Note: Descriptions are shown in the official language in which they were submitted.
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
PRESSURE SWING CATALYST REGENERATION PROCEDURE FOR
FISCHER-TROPSCH CATALYST
FIELD OF THE INVENTION
The present invention relates to a process for the preparation of hydrocarbons
from
synthesis gas, (i.e., a mixture of carbon monoxide and hydrogen), typically
labeled the
Fischer-Tropsch process. More particularly, the present invention relates to a
regeneration
method for a Fischer-Tropsch catalyst. Still more particularly, the present
invention relates
to the use of a regeneration pressure lower than the Fischer-Tropsch reaction
pressure to
remove hydrocarbon deposits and regenerate a deactivated Fischer-Tropsch
catalyst.
BACKGROUND OF THE INVENTION
Large quantities of methane, the main component of natural gas, are available
in many
areas of the world. However, most natural gas is situated in areas that are
geographically
remote from population and industrial centers. The costs of compression,
transportation, and
storage make the use of this remote gas economically unattractive. To improve
the economics
of natural gas use, much research has focused on the use of methane as a
starting material for
the production of higher hydrocarbons and hydrocarbon liquids.
As a result, various technologies for the conversion of methane to
hydrocarbons have
evolved. The conversion is typically carried out in two steps. In the first
step methane is
reformed with water or partially oxidized with oxygen to produce carbon
monoxide and
hydrogen (i.e., synthesis gas or syngas). In a second step, the syngas is
converted to
hydrocarbons.
This second step, the preparation of hydrocarbons from synthesis gas is well
known in
the art and is usually referred to as Fischer-Tropsch synthesis, the Fischer-
Tropsch process, or
Fischer-Tropsch reaction(s). The Fischer-Tropsch reaction involves the
catalytic
hydrogenation of carbon monoxide to produce a variety of products ranging from
methane to
heavy hydrocarbons (up to C80 and higher) as well as a variety of oxygenated
hydrocarbons.
The methanation reaction was first described in the early 1900's, and the
later work by Fischer
and Tropsch dealing with higher hydrocarbon synthesis was described in the
1920's.
Catalysts for use in such synthesis usually contain a catalytically active
metal of Groups
8, 9, 10 (in the New notation of the periodic table of the elements, which is
followed
1
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
throughout). In particular, iron, cobalt, niclcel, and ruthenium have been
used as the
catalytically active metals. Cobalt, iron and ruthenium have been found to be
most suitable for
catalyzing a process in which synthesis gas is converted to primarily
hydrocarbons having five
or more carbon atoms (i.e., where the C5+ selectivity of the catalyst is
high). Additionally, the
catalysts often contain one or more promoters and a support or carrier
material. Ruthenium is a
widely used promoter for cobalt catalysts.
The Fischer-Tropsch synthesis reactions are highly exothermic and reaction
vessels
must be designed for adequate heat exchange capacity. Because the feed streams
to Fischer-
Tropsch reaction vessels are gases, while the product streams include liquids,
the reaction
vessels must have the ability to continuously produce and remove the desired
range of liquid
hydrocarbon products. The first major commercial use of the Fischer-Tropsch
process was in
Germany during the 1930's. More than 10,000 B/D (barrels per day) of products
were
manufactured with a cobalt based catalyst in a fixed-bed reactor. This worlc
was described by
Fischer and Pichler in German Patent 731,295 issued August 2, 1936.
Motivated by the hope of producing high-grade gasoline from natural gas,
research on
the possible use of the fluidized bed for Fischer-Tropsch synthesis was
conducted in the United
States in the mid-1940s. Based on laboratory results, Hydrocarbon Research,
Inc. constructed a
dense-phase fluidized bed reactor, the Hydrocol unit, at Carthage, Texas,
using powdered iron
as the catalyst. Due to disappointing levels of conversion, scale-up problems,
and rising natural
gas prices, operations at this plant were suspended in 1957. Research
continued, however, on
developing Fischer-Tropsch reactors, such as slurry-bubble columns, as
disclosed in U.S Patent
5,348,982. Despite significant advances, certain areas of the Fischer-Tropsch
technology still
have room for improvement. One potential technology in need of improvement
relates to
regeneration of the Fischer-Tropsch catalyst.
After a period of time in operation, a catalyst will become deactivated,
losing its
effectiveness for synthesis gas conversion to a degree that makes it
uneconomical at best and
inoperative at worst. At this point, the catalyst can be either replaced or
regenerated. Because
the catalysts tend to be relatively expensive, regeneration is preferred over
replacement.
Catalyst systems can become deactivated by a number of processes, including
coking,
sintering, oxidation, and poisoning. The process chiefly responsible for
deactivation varies
among catalyst systems. Therefore, the preferred method for regeneration tends
to depend on
the catalyst system to be regenerated.
Research is continuing on the development of more efficient Fischer-Tropsch
catalyst
2
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
systems and catalyst systems that can be more effectively regenerated. In
particular, a number
of studies describe the use of various gases, including hydrogen, air, and
carbon monoxide to
regenerate a variety transition metal containing Fischer-Tropsch catalyst
systems.
U.S. Patent 3,958,957, issued on May 25, 1976, describes a carbon-alkali metal
catalyst,
used for conversion of synthesis gas to methane and higher hydrocarbons at a
pressure of 100 -
1500 psig and a temperature of 300 - 550 F at a typical gas hourly space
velocity of 1000
volumes gas/hr/volume catalyst. The carbon-alkali metal catalyst can be
regenerated with air
oxidation.
U.S. Patent 4,151,190, issued on Apri124, 1979, describes a catalyst
comprising at least
one of a sulfide, oxide, or metal of Mo, W, Re, Ru, Ni, or Pt, at least one of
a hydroxide, oxide,
or salt of Li, Na K, Rb, Cs, Mg, Ca, Sr, Ba, or Th, and a support, used for
conversion of
synthesis gas with an H2:CO ratio of 0.25-4.0, preferably 0.5 - 1.5, to C2 -
C4 hydrocarbons at a
pressure of 15-2000 psia and a temperature of 250-500 C at a typical gas
hourly space velocity
of 300 v/hr/v. This catalyst can be regenerated by contacting it with hydrogen
gas at 500-600
C for 16 hours.
