Note: Descriptions are shown in the official language in which they were submitted.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
1
A MICROFLUIDIC SYSTEM (MS)
TECHNICAL FIELD
The present invention relates to a microfluidic device, which can be
interfaced
to a mass spectrometer (MS). The device comprises a microchannel structure
having a first port (inlet port) and a second port (outlet port). A sample to
be
analysed is applied to the first port and presented to the mass spectrometer
in
the second port. This second port will be called an MS-port. There may be
additional inlet and outlet ports. During passage through the microchannel
structure the sample is prepared to make it suitable for analysis by mass
spectrometry.
The sample presented in an MS-port will be called an MS-sample. An analyte
in an MS-sample is an MS-analyte. "Sample" and "analyte" without prefix will
primarily refer to a sample applied to an inlet port.
Conductive and non-conductive properties are with respect to conducting
electricity.
The invention concerns mass spectrometry in which the MS-samples are
subjected to Energy Desorption/lonisation from a surface by input of energy
(EDI MS). Generically this kind of process will be called EDI and the surface
an EDI-surface in the context of the invention. Typicallly EDIs are thermal
desorption/ionisation (TDI), plasma desorption/ionisation (PDI) and various
kinds of irradiation desorption/ionisation (1D1) such as by fast atom
bombardment (FAB), electron impact etc. In the case a laser is used the
principle is called laser desorption/ionisation (LDI). Desorption may be
assisted by presenting the MS analyte together with various helper
substances or functional groups on the surface. Common names are matrix
assisted laser desorption/ionisation (MALDI) including surface-enhanced laser
desorption/ionisation (SELDI). For MALDI see the publications discussed
under Background Publications below. For SELDI see WO 0067293
(Ciphergen Biosystems).
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
2
The invention also concerns electron spray ionisation mass spectrometry (ESI
MS).
The term "EDI-area" comprises the EDI-surface as such and the part of a
substrate covered by this surface, e.g. the part of the substrate that is
under
the EDI-surface. Compare the description of figure 5.
The term "microformat" means that in at least a part of a microchannel
structure the depth and/or width is in the microformat range, i.e. < 103 Vim,
preferably < 102 Vim. The depth and/or width are within these ranges
essentially everywhere between an inlet port and an outlet port, e.g. between
a sample inlet port and an MS-port. The term "microchannel structures"
includes that the channels are enclosed in a substrate.
The term "microfluidic device" means that transport of liquids and various
reagents including analytes are transported between different parts within the
microchannel structures by a liquid flow.
BACKGROUND PUBLICATIONS.
For some time there has been a demand for microfluidic sample handling and
preparation devices with integrated MS-ports. This kind of devices would
facilitate automation and parallel experiments, reduce loss of analyte,
increase reproducility and speed etc.
~ WO 9704297 (Karger et al) describes a microfluidic device that has an
outlet port that is claimed useful when conducting electrospray ionisation
mass spectrometry (ESI MS), atmospheric pressure chemical ionisation
mass spectrometry (APCI MS), matrix assisted laser desorption/ionisation
mass spectrometry (MALDI MS) and a number of other analytical
principles.
~ US 6,110,343 (Ramsey et al) describe an electrospray interface between
a microfluidic device and a mass spectrometer.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
3
~ US 5,969,353 (Hsieh) describes an improved interface for electrospray
ionization mass spectrometry. The interface is in the form of an
electrospray tip connected to a microchannel structure of a chip.
~ US 5,197,185 (Yeung et al) describes a laser-induced vaporisation and
ionization interface for directly coupling a microscale liquid based
separation process to a mass spectrometer. A light-adsorbing component
may be included in the eluting liquid in order to facilitate vaporisation.
~ US 5,705,813 (Apffel et al) and US 5,716,825 (Hancock et al) describe a
microfluidic chip containing an MS-port. After processing a sample within
the chip the sample will appear in the MS-port. The whole chip is then
placed in an MALDI-TOF MS apparatus. The microfluidic device
comprises
(a) an open ionisation surface that may be used as the probe surface in
the vaccum gate of an MALDI-TOF MS apparatus (column 6, lines 53
58 of US 5,705,813) or
(b) a pure capture/reaction surface from which the MS-analyte can be
transferred to a proper probe surface for MALDI-TOF MS (column 12,
lines 13-34, of US 5,716,825).
These publications suggest that means for transporting the liquid within a
microchannel structure of the device are integrated with or connected to the
device. The means given are electrical connections, pumps etc. These kinds
of transporting means impose an extra complexity on the design and use,
which in turn may negatively influence the production costs, easiness of
handling etc of these devices.
Although both US 5,705,813 (Apffel et al) and US 5,716,825 (Hancock et al)
explicitly concern microfluidic devices, they are scarce about
~ the proper fluidics around the MALDI ionisation surface,
~ the proper crystallisation on the MALDI ionisation surface,
the proper geometry of the port in relation to crystallisation, evaporation,
the incident laser beam etc,
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
4
~ the conductive connections to the MALDI ionisation surface for MALDI MS
analysis.
These features are important in order to manage with interfacing a
microfluidic deuce to an MALDI mass spectrometer.
WO 9704297 (Karger et al) and PCT/SE01/02753 (Gyros AB) suggest a
radial or spoke arrangement of the microchannel structures of a microfluidic
device.
WO 9721090 (Mian et al) (page 30, lines 3-4, and page 51, line 10) and WO
0050172 (Burd Mehta) (page 55, line 14) suggest in general terms that their
microfluidic systems might be used for preparing samples that are to be
analysed by mass spectrometry. WO 9721090 is explicitly related to a system
in which centrifugal force is used for driving the liquid flow.
A number of publications referring to the use of centrifugal force for moving
liquids within microfluidic systems have appeared during the last years. See
for instance WO 9721090 (camera Bioscience), WO 9807019 (camera
Bioscience) WO 9853311 (camera Bioscience), WO 9955827 (Gyros AB),
WO 9958245 (Gyros AB), WO 0025921 (Gyros AB), WO 0040750 (Gyros
AB), WO 0056808 (Gyros AB), WO 0062042 (Gyros AB), WO 0102737
(Gyros AB), WO 0146465 (Gyros AB), WO 0147637, (Gyros AB), WO
0154810 (Gyros AB), WO 0147638 (Gyros AB),
US S.N. 60/315,471 and the corresponding International Patent Application
discuss various designs of microfluidic functions, some of which can be
applied to the present invention.
See also Zhang et al. "Microfabricated devices for capillary electrophoresis
electrospray mass spectrometry", Anal. Chem. 71 (1999) 3258-3264 and
references cited therein.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
Kido et al., ("Disc-based immunoassay microarrays", Anal. Chim. Acta 411
(2000) 1-11 ) has described microspot immunoassays on a compact disc
(CD). The authors suggest that a CD could be used as a continuous sample
collector for microbore HPLC and subsequent detection for instance by
5 MALDI MS. In a preliminary experiment a piece of a CD manufactured in
polycarbonate was covered with gold and spotted with a mixture of peptides
and MALDI matrix.
In an International Type Search Report compiled for the priority application
US 6,191,418 (Hinsgaul et al), US 4,279,862 (Bretaudiere et al), and US
5,869,830 (Franzen et al) have labelled X/Y. None of these publications
concerns problems associated with microfluidic devices and their interfacing
with mass spectrometers. US 6,191,418 (Hinsgaul et al) describes a circular
arrangement of electrospray tips that can be interfaced one by one to an MS
apparatus by rotating the arrangement. The tips are connected to
chromatographic columns through which a liquid flow is applied by external
means. US 4,279,862 (Bretaudiere et al) describes a circular disc comprising
an outwardly directed flow system comprising (a) a unit in which mixing is
caused by creating turbulence in a flow which is driven by centrifugal force,
and (b) an ending measuring chamber. US 5,869,830 (Franzen et al)
decribes exact mass determination of MS-analytes that are presented in a
conventional way in an MALDI MS apparatus together with a reference
compound.
OBJECTS OF THE INVENTION.
~ A first object is to provide improved means and methods for transporting
samples, analytes including fragments and derivatives, reagents etc in
microfluidic devices that are capable of being interfaced with a mass
spectrometer.
~ A second object is to provide improved microfluidic methods and means
for sample handling before presentation of a sample analyte as an MS-
analyte. Sub-objects are to provide an efficient concentration, purification
and/or transformation of a sample within the microfluidic device while
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
6
maintaining a reproducible yield/recovery, and/or minimal loss of precious
material.
~ A third object is to provide improved microfluidic methods and means that
will enable efficient and improved presentation of an MS-sample/MS
analyte. This object in particular applies to MS-samples that are presented
on an EDI-surface, or via electrospray ionisation (ESI-tips).
~ A fourth object is to enable reproducible mass values from an MS-sample
that is presented on an EDI-surface that is present on a microfluidic device
in which a liquid flow is caused by inertia force..
~ A fifth object is to provide improved microfluidic means and methods for
parallel sample treatment before presentation of the MS-analyte to mass
spectrometry. The improvements of this object refer to features such as
accuracy in concentrating, in chemical transformation, in required time for
individual steps and for the total treatment protocol etc. By parallel sample
treatment is meant that two or more sample treatments are run in parallel
in different microchannel structures within the same microfluidic device.
