Note: Descriptions are shown in the official language in which they were submitted.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-1-
ANTIBACTERIAL AGENTS
Field of the Invention
This invention relates to antibacterial agents having a quinazolindione core
structure, processes for their preparation, and methods for their use.
Background of the Invention
Antibiotic resistance is a worldwide problem with catastrophic potential.
A Task Force co-chaired by the United States Centers for Disease Control
(CDC),
Food and Drug Administration (FDA), and National Institutes of Health (NIIII)
recently addressed this important issue, observing that drug resistant
pathogens
are a growing menace to all people, regardless of race, age, gender, or
socioeconomic background. The Task Force noted that a number of microbes
responsible for infections in humans are rapidly developing resistance to
existing
drugs. For example, according to the Task Force, in the United States alone,
up to
30 percent of the Staphylococcus pneumoniae infections (skin, bone, lung, and
bloodstream infections) are no longer susceptible to penicillin in some areas.
Up
to 11 percent of S. pneumoniae are resistant to third generation cephalosporin
antibiotics. Significantly, resistance of S. pneumoniae to the
fluoroquinolones, a
newer class of potent antibiotics, has also been reported.
Exemplified by ciprofloxacin A, the fluoroquinolones are bacterial
inhibitors that apparently exert their effect by inhibiting bacterial DNA
gyrase and
topoisomerase IV
OH
Hl
A
The consequences of antibiotic resistance, particularly fluoroquinolone
resistance, can be fatal for some individuals. In a case reported from
Denmark, a
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-2-
62-year-old woman diagnosed with food poisoning from ciprofloxacin-resistant
Salrnonella died after undergoing antibiotic treatment using that drug.
The dramatic and lethal emergence of antibiotic resistance typified by this
and other reports has spurred the U.S. Task Force to call for the
implementation of
a public health action plan to combat antimicrobial resistance. As a vital
component of that plan, there is a need for the development of new products
that
will prevent the continued emergence of antibiotic resistance generally, and
that
will prevent and treat colonization and infection of resistant organisms in
patients.
Summary of the Invention
The present invention provides compounds meeting these and other needs.
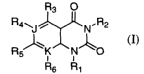
Accordingly, there is provided a compound of the invention which is a compound
of Formula I:
R~ O
Ra~J r ' ~N. R2
RS~K N' \O
Rs R1
I
or a tautomer or pharmaceutically acceptable salt thereof wherein:
R1 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R~, is H,
0
I I
-C-R~,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-3-
O
I I
-C-OR~,
O
II
-C-NR~, wherein R~ is
CI-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3, R4, and R6 independently are H,
OH,
(O)nC I -C~ alkyl and substituted alkyl,
(O)nC~,-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or I,
halo,
N02,
CN,
NRaRb, wherein Ra and Rb are each independently H,
C ~ -C~ alkyl and substituted alkyl,
C~-C~ alkenyl and substituted alkenyl,
CZ-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
I I
-C-OR~,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-4-
O
I I
-C-SR~,
O
I)
-C-R~, wherein R~ is
C1-C7 alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above; '
O
Ii
-C-NRaRe, wherein Rd and Re are independantly H,
C1-C~ alkyl and substituted alkyl,
CZ-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R1 and R6 taken together with the atoms to which they are attached form a
5, 6, 7, or 8 membered ring having from 0 to 3 heteroatoms
selected from N, O, and S, wherein said ring is optionally
substituted by one or more substituents;
RS is hydrogen,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-5-
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
ORS,
O
I I
-C-R
O
II
-OC-R~,
O
I I
-OC- OR~,
O
II
-C-OR~,
O
I I
-NC-ORS
O
I I
-C-sR~,
SR~,
O
T
-S-R
O
II
OR~,
I I
O
O
Ii
-~~ R~,
O
wherein R~ is defined
as above,
O
I I
-O ~-F,
O
O
I I
'O~ -CF3,
O
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-6-
O
I I
(Z)p-C-NRdRe, wherein Z is O or N Rd and Re are defined as
above and p is 0 or l;
halo,
N02,
CN,
NR~Rg wherein Rf and Rg are defined as for Ra and Rb above;
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl,
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
I S bicycIic heterocyclic, or spiro heterocyclic can be substituted; and
wherein J and I~ independently are C or N, provided that when J or K is N,
Rq. or R6 is absent at that position.
The invention also provides a compound of Formula II:
R3 O
R4 ~ I NR2
R5 ~ N' 'O
Rs R1
)x
or a tautomer or pharmaceutically acceptable salt thereof wherein:
RI is H,
CI-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyI,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
or heteroaryl and substituted heteroaryl;
R~isH,
O
I I
-C-R~,
O
I I
-C-ORS,
O
II
-C NR~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyI and substituted heterocycloalkyl,defined
as above;
R3, Rq., and Rg independently are H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
NRaRb, wherein Ra and Rb are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
C5-Cg cycloalkenyl and substituted cycloalkenyl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
_g_
aryl and substituted aryl, or
O
I I
-C- OR~,
O
I I
-C- SR~,
O
II
-C- R~, wherein R~ is
C1-C~ alkyl and substituted
alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
I S aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
I I
-C-NRdRe, wherein Rd and Re are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-9-
R1 and R6 taken together with the atoms to which they are attached to
form a 5, 6, 7, or ~ membered ring having from 0 to 3 heteroatoms
selected from N, O, and S, wherein said ring is optionally
substituted by one or more substituents;
RS is hydrogen,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
OR~,
O
I1 '
-C-R~,
O
II
I 5 -OC-R~,
O
I I
-OC- OR~,
O
I I
-NC- OR~,
O
Ii
-C-OR~,
O
II
-C-SRS,
SR~,
O
T
-s-R~,
O
II
I I OR~,
O
O
I I
- ~ R~,
O
wherein R~ is defined as above,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-10-
O
I(
-O~ -F,
O
O
II
-O ~-CF3,
O
O
I I
1'S (Z)p C-NRdRe, wherein Z is O or N Rd and Re are defined as
above and p is 0 or 1;
halo,
N02,
CN,
NRfRg, wherein Rf and Rg are defined as for Ra and Rb above;
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl, or
bicyclic heterocyclic or spiro heterocyclic;
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted.
The present invention also provides a compound of Formula III:
R3 O
R~ / NR2
R5 ~N~N~O
i
Rj
III
or a tautomer or pharmaceutically acceptable salt thereof wherein:
R 1 is H,
C 1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-I I-
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R~ is H,
O
I I
-C-R~,
O
II
-C-ORS,
O
Ii
-C-NR~, wherein R~ is
, _ C1-C~ alkyl and substituted alkyl,
CZ-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3, and R,~ independently are H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN, '
NRaRb, wherein Ra and Rb are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-12-
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
I I
-C- OR~,
O
Ii
-C- SR~,
O
I1
-C- R~, wherein R~ is
C1-C~ alkyl and substituted
alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
I I
-C-NRdRe, wherein Rd and Re are independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-13-
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
RS is hydrogen,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
OR~
O
I I
-C-R~,
O
I I
-OC-R~,
O
II
-OC- OR~,
O
I I
NC- OR~,
O
I I
-C-OR~,
O
II
-C-SRS,
SR~,
O
T
-S-R~,
O
II
i I OR~,
O
O
I I
R
O
wherein R~ is defined as above,
O
I I
-O I-F
O
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-14-
O
I I
O
O
I I
(Z)P C-NRdRe, wherein Z is O or N Rd and Re are defined as
above and p is 0 or 1;
halo,
NO2,
CN,
NRfRg, wherein Rf and Rg are defined as for Ra and Rb above;
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl, or
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted.
The present invention also provides a compound of Formula IV:
R3 O
N' I ~NR2
R5 ~ N' 'O
Rs R1
IV
or a pharmaceutically acceptable salt thereof wherein:
RI is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyI and substituted aIkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-15-
or heteroaryl and substituted heteroaryl;
R2 is H,
O
i1
-C-R~,
O
I I
-C-OR~,
O
II
-C-NR~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3 and Rg independently are H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ aIkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
NRaRb, wherein Ra and Rb are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
C5-Cg cycloalkenyl and substituted cycloalkenyl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-16-
aryl and substituted aryl, or
O
I I
-C-OR~,
O
II
-C-SR~,
O
I I
-C-R~, wherein R~ is
C 1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted aIkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
I I
-C-NRdRe, wherein Rd and R~ are independently H,
C1-C7 alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R1 and R6 can be taken together with the atoms to which they are attached
form a 5, 6, 7, or 8 membered ring having from 0 to 3 heteroatoms
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-17-
selected from N, O, and S, wherein said ring is optionally
substituted by one or more substituents;
R5 is hydrogen,
C1-C7 alkyl and substituted alkyl,
C2-C7 alkenyl and substituted alkenyl,
C2-C7 alkynyl and substituted alkynyl,
OR~>
O
I I
-C-R~,
O
I I '
-OC-R~,
O
I1
-OC- OR~,
O
I I
-NC- OR~,
O
i1
-C-OR~,
O
II
-C-SR~,
SR~,
O
T
-S-R~,
O
I I
I I OR~,
O
O
i1
R~,
O
wherein R~ is defined as above,
O
I I
-O~ F,
O
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-18-
O
I I
-O, -CF3,
O
O
I I
(Z)p C-NRdRe, wherein Z is O or N Rd and Re are defined as
IO above and p is 0 or l;
halo,
N02,
CN,
NRfRg, wherein Rf and Rg are defined as for Ra and Rb above;
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl,
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted.
The present invention also provides a compound of Formula V:
R3 O
N' I ~NR2
R "N"N' \O
5
R~
V
or a tautomer or pharmaceutically acceptable salt thereof wherein:
R1 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-19-
or heteroaryl and substituted heteroaryl;
R~ is H,
O
I I
S -C-R~,
O
I I
-C-ORS,
O
II
-C-NR~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C~-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3 is H,
OH,
(O)nC1-C~ alkyl and substituted alkyl,
(O)nC~-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
NO~,
CN,
NRaRb, wherein Ra and Rb are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C~-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
Cg-Cg cycloalkenyl and substituted cycloalkenyl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-20-
aryl and substituted aryl, or
O
I I
-C-OR~
O
II
-C-SR~,
O
I I
-C-R~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
I I
-C-NRdRe, wherein Ra and Re are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
RS is hydrogen,
C1-C~ alkyl and substituted alkyl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-21-
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
OR~,
O
S I I
-C-R~,
O
Ii
-OC-R~,
O
II
-OC- OR~,
O
I I
-NC- OR~,
O
!)
-C-OR~,
O
I I
-C-SRS,
S R~,
O
T
-S-R~,
O
I I
OR~,
O
O
i1
R
-~
~
O
wherein R~ is defined as above,
O
I(
-.OI L-F
O
O
I I
-O ~-CF3,
O
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-22-
O
Ii
(Z)p C-NRdRe, wherein Z is O or N Rd and Re are defined as
above and p is 0 or 1;
halo,
NO2,
CN,
NRfRg, wherein Rf and Rg are defined as for Ra and Rb above;
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl,
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted.
The invention also provides a compound of formula VI:
R3 O
Ra / NR2
Rf ~ N \ I N' '_O
i i
Rs Rs R1
VI
or a tautomer or pharmaceutically acceptable salt thereof wherein:
R1 is H,
C1-C~ alkyl and substituted alkyl,
C~-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R2 is H,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-23-
O
I I
-C-R~,
O
II
-C-OR~,
O
I I
-C-NR~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3, R4, and Rg independently are H,
OH,
(O)nC 1-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or l,
halo,
N02,
CN,
NRaRb, wherein Ra and Rb are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycIoalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-24-
O
I I
-C- OR~,
O
II
-C- SR~,
O
I I
-C- R~, wherein R~ is
CI-C~ alkyl and substituted
alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
II
-C-NRdRe, wherein Rd and Re are independantly H,
CI-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R1 and R6 taken together with the atoms to which they are attached frm a
5, 6, 7, or 8 membered ring having from 0 to 3 heteroatoms
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-25-
selected from N, O, and S, wherein said ring is optionally
substituted by one or more substituents; and
Rf and Rg are defined as for Raand Rb above.
The invention also provides a compound of formula VII:
R3 O
R4 ~ ~ NH
Rf~N ~N~N~O
i i
R9 R1
VII
or a tautomer or pharmaceutically acceptable salt thereof wherein:
R1 is H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heterocyclic and substituted heterocyclic,
or heteroaryl and substituted heteroaryl;
R2 is H,
O
I I
-C-R~,
O
i1
-C-OR~,
O
I I
-C-NR~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-26-
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3, and Rq. independently are H,
OH,
(O)nC1-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
NO2,
CN,
NRaRb, wherein Ra and Rb are each independently
H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
O
I I
-C-OR~,
O
i1
-C-SR~,
2S O
1 I
-C-R~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-27-
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
I I
-C-NRdRe, wherein Rd and Re are independently H,
C1-C7 alkyl and substituted alkyl,
C2-C7 alkenyl and substituted alkenyl,
C3-C7 cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 4, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents; and
Rf and Rg are defined as for Raand Rb above.
The invention also provides a compound of formula VIII:
R3 O
Ra / NR2
R5 \ N' 'O
X~-~Rn
m
VIII
or a tautomer or pharmaceutically acceptable salt thereof wherein:
R~ is H,
O
I I
-C-R~,
O
I I
-C-OR~,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-28-
O
I I
-C-NR~, wherein R~ is
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3 and R4 independently are H,
OH,
(O)nC1-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
NRaRb, wherein Ra and Rb are each independently H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
C5-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted aryl, or
~O
I I
-C-OR~,
O
Ii
-C-SR~,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-29-
0
I I
-C-R~, wherein R~ is
C 1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
II
-C-NRdRe, wherein Rd and Re are independantly H,
C1-C~ alkyl and substituted alkyl,
C~-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 4, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
RS is hydrogen,
C 1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C~-C~ alkynyl and substituted alkynyl,
OR~,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-30-
O
I I
-C-R~,
O
II
-OC-R~,
O
I I
-OC- OR~,
O
I I
-NC- OR~,
O
I I
-C-OR~,
O
I I
-C-SR~,
SR~,
O
T
-S-R~,
O
II
I I OR~'
O
O
II
R
O
wherein R~ is defined as above,
O
I I
-O ~-F,
O
O
I I
-O i -CF3,
O
O
I I
(Z)p-C-NRdRe, wherein Z is O or N Rd and Re are defined as
above and p is 0 or 1;
halo,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-31-
N02,
CN,
NRfRg, wherein Rf and Rg are defined as for Ra and Rb above;
aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl,
bicyclic heterocyclic or spiro heterocyclic,
wherein fused aryl, fused heterocyclic, fused heteroaryl,
bicyclic heterocyclic, or spiro heterocyclic can be substituted;
X and Y each independently are O, CH2, CH(C1-C7 alkyl), C(Cl-C~ alkyl)2,
C C
C(C3-C6 cycIoalkyI), , wherein is a C3 C6
cycloalkyl, NH, N(C~-C~ alkyl), S, SO, or 502;
m is 0-14;
Rh is H,
OH,
(O)nCl-C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyl,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1,
halo,
N02,
CN,
NR~Rk,
wherein R~and Rkindependently are H,
Cl-C~ alkyl and substituted alkyl,
CZ-C~ alkenyl and substituted alkenyl,
C2-C~. aIkynyl and substituted alkynyl,
O
I I
-C-CI-C~ alkyl and substituted alkyl, or
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-32-
R~ and Rk taken together with the nitrogen to which they are
attached form a 3- to 7-membered ring containing from 1 to 3 heteroatoms
selected from N, O, and S, said ring being unsubstituted or substituted with
1, 2, 3, or 4 substituent groups.
The invention also provides a compound of formula IX:
R3 O
R4y i N.R2
R'-V \V~K N' \O
R6 R1
IX
or a pharmaceutically acceptable salt thereof wherein:
RI is H,
C I -C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
IS aryl and substituted aryl,
heterocyclic and substituted heterocychc,
or heteroaryl and substituted heteroaryl;
R2 is H,
O
-C-R~,
O
II
-C-ORS,
O
C-NR~, wherein R~ is
CI-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-33-
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
R3, R4, and R6 independently are H,
OH,
(O)nC I -C~ alkyl and substituted alkyl,
(O)nC2-C~ alkenyl and substituted alkenyI,
(O)nC2-C~ alkynyl and substituted alkynyl,
wherein n is 0 or 1, ,
halo,
N02,
CN,
NRaRb, wherein Ra and Rb are each independently H,
CI-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C2-C~ alkynyl and substituted alkynyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
CS-Cg cycloalkenyl and substituted cycloalkenyl,
aryl and substituted
aryl, or
O
l l
-C- OR~,
O
I I
-C- SR~,
O
I I
-C- R~, wherein R~ is
Cl-C~ alkyl and substituted
alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-34-
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl,defined
as above;
O
II
-C-NRdRe, wherein Rd and Re are independantly H,
C1-C~ alkyl and substituted alkyl,
C2-C~ alkenyl and substituted alkenyl,
C3-C~ cycloalkyl and substituted cycloalkyl,
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl;
aryl and substituted aryl,
heteroaryl and substituted heteroaryl,
heterocycloalkyl and substituted heterocycloalkyl, or
Ra and Rb taken together with the nitrogen to which they are
attached form a 4, 5, 6, 7, or 8 membered ring having from 0 to
3 heteroatoms selected from N, O, and S, wherein said ring is
optionally substituted by one or more substituents;
R1 and R6 taken together with the atoms to which they are attached form a
5, 6, 7, or 8 membered ring having from 0 to 3 heteroatoms
selected from N, O, and S, wherein said ring is optionally
substituted by one or more substituents;
WZ
R'-Z' \ I'
is aryl or fused aryl,
heterocyclic or fused heterocyclic,
heteroaryl or fused heteroaryl,
bicyclic heterocyclic or spiro heter~cyclic,
wherein fused aryl, fused heterocyclic, fused
heteroaryl, bicyclic heterocyclic, or spiro heterocyclic can
be substituted;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-35-
V is N, CH, or C, provided that when Z is N or CH, "- -" is absent
and when Z is C, "- -" is a double bond;
zis0,1,2,or3;
V' is O, S, NH2, NHR", wherein R" is Cl-C~ alkyl and substituted
alkyl;
R' is
O
I I
R~ C,
O
I I
R~ OC,
O
I I
R~NH-C,
O
I I
R~S-C,
O
T
R~ S,
O
l l
R~O- I
0
O
I I
Rc I~
0
wherein R~ is defined as above,
O
I I
F- ~ I,
O
O
I I
CF3- ~; and ,
O
wherein J and K independently are C or N, provided that when J or K is N,
R4 or R6 is absent at that position.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-36-
The invention also provides a compound which is
7-(6-amino-3-aza-bicyclo[3.1.0]hex-3-yl) -6-fluoro-3H 1-
methylcyclopropyl-1H-quinazoline-2, 4-dione (la,, 5oc, hoc) hydrochloride,
1-Cyclopropyl-6-fluoro-8-methyl-7-[(R)-3-((S)-1-methylaminoethyl)-
pyrrolidin-1-yl]-1H-quinazolinedione,
1-Cyclopropyl-6-fluoro-8-methoxy-7-[ (R)-3-((S )-1-methylaminoethyl)-
pyrrolidin-1-yl]-1H-quinazolinedione,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yI]-I-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride,
1-Cyclopropyl-7-dimethylamino-6-fluoro-8-methyl-1H-quinazolinedione,
7-((S)-3-Amino-pyrrolidin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-IH
quinazolinedione trifluoroacetic acid,
7-(3-[ 1-Amino-1-(2-fluorophenyl)methyl]-pyrrolidin-1-yl }-1-cyclopropyl-
6-fluoro-6-methyl-IH quinazolinedione hydrochloride,
1-Cyclopropyl-8-methyl-7-[(R)-3-((S)-1-methylaminoethyl)pyrrolidin-1-
yl]-1H pyrido[4,3-d]pyrimidinedione hydrochloride,
7-((S)-3-Aminopyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-1H-pyrido[2,3-
d]pyrimidinedione hydrochloride,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-IH-
pyrido[2,3-d]pyrimidinedione hydrochloride,
7-((S)-3-Aminopyrrolidin-1-yl)-8-fluoro-5-methyl-5,6-
dihydropyrrolo[3,2,1-ij]quinazoline-1,3-dione hydrochloride,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl)-8-fluoro-5-methyl-5,6-
dihydropyrrolo[3,2,1-i,j] quinazoline-1,3-dione hydrochloride,
8-((S)-3-Aminopyrrolidin-1-yl)-9-fluoro-5-methyl-6,7-
dihydropyrido[3,2,1-ij]quinazoline-1,3-dione hydrochloride,
8-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-I-yl)-9-fluoro-5-methyl-6,7-
dihydro-5H-pyrido[3,2,1-i,j] quinazoline-1,3-dione hydrochloride,
1-(I -Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)cyclopropanecarbonitrile,
1-( I -Cyclopropyl-6-fJuoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)cyclopropanecarboxylic acid amide,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-37-
7-Amino-9-[9-(R)-3-((S)-I -aminoethyl)pyrrolidin-1-yl)-8-fluoro-3-
methyl-2,3-dihydro-I-oxa-3a,5-diazaphenalene-4,6-dione hydrochloride,
7-((3aR, 6aS)- and (3aS, 6aR)-4-Aminohexahydrocyclopenta[c]pyrrol-2-
yl-1-cyclopropyl-6-fluoro-8-methyl-1H quinazolinedione, hydrochloride,
7-((3aR, 6aS)- and (3aS, 6aR)-4-Aminohexahydrocyclopenta[c]pyrrol-2-
yl-1-cyclopropyl-6-fluoro-8-methoxy-1H quinazolinedione hydrochloride,
7-[3-(I-Amino-2-fluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride,
7-[3-(AminocyclopropylmethyI)-pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
8-methyl-1H-quinazolinedione hydrochloride,
7-(3-Aminomethyl-4-fluoromethylpyrrolidin- I -yl)-I -cyclopropyl-6-fluoro-
8-methyl-1H-quinazolinedione,
7-(5-Aminomethylthiophen-3-yl)-I -cyclopropyl-6-fluoro-8-methyl-1 H
quinazoline- 2,4-dione hydrochloride,
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methyl-1H quinazolinedione,
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione hydrochloride,
7-(4-Amino-5,6-dihydro-4H-cyclopenta[b]thiophen-2-yl)-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione hydrochloride,
7-[(R)-3-( I -Aminocyclopropyl)pyrrolidin-1-yl]-1-cycIopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride,
7-(4-Amino-5,6-dihydro-4H 4,5,6,7-tetrahydrobenzo[b]-thiophen-7-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione hydrochloride,
1-cyclopropyl-6-fluoro-8-methyl-7-(7-methyl-4,5,6,7-
tetrahydrothieno[2,3-c]pyridin-2-yI)-1H-quinazolinedione,
1-Cyclopropyl-6-fluoro-8-methyl-7-(4-methyl-5,6-dihydro-4H-thieno[2,3-
c]pyrrol-2-yl)-IH-quinazolinedione hydrochloride,
7-[[(3S, 4R)-3-(R)- and (3R, 4S)-3-(S)]-1-amino-2,2,2-trifluoroethyl)-4-
hydroxypyrrolidin-1-yI]-1-cyclopropyl-6-fluoro-8-methyl-1H quinazolinedione,
7-[3-(Aminooxazol-4-ylmethyl)pyrrolidin-1-yl]-1 cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-38-
7-(3R, 4S)- and 7-((3S, 4R)-3-Aminomethyl-4-fluoropyrrolidin-I-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H quinazoloinedione hydrochloride,
7-(3-Aminohexahydrofuro[2,3-c]pyrrol-5-yl)-1-cyclopropyl-6-fluoro-8-
methyl-IH-quinazolinedione hydrochloride,
7-[4-(Aminoethyl)-3,3-dimethylpyrrolidin-1-yl]-1-cylcopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride,
7-(4-Aminooctahydroisoindol-2-yl)-I-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione hydrochloride,
7-[(R)-3-((S)-I -Aminoethyl)pyrrolidin- I -yl]-1-cyclopropyl-6-fluoro-8-
fluoromethoxy-1H quinazolinedione hydrochloride,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-I -yl]-1-cyclopropyl-8-
difluoromethyl-6-fluoro-1H-quinazolinedione hydrochloride,
7-[5-(1-Aminocyclopropyl)thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride,
7-[(R)-3-(1-Aminocyclopropyl)pyrrolidin-1-yl]-1-cyclopropyl-8-
diflouromethoxy-6-fluoro-1H-quinazolinedione,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin- I -yl]-1-cyclopropyl-6-fluoro-8-
difluoromethoxy-IH quinazolinedione hydrochloride,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-I -yl]-1-cyclopropyl-6-fluoro-5,8-
dimethyl-LH quinazolinedione hydrochloride,
1-Cyclopropyl-8-difluoromethoxy-7-((R)-I -methyl-2,3-dihydro-1H-
isoindol-5-yl)-1H quinazolinedione hydrochloride,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-8-
difluoromethoxy-1H-quinazolinedione hydrochloride,
7-((3R, 4S)- and (3S, 4R)-3-Aminomethyl-4-trifluoromethylpyrrolidin-1-
yl)-1-cyclopropyl-8-difluoromethoxy-6-fluoro-1H-quinazolinedione
hydrochloride,
1-Cyclopropyl-6-fluoro-8-methoxy-7-[3(R)-(1 (S)-
methylaminoethyl)pyrrolidin-1-yl]-I H-quinazolinedione,
I-Cyclopropyl-6-fluoro-8-methyl-7-[3(R)-(1 (S)-
methylaminoethyl)pyrrolidin-I -yl]-1H-quinazolinedione,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-39-
7-(3-Aminopiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-1H
quinazolinedione,
1-Cyclopropyl-6-fluoro-8-methoxy-7-(octahydropyrrolo[3,4-c]pyridin-2-
yl)-1H-quinazolinedione,
7-((S)-3-Aminopyrrolidin-I-y1)-1-cyclopropyl-6-fluoro-8-methyl-1 H-
quinazolinedione,
7-(3-Aminopiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methyl-1 H
quinazolinedione,
1-Cyclopropyl-6-fluoro-8-methyl-7-(octahydropyrrolo[3,4-c]pyridin-2-yl)-
1H quinazolinedione,
7-(3(S)-Aminopyrrolidin-I-yl)-l -cyclopropyl-6-fluoro-8-methoxy-1 H-
quinazolinedione,
7-(3-Aminomethyl-3-methylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-1H-quinazolinedione,
7-(3-Aminomethylpyrrolidin-1-yl)-I-cyclopropyl-6-fluoro-8-methoxy-1H
quinazolinedione,
7-[3(R)-( 1-Amino-1-methylethyl)-pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
8-methoxy-1H quinazolinedione,
7-(3-Aminomethylpiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione,
7-(3-Aminomethyl-3-benzylpyrrolidin-1-yl)-I -cyclopropyl-6-fluoro-8-
methoxy-1H quinazolinedione,
I-Cyclopropyl-6-fluoro-8-methoxy-7-(octahydropyrrolo[3,4-b]pyridin-6-
yl)-1H-quinazolinedione,
7-(l-Amino-5-aza-spiro[2.4]hept-5-yl)-1-cyclopropyl-6-fluoro-8-methoxy-
1H-quinazolinedione,
7-(3-Aminomethyl-3-methylpyrrol i di n-1-yl )-1-cycl opropyl-6-fluoro-8-
methyl-IH quinazolinedione,
7-[3(R)-(1-Amino-1-methylethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
8-methyl-1H-quinazolinedione,
1-Cyclopropyl-6-fluoro-8-methyl-7-(octahydropyrrolo[3,4-b]pyridin-6-yl)-
1H-quinazolinedione,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-40-
7-(3a-Aminomethyloctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione,
7-(3S, 4R)- and 7-((3R, 4S)-3-Amino-4-fluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione,
l -Cyclopropyl-7-[3 (R)-( 1-ethylaminoethyl)pyrrol idin-1-yl]-6-fluoro-8-
methoxy-1H-quinazolinedione,
7-(3a-Aminomethyloctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-1H quinazolinedione,
7-(3S, 4R)- and 7-((3R, 4S)-3-Amino-4-fluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione,
1-Cyclopropyl-7-[(R)-3-((S)-1-ethylaminoethyl)pyrrolidin-1-yl]-6-fluoro-
8-methyl-1H quinazolinedione,
7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl -1H-quinazolinedione hydrochloride; or
a pharmaceutically acceptable salt thereof.
The invention also provides a compound of the invention which is:
7-[3-Aminocyclopropyl)-4-trifluoromethylpyrrolidin-1-yI]-1-cyclopropyl-
6-fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(3-hydroxymethylpyrrolidin-1-yl)-8-methoxy-5-
methyl-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-8-methoxy-5-methyl-7-pyrrolidin-1-yl-1 H-
quinazolinedione;
7-[3-Aminopyrrolidin-1-yl]-l -cyclopropyl-6-fluoro-8-methoxy-5~methyl-
1H-quinazolinedione;
7-[3-(2-Amino-I-hydroxyethyl)pyrrolidin-1-yI]-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-1H-quinazolinedione;
7-[3-(2-Amino-1-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-8-methoxy-5-methyl-7-morpholin-4-yl-1H
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-41-
1-Cyclopropyl-6-fluoro-8-methoxy-5-methyl-7-piperazin-1-yl-1H-
quinazolinedione;
-Cyclopropyl-7-{ 3-[(2,4-difluorophenyl)hydroxymethyl]-4-
fluoropyrrolidin-1-yl }-6-fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
1-Cyclopropyl-7-{ 3-[(4-fluorophenyl)hydroxymethyl]-4-fluoropyrrolidin-
1-yl}-6-fluoro-8-methoxy-5-methyl-IH quinazolinedione;
1-CycIopropyl-6-fluoro-7-(4-hydroxyoctahydroisoindoI-2-yI)-8-methoxy-
5-methyl-1H-quinazolinedione;
I-Cyclopropyl-6-fluoro-7-(4-hydroxyhexahydrocyclopent[c]pyrrol-2-yl)-
8-methoxy-5-methyl-1H-quinazolinedione;
5-Amino-7-[3-aminocyclopropyl)-4-trifluoromethylpyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-1H quinazolinedione;
5-Amino-1-cyclopropyl-6~fluoro-7-(3-hydroxymethylpyrrolidin-1-yl)-~-
methoxy-1H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro~8-methoxy-7-pyrrolidin-1-yl-1H
quinazolinedione;
5-Amino-7-[3-aminopyrrolidin- I -yl]-1-cyclopropyl-6-fluoro-8-methoxy-
1H-quinazolinedione;
5-Amino-7-[3-(2-amino-1-hydroxyethyl)pyrrolidin-1-yl]-I -cyclopropyl-6-
fluoro-8'-methoxy-1H-quinazolinedione;
5-Amino-7-[3-(2-amino-1-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-1H quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-8-methoxy-7-morpholin-4-yl-1H-
quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-8-methoxy-7-piperazin-1-yl-1H-
quinazolinedione;
5-Amino-1-cyclopropyl-7-{ 3-[(2,4-difluorophenyl)hydroxymethyl]-4-
fluoropyrrolidin-1-yl }-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-1-cyclopropyl-7-{ 3-[(4-fluorophenyl)hydroxymethyl]-4-
fluoropyrrolidin-1-yl}-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-(4-hydroxyoctahydroisoindol-2-yl)-8-
methoxy-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-42-
5-Amino-1-cyclopropyl-6-fluoro-7-(4-
hydroxyhexahydrocyclopent[c]pyrrol-2-yl)-8-methoxy-1H-quinazolinedione;
7-[3-Aminocyclopropyl)-4-trifluoromethylpyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-5-hydroxy-8-methoxy-1H-quinazolinedione;
I-Cyclopropyl-6-fluoro-7-(3-hydroxymethylpyrrolidin-1-yl)- 5-hydroxy-8-
methoxy-1H-quinazolinedione;
I-Cyclopropyl-6-fluoro-5-hydroxy-8-methoxy-7-pyrrolidin-1-yl-1H
quinazolinedione;
7-[3-Aminopyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-hydroxy-8-methoxy-
1H-quinazolinedione;
7-[3-(2-Amino-1-hydroxyethyl)pyrrolidin-I-yl]-1-cyclopropyl-6-fluoro-5-
hydroxy-8-methoxy-1H-quinazolinedione;
7-[3-(2-Amino-1-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-5-hydroxy-8-methoxy-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-5-hydroxy-8-methoxy-7-rnorpholin-4-yl-1H-
quinazolinedione;
1-Cycl opropyl-6-fluoro-5-hydroxy-8-methoxy-7-piperazin-1-yl-1 H-
quinazolinedione;
1-Cyclopropyl-7-{ 3-[(2,4-difluorophenyl)hydroxymethyl]-4-
fluoropyrrolidin-1-yl}-6-fluoro-5-hydroxy-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-7-{ 3-[(4-fluorophenyl)hydroxymethyl]-4-fluoropyrrolidin-
1-yl }-6-fluoro-5-hydroxy-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxyoctahydroisoindol-2-yl)- 5-hydroxy-
8-methoxy-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxyhexahydrocyclopent[c]pyrrol-2-yl)-
5-hydroxy-8-methoxy-1H-quinazolinedione;
7-[3-(1-Aminocyclopropyl)-4-trifluoromethylpyrrolidin-1-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(3-hydroxymethylpyrrolidin-
1-yl)-8-methoxy-1H-quinazolinedione;
7-(3-Aminopyrrolidin-1-yl)-1-cyclopropyl-5-difluoromethyl-6-fluoro-8-
methoxy-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-43-
7-[3-( 1-Amino-2-hydroxyethyl)-4-fluoropyrrolidin-l -yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-1H-quinazoIinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-7-pyrrolidin-1-yl-
1H quinazolinedione;
7-[3-(2-Amino-1-hydroxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-1H-quinazolinedione;
1-Cycl opropyl-5-difluoromethyl-6-fluoro-8-methoxy-7-piperazin- I -yI- I H
quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-7-morpholin-4-yl-
1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-{ 3-fluoro-4-[(4-fluorophenyl)-
hydroxymethyl]pyrrolidin-1-yl }-8-methoxy-1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-{ 3-[(2,4-
difluorophenyl)hydroxymethyl]-4-fluoropyrrolidin-1-yl } -6-fluoro-8-methoxy-1
H
quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(4-hydroxyoctahydroisoindol-
2-yl)-8-methoxy-1H-quinazolinedione;
I-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(4-hydroxyhexahydro-
cyclopenta[c]pyrrol-2-yl)-8-methoxy-1H-quinazolinedione;
7-(4-Aminooctahydrocyclohepta[c]pyrrol-2-yl)-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(3-hydroxymethylpyrrolidin-1-yl)-5,8-dimethyl-
1H-quinazolinedione;
7-(3-Aminopyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-5,8-dimethyl-1H-
quinazolinedione;
7-[3-( I -Amino-2-hydroxyethyl)-4-fluoropyrrolidin-1-yI]-1-cyclopropyl-6-
fluoro-5,8-dimethyl-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-5,8-dimethyl-7-pyrrolidin-1-yl-I H-
quinazolinedione;
7-[3-(2-Amino-1-hydroxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
5,8-dimethyl-1H quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-44-
I -Cyclopropyl-6-fluoro-5,8-dimethyl-7-piperazin-1-yl-1H-
quinazolinedione;
1-Cyclopropyl-6-fluoro-5,8-dimethyl-7-morpholin-4-yl-1H-
quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-fluoro-4-[(4-fluorophenyl)hydroxymethyl]-
pyrrolidin-1-yl }-5,8-dimethyl-1H-quinazolinedione;
1-Cyclopropyl-7-{ 3-[(2,4-difluorophenyl)hydroxymethyl]-4-
fluoropyrrolidin-1-yl}-6-fluoro-5,8-dimethyl-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxyoctahydroisoindol-2-yl)-5,8-
dimethyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-
5,8-dimethyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-
5,8-dimethyl-1H-quinazolinedione;
7-[3-(1-Aminocyclopropyl)-4-trifluoromethylpyrrolidin-I-y1]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-1H-quinazolinedione;
I-Cyclopropyl-6-fluoro-7-(3-hydroxymethylpyrrolidin-1-yl)-5-methoxy-8-
methyl-1H-quinazolinedione;
7-(3-Aminopyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-5-methoxy-8-methyl-
1H-quinazolinedione;
7-[3-(1-Amino-2-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-1H-quinazolinedione;
I -Cyclopropyl-6-fluoro-5-methoxy-8-methyl-7-pyrrolidin-1-yl-1H-
quinazolinedione;
7-[3-(2-Amino-1-hydroxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
methoxy-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-6-fluoro-5-methoxy-8-methyl-7-piperazin-1-yl-1H-
quinazolinedione;
1-Cyclopropyl-6-fluoro-5-methoxy-8-methyl-7-morpholin-4-yl-1H-
quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-fluoro-4-[(4-fluorophenyl)hydroxymethyl]-
pyrrolidin-1-yl }-5-methoxy-8-methyl-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-45-
1-Cyclopropyl-7-{ 3-[(2,4-difluorophenyl)hydroxymethyl]-4-
fluoropyrrolidin-I-yl }-6-fluoro-5-methoxy-8-methyl-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxy-octahydro-isoindol-2-yl)-5-
methoxy-8-methyl-1 H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-
5-methoxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-
5-methoxy-8-methyl-1H-quinazolinedione;
7-(3-Aminomethylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-5-
methyl-1H quinazolinedione;
7-[3-( 1-Aminocyclopropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-IH quinazolinedione;
7-[3-(1-Amino-1-methylethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-1H-quinazolinedione;
7-[3-(1-Amino-2-fluoroethyl)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-1H-quinazolinedione;
7-[3-( I -Amino-2,2-difluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
8-methoxy-5-methyl-1H quinazolinedione;
7-[3-( 1-Amino-2,2,2-triouoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
7-[3-( I -Aminoethyl)-4-fluoropyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-1H-quinazolinedione;
7-{ 3-[Amino-(2,6-difluorophenyl)methyl)pyrrolidin-I -yl }-1-cyclopropyl-
6-fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
7-(3-Aminomethyl-4-fluoromethylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-
8-methoxy-5-methyl-1H quinazolinedione;
7-[3-(Aminothiazol-2-yl-methyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
8-methoxy-5-methyl-1 H-quinazolinedione;
7-[3-(Arninocyclopropylmethyl)pyrrolidin-I -yl]-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-1H-quinazolinedione;
7-(4-Ami nooctah ydroi soi ndol-2-yl )- I -cycl opropyl-6-f1 uoro-8-methox y-5-
methyl-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-46-
7-(4-Aminooctahydrocyclohepta[c]pyrrol-2-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-7-[3-( 1-hydroxycyclopropyl)pyrrolidin-1-yl]-8-
methoxy-5-methyl-1H-quinazolinedione;
7-(4-Aminohexahydrocyclopenta[c]pyrrol-2-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-5-methyl-IH-quinazolinedione;
7-[3-(Aminooxazol-4-yl-methyl)pyrrolidin-1-yl]-I -cyclopropyl-6-fluoro-
8-methoxy-5-methyl-1H-quinazolinedione;
5-Amino-7-(3-aminomethylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-1H quinazolinedione;
S-Amino-7-[3-(1-aminocyclopropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-[3-( 1-amino-1-methylethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-1 H-quinazolinedione;
5-Amino-7-[3-(1-amino-2-fluoroethyl)pyrrolidin-I-yl]-1-cyclopropy1-6-
fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-[3-(I-amino-2,2-difluoroethyl)pyrrolidin-1-yI]-1-cyclopropyl-
6-fluoro-8-methoxy-1 H-quinazolinedione;
5-Amino-7-[3-(1-amino-2,2,2-trifluoroethyl)pyrrolidin-1-yl]-I-
cyclopropyl-6-fluoro-8-methoxy-1H quinazolinedione;
5-Amino-7-[3-(1-aminoethyl)-4-fluoropyrrolidin-I-yl]-1-cyclopropy1-6-
fluoro-8-methoxy-1H quinazolinedione;
5-Amino-7-{ 3-[amino-(2,6-difluorophenyl)methyl]pyrrolidin-1-yl }-1-
cyclopropyl-6-fluoro-8-methoxy-1 H-quinazolinedione;
2S 5-Amino-7-(3-aminomethyl-4-fluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-[3-(aminothiazol-2-yl-methyl)pyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-8-methoxy-1H=quinazolinedione;
5-Amino-7-[3-(aminocyclopropylmethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-(4-aminooctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-47-
5-Amino-7-(4-aminooctahydrocyclohepta[c]pyrrol-2-yl)-1-cyclopropy1-6-
fluoro-8-methoxy-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-[3-( 1-hydroxycyclopropyl)pyrrolidin-
1-yl]-8-methoxy-1H quinazolinedione;
5-Amino-7-(4-aminohexahydrocyclopenta[c]pynrol-2-yl)-1-cyclopropy1-6-
fluoro-8-methoxy-1H quinazolinedione;
5-Amino-7-[3-(aminoox azol-4-yl-methyl)pyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-8-methoxy-1H-quinazolinedione;
7-(3-Aminomethylpyrrolidin-1-yl)-I-cyclopropyl-5-diftuoromethyl-6-
fluoro-8-methyl-1H-quinazolinedione;
7-[3-( 1-Aminocyclopropyl)pyrrol i din-I -yl]-1-cycl opropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
7-[3-(1-Amino-1-methylethyl)pyrrolidin-1-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-[3-(1-Arnino-2-fluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-2,2-difluoroethyI)pyrrolidin-I-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
7-[3-(1-Amino-2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
7-[3-( 1-Amino-ethyl)-4-fluoro-pyrrolidin-I-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-{ 3-[(2,6-
difluorophenyl)hydroxymethyl]-pyrrolidin-1-yl }-6-fluoro-8-methyl-1H-
quinazoIinedione;
7-(3-Aminomethyl-4-fluoromethylpyrrolidin-1-yl)-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
7-[3-(Aminothiazol-2-yl- methyl)pyrrolidin-1-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-(Amino-cyclopropyI-methyl)-pyrroIidin-1-yl]-1-cyclopropyI-5-
difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-48-
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-[3-( 1-hydroxycyclopropyl)-
pyrrolidin-1-yl]-8-methyl-1H-quinazolinedione;
7-[3-(Aminooxazol-4-ylmethyl)pyrrolidin-I -yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-(3-Aminomethylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-S,8-dimethyl-
1H quinazolinedione;
7-[3-( 1-Aminocyclopropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-S,8-
dimethyl-1H-quinazolinedione;
7-[3-( 1-Amino-2-fluoroethyl)pyrrolidin-I -yI]-1-cyclopropyl-6-fluoro-S,8-
dimethyl-1H-quinazolinedione;
7-[3-( 1-Amino-2,2-difluoroethyl)pyrrolidin-1-yl]-I-cyclopropyl-6-fluoro-
S,8-dimethyl-1H-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-S,8-dimethyl-1H quinazoIinedione;
1 S 1-Cyclopropyl-7-{ 3-[(2,6-difluorophenyl)hydroxymethyl]pyrrolidin-1-yl }-
6-fluoro-S,8-dimethyl-1H quinazolinedione;
7-(3-Aminomethyl-4-fluoromethylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-
S,8-dimethyl-1H-quinazolinedione;
7-[3-(Aminothiazol-2-ylmethyl)pyrrolidin-I -yl]-1-cyclopropyl-6-fluoro-
S,8-dimethyl-1H-quinazolinedione;
7-[3-(Aminocyclopropylmethyl)pyrrolidin-I -yl]-1-cyclopropyl-6-fluoro-
S,8-dimethyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-[3-( 1-hydroxycyclopropyl)pyrrolidin-1-yl]-S,8-
dimethyl-1H quinazolinedione;
2S 7-[3-(Aminooxazol-4-ylmethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
S,8-dimethyl-1H-quinazolinedione;
S-Amino-7-(3-aminomethylpyrrolidin-1-yI)-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione;
S-Amino-7-[3-( 1-aminocyclopropyl)pyrrolidin-l -yl]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
S-Amino-7-[3-( 1-amino-1-methylethyl)pyrrolidin-l -yl]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-49-
5-Amino-7-[3-( 1-amino-2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-( 1-amino-2-fluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-( 1-aminoethyl)-4-fluoropyrrolidin- I -ylJ-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-( 1-amino-2,2-difluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-8-methyl-1H quinazolinedione;
5-Amino-1-cyclopropyl-7-{ 3-[(2,6-
difluorophenyl)hydroxymethyl]pyrrolidin-1-yl}-6-fluoro-8-methyl-1H-
quinazolinedione;
5-Amino-7-(3-aminomethyl-4-fluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-(aminothiazol-2-ylmethyl)pyrrolidin-1-y1]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-[3-( 1-hydroxycyclopropyl)pyrrolidin-
1-yl]-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-(aminooxazol-4-ylmethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
7-[3-(1-Amino-2-fluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
hydroxy-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-2,2-difluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
5-hydroxy-8-methyl-1H quinazolinedione;
7-[3-(1-Amino-2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
7-[3-(AminothiazoI-2-ylmethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
hydroxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-5-hydroxy-7-[3-( 1-
hydroxycyclopropyl)pyrrolidin-1-yI]-8-methyl-1 H-quinazolinedione;
7-[3-(Aminooxazol-4-ylmethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
hydroxy-8-methyl-1H quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-50-
7-[3-( 1-Aminocyclopropyl)pyrrolidin-1-yl]-I-cyclopropyl-6-fluoro-5-
methoxy-8-methyl-IH quinazolinedione;
7-[3-( 1-Amino-2-fluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
methoxy-8-methyl-1H quinazolinedione;
7-(3-Aminomethylpyrrolidin-I-yl)-1-cyclopropyl-6-fluoro-5-methoxy-8-
methyl-1 H-quin azolinedione;
7-[3-( 1-Amino-2,2-difluoroethyl)pyrrolidin-1-yl]- I -cyclopropyl-6-fluoro-
5-methoxy-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl)pyrrolidin-I-yl]-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-1H-quinazolinedione;
7-[3-( 1-Aminoethyl)-4-fluoropyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
methoxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-7-{ 3-[(2,6-difluorophenyl)hydroxymethyl]pyrrolidin-1-yl }-
6-fluoro-5-methoxy-8-methyl-1H-quinazolinedione;
7-(3-Aminomethyl-4-fluoromethylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-
5-methoxy-8-methyl-1H-quinazolinedione;
7-[3-(Aminothi azol-2-ylmethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
methoxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-[3-( 1-hydroxycyclopropyl)pyrrolidin-1-yl]-5-
methoxy-8-methyl-1H quinazolinedione;
7-[3-(Aminooxazol-4-ylmethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
methoxy-8-methyl-1H quinazolinedione;
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-(4-Amino-5,5-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH quinazolinedione;
7-[3-( 1-Amino-2-hydroxyethyl)pyrrolidin-1-yl]-I -cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-51-
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH quinazolinedione;
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-
tetrahydro-benzo[b]thiophen-2-yl)-8-methyl-1H-quinazolinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b)thiophen-2-yl)-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-[4-( I -Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
I-cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[4-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-
cycIopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH quinazolinedione;
7-(5-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
I -Cyclopropyl-5-difluoromethyl-6-fluoro-7-(5-hydroxymethyl-4,5,6,7-
tetrahydro-benzo[b]thiophen-2-yl)-8-methyl-7H-quinazolinedione;
7-[5-( I -Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b)thiophen-2-
yl]-I-cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH quinazolinedione;
7-[5-( I -Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-lH-quinazolinedione;
7-[5-( 1-Amino-2-hydroxyethyI)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1- cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-I-yl)-l-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione; .
7-[3-( 1-Amino-3,3,3-trifluoropropyl)pyrrolidin-1-yl]-l -cyclopropyl-5-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-( I -Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-S2-
7-[4-( 1-Aminoethyl)-3,3-difluoropyrrolidin-1-yl]-I -cyclopropyl-S-
difluoromethyl-6-fluoro-8-methyl-IH quinazolinedione;
7-(3-Amino-4-ethylpiperidin-1-yl)-1-cyclopropyl-S-difluoromethyl-6-
fluoro-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-S-
difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethoxypyrrolidin-1-yl)-1-cyclopropy1-S-
difluoromethyl-6-fluoro-8-methyl-IH quinazolinedione;
7-[3-Aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-
S-difluoro-methyl-b-fluoro-8-methyl-IH-quinazolinedione
7-[3-( 1-Amino-2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-yl]-I -
cyclopropyl-S-difluoromethyl-6-fluoro-8-methyl-IH quinazolinedione;
7-[3-( 1-Amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yI]-1-
cyclopropyl~S-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
1S 7-[3-(I-Amino-3,3-difluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-S-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-1-yl)-S-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-S-difluoromethyl-7-[7-( 1,2-dihydroxyethyl)-S-
azaspiro[2.4]hept-S-yl]-6-fluoro-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(S,S-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-S-difluoromethyl-6-fluoro-8-methyl-IH-
quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoro-1-trifluoromethylethyl)pyrrolidin-l -yl]-1-
2S cyclopropyl-S-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-Aminomethyl-4-(3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-
yl]-1-cyclopropyl-S-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-4,4,4-trifluoro-3-trifluoromethylbut-2-enyl)pyrrolidin-1-
yl]-1-cyclopropyl-S-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-[3-(1-Amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-1-yl]-1-
cyclopropyl-S-difluoromethyl-6-fluoro-8-methyl-7H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-53-
7-[3-( 1-Amino-2-hydroxyethyl)-4-fluoropyrroIidin-1-yI]-I -cyclopropyI-5-
difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl)-4-fluoropyrrolidin-1-yl]-I -
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-(3-Aminomethyl-4-difluoromethoxypyrrolidin-I -yl)-I -cyclopropyl-S-
difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
7-(4-Aminomethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
I-cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
I0 cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
I -Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrro1-2-
yl)-5-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[5-(1-Aminoethyl)thiophen-3-yI]-I-cyclopropyl-5-difluoromethyl-6-
fluoro-8-methyl-1H-quinazolinedione;
15 7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-5-difluoromethyl-6-
fluoro-8-methyl-IH-quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-I-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
20 tetrahydrobenzo[b]thiophen-2-yl)-5-difluoromethyl-6-fluoro-8-methyl-IH
quinazolinedione;
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-I -cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-7H-quinazolinedione;
7-(4-Amino-5,S-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
25. cyclopropyl-5-difluoro-methyl-6-fluoro-8-methoxy-7H-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-
yl)-5-difluoro-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-(1-Amino-2-hydroxyethyl)pyrrolidin-1-yl]-I-cyclopropyl-5- .
