Note: Descriptions are shown in the official language in which they were submitted.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
TITLE
An adhesive composition
FIELD OF THE INVENTION
The present invention relates to adhesive compositions, the process for their
preparation and medical devices including such.
BACKGROUND OF THE INVENTION
In medical devices, such as wound dressings and ostomy appliances, it is often
desired that the device is moisture absorbing and vapour permeable. These
devices often comprise a moisture absorbing adhesive coated on a backing
layer. Much effort has been made to optimise the moisture absorbing and
moisture transporting properties of the construction.
The use of hydrocolloids as absorbers in matrices such as adhesives are well-
known in medical devices. The hydrocolloids have superior properties when it
comes to absorption of aqueous liquids. Hydrocolloids are e.g. incorporated
into
absorbent articles such as sanitary napkins, in wound dressings and in ostomy
appliances. In US Pat. No. 3,339,546 a bonding composition of polyisobutylene
and hydrocolloids is disclosed. Similarly adhesive compositions based on block
copolymers are disclosed in US Pat. No. 4,367,732. The adhesives are intended
to be used on the skin or the mucosa.
When incorporated into adhesive, hydrocolloids in the form of particles are
preferred. Traditionally, hydrocolloids may be any type of water soluble or
swella-
ble material. Both natural polymers or derivatives thereof like carboxy methyl
cellulose (CMC), pectin and guar gum, and synthetic polymers like polyacrylic
acid and polyvinyl pyrilidone (PVP) may be used.
The physical form of hydrocolloids are relatively coarse and irregular
particles,
typically about 60-100 pm. The traditional hydrocolloids are hence fairly easy
to
produce by conventional techniques like milling and their coarse size support
safe handling in production, by minimising dust formation.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
2
Adhesives with hydrocolloid particles incorporated are well-known in the art
e.g.
from International Patent Application No. WO 91/09633 which discloses an
adhesive composite comprising a swollen hydrocolloid dispersed in a pressure
sensitive adhesive matrix. The swollen hydrocolloids are in the form of
irregular
particles of different shapes. As the hydrocolloid is swollen, the absorbent
capac-
ity of the adhesive composite will be limited.
Usually, the hydrocolloid particles are in a dry, non-swollen state when
incorpo-
rated in the adhesive matrix.
When hydrocolloid particles are incorporated into adhesives, the properties of
the resulting adhesives change. Typically the hardness of the adhesive will
increase with the addition of hydrocolloid. This is often undesired in medical
devices as it is preferred to have soft and skin-friendly adhesives being
capable
of following the movements of the skin. Furthermore, it is a disadvantage that
the
processing of the adhesive will be more difficult when working with
hydrocolloid-
containing adhesives. Due to the increased hardness of the adhesive the tacki-
ness of the adhesive matrix decrease and may prior to addition of
hydrocolloids
need to be increased by increasing the adhesiveness of the composition,
enhancing the risk of damaging the skin when the device is removed.
When a hydrocolloid containing adhesive is used in medical devices, such as
wound dressings or ostomy appliances, the hydrocolloids will also serve as
moisture transport from the skin or a wound through to the top the device. In
order to optimise the moisture transporting properties of the device a very
permeable backing layer is normally used. However, a limiting factor in the
moisture transport is often related to the interface between the film and the
hydrocolloid particles of the adhesive. To achieve an adhesive with moisture
transporting properties, an amount of at least 25% w/w hydrocolloids is
needed.
From European Patent Application No. 528 191 is disclosed an adhesive compo-
sition comprising hydrocolloids. The particle size of the hydrocolloid is
between
1-1000 microns, but preferably an average of 50 microns or less. This size is
obtained by pulverising the hydrocolloid, and the resulting powder was then
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
3
sieved to obtain an average particle size of 50 microns or less. Particles
obtained
by milling or pulverising will be irregular and sharp-edged, and furthermore,
handling of very fine particles may be difficult due to dust problems.
DE Patent Application No. 44 34 171 discloses an absorbent adhesive with
absorbent particles incorporated. The particle size is between 10 and 1000
microns, or at least smaller than the thickness of the adhesive layer.
The size of the hydrocolloid particles may be a limiting factor on the
thickness of
adhesive coatings, as the thickness of the coating cannot be smaller than two
to
three times the particle size. Very thin coatings may be desired in order to
achieve a more flexible product as well as the breathability of the product
may be
enhanced.
Thus, there is still a need for a skin-friendly adhesive composition being
capable
of absorbing and transporting moisture, having a good tack, being easy to
separate and process.
It has surprisingly been shown that by replacing at least a part of the
absorbent
particles of the adhesive composition with microcolloid particles, improved
properties of the adhesive are achieved.
BRIEF DESCRIPTION OF THE INVENTION
The invention relates to adhesive compositions comprising a polymeric matrix
and absorbent particles.
The invention further relates to a medical device comprising an adhesive compo-
sition comprising a polymeric matrix and absorbent particles.
The invention also relates to a process for preparing an adhesive composition
comprising a polymeric matrix and absorbent particles.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
4
BRIEF DESCRIPTION OF THE DRAWING
The invention is explained more in detail with reference to the drawing in
which
Figure 1 shows the moisture transmission over time of adhesive compositions.
DETAILED DESCRIPTION OF THE INVENTION
The invention relates to an adhesive composition comprising a polymeric matrix
and absorbent particles wherein at least a part of the absorbent particles are
microcolloid particles having a substantially rounded or spherical shape.
