Note: Descriptions are shown in the official language in which they were submitted.
CA 02444749 2005-04-04
1
Golf Ball With Multi-Layer Cover
Utilizing Polyurethane Materials
Field of the Invention
s The present invention relates generally to golf balls, and more
particularly to golf balls having multi-layer covers that utilize one or more
polyurethane materials. Preferably, the cover layers exhibit hardnesses less
than 60 as measured on the Shore D hardness scale. In a further aspect, the
present invention relates to golf balls having multi-layer covers in which
each
of the individual cover layers have the same, or similar, hardness.
Background of the Invention
Top grade golf balls sold in the United States generally comprise a
central core with one or more cover layers formed thereover. A golf ball
cover is particularly influential in effecting the compression (feel) and
durability (i.e., impact resistance, etc.) of the resulting ball. Various
cover
compositions have been developed in order to optimize desired properties of
the resulting golf balls.
Figs. 1 and 2 illustrate a conventional golf ball 10 having a single cover
layer 14 molded about a golf ball core 16. Fig. 2 illustrates (in an
exaggerated view) stress lines 12 extending partially, or entirely across the
thickness of the cover layer 14. Stress lines 12 typically result in a crack
or
fracture across the thickness of the golf ball cover. Figs. 1 and 2 illustrate
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
2
one problem that may occur when a very thick, single layer cover is formed
about a golf ball core.
Although not wishing to be bound to any particular theory, it is believed
that stress lines in a golf ball cover, such as stress lines 12 in cover 14,
result
from repeated strikes with a golf club, particularly drivers, and temperature
effects. Stress lines often serve as initiation sites for crack or fracture
propagation in a golf ball cover material. Such cracks or fractures, and their
related stress lines, are undesirable in golf ball covers. Moreover, it is
particularly undesirable for such stress lines and the resulting cracks or
fractures to extend across the entire thickness of a golf ball cover since
such
damage significantly impairs golf ball performance. And, such cracks or
fractures greatly reduce the durability of a golf ball cover.
When a multi-layer cover is employed, each cover layer traditionally
has had a significantly different Shore D hardness than an adjacent cover
layer in order to impart to the golf ball a particular desired combination of
spin
and distance characteristics. Although the use of a multi-layer cover
configuration reduces the tendency of stress lines, and thus cracks and
fractures, propagating across the entire thickness of the cover, such multi-
layer arrangement of cover materials, each having its own particular set of
properties and characteristics, has associated design and manufacturing
problems.
For instance, in order to produce a multiple cover layer golf ball that
exhibits a desired set of performance characteristics, it is necessary to
design
and anticipate an overall performance profile for the set of cover layers.
This
involves analyzing each of the individual cover layers and any and all effects
between the individual cover layers. Even if such daunting design analysis is
performed, the increased number of variables may lead to unanticipated
difficulties in manufacturing or with the final product golf ball.
In addition, although, once again, not wishing to be bound to any
particular theory, it is believed that although a multiple cover layer
configuration may reduce the tendency for cracks or fractures that extend
through the entire thickness of the cover, such configuration may lead to an
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
3
increase in the number or frequency of fractures, particularly in applications
in which the various cover layers constituting the multi-layer cover each have
different physical properties such as hardness and flexural characteristics.
Accordingly, there is a need for an improved golf ball which is less
susceptible to cracking or fracturing across the thickness of the cover than
currently known single cover layer golf balls. And, there is a need for an
improved multiple cover layer golf ball that is simpler to design and
manufacture, and which is less susceptible to cracking or fracturing of one or
more of the individual cover layers that constitute the multiple cover layer
of
the ball.
Brief Summary of the Invention
The present invention relates to new and improved golf balls which
overcome the above-referenced problems and others. In a first aspect, the
present invention provides a golf ball comprising a core, a first cover layer
disposed about the core, and a second outermost cover layer disposed on
the first cover layer. The first inner cover layer comprises a majority
proportion by weight of a polyurethane. The first cover layer exhibits a Shore
D hardness of less than 60. The second outermost cover layer comprises a
majority proportion by weight of a polyurethane, and exhibits a Shore D
hardness of less than 60.
In another aspect, the present invention provides a golf ball comprising
a core, a first cover layer disposed about the core, and a second outermost
cover layer disposed on the first cover layer. The first inner cover layer
comprises a majority proportion by weight of a polyurethane and exhibits a
Shore D hardness less than 60. The second outermost cover layer exhibits a
Shore D hardness less than 60.
In yet another aspect, the present invention provides a golf ball
comprising a core, a first cover layer disposed about the core, and a second
outermost cover layer disposed on the first inner cover layer. The inner cover
layer exhibits a Shore D hardness of less than 60. And, the second
outermost cover layer exhibits a Shore D hardness of less than 60.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
4
Furthermore, the second outermost cover layer comprises a majority
proportion by weight of a polyurethane.
Other features and objectives of the present invention will be pointed
out in more detail hereinafter.
Brief Description of the Drawings
The following is a brief description of the drawings which are
presented for the purposes of illustrating the invention and not for the
purposes of limiting the same.
Figure 1 is a perspective view of a conventional single cover layer golf
ball.
Figure 2 is a partial cross sectional view of the golf ball depicted in
Figure 1, taken across line 2-2, illustrating stress lines extending partially
or
entirely across the thickness of the golf ball cover.
Figure 3 is a partial cross section of a preferred embodiment solid golf
ball with a dual layer cover according to the present invention.
Figure 4 is a partial cross section of a preferred embodiment solid golf
ball with a three layer cover according to an alternative embodiment of the
present invention.
Detailed Description of the Preferred Embodiments
The present invention generally provides a golf ball having a multiple
layer cover in which one or more properties of each of the individual cover
layers are matched so that certain desired properties are the same, or
substantially so. In several of the preferred embodiments described herein,
the individual cover layers all have the same, or nearly the same, Shore D
hardness. The present invention includes matching of one or more further
properties or characteristics of individual cover layers in a multiple cover
layer
assembly utilized in a golf ball. The present invention is embodied in golf
balls having multi-layer cover assemblies comprising two, three, or more
cover layers.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
In a particularly preferred aspect, the golf ball of the present invention
has a solid core having a coefficient of restitution (COR) of at least about
0.650 in combination with a thick, relatively soft cover assembly which is
formed from two or more layers. Each cover layer has a Shore D hardness
5 within 5 points, and preferably within 2 points, of the Shore D hardness of
all
other cover layers.
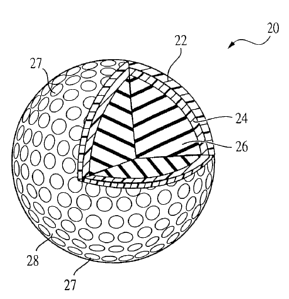
Referring to the drawings and particularly to Fig. 3, a first preferred
embodiment of a golf ball according to the present invention is shown and is
designated as 20. The golf ball 20 has a core 26. The core 26 can consist of
a solid or wound core and can consist of one or more layers.
A multi-layer cover surrounds the core 26. The multi-layer cover
includes a non-dimpled inner cover layer 24 and a dimpled outer cover layer
22. The outer cover layer 22 defines a plurality of dimples 27 and an outer
surface 28 to form an unfinished golf ball.
A thin primer coat (not shown) is preferably applied to the outer
surface of cover layer 22. A thin top coat (not shown) also preferably
surrounds the primer coat to form a finished ball. Optionally, one or more
pigmented paint coat(s) can be substituted for the primer coat and/or top
coat. In one preferred embodiment, the core 26 is relatively soft, with a PGA
compression of about 85 or less, preferably about 20 to 85, and more
preferably about 40 to 60. PGA compression is described and defined herein
below.
The multi-layer cover has an overall cover thickness of at least about
3.6 mm (0.142 inches). It is particularly preferred that the cover thickness
be
at least 3.8 mm (0.150 inches). Particularly good results are obtained when
the cover has a thickness of at least 4.0 mm (0.157 inches). In certain
circumstances, such as when a harder compression and harder feel may be
desired, it is useful to employ a cover having a thickness of at least 4.5 mm
(0.177 inches). The thickness of the individual cover layers may vary
depending upon the thicknesses of the other cover layers and the desired
overall cover layer thickness. It may, in some applications, be desirable to
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
6
form the outermost cover layer to be relatively thick due to the presence of
the dimples.
As used herein, "overall cover thickness" is the thickness of the multi-
layer cover as measured from the inner diameter of the innermost cover to
the outer surface of the outermost or exterior cover at a land (i.e. non-
dimpled) area. The "cover layer thickness" of any particular cover layer is
the
thickness of the layer from its inner diameter to its outer surface. If the
outer
surface of the layer is dimpled, the measurement is made to a land area of
the cover layer.
The preferred golf ball of the present invention preferably exhibits a
difference between the coefficient of restitution of the ball and the
coefficient
of restitution of the core of at least about 0.025, preferably at least 0.035,
and
more preferably at least 0.045. The golf balls exhibit an unexpectedly long
distance upon impact or drives given their coefficient of restitution.
In one preferred embodiment, each layer of the multi-layer cover
assembly has a Shore D hardness of less than 60, more preferably less than
55, and most preferably less than 50.
The golf balls of the present invention can be produced by molding
processes currently well known in the golf ball art. Specifically, the golf
balls
can be produced by injection molding or compression molding the cover
compositions described herein about wound or solid molded cores to produce
a golf ball having a diameter of about 1.680 to about 1.800 inches and
weighing about 1.620 ounces. The standards for both the minimum diameter
and maximum weight of the balls have been established by the United States
Golf Association (U.S.G.A.).
Although both solid core and wound cores can be utilized in the
present invention, as a result their lower cost and superior performance,
solid
molded cores are preferred over wound cores.
The term "solid cores" as used herein refers not only to one piece
cores but also to those cores having a separate solid layer beneath the cover
and above the core as in U.S. Patent No. 4,431,193, and other multi-layer
CA 02444749 2005-04-04
7
and/or non-wound cores. The compositions of suitable cores that can be
incorporated into the present invention are presented in more detail below.
The layers of the multi-layer cover may be formed from generally the
same resin composition, or may be formed from different resin compositions
with similar hardnesses. For example, one cover layer may be formed from
an ionomeric resin of ethylene and methacrylic acid, while another layer is
formed from an ionomer of ethylene and acrylic acid. One or more cover
layers may contain polyamides or polyamide-nylon copolymers or intimate
blends thereof. Furthermore, poiyurethanes, Pebax polyetheramides,
Hytrel polyesters, and/or thermosetting polyurethanes can be used. In order
to visibly distinguish the layers, various colorants, metallic flakes,
phosphorous, florescent dyes, florescent pigments, etc. can be incorporated
in the resin. Preferably, the various cover layers that comprise ionomer are
made of at least 50 weight % ionomer based upon 100 parts by weight of
resin composition, and more preferably 75 or more weight % ionomer.
However, as explained in greater detail herein, it is preferred that at least
one
of the cover layers of the multi-layer cover comprise a polyurethane, and
most preferably, at least 50% by weight. Additional description and details of
suitable materials for cover layers are provided herein.
Preferable cover materials for use as inner or outer cover layers
include, for example, zinc, sodium and lithium ionomers, and blends of
ionomers with harder non-ionic polymers such as nylon, polyphenyiene
oxide, metallocene catalyzed polyolefins, and other compatible
thermoplastics. A wide array of nylon materials or blends thereof can be
incorporated in the present invention golf ball covers. This is described in
greater detail herein. Moreover, various suitable ionomers are further
described below. Furthermore, examples of cover compositions which may
be used are set forth in detail in Canadian Patent No. 2,078,842 and U.S.
Patent No. 5,688,869.
CA 02444749 2005-04-04
8
The cover compositions are not limited in any way to the compositions set
forth
in said copending applications.
A further embodiment of a golf ball according to the present invention
is shown in Fig. 4, and is designated as golf ball 30. The ball 30 has a core
38, as is illustrated in Fig. 3. The core 38 preferably has a PGA compression
of about 85 or less, preferably 20 to 85, and more preferably 40 to 60.
A multi-layer cover having three layers is formed over the core 38 to
produce an unfinished golf ball. In the embodiment shown, the cover
includes an inner cover layer 36, an intermediate inner cover layer 34 and an
outer cover layer 32. Again, as in the embodiment illustrated in Fig. 3,
further
finished coat(s) (not shown) can be included to produce a finished golf ball.
The inner, intermediate, and outer cover layers 36, 34 and 32 respectively,
preferably exhibit substantially the same Shore D hardness. Restated, the
difference between the Shore D hardness of any two of these cover layers is
i5 preferably 5 or less, and most preferably is 2 or less. Preferably, each of
the
cover layers 36, 34 and 32 has a Shore D hardness of Iess than 60, more
preferably less than 55, and most preferably less than 50.
The overall thickness of the multi-layer cover assembly shown in Fig. 4
can be the same as the thickness of the multi-layer cover assembly of the
embodiment of Fig. 3. The thickness of the individual cover layers may vary
depending upon the thicknesses of the other cover layers and the desired
overall cover layer th:ckness. It may, In some applications, be desirable to
form the outermost cover layer to be relatively thick due to the presence of
the dimples. The three cover layers 36, 34 and 32 can be formed from the
same or different resin compositions, and preferably comprise ionomer,
ionomer blends, polyurethanes, polyureas, polyurethane blends, etc.
A detailed description of the various components and materials utilized
in the present invention golf balls is set forth below after a description of
various golf ball properties and their measurement. Moreover, further
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
9
multiple cover layers are also included as being within the scope of the
present invention.
As used herein, "Shore D hardness" of a cover is measured generally
in accordance with ASTM D-2240, except the measurements are made on
the curved surface of a molded cover, rather than on a plaque. Furthermore,
the Shore D hardness of the cover is measured while the cover remains over
the core. When a hardness measurement is made on a dimpled cover,
Shore D hardness is measured at a land area of the dimpled cover.
Two principal properties involved in golf ball performance are
resilience and PGA compression. Resilience is determined by the coefficient
of restitution (C.O.R.), i.e. the constant "e" which is the ratio of the
relative
velocity of an elastic sphere after direct impact to that before impact. As a
result, the coefficient of restitution ("e") can vary from 0 to 1, with 1
being
equivalent to a perfectly or completely elastic collision and 0 being
equivalent
to a perfectly or completely inelastic collision.
Resilience (C.O.R.), along with additional factors such as club head
speed, angle of trajectory and ball configuration (i.e., dimple pattern)
generally determine the distance a ball will travel when hit. Since club head
speed and the angle of trajectory are factors not easily controllable by a
manufacturer, factors of concern among manufacturers are the coefficient of
restitution (C.O.R.) and the surface configuration of the ball.
The coefficient of restitution (C.O.R.) in solid core balls is a function of
the composition of the molded core and of the cover. In balls containing a
wound core (i.e., balls comprising a liquid or solid center, elastic windings,
and a cover), the coefficient of restitution is a function of not only the
composition of the center and cover, but also the composition and tension of
the elastomeric windings.
