Note: Descriptions are shown in the official language in which they were submitted.
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
1
ALUMINIUM-WETTABLE POROUS CERAMIC MATERIAL
Field of the Invention
The invention relates to a ceramic material which
can be utilised for the manufacture of aluminium-wettable
and aluminium-wetted ceramic components, in particular
for use in aluminium production, for example as cathodes,
sidewalls and other cell components which during use are
exposed to molten aluminium, electrolyte and/or corrosive
gases.
Background of the-Invention
Aluminium is produced conventionally by the Hall-
Heroult process, by the electrolysis of alumina dissolved
in cryolite-based molten electrolytes at temperatures up
to around 950°C. A Hall-Heroult reduction cell typically
has a steel shell provided with an insulating lining of
refractory material, which in turn has a lining of carbon
which contacts the molten constituents and corrosive
gases. Conductor bars connected to the negative pole of a
direct current source are embedded in the carbon cathode
forming the cell bottom floor. The cathode is usually an
anthracite based carbon lining made of prebaked cathode
blocks, joined with a ramming mixture of anthracite, coke
and coal tar, or with glue.
It has long been recognised that it would be
desirable to make (or coat or cover) the cathode of an
aluminium electrowinning cell with a refractory boride
such as titanium diboride that would render the cathode
surface wettable to molten aluminium which in turn would
lead to a series of advantages. Many difficulties were
encountered in producing refractory boride coatings which
meet up to the rigorous conditions in an aluminium
electrowinning cell. Nevertheless, such coatings applied
from slurries to carbon bodies have been developed. The
most recent slurry-applied coatings are disclosed in
WO01/42168 (de Nora/Duruz) and W001/42531 (Nguyen/Duruz/
de Nora).
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
2
US Patent 5,981,081 (Sue) discloses wear and
corrosion resistant coatings made of transition metal
boride particles dispersed in a matrix of nickel, cobalt
or iron. The coatings are applied by explosion or plasma
spraying a mixture of powders of a transition metal
boride and a boron containing alloy on a metal substrate
and heat treating.
Previously, it had been proposed to replace the
carbon material of the cathodes of aluminium production
cells with ceramic material. For example, US Patent
4,560,448 (Sane/Wheeler/Kuivila) discloses a porous
component made of aluminium repellent material covered
with an aluminium-wettable metal boride coating which
during use is maintained by saturating the molten
aluminium infiltrating the porous component with coating
constituents. US Patent 4,650,552 (de Nora/Gauger/
Fresnel/Adorian/Duruz) discloses an aluminium production
cell component produced from a powder mixture of alumina
and aluminium. US Patent 4,600,481 (Sane/Wheeler/Gagescu/
Debely/Adorian/Derivaz) discloses a component of an
aluminium production cell which is made of an openly
porous matrix, e.g. an alumina matrix, filled with molten
aluminium. The openly porous matrix may comprise an
aluminium-wettable coating made of a boride or nickel.
The infiltration of the matrix with aluminium is carried
out at a temperature of 1000° to 1500°C.
Materials made of a ceramic matrix infiltrated with
metal have also been described in the following
references. US Patents 4,935,055 (Aghajanian/Claar),
5,194,202 (Yun/Marra/Gurganus/Kelsey) and 5,6'76,907
(Ritland/Readey/Stephan/Rulis/Sibold) disclose different
methods of infiltrating a ceramic structures, e.g. A12O3,
SiN or SiC, with molten aluminium. US Patent 5,043,182
(Schultze/Schindler/Deisenroth) discloses a porous A1203-
A12Ti05 structure infiltrated under pressure with a
molten aluminium alloy.
US Patent 5,007,475 (Kennedy/Aghajanian) discloses a
ceramic structure, e.g. alumina, infiltrated by molten
aluminium with the aid of an infiltration enhancer
consisting of a metal/gas combination selected from Mg/N,
Sr/N, 2n/0 and Ca/N to which the alumina structure is
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
3
exposed before and during infiltration, It is also
contemplated in this patent to use ceramic structures
described in US Patent 4,713,360 (Newkirk/Di~io) that
discloses porous ceramic structures obtained by oxidising
aluminium metal with additives selected from Mg, Vin, Si,
Na, Li, Ca, B, P, Y, rare earth metals, and possibly non-
functional diluents or impurities, such as Mn, Fe, Cu and
W, in an amount of much less than 1% of the structure.
Objects of the Invention
An object of the invention is to provide an
aluminium-wettable component for a cell for the
production of aluminium from alumina dissolved in a
fluoride-based molten electrolyte.