difluoromethyl-6-fluoro-8-methoxy-7H-quinazolinedione;
30 1-Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydroisoindol-2-y1)-5-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-54-
7-[3-( I =Amino-2-hydroxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-
tetrahydro-benzo[b]thiophen-2-yl)-8-methooxy-IH-quinazolinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
I -cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[4-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[4-(I-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2
yl]-1-cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione
7-(5-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-I
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-7H-quinazolinedione;
l -Cyclopropyl-5-difluoromethyl-6-fluoro-7-(5-hydroxymethyl-4,5,6,7-
tetrahydro-benzo[b]thiophen-2-yl)-8-methoxy-1H-quinazolinedione;
7-[5-( l -Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-I -cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione
7-[5-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[5-(1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-y1]
1- cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[5-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-S-difluoromethyl-6-fluoro-8-rnethoxy-7H-quinazoli nedione;
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-7H-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-l -yl]-I -
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-SS-
7-[4-( 1-Aminoethyl)-3,3-difluoropyrrolidin-1-yl]-1-cyclopropyl-S-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Amino-4-ethylpiperidin-1-yl)-1-cyclopropyl-S-difluoromethyl-6-
fluoro-8-methoxy-IH quinazolinedione;
7-[3-( l -Amino-2-trifluoromethoxyethyl)pyrrolidin-I -yl]-1-cyclopropyl-S-
difluoromethyl-6-fluoro-8-methoxy-IH quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethoxypyrrolidin-1-yl)-1-cyclopropy1-S-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-Aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-
S-difluoro-methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-2-difluoromethyl-3,3-difluoropropyl)pyrrolidin- I -yI]-1-
cyclopropyl-S-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-S-difluoromethyl-6-fluoro-8-methoxy-IH quinazolinedione;
1S 7-[3-(1-Amino-3,3-difluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-S-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
I -Cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-I -yl)-S-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-S-difluoromethyl-7-[7-( 1,2-dihydroxyethyl)-S-
azaspiro[2.4]hept-S-yl]-6-fluoro-8-methoxy-IH-quinazolinedione;
I -Cyclopropyl-7-(S,S-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-S-difluoromethyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoro-I -trifluoromethylethyl )pyrrolidin-1-yl]-1-
2S cyclopropyl-S-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-Aminomethyl-4-(3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-
yl]-1-cyclopropyl-S-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-4,4,4-trifluoro-3-trifluoromethylbut-2-enyl)pyrrolidin-1-
yl]-1-cycIopropyl-S-difluorornethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-(1-Amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-1-yl]-1-
cyclopropyl-S-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-56-
7-[3-( I -Amino-2-hydroxyethyl)-4-fluoropyrrolidin-I -yl)-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( I -Amino-2,2,2-trifluoroethyl)-4-fluoropyrrol idin-1-yl]- I -
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Aminomethyl-4-difluoromethoxypyrrolidin-1-yl)-1-cyclopropyl-5-
difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Aminornethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
1-cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-I-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH quinazolinedione;
l -Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrro1-2-
yl)-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[S-( 1-Aminoethyl)thiophen-3-yl]-I -cyclopropyl-5-difluoromethyl-6-
fluoro-8-methoxy-1H quinazolinedione;
7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-5-difluoromethyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-I-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
I -Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-1-cyclopropyl-5,8-
dimethyl-6-fluoro-IH-quinazolinedione;
7-(4-Amino-5,S-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-5,8-dimethyl-6-fluoro-IH quinazolinedione;
7-(5,5-Difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-cyclopropyl-5,8-
dimethyl-6-fluoro-1H-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[3-( I -Amino-2-hydroxyethyl)pyrrolidin-I -yl]-l -cyclopropyl-S-dimethyl-
6-fluoro-IH-quinazolinedione;
7-[3-( I -Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-I -yl]-I -
cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-57-
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
1-Cyclopropyl-5,8-dimethyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-
tetrahydro-benzo[b]thiophen-2-yl)-IH-quinazolinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-I -
cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[4-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
I 0 I -cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[4-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-(5-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
15 I -Cyclopropyl-5,8-dimethyl-6-fluoro-7-(5-hydroxymethyl-4,5,6,7-
tetrahydro-benzo[b]thiophen-2-yl)-1H-quinazolinedione;
7-[5-( l -Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-5,8-methyl-6-fluoro-IH-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-cyclopropyl-5,8-
20 dimethyl-6-fluoro-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin- I -yl]-I -
cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoropropyl )pyrrolidin-1-yl ]-I -cyclopropyl-5,8-
dimethyl-6-fluoro-IH-quinazolinedione;
25 7-[3-(1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-cyclopropyl-5,8-
dimethyl-6-fluoro-1H-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-1-
30 cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[3-( l -Amino-3,3,3-trifluoropropyl)pyrrol idin-l -yl]-l -cyclopropyl-5,8-
dimethyl-6-fluoro-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-5 8-
7-[4-( 1-Aminoethyl)-3,3-difluoropyrroIidin-I -yl]-1-cyclopropyI-5,8-
dimethyl-6-fluoro-IH-quinazolinedione;
7-(3-Amino-4-ethylpiperidin-1-yl)-1-cyclopropyl-5,8-dimethyl-6-fluoro-8-
1H-quinazolinedione;
7-[3-( l -Amino-2-trifluoromethoxyethyl)pyrrolidin-I -yl]-I -cyclopropyl-
5,8-dimethyl-6-fluoro-IH quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethoxypyrrolidin-I -yl)-1-cyclopropyl-5,8-
dimethyl-6-fluoro-IH-quinazolinedione;
7-[3-Aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-I -yl]-1-cyclopropyl-
5,8-dimethyl-6-fluoro-IH-quinazolinedione
7-[3-( 1-Amino-2,2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-yl]-I-
cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
7-[3-( I -Amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin- I -yl]- I -
cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
7-[3-(I-Amino-3,3-difluoropropyl)pyrrolidin-1-yI]-1-cyclopropyl-5,8-
dimethyl-6-fluoro-IH-quinazolinedione;
1-Cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-I -yl)-5,8-
dimethyl-6-fluoro-IH-quinazolinedione;
I -Cyclopropyl-5,8-dimethyl-7-[7-( 1,2-dihydroxyethyl)-5-
azaspiro[2.4]hept-5-yl]-6-fluoro-1H-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoro-1,1,1-trifluoromethylethyl)pyrrolidin-I -yl]-
l -cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[3-Aminomethyl-4-(3,3,3-trifluoro-2,2,2-
trit7uoromethylpropyl)pyrrolidin-I-yl]-I -cyclopropyl-5,8-dimethyl-6-fluoro-IH-
quinazolinedione;
7-[3-( I -Amino-4,4,4-trifluoro-3,3,-trifluoromethylbut-2-enyl)pyrrolidin-1-
yl]-1-cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
7-[3-(1-Amino-4,4,4-trifluoro-3,3,3-trifluoromethylbutyl)pyrrolidin-I-yl]-
I -cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-59-
7-[3-( 1-Amino-2-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-cyclopropyl-
5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl)-4-fluoropyrrolidin-1-yl]-1-
cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-(3-Aminomethyl-4,4-difluoromethoxypyrrol idin-1-yl)-1-cyclopropyl-
5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-(4-Aminomethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
1-cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
cyclopropyl-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrrol-2-
yl)-5,8-dimethyl-6-fluoro-IH-quinazolinedione;
7-[5-( 1-Aminoethyl)thiophen-3-yl]-1-cyclopropyl-5,8-dimethyl-6-fluoro-
1H-quinazolinedione;
7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-5,8-dimethyl-6-fluoro-
IH-quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5,8-dimethyl-6-fluoro-1H-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-5,8-dimethyl-6-fluoro-7H-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-1-cyclopropyl-5-methyl-
6-fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Amino-5,5-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-cyclopropyl-5-
methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-2-hydroxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-5-methyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( I -Ami no-3,3,3-trifl uoro-2,2,2-trifl uoromethylpropyl)pyrrol idin-1-
yl]-1-cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-60-
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-5-methyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-tetrahydro-
benzo[b]thiophen-2-yl)-8-methoxy-IH-quinazoIinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-1H quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH quinazolinedione;
7-[4-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[4-( l -Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-5-methyl-6-fluoro-8-methxoy-IH quinazolinedione;
7-(5-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
Z 5 1-Cyclopropyl-5-methyl-6-fluoro-7-(5-hydroxymethyl-4,5,6,7-tetrahydro-
benzo[b]thiophen-2-yl)-8-methoxy-IH-quinazolinedione;
7-[5-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-cyclopropyl-5-
methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-l -yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-5-
methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-(1-Amino-3,3,3-trifluoro-2,2,2-trifluoromethylpropyl)pyrrolidin-I-
yl]-1-cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Aminomethyl-4,4,4-trifluoromethylpyrrolidin-l -yl)-1-cyclopropyl-5-
methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoropropyl )pyrroiidin-1-yl ]- l -cyclopropyl-5-
methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-61-
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-I -yl)-1-cyclopropyl-S-
methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrol idin-1-yl]-1-
cyclopropyl-S-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( l -Amino-3,3,3-trifluoropropyl)pyrrolidin- l -yl]-1-cyclopropyl-S-
methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[4-( 1-Aminoethyl)-3,3-difluoropyrrol idin- I -yl]-1-cyclopropyl-S-methyl-
6-fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Amino-4-ethylpiperidin-I-y1)-I-cyclopropyl-S-methyl-6-fluoro-8-
methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-S-
methyl-6-fluoro-8-methoxy-IH quinazolinedione;
7-(3-Aminomethyl-4,4,4-trifluoromethoxypyrrolidin-1-yl)-1-cyclopropyl-
S-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
1 S 7-[3-Aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-
S-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-2,2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-S-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3-fluoro-2-fluoromethylpropyl )pyrrolidin-1-yl]- I
cyclopropyl-S-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3,3-difluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-S-
methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrol idin-1-yl )-S-methyl-
6-fluoro-8-methoxy-7H-quinazolinedione;
2S l -Cyclopropyl-S-methyl-7-[7-( l ,2-di hydroxyethyl)-S-azaspiro[2.4]hept-S-
yl]-6-fluoro-8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-7-(S,S-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-S-methyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
7-[3-(1-Amino-2,2,2-trifluoro-l,l,l-trifluoromethylethyl)pyrrolidin-I-yl]-
1-cyclopropyl-S-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-62-
7-[3-Aminomethyl-4-(3,3,3-trifluoro-2-trifluoromethylpropyI)pyrrolidin-I-
yl]-I -cyclopropyl-5-methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-4,4,4-trifluoro-3,3,3-trifluoromethylbut-2-enyl)pyrrol idin-
1-yl)-1-cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( l -Amino-4,4,4-trifluoro-3,3,3-trifluoromethylbutyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-2-hydroxyethyl)-4-fluoropyrrolidin-l -yl]-1-cyclopropyl-5-
methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl)-4-fluoropyrrol idin-I -yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Aminomethyl-4,4-difluoromethoxypyrrolidin-1-yl)- l -cyclopropyl-5-
methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Aminomethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
1-cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c)pyrroI-2-yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrrol-2-
yl)-5-methyl-6-fluoro-8-methoxy-7H quinazolinedione;
7-[5-( 1-Aminoethyl)thiophen-3-yl)-1-cyclopropyl-5-methyl-6-fluoro-8-
methoxy-IH-quinazolinedione;
7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-5-methyl-6-fluoro-8-
me°thoxy-IH-quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b)thiophen-2-yl)-1-
cyclopropyl-5-methyl-6-fluoro-8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b)thiophen-2-yl)-5-methyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
5-Amino-7-(4-amino-5,5-di fluorooctahydroisoindol-2-yl)-1-cyclopropyl-
6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-(4-amino-5,5-difluorohexahydrocyclopenta[c)pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-63-
S-Amino-7-(S,S-difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
S-Amino-7-(S,S-difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-y1)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
S-Amino-7-[3-( 1-amino-2-hydroxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
S-Amino-7-[3-( 1-amino-3,3,3-trifluoro-2,2,2-
trifluoromethylpropyl)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
S-Amino-7-(4-aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
S-Amino-1-cyclopropyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-8-methyl-IH-quinazolinedione;
S-Amino-7-[4-( 1-aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
1 S cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
S-Amino-7-[4-(2-amino-1-hydroxyethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
S-Amino-7-[4-( 1-amino-2-hydroxyethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
S-Amino-7-[4-( 1-amino-2,2,2-trifluoroethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione;
2S S-Amino-7-(5-aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
S-Amino-1-cyclopropyl-6-fluoro-7-(S-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-8-methyl-IH-quinazolinedione;
S-Amino-7-[S-(1-amino-2,2,2-trifluoroethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-64-
5-Amino-7-(3-aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-I -
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Ami no-7-[3-( 1-amino-2,2,3,3,3-pentafluoropropyl )pyrrol idin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-( 1-amino-3,3,3-trifluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-(1-amino-3,3,3-trifluoro-2,2,2-
trifluoromethylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
5-Amino-7-(3-aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-( 1-amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-I -yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-( 1-amino-3-trifluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-( 1-amino-3,3,3-trifluoro-2,2,2-
trifluoromethylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione;
5-Amino-7-[4-( 1-aminoethyl)-3,3-difluoropyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-(3-amino-4-ethylpiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methyl-IH-quinazolinedione;
5-Amino-7-[3-( 1-amino-2,2,2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-(3-aminomethyl-4-trifluoromethoxypyrrolidin-l-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-( l -amino-2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-( l -amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yl]-l -
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-65-
5-Amino-7-[3-( 1-amino-3,3-difluoropropyl)pyrrolidin-1-yI]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-1-cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-1-yI)-
6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-1-cyclopropyt-7-[7-( l ,2-dihydroxyethyl)-5-azaspiro[2.4]hept-5-
yl]-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino- I -cyclopropyl-7-(5,5-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yI)-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-( 1-amino-2,2,2-trifluoro-1-trifluoromethylethyl)pyrrolidin-
1-yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-aminomethyl-4-(3,3,3-trifluoro-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione;
5-Amino-7-[3-( 1-amino-4,4,4-trifluoro-3-trifluoromethyl-but-2-
enyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-[3-(1-amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-
1-yl]-l -cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-7-[3-( 1-amino-2-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-7H-quinazolinedione;
5-Amino-7-[3-(1-amino-2,2,2-trifluoroethyl)-4-fluoropyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Ami no-7-(3-aminomethyl-4-difluoromethoxypyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-(4-aminomethyl-5,5-difluoro-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
5-Amino-7-(4-amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-7-(5,5-difluoro-4-
hydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-6-fluoro-8-methyl-IH-
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-66-
S-Amino-7-[5-( 1-aminoethyl)thiophen-3-yl]-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione;
5-Amino-7-(5-aminomethylthiophen-3-yl)-1-cyclopropyl-6-fluoro-8-
methyl-IH-quinazolinedione;
5-Amino-7-(4-amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl)-1-cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methyl-IH-quinazolinedione;
5-Amino-7-(4-amino-5,S-difluorooctahydroisoindol-2-yl)-1-cyclopropyl-
6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-(4-amino-5,S-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H quinazolinedione;
5-Amino-7-(5,5-difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
5-Amino-7-(5,5-difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-y1)-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
5-Amino-7-[3-( 1-amino-2-hydroxyethyl)pyrrolidin-1-yl]- I -cyclopropyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
5-Amino-7-[3-( I -amino-3,3,3-trifluoro-2-
trifluoromethylpropyl)pyrrolidin-l-yl]-1-cyclopropyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
5-Amino-7-(4-aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-8-methoxy-IH-quinazolinedione;
5-Amino-7-[4-( 1-aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
5-Amino-7-[4-(2-amino- I -hydrox yethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-67-
S-Amino-7-[4-( 1-amino-2-hydrox yethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione;
S-Amino-7-[4-( I -amino-2,2,2-trifluoroethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-l -cyclopropyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
S-Amino-7-(S-aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
S-Amino-1-cyclopropyl-6-fluoro-7-(S-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-8-methoxy-lH-quinazolinedione;
S-Amino-7-[S-( 1-amino-2,2,2-trifluoroethyl)-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methoxy-1H
quinazolinedione;
S-Amino-7-(3-aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-
1S cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
S-Amino-7-[3-( 1-amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
S-Amino-7-[3-( 1-amino-3,3,3-trifluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
S-Amino-7-[3-(1-amino-3,3,3-trifluoro-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
S-Amino-7-(3-aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
2S S-Amino-7-[3-(1-amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
S-Amino-7-[3-( I -amino-3,3,3-trifluoropropyl )pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
S-Amino-7-[3-( 1-amino-3,3,3-trifluoro-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-68-
5-Amino-7-[4-( 1-aminoethyl)-3,3-difluoropyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-8-methoxy-IH-quinazolinedione;
S-Amino-7-(3-amino-4-ethylpiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-IH-quinazolinedione;
S-Amino-7-[3-( 1-amino-2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-IH-quinazolinedione;
S-Amino-7-(3-aminomethyl-4-trifluoromethoxypyrrolidin-1-yl )-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
S-Amino-7-[3-aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-1-yI]-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-[3-( 1-amino-2-difluoromethyl-3,3-difluoropropyl )pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-[3-( 1-amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-[3-(l-amino-3,3-difluoropropyl)pyrrolidin-1-yl]-I-
cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
5-Amino-1-cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-1-yl)-
6-fluoro-8-methoxy-1H quinazolinedione;
5-Amino-l -cyclopropyl-7-[7-( 1,2-dihydroxyethyl)-S-azaspiro[2.4]hept-5-
yl]-6-fluoro-8-methoxy-IH quinazolinedione;
5-Amino-1-cyclopropyl-7-(5,S-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methoxy-IH-quinazolinedione;
S-Amino-7-[3-( 1-amino-2,2,2-trifluoro-l -trifluoromethylethyl)pyrrolidin-
1-yl]-1-cycIopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
S-Amino-7-[3-aminomethyl-4-(3,3,3-trifluoro-2-
trifluoromethylpropyl)pyrrol idin-1-yl]-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione;
5-Amino-7-[3-( l -amino-4,4,4-trifluoro-3-trifluoromethyl-but-2-
enyl)pyrrol idin-1-y1]-1-cyclopropyl-6-fluoro-~-methoxy-IH-quinazolinedione;
S-Amino-7-[3-(l-amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-
1-yl]-I-cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-69-
5-Amino-7-[3-( 1-amino-2-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
5-Amino-7-[3-( 1-amino-2,2,2-trifluoroethyl)-4-fluoropyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-(3-aminomethyl-4-difluoromethoxypyrroIidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-7-(4-aminomethyl-S,5-difluoro-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione;
5-Amino-7-(4-amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
5-Amino-1-cyclopropyl-7-(5,5-difluoro-4-
hydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-6-fluoro-8-methoxy-1H-
quinazolinedione;
5-Amino-7-[5-(1-aminoethyl)thiophen-3-yl]-1-cyclopropyl-6-fluoro-8-
methoxy-IH-quinazolinedione;
5-Amino-7-(5-aminomethylthiophen-3-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-IH quinazoIinedione;
5-Amino-7-(4-amino-S,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl)-1-cyclopropyl-6-fluoro-8-methoxy-lH quinazolinedione;
5-Amino-1-cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methoxy-1H-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-
5-hydroxy-8-methyl-IH-quinazolinedione;
7-(4-Amino-5,5-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl -6-fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-
5-hydroxy-8-methyl-1H-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2-hydroxyethyl)pyrrolidin-1-y1]-l -cyclopropyl-6-fluoro-S-
hydroxy-8-methyl-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-70-
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-JH-quinazolinedione;
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-l -
cyclopropyl-6-fluoro-S-hydroxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-tetrahydro-
benzo[b]thiophen-2-yl)-S-hydroxy-8-methyl-JH-quinazolinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-S-hydroxy-8-methyl-IH-quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-6-fluoro-S-hydroxy-8-methyl-IH-quinazolinedi one;
7-[4-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-6-fluoro-S-hydroxy-8-methyl-IH-quinazolinedione;
7-[4-( 1-Amino-2,2,2-trifluoroethyl)-4.,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-6-fluoro-S-hydroxy-8-methyl-IH-quinazolinedione;
IS 7-(S-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-S-hydroxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(S-hydroxymethyl-4,5,6,7-tetrahydro-
benzo[b]thiophen-2-yl)-S-hydroxy-8-methyl-1H-quinazolinedione;
7-[S-( 1-Amino-2,2,2-trifluoroethyl )-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-6-fluoro-S-hydroxy-8-methyl-1H-quinazolinedione;
7-[S-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-S-hydroxy-8-methyl-IH-quinazolinedione;
7-[S-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1- cyclopropyl-6-fluoro-S-hydroxy-8-methyl-JH-quinazolinedione;
2S 7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-S-hydroxy-8-methyl-JH-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-1-cyclopropy-
6-fluoro-S-hydroxy-8-methyl-JH-quinazolinedione;
7-[3-( l -Amino-3,3,3-trifluoropropyl)pyrrolidin-1-yl]-l -cyclopropyl-6-
fluoro-S-hydroxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-flaoro-S-hydroxy-8-methyl-lH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-71-
7-[4-( 1-Aminoethyl)-3,3-difluoropyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
5-hydroxy-8-methyl-IH-quinazolinedione;
7-(3-Amino-4-ethylpiperidin-1-yl)-1-cyclopropyl-6-fluoro-5-hydroxy-8-
methyl-IH quinazolinedione;
7-[3-( 1-Amino-2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethoxypyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-[3-AminomethyI-4-(2,2,2-trifluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
IS 7-[3-(1-Amino-3,3-difluoropropyl)pyrrolidin-I-yl]-1-cyclopropyl-6-
fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
I -Cyclopropyl-7-(3,3-difluoro-4.-hydroxymethylpyrrolidin-1-yl)-6-fluoro-
5-hydroxy-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-[7-( 1,2-dihydroxyethyl)-5-azaspiro[2.4]kept-5-yl]-6-
fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-S-hydroxy-8-methyl-1H-
quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoro-1-trifluoromethylethyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
7-[3-Aminomethyl-4-(3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-4,4,4-trifluoro-3-trifluoromethylbut-2-enyl)pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
7-[3-(1-Amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-72-
7-[3-( I -Amino-2-hydroxyethyl)-4-fluoropyrrolidin-I -yl]-1-cyclopropyl-6-
fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl)-4-fluoropyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-(3-Aminomethyl-4-difluoromethoxypyrrolidin-I -yl)-1-cyclopropyl-6-
fluoro-5-hydroxy-8-methyl-IH quinazolinedione;
7-(4-Aminomethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
1-cyclopropyl-6-fluoro-5-hydroxy-8-methyl-IH quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrrol-2-
yl)-6-fluoro-5-hydroxy-8-methyl-IH-quinazolinedione;
7-[5-(1-Aminoethyl)thiophen-3-yl]-1-cyclopropyl-6-fluoro-5-hydroxy-8-
methyl-1H-quinazolinedione;
7-(5-Aminornethylthiophen-3-yl)-I-cyclopropyl-6-fluoro-5-hydroxy-8-
methyl-IH-quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-5-hydroxy-8-methyl-7H-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-5-hydroxy-8-methyl-IH
quinazolinedione;
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-I -cyclopropyl-6-fluoro-
5-methoxy-8-methyl-IH quinazolinedione;
7-(4-Amino-5,5-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl -6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-
5-methoxy-8-methyl-IH-quinazolinedione;
7-(S,5-Difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-(1-Amino-2-hydroxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-5-
methoxy-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-73-
7-[3-( 1-Amino-3,3,3-triouoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-emthoxy-8-methyl-IH-quinazolinedione;
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-5-methoxy-8-methyl-1H-quinazolinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-1H-quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[4-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[4-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-6-fluoro-5-methoxy-8-methyl-1H quinazolinedione;
7-(5-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-~H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(5-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-5-methoxy-8-methyl-1H-quinazolinedione;
7-[5-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-l-cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[5-( 1-Aminoethyl )-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[5-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1- cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-1-cyclopropy-
6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoropropyl)pyrrolidin- l -yl]-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-74-
7-[4-( I -Aminoethyl)-3,3-difluoropyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-
5-methoxy-8-methyl-IH-quinazolinedione;
7-(3-Amino-4-ethylpiperidin-1-yl)-l -cyclopropyl-6-fluoro-5-methoxy-8-
methyl-IH quinazolinedione;
7-[3-( 1-Amino-2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-1H quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethoxypyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-Aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-1-yl]-I-cyclopropyl-
6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-( I-Amino-2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-(1-Amino-3,3-difluoropropyl)pyrrolidin-1-yl]-I-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-1-yl)-6-fluoro-
5-methoxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-7-[7-( I ,2-dihydroxyethyl)-5-azaspiro[2.4]kept-5-yl]-6-
fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-5-methoxy-8-methyl-1H-
quinazolinedione;
7-(3-( I -Amino-2,2,2-trifluoro-1-trifluoromethylethyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-(3-Aminomethyl-4-(3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-4,4,4-trifluoro-3-trifluoromethyl-but-2-enyl)pyrroli~din-1-
yl]-I -cyclopropyl-6-fluoro-5-methoxy-8-methyl-lH-quinazolinedione;
7-[3-(1-Amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-7H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-75-
7-[3-( 1-Amino-2-hydroxyethyl)-4-fluoropyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl )-4-fluoropyrrol idin-1-yl]-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-IH-quinazolinedione;
7-(3-Aminomethyl-4-difluoromethoxypyrroIidin-1-yl)-1-cyclopropyl-6-
fluoro-5-methoxy-8-methyl-IH quinazolinedione;
7-(4-Aminomethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
1-cyclopropyl-6-fluoro-5-methoxy-8-methyl-IFI quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-5-methoxy-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrrol-2-
yl)-6-fluoro-5-methoxy-8-methyl-IH quinazolinedione;
7-[5-( 1-Aminoethyl)thiophen-3-yl]-1-cyclopropyl-6-fluoro-5-hydroxy-8-
methyl-1H-quinazolinedione;
7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-6-fluoro-5-methoxy-8-
methyl-IH-quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-S-methoxy-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-5-methoxy-8-methyl-IH-
quinazolinedione;
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-
8-methyl-IH-quinazolinedione;
7-(4-Amino-5,5-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-
8-methyl-1H-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-[3~(l-Amino-2-hydroxyethyl)pyrrolidin-I-yI]-1-cyclopropyI-6-fluoro-8-
methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-76-
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-I -yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-8-methyl-1H-quinazolinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
l 0 1-cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[4-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[4-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
15 7-(S-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(5-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-8-methyl-1H quinazolinedione;
7-[5-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
20 yl]-1-cyclopropyl-6-fluoro-8-methyl-IH quinazolinedione;
7-[5-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-[5-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1- cyclopropyl-6-fluoro-8-methyl-IH quinazolinedione;
25 7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-I-yl)-1-cyclopropyl-6-
fluoro-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoropropyl)pyrrolidin-1-yl]-l -cyclopropyl-6-
30 fluoro-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-l -yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
_77_
7-[4-( I -Aminoethyl)-3,3-difluoropyrrol idin-1-yl]-I -cyclopropyl-6-fluoro-
8-methyl-IH-quinazolinedione;
7-(3-Amino-4-ethylpiperidin-l -yl)-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
7-[3-( 1-Amino-2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methyl-IH-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethoxypyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-8-methyl-IH-quinazolinedione;
7-[3-Aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-I-yl]-1-cyclopropyl-
6-fluoro-8-methyl-1H-quinazolinedione;
7-[3-( I -Amino-2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-7H-quinazolinedione;
7-[3-( 1-Amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH quinazolinedione
7-[3-(I-Amino-3,3-difluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-1-yl)-6-fluoro-
8-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-[7-( 1,2-dihydroxyethyl)-5-azaspiro[2.4]kept-5-yl]-6-
fluoro-8-methyl-1H-quinazolinedione;
I -Cyclopropyl-7-(5,5-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yI)-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-( l -Amino-2,2,2-trifluoro-1-trifluoromethylethyl)pyrrolidin-1-yl]-I
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-Aminomethyl-4-(3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-I -
yl]-1-cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-4,4,4-trifluoro-3-trifluoromethyl-but-2-enyl)pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
7-[3-(l-Amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-78-
7-[3-( 1-Amino-2-hydroxyethyl)-4-fluoropyrrolidin-I -yl]-1-cyclopropyl-6-
fluoro-8-methyl-IH-quinazolinedione;
7-[3-( l -Ami no-2,2,2-trifluoroethyl )-4-fluoropyrrol idin-1-yl]-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-(3-Aminomethyl-4-difluoromethoxypyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-8-methyl-IH-quinazolinedione;
7-(4-Aminomethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
1-cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
l -Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrro1-2-
yl)-6-fluoro-8-methyl-IH-quinazolinedione;
7-[S-( I -Aminoethyl)thiophen-3-y1 J-1-cyclopropyl-6-fluoro-8-methyl-IH
quinazolinedione;
7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-IH quinazolinedione
I -Cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methyl-IH-quinazolinedione
7-(4-Amino-5,5-difluorooctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-
8-methoxy-1H-quinazolinedione;
7-(4-Amino-5,5-difluorohexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyoctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-
8-methoxy-1H-quinazolinedione;
7-(5,5-Difluoro-4-hydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-2-hydroxyethyl)pyrrolidin-l -yl]-I -cyclopropyl-6-fluoro-8-
methoxy-IH quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-trio uoromethylpropyl)pyrrol idin-I -yl]-I -
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-79-
7-(4-Aminomethyl-4,5,6,7-tetrahydrobenzojb]thiophen-2-yl)-1-
cyclopropyI-6-fluoro-8-methoxy-IH quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxymethyl-4,5,6,7-tetrahydro-
benzo[b]thiophen-2-yl)-8-methoxy-IH quinazolinedione;
7-[4-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[4-(2-Amino-1-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
7-[4-( 1-Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1-cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-j4-( I -Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-1-cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
7-(5-Aminomethyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(5-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-8-methoxy-IH-quinazolinedione;
7-[5-( 1-Amino-2,2,2-trifluoroethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-
yl]-l-cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
7-[5-( 1-Aminoethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[5-( I -Amino-2-hydroxyethyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-
1- cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethylpyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-8-methoxy-7H-quinazolinedione;
7-[3-(1-Amino-2,2,3,3,3-pentafluoropropyl)pyrrolidin-1-yl]-I-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[4-( l -Aminoethyl)-3,3-difluoropyrrol idin-1-yl]-l -cyclopropyl-6-fluoro-
8-methoxy-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-80-
7-(3-Amino-4-ethylpiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-IH-
quinazolinedione;
7-[3-( 1-Amino-2-trifluoromethoxyethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
7-(3-Aminomethyl-4-trifluoromethoxypyrroiidin-1-y1)-1-cyclopropyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
7-[3-Aminomethyl-4-(2,2,2-trifluoroethyl)pyrrolidin-l -yl]-1-cyclopropyl-
6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-( 1-Amino-2-difluoromethyl-3,3-difluoropropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3-fluoro-2-fluoromethylpropyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-3,3-difluoropropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-7-(3,3-difluoro-4-hydroxymethylpyrrolidin-1-yl)-6-fluoro-
8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-7-[7-( 1,2-dihydroxyethyl)-5-azaspiro[2.4]hept-5-yl]-6-
fluoro-8-methoxy-IH quinazolinedione;
I -Cyclopropyl-7-(5,5-difluoro-4-hydroxymethyl-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methoxy-lH quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoro-1-trifluoromethylethyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione;
7-[3-Aminomethyl-4-(3,3,3-trifluoro-2-trifluoromethylpropyl)pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-8-methoxy-IH quinazolinedione;
7-[3-(1-Amino-4,4,4-trifluoro-3-trifluoromethylbut-2-enyl)pyrrolidin-1-
yl]-1-cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( I -Amino-4,4,4-trifluoro-3-trifluoromethylbutyl)pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Ami no-2-hydroxyethyl)-4-fluoropyrrol idin-l -yl]-1-cyclopropyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
7-[3-( 1-Amino-2,2,2-trifluoroethyl )-4-fluoropyrrol idin-1-yl ]- l -
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
_81_
7-(3-Aminomethyl-4-difluoromethoxypyrrolidin-1-yl)-1-cyclopropyl-6-
fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Aminomethyl-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-
1-cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
7-(4-Amino-5,5-difluorooctahydrocyclohepta[c]pyrrol-2-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-7-(5,5-difluoro-4-hydroxyoctahydrocyclohepta[c]pyrrol-2-
yl)-6-fluoro-8-methyl-IH-quinazolinedione;
7-[5-( 1-Aminoethyl)thiophen-3-yl]-1-cyclopropyl-6-fluoro-8-methoxy-
IH quinazolinedione;
7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione;
7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl )-1-
cyclopropyl-6-fluoro-8-methoxy-IH-quinazolinedione;
1-CycIopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methoxy-IH-quinazolinedione;
1-Cyclopropyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)pyrrolidin-1-
yl]-6-fluoro-5,8-dimethyl-1 H-quinazolinedione;
1-Cyclopropyl-b-fluoro-5,8-dimethyl-7-[3-(3,3,3-trifluoro-1,2-dihydroxy-
2-trifluoromethylpropyl)pyrrolidin-1-yl]-IH-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-[hydroxyl 1-
hydroxycyclopropyl)methyl]pyrrolidin-1-yl }-5,8-dimethyl-1 H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-[hydroxyl 1-
hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-5,8-dimethyl-1 H-quinazolinedione;
7-[3-(1-Amino-2-hydroxy-2-methylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-
6-fluoro-5,8-dimethyl-1 H-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-hydroxy-2-
trifluoromethylpropyl)pyrrolidin-l -yl]-1-cyclopropyl-6-fluoro-5,8-dimethyl-1
H-
quinazolinedione;
7-{3-[Amino-(1-hydroxycyclopropyl)methyl]pyrrolidin-1-yl}-1-
cyclopropyl-6-fluoro-5,8-dimethyl-1 H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-82-
7-{ 3-[Amino-( 1-hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-I-
cyclopropyl-6-fluoro-S,8-dimethyl-1H-quinazolinedione;
1-Cyclopropyl-7-(4,S-dihydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-6-
fluoro-5,8-dimethyl-1H-quinazolinedione;
1-Cyclopropyl-7-(4,S-dihydroxyoctahydroisoindol-2-yl)-6-fluoro-S,8-
dimethyl-1H-quinazolinedione;
1-Cyclopropyl-7-(4,S-dihydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-6-
fluoro-5,8-dimethyl-1H quinazolinedione;
1-Cyclopropyl-7-(4,S-dihydroxydecahydrocycloocta[c]pyrrol-2-yl)-6-
IO fluoro-S,8-dimethyl-lH-quinazolinedione;
S-Amino-1-cyclopropyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)pyrrolidin-1-
yl]-6-fluoro-8-methyl-1 H-quinazolinedione;
S-Amino-1-cyclopropyl-6-fluoro-8-methyl-7-[3-(3,3,3-trifluoro-1,2-
dihydroxy-2-trifluoromethylpropyl)pyrrolidin-I-yl]-1H-quinazolinedione;
1S S-Amino-1-cyclopropyl-6-fluoro-7-{3-[hydroxy-(I-
hydroxycyclopropyl)methyl]pyrrolidin-1-yl } -8-methyl-1 H-quinazolinedione;
S-Amino-1-cyclopropyl-6-fluoro-7-{ 3-[hydroxy-( 1-
hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-8-methyl-1 H-quinazolinedione;
S-Amino-7-[3-( 1-amino-2-hydroxy-2-methylpropyl)pyrrolidin-1-yl]-1-
20 cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
S-Amino-7-[3-( 1-amino-3,3,3-trifluoro-2-hydroxy-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-I -cyclopropyl-6-fluoro-8-methyl-I H-
quinazolinedione;
S-Amino-7-{ 3-[amino-( 1-hydroxycyclopropyl )methyl]pyrrolidin-1-yl } -1-
2S cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione;
S-Amino-7-{ 3-[amino-( I -hydroxycyclopentyl)methyl]pyrrolidin-1-yl } -1-
cyclopropyl-6-fluoro-8-methyl-1 H-quinazoIinedione;
S-Amino-1-cyclopropyl-7-(4,S-dihydroxyhexahydrocyclopenta[c]pyrro1-2-
yl)-6-fluoro-8-methyl-I H-quinazolinedione;
30 S-Amino-1-cyclopropyl-7-(4,S-dihydroxyoctahydroisoindol-2-yl)-6-
fluoro-8-methyl-I H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-83-
5-Amino-1-cyclopropyl-7-(4,5-dihydroxyoctahydrocyclohepta[c]pyrrol-2-
yl)-6-fluoro-8-methyl-1 H-quinazolinedione;
5-Amino-I -cyclopropyl-7-(4,5-dihydroxydecahydrocycloocta[c]pyrrol-2-
y1 )-6-fluoro-8-methyl-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-[3-( 1,2-dihydroxy-2-
methylpropyl)pyrrolidin-1-y1]-6-fluoro-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-7-[3-(3,3,3-trifluoro-
1,2-dihydroxy-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-{ 3-[hydroxy-( 1-
l0 hydroxycyclopropyl)methyl]pyrrolidin-1-yl}-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-{ 3-[hydroxy-( I -
hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-8-methyl-1H-quinazolinedione;
7-[3-( 1-Amino-2-hydroxy-2-methylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-
5-difluoromethyl-6-fluoro-8-methyl-1H-quinazolinedione;
15 7-[3-(I-Amino-3,3,3-trifluoro-2-hydroxy-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-I-cyclopropyl-5-difluoromethyl-6-fluoro-
8-methyl-1H-quinazolinedione;
7-{ 3-[Amino-( I -hydroxycyclopropyl)methyl]pyrrolidin-l -yl }-l -
cycIopropyl-5-difluoromethyl-6-fluoro-8-methyl-1 H-quinazolinedione;
20 7-{3-[Amino-(1-hydroxycyclopentyl)methyl]pyrrolidin-1-yl}-1-
cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(4,5-
dihydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-6-fluoro-8-methyl-1H-
quinazolinedione;
25 1-Cyclopropyl-5-difluoromethyl-7-(4,5-dihydroxyoctahydroisoindol-2-yl)-
6-fluoro-8-methyl-1 H-quinazoli nedione;
1-Cyclopropyl-5-difluoromethyl-7-(4,5-
dihydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-6-fluoro-8-methyl-1 H-
quinazolinedione;
30 1-Cyclopropyl-5-difluoromethyl-7-(4,5-
dihydroxydecahydrocycloocta[cJpyrrol-2-yl)-6-fluoro-8-methyl-1 H-
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-84-
1-Cyclopropyl-6-fluoro-7-(3-fluoro-4-hydroxymethylpyrrolidin-1-yl)-5,8-
dimethyl-1H-quinazolinedione;
l -Cyclopropyl-7-(3,4-dihydroxypiperidin-1-yl)-6-fluoro-5,8-dimethy1-1 H-
quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(3-hydroxymethyl-4-methylpiperidin-1-yl)-5,8-
dimethyl-1H-quinazolinedione;
1-Cycl opropyl -7-[3-( 1,2-di h ydrox y-2-meth ylpropyl )-4-fl uoropyrrol i d
i n-1-
yl]-6-fluoro-5,8-dimethyl-IH-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopropyl)methyl]pyrrolidin-1-yl}-5,8-dimethyl-1H quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-fluoro-4-(hydroxy-(1-
hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-5,8-dimethyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-[3-fluoro-4-(3,3,3-trifluoro-1,2-dihydroxy-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-5,8-dimethyl-IH-quinazolinedione;
1-Cyclopropyl-7-(4-ethyl-3-hydroxypiperidin-1-yl)-6-fluoro-5,8-dimethyl-
1H-quinazolinedione;
l -Cyclopropyl-7-(4-ethyl-3-hydroxymethylpiperidin-1-yl)-6-fluoro-5,8-
dimethyl-1 H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(3-hydroxy-4-methylpiperidin-1-yl)-5,8-
dimethyl-1H-quinazolinedione;
l -Cyclopropyl-6-fluoro-5,8-dimethyl-7-[3-(2,2,2-trifluoro-I -
hydroxyethyl)pyrrolidin-1-yl]-1 H-quinazolinedione;
1-Cyclopropyl-7-[3-( 1,2-dihydroxyethyl)-4-fluoropyrrolidin-1-yl]-6-
fluoro-5,8-dimethyl-l H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(4-hydroxy-3-hydroxymethylpiperidin-1-yl)-
5,8-dimethyl-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-(3-fluoro-4-hydroxymethylpyrrolidin-
1-yl)-8-methyl-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-7-[3-( 1,2-dihydroxyethyl )-4-fluoropyrroli di n-1-
yl]-6-fluoro-8-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-8-methyl-7-[3-(2,2,2-trifluoro-1-
hydroxyethyl)pyrrolidin-I-yl]-1H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-85-
5-Amino-1-cyclopropyl-7-(3,4-dihydroxypiperidin-1-yl)-6-fluoro-8-
methyl-1H-quinazolinedione;
l -Cycl opropyl-6-fl uoro-7-(4-h ydrox y-3-h ydrox ymeth y1 pi peri d i n- I -
yl )-
5,8-dimethyl-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-(3-hydroxymethyl-4-methylpiperidin-
1-yl)-8-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)-4-
fluoropyrrolidin-1-yl]-6-fluoro-8-methyl-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-{ 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopropyl)methyl]pyrrolidin-1-yl}-8-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-(3-hydroxy-4-methylpiperidin-1-y1)-8-
methyl-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-7-(4-ethyl-3-hydroxypiperidin-1-yl)-6-fluoro-8-
methyl-1H-quinazolinedione;
IS 5-Amino-1-cyclopropyl-7-(4-ethyl-3-hydroxymethylpiperidin-1-yl)-6-
fluoro-8-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-[3-fluoro-4-(3,3,3-trifluoro-1,2-
dihydroxy-2-trifluoromethylpropyl)pyrrolidin-1-yl]-8-methyl-1 H-
quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-{3-fluoro-4-[hydroxy-(1-
hydroxycyclopentyl)methyl]pyrrol idin-1-yl } -8-methyl-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-{ 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopropyl)methyl]pyrrolidin-1-yl }-8-methyl-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)-4-
fluoropyrrolidin-1-yl]-6-fluoro-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(3-hydroxymethyl-4-
methylpiperidin-1-yl)-8-methyl-1 H-quinazolinedione;
l -Cyclopropyl-5-difluoromethyl-7-(3,4-dihydroxypiperidin-l -yl)-6-fluoro-
8-methyl-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(3-fluoro-4-
hydroxymethyIpyrrolidin-1-yl)-8-methyl-1 H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-86-
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7- { 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-[3-fluoro-4-(3,3,3-trifluoro-
1,2-dihydroxy-2-trifluoromethylpropyl) pyrrolidin-1-yl]-8-methyl-IH-
quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(4-ethyl-3-hydroxymethylpiperidin-1-
yl)-6-fluoro-8-methyl-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(4-ethyl-3-hydroxypiperidin-1-yl)-6-
fluoro-8-methyl-IH-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(4-hydroxy-3-
hydroxymethylpiperidin-l -yl)-8-methyl-1 H-quinazolinedi one;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(3-hydroxy-4-
methylpiperidin-1-yl)-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-[3-( 1,2-dihydroxyethyl)-4.-
fluoropyrrolidin-1-yl]-6-fluoro-8-methyl-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-8-methyl-7-[3-(2,2,2-trifluoro-1-
hydroxyethyl)pyrrolidin-1-yl]-1 H-quinazolinedione;
1-Cyclopropyl-7-[3-( I ,2-dihydroxy-2-methylpropyl)-pyrrolidin-1-yl]-6-
fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-8-methoxy-5-methyl-7-[3-(3,3,3-trifluoro-1,2-
dihydroxy-2-trifluoromethylpropyl)pyrrolidin-1-yl]-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-[hydroxy-( 1-hydroxycyclopropyl)methyl]-
pyrrolidin-l -yl }-8-methoxy-5-methyl-1 H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-{ 3-[hydroxy-( 1-hydroxycyclopentyl)-
methyl]pyrrol idin-l -yl } -8-methoxy-5-methyl-1 H-quinazol inedione;
7-[3-( 1-Amino-2-hydroxy-2-methylpropyl)-pyrrolidin-1-yl]-1-
cyclopropyl-6-fluoro-8-methoxy-5-methyl-1 H-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-hydroxy-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-l-cyclopropyl-6-fluoro-8-methoxy-5-
methyl-1 H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
_87_
7-{ 3-[Amino-( I-hydroxycyclopropyl)methyl]pyrrolidin-1-yl }-l -
cyclopropyl-6-fluoro-8-methoxy-5-methyl-1 H-quinazolinedione;
7-{ 3-[Amino-(1-hydroxycyclopentyl)methyl)pyrrolidin-1-yl }-1-
cyclopropyl-6-fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
1-Cyclopropyl-7-(4,5-dihydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-6-
fluoro-8-methoxy-5-methyl-IH-quinazolinedione;
1-Cyclopropyl-7-(4,5-dihydroxyoctahydroisoindol-2-yl)-6-fluoro-8-
methoxy-5-methyl-1 H-quinazolinedione;
1-Cyclopropyl-7-(4,5-dihydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-6-
fluoro-8-methoxy-5-methyl-IH-quinazolinedione;
l -Cyclopropyl-7-(4,5-dihydroxydecahydrocycloocta[c]pyrrol-2-yl)-6-
fluoro-8-methoxy-5-methyl-1H-quinazolinedione;
5-Amino-1-cyclopropyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)pyrrolidin-1-
yl]-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-I-cyclopropyl-6-fluoro-8-methoxy-7-[3-(3,3,3-trifluoro-1,2-
dihydroxy-2-trifluoromethylpropyl)pyrrolidin-I -yl]-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-{ 3-[hydroxy-( 1-
hydroxycyclopropyl)methyl]-pyrrolidin-I -yl }-8-methoxy-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-{ 3-[hydroxy-(I-
hydroxycyclopentyl)methyl]-pyrrolidin-1-yl}-8-methoxy-lH-quinazolinedione;
5-Amino-7-[3-( 1-amino-2-hydroxy-2-methylpropyl)pyrrolidin-I -yl]-1-
cyclopropyl-6-fluoro-8-methoxy-l H-quinazolinedione;
5-Amino-7-[3-( l -amino-3,3,3-trifluoro-2-hydroxy-2-
trifluoromethylpropyl )pyrrol idin-1-yl]-1-cyclopropyl-6-fl uoro-8-methoxy-1 H-
quinazolinedione;
5-Amino-7-{ 3-[amino-( 1-hydroxycyclopropyl)methyl]pyrrolidin-1-yl }-1-
cyclopropyl-6-fluoro-8-methoxy-l H-quinazolinedione;
5-Amino-7-{ 3-[amino-( 1-hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-1-
cyclopropyl-6-fluoro-8-methoxy-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-7-(4,5-dihydroxyhexahydrocyclopenta[c]pyrro1-2-
yl)-6-fluoro-8-methoxy-1H quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
_g8_
5-Amino-1-cyclopropyl-7-(4,5-dihydroxyoctahydroisoindol-2-yl)-6-
fluoro-8-methoxy-1 H-quinazolinedione;
S-Amino-1-cyclopropyl-7-(4,5-dihydroxyoctahydrocyclohepta[c]pyrro1-2-
yl)-6-fluoro-8-methoxy-1H-quinazolinedione;
S-Amino-l -cyclopropyl-7-(4,5-dihydroxydecahydrocycloocta[c]pyrrol-2-
yl)-6-fluoro-8-methoxy-1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-[3-( 1,2-dihydroxy-2-
methylpropyl)pyrrolidin-1-yl]-6-fluoro-8-methoxy-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-7-[3-(3,3,3-trifluoro-
1,2-dihydroxy-2-trifluoromethylpropyl)pyrrolidin-1-yl]-I H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-{ 3-[hydroxy-( 1-hydroxy-
cyclopropyl)methyl]pyrrolidin-1-yl}-8-methoxy-1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-{ 3-[hydroxy-( 1-
hydroxycyclopentyl)methyl]pyrrolidin-1-yl } -8-methoxy-1 H-quinazolinedione;
7-[3-(I-Amino-2-hydroxy-2-methylpropyl)pyrrolidin-1-yl]-1-cyclopropyl-
5-difluoromethyl-6-fluoro-8-methoxy-1 H-quinazolinedione;
7-[3-( 1-Amino-3,3,3-trifluoro-2-hydroxy-2-
trifluoromethylpropyl)pyrrolidin-1-yl]-l -cyclopropyl-5-difluoromethyl-6-
fluoro-
8-methoxy-1 H-quinazolinedione;
7-{3-[Amino-(1-hydroxycyclopropyl)methyl]pyrrolidin-1-yl}-1-
cyclopropyl-S-difluoromethyl-6-fluoro-8-methoxy-I H-quinazolinedione;
7-{ 3-[Amino-(1-hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-1-
cyclopropyl-S-difluoromethyl-6-fluoro-8-methoxy-l H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(4,S-
dihydroxyhexahydrocyclopenta[c]pyrrol-2-yl)-6-fluoro-8-methoxy-1H-
quinazolinedione;
l -Cyclopropyl-5-difluoromethyl-7-(4,5-dihydroxyoctahydroisoindol-2-yl)-
6-fluoro-8-methoxy-1 H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(4,5-
dihydroxyoctahydrocyclohepta[c]pyrrol-2-yl)-6-fluoro-8-methoxy-1H-
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-89-
1-Cyclopropyl-5-difluoromethyl-7-(4,5-
dihydroxydecahydrocycloocta[c]pyrrol-2-yl)-6-fluoro-8-methoxy-1 H-
quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(3-fluoro-4-hydroxymethylpyrrolidin-1-yl)-8-
methoxy-5-methyl-1 H-quinazolinedione;
1-Cyclopropyl-7-[3-( 1,2-dihydroxyethyl)-4-fluoropyrrolidin-1-yl]-6-
fluoro-8-methoxy-5-methyl-1 H-quinazolinedione;
l -Cycl opropyl -6-fluoro-8-methox y-5-methyl-7-[3-(2,2,2-tri fl a oro-1-
hydroxyethyl)pyrrolidin-1-yl]-1 H-quinazolinedione;
I 0 1-Cyclopropyl-7-(3,4-dihydroxypiperidin-1-yl)-6-fluoro-8-methoxy-S-
methyl-1 H-quinazolinedione;
I -Cyclopropyl-6-fluoro-7-(4-hydroxy-3-hydroxymethylpiperidin-I -yl)-8-
methoxy-5-methyl-1 H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(3-hydroxy-4-methylpiperidin-1-yl)-8-methoxy-
15 5-methyl-1H-quinazolinedione;
1-Cyclopropyl-6-fluoro-7-(3-hydroxymethyl-4-methylpiperidin-I -yl)-8-
methoxy-5-methyl-l H-quinazolinedione;
1-Cyclopropyl-7-(4-ethyl-3-hydroxymethylpiperidin-1-yl)-6-fluoro-8-
methoxy-5-methyl-1 H-quinazolinedione;
20 1-Cyclopropyl-7-(4-ethyl-3-hydroxypiperidin~1-yl)-6-fluoro-8-methoxy-5-
methyl-IH-quinazolinedione;
1-Cyclopropyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)-4-fluoropyrrolidin-1-
yl]-6-fluoro-8-methoxy-5-methyl-1 H quinazolinedione;
1-Cyclopropyl-6-fluoro-7-[3-fluoro-4-(3,3,3-trifluoro-l ,2-dihydroxy-2-
25 trifluoromethylpropyl)-pyrrolidin-1-yl]-8-methoxy-5-methyl-1H-
quinazolinedione;
1-Cyclopropyl-6-fluoro-7- { 3-fluoro-4-[hydroxy-( I -
hydroxycyclopropyl)methyl]-pyrrolidin-1-yl }-8-methoxy-5-methyl-1H-
quinazolinedione;
30 I -CyclopropyI-6-fluoro-7- { 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopentyl)methylJpyrrolidin-l -yl } -8-methoxy-5-methyl-1 H-
quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-90-
5-Amino-1-cyclopropyl-6-fluoro-7-(3-fluoro-4-hydroxymethylpyrrolidin-
l -yl)-$-methoxy-I H-quinazolinedione;
5-Amino-1-cyclopropyl-7-(3-(1,2-dihydroxyethyl)-4-fluoropyrrolidin-I -
yl]-6-fluoro-8-methoxy-1 H=quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-8-methoxy-7-[3-(2,2,2-trifluoro-1-
hydroxyethyl)pyrrolidin-1-yl]-IH-quinazolinedione;
5-Amino-1-cyclopropyl-7-(3,4-dihydrox ypiperidin-1-yl )-6-fluoro-8-
methoxy-1H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-(4-hydroxy-3-hydroxymethylpiperidin-
1-yl)-8-methoxy-1 H-quinazolinedione;
5-Amino-l -cyclopropyl-6-fluoro-7-(3-hydroxy-4-methylpiperidin-1-yl)-8-
methoxy-1 H-quinazolinedione;
5-Amino-I -cyclopropyl-6-fluoro-7-(3-hydroxymethyl-4-methylpiperidin-
I-yl)-8-methoxy-1H-quinazolinedione;
5-Amino-1-cyclopropyl-7-(4-ethyl-3-hydroxymethylpiperidin-I-yl)-6-
fluoro-8-methoxy-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-7-(4-ethyl-3-hydroxypiperidin-1-yl)-6-fluoro-8-
methoxy-1H quinazolinedione;
5-Amino-l -cyclopropyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)-4-
fluoropyrrolidin-l-yl]-6-fluoro-8-methoxy-1H-quinazolinedione;
5-Amino-I -cyclopropyl-6-fluoro-7-[3-fluoro-4-(3,3,3-trifluoro-1,2-
dihydroxy-2-trifluoromethylpropyl)pyrrolidin-I -yl]-8-methoxy-1 H-
quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7-{ 3-fluoro-4-[hydroxy-( I -
hydroxycyclopropyl)methyl]pyrrolidin-1-yl }-8-methoxy-1 H-quinazolinedione;
5-Amino-1-cyclopropyl-6-fluoro-7- { 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopentyl)methyl]pyrrolidin-1-yl }-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(3-fluoro-4-
hydroxymethylpyrrolidin-1-yl)-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-[3-( 1,2-dihydroxyethyl)-4
fluoropyrrolidin-1-yl]-6-fluoro-8-methoxy-1 H-quinazolinedione;
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-91-
1-Cyclopropyl-5-difluoromethyl-6-fluoro-8-methoxy-7-[3-(2,2,2-trifluoro-
1-hydroxyethyl)pyrrolidin-1-yl]-IH-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(3,4-dihydroxypiperidin-1-yl)-6-fluoro-
8-methoxy-I H-quinazoIinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(4-hydroxy-3-
hydroxymethylpiperidin-1-yl)-8-methoxy-1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-(3-hydroxy-4-
methylpiperidin-1-yl)-8-methoxy-1H quinazolinedione;
I -CyclopropyI-5-difluoromethyl-6-fluoro-7-(3-hydroxymethyl-4-
l0 methylpiperidin-1-yl)-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(4-ethyl-3-hydroxymethylpiperidin-1-
yl)-6-fluoro-8-methoxy-1H quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-7-(4-ethyl-3-hydroxypiperidin-1-yl)-6-
fluoro-8-methoxy-1 H-quinazolinedione;
15 1-Cyclopropyl-5-difluoromethyl-7-[3-( 1,2-dihydroxy-2-methylpropyl)-4-
fluoropyrrolidin-1-yl]-6-fluoro-8-methoxy-1 H-quinazolinedione;
1-Cyclopropyl-S-difluoromethyl-6-fluoro-7-[3-fluoro-4-(3,3,3-trifluoro-
1,2-dihydroxy-2-trifluoromethylpropyl)pyrrolidin-1-yl]-8-methoxy-1 H-
quinazolinedione;
20 1-Cyclopropyl-5-difluoromethyl-6-fluoro-7- { 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopropyl)methyl]pyrrolidin-1-yl }-8-methoxy-1H-quinazolinedione;
1-Cyclopropyl-5-difluoromethyl-6-fluoro-7-{ 3-fluoro-4-[hydroxy-( 1-
hydroxycyclopentyl)methyl]pyrrolidin-l -yl }-8-methoxy-IH-quinazolinedione; or
a pharmaceutically acceptable salt thereof.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-92-
The invention also provides a pharmaceutical composition comprising a
compound of one of the above-mentioned Formulas admixed with a can-ier,
diluent, or excipient.
The invention also provides a method of treating a bacterial infection in a
mammal comprising administering to the mammal in need thereof an antibacterial
effective amount of a compound of one of the above-mentioned Formulas.
The invention also provides a method of inhibiting a bacterial
topoisomerase in a mammal comprising administering to the mammal in need
thereof an effective amount of a compound of one of the above-mentioned
Formulas.
The invention also provides a method of inhibiting a bacterial DNA gyrase
in a mammal comprising administering to the mammal in need thereof an
effective amount of a compound of one of the above-mentioned Formulas.
The invention also provides a method of inhibiting a bacterial
topoisomerase IV in a mammal comprising administering to the mammal in need
thereof an effective amount of a compound of one of the above-mentioned
Formulas.
The invention also provides a method of inhibiting a quinolone-resistant
bacteria in a mammal comprising administering to the mammal an effective
amount of a compound of one of the above-mentioned Formulas.
The invention also provides a method of inhibiting a quinolone resistant
bacterial DNA gyrase in a mammal comprising administering to the mammal an
effective amount of a compound of any of Formulas I-VIII.
A method of inhibiting a quinolone resistant bacterial topoisomerase in a
mammal comprising administering to the mammal an effective amount of a
compound of any of Formulas I-VIII.
The invention also provides a process for preparing a compound of
formula IX, wherein Ra, R~, R3, R4, R6, J, K, V, V', z, and R' are as defined
above
and R5~ is halo, comprising:
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-93-
(a) coupling compound IXA wherein M is n-Bu3Sn with compound
IXB wherein RS~ is halo in the presence of Pd° to provide
the RS-coupled product IXC;
R3 O
R, V ~V_M + R4~3, ~.R2
R5/ 'IC N- 'O
Rs Ry
IXA IXB
R3 O
Ray i N.R2
w
R'-V' V tC N O
z
Rs Ry
IXC
(b) removing the R' group in IXC to provide compound IXD; and
R3 O R3 O
R4~~, N.R2 R4~~~ N.R2
z V R R O H_V, ~V~IC N_ '-O
i ~ ~
s i /z~ Rs Ry
IXC !XD
The invention also provides a process for preparing a compound of
formula IX, wherein R~, R3, R4, R6, J, K, V', z, and R' are as defined above
and
R5~ is halo, comprising:
(a) coupling compound IXA' with compound IXB' in the presence of
base to provide the RS-coupled product IXC';
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-94-
R3 O
~N_H R4~~~ N.R2
R'-V' 7~~ + ~
F~K N_ 'O
Rs Rf
IXA' IXB'
R3 O
R4~~, N.R2
R'-V' \N~K N- '-O
~z
Rs R~
iXC'
and
(b) removing the R' group in IXC' to provide compound IXD'
R3 O R3 O
R4~3, N.R2 R4~3, N.R2
R'-V' z ~N~fe N' 'O ~ H_V~ z ~N~K N O
i ~ Rs R1 / ~ Rs R~
IXC' IXD'
Detailed Description of the Invention
The following definitions are used, unless otherwise described: "Ph" is
phenyl; halo is fluoro, chloro, bromo, or iodo. Alkyl, alkoxy, alkenyl,
alkynyl, etc.
denote both straight and branched groups; but reference to an individual
radical .
such as "propyl" embraces only the straight chain radical, a branched chain
isomer
such as "isopropyl" being specifically referred to.
The term "alkyl" means a straight or branched hydrocarbon radical having
from l to 7 carbon atoms and includes, for example, methyl, ethyl, n-propyl,
isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-pentyl, n-hexyl, n-
heptyl, and
the like.
The term "C2-C~ alkenyl" means a straight or branched hydrocarbon
radical having from 1 to 3 double bonds. Examples include ethenyl, 2-propen-
1-yt, 1,3-butadien-1-yl, 3-hexen-1-yl, 5-octen-2-yl, 2-isopropyl-3,5-octadien-
l-yl,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-95-
cis-3-hexen-1-yl, and trans-2-hepten-1-yl, and the like. Preferred alkenyl
groups
include C2-C6 alkenyls such as ethenyl, 2-propen-1-yl, 2-buten-1-yl, and 3-
penten-1-yl, and the like.
The term "C2-C~ alkynyl" means a straight or branched hydrocarbon
radical having from 1 to 3 triple-bonds. Examples include ethynyl, propynyl,
3-butyn-I-yl, 4-hexyn-1-yl, and 5-heptyn-3-yl, and the like. Preferred alkynyl
groups are C2-C6 alkynyls such as ethynyl, propynyl, 3-butyn-I-yl, and 5-hexyn-
1-yl, and the like.
The alkyl, alkenyl, and alkynyl groups can be substituted with one or
more groups selected from halo, hydroxy, cyano, C I -Cb alkoxy, vitro,
nitroso,
amino, CI-C6 alkylamino, di-CI-C6 alkylamino, carboxy, CI-Cg alkoxycarbonyl,
aminocarbonyl, halomethyl, dihalomethyl, trihalomethyl, haloethyl,
dihaloethyl,
trihaloethyl, tetrahaloethyl, pentahaloethyl, thiol, (C~-C4)alkylsulfanyl, (C~-
Cø)alkylsulfmyl, and aminosulfonyl, -NH-SOZ-NH2, -O-S02-NH2-,
I 5 NH
I!
-NH-SO~-NH2, -NH-C-NH2, (C~-C6)dialkylthio, -NH-SO~-R, where R
is (C~-C6)alkyl, and aryl, as defined below. Examples of substituted alkyl
groups
include fluoromethyl, difluoromethyl, trifluoromethyl, tribromomethyl,
hydroxymethyl, 3-methoxypropyl, 3-carboxypentyl, 3,5-dibromo-6-
aminocarbonyldecyl, and 4-ethylsulfinyloctyl. Examples of substituted alkenyl
groups include 2-bromoethenyl, 1-amino-2-propen-I-yl, 3-hydroxypent-2-en-1-yl,
4-methoxycarbonyl-hex-2-en-1-yl, and 2-vitro-3-bromo-4-iodo-oct-5-en-I-yl.
Typical substituted alkynyl groups include 2-hydroxyethynyI, 3-dimethylamino-
hex-5-yn-1-yl, and 2-cyano-hept-3-yn-I-yl.
The term "cycloalkyl" means a hydrocarbon ring containing from 3 to
12 carbon atoms, for example, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
cycloheptyl, cycloctyl, decalinyl, norpinanyl, and adamantyl. Where possible,
the
cycloalkyl group may contain double bonds, for example, 3-cyclohexen-1-yl. The
cycloalkyl ring may be unsubstituted or substituted by one or more
substituents
selected from alkyl, alkoxy, thioalkoxy, hydroxy, thiol, vitro, halogen,
amino,
alkyl and dialkylamino, formyl, carboxyl, CN, -NH-CO-R, -CO-NHR, -C02R,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-96-
-COR, wherein R is defined as above, aryl, heteroaryl, wherein alkyl, aryl,
and
heteroaryl are as defined herein, or as indicated above for alkyl, alkenyl,
and
alkynyl substitutents. Examples of substituted cycloalkyl groups include
fluorocyclopropyl, 2-iodocyclobutyl, 2,3-dimethylcyclopentyl, 2,2-
dimethoxycyclohexyl, and 3-phenylcyclopentyl.
The term "heterocyclic" means a monocyclic, fused, bridged, or spiro
bicyclic heterocyclic ring systems. Monocyclic heterocyclic rings contain from
about 3 to 12 ring atoms, with from 1 to 5 heteroatoms selected from N, O, and
S,
and preferably from 3 to 7 member atoms, in the ring. Bicyclic heterocyclics
contain from about 5 to about 17 ring atoms, preferably from 5 to 12 ring
atoms.
Bicyclic heterocyclic rings may be fused, spiro, or bridged ring systems.
Examples of heterocyclic groups include cyclic ethers (oxiranes) such as
ethyleneoxide, tetrahydrofuran, dioxane, and substituted cyclic ethers,
wherein the
substituents are those described above for the alkyl and cycloalkyl groups.
Typical
substituted cyclic ethers include propyleneoxide, phenyloxirane (styrene
oxide),
cis-2-butene-oxide (2,3-dimethyloxirane), 3-chlorotetrahydrofuran, 2,6-
dimethyl-
1,4-dioxane, and the like. Heterocycles containing nitrogen are groups such as
pyrrolidine, piperidine, piperazine, tetrahydrotriazine, tetrahydropyrazole,
and
substituted groups such as 3-aminopyrrolidine, 4-methylpiperazin-1-yl, and the
like. Typical sulfur containing heterocycles include tetrahydrothiophene,
dihydro-
1,3-dithiol-2-yl, and hexahydrothiophen-4-yl and substituted groups such as
aminomethyl thiophene. Other commonly employed heterocycles include dihydro-
oXathiol-4-yl, dihydro-1H-isoindole, tetrahydro-oxazolyl, tetrahydro-
oxadiazolyl,
tetrahydrodioxazolyl, tetrahydrooxathiazolyl, hexahydrotriazinyl, tetrahydro-
oxazinyl, morpholinyl, thiomorpholinyl, tetrahydropyrimidinyl, dioxolinyl,
octahydrobenzofuranyl, octahydrobenzimidazolyl, and octahydrobenzothiazolyl.
For heterocycles containing sulfur, the oxidized sulfur heterocycles
containing SO
or S02 groups are also included. Examples include the sulfoxide and sulfone
forms of tetrahydrothiophene.
The term "aryl" means a cyclic or polycyclic aromatic ring having from
5 to 12 carbon atoms, and being unsubstituted or substituted with one or more
of
the substituent groups recited above for alkyl, alkenyl, and alkynyl groups.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-97-
Examples of aryl groups include phenyl, 2,6-dichlorophenyl, 3-methoxyphenyl,
naphthyl, 4-thionaphthyl, tetralinyl, anthracinyl, phenanthrenyl,
benzonaphthenyl,
fluorenyl, 2-acetamidofluoren-9-yl, and 4'-bromobiphenyl.
The term "heteroaryl" means an aromatic cyclic or polycyclic ring system
having from 1 to 4 heteroatoms selected from N, O, and S. Typical heteroaryl
groups include 2- or 3-thienyl, 2- or 3-furanyl, 2- or 3-pyrrolyl, 2-, 4-, or
5-imidazolyl, 3-, 4-, or 5-pyrazolyl, 2-, 4-, or 5-thiazolyl, 3-, 4-, or 5-
isothiazolyl,
2-, 4-, or 5-oxazolyl, 3-, 4-, or 5-isoxazolyl, 3- or S-1,2,4-triazolyl, 4- or
5-1,2,3-triazolyl, tetrazolyl, 2-, 3-, or 4-pyridinyl, 3-, 4-, or S-
pyridazinyl,
2-pyrazinyl, 2-, 4-, or 5-pyrimidinyl, 2-, 3-, 4-, 5-, 6-, 7-, or 8-
quinolinyl, I-, 3-,
4-, 5-, 6-, 7-, or 8-isoquinolinyl, 2-, 3-, 4-, 5-, 6-, or 7-indolyl, 2-, 3-,
4-, 5-, 6-, or
7-benzo[b]thienyl, 2-, 4-, 5-, 6-, or 7-benzoxazolyl, 2-, 4-, 5-, 6-, or
7-benzimidazolyl, 2-, 4-, 5-, 6-, or 7-benzothiazolyl. The heteroaryl groups
may
be unsubstituted or substituted by 1 to 3 substituents selected from those
described
I S above for alkyl, alkenyl, and alkynyl, for example, cyanothienyl and
formylpyrrolyl.
