-b]pyridine
The title compound was obtained as colorless crystals
.~s from 4-(2-chlorophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[.3,4-b]pyridine, dimethylaminopyridine and 4-pentenoyl
chloride in the same manner as in Example 204.
MP :176-177°C .
Anal. Calcd. for:CzIHZ1C1N40:C,66.22;H,5.56N,14.71.
ao Found:C,66.15;H,5.63;N,14.55.
MS (EI ) : 380 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.95(3H,t,J=7.3Hz), 1.67(2H,m),
2.34-2.49(4H,m), 3.00(2H,t,J=7.6Hz), 4.96(lH,d,J=10.6Hz),
5.02(lH,d,J=27.1Hz), 5.36(lH,s), 5.82(lH,m), 7.30-7.35(3H,m),
a.s 7.46(lH,d,J=7.8Hz), 7.83(lH,s), 10.39(lH,brs).
Example 359
5-Cyano-4,7-dihydro-4-(6-methylpyridin-2-yl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butyrate, 6-
3o methylpyridine-2-aldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:177-181°C.
Anal. Calcd. for:C16H17N5 4/5 HZo:C,65.42;H,6.38;N,23.84.
207
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Found:C,65.52;H,6.31;N,24.19.
MS(EI) :279 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.95(3H,t,J=7.6Hz), 1.66(2H,m),
2.41(2H,m), 2.43(3H,s), 4.94(lH,s), 6.98(lH,d,J=7.6Hz),
7.06(lH,d,J=7.5Hz), 7.21(lH,s), 7.62(lH,dd,J=7.6 and 7.7Hz),
9.71(lH,brs), 12.09(lH,brs).
Example 360
4-(5-Cyano-4,7-dihydro-6-propyl-2H-pyrazolo[3,4-
b]pyridine)pyridine-N-oxide
to The title compound was prepared from methyl butyrate,
pyridine-4-aldehyde-N-oxide and 3-aminopyrazole in the same
manner as in Example 94.
MP:110-115°C.
Anal. Calcd. for:C15H15N50:C,62.O1;H,6.18;N,24.11.
Is. Found:C,61.94;H,5.85;N,23.73.
MS(EI):283(M+).
1H-NMR (400MHz,DMSO-d6)~(ppm): 0.91(3H,t,J=7.3Hz), 1.62(2H,m),
2.36(2H,m), 4.98(lH,s), 7.18(2H,d,J=6.6Hz), 7.31(lH,s),
8.14(2H,d,J=6.3Hz), 9.86(lH,brs), 12.2(lH,brs).
2o Example 361
5-Cyano-4,7-dihydro-4-(3-(4-morpholinomethyl)phenyl)-6-propyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butyrate, 3-
(4-morpholinomethyl)benzaldehyde and 3-aminopyrazole in the
z5 same manner as in Example 94.
MS(EI) :363 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.92(3H,t,J=7.3Hz), 1.63(2H,m),
2.30(4H,m), 2.30(2H,m), 3.40(2H,s), 3.53(4H,m), 4.86(lH,s),
7.05(lH,d,J=7.8Hz), 7.10(lH,d,J=7.6Hz), 7.14(lH,s), 7.19(lH,s),
30 7.23(lH,dd,J=7.5 and 7.6Hz), 9.70(lH,brs), 12.10(lH,brs).
Example 362
4-(3-Bromophenyl)-5-cyano-4,7-dihydro-6-propyl-2H-
pyrazolo[3,4-b]pyridine
208
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
The title compound was prepared from methyl butyrate, 3-
bromobenzaldehyde and 3-aminopyrazole in the same manner as in
Example 94.
MP: 202-205°C .
s Anal. Calcd. for:C16H1sBrN4:C,55.99;H,4.41;N,16.32.
Found:C,55.82;H,4.46;N,17.03.
MS(EI) :343(M+) .
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.91(3H,t,J=7.3Hz), 1.63(2H,m),
2.37(2H,m), 4.92(lH,s), 7.I8(lH,d,J=7.9Hz), 7.25(lH,s),
l0 7.28(lH,d,J=7.8Hz), 7.33(lH,s), 7.39(lH,d,J=8.3Hz),
9.80(lH,brs), 12.18(lH,brs).