The number of parallel runs may be more than five, such as more than 10,
50, 80, 100, 200, 300 or 400 runs. Particular important numbers of parallel
samples are below or equal to the standard number of wells in microtiter
plates, e.g. 96 or less, 384 or less, 1536 or less, etc
~ A sixth object is to provide a cheap and disposable microfluidic device unit
enabling parallel sample treatments and having one or more MS-ports that
are adapted to a mass spectrometer.
SUMMARY OF THE INVENTION.
The present inventors have recognized that several of the above-mentioned
objects can be met in the case inertia force is used for transportation of a
liquid within a microfluidic device as defined in this specification. This is
applicable to liquid, such as washing liquids and liquids containing at least
one of (a) the analyte including derivatives and fragments thereof, (b) a
reagent used in the transformation of the sample/analyte, etc.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
7
The present inventors have also recognized that the optimisation of an EDI-
area in a microfluidic device is related to
(a) the design and/or positioning of a conductive layer in the EDI-area,
and/or
(b) the need of a calibrator area associated with an EDI MS-port, and/or
(c) the need of a proper conductive connection to the EDI-area for MS
analysis.
The proper conductive connection will support the proper voltage and/or
charge transport at the EDI-area, for instance. Improper conductive properties
may negatively affect the mass accuracy, sensitivity, resolution etc. The
importance of (a)-(c) increases if there is a plurality of microchannel
structures in the microfluidic device.
DETAILED DESCRIPTION OF THE INVENTION
The first aspect of the invention is a method for presenting an analyte of a
liquid sample as an MS-analyte to a mass spectrometer. The method is
characterized in
(a) comprising the steps of:
(i) applying the liquid sample to a sample inlet port (I) of a microchannel
structure (I) of a microfluidic device, said structure also comprising an
MS-port,
(ii) transporting the analyte by a liquid flow in microchannel structure (I)
thereby transforming the analyte to an MS-analyte, and
(iii) presenting the MS-analyte to a mass spectrometer via the MS-port,
and
(b) using inertia force for driving said liquid flow within at least a part of
microchannel structure (I).
A second aspect of the invention is a microfluidic disc which is characterized
in comprising:
(a) an axis of symmetry perpendicular to the plane of the disc,
(b) a microchannel structure (I) oriented radially with a liquid flow
direction
from an inner inlet port towards the periphery of the disc and comprising
an MS-port and a sample inlet port (I)
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
8
This aspect comprise that the MS-port may be an ESI MS-port or an EDI-MS-
port. In case of EDI MS-ports.
The method aspect may also include the mass spectrometric method applied,
i.e. the innovative methods may also include the actual collection of a mass
spectrum and analysis thereof, for instance in order to gain molecular weight
and structure information about an analyte.
The various innovative embodiments of the aspects of the invention are
further defined as discussed below.
Liquid flow
The liquid flow used for transport of analyte, analyte-derived entities,
reagents
etc within the microchannel structures may be driven by electrokinetic forces
and/or by non-electrokinetic forces. Typical non-electrokinetic forces are
inertia force, such as centrifugal force, capillary forces, forces created by
pressure differences etc. For microfluidic devices having circular forms as
discussed in this specification it is preferred to drive a liquid flow by
spinning
the device, i.e. by centrifugal force, in at least a part of each microchannel
structures, for instance for the transport into the MS-port. The term "forces
created by pressure differences" includes hydrostatic pressure created within
certain kinds of microchannel structures by the combined action of spinning
and application of a series of liquid aliquots (see below and WO 0146465
(Gyros AB)).
At the priority date the most important inertia force to be used in the
innovative device and method is centrifugal force, i.e. spinning of the device
in order to accomplish an outward transportation of liquid towards the
periphery of the disc. The spinning axis coincides with the axis of symmetry
of
the disc.
Inertia force, such as centrifugal force, may be combined with one or more
other kinds of driving forces. The combination may be in the same part of a
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
9
microchannel structure. The combination may also mean that inertia forcein
form of centrifugal force is utilized for transport in a part where the flow
shall
be directed outwards towards the periphery of a circular disc and other forces
in some other part for creating a flow inwards or more or less parallel to the
periphery of a disc.
Capillary force may typically be used to transport a liquid aliquot from an
inlet
port into a microchannel associated with the inlet port. This kind of
rriicrochannels may be directed inwards towards the center of a disc or more
or less perpendicular thereto.
It may be beneficial to include a pulse giving increased flow for over-coming
inter-channel variations in flow resistance, in particular when initiating
flow
and/or when the liquid is to pass through branchings and curvatures.
The sample and its processing.
The sample applied to an inlet port may contain one or more analytes, which
may comprise lipid, carbohydrate, nucleic acid and/or peptide structure or any
other organic structure. The analyte may also comprise an inorganic
structure. The sample treatment protocol to take place within the
microchannel structure typically means that the sample is transformed to one
or more MS-samples in which
(a) the MS-analyte is a derivative of the starting analyte and/or
(b) the amounts) of non-analyte species have been changed compared to
the starting sample, and/or
(c) the relative occurrence of different MS-analytes in a sample is changed
compared to the starting sample, and/or
(d) the concentration of an MS-analyte is changed relative the corresponding
starting analyte in the starting sample, and/or
(e) sample constituents, such as solvents, have been changed and/or the
analyte has been changed from a dissolved form to a solid form, for
instance in a co-crystallised form..
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
Item (a) includes digestion into fragments of various sizes and/or chemical
derivatization of an analyte. Digestion may be purely chemical or enzymatic.
Derivatization includes so-called mass tagging of either the starting analyte
or
of a fragment or other derivative formed during a sample treatment protocol,
5 which takes place in the microchannel structure. Items (b) and/or (c)
include
that the sample analyte has been purified and/or concentrated. Items (a)-(d),
in particular, apply to analytes that are biopolymers comprising carbohydrate,
nucleic acid and/or peptide structure.
10 The sample is typically in liquid form and may be aqueous.
The sample may also pass through a microchannel structure without being
changed. In this case the processing within a microchannel structure only
provides a form for dosing of the analyte to the mass spectrometer.
FIGURES.
Figure 1-3 illustrate various microchannel structures that have an MS-port.
Figure 4 illustrates an MS-port in form of an electrospray (sideview).
Figures 5a-f illustrate various designs and positions of the conductive layer
(I) in MS-ports containing an EDI-surface (cross-sectional sideview of two MS-
ports). .
Figure 6 illustrates an arrangement around EDI MS-ports with layer (I) and
conductive connections (transparent lid, seen from above).
Figures 7a-b illustrate a variant of an EDI-port with a transparent lid (seen
from above and in a cross-sectional side-view, respectively.
Figures 8a-b illustrate a variant of microchannel structures suitable to be
interfaced with MALDI MS and an optimal arrangement on a full circular
microfluidic disc (CD).
The microchannel structures represented in figures 1-8 are present in planar
microfluidic devices.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
11
THE MICROFLUIDIC DEVICE.
The microfluidic device comprises one or more microchannel structures
having an inlet port for application of a liquid sample and an MS-port for
release and presentation of an MS-analyte to a mass spectrometer. These
kinds of ports may coincide in a microchannel structure. There may also be
separate inlet ports for application of solvents and reagents and separate
outlet ports or waste chambers/cavities for withdrawal of other components
that are added and/or produced in the structure. Two or more microchannel
structures may have a common inlet port. Depending on the particular design
of the device some of the ports may be closed during the sample treatment
but opened later on, for instance in order to enable proper release and
presentation of the MS-analyte.
The distance between two opposite walls in a microchannel is typically <_ 1000
Vim, such as s 100 wm, or even <_ 10 Vim, such as < 1 Vim. Functional channel
parts (chambers, cavities etc) typical have volumes that are <_ 500 p1, such
as
<_ 100 ~I and even <_ 10 ~,I such as _< 1 ~I. In important variants these
volumes
may be < 500 n1 such as <_ 100 n1 or <_ 50 n1.. The depths of these parts may
be in the interval <_ 1000 pm such as <_ 100 ~,m such as _< 10 ~.m or even 5 1
Vim. The lower limits (width and depth) are always significantly greater than
the largest of the reagents and analytes (including fragments and derivatives)
that are to be transported within the microchannel structure. The lower limits
of the different channel parts are typically in the range 0.1-0.01 Vim. The
aspect ratio (depth to width) may be >_ 1 or <_ 1 in all parts or in only a
part of a
microchannel structure.
Preferred microfluidic devices typically comprise one, two or more, preferably
more than 5, microchannel structures. In the preferred variants, the device is
formed by covering a substrate surface exposing parts of the microchannel
structures with a lid comprising the remaining parts, if any, of the
microchannel structures. The lid will prevent or minimise undesired
evaporation of liquids as well as facilitate transport of liquids.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
12
A microchannel structure preferably extends in a plane that is common for
several microchannel structures. In addition there may be other
microchannels that extend in other directions, primarily perpendicular to the
common plane. Such other microchannels may function as sample or liquid
application areas or connections to microchannel structures that are not
located in the common plane, for instance.
The microfluidic devices may be disc-formed and have various geometries,
with the circular form being the preferred variant (CD-form). Other variants
of
discs like the circular form may have an axis of symmetry that is at least 3-
or
at least 6-numbered. Circular forms typically have radii (r) >_ 10% or <_ 300
of the radii of a conventional CD with the conventional CD-format being the
preferred.
