Note: Descriptions are shown in the official language in which they were submitted.
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
1
CELL FOR THE ELECTROWINNING OF ALUMINIUM
OPERATING WITH METAL-BASED ANODES
Field of the Invention
The invention relates to a cell for the
electrowinning of aluminium from alumina dissolved in a
crustless fluoride-containing molten electrolyte at a
temperature below 930°C, as well as the production of
aluminium in such cell.
Background of the Invention
The production of aluminium today utilises cells for
the electrolysis of alumina dissolved in cryolite with
an excess of approximately 10 weighto aluminium
fluoride, operating at a temperature of approximately
950°C, utilising carbon anodes.
Several patents have been. filed. and many granted
concerning anode and cathode materials, shape, cell
designs, operating conditions etc., and many solutions
to specific problems have been proposed. However, no
overall arrangement has heretofore been proposed which
meets up to all the practical requirements for the
industrial production of aluminium with low
contamination.
Most metal anodes suggested until now, except anodes
covered with a protective cerium-based coating, are
highly soluble in the electrolyte utilised contaminating
the aluminium produced, and have other drawbacks such as
low electrical conductivity, short life and high COSt.
US Patents 4,614,569 (Duruz/Derivaz/Debely/
Adorian), 4,966,674 (Bannochie/Sheriff), 4,683,037 and
4,680,094 (both in the name of Duruz) describe metal
anodes for aluminium electrowinning coated with a
protective coating of cerium oxyfluoride, formed in-situ
in the cell or pre-applied, this coating being
maintained by the addition of~small amounts of cerium to
the molten cryolite.
EP Patent application 0 306. 100 and US Patents
5,069,771, 4,960,494 and 4,956,068 (all in the name of
Nyguen/Lazouni/Doan) disclose ,aluminium production
anodes having an alloy substrate protected with an
oxygen barrier layer that is covered with a copper-
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
2
nickel layer for anchoring a cerium oxyfluoride
operative surface coating.
Several improvements' of 'the cathodic side of
aluminium production cells have been disclosed in the
following patents.
W001/42168 (de Nora/Duruz)..and W001/42531 (Nguyen/
Duruz/de Nora) describe~a carbon-containing component of
a cell for the production of aluminium by the
electrolysis of alumina dissolved in a cryolite-based
molten electrolyte, which cell component is protected
from attack lay liquid and/or gaseous components of the
electrolyte or products, such as aluminium, produced
during cell operation by a slurry-applied aluminium-
wettable coating. PCT publications W096/07773 (de Nora)
and W098/53120 (Berclaz/de Nora) disclose cells for the
production of aluminium having a horizontal cathode
bottom covered with a slurry-applied aluminium-wettable
coating.
It has also been proposed to stabilise the cathodic
aluminium pool of conventional aluminium production
cells by placing bodies onto the cathode bottom
US Patents 5,472,578 and 5,865,981 (both in the name
of de Nora) disclose a cell for the production of
aluminium containing grids made of side-by-side upright
or inclined walls whose bottom ends stand on a ceramic-
coated carbon cell bottom covered by the pool of molten
aluminium. Each grid has generally vertical through-
openings dimensioned to allow the molten cell content to
occupy the inside of the through-openings.
US Patent 4,600,481, (Sane/Wheeler/Gagescu/Debely/
Adorian/Derivaz) and 4,650,552 (de Nora/Gauger/Fresnel/
Adorian/Duruz) describe aluminium-wettable composite
materials for use in contact~with molten aluminium in an
aluminium production cell. The composite materials are
made of alumina and aluminium in particular with TiB2.
Slabs of this material may be used to cover a carbon
cathode bottom of a conventional aluminium production
cell.
Objects of the Inyention
One object of the invention is to provide an
aluminium electrowinning cell incorporating metal-based
anodes that can be operated without excessive
contamination of the produced aluminium.
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
3
Another object of the invention is to provide an
aluminium electrowinning cell ,that can achieve high
productivity, low contamination of the product
aluminium, and whose compbnents resist corrosion and
wear.
Yet another object of the invention is to provide an
aluminium electrowinning cell including metal-based
anodes which remain substantially insoluble under the
cell operating conditions.
An overall object of the invention is to provide a
cell for the electrowinning of aluminium from alumina
dissolved in a fluoride-containing molten electrolyte
which overcomes the various drawbacks of the previous
proposals.
