Note: Descriptions are shown in the official language in which they were submitted.
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
PRESSURE TRANSDUCED CHEMICAL ASSAY AND METHOD
This application claims the benefit of U.S. provisional application number
60/294861 incorporated herein by reference in its entirety. This invention was
made with.
Government support under Contract No. 68D60024 awarded by the US EPA and
Contract
No. R43 AI 43806-O1 awarded by the MAID. The Government has certain rights in
the
invention.
Field of the Invention
The field of the invention is test systems for analytes.
Background
Analysis of an analyte in a sample generally includes detection of the
analyte.
Detection may be classified as either direct or indirect. Direct detection
typically includes
observations that are readily apparent without any further chemical reaction.
Indirect
detection, on the other hand, often requires a chemical reaction before
becoming readily
apparent.
Indirect detection may include reactions that cause changes in mass. Devices
that
detect changes in mass, however, are often not amenable to certain types of
samples.
Another indirect detection involves events measured using labels that are
radioisotopes.
Such techniques, however, raise safety concerns.
Indirect detection may also include reactions that cause a change in color,
fluorescence, and luminescence. Such changes, however, may be so slight that
they are
not readily detectable by the human eye. Slight changes in color of many known
systems
may be due to relatively small concentrations of analytes present in the
samples. In
addition, color changes and other photometric characteristics may be spread
over too large
an area to be very useful. Although devices and chemical reactions may be used
to
amplify signals, noise is often amplified as well.
Problems with detection are addressed by US Patent 5518895 issued to Thorpe et
al. (May 1996). The '895 patent is directed toward a device and method that
detects
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
microorganisms in blood and other body fluids by measuring pressure changes
within a
sealable container. By measuring pressure changes, the accuracy of the
analysis may be
increased. Accuracy, however, may be negatively impacted by cleanliness and
sterility of
the container. Another problem may involve loss of gas caused by not sealing
the
container quickly enough after adding the reactant that causes the gas to be
evolved.
U.S. Patent 6287851 issued to Delwiche et al. (September 2001) teaches a
sensor
comprising a chamber having an inlet adapted to admit a liquid sample which
gets pumped
into a reaction cell using a pump. Problems exist in this system generally
because it
requires that a liquid sample enter the reaction cell. First, a liquid sample
may not always
be available. Second a sufficient volume of liquid may not be available to
enable pumping
to occur. Third, the existence of a pump and porous membrane to separate the
liquid
portion from the gaseous portion adds complexity to the sensor design.
Thus, there is a need for less complicated detection devices and methods,
especially for low concentration analytes.
Summary of the Invention
The present invention is directed toward analyzing an analyte contained on a
collection strip. The analyte generally reacts with a reagent in a chamber.
The reaction
evolves a gas. A piezoelectric crystal, polymer or other material that
produces an
electrical signal in response to pressure changes caused by the gas is in
gaseous
communication with the chamber and the reaction.
There are some apparent disadvantages to using a collection strip. One
disadvantage is that the analyte and/or reagent may bind to the strip and
thereby distort the
analysis. Another disadvantage is that capillary action may distort the
analysis because
the analyte on the strip may be impeded from wicking across the strip to the
area where
the reagent exists. Certain analytes may not reach the reagent at all.
The apparent disadvantages of using a collection strip are outweighed by less
apparent advantages that result from the use of a collection strip in the
inventive assay.
2
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
Various objects, features, aspects, and advantages of the present invention
will
become more apparent from the following detailed description of preferred
embodiments
of the invention, along with the accompanying drawings in which like numerals
represent
like components.
Brief Description of The Drawing
Figure 1 is a schematic vertical cross section of a fiirther exemplary assay
system.
Figure 2 is a schematic view of an exemplary test strip.
Figure 3 is a schematic of an exemplary "sandwich" configuration in an
exemplary
assay system.
Detailed Description
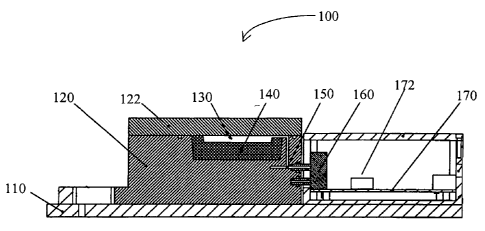
In Figure 1, an assay system 100 generally comprises a base 110 and a housing
120, which forms together with lid 122, a chamber 130. Disposed within the
chamber 130
is a strip holder 140 that receives a strip (not shown). Chamber 130 is in
gaseous
communication with the piezoelectric crystal 160 via gas conduit 150. The
piezoelectric
crystal 160 produces an electric signal that is processed in a circuitry (not
shown) compris-
ing a microprocessor 172 on the circuit board 170.
