Note: Descriptions are shown in the official language in which they were submitted.
CA 02437412 2004-04-21
DELAYED RELEASE MICROTABLET OF I3-PHENYLPROPIOPHENONE
DERIVATIVES
The present invention relates to cylindrical microtablets of 13-
phenylpropiophenone
derivatives with a high content and density of active ingredient and a delayed
release
which is independent of the compressive force, with no release-delaying
ancillary
substances.
Reference to 13-phenylpropiophenone derivatives hereinbefore and hereinafter
always
includes and particularly means their physiologically or pharmacologically
acceptable
salts, preferably the hydrochloride.
In the prior art the release of active ingredient from tablets is delayed
either by a release-
delaying matrix in which the active ingredient is embedded, or by a release-
delaying
coating through which the digestive fluid diffuses in and the active
ingredient diffuses
out.
Both principles have considerable disadvantages. For example, matrix tablets
contain
relatively large amounts of ancillary substances so that the volume of the
tablet for a
given dose of active ingredient is relatively large, which is unpleasant for
the patient. On
the other hand, film-coated tablets are elaborate to produce and, in
particular,
mechanically sensitive. The slightest damage to the lacquer film leads to the
risk of
sudden release of the entire content of active ingredient (dose dumping),
which is
extremely undesirable (local and temporal overdose with adverse side effects;
short total
action time).
Both matrix and film-coated delayed release tablets normally have diameters of
about 6 to
12 mm or more and are therefore unable to pass through the closed pylorus. The
release
and absorption of their total content of active ingredient concentrated at one
site in the
gastrointestinal tract depends on the conditions prevailing at this site,
which results in
wide interindividual and intraindividual variation in the plasma level.
This variation is less with multiple unit delayed release forms because the
units are
distributed uniformly along the gastrointestinal tract and can also pass
through the closed
pylorus. Usually employed as multiple unit forms are pellets with a diffusion
lacquer
packed into hard gelatin capsules. It is possible to produce matrix pellets
only with very
CA 02437412 2004-04-21
2
low doses of medicinal substances because, owing to the large surface area,
even more
matrix substance would be required than for the bolus delayed release tablet.
For example, the Patent Applications GB 2 176 999 and WO 92/04013 disclose
small
matrix delayed release tablets which likewise contain relatively large amounts
of release-
s delaying ancillary substances. The Patent Application EP 22 17 32 claims
delayed
release tablets of active ingredients with low solubility, which contain 60-
80% active
ingredient in addition to at least four auxiliaries. The release from these
bolus forms is, as
described in the patent, highly dependent on the granulation process and the
equipment
used for manufacture.
It is furthermore generally known that an increase in the compressive force in
tablet
production is associated with a slowing of the release of active ingredient.
This applies
both to fast release tablets and to delayed release tablets (Patent
Application WO
92/00064). Since the compressive forces fluctuate, despite the most up to date
machine
engineering, the resulting release rates vary. An additional factor is the
variation between
batches in the compression properties, which derives from the variability in
the granules
to be compressed. Differences in the particle size, porosity, surface
structure, wettability
etc. may have a large effect on the compression properties and the delaying of
release.
It is an object of the present invention to overcome the disadvantages of the
prior art, ie.
to develop propafenone and diprafenone tablets with a small size, high content
and
density of active ingredient and release of active ingredient which is
independent of the
compressive force and uniform over a lengthy period.
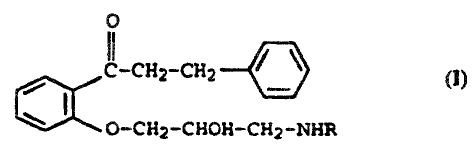
Accordingly, the invention provides, in accordance with a first aspect, a
cylindrical
delayed release microtablet with a convex or flat upper side and lower side of
0
~..~H~_~z /
~ (1)
W
o-ct~z-cHaH-cxz-NHR
(3-phenylpropiophenone derivatives of the formula I as active ingredient where
R is n
propyl or 1,1-dimethylpropyl, and their pharmacologically acceptable salts,
wherein
CA 02437412 2004-04-21
(a) the height and diameter are, independently of one another, 1-3 mm,
(b) the active ingredient content is in the range from 81 to 99.9% of the
weight of the
microtablet, but not taking into account the weight of any coating which is
present,
(c) the release of active ingredient in the USP paddle method at 50 rpm is 80%
as a
maximum after 3 hours and as a minimum after 24 hours,
(d) the release rate is virtually independent of the pressure when compressing
the
microtablets, and
(e) the microtablet contains no release-delaying ancillary substance but 0.1-
5% by
weight of a lubricant and 0-18.9% by weight of other conventional ancillary
substances.