Preferred aromatic fused heterocyclic rings of from 8 to 10 atoms include
but are not limited to 2-, 3-, 4-, 5-, 6-, 7-, or 8-quinolinyl, I-, 3-, 4-, 5-
, 6-, 7-, or
8-isoquinolinyl, 2-, 3-, 4-, 5-, 6-, or 7-indolyl, 2-, 3-, 4-, 5-, 6-, or
7-benzo[b]thienyl, 2-, 4-, 5-, 6-, or 7-benzoxazolyl, 2-, 4-, 5-, 6-, or
7-benzimidazolyl, 2-, 4-, 5-, 6-, or 7-benzothiazolyl. Heteroaryl also
includes 2-
and 3- aminomethylfuran, 2- and 3- aminomethylthiophene and the like.
It will be appreciated by those skilled in the art that compounds of the
invention having one or more chiral centers may exist in and be isolated in
optically active and racemic forms. Some compounds may exhibit polymorphism.
It is to be understood that the present invention encompasses any racemic,
optically-active, polymorphic, geometric, or stereoisomeric form, or mixtures
thereof, of a compound of the invention, which possess the useful properties
described herein, it being well known in the art how to prepare optically
active
forms (for example, by resolution of the racemic form by recrystallization
techniques, by synthesis from optically-active starting materials, by chiral
synthesis, or by chromatographic separation using a chiral stationary phase).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-9S-
A "prodrug" is an inactive derivative of a drug molecule that requires
a chemical or an enzymatic biotransformation in order to release the active
parent
drug in the body.
"Tautomers" are structural isomersthat are conceptually related by the shift
of a H or labile group (such as an acetoxy group) and one or more II bonds. A
compound of the present invention exists in two tautomeric forms, depicted
below:
R3 O R3 OR2
R4~J ~ I NR2 R4~~ ~ I ~ N R2= H, Ac, etc.
R5 'K N~O R ~K NI '_O
Rs R1 Rs R1
Specific and preferred values for compounds of Formula I are listed below
for radicals, substituents, and ranges are for illustration purposes only, and
they do
not exclude other defined values or other values within defined ranges for the
radicals and substituents.
A specific value for J is C. Another specific value for J is N.
A specific value for K is C. Another specific value for K is N.
A specific value for R1 is methyl. Another specific value for R1 is ethyl,
isopropyl, cyclopropyl, t-butyl, 2-fluorocyclopropyl, 1- or 2-
methylcyclopropyl,
cyclopropylmethyl, vinyl, phenyl or substituted phenyl, heteroaryl or
substituted
heteroaryl.
A specific value for R~ is H.
A specific value for each of R3, Rq., and R6 is H, OH, (O)nCl-C~ alkyl
and substituted alkyl, (O)nC2-C~ aIkenyl and substituted alkenyl, (O)nC2-C~
alkynyl and substituted alkynyl, wherein n is 0 or l ; halo, N02, CN, NRgRh,
wherein Rg and Rh independently are H, C1-C~ alkyl and substituted alkyl, C2-
C~
alkenyl and substituted alkenyl, C2-C~ alkynyl and substituted alkynyl, -CO-
C1-C~ alkyl and substituted alkyl, or Ra and R,, taken together with the
nitrogen to
which they are attached form a 3- to 7-membered ring containing from 1 to
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-99-
3 heteroatoms selected from N, O, and S, said ring being unsubstituted or
substituted with 1, 2, 3, or 4 substituent groups.
A specific value for RS is C1-C~ alkyl and substituted alkyl, C2-C~
alkenyl and substituted alkenyl, C2-C~ alkynyl and substituted alkynyl, -
C02Ra,
' O O
wherein Ra is defined as above, -OCOZR~, -C-SRS ~ -O-C-R~,~ORb,
wherein Rb is defined as above, -(Z)PCONR~Rd, wherein Z is N Or O, p is 0 or
1,
and R~ and Rd are defined as above; halo, N02, CN, NR;R~, wherein R; and R~
independently are H, C1-C~ alkyl and substituted alkyl, C2-C~ alkenyl and
substituted alkenyl, C2-C~ alkynyl and substituted alkynyl, CO-C1-C~ alkyl and
I O substituted alkyl, or R; and R~ taken together with the nitrogen to which
they are
attached form a 3- to 7-membered ring containing from 1 to 3 heteroatoms
selected from N, O, and S, said ring being unsubstituted or substituted with
1, 2, 3,
or 4. substituent groups; aryl, fused aryl, heterocyclic, fused heterocyclic,
bicyclic
heterocyclic, or spiro heterocyclic, wherein fused aryl, fused heterocyclic,
bicyclic
heterocyclic, or spiro heterocyclic can be substituted.
Further examples of typical heterocycles, fused bicyclic or spiro
heterocycles, and heteroaryl groups that are specific values for RS are listed
below
in Table 1. In Table l, "N ~ " indicates the point of attachment. It is
additionally to be understood that the "N ~" point of attacment in the groups
disclosed in the table may be replaced by a "CH2'~ " or "=CH ~ " point
H,
of attachment, such as in . Morevoer, many of the entries
depicted in Table 1 incorporate additional functionality such as primary and
secondary amino groups, hydroxy groups, and thio groups. These additional
functional groups can be protected by protecting groups known in the art,
according to methods known in the art, as provided below.
N
H
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-100-
Table I
H
H~ HN N
Nv". ~'_~~N"",. N""" N/'~N."". Me-N~N."".
HN\-~N~~ ~~r
HN'~~~N~ HN~~~Nv,, H2N ~N."".
MeO~~
H2N
HZN -~~N~ H Ns~\~~N~ ~N~ H2N /~N~
Me
Me~N ~N Me~N~ H N ~
H~N~~~ H~N~~~ H ' 'N"",. 2 ~~N"""
Me Me Me Mme M '~/e
Me~N -~N
H2N N."". H2N N~,~ H N."". H N."".
r
H
2
H N N~ H2N N~ H2N Me N~ ~N~ HN'~~N."".
M ,,//[[~~,,e
I H2N
~~N~ ~ ~N""" H2N H N
HN HN N."". ~ 2 'N~~r
N~~~
H2
H2N~WN."". N N.,.". / ~N."". ~N."". N"".
NN H2 ~ ~N
N
HO HO ~ ~ HO''~N Or,SVN."".
~N~~,. ~N~~~ N""" ~N""°, n= 0 1, 2
HZN ~~// Me
N."". H2N N."". HN N."". H ~N""' HN N~ .
-C
Me Me
HEN Me H N Ph NH2 NH2 H N
~N."". 2 ~N
~~/N~ FsC~N~ Me N."".
Me
H2N NH2 OH
F~~,\~ """ F F
F ,~/N
F ~~~\~N""" F
S
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-101-
Me H2N
HaN H2N ~ H2N
~N H2N N."". N."". N."", ~N."".
Me Me Me Me Me Me
Me0 HO O H2N H2N Me
H2N H2N H2N ~N."...
vN."". vN."". N"". N.r.,.
Me
OMe MeF Me0 F H N
'~ 2 ~/
H2N/ / ' ."". H2N N H2N N."". H2N N..~".
~N ~",. ' 'N"",.
F
Me H2N H2N
H2N/~/~N""" H2N N"~ H2N/~~N""" ~N'""' ~N"""
F3~ Me0 Me0 F
H2N NH2 H N~N~ Me Me
CN~~" HO
~N N"",. 0J N."".
H2N Me Me
N."". MeHN H2 ~/N
H2N~N~ EtHN
N."". ~N~~~ N~",.
i
N"',' HO ~ ~ Me
H2N \v/~ /~ ,~N~""~ H2N ~ H2N N."".
HO H2N N."". N F
Me Me
H2N N.r", H2N ~N."". H2N H2N N"",. HEN N
N."". """
F~~,~ F
F F F F F
Et0 O H2N O Me Me
w
H2N N~ H2N N~ HN~ H2N ~ H N ~N
2
H2N S N ~ ~ ~N %---'
H2N N N H2N N O'i'N
Me
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-102-
lW
r
H2N
H2N ~ ~ H2N ~ N."".
N H2N ~N."". N."". F
S H2N
S ~ \ I ~ ~ I ~ N N.~".
S
r
H
N ~ I N\ I
I ~ N ~N~ H N N~ N"'"' N."",
2
i
S
Me ~ I \ I H2N N I N ~ I
HN~ HN ~N.~". ~N~,..
Me H2N
S S I S \
Me \ I \
NH H2N Me~
2 Me NH2
N
i F F H O FsC FaC F
H~N H~N N~ H~N H~N N."".
H N.,;". H N."". N N~ H N."". H
H
F F NH2 NH2
OH F F ~
H.O ~ H_N , ~ I S" w
H
~N N S NH2 /
OH
H-O
F3C CF N."".
3
In compounds of Formula 1, a preferred value for J is C. Another
preferred value for J is N. A preferred value for K is C. Another preferred
value
for K is N. A preferred value for R1 is cyclopropyl. Another preferred value
for
Rl is 2-fluorocyclopropyI, 1- or 2-methyIcycIopropyl, or cyclopropyImethyl. A
preferred value for R~ is H. A preferred value for R3 is H. Another preferred
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-103-
value for R3 is methyl. Another preferred value for R3 is R Another preferred
value for R3 is methoxy. Another preferred value for R3 is NHS. A preferred
value for R4 when J is C is H. A preferred value for R4 when J is C is F
Another
preferred value for R4 when J is C is Cl. A preferred value for RS is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl. Another preferred value for RS is
1-
piperidinyl or substituted 1-piperidinyl, or 1-piperizinyl or substituted 1-
piperizinyl. Other preferred values for RS include heterocycles and heteroaryl
groups such as those known in the quinolone art, for instance, as found in J.
Med.
Chem., 1992;35:1764; J. Med. Chem., 1996;39:3070; Synlett.,1996:1097; and
NH2
J. Med: Chem., 1986;29:445; or, for example, S . A preferred value
for R6 when K is C is H. Another preferred value for R6 when K is C is Cl-C4
alkyl and substituted alkyl, halo, OH, or -O-C1-C4 alkyl and substituted -O-
C1-C4 alkyl, OCF3, OCHFZ, OCH2F, OCH2CF3, OCHZCHFa, or OCH2CHZF.
A preferred group of compounds of Formula I are compounds wherein J
and K are C; R1 is methyl, ethyl, cyclopropyl, t-butyl, 2-fluorocyclopropyl;
R2 is
H; R3 is H, F, Me, or NH2; R4 is F or Cl; RS is 1-pyrrolidinyl or substituted
1-
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted
l -piperizinyl
NH2
or S ; and R6 is F, Cl, methyl, methoxy, OCF3, OCHF~, OCHZF,
OCH~CF3, OCH2CHF2, or OCHzCH~F
Another preferred group of compounds of Formula I are compounds
wherein J is N, K is C; R1 is cyclopropyl; R~ is H; R3 is H, F, Me, or NH2; R4
is F
or Cl; and RS is 1-pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl
or
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-104-
substituted 1-piperidinyl, 1-piperizinyl or substituted 1-piperizinyl, or
NH2
and R6 is F, Cl, methyl, methoxy, or OCF3..
Another preferred group of compounds of Formula I are compounds
wherein J is C; K is N; R1 is cyclopropyl; R2 is H; R3 is H, F, Me, or NH2; R4
is F
or Cl; and RS is 1-pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl
or
substituted 1-piperidinyl, 1-piperizinyl or substituted 1-piperizinyl, or
NH2
and R6 is F, Cl, methyl, methoxy, or OCF3.
Another preferred group of compounds of Formula I are compounds
wherein J is N; K is N; R1 is cyclopropyl; R~ is H; R3 is H, F, Me, or NHz;
and RS
is 1-pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl or substituted
1-
NH2
piperidinyl, 1-piperizinyl or substituted 1-piperizinyl, or S ; and R6 is
F, Cl, methyl, methoxy, or OCF3.
Representative compounds of the invention which are compounds of
Formula 1 are shown below in Table 2-I.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-l OS-
Table 2-I
0 0
0
F i NH F ~ I NH F ~ I NH
\ I \ N~p ~N \ N~O
H2N~N CI ~ O CI ~ H2N Me CI
O O O
A, \ I ~ H F i I NH N F \ J
H2N~N CI ~ O Me N~N \ N~O H-N~N CI j O
1 -(~J, CI ~ ~.J I
F
F O NH2 O O
F i NH H F ~ I NH
H-N N \ I N- 'O H~N~N \ N~O H2N--(~,N \ I N O
F ~ F C! CH(Me)2
O O
F r O
\ I F ~ NH F ~ I NH
H2N~N CI N O H Ne Me N \ I N~O H2N~N \ N~O
OMe
O O
\ I Me F w I
H2N~N N O H N~N N N O
~Iz
Me
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-106-
0 0 0
F ~ NH ' F ~ NH F , W
~NH
Me, _ N ! ~ N~O Me, _ N ! s N O HO N ~ N O
HZN~u~~, Me ~ HZN~ O ~ HO~ Me
Me
O O O
F
F ~ NH NHz F I ~ NH
H N I ~ N~O ~ ~ N O ~ II WI ~ N O
HO ~ S Me ~ HN~ Me
Me
Me
Me O Me O Me O
F \ F
NH Me I ~ NH F ~ NH
Me N / N O N / N O Me ~ ,
H2N~ Me ~ HzN~ ,O H N~N N ~ O
Me
O O Me O
F ~ F ~ NH F ~ NH
N I N O F3C N I ~ N~p /~( ~N~ I '~ N' '_O
H2N~ Me0 ~ HZN Me ~ HO~ Me
F
Me O O NHz O
F ~ NH ~ NH F I ~ NH
F N I ~ N~O ~N I ~ N~O Me~N ~ N~O
H2N Me ~ H2N~ ~ Me ~ H2N/~'~ O_ ~
v 'Me
Representative compounds of the present invention, which are
encompassed by Formula I include, but are not limited to the compounds in
Table 2 and their pharmaceutically acceptable acid or base addition salts, or
amide
or prodrugs thereof.
In compounds of Formula II, a preferred value for R1 is cyclopropyl.
Another preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-
methylcyclopropyl,
or cyclopropylmethyl. A preferred value for R~ is H. A preferred value for R3
is
H. Another preferred value for R3 is ft Another preferred value for R3 is
methyl.
Another preferred value for R3 is methoxy. Another preferred value for R3 is
NH2. A preferred value for Rq. is R Another preferred value for R4 is Cl. A
preferred value for RS is 1-pyrrolidinyl or substituted l-pyrrolidinyl.
Another
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-l 07-
preferred value for RS is 1-piperidinyl or substituted 1-piperidinyl, or I-
piperizinyl or substituted 1-piperizinyl. Other preferred values for RS
include
heterocycles and heteroaryl groups such as those known in the quinolone art,
for
instance, as described above for compounds of Formula I. A preferred value for
R6 is H. Another preferred value for R6 is C~-C4 alkyl and substituted alkyl,
halo,
OH, or -O-CI-Cq. alkyl and substituted -O-CI-Cq. alkyl, OCF3, OCHF2, OCHZF,
OCH~CF3, OCH~CHF~, or OCHaCH2F.
A preferred group of compounds of Formula II are compounds wherein RI
is cyclopropyl; R2 is H; R3 is H, F, Me, OMe, or NHS; Rq. is F or Cl; RS is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl or substituted I-
NH2
piperidinyl, 1-piperizinyl or substituted 1-piperizinyl, or S ; and R6 is
F, Cl, methyl, methoxy, OCF3, OCHF2, OCHZF, OCH~CF3, OCH2CHF2, or
OCHZCHZft
Another~preferred group of compounds of Formula II are compounds
wherein RI is cyclopropyl; R2 is H; R3 is H, F, Me, or NH2; Rq. is F; RS is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyI or substituted I-
piperidinyl; 1-piperizinyl or substituted 1-piperizinyl; and R6 is methyl,
methoxy,
or OCF3.
Another preferred group of compounds of Formula II are compounds
wherein RI is cyclopropyl; R2 is H; R3 is H, F, Me, or NHZ; Rq. is F; RS is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, or 1-piperidinyl or substituted I-
piperidinyl; and R6 is F, Cl, methyl, methoxy, or OCF3.
Another preferred group of compounds of Formula II are compounds
wherein R1 is cyclopropyl; R2 is H; R3 is H, F, Me, or NHS; R4 is F; R5 is 1-
pyrrolidinyl; and R6 is F, CI, methyl, methoxy, or OCF3.
Representative compounds of the invention which are compounds of
Formula 1 are also depicted in Table 2-I.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-10~-
In compounds of Formula III, a preferred value for R1 is cyclopropyl.
Another preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-
methylcyclopropyl,
or cyclopropylmethyl. A preferred value for R2 is H. A preferred value for R3
is
H. Another preferred value for R3 is F. Another preferred value for R3 is
methyl.
Another preferred value for R3 is methoxy. Another preferred value for R3 is
NH2. A preferred value for R4 is F Another preferred value for Rq. is CI. A
preferred value for RS is 1-pyrrolidinyl or substituted 1-pyrrolidinyl.
Another
preferred value for RS is 1-piperidinyI or substituted 1-piperidinyl, or 1-
NH2
piperizinyl or substituted 1-piperizinyl, or S . Other preferred values
for RS include heterocycles and heteroaryl groups such as those known in the
quinolone art, for instance, as described above for compounds of Formula I.
A preferred group of compounds of Formula III are compounds wherein
R1 is cyclopropyl; R3 is H, F, Me, or NH2; Rq. is F or Cl; and RS is 1-
pyrrolidinyl
or substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-
NH2
piperizinyl or substituted 1-piperizinyl, or
Another preferred group of compounds of Formula III are compounds
wherein R1 is cyclopropyl; R2 is H; R3 is H, F, Me, OMe, or NH2; Rq. is F; and
RS is 1-pyrrolidinyl or substituted l-pyrrolidinyl, l-piperidinyl or
substituted l-
piperidinyl, 1-piperizinyl or substituted 1-piperizinyl.
Another preferred group of compounds of Formula III are compounds
wherein R1 is cyclopropyl; R~, is H; R3 is H, F, Me, or NHS; R4 is F; and RS
is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, or I-piperidinyl or substituted 1=
piperidinyl.
Another preferred group of compounds of Formula III are compounds
wherein R1 is cyclopropyl; R2 is H; R3 is H, F, Me, or NHS; R4 is F; and RS is
1-
pyrrolidinyl or substituted 1-pyrrolidinyl.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-109-
Representative compounds of the invention which are compounds of
Formula III are shown below in Table 2-III.
Table 2-III
0 0 0
F i NH F / NH F \ I NH
,I ~ II
H2N~N N N~O ~N~N~O HEN M~N N N O
O O
O
F ~ NH F , NH H F ~ I NH
wI H ~ w
N N N~O ~ ~ ~ I ~ .N N N N O
H N~ Me N~N N N~O H ~ , F
I
F
F O NH2 O O
H F ~ I NH H F \ I NH F ~ I NH
i
.N N N N O
H-N~N N N~O H '~ H2N~N N N O
CH(Me)2
O
O O
NH F ,- NH NH
F / ~ F
H N N N N- 'O Me
2 Me -~~~~ HZN N N N~O
H2N~N N N~O
O O
F \ I NH Me F \ I NH
H2N~N N O H N~N N N~O
2
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-110-
Table 2-IIII Continued
0 o p
NH ~ NH ~ NH
Me F ~ r ~O Me N ~ N O HO F ~ r
~---~ N~ N N ~' ~--~ N~ N N O
H2N a ~ H2N V HO--i a
O O O
F F I ~ NH
F I ~ NH NH2 I ~ NH ~ ~
HO r w N N_ '-O I ~ N~N~O
~N~ N O ~ ~ ~ ~ HN
HO--i 'J S
Me
Me O Me ~ Me O
F
F ~ NH I ~ NH F ~ NH
Me I r N~O Me N N N"-O Me ~ r
~--~ N~ N N N N O
HzN~ HaN HZN
O O Me O
/~~NH ~ NH
N JI N N~O F3C F I r N I N N O
N N N O
HzN~ H2N HO~
F
Me O O NHZ O
F ~ NH ~ NH F I ~ NH
I N N~O N i N N~O Me r
F N ~ ~N N~O
HpN HpN H2N' V N
Representative compounds of the present invention, which are
encompassed by Formula III include, but are not limited to the compounds in
Table 2-III and their pharmaceutically acceptable acid or base addition salts,
or
amide or prodrugs thereof.
In compounds of Formula IV, a preferred value for Rj is cyclopropyl.
Another preferred value for R1 is 2-fluorocyclopropyl, 1- or 2.-
methylcyclopropyl,
or cyclopropylmethyl. A preferred value for R~ is H. A preferred value for R3
is
H. Another preferred value for R3 is ft Another preferred value for R3 is
methyl.
Another preferred value for R3 is methoxy. Another preferred value for R3 is
NHS. Apreferred value for RS is 1-pyrrolidinyl or substituted 1-pyrrolidinyl.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-111-
Another preferred value for RS is 1-piperidinyl or substituted 1-piperidinyl,
or I
NH2
piperizinyl or substituted 1-piperizinyl, or S . Other preferred values
for RS include heterocycles and heteroaryl groups such as those known in the
quinolone art, for instance, as described above for compounds of Formula I. A
preferred value for R6 is H. Another preferred value for R6 is C~-Cq. alkyl
and
substituted alkyl, halo, OH, or-O-C1-Cq. alkyl and substituted -O-C1-Cq.
alkyl,
OCF3, OCHF2, OCH2F, OCH2CF3, OCH~CHFZ, or OCHZCH~F.
A preferred group of compounds of Formula IV are compounds wherein
R1 is cyclopropyl; R2 is H; R3 is H, methyl, NH2, or methoxy; RS is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-
NH2
piperidinyl, 1-piperizinyl or substituted 1-piperizinyl, or s ; and R6 is
F, CI, methyl, methoxy, OCF3, OCHF2, OCHZF, OCHZCF3, OCH~CHF~, or
OCH~CH~ft
Another preferred group of compounds of Formula IV are compounds
wherein RI is cyclopropyl; R2 is H ; R3 is H, methyl, or methoxy; RS is 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-
piperidinyl, 1- piperizinyl or substituted 1-piperizinyl; and Rg is F, Cl,
methyl,
methoxy, or OCF3.
Another preferred group of compounds of Formula IV are compounds
wherein RI is cyclopropyl; R2 is H; R3 is H; RS is 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl, or I-piperidinyl or substituted l-piperidinyl; and R~ is F, Cl,
methyl,
methoxy, or OCF3.
Another preferred group of compounds of Formula IV are compounds
wherein RI is cyclopropyl; R2 is H; R3 is H; RS is 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl, or 1-piperidinyl or substituted 1-piperidinyl; and R6 is F, CI,
methyl,
methoxy, or OCF3.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-112.-
Representative compounds of the invention which are compounds of
Formula l are shown below in Table 2-IV.
Table 2-IV
0 0 0
N' NH N~ I NH N~ I ~NH
W I w N~O H N~~ N~N~O
H2N~N CI N O CI 2 Me '-J CI
O O
O
N I ~NH N~ NH H N I ~NH
~~N ~ N' \O /N N ~ I N~O H.N N ~ N- 'O
H2N~ CI ~ Me ~ CI ~ CI / F
I
F
F O NH2 O O
N' NH H N I '~ N' I NH
-N N ~ I ~ N~O H.N N~N O N ~ N~O
H ~ H2N
F ~ F ~ ~J CI CH(Me)2
O
O O
N/ I '~ N/ I
H2N~N CI N O Me Me N W I N~O H2N~N ~ N O
H2N'~J OMe
O O
NH Me ~ I NH
H2N~N O H N~N N N_ 'O
~/2
Me
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-113-
Table 2-IV Continued
0 0 0
N ~ ~NH N ~ ~NH N ~ NH
Me N I / N~O Me N' I / N O HO~ N~ I / N_ '_O
HZN v Me ~ HzN V O ~ HO~ Me
Me
O O O
N ~ NH NH2 N ~ NH N ~ ~NH
HO
~N I / N~O ~ I / N~O HN ' \ I / N~O
HO-' ~J ~ S Me ~ / Me
Me
Me
Me O Me O Me O
N ~ NH N ~ NH N
Me
~ ~N I / N O Me~ /~ N ~ / N O Me N I
~-( ~ /'~(~ O
HzN~ Me ~ HzN~ Meo~ H2N
O O Me O
N ~ 'NH N ~ NH N ~ NH
N I / N~O F3C I / ~ I /
~N N O ~N N O
H2N~ Me0 ~ HZN/ ~J Me ~ HO~ ~ Me
F
Me O O NH2 O
N ~ NH N ~ NH N ~ NH
F N I / N~O ~N I / N~O Me N I / N~O
H2N Me ~ H2N/ V Me ~ HaN O
Me
Representative compounds of the present invention, which are
encompassed by Formula IV include, but are not limited to the compounds in
Table 2-IV and their pharmaceutically acceptable acid or base addition salts,
or
amide or prodrugs thereof.
In compounds of Formula V, a preferred value for R1 is cyclopropyl.
Another preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-
methylcyclopropyl,
or cyclopropylmethyl. A preferred value for R2 is H. A preferred value for R3
is
H. Another preferred value for R3 is F. Another preferred value for R3 is
methyl.
Another preferred value for R3 is methoxy. Another preferred value for R3 is
NHS. A preferred value for RS is 1-pyrrolidinyl or substituted 1-pyrrolidinyl.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-114-
Another preferred value for RS is 1-piperidinyl or substituted 1-piperidinyl,
or 1-
NH2
piperizinyl or substituted 1-piperizinyl, or S . Other preferred values
for RS include heterocycles and heteroaryl groups such as those known in the
quinolone art, for instance, as described above for compounds of Formula T.
A preferred group of compounds of Formula V are compounds wherein R1
is cyclopropyl; R2 is H; R3 is H, methyl, NHS, or methoxy; and RS is l-
pyrroIidinyI or substituted 1-pyrrolidinyI, I-piperidinyl or substituted 1-
NH2
piperidinyl, 1-piperizinyl or substituted 1-piperizinyl, or
Another preferred group of compounds of Formula V are compounds
wherein R1 is cyclopropyl; R2 is H; R3 is H, methyl, NHS, or methoxy; and RS
is
1-pyrrolidinyl or substituted 1-pyrrolidinyl, or 1-piperidinyl or substituted
1-
piperidinyl.
Another preferred group of compounds of Formula V are compounds
wherein RI is cyclopropyl; R2 is H; R3 is H, methyl, NHS, or methoxy; and RS
is
l5 1-pyrrolidinyl or substituted 1-pyrrolidinyl.
Another preferred group of compounds of Formula V are compounds
wherein Rl is cyclopropyl; R3 is H, methyl, NHS, or methoxy; and RS is
substituted 1-pyrrolidinyl.
Representative compounds of the invention which are compounds of
Formula V are shown below in Table 2-V.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-115-
Table 2-V
0 0 0
N' NH N ~ I NH ~ i NH
~ N N~O ~lN N N O
H N~N N N~O H2N Me '-
O O
O
NH H N' NH H N\ I NH
~N~ N N O ~ ~ ~ .N N N N_ 'O
Me'N~N N N' 'O H
H2N~ ~ F
F
F O NH2 O O
H N' I NH H ~~~NH N' I NH
.N ~
N N N O
H-N~N N N O H ~ HZN~N N N' \O
CH(Me)2
O
O O
N' I NH
N' NH N ~ i NH
H2N N N N O Me Me ~
H N N N N~O
H2N N N N~O
O
NH
Me I
H2N~N N N~O
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-I l6-
Table 2-V Continued
0 0
N~NH Me O N w NH
HO
Me N ~j N O Me ~~ ~N~~N N~O
H2N~ ~N N N O HO-i LJ
HZ / ~/N
O O
Me O ~ ~
NH2 N~NH N~NH
i ~
N N' '-O
N ~ O ~ N N O HN
F H2N ~ S ~ Me~
O O O
N~NH N ~ NH N~NH
N~N N~O F30 ~ ~ ~N'~N~N"_O
HpN uN N N O H2~
H2N
F
Me O
N~NH .
N~N N- '-O
i-10~
Representative compounds of the present invention, which are
encompassed by Formula V include, but are not limited to the compounds in
Table 2-V and their pharmaceutically acceptable acid or base addition salts,
or
amide or prodrugs thereof.
In compounds of Formula VI, a preferred value for RI is cyclopropyl.
Another preferred value for RI is 2-fluorocyclopropyl, I- or 2-
methylcyclopropyl,
or cyclopropylmethyl. A preferred value for R~ is H. A preferred value for R3
is
H. Another preferred value for R3 is methyl. Another preferred value for R3 is
methoxy. Another preferred value for R3 is NHS. A preferred value for Rø is P
Another preferred value for Rq. is Cl. A preferred value for R f and Rg,
together
with the nitrogen to which they are attached, is I-pyrrolidinyl or substituted
1-
I S pyrrolidinyl. Another preferred value for R f and Rg, together with the
nitrogen to
which they are attached, is l-piperidinyl or substituted 1-piperidinyl, or 1-
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-117-
piperizinyl or substituted 1-piperizinyl. Other preferred values for Rf and
Rg,
together with the nitrogen to which they are attached, include heterocycles
and
heteroaryl groups such as those known in the quinolone art, for instance, as
described above for compounds of Formula I. A preferred value for R6 is H.
Another preferred value for R6 is CI-C4 alkyl and substituted alkyl, halo, OH,
or
O-C 1-C4 alkyl and substituted -O-C 1-Cq. alkyl, OCF3, OCHF2, OCH2F,
OCH~CF3, OCHZCHF~, or OCH2CHZft
A preferred group of compounds of Formula VI are compounds wherein
R1 is cyclopropyl; R2 is H; R3 is H, methyl, NHS, or methoxy; Rq. is F or Cl;
Rf
and Rg, together with the nitrogen to which they are attached, are 1-
pyrrolidinyl
or substituted I-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1
piperizinyl or substituted l-piperizinyl; and R6 is F, Cl, methyl, methoxy,
OCF3,
OCHFa, OCHZF, OCH~CF3, OCH2CHF2, or OCH2CHaft
Another preferred group of compounds of Formula VI are compounds
wherein RI is cyclopropyl; R2 is H; R3 is H, methyl, NH2, or methoxy; Rq. is
F;
Rf and Rg, together with the nitrogen to which they are attached, are 1-
pyrrolidinyl or substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-
piperidinyl, 1-piperizinyl or substituted 1-piperizinyl; and R6 is methyl,
methoxy,
OCF3, OCHF2, OCH2F, or OCH~CF3.
Another preferred group of compounds of Formula VI are compounds
wherein R1 is cyclopropyl; R2 is H; R3 is H, methyl, NH2, or methoxy; Rq. is
F;
R f and Rg, together With the nitrogen to which they are attached, are I-
pyrrolidinyl or substituted 1-pyrrolidinyl, or I-piperidinyl or substituted 1-
piperidinyl; and R6 is methyl, methoxy, or OCF~.
Another preferred group of compounds of Formula VI are compounds
wherein R1 is cyclopropyl; R2 is H; R~ is H, methyl, NH2, or methoxy; Rq. is
F;
R f and Rg, together with the nitrogen to which they are attached, are l -
pyrrolidinyI or substituted I-pyrroIidinyl; and R6 is F, CI, methyl, methoxy,
or
OCF~.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-I I8-
Representative compounds of the invention which are compounds of
Formula VI are also shown in Table 2-I.
In compounds of Formula VII, a preferred value for RI is cyclopropyl.
S Another preferred value for R1 is 2-fluorocyclopropyl, 1- or 2-
methylcyclopropyl,
or cyclopropylmethyl. A preferred value for R3 is H. Another preferred value
for
R3 is methyl. Another preferred value for R3 is methoxy. Another preferred
value for R3 is NHS. A preferred value for R4 is F. Another preferred value
for
R4 is Cl. A preferred value for Rg and Rh, together with the nitrogen to which
they are attached, is 1-pyrrolidinyl or substituted 1-pyrrolidinyI. Another
preferred
value for Rg and Rh, together with the nitrogen to which they are attached, is
1-
piperidinyl or substituted 1-piperidinyl, or 1-piperizinyl or substituted I-
piperizinyl. Other preferred values for Rg and Rh, together with the nitrogen
to
which they are attached, include heterocycles and heteroaryl groups such as
those
1 S known in the quinolone art, for instance, as described above for compounds
of
Formula I.
A preferred group of compounds of Formula VII are compounds wherein
R1 is cyclopropyl; R3 is H, methyl, NHS, or methoxy; R4 is F or CI; and Rg and
Rh, together with the nitrogen to which they are attached, are I-pyrralidinyl
or
substituted 1-pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-
piperizinyl
or substituted 1-piperizinyl.
Another preferred group of compounds of Formula VII are compounds
wherein RI is cyclopropyl; R~ is H, methyl, NHS, or methoxy; Rq is F; and Rg
and Rh, together with the nitrogen to which they are attached, are I-
pyrrolidinyl
2S or substituted 1-pyrrolidinyl, or I -piperidinyl or substituted l -
piperidinyl.
Another preferred group of compounds of Formula VII are compounds
wherein RI is cyclopropyl; R3 is H, methyl, NHS, or methoxy; R4 is F; and Rg
and Rh, together with the nitrogen to which they are attached, are I-
pyrrolidinyl
or substituted 1-pyrrolidinyl.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-119-
Another preferred group of compounds of Formula VII are compounds
wherein RI is cyclopropyl; R3 is H, methyl, NHS, or methoxy; Rq. is F; Rg and
Rh,
together with the nitrogen to which they are attached, are substituted I-
pyrrolidinyl.
Representative compounds of the invention which are compounds of
Formula VII are shown in Table 2-III.
In compounds of Formula VIII, a preferred value for m is 0 or 1. A
preferred value for X is O. Another preferred value for X is CHI or CH(C1-C~
alkyl). A preferred value for Y is CHI or CH(C~-C~ alkyl). Another preferred
value for Y ~s C(C~-C~ alkyl)2, C(C3-C6 cycloalkyl), , wherein is a
C3-C6 cycloalkyl. Another preferred value for Y is NH or N(Ca-C~ alkyl).
C
Another preferred value for Y is C(C~-C~ alkyl)Z, C(C3-C6 cycloalkyl), ~~~//)
,
C~ I
wherein ~ is a C3-C6 cycloalkyl. A preferred value for R~ is CI C~ alkyl. A
preferred value for Ra is H. A preferred value for R3 is H. Another preferred
value for R3 is methyl. Another preferred value for R3 is methoxy. Another
preferred value for R3 is NHS. A preferred value for Rq. is R Another
preferred
value for Rq. is Cl. A preferred value for RS is 1-pyrrolidinyl or substituted
1-
pyrrolidinyl. Another preferred value for R5 is 1-piperidinyl or substituted 1-
NH2
piperidinyl, or 1-piperizinyl or substituted 1-piperizinyl, or S . Other
preferred values for RS include heterocycles and heteroaryl groups such as
those
known in the quinolone art, for instance, as described above for compounds of
Formula I.
A preferred group of compounds of Formula VIII are compounds wherein
m is 1; X is O or CH~; Y is CH~, CH(C,-C~ alkyl), NH, or N(C~-C~ alkyl); Rh is
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-120-
methyl; R2 is H; R3 is H; Rq. is F or Cl; and RS is 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl, 1-piperidinyl or substituted 1-piperidinyl, 1-piperizinyl or
substituted
1-piperizinyl.
Another preferred group of compounds of Formula VIII are compounds
wherein X is O or CHI; Y is CH2, CH(C1-C~ alkyl), NH, or N(C~-C~ alky 1); Rh
is
methyl; RZ is H; R3 is H; and RS is 1-pyrrolidinyl or substituted I-
pyrrolidinyl, or
1-piperidinyl or substituted 1-piperidinyl,
Another preferred group of compounds of Formula VIII are compounds
wherein X is O or CH2; Y is CHa, CH(C~-C~ alkyl), NH, or N(C,-C~ alkyl); Rh is
methyl; R~ is H; R3 is H; Rq. is F or Cl; and RS is 1-pyrrolidinyl or
substituted 1-
pyrrolidinyl.
Another preferred group of compounds of Formula VIII are compounds
wherein X is O or CH2; Y is CHI, CH(C~-C~ alkyl), NH, or N(C~-C~ alkyl); Rh is
methyl; R2 is H; R3 is H; and RS is substituted 1-pyrrolidinyl.
I S Representative compounds of the invention which are compounds of
Formula VIII axe shown below in Table 2-VIII.
Table 2-VIII
O NH2 O
F
NH F ~ NH
H2N N ~ I N' \-O Me N I ~ N- '-O
Me H2N~~ O_ ~
-Me
Representative compounds of the present invention, which are
encompassed by Formula 2-VIII include, but are not limited to the compounds in
Table 2-VIII and their pharmaceutically acceptable acid or base addition
salts, or
amide or prodrugs thereof.
Illustrations of typical preparations of compounds of the present invention
having Formula I are shown in the following synthetic schemes. Typical
heterocyclic and aromatic side chains defined by RS in Formula I are prepared
as
are also described. All of the 3-hydridoquinazolinediones of the invention may
be
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-121-
prepared from appropriately substituted benzoic acid starting materials.
Protecting
groups (referred to in the schemes as "Pro") may be used when appropriate
throughout many of the schemes. Although specifically noted in certain
schemes,
the appropriate use and choice of protecting groups is well-known by those
skilled
in the art, and is not limited to the specific illustrations shown below. It
should
also be understood that such protecting groups not only serve to protect
chemically reactive sites, but also to enhance solubility or otherwise change
physical properties of the underlying invention compound. A number of general
reactions such as oxidations and reductions are not shown in detail in the
schemes,
but can be carried out by standard methods well-known to those skilled in the
art.
In general, the starting materials used in the following schemes are obtained
from
commercial sources, or are readily prepared by standard methods. All cited
published articles, patents, books, and the like are incorporated herein by
reference.
The following Schemes are organized in two parts. The first part
summarizes synthetic approaches to the core structures represented by
compounds
of Formulas I-VIII, depicted generally by the reference structure in the
following
drawing. The second part summarizes synthetic approaches to the preparation of
particular RS groups that can be attached to the core structures. The meaning
of
"core structure" and "sidechain" is demonstrated in the following drawing by
structure X. As will be seen below, there are two general approaches to
preparing
compounds of the present invention. In the first general approach, the core
structure is prepared and then the RS sidechain is attached to the core
structure to
provide a compound of the invention. In the second general approach, the R5
sidechain is attached to a precursor of the core structure and then the core
structure is prepared by intramolecular cyclization. Both approaches are
disclosed
in the following section.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-122-
R5 Sidechain
0
NH
R3 ~ ~
Ra / N. R2 H~,, N \ N- 'O
N~O H_Nv
H
R6 Rf H ~--~.--
Reference Structure
x
Core Structure
Core Structures
The core structures of the compounds of the invention can be prepared
from appropriately substituted benzoic acid derivatives. Thus compounds of
formula I wherein J is C or N, K is C, and R6 is C~-C~ alkyl or substituted
alkyl
can be prepared as summarized in Scheme 1, wherein the R~ and RS sidechain are
attached subsequent to cyclization to form the dione ring. Thus, the
anthranilic
acid derivative can be converted to the corresponding alkoxy benzamide ester.
The amide can be treated with a carbon monoxide equivalent such as phosgene to
provide the 3-N-methoxy quinazolinedione derivative. Deprotonation of the
position 1 amide moiety in the dione, followed by alkylation using an
alkylating
agent such as R,X, wherein R~ has any of the meanings provided herein, can
give
rise to the R~ alkylated quinazolinedione derivative. An RS sidechain can be
attached to quinazolinedione core structure using base or a palladium catalyst
according to methods available to the skilled artisan. Finally, the 3-methoxy
group can be removed by hydrogenation to provide the 3-hydridoquinazolinedione
compound.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-123-
Scheme 1
R Rs O Ra O Ra O
~JI~OH "amide formation" R wJ ~ N-OMe "CO" equivalent R4~J ~ N.OMe
R ~K~ NH2 ~ H ~ I
s t s Rs K NH2
R ~s R5 K N O
R Rs H
J= C, N; K= C
Rs= Halo base
R~X
3 R3 O
Rs O 4 R O ~NH
a R ~ .OMe
R ~J~NH "reduction" ~~ ~ R ~~~ Me
I
~N~~ N~O ~ GN K~ N O Rs Rs R7 O
Rs R~ Rs R~
Scheme 2 provides a particular example of this approach. Thus, upon
treatment with carbonyl diimidazole and O-methylhydroxy amine, 4,5-
difluoroanthranilic acid can be converted to the N-methoxy benzamide
derivative.
Upon treatment with phosgene, the N-methoxy benzamide derivative undergoes
cyclization to provide methoxyquinazolinedione. AIkyIation of at the I-
position
using bromomethylcyclopropane in the presence of base affords the Rl
substituted
compound. Coupling of an amine at the RS position provides the RS coupled
compound, which can be converted to the 3-hydrido compound under
hydrogenation conditions.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-124-
Scheme 2
0
0
F I j OH CDI/THF, ref( F I ~ ~.OMe phosgene F ~ O N,OMe
R5 NH2 CH30NH2 ' HCI
R5~NH2 dioxane, reflux R5 ~ N~O
Et3N, reflux H
R5= F
NaH
DMF
Br
O H
F ~ N.OMe BocHN~~~yNH O
F ~ .OMe
H~N , N~O ~ H ~ N
R5 ~ N' 'O
BocHN'~ H
H2
Raney Ni
MeOH
O
O
F ~ .H F ~ N.H
N deprotection H,, N I ~ N~O
H, N'~.
H~~N / N ~O
BocHN'~ H hl
H
Scheme 2-A provides an alternative approach to quinazolinedione ring
formation. Urea formation at the nitrogen of the cyclopropylamine moiety in
the
starting compound can be effected using chlorosulfonyl isocyanate. Heating of
the urea in refluxing toluene, followed by removal of the protecting group,
gives
the invention compound.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-125-
Scheme 2-A
Me
~O
F ~ O
chlorosulfonyl isocyanate
BocHNn,.~N ~ NH CH2CI2 BocHNn,.
CI ~ O, _NH2
toluene, reflux
O
F .H F ~ N.H(Pro)
~N deprotection ~
H'N", N '~ N~O ~ BocHNn~~~N N~O
H ~ CI ~ CI
A characteristic of the approach outlined in Schemes 1, 2, and 2-A is the
generation of the 3-hydrido group subsequent to the introduction of the RS
sidechain. Scheme 3 provides an alternative approach to compounds of the
invention wherein J is N or C, and wherein the RS sidechain is attached
subsequent to the generation of 3-hydrido group. Thus, the nicotinic acid
derivative can be converted to the nicotinamide derivative via an acid
chloride or
acid anhydride intermediate. The acid chloride or anhydride intermediate can
be
converted to a urea deriviative upon treatment with oxalyl chloride or an
equivalent, followed by addition of R~NH~, wherein R1 has any of the meanings
provided herein. Cyclization of the urea can occur upon treatment of the
compound with base in the prirsence or absence of a chelating agent, to
provide a
quinazolinedione. An R5 sidechain can be attached to the dione as provided in
Scheme 2.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-126-
Scheme 3
0 0
1) (GOCI)2, CHzCl2, O O
JI ~ OH DMF J ~ NHZ 1) (COCI)2, CICHZCH2CI II
-. I reflux J ~ N~N
Rs GH3 C! 2) NH3 (g) Rs C 3 GI 2) cyclopropylamine Rs i i CI H H
CH2CI2 CH3
J= N, C
Rs- CI
KN[Si(CH3)~]z
1 B-crown-6
THF
O
J ~ N.H
I
Rs ~ N~O
CH3
Scheme 4 provides a particular approach to invention compounds wherein
RS is aryl. Thus, the acid is treated with acid and methanol to provide the
methyl
ester. Conversion to the RS aminated compound occurs using using benzyl amine
in the presence of triethylamine and DMSO. Removal of the benzyl group using
Pd/C provides the RS primary amine. The ester moiety is then saponified to the
acid, and the RS amine is converted to a bromide using CuBr and t-BuNO~.
l0 Conversion of the acid moiety to an amide, followed by generation of the
acyl
isocyanate and treatment with cyclopropylamine (RINH2) provides the urea,
which is treated with base as provided in Scheme 2-A to give a
quinazolinedione
with an RS bromo group. An aryl group can be coupled at the RS-position using
an aryl stannane in the presence of a palladium catalyst under conditions
known to
those skilled in the art to provide a compound of the invention.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-127-
Scheme 4
0 0 0
F
~ OH HCI (g) ~ MeOH F I w O.CH3 _BnNH2 F I w O.CH3
Rs / F 0 °C to reflux Rs ~ F Et3N / DMSO ~N ~ F
CH3 CH3 100 °C I -~ H CH3
Rs= F
Pd/C
MeOH, reflux
O O O
F tBuNO CuBr F F ,CH
~OH ~ I ~ ~OH Saponification ~ ~ 0
Br ~ F MeCN H2N ~ F ' H2N ~ F
CH3 CH3 CH3
1 ) (COCI)2
2) NH3 (9)
O O
F ~ _ 1 ) (COCI)2 F ~ _N N O
NH2 DCE, 90 °C ~ H H KN[Si(CH3)a12 F ~ ~H
Br ~ F 2) cyclopropylamine Br ~ F 18-crown-6 ~ / 'N
CH3 CH2CI2 CH3 THF Br N O
CH3
aryl stannane
Pd2(dba)3
Ph3As
O
F ~ N.H
Ar J ~ N~O
CH3
Seheme 5 provides another variation of this approach wherein R5 is iodo.
Attachment of an iodo group at the RS-position can be effected via
diazotization of
the R5 amine in the starting compound using isoamyl nitrite in the presence of
CuI. Saponification of the ester moiety, followed by the series of
transformations
provided earlier can provide the RS iodo quinazolinedione compound. Attachment
of an aryl, alkyl, or heterocycloalkyl group to the RS-position of the
compound
can be achieved using procedures available to those skilled in the art.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-128-
Scheme 5
0
F ~ .CH3 O O
isoamyl nitrite F ~ ~ p'CH3 2N NaOH l MeOH F ( ~ OH
Rs F Cul, CH3GN R ~ F o
CH3 o s 60 C Rs
rt to 50 C CH3 CH3
Rs' NH2 Rs= ! Rs= I
1 ) (COCI)2
CH2CI2, DMF
2) NH3(9)
THF! Et20
-78 °C to rt
O O 1 ) (COCI)2, O
F ! ~ _N~N~ CICH2CH2CI, reflux F W NH2
R ~ F 2) cyclopropylamine, Rs I ~ F
NaH s CH dioxane
CH
Rs- I
THF l DMF Rs= I
F ~ N.H
reflux
RS ~ N' 'O
CH3
Rs- I
Scheme 6 provides an approach to invention compounds wherein R6 is a
fluorinated alkyl group. Thus, treatment of 2,4,5 trifluorobenzoic acid with
lithium hexamethyldisilylazide and dimethyl formamide gives the R6 aldehyde,
which, when treated with (diethylamino) sulfur trifluoride (DAST) gives the R6
difluoromethyl compound. Ring closure to provide the quinazolinedione scaffold
is achieved as provided in earlier schemes.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-129-
Scheme 6
0 0 0 0
1) n-BuLi, THF F
OH 1 COCI CH CI F ~
F ~ pH Me3SiNHSiMe3 I ~ ) ~ )z~ 2 z. N N
I i R ~- F DMF ~ I , H H
Rs F s RS ~ 'F
Rs 2) DMF F F 2) cyclopropylurea,
benzene reflex F F
3) (diethylamino)sulfur trifluoride
Rs= F NaH
Rs =H
THF/DMF
60 °C
O
F ~ N.H
Rs I ~ N~O
F F
Scheme 7 provides an approach to compounds of the invention wherein RS
is a substituted cyclopropyl group. The pare position in ethyl (2,4,5
trifluoro-3-
methyl) benzoate is activated relative to the ortho or mete position. Thus,
reaction
of the starting compound with the shown cyano ester in the presence of base
provides the pare addition product. Saponification of the t-butyl ester,
followed
by decarboxylation under acidic conditions provides the RS cyanomethyl
compound. Treatment of the RS cyanomethyl compound with benzyl triethyl
ammonium chloride and 1,2 dibromethane gives the RS cyanopropyl compound.
Quinazolinedione formation then occurs via cyclization upon treatment with
base
to provide the target compound.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-130-
Scheme 7
0 0 0
R° ~ OEt K2C03, DMSO R4 ~ OEt R4 ~ OEt
NC ~ , p-TsO~ NC
RS F O ~ ~F reflux F
CH3 tBU~O O CH3 CH3
NC Ot-Bu
R4= F RQ= F
RS= F R4= F PhCH2N(Et)3CI
10N NaOH
BrCH2CH2Br
O O'I /~ 1 ) (COCI)2, DMF O
Ra ~ N~N~ CH~CI2 R4 w OH
NC ~ r H H '
F 2) cyclopropyl urea NC ~ F
benzene, reflux CH
NaH CH3 Ra= F
R4= F
DMF
O -20 °C to reflux
O
R4 ~ N,H OR4 ~ N,H
NC ~ / N ~O ~ N NaOH, N202
CH3 ~ room temperature HzN ~ N~O
CH3
R4= F R4= F
Schemes 8A-C provide approaches to invention compounds wherein R6 is
an alkoxy group and J may be N or C. Scheme 8-A summarizes the general
approach. Esterification of the meta-hydroxy benzoic acid derivative, followed
by
O-alkylation of the meta-hydroxy group using the t-butyl ester of bromoacetic
acid provides the shown aryl ether. Hydrolysis of the t-butyl ester, followed
by
fluormation gives the fluoromethoxy compound. Quinazolinedione ring
formation can be effected as provided in earlier schemes.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-131-
Scheme 8-A
R3 O R3 O R3 O
4 4
R ~J ~ OH "esterification" R ~ R ~
J ~ FOR Base, DMF ~ ~ ~OR
s I ~
R R Rs I ~ R~ O Rs i R~
OH OH Br~O~ t Bu O
~O~t Bu
O
ester hydrolysis
R3 O R3 O R3 O
'~ 4
R ~J ~ NH2 "amide formation" R ~J ~ OR XeF2 R ~J ~ OR
Rs I i R~ ~ Rs i , R~ . i
CH2Ci2 Rs ~ R~
O O O
FJ FJ ~OH
O
"urea formation"
R3 O O R3 O
4
R ~ i w H~H'R~ ..base" R \J! / ~H
Rs ~ R~ Rs N O
0
FJ F
Scheme 8-B provides a particular example of the Scheme 8A approach. In
this variant, fluorination is effected using xenon difluoride (XeF2) in a
chlorinated
solvent (Shaw, et. al, J. Am. Chem. Soc. 91, 1563 (1969).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-132-
Scheme 8-B
0 0 0
F
OH HZSOQ / MeOH F ~ O~CH3 NaN, DMF F ~ ~ O~CH3
F I F refl~ F I ~ F ' O F ~ F
OH pH Br~O. t Bu O
0 °C to rt ~O~t-8u
O
TFA
CH2CIz
O O O
F
~NH2 NH3, MeOH, rt F w O~CH3 XeF , CH Ct F ~ O~CH3
z z 2
F F F ~ F ' F ~ F
O O O
FJ FJ ~OH
1 ) (COCI)z, CICH2CH2CI
reftux
2) cyclopropylamine, dioxane
O O'' O
F I ~ H~H~ NaH, THF, reflux F ~ N-H
F O F F ~ N- '_O
FJ J
F
Scheme 8-C provides an approach to the synthesis of an invention
compound with an R6 difluoroalkoxy group. Thus, 2,4 difluoro-3-methoxy
benzoic acid is converted to the amide. The methoxy group is then converted to
a
phenol using boron tribromide. O-alkylation of the phenol with
dichlorodifluoromethane in the presence of base, followed by hydrogentaion,
provides the difluoromethoxy compound. Formation of the quinazolinedione ring
scaffold can then be achieved as provided earlier.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-133-
Scheme 8-C
0 0 0
X \ 1) (COCI)2, CH2CI2 X
I W ~NH X I W
OH DMF~ I 2 BBr3, CH2Ci2 NH2
~ i
Y F 2) NH3(g), THF l EtzO Y F Y F
H20 OH
O~CH3 CHs
X=H, F ~ 1) K2C03, DMF
Y= F, Br
CI2F2C
-78 °C to i 10 °C
2) Hydrogenation
O O ~ O
X N~N,~ 1) (COCI)Z, CICH2CH2C1 X
reflux I ~ 'NH2
I H H
Y ~ F 2) cyclopropylamine, Y ~ F
O~F dioxane, rt O~F
F IF
NaH
THF/DMF
reflux
O
X ~ N.H
Y I ~ N' '_O
F~O
F
Scheme 9 provides an approach to the synthesis of tricyclic invention
compounds. Thus, the indole starting material can be converted to the shown
methyl ester using standard procedures. Removal of the thioethyl group using
Raney Nickel is followed by reduction of the indole double bond using
trifluoroacetic acid/triethyl silane to provide the cyclization precursor.
Treatment
of the cyclization precursor with trifluoroacetic acid and potassium
isocyanate in a
chlorinated solvent gives the tricyclic invention compound.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
- I 34-
Scheme 9
F Ce F Ce
F S CO S
Pd(OAc l Et N F ~ Raney Ni F
CH3 )2 s ~ \ CH3 EtOH, reflux F
\ CH3
diphenylphosphinopropane / . ~ .i
Br H MeOH, 100 °C HsC H
'0 O H3C'O O
TFA
Et3SiH, 50 °C
O F
F W N'H ICNCO / CH2CI2 F
/ CHs
F ~ N~O TFA
H3C'O O H
Scheme 10 provides an approach to compounds of the invention wherein
R3 is alkyl or alkoxy as defined herein. Thus, a compound wherein R3 is H and
R6
is alkyl or alkoxy can be treated with a base, such as lithium diisopropyl
amide
(although other bases may be used), followed by an alkylating or acylating
agent.
Alkylating agents (such as alkyl halides, -mesylates, triflates, and the like)
and
acylating agents (such as acid halides, acid anhydrides, phosphoryl halides,
and
sulfonyl halides, among others) are well known in the art and many are
commercially available, for instance from a supplier such as Aldrich, and are
listed in the Aldrich Handbook of Fine Chemicals, 2002-2003. Other alkylating
or acylating agents can be prepared as needed according to methods available
to
the skilled artisan. An R5 sidechain can then be attached to the resulting
l5 compound wherein R3 is an alkyl, acyl, or other group.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-135-
Scheme 10
R3 O R3 O
F / 1 ) Base F i NH
R ~ N O 2) Alkylating or Acylating Agent R5 \ N' 'O
Rs ~ (E+) Rs
Rs= H Rs= E
R5= F R5= F
Rs= C1-Cs alkyl, O(C1-Cs alkyl) Rs° C7-Cs alkyl, O(C~-Cs alkyl)
Other approaches to the preparation of the invention compounds are
5 available to the skilled artisan. For example, a general synthetic strategy
for core
formation suggested by WO 01/53273, which is assigned to the same assignee as
the instant application, can be substantially modified and adapted to the
synthesis
of the invention compounds disclosed herein. Thus, Scheme 11 discloses another
approach to the invention compounds wherein the Rland RS sidechains are
attached prior to cyclization to form the quinazolinedione ring. As provided
by
the scheme, a difluoro substituted benzoic acid wherein one or both of J or K
may
be N is reacted with oxalyl chloride or an equivalent acylating reagent (such
as an
acid anhydride), and the acid halide or anhydride is reacted with an alcohol
(ZOH)
to afford the respective ester (Z is C1-C6 alkyl such as methyl, ethyl,
isopropyl,
etc.). The ester is reacted with an amine, for example, a heterocyclic amine,
to
produce the desired 4-heterocycIic phenyl derivative. Alternatively,
carbocycles
and aryls may also be introduced at this 4-position using palladium catalyzed
couplings of tin or boronate carbocycles and aryls, with starting materials
containing a Br, I, or triflate at the 4-position as described by Suzuki A.,
Pure
Appl. Chem., 1994;66(2):213-222 and Stille J.K., Angew. ehena.
1986;98(6):504-S I 9.
Reaction of the 2-fluQro benzoic acid analog with a primary amine R1NH2
affords the corresponding anthranilic acid ester. The ester group is readily
hydrolyzed by reaction with an acid such as hydrochloric acid or a base such
as
sodium hydroxide to give the corresponding polysubstituted anthranilic acid.
The
acid is then coupled to a source of NH3, typically protected by a protecting
group
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
- I 36-
(Pro), to provide the corresponding amide. The amide may then undergo
cyclization in the presence of a carbon monoxide equivalent such as phosgene
to
provide the quinazolinedione. At this point, the protecting group may be
removed
to give the 3-hydridoquinazolinedione of Formula I, which may be a final
product
S of the invention, or may be further derivatized. The protecting group (Pro)
of the
N-protected quinazolinedione is removed by conventional methods such as
hydrogenation, treatment with acid, lewis acid, or base, or metal catalysis to
afford
the shown invention compound A.
If R3 is a leaving group such as F, it may be activated towards
displacement with a nucleophile HY Pro' where Y is NH or O. Other R3 groups
such as chlorine, bromine, or sulfonyl are also good leaving groups. The
displacement generally is carned out in a solvent such as ethanol, DMSO, DMF,
THF, and at a temperature of about 0°C to 120°C.
The protecting groups (Pro and Pro') may be selectively removed by
IS hydrogenation, acid or base treatment, metal catalysis, or other standard
methods.
When Pro or Pro' is methoxy or benzyl, either of these groups may be removed
with Pd/C and hydrogen. A t-butyl oxycarbonyl group may be removed by
alcoholic HCI, TFA, or TFA in dichloromethane, ethyl acetate or diethyl ether.