On devices having circular forms or other forms having an axis of symmetry,
an MS-port typically is located at a larger radial distance from the axis of
symmetry than an inlet port, a common distribution system/channel etc of a
microchannel structure. In case there are more than one inlet ports they may
be placed at different radial distances from the axis of symmetry. The flow
direction for each microchannel structure is from an inner application area
(inlet port, common distribution system or channel etc) towards an outlet
port,
typically an MS-port, at the periphery of the disc. The microchannel
structures
may be arranged in the form of one or more concentric circles
(annular/circular arrangements) around the axis of symmetry of a disc. The
MS-ports in each circle are at the same radial distance from the axis of
symmetry.
By the term "radially directed microchannel structure" means that the
microchannel structure has an inlet port or a common distribution unit that is
closer to the spinning axis (axis of symmetry) than an outlet port, typically
the
MS-Port. The term does not take into account the design or direction of part
structures.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
13
Each microchannel structure may comprise parts that differ with respect to
function. In addition to the inlet ports, MS-ports, transportation
conduits/channels there may be one or more parts that function as
(a) application zone/port for reagents and liquids other than sample liquid
S (second inlet port),
(b) additional MS-ports,
(c) reaction zone, for instance for derivatization of an analyte discussed
above (digestion, tagging etc).
(d) pressure creating zone (for instance hydrostatic pressure),
(e) volume defining zone,
(f) mixing zone,
(g) zone for separating and/or concentrating and/or purifying the analyte or a
derivative or fragment thereof, for instance by capillary electrophoresis,
chromatography and the like,
(h) waste conduit/chamber/ cavity (for instance in the form of an outlet
port),
(i) zone for splitting a liquid flow, etc.
Each of these parts may have the same or different cross-sectional
dimensions as a preceding and/or a subsequent part of the microchannel
structure.
The sizes of the various parts (a)-(i) depend on number of factors, such as
the sample, reagents used, washing, process protocol, desired sensitivity,
type of mass spectrometer etc. Typical sizes are found in the range of 1 n1 to
1000 ~I, mostly below 1 ~I such as below 500 n1 or even below 100 n1 such as
below 25 or 10 n1 (volume defining unit, reactor part, separation part etc).
Repeated application of a liquid, e.g. a sample, a washing liquid, a
desorption
liquid etc to the same inlet port may replace the need for a larger volume
defining unit.
Splitting of a liquid flow may be located to an upstream part and associated
with the inlet so that a starting sample is divided in several aliquots, each
of
which is then processed in parallel within the device of the invention.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
14
Except for the presence of an MS-port, useful microchannel structures have
been described in a number of previous patent publications. See the
background publications discussed above.
Between parts having different functions there may be valves that can be
overcome by increasing the force driving the liquid flow. For variants
utilizing
spinning, this may for instance be accomplished by increasing the spinning
and/or utilizing pressure built up within the structure due to addition of a
new
portion of liquid combined with spinning. See for instance WO 0040750
(Gyros AB) and WO 0146465 (Gyros AB). Valves may be based on capillary
junctions (WO 9807019 (camera Bioscience)) or hydrophobic breaks (WO
9958245 (Gyros AB), WO 0185602 (Gyros AB & Amic AB) or on. thermic
properties of the valve material. The latter kind of valves may be illustrated
by
so called sacrificing valves (WO 9853311 (camera Bioscience)) for instance
containing a plug of wax-like material, or reversible valves, for instance
containing a thermoreversible polymer in the form of a plug (WO 0102737
(Gyros AB)).
One kind of microchannel structures used according to the invention
comprises a zone in which separation and/or concentration and/or purification
of the analyte or an analyte-derived entity can take place. This zone is
located
either before or in the MS-port. Examples of analyte-derived entities are
fragments and derivatives of the analyte. This kind of functionality may be
particularly important for samples containing low concentrations of analytes,
complex mixtures of analytes or high concentrations of interfering substances
that may negatively affect the resolution and/or sensitivity of the MS-
analysis.
The principles utilized for separation, concentration, purification,
derivatization, fragmentation etc in the invention are similar to those that
are
used in the life science area, e.g. separations based on size exclusion and/or
on differences in binding to a ligand structure are applicable. Accordingly, a
separation zone may contain a separation medium that is capable of binding
the analyte or an analyte-derived entity but not the contaminants, or vice
versa. The separation medium is typically in particle/bead form, the surface
of
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
the separation zone, or a monolithic plug (porous) that permits through flow.
If
the analyte or the analyte-derived entity becomes bound, a liquid having the
proper desorption characteristics for the bound entity is subsequently allowed
to pass through the zone whereupon the bound entity is released and
5 transported downstream. This transport may be directly to the MS-port or to
a
zone in which a further preparation step is accomplished. Washing steps may
be inserted between the sample liquid and the desorption liquid. The
separation medium may be soluble or insoluble during the binding step.
Soluble separation media are typically insolubilized after binding a desired
10 substance. The principles are well known in the field of macroscopic
separations.
Binding as discussed in the preceding paragraph typically means affinity
binding or covalent binding to the separation medium. Covalent binding is
15 typically reversible, for instance by thiol-disulfide exchange. Affinity
binding ( _
affinity adsorption) can be illustrated with:
(a) electrostatic interaction that typically requires that the ligand and the
entity
to be bound have opposite charges,
(b) hydrophobic interaction that typically requires that the ligand and the
entity
to be bound comprises hydrophobic groups,
(c) electron-donor acceptor interaction that typically requires that the
ligand
and the entity to be bound have an electron-acceptor and electron-donor
group, respectively, or vice versa, and
(d) bioaffinity binding in which the interaction is of complex nature,
typically
involving a mixture of different kinds of interactions and/or groups.
Ion exchange ligands may be cationic (= anion exchange ligands) or anionic
(= cation exchange ligands). Typical anion exchange ligands have positively
charged nitrogen, the most common ones being primary, secondary, tertiary
or quarternary ammonium ligands, and certain amidinium groups. Typical
cation exchange ligands have negatively charged carboxylate groups,
phosphate groups, phosphonate groups, sulphate groups and sulphonate
groups.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
16
Bioaffinity binding includes that the analyte or the analyte-derived entity is
a
member of a so-called bioaffinity pair and the ligand is the other member of
the pair. Typical bioaffinity pairs are antigen/hapten and an antibody/antigen
binding fragment of the antibody; complementary nucleic acids;
immunoglobulin-binding protein and immunoglobulin (for instance IgG or an
Fc-part thereof and protein A or G), lectin and the corresponding
carbohydrate, etc. The term "bioaffinity pair" includes affinity pairs in
which
one or both of the members are synthetic, for instance mimicking a native
member of a bioaffinity pair.
If the analyte in a sample has peptide structure or nucleic acid structure or
in
other ways has a pronounced hydrophobicity, the separation medium may be
of the reverse phase type (hydrophobic) combined with using desorption
liquids (eluents) that are organic, for instance acetonitrile, isopropanol,
methanol, and the like. Depending on the particular sample and the presence
of analytes or analyte-derived entities, which have a common binding
structure, a group-specific separation medium may be utilized. The separation
medium may thus, like a reverse phase adsorbent, result in an MS-sample
that has a reduced concentration of salt, i.e. in desalting.
In each microchannel structure there may be two or more separation zones
utilizing the same or different principles such as size and charge. For
amphoteric substances such as proteins and peptides the latter principle may
be illustrated with isoelectric focusing.
After a separation step comprising binding to a separation medium the
concentration of an analyte or an analyte-derived entity in the desorption
liquid after passage of the separation medium is typically higher than in the
starting sample. The increase may be with a factor > 10°, for instance
in the
interval 10~-106, such as 10'-104.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
17
As already mentioned a separation zone may be combined with zones for
derivatization including fragmentation. There may also be microchannel
structures that have a derivatization zone but no separation zone.
U.S S.N. 60/322,621 and the corresponding International Application
describes the use of the above-mentioned affinity principles in an assay
without explicitly referring to mass spectrometry.
Figure 1 illustrates a microchannel structure that comprises (a) an inlet port
(1 ) for liquids including the sample liquid, (b) an MS-port (2) comprising
for
instance an EDI-surface, (c) a flow conduit (3) between the inlet port (1 )
and
the MS-port (2). The MS-port may be open or covered. The flow conduit (3)
may have a zone (4) containing an adsorbent for separation/concentration. If
there are several microchannel structures in a device there may be a
common application area/channel with openings for the inlet ports (not
shown). The MS-port may be an EDI MS-port, an eletrospray MS-port.
The structure of figure 1 may be present on a circular disc with the inlet
port
(1 ) closer to the centre than the MS-port (2). If the MS-port is an EDI-MS
port
and liquid is transported through the conduit (3) by spinning the disc, liquid
will leave the MS-port either as drops or by evaporation depending on the
vapour pressure of the liquid and/or the spinning speed. A lower vapour
pressure and an increased spinning speed will promote drop formation while
a higher vapour pressure and a decreased spinning speed will promote
evaporation of the liquid and crystallisation of the MS-analyte in the mS-
port.
A too low spinning speed and a too low vapour pressure will increase the risk
for deposition of material in the conduit (3).