Example 363
5-Cyano-4,7-dihydro-4-(4-fluoro-2-chlorophenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
15 The title compound was prepared from methyl butyrate, 2-
chloro-4-fluorobenzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:209-212°C.
Anal. Calcd. for:C16H14C1FN4: C,60.67;H,4.45;N,17.69.
zo Found:C,60.48;H,4.48;N,17.87.
MS(EI):316(M+).
1H-NMR (400MHz,DMSO-d6)b(ppm): 0.93(3H,t,J=7.3Hz), 1.64(2H,m),
2.39(2H,m), 5.33(lH,s), 7.17-7.40(3H,m), 7.41(lH,dd,J=2.7 and
6.lHz), 9.85(lH,brs), 12.17(lH,brs).~
z5 Example 364
5-Cyano-4,7-dihydro-4-(3-(morpholin-4-yl)phenyl)-6-propyl-2H-
pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl butyrate, 3-
(morpholin-4-yl)benzaldehyde and 3-aminopyrazole in the same
3o manner as in Example 94.
MP:196-200°C.
Anal. Calcd. for:CzoHz3N50:C,68.47;H,6.63;N,20.04.
Found:C,68.41;H,6.77;N,20.16.
209
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
MS(EI):349(M+).
sH-NMR (400MHz,DMSO-d6)b(ppm): 0.92(3H,t,J=7.3Hz), 1.63(2H,m),
2.32(2H,m), 3.05(4H,t,J=4.6Hz), 7.71(4H,t,J=4.6Hz), 4.80(lH,s),
6.59(lH,d,J=7.5Hz), 6.74(lH,m), 6.76(lH,s), 7.13(lH,dd,J=7.8
s and 7.8Hz), 7.21(lH,s), 9.67(lH,brs), 12.02(lH,brs).
Example 365
5-Cyano-4,7-dihydro-4-(3-(morpholin-4-yl)phenyl)-6-isopropyl-
2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl isobutyrate,
so 3-(morpholin-4-yl)benzaldehyde and 3-aminopyrazole in the same
manner as in Example 94.
MP:254-257°C.
Anal. Calcd. for:CzoH~3N50:C,68.47;H,6.63;N,20.04.
Found:C,68.56;H,6.73;N,20.30.
15 MS(EI) :349 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 1.20(3H,d,J=7.0Hz),
1.22(3H,d,J=7.lHz), 3.02(2H,m), 3.04(4H,t,J=4.8Hz),
3.70(4H,t,J=4.8Hz), 4.78(lH,s), 6.59(lH,d,J=7.6Hz), 7.74(lH,s),
7.13(lH,dd,J=7.5 and 8.lHz), 7.22(lH,s), 9.48(lH,brs),
zo 12.09(lH,brs).
Example 366
5-Cyano-6-cyclopropyl-4,7-dihydro-4-(3-(morpholin-4-
yl)phenyl)-2H-pyrazolo[3,4-b]pyridine
The title compound was prepared from methyl
25 cyclopropanecarboxylate, 3-(morpholin-4-yl)benzaldehyde and 3-
aminopyrazole in the same manner as in Example 94.
MP:>260°C.
MS(EI) :347 (M+) .
1H-NMR (400MHz,DMSO-d6)8(ppm): 0.86(4H,m), 1.93-1.98(lH,m),
30 3.05(4H,t,J=4.6Hz), 3.70(4H,t,J=4.6Hz), 4.79(lH,s),
6.56(lH,d,J=7.5Hz), 6.74(lH,s), 6.77(lH,s), 7.13(lH,dd,J=7.8
and 7.8Hz), 7.20(lH,s), 8.98(lH,brs),12.09(lH,brs).
The compounds of the above-described Examples are as
210
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
follows.
