Note: Descriptions are shown in the official language in which they were submitted.
CA 02439102 2003-08-22
Synthesis apparatus and method of manufacturing the same
The present invention relates to a synthesis apparatus, especially for the use
incombinatorial chemistry (e.g., solid phase synthesis) as well as to a method
of
manufacturing the same.
For the production of a multitude of chemicals compounds various methods and
apparatuses have been developed in the past, which minimize the time for the
synthesis of this multitude of chemical compounds and if possible
simultaneously
automatize all procedures and miniaturize the sizes of the apparatuses. Here
paralleling of the synthesis procedures for the various chemical products
plays an
important role, i.a., all syntheses should work according to one synthesis
protocol.
It has become obvious that syntheses on solid phase are especially well suited
to
fulfill the above-mentioned requirements. First developments in the field of
solid
phase synthesis have been made by Merrifield et all (R.B. Merrifield, J. Am.
Chem. Soc. 85, 2149-2154 (1963)).
A synthesis concept used for the generation of a multitude of chemical
compounds is the so-called combinatorial chemistry. Which embraces a whole
range of techniques which are able to produce in a few, often automatized
reaction series a multitude of different compounds (so-called compound
depositories) (see, e.g., M.A: Gallop et al, J. Med. Chem. 37 (1994), 1233-
1251;
E.M. Gorden et al, J. Med. Chem. 37 (1994), 1385-1401). Here, too, the
reactions
are preferably conducted on solid phase for practical reasons. The support
materials are usually transversally interlaced polymers in particle form (so-
called
beads of polystyrol, or polyethyleneglycoUpolystyrene resin). Starting from a
functionalized surface, the desired structures are made-up in multiple
synthesis
steps. An overview over the synthesis of compound depositories on solid phase
as well as in solutions is given by L.A. Thompson and J.A. Ellman, Chem. Rev.
96
(1996), 555-600. After finishing a combinatorial solid phase synthesis the
products
are separated in general from the solid phase, i.e., by separating an instable
bonding between the final product and the support resin. Here often linkers
are
used, which function as a bonding member between the support resin and the
desired chemical compound. (With regard to solid phase support, linker and
suitable reactions, see, e.g., Florenzio Zaragoza Dorwald, Organic Synthesis
on
Solid Phase, Supports, Linkers, Reactions, Wiley-VCH, Weinheim 2000)
CA 02439102 2003-08-22
2
Besides the already mentioned beads planar supports also have an important
role
in the solid phase synthesis. Here, the most important materials are glass,
membranes of cellulose and other polymer materials. For synthesis purposes,
the
materials should be porous, mechanically and chemically stable but also easy
to
be separated, in order to achieve an as high as possible synthesis capacity
per
(surface) area unit and to guarantee universal handling. These requirements
are
only fulfilled by membranes of natural (like cellulose) and synthetic origin.
The
latter are made , e.g., of polyethylene superimposed by polystyrene which in
turn
is functionalized by aminomethylising, or of polypropylene , which is encoated
with
cross-linked polyhydroxypropylacrylat (Michal Lebl, Biopolymers (Peptide
Science) 47, 397-404 (1998)). Also, the use of transversally interlaced
functionalized Teflon~ membranes for the synthesis of combinatorial
depositories
is known (M. Stankova, S. Wade, K.S. Lam, M. Lebl, Peptide Research 7, 292-
298 (1994)). Further polymer compounds of membranes for the synthesis of
oligopeptides and nucleotides are described in US-A-4 923 901.
Besides the support used for the solid phase synthesis the choice of the
geometry
of the synthesis reactor also plays an important role for the automatizing and
miniaturizing. Here the synthesis areas should be generally arranged in a
uniform
grid and should be position-addressable. Usually vessels are used in which the
solid phase material (beads, membrane pieces etc.) are introduced, the vessels
being firmly bonded to each other by casting. In order to facilitate the use
of
effective robots for pipetting or the like, the vessels should be open on the
upper
face. e.g., a microtiterplate complies with these requirements. If membranes
are
used also a whole membrane sheet can be looked upon as a planar synthesis
reactor.
In the latter case especially the spot synthesis is appliable, which is also
applied
in the case of cellulose (Michal Lebl, Biopolymers (Peptide Science) 47, 397-
404
(1998). In the spotting technique, the test material is transferred from a
microtiterplate onto the support by means of transfer pins or multiple
pipetters.
Here the transfer pins are immersed in the test fluid - the drop of test fluid
adhering to the point of the transfer pin is then deposited on the support. By
varying the size of the pin, varying volumes of test fluid can be transferred.
Sharn
et al. describe the spot synthesis of a combinatorial 1,3,5-triazine compound
depository on various functionalized polypropylene membranes (D. Scharn, H.