A test strip generally receives a sample comprising an analyte. As used
herein, the
term "analyte" refers to the at least one substance of which the presence or
concentration is
be determined. Therefore, contemplated analytes include single molecules, homo-
and
heterodimers, -trimers and -multimers, molecular assemblies (e.g., K-ATPase,
microtubuli, etc.), molecular superstructures (e.g., biological membranes),
and various
organisms. Particularly preferred analytes include peptides of various
molecular weights,
nucleic acids (e.g., RNA, DNA, PNA, etc.), carbohydrates, hormones,
pharmaceutical
agents, lipids, bacteria, viruses, viroids, mammalian cells, parasites,
chelated metal ions, or
haptenic organic molecules.
It should be especially appreciated that the definition of analyte
particularly
includes complexes of the aforementioned substances and structures with a
first
3
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
compound, wherein the complex formation may be due to a covalent bond, a non-
covalent
bond (e.g., electrostatic, ionic, hydrophobic interaction, etc.), or any
reasonable
combination thereof. Particularly contemplated first compounds include various
peptides,
antibodies or their fragments, nucleic acids, enzyme, and metal ion chelators.
Furthermore,
it should be appreciated that contemplated first compounds may further include
an enzyme
that produces a gas when the enzyme reacts with the reagent. There are
numerous methods
of coupling an enzyme to a substrate, and all known methods are contemplated
suitable for
use in conjunction with the teachings herein.
It is generally contemplated that all enzymes that catalyze a reaction that
produces
a gaseous product are suitable, however, especially preferred enzymes include
peroxidases, ureases, carbonic anhydrases and catalases from various sources.
Further
contemplated enzymes include decarboxylases (and particularly amino acid
decarboxylases, oxalate DC, pyruvate DC, etc.), various dehydrogenases
(pyruvate DH,
isocitrate DH), and oxidases. Moreover, it should be recognized that more than
one
enzyme may be employed in the generation of a gas. For example, an enzyme
coupled to
the first compound may produce a first product, which is then substrate for
one or more
subsequent enzymatic and/or non-enzymatic reactions in which a gas is formed.
A chamber 130 receives a test strip, and the chamber is preferably sealed
during
the reaction such that the electric signal is sufficient to provide a
qualitative assessment of
the analyte (e.g., partially sealed). On the other hand, the chamber may be
sealed during
the reaction such that the electric signal is sufficient to provide a
quantitative assessment
of the analyte (e.g., hermetically sealed).
With respect to the chamber volume, it is contemplated that various volumes
are
suitable. However, it is generally preferred that the volume of the chamber
relative to the
sample allows a relatively rapid increase in pressure where the chamber is at
least partially
sealed. Consequently, it is preferred that the chamber volume is no greater
than S times,
and more preferably no greater than 3 times the volume of the sample that is
applied to the
test strip. It should be appreciated that the volume of the chamber relative
to the sample is
not meant to be a limitation, and as such the chamber volume may be 20, 50, or
even 100
times or more than that of the sample.
4
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
A piezoelectric crystal 160, or other suitable material, is in gaseous
communication
with the chamber 130. The piezoelectric crystal 160 generates an electrical
signal in
response to a pressure change in the chamber caused by a reaction between the
analyte and
a reagent.
The term "reagent" as used herein refers to any substance or substrate that in
a
chemical reaction (preferably as substrate or cosubstrate of contemplated
enzymes) will
produce a gaseous compound. The term "gaseous compound" as used herein
includes all
compounds that have in their isolated form at atmospheric pressure a boiling
point of
lower than 25°C. Depending on the enzyme, contemplated substrates may
vary
considerably. However, especially preferred substrates include H202, urea,
HC03-, various
amino acids, glucose, ethanol, salicylate, etc.
A microprocessor 172 is preferably located on a circuit board 170. A
microprocessor may be any appropriate microprocessor (i.e., Intel Pentium 4,
AMD
Athlon, and so on). Depending on the particular configuration and point of
use,
contemplated configurations may include a microprocessor that transforms the
electrical
signal from the piezoelectric material into a user readable output, wherein
the
microprocessor may or may not be at least partially enclosed in the chamber.
In a preferred method of performing an assay, a sample is mixed with a
solution
comprising an antibody that binds the analyte with relatively high affinity
(i.e., KD<106M'
') to form a complex, wherein the antibody is further coupled to catalase. The
resulting
mix is applied to the sample receiving area of the test strip and moves
(predominantly via
capillary action) towards the wick on the opposite end of the test strip. The
screening area
on the test strip comprises immobilized analyte in a concentration effective
to remove
substantially all of the antibody that did not bind to the analyte in the
sample (or excess
antibody). As the mix passes the screening area, only complexes between the
antibody and
the analyte will pass towards the capture zone, while free antibodies
(antibody without
analyte) will be substantially retained in the screening area. The capture
zone on the strip
comprises immobilized antibodies that bind the complexes with relatively high
affinity.
