Note: Descriptions are shown in the official language in which they were submitted.
CA 02441713 2003-09-18
Rubber Reduction
Field of the Invention
The invention generally relates to rubber reduction, and more particularly to
rubber reduction using solvent extraction techniques at a subcritical
temperature.
Background of the Invention
It is estimated that in many countries the number of used tires produced per
year is approximately equal to the population of the country. As an example
there
are more than 250 million used tires produced annually in the United States.
Methods
of dealing with these used tires can generally be placed in two categories;
disposal
and reclamation. The former group includes land filling and stock piling which
are
increasingly unacceptable options for a multitude of reasons. Within the
latter group
are approaches that use the tires in close to their original state with
possibly some
physical processing. Examples of uses within the above group include use as
vibration and debris dampening mats as may be used in drilling operations or
filler
material for road construction and burning as a source of energy. Burning has
at
times and in certain areas represented up to 40% of the tires being discarded.
Most
of the applications in the above group represent a limited volume of tires and
do not
exploit the economic value imparted on the raw materials during the original
fabrication of the tire.
Another group of reclamation methods look to extracting increased value from
the constituent materials within a tire. The major constituents include
synthetic and
natural rubber, carbon black and steel and minor constituents include sulphur
and
any stabilizers. The processes within this group may be referred to as
reduction
processes where the tire is being reduced to its constituents.
Reclamation of natural rubber using water, and more particularly steam, is
known in the art. It has been disclosed that natural rubber can be reclaimed
by
processing with steam at temperatures above 100°C. The disclosed
pressures
include the saturated water vapour pressure. The disclosed methods provide for
the
devulcanization of natural rubber and possibly some depolymerization,
depending on
the particular reaction conditions.
CA 02441713 2003-09-18
It however became common practice to reclaim natural rubber at
temperatures around 200°C and pressures of around 200 psi. It was
subsequently
determined that synthetic rubbers could not be reclaimed using these latter
conditions. D.S. LeBeau, discusses the reclamation of rubber that includes
styrene-
butadiene rubber (SBR), a synthetic rubber, using steam in Science and
Technology
of Reclaimed Rubber, Rubber Chemistry and Technology 40, 1967, 217-237. Figure
1, Figure 1 of LeBeau, illustrates effect of steam treatment on natural rubber
and
SBR. Natural rubber softens i.e. the viscosity is lowered and can be reclaimed
when
exposed to 200 psi steam. However, SBR experiences a short-lived softening
that is
followed by an extended hardening. LeBeau notes that the rate of hardening
increases with increasing temperature. LeBeau further notes that the
reclamation of
synthetic rubbers therefore requires reclaiming agents or catalysts.
One process used for the reduction of used tires that include synthetic
rubbers is pyrolysis. In a typical pyrolysis process the tires are subjected
to
temperatures between 600 and 900°C in either an inert atmosphere or a
vacuum.
This process produces light oils and char where the char contains carbon black
and
pyrolytic carbon formed by the carbonization of rubber hydrocarbon.
Pyrolysis is generally not seen as a desirable reduction process. With the
rubber hydrocarbons being either reduced to light oils or carbonized to char
the end
products of a pyrolysis process do not retain much of the economic value
associated
with the original rubber hydrocarbon and carbon black.
Reduction processes typically include devulcanization and depolymerization
steps or processes. The devulcanization process breaks sulphur-sulphur and
sulphur-carbon bonds that cross-link rubber molecules. The devulcanization
process
produces a solid residue where the mass of the solid residue is approximately
100%
of the original mass of tire. The solid residue contains rubber hydrocarbon
and
carbon black where rubber hydrocarbon includes any hydrocarbon with a
molecular
weight above that of oil that originates from the initial rubber. The rubber
hydrocarbon has an average molecular weight that is generally less than the
initial
rubber but much greater than oil, where oil has an average molecular weight of
approximately 500 or less.
The depolymerization process reduces the average molecular weight of the
rubber hydrocarbon by breaking carbon-carbon bonds of the rubber hydrocarbon
2
CA 02441713 2003-09-18
until, at completion, the rubber hydrocarbon has been reduced to oil. Thus the
depolymerization process, at completion, reduces the molecular weight from
around
200,000 to 500. At the end of the depolymerization process the mass of solid
residue is approximately 40% of the initial mass of tire. At this point the
solid residue
is substantially only carbon black with the rubber hydrocarbon being
completely
reduced to oil.
