Note: Descriptions are shown in the official language in which they were submitted.
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
BUOYANT ELECTROLYSIS DEVICE FOR TREATING A RESERVOIR OF WATER
RELATED APPLICATION
This application incorporates by reference U.S. Provisional Application No.
60/269,048, filed February 15, 2001.
FIELD OF THE INVENTION
This invention relates to an electrolysis device having an electrolysis cell
for
treating a reservoir of water or other electrolyte solution.
BACKGROUND OF THE INVENTION
The worldwide population uses water daily for drinking, cooking, bathing,
cleaning, and other personal uses. In many countries, the supply of water is
made
relatively safe for consumption or for contact with the body through municipal
water
treatments. Such municipal treatment usually uses chemicals, such as chlorine
or ozone,
to treat the water to destroy harmful microorganisms in the water.
Nevertheless, these
supplies are not completely effective at killing all of the bacteria and other
pathogens, and
can become contaminated with bacteria and other pathogens as a result of
faulty treatment
operations. In a variety of circumstances, these contaminants must be removed
or
neutralized before the water can be used. For example, in many medical
applications and
in the manufacture of certain electronic components, extremely pure water is
required.
As a more common example, any harmful contaminants must be removed from water
before consumption or use for bathing. Despite modern water purification
means, the
general population is at risk, and in particular infants and persons with
compromised
immune systems are at considerable risk. In many countries, a substantial
proportion of
the population of this planet does not have "running water; that is, a supply
of reasonably
fresh, safe water that can be delivered into the community, or into the
individual homes,
and can only obtain a supply of water for drinking, cooking, bathing, etc.
from local water
sources, such as lakes, ponds, streams, rivers, wells, cisterns, springs, etc.
Even the
freshest of these water sources has some level of harmful bacteria and other
pathogens.
Very often these water sources can be highly polluted and can contain
extremely high
1
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
level of harmful microorganisms and pathogens. There are deadly consequences
associated with exposure to contaminated water, caused by increasing
population
densities, increasingly scarce water resources, and often no community water
treatment
utilities. It is common for sources of drinking water to be in close proximity
to human
and animal waste, such that microbiological contamination is a major health
concern. As
a result of waterborne microbiological contamination, an estimated six million
people die
worldwide each year, half of which are children under 5 years of age.
In 1987, the U.S. Environmental Protection Agency (EPA) introduced the "Guide
Standard and Protocol for Testing Microbiological Water Purifiers". The
protocol
establishes minimum requirements regarding the performance of drinking water
treatment
systems that are designed to reduce specific health related contaminants in
public or
private water supplies. The requirements are that the effluent from a water
supply source
exhibits 99.99% (or equivalently, 4 log) removal of viruses and 99.9999% (or
equivalently, 6 log) removal of bacteria against a challenge. Because of the
prevalence of
Escherichia coli (E. coli, bacterium) in water supplies, and the risks
associated with its
consumption, this microorganism is used as the bacterium in the majority of
studies.
It is known that the containers used for holding the water supply can also
become
contaminated with bacteria and other pathogens, such that, even when fresh,
safe water is
placed for holding into the container, the water can become contaminated (or
re-
contaminated) by the container itself. Furthermore, the user's containers of
the water,
such as baths, tubs, drinking water pitchers, etc. can become contaminated and
can retain
a biofilm on the surface of the container, even though cleansed with water and
common
detergents.
An effective means for treating water and other electrolyte solutions to kill
microorganisms and other pathogens therein employs an electrolysis cell
whereby the
solution (e.g., water) passes in between or over a set of electrodes across
which an
electrical current is applied. The electrical current passing between the
electrodes and
through the solution can convert chloride ions (residual or added, such as by
adding salt,
NaCI) into one or more chlorine biocidal agents that are effective in killing
bacteria,
viruses, parasites, protozoa, molds, spores, and other pathogens in the
solution. Examples
of electrolysis cells and methods for electrolyzing water are disclosed in
U.S. Patent
2
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
3,616,355 (Themy et al., issued Oct. 26, 1971, U.S. Patent 4,062,754 (Eibl),
issued Dec.
13, 1977, U.S. Patent 4,100,052 (Stillman), issued Jul. 11, 1978, U.S. Patent
4,761,208
(Gram et al.), issued Aug. 2, 1988, US 5,313,589 (Hawley), issued May 24,
1994, and
U.S. 5,954,939 (Kanekuni et al.), issued Sep. 21, 1999.
Much of the world's water supply for cooking, bathing, drinking, cleaning, and
recreation (for example, swimming pool and spa water) is contained as a
reservoir of
water, such as tanks, tubs, water pitchers, as well as ponds, cisterns, lakes,
and others.
Therefore, of specific interest are reservoirs of water contaminated with
harmful bacteria
and other unhealthy microorganisms, or that are contained within reservoir
containers
(tubs, pitchers, and the like) that are contaminated with these same
pathogens. Various
attempts have been made to treat such reservoirs of water, but none have been
completely
effective. It is known to treat swimming pools for the growth of algae and for
potential
microorganism with only limited success. U.S. Patent 4,337,136 issued to
Dahlgren (Jun.
29, 1982) discloses a device having a pair of silver-copper electrodes
depending from the
bottom of a floating container, and containing a 12-volt battery. The device
sacrifices
silver ions from the electrodes into the water that can allegedly attack
bacteria in the
water. U.S. Patent 5,013,417, issued to Judd, Jr. (May 7, 1991) discloses a
device that
floats inside the skimmer of a pool, having attached to its bottom a pair of
copper/silver
disks that are spaced apart sufficiently for unobstructed flow of water
between the disks.
The device can be powered by photovoltaic cells or batteries. Other examples
of floating
devices having sacrificial anodes to treat swimming pool water are disclosed
in U.S.
Patents 5,059,296 (issued Oct. 22, 1991) and 5,085,7532 (issued Feb. 4, 1992),
which
disclose floating solar powered water purifiers having a purification cell
below the
surface of the water to be treated. None of these references teaches an
electrolysis device
that is reliably and completely effective in killing microorganisms in the
reservoir of
water.
Another means of treating a reservoir of water is described in WO 00/71783,
published Nov. 30, 2000, describes a portable disinfection device having an
annular
electrolysis cell in which a batch of brine solution is electrolyzed to form
an electrolyzed
brine solution for use in sterilizing a substance or a container of untreated
water. The
3
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
portable disinfection device is described as a "pen" purification device for
personal water
purification.
Despite the many advances in the technology of electrolyzing waters and other
electrolytic solutions, there remains a need for more effective, more
efficient, more
portable, and more affordable electrolysis devices and techniques for the
treatment of the
world's water supplies for safe and healthy living.