Figure 2 illustrates another variant of a microchannel structure. It has two
inlet ports (5,6) that may be used for application of sample, washing liquid
and desorption liquid. One of the inlet ports (5) is connected to an
application
area/channel (7) that may be common to several microchannel structures in
the same device. This first inlet port (5) is connected to one of the shanks
(8)
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
18
of a U-shaped channel via the application area/channel (7). The other inlet
port (6) is connected to the other shank of the U. In the lower part of the U
there is an exit conduit (9) leading to an MS-port (10). The exit conduit (9)
may comprise a zone (12) containing a separation medium. From the MS-port
(10) there may be a waste channel (13) leading to a waste channel (14) that
may be common for several microchannel structures in the same device.
Conduit (9) may comprise a valve function, for instance in the form of a
hydrophobic break, upstream a possible separation zone (12).
The microchannel structure of figure 2 is also adapted to a circular disc and
driving liquid flow by spinning the disc. The application channel (7) is at a
shorter radial distance from the centre of the disc than waste channel (14).
Figure 3 illustrates a microchannel structure which comprises a separate
sample inlet port (14), an MS-port (15) and therebetween a structure that may
be used for sample preparation. In this variant there is a volume-defining
unit
comprising a metering microcavity (16) between the sample inlet port (14) and
MS-port (15) with an over-flow conduit (17) that ends in a waste chamber
(25a) that may be common for several microchannel structures. At the lower
part of the metering microcavity (16) there is a first exit conduit (18)
leading to
one of the shanks (19) of a U-shaped channel. The other shank (20) of this U
may be connected to an inlet port (21 ) for washing and/or desorption liquids.
At the lower part of the U-shaped channel there may be a second exit conduit
(22) leading into one of the shanks (23) of a second U-shaped channel. The
other shank (24) may be connected to a waste channel (25b) that after a bent
(26) may end in a waste chamber (25a). At the lower part of the second U-
formed channel there may be a third exit conduit (27) leading into the MS-port
(15) that may contain an EDI-surface or an electrospray unit. In order to
control the flow in the microchannel structure, valve functions may be located
(a) in the first exit conduit (18), for instance immediately downstream the
volume-defining unit (16),
(b) possibly also in the second exit conduit (22), for instance immediately
after
the first U,
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
19
(c) in the third exit conduit (27), for instance immediately after the second
U,
and
(d) in association with the connection between the overflow channel (17) and
the waste channel (25b).
The valves may be of the types discussed above with preference for
hydrophobic breaks. A suitable adsorbent (28) as discussed above may be
placed in the second exit conduit (23) and may also function as a valve. In
the
case the adsorbent is in the form of particles they are preferably kept in
place
by a constriction of the inner walls of the conduits.
The structure presented in figure 3 is adapted for transporting liquid with
centrifugal forces, i.e. with the structure present in a disc and oriented
radially
outwards from the centre of the disc. At the start of an experiment the
metering cavity (16) is filled up with sample liquid at least to the
connection
between the over-flow channel (17) and the metering cavity (16), for instance
by capillary action. Liquid will enter the overflow channel (17). By first
overcoming the valve function between the overflow channel (17) and the
waste channel (25a), excess liquid will pass into the waste channel (25a). By
then overcoming the valve function in the first exit conduit (18), the liquid
in
the metering microcavity (16) will pass into the first U and down through the
adsorbent (28) where the analytes are captured. The liquid now being
essentially devoid of analyte will then halt at the bottom of the second U. In
the next step, one or more aliquots of a washing liquid may be applied
through either of the inlet ports (14,21 ), i.e. through the second shank (20)
of
the first U or via the same inlet port (14) as the sample. A washing liquid
will
pass through the adsorbent (28), collect in the bottom of the second U and
push the liquid already present into the waste chamber/channel (25a,b).
Subsequently, a desorption liquid is applied through either of the two inlet
ports (14,21 ) and passed through the adsorbent (18) where it releases the
analyte and into the bottom of the second U where it pushes the washing
liquid into the waste chamber/channel (25a,b). The desorption liquid
containing released analyte is then passed into the MS-port (15) from the
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
bottom of the second U by overcoming the valve function in the third exit
conduit (27).
The operations are preferably carried out while spinning the disc. If the
valves
S are in the form of hydrophobic breaks they can be passed by properly
adapting the g-forces, i.e. by the spinning. By properly balancing the
hydrophilicity/hydrophobicity of a liquid, passage or non-passage through a
valve may be controlled without changing the spinning speed. This is
illustrated by utilizing a hydrophobic break as the valve in the third exit
conduit
10 (27) combined with utilizing water-solutions as samples and washing liquids
and liquids containing organic solvents as desorption liquids. In the
alternative, valves that are opened by external means can be used. By
placing the outlet of the first exit conduit (18) at a shorter radial distance
from
the axis of symmetry than the lowest part of the metering microcavity (16)
15 particulate matters, if present in the sample, will sediment and be
retained in
the volume-defining unit when the metering microcavity (16) is emptied
through the first exit conduit (18).
Calibrator areas (29) are shown in each of figures 1-3. Each calibrator area
20 may be connected to a common area for application of a calibrator
substance.
These kind of flow systems has been described in WO 0040750 (Gyros AB)
and WO 0146465 (Gyros AB) which are hereby incorporated by reference.
In certain variants the inlet port for the sample and the MS-port may
coincide.
In this case the MS-port preferably comprises the surface on which the
analyte can be collected (adsorbed). Remaining liquid and washing liquids, if
used, are passed into the microchannel structure that then will function as a
waste channel and possibly contain a separate outlet port particularly adapted
for wastes and the like, or a waste chamber. In order to accomplish a
concentrating and/or separating effect the surface may expose structures
selectively binding/capturing the analyte as discussed above for a separation
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
21
zone. This variant also encompasses that there may be a separate inlet port
for washing and desorption liquids and microchannel part communicating with
the combined sample and MS-port.
The MS-port
The MS-port typically has an conductive part. The conductive part may for
instance be present in an EDI area or in a nozzle suitable for electrospray
ionisation, for instance a nanospray, or in any other form device that is used
to present a sample to a mass spectrometer. An electrospray nozzle provides
an orifice for instance in the form of a tip with a through-passing hole.
Various
kinds of sample presentation devices have been described in the publications
discussed above.
There may be a valve in the microchannel before its inlet to the MS-port.
The term conductive material includes semi-conductive material, although
materials having a conductivity that is larger than silicon or larger than
germanium are preferred. A typical conductive material comprise:
(a) metals such as copper, gold, platinum etc, mixtures of metals (alloys),
such as stainless steel etc,
(b) conductive metal oxides and mixtures thereof, such as indium oxide, tin
oxide, indium tin oxide etc,
(c) conductive polymers which includes polymers that are conductive as such
and conductive composites containing a non-conductive polymer and a
conductive material, for instance according to a)-c) and other conductive
composites, etc.
ESI MS-ports
Figure 4 illustrates an MS-port suitable for electrospray ionisation in a mass
spectrometer. This kind of port may be located where an MS-port has been
indicated in any of the structures given in figures 1-3. The MS-analyte may
thus be collected in an MS-port comprising a collection zone (30), which zone
is in fluid communication via the electrospray conduit (31 ) with the outlet
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
22
orifice (32). The electrospray conduit may be in the form of a tip. The MS-
analyte is entering the MS-port via conduit (33). The orifices (32) of the
electrospray arrangement are preferably positioned on the edge of a disc with
one, two or more orifices per microchannel structure. Typical disc-forms have
been discussed above. In use an electrospray orifice is matched to the
sampling orifice of a mass spectrometer and liquid in the collection zone (30)
is sprayed into the mass spectrometer. In a preferred variant the disc is
circular. The arrangement of the electrospray tips is preferably annular
around the centre of the disc. The orifices are preferably located at the edge
of the disc with a radial spray direction. The electrospray orifices may
alternatively be in one planar side of the microfluidic device with a spray
direction having a component that is perpendicular to the side. Annular
arrangements preferably at the edge of a circular disc will simplify accurate
positioning of the electrospray orifices relative to the sample application
opening of a mass spectrometer.
Electrospray units suitable for electrospray ionization mass spectrometry (ESI
MS), for instance adapted to the nanospray format, are mostly formed in
capillaries made of glass or fused silica, or polymer material like silicon.
The
tubings are typically of cylindrical geometry with tip internal diameters in
the 5-
20 ~m range. The word nanospray means that the liquid transferred out of the
tubing is in the nanoliter per minute range. Suitable rates for transfer of
liquid
to the mass spectrometer can be found in the interval of 1-1000 nl/min, e.g.
in
the interval 10-500 nl/min. By infusion (no external force), only a few
nanoliters per minute (5-25 nl/min) is transported out of the tubing while
with
applied external pressure 50-500 nl/min is more common.
A liquid solution suitable for ESI MS analysis comprises an organic
solvent:water mixture and includes a lower concentration of acid or base. The
composition is important especially with regard to surface tension and
conductivity. A low surface tension and a low conductivity are desirable in
order to obtain an efficient desolvation and ionization process and a stable
spray. If the sample is dissolved in water only, a so-called make-up solvent
is
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
23
preferably added (external delivery). A make-up solvent is typically
configured
co-axially (sheat-flow) around the nanospray tip. A make-up gas (typical N2)
may be added (e.g., co-axially) to assist the desolvation process. Creation of
a suitable liquid composition of the MS-sample may be part of the sample
S preparation process taking place upstream the MS port in other parts of
microfluidic device.
