Note: Descriptions are shown in the official language in which they were submitted.
CA 02442467 2003-09-25
P07850
LeA 36,236
A PROCESS FOR QUENCHING A GASEOUS REACTION MIXTURE
DURING THE GAS PHASE PHOSGENATION OF DIAMINES
Field of the Invention
The present invention provides a process for quenching a gaseous reaction
mixture
during the phosgenation of diamines in the gas phase to produce diisocyanates,
wherein the gas mixture contains at least diisacyanate, phosgene and hydrogen
chloride. Quenching is achieved by injecting a quenching liquid into the gas
mixture.
Background of the Invention
The preparation of diisocyanates by reacting diamines with phosgene in the gas
phase is described, for example, in EP 0 289 840. The diisocyanates formed in
a
cylindrical reaction chamber, such as a tubular reactor, are not thermally
stable at
the reaction temperatures of 300 to 500°C. Rapid cooling of the
reaction gases
after the phosgenation reaction to temperatures below 150°C is
therefore needed
to avoid the formation of undesired secondary products due to the thermal
decomposition of diisocyanate or by further reaction. For this purpose, in EP
0
289 840; the gaseous mixture continually leaving the reaction chamber, which
contains, inter olio, diisocyanate, phosgene and hydrogen chloride, is passed
into
an inert solvent, e.g. dichlorobenzene. The disadvantage of this process is
that the
rate of flow at which the gas mixture is passed through the solvent bath has
to be.
relatively low because at too high rates of flow the solvent and the compounds
dissolved therein would be carried over. In a subsequent step, the liquid
compounds have to be separated from the gas. Another disadvantage is that, due
to
the low rates of flow and a small heat transfer term, large solvent containers
have
to be used to produce the cooling effect.
Furthermore, processes are known which use heat exchangers and/or expand the
gases into a vacuum to cool the reaction gases. The disadvantage of heat
CA 02442467 2003-09-25
P07850
-2-
exchangers is that, due to poor heat transfer, large exchange surfaces and
thus
large heat exchangers are required for effective cooling. In addition,
deposits of
solids on the relatively cold surfaces of the heat exchangers takes place due
to
secondary reactions of the gas mixture on these surfaces; such as e.g. decom-
position or polymerization. The transfer of heat is further impaired by .these
deposits and this leads to a higher residence time and thus results in a
further
increase in secondary product formation. On top of that, undesired shutdown
times
are produced for the entire plant due to cleaning of the cooling .stage.
Summary of the Invention
The present invention reduces or eliminates the disadvantages inherent in the
art
such as those mentioned above when rapidly cooling the gaseous reaction
mixture
present during the gas phase phosgenation of diamiries to produce
diisocyanates to
a temperature at which the relevant reaction product is thermally stable. At
the
same time, the formation of undesired secondary products is suppressed.
Brief Description Of The Figures
The present invention will now be described for purposes of illustration and
not
limitation in conjunction with the figures, wherein:
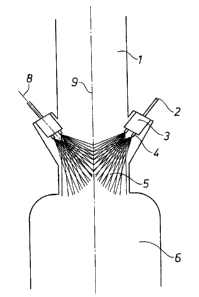
Figure 1 shows a schematic cross-section through a first embodiment of the
quenching zone; and
Figure 2 illustrates a schematic cross-section through a second embodiment of
the
quenching zone.
Detailed Description of the Invention
The present invention will now be described for purposes of illustration and
not
limitation. Except in the operating examples, or where otherwise indicated,
all
CA 02442467 2003-09-25
P07850
_3 _
numbers expressing quantities, percentages and so forth in the specification
are to
be understood as being modified in all instances by the term "about."
The present invention provides a process for quenching a gaseous reaction
mixture
during the phosgenation of diamines in the gas phase to produce diisocyanates,
wherein the gaseous reaction mixture contains at least a diisocyanate,
phosgene
and hydrogen chloride, by injecting a quenching liquid into the gas mixture
continuously flowing out of a cylindrical reaction zone into the downstream
cylindrical quenching zone, wherein the quenching liquid is injected with the
aid
of at least two spray nozzles arranged at the entrance to the quenching zone
at
equal distances along the circumference of the quenching zone.
