Note: Descriptions are shown in the official language in which they were submitted.
CA 02449549 2003-11-17
HYBRID FILM TYPE SENSOR
Field of the Invention
[oooi] The invention is directed toward a sensor having improved
repeatability and efficiency.
Background of the Invention
jo0o2) Film based techniques have generally been investigated for a
wide variety of sensors, as reported by Wenyi et al., 1997; Hughes et al.,
1997;
Staley, 1996; Agbor et al., 1995; Tan and Tan, 1995; Menil et al., 1994;
Kunnecke
et al., 1994; Creasey and Vamey, 1994; Geistlinger, 1993; Ishiji et al., 1993;
Najafi et al., 1992; Hampp et al., 1992; Nakano and Ogawa, 1994; Yamazoe and
Miura, 1994; and, Madou and Otagawa, 1989. While solid-state gas sensors may
have the advantage of being able to operate at elevated temperatures, they
also
may have the disadvantages of slow response and recovery time and a high
intemal operating temperature as reported by Liu et al., 1993; and Narducci et
al.,
1993. More recent literature (Schwebel et af., 1997; Sheng et al., 1997;
Micocci
et al., 1997) eludes to more substantial development work yet to be done.
(00031 In Kinlen et al., 1994, a Nafion -coated metal oxide pH sensor is
generally characterized as having sputtered iridium oxide sensing and
silver/silver
chloride reference electrodes on alumina ceramic substrates. Nafion may have
been used as a cation-selective ionomer coating in order to decrease the
oxidation-reduction error typically affecting the performance of metal oxide
pH
electrodes. In Yasuda et al., 1994, the use of Nafion as polymer-electrolyte
for a
thin-film CO sensor is generally described with macro-sized, sputtered Pt
sensing
and counter electrodes and a smaller, sputtered Au electrode as a reference
electrode. A 5 wt% n-propyl alcohol solution of Nafion (DuPont, 1100 EW) may
be used to form the polymer electrolyte film over the electrodes by casting.
The
polymer is usually washed and protonated in aqueous sulfuric acid prior to
CA 02449549 2003-11-17
-2-
casting. A theorized lifetime of this sensor is normally less than one month.
During this time, the CO oxidation current typically decreases steadily down
to a
few percent of its original value without any period of stable measurement
signal.
The lifetime of the device may be extended up to three years by laminating the
polymer electrolyte layer with a cast perfluorocycloether-polymer film in
order to
keep the CO permeability coefficient through Naflon constant. Theoretical
calculations often reflect the drift rate of the signal could be significantiy
reduced
under these conditions.
1ooo4] A description of typical state-of-the-art hydrated solid polymer
electrolyte or ionomer sensors and sensor cells is generally described by
Kosek
et al. U.S. Patent 5,527,446; LaConti and Griffith, U.S. Patent 4,820,386;
Shen et
al., U.S. Patent 5,573,648; and, Stetter and Pan, U.S. Patent 5,331,310. These
sensor cells, based on hydrated solid polymer electrolyte or ionomer
technology,
may have several advantages over conventional electrochemical sensor cells.
The catalytic electrodes are normally bonded directly to both sides of a
proton
conducting solid polymer ionomer membrane providing a stable electrode to
electrolyte interface. One side of the electrolyte membrane is usually flooded
with
distilled water, making the sensor cell self-humidifying and independent of
extemal humidity. Since no corrosive acids or bases are generally used in the
sensor cell, a lifetime of over 10 years may be experienced for solid polymer
ionomer sensor cells. Finally, the sensor cells may be easy to maintain and
may
be ideal for use in remote, unattended environments because maintenance
typically entails little more than addition of water to the reservoir in the
sensor
housing every several months and monthly calibration checks.
[ooos] A disadvantage of the state-of-the-art sensors described above
may be that the signal-to-noise ratio is not be conducive to detection of very
low
concentrations (parts per billion, ppb) of important environmental and
biomedical
gases and vapors. Also, response time may be relatively slow, and
reproducibility
between sensors and sensor cells may be difficult to achieve. Also, they are
relatively costly.
CA 02449549 2003-11-17
-3-
[ooos] Recently, miniaturized thick- and thin-film type sensors have been
developed where the solid ionomer membrane often acts as a conduit between
the gas to be detected (sample gas) and the sensing electrode (Yasuda et al.,
1994). The sample gas usually permeates through the membrane itself where a
3-phase contact area is established. A disadvantage with this configuration
may
be that the solid ionomer membrane water content often controls the gas
permeation rate as well as proton conductivity. As the humidity increases, the
membrane water content typically increases. This may cause an increase in the
gas diffusion rate as well as proton conductivity and sensor signal response.