Summary of the Invention
The invention proposes a cell for the electrowinning
of aluminium from alumina dissolved in a fluoride
containing molten electrolyte. This invention can be
implemented in a conventional cell or can be applied to
cells of new design.
The cell of the invention comprises a horizontal
carbon cathode bottom having an aluminium-wettable
surface coating and a series of plates made of
aluminium-wettable reticulated porous material,
typically foams, filled with aluminium and placed flat
on the aluminium-wettable surface coating.
During use, a thin bottom layer of aluminium wets
the aluminium-wettable surface coating on top of the
cathode bottom, usually the entire or substantially the
entire surface coating, and a bottom part of the porous
aluminium-filled plates.
The application of an aluminium-wettable coating on
the carbon cathode bottom underneath the aluminium
wettable reticulated porous plates leads to a singular
improvement over prior art configurations.
Indeed, as opposed to the configuration disclosed in
US Patents 4,600,481 and 4,650,552 mentioned above in
which the aluminium-wetted ceramic foams rest directly
on the aluminium-repellent carbon bottom of the cell, in
the cell according to the present invention the
aluminium-wettable porous plates rest on an aluminium-
wettable coating which during use leads the aluminium to
form a layer between the aluminium-filled porous plates
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
4
and the aluminium-wettable coating. This aluminium layer
covers and wets substantially the entire surface of the
aluminium-wettable coating and wets also the bottom part
of the aluminium-filled porous plates, whereby a
continuous and. substantially improved electrical contact
is formed between the cathode bottom and the above
located cathodic aluminium.
During use, a top layer of aluminium which is formed
above the porous aluminium-filled plates and is covered
with the electrolyte, provides an active cathode surface
on which aluminium is cathodically reduced.
In a main embodiment of the invention, the cell
comprises a series of metal-based anodes located above
and parallel to the surface of the top layer of
aluminium. Especially for cell operation above about
910°-930°C, each anode can have a metal-based anode
substrate protected with an electrochemically active
coating made of one or more cerium compounds that is
maintained by the presence of cerium species in the
electrolyte, as disclosed in US Patents 4,614,569,
4,966,674, 4,683,037, 4,680,094, 5,069,771, 4,960,494
and 4,956,068 mentioned above, and that prevents
unacceptable contamination of the product aluminium by
anode materials.
Other suitable metal-based anode materials
optionally coated with the above cerium-based coating,
include iron and nickel based alloys which may be heat-
treated in an oxidising atmosphere as disclosed in
WO00/0&802, W000/06803 (both in the name of
Duruz/de Nora/Crottaz), WO00/06804 (Crottaz/Duruz),
W001/42535 (Duruz/de Nora); W001/42534 (de Nora/Duruz)
and W001/42536 (Duruz/Nguyen/de Nora). Further oxygen-
evolving anode materials are disclosed in W099/36593,
W099/36594, W000/06801, WO00/06805, WO00/40783 (all in
the name of de Nora/Duruz), W000/06800 (Duruz/de Nora),
W099/36591 and W099/36592 (both in the name of de Nora).
Alternatively, the anodes can be consumable carbon
anodes on which, during operation, CO~ is formed.
The anodes may be spaced above the surface of the
top layer of aluminium by a reduced anode-cathode
distance (ACD) in the range .of 20 to 40mm. Such a
reduced ACD permits cell operation with an increased
electrolysis current density of about 0.6 to 1.2 A/cm2 at
the surface of the anodes. The increased current density
produces sufficient heat to maintain cell stability
while producing more aluminium.
CA 02437687 2003-08-05
- 5 -
Usually, the bottom layer of aluminium has a
thickness in the range of 0.5 to 10 mm. The top layer of
aluminium may have a thickness in the range of 5 to 100
mm and can even form a pool.
Preferably, the porous aluminium-filled plates have
a thickness in the range of 10 to 100 mm.
The plates can be made of the materials disclosed in
the aforementioned US Patents 4,600,481 and 4,650,552.
Preferably, the plates are made of a reticulated ceramic
material that is inert and resistant to molten aluminium
having at its surface an aluminium-wetting agent, in
particular a metal oxide that is reactable with molten
aluminium as described below.