The tip geometry is important for a stable spray. Preferably the tip is pulled
from a cylindrical tubing which result in an oblong tip with a conical shape.
The outer diameter of the tubing near the orifice then becomes of similar
dimension as the internal diameter.
In order to induce a spray from an electrospray typ (towards the inlet of the
mass spectrometer) a voltage has to be applied on the tip. Therefore the tip
has to be made conductive. Different kind of metals can be deposited by
different techniques onto the tip (or part of the tubing). Important aspects
here
regard the stability (life-time) of the metallized tip since the voltage
applied as
well as different solvents affects its stability. Other possibilities also
exist, e.g.,
an electrode can be inserted into the tip whereby a voltage can be applied to
induce electrospray. Another alternative is to make the tip in a material
comprising any of the above-mentioned conductive materials. Typical
voltages used in nanospray range between 500-2000 volts.
Typical electrospray nozzles are available from a number of manufacturers,
for instance New Objective, MA, U.S.A. A variant that is believed to have
advantages for microfluidic devices is presented in PCT/SE01/02753 (Gyros
AB). See also WO 9704297 (Karger et al), US 5,969,353 (Hsieh) and US
6,110,343 (Ramsey et al) discussed above.
EDI MS-ports
The MS-port may also be used for EDI-MS and will then contain an EDI-area.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
24
Upward and downward directions when used in the context of EDI areas refer
to the directions defined in the figures irrespective of how the area is
positioned in a mass spectrometer.
EDI MS ports may be adapted to different EDI mass spectrometry variants,
for instance Time of Flight (TOF), Quadropole, Fourier-Transformed Ion
Cyclotron Resonance (FT-ICR), ion trap etc.
The EDI MS-port requires a free passage for the release of the ions created
during desorption/ionisation and thus has an opening straight above the EDI-
surface. This opening should be coaxial with and cover the EDI-surface. In
other words the EDI MS port is typically in form of a well or depression with
the EDI-surface at the bottom and in fluid communication at least with
upstream parts of the corresponding microchannel structure. This includes
that the opening may be covered during the sample treatment within the
microfluidic device but subsequently opened to enable desorption/ionisation
and possibly also evaporation of solvents. If an IDI principle is used the
opening should also provide space for the incident irradiation.
An EDI-surface may in principle have any geometric form although preferred
forms should be as compact as possible, for instance regular forms, such as
squares and square-like forms, and rounded forms, such as circular and
circle-like forms. The size of an EDI-surface preferably is the same as a
circle
with a diameter in the interval of 25-2000 Vim. There may be advantages if the
cross-sectional area of the incident beam used for irradiation is able to
encompass the complete EDI-surface or as much as possible, for instance
more than 25 % or more than 50 %.
An EDI-area comprises a conductive layer (layer I), for instance a metal layer
of copper, gold, platinum, stainless steel etc or a layer of any other
conductive
material of the kinds discussed above. Layer (I) may coincide with the EDI-
surface or be parallel thereto. Layer (I) has a conductive connection for
supporting the proper voltage and charge transport at the EDI-surface. The
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
complete EDI-area from the lowest part to the EDI-surface may be made of
conductive material, i.e. correspond to layer (I). In the case the
microfluidic
device comprises more than one microchannel structure with an EDI MS-port,
layer (I) of one EDI MS-port may be part of a common continuous conductive
S layer which extends into and encompasses layer (I) of two or more of the
other EDI MS-ports. In preferred variants the common continuous layer
comprises layer (I) of all EDI-MS ports of a microfluidic device. The common
conductive layer may be essentially planar. The common conductive layer
may have depressions corresponding to the EDI-surfaces and/or to other
10 parts of the microchannel structures of the innovative device. Typical
variants
are that the common conductive layer is positioned
(a) on top of the microfluidic device or
(b) between two substrates that are joined together to form the enclosed
microchannel structures of a microfluidic device.
15 In both variants the common conductive layer extends into the inner walls
and
layer (I) of the MS-ports. The MS-ports correspond to depressions.
The exact geometric shape of layer (I) outside the MS-port depends on the
particular device and practical ways of its manufacture. For instance a
20 common conductive layer may have an annular or arc-like form in case the
MS-ports are annularly arranged.
In one innovative variant, the EDI-area comprises a non-conductive layer
(layer (II)), which covers the conductive layer (I). Layer (II) in one EDI-
area
25 may extend into and encompass layer (II) in two or more of the other EDI-
areas as described for layer (I).
In another innovative variant the device has a separate conductive layer (III)
positioned above the common plane defined by the surface of each EDI-area
of a device and not connected to layer (I) in different EDI MS-ports. Layer
(III)
has openings matching the EDI-surfaces and permitting irradiation of these
surfaces and escape of ions through the openings.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
26
These innovative variants of EDI-areas are schematically illustrated in
figures
5a-f, each of which shows a cross-sectional view across the EDI-areas of two
MS-ports in a microfluidic device according to the invention. The EDI-surfaces
are referenced as (51 ) and the EDI-areas as (52) (within the dotted vertical
lines, a and b in figure 5f). Each MS-port comprises the EDI-area plus the
corresponding depression. The conductive layers (54) are hatched. It is
apparent that each EDI-area comprises a conductive layer (I) (53).
Figure 5a shows a common continuous conductive layer (54) at the bottom of
the device which layer encompasses layer (I) (53) of each EDI-area (52). A
non-conductive layer (II) (55) is placed between layer (I) (53) and the EDI-
surface (51 ). Figure 5b shows a variant, which is similar to the variant in
figure 5a, but the common continuous conductive layer is embedded within
the device. Non-conductive layer (II) (55) is present. In figure 5c there is a
common continuous conductive layer (54) comprising the EDI-surfaces and
layer (I). In figure 5d there is no common continuous conductive layer. Layer
(I) (53) for different MS-ports are isolated from each other and correspond to
EDI-surfaces. Figures 5e shows a variant in which there is a separate
continuous conductive layer (54) above layer (I) (53) of the EDI-areas. This
conductive layer (54) has openings (56) corresponding to the openings of
each MS-port and may be a surface layer on the upper or lower side of a lid
covering the microchannel structures. Figure 5f shows a variant in which
there is a common continuous conductive layer comprising layer (I). The EDI-
surfaces coincides with layer (I) in the MS-ports. The continuous layer also
encompasses the inner walls of the MS-ports. The MS-ports appear as
depressions in the common conductive layer.
For variants in which the open microchannel structures have been fabricated
in a base substrate and covered by a lid, the base substrate may consist of
conductive material and correspond to layer (I). In these variants the lid may
comprise a non-conductive or conductive material.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
27
Figure 6 illustrates an arrangement of MS-ports on a circular disc (with a
transparent lid), in which layer (I) (34) of each MS-port has a connection for
conductivity (35) with a peripheral conductive layer (36) which is closer to
the
edge of the disc than the MS-ports. In this variant each microchannel
S structure (37) comprises an MS-port and extends upstream to an inlet port
(38). Layer (I) (34), the connections (35) and layer (36) may be interpreted
as
a continuous conductive layer.
Figures 7a-b illustrate an MS-port in which the opening above an EDI-surface
is defined by a hole (39) in a lid (40) which in this case is transparent. The
incoming microchannel (41 ) opens to a circular area (42) with a diameter,
which is less than the diameter of the hole (39). Layer (I) (43), EDI-area
(44),
EDI-surface (45) are between the two dotted lines. Layer (I) extends into a
common conductive layer (46). This design in which the MS-port provides an
opening, which is greater than the EDI-area will facilitate for an incident
beam
to cover any spot of the EDI-surface. In preferred variants the microchannel
(41 ) extends into the bottom of the MS-port as an open microchannel of
constant depth. Seen from above the microchannel may be widening like an
expanding droplet.
A conductive layer per se may function as a conductive connection or there
may be distinct connections (35) to layer (I). See figure 6.
In certain variants the lid that covers the microchannel structures also
covers
the EDI-surfaces. For these variants the lid is removable at least at the MS-
ports. After processing of a sample in an upstream part of a microchannel
structure and transportation of the treated sample to the covered MS-port, the
lid is removed thereby permitting evaporation of solvents from the MS-port
and irradiation in order to accomplish desorption/ionisation of MS-analyte
molecules.
Liquids entering the MS-ports.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
28
During transport through a microchannel structure the solvent composition
may be changed to fit the particular kind of mass spectrometer used. In the
case of microchannel structures comprising EDI MS-ports and separation
zones containing a separation medium, a compound (= EDI-matrix) that upon
co-crystalisation with the analyte or analyte-derived entity assists
desorption/ionisation may be (a) included in the desorption liquid, (b)
included
in another liquid that is also guided to the MS-port, or (c) predispensed to
the
EDI-surface or dispensed to this surface after the analyte or analyte-derived
entity has been deposited on the EDI-surface. There may also be included
compounds that facilitate crystallization on the EDI-surface. Both kinds of
helper compounds may be included even there is no separation zone.
Calibration of the mass scale.
To ensure accurate mass determination, calibrator areas (spots) containing a
compound of known molecular weight (standard, calibrator substance) may
be present in the proximity of an MS-port. Calibrator areas (29) are shown in
figures 1-3. Alternatively, the standards may be included in the sample or
added to an EDI-area before desorption/ionisation (internal calibrator). The
choice of calibrator substance, its amount etc will depend on its use as an
external or internal calibrator, the MS-analyte and its concentration etc.