Allylic oxycarbonyl groups may be removed by PhSiH3 and Pd catalyst. Solvents
such as alcohol, THF, alcohol/THF, alcohol/THF/DMF, diethyl ether, etc. are
generally employed in such protecting group cleavage reactions.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-137-
Scheme 11
Rs O Rs O
/\ ,NH
R4~Jl ~ OH 1 ) oxalyl chloride R4 I ~ O ~Z
F~K F 2) ZOH F ~ F
I Base, heat
Rs Rs
R3 O R3 O
ester hydrolysis R4 I ~ OZ R1 NH2 R4
~OZ
~N ~ NH Base, heat ~N ~ .~ F
I
Rs Ry ~ R
s
R3 O H R3 O
R4 ~ OH H.N.H(Pro) R4 ~ .Pro
'H "GO Equivalent"
~~ i H coupling ~ ~ NH
Rs R1 ~ Rs R1
R3 O R3
R O
N. (Pro) Ra
Pro Removal ~ NH
N O ~N ~ N~O
Rs R1 ~ Rs R1
A
Pro'~Y O
R4 N.(Pro) Pro'
N ~ N' \O Rem~-oval
I I
Rs R~
YH O YH O
R4 I ~ N,Pro Pro R4 I W
~NH
~N ~ N' '-O Removal ~N ~ N' 'O
Rs R1 I Rs R1
B A
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-I38-
In Scheme 12, an ortho-aminobenzoic acid wherein one or both of J or K
may be N is utilized as the starting material, and is alkylated on the amino
group.
For example, when RI is cyclopropyl, the alkylation is carried out according
to
the method of Gillaspy (Tetrahedon Letters, 1995:7399) to provide the
cyclopropyl amine. When RI is phenyl or substituted phenyl, the respective
amine
is prepared from the ortho-fluorobenzoic acid using a base, such as, lithium
diisopropylamide or lithium hexamethyl disilazide, and the appropriate aryl
amine
(R1NH2). When R1 is any alkyl group, such as t-butyl or isopropyl, the R1 can
be
introduced by reacting the amine and ortho halo benzoic acid with a Cu
catalyst
such as copper bronze, or cuprous acetate in the presence of a base such as
potassium acetate, triethylamine, or pyridine.
The resulting R1-substituted amino benzoic acid is then coupled to
ammonia in an appropriately protected form to provide the corresponding
benzamide using methods described in the literature. The corresponding amide
can be further reacted, if RS is a leaving group such as fluoro, with various
heterocyclic amines (e.g., piperidine or pyrrolidine) to form the desired
4-heterocyclic benzamide derivative. Alternatively, carbocycles and aryls
(e.g.,
cyclobutyl or phenyl) may also be introduced at this 4.-position using
palladium
catalyzed couplings of tin or boronate carbocycles and aryls, if the starting
material contains a Br, I, or triflate at the 4.-position.
The 4-substituted benzamide derivative is then cyclized to generate the
quinazolinedione by reaction with carbonyldiimidazole (CDI), phosgene,
triphosgene or the like in ethereal solvents such as diethyl ether,
chlorinated
hydrocarbons such as dichloromethane, or aromatic hydrocarbons such as
toluene,
in the presence of a base such as triethylamine or sodium bicarbonate
(NaHC03).
Alternatively, the corresponding amide is first cyclized and then the RS
halo group is displaced by reaction with a carbocyclic amine to afford the
same
product. Deprotection by conventional methods provides the invention
compound A.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-139-
Scheme 12
R3 O Et-O OTMS
R4~Jl ~ OH
R5~K~ NH2
Rs NaBH3CN
R3 O
R4 ~ OH
R3 O LDA, R1NH2 I
R4 R5 ~ NH
I w ~OH
Rs Ry
R5 ~ F
Rs RyNH2, Cu catalyst
R3 O H2N-H(Pro)
R4 ~ OH "Coupling"
I
R5 ~ X
Rs
R3 O
X= CI, Br, I
NH R4 i w NH(Pro)
O R5 ~ NH
R~ I ~ NH(Pro) Base, Heat Rs Ri
N ~ NH
Rs R1
Triphosgene
Triphosgene
R3 O
'NH R
R3 O 4 ~ N-H(Pro)
R
N-H(Pro) R5 N O
I ~ Base, Heat Rs Ri
N ~ N~O
i
Rs R1
-'' Pro Removal
R3 p
R4 W N,H
N I ~ NI \O
i
Rs R1
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-140-
Scheme 13 illustrates alkylation at the l-position of a quinazolinedione to
provide invention compounds wherein Rl is alkyl. Thus, a 2-aminobenzoic acid
wherein one or both of J or I~ may be N is reacted with an N-protected amine
to
provide the corresponding N-protected amide. This intermediate can then be
reacted with a carbon monoxide equivalent such as phosgene or phosgene/base in
an ethereal solvent, or with a phosgene equivalent such as triphosgene in a
chlorinated hydrocarbon such as dichloromethane, to give the quinazolinedione.
The alkylation of the quinazolinedione to provide a 1-alkylated-
quinazolinedione
is accomplished by reaction with an alkyl halide as described by Bouzard,
supra.,
1990. Typically, such reactions are carried out in THF, ether, DMSO, an
alkanol,
or DMF, and in the presence of a base. Typical alkyl halides (R1X where X is
halo) include ethyl iodide, ethyl bromide, cyclopropyl iodide, n-decyl
bromide,
and the like. Typical bases include sodium hydride, potassium carbonate, and
the
like. Conversion of the 1-alkylated-quinazolinedione to other invention
compounds (and removal of protecting groups such as benzyl) can be carned out
as provided earlier to give the corresponding 1-alkyl (R1 = alkyl) 3-hydrido
compounds such as A.
Scheme 13
R3 O Rs O
Raw ~ H2N-Pro R
;r~OH 4~~ ~ I NH-Pro
R5 ~tC~NH2 R ' 'K NH Equivlent
R s ~ z
s Rs
R3 O RiX, Base R3 O
RQ~~, N.Pro R4~J~ N.Pro
Heat
R5 K N O R5 K N O
Rs H Rs R1
Displacement of leaving groups located at the R$-position of the
quinazolinedione (e.g., RS = halo) as shown above is not limited to nitrogen
heterocycles. Other nucleophiles (Nu) such as CH30-, N3-, R'R"NH, R'-NH2,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-141-
and R°S- (where R' and R" are each independently (C~-C~)alkyl) also
displace a
leaving group such as F, Cl, or N02 at the RS-position as provided in earlier
schemes. When the leaving group is a triflate or higher halide (Br or I),
organotin
reagents or organoboronates may be used with palladium catalysts to deliver a
carbon nucleophile. The methodology generally disclosed in Scheme 14 may be
adapted to follow the coupling methodology of StilIe et al., Angew. Chem. Int.
Ed.
Eng., 1986;25:508, further exemplified by Mitchell (Synthesis, 1992:803). It
is to
be understood that in Scheme 14, one or both of J or K may be N.
Scheme 14
I-i3 O R3 O
R4~~ ~ N,H(Pro) RS~~ ~ N.H(Pro)
I ,. -. ,1
R~K N~O Nu K N~O
Rs R1 R6 R1
Deprotection
R3 O
R~
N.H
II ~
Nu K N' '-O
Rs R1
All of the chemistry depicted and described in Schemes 11 to 14 is
applicable to make compounds of Formula I wherein J and K both are carbon, or
where one or both of J and K are N. When either J or K is nitrogen, the
displacement reactions described above may be even more facile than when
J and K are both carbon.
As stated above, compounds of Formula I wherein one or both of J or K
are nitrogen may be prepared by Schemes 11-14, or alternatively, by routes
which
take advantage of the activation of leaving groups ortho and para to the J
and/or
K nitrogen atom. Such routes will systematically introduce RS and R1 groups as
desired. This methodology also applies to cases in Formula I where K-R6 is C-H
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-142-
or C-F and J-R4 is C-F. Such systematic substitutions are illustrated in
Scheme 15.
For example, a pyridine amide has leaving groups such as halo on both
sides of the nitrogen. Such groups are generally Cl, but Br, I, F, alkylthio,
and
sulfoxides, such as methyl sulfoxides, are also good leaving groups for such
compounds. These leaving groups may be sequentially displaced based on
reactivity. In Scheme 15, where J = N or J-R4 = CF, the 4-chloro (para to the
aminocarbonyl group) is displaced preferentially (relative to the 2-chloro
group)
using a nucleophilic amine such as diethylamine, pyrrolidine,
methylpiperazine,
and the like to give the corresponding amino substituted analog. This analog
can
then undergo reaction with R1NH2 to displace the second leaving group (e.g.,
the
2-chloro group). The resulting 2,6-disubstituted-pyridylamide is then reacted
with
carbonyl diimidazole (CDI), phosgene, or other carbon monoxide equivalents to
form the cyclized quinazolinedione product.
Scheme 15
O NH O
\ NH2 ~ ~ \ NH
2
CI N CI N N CI
Base
J= N, CF
Ri NH2 Base
O
O
~H J ~ NH
J \ ~N I 2
w ~ .~ . ..CO.. ~N~N NH
N N N O
Equivalent R
R 1
i
Tricyclic compounds (i.e., where Rl and R6 in Formula I are taken
together with the atoms to which they are attached to form a ring) can be
prepared
according to Schemes 16-19. Schemes 16-19 differ in the introduction of the R1
substitutions in Structures B and C wherein RS is as defined above for Formula
I.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-143-
In Scheme 16, the ortho-fluoro vitro compound (other leaving groups such as
chlorine,.bromine, and sulfonyl may also be employed in place of fluoro)
undergoes reaction with the shown ester. The vitro group is then reduced
using,
for example, Raney Ni, H2 over Pd/C, or an active metal in acid such as iron
or tin
in HCl or acetic acid. The newly formed amine readily cyclizes with the ester
(other acid analogs may be employed such as thioesters, amides, and the Iike).
The
cyclized product is then reduced with hydride reducing agents such as LiAlH4
and
the Like to produce the dihydroquinoline derivative, which in turn is reacted
with
chloral hydrate and then an acid to form a dione ring. The dione ring is
subsequently opened using, for example, sodium hydroxide and hydrogen
peroxide to give the benzoic acid. The quinazolinedione ring is then prepared
using the chemistry described earlier to give a precursor B to the invention
compound.
Scheme 16
R O R3 R3
3 X
R4 OMe Ra Ra
1) ~ \ \
Rh I '~ NH reduction I ~ NH
R5 ~ ~ N02 R5 X ~O ~ R5 X
2) reduction
F
Rh Rn
X= O, S, NH
1 ) Chloral 2) H2S04
NH20H'HCI
Ra O Rs O Rs
R4 I \ N~H R4 I \ OH NaOH Ra
Rs / N~O f Rs / NH f H O R
X~ X-
'R( h ~Rh
IS
In a similar series of reactions depicted in Scheme 17, the ortho-fluoro
vitro compound is reacted with an oc-nucleophile substituted ketone, and the
resulting product is likewise reduced. In this sequence, the resulting aniline
forms
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-14.4-
a cyclic imine, which is further reduced with H2 over Pd/C or by chemical
hydride reducing agents such as sodium borohydride or sodium cyanoborohydride
to give the dihydroquinoline. Such reductive aminations are well-known in the
art
and are typically performed in THF, alcohol, water alcohol mixtures, or in
water
DMF mixtures. The remaining steps to produce C follow those of Scheme 16.
When the RS substituent is a leaving group (e.g., RS = halo), compounds B and
C
may be further reacted with nucleophiles (such as pyrrolidine or piperidine)
to
give compounds of Formula I as in the previous schemes. Also, as indicated in
Schemes 17-19, R,,~ can have any of the meanings disclosed for R~,.
Scheme 17
0
R X R3 R3
s ~ R~,
Ra 1) R7 Ra Ra
reduction
N ~ NH
w
Rs N02 2) reduction R5 x~R , R5 X~R ,
n n
Rn Rn
1 ) Chloral 2) H2S04
NH20H~HCI
Rs O Rs O Rs
R4 ,H R4 NaOH R,
J w ~N ! W ~OH
N~O Schemes ~ NH H202
RS x~Rn' 2, 3, or 5 RS X~Rn,
Rn ~R _n
C
In Scheme 18, an R3 amino group is attached to the tricyclic compound via
nitration and reduction.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-145-
Scheme 18
Rs O N02 p NH2 O
F I ~ N'H H2gp~ F W N~H H2, Raney Ni F ~ N~H
~i ~ ~
F N O i<N03 F I '~ N' 'O THF l MeOH F I ~ N- \O
Ov 'CH3 ~ ~ _ ~
Ov 'CH3 O V 'CH3
R3= H
As depicted in Scheme 19, target tricylic compounds such as C are
prepared in a slightly different manner. In this variant approach, XH (wherein
X is
O, S, or NH, for example) is attached to the phenyl ring of the starting
aniline, and
a leaving group L (such as halo) is attached alpha to the ketone reactant. The
nucleophile may be activated with bases such as sodium hydride or potassium
hydride, triethylamine or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), or sodium,
potassium, or cesium carbonate to displace the leaving group L. In this
sequence,
the resulting aniline forms a cyclic imine, which is further reduced with H2
on
Pd/C or by chemical hydride reducing agents such as sodium borohydride or
sodium cyanoborohydride to give a dihydroquinoline. Such reductive aminations
are well-known in the art and are typically performed in THF, alcohol, water
alcohol mixtures, or in water/DMF mixtures. The dihydroquinoline intermediate
is
reacted with chloral hydrate, and then an acid to form the dione ring. The
dione
ring is subsequently opened by reaction with a base, for example sodium
hydroxide and hydrogen peroxide, to give the benzoic acid.
The quinazolinedione ring is then prepared by first forming an ester on the
benzoic acid as provided earlier, followed by reaction of the benzoic acid
ester
with chlorosulfonylisocyanate or the like at temperatures of 0°C and
below,
followed by treatment with a base such as triethylamine or
diisopropylethylamine
to provide compounds of structure C. When RS is a leaving group such as CI or
F,
the invention compound precursor B (in Scheme 16) and the invention compound
D (in Scheme 19) can be prepared by coupling to the RS a side chain, e.g.,
various
heterocyclic amines C~ to produce the desired derivatives. Alternatively,
carbocycles and aryls may also be introduced as R5 side chains using palladium
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
- I 46-
catalyzed couplings with tin or bornate carbocycles and aryls, for example
when
RS is a Br, I, or triflate.
Scheme 19
0
Rs R3
Rs ~ Rn, Ra Ra
R4 Rn ~ I w
reduction
i Base R5 ~N R5 NH
R5 X NH2 X~Rn, X~Rn,
Rn Rn
X= OH, SH, NH2 X= O, S, NH X= O, S, NH
Chloral,
NH20H~HCI
O Rs O R3 OH
R4 ~ ~ OH NaOH R4 ~ ~ O H2SO4 R6 I ~ N~
s ~
R5 / NH H202 R / N R ~ N- 'O
5
X~Rn, X~Rn, X~R ,
n
Rn X= O, S, NH Rn Rn
X=O, S, NH X=O, S, NH
esterification (HOZ)
Rs O Ra O
Ra ~ y) chlorosulfonyl- R4
'OZ isocyante ~ ~ ~NH
R5 NH 2) vase R5 N O NH
X ~ Rn~ X ~ Rn, I
Rn Rn
X~ O, S, NH C
X= O, S, NH Rs O
Ra ~ NiH
N ~ N' 'O
X
Rn,
X= O, S, NH Rn
5 D
It should be noted from Schemes 16-19 that Rh and Rh~ will form chiral
centers, giving R and S enantiomers and diastereomers. Such enantiomers or
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-147-
diastereomers may be separated, if desired, by chiral HPLC at any stage.
Resolution of any of the intermediates may be performed with techniques of
fractional crystallization using mandelic acid, tartaric acid, or other
chiral,
optically pure acid resolving agents. Chiral benzylic amines (such as a-
methylbenzyl amine) can be used in the preparation of starting materials for
the
above schemes, and chiraI amides may also be prepared using chiral acids, such
as
mandelic acid and the like. The isomers can then be separated and the chiral
amine can be hydrogenated, or the chiral amide can be hydrolyzed.
Scheme 20 illustrates synthesis of compounds of Formula I wherein one or
both of R4 and R6 are halo via halogenation. The halogenation is carried out
on a
2-aminobenzoic acid where one or both of R4 and R6 is hydrogen. If both R4 and
R6 in the benzoic acid are H, then halogenation can be accomplished at both
positions selectively or simultaneously. Thus, for example, chlorination at R4
or
Rb is achieved by reaction of the benzoic acid with N-chlorosuccinimide,
t-butylhypochlorite, chlorine gas, and the Iike. Similarly, bromination at R4
and
R6 can also be accomplished by reaction of the benzoic acid with Br2,
N-bromosuccinimide, and the like. Such halogenations are well-known in the
art.
Halogenation thus provides the respective mono- or dihalo-compound. The
halogenated benzoic acid then can be further reacted as shown in earlier
schemes to provide the quinazolinediones of Formula I.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-148-
Scheme 20
R3 O R3 O R3 O
Ra \ OH halogenatio_n T , \ OH ~ Ra I w N~H(Pro)
where ~ / H
s NH Ra/Rs = H RS NH RS ~ ~NH
Rs Rt Y Ri Rs Ry
T, Y = CI, Br, F, H
H2N-H{Pro) "Coupling" R
3
Ra \
H {Pro)
R3 O R ~~ N' 'O
Ra ~ ~H(Pro) 3 O 5 I
~N T ~H(Pro) Rs R1
RS I ~ NH halogenation i j H ~ or
I where NH
Rs Rt Ra/Rs = H R5 Y I R3 O
R.1 Ra
~ i H \ ~NH(Pro)
Base, heat T, Y = CI, Br, F, H
iH
Rs Ri
R3 O R3
Ra N~H(Pro) T O ~ ( )
H Pro
H halogenati~n I \ ~N
N NH where ~ ~ H
R R Ra/Rs = H N Y ~ H2
s i
R~
T, Y = CI, Br, F, H
R3 O
Ra \ _ ~H(Pro)
N deprotection goratula I
N~O
RS I
Rs R1
In Scheme 21, compounds where R6 is H are halogenated as described
above to provide the corresponding 3-halo-2-aminobenzoic acid (Y = halo). This
intermediate can then be diazotized by reaction of the 2-amino group with,
sodium
nitrite or t-butyl nitrite, which is then converted to a 2,-halobenzoic acid
in the
presence of an appropriate sodium, potassium, or copper salt such as sodium
iodide, potassium chloride, or the like. The resulting 2,3-dihalobenzoic acid
(where X and Y both are halo) is then converted to the 3-halo-2-aminobenzoic
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-149-
acid by reaction with an amine Rl NH2 in the presence of a copper catalyst.
The
3-halo-2-aminobenzoic acid is converted to an amide and cyclized to the
corresponding 8-halo-quinazolindione.
Scheme 21
R3 O R3 O
R4 ~ OH halogenation R4 i OH
R \ I NH 5 ~ I NH Y= CI, Br
~ w 2 R 2
R6 Y
diazotization
KI, CuBr, etc.
R3 O
R4 R3 O
i I ,OH NH2Ri Ra
'OH
X= CI, Br, I
R5 Y RH Cu catalyst R5 ~ X
5 . ' Y
Compounds of Formula I wherein R4 and/or R6 is halo such as chloro ar
bromo are readily dehalogenated by reaction with metal catalysts under
hydrogen
pressure (Scheme 22). Suitable catalysts include the many variations of Pd on
l0 carbon, Raney nickel, or other reagents that are well-known to effect such
dehalogenation.
Scheme 22
i-i3 O R3 O
R4 N.H(Pro) ~ N.H(Pro)
Pd/C
N i O H2 ' i i O
Rs Ri R1
R4 and/or R6 = CI, Br
Compounds of Formula I wherein R4 and/or R6 are hydrogen can be
l 5 halogenated to give the mono- or dihalo compound (e.g., R4 or R6 = Cl or
Br).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-150-
The invention compounds are preferably prepared by first halogenating a
benzoic
acid derivative, and then cyclizing the halogenated benzoate (Scheme 23). If
both
Rq. and R6 are H, then halogenation can be accomplished at both positions
selectively or simultaneously. Halogenations can be carried out as described
above for Scheme 20. The resulting compound is then converted to the
2-substituted-aminobenzoic acid as depicted in Scheme 1 l, which is
subsequently
cyclized.
Scheme 23
Rs O Rs O
R4 ' ~ OZ "halogenation_s" X ( ~ OZ
N ~ F Where R41R6 N '~ F
=H ~ Y
R6 X,Y = H, F, CI or Br
RiNH2 'base, heat
Z is an ester forming
group such as alkyl or benzyl R3 O
OZ
N ~ NH
Y R1
I O Invention compounds of Formula I can also be prepared as shown in
Scheme 24. Substituted benzoic acids can be converted into esters (where Z is
an
ester forming group such as alkyl or benzyl) by a number of methods known by
those skilled in the art. The ester is reacted with an isocyanate such as
trimethylsilylisocyanate, chlorosulfanyl isocyanate, and chlorocarbonyl
isocyanate, followed by treatment with a base such as triethylamine, sodium
t-butoxide or the like, to provide a quinazolinedione. This compound may be
further reacted, when RS is a leaving group such as fluoro, with various
heterocyclic amines. Again, carbocycles and aryls may also be introduced at RS
if
RS is a Br, I, or trif7ate, using palladium catalyzed couplings of tin or
boronate
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-I51-
carbocycles and aryls. Removal of any protecting groups (Pro) by normal means
provides invention compounds such as A.
Scheme 24
Rs O Ra O
.O
R4 I \ OH "esterification" R4 I \ OZ RAN C
R5 ~ NH ZOH/H+ ~ NH R = -H, TMS, -COCI
R I
I 5
Rs Rf Rs Ri
R3 O NH
R3
~NH pro-N I R4 \ O
R5 N ~O H
N O
Rs R~
Rs R1
Pro-N
H
R3 O
"Deprotection" Ra \
'NH
~ N ~O
I Rs R1
H2N
A
Invention compounds of Formula I having a cyano substituent can be
prepared as shown in Scheme 25. For example, a benzoic acid wherein R6 is
hydrogen can be metallated with a strong base such as lithium
hexamethyldisilazide or lithium diisopropylamine. The resulting metallated
intermediate can then be quenched with dimethylformamide or an equivalent to
provide an aldehyde. The aldehyde can be converted to an oxime by reaction
with
an alkoxy amine. Other electrophiles such as alkyl halides, activated amides,
esters and halide sources such as 1,2-dichloro-tetrafluoroethane may also be
employed to provide other R6-substituted benzoic acid derivatives that can be
used
to make the invention compounds. The oxime is then converted into a cyano
group under the cyclization conditions required to form the quinazolinedione
ring
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-152-
system (e.g., reaction with phosgene or triphosgene or the like). Deprotection
provides invention compounds where R6 is -CN.
Scheme 25
Rs O R3 O R3 O
R4 I ~ OH 1 ) Metallation R4 I ~ OH NH20R' Ra I
'OW
F ~ F 2) DMF F '~ F F ~ F
1 ) oxalyl
Rs O~ N ~ chloride
Re - H OR' 2) ZOH
R3 O R3 O GNH R3 O
R4 ~ OZ RiNH2 R4 ~ OZ R4 I ~ OZ
I .-I
~N ~ NH Base, Heat ~N ~ F Base, Heat F ~ F
I N~ R~ ~I N~ N~
OR' OR' OR'
Ester Hydrolysis
RS O R3 O
R4 ~ OH Ra ~ N-H(Pro)
I (H)Pro-NH2 I
GN N ~ RH coupling GN N ~ RH ~,COw
Equivalent
OR' OR'
R3 O R3 O
R4 ~ .H RQ .H(Pro)
I '~ Pro Removal
~N ~ N O ~ ~N '~ N O
CN R~ ~~ CN Ri
S Another alternative for preparing invention compounds is illustrated in
Scheme 26. Appropriately substituted benzoic acids can be converted into
benzamides by any number of methods as described above. A benzamide can then
be treated with oxalyl chloride in a chlorinated solvent such as
dichloroethane or
an equivalent to provide an isocyanate. The isocyanate is reacted with a
substituted primary amine to give a benzoyl-substituted urea. This
intermediate
can be cyclized to form a quinazolinedione ring system by reaction with sodium
hydride, potassium hexamethyldisilazane or other non-nucleophilic bases,
generally in a solvent such as tetrahydrofuran (THF)/dimethylformamide, THF
with 18-crown-6, THF/dioxane, THF/glyme, THF/diglyme, dimethoxyethane/
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-I53-
toluene or an equivalent. The resulting quinazolinedione ring system can then
be
readily coupled with an appropriately substituted heterocyclic amine (such as
those noted above in Table I ) by reaction in the presence of a base such as
triethyl
amine, diisopropylethyl amine, tetramethyl guanidine, and the like in solvents
such as dimethyl sulfoxide, dimethylformamide, dimethylacetamide, sulfolane or
the equivalent. Any protecting group associated with the heterocyclic amine
side
chain is then removed by methods known to those skilled in the art to provide
invention compounds having Structure A.
Scheme 26
R3 O Ra O Rs O O
R4 I ~ OH "amide formation" R4 I ~ NH2 oxalyl chloride Ra ~ N.C
X ~ X' X '~ X' X ' '~ X'
Rs Rs Rs
X, X'= halo
R~NH2
R3 O ~NH R3 O R3 O O
N ~~./) 4
R I ~ ~H R w NH base R4 ~ N~N-R~
N N O X ! ~ N~O X J ~ X'H H
Rs R~ Base, Rs R~ Rs
I 0 A heat
Tricyclic compounds (i.e., where RI and R6, together with the atoms to
which they are attached, form a carbocyclic ring in invention compounds of
Formula I) can be prepared according to Scheme 27 where a palladium mediated
carbon monoxide insertion on a quinaline (X = Br, I, or triflate) under well-
precedented conditions gives rise to an ester. The quinoline ring can then be
hydrogenated to provide a tetrahydroquinoline by standard hydrogenation
conditions. The remainder of Scheme 27 follows the approach of earlier schemes
to provide invention compounds such as G, substituted with a displaceable
substituent at RS (e.g., halo). These compounds may be further reacted with
nucleophiles such as amines from Table 1 to give compounds of Formula I.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-154-
Scheme 27
R3 R3 O R3 O
R4 ~ X R4 ~ OR R° ~ OR
CO insertion ~ reduction
RS ~ N ~ R5 ~ N " RS '~ NH
Rn~ ~ Rn, Rn,
Rh Rn Rh
1 ) chlorosulfonyl- or K-N=C=O, HOAc
isocyanate
2) base
R3 O
R° ~ NH
R5 I ~ N~O
Rn,
Rh
G
Compounds of Formula I where K is N may be prepared by the routes
shown as illustrated earlier or as indicated in Scheme 28, which follows
closely
the chemistry shown in Schemes 3 and 24. The leaving groups (e.g. halo) ortho
and para to the carboxyl group of the pyridyl starting material are highly
activated. The two leaving groups ortho to the pyridine nitrogen are generally
chlorine, but fluorine, alkylthiol, and sulfoxides such as methylsulfoxide are
also
good leaving groups for such compounds. The urea intermediate readily cyclizes
to the bicyclic system. The RS halo leaving group is readily displaced by
reaction
with an amine ~NH
Scheme 28
R3 O Ra O R3 ~ O
R4 I ~ OH "amide formation" R4 I ~ NH2 oxalyl chloride Ra
R5 N CI R5 N CI R5 N CI
RS= F, CI, Br, I
RtNH2 ~.
R3 O R3 O O
R4 I ~ NH base R4 I ~ N~N.R~
~~H H
R5 N N O R5 N CI
R~
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-155-
Scheme 29 illustrates the synthesis of benzoic acid starting materials
wherein RS is bromo. The carboxylic acid moiety of the difluoro substituted
benzoic acid first is converted to an ester moiety, by conversion to an acid
halide
or mixed anhydride using an acid anhydride, mixed anhydride, or acid halide
such
as oxalyl chloride or the like, followed by treatment with an alkanol. The
resulting
ester is then reacted with 4-methoxybenzylamine or an equivalent to produce
the
desired 4-substituted benzoic acid derivative. This intermediate is reacted
with
triethylsilane and trifluoroacetic acid in a chlorinated solvent such as
dichloromethane or an equivalent to provide an aniline derivative.
Alternatively,
one skilled in the art might also employ transition metal catalysis. The
resulting
aniline is then subjected to diazotization and converted to a bromide by
treatment
with cuprous bromide. The ester is then hydrolyzed by well-known methods to
the
desired benzoic acid, which can be used as a starting material to make
compounds
of the invention.
Scheme 29
R3 O R3 O ~ ~ NH2 Ra O
R4 , ~ OH esterification R4 I ~ OZ Me0 ~ R4 I W OZ
F ~ F F / F dimethylsulfoxide ~ N / F
heat
Rs Rs . . _., I / H Rs
Z=_ (01_Cs~alkyl
triethyisilane
dichloromethane
trifluoacetic acid
R3 O R3 O R3 O
R RQ t-butyinitrite R4
a ( ~ OH Saponification ~ ~OZ , ~ OZ
Br / F ~ Br ! / F CuBr HZN I / F
Rs Rs Rs
In Scheme 30, 4-bromo-2-fluorobenzoic acid derivatives can be
selectively chlorinated by treatment with chlorine gas in chlorosulfonic acid
at a
temperature of 40°C-100°C.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-156-
Scheme 30
R3 O R3 O
R4 I ~ OH C12 R4 I ~ OH
Br ~ F CIS03H gr ~ F
Rs CI
Rs=H
Scheme 31 illustrates the use of 4-bromobenzoic acids, in particular, as
starting materials to make invention compounds wherein RS is aryl. The
4-bromobenzoic acids are converted into benzamides by any number of methods
known in the art. A benzamide is reacted with oxalyl chloride in a chlorinated
solvent such as dichloroethane or an equivalent to provide an isocyanate. The
isocyanate is reacted with a substituted primary amine to give a benzoyl
substituted urea. This intermediate can be cyclized to form a quinazolinedione
ring system by reaction with sodium hydride, potassium hexamethyldisilazide or
other non-nucleophilic bases in tetrahydrofuran/dimethylformamide,
tetrahydrofuran with 18-crown-6, toluene/dioxane, tetrahydrofuran/glyme,
tetrahydrofuran/diglyme, glymeltoluene or an equivalent. The resulting
3-aminoquinazolinedione ring system can then be readily coupled with a
stannane
or boronic acid derivative of a substituted aryl such as phenyl or substituted
aromatic heterocycle (Ar).
Alternatively, the 3-position can be protected with a protecting group such
as a tert-butyl carbamate, trifluoroacetamide, or 2,5-dimethoxybenzyl group.
Protecting groups of these types are well known in the art. The 3-protected
quinazolinedione ring system can then be coupled via palladium catalysis with
an
aromatic (Ar) stannane or boronic acid.
Each of the outlined routes, upon deprotection by standard procedures,
provides invention compounds of Formula I where RS is aryl such as phenyl or
substituted phenyl, or heteroaryl such as pyridyl or substituted pyridyl.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-157-
Scheme 31
R3 O R3 O R3 O O
Ra ~ ~ OH "amide formation" Ra ~ ~ NH2 oxalyl chloride Ra I w N-C
Br ~ F Br ~ F Br ~ F
Rs Rs Rs
R~NHZ
R3 O R3 O O
Ra I ~ N(Pro) Protection Ra I ~ N~N.H
Br . ~ N~O ~ cyclization Br ~ F H Ri
Pd coupling Rs R' Rs
R3 O R3 O
"Protection" Ra ~ N(Pro) Ra ~ NH
APro R R O "Deprotection" Ar R R O
s i s
H
R3 O R3 O "Deprotection"
Ra ~ N.Pro' Ra ~ N.Pro'
Pd coupling
Br N O A~ N O
Rs Ry Pro Rs R~
Compounds of Formula I wherein RS is a heteroaryl group are
alternatively prepared as illustrated in Scheme 32, where RS is a substituted
thiazole. A 3-protected 7-bromoquinazolinedione is reacted with 1-tributyl-
ethoxyvinyltin in the presence of a palladium catalyst. The resulting
RS-substituted adduct is reacted with a brominating reagent such as n-
bromosuccinimide and the like in tetrahydrofuran/H20 to provide an
a-bromoketone moety as R5. Reaction of this intermediate with a thioamide (or
thiourea) in a polar solvent such as dimethyl formamide, dimethylacetamide, or
ethanol, and at an elevated temperature of about 80°C to 120°C,
effects
cyclization to form a thiazolyl group. Deprotection by known methods can then
be
applied to provide invention compounds of Formula I wherein RS is the
heteroaryl, optionally substituted with T, which is H, alkyl, substituted
alkyl,
NH2, NH-alkyl and N-dialkyl.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-158-
Scheme 32
R3 O SnBus Rs p
R4 \ ~H(Pro) ~ R4 ~H(Pro)
~N
'~N Et0 \ n-bromosuccinamide
N~O Pd cata y Et0
N O tetrahydrofuran
Rs R1 Rs R1 H20
R~ = Br, I, or OTf
O SII R3 O
Ra ~ N~H(Pro) T~NH R4 ~ ~H(Pro)
S ~ ~ N~O "polar solvent" Br ~ N O
~N ~ ~ heat p
T Rs R1 Rs R1
°deprotection" ~ Rs
O
N~H
N~O
Rs R1
T= H, alkyl, substituted alkyl, NH2, NH-alkyl and N?dialkyl.
Alternatively, RS-aromatic or heterocyclic aromatic compounds of
Formula I are prepared as shown in Scheme 33. A 4-bromo-2-fluorobenzoic acid
first undergoes esterification (Z is alkyl or benzyl), and then the ester is
reacted
with a substituted primary amine (R1NH2) in dimethylsulfoxide (DMSO) at
elevated temperature of about 100°C to provide an anthranilic ester.
The amine
moiety of the antranilic ester is then reacted with an appropriately protected
source of NH2 to provide the corresponding amide. The resulting amido aniline
is
then cyclized by reaction with phosgene, CDI, or the like, to generate the
quinazolinedione. The cyclization typically is carried out in a solvent, such
as
ethereal solvents, chlorinated hydrocarbons such as chloroform, or aromatic
hydrocarbons such as toluene, and in the presence of a base such as
triethylamine
or NaHC03. The quinazolinedione ring system is then further modified as
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-159-
described in earlier schemes to provide the desired R5-aryl (Ar) compound of
Formula I.
Scheme 33
R3 O R3 O R3 O
R4 ~ ~ OH R4 ~ OZ R~NH2 R4 ~ OZ
HOZ
Br ~ 'F ~ gr ~ ~F DMSO Br ~ ~NH
Rs "esterification° Rs heat R R
s 1
H2N-H(Pro)
R3 O R3 O R3 O
R4 ~ .H R4 ~H(Pro) Ra ~H(Pro)
w ~ ~~ph~.. ( ~ ~N
H
Ar ~ N ~O Br ~ N ~O Br ~ NH
Rs R1 Rs R1 Rs R1
As noted above, RS in Formula I is referred to as the "side chain" of the
quinazolinedione nucleus. The RS side chains are any of those typically found
on
the quinolone antibiotics. Most of the RS side chain reactants required to
make
the Formula I compounds are readily available from commercial sources. Typical
RS side chains are shown in Table 1 above.
The synthesis of the RS side chains can be accomplished by standard
synthetic methods, for example as shown in the following schemes or as found
in
the literature. Those of ordinary skill in the art will be able to make any of
the
starting materials required to prepare the invention compounds, although most
are
available from commercial sources. Some of the sidechains were prepared as
disclosed in WO 01!53273 or as otherwise provided in the following schemes.
Scheme A1 generally illustrates the synthesis of typical pyrrolidines,
which are preferred side chains (RS) for invention compounds of Formula I. In
Scheme A1, appropriately activated enones can undergo [3+2]cycloadditions
under the conditions described by Tsuge et al. (Recent advances in azomethine
ylid chemistry. In: Advances in Heterocyclic Chemistry [Katritsky A., ed.]
San Diego: Academic Press, 231-349). These reactions are carried out in a
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-160-
chlorinated hydrocarbon solvent such as dichloromethane, chloroform, or
dichloroethane and the like, and in the presence of a catalytic acid such as
trifluoacetic acid, to provide substituted pyrrolidines (wherein SI, S2, S3,
and S4
independently are alkyl, substituted alkyl, aryl, amino, alkyl and
dialkylamino).
These intermediates can then be treated with hydroxylamine or any O-alkylated
or
arylated hydroxylamine under a variety of conditions known to those skilled in
the
art to provide oximated substituted pyrrolidines. The oximes are reduced to
amines with lithium aluminum hydride, diisobutylaluminum hydride, borane, or
by selective catalytic hydrogenation. The resulting primary amines can then be
protected using a number of methods as described in "Protecting Groups in
Organic Synthesis" by Theodore Green (supra). The benzylic pyrrolidine can
then
be deprotected by hydrogenation, and the resulting pyrrolidine used in the
preparation of 7-cyclic amino substituted 3-aminoquinazolinediones of Formula
I
as shown in the schemes above.
I S Scheme A1
Ph 1 ~ H~~' N
SZ I S
S' S2 MeO~N~TMS S~ ~Ph hydroxylamine . S~ 2 Ph
N 'N~
TFA, chlorinated
S4 S3 hydrocarbon S4 S3 ' S4 S3
reduction
Pro~NH
S2 Pro~N S NH~
2 2
S~ NH Hydrogenation S~ ~Ph . Amine S~ Ph
N N-~
S4 S3 S4 S Protection Sa~
3 3
In Scheme A2, enoates (or vinylogous nitriles) may also be used in
[3+2]cycloadditions to provide 3- and 4-substituted pyrrolidines. The ester
(or
nitrite) functionality can then be treated directly with alkyl lithium, -
aluminum, or
reagents at low temperature (0°C) to provide S I substituted carbonyl
groups, or
the ester (Z is alkyl or benzyl) can be hydrolyzed under conditions usually
employed by those skilled in the art to provide carboxylic acids. This
intermediate
can then be transformed into an acid chloride with oxalyl chloride and
catalytic
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-161-
dimethylformamide in solvents such as dichloromethane or chloroform or into an
activated amide (Singh J., Satyamurthi N., Aidhen L, Singh J., Prakt. Chern.
[Weinheim, Ger.], 2000;342(4):340-347). An acid chloride can be converted into
a ketone using organocopper reagents (Lipshutz B.H., Sengupta S., Org. React.
[N.Y.], 1992;41:135-631), and Weinreb amides can be converted into ketones
according to the methods described by Weinreb (Tetrahedron Lett.,
1981;22(39):3815-3818). The resulting ketones can then be converted into
pyrrolidines (optionally substituted with S2, S3, and S4) as described in
Scheme Al. As noted above, pyrrolidines and substituted pyrrolidines are
preferred R5 groups in Formula I.
Scheme A~
O Ph1 O S O
ZO S2 MeO~N~TMS ZO 2 N JPh Hydro ysis HO SZ N~Ph
TFA, chlorinated
S4 S3 hydrocarbon S4 Ss S4 Ss
O hydrogenation
S2 Si Li, acid chloride
ZO ~NH diethyl or
g //I~~, ether, Weinreb amide
formation
S3 _780
Pro
NH O O
S2 S2 Organocopper S2
S1 ~ Scheme A1 g~ N~Ph Reagent ?( N~Ph
NH .--
or
S4 Sa S4 S3 Organo Grignard S4 S3
Reagent
X = CI or -N(Me)OMe
In Scheme A3, 3-carboxylic acid N-benzyl substituted pyrrolidines
(Scheme A2) can be converted into amides by a number of methods well-known
to those who practice the art of organic synthesis. The amides can then be
treated
with lithium aluminum hydride in an ethereal solvent such as diethyl ether or
tetrahydrofuran or the like to provide amines that can be protected as
described in
Scheme Al . Hydrogenation with palladium catalysis provides an appropriately
substituted pyrrolidine (S2, S3, S4 are independently alkyl, lower alkyl,
aryl, etc.).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-162-
Scheme A3
O O Lithium
S2 amide S2 Aluminum S2
HO N~Ph formation H2N N~Ph Hydride H2N~~~N~Ph
Sa ~ S4 ethereal
S3 s3 SOIVent 4 S3
Amine
Protection
S2 S2 Ph
ProHN'~~NH Hydrogenation ProHN'~~N~
S4//I[[53~~' S4 Is~'.3
In Scheme A4, a 3-carboxypyrrolidinone [Culbertson T.P.,
Domagala J.M., Nichols J.B., Priebe S., Skeean R.W., J. Med. Chem.,
1987;30(10):1711-1715] can be converted into an acid chloride or Weinreb amide
in the same fashion as that defined in Scheme A3. The acid chlorides are
reacted
with an organocopper reagent or an organo Grignard reagent (for a Weinreb
amide) to provide a ketone that can be manipulated as described in Scheme A1
to
provide an appropriately substituted pyrrolidine with a variety of
substitutions at
S1 (alkyl, lower alkyl, substituted alkyl, aryl). The use of S-methylbenzyl
(or
R-methylbenzyl) as a protecting group for the pyrrolidine nitrogen allows for
the
separation of enantiomers and diastereomers at any step in the reaction
sequence.
Scheme A4
0 0
HO ph acid chloride ph Organocopper O
~N--~ °r. X N-/ Rep Ph
Weinreb amide ~~ or SI N-/
O formaUOn O Organo Grignard
Reagent O
X = Cl or -N(Me)OMe
hydroxylamine
Pro NH2 HO
NH Scheme A 1 lithium aluminum 'N
-S Ph hydride 1
S 1 ~NH 1 N ~ ~E S Ph
or 1 N
borane
O
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-163-
In a particular variant of this approach as provided in Scheme A4.1, the
ketone is formed from the Weinreb amide starting compound. Oxime formation
and reduction provides the primary amine, and removal of the protecting group
provides the target compound
Scheme AI4.1
~Ph
O OMe N O N~ ,O
Me n'BuLi, C ~~ ~ ~ Nv N
O O PhCH20NHz' HCI
N O N
I THF ',,~ pyridine, reflux O N
N3C'~~~~Ph N C Ph I
H3C''~~~ Ph
HzN N HzN N.
BH3~THF
~--O ammonium formate
i
N PdIC, MeOH N
H reflux H3C"'~~ph
In Scheme A5, the protected dihydropyrrole undergoes [3+2]
cycloaddition with the imine oxide generated ire situ from hydroxylbenzyl
amine
to provide the bicyclic intermediate. Removal of the benzyloxycarbonyl group
and cleavage of the N-O bond can be effected under hydrogenation conditions to
provide the target sidechain.
Scheme AS
'Ph
(N
F3CCH(OEt)OH O' ~,'CF3 H2, Pd/C, MeOH HO F3C NH2
h PhCH2NHOH ' HCI N
Et3N ~ N
O O~Ph H
A [3+2] cycloaddition approach is employed as the first step in an
approach to a [3.3.0] heterocyclic sidechain as depicted in Scheme A6 starting
from the N-benzyl protected dihydropyrrole. Cleavage of the N-O bond of the
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-164-
cycloaddition product using lithium aluminum hydride gives the shown
pyrrolidinol derivative. Boc protection of the primary amine, followed by
removal of the THP protecting group, gives the shown pyrrolidinyl diol.
Conversion of the primary alcohol to a leaving group using DAST and
intermolecular cyclization gives the fused bicyclic compound. The benzyl
protecting group is then removed.
Scheme A6
HZN
n O~N HO
~O~NOZ OTHP ~IAIH4, Et20 OTHP
N
Et3N, N N
benzene
PhNCO, y
reflux i
BoczO
BocHN BocHN
O HO HO~OTHP
NHBoc H ~(~
DAST, ~~OH PPTS, EtO /
CH2C12 /
N ' '
~ N N
ammonium formate
Pd/C
MeOH, 70 C
O NHBoc
N
H
Scheme A7 provides an approach to the synthesis of a sidechain
incorporating a thiophene core. Treatment of the thiophene starting compound
with bromine in the presence of sodium acetate provides the bromide. The keto
moiety is then converted to the benzyl oxime, which is reduced to the primary
amine using borane. A chloromethyl group is then attached to the 2-position
using HCI in the presence of formaldehyde. Protection of the primary amine,
with
concomittant cyclization via displacement of the chloride provides the
bicyclic
compound. Stannylation using butyl lithium, followed by quenching with tri-n-
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-165-
butyltin chloride provides the target compound, ready for coupling to the
quinazolinedione core.
Scheme A7
Ph
O
O CH3 NaOAc, Br2 CH3 phCH20NH2' HCI NO
Br ~ ' ----~ CH3
acetic acid g MeOH, reflux
S gr ~ \ BH3'THF
S
50 °C
CH3 HzN HZN
N-Soc Boc20, Et3N CH3 HCI, (CHzO)° CH3
CI Br ~
Br~ Br~
S S S
1 ) n-BuLi
2) Bu3SnCl
CH3
N~Boc
Bu3Sn S
In Scheme A8, 2-thiophenacetonitrile is treated with 1,2 dibromoethane
in the presence of base to provide the cyclopropanecarbonitrile.
Saponification of
the nitrile group with sodium hydroxide provides the cyclopropane carboxylic
IO acid compound. The acid is then converted to the protected amine. Formation
of
the stannane can be effected using n-butyl lithium and tri-n-butyl stannyl
chloride.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-166-
Scheme A8
0
/ \ CN PhCH2Et3NBr / \ CN NaOH, EtOH // \
---~- yOH
S CH2CIZ, NaOH S retlux S
BrCH2CH2Br
DPPA, Et3N
L BuOH, reflux
/ \ NHBoc 1) ~BuLi / \ NHBoc
Bu3Sn S
2) Bu3SnCl S
In Scheme A9, the oxime in the fused thiophene starting compound is
reduced with borane. The resulting primary amine is then protected, and the
resulting protected compound is then converted to the stannane as provided in
the
previous scheme.
Scheme A9
,OH
N NHZ HN~Pro HN-Pro
BH3'THF Ph3CCl,1) n-BuLi
~ Et3N
n reflux ~ 1 ~n CH~ / ' ~n Bu Sn
S S S 2) BuaSnCl s S n
or8oc20,THF
Et3N
1 ~ n= 1, 2, 3 Et20
In Scheme AIO, the fused thiophen-piperidinyl amine is protected as
provided in earlier schemes.
Scheme A10
/ \ NH PhsCBr, Et3N / \ N Tr 1 ) ~-BuLi ~ \ N Tr
S ~ CH3 CH2CI2 S CH3 2) Bu3SnCl BuaSn S CH3
In Scheme A11, the carboxylic acid moiety of the pyrrolidinyl starting
compound is converted to a Weinreb amide, which undergoes reaction with
cyclopropyl lithium to provide the corresponding ketone. The ketone is
converted
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-167-
to a primary amino group via formation of the oxime, and then hydrogenation in
the presence of Raney Nickel to provide the target compound.
Scheme All
0 0
O Me(Me0)NH ' HCI MeO~N~ O n-BuLi, D-Br
HO
N ' Et N, EDAC ' HCI Me N ' THF, -78 °C to rt
O 3 O
/ \ CHzCIz / \
O
N.OH
I ,,O
NHz H Rane Ni O HONH ' HCI ~~~ -!~N
z, Y ~~~N , z O
'~~NH '
MeOH O pyridine, 90 °C / \
In Scheme A12, the starting compound undergoes reaction with N,N
dibenzylacrylamide to provide the shown pyrrolidinyl ketone. Cyclopropanation
of the ketone moiety provides the target compound after removal of the
protecting
groups (Angew. Chemie Int. Ed. Eng. 1996, 35, 413).
Scheme A12
Ph'
O Ph1
OMe ~/O ph N /N
+ N,-/ TFA, CHZCIz ph EtMgBr, Ti(Oi-Pr)4
N~SiMe3 ( N ~ Ph
H C ph \ CH3 N
U ~ ~ ~G"3
0
Hz, Pd/C N"z
acetic acid
H
Scheme A12, provides a synthesis for an additional pyrrolidinyl sidechain
using techniques known to those skilled in the art. In one approach, the acid
moiety in the starting compound is treated with isobutyl chloroformate in the
presence of base to form the mixed anhydride. Conversion of the mixed
anhydride to the diazomethyl compound using 1-methyl-3-vitro-1-
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-16~-
nitrosoguanidine and KOH, followed by treatment with HBr/HOAc, gives rise to
oc-bromomethyl ketone. The alpha-bromomethyl ketone is readily converted to
the fluoromethyl ketone using a fluorine source such as KF. Reductive
amination
of using benzylamine and a reducing agent such as sodium triacetoxyborohydride
provides the fluoroethylaminopyrrolidinone derivative. Reduction of the amide
moiety in the pyrrolidinone, followed by hydrogenolysis to remove the benzyl
moieties provided the target compound.
Scheme A12
HO Br F
O 1) 4-methylmorpholine
isobutyl chloroformate O O
THF/dioxane, -10 °C KF, 18-crown-6
N
O 2) diazomethane O N CH CN 80 °C O N
1:1 HBrIAcOH
PhCH2NH2
NaB(OAc)3H
CICH2CH2CI
0°C tort
F F
F H
H2, Pd/C l-iAIH4, THF ' /~-N
NH2 H2S04
M ~ ' 0 °c to rt
O N
N
H
In Scheme A13, the Weinreb amide is prepared from the corresponding
carboxylic acid starting compound as provided earlier. Formation of the phenyl
ketone from the Weinreb amide is effected via addition of fluorophenyl
lithium.
Oxime formation, followed by reduction, provides the primary amine. Lithium
aluminum hydride reduction of the amide moiety is followed by protection of
the
primary amine. The pyrrolidinyl nitrogen is then deprotected as provided in
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-169-
Scheme A4 and then is reprotected. A series of purifications and deprotection
procedures provides the target compound.
Scheme A13
0
O Br
HO N CHa Me(Me0)NH ' HCI MeO~N CH3 I i
vN F
O / ~ Et3N, EDAC ' HCI Me
CH2CI2 O / ~ rrBu°Li, THF
-7$ C to rt
F O
F N.OH
\ I CH HONH2 ' HCI ~ % N CH3
3
N pyridine, 90 °C O /
HZ, Raney Ni O /
MeOH/THF
F NHZ F
1) LiAIH4, THF, NHBoc
N CH3 refiux I \ CH3 PhvO CI
-- N
O / \ 2j Bac20, CHZCIZ
CHZCi2
0°C tort
F F NHBoc
NH2 ~ ) HCI(g), CH2CI2 1 \ . H2, Pd/C
NH 2) basic Amberlite ~~~%~NH MeOH
resin/MeOH
In Scheme A14, the starting ester is reduced to the primary alcohol using
sodium borohydride in the presence of lithium chloride. Mesylation of the
alcohol, followed by treatment with tetrabutyl ammonium fluoride, provides the
l0 primary fluoro compound. Alkylation of the lactone moiety using lithium
diisopropyl amide and chloromethyl benzyl ether provides the
fluoromethylbenzyl
ether as a mixture of diastereomers. The diastereomers are separated, and the
benzylether protecting group is selectively removed to afford the primary
alcohol.
The lactone is then reduced with LAH. Mesylation of the primary alcohol,
I S followed by azide displacement and reduction provides the target compound.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-170-
Scheme A14
0 0 0
CH3 LiCI, NaBH4 CH3 CH3S02CI, Et3N N CH3
N
Me0 N THF EtOH HO~ CH2CI2 Ms0
s\
0
BuyNFxH20
THF, reflux
A /O, O
HO~ CHa Hz, Pd/C O ~ CH3
I~.N ~ ~ O~ CH3 LDA, PhCH20CH2Cl [ 'N
F~~ MeOH i N . FF
F~~ ~ \ THF, -78 °C to rt ~ ~ \
i
LiAIH4
THF, reflux
HO CHs MsO~ CHs NaN3, DMF N3 CH3
~N ' CH3SOpCl, Et3N N ~N
F~~ ~ \ CH2CIp Fw~ ~ \ 9~ Fwo / \
Hp, PdlC
THF
HZN
~NH
F~
It should be recognized from all of the above schemes that substitutions on
ring systems such as a pyrrolidine, piperazine, or piperidine ring will form
chiral
centers giving R and S enantiomers and diastereomers. Such enantiomers or
diastereomers may be separated, if desired, by chiral HPLC at any stage.
Resolution of any of the intermediates may be performed with techniques of
fractional crystallization using mandelic acid, tartaric acid, or other
chiral,
optically pure acid resolving agents. Chiral benzylic amines can be used in
the
preparation of starting materials for the above schemes, and chiral amides may
also be prepared using chiral acids, such as mandelic acid and the like. The
isomers can then be separated and the chiral amine can be hydrogenated, or the
chiral amide can be hydrolyzed.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-171-
Some of the compounds of Formula I are capable of further forming
pharmaceutically acceptable acid-addition and/or base salts. All of these
forms are
within the scope of the present invention.
Pharmaceutically acceptable acid addition salts of the compounds of
Formula I include salts derived from nontoxic inorganic acids such as
hydrochloric, nitric, phosphoric, sulfuric, hydrobromic, hydroiodic,
hydrofluoric,
phosphorous, and the like, as well as the salts derived from nontoxic organic
acids, such as aliphatic mono- and dicarboxylic acids, phenyl-substituted
alkanoic
acids, hydroxy alkanoic acids, alkanedioic acids, aromatic acids, aliphatic
and
aromatic sulfonic acids, etc. Such salts thus include sulfate, pyrosulfate,
bisulfate,
sulfite, bisulfite, nitrate, phosphate, monohydrogenphosphate,
dihydrogenphosphate, metaphosphate, pyrophosphate, acetate, trifluoroacetate,
propionate, caprylate, isobutyrate, oxalate, malonate, succinate suberate,
sebacate,
fumarate, maleate, mandelate, benzoate, chlorobenzoate, methylbenzoate,
dinitrobenzoate, phthalate, benzensoulfonate, toluenesulfonate, phenylacetate,
citrate, lactate, maleate, tartrate, methanesulfonate, and the like. Also
contemplated are salts of amino acids such as arginate and the like and
gluconate,
galacturonate (see, for example, Berge S.M. et al., "Pharmaceutical Salts,"
Journal of Pharmaceutical Science, 1977;66:1-19).
The acid addition salt of said basic compounds are prepared by contacting
the free base form with a sufficient amount of the desired acid to produce the
salt
in the conventional manner.
Pharmaceutically acceptable base addition salts are formed with metals or
amines, such as alkali and alkaline earth metals or organic amines. Examples
of
metals used as cations are sodium, potassium, magnesium, calcium, and the
like.
Examples of suitable amines are N,N'-dibenzylethylenediamine, chloroprocaine,
choline, diethanolamine, dicyclohexylamine, ethylenediamine,
N-methylglucamine, and procaine (see, for example, Berge S.M., supra., 1977).
The base addition salts of said acidic compounds are prepared by
contacting the free acid form with a sufficient amount of the desired base to
produce the salt in the conventional manner.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-172-
Certain of the compounds of the present invention can exist in unsolvated
forms as well as solvated forms, including hydrated forms. In general, the
solvated
forms, including hydrated forms, are equivalent to unsolvated forms and are
intended to be encompassed within the scope of the present invention.
Certain of the compounds of the present invention possess one or more
chiral centers and each center may exist in the R(D) or S(L) configuration.
The
present invention includes all enantiomeric and epimeric forms, as well as the
appropriate mixtures thereof.
The compounds of the present invention can be prepared and administered
in a wide variety of oral and parenteral dosage forms. Thus, the compounds of
the
present invention can be administered by injection, that is, intravenously,
intramuscularly, intracutaneously, subcutaneously, intraduodenally, or
intraperitoneally. Also, the compounds of the present invention can be
administered by inhalation, for example, intranasally. Additionally, the
compounds of the present invention can be administered transdermally. It will
be
obvious to those skilled in the art that the following dosage forms may
comprise
as the active component, either a compound of Formula I or a corresponding
pharmaceutically acceptable salt of a compound of Formula I. The formulations
typically will comprise from about 1 to about 95 percent by weight of the
active
invention compound.
For preparing pharmaceutical compositions from the compounds of the
present invention, pharmaceutically acceptable carriers can be either solid or
liquid. Solid form preparations include powders, tablets, pills, capsules,
cachets,
suppositories, and dispersible granules. A solid carrier can be one or more
substances which may also act as diluents, flavoring agents, binders,
preservatives, tablet disintegrating agents, or an encapsulating material.
In powders, the carrier is a finely divided solid which is in a mixture with
the finely divided active component.
In tablets, the active component is mixed with the carrier having the
necessary binding properties in suitable proportions and compacted in the
shape
and size desired.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-173-
The powders and tablets preferably contain from five or ten to about
seventy percent of the active compound. Suitable carriers are magnesium
carbonate, magnesium stearate, talc, sugar, lactose, pectin, dextrin, starch,
gelatin,
tragacanth, methylcellulose, sodium carboxymethylcellulose, a low melting wax,
cocoa butter, and the like. The term "preparation" is intended to include the
formulation of the active compound with encapsulating material as a Garner
providing a capsule in which the active component with or without other
carriers,
is surrounded by a carrier, which is thus in association with it. Similarly,
cachets
and lozenges are included. Tablets, powders, capsules, pills, cachets, and
lozenges
can be used as solid dosage forms suitable for oral administration.
For preparing suppositories, a low melting wax, such as a mixture of fatty
acid glycerides or cocoa butter, is first melted and the active component is
dispersed homogeneously therein, as by stirring. The molten homogenous mixture
is then poured into convenient sized molds, allowed to cool, and thereby to
solidify.
Liquid form preparations include solutions, suspensions, and emulsions,
for example, water or water propylene glycol solutions. For parenteral
injection
liquid preparations can be formulated in solution in aqueous polyethylene
glycol
solution.
Aqueous solutions suitable for oral use can be prepared by dissolving the
active component in water and adding suitable colorants, flavors, stabilizing
and
thickening agents as desired.
Aqueous suspensions suitable for oral use can be made by dispersing the
finely divided active component in water with viscous material, such as
natural or,
synthetic gums, resins, methylcellulose, sodium carboxymethylcellulose, and
other well-known suspending agents.
Also included are solid form preparations which are intended to be
converted, shortly before use, to liquid form preparations for oral
administration.
Such liquid forms include solutions, suspensions, and emulsions. These
preparations may contain, in addition to the active component, colorants,
flavors,
stabilizers, buffers, artificial and natural sweeteners, dispersants,
thickeners,
solubilizing agents, and the like.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-174-
The pharmaceutical preparation is preferably in unit dosage form. In such
form the preparation is divided into unit doses containing appropriate
quantities of
the active component. The unit dosage form can be a packaged preparation, the
package containing discrete quantities of preparation, such as packeted
tablets,
capsules, and powders in vials or ampoules. Also, the unit dosage form can be
a
capsule, tablet, cachet, or lozenge itself, or it can be the appropriate
number of any
of these in packaged form.
The quantity of active component in a unit dose preparation may be varied
or adjusted from 0.1 mg to 100 mg preferably 0.5 mg to 100 mg according to the
particular application and the potency of the active component as determined
by a
skilled physician. The composition can, if desired, also contain other
compatible
therapeutic agents.
