Note: Descriptions are shown in the official language in which they were submitted.
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
CANCER TREATMENT METHOD AND COMPOSITIONS
Cross-Reference to Related Applications)
This application claims priority to U.S. Provisional Patent Application
Serial No. 60/296098, filed June 5, 2001, entitled "THERAPEUTIC REGIMEN
UTILIZING COMPOUNDS OF THE GINGER FAMILY TO TREAT
ESTABLISHED CANCERS".
Field of the Invention
The present invention relates generally to therapeutic compositions and
methods of use in treating cancer, and more specifically the present invention
relates to therapeutic compositions and methods of use which are effective in
arresting the growth of tumors, shrinking the size of established tumors, or
clearing tumors entirely in mammalian cancers, for example, of the skin and
other organ tissues.
Background of the Invention
Carcinoma is one of the most serious diseases threatening human and
animal health and life. Predominant treatments for cancer patients are
radiotherapy and chemotherapy. Both treatments have certain known toxicity or
side effects on humans while suppressing cancer cell growth or killing cancer
cells. Extensive investigations have been carried out in order to find
effective
anti-carcinogen compounds with minimum side effects and toxicity.
Sunlight, which includes ultraviolet wavelength components, is a known
causal factor of certain mammalian skin cancers. Similarly, a host of chemical
compounds are known causal factors, for initiation or promotion, of certain
mammalian cancers of the skin and other organ tissues.
Prior studies have examined the chemo-preventive effects of ginger and
focused on the effect of ginger extracts on phorbol ester, that is 12-O-
tetradecanoyl-phorbol-13-acetate(TPA), or TPA-induced tumor promotion.
Topical pre-application of an ethanol extract of the ginger root was shown to
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
inhibit TPA induced epidermal onuthine decarboxylase (ODC) activity and
protein expression, and TPA-induced epidermal lipo-oxygenase activity in the
skin of mice. In addition, topical application of the ginger extract prior to
TPA-
induced tumor promotion inhibited the development of tumors in 7,12-
dimethylbenz[a]anthracene ( DMBA )-initiated mice. In a more recent study, 6-
gingerol was used to investigate its protective effects against TPA-induced
skin
tumor formation in mice. The results indicated that the 6-gingerol inhibited
TPA-induced skin tumor promotion in addition to inhibiting ear edema and
epidermal ODC activity. There is evidence to suggest that 6-gingerol has
strong
antioxidant capacity which contributes to its anti-cancer effects. At least
two
recent studies suggested that 6-gingerol and 6-paradol suppressed
proliferation of
human cancer cells through the induction of apoptosis. These reports focused
on
the use of 6-gingerol and 6-paradol as potential chemo-preventive agents and
none of the existing studies used either compound to treat existing cancers or
in
1 S combination with each other or with TPA to reduce the size of an
established
tumor. Aside from these studies little is known about the cellular or
molecular
mechanisms by which gingerol or paradol may exert anti-carcinogenic effects.
See for example, Y.-J. Surly Mutation Research, 428, (1999) 305-327.
There remains a need for therapeutic compositions and methods for
treating cancer, especially for compositions and methods which are effective
in
arresting the growth of established skin cancer tumors, shrinking the size of
established tumors, or completely eliminating established skin cancer tumors,
in
mammals including humans. There is also a need for pharmacological tools for
further study of the physiological processes associated with mammalian cancer.
Summary of the Invention
Applicants have unexpectedly discovered therapeutic compositions and
treatment methods which can arrest established tumor growth, shrink the size
of
established tumors, or completely eliminate established tumors in mammalian
skin cancer. The present invention provides in embodiments a therapeutic
regimen which uses compounds of the Ginger (Zingiber officinale Roscoe,
Zingiberaceae) family to treat existing or established cancers. The
therapeutic
2
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
compositions, in embodiments, can include one or more phenolic compounds,
such as either or both 6-gingerol and 6-paradol. In embodiments, the
therapeutic
compositions, can include a tumor promoting compound, such as phorbol
myristate acetate known as TPA, in combination with one or more of the above
mentioned phenolic compounds. The resulting mixtures are synergistically
highly effective and useful in methods for treating tumors, for example,
treatment of established mammalian skin cancers. It was also unexpectedly
discovered that any combination of the TPA tumor promoter compound, and
either of the phenolic compounds, 6-gingerol and 6-paradol, when administered
at any suitable dose level was highly effective in arresting and shrinking
tumors
but was found to be non-toxic and had no adverse side-effects in mammals, for
example in mice, where there was observed no extraordinary mortality or
disease
symptoms associated with the treatment regimens.
Accordingly the present invention includes:
A pharmaceutical composition comprising a combination of a compound
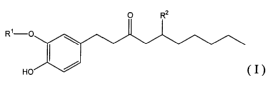
of the formula ( I )
O RZ
R~-O
Ho ~ (I)
wherein Rl is C1-C6 alkyl and RZ is independently -H or -OH, or a
pharmaceutically acceptable salt thereof; and a phorbol compound; and a
pharmaceutically acceptable Garner;
A pharmaceutical composition comprising 6-gingerol and 12-O-
tetradecanoyl-phorbol-13-acetate, and a pharmaceutically acceptable Garner;
A pharmaceutical composition comprising 6-paradol and 12-O-
tetradecanoyl-phorbol-13-acetate, and a pharmaceutically acceptable carrier;
and
A method for the treatment of cancer, such as chemically or radiation
induced or promoted skin tumors, comprising:
3
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
administering to a mammal in need of such treatment a therapeutically
effective amount of a combination, such as together, separately, or
sequentially,
of a compound or compounds of the formula ( I )
O Rz
R' -O
HO
wherein R' is C1-C6 alkyl and RZ is independently -H or -OH, or a
pharmaceutically acceptable salt thereof; and a phorbol compound, such as
phorbol diester, and the like compounds.
The invention also provides a pharmaceutical composition comprising a
compound of the above formula ( I ), or a pharmaceutically acceptable salt
thereof, and a phorbol compound, in combination with a pharmaceutically
acceptable diluent or carrier.
The invention also provides for any of the above compounds or
combinations thereof for use in medical therapy, for example, in treating
various
mammalian tumors, as well as the use of a compound of formula ( I ) for the
manufacture of a medicament useful for the treatment of cancer, such as
various
tumors in a mammal, such as a human.
These and other aspects of the present invention are illustrated herein.
Definitions
The following definitions) are used, unless otherwise described:
Alkyl, alkoxy, alkenyl, alkynyl, etc. denote both straight and branched
groups; but reference to an individual radical such as "propyl" embraces only
the
straight chain radical, a branched chain isomer such as "isopropyl" being
specifically referred to.
"Combination" in the context of administration means combined
treatment where the active compounds are provided to the mammal, for example,
together, separately, or sequentially, but within such a time frame that the
compound combination effectively acts together or in concert.