Figure 2a is schematic graph of the % completion v. time for a typical
pyrolysis process. In a typical pyrolysis process a devulcanization process
202 and a
depolymerization process 204 occur substantially in parallel. As such the two
processes are complete at approximately the same time i.e. t~~tp. Figure 2b
shows a
non-pyrolysis process in which the devulcanization process 206 is separated
from
the depolymerization process 208. At time t~ the devulcanization process 206
is
complete while the depolymerization process 208 is only a fraction of the way
to
completion.
An alternative approach for reducing tires uses solvent extraction techniques.
Solvent extraction uses elevated temperatures and pressures in the presence of
a
solvent to at least devulcanize and often depolymerize the rubber. In almost
all of
the work in this area processing is conducted at a temperature and pressure
that are
above the critical values of these parameters for the particular solvent in
which
processing is being conducted.
Supercritical reaction conditions are defined as having a reaction temperature
that is above the critical temperature and a pressure that is above the
critical
pressure. Supercritical reactions have been performed using a variety of
solvents
including alcohols, organic solvents and water. Much of the work using
supercritical
reaction conditions has been directed to extensive depolymerization of the
rubber. In
particular work has been directed to the reduction of the rubber to oil.
Supercritical
processing has been found to be advantageous in these cases as it provides a
feasible reaction rate for the required depolymerization reactions. However,
as the
resulting oil will generally be used as a fuel the economic value of the
rubber is
reduced to a level well below that of that imparted to the tire during initial
processing.
Recently there has been work directed to the devulcanization of rubber while
mitigating depolymerization of the devulcanized hydrocarbons. By maintaining
the
hyrodrocarbon chain length near its original value a higher proportion of the
CA 02441713 2003-09-18
economic value imparted to the rubber during initial processing is maintained.
United
States Patent 6,548,560 to Kovalak et al. discloses the use of subcritical
processing
with a solvent selected from alcohols and ketones while United States Patent
5,891,926 to Hunt et al. discloses subcritical processing conditions with the
use of 2-
butanol as a solvent. The use of the above solvents allowed processing
temperatures below 300°C which Kovalak et al. teach as important in
reducing the
amount of polymer degradation. Thus their focus is on solvents that have a
critical
temperature between 200 and 350°C. Hunt et al. and Kovalak et al. do
not however
disclose complete devulcanization of the rubber.
Many organic solvents are however costly and have properties that make
them less than desirable with regard to health and safety considerations. For
example, 2-butanol is flammable, has a low flash point and is an irritant.
Summary of the Invention
The invention is directed to a method for the reduction of rubber. An object
of
the invention is to mitigate one or more disadvantages in the prior art. A
further
object of the invention is to provide a method for the reduction of rubber
that includes
synthetic rubber using a solvent that includes water where at least a portion
of
reaction product has a molecular weight that is larger than oils.
According to one aspect of the invention a method of reducing vulcanized
rubber is provided. The method comprises the steps of heating the rubber,
wherein
the rubber includes synthetic rubber, in the presence of a solvent, wherein
the
solvent includes water to a temperature below a critical temperature of the
solvent,
providing a pressure that is at least equal to a saturated vapour pressure of
the
solvent at the temperature, and maintaining the temperature and the pressure
for a
time sufficient to devulcanize the rubber and produce a reaction product that
is
primarily a solid phase and includes rubber hydrocarbon.
According to another aspect of the invention a method of reducing a
vulcanized tire is provided. The method comprises the steps of heating the
tire,
wherein the tire includes synthetic rubber, in the presence of a first
solvent, wherein
the first solvent includes water, to a temperature below a critical
temperature of the
first solvent, providing a pressure that is at least equal to a saturated
vapour pressure
of the solvent at the temperature, maintaining the temperature and the
pressure for a
4
CA 02441713 2003-09-18
time sufficient to devulcanize the tire and produce a reaction product that is
primarily
a solid phase and includes rubber hydrocarbon, washing and drying the solid
phase
of the reaction product, dissolving the rubber hydrocarbon in a second
solvent, the
second solvent being appropriate for the dissolution of rubber hydrocarbon
therein,
separating the carbon black from the reaction product and separating the
second
solvent from the rubber hydrocarbon.
Other aspects and advantages of the invention, as well as the structure and
operation of various embodiments of the invention, will become apparent to
those
ordinarily skilled in the art upon review of the following description of the
invention in
conjunction with the accompanying drawings.