In the adhesive composition of the present invention at least a part of the
absorb-
ent particles is present in a finely divided state, for instance as micronised
parti-
cles of size less than 20 pm, hereafter named microcolloids. It has
surprisingly
been found that by embedding microcolloids in an adhesive the adhesive compo-
sition will achieve reduced hardness and increased moisture transport
properties
compared to hydrocolloid adhesives. It has additionally been found that the
compositions of the present invention are more sturdy to variations in
production
methods than traditional compositions and lead to reduced viscosity in for
instance hot melts.
The microcolloid particles may have a particle size of less than 20 microns.
The microcolloid particles may preferably have a particle size of less Than 10
microns.
The microcolloid particles may more preferred have a particle size of less
than 6
microns.
The microcolloid particles may most preferred have a particle size of less
than 2
microns.
In one embodiment of the invention the microcolloid particles have a particle
size
of less than 1,5 microns, more preferred less than 1 micron.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
The invention relates further to an adhesive composition consisting
essentially of
a polymeric matrix and absorbent particles wherein at least a part of the
absorb-
ent particles are microcolloid particles having a particle size of less than
20
microns.
Depending on the method of preparation, the particle size of the microcolloid
particles will normally follow a normal distribution curve, meaning the
majority of
the particles are having essentially the same size, but a minor part of the
parti-
cles will be smaller or larger.
In the adhesive composition according to the invention it is preferred that 5-
100
w/w, more preferred 10-100 % w/w, even more preferred 25-100 % wlw and
most preferred 50-100 % w/w of the absorbent particles are microcolloid
particles.
It is preferred that at least 75%, more preferred 90% and most preferred 95%
of
the microcolloid particles have a particle size below 20 pm, more preferably
below 10 pm and even more preferably below 6 pm, and most preferred below 2
pm.
A predominant share of the absorbent particles of the composition of the inven-
Lion may be microcolloid particles. In one embodiment of the invention 100 %
of
the absorbent particles are microcolloid particles.
Absorbent particles for incorporation into adhesives are often hydrocolloid
parti-
cles.
The physical form of hydrocolloids used today are relative coarse and
irregular
particles, typically about 60-100 pm and in the form of a dry powder. These
are
fairly easy to produce by conventional techniques like milling and the
relative
coarse particle size makes it possible to handle them at production scale
without
serious dust problems.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
6
When handling hydrocolloid particles with a substantial amount of fine
particles,
a powderous formulae will often give rise to dust problems, as well as it will
be
difficult and explosive to produce particles of such a size by the traditional
methods, due to dust and handling problems. Furthermore, particles of a size
of
less than 20 microns may be inhaled into the lungs and may cause health
hazards. Hence, such powders are not commercially available. The microcolloid
particles of the composition according to the invention may be prepared e.g.
by
milling, but will preferably be prepared by an emulsion process, and will be
deliv-
ered in a carrier liquid in the form of a paste or a liquid suspension, or the
micronised particles may be agglomerated into granules or potentially in a
free
flowing powderous form.
The microcolloid particles may preferably have a substantially rounded or
spheri-
cal shape. A special advantage of such particles is that they often may be
closer
packed providing high bulk density, as well as the rounded shape may influence
the viscosity and processability of the adhesive.
The microcolloid particles may have a homogeneous, essentially monodisperse
size distribution.
The invention further relates to an adhesive composition comprising a
polymeric
matrix and absorbent particles wherein at least a part of the absorbent
particles
are in the form of microcolloid particles wherein the microcolloid particles
are in a
stabilised form.
Preferably, the microcolloids are stabilised by low-molecular weight
surfactants.
The polymeric matrix may be a continuous phase and the polymeric matrix may
preferably be hydrophobic.
In one embodiment of the invention the polymeric matrix may be hydrophilic.
The polymeric matrix may preferably be a skin-friendly adhesive, optionally an
adhesive for medical use.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
7
The invention also relates to a medical device comprising a composition
compris-
ing a polymeric matrix and absorbent particles wherein at least a part of the
absorbent particles are microcolloids having a substantially rounded or
spherical
shape.
The smaller size and/or the rounded shape of the microcolloid particles in the
composition of the invention may influence the rheological properties of the
invention. The viscosity and processing temperature will be lower than when
using traditionally sized hydrocolloides, rendering it possible to prepare
thinner
coatings, e.g. adhesives with a thickness of less than 50 pm, and bevelling of
the
edge of a thick adhesive as well as the adhesive as such may be easier to
mould. The tack of the adhesive may also enhance, due to the softer properties
of the adhesive.
Due to their smaller size and preferably rounded shapes the microcolloids and
adhesives based on those are able to pass through narrow nozzles, dies and
valves. This separates the microcolloids and microcolloid based adhesives from
hydrocolloids and hydrocolloid based adhesives, that will block the nozzles,
dies
and valves or at least increase the viscosity of the adhesives to levels
beyond
normal processing temperatures. The increase in viscosity combined with possi-
ble blocking of narrow nozzles, dies and valves renders the use of standard
hydrocolloid based adhesives difficult or impossible in equipment for
application
of the adhesives in very thin layers by coating with slot dies, spray, swirl,
controlled coat or melt-blowing dies and equipment as supplied by companies as
Nordson or Rubatech.