Material from which the microfluidic device is manufactured.
The microchannel structures are typically fabricated in inorganic and/or
organic material, preferably plastics or other organic polymers. The material
may be conductive or non-conductive as already discussed. Certain parts of a
microchannel structure may be metalized.
Suitable organic polymers may derive from polymerisation of monomers
comprising unsaturation, such as carbon-carbon double bonds and/or carbon-
carbon-triple bonds. The monomers may, for instance, be selected from
mono- , di and poly/oligo-unsaturated compounds, e.g. vinyl compounds and
other compounds containing unsaturation.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
29
Another type of organic polymers that may be used is based on condensation
polymers in which the monomers are selected from compounds exhibiting two
or more groups selected among amino, hydroxy, carboxy etc groups. The
plastics contemplated are typically polycarbonates, polyamides, polyamines,
polyethers etc. Polyethers include the corresponding silicon analogues, such
as silicone rubber.
The polymers are preferably in cross-linked form.
The plastics may be a mixture of two or more different polymer(s)/
copolymer(s).
At least a part of the microchannel structure may have a surface that has
been derivatised and/or hydrophilized, for instance by being coated with a
non-ionic hydrophilic polymer according to the principles outlined in WO
0147637 (Gyros AB) or by treatment in gas plasma. Typical gas plasma
treatments utilize non-polymerisable gases, for instance as outlined in WO
0056808 (Gyros AB). A hydrophilized surface may also be funtionalized in
order to introduce one or more functional groups that are capable of
interacting with the sample analyte, an analyte-derived compound or one or
more of the reagents added. Surfaces may be made of copper, gold,
platinum, stainless less etc, for instance by metallization, in order to
enable a
desired derivatization or for providing a conductive surface, for instance in
an
MS-port. Gold surfaces for instance may be derivatized by reaction with thiol-
containing compounds that have a desired functionality, for instance
hydrophilicity.
The optimal water contact angle for the surfaces within a structure depends
on the protocols to be carried out, the dimensions of the microchannels and
chambers, composition and surface tension of the liquids, etc. As a rule of
thumb, the surface of one, two, three or four of the inner walls (side-walls,
bottom or top), of a microchannel in a microfluidic device have to be wettable
the liquid used, preferably aqueous liquids, such as water. Preferred water
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
contact angles are _< 40° or <_ 30°, such as <_ 25° or <_
20°. These figures refer
to values obtained at the temperature of use, primarily room temperature.
It is believed that the preferred variants of the inventive microfluidic
devices
5 will be delivered to the customer in a dry state. The surfaces of the
microchannel structures of the device therefore should have a hydrophilicity
sufficient to permit the aqueous liquid to be used to penetrate different
parts
of the channels of the structure by capillary forces (self-suction). This of
course only applies if a valve function at the entrance of the particular part
10 has been overcome.
Best Mode
The best mass spectrometric results accomplished at the priority date have
been obtained for the variant described in example 4 below.
The best mode at the filing date is illustrated by example 5.
The invention is further defined in the appending claim and will now be
illustrated with a non-limiting experimental part.
The following patents and patent applications have been referenced in this
specification and hereby incorporated by reference:
WO 9116966 (Pharmacia Biotech AB), WO 9704297 (Karger et al), WO
9721090 (camera Bioscience), WO 9807019 (camera Bioscience) WO
9853311 (camera Bioscience), WO 9955827 (Gyros AB), WO 9958245
(Gyros AB), WO 0025921 (Gyros AB), WO 0040750 (Gyros AB), WO
0056808 (Gyros AB), WO 0062042 (Gyros AB), WO 0102737 (Gyros AB),
WO 0146465 (Gyros AB), WO 0147637, (Gyros AB), WO 0154810 (Gyros
AB), WO 0147638 (Gyros AB), WO 0185602 (Amic AB & Gyros AB),
PCT/SE01/022753 (Gyros AB & Amic), US 5969353 (Hsie), and US
6,110,343 (Ramsey et al), and U.S. S.N. 60/315,471, U.S. S.N. 60/322,621
and corresponding International Applications.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
31
EXPERIMENTAL PART
EXAMPLE 1. Gold at different positions in a CD
Gold patterning Sensitivity* Charging of
substrate**
No gold Poor Yes
Gold on all sides Good No
Gold on upper side Good No
Gold on bottom side Good Yes
Isolated gold spots on the Good Yes
upper
side
Gold spots on the upper side.Good No
Every spot being conductively
connected contact with the
adapter
plate through an individual
gold
string or a common gold area.
Good = sensitivity for an in-solution tryptic digest of BSA comparable to the
sensitivity obtained on a conventional stainless steel target
..
Charging is observed as significant mass shift ( >_1 Da) upon repeated laser
desorption/ionization and/or loss of signal.
This table shows the results form a summary of experiments performed
before the priority date in order to optimise the design of the CD-MALDI
interface. Gold was sputtered at various positions of the CD and the MALDI
characteristics were studied for a tryptic digest of Bovine Serum Albumin
(BSA). The CD was placed on a metal adapter inserted into the ion source.
The gold was hence patterned in various ways to determine the importance of
electrical contact between the MALDI ports and the adapter plate.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
32
EXAMPLE 2. Planar CD and structured removable lid.
This example shows a planar CD in combination with a lid in which the
microfluidic structures are present. The structured lid was achieved through
casting Memosil (Hereaus, Germany) against a nickel-coated master. The
microfluidic structure employed in this example is shown in figure 2.
The structured lid is attached to the CD by adhesion forces. The surface
facing the lid should be hydrophilic as the presented invention utilizes
capillary action to fill the microfluidic structures. This is especially
important as
the moulded lid, being a type of silicon rubber is hydrophobic.
The upper side of the CD was covered with gold using a DC Bias magnetron
sputtering method (1 * 10-5 torr, Ar plasma and titan as adhesion layer) and
made hydrophilic according to the following procedure; The gold sputtered
side was cleaned by rinsing with ethanol, followed by an oxygen plasma
treatment (Plasma Science PS0500,). After plasma cleaning a self-
assembled monolayer (SAM) of hydroxylthiol was formed on the gold surface.
The hydroxylthiol was 11-mercapto-1-undecanol (Aldrich, Milwaukee, WI) and
used at a concentration of 2mM in degassed ethanol. To obtain a well-
organized SAM, the gold sputtered disc was immersed in the thiol solution
over night. After the thiol adsorption the CD was sonicated in ethanol for ca
2
min.
T.he lid, containing the microfluidic channels, was attached to the CD by
adhesion forces. A second piece of polymeric material was mounted at a
position of 180° from the structured lid as a counterbalance. Reversed
phase
beads (Source 15 RPC, Amersham Pharmacia Biotech, Sweden) with a
diameter of 15 p,m were packed into the individual structures using the
filling
port present in the common distribution channel. The slurry, containing the
beads, was drawn into the individual channels by capillary action. Eighteen
parallel reversed phase columns were formed when the disc was spun at
3000 rpm for 1 minute. The columns were rinsed with water containing 0.1
l'FA (trifluoroacetic acid, Aldrich)) two times. The rinsing was performed at
an
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
33
rpm of 2500 for ca 1 min. 200 nL of in-solution tryptic digest of BSA was
added to individual channels through the sample inlet. The following
procedure was used for digestion. The BSA (Sigma) was dissolved to a final
concentration of 4.75 pmol/~.I in 0.1 ammonium bicarbonate buffer at pHB.
The enzyme-modified trypsin (Promega Corp., Madison, WI) was added and
dissolved at a ratio of BSA/trypsin 20:1. The sample was incubated at
37°C
for 4 hours and then stored at -20 °C until used.
The sample was allowed to pass over the reversed phase columns at 1500
rpm. A second rinsing/washing step was performed as above using water
containing 0.1 % TFA. Finally the peptides were eluted using 200 nL eluent
consisting 50% isopropanol, 50 % water and a-cyano-4-hydroxycinnamic acid
(Aldrich) below saturation. The eluent was prepared by saturating a
water:isopropanol (50%) mixture with a-cyano-4-hydroxycinnamic acid. To
100 ~.L of this mixture 200 ~L of 50% water:50% isopropanol was added,
resulting in an eluent saturated to approximately 2/3 with a-cyano-4-
hydroxycinnamic acid.
The presentation of the sample in the MALDI port was performed in two
different ways.
a) In the first example a full structure was utilised (figure 2). Eluent from
the column was collected in the container placed at an outer radial
position relative of the reversed phase column. When the lid was
removed the liquid quickly evaporated leaving co-crystallized matrix
and peptides on the gold sputtered surface. The disc was cut in halves
to fit in the MALDI ionisation interface.
b) The moulded structure was cut directly after the packed column leaving
an open-ended microstucture. The eluent was allowed to pass the
column at a pre-determined speed (1500 rpm) in order to generate a
controlled evaporation of the solvent at the MALDI port, and hence the
formation of co-crystallized matrix and peptides suitable for MALDI
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
34
analysis. The disc was cut in halves to fit in the MALDI ionisation
interface.
EXAMPLE 3. Structured CD and site-specific elution
This example employs a CD with integrated microfluidic structures, a thin ( ~0
~.m) lid with holes at positions matching the MALDI port in the CD. The
microfluidic structure employed in this example is shown in figure 1.
