Note: Descriptions are shown in the official language in which they were submitted.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-1_
WELL SERVICE FLUID AND METHOD OF MAKING AND USING THE SAME
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority to U.S. Provisional Patent
Application Serial No.
60/282,732 filed April 10, 2001.
FIELD OF THE INVENTION
[0002] The present invention relates to a sandstone water wetting viscoelastic
surfactant and
polymer based composition for well fracturing fluids. More specifically, it
relates to a
viscoelastic anionic surfactant in combination with relative permeability
modifier polymer based
compositions for selectively increasing hydrocarbon production (oil and or
gas) while
simultaneously maintaining ur reducing the flow of subterranean aqueous fluids
into a well,
thereby improving hydrocarbon production efficiency.
BACKGROUND OF THE INVENTION
[0003] Various types of wellbore fluids are used in operations related to the
development,
completion, and production of natural hydrocarbon reservoirs. The operations
include fracturing
subterranean formations, modifying the permeability of subterranean
formations, or sand control.
Of particular interest with regard to the present inventions are fluids that
could be used
simultaneously for fracturing and controlling water applications during the
life cycle of a
hydrocarbon well, e.g., a well for extracting oil or nattual gas from the
Earth, wherein the
producing well commonly also yields water. In these instances, the amount of
water produced
from the well tends to increase over time with a concomitant reduction of
hydrocarbon
production. Frequently, the production of water becomes so profuse that
remedial measures
have to be taken to decrease the water/hydrocarbon production ratio. As a
final consequence of
the increasing water production, the well has to be abandoned. If the
formations are
hydraulically fractured with the objective of increasing oil recovery, water
production will also
be increased, further reducing the productive life of the well.
[0004] The chemicals that can be used to modify the permeability of
subterranean reservoirs, and
hence the undesirable water production, must preferentially be easily pumped
(i.e. have a low
viscosity) so that they may be easily placed into the reservoir sufficiently
far from the wellbore
so as to be effective. It is also desirable for the chemicals or mixtures to
reduce the permeability
of the reservoir to flooding fluids (water) while simultaneously retaining
most of the
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-2-
hydrocarbon permeability. Additionally, their placement should be selective in
that they are
placed into and reduce the permeability of flooding or driving fluids (water)
without significantly
affecting the less permeable strata. Numerous attempts at resolving these
issues have been
attempted over the years.
[0005] In many cases, a principal component of well water control fluids have
been gelling
compositions, usually based on polymers or cross-linked polymers. Water
control fluids must
selectively stop water production without detrimental effects to the oil
production. Initially the
preferred materials for this purpose were relative permeability modifiers
(RPMs) such as
polyacrylamide solutions. These polymers have long molecular chains that,
after being injected,
adhere loosely to the pore spaces, producing a drag on water production
(resist water flow)
without detrimental effects to the hydrocarbon (oil or gas) production.
Unfortunately, these
same compounds alone are shear sensitive and have their molecular weight
(length of the chain)
reduced when sheared, siguficantly affecting their ability to control/reduce
water flow.
[0.006] New generations of RPM materials have been developed that are less
sensitive to shear
and contain charged sites that could be adsorbed to the rock so that they
remain in place for
longer periods of time. These compounds are capable of reducing the relative
permeability to
water by 2 to 100 fold, depending on the differential pressure. However, for
them to anchor
properly to the rock these new RPM materials must be water wet and free of oil
residues, owing
to the fact that these fluids usually incorporate in their formulation a muW
al solvent and water-
wetting surfactants or detergents. Unfortunately, these compounds also are
pour at controlling
water in a fracture in heavy oil or asphaltenic crudes with deposits.
[0007] In attempting to improve water control down the wellbore and in the
reservoir, it is
important to understand the effects water has on the surrounding fluids. Water
production is
directly proportional to the mobility ratio of the formation fluids and
therefore to their viscosities
and relative permeability. Water at downhole conditions will have a viscosity
ranging from 0.2
cps to 1 cps, depending on the well temperature. On the other hand, oil is
encountered around
the world with viscosities that could be as high as 5000 cps. Consequently,
whenever the oil/
water viscosity ratio is higher than 10, these RPM solutions become
ineffective. In order to
overcome this situation and improve water control, higher gel strengths have
been suggested and
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-3-
pore blocking systems were developed (such as delayed cross-linked. pc.~lymer
solutions).
However, these compounds have low viscosity while being pumped and develop
high viscosity
3-dimensional gels over time.
[0008] Such pore-blocking systems could also preferentially block the oil-
producing zone so
they could not be used in fracturing treatments, therefore, to overcome this
potential problem,
rely on selective placement within the pore system for successfully blocking
only the water
production. This has been attempted at matrix rates (radial injection) by
either mechanical
methods when the water zone is known, or by reducing the viscosity of these
fluids to water
viscosity (very low polymer concentration) making the gel almost transparent
to water so it can
preferentially follow the water production paths in the reservoir instead of
oil paths. Moreover,
to achieve this and simultaneously obtain deep penetration into the reservoir,
the fluids are
pumped at very low injections rates (meaning that these treatments could last
several days or
even weeks). Unfortunately, sandstone forniations are extremely heterogeneous,
allowing these
fluids to easily invade the oil-producing zone, blocking the pores with
overall detrimental effects
to oil or other hydrocarbon. production.
[0009] Gels which are formed by polyacrylamide (U.S. Patent No. 3,490,533) or
polysaccharides
(U.S. Patent No. 3,581,524; U.S. Patent No. 3,908,760; U.S. Patent No.
4,048,079) with cations
have been used as permeability modifiers for subterranean reservoirs. However,
their application
has been limited to reservoirs with low ambient temperature (less than 70
°C). Modifications of
these compounds, such as sulfomethylated melamine gels (U.S, Patent No.
4,772,641) have been
made, but fail to address all of the needs of an effective well fracturing
fluid. Additionally,
numerous difficulties have been encountered with the use of such gel-forming
chemicals, such as
premature gel forniation with concomitant plugging of the reservoir strata
near the wellbore;
decomposition of polyacrylamides and/or polysaccharides at elevated
temperatures leading to
destruction of gel character and loss of any permeability modifying
attributes; and, over cross-
linking and syneresis of the gel at elevated temperatures, thereby reducing
the effectiveness of
the gel as a permeability modifier.