4
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
"A therapeutically effective amount" means the concentration or quantity
or level of the compound of the present invention that can attain a particular
medical end, such as control or destruction of cancer cells, virally-infected
cells,
or viruses without producing unacceptable toxic symptoms. The term "safe and
effective amount" refers to the quantity of a component which is sufficient to
yield a desired therapeutic response without undue adverse side effects, such
as
toxicity, irntation, or allergic response, commensurate with a reasonable
benefit/risk ratio when used in the manner of this invention. The specific
"safe
and effective amount" will vary with such factors as the particular condition
being treated, the physical condition of the patient, the type of mammal being
treated, the duration of the treatment, the nature of concurrent therapy, if
any,
and the specific formulations employed and the compounds) selected or their
salts.
"In need of such treatment" means a mammal having cancer or cancerous
condition, such as a skin tumor.
"Free of toxic effects" means, for example, in treated mammals, such as
mice, there was observed no mortality or disease symptoms associated with the
treatment regimens. Most drugs used for cancer chemotherapy are generally
toxic to both cancer cells and normal cells and the drug treatment can cause
undesired and often lethal side effects. In the treatment regimes of the
present
invention there was observed no evidence of normal cell death or inflammation.
Further, no mice died as a result of the treatment. Most of the treated mice
appeared energetic and healthy, and free of weight loss.
"Cancer" refers to all types of cancers, or neoplasms, or benign or
malignant tumors. In one embodiment, those cancers that attack normal healthy
blood cells or bone marrow are contemplated by the present invention.
Preferred
cancers for treatment using methods provided herein include carcinoma.
"Carcinoma" can mean a benign or malignant epithelial tumor and includes, but
is not limited to, breast carcinoma, prostate carcinoma, non-small cell lung
carcinoma, colon carcinoma, CNS carcinoma, melanoma carcinoma, ovarian
carcinoma, or renal carcinoma.
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
"Treatment of cancer" refers to application, such as administering or
contacting the abovementioned "cancer" conditions with the compound
combinations and methods of the present invention to cause, for example, the
growth of tumors to arrest, to shrink the size of a tumor mass associated with
mammalian cancers, for example, of the skin or other tissue, or to cause the
cancerous tumor cells or tissue to completely remit or die and optionally
fully
disappear from the host mammal. A preferred host mammal is a human.
"Potentiator" or "potentiators" are materials that affect the immune
system or enhance the effectiveness of compounds or methods of the present
invention, see for example, U.S. Patent No. 6,177,460. In the present
invention,
for example, the tumor promoting compound such as a phorbol compound can
be a potentiator for any of the phenol compounds, such as 6-gingerol or 6-
paradol, individually or in combination, to synergistically render them more
effective as retrograde-carcinoma treatment compositions or cancer treatment
compositions.
"Anti-cancer" means used against cancer or tending to arrest cancer in the
treatment of cancerous cells or tissues. The compound combinations and
treatment methods of the present invention provide potent anti-cancer regimens
as illustrated herein.
"Retrograde-carcinoma" means moving, occurring, or performing in a
backward direction or opposite to the usual direction or course of cancerous
tissue. The present invention provides compositions and methods for
transforming malignant cancerous cells or tissue, such as skin tumors, into a
retrograde-carcinoma, that is cancerous tissue which has, for example, any or
all
of the following properties and characteristics: a slowed growth rate, a no
growth
rate, a reduced cell size or tumor size, a complete shrinkage of necrotic
cells or
tissue, or a sloughing-off or expulsion of the necrotic cells or tissue.
"Apoptosis", also known as "programmed cell death," is a series of
programmed steps that cause a cell to die via "self digestion" without
rupturing
and releasing intracellular contents, for example, the nucleus, chromosomes,
refractile bodies, etc., into the local, that is, surrounding tissue
environment.
Manifestations of cell apoptosis include shrinking of the cell's cytoplasm and
6
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
chromatin condensation. The compositions and treatment methods of the present
invention provide an effective approach to selectively achieving apoptosis in
cancerous cell lines or cancerous tissue and without harming surrounding
healthy
cells or healthy tissue.
"Phorbol" is l,la-beta,lb-alpha,4,4a,7a-beta,7b,8,9,9a-decahydro-4a-
alpha,7b-alpha,9-beta,9a-alpha-tetrahydroxy-3-(hydroxymethyl)-1,1,6,8-alpha-
tetramethyl-SH-cyclopropa(3,4)benz[1,2-a]azulen-5-one of the formula
The Merck Index, 9t" Edition, at page 7138 under "Phorbol", indicates that
Phorbol is the parent alcohol of tumor promoting compounds in croton oil, and
that phorbol diesters are potent co-carcinogens. A co-carcinogen is an agent
that
aggravates the carcinogenic effects of another substance.
A "gingerol compound" is any of the pungent naturally occurring
compounds in the ginger plant family, or synthetic or semi-synthetic
equivalents
thereof, obtained for example from ginger root, such as, 6-gingerol and the
like
compounds illustrated herein.
A "paradol compound" is any of the pungent naturally occurring
compounds in the ginger plant family, or synthetic or semi-synthetic
equivalents
thereof, obtained for example from ginger root, such as, 6- paradol and like
compounds illustrated herein.
"Phorbol ester" is a compound or mixture of compounds with a phorbol
carbocyclic ring system wherein one or more of the hydroxy groups has been
acylated to form an ester, for example, the diester phorbol myristate acetate
or
12-O-tetradecanoyl-phorbol-13-acetate is of the formula
7
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
It is readily evident to one of ordinary skill in the art that the myristate
and acetate ester groups or other hydroxy groups can be other known esters,
for
example, with from C1-CZO atoms. Phorbol esters can be prepared by acylating
one or more of the hydroxy groups of phorbol with, for example, a C1-Czo
saturated or unsaturated carboxylic acid or equivalent reactant. Preferred
acids
for forming esters, in embodiments, are, for example, tridecanoic acid or
acetic
acid.
The indefinite articles "a" and "an" mean "at least one" or "one or more"
when used in this application including the claims unless indicated otherwise.
Detailed Description
The present invention provides in embodiments a method for the
treatment of cancer, comprising:
administering to a mammal in need of such treatment a therapeutically
effective amount of a combination of a compound of the formula ( I )
O Rz
R'-O
Ho ~ (I)
wherein R' is CI-C6 alkyl and RZ is independently -H or -OH, or a
pharmaceutically acceptable salt thereof; and a phorbol compound, such as a
phorbol ester or diester. The phorbol ester can be, for example, 12-O-
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
tetradecanoyl-phorbol-13-acetate. "Phorbol" and "phorbol ester" are known to
those in the art with the above mentioned formulas, and can include other
structurally related phorbol compounds.