A
method for controlling or fixing the water content of the membrane may be to
have a water reservoir on the back side of the membrane, directly opposite to
where the film type electrodes and non-conductive supportive substrate are
located. However, the back side of the membrane is often required to be free
of
liquid so that the sample gas can diffuse through the membrane to the sensing
electrode.
[ooo7] U.S. Patent No. 4,812,221 to Madou et al. ("Madou") typically
relates to a gas sensor having a porous member located in a passage adjacent
and generally in contact with a sensing electrode. The pore size of the porous
member may be controlled by varying the processing parameters, such as
current, hydrogen fluoride concentration, and the like. In addition, Madou
appears to indicate a number of other steps for providing the gas sensor, such
as
sizing the pores in the porous membrane, sizing the pores of the sensing
electrode, and selecting the materials of the electrodes. A problem often
associated with a sensor provided in accordance with Madou is difficulty in
repeatabiiity and/or reproducibility due to the numerous variations from one
sensor to another. Another difficulty may be problems or costs in
manufacturing
sensors due to the quantity of steps, where it is often believed that
manufacturing
becomes more expensive as the quantity of steps is increased. Another possible
disadvantage is that the permeability coefficient of the filter in Madou is
not
disclosed to be utilized for determining the membrane's thickness and other
CA 02449549 2003-11-17
-4-
physical characteriztics, which optimizes the sensor's response time. Still a
further possible disadvantage is that Madou's filter is inert and does not
react, if
desired, with the gas that diffuses through the membrane.
[ooos] What is desired, therefore, is a sensor having improved
repeatability. Another desire is to provide a sensor having improved response
time. A further desire is to provide a sensor having an improved signal to
noise
ratio. A still further desire is to provide a sensor that is easy to
manufacture with
reduced costs while maintaining or improving its operational and manufacturing
efficiency.
Summary of the Invention
[ooo9] This invention is directed toward a controllable and reproducible
gas sensor configuration having a three-phase contact area, whereby the sample
gas diffuses to the sensing electrode and solid proton conductive membrane
through openings, holes or slits that extend through the non-conductive
supportive substrate as well as a solid diffusion membrane.
[oooioj This invention is further directed toward a gas sensor where the
gas diffusion process is decoupled from the proton conduction process. Hence,
the invention controls gas flow via the holes in the substrate and not merely
through the diffusion membrane that is in contact with the sensing electrode,
which is known in traditional sensors. Therefore, the invention may use, in
addition to the substrate holes, a non-electrolytic or an electrolytic
membrane to
control gas flow. The gas diffusion is controlled by through openings of known
area in the substrate or in the substrate and an additional rate limiting gas
diffusion barrier film, eg: polyethiene, while proton conduction takes place
only
through a proton conductive electrolyte layer, e.g., a Nafion membrane.
[OOo11j The invention is also directed toward utilizing a method of mass
producing film type gas sensors by stacking a number of component layers to
CA 02449549 2007-01-22
form a series of adjacent sensors which are subsequently separated into
individual sensors.
[00012] The invention is still further directed toward a gs sensor
utilized in conjunction with a gas sensor control circuit.
[00013] The invention is also directed toward a gas sensor utilized in
a gas sensing instrument.
[00014] The invention achieves the foregoing and other objects by
providing a sensor cell having a substrate with a first surface and a second
surface, a sensing electrode in contact with the substrate, and an opening
extending from the first surface to the second surface proximate to the
sensing
electrode for controlling a gas flow. The invention also includes a gas
diffusion
membrane being of electrolytic material in contact with the sensing electrode
and placed within the opening and between the gasses to be detected and the
sensing electrode.
[00015] In a preferred embodiment, the thickness of the gas
diffusion membrane is calculated based upon a parameter selected from the
group consisting of a material of said gas diffusion membrane, a difference in
partial pressure of the gas across said gas diffusion membrane, a permeability
coefficient of said gas diffusion membrane, and combinations thereof. More
preferably, the gas diffusion membrane has a thickness determined from a
permeability coefficient of the gas diffusion membrane.