[0010] Recently, the use of cross-linked gels that suffer controlled syneresis
(gel contraction
with extrusion of water) that partially unblock some of the pores spaces has
been attempted.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-4-
However, this effect happens in both water and oil pores, thereby eliminating
any water control
effect.
[0011 ] More recently, cationic viscoelastic surfactants such as N-erucyl-N,N-
bis (2-
hydroxyethyl)-N- methylammonium chloride (which exlubits oil wetting tendency
in sandstones)
have been frequently documented for application as an aid in water control in
well fracturing
fluids. This surfactant and similar surfactants are identified in lv.T.S.
Patent No. 4,695,389; U.S.
Patent No. 4,725;372, U.S. Patent No. 5,551,516, U.S. Patent No. 5,964,295,
and U.S. Patent No.
6,194,356 B1. Unfortunately being cationic and oil wetting, some of the
benefits are lost as
detailed earlier.
[0012] In many cases, a principal component of wellbore fracturing fluids are
gelling
compositions, usually based on polymers and more recently on viscoelastic
surfactants. The
complete development of fracturing fluid and required properties are fully
explained in SPE
37359 (Rae, P.; Di Lullo, G., "Fracturing Fluids and Breaker Systems: A Review
of the State of
the Art", Soc. Pet. Engi~., 37359, 1996).
[0013] The three dimensional gels produced by viscoelastic surfactants are
preferred as well
fracturing fluids when compared with other polymer linear or cross-linked gels
even though they
are more expensive because of their ability to support/transport solids at low
viscosities and
because 'they break cleanly (their viscosity reverts to that of water) in the
presence of
hydrocarL~ons, thus producing little or no damage to the sand pack (proppant
pack) and to the
formation rock, thereby yielding higher well production rates.
[0014] The viscoelasticity of the surfactant solutions appears and forms
rapidly on mixing the
various components which are usually rilixed and propoutioned continuously
during. the
fracturing process. However, being shear thinning fluids they can be easily
placed down the
well. Viscoelastic gels are solid/polymer free fluids, and therefore their
filtration into the
formation matrix during the fracturing process is strictly dependent on the
fluid leak-off
viscosity. Fracturing fluids (irrespective of their chemistry) whose leak-off
is controlled by
viscosity, are less efficient than wall-building fluids, being at least one
order of magnitude (10
times) worse than polymer based gels (which act to control their filtration
process through
polymer filter "cake" at the forniation face). Thus, viscoelastic gels are
relatively inefficient
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-5-
fracturing fluids and their use is limited to small and medium size fracture
treatments. This
could be overcome by the addition of solids or fluid loss control additives
but with detrimental
damaging effects to the sand/proppant pack. Another option could be the use of
higher
surfactant concentration to generate higher viscosities than those required to
transport the
sand/proppants at surface conditions, but this approach make the fluids very
expensive and also
surface handling or placement more difficult, especially when fluids are batch
mixed. If this
approach is acceptable, any application of viscoelastic surfactant gels that
requires their
transport or placement after their preparation would benefit from a method of
controlling their
viscosities, filtration properties, and gel times.
[0015] In addition to the reduced damage to the sand pack and to the rock,
another possible
benefit of using viscoelastic gels is controlling water production.
Viscoelastic gels do not break
easily in the presence of water, therefore any gel that infiltrated a water
zone will maintain its
viscoelastic properties, and being a shear thinning fluid under static
conditions, will develop
extremely high viscosities (over 10000 cps) that will block the pores and
prevent water flow.
However, another important factor in controlling water and hydrocarbon flow
through a porous
media is the wettability of the rock. Sandstone formation needs to be water
wet for higher
hydrocarbon flow, as oil wet sandstone rocks favor water flow. Cationic
surfactants usually oil
wet sandstone so the benefit obtained from blocking water in the water
saturated pores are partly
overcome by favoring water flow in the recently oil wet pores. Because
viscoelastic surfactant
properties/viscosity are affected by other chemicals (glycols, alcohols,
mutual solvents and other
surfactants but not limited to these families) it is quite difficult to
overcome this detrimental oil
wetting effect.
[0016] Thus, it can be seen that there is a need for improved compositions for
wellbore water
controlling fluids, especially fracturing fluids based on water wetting
viscoelastic surfactants due
to their higher hydrocarbon production potential (reduced formation damage)
and to their
potential water control properties. Moreover, it is desirable to reduce the
viscoelastic gel
filtration process of such compositions by either irZ-sitar viscosity
generation, or by the addition
of water control chemicals or polymers that do not affect the viscoelastic
properties of the gel
and the rock oil relative permeability such as an RPM type material, in order
to reduce the cost
and allow for its application in bigger treatments. Providing such
compositions with high and
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
long lasting viscosity in the water-saturated pores and with water wetting
properties in order to
reduce water flow improves the oil/water ratio, and ultimately hydrocarbon
(oil/gas) recovery,
after fracturing operations within the hydrocarbon wells.
SUMMARY OF THE INVENTION
[0017] The invention relates to the synergistic effect obtained by a
combination of anionic
surfactant gels and amphoteric polymeric materials to be used as non-damaging
fracturing fluids
with long lasting water control properties.
[0018] An embodiment of the present invention is a viscous fluid which is
particularly useful in
transporting particulate through a conduit. The viscous fluid of the invention
has application for
conventional well fracturing, coiled tubing fracturing, and frac packing,
concurrently with water
control.
[0019] Another aspect of this invention, as described, is the use of
amphoteric polymeric
materials, typically RPMs, improving the leak-off properties of the VAS gel as
a fracturing fluid.
The VAS gel, in turn, cleans the formation from oil residues in order to
improve the anchoring of
the RPM to the forniation minerals.
[0020] A further synergistic effect attributed to the specific combination of
this invention relates
to duration of the treatment. While the VAS gel formed in the water pores
could readily flow or
move under higher pressures, such as found in a wellbore, the RPM anchored to
the rock aids in
preventing such movement, allowing the treatment to last longer. Additionally,
the viscoelastic
fluid breaks by dilution with formation water, so this anchoring process
prevents intermixing
within the rock pores.
[0021] The VAS gel that breaks in the oil containing pores in turn leaves the
formation water
wet favoring oil flow and anchored to the RPM of the fluid for future water
control when the
water level rises within the reservoir.
[0022] The combined gel of the present invention treats all four aspects of
the fracture in an
equal manner, regardless of heterogeneities, as a result of higher injection
rates and pressures
from fracturing in comparison with radial matrix treatments. Within a short
period of time, the
leak-off fluid begins to break in the oil pores and starts to receive a drag
force in the water
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
saturated pores, favoring the preferential propagation of the fracture within
the oil producing
zone.