In embodiments the compound of the formula ( I ) can be where Rl is
methyl and RZ is -OH, for example, of the formula ( II )
O OH
H3C-O
Ho ~ (I~
In embodiments the compound of the formula ( I ) can be where R' is
methyl and RZ is -H, for example, the compound of the formula ( III )
0
H3C-O
Ho ~ (III)
In still other embodiments the combination of the compound of formula
I ) can be a mixture of two compounds where the first compound has Rl =
methyl and R2 = -H, and the second compound has R' = methyl and RZ = -OH,
that is, a mixture of the above mentioned compounds of the formulas ( II ) and
III ). The mixture of compounds of the formulas ( II ) and ( III ) can be
administered in a weight ratio of from about 100:1 to about 1:100, and
preferably from about l :l to about 4:1. The weight ratio of the compound or
compounds of the formula ( I ) and the tumor promoting compound can be from
about 500:1 to about 10:1, for example, when compound ( I ) was 6-gingerol
and the tumor promoting compound was phorbol, a weight ratio of about 60:1
was effective to arrest and shrink mouse tumors.
In embodiments, the combination can be administered topically, for
example, by painting or applying the combination, that is, together,
severally, or
sequentially, and directly onto a skin tumor mass. Other examples of topical
application include applying the mixture dispersed or dissolved in a suitable
9
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
Garner. Other examples of effective administration include, for example, intra-
corporally, that, is within the tumor mass itself, such as by injection or
implantation of a suitably adapted dosage form.
The compositions and treatment regimens of the present invention can
arrest, that is partially or completely, stop or suspend, tumor growth and
tumor
cell proliferation, and prevent the spread of the treated tumor mass. The
compositions and treatment regimens of the present invention can, for example,
as a result of the above-mentioned arresting of the tumor growth and little or
no
toxic effects on healthy or non-diseased tissue, can cause a reduction in the
size
of a treated tumor. For example, the reduction in the size, or shrinkage, of a
treated tumor mass can be from about 10 to about 100 percent compared to the
size of the tumor before treatment.
The compositions and treatment regimens of the present invention can
have, for example, very low or no apparent toxic effects on healthy cells or
1 S tissues. In embodiments, the compositions and treatment regimens of the
present
invention can be, for example, entirely free of toxic effects on the treated
mammal, that is, there are no apparent ill-effects on surrounding healthy
cells or
tissue nor is there any apparent ill-effects, systemic or otherwise, on the
treated
mammal. Thus the present invention overcomes the above mentioned toxicity
limiting aspects of other available cancer treatment methods. The compositions
and treatment regimens of the present invention can used for the treatment of
mammals, such as, a mouse, a dog, a farm animal, or a human.
In embodiments the present invention provides a method of inhibiting the
growth of established cancer cells, such as established skin tumors,
comprising:
contacting the cancer cells with a cancer cell growth inhibiting amount of a
mixture of compounds of the formula ( I )
O RZ
R'-O
Ho ~ (I)
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
wherein Rl is Cl-C6 alkyl and RZ is independently -H or -OH, or a
pharmaceutically acceptable salts thereof; and a phorbol compound, such as
phorbol or a phorbol ester.
In embodiments, the present invention also provides a method for the
treatment of cancer, comprising: administering to a mammal in need of such
treatment a therapeutically effective amount of a combination of 6-gingerol, 6-
paradol, and 12-O-tetradecanoyl-phorbol-13-acetate. The combination can
comprise from about 60 to about 90 weight percent of 6-gingerol, from about 10
to about 40 weight percent of 6-paradol, and from about 1 to about 5 weight
percent of the tetradecanoyl phorbol acetate.
In embodiments the present invention also provides a method for the
treatment of cancer, comprising: administering to a mammal in need of such
treatment a therapeutically effective amount of a combination of 6-gingerol
and
12-O-tetradecanoyl-phorbol-13-acetate. The combination can comprise from
about 95 to about 99 weight percent of 6-gingerol, and from about 1 to about 5
weight percent of the phorbol acetate.
In embodiments the present invention also provides a method for the
treatment of cancer, comprising: administering to a mammal in need of such
treatment an effective amount of a combination of 6-paradol and 12-0-
tetradecanoyl-phorbol-13-acetate. The combination can comprise from about 90
to about 99 weight percent of 6-paradol, and from about 1 to about 5 weight
percent of the phorbol acetate.
In embodiments, the present invention also provides a method for the
treatment of cancer, comprising: administering to a mammal in need of such
treatment a therapeutically effective amount of a combination of 6-gingerol
and
6-paradol. The combination can comprise from about 60 to about 90 weight
percent of 6-gingerol, from about 10 to about 40 weight percent of 6-paradol.
The combination can comprise, for example, a relative weight ratio of from
about 60 to about 90 parts of 6-gingerol to from about 10 to about 40 parts of
6-
paradol.
The foregoing treatment regimes can, in embodiments result in a
substantial shrinkage of treated cancers, for example, in amounts of from
about
11
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
to about 100 percent compared to a comparable or control untreated
cancerous cell or tissue. The treatment can result in partial disappearance of
the
treated cancer. The treatment can, in embodiments, result in the complete
disappearance of the treated cancers, such as found in chemically or radiation
5 induced skin tumors.
The present invention can further comprise treating cancerous tumors that
have been initiated in a mammal by UV radiation, such as contained in natural
or
synthetic light sources, a carcinogenic compound, or a combination thereof,
followed by administrating one or more of the above mentioned therapeutic
10 combination of compounds. Thus it is evident that the compositions and
treatment methods of the present invention can be used to remediate the
effects
of, for example, environmental or occupational causal agents of skin cancer.
It is
also believed that the compositions and treatment methods of the present
invention can also be used to prevent, protect, or mitigate from the effects
of
causal agents of skin cancer. The compositions and treatment methods of the
present invention can be used for their prophylactic properties in protecting
or
reducing the incidence of cancer in at-risk mammalian populations. Examples of
UV radiation induced skin cancers can occur, for example, in humans with
occupations or recreations which involve prolonged, and extensive direct or
indirect exposure to sun light or to artificial light with comparable UV
spectral
components. Chemically induced carcinoma is also known in mammals, for
example, the compound 7,12-dimethylbenz[a]anthracene of the formula
/ \
\ \ /
is highly effective in inducing skin tumors in mammals, such as mice. Thus the
present invention provides method for the treatment of ultraviolet light or
chemically induced tumors, such as skin tumors, comprising: administering to a
mammal in need of such treatment an effective amount of 6-gingerol, 6-paradol,
or mixtures thereof.
12
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
In embodiments, the present invention also provides a method for the
treatment or prevention of UV light or chemically induced tumors, comprising:
administering to a mammal in need of such treatment or prevention an effective
amount of 6-gingerol or 6-paradol.