J
Example 290 Br Example 291 Example 292 % Exampie 293
0
I ~ c1 ~ \ I '~ c1 I \
NC ~ S NC ~ O
I ' NH I \ NH
N N N N
H H
Example 294 Example 295 Example 296 Example 297
c1 ~ I I ~ c1 ~
_ ~ _
NC NC v v
H I ~ NH ~ I I ~ NH
N N N N
H H
CI
Example 298 - Example 299 Example 300 Example 301
I
C1 I / C10H I ~ CI
_ NC NC
NC I ' NH I N NH I N NH
N N H H
H
Example 302 Example 303 Example 304
Example 305
I
~ c1 I i
NC -CI
I N~NvNH NC I i w
NH
H N N
H
Example 306 Example 307 Example 308 Example 309
211
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
\ \
CI I / CI
NC ~ NC _ v
' NH ~ ~ NH NH
N N N N
H H
Example 310 Example 311 Example 312 Example 313
\ \ \
I / c1 I / c1 I / c1
NC NC NC
~ NH ~ r NH ~ wNH
N ~N N ~N N~N
H H H
Example 314 ~ Example 315 Example 316 Example 317
\ CN / ~N
/ Br \ ~NO
NC NC
~wNH ~ ~~~NH
N ~N N ~N
H H
Example 318 Example 319 Example 320 Example 321
Example 322 Example 323 , Example 324 Example 325
I ~N I \ / rN,
/ / CI \ ENO
NC _ NC O~O
NH ~ ~ \ N IO ~ ~ ~ NH
NON NON ~p NON
H H H H
Example 326 Example 327 Example 328 Example 329
212
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
\ / ~K / ..N /
CI \ \ O \ \ O \
NC _ O~ NC _ NC NC
~~ 'NH p ~ ~\ ,NH ~ ~' iNH ~ ~~ ~NH
N N ~ N N N N N N
H H H H
Example 330 Example 331 Example 332 Example 333
/
~CI
NC _
H ~ ~ NH
N N
H
o~ Example 335 Example 336 txample 331
Example 334
\ \ \ / .-K
0
/ NOZ I / NOZ I / NOZ O \ ~N
NC ~ NC ~ NC
\ NH ~ ~ \NH ~ ~ 'NH O ~ ~ \NH
N N N N N N N N
H H H H
Example 338 Example 339 Example 340 Example 341
Example 342 Example 343 Example 344 Example 345
\ / ~N, \ CN
/ CI ~ ~N O I / gr / CI
NC NC NC NC I ~ NH
~/-'NH I ~/'NH I ~/'NH ~N
I ~N I ~N I ~N N
H
Example 346 Example 347 Example 348 Example 349
213
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
I~ I~ L~ I~
'CI 'c1 'CI 'c1
NC ~ / NC ~ \N NC
N I ~ NH \ I O I ~ NH ~N I \ NH ~N I ~ NH
N N N N N N N N
H H H H
Example 350 Example 351 Example 352 Example 353
\ l ~~l l ~~l l
I ~ N02 \ ~N O ~ ~N O ~ I CI
O I \ NH ~O I ~ NH NC I ~ N O NC I I ~N
H~N H~N H N H ~ ~ H
O
Example 354 Example 355 Example 356 Example 357
I ~ -~ N
CI \ N \ I ~O
NC p
I ~' N NC ~ NC
N ~N I ~ NH I ~ NH
H ~ N N N N
H H
Example 358 Example 359 Example 360 Example 361
Br
\I \I
NC NC _
NH I ~ NH
N N N N
H H
Example 362 Example 363 Example 364 Example 365
Example 366
214
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
Formulation Example 1
The compound of Example 1 (0.5 part), lactose (25 parts),
crystalline cellulose (35 parts) and corn starch (3 parts)
were thoroughly mixed and kneaded well with a binder made of
corn starch (2 parts). The kneaded product was passed through
a 16 mesh sieve, dried in an oven at 50°C and passed through a
4 mesh sieve. The kneaded powder thus obtained, corn starch (8
parts), crystalline cellulose (11 parts) and talc (9 parts)
were thoroughly mixed and compression-punched to give tablets
to containing 0.5 mg of the active ingredient per tablet.
Formulation Example 2
The compound of Example 1 (1.0 ing) and sodium chloride
(9.0 mg) were dissolved in water for injection and the
solution was filtered to remove pyrogen. The filtrate was
transferred into ampoules under sterile conditions. After
sterilization, the ampoules were weld-sealed to give
injections each containing 1.0 mg of the active ingredient.
The effects of the compounds of the present invention on
glycogen synthase kinase-3 beta (GSK-3(3) were evaluated and
ao confirmed as follows.