Another object of the invention is to provide an
aluminium-wetted component which is highly conductive and
resistant to molten electrolyte for use as a cathode in a
drained cell or in a cell operating with a shallow or
deep aluminium pool or as a cell sidewall or another
component which is exposed to molten aluminium,
electrolyte and/or corrosive gases, or as a lining for
protecting other cell components against molten
electrolyte, or for making other cell components
aluminium-wettable.
A further obj ect of the invention is to provide an
aluminium-wettable or aluminium-wetted component which
can be made from readily available materials.
Yet another object of the invention is to provide an
aluminium-wettable component which can be wetted with
aluminium outside an aluminium production cell or in-situ
by exposure to cathodic molten aluminium.
Another object of the invention is to provide an
aluminium-wetted component that retains its protective
and wettability properties even when exposed to highly
oxidising and/or corrosive environments.
Yet a further obj ect of the invention is to provide
a ceramic-based or a ceramic-metal material which can be
used in an oxidising and/or corrosive media at elevated
temperature.
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
4
Summary of the Invention
A first aspect of the invention relates to an
aluminium-wettable component of a cell for the
electrowinning of aluminium from alumina dissolved in a
fluoride-based molten electrolyte. The component
comprises an openly porous or reticulated ceramic
structure whose surface during use is exposed to and
wetted by molten aluminium. The structure is made of a
ceramic material inert and resistant to molten aluminium
and an aluminium-wettable material that comprises metal
oxide and/or partly oxidised metal which is/are reactable
with molten aluminium to form a surface layer containing
alumina, aluminium and metal derived from the metal oxide
and/or partly oxidised metal.
The inert and resistant ceramic material may
comprise at least one oxide selected from oxides of
aluminium, zirconium, tantalum, titanium, silicon,
niobium, magnesium and calcium and mixtures thereof, as a
simple oxide and/or in a mixed oxide, for example an
aluminate of zinc (2nAlO4) or titanium (TiAl05). Other
suitable inert and resistant ceramic materials can be
selected amongst nitrides, carbides and borides and
oxycompounds, such as aluminium nitride, AlON, SiAlON,
boron nitride, silicon nitride, silicon carbide,
aluminium borides, alkali earth metal zirconates and
aluminates, and their mixtures.
Usually, the reaction of the metal oxide and/or
partly oxidised metal with molten aluminium involves the
reduction of the metal oxide and/or partly oxidised metal
and the oxidation of aluminium. For the metal oxide
and/or partly oxidised metal to be reducible by molten
aluminium, it is necessary that such a metal be more
electronegative than aluminium. For example, the metal of
the metal oxide and/or partly oxidised metal reducible by
molten aluminium is selected from manganese, iron,
cobalt, nickel, copper and zinc and combinations thereof.
The concentration of reactable metal oxide and/or
partly oxidised metal at the surface of the ceramic
structure affects the speed at which the structure is
wetted by molten aluminium. The surface of the ceramic
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
structure should contain the reactable metal oxide and/or
partly oxidised metal in an amount of at least 2 to 3
weight%, preferably at least 5 to 25 weighto of the
material making the surface of the Ceramic structure.
5 When the ceramic structure comprises a coating of the
aluminium wettable material as described hereafter, the
coating may comprise much more metal oxide and/or partly
oxidised metal, e.g. up to 50 or even 80 weight% or
possibly even more. The electronegativity of the metal of
the reactable metal oxide and/or partly oxidised metal
also affects the speed of aluminium wetting. The fastest
wetting of the ceramic structure is achieved when the
metal of the readable metal oxide and/or partly oxidised
metal is selected from copper, nickel, cobalt, manganese
and iron.
Zn one embodiment of the invention, the openly
porous or reticulated ceramic structure comprises a
coating of the aluminium-wettable material on the inert
and resistant ceramic material. In other words, the
openly porous or reticulated ceramic structure consists
of a skeleton of the inert and resistant ceramic material
coated with the aluminium-wettable material.
Such aluminium-wettable coating is usually a slurry-
applied coating comprising particles of the metal oxide
and/or partly oxidised metal reactable with molten
aluminium in a dried colloidal carrier selected from
alumina, ceria, lithia, magnesia, silica, thoria, yttria,
~irconia, titanium oxide and ainc oxide, and precursors
and mixtures thereof. Further details of such slurry-
applied coatings are disclosed in W001/42168 (de Nora/
Duruz), which describes such coatings on solid
substrates.