U.S. Patent 4,738,948, issued on April 19, 1988, describes a catalyst
comprising cobalt
and ruthenium at an atomic ratio of 10-400, on a refractory carrier, such as
titania or silica. The
catalyst is used for conversion of synthesis gas with an H2:CO ratio of 0.5-
10, preferably 0.5 -
4, to C5 - C40 hydrocarbons at a pressure of 80-600 psig and at a temperature
of 160-300 C, at
a gas hourly space velocity of 100-5000 v/hr/v. This catalyst can be
regenerated by contacting
it with hydrogen gas at 150-300 C, preferably 190-260 C, for 8-10 hours.
U.S. Patent 5,728,918, issued on March 17, 1998, describes a catalyst
comprising cobalt
on a support, used for conversion of synthesis gas with an H2:CO ratio of 1-3,
preferably 1.8-
2.2, to C5+ hydrocarbons at a pressure of 1-100 bar and at a temperature of
150-300 C, at a
typical gas hourly space velocity of 1000-6000 v/hr/v. This catalyst can be
regenerated by
contacting it with =a gas containing carbon monoxide and less than 30%
hydrogen, at a
temperature more than 10 C above Fischer-Tropsch conditions and in the range
100-500 C,
and at a pressure of 0.5-10 bar, for at least 10 minutes, preferably 1-12
hours. The contact time
period depends on temperature and gas hourly space velocity. The 5,728,918
patent also
teaches an activation procedure, which may include a first step of contacting
the catalyst with a
gas containing molecular oxygen, preferably air, at 200-600 C, at atmospheric
pressure, for
more than 30 minutes, and preferably for 1-48 hours.
U.S. Patent 4,595,703, issued on June 17, 1986, describes a catalyst
comprising cobalt
3
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
or thoria promoted cobalt on a titania support, used for conversion of
synthesis gas with an
H2:CO ratio of 0.5-4, preferably 2-3, to Clo+ hydrocarbons at a pressure of
preferably 80-600
psig, and at a temperature of 160-290 C, at a gas hourly space velocity of
100-5000 v/hr/v.
This catalyst can be regenerated by contacting it with hydrogen gas, or a gas
which is inert or
non-reactive at stripping conditions such as nitrogen, carbon monoxide, or
methane, at a
temperature substantially the same as Fischer-Tropsch conditions. If it is
necessary to remove
coke deposits from the catalyst, the catalyst can be contacted with a dilute
oxygen-containing
gas, at oxygen partial pressure of at least 0.1 psig, at 300-550 C, for a
time sufficient to
remove coke deposits, followed by contact with a reducing gas containing
hydrogen, at a
temperature of 200-575 C and'at a pressure of 1-40 atmospheres, for 0.5-24
hours.
U.S. Patent 4,585,798 issued on April 29, 1986, describes a catalyst
comprising cobalt
and ruthenium in an atomic ratio greater than about 200:1 and, preferably, a
promoter, such as a
Group IIIB or IVB metal oxide, on an alumina support, used for conversion of
synthesis gas to
hydrocarbons at a pressure of preferably 1-100 atmospheres and at a
temperature of 160-350 C,
at a gas hourly space velocity less than 20,000 v/hr/v, preferably 100-5000
v/hr/v, especially
1000-2500 v/hr/v, which is activated prior to use by reduction with hydrogen
gas, followed by
oxidation with diluted air, followed by further reduction with hydrogen gas.
Despite the vast amount of research effort in this field, currently known
methods of
regeneration of Fischer-Tropsch catalysts are not always sufficiently
effective for a particular
catalyst system. Among the main deactivation mechanisms for cobalt based
catalysts are sulfur
poisoning [e.g. R.L. Espinoza, et al, Applied Catalysis A:General 186
(1999)13], metal
oxidation [e.g. D. Schanke et al, Catal. Lett. 34 (1995) 269] and surface
condensation of heavy
hydrocarbons [e.g. E. Iglesia et el, J. Catal. 143 (1993) 345]. The removal of
heavy
hydrocarbons, deposited in the pores of a used catalyst, is therefore one of
the challenges to
efficient commercialization of slurry bed technology for the Fischer-Tropsch
reaction.
In a slurry bed reactor, the Fischer-Tropsch catalyst particles are suspended
in liquid
reaction products (heavy hydrocarbons), predominantly wax. These heavy
hydrocarbons may
include heavy hydrocarbons formed in the Fischer-Tropsch reaction. In a fixed
bed reactor, the
catalyst particles, though not suspended in heavy hydrocarbons, will contain
and/or become
coated with heavy hydrocarbons as reaction proceeds. One of the deactivation
mechanisms of
the catalyst is the deposition of very heavy hydrocarbons into the catalyst
pores and/or on the
surface of the catalyst particles. Hydrogen gas, conventionally maintained at
reaction pressure,
has been used to remove a portion of this material through hydrogenation of
the heavy
4
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
hydrocarbon. However, this method has the disadvantages that hydrogenation may
be
incomplete and that the hydrogenated hydrocarbon may remain deposited in the
pores of the
catalyst and/or on the suiface of the catalyst particles. Also, a certain
degree of hydrogenolysis,
that is, destruction of valuable heavy hydrocarbons may occur, producing
gaseous
hydrocarbons of lower commercial value.
Hence, there is still a great need to identify new regeneration methods which
can be
used concurrently and/or periodically with contacting regenerated catalyst
with synthesis gas,
so as to maximize the regenerated catalyst activity and thus enhance the
process economics.