Objects of the present invention include: providing an improved electrolysis
device for electrolyzing water and other electrolytic solutions stored or
handled in
containers, tanks, and any other reservoir (including small ponds; cisterns,
etc.);
providing an electrolysis device that is both effective in electrolyzing water
from the
reservoir, and safe to persons who use or benefit from the device, including
children and
infants; providing a self-powered electrolysis device for treating a reservoir
of water,
which can operate away from (and in the absence of) conventional household
electrical
currents; providing an electrolysis device that is self-contained and self-
powered, that
both effectively and reliably electrolyzes water, and is affordable to
consumers in most
income brackets; providing an electrolysis device that can effectively kill
bacteria and
other pathogens in the water source, as well as bacteria and other pathogens
that are
resident on the surfaces of the water container and that can contaminate, or
re-
contaminate, the water source; providing an electrolysis device that is mobile
within the
reservoir of water or can ensure the necessary diffusion of biocidal active
via movement,
propulsion, or water jets, to provide the biocidal benefits throughout the
reservoir of
water; providing an improved electrolysis device having a buoyant body and an
electrolysis cell having close-spaced electrodes that provide efficient
conversion of
chloride ions in the source water into biocidal oxidant agents at low power
requirements;
providing a method for sterilizing a reservoir of water or electrolysis
solution which can
continue to sterilize the reservoir in case of a re-contamination from an
outside source;
and providing an improved method of bathing infants and small children that
virtually
eliminates harmful and unhealthy microorganisms and other pathogens from the
bathing
water.
4
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
SUMMARY OF THE INVENTION
The invention provides a self-powered electrolysis device, for placement into
a
reservoir of an electrolytic solution containing chloride ions, to electrolyze
the
electrolytic solution, comprising:
(1) a buoyant body,
(2) an electrolysis cell comprising a pair of electrodes defining a cell
passage formed
there between through which the electrolytic solution can flow, the cell
passage
having an inlet and an outlet, wherein the cell inlet is in fluid
communication with the
reservoir electrolytic solution, and wherein the cell passage forms a gap
between the
pair of electrodes having a gap spacing between about 0.1 mm to about 5.0 mm,
and
(3) an electrical current supply for applying electrical current between the
pair of
electrodes.
The electrolysis device can further comprise a means for pumping the reservoir
water through the cell passage,
The invention also provides a self-powered, self-propelled buoyant
electrolysis
device, for placement into a reservoir of an electrolytic solution containing
chloride ions
to electrolyze the electrolytic solution, comprising:
(1) a buoyant body,
(2) an electrolysis cell comprising at least a pair of electrodes defining a
cell passage
farmed there between through which the electrolytic solution can flow, the
cell
passage having an inlet and an outlet, wherein the cell inlet is in fluid
communication
with the reservoir electrolytic solution,
(3) an electrical current supply for applying electrical current between the
electrodes,
and
(4) a means of propulsion for moving the buoyant electrolysis device within
the
reservoir of water.
Preferably the electrolysis cell is contained within the buoyant body of the
self-
propelled buoyant device. The electrolysis cell can also be positioned on an
outside,
submerged surface of the buoyant body, whereby reservoir water passes into the
inlet of
the electrolysis cell as the buoyant body moves within the reservoir of water.
The self-
propelled buoyant electrolysis' device can further comprise a means for
pumping the
5
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
reservoir water through the cell passage, which can be the same means as the
propulsion
means. In a preferred embodiment, the propulsion means comprises a rotating
impeller
driven by an electric motor that is powered by an electrical current supply.
Preferably,
the buoyant body can be positively buoyant in the electrolytic solution,
whereby the
device is at least partially exposed above the surface of the reservoir
electrolytic solution.
The invention also includes a method of disinfecting a reservoir of an
electrolytic
solution containing halide ions, and optionally a reservoir which can be
repeatedly
contaminated with microorganisms, with a self-powered electrolysis device,
comprising:
1) providing a reservoir of contaminated water;
2) treating at least a portion of the reservoir water with self-powered
buoyant
electrolysis device, thereby disinfecting the water; and, optionally
3) re-treating at least a portion of the reservoir water with the electrolysis
device,
in response to a re-contamination of the water with microorganisms, thereby re-
disinfecting the water.
A preferred method continuously treats the reservoir of electrolytic solution
with
the electrolysis device, thereby preventing a re-contamination of the
reservoir. A
preferred method treats the reservoir solution by passing at least a portion
of the reservoir
solution to the electrolysis device, electrolyzing the portion of reservoir
water in an
electrolysis cell of the electrolysis device, thereby forming an effluent of
electrolyzed
water comprising a quantity of mixed oxidant material, discharging the
effluent into the
reservoir of water, and dispersing the effluent throughout the reservoir of
water, thereby
disinfecting the reservoir. An optional method of the present invention
provides a local
source of halide ions that is mixed with the portion of the reservoir solution
passing to the
electrolysis cell, and electrolyzed in the electrolysis cell, thereby forming
an effluent of
electrolyzed water comprising a quantity of mixed oxidant material that is
greater than a
quantity of mixed oxidant material formed by electrolyzing the portion of the
reservoir
solution only.
6
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
BRIEF DESCRIPTION OF THE DRAWINGS
The various advantages of the present invention will become apparent to
skilled
artisans after studying the following specification and by reference to the
drawings in
which:
S Fig. 1 shows a planar electrolysis cell used in an electrolysis device of
the present
invention.
Fig. 2 shows an alternative electrolysis cell used in an electrolysis device
of the
present invention.
Fig. 3 shows yet another alternative electrolysis cell used in an electrolysis
device
of the present invention.
Fig. 4 shows one embodiment of a device of the present invention, comprising
the
electrolysis cell of Fig. 1 taken through line 4-4.
Fig. 5 shows another embodiment of a device of the present invention,
comprising
the electrolysis cell of Fig. 3 taken through line 5-5.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
"Self-powered" means that a device comprises the source of electrical or other
power necessary for the defined functions of the device, which can include,
but are not
limited to, the electrical current supply for the electrolysis cell, the power
for any
pumping means, the power for any propulsion means, the power for any
indication or
control means, etc.
"Self-contained" means that the device and all its elements are substantially
contained as a single article or unit, and do not require physical connection
outside the
reservoir with external power or propulsion means through wires, tethers, etc.
"Buoyant" means positively buoyant (i.e., the body and/or device will float to
the
surface of the reservoir electrolytic solution) and neutrally buoyant (i.e.,
the body and/or
device will remain submerged and substantially stationary in the reservoir
electrolytic
solution). A non-buoyant body and/or device will sink quickly in the reservoir
electrolytic solution.
7
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
"Fluid communication" means that electrolytic solution can flow between the
two
objects between which the fluid communication is defined.
"Sterilization" means the destruction al all microbial life, including
bacterial
spores.
"Disinfection" means the elimination of nearly all microbial forms, but not
necessarily all. Disinfection does not ensure overkill and lacks the margin of
safety
achieved by sterilization.
Electrolytic Solution
In its broadest use in the present invention, an electrolytic solution is any
chemically compatible solution that can flow through the passage of the
electrolysis cell,
and that contains sufficient electrolytes to allow a measurable flow of
electricity through
the solution. Water, except for deionized water, is a preferred electrolytic
solution, and
can include: sea water; water from rivers, streams, ponds, lakes, wells,
springs, cisterns,
etc., mineral water; city or tap water; rain water; and brine solutions.
