Note: Descriptions are shown in the official language in which they were submitted.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
ANTIBACTERIAL AGENTS
Field of the Invention
This invention relates to antibacterial agents having a pyrido[1,2-
a]pyrimidine
core structure and methods for their use.
S Background of the Invention
Antibiotic resistance is a worldwide problem with catastrophic potential. A
Task Force co-chaired by the United States Centers for Disease Control (CDC),
Food
and Drug Administration (FDA), and National Institutes of Health (NIH)
recently
addressed this important issue, observing that drug resistant pathogens are a
growing
menace to all people, regardless of race, age, gender, or socioeconomic
background.
The Task Force noted that a number of microbes responsible for infections in
humans
are rapidly developing resistance to existing drugs. For example, according to
the
Task Force, in the United States alone, up to 30 percent of the Staphylococcus
pneumoniae infections (skin, bone, lung, and bloodstream infections) are no
longer
susceptible to penicillin in some areas. Up to 11 percent of S. pneurnoniae
are
resistant to third generation cephalosporin antibiotics. Significantly,
resistance of S
pneumoniae to the fluoroquinolones, a newer class of potent antibiotics, has
also been
reported.
Exemplified by ciprofloxacin A, the fluoroquinolones are bacterial inhibitors
that apparently exert their effect by inhibiting DNA gyrase and topoisomerase
IV
OH
l~
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
A
The consequences of antibiotic resistance, particularly fluoroquinolone
resistance, can be fatal for some individuals. In a case reported from
Denmark, a 62-
year-old woman diagnosed with food poisoning from ciprofloxacin-resistant
Salmonella died after undergoing antibiotic treatment using that drug.
The dramatic and lethal emergence of antibiotic resistance typified by this
and
.,
other reports has spurred the U.S. Task Force to call for the implementation
of a
public health action plan to combat antimicrobial resistance. As a vital
component of
that plan, there is a need for the development of new products that will
prevent the
continued emergence of antibiotic resistance generally, and that will prevent
and treat
colonization and infection of resistant organisms in patients.
Summary of the Invention
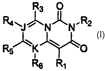
The present invention provides compounds having pyrido[1,2-c] pyrimidine
core structures that meet these and other needs. Accordingly, there is
provided a
compound of the invention which is a compound of Formula I:
R3 O
R4~~~N~N.R2
R5 K~O
Rs R~
or a pharmaceutically acceptable salt thereof wherein:
R1 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
3
or heteroaryl and substituted heteroaryl;
Rz is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic,and substituted heterocyclic,
heteroaryl and substituted heteroaryl,
halo,
N02,
NO,
CN,
ORa,
O
I(
-OCRs, wherein Ra is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
I I
-C-ORb,
O
I I
-C-SRb,
O
C-Rb, wherein Rb is H,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
4
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
, heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
0
I I
-C-NR~Rd, wherein R~ and Rd are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
,~
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
NReRf, wherein
Re and Rf are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
0
II
-C-ORb,
O '
I I
-C-SRb,
O
C-Rb, wherein Rb is defined as above;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
.
-C-NR~Ra, wherein R~ and Rd are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Re and Rf are taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R3, Rq., and R6 independently are H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
NReRf, wherein Re and Rf are defined as above; or
R1 and R6 taken together with the atoms to which they are attached form a 5,
6, 7, or 8 membered ring having from 0 to 3 heteroatoms selected from
N, O, and S, wherein said ring is optionally substituted by one or more
substituents;
RS is hydrogen;
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
ORa, wherein Ra is defined as above,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
6
O
I I
-OC- ORb,
O
11
-C-ORb,
O
I I
-C-SRb,
SRb,
O
T
-s Rb
O
11
-'-ORb
O
O
II
- ~ F,
O
O
I I
- ~ CF3,
O
O
II
-C-Rb, wherein Rb is defined~as above
O
l I
-C-NR~Rd, wherein Its and Rd are defined as above;
halo,
N02,
CN,
NReRf, wherein Re and Rf are defined as above;
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl, .
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
7
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted; and
wherein J and K independently are C or N, provided that when
J or K is N, R4 or R6 is absent at that position.
The invention also provides a compound of Formula II;
R3 O
Ra ~ N~N.R2
R5 ~ ~ O
R6 R~
II
or a pharmaceutically acceptable salt thereof wherein:
Rl is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C7 cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R2 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
8
heteroaryl and substituted heteroaryl,
halo,
N02,
NO,
ORa,
O
I I
-OCRs, wherein Ra is H,
C1-C7 alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
I I
-C-ORb,
O
I I
-C-SRb,
O
I)
-C-Rb, wherein Rb is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
O
I I
-C NR~Rd, wherein R~ and Rd are independantly H,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
9
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
NReRf, wherein
Re and Rf are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
I I
-C-ORb,
O
I I
-C-SRb,
O
I!
-C-Rb, wherein Rb is defined as above;
-C-NR~Rd, wherein R~ and Rd are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Re and Rf taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
R3, R4, and Rg independently are H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
10 NReR~, wherein Re and Rf are defined as above; or
R1 and Rg taken together with the atoms to which they are attached form a 5,
6, 7,,or 8 membered ring having from 0 to 3 heteroatoms selected from
N, O, and S, wherein said ring is optionally substituted by one or more
substituents;
RS is hydrogen,
C 1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
ORa, wherein Ra is defined as above,
O
I I
-OC- ORb,
O
I I
-C-ORb,
O
I I
-C-SRb,
SRb,
O
T
-S-Rb,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
11
O
I I
- ~-ORb,
O
O
I I
I F'
. O
O
II
- ~--CF3
O
O
I I
-C-Rb, wherein Rb is defined as above
O
I I
-C NR~Rd, wherein R~ and Rd are defined as above;
halo,
N02,
CN,
NReRf, wherein Re and Rf are as defined above,
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl, or
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted.
The invention also provides a compound of Formula III:
H O
R4 , N~N.R2
R5 ~N ~0
R~
III
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
12
or a pharmaceutically acceptable salt thereof, wherein:
Rl is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R2 is H,
C1-C~ alkyl and substituted alkyl,
C~-C~ alkenyl and substituted alkenyl,
C~-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
heteroaryl and substituted heteroaryl,
halo,
N02,
NO,
CN,
~Ra,
-OCRs, wherein Ra is H,
C 1-C~ alkyl and substituted alkyl,
Ca-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
13
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
II
-C-ORb,
O
II
-C-SRb,
O
I I
--C-Rb, wherein Rb is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
O
I I
-C-NR~Rd, wherein R~ and Rd are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
NReRf, wherein
Re and Rf are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
14
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
11
-C-ORb
O
11
-C-S~
O
II
-C-Rb, wherein Rb is defined as above;
O
If
-C-NR~Ra, wherein R~ and Ra are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Re and Rf are taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
Rq. is H,
OH,
(O)nC 1-C7 alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
NReRf, wherein Re and Rf are defined as above;
RS is hydrogen,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
ORa, wherein Ra is defined as above,
S O
-OC- ORb,
O
i1
10 --C-ORb,
O
II
-C-SRb,
SRb,
15 0
T
-S-Rb
O
Il
- ~ ORb,
O
0
Il
- i F,
O
- ~-CF3,
O
O
II
-C-Rb, wherein Rb is defined as above
O
I I
--C-NR~Rd, wherein R~ and Rd are defined as above;
halo,
N02,
CN,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
16
NReRf, wherein Re and Rf are defined as above,
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl, or
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted.
The invention also provides a compound of Formula IV:
H O
N~N~N~R2
R ~ \ O
s
Rs R1
IV
or a pharmaceutically acceptable salt thereof, wherein:
Rl is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
Ca-C~ alkynyl and substituted alkynyl,
C3-C~ cycIoaIkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R2 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
17
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
heteroaryl and substituted heteroaryl,
halo,
N02,
NO,
CN,
OR.a,
O .
i1
-OCRs, wherein Ra is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
I I
-C-ORb,
O
Ii
-C-SRb,
O
I I
-C-Rb, wherein Rb is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
18
O
I I
-C-NR~Rd, wherein R~ and R~ are independently H,
C 1-C~ alkyl and substituted alkyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
Ca-C~ alkenyl and substituted alkenyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
NR~Rf, wherein
Re and Rf are each independently H,
C 1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
I I
-C-ORb,
O
I I
-C-SRb,
O
I I
--C-Rb, wherein Rb is defined as above;
O
I I
-C NR~Rd, wherein R~ and Rd are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
19
Re and Rf are taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or ~ membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R5 is hydrogen,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
ORa, wherein Ra is defined as above,
O
-OC- ORb,'
O
I)
-C-ORb,
O
I I
-C-SRb,
SRS,
O
-S-Rb,
O
II
- ~-ORb
O
O
II
O
- ~-CF3,
O
O
I I
--C-Rb, wherein Rb is defined as above
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
-C-NR~Rd, wherein R~ and Rd are defined as above;
halo,
5 NOa,
CN,
NReRf, wherein Re and Rf are defined as above,
aryl or fused aryl,
heterocyclic or fused heterocyclic,
10 heteroaryl or fused heteroaryl,
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted;
R6 is H,
15 OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC~-C~ alkenyl and substituted alkenyl,
(O)nC~,-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
20 halo,
N02,
CN,
NReRf, wherein Re and Rf are defined as above; or
R1 and R6 taken together with the atoms to which they are attached
form a 5, 6, 7, or 8 membered ring having from 0 to 3 heteroatoms selected
from N, O, and S, optionally substituted by one or more substituents.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
21
The invention also provides a compound of Formula V:
H O
N~N~N'R2
RS~N~~O
R~
V
or a pharmaceutically acceptable salt thereof, wherein:
Rl is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
, aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R2 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
heteroaryl and substituted heteroaryl,
halo,
N02,
NO,
CN,
ORa,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
I 22
O
I I
-OCRs, wherein Ra is H,
C1-C~ alkyl and substituted alkyl,
S C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
-C-ORb,
O
II
-C-SRb,
O
I I
-C-Rb, wherein Rb is H,
C1-C~ alkyl and substituted alkyl,
Ca-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
O
I I
-C-NR~Rd, wherein R~ and Rd are independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
23
NReRf, wherein
Re and Rf are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
S C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
I)
-C-ORb,
O
I I
-C-SRb,
O
-C-Rb, wherein Rb is defined as above;
O
I I
-C NR~Ra, wherein R~ and Rd are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Re and Rf are taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
RS is hydrogen,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
ORa, wherein Ra is defined as above,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
24
O
I!
-OC- ORb,
O
S I I
-C-ORb,
O
I I
-C-SRb,
I 0 SRb,
O
T
-S Rb
O
15 I)
- ~-ORb,
O
- i: F,
O
O
I I
- ~-CF3,
O
O
II .
--C-Rb, wherein Rb is defined as above
O o
-C NR~Ra, wherein R~ and Rd are defined as above;
halo,
N02,
CN,
NReRf, wherein Re and Rf are defined as above,
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl, or
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted.
The invention further provides a compound of Formula VI
R3 O
R4 / N~N.R2
Rs~N \ \ O
Rn Rs R1
VI
or a pharmaceutically acceptable salt thereof, wherein:
Rl is H,
C1-C~ alkyl and substituted alkyl,
10 C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
15 or heteroaryl and substituted heteroaryl;
RZisH,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
20 C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
heteroaryl and substituted heteroaryl,
halo,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
26
NOa,
NO,
CN,
ORa,
O
-OCRs, wherein Ra is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
II
-C-ORb,
O
II
-C-SRb,
-C Rb, wherein Rb is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
O
II
-C NR~Rd, wherein R~ and Ra are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
27
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
S NReRf, wherein
R~ and Rf are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
' C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
(I
-C-ORb,
O
I I
-C-SRb, '
O
I I
-C-Rb, wherein Rb is defined as above;
O
i1
-C-NR~Rd, wherein R.~ and Rd are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Re and Rf are taken together with the nitrogen to which they are
attached form a 4, S, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R3, Rq., and R6 independently are H,
OH,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
28
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN, or
NR~Rf, wherein Re and Rf are defined as above; and
Rg and Rh are defined as for Reand Rfabove.
~10
The invention also provides a compound of Formula VII
R3 O
Ra / N~N.R2
R9~N ~N~O
Rh R1
VII
or a pharmaceutically acceptable salt thereof, wherein:
15 Rl is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
20 aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R2 is H,
C1-C~ alkyl and substituted alkyl,
25 C2-C~ alkenyl and substituted alkenyl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
29
Ca-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
heteroaryl and substituted heteroaryl,
halo,
NOz,
NO,
CN,
ORa,
O
I I
-OCRs, wherein Ra is H,
C1-C~ alkyl and substituted alkyl,
1 S C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
-C-ORh,
O
I I
-C-SRb,
O
I I
-C-Rb, wherein Rb is H,
C1-C7 alkyl and substituted alkyl,
C~-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
O
I I
S -C-NR~R~, wherein R~ and R~ are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
10 heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
NReRf, wherein
Re and Rf are each independently H,
C1-G~ alkyl and substituted alkyl,
15 C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
20 O
I I
-C-ORb
O
II
25 -C-SRb,
O
(I
-C-Rb, wherein Rb is defined as above;
O
30 II
--C NR~Rd, wherein R~ and Rd are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
31
heterocycloalkyl and substituted heterocycloalkyl, or
Re and Rf are taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R3 and R4 independently are H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN, or
1 S .~ NReRf, wherein Re and Rf are defined as above; and
Rg and Rh are defined as for Re and Rf above.
The invention further provides a compound of Formula VIII:
R4 , NJ~N.R~
R ~ \ O
s
X ~ Ri
VIII
or a pharmaceutically acceptable salt thereof, wherein:
R2 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
Ca-C~ alkynyl and substituted alkynyl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
32
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
heteroaryl and substituted heteroaryl,
halo,
NO2,
NO,
CN,
ORa,
-OCRs, wherein Ra is H,
Cl-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,
O
I I
-C-ORb,
O
I I
-C-SRb,
-C-Rba v~'herein Rb is H,
Cl-C~ alkyl and substituted alkyl,
Ca-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
33
O
I I
-C NR~R~, wherein R~ and Ra are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
NReRf, wherein
Re and Rf are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
1 S C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
I I
-C-ORb,
O
I I
-C-
O
II
-C-Rb, wherein Rb is defined as above;
O
I I
-C NR~Rd, wherein R~ and Rd are defined as above;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
34
Re and Rf are taken together with the nitrogen to which they are
attached form a 4, S, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R3 and R4 independently are H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
NReRf, wherein Re and Rf are defined as above,
RS is hydrogen,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
ORa, wherein Ra is defined as above,
-OC- ORb,
O
I I
--C-~Rb~
O
I I
-C-SRb,
SRb,
-S-Rb,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
O
II
- ~ ~ ORb,
5 O
O
II
10 , -0 F,
O
I I
15 - ~-CF3,
O
O
20 I I
-C-Rb, wherein Rb is defined as above
' O
I I
-C NR~Rd, wherein R~ and Rd are defined as above;
25 halo,
N02,
CN, .
NReRf as defined above,
aryl or fused aryl,
30 heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl,
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted; and
35 X and Y each independently are O, CH2, CH(C1-C7 alkyl), NH,
N(C1-C7 alkyl), S, SO, or SOa;
m is 0-14;
R~isH,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
36
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or l,
halo,
N02,
CN,
~' kRm,
wherein Rk and Rm independently are H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
O
I I
-C--C1-C~ alkyl and substituted alkyl, or
Rk and Rm taken together with the nitrogen to which
they are attached form a 3- to 7-membered ring containing
from 1 to 3 heteroatoms selected from N, O, and S, said ring
being unsubstituted or substituted with l, 2, 3, or 4 substituent
groups.