In therapeutic use as agents for the treatment of infections caused by a
bacteria, the compounds utilized in the pharmaceutical method of this
invention
are administered at the initial dosage of about 0.01 mg to about 500 mg/kg
daily.
A daily dose range of about 0.01 mg to about 100 mg/kg is preferred. The
dosages, however, may be varied depending upon the requirements of the
patient,
the severity of the condition being treated, the compound being employed.
Determination of the proper dosage for a particular situation is within the
skill of
the art. Generally, treatment is initiated with smaller dosages which are less
than
the optimum dose of the compound. Thereafter, the dosage is increased by small
increments until the optimum effect under the circumstances is reached. For
convenience, the total daily dosage may be divided and administered in
portions
during the day, if desired.
Certain of the compounds of the present invention possess one or more
chiral centers and each center may exist in the R or S configuration. The
present
invention includes all diastereomeric, enantiomeric, and epimeric forms as
well as
the appropriate mixtures thereof. Additionally, the compounds of the present
invention may exist as geometric isomers. The present invention includes all
cis,
trans, syn, anti, entgegen (E), and zusammen (Z) isomers as well as the
appropriate mixtures thereof.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-175-
The ability of a compound of the invention to inhibit bacterial growth,
demonstrate in vivo activity, and enhanced pharmacokinetics are demonstrated
using pharmacological models that are well known to the art, for example,
using
models such as the tests described below.
Test A--Antibacterial Assay
The compounds of the present invention were tested against an assortment
of Gram- negative and Gram-positive organisms using standard microtitration
techniques (Cohen, et al., Antimicrob. Agents Chernother., 1985;28:766;
Heifetz,
et al., Antimicrob. Agents Chemother., 1974;6:124). The results of the
evaluation
are shown in Table 3 and are compared to ciprofloxacin.
Table 3
Antibacterial and E. coli gyrase Activities
Compound Minimum E.
Inhibitory coli
Concentrations
~.g/mL
Number or Gram Gram gyrase
Negatives Positives
Structure E. coliE. E. coli E. S. S. ICso
MC coli Tol faecalisaureus (u,M)
C pyogenes
4100 B90 RB I 29213 C203
2 8.0 0.5 0.25 0.25 0.5 0.03 2.1
3 32.0 2.0 0.5 2.0 0.13 7.0
4b 4.0 0.13 0.13 0.13 0.25 0.015 0.6
7j 8.0 0.25 0.06 0.25 0.13 0.03 0.8
19f 4.0 0.25 0.13 0.06 O.I3 0.06 I.8
22j 8.0 1.0 I.0 2.0 1.0 0.25 1.4
26b 2.0 0.25 0.13 0.13 0.25 0.03 1.6
23b 16.0 0.13 <0.06 1.0 0.5 2.0 1.0
16d >64.0 16.0 4.0 1.0 I.0 0.5 25.0
41c ~ 2.0 0.25 0.06 0.03 0.03 0.008 0.6
Ciprofloxacin<0.02 <0.01 <0.010.5 0.5 0.5 0.2
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-176-
Test B-DNA gyrase assay
The effects of test agents on the activity of DNA gyrase was determined by
the supercoiling inhibition assay, following reaction conditions recommended
by
the enzyme supplier (Lucent, Ltd., Leicester, UK), as follows: Reactions are
performed in buffer G (35 mM Tris-HCI (pH 7.5), 24 mM KCI, 4 mM MgCl2,
2 mM DTT, 1.8 mM spermidine, 1 mM ATP, 0. I mg/mL bovine serum albumin).
Relaxed plasmid pBR322 (0.25 p.g, Lucent, Ltd., Leicester, UK) is reacted with
I U E. coli gyrase (Lucent, Ltd., Leicester, UK), in the absence or presence
of
drugs, for 30 minutes at 37°C. Reactions were.stopped by the addition
of SDS and
proteinase K to respective final concentrations of 1 % and 0.5 mglmL. After an
additional 30 minutes at 37°C, one-tenth volume of lOX loading buffer
(0:3%
bromophenol blue, 16% Ficoll, 10 mM Na2HP04) was added, and reactions were
loaded onto agarose gels and electrophoresed as described for intercalation
assays
(Y Pommier et al. Nucleic Acids Research 1987, 1 S, 6713-6731.). The
concentration of drug inhibiting 50% of the supercoiling activity of DNA
gyrase
was measured and is given as an IC50 in Table 3.
Test C-In Vivo Activity (Mouse)
The in vivo activity was obtained when the compounds were tested
according to the procedure of Miller, et a1. (Proc. Soc. Exp. Biol. Med.,
1944;57:261). The median protective dose (PD50) was determined in mice given
lethal systemic infections, as depicted in Table 4. Compounds 2 and 4b are
compared to ciprofloxacin.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-177-
Table 4
In Vivo Median Protective Dose (PDSp) in Mice (PO)
Compound Number or Organism PDSO (mg/kg)
Structure
2 S. pyogenes 10.8
4b S. pyogenes . 3.6
Ciprofloxacin S. pyogenes > 100.0
Test D-Cross Resistance Antibacterial Assay
The compounds of the present invention were tested against an assortment
of ciprofloxacin resistant E. coli and S. aureus organisms described below
using
standard microtitration techniques (Cohen, et aL, Antimicrob. Agents
Chemother.,
1985;28:766; Heifetz, et al., Antimicrob. Agents Chemother., 1974;6:124). The
results of the evaluation are shown in Table 5 compared to ciprofloxacin.
N. gonorrhoeae and S. aureus organisms:
N. gonorrhoeae 2637 (N.g. 2637) is a derivative of Neisseria gonorrhoeae MS 11
l0 containing a TAC-LAC recA to allow for control of homologous
recombination [Tonjum T. et al. Molecular Mierobiology 1995, 16, 451-
64].
N. gonorrhoeae 2709 (N.g. 2709): Isogenic to N. gonorrhoeae 2637 contains
gyrA quinolone- resistant determining region (QRDR) mutations (S91F
D95G).
N. gonorrhoeae 2693 (N.g. 2693): Isogenic to N. gonorrhoeae 2709 containing
par-C QRDR mutations [ P88S and E91 K].
S. aureus UC-76: Typical sensitive laboratory strain (Wild type).
S. aureus 2552: Isogenic to S. aureus UC-76, with upregulated norA pump.
S. aureus 2554: Isogenic to S. aureus 2552, with point mutation at position 80
of
grlA subunit.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-178-
S. aureus 2558: Isogenic to S. aureus 2554, with point mutation at position 84
of
gyrA subunit.
Table 5
Antibacterial Activities Against Ciprofloxacin Resistant Strains
Compound Minimum Inhibitory Concentrations ~,g/mL
Number or N. g. N. g. N. g. S. aureus S. aureus S. aureus S. aureus
Structure 2637 2709 2693 UC-76 2552 2554 2558
2 0.5 4.0 8.0 0.25 0.5 0.5 1.0
(8x) (16x) (2x) (2x) (4x)
4b 0.25 2.0 2.0 0.13 0.5 0.25 1.0
(8x) (8x) (4x) (2x) (8x)
7j 0.13 0.5 1.0 0.06 0.06 0.06 0.06
(4x) (8x) (Ix) (lx) (lx)
Ciprofloxacin0.002 0.06 2.0 0.13 2 2 64
(30x) (1000x) (15x) (15x) (123x)
Test E-Pharmacokinetic Behavior or Quinazolinediones vs. 3-
Aminoquinazolinediones
The compounds of the present invention were tested for pharmacokinetic
behavior against the structurally related 3-aminoquinazolinediones in rats,
dogs
and monkeys. The representative result of the evaluation for compound 4b is
shown in Table 6 compared to a structurally similar 3-aminoquinazolinedione.
Male Wistar Rats
In this study, compounds were administered to male Wistar rats and were
dosed at 1 mg/kgIV infusion in DSW over 5 minutes. Blood samples were drawn
at 0, 0.083, O.I67, 0.25, 0.5, l, 2, 4, 6, 8, 12, and 24 hours post dose.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-179-
Male Beagle Dogs
Male Beagle Dogs were dosed with compound dissolved in DSW at
Smg/kg IV Infusion over 15 minutes. Blood samples will be collected at 0,
0.167,
0.25, 0.33, 0.5, I, 2, 4, 6, 8, 12 and 24 hours post dose.
In Monkeys
Male Cynomologous Monkeys were dosed with compound dissolved in
DSW at Smg/kg IV Infusion over IS minutes. Blood samples were collected at 0,
0.167, 0.25, 0.33, 0.5, 1, 2, 4, 6, 8, 12 and 24 hours post dose.
Plasma from all samples were harvested following centrifugaltion and
stored frozen until plasma concentrations were determined using liquid
chromatographylmass spectroscopy methods. The concentrationltime profile for
each compound in each animal species was analyzed using non-compartmental
pharmacokinetic analysis approach. In the table, clearance is defined as the
volume of fluid cleared of drug from the body per unit of time. Clearance is a
quantitative assessment of drug elimination. Drug elimination is the
irreversible
removal of drug from the body by all routes of elimination.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-180-
Table 6: Comparative Pharmacokinetic Behavior of quinazolinediones and 3
Aminoquinazolinediones in Rats, Dogs and Monkeys
Pharmacokinetic Rat Dog Monkey
parameters
O
lf 4
lif
h
2
h
F ~ N.NH2 - 2. 2.2
ours)
a
e (
N N i i N.~O Clearance
62 21 24
H2N~ Me ~ (mUmin/leg)
O
F .H half-life (hours) ; 6.3 4.1
N 1.9
~N ~ NCO Clearance 47 8 7 17
H2N' ~J Me ~ (mUmin/kg) .
4b
The antibacterial agents described in this invention display Gram-negative
and Gram-positive activity. The compounds also show inhibition of bacterial
DNA
gyrase.
Finally, the compounds demonstrate in vivo protective activity in mice and
are not highly cytotoxic to mammalian cells indicating selectivity for
bacteria.
Representative examples of methods for preparing compounds of the
invention are set forth below.
Example 1
a) 2-Amino-4,5-dit7uoro- N-methoxybenzamide
4,5-Difluoroanthranilic acid (5.0 g, 20.0 mmol) and carbonyl diimidazole
(5.63 g, 32.0 mmol) were combined in 200 mL of dry tetrahydrofuran and heated
to reflux for 8 hours. O-methylhydroxylamine hydrochloride (2.42 g, 20.0 mmol)
and triethylamine (4.95 mL, 35.0 mmol) were added to the cooled mixture and it
was returned to reflux for 18 hours. The mixture was cooled and concentrated
to
give a solid. The solid was dissolved in chloroform and washed with 1 N
hydrochloric acid, saturated sodium bicarbonate and brine. The organic layer
was
dried over magnesium sulfate and concentrated to afford 3.96 g of a solid. The
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-181-
solid was purified by chromatography (SiO~, chloroform to Chloroformlmethanol
95/5) to afford 2.5 g of the title compound. 'H NMR (400 MHz, d6-DMSO) 8
11.45 (bs, 1 H), 7.36 (da, 1 H), 6.65 (dd, 1 H), 6.49 (bs, 2H), 3.65 (s, 3H),
MSCI:
mlz = 203 (MH+).
b) 6, 7-Difluoro-3-methoxy-1H-quinazoline-2, 4-dione
A 12.5% solution of phosgene in toluene (4.6 mL, 5.3 mmol) was added to
a solution of 2-amino-4,5-difluoro-N methoxybenzamide (1.07 g, 5.3 mmol,
Example 1 a) in 30 mL of dioxane. The solution was heated at reflux for 20
hours,
then poured into 100 mL of water. The aqueous mixture was extracted with ethyl
acetate and the combined organic layers were washed with water, brine and
dried
over magnesium sulfate. The solution was concentrated to give 1.16 g of the
title
compound. H' NMR (400 MHz , CDCI3) S I 1.50 (bs, I H), 7.80 (m, 1 H), 6.99 (m,
1H), 3.97 (s, 3H); MSCI: m/z = 229 (MH+).
c) 6,7-Difluoro-3-methoxy-1-methylcyclopropyl-1H-quinazolinedione
A solution of 6,7-difluoro-3-methoxy-1H-quinazolinedione (1.15 g, 5.0
mmol, Example 1 b) in N,N dimethylformamide (20 mL) was added to a
suspension of sodium hydride (0.24 g, 6.0 mmol) in N,N-dimethylformamide (15
mL) and stirred for 45 minutes. Bromomethylcyclopropane (0.73 mL, 7.6 mmol)
was added and the mixture stirred at 25 °C for l 8 hours. The reaction
was
quenched with water (1 mL) and concentrated to provide an oil that was
dissolved
in chloroform. The solution was washed with water, brine, dried over magnesium
sulfate and concentrated to a solid that was purified by flash silica gel
chromatography (chloroform then 97:3 chloroform:methanol) to afford the title
compound (0.86 g). ' HNMR (400 MHz, CDC13) 8 8.06 (t, 1 H), 7.15 (dd, 1 H),
4.05 (s, 3H), 4.01 (d, 2H), 1.23-1.15 (m, 1 H), 0.62-0.51 (m, 4H); MSCI: mlz =
283 (MH'~).
d) 3-(1-Cyclopropylmethyl-6-fluoro-3-methoxydioxo-1,2,3,4-tetrahydro-
quinazolin-7-yl)-3-azabicyclo(3.1.0]hex-6-yl]carbamic arid tent-butyl ester
(la, Sa, 6a)
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-182-
(3-Azabicyclo[3.1.0]hex-6-yl)carbamic acid tert-butyl ester(la, Sa, 6a)
(0.16 g, 7.8 mmol, [Eur. Pat. Appl. EP 413455 A2]) was added to a solution of
6,7-difluoro-I-methylcyclopropyl-3-methoxy-1H-quinazoline-2, 4-dione (0.15 g,
5.3 mmol, Example Ic) and triethylamine (0.12 mL, 0.9 mmol) in acetonitrile
(15
mL). The solution was heated at reflux for 17 hours, cooled, and concentrated
to a
solid. The solid was dissolved in chloroform, and the resulting solution was
washed successively with 1N hydrochloric acid, saturated sodium bicarbonate,
and brine. The solution was dried over magnesium sulfate and concentrated to
give a solid that was purified by flash silica gel chromatography (chloroform
then
97:3 chloroform:methanol) to afford the title compound (0.18 g):'H NMR (400
MHz, CDCl3) 8 7.43 (d, 1 H), 6.24 (d, 1 H), 4.77 (bs, 1 H), 4.05-3.83 (m, 7H),
3.68-
3.58 (m, 2H), 2.44 (s, 1H), 1.91 (s, 2H), 1.45 (s, 9H), 1.23-1.15 (m, IH),
0.62-0.51
(m, 4H); MSCI: m/z --.4.61 (MH+).
e) 3-(1-Cyclopropylmethyl-6-fluorodioxo-1,2,3,4-tetrahydroquinazolin-7-yl)-
3 azabicyclo[3.1.0]hex-6-yl]carbamic acid tent-butyl ester (la, Sa, 6a)
3-(1-Cyclopropylmethyl-6-fluoro-3-methoxydioxo-I,2,3,4-
tetrahydroquinazolin-7-yl)-3-azabicyclo[3.1.0]hex-6-yl]carbamic acid tert-
butyl
ester (1 a, Sa, 6a) (0.16 g, 0.34 mmol, Example 1d) in methanol (25 mL) was
treated with Raney nickel (1 g) and placed under 50 pounds per square inch
(psi)
of hydrogen for 96 hours at room temperature. The mixture was filtered and
concentrated to afford the title compound (0.l 1 g): 'H NMR (CDCl3) rj 7.93
(bs,
1 H), 7.66 (d, 1 H), 6.26 (d, 1 H), 4.78 (bs, l H), 4.01-3.90 (m, 4H), 3.72-
3.55 (m,
2H), 3.13-3.06 (m, 1 H), 2.55-2.40 (m, 1 H), l .45 (s, 9H), l .23-1.15 (m, 1
H), 0.62-
0.51 (m, 4H); MSCI: mlz = 431 (MH+).
f) 7-(6-amino-3-aza-bicyclo[3.1.0]hex-3-yl)-6-fluoro-3H-1-methylcyclopropyl-
1H-quinazoline-2, 4-dione (loc, Sa, 6a) hydrochloride
Hydrogen chloride gas was bubbled into a solution of 3-(1-
cyclopropylmethyl-6-fluorodioxo-1,2,3,4-tetrahydroquinazolin-7-yl)-3-
azabicyclo[3.1.0]hex-6-yl]carbamic acid tent-butyl ester (1 a, 5a, 6a) (0.05
g, l .l
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-183-
mmol, Example 1e) in dichloromethane (30 mL) at 0°C. The suspension was
stirred for 2 hours and filtered to afford the title compound (0.04 g); mp
>250 °C:
'H NMR (DMSO-d6) 8 11.30 (bs, 1 H), 8.37 (bs, 3H), 7.49 (d, 1 H), 6.41 (d,
2H),
3.96 (d, 2H), 3.85-3.77 (m, 2H), 3.60-3.55 (m, 2H), 2.13 (s, 2H), 1.25-1.15
(m,
I H), 0.50-0.46 (m, 2H), 0.45-0.35 (m, 2H); MSCI: m/z 331 (MH+)
Example 2
1-Cyclopropyl-6-fluoro-8-methyl-7-((R)-3-((S)-I-
methylaminoethyl)pyrrolidin-1-yl]-1H-quinazolinedione
IO I-Cyclopropyl-6,7-difluoro-8-methyl-IH-quinazolinedione (0.2 g, 0.79
mmol, [PCT Int. Appl. WO 0153273 AI]) and methyl-((R)-(S)-I-pyrrolidinyl-3-
ylethyl)amine (0.31 g, 2.4 mmol, [J. Het. Chem., 1992, 29,1481]) in dimethyl
sulfoxide (1 mL) was heated at 80 °C for 6 hours. The solution was
diluted with
water (4 mL) and saturated ammonium chloride ( 1.5 mL) and stirred for 2
hours.
I S The mixture was filtered and dried to afford the title compound (0.23 g);
mp >250
°C: 'H NMR (CDCl3) 8 7.33 (d, 1H), 3.60-3.50 (m, 1H), 3.45-3.20 (m,
4H), 3.16-
3.08 (m, 1H), 2.55-2.38 (m, SH), 2.35 (s, 3H), 2.60-2.00 (m, 1H), 1.72-1.64
(m,
IH), 1.21 (d, 3H), 1.05-0.95 (m, 2H), 0.58-0.42 (m, 2H); MSCI: mlz 361 (MH+).
20 Example 3
1-Cyclopropyl-6-fluoro-8-methoxy-7-[(R)-3-((S)-1-
methylaminoethyl)pyrrolidin-1-yl]-1H-quinazolinedione
Utilizing the same procedure as described in Example 2, from reaction of
1-cyclopropyl-6,7-difluoro-8-methoxy-1H-quinazolinedione [PCT Int. Appl. WO
25 0153273 A1 Al] and methyl-((R)-(S)-1-pyrrolidinyl-3-ylethyl)amine; mp 162-
164 °C:'H NMR (CDCl3) b7.23 (d, 1H), 3.73-3.61 (m, 1H), 3.58-3.24 (m,
8H),
3.17-3.10 (m, l H), 2.27 (s, 3H), 2.16-2.04 (m, 1 H), 2.00-1.92 (m, 1 H), l
.62-1.50
(m, 1 H), 1.03 (d, 3H), I .OS-0.95 (m, I H), 0.86-0.78 (m, 1 H), 0.60-0.52 (m,
1 H),
0.50-0.42 (m, 1 H); MSCI: m/z = 377 (MH+).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-184-
Example 4
a) {(S)-1-[(R)-1-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydro-
quinazolin-7-yl)pyrrolidin-3-yl]ethyl}carbamic acid tert-butyl ester
I-Cyclopropyl-6,7-difluoro-8-methyl-IH quinazolinedione (1.5 g, 6.0
mmol) and (R)-(S)-1-pyrrolidin-3-ylethyl)carbamic acid tert-butyl ester (1.78
g,
15.4 mmol, [J. Het. Chem. 1992, 29, 48I]) in dimethyl sulfoxide (5 mL) was
heated at 90 °C for 10 days. The solution was diluted with water (16
mL) and
saturated ammonium chloride (4 mL) and stirred for 2 hours. The solid was
collected by filtration, dried and purified by flash silica gel chromatography
IO (chloroform then 98:2 chloroformlmethanol) to afford the title compound
(0.71
g) as a white solid:'H NMR (CDCl3) 8 8.13 (bs, IH), 7.52 (d, IH), 4.48 (d,
1H),
3.80-3.70 (m, 1 H), 3.68-3.59 (m, 1 H), 3.51-3.43 (m, I H), 3.44-3.37 (m, 2H),
3.36-3.29 (m, 1 H), 2.39 (s, 3H), 2.32-2.23 (m, 1 H), 2.12-2.04 (m, 1 H), 1.78-
1.66
(m, 1 H), I .43 (s, 9H), I .21 (d, 3H), 1.23- I .17 (m, 1 H), 1.12-1.04 (m, 1
H), 0.64-
0.56 (m, 2H); MSCI: mlz 447 (MH+)
b) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride
Hydrogen chloride gas was bubbled into a solution of {(S)-1-[(R)-1-(1-
cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)pyrrolidin-
3-yl]ethyl }carbamic acid tert-butyl ester (0.69 g, I.5 mmol, Example 4a) in
dichloromethane (30 mL) at 0 °C. The suspension was stirred for 2 hours
and
filtered to afford the title compound (0_48 g); mp 200-202 °C: 'H NMR
(DMSO-
d6) 8 1 I .28 (s, 1 H), 7.84 (bs, 3H), 7.35 (d, l H), 3.60-3.50 (m, 1 H), 3.50-
3.20 (m,
4H) 2.40-2.22 (m, 4H), 2.10-2.02 (m, 1 H), 1.75-1.65 (m, 1 H), 1.24 (d, 3H),
1.05-
0.98 (m, 2H), 0.55-0.45 (m, 2H); MSCI: mlz 347 (MHO).
Example 5
1-Cyclopropyl-7-dimethylamino-6-fluoro-8-methyl-1H-
quinazolinedione
1-Cyclopropyl-6,7-difluoro-8-methyl-1H-quinazolinedione (1.5 g, 6.0
mmol) in N,N-dimethylformamide (5 mL) was heated at 90 °C for 10 days.
The
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-185-
solution was diluted with water (I6 mL) and saturated ammonium chloride (4
mL), and stirred for 2 hours. The solid was collected by filtration, dried,
and
purified by flash silica gel chromatography (chloroform then 98:2
chloroform/methanol) to afford the title compound (0.66 g); mp 238-239
°C; 1H
NMR (CDC13) 8 8.02 (bs, 1 H), 7.56 (d, 1 H), 3.36-3.3 I (m, 1 H), 2.96 (d,
6H), 2.47
(s, 3H), 1.16-1.10 (m, 2H), 0.64-0.60 (m, 2H); MSCI: m/z 278 (MH+)
Example 6
a) 4-((S)-3-tart-Butoxycarbonylaminopyrrolidin-1-yl)-3-chloro-2-(1-
cyclopropyl-ureido)-5-fluorobenzoic acid ethyl ester
To a solution of 4-((S)-3-tart-butoxycarbonylaminopyrrolidin-1-yl)-3-
chloro-2-cyclopropylamino-5-fluorobenzoic acid ethyl ester (0.36 g, 0.82 mmol,
[PCT Int. Appl. WO 0153273 A1]) in dichloromethane (10 mL), under a nitrogen
atmosphere, at 0 °C was added chlorosulfonyl isocyanate (0.14 mL, 1.63
mmol)
dropwise via syringe. After I.5 hours, the reaction mixture was diluted with
dichlromethane, washed with saturated sodium bicarbonate, water, and brine.
The
organic layer was dried over magnesium sulfate, filtered, and filtrate
concentrated
to afford 4-((S)-3-tart-butoxycarbonyl aminopyrrolidin-1-yl)-3-chloro-2-(I-
cyclopropylureido)-5-fluorobenzoic acid ethyl ester (0.323 g) as an oil. MSCI:
m/z
= 485 (MHO).
b) [(S)-1-(8-Chloro-1-cyclopropyl-6-tluorodioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)pyrrolidin-3-yl)carbamic acid tent-butyl ester
A solution of 4-((S)-3-tart-butoxycarbonylaminopyrrolidin-1-yl)-3-chloro-
2-(1-cyclopropylureido)-5-fluorobenzoic acid ethyl ester (0.323 g, 0.66 mmol,
Example 6a) in toluene ( 10 mL) was refluxed for 24 hours. The reaction
mixture
was concentrated and the resulting residue purified by flash silica gel
chromatography ( 1: I ethyl acetatelhexanes) to afford the title compound
(0.056 g)
as a white solid: MSCI: m1z 439 (MH+).
c) 7-((S)-3-Aminopyrrolidin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1H-
quinazolinedione triftuoroacetate
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-186-
To a solution of [(S)-1-(8-chloro-1-cyclopropyl-6-fluorodioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)pyrrolidin-3-yl]carbamic acid tert-butyl ester
(0.047 g,
0.11 mmol, Example 6b) in dichloromethane ( 1 mL) was added trifluoroacetic
acid (1 mL). After 30 minutes, the precipitate was collected by filtration,
washed
with hexanes, and dried to afford the title compound (0.031 g); mp I 17-I 18
°C:
MSCI: m1z 339 (MH+).
Example 7
a) 5-Oxo-1-((S)-1-phenylethyl)pyrrolidine-3-carboxylic acid
methoxymethylamide
To a solution of 5-oxo-1-((S)-1-phenylethyl)pyrrolidine-3-carboxylic acid
(20.4 g, 87.5 mmol, [J. Het. Chem. 1992, 29, 1481 ]) in dichloromethane (200
mL)
at 0 °C was added triethylamine (I8.3 mL, 131 mmol), N,O-
dimethylhydroxylamine hydrochloride (10.2 g, 105 mmol), and N-(3-
dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (20 g, 105 mmol).
The reaction mixture was slowly warmed to room temperature. After 20 hours,
the
organic solution was washed with saturated sodium bicarbonate, water, and
brine.
The organic layer was dried over magnesium sulfate and concentrated. The
resulting residue was purified by flash silica gel chromatography (5:95
isopropanol: dichloromethane) to afford the title compound (22.2 g) as a clear
oil:
MSCI: mlz 246 (MH+).
b) 4-[1-(2-Fluorophenyl)methanoyl]-1-((S)-1-phenylethyl)pyrrolidin-2-one
To a solution of l-bromo-2-fluorobenzene (7.68 g, 43.8 mmol) in
tetrahydrofuran ( 100 mL) under nitrogen atmosphere at -78 °C was added
n-
butyllithium (1.6 M in hexanes, 30.2 mL, 48.2 mmol) slowly over 30 minutes.
After 1 hour, 5-oxo-1-((S)-1-phenylethyl)pyrrolidine-3-carboxylic acid
methoxymethylamide (9.68 g, 35.1 mmol, Example 7a) was added via carrnula, as
a solution in tetrahydrofuran (25 mL). After 30 minutes, the reaction mixture
was
warmed to room temperature for 1 hour. The reaction mixture was diluted with
ethyl acetate and washed with saturated ammonium chloride, water and brine.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-187-
The organic layer was dried over magnesium sulfate and concentrated to afford
the title compound (9.70 g) as a clear brown oil: MSCI: mlz 312 (MHi~).
c) 4-[1-(2-Fluorophenyl)-1-hydroxyiminomethyl]-1-((S)-1-
phenylethyl)pyrrolidin-2-one
To a solution of 4-[1-(2-fluorophenyl)methanoyl]-1-((S)-1-
phenylethyl)pyrrolidin-2-one (8.86 g, 28.45 mmol, Example 7b) in pyridine (20
mL) was added hydroxylamine hydrochloride (2.57 g, 36.9 mmol). The reaction
was heated to 90 °C for 16 hours, and diluted with ethyl acetate. The
organic
layer was washed twice with 1N hydrochloric acid, water and brine. The organic
layer was dried over magnesium sulfate and concentrated. The resulting residue
was purified by flash silica gel chromatography (ethyl acetate) to give the
title
compound (7.35 g) as a brown oil: MSCI: rnlz 327 (MHO).
d) 4-[1-Amino-1-(2-fluorophenyl)methyl]-1-((S)-1-phenylethyl)pyrrolidin-2-
one
To a solution of 4-[1-(2-fluorophenyl)-1-hydroxyiminomethyl]-1-((S)-1-
phenylethyl)-pyrrolidin-2-one (7.35 g, 22.5 mmol, Example 7c) in methanol (50
mL) and tetrahydrofuran (50 mL) was added Raney nickel (5 g). Hydrogen was
introduced to the reaction mixture at high pressure (48 psi) for 72 hours. The
reaction mixture was then filtered through celite, washed with methanol, and
the
combined filtrate concentrated under vacuum to afford the title compound (6.23
g)
as a brown oil: MSCI: m/z 313 (MH+).
e) {1-(2-Fluorophenyl)-1-[1-((S)-1-phenylethyl)pyrrolidin-3-
yl]methyl}carbamic acid tent butyl ester
To a solution of 4-[l-amino-1-(2-fluorophenyl)methyl]-1-((S)-1-
phenylethyl)pyrrolidin-2-one (6.23 g, 19.9 mmol, Example 7d) in
tetrahydrofuran
(25 mL) was added lithium aluminum hydride (1 M in tetrahydrofuran, 39 mL).
The reaction mixture was refluxed for 4 hours, cooled to room temperature, and
quenched with saturated ammonium chloride. The mixture was diluted with ethyl
acetate, washed with saturated ammonium chloride, water, and brine. The
organic
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-188-
layer was dried over magnesium sulfate and concentrated. The resulting residue
was dissolved in dichloromethane (25 mL) and while stirring, di-tart-butyl
dicarbonate (5.66 g, 25.9 mmol) added. After 1 hour, the reaction mixture was
concentrated and the resulting residue purified by flash silica gel
chromatography
S (ethyl acetate) to afford the title compound (10.2 g) as an oil: MSCI: m/z
399
(MHO).
f) 3-[1-tent-Butoxycarbonylamino-1-(2-fluorophenyl)methyl]pyrrolidine-1-
carboxylic acid benzyl ester
l0 To a solution of { 1-(2-fluorophenyl)-1-[1-((S)-1-phenylethyl)pyrrolidin-3-
yl]methyl }-carbamic acid tart butyl ester (5.65 g, 14.2 mmol, Example 7e) in
dichloromethane (50 mL) at 0 °C was added benzyl chloroformate (3.04
mL, 21.3
mmol). The reaction mixture was heated to reflux for 3 hours, cooled to room
temperature, and concentrated. The resulting residue was purified by flash
silica
15 gel chromatography (1:1 ethyl acetate/hexanes) to give the title compound
(7.5 g)
as an oil: MSCI: mlz 429 (MH+).
g) [1-(2-Fluorophenyl)-1-pyrrolidin-3-ylmethyl]carbamic acid tart-butyl ester
To a solution of 3-[1-tart-butoxycarbonylamino-1-(2-fluorophenyl)
20 methyl]pyrrolidine-1-carboxylic acid benzyl ester (7.50 g, 17.5 mmol,
Example
7f) in methanol (50 mL) was added 20% Pd/C (0.5 g). Hydrogen was introduced
to the reaction mixture at high pressure (48 psi) for 72 hours. The reaction
mixture
was altered through Celite, washed with methanol, and the combined filtrates
concentrated under vacuum to afford the title compound (2.55 g) as an oil:
MSCI:
25 mlz 295 (MH+).
h) C-[1-(2-Fluorophenyl)-1-pyrrolidin-3-yl]methylamine
To a solution of [1-(2-fluorophenyl)-1-pyrrolidin-3-ylmethyl]carbamic
acid tart-butyl ester (1.60 g, 5.43 mmol, Example 7g) in dichloromethane (10
mL)
30 was added a solution of gaseous hydrogen chloride (2M in ether, 10 mL).
After 2
hours, the reaction mixture was concentrated and the resulting solid stirred
in
methanol with Amberlite resin (basic). After 1 hour, the mixture was filtered
and
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-189-
the filtrate concentrated to afford the title compound (1.22 g) as an oil:
MSCI: m/z
195 (MH+).
i) [1-[1-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-
7-yl)pyrrolidin-3-yl]-1-(2-fluorophenyl)methyl)carbamic acid tert-
butyl ester
In a sealed tube, a mixture of 1-cyclopropyl-6,7-difluoro-8-methyl-1H-
quinazolinedione (0.350 g, 1.39 mmol), C-[1-(2-fluorophenyl)-1-pyrrolidin-3-
yl]methylamine (0.61 g, 3.15 mmol, Example 7h) and triethylamine (0.480 mL,
3.48 mmol) was stirred in dimethyl sulfoxide (1.0 mL) at 130 °C. After
24 hours,
the reaction mixture was cooled to room temperature and di-tert-butyl
dicarbonate
(1.52 g, 6.95 mmol) added. After 1 hour, the reaction mixture was diluted with
ethyl acetate, washed with saturated sodium bicarbonate, water, and brine. The
organic layer was dried over magnesium sulfate and concentrated. The resulting
residue was purified by flash silica gel chromatography (80:19:1
dichloromethane:
isopropanolariethylamine) to afford the title compound (0.047 g) as a glassy
solid:
MSCI: m1z 527 (MHO).
j) 7-(3-[1-Amino-1-(2-t7uorophenyl)methyl]pyrrolidin-1-yl}-1-cyclopropyl-6-
fluoro-6-methyl-1H-quinazolinedione hydrochloride
To a solution of [ I -[ 1-( 1-cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)pyrrolidin-3-yl]-1-(2-fluorophenyl)methyl]carbamic
acid tert-butyl ester (0.037 g, 0.071 mmol, Example 7i) in dichloromethane (1
mL) was added a hydrogen chloride solution (2M in ether, 1 mL). After 16
hours,
the precipitate was collected by filtration, washed with hexanes, and dried to
afford the title compound (0.019 g); mp 211-213 °C: MSCI: rnlz 427
(MH+).
Example 8
a) 4,6-Dichloro-5-methylnicotinamide
To a solution of 4,6-dichloro-5-methylnicotinic acid (3.0 g, 14.6 mmol, [J.
Het. Chem., 1999, 36, 953]) in dichloromethane (50 mL) was added oxalyl
chloride (2.5 mL, 29.1 mmol) and dimethylformamide (0.1 mL). After 90
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-l 90-
minutes, the reaction mixture was concentrated and the resulting residue
redissolved in dichloromethane (25 mL). This solution was slowly added over IO
minutes to a stirred ether solution saturated with gaseous ammonia at 0
°C. After
30 minutes, the reaction mixture was allowed to warm to room temperature over
a
2 hour period, then concentrated. The solid was triturated in ether/hexanes,
collected by filtration, washed with hexanes and dried afford the title
compound
(3.2 g) as a beige solid: MSCI: m1z 206 (MH+).
b) 1-Cyclopropyl-3-[1-(4,6-dichloro-5-methylpyridin-3-yl)methanoyl]urea
I O To a solution of 4,6-dichloro-5-methylnicotinamide (3.0 g, 14.6 mmol,
Example 8a) in 1,2-dichloroethane (50 mL) was added oxalyl chloride (1.91 mL,
22.0 mmol). The reaction mixture was refluxed for 4 hours, cooled to room
temperature, and concentrated. The resulting residue was dissolved in
dichloromethane and while stirring at 0 °C, cyclopropylamine (1.52 mL,
22.0
I S mmol) was added dropwise over 10 minutes via syringe. After 30 minutes,
the
reaction mixture was warmed to room temperature and stirred for an additional
16
hours. The reaction mixture was washed with saturated sodium bicarbonate,
water, and brine. The organic layer was dried over magnesium sulfate and
concentrated. The resulting residue was purified via flash silica gel
20 chromatography (ethyl acetate) to afford the title compound (4.6 g) as a
beige
solid: MSCI: m/z 289 (MH+).
c) 7-Chloro-1-cyclopropyl-8-methyl-1H-pyrido[4,3-d]pyrimidinedione
To a solution of 1-cyclopropyl-3-[1-(4,6-dichloro-5-methylpyridin-3-
25 yl)methanoyl]urea (4.60 g, I5.9 mmol, Example 8b) in tetrahydrofuran (100
mL)
at -20 °C was added potassium bis(trimethylsilyl)amide (0.5 M in
toluene, 64 mL)
dropwise over 20 minutes. After 15 minutes, the reaction mixture was warmed to
room temperature and 18-crown-6 ether (0.84 g, 3.18 mmol) added. The reaction
mixture was refluxed for 4 hours, cooled to room temperature, and diluted with
30 ethyl acetate. The organic layer was washed with l N hydrochloric acid,
water,
and brine. The organic layer was dried over magnesium sulfate and
concentrated.
The resulting residue was purified by flash silica gel chromatography (ethyl
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-191-
acetate) to give the title compound (2.82 g) as a beige solid: MSCI: m/z 252
(MHO).
d) {(S)-1-[(R)-1-(I-Cyclopropyl-8-methyldioxo-1,2,3,4-tetrahydropyrido[4,3
d'Jpyrimidin-7-yl)pyrrolidin-3-y1]ethyl}methylcarbamic acid tart-butyl ester
In a sealed tube, 7-chloro-1-cyclopropyl-8-methyl-1H-pyrrido[4,3
d]pyrimidinedione (0.15 g, 0.59 mmol, Example 8c) and methyl-((R)-(S)-1-
pyrrolidin-3-ylethyl)amine (0.22 g, 1.79 mmol, [J. Het. Claena. 1992 29,1481
])
was stirred in dimethyl sulfoxide (1mL) at 120 °C. After 6 hours, the
reaction
mixture was cooled to room temperature, and di-tart-butyl dicarbonate (0.65 g,
2.98 mmol) added. After an additional hour, the reaction mixture was diluted
with
ethyl acetate, washed with saturated sodium bicarbonate, water, and brine. The
organic layer was dried over magnesium sulfate and concentrated. The residue
was purified via flash silica gel chromatography (ethyl acetate) to afford the
title
compound (0.177g) as a clear, glassy solid: MSCI: rrzlz 444 (MH+)
e) 1-Cyclopropyl-8-methyl-7-[(R)-3-((S)-1-methylaminoethyl)pyrrolidin-1-yl]-
1H-pyrido[4,3-d]pyrimidinedione hydrochloride
The reaction of hydrogen chloride with {(S)-1-[(R)-1-(1-cyclopropyl-8-
methyldioxo-1,2,3,4-tetrahydropyrido[4,3-d]pyrimidin-7-yl)pyrrolidin-3-
yl]ethyl}methylcarbamic acid tart-butyl ester (0.17 g, 0.38 mmol, Example 8d)
as
described in Example 7j afforded the title compound (0.14 g); mp 217-219
°C:
MSCI: m1z 344 (MH+).
Example 9
a) [(S)-1-(1-Cyclopropyl-6-fluorodioxo-1,2,3,4-tetrahydropyrido[2,3-
d]pyrimidin-7-yl)pyrrolidin-3-yl]carbamic acid tent-butyl ester
A solution of l-cyclopropyl-6-fluoro-7-methanesulfanyl-IH-pyrido[2,3-
d]pyrimidinedione (0.15 g, 0.5 mmol, [PCT Int. Appl. WO 0153273 AI]), (S)-3-
pyrrolidinylcarbamic acid tart-butyl ester (0.28 g, 1.5 mmol, [J. Med. Che~n.
1992,
35, 1764]), triethylamine (0.12 mL, 1.5 mmol) and acetonitrile (10 mL) was
heated at reflux for 6 hours and then stirred at room temperature for 18
hours. The
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-192-
solution was concentrated and the residue partitioned between ethyl acetate
and
water. The organic layer was washed with water, dried over magnesium sulfate,
and concentrated to a residue that was purified by flash silica gel
chromatography
(95:5 dichloromethane !ethanol) to afford the title compound (0.17 g); mp 220-
222 °C.
b) 7-((S)-3-Aminopyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-1H-pyrido[2,3-
d]pyrimidinedione hydrochloride
A solution of [(S)-1-(1-cyclopropyl-6-fluorodioxo-1,2,3,4-
tetrahydropyrido[2,3-d]pyrimidin-7-yl)pyrrolidin-3-yl]carbamic acid tart-butyl
ester (0.135 g, 0.33 mmol, Example 9a) in ethanol (5 mL) was treated with
ethanol (1 mL) saturated with hydrogen chloride gas. The mixture was heated at
reflux for 0.5 hours, stirred at room temperature for 18 hours, and then
concentrated. The solid was then triturated in ethyl ether, collected by
filtration,
washed with ether and dried to give the title compound (0.11 g); mp >280
°C.
Example 10
a) {(S)-1-[(R)-1-(1-Cyclopropyl-6-fluorodioxo-1,2,3,4-tetrahydropyrido[2,3-
d]pyrimidin-7-yl)pyrrolidin-3-yl]ethyl}carbamic acid tart-butyl ester
Reaction of 1-cyclopropyl-6-fluoro-7-methanesulfanyl-1H pyrido[2,3-
d]pyrimidinedione (0.15 g, 0.5 mmol, [PCT Int. App!. WO 0153273 A1] ) with
((R)-(S)-1-pyrrolidin-3-ylethyl)carbamic acid tart-butyl ester (0.32 g, 1.5
mmol,
[J. Het. Chem. 1992, 29, 1481 ] ) as described in Example 9a gives the title
compound (0.17 g); mp 249-251 °C.
b) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-t7uoro-1H-
pyrido[2,3-d]pyrimidinedione hydrochloride
The reaction of {(S)-1-[(R)-1-(1-cyclopropyl-6-fluorodioxo-1,2,3,4-
tetrahydropyrido[2,3-d]pyrimidin-7-yl)pyrrolidin-3-y1]ethyl}carbamic acid tert-
butyl ester (0.126 g, 0.29 mmol, Example !0a) with hydrogen chloride as
described in Example 9b gave the title compound (0.10 g); mp >280 °C.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-193-
Example 11
a) 3-Ethylsulfanyl-4,5-difluoro-2-methyl-1H-indole-7-carboxylic acid methyl
ester
A solution of 9.5 g (30.7 mmoles) of 7-bromo-3-ethylsulfanyl-4,5-
difluoro-2-methyl-1H-indole [Chem. Pharm. Bull. 1990, 38, 2459] in 300 mL of
methanol was treated with 0.35 g (I.5 mmol) of palladium acetate, 0.93 g (2.25
mmol) of diphenylphosphinopropane and 7.7 g (10.7 mL, 76 mmol) of
triethylamine. The resulting mixture was pressurized to 500 psi with carbon
monoxide and heated at 100 °C for 12 hours. The solvent was removed irv
vacuo
and the residue was chromatographed on flash grade silica gel (230-400 mesh)
eluting with dichloromethane to give 0.6 g of starting material and 7.2 g of
the
title compound, mp 110-112 °C.
b) 4,5-Difluoro-2-methyl-1H-indole-7-carboxylic acid methyl ester
A solution of 5.2 g (18.2 mmol) of 3-ethylsulfanyl-4,5-difluoro-2-methyl-
1H-indole-7-carboxylic acid methyl ester (Example 11 a) in 150 mL of ethanol
was treated with 30 g of Raney-nickel and the resulting suspension was heated
at
reflux for 2 hours. An additional 10 g of Raney-nickel was added and the
heating
was continued for an additional 2 hours. The solid was removed by filtration
and
washed with ethanol. The ethanolic filtrates were combined and concentrated in
vacuo to dryness and used as is for the next step. The yield of the title
compound
was 4.0 g.
c) 4,5-Difluoro-2-methyl-2,3-dihydro-1H-indole-7-carboxylic acid methyl
ester
A suspension of 3.25 g (14.3 mmol) of 4,5-difluoro-2-methyl-IH-indole-7-
carboxylic acid, methyl ester (Example 11 b) in 50 mL of trifluoroacetic acid
was
heated to 50 °C and the resulting solution treated dropwise with 3.3 g
(4.6 ml,
28.6 mmol) of triethylsilane. The reaction was heated at 50 °C for 3
hours and the
solvent removed in vacuo. The residue was then dissolved in methanol, which
was
also removed if? vacuo. The resulting residue was triturated with hexane (2 x
30
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-I 94-
mL) then ether, both of which were removed in vacuo to give 3.1 g of the title
compound, mp 50-52 °C.
d) 7,8-Difluoro-5-methyl-5,6-dihydro-5H-pyrrolo[3,2,1-i~j]quinazoline-1,3-
dione
A solution of 3.2 g (14.3 mmol) of 4,5-difluoro-2-methyl-2,3-dihydro-1H
indole-7-carboxylic acid methyl ester (Example 1 lc) in 60 mL of
dichloromethane
was treated with 4.06 g (50 mmol) of 96°70 potassium cyanate. The
resulting
suspension was stirred at room temperature for IO minutes and treated with 5.7
g
(3.85 mL, 50 mmol) of trifluoroacetic acid. There was a slight exotherm
initially.
The reaction was stirred at room temperature for 18 hours. A complete solution
after 1 hour was followed by the formation of a heavy precipitate. The
reaction
was concentrated in vacuo and the residue was triturated with ether/water (200
mL
of each). The solid was removed by filtration, washed with water, diethyl
ether
and dried in vacuo to give 3.2 g of the title compound, mp 239-241 °C.
e) [(S)-1-(8-Fluoro-5-methyl-1,3-dlOXO-2,3,5,6-tetrahydro-1H-pyrrolo[3,2,1-
i~j]quinazolin-7-yl)pyrrolidin-3-yl]carbamic acid tart-butyl ester
A solution of 0.12 g (0.5 mmol) of 7,8-difluoro-5-methyl-5,6-dihydro-SH-
pyrrolo[3,2,1-ij]quinazoline-1,3-dione (Example l 1 d) in 5 mL of dimethyl
sulfoxide was treated with 0.37 g (2.0 mmol) of (S)-3-pyrrolidinylcarbamic
acid
tart-butyl ester [J. Med. Chem.1992, 35, 1764] and the reaction mixture
stirred at
room temperature for 40 hours. The reaction was diluted to 50 mL with ice and
water and extracted with ethyl acetate (2 x 40 mL). The combined organics were
washed with water (2 x 30 mL), dried with magnesium sulfate, filtered and
evaporated in vacuo. The residue was chromatographed over flash grade silica
gel
(230-400 mesh) eluting with dichloromethane/ ethyl acetate/ethanol (80:20:10)
to
give 0.19 g of the title compound, mp 203-205 °C
f) 7-((S)-3-Aminopyrrolidin-1-yl)-8-fluoro-5-methyl-5,6-dihydropyrrolo[3,2,1-
i~j]quinazoline-1,3-dione hydrochloride
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-19S-
A solution of 0.19 g (0.47 mmol) of [(S)-1-(8-fluoro-S-methyl-1,3-dioxo-
2,3,5,6-tetrahydro-1 H-pyrrolo[3,2,1-i,j]quinazoIin-7-yl)pyrrolidin-3-
yl]carbamic
acid tert-butyl ester (Example l 1e) in S mL of ethanol was treated with 1 mL
of
ethanol saturated with hydrogen chloride gas and the solution stirred at room
S temperature overnight. The solvent was removed in vacuo and the residue
dissolved in water, filtered through a fiber glass pad to clarify and the
filtrate
lyophilized to give 0.148 g of the title compound, mp 213-21 S °C.
Example 12
a) ((S)-1-[(R)-1-(8-Fluoro-5-methyl-1,3-dioxo-2,3,5,6-tetrahydro-1H-
pyrrolo[3,2,1-i~j]quinazolin-7-yl)pyrrolidin-3-yl]ethyl}carbamic acid tert-
butyl ester
A solution of 0.125 g (0.S mmoles) of 7,8-difluoro-S-methyl-S,6-dihydro-
SH pyrrolo[3,2,1-ij]quinazoline-1,3-dione (Example l 1d), 0.43 g (2.0 mmoles)
of
1S ((R)-(S)-1-pyrrolidin-3-ylethyl)carbamic acid tert-butyl ester [J. Het.
Chem.1992,
29, 1481 ] and 5 mL of dimethyl sulfoxide was heated at 100 °C for 24
hours. The
reaction was cooled to room temperature, diluted to SO mL with ice and water
and
extracted with ethyl acetate (2 x 30 mL). The combined organic extracts were
washed with water (2 x 2S mL), dried with magnesium sulfate, filtered and
concentrated in vacuo. The residue was chromatographed over flash grade silica
gel (230-400 mesh) eluting with dichloromethane /ethanol (90:10) to give 0.19
g
of the title compound as a foam which was used "as is" for the next step.
b) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl)-8-fluoro-5-methyl-5,6-
2S dihydropyrrolo[3,2,1-i~j]quinazoline-1,3-dione hydrochloride
A solution of 0.19 g (0.42 mmol) of ((S)-1-[(R)-1-(8-Fluoro-S-methyl-1,3-
dioxo-2,3,5,6-tetrahydro- I H-pyrrolo[3,2,1-i, j]quinazolin-7-yl)pyrrolidin-3-
yl]ethyl }carbamic acid tert-butyl ester (Example 12a) in S mL of ethanol was
treated with 2 mL of ethanol saturated with hydrogen chloride gas and the
mixture
stirred at room temperature for 18 hours. The solvent was removed in vacuo and
the residue triturated with ethyl acetate. The resulting solid was removed by
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-l 96-
filtration, washed with ethyl acetate and dried in vacuo affording 0.14 g of
the title
compound, mp 203-205 °C.
Example 13
b) [(S)-1-(9-Fluoro-S-methyl-1,3-dioxo-2,3,6,7-tetrahydro-1H, SH-
pyrido[3,2,1-ij]quinazolin-8-yl)pyrrolidin-3-ylJcarbamic acid tert-butyl ester
Reaction of 8,9-difluoro-5-methyl-6,7-dihydro-SH-pyrido[3,2,1-
i,j]quinazoline-1,3-dione (0.125 g, 0.5 mmol, [PCT Int. Appl. WO 0153273 Al])
with (S)-3-pyrrolidinylcarbamic acid tert-butyl ester [J. Med Chem. 1992, 35,
1764] (0.37 g, 2.0 mmol) as described in Example 12a (except that reaction was
carried out at 110 °C for 18 hours) gave the title compound (0.11 g).
b) 8-((S)-3-Aminopyrrolidin-1-yl)-9-fluoro-S-methyl-6,7-dihydropyrido[3,2,1-
i~j]quinazoline-1,3-dione hydrochloride
A solution of 0.11 g (0.26 mmol) of [(S)-1-(9-fluoro-5-methyl-1,3-dioxo-
2,3,6,7-tetrahydro-1H, SH-pyrido[3,2,1-i,j]quinazolin-8-yl)pyrrolidin-3-
yl]carbamic acid tert-butyl ester (Example 13a) in 5 mL of ethanol was treated
with 1 mL of ethanol saturated with hydrogen chloride gas and the reaction
stirred
at room temperature for 18 hours. The solvent was removed in vacuo and the
residue dissolved in water, filtered through a fiber glass pad to clarify and
the
filtrate lyophilized to give 0.07 g of the title compound, mp 134-136
°C.
Example 14
a) {(S)-1-[(R)-1-(9-Fluoro-S-methyl-I,3-dioxo-2,3,6,7-tetrahydro-1H, SH-
pyrido[3,2,1-i,j]quinazolin-8-yl)pyrrolidin-3-yl]ethyl}carbamic acid tert-
butyl
ester
The reaction of 8,9-difluoro-5-methyl-6,7-dihydro-SH-pyrido[3,2,1-
i~j]quinazoline-1,3-dione (0.125 g, 0.5 mmol, [PCT Int. Appl. WO 0153273 Al])
with ((R)-(S)-1-pyrrolidin-3-ylethyl)carbamic acid tert-butyl ester (0.22 g,
1.0
mmol, [J. Het. Chena.1992, 29, 1481]) and 1,1,3,3-tetramethylguanidine (0.125
mL, 1.0 mmol) in dimethyl sulfoxide (3 mL) was heated at 110 °C for 18
hours.
The reaction was cooled to room temperature, diluted to 50 mL with ice and
water
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
- I 97-
and extracted with ethyl acetate (2 x 30 mL). The combined organics were
washed
with water (2 x 25 mL), dried with magnesium sulfate, filtered and
concentrated
in vacuo. The residue was chromatographed over flash grade silica gel (230-400
mesh) eluting with dichloromethane !ethanol (90:10) to provide the title
compound (0.I 1 g); mp I23-125 °C.
b) 8-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl)-9-fluoro-5-methyl-6,7-dihydro-
SH-pyrido[3,2,1-i~j]quinazoline-1,3-dione hydrochloride
A solution of 0.1 I g (0.25 mmol) {(S)-1-[(R)-1-(9-fluoro-5-methyl-1,3-
l0 dioxo-2,3,6,7-tetrahydro-1H, SH-pyrido[3,2,I-i~j]quinazolin-8-yl)pyrrolidin-
3-
yl]ethyl }carbamic acid, tent-butyl ester (Example 14a) in 5 mL of ethanol was
treated with 2 mL of ethanol saturated with hydrogen chloride gas. A
precipitate
formed and the mixture was then stirred at room temperature for 18 hours. The
solvent was removed in vacuo and the residue triturated with ethyl ether. The
resulting solid was removed by filtration, washed with ether and dried in
vacuo to
give 0.065 g of the title compound, mp 276-278 °C.
Example 15
a) 4-(1-tert-Butoxycarbonyl-1-cyanomethyl)-2,5-difluoro-3-methylbenzoic
acid ethyl ester
A solution of 2,4,5-trifluoro-3-methylbenzoic acid ethyl ester (20 g, 92
mmol, [PCT Int. App!. WO 0153273 A 1 ]), potassium carbonate (30.4 g, 220
mmol) and tert-butylcyanoacetate (15.5 g, I 10 mmol) in dirnethyl sulfoxide
(I20
mL) was heated for 2 hours at 65-70 °C. The resulting mixture was
poured into a
stirred mixture bf ice water and ethyl acetate (2: l ) and acidified to pH 3
with
aqueous 6N hydrochloric acid. The aqueous layer was separated and the organic
layer washed with water and brine. The organic extract was then dried over
sodium sulfate and concentrated to afford the title compound (3I g): 'H NMR
(200 MHz, CDC13): 8 7.63-7.50 (m, 1 H), 5.15 (s, 1 H), 4.41 (q, 2H), 2.38 (d,
3H),
1.50 (s, 9H), 1.41 (t, 3H).
b) 4-Cyanomethyl-2,5-difluoro-3-methylbenzoic acid ethyl ester
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-198-
4-( 1-tert-Butoxycarbonyl- I -cyanomethyl)-2,5-difluoro-3-methylbenzoic
acid ethyl ester (10 g, 29.5 mmol, Example 15a) and a catalytic amount of p-
toluenesulfonic acid (200 mg) in toluene (60 mL) was refluxed for 6 hours,
then
poured into ice water. The organic layer was separated, washed with water,
brine
and dried over sodium sulfate and concentrated. The resulting residue was
purified
by flash silica gel chromatography (1:5 ethyl acetate/hexanes) to afford the
title
compound (5.3 g): 'H NMR (200 MHz, CDCl3): b 7.59-7.45 (m, 1H), 4.40 (q,
2H), 3.78 (s, 2H), 2.39 (d, 3H), 1.41 (t, 3H).
c) 4-(1-Cyanocyclopropyl)-2,5-difluoro-3-methylbenzoic acid
Benzyltriethylamrnonium chloride (0.937 g, 4.2 mmol) and aqueous 10 N
sodium hydroxide (8.2 mL) were added to a mixture of 4-cyanomethyl-2,5-
difluoro-3-methylbenzoic acid, ethyl ester (1.0 g, 4.2 mmol, Example 15b) and
1,2-dibromoethane (1.67 g, 8.9 mmol) at 10 °C. The resulting mixture
was stirred
at room temperature for 2 hours, then acidified with aqueous 6N hydrochloric
acid
and extracted with ethyl acetate. The organic layer was washed with water and
brine, dried over sodium sulfate and concentrated to afford the title compound
(1
g), which was used without further purification.
d) 1-{1-[4-(1-Cyanocyclopropyl)-2,5-difluoro-3-methylphenyl]methanoy1}-3-
cyclopropyl urea
A solution of 4-(1-cyanocyclopropyl)-2,5-difluoro-3-methylbenzoic acid
(1 g, 4.21 mmol, Example ISc) in dichloromethane (IS mL) at 0 °C was
treated
with oxalyl chloride (5 mL) followed by N,N dimethylformamide (3 drops). The
mixture was stirred at room temperature for 1 hour then concentrated in vacuo.
The residue was dissolved in benzene (15 mL), treated with cyclopropyl urea
(0.421 g, 4.21 mmol) in benzene (10 mL), and refluxed overnight. The resulting
mixture was concentrated and diluted with ethyl acetate. The solution
was~washed
with water and brine, dried over sodium sulfate and concentrated. The
resulting
residue was purified by flash silica gel chromatography (1:1 ethyl
acetate/hexanes) to afford the title compound (0.94 g): 'H NMR (200 MHz,
CDCI3): 8 9.01 (bd, 1 H), 8.50 (bs, 1 H), 7.62-7.46 (m, I H), 2.87-2.69 (m, 1
H),
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-199-
2.51 (d, 3H), 1.96-1.81 (m, 2H), 1.45-1.31 (m, 2H), 0.91-0.78 (m, 2H), 0.71-
0.56
(m, 2H).
e) 1-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)cyclopropanecarbonitrile
A solution of 1-{ 1-[4-(1-cyanocyclopropyl)-2,5-difluoro-3-
methylphenyl]methanoyl }-3-cyclopropyl urea (0.640 g, 2 mmol, Example 15d) in
tetrahydrofuran (20 mL) and N,N dimethylformamide ( 1 mL) at -20 °C was
treated with sodium hydride (60% dispersion in mineral oil, 0.280 g, 7 mmol).
The resulting mixture was stirred at room temperature for 30 minutes, then
refluxed for 3 days. The mixture was then diluted with ethyl acetate, washed
with
brine, dried over sodium sulfate and concentrated. The resulting residue was
purified by~column chromatography (3:2 ethyl acetate/hexanes) to afford the
title
compound (0.55 g): ' H NMR (200 MHz, CDC13): 8 9.47 (bs, 1 H), 7.70 (d, 1 H),
IS 3.50-3.33 (m, 1H), 2.79 (s, 3H), 2.00-1.82 (m, 2H), 1.48-1.33 (m, 2H), 1.30-
1.08
(m, 2H), 0.69-0.51 (m, 2H).
fj 1-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)cyclopropanecarboxylie acid amide
A solution of 1-(1-cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)cyclopropanecarbonitrile (0.300 g, 1 mmol, Example
l Se) in aqueous IN sodium hydroxide (2 mL) was treated with hydrogen peroxide
(27% w/w, 0.252 g, 2 mmol) over 5 minutes at room temperature. The resulting
mixture was stirred for 30 minutes and acidified to pH 2 with aqueous 6N
hydrochloric acid. The precipitate was collected by filtration to afford the
title
compound (0.204 g): ' H NMR (200 MHz, DMSO-d6): 8 7.44 (d, 1 H), 7.05 (bs,
1 H), 6.63 (bs, 1 H), 3.48-3.24 (m, 2H), 2.52 (s, 3H), 1.71-I .50 (m, 2H),
1.20-0.85
(m, 4H), 0.76-0.58 (m, I H), 0.50-0.31 (m, 1 H).