[0023] Additional objects, features, and advantages will be apparent in the
written description,
which follows.
DESCRIPTION OF THE FIGURES
[0024] The following figures form part of the present specification and are
included to further
demonstrate certain aspects of the present invention. The invention may be
better understood by
reference to one or more of these figures in combination with the detailed
description of specific
embodiments presented herein. The components in the figures are not
necessarily to scale, with
the emphasis instead being placed upon clearly illustrating principles of the
present invention.
[0025] Figure 1 describes the rheology of the Elastrafrac with a phosphate
salt, indicating that
the viscosity is exceptionally good for fracture creation and expansion. The
fluid is shear
thinning (viscosity drops as the shear rate increases) for low friction
pressures and the fluid have
n' < 0.6 and K' > 0.01 which is better than adequate for sand/proppant
transport. '
[0026] Figure 2 describes the temperaW re and velocity of the shear rate using
M-Aquatrol with a
phosphate salt. Notice that the viscosity and its rheology are similar to the
gel without the
Aquatrol, and the fluid is viscoelastic and shear thinning with no detrimental
effects to the gel by
the addition of Aquatrol.
[0027] Figure 3 describes the development of viscosity vs. pH and shows how
the viscosity can
be increased or reduced when the pH is adjusted to an optimum value (usually
the formation
pH).
[0028] Figure 4 shows a table that indicates the filtrate volumes vs. time for
an Elastrafrac fluid
with and without the addition of Aquatrol Concentrate. As can be seen, the
fluid's viscosity with
and without Aquatrol C, and pre- and post-filtering are similar, however the
filtration efficiency
measured by a calculated C« value (fluid-loss coefficient) is over 100 times
better (i.e. smaller)
for the ElastraFrac formulation containing Aquatrol.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-s-
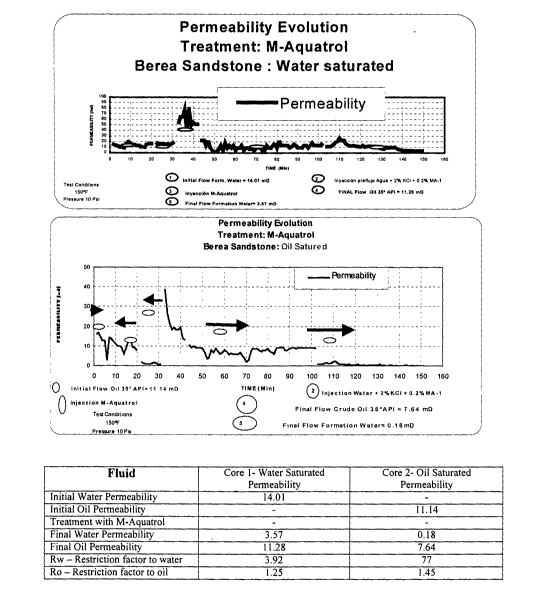
[0029] Figure 5 contains two graphs and a table exhibiting the combined effect
of controlling
water without detrimental effects on oil permeability on a dual parallel core
filtration test using
M-Aquatrol.
DEFINITIONS
[0030) The following definitions are provided in order to aid those skilled in
the art in
understanding the detailed description of the present invention.
[0031 ] The term "amphoteric" refers to surfactants that have. both positive
and negative charges.
The net charge of the surfactant can be positive, negative, or neutral,
depending on the pH of the
solution.
[0032] The term "anionic" refers to those viscoelastic surfactants that
possess a net negative
charge.
[0033] The term "fracturing" refers to the process and methods of breaking
down a geological
formation, i.e. the rock fornzation around a well bore, by pumping fluid at
very high pressures, in
order to increase production rates from a hydrocarbon reservoir. The
fracturing methods of this
invention use otherwise conventional techniques known in the art.
[0034] The term "proppant" refers to a granular substance suspended in the
fracturing fluid
during the fracturing operation, which serves to keep the formation from
closing back down
upon itself once the pressure is released. Proppants envisioned by the present
invention include,
but are not limited to, conventional proppants familiar to those skilled in
the art such as sand,
20-40 mesh sand, resin-coated sand, sintered bauxite, glass beads, and similar
materials.
[0035] The abbreviation "RPM" refers to relative permeability modifiers.
[0036] The term "surfactant" refers to a soluble, or partially soluble
compound that reduces the
surface tension of liquids, or reduces inter-facial tension between two
liquids, or a liquid and a
solid by congregating and orienting itself at these interfaces.
[0037] The term "viscoelastic" refers to those viscous fluids having elastic
properties, i.e., the
liquid at least partially returns to its original form when an applied stress
is released.
[0038] The phrase "viscoelastic surfactants" or "VES" refers to that class of
compounds which
can form micelles (spherulitic, anisometric, lamellar, or liquid crystal) in
the presence of counter
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-9-
ions in aqueous solutions, thereby imparting viscosity to the fluid.
Anisometric micelles in
particular are prefe~~red, as their behavior in solution most closely
resembles that of a polymer.
[0039] The abbreviation "VAS" refers to a Viscoelastic Anionic Surfactant,
useful for fracturing
operations and frac packing. As discussed herein, they have an anionic nature
with preferred
counterions of potassium, ammonium, sodium, calcium or magnesium. .
DESCRIPTION OF THE INVENTION
[0040] One embodiment of the invention is directed towards a fluid for use as
a well fracturing
fluid,. the fluid comprising a liquid cawier, a surfactant, and an amphoteric
polymer.
[0041] The liquid carrier can generally be any liquid caixier suitable for use
in oil and gas
producing wells. A presently preferred liquid carrier is water. The liquid
carrier can comprise
water, can consist essentially of water, or can consist of water. Water will
typically be a major
component by weight of the fluid. The water can be potable or non-potable
water. The water
can be brackish or contain other materials typical of sources of water found
in or near oil fields.
For example, it is possible to use fresh water, brine, or even water to which
any salt, such as an
alkali metal or alkali earth metal salt (NaC03, NaCI, KC1, etc:) has been
added. The liquid
carrier is preferably present in an amount of at least about 80% by weight.
Specific examples of
the amount of liquid carrier include 80%, 85%, 90%, and 95% by weight.
The.canier liquid can
be a VAS gel.