The slurry-applied aluminium-wettable coating may
further comprise particles of at least one compound
selected from metal borides, carbides and nitrides. For
example, the aluminium-wettable coating comprises the
particles of the metal oxide and/or partly oxidised metal
reactable with molten aluminium and particles of titanium
diboride in dried colloidal alumina.
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
6
Particles of the metal boride, carbide or nitride
may be covered with mixed oxides of metal derived from
the dried colloidal carrier and metal derived from the
metal boride, carbide or nitride. To improve the
structure of the coating, the slurry-applied aluminium-
wettable coating can be obtained from a slurry comprising
metal oxide particles that combine upon heat treatment
with metal derived from the dried colloidal Carrier to
form mixed oxides which are miscible with the mixed
oxides covering the particles of the metal boride,
carbide or nitride. Suitable slurries producing such a
coating are disclosed in WO01/42531 (Nguyen/Duru~/de
Nora), which describes such coatings on solid substrates.
Tn another embodiment of the invention, the openly
porous ceramic structure is made of a composition which
comprises a mixture of the inert and resistant ceramic
material and the aluminium-wettable ceramic material.
Such a ceramic structure should comprise a sufficient
amount of inert and resistant ceramic material that upon
contact/reaction of the aluminium-wettable ceramic
material with molten aluminium, the overall ceramic
structure retains sufficient mechanical properties.
Usually, the aluminium-wettable material makes up less
than 15 weighto, usually less than l0 weighto, of the
ceramic structure.
Furthermore, the openly porous ceramic structure may
be formed on a reinforcing metal skeleton, in particular
a metal mat. Suitable metals for such a skeleton include
iron and iron alloys and other metals which are
mechanically resistant at elevated temperature.
For some applications, it may be advantageous to use
internal inserts acting as ballast inside a component
made of the ceramic structure, for instance to secure the
ceramic structure on the bottom of an aluminium
production cell as disclosed in Figs. 2 and 3 of US
Patent 5,651,874 (de Nora/Sekhar). The internal inserts
may be made of iron or iron alloys or other heavy
materials. A reinforcing metal can also act as ballast.
The component of the invention has numerous
applications some of which are set out hereafter.
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
7
For instance, the component may be a cathode or a
cathode lining, for example plate- or wedge-shaped, on a
cathode body, in particular made of carbon material. The
component can also be an aluminium pool stabiliser in the
form of a plate having a density which is either lower
than that of molten aluminium so that it can float at the
surface of the aluminium pool, or higher than that of
molten aluminium so that it can rest at the bottom of the
aluminium pool. All of the aforementioned components,
which are exposed during use to the product aluminium,
can be placed as such in the cell and wetted during use.
Such components may be top coated with a highly
aluminium-wettable start-up layer, for example as
disclosed in WO01/42168 (de Nora/Duruz).
On the other hand, for certain applications the
components may need to be wetted with molten aluminium
before use. Therefore, the aluminium-wettable component
can constitute a skeleton which can be infiltrated with
molten aluminium to form for example a cell sidewall or a
sidewall lining, or a wedge-shaped connecting body for
joining the surface of a cell bottom to an adjacent
sidewall at the periphery of the cell bottom.
The invention also relates to an aluminium-wetted
component of a cell for the electrowinning of aluminium.
The aluminium-wetted component comprises an openly porous
or reticulated ceramic structure which has a surface
layer containing alumina, aluminium and another metal,
e.g. iron, copper or nickel. Such component is obtainable
by exposing to molten aluminium an openly porous or
reticulated aluminium-wettable component made of ceramic
material inert and resistant to molten aluminium, e.g.
alumina, and an aluminium-wettable material that
comprises metal oxide and/or partly oxidised metal, e.g.
iron, copper or nickel as oxides and/or partly oxidised
metals, which is/are reactable with molten aluminium as
described above.
The component comprises an openly porous or
reticulated ceramic structure whose surface during use is
exposed to and wetted by molten aluminium. The structure
is made of a ceramic material inert and resistant to
molten aluminium and an aluminium-wettable material that
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
8
comprises metal oxide and/or partly oxidised metal which
is/are reactable with molten aluminium to form a surface
layer containing alumina, aluminium and metal derived
from the metal oxide and/or partly oxidised metal.
Usually, aluminium-wetted components are completely
filled and covered with aluminium that shields their
openly porous or reticulated ceramic structure from
exposure to molten electrolyte and/or corrosive gases
during use.
The aluminium-wetted component may be a cathode or a
cathode lining or an aluminium pool stabiliser wetted by
aluminium before or during use. The component may be a
cell sidewall or a sidewall lining or a wedge-shaped body
for joining the surface of a cell bottom to an adjacent
sidewall, all wetted by aluminium before use.