Example 16
a) 8,9-Difluoro-3-methyl-7-nitro-2,3-dihydro-1-oxa-3a,S-diazaphenalene-4,6-
dione
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-200-
A solution of 0.54 g (2.0 mmol) of 5-amino-8,9-difluoro-3-methyl-2,3-
dihydro-1-oxa-3a,5-diazaphenalene-4,6-dione (WO 0153273) in 5 mL of 98%
sulfuric acid was treated portionwise with 0.30 g (3.0 mmol) of potassium
nitrate
at room temperature. After the addition was complete, the reaction was stirred
overnight. The solution was then poured onto 50 g of ice and water and
stirred.
The resulting coarse precipitate was removed by filtration, washed with water
and
dried in vacuo to give 0.50 g of material that was chromatographed over silica
gel
eluting with dichloromethane/ethanol (90/10) to give 0.23 g of the title
compound,
mp 286-288 °C.
b) 7-Amino-S,9-difluoro-3-methyl-2,3-dihydro-1-oxa-3a,5-diazaphenalene-
4,6-dione
A solution of 0.48 g ( 1.6 mmol) of 8,9-difluoro-3-methyl-7-nitro-2,3-
dihydro-1-oxa-3a,5-diazaphenalene-4,6-dione (Example 16a) in a mixture of 50
mL of tetrahydrofuran/methanol (45-5) was treated with 0.1 g of Raney-nickel
and shaken in a hydrogen atmosphere at 22 °C and pressures of 21-49 psi
for 22
hours. The catalyst was removed by filtration and the solvent removed in vacuo
to
give 0.42 g of the title compound, mp >280 °C.
c) {(S)-1-[(R)-1-(7-Amino-8-fluoro-3-methyl-4,6-dioxo-2,3,5,6-tetrahydro-4H-
1-oxa-3a,5-diazaphenalen-9-yl)pyrrolidin-3-yl]ethyl}carbamic acid tert-butyl
ester
A solution of 0.20 g (0.75 mmol) of 7-amino-8,9-difluoro-3-methyl-2,3
dihydro-1-oxa-3a,5-diazaphenalene-4,6-dione (Example 16b), 0.32 g (1.5 mmol)
of (R)-(S)-1-pyrrolidin-3-ylethyl)carbamic acid tert-butyl ester [J.
Heterocyel.
Chem. 1992, 29(6), 1481 ], 0.3 g (3.0 mmol) of triethylamine and 10 mL of
acetonitrile was heated at reflex for 12 hours. The solvent was removed in
vacuo
and the residue partitioned between dichloromethane/water (75 mL each).'The
organic layer was then washed with water, dried with magnesium sulfate,
filtered
and concentrated to give 0.33 g of material that was chromatographed over
silica
gel eluting with dichloromethane/ethanol (97:3) to give 0.23 g of the title
compound, mp 136-138 °C.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-201-
d) 7-Amino-9-[9-(R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-8-Flnoro-3-methyl-
2,3-dihydro-1-oxa-3a,5-diazaphenalene-4,6-dione hydrochloride
A solution of 0.23 g (0.5 mmol) of {(S)-1-[(R)-1-(7-amino-8-fluoro-3-
methyl-4,6-dioxo-2,3,5,6-tetrahydro-4H-1-oxa-3a,5-diazaphenalen-9-
yl)pyrrolidin-3-yI]ethyl}carbamic acid tart-butyl ester (Example 16c) in 2 mL
of
ethanol was treated with 1 mL of ethanol saturated with hydrogen chloride gas
and the mixture stirred at room temperature for 18 hours. The solvent was then
removed in vacuo and the residue triturated with ethanol/ether (5 mL of a 1:1
solution). The solid was removed by filtration, washed with ethanol/ether
(1:1)
and dried in vacuo to give 0.21 g of the title compound, mp 223-225 °C.
Example 17
a) [(3aR,6aS)- and (3aS,6aR)-2-(1-Cyclopropyl-6-fluoro-8-methoxydioxo-
I 5 1,2,3,4-tetrahydroquinazolin-7-yl)octahydrocyclopenta[c]pyrrol-4-
yl]carbamic acid tart-butyl ester
A solution of 0.27 g (1.0 mmol) of 1-cyclopropyl-6,7-difluoro-8-methoxy-
1H-quinazolinedione, 0.45 g (2.0 mmol) of ((3aS, 6aR)- and (3aR,6aS)-2-
benzyloctahydrocyclopenta[c]pyrrol-4-yl)carbamic acid tart-butyl ester [US
5580872], 0.39 g (3.0 mmol) of diisopropylethylamine (Hunig's base) and 1.25
mL of dimethyl sulfoxide was heated at 90 °C for 18 hours. The reaction
mixture
was diluted to l 5 mL with water and extracted with ethyl acetate (2 x 20 mL).
The
combined organic extracts were washed with water (2 x 20 mL), dried with
magnesium sulfate, filtered and concentrated in vacuo. The residue (0.75 g)
was
chromatographed over silica gel eluting with dichloromethanelethyl acetate
(80:20) to provide 0.18 g of the title compound, mp 100-102°C.
b) 7-((3aR, 6aS)- and (3aS, 6aR)-4-Aminohexahydrocyclopenta[c]pyrrol-2-yl-
1-cyclopropyl-6-fiuoro-8-methoxy-1H-quinazolinedione hydrochloride
A solution of 0.17 g (0.36 mmol) of [(3aR,6aS)- and (3aS, 6aR)-2-(1-
cyclopropyl-6-fluoro-8-methoxydioxo-1,2,3,4-tetrahydroqui nazolin-7-
yl)octahydrocyclopenta[c]pyrrol-4-yl]carbamic acid tart-butyl ester (Example
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-202-
17a) in 2 mL of ethanol was treated with 1 mL of ethanol saturated with
hydrogen
chloride gas and the reaction stirred at room temperature for 18 hours. The
solvent
was removed in vacuo and the residue dissolved in water, filtered through a
fiberglass pad and lyophilized to give 0.15 g of the title compound, mp 233-
235
°C.
Example 18
a) [(3aR, 6aS)- and (3aS, 6aR)-2-(~-Cyclopropyl-6-fluoro-8-methyldioxo-
1,2,3,4-tetrahydroquinazolin-7-yl)octahydrocyclopenta[c]pyrrol-4-
yl]carbamic acid tart-butyl ester
A solution of 0.25 g (1.0 mmol) of I-cyclopropyl-6,7-difluoro-8-methyI-
1H-quinazolinedione, 0.45 g (2.0 mmol) of ((3aS, 6aR)- and (3aR, 6aS)-2-
benzyloctahydrocyclopenta[c]pyrrol-4-yl)carbamic acid tent-butyl ester, 0.24 g
(2.0 mmol) of 1,1,3,3-tetramethylguanidine and 1.5 mL of dimethyl sulfoxide
was heated at 90 °C for 18 hours. The reaction mixture was diluted to
15 mL, with
water and the resulting solid removed by filtration, washed with water and
dissolved in ethyl acetate. After drying with magnesium sulfate and filtering,
the
solvent was removed in vacuo and the residue chromatographed over silica gel
eluting with dichloromethanelethyl acetate (80:20) to give 0.078 g of the
title
compound, MSCI: m/z 459 (MH+).
b) 7-((3aR, 6aS)- and (3aS, 6aR)-4-Aminohexahydrocyclopenta[c]pyrrol-2-yl-
1-cyclopropyl-6-f7uoro-8-methyl-1H-quinazolinedione hydrochloride
A solution of 0.078 g (0.17 mmol) of [(3aR, 6aS)- and (3aS, 6aR)-2-(1-
cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)octahydrocyclopenta[c]pyrrol-4-yl]carbamic acid tart-butyl ester (Example
18a) in 2 mL of ethanol was treated with l mL of ethanol saturated with
hydrogen
chloride gas and the reaction stirred at room temperature for 18 hours. The
solvent
was removed in vacuo and the residue dissolved in water, filtered through a
fiberglass pad and lyophilized to give 0.07 g of the title compound, mp 208-
210
°C.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-203-
Example 19
a) 1-Benzyl-3-(2-bromoacetyl)pyrrolidin-2-one
To a solution of I-benzyl-2-oxopyrrolidine-3-carboxylic acid (10 g, 45.7
mmol [United States Patent No. 5,175,157] in tetrahydrofuran/dioxane (300
mL/60 mL) at -10 °C was added 4-methylmorpholine (6.5 mL, 59.4 mmol)
followed by isobutyl chIoroformate (7.10 mL, 54.8 mmol). After 10 minutes, a
white precipitate was filtered off and washed with tetrahydrofuran. The
filtrate
and wash were poured into a new Erlenmyer flask and kept at 0 °C. To
this
mixture was added a solution of diazomethane (1.1M in ether, 55 mL). After 15
minutes, a 1:1 hydrobromic acid (48%)/acetic acid solution was added dropwise
until gas evolution ceased. After 15 minutes, the reaction mixture was diluted
with ethyl acetate and washed with saturated aqueous sodium bicarbonate. The
combined organic layers were dried over magnesium sulfate, filtered and
concentrated. The residue mixture was purified by chromatography (99:1
dichIoromethane/methanol) to give the title compound (26.6 g); MSCI: m/z
296,298 (MH+).
b) 1-Benzyl-3-(2-fluoroacetyl)pyrrolidin-2-one
To a solution of I-benzyl-3-(2-bromoacetyl)pyrrolidin-2-one (2.2 g, 7.43
mmol, Example 19a) in acetonitrile (25 mL) was added 18-crown-6 (0.980 g, 3.72
mmol) and potassium fluoride (spray dried) (2.16 g, 37.2 mmol). The reaction
mixture was immersed in an oil bath at 80 °C. After 1 hour, the mixture
was
cooled to room temperature and partitioned between water and ethyl acetate.
The
organic layer was dried over magnesium sulfate, filtered and concentrated. The
residue was purified by chromatography (99:1 dichloromethane/methanol then
98:2 dichloromethane/methanol) to afford the title compound (0.515 g): 'H NMR
(400 MHz, CDCl3) b 7.36-7.19 (m, SH), 4.93 (m, 2H), 4.46 (m, 2H), 3.61 (m,
1 H), 3.48 (m, 2H), 2.74 (m, 2H).
c) 1-Benzyl-3-(1-benzylamino-2-fluoroethyl)pyrrolidin-2-one
Benzylamine (3.90 mL, 35.7 mmol) was added to a solution of I-benzyl-3-
(2-fluoroacetyl)pyrrolidin-2-one (7.00 g, 29.8 mmol, Example 19b) in 1,2-
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-204-
dichloroethane (150 mL). The solution was cooled to 0 °C and sodium
triacetoxyborohydride (8.20 g, 38.7 mmol) was added. The reaction mixture was
warmed to room temperature and, stirred overnight, then washed with aqueous
sodium bicarbonate solution and brine. The organic layer was dried over
magnesium sulfate, filtered and concentrated. The residue was purified by
chromatography (99:1 to 97:3 dichloromethane/methanol) to afford the title
compound (7.2 g) as a mixture of diastereomers: MSCI: m/z 327 (MHO)
d) Benzyl-[1-(l.-benzylpyrrolidin-3-yl)-2-fluoroethyl]amine
To a solution of I-benzyl-3-(1-benzylamino-2-fluoroethyl)pyrrolidin-2-
one (7.2 g, 22 mmol, Example 19c) in tetrahydrofuran (100 mL) at 0 °C
was
added lithium aluminum hydride (1M in tetrahydrofuran, 22 mL) dropwise. After
I hour, the mixture was warmed to room temperature and quenched after an
additional 30 minutes by the addition of 0.84 mL water, 0.84 mL 15% sodium
hydroxide solution and 2.5 mL water. The reaction mixture was filtered and
concentrated. The crude residue was purified by chromatography (97:3 to 90:10
dichloromethane/methanol) to give the title compound (2.5 g) as a mixture of
diastereomers. MSCI: m/z = 313 (MH+).
e) 2-Fluoro-1-pyrrolidin-3-ylethylamine
To a solution of benzyl-jl-(1-benzylpyrrolidin-3-yl)-2-fluoroethyl]amine
(2.5 g, 8.0 mmol, Example 19d) in methanol (50 mL) was added 20% palladium
on carbon (200 mg). Hydrogen gas was introduced to the reaction mixture at
high
pressure (4$ psi) for 24 hours, at which time sulfuric acid (3 drops) was
added.
After hydrogenation for an additional 24 hours, the reaction mixture was
filtered
through Celite, washed with methanol, and the combined filtrate concentrated
under vacuum to afford the title compound (1.0 g): MSCI: mlz 133 (MH+).
fj 7-[3-(1-Amino-2-fluoroethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-tluoro-8-
methyl-1H-quinazolinedione hydrochloride
l-Cyclopropyl-6,7-difluoro-8-methyl-1H quinazolinedione (0.97 g, 3.83
mmol), 2-fluoro-I-pyrrolidine-3-ylethylamine (0.66 g, 4.98 mmol, Example 19e),
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-20S-
and 1,1,3,3-tetramethylguanidine (0.96 mL, 7.66 mmol) in dimethyl sulfoxide (1
mL) was heated to 90 °C for 16 hours. The solution was diluted with
ethyl acetate
and washed with saturated sodium bicarbonate, water and brine. The organic
layer was dried over magnesium sulfate, filtered, and concentrated. The
resulting
S residue was then purified by flash silica gel chromatography (90:10
dichloromethane/ methanol) to afford a yellow residue. The residue was
dissolved
in dichloromethane (2 mL) and gaseous hydrogen chloride solution (1 mL, 2.0 M
in ether). The resulting precipitate was filtered to afford the title compound
(0.092 g); mp 218-221 °C, MSCI: m/z 365 (MH+).
Example 20
a) 3-(Methoxymethylcarbamoyl)pyrrolidine-1-carboxylic acid benzyl ester
To~a solution of pyrrolidine-1,3-dicarboxylic acid benzyl ester (5.19 g,
20.8 mmol, [WO 9706802]) in dichloromethane (100 mL) was added
1 S triethylamine (4.35 mL, 31.2 mmol), N,O-dimethylhydroxylamine
hydrochloride
(2.44 g, 25.0 mmol), and N (3-dimethylaminopropyl)-N'-ethylcarbodiimide
hydrochloride (4.79 g, 25.0 mmol). After S hours, the reaction mixture was
washed with saturated sodium bicarbonate, water, and brine. The organic layer
was then dried over magnesium sulfate, filtered, and the filtrate
concentrated. The
resulting residue was purified by flash silica gel chromatography
(hexanes/ethyl
acetate) to give the title compound (4.30 g) as a yellow solid: MSCI: m/z 293
(MH+).
b) 3-Cyclopropanecarbonylpyrrolidine-1-carboxylic acid benzyl ester
2S To a solution of cyclopropylbromide (1.76 mL, 22.0 mmol) in
tetrahydrofuran (SO mL) under nitrogen atmosphere at -78 °C was added a
1.6 M
solution of n-butyllithium (16.5 mL, 26.4 mmol) in hexanes slowly over 1S
minutes. After 1 hour, 3-(methoxymethyl-carbamoyl)pyrrolidine-1-carboxylic
acid benzyl ester (4.29 g, 14.7 mmol, Example 20a) was added as a solution in
tetrahydrofuran (2S mL). After 30 minutes, the reaction mixture was warmed to
room temperature for 1 hour. The reaction mixture was diluted with ethyl
acetate,
washed with saturated ammonium chloride, water, and brine. The organic layer
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-206-
was dried over magnesium sulfate, filtered, and the filtrate concentrated. The
resulting residue was purified by flash silica gel chromatography
(hexanes/ethyl
acetate) to give the title compound (1.91 g) as a yellow oil: MSCI: m/z 274
(MH+).
S
c) 3-(Cyclopropylhydroxyiminomethyl)pyrrolidine-1-carboxylic acid benzyl
ester
To a solution of 3-cyclopropanecarbonylpyrrolidine-1-carboxylic acid
benzyl ester ( 1.91 g, 6.99 mmol, Example 20b). in pyridine ( 10 mL) was added
hydroxylamine hydrochloride (0.S8 g, 8.4 mmol). The reaction was heated to 90
°C for 6 hours, and diluted with ethyl acetate. The organic layer was
washed
twice with 1N hydrochloric acid, water, and brine. The organic layer was dried
over magnesium sulfate, filtered, and the filtrate concentrated. The resulting
residue was purified by flash silica gel chromatography (ethyl acetate) to
give the
1S title compound (1.60 g) as a brown oil: MSCI: m/z 289 (MH+).
d) C-Cyclopropyl-C-pyrrolidin-3-ylmethylamine
To a solution of 3-(cyclopropylhydroxyiminomethyl)pyrrolidine-1-
carboxylic acid benzyl ester (1.60 g, S.S3 mmol, Example 20c) in methanol (SO
mL) was added Raney nickel (1 g). Hydrogen was introduced to the reaction
mixture at high pressure (47 psi) for 72 hours, then the reaction mixture was
filtered through Celite, washed with methanol, and the combined filtrates
concentrated under vacuum to afford the title compound (1.32 g) as a brown
oil:
MSCI: m/z 141 (MH+).
e) 7-[3-(Aminocyclopropylmethyl)pyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride
Utilizing the same procedure as described in Example 19f, from reaction
of 1-cyclopropyl-6,7-difluoro-8-methyl-1H-quinazolinedione (0.83 g, 3.30
mmol),
C-cyclopropyl-C-pyrrolidin-3-ylmethylamine (0.69 g, 4.95 mmol, Example 20d),
and 1,1,3,3-tetramethylguanidine (1.24 mL, 9.90 mmol) to afford the title
compound (0.040 g); mp 191-193 °C; MSCI: m/z 373 (MH+).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-207-
Example 21
a) 4-Hydroxymethyl-1-((S)-1-phenylethyl)pyrrolidin-2-one
To a solution of 5-oxo-1-(1-phenylethyl)pyrrolidine-3-carboxylic acid
methyl ester (11.17g, 45.17 mmol, [J. Het. Chem.1992, 29, 1481]) in
tetrahydrofuran ( 100 mL) was added lithium chloride (3.83 g, 90.34 mmol),
sodium borohydride (3.42 g, 90.34 mmol), and ethanol (200 mL). After 20 hours,
saturated ammonium chloride (50 mL) was added and the reaction mixture
concentrated under vacuum. The resulting residue was dissolved in ethyl
acetate
and washed with saturated ammonium chloride, water, and brine. The organic
layer was dried over magnesium sulfate, filtered, and the filtrate
concentrated to
give the title compound (8.61 g): MSCI: m/z 220 (MH*).
b) Methanesulfonic acid 5-oxo-1-((S)-1-phenylethyl)pyrrolidin-3-ylmethyl
ester
To a solution of 4-hydroxymethyl-I-((S)-1-phenylethyl)pyrrolidin-2-one
(5.35 g, 24.39 mmol, Example 21 a) in dichloromethane (25 mL) at 0 °C
was
added triethylamine (4.42 mL, 31.7 mmol) and methanesulfonyl chloride (1.93
mL, 25.0 mmol). After 15 minutes the reaction mixture was warmed to room
temperature for 4 hours. The reaction mixture was diluted with dichloromethane
and washed with saturated sodium bicarbonate, water, and brine. The organic
layer was then dried over magnesium sulfate, filtered, and the filtrate
concentrated. The resulting residue was purified by flash silica gel column
chromatography (1:1 hexanes/ethyl acetate to ethyl acetate gradient) to give
the
title compound (7.31 g) as a yellow oil: MSCI: m/z 298 (MH+).
c) 4-Fluoromethyl-1-((S)-1-phenylethyl)pyrrolidin-2-one
To a solution of methanesulfonic acid 5-oxo-1-(1-phenylethyl)pyrrolidin
3-ylmethyl ester (7.30 g, 24.5 mmol, Example 21b) in tetrahydrofuran (100 mL)
was added tetrabutylammonium fluoride hydrate (9.64 g, 36.9 mmol). The
reaction mixture was refluxed for 12 hours, cooled to room temperature, and
concentrated. The resulting residue was purified by flash silica gel
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-208-
chromatography (ethyl acetate) to give the title compound (4.02 g) as a yellow
oil:
MSCI: m/z 222 (MHO).
d) (3S, 4R)- and (3R, 4S)-3-Benzyloxymethyl-4-fluoromethyl-1-((S)-1-
phenylethyl)pyrrolidin-Z-one
To a solution of 4-fluoromethyl-1-(1-phenylethyl)pyrrolidin-2-one (4.0 g,
18.17 mmol, Example 21c) in tetrahydrofuran (100 mL) under nitrogen
atmosphere at -78 °C was added lithium diisopropylamide (2 M in
heptane/tetrahydrofuran/ethylbenzene, 10.9 mL, 21.8 mmol) slowly over 15 min.
After I hour, benzyl chloromethyl ether (3.03 mL, 21.80 mmol) was added. After
30 minutes, the reaction mixture was warmed to room temperature for 1 hour.
The reaction mixture was diluted with ethyl acetate, washed with saturated
ammonium chloride, water, and brine. The organic layer was then dried over
magnesium sulfate, filtered, and the filtrate concentrated. The resulting
residue
was purified by flash silica gel chromatography (hexanes/ethyl acetate) to
provided the title compound (1.91 g) as a yellow oil: MSCI: m/z 342 (MH+).
e) (3S, 4R)- and (3R, 4S)-4-Fluoromethyl-3-hydroxymethyl-1-((S)-1-
phenylethyl)pyrrolidin-2-one
To a solution of (3S, 4R)- and (3R, 4S)-3-benzyloxymethyl-4-
fluoromethyl-1-((S)-1-phenylethyl)pyrrolidin-2-one (4.95 g, 14.5 mmol, Example
21 d) in methanol (50 mL) was added 20% palladium on carbon (0.5 g). Hydrogen
gas was introduced to the reaction mixture at high pressure (48 psi) for 2
hours,
then the reaction mixture was filtered through diatomaceous earth, washed with
methanol, and the combined filtrate concentrated under vacuum to afford the
title
compound (3.50 g) as an oil: MSCI: mlz 252 (MH+).
f7 [(3R, 4R)- and (3S, 4S)- 4-Fluoromethyl-1-((S)-1-phenylethyl)pyrrolidin-3-
yl]methanol
To a solution of (3S, 4R)- and (3R, 4S)-4-fluoromethyl-3-hydroxymethyl-
1-((S)-I -phenylethyl)pyrrolidin-2-one (3.50 g, l 3.93 mmol, Example 21 e) in
tetrahydrofuran (50 mL) was added lithium aluminum hydride (1M in
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-209-
tetrahydrofuran, 28 mL, 28 mmol) slowly over 10 min. The reaction mixture was
refluxed for 3 hours and cooled to room temperature. While stirring, water ( 1
mL), 1 S%~ sodium hydroxide solution (1 mL), and water (3 mL) were added.
After 1 S min, magnesium sulfate was added and the mixtui~e was filtered. The
S solid was washed with tetrahydrofuran and the combined filtrate concentrated
under vacuum to give the title compound (3.35 g) as a clear oil: MSCI: m/z 238
(MH+).
g) (3R, 4R)- and (3S, 4S)-4-Fluoromethyl-1-((S)-1-phenylethyl)pyrrolidin-3-
yl-methanesulfonic acid methyl ester
To a solution of [(3R, 4R)- and (3S, 4S)- 4-fluoromethyl-I-((S)-I-
phenylethyl)pyrrolidin-3-yI]methanol (3.35 g, 14.12 mmol, Example 21f) in
dichloromethane (2S mL) at 0 °C was added triethylamine (2.95 mL, 21.18
mmol)
and methanesulfonyl chloride ( 1.20 mL, 15.53 mmol). After 1 S minutes the
reaction mixture was warmed to room temperature for 4 hours. The reaction
mixture was then diluted with dichloromethane and washed with saturated sodium
bicarbonate, water, and brine. The organic layer was subsequently dried over
magnesium sulfate, filtered, and the filtrate concentrated. The resulting
residue
was purified by flash silica gel chromatography (1:l hexanes/ethyl acetate to
ethyl
acetate gradient) to give the title compound (3.41 g) as a yellow oil; MSCI:
m/z
316 (MH+).
h) (3R, 4R)- and (3S, 4S)-3-Azidomethyl-4-fluoromethyl-1-((S)-1-
phenylethyl)pyrrolidine
2S To a solution of (3R, 4R)- and (3S, 4S)-4-fluoromethyl-1-((S)-I-
phenylethyl)pyrrolidin-3-yl-methanesulfonic acid methyl ester (3.41 g, 10.8
mmol, Example 21g) in N,N-dimethylformamide (I0 mL) was added sodium
azide (2.81 g, 43.3 mmol). The reaction mixture was heated to 90 °C for
16
hours, cooled to room temperature, and diluted with ethyl acetate. The organic
mixture was washed twice with water and brine. The organic layer was dried
over
magnesium sulfate, filtered, and concentrated. The resulting residue was
purified
by flash silica gel chromatography (1:1 hexanes/ethyl acetate to ethyl acetate
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-210-
gradient) to give the title compound (1.93 g) as a yellow oil; MSCI: m/z 263
(MH+).
i) (3R, 4R)- and (3S, 4S)-3-Azidomethyl-4-0uoromethylpyrrolidine-1-
carboxylic acid benzyl ester
To a solution of (3R, 4R)- and (3S, 4S)-3-azidomethyl-4-fluoromethyl-1-
((S)-1-phenylethyl)pyrrolidine (1.93 g, 7.36 mmol, Example 21h) in 1,2-
dichloroethane (50 mL) was added benzyl chloroformate (1.57 mL, 11.04 mmol).
The reaction mixture was refluxed for 4 hours, cooled to room temperature, and
concentrated under vacuum. The resulting residue was then purified by flash
silica gel chromatography (1:1 hexaneslethyl acetate to ethyl acetate
gradient) to
give the title compound (2.58 g) as a oil: MSCI: m/z 293 (MH+).
j) C-((3S, 4R)- and (3R, 4S)-4-Fluoromethylpyrrolidin-3-yl)methylamine
To a solution of (3R, 4R)- and (3S, 4S)-3-azidomethyl-4-
fluoromethylpyrrolidine-1-carboxylic acid benzyl ester (2.58 g, 8.84 mmol,
Example 21i) in tetrahydrofuran (100 mL) was added 10°1o Pd/C
(0.39 g).
Hydrogen was introduced to the reaction mixture at high pressure (48 psi) for
5
days, then the reaction mixture was filtered through Celite, washed with
methanol,
and the combined filtrate concentrated under vacuum to afford the title
compound
(1.36 g) as an oil: MSCI: m/z 133 (MH+)
k) 7-((3S, 4R)- and (3R, 4S)-3-Aminomethyl-4-fluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione
Utilizing the same procedure as described in Example 19f, from reaction
of I-cyclopropyl-6,7-difluoro-8-methyl-1H quinazolinedione (0.26 g, I.03
mmol),
G((3S, 4R)- and (3R, 4S)-4-fluoromethylpyrroIidin-3-yl)methylamine (0.27 g,
2.06 mmol, Example 21i), and 1,1,3,3-tetramethylguanidine (0.25 mL, 2.06
mmol) afforded the title compound (0.0073 g); mp 175-178 °C: MSCI: m/z
365
(MH+).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-211-
Example 22
a) 4-Bromo-2,5-difluoro-3-methylbenzoic acid
An ice-chiIIed solution of acetonitrile (40 mL) and tart-butyl nitrite (5.9
mL, 49.6 mmol) was treated with copper(II) bromide (8.9 g, 39.8 mmol) and
allowed to stir. After 15 minutes, a solution of 4-amino-2,5-difluoro-3-
methylbenzoic acid [PCT Int. Appl. Ser. No. WO 96/05192 A1](6.2 g, 33.1
mmol) in acetonitrile (300 mL) was added by addition funnel, and the reaction
mixture warmed to room temperature and stirred for 19 hours. The mixture was
concentrated in vacuo, and the resulting residue dissolved in ethyl acetate
and
washed with 1 N hydrochloric acid, water and brine. The combined organic
layers were dried over magnesium sulfate, fltered, and concentrated in vacuo
to
afford the title compound (6.8 g): 'H NMR (400 MHz, DMSO-d6) b 13.62 (bs,
1 H), 7.61 (dd, 1 H), 2.33 (d, 3H).
I S b) 4-Bromo-2,5-difluoro-3-methylbenzamide
To a solution of 4-bromo-2,5-difluoro-3-methylbenzoic acid (6.8 g, 27
mmol, Example 22a) in dichloromethane (90 ml) was added oxalyl chloride (3.5
mL, 40 mmol) and 10 drops of N,N-dimethylformamide. The reaction mixture
was stirred for 15 hours at room temperature and concentrated in vacuo. The
resulting residue was dissolved in dichloromethane (100 mL) and concentrated
in
vacuo. The residue was re-dissolved in dichloromethane, cooled to 0°C
and
ammonia gas bubbled through the solution for 15 minutes. The mixture was
allowed to warm to room temperature and stirred for l hour. The mixture was
partitioned between aqueous saturated sodium bicarbonate and ethyl acetate.
The
aqueous phase was extracted with ethyl acetate. The combined organic layers
were dried over magnesium sulfate, filtered, and concentrated in vacuo to
afford
the title compound (6.8 g): MSCI: mlz 2S0 (MH+);'H NMR (400 MHz, CDCI3)
8 7.82 (bs, 1 H), 7.77 (bs, 1 H), 7.44 (dd, I H), 2.33 (d, 3H).
c) 1-(4-Bromo-2,5-difluoro-3-methylbenzoyl)-3-cyclopropylurea
To a solution of 4-bromo-2,5-difluoro-3-methylbenzamide (6.8 g, 27.2
mmol, Example 22b) in l, 2-dichloroethane (60 mL) was added oxalyl chloride
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-212-
(4.7 mL, 53.9 mmol) and the resulting mixture heated at 90 °C for 2
hours. The
reaction mixture was then cooled to room temperature, and concentrated in
vacuo.
The resulting residue was dissolved in dichloromethane (50 mL), concentrated
in
vacuo and re-dissolved in dichloromethane (50 mL), cooled to 0 °C and
treated
with cyclopropylamine (2.8 mL, 40.4 mmol). The reaction mixture was warmed
to room temperature for 1 hour and partitioned between ethyl acetate and
saturated
sodium bicarbonate. The aqueous phase was extracted with ethyl acetate. The
combined organics layers were dried over magnesium sulfate, filtered, and
concentrated in vacuo to obtain the title compound (8.0 g): IH NMR (400 MHz,
CDCl3) ~ 8.59 (bs, 1 H), 8.46 (bs, 1 H), 7.60 (m, 1 H), 2.76 (m, 1 H), 2.41
(d, 3H),
0.81. (m, 2H), 0.62 (m, 2H).
d) 7-Bromo-1-cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione
To a solution of 1-(4-bromo-2,5-difluoro-3-methylbenzoyl)-3-
cyclopropylurea (3.8 g, I 1.4 mmol, Example 22c) in tetrahydrofuran (40 mL) at
0
°C was added potassium bis(trimethylsilyl)amide (57 mL, 28.5 mmol, 0.5
M in
toluene) over 15 minutes. The reaction mixture was warmed to room temperature,
and 18-crown-6 (1.30 g, 4.92 mmol) added. The mixture was heated at 80
°C for
3 hours, cooled to room temperature, diluted with ethyl acetate, and washed
with 1
N hydrochloric acid. The aqueous phase was extracted with ethyl acetate and
the
combined organic layers dried over magnesium sulfate, filtered, and
concentrated
in vacuo. The residue was purified by flash silica gel chromatography (hexanes
to
40:60 hexaneslethyl acetate gradient) to afford the title compound (2.0 g);
MSCI:
m1z 3 I 3 (MH+); 'H NMR (400 MHz, CDCI3) b 8.42 (bs, 1 H), 7.71 (d, I H), 3.40
(m, 1 H), 2.73 (s, 3H), 1.18 (m, 2H), 0.63 (m, 2H).
e) 4-Bromothiophene-2-carbaldehyde O-benzyloxime
To a solution of 4-bromothiophene-2-carboxaldehyde (10.3 g, 53.9 mmol)
in ethanol (100 mL) was added O-benzylhydroxylamine hydrochloride (13.0 g,
81.4 mmol) followed by pyridine (7.0 mL). The reaction mixture was heated at
$0 °C for 20 hours, cooled to room temperature and concentrated in
vacuo. The
resulting residue was partitioned between water and ethyl acetate. The aqueous
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-2I3-
phase was extracted with ethyl acetate (3 times), the combined organic layers
washed with brine, dried over magnesium sulfate, filtered and concentrated in
vacuo. Purification by flash silica gel chromatography (hexanes to 50:50
hexanes/ethyl acetate gradient) afforded the title compound (16 g) as a yellow
liquid; MSCI: rnlz 296 (MHO).
t7 C-(4-Bromothiophen-2-yl)methylamine
To a solution of 4-bromothiophene-2-carbaldehyde O-benzyloxime (8.0 g,
27 mmol, Example 22e) in tetrahydrofuran (20 mL) was added borane-
tetrahydrofuran complex (60 mL, 60 mmol, 1.0 M in tetrahydrofuran), and the
mixture heated at 70 °C for 20 hours. The reaction mixture was cooled
to room
temperature and 1 N sodium hydroxide added. The mixture was extracted with
ethyl acetate (3 times), the organic layers combined and washed with brine,
dried
over magnesium sulfate, filtered, and concentrated in vacuo to afford the
title
compound; MSCI: m/z 192 (MH+).
g) (4-Bromothiophen-2-ylmethyl)carbamic acid tart-butyl ester
To a solution of C-(4-bromothiophen-2-yl)methylamine (5.2 g, 27.1 mmol,
Example 22f) in dichloromethane (100 mL) was added di-tart-butyl Bicarbonate
(8.8 g, 40.3 mmol) followed by triethylamine (15 mL). After 4 hours, 1 N
hydrochloric acid was added and the mixture extracted with dichloromethane.
The combined organic layers were washed with brine, dried over magnesium
sulfate, filtered and concentrated in vacuo. Purification by flash silica gel
chromatography (hexanes to 80:20 hexanes/ethyl acetate gradient) affords the
title
compound (3.8 g): ' H NMR (CDC13) 8 7.10 (s, 1 H), 6.86 (s, 1 H), 4.92 (bs, 1
H),
4.42 (m, 2H), 1.46 (s, 9H).
h) (4-Tribntylstannylthiophen-2-ylmethyl)carbamic acid, tent-butyl ester
To a solution of (4-bromothiophen-2-ylmethyl)carbamic acid tart-butyl
ester (2.1 g, 7.I9 mmol, Example 22g) in diethyl ether (15 mL) at -78
°C was
added methyllithium (5.1 mL, 7.14 mmol, 1.4 M in diethyl ether). After 20
minutes, n-butyllithium (14 mL, 22.4 mmol, 1.6 M in hexanes) was added, and
the
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-214-
mixture stirred for 1 hour then treated with tributyltin chloride (7.8 mL,
28.8
mmol). After 3 hours, the mixture was warmed to room temperature and
partitioned between ethyl acetate and water. The aqueous layer was extracted
with ethyl acetate and the organic layers combined, washed with brine, dried
over
magnesium sulfate, filtered and concentrated in vacuo. Purification by flash
silica
gel chromatography (1:99 triethylamine/hexanes to 1:50:19 triethylamine/
hexanes/ ethyl acetate gradient) afforded the title compound (1.8 g): 'H NMR
(400 MHz, CDCI3) 8 7.19 (s, 1H), 6.94 (s, 1 H), 4.84 (bs, 1 H), 4.51 (bs, 2H),
1.59-
1.41 (m, 15H), 1.40-1.28 (m, 6H), 1.05-0.92 (m, 6H), 0.89 (m, 9H).
i)[4-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)-thiophen-2-ylmethyl]carbamic acid tent-butyl ester
To a slurry of 7-bromo-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione (0.250 g, 0.798 mmol, Example 22d) in toluene (2 mL) was
added tris(dibenzylideneacetone)dipalladium(0) (0.150 g, 0.164 mmol) and
triphenylarsine (0.200 g, 0.653 mmol). After 10 minutes, (4-
tributylstannylthiophen-2-ylmethyl)carbamic acid tart-butyl ester (1.00 g,
1.99
mmol, Example 22h) in toluene (3 mL) was added and the mixture heated at 110
°C for 24 hours. The mixture was then cooled, diluted with ethyl
acetate and
poured into 10% aqueous potassium fluoride. After 1.5 hours, the mixture was
filtered through diatomaceous earth. The recovered organics were washed with
brine, dried over magnesium sulfate, filtered and concentrated in vacuo. The
residue was triturated with hexanes and the resulting solid purified by flash
silica
gel chromatography (dichloromethane to 50:50 dichloromethane:ethyl acetate
gradient) to afford the title compound (0.191 g); MSCI: m1z 446 (MH+);'H
NMR (400 MHz, CDCl3) b 8.53 (bs, l H), 7.68 (d, l H), 7.21 (s, 1 H), 6.93 (s,
1 H),
4.98 (bs, 1 H), 4.5 I (bs, 2H), 3.36 (m, 1 H), 2.43 (s, 3H), 1.46 (s, 9H), 1.
l 8 (m,
2H), 0.80 (m, 2H).
j) 7-(5-Aminomethylthiophen-3-yl)-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazoline- 2,4-dione hydrochloride
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-215-
Hydrogen chloride gas was bubbled into a cooled solution (0 °C) of
[4-(I-
cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-yl)-thiophen-
2-ylmethyl]carbamic acid tart-butyl ester (0.191 g, 0.429 mmol, Example 22i)
in
methanol ( 10 mL). The reaction mixture was warmed to room temperature and
stirred for 20 hours. The mixture was then concentrated in vacuo, and the
resulting solid washed with hexanes and dried to afford the title compound
(0.109
g); mp 205-208 °C: 'H NMR (400 MHz, DMSO-d6) 8 8.37 (bs, 3H), 7.71 (s,
IH),
7.53 (d, 1 H), 7.30 (s, 1 H), 4.28 (bs, 2H), 3.30 (m, 1 H), 2.36 (s, 3H), 1.02
(m, 2H),
0.60 (m, 2H).
Example 23
a) 7-[4-(tart-ButyldimethyIsilanyloxy)-S,5-difluoro-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione
To a slurry of 7-bromo-1-cyclopropyl-6-fluoro-8-methyl-1H
quinazolinedione (1.03 g, 3.29 mmol, Example 22d) in toluene (8 mL) was added
tris(dibenzylideneacetone)dipalladium(0) (0.301 g, 0.332 mmol) and
triphenylarsine (0.403 g, I.32 mmol). After I O min, tart-butyl(5,5-difluoro-2-
tributylstannyl-4,5,6,7-tetrahydrobenzo[b]thiophen-4-yloxy)dimethylsilane (3.9
g,
6.57 mmol, [WO 01/32655]) in toluene (6 mL) was added and the resulting slurry
heated at I 10 °C for 20 hours. The mixture was cooled, diluted with
ethyl
acetate, and poured into 10% aqueous potassium fluoride. After 3 hours, the
mixture was filtered through diatomaceous earth. The recovered organics were
washed with brine, dried over magnesium sulfate, filtered, and concentrated in
vacuo. The residue was triturated with hexanes and the resulting solid
purified by
flash silica gel chromatography (hexanes to 50:50 hexanes/ethyl acetate
gradient)
to afford the title compound (1.5 g): MSCI: m/z 537 (MH+);'H NMR (400 MHz,
CDC13) 8 8.13 (s, 1 H), 7.71 (d, 1 H), 6.87 (s, 1 H), 4.77 (m, 1 H), 3.37 (m,
1 H),
3.06 (m, 2H), 2.52 (s, 3H), 2.55-2.46 (m, 1 H), 2.27 (m, 1 H), 1.23-1.13 (m,
2H),
0.91 (s, 9H), 0.71 (m, 2H), 0.19 (s, 3H), 0.18 (s, 3H).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-216-
b) 1-Cyclopropyt-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-6-fluoro-8-methyl-1H-quinazolinedione
To a cooled solution (0 °C) of 7-[4-(tart-butyldimethylsilanyloxy)-
5,5-
difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl]-1-cyclopropyl-6-fluoro-8
methyl-IH quinazolinedione (0.70 g, 1.30 mmol, Example 23a) in tetrahydrofuran
(13 mL) was added tetrabutylammonium fluoride (5.2 mL, 5.20 mmol, 1M in
tetrahydrofuran). After 1 hour, the reaction mixture was warmed to room
temperature and partitioned between ethyl acetate and saturated ammonium
chloride. The combined organic extracts were washed with brine, dried over
magnesium sulfate, filtered, and concentrated ira vacuo. The resulting residue
was
purified by flash silica gel chromatography (hexanes to ethyl acetate
gradient) to
afford the title compound (0.48 g): MSCI: m/z 423 (MH+); 1H NMR (400 MHz,
CDCI3) 8 8.27 (s, 1H), 7.70 (dd, 1H), 7.02 (s, 1H), 4.83 (m, 1H), 3.35 (m,
1H),
3.07 (m, 2H), 2.52 (s, 3H), 2.48 (m, 2H), 2.31 (m, 1H), 1.18 (m, 2H), 0.69 (m,
2H).
Example 24
a) 2-(1-Cyclopropyl-6-f7uoro-8-methyldioxo-1,2,3,4-tetrahydro-quinazolin-7-
yl)-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-4-yl phosphoric acid
Biphenyl ester
To a solution of 1-cyclopropyl-7-(5,5-difluoro-4-hydroxy-4,5,6,7-
tetrahydro-benzo[b]thiophen-2-yl)-6-fluoro-8-methyl-1H-quinazolinedione (0.80
g, 1.89 mmol, Example 23b) in dichlorornethane (35 mL) was added
diphenylphosphoryl azide (0.81 mL, 3.76 mmol) followed by 1,8-
diazabicyclo[5.4.0]undec-7-ene (0.65 mL, 4.35 mmol). After 24 hours, the
reaction mixture was partitioned between ethyl acetate and saturated ammonium
chloride. The organics were washed with brine, dried over magnesium sulfate,
filtered, and concentrated in vacuo. The resulting residue was purified by
flash
silica gel chromatography (hexanes to ethyl acetate gradient) affording the
title
compound (0.73 g); MSCI: m~z 655 (MH+);'H NMR (400 MHz, CDC13) 8 8.03
(s, 1 H), 7.73 (d, 1 H), 7.34 (m, 2H), 7.24-7.18 (m, SH), 7.l 2-7.04 (m, 3H),
6.90 (s,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-217-
1 H), 5.66 (m, 1 H), 3.38 (m, 1 H), 3.09 (m, 2H), 2.50-2.36 (m, 2H), 2.40 (s,
3H),
1.11 (m, 2H), 0.63 (m, 2H).
b) 7-(4-Azido-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
S cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione
To a solution of phosphoric acid 2-(1-cyclopropyl-6-fluoro-8-
methyldioxo-1,2,3,4-tetrahydroquinazolin-7-yl)-S,S-difluoro-4,5,6,7-
tetrahydrobenzo[b]thiophen-4-ylphosphoric acid diphenyl ester (0.73 g, 1.12
mmol, Example 24a) in dimethyl sulfoxide (11 mL) was added sodium azide (0.72
g, I 1.1 mmol) and the reaction mixture heated at 8S °C for 22 hours.
The reaction
mixture was cooled to room temperature, partitioned between water and ethyl
acetate and extracted with ethyl acetate. The combined extracts were dried
over
magnesium sulfate, filtered, and concentrated in vacuo. The resulting residue
was
purified by flash silica gel chromatography (hexanes to SO:SO ethyl
1S acetate/hexanes gradient) to afford the title compound (0.46 g); MSCI: m/z
448
(MH+); ! H NMR (400 MHz, CDCl3) S 8.48 (s, 1 H), 7.72 (d, 1 H), 6.95 (s, 1 H),
4.63 (m, 1 H), 3.36 (m, 1 H), 3.10 (m, 2H), 2.S 1 (s, 3H), 2.49-2.33 (m, 2H),
1.19
(m, 2H), 0.70 (m, 2H).
c) [2-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-4-yl]carbamic acid tert-
butyl ester
To a solution of 7-(4-azido-S,S-difluoro-4,5,6,7-
tetrahydrobenzo[b]thiophen-2-yl)-1-cyclopropyl-6-fluoro-8-methyl-1H-
2S quinazolinedione (0.S0 g, 1.12 mmol, Example 24b) in ethanol (10 mL) and
dichloromethane (3 mL) was added palladium hydroxide (0.1S8 g, 0.225 mmol,
20 wt. % on carbon), di-tart-butyl dicarbonate (1.2 g, S.S mmol), and
triethylsilane
(1.44 mL, 9.02 mmol). After 20 hours, the reaction mixture was filtered
through
diatomaceous earth. The recovered organics were washed with water, brine,
dried
over magnesium sulfate, filtered, and concentrated in vacuo. The resulting
residue
was then purified by flash silica gel chromatography (hexanes, then SO:SO
hexanes:ethyl acetate gradient) to afford the title compound (0.34 g) as a
white
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-218-
solid: MSCI: rr~lz 522 (MH+); 1H NMR (400 MHz, CDC13) 8 8.51 (s, 1H), 7.69
(d, J = 8.3, 1 H), 6.86 (s, 1 H), 5.21 (m, 1 H), 5.05 (m, 1 H), 3.35 (m, 1 H),
3.05 (m,
2H), 2.49 (s, 3H), 2.45 (m, 2H), 1.47 (s, 9H), 1.17 (m, 2H), 0.68 (m, 2H).
d) 7-(4-Amino-5,5-difluoro-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione hydrochloride
Hydrogen chloride gas was bubbled into a cooled solution (0 °C) of
[2-(1-
cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-yl)-5,5-
difluoro-4,5,6,7-tetrahydro-benzo[b]thiophen-4-yl]carbamic acid tert-butyl
ester
(0.37 g, 0.713 mmol, Example 24c) in a mixture of methanol (5 mL) and
dichloromethane (5 mL) for 15 minutes. The reaction mixture was then warmed
to room temperature and stirred for 3 hours. The mixture was concentrated in
vacuo, and the resulting solid triturated with hexanes and dried to provide
the title
compound (0.308 g); mp 245-250 °C: aH NMR (400 MHz, DMSO- d6) S 11.53
(s, 1 H), 9.11 (bs, 2H), 7.56 (d, 1 H), 7.43 (s, 1 H), 5.01 (m, 1 H), 3.30 (m,
1 H),
3.09-3.02 (m, 2H), 2.63-2.50 (m, 2H), 2.44 (s, 3H), 1.03 (m, 2H), 0.60 (m,
2H);
MSCI: mlz 422 (MH+).
Example 25
a) 2,4,5-Trifluoro-3-methylbenzoic acid methyl ester
A solution of 2,4,5-trifluoro-3-methylbenzoic acid (32 g, 0.17 mol,
[Japanese Appl. JP 95-219069]) in methanol (1000 mL) was cooled to 0 °C
and
saturated with hydrogen chloride gas. The resulting solution was stirred at
room
temperature for 1 hour, then refluxed overnight. The solvent was removed in
vacuo and the residue was partitioned between ether ( 1000 mL) and water (200
mL). The organic layer was separated, washed with brine, dried over sodium
sulfate, and concentrated. The residue was purred by column chromatography
(1:9 ethyl acetate/hexanes) to afford the title compound (32.4 g): 'H NMI~
(200
MHz, CDCl3) 8 7.62 (m, 1H), 3.93 (s, 3H), 2.27 (m, 3H).
b) 4-Benzylamino-2,5-dit7uoro-3-methylbenzoic acid methyl ester
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-219-
A solution of 2,4,5-trifluoro-3-methylbenzoic acid methyl ester (26.5 g,
130 mmol, Example 25a), benzylamine (27.8 g, 260 mmol), and triethylamine
(65.6 g, 650 mmol) in 250 mL of dimethylsulfoxide was heated at 100 °C
for 18
hours, then cooled to room temperature. Ethyl acetate (1000 mL) and water (200
mL) were added, and the organic layer was separated, washed with brine, dried
over sodium sulfate, and concentrated. The residue was purified by column
chromatography (1:6 ethyl acetate/hexanes) to afford the title commpound (30.2
g):'H NMR (200 MHz, CDCI3) 8 7.45 (dd, 1H), 7.32 (m, 5H), 4.59 (s, 2H), 4.05
(bs, 1 H), 3.87 (s, 3H), 2.10 (d, 3H).
c) 4-Amino-2,5-ditluoro-3-methylbenzoic acid methyl ester
A suspension of 20°lo palladium on carbon (20 g), 4-benzylamino-
2,5-
difluoro-3-methylbenzoic acid methyl ester (24.7 g, 0.085 mol, Example 25b),
ammonium formate (26.8 g, 0.425 mol) and methanol (500 mL) was heated at
reflux for 4 hours. The reaction mixture was cooled to room temperature,
filtered
though diatomaceous earth and the solvent removed in vacuo to give a solid
which
was recrystallized from ethyl acetate/hexanes to afford the title compound
(14.6
g): ' H NMR (200 MHz, CDC13) 8 7.47 (dd, 1 H), 4.23 (bs, 1 H), 3.87 (s, 3H),
2.10
(d, 3H).
d) Z,5-Difluoro-4-iodo-3-methylbenzoic acid methyl ester
A room temperature suspension of 4-amino-2,5-difluoro-3-methylbenzoic
acid methyl ester (S.0 g, 25.0 mmol, Example 25c) and CuI (7.0 g, 37.5 mmol)
in
acetonitrile (250 mL) was treated dropwise with isoamyl nitrite (5.85 g, 50.0
mmol). The mixture was stirred at room temperature for 1 hour, then heated to
50
°C for 1 hour. The solvent was removed in vacuo and the residue was
dissolved in
ethyl acetate (500 mL), washed with 1N hydrochloric acid (50 mL) and brine (2
x
50 mL). After drying over sodium sulfate and concentrating in vacuo, the
residue
was purified by column chromatography (1:10 ethyl acetate/hexanes) to afford
the
title compound (7.2 g);'H NMR (200 MHz, CDC13) 8 7.47 (dd, 1H), 3.93 (s, 3H),
2.46 (d, 3H).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-220-
e) 2,5-Difluoro-4-iodo-3-methylbenzoic acid
A solution of 2,5-difluoro-4-iodo-3-methylbenzoic acid methyl ester (3.74
g, I2.0 mmol, Example 25d) in a mixture of 2 N sodium hydroxide (50 mL)~and
methanol (50 mL) was heated at 60 °C for 2 hours, then cooled to room
temperature. The methanol was removed in vacuo and the solution acidified with
2 N HCI to pH 3. The white precipitate was collected by filtration, washed
with
water and dried to give the title compound as a white solid (3.3 g).'H NMR
(200
MHz, CDC13) 8 13.57 (bs, 1H), 7.50 (dd, 1H), 2.39 (d, 3H).
f7 2,5-Difluoro-4-iodo-3-methylbenzamide
A mixture of 2,5-difluoro-4-iodo-3-methylbenzoic acid (2.98 g, 10 mmol,
Example 25e) and oxalyl chloride (1.52 g, 12 mmol) in 20 mL of dichloromethane
was treated with 2 drops of dimethyl formamide, and stirred at room
temperature
for 2 hours. The mixture was concentrated in vacuo and the residue dissolved
in
dry tetrahydrofuran ( 10 mL). This solution was slowly added to a - 78
°C solution
of diethyl ether (40 mL) saturated with gaseous ammonia. After the addition,
the
mixture was warmed to room temperature and stirred for 30 minutes. Ethyl
acetate
(100 mL) and water (20 mL) were added and the organic layer washed with brine,
dried over sodium sulfate and concentrated in vacuo providing 2.97 g of the
title
compound as a white solid. 'H NMR (200 MHz, CDC13) 8 7.68 (dd, 1H), 6.69 (bs,
I H), 6.01 (bs, 1 H), 2.48 (d, 3H).
g) 1-Cyclopropyl-3-(2,5-ditluoro-4-iodo-3-methylbenzoyl)urea
A room temperature solution of 2,5-difluoro-4-iodo-3-methylbenzamide
(2.97 g, 10 mmol, Example 25f) in 1,2-dichloroethane (20 mL) was treated
dropwise with oxalyl chloride (3.80 g, 30 mmol). The mixture was stirred at
room
temperature for 1 hour, refluxed for 4 hours and concentrated in vacuo. The
residue was dissolved in 40 mL of dioxane, cooled to 5 °C and treated
dropwise
with a solution of cyclopropylamine (1.14 g, 20 mmol) in dioxane (10 mL). The
mixture was warmed slowly to room temperature, stirred for 3 hours and the
solvent removed in vacuo. The residue was purified by column chromatography
(I: I00 ethyl acetate/chloroform) to provide the title compound (2.98 g). 'H
NMR
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-221-
(200 MHz, CDC13) 8 8.58 - 8.48 (m, 2H), 7.56 (dd, IH)~, 2.78 (m, 1H), 2.49 (d,
3H), 0.83 (m, 2H), 0.65 (m, 2H).
h) 1-Cyclopropyl-6-fluoro-7-iodo-8-methyl-1H-quinazolinedione
To a 0 °C solution of 1-cyclopropyl-3-(2,5-difluoro-4-iodo-3-
methylbenzoyl)urea (8.39 g, 22.1 mmol, Example 25g) in tetrahydrofuran (100
mL) and dimethylformamide (5 mL) was added, portionwise, sodium hydride
(1.86 g, 77.3 mmol, 60% dispersion in mineral oil). The mixture was stirred at
room temperature for 30 minutes, then refluxed 18 hours. After cooling, the
mixture was poured onto ice, and the resulting solution acidified with 1N
hydrochloric acid to pH 5. After extracting with ethyl acetate (500 mL), the
organic layer was washed with brine, dried over sodium sulfate and
concentrated
in vacuo. The residue was purified by column chromatography (1:4 ethyl
acetate/chloroform) to afford the title compound (4.20 g). 'H NMR (200 MHz,
CDC13) 8 8.64 (bs, 1H), 7.62 (d, 1H), 3.40 (m, 1H), 2.79 (s, 3H), 1.18 (m,
2H),
0.61 (m, 2H).
i) 5,6-Dihydrocyclopenta[b]thiophen-4-one oxime
A mixture of 5,6-dihydrocyclopenta[b]thiophen-4-one (11.7 g, 85 mmol,
(Russ. J. Org. Cheyn.1998, 34(7), 1019]), hydroxylamine hydrochloride (9.03 g,
0.13 mol) and methanol (150 mL) was heated at 70 °C overnight. The
solvent was
removed in vacuo and the residue was dissolved in ethyl acetate (500 mL),
washed with water, dried with sodium sulfate and concentrated in vacuo. The
residue was purified by column chromatography ( I :3 ethyl acetate/hexanes) to
2S afford the title compound (10.7 g).'H NMR (400 MHz, DMSO-d6) b 10.42 (s,
1 H), 7.56 (d, 1 H), 7.00 (d, 1 H), 3.12 (m, 2H), 3.02 (m, 2H); MSCI: mlz 154
(MH+)
j) 5,6-Dihydro-4H-cyclopenta[b]thiophen-4-ylamine
A mixture of 5,6-dihydrocyclopenta[b]thiophen-4-one oxime (10.0 g,
0.065 moI, Example 25i) and borane tetrahydrofuran complex (650 mL, 0.65 mol,
1 M in tetrahydrofuran) was refluxed for 18 hours. The reaction mixture was
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-222-
acidified with 4 N HCl and stirred at 70 °C for 1 hour. After cooling
to room
temperature, the mixture was washed with diethyl ether, and the aqueous phase
was adjusted to pH 10 with 2N sodium hydroxide and extracted with ethyl
acetate.
The organic extracts were washed with brine, dried over sodium sulfate and
concentrated in vacuo. The residue was then purified by column chromatography
(9:1 dichloromethanelmethanol) to afford the title compound (3.0 g). 1H NMR
(400 MHz, DMSO-d6) 8 7.34 (d, 1 H), 6.92 (d, 1 H), 4.18 (m, 1 H), 3.30 (bs,
2H),
2.92 (m, 1 H), 2.73 (m, 2H), 1.98 (m, 1 H).
k) (5,6-Dihydro-4H-cyclopenta[b]thiophen-4-yl)tritylamine
A mixture of S,6-dihydro-4H cyclopenta[b]thiophen-4-ylamine (2.54 g,
18.3 mmol, Example 25j), triphenylmethyl chloride (5.60 g, 20.1 mmol),
triethylamine (2.77 g, 27.4 mmol) and dichloromethane (1S0 mL) was stirred at
room temperature for 18 hours. The mixture was diluted with dichloromethane
(200 mL), washed with brine, dried with sodium sulfate and concentrated in
vacuo. The residue was purified by column chromatography ( 1:30 ethyl
acetate/hexanes) to afford the title compound (6.90 g).'H NMR (400 MHz,
CDC13) $ 7.65 - 7.20 (m, 1 SH), 7.08 (d, 1 H), 6.39 (d, 1 H), 4.18 (m, 1 H),
2.76 (m,
1 H), 2.52 (m, 1 H), 2.00 (m, 1 H), 1.96 (bs, 1 H), 1.68 (m, 1 H).
I) I-Cyclopropyl-6-fluoro-8-methyl-7-[4-(tritylamino)-5,6-dihydro-4H-
cyclopenta[b]thiophen-2-yl]-1H-quinazolinedione
A - 78 °C solution of (S,6-dihydro-4H cyclopenta[b]thiophen-4
yl)tritylamine (1.6 g, 4.2 mmol, Example 2Sk) in tetrahydrofuran (SO mL) was
2S treated dropwise with n-butyllithium (4.2 mL, 10.5 mmol, 2.5 M in hexane),
warmed to - 10 °C and stirred for 3 hours. The reaction was cooled to -
78 °C,
treated dropwise with a solution of n-tributyltin chloride (1.64 g, 5.04 mL)
in
tetrahydrofuran (S mL) and allowed to warm to room temperature. After
partitioning between ethyl acetate and water, the aqueous layer was extracted
with
ethyl acetate and the organic layers combined and washed with brine, dried
over
sodium sulfate and concentrated in vacuo. The resulting stannane was used
immediately without purification by dissolving it in toluene (100 mL) and
treating
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-223-
with 1-cyclopropyl-6-fluoro-7-iodo-8-methyl-1H-quinazolinedione (0.49 g, 1.36
mmol, Example 25h), triphenylarsine (0.165 g, 0.54 mmol),
dichlorobis(triphenylphosphine)palladium(II) (0.098 g, 0.14 mmol), and
copper(I)
iodide (0.027 g, 0.14 mmol). The mixture was heated under a nitrogen
atmosphere at 95 °C for 24 hours., then cooled to room temperature. The
mixture
was treated with ethyl acetate (500 mL) and 15% potassium fluoride (20 mL) and
stirred at room temperature for 1 hour, then filtered though Celite. The
aqueous
layer was extracted with ethyl acetate (2 x 100 mL) and the combined organic
extracts washed with brine, dried over sodium sulfate and concentrated in
vacuo.