The polycarbonate CD was covered with gold as described above. The side
was hydrophilized using the thiolprocedure described above. The lid
(SkultunaFlexible, Skultuna, Sweden), having, pre-drilled holes, was attached
to the CD through heat pressing at 135°C.
Reversed phase beads (Source 15 RPC) with a diameter of 15 ~m were
packed in the individual structures using capillary forces in combination with
centrifugation. The columns were rinsed with ethanol and spun to dryness
before 23 fmol of tryptically digested BSA was added and spun down using
700 rpm. The tryptic digest of BSA was generated according to the
procedure described above. After sample addition, the column was rinsed
twice with water. a-cyano-4-hydroxycinnamic acid was mixed in an organic
solvent of acetonitrile/water 3:7 containing 0,1% TFA to a saturation of 2/3
and 250 n1 was used to elute the sample from the 3 n1 packed column.
The crystals obtained after evaporation of the organic/water mixture
contained co-crystallized peptides. Eight singly charged peptide peaks were
present in the mass spectrum obtained.
EXAMPLE 4. Parallel sample preparation in a product CD.
Description of the microfluidic disc (CD)
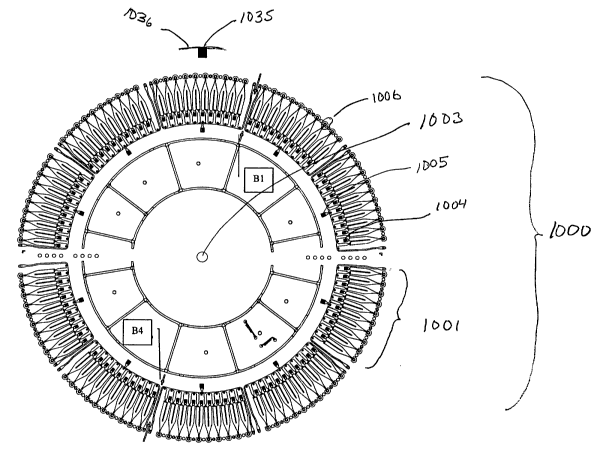
Figure 8a illustrate a product microfluidic device (CD) (1000) comprising 10
sets (1001 ) of identical microchannel structures (1002) arranged annularly
around the spinning axis (axis of symmetry) (1003) of a circular disc (1000).
Each set comprises 10 microchannel structures. Each microchannel structure
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
is oriented radially with an inlet port (1004,1005) located at shorter radial
distance than an outlet port (MS-port) (1006). The MS-ports are ~0.9 cm from
the edge of the disc (not shown). The disc was of the same size as a
conventional CD. The CD has a home mark (1035) at the edge (1036) for
5 positioning the disc when dispensing liquids.
The final device comprises a bottom part in plastic material that contains the
uncovered form of the microchannel structures given in figure 8a. The
microchannel structures are covered with a lid in which there are circular
10 holes (1007,1008,1009,1010,1011,1012 in figure 7b) that will function as
inlets (1007,1008) or outlets (1009,1010) in the final microfluidic device or
as
separate claibrator areas (1011,1012). The bottom part with its
microstructures is made of plastics and has been manufactured by a
moulding replication process. The surface with the uncovered form of the
15 microchannel structures has been hydrophilised in accordance WO 0056808
(Gyros AB). The lid was thermo laminated to the bottom part in accordance
with WO 0154810 (Gyros AB).
Figure 8b shows in enlarged form a set (1001 ) of 10 microchannel structures
20 (1002). Each microchannel structure has a sample inlet port (1005) and one
common inlet port (1004) for other liquids. At the bottom of each of these two
inlet ports (1004,1005) there are ridges/grooves (1013) directed inwards the
microchannel structure. The sample inlet port (1005) is connected to one
(1014) of two inwardly/upwardly directed shanks (1014,1015) of a Y-shaped
25 sample reservoir (1016). The inlet port (1004) for other liquids is common
for
all microchannel structures in a set and is connected to a common distribution
manifold (1017) with one reservoir/volume defining unit (1018) for other
liquids than sample connected to the other upwardly directed shank (1015) of
each sample reservoir (1016). The distribution manifold (1017) has one waste
30 outlet port (1009) at each flank of the set. The downward shank (1019) of
the
Y-like sample reservoir (1016) leads to an outlet port (MS port) (1006) and
comprises a bed (1020) of chromatography particles (RPC, reversed phase
chromatography) held against a dual depth (1021 ) (from 100 ~m to 10 ~m to
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
36
20 ~,m in the flow direction), of the outer part of the downward
shank/microchannel (1019). The microchannel corresponding to the
downward shank (1021 ) will end in the bottom (1022) of the outlet port (MS
port) (1006) as a widening groove (drop-like seen from above)(1023), which
will function as a crystallization area.
Each volume-defining unit (1018) for other liquids is surrounded by anti-
wicking means (1024,1025) that will prevent wicking of liquid between the
volume-defining units (1018). The anti-wicking comprises both (a) a geometric
change (1024) in edges going between the volume defining units (1018) or
from a volume defining unit (1018) to a waste outlet port (1009) and a
hydrophobic surface break (1025, rectangle).
Valve functions in the form of local hydrophobic surface breaks (rectangles,
1026, 1027) are present in the waste channels (1028) of the distribution
manifold (1017) before the outlet openings (1009) at the flanks, and in each
microconduit (1029) between a volume defining unit (1018) for other liquids
and the upwardly directed shank (1015) of the sample reservoir (1016). The
valve function (hydrophobic surface break) (1027) may be positioned before,
across or immediately after the joint between the microconduit (1029) and the
upward shank (1015) of the sample reservoir (1016). Despite the sharp
change in lateral dimension at the joint between the microconduit (1029) and
the upward shank (1015), the hydrophobic surface break (1027) was
imperative for the valve function.
Local hydrophobic surface breaks (1030,1031, rectangles) for directing liquid
into the structure are present at the inlet openings (1007,1008).
Furthermore, a U- (horse-shoe) shaped local hydrophobic surface break
(1032) is positioned at the outlet opening (1010 of each outlet port (1006, MS-
port) for preventing liquid exiting into the port from spreading onto the top
of
the disc.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
37
The hydrophobic surface breaks (1026,1027,1030,1031) were applied before
an upper substrate (lid) was laminated to the surface of the bottom substrate
comprising the microchannel structures in open form. The hydrophobic
surface break (1032) was applied after lamination and gold sputtering.
The openings (10011,1012) in the lid are calibration areas for calibration
substance. The surface within the circles is the top of the bottom part. One
(1012) of them comprises a depression (1033) that mimics the widening
groove (1022) of an MS-port (1006)
Before application of the local hydrophobic surface area (1032) around the
opening (1010) the top of the lid was sputtered with gold at least as a
continuous layer in-, around-, and between the openings including the
calibrator areas (1010). A continuous gold film thus were connecting the
bottom and the walls of the MS-ports (1006) and the calibrator areas . Other
parts of the lid (but not the whole lid), besides the areas in and around the
MS-ports and calibrators, were also covered with gold. The aim has been to
cover as much lid area as possible with gold as long as the gold layer do not
interfere with microfluidic- and instrumental functions, e.g. the gold is not
allowed to cover the rim of the lid (CD) as it upsets the home-positioning of
the CD or the inlets (1007,1008) of the microfluidic structures since it
affects
the capillary force by an increased hydrophobicity (liquid would then be more
difficult to fill up the channels). Other conducting materials than gold could
also be beneficial for this application, for instance at the filing date
indium tin
oxide was sputtered onto the lid and was shown promising for this application.
Since indium tin oxide is much more transparent than gold and relatively
hydrophilic the whole lid could be covered (i.e. no mask would be necessary
for sputtering the conductive layer) without concern for microfluidics and
instrumental aspects. Therefore the manufacturing and production process
would be more simple and cheap.
The depth in the microchannel structures is the same (100 Vim) and constant
from the inlet openings (1007,1008) to the dual depths (1021 ).
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
38
Loading of RPC-particles. The distribution manifold (1017) is filled with a
suspension of RPC-particles via the common inlet port (1004). After filling,
the
suspension will be present between the inlet port (1004) and the valves
(1026) at the flanking waste openings (1009). Upon spinning at a first speed,
S excess waste suspension will leave the distribution manifold (1017) via the
flank openings (1009) while air will enter the manifold via the common inlet
(1004). Defined aliquots (about 0,2 ~I) of the suspension will be retained in
the volume-defining units (1018). The anti-wicking means (1024,1025)
surrounding the volume-defining units (1018) will assist in retaining the
defined volume in each volume-defining unit. When the spinning speed is
increased, the aliquots in the volume-defining units (1018) will break through
the valves (1027), pass through upward shanks (1015) and the Y-shaped
sample reservoirs (1016) and out through the downward shank (1019). The
particles will be collected as a packed bed (1020) against the dual depth
(1021 ), and the liquid will pass out through the outlet opening (1012) where
it
leaves the system.
Filling of the distribution manifold (1017) including the volume defining
units
(1018) through the common inlet port (1004) is solely by capillary force.
Experimental
A model protein consisting of bovine serum albumin (BSA) in 50 mM
ammonium bicarbonate buffer, pH 8, was reduced and alkylated according to
standard protocol and in-solution digested with trypsin. The reaction was
quenched by adding trifluoroacetic acid (TFA) to a final concentration of 0.1
and transferred to a micro plate for subsequent sample processing on-CD, as
described above.