In one embodiment, the microtablet, in vivo, results in a pronounced plasma
level plateau
with a PTF < 75% and the bioavailability of the microtablet does not depend on
the intake
of food.
The active ingredient may be propafenone hydrochloride and the height and
diameter of
the microtablet may be approximately the same.
In accordance with another aspect, the invention provides a gelatin capsule
which
contains 3-200 microtablets in accordance with the first aspect with identical
or different
release rates.
According to another aspect, the invention provides a process for producing a
cylindrical
delayed release microtablet in accordance with the first aspect, the
microtablet comprising
a homogeneous mixture of 81-99.9% by weight of the granulated active
ingredient with a
particle size below 1 mm, 0.1-5% by weight of a lubricant and 0-18.9% by
weight of
other conventional ancillary substances which do not delay release, the
process
comprising compressing the microtablet in a cylindrical mold with a height and
diameter
each of 1.3 mm, and removing the microtablet from the mold.
CA 02437412 2004-04-21
4
We have found that this object is achieved by the microtablets according to
the first
aspect of the invention. This is because it has been found, surprisingly, that
it is possible
in the present case to produce delayed release tablets without release-
delaying ancillary
substances. This is all the more surprising because other medicinal substances
with a
water solubility similar to that of propafenone hydrochloride (0.7%), for
example
cimetidine hydrochloride or paracetamol, are 90% released in 1 hour from the
same
preparation.
By comparison with other substances, propafenone HCl is extremely difficult to
compress. A bolus tablet with commercial dosages of 150-300 mg and an active
ingredient content above 80% cannot be produced under production conditions.
By
contrast, the microtablets according to the invention can, surprisingly, be
produced at a
relatively high machine speed without problems concerning friability and
hardness, and
specifically with active ingredient contents in the range from 81 to 99.9,
preferably 85 to
99.5, % by weight and with an active ingredient density above 1. Such high
contents of
active ingredients of this type in tablets have not previously been reached.
The microtablets according to the invention are cylindrical with a flat or
convex upper
side and lower side and with a diameter and height which are preferably
approximately
equal and, independently of one another, from 1 to 3, preferably 1.5 to 2.5
mm.
It was furthermore not predictable that the release of active ingredient is,
in contrast to
usual experience, virtually independent of the pressure when compressing the
microtablets and, moreover, over a wide range of pH of the medium. "Virtually
independent" means that the effect can be neglected for practical purposes.
This ensures
release at a constant rate. It is adjusted via the size of the microtablet and
possibly by
additives which increase the release rate so that the release of active
ingredient after 3,
preferably 5, hours is not more than 80% and after 24, preferably 15, hours is
not less
than 80%. Surprisingly, the microtablets according to the invention also
display distinct
advantages in vivo unlike conventional delayed release forms such as a bolus
delayed
release form with similar in vitro release. Despite the short half life, a
pronounced blood
level plateau develops (Fig. 11). The fluctuations in the blood level are
considerably less
with the microtablets. This is evident from the t~s% (period in the dosage
interval during
CA 02437412 2004-04-21
which the plasma levels are at least 75% of the maximum level), which is 8 to
9 hours
with the microtablets according to the invention compared with 5 to 6 hours
with the
bolus delayed release form, and from the PTF (peak to trough fluctuation; cf.
H. P. Koch
and W. A. Ritschel, Synopsis der Biopharmazie and Pharmakokinetik, Ecomed-
Verlagsgesellschaft mbH, Landsberg and Munchen, 1986)
Cmax - Cm;" for the AUC, cf. J. K. Aronson
PTF (%) = x100 et al., Europ. J. of Clinical
AUC Pharmacology 35 (1988), 1-7.