Another aspect of the invention is a cell for the
electrowinning of aluminium from alumina dissolved in a
fluoride-based electrolyte, comprising one or more
aluminium-wettable and/or aluminium-wetted components
described above.
The cell may in particular comprise a cathode or a
cathode bady whose surface is lined with a cathode lining
as disclosed above. The cathode body and the cathode
lining may be joined through a bonding layer, in
particular a slurry-applied refractory boride layer as
disclosed in WOOl/42168 (de Nora/Duruz) and W001/42531
(Nguyen/Duruz/de Nora). For example, the lined cathode
surface is part of a horizontal or inclined cathode
bottom, in particular a horizontal cathode bottom lined
with a wedge-like cathode lining forming an aluminium-
wettable drained sloping cathode surface thereon.
Alternatively, the cathode body may be located above a
cell bottom that is arranged to collect molten aluminium
produced on and drained from the cathode lining.
Further aspects of the invention relate to uses of
the above described material in fields other than the
field of aluminium electrowinning.
One further aspect of the invention relates to a
composite ceramic-based material which comprises an
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
9
openly porous or reticulated ceramic structure whose
surface during use is exposed to and wetted by molten
aluminium. This structure is made of a ceramic material
inert and resistant to molten aluminium and an aluminium-
wettable material that comprises metal oxide and/or
partly oxidised metal selected from partly oxidised or
oxide of copper, n~.ckel, cobalt, manganese and iron and
mixtures thereof, which is/are reactable with molten
aluminium to form a surface layer containing alumina,
aluminium and metal derived from the metal oxide and/or
partly oxidised metal.
Such a material may be used, for instance, for the
manufacture of components or linings of apparatus for
treating molten aluminium, in particular for purifying
molten aluminium or separating alloying metals from an
aluminium alloy. Further details of such apparatus can be
found in WO00/&3630 (Holz/Duruz).
A yet further aspect of the invention relates to a
composite ceramic-metal material which comprises, as
before, an openly porous or reticulated ceramic structure
which has a surface layer containing alumina, aluminium
and another metal. The composite ceramic-metal material
is obtainable by exposing to molten aluminium a composite
material made of a ceramic material inert and resistant
to molten aluminium and an aluminium-wettable material
that comprises a metal oxide and/or a partly oxidised
metal selected from copper, nickel, cobalt, manganese and.
iron and mixtures thereof, which is/are reactable with
molten aluminium to form a surface layer containing
alumina, aluminium and metal derived from the metal oxide
and/or partly oxidised metal.
Such a material may be used for the manufacture of
aluminium-wetted components for applications in high
temperature oxidising or corrosive gases, in particular
oxygen and/or fluorine-containing gases, or liquids, such
as fluorine-containing liquids or molten metal, in
particular molten aluminium.
In particular, the aluminium-wetted components may
be used in apparatus for treating molten aluminium. The
components may also be used at temperatures below the
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
melting point of aluminium as electrodes, heating
elements, structural materials, metallurgical crucibles
for containing molten metals other than aluminium,
anodes, furnace fixtures, molds etc. Due to the capacity
5 of the ceramic structure to retain molten aluminium
within its pores and on its surface by capillary effect,
the aluminium-wetted components may be ta.sed in chemically
aggressive environments at temperatures above the melting
point of aluminium, for instance as linings in furnaces,
10 providing the components are not exposed to substantial
mechanical wear.
Brief Description of the Drawings
The invention will be further described with
reference to the accompanying schematic drawings, in
which Figures 1, 2 and 3 illustrate cells of different
configurations fitted with aluminium-wetted components of
the invention.
Detailed Description
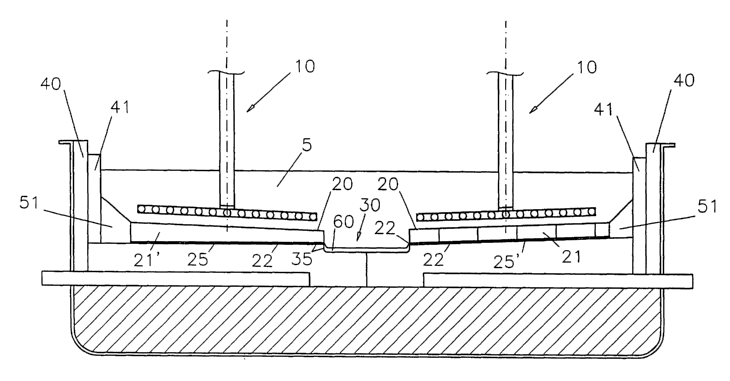
Figure 1 shows an aluminium production cell of
drained configuration. The cell comprises non-carbon
metal-based anodes 10, for example as disclosed in
WO00/40781 and WO00/40782 (both in the name of de Nara),
which are spaced apart from correspondingly sloped facing
cathode surfaces 20, for example as disclosed in
W000/63463 (de Nora), in a fluoride-based molten
electrolyte 5.