The coefficient of restitution is the ratio of the outgoing velocity to the
incoming velocity. In the examples of this application, the coefficient of
restitution of a golf ball was measured by propelling a ball horizontally at a
speed of 125 1 feet per second (fps) against a generally vertical, hard, flat
steel plate and measuring the ball's incoming and outgoing velocity
CA 02444749 2005-04-04
*
electronically. Speeds were measured with a pair of Ohler Mark 55 ballistic
screens, which provide a timing pulse when an object passes through them.
The screens are separated by 36 inches and are located 25.25 inches and
61.25 inches from the rebound wall. The ball speed was measured by timing
5 the pulses from screen 1 to screen 2 on the way into the rebound wall (as
the
average speed of the ball over 36 inches), and then the exit speed was timed
from screen 2 to screen I over the same distance. The rebound wall was
tilted 2 degrees from a vertical plane to allow the ball to rebound slightly
downward in order to miss the edge of the cannon that fired it.
10 As indicated above, the incoming speed should be 125 +/- 1 fps.
Furthermore, the correlation between COR and forward or incoming speed
has been studied and a correction has been made over the +/- 1 fps range so
that the COR is reported as if the ball had an incoming speed of exactly
125.0 fps.
The coefficient of restitution must be carefully controlled in all
commercial golf balls if the ball is to be within the specifications regulated
by
the United States Golf Association (U.S.G.A.). Along this line, the U.S.G.A.
standards indicate that a "regulation" ball cannot have an initial velocity
(i.e.,
the speed off the club) exceeding 255 feet per second in an atmosphere of
75 F when tested on a U.S.G.A. machine. Since the coefficient of restitution
of a ball is related to the ball's initial velocity, it is highly desirable to
produce
a ball having sufficiently high coefficient of restitution to closely approach
the
U.S.G.A. limit on initial velocity, while having an ample degree of softness
(i.e., hardness) to produce enhanced playability (i.e., spin, etc.).
As indicated above, PGA compression is another important property
involved in the performance of a golf ball. The compression of the ball can
affect the playability of the ball on striking and the sound or "click"
produced.
Similarly, compressidn can effect the "feel" of the ball (i.e., hard or soft
responsive feel), particularly in chipping and putting.
Moreover, while compression itself has little bearing on the distance
performance of a ball, compression can affect the playability of the ball on
striking. The degree of compression of a ball against the club face and the
*Trade-mark
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
11
softness of the cover strongly influences the resultant spin rate. Typically,
a
softer cover will produce a higher spin rate than a harder cover.
Additionally,
a harder core will produce a higher spin rate than a softer core. This is
because at impact a hard core serves to compress the cover of the ball
against the face of the club to a much greater degree than a soft core thereby
resulting in more "grab" of the ball on the clubface and subsequent higher
spin rates. In effect the cover is squeezed between the relatively
incompressible core and clubhead. When a softer core is used, the cover is
under much less compressive stress than when a harder core is used and
therefore does not contact the clubface as intimately. This results in lower
spin rates.
The term "compression" utilized in the golf ball trade generally defines
the overall deflection that a golf ball undergoes when subjected to a
compressive load. For example, PGA compression indicates the amount of
change in golf ball's shape upon striking. The development of solid core
technology in two-piece balls has allowed for much more precise control of
compression in comparison to thread wound three-piece balls. This is
because in the manufacture of solid core balls, the amount of deflection or
deformation is precisely controlled by the chemical formula used in making
the cores. This differs from wound three-piece balls wherein compression is
controlled in part by the winding process of the elastic thread. Thus,
two-piece and multi-layer solid core balls exhibit much more consistent
compression readings than balls having wound cores such as the thread
wound three-piece balls.
In the past, PGA compression related to a scale of from 0 to 200 given
to a golf ball. The lower the PGA compression value, the softer the feel of
the
ball upon striking. In practice, tournament quality balls have compression
=. ratings around 50 to 110, and preferably around 80 to 100.
In determining PGA compression using the 0 to 200 scale, a standard
force is applied to the external surface of the ball. A ball which exhibits no
deflection (0.0 inches in deflection) is rated 200 and a ball which deflects
2/10th of an inch (0.2 inches) is rated 0. Every change of 0.001 of an inch in
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
12
deflection represents a 1 point drop in compression. Consequently, a ball
which deflects 0.1 inches (100 x 0.001 inches) has a PGA compression value
of 100 (i.e., 200 to 100) and a ball which deflects 0.110 inches (110 x 0.001
inches) has a PGA compression of 90 (i.e., 200 to 110).
In order to assist in the determination of compression, several devices
have been employed by the industry. For example, PGA compression is
determined by an apparatus fashioned in the form of a small press with an
upper and lower anvil. The upper anvil is at rest against a 200-pound die
spring, and the lower anvil is movable through 0.300 inches by means of a
crank mechanism. In its open position the gap between the anvils is 1.780
inches allowing a clearance of 0.100 inches for insertion of the ball. As the
lower anvil is raised by the crank, it compresses the ball against the upper
anvil, such compression occurring during the last 0.200 inches of stroke of
the
lower anvil, the ball then loading the upper anvil which in turn loads the
spring.
The equilibrium point of the upper anvil is measured by a dial micrometer if
the anvil is deflected by the ball more than 0.100 inches (less deflection is
simply regarded as zero compression) and the reading on the micrometer dial
is referred to as the compression of the ball. In practice, tournament quality
balls have compression ratings around 80 to 100 which means that the upper
anvil was deflected a total of 0.120 to 0.100 inches.
An example to determine PGA compression can be shown by utilizing
a golf ball compression tester produced by Atti Engineering Corporation of
Newark, N.J. The value obtained by this tester relates to an arbitrary. value
expressed by a number which may range from 0 to 100, although a value of
200 can be measured as indicated by two revolutions of the dial indicator on
the apparatus. The value obtained defines the deflection that a golf ball
undergoes when subjected to compressive loading. The Atti test apparatus
consists of.a lower movable platform and an upper movable spring-loaded
anvil. The dial indicator is mounted such that it measures the upward
movement of the spring- loaded anvil. The golf ball to be tested is placed in
the lower platform, which is then raised a fixed distance. The upper portion
of
the golf ball comes in contact with and exerts a pressure on the spring-loaded
CA 02444749 2005-04-04
13
anvil. Depending upon the distance of the golf ball to be compressed, the
upper anvil is forced upward against the spring.
Alternative devices have also been employed to determine
compression. For example, Applicant also utilizes a modified Riehle*
Compression Machine originally produced by Riehle Bros. Testing Machine
Company, Philadelphia, Pennsylvania to evaluate compression of the various
components (i.e., cores, mantle cover balls, finished balls, etc.) of the golf
balls. The Riehle compression device determines deformation in thousandths
of an inch under a load designed to emulate the 200 pound spring constant of
the Atti or PGA compression testers. Using such a device, a Riehle
compression of 61 corresponds to a deflection under load of 0.061 inches.
Additionally, an approximate relationship between Riehle compression
and PGA compression exists for balls of the same size. It has been
determined by Applicant that Riehle compression corresponds to PGA
compression by the general formula PGA compression equals 160 minus
Riehle compression. Consequently, 80 Riehle compression corresponds to
80 PGA compression, 70 Riehle compression corresponds to 90 PGA
compression, and 60 Riehle compression corresponds to 100 PGA
compression. For reporting purposes, Applicant's compression values are
usually measured as Riehle compression and converted to PGA compression.
Furthermore, additional compression devices may also be utilized to
monitor golf ball compression so long as the correlation to PGA compression
is known. These devices have been designed, such as a Whitney Tester, to
correlate or correspond to PGA compression through a set relationship or
formula.
Core
The core which is used to form the golf balls of the present invention
can be solid, foamed, wound, hollow or liquid. The core can be uriitary, or
can have two or more core layers. A solid core or solid layer of a multi-layer
core can be thermosetting or thermoplastic. Preferably, the core is solid and
is formed from a thermoset material.
*Trade-mark
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
14
Solid cores of the more preferred embodiment of the present invention
can be manufactured using relatively conventional techniques. In this regard,
the core compositions of the invention may be based on polybutadiene,
natural rubber, metallocene catalyzed polyolefins such as Exact (Exxon
Chem. Co.) and Engage (Dow Chem. Co.), polyurethanes, other
thermoplastic or thermoset elastomers, and mixtures of one or more of the
above materials with each other and/or with other elastomers. The core may
be formed from a uniform composition or may be a dual or multi-layer core.
The core may be foamed or unfoamed.
It is preferred that the base elastomer have a relatively high molecular
weight. Polybutadiene has been found to be particularly useful because it
imparts to the golf balls a relatively high coefficient of restitution.
Polybutadiene can be cured using a free radical initiator such as a peroxide,
or can be sulfur cured. A broad range for the molecular weight of preferred
base elastomers is from about 50,000 to about 500,000. A more preferred
range for the molecular weight of the base elastomer is from about 100,000
to about 500,000. As a base elastomer for the core composition,
cis-1-4-polybutadiene is preferably employed, or a blend of
cis-1-4-polybutadiene with other elastomers may also be utilized. Most
preferably, cis-1-4-polybutadiene having a weight-average molecular weight
of from about 100,000 to about 500,000 is employed. Along this line, it has
been found that the high cis-1-4-polybutadienes manufactured and sold by
Bayer Corp., Germany, under the trade name Taktene 220 or 1220 are
particularly preferred. Furthermore, the core may be comprised of a
crosslinked natural rubber, EPDM, metallocene catalyzed polyolefin, or
another crosslinkable elastomer.
When polybutadiene is used for golf ball cores, it commonly is
crosslinked with an unsaturated carboxylic acid co-crosslinking agent. The
unsaturated carboxylic acid component of the core composition typically is
the reaction product of the selected carboxylic acid or acids and an oxide or
carbonate of a metal such as zinc, magnesium, barium, calcium, lithium,
sodium, potassium, cadmium, lead, tin, and the like. Preferably, the oxides of
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
polyvalent metals such as zinc, magnesium and cadmium are used, and
most preferably, the oxide is zinc oxide.
Exemplary of the unsaturated carboxylic acids which find utility in the
core compositions are acrylic acid, methacrylic acid, itaconic acid, crotonic
5 acid, sorbic acid, and the like, and mixtures thereof. Preferably, the acid
component is either acrylic or methacrylic acid. Usually, from about 5 to
about 40, and preferably from about 15 to about 30 parts by weight of the
carboxylic acid salt, such as zinc diacrylate, is included in the core
composition. The unsaturated carboxylic acids and metal salts thereof are
10 generally soluble in the elastomeric base, or are readily dispersible.
The free radical initiator included in the core composition is any known
polymerization initiator (a co-crosslinking agent) which decomposes during
the cure cycle. The term "free radical initiator" as used herein refers to a
chemical which, when added to a mixture of the elastomeric blend and a
15 metal salt of an unsaturated, carboxylic acid, promotes crosslinking of the
elastomers by the metal salt of the unsaturated carboxylic acid. The amount
of the selected initiator present is dictated only by the requirements of
catalytic activity as a polymerization initiator. Suitable initiators include
peroxides, persulfates, azo compounds and hydrazides. Peroxides which are
readily commercially available are conveniently used in the present invention,
generally in amounts of from about 0.1 to about 10.0 and preferably in
amounts of from about 0.3 to about 3.0 parts by weight per each 100 parts of
elastomer.
Exemplary of suitable peroxides for the purposes of the present
invention are dicumyl peroxide, n-butyl 4,4'-bis (butylperoxy) valerate,
1,1-bis(t-butylperoxy)-3,3,5-trimethyl cyclohexane, di-t-butyl peroxide and
2,5-di-(t-butylperoxy)-2,5 dimethyl hexane and the like, as well as mixtures
thereof. It will be understood that the total amount of initiators used will
vary
depending on the specific end product desired and the particular initiators
employed.
Examples of such commercially available peroxides are Luperco 230
or 231 XL sold by Atochem, Lucidol Division, Buffalo, N.Y., and Trigonox
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
16
17/40 or 29/40 sold by Akzo Chemicals, America, Chicago, III. In this regard
Luperco 230 XL and Trigonox 29/40 are comprised of
1,1-bis(t-butylperoxy)-3,3,5-trimethyl cyclohexane. The one hour half life of
Luperco 231 XL is about 112 C., and the one hour half life of Trigonox
29/40 is about 129 C.
The core compositions of the present invention may additionally
contain any other suitable and compatible modifying ingredients including,
but not limited to, metal oxides, fatty acids, and diisocyanates and
polypropylene powder resin. For example, Papi 94, a polymeric
diisocyanate, commonly available from Dow Chemical Co., Midland, Mich., is
an optional component in the rubber compositions. It can range from about 0
to 5 parts by weight per 100 parts by weight rubber (phr) component, and
acts as a moisture scavenger. In addition, it has been found that the addition
of a polypropylene powder resin results in a core which is hard (i.e. exhibits
high PGA compression) and thus allows for a reduction in the amount of
crosslinking co-agent utilized to soften the core to a normal or below normal
compression.
Furthermore, because polypropylene powder resin can be added to a
core composition without an increase in weight of the molded core upon
curing, the addition of the polypropylene powder allows for the addition of
higher specific gravity fillers, such as mineral fillers. Since the
crosslinking
agents utilized in the polybutadiene core compositions are expensive and/or
the higher specific gravity fillers are relatively inexpensive, the addition
of the
polypropylene powder resin substantially lowers the cost of the golf ball
cores
while maintaining, or lowering, weight and compression.
The polypropylene (C3H5) powder suitable for use in the present
invention has a specific gravity of about 0.90 g/cm3, a melt flow rate of
about
4 to about 12 and a particle size distribution of greater than 99% through a
20
mesh screen. Examples of such polypropylene powder resins include those
sold by the Amoco Chemical Co., Chicago, Ill., under the designations "6400
P", "7000 P" and "7200 P". Generally, from 0 to about 25 parts by weight
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
17
polypropylene powder per each 100 parts of elastomer are included in the
present invention.
Various activators may also be included in the compositions of the
present invention. For example, zinc oxide and/or magnesium oxide are
activators for the polybutadiene. The activator can range from about 2 to
about 30 parts by weight per 100 parts by weight of the rubbers (phr)
component.
Moreover, reinforcement agents may be added to the core
compositions of the present invention. Since the specific gravity of
polypropylene powder is very low, and when compounded, the polypropylene
powder produces a lighter molded core, when polypropylene is incorporated
in the core compositions, relatively large amounts of higher specific gravity
fillers may be added so long as the specific core weight limitations are met.
As indicated above, additional benefits may be obtained by the incorporation
of relatively large amounts of higher specific gravity, inexpensive mineral
fillers such as calcium carbonate. Such fillers as are incorporated into the
core compositions should be in finely divided form, as for example, in a size
generally less than about 30 mesh and preferably less than about 100 mesh
U.S. standard size. The amount of additional filler included in the core
composition is primarily dictated by weight restrictions and preferably is
included in amounts of from about 10 to about 100 parts by weight per 100
parts rubber.