Microcolloid based adhesives can be applied to different substrates in very
thin
open networks, the networks comprises of very thin adhesive strings
overlapping
each other forming a random open networks of adhesive on the substrate.
With this technology it is possible to coat substrates with very open network
structures of adhesive and obtain very low, but still sufficiently adhesive,
coating
weights.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
The open and thin networks of adhesives can be produced by a variety of
technologies such as spraying the adhesives as particles or pieces of adhesive
onto the surface of the substrate thereby creating an open layer of adhesive
on
the substrate surface. The adhesive may also be applied in thin strings, which
are pressed through small holes in dies. The adhesive string can be applied as
both parallel lines of adhesive, vibrated strings forming an open random
coating
such as the ones created by the Controlled coat dies from Nordson, and as
overlapping circles creating an open network of adhesive on the substrate. The
later can be produced by the swirl dies from Nordson or Rubatech.
The microcolloid based adhesives can also be applied as a random network of
very thin pieces of adhesive fibres such a pattern can be created by turning
up
the airflow on a standard die for swirling adhesive strings the power of the
increased airflow tears the adhesive string and dispense it as short thin
overlap-
ping adhesive fibres on the substrate thus creating a random open network-
structure of adhesive.
Hydrocolloid based adhesives cannot be applied by the methods mentioned
above, or at least not as thin coatings as the microcolloid based adhesives.
The
nozzles, dies and valves used in these processes have very small holes which
will be blocked by the large hydrocolloid particles or the significant rise in
viscos-
ity created by incorporating the hydrocolloids.
Dies and nozzles with larger holes can of course be constructed and used but
this will result in much thicker adhesive strings which again may render it
difficult
to obtain the very small coating weights. Furthermore, the thick strings may
feel
both uncomfortable upon direct skin contact and be displeasing to the eye.
Very thin coatings of microcolloid based adhesives may also be obtained with
coating by the use of slot dies. Normally the thickness of the adhesive
containing
standard size hydrocolloids is limited by the size of these particles. The
adhesive
layer can only be as thin as the largest of the hydrocolloid particles and not
even
that if a smooth surface of the coating is to be achieved.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
9
With microcolloid based adhesives coatings much thinner than the particle size
of hydrocolloids may be achieved. Furthermore, it has been shown that when
very thin layers of microcolloid based adhesives are coated with slot dies on
porous substrates such as a foam, e.g. polyurethane foam, a porous adhesive
film is achieved due to the thin adhesive film rupturing over the pores.
This opens up the possibility of creating porous adhesive coatings on porous
substrates with slot dies. Porous coatings from slot dies opens up a range of
new
possibilities because slot dies offers very good control over the thickness of
the
adhesive layer and the amount of adhesive that is deposited on the substrate.
The porous character of the adhesive coating on porous substrates allows
fluids
such as wound exudates to pass through the layer quickly. The adhesive layer
may thus serve as a wound contact layer.
The microcolloid based adhesives renders it possible to produce a highly
absorb-
ent and permeable adhesive that can be coated in open networks for even higher
permeability to be achieved or for reducing the amount of adhesive used.
The improved rheological properties of the composition according to the inven-
tion may render it easily possible to process the adhesive by methods such as
injection moulding or vacuum moulding.
In one embodiment of the invention the composition may be in the form of a
foam.
The combination of improved rheological and cohesive properties of
microcolloid
adhesives creates the opportunity of producing stable, yet moisture absorbent
and transmitting, porous or foamed adhesives. Foamed adhesives are especially
interesting as they may provide improved cost, improved moisture handling
properfiies, improved adhesion to flexible andlor uneven substrates and poten-
tially improved skin compatibility.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
The foamed adhesive may be characterised by porosity, stability, open/closed
or
mixed cell structure and cell size distribution. The higher porosity, the
softer and
more flexible an adhesive is produced with minimal base adhesive consumption.
It is preferred that the porosity is between 10 and 80%, and more preferred
between 40 and 70 %. Open cells will yield a higher moisture absorption and
transmission rate. Closed cells will provide the best physical stability.
Foamed
adhesive with small sized cells may be preferred.
The foamed adhesive may be produced by mechanical introduction and disper-
sion of a suitable expanding moiety, e.g. compressed air, nitrogen, carbon
dioxide, argon or other gasses or low boiling point liquids. Suitable
equipment
may include the "FoamMelt ~" and "FoamMix ~" machines available from the
Nordson Corporation.
The foamed adhesive may alternatively be produced by compounding the
adhesive with a suitable chemical blowing agent, which may generate gas
bubbles by a variety of mechanisms. These mechanisms include, but are not
limited to chemical reaction, thermal decomposition or chemical degradation,
volatilisation of low boiling materials, expansion of gas filled materials or
by a
combination of these methods.
The term chemical blowing agent is used herein to cover the use of single or
multiple component chemicals in a mixture or paste. Suitable chemical blowing
agents include the carbonates of alkali metals, such as ammonium carbonate,
ammonium bicarbonate, sodium carbonate, sodium bicarbonate, and calcium
carbonate. Improved gas generation may be obtained by preparing a mixture of
carbonates of alkali metals and various organic acids. Other suitable blowing
agents includes expanding spheres such as "Expancel ~" available from Akzo
Nobel.