The inert and resistant ceramic material may
comprise at least one oxide selected from oxides of
aluminium, zirconium, tantalum, titanium, silicon,
niobium, magnesium and calcium and mixtures thereof, as
a simple oxide and/or in a mixed oxide, for example an
aluminate of zinc (ZnAl04) or titanium (TiA105). Other
suitable inert and resistant ceramic materials can be
selected amongst nitrides, carbides, borides and
oxycompounds, such as aluminium nitride, AlON, SiAlON,
boron nitride, silicon nitride, silicon carbide,
aluminium borides, alkali earth metal zirconates and
aluminiumates, and their mixtures.
The porous aluminium-filled plates preferably have a
surface layer containing alumina, aluminium and a
further metal, such as copper, iron and/or nickel. This
surface layer is producible by exposing to molten
aluminium the surface of aluminium-wettable plates which
contains before use metal oxides, such as copper, iron
and/or nickel oxides, that are reactable with molten
aluminium. Other useful metal oxides that are suitable
for reaction with molten aluminium are disclosed in
W001/42168 (de Nora/Duruz) and WO01/42531 (Nguyen/Duruz/
de Nora).
Likewise, the aluminium-wetted surface coating on
the carbon cathode preferably has a surface layer
containing alumina, aluminium and a further metal, such
as copper, iron and/or nickel. This surface layer is
producible by exposing to molten aluminium an aluminium-
wettable surface coating which contains before use metal
oxides, such as copper, iron and/or nickel oxides, that
are reactable with molten aluminium, as disclosed in the
references mentioned above (W001/42168 and W001/42531).
These references teach that in order to avoid contact
between aluminium infiltrated into the aluminium-wetted
coating and the substrate, a sub-layer of boride, for
instance as disclosed in US Patents 5,364,513 and
5,651,874, which is inert and impervious to molten
aluminium, or an aluminium-repellent layer can be used
to anchor the coating onto the substrate.
AMFNnFI~ SHFFT
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
6
In one embodiment, the coated metal structure of
each anode has a horizontal expanse and is foraminate
for guiding therethrough an electrolyte circulation from
and to the electrochemically active coating. Suitable
anode designs are disclosed. in WO00/40781 and WO00/40782
(both in the name of de IVora) .
The electrolyte may be at a temperature below 960°C,
typically 860° to 930°C. The electrolyte may comprises
cryolite and, in addition to. cryolite, an excess of AlF3
in an amount of 15 to 30 weight% of the cryolite.
Electrolyte on the electrochemically active coating
is preferably substantially saturated with alumina.
Substantial alumina saturation can be achieved by using
means for distributing alumina over a large area of the
electrolyte, such as a plurality of alumina point
feeders or a device for spraying alumina over the molten
electrolyte, as disclosed in WO00/06804 (de
Nora/Berclaz).
The cathode bottom may comprise a reservoir, for
example located centrally in the cell, for collecting
product aluminium. Also, The, porous aluminium-filled
plates may be arranged so that the top layer of
aluminium located thereon drains. into the reservoir.
The cell, in particular when it is retrofitted, may
comprise a sideledge of frozen .electrolyte and/or a
crust of frozen electrolyte. However, the cell may also
be operated with a crustless and ledgeless molten
electrolyte, i.e. in an entirely molten state.
The invention also relates. to a method of producing
aluminium in a cell as described above. The method
comprises feeding alumina to the electrolyte and passing
an electrolysis current between the electrochemically
active anode coatings and the top layer of aluminium to
evolve gas, in particular oxygen, on the anodes and
cathodically reduce aluminium.
A further aspect of the invention relates to a cell
structure of a cell for the electrowinning of aluminium
from alumina dissolved in a~fluoride-containing molten
electrolyte. The structure Comprises a horizontal carbon
cathode bottom having an aluminium-wettable surface
coating; and a series of plates made of aluminium-
wettable reticulated porous material placed flat on the
aluminium-wettable surface coating.
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
7
In a preferred embodiment, the cell structure
comprises a series of metal-based anode substrates
located above and parallel ,to the horizontal carbon
bottom. Each anode substrate is protected with an
electrochemically active coating made of one or more
cerium compounds. Other metal-based anodes can also be
used as mentioned above.
It is also possible to use consumable carbon anodes
instead of cerium-based ~or other metal-based anodes.