Ot
which has a value for the microtablets which is only about half that for the
bolus forms, in
particular less than 75, preferably less than 60, %. The microtablets
accordingly increase
therapeutic safety because excessive peaks of plasma levels and the side
effects caused
thereby do not occur, the plasma level does not fall below the minimum
effective level,
and the bioavailability of this form is unaffected by food intake, in contrast
to the bolus
delayed release form.
The AUC found for the bolus delayed release form is 50% higher when fasting.
In general, the microtablets show smaller intra- and inter-individual
differences by
comparison with the bolus delayed release form.
The microtablets according to the invention furthermore have the advantage
that when
introduced into gastric or intestinal fluid they show no tendency to stick or
adhere. This
ensures that they pass as individual articles through the gastrointestinal
tract and,
moreover, do not become attached to the wall of the stomach or intestine and
induce
irntation. Sticking or adhesion properties of this type are displayed, for
example, by
small articles with hydrophilic release-delaying polymers (cf. WO 92/04013).
The production of delayed release forms with hydrophilic release-delaying
polymers
often requires the use of organic solvents during granulation so that swelling
does not
start even during this process. It is possible entirely to dispense with this
in the
production of the microtablets according to the invention.
CA 02437412 2004-04-21
Presentations with hydrophilic release-delaying polymers additionally have the
disadvantage that, because of the tendency to sorption and swelling, they are
sensitive to a
change in humidity during storage. These formulations are damaged by high
humidities
in particular. The microtablets according to the invention are stable even at
relatively
high humidities because of the insensitivity of the materials used. Even after
storage at
93% rel. humidity for 21 days the water uptake is less than 1%, and no visible
change is
detectable.
The microtablets according to the invention are produced in conventional
pharmaceutical
equipment by the following steps: granulation, drying, mixing, tabletting.
The particle size of the active ingredient is, within the conventional
pharmaceutical range,
of only minor or no importance in the production of the microtablets according
to the
invention, against all expectations. This means that it is possible to convert
propafenone
hydrochloride and diprafenone hydrochloride of different particle sizes into
products of
the same quality.
Granulation and drying are preferably carried out in a fluidized bed. However,
the
agglomeration can also be carried out in a horizontal or vertical mixer.
After the wet granules have been passed through a screen of suitable mesh
width they are
dried either in a circulating air dryer or in a fluidized bed. The particle
size of the
granules should be below 1 mm, preferably below 0.8 mm.
It is possible to employ all conventional binders or adhesives for the
agglomeration, eg.
polyvinylpyrrolidone, vinylpyrrolidone/vinyl acetate copolymers, gelatin,
hydroxypropylmethylcellulose, hydroxypropylcellulose, polymers of methacrylic
acid
and its esters. It is possible to dispense with the use of a binder by using a
solution of
active ingredient as granulation liquid. Water without additives is preferred
as granulation
liquid.
After the granules have been dried to the defined water content, 0.1-5,
preferably 0.3-2,
by weight of a lubricant for the tabletting are mixed in homogeneously. It is
likewise
possible to use for this purpose all conventional substances such as talc,
magnesium
stearate, calcium stearate, stearic acid, calcium behenate, glycerin
palmitostearate, sodium
CA 02437412 2004-04-21
acetate, polyethylene glycol, sodium stearate [sic] fumarate. In addition, up
to 18.9% by
weight of other conventional ancillary substances can be added, for example
colorants,
stabilizers, fillers, wetting agents, flow regulators but no release-delaying
agents.
The tabletting takes place in a suitable tabletting machine equipped with
multiple
microtablet punches. The resulting microtablets have a cylindrical shape with
flat or
convex surface [sic]. The height and the diameter can be varied independently
of one
another. It is often expedient, to increase the apparent density and improve
flowability, to
match the height of the microtablets to the diameter.