Experimental Example 1: GSK-3(3-inhibitory activity
CREB phosphopeptide (4.6 nmol), rabbit GSK-3(3 (0.5 unit),
ATP (5 nmol), [y-3zP]ATP (12.3 kBq) and a test compound were
reacted in a GSK-3(3.buffer solution (25 ~,L) (20 mmol/L Tris-HCl
z5 (pH 7.5), 10 mmol/L magnesium chloride, 5 mmol/L
dithiothreitol) containing to dimethyl sulfoxide, at 30°C for
minutes. The reaction product (10 ~uL) was adsorbed on.a P81
ion-exchange paper, and the paper was washed with phosphoric
acid (100 mmol/L) and measured for cpm on a scintillation
3o counter. As a result, the compounds of the present invention
showed the IC5o values of 1 to 1000 nmol/L. For example, the
IC5o values of the compounds of Examples l, 14, 27, 66 and 140
were 210, 170, 25, 51 and 24 nmol/L, respectively.
215
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
CREB Phosphopeptide is Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-
Arg-Pro-Ser(P)-Tyr-Arg.
Experimental Example 2: GSK-3(3-inhibitory activity in rat
cultured hippocampal neurons
s Hippocampal neurons were obtained from rat embryos on
the 18th day after conception. After culturing the hippocampal
neurons for 7 days, the neurons were treated with amyloid (3
(25-35) (20 ~umol/L) and a test compound (GSK-3(3 inhibitor) and
the culture was continued for 3 hours, whereby phosphorylation
to of Tau protein was induced. After the completion of culture,
the level of phosphorylation of Tau protein was determined by
EIA method using phosphorylated Tau-recognizing antibody
(phosphorylated site by GSK-3(3) and the inhibitory effect of
the GSK-3(3 inhibitor on the neurons was evaluated. Fig. 1
Is shows the GSK-3(3-inhibitory activity of the compounds of
Example 47 and Example 137.
Experimental Example 3: Effect on amyloid [3-induced
cytotoxicity in rat cultured hippocampal neurons
Hippocampal neurons were obtained from rat embryos on
ao the 18th day after conception. After culturing the hippocampal
neurons for 7 days, the neurons were treated with amyloid (3
(25-35) (20 ~umol/L) and a test compound (GSK-3(3 inhibitor) and
the culture was continued for 24 hours, whereby cytotoxicity
(decreased activity of intracellular dehydrogenases) was
zs induced. After the completion of culture, activity of
intracellular dehydrogenases was determined and the effect of
the GSK-3(3 inhibitor on the amyloid [3-induced cytotoxicity was
evaluated. Fig. 2 shows the effect of the compounds of Example
66 on amyloid (3-induced cytotoxicity.
3o Experimental Example 4: GSK-3(3-inhibitory effect in gerbil
brain ischemia model
A test compound (GSK-3/3 inhibitor) was intraperitoneally
administered to gerbils and 30 minutes later, brain ischemia
216
CA 02437215 2003-07-29
WO 02/062795 PCT/JP02/00829
was created by shutting off (for 4 minutes) all carotid
arteries, whereby phosphorylation of Tau protein in the brain
was induced. Three hours after the brain ischemia, the
hippocampus.was obtained from the gerbil brain and the level
s of phosphorylation of Tau protein was determined by Western
blot using phosphorylated Tau-recognizing antibody
(phosphorylated site by GSK-3(3), based on which the GSK-3(3-
inhibitory effect of the GSK-3(3 inhibitor in the gerbil brain
was evaluated. Fig. 3 shows the GSK-3(3-inhibitory effect of
.to the compounds of Example 27 in gerbil brain ischemia model.
Industrial Applicability
The compounds of the present invention show a selective
and strong inhibitory action on glycogen synthase kinase-3
15 beta (GSK-3(3), and are useful as medicaments for prevention
and/or treatment of diabetes, diabetic complications and
neurodegenerative diseases (Alzheimer's disease, ischemic
cerebrovascular disorder, Down's syndrome, cerebral ischemia
due to cerebral amyloid angiopathy, progressive supranuclear
ao paralysis, subacute sclerosing panencephalitic Parkinsonism,
postencephalitic Parkinsonism, boxer's encephalopathy,
Parkinson dementia complex of Guam, Lewy body disease, Pick's
disease, corticobasal degeneration, frontotemporal dementia,
ATDS encephalopathy, Huntington's disease, manic-depressive
a5 psychosis and the like), or as immunopotentiators.
This application is based on patent application Nos.
2001-304707, 2001-26379 and 2001-081238 filed in Japan, the
contents of which are hereby incorporated by reference.
217