Wenschuh, U. Reineke, J. Schneider-Mergener, L. Germeroth, J.Comb. Chem. 2,
CA 02439102 2003-08-22
3
361-369 (2000)).
A disadvantage of the spotting technique is that during depositing the fluid a
concentration gradient can from around the spot. Furthermore, sequential
adding
of multiple reagents for one reaction step is not possible, if these are to
form a
homogeneous reaction mixture. Also, an individual (spotlike) treatment of the
single reaction spots (membrane areas) is not possible or only very difficult
by
means of batch reagents like washing solutions.
If reaction vessels are used, e.g., microtiterplates, it is advantageous if
the support
material is fixed in the reaction vessel. This prevents an uncontrolled
floating in
the reagent solution. Fixing guarantees that the support is entirely soaked
and
that thus there is about the same reagent concentration throughout. Even
during
working under reduced pressure and by using so-called plate washers fixation
is
advantageous. Here membrane pieces are usually to be preferred over beads,
since based on the absolute capacity of synthesis a membrane unit is
equivalent
to a multitude of bead units, i.e., a great deal of beads are necessary to
achieve a
synthesis capacity comparable to that of a piece of membrane in the size of
the
bottom of a microtiterplate cavity. The handling of such beads is difficult
and
especially its fixing is very awkward.
WO-A-94/05394 describes various possibilities of the fixing of solid phase
supports. On the one hand a multi-layered support (three plates) is suggested
in
which a reaction vessel forms by corresponding apertures in the topmost
layers.
In the bottom area beads are fixed by a suitable adhesive. This is very
inconvenient, since - as already mentioned - the handling of very small
particles is
necessary. Furthermore the synthesis conditions and the reagents used have to
be adjusted to the adhesive used. Also it cannot be ruled out that the
adhesive
negatively influences synthesis properties of the beads.
An alternative described in WO-A-94/05394 suggests the superimposing of a
polymerfilm in the bottom area of the multi-layered reaction vessel. Here the
bottom plate of polyethylene is provided in multiple steps with acid chloride
functionalities, in order for, e.g., a methacrylamide polymer to be applied on
which
in its turn a polyacryalmide gel is superimposed which is transversally
interlaced
with bisacrylamide and has additionally to be functionalized, in order for
e.g., a
suitable linker or spacer to be chemically bonded for solid phase synthesis.
Such
a construction of a polymer film suitable for the solid phase synthesis thus
CA 02439102 2003-08-22
4
embraces several chemical steps and is thus quite demanding. Furthermore
during the construction of the multi-layered reaction vessel attention has to
be
paid to a sufficient tightness in the intermediate area between the individual
plates.
A further possibility which is described in WO-A-94/05394 starts from the use
of a
substrate in form of a sintered polyethylene disc with a diameter of 1/4" and
a
thickness of 1/8". This is coated with a thin, hydrophilic, polar, multi-
functionalized
polymer film (HPMP) and is pressed into the recess of a plate so that the
whole
reaction space is filled. Pressing the film into the recess is to prevent it
from falling
out while a taper in the bottom area preventing it from slipping out
downwardly.
Furthermore one or more channels are provided in order to produce a vacuum
and thus a fluid transport is facilitated. The size of the disc is an obstacle
to the
miniaturization of the apparatus, as well as fluid transport by suction is
difficult on
the apparatus side and can also be miniaturized only with great efforts.
US-A-6 063 338 discloses a microtiterplate, which contains a cycloolefin for
spectroscopic purposes and is also said to be suitable for solid phase
synthesis.
This document suggests, i.a., that the inner walls and bottoms of the cavities
should be functionalized in order to immobilize components for solid phase
synthesis. Disadvantageous of such an approach is the low synthesis capacity,
which is only achieved by surface treatment.
WO-A-99/32219 describes a solid phase system working in parallel, in which
whole membranes are pressed in between plates with apertures laying over each
other and having cylindrical nozzles on the top and bottom side, in order to
achieve a pump system running from the top side to the bottom side.
Furthermore,
beads are suggested for solid phase supports, which are introduced into
vessels,
formed by the recesses of a plate with an incorporated fritted bottom. Pumping
the
fluid at least guarantees that a certain fixing in the bottom area is
possible, i.e., a
continuous floating of the particles is prevented. But such a pump system is
very
demanding and miniaturization is only very difficult to be achieved.
Especially if
membrane sheets are used attention has to be paid that a sealing of one flow
channel against the adjacent one is achieved. Also the use of valves is
suggested
which cause an additional effort on the apparatus side.