The cell bottom 25,25', for example made of carbon
material, is covered with aluminium-wetted cathode
linings 21,21' which form drained aluminium-wetted
sloping cathode surfaces 20 according to the invention,
different embodiments being shown in the right and the
left hand part of Figure 1. As shown, the cathode
surfaces 20 slope down towards the middle of the cell
bottom 25,25'. On the left-hand side of Figure 1, the
cell bottom 25 is horizontal whereas the cathode lining
21' covering it is a wedge with a small angle forming a
sloping cathode surface 20 above the horizontal cell
bottom 25. On the right-hand side of Figure 1, the cell
bottom 25' is at a slope and covered with cathode lining
plates (tiles) 21 of uniform thickness and which form a
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
11
sloping cathode surface 20 parallel to the sloping cell
bottom 25'.
The cell bottom 25,25' is only partly covered with
the cathode lining 21,22", leaving a central channel 30
formed by the cell bottom 25,25' and the adjacent cathode
linings 21,21' which are spaced in the middle of the cell
by channel 30. This channel 30 serves to collect product
molten aluminium 60 from the sloping cathode surfaces 20.
The cell bottom 25,25', in particular where it forms
part of the aluminium-collection channel is preferably
protected with an aluminium wettable layer 35, for
example a slurry-applied refractory boride layer as
disclosed in W001/42168 (de Nora/Duruz) or W001/42531
(Nguyen/Duruz/de Nora). Such a slurry-applied layer 35 is
also wetted by molten aluminium 22 that wets also the
bottom of the cathode linings 21,21' providing a
continuous and optimal electrical contact.
As shown in Figure 1, the cell comprises sidewalls
40, for example made of silicon carbide, which are
protected with an aluminium-wetted sidewall lining 41
according to the invention. The sidewall lining 41 is
completely filled with molten aluminium retained in. its
pores by capillary effect. The sidewall lining 41 extends
vertically from the cell bottom 25,25' to above the
surface of the molten electrolyte 5, and completely
shields the sidewalk 40 from molten electrolyte 5.
The aluminium-wetted sidewall lining 41 and cathode
linings 21,21' are joined through generally wedge-shaped
aluminium-filled bodies 51 according to the invention
located on the periphery of cell bottom 25,25'.
Thus, all the structural elements except anodes 10
are completely shielded from the molten electrolyte 5 by
molten aluminium retained in and on the aluminium-wetted
components according to the invention, or by the layer of
molten aluminium 60 collected in channel 30. Such a cell
configuration utilising these cell materials permits use
of the electrolyte 5 which is entirely in a molten state,
i.e. without frozen electrolyte ledges along the
sidewalls 40 and without a frozen electrolyte crust at
the surface of the electrolyte 5.
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
12
Figure 2, where the same reference numerals are used
to designate the same elements, illustrates inventive
cell components in another cell according to the
invention.
The cell shown in Figure 2 has a horizontal cell
bottom 25 which is covered with an aluminium-wetted
cathode lining 21 according to the invention of uniform
width forming a horizontal drained cathode surface 20.
The sidewalls 40 of the cell are covered with an
aluminium-wetted wedge-shaped sidewall lining 41' that
extends from the periphery of the cell bottom 25 to above
the surface of the molten electrolyte 5.
The cell bottom 25 comprises in the middle of the
cell, a channel 30 for collecting product aluminium 60
drained from the adjacent aluminium-wettable cathode
surfaces 20.
The aluminium collection channel 30 is preferably
coated with a slurry-applied refractory boride layer 35
as described above. The slurry-applied layer 35 is wetted
by molten aluminium 22 that wets also the bottom of the
aluminium-wetted cathode lining 21.
Similarly to the cell shown in Figure 1, all the
internal structural elements except anodes 10 are
completely shielded from the molten electrolyte 5 by
molten aluminium retained in and on the aluminium-wetted
components according to the invention or by the layer of
molten aluminium 60 collected in channel 30.