SUIVIMARY OF THE INVENTION
This invention relates to a process and catalyst for producing hydrocarbons,
and
includes a method for catalyst regeneration. The Fischer-Tropsch synthesis
process includes
contacting a feed stream comprising hydrogen and carbon monoxide with a
catalyst in a
reaction zone maintained at conversion-promoting conditions effective to
produce an effluent
stream comprising hydrocarbons.
The regeneration process comprises contacting a deactivated Fischer-Tropsch
catalyst
with a regeneration gas under regeneration-promoting conditions, for a period
of time sufficient
to reactivate the Fischer-Tropsch catalyst. The pressure is preferably
substantially less than the
mean Fischer-Tropsch reaction pressure. More specifically, the regeneration-
promoting
conditions in accordance with the present invention include a temperature
between about 250 C
and 350 C and a pressure between about 0 psig (0.1 MPa) and about 350 psig
(2.5 MPa).
Contact with the regeneration gas is maintained for a period of time
sufficient to reactivate the
Fischer-Tropsch catalyst. The regeneration gas is preferably selected from the
group consisting
of hydrogen-containing gases, oxygen-containing gases, steam, inert gases such
as nitrogen,
and non-explosive combinations thereof. The volume ratio of the regeneration
gas to the
deactivated Fischer-Tropsch catalyst is preferably at least about 1 Standard
Liter per hour per
gram of catalyst. It will be understood that in some instances the
regeneration gas may be
generated by passing a liquid through a heating zone before contact with the
catalyst; and it will
be further understood that references herein to regeneration gas include
streams that may
contain some liquid.
This invention further includes a process of cycling between the synthesis
process and
the regeneration process.
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
BRIEF DESCRIPTION OF THE DRAWINGS
For a detailed description of the preferred embodiment of the present
invention,
reference will now be made to the accompanying drawings, wherein:
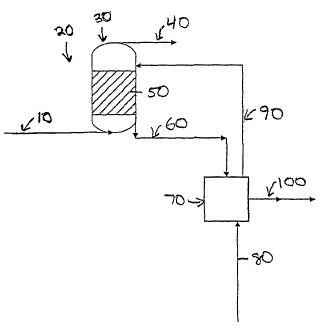
Figure 1 is a schematic diagram of a coupled reaction/regeneration process,
particularly
suited to slurry bed reactors, according to an embodiment of the present
invention;
Figure 2 is a schematic diagram of a second coupled reaction/regeneration
process,
particularly suited to fixed bed reactors, according to an embodiment of the
present invention;
Figure 3 is a plot of measured catalyst activity as a function of time in a
process
including a regeneration procedure according to an embodiment of the present
invention, where
the thick vertical lines indicate the beginning of the regeneration procedure.
DETAILED DESCRIPTION OF A PREFERRED EMBODIMENT
Synthesis Reaction
The feed gases charged to the synthesis process that precedes the present
regeneration
process comprise hydrogen, or a hydrogen source, and carbon monoxide. HZ/CO
mixtures
suitable as a feedstock for conversion to hydrocarbons according to the
synthesis process can
be obtained from light hydrocarbons such as methane by means of steam
reforming, partial
oxidation, or other processes known in the art. It is preferred that the molar
ratio of hydrogen
to carbon monoxide in the feed be greater than 0.5:1 (e.g., from about 0.67:1
to 2.5:1). The
feed gas stream may contain hydrogen and carbon monoxide in a molar ratio of
about 2:1. The
feed gas stream may also contain carbon dioxide. The feed gas stream should
contain a low
concentration of compounds or elements that have a deleterious effect on the
catalyst, such as
poisons. For example, the feed gas may need to be pre-treated to ensure that
it contains low
concentrations of sulfur or nitrogen compounds such as hydrogen sulfide,
ammonia and
carbonyl sulfides.
The feed gas is contacted with the catalyst in a reaction zone. Mechanical
arrangements
of conventional design may be employed as the reaction zone including, for
example, fixed
bed, fluidized bed, slurry phase, slurry bubble column, reactive distillation
column, or
ebulliating bed reactors, among others, may be used. The size and physical
form of the catalyst
may vary, depending on the reactor in which it is to be used.
Catalyst Support
The active catalyst components are preferably carried or supported on a
suitable
support. Suitable supports include titania, titania/alumina, zirconia,
alumina, silica,
silica/alumina, and the like. Further, suitable supports include those
disclosed in commonly
6
CA 02445918 2006-09-27
assigned U.S. Patent 6,368,997, issued from U.S. Application Serial No.
09/314,921, entitled "Fischer-Tropsch Catalysts and Processes Using Fluorided
Supports; U.S. Patent No. 6,365,544, issued from U.S. Application Serial No.
09/314,920, entitled "Fischer-Tropsch Processes and Catalysts Using Fluorided
Alumina Supports", and U.S. Patent No. 6,730,708, entitled "Fisher-Tropsch
Processes and Catalysts Using Aluminum Borate Supports". Thus, suitable
supports further may include fluorided metal oxides, fluorided alumina,
aluminum fluoride, borated alumina, and aluminum borate.
Metals can be supported on aluminum fluoride or on fluorided alumina in
a variety of ways. For example, U.S. Patent No. 4,766,260 discloses the
preparation of metals such as cobalt on a fluorided alumina support using
impregnation techniques to support the metal. U.S. Patent No. 5,559,069
discloses the preparation of a multiphase catalyst composition comprising
various metal fluorides including cobalt fluoride homogeneously dispersed with
aluminum fluoride. PCT Int. Publ. No. 97/19751 discloses the preparation of
multiphase catalyst compositions comprising metallic ruthenium homo-
geneously dispersed with various metal fluorides including aluminum fluoride.
Phases of aluminum fluoride such as eta, beta, theta and kappa can be
prepared as described in U.S. Patent 5,393,509, U.S. Patent 5,417,954, and
U.S. Patent 5,460,795.