In embodiments there is provided a method of treating cancer
comprising: simultaneously inhibiting activator protein (AP-1) and inducing
apoptopsis in cancerous cells or tissue. The simultaneous inhibition of
activator
protein (AP-1) and induction of apoptopsis, in embodiments of the present
invention, can be accomplished by, for example, contacting the cancer with a
therapeutically effective amount of a combination of either or both 6-gingerol
and 6-paradol, and a phorbol diester. Although not wanting to be limited by
theory it is believed that 6-gingerol inhibits AP-1 activation and that 6-
paradol
induces apoptosis, while the phorbol compound, such as a phorbol diester such
as TPA, in the combination with 6-paradol potentiates, that is increases the
level
of apoptosis. Other approaches to inhibiting AP-1 and apoptosis are known, see
for example, Huang, C., Ma, W. Y., Goranson, A., and Dong, Z., in
Carcinogenesis, 20: 237-42, 1999 (resveratrol suppression of cell
transformation
and induction of apoptosis through a p53-dependent pathway); and Dong, Z., in
Biofactors, 12: 17-28, 2000 ( effect of food factors on signal transduction
pathways).
In embodiments the administration or contacting of the abovementioned
treatment methods can further comprise delivering the combination in any
suitable Garner or diluent.
The present invention provides in embodiments a cancer treatment or
prevention lotion or ointment formulation comprising: a compound of the
formula ( I )
O R2
R'-O
Ho (I)
13
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
wherein R' is Cl-C~ alkyl and RZ is independently -H or -OH, or a
pharmaceutically acceptable salt thereof; a tumor promoting phorbol compound;
and a carrier or diluent. The lotion formulation containing a compound of
formula ( I ) and a phorbol compound is a remedial formulation for use with
existing or established skin tumors and in embodiments can comprise, for
example, a mixture of from about 60 to about 90 weight percent of 6-gingerol
and from about 10 to about 40 weight percent of 6-paradol, or pharmaceutically
acceptable salts, and from about 1 to about 5 weight percent of a tumor
promoting phorbol compound. Other optional ingredients include, for example,
W light absorbing compounds or blocking compounds, emollients, and the like
additives, reference for example, U.S. Patent No. 6,020,323, especially in
section
4.7, and references therein. The substances of the present invention also find
use
in topically administered compositions, such as those preparations for the
treatment of established skin cancer tumors. Beyond purely therapeutic
applications, the compound combinations of the present invention may have
utility in supplementing the protective action of cosmetic compositions, such
as
sunscreen or suntan lotions. The incorporation of the active substances of the
present invention in cosmetic formulations is specifically contemplated both
for
the purpose of preserving and protecting the skin, as well as treating a
medical
condition, such as established skin tumors. In sunscreen or suntan lotion
formulations it is advantageous to include an effective amount of the compound
combination of the present invention along with other conventional sunscreen
agents. In embodiments an effective amount of active substance is present to
provide, for example, about 1 microgram to about 100 milligrams per kilogram
of the mammal, preferably from about 0.01 mg to about 10 mg per kilogram of
the mammal, and most preferably about 0.1 mg to about 1 mg per of the
mammal. The cosmetic compositions, may contain conventional ingredients
known to those of ordinary skill in the art, such as those described in Kirk-
Othmer, Encyclopedia of Chemical Technology, Third Edition (1979), Vol. 7,
pages 143-176. In sunscreen preparations, the addition of the compound
combinations of the present invention increases the minimum erythemal dose
(MED) and, consequently, the sun protection factor (SPF). Specific
ingredients,
14
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
including typical sunscreens, are listed in, for example, the above mentioned
Kirk-Othmer Encyclopedia, at pages 153-154. In addition, topical preparations
and cosmetic formulations may be prepared as described in U.S. Pat. Nos.
4,199,576, 4,136,165, and 4,248,861, the disclosures of which are incorporated
by reference herein in their entirety. It is apparent to those of ordinary
skill in the
art that the resulting compositions can be in many forms, including, but not
limited to, for example, solutions, lotions, creams, pastes, emulsions,
sprays, or
aerosols.
The compositions and treatment methods of the present invention provide
methods of treating established cancerous tumors wherein the cancer can be,
for
example, melanocarcinoma, colorectal cancer, hepatocarcinoma, breast cancer,
prostate cancer, renal cancer, gastric cancer, esophagus cancer, lung cancer,
leukemia, tongue cancer, pancreas cancer, and like cancers, and co-occurrences
thereof.
The gingerol, paradol, and phorbol compounds can be, but are not limited
to, those obtained by synthesis, semi-synthetic, by isolation from readily
available natural product sources, or combinations thereof. Phorbol is
typically
used experimentally as a tumor promoter and not as a therapeutic agent.
However, preliminary work in vitro unexpectedly indicated that TPA when
combined with either or both 6-gingerol and 6-paradol there was induced
massive tumor cell death in what appeared to be a synergistic effect.
Therefore
corroborative in vivo studies were accomplished where a TPA was combined
with either or both 6-gingerol and 6-paradol or a pharmaceutically acceptable
salt
thereof, for example, subcutaneously, topically, intra-cutaneously, and the
like
administration routes. The results suggested that the growth of several human
cancer cell lines, as measured by blocking of tumor phenotype expression, was
inhibited in a concentration dependent manner when the cell lines were treated
with increasing concentrations of 6-gingerol.
The invention provides a compound or compounds of the above
mentioned formula ( I ), and a tumor promoting compound, such as phorbol and
like compounds, for use in medical therapy, or alternatively, as a tool in the
study
and treatment of existing cancers, such as established tumors in humans.
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
It will be appreciated by those skilled in the art that compounds of the
invention having a chiral center may exist in and be isolated in optically
active
and racemic forms. Some compounds may exhibit polymorphism. It is to be
understood that the present invention encompasses any racemic, optically-
active,
polymorphic, or stereoisomeric form, or mixtures thereof, of a compound of the
invention, which possess the useful properties described herein, it being well
known in the art how to prepare optically active forms, for example, by
resolution of the racemic form by recrystallization techniques, by synthesis
from
optically-active starting materials, by chiral synthesis, or by
chromatographic
separation using a chiral stationary phase, and how to determine optical
activity
using the standard tests described herein, or using other similar tests which
are
well known in the art.
Specific and preferred values listed below for radicals, substituents, and
ranges, are for illustration only; they do not exclude other defined values or
other
values within defined ranges for the radicals and substituents. Specifically,
(C,-
C6)alkyl can be methyl, ethyl, propyl, isopropyl, butyl, iso-butyl, sec-butyl,
pentyl, 3-pentyl, or hexyl.
In the compound of the formula ( I ) a specific value for R1 is methyl or -
CH3, and a specific value for RZ is hydroxyl or -0H, and which values taken
together are 6-gingerol.