Thus, the amount of bound complexes (bearing a gas producing enzyme) in the
capture
zone is substantially identical with the amount of analyte. A substrate
solution is then
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
applied to the capture zone and at least the capture zone is placed in the
chamber to detect
evolving gas from the reaction between the reagent and the analyte.
In a preferred aspect of the inventive subject matter, the test strip has a
configuration as depicted in Figure 2. Here, the test strip 200 is
manufactured on a base of
MylarTM (not shown) onto which (from left to right) a sample receiving zone
210 is
juxtaposed to an area of nitrocellulose 220, which is adjacent to a screening
zone 230. The
screening zone 230 is followed by another nitrocellulose area 220 juxtaposed
to a capture
zone 240, a nitrocellulose area 220, and a wick 250. Thus, a sample applied to
the sample
receiving zone 210 will travel through the nitrocellulose area via the
screening zone and
capture zone to the wick by virtue of capillary action.
In alternative aspects, a "sandwich format" (e.g., a Garner material with
capture
antibodies binds the antigen, which binds in turn a detector antibody) may be
employed
for detection of a target antigen. For example, particularly contemplated
carriers include
nitrocellulose membranes (with optional backing such as a sticky MylarTM
membrane) or
other membranes to which antibodies can be coupled. It is further preferred
that suitable
membranes are blocked after application of the capture reagent to minimize non-
specific
binding of subsequent reagents and/or sample. The sample containing the
antigen can then
be applied to the capture zone (e.g., via capillary action or pipetting) and
incubated for an
appropriate amount of time. A washing step will remove unbound antigen, and
subsequently detector reagent can be added over the capture zone comprising
the antigen.
After incubation for an appropriate amount of time, the carrier is washed and
the capture
zone placed in a sealable test chamber. Substrate solution is added, and the
capture zone is
coupled to a pressure sensor in gaseous communication to record evolution of
gas (signal
being measured in mV). Thus, an exemplary sandwich could have a configuration
as
depicted in Fig. 3. Here, the sandwich 300 comprises a backing 310 onto which
a
nitrocellulose membrane 320 is attached. Bound to the membrane 320 is a
plurality of
capture antibodies 330 that bind the analyte 340. The analyte is detected with
a detection
antibody 350, which is coupled to an enzyme.
6
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
Examples
A lateral-flow assay was constructed based on commercially available reagents
and
enzyme conjugates. The assay was developed with a screening zone and a capture
zone, in
order to generate signals that were directly proportional to the concentration
of the test
substrate atrazine. This assay was evaluated in a pressure sensing test bed
for the ability to
respond to atrazine in spiked buffer samples. The test system produced signal
differentials
of approximately 3.5-fold for samples with 1 ppm atrazine compared to negative
samples.
Biologicalllmmunochemical Reagents
The antibody against atrazine was obtained from Biostride, Inc. (Palo Alto,
CA).
Atrazine-BSA also was obtained from Biostride. The Goat anti-Rabbit IgG was
obtained
from Jackson ImmunoResearch Laboratories (West Grove, PA). All other chemicals
were
obtained from Sigma Chemical, Co. (St. Louis, MO), Aldrich (St. Louis, MO),
Pierce
Chemical Co. (Rockville, IL) or Pharmacia (Piscataway, NJ).
Pressure Sensing Components
The multimeter and various electrical components were obtained from Radio
Shack. The pressure chamber, the assay strip holder and the mounted pressure
sensor were
fabricated by J M Speciality Parts (San Diego, CA). The piezofilm was obtained
from
AMP, Inc (Folsom, CA). The pressure sensors were obtained from SenSym, Inc.
(Sunnyvale, CA).
Enzymes and Antibodies
Urease, carbonic anhydrase, catalase, peroxidase, and their respective
substrates
were purchased from Sigma. The enzymes were purified and biotinylated
following
standard protocols known in the art. Similarly, anti-atrazine antibodies were
purchased
from Sigma and conjugated with streptavidin following standard protocols known
in the
art.
Conjugate Formation
7
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
Streptavidin-containing antibodies and biotin-containing enzymes ware
conjugated
by mixing (with excess of biotinylated enzyme). The mixture is incubated at
room
temperature for 2 hr and then overnight at 4°C with gentle shaking.
After incubation, the
mixture is run on a Sepharose 5300 column and the leading edge of the first
peak is
collected. The collected fractions were evaluated for the presence of antibody
reactive
with atrazine in an ELISA under standard conditions. The results from ELISA
analysis
indicated that antibody and enzyme had formed conjugates, since antibody
activity was
found in the pools made from the leading edge of the elution profiles of both
urease and
catalase.