Brief Description of the Drawings
The invention will be described with reference to the accompanying drawings,
wherein:
Figure 1 is a schematic diagram of viscosity v. time for natural rubber and
SBR;
Fig. 2a is a schematic graph illustrating % completion v. time for a pyrolysis
reduction process;
Fig. 2b is a schematic graph illustrating % completion v. time for a non-
pyrolysis reduction process;
Fig. 3 is a schematic graph of mass of solid residue vs. stages of reduction
process;
Fig. 4 is a flow chart of a process for reducing tires according to an
embodiment of the invention;
Fig. 5 is a flow chart of a process for reducing tires according to another
embodiment of the invention; and
Fig. 6 is a flow chart of a process for reducing tires according to a further
embodiment of the invention.
5
CA 02441713 2003-09-18
Like numerals identify like features within the drawings.
Detailed Description of the Invention
An embodiment of the current invention provides a method for the reduction
of rubber using subcritical water i.e. the reduction process occurs at a
temperature
below the critical temperature of water. The term "critical temperature of
water" as
used herein is defined as the temperature above which steam cannot be
liquefied by
the application of pressure. The critical temperature of water is
374°C. The method
allows for at least some separation of the devulcanization and
depolymerization
processes, using a low cost, non-flammable, non-toxic and environmental
friendly
solvent.
Process parameters for subcritical fluid techniques include pressure,
temperature, solvent and time. Without being bound by theory it appears that
operation of the process at temperatures below the critical temperature of the
solvent
allows for the separation of the devulcanization and the depolymerization
processes.
The rate of depolymerization process is lowered to an extent where it is
effectively
separated from the step of devulcanization. This allows improved control
regarding
the extent to which depolymerization is allowed to proceed. In an embodiment
of the
invention the reaction time and temperature are varied to provide
substantially
complete devulcanization and a desired amount of depolymerization.
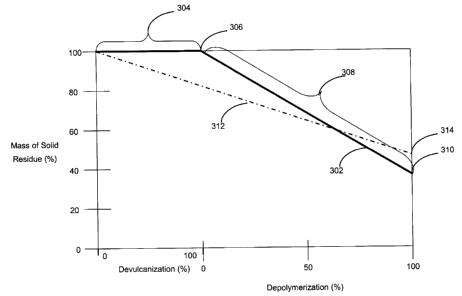
Figure 3 presents a schematic graph of mass of solid residue v. stages in the
tire reduction process. A line 302 represents a non-pyrolysis process while a
line
312 represents a typical pyrolysis process. The line 312 i.e. a typical
pyrolisis
process is complete at a point 314 where rubber hydrocarbon have been reduced
to
light oils and some has been carbonized to char.
The line 302 contains a devulcanization portion 304 and a depolymerization
portion 308. At a point 306 the initial rubber has undergone substantially 100
devulcanization. At the point 306 the mass of solid residue is essentially 100
% with
the solid residue containing rubber hydrocarbon and carbon black. During the
depolymerization portion 308 the average molecular weight of the rubber
hydrocarbon is reduced. At the end of the depolymerization process the rubber
hydrocarbon is substantially a mixture of oils and carbon black, where the
oils
generally have average molecular weights of less than about 500. The solid
residue
CA 02441713 2003-09-18
at the end of the depolymerization process i.e. at a point 310 is
substantially carbon
black. The variance between the points 310 and 314 represents the formation of
char in the pyrolysis process.
A flow chart of a reduction process according to an embodiment of the
invention is presented in Figure 4. Rubber feedstock is provided to a reactor
suitable
for temperatures and pressures appropriate for subcritical treatment with a
solvent at
step 402. The rubber feedstock may be any vulcanized rubber that includes
synthetic rubber. Typical synthetic rubbers include synthetic polyisoprene
rubber,
polybutadiene rubber, styrene-butadiene rubber, isoprene-butadiene rubber,
styrene-
isoprene rubber, styrene-isoprene-butadiene rubber, nitrite rubber, bromobutyl
rubber
and chlorobutyl rubber. The above list of rubbers is not meant to be limiting.
Other
appropriate rubbers will, upon consideration of the above list, be apparent to
one of
skill in the art. In the current exemplary embodiment the rubber feedstock is
used
automobile tires.
In the current exemplary embodiment the automobile tires used as rubber
feedstock are shredded. The shredding process produces pieces or rubber with
anisotropic dimensions. The pieces are between about 1 and about 4 mm thick
and
had a surface area of between about 0.5 and about 5 cm2. The pieces are more
typically between about 1 and about 2 mm thick and have a surface area of
between
about 1 and about 2 cm2. While the rubber feedstock has been shredded in the
above embodiment the invention is by no means limited to a particular size of
rubber
feedstock. For example, the method of the invention can also be applied to
whole
tires. Thus, the method of the invention is independent of the size of rubber
feedstock.