[00016] In a further preferred embodiment, the gas diffusion
membrane is proton exchange membrane, especially an anion, hydroxide ion
exchange membrane. Alternatively, it can be an electrolytic material.
Brief Description of the Drawings
[00017] Figure 1 shows a schematic top view of the non-conductive
supportive substrate.
CA 02449549 2003-11-17
-g-
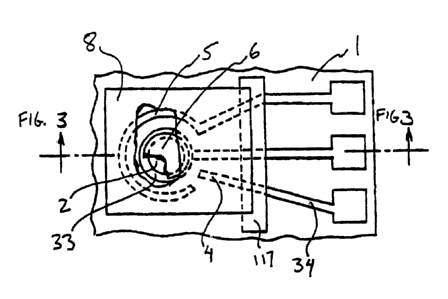
[ooo1s] Figure 2 shows a film type electrochemical sensor cell with Pt/Air
(02) reference.
[o0019] Figure 3 shows a film type electrochemical sensor cell with a
polymeric gas-diffusion layer over the sensing electrode membrane.
[0002o] Figure 4 shows a gas sensor control circuit.
[00021] Figure 5 shows a gas sensor utilized in a gas sensing instrument.
Detailed Description of the Invention
[00022] The present invention overcomes the limitations of the sensors
mentioned above by uniquely combining an advanced solid polymer ionomer
membrane configuration with a film type electrode on a non-conductive
supportive
substrate. The substrate has diffusion openings or holes having a known area
which permit easy access of the sample gas, through a diffusion membrane, to a
sensing electrode contact area. The sensor configuration provides a three
phase
contact area at the interface of the membrane, the sensing electrode, and the
gas
being detected. This design utilizes the precision of solid-state device
fabrication
techniques to yield inexpensive, low maintenance, highly sensitive, rapidly
responsive, and reproducible sensor devices for environmental, industrial, and
biomedical monitoring.
[00023] Figure 1 shows the top view of a substrate (1), which is preferably
non-conductive and may be a sheet or film of ceramic or alumina, having holes
(2). Holes (2) are shown to be distributed in generally parallel rows but such
an
arrangement of uniformity is not required for proper operation of the sensor.
The
distance between holes (2) in the parallel rows and the distance between the
rows
determine the dimensions of the sensor. In a preferred embodiment, holes (2)
are
ideally punched in a single step, while the alumina plate is still soft, in
the "green"
stage of substrate fabrication, prior to high-temperature sintering. In other
CA 02449549 2003-11-17
-7-
embodiments, holes (2) are created using laser ablation or use of soluble
fillers.
In further embodiments, any manner for providing holes (2) is envisioned.
1000241 Conducting leads 32, 33, and 34 and thick- and thin-film
electrodes are formed on substrate (1) for multiple electrodes. Leads 32-34
may
be provided using screen printing or lithographic techniques or any other
manner
for providing leads 32-34 on substrate (1).
[00025] A typical sensor design utilizing this method is shown in Figure 2,
which has reference electrode (4) and a Pt counter electrode (5): Reference
electrode material includes Platinum or Pt/Air (02) and counter electrode
material
includes Platinum. The contact for the sensing electrode (6) is a ring
concentric
to the hole. This ring can be made of smooth, rough or platinized platinum. In
other embodiments, this ring may be Platinum, Gold, Titanium, Tantalum,
Zirconium, Tungsten, or Niobium. Some platinization of the ring may provide
better contact. Simultaneous platinization of electrodes can be performed by
customized electrolytic plating on properly masked multi-sensor plates.
[000261 The sensing or working electrode (6) may be a disc of Teflon -
bonded or Nafion-bonded platinum or other electrocatalyst. A number of discs
are
deposited on an ionomer film, such as Nafion electrolyte membrane (8) at
uniform
distances from each other, for instance, by decal transfer, silk printing,
spray
painting, artist brush lettering, or by any approach which lends itself to
uniform
deposition of a design on a transfer substrate without waste. The discs'
distances
from center to center are the same as for the holes of Figure 1. The diameter
of
the sensing or working electrode disc is larger than the diameter of the hole
in
Figure 1 to allow for contact between the disc and the sensing electrode
support
ring of Figure 2. Instead of a single large hole per sensor of Figure 1(which
requires the use of the substrate to control diffusion of the analyte), a
series of
smaller openings may be used, with small enough diameters to control diffusion
independently of the analyte flow. The areas of the openings are chosen so as
to
control diffusion of the sample gas toward the sensor and to maintain a
constant
CA 02449549 2003-11-17
-8-
diffusion rate independent of any changes in the sample gas flow rate. By
using a
number of these diffusion-controlling orifices, a reasonably large signal may
be
obtained. The range for the length to diameter ratio for these orifices is
2/20 with
a preferred ratio of 3/10.