To prevent the electrolyte 5 from freezing along the
sidewall lining 41' and on the surface of the electrolyte
5, the cell is thermally well insulated. As shown in
Figure 2, the cell is fitted with an insulating cover 45
above the molten electrolyte 5. Further details of
suitable covers are disclosed in WO01/3I086 (de Nora/
Duruz ) .
The anodes 10 are preferably made of electrolyte
resistant inert metal-based material. Suitable metal-
based anode materials include iron and nickel based
alloys which may be heat-treated in an oxidising
atmosphere as disclosed in WO00/06802, WO00/06803 (both
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
13
in the name of Duruz/de Nora/Crottaz), WO00/06804
(Crottaz/Duruz), W001/42535 (Duruz/de Nora), WO01/42534
(de Nora/Duruz) and WO01/42536 (Duruz/Nguyen/de Nora).
Further oxygen-evolving anode materials are disclosed in
WO99/36593, W099/36594, WO00/06801, WO00/06805,
WO00/40783 (all in the name of de Nora/Duruz), WO00/06800
(Duruz/de Nora), W099/36591 and W099/36592 (both in the
name of de Nora).
To reduce the dissolution of the anodes 10 in the
electrolyte, the cell may be operated with an electrolyte
5 at reduced temperature, typically from about 830° to
930°C, preferably from 850° to 910°C. Operating with an
electrolyte at reduced temperature reduces the solubility
of oxides, in particular of alumina. Therefore, it is
advantageous to enhance alumina dissolution in the
electrolyte 5.
Enhanced alumina dissolution may be achieved by
utilising an alumina feed device which sprays and
distributes alumina particles over a large area of the
surface of the molten electrolyte 5. Suitable alumina
feed devices are disclosed in greater detail in
WO00/63464 (de Nora/Berclaz). Furthermore, the cell may
comprise means (not shown) to promote circulation of the
electrolyte 5 from and to the anode-cathode gap to
enhance alumina dissolution in the electrolyte 5 and to
maintain in permanence a high concentration of dissolved
alumina close to the active surfaces of anodes 10, for
example as disclosed in WO00/40781 (de Nora).
During operation of the cells shown in Figures 1 and
2, alumina dissolved in the electrolyte is electrolysed
to produce oxygen on the anodes 10 and aluminium 60 on
the drained cathode surfaces 20. The product aluminium 60
drains from the cathode surfaces 20 into the collection
channel 30 from where it can be tapped or evacuated into
an aluminium reservoir (not shown), for example as
disclosed in WO00/63463 (de Nora).
Figure 3 where the same reference numerals are used
to designate the same elements, illustrates a retrofitted
cell utilising aluminium-wetted components according to
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
14
the invention and conventional consumable carbon. anodes
10'.
The cell bottom 25 is horizontal and protected from
wear with an aluminium-wetted cathode lining 21 according
to the invention forming a drained cathode surface 20.
The cell sidewalk 40 are covered with a sidewall lining
41 according to the invention, extending from the cell
bottom to above the surface of the molten electrolyte 5.
The aluminium-wetted sidewall lining 41 and the
aluminium-wetted cathode linings 21 are joined through
generally wedge-shaped bodies 51 according to the
invention.
The cell bottom 25 is covered with a slurry-applied
refractory boride layer 35 wetted by molten aluminium 22
that wets also the bottom of aluminium-wetted cathode
lining 21.
The cell bottom 25 comprises in the middle of the
cell, a channel 30 for collecting product aluminium 60
drained from the adjacent aluminium-wettable cathode
surfaces 20.
Unlike the cell shown in Figures 1 and 2, the cell
shown in Figure 3 operates with a frozen electrolyte
crust 70 and ledge 71.
During operation of the cell shown in Figure 3,
alumina is dissolved into the electrolyte 5 and
electrolysed between the carbon anodes 10' and the
drained cathode surface 20 to produce C02 at the carbon
anodes 10' and aluminium which is drained into channel
30.
In a variation, a retrofitted cell without an
aluminium collection groove may operate with a shallow
aluminium cathodic pool with little motion of molten
aluminium in the shallow cathadic pool. Consequently, the
inter-electrode distance may also be reduced which leads
to a reduction of the cell voltage and energy savings.
Furthermore, compared to conventional deep pool cells, a
smaller amount of molten aluminium is needed to operate
the cell which substantially reduces the costs involved
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
with immobilising large aluminium stocks in aluminium
production plants.