A solvent is provided to the reactor at step 404. In the current exemplary
embodiment the solvent is water. The amount of solvent provided is sufficient
to
provide the saturated solvent vapour pressure at the reaction temperature
while
maintaining some solvent in the reactor. In the current exemplary embodiment
the
solvent within the reactor is sufficient for the tire feedstock to be immersed
in the
solvent at the reaction temperature and remains so for the duration of the
reduction
process. In an alternative embodiment the rubber feedstock is placed in a
basket
such that the rubber is above the level of the solvent for the duration of the
reduction
process.
7
CA 02441713 2003-09-18
The reactor is heated to the reaction temperature, T, at step 406. To maintain
a subcritical reaction the reaction temperature must be less than the critical
temperature of the solvent. In the current exemplary embodiment the
temperature is
less than 374°C. In an embodiment of the invention the reaction
temperature is
preferably between about 260°C and about 370°C. The reaction
temperature is more
preferably between about 290°C and about 320°C.
Prior to attaining the desired reaction temperature air present in the reactor
is
purged. In the current exemplary embodiment the air present in the reactor is
purged
with steam generated during the heating step. Other appropriate means for
purging
air from the reactor, including purging with an inert gas, will be apparent to
those of
skill in the art. Once the air has been purged the reactor is sealed from the
external
environment.
A pressure is provided to the reactor at step 408. This pressure is equal to
or
greater than the saturated solvent vapour pressure at the reaction
temperature. In
the current exemplary embodiment the reaction pressure is the saturated water
vapour pressure at the reaction temperature. This pressure is solely created
by
steam present in the reactor. For example, for a reaction temperature of
300°C the
pressure is 1230 psig. In an alternative embodiment pressures greater than the
saturated solvent vapour pressure are provided by the application of an inert
gas to
the reaction chamber. Inert gases appropriate for this pressurization of the
reactor
include, but are not limited to, nitrogen and argon. Other means of providing
pressures above the saturated water vapour pressure will be apparent to those
of
skill in the art. The use of means to provide a pressure that are independent
of the
generation of vapour allows control of the fraction of vapour present in the
reactor.
The reaction is continued at step 410 for time t to produce a reaction
product.
The reaction product produced according to the method of the current exemplary
embodiment of the invention includes rubber hydrocarbon, carbon black and
sulphur.
The reaction product may also include, depending on the exact reaction
parameters,
oil from at least partial depolymerization of the rubber hydrocarbon, and
vulcanized
rubber feedstock. The reaction product can generally be divided into a solid
and
liquid phase. The solid phase contains the rubber hydrocarbon, carbon black
and
any vulcanized rubber. The liquid phase contains any oil that is produced. The
liquid
phase will be present as a slurry with the water. While not being limited by
theory it
8
CA 02441713 2003-09-18
is believed sulphur is dissolved in the water during the reaction. The sulphur
then
precipitates from the water upon cooling and is deposited on the solid phase.
A flow chart of a reduction process according to another embodiment of the
invention is presented in Figure 5. The process includes the steps presented
in
Figure 4. The process according to this embodiment of the invention further
includes
the washing and drying the solid phase at step 501. In this embodiment the
solid
phase is washed with water. At step 502 the solid phase of the reaction
product is
treated with a solvent. In this embodiment this solvent is cyclohexane. The
use of
other solvents appropriate for the dissolution of rubber hydrocarbon,
including
toluene, is also within the scope of the invention. The use of other solvents
and
mixtures of solvents as appropriate for the dissolution of the rubber
hydrocarbon,
which may include hydrocarbons of varying molecular weight will be apparent to
one
skilled in the art. Carbon black is then separated at step 504 using
filtration methods.
It will be apparent to one skilled in the art that any appropriate techniques
may be
implemented for the separation of the carbon black form the dissolved reaction
product. It will also be apparent that step 504 may be omitted for those
rubber
feedstocks that do not include carbon black.
A flow chart of a reduction process according to a further embodiment of the
invention is presented in Figure 6. The process includes the steps presented
in
Figure 5. The process according to this embodiment of this invention further
includes
the step of separating the solvent from the rubber hydrocarbon. Techniques
appropriate for separating the solvent from the rubber hydrocarbon will be
apparent
to one skilled in the art. In this embodiment evaporation is used to separate
the
solvent from the rubber hydrocarbon.