During operation of cells with carbon anodes, C02 is
formed at the anodes' surface instead of 02.
Brief Description of Drawincts
The invention will be further described with
reference to the accompanying. schematic drawings, in
which:
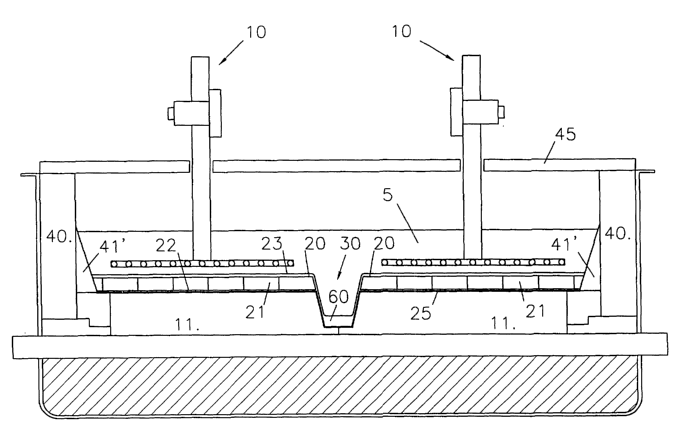
- Figure 1 is a cross-sec~tion~ through a drained cell
of the invention with metal-based anodes; and
- Figure 2 is a cross-section through another
drained cell of the invention with carbon anodes.
Detailed Description
The cell shown in Figure 1 has a horizontal carbon
cathode bottom 11 whose surface is protected with an
aluminium-wettable surface coating 25. The aluminium-
wettable surface coating 25 is covered with a series of
plates 21 made of aluminium-wettable reticulated porous
material filled with aluminium. These plates 21 form a
horizontal drained cathode surface 20 on which a top
layer of aluminium 23 is produced, during use. A bottom
layer of molten aluminium 22 wets substantially the
entire aluminium-wettable surface coating 25 and a
bottom part of plates 21.
The cathode bottom ll.comprises in the middle of
the cell, a channel 30 for collecting product aluminium
60 drained from the adjacent aluminium-wettable cathode
surfaces 20. The aluminium collection channel 30 is
preferably coated with a slurry-applied refractory
boride layer as described above.
The cell is fitted with metal-based anodes 10 on
which during use oxygen is evolved:
The anodes 10 are resistant to the electrolyte 5
and to oxygen and other gases evolved during use, for
example by being protected with a cerium oxyfluoride-
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
8
based coating as disclosed in US Patents 4,614,569,
4,966,674, 4,683,037, 4,680,094, 5,069,771, 4,960,494
and 4,956,068 mentioned above. Alternatively, anodes 10
can be made of other suitable metal-based anode
materials as mentioned above.
The cell comprises siclewalls 40, for example made
of silicon carbide, whlCh are covered with an aluminium-
wetted wedge-shaped sidewall lining 41' that extends
from the periphery of the cathode~bottom 11 to above the
surface of the molten electrolyte 5 to shield the
sidewalls 40 from molten~elect.rolyte 5. The sidewall
lining 41' can be made of the same material as plates 21
and can be completely filled with molten aluminium
retained in the material's pores by capillary effect.
To prevent the electrolyte 5 from freezing along
the sidewall lining 41' and on the surface of the
electrolyte 5, the cell is thermally well insulated. As
shown, the cell is fitted with an insulating cover 45
above the molten electrolyte 5. Details of suitable
covers are disclosed in WO01/31086 (de Nora/Duruz).
To reduce the dissolution of the anodes 10 in the
electrolyte, the cell in.ay be operated with an
electrolyte 5 at reduced temperature, typically from
about 730° to 960°C, preferably from 860° to
930°C.
Operation with an, electrolyte at reduced
temperature reduces the solubility of oxides, including
alumina. Therefore, it is advantageous to enhance
alumina dissolution in the electrolyte 5.
Enhanced alumina dissolution may be achieved by
utilising an alumina feed device which sprays and
distributes alumina particles over a large area of the
surface of the molten electrolyte 5. Suitable alumina
feed devices are disclosed in greater detail in
WO00/63464 (de Nora/Berclaz). Alternatively, alumina may
be supplied by several conventional point feeders
distributed of the molten electrolyte 5. Furthermore,
the cell may comprise means (not shown) to promote
circulation of the electrolyte 5 from and to the anode-
cathode gap to enhance alumina dissolution in the
electrolyte 5 and to maintain in permanence a high
concentration of dissolved alumina close to the active
surfaces of anodes 10, for example as disclosed in
W000/40781 (de Nora).