Electrolytic
solutions can also include blood, plasma, urine, polar solvents, electrolytic
cleaning
solutions, beverages, and others. An electrolytic solution of the present
invention is
chemically compatible if it does not chemically explode, burn, rapidly
evaporate, or if it
does not rapidly corrode, dissolve, or otherwise render the electrolysis
device unsafe or
inoperative, in its intended use.
Preferred are electrolytic solutions that contain a residual amount of halide
ions,
including chloride, fluoride, bromide, and iodide, and more preferably
chloride ions.
During electrolysis, which is described in more detail below, the halide ions
can be
converted to biocidally-effective mixed oxidants that include various halide
oxidants.
Preferred devices of the present invention comprise an electrolysis cell that
is very
effective in converting the reservoir solution containing low levels of
residual halide ions
into an effluent solution (that is, the electrolyzed solution that is
discharged from the
outlet of the cell) containing a higher level of the biocidal mixed oxidants.
Such reservoir
solutions containing residual halide ions can comprise 35,000 ppm (sea water)
or less,
preferably less than 1,000 ppm, more preferably less than about 400 ppm, and
most
preferably less than 200 ppm, of halide ions. Of course, reservoir solutions
containing the
8
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
higher levels of residual halide ions also are more efficiently converted into
an effluent
solution having even larger amounts of the mixed oxidants. This is due in part
because
the conductivity of the electrolysis solution increases with the concentration
of halide
ions, thereby enabling a greater current flow across the passage gap between
the pair of
electrodes under a constant voltage potential. In general, to produce the same
amount of
mixed oxidants at a fixed power (current and voltage potential), an
electrolysis solution
having a higher concentration of halide ions will require a substantially
larger gap
spacing, compared to an electrolysis solution having lower concentrations of
the halide
ions.
Preferably the electrolytic solution has a specific conductivity p of greater
than
100 ~S/cm, preferably more than 150 ~S/cm, even more preferably more than 250
~S/cm,
and most preferably more than 500 wS/cm.
Bodv
The devices of the present invention have a body into, or onto, which the
other
elements are positioned. A body can be any open or closed object that can
contain one or
more of the other elements of the electrolysis device, including an
electrolysis cell, an
electrical current supply, a pumping means, a propulsion means, and a local
source of
halide ions. The body can be made of any material that is compatible with the
reservoir
electrolysis solution, and the device's use. For use in water, the body is
preferably made
of plastics, including PVC, polyethylene, polypropylene, other polyolefins,
foam plastics,
rubberized plastics, and Styrofoam; metals including tin, aluminum, steel, and
others; and
can even use wood or paper board including coated paperboard, depending upon
the use.
Preferred are durable, resilient plastics that can help to protect the
internal components
from external impact and forces that might otherwise damage them.
The body can be made in almost any shape, including spheres and ovals, cubes,
and rectilinear shapes. A preferred shape is that of a play toy, such as a
boat, duck,
whale, or other shape, for use in an infant bath tub.
Preferred devices comprise a housing that is sealed or is sealable to prevent
electrolytic solution from entering the housing, except as intended (such as
through the
inlet port). The body is preferably a closed body having a confined space
within the body
9
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
to contain one or more of the other components of the electrolysis device, and
is most
preferable water-proof to prevent the solution (e.g., water) from the
reservoir from
entering into the body (except through the passage of the electrolysis cell),
thereby
preventing short circuiting or other damage to an electrical current supply,
and any
pumping means, propulsion means, etc. The body can have an opening through its
outer
surface through which electrolysis solution can pass through to the
electrolysis cell
contained therein. The body can have at least one sealed or sealable
compartment therein
into which the electrical current supply, such as a set of dry cell batteries,
are placed. The
body can have one or more removable covers for openings, through which
components,
such as batteries, can be removed, installed, or replaced, and which can be
made liquid
sealable. The sealed or sealable compartment within the body serves to prevent
liquid,
such as the electrolysis solution, from entering, and ensures buoyancy. The
internal
volume of the body should be sized to provide both a space for the components,
and air
space sufficient to make the device buoyant, taking into account the combined
weight of
the body and its components. For positively buoyant devices, a target maximum
submersion of the device is about 80%, which means the volume of the device
that is
below the surface of the water should be 80% or less. The weight of the device
should be
80% or less of the weight in water that the volume of the device will occupy.
Small
devices that are more convenient to handle can advantageously use miniaturized
pumps,
electrolysis cells, and battery sets that deliver high productivity and
efficiency.
When the electrolysis cell is positioned inside the body, the cell inlet is
placed into
fluid communication with the reservoir solution via at least one opening in
the outer
surface of the body, and a tube or duct that connects the outer opening with
the inlet of
the cell. Likewise, the body can have an outlet opening that is in fluid
communication
between the outlet of the cell and the reservoir.
Electrolysis Cell
The electrolysis cell is the most important functional component of the
device.
The electrolysis cell generates biocidal agents by passing electrical current
through an
electrolytic solution that is positioned within or flows through the cell, and
more
particularly, from the halide ions contained in, or added to, the reservoir
electrolytic
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
solution. The electrolysis cell comprises at least a pair of electrodes,
between which
passes the electrolysis solution. A cell passage is the space between the pair
of
electrodes, and has the shape defined by the confronting surfaces of the pair
of electrodes.
The cell passage has a cell gap, which is the perpendicular distance between
the two
confronting electrodes. Ordinarily, the cell gap will be substantially
constant across the
confronting surfaces of the electrodes.
Generally, the electrolysis cell will have one, or more, inlet openings, in
fluid
communication with each cell passage, and one, or more, outlet openings, also
in fluid
communication with the passage. The inlet opening is also in fluid
communication with
the reservoir solution, such that the reservoir solution can flow into the
inlet, through the
passage, and from the outlet of the electrolysis cell. The effluent solution
(the
electrolyzed solution exiting from the passage) is typically returned to
reservoir, thereby
treating the reservoir solution with the generated biocidal agents.