The embodiments of the invention as outlined above are applicable to and
appropriate for batch, semi-continuous and continuous processes.
While the currently preferred solvent is water a mixture of another solvent or
solvents with water is also encompassed. In an alternative embodiment of the
invention the extraction solvent is a mixture of solvents, where the mixture
includes
water such that the water plays a role in the extraction/ devulcanization
process. For
example, mixtures of solvents that included 10 weight percent and 30 weight
percent
ethanol in water were used in reduction processes conducted at temperatures of
280°C, and 290°C and above. It was found that the %
devulcanization was
9
CA 02441713 2003-09-18
approximately the same when using the mixed solvent compared to 100% water. It
is contemplated that alcohols and organic solvents are applicable for mixture
with
water to form the extraction solvent.
The invention is illustrated by the following examples that are merely for the
purpose of illustration and are not to be regarded as limiting the scope of
the
invention or the manner in which it can be practiced.
Examples 1-14
In all of the examples the rubber feedstock was from used automobile tires.
The tires were processed to remove any steel and fibre belts contained
therein. The
rubber was shredded to produce chips or shavings having an irregular shape.
The
chips and shavings were generally between about 1 and about 4 mm thick and had
a
surface area of between about 0.5 to about 5 cm2. The majority of chips and
shavings were between about 1 and about 2 mm thick and had a surface area of
between about 1 to about 2 cm2.
A reactor capable of operating at temperatures of up to 500°C and
pressures
of up to 5000 psi was used in all experiments. The reactor was charged with
between about 20 and about 30 g of rubber and about 100 ml of water. In most
experiments the rubber sample was immersed in water in the reactor. In some
experiments the rubber sample was held in a perforated basket above the water.
Heating was initiated with a valve on the reactor remaining open. This valve
was
closed after the vessel had reached a temperature greater than 100°C.
This allowed
for the purging of air from the reactor with steam generated during heating of
the
reactor. The temperature was raised to the desired reaction temperature and
the
reaction continued for either one or five hours. At the end of the desired
reaction
time the reactor was cooled to room temperature and the contents removed.
The nature and relative amount of reaction products present at the end of a
reaction are a function of the particular reaction conditions. The reaction
products
may include vulcanized rubber, rubber hydrocarbon, oil and carbon black. The
vulcanized rubber, rubber hydrocarbon and carbon black were present in a solid
phase while the oil was present in a liquid phase. The solid phase was washed
with
water and dried to constant weight in an oven at 100°C. This washing
step removed
any sulphur that was on the reaction product.
CA 02441713 2003-09-18
The yield of the rubber reduction process of examples 1-14 is presented in
Table 1. The yield (%) is defined by the following expression
S Yield(%) _ ~ mass of solid phase ~ * 100
mass of rubber feedstock
The yield as calculated above will decrease with depolymerization. It is
apparent
from Table 1 that depolymerization starts to occur at around 320°C.
Example 7 was
run with the rubber feedstock suspended in the vapour. The extent of
depolymerization increased with increases in the reaction temperature and with
increases in the reaction time.
The rubber hydrocarbon of examples 6, 8 and 9 was analysed for sulphur
content. The sulphur content of the rubber hydrocarbon was 0.19, 0.18 and
0.22%
for examples 6, 8 and 9, respectively. Therefore, the measured sulphur content
is
lower than that of the rubber feedstock indicating that sulphur is extracted
from the
rubber.
Example TEMPERATURE DURATION YIELD
(C) (hours) (%)
1 260 1 100
2 270 1 100
3 280 1 100
4 290 1 100
5 290 5 100
6 300 1 100
7 300 1 100
8 320 1 95
9 320 5 86
10 330 5 78
11 340 5 63
12 350 5 50 I
13 360 5 45 ',
14 370 5 44
11
CA 02441713 2003-09-18
Table 1 Percent devulcanization as a function of reaction time and temperature
A multi-step separation process was used to separate the various
components of the solid phase. The solid phase was mixed with cyclohexane,
heated to and held at 60°C and stirred for up to 1 hour. During this
process the
rubber hydrocarbon dissolved in the cyclohexane. The mixture was passed
through
a wire mesh strainer to remove any vulcanized rubber, which would not be
dissolved
by the cyclohexane. Any vulcanized rubber that was removed during this
filtering
step was weighed and the mass compared to the initial mass of rubber. The
fraction
of the sample that was devulcanized was calculated as a percentage. The
results
are presented in Table 2.