Another element in the control of release besides the size of the microtablets
is the
addition of wetting agents which increase the rate of dissolution. Wetting
agents which
can be used are, on the one hand, surfactants such as polyoxyethylene fatty
acid esters,
polyoxyethylene fatty alcohol ethers, fatty acid salts, bile acid salts, alkyl
sulfates or
ethylene oxide/propylene oxide block copolymers or, on the other hand,
genuinely water-
soluble substances such as polyethylene glycols, urea, sodium chloride,
sorbitol,
mannitol, glycine, nicotinamide, or salts of citric acid, tartaric acid or
phosphoric acid. In
this case the rate of release increases in parallel with the rise in the
wetting agent
concentration.
The wetting agent can be incorporated into the granules or else be
subsequently mixed in
together with the lubricant. This is, of course, possible only with solid
wetting agents.
The wetting agent concentration is 0.1-15, as a rule 1-10, % of the total
mass.
To increase the rate of erosion of the active ingredient from the tablet
surface, and thus
the release of active ingredient, it is also possible to use disintegrants in
concentrations of
0.001-0.5, preferably 0.01-0.1, %, which are far below the conventional
concentrations.
As a rule, the microtablets can be packed into gelatin capsules directly using
conventional
filling machines. It may occasionally be advantageous for the microtablets,
before the
packing, to be provided with a readily soluble film coating which does not
influence the
release.
In addition, it is in many cases expedient to combine delayed release with
instant release
or not so delayed release microtablets. This results in release of an initial
dose at once,
CA 02437412 2004-04-21
followed by the slow release of the maintenance dose. . Modern capsule filling
machines
are able to meter two products into one capsule without problems.
The instant release microtablet differs from the delayed release microtablet
in that it
contains conventional amounts of disintegrant, swelling agent, pore former,
which bring
S about rapid disintegration of the microtablet into small fragments and rapid
dissolution of
the active ingredient.
The microtablets of the examples always had a diameter and height each of 2
mm, and the
density of active ingredient was always more than 1.
EXAMPLES
Example 1 (Fig. 1)
Propafenone delayed release microtablets
Composition
Propafenone HCl 6.25 mg (96%)
Hydroxypropylinethylcellulose 0.20 mg
Magnesium stearate 0.05 mg
Total weight 6.50 mg
30kg of propafenone HCl were granulated with 10 kg of a 10% strength
hydroxypropylmethylcellulose solution (Pharmacoat~ 603) and dried in a
fluidized bed
granulator. The granules were passed through a screen of suitable mesh width
and then
mixed in a plowshare mixer with the stated amount of magnesium stearate.
The microtablets were produced in a rotary tabletting machine equipped with
multiple
microtablet punches.
The number of microtablets corresponding to the dose to be administered was
packed into
hard gelatin capsules using a suitable capsule filling machine.
CA 02437412 2004-04-21
9
Table 1
Results of studies on volunteers with propafenone HCl microtablets of Example
1 and a
bolus delayed release form according to the comparative test (n = 18, dose:
400 mg of
propafenone HCI, repeated administration)
Microtablets Bolus delayed
release
form
fastin non-fasting fasting non-fasting
AUC ng~h 5 500 5 500 6 900 4 700
ml
t~s % (h) 8-9 8-9 5-6 5-6
PTF% 52 56 88 106
n = number of volunteers
ng = nanogram
h = hours
Example 2 (Fig. 2)
Propafenone delayed release microtablets
Composition
Propafenone HCl 5.92 mg (91 %)
Hydroxypropylmethylcellulose 0.20 mg
Poloxamer 188 (USP) 0.33 mg
Magnesium stearate 0.05 mg
Total weight 6.5 mg
Production took place as in Example 1. The required amount of poloxamer 188
together
with the magnesium stearate were mixed with the granules in a plowshare mixer.
1 S Example 3 (Fig. 3)
Propafenone delayed release microtablets
Composition
Propafenone HCl 5.61 mg (86%)
Hydroxypropylmethylcellulose0.19 mg
Poloxamer188 0.65 mg
Magnesium stearate 0.05 mg
Total weight 6.5 mg
Production took place as in Example 2.