[0042] The pH of the fluid can generally be any pH compatible with downhole
formations. The
pH is presently preferred to be about 6.5 to about 10Ø The pH can be about
the same as the
formation pH.
[0043] The surfactant can generally be any surfactant. The surfactant is
preferably viscoelastic.
The surfactant is preferably anionic. The anionic surfactant can be an alkyl
sarcosinate. The
alkyl sarcosinate can generally have any number of carbon atoms. Presently
preferred alkyl
sarcosinates have about 12 to about 24 carbon atoms. The alkyl sarcosinate can
have about 14 to
about 18 carbon atoms. Specific examples of the number of carbon atoms include
12, 14, 16, 18,
20, 22, and 24 carbon atoms.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-10_
[0044] The anionic surfactant can have the chemical formula RICON(R2)CHZX,
wherein Rl is a
hydrophobic chain having about 12 to about 24 carbon atoms, R2 is hydrogen,
methyl, ethyl,
propyl, or butyl, and X is carboxyl or sulfonyl. The hydrophobic chain can be
an alkyl group, an
alkenyl group, an alkylarylalkyl group, or an alkoxyalkyl group. Specific
examples of the
hydrophobic chain include a tetradecyl group, a hexadecyl group, an
octadecentyl group, an
octadecyl group, and a docosenoic group.
[0045] The surfactant can generally be present in any weight percent
concentration. Presently
preferred concentrations of surfactant are about 0.1% to about 15% by weight.
A presently more
preferred concentration is about 0.5% to about 6% by weight. Laboratory
procedures can be
employed to determine the optimum concentrations for any particular situation.
[0046] The amphoteric polymer can generally be any arnphoteric polymer. The
amphoteric
polymer can be a nonionic water-soluble homopolysaccharide or an anionic water-
soluble
polysaccharide. The polymer can generally have any molecular weight, and is
presently
preferred to have a molecular weight of at least about 500,000.
[0047] The polymer can be a hydrolyzed polyacrylamide polymer. The polymex can
be a .
scleroglucan, a , modified scleroglucan, or a scleroglucan modified by contact
with glyoxal or
glutaraldehyde. The scleroglucans are nonionic water-soluble
homopolysaccharides, or water-
soluble anionic polysaccharides, having molecular weights in excess of about
500;000, the
molecules of which consist of a main straight chain formed of D-glucose units
which are bonded
by (3-1,3-bonds and one in three of which is bonded to a side D-glucose unit
by means of a (3-1,6
bond. These polysaccharides can be obtained by any of the known methods in the
art, such as
fermentation of a medium based on sugar and inorganic salts under the action
of a
microorganism of Sclerotium type A. A more complete description of such
scleroglucans and
their preparations may be found, for example, in U.S. Patent Nos. 3,301,848
and 4,561,985. In
aqueous solutions, the scleroglucan chains are combined in a triple helix,
which explains the
rigidity of the biopolymer, and consequently its features of high viscosity-
increasing power and
resistance to shearing stress.
[004S] It is possible to use, as source of scleroglucan, the scleroglucan
which is isolated from a
fermentation medium, the product being in the form of a powder or of a more or
less
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-11-
concentrated solution in an aqueous and/or aqueous-alcoholic solvent.
Scleroglucans
customarily used in applications in the petroleum field are also preferred
according to the present
invention, such as those which are white powders obtained by alcoholic
precipitation of a
fermentation broth in order to remove residues of the producing organism
(mycelium, for
example). Additionally, it is possible to use the liquid reaction mixture
resulting from the
fermentation and containing the scleroglucan in solution. According to the
present invention,
further suitable scleroglucans are the modified scleroglucan which result from
the treatment of
scleroglucans with a dialdehyde reagent (glyoxal, glutaraldehyde, and the
like), as well as those
described in LT.S. Patent No. 6,162,449 ((3-1,3-scleroglucans with a cross-
linked 3-dimensional
structure produced by Scle~°otiunt rolfsii).,
[0049] The polymer can be Aquatrol V (a synthetic compound which reduces water
production
problems in well production; described in U.S. Patent No. 5,465,792); AquaCon
(a moderate
molecular weight hydrophilic terpolymer based on polyacrylamide capable ' of
binding to
formation surfaces to enhance hydrocarbon production; described in U.S. Patent
No. 6,228,812)
and Aquatrol C (an amphoteric polymeric material). Aquatrol V, Aquatrol C, and
AquaCon are
commercially available from BJ Services Company.
[0050] The polymer can be a terpolymer synthesized from an anionic monomer, a
cationic
monomer, and a neutral monomer. The monomers used preferably have similar
reactivities so
that the resultant amphoteric polymeric material has a random distribution of
monomers. The
anionic monomer can generally be any anionic monomer. Presently preferred
anionic monomers
include acrylic acid, methaciylic acid, 2-acrylamide-2-methylpropane sulfonic
acid, and malefic
anhydride. The cationic monomer can generally be any cationic monomer.
Presently prefeiTed
cationic monomers include dimethyl-diallyl annnonium chloride, dimethylamino-
ethyl
methacrylate, and allyltrimethyl ammonium chloride. The neutral monomer can
generally be
any neutral monomer. Presently prefeiTed neutral monomers include butadiene, N-
vinyl-2-
pywolidone, methyl vinyl ether, methyl acrylate, malefic anhydride, styrene,
vinyl acetate,
acrylamide, methyl methacrylate, and acrylonitrile. The polymer can be a
terpolymer
synthesized from acrylic acid (AA), dimethyl diallyl ammonium chloride
(DMDAC), and
acrylamide (AM). The ratio of monomers in the terpolymer can generally be any
ratio. A
presently preferred ratio is about 1:1:1.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
- 12-
[0051 ] Another presently preferred amphoteric polymeric material (hereinafter
"polymer 1 ")
includes approximately 30% polymerized AA, 40% polymerized AM, and 10%
polymerized
DMDAC with approximately 20% free residual DMDAC which is not polymerized due
to lower
relative reactivity of the DMDAC monomer. The stuuctural formula for a
preferred amphoteric
polymeric material is shown below:
AA AM DMDAC
CHI CH CHI CH CHZ CH CH CHI
COZ- ~ONH~ H~~ CHZ
Cl-
K+ CH;~N\CH;
[0052] The fluid can further comprise one or more additives. The fluid can
fiirther comprise a
base. The fluid can further comprise a salt. The fluid can further comprise a
buffer. The fluid
can fiu-ther comprise a relative pernzeability modifier. The fluid can further
comprise
methylethylamine, monoethanolamine, triethylamine, triethanolamine, sodium
hydroxide,
potassium hydroxide, potassium carbonate, sodium chloride, potassium chloride,
potassium
fluoride, KH?PO~, or K~HP04. The fluid can further comprise a proppant.