The foamed adhesive may be coated or otherwise shaped by a wide number of
possible processes including reverse roll coating, slot die coating and
different
moulding techniques such as injection and vacuum moulding. The foamed
adhesive may by further shaped by e.g. cutting or bevelling and if may be
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
11
laminated onto other materials. The foamed adhesive may be coated into thin
layers, as well as it may be moulded into three-dimensional structures.
A medical device according to the invention is typically in the form of a
laminate
comprising a backing layer, a layer of adhesive and which optionally is
covered in
part or fully by one or more release liners or cover films to be removed
before
use. The device may further comprise a secondary backing layer to be removed
before use.
In one embodiment of the invention the polymeric matrix is a soft and
mouldable
paste. Such paste may be useful e.g. for sealings around a stoma or filling
agent
for wounds.
The adhesive composition may be placed on a backing layer.
The backing layer of the device according to the invention may be any layer,
such as a polyurethane film, foam or non-wowen or combination of films or
layers
which, in combination with the adhesive, shows the desired characteristics of
the
device according to the invention. The film may e.g. be produced from a
polyole-
finic material, PVAI, polyester, polyamid, silicones, Teflon, polyurethane
material or polyethylene or copolymers or blends thereof.
The film may be biodegradable or solubilised under certain conditions.
A preferred material for the backing layer may be polyurethane in the form of
a
film or a foam or combinations of such e.g. in the form of laminates.
The skin-contacting surface of the device may be covered by one or more
release liners.
Release liners which are especially suitable for use with the device of the
inven-
tion can be made of kraft papers, polyethylene, polypropylene, polyester or
composites of any of these materials. The liners are preferably coated with
release agents such as fluorochemicals or silicones. The release liner may, if
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
12
present, be removed before, during, or after application. If only removed
after
application, the release liner may act as a handle during application.
The adhesive layer may be in the form of a continuous layer or a pattern or
the
adhesive may only be situated on a part of the skin-facing side of the device,
e.g.
on a flange around the central part of the device to form an island dressing
with a
non-adhesive or low-adhesive absorbent pad at the central part of the
dressing.
The adhesive composition may be in the form of an absorbent pad.
The medical device according to the invention may e.g. be a wound care device,
continence device, breastcare device, an ostomy appliance or any adhesive
device for application to the skin.
The medical device of the invention may be a dermatological dressing.
The medical device may be a sealing paste for ostomy.
The medical device may be an ostomy appliance.
The medical device may be in the form of a spray, e.g. for use as a primer
coating on contoured areas or for breathable and conformable spray adhesives.
The invention further relates to a process for preparing an adhesive
composition
comprising a polymeric matrix and microcolloid particles wherein the
microcolloid
particles are dispersed in one of the components of the polymeric matrix
before
combining further with the rest of the components of the polymeric matrix.
The microcolloid particles may be incorporated into the polymeric matrix in
the
form of a paste or a liquid dispersion. The dispersing liquid may optionally
be
removed subsequently.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
13
The microcolloid particles may be incorporated in the form of agglomerates or
granules which are broken down before or during the adhesive manufacturing
process.
The microcolloid may be in a stabilised form, preferably as a dispersion.
In one embodiment of the invention the composition may be foamed by mechani-
cally introducing an expanding moiety into the composition.
In another embodiment of the invention the composition may be foamed by intro-
ducing a chemical blowing agent.
The adhesive composition may be produced by hot-melt mixing in continuous or
conventional batch processing. In a batch mixing process a premix of the
polymeric matrix may be prepared and subsequently the microcolloid particles,
suspended in one or more of the matrix components, are dispersed in the
polymeric matrix. The microcolloid particles may alternatively be applied in
the
form of a dispersion in a volatile solvent which solvent may be removed during
or
after mixing with the other components of the composition or evaporated in-
situ
when applying the adhesive. Traditional hydrocolloids may be added subse-
quently in the form of a powder.
Continuous mixing in single or double screw extruders may be used
alternatively.
The present invention provides a method of formulating, producing and using
adhesives where the addition of moisture regulating microcolloids provides an
improved moisture transmission rate compared to state of the art technology
and/or where the rheological properties of the adhesive matrix are less
affected
than by addition of traditional hydrocolloids. Furthermore the invention
provides
means of producing breathable hydrocolloid adhesives with lower coat weight
than present technology.
The adhesive matrix may comprise any suitable adhesive known per se, such as
a pressure sensitive adhesive, which is defined by the Pressure Sensitive Tape
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
14
Council (Glossary of Terms Used in Pressure Sensitive Tape Industry, PSTC,
Glenview, 111. 1959) to be adhesives "which in dry form are aggressively and
permanently tacky at room temperature and firmly adhere to a variety of
dissimi-
lar surfaces upon mere contact without the need of more than finger or hand
pressure." These adhesives "have a sufficiently cohesive holding and elastic
nature so that, despite their aggressive tackiness, they can be handled with
the
fingers and removed from smooth surfaces without leaving a residue." Because
pressure sensitive adhesives vary in their strength and adhesiveness, their
selec-
tion for use in the composition will depend on the final application desired.
THE MICROCOLLOID
Microcolloids are a new group of water absorbent particles for adhesives.
Micro-
colloids are distinguished from hydrocolloids by having a very fine particle
size,
preferably below 20pm, more preferably below 10pm and even more preferably
below 6pm, and most preferred below 2 pm. They often have a predominately
spherical geometry.