Nevertheless, these aluminium-wetted cathode linings
can also be used in deep pool cells operating with a
5 frozen electrolyte ledge and/or an electrolyte crust
above the molten electrolyte. Furthermore, one or more
large aluminium-wetted conductive plates according to the
invention made from a low density openly porous or
reticulated ceramic structure may be put into the
10 aluminium pool so that the plates float at the surface of
the aluminium pool to restrain aluminium motion and
stabilise the aluminium pool. Thus, use of stabiliser
plates in a deep aluminium pool permits a reduction of
the inter-electrode distance.
15 In further variations of the above cells only one or
some of the above described cell components according to
the invention, i.e. cathode lining 21,21', sidewall
lining 41,41', wedge-shaped bodies 51 and stabiliser
plates, may be used in an aluminium production cell, in
different combinations.
The invention will be further described in the
following examples.
Example 1
An openly porous alumina structure (10 pores per
inch which is equivalent to about 4 pores per centimetre)
was rendered aluminium-wettable by coating it with two
slurry-applied layers of different composition.
The first slurry of the first layer was made of 60
weight% particulate needle-shaped surface-oxidised TiB~
(-325 mesh) having a Ti02 surface oxide film, 3.3 weight%
aluminium-wetting agent in the form of particulate Fe203
(-325 mesh) and 3 . 3 weight o Ti02 powder (-325 mesh) in 33
weighto colloidal A1203 (NYACOL~ Al-20, a milky liquid
with a colloidal particle size of about 40 to 60
nanometer). When this slurry is heat treated, the
colloidal alumina reacts with a Ti02 surface oxide and
the Ti02 powder to form a mixed oxide matrix of A1203 and
Ti02 throughout the coating, this matrix containing and
bonding the TiB2 particles and the Fe203 particles.
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
16
The second slurry was made of 33 weight% of partly
oxidised copper particles, 37 weighto of a first grade of
colloidal alumina (NYACOL~ A1-20) and 30 weight% of a
second grade of colloidal alumina (CONDEA° 10/2 Sol, a
clear, opalescent liquid with a colloidal particle site
of about 10 to 30 nanometer).
An aluminium-wettable coating was applied onto the
porous alumina structure by dipping this structure into
the first slurry followed by drying for 4 hours at 40°C
and dipping it into the second slurry followed by drying
for 15 hours are 40°C. The coated alumina structure was
then heat treated for 3 hours in air at 700°C to
consolidate the coating.
The resulting structure is aluminium-wettable and is
suitable to be wetted by aluminium before use or it can
be wetted in-situ when used as a cathode.
The aluminium-wettable porous structure was wetted
with aluminium by dipping it in molten aluminium at
850°C. After 20 hours the wetted porous structure was
extracted from the molten aluminium and allowed to cool
down to room temperature.
Examination of the aluminium-wetted porous structure
showed that it was completely filled with aluminium
retained in the pores by the wettability of the structure
and the capillary effect, and covered over the outer
surface with aluminium.
The electrical resistivity of the aluminium-wetted
structure was of the order of the resistivity of metal
aluminium (2.65 ~.~SZ.cm) , whereas before wetting the
structure had a resistivity of 35 to 45 kS2.cm.
Such a wetted alumina structure can be used for
various applications in an aluminium electrowinning cell,
in particular as a cathode or cathode lining, a cell
sidewall or a sidewall lining, or as a non current
carrying component of the cell bottom which is exposed to
molten aluminium and/or electrolyte.
CA 02438526 2003-08-14
WO 02/070783 PCT/IB02/00668
17
Example 2
An aluminium-wettable ceramic structure was made of
a mixture of material inert and resistant to molten
aluminium, i.e. alumina and titania, and aluminium-
wettable material, i.e. copper oxide. The oeramic
structure was prepared by coating a polyurethane foam
with a slurry of ceramic particles followed by a heat
treatment.
The slurry of ceramic material consisted of a
suspension of 40 g particulate A1203 with an average
particle size of 10 to 20 micron, 2.5 g of particulate
Cu0 with a particle size of less than about 45 micron,
2.5 g of particulate Ti02 with a particle size of less
than about 45 micron in a colloidal alumina carrier
consisting of 93 g deionised water and 6.6 g colloidal
alumina particles with a collaidal particle size of about
10 to 30 nanometer.
A polyurethane foam having 10 to 20 pores per inch
(equivalent to about 4 to 8 pores per centimetre) was
dipped into the slurry and dried in air at 40° to 50°C
for 20 to 30 minutes. The dipping was repeated three
times.