When the anodes 10 are protected with a cerium
oxyfluoride-based coating; an amount of cerium species
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
9
is preferably maintained in the electrolyte to maintain
the coatings.
During operation of the cells shown in Figure 1,
alumina dissolved in the electrolyte is electrolysed to
produce oxygen on the anodes l0 and aluminium at the
cathodes that is incorporated into the top aluminium
layer 23 on the drained cathode surfaces 20. Aluminium
60 from the top layer 23 drains into the collection
channel 30 from where it can be tapped.
Figure 2 where the same reference numerals are used
to designate the same elements, illustrates a
retrofitted cell utilising conventional consumable
carbon anodes 10' and operating with a frozen
electrolyte crust 70 and ledge 71 that Covers sidewalls
40, lining 42 and wedges 52.
In a variation, aluminium-wettable plates of larger
size than shown in Figures 2 and 2 may be used, each
larger plate extending over a significant part of a
cathode block 12, in particular over the entire length
across the cell of the a cathode block 11, preferably
extending also over part of the channel 30 as disclosed
in PCT/IB01/00953 (de Nora).
In a further variation, a retrofitted cell without
an aluminium collection groove may operate with a top
layer of aluminium that forms a cathodic aluminium
shallow pool. Consequently, the inter-electrode distance
may also be reduced which leads to a reduction of the
cell voltage and energy savings. Furthermore, compared
to conventional deep pool cells, a smaller amount of
molten aluminium is needed to operate the cell which
substantially reduces the costs involved with
immobilising large aluminium inventories in aluminium
production plants.
The production of aluminium-wettable reticulated
porous material suitable to b~e used as a cathode plate
for a cell of the invention will be further described in
the following examples.
Examp 1 e. 1
An openly porous alumina structure (10 pores per
inch which is equivalent to about 4 pores per
centimetre) was rendered aluminium-wettable by coating
it with two slurry-applied layers of different
composition.
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
The first slurry of the first layer was made of 60
weight% particulate needle-shaped surface-oxidised TiB2
(-325 mesh) having a TiO~ surface oxide film, 3.3
weighto aluminium-wetting agent in the form of
5 particulate Fe203 (-3~5 mesh) and 3.3 weight% TiO~ powder
(-325 mesh) in 33 weighs% colloidal A1203 (NYACOL~ A1-
20, a milky liquid with a colloidal particle size of
about 40 to 60 nanometer). When this slurry is heat
treated, the colloidal alumina reacts with a Ti02
10 surface oxide and the Ti02 powder to form a mixed oxide
matrix of A1203 and TiO~ throughout the coating, this
matrix containing and bonding the TiB~ particles and the
Fe~03 particles .
The second slurry was made of 33 weighto of partly
oxidised copper particles, 37 weight% of a first grade
of colloidal alumina (NYACOL~ Al-20) and 30 weight% of a
second grade of colloidal alumina (CONDEA~ 10/2 Sol, a
clear, opalescent liquid with a colloidal particle size
of about 10 to 30 nanometer).
An aluminium-wettable coating was applied onto the
porous alumina structure by dipping this structure into
the first slurry followed by drying for 4 hours at 40°C
and dipping it into the second slurry followed by drying
for 15 hours are 40°C. The coated alumina structure was
then heat treated for 3 hours in air at 700°C to
consolidate the coating. '
The resulting structure is aluminium-wettable and
is suitable to be wetted by aluminium before use or it
can be wetted in-situ when used as a cathode plate for a
cell of the invention.
The aluminium-wettable porous structure was wetted
with alumina by dipping it in molten aluminium at 850°C.
After 20 hours the wetted porous structure was extracted
from the molten aluminium and allowed to cool down to
room temperature.
Examination of the , aluminium-wetted porous
structure showed that it was completely filled with
aluminium retained in the pores by the wettability of
the structure and the capillary effect, and covered over
the outer surface with aluminium.