The preferred fillers are relatively inexpensive and heavy and serve to
lower the cost of the ball and to increase the weight of the ball to closely
approach the U.S.G.A. weight limit of 1.620 ounces. However, if thicker
cover compositions are to be applied to the core to produce larger than
normal (i.e. greater than 1.680 inches in diameter) balls, use of such fillers
and modifying agents will be limited in order to meet the U.S.G.A. maximum
weight limitations of 1.620 ounces. Limestone is ground calcium/magnesium
carbonate and is used because it is an inexpensive, heavy filler. Ground
flash filler may be incorporated and is preferably 20 mesh ground up center
CA 02444749 2005-04-04
18
stock from the excess flash from compression molding. It lowers the cost
and may increase the hardness of the ball.
Fatty acids or metallic salts of fatty acids may also be included in the
compositions, functioning to improve moldability and processing. Generally,
free fatty acids having from abut 10 to about 40 carbon atoms, and preferably
having from about 15 to about 10 carbon atoms, are used. Exemplary of
suitable fatty acids are stearic acid and linoleic acids, as well as mixtures
thereof. An example of a suitable metallic salt of a fatty acid is zinc
stearate.
When included in the core compositions, the metallic salts of fatty acids are
present in amounts of from about 1 to about 25, preferably in amounts from
about 2 to about 15 parts by weight based on 100 parts rubber (elastomer).
It is preferred that the core compositions include stearic acid as the fatty
acid
adjunct in an amount of from about 2 to about 5 parts by weight per 100 parts
of rubber.
Diisocyanates may also be optionally included in the core
compositions. When utilized, the diisocyanates are included in amounts of
from about 0.2 to about 5.0 parts by weight based on 100 parts rubber.
Exemplary of suitable diisocyanates is 4,4'-diphenylmethane diisocyanate
and other polyfunctional isocyanates known in the art.
Furthermore, the dialkyl tin difatty acids set forth in U.S. Patent No.
4,844,471, the dispensing agents disclosed in U.S. Patent No. 4,838,556,
and the dithiocarbamates set forth in U.S. Patent No. 4,852,884 may also be
incorporated into the polybutadiene compositions of the present invention.
The specific types and amounts of such additives are set forth in the above
identified patents.
The core compositions of the invention which contain polybutadiene
are generally comprised of 100 parts by weight of a base elastomer (or
rubber) selected from polybutadiene and mixtures of polybutadiene with other
elastomers, 15 to 25 parts by weight of at least one metallic salt of an
unsaturated carboxylic acid, and 0.5 to 10 parts by weight of a free radical
initiator.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
19
As indicated above, additional suitable and compatible modifying
agents such as particulate polypropylene resin, fatty acids, and secondary
additives such as pecan shell flour, ground flash (i.e. grindings from
previously manufactured cores of substantially identical construction), barium
sulfate, zinc oxide, etc. may be added to the core compositions to adjust the
weight of the ball as necessary in order to have the finished molded ball
(core, cover and coatings) to closely approach the U.S.G.A. weight limit of
1.620 ounces.
In producing solid golf ball cores utilizing the present compositions, the
ingredients may be intimately mixed using, for example, two roll mills or an
internal mixer until the composition is uniform, usually over a period of from
about 5 to about 20 minutes. The sequence of addition of components is not
critical. A preferred blending sequence is as follows.
The elastomer, polypropylene powder resin (if desired), fillers, zinc
salt, metal oxide, fatty acid, and the metallic dithiocarbamate (if desired),
surfactant (if desired), and tin difatty acid (if desired), are blended for
about 7
minutes in an internal mixer such as a Banbury (Farrel Corp.) mixer. As a
result of shear during mixing, the temperature rises to about 200 F. The
initiator and diisocyanate are then added and the mixing continued until the
temperature reaches about 220 F whereupon the batch is discharged onto a
two roll mill, mixed for about one minute and sheeted out.
The sheet is rolled into a"pig" and then placed in a BarwellT"'
preformer and slugs are produced. The slugs are then subjected to
compression molding at about 320 F for about 14 minutes. After molding,
the molded cores are cooled, the cooling effected at room temperature for
about 4 hours or in cold water for about one hour. The molded cores can be
subjected to a centerless grinding operation whereby a thin layer of the
molded core is removed to produce a round core having a diameter of 1.2 to
1.5 inches. Alternatively, the cores are used in the as-molded state with no
grinding needed to achieve roundness.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
The mixing is desirably conducted in such a manner that the
composition does not reach incipient polymerization temperatures during the
blending of the various components.
Usually the curable component of the composition will be cured by
5 heating the composition at elevated temperatures on the order of from about
275 F to about 350 F, preferably and usually from about 290 F to about
325 F, with molding of the composition effected simultaneously with the
curing thereof. The composition can be formed into a core structure by any
one of a variety of molding techniques, e.g. injection, compression, or
10 transfer molding. When the composition is cured by heating, the time
required for heating will normally be short, generally from about 10 to about
20 minutes, depending upon the particular curing agent used. Those of
ordinary skill in the art relating to free radical curing agents for polymers
are
conversant with adjustments of cure times and temperatures required to
15 effect optimum results with any specific free radical agent.
After molding, the core is removed from the mold and the surface
thereof optionally is treated to facilitate adhesion thereof to the covering
materials. Surface treatment can be effected by any of the several
techniques known in the art, such as corona discharge, ozone treatment,
20 sand blasting, and the like. Preferably, surface treatment is effected by
grinding with an abrasive wheel.
In addition to using solid molded cores, wound cores may also be
incorporated in the golf balls of the present invention. Such wound cores
would include a generally spherical center and a rubber thread layer, or
windings, enclosing the outer surface of the center.
In this regard, the generally spherical center of the wound cores may
be a solid center or a liquid center. The solid center can consist of one or
more layers. For example, the solid center can comprise a molded
polybutadiene rubber'sphere which, although smaller in size, is of similar
construction to the molded cores in the two-piece molded golf balls described
above.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
21
Suitable solid centers used in the invention are not particularly limited
to, but include those made of vulcanized rubber. Such solid centers may be
prepared by adding to butadiene rubber, additives such as vulcanizing
agents, accelerators, activating agents, fillers, modifiers and aids and then
subjecting the mixture to vulcanization and molding.
The solid center (whether of single unitary construction or of multi-
layers) generally is from 1 to 1.5 inches in diameter, preferably 1.0625 to
1.42 inches, with a weight of 15 grams to 36 grams, preferably 16.5 to 30
grams.
Alternatively, a liquid center can be incorporated into the wound core
of the present invention. The liquid center consists of a hollow spherical bag
or sack of conventional vulcanized rubber filled with a liquid, paste or gel.
Examples of such a liquid include water, glycerin, sugar-water solutions,
corn-syrup, saline solutions, oils, etc. and/or combinations thereof. Examples
of pastes can be produced by adding clay, sodium sulfate, barytes, barium
sulfate to a minor amount of ethylene glycol in water. Examples of suitable
gels include hydrogels, cellulose gels, water gels, etc. The specific gravity
of
the liquid is, in general, .6 to 3 and the specific gravity of the paste is
from .6
to 3 and the gels from .6 to 3. The bag or sack is, in general, from 0.05 to
0.150 inches in thickness, preferably 0.08 to 0.105 inches in thickness.
The liquid center generally is from 1 to 1.25 inches in diameter,
preferably 1.0625 to 1.14 inches, with a weight of 5.5 to 25.5 grams,
preferably 15 to 21 grams.
The wound core is formed by winding conventional thread rubber
around the outer periphery of the solid or liquid center. The thread rubber
may include, for example, those prepared by subjecting natural rubber, or a
blend of natural rubber and polyisoprene rubber to vulcanization and molding.
The winding process is under high tension to produce a threaded layer over
the solid or liquid center. Conventional techniques may be employed in
winding the thread rubber and known compositions may be used. Although
the thread rubber is not limited with respect to specific gravity, dimension
and
gage, it usually has a specific gravity of .9 to 1.1, a width of .047 to .094
and
a gage of .012 to .026.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
22
The rubber thread layer has a radial thickness of 0.10 to .315 inches
and comprises a wound core having an outer diameter of 1.52 to 1.63 inches.
The overall weight of the wound core is 33 to 44 grams, preferably 35 to 39
grams.
Multi-Layer Covers
As indicated above, cover layers of the present invention golf ball
preferably but not necessarily comprise an ionomer resin. High or low acid
ionomers, or ionomer blends can be used, along with polyurethane, polyurea
and blends thereof. The high acid ionomers which may be suitable for use in
formulating the cover compositions are ionic copolymers which are the metal,
i.e., sodium, zinc, magnesium, lithium, etc., salts of the reaction product of
an
olefin having from about 2 to 8 carbon atoms and an unsaturated
monocarboxylic acid having from about 3 to 8 carbon atoms. Preferably, the
ionomeric resins are copolymers of ethylene and either acrylic or methacrylic
acid. In some circumstances, an additional comonomer such as an acrylate
ester (i.e., iso- or n-butylacrylate, etc.) can also be included to produce a
softer terpolymer. The carboxylic acid groups of the copolymer are at least
partially neutralized (i.e., approximately 10% to 100%, and preferably 30% to
70%) by the metal ions. Each of the high acid ionomer resins contains
greater than about 16% by weight of a carboxylic acid, and preferably from
about 17% to about 25% by weight of a carboxylic acid, and more preferably
from about 18.5% to about 21.5% by weight of a carboxylic acid.
The high acid ionomeric resins available from Exxon under the
designation lotek , are somewhat similar to the high acid ionomeric resins
available under the Surlyn trademark. However, since the lotek ionomeric
resins are sodium, lithium or zinc salts of poly(ethylene-acrylic acid) and
the
Surlyn resins are zinc, sodium, lithium, etc. salts Qf
poly(ethylene-methacrylic acid), distinct differences in properties exist.
Non-limiting examples of the high acid methacrylic acid based
ionomers suitable for use in accordance with this invention include Surlyn
8140(Na), 8220 (Na), 8240 (Na), 9120 (Zn), 9220 (Zn), AD8181 (Li), AD8530
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
23
(Zn), AD8531 (Na) and SEP 671 (Li). Table 1, set forth below, lists
properties for two of these materials.
Table 1
Surlyn Resins
SURLYN 8140 SURLYN 120
(19 wt% acid) (19 wt% acid)
IONOMER
Cation Na Zn
Melt Flow Index, g/10 min. 2.60 1.30
Specific gravity 0.96 0.97
MP, C 88 85
FP, C 49 50
MECHANICAL
PROPERTIES
Tensile Strength, kpsi (MPa) 5.0 (34.5) 3.8 (26.2)
Yield Strength, kpsi (MPa) 2.8 (19.3) 2.4 (16.6)
Elongation, % 340 280
Flex Mod, kpsi (MPa) 71(490) 84 (440)
Shore D Hardness 70 69
Examples of the high acid acrylic acid based ionomers suitable for use
in the present invention also include lotek high acid ethylene acrylic acid
ionomers produced by Exxon such as 1001, 1002, 959, 960, 989, 990, 1003,
1004, 993, and 994. In this regard, lotek 959 is a sodium ion neutralized
ethylene-acrylic acid copolymer. According to Exxon; lotek 959 and 960
contain from about 19.0 to about 21.0% by weight acrylic acid with
approximately 30 to about 70 percent of the acid groups neutralized with
sodium and zinc ions, respectively. The physical properties of these and
.other' high acid acrylic acid based ionomers are setJorth in Table 2 as
follows:
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
24
Table 2
lotek Resins
PROPERTY 1001 1002 959 1003 1004 960
Melt index, 1.00 1.60 2.00 1.10 2.00 1.80
g/10 min.
Cation Na Na Na Zn Zn Zn
Melting point, 183.0 183.0 172.0 180.0 180.5 174.0
F
Crystallization 107.0 110.0 106.0 125.0 126.5 120.0
point, F
Vicat
Softening 125.0 125.0 130.0 133.0 131.0 131.0
Point, F
Tensile 34.4 31.7 4600 24.8 20.6 3500
Break MPa MPa psi MPa MPa psi
Tensile 21.8 22.5 14.9 14.0
Yield MPa MPa ~ MPa MPa
1% Secant 356 418 350 145 128 140
Modulus MPa MPa MPa MPa MPa MPa
Elongation 341 348 325 387 437 430
@@ Break, %
Hardness, 63 62 66 54 53 57
Shore D
Flexural 365 380 66,000 147 130 27,000
Modulus MPa MPa psi MPa MPa psi
.9558 .9557 .968 .9715 .9691 .980
Density g/cm' g/cm' g/cm' g/cm' g/cm' g/cm'
lotek Resins
EX 989 EX 993 EX 994 EX 990
Mett index g/10min 1.30 1.25 1.32 1.24
Moisture ppm 482 214 997 654
Cation Type Na Li K Zn
M+ content by Wt% 2.74 0.87 4.54 0.00
AAS =
Zn content by Wt% 0.00 0.00 0.00 3.16
AAS
Density kg/m3 959 945 976 977
Vicat softening C 52.5 51.0 50.0 55.0
point
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Crystallization C 40.1 39.8 44.9 54.4
point
Melting point C 82.6 81.0 80.4 81.0
Tensile at yield MPa 23.8 24.6 22.0 16.5
5 Tensile at MPa 32.3 31.1 29.7 23.8
break
Elongation at % 330 260 340 357
break
196 secant MPa 389 379 312 205
10 modulus
Flexural MPa 340 368 303 183
modulus
Abrasion mg 20.0 9.2 15.2 20.5
resistance
15 Hardness - 62 62.5 61 56
Shore D
Zwick rebound % 61 63 59 48
Furthermore, as a result of the development by the assignee of this
application of a number of new ionomers neutralized to various extents by
20 several different types of metal cations, such as by manganese, lithium,
potassium, calcium and nickel cations, several new ionomers and/or ionomer
blends besides sodium, zinc and magnesium high acid ionomers or ionomer
blends are now available for golf ball cover production. In particular it has
been found that new cation neutralized high acid ionomer blends produce
25 inner cover layer compositions exhibiting enhanced hardness and resilience
due to synergies which occur during processing. Consequently, the metal
cation neutralized high acid ionomer resins recently produced can be blended
to produce substantially higher C.O.R.'s than those produced by the low acid
ionomer inner cover compositions presently commercially available.
More particularly, several new metal cation neutralized high acid
ionomer resins have been produced by the inventor by neutralizing, to
various extents, high acid copolymers of an alpha-olefin and an alpha,
beta-unsaturated carboxylic acid with a wide variety of different metal cation
salts. It has been found that numerous new metal cation neutralized high
acid ionomer resins can be obtained by reacting a high acid copolymer (i.e. a
copolymer containing greater than 16% by weight acid, preferably from about
17 to about 25 weight percent acid, and more preferably about 20 weight
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
26
percent acid), with a metal cation salt capable of ionizing or neutralizing
the
copolymer to the extent desired (i.e. from about 10% to 90%).