After dipping, the foam was dried in air at 50°C for
4 to 5 hours . The foam contained about 0 . 3 to 0 . 5 g/cm3
of the dried slurry. The drying was followed by a heat
treatment at about 850° to 1000°C in air for 4 to 5 hours
to eliminate the polyurethane foam and consolidate the
ceramic material formed from the slurry into a self-
sustaining foam. This heat treatment was followed by an
aluminisation treatment by immersion in molten aluminium
for 2 hours in molten aluminium at 850°C.
The aluminised foam was extracted from the molten
aluminium, allowed to cool to room temperature and cut
perpendicular to a surface.
Examination of the aluminised foam showed that the
polyurethane foam had disappeared. The Ti02 had reacted
with A1203 in the ceramio foam to form a titanium-
aluminium mixed oxide matrix. Cu0 present at the surface
of the ceramic foam had reacted with molten aluminium to
08-02-2003 . CA 02438526 2003-08-14
IB020066~
- 18 -
produce an aluminium-wetted surface layer of A1203 and an
alloy of copper and aluminium. The pores of the ceramic
foam were completely filled with molten aluminium.
In a variation, the heat treatment step and the
aluminisation step are carried out simultaneously as a
single step. In a further variation, the copper oxide of
the ceramic structure is replaced partly or completely
with iron oxide and/or nickel oxide.
Example 3
An aluminium-wettable openly porous ceramic
structure as in Example 1 was tested as cathodic material
for aluminium production.
The aluminium-wettable ceramic structure was placed
on tine bottom of a graphite receptacle having an inner
diameter of 85 mm. The structure was covered with 120 g
aluminium. The receptacle and its content was heated at a
rate of 120°C/hour. At a temperature of 700°C, the
aluminium had formed an aluminium pool on which the
ceramic structure was floating. The temperature was
further increased to about 850°C and then maintained for
4 hours so that the molten aluminium completely
aluminised and wet the ceramic structure.
After aluminisation, an amount of 1.5 kg
electrolytic molten bath consisting of 68 weight
cryolite, 28 weight$ aluminium fluoride and 4 weight
dissolved alumina was poured into the receptacle on top
of the aluminium pool and aluminium-wetted ceramic
structure. A carbon anode was dipped into the electrolyte
to face the floating ceramic structure which formed both
an aluminium pool stabiliser and a cathode surface. An
electrolysis current was passed between the anode and the
graphite receptacle at a current density of about 0.8
A/cm2 at the anode. A constant cell voltage of about 4 to
4,.2 volt was measured throughout electrolysis.
After 10 hours, electrolysis was interrupted and the
floating aluminium-wetted ceramic structure extracted
from the graphite receptacle.
AMENDED SHEET
0$-02-2003 CA 02438526 2003-08-14 ~B020066i
- 19 -
The ceramic structure was allowed to cool down to
room temperature and cut perpendicular to one of its
surfaces. Examination of the ceramic structure showed
that it was still completely wetted by and filled with
molten aluminium. The ceramic structure itself had
remained unchanged demonstrating its stability and
suitability as cathode material.
Example 4
An openly porous silicon carbide structure (30 pores
per inch which is equivalent to about 12 pores per
centimetre) was rendered aluminium-wettable by coating it
with a slurry-applied layer.
The slurry consisted of 75 g surface oxidised iron
particles (-325 mesh), 75 g Silica sol Nyacol 830 (a
milky aqueous liquid containing 32 weight$ colloidal
silicon hydroxide that is converted into silica upon heat
treatment) and 0.35 g of an aqueous solution containing
15$ PVA (polyvinyl alcohol) that was used to adjust the
viscosity of the slurry.
The openly porous structure was dipped onto the
slurry and then dried for 30 min. at 60°C. The
impregnated porous structure contained 0.278 g/cm3 of
dried slurry including 0.214 g/cm3 surface oxidised iron
particles.
The resulting structure was aluminium-wettable and
suitable to be wetted by aluminium before use or in-situ
when used for example as a cathode.
The aluminium-wettable porous structure was wetted
with aluminium by dipping it in molten aluminium at
850°C. After 15 hours the wetted porous structure was
extracted from the molten aluminium and allowed to cool
down to room temperature.
Examination of the aluminium-wetted porous structure
showed that it was filled with aluminium retained in the
pores by the wettability of the structure and the
capillary effect, and covered over the outer surface with
aluminium. The pares had an aluminium filling ratio that
was greater than 90 volt.
AMENDED SHEET
08-02-2003 . CA 02438526 2003-08-14
- 20 -
The aluminium-wetted porous structure can be used as
cathodic material like in Example 3.
AMENDED SHEET