In addition to phosgene, hydrogen chloride and the major product,
diisocyanate,
the gaseous reaction mixture may also contain further isocyanates produced as
secondary products, as well as nitrogen and/or organic solvent.
Examples of diisocyanates prepared by the gas phase phosgenation of diamines
include, but are not limited to, hexamethylene diisocyanate (HDI), isophorone
diisocyanate (IPDI), naphthylene diisocyanate (NDI), toluene diisocyanate
(TDI),
dipenylmethane diisocyanate and dicyclohexylmethane diisocyanate (HMDI).
One advantage of the process according to the present invention is that the
desired
rapid cooling of the gas mixture which contains a diisocyanate, hydrogen
chloride
and excess phosgene from 300 to 400°C to a maximum of 150°C on
leaving the
reactor is produced by the spraying of a suitable quenching liquid. The
contact
time during which cooling takes place is reduced to from 0.2 to 3 s.
Spraying of the liquid is performed with conventional spray nozzles or via
openings, such as slits or holes, at the exit from the reaction zone or the
entrance
to the quenching zone. If only two spray nozzles are provided, these are
preferably
CA 02442467 2003-09-25
PO7850
_q._
arranged diametrically opposite to each other. The spray nozzles may
preferably
be individual nozzles. More preferably, however, nozzle heads, each with at
least
two individual nozzles, are used, wherein single substance nozzles are
preferably
chosen.
Another advantage of the process of the present invention is that the
quenching
liquid is sprayed into the gas stream in such a way that the hot reaction gas
does
not make contact with the relatively cold surfaces of the quenching zone or
the
nozzles and their pipes. Only after the gas mixture has cooled to the stable
temperature range for the particular diisocyanate does it come into contact
with
the relatively cold walls of the quenching zone or other components.
The spray nozzles are preferably arranged independently of each other in such
a
way that the direction of flow of each quenching liquid is preferably at an
angle of
0° to 50°, more preferably 20° to 35°, to the
direction of flow of the gas mixture.
The direction of flow of the gas mixture is substantially along the axis of
the
cylindrical reaction zone or of the quenching zone. If the tubular reactor is
arranged in an upright position, the gas flows from top to bottom through the
reaction zone and the downstream quenching zone: In the same way, the
direction
of flow of the quenching liquid is along the axis of the spray nozzle. The
cone
angle of the spray nozzles, independently of each other, is preferably
20° to 90°,
more preferably 30° to 60°. In one embodiment, the direction of
flow of all those
nozzles which axe arranged in one plane have the same angle to the direction
of
flow of the gas mixture and the same cone angle.
Suitable quenching liquids are organic solvents or a mixture of different
organic
solvents which do not react with the diisocyanate formed. The choice of
solvent is
also determined, ihter alia, by the solubility of phosgene. Suitable solvents
are, for
example, toluene, chlorotoluene, xylene and chloronaphthalene.
CA 02442467 2003-09-25
P07850
-5-
Monochlorobenzene and o-dichlorobenzene are especially suitable. A solution of
the diisocyanate formed in one of these organic solvents may also be used. In
this
case, the proportion of solvent is preferably 40 to 90 vol.%. The temperature
of
the quenching liquid is preferably 100 to 170°C.
The quenching zone downstream of the cylindrical reaction zone is also
cylindrical. The diameter of the quenching zone may be chosen to be
substantially
identical to that of the reaction zone or larger than that of the reaction
zone. The
reaction zone is preferably a tubular reactor without baffles.