Conventional
proppants will be familiar to those skilled in the ant and include sand, resin
coated sand sintered
bauxite and similar materials. The proppant can be suspended in the fluid.
[0053] Relative peuneability modifiers can be added to the fluids to further
improve water shut
off properties. These compounds are polymers that are water-soluble and
improve the leak-off
viscosity of the fracturing fluid.
[0054] A specific example of a treating fluid is as follows: (a) 11% KC1 by
weight; (b) 2.5
surfactant by weight; (c) I.6% buffer (potassium carbonate in water (45% by
weight potassium
carbonate)) by volume, and (d) 1.0% of 10% (by weight) Polymer 1 solution. The
rheology of
this formulation is shown in Table 1 and Figures 1-2.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-13-
[0055] An additional embodiment of the invention involves the use of any of
the above
described fluids in a method of fracturing a subterranean formation. The
method can comprise
providing a fluid comprising a liquid carrier, a viscoelastic anionic
surfactant, and an amphoteric
. polymer, pumping the fluid through a wellbore, and contacting the fluid and
the subterranean
formation to fracture the formation.
[0056] A further additional embodiment of the invention involves the use of
airy of the above
described fluids in a method of reducing the amount of water produced from a
subterranean oil
producing formation. The method can comprise providing a fluid comprising a
liquid carrier, a
viscoelastic anionic surfactant, and an amphoteric polymer, pumping the fluid
through a
wellbore, contacting the fluid and the subterranean formation, and obtaining
product from the
formation. The weight percent of water in the product is less than the weight
percent of water in
product produced from a similar formation that was not contacted with the
fluid. The fluid can
further comprise a relative permeability modifier. The CW of the similar
forniation that was not
treated with the fluid ("untreated C,~") is preferably greater than the C,~.
of the formation treated
with the fluid ("treated C«"). The ratio of the untreated C,v to the treated
Cw is preferably at least
about 2, at least about 5, at least about 10, at least about 20, at least
about 30, at least about 40, at
least about 50, at least about 60, at least abOLlt 70, at least about 80, at
least about 90, at least
about 100, at least about 150, or at least about 200.
[0057] According to other embodiments of the invention, there is provided a
fracturing fluid
comprising anionic viscoelastic surfactants which viscosify and its leak-off
viscosity can be
enhanced while the fluid is injected in the pores of the rock, providing water
shut off and
favoring oil/gas flow and allowing non damaging polymers such as relative
permeability
modifiers to be included in the fornmlations without adversely affecting the
gel viscosity but
improving the gel filtration efficiency and its water control properties.
[0058] Some embodiments of the invention take advantage of the natural pH
change at the
formation rock to cause an increase in the gel viscosity at the formation
pores to block water
production, which is discussed herein. For example, in its use the fluid is
designed for optimum
viscosity at the same pH of the formation water/rock. However it is piunped at
a pH that is lower
or higher than the formation pH (0.3 to 1 unit) through a wellbore and into a
surrounding
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-14-
fornzation having an aqueous zone and a hydrocarbon zone. The fluid is then
allowed to contact
the aqueous zone and the hydrocarbon zone. Contact with the hydrocarbon zone
serves to thin
the fluid since the surfactant gel is thinned by hydrocarbons. While contact
with the water zone
or water saturated pores will lower the gel pH to that of the formation
increasing its
viscoelasticity and viscosity. Additionally, if an RPM polymer is included in
the formulation it
will adhere to the water wet rock and induce a drag, or friction force on
water, reinforcing the
viscoelastic gel structure and also lubricating oil production, ser<ling to
preferentially block the
flow of water from that portion of the formation. Consequently oil production
is unaffected
while water flow is preferentially shut off.
[0059] The amphoteric polymeric material is characterized by the presence of
both positively
and negatively charged components along. the polymer chain. This nature of the
polymeric
material is believed to account for the polymeric material's ability to
strongly bond to the
formation while exhibiting a hydrophilic character capable of forming a strong
hydrogen bond to
water causing a drag or a higher friction pressure on water flowing through
the capillaries or
openings of the formation. By whatever mechanism, the mobility of formation
water is greatly
reduced by ahe amphoteric polymeric material without restricting the
production of oil or gas to
any appreciable extent.
[0060] Additional description of various embodiments of the invention are
provided below. The
description with respect to "well-treating solution", and "viscous fluid" is
applicable, with or
without modifications, to the well service fluid in accordance with
embodiments of the
invention.. It should be noted that any number disclosed herein should be
understood as to mean
an approximate value, regardless of whether the word "about" or "approximate"
is used in
describing the number.
[0061 ] A presently preferred well treating solution for changing the relative
permeability of a
formation to water can be prepared by adding the amphoteric polymeric material
to VAS cawier
liquid with the amphoteric polymeric material being present at about 1.0% to
about 10% by
volume, depending upon the permeability.
[006?] The resulting treating solution can be injected into the formation at
pumping rates and
treating pressures above the fracture gradient of the formation. The volume of
treating solution
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-15-
used is based on the desired fracture geometry, the thickness of the zone to
be treated, the
porosity of the formation being treated, and other factors.
[0063] The viscous fluids of the invention can be used for transporting
particulate through a
conduit to a subterranean location. In one form, the fluids comprise an
aqueous base, a
surfactant comprising an alkyl sarcosinate having from about 12 to about 24
carbon atoms and a
buffer for adjusting the pH of the combined aqueous base and surfactant at or
for the formation
pH. ~ The alkyl sarcosinate is preferably present at about 0.5% to about 10%
by weight, based
upon the weight of the total fluid. The pH of the viscous fluid is preferably
adjusted with the
buffer to about 6.5 to about 10.0 for most formations.
[0064] The viscous fluids of the invention can also include an additional
source of anions in
addition to those furnished by the surfactant. The additional source of anions
can be a co-
surfactant such as any ionic or anionic undiluted surfactant.
[0065] In the method of fracturing a subterranean formation of the invention,
an aqueous base
fluid is combined with a surfactant comprising an alkyl sarcosinate having
from about 12 to
about 24 carbon atoms. The combined fluid is buffered to thereby adjust the pH
of the combined
aqueous base and surfactant at or for the formation pH, thereby creating a
viscous fluid capable
of supporting proppant. The viscous fluid is pumped through a wellbore and
into a surrounding
formation at a pressure sufficient to fracture the formation.