The microcolloids of the present invention are less suitable to handle in the
form
of a powder due the dust problems caused by the very fine particle size and
hence they are often used in formulated form. This may be as a liquid
dispersion,
a paste or a meltable block of particles, where the microcolloids are
dispersed in
one or more of the components of the final adhesive matrix, e.g. plasticisers,
low
molecular weight polymers, or a tackifying resin. Alternatively the
microcolloids
may be in the form of a solid or semi solid granulate which is broken down
during
processing of the adhesive. The microcolloid granulate may further comprise
one
or more of the adhesive components such as the elastomer, high melting tackify-
ing resin or traditional granulate binders. Or the microcolloids are in the
form of a
dispersion in a volatile solvent which solvent may be removed during
production
of the final adhesive composition or device or evaporated in situ when the
adhesive is used.
The microcolloids of the present invention may comprise of any type of water
soluble or swellable material which can be formulated into ultra-fine
particles.
Varieties of microcolloids within the scope of the present invention include
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
synthetic polymers prepared from single or multiple monomers, naturally occur-
ring hydrophilic polymers or chemically modified naturally occurring
hydrophilic
polymers. The hydrocolloid polymers may be linear or crosslinked. This include
natural or chemically modified natural polymers like cellulosics such as CMC,
chitosan, pectin, guar gum, starches or dextrines, collagenes and gelatine and
synthetic polymers like polyacrylic acid, polyvinylealcohol/acetate,
polyhydroxyal-
kyl acrylates and methacrylates, polyacrylamides, polystyrene sulfonates,
polyvi-
nyl pyrilidone, polyglycols, copolymers, grafts of such, copolymers or
compositions of such.
The ultra fine particle size may be obtained by physical means; wet milling,
sonication, high shear, but preferably the microcolloid particles are produced
by
some means of an emulsion process to provide very fine spherical particles.
One
way of producing microcolloids is to emulsify an aqueous solution of the
polymer
in a hydrophobic liquid and subsequently distilling of the water. Another way
is to
use the product from an emulsion polymerisation process.
In general it is desirable that the microcolloids is substantially free of
volatile
components which will evaporate during processing of the adhesive.
It is desirable that the microcolloids is prepared on a stabilised form, which
will
allow raw material storage, mixing and processing without significant
instability.
In a preferred embodiment of the invention the stabilised microcolloids are in
the
form of a dispersion and stabilised by low-molecular weight surfactants.
The stabilised microcolloid dispersion may further comprise polymeric steric
stabilisers in order to secure the desired theological properties.
It is well know to stabilise and remove volatile components and dehydrate
polymer suspensions by distillation, for example as describe in e.g. US
4,052,353. Further stabilisation may be obtained by use of suitable polymeric
stabilisers for example as described in US 4,339,371.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
16
THE HYDROCOLLOID
The physical form of hydrocolloids are relatively coarse and irregular,
typically
particles with a diameter of about 60-100 pm and usually supplied in the form
of
a dry powder. These hydroco!loids are hence fairly easy to produce by conven-
tional techniques like milling and their coarse size support safe handling in
production, by minimising dust formation.
In one embodiment of the invention the adhesive composition comprises both
hydrocolloid particles and microcolloid particles. This may be desirable for
obtain-
ing some of the good characteristics of the microcolioid adhesive, obtaining
synergistic effects, or simply for optimising costs.
Suitable hydrocolloids for incorporation in the adhesive compositions of the
invention are selected from naturally occurring hydrocolloids, semisynthetic
hydrocolloids and synthetic hydrocolloids. Varieties of hydrocolloids within
the
scope of the present invention include synthetic polymers prepared from single
or multiple monomers, naturally occurring hydrophilic polymers or chemically
modified naturally occurring hydrophilic polymers. The hydrocolloid polymers
may be linear or cross-linked.
More particularly, the hydrocolloids are preferably selected from guar gum,
locust
bean gum (LBG), pectin, alginates, gelatine, xanthan and/or gum karaya; cellu-
lose derivatives (e.g. salts of carboxymethylcellulose such as sodium carboxy-
methylcellulose, methylcellulose and hydroxypropylmethylcellulose) and/or
sodium starch glycolate and/or polyvinylalcohol and/or polyethylene glycol,
polyhydroxyalkyl acrylates and methacrylates, polyacrylamides, polyacrylic
acid,
polystyrene sulfonates, natural or synthetically modified polysaccarides, such
may be alginates, pectins, xantan gums, guar gum, chitosan, carboxy methyl
cellulose and hydroxy ethyl or hydroxypropyl cellulose.
THE PRESSURE SENSITIVE ADHESIVE OR PASTE
In medicinal application, the pressure sensitive adhesive is suitably tacky at
room
temperature as well as at skin temperature of the user. Also, the adhesive
must
be dermatologically acceptable, i.e., after continuous contact with skin,
there is
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
17
little or no adhesive residue upon removal and there is no significant
reaction
with skin during or after adhesion.
The strength of the pressure sensitive adhesive phase of the composition
depend on the type of pressure sensitive adhesive chosen. The adhesives must
provide sufficient adhesive strength to adhere the microcolloid containing
compo-
sition of the invention to the skin of the user.