The base copolymer is made up of greater than 16% by weight of an
alpha, beta-unsaturated carboxylic acid and an alpha-olefin. As indicated
above, a softening comonomer can be included in the copolymer. Generally,
the alpha-olefin has from 2 to 10 carbon atoms and is preferably ethylene,
and the unsaturated carboxylic acid is a carboxylic acid having from about 3
to 8 carbons. Examples of such acids include acrylic acid, methacrylic acid,
ethacrylic acid, chloroacrylic acid, crotonic acid, maleic acid, fumaric acid,
and itaconic acid, with acrylic acid being preferred.
The softening comonomer that can be optionally included in the inner
cover layer for the golf ball of the invention may be selected from the group
consisting of vinyl esters of aliphatic carboxylic acids wherein the acids
have
2 to 10 carbon atoms, vinyl ethers wherein the alkyl groups contains I to 10
carbon atoms, and alkyl acrylates or methacrylates wherein the alkyl group
contains 1 to 10 carbon atoms. Suitable softening comonomers include vinyl
acetate, methyl acrylate, methyl methacrylate, ethyl acrylate, ethyl
methacrylate, butyl acrylate, butyl methacrylate, or the like.
Consequently, examples of a number of copolymers suitable for use to
produce the high acid ionomers included in the present invention include, but
are not limited to, high acid embodiments of an ethylene/acrylic acid
copolymer, an ethylene/methacrylic acid copolymer, an ethylene/itaconic acid
copolymer, an ethylene/maleic acid copolymer, an ethylene/methacrylic
acid/vinyl acetate copolymer, an ethylene/acrylic acid/vinyl alcohol
copolymer, etc. The base copolymer broadly contains greater than 16% by
weight unsaturated carboxylic acid, from about 39 to about 83% by weight
ethylene and from 0 to about 40% by weight of a softening comonomer.
Preferably, the copolymer contains about 20% by weight unsaturated
carboxylic acid and about 80% by weight ethylene. Most preferably, the
copolymer contains about 20% acrylic acid with the remainder being
ethylene.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
27
Along these lines, examples of the preferred high acid base
copolymers which fulfill the criteria set forth above, are a series of
ethylene-acrylic copolymers which are commercially available from Dow
Chemical Company, Midland, Michigan, under the Primacor designation.
These high acid base copolymers exhibit the typical properties set forth
below in Table 3.
Table 3
Typical Properties of Primacor
Ethylene-Acrylic Acid Copolymers
MELT TENSILE FLEXURAL VICAT
PERCENT DENSITY, SHORED
GRADE ACID g/cc INDEX, YD.ST MODULUS SOFT PT HARDNESS
g/10min (psi) (psi) (C)
ASTM D-792 D-1238, D-638 D-790 D-1525 D-2240
190 c
5980 20 0.96 300 4800 43 50
5990 20 0.96 1300 650 2600 40 42
5981 20 0.96 300 900 3200 46 48
5983 20 0.96 500 850 3100 44 45
5991 20 0.95 2600 635 2600 38 40
Due to the high molecular weight of the Primacor'O 5981 grade of the
ethylene-acrylic acid copolymer, this copolymer is the more preferred grade
utilized in the invention.
The metal cation salts utilized in the present invention are those salts
which provide the metal cations capable of neutralizing, to various extents,
the carboxylic acid groups of the high acid copolymer. These include
acetate, oxide or hydroxide salts of lithium, calcium, zinc, sodium,
potassium,
nickel, magnesium, and manganese.
Examples of such lithium ion sources are lithium hydroxide
monohydrate, lithium hydroxide, lithium oxide and lithium acetate. Sources
for the calcium ion include calcium hydroxide, calcium acetate and calcium
oxide. Suitable zinc ion sources are zinc acetate dihydrate and zinc acetate,
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
28
a blend of zinc oxide and acetic acid. Examples of sodium ion sources are
sodium hydroxide and sodium acetate. Sources for the potassium ion
include potassium hydroxide and potassium acetate. Suitable nickel ion
sources are nickel acetate, nickel oxide and nickel hydroxide. Sources of
magnesium include magnesium oxide, magnesium hydroxide, and
magnesium acetate. Sources of manganese include manganese acetate
and manganese oxide.
The new metal cation neutralized high acid ionomer resins are
produced by reacting the high acid base copolymer with various amounts of
the metal cation salts above the crystalline melting point of the copolymer,
such as at a temperature from about 200 F to about 500 F, preferably from
about 250 F to about 350 F under high shear conditions at a pressure of from
about 10 psi to 10,000 psi. Other blending techniques may also be used.
The amount of metal cation salt utilized to produce the new metal cation
neutralized high acid based ionomer resins is the quantity which provides a
sufficient amount of the metal cations to neutralize the desired percentage of
the carboxylic acid groups in the high acid copolymer. The extent of
neutralization is generally from about 10% to about 90%.
When the acid groups of copolymers of acrylic acid and ethylene sold
by Dow Chemical Co. (Midland, MI) and designated as Primacor' 5981 were
neutralized to various weight percentages using a number of different
cations, a number of different high acid ionomer resins were produced. Due
to differences in the nature of the cation salts, the amount of cation salts
utilized, etc., the new high acid ionomer resins produced differed
substantially
in the extent of neutralization and in melt indices, as well as in resilience
(i.e.
C.O.R.) and hardness values.
For the purpose of determining the weight percent of neutralization of
the carboxylic acid groups in the acrylic acid/ethylene copolymer after
reacting with various cation salts, it was assumed that one mole of sodium
(Na'), potassium (K'), and lithium (Li+) neutralized one mole of acrylic acid,
and that one mole of zinc (Zn2+), magnesium (Mg2+), manganese (Mnz'),
calcium (Ca2+) and nickel (Niz') neutralized two moles of acrylic acid. The
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
29
calculations of neutralization were based upon an acrylic acid molecular
weight of 79 g/m, giving 0.2778 moles per 100 grams of copolymer.
As indicated below in Table 4, the various cation salts were added in
variable amounts to the 20 weight percent acrylic acid/ethylene copolymer in
order to determine the optimal level of neutralization for each of the
cations.
In Table 4, NaOH refers to sodium hydroxide (formula weight of 40). MnAc
refers to manganese acetate tetrahydrate having a formula weight of 245.
LiOH is lithium hydroxide, fwt = 24. KOH is potassium hydroxide, fwt = 56.
ZnAc is zinc acetate dihydrate, fwt = 219.5. MgAc is magnesium acetate
tetrahydrate, fwt = 214.4. CaAc is calcium acetate, fwt = 158. MgO is
magnesium oxide, fwt = 40.3. NiAc is nickel acetate, fwt = 176.8. All of
these cation salts are solids at room temperature.
The specific cation salts were added in differing amounts with the 20
weight percent acrylic acid/ethylene copolymer (i.e. the Primacor 5981) to
an internal mixer (Banbury type) for the neutralization reaction. The only
exception was calcium acetate, which, due to problems encountered in solid
form, was added as a 30 wt % solution in water.
In the neutralization reaction, the cation salts solubilized in the
Primacor 5981 acrylic acid/ethylene copolymer above the melting point of
the copolymer and a vigorous reaction took place with a great deal of
foaming occurring as the cation reacted with the carboxylic acid groups of the
acrylic acid/ethylene copolymer and the volatile by-products of water (in the
case of oxides or hydroxides) or acetic acid (when acetates are used) were
evaporated. The reaction was continued until foaming ceased (i.e. about 30 -
45 minutes at 250 to 350 F), and the batch was removed from the Banbury
mixer. Mixing continued of the batch obtained from the mixer on a hot
two-roll mill (175 to 250 F) to complete the neutralization reaction. The
extent of the reaction was monitored by measuring melt flow index according
to ASTM D-1238-E. As indicated below, the neutralized products exhibited
different properties depending upon the nature and amount of the cation salts
utilized.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Table 4
Formulation Wt % Wt % Shore D
No. Cation Salt Neutralization Melt Index C.O.R. Hardness
1(NaOH) 6.98 67.50 0.90 0.80 71
5 2(NaOH) 5.66 54.00 2.40 0.81 73
3(NaOH) 3.84 35.90 12.20 0.81 69
4(NaOH) 2.91 27.00 17.50 0.81 (brittle)
5(MnAc) 19.60 71.70 7.50 0.81 73
6(MnAc) 23.10 88.30 3.50 0.81 77
10 7(MnAc) 15.30 53.00 7.50 0.81 72
8(MnAc) 26.50 106.00 0.70 0.81 (brittle)
9(LiOH) 4.54 71.30 0.60 0.81 74
10(LiOH) 3.38 52.50 4.20 0.82 72
11(LiOH) 2.34 35.90 18.60 0.81 72
15 12(KOH) 5.30 36.00 19.30 Broke 70
13(KOH) 8.26 57.90 7.18 0.80 70
14(KOH) 10.70 77.00 4.30 0.80 67
15(ZnAc) 17.90 71.50 0.20 0.81 71
16(ZnAc) 13.90 53.00 0.90 0.80 69
20 17(ZnAc) 9.91 36.10 3.40 0.79 67
18(MgAc) 17.40 70.70 2.80 0.81 74
19(MgAc) 20.60 87.10 1.50 0.81 76
20(MgAc) 13.80 53.80 4.10 0.81 74
21(CaAc) 13.20 69.20 1.10 0.81 74
25 22(CaAc) 7.12 34.90 10.10 0.81 70
Controls: 50/50 Blend of loteks 8000/7030 C.O.R. =.810/65 Shore D Hardness
DuPont High Acid Surtyn 8422 (Na) C.O.R. =.811l70 Shore D Hardness
DuPont High Acid Sur" 8162 (Zn) C.O.R. = .807/65 Shore D Hardness
Exxon High Acid lotek EX-960 (Zn) C.O.R. =.796/65 Shore D Hardness
30 Formulation Wt % Wt %
No. Cation Salt Neutralization Melt Index C.O.R.
23 (MgO) 2.91 53.50 2.50 0.81
24(MgO) 3.85 71.50 2.80 0.81
25(MgO) 4.76 89.30 1.10 0.81
26(MgO) 1.96 35.70 7.50 0.81
CA 02444749 2005-04-04
31
Control tor Formulations 23 - 26 is 50150 lotek 8000/7030, C.O.R. = .814
Formulation 26 C.O.R. was normalized to that controt accordingly.
Wt %
Formulation Cation Wt % Shore D
No. Salt Neutralization Melt Index C.O.R. Hardness
27(NiAc) 13.04 61.10 0.20 0.80 71
28(NiAc) 10.71 48.90 0.50 0.80 72
29(NiAc) 8.26 36.70 1.80 0.80 69
30(NiAc) 5.66 24.40 7.50 0.79 64
Control for formulation No. 27 - 30 is 50/50 lotek 8000/7030. C.O.R. - .807
When compared to low acid versions of similar cation neutralized
ionomer resins, the new metal cation neutralized high acid ionomer resins
exhibit enhanced hardness, modulus and resilience characteristics. These
are properties that are particularly desirable in a number of thermoplastic
fields, including the field of golf ball manufacturing.
As will be further noted in the examples below, either or both high and
low acid ionomer resins may be used in the cover compositions so long as
the molded cover layers have a Shore D hardness of 60 or less, and more
preferably 55 or less.
For example, one or more low acid (i.e. 16 weight % and/or less) hard
ionomers may be included in the present invention. The hard (high modulus)
ionomers suitable for use in the present invention include those ionomers
having a hardness greater than 50 on the Shore D scale as measured in
accordance with ASTM method D-2240, and a flexural modulus from about
15,000 to about 70,000 psi as measured in accordance with ASTM method
D-790.
The hard ionomer resins utilized to produce the cover compositions are
ionic copolymers which are the sodium, zinc, magnesium or lithium salts of
the reaction product of an olefin having from 2 to 8 carbon atoms and an
unsaturated monocarboxylic acid having from 3 to 8 carbon atoms. The
carboxylic acid groups of the copolymer may be totally or partially (i.e.
approximately 15-75 percent) neutralized.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
32
Preferably, the hard ionomeric resins are copolymers of ethylene and
either acrylic and/or methacrylic acid, with copolym.ers of ethylene and
acrylic
acid the most preferred. In addition, two or more types of hard ionomeric
resins may be blended into the cover compositions in order to produce the
desired properties of the resulting golf balls.
Examples of commercially available hard ionomeric resins which may
be utilized in the present invention include the hard sodium ionic copolymer
sold under the trademark Surlyn 8940 and the hard zinc ionic copolymer
sold under the trademark Surlyn 9910. Surlyn 8940 is a copolymer of
ethylene with methacrylic acid with about 15 weight percent acid which is
about 29% neutralized with sodium ions. This resin has an average melt flow
index of about 2.8. Surlyn 9910 is a copolymer of ethylene and methacrylic
acid with about 15 weight percent acid which is about 58% neutralized with
zinc ions. The average melt flow index of Surlyn 9910 is about 0.7. The
typical properties of Surlyn 9910 and 8940 are set forth below.
Table 5
Typical Properties of Commercially Available Hard
Surlyn Resins Suitable for Use in the Present Invention
ASTM D 8940 9910 8920 8528 9970 9730
Cation Type Sodium Zinc Sodium Sodium Zinc Zinc
Melt flow index,
gms/10 min. D01238 2.8 0.7 0.9 1.3 14.0 1.6
Specific Gravity,
g/cm' D-792 0.95 0.97 0.95 0.94 0.95 0.95
Hardness, Shore D D-2240 66 64 66 60 62 63
Tensile Strength,
(kpsi), MPa D-638 (4.8) (3.6) (5.4) (4.2) (3.2) (4.1)
33.1 24.8 37.2 29.0 22.0 28.0
Elongation, % D-638 470 290 350 450 460 460
Flexural Modulus,
(kpsi) MPa D-790 (51) (48) (55) (32) (28) (30)
350 330 380 220 190 210
Tensile Impact (23 C)
KJ/m, (ft.-Ibslin2) D-18225 1020 1020 865 1160 760 1240
(485) (485) (410) (550) (360) (590)
Vicat Temperature, 'C D-1525 63 62 58 73 61 73
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
33
In addition, examples of the more pertinent acrylic acid based hard
ionomer resins suitable for use in the present invention sold under the lotek
trademark by the Exxon Corporation include lotek 4000 (formerly Escor
4000), lotek 4010, lotek 8000 (formerly Escor 900), lotek 8020, and
lotek 8030. The typical properties of the lotek hard ionomers are set forth
below in Table 6.