[000271 Over the empty alumina surface (the surface with no printed leads
and electrodes) a gas-permeable diffusion film (9) is deposited. Film (9) is
made
to conform to the sensing electrode over holes (2), as shown in Figure 3, or
hangs loose over sensing electrode (6). The substrate (with multiple arrays of
printed conductors), the Nafion membrane (with multiple sensing electrode
discs),
and the gas-permeable film are arranged as shown in the schematic
representation of Figure 3. After all the components are unitized, the
resulting
structure is cut in individual sensor units.
[0002al Because holes (2) control the diffusion, or flow, of gas that diffuse
toward sensing electrode (6), membrane (9) is of a polymeric material.
Polymeric
is defined to be either electrolytic, such as an ionomeric film, or non-
electrolytic.
Depending upon the application or experiment, membrane (9) may be of a non-
electrolytic material when no reaction, or interference, is desired between
membrane (9) and sensing electrode (6). In other embodiments, membrane (9)
may be of an electrolytic material when reaction between membrane (9) and
sensing electrode (6) is desired. In further embodiments, membrane (9),
whether
electrolytic or non-electrolytic, may, in addition to holes (2), provide a
second
structure to control gas diffusion. However, it is understood that holes (2)
provide
the primary gas diffusion structure to the invention.
[000291 Permeability of membrane (9), which is an indication of the
diffusion rate of a gas through membrane (9), may be expressed as a
coefficient,
which is a function of the gas to be diffused through membrane (9) and the
material selected for membrane (9).
CA 02449549 2003-11-17
-9-
[000301 In other words, the permeability coefficient is an indication of the
amount of molecules that may move through membrane (9) with a certain
thickness, at a certain temperature, under a certain pressure, and in a given
time.
Hence, a user selects membrane (9) for use with the invention. A first step is
for
the user to determine the gas to be diffused and the type of material, based
on
reactive properties with the gas, to be used in the sensor. Then, based on the
permeability coefficient of the material and gas to be diffused, the user
determines the thickness, surface area of membrane (9), and other physical
characteristics of membrane (9).
[00031] By selecting membrane (9), the sensor is completed quickly and
easily, thereby reducing manufacturing costs. Further, the sensor has enhanced
repeatability because membrane (9) is generally of a similar material,
thickness,
surface, area, and permeability coefficient from sensor to sensor. Hence,
membrane (9) is controlled during manufacturing in an improved manner over the
prior art.
[000321 It is understood that the thickness of membrane (9) is merely one
physical characteristic that may be determined from the permeability
coefficient
and that other characteristics may be calculated.
[000331 The formula for determining the thickness of membrane (9) based
on the permeability coefficient is:
1000341 i=PdxAxaP/d or d=PdxAxaP/I
The flux of gas through the gas permeable membrane (9), and thus the measured
amount of current i, depends on the difference in partial pressure AOP of the
gas
across the membrane (9). The area A is equal to the geometric sensing
electrode
area and d is the membrane (9) thickness. Pd is the permeability coefficient.
CA 02449549 2003-11-17
-10-
[00035] The formula for determining the volume of gas diffusing through
membrane (9), per second, based on the permeability coefficient is:
[00036] Fd = Pd x Ac
where Fd is the flow density, measured in mol/cm2sec, assuming
a 1 cm thick membrane, standard pressure of I atm, and
standard room temperature. Pd is the permeability coefficient,
which may be measured in units of cm/sec. Ac - c1- co is the
concentration difference across the membrane, measured in
mol/cm3, where Ac is the difference between the two
concentrations on both sides of the membrane. cl is the
concentration of the material inside the membrane, which is
preferably located on a surface of the sensing electrode. co is the
concentration outside of the membrane, which is preferably the
gas sample, surrounding air outside the chamber, or the gas
flowing through a GC (in which we want to measure how much of
a specific gas, for example H2S, is contained in that gas sample).