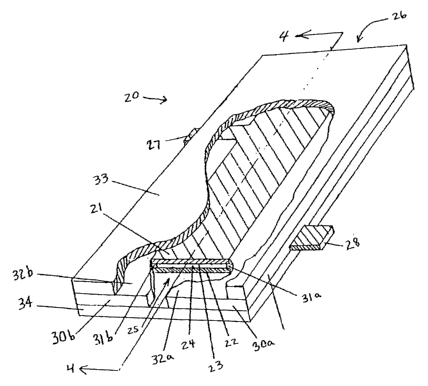
Fig. 1 shows a planar electrolysis cell 20 that can be used in an electrolysis
device
of the present invention. The cell comprises an anode 21 electrode and a
cathode 22
electrode. The electrodes are held a fixed distance away from one another by a
pair of
opposed non-conductive electrode holders 30a and 30b having electrode spacers
31a and
31b that space the confronting longitudinal edges of the anode and cathode
apart by a
spacing gap 23, thereby forming a passage 24 between the electrodes. The
passage 24
has a cell inlet 25 and an opposed cell outlet 26 through which the
electrolysis solution
can pass into and out of the cell. Reservoir solution flows into the cell
between an
expanding flow inlet formed between the extended inlet portions 32a and 32b of
the
electrode holders 30a and 30b, and into the cell passage 24. The assembly of
the anode
and cathode, and the opposed plate holders are held tightly together between
non-
conductive anode cover 33 (shown partially cut away) and cathode cover 34 by a
retaining means (not shown) that can comprise non-conductive, water-proof
adhesive,
bolts, or other means, thereby restricting exposure of the two electrodes only
to
electrolysis solution that flows through the passage 24. Anode lead 27 and
cathode lead
28 extend laterally and sealably through channels made in the electrode
holders 30b and
30a, respectively.
m
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
Fig. 2 shows an alternative electrolysis cell of the present invention. The
cell
comprises a curled anode 21 and a curled cathode 22. The outer surface of the
cathode 22
and the inner surface of the curled anode 21 are confronting and form a
passage 24 there
between. The electrodes are formed to provide a uniform gap spacing between
the
electrodes across their entire confronting surfaces. Electrolytic solution can
flow into and
out of the passage of the cell through any of the openings into the cell along
edges 36b,
36c, and 36d. Alternatively, the cell plates can be sealed along the edge 36b
to provide a
cell having an inlet and outlet openings 36c or 36d. The electrodes are held
in their
confronting spaced position by a plurality of electrode spacers 31 positioned
along the
periphery of the passage 24. Usually, a planar base for the cell (not shown)
is attached to
the curled edges 36a of the electrodes, which also helps to stabilize the
electrodes from
flexing and separating from one another. Anode lead 27 and cathode lead 28 are
used to
attach the electrical current supply to the cell.
Another preferred cell embodiment can comprise a pair of electrodes open to
the
flow of solution in from and out toward any direction. An example of such an
electrical
cell is shown for illustration in Fig. 3, wherein spacers 31 are positioned
along the
periphery of the passage 24 to maintain the gap spacing between the
electrodes. So long
as the gap spacing is sufficient to provide a flow of liquid through the
electrolyzed cell
passage, sufficient amounts of mixed oxidant agents can be produced to
effectively treat
the reservoir solution. Although the cell in Fig. 3 is shown with rectangular
electrodes,
the electrodes can be provided in other shapes, including circles, oval and
squares. A
funnel member 86 is shown affixed to the electrolysis cell, adjacent to the
cathode 22,
although it can be affixed to either or both electrodes. In Fig. 3, a base 35
is attached to
the upper surface of the anode 21, which can then be easily affixed to an
outer surface of
the body 16. The funnel member 86 is also shown attached to the entire
periphery of the
cathode, but can be attached to one side, or to two or more sides. The funnel
member
helps to force liquid from the reservoir that enters the expanded funnel
opening 87 and
into the inlet of the cell as the cell, which is mounted to a body 16 and
connected to an
electrical current supply 50, is moved or propelled through the reservoir (as
shown by
direction 90 in Fig. 5), or as reservoir solution is moved past the cell.
12
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
Electrodes
An electrode can generally have any shape that can effectively conduct
electricity
through an electrolytic solution between itself and another electrode, and can
include a
planar electrode, an annular electrode, a spring-type electrode, and a porous
electrode.
Another preferred electrode forms are curled plates such as shown in Fig. 2.
Generally,
the anode and cathode electrodes, as well as any ancillary electrodes
positioned there
between, are shaped and positioned such that there is a uniform gap between a
cathode
and an anode electrode pair. Consequently, a pair of planar electrodes will be
preferably
co-extensive and parallel to, or separated by a constant gap spacing from, one
another.
Planar electrodes, such as shown in Fig. 1, are commonly used. The aspect
ratio
of an electrolysis cell employing planar electrodes is defined by the ratio of
the length of
the anode along the flow path of the solution, to the width of the anode,
transverse to the
flow path. Generally, the aspect ratio of the electrolysis cell is between 0.2
and 10,
though more preferably is between 0.1 and 6, and most preferably between 2 and
4.
The pair of electrodes, both the anode and the cathode, are generally
metallic,
conductive materials, though non-metallic conducting materials, such as
carbon, can also
be used. The materials of the anode and the cathode can be the same, but can
advantageously be different. The electrodes are preferably dimensionally and
spatially
stable, to avoid excessive bending, flexing, warping, and gapping of the
electrodes during
use, thereby maintaining a constant gap spacing between the confronting
electrodes. To
minimize corrosion, chemical resistant metals are preferably used. Examples of
suitable
electrodes are disclosed in US Patent 3,632,498 and U.S. Patent 3,771,385.
Preferred
anode metals are stainless steel, platinum, palladium, iridium, ruthenium, as
well as iron,
nickel and chromium, and alloys and metal oxides thereof. More preferred are
electrodes
made of a valve metal such as titanium, tantalum, aluminum, zirconium,
tungsten or
alloys thereof, which are coated or layered with a Group VIII metal that is
preferably
selected from platinum, iridium, and ruthenium, and oxides and alloys thereof.
Particularly preferred is an anode made of titanium core and coated with, or
layered with,
ruthenium, ruthenium oxide, iridium, iridium oxide, and mixtures thereof,
having a
thickness of at least 0.1 micron, preferably at least 0.3 micron. The
electrode can have a
thickness of about 5 mm or less, though more preferably about 0.1 mm to about
2 mm.
13
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
For many applications, a metal foil having a thickness of about 0.03 mm to
about
0.3 mm can be used. Foil electrodes should be made stable in the cell so that
they do not
warp or flex in response to the flow of liquids through the passage that can
interfere with
proper electrolysis operation. The use of foil electrodes is particularly
advantageous
when the cost of the device must be minimized, or when the lifespan of the
electrolysis
device is expected or intended to be short, generally about one year or less.
Foil
electrodes can be made of any of the metals described above, and are
preferably attached
as a laminate to a less expensive base metal, such as tantalum, stainless
steel, and others.
The electrolysis cell of this embodiment can be positioned inside the body, on
the
outside surface of the body, or partially on the outside and the inside.
Preferably, the cell
is positioned inside the body of the device to avoid contact by the electrodes
and the
circuitry with the hands or body of the user or with other non-compatible
objects in the
environment.
The electrolysis cell can also comprise a batch-type cell that electrolyses a
volume
of the electrolytic solution (such as water). The batch-type cell comprises a
batch
chamber having a pair of electrodes. The batch chamber is filled with water
from the
reservoir, which is then electrolyzed and returned back to the reservoir. The
electrodes
preferably comprise an outer annular anode and a concentric inner cathode.