Table 6
Typical Properties of lotek lonomers
Resin ASTM
Properties Method Units 4000 4010 8000 8020 8030
Cation Type zinc zinc sodium sodium sodium
Melt Index 0-1238 m~10 2.50 1.50 0.80 1.60 2.80
Density D-1505 kg/m' 963.00 963.00 954.00 960.00 960.00
Melting Point D-3417 C 90.0 90.0 90.0 87.5 87.5
Crystallization Point D-3417 C 62 64 56 53 55
Vicat Softening Point D-1525 C 62 63 61 64 67
% Wt Acrylic Acid 16 11
% of Acid Groups
cation neutralized 30 40
Plaque
Properties (3 mm thick, 4000 4010 8000 8020 8030
compression molded)
Tensile at break D-638 MPa 24.0 26.0 36.0 31.5 28.0
Yield point D-638 MPa none none 21.0 21.0 23.0
ElongaGon at break D-638 % 395 420 350 410 395
1"/u Secant modulus D-638 MPa 160 160 300 350 390
Shore Hardness 0 D-2240 - 55 55 61 58 59
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
34
Filrn Properties (50 micron filrn
2.2:1 Blow-up ratio) 4000 4010 8000 8020 8030
Tensile at Break MD D-882 MPa 41 39 42 52 47.4
TD D-882 MPa 37 38 38 38 40.5
Yield Point MD D-882 MPa 15 17 17 23 21.6
TD D-882 MPa 14 15 15 21 20.7
Elongation at Break MD D-882 % 310 270 260 295 305
TD D-882 % 360 340 280 340 345
1% Secant modulus MD D-882 MPa 210 215 390 380 380
TD D-882 MPa 200 225 380 350 345
Dart Drop Impact D-1709 g/micron 12.40 12.50 20.30
Resin Properties ASTM Method Units 7010 7020 7030
Cation type zinc zinc zinc
Melt Index D-1238 g/10 min. 0.80 1.50 2.50
Density D-1505 kg/m' 960 960 960
Melting Point D-3417 C 90 90 90
Crystallization Point D-3417 C - - --
Vicat Softening Point D-1525 C 60 63 62.5
% Wt Acrylic Acid -- - --
% of Acid Groups
cation neutralized
Plague Properties (3 mm thick, ASTM Method Units 7010 7020 7030
compressionmolded)
Tensile at break D-638 MPa 38 38 38
Yield Point D-638 MPa none none none
Elongation at break D-638 % 500 420 395
1% Secant modulus D-638 MPa - -- --
Shore Hardness D D-2240 - 57 55 55
In addition to the above, non-ionomeric materials can also be blended
with the ionomers, or used separately, to produce the cover layer of the
invention. Non-limiting examples of materials that can be utilized include
ethylene-ethyl acrylate, ethylene-methyl acrylate, ethylene-vinyl acetate, low
density polyethylene, linear low density polyethylene, metallocene catalyzed
polyolefins such as Engage polyolefins available from Dow Chemical and
Exact polyolefins available from Exxon, non-ionomeric acid copolymers such
as Primacor , available from Dow Chemical, and Nucrel , available from
CA 02444749 2005-04-04
DuPont, and a variety of thermoplastic elastomers, including Kraton ,
available from Shell, Santoprene , available from Monsanto, and Hytrelg,
available from DuPont, etc. Furthermore functionalized EPDM, such as
maleated EPDM, nylon, and nylon-ionomer graft copolymers.
5 A wide array of nylon-containing or nylon-based materials may be
incorporated into the various cover layers of the present invention golf ball.
Preferred nylon materials for utilizing in the present invention golf ball are
described in U.S. Patent No. 5,886,103.
Moreover, a wide array of polyurethane materials can be utilized in one
10 or more cover layers of the present invention golf balls. Before turning
attention to the specifics of such materials, it is instructive to review the
features and terminology associated with polyurethanes.
Polyurethanes are polymers which are used to form a broad range of
products. They are generally formed by mixing two primary ingredients
15 during processing. For the most commonly used polyurethanes, the two
primary ingredients are a polyisocyanate (for example, diphenylmethane
diisocyanate monomer ("MDI") and toluene diisocyanate ( TDI") and their
derivatives) and a polyol (for example, a polyester polyol or a polyether
polyol).
20 A wide range of combinations of polyisocyanates and polyols, as well
as other ingredients, are available. Furthermore, the end-use properties of
polyurethanes can be controlled by the type of polyurethane utilized, i.e.,
whether the material is thermoset (cross linked molecular structure) or
thermoplastic (linear molecular structure).
25 Crosslinking occurs between the isocyanate groups (-NCO) and the
polyol's hydroxyl end-groups (-OH). Additionally, the end-use characteristics
of polyurethanes can also be controlled by different types of reactive
chemicals and processing parameters. For example, catalysts are utilized to
control polymerization rates. Depending upon the processing method,
30 reaction rates can be very quick (as in the case for some reaction
injection
molding systems (i.e., "RIM")) or may be on the order of several hours or
CA 02444749 2005-04-04
36
longer (as in several coating systems). Consequently, a great variety of
polyurethanes are suitable for different end-users.
Polyurethane has been used for golf balls and other game balls as a
cover material. Polyurethanes are typically classified as thermosetting or
thermoplastic. Commercially available polyurethane golf balls have been
made of thermoset polyurethanes. A polyurethane becomes irreversibly "set"
when a polyurethane prepolymer is crosslinked with a polyfunctional curing
agent, such as polyamine and polyol. The prepolymer typically is made from
polyether or polyester. Diisocyanate polyethers are preferred because of
their water resistance.
The physical properties of thermoset polyurethanes are controlled
substantially by the degree of crosslinking. Tightly crosslinked polyurethanes
are fairly rigid and strong. A lower amount of crosslinking results in
materials
that are flexible and resilient. Thermoplastic polyurethanes have some
crosslinking, but purely by physical means. The crosslinking bonds can be
reversibly broken by increasing temperature, as occurs during molding or
extrusion. In this regard, thermoplastic polyurethanes can be injection
molded, and extruded as sheet and blown film. They can be used to up to
about 350 F and are available in a wide range of hardnesses.
U.S. Patent No. 5,006,297 indicates that while thermoplastic and
thermosetting polyurethanes are known, thermosets have been found to
produce better cover stocks for golf balls. Additionally, while thermoplastic
polyurethanes can be used to form game balls, they lack the scuff and cut
resistance of a crosslinked polyurethane. Similarly, thermoplastic
polyurethanes do not readily crosslink.
Polyurethanes typically are formed by reacting a polyol with a
polyisocyanate. In some cases, the polyisocyanate is in the form of a
polyurethane prepolymer formed from a polyether or polyester and a
polyisocyanate. The polyol or polyamine is typically referred to as a "curing"
agent. Examples of reactants used to form polyurethanes by this technique
are discussed in U.S. Patent No. 5,006,297. In other cases a polyester or
acrylic polyol is reacted with a polyisocyanate.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
37
Two types of polyisocyanates are predominantly used to make
polyurethanes, diphenylmethane diisocyanate monomer (MDI) and its
derivatives, and toluene diisocyanate (TDI) and its derivatives.
MDI is the most widely used polyisocyanate. Both rigid and flexible
foams, reaction injection moldings, elastomers, coatings, and casting
compounds are made from MDI. There are three basic grades of MDI:
polymeric MDI, pure MDI, and pure MDI derivatives.
Polymeric MDI is used in both cellular and non-cellular products.
However, because of the high thermal insulation properties possible with
polymeric MDI, its main use is in closed-cell, rigid foam insulation for the
construction and refrigeration industries. Other uses are high-resilience (HR)
flexible foam, carpet backing, and binders.
Pure MDI, which is produced from polymeric MDI, is a low-melting-
temperature (about 100 F) solid. Its primary use is in thermoplastic and cast
elastomers. It also is used as an additive for synthetic fibers to achieve
high
fiber tenacity and elongation.
Pure MDI derivatives are tailored to provide specific processing and
reaction characteristics. A major use for these solvent-free liquids is in
reaction injection molding (RIM), but they also find application in integral
skin
moldings, semi-flexible moldings, and cast elastomers.
Toluene diisocyanate, TDI, is used almost exclusively to make flexible
foam. TDI, however, also finds some use in elastomers, sealants, and
coatings. TDI's generally are water-white liquids which have much higher
isocyanate (-NCO) contents than any MDI, but lower molecular weights.
MDI and TDI also are blended, particularly for producing flexible
molded foams. The free-flowing, brown liquid blends have nearly as high
isocyanate contents as TDI.
Urethanes obtained from aromatic diisocyanates undergo slow
oxidation in the presence of air and light, causing discoloration, which is
unacceptable in some applications. Polyurethanes obtained from aliphatic
diisocyanates are color-stable, although it is necessary to add antioxidants
and uv-stabilizers to the formulation to maintain the physical properties with
time. The least costly aliphatic diisocyanate is hexamethylene diisocyanate
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
38
(HDI), which is obtained by phosgenating the nylon intermediate
hexamethylenediamine. Because of its low boiling point, HDI is mostly used
in form of its derivatives, such as biurets, allophanates, dimers, or trimers.
It
is contemplated that isophorone diisocyanate (IPDI) and its derivatives; and
hydrogenated MDI (HMDI) and cyclohexame diisocyanate (CHDI) may be
used in the formulations described herein.
A wide array of isocyanates may be used in forming polyurethanes for
use in the present invention, such as p-phenylene diisocyanate (PPDI) (CAS
Registry No. 104-49-4); toluene diisocyanate (TDI) (CAS Registry No. 1321-
38-6); 4,4'-methylenebis-(phenylisocyanate) (MDI) (CAS Registry No. 101-
68-8); polymethylene polyphenyl isocyanate (PMDI) (CAS Registry No. 9016-
87-9); 1,5-naphthalene diisocyanate (NDI) (CAS Registry No. 3173-72-6);
bitolylene diisocyanate (TODI) (CAS Registry No. 91-97-4); m-xylyiene
diisocyanate (XDI) (CAS Registry No. 3634-83-1); m-tetramethyl-xylylene
(TMXDI) (CAS Registry No. 58067-42-8); hexamethylene diisocyanate (HDI)
(CAS Registry No. 822-06-0); 1,6-diisocyanato-2,2,4,4-tetra-methylhexane
(TMDI) (CAS Registry No. 83748-30-5); 1,6-diisocyanato-2,4,4-
trimethylhexane (TMDI) (CAS Registry No. 15646-96-5); trans-cyclohexane-
1,4-diisocyanate (CHDI) (CAS Registry No. 2556-36-7); 1,3-bis(isocyanato-
methyl)cyclohexane (HXDI) (CAS Registry No. 38661-72-2); 3-isocyanato-
methyl-3,5,5-trimethylcyclo-hexyl isocyanate (IPDI) (CAS Registry No. 4098-
71-9); dicyclohexylmethane diisocyanate (HMDI) (CAS Registry No. 5124-30-
1).
Two basic types of polyols are used in polyurethanes systems:
polyesters and polyethers. Polyethers are the most widely used.
Often in referring to polyols, their functionality is specified. The
functionality pertains to the number of reactive sites, which in turn,
controls
crosslinking. The more crosslinked (higher functionality), the more rigid will
be the polyurethane. Functionality is controlled by the initiator used to
manufacture the polyol. Glycerine, for example, is commonly used to initiate
triol (3 functional) polyols. To this initiator is added an oxide such as
propylene oxide, ethylene oxide, or a combination, to extend the molecular
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
39
chain and tailor final processing and performance characteristics of the
polyol. Triols typically are used to produce flexible foams; diols are used
for
elastomers, coatings, and sealants; and tetrols typically are used for rigid
foams.
Polyether-based polyols have greater resistance to hydrolysis.
Polyether polyols can be modified by the in-situ polymerization of
acrylonitrile/styrene monomers. The resulting graft polyols generally produce
flexible foams with improved load-bearing properties as well as greater
tensile and tear strengths. Depending on the backbone on which these vinyl
monomers are grafted, a wide range of performance characteristics can be
developed.
Polyether polyols are high molecular weight polymers that range from
viscous liquids to waxy solids, depending on structure and molecular weight.
Most commercial polyether polyols are based on the less expensive ethylene
or propylene oxide or on a combination of the two. Block copolymers are
manufactured first by the reaction of propylene glycol with propylene oxide to
form a homopolymer. This polymer upon further reaction with ethylene oxide
to form the block copolymer. Because primary hydroxyl groups, resulting
from the polymerization of the ethylene oxide, are more reactive than
secondary hydroxyl groups, the polyols produced in this manner are more
reactive. Random copolymers are obtained by polymerizing mixtures of
propylene oxide and ethylene oxide. The viscosity of polyether polyols
increases with hydroxyl equivalent weight. The higher molecular weight
polyether polyols are soluble in organic solvents. Poly(propylene oxide) is
soluble in water up to a molecular weight of 760, and copolymerization with
ethylene oxide expands the range of water solubility.
Polyester polyols yield polyurethanes with greater strength properties,
wear resistance, and thermal stability than polyether polyurethanes, and they
can absorb more energy. These materials, however, are generally more
expensive than polyethers.
Polyester polyols are based on saturated aliphatic or aromatic
carboxylic acids and diols or mixtures of diols. The carboxylic acid of choice
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
is adipic acid because of its favorable cost/performance ratio. For
elastomers, linear polyester polyols of about 2000 mol wt are preferred.
Branched polyester polyols, formulated from higher functional glycols, are
used for foam and coatings applications. Phthalates and terephthalates are
5 also used.
Polyester polyols are typically classed by molecular weight. Low
molecular weight polyols (less than 1500) are used in coatings, casting
compounds, and rigid foams. Medium molecular weight polyols (1550 to
2500) are used in elastomers. And, high molecular weight polyols (greater
10 than 2500) are used in flexible foams.
Thermoset polyurethanes are typically crosslinked and cannot be
repeatedly thermoformed. On the other hand, thermoplastic polyurethanes
are similar to other thermoplastics in that they can be repeatedly plasticized
by the influence of temperature and pressure.
15 The crosslinkable thermoplastic polyurethane used to form a game ball
according to the present invention is initially a thermoplastic, and in this
state
can be melted and solidified repeatedly. However, the material can be
readily crosslinked, thereby increasing its hardness and providing that it
cannot be reversibly melted without thermal degradation.
20 The melt viscosity of a thermoplastic polyurethane (TPU) depends on
the weight-average molecular weight and is influenced by chain length and
branching. TPUs are viscoelastic materials, which behave like a glassy,
brittle solid, an elastic rubber, or a viscous liquid, depending on
temperature
and time scale of measurement. With increasing temperature, the material
25 becomes rubbery because of the onset of molecular motion. At higher
temperatures a free-flowing liquid forms.
The melt temperature of a polyurethane is important for processibility.
Melting should occur well below the decomposition temperature. Below the
glass-transition temperature (T9), the molecular motion is frozen, and the
30 material is only able to undergo small-scale elastic deformations. For
amorphous polyurethane elastomers, the T9 of the soft segment is about -50
CA 02444749 2005-04-04
41
to -60 C, whereas for the amorphous hard segment, T9 is in the 20-100 C
range.
The choice of macrodiol influences the low temperature performance,
whereas the modulus, i.e. hardness, stiffness, and load-bearing properties,
increases with increasing hard-segment content.