[00037] The formula for determining the concentration of gas within
membrane (9) as a function of time based on the permeability coefficient is:
[00038] cl (t) = ca X (1 - e"u)
T= V/(A x Pd)...is the time constant of the equation above
A is the geometrical area of the membrane
V is the volume of the material in front of the membrane
Pd is the permeability coefficient
In this example cl is a function of time and changes from zero at
c, (o)tocl (fort=inflnity) = co
CA 02449549 2003-11-17
-11-
[00o3s] The following is a non-exhaustive list of possible materials and
permeability coefficients that may be used for membrane (9):
Table 1.
Temperature Permeability coefficient
Gas deg C Glassine Polyethylene
Carbon dioxide 30 9.7 28.1
Hydrogen suiphide 30 9.7 43.0
Oxygen 30 10.5 6.9
Nitrogen 0 11.2 0.25
30 9.4 2.1
50 9.3 7.4
70 8.4 22.0
Units cc x cm x 1010/sq cm x sec x dcm Hg at Standard Temperature and Pressure
Table 2.
Katio
Material P02 PC02 (CO2/02) PH2O
Polyacrylonitrile 0.0003 0.0018 6.0 300
Polymethacrylonitrile 0.0012 0.0032 2.7 410
Polyvinylidene chloride 0.0053 0.029 5.5 1
Barex (Sohio Co.)* 0.0054 0.018 3.3 660
Polyethylene terephthalate 0.035 0.17 4.9 175
Nylon 6 0.038 0.16 4.2 275
Polyvinyl chloride
(unplasticized) 0.045 0.16 3.6 275
Polyethylene (0.964 density) 0.40 1.80 4.5 12
Cellulose acetate 0.80 2.40 3.0 6,800
(unplasticized)
Butyl rubber 1.30 5.18 4.0 120
Polycarbonate 1.40 8.0 5.7 1,400
Polypropylene (0 907 density) 2.20 9.2 4.2 65
Polystyrene 2.63 10.5 3.8 1,200
Neoprene 4.0 25.8 6.5 910
Teflon 4.9 12.7 2.6 33
Polyethylene (0.922 density)
Poly (2,6 dimethyl 15.8 75.7 4.8 4,060
phenylene oxide)
Natural rubber 23.3 153 6.6 2,600
Poly (4 methyl pentene 1) 32.3 92.6 2.9 ---
Poly dimethyl siloxane 605 3,240 5.3 40,000
CA 02449549 2003-11-17
-12-
Temperature is 30 C for all of the above
*High acrylonitri{e copolymer
Units cc x cm x 10'10/sq cm x sec x dcm Hg at Standard Temperature and
Pressure
Table 3.
0, Permeabilit~
Polymer Membrane 10' [mol m/s m Pa]
PTFE. 0.25
Mylar 0.0063
Nylon 6 0.013
PVA10'10 0.033
PVC 0.047
Methyl cellulose 0.233
Cellulose acetate 0.267
PE, high density 0.33
Polystyrene 0.4
Natural rubber 8
Fluorosilicone 36.6
PDMS 167
Table 4.
Permeability X1010 moles/sec
Polymer 02 CO2 H20
Dimethyl silicone rubber 804 4200 50,000
Natural rubber 32.0
Polyethylene (low density) 10.7 33.0 444
Teflon 7.6 21.0 46
Polystyrene 1.6 4.0 -----
Polypropylene 1.34 4.5 100
Mylar 0.025 0.046 70
For 1-mil thick membranes, 1 cm2, at room temperature with a differential
pressure of 760
mm across the membrane.
[oooao] The following is a non-exhaustive list of possible materials and
thicknesses that may be used for membrane (9):
Table 5. Electrode output and response time as a function of cathode area,
membrane, and membrane thickness
CA 02449549 2003-11-17
-13-
97.5% Response
Output Time
Electrode Membrane Thickness (air) (Air-- Nitrogen)
Galvanic Teflon 0.5 mil 4.40 a 2.0 sec
(55-mil cathode) " 1.0 mil 1.63 pa 8.0 sec
Polypropylene 0.5 mil 0.80 ya 10.0 sec
" 1.0 mil 0.222 pa 30.0 sec
Polarographic Polypropylene 0.5 mil 0.80 na 18.0 sec
(1.0-mil cathode) " 1.0 mil 0.41 na 45.0 sec
Teflon 0.125 mil 8.3 na 0.3 sec
Teflon 0.25 mil 4.2 na 0.6 sec
Teflon 0.375 mil 3.75 na 1.0 sec
Teflon 0.5 mil 2.70 na 2.2 sec
Teflon 0.75 mil 2.34 na 7.0 sec
Teflon 1.0 mil 1.78 na 17.0 sec
Fermentation Special 110 na 45.0 sec
(Polarographic Silicone
withl0-mil Composite
cathode)
[00041] The film type sensor configuration described above is integrated
with a potentiostat and a voltage of approximately +0.1 V is applied to the Pt
sensing electrode with respect to a Pt/Air (02) reference. This corresponds to
an
applied potentiostatic voltage of approximately 1.16 V with respect to a
normal
hydrogen electrode (NHE).