Aluminas that have been treated with fluosilicic acid (HZSiF6) such as
those described in European Patent Application EP 497,436 can also be used as
a support. The support disclosed therein comprises from about 0.5 to about 10
weight percent of fluorine, from 0.5 to about 5 weight percent of silica and
from about 85 to about 99 weight percent of alumina.
Catalyst
Catalysts which are contemplated to be regenerated by the present
method include any of the Fischer-Tropsch catalysts known in the art, such as
cobalt, ruthenium, cobalt/ruthenium, cobalt/rhenium, other cobalt/promoter
systems, iron, and nickel. In particular, the catalyst may include cobalt and
ruthenium. The amount of cobalt and ruthenium present in the catalyst may
vary widely. Typically, the catalyst comprises cobalt and ruthenium in an
amount from about 1 to 50% weight (as the metal) of the total weight of
catalytic metal and support, preferably from about 1 to 30% by weight, and
more preferably from about 1 to 25% by weight. Ruthenium is added to the
support in a concentration sufficient to provide a weight ratio of elemental
ruthenium to elemental cobalt of from about 0.001:1 to about 0.25:1, and
preferably from about 0.001:1 to about 0.05:1 (dry basis).
7
CA 02445918 2006-09-27
The catalyst may also contain other promoters. When the catalytic metal
is iron, cobalt, nickle, and/or ruthenium, suitable promoters include at least
one
promoter selected from the group consisting of Group 1 metals (i.e., Na, K,
Rb,
Cs), Sr, Group 11 metals (i.e., Cu, Ag, and Au) Sc, Group 4 metals (i.e., Ti,
Zr
and Hf), Group 5 metals (i.e., V, Nb and Ta), and Rh, Pd, Os, Ir, Pt and Re.
Preferably, any additional promoters for the cobalt and/or ruthenium catalysts
are selected from Sc, Ti, Zr, Hf, Rh, Pd, Os, Ir, Pt, Re, Nb, Cu, Ag and Ta.
Preferably, any additional promoters for the iron catalysts are selected from
Na, K, Rb, Cs and Sr. The amount of additional promoter, if present, is
typically
between 0.001 and 40 parts by weight per 100 parts of carrier. Further, the
amount of promoter is preferably added to a cobalt-containing catalyst in a
concentration sufficient to provide a weight ratio of elemental promoter to
elemental cobalt of from about 0.00005:1 to about 0.5:1, and preferably from
about 0.0005:1 to about 0.01:1 (dry basis).
It will be understood that the promoter is not limited to those listed
above and may be any known Fischer-Tropsch promoter, including those
disclosed in any of the above-referenced patents and publications. The
promoter is preferably one that improves the activity of a catalyst in the
Fischer-Tropsch reaction. Further, suitable promoters, and concentrations
thereof, include those disclosed in commonly assigned U.S. Patent 6,333,294,
entitled "Fischer-Tropsch Processes and Catalysts Using Promoters", and U.S.
Patent 6,727,289 entitled "Boron Promoted Catalysts and Fischer-Tropsch
Processes". Thus suitable promoters include boron, vanadium, phosphorous,
manganese, and alkali metals.
Catalysts which may be regenerated by the present method may be
prepared by any of the methods known to those skilled in the art. By way of
illustration and not limitation, such methods include impregnating the
catalytically active compounds or precursors onto a support, extruding one or
more catalytically active compounds or precursors together with support
material to prepare catalyst extrudates, and/or precipitating the
catalytically
active compounds or precursors onto a support. Accordingly, the supported
catalysts of the present invention may be used in the form of powders,
particles, pellets, monoliths, honeycombs, packed beds, foams, and aerogels.
The most preferred method of preparation may vary, as will be
recognized by those skilled in the art, depending for example on the desired
catalyst particle size. Those skilled in the art will be able to select the
most
suitable method for a given set of requirements.
8
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
One method of preparing a supported metal catalyst (e.g., a supported cobalt,
cobalt/ruthenium, or cobalth-uthenium/promoter catalyst) is by incipient
wetness impregnation
of the support with an aqueous solution of a soluble metal salt such as
nitrate, acetate,
acetylacetonate or the lilce. Another method of preparing a supported metal
catalyst is by a
melt impregnation technique, which involves preparing the supported metal
catalyst from a
molten metal salt. One preferred method is to impregnate the support with a
molten metal
nitrate (e.g., Co(NO3)2=6H20). Alternatively, the support can be impregnated
with a solution of
a zero valent metal precursor. One preferred method is to impregnate the
support with a
solution of zero valent cobalt such as Co2(CO) 8, Co4(CO)2 or the like in a
suitable organic
solvent (e.g., toluene). Suitable ruthenium compounds are the common water
soluble ones,
e.g., ruthenium heptoxide (Ru207) and ammonium perruthenate (NH4RuO4).
The impregnated support is dried and reduced with hydrogen or a hydrogen
containing gas. The hydrogen reduction step may not be necessary if the
catalyst is prepared
with zero valent cobalt. In another preferred method, the impregnated support
is dried,
oxidized with air or oxygen and reduced in the presence of hydrogen.
Typically, at least a portion of the metal(s) of the catalytic metal component
(a) of the
catalysts of the present invention is present in a reduced state (i.e., in the
metallic state).
Therefore, it is normally advantageous to activate the catalyst prior to use
by a reduction
treatment, in the presence of hydrogen at an elevated temperature. Typically,
the catalyst is
treated with hydrogen at a temperature in the range of from about 75 C to
about 500 C, for
about 0.5 to about 24 hours at a pressure of about 1 to about 75 atm. Pure
hydrogen may be
used in the reduction treatment, as may a mixture of hydrogen and an inert gas
such as nitrogen,
or a mixture of hydrogen and other gases as are known in the art, such as
carbon monoxide and
carbon dioxide. Reduction with pure hydrogen and reduction with a mixture of
hydrogen and
carbon monoxide are preferred. The amount of hydrogen may range from about 1%
to about
100% by volume.