The invention also provides a compound which is:
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-[3-( 1-
methylaminoethyl)pyrrolidin-1-yl]pyrido [ 1,2-c]pyrimidine-1, 3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-pyrrolidin-1-yl-pyrido[1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-6-[3-(1-hydroxyethyl)pyrrolidin-1-yl-5-
methylpyrido [ 1,2-c]pyrimidine-1,3-dione;
' 2-Amino-6-[(R)-3-(1-aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
37
2-Amino-6-((3S, 4S) and (3R,4R)-3-aminomethyl-4-fluoropyrrolidin-1-yl)-4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[(R)-3-((S)-1-amino ethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
5-methyl-pyrido[1,2-c]pyrimidine-1,3-dione; hydrochloride; and
2-Amino-6-[4-( 1-amino ethyl)-3,3-dimethylpyrrol idin-1-yl]-4-cyclopropyl-7-
fluoro-5-methyl-pyrido[1,2-c]pyrimidine-1,3-dione; hydrochloride;
2-Amino-4-cyclopropyl-7-fluoro-S-methyl-6-[3-( 1-
methylaminoethyl)pyrrolidin-1-yl]-pyrido [ 1,2-c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-pyrrolidin-1-ylpyrido[1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-6-[3-(1-hydroacyethyl)pyrrolidin-1-yl-5-
methyl-pyrido[1,2-c]pyrimidine-1,3-dione;
2-Amirio-6-[(R)-3-((R)-1-amino ethyl)-pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
1 S 2-Amino-6-[(R)-3 -((S)-1-aminoethyl)-pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
2-Amino-6-[4-(1-aminoethyl)-3,3-dimethyl-pyrrolidin-1-yl]-4-
cyclopropropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione
hydrochloride;
2-Amino-6-(3-aminopyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-a]pyrimidine-1,3-dione hydrochloride;
2-Amino-6-[3-( 1-amino ethyl)-4-methylpyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
2-Amino-6-[3-( 1-amino-1-phenylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6- { 3-[ 1-amino-1-(2-fluorophenyl)methyl]pyrrolidin-1-yl } -4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6- { 3-[ 1-amino-1-(4-fluorophenyl)methyl]pyrrolidin-1-yl } -4-
cyclopropyl-7-fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
38
2-Amino-6-{3-[ 1-amino-1-(2,4-difluorophenyl)methyl]pyrrolidin-1-yl}-4-
cyclopropyl-7-fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6- {3-[ 1-amino-1-(2,6-difluorophenyl)methyl]pyrrolidin-1-yl}-4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6- { 3-[ 1-amino-1-(2,4,6-trifluorophenyl)methyl]pyrrolidin-1-yl } -4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-aminopropyl)pyrrol idin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-methylpropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-cyclopropylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
5-methylpyrido [ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminocyclopropyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-1-phenylmethyl)-4-fluoropyrrolidin-1-yl]-4-
cyclopropyl-7-fluoro-S-methylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-fluoroethyl)pyrroli din-1-yl]-4-cyclbpropyl-7-
fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2,2-difluorocyclopropyl)pyrrolidin-1-yl]-4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-2-fluorocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-1-methyl ethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
39
2-Amino-6-[3-( 1-aminoethyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido(1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-1-pyridin-2-ylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-fluorocycl opropyl)-4-fluoropyrrolidin-1-yl]-4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-1-pyridin-3-ylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-1-pyridin-4-ylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(4-aminohexahydrocyclopenta[c]pyrrol-2-yl)-4-cyclopropy1-7-
fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(4-amino-S-fluorohexahydrocyclopenta[c]pyrrol-2-yl)-4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(3-phenylpyrrolidin-1-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(3-pyridin-2-ylpyrrolidin-1-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(3-pyridin-3-ylpyrrolidin-1-
' yl)pyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(3-pyridin-4-ylpyrrolidin-1-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(piperazin-1-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(4-methylpiperazin-1-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
N {1-[1-(2-Amino-4-cyclopropyl-7-fluoro-5-methyl-1,3-dioxo-2,3-dihydro-
1 H-pyrido[ 1,2-c]pyrimidin-6-yl)pyrrolidin-3-yl] ethyl } methanesulfonamide,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-octahydropyrrolo[3,4-b]pyridin-
6-yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(7-amino-S-azaspiro[2.4]hept-S-yl)-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(thiophen-3-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-( 1-methyl-2; 3-dihydro-1 H-
isoindol-5-yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-6-(7-hydroxymethyl-5-azaspiro[2.4]hept-5-
10 yl)-S-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(3-amino-4-methoxyiminopyrrolidin-1-yl)-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(3-
methylaminomethylpyrrolidin-1-yl)pyrido[1,2-c] pyrimidine-1,3-dione;
15 2-Amino-6-(5-aminomethylthiophen-3-yl)-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3 -( 1-amino ethyl)-3-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(5-aminomethylthiophen-3-yl]-4-cyclopropyl-7-fluoro-S-
20 methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(5-aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(4-aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-e] pyrimidine-1,3-dione;
25 2-Amino-6-(4-aminomethylthiophen-3-yl]=4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-c]pyrrol-3-yl)-7-fluoro-
5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
41
2-Amino-4-cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-c]pyrrol-2-yl)-7-fluoro-
5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(6-methyl-5,6-dihydro-4H
thieno[2,3-c]pyrrol-2-yl)pyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methyl-6-(6-methyl-5,6-dihydro-4H
thieno[2,3-c]pyrrol-3-yl)pyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(5-pyridin-4-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino=4-cyclopropyl-7-fluoro-5-methyl-6-(5-pyridin-3-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(5-pyridin-2-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(4-pyridin-4-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methyl-6-(4-pyridin-3-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-(4-pyridin-2-ylthiophen-3-
yl)pyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methyl-6-pyridin-4-ylpyrido[ 1,2-c]
pyrimidine-1,3-dione;
2-Amino-6-(4-aminomethylpyridin-2-yl)-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(5-aminomethylpyridin-2-yl)-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(6-aminomethylpyridin-2-yl)-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(S-aminomethylpyrimidin-2-yl]-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
42
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-pyrimidin-2-ylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amirio-6-(2-aminomethylpyrimidin-5-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-pyrimidin-5-ylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-pyrazin-2-ylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-6-(6-aminomethylpyrazin-2-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(6-aminomethylpyridin-2-yl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(2-aminomethylpyridin-3-yl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-e] pyrimidine-1;3-dione;
2-Amino-6-[5-(2-aminomethylpyridin-3-yl)thiophen-3-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-eJ pyrimidine-1,3-dione;
2-Amino-6-[5-(2-aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-S-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[S-(6-aminomethylpyridin-2-yl)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-S-methylpyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-6-[5-(6-aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[4-(2-aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-S-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(2-aminomethylpyridin-3-y~)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-S-methylpyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[4-(6-aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-e] pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
43
2-Amino-4-cyclopropyl-7-fluoro-S-methyl-6-(4-pyridin-4-ylthiophen-2-
yl)pyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[4-(1-aminoethyl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[(R)-3-(1-aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-rriethylpyrido [ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-((3S, 4S) and (3R,4R)-3-aminomethyl-4-fluoropyrrolidin-1-yl)-4-
cyclopropyl-7-fluoro-S-methylpyrido [ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-(3-aminomethylpyrrolidin-1-yl)-4-cyc lopropyl-7-fluoro-S-
methylpyrido-[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-(7-amino-S-azospiro[2.4]hept-5-yl)-4-cyclopropyl-7-fluoro-S-
methylpyrido[ 1,2-c]primidine-1,3-dione;
2-Amino-6-[3-(aminooxazol-4-yl-methyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methylpyrido [ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-(4-aminooctahydroisoindol-2-yl)-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-1-methyl ethyl)pyrrolidine-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione; and
6-Chloro-4-cyclopropyl-7-fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-[3-( 1-
methylaminoethyl)pyrrolidin-1-yl]pyrido[ 1,2-a]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5- methoxy -6-pyrrolidin-1-yl-pyrido[1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-6-[3-( 1-hydroxyethyl)pyrrolidin-1-yl-5-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[(R)-3-( 1-aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-((3S, 4S) and (3R,4R)-3-aminomethyl-4-fluoropyrrolidin-1-yl)-4-
cyclopropyl-7-fluoro-5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
44
2-Amino-6-[(R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
S- methoxypyrido[1,2-c]pyrimidine-1,3-dione; hydrochloride; and
2-Amino-6-[4-(1-aminoethyl)-3,3-dimethylpyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5- methoxypyrido[1,2-c]pyrimidine-1,3-dione; hydrochloride;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-[3-(1-
methylaminoethyl)pyrrolidin-1-yl]-pyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-pyrrolidin-1-ylpyrido [ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-6-[3-( 1-hydroxyethyl)pyrrolidin-1-yl-S-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[(R)-3-((R)-1-aminoethyl)-pyrrolidin-1-yl]-4-cyclopropy1-7-
fluoro-5- methoxypyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
2-Amino-6-[(R)-3-((S)-1-aminoethyl)-pyrrolidin-1-yl]-4-cyclopropy1-7-
fluoro-5- methoxypyrido[1,2-a]pyrimidine-1,3-dione hydrochloride;
I 5 2-Amino-6-[4-( 1-aminoethyl)-3,3-dimethyl-pyrrolidin-1-yl]-4-
cyclopropropyl-7-fluoro-5- methoxypyrido[1,2-a]pyrimidine-1,3-dione
hydrochloride;
2-Amino-6-(3-aminopyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
2-Amino-6-[3-( 1-aminoethyl)-4-methylpyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5--methoxypyrido[I,2-a]pyrimidine-1,3-dione hydrochloride;
2-Amino-6-[3-( I -amino-1-phenylmethyl)pyrrolidin- I -yl]-4-cyclopropyl-7-
fluoro-5- methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6- { 3-[ 1-amino-1-(2-fluorophenyl)methyl]pyrrolidin-1-yl } -4-
cyclopropyl-7-fluoro-5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;~
2-Amino-6- { 3-[ 1-amino-1-(4-fluorophenyl)methyl] pyrrolidin-1-yl } -4-
cyclopropyl-7-fluoro-S- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-{3-[1-amino-1-(2,4-difluorophenyl)methyl]pyrrolidin-1-y1}-4-
cyclopropyl-7-fluoro-S-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
2-Amino-6- {3-[ 1-amino-1-(2,6-difluorophenyl)methyl]pyrrolidin-1-yl }-4-
cycl opropyl-7-fluoro-5-methoxypyrido [ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-{3-[ 1-amino-1-(2,4,6-trifluorophenyl)methyl]pyrrolidin-1-yl }-4-
cyclopropyl-7-fluoro-S-methoxypyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-methylpropyl)pyrro lidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-2-cyclopropylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-
10 7-fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
5-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminocyclopropyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-S-methoxypyrido[1,2-e] pyrimidine-1,3-dione;
15 2-Amino-6-[3-( 1-amino-1-phenylmethyl)-4-fluoropyrro lidin-1-yl]-4-
cyclopropyl-7-fluoro-5-methoxypyrido( 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-fluoro ethyl)pyrrol i din-1-yl]-4-cycl opropyl-7-
fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2,2-difluorocyclopropyl)pyrrolidin-1-yl]-4-
20 cyclopropyl-7-fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-fluorocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methoxypyrido[1,2-e] pyrimidine-1,3-dione;
25 2-Amino-6-[3-(1-amino-1-methylethyl)pyrrolidin-1-yl]-4-cyclopropy1-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminoethyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropy1-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
46
2-Amino-6-[3-( 1-amino-1-pyridin-2-ylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-2-fluorocyclopropyl)-4-fluoropyrrolidin-1-y1]-4-
cyclopropyl-7-fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-1-pyridin-3-ylmethyl)pyrroIidin-1-y1]-4-cycIopropyl-
7-fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-1-pyridin-4-ylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-S-methoxypyrido[I,2-cJ pyrimidine-1,3-dione;
2-Amino-6-(4-aminohexahydrocyclopenta[c]pyrrol-2-yl)-4-cyclopropy1-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(4-amino-S-fluorohexahydrocyclopenta[c]pyrrol-2-yl)-4-
cyclopropyl-7-fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(3-phenylpyrrolidin-1-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(3-pyridin-2-ylpyrrolidin-1-
yl)pyridojl,2-e] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(3-pyridin-3-ylpyrrolidin-1-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(3-pyridin-4-ylpyrrolidin-1-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cycIopropyl-7-fluoro-S-methoxy-6-(piperazin-I -yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(4-methylpiperazin-1-
yl)pyrido [1,2-c] pyrimidine-1,3-dione;
2S N {1-[1-(2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-1,3-dioxo-2,3-dihydro-
1H pyrido[1,2-c]pyrimidin-6-yl)pyrrolidin-3-yl]ethyl}methanesulfonamide,
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-octahydropyrrolo[3,4-
b]pyridin-6-yl)pyrido[1,2-c] pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
47
2-Amino-6-(7-amino-5-azaspiro [2.4] hept-5-yl)-4-cycl opropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-(thiophen-3-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-( 1-methyl-2, 3-dihydro-1 H
isoindol~5=yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-6-(7-hydroxymethyl-5-azaspiro[2.4]hept-5-
yl)-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(3-amino-4-methoxyiminopyrrolidin-1-yl)-4-cyclopropyl-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-(3-
methylaminomethylpyrrolidin-1-yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(5-aminomethylthiophen-3-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminoethyl)-3-fluoropyrrolidin-1-yl]-4-cyclopropy1-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(5-aminomethylthiophen-3-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(5-aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(4-aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(4-aminomethylthiophen-3-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] .pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-c]pyrrol-3-yl)-7-fluoro-
5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-c]pyrrol-2-yl)-7-fluoro-
5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
48
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(6-methyl-5,6-dihydro-4H-
thieno[2,3-c]pyrrol-2-yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(6-methyl-5,6-dihydro-4H-
thieno[2,3-c]pyrrol-3-yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(S-pyridin-4-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-(5-pyridin-3-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-(5-pyridin-2-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-(4-pyridin-4-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-(4-pyridin-3-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-(4-pyridin-2-ylthiophen-3-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-pyridin-4-ylpyrido[ 1,2-c]
pyrimidine-1,3-dione;
2-Amino-6-(4-aminomethylpyridin-2-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-6-(S-aminomethylpyridin-2-yl)-4-cyclopropyl~7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(6-aminomethylpyridin-2-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-(S-aminomethylpyrimidin-2-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido[1,2-e] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-pyrimidin-2-ylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
49
2-Amino-6-(2-aminomethylpyrimidin-5-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-pyrimidin-5-ylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-pyrazin-2-ylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-6-(6-aminomethylpyrazin-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(6-aminomethylpyridin-2-yl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyridojl,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(2-aminomethylpyridin-3-yl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[5-(2-aminomethylpyridin-3-yl)thiophen-3-yl]-4-cyclopropyl-7-
fluoro-5-methoxypyridojl,2-c] pyrimidine-1,3-dione;
2-Amino-6-[S-(2-aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[5-(6-aminomethylpyridin-2-yl)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[5-(6-aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[4-(2-aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-5-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[3-(2-aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-
fluoro-5-methoxypyrido[ 1,2-c] pyrimidine-1,3-dione;
2-Amino-6-[4-(6-aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[1,2-c] pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-7-fluoro-S-methoxy-6-(4-pyridin-4-ylthiophen-2-
yl)pyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
2-Amino-6-[4-( 1-aminoethyl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[(R)-3-(1-aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-((3S, 4S) and (3R,4R)-3-aminomethyl-4-fluoropyrrolidin-1-yl)-4-
cyclopropyl-7-fluoro-5-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-(3-aminomethylpyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-S-
methoxypyrido-[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-(7-amino-5-azospiro[2.4]hept-5-yl)-4-cyclopropyl-7-fluoro-S-
10 methoxypyrido(1,2-c]primidine-1,3-dione;
2-Amino-6-[3-(aminooxazol-4-yl-methyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[ 1,2-a]pyrimidine-1,3-dione;
2-Amino-6-(4-aminooctahydroisoindol-2-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
15 2-Amino-6-[3-(1-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5,8-
dimethylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S-
methoxy-8-methylpyrido[1,2-c]pyrimidine-1,3-dione;
2,8-Diamino-6-[3-(1-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
20 methylpyrido[1,2-a]pyrimidine-1,3-dione;
2,8-Diamino-6-(3-aminomethyl-4-fluoropyrrolidin-1-yl)-4-cyclopropyl-7-
fluoro-5-methylpyrido[ 1,2-a]pyrimidine-1,3-dione;
2-Amino-6-(3-aminomethyl-4-fluoropyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-
5,8-dimethylpyrido[1,2-a]pyrimidine-1,3-dione;
25 2-Amino-6-[3-(1-amino-2,2-difluoroethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5,8-dimethylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-2,2-difluoroethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methoxy-8-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
51
2-Amino-6-(4-aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-4-
cyclopropyl-7-fluoro-5-methoxy-8-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-(4-aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-4-
cyclopropyl-7-fluoro-5,8-dimethylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-(4-aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-4-
cyclopropyl-7-fluoro-5-methylpyrido [ 1,2-c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-6-[3-( 1,2-dihydroxyethyl)pyrrolidin-1-yl]-7-fluoro-5-
methoxy-8-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-6-[3-( 1,2-dihydroxyethyl)pyrrolidin-1-yl]-7-fluoro-
S,8-dimethylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-6-[3-( 1,2-dihydroxyethyl)pyrrolidin-1-yl]-7-fluoro-5-
methylpyrido[1,2-a]pyrimidine-1,3-dione;
2-Amino-4-cyclopropyl-6-[3-(1,2-dihydroxyethyl)pyrrolidin-1-yl]-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-hydroxyethyl)pyrrolidin-1-yl]-4-cyc lopropyl-7-
fluoro-5-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-( 1-amino-2-hydroxyethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methoxy-8-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-2-hydroxyethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5,8-dimethylpyrido[1,2-c]pyrimidine-1,3-dione;
2-Amino-6-[3-(1-amino-1-methylethyl)pyrrolidine-1-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[1,2-c]pyrimidine-1,3-dione; and
6-Chloro-4-cyclopropyl-7-fluoro-5-methoxypyrido[ 1,2-c]pyrimidine-1,3-
dione;
6-Chloro-4-cyclopropyl-7-fluoro-5~methoxy-8-methylpyrido[1~,2-
c]pyrimidine-1, 3-dione;
6-Chloro-4-cyclopropyl-7-fluoro-5,8-dimethylpyrido[1,2-c]pyrimidine-1,3-
dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
52
2-Amino-6-chloro-4-cycl opropyl-7-fluoro-5-methoxypyrido [ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxy-8-methylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
2-Amino-6-chloro-4-cyclopropyl-7-fluoro-5,8-dimethylpyrido[ 1,2-
cJpyrimidine-1,3-dione;
8-Amino-6-hloro-4-cyclopropyl-7-fluoro-S-methylpyrido[ 1,2-c]pyrimidine-
1,3-dione;
2, 8-D iamino-6-hloro-4-cyclopropyl-7-fluoro-5-m ethylpyrido [ 1,2-
c]pyrimidine-1,3-dione;
or a pharmaceutically acceptable salt thereof.
The invention also provides a compound which is:
4-Cyclopropyl-7-fluoro-5-methyl-6-[3-( 1-methylaminoethyl)pyrrolidin-1-
ylJpyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-pyrrolidin-1-ylpyrido[1,2-cJpyrimidine-
1,3-dione;
4-Cyclopropyl-7-fluoro-6-[3-( 1-hydroxyethyl)pyrrolidin-1-yl-5-
methylpyrido[ 1,2-a]pyrimidine-1,3-dione;
6-[(R)-3-((R)-1-Aminoethyl)-pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
6-[(R)-3-((S)-1-Aminoethyl)-pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
6-[4-(1-Aminoethyl)-3,3-dimethylpyrrolidin-1-yl]-4-cyclopropropyl-7-fluoro-
5-methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
6-(3-Aminopyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-5-methyl-pyrido[ 1,2-
c]pyrimidine-1,3-dione hydrochloride;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
53
6-[3'-(1-Aminoethyl)-4-methylpyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride;
6-[3-(1-Amino-1-phenylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-{3-[1-Amino-1-(2-fluorophenyl)methyl]pyrrolidin-1-yl}-4-cyclopropyl-7-
fluoro-5-rriethylpyrido[1,2-c]pyrimidine-1,3-dione;
6-{3-[ 1-Amino-1-(4-fluorophenyl)methyl]pyrrolidin-1-yl}-4-cyclopropyl-7-
fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6- { 3-[ 1-Amino-1-(2,4-difluorophenyl)methyl] pyrrolidin-1-yl } -4-cyc
lopropyl-
7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione;
6-{3-[1-Amino-1-(2,6-difluorophenyl)methyl]pyrrolidin-1-yl}-4-cyclopropyl-
7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione;
6- { 3-[ 1-Amino-1-(2,4, 6-tri fluorophenyl)methyl]pyrrolidin-1-yl } -4-
cyclopropyl-7-fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Aminopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-2-methylpropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-a]pyrimidine-1,3-dione;
6-[3-(1-Amino-2-cyclopropylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
5-methylpyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Aminocyclopropyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
5-methylpyrido[ 1,2-a]pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-phenylmethyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-2-fluoroethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
54
6-[3-(1-Amino-2,2-difluorocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-cJpyrimidine-1,3-dione;
6-[3-( 1-Amino-2-fluorocyclopropyl)pyrrolidin-1-yl] -4-cyc lopropyl-7-fluoro-
5-methylpyri do [ 1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-methylethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido [ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Aminoethyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-pyridin-2-ylmethyl)pyrrolidin-1-yl]-4-cyc lopropyl-7-fluoro-
5-methylpyrido[1,2-c] pyrimidine-1,3-dione;
6-[3-(1-Amino-2-fluorocyclopropyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5-methylpyrido [ 1,2-c] pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-pyri din-3-ylmethyl)pyrrolidin-1-ylJ-4-cycl opropyl-7-fluoro-
5-methylpyrido[ 1,2-a]pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-pyridin-4-ylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-(4-Aminohexahydrocyclopenta[c]pyrrol-2-yl)-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-cJpyrimidine-1,3-dione;
6-(4-Amino-5-fluorohexahydrocyclopenta[c]pyrrol-2-yl)-4-cyclopropyl-7-
fluoro-5-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(3-phenylpyrrolidin-1-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(3-pyridin-2-ylpyrrolidin-1-yl)pyrido[1,2-
a]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(3-pyridin-3-ylpyrrolidin-1-yl)pyrido[1,2-
c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
4-Cyclopropyl-7-fluoro-5-methyl-6-(3-pyridin-4-ylpyrrolidin-1-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(piperazin-1-yl)pyrido[ 1,2-c]pyrimidine-
1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(4-methylpiperazin-1-yl)pyrido[ 1,2-
cJpyrimidine-1,3-dione;
N {1-[1-(4-Cyclopropyl-7-fluoro-5-methyl-1,3-dioxo-2,3-dihydro-1H
pyrido[1,2-c]pyrimidin-6-yl)pyrrolidin-3-yl]ethyl}methanesulfonamide;
4-Cyclopropyl-7-fluoro-5-methyl-6(octahydropyrrolo[3,4-bJpyridin-6-
10 yl)pyrido[1,2-a]pyrimidine-1,3-dione;
6-(7-Amino-5-azaspiro[2.4]kept-5-yl)-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(thiophen-3-yl)pyrido[ 1,2-c]pyrimidine-
1,3-dione;
15 4-Cyclopropyl-7-fluoro-5-methyl-6-(1-methyl-2,3-dihydro-1H isoindol-5-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-6-(7-hydroxymethyl-5-azaspiro[2.4]hept-5-y1)-5-
methylpyrido[1,2-cJpyrimidine-1,3-dione;
6-(3-Amino-4-methoxyiminopyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-S-
20 methylpyrido[1,2-c]pyrimidine-1,3-dione;
4-cyclopropyl-7-fluoro-5-methyl-6-(3-methylaminomethylpyrrolidin-1-
yl)pyrido[ 1,2-c]pyrimidine-1,3-dione;
6-( .5-Aminomethylthiophen-3-yl)-4-cyclopropyl-7-fluoro-5-methylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
2 S 6-[3-( 1-Aminoethyl)-3-fluoropyrroli din-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-aJpyrimidine-1,3-dione;
6-(S-Aminomethylthiophen-3-yl]-4-cyclopropyl-7-fluoro-5-methylpyrido[1,2-
c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
56
6-(S-Aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-5-methylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
6-(4-Aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-S-methylpyrido [ 1,2-
c]pyrimidine-1,3-dione;
6-(4-Aminomethylthiophen-3-yl]-4-cyclopropyl-7-fluoro-S-methylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-c]pyrrol-3-yl)-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-c]pyrrol-2-yl)-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(6-methyl-5,6-dihydro-4H thieno[2,3-
c]pyrrol-2-yl)pyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(6-methyl-5,6-dihydro-4H thieno[2,3-
c]pyrrol-3-yl)pyrido[I,2-c]pyrimidine-1,3-dione;
1 S 4-Cyclopropyl-7-fluoro-5-methyl-6-(5-pyridin-4-ylthiophen-3-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-S-methyl-6-(5-pyridin-3-ylthiophen-3-yl)pyrido[1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(5-pyridin-2-ylthiophen-3-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-S-methyl-6-(4-pyridin-4-ylthiophen-3-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-S-methyl-6-(4-pyridin-3-ylthiophen-3-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-(4-pyridin-2-ylthiophen-3-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-pyridin-4-ylpyrido [ 1,2-a]pyrimidine-1,3-
dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
57
6-(4-Aminomethylpyridin-2-yl]-4-cyclopropyl-7-fluoro-S-methylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
6-(S-Aminomethylpyridin-2-yl]-4-cyclopropyl-7-fluoro-5-methylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
6-(6-Aminomethylpyridin-2-yl]-4-cyclopropyl-7-fluoro-S-methylpyrido[ 1,2-
c]pyrimidine-1,3-dione;
6-(5-Aminomethylpyrimidin-2-y1]-4-cyclopropyl-7-fluoro-5-
methylpyrido [ 1,2-c] pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-pyrimidin-2-ylpyrido[1,2-c] pyrimidine-
1,3-dione;
6-(2-Aminomethylpyrimidin-5-yl]-4-cyclopropyl-7-fluoro-5- ' . .