[00042] Gas samples of air and 7.4 ppm SO2 in air are introduced into the
sampling port of the fixture described above. The gas flow is approximately _
60 cm3/min and temperature is approximately 25 C. The sample gas diffuses
through the 80-mil hole in the non-conductive substrate and electrochemically
reacts at the exposed sensing electrode/solid ionomer electrolyte surface.
Humidification is provided by the liquid water in the reservoir which soaks
the
opposite, or back side of the membrane where the electrode structures are
located.
[00043] The background response signal with air is 30 nanoamps (nA).
The response signal with 7.4 ppm SO2 in air is 135 nA. This corresponds to a
net
CA 02449549 2003-11-17
-14-
response signal for 7.4 ppm S02 in air of 105 nA or 14.2 nA/ppm per 80-mil
hole.
It is possible to increase the magnitude of signal and signal-to-noise ratio
by
increasing the number of holes in the substrate above the integral sensing
electrode structure.
[00044] It is also possible, with this configuration, to detect other
oxidizable or reducible gases such as CO, NO, NO2, H2S, ozone, C02, hydrogen,
hydrazine, ammonia, HCI, alcohols and acetone.
[00045] Referring to Figures 4 and 5, a block diagram of the sensor
control circuit (13) is shown. The sensor control circuit (13) is designed to:
1) control the potential of the sensing electrode (6) at a predetermined
voltage
(the "potentiostatic voltage", or "Epot'); 2) measure the temperature; 3)
convert the
gas concentration-related current to a temperature-compensated voltage signal;
and 4) provide properly amplified voltage to the data acquisition/storage
microprocessor (14). An on-board micro power-regulated power supply (16) uses
the microprocessor's (14) power supply to provide the required 1-3.9 volts for
the
sensor circuitry. The DC power can be supplied by a 6-V battery (16d) or an AC
adaptor (16e).
[ooo46] The control amplifier portion (17b) of the sensor control circuit (13)
consists of a micro power operational amplifier (e.g., MAX407 or LM6062). The
sensing (6), counter (5) and reference (4) electrode portions of the sensor -
assembly (1) are in the feedback loop of the control amplifier (1 7b) as shown
in
Figure 4, a standard configuration for potentiostat circuits. An adjustable
voltage
divider (17a) allows the polarizing voltage (Epot) to be set at a
predetermined
voltage range such as 0 to 50 W. This signal is compared to the reference
electrode (4) voltage (which appears with it at the summing junction) by the
control amplifier (1 7b) of the sensor control circuit (13). The latter
adjusts the
current through the sensor cell (10) to minimize the difference between the
Epot
and the reference electrode (4) voltages.
CA 02449549 2003-11-17
-15-
[00047] The resulting sensor cell assembly (19) current (flow of electrons
from sensing electrode (6) to counter electrode (5)), which is linearly
related to the
concentration of gas, is transformed into a voltage signal by the current-to-
voltage
converter (15a). Temperature compensation of the sensor signal is effected in
the
next stage of amplification (15b) using a thermistor (18a) which is positioned
in
the gas sensor housing (10). The last stage of amplification (15c) provides,
if
required, inversion of the voltage signal as well as gain adjustment, to
permit
calibration for normal variations in sensitivity among sensors. The same type
of
micro power operational amplifier is used for these stages (15a), (15b), (15c)
as
for the control amplifier (15b). The transformed current signal is directed to
an
A/D channel on the data acquisition board of the microprocessor (14).
lo0048] Power for the sensor control circuit (13) is provided by a Duracell
6-V battery (16d) (PX 28A or 28L) through a micro power-regulated power supply
(16). The power supply (16) utilizes a voltage inverter (e.g., ICL 7660) (16a)
to
convert the positive battery voltage to a negative voltage of the same
magnitude,
and a positive voltage regulator (e.g., MAX663) (16c) and negative voltage
regulator (e.g., MAX 664) (16b) to provide a stable t3.9 volts.