Alternatively, the cell can comprise a batch-continuous-type cell that
electrolyses a
volume of water, a portion of which flows into the chamber and a portion of
which flows
out of the chamber during the step of electrolyzing the water contained within
the
chamber. Preferably, the reservoir water is mixed with a local source of
halide ions to
generate proportionally greater amounts of mixed oxidants. An example of a
suitable
batch cell, along with a halide salt supply and electrical circuitry to
control the
electrolysis of the salt solution, are disclosed in WO 00/71783-A1, published
Nov. 30,
2000, incorporated herein by reference.
Electrical Current Supply
Operation of the electrolysis cell requires an electrical current supply to
provide a
flow of current across the passage of flowing water, between the electrodes. A
preferred
electrical current supply is a battery or set of batteries, preferably
selected from an
14
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
alkaline, lithium, silver oxide, manganese oxide, or carbon zinc battery. The
batteries can
have a nominal voltage potential of 1.5 volts, 3 volts, 4.5 volts, 6 volts, or
any other
voltage that meets the power requirements of the electrolysis device. Most
preferred are
common-type batteries such as "AA" size, "AAA" size, "C" size, and "D" size
batteries
having a voltage potential of 1.5 V. Two or more batteries can be wired in
series (to add
their voltage potentials) or in parallel (to add their current capacities), or
both (to increase
both the potential and the current). Rechargable batteries are advantageously
employed.
An alternative electrical current supply can be a rectifier of household
current,
which converts 100-230 volt AC current to the required DC current. Another
alternative
is a solar cell that can convert (and store) solar power into electrical
power. Solar-
powered photovoltaic panels can be used advantageously when the power
requirements of
the electrolysis cell draws currents below 2000 milliamps across voltage
potentials
between 1.5 and 9 volts.
In one embodiment, the electrolysis cell can comprise a single pair of
electrodes
having the anode connected to the positive lead and the cathode connected to
the negative
lead' of the battery or batteries. A series of two or more electrodes, or two
or more cells
(generally, a pair of electrodes) can be wired to the electrical current
source. Arranging
the cells in parallel, by connecting each cell anode to the positive
terminals) and each
cell cathode to the negative terminal(s), provides that the same electrical
potential
(voltage) from the electrical current supply will pass across each cell, and
that the total
current of the electrical current supply will be divided (evenly or unevenly)
between the
two or more electrode pairs of cells. Arranging two cells (for example) in
series, by
connecting the first cell anode to the positive terminal, the first cell
cathode to the second
cell anode, and the second cell cathode to the negative terminal, provides
that the same
electrical current from the electrical current supply will pass across each
cell, and that the
total voltage potential of the electrical current supply will be divided
(evenly or unevenly)
between the two cells.
The electrical current supply can further comprise a circuit for periodically
reversing the output polarity of the battery or batteries in order to maintain
a high level of
electrical efficacy over time. The polarity reversal minimizes or prevents the
deposit of
scale and the plating of any changed chemical species onto the electrode
surfaces.
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
In addition to the electrolysis cell and any pumping means or propulsion
means,
the electric current supply can also provide power optional control circuits,
including
indicating light(s), to control the timing and duration of the electrical
operations of the
device. The control system can automatically shut off the current to the
electrolysis cell,
pumping means, or propulsion means, or any combination thereof, after a period
of time,
and can operate the indicator lights to indicate when the device is operating,
when the
device should be turned off, when the reservoir water is sterilized safe, and
when the
battery life runs low. Alternatively, the current to the electrolysis cell and
other electrical
components can simply be wired in series to an on-off switch, with an
indicator light to
show that power is being delivered to the components.
Operation of the Electrolysis Cell
The chemistry of the conversion of halide ions to biocidal agents proceeds as
electrical energy is applied between the pair of electrodes and through the
electrolytic
solution. Since chloride is the most prevalent halide in most waters, the
description of the
electrolysis cell chemistry and operation will be described with respect to
converting
chloride to chlorine, although it should be understood that other halides,
especially
bromide and iodide, would function and respond similarly to chloride.
Similarly, since
water (such as tap water) is a particularly preferred electrolytic solution,
the description
below will describe the use of water having a residual amount of chloride
ions, although
it should be understood that other electrolytic solutions can be used.
Water containing residual amounts of chloride ions is electrolyzed as it
passes
between the anode (the positively charged electrode of the pair) and the
cathode (the
negatively charged electrode). Two of the reactions that occur at the anode
electrode are
set forth below as equations 1 and 2.
2C1- -~ C12 + 2e-
H20 -~ 1/202 + 2H++ 2e (2)
One of the reactions that occurs at the cathode is set forth as equation 3.
2H20 +2e- -~ H2 + 20H- (3)
16
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
Furthermore, chlorine molecules can be converted to hypochlorous acid and
hypochlorite ions as set forth in equations 4 and 5, respectively.
C12 + H20 ~HOCI + Cl- + H+ (4)
HOCI ~ OCl- + H+ (5)
The chlorine gas that is generated dissolves or diffuses into the water to
generate
free chlorine in the form of hypochlorous acid, hypochlorous acid ions, and
hypochlorite
ions. It is believed that other various mixed oxidant species that can form
include
chlorine dioxide (C102), other chloro-oxides molecules, oxide molecules
including ozone,
hydrogen oxide (H202) and free radicals (oxygen singlet, hydroxyl radicals)
and ions
thereof. Such mixed oxidants are demonstrated and described in U.S. Patent
3,616,355
(issued 10/26/71) and U.S. Patent 4,761,208 (issued 8/2/88). These types of
mixed
oxidants are very effective biocidal agents, but have very short lifespans,
lasting from a
fraction of a second to minutes under ordinary, ambient conditions.
Consequently,
generating these biocidal agents at the point of use ensures the most
effective use of the
biocidal species. Furthermore, generating the biocidal agents continuously
throughout
the use of the solution, such as in a bathtub, is highly effective in avoiding
any re-
contamination of the water by other objects that are associated with the bath,
such as play
toys, sponges, and wash cloths, or from soil on the body of the infant or
bather.
For effective treatment of the harmful microorganisms in the reservoir
solution,
including those in the solution passing through the electrolysis cell, as well
as the
reservoir solution that is treated by the residual mixed oxidants in cell
effluent, the
concentration of mixed oxidants in the electrolysis cell effluent, as measured
by the DPD
method, is at least 0.1 mg per liter (about 0.1 ppm) of electrolysis cell
effluent, preferably
0.2 mg per liter (about 0.2 ppm), more preferably at least 1 mg per liter
(about 1 ppm),
and most preferably at least 5 mg per liter (about 5 ppm).
An important consideration for small, portable electrolysis devices, and
particlarly
for those devices of the present invention, is the productivity of the
electrical power of the
device. When battery power is used, it is important to provide the greatest
possible
production of mixed oxidant agents for each watt of power consumed. This
ensures long
17
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
battery life, greater consumer convenience, smaller and more portable devices,
and
greater consumer value.
The productivity of an electrolysis cell is expressed by equation I,
r) _ (CCl * Q)/(I*V) (I)
wherein:
~ units are micrograms of chlorine per minute, per watt of power used;
CCl is the concentration of the generated chlorine equivalent, as determined
by
the DPD Method, in milligrams per liter (mg/1);
I is the electric current in amps;
Q is the volumetric flow rate in milliliters per minute (ml/m); and
V is electric potential across the cell in volts.