[0066] The viscous fluids of the invention can also be used in a method for
reducing the amount
of water produced from a subterranean oil producing formation. An aqueous base
fluid is
combined with a surfactant comprising an alkyl sarcosinate having from about
12 to about 24
carbon atoms. The combined fluid is buffered to thereby adjust the pH of the
combined aqueous
base and surfactant sufficiently to produce a viscous fluid. The viscous fluid
is pumped through
a wellbore and into a surrounding formation having an aqueous zone and a
hydrocarbon zone,
the aqueous zone comprising water. The viscous fluid is then allowed to
contact the aqueous
zone and the hydrocarbon zone. Contact with the hydrocarbon zone serves to
thin the viscous
fluid while contact with the aqueous zone serves to preferentially block the
flow of water from
that portion of the formation.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-16-
[0067] The viscoelastic surfactant fluid is useful as a fracturing fluid with
improved efficiency.
Specifically, the use of this fluid in fracturing a formation will
simultaneously enhance oil
production while simultaneously drastically minimizing or completely stopping
water
production.
[0068] In a prefewed form, the viscous fluids of the invention comprise water,
a base, a
surfactant comprising an alkyl sarcosinate having from about 12 to about 24
carbon atoms in the
alkyl group, and a buffer for adjusting the pH, of the combined aqueous base
and surfactant at or
for the formation pH. As will be explained in detail, the fluids of the
invention can be optimized .
for viscosity and for the formation pH in order to reduce ion exchange at the
formation, thereby
avoiding clay dispersion and swelling. The water used in formulating the
fluids can be fresh
water or light brines from any convenient source. The particularly preferred
alkyl sarcosinates
used as the surfactant have an alkyl group of about 14 to about 18 carbon
atoms.
[0069] Sarcosine (N-methylglycine) is a naturally occurring amino acid found
in starfish, sea
urchins and crustaceans. It can be purchased from a variety of commercial
sources, or
alternately produced by a number of synthetic routes lmo~.vn in the art
including thermal
decomposition of caffeine in the presence of barium hydroxide (Arch. Phar~m.
232: 601, 1894);
(Bzrll. Claer~~. Soc. Japan, ~9: 2535, 1966); and numerous others (T. Shirai
in Synthetic
Production and Utilization of Amino Acids; T. Ivaneko, et al., Eds.; Wiley;
New fork: pp. 184-
186, 1974). Sodium sarcosinate is manufacW red commercially from formaldehyde,
sodium
cyanide and methyl amine (U.S. Patent Nos. 2,720,540 and 3,009,954). ' The
preferred
sarcosinate are the condensation products of sodium sarcosinate and a fatty
acid chloride. The
fatty acid chloride is reacted with sodium sarcosinate under carefully
controlled alkaline
conditions (i.e. the Schotten-Bauman reaction) to produce the fatty
sarcosinate sodium salt which
is water soluble. Upon acidification, the fatty sarcosine acid, which is also
water insoluble, is
formed and may be isolated from the reaction medium. The aryl sarcosines may
be neutralized
with bases such as the salts of sodium, potassium, ammonia, or organic bases
such as
triethanolamine in order to produce aqueous solutions. The preferred
sarcosinates of the
invention can be represented structurally as:
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-17-
O
Rl NIX
n2
[0070] wherein R, is a hydrophobic moiety of alkyl, alkenyl, alkylarylalkyl,
alkoxyalkyl, and the
like, wherein alkyl and alkenyl represent groups that contain about 12 to
about 24 carbon atoms
which may be branched or straight chained. Representative long chain alkyl
groups include, but
are not limited to, tetradecyl, hexadecyl, octadecentyl (oleyl), octadecyl
(stearyl), and docosenoic
functionalities. R2 is hydrogen, methyl, propyl, butyl, or ethyl. X is
carboxyl or sulfonyl.
[0071 ] One particular surfactant selected for use in the method of the
invention is an anionic
sarcosinate surfactant available commercially from BJ Services Company as "M-
Aquatrol"
(MA). The MA-1 sarcosinate is a viscous liquid surfactant with at least 94%
oleoyl sarcosine.
For hydraulic fracturing, a sufficient quantity of the sarcosinate is present
in aqueous solution to
provide sufficient viscosity to suspend proppant during placement. The
surfactmt is preferably
present at about 0.5% to about 10% by weight, most preferably at about 0.5% to
about 6% by
weight, based upon the weight of the total fluid. Laboratory procedures can be
employed to
determine the optimum concentrations for any particular job.
[0072] The surfacant can be added to an aqueous solution in which there is
typically dissolved a
quantity of at least one water soluble salt to effect formation stability.
Typical water-soluble
salts include potassium chloride, sodium chloride and the like. Formation
stability is typically
achieved with only small concentrations of salt. The water-soluble salts may
be considered part
of the "buffer" for adjusting the pH of the combined aqueous base and
surfactant in the method
of the present invention. The viscosity of the fluids of the invention are
improved significantly
by the addition of certain additional anions to the surfactant-laden solution.
The pH can be
adjusted, for example, by the addition of alkali metal, carbonate, phosphate
or borate, or organic
amines, especially alkanol amines such as mono-, di- or triethanolamine.
[0073] High temperature stability of the fluids in question is achieved if
selecting specific anion,
such as phosphate or fluoride ions instead of chlorides, preferably provided
in the form of an
inorganic phosphate or fluoride salt or a fluoride acid such as fluosilicic
acid (H2SiF6). The
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-18-
fluoride salt concentration can be about 0.5% to about 10% by weight, and more
preferably
about 3% to about 7% by weight, based upon the total weight of the fluid.
Typical fluoride salts
include ammonium bifluoride and potassium fluoride. The pH of the surfactant-
fluoride salt
solution can be adjusted to about 6.5 to about 10. The pH can be adjusted with
the same bases as
discussed above.
[0074] Each salt will produce a peak viscosity at a different pH. The fluids
of invention are
optimized for viscosity a.nd formation pH as will be discussed with respect to
the laboratory
analyses which follow.