[00049] The film type gas or vapor sensing instrument (12), as shown in
Figure 5, includes the sensor cell assembly (19), potential-control circuitry
(13),
power supply (16), and the microprocessor (14) with the data acquisition-
recording unit. The sensing instrument (12) is preferably battery operated,
and
has the ability to sample the gas or vapor and temperature signals at
intervals and
store in the random access memory (RAM) on the data acquisition board days to
weeks of data. The data acquisition circuit microprocessor is programmed to
sample and store the gas concentration signals at preset intervals. Data are
off-
loaded to a personal computer by accessing the microprocessor through an
RS232 port.
CA 02449549 2003-11-17
-16-
[oo050j The sensor cell assembly (19) and its potential-control circuit (13)
are integrated with a battery-operated microprocessor (14) of 32K memory,
which
samples the sensor signal as well as temperature and other signals at 10-, 20-
, or
30-second intervals and stores an average value at intervals of 2, 5, or
minutes according to a programmable protocol. The data acquisition/storage
unit in the microprocessor (14) can record 8 days of data, storing at 2-minute
intervals, or up to 40 days storing at 10-minute intervals. In clinical
testing to
date, a 2-minute interval is suitable for one-day clinical studies and a 10-
minute
interval is appropriate for extended use. The microprocessor (14) with data
acquisition/logic circuit can be programmed to sample more than one analog
signal from the control circuit (13), and to convert these to digital signals
and store
them (i.e., gas concentration and temperature) at preset intervais together
with
real-time data. Data are off-loaded to a personal computer by accessing the
microprocessor (14) through an RS232 port. After downloading, the digital data
are converted to engineering units of gas concentration and temperature, and
can
be graphed by a menu-driven Lotus 123 spreadsheet. Through a potentiometer
in the gain amplifier circuit (15c), the device can be calibrated with
calibrated gas
samples, to indicate gas concentrations in the ambient. The potential-control
circuit (13) shown in Figure 4 is powered, in a preferred embodiment, by six
11/2
volt AA-size batteries (16d). A typical microprocessor (14) with data
acquisition-
recording capability that has been successfully used is sold by ONSET
Computers, Falmouth, MA, under the product name of "Tattletale Lite ." The
sensor cell assembly (19) with its control circuit (13) is also designed to
yield a
current or voltage signal proportional to gas flux that could be used to
continuously transmit the data to a remote receiving device or central
monitoring
station or unit.
[ooo51] The sensing electrodes can be organized in multiple arrays or sets
containing a necessary number of counter or reference electrodes. Reference
electrodes such as Pt/air (02), Pt02, or dynamic hydrogen electrode as
described
by Giner (1964) may be employed. Electrically driven 3- or 2-electrode film
type
CA 02449549 2007-01-22
-17-
configurations may be employed using potentiostatic or potentiodynamic control
of the potential of the sensing electrode (6). While the potentiostatic
technique is
a steady-state measurement, the potentiodynamic technique is a transient-type
measurement method. In practice, the potentiodynamic process consists of four
basic steps: a) hold sensing electrode potential constant with respect to the
reference electrode to form a well-defined oxide layer; b) place the sensing
electrode at open circuit to react the oxide layer with the gas for a pre-
determined
time, during which the sensing electrode rest potential shifts, depending on
reactant concentration; c) resume potentiostatic control of the sensing
electrode,
changing the potential back to the initial potential and electrochemically
regenerating the oxide layer; and d) measure the current (or charge) necessary
to regenerate the oxide layer. The primary advantages of the potentiodynamic
method are increased sensitivity, specificity, and stability. Two-electrode
configurations require a reversible or stable counter-reference electrode such
as
Pt/air (02), Pt02 or Pt/H2 which has a higher BET (Brunauer, Emmett, Teller)
surface area (25 m2/g or larger) and/or larger geometric surface areas than
the
sensing electrode.
[00052] Electrochemically reversible electrodes may be used in 3 or 2
electrode configurations, but especially in a 2 electrode arrangement where
the
counter electrode also acts as a reference electrode. Electrochemically
reversible
electrodes are constructed of stable catalyst materials and usually have a
relatively large electrochemical active surface area so that they remain
stable and
their potential is not perturbed by small current flow. Examples include Pt02
and
Ag/AgCI electrodes.