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-pyrimidin-S-ylpyrido[ 1,2-a]pyrimidine-
1,3-dione;
4-Cyclopropyl-7-fluoro-5-methyl-6-pyrazin-2-ylpyrido[ 1,2-c]pyrimidine-1,3-
dione;
6-(6-Aminomethylpyrazin-2-yl]-4-cyclopropyl-7-fluoro-5-methylpyrido[ 1;2-
a]pyrimidine-1,3-dione;
6-[3-(6-Aminomethylpyridin-2-yl)pyrrolidin-1-yl]-4-cyc lopropyl-7-fluoro-S-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-(2-Aminomethylpyridin-3-yI)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S-
methylpyrido [ 1,2-c]pyrimidine-1,3-dione;
6-[5-(2-Aminomethylpyridin-3-yl)thiophen-3-yl]-4-cyclopropyl-7-fluoro-S-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[5-(2-Aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[5-(6-Aminomethylpyridin-2-yl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
58
6-[5-(6-Aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
6-[4-(2-Aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-?-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-(2-Aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-?-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[4-(6-Aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-?-fluoro-5-
methylpyrido[1,2-c] pyrimidine-1,3-dione;
4-Cyclopropyl-?-fluoro-5-methyl-6-(4-pyridin-4-ylthiophen-2-yl)pyrido[ 1,2-
c]pyrimidine-1,3-dione; and
6-[4-( 1-Aminoethyl)thiophen-2-yl]-4-cyclopropyl-?-fluoro-5-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-?-fluoro-S-methoxy-6-[3-( 1-methylaminoethyl)pyrrolidin-1-
yl]pyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-?-fluoro-S-methoxy-6-pyrrolidin-1-ylpyrido[ 1,2-a]pyrimidine-
1,3-dione;
4-Cyclopropyl-?-fluoro-6-[3-( 1-hydroxyethyl)pyrrolidin-1-yl-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[(R)-3-((R)-1-Aminoethyl)-pyrrolidin-1-yl]-4-cyclopropyl-?-fluoro-S-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[(R)-3-((S)-1-Aminoethyl)-pyrrolidin-1-yl]-~-cyclopropyl-?-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[4-(1-Aminoethyl)-3,3-dimethylpyrrolidin-1-yl]-4-cyclopropropyl-7-fluoro-
S- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-(3-Aminopyrrolidin-1-yl)-4-cyclopropyl-?-fluoro-5-methoxypyrido[1,2-
c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
59
6-[3-(1-Aminoethyl)-4-methylpyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Amino-1-phenylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6- { 3-[ 1-Amino-1-(2-fluorophenyl)m ethyl]pyrrol idin-1-yl } -4-cyc lopropyl-
7-
fluoro-5-methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6- { 3-[ 1-Amino-1-(4-fluorophenyl)methyl] pyrro lidin-1-yl } -4-cycl opropyl-
7-
fluoro-5-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-{3-[ 1-Amino-1-(2,4-difluorophenyl)methyl]pyrrolidin-1-yl}-4-cyclopropyl-
7-fluoro-S-methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-{3-[1-Amino-1-(2,6-difluorophenyl)methyl]pyrrolidin-1-yl}-4-cyclopropyl-
7-fluoro-5-methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-{3-[1-Amino-1-(2,4,6-trifluorophenyl)methyl]pyrrolidin-1-yl}-4-
cyclopropyl-7-fluoro-5-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
1 S 6-[3-( 1-Aminopropyl)pyrrolidin-1-yl]-4-cycl opropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Amino-2-methylpropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Amino-2-cyclopropylmethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
5- methoxypyrido[1,2-a]pyrimidine-1,3-dione;
6-[3-( 1-Aminocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Aminocyclopropyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-
S- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-(3-(1-Amino-1-phenylmethyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Amino-2-fluoroethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-S
methoxypyrido[ 1,2-a]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
6-[3-(1-Amino-2,2-difluorocyclopropyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-S-methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-2-fluorocyclopropyl)pyrrolidin-1-ylJ-4-cyclopropyl-7-fluoro-
5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-methyl ethyl)pyrrol i din-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Aminoethyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
10 methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-pyridin-2-ylmethyl)pyrrolidin-1-yl]-4-cycl opropyl-7-fluoro-
5- methoxypyrido[1,2-c] pyrimidine-1',3-dione;
6-[3-(1-Amino-2-fluorocyclopropyl)-4-fluoropyrrolidin-1-yl]-4-cyclopropyl-
7-fluoro-5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
15 6-[3-( 1-Amino-1-pyridin-3-ylmethyl)pyrrolidin-1-yl]-4-cyc l opropyl-7-
fluoro-
5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-1-pyridin-4-ylmethyl)pyrrolidin-1-ylJ-4-cyclopropyl-7-fluoro-
5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-(4-Aminohexahydrocyclopenta[c]pyrrol-2-yl)-4-cyclopropyl-7-fluoro-5-
20 methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-(4-Amino-5-fluorohexahydrocyclopenta[c]pyrrol-2-yl)-4-cyclopropyl-7-
fluoro-5- methoxypyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(3-phenylpyrrolidin-1-yl)pyrido[1,2-
c]pyrimidine-1,3-dione;
25 4-Cyclopropyl-7-fluoro-5-methoxy-6-(3-pyridin-2-ylpyrrolidin-1-
yl)pyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(3-pyri din-3-ylpyrrolidin-1-
yl)pyrido[1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
61
4-Cyclopropyl-7-fluoro-5-methoxy-6-(3-pyridin-4-ylpyrrolidin-1-
yl)pyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(piperazin-1-yl)pyrido [ 1,2-
c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(4-methylpiperazin-1-yl)pyrido [ 1,2-
c]pyrimidine-1,3-dione;
N- { 1-[ 1-(4-Cyclopropyl-7-fluoro-5-methoxy-1, 3-dioxo-2,3-dihydro-1 H
pyrido[ 1,2-c]pyrimidin-6-yl)pyrrolidin-3-yl] ethyl } methanesulfonamide,
4-Cyclopropyl-7-fluoro-5-methoxy-6(octahydropyrrolo[3,4-b]pyridin-5-
yl)pyrido[1,2-c]pyrimidine-1,3-dione;
6-(7-Amino-5-azaspiro[2.4]hept-5-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(thiophen-3-yl)pyrido[I,2-c]pyrimidine-
1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(1-methyl-2,3-dihydro-1H isoindol-5-
yl)pyrido[1,2-c] pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-6-(7-hydroxymethyl-5-azaspiro[2.4]hept-5-y1)-5-
methoxypyridoj 1,2-c]pyrimidine-1,3-dione;
6-(3-Amino-4-methoxyiminopyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
4-cyclopropyl-7-fluoro-5-methoxy-6-(3-methylaminomethylpyrrolidin-1-
yl)pyrido[ 1,2-c]pyrimidine-1,3-dione;
6-(5-Aminomethylthiophen-3-yl)-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Aminoethyl)-3-fluoropyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-(5-Aminomethylthiophen-3-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
62
6-(5-Aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido( 1,2-c]pyrimidine-1,3-dione;
6-(4-Aminomethylthiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-cJpyrimidine-1,3-dione;
6-(4-Aminomethylthiophen-3-yl]-4-cyclopropyl-7-fluoro-5~
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-c]pyrrol-3-yl)-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-6-(5,6-dihydro-4H thieno[2,3-cJpyrrol-2-yl)-7-fluoro-5-
methoxypyridojl,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(6-methyl-5,6-dihydro-4H thieno[2,3-
a]pyrrol-2-yl)pyrido[ 1,2-cJpyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-S-methoxy-6-(6-methyl-5,6-dihydro-4H thieno[2,3-
a]pyrrol-3-yl)pyrido[ 1,2-cJpyrimidine-1,3-dione;
1 S 4-Cyclopropyl-7-fluoro-5-methoxy-6-(S: pyridin-4-ylthiophen-3-
yl)pyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(5-pyridin-3-ylthiophen-3-
yl)pyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(S-pyridin-2-ylthiophen-3-
yl)pyrido[1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(4-pyridin-4-ylthiophen-3-
yl)pyrido[ 1,2-a]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(4-pyridin-3-ylthiophen-3-
yl)pyrido[ 1,2-cJpyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-(4-pyridin-2-ylthiophen-3-
yl)pyrido[ 1,2-a]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-pyridin-4-ylpyrido[ 1,2-c]pyrimidine-
1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
63
6-(4-Aminomethylpyridin-2-yl]-4-cyclopropyl-7-fluoro-5-methoxypyrido [ 1,2-
c]pyrimidine-1,3-dione;
6-(5-Aminomethylpyridin-2-yl]-4-cyclopropyl-7-fluoro-5-methoxypyrido[1,2-
c]pyrimidine-1,3-dione;
6-(6-Aminomethylpyridin-2-yl]-4-cyclopropyl-7-fluoro-S-methoxypyrido[ 1,2-
c]pyrimidiile-1,3-dione;
6-(5-Aminomethylpyrimidin-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-pyrimidin-2-ylpyrido[ 1,2-c]pyrimidine-
1,3-dione;
6-(2-Aminomethylpyrimidin-5-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-7-fluoro-5-methoxy-6-pyrimidin-5-ylpyrido[ 1,2-c]pyrimidine-
1,3-dione;
4-Cyclopropyl-7-fluoro-S-methoxy-6-pyrazin-2-ylpyri do [ 1,2-c]pyrimidine-
1,3-dione;
6-(6-Aminomethylpyrazin-2-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido [ 1,2-c]pyrimidine-1,3-dione;
6-[3-(6-Aminomethylpyridin-2-yl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-(2-Aminomethylpyridin-3-yl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido [ 1,2-c]pyrimidine-1,3-dione;
6-[5-(2-Aminomethylpyridin-3-yl)thiophen-3-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-(S-(2-Aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
6-[5-(6-Aminomethylpyridin-2-yl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[ 1,2-c]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
64
6-[5-(6-Aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[4-(2-Aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-S-
methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-(2-Aminomethylpyridin-3-yl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido [ 1,2-c]pyrimidine-1,3-dione;
6-[4-(6-Aminomethylpyridin-2-yl)thiophen-3-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c] pyrimidine-1,3-dione;
6-[3-( 1-Amino ethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5, 8-
dimethylpyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-methoxy-8-
methylpyrido[ 1,2-cJpyrimidine-I,3-dione;
8-Amino-6-[3-( I-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methylpyrido[ 1,2-aJpyrimidine-1,3-dione;
8-Amino-6-(3-aminomethyl-4-fluoropyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-
S-methylpyrido[1,2-c]pyrimidine-1,3-dione;
6-(3-Aminomethyl-4-fluoropyrrolidin-I-yl)-4-cyclopropyl-7-fluoro-5,8-
dimethylpyrido[ 1,2-a]pyrimidine-1,3-dione;
6-[3-(1-Amino-2,2-difluoroethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5,8-
dimethylpyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-(1-Amino-2,2-difluoroethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxy-8-methylpyrido[ I,2-c]pyrimidine-1,3-dione;
6-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-4-cyclopropyl-
7-fluoro-5-methoxy-8-methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
6-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-4-cyclopropyl-
7-fluoro-5,8-dimethylpyrido[1,2-c]pyrimidine-1,3-dione;
6-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-4-cyclopropyl-
7-fluoro-5-methylpyrido[ 1,2-a]pyrimidine-1,3-dione;
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
4-Cyclopropyl-6-[3-( 1,2-dihydroxyethyl)pyrrol idin-1-yl]-7-fluoro-S-methoxy-
8-methylpyrido[1,2-c]pyrimidine-1,3-dione;
4-Cycl opropyl-6-[3-( 1,2-dihydroxyethyl)pyrrol idin-1-yl]-7-fluoro-5, 8-
dimethylpyrido[ 1,2-c]pyrimidine-1,3-dione;
5 4-Cyclopropyl-6-[3-(1,2-dihydroxyethyl)pyrrolidin-1-yl]-7-fluoro-S-
methylpyrido[ 1,2-c]pyrimidine-1,3-dione;
4-Cyclopropyl-6-[3-( 1,2-dihydroxyethyl)pyrrol idin-1-yl]-7-fluoro-S-
methoxypyrido[ 1,2-a]pyrimidine-1,3-dione;
6-[3-( 1-Amino-2-hydroxyethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
10 methoxypyrido[1,2-c]pyrimidine-1,3-dione;
6-[3-( 1-Amino-2-hydroxyethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-fluoro-5-
methoxy-8-methylpyrido[ 1,2-a]pyrimidine-1,3-dione;
6-[3-( 1-Amino-2-hydroxyethyl)pyrrol idin-1-yl]-4-cyc I opropyl-7-fluoro-5, 8-
dimethylpyrido[ 1,2-c]pyrimidine-1,3-dione;
15 4-Cyclopropyl-7-fluoro-5-methoxy-6-(4-pyridin-4-ylthiophen-2-
yl)pyrido[1,2-c]pyrimidine-1,3-dione; and
6-[4-( 1-Aminoethyl)thiophen-2-yl]-4-cyclopropyl-7-fluoro-5-
methoxypyrido[1,2-c]pyrimidine-1,3-dione; or
a pharmaceutically acceptable salt thereof.
The invention also provides a pharmaceutical composition comprising a
compound of one of the above-mentioned Formulas admixed with a carrier,
diluent,
or excipient.
The invention also provides a method of treating a bacterial infection in a
mammal comprising administering to the mammal in need thereof an antibacterial
effective amount of a compound of one of the above-mentioned Formulas.
The invention also provides a method of inhibiting a bacterial topoisomerase
in a mammal comprising administering to the mammal in need thereof an
effective
amount of a compound of one of the above-mentioned Formulas.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
66
The invention also provides a method of inhibiting a bacterial DNA gyrase in
a mammal comprising administering to the mammal in need thereof an effective
amount of a compound of one of the above-mentioned Formulas.
The invention also provides a method of inhibiting a bacterial topoisomerase
IV in a mammal comprising administering to the mammal in need thereof an
effective
amount of a compound of one of the above-mentioned Formulas.
The invention also provides a method of inhibiting a quinolone-resistant
bacteria in a mammal comprising administering to the mammal an effective
amount
of a compound of one of the above-mentioned Formulas.
The invention also provides a process for preparing a compound of Formula I
wherein R2 is H or NH2 and R6 is C~-C7 alkyl and substituted alkyl,
comprising:
(a) ~ reacting amide 2 with a carbon monoxide equivalent such as phosgene
to form bicyclic compound 3;
Ra Rs O
R4 I %N NH2 "CO" Equivalent _R4 ~ N~NH
R5 K~O R5 ~K ~ O
R R '
6 1
Rs R1 3
(b) coupling an amine to bicyclic compound 3 to form compound C1;
R3 O
R4 i N~NH
3
N H N \~~~0
I I Rs R1
C1
and optionally
(c) aminating bicyclic compound 3 to form compound B1.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
67
R3 O
"amination" R4 / N~N-NH2
C1 N ~K~O
Rs R~
B1
The invention also provides a process for preparing a compound of Formula I
wherein R2 is H or NH2 and R6 is C1-C~ alkoxy and substituted alkoxy,
comprising:
(a) reacting substituted pyridine compound 6 with hydrazine to from
hydrazine derivative 7;
F NH-NH2
F w N NH2-NHS F ~ N
t-Bu ~O ~ / F ' t-Bu ~O I /. F
g OMe 7 OMe
(b) reacting hydrazine derivative 7 with oxygen in the presence of base to
form compound 8;
NH-NHZ F
F I ~ N Oa/Base 'N
t-Bu~O / F t Bu~O / F
OMe
7 OMe 8
(c) reacting compound 8 with the anion of cyclopropyl acetonitrile to form
compound 9;
F ~N
~CN t-Bu,O I / CN
8 Base
O a
9
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
68
(d) converting the nitrile moiety in compound 10 to an amide and
cyclizing according to step a in the previous process.
1 ) H+ F ~ w N
CN
2) "X-,. X
OMe
U
5
(e) converting the nitrite moiety in compound 10 to an amide and
cyclizing as described for step (a) of the previous process.
Detailed Description of the Invention
The following definitions are used, unless otherwise described: "Ph" is
10 phenyl; halo is fluoro, chloro, bromo, or iodo. Alkyl, alkoxy, alkenyl,
alkynyl, etc.
denote both straight and branched groups; but reference to an individual
radical such
as "propyl" embraces only the straight chain radical; a branched chain isomer
such as
"isopropyl" being specifically referred to.
The term "alkyl" means a straight or branched hydrocarbon radical having
from 1 to 7 carbon atoms and includes, for example, methyl, ethyl, n-propyl,
isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-pentyl, n-hexyl, n-
heptyl, and the
like.
The term "C2-C~ alkenyl" means a straight or branched hydrocarbon radical
having from 1 to 3 double bonds. Examples include ethenyl, 2-propen-1-yl, 1,3-
butadien-1-yl, 3-hexen-1-yl, 5-octen-2-yl, 2-isopropyl-3,5-octadien-1-yl, cis-
3-hexen-
1-yl, and trans-2-hepten-1-yl, and the like. Prefer ed alkenyl groups include
C2-C6
alkenyls such as ethenyl, 2-proper-1-yl, 2-buten-1-yl, and 3-penten-1-yl, and
the like.
The term "C2-C~ alkynyl" means a straight or branched hydrocarbon radical
having from 1 to 3 triple-bonds. Examples include ethynyl, propynyl, 3-butyn-1-
yl, 4-
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
69
hexyn-1-yl, and 5-heptyn-3-yl, and the like. Preferred alkynyl groups are C2-
C6
alkynyls such as ethynyl, propynyl, 3-butyn-1-yl, and 5-hexyn-1-yl, and the
like.
The alkyl, alkenyl, and alkynyl groups can be substituted with one or
more groups selected from halo, hydroxy, cyano, C 1-C6 alkoxy, nitro, nitroso,
amino,
C1-C6 alkylamino, di-C1-C6 alkylamino, carboxy, C1-C6 alkoxycarbonyl,
aminocarbonyl, halomethyl, dihalomethyl, trihalomethyl, haloethyl,
dihaloethyl,
trihaloethyl, tetrahaloethyl, pentahaloethyl, thiol, (C1-C4)alkylsulfanyl, (C,-
C4)alkylsulfinyl, and aminosulfonyl, -NH-SOa-NH2, -O-S02-NH-,
N
II
NH-SOa NH2, -NH-C NH2, (Ci-C6)dialkylthio, -NH-S02-R, where R is
(C1-C6)alkyl. Examples of substituted alkyl groups include fluoromethyl,
difluoromethyl, trifluoromethyl, tribromomethyl, hydroxymethyl; 3-
methoxypropyl,
3-carboxypentyl, 3,5-dibromo-6-aminocarbonyldecyl, and 4-ethylsulfinyloctyl.
Examples of substituted alkenyl groups include 2-bromoethenyl, 1-amino-2-
propen-
1-yl, 3-hydroxypent-2-en-1-yl, 4-methoxycarbonyl-hex-2-en-1-yl, and 2-nitro-3-
bromo-4-iodo-oct-5-en-1-yl. Typical substituted alkynyl groups include 2-
hydroxyethynyl, 3-dimethylamino-hex-5-yn-1-yl, and 2~-cyano-kept-3-yn-1-yl.
The term "cycloalkyl" means a hydrocarbon ring containing from 3 to
12 carbon atoms, for example, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
cycloheptyl, cycloctyl, decalinyl, norpinanyl, and adamantyl. Where possible,
the
cycloalkyl group may contain double bonds, for example, 3-cyclohexen-1-yl. The
cycloalkyl ring may be unsubstituted or substituted by one or more
substituents
selected from alkyl, alkoxy, thioalkoxy, hydroxy, thiol, nitro, halogen,
amino, alkyl
and dialkylamino, formyl, carboxyl, CN, -NH-CO-R, -CO-NHR, -C02R, -COR,
wherein R is defined as above, aryl, heteroaryl, wherein alkyl, aryl, and
heteroaryl are
as defined herein, or as indicated above for alkyl, alkenyl, and alkynyl
substitutents.
Examples of substituted cycloalkyl groups include fluorocyclopropyl, 2-
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
iodocyclobutyl, 2,3-dimethylcyclopentyl, 2,2-dimethoxycyclohexyl, and 3-
phenylcyclopentyl.
The term "heterocyclic" means a monocyclic, fused, bridged, or spiro bicyclic
heterocyclic ring systems. Monocyclic heterocyclic rings contain from about 3
to
5 12 ring atoms, with from 1 to 5 heteroatoms selected from N, O, and S, and
preferably from 3 to 7 member atoms, in the ring. Bicyclic heterocyclics
contain from
about 5 to about 17 ring atoms, preferably from S to 12 ring atoms. Bicyclic
heterocyclic rings may be fused, spiro, or bridged ring systems. Examples of
heterocyclic groups include cyclic ethers (oxiranes) such as ethyleneoxide,
10 tetrahydrofuran, dioxane, and substituted cyclic ethers, wherein the
substituents are
those described above for the alkyl and cycloalkyl groups. Typical substituted
cyclic
ethers include propyleneoxide, phenyloxirane (styrene oxide), cis-2-butene-
oxide
(2,3-dimethyloxirane), 3-chlorotetrahydrofuran, 2,6-dimethyl-1,4-dioxane, and
the
like. Heterocycles containing nitrogen are groups such as pyrrolidine,
piperidine,
TS piperazine, tetrahydrotriazine, tetrahydropyrazole, and substituted groups
such as 3-
aminopyrrolidine, 4-methylpiperazin-1-yl, and the like. Typical sulfur
containing
heterocycles include tetrahydrothiophene, dihydro-1,3-dithiol-2-yl, and
hexahydrothiophen-4-yl and substituted groups such as aminomethyl thiophene.