Catalysis
The Fischer-Tropsch process is typically run in a continuous mode. In this
mode, the
gas hourly space velocity through the reaction zone may range from about
100 volumes/hour/volume catalyst (v/hr/v) to about 15,000 v/hr/v, preferably
from about
2000 v/hr/v to about 10,000 v/hr/v. The reaction zone temperature is typically
in the range
from about 160 C to about 300 C. Preferably, the reaction zone is operated at
conversion
promoting conditions at temperatures from about 190 C to about 260 C. The
reaction zone
9
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
pressure is typically in the range of about 80 psig (653 kPa) to about 1000
psig (6994 kPa),
preferably from 80 psig (6531cPa) to about 600 psig (4237 kPa), more
preferably from about
140 psig (1066 kPa) to about 450 psig (2858 kPa), more preferably from about
300 psig to
about 450 psig.
The products resulting from Fischer-Tropsch synthesis will have a range of
molecular
weights. Typically, the carbon number range of the product hydrocarbons will
start at methane
and continue to the limits observable by modern analysis, about 50 to 100
carbons per
molecule. The catalyst of the present process is particularly useful for
making hydrocarbons
having five or more carbon atoms, especially when the above-referenced
preferred space
velocity, temperature and pressure ranges are employed.
Regeneration
Catalysts, such as those described above, tend to become deactivated. The
present
method of regenerating a catalyst preferably includes passing the catalyst
from a reaction zone
maintained at a reaction pressure to a regeneration zone maintained at a
regeneration pressure
less than the reaction pressure. The regeneration pressure is preferably
between about 10 psig
and about 350 psig, more preferably between about 50 psig and about 350 psig.
A portion of
the heavy hydrocarbons is preferably removed from the catalyst surface via
vaporization. The
regeneration pressure is preferably set at a predetermined value selected to
effect volatilization
of a portion of the heavy hydrocarbon that would otherwise be present as
liquids at normal
operating pressure. In combination, or alternatively, the regeneration
pressure is preferably set
at a predetermined value selected to effect volatilization of heavier than
C40=
It will be understood that reaction pressure, as used herein, may be the mean
reaction
pressure. Further, it will be understood that regeneration pressure, as used
herein, may be the
mean regeneration pressure. The mean may be a time average taken at a fixed
location in the
reactor. Alternatively or in combination, the mean may be a spatial average
taken at a fixed
time.
Further, it will be appreciated that heavy hydrocarbons may include different
molecular
weight hydrocarbons according to the system. Heavy hydrocarbons, as used
herein preferably
include those hydrocarbons with a molecular weight of at least 500.
Alternatively, or in
combination, very heavy hydrocarbons, as used herein, preferably include those
hydrocarbons
including 40 or more carbon atoms.
Regeneration preferably includes contacting the catalyst with a regeneration
gas in a
regeneration zone. The regeneration gas may be any conventional regeneration
gas, preferably
a regeneration gas for a Fischer-Tropsch catalyst. Thus, the regeneration gas
is preferably
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
chosen from among hydrogen-containing gases, such as hydrogen gas, oxygen-
containing
gases, such as air and oxygen gas, steam, inert gases such as nitrogen and the
lilce, and non-
explosive combinations thereof. For example, one preferred regeneration gas
includes steam
and hydrogen. The hydrogen is preferably present with the steam in an amount
not exceeding
5% by volume of the total regeneration gas.
Fischer-Tropsch synthesis conditions may be maintained, but preferably the
pressure is
reduced and the temperature is raised during regeneration. The temperature is
preferably
increased by an amount between 0 C and 150 C from the operating temperature of
the Fischer-
Tropsch synthesis. Regeneration temperatures can thus range from about 200 C
to about 450
C, more preferably from about 220 C to about 450 C, preferably from about 250
C to about
350 C. Likewise, the pressure is preferably reduced by between 10 psig and
about 300 psig
from the pressure of the Fischer-Tropsch synthesis. Pressures can thus range
from about 0
psig to about 350 psig. Contact between the regeneration gas and the catalyst
is maintained for
at least 5 minutes and preferably for at least 4 hours. At least 0.25 Standard
L/hr, preferably at
least 0.5 Standard L/hr, and most preferably at least 1 Standard L/hr/ of
regeneration gas are
used for each gram of catalyst.
Under these regeneration conditions, it has been found that the activity of
certain
catalysts will return to levels at or exceeding 50% of the pre-deactivation
activity.
Regeneration Using Steam
In a regeneration process in which the regeneration gas includes steam, there
is an
optimal temperature range. It has been discovered that at lower temperatures,
the steam does
not regenerate the catalyst. At high temperatures, the steam causes
detrimental selectivity
changes to the catalyst. Steam is an oxidizing agent that can oxidize cobalt
metal sites on the
catalyst to cobalt oxide, which is not catalytic for Fischer-Tropsch
synthesis. There is
significant literature stating that water or steam has a significant
deactivating effect on the
Fischer-Tropsch reaction. Hence, it was expected that steam would deactivate
the catalyst
further. Contrary to these expectations, it has been discovered that there
exists a temperature
range in which contact with steam can cause an increase in catalytic activity.
Depending on the
catalyst and the degree of deactivation, it has been found that activity can
be increased to at
least 50% of the pre-deactivation activity and sometimes as much as 100% of
the pre-
deactivation activity.
While not wishing to be bound by any theory, it is believed that steam may
oxidize coke on the
catalyst surface and/or remove the heavy hydrocarbons on the catalyst surface.
If the primary
11
CA 02445918 2006-09-27
deactivation mechanism is coking and/or deposition of heavy hydrocarbons,
then steam will work at least as well as hydrogen gas. Hydrogen gas may work
better in cases where oxidation is one of the mechanisms of deactivation.