The productivity 'q of the electroytic device used in accordance with the
present
invention is typically greater than 100, and more typically greater than 250.
In preferred
embodiments of the electrolysis cell, the productivity r) is more than about
500, and more
preferably more than about 1000, when the reservoir water has a concentration
of halogen
ions of more than 0.001% (10 ppm) and less than 0.1%. Preferably, the
electrolysis
device has the above-described efficiencies when the electric current is
between about
100 milliamps and about 2000 milliamps, with typical current densities of
between about
5 milliamps / cm2 and 100 milliamps / cm2 of exposed anode electrode surface,
and more
preferably between about 10 milliamps and 50 milliamps / cm2. Since the
electrical
potentials required to convert chloride to chlorine is about 1.36V, a voltage
potential
greater than 1.36V across the passage will generate a proportionally greater
amount of
mixed oxidants from the chloride ions. The voltage potential maintained
between any
pair of anode and cathode electrodes must be generally greater than 1.36V, and
generally
less than about 12 volts, and is preferably between about 2.0V and 6V, and
more
preferably between about 3V and 4.5V. For self-powered self-contained devices,
batteries are the preferred electrical current sources. To achieve the
extended life from a
set of batteries, the device is preferably designed to draw a total power of
20 watts or less,
preferably 5 watts or less, more preferably 2.5 watts or less, and most
preferably 1 watt or
less, across the electrode pairs of the cell.
18
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
Generally, the electrolysis cell has a cell gap spacing greater than about
0.05 mm,
preferably greater than 0.10 mm, more preferably greater than 0.15 mm, end
most
preferably greater than about 0.20 mm, and a cell gap spacing less than about
5 mm,
preferably less than about 2.0 mm, more preferably less than about 0.80 mm,
and most
preferably less than about 0.50 mm.. The more preferable cell gap spacings are
for use
with electrolytic solutions that contain a concentration of halide ions of
less than about
200 ppm, and a specific conductivity p of greater than about 250 pS/cm.
The residence time between the inlet and outlet of the anode and cathode pair
is
generally less than 10 seconds and preferably is less than 5 seconds, in more
preferred
embodiments, between about 0.01 seconds and about 1.5 seconds, and most
preferably
between 0.05 and about 0.5 seconds. The residence time can be approximated by
dividing the total volume of the passage between the anode and cathode pair by
the
average flow rate of water through the electrolysis cell.
Operation and effectiveness of the electrolysis device requires that the
reservoir
solution passes through the electrolysis cell in a quantity sufficient to
generate an
effective production of the biocidal mixed oxidants for the intended purpose.
In general,
without some means of moving the reservoir solution through the cell, as
opposed to just
filling the cell, low levels of the mixed oxidants will be produced. Water
from the
reservoir can be moved through the electrolysis cell by pumping through the
cell, by
movement of the device body through the reservoir, such as by hand, by
propulsion, or by
pulling or pushing the device through the reservoir by a tether or at the end
of a handle.
Alternatively, the device can be placed into an area of the reservoir where
there is water
flow sufficient to pass through the cell.
Operation in a Reservoir of Electrolytic Solution
In the operation of the present electrolysis device in a reservoir, it is not
necessary
that the entire volume of reservoir water pass through the electrolysis cell.
Because of the
high biocidal activity of the high concentration of mixed oxidants in the
effluent of the
electrolysis cell (a concentration substantially higher than needed to destroy
the
population of microorganisms in the reservoir solution), a water volume less
that the total
volume of the reservoir will need to pass through the device to ensure that
all the
19
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
microorganisms in the reservoir solution have been destroyed. Generally only
about 25%
or less, and preferably only 10°Io or less, of the total volume of the
reservoir will need to
be passed through the electrolysis cell.
The electrolysis device of the present invention can neutralize at least about
4 log,
and preferable at least about 6 log, and more preferably at least about 8 log
of the
microorganisms in the electrolysis solution that passes through the
electrolysis device.
The log neutralization is intended to refer to the difference between the live
microorganisms that enter the electrolysis device and those that exit the
electrolysis
device. For example, an 8 log neutralization is intended to refer to a
situation where no
live microorganisms are present in the water at the exit of the electrolysis
device when
10g live microorganisms were present in the water of the inlet to the
electrolysis device.
Similarly, the electrolysis device of the present invention can neutralize at
least about 4
log, and preferable at least about 6 log, and more preferably at least about 8
log of the
microorganisms in the reservoir of electrolysis solution that has been treated
with the
electrolysis device.
Pumping-Means
The device is preferably provided with a pump means for pumping the reservoir
water through the cell passage. The pumping means can provide three functions:
to move
electrolytic solution from the reservoir through the electrolysis cell, where
mixed
oxidants can be generated from halide ions when electric current is passed
through the
cell; to expel and disperse the effluent solution containing the mixed
oxidants back into
the reservoir; and to provide movement (propulsion) of the device through the
reservoir in
response to the force of the effluent solution leaving the device.
A preferred pumping means comprises a pump having a rotating impeller,
mounted inside the buoyant body, and having a pump inlet in fluid
communication with
the reservoir solution, and a pump outlet in fluid communication with the
inlet of the
electrolysis cell. Self-priming pumps, such as peristalsis pumps, can be used.
The pump
is preferably driven by an electric, direct drive motor that is powered by a
battery,
although other power means to drive the pump, such as mechanical wind-up
springs or
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
photovoltaic panels can be used. Preferably, the pump electric motor draws
power of the
same voltage potential as the electrolysis cell.
The direction of the discharge of the effluent can affect both the dispersion
of the
mixed oxidants into the reservoir, and the movement of the device through the
reservoir.
For dispersion purposes, a discharge angle of about 45° downward from
horizontal has
been found optimum. For propulsion purposes, a discharge angle of from
0° to about 30°
works well. Straight-ahead propulsion is generally achieved by directing the
discharge
outward and straight backward in a direction opposite the center of gravity of
the device
(hereinafter, the "straight back direction"). Preferred is a propulsion means
that turns the
device in sweeping circles, achieved by angling the discharge from between
about 10° to
about 80° from the straight-back direction.
The pump can have a throughput of between 0.05 liters solution per minute, up
to
about 10 liters per minute. Higher pumping rates are possible, depending upon
the size of
the buoyant device, and the capacity of any electric current supply. For
devices that are
easily portable and powered by conventional alkaline batteries, a preferred
pumping
capacity is between 0.1 and 5 liters per minute, and more preferably between
0.2 and 2
liters per minute.
While the entire volume of the pump means can be directed fully through the
electrolysis cell, the pump discharge can be divided, with one portion passing
through the
electrolysis cell and the remaining portion by-passing the electrolysis cell.
This enables a
device to deliver a certain mass rate of electrolytic solution through the
electrolysis cell,
while using the by-passing portion of the pumped solution for propelling the
device.