Other commonly employed heterocycles include dihydro-oxathiol-4-yl, dihydro-1H
20 isoindole, tetrahydro-oxazolyl, tetrahydro-oxadiazolyl,
tetrahydrodioxazolyl,
tetrahydrooxathiazolyl, hexahydrotriazinyl, tetrahydro-oxazinyl, morpholinyl,
thiomorpholinyl, tetrahydropyrimidinyl, dioxolinyl, octahydrobenzofuranyl,
octahydrobenzimidazolyl, and octahydrobenzothiazolyl. For heterocycles
containing
sulfur, the oxidized sulfur heterocycles containing SO or S02 groups are also
25 included. Examples include the sulfoxide and sulfone forms of
tetrahydrothiophene.
The term "aryl" means a cyclic or polycyclic aromatic ring having from 5 to
12 carbon atoms, and being unsubstituted or substituted with one or more of
the
substituent groups recited above for alkyl, alkenyl, and alkynyl groups.
Examples of
aryl groups include phenyl, 2,6-dichlorophenyl, 3-methoxyphenyl, naphthyl,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
71
4-thionaphthyl, tetralinyl, anthracinyl, phenanthrenyl, benzonaphthenyl,
fluorenyl, 2-
acetamidofluoren-9-yl, and 4'-bromobiphenyl.
The term "heteroaryl" means an aromatic cyclic or polycyclic ring system
having from 1 to 4 heteroatoms selected from N, O, and S. Typical heteroaryl
groups
include 2- or 3-thienyl, 2- or 3-furanyl, 2- or 3-pyrrolyl, 2-, 4-, or 5-
imidazolyl, 3-, 4-,
or S-pyrazolyl, 2-, 4-, or 5-thiazolyl, 3-, 4-, or S-isothiazolyl, 2-, 4-, or
5-oxazolyl, 3-,
4-, or 5-isoxazolyl, 3- or 5-1,2,4-triazolyl, 4- or S-1,2,3-triazolyl,
tetrazolyl, 2-, 3-; or
4-pyridinyl, 3-, 4-, or 5-pyridazinyl, 2-pyrazinyl, 2-, 4-, or S-pyrimidinyl,
2-, 3-, 4-,
5-, 6-, 7-, or 8-quinolinyl, 1-, 3-, 4-, 5-, 6-, 7-, or 8-isoquinolinyl, 2-, 3-
, 4-, S-, 6-, or
7-indolyl, 2-, 3-, 4-, 5-, b-, or 7-benzo[b]thienyl, 2-, 4-, S-, 6-, or 7-
benzoxazolyl, 2-,
4-, 5-, 6-, or 7-benzimidazolyl, 2-, 4-, 5-, 6-, or 7-benzothiazolyl. The
heteroaryl
groups may be unsubstituted or substituted by 1 to 3 substituents selected
from those
described above for alkyl, alkenyl, and alkynyl, for example, cyanothienyl and
formylpyrrolyl.
Preferred aromatic fused heterocyclic rings of from 8 to 10 atoms include but
are not limited to 2-, 3-, 4-, 5-, 6-, 7-, or 8-quinolinyl, 1-, 3-, 4-, 5-, 6-
, 7-, or
8-isoquinolinyl, 2-, 3-, 4-, 5-, 6-, or 7-indolyl, 2-, 3-, 4-, S-, 6-, or 7-
benzo[b]thienyl,
2-, 4-, 5-, 6-, or 7-benzoxazolyl, 2-, 4-, 5-, 6-, or 7-benzimidazolyl, 2-, 4-
, 5-, 6-, or
7-benzothiazolyl. Heteroaryl also includes 2- and 3- aminomethylfixran, 2- and
3-
aminomethylthiophene and the like.
It will be appreciated by those skilled in the art that compounds of the
invention having one or more chiral centers may exist in and be isolated in
optically
active and racemic forms. Some compounds may exhibit polymorphism. It is to be
understood that the present invention encompasses any racemic, optically-
active,
polymorphic, geometric, or stereoisomeric form, or mixtures thereof, of a
compound
of the invention, which possess the useful properties described herein, it
being well
known in the art how to prepare optically active forms (for example, by
resolution of
the racemic form by recrystallization techniques, by synthesis from optically-
active
starting materials, by chiral synthesis, or by chromatographic separation
using a chiral
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
72
stationary phase) and how to determine activity or cytotoxicity using the
standard
tests described herein, or using other similar tests which are well known in
the art.
A "prodrug" is an inactive derivative of a drug molecule that requires
a chemical or an enzymatic biotransformation in order to release the active
parent
drug in the body.
Specific and preferred values for compounds of Formula I are listed below for
radicals, substituents, and ranges are for illustration purposes only, and
they do not
exclude other defined values or other values within defined ranges for the
radicals
and substituents.
A specific value for J is C. Another specific value for J is N.
A specific value for K is C. Another specific value for K. is N.
A specific value for R1 is methyl. Another specific value for R1 is ethyl,
isopropyl, cyclopropyl, t-butyl, 2-fluorocyclopropyl, 1- or 2-
methylcyclopropyl,
cyclopropylmethyl, vinyl, phenyl or substituted phenyl, heteroaryl or
substituted
heteroaryl.
A specific value for RZ is H. Another specific value for R2 is OH. Another
specific value for R~ is NH2. Another specific value for R2 is C1-C~ alkyl and
A
substituted alkyl, C2-C~ alkenyl and substituted alkenyl, Ca-C~ alkynyl and
substituted alkynyl, C3-C~ cycloalkyl and substituted cycloalkyl, aryl and
substituted
aryl, heterocyclic and substituted heterocyclic, heteroaryl and substituted
heteroaryl,
halo, N02, NO, CN, ORa, -COaRa, wherein Ra is H, C 1-C~ alkyl and substituted
alkyl, C1-C~ alkenyl and substituted alkenyl, C3-C~ cycloalkyl and substituted
cycloalkyl, aryl and substituted aryl, heteroaryl and substituted heteroaryl,
O
heterocycloalkyl and substituted heterocycloalkyl; -COaRb, -C-SRb, O=C-SRb,
-CORb, wherein Re is H, C1-C~ alkyl and substituted alkyl, C1-C~ alkenyl and
substituted alkenyl, C3-C~ cycloalkyl and substituted cycloalkyl, aryl and
substituted
aryl, heteroaryl and substituted heteroaryl, heterocycloalkyl and substituted
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
73
heterocycloalkyl; -C02NR~Ra, wherein R~ and Rd are independently H, C1-C~
alkyl
and substituted alkyl, C1-C~ alkenyl and substituted alkenyl, C3-C~ cycloalkyl
and
substituted cycloalkyl, aryl and substituted aryl, heteroaryl and substituted
heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl; NReRf, wherein Re and Rf
are each
independently H, C1-C~ alkyl and substituted alkyl, C3-C~ cycloalkyl and
substituted
O
cycloalkyl, aryl and substituted aryl, or -CO2Rb, -C-SRb ~ ~ORb, "vherein Rb
is
defined as above; -CONR~Ra, wherein R~ and Rd are defined as above; aryl and
substituted aryl, heteroaryl and substituted heteroaryl, heterocycloalkyl and
substituted heterocycloalkyl, or R~ and Rf are taken together with the
nitrogen to
which they are attached to form a 3 to 7 membered ring, said ring being
substituted or
unsubstituted with l, 2, 3, or 4 substitutent groups.
A specific value for each of R3, R4, and R6 is H, OH, (O)nCl-C7 alkyl and
substituted alkyl, (O)nC2-C~ alkenyl and substituted alkenyl, (O)nC2-C7
alkynyl arid
substituted alkynyl, wherein n is 0 or 1; halo, N02, CN, NRsR;,, wherein Rg
and R;,
independently are H, C1-C~ alkyl and substituted alkyl, C2-C~ alkenyl and
substituted alkenyl, C2-C~ alkynyl and substituted alkynyl, -CO-C1-C7 alkyl
and
substituted alkyl, or Rg and R;, taken together with the nitrogen to which
they are
attached form a 3- to 7-membered ring containing from 1 to 3 heteroatoms
selected
from N, O, and S, said ring being unsubstituted or substituted with 1, 2, 3,
or 4
substituent groups. ,
A specific value for RS is C 1-C~ alkyl and substituted alkyl, C2-C~ alkenyl
and substituted alkenyl, C2-C~ alkynyl and substituted alkynyl, -COZRa,
wherein Ra
0
is defined as above, -CO2Rb, -C-SRb, -CORb, wherein Rb is defined as above,
-CONR.~Ra, wherein R~ and Rd are defined as above; halo, N02, CN, NR;R~,
wherein R; and R~ independently are H, C1-C~ alkyl and substituted alkyl, C2-
C~
alkenyl and substituted alkenyl, Ca-C~ alkynyl and substituted alkynyl, CO-C1-
C~
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
74
alkyl and substituted alkyl, or R; and R~ taken together with the nitrogen to
which they
are attached form a 3- to 7-membered ring containing from I to 3 heteroatoms
selected from N, O, and S, said ring being unsubstituted or substituted with
1, 2, 3, or
4 substituent groups; aryl, fused aryl, heterocyclic, fused heterocyclic,
bicyclic
heterocyclic, or spiro heterocyclic, wherein fused aryl, fused heterocyclic,
bicyclic
heterocyclic, or spiro heterocyclic can be substituted.
Further examples of typical heterocycles, fused bicyclic or spiro
heterocycles,
and heteroaryl groups that are specific values for RS are listed below in
Table I . In
Table I, "~" indicates the point of attachment.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
Table 1
H
H~ HN N
N~~~ ~~_~N~~~ N."", Nr~N~~r Me-N~N~~~
H2N
HN'~N HN'\~N~ HNI~~N~" ~N~
Me0lN
. . HaN ~ ~ HaN ~ ~.
HaN -~~N~ H N~~~UN~ N N
a Me
Me.N /~N Me~N '~-~
H~N~~~ H~N,~~ H~N~~~ HaN ~~N~~~
Me Me Me Me M 'tee
Me~N ~N
HaN N~," HaN N.....' H N~ H N~M
a
H
H2N HaN N
~N~ ~~~N,"", HaN Me N""" ~~N""" HN'~~N."".
M ~e
t~ HZN
I~ N~~' ~ ~N~",. HZN , H N
HN~ HN a N
N'M N."",
Ha
H2N~~~N."". N N...... ~ ~N"". ~N.".,. N."",
L~ HN HZ ~ ~/N
N
HO HO ~ I HO'~N O~ ~N.".,.
~N~ ~N~ NW . ~N~ n= 0, 1, 2
HaN Me
N."". H2N ~N~~~ HN~N~~~ HN 'N"",. HN~N~~~
M/ M
H2N H N Ph NH2 ~ NHa H N
~N."". 2 N~ a
N~ Fs0 N~ Me N."".
Me
HzN
F F NH2 F OH
F,~\~N."".
.~/ F ~N~~r F
S
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
76
Me H2N
H2N H2N ~ HzN
~ HZN ~~ N."". ~N~~~ N~",.
~N~~~ N~~~
Me Me Me Me
Me Me
Me0 HO O HzN HzN Me
H2N H2N N."". N."". HzN ~N~~,.
N~ N~~
Me
OMe MeF Me0 F H N
z
HzN~N~ HzN N HzN N~ HzN ~ N."".
N F
Me HZN H2N
HzN~~~N''"" HZN N'~' HzN/~~N'""' ~N'""' ~N""''
F3C Me0 Me0 F
HzN ~ NHz H N'~N"'~ Me Me
z CN~~~ HO
~N N"". 0J N""..
H2N Me Me
HzN/~N~ EtHN
N"~'' MeHN H2 ~/N
N."". ~N-"". N"""
N~''~ HO~~N,~,~" ~ HzN Me H2N
HzN ~~,!~ H N N"""
HO z N"". N~ F~~,
Me
HzN N HzN ~N"~ HzN HzN N~",. HzN N."".
""" N."".
F~V~ F
F F F F F
Et0 O H2N O Me Me
HzN HzN HN ~ \ H2N ~ \ H N
~N~ ~N~ / , z
HzN S~ ~ ~ ~~ N %"""'
H2N N N H2N N O~N
Me
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
77
F
H2N
H2N N."". H2N H2N ~N N-"".
vN ."". F
v
_ S H2N \
S \ I / I I ~ N N"",.
S
H
I \ N \ I N~ I ~ I
N
~ N N."". N."", N."", N"".
H2N
S i
S
Me ~ I \ I H2N ~N I N ~ I
HN~ HN ~N",~ ~N~
Me H2 ~~'N
S S I S \
Me
H2N Me~
NH2 Me NH2
N F3C F
F F H_O FsC F
H'N H~N H_ H~N H_N N,"".
H N."", H ~N."". N ~N~ H N""" H
~H
F F NH2 NH2
OH F F I
H,O N H_N ~S S I \
~"" H
'N NH2
In compounds of Formula 1, a preferred value for J is C. Another preferred
$ value for J is N. A preferred value for K is C. Another preferred value for
K is N. A
preferred value for R1 is cyclopropyl. Another preferred value for Rl is 2-
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
78
fluorocyclopropyl, 1- or 2-methylcyclopropyl, or cyclopropylmethyl. A
preferred
value for R2 is NH2. Another preferred value for R~ is H. Another preferred
value for
R2 is H. A preferred value for R3 is H. Another preferred value for R3 is
methyl.
Another preferred value for R3 is methoxy. Another preferred value for R3 is
NHZ. A
preferred value for R4 when J is C is F. Another preferred value for R4 when J
is C is
Cl. A preferred value for RS is 1-pyrrolidinyl or substituted 1-pyrrolidinyl.
Another
preferred value for RS is 1-piperidinyl or substituted 1-piperidinyl, or 1-
piperizinyl or
substituted 1-piperizinyl. Other preferred values for RS include heterocycles
and
heteroaryl groups such as those known in the quinolone art, for instance, as
found in
J. Med. Chem., 1992;35:1764; J. Med. Chem., 1996;39:3070; Synlett.,1996:1097;
and
.I. Med. Chem., 1986;29:445. A preferred value for R6 when K is C is H.
Another
preferred value for R6 when K is C is C,-C4 alkyl and substituted alkyl, halo,
OH, or
-O-C1-C4 alkyl and substituted -0-C1-C4 alkyl, OCF3, OCHFZ, OCHzF, OCH2CF3,
OCH2CHFa, or OCH2CHaF.
A preferred group of compounds of Formula I are compounds wherein J and
K are C; R1 is methyl, ethyl, cyclopropyl, t-butyl, 2-fluorocyclopropyl; R2 is
H, OH,
or NHZ; R3 is H; R4 is F or Cl; RS is 1-pyrrolidinyl or substituted 1-
pyrrolidinyl, 1-
piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or substituted 1-
piperizinyI or
NH2
and R6 is F, Cl, methyl, methoxy, OCF3, OCHF2, OCHaF, OCH2CF3,
OCH2CHF2, or OCHzCH2F.
Another preferred group of compounds of Formula I are compounds wherein J
is N, K is C; R1 is cyclopropyl; R~ is H or NH2; R3 is H; RS is 1-pyrrolidinyl
or
substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-
piperizinyl or
substituted I-piperizinyl; and R6 is methyl, methoxy, OCF3, OCHFa, OCH2F,
OCHzCF3, OCH2CHF2, or OCHaCH2F.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
79
Another preferred group of compounds of Formula I are compounds wherein J
is C; K is N; R1 is cyclopropyl; RZ is H or NHZ; R3 is H; R4 is F or Cl; and
RS is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-
piperidinyl, 1-
piperizinyl or substituted 1-piperizinyl.
S Another preferred group of compounds of Formula I are compounds wherein J
is N; K is N; R1 is cyclopropyl; R2 is H or NH2; R3 is H; and RS is 1-
pyrrolidinyl or
substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-
piperizinyl or
substituted 1-piperizinyl.
Representative compounds of the invention which are compounds of Formula
1 are shown below in Table 2-I.
Table 2-I
O
F r N~N.NH2 H2
~N ~ ~ O Me H
w
M MeHN
Example 4g Example 4f
O O
F , N.~N.NH~ F , N~N,NHZ
Me N ~ ~ O Me N ~ ~ O
w
HO~~ ~ H2N Me
Me U
Example 4h Me Example 5c
Representative compounds of the present invention, which are encompassed
1 S by Formula I include, but are not limited to the compounds in Table 2-I
and their
pharmaceutically acceptable acid or base addition salts, or amide or prodrugs
thereof.
In compounds of Formula II, a preferred value for R1 is cyclopropyl. Another
preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-methylcyclopropyl, or
cyclopropylmethyl. A preferred value for RZ is NHZ. Another preferred value
for R2 is
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
H. Another preferred value for RZ is OH. A preferred value for R3 is H.
Another
preferred value for R3 is methyl. Another preferred value for R3 is methoxy.
Another preferred value for R3 is NH2. A preferred value for Rq, is F. Another
preferred value for R4 is Cl. A preferred value for RS is 1-pyrrolidinyl or
substituted
5 1-pyrrolidinyl. Another preferred value for RS is 1-piperidinyl or
substituted 1-
piperidinyl, or 1-piperizinyl or substituted 1-piperizinyl. Other preferred
values for
NH2
RS include heterocycles and heteroaryl groups such as S , or such as those
known in the quinolone art, for instance, as described above for compounds of
Formula I. A preferred value for R6 is H. Another preferred value for R6 is C,-
C4
10 alkyl and substituted alkyl, halo, OH, or -O-C I -C4 alkyl and substituted -
O-C 1-C4
alkyl, OCF3, OCHFa, OCHZF, OCHaCF3, OCHzCHFa, or OCH2CHZF.
A preferred group of compounds of Formula II are compounds wherein Rl is
cyclopropyl; RZ is H, OH or NH2; R3 is H; R4 is F or Cl; RS is 1-pyrrolidinyl
or
substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-
piperizinyl or
NH2
15 substituted 1-piperizinyl or S ; and R6 is F, Cl, methyl, methoxy, OCF3,
OCHF2, OCH2F, OCHaCF3, OCHaCHFa, or OCH2CHaF.
Another preferred group of compounds of Formula II are compounds wherein
Rl is cyclopropyl; R2 is H or NH2; R3 is H; R~. is F; RS is 1-pyrrolidinyl or
substituted I-pyrrolidinyl, 1-piperidinyl or substituted I-piperidinyI; and R6
is
20 methyl, methoxy, OCF3, OCHFZ, OCH2F, OCH2CF3, OCH2CHFa, or OCH2CHaF.
Another preferred group of compounds of Formula II are compounds wherein
Rl is cyclopropyl; R2 is H or NHZ; R3 is H; R4 is F; RS is 1-pyrrolidinyl or
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
81
substituted 1-pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl; and
R6 is
methyl, methoxy, OCF3, OCHF2, OCHZF, OCHaCF3, or OCHZCHFa.
Another preferred group of compounds of Formula II are compounds wherein
RI is cyclopropyl; R2 is H or NH2; R3 is H; R4 is F; RS is 1-pyrrolidinyl or
S substituted 1-pyrrolidinyl, 1-piperidinyl or substituted I-piperidinyl, 1-
piperizinyl or
substituted 1-piperizinyl; and R6 is methyl, methoxy, OCF3, OCHF2, OCHaF, or
OCHaCF3.
Representative compounds of the invention which are compounds of Formula
1 are also shown in Table 2-I.
In compounds of Formula III, a preferred value for R1 is cyclopropyl. Another
preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-methylcyclopropyl, or
cyclopropylmethyl. A preferred value for RZ is NH2. Another preferred value
for Ra
is H. Another preferred value for R2 is OH. A preferred value for R4 is F.
Another
I S preferred value for Rq, is CI. A preferred value for RS is 1-pyrrolidinyl
or substituted
1-pyrrolidinyl. Another preferred value for RS is 1-piperidinyl or substituted
1-
piperidinyl, or 1-piperizinyl or substituted 1-piperizinyl. Other preferred
values for
NN2
RS include heterocycles and heteroaryl groups such as ~ , or such as those
known in the quinolone art, for instance, as described above for compounds of
~ Formula I.
A preferred group of compounds of Formula III are compounds wherein RI is
cyclopropyl; R2 is H, OH, or NH2; R3 is H; R4 is F or Cl; and RS is 1-
pyrrolidinyl or
substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, I-
piperizinyl or
NH2
substituted 1-piperizinyl, or
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
82
Another preferred group of compounds of Formula III are compounds wherein
Rl is cyclopropyl; R2 is H OH, or NHZ; R3 is H; Rq. is F; and RS is 1-
pyrrolidinyl or
substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-
piperizinyl or
substituted 1-piperizinyl.
Another preferred group of compounds of Formula III are compounds wherein
Rl is cyclopropyl; R2 is H or NHa; R3 is H; Rq. is F; and RS is 1-pyrrolidinyl
or
substituted 1-pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl.
Another preferred group of compounds of Formula III are compounds wherein
Rl is cyclopropyl; R2 is H or NHZ; R3 is H; R4 is F; and RS is 1-pyrrolidinyl
or
substituted 1-pyrrolidinyl.
Representative compounds of the invention which are compounds of Formula
III are shown below in Table 2-III.
Table 2-III
O O
F ~, N~N.NH2 F / N~N,NH2
Me H
~N N ~O ~~N N O
MeHN
III-a III-b
O O
F , N~N.NH2 F , N~N.NH~
Me ~ ~ Me
~~N N ~O N N 0
HO ~ H2N MM~
III-c III-d
Representative compounds of the present invention, which are encompassed
by Formula III include, but are not limited to the compounds in Table 2-III
and their
pharmaceutically acceptable acid or base addition salts, or amide or prodrugs
thereof.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
83 .