In the compound of the formula ( I ) a specific value for R~ is methyl or -
CH3, and a specific value for RZ is hydrogen, and which values taken together
are
6-paradol.
A preferred compound of the invention is a compound of the formula
(II)
O OH
HOC-O
Ho ( II );
or a pharmaceutically acceptable salt thereof.
16
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
In cases where compounds are sufficiently basic or acidic to form stable
nontoxic acid or base salts, administration of the compounds as salts may be
appropriate. Examples of pharmaceutically acceptable salts are organic acid
addition salts formed with acids which form a physiological acceptable anion,
for
example, tosylate, methanesulfonate, acetate, citrate, malonate, tartarate,
succinate, benzoate, ascorbate, a-ketoglutarate, a-glycerophosphate, and the
like
acids. Suitable inorganic salts may also be formed, including hydrochloride,
sulfate, nitrate, bicarbonate, and carbonate salts. Pharmaceutically
acceptable
salts may be obtained using standard procedures known in the art, for example
by reacting a sufficiently basic compound such as an amine with a suitable
acid
affording a physiologically acceptable anion. Alkali metal, for example,
sodium,
potassium or lithium, or alkaline earth metals, for example calcium salts can
also
be made.
The compounds of formula ( I ) can be formulated as pharmaceutical
compositions and administered to a mammalian host, such as a human patient, in
a variety of forms adapted to the chosen route of administration, that is,
orally or
parenterally, by intravenous, intramuscular, topical, or subcutaneous routes.
Thus, the present compounds may be systemically administered, for
example, topically, in combination with a pharmaceutically acceptable vehicle
such as an inert diluent or an assimilable edible carrier. The compounds may
be
enclosed or formulated into a variety of dosage forms such as hard or soft
shell
gelatin capsules, compressed into tablets, or incorporated directly with a
food as
part of a patient's diet. For oral therapeutic administration, the active
compound
may be combined with one or more excipients and used in the form of ingestible
tablets, buccal tablets, troches, capsules, elixirs, suspensions, syrups,
wafers, and
the like. Such compositions and preparations should contain at least 0.1
percent
of active compound. The percentage of the compositions and preparations may,
of course, be varied and may conveniently be between about 2 to about 60
percent of the weight of a given unit dosage form. The amount of active
compound in such therapeutically useful compositions is such that an effective
dosage level will be obtained.
17
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
The tablets, troches, pills, capsules, and the like may also contain, for
example, the following: binders such as gum tragacanth, acacia, corn starch or
gelatin; excipients such as dicalcium phosphate; a disintegrating agent such
as
corn starch, potato starch, alginic acid and the like; a lubricant such as
magnesium stearate; and a sweetening agent such as sucrose, fructose, lactose,
or
aspartame, or a flavoring agent such as peppermint, oil of wintergreen, or
cherry
flavoring may be added. When the unit dosage form is a capsule, it may
contain,
in addition to materials of the above type, a liquid carrier, such as a
vegetable oil
or a polyethylene glycol. Various other materials may be present as coatings
or
to otherwise modify the physical form of the solid unit dosage form. For
instance, tablets, pills, or capsules may be coated with gelatin, wax, shellac
or
sugar and the like. A syrup or elixir may contain the active compound, sucrose
or fructose as a sweetening agent, methyl and propylparabens as preservatives,
a
dye and flavoring such as cherry or orange flavor. Of course, any material
used
in preparing any unit dosage form should be pharmaceutically acceptable and
substantially non-toxic in the amounts employed. In addition, the active
compound may be incorporated into sustained-release preparations and devices.
The active combination of compounds may also be administered
intravenously or intraperitoneally by infusion or injection. Solutions of the
active combination of compounds or their salts can be prepared in water, and
optionally mixed with a nontoxic surfactant or co-solvent. Dispersions can
also
be prepared in glycerol, liquid polyethylene glycols, triacetin, and mixtures
thereof and in oils. Under ordinary conditions of storage and use, these
preparations contain a preservative to prevent the growth of microorganisms.
The pharmaceutical dosage forms suitable for injection or infusion can
include sterile aqueous solutions or dispersions or sterile powders comprising
the
active ingredient which are adapted for the extemporaneous preparation of
sterile
injectable or infusible solutions or dispersions, optionally encapsulated in
liposomes. The ultimate dosage form should be sterile, fluid and stable under
the conditions of manufacture and storage. The liquid carrier or vehicle can
be a
solvent or liquid dispersion medium comprising, for example, water, ethanol, a
polyol, for example, glycerol, propylene glycol, liquid polyethylene glycols,
and
18
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
the like, vegetable oils, nontoxic glyceryl esters, and suitable mixtures
thereof.
The proper fluidity can be maintained, for example, by the formation of
liposomes, by the maintenance of the required particle size in the case of
dispersions or by the use of surfactants. The prevention of the action of
S microorganisms can be brought about by various antibacterial and antifungal
agents, for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal,
and the like. In many cases, it will be preferable to include isotonic agents,
for
example, sugars, buffers or sodium chloride. Prolonged absorption of the
injectable or topical compositions can be brought about by the use in the
compositions of agents delaying absorption, for example, aluminum
monostearate and gelatin.
Sterile injectable solutions are prepared by incorporating the active
compound in the required amount in the appropriate solvent with various of the
other ingredients enumerated above, as required, followed by filter
sterilization.
In the case of sterile powders for the preparation of sterile injectable
solutions,
the preferred methods of preparation are vacuum drying and the freeze drying
techniques, which yield a powder of the active ingredient plus any additional
desired ingredient present in the previously sterile-filtered solutions.
For topical administration, the present active combination of compounds
may be applied in pure form, that is, without diluents or Garner liquids.
However, it will generally be desirable to administer them to the skin as
compositions or formulations, in combination with a dermatologically
acceptable
carrier, which may be a solid or a liquid. Useful solid carriers include
finely
divided solids such as talc, clay, microcrystalline cellulose, silica,
alumina, and
the like. Useful liquid Garners include water, alcohols or glycols or water-
alcohol and glycol blends, in which the present compounds can be dissolved or
dispersed at effective levels, optionally with the aid of non-toxic
surfactants.
Adjuvants such as fragrances and additional antimicrobial agents can be added
to
optimize the properties for a given use. The resultant liquid compositions can
be
applied from absorbent pads, used to impregnate bandages and other dressings,
or sprayed onto the affected area using pump-type or aerosol sprayers.
Thickeners such as synthetic polymers, fatty acids, fatty acid salts and
esters,
19
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
fatty alcohols, modified celluloses or modified mineral materials can also be
employed with liquid carriers to form spreadable pastes, gels, ointments,
soaps,
and the like, for application directly to the skin of the user.