A wide array of crosslinkable thermoplastic polyurethanes can be used
*
in the present invention. For example, EBXL-TPU is a thermoplastic
polyurethane recently made available from Zylon Polymers, 23 Mountain
Avenue, Monsey, New York 10952. EBXL-TPU is a pelletized, medical
grade, polyether or polyester based thermoplastic polyurethane, reactor
modified to allow crosslinking by ionizing radiation. It is a low melt index
material suitable for extrusion into profiles, film and sheet, or injection
molding. Once crosslinked, the material combines the ease of processing
and toughness of TPU with the improved resistance to water, solvents and
elevated temperatures characteristic of thermoset materials. Table 7 below,
sets forth details of this preferred material.
*Trade-mark
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
42
TABLE 7
EBXL-TPU
Typical Physical Properties
PROPERTY VALUE UNITS
Radiation 12.5 - 15 MegaRads
Shore Hardness 80 Shore A
Specific Gravity 1.04 grlcc
Tensile Strength 5000 psi
Ultimate Elongation 425 %
Compression set, 50 %
70hrs@ 100degC
Melt Flow Index 2 gms/10 min
FLUID RESISTANCES
Water, no effect
24hrs@ 23C
Isopropyl Alcohol, no effect
100% 24 hrs @ 23 C
Tetrahydrofuran, swells, does not dissolve
24hrs@ 23 C
A further preferred class of crosslinkable thermoplastic polyurethanes
is a commercially available polyurethane from BASF, designated as
Elastollan . Properties of several specific formulations of Elastollan
polyurethanes are set forth in Table 8 below.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Q O~ N Ln O NO aO0 C) (D U-) C) ttl
+1 V 0 C) O C) O LLn ap U') L[) r-
M U') (p Ln M N V
r- M Ln
Q c0 ' N O LD CD cC) O tf) O O O tl)
+1 V O N O ('') O N 0) Ntf) U-)
c0 V 0 c0 00 V N~
CO (O C) V
Q f~ N O O N O M (D tC) O U') O O
p +I V 0 N 0 M O (D Or- tn
p O oo N o0 V N
(O LA M V
Q (O N 00 OO 00 O O OLf) tf)
c- +I V O N O m O (0 I- 00 N 1-
LO M N Q) (Y) 'It O
lf) U) N
~
Q V N N (fl O N O O O tf) O O U)
y~ +1 +1 M O ~ V) N 0 0) (p O tf)
Q) u') 1- N r- 0 aO
O V tf) C)
Q C) N N 1- 0 O O I- 0 u') U) tn LO Ln
p +I +I M O <-- O - 0 1- 1- N
Q) ~ N M tn L() tA
~ O V U') N
co
N N M O CO O (N O O O tn O O
17 +I MO ~ 0 .--lA V h 00 M
(O a0 .- t~ CO (D
00 ~ 00 C -
F-
Q N' N O N 0 O O O tf) OU) U')
p +I M O Vj O - O O V O) N
00 e-- O i~ CO tf) (O tf)
CO V
V N' O O C) O Cl) 0 O O O
M O N 00 Iq a0 (p ~
Q ~ (O Lf1 (O 1- V c
ti d
~ Gl
'
N
N V N _N N N_ V()
0 N _ ~ V~ ~t (O W (D~
Q 0 C) C) 0 0 QQ ~~ $ U
~ p-- o
O
y'-
a L~ y
' Qp cu y fO Fn m'N o o E=Q ~~
o a o a a
a ~ E u) oLN
0 N MV
(n Y ~ W N
d)
S
U a
y 0) 2.
O
C C ~ ; O
O O U N O
N Y ,C C - O
f
L L' ~ d~ a
0) 0)
O (p C C ,~' ~ L U1 ~ L y
C fA O O v tJl ~
N fD 2 N7FD
y C 01 N ~
U) N t5 o 0 0 0 a) y~
U~ ~ O N N O O N N 0 i- 2 U
0 v c ~n U) oom~ c rn ~ '~
a
L ~ a (9 U) U) v t:! 2 C (U ~ W
0- a cn = ~ -- w ~ ~ Q o
z
Ln o Ln
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
44
Elastollan 1100 series of products are polyether-based thermoplastic
polyurethanes. They exhibit excellent low temperature properties, hydrolysis
resistance and fungus resistance. These products can be injection and blow
molded and extruded.
BASF indicates that Elastollan 1175AW, 80A, 90A and 95A are
suitable for extrusion. And, Elastollan 1175AW to 1174D are suitable for
injection molding. BASF further provides that a grade should be dried before
processing. Elastollan can be stored for up to 1 year in its original sealed
container. Containers should be stored in a cool, dry area. Elastollan TPU's
from BASF are commercial TPU's but will not crosslink using irradiation
unless a particular reactive co-agent such as LiquiflexTM H, described below,
is added. Nearly any other commercially available TPU such as Urepan ,
Pellethane , Morthane , Desmopan , etc. can be used provided it is
compounded with a co-agent that readily crosslinks with radiation.
LiquiflexTM is a commercially available hydroxyl terminated
polybutadiene (HTPB), from Petroflex. It is believed that this co-agent
enables the thermoplastic polyurethane to crosslink upon exposure to
radiation. It is believed that the previously noted thermoplastic polyurethane
EBXL-TPU from Zylon contains a co-agent similar to LiquiflexT""
In accordance with the present invention, it is most preferred that at
least one of the cover layers of a multi-layer assembly comprise a
polyurethane. More preferably, the one or more cover layers comprise a
majority proportion, by weight, of a polyurethane.
As indicated above, numerous ways are known to induce crosslinking
in a polymer by free radical initiation, including peroxide initiation and
irradiation. The golf ball covers of the present invention preferably are
crosslinked by irradiation, and more preferably light rays such as gamma or
UV irradiation. Furthermore, other forms of particle irradiation, including
electron beam also can be used. Gamma radiation is preferred as golf balls
or game balls can be irradiated in bulk. Gamma penetrates very deep but
also increases crosslinking of the inner core and the compression of the core
has to be adjusted to allow for the increase in hardness.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Electron beam techniques are faster but cannot be used for treating in
bulk as the electron beam does not penetrate very deep and the product
needs to be rotated to obtain an even crosslink density.
The type of irradiation to be used will depend in part upon the
5 underlying layers. For example, certain types of irradiation may degrade
windings in a wound golf ball. On the other hand, balls with a solid core
would not be subject to the same concerns. However, with any type of core,
certain types of irradiation will tend to crosslink and thus harden the core.
Depending upon whether this type of effect is sought or is to be avoided, the
10 appropriate type of irradiation can be selected.
The level of radiation employed depends upon the desired end
characteristics of the final game ball, e.g. golf ball, cover. However,
generally
a wide range of dosage levels may be used. For example, total dosages of
up to about 12.5, or even 15 Mrads may be employed. Preferably, radiation
15 delivery levels are controlled so that the game ball is not heated above
about
80 C (176 F) while being crosslinked.
The layers of the cover may be formed from generally the same resin
composition, or may be formed from different resin compositions with similar
hardnesses. For example, one cover layer may be formed from an ionomeric
20 resin of ethylene and methacrylic acid, while another layer is formed from
an
ionomer of ethylene and acrylic acid. One or more cover layers may contain
polyamides or polyamide-nylon copolymers or intimate blends. Furthermore,
polyurethanes, pebax, or thermosetting polyurethane can be used.
In order to visibly distinguish the layers, a wide variety of agents such
25 as phosphorous, florescent dies, florescent pigments, etc. can be used.
Additional materials may also be added to the cover (or inner and outer
cover layers) of the present invention as long as they do not substantially
reduce the playability properties of the ball. Such materials include dyes
(for
example, Ultramarine BIueTM sold by Whitaker, Clark, and Danie.ls of South
30 Plainsfield, N.J.) (see U.S. Patent No. 4,679,795), optical brighteners,
pigments such as titanium dioxide, zinc oxide, barium sulfate and zinc
sulfate; UV absorbers; antioxidants; antistatic agents; and stabilizers.
CA 02444749 2005-04-04
46
Moreover, the cover compositions of the present invention may also contain
softening agents such as those disclosed in U.S. Patent Nos. 5,312,857 and
5,306,760, including plasticizers, metal stearates, processing acids, etc., as
long as the desired properties produced by the golf ball covers of the
invention
are not impaired.
Moreover, since there are various hues of white, i.e. blue white, yellow
white, etc., trace amounts of blue pigment may be added to the cover stock
composition to impart a blue white appearance thereto. However, if different
hues of the color white are desired, different pigments can be added to the
cover composition at the amounts necessary to produce the color desired.
In addition, it is within the purview of this invention to add to the cover
compositions of this invention compatible materials such as antioxidants (i.e.
Santonox R), antistatic agents, stabilizers and processing aids. The cover
compositions of the present invention may also contain softening agents,
such as plasticizers, etc., and reinforcing materials such as glass fibers and
inorganic fillers, as long as the desired properties produced by the golf ball
covers of the invention are not impaired.
In one preferred form of the invention, the inner cover layer or inner
cover layers contain filler materials. More particularly, filler materials are
included in order to affect moment of inertia and spin of the golf ball, for
example. Suitable filler materials for the inner cover layer or layers of the
golf
ball include, but are not limited to, clay, talc, asbestos, graphite, glass,
mica,
calcium metal silicate, barium sulfate, zinc sulfide, aluminum hydroxide,
silicates, diatomaceous earth, carbonates such as calcium carbonate,
magnesium carbonate and the like, metals such as titanium, tungsten,
aluminum, bismuth, nickel, molybdenum, iron, copper, brass, boron, bronze,
cobalt and beryllium, and alloys of the above metals, metal oxides such as
zinc oxide, iron oxide, aluminum oxide, titanium oxide, magnesium oxide,
zirconium oxide and the like, particulate synthetic plastic such as high
molecular weight polyethylene, polystryene, polyethylene ionomer resins and
the like, particulate carbonaceous materials such as carbon black, natural
bitumen and the like, as well as cotton flock, cellulose flock, and leather
fiber_
CA 02444749 2005-04-04
47
Dark colored fillers generally are not preferred for use at the outer
surface of the ball if a white ball is desired. Thus, a two-layer cover in
which
a non-white filler is only present in the inner cover layer can be employed.
The amount of filler employed is primarily a function of weight
restrictions. For example, weight may be removed from the core and placed
in the inner and/or outer cover. This added weight will change the moment of
inertia of the ball thereby potentially altering performance. Whereas
typically
the specific gravity of the cover iayer or layers is about 0.95 - 1.00, it may
be
desirable to increase the specific gravity of one or more of the cover layers
to
greater than 1.0, preferably 1.1 - 2Ø
Furthermore, optical brighteners, such as those disclosed in U.S.
Patent No. 4,679,795, may also be included in the cover composition of the
invention. Examples of suitable optical brighteners which can be used in
accordance with this invention are Uvitex OB as sold by the Ciba-Geigy
Chemical Company, Ardsley, N.Y. Uvitex OB thought to be 2,5-Bis(5-tert-
butyl-2-benzoxazoyl)-thiophene. Examples of other optical brighteners suitable
for use in accordance with this invention include Leucopure EGM as sold by
Sandoz, East Hanover, N.J. 07936. Leucopure EGM is thought to be 7-(2n-
naphthol(1,2-d)-triazol-2y1)3-phenyl-coumarin. Phorwhite K-20G2 is sold by
Mobay Chemical Corporation, P.O. Box 385, Union Metro Park, Union, N.J.
07083, and is thought to be a pyrazoline derivative. Eastobrite OB-1 is
2,2'(1,2-ethenediyldi-4, 1-phenylene)bisbenzoxazole and is available from
Eastman Chemical Company.
Moreover, since many optical brighteners are colored, the percentage
of optical brighteners utilized must not be excessive in order to prevent the
optical brightener from functioning as a pigment or dye in its own right.
The percentage of optical brighteners which can be used in
accordance with this invention is from about 0.01 % to about 0.5% as based
on the weight of the polymer used as a cover stock. A more preferred range
is from about 0.05% to about 0.25% with the most preferred range from
about 0.10% to about 0.20% depending on the optical properties of the
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
48
particular optical brightener used and the polymeric environment in which it
is
a part.
Generally, the additives are admixed with an ionomer to be used in the
cover composition to provide a masterbatch (M.B.) of desired concentration
and an amount of the masterbatch sufficient to provide the desired amounts
of additive is then admixed with the copolymer blends.
The above cover layer compositions, when combined with soft cores at
the cover layer thicknesses described herein, produce golf balls having a
relatively low spin in combination with good click and feel.
The cover compositions and molded balls of the present invention may
be produced according to conventional melt blending procedures. In this
regard, the ionomeric resins are blended along with the masterbatch
containing the desired additives in a Banbury type mixer, two-roll mill, or
extruded prior to molding. The blended composition is then formed into slabs
or pellets, etc. and maintained in such a state until molding is desired.
Alternatively a simple dry blend of the pelletized or granulated resins and
color masterbatch may be prepared and fed directly into the injection molding
machine where homogenization occurs in the mixing section of the barrel
prior to injection into the mold. Additives such as the fillers, etc., are
added
and uniformly mixed before initiation of the molding process.
The golf balls of the present invention can be produced by molding
processes currently well known in the golf ball art. Specifically, the golf
balls
can be produced by conventional molding techniques, such as by injection
molding or compression molding the novel cover compositions over the soft
polybutadiene cores to produce a golf ball having a diameter of about 1.680
inches or greater, preferably at least 1.70 inches, and weighing about 1.620
ounces. Larger molds are utilized to produce the thicker covered oversized
golf balls. For injection-molded cover layers having a thickness of up to
about 3.0 mm, it may be preferable to mold the cover in a single step. For
covers and for cover layers of 3.0 mm or more, it generally is preferable for
reasons of both processability and uniformity to mold the cover in two layers.
In compression molding, it may be appropriate to mold a thicker cover in a
CA 02444749 2005-04-04
49
single layer. In compression molding, the cover composition is formed via
injection at about 380 F to about 450 F into smooth surfaced hemispherical
shells which are then positioned around the core in a dimpled golf ball mold
and subjected to compression molding at 200 to 300 F for 2 to 10 minutes,
foliowed by cooling at 50 to about 70 F for 2 to 10 minutes, to fuse the
shells
together to form an unitary ball. In addition, the golf balls may be produced
by injection molding, wherein the cover composition is injected directly
around the core placed in the center of a golf ball mold for a period of time
at
a mold temperature of from 50 to about 100 F. After molding the golf balls
produced may undergo various further finishing steps such as flash trimming,
priming, marking, finish coating and the like as is well known and is
disclosed, for example in U.S. Patent No. 4,911,451.
A preferred method of forming a golf ball according to the present
invention is forming one or more layers via a fast-chemical-reaction process.
Specifically, the preferred method of forming a fast-chemical-reaction-
produced component for a golf ball according to the invention is by reaction
injection molding ("RIM"). RIM is a process by which highly reactive liquids
are injected into a closed mold, mixed usually by impingement and/or
mechanical mixing in an in-line device such as a "peanut mixer," where they
polymerize primarily in the mold to form a coherent, one-piece molded article.