In compounds of Formula IV, a preferred value for R1 is cyclopropyl. Another
preferred value for Rl is 2-fluorocyclopropyl, 1- or 2-methylcyclopropyl, or
cyclopropylmethyl. A preferred value for Rz is NHz. Another preferred value
for Rz is
H. Another preferred value for R2 is OH. A preferred value for RS is 1-
pyrrolidinyl
or substituted 1-pyrrolidinyl. Another preferred value for R5 is 1-piperidinyl
or
substituted 1-piperidinyl, or 1-piperizinyl or substituted 1-piperizinyl.
Other preferred
NH2
values for RS include heterocycles and heteroaryl groups such as S , or
such as those known in the quinolone art, for instance, as described above for
compounds of Formula I. A preferred value for R6 is H. Another preferred value
for
Rg is Ci-C4 alkyl and substituted alkyl, halo, OH, or -O-C1-Cq. alkyl and
substituted
-O-C1-Cq. alkyl, OCF3, OCHFz, OCHzF, OCHzCF3, OCHzCHFz, or OCH2CHzF.
A preferred group of compounds of Formula IV are compounds wherein R1 is
cyclopropyl; Rz is H, OH, or NHz; RS is 1-pyrrolidinyl or substituted 1-
pyrrolidinyl,
1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or substituted 1-
piperizinyl or
NH2
I ~ ; and Rg is F, Cl, methyl, methoxy, OCF3, OCHFz, OCHzF, OCHzCF3,
OCHzCHFz, or OCHzCH2F.
Another preferred group of compounds of Formula IV are compounds
wherein Rl is cyclopropyl; Rz is H or NHz; R$ is 1-pyrrolidinyl or substituted
1-
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted 1-
piperizinyl; and Rg is methyl, methoxy, OCFs, OCHFz, OCHZF, OCH2CF3,
OCHzCHFz, or OCHzCH2F.
Another preferred group of compounds of Formula IV are compounds
wherein Rl is cyclopropyl; Rz is H, OH, or NHz; and RS is 1-pyrrolidinyl or
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
84
substituted 1-pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl; and
R6 is
methyl, methoxy, OCF3, OCHF2, OCH2F, OCHZCF3, OCH2CHF2, or OCH2CHZF
Another preferred group of compounds of Formula IV are compounds
wherein R1 is cyclopropyl; Ra is H or NH2; RS is 1-pyrrolidinyl or substituted
1-
pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl and R6 is methyl,
methoxy,
OCF3, OCHF2, OCH2F, or OCHZCF3.
Representative compounds of the invention which are compounds of Formula
1 are shown below in Table 2-IV.
Table 2-IV
O O
N~N~N.NH2 N~N~N~NH~
~N w w O Me H N w W O
w
MeHN~~ Me
IV-a IV-b
OII O
N~N~N.NH2 ~ N~N~NH~
Me N w w O Me N w w O
w
HO~~ Me H2N Me
U MMe
IV-c IV-d
Representative compounds of the present invention, which are encompassed
by Formula IV include, but are not limited to the compounds in Table 2-IV and
their
pharmaceutically acceptable acid or base addition salts, or amide or prodrugs
thereof.
In compounds of Formula V, a preferred value for R1 is cyclopropyl. Another
preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-methylcyclopropyl, or
cyclopropylmethyl. A preferred value for RZ is NH2. Another preferred value
for Ra is
H, Another preferred value for Rz is OH, A preferred value for RS is 1-
pyrrolidinyl
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
or substituted 1-pyrrolidinyl. Another preferred value for RS is 1-piperidinyl
or
substituted 1-piperidinyl, or 1-piperizinyl or substituted 1-piperizinyl.
Other preferred
NH2
values for RS include heterocycles and heteroaryl groups such as S , or
such as those known in the quinolone art, for instance, as described above for
S compounds of Formula I.
A preferred group of compounds of Formula V are compounds wherein R1 is
cyclopropyl; Ra is H, OH, or NH2; and RS is 1-pyrrolidinyl or substituted 1-
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted 1-
NH2
piperizinyl, or
10 Another preferred group of compounds of Formula V are compounds wherein
R1 is cyclopropyl; R2 is H or NHZ; and RS is 1-pyrrolidinyl or substituted 1
pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl.
Another preferred group of compounds of Formula V are compounds wherein
Rl is cyclopropyl; R2 is H or NH2; and RS is 1-pyrrolidinyl or substituted 1-
15 pyrrolidinyl.
Another preferred group of compounds of Formula V are compounds wherein
R1 is cyclopropyl; Ra is H or NH2; and RS is substituted 1-pyrrolidinyl.
Representative compounds of the invention which are compounds of Formula
V are shown below in Table 2-V.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
86
Table 2-V
O O
NnN~N.NH2 N~N~N'NH2
s
N~N ~ 0 Me H N~N ~ 0
MeHN
c~
V-a V-b
O'' O
N~N.~N.NH2 N~N~N.NH2
Me N~N ~ O Me N~N ~ 0
w
HO~~ HZN
MMe
V-c V-d
Representative compounds of the present invention, which are encompassed
S by Formula IV include, but are not limited to the compounds in Table 2-V and
their
pharmaceutically acceptable acid or base addition salts, or amide or p rodrugs
thereof.
In compounds of Formula VI, a preferred value for R1 is cyclopropyl. Another
preferred value for Rl is 2-fluorocyclopropyl, 1- or 2-methylcyclopropyl, or
cyclopropylmethyl. A preferred value for RZ is NHz. Another preferred value
for RZ is
H. Another preferred value for R2 is OH. A preferred value for R3 is H.
Another
preferred value for R3 is methyl. Another preferred value for R3 is methoxy.
Another preferred value for R3 is NHa. A preferred value for R4 is F. Another
preferred value for R4 is Cl. A preferred value for Rg and Rh, together with
the
nitrogen to which they are attached, is 1-pyrrolidinyl or substituted 1-
pyrrolidinyl.
Another preferred value for Rg and Rh, together with the nitrogen to which
they are
attached, is 1-piperidinyl or substituted 1-piperidinyl, or 1-piperizinyl or
substituted
1-piperizinyl. Other preferred values for Rg and Rh, together with the
nitrogen to
which they are attached, include heterocycles and heteroaryl groups such as
those
known in the quinolone art, for instance, as described above for compounds of
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
87
Formula I. A preferred value for R6 is H. Another preferred value for R6 is C1-
C4
alkyl and substituted alkyl, halo, OH, or -O-Cl-Cq. alkyl and substituted -O-
C1-Cq.
alkyl, OCF3, OCHFa, OCH2F, OCH2CF3, OCHzCHF2, or OCHzCHZR
A preferred group of compounds of Formula VI are compounds wherein R1 is
cyclopropyl; R2 is H, OH, or NHa; R3 is H; R4 is F or Cl; Rg and Rh, together
with
the nitrogen to which they are attached, are 1-pyrrolidinyl or substituted 1
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted 1-
piperizinyl; and R6 is F, Cl, methyl, methoxy, OCF3, OCHF2, OCH2F, OCH2CF3,
OCH2CHF2, or OCH2CHZR
Another preferred group of compounds of Formula VI are compounds
wherein R1 is cyclopropyl; R2 is H or NHa; R3 is H; R4 is F; Rg and Rh,
together
with the nitrogen to which they are attached, are 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted 1-
piperizinyl; and R6 is methyl, methoxy, OCF3, OCHF2, OCHaF, OCHZCF3,
OCH2CHF2, or OCHaCH2F.
Another preferred group of compounds of Formula VI are compounds
wherein Rl is cyclopropyl; RZ is H or NHZ; R3 is H; R4 is F; Rg and Rh,
together
with the nitrogen to which they are attached, are 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl; and R6 is methyl,
methoxy,
OCF3, OCHFZ, OCH2F, OCH2CF3, OCHZCHFa, or OCH2CH2F.
Another preferred group of compounds of Formula VI are compounds
wherein Rl is cyclopropyl; R2 is H or NH2; R3 is H; R4 is F; Rg and Rh,
together
with the nitrogen to which they are attached, are 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl; and R6 is methyl,
methoxy,
2S OCF3, OCHF2, OCH2F, OCH2CF3, or OCH2CHF2.
Representative compounds of the invention which are compounds of Formula
VI are shown in Table 2-I.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
88
In compounds of Formula VII, a preferred value for R1 is cyclopropyl.
Another preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-
methylcyclopropyl, or
cyclopropylmethyl. A preferred value for R2 is NH2. Another preferred value
for R2 is
H. Another preferred value for Ra is OH. A preferred value for R3 is H.
Another
preferred value for R3 is methyl. Another preferred value for R3 is methoxy.
Another preferred value for R3 is NH2. A preferred value for R4 is R Another
preferred value for R4 is Cl. A preferred value for Rg and Rh, together with
the
nitrogen to which they are attached, is 1-pyrrolidinyl or substituted 1-
pyrrolidinyl.
Another preferred value for Rg and Rh, together with the nitrogen to which
they are
attached, is 1-piperidinyl or substituted 1-piperidinyl, or 1-piperizinyl or
substituted
1-piperizinyl. Other preferred values for Rg and Rh, together with the
nitrogen to
which they are attached, include heterocycles and heteroaryl groups such as
those
known in the quinolone art, for instance, as described above for compounds of
Formula I.
A preferred group of compounds of Formula VII are compounds wherein R1
is cyclopropyl; RZ is H, OH, or NHZ; R3 is H; Rq, is F; and Rg and Rh,
together with
the nitrogen to which they are attached, are 1-pyrrolidinyl or substituted 1-
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted 1-
piperi.zinyl.
Another preferred group of compounds of Formula VII are compounds
wherein R1 is cyclopropyl; R2 is H; OH, or NHZ; R3 is H; Rq. is F; and Rg and
Rh,
together with the nitrogen to which they are attached, are 1-pyrrolidinyl or
substituted
1-pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl.
Another preferred group of compounds of Formula VII are compounds
wherein R1 is cyclopropyl; R2 is H or NHa; R3 is H; R4 is F; and Rg and Rh,
together
with the nitrogen to which they are attached, are 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
89
Another preferred group of compounds of Formula VII are compounds
wherein R1 is cyclopropyl; Ra is H or NH2; R3 is H; Rq. is F; Rg and Rh,
together
with the nitrogen to which they are attached, are substituted 1-pyrrolidinyl.
Representative compounds of the invention which are compounds of Formula
VI are shown in Table 2-III.
In compounds of Formula VIII, a preferred value for m is 0 or 1. A preferred
value for X is O. Another prefered value for X is CH2 or CH(C~-C7 alkyl). A
preferred value for Y is CHa or CH(Cl-C7 alkyl). Another preferred value for Y
is NH
or N(C1-C7 alkyl). A preferred value for R; is C1-C7 alkyl. A preferred value
for R~ is
NHZ. Another preferred value for R2 is H. Another preferred value for Ra is
OH. A
preferred value for R3 is H. Another preferred value for R3 is methyl. Another
preferred value for R~ is methoxy: Another preferred value for R3 is NH2. A
preferred value for R4 is F. Another preferred value for R4 is Cl. A preferred
value
for RS is 1-pyrrolidinyl or substituted 1-pyrrolidinyl. Another preferred
value for RS
is 1-piperidinyl or substituted 1-piperidinyl, or 1-piperizinyl or substituted
1-
piperizinyl. Other preferred values for RS include heterocycles and heteroaryl
groups
NHS
such as S , or such as those known in the quinolone art, for instance, as
described above for compounds of Formula I.
A preferred group of compounds of Formula VIII are compounds wherein m
is 1; X is O or CH2; Y is CH2, CH(CI-C7 alkyl), NH, or N(C,-C7 alkyl); R~ is
methyl;
R2 is H, OH, or NH2; R3 is H; Rq, is F or Cl; and RS is 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted 1-
piperizinyl.
Another preferred group of compounds of Formula VIII are compounds
wherein X is O or CHZ; Y is CHa, CH(C,-C7 alkyl), NH, or N(C1-C7 alkyl); R~ is
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
methyl; R2 is H or NH2; R3 is H; and RS is 1-pyrrolidinyl or substituted 1-
NH2
pyrrolidinyl, or I-piperidinyl or substituted 1-piperidinyl, or S
Another preferred group of compounds of Formula VIII are compounds
wherein X is O or CH2; Y is CH2, CH(C1-C7 alkyl), NH, or N(C1-C7 alkyl); R~ is
5 methyl; RZ is H or NH2; R3 is H; R4 is F or Cl; and RS is 1-pyrrolidinyl or
substituted
1-pyrrolidinyl.
Another preferred group of compounds of Formula VIII are compounds
wherein X is O or CHa; Y is CHa, CH(C1-C7 alkyl), NH, or N(C~-C7 alkyl); R~ is
methyl; R2 is H or NHZ; R3 is H; and RS is substituted I-pyrrolidinyl.
10 Representative compounds of the invention which are compounds of Formula
VIII are shown below in Table 2-VIII.
Table 2-VIII
O O
N~N.NH2 ~ / N~N.NHZ
N ~ ~ 0 Me H N W ~ 0
O~e MeHN~~ O~
Me
VIII-a VIII-b
O O
N~N.NH2 ~ N~N~NH2
Me N ~ ~ 0 Me N
HO~~ ~~~~ H2N
Me Me Me
Me
VIII-c VIII-d
15 Representative compounds of the present invention, which are encompassed
by Formula 2-VIII include, but are not limited to the compounds in Table 2-
VIII and
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
91
their pharmaceutically acceptable acid or base addition salts, or amide or
prodrugs
thereof.
Processes and novel intermediates for preparing compounds of Formula I-VIII
are provided as further embodiments of the invention and are illustrated by
the
following procedures in which the meanings of the generic radicals are as
given
above unless otherwise qualified. In some cases, protecting groups may have
been
used to allow synthetic manipulation of one functional group in the presence
of other
functional groups. It is therefore to be noted that, although not specifically
noted in
Schemes 1 - 7, the appropriate use and choice of protecting groups is well-
known by
one skilled in the art, and is not limited to the specific examples below. It
is also to be
understood that such groups not only serve to protect chemically reactive
sites, but
also to enhance solubility or otherwise change physical properties. A good
general
reference for protecting group preparation and deprotection is Greene,
Theodora,
Pr~tective Groups in Organic Synthesis; Wiley: New York, USA, 1991.
Further in regards to Schemes 1-7, a number of general reactions such as
oxidations and reductions, etc. are not shown in detail but can be done by
methods
understood by one skilled in the art. General transformations are well-
reviewed in
Larock, Richard. Comprehensive Organic Transformations; Wsiley: New York,
USA, 1999, and the series "Compendium of Organic Synthetic Methods" published
by Wiley-Interscience. In general, the starting materials are obtained from
commercial sources unless otherwise indicated.
Structures encompassed by Formula I wherein J is C and K is C or N can be
prepared as described in Scheme 1. It is to be understood that when K is N in
any of
the compounds shown in Scheme 1, R6 is absent. When K is G in Scheme 1, R6 can
be as defined above. It is additionally to be understood that compounds shown
in
Scheme 1 wherein K is N can be prepared according to the method disclosed in
WO
91/16894. Thus, nitrite 1 (Qun Li et aI. Heterocycles (1999), 51(6), 1345-
1353.) can
be converted into amide 2 by partial hydrolysis using any number of methods
known
by those skilled in the art. Treatment of amide 2 with a carbon monoxide
equivalent
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
92
such as phosgene provides a pyrido[1,2-c~pyrimidine ring system 3. The
amination of
the 2-position NH of 3 can be achieved using any number of aminating reagents
as
described by Kloetzer (Sci. Pharm.,1984; 52: 46-50). The intermediatewherein
RS is
Cl or F (Compound Al), can be coupled with various heterocyclic amines to
produce
the desired derivatives. Alternatively, if RS is a bromo, iodo or triflate
group, various
carbocyclic and aryl moieties may be introduced at RS using palladium-
catalyzed
coupling procedures using appropriately substituted tin or boronate reagents.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
93
Scheme 1
R3 R3
R4 I ~ N Ra ~ N NH2
R5 K~CN R5 K~O
Rs 'R~ Rs R~ "phosgene
1 ~ equiv."
2
R3 O R3 O
R4 ~ N~N'NH2 "amination" R4 ~ N~NH
R5 ~K~O ' R5 ~K~O
Rs R' Rs R' 3
A1
NH ~NH
Rs O R3 O
,.
R4 ~ N~N'NH2 Rs N~NH
"amination"
N ~K'~O ~ N ~K~O
Rs R~ Rs R~
B1 C1
R3 O aryl stannane R3 O
or ~ .NHBoc
Boc2O R4 i N~N.NHBoc aryl boronic acid R4 ~ N N
dioxane R5 ~J'~O Ar ~J ~ O
Pd catalysis ,
Rs R~ Rs R~
acid
R3 O
R4 , N~N.NHZ
Ar ~J ~O
Rs R~
D1
It is to be recognized from Scheme 1 that R1 and R6, taken together, may
form a ring having substiiuents, providing chiral centers that may give rise
to R and
5 S enantiomers as well as diastereomers. It is also to be recognized that RS
sidechains
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
94
(Compounds B1, C1, and D1) may also, with appropriate substitution, have
chiral
centers giving R and S enantiomers and diastereomers. Such enantiomers or
diastereomers may be separated by any of the methods indicated above at any
stage.
Resolution of any intermediate may also be accomplished by fractional
crystallization
using mandelic acid, tartaric acid, or other chiral, optically pure acid or
base bearing
resolving agents. The isomers can then be separated and the chiral amide
hydrolyzed.
An additional approach to the synthesis of an intermediate useful in the
preparation of compounds of Formula I wherein J and K are C is summarized in
Scheme 2. Thus, substituted pyridine 6 (Qun Li et al. J. Med. Chem. 1996,
39(16),
3070-3088) is converted into 11 (Al in Scheme 1) in a fashion similar to that
described above. (Qun Li et al. Heterocycles 1999, 51 (6), 1345-1353.).
Intermediate 6 is then treated with hydrazine in alcoholic solvent to provide
7.
Treatment of 7 with oxygen in the presence of a base such as NaOH provides
compound 8. Upon treatment with metalated cyclopropyl acetonitrile, compound 8
generates compound 9. Reaction of compound 10 with an acid-for example, HCl or
trifluoacetic acid-and subsequent conversion of the resulting phenol to the
triflate or
alternatively, treatment with a source of Br, Cl, such as phosphorous
oxychloride, or
the like, provides 11. This intermediate may then be converted into 11(A1) as
described in Scheme 1.
ao
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
Scheme 2
F NH-NH2
F
F w N NH2-NH2 ~ F w N 02/Base ~ 'N
t-Bu ~O ( / F t-Bu ~O I / F t Bu ~O / F
OMe
6 OMe 7 OMe 8
~CN
Base
O
F / N~N.NH2 F ~ + F w
'N 1 ) H 'N
;I ~ ~ O ~ Scheme 1 CI ~ , CN t-Bu~O I / CN
OMe~ OMe~ 2) POCI3 OMe
11 (A1 ) 10 99
5 Structures encompassed by Formula I wherein J is N and I~ is C can be
prepared as described in Scheme 3. It is to be understood that when J is N in
any of
the compounds shown in Scheme 3, R4 is absent. Thus, dichorodiazine I2 can
undergo reaction with metalated cyclopropyl acetonitrile to provide compound
13.
Using the methodology summarized in Scheme 1, compound 13 can undergo
10 cyclization using a carbon monoxide equivalent to provide bicyclic system
14.
Compound 14 can be converted to various compounds of the invention bearing an
RS
substituent, also according to Scheme 1.
Scheme 3
O
N'~N ~CN N \ N Scheme 1 N~N~N~H
CI / CN CI \ \ O
CI ~CI Base R
R R
15- 12 13 14
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
96
Structures encompassed by Formula I wherein J is N and K is N can be
prepared as described in Scheme 4. It is to be understood that when J is N in
any of
the compounds shown in Scheme 3, R4 is absent. Thus, dichlorotriazine 15
[Agric.
Biol. Chem. 1982, 46(6), 1439]
can undergo reaction with metalated cyclopropyl acetonitrile to provide
compound
16. Using the methodology summarized in Scheme 1, compound 16 can undergo
cyclization using a carbon monoxide equivalent to provide bicyclic system 17.
Compound 17 can be converted to various compounds of the invention bearing an
RS
substituent, also according to Scheme 1.
Scheme 4
O
N~N ~CN NON Scheme 1 N~N~N~H
~ CN ~~
CI~N~CI Base CI N CI' 'N ~ O
16 17
15 Structures encompassed by Formula VIII can be prepared as summarized in
Schemes S and 6. Scheme 5 provides an approach to Formula VIII compounds
wherein m is 1, X is O or CHZ, and Y is CH-Me. Compound 25a wherein X and Y
are CHa can be prepared from compound I8a. Thus, compound I8a can be
metallated according to methods known to those skilled in the art via
deprotonation
using an alkyl lithium reagent, followed by "quenching" with a borane,
stannane,
zinc, or copper reagent, or the like, to provided metallated intermediate 19a
wherein
V is a borane, stannane, or other organometallic moiety known in the art.