[0075] In the method of fracturing a formation using the formulations of the
invention, an
aqueous base fluid is combined with an anionic surfactant comprising an alkyl
sarcosinate
having from about 12 to about 24 carbon atoms, and alternatively a
viscoelastic polymer such as
an RPM. Standard mixing procedures known in the art can be employed since
heating of the
solution or special agitation procedures are not normally required. The
aqueous base has been
buffered with a buffer to thereby adjust the pH of the combined aqueous base
and surfactant
above about 6.5, thereby creating a viscous fluid capable of supporting
proppant. The proppant
can be added and the viscous fluid can then be pumped through a wellbore and
into a
surrounding formation at a pressure sufficient to fracture the formation.
Typically, the viscous
fluid can be allowed to contact the formation for a period of tine sufficient
to increase the
viscosity in the water saturated pores, while in the oil pores it will thin
immediately and therefore
no breakers are required.
[0076] These effects cannot be easily achieved when cationic surfactants are
used. Due to the
fact that cationic surfactants are not pH dependent with regards to viscosity,
their viscosity
remains within a narrow, unadjustable range, thereby limiting their utility.
The anionic
surfactants of the present invention overcome this problem by being pH
dependent with regards
to viscosity, thereby allowing for their viscosity to be adjusted to the
desired value by altering
the pH appropriately.
[0077] The fluid of the present invention may also be used as asphaltene-
dispersing agents.
Asphaltenes are constituents of crude oils, usually present as colloidal
dispersions stabilized by
resins in the oil. While examples of asphaltene-dispersing agents are know in
the art (e.g. U.S.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
- 19-
Patent No. 5,948,237), the sarcosinate anionic surfactant of the invention in
combination with
RPM type materials produces a synergistic effect in this regard. Specifically,
these compomds
in combination form an excellent asphaltene-dispersant, thereby aiding in the
cleaning of rocks,
pipes, valves, conveying devices, and the like by removing heavy oil deposits
and asphaltenes
themselves.
[0075] The fluids of the invention can also be used as selective water control
additives. The
viscous fluids can be pumped into a water rich sector of a producing interval:
Once placed, the
gel viscosity will prevent formation water flow through that portion of the
reservoir. On the
other hand, gel pumped into the oil rich sector of the formation reservoir
will immediately thin
on contact with the oil contained within the reservoir. Consequently, oil
production will be
uninhibited while water flow will be preferentially stopped or significantly
reduced.
[0079] For fracturing applications, the fluids of the invention are typically
pumped downhole at
or slightly above the formation pH. Preferably, when the fluids of the
invention are used for
water control purposes, the fluids are pumped downhole at about 3/10 of a pH
unit less or more
than the formation material pH depending on the anion portion of the salt used
as counter cation.
The fluid is thus pumped in a thinned state, reducing the friction pressure of
the pumping job.
Upon contacting the formation material, the pH of the fluid increases,
resulting in complete
gellation of the fluid at the forniation location rather than at the well
surface. '
[0050] The following examples are included to demonstrate prefeiTed
embodiments of the
invention. It should be appreciated by those of skill in the aut that the
teclmiques disclosed in the
examples which follow represent techniques discovered by the inventors to
function well in the
practice of the invention, and thus can be considered to constitute preferred
modes for its
practice. However, those of skill in the art should, in light of the present
disclosure, appreciate
that many changes can be made in the specific embodiments which are disclosed
and still obtain
a like or similar result without departing from the spirit and scope of the
invention.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-20-
EXAMPLES
Example 1: General methods
[0081 ] Each of the components of the viscous fluids of the invention is
readily soluble in water
or light brines at pH higher than 4.5. The fluid is easily prepared in mixing
equipment that
allows circulation or agitation, i.e. blenders, tanks equipped with augers or
tanks that are
connected to a circulating pump, such as an acid transport trailer or in
displacement tanks of
cementing units.
[0082] A prefeiTed mixing procedure is as follows.
[0083] 1) Load the fresh water necessary to prepare the desired volume of
viscous fluid a
clean tank.
[0084] 2) while agitating or circulating, slowly add and dissolve the required
amount of the
salt, for example, KC1, KF, KHZPO~ and/or K2HP04.
[0085] The salt concentration will depend on the type of salt used and the
fnal pH, as part of the
"additional canons requirement for optimum viscosity as discussed above. The
following table
can be used as a guideline. Unless otherwise stated, the percentages are by
weight of total fluid
solution.
Salt Preferred ConcentrationMore Prefewed Concentration' Preferred
Type for Visco Fluid for Visco Fluid pH
KC1 >(% 11 -12% 7.3-8.5
NH4C1 > 2% 3 - 5% 7 - 8.5
KF >_5% 6.S-8% 8-10
ABF >_1.5% 2.5 - 3% 8 -'9.5
[0086] 3. Add the required volume of pH adjuster/buffer solution.
[0087] 4. Add the MA-l and mix slowly mix (to avoid foam) for 5-10 minutes or
until it
disperses well to produce a uniform linear gel.
[0088] 5. Add the remainder of the buffer for completed activation and full
viscosity
development.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-21 -
[0089] In the case of KF, the anionic surfactant is supplied in the acid form
is not soluble in field
water. The pH of the water in the mixing tank must be alkaline enough before
the addition of
MA- 1, so that when the MA-1 is added, the pH is at least above 4.5 and
preferentially above 7Ø
This buffering of the mixing water will prevent waxing out of the surfactant
and speeds the
mixing process. Usually with field water, the addition of 40% solution of base
such as KOH at
one-half of the concentration of MA-1 will neutralize the pH of the mixing
water when the MA-1
is added.
[0090] The tables below are examples of the viscosity optimization process
using 4% MA-1
(sarcosinate surfactant) for a target forniation pH of 8.0 using KC1 and KF
with field water. The
BF-7L is a commercially available buffer commonly used in the formulation of
fracturing fluids
which allows adjustment of pH levels in fracturing gels to about 8.5 to about
9.75, thereby
preventing the fall in pH that occurs as fluid temperatures increase. BF-7L is
commercially
available from BJ Services.
Example 2: Fluids containing potassium chloride
[0091] Formation pH: 8.0 at 180° F (82 °C); design for a pH of
8.5 to 9.0 at the well surface.
Fluid viscosity requirements = 200 cps at 180° F (S2 °C)
and 40 sec.