It is believed that there are at least two general classes of catalyst where
coking may be the key deactivation mechanism. The first class of catalyst is
cobalt-containing supported catalysts where the support has high acidity. Acid
site coking is well known in the art as a mechanism of the coking reaction. As
an example, fluorided supports are highly acidic. The second class of catalyst
is
cobalt-containing catalysts having a high initial activity. A high initial
activity
occurs when the initial CO conversion is greater than about 60%. With high
activity, localized surface temperatures on a catalyst particle can be very
high,
aiding in the coking mechanism.
Reaction/Regeneration Cycle
According to an embodiment of the present invention, a process for
cycling between reaction and regeneration includes applying a pressure swing
condition to a catalyst. Pressure swing, as termed herein, designates a cycle
in
pressure from a reaction pressure to a lower regeneration pressure and back to
the reaction pressure.
Referring to Figure 1, a cyclical process preferably includes passing a
feed stream 10 to a reaction zone 20. Feed stream 10 preferably includes
synthesis gas. Reaction zone 20 preferably includes a reactor 30. Reactor 30
is
preferably a slurry bed reactor. Slurry bed reactors are known and are
described for example in U.S. Patent 5,348,982.
A light product 40 preferably exits from reactor 30 during operation,
preferably from near the top of the reactor. Light product 40 may include
water, a byproduct of Fischer-Tropsch synthesis, and un-reacted synthesis gas
components, that is carbon monoxide and hydrogen. Light product 40 may
further include any reaction products that are typically gaseous under Fischer-
Tropsch reaction conditions.
Reactor 30 preferably contains a catalyst slurry 50. Slurry 50 includes
solid catalyst, preferably particulate catalyst, in a liquid suspension. Used
slurry
60 preferably exits reactor 30 during operation. Used slurry 60 includes
catalyst particles, some of which may be deactivated by heavy hydrocarbons.
Used slurry 60, containing used catalyst, preferably passes to
regenerator 70. Regenerator 70 is preferably a variable-pressure
regeneration unit. A rejuvenation gas 80 preferably passes into regenerator
70. A described in more detail above in the section entitled "Regeneration",
the rejuvenation gas, also termed regeneration gas, is preferably any Fischer-
12
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
Tropsch regeneration gas, including, but not limited to, a hydrogen-containing
gas, and steam.
The pressure of the regenerator is preferably maintained so as to volatilize
heavy hydrocarbons.
Further, as described in more detail above, in the section entitled
"Regeneration", when the
regeneration gas is hydrogen, the hydrogen may hydrogenate one or more heavy
hydrocarbons
deposited on the catalyst.
Regenerator 70 is operated in semi-batch mode. After the used slurry 60 enters
the
regenerator, the rejuvenation gas is started and the pressure of the vessel is
reduced to pressure
sufficient to volatilize heavy hydrocarbons. After a sufficient treatment, the
pressure in the
regenerator 70 can be raised and the rejuvenated catalyst (90) passes back to
the reactor 30
preferably still in a slurry.
Alternatively, or in combination reactor slurry 100 is passed from regenerator
70 to a
filtration unit (not shown.)
Referring now to Figure 2 an alternative embodiment of the present invention
more
suitable to reactors other than slurry reactors is shown. However, this
embodiment could be
used with any reactor type. A feed stream 200, preferably including synthesis
gas, feeds a
reaction zone 210 through a valve 220. Reaction zone 210 contains a reactor
230. A product
stream 240 from the reactor may include water, a byproduct of Fischer-Tropsch
synthesis, un-
reacted synthesis gas components, that is carbon monoxide and hydrogen and
hydrocarbon
products produced under Fischer-Tropsch reaction conditions.
Reactor 230 contains a solid catalyst. The catalyst deactivates over a period
of time.
When the activity of the catalyst falls to a pre-determined level, valve 220
is closed separating
feed stream 200 from reactor 230. Simultaneously, valve 250 is opened letting
a regeneration
gas stream 260 into reactor 230. Reactor 230 may be de-pressurized and heated
(if desirable) to
the desired regeneration conditions. As described in more detail above in the
section entitled
"Regeneration", the rejuvenation gas, also termed regeneration gas, is
preferably any Fischer-
Tropsch regeneration gas, including, but not limited to, a hydrogen-containing
gas, and steam.
The pressure during regeneration is preferably maintained so as to volatilize
heavy
hydrocarbons.
After a suitable length of time, reactor 230 is cooled (if desirable) to
reaction
temperature and valve 250 is closed separating regeneration gas stream 260
from reactor 230.
Simultaneously, valve 220 is opened letting in feed stream 200 to reactor 230.
Reactor 230 is
subsequently pressurized to the desired reaction pressure. By this process,
reactor 230 is cycled
between conditions which promote the Fischer-Tropsch reaction and conditions
which promote
catalyst regeneration.
13
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
It will be appreciated that while the Fischer-Tropsch reaction has been
described with
respect to the production of hydrocarbons, the Fischer-Tropsch reaction, or
Fischer-Tropsch
process, may include any catalytic transformation of synthesis gas to form any
organic reaction
product, that is containing at least one specie of organic molecule. Organic
molecules include
molecules that contain carbon and hydrogen, such as hydrocarbons, oxygenates,
and the lilce.
Hydrocarbons include molecules limited to carbon and hydrogen, such as
paraffins (alkanes),
straight-chain paraffins, olefins (alkenes), and the like. Oxygenates include
molecules that
include carbon, hydrogen, and oxygen, such as alcohols, and the like.
Further, it will be understood that while reaction has been described with
respect to the
Fischer-Tropsch catalysis, the present process may be applied to any reaction
or reaction
system that involves the contact of a catalyst with heavy hydrocarbons.
Exemplary reactions
and systems for which the present process is contemplated include methanol
synthesis,
hydrogenation reactions, and petroleum residue hydroprocessing.
Without further elaboration, it is believed that one skilled in the art can,
using the
description herein, utilize the present invention to its fullest extent. The
following
embodiments are to be construed as illustrative, and not as constraining the
scope of the present
invention in any way whatsoever.