Intermediate 19a can undergo reaction with a compound such as with
commercially
available 2-methyl-2-vinyl oxirane 2.0a (Aldrich Handbook of Fine Chemicals
and
Laboratory Equipment, 2002) under palladium catalyzed conditions or in a SN2'
fashion to provide vinyl compound 21 a. Hydrogenation of the double bond in
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
97
compound 21a gives rise to the butanol derivative 22a. Conversion of the
alcohol
moiety in compound 22a to a leaving group such as a halide, mesylate,
tostlate,
triflate, etc., followed by treatment with cyanide, will provide the nitrite
compound
23a. Base treatment of nitrite 23a can give rise to the bicylic intermediate
24a, which
can be converted to the compound of Formula VIII 25a as provided earlier in
the
instant application.
Scheme 5 '
Me p
F , 1)AIkyILlthium F / N ~ 20a
'N
Me-~--O ~ I F 2) borane, or Me~O ~ I F
stannane, etc.
Me H Me V
18a 19a
F N 1 ) HX or MsCI, etc.
MeF / N 1) ~Hl Me / I
Me-f -O ~ F
OAc 2) Deprotect Me-I-O \ F OH 2) CN'
Me ~ Me
Me Me
21a 22a
F / N F ~ N
Me \ I Cyclize Me ~ I CN
Me-~-O F Me~-O ________
Me CN Me Me
Me
23a 24a
O
F ~ NRZ
______________., R5
Me
25a
As summarized in Scheme 6, compounds of formula VIII wherein X is O can
be prepared in a similar fashion. Treatment of phenol 18b with base can
provide the
phenoxide 19b. Reaction of compound 19b with a compound such as 20b can give
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
98
rise to the vinyl derivative 21b. Hydrogenation of the double bond in compound
21b
followed by the series of steps provided in Schem Se provides 25b.
Scheme 6
TMS~~OAc 20b
IIN
MeF \ N MeF \
Me-I--O F Me-~-0 F
Me H Me O
18b 19b
F
Me / 'N F ~ N
F Me
Me-j-0 O OH Me--~-O \ F OH
Me 0
Me
21 b 22b
F ~ N MeF ~ N
Me-+-0 \ ' F CN Me--I-0 ~ ' CN ___________..
Me O~ Me ~~Me
Me
23b 24b
O
F ~ N~NRZ
.______________.,. R5 ~ \ O
O Me
25b
Schemes 7-9 summarize the preparation of RS sidechains for compounds of
Formula I wherein RS is a variously substituted nitrogen-containing
heterocycle,
wherein the point of attachment is the nitrogen of the heterocycle. According
to
Scheme 7, appropriately activated enones can undergo [3+2] cycloadditions
under the
conditions described by Tsuge et al. (See Recent advances in azomethine ylid
chemistry : Advances in Heterocyclic Chemistry (Katritsky, A. ed.) Academic
Press;
San Diego USA, 23I-349). Thus, compound 26 can undergo reaction with compound
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
99
27 in a chlorinated hydrocarbon such as dichloromethane, chloroform,
dichloroethane
or the like, in the presence of a catalytic acid such as trifluoacetic acid,
to provide a
substituted pyrrolidine 28, wherein S1, Sz, S3, S4 are each independently
alkyl,
substituted alkyl, or aryl. Pyrrolidine 28 can then be reduced using sodium
borohydride or a similar reducing agent under a variety of conditions known to
those
skilled in the art to provide alcohol-substituted pyrrolidine 29. Pyrrolidine
29
subsequently can be deprotected by hydrogenation and the resulting pyrrolidine
30
can be used in the preparation of 2-aminopyrido[1,2-c]pyrimidines and
congeners as
shown in Scheme 1.
Scheme 7
p O OH
S1 S2 S~ S2 N~Ph reduction ~ S~ S2 ~Ph
acid, chlorinated 'N
4 3 hvrlrnn.~rhnn
$ $. S4 Sv S4 e_
28 29
26
Hydrogenation
Ph
MeO~N~TMS OH
S2
27 S~
/~NH
Sa Ss
In Scheme 8, a 3-carboxypyrrolidinone 31 [Culbertson, Townley P.;
Domagala, John M.; Nichols, Jeffery B.; Priebe, Stephen; Skeean, Richard W. J.
Med. Chem. 1987, 30(10), 1711] can be converted into an acid chloride or
Weinreb
15 amide 32 [Nahm, Steven; Weinreb, Steven M.. Tetraheelron Lett. 1981,
22(39),
3815]. Acid chlorides and Weinreb amides 32 can be converted into ketones 33
(S1 is
defined as in Scheme 2) by treatment with an organocopper reagent or Grignard
reagent, respectively. These intermediates 33 can then be treated with
hydroxylamine
or any O-alkylated or -arylated hydroxylamine under a variety of conditions
known
20 to those skilled in the art to provide oximated substituted pyrrolidines
34. The
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
100
oximes 34 can then be reduced to amines 35 with lithium aluminum hydride,
diisobutylaluminum hydride, borane or by selective catalytic hydrogenation by
those
skilled in the art. The resulting primary amines can then be protected using a
number
of methods as decribed in Protective Groups in Organic Synthesis by Green and
Wuts. The benzylic pyrrolidine 35 can then be deprotected by hydrogenation and
the
resulting pyrrolidine 36 used in the preparation of pyrido[1,2-cJpyrimidines
and
congeners as shown in Scheme 1. The use of S methylbenzyl (or R-methylbenzyl)
as
a protecting group for the pyrrolidine nitrogen allows for the separation of
enantiomers and diastereomers at any step in the reaction sequence.
Scheme 8
O acid chloride O Organocopper O
HO N~Ph or X . N~Ph Reagent gy N~Ph
~Me Weinreb amide Me or . ~Me
31 O formation 32 O Organo Grignard 33 O
Reagent
X = CI or -N(Me)OMe
hydroxylamine
Pro NH HO.~
NH 2 lithium aluninum N
1 ) protection g~ ~Ph hydride S~ i Ph
N ~ -1
NH 2) deprotection ~Me or N ~Me
36 35 borane 34 0
Scheme 9 provides a synthesis for an additional pyrrolidinyl sidechain,
compound 42, using techniques known to those skilled in the art. In one
approach,
acid 37 was treated with isobutyl cholorformate in the presence of base to
form the
mixed anhydride. Conversion of the mixed anhydride to the diazomethyl compound
using 1-methyl-3-nitro-1-nitrosoguanidine and KOH, followed by treatment with
HBr/HOAc, gave rise to a-bromomethyl ketone 38. Alpha-bromomethyl ketone 38
was readily converted to the fluoromethyl ketone 39 using a fluorine source
such as
KF. Reductive amination of 39 using benzylamine and a reducing agent such as
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
101
sodium triacetoxyborohydride provided fluoroethylamino derivative 40.
Reduction
of the amide moiety in compound 40, followed by hydrogenolysis to remove the
benzyl moieties provided the target compound 41.
Scheme 9
Br F
HO
O
1. Acid Chloride or Mixed Anhydride formation ~ Fluorination O
O 2. Diazo formation
N 3. HX/H+ (1:1) N ~ N
/ 38
37 39
F
F
BnNH2~ [H) NH 1. [H)
2. Hy rogenation -' NHz
N O
N
H
/
41
Certain compounds of Formula I are also useful as intermediates for preparing
other compounds of Formula I. Thus, a compound wherein R2 is NRa, can be
metabolized to form another compound of the invention wherein R2 is H. This
10 conversion can occur under physiological conditions. To that end, both the
non-
metabolized compound of the invention and the metabolized compound of the
invention--that is, the compound wherein Rz is NRa and the compound wherein Ra
is
H--can have antibacterial activity.
Some of the compounds of Formula I are capable of further forming
15 pharmaceutically acceptable acid-addition and/or base salts. All of these
forms are
within the scope of the present invention. Thus, pharmaceutically acceptable
acid
addition salts of the compounds of Formula I include salts derived from
nontoxic
inorganic acids such as hydrochloric, nitric, phosphoric, sulfuric,
hydrobromic,
hydriodic, hydrofluoric, phosphorous, and' the like, as well as the salts
derived from
20 nontoxic organic acids, such as aliphatic mono- and dicarboxylic acids,
phenyl-
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
102
substituted alkanoic acids, hydroxy alkanoic acids, alkanedioic acids,
aromatic acids,
aliphatic and aromatic sulfonic acids, etc. Such salts thus include sulfate,
pyrosulfate,
bisulfate, sulfite, bisulfate, nitrate, phosphate, monohydrogenphosphate,
dihydrogenphosphate, metaphosphate, pyrophosphate, acetate, trifluoroacetate,
propionate, caprylate, isobutyrate, oxalate, malonate, succinates suberate,
sebacate,
fumarate, maleate, mandelate, benzoate, chlorobenzoate, methylbenzoate,
dinitrobenzoate, phthalate, benzensoulfonate, toluenesulfonate, phenylacetate,
citrate,
lactate, maleate, tartrate, methanesulfonate, and the like. Also contemplated
are salts
of amino acids such as arginate and the like and gluconate, galacturonate
(see, for
example, Berge S.M. et al., "Pharmaceutical Salts," .lournal of Pharmaceutical
Science, 1977;66:1-19).
The acid addition salt of said basic compounds are prepared by contacting the
free base form with a sufficient amount of the desired acid to produce the
salt in the
conventional manner.
Pharmaceutically acceptable base addition salts are formed with metals or
amines, such as alkali and alkaline earth metals or organic amines. Examples
of
metals used as cations are sodium, potassium, magnesium, calcium, and the
like.
Examples of suitable amines are N,N'-dibenzylethylenediamine, chloroprocaine,
choIine, diethanolamine, dicyclohexylamine, ethylenediamine, N-
methylglucamine,
and procaine (see, for example, Berge S.M., supra., 1977).
The base addition salts of said acidic compounds are prepared by contacting
the free acid form with a sufficient amount of the desired base to produce the
salt in
the conventional manner.
Certain of the compounds of the present invention can exist in unsolvated
forms as well as solvated forms, including hydrated forms. In general, the
solvated
forms, including hydrated forms, are equivalent to unsolvated forms and are
intended
to be encompassed within the scope of the present invention.
Certain of the compounds of the present invention possess one or more chiral
centers and each center may exist in the R(D) or S(L) configuration. The
present
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
103
invention includes all enantiomeric and epimeric forms, as well as the
appropriate
mixtures thereof.
The compounds of Formula I can be Formulated as pharmaceutical
compositions and administered to a mammalian host, such as a human patient in
a
variety of forms adapted to the chosen route of administration, i.e., orally
or
parenterally, by intravenous, intramuscular, topical or subcutaneous routes.
Thus, the present compounds maybe systemically administered, e.g., orally,
in combination with a pharmaceutically acceptable vehicle such as an inert
diluent or
an assimilable edible carrier. They may be enclosed in hard or soft shell
gelatin
capsules, may be compressed into tablets, or may be incorporated directly with
the
food of the patient's diet. For oral therapeutic administration, the active
compound
may be combined with one or more excipients and used in the form of ingestible
tablets, buccal tablets, troches, capsules, elixirs, suspensions, syrups,
wafers, and the
Like. Such compositions and preparations should contain at least 0.1 % of
active
compound. The percentage of the compositions and preparations may, of course,
be
varied and may conveniently be between about 2 to about 60% of the weight of a
given unit dosage form. The amount of active compound in such therapeutically
useful compositions is such that an effective dosage level will be obtained.
The tablets, troches, pills, capsules, and the like may also contain the
following: binders such as gum tragacanth, acacia, corn starch or gelatin;
excipients
such as dicalcium phosphate; a disintegrating agent such as corn starch,
potato starch,
alginic acid and the like; a lubricant such as magnesium stearate; and a
sweetening
agent such as sucrose, fructose, lactose or aspartame or a flavoring agent
sash as
peppermint, oil of wintergreen, or cherry flavoring may be added. When the
unit
dosage form is a capsule, it may contain, in addition to materials of the
above type, a
liquid carrier, such as a vegetable oil or a polyethylene glycol. Various
other
materials may be present as coatings or to otherwise modify the physical form
of the
solid unit dosage form. For instance, tablets, pills, or capsules may be
coated with
gelatin, wax, shellac or sugar and the like. .A syrup or elixir may contain
the active
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
104
compound, sucrose or fructose as a sweetening agent, methyl and propylparabens
as
preservatives, a dye and flavoring such as cherry or orange flavor. Of course,
any
material used in preparing any unit dosage form should be pharmaceutically
acceptable and substantially non-toxic in the amounts employed. In addition,
the
S active compound may be incorporated into sustained-release preparations and
devices.
The active compound may also be administered intravenously or
intraperitoneally by infusion or injection. Solutions of the active compound
or its salts
can be prepared in water, optionally mixed with a nontoxic surfactant.
Dispersions
can also be prepared in glycerol, liquid polyethylene glycols, triacetin, and
mixtures
thereof and in oils. Under ordinary conditions of storage and use, these
preparations
contain a preservative to prevent the growth of microorganisms.
The pharmaceutical dosage forms suitable for injection or infusion can include
sterile aqueous solutions or dispersions or sterile powders comprising the
active
ingredient which are adapted for the extemporaneous preparation of sterile
injectable
or infusible solutions or dispersions, optionally encapsulated in liposomes.
In all
cases, the ultimate dosage form must be sterile, fluid and stable under the
conditions
of manufacture and storage. The liquid carrier or vehicle can be a solvent or
liquid
dispersion medium comprising, for example, water, ethanol, a polyol (for
example,
glycerol, propylene glycol, liquid polyethylene glycols, and the like),
vegetable oils,
nontoxic glyceryl esters, and suitable mixtures thereof. The proper fluidity
can be
maintained, for example, by the formation of liposomes, by the maintenance of
the
required particle size in the case of dispersions or by the use of
surfactants. The
prevention of the action of microorganisms can be brought about by various
antibacterial and antifungal agents, for example, parabens, chlorobutanol,
phenol,
sorbic acid, thimerosal, and the like. In many cases, it will be preferable to
include
isotonic agents, for example, sugars, buffers or sodium chloride. Prolonged
absorption of the injectable compositions can be brought about by the use in
the
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
105
compositions of agents delaying absorption, for example, aluminum monostearate
and gelatin.
Sterile injectable solutions are prepared by incorporating the active compound
in the required amount in the appropriate solvent with various of the other
ingredients
enumerated above, as required, followed by filter sterilization. In the case
of sterile
powders for the preparation of sterile injectable solutions, the preferred
methods of
preparation are vacuum drying and the freeze drying techniques, which.yield a
powder of the active ingredient plus any additional desired ingredient present
in the
previously sterile-filtered solutions.
For topical administration, the present compounds may be applied in pure
form, i.e., when they are liquids. However, it will generally be desirable to
administer
them to the skin as compositions or Formulations, in combination with a
dermatologically acceptable carrier, which may be a solid or a liquid.
Useful solid Garners include finely divided solids such as talc, clay,
microcrystalline cellulose, silica, alumina and the like. Useful liquid
carriers include
water, alcohols or glycols or water-alcohol/glycol blends, in which the
present
compounds can be dissolved or dispersed at effective levels, optionally with
the aid of
non-toxic surfactants. Adjuvants such as fragrances and additional
antimicrobial
agents can be added to optimize the properties for a given use. The resultant
liquid
compositions can be applied from absorbent pads, used to impregnate bandages
and
other dressings, or sprayed onto the affected area using pump-type or aerosol
sprayers.
Thickeners such as synthetic polymers, fatty acids, fatty acid salts and
esters,
fatty alcohols, modified celluloses or modified mineral materials can also be
employed with liquid carriers to form spreadable pastes, gels, ointments,
soaps, and
the like, for application directly to the skin of the user.
Examples of useful dermatological compositions which can be used to deliver
the compounds of Formula I to the skin are known to the art; for example, see
Jacquet
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
106
et al. (U.5. Pat. No. 4,608,392), Geria (U.5. Pat. No. 4,992,478), Smith et
al. (U.5.
Pat. No. 4,559,157) and Wortzman (U.5. Pat. No. 4,820,508).
Useful dosages of the compounds of Formula I can be determined by
comparing their in vitro activity, and in vivo activity in animal models.
Methods for
the extrapolation of effective dosages in mice, and other animals, to humans
are
known to the art; for example, see U.S. Pat. No. 4,938,949.
Generally, the concentration of the compounds) of Formula I in a liquid
composition, such as a lotion, will be from about 0.1-2S wt-%, preferably from
about
O.S-10 wt-%. The concentration in a semi-solid or solid composition such as a
gel or a
powder will be about 0.1-5 wt-%, preferably about O.S-2.S wt-%.
The amount of the compound, or an active salt or derivative thereof, required
for use in treatment will vary not only with the particular salt selected but
also with
the route of administration, the nature of the condition being treated and the
age and
condition of the patient and will be ultimately at the discretion of the
attendant
physician or clinician.
In general, however; a suitable dose will be in the range of from about O.S to
about 100 mg/kg, e.g., from about 10 to about 7S mg/kg of body weight per day,
such
as 3 to about 50 mg per kilogram body weight of the recipient per day,
preferably in
the range of 6 to 90 mg/kg/day, most preferably in the range of 1 S to 60
mg/kg/day.
The compound may conveniently be administered in unit dosage form; for
example, containing S to 1000 mg, conveniently 10 to 750 mg, most
conveniently, S0
to 500 mg of active ingredient per unit dosage form.
Ideally, the active ingredient should be administered to achieve peak plasma
concentrations of the active compound of from about O.S to about 7S ~,M,
preferably,
2S about 1 to SO ~M, most preferably, about 2 to about 30 ~M. This may be
achieved,
for example, by the intravenous injection of a O.OS to S% solution of the
active
ingredient, optionally in saline, or orally administered as a bolus containing
about 1-
100 mg of the active ingredient. Desirable blood levels may be maintained by
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
107
continuous infusion to provide about 0.01-5.0 mg/kglhr or by intermittent
infusions
containing about 0.4-15 mg/kg of the active ingredient(s).
The desired dose may conveniently be presented in a single dose or as divided
doses administered at appropriate intervals, for example, as two, three, four
or more
sub-doses per day. The sub-dose itself may be further divided, e.g., into a
number of
discrete loosely spaced administrations; such as multiple inhalations from an
insufflator or by application of a plurality of drops into the eye.
The ability of a compound of the invention to inhibit bacterial growth is
demonstrated using pharmacological models that are well known to the art, for
example, using models such as the tests described below.
Test A--Antibacterial Assay
The compounds of the present invention were tested against an assortment of
Gram- negative and Gram-positive organisms using standard microtitration
techniques (Cohen, et al., Antimicrob. Agents Chemother, 1985;28:766; Heifetz,
et al., Antimicrob. Agents Chemother., 1974;6:124). The results of the
evaluation are
shown in Table 3 and are compared to ciprofloxacin.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
108
Table 3
Antibacterial and E. coli gyrase Activities
Compound Minimum Inhibitory Concentrations pg/mL E. coli
Number or Gram Negatives Gram Positives gyrase
Structure E. coli E. E. coli E. S S. ICso
MC coli Tol C faecalis aureus pyogenes (p,M)
4100 B90 RB1 29213 C203
4f 2.0 0.130.03 0.06 0.25 0.015 1.0
4g 16.0 0.25<0.06 2.0 1.0 1.0 1.7
4h 16.0 0.13<0.06 2.0 0.5 2.0 1.2
4i 0.5 0.030.015 0.06 0.06 0.015 0.64
4j 2.0 0.250.13 0.5 0.03 1.5
Sb 0.5 0.060.03 0.13 0.25 0.008 0.5
Sc 16.0 2.0 0.5 2.0 2.0 0.5 0.7
Ciprofloxacin <0.02 <0.01 0.5 0.5 0.5 0.2
<0.01
Test B-DNA gyrase assay
The effects of test agents on the activity of DNA gyrase was determined by
the supercoiling inhibition assay, following reaction conditions recommended
by the
enzyme supplier (Lucent, Ltd., Leicester, UK), as follows: Reactions are
performed in
buffer G (35 mM Tris-HCl (pH 7.5), 24 mM KCI, 4 mM MgCl2, 2 mM DTT, 1.8 mM
spermidine, 1 mM ATP, 0.1 mglmL bovine serum albumin). Relaxed plasmid pBR322
(0.25 pg, Lucent, Ltd., Leicester, UK) is reacted with 1 U E. coli gyrase
(Lucent, Ltd.,
Leicester, UK), in the absence or presence of drugs, for 30 minutes at
37°C.
Reactions were stopped by the addition of SDS and proteinase K to respective
final
concentrations of 1 % and 0.5 mg/mL. After an additional 30 minutes at
37°C,
one-tenth volume of lOX loading buffer (0.3% bromophenol blue, 16% Ficoll,
10 mM Na2HP~4) was added, and reactions were loaded onto agarose gels and
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
109
electrophoresed as described for intercalation assays (Y Pommier et al.
Nucleic
Acids Research 1987, I5, 6713-6731.). The concentration of drug inhibiting 50%
of
the supercoiling activity of DNA gyrase was measured and is given as an ICSO
in
Table 4.The in vivo activity was obtained when the compounds were tested
according
to the procedure of Miller, et al. (Proc. Soc. Exp. Biol. Med., 1944;57:261).
The
median protective dose (PD50) was determined in mice given lethal systemic
infections, as depicted in Table 4. Compound 4f is compared to ciprofloxacin.