The pressure sensitive adhesive may comprise polymeric adhesive compositions
prepared from a combination of monomers, homopolymers, copolymers, A-B-A
block copolymers, A-B block copolymers, tackifiers and plasticisers, or blends
thereof to produce polymeric adhesive compositions containing polyolefins,
polyacrylates, silicone adhesives, natural or synthetically derived rubber
based
adhesives, or polyvinyl ethers.
Preferably, the pressure sensitive adhesives useful in the composition may be
hydrophobic allowing the adhesives of the pressure sensitive adhesive to
resist
absorbing moisture or other body exudates gathering at the skin or skin
opening
during use. The composition retains its strong adhesiveness even in the
presence of water or exudates since the pressure sensitive adhesive is
unaffected and not plasticised by these agents. Excess moisture is taken away
from the skin surface by the microcolloids having a high moisture vapour trans-
mission rate. This decreases the risk of adhesion loss due to pooling of
moisture
on the skin-facing side of the adhesive.
Preferred adhesives are A-B f1 block copolymer pressure-sensitive adhesives,
such as polystyrene-polybutadiene-polystyrene (S-B-S), polystyrene-
polyisoprene-polystyrene (S-I-S), polystyrene-poly(ethylene/butylene)-polysty
rene (S-EB-S), and polystyrene-poly(ethylene/propylene)-polystyrene (S-EP-S)
polymers. Other useful adhesives are described in the literature. In the
Handbook of Pressure Sensitive Adhesive Technology 2nd Ed., Satas, Editor,
(Von Nostrand Reinhold, New York 1989), a number of types of useful pressure
sensitive adhesives are discussed: A-B-A block copolymer adhesives,
amorphous poly alpha olefins, natural rubbers, polyisoprene, butyl rubber,
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
18
polyisobutylene, silicones, polychloroprene, acrylic adhesives and acrylic
disper-
sions and polyvinylethers. Any of these pressure sensitive adhesives, or
blends
thereof, may be used to form the polymeric matrix into which the microcolloids
may be dispersed.
The polymeric matrix may further comprise tackifiers, A-B block copolymers,
low
molar weight rubber, plasticisers etc. or blends thereof in order to adjust
the
elastic and coherent properties of the polymeric matrix.
The polymeric matrix of the sealing or absorption paste may be polymeric
compositions prepared from a a combination of polyolefines in molecular
weights
enabling the mouldable nature into which the hydrocolloids and microcolloids
according to the invention are embedded in levels of typically 10 to 60
percent by
weight. An alternative composition may be based on the block copolymers of the
A-B-A nature described in association with the adhesives.
The polymeric matrix of the absorbent may be any continuos phase of soft and
expandable type. Preferred are rubber or elastomeric types and most preferred
are types derived from the block coplymers.
Alternatively may compositions in which moisture may lead to softening of the
structure be applicable. Examples may be acrylics, polyvinyl pyrrolidone,
polyvi-
nyl alcohol or acetate, polyethylen glycol, copolymers or blends comprising
such.
OPTIONAL COMPONENTS
In some aspects of the invention it may be desirable to prepare the pressure
sensitive adhesive composition of the invention with other solids, e.g. non
absorbing particles or pigments. E.g. a combination of microcolloids and more
coarse and/or irregular particles may be useful to formulate a high module
pressure sensitive adhesive or pastes with moisture absorption and
transmission
rates above the level obtainable by traditional methods.
The addition of a variety of biologically active materials into the
microcolloid
composition of the present invention may be desirable. The microcolloid
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
19
compositions may be used for a tailor-fit controlled release of both
hydrophobic
materials, typically released through the hydrophobic polymer matrix or hydro-
philic components typically released through the microcolloid phase.
Hence, the composition of the invention may comprise one or more pharmaceuti-
cally or biologically active ingredients.
The adhesive composition according to the invention may comprise wound
heating associated indicators) such as indicators of pH, partial pressure of
02,
temperature, radical mechanisms or biotechnological assays, e.g. indicating
formation of collagen.
It is also advantageous that a medical device according to the invention
comprises wound healing associated indicator(s), cushions or similar device
for
treatment or prophylaxis of formation of wounds and/or skin abnormalities.
This opens for a combined medical treatment of a wound or skin and an easy
and sterile application of the active ingredients, e.g. by incorporating
active ingre-
dients such as a cytokine such as growth hormone or a polypeptide growth
factor
giving rise to the incorporation of such active substances in a form being apt
to
local application in a wound in which the medicament may exercise its effect
on
the wound, other medicaments such as bacteriostatic or bactericidal compounds,
e.g. iodine, iodopovidone complexes, chloramine, chlorohexidine, silver salts
such as sulphadiazine, silver nitrate, silver acetate, silver lactate, silver
sulphate,
silver-sodium-thiosulphate or silver chloride, silver-complexes, zinc or salts
thereof, metronidazol, sulpha drugs, and penicillins, tissue-healing enhancing
agents, e.g. RGD tripeptides and the like, proteins, amino acids such as
taurine,
vitamins such as ascorbic acid, D-vitamine derivatives, enzymes for cleansing
of
wounds, e.g. pepsin, trypsin and the like, proteinase inhibitors or
metalloprote-
inase inhibitors such as Illostat or ethylene diamine tetraacetic acid,
cytotoxic
agents and proliferation inhibitors for use in for example surgical insertion
of the
product in cancer tissue and/or other therapeutic agents which optionally may
be
used for topical application, antioxidants, fungicides, nicotine,
nitroglycerine,
antiinflamatory drugs, NSAIDS such as ibuprofen, ketoprofen or diclofenac,
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
cortico steroids, pain relieving agents such as lidocaine or chinchocaine,
emollients, retinoids or agents having a cooling effect which is also
considered
an aspect of the invention.