Example 1
A fixed bed reactor was used for the first example. The fixed bed was a 1 in.
tubular
reactor packed with 6 grams of catalyst and about 30 grams of diluent (glass
beads). A
thermocouple in the middle of the bed recorded the temperature. Synthesis gas
with a ratio of
about 2 H2/CO (mol/mol) was fed to the reactor such that the space velocity
was about 2
standard liters of syngas/hr/gram of catalyst. The temperature during the
reaction was held
constant at about 225 C. The pressure was held constant at about 350 psig at
the bed outlet
during the reaction. Two liquid products were obtained, the heavy waxy
hydrocarbon (mostly
C20+) and a mixture of water and light hydrocarbon. A standard dry gas meter
measured the
off-gas rate. The composition of the off-gas was measured by gas
chromatography. In the
attached data, the catalyst was 19% Co, 0.1 % Ru on Alumina. The catalyst
average particle
size was 20 microns.
With reference to Figure 3, which shows the measured results, it can be seen
that
initially the catalyst activity produced a conversion of about 70%. The
conversion fell fairly
rapidly to 15% within 168 hours. At that point, the regeneration procedure was
started. The
14
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
feed was stopped and steam containing H2 in the amount of 7% by volume was fed
to the
reactor. The steam was formed by vaporizing water in a line. Hydrogen gas was
combined
with the steam after vaporization. The W.H.S.V. was 5 grams water per gram
catalyst per hour.
The temperature in the reactor was maintained at about 300 C. The pressure in
the reactor was
maintained at about 50 psig. The regeneration gas was fed for 4 hours. At the
conclusion of
the regeneration procedure, the regeneration gas was stopped. The temperature
was lowered
back to the reaction temperature of about 225 C. Syngas feed was resumed. The
catalyst
activity and selectivity as measured returned to the values at pre-
deactivation levels. After
about an additional 300 hours, the conversion fell to 32%. The catalyst was
again regenerated
in the manner described above. This procedure was repeated several more times.
Regeneration
was effective each time in the initial activity and selectivity.
In Figure 3 the thick vertical lines indicate the beginning of the
regeneration procedure.
The slight increase in activity level preceding each application of the
regeneration procedure is
not believed to be a general phenomenon. Causes might include slight
adjustments in reaction
conditions that occurred in some cases prior to regeneration. It can be seen
in Figure 3 that the
conversion after regeneration was at least 70% each time the catalyst was
regenerated. The
data in Figure 3 suggest that the activity falls again less than 50 hours
after regeneration.
However, the data in Figure 3 also suggest that the activity is raised to a
higher baseline value
after each regeneration procedure. One way to keep the activity closer to its
post-regeneration
value for a longer period of time is to utilize a continuous regenerator. In a
continuous
regenerator, the catalyst is sent into a regenerator vessel that continuously
applies steam.
Regenerated catalyst is cycled back into the reaction zone in the Fischer-
Tropsch reactor online.
Thus, the average time since last regeneration of the catalyst may be
minimized. In this way,
the Fischer-Tropsch synthesis operates at a level closer to, for example, the
high conversion
peaks of the graph shown in Figure 3
Exam.ple 2
A fixed bed reactor was used. The fixed bed was a 1 in. tubular reactor packed
with 6
grams of catalyst and about 30 grams of diluent (glass beads). A thermocouple
in the middle of
the bed recorded the temperature. Synthesis gas with a ratio of about 2 H2/CO
(mol/mol) was
fed to the reactor such that the space was about 2 standard liters of
syngas/hr/gram of catalyst.
The temperature during the reaction was held constant at about 225 C. The
pressure was held
constant at about 350 psig at the bed outlet. Two liquid products were
obtained, the heavy
waxy hydrocarbon (mostly C20+) and a mixture of water and light hydrocarbon. A
standard dry
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
gas meter measured the off-gas rate. The composition of the off-gas was
measured by gas
chromatography. In the attached data, the catalyst was 19% Co, 0.1 % Ru on
Alumina. The
catalyst average particle size was 20 microns. Regeneration was started after
the feed was on
stream for a duration of between about 500 and 1000 hours. The feed was
stopped and a
regeneration gas was fed to the reactor. The first three rows of Table 1
summarize results for a
regeneration gas including about 93 % steam and about '7 % hydrogen gas. The
last row of
Table 1 summarizes results for hydrogen gas as the regeneration gas.
In the cases of a regeneration gas including 93% steam and 7% hydrogen gas,
the steam
was formed by vaporizing water in a line. The W.H.S.V. was about 5 grams water
per gram
catalyst per hour. Hydrogen gas was combined with the steam after
vaporization. In each case,
the regeneration gas was fed for 4 hours. The pressure of the reaction zone
was maintained at
about 50 psig during regeneration. At the conclusion of the regeneration
procedure, the
regeneration gas was stopped. The temperature was lowered back to the reaction
temperature
of about 225 C. Syngas feed was resumed. The procedure was repeated, varying
the hours the
syngas feed was on stream in the range 500-1000 hours. The level of initial
catalyst activity
measured after regeneration, as the fraction of CO% conversion regained after
a single
regeneration is measured and displayed in Table 1.
In all the steam cases, selectivity to C5+ and methane after regeneration was
the same as
the initial runs with the catalyst. However, in the case of H2 gas at 350 C,
the small conversion
increase that occurred after regeneration came almost entirely in the form of
methane. This is
consistent with either sintering of the Co particles during the regeneration
or preferential
regeneration of methane producing sites.
Table 1 shows that there is an optimal temperature region for catalyst
regeneration
using a steam-containing gas. This optimal region is 200 C and 350 C, more
optimally
between 250 and 350 C and preferably close to 300 C. Referring again to Table
1, the poor
performance of H2 gas may have been due to sintering of the Co particles at a
high temperature
of 350 C. The optimal temperature for H2 gas may be lower than 350 C.