The electrical resistivity: of the aluminium-wetted
structure was of the order,of the resistivity of metal
aluminium (2.&5 ~,S~.cm), whereas before wetting the
structure had a resistivity of 35 to 45 kS2.cm.
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
11
Example 2
An aluminium-wettable ceramic structure for use as
a cathode plate in a cell according to the invention was
made of a mixture of material inert and resistant to
molten aluminium, i.e. , alumina_ and titania, and
aluminium-wettable material, ~i.e, copper oxide. The
ceramic structure was prepared by coating a polyurethane
foam with a slurry of ceramic particles followed by a
heat treatment.
The slurry of ceramic material consisted of a
suspension of 40 g particulate A1203 with an average
particle size of 10 to 20 micron, 2.5 g of particulate
Cu~ with a particle size of less than about 45 micron,
2.5 g of particulate Ti02 with a particle size of less
than about 45 micron in a colloidal alumina carrier
consisting of 93 g deionised water and 6.6 g colloidal
alumina particles with a colloidal particle size of
about 10 to 30 nanometer. ~ .
A polyurethane foam having 10 to 20 pores per inch
(equivalent to about 4 to 8 pores per centimetre) was
dipped into the slurry and dried in air at 40° to 50°C
for 20 to 30 minutes. The dipping was repeated three
times.
After dipping, the foam was. died in air at 50°C
for 4 to 5 hours. The foam contained about 0.3 to 0.5
g/cm3 of the dried slurry. The drying was followed by a
heat treatment at about 850° to 1000°C in air for 4 to 5
hours to eliminate the polyurethane foam and consolidate
the ceramic material formed from the slurry into a self-
sustaining foam. This heat treatment was followed by an
aluminisation treatment by immersion in molten aluminium
for 2 hours in molten aluminium at 850°C.
The aluminised foam was extracted from the molten
aluminium, allowed to cool to room temperature and cut
perpendicular to a surface.
Examination of the aluminised foam showed that the
polyurethane foam had disappeared. The Ti02 had reacted
with A1203 in the ceramic foam to form a titanium-
aluminium mixed oxide matrix. Cu0 present at the surface
of the ceramic foam had reacted with molten aluminium to
produce an aluminium-wetted surface layer of A1203 and
an alloy of copper and aluminium. The pores of the
ceramic foam were completely filled with molten
aluminium.
CA 02437687 2003-08-05
WO 02/070785 PCT/IB02/00670
12
In a variation, the heat treatment step and the
aluminisation step are carried out simultaneously as a
single step. In a further variation, the copper oxide of
the ceramic structure is replaced partly or completely
with iron oxide and/or nickel oxide.
Example ' 3.
An openly porous' silicon carbide structure (30
pores per inch which is equivalent to about 12 pores per
centimetre) for use as ,a. cathode plate in a cell
according to the invention was rendered aluminium-
wettable by coating it with a slurry-applied layer.
The slurry consisted of 75 g surface oxidised iron
particles (-325 mesh), 75 g Silica sol Nyacol 830 (a
milky aqueous liquid containing 32 weight% colloidal
silicon hydroxide that is converted into silica upon
heat treatment) and 0.35 g. of an aqueous solution
containing 15% PVA (polyvinyl alcohol) that was used to
adjust the viscosity of the slurry.
The openly porous structure was dipped onto the
slurry and then dried for 30 min. at 60°C. The
impregnated porous structure contained 0.278 g/cm3 of
dried slurry including 0.214 g/cm3.surface oxidised iron
particles. . .
The resulting structure was aluminium-wettable and
suitable to be wetted by aluminium before use or in-situ
when used as a cathode.
The aluminium-wettable.porous~.structure was wetted
with aluminium by dipping it. in molten aluminium at
850°C. After 15 hours~the wetted porous structure was
extracted from the molten aluminium and allowed to cool
down to room temperature.
Examination of the.aluminium-wetted porous
structure showed that it was filled with aluminium
retained in the pores by the wettability of the
structure and the capillary effect,~and covered over the
outer surface with aluminium. The pores had an aluminium
filling ratio that was greater than 90 vol%.
The aluminium-wetted.mate,rials of Examples 1 to 3
can also be used to produce a sidewall lining or another
cell component exposed to at least one of molten
aluminium, molten electrolyte and oxidising or corrosive
gas such as anodically produced oxygen.