For KCl determination:
4% MA-1 + 2% BF-7L hCl % pH gel at 80 F Viscosity at
by weight(27 C) 40 sec and
+ 0.4% EF-7L 80 F (27 C),
CPS
4% MA-1 + 2.0% BF-7L10 8.5 1281
4% MA-1 + 2.0% BF-7L11 8.6 1565 '
4% MA-1 + 2.0% BF-7L12 8.6 1708
4% MA-1 + 2.0% BF-7L13 8.7 1512
[0092] The 12% KCl systems produced 285 cps at 40 sec I and a pH of 8.2 when
tested at 180° F
(82 °C), therefore a system with 3.5 MA-1 may be sufficient for this
treatment.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
- 22 -
Example 3: Fluids containin~~otassium fluoride
[0093] Formation pH: 8 at 180 °F (82 °C); design for 8.5 to 9.0
at the well surface. Fluid
viscosity requirements = 200 Cps at 40 sec.
For KF determination
4% MA-1 + 2% BF-7L KCl % pH gel at 80 F + Viscosity at
by weight0.4% 40 sec
EF-7L and 80 F, CPS
4% MA-1 + 2.0% BF-7L7 8.91 1424
4% MA-1 + 2.0% BF-7L8 8.95 2277
4% MA-1 + 2.0% BF-7L9 8.96 996
[0094] The 8% KF system produced 452 cps at 40 sec-1 and a pH of 8.6 when
tested at 180 °F
(82 °C), therefore a system with 3% MA-1 may be sufficient for this
treatment.
[0095] As indicated in the above examples, the formulations utilizing KF
produce about twice
the viscosity of similar formulations that contain KCI. However, KF is not
available in all
geographic locations. The formulations may use a combination of ammonium
bifluoride
(NH4HF2; ABF) and potassium hydroxide in order to produce KF in solution.
Because the
formulations are mixing cations (potassium and ammonium), it is more difficult
to optimize the
viscosity and pH.
Example 4: Field mixing surfactant solutions
[0096] Each component of the surfactant fluid is readily soluble in water or
light brines at pH
higher than 4.5. The fluid is easily prepared in mixing equipment that allow
circulation' or
agitation, i.e. blenders, tanks equipped with augers or tanks that are
connected to a circulating
pump, such as an acid transport trailer or in displacement tanla of cementing
units. The
following procedures can be used to prepare a fluid of the present invention.
[0097] 1. Load the fresh water necessary to prepare the desired volume of
fluid into a clean
tank.
[0098] 2. While agitating or circulating, slowly add and dissolve the required
amount of the
salt; see Note 1 below.
[0099] 3. Add the required volume of pH adjuster/buffer solution; see Note 2
below.
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
- 23 -
[0100] 4. Add the surfactant and mix slowly mix (to avoid foam) for 5-10
minutes or until it
disperses well to produce a uniform linear gel.
[0101] 5. Add the cowect concentration of buffer for either 1) complete
activation and full
viscosity development or 2) for partial activation and low viscosity
development
in some water control applications; see Note 3 below.
[0102] 6. Add the required volume or weight of polymer; see note 4 below.
[0103] Note 1: The salt concentration will depend on the type of salt used and
the final pH, as
part of the counter cation requirements for optimum viscosity is provided by
the pH
adjuster/buffer system used. The following table can be used as a guideline.
Salt Preferred ConcentrationMore Preferred ConcentrationPreferred
Type for Viscoelasticityfor Viscoelasticity pH
KC1 >_6% 11-12% 7.3-8.5
KF >5% 6.5-8% 8-10
~zPC~t >_ 10% 12 - 15% 6.6 - 9
~aHP~a >_ 14% 15 - 19% 7 - 9.5
[4104] Note 2: The surfactants are commercially available in acid and salt
form. The surfactant.
in the acid form is not soluble in water, and therefore the pH of the water in
the tank preferably is
sufficiently alkaline (adjusting accordingly) before the addition of
surfactant so that when the
surfactant is added, the pH is at least above 4.5 and preferentially 7. In
this manner, waxing out
(i.e. exceeding the solubility) of the surfactant (which can lead to
significantly longer mixing
times) will be reduced or eliminated.
[0105] Note 3: The fluids are preferably pumped downhole at about 0.3 to about
1 pH unit more
or less than the formation water pH, depending upon the salt used in the
formulation as KC1 salt
produce higher viscosity with lower pH while the other salts produce higher
viscosities with
higher pHs. The fluid is thus pumped in a thinner state, reducing the friction
presslu~e of the
pumping job. Upon contacting the formation, the pH of the fluid adjusts to the
formation rock
pHs, resulting in complete gellation of the fluid at the formation rather than
at the well surface or
in the fracture. The preferred pH adjusters or buffers are functional buffers
or potassium base
additives for improved viscosity. However, other cation base alkaline
materials could also be
CA 02443977 2003-10-07
WO 02/084075 PCT/US02/11147
-24-
used such as NaOH or NaZC03, as well as organic basis such as
methylethylamine,
monoethanolamine, and triethylamine.
[0106] Note 4: Depending upon the RPM polymer used, the pH of the solution may
be altered.
This needs to 'be preferentially added prior to the addition of the buffer.
Additionally, the
polymer added may be in either solid or liquid fornz.
[0107] An invention has been provided with several advantages. The fluids
described herein
have the ability to add more control to a fracture, specifically controlling
the water while
increasing the efficiency of the frac fluid. The fluids of the invention have
the ability to suspend
proppant for hydraulic fracturing operations and yet possess superior clean-up
possibilities. The
fluids can also be used fur gravel pack jobs, filter cake removal, sand or
proppant clean jobs, for
water control purposes and for other applications. The fluids are economical
to produce and
offer superior properties as compared to existing products available in the
market. The fluids are
more temperature tolerant than previously available surfactant based systems.
The KCl systems
described above can generally be used fur temperatures up to about 230
°F (110 °C) with the ILF
or phosphate systems being useful up to about 260 °F (127 °C).
The fluids also exhibit reduced
friction pressures during pumping.
[0108] All of the processes disclosed and claimed herein can be made and
executed without
undue experimentation in light of the present disclosure. While the
compositions and methods of
this invention have been described in terms of preferred embodiments, and in a
limited number
of forms, it will be apparent to those of skill in the art that variations may
be applied to the
processes and in the steps or in the sequence of steps of the methods
described herein without
departing from the concept, spirit and scope of the invention. More
specifically, it will be
apparent that certain agents which are chemically related may be substituted
for the agents
described herein while the same or similar results would be achieved. All such
similar
substitutes and modifications apparent to those skilled in the art are deemed
to be within the
spirit, scope and concept of the invention.