EXAMPLES
EXAMPLE 1:
Microcolloids were obtained from Ciba Specialty Chemicals in the form of
"Collafix PP80" which is a 50% w/w dispersion of particles with a size of
about
0.5-1 pm cross-linked polyacrylic acid based copolymer which intended use was
as "Prepaste adhesive for wallpaper".
acResin A 258, UV-curable acrylic adhesive (BASF)
Collafix PP80, 50% w/w microcol(oids,, Carrier oil: Aliphatic hydrocarbon.
Ciba
Specialty Chemicals
PU Backing film, Moisture Permeable (MTR app.12-13.000 g/m2/24 hours)
1 part acResin A258 was mixed on a standard laboratory mixer with 2 parts
Collafix PP80. The carrier oil was removed by placing the mixture in a vacuum
oven over night, producing a acResin:microcolloid mix of approximately 1:1,
Mixture A
1 part of A was mixed with 4 parts of ethanol to produce solution B and 1 part
acResin was dissolved in ethanol to produce solution C.
Solution B & C were mixed to produce a range of microcolloid dosages, coated
on the PU backing by means of standard bar coating to produce a solvent coat
of
about 1 OOpm (wet) resulting in a dry adhesive coat of about 20pm. The
adhesive
was cured by UV radiation after the ethanol had been allowed to evaporate.
Moisture transmission and peel was measured, and the results are shown in
Table 1.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
21
TABLE 1
20pm acResin/Collafix on PU
Sample 1,1 1,2 1,3 1,4 1,5 1,6 1,7 1,8 1,9 0*
Microcolloids0 2 4 7 10 15 22 33 50
Peel from 7,5 6 6,2 5,6 5,8 3,8 3,2 2 1,3
steel
(N/20mm) 23C
Transmission**2 2,8 2,7 2,7 2,9 3,4 5,2 12 12 13
(kglm2/24hours)
*: Backing without adhesive
**: Measured in a standard Pattington cup with 0.9% NaCI, 37 °C, 15% RH
Major moisture transmission improvements are obtained by addition of microcol-
loids to the adhesive matrix and it is possible to prepare very thin
transmitting
coatings of adhesive.
The adhesive may be modified by standard techniques, e.g. addition of
softeners
or resins to adjust the adhesive properties.
EXAMPLE 2
Microcolloids were obtained from Ciba Specialty Chemicals in the form of
Salcare SC91 but without the activating surfactant and as a 60% w/w dispersion
of 0.5-1 Nm cross-linked polyacrylic acid based copolymer particles, which
intended use was as rheology modifier for personal care products.
Salcare SC91, w/o activating surf., 60% Solids, microcolloids, Ciba Specialty
Chemicals
Kraton 1107CU, Styrene-Isoprene-Styrene polymer, Shell
Arkon P115, Tackifying resin, Arakawa Chemical Industries, LTD
Parafluid PL500, white mineral oil, PARAFLUID Mineraloelgesellschaft mbH
Aquasorb A500, Hydrocolloid, Crosslinked CMC, Aqualon
PU Backing film, Moisture Permeable (MTR app.12-13.000 g/m2/24 hours)
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
22
The microcolloids were neutralised by addition of 5M NaOH to the dispersion
until the solution pH where above 9 in order to maximise swelling, and water
was
removed by vacuum distillation.
2 parts of the neutralised microcolloids were diluted with 1 part petroleum
spirit
(60-80°C Bp) to make up Solution D
1 part Kraton 1107 was dissolved in 9 parts petroleum spirit (60-80°C
Bp) to
make up Solution E
1 part Arkon P115 was dissolved in 1 part petroleum spirit (60-80°Bp)
to make
up Solution F
Solutions D, E, F, parafluid PL500 and Aquasorb A500 were mixed according to
Table 2 below, coated onto siliconised paper frames and the petroleum spirit
was
allowed to evaporate.
TABLE 2
w/w Solids
Adhesive A1 Adhesive A2 Adhesive A3
Kraton 1107 15% 15% 24% (15 parts)
Arkon P115 22,5% 22,5% 36% (22,5 parts)
Mineraloil 25%* 25%** 40%** (25 parts)
Microcolloid 37,5% 0% 0%
Aquasorb A5000% 37,5% 0%
Total 100% 100% 100%
* From the microcolloid dispersion
** Parafluid PL500
The three adhesives A1, A2 and A3 have the same polymeric matrix and differs
only in dispersed phase, so A1 is the microcolloid adhesive, A2 is the
traditional
hydrocolloid adhesive and A3 is the control without dispersed phase.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
23
The dried adhesives were removed from the paper frame and warm pressed to
200Nm thickness and laminated onto the permeable PU backing.
The following parameters were measured: Absorption speed, absorption
capacity, moisture vapour transmission rate, peel, adhesive tack (1 mm
adhesive,
no backing) and rheological properties (1 mm adhesive, no backing). The
methods and results are shown below.
Absorption Speed:
Absorption speed was measured by mounting a 2'/Z x 2'/2 cm sample of adhesive
on a small glass slide, submersing the adhesive in a 0.9% NaCI solution and
measuring the weight gain as function of time. The results are shown in Table
3.