Sample and reagents were transferred from micro plates (containing typical
volumes of 5 to 100 NI) to CD by a robotic arm. The robotic arm holds 10
capillaries where sample and reagents are contained inside during transfer.
The volume of sample/reagents aspirated into the capillaries and later
dispensed onto the CD is driven by syringe pumps and controlled by software
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
39
(as are the robotic arm). Aspiration and dispersion rates are typical in the
0.5-
pl/sec rate. Once the liquid is dispensed onto the CD, at respective inlet
port, it is drawn, by capillary force, into respective common/micro structure.
5 The instrument for performing the experiment was a CD microlaboratory
(Gyrolab Workstation, Gyros AB, Uppsala, Sweden). This instrument is a fully
automated robotic system controlled by application-specific software.
Microplates containing samples or reagents are stored in a carousel within the
system.A high precision robot transfers samples from microplates or
10 containers into the microworld of the CD. CDs are moved to the spinning
station for the addition of samples and reagents. An application-specific
method within the software controls the spinning at precisely controlled
speeds controls the movement of liquids through the microstructures as the
application proceeds. The CDs are transferred to a MALDI mass
spectrometer for analysis and identification.
In order to reduce eventual carry-over between individual microchannel
structures, i.e., if part of sample remains inside the capillary after
dipensing it
onto CD it might contaminate the sample following and has therefore to be
properly washed away, the following wash procedure was applied:
1. 20 p1 of water was flushed through all capillaries.
2. 4 p1 of 50% ethanol in water was then aspirated into the capillaries and
dispensed to waste, this was repeated four times using 4.5 NI in the last
two cycles.
3. Finally, 4 p1 of 0.1 % TFA was aspirated and dispensed to waste, this
was repeated four times using 4.5 NI in the last two cycles.
Operation method:
The following scheme gives an overview of a typical spin program for running
multiplex samples on a CD for the above-mentioned MALDI application.
The CD is the one described above. A ramp (see below) indicates an
acceleration phase, deceleration phase, or a constant rpm value.
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
The CD was applied in an instrument from Gyros AB.
1. First spin. The purpose here is to restore ("re-pack") the chromatographic
columns.
Order Ramp (rpm) Time~sec) Order Ramp (rpm) Time (sec)
1 7000 2 3 0 2
2 7000 30
5
2. Conditioning of commonlindividual microstructures and reversed-phase
columns. 3.8 NI (per 10 structures) of 50% acetonitrile in water is dispensed
into each common inlet port (1004). The first ramp (no spin) is a lag period
as
for the liquid to completely fill up the common channel.
Order Ramp (rpm) Time sec Order Ramp rpm) Time (sec)
1 0 5 6 0 2
2 700 7 7 8000 2
3 700 2.5 8 8000 30
4 1600 0.15 9 0 2
5 1600 20
3. Conditioning of individual microstructures. This item differs from the one
above (no 2) by addressing other parts of the microchannel structures not
accessible by the procedure mentioned in item 2. The purpose is to more
completely re-wett any microstructure. 400 n1 of 50% acetonitrile in water is
dispensed per microchannel structure through each inlet ports (1005).
Order Ramp (rpm) Time sec Order Ramp Lpm) Time sec
1 8000 2 3 0 2
2 8000 30
4. Conditioning of commonlindividual microstructures and reversed-phase
columns. 3.8 NI (per 10 structures) of 0.1 % trifluoroacetic acid (TFA) in
water
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
41
is dispensed into each common inlet port (1004). The first ramp (no spin) is a
lag period as for the liquid to completely fill up the common channel.
Order Ram~~rpm) Time sec Order Ramp (rpm) Time (sec)
1 0 5 5 1600 20
2 700 7 6 8000 2
3 700 2.5 7 8000 30
4 1600 0.15 8 0 2
5. Sample transfer. 1-10 p1 of sample is applied into each inlet port (1005)
(total 100 identical micro structures and therefore 100 samples per CD). The
first ramp (no spin) is a lag period as for the liquid to completely fill up
the
common channel.
Order Ramp (rpm) Time (sec) Order Ramp (rpm) Time sec
1 0 5 7 1200 20
2 1800 0.3 8 2500 0.25
3 1000 0.2 9 1500 0.2
4 1000 30 10 1500 20
5 2000 0.2 11 0 2
6 1200 0.2
6. Desaltinglwashing of sample. 3.8 p1 (per 10 structures) of 5-10% organic
solvent/ 0.1 % trifluoroacetic acid (TFA) in water is dispensed into each
common inlet port (1004). The first ramp (no spin) is a lag period as for the
liquid to completely fill up the common channel.
Order Ramp rpm) Time sec Order Ramp (rpm) Time sec
1 0 5 5 1600 20
2 700 7 6 8000 2
3 700 2.5 7 8000 30
4 1600 0.15 8 0 2
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
42
7. Sample elution and peptide-matrix cocrystallization on MALDI target area
on CD.
Eluent consists of 50% acetonitrile/0.1 % TFA in water wherein the MALDI
matrix (1.5 mg/ml of a-cyanohydroxycinnamic acid) is dissolved. 4.1 p1 of
eluent (per 10 structure) is applied into each common inlet port (1004).
Order Ram r m Time sec Order Ram r m Time sec
1 0 2 15 300 4
2 600 0.1 16 1600 0.2
3 600 7 17 1600 0.1
4 1400 0.14 18 1200 0.07
5 1400 0.25 19 1200 0.4
6 300 0.22 20 1000 0.05
7 300 4 21 1000 1
8 1400 0.2 22 800 0.05
9 1400 0.1 23 800 90
300 0.2 24 1200 0.1
11 300 4 25 1200 1.9
12 1400 0.2 800 0.1
13 1400 0.1 800 90
14 300 0.1 0 2
The CD (or more exactly half of it) was subsequently fixed to a steel target
holder and inserted into a MALDI TOF instrument (Bruker Biflex) for running
10 mass spectrometry.
Results:
The molecular mass of the peaks was identified as BSA peptides by a
database search (NCBI). The mass spectra typically showed ten peaks which
were identified as BSA peptides. High sensitivity was attainable using the CD
for sample concentration and preparation. High mass resolution and accuracy
were also demonstrated.
Comments on the design of the MS-port
Meanwhile the peptides are eluted from the chromatographic column with an
organic:aqueous solvent containing the MALDI matrix, the liquid flows into the
MS-port (i.e., the MALDI target area) by centrifugal force. Once a liquid
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
43
element (droplet) enters this open area (restricted by the walls of the lid
and
the upper surface of the bottom substrate) the solvent quickly evaporates and
peptides and matrix cocrystallizes on the surface. In order to make this
process more robust, i.e., to stronger retain the liquid element while
spinning
is performed, a hydrophobic pattern was created surrounding the MS-port
(then considered a more hydrophilic area). This process of hydrophobic
patterning and its flow restriction effect is similar to the process and
effect of
creating hydrophobic breaks, the difference here being that the hydrophobic
pattern surrounding the MS-port is created after the lid has been laminated
onto the CD and after the application of the conductive layer. This
hydrophobic area has a U-shape (horseshoe) configuration and covers part of
the MS-port and part of the lid surface and its wall. Since the liquid element
is
repelled from this hydrophobic area the droplet preferably stays on the more
hydrophilic area during crystallization. In addition to this the crystals are
formed on a smaller surface area at some distance away from the walls of the
lid. This means that the analyte concentration will be further enhanced (and
therefore a higher sensitivity can potentially be reached in the subsequent
mass spectrometry analysis) compared to if the crystals were deposited on a
larger surface where the sample would more spread out. Also, with
automated MALDI analysis it is preferable to have a smaller surface area
where the crystals are found as for the laser to more efficiently cover that
particular area in a shorter time period (assuming heterogeneous crystal
formation, i.e., no "sweet spot"). Moreover, by having the crystals at some
distance away from the lid wall less electric field strength disturbances are
expected during MALDI analysis due to a non-homogenous field close to the
wall. If so, less mass accuracy and resolution is expected. The same would
be true for crystals found at different height levels attached to the wall of
the
lid, i.e., less mass accuracy and resolution would be expected if the crystals
were to be irradiated by the laser at different heights along the wall.
Finally,
any influence of "laser-shadow" by the wall will be diminished.
Certain innovative aspects of the invention are defined in more detail in the
appending claims. Although the present invention and its advantages have
CA 02442345 2003-09-17
WO 02/075776 PCT/SE02/00539
44
been described in detail, it should be understood that various changes,
substitutions and alterations can be made herein without departing from the
spirit and scope of the invention as defined by the appended claims.
Moreover, the scope of the present application is not intended to be limited
to
the particular embodiments of the process, machine, manufacture,
composition of matter, means, methods and steps described in the
specification. As one of ordinary skill in the art will readily appreciate
from the
disclosure of the present invention, processes, machines, manufacture,
compositions of matter, means, methods, or steps, presently existing or later
to be developed that perform substantially the same function or achieve
substantially the same result as the corresponding embodiments described
herein may be utilized according to the present invention. Accordingly, the
appended claims are intended to include within their scope such processes,
machines, manufacture, compositions of matter, means, methods, or steps.