The residue was purified by column chromatography (3:7 ethyl acetate/hexanes)
to afford the title compound (0.48 g);'H NMR (400 MHz, CDC13) 8 8.22 (bs,
1 H), 7.76 (d, 1 H), 7.68 - 7.18 (m, 15H), 6.10 (s, 1 H), 4.24 (m, 1 H), 3.40
(m~ 1 H),
2.88 (m, 1 H), 2.62 (m, 1 H), 2.49 (s, 3H), 2.18 (m, 1 H), 1.86 (m, 1 H), 1.24
(m,
2H), 0.76 (m, 2H).
m) 7-(4-Amino-5,6-dihydro-4H-cyclopenta[b]thiophen-2-yl)-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione hydrochloride
Hydrogen chloride gas was bubbled through a 0 °C solution of 1-
cyclopropyl-6-fluoro-8-methyl-7-[4-(tritylamino)-5,6-dihydro-4H-
cyclopenta[b]thiophen-2-ylJ-1H-quinazolinedione (0.48 g, 0.78 mmol, Example
251) in diethyl ether (80 mL) and methanol (40 mL) for 30 minutes. After
stirring
at room temperature overnight, the solvent was removed in vacuo and the
residue
chromatographed on a silica gel column eluting with dichloromethane/methanol
(85:15) to give the title compound (0.21 g): mp 233 - 235 °C; iH NMR
(400
MHz, DMSO-d6) 8 7.59 (d, 1 H), 7.17 (s, 1 H), 4.56 (m, 1 H), 3.36 (m, 1 H),
3.15
(m, 1 H), 2.96 (m, 1 H), 2.82 (m, 1 H), 2.48 (s, 3H), 2.24 (m, 1 H), 1.04 (m,
2H),
0.62 (m, 2H). MSCI: m/z 372 (MH+), 355 (MH+ - NH3).
Example 26
a) {1-[(R)-1-(1-cyclopropyl-6-Fluoro-8-methyldioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)pyrrolidin-3-yl]cyclopropyl}carbamic acid tert-
butyl ester
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-224-
A solution of 1-cyclopropyl-6,7-difluoro-8-methyl-1H-quinazolinedione
(0.50 g, 2.0 mmol), ((R)-I-pyrrolidin-3-yIcyclopropyI)carbamic acid tart-butyl
ester (0.38 g, 1.7 mmol [US Pat. 5,849,757]), 1,1,3,3-tetramethylguanidine
(0.39
g, 3.4 mmol) and dirnethyl sulfoxide (0.7 mL) was heated in a sealed tube at
75-80
°C for 80 hours. The mixture was cooled, diluted with water and
extracted with
ethyl acetate. The combined organic extracts were dried with sodium sulfate,
filtered and concentrated in vaeuo. The residue was purified by preparative
thin
layer chromatography (8 : 92 methanol/dichloromethane) to afford the title
compound (0.23 g): 'H NMR (400 MHz, CDCl3) cS 9.00 (bs, 1H), 7.50 (d, I H),
5.05 (bs, 1 H), 3.70-3.58 (m, 1 H), 3.55-3.46 (m, 1 H), 3.40-3.26 (m, 3H),
2.35 (s,
3H), 1.81-1.68 (m, 1H), 1.50 (m, 2H), 1.41 (s, 9H), 1.25-1.18 (m, 1H), 1.11-
1.00
(m, 1 H), 0.90-0.51 (m, 6H).
b) 7-[(R)-3-(1-Aminocyclopropyl)pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride
Hydrogen chloride gas was bubbled through a 0 °C solution of { I-
[(R)-1-
( 1-cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)pyrrolidin-3-yl]cyclopropyl}carbamic acid tent-butyl ester (0.23 g, 0.51
mmol,
Example 26a) in anhydrous diethyl ether (IS mL) for 10 minutes. The resulting
suspension was slowly warmed to room temperature and stirred for 5 hours. The
solid was removed by filtration, washed with dichloromethane (10 mL) and dried
in vacuo to afford the title compound (0.12 g): mp 202-203 °C;'H NMR
(400
MHz, DMSO-d6) b 8.52 (bs, 3H), 7.38 (d, l H), 3.60-3.50 (m, 1 H), 3.45-3.10
(m,
4H), 2.70-2.60 (m, I H), 2.35 (s, 3H), 2.10-1.96 (m, 1 H), I .70-1.60 (m, 1
H), 1.10-
0.82 (m, 6H), 0.60-0.45 (m, 2H).
Example 27
a) 4,5,6,7-Tetrahydrobenzo(b]thiophen-7-yl)carbamic acid tart-butyl ester
Di-tart-butyldicarbonate (0.79 g, 3.6 mmol) was added to a room
temperature solution of 4,5,6,7-tetrahydrobenzo[b]thiophen-7-ylamine (0.37 g,
2.4
mmol [Eur. J. Med. Chem. Chim. Ther. 1998, 33,. 867], triethylamine (0.41 g,
4.0
mmol) and dry diethyl ether (15 mL). After stirring for 1 hour, the reaction
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-225-
mixture was diluted with diethyl ether, washed with water, 2 N hydrochloric
acid,
saturated bicarbonate solution and brine. The diethyl ether solution was dried
with
sodium sulfate, concentrated in vaeuo and the residue purified by flash
chromarography on silica gel (hexane/ethyl acetate 10:1 ) to provide the title
compound (0.59 g) as a colorless solid.'H NMR (400 MHz, CDCl3) 8 7.12 (d,
1 H), 6.73 (d, 1 H), 4.98 - 4.48 (m, 2H), 2.70 - 2.52 (m, 2H), 2.18 - 2.04 (m,
1 H),
1.9I - I.80 (m, 3H), 1.47 (s, 9H).
b) (2-Tributylstannanyl-4,5,6,7-tetrahydrobenzo[b]thiophen-7-yl)carbamic
acid, tert-butyl ester
A -30°C solution of (4,5,6,7-tetrahydrobenzo[b]thiophen-7-
yl)carbamic
acid tert-butyl ester (0.127 g, O.S mmol, Example 27a) in anhydrous
tetrahydrofuran (3 mL) was treated dropwise with n-butyllithium (0.5 mL of a
2.5
M hexane solution, 1.25 mmol) under a nitrogen atmosphere. After stirring for
1 h
at -30 °C, the mixture was cooled to -70 °C, treated with neat
tri-n-butyltin
chloride (407 mg, 1.25 mmol) and allowed to warm to 0°C. The reaction
mixture
was diluted with diethyl ether and water and the organic layer was washed with
water, brine, dried over sodium sulfate and concentrated under vacuum.
Purification of the residue by flash chromatography on silica gel
(hexane/ethyl
acetate/triethylamine 200:10:1) gave the title compound (0.180 g).'H NMR (400
MHz, CDC13) 8 6.79 (s, 1 H), 4.98 - 4.50 (m, 2H), 2.71 - 2.53 (m, 2H), 2.15 -
2.03 (m, 1 H), 1.90 -1.70 (m, 3H), 1.62 - 1.50 (m, 6H), 1.47 (s, 9H), 1.38 -
1.28
(m, 6H), 1.10 - 1.03 (m, 6H), 0.89 (t, 9H).
c) [2-(1-cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)-4,5,6,7-tetrahydrobenzo[b]thiophen-7-yl]carbamic acid tert-butyl ester
A mixture of (2-tributylstannanyl-4,5,6,7-tetrahydrobenzo[b]thiophen-7-
yl)carbamic acid tert-butyl ester (0.180 g, 0.33 mmol, Example 27b), 1-
cyclopropyl-6- fluoro-7-iodo-8-methyl-1H-quinazolinedione (0.119 g, 0.33 mmol,
Example 25h), dichlorobis(triphenylphosphine) palladium (II) (0.023 g, 0.033
mmol), and triphenylarsine (0.031 g, O.l mmol) in anhydrous toluene (6 mL) was
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-226-
stirred under nitrogen at 95 °C for 20 hours. After cooling to room
temperature,
diethyl ether (1S mL) was added, followed by 15 % aqueous potassium fluoride
solution and stirring was continued for 1 hour at room temperature. The
mixture
was filtered through Celite and the organic layer was washed with water, dried
with sodium sulfate and concentrated in vacuo. Purification by flash
chromatography on silica gel (hexane/ethyl acetate 1:1) gave the title
compound
(102 mg) as colorless crystals. 'H NMR (400 MHz, CDCl3) 8 8.28 (bs, 1H), 7.67
(d, 1 H), 6.72 (s, 1 H), 5.02 - 4.62 (m, 2H), 3.38 (m, 1 H), 2.71- 2.68 (m,
2H), 2.51
(s, 3H), 2.22 - 2.10 (m, 1H), 1.97 -1.73 (m, 3H), 1.47 (s, 9H), 1.21 -1.13 (m,
ZH), 0.73 - 0.63 (m, 2H).
d) 7-(4-Amino-5,6-dihydro-4H-4,5,6,7-tetrahydrobenzo[b]thiophen-7-yl)-1-
cyclopropyl-6-fluoro-8-methyl-1H-quinazolinedione hydrochloride
A stream of gaseous hydrogen chloride was bubbled through a 0 °C
solution of 2-(1-cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)-4,5,6,7-tetrahydrobenzo[b]thiophen-7-yl]carbamic
acid
tert-butyl ester (0.102 g, 0.21 mmol, Example 27c) in anhydrous diethyl ether
(I0
mL) for 40 minutes. The resulting precipitate was removed by filtration,
washed
with anhydrous diethyl ether and dried in vacuo to provide the title compound
(0.036 g): mp > 220 °C (dec.);'H NMR (400 MHz, DMSO-d6) 8 1 I.55 (bs,
1H),
8.40 (bs, 3H), 7.57 (d, 1 H), 7.01 (s, 1 H), 4.58 (m, 1 H), 3.30 (m, 1 H),
2.75 - 2.65
(m, 2H), 2.40 (s, 3H), 2.18 - 2.05 (m, 1 H), 2.02 - 1.90 (m, 2H), 1.87 - 1.70
(m,
1H), 1.03 (m, 2H), 0.58 (m, 2H).
Example 28
a) 7-Methyl-6-trityl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine
A solution of 7-methyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine (1.50 g,
9.94 mmol [J. Med Chem.1989, 32, 1242.]) in dichloromethane (20 mL) was
treated, portionwise, with trityl bromide (3.86 g, 11.9 mmol) at room
temperature
followed by triethylamine (2.20 mL, 15.8 mmol). The reaction mixture was
stirred
at room temperature for 4 hours, washed with water (2 x 40 mL), dried over
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-227-
sodium sulfate, filtered, and the solvent removed under reduced pressure. The
residue was chromatographed on silica gel (ethyl acetate/hexane 5:95) to
provide
3.45 g of the title compound. 'H NMR (400 MHz, CDCl3) 8 7.50-7.07 (m, 15H),
6.90 (d, 1H), 6.33 (d, IH), 4.60 (q, 1H), 3.50-3.33 (m, 2H), 2.03-1.95 (m,
1H),
1.63-1.53 (m, 1H), 1.36 (d, 3H).
b) 7-Methyl-2-tri-n-butyIstannanyl-6-trityl-4,5,6,7-tetrahydrothieno[2,3-
c]pyridine
Under a nitrogen atmosphere, a -78 °C solution of 7-methyl-6-
trityl-
4,5,6,7-tetrahydro-thieno[2,3-c]pyridine (0.11 g, 0.26 mmol, Example 28a) in
anhydrous tetrahydrofuran (0.50 mL) was treated with fa-butyllithium (0.20 mL
of
a 2.5M hexane solution, 0.50 mmol) and stirred at -78 °C for one hour.
Tri n-
butylstannyl chloride (0.1 mL, 0.37 mmol) was then added and the reaction
mixture was stirred at -78 °C for one hour then allowed to come to room
temperature over 4.5 hours. The reaction was quenched with methanol (3 mL)
and concentrated under reduced pressure. The residue was chromatographed over
silica gel eluting with ethyl acetate/hexane/triethylamine (5:95:0.5) to give
0.087 g
of the title compound. 'H NMR (400 MHz, CDC13) b 7.55-7.03 (m, 15H), 6.37 (s,
1 H), 4.70-4.58 (m, 1 H), 3.52-3.33 (m, 2H), 2.05-1.93 (m, 1 H), 1.63-I .17
(m,
13H), 1.07-0.82 (m, 18H).
c) 1-Cyclopropyl-6-fluoro-8-methyl-7-(7-methyl-6-trityl-4,5,6,7-
tetrahydrothieno[2,3-c]pyridin-2-yl)-1H-quinazolinedione
Under a nitrogen atmosphere, a mixture of 7-methyl-2-tri-n-
butylstannanyl-6-trityl-4,5,6,7-tetrahydrothieno[2,3-c)pyridine (1.86 g, 2.72
mmol, Example 28b), 1-cyclopropyl-6-fluoro-7-iodo-8-methyl-1H-
quinazolinedione (0.448 g, 1.24 mmol, Example 25h), triphenylarsine (0.160 g,
0.522 mmol) and tris(dibenzylideneacetone)dipalladium(0) (0.I 15 g, O.I2~
mmol)
in anhydrous toluene (2 mL) was heated with stirring at 95 °C for 19
hours. After
cooling to room temperature, the reaction mixture was diluted with ethyl
acetate
(20 mL) and l5% w/v aqueous potassium fluoride solution (I5 mL) and stirred
for
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-228-
2 h. The mixture was filtered through Celite and washed with ethyl acetate.
The
organic phase was then dried over anhydrous sodium sulfate, filtered and
concentrated under reduced pressure. The residue was purified by silica gel
chromatography eluting with ethyl acetate/hexane/triethylamine gradient
(30:70:1
to 50:50:1) and gave the title compound (0.551 g). 'H NMR (400 MHz, CDCI3) 8
8.07 (bs, 1 H), 7.67 (d, 1 H), 7.55-7.08 (m, 1 SH), 6.27 (s, 1 H), 4.70-4.62
(m, 1 H),
3.63-3.47 (m, 2H), 3.42-3.32 (m, 1H), 2.47 (s, 3H), 2.02-1.93 (m, 2H), 1.55
(d,
3H), 1.23-1.13 (m, 2H), 0.73-0.63 (m, 2H).
d) 1-Cyclopropyl-6-tluoro-8-methyl-7-(7-methyl-4,5,6,7-tetrahydrothieno[2,3-
c]pyridin-2-yl)-1H-quinazolinedione
Gaseous hydrogen chloride was bubbled through a 0 °C suspension of
1-
cyclopropyl-6-fluoro-8-methyl-7-(7-methyl-6-trityl-4,5,6,7-
tetrehydrothieno[2,3-
c]pyridin-2-yl)-1H-quinazolinedione (0.551 g, 0.878 mmol, Example 28c) in
diethyl ether for 25 minutes and then the mixture was warmed to room
temperature and stirred overnight. The resulting solid was isolated by
filtration,
suspended in dichloromethane (10 mL), and treated with triethylamine (2 mL).
The mixture was concentrated under reduced pressure and the residue was
purified by column chromatography on silica gel eluting with
dichloromethanelmethanol/triethylamine (90:10:1). The product was then
triturated with methanol to give the title compound (0.070 g). 'H NMR (400
MHz, CD30D) 811.58 (s, 1 H), 9.83 (bs, 1 H), 7.58 (d, 1 H), 7.10 (s, 1 H),
4.83-4.70
(m, 1 H), 3.65-3.52 (m, 1 H), 3.45-3.30 (m, 2H), 3.02-2.90 (m, 2H), 2.45 (s,
3H),
1.65 (d, 3H), 1.12-0.98 (m, 2H), 0.75-0.55 (m, 2H); MSCI: m/z 386 (MH+)
Example 29
a) 1-(5-Bromothiophen-3-yl)ethanone
To a solution of 3-acetylthiophene (10.3 g, 82 mmol) in acetic acid (50
mL) was added sodium acetate (I0.0 g, 122 mmoI) followed by bromine (4.5 mL,
86 mmol) dropwise over 30 minutes. The mixture was allowed to stir at room
temperature overnight. Water (150 mL) was added and the reaction mixture was
stirred for 2 hours before the resulting solid was collected by filtration,
washed
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-229-
with water, and hexane to give 7.67 g of the title compound. 'H NMR (200 MHz,
CDCl3) $ 7.93 (d, 1 H), 7.50 (d, 1 H), 2.48 (s, 3H).
b) 1-(5-Bromothiophen-3-yl)ethanone O-benzyI oxime
S A solution of 1-(S-bromothiophen-3-yl)ethanone (2.1 g, 10 mmol,
Example 29a) in methanol (20 mL) was treated with O-benzylhydroxylamine
hydrochloride ( 1.76 g, 11 mmol) and refluxed for 3 hours. The solvent was
removed in vacuo and the resulting residue purified by column chromatography
(8:1 hexane/ethyl acetate) to give 2.8 g of the title compound.'H NMR (200
MHz, CDCl3) b 7.45-7.20 (m, 7H), S.15 (s, 2H), 2.1 S (s, 3H).
c) 1-(5-Bromothiophen-3-yl)ethylamine
A solution of 1-(S-bromothiophen-3-yl)ethanone O-benzyl oxime (2.7 g,
8.74 mmol, Example 29b) in tetrahydrofuran (30 mL) was treated with a solution
of borane-tetrahydrofuran complex (20 mL, 1M in THF) and heated at SO
°C for
24 hours. Methanol (2S mL) was added and the solvent removed in vacuo. The
resulting residue was purified by column chromatography (S-10% methanol /
chloroform) to give 0.88 g of the title compound.'H NMR (200 MHz, CDCl3) 8
7.02 (m, 2H), 4.10 (q, 1 H), 1.6S (bs, 2H), l .37 (d, 3H).
d) 1-(5-Bromo-2-chloromethylthiophen-3-yl)ethylamine hydrochloride
A solution of 1-(S-bromothiophen-3-yl)ethylamine (2.0S g, 10 mmol,
Example 29c) in concentrated hydrochloric acid (30 mL) was treated with
paraformaldehyde (1.0 g, 33 mmol) and the reaction mixture was stirred at room
2S temperature for 3 hours. After cooling to S °C and stirring for 2
hours, the
resulting solid was collected by filtration and washed with small amounts of
concentrated hydrochloric acid to give 1.84 g of the title compound.'H NMR
(200 MHz, DMS~-d6) 8 8.64 (bs, 3H), 7.54 (s, 1H), 5.12 (q, 2H), 4.61 (m, 1H),
1.48 (d, 3H).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-230-
e) 2-Bromo-4-methyl-4,6-dihydrothieno[2,3-c]pyrroIe-S-carboxylic acid tert-
butyl ester
A suspension of 1-(S-bromo-2-chloromethylthiophen-3-yl)ethylamine
hydrochloride (1.84 g, 6.35 mmol, Example 29d) in tetrahydrofuran (80 mL) was
S treated with triethylamine (2 mL) and stirred at room temperature for 2
hours. Di-
tent-butyl dicarbonate ( 1.66 g, 7.6 mmol) was added and the reaction mixture
was
stirred at room temperature for 18 hours. The solid was removed by filtration,
the
filtrate concentrated in vacuo and the residue purified by silica column
chromatography eluting with hexane%thyl acetate (16:1 to 8:1) to give 1.S g of
the
title compound. 'H NMR (200 MHz, CDC13) ~ 6.80 (d, 1 H), 5.00-4.80 (m, 1 H),
4.70-4.40 (m, 2H), 1.60-1.30 (m, 12H).
t7 4-Methyl-2-tributylstannanyl-4,6-dihydrothieno[2,3-c]pyrrole-5-carboxylic
acid, tert-butyl ester
I S Under a nitrogen atmosphere, to a -78 °C solution of 2-bromo-4-
methyl-
4,6-dihydro-thieno[2,3-c]pyrrole-S-carboxylic acid tent-butyl ester (1.S g,
4.7
mmol, Example 29e) in diethyl ether (SO mL) was added n-butyllithium (2.S M, S
mL, I2.S mmoI) and the mixture was allowed to stir for 10 minutes. Tri-n-
butylstannyl chloride (3.S g, 10.5 mmol) was then added and after stirring at -
78
°C for 40 minutes, methanol (30 mL) was added and the solvent removed
in
vacuo. The residue was partitioned between ethyl acetate and water ( l00 mL
each), and the organic layer washed with water, dried with sodium sulfate and
concentrated in vacuo. The residue was purified by column chromatography (16:I
hexane/ethyl acetate with O.S% triethylamine) to give 2.3 g of the title
compound.
2S 'H NMR (200 MHz, CDCl3) 8 6.80 (d, 1 H), 4.80-5.00 (m, 1 H), 4.70- 4.50 (m,
2H), 1.60- 0.60 (m, 39H).
g) 2-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yI)-4-methyl-4,6-dihydrothieno[2,3-cJpyrrole-5-carboxylic acid tent-butyl
ester
To a mixture of 4-methyl-2-tributylstannanyl-4,6-dihydrothieno[2,3-
cJpyrrole-S-carboxylic acid ter-t-butyl ester (0.96 g, 1.82 mmol, Example 29f)
and
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-231-
1-cyclopropyl-6-fluoro-7-iodo-8-methyl-1H-quinazolinedione (0.275 g, 0.76
mmol, Example 25h) in toluene (8 mL) was added
dichlorobis(triphenylphosphine)palladium(II) (0.053 g, 0.076 mmoI) and
triphenylarsine (0.097 g, 30 mmol). The mixture was heated at 100 °C in
a sealed
tube for 4 hours. The mixture was concentrated in vacuo and the residue
purified
by column chromatography (2:1 hexane/ethyl acetate, and 1:1 hexane/ethyl
acetate) to give 0.33 g of the title compound. 1H NMR (200 MHz, CDC13) 8 8.86
(bs, 1 H), 7.74 (d, 1 H), 6.82 (d, 1 H), S. I O-4.90 (m, 1 H), 4.80-4.60 (m,
2H), 3.39
(m, 1H), 2.54 (s, 3H), 1.53 (m, 12H), 1.30-1. 10 (m, 2H), 0.68 (m, 2H).
h) 1-Cyclopropyl-6-fluoro-8-methyl-7-(4-methyl-5,6-dihydro-4H-
thieno[2,3-c]pyrrol-2-yl)-1H-quinazolinedione hydrochloride
A stream of hydrogen chloride gas was bubbled into a solution of 2-(1-
cyclopropyl-6-fluoro-8-methyIdioxo- I ,2,3,4-tetrahydroquinazolin-7-yl)-4-
methyl-
4,6-dihydrothieno[2,3-c]pyrrole-5-carboxylic acid tert-butyl ester (0.30 g,
0.637
mmol, Example 29g) in a solvent mixture of dichloromethane ( 10 mL) and
diethyl
ether (25 mL). The resulting solution was cooled to 0-5 °C for 1 hour
and then
stirred at room temperature for 2 hours. The solid was collected by filtration
and
washed with diethyl ether to provide 0.235 g of the title compound.'H NMR (200
MHz, DMS O-d6) 8 11.60 (s, 1 H), 10.54 (bs, 1 H), l 0.0 I (bs, 1 H), 7.60 (d,
1 H),
7.17 (s, 1H), 4.92 (m, 1H), 4.58 (m, 2H), 3.33 (m, 1H), 2.46 (s, 3H), 1.61 (d,
3H),
0.90-1.04 (m, 2H), 0.64 (m, 2H). MSCI: m/z 371 (MH+).
Example 30
a) (3S, 3aS, 6aR and 3R, 3aR, 6aS)-2-Benzyl-3-trifluoromethylhexahydro-
pyrrolo[3,4-d]isoxazole-5-carboxylic acid benzyl ester
A mixture of N benzylhydroxylamine (7.6 g, 10 mmol), 1-ethoxy-2,2,2-
trifluoromethyl-ethanol (1.6 g, 90%, 10 mmol ) and triethylamine (1.5 mL) in
benzene (50 mL) was refluxed for 2 hours. After cooling to room temperature,
2,5-dihydropyrrole-l-carboxylic acid benzyl ester (2.0 g, 10 mmol) was added
and
the reaction mixture was refluxed for 24 hours. The solvent was removed irz
vacuo
and the residue triturated with hexane/ethyl acetate ( 100 mL of a 2:1
mixture).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-232-
The solid was removed by filtration and the filtrate concentrated under
reduced
pressure. The residue was purif ed by column chromatography (8:1 hexane%thyl
acetate) to give 3.2 g of the title compound.'H NMR (400 MHz, CDCI3) ~ 7.20
(m, l OH), 5.20 (s, 2H), 4.65 (m, 1 H), 4.30 (m, 1 H), 4.05 (m, 1 H), 3.75 (m,
2H),
3.10-3.50 (m, 4H).
b) ([(3R, 4S)-4-(R)- and (3S, 4R)-4-(S)]-1-Amino-2,2,2-
trifluoroethyl)pyrrolidin-3-of
A suspension of (3S, 3aS, 6aR)- and (3R, 3aR, 6aS)-2-benzyl-3-
trifluoromethylhexahydropyrrolo[3,4-d]isoxazole-5-carboxylic acid benzyl ester
(1.7 g, 4.18 mmol, Example 30a) and 1.0 g of palladium on carbon (10% Pd, 50%
water) in methanol (70 mL) was shaken under a hydrogen atmosphere at 50 psi
for 20 hours. The catalyst was removed by filtration and the solvent removed
in
vacuo to give 0.78 g of the title compound.'H NMR (400 MHz, DMSO-d6) 8 4.40
(m, 1 H), 3.30 (m, 1 H), 3.20-2.80 (m, 4H), 2.10 (m, 1 H). MSCI: m/z 184
(MHO).
c) 7-[[(3S, 4R)-3-(R)- and (3R, 4S)-3-(S)]-1-amino-2,2,2-trifluoroethyl)-4-
hydroxypyrrolidin-1-ylj-1-cyclopropyl-6-fluoro-8-methyl-IH-
quinazolinedione
A mixture of ([(3R, 4S)-4-(R)- and (3S, 4R)-4-(S)]-1-amino-2,2,2-
trifluoroethyl)pyrrolidin-3-of (0.70 g, 3.8 mmol, Example 30b), 1-cyclopropyl-
6,7-difluoro-8-methyl-1H-quinazolinedione (0.90 g, 3.6 mmol) and triethylamine
(0.5 mL) in 5 mL of dimethyl sulfoxide was heated at 110 °C for 40
hours. The
reaction mixture was then diluted with 50 mL of ethyl acetate and 50 mL of
water.
The organic layer was separated and the water layer was extracted with ethyl
acetate (50 mL x 3). The combined organic layers were washed with water. The
solvent was then removed in vacuo and the residue purified by column
chromatography (5% methanol in chloroform, then 10% methanol in chloroform)
to give 0.518 g of the title compound.'H NMR (400 MHz, DMSO-d6) 811.25 (s,
1 H), 7.25 (d, 1 H), 5.10 (d, 1 H), 4.50 (m, 1 H), 3.90 (d, 1 H), 3.75 (m, 1
H), 3.40 (m,
1 H), 3.30 (m, l H), 3.25 (m, 2H), 3. ~ 0 (d, 1 H), 2.28 (s, 3H), 2.20 (m, l
H), 2.10 (s,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-233-
1 H), 1.10 (m, 1 H), 1.00 (m, 1 H), 0.58 (m, 1 H), 0.52 (m, 1 H). ' 9F NMR
(376 MHz,
DMSO-d6) ~ -75 (s, 3F), -129 (s, 1F). MSCI: m/z 416(MH~).
Example 31
a) 4-(Oxazole-4-carbonyl)-1-((S)-1-phenylethyl)pyrrolidin-2-one
To a -78 °C solution of oxazole (10.30 g, 149.10 mmol) in
tetrahydrofuran
(150 mL) was added n-butyl lithium (2.5 M in hexane, 53.7 mL, 134.19 mmol).
The solution was stirred at -78 °C for 3 hours and treated with a
solution of 5-oxo-
1-((S)-1-phenylethyl)-pyrrolidine-3-carboxylic acid methoXymethylamide (8.24
g,
29.8 mmol, Example 7a) in tetrahydrofuran (50 mL). The reaction was allowed to
warm to room temperature and stir for an additional 3 hours. Water was added
followed by saturated ammonium chloride solution, and the mixture extracted
with ethyl acetate. The combined organic layers were dried over sodium
sulfate,
filtered and concentrated under reduced pressure. The residue was
I5 chromatographed over silica gel (hexanes/ethyl acetate, I :4) to afford
1.96 g of the
title compound as a mixture of isomers (~1:1 ratio). 1St Isomer'H NMR (400
MHz, CDCl3) 8 7.88 (s, 1 H), 7.40-7.21 (m, 6H), 5.50 (q, 1 H), 4.18-4.10 (m, 1
H),
3.66-3.62 (m, 1 H), 3.35-3.28 (m, l H), 2.95-2.75 (m, 2H), 1.52 (d, 3H). 2-dd
Isomer'H NMR (400 MHz, CDC13) 8 7.85 (s, I H), 7.40-7.21 (m, 6H), 5.50 (q,
1 H), 4.30-4.20 (m, 1 H), 3.78-3.67 (m" 1 H), 3.29-3.18 (m, 1 H), 2.95-2.75
(m, 2H),
1.55 (d, 3H).
b) 4-(Benzyloxyiminooxazol-4-ylmethyl)-1-((S)-1-phenylethyl)-pyrrolidin-2-
one
A mixture of 4-(oxazole-4-carbonyl)-1-((S)-1-phenylethyl)pyrrolidin-2-
one (0.5 g, 1.76 mmol, Example 31 a) and O-benzylhydroxylamine hydrochloride
(0.42 g, 2.64 mmol) in pyridine (5 mL) was refluxed for 5 hours and cooled to
room temperature. The mixture was diluted with water and extracted with 'ethyl
acetate (2 x 20 ml). The combined organic layers were washed with saturated
sodium bicarbonate solution, dried with sodium sulfate, filtered and
concentrated
in vacuo. The residue was chromatographed over silica gel, eluting with
hexanes:ethyl acetate ( 1:2), to give 0.41 g of the title compound as a
mixture of
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-234-
isomers. ' H NMR (400 MHz, CDC13) 8 7.75 - 7.60 (m, 1 H), 7.40-7.12 (m, ~l 1
H),
5.60-5.45 (m, 1 H), 5.30-5.05 (m, 2H), 4.30-4.16 (m, 1 H), 3.88-2.60 (m, 4H),
1.50,
1.49, 1.45 - 1.30 (m, 3H).
c) C-Oxazol-4-yl-C-[1-((S)-1-phenylethyl)pyrrolidin-3-yl]methylamine
To a 0 °C solution of 4-(benzyloxyiminooxazol-4-ylmethyl)-1-((,S~-
1-
phenylethyl)-pyrrolidin-2-one (3.16 g, 8.11 mmol, Example 31b) in
tetrahydrofuran (80 mL) was added a 1.0 M solution of borane-tetrahydrofuran
complex (24.3 mL, 24.3 mmol) and the reaction stirred at room temperature for
21
hours. The solvent was evaporated and the residue was taken up in water (5 mL)
and extracted with chloroform. The combined organic layers were evaporated
under reduced pressure and the residue dissolved in 80°lo aqueous
ethanol, treated
with triethylamine (20 mL) and then heated to reflux for two hours. The
mixture
was concentrated to remove the organic solvents and the aqueous mixture
extracted with dichloromethane. The organic extracts were then combined, dried
over sodium sulfate and concentrated in vacuo. The resulting residue was
purified
using silica gel column chromatography (chloroform/methanol, 9:1 ) to obtain
the
title compound (2.5 g) as a mixture of isomers.'H NMR (400 MHz, CDCI3) b
7.55 - 7.54 (2 m, 1 H), 7.40-7.15 (m, 7H), 7.10 -7.05 (m, I H), 4.02-3.88 (m,
1 H),
3.25-3.10 (m, IH), 2.90-2.20 (m, SH), 2.00-1.60 (m, 2H), 1.60-1.35 (m, 3H).
d) C-Oxazol-4-yl-C-pyrrolidin-3-ylmethylamine
A mixture of C-oxazol-4-yl-C-[1-((S)-1-phenylethyl)pyrrolidin-3-
yl]methylamine (2.44 g, 8.99 mmol, Example 31 c), ammonium formate (2.83 g,
45.0 mmol) and 10% palladium on carbon (2.87 g) in methanol (45 mL) was
heated at reflux for 5 hours. After filtering through Celite, the filtrate was
concentrated under reduced pressure to obtain the title compound (0.9 g). 'H
NMR (400 MHz, CDC13) $ 7.66 - 7.64 (m, 1 H), 7.13 - 7. I 1 (m, I H), 4. I 8 -
4.10
(m, 1H), 3.50-3.40 (bs, 3H), 3.40-3.25 (m, 3H), 3.08-2.80 (m, 2H), 2.30-2.05
(m,
1H), 1.90-1.60 (m, 1H).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-235-
e) 7-[3-(Aminooxazol-4-ylmethyl)pyrrolidin-I-yl]-I-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione
The title compound was obtained as a white solid (0.21 g) from 1-
cyclopropyl-6,7-difluoro-8-methyl-1H-quinazolinedione (0.66 g, 2.60 mmol) and
C-oxazol-4-yl-C-pyrrolidin-3-ylmethylamine (1.09 g, 6.5 mmol, Example 31d)
according to the method described for Example 2. 1H NMR (400 MHz, CDCl3) 8
7.63 - 7.61 (m, 1 H), 7.60-7.45 (m, 1 H), 7.13 - 7.11 (m, 1 H), 4.13-4.10 (m,
1 H),
3.60 (6s, 3H), 3.35-3.25 (m, 2H), 2.81-2.68 (bs, 3H), 2.41 - 2.39 (m, 3H),
2.30-
1.86 (m, 3H), 1.25-1.05 (m, 2H), 0.60 (bs, 2H). MSCI: m/z 400 (MH+)~
Example 32
a) [(3R,4S)- and (3S, 4R)-I-(I-Cyclopropyl-6-fluoro-8-methoxydioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)-4-fluoropyrrolidin-3-ylmethyl]carbamic acid tert-
butyl ester
The title compound, as a mixture of two isomers (~2:1), was obtained as a
white solid (0.052 g) from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione (0.49 g, 1.832 mmol) and (3S, 4S) and ((3R, 4R)-4-
fluoropyrrolidin-3-ylmethyI)carbamic acid ter-t-butyl esters (0.60 g, 2.747
mmol,
[J. Med. Chem. 1990, 33, 1344]). 1H NMR (400 MHz, CDCI3) 8 9.10-8.82 (bs,
1 H), 7.47 (m, 1 H), 5.12 (d, 1 H), 5.00 - 4.85 (bm, 1 H), 4.10-4.05 (m, 1 H),
3.73-
3.60 (m, 2H) 3.52 - 3.48 (m, 3H), 3.32-3.08 (m, 2H), 2.72-2.60 (m, 1 H), 2.50-
2.35, 2.15-2.00, 1.75-1.65 & 1.65-1.50 (4 x m, 2H), 1.46 (s, 9H), 1.25-0.95
(m,
2H), 0.75-0.45 (m, 2H). MSCI: m/z 467 (MH+)
b) 7-(3R, 4S)- and 7-((3S, 4R)-3-Aminomethyl-4-fluoropyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazoloinedione hydrochloride
A solution of (3R,4S) and [(3S, 4R)-1-(1-cyclopropyl-6-fluoro-8-
methoxydioxo-1,2,3,4-tetrahydroquinazolin-7-yI)-4-fluoropyrroIidin-3-
ylmethyl]carbamic acid tent-butyl ester (0.052 g, 0.112 mmol, Example 32a) in
dichloromethane was saturated with hydrogen chloride gas and stirred at room
temperature for l 8 hours. The solvent was removed under reduced pressure to
give the title compound as a white solid in a ~2:1 mixture isomers (0.045
g).'H
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-236-
NMR (400 MHz, CD30D) 8 7.40 (m, 1 H), 5.25 (d, I H), 4.23-3.96 (m, 2H), 3.85-
3.64 (2 x m, 2H), 3.62 (s, 3H), 3.51-3.48 (m, 1H), 2.85-2.72 (m, 1H), 2.65-
2.S5,
2.30-2.20, 1.85-1.70 &1.50-I.35 (4 x m, 2H), 1.15-0.88 (m, 2H), 0.72-0.56 (m,
2H). MSCI: m/z 367 (MH+).
Example 33
a) 5-benzyl-3-(tetrahydropyran-2-yloxymethyl)-4,5,6,6x-tetrahydro-3aH-
pyrrolo[3,4-d']isoxazole
To a solution of 1-benzyl-2,5-dihydro-1 H-pyrrole ( 13.5 g, 84.8 mmol) in
benzene (150 mL) was added 2-(2-nitroethoxy)tetrahydropyran (37 g, 21 I.2
mmol) and triethylamine (5.4 mL, 38.4 mmol). The solution was heated to reflex
and phenyl isocyanate (37.8 mL, 347.8 mmol) was slowly added over 2 hours.
After the addition was complete, the mixture was refluxed overnight and the
resulting precipitate removed by filtration. The filtrate was concentrated in
vacuo
I S and the residue purified by column chromatography eluting with ethyl
acetate:hexanes (1:4), to obtain 19.5 g the title compound. 'H NMR (200 MHz,
CDCl3) 8 7.38-7.18 (m, 5H), 5.10-4.98 (m, 1 H), 4.68-4.58 (bs, 1 H), 4.50-4.18
(m,
2H), 3.$6-3.42 (m, 5H), 3.25-3.05 (m, 2H), 2.44-2.24 (m, 2H), 1.82-1.38 (m,
6H).
b) 4-[1-Amino-2-(tetrahydropyran-2-yloxyethyl]-1-benzylpyrrolidin-3-of
To a 5 °C solution of 5-benzyl-3-(tetrahydropyran-2-yloxymethyl)-
4,5,6,6a-tetrahydro-3aH-pyrrolo[3,4-c~isoxazole (8.80 g, 27.8 mmol, Example
33x) in diethyl ether (150 mL) was added, portionwise, lithium aluminum
hydride
(2.58 g, 68.0 mmol). The mixture was stirred at 5 °C for 1 hour and
then at room
temperature for 1 hour. The mixture was then retooled to 5 °C and
treated
successively, dropwise, with water (2.6 mL), 3N sodium hydroxide (2.6 mL) and
water (7.7 mL). The mixture was then diluted with chloroform and stirred at
room
temperature for 2 hours. The mixture was filtered through Celite, and the
filter
cake was washed copiously with chloroform. The combined filtrates were dried
over sodium sulfate and evaporated. The residue was triturated with diethyl
ether,
the solid removed by filtration, washed with ether and dried in vacuo to give
7.3 g
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-237-
of the title compound. 'H NMR (200 MHz, CDCl3) 8 7.35-7.28 (m, SH), 4.62-
4.52 (bs, 1 H), 4.46-4.35 (bs, 1 H), 3.86-3.12 (m, 8H), 3.00-2.88 (m, 1 H),
2.65-2.48
(m, 2H), 2.35-2.18 (m, 1 H) 1.80-1.40 (m, 9H).
c) [1-(1-Benzyl-4-hydroxypyrolidin-3-yl)-2-(tetrahydropyran-2-yloxy)ethyl]-
carbamic acid tart-butyl ester
To a solution of 4-[1-amino-2-(tetrahydropyran-2-yloxyethyl]-1-
benzylpyrrolidin-3-of (7.3 g, 22.8 mmol, Example 33b) in chloroform (60 mL)
was added di-tart-butyl dicarbonate (4.97 g, 22.8 mmol). The mixture was
stirred
at room temperature for 3 hours, and the solvent was removed in vacuo. The
residue was purified by column chromatography on silica gel (5% methanol in
ethyl acetate) to obtain 7.8 g of the title compound.'H NMR (200 MHz, CDCl3) 8
7.32-7.25 (m, SH), 5.15-4.85 (m, 1 H), 4.62-4.58 (bs, 1 H), 4.36-4.20 (m, 1
H),
4.00-3.35 (m, 7H), 3.26-3.05 (bs, 1H), 3.00-2.88 (m, 1H), 2.70-2.28 (m, 4H),
1.80-1.48 (m, 6H), 1.42 (s, 9H).
d) [1-(1-Benzyl-4-hydroxypyrrolidin-3-yl)-2-hydroxyethyl]carbamic acid tert-
butyl ester
A solution of [I-(1-benzyl-4-hydroxypyrolidin-3-yl)-2-(tetrahydropyran-
2-yloxy)ethyI]-carbamic acid tart-butyl ester (7.8 g, I8.6 mmol, Example 33c)
in
ethanol (75 mL) was treated, portionwise, with pyridinium p-toluene sulfonate
(5.92 g, 23.6 mmol). The mixture was heated to 85 °C for 18 hours and
the solvent
removed in vacuo. The residue was then purified by column chromatography on
silica gel (10°lo methanol in ethyl acetate) to obtain 7.5 g of
tosylate salt. This
solid was suspended in chloroform, treated with potassium carbonate (2.6 g)
and
stirred at room temperature for I hour. The organic Iayer was concentrated,
filtered through Celite and evaporated in vacuo to dryness to obtain 4.3 g of
the
title compound.'H NMR (200 MHz, CDC13) 8 7.36-7.28 (m, 6H), 5.68-5.56 (d,
1 H), 4.42-4.28 (bs, 1 H), 3.98-3.58 (m, 6H), 2.82-2.40 (m, SH), 1.43 (s, 9
H).
e) (5-Benzylhexahydrofuro[2,3-c]pyrrol-3-yl)carbamic acid tart-butyl ester
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-238-
A -70 °C suspension of [1-(1-benzyl-4-hydroxypyrrolidin-3-yl)-2-
hydroxyethyl]- carbamic acid tart-butyl ester (4.35 g, 13.0 mmol, Example 33d)
in
dichloromethane was treated, dropwise, with (diethylamino)sulfur trifluoride
(DAST) (1.7 mL, 12.90 mmol). After stirring at -70 °C for half an hour,
the
solution was allowed to warm tol5 °C. The solvent was removed in vacuo
and the
residue purified by column chromatography on silica gel eluting with a mixture
of
ethyl acetate:hexanes (1:2) to obtain 1.9 g of the title compound. IH NMR (200
MHz, CDCI3) b 7.32-7.26 (m, SH), 4.82-4.70 (m, 1H), 4.68-4.58 (t, 1H), 4.20-
4.10 (m, 1 H), 4.04-3.92 (m, 1 H), 3.72-3.60 (m, 1 H), 3.50 (s, 2H), 2.92-2.70
(m,
2H), 2.65-2.55 (m, 1 H), 2.45-2.26 (m, 2H), I .43 (s, 9H).
f) (Hexahydrofuro[2,3-c]pyrrol-3-yl)carbamic acid tart-butyl ester
A suspension of (5-benzylhexahydrofuro[2,3-c]pyrrol-3-yl)carbamic acid
tart-butyl ester (1.7 g, 5.3 mmol, Example 33e), ammonium formate (1.9 g, 30
mmol) and IO% palladium-carbon (I.7 g) in dry methanol (20 mL) was heated at
70 °C for 1 hour. The cooled mixture was then filtered through Celite
and the
solvent removed in vacuo affording 1.2 g of the title compound. 'H NMR (200
MHz, CDCl3) 8 4.84-4.75 (m, 1 H), 4.68-4.60 (t, 1 H), 4.08-3.98 (m, I H), 3.96-
3.85 (m, I H), 3.60-3.46 (m, I H), 3.10 (d, 1 H), 2.96 (d, 2H), 2.78-2.68 (dd,
I H),
2.60-2.48 (m, 1 H), 1.85-1.75 (m, 1 H), 1.45 (s, 9H).
g) [5-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)hexahydrofuro[2,3-c]pyrrol-3-yl]carbamic acid tent-butyl ester
A solution of 1-cyclopropyl-6,7-difluoro-8-methyl-1H-quinazolinedione
(0.55 g, 2.I9 mmol), (hexahydrofuro[2,3-c]pyrrol-3-yl)carbamic tart-butyl
ester
(1.0 g, 4.38 mmol, Example 33fj and triethylamine (1.2 mL, 8.53 mmol) in
dimethylsulfoxide (2 mL) was heated at 110 °C for 4 days and 120
°C for 3 days.
The cooled mixture was diluted with water (50 mL) and extracted with ethyl
acetate (2x 100 mL). The combined extracts were washed with water (2x 100 mL),
brine ( I x 100 mL), dried with sodium sulfate and concentrated in vacuo. The
residue was purified by column chromatography on silica gel eluting with ethyl
acetate:hexanes (I:1) yielding 0.29 g of the title compound. 1H NMR (200 MHz,
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-239-
CDC13) 8 8.2 (s, 1H), 7.57 (d, 1H), 4.92-4.75 (m, 2H), 4.25-4.08 (m, 2H), 3.78-
3.28 (m, 5H), 2.90-2.76 (m, 1H), 2.50 (s, 3H), 1.70-1.58 (m, 1H), 1.46 (s,
9H),
1.18-1.08 (m, 2H), 0.68-0.56 (m, 2H).
h) 7-(3-Aminohexahydrofuro[2,3-c]pyrrol-5-yl)-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride
Hydrogen chloride gas was bubbled through a 5 °C solution of [5-(1-
cycIopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)hexahydrofuro[2,3-c]pyrrol-3-yl]carbamic acid, tert-butyl ester (0.29 g,
0.63
mmol, Example 33g) in a mixture of dichloromethane (6 mL) and diethyl ether
(40 mL) for 15 minutes. After stirring at 10-15 °C for 1 hour, the
solid was
collected by filtration and washed with diethyl ether to obtain 0.17 g of the
title
compound. 1H NMR (200 MHz, DMSO-d6) ~ 8.28-8.18 (m, 2H), 7.42 (d, 1H),
4.85-4.75 (m, I H), 4.16-4.02 (m, I H), 3.85-3.70 (m, 2H), 3.58-3.25 (m, 6H),
3.02-
2.88 (m, 1H), 2.43 (s, 3H), 1.10-0.98 (m, 2H), 0.58-0.45 (m, 2H). MSCI: m/z
361
(MH+).
Example 34
a) {1-[1-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-
7-yl)-4,4-dimethylpyrrolidin-3-yl]ethyl}carbamic acid tert-butyl ester
A solution of 1-cyclopropyl-6,7-difluoro-8-methyl-IH quinazolinedione
(0.44 g, 1.74 mmol), (1-(4,4-dimethypyrrolidin-3-yl)ethyl]carbamic acid tert-
butyl ester (0.85 g, 3.51 mmol, [PCT Int. applic. WO 0153273 Al ) and
triethylamine (0.98 mL, 6.98 mmol) in dimethylsulfoxide (2 mL) was heated in a
sealed tube at 110 °C for 40 hours. The cooled reaction was diluted
with water (50
mL) and extracted with ethyl acetate (2x 100 mL). The combined extracts were
washed with water (2x 100 mL), brine ( 100 mL), dried with sodium sulfate and
concentrated in vacuo. The residue was then purified by column chromatography
on silica gel eluting with ethyl acetate:hexanes (1:2) to give 0.4 g of the
title
compound. 1H NMR (200 MHz, CDCl3) 8 8.12-8.02 (bs, 1H), 7.50 (d, 1H), 4.60-
4.48 (m, 1 H), 4.00-3.82 (m, I H), 3.80-3.66 (t, 1 H), 3.58-3.45 (m, 2H), 3.38-
3.26
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-240-
(m, 1H), 3.15 (d, 1H), 2.37 (s, 3H), 2.00-1.82 (m, 1H), 1.43 (s, 9H), 1.25 (d,
3H),
1.17 (d, 6H), 1.30-1.I0 (m, 2H), 0.74-O.S6 (m, 2H).
b) 7-[4-(Aminoethyl)-3,3-dimethylyrrolidin-1-yl]-1-cylcopropyl-6-fluoro-8-
methyl-1H-quinazolinedione hydrochloride
Hydrogen chloride gas was bubbled through a S °C solution of { 1-
[1-(1-
cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazoIin-7-y1)-4,4-
dimethylpyrrolidin-3-yl]ethyl}carbamic acid tent-butyl ester (0.48 g, 1 mmol,
Example 34a) in a mixture of dichloromethane (20 mL) and ether (SO mL) for 1S
minutes. After stirring for 1 hour, the resulting solid was removed by
filtration,
washed with ether and dried to obtain 0.37 g of the title compound. 'H NMR
(200
MHz, DMSO-d6) 8 8.25-8. I O (bs, 2H), 7.35 (d, 1 H), 3.82-3.56 (m, 2H), 3.46-
3.22
(m, 4H), 3.0S (d, 1H), 2.38 (s, 3H), 2.22-2.02 (m, IH), 1.36 (d, 3H), 1.12 (d,
6H),
1.20-1.00 (m, 2H), 0.62-0.38 (m, 2H). MSCI: m/z 37S (MH+).
Example 35
a) [2-(1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)octahydroisoindol-4-yl]carbamic acid tart-butyl ester
A mixture of l-cyclopropyl-6,7-difluoro-8-methyl-1H-quinazolinedione
(0.40 g, I.SO mmol), (octahydroisoindol-4-yl)carbamic acid tent-butyl ester
(1.l g,
4.50 mmol, [Patent applic. WO 96/9637495]) and 1,1,3,3-tetramethylguanidine
(0.S6 mL, 4.S mmol) in dimethyl sulfoxide (1.S mL) was heated at 80 °C
for four
days. The mixture was cooled, diluted with water and extracted with ethyl
acetate. The combined organic extracts were dried over sodium sulfate,
filtered
2S and concentrated under vacuum. The residue was purified by column
chromatography (1:1 hexane/ethyl acetate, O.S% triethylamine) to afford the
title
compound (0.217 g). 'H NMR (400 MHz, CDC13): 8 8.23 (bs, 1H), 7.56 (d, 1H),
4.45-4.42 (m, 1 H), 3.95-3.81 (m, 3H), 3.37-3.33 (m, 1 H), 3.22-3.18 (m, 1 H),
2.95-
2.83 (m, 2H), 2.34 (s, 3H), 2.18-2.11 (m, 2H), I.82-1.78 (m, 2H), 1.62-1.SS
(m,
2H), 1.35 (s, 9H), 1.01-0.94 (m, 3H), 0.71-O.SS (m, 2H). MSCI: mlz 473 (MH+).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-241-
b) 7-(4-Aminooctahydroisoindol-2-yI)-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione hydrochloride
[2-( 1-Cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-7-
yl)octahydroisoindol-4-yl~carbamic acid tent-butyl ester (0.217 g, 0.46 mmol,
Example 35a) was dissolved in ether (20 mL), cooled in an ice bath, and
hydrogen
chloride gas was bubbled through the solution for 15 minutes. The mixture was
stirred at 5 °C for four hours then filtered to provide 0.147 g of the
title compound
as a solid. 'H NMR (440 MHz, DMSO): 8 8.24 (bs, 3H), 7.35 (d, 1H), 3.95-3.90
(m, 2H), 3.44-3.41 (m, 2H), 3.31-3.28 (m, 1H), 3.03-2.89 (m, 2H), 2.41-2.22
(m,
4H), 1.78-1.61 (m, 2H), 1.59-1.55 (m, 3H), 1.37-1.04 (m, 4H), 0.60-0.56 (m,
1H),
0.47-0.44 (m, 1H). MSCI: m/z 373 (MH+).
Example 36
a) 2,4,5-Trifluoro-3-hydroxybenzoic acid methyl ester
To a solution of 2,4,5-trifluoro-3-hydroxybenzoic acid (10.86 g, 56.56
mmoI) in methanol (100 mL) was added concentrated sulfuric acid (1.50 mL). The
reaction mixture was heated at reflux for 5 hours and the solvent removed in
vacuo. The residue was dissolved in dichloromethane (600 mL), washed with
brine (3x500 mL), dried over sodium sulfate, and concentrated under reduced
pressure to give the title compound as white crystals (10.75 g). 'H NMR (400
MHz, DMSO-d6) 811.40 (s, 1 H), 7.38 (m, 1 H), 3.82 (s, 3 H).
b) 3-tent-ButoxycarbonyImethoxy-2,4,5-trifluorobenzoic acid methyl ester
A 0 °C solution of 2,4,5-trifluoro-3-hydroxybenzoic acid methyl
ester
(9.82 g, 47.67 mmol, Example 36a) in N,N dimethylformamide (120 mL) was
treated portionwise with sodium hydride (2.30 g, 57.2 mmol, 60% in mineral
oil).
After stirnng at 0 °C for 20 min., tert-butyl bromoacetate (7.90 mL,
52.4 mmol)
was added, and the mixture stirred at room temperature for 18 hours. The
reaction
mixture was adjusted to pH 8.0 by the addition of saturated ammonium chloride
and extracted with dichloromethane (800 mL). The organic layer was washed with
brine (3x600 mL), dried over sodium sulfate and the solvent removed in vacuo.
The residue was purified by flash chromatography (dichloromethane) to yield
the
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-242-
title compound (I5.0 g). 'H NMR (400 MHz, CDCI~) ~ 7.50 (m, 1 H), 4.72 (s, 2
H), 3.92 (s, 3 H), 1.44 (s, 9 H).
c) 3-Carboxymethoxy-2,4,5-trifluorobenzoic acid methyl ester
To a solution of 3-tert-butoxycarbonylmethoxy-2,4,5-trifluorobenzoic acid
methyl ester (15.00 g, Example 36b) in dichloromethane (100 mL) was added
trifluoroacetic acid (50 mL) and the mixture was stirred at room temperature
for 4
hours. The mixture was concentrated in vacuo and the residue crystallized
(hexane/dichloromethane) to afford the title compound as white crystals (
10.95 g).
'H NMR (400 MHz, DMSO-d6) 8 13.28 (bs, 1 H), 7.62 (m, 1 H), 4.90 (s, 2 H),
3.86 (s, 3 H).
d) 2,4,5-Trifluoro-3-fluoromethoxybenzoic acid methyl ester
To a solution of 3-carboxymethoxy-2,4,5-trifluorobenzoic acid methyl
ester (2.49 g, 9.43 mmol, Example 36c) in dichloromethane (60 mL) was added
xenon difluoride ( 2.38 g, 14.1 mmol) and the mixture was stirred at room
temperature for 18 hours. The reaction mixture was washed with saturated
aqueous sodium bicarbonate (2x50 mL), brine (2x50 mL), dried with sodium
sulfate and concentrated in vacuo. The residue was purified by flash
chromatography (1:l dichloromethane/hexanes) to give the title compound (1.00
g). ' H NMR (400 MHz, CDCI3) ~ 7.64 (m, 1 H), 5.68 (d, 2 H), 3.98 (s, 3 H).
e) 2,4,5-Trifluoro-3-fluoromethoxybenzamide
To a solution of 2,4,5-trifluoro-3-fluoromethoxybenzoic acid methyl ester
(1.00 g, 4.20 mmol, Example 36d) in methanol (5 mL) was added aqueous
ammonia (25 mL). The mixture was stirred at room temperature for 18 hours and
extracted with dichloromethane (3x20 mL). The combined organic layers were
dried over sodium sulfate, concentrated in vacuo and the residue purified by
chromatography (dichloromethane to 95:5 dichloromethane/methanol gradient) to
give the title compound (0.65 g). 'H NMR (400 MHz, CDC13) S 7.84 (m, 1 H),
6.60 (bs, l H), 5.90 (bs, 1 H), 5.68 (d, 2 H).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-243-
f71-Cyclopropyl-3-(2,4,5-tritluoro-3-fluoromethoxybenzoyl)urea
A solution of 2,4,5-trifluoro-3-fluoromethoxybenzamide (6.65 g, 2.91
mmol, Example 36e) in 1,2-dichloroethane (12 mL) was treated dropwise with
oxalyl chloride (0.78 mL, 8.73 mmol). The mixture was stirred at room
temperature for 1 hour, heated at reflux for 4 hours, and the solvent was
removed
under reduced pressure to give 2,4,5-trifluoro-3-fluoromethoxybenzoyl
isocyanate, which was dissolved in dioxane (10 mL) and treated with a solution
of
cyclopropylamine (0.62 mL, 8.73 mmol) in dioxane (2 mL). The mixture was
warmed to room temperature for 18 hours and concentrated in vacuo. The residue
was dissolved in ethyl acetate, washed with brine, dried over sodium sulfate
and
evaporated. This residue was purified by chromatography (9:1 methylene
chloride/methanol) to give the title compound (0.88 g). 'H NMR (400 MHz,
CDCl3) 8 8.62 (d, 1H), 8.40 (bs, 1H), 7.70 (m, 1 H), 5.69 (d, 2 H), 2.78 (m, I
H),
0.82 (m, 1 H), 0.62 (m, 1 H).
g) 1-Cyclopropyl-6,7-difluoro-8-fluoromethoxy-1H-quinazolinedione
A solution of 1-cyclopropyl-3-(2,4,5-trifluoro-3-
fluoromethoxybenzoyl)urea (0.88 g, 2.90 mmol, Example 36f) in tetrahydrofuran
(35 mL) was treated portionwise with sodium hydride (0.35 g, 8.82 mmol, 60% in
mineral oil). The mixture was stirred at room temperature for 30 min. and then
heated at reflux overnight. The cooled reaction mixture was adjusted to pH 8.0
by
the addition of a saturated solution of ammonium chloride and extracted with
dichloromethane (3x50 mL). The combined organic layers were washed with
water, dried with sodium sulfate and concentrated in vacuo. The residue was
purified by chromatography (9:1 methylene chloride/methanol) to give the title
compound (0.56 g). ' H NMR (400 MHz, CDCl3) & 8.20 (bs, 1 H), 7.82 (t, 1 H),
5.64 (d, 2 H), 3.38 (m, I H), 1.20 (m, 2 H), 0.78 (m, 2 H).
h) {(S)-1-[(R)-1-(1-Cyclopropyl-6-fluoro-8-fluoromethoxydioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)pyrrolidin-3-yl]ethyl}carbamic acid tert-butyl ester
A mixture of 1-cyclopropyl-6,7-difluoro-8-fluoromethoxy-IH
1
quinazolinedione (0.160 g, 0.56 mmol, Example 36g), ((S)-(R)-1-pyrrolidin-3-
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-244-
ylethyl)carbamic acid tert-butyl ester (0.240 g, 1.12 mmol), triethylamine
(0.23
mL, 1.68 mmol) and dimethyl sulfoxide (4 mL) was heated at 90°C for 2
hours.