EP-A-0 608 779 discloses an apparatus for the peptide synthesis, providing a
microtitierplate in which membrane pieces are clamped in the individual
cavities
CA 02439102 2003-08-22
and are thus fixed. Clamping is achieved in that the diameter of the pieces is
chosen so that it is somewhat greater than the diameter of the cavities. Here
it is
especially disadvantageous that in the margin area between the membrane and
the microtiterplate bottom hollow spaces form which are an obstacle to an
5 unhindered fluid exchange. Furthermore, it cannot be prevented that the
pieces
are set free from the cavities during certain procedures, e.g., during vacuum
drying. Also, for such a fixation a certain thickness of the membrane is
necessary
since otherwise if small and thin membrane pieces are used, as are, e.g.,
necessary in a 96 microtiterplate, the edges of the membrane pieces can roll
up
on contact with the fluid and thus the fixing effect is removed.
The object of the present invention is to provide an improved synthesis
apparatus
or device, in which a solid phase support is fixed in the reaction vessel as
well as
a method of manufacturing the same. This object is attained by the features of
the
independent claims. Preferred embodiments are described in the dependent
claims.
The invention starts from the basic idea to equip the synthesis apparatus
essentially with a vessel with wall and/or bottom areas as well as a solid
phase
support for the use in the solid phase synthesis whereby preferably by thermal
effects on a relatively limited area of the solid phase support a fixing of
the
support at at least one of the vessel areas is achieved.
According to a preferred embodiment of the present invention; chemically
functionalized membranes having a polymer are used as solid phase support.
Preferably a multitude of vessels are arranged in an uniform grid and are
bonded
with each other by casting or an integral housing. This arrangement is
advantageously of plastic, especially preferred is a microtiterplate. The
bonding
between membrane and inner surface of a vessel is preferably achieved by a
spot
welding method, i.e., both materials should ideally have thermoplastic
properties
and should form a stable bonding with each other by a melting process.
However,
e.g. in the case of teflon membranes and cellulose, it is surprisingly
sufficient if
only the plastic vessel has thermoplastic properties. The pasting or welding
(fixing) is amazingly stable both against mechanical or chemical impacts, so
that
normally removal is only possible if the membrane is destroyed. Furthermore
the
plastics used should be stable against the chemicals and solvents used for the
chemical synthesis. Furthermore, a certain thermal stability is advantageous.
CA 02439102 2003-08-22
6
Polypropylene has shown to be an especially well suited material for plastic
vessels. Polypropylene is inert against almost all organic solvents and also
stable
against aggressive reagents. The usable thermal range is usually between about
-
80°C and 100°C. As vessels, various in size standardized
polypropylene
microtiterplates (PP-MTP) are available. These can be obtained with a
different
number of cavities and volumes on a large scale. At the moment polypropylene
MTPs are available with 24, 96, 384 or 1536 cavities and volumes from 8,u1 to
2.7m1 and a bottom area from 1.56mm2 to 700mm2 per cavity. Various porous,
absorbent polypropylene and teflon membranes i.a. have proved to be suitableas
reactive phase. Polypropylene membranes with various loading densities of
reactive functionality (80 - 2500 nmol/cm2) are purchasable. These membranes
are available with hydroxyl- and amino groups as functional groups.
In the following preferred embodiments of the present invention are described
with
respect to the drawings in more detail:
Figure 1 shows a schematic cross-sectional view of the synthesis apparatus
according to the present invention;
Figure 2 shows the loss of synthesis amount of a fixed membrane in
comparison to a non-fixed membrane by means of the ratio of the
vitrified area to the total area; and
Figure 3 shows a 384 microtiterplate during various cycles in the removal of
reagent remainders
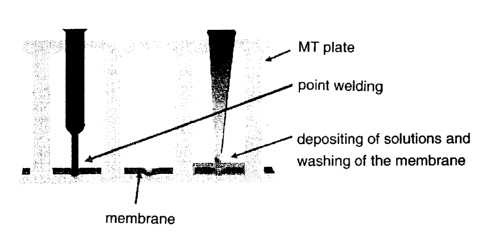
In the embodiment according to the present invention shown in Figure 1 the
membrane is fixed in the bottom area of the vessel. However the membrane could
also or additionally be fixed to the wall areas of the vessel or cavities. The
geometry of the membrane piece can advantageously adapted to the cavity,
especially to the bottom of the cavity in such a manner that the forms are
essentially the same (e.g., round membrane pieces for round cavities) and the
walls are hardly or not at all in contact with the membrane.
A preferred embodiment of the manufacturing method according to the present
invention of the described synthesis vessels starts with the stamping of the
membrane into the microtiterplates (MTP). Here ,e.g., a stamping machine can
be
used wherein the MTP is located below the stamping blade(s). Such stamping
machines are described, e.g., in US-A-5 146 794. During stamping the membrane
cutouts (or membrane pieces) fall preferably directly into the cavities of the
MTP.
CA 02439102 2003-08-22
7
The membrane cutouts are dimensioned in a manner that they do not tilt in the
cavities but that they are slightly smaller than the internal size of the
cavities.