Table 4
In Vivo Median Protective Dose (PDSp) in Mice (PO)
Compound Number or Structure Organism PDSO (mg/kg)
4f S pyogenes 1.2
4i S. pyogenes 2.1
Ciprofloxacin S. pyogenes . >I00
Test C-Cross Resistance Antibacterial Assay
The compounds of the present invention were tested against an assortment of
ciprofloxacin resistant E. coli and S. aureus organisms described below using
standard microtitration techniques (Cohen, et al., Antimicrob. Agents
Chemother.,
1985;28:766; Heifetz, et al., Antimicrob. Agents Chemother, 1974;6:124). The
results
of the evaluation are shown in Table 5 compared to ciprofloxacin.
N. gonorrhoeae and S. aureus organisms:
N. gonorrhoeae 2637 (N.g. 2637) is a derivative of Neisseria gonorrhoeae MS11
containing a TAC-LAC recA to allow for control of homologous
recombination [Tonjum T. et al. Molecularlllicrobiology 1995,16, 451-64].
N. gonorrhoeae 2709 (N.g. 2709): Isogenic to N. gonorrhoeae 2637 contains gyrA
quinolone- resistant determining region (QRDR) mutations (S91F D95G).
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
110
N. gonorrhoeae 2693 (N.g. 2693): Isogenic to N. gonorrhoeae 2709 containing
parC
QRDR mutations [ P88S and E91KJ.
S aureus UC-76: Typical sensitive laboratory strain (Wild type).
S. aureus 2552: Isogenic to S. aureus UC-76, with upregulated norA pump.
S S. aureus 2554: Isogenic to S aureus 2552, with point mutation at position
80 of grlA
subunit.
S. aureus 2558: Isogenic to S aureus 2554, with point mutation at position 84
of ~rA
subunit.
Table 5
Antibacterial Activities Against Ciprofloxacin Resistant Strains
Compound . Minimum Inhibitory Concentrations ~glmL
Number or N. g. N. g. N. g. S. aureus S. aureus S. aureus S. aureus
Structure 2637 2709 2693 UC-76 2552 2554 2558
4f 0.25 0.5 1.0 0.06 0.5 0.13 0.5
4h 0.5 1 1 1
4i ~ 0.03 0.13 0.13 0.06 0.06 0.06 0.13
5b 0.13 0.25 0.25 0.06 0.25 0.13 1
Ciprofloxacin0.002 0.06 2.0 0.13 2 2 64
The antibacterial agents described in this invention display Gram-negative and
Gram-positive activity. The compounds also show inhibition of bacterial 1~NA
gyrase.
Finally, the compounds demonstrate in vivo protective activity in mice and are
not highly cytotoxic to mammalian cells indicating selectivity for bacteria.
1 S The invention will now be demonstrated by the following non-limiting
examples.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
111
Example 1
2-(4-Chloro-5-fluoro-3-methylpyridin-2-yl)-2-cyclopropylacetamide
~N 0
l
CI ~ NH2
Me
U
2-(4-Chloro-5-fluoro-3-methyl-2-pyridinyl)cyclopropylacetonitrile (10.0 g,
S 44.5 mmol, [Qun Li et al. Heterocyeles 1999, 51 (6), 1345-1353]) was added
to a
stirred solution of sulfuric acid (10 mL) and acetic acid (30 mL). The
resulting
mixture was heated for 2 hours at 100 °C. The mixture was then cooled
and made
alkaline with ammonia to pH 9. The product was extracted with chloroform (3 x
60mL). The extracts were combined, dried over sodium sulfate and evaporated to
afford the title compound (8.6 g). MSCI: m/z = 243 (MH+).
Example 2
6-Chloro-4-cyclopropyl-7-fluoro-5-methyl-pyrido[1,2-c]pyrimidine-1,3-dione
0
N~NH
CI ~ ~ O
Me
To a -60 °C solution of 2-(4-chloro-5-fluoro-3-methylpyridin-2-yl)-
2-
cyclopropylacetamide (2.7 g, 11.0 mmol (Example 1)) and triphosgene (7.2 g,
24.0
mmol) in dichloromethane (250 mL) was added portionwise potassium tert-
butoxide
(4.5 g, 40.0 mmol). After the addition was complete, the mixture was stirred
at - 50
°C for 30 minutes. Water was then added at this temperature. The
organic layer was
separated, washed with brine (3x), dried over sodium sulfate, filtered, and
evaporated.
The residue was purified by column chromatography (7:3 ethyl acetate/hexanes)
to
afford the title compound (2 g).'H NMR (200 MHz, CDC13) 8 8.95 (bs, 1H), 8.16
(d,
1H), 2.66 (s, 3H), 1.75-1.71(m, 1H), 1.11-1.01 (m, 2H), 0.46-0.37 (m, 2H).
MSCI:
mlz = 269 (MH+).
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
112
Example 3
2-Amino-6-chloro-4-cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-
dione
O
F / N~N.NH2
CI ~ ~ O
Me
U
To a 5 °C solution of 6-chloro-4-cyclopropyl-7-fluoro-5-methyl-
pyrido[1,2-
c]pyrimidine-1,3-dione (0.76 g, 2.8 mmol (Example 2)) in a mixture of dry
tetrahydrofuran (20 mL) and dry dimethylformamide (10 mL) was added sodium
hydride (0.12 g, 3.0 mmol, 60% mineral dispersion) portionwise. After stirring
at
room temperature for 30 minutes, 2,4-dinitrophenylhydroxylamine (0.65 g, 3.00
mmol) was added. The mixture was then heated at 80 °C for 45 minutes,
cooled to
ambient temperature, poured into ice water, and extracted with
dichloromethane. The
combined extracts were washed with brine, dried with sodium sulfate and
concentrated. The residue was purified by column chromatography (9:1 ethyl
acetate/hexanes) to afford the title compound (0.635 g). MSCI: rn/z = 284
(MH+).
Example 4
General Procedure
A solution of 2-amino-6-chloro-4-cyclopropyl-7-fluoro-S-methyl-pyrido[1,2-
c]pyrimidine-1,3-dione (Example 3) and a substituted pyrrolidine (3 eq.) in
dimethyl
sulfoxide was heated at 60 °C for 5 hours. The mixture was cooled to
ambient
temperature, diluted with water and filtered. The product was purified by
column
chromatography to afford each of the following title compounds:
(a) {(R)-1-[(R)-1-(2-Amino-4-cyclopropyl-7-fluoro-S-methyl-1,3-dioxo-2,3-
dihydro-1H pyrido[1,2-c]pyrimidin-6-yl)-pyrrolidin-3-yl]ethyl}carbamic acid,
tert
butyl ester (MSCI: m/z = 462 (MH+)) from ((R)-(R)-1-pyrrolidinin-3-yl
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
113
ethyl)carbamic acid, tent-butyl ester [D.R Johnson et al. J. Heterocycl. Chem.
1992,
29(6), 1481-8].
(b) {(S)-1-[(R)-1-(2-Amino-4-cyclopropyl-7-fluoro-5-methyl-1,3-dioxo-2,3-
dihydro-lH~pyrido[1,2-c]pyrimidin-6-yl)-pyrrolidin-3-yl]ethyl}carbamic acid,
tert-
butyl ester (MSCI: m/z = 462(MH+)) from ((S)-(R)-1-pyrrolidinin-3-yl-
ethyl)carbamic acid, tert-butyl ester [ester [Don R Johnson et al. J.
Heterocycl. Chem.
1992, 29(6), 1481-8].
(c) {1-[1-(2-Amino-4-cyclopropyl-7-fluoro-5-methyl-1,3-dioxo-2,3-dihydro-
1-H pyrido[1,2-c]pyrimidine-6-yl)-4,4-dimethylpyrrolidin-3-yl]-ethyl}carbamic
acid, tert-butyl ester (MS (EI, M+1) m/z = 490) from [1-(4,4-dimethyl-
pyrrolidin-3-
yl)ethyl]carbamic acid, tent-butyl ester [U.S. Provisional App. Ser. No.
60/241267,
filed Oct. 18, 2000, Warner Lambert Docket No. A0000072].
(d) [1-(2-Amino-4-cyclopropyl-7-fluoro-5-methyl-1,3-dioxo-2,3-dihydro-1H
pyrido[1,2-c]pyrimidin-6-yl)pyrrolidin-3-yl]carbamic acid, tert-butyl ester
(MSCI:
m/z = 434(MH+) from pyrrolidin-3-yl-carbamic acid, tent-butyl ester.
(e) f 1-[1-(2-Amino-4-cyclopropyl-7-fluoro-5-methyl-1,3-dioxo-2,3-dihydro-
1H pyrido[1,2-c]pyrimidin-6-yl)-4-methylpyrrolidin-3-yl]-ethyl}carbamic acid,
tert-
butyl ester (MSCI: m/z = 476 (MH+)) from [1-(4-methylpyrrolidin-3-yl)-
ethyl]carbamic acid tent-butyl ester [U.S. Provisional App. Ser. No.
60/241267, filed
oct. l s, 2000].
(f) 2-Amino-4-cyclopropyl-7-tluoro-5-methyl-6-[3-(1-
methylaminoethyl)pyrrolidin-1-yl]pyrido[1,2-c]pyrimidine-1,3-dione (mp = 95-97
°C; MSCI: m/z = 376 (MH+)) from methyl-(1-pyrrolidin-3-yl-ethyl)amine
[J.S.
Plummer et al. Tetrahedron Lett. 1993, 34(47), 7529-32].
(g) 2-Amino-4-cyclopropyl-7-fluoro-5-methyl-6-pyrrolidin-1-yl-pyrido[1,2
c]pyrimidine-1,3-dione (mp = 202-204 °C; MSCI: m/z = 319(MH+)) from
pyrrolidine.
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
114
(h) 2-Amino-4-cyclopropyl-7-fluoro-6-[3-(1-hydroxyethyl)pyrrolidin-1-yl-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione (mp = 172-174 °C; MSCI: m/z =
363
(MH+)) from 1-pyrrolidin-3-yl-ethanol (Example 6A2)..
(i) 2-Amino-6-[(R)-3-(1-aminocyclopropyl)pyrrolidin-1-yl]-4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione (1H NMR (200
MHz, CDC13) 8 8.20 (d, 1H), 5.42 (bs, 2H), 3.86-3.80 (m, 1H), 3.71-3.63 (m,
1H),
3.52-3.44 (m, 2H), 2.27 (s, 3H), 2.12-2.00 (m, 2H), 1.86-1.72 (m, 2H), 1.62
(6s, 2H),
1.18-1.07 (m, 1H), 0.96-0.83 (m, 1H), 0.67-0.62 (m, 2H), 0.58-0.49 (m, 2H),
0.40-
0.29 (m, 2H). MSCI: m/z = 374 (MH+).) from (R)-1-pyrrolidin-3-
ylcyclopropylamine [Chem. Pharm. Bull. 1994, 42, 1442.].
(j) 2-Amino-6-((3S, 4S) and (3R,4R)-3-aminomethyl-4-fluoropyrrolidin-
1-yl)-4-cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione ('H NMR
(200 MHz, CDC13) ~ 8.21 (d, 1H), 5.42 (bs, 2H), 5.31 (dd, 1H), 4.07 (dd, 1H),
3.84-
3.76 (m, 1H), 3.75-3.47 (m, 3H), 3.14-3.08 (m, 1H), 3.02-2.94 (m, 1H), 2.33
(s, 3H),
1.83-1.78 (m, 1H), 1.70 (bs, 2H), 1.22-1.09 (m, 1H), 0.93-0.80 (m, 2H), 0.40-
0.29 (m,
1H); MSCI: m/z =366 (MH+)) was prepared from a mixture of (3S, 4S) and (3R,
4R)-4-fluoropyrrolidin-3-yl)methylamine [J. Med. Chem. 1990 33, 1344.].
(k) 2-Amino-6-(3-aminomethylpyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-5-
methylpyrido-[1,2-c]pyrimidine-I,3-dione ('H NMR (400 MHz, DMSO) 8 8.19 (d,
1H), 5.71 (bs, 2H), 3.74-3.50 (m, 3H), 3.49-3.38 (m, 1H), 3.31 (bs, 2H), 2.97
(d, 2H),
2.95-2.91 (m, 1H), 2.25 (s, 3H), 2.19- 2.09 (m, 2H), I.72-1.65 (m, 1H), 0.91-
0.81 (m,
2H), 0.29-0.22 (m, 2H); MSCI: m/z = 348 (MH+)) was prepared from pyrrolidin-3-
ylmethylamine [J. Org. Chem. 1961, 26, 4955.].
(1) 2-Amino-6-(3-aminopyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-S-
methylpyrido[1,2-c]pyrimidine-1,3-dione (1H NMR (400 MHz, CDC13) ~ 8.20 (d,
1 H), 5.40 (bs, 2H), 3.85 - 3.60 (m, 4H), 3.35 - 3.30 (m, 1 H), 2.35 (s, 3H),
2.30 - 2.15
(m, 1H), 1.85 -1.75 (m, 2H), 1.25 (bs, 2H), 1.00 - 0.95 (m, 2H), 0.38 - 0.30
(m, 2H).
MSCI: m/z = 334 (MH+)) was prepared from pyrrolidin-3-ylamine [JP 03133954,
1991].
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
115
(m) 2-Amino-6-(7-amino-5-azaspiro[2.4]hept-5-yl)-4-cyclopropyl-7
fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione (1H NMR (400 MHz, CD30D) 8
8.20 (d, 1H), 4.90 (bs, 2H), 4.40 - 4.30 (m, 1H), 4.20 = 4.13 (m, 1H), 3.75 -
3.40 (m,
3H), 3.25 (s, 1H), 2.40 (s, 3H), 1.75 - 1.68 (m, 1H), 1.25 (s, 2H), 1.13 -
0.70 (m,
7H). MSCI: m/z = 360 (MH+)) was prepared using 5-azaspiro[2.4]hept-7-ylamine
[PCT Int. Appl. WO 9637470, 1996].
(n) 2-Amino-6-[3-(aminooxazol-4-yl-methyl)pyrrolidin-1-yl]-4-
cyclopropyl-7-fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione (1H NMR (400
MHz, CDC13) 8 8.20 (t, 1H), 7.68 (s, 1H), 7.09 (d, 1H), 5.45 (bs, 2H), 4.12-
4.00 (m,
1H), 3.85-3.70 (m, 2H), 3.69-3.45 (m, 2H), 2.80-2.65 (m, 1H), 2.35-2.30 (d,
3H),
2.09-1.95 (m, SH), 1.10-0.80 (m, 2H), 0.31-0.22 (m, 2H). MSCI: m/z = 41 S
(MH+))
was prepared using oxazol-4-yl-C-pyrrolidin-3-ylmethylamine (Example 6B4).
(o) 2-Amino-6-(4-aminooctahydroisoindol-2-yl)-4-cyclopropyl-7-fluoro-
5-methylpyrido[1,2-c]pyrimidine-1,3-dione (1H NMR (400 MHz, d4-MeOH) 8 8.23
(d, 1H), 4.90 (bs, 4H), 4.16-4.01 (m, 2H), 3.60 (m, 1H), 3.35 (s, 2H), 3.20
(d, 1H),
2.85-2.75 (m, 1H), 2.40 (s, 3H), 1.98-1.82 (m, 2H), 1.80-1.60 (m, 3H), 1.58-
1.30 (m,
2H), 1.15-1.00 (m, 1H), 0.90-0.78 (m, 1H), 0.40-0.23 (m, 2H). MSCI: m/z = 388
(MH+)) was prepared from octahydroisoindol-4-ylamine [M. Ogata et al. Eur.
Pat.
Appl. (1990), EP 359172].
(p) 2-Amino-6-[3-(1-amino-1-methylethyl)pyrrolidine-1-ylJ-4-
cyclopropyl-7-fluoro-5-methylpyridojl,2-c]pyrimidine-1,3-dione (1H NMR (400
MHz, CDC13) 8 8.18 (d, 1H), 5.40 (s, 2H), 3.91-3.80 (m, 1H), 3.75-3.63 (m,
1H),
3.50-3.39 (m, 3H), 2.40-2.28 (s, 4H), 2.10-1.90 (m, 1H), 1.88-1.73 (m, 2H),
1.32-1.08
n
'l (m, 8H), 0.92-0.80 (m, 1H), 0.40-0.25 (m, 2H). MSCI: m/z = 376 (MH+)) was
prepared using 1-methyl-1-pyrrolidin-3-ylethylamine [WO 01/53273 A1J.
Example 5
Hydrogen chloride gas was bubbled into ice-cold anhydrous diethyl ether and
the resulting saturated diethyl ether solution was added to a solution of a
compound
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
116
of Example 4 in dry dichloromethane to form a suspension. The suspension was
sealed and stirred at room temperature for 1 hour. The resulting solids were
filtered
and washed with diethyl ether/hexanes (2:1). The solid was dried under vacuum
to
afford the following title compounds:
(a) 2-Amino-6-[(R)-3-((R)-1-amino-ethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione, hydrochloride (mp = 218-
221°C;
MSCI: m/z = 362 (MH+)) from {(R)-1-[(R)-1-(2-amino-4-cyclopropyl-7-fluoro-5-
methyl-1,3-dioxo-2,3-dihydro-1H pyrido[1,2-c]pyrimidin-6-yl)pyrrolidin-3-
yl]ethyl}carbamic acid, tert-butyl ester (Example 4a).
(b) 2-Amino-6-[(R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methyl-pyrido[1,2-c]pyrimidine-1,3-dione, hydrochloride (mp = 218-220
°C; MSCI: m/z = 362 (MH~) from {(S)-1-[(R)-1-(2-amino-4-cyclopropyl-7-
fluoro-5-
methyl-1,3-dioxo-2,3-dihydro-1H pyrido[1,2-c]pyrimidin-6-yl)-pyrrolidin-3-yl]-
ethyl}carbamic acid, tent-butyl ester (Example 4b).
(c) 2-Amino-6-[4-(1-amino-ethyl)-3,3-dimethylpyrrolidin-1-yl]-4-
cyclopropropyl-7-fluoro-S-methyl-pyrido[1,2-c]pyrimidine-1,3-dione,
hydrochloride
(mp = 238-240 °C ; MSCI: m/z = 390 (MH+)) from { 1-[1-(2-amino-4-
cyclopropyl-7-
fluoro-S-methyl-1,3-dioxo-2,3-dihydro-1-H pyrido[1,2-c]pyrimidine-6-yl)-4,4-
dimethylpyrrolidin-3-yl]ethyl}carbamic acid, tert-butyl ester (Example 4c)
(d) 2-Amino-6-(3-amino-pyrrolidin-1-yl)-4-cyclopropyl-7-fluoro-5-
methylpyrido[1,2-c]pyrimidine-1,3-dione hydrochloride (mp = 246-248 °C;
MSCI
m/z= 334 (MH+)) from [1-(2-amino-4-cyclopropyl-7-fluoro-S-methyl-1,3-dioxo-2,3-
dihydro-1H pyrido[1,2-c]pyrimidin-6-yl)-pyrrolidin-3-yl]carbamic acid, tert-
butyl
ester (Example 4d)
(e) 2-Amino-6-[3-(1-aminoethyl)-4-methylpyrrolidin-1-yl]-4-cyclopropyl-7-
fluoro-5-methylpyrido[1,2-c]pyrimidine-1,3-dione, hydrochloride (mp = 238-
240°C ;
MSCI: mlz = 376 (MH~) from {1-[1-(2-amino-4-cyclopropyl-7-fluoro-5-methyl-1,3-
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
117
dioxo-2,3-dihydro-1H pyrido[1,2-c]pyrimidin-6-yl)-4-methylpyrrolidin-3-
yl]ethyl}carbamic acid, tert-butyl ester (Example 4e)
Example 6
Amine Side Chain Synthesis
Example 6A1
1-(1-Benzylpyrrolidin-3-yl)ethanol
OH
Me
N
To a 5°C solution of 3-acetyl-1-benzylpyrrolidine (1g, 4.9 mmol
[L.E.
Overman, J. Am. Chem. Soc. 1983,105, 6622]) in methanol (10 mL) was added
portionwise sodium borohydride (0.37g, 9.8 mmol). After stirnng at room
temperature for 17 hours, the mixture was concentrated, diluted with ethyl
acetate,
washed with water, brine, dried with sodium sulfate and concentrated to afford
the
title compound (0.75g). MSCI: mlz = 206 (MH+).
Example 6A2
(1-Pyrrolidin-3-yl)ethanol
OH
Me
N
H
A mixture of 1-(1-benzylpyrrolidin-3-yl)ethanol (4.0 g, 19 mmol), 10% Pd-C
(2g) and ammonium formate (4.8 g, 76 mmol) in methanol (40 mL) was refluxed
for
3 hours. The mixture was then allowed to cool and diluted with dichloromethane
(80
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
118
mL) and filtered. The filtrate was concentrated to afford the title compound
(1.97g).
MSCI: mlz = 116 (MH+).