Alternatively, an electrolysis device can comprise a pumping means which
discharges through the electrolysis cell, with a portion of the discharged
effluent from the
electrolysis cell being recirculated back to the inlet of the pump, to provide
a continuous
recycle of a portion of the effluent back through the inlet of the cell. This
arrangement
can increase the concentration of the resulting mixed oxides in the effluent
discharged
from the electrolysis cell.
21
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
Local Source of Halide Ion
An optional embodiment of the present invention includes an electrolysis
device
comprising a local source of halide ions, and a means for delivering the local
source of
halide ions to a portion of the reservoir water in fluid communication with
the cell inlet.
This embodiment is advantageously used in those situations when the reservoir
water has
a very low concentration, or even no, halide ions, thereby increasing the
production of
mixed oxidants in the effluent as compared to the production of mixed oxidants
from the
reservoir solution alone. Preferably, all the local source of halide ion
passes through the
electrolysis cell, to maximize the conversion of the local source of halide
ion to mixed
oxidants, and to limit adding salts to the reservoir generally. The local
source of halide
ions can supplement the ordinary levels of halide ion in many water sources,
such as tap
water, to generate extraordinarily high concentrations of mixed oxidants in
the effluent.
The local source of halide ions can be a concentrated brine solution, a salt
tablet in
fluid contact with the reservoir of electrolytic solution, or both. A
preferred localized
source of halide ions is a solid form, such as a pill or tablet, of halide
salt, such as sodium
chloride (common salt). The means for delivering the local source of halide
ions can
comprise a salt chamber comprising the halide salt, preferably a pill of
tablet, through
which a portion of the reservoir water will pass, thereby dissolving a portion
of the halide
salt into the portion of water. The salted portion of water then passes into
the electrolysis
cell. The salt chamber can comprise a salt void that is formed in the buoyant
body and
positioned in fluid communication with the portion of water that will pass
through the
electrolysis cell.
A brine solution can be provided within a brine chamber that is position in
fluid
communication with the inlet port of the electrolysis cell via a tube, such
that a flow of
brine solution will be induced through the tube by venturi suction in response
to the flow
of water through the inlet port, whereby a constant proportion of brine
solution is
deli vered.
Other halide salts with a substantially lower solubility in water can be
advantageously used to control the rate of dissolution of halide salt.
Preferred salts for
use as a solid form of the local source of halide ion are the less soluble
salts, such as
calcium chloride, magnesium chloride, potassium chloride and ammonium
chloride. The
22
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
pill can also be formulated with other organic and inorganic materials to
control the rate
of dissolution of the sodium chloride. Preferred is a slow dissolving salt
tablet, to release
sufficient halide ions to effect the conversion of an effective amount of
mixed oxidant
biocidal agents. The release rate halide ion is typically between 0.01 to 0.3
mg halide ion
for each liter of reservoir water treated. The halide pill can be a simple
admixture of the
salt with the dissolution restricting materials, which can be selected from
various well-
known encapsulating materials.
The following specific embodiments of the present invention are intended to
exemplify, but in no way limit, the operation of the present invention.
Embodiment I
An example of a self-contained, self-propelled buoyant electrolysis device is
shown in cross section in Fig. 4. The duck electrolysis device 10 has a
buoyant body 12
made into the form of a duck. The body has a substantially continuous outer
surface 13
and a hollow interior 14. The body is molded from a rubberized PVC plastic.
Within the
interior of the body, mounted to the base 16 is an electrically-driven motor
44 (model
RE260, LMP Inc., Jersey City, N.J.) that drives a pump 40 having impeller 41
(model
IIVVIPELR-S, Swampworks Mfg., Springfield, MO). The inlet 42 to the pump is
positioned directly against an inlet opening 17 in the base 16 of the body to
provide fluid
communication between the reservoir 100 of water and the inlet 42 to the pump.
The
periphery of the pump outboard of the pump inlet is sealed to the base 16 with
a water-
proof adhesive 70 to prevent any leakage of reservoir water into the body of
the device.
The discharge 43 of the pump is connected via'/a inch Tygon tubing 60 to the
inlet 25 of
an electrolysis cell 20 mounted within the buoyant body. An electrolysis cell
of the type
shown in Fig. 1 is shown in Fig. 4 in cross section taken through line 4-4 of
Fig. 1. The
electrolysis cell 20 has an anode plate 21 made of titanium with a coating of
ruthenium
oxide (1.45 mm thick) and measuring 7.2 cm long in the direction of fluid
flow, and 2.7
cm wide (transverse to the fluid flow path), and a cathode plate 22 made of
stainless steel
(1.45 mm thick), having the same dimensions as the anode and positioned
parallel to and
coterminous with the anode. The anode and the cathode are separated by a gap
spacing of
0.20 mm, and define a passage 24 there between. The outlet 26 of the
electrolysis cell
23
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
discharges to one end of a '/a inch Tygon tube 61, with the other end of the
tube
penetrating through a rear port 18 in the duck body near the rear end of the
base 16,
which is sealed with water-proof adhesive at the penetration opening in the
base to
prevent leakage of the reservoir water into the body. The anode lead 27 and
the cathode
lead 28 are connected via wiring to the positive and negative terminals,
respectively, of
an electrical current supply 50, consisting of two "AA" alkaline batteries
(each 1.5V)
arranged in series to provide a 3.0V potential electrical supply. The
aforementioned
pump motor 44 is also wired with the batteries, in parallel to and downstream
from the
electrolysis cell, to receive the same 3 volt potential. With a 3 volt
potential, the
electrolysis cell draws about 0.20 amps, while the motor 44 draws about 200
milliamps in
driving the pump 40 to pump 400 ml per minute of water through the
electrolysis cell 20.
In addition, an indicator lamp 80 (model 160-1127-ND, Digi-Key) is wired in
line
between the pump motor and the positive terminal of the batteries, to glow
when current
flows. This serves as an indicator to the user that the electrolysis device is
functioning.
Further, an on-off switch 82 is wired just downstream from the positive
terminal to turn
on and shut off the current to the pump motor 44 and the electrolysis cell 20.
The
indicator lamp 80 and the on-off switch 82 are positioned to extend through
the body as
shown in Fig. 4.
A 20-liter capacity plastic tub of water is filled with about 10 liters of
water from
a stream that contains E. coli bacteria. The stream water has a residual
chloride level of
80 ppm. The water temperature is adjusted to 28°C to make the water
comfortable for an
infant. A 110 ml water sample is collected (Sample A) of the before-treatment
water, in a
sterile 125 ml polypropylene bottle with cap for a baseline reading of
microbial
contamination and residual chlorine in the water.
An additional 20 cm length of Tygon tube is attached to the rear port 18, for
sampling the electrolyzed water discharged from the device. The duck
electrolysis device
is placed floating onto the surface of the tub water, with the sampling tube
discharge end
positioned outside the plastic tub, toward a drain. The switch is pushed to
the "on"
position, and the device operates (i.e., it pumps reservoir water through the
electrolysis
cell with current passing between the electrodes). After 30 seconds, a 110 ml
water
sample is collected (Sample B) of the effluent discharged directly from the
device, into a
24
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
sterile 125 ml polypropylene bottle. The switch is pushed to the "off '
position, and the
sampling tube is removed from the rear port 18 of the device.