The cooled reaction mixture was diluted with ethyl acetate (20 mL) and washed
with brine (3x20 mL). The combined organic layers were dried over sodium
sulfate and concentrated, and the residue was purified by flash chromatography
(9:1 methylene chloridelmethanol) to give the title compound (0.29 g).'H NMR
(400 MHz, CDCl3) 8 8.00 (bs, 1 H), 7.56 (d, 1 H), 5.50-5.20 (m, 2 H), 4.50 (d,
1
H), 3.80-3.50 (m, 5 H), 3.25 (m, 1 H), 2.20 (m, 1 H), 2.05 (m, 1H), 1.62 (m, 1
H),
1.43 (s, 9 H), 1.25 (d, 3 H), 1.20-1.00 (m, 2 H), 0.70-0.60 (m, 2 H).
i) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yI]-1-cyclopropyl-6-fluoro-S-
fluoromethoxy-1H-quinazolinedione hydrochloride
A 0 °C solution of {(S)-1-[(R)-1-(1-cyclopropyl-6-fluoro-8-
fluoromethoxydioxo-1,2,3,4-tetrahydroquinazolin-7-yl)pyrrolidin-3-
yl}ethyl}carbamic acid tert-butyl ester (0.286 g, Example 36h) in diethyl
ether
was saturated with hydrogen chloride gas. The resulting mixture was stirred at
room temperature for 2 hours and the solvent removed in vacu~ to give the
title
compound (0.250 g).'H NMR (400 MHz, DMSO-d6) 8 11.30 (s; l H), 8.08 (bs,
3H), 7.40 (d, 1 H), 5.60- 5.40 (m, 2 H), 3.70-3.40 (m, 4 H), 3.25 (m, 1 H),
3.10
(m, 1 H), 2.30 (m, 1 H), 2.06 (m, 1 H), 1.62 (m, 1 H), 1.25 (d, 3 H), I .04
(m, 1 H),
0.92 (m, 1 H), 0.70 (m, I H), 0.60 (m, I H).
Example 37
a) 3-Ditluoromethyl-2,4,5-trifluorobenzoic acid
Under a nitrogen atmosphere, a -30 °C solution of
hexamethyldisilazane
(5.5 g, 34 mmol) in anhydrous tetrahydrofuran (30 mL) was treated with n-
butyllithium (17.2 mL of 2.5 M hexane solution, 34 mmol). After 30 min at -
30°C, the mixture was cooled to -50 °C and a solution of 2,4,5-
trifluorobenzoic
acid (3.0 g, 17 mmol) in tetrahydrofuran (20 mL) was added by syringe, and the
mixture was stirred at -10 °C for 2 hours. The mixture was cooled to -
30 °C,
anhydrous N,N dimethylformamide (3.8 mL, 38 mmol) was added, and the
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-245-
mixture was allowed to warm to 0 °C for 1 hour. Saturated aqueous
ammonium
chloride solution was added and mixture acidified with 2 N hydrochloric acid
and
extracted with ethyl acetate. The organic extracts were combined and washed
with
water, brine, dried with sodium sulfate and concentrated under vacuum to give
3.47 g of 3-formyl-2,4,5-trifluorobenzoic acid, which was used for the next
step
without further purification. A room temperature solution of this intermediate
in
dichloromethane was treated with (diethylamino)sulfur trifluoride (DAST) (13.7
g, 85 mmoI), and the reaction mixture was stirred at room temperature for 24
hours. After cooling to 5 °C, the reaction was quenched with ice
(exothermic
reaction!), washed with water and treated with aqueous ammonia for 1 hour at
room temperature. The aqueous layer was separated, acidified with 2 N
hydrochloric acid and extracted with dichloromethane. The organic extracts
were
dried over sodium sulfate, concentrated under vacuum and extracted with hot
hexanes. The hexane layers were combined and concentrated in vacuo and the
residue recrystallized from hexanes l ethyl acetate (10:1) to provide 0.98 g
of the
title compound as colorless crystals. 'H NMR (400 MHz, CDCI3) ~ 11.8-10.0 (bs,
1H), 8.02 (m, 1H), 6.98 (t, 1H).
b) 1-Cyclopropyl-3-(3-difluoromethyl-2,4,5-trifluorobenzoyl)urea
To a solution of 3-difluoromethyl-2,4,5-trifluorobenzoic acid (0.46 g, 2
mmol, Example 37a) in anhydrous dichloromethane (10 mL) was added oxalyl
chloride (0.63 g, 5 mmol) followed by N,N-dimethylformamide (1 drop). The
mixture was stirred for 2 hours and the solvent removed in vacuo. The residue
was dissolved in anhydrous benzene and cyclopropylurea (0.4 g, 4 mmol)was
added. The mixture was refluxed for 3 hours and diluted with ethyl acetate,
washed with water and brine, dried with sodium sulfate and concentrated.
Purification by flash chromatography on silica gel (hexane/ethyl acetate 3:1 )
provided 0.33 g of the title compound as a colorless solid.'H NMR (400 MHz,
CDCl3) 8 9.00 (d, 1 H), 8.38 (bs, 1 H), 7.96 (m, 1 H), 6.94 (t, 1 H), 2.73 (m,
1 H),
0.82 (m, 2H), 0.62 (m, 2H).
c) 1-Cyclopropyl-8-difluoromethyl-6,7-difluoro-1H-quinazolinedione
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-246-
Under a nitrogen atmosphere, a 0-5 °C solution of I-cyclopropyl-3-
(3-
difluoromethyl-2,4,5-trifluorobenzoyl)urea (0.33 g, 1.1 mmol, Example 37b) in
anhydrous tetrahydrofuran ( 10 mL) and N,N-dimethylformamide (0.5 mL) was
treated portionwise with sodium hydride (0.16 g of 60 % oil dispersion, 3.9
mmol). The mixture was stirred at room temperature for 30 min and at 60
°C for 3
hours. After cooling, the mixture was quenched with ice, quenched with 2 N
hydrochloric acid and extracted with ethyl acetate. The organic extracts were
washed with water, dried with sodium sulfate and concentrated in vacuo
affording
0.38 g of the title compound as a pale yellow solid. 'H NMR (400 MHz, CDC13) 8
8.60 (bs, IH), 8.06 (m, 1H), 7.40 (t, 1H), 3.31 (bs, 1H), 1.20 (m, 2H), 0.71
(m,
2H).
d) {(S)-1-[(R)-1-(1-Cyclopropyl-8-difluoromethyl-6-fluorodioxo-1,2,3,4-
tetrahydroquinazolin-7-yI)pyrrolidin-3-yl]ethyl}carbamic acid tert-butyl ester
A solution of 1-cyclopropyl-8-difluoromethyl-6,7-difluoro-1H-
quinazolinedione (0.29 g, 1.0 mmol, Example 37c), ((S)-(R)-1-pyrrolidin-3-
ylethyl)carbamic acid tert-butyl ester (0.43 g, 2 mmol), triethylamine (0.3 g,
3
mmol) and anhydrous dimethylsulfoxide (2 mL) was stirred at 80 °C fox 4
hours.
The cooled reaction was diluted with water and then extracted with ethyl
acetate.
The organic layers were combined and washed with water, brine, dried with
sodium sulfate and concentrated in vacuo. Purification of the residue by flash
chromatography on silica gel (dichloromethane / diethyl ether 2:1 ) gave 0.42
g of
the title compound as a colorless solid. 'H NMR (400 MHz, CDCl3) 8 8.34 (bs,
1 H), 7.71 (d, 1 H), 6.76 (t, 1 H), 4.50-4.40 (m, 1 H), 3.80-3.33 (m, 6H),
2.34-2.22
(m, 1 H), 2.12-2.06 (m, 1 H), 1.80-1.68 (m, 1 H), 1.43 (s, 9H), 1.28-1.12 (m,
SH),
0.66-0.52 (m, 2H).
e) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-8-
difluoromethyl-6-fluoro-1H-quinazoIinedione hydrochloride
A 0 °C solution of {(S)-1-[(R)-1-(1-cyclopropyl-8-difluoromethyl-6-
fluorodioxo-1,2,3,4-tetrahydroquinazolin-7-yl)pyrrolidin-3-yl]ethyl}carbamic
acid
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
_2q.7_
tert-butyl ester (0.42 g, 0.87 mmol, Example 37d) in dichloromethane (20 mL)
was treated with a stream of gaseous hydrogen chloride for 40 minutes. The
resulting precipitate was removed by filtration, washed with dichloromethane
and
dried in vacuo to give 0.32 g of the title compound as colorless crystals. tH
NMR
(400 MHz, DMSO-d6) 8 11.44 (s, 1H), 8.09 (bs, 3H), 7.58 (d, IH), 7.18 (t, 1H),
3.64-3.35 (m, 4H), 3.28-3.11 (m, 2H), 2.43-2.30 (m, 1 H), 2.12-1.98 (m, 1 H),
1.76-
1.64 (m, 1H), 1.24 (d, 3H), 1.16-0.97 (m, 2H), 0.61-0.43 (m, 2H). MSCI: m/z
381
(M+)
Example 38
a) 1-Thiophen-2-ylcyclopropanecarbonitrile
A stirred mixture of 2-thiapheneacetonitrile (2.5 g, 20 mmol),
benzyltriethylammonium bromide (0.54 g, 2.0 mmol), dichloromethane (20 mL),
and 50% aqueous sodium hydroxide solution (8 g, 200 mmol) was cooled to 0
°C
and treated dropwise with 1,2-dibromoethane (2.07 mL, 24 mmol). The mixture
was allowed to warm to room temperature and stir for two days. After diluting
with dichloromethane (20 mL) and water (30 mL), the aqueous layer was
extracted with dichloromethane (3x40 mL) and the combined organics were
washed with water (30 mL), brine (30 mL), dried with sodium sulfate and
concentrated in vacuo. The dark residual oil was purified by flash
chromatography
(10/90 ethyl acetate/hexane) to give the title compound as a light brown oil
(1.40
g ). 1H NMR (200 MHz, CDC13) 8 7.40 (dd, l H), 7.20 (dd, 1 H), 6.95 (dd, 1 H),
1.55 (m, 2H), 1.45 (m, 2H).
2S b) 1-Thiophen-2-ylcyclopropanecarboxylic acid
A 5 °C solution of 1-thiophen-2-ylcyclopropanecarbonitrile (12.3
g, 82.6
mmol, Example 38a) in ethanol (150 mL) was treated with 6 N sodium hydroxide
(100 mL) and stirred at 0 °C for 5 minutes. The reaction mixture was
then'heated
at reflux for 4 hours, the mixture was cooled and the ethanol was removed in
vacuo. The basic, aqueous residue was acidified with 6 N hydrochloric acid and
extracted with ethyl acetate (3x l00 mL). The combined organics were dried
over
sodium sulfate and the solvent was removed in vacuo to give the title compound
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-248-
as white crystals (13.60 g). 1H NMR (400 MHz, DMSO-d6) 8 12.5 (bs, 1H), 7.40
(dd, 1H), 7.00-6.90 (m, 2H), 1.60 (m, 2H), 1.28 (m, 2H).
c) (1-Thiophen-2-ylcyclopropyl)carbamic acid tart-butyl ester
A room temperature solution of 1-thiophen-2-ylcyclopropanecarboxylic
acid (3.20 g, 19.0 mmol, Example 38b) in tart-butanol (50 mL) was treated
dropwise with diphenylphosphoryl azide (5.48 mL, 24.8 mmol) followed by
triethylamine (4.23 mL, 30.4 mmol). The mixture was stirred at room
temperature
for 2 hours and then heated at reflux for 20 hours. The solvent was removed in
vacuo and the residue chromatographed on silica gel ( 10/90 ethyl
acetate/hexane)
to yield the title compound (3.20 g). 'H NMR (200 MHz, CDCl3) 8 7.10 (dd, 1H),
6.90 (dd, 1 H), 6.80 (dd, 1 H), 5.35 (bs, 1 H), 1.45 (s, 9H), 1.30 (m, 2H),
1.22 (m,
2H).
d) [1-(5-Z'ributylstannylthiophen-2-yl)cyclopropyl]carbamic acid tart butyl
ester
A -78 °C solution of compound (1-thiophen-2-ylcyclopropyl)carbamic
acid tart-butyl ester (1.24 g, 5.00 mmol, Example 38c) in tetrahydrofuran (30
mL)
was treated dropwise with n-butyllithium (2.5 M in hexanes, 5 mL, 12.5 mmol),
warmed to -20 °C and stirred for 3 hours. After recooling to -78
°C, a solution of
tri-n-butyltin chloride (1.9 g, 6.0 mmol) in tetrahydrofuran (8 mL) was added
dropwise. The reaction mixture was warmed to room temperature, and partitioned
between ethyl acetate and water. The aqueous layer was extracted with ethyl
acetate, and the combined extracts washed with water, brine, dried with sodium
sulfate, filtered, and concentrated. The residue was purified by silica gel
chromatography (4/96 ethyl acetate/hexane and 0.5% triethylamine) to provide
the
title compound (1.08 g) as a colorless oil. 1H NMR (400 MHz, CDC13) b 6.94 (d,
1H), 6.92 (d, 1H), 5.40 (bs, 1H), 1.55 (m, 6H), 1.45 (s, 9H), 1.35 (m, 6H),
1.26
(m, 4H), 1.07 (m, 6H), 0.90 (t, 9H).
e) {1-[5-(1-cyclopropyl-6-fluoro-8-methyldioxo-1,2,3,4-tetrahydroquinazolin-
7-yl)thiophen-2-yl]cyclopropyl}carbamic acid tart-butyl ester
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-249-
A mixture of [1-(5-tri-n-butylstannylthiophen-2-yl)cyclopropyl]carbamic
acid tert-butyl ester (0.74 g, 1.4 mmol, Example 38d), I-cyclopropyl-6-fluoro-
7-
iodo-8-methyl-IH-quinazoIinedione (0.50 g, 1.4 mmol, Example 25h),
dichlorobis(triphenylphosphine)palladium(II) (0.11 g, 0.16 mmol), and
triphenylarsine (0.165 g, 0.54 mmol) in toluene (20 mL) was heated at 90-95
°C
for 24 hours. After evaporation of the solvent, the residue was purified by
chromatography (1:l ethyl acetate/hexane) to give the title compound (0.40 g).
1H
NMR (400 MHz, CDCl3) 8 8.38 (bs, 1 H), 7.70 (d, 1 H), 6.90 (d, 1 H), 6.84 (d,
1 H),
5.40 (bs, 1H), 3.38 (m, 1H), 2.50 (s, 3H), 1.45 (s, 9H), 1.40-1.20 (m, 6H),
0.78 (m,
2H).
fj 7-(5-(1-Aminocyclopropyl)thiophen-2-yl]-1-cyclopropyl-6-fluoro-8-methyl-
1H-quinazolinedione hydrochloride
A 0 °C solution of { 1-[5-(1-cyclopropyl-6-fluoro-8-methyldioxo-
1,2,3,4-
IS tetrahydro-quinazolin-7-yI)thiophen-2-yl]cyclopropyljcarbamic acid tert-
butyl
ester (0.209 g, Example 38e) in dichloromethane was saturated with hydrogen
chloride gas. After stirring at room temperature overnight, the resulting
precipitate
was removed by filtration, washed with dichloromethane and dried in vacuo to
give the title compound (0.150 g). 'H NMR (400 MHz, DMSO-d6) 8 1 I .b0 (s,
1 H), 9.00 (bs, 3H), 7.60 (d, I H), 7.40 (d, 1 H), 7.20 (d, 1 H), 3.34 (m, 1
H), 2.40 (s,
3H), 1.50 (m, 2H), 1.38 (m, 2H), 1.05 (m, 2H), 0.62 (m, 2H).
Example 39
a) 1-Cyclopropyl-3-(3-dif7uoromethoxy-2,4,5-trifluorobenzoyl)urea
A solution of 3-difluoromethoxy-2,4,5-trifluorobenzamide (80g, 330
mmol, EP 352123 A2), oxalyl chloride (126.6g 990 mmoles) and 1,2-
dichloroethane (800m1) was heated at reflux for 4 hours. The solvent was
removed in vacuo and the residue was dissolved in dry 1,4-dioxane (700m1) and
cooled to ~5°C. CyclopropyIamine (36.5 g, 640 mmol) was added and the
reaction
mixture was stirred at room temperature for 16 hours. The solvent was removed
in vacuo and the residue was dissolved in ethyl acetate, washed with brine,
dried
with sodium sulfate and concentrated in vacuo. The residue was triturated with
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-2so-
hexanes, and the solid was removed by filtration, washed with hexanes and
dried
to give 88 g of the title compound as a light yellow semi solid. 'H NMR
(400MHz, DMSO-d6): 8 11.00 (s, 1 H), 8.2 (s, 1 H), 7.8 (m, 1 H), 7.2s (t, 1
H), 2.63
(s, IH), 0.6s (m, 2H), O.sO (m, 2H)
b) 1-Cyclopropyl-8-difluoromethoxy-6,7-difluoro-1H-quinazolinedione
Under a nitrogen atmosphere, a solution of 1-cyclopropyl-3-(3-
difluoromethoxy-2,4,s-trifluorobenzoyl)urea (87 g, 277 mmol, Example 39a) in
anhydrous tetrahydrofuran (750 ml) and dimethylformamide (7sm1), was treated
portionwise over 4s minutes, with sodium hydride (38 g, 60% in mineral oil,
9s0
mmol). After heating at reflux for 2 hours, the reaction mixture was cooled to
room temperature, diluted with 5 °C water, acidified with 2N
hydrochloric acid
and extracted with ethyl acetate. The organic extract was washed with a 10%
aqueous sodium carbonate, water, brine, dried with sodium sulfate and
1 s concentrated in vacuo. Purification of the residue by flash chromatography
on
silica gel (hexane/ethyl acetate 2:1 ) afforded 25 g of the title compound as
a Light
yellow solid.
' H NMR (400 MHz, CDC13) 8 9.00 (bs, I H), 7.9s (dd, 1 H), 6.68 (t, I H), 3.23
(m,
1 H), 1.21 (m, 2H), 0.78 (m, 2H).
c) 1-((S)-1-Phenylethyl)pyrrolidine-3-carboxylic acid dibenzylamide
N,N-Dibenzylacrylamide (79.5 g, 0.317 mol, [WO 9801417]) and N
(methoxymethyl)-N-(trimethylsilylmethyl)-(S)-a-methylbenzylamine (103 g, 4I2
mmoI) were dissolved in dichloromethane ( 1 s00 mL) and cooled to 0 °C.
Trifluoroacetic acid (IM in dichloromethane, 27 mL) was added over a period of
20 minutes and the resulting reaction mixture was stirred at room temperature
overnight. The mixture was washed with aqueous sodium bicarbonate, brine,
dried over sodium sulfate and concentrated. The residue was purified by flash
chromatography (10:2:0.1 heptanelethyl acetate/triethylamine) to afford the
title
compound (97.7 g), which was used in the following reaction without further
purification.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-251-
d) Dibenzyl-{1-[(R)-1-((S)-1-phenylethyl)pyrrolidin-3-yl]cyclopropyl}amine
Ethylmagnesium bromide (3M in ether, 178 mL) was added to dry
tetrahydrofuran ( 1400 mL) and the solution was cooled to -78 °C under
a nitrogen
atmosphere. A solution of titanium tetraisopropoxide (66.0 mL, 0.228 mol) in
dry
tetrahydrofuran (150 mL) was then added while maintaining the temperature
below -68 °C. After the addition was complete, the solution was stirred
for three
minutes and then 1-((S)-1-phenylethyl)pyrrolidine-3-carboxylic acid
dibenzylamide (86.6 g, 0.218 mmol, Example 39c) dissolved in dry
tetrahydrofuran (150 mL) was added, maintaining the temperature below -68
°C.
The reaction mixture was allowed to warm to room temperature, stirred for 1
hour, then heated at reflux for 1 hour. The reaction mixture was then cooled
to 8
°C, and ethylmagnesium bromide (3M in ether, I50 mL) was added followed
by
the rapid addition of titanium tetraisopropoxide (55.6 mL, 192 mmol) in
tetrahydrofuran ( 150 mL). The resulting mixture was stirred at room
temperature
for 1 hour before being quenched with aqueous ammonium chloride (3000 mL)
and water (800 mL). The mixture was filtered through Celite, rinsed with ether
and the organic layer separated. The mixture was made basic (pH 8.5) with
sodium hydroxide and extracted with ether. The combined organic layers were
combined and dried over sodium sulfate, concentrated and purified by flash
chromatography (10:1:0.1 heptane/ethyl acetate/triethylamine) to provide the
title
compound (31.3 g) as colorless crystals: mp 76-76.5 °C.
e) (R)-1-pyrrolidin-3-yl-cyclopropylamine
20% palladium on carbon (0.25 g) was added to a solution of dibenzyl-{ I-
[(R)-1-((S)-I-phenylethyl)pyrrolidin-3-yl]cyclopropyl}amine (1.0 g, 2.4 mmol,
Example 39d) in glacial acetic acid (50 mL) and the reaction vessel
pressurized
with hydrogen gas (48 psi) overnight. The mixture was then filtered,
concentrated
in vacuo and the residual dissolved in methanol (20 mL) and stirred with I1ZA-
400-OH basic ion exchange resin. The mixture was filtered after 1 hour and the
filtrate concentrated to give the title compound (0.307 g): IH NMR (CD30D) 8
3.46-3.37 (m, 2H), 3.24 (m, 1 H), 3.13 (dd, l H), 2.25-2.09 (m, 2H), l .88 (m,
2H),
0.73-0.59 (m, 4H).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-252-
f~ 7-[(R)-3-(1-Aminocyclopropyl)pyrrolidin-x-y1]-1-cyclopropyl-8-
diflouromethoxy-6-fluoro-1H-quinazolinedione
1-Cyclopropyl-8-difluoromethoxy-6,7-difluoro-1 H-quinazolinedione
(0.49 g, 1.62 mmol, Example 39b) and (R)-1-pyrrolidin-3-ylcyclopropylamine
(0.31 g, 2.4 mmol, Example 39e) in dimethyl sulfoxide (5 mL) were heated at 90
°C for 5 hours. The solution was diluted with brine and extracted with
ethyl
acetate. The organic layers were then combined, dried over magnesium sulfate,
filtered and concentrated. The residue was purified by flash silica gel
chromatography (2-5% methanolldichloromethane) to afford the title compound
(0.51 g) as a solid, which was re-crystallized to give an analytically pure
sample:
mp 2I 0-2I 4 °C; ' H NMR (DMSO-d6) 8 7.46 (d, 1 H), 6.79 (t, 1 H), 3.77
(m, 1 H),
3.65 (dt, IH), 3.48 (m, 2H), 3.35 (bs,1H), 3.12 (m, 1H), 2.05-1.88 (m, 2H),
1.78
(m, 1 H), I . I 1 (m, 1 H), 0.96 (m, 1 H), 0.73-0.58 m, 2H), 0.5I -0.42 m,
4H).
Example 40
a) {(S)-1-[(R)-1-(1-Cyclopropyl-6-fluoro-8-ditluoromethoxydioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)pyrrolidin-3-yl]ethylJcarbamic acid tert-butyl ester
A mixture of I-cyclopropyl-6,7-difluoro-8-difluoromethoxy-1H-
quinazolinedione (0.200 g, 0.66 mmol, Example 39b), ((S)-(R)-1-pyrrolidin-3-
ylethyl)carbamic acid tent-butyl ester (0.225 g, 1.97 mmol) and dimethyl
sulfoxide
(2 mL) was heated at 90 °C for 1.5 hours. The solution was then treated
with
saturated ammonium chloride, stirred for 1 hour then filtered. The collected
solid
was washed with water and dried to afford the title compound (0.129 g). MSCI:
m/z 497 (M~)
b) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-t7uoro-8-
difluoromethoxy-1H-quinazolinedione hydrochloride
A solution of {(S)-1-[(R)-1-(1-Cyclopropyl-6-fluoro-8-
difluoromethoxydioxo-I,2,3,4-tetrahydroquinazolin-7-yl)pyrrolidin-3-
yl]ethyl }carbamic acid ter-t-butyl ester (0.129 g, Example 40a) in methanol
(3 mL)
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-253-
was treated with a 2 M diethyl ether solution of hydrochloric acid (4 mL, $
mmol)
and allowed to stir for 6 hours. The mixture was then concentrated in vacuo,
re-
dissolved in water and lyophilized to provide a solid (0.095 g). mp >250
°C;
MSCI: m/z 399 (MH+).
Example 41
a) 1-Cyclopropyl-6,7-di~luoro-5,8-dimethyl-llY-quinazolinedione
A solution of 1-cyclopropyl-6,7-difluoro-8-methyl-1H quinazolinedione
(0.50 g, 2.0 mmol) in tetrahydrofuran (10 mL) was cooled to -20 °C and
treated
with a 2.0 M tetrahydrofuran solution of lithium diisopropylamine (3.1 mL, 6.3
mmol).. The mixture was allowed to stir for 1 hour then cooled to -78
°C and
treated with iodomethane (0.31 mL, 5.0 mmol). After stirring for 1 hour, the
mixture was poured into saturated ammonium chloride and extracted with ethyl
acetate. The extracts were combined, dried with sodium sulfate and purified
via
1 S silica column chromatography (hexanes/ethyl actetate) to provide a solid
(0.206
g). MSCI: m/z 267 (MHO).
b) {(S)-1-[(R)-1-(1-Cyclopropyl-6-fluoro-5,8-dimethyldioxo-1,2,3,4-
tetrahydro-quinazolin-7-yl)pyrrolidin-3-yl]ethyl]carbamic acid tart-butyl
ester
1-Cyclopropyl-6,7-difluoro-5,8-dimethyl-1H-quinazolinedione (0.20 g,
0.75 mmol, Example 41a), 1,1,3,3-tetramethylguanidine (0.37 mL, 3.0 mmol) and
((S)-(R)-1-pyrrolidin-3-ylethyl)carbamic acid tart-butyl ester (0.68 g, 6.0
mmol) in
dimethyl sulfoxide (2 mL) were heated at 90 °C for 2 days. The solution
was
diluted with saturated ammonium chloride and extracted with ethyl acetate. The
organic layers were combined, dried over magnesium sulfate, filtered and
concentrated. The residue was purified by flash silica gel chromatography (1:1
hexanes:ethyl acetate) to afford the title compound (0.215 g). MSCI: m/z 461
(MH )
c) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-tluoro-5,8-
dimethyl-1H-quinazolinedione hydrochloride
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-254-
A solution of {(S)-1-[(R)-1-(1-cyclopropyl-6-fluoro-5,8-dimethyldioxo-
1,2,3,4-tetrahydro-quinazolin-7-yI)pyrrolidin-3-yl]ethyl}carbamic acid tert-
butyl
ester (0.22 g, 0.75 mmol, Example 41b) in a mixture of methanol (3 mL) and
dichloromethane (3 mL) was treated with a 2.0 M diethyl ether solution of
S hydrogen chloride (4 mL, 8 mmol) and allowed to stir for 6 hours. The
mixture
was concentrated in vacuo, re-dissolved in water and lyophilized to provide a
solid (0.095 g); mp 207 °C; MSCT: m/z 461 (MH+).
Example 42
a) 2,4-Dibromo-3-difluoromethoxybenzamide
To a suspension of 2,4-dibromo-3-difluoromethoxybenzoic acid (73.3 g,
210 mmol, [WO 9921849 A1]), N,N dimethylformamide (1.0 ml) and
dichloromethane (700 ml) was added dropwise, oxalyl chloride (40.3 g, 320
mmol). After heating at reflux for 5 hours and stirring at room temperature
for 2
hours, the solvent was removed in vacuo. The residue was dissolved in
anhydrous
tetrahydrofuran (500 ml) and added to a -70 °C solution of diethyl
ether saturated
with ammonia gas. The reaction mixture was stirred at room temperature for 2
hours and evaporated under vacuum. The residue was dissolved in ethyl acetate,
washed with water, brine, dried over sodium sulfate, filtered and evaporated
in
vacuo to give 58.0 g of the title compound as a white solid, mp 186
°C.'H NMR
(400 MHz, CDC13): 8 7.65 (d, 1 H); 7.39 (d, I H); 6.62 (t, 1 H); 6.0 (bs, 2H).-
b) 1-Cyclopropyl-3-(2,4-dibromo-3-difluoromethoxybenzoyl)urea
To a suspension of 2,4-dibromo-3-difluoromethoxybenzamide (58.0 g, 168
mmol, Example 42a) in 1,2-dichloroethane (600 ml) was added oxalyl chloride
(53.0 g, 420 mmol). After stirring for 1 hour at room temperature, the mixture
was
refluxed for 5 hours, cooled and the solvent removed in vacuo. The oily
residue
was dissolved in dioxane (500 ml), cooled to 0 °C and treated with a
solution of
cyclopropylamine (20.0 g, 350 mmol) in dioxane (50 ml). After stirring at room
temperature for 18 hours, the solvent was removed in vacuo and the residue
triturated with a mixture of diethyl ether and hexanes. The resulting solid
was
collected by filtration, washed with hexanes and dried in vacuo to give 60.8 g
of
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-255-
the title compound as a white solid, mp 170° C. 1H NMR (400 MHz, CDCl3)
8
8.45 (s, 1 H); 8.25 (s, 1 H); 7.65 (d, 1 H); 7.25 (d, 1 H); 6.65 (t, 1 H);
2.65 (m, 1 H);
0.80 (m, 2H); 0.62 (m, 2H).
c) 7-Bromo-1-cyclopropyl-8-difluoromethoxy-1H-quinazolinedione
To a 0 °C solution of 1-cyclopropyl-3-(2,4-dibromo-3-
difluoromethoxy
benzoyl)urea (60.0 g, 140 mmol, Example 42b) in anhydrous tetrahydrofuran (600
ml) was added dropwise, at room temperature, potassium
bis(trimethylsilyl)amide
(700 ml, 0.5 M in toluene, 0.35 mol). After stirring for 20 minutes, 18-crown-
6
( 16.8 g) was added and the reaction heated to reflux for 2.5 hours. The
solvent
was removed in vacuo and the residue partitioned between ethyl acetate and 1N
hydrochloric acid. The organic layer was washed with water, brine, dried with
magnesium sulfate, filtered and evaporated under vacuum. Purification of the
residue by chromatography on silica gel (hexane/ethyl acetate) yielded 13.6 g
of
the title compound as a white solid, mp 244° C.'H NMR (CDC13): b 8.17
(s, 1H);
7.88 (d, 1H); 7.48 (d, 1H); 6.45 (t, 1H); 3.39 (m, 1H); 1.22 (m, 2H); 0.65 (m,
2H).
d) 1-Cyclopropyl-8-difluoromethoxy-7-((R)-1-methyl-Z-trityl-2,3-dihydro-1H-
isoindol-5-yl)-1H-quinazolinedione
To a suspension of 2-[(1R)-1-methyl-2-trityl-2,3-dihydro-1H 5-
isoindolyl]-1,3,6,2-dioxazaborocane (0.375 g, 0.768 mmol, [EP 1031569]) in
ethyl acetate (1.8 mL) and water (0.8 mL) was added 7-bromo-1-cyclopropyl-8-
difluoromethoxy-1H-quinazolinedione (0.10 g, 0.288 mmol, Example 42c),
sodium carbonate (0.128 g, 1.21 mmol) and bis(triphenylphosphine)palladium(II)
chloride (0.040 g, 0.057 mmol). The resulting mixture was heated at 80
°C for 24
hours. The mixture was cooled to room temperature and partitioned between
dichloromethane and water. The recovered organics were washed with brine,
dried over magnesium sulfate, filtered, and concentrated in vacuo. The
resulting
residue was purified by flash silica gel chromatography (100:0 to 50:50
hexanes:ethyl acetate) to afford the title compound (0.192 g) as a yellow
solid:
MSCI: m1z 642 (MH+).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-256-
e) (R)-S-(1-Cyclopropyl-8-difluoromethoxydioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)-1-methyl-1,3-dihydroisoindole-2-carboxylic acid
tert-butyl ester
Hydrogen chloride gas was bubbled into a 0 °C solution of 1-
cyclopropyl-
8-difluoro-methoxy-7-((R)-l-methyl-2-trityl-2,3-dihydro-1H-isoindol-5-yl)-1H
quinazolinedione (0.192 g, 0.299 mmol, Example 42d) in methanol (5 mL) and
dichloromethane (5 mL) for 20 minutes. The reaction mixture was warmed to
room temperature and stirred for 24 hours. The solvent was removed in vacuo,
and the resulting solid was treated with dichloromethane (10 mL),
triethylamine
(0.30 mL), and di-tert-butyl dicarbonate (0.323 g). After 24 hours at room
temperature, the reaction mixture was concentrated in vacuo and the resulting
residue was purified by preparatory thin layer chromatography eluting with 20%
ethyl acetate in dichloromethane to provide the title compound (0.033 g, 25%)
as
a yellow solid: MSCI: m/z 500 (MH+).
f7 1-Cyclopropyl-8-difluoromethoxy-7-((R)-1-methyl-2,3-dihydro-1H-
isoindol-S-yl)-1H-quinazolinedione hydrochloride
Hydrogen chloride gas was bubbled into a 0 °C solution of (R)-5-(1-
cyclopropyl-8-difluoromethoxydioxo-I ,2,3,4-tetrahydroquinazolin-7-yl)-1-
methyl-1,3-dihydro-isoindole-2-carboxylic acid tert-butyl ester (0.033 g,
0.066
mmol, Example 42e) in methanol (3 mL) and dichloromethane (6 mL) for 15
minutes. The mixture was warmed to room temperature and stirred for 4 hours.
The mixture was concentrated in vacuo, and the resulting solid was washed with
hexanes and dried to afford the title compound (0.022 g, 85%) as a yellow
solid,
mp l 80-185 °C: MSCI: m/z 400 (MH+)
Example 43
a) 2,4-Difluoro-3-methoxybenzamide
A 0 °C suspension of 2,4-difluoro-3-methoxybenzoic acid (147.8 g,
786
mmol, PCT Int. Appl. WO 9914214) in dichloromethane (1.5 L) was treated with
oxalyl chloride (109.8 g, 865 mmol) followed by dimethylformamide (2 ml). The
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-257-
mixture was stirred at room temperature for 16 hours and the solvent removed
in
vacuo. The residual oil was dissolved in anhydrous tetrahydrofuran (500 ml)
and
added to a -70 °C solution of ether ( 1.5 L) saturated with ammonia
gas. After the
addition was complete, the reaction was allowed to come to room temperature
where it was stirred for 1 hour. After diluting with ethyl acetate, the
mixture was
washed with water, brine, dried with magnesium sulfate, filtered and
concentrated
in vacuo to give 137.5 g of the title compound, mp I I8-I20 °C.'H NMR
(200
MHz, DMSO-d6): 8 7.77 (bs, 1H), 7.70 (bs, 1H), 7.32-7.43 (m, 1H), 7.16-7.26
(m,
1H), 3.93 (s, 3H).
b) 2,4-Difluoro-3-hydroxybenzamide
A -70 °C solution of 2,4-diftuoro-3-methoxybenzamide (124.0 g, 663
mmoI, Example 43a) in methylene chloride (2.5 L) was treated dropwise, over 1
hour, with boron tribromide (338.0 g, 1350 mmol). The reaction was stirred at
room temperature for 18 hours, cooled to -70 °C, quenched with water
and diluted
with ethyl acetate, tetrahydrofuran and brine. The organic layer was separated
and
the aqueous layer was extracted with a mixture of ethyl acetate and
tetrahydrofuran (2:1 ). The combined extracts were dried over anhydrous sodium
sulfate, filtered and concentrated in vacuo affording 102.6 g of the title
compound,
mp 161-162 °C.'H NMR (400 MHz, DMSO-d6): 8 10.41 (s, 1H), 7.69 (bs,
1H),
7.62 (bs, 1 H), 7.03-7.15 (m, 2H).
c) 3-Difluoromethoxydifluorobenzamide
In a steel bomb, a -70 °C mixture of 2,4-difluoro-3-
hydroxybenzamide
(173.0 g, 1000 mmol, Example 43b), potassium carbonate (165.6 g, 1200.0 mmol)
and dimethylformamide (500 ml) was treated with a solution of
dichlorodifluoromethane (1200 g, 1400 mmol) in N>N-dimethylformamide (800
ml). The reaction mixture was heated at 110 °C for 41 h, cooled to room
temperature and the contents of the steel bomb added to a mixture of ethyl
acetate
(2 L) and water (4 L). The mixture was adjusted to pH 2 with 6N hydrochloric
acid, the organic layer was separated and aqueous layer was extracted
copiously
with ethyl acetate. The combined organics were washed with brine, dried with
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-258-
sodium sulfate, filtered and concentrated under vacuum. Recrystallization of
the
residue (hexane/ethyl acetate) yielded 159.6 g of the title compound, mp 124
°C.
1H NMR (400 MHz, DMSO-d6): 8 7.87 (bs, 1H), 7.79 (bs, 1H), 7.59-7.70 (m,
1 H), 7.32-7.43(m, 1 H), 7.28 (t, 1 H).
d) 1-Cyclopropyl-3-(3-difluoromethoxydifluorobenzoyl)urea
A solution of 3-difluoromethoxydifluorobenzamide (60.0 g, 257 mmol,
Example 43c), oxalyl chloride (98.0 g, 773 mmol) and 1,2-dichloroethane (330
ml) was heated at reflux for 4 hours. The solvent was removed in vacuo to give
an oil that was used without further purification. A 0 °C solution of
the oil in
dioxane (330 mL) was treated rapidly dropwise with a solution of
cyclopropylamine (33 ml, 470 mmol) in dioxane (330 ml). After stirring at room
temperature for 1.5 hours, the mixture was diluted with water, extracted with
ethyl
acetate and the organic extracts washed with brine, dried with sodium sulfate,
filtered and evaporated under in vacuo to give 66.0 g of the title compound,
mp
128-130 °C. 1H NMR (400 MHz, DMSO-d6): 810.90 (s, 1H), 8.27 (d, 1H),
7.58-
7.69 (m, 1 H), 7.37-7.47 (m, 1 H), 7.27 (t, 1 H), 2.63-2.75 (m, 1 H), 0.62-
0.75 (m,
2H), 0.53-0.59 (m, 2H).
e) 1-Cyclopropyl-8-difluoromethoxy-7-fluoro-1F1-quinazolinedione
A solution of 1-cyclopropyl-3-(3-difluoromethoxydifluorobenzoyl)urea
(60.0 g, 195 mmol, Example 43d) in a mixture of tetrahydrofuran (1 L) and N,N
dimethylformamide (75 ml) was treated portionwise with sodium hydride (30.0 g,
60°Io in mineral oil, 750 mol). After heating at reflux for 5 hours,
the cooled
reaction was poured over ice, adjusted to pH 2 with 6N hydrochloric acid and
extracted with ethyl acetate. The organic extracts were washed with brine,
dried
with sodium sulfate, filtered and concentrated in vacuo. Purification of the
residue by chromatography on silica gel (hexane/ethyl acetate 1:1) afforded
21.0 g
of the title compound, mp 215-221 °C.'H NMR (400 MHz, CDCI3) 8 8.80 (s,
1 H), 8.16 (m, 1 H), 7.14 (m, 1 H), 6.42 (t, 1 H), 3.24 (m, 1 H), 1.21 (m,
2H), 0.71
(m, 2H).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-259-
t7 {(S)-1-[(R)-1-(1-Cyclopropyl-8-difluoromethoxydioxo-1,2,3,4-
tetrahydroquinazolin-7-yl)pyrrolidin-3-ylJethyl}carbamic acid tart-butyl ester
A mixture of 1-cyclopropyl-8-difluoromethoxy-7-fluoro-1H-
quinazolinedione (0.2 g, 0. mmol, Example 43e), ((S)-(R)-1-pyrrolidin-3-
ylethyl)carbamic acid tart-butyl ester (0.225 g, 1.97 mmol) and dimethyl
sulfoxide
(2 mL) was heated at 90 °C for 1.5 hours. The solution was treated with
saturated
ammonium chloride, stirred for 1 hour and filtered. The collected solid was
washed with water and dried to afford the title compound (0.317 g). MSCI: m/z
481 (MH+).
g) 7-[(R)-3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-8-
ditluoromethoxy-1H-quinazolinedione hydrochloride
A solution of {(S)-1-[(R)-1-(1-cyclopropyl-8-difluoromethoxydioxo-
1,2,3,4-tetrahydroquinazolin-7-yl)pyrrolidin-3-yl]ethyl}carbamic acid tart-
butyl
ester (0.317 g, Example 43f) in methanol (2 mL) was treated with a 2 M diethyl
ether solution of hydrogen chloride (S mL, 10 mmol) and allowed to stir for 1
hour. The mixture was concentrated in vacuo, re-dissolved in water and
lyophilized to provide a solid (0.190 g); mp >250 °C; MSCI: m/z 381
(MH+).
Example 44
a) [(3R, 4S)- and (3S, 4R)-1-(1-Cyclopropyl-8-difluoromethoxy-6-fluorodioxo-
1,2,3,4-tetrahydroquinazolin-7-yl)-4-trifluoromethylpyrrolidin-3-
ylmethyl]carbamic acid tart-butyl ester
A mixture of 1-cyclopropyl-6,7-difluoro-8-difluoromethoxy-1H
quinazolinedione (0.20 g, 0.66 mmol, Example 39b), ((3S, 4S)- and (3R, 4R)-4-
trifluoromethylpyrrolidin-3-ylmethyl)carbamic acid, tart-butyl ester (0.225 g,
1.97
mmol, [Bioorg. Med. ahem. Lett. 1998, 8, 2833]), triethylamine (0.28 mL, 2.0
mmol) and dimethyl sulfoxide (1 mL) was heated at 90 °C for 2 hours.
The
solution was treated with saturated ammonium chloride, stirred for 2 hours and
filtered. The collected solid was washed with water and dried to afford the
title
compound (0.372 g). MSCI: mlz 553 (MH+).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-260-
b) 7-((3R, 4S)- and (3S, 4R)-3-Aminomethyl-4-trifluoromethylpyrrolidin-1-
yl)-1-cyclopropyl-8-difluoromethoxy-6-fluoro-1H-quinazolinedione
hydrochloride
A solution of [(3R, 4S)- and (3S, 4R)-1-(1-Cyclopropyl-8-
difluoromethoxy-6-fluorodioxo-1,2,3,4-tetrahydroquinazolin-7-yl)-4-
trifluoromethylpyrrolidin-3-ylmethyl]carbamic acid tart-butyl ester (0.317 g,
Example 44a) in methanol (5 mL) was treated with a 2 M diethyl ether solution
of
hydrogen chloride (6 mL, 12 mmol) and allowed to stir for 4 hours. The mixture
was concentrated irz vaeuo, re-dissolved in water and lyophilized to provide a
solid (0.298 g). mp 189 - 190 °C; MSCI: m/z 453 (MH+).
General Procedure for Examples 45 - 68: Array Chemistry:
In a 2-dram vial, a 0.33 M solution of the template (0.300 mL, 1 mmol, [1-
cyclopropyl-6,7-difluoro-8-methyl-1H-quinazolinedione or 1-cyclopropyl-6,7-
difluoro-8-methoxy-1H-quinazolinedione]) was treated with a 1 M solution of
300
p,L of side chain (0.30 mL, 0.3 mmol) and 1,1,3,3-tetramethylguanidine (0.024
mL, 0.2 mmol). The mixture was shaken at 90 °C for 20 hours. Upon
completion, the solution was concentrated using a Genevac concentrator (HT-
12).
The products were 1-iltered through a silica gel plug eluting with 20% ethyl
acetate
in methylene chloride, to provide clean product.
Any product that used ethyl-[(S)-(R)-1-pyrrolidin-3-ylethyl]amine or
methyl-[(S)-(R)-1-pyrrolidin-3-ylethyl]amine, as the side chain, was treated,
prior
to purification, with a 0.3 M solution of di-t-butyl dicarbonate (0.069 mL,0.3
mmol) in dichloromethane overnight at room temperature. The mixture was then
concentrated using a Genevac concentrator (HT-12) and the product purified by
filtration through a silica gel plug eluting with 20% ethyl acetate in
dichloromethane.
The product was then treated with 2 ml of a saturated solution hydrogen
chloride in methanol, at room temperature, overnight. The solution was then
concentrated, as above, and purified by high pressure liquid chromatography
(gradient: l0 to 100% 3% n-propanol in acetonitrile / 3% aqueous n-propanol).
The compound was analyzed by LC-MS.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-261-
Example 45
I-Cyclopropyl-6-fluoro-8-methoxy-7-[3(R)-(1(S)-
methylaminoethyl)pyrrolidin-1-yl]-1H-quinazolinedione from 1-cyclopropyl-
6,7-difluoro-8-methoxy-1H-quinazolinedione and [3(R)-(1(S)-
methylaminoethyl)pyrrolidin-1-yl]carbamic acid tart-butyl ester; MSCI: m/z 377
(MH+).
Example 46
1-Cyclopropyl-6-fluoro-8-methyl-7-[3(R)-(1 (S)-
methylaminoethyl)pyrrolidin-1-yl]-1H-quinazolinedione, from 1-cyclopropyl-
6,7-difluoro-8-methyl-1H-quinazolinedione and [3(R)-(1(S)-
methyIaminoethyl)pyrrolidin-1-yl]carbamic acid tart-butyl ester; MSCI: m/z 361
(MH+).
Example 47
7-(3-Aminopiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione and piperidin-3-yl-carbamic acid tart-butyl ester [J. Med.
Chem.
1995, 38(22), 4478.]; MSCI: m/z 349 (MH+).
Example 48
1-Cyclopropyl-6-fluoro-8-methoxy-7-(octahydropyrrolo[3,4-c]pyridin-
2-yl)-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione and octahydopyrrolo[3,4-c]pyridine-5-carboxylic acid tart-
butyl
ester [WO 0153273 Al]; MSCI: m/z 375 (MH+)
Example 49
7-((S)-3-Aminopyrrolidin-1-yI)-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methyl-1H
quinazolinedione and (S)-pyrrolidin-3-ylcarbamic acid tart-butyl ester; MSCI:
mlz
319 (MH+)
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-262-
Example 50
7-(3-Aminopiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methyl-1H-
quinazolinedione from 1-cyclopropyl-6,7-difluoro-8-methyl-1H
S quinazolinedione and piperidin-3-ylcarbamic acid tert-butyl ester; MSCI: mlz
333
(MH+).
Example 51
1-Cyclopropyl-6-fluoro-8-methyl-7-(octahydropyrrolo[3,4-c]pyridin-2-
yl)-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methyl-1H
quinazolinedione and octahydropyrrolo[3,4-c]pyridine-1-carboxylic acid tert-
butyl ester [WO O1S3273 A1]; MSCI: mlz 3S9 (MH+).
Example S2
1 S 7-(3(S)-Aminopyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-1H-
quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione and 3(S)-pyrroIidin-3-ylcarbamic acid tert-butyl ester; MSCI:
m/z 33S (MH+).
Example S3
7-(3-Aminomethyl-3-methylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-lIl-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione and (3-methylpyrrolidin-3-ylmethyl)carbamic acid tert-butyl
ester [J. Med. Chem. 1992, 35(2), 361 ]; MSCI: mlz 363 (MH+).
2S
Example 54
7-(3-Aminomethylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-
1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione and (pyrrolidin-3-ylmethyl)carbamic acid tent-butyl ester [EP
591030 A2]; MSCI: m/z 349 (MH+).
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-263-
Example 55
7-[3(R)-(1-Amino-1-methylethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methoxy-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-
methoxy-1H quinazolinedione and (3(R)-methylpyrrolidin-3-ylmethyl)carbamic
acid tart-butyl ester; MSCI: m/z 377 (MH+)
Example 56
7-(3-Aminomethylpiperidin-1-yl)-1-cyclopropyl-6-fluoro-8-methoxy-
IH-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione and piperidine-3-ylmethylcarbamic acid tart-butyl ester;
MSCI:
m/z 363 (MH+).
Example 57
7-(3-Aminomethyl-3-benzylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H
quinazolinedione and (3-benzylpyrrolidin-3-ylmethyl)carbamic acid, tent-butyl
ester [WO 0153273 A1]; MSCI: m/z 440 (MH+).
Example 58
1-Cyclopropyl-6-fluoro-8-methoxy-7-(octahydropyrrolo[3,4-b]pyridin-
6-yl)-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H
quinazolinedione and octahydropyrrolo(3,4-b]pyridine-1-carboxylic acid tert-
butyl ester [JP 2001213878 A2]; MSCI: m/z 375 (MH+).
Example 59
7-(1-Amino-5-aza-spiro[2.4]hept-5-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H
quinazolinedione and (S-azaspiro[2.4]kept-1-yl)carbamic acid tart-butyl ester
[EP
550016 Al]; MSCI: m/z 361 (MH+)
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-264-
Example 60
7-(3-Aminomethyl-3-methylpyrrolidin-1-yl)-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methyl-1H-
quinazolinedione and (3-methylpyrrolidin-3-yImethyI)carbamic acid tert-butyl
ester; MSCI: m/z 347 (MH+)
Example 61
7-[3(R)-(1-Amino-1-methylethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-
fluoro-8-methyl-1H-quinazolinedione from 1-cyclopropyl-6,7-difluoro-8-
methyl-1H-quinazolinedione and [(R)-1-methyl-1-pyrrolidin-3-ylethyl]carbamic
acid tert-butyl ester [J. Med. Chem. 1994, 37(6), 733]; MSCI: mlz 3361
(MH+).
Example 62
1-Cyclopropyl-6-fluoro-8-methyl-7-(octahydropyrrolo[3,4-b]pyridin-
6-yl)-1H-quinazolinedione from 1-cyclopropyl-6,7-difluoro-8-methyl-1H-
quinazolinedione and octahydropyrrolo[3,4-b]pyridine-1-carboxylic acid tert-
butyl ester; MSCI: m7z 359 (MH+).
Example 63
7-(3a-Aminomethyloctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-8-
methyl-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methyl-1H-
quinazolinedione and (octahydroisoindol-3a-ylmethyl)carbamic acid tert-butyl
ester [WO 0153273 AI]; MSCI: mlz 387 (MH+)
Example 64
7-(3S, 4R)- and 7-((3R, 4S)-3-Amino-4-t7uoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-fluoro-8-methoxy-1H-quinazolinedione, from 1-cyclopropyl-6,7-
difluoro-8-methoxy-1H quinazolinedione and (3R,4S)- and (3S,4R)-4-
fluoromethylpyrrolidin-3-yl)carbamic acid tent-butyl ester [Bioorg. Med. Chem.
Lett. 1998, 8(75), 1953]; MSCI: m/z 367 (MH+)
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-265-
Example 65
1-Cyclopropyl-7-[(R)-3-((S)-1-ethylaminoethyl)pyrrolidin-1-yl)-6-
fluoro-8-methoxy-1H-quinazolinedione from 1-cyclopropyl-6,7-difluoro-8-
methoxy-IH quinazoIinedione and ethyl-[(S)-(R)-1-pyrroIidin-3-ylethyl]amine
[J.
Med. Chern. 1993, 36(7), 871 ]; MSCI: mlz 391 (MH+)
Example 66
7-(3a-Aminomethyloctahydroisoindol-2-yl)-1-cyclopropyl-6-fluoro-8-
methoxy-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-methoxy-1H-
quinazolinedione and (octahydroisoindol-3a-ylmethyl)carbamic acid tert-butyl
ester; MSCI: m/z 403 (MH+).
Example 67
7-(3S, 4R)- and 7-((3R, 4S)-3-Amino-4-tluoromethylpyrrolidin-1-yl)-1-
cyclopropyl-6-tluoro-8-methyl-1H-quinazolinedione, from 1-cyclopropyl-6,7-
difluoro-8-methyl-1H-quinazolinedione and (3S, 4R)- and (3R, 4S)-4-
fluoromethylpyrrolidin-3-yl)carbamic acid tent-butyl ester [Bioorg. Med.
Chern.
Lett. 1998, 8(15), 1953] ; MSCI: m/z 351 (MH+).
Example 68
1-Cyclopropyl-7-[(R)-3-((S)-1-ethylaminoethyl)pyrrolidin-1-yl]-6-
fluoro-8-methyl-1H-quinazolinedione, from 1-cyclopropyl-6,7-difluoro-8-
methyl-1H-quinazolinedione and ethyl-[(S)-(R)-1-pyrrolidin-3-ylethyl]amine;
MSCI: mlz 375 (MH+).
Example 69
a) 1=Cyclopropyl-6,7-difluoro-8-methoxy-5-methyl-1H-quinazolinedione
A solution of 1-cyclopropyl-6,7-difluoro-8-methoxy-IH-quinazolinedione
( 1.0 g, 3.7 mmol) in tetrahydrofuran ( 18 mL) was cooled to -30 °C and
treated
with a 2 M heptaneltetrahydrofuran/ethylbenzene solution of lithium
diisopropylamine (5.6 mL, 11 mmol). The mixture Was allowed to stir for 2
hours
then iodomethane (0.28 mL, 4.5 mmol) was added. After stirring for 1 hour and
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-266-
warming to 0 °C, the mixture was quenched into water and extracted with
ethyl
acetate. The extracts were combined, dried with magnesium sulfate and purified
via silica column chromatography (98:2 methylene chloride/methanol) to provide
a solid (0.650 g). MSCI: m/z 283 (MH+).
b) {(S)-1-[(R)-1-(1-Cyclopropyl-6-fluoro-8-methoxy-5-methyldioxo-1,2,3,4-
tetrahydro-quinazolin-7-yl)pyrrolidin-3-ylJethyl}carbamic acid tert-butyl
ester
1-Cyclopropyl-6,7-difluoro-8-methoxy-5-methyl-1 H-quinazolinedione
(0.19 g, 0.67 mmol, Example 69a), 1,1,3,3-tetramethylguanidine (0.17 mL, 1.4
mmol) and ((S)-(R)-1-pyrrolidin-3-ylethyl)carbamic acid tert-butyl ester (0.29
g,
1.4 mmol) in dimethyl sulfoxide ( 1.5 mL) were heated at 90 °C
overnight. The
solution was diluted with brine and extracted with ethyl acetate. The organic
layers were then combined dried over magnesium sulfate, filtered and
concentrated. The residue was purified by flash silica gel chromatography (3:2
ethyl acetate/hexanes then 7:3 ethyl acetate/hexanes) to afford the title
compound
(0.280 g). MSCI: mlz 477 (MH+).
c) 3-((S)-1-Aminoethyl)pyrrolidin-1-yl]-1-cyclopropyl-6-t7uoro-8-methoxy-5-
methyl -1H-quinazolinedione hydrochloride
To a solution of {(S)-1-[(R)-I-(I-Cyclopropyl-6-fluoro-8-methoxy-5-
methyldioxo-1,2,3,4-tetrahydro-quinazolin-7-yl)pyrrolidin-3-yl}ethyl }carbamic
acid tert-butyl ester (280 g, Example 69b) in dichloromethane (5 mL) was
bubbled in hydrochloric and the resulting saturated solution was allowed to
stir
for 3 hours. The mixture was then concentrated, triturated with diethyl ether
and
dried to afford the title compound (0.21 g); mp 200-210 °C; MSCI: m/z
377
(MH+).
The following additional examples illustrate typical formulations for use
according to the invention.
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-267-
Example 70
The following illustrates representative pharmaceutical dosage forms,
containing a compound of Formula I ("Invention Compound"), for therapeutic or
prophylactic use in humans.
(i) Tablet mg/tablet
'Invention Compound' 25 . 0
Lactose 50.0
Corn Starch (for mix) 10.0
Corn Starch (paste) 10.0
Magnesium Stearate ( 3.0
1 %)
300.0
The biphenylsulfonamide, lactose, and corn starch (for mix) are blended to
uniformity. The corn starch (for paste) is suspended in 200 mL of water and
heated with stirring to form a paste. The paste is used to granulate the mixed
powders. The wet granules are passed through a No. 8 hand screen and dried at
80°C. The dry granules are lubricated with the 1 % magnesium stearate
and
pressed into a tablet. Such tablets can be administered to a human from one to
four
times a day for treatment of pathogenic bacterial infections.
(ii) Tablet mg/capsule
'Invention Compound 10 . 0
Colloidal Silicon Dioxide1.5
Lactose 465.5
Pregelatinized Starch 120.0
Magnesium Stearate ( 1 3.0
%)
600.0
(iii) Preparation for
Oral Solution Amount
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-268-
'Invention Compound' 400 mg
Sorbitol Solution (70 40 mL
% N.F.)
Sodium Benzoate 20 mg
Saccharin 5 mg
Cherry Flavor 20 mg
Distilled Water q.s. 100 mL
The sorbitol solution is added to 40 mL of distilled water, and the
biphenylsulfonamide is dissolved therein. The saccharin, sodium benzoate,
flavor,
and dye are added and dissolved. The volume is adjusted to 100 mL with
distilled
water. Each milliliter of syrup contains 4 mg of invention compound.
(iv) Parenteral Solution
In a solution of 700 mL of propylene glycol and 200 mL of water for
injection is suspended 20 g of an invention compound. After suspension is
complete, the pH is adjusted to 6.5 with 1 N hydrochloric acid, and the volume
is
made up to 1000 mL with water for injection. The Formulation is sterilized,
filled
into 5.0 mL ampoules each containing 2.0 mL, and sealed under nitrogen.
(v) Injection 1 (1 mg/mL) Amount
'Invention Compound' 1.0
Dibasic Sodium Phosphate 12.0
Monobasic Sodium Phosphate 0.7
Sodium Chloride 4.5
1.0 N Sodium hydroxide solution q.s.
(pH adjustment to 7.0-7.5)
Water for injection q.s. ad 1 mL
CA 02446963 2003-11-10
WO 02/102793 PCT/IB02/01768
-269-
(vi) Injection 2 (10 mg/mL) Amount
'Invention Compound' 10.0
Dibasic Sodium Phosphate 1.1
Monobasic Sodium Phosphate 0.3
Polyethylene glyco 400 200.0
0.1 N hydrochloric acid solutionq.s.
(pH adjustment to 7.0-7.5)
Water for injection q.s. ad I
mL
(vii) Injection 2 (10 mg/mL) Amount
.
'Invention Compound' 20.0
Oleic Acid 10.0
Trichloromonofluoromethane 5,000.0
Dichlorodifluoromethane 10,000.0
Dichlorotetrafluoroethane 5,000Ø
All patents, and patent documents are incorporated by reference herein, as
though individually incorporated by reference. The invention and the manner
and
process of making and using it, are now described in such full, clear, concise
and
exact terms as to enable any person skilled in the art to which it pertains,
to make
and use the same. It is to be understood that the foregoing describes
preferred
embodiments of the present invention and that modifications may be made
therein
without departing from the spirit or scope of the present invention as set
forth in
the claims. To particularly point out and distinctly claim the subject matter
regarded as invention, the following claims conclude this specification.