Table 1
Regeneration Gas Temperature of Regeneration Fraction of Conversion
( C) Regained
93% steam/7% H2 250 10
93% steam/7% H2 300 95
93% steam/7% H2 350 0
H2 gas (no steam) 350 10
16
CA 02445918 2006-09-27
Example 3
A slurry reactor was used. The catalyst particle size was about 20
microns in diameter. The impeller speed was 600 rpm during reaction and
regeneration. The impeller acts to suspend the catalyst particles in the
solution
and keeps them from settling. The catalyst was 20 wt % Co, 0.1 wt % Re on a
fluorided alumina support. Suitable fluorided alumina supports are disclosed,
for example, in U.S. Patent 6,368,997, commonly owned with the present
application. The slurry was approximately 15 wt % catalyst. The Fischer-
Tropsch reaction was carried out at a pressure of about 350 psig (2.5 MPa) and
a temperature of about 225 C. Table 2 shows that initially the catalyst
activity
produced a conversion of about 60%. The conversion fell fairly rapidly to 15%
within 1014 hours. At that point, the regeneration procedure was started. The
feed was stopped and steam containing about 5% H2 by volume was fed to the
reactor. The steam was formed by vaporizing water in a line. The W.H.S.V. was
about 3 grams water per gram catalyst per hour. The temperature in the
reactor was maintained at about 300 C. The pressure in the reactor was
maintained at about 50 psig. The regeneration gas was fed for 4 hours. At the
conclusion of the regeneration procedure, the regeneration gas was stopped.
The temperature was lowered back to the reaction temperature of about
225 C. Syngas feed was resumed. The initial catalyst activity as measured was
regained to 50%. The initial selectivity as measured returned to a level close
to
the initial level.
Table 2
Time on Steam (hours) CO Conversion (mole %)
0 60
1014 15
Post-regeneration 50
Example 4
A slurry reactor was used. The catalyst particle size was about 50
microns in diameter. The impeller speed was 1000 rpm during reaction and
regeneration. The impeller acts to suspend the catalyst particles in the
solution
and keeps them from settling. The catalyst was 21 wt % Co and 0.02 wt. % Ru
on a gamma alumina support. The slurry was approximately 6 wt %
catalyst. The Fischer-Tropsch reaction was carried out at a pressure of
about 300 psig (2.1 MPa) and a temperature of about 230 C. Table 3 shows
that initially the catalyst activity produced a conversion of about 44%. The
conversion fell to 27% within 325 hours. At that point, the regeneration
procedure was started. The feed was stopped and nitrogen was fed to
17
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
the reactor. The G.H.S.V. was about 1 Standard liters of Nitrogen per hour per
gram of
catalyst. The temperature in the reactor was maintained at about 240 C. The
pressure in the
reactor was maintained at about 50 psig. The regeneration gas was fed for 24
hours. At the
conclusion of the regeneration procedure, the regeneration gas was stopped.
The temperature
was lowered back to the reaction temperature of about 230 C. Syngas feed was
resumed. The
conversion exhibited by the regenerated catalyst was 47%. The selectivity as
measured
returned to a level close to the initial level.
Table 3
Time on Stream (hours) CO Conversion (mole %)
0 44%
325 27%
Post-regeneration 47%
Example 5
A slurry reactor was used. The catalyst particle size was about 50 microns in
diameter.
The impeller speed was 1000 rpm during reaction and regeneration. The impeller
acts to
suspend the catalyst particles in the solution and keeps them from settling.
The catalyst was 21
wt % Co and 0.02 wt.% Ru on a gamma alumina support. The slurry was
approximately 6 wt
% catalyst. The Fischer-Tropsch reaction was carried out at a pressure of
about 450 psig (3.2
MPa) and a temperature of about 210 C. Table 4 shows catalyst activity at the
start of
reaction, after deactivation and after regeneration in temis of relative
activity. The catalyst
activity at the start of reaction is designated as 100%. The catalyst relative
activity fell to 33%
or a third of the initial activity within 450 hours. At that point, the
regeneration procedure was
started. The carbon monoxide feed was stopped. The reactor was depressurized
under
hydrogen flow to 125 psig. The G.H.S.V. was about 1 Standard liters of
hydrogen per hour per
gram of catalyst. The reactor temperature was then decreased to the desired
regeneration
temperature. The regeneration gas was fed for 24 hours. At the conclusion of
the regeneration
procedure, the temperature was lowered back to the reaction temperature of
about 210 C.
Syngas feed was resumed.
Table 4 shows the catalyst relative activity as a function of the regeneration
temperature. After a regeneration temperature of 227 C, the catalyst had
regained 45% of its
initial activity. After a regeneration temperature of 271 C, the catalyst had
regained 71% of its
initial activity.
18
CA 02445918 2003-10-29
WO 02/092227 PCT/US02/15460
Table 4
Description Relative Activity
Initial Catalyst Activity 100%
Catalyst Activity at 450 hours 33%
Activity after regeneration at 227 C 45%
Activity after regeneration at 271 C 71%
Without further elaboration, it is believed that one skilled in the art can,
using the
description herein, utilize the present invention to its fullest extent. The
embodiments
described herein are to be construed as illustrative, and not as constraining
the scope of the
present invention in any way whatsoever. Furthermore, various modifications
can be made
without departing from the scope of the present invention. For example, while
the present
method has been described as a batch process, it will be understood that it
can be carried out on
a continuous basis, using known technologies for continuously treating
catalyst.
The complete disclosures of all patents, patent documents, and publications
cited herein
are incorporated by reference in their entirety.
While preferred embodiments of this invention have been shown and described,
modifications thereof can be made by one skilled in the art without departing
from the spirit or
teaching of this invention. The embodiments described herein are exemplary
only and are not
limiting. Many variations and modifications of the process are possible and
are within the scope
of the invention. Accordingly, the scope of protection is not limited to the
embodiments
described herein, but is only limited by the claims which follow, the scope of
which shall
include all equivalents of the subject matter of the claims.
19