Test Strip Components and Preparation
Nitrocellulose is lightly marked with pencil to indicate the location of the
screening and capture zones. The screening zone material is diluted in 10 mM
Tris (pH
8.5) to give a concentration of 1 mg/ml. Approximately 10 ~l of the solution
is spotted in
the appropriate zone. The antibody used for the capture zone is diluted in 10
mM Tris (pH
8.5) to give a concentration of 1 mg/ml. Approximately 8 ~l of the solution is
spotted in the
appropriate zone. After drying at 45°C for 15 min, the nitrocellulose
is placed in excess 10
mM Tris buffer with 10 mg/ml BSA. The nitrocellulose is blocked for 2 hrs at
room
temperature, after which it is blotted with paper towels and placed at
45°C for 1 hr. The
sample pad material is blocked and dried following known procedures. A strip
of sticky
mylar (2 mil thickness) is placed on a flat surface. A blocked strip of
nitrocellulose is
placed on the mylar, leaving some sticky surface at both ends of the strip.
The sample pad
is placed to the left of the screening zone and the absorbent pad is placed at
the other end.
The materials are pressed gently into the mylar strip. The completed strips
are stored in a
desiccator at room temperature.
Assay Procedure
A strip is removed from the desiccator and placed on a flat surface. The
antibody-
catalase conjugate (at the appropriate dilution made in 10 mM Tris [pH 8.5]
with 1 mg/ml
BSA) is added to the strip to the left of the screening zone. Approximately
250 ~l of the
Tris/BSA solution is added to the sample pad. The liquid is allowed to wick to
the sample
8
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
absorbent pad for 20 - 30 min, depending on the strip's wicking rate. The
capture zone and
the screening zone are cut from the strip and tested in the pressure sensing
test bed.
Atrazine was spiked into the antibody-catalase conjugate at the appropriate
level and
incubated at room temperature for a minimum of 2 hrs before testing.
The test bed is turned on and allowed to stabilize for 10 min prior to use.
The test
chamber is cleaned with dH20 and 70 % Isopropyl alcohol. 300 ~l of the enzyme
substrate
solution is added to the chamber. The chamber is sealed and the tubing is
connected to the
sensor test bed. A timer is started and the initial pressure reading (in mV)
is recorded. The
chamber is rotated gently for 15 sec, and is rotated for 30 sec at 1 min and
again at 3 min.
A reading is taken at 5 min.
Results
The average (0 1.0 standard deviation) change in pressure was determined in
millivolts produced during a 5 min testing period. The obtained data (and
further data, not
shown) confirm that contemplated pressure-based assays are responsive to
concentrations
of the analyste in a sample with a sensitivity of lower limit of detection in
the order of 10
ppb at a signal differential of approximately 3.5-fold.
ATRAZINE CAPTURE
LEVEL (ppm) ZONE (mV)*
0 3 . 5 ~ 1.0
(n=S )
0.01 3.9 0 0.3 (n=3)
0.1 6.5~1.3(n=3)
1.0 11.7 ~ 1.1 (n=3)
Alternative Assay Procedure (Sandwich Format)
9
CA 02448762 2003-11-27
WO 02/099430 PCT/US02/12052
In an alternate immunoassay strip format, nitrocellulose was spotted with
capture
reagent and then blocked as described above. The blocked strip was mounted on
a sticky
mylar backing, but the sample and wick materials were not attached. Antigen
was allowed
to migrate through the capture zone, wherein the antigen was applied to the
end nearest the
capture zone. After a brief rinse with deionized water, the strip was placed
flat and
detector reagent was added over the capture zone. After 15-30 minute
incubation, the strip
was washed with deionized water. The capture zone was cut from the strip and
placed in a
sealable test chamber. Substrate solution was added, the chamber was sealed
and the
system connected to the pressure sensor to record evolution of gas (signal
being measured
in mV). The results obtained for this alternative biosensor suggest the
ability to detect
levels of extracted antigen associated with 10-100 oocysts per mL.
Thus, specific embodiments and applications of pressure transduced chemical
assays and methods have been disclosed. It should be apparent, however, to
those skilled
in the art that many more modifications besides those already described are
possible
without departing from the inventive concepts herein. The inventive subject
matter,
therefore, is not to be restricted except in the spirit of the appended
claims. Moreover, in
interpreting both the specification and the claims, all terms should be
interpreted in the
broadest possible manner consistent with the context. In particular, the terms
"comprises"
and "comprising" should be interpreted as referring to elements, components,
or steps in a
non-exclusive manner, indicating that the referenced elements, components, or
steps may
be present, or utilized, or combined with other elements, components, or steps
that are not
expressly referenced.