It is apparent from Table 2 that there is a change in the nature of
devulcanization between about 280°C and about 290°C. At reaction
temperatures
below 290°C there is minimal devulcanization occurring. Between 260 and
280°C
rubber hydrocarbon and carbon black were removed from the surface of the
pieces
of rubber. The remainder of the pieces of rubber remained elastic. Thus the
pieces
experienced surface devulcanization. When reacted at 290°C for one hour
there was
31 % devulcanization indicating bulk devulcanization is occurring. When either
the
reaction time is increased to five hours at a reaction temperature of
290°C (Example
5) or the reaction temperature is raised to 300°C (Example 6) or above
the sample
underwent complete bulk devulcanization. Thus there is a transition from
surface to
bulk devulcanization.
Example TEMPERATURE DURATION DEVULCANIZATION
(C) (hours) (%)
1 260 1 <1
2 270 1 <1
3 280 1 3
4 290 1 31
5 290 5 100
6 300 1 100
7 300 1 100
8 320 1 100
9 320 5 100
10 330 5 100
12
CA 02441713 2003-09-18
Table 2 Percent devulcanization as a function of reaction time and temperature
The mixture that passed through the strainer was passed through filter paper
to separate out the carbon black. The extracted carbon black was characterized
by
measuring its surface area. Nitrogen adsorption on the carbon black was
measured
at 77°K and a BET equation was used to quote a surface area. A surface
area of
60m2/g measured for the carbon black of Example 8. The extracted carbon black
approaches that used in the manufacture of tires.
Finally, the cyclohexane was evaporated to leave the rubber hydrocarbon.
The molecular weight of the rubber hydrocarbon was determined using gel
permeation chromatography. The weight average molecular weight of the rubber
hydrocarbon is presented in Table 3. There is minimal depolymerization
occurring
until a reaction temperature of 320°C.
Example TEMPERATURE DURATION MOLECULAR WEIGHT
(C) (hours) (weight average)
4 290 1 98,000
6 300 1 99,000
7 300 1 102,200
8 320 1 43,600
10 330 5 11,900
Table 3 Weight average molecular weight as a function of reaction time and
temperature
The method according to the various embodiments of the invention allow for
various end products. First, end products could be obtained directly from the
reactor
without subsequent separation. For reaction conditions that produce complete
devulcanization with minimal depolymerization the reaction product will have a
carbon black to rubber hydrocarbon ratio that is approximately equal to that
of the
original rubber. Thus the reaction product can be used as an extender for
rubber
formulations. Second, the end product could be rubber hydrocarbon extracted
from
the reaction product and has had its carbon black removed. It is further
possible to
set the reaction conditions to tailor the molecular weight of the rubber
hydrocarbon.
13
CA 02441713 2003-09-18
Third, the carbon black that has been isolated from the rubber hydrocarbon can
be
isolated as an end product. One can also provide a mixture, of a specified
ratio of
the carbon black and rubber hydrocarbon. For example, carbon black with 10-20%
rubber hydrocarbon could be used to facilitate the mixing of carbon black in
rubber
formulations. Finally, a low temperature reaction could be used to produce
surface
modified crumb rubber.
It will be apparent to one skilled in the art that tires can include any
vulcanized
rubber tire as may be used for transportation purposes. It will also be
apparent to
one of skill in the art that other vulcanized rubber products that include
synthetic
rubber may be used as a feedstock.
It will also be apparent to one of skill in the art that the processing
conditions
presented above are with respect to one embodiment of the invention. The
invention
encompasses those processing parameters i.e. temperature, pressure, time that
allow for subcritical processing of rubber with a solvent that includes water.
While the invention has been described according to what is presently
considered to be the most practical and preferred embodiments, it must be
understood that the invention is not limited to the disclosed embodiments.
Those
ordinarily skilled in the art will understand that various modifications and
equivalent
structures and functions may be made without departing from the spirit and
scope of
the invention as defined in the claims. Therefore, the invention as defined in
the
claims must be accorded the broadest possible interpretation so as to
encompass all
such modifications and equivalent structures and functions.
Variations in the present invention are possible in light of the description
of it
provided herein. While certain representative embodiments and details have
been
shown for the purpose of illustrating the invention, it will be apparent to
one skilled in
the art that various changes and modifications can be made therein without
departing
from the scope of the invention.
14