Examples of useful dermatological compositions which can be used to
deliver the compounds of formula ( I ) to the skin are known to the art; for
example, see Jacquet et al., U.S. Pat. No. 4,608,392; Geria, U.S. Pat. No.
4,992,478; Smith et al., U.S. Pat. No. 4,559,157; and Wortzman U.S. Pat. No.
4,820,508.
Useful dosages of the compounds of formula ( I ) and a phorbol
compound can be determined by comparing their in vitro activity, and in vivo
activity in animal models. Methods for the extrapolation of effective dosages
in
mice, and other animals, to humans are known to the art; see for example, U.S.
Pat. No. 4,938,949. Generally, the concentration of the compounds) of formula
( I ) and a phorbol compound in a liquid composition, such as a lotion, will
be
from about 0.1-25 weight percent, preferably from about 0.5-10 weight percent.
The concentration in a semi-solid or solid composition such as a gel or a
powder
can be, for example, about 0.1-5 weight percent, and preferably about 0.5 -
2.5
weight percent. The amount of the active combination of compounds, or active
salts or derivatives thereof, required for use in treatment will vary not only
with
the particular salt selected but also with the route of administration, the
nature of
the condition being treated and the age and condition of the patient and will
be
ultimately at the discretion of the attendant physician or clinician. In
general,
however, a suitable dose will be in the range of from about 0.5 to about 100
mg/kg, for example, from about 10 to about 75 mg/kg of body weight per day,
such as 3 to about 50 mg per kilogram body weight of the recipient per day,
preferably in the range of 6 to 90 mg/kg/day, most preferably in the range of
15
to 60 mg/kg/day. The compounds can be conveniently administered in unit
dosage form; for example, containing 5 to 1,000 mg, conveniently 10 to 750 mg,
and most conveniently, 50 to 500 mg of active ingredient per unit dosage form.
In embodiments the active ingredients include either or both 6-gingerol and 6-
paradol or the like compounds, in combination with a tumor promoting
compound such as phorbol ester compound, and where the 6-gingerol and 6-
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
paradol or the like compounds are present, individually or combined, in a
relative amount of about 100 to about 500 fold weight percent greater than the
phorbol ester compound.
Ideally, the active ingredient should be administered to achieve peak
plasma concentrations of the active compound of from about 0.5 to about 75
micromolar (~.M), preferably, about 1 to 50 ~.M, and most preferably, about 2
to
about 30 p,M. This may be achieved, for example, by the intravenous injection
of a 0.05 to 5 percent solution of the active ingredient, optionally in
saline, or
orally administered as a bolus containing about 1 to about 100 mg of the
active
ingredient. Desirable blood levels may be maintained by continuous infusion to
provide about 0.01-5.0 mg/kg/hr or by intermittent infusions containing about
0.4-15 mg/kg of the active ingredient(s).
The desired dose may conveniently be presented in a single dose or as
divided doses administered at appropriate intervals, for example, as two,
three,
four or more sub-doses per day. The sub-dose itself may be further divided,
for
example, into a number of discrete loosely spaced administrations; such as
multiple inhalations from an insufflator or by application of a plurality of
drops
into the eye.
The ability of a compound of the invention to act as anti-tumor or
retrograde-carcinoma therapy may be determined using pharmacological models
which are well known to the art, or using Test A described below.
Test A. Experimental results generally from Test A for representative
compounds or combinations of compounds of the invention are shown generally
in Table 1 and specifically in Table 2 and as described below. These results
demonstrate that compound combinations of the invention have retrograde-
carcinoma activity as illustrated herein. Accordingly the compound
combinations of the invention are useful as therapeutic agents for the
treatment
of skin cancers, such as skin tumors. Additionally, compounds or combinations
of the invention may be useful as pharmacological tools for the further
investigation of cancer initiation, cancer promotion, and inhibition of cancer
proliferation.
21
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
The compounds or compound combinations of the invention can also be
administered in combination with other therapeutic agents that are effective
for
controlling cancerous cell or tissue growth conditions. The invention will now
be illustrated by the following non-limiting Examples.
Example 1.
RETROGRADE-CARCINOMA ACTIVITY IN VIVO STUDIES; T~VO-
STAGE CHEMICAL INITIATION-PROMOTION OF MOUSE TUMORS
In a two-stage initiation-promotion model, live mice skin tumors were
initiated
by a single application of the known carcinogen, the above mentioned 7,12-
dimethylbenz[a]anthracene (DMBA), followed by optional repeated topical
applications of the abovementioned known tumor promoter compound TPA.
Three mice groups consisted o~
Group 1 - JNK1 knockout mice that were treated with DMBA followed
by TPA applications and designated as (JNK1 -/-)+TPA.
Group 2 - JNK1 knockout mice that were treated with DMBA but not
with TPA applications and designated as (JNK1 -/-) No TPA.
Group 3 - JNK1 wild type mice that were treated with DMBA followed
by TPA applications and designated as (JNK1 +/+) + TPA.
Each mouse had from 1 to 8 tumors with the largest tumor measured
among all mice at 144 mm2 and the smallest at 1 mm2. Prior to therapeutic
treatment Group 1 mice had significantly more tumors than mice in Groups 2
and 3. The tumors on the mice in Group 2 were significantly larger than those
mice in Groups 1 and 3.
Therapeutic treatment included various amounts of either or both of the
phenolic compounds and optionally in admixture with TPA. For example, 6-
paradol in an amount of from about 25 to about 200 nanomol per mouse alone,
that is, without TPA, or in admixture with TPA in an amount S nanograms per
mouse; or 6-gingerol in an amount of from about 100 to about 350 nanomols per
mouse alone, that is without TPA, or in admixture with TPA in an amount S
nanograms per mouse; were applied by pipetting the mixture of compounds in an
acetone solution onto the tumor mass. Other common modes of application can
22
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
include, for example, painting on a lotion with a brush; spraying an aerosol
spray
or atomized liquid; taping on a bandage medicated with one or more of the
active
combination of ingredient compounds; subcutaneous injecting; and the like
methods. In one study the phenolic compounds in combination with a TPA
were applied on alternate days, that is every other day, for example: day 1 -
paradol and TPA; day 2 - gingerol and TPA; day 3 - paradol and TPA; day 4 -
gingerol and TPA; etc. In another study TPA was entirely excluded from the
above mentioned alternate day treatment regime and instead there was
administered a combination of gingerol and paradol five times per week,
Monday through Friday, for 5 weeks. Tumors were measured and counted once
a week and mice were photographed each week.