Example 6B~
4-(Oxazole-4-carbonyl-1-((S)-1-phenylethyl)pyrrolidin-2-one
O N1
\ O
O~N
Me~;~Ph
To a cooled solution (-78 °C) of oxazole (10.3 g, 149.1 mmol) in
tetrahydrofuran (150 mL) was added n-butyl lithium (2.5 M in hexane, 53.7 mL,
134.2 mmol). The solution was stirred at -78 °C for three hours. To the
cold solution
was added a solution of 5-oxo-1-((S)-1-phenylethyl)pyrrolidine-3-carboxylic
acid,
methoxymethylamide (8.2 g, 29.8 mmol [WO 0031062]) in tetrahydrofuran (50
mL). The mixture was warmed to ambient temperature and allowed to stir three
hours
followed by treatment with water, saturated aqueous ammonium chloride, and
extraction with ethyl acetate. The combined organic layers were dried over
sodium
sulfate, filtered and the solvent removed under reduced pressure. The
resulting
residue was purified by silica gel column chromatography, eluting with
hexane:ethyl
acetate (1:4) to obtain 1.96 g of the title compound as a 1:1 mixture of
isomers.
1St Isomer: 1H NMR (400 MHz, CDC13) S 7.88 (s, 1H), 7.40-7.21 (m, 6H),
5.50 (q, 1H), 4.18-4.10 (m, 1H), 3.66-3.62 (m, 1H), 3.35-3.28 (m, 1H), 2.95-
2.75 (m,
2H), 1.52 (d, 3H).
2°a Isomer:lH NMR (400 MHz, CDC13) 8 7.85 (s, 1H), 7.40-7.21 (m, 6H),
5.50 (q, 1H), 4.30-4.20 (m, 1H), 3.78-3.67 (m, 1H), 3.29-3.18 (m, 1H), 2.95-
2.75 (m,
2H), 1.55 (d, 3H).
Example 6B2
4-(Benzyloxyiminooxazol-4-ylmethyl)-1-((S)-1-phenylethyl)pyrrolidin-2-one
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
119
BnON N1
\ O
Or'N
Me~~~Ph
4-(Oxazole-4-carbonyl-1-((S)-1-phenylethyl)pyrrolidin-2-one (0.5 g, 1.76
mmol) [Example 6B1] and O-benzylhydroxylamine, hydrochloride (0.42 g, 2.64
mmol) in pyridine (5 mL) were refluxed for five hours and then cooled to room
temperature. The reaction mixture was diluted with water, washed with
saturated
sodium bicarbonate and extracted with ethyl acetate. The combined organic
layers
were dried over sodium sulfate, filtered and concentrated under reduced
pressure. The
resulting residue was purified by silica gel column chromatography eluting
with a
mixture of 1:2 mixture of hexanes:ethyl acetate to provide 0.41 g of the title
compound. 1H NMR (400 MHz, CDC13) 8 7.75-7.60 (m, 1H), 7.40-7.12 (m, 11H),
5.60-5.45 (m, 1H), 5.35-5.23 (m, 1H), 5.20-5.08 (m, 1H), 4.30-4.16 (m, 1H),
3.88-
2.60 (m, 4H), 1.50-1.30 (m, 3H).
Example 6B3
C-Oxazol-4-yl-C-[1-((S)-phenylethyl)pyrrolidin-3-yl]methylamine
HZN N O
N
Me~ ~Ph
To a cooled solution (0 °C) of 4-(benzyloxyiminooxazol-4-ylmethyl)-1-
((S)-1-
phenylethyl)pyrrolidin-2-one (3.16 g, 8.11 mmol) [Example 6B2] in
tetrahydrofuran
(80 mL) was added borane tetrahydrofuran complex (1M, 24 mL). The mixture was
warmed to room temperature and stirred for 21 hours. The mixture was
concentrated,
and the residue was mixed with water (5 mL) and extracted with chloroform. The
combined organic extracts were concentrated under reduced pressure, dissolved
in
80% aqueous ethanol, treated with triethylamine (20 mL) and then heated under
reflux for two hours. The solvent was evaporated, and the residue was
extracted with
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
120
dichloromethane. The combined extracts were then dried over sodium sulfate and
the
solvent removed in vacuo. The resulting residue was purified by silica gel
column
chromatography, eluting with 9:1 chloroform:methanol to give the title
compound.
(2.5 g).1H NMR (400 MHz, CDCl3) 8 7.55 (s, 1H), 7.40-7.15 (m, 5H), 7.05 (s,
1H),
4.02-3.88 (m, 1H), 3.25-3.10 (m, IH), 2.90-2.20 (m, 5H), 2.00-1.50 (m,
4H),1.35 (m,
3H).
Example 6B4
C-Oxazol-4-yl-C-pyrrolidin-3-ylmethylamine
O_~N
L_~N
' H2N
To the solution of C-Oxazol-4-yl-C-[1-((S)-phenylethyl)pyrrolidin-3-
yl]methylamine (2.44 g, 8.99 mmol) [example 6B3] and ammonium fonnate (2.83 g,
44.9 mmol) in methanol (45 mL), was added portionwise 10% palladiumlcarbon
catalyst under a nitrogen atmosphere. The mixture was then refluxed for five
hours
and filtered through a pad of Celite and washed with methanol. The combined
organics were concentrated under reduced pressure to obtain the title compound
(0.9
g). 1H NMR (400 MHz, CDC13) b 7.66 (s, 1H), 7.11 (s, 1H), 5.60-4.50 (bs, 3H),
4.18-4.10 (m, IH), 3.60-3.25 (m, 3H), 3.08-2.80 (m, 2H), 2.30-1.80 (m, 2H).
Example 7
4-tart-Butoxy-2,5-difluoro-3-methoxypyridine
F ~N
f-Bu~~ ~ i F
OMe
A mixture of 4-tent-butoxy-2,3,6-trifluoro-5-methoxypyridine (1.5 g. 6.4
mmol) and hydrazine monohydrate (1.7 mL, 34 mmol [Qun Li et al. J. Med. Chem.
1996, 39(16), 3070-3088]) in n-propanol (10 mL) was heated to reflux for 3
hours.
The solvent was removed in vacuo and the residue was dissolved in
dichlromethane,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
121
washed with water and concentrated to afford crude (4-tert-butoxy-3,6-difluoro-
S-
methoxypyridin-2-yl)hydrazine (1.4 g, 90%). 1.2 g of the material was
redissolved in
methanol (12 mL~ and 20% aqueous sodium hydroxide (2.S mL). Oxygen was passed
through the solution with vigorous stirring for 20 hours. The deep blue
solution was
S diluted with water (12 mL) and extracted with hexane (2x 30 mL). The
combined
organics were washed with brine, dried over sodium sulfate and concentrated in
vacuo. The residue was purified by column chromatography, eluting with ethyl
acetate/hexane (1:16) to afford the title compound (0.9 g). 'H NMR (400 MHz,
CDCI~) 8 7.74 (rn, 1H), 3.93 (m, 3H), 1.41 (s, 9H). MSCI: m/z = 218 (MH+).
Example 8
(4-tert-Butoxy-5-fluoro-3-methoxypyridin-2-yl)cyclopropylacetonitrile
~N
f-Bu.O ~ i CN
OMe
A-78 °C solution of diisopropylamine (1.3 mL, 9.0 mmol), under a
nitrogen
1S atmosphere in dry tetrahydrofuran (20 mL), was treated dropwise with 2.S N
n-
butyllithium (3.6 mL, 9.0 mmol) and stirred for 10 minutes. The solution was
treated
dropwise with cyclopropylacetonitrile (0.8 g, 3.S mmol) and stirred at - 78
°C for 1 S
minutes. A solution of 4-tert-butoxy-2,S-difluoro-3-methoxypyridine (0.8 g,
3.S
mmol)[Example 7] in dry tetrahydrofuran (S mL) was added and stirred for 1
hour at
-78 °C followed by 1 hour at 0 °C. The mixture was quenched with
saturated
ammonium chloride and extracted with diethylether. The combined extracts were
washed with brine, dried over sodium sulfate, and the solvent removed in
vacuo. The
residue was purified by column chromatography, eluting with ethyl
acetate/hexanes
(1:4) to afford the title compound (0.9 g). 1H NMR (400 MHz, CDC13) 8 8.22 (s,
1H),
2S 3.98 (s, 3H), 3.77 (d, 1H), 1.SS-1.48 (m, 1H), 1.42 (s, 9H), 0.62-O.S4 (m,
2H), O.SO-
0.42 (m, 1H), 0.35-0.30 (m, 1H). MSCI: m/z = 279 (MH+).
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
122
Example 9
(4-Chloro-5-fluoro-3-methoxypyridin-2-yl)cyclopropylacetonitrile
F ~N
C~ I ~ CN
OMe
A solution of (4-tent-butoxy-S-fluoro-3-methoxypyridin-2-
yl)cyclopropylacetonitrile (0.9 g, 3 mmol) [Example 8] and trifluoroacetic
acid (2
mL) in dichloromethane was stirred at room temperature for 25 minutes. The
solvent
was removed in vacuo and the oily residue dissolved in 1 S mL of
dichloromethane
and 2 mL of dimethylformamide. The resulting solution was cooled in an ice-
bath and
treated dropwise with phosphoryl chloride (2.4 mL). The solution was allowed
to
warm to ambient temperature and stirred overnight. The mixture was then poured
onto ice and extracted with dichloromethane (2x 20 mL). The combined extracts
were
washed with water, saturated sodium bicarbonate, water, dried over sodium
sulfate,
and concentrated in vacuo. The residue was purified by column chromatography,
eluting with ethyl acetate/hexane (1:4) to provide the title compound (0.4 g,
60%). 1H
NMR (400 MHz, CDC13) 8 8.32 (s. 1H), 4.05 (s, 3H), 3.77 (d, 1H), 1.58-1.49 (m,
1H), 0.80-0.71 (m, 1H), 0.68-0.55 (m, 2H), 0.52-0.43 (m, 1H). MSCI: mlz = 241
(MH+). ,
Example 10
(4-Chloro-5-fluoro-3-methoxypyridin-2-yl)-2-cyclopropylacetamide
F ~ N NH2
CI ~ O
OMe
The title compound (0.88 g) was prepared from (4-chloro-5-fluoro-3-
methoxypyridin-2-yl)cyclopropylacetonitrile (I.0 g, 4.I5 mmol)[Example 91
using
the procedure used to prepare Example 1.'H NMR (400 MHz, CDC13) b 8.33 (s,
1H),
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
123
6.89 (bs, 1H), 5.66 (bs, 1H), 3.94 (s, 3H), 3.26 (d, 1H), 1.46-1.43 (m, 1H),
0.81-0.70
(m, 1H), 0.60-0.44 (m, 2H), 0.31-0.22 (m, 1H). MSCI: m/z = 259 (MH+).
Example 11
6-Chloro-4-cyclopropyl-7-fluoro-5-methoxypyrido[1,2-c]pyrimidine-1,3-dione
The title compound (0.15 g) was prepared from (4-chloro-5-fluoro-3-
methoxypyridin-2-yl)cyclopropylacetamide (0.88 g, 3.40 mmol [Example 10]) and
triphosgene (2.10 g, 7.20 mmol) using a similar procedure to that of Example
2.'H
NMR (400 MHz, CDCI3) 8 9.12 (bs, 1H), 8.13 (d, 1H), 3.82 (s, 3H), 1.33-1.24
(m,
1H), 1.05-0.98 (m, 2H), 0.71-0.60 (m, 2H). MSCI: m/z 285 (MH+).
Example 12
2-Amino-6-chloro-4-cyclopropyl-7-fluoro-5-methoxypyrido[1,2-c]pyrimidine-
1,3-
dione
NH2
CI
Me
The title compound (0.09 g) was prepared from 6-chloro-4-cyclopropyl-7-
fluoro-5-methoxypyrido[1,2-c]pyrimidine-1,3-dione (0.12 g, 0.42 mmol [Example
11]) and 2,4-dinitrophenylhydroxylamine (0.11 g, 0.5 mmol) using the procedure
used to prepare Example 3. 1H NMR (400 MHz, CDC13) 8 8.17 (d, 1H), 5.52 (bs,
2H), 3.84 (s, 3H), 1.90-1.82 (m, 1H), 1.07-1.00 (m, 2H), 0.62-0.55 (m, 2H).
MSCI:
m/z 300 (MH+).
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
124
Example I3
2-Amino-4-cyclopropyl-7-fluoro-5-methoxy-6-[(R)-3-((S)-1-
methylaminoethyl)pyrrolidin-1-yl]pyrido(1,2-c]pyrimidine-1,3-dione
O
F / N~N.NH2
Me hi N ~ ~ O
MeHN~~ O a
The title compound (0.04 g) was prepared from 2-amino-6-chloro-4-
cyclopropyl-7-fluoro-5-methoxypyrido[1,2-c]pyrimidine-1,3-dione (0.09 g, 0.30
mmol [Example 12]), and methyl-((S)-(R)-1-pyrrolidin-3-ylethyl)amine (0.11 g,
0.90
mm01) using the general procedure of Example 4. 1H NMR (400 MHz, CDCl3) S 8.20
(d, 1H), 5.42 (s, 2H), 3.89-3.58 (m, 5H), 3.22 (s, 3H), 2.67-2.58 (m, 1H),
2.46 (s, 3H),
2.22-2.02 (m, 2H), 1.80-1.61 (m, 2H), 1.21 (d, 3H), 1.10-1.00 (m, 1H), 0.84-
0.76 (m,
1H), 0.52-0.44 (m, 1H), 0.4'0-0.32 (m, 1H). MSCI: m/z = 392 (MH+).
Example 14
1-Benzyl-3-(2-bromoacetyl)pyrrolidin-2-one
Br
O
N~O
To a solution of 1-benzyl-2-oxopyrrolidine-3-carboxylic acid (10 g, 45.7
mmol [US S 175157]) in tetrahydrofuran/dioxane (300 mL/60 mL) at -10 °C
was
added 4-methylmorpholine (6.5 mL, 59.4 mmol) followed by isobutyl
chloroformate
(7.10 mL, 54.8 mmol). After 10 minutes, a white precipitate was filtered off
and
washed with tetrahydrofuran. The filtrate and wash were poured into an
Erlenmyer
flask and kept at 0 °C. To this mixture was added a solution of
diazomethane (1.1M
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
125
in ether, 55 mL). After 15 minutes, a 1:1 hydrobromic acid (48%)/acetic acid
solution was added dropwise until gas evolution ceased. After 15 minutes, the
reaction mixture was diluted with ethyl acetate and washed with saturated
aqueous
sodium bicarbonate. The combined organic layers were dried over magnesium
sulfate, filtered and concentrated. The crude reaction mixture was then
purified by
chromatography (99:1 dichloromethane/methanol) to give the title compound
(26.6
g). MSCI: m/z = (MH''~ 296,298.
Example 15
1-Benzyl-3-(2-fluoroacetyl)pyrrolidin-2-one
F
0
N'~0
(\
To a solution of 1-benzyl-3-(2-bromoacetyl)pyrrolidin-2-one (2.2 g, 7.43
mmol, Example 14) in acetonitrile (25 mL) was added 18-crown-6 (0.980 g, 3.72
mmol) and potassium fluoride (spray dried) (2.16 g, 37.2 mmol). The reaction
mixture was immersed in an oil bath at 80 °C. After 1 hour, the mixture
was cooled
to room temperature and partitioned between water and ethyl acetate. The
organic
layer was dried over magnesium sulfate, filtered and concentrated. The crude
residue
was then purified by chromatography (99:1 dichloromethane/methanol then 98:2
dichloromethane/methanol) to afford the title compound (0.515 g): 1H NMR
(C1~C13)
b 7.36-7.19 (m, SH), 4.93 (m, 2H), 4.46 (m, 2H), 3.61 (m, 1H), 3.48 (m, 2H),
2.74
(m, 2H).
Example 16
1-Benzyl-3-(1-benzylamino-2-fluoroethyl)pyrrolidin-2-one
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
126
Benzylamine (3.9 mL, 35.7 mmol) was added to a solution of 1-benzyl-3-(2-
fluoro-acetyl)pyrrolidin-2-one (7.00 g, 29.8 mmol, Example 15) in
dichloroethane
(ISO mL). The solution was cooled to 0 °C and sodium
triacetoxyborohydride (8.20
g, 38.7 mmol) added. The reaction mixture was warmed to room temperature and
stirred overnight, then washed with aqueous sodium bicarbonate solution and
brine.
The organic layer was then dried over magnesium sulfate, filtered and
concentrated.
The residue was purified by chromatography (99:1 to 97:3
dichloromethane/methanol) to afford the title compound (7.2 g) as a mixture of
diastereomers. MSCI: m/z 327 (MH~.
Example 17
Benzyl[1-(1-benzylpyrrolidin-3-yl)-2-fluoroethyl) amine
To a solution of 1-benzyl-3-(1-benzylamino-2-fluoroethyl)pyrrolidin-2-one
(7.2 g, 22 mmol, Example 16) in tetrahydrofuran (100 mL) at 0°C was
added lithium
aluminum hydride (1M in tetrahydrofuran, 22 mL) dropwise. After 1 hour, the
mixture was warmed to room temperature and then quenched after 30 minutes with
0.84 mL water, 0.84 mL IS% sodium hydroxide solution and 2.5 mL water. The
reaction mixture was then filtered and concentrated. The crude residue was
purified
by chromatography (97:3 to 90:10 dichloromethane/methanol) to give the title
compound (2.5 g) as a mixture of diastereomers. MSCI: m/z = 313 (MH+).
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
127
Example 18
2-Fluoro-1-pyrrolidin-3-ylethylamine
F
NH2
N~
H
To a solution of benzyl[1-(1-benzylpyrrolidin-3-yl)-2-fluoroethyl]amine (2.5
g, 8.0 mmol, Example 17) in methanol (50 mL) was added 20% palladium on carbon
(200 mg). Hydrogen was introduced to the reaction mixture at high pressure (48
psi)
for 24 hours, at which time sulfuric acid (3 drops) was added. After an
additional 24
hours, the reaction mixture was filtered through Celite, washed with methanol,
and
the combined filtrates concentrated under vacuum to afford the title compound
(1.0
g): MSCI: m/z 133 (MH~.
Example 19
The following illustrates representative pharmaceutical dosage forms,
containing a compound of Formula I (Compound 4f), for therapeutic or
prophylactic
use in humans.
(i) Tablet mg/tablet
'Compound 4f 2 5 . 0
Lactose 50.0
Corn Starch (for mix) 10.0
Corn Starch (paste) 10.0
Magnesium Stearate ( 1 %) 3.0
300.0
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
128
The biphenylsulfonamide, lactose, and corn starch (for mix) are blended to
uniformity. The corn starch (for paste) is suspended in 200 mL of water and
heated
with stirnng to form a paste. The paste is used to granulate the mixed
powders. The
wet granules are passed through a No. 8 hand screen and dried at 80°C.
The dry
granules are lubricated with the 1% magnesium stearate and pressed into a
tablet.
Such tablets can be administered to a human from one to four times a day for
treatment of pathogenic bacterial infections.
(ii) Tablet mg/capsule
'Compound 4f 10 . 0
Colloidal Silicon Dioxide 1.5
Lactose 465.5
Pregelatinized Starch 120.0
Magnesium Stearate (1%) 3.0
600.0
(iii) Preparation for
Oral Solution Amount
'Compound 4f 4 00 mg
Sorbitol Soluition (70 % 40 mL
N.F.)
Sodium Benzoate 20 mg
Saccharin 5 mg
Cherry Flavor 20 rng
Distilled Water q.s. 100 mL'
The sorbitol solution is added to 40 mL of distilled water, and the
biphenylsulfonamide is dissolved therein. The saccharin, sodium benzoate,
flavor,
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
129
and dye are added and dissolved. The volume is adjusted to 100 mL with
distilled
water. Each milliliter of syrup contains 4 mg of invention compound.
(iv) Parenteral Solution
In a solution of 700 mL of propylene glycol and 200 mL of water for injection
is suspended 20 g of 2-Amino-4-cyclopropyl-7-fluoro-S-methyl-6-[3-(1-
methylamino-ethyl)-pyrrolidin-1-yl]-pyrido[1,2-c]pyrimidine-1,3-dione
(Compound
4f). After suspension is complete, the pH is adjusted to 6.5 with 1 N
hydrochloric
acid, and the volume is made up to 1000 mL with water for injection. The
Formulation is sterilized, filled into 5.0 mL ampoules each containing 2.0 mL,
and
sealed under nitrogen.
(v) Injection 1 (1 mglmL) Amount
'Compound 4f 1 .0
Dibasic Sodium Phosphate I2.0
Monobasic Sodium Phosphate 0.7
Sodium Chloride 4.5
1.0 N Sodium hydroxide solution q.s.
(pH adjustment to 7.0-7.5)
Water for injection q.s. ad 1 mL
_(vi) Injection 2 (10 mg/mL) Amount
'Compound 4f 10.0
Dibasic Sodium Phosphate 1.1
Monobasic Sodium Phosphate 0.3
Polyethylene glyco 400 200.0
0.1 N hydrochloric acid solution q.s.
(pH adjustment to 7.0-7.5)
Water for injection q.s. ad 1 mL
CA 02446697 2003-11-06
WO 02/096908 PCT/IB02/01598
130
_(vii) Injection 2 (10 mg/mL) Amount
'Compound 4f - 20.0
Oleic Acid 10.0
Trichloromonofluoromethane 5,000.0
Dichlorodifluoromethane 10,000.0
Dichlorotetrafluoroethane 5,000Ø
All patents, and patent documents are incorporated by reference herein, as
though individually incorporated by reference. The invention has been
described with
reference to various specific and preferred embodiments and techniques.
However, it
should be understood that many variations and modifications may be made while
remaining within the spirit and scope of the invention.