The RIM process usually involves a rapid reaction between one or more
reactive components such as polyether - or polyester - polyol, polyamine, or
other material with an active hydrogen, and one or more isocyanate -
containing constituents, often in the presence of a catalyst. The constituents
are stored in separate tanks prior to molding and may be first mixed in a mix
head upstream of a mold and then injected into the mold. The liquid streams
are metered in the desired weight to weight ratio and fed into an impingement
mix head, with mixing occurring under high pressure, e.g., 1,500 to 3,000 psi.
The liquid streams impinge upon each other in the mixing chamber of the mix
head and the mixture is injected into the mold. One of the liquid streams
typically contains a catalyst for the reaction. The constituents react rapidly
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
after mixing to gel and form polyurethane polymers. Polyureas, epoxies, and
various unsaturated polyesters also can be molded by RIM.
RIM differs from non-reaction injection molding in a number of ways.
The main distinction is that in RIM a chemical reaction takes place in the
5 mold to transform a monomer or adducts to polymers and the components
are in liquid form. Thus, a RIM mold need not be made to withstand the
pressures which occur in a conventional injection molding. In contrast,
injection molding is conducted at high molding pressures in the mold cavity
by melting a solid resin and conveying it into a mold, with the molten resin
10 often being at about 150 to about 350 C. At this elevated temperature, the
viscosity of the molten resin usually is in the range of about 50,000 to about
1,000,000 centipoise, and is typically around 200,000 centipoise. In an
injection molding process, the solidification of the resins occurs after about
10
to about 90 seconds, depending upon the size of the molded product, the
15 temperature and heat transfer conditions, and the hardness of the injection
molded material. Subsequently, the molded product is removed from the
mold. There is no significant chemical reaction taking place in an injection
molding process when the thermoplastic resin is introduced into the mold. In
contrast, in a RIM process, the chemical reaction causes the material to set
20 in less than about 5 minutes, often in less than 2 minutes, preferably in
less
than one minute, more preferably in less than 30 seconds, and in many
cases in about 10 seconds or less.
If plastic products are produced by combining components that are
preformed to some extent, subsequent failure can occur at a location on the
25 cover which is along the seam or parting line of the mold. Failure can
occur
at this location because this interfacial region is intrinsically different
from the
remainder of the cover layer and can be weaker or more stressed. The
present invention is believed to provide for improved durability of a golf
ball
cover layer by providing a uniform or "seamless" cover in which the
30 properties of the cover material in the region along the parting line are
generally the same as the properties of the cover material at other locations
on the cover, including at the poles. The improvement in durability is
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
51
believed to be a result of the fact that the reaction mixture is distributed
uniformly into a closed mold. This uniform distribution of the injected
materials eliminates knit-lines and other molding deficiencies which can be
caused by temperature difference and/or reaction difference in the injected
materials. The process of the invention results in generally uniform molecular
structure, density and stress distribution as compared to conventional
injection-molding processes.
The RIM process used in forming components of a multi-layered golf
ball disclosed herein is substantially different from, and advantageous over,
the conventional injection and compression molding techniques.
First, during the RIM process of the present application, the chemical
reaction, i.e., the mixture of isocyanate from the isocyanate tank and polyol
from the polyol tank, occurs during the molding process. Specifically, the
mixing of the reactants occurs in the recirculation mix head and the after
mixer, both of which are connected directly to the injection mold. The
reactants are simultaneously mixed and injected into the mold, forming the
desired component.
Typically, prior art techniques utilize mixing of reactants to occur before
the molding process. Mixing under either compression or injection molding
occurs in a mixer that is not connected to the molding apparatus. Thus, the
reactants must first be mixed in a mixer separate from the molding apparatus,
then added into the apparatus. Such a process causes the mixed reactants
to first solidify, then later melt in order to properly mold.
Second, the RIM process requires lower temperatures and pressures
during molding than does injection or compression molding. Under the RIM
process, the molding temperature is maintained at about 100 F to about
120 F in order to ensure proper injection viscosity. Compression molding is
typically completed at a higher molding temperature of about 320 F (160 C).
Injection molding is completed at even a higher temperature range of from
about 392 F to about 482 F (200 to 250 C). Molding at a lower temperature
is beneficial when, for example, the cover is molded over a very soft core so
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
52
that the very soft core does not melt or decompose during the molding
process.
Third, the RIM process creates more favorable durability properties in a
golf ball than does conventional injection or compression molding. The
preferred process of the present invention provides improved durability for a
golf ball cover by providing a uniform or "seamless" cover in which the
properties of the cover material in the region along the parting line are
generally the same as the properties of the cover material at other locations
on the cover, including at the poles. The improvement in durability is due to
the fact that the reaction mixture is distributed uniformly into a closed
mold.
This uniform distribution of the injected materials eliminates knit-lines and
other molding deficiencies which can be caused by temperature difference
and/or reaction difference in the injected materials. The RIM process of the
present invention results in generally uniform molecular structure, density
and
stress distribution as compared to conventional injection molding processes,
where failure along the parting line or seam of the mold can occur because
the interfacial region is intrinsically different from the remainder of the
cover
layer and, thus, can be weaker or more stressed.
Fourth, the RIM process is relatively faster than the conventional
injection and compression molding techniques. In the RIM process, the
chemical reaction takes place in under 5 minutes, typically in less than two
minutes, preferably in under one minute and, in many cases, in about 30
seconds or less. The demolding time of the present application is 10 minutes
or less. The molding process alone for the conventional methods typically
takes about 15 minutes. Thus, the overall speed of the RIM process makes it
advantageous over the injection and compression molding methods.
The present invention is further illustrated by the following examples in
which the parts of the specific ingredients are by weight (pbw). It is to be
understood that the present invention is not limited to the examples, and
various changes and modifications may be made in the invention without
departing from the spirit and scope thereof.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
53
Examples
Example 1 - Thick, Single Cover Oversize Balls
A number of golf ball cores having Formulation A, shown below, were
prepared.
Core Formulation A
Material Parts by
Weight
SMR - CV 601 25.00
Taktene 2202 73.50
HI-SIIT"" 234 LD3 6.00
Zinc Oxide4 5.00
Barytes #225 80.00
Stearic Acid6 1.60
Agerite SuperliteTM' 1.60
Ti02 Rutile 20208 3.00
CircoliteTM Oil9 5.00
Red pigment10 3.00
Sulfur (insol)11 3.14
SantocureTM N.S.12 1.28
Methyl Zimate13 0.27
D.P.G.14 0.68
206.07
Natural rubber, Muehlstein, Norwalk, CT
' Synthefic polybutadiene, Bayer Corp., Akron, OH
Precipitated hydrated silica,
PPG Industries, Pittsburgh, PA
' Zinc Corp. of America, Monaca, PA
Hannick Chemical, Akron, OH
' HanNick Chemical, Akron, OH
R.T. Vanderbilt, Norwalk, CT
Harwick Chemical, Akron, OH
Sun Oil, Philadelphia, PA
f0 Stauffer Chemical, Westport, CT
" Stauffer Chemical, Westport, CT
'Z R.T. Vanderbilt, Norwalk, CT
" R.T. Vanderbilt, Norwalk, CT
14 1,3-Diphenylguanidine (accelerator)
R.T. Vanderbilt, Norwalk, CT
One to two dozen cores were made having an average diameter of
36.3 mm (1.430 inches) (Example 1-1). One to two dozen cores having an
average diameter of 37.3 mm (1.470 inches) also were made (Example 1-2).
The 36.3 mm diameter cores were cured at 320 F for 12 minutes, followed by
six minutes of cooling using cold water. The cores having a 37.3 mm
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
54
diameter were cured at 320 F for 12 minutes, followed by six minutes of
cooling using cold water.
The cores were covered with a thick, single layer of an ionomeric
cover material to produce an unfinished golf ball 43.7 mm (1.72 inches) in
diameter. The ionomeric cover material consisted of Cover Formulation W,
shown below:
Cover Formulation W
Parts by weight White Masterbatch - Parts by weight
lotek 8000 70.6 lotek 7030 100.
lotek 7010 19.9 Unitane 0-110 31.72
White MasterBatch 9.5 Ultra Marine BIueTM 0.6
Eastobrite OB-1 0.35
Santonox R 0.05
As shown in Table 9 below, the golf balls with a 36.3 mm average core
diameter had an overall average weight of 43.5 grams, an average cover
thickness of 3.68 mm (0.145 inches), an average PGA compression of 78,
and an average coefficient of restitution (C.O.R.) of 0.744 (Example 1-1).
The golf balls with 37.3 mm (1.470 inch) average core diameters had an
average weight of 44.4 grams, an average cover thickness of 3.18 mm
(0.125 inches), an average PGA compression of 48, and an average
coefficient of restitution of 0.732 (Example 1-2). These thick covered, two
piece oversized golf balls have excellent feel due to the combination of a
hard cover and a very soft core, and could be used (due to their low average
C.O.R.'s) as restricted flight golf balls.
A number of golf ball cores having Core Formulation B, shown below,
were formed.
Core Formulation B
Parts by
Material Weight
Cariflex BR-1220' 67.35
Taktene 2202 27.50
NatsynTM 22003 5.15
Zinc Oxide4 6.53
Limestone 5 8.25
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Poly ProTM 20 Mesh6 6.19
Regrind' 19.59
Zinc Stearate 8 14.78
Zinc Diacrylate9 19.24
5 Luperco 230XL or Trigonox 17/4010 1.48
~ Polybutadiene 6 Amoco Chemical,
Muehlstein, Norwalk, CT ' golf ball core regrind
2 Synthetic Polybutadiene 8 Synpro, Cleveland, OH
10 Bayer Corp., Akron, OH 9 Rockland React Rite,
(internal source) Rockland, GA
Natural Rubber 10 Peroxide, R.T. Vanderbilt,
Muehlstein, Norwalk, CT Norwalk, CT
Zinc Corp of America,
15 Monaca, PA
Lee Lime, Lee, MA
The cores were cured for 15 minutes at 310 F followed by 7 minutes
of cooling using cooling water. Cores having average diameters of 36.3 mm
(1.430 inches) (Example 1-4) and of 37.3 mm (1.470 inches) were formed
20 (Example 1-3).
Cores having an average diameter of 39.2 mm (1.545 inches) also
were formed (Example 1-C1) as a control. These cores are representative of
the size of cores in standard size, oversized golf balls.
The cores of Examples 1-3, 1-4 and 1-Cl were covered with a single
25 layer of the same ionomeric cover material as was used in Examples 1-1 and
1-2. The 36.3 mm and 37.3 mm diameter cores resulted in thick covered,
oversized golf balls having an overall diameter of 43.7 mm (1.72 inches)
(Examples 1-3 and 1-4). Similarly, the 39.2 mm cores were used to form golf
balls having a diameter of 43.8 mm (1.725 inches) (Example 1-Cl).
30 The golf balls made from 36.3 mm cores (Example 1-4) had a final
weight of 44.5 grams, a cover thickness of 3.68 mm (0.145 inches), a PGA
compression of 112 and a coefficient of restitution of 0.811. The balls made
from 37.3 mm cores (Example 1-3) had a weight of 45.1 grams, a cover
thickness of 3.18 mm (0.124 inches), a PGA compression of 105, and a
35 coefficient of restitution of 0.809. The normal cover thickness control
balls
having 39.2 mm cores (Example 1-Cl) had an overall weight of 46.0 grams,
a cover thickness of 2.29 mm (0.090 inches), a PGA compression of 93, and
a coefficient of restitution of 0.812.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
56
Example 2 - Thick Covered Multi-Layer Golf Balls, Standard Size
A number of 32.8 mm (1.29 inch) average diameter golf ball cores
were made using Core Formulation C, shown below. The curing process
was the same as the sulfur curing process described above in Example 1.
The cores were used to make four different types of golf balls having the
cover compositions and thicknesses shown in Table 9 as Examples 2-1 to
2-4. The lotek 959/960 cover formulation (Cover Formulation X utilized)
also is shown below.
Core Formulation C Cover Formulation X
Parts by Weight Parts by Weight
Cariflex BR 1220 80 lotek 959 45.3
SMR CV 60 20 lotek 960 45.3
Zinc Oxide 5 White MasterBatch 9.4
Limestone 110 (see formulation in Ex. 1)
Stearic Acid 1.6
Agerite SuperliteTM 1.6
CircoliteTM Oil 5
Sulfur 3.14
Santocure N.S. 1.28
Methyl Zimate 0.28
D.P.G. 0.68
228.58
The resulting average PGA compression and coefficient of restitution
of the golf balls also is shown in Table 9. A control example using a standard
size, two piece ball core, i.e. 39.2 mm (1.545 inch) core, having Core
Formulation I, shown below, and a single cover layer with a thickness of 1.78
mm (0.070 inches) also was formed. The physical properties of the resulting
balls are shown in Table 9 as 2-Cl.
Core Formulation I
Parts by Weight
Cariflex BR-1220 70.80
Taktene 220 29.20
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
57
Zinc Oxide 6.93
Limestone 18.98
Poly ProTM 20 Mesh 2.55
Regrind 20.07
Zinc Stearate 20.07
ZDA 30.29
Blue masterbatch 0.01
Luperco 231 -XL or Trigonox 29/402 0.90
Z peroxide, R.T. Vanderbilt, Norwalk, CT
The very thick covered balls (2-1 to 2-4) had the same, or substantially
the same, overall compression (i.e. 105-116 PGA) as the thin covered control
(i.e., 2-Cl, 105 PGA) even though the thick covers were more than double,
and in some instances nearly triple, the thickness of the control.
Example 3 - Thick Covered Multi-Layer Balls, Standard Size
A number of sulfur-cured golf ball cores having an average diameter of
32.5 mm (1.28 inches) and the formulation shown below were formed:
Core Formulation D
Materials ~hr
Cariflex BR-1220 80
SMR CV-60 20
Zinc Oxide 5
Limestone 20
Stearic Acid 1.6
CircoliteT"' oil 5
Sulfur 3.14
SantocureTM N.S. 1.28
Methyl Zimate 0.28
D. P. G. 0.68
Agerite WhiteTM' 0.8
1 R.T. Vanderbilt, Norwalk, CT
The cores were cured for 12 minutes at 320 F, followed by cooling for
six minutes with cooling water. The sulfur-cured cores (Examples 3-3, 3-4,
3-7 and 3-8) had an average surface Shore A hardness of 71, an average
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
58
surface Shore C hardness of 35 and an average surface Shore D hardness
of 21.
A number of peroxide-cured cores having an average diameter of 32.5
mm (1.28 inches) and Core Formulation B, shown above were formed. The
cores were cured for 15 minutes at 310 F, followed by cooling for seven
minutes using cooling water. The cores (Examples 3-1, 3-2, 3-5 and 3-6) had
the PGA compression and COR values shown in Table 6.