The pump switch is again pushed to the "on" position. The pump immediately
begins pumping reservoir water through the electrolysis cell, and from the
rear port and
out into the reservoir of water, thereby providing forward propulsion to the
buoyant
device. The pump and electrolysis cell operate for 5 minutes, during which
time the
buoyant duck device propels itself about the surface of the water in the bath
tub. The
currents drawn on the pump and the electrolysis cell are determined to be
constant over
this period of time. The switch is then pushed to the "off ' position, cutting
off current to
the pump motor and to the electrolysis cell. The tub water is quickly stirred
with a paddle
(which has been sterilized to prevent a re-contamination of the treated water)
to ensure
that the resulting batch of electrolyzed water is homogenous. A third 110 ml
sample of
the resulting electrolyzed reservoir water 100 (Sample C) is placed into a 125
ml
polypropylene bottle with cap for a reading of microbial contamination and
residual
chlorine in the treated water. The results are shown in Table A.
The number of E coli microorganisms in the 100 ml samples is measured using
any one of a number of methods known in the art. For example, US Patent
4,925,789,
incorporated herein by reference, describes a suitable test. In addition, the
residual
chlorine (mixed oxidants) present in the 110 ml sample collected at the outlet
of the
electrolysis device can be measured using the DPD (N, N diethyl-p-
phenylenediamine)
Colorimetric Test Method. This method is well known in the art, and is set
forth by way
of example in International Organization for Standardization, Water Quality,
ISO
Standard 7393-2:1985, the substance of which is incorporated herein by
reference. A
suitable DPD reagent for use with the DPD Colorimetric Method is catalog no.
21055-69
manufactured by the Hatch, Company of Loveland, Colorado. A suitable
colorimeter is
model no. DR/890 manufactured by the Hatch Company of Loveland, Colorado.
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
Table A
Sample Chlorine level,Microbial
ppm by DPD count
(organism/liter)
A 0.0 > 10'
B 0.6 none
C 0.12 none
The productivity r1 of the electrolysis cell (from Sample B) as determined by
equation I is
400.
A mother may often put her hands into the water, after having touched a
bacterially
contaminated surface outside the tub. Also, bacteria and other pathogens can
inhabit bath
sponges, cloths, and even the surface of other play toys. Nevertheless, any
object
contaminated with bacteria or other pathogen that is introduced into the
electrolyzed
reservoir solution is immediately sterilized by the continuous electrolyzing
action of the
device, thereby preventing a re-contamination of the reservoir.
In another embodiment of the invention, a long length tube like the above
sampling tube can be attached to the rear port 18 and left in place while the
device
operates. The discharge of water from the end of the length of tube will cause
the
discharge end of the tube to move about, and back and forth, like a snake,
below the
water surface, thereby distributing the cell effluent throughout the
reservoir.
Embodiment II
An example of a self-powered buoyant electrolysis device with a close-spaced
gap
between the electrodes is shown in partial cross section in Fig. 5. Fig. 5
shows an
electrolysis device 10 having a buoyant body 12 made into the form of a boat.
The body
is made from PVC plastic. Mounted on the exterior of the base 16 of the
buoyant body is
an electrolysis cell 20 of the type shown in Fig. 3 (shown in Fig. 5 in cross
section taken
through line 5-5 of Fig. 3), having a planar anode plate 21 and a confronting
planar
cathode plate 22. The anode plate is made of titanium with an iridium oxide
coating (0.4
microns thick) and measuring 7:2 cm long and 2.7 cm wide. The cathode plate is
made of
stainless steel (1.45 mm thick), having the same length and width dimensions
as the
26
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
anode. The cathode plate have a constant gap spacing of 0.40 mm between the
two
electrodes. An electrical current supply 50 consisting of two "AA" alkaline
batteries
(each 1.5V) is positioned inside the body, and are wired in series to provide
a 3.0V
potential current supply across the electrodes. Wires connect the batteries to
anode lead
27 and cathode lead 28, which are extending up through the base 16 into the
interior of
the body 12. The funnel member 86 affixed to the bottom of the cell forces
water brought
into the funnel opening 87 into the cell passage as the buoyant boat device is
moved in
direction 90 through the reservoir.
The device can be used to electrolyze water with substantially the same
effectiveness as described in Embodiment I for the self-propelled, self-
powered buoyant
electrolysis device. In the present embodiment, once the device 10 is placed
in the
reservoir of water, an electric current is established across the pair of
electrodes 21 and 22
as water floods into the passage 24. With periodic stirring of the tub water
by hand, or
movement of the device by hand, or preferably by an extended handle attached
to the
device (not shown) through the reservoir water for several minutes, sufficient
water will
pass between the pair of electrodes with the defined spacing to generate an
effective level
of biocidal mixed oxidants for sterilization of the bath water.
Uses of Electrolyzed Water
The electrolyzed water that exits the electrolysis device 20 can effectively
disinfect
or sterilize the reservoir water, making the reservoir solution useful as a
source of potable
water, bathing water, or as a source of sterile water (i.e., water in which
microorganism
have been neutralized), for manufacturing products or for cleaning
manufacturing
equipment and for numerous other uses. The electrolyzed reservoir water can
also be
added to other sources of water to sanitize them (e.g., to neutralize the
microorganisms in
standing water found in pools, saunas, cooling towers, etc.) Further, the
electrolyzed
reservoir water can be used to neutralize microorganisms located on organic
and
inorganic surfaces, body surfaces (e.g., hands, feet, face, etc.), hard and
soft surfaces,
eating utensils and food contact surfaces, sinks, countertops, faucets,
floors, soft surfaces,
fabrics, clothing, and other hard and soft surfaces.
27
CA 02445652 2003-10-28
WO 02/064511 PCT/US02/04616
A preferred embodiment comprises a device for treating the bath water for
babies.
Babies require frequent bathing, including the time between the birth and the
age of 6
months when the immune system is underdeveloped and susceptible to bacteria
and other
pathogens. The water in which the baby is bathed can be a significant source
of
microorganisms that can cause illness, especially diarrhea, by contact with
the mucous
areas or by unintended ingestion of the bath water by the baby. Sterilization
of the bath
water before and during the bathing greatly reduces, and can eliminate,
illness caused by
the bath water.
It is highly preferred to use the electrolyzed reservoir water immediately
after the
electrolysis, since the beneficial biocidal mixed oxidants have a short life
span.
Preferably, the reservoir water, when used for disinfection, sanitization or
sterilization, is
used within about 15 minutes, preferably within about 5 minutes, more
preferably within
about 1 minute, and most preferably almost immediately, after electrolysis.
The various advantages of the present invention will become apparent to those
skilled in the art after a study of the foregoing specification and following
claims.
28