It has proved useful, to spray the MTPs to be provided with membrane pieces
with
a suitable fluid, e.g., ethanol, since otherwise the membrane pieces -
assumably
due to static charging - partially adhere to the walls of the cavities instead
of the
bottom.
For fixing the membranes to the MTP a thermal method is surprisingly
particularly
advantageous. Here preferably a metal point (e.g., electrically) heated to
450°C
and having a diameter of about 0.3mm is pressed for 0.8s onto the membrane
which is on the bottom of a cavity of the MTP. This procedure can be
automatized
with common robots. During welding a punctual melting of the membrane (in case
of a polypropylene membrane) and the underlying MTP material (e.g., PP-MTP)
occurs. During the following hardening a stable bonding between the two
components forms.
It has been shown that the membrane looses its porosity in a relative small
area
around the welding point due to the thermal melting of the material. The area
of
thermoplastic deformation where a considerable synthesis yield can no longer
be
expected is about 0.7mm around the center of the melting or welding point. The
percentual yield loss is, however, surprisingly negligibly small in comparison
to a
non-fixed membrane cutout of the same size. It is generally dependent from the
size of the membrane piece and the MTPused. For a commercial 1536 MTP, the
geometrically determined loss is preferably less than 5%.
The thus manufactured multi-synthesis plates can be cleaned with suitable
organic solvents and then be dried prior to their use. During the cleansing
step
thermal decomposition products formed during melting are removed.
Due to the use of a synthesis apparatus with solid phase support according to
the
present invention, it is possible to use usual pipetting robots, dispensing
automats
and plate washer as well as vacuum drying. Furthermore multiple addition and
the
suction of reagent solutions is possible. Due to the point welding, the
membrane
pieces are very well washed by wash and other solutions and no reservoir forms
between the solid phase support and bottom area. Furthermore the apparatus
according to the present invention is very variable with respect to the
desired
synthesis amount by using various MTPs or membranes. The synthesis mass can
CA 02439102 2003-08-22
8
very well be adjusted by the area of the membrane. Due to vitrifying the
contact
point in the welding of the membrane, visual control of the welding quality,
advantageously on the back side of a MTP, is possible. A further advantage is
that the membrane can be functionalized batchwise before stamping.
A preferred use of the synthesis apparatus according to the present invention
is in
the field of the combinatorial chemistry, as a multitude of various compounds
can
be obtained in a very short period of time and with comparatively simple means
due to parallelizing and miniaturizing.
Examples
Example 1 loss of synthesis amount of a fixed membrane in comparison with a
non-fixed membrane.
a) The geometrical loss (ratio of the vitrified area to the total area
according to
Figure 2 by fixing by an metal needle) was determined for a 384 PP-MTP
and a with an amino-group functionalized membrane from the manufacturer
AIMS Scientific Products GbR, Braunschweig (800 nmol/cm2) to be less
than 5%.
b) The loss of chemical yield under the same conditions as in a) was
determined by coupling of amino acids according to standard peptide
synthesis and following Fmoc analysis. Here membrane discs having a
diameter of 3mm or membrane squares having a side length of 1.05mm
are laid in a 384 MTP of the company Greiner or a 1536 MTP and fixed or
welded with a hot metal needle. On the membrane areas, Fmoc-Alanine
has been coupled according to standard synthesis protocols (Fields et al.
1990) and the loading of the membrane has been determined by Fmoc-
analysis. The Fmoc protection group has been separated by 20%
piperidine and the amount of the Fmoc-group has been determined
photometrically (extinction coefficient: 7800M-'cm-'). For the membrane
area in the 284 MTP an average load of 52nmol has been determined. The
calculated material amount was 57nmol. For the membrane area in a 1536
MTP an average of 8.5nmol has been determined at a calculated amount
of 8.6nmol.
Example 2 Removing of reagent remainders
CA 02439102 2003-08-22
9
During washing cycles, membrane areas soaked with reagent solutions
(fluorescine) are washed. A membrane with a diameter of 0.3mm has been fixed
in 8 wells of a 384 PP-MTP of the company Greiner Bio-one (Frickenhausen) and
loaded with 3N1 of a 0.1 mg/ml fluorescine solution (= 220nmol, 0.073mo1/I)
and the
fluorescence (50ms, 560nm) has been determined by means of a LUMI-Imager~
of the company Roche Diagnostics GmbH, Mannheim. Then, it was washed by
means of a Embla 384 Plate Washer of the company Molecular Devices
(Ismaningen) with ethanol (+0.1 % TFA) and then the fluorescence was
immediately measured again in the LUMI-Imager. The following washing program
has been used:
~ 20N1 of the solution were filled in;
~ waiting time 10s
~ suction (2mm/s advance, 2s waiting time)
After at most 4 of such washing cycles no difference to the non-treated
membranes could be detected as shown in Figure 3.