CA 02437412 2004-04-21
Example 4 (Fig. 4)
Propafenone delayed release microtablets
Composition
Propafenone HCl 6.0 mg (86%)
5 Hydroxypropylmethylcellulose0.2 mg
Calcium hydrogen phosphate 0.613 mg
Monoglyceride (Myvatox ~) 0.15 mg
Crosslinked polyvinylpyrrolidone0.007 mg
Magnesium stearate 0.03 mg
10 Total weight 7.0 mg
Production took place as in Example 2.
Example 5 (Fig. 5)
Propafenone delayed release microtablets
Composition
Propafenone HCl 5.70 mg (81 %)
Gelatin 0.18 mg
Calcium hydrogen phosphate 0.38 mg
NaCI 0.70 mg
Magnesium stearate 0.04 mg
Total weight 7.0 mg
Production took place as in Example 1. A 10% strength gelatin solution was
used as
granulating agent. The amount of NaCI was mixed in with the magnesium
stearate.
Example 6 (Fig. 6)
Propafenone delayed release microtablets
Composition
Propafenone HCl 5.83 mg (83%)
Hydroxypropylmethylcellulose0.17 mg
13-Cyclodextrin 0.9 mg
Magnesium stearate 0.1 mg
Total weight 7.0 mg
Production took place as in Example 2.
CA 02437412 2004-04-21
11
Example 7 (Fig. 7)
Gelatin capsules with propafenone delayed release microtablets and propafenone
instant
release microtablets
To achieve a higher initial release, 14 instant release microtablets and 55
delayed release
microtablets were packed into hard gelatin capsules in a suitable capsule
filling machine.
Composition of the instant release microtablets
Propafenone HCl 6.05 mg (93%)
Hydroxypropylmethylcellulose0.20 mg
Sodium carboxymethylstarch 0.20 mg
Magnesium stearate 0.05 mg
Total weight 6.5 mg
The instant release microtablets were produced as in Example 2.
The delayed release microtablets were produced as in Example 1.
Example 8 (Fig. 8)
Propafenone delayed release microtablets
Composition
Propafenone HCl 6.48 mg (99.7%)
Magnesium stearate 0.02 mg
Total weight 6.50 mg
Propafenone hydrochloride and magnesium stearate were mixed in a plowshare
mixer and
subsequently compressed to microtablets.
The in vitro release plots (Figs. 1 to 10) were determined using a USP paddle
apparatus
with 0.08 molar HCl in the first two hours and then phosphate buffer pH 6.8.
The paddle
rotated at 50 rpm.
Comparative test
Propafenone delayed release bolus film-coated tablet
CA 02437412 2004-04-21
12
Composition
Propafenone HCl 450.0
mg
Sodium alginate 112.0
mg
Microcrystalline cellulose type PH 101 37.0 mg
Copolymers of acrylic and methacrylic esters
with a small
content of quaternary ammonium groups (Eudragit15.0 mg
~ RS)
Gelatin 55.0 mg
Magnesium stearate 3.5 mg
Microcrystalline cellulose type PH 102 12.5 mg
Readily soluble film coating 15.0 mg
Total weight 700.0
mg
Propafenone hydrochloride, sodium alginate, microcrystalline cellulose (type
PH 101 )
and Eudragit RS were mixed in a vertical mixer and granulated with 20%
strength gelatin
solution. The wet granules were dried in a fluidized bed dryer with inlet air
at 60°C.
After passing through a screen of suitable mesh width, magnesium stearate and
microcrystalline cellulose (type PH 102) were admixed in a horizontal mixer
and
subsequently the mixture was compressed to oblong tablets (dimensions 18X8.7
mm) in a
rotary tabletting machine. The readily soluble coating was applied in a
horizontal coater.
Determination of in vitro release in a paddle apparatus at 50 rpm produced the
following
results (in %):
1 st hour 3
.8
2nd hour 5.5
3rd hour 23.7
4th hour 43.0
6th hour 75.4
8th hour 89.5
The in vitro release from the delayed release bolus film-coated tablet is thus
similar to
that of the delayed release microtablets according to the invention.
Nevertheless, the in
vivo release is entirely different and, in fact, better according to the
invention, cf. drug
levels shown in Fig. 11