Table 1 summarizes the trends observed for changes in mouse tumor size
in three different mouse-type groups following treatment with compounds of the
formula ( I ) of the present invention with or without TPA present. It is
evident
that Group 2, with only four mice with one tumor each and a total of only 4
tumors, while Group 2 had a 100% tumor size reduction response the percentage
range of size reduction was smaller, 15-65 percent, compared to Groups 1 and 3
which treatment groups included a phorbol compound and both Groups l and 3
provided a broader range of size reduction, 11-100 percent.
Table 1. Aggregate tumor size reduction in mice.
total % % range
Mouse tumors tumors of size tumors tumors
Group per reduced reductionwith increase
no
group in size change d in size
Group 1 38 86.8 11-100 7.9 5.3
JNK1 (-/-)
+ TPA
Group 2 4 100 15-65 0 0
JNK 1 (-/-)
no TPA
Group 3 21 61.9 11-100 14.3 23.8
JNKI (+/+)
+ TPA
23
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
Table 2 provides the data of the changes in tumor size as observed in
individual mice within the different mouse-type groups that is summarized in
Table 1 with or without TPA treatment. A "mouse number" corresponds to an
arbitrary individual mouse identification number. A "mouse number" having
multiple data entries indicates that mouse had a corresponding equivalent
number of tumors subjected to the indicated treatment regimen and observation.
The indicated treatment regimen for each group corresponds to that indicated
in
Table 1 summarized above. The result illustrate that the compounds of formula
( I ) in combination with a tumor producing compound, such as a phorbol
provide an effective tumor treatment method including arresting, shrinking,
and
in embodiments complete eradication of skin tumors. Time sequenced color
photographs and tumor size measurement of the tumors in the treated mice over
a six week period illustrated the clear effectiveness of the compound
combination.
Table 2. Size reduction of individual mice tumors.hz
MOUSE Week Week Week Week Week Week OVERALL
No. 1 2 3 4 5 6 % change
size size size size size size tumor size
Grou
1
13 24.75 23.1 22 20.25 9 - -63.636
7 6 3 3 2 - -71.429
4 3.5 1 1 1 - -75
2.25 1 1 1 1 - -55.556
19 9 10 10 12 9 - 0
9 4.5 1 0 0 - -100
7.5 4 2.25 2.25 1 - -86.667
1 2.25 1 1 1 - 0
39 15 15 9 6.25 4 -89.744
36 10 5.25 4 2.25 2.25 -93.75
3.5 3.5 3.75 1.44 1 0.25 -92.857
36 56 66 38.5 25 24 20.25 -63.839
42 33 39 32.5 35.75 32.5 -22.619
32.5 12 10.5 8 8 6.25 -80.769
6 3 5 4 1 1 -83.333
4 2 1 1 1 1 -75
1 2 2.25 2.25 2.25 2.25 0
37 63 24.75 24 24 20 20 -68.254
18 18 ~ 18 17.5 17.5 16 ~ -11.111
~ ~
24
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
1 1 0 0 0 0 -100
38 37.5 36 41.25 37.05 36 27 -28
32.5 30.25 31.5 31.5 27 24 -26.154
27 24 25 16 24 20 -25.926
18 9 7.5 6 10.5 10.5 -41.667
2.25 6 7.5 4 4 4 77.778
54 20 22 18 18 18 9 -55
15.75 12 13.5 9 9 7.5 -52.381
8 8 7 6 6 3.75 -53.125
2 2 1 1 1 1 -50
65 90 80 71.25 40 35.75 33 -63.333
12.25 12 9 14 22 0 -100
66 42 31.5 40 45 31.5 32.5 -22.619
14 13.5 12 12 12 12 -14.286
9 3 4 2 2 2 -77.778
1 2.25 2.5 2 2 2 100
69 16 9 9 7.5 6 6 -62.5
2 1 1 1 1 1 -50
1 0.25 0.25 0.25 0 0 -100
Grou
2
27 39 23.1 15.75 19.2 15.75 15.75 -59.615
56 144 132.25126.5 121 121 121 -15.972
67 25 22.5 8 8.75 8.75 8.75 -65
68 49 49 49 32.5 32.5 29.25 -40.306
Grou
3
76 14 14 15 17.5 17.5 16.5 0
9 9 12.25 10 8 8 -11.111
6 6 7 8.75 7 2.25 -62.5
6 6 4 4 1 1 -83.333
90 4 2.25 4 1 1 -75
89 42.25 36 24 24 22 8.75 -79.29
9 9 9 9 1 0 -100
83 48.75 12 10 8.75 6 6 -87.692
100 9 6 3.75 3 2.25 -75
108 9 9 9 18 18 16 77.778
9 2.25 2.25 5 5 9 0
105 9 9 4 6 6 12.25 36.111
6.25 1 0 0 0 0 -100
4 4 3 5 4 4 0
113 15 12 12 14 14 25 66.667
135 65 44 40.5 32.5 32.5 32.5 -50
16 9 7.5 10 10 9 -43.75
136 48 48.75 35.75 32.5 48 65 35.417
48 37.5 27 31 30.25 27.5 -42.708
139 48 35 45 99 84 95 97.917
48 33 27 42 42 0 -100
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
1. Negative numbers indicate the percentage decrease in tumor size;"0"
indicates no change; and positive numbers indicates the percentage
increase in size.
2. "Size" is the tumor volume in cubic millimeters (mm3) measured with a
micrometer.
Example 2.
RADIATION INITIATED MOUSE TUMORS Healthy mice were irradiated
with ultraviolet (UV) light. For W-induced skin carcinogenesis, the back of
each mouse was shaved each week and then exposed to ultraviolet B radiation
emitted by banks of six FS40 Westinghouse fluorescent sun lamps for 30 minute
per day per 5 days per week. The dose rate of the lamp output at the distance
from the backs of the mice was approximately 6.94 Joules per square meter per
second (J/m2/sec) as measured with a UVX Digital radiometer with a UVX-31
sensor. The FS40 lamp emits a continuous spectrum wavelength range of about
280 to about 340 nanometers. The irradiation was continued for 30 weeks for an
approximate total dose of 1.87 x 106 Joules per square meter (J/m2).
Example 3.