The process according to the present invention has the further advantage that
cooling of the reaction gases takes place rapidly, preferably within 0.2 to 3
s,
immediately after reaction has taken place, because the gas stream flowing out
of
the reactor does not have to be slowed down and/or passed into a container but
is
passed directly through a stream of an atomized quenching liquid. In addition,
the
quenching zone is designed in such a way and the nozzles are mounted in such a
way that the hot gas mixture does not make contact with any of the relatively
cold
surfaces in the quenching zone. For this purpose; for example, the diameter of
the
cylindrical quenching zone may be larger than the diameter of the reaction
zone.
In another embodiment of the process of the present invention, quenching of
the
reaction gases may also take place in several steps, preferably in two steps.
In this
case, each quenching step includes at least two spray nozzles at equal
distances
along the circumference of the quenching zone. The same quenching liquids may
be used in the different quenching steps. More preferably, however, in a two-
step
quenching process, different quenching Liquids are used in the two steps, that
is an
organic solvent, preferably monochlorobenzene or o-dichlorobenzene, in the
first
step and a solution of the diisocyanate formed in the organic solvent which
was
used in the first quenching step in the second step. The proportion by volume
of
the solvent is preferably 40 to 90
CA 02442467 2003-09-25
P07850
-6-
In the following description, the process according to the present invention
is
explained in more detail making reference to the figures.
Figure 1 shows a cylindrical reaction zone L, through which the gaseous
mixture
flows from top to bottom along the broken line 9. On leaving reaction zone 1,
the
gas mixture flows through a similarly cylindrical quenching zone 5. In the
quenching zone 5 there are two nozzle heads 3; each with two individual
nozzles
4, located diametrically opposite to each other. The quenching liquid is
supplied to
nozzle head 3 via a pipe 2. Nozzles 4 and nozzle head 3 preferably are
arranged so
that the direction of flow of the quenching liquid (shown by broken line 8)
and
that of the gas stream 9 are at an angle of 0° to 50°, more
preferably 20° to 35°, to
each other and thus the hot gas mixture does not make contact with the colder
nozzles and nozzle head. In the quenching zone 5, cooling of the reaction gas
takes place by evaporation of the atomized liquid. The remaining liquid and
the
cooled reaction gas pass into the liquid collection container 6 located below
the
quenching zone, this container acting simultaneously as a pump-tank and as
apparatus to separate gas and liquid.
The embodiment of the quenching zone shown in Fig. 2 corresponds in principle
to the embodiment shown in Fig. 1. Identical or similar components therefore
have the same reference numbers as in Fig: I . The embodiment shown in Fig. 2
differs from that shown in Fig. 1 in that the diameter of the quenching zone 5
is
greater than that of the tubular reactor I.
EXAMPLE
Out of a vertically arranged tubular reactor with a diameter of 260 mm flowed
6700 kg/h of a gas mixture of isophorone diisocyanate, hydrogen chloride and
excess phosgene at 400°C, at a pressure of 1000 to 1800 mbax with a
speed of 1 f
m/s into a downstream quenching zone with a diameter of 510 mm. The tubular
widening from the reactor to the quenching zone was designed with an angle of
CA 02442467 2003-09-25
P07850
:7_
75° to the vertical. Within the widening section, four individual
nozzles were
mounted at equal distances along the circumference, spraying out 130 x 103
kg/h
of a solution of isophorone diisocyanate in monochlorobenzene, in a ratio by
volume of 20:80, and at a temperature of 120°C. The direction of flow
of the
quenching liquid was at 35° to the direction of flow of the gas
mixture. The cone
angle of the nozzles was 30°. Cooling of the reaction gas took place in
the
quenching zone. The condensable constituents dissolved in the solution and the
amount of monochlorobenzene required for cooling purposes evaporated. The
liquid/gas mixture passed into a separator. After a contact time of 0:8 to 1.3
seconds, the temperature of the concentrated-up isophorone diisocyanate
solution
collected in the separator was 140°C: The gas flowing out of the
separator had a
temperature of 145°C.
Although the invention has been described in detail in the foregoing for the
purpose
of illustration, it is to be understood that such detail is solely for that
purpose and
that variations can be made therein by those skilled in the art without
departing from
the spirit and scope of the invention except as it may be limited by the
claims.