A number of standard size "control" cores were made having a
diameter of 39.2 mm (1.545 inches) and having Core Formulation F, shown
below, were formed (Examples 3-Cl and 3-C2).
Core Formulation F
Parts by Weight
Cariflex BR-1220 70.37
Taktene 220 29.63
Zinc Oxide 6.67
Limestone 24.07
Poly ProTM 20 Mesh 8.89
Regrind 17.04
Zinc Stearate 18.52
Zinc Diacrylate 27.41
Luperco 231-XL or Trigonox 17/40 0.9
Furthermore, a number of "control" cores having a diameter of 39.2
mm (1.545 inches) and having Core Formulation G, shown below, were
formed (Examples 3-C3 and 3-C4).
Core Formulation G
Parts by Weight
Cariflex BR-1220 73.33
Taktene 220 26.67
Zinc Oxide 22.33
Regrind 10
Zinc Stearate 20
Zinc Diacrylate 26
Luperco 231 -XL or Trigonox 17/40 0.9
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
59
The 32.5 mm cores were covered with a thick multi-layer cover, i.e.
3.35 mm (0.132 inch) thick layer of ionomer followed by a 1.78 mm (0.070
inch) thick layer of the same or a different ionomer. The covers had a"422
tri" dimple pattern, which is the same dimple pattern as is used on the Top-
Flite Hot XL (1995), tour trajectory ball. The compression and coefficient of
the cores, balls having the first cover layer, and balls having the second
cover layer, as well as the finished balls, was obtained and is shown in Table
9. The control cores were covered with a single layer of ionomer having a
thickness of 1.78 mm (0.070 inches).
The results demonstrate that thick covered multi-layer golf balls can be
produced having comparable compression and C.O.R. values as existing
multi-layer golf balls.
Example 4
A number of thermoplastic golf ball cores containing 100 parts by
weight Exact 4049 (Exxon Chemical Co.), a metallocene catalyzed
polyolefin and 60 parts by weight of tungsten powder were formed (Core
Formulation H, Examples 4-1, 4-2, 4-5 and 4-6). The cores were cured for 5
minutes at 320 F followed by cooling using cooling water for 7 minutes. The
cores had an average weight of 23.3 grams and an average diameter of 32.5
mm (1.28 inches). The cores were covered with a 3.35 mm (0.132 inch) thick
layer of ionomer, followed by a second cover having a thickness of about
1.78 mm (0.070 inches). The inner and outer cover layers had the
formulations Y and Z as shown in Table 9. Cover formulation Y is as follows:
Cover Formulation Y
Parts by Weight
lotek 1002 45.3
lotek 1003 45.3
White MasterBatch 9.4
(see formulation in Ex. 1)
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Cover formulation Z is as follows:
Cover Formulation Z
Parts by Weight
lotek 8000 70.6
5 lotek 7010 19.9
White MasterBatch 9.5
(see formulation in Ex. 1)
A number of crosslinked cores were made using 100 parts by weight of
Exact 4049 (Exxon Chemical Co., Houston, Texas), which is a metallocene
10 catalyzed polyolefin, 60 parts by weight of tungsten powder and 5 parts by
weight Trigonox 17/40 (Core Formulation J, Examples 4-3, 4-4 and 4-7).
The cores were cured for 14 minutes at 320 F followed by cooling with
cooling water for 7 minutes. The cores had a weight of 23.6 grams. The
cores had a diameter of 32.5 mm (1.28 inches), and were covered with the
15 same types and thicknesses of cover materials as were used for the
thermoplastic cores. The cover materials are shown in Table 9. The outer
covers of Example 4 employed a "422 Hex" dimple pattern, which is the
same dimple pattern as is used on the Top-Flite XL (1996), regular
trajectory ball.
20 The compression and coefficient values for the balls having a single
cover layer, both cover layers, and finished products were determined and
are shown in Table 9. As shown by the results, the thick covered balls,
having metallocene catalyzed polyolefin cores, give relatively soft
compression versus the thick covered balls having polybutadiene cores, and
25 demonstrate the variety of properties which are possible with the novel
constructions of the invention. The balls of this Example which have cores
made of metallocene catalyzed polyolefin would be useful as range or
practice balls, as they have a soft feel and high spin, as well as a very
durable, hard cover.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Z a Q ~,
cn E . . . . . . . ! . . . . . . r' . .
O N Qi N M ~ (D O ~ an0 ! A Orl
U~ n ~ m m r n n ~+
~ aQ
w E(7 N N 8 0 fD O N o ~D n v t0 ~D ~D eD
2 U a O.-
Z
LL
0 O O N O
> M v N O ~U
> v v v o a
0
Q W Qi O tD N QOi
U2~ n n n cr0 r n
w
> E('J N O o N O aD tp N N
p U a ~ o N o 0 0
U~y
LL H
w
f- C
D E 2 g m 0 oc m co ~. - ~ ~. . w ~
. . . . . . - - - -
H E ci ci 6 ni
oi
0 -
_ ~ .
~ ~ 3 3 3 3 3 3~ x x x x 3 x 3 x 3 x
m ~ n ~ ~ Q
w aQ
> E a ! N N N N O> Oi
p r n ao m
U~v
LL N
w
Z t E cO v ~i ~D ~i vNi ~ ~ cNn rNi m m ~
- ~- E vi oi
d
a
~ 3 3 x x x x x x 3 3 3 3
o~
U x N N N N ~O
... . . . . . . . . . . n n n n r
Ea
o
w U a . . . . . . . . ~ . ~ n n n W
0
U N N v O N O r n n n O v
N E M M M M N n n n n N N ill V1 N N Y1 N N N
< m a0 (a U U U U - M a0 0 0 m m O a LL
X tV c~) V U .- N M V U .- N M R N t0 r cg
LLI St .- .- .- .-- (V fV fV N N ~) t%) f') Cl 1") Pl t~) M Cl
C) LO
Q
~- . (V
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
v O tn IO rv
, . . m m m m m w ,
OI O O~i v u0'1 <~O ~ V Vl ~
r~ m r h n n n n r r
o ~ ~ o 0 0 ~ o ~ o
o 0
M O (OV ~ ~ OOi O C O
N N ~O <D N ~ N - O~ N
V V V O O V V ?'~ __
E , E E
a E o 0
- oMO
HH
og y
Ydvd
('i CI N t~f (V O O O O
O CO In O O O O O W W O~I i 11
O~ O~ 01 O
X N
~ m m m m nro I~ m m m
N
~
3 X ~ T } } T N N N
M ~n o ~ m v u'~ v
. . , n I~ n n n r n o
y W
O O
u O
U ~
~ryl-
U
N O L
aQo~
cw~ m oi <n M ci
..H
u u n
I-~
. T N } N Y N N
ryU
D D
n n f'
c6
W
O Y p O
rn m a"o v a+ u
~th o W
D ry M j
fV'1 (VV fV 10 N ~ In Vl N N W 01 W W Ol m
O! Oi O! (V (V fV N (V tV fV D 10 p U
th t~/ c'~ M tn t~l th t7 f7 t'1 Ox l7 K x
CI O 0 O O O
r y
a0 U n a
D~ ~ D D d C
C> l~) ~ o CCI U
u. c9 c9 x x -1 ~ x x -, u' q~ u u 9,~
5, 95,
L ~ p D
a_?. a d_T ~=
O N N O O
N t7 V a d a
U U U ~ ~ ~ 11 1 11
M ~i t7 V V V O d o O Q f~ U O LL(.~
LO LO
r f~
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
63
Example 5A
The balls of Examples 3-1, 3-2, 3-7, and 4-1 to 4-6 were spin tested
under the following conditions:
Miyamae Driving Machine
Club: Top-Flite Custom 9 iron
Club Head Speed: 105 fps
The results are shown in Table 6 above.
The balls of Examples 3-1, 3-2, 3-7, 4-5 and 4-6 were distance tested
and were compared with the 1995 Top-Flite Hot XL golf balls. The distance
test conditions are provided below:
Club Name: Top-Flite Tour 10.5
Club Head Speed: 160 ft/sec
Launch Angle - degrees: 9.5
The distance test results are shown below in Table 10.
Table 10
Traj Flight Carry Ctr Total Total
Ball deg. Time Carry Diff Dev' Roll Dist Diff
sec yds yds yds yds yds yds
3-1 12.50 10.00 244.40 0.00 -2.25 11.30 255.60 0.00
3-7 12.80 10.00 237.70 -6.60 -1.75 9.40 247.10 -8.50
3-2 12.10 10.00 227.40 -17.00 -3.04 19.20 246.60 -9.10
4-5 10.90 10.00 225.50 -18.90 -6.54 10.20 235.70 -20.00
4-6 11.30 9.90 226.80 -17.50 -6.71 11.70 238.50 -17.10
Hot
XL 11.70 10.00 237.80 -6.60 -4.75 13.10 250.90 -4.80
(1995)
' Deviation from center
The longest ball is that of Example 3-1. This result is surprising,
particularly in view of the fact that this ball has a COR of 0.806, while the
1995 Top-Flite Hot XL ball has a COR of 0.812 0.003. The ball of
Example 3-2 also had a surprisingly long total distance given its low COR of
.796.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
64
Example 5B
Distance tests were conducted for the balls of Examples 3-1, 3-2 and
4-1 to 4-4 under slightly different conditions, which were the following:
Club Name: Top-Flite Tour 10.5
Club Head Speed: 155 ft/sec
Launch Angle - degrees: 9.6
The distance test results are shown below in Table 11.
Table 11
Traj Flight Carry Ctr Total Total
Ball deg. Time Carry Diff Dev Roll Dist Diff
sec yds yds yds yds yds yds
4-1 11.50 9.90 227.10 -12.20 2.08 13.10 240.20 -12.90
4-2 11.90 10.00 226.10 -13.10 1.96 13.40 239.50 -13.60
4-3 11.80 10.00 228.00 -11.30 2.54 12.10 240.10 -13.00
4-4 11.80 10.00 227.40 -11.90 0.63 11.10 238.50 -14.60
3-1 12.00 10.00 239.30 0.00 2.10 13.80 253.10 0.00
3-2 12.00 10.00 233.40 -5.90 2.79 11.10 244.50 -8.60
Hot XL 12.40 10.00 239.00 -0.20 2.46 13.80 252.90 -0.20
(1995)
Once again, the ball of Example 3-1 is the longest. The ball of
Example 3-2 again had a surprisingly long total distance given its low COR.
Example 6
A number of standard size, control golf ball cores having an average
diameter of 39.2 mm (1.545 inches) and a weight of 36.7g were formed using
Core Formulation K, shown below.
Core Formulation K
Parts by Weight
Cariflex 1220 70
Taktene 220 30
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
Zinc Oxide 6.7
Zinc diacrylate 27.4
Zinc Stearate 18.5
Limestone 24
5 Poly ProTM 20 Mesh 8.9
Regrind 17
Trigonox 17/40 0.9
The cores were cured for 11 1/2 minutes at 320 F, and were then
cooled using cooling water for about 7 minutes. The cores had a PGA
10 compression of 95 and a COR of 0.770.
A number of golf ball cores having Core Formulation L (shown below)
and average diameters of 34.8 mm (1.37 inches) and 39.9 mm (1.57 inches)
were formed. The cores were cured for 12 minutes at 320 F, followed by
cooling using cooling water for about 6 minutes.
15 Core Formulation L
Parts by Weight
Cariflex 1220 100
Stearic Acid 2
Zinc Oxide 4
20 Barytes 52
Hi-SiIT"' 2331 7.5
VanoxTM 12902 1
Sulfur 5.25
DuraxTM3 1.75
25 DOTG4 1
BismateT""5 2.8
' P.P.G. Industries
2R.T. Vanderbilt
30 'R.T. Vanderbilt
'R.T. Vanderbilt
5R.T. Vanderbilt
The cores of control Examples 6-Cl, 6-C2 and 6-C3 were covered
with a single cover layer having a thickness of 1.78 mm (0.07 inches). The
35 control cores were covered with the cover formulations shown in Table 12,
which are the same as cover formulations W-Z in Examples 1-4. The cores
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
66
of Examples 6-1 through 6-10 were covered with inner and outer covers
having the cover formulations and thicknesses shown in Table 12. All of the
balls of the present invention and the control balls were distance tested
using
a 5-iron at 128 feet per second and a driver at 160 feet per second.
As shown in Table 12, while the thick covered balls of the present
invention had substantially lower coefficients of restitution than the control
balls, their distance was only slightly shorter. Thus, the golf balls of the
invention provide a greater distance per point of COR as compared to the
control balls.
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
.ry+ N
m m m n r r cp n O rn m O
O V N V a a 7 V N N N ~ V ~A
a, F- ~ N N N N' N N N N N N N N
N
_: Q
'~ ~ V V O~ N C) n) .- N ~A V N V1
~ U~ N N N Z{- N N N N N N N N N
- ~ c m 0 tr0 cm0 ~ ~
~
O CO N
O r m y Z y N (p r m 0) O N
c\j
~ s- U'. ~ c0 ~D ~N c0 ~O m m cO m ~ ~ ~
C.
o CD N V 01 ~ fD 1n r 'I r O) C) N 't
~- rn f0 v~ n m m In o 0 m m ~ r
U X r n n n n n r m m r N. n r-
~ a
m o o o ~ o 0 0 0 0 ' 00
~ 0
a
~
r
~ o 0 0 0 0 0 0 0 0 0 0 0 0
C m o J O O O ll~ r m N ~ c+) N
V ln
~ N V V ~ ~ N ln 1n ~A ~A 1~
co
v ~ a v v v v v a v v v v
Y y
~ v N E m m m m o 0 0o m co m m o 0
~ r r n rl: v v r r t,-: r r v v
> IL- c E - - - - - - - - - - - -
0
U
~
:3 (D
O
N N N N >- } } } X X X X X
N
~
r--
(D ~ p o ~ Q 04 (D CO W Nv Q Q a m m m
(Q U x z n n r r r r z z
~
a
d U Z N. ~ amo n oNO 0 Z z W ~ ~ m
" Q m m r ~ c y a o Q Q m c ~ n
~
3 a z~~ M ; ; ; z z M M ;~
tl!
y
m
C
Y
E d 0 ~ a 4 $ 0
~o w
E z Mcn M cl) Mcn Z zri m~m
cd E ~ N N N 00) Ooi N Q Q N N OOi 00)
(D E z m m c 'n fi m M Z Z Cc)) c=rni cmo M
~
0
U
a>
c
a~ aD
o a
L~U 1~' Y J J J J J J Y Y J J J J
x .- N m C>
W U N M U U r rn
cd ~n ro to (c (D co to (c ID (D (b (6
LO LO
CA 02444749 2003-10-20
WO 02/085464 PCT/US02/12861
68
The foregoing description is, at present, considered to describe
the preferred embodiments of the present invention. However, it is
contemplated that various changes and modifications apparent to those
skilled in the art, may be made without departing from the present invention.
Therefore, the foregoing description is intended to cover all such changes
and modifications encompassed within the spirit and scope of the present
invention, including all equivalent aspects.