RETROGRADE-CANCER ACTIVITY AND ABSENCE OF
CYTOTOXICITY IN VIVO STUDIES The data demonstrate the effectiveness
of a method of skin cancer treatment that uses topical application of a
combination of 6-gingerol and 6-paradol. In these experimental mice, the
combination of 6-gingerol and 6-paradol appears to be highly effective in
arresting ultraviolet-induced skin tumor growth and appears to cause a marked
reduction in established tumor size. Following treatment of established tumors
with these compounds, the tumors became extremely dry and hard as observed in
the related experiments for treating chemically induced tumors. The color of
the
tumors before treatment were typically pinkish-red. The color of the tumors
during and after treatment changed to a white, dark gray, or black. The mice
that
were treated with a combination of 6-gingerol and 6-paradol compounds in
accordance with the present invention showed an average decrease in tumor size
of about 55 percent mass volume (cubic millimeters) after 2 weeks of treatment
26
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
compared to control or untreated tumors which averaged an increase in size of
about 28 percent mass volume (cubic millimeters). There was a single mouse
which did not respond to the treatment regimen. The combination of compounds
had no apparent toxic effects on any of the mice. Also the compounds do not
S appear to be toxic in cell culture as assessed by the MTT assay. The MTT
assay
was performed to test the cytotoxicity. The JB6 cells were maintained in
exponential growth in Eagles Minimal Essential Medium (MEM) supplemented
with 5% heat-inactivated fetal bovine serum (FBS). The cells were added in a
0.1 mL volume of FBS/MEM to each well of a 96 well plate and cultured for 24
hours. After 24 hour, 0.1 mL of 0.1 percent FBS/MEM containing one of
several combinations of compounds was added to designated wells. The TPA
was added as a 10 microliter aliquot to designated wells. Cells were incubated
in the presence of these chemicals for 1 S hours, 19 hours, or 36 hours. Media
were removed by fast inversion of the plate and 20 microliters of 5 mg/mL MTT
substrate was added into each well. Cells were incubated for an additional 4
to 6
hours and then DMSO ( 100 microliters per well) was added and the plate was
gently rotated for 10 minutes. Absorbance at 570 nanometers was read using an
Immunoreader BioTek EL311 S model plate reader. The results were expressed
as percentage of the control.
1 ) control = no cells, that is media only; for background assessment; n =
6 wells
2) control cells = no chemical additives, media only; n = 6 wells
3) 100 micromolar gingerol; n = 4 wells
4) 200 micromolar gingerol; n = 4 wells
5) 400 micromolar gingerol; n = 4 wells
6) 12.5 micromolar paradol; n = 4 wells
7) 25 micromolar paradol; n = 4 wells
8) 50 micromolar paradol; n = 4 wells
9) 100 micromolar gingerol + 12.5 micromolar paradol; n = 4 wells
10) 200 micromolar gingerol + 25 micromolar paradol; n = 4 wells
11) 400 micromolar gingerol + 50 micromolar paradol; n = 4 wells
12) 20 nanogram per mL TPA; n = 4 wells
27
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
13) 20 nanogram per mL TPA + 200 micromolar gingerol; n = 4 wells
14) 20 nanogram per mL TPA + 25 micromolar paradol; n = 4 wells
Results from an MTT assay indicated that compounds, such as 6-gingerol
or 6-paradol alone or in combinations with TPA are non-toxic, that is, they
have
no effect on the proliferation of cells in culture except at very high
concentrations, for example, above about from about 400 to about S00
micromolar of either or both 6-gingerol or 6-paradol, and from above about 50
nanograms per milliliter of TPA.
Cell lines. The human cancer cell lines, obtained from human cancer patients,
included skin cancer cells (SK-OV-3), colon adenocarcinoma (HCT-15), 2
colorectal adenocarcinoma epithelial cell lines (DLD-1 and HCT-116) and breast
cancer cells (T47D).
Example 4.
RETROGRADE CANCER IN VITRO STUDIES The results show that 6-
gingerol effectively blocks tumor phenotype expression in a concentration
dependent manner in a variety of human tumor cell lines. 6-Gingerol was highly
effective in preventing phenotype expression of SK-OV-3 human skin cancer
cells, HCT-15 human colon adenocarcinoma cells, HCT 116 human colorectal
adenocarcinoma epithelial cells, and DLD-1 human colorectal adenocarcinoma
epithelial cells. 6-gingerol was also effective in inhibiting phenotype
expression
of T47D breast cancer cells. The in vitro results demonstrate and in vivo
results
suggest the potential of these compound combinations, for example, for
preventing or treating established tumors, for example, in humans.
Example 5.
The following illustrate representative pharmaceutical dosage forms
containing a compound or combination of compounds of the formula ( I ) and
optionally a phorbol compound, for therapeutic use in humans. "Compound X"
refers to 6-gingerol. "Compound Y" refers to 6-paradol. "Compound Z" refers
to above illustrated phorbol diester.
28
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
In the exemplary formulations that follow, 'Compounds X + Y + Z' can
be present in a formulation or dosage form, for example, in a relative weight
ratio of from about 100:100:1 to about 500:500:1. 'Compounds X + Y' can be
present in a formulation or dosage form in a relative weight ratio of from
about
1:1 to about 4:1, and optionally without compound Z present. Compound X may
be effective and optionally used without either compound Y or compound Z
present.
(i) Tablet 1 mg/tablet
'Compounds X+Y+Z' 100.0
Lactose 77.5
Povidone 15.0
Croscarmellose sodium 12.0
Microcrystalline cellulose 92.5
Magnesium stearate 3.0
300.0
(ii) Tablet 2 mg/tablet
'Compounds X+Y+Z' 20.0
Microcrystalline cellulose 410.0
Starch 50.0
Sodium starch glycolate 15.0
Magnesium stearate 5.0
500.0
(iii) Capsule mg/capsule
'Compounds X+Y+Z' 10.0
Colloidal silicon dioxide 1.5
Lactose 465.5
Pregelatinized starch 120.0
Magnesium stearate 3.0
600.0
(iv) Injection 1 (1 mg/ml) mg/ml
'Compounds X+Y+Z' 1.0
Dibasic sodium phosphate 12.0
Monobasic sodium phosphate 0.7
Sodium chloride 4.5
1.0 N Sodium hydroxide solution
(pH adjustment to 7.0-7.5) q.s.
Water for injection q.s. ad 1
mL
29
CA 02449450 2003-12-03
WO 02/098399 PCT/US02/17705
(v) Injection 2 (10 mg/ml) mg/ml
'Compounds X+Y+Z' 10.0
Monobasic sodium phosphate 0.3
Dibasic sodium phosphate 1.1
Polyethylene glycol 400 200.0
O1 N Sodium hydroxide solution
(pH adjustment to 7.0-7.5) q.s.
Water for injection q.s. ad 1
mL
(vi) Aerosol mg/can
'Compounds X+Y+Z' 20.0
Oleic acid 10.0
Trichloromonofluoromethane 5,000.0
Dichlorodifluoromethane 10,000.0
Dichlorotetrafluoroethane 5,000.0
(vii) Lotion mg/gram
'Compounds X+Y+Z' 20.0
UV absorber or Mocker 50.0
Oleic acid 10.0
Mineral oil 1,000
The above formulations may be obtained by conventional preparative
procedures known in the pharmaceutical art.
All publications, patents, and patent documents above are incorporated
by reference herein, as though individually incorporated by reference. The
invention has been described with reference to various specific and preferred
embodiments and techniques. However, it should be understood that many
variations and modifications may be made while remaining within the spirit and
scope of the invention.