TABLE 3
Absorption speed, after 30 min. in 0.9% NaCI, 37°C
Adhesive Absorption speed
A1) Microcolloid adhesive 98000 g/m2/24 hours
A2) Hydrocolloid control 19000 g/m2/24 hours
A3) No dispersed phase 1000 g/m2/24 hours
control
As shown in Table 3 the microcolloid adhesive provides superior initial
absorption, which is desired, e.g. in order to get good and fast adhesion to
wet or
moist surfaces.
Absorption Capacity:
Absorption capacity is measured in the same way as absorption speed, only
after
longer time. Apart the actual weight gain, the cohesion of the swelled
adhesive is
important in order to render it possible to remove the adhesive without
leaving
residues. The results are shown in Table 4.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
24
TABLE 4
Absorption capacity, w/w, after 24 hours in 0.9% NaCI, 37°C
Adhesive Absorption capacityCharacter
A1) Microcolloid adhesive1740 %, Good cohesion
A2) Hydrocolloid control800 %, Poor-fair cohesion
A3) No dispersed phase 20 % Very good cohesion
control
As can be seen from the table, this type of microcolloids provide a high
swelling
capacity, and most importantly combined with good cohesion.
Moisture transmission:
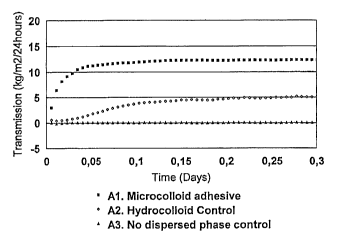
Moisture transmission is measured by an inverted Pattington cup method.
The moisture vapour transmission is measured as g/m2/24hours in 0.9% NaCI,
37°C, 15%RH. The results are shown in Figure 1.
As disclosed in Figure 1 the microcolloid adhesive does not only provide
higher
moisture transmission, 12-13000 g/m2124 hours (same as backing), compared to
about 5000 g/m2/24 hours for the hydrocolloid control, but also the lack time
before maximum transmission is reached is much shorter.
Peel
Peel adhesion is measured as 90° peel from steel plates, at
23°C, 50% RH,
304mm/min, 25mm., Instron model 5564 tensile tester (PSTC-2). The results are
shown in Table 5.
TABLE 5
Adhesive Peel adhesion
A1 ) Microcolloid adhesive 14.2 N
A2) Hydrocolloid control 0.9 N
As can be seen from the table, the peel adhesion of the microcolloid adhesive
is
much higher than that of the hydrocolloid control.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
Adhesive tack
The adhesive tack is measured as probe tack with a 5mm teflon probe, at
23°C,
50% RH. (Instron model 5564 tensile tester). The results are shown below in
Table 6.
TABLE 6
Adhesive Adhesive tack
A1) Microcolloid adhesive 7.8 mJ
A2) Hydrocolloid control 1.2 mJ
As disclosed in the table, the adhesive tack is much improved when comparing
the microcolloid adhesive with the hydrocolloid control.
EXAMPLE 3
Preparation of foamed adhesive with microcolloids.
MicroColloids DP199-9086 were obtained from Ciba Specialty Chemicals, as a
55% w/w dispersion of 0.5-1 pm cross-linked poly acrylic acid based copolymer
particles.
DP199-9086, 55% Solids, Ciba Specialty Chemicals
Quintac 3433N, Styrene-Isoprene-Styrene polymer, Nippon Zeon Ltd.
Arkon P115, Tackifying resin, Arakawa Chemical Industries, LTD
PU Backing film, Moisture Permeable (MTR app.12-13.000 glm2/24 hours)
The adhesive was hot melt processed in a Herman Linden z-blade mixer
(Machine type LK 110.5) with an circulating oil temperature at 160 °C.
The ingredients of Table 7 were mixed:
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
26
TABLE 7
Quintac 3433N 100 parts w/w
Arkon P125 112 parts w/w
DP199-9086 122 parts w/w
After the processing of the adhesive the bulk adhesive was placed in a
FoamMelt
130 from Nordson, which is designed to force gas (in this case inert Nitrogen)
into dispersion in the moulded adhesive.
The temperature in the chamber, where the adhesive was melted, was 170
degree C. After the adhesive was melted the density controller was adjusted.
After 20 min. of circulation the density was constant and the foamed adhesive
were dispensed onto a siliconised paper. Just after application the gas
expands
to create a closed-cell foam. A soft and stable foam with excellent adhesive
properties was obtained.
The foamed adhesive was laminated on to a PU backing. The thickness of the
foam layer was: 0,3 mm, 0,6 mm, 1,0 mm and 2,0 mm.
Compared to unfoamed adhesives, the foamed adhesive was provided the
following benefits:
- increased adhesion to flexible substrates
- increased flexibility
- lower pain upon removal from the skin
- improved soft feel
- reduced adhesive consumption
The porosity of the 2,0 mm foamed adhesive was determined immediately after
preparation and again after 2 weeks stored at 40 degrees C. The results are
shown in Table 8.
CA 02438875 2003-08-21
WO 02/066087 PCT/DK02/00116
27
TABLE 8
Initial porosity Porosity after 2 weeks
47,3 % 46,9
The results of Table 8 show a very good stability of the foamed adhesive.