Note: Descriptions are shown in the official language in which they were submitted.
CA 02449621 2003-12-09
. - -. . -. . - .. :.. --SYSTEM A_ND~.. METHOD.:.FOR. NONINVASI~7E .
HENLATOCRIT MONITORING .
' ... .. , BACKGI30UND
. 1. The Field of the Invention.
This invention relates to systems and methods..for .
noW nvasively.measuring one. or m~re.biologic constituent
values. More particularly, the ;present invention relates
to noninvasive spectrophotometric systyems and methods for
.'15 ~~quantitatively ~~arici ~'con~'inuous'ly imoniior'iiig the"~ematoerit'
""
.and : other blood -parameters :of .a s ub~.e.ct.,.. . .. . ...
2. The Prior Art.
Modern medical practice utilizes a number of
procedures and indicators to assess a patient's condition.
One of these indicators is the patient's hematocrit.
Hematocrit (often abbreviated as Hct) is the volume,'
expressed as a percentage, of the patient's blood which is
occupied by red corpuscles (commonly referred to as red
blood cells).
- Human blood consists principally of liquid plasma
(which is comprised of over 90 o water with more than 300
other constituents such as proteins, lipids, salts, etc.)
and three different corpuscles. 'The three corpuscles found
in blood are red corpuscles, white corpuscles, and
platelets.
The chief function of red corpuscles is to carry
oxygen from the lungs to the body tissues and carbon
dioxide from the tissues to the lungs. This critical life
supporting function is made possible by hemoglobin which is
the principal active constituent of red corpuscles. In the
lungs, hemoglobin rapidly absorbs oxygen to form
oxyhemoglobin which gives it a bright scarlet color. As
the red corpuscles travel to the tissues, the oxyhemoglobin
CA 02449621 2003-12-09
D
_ .
~" ..- ~ releases .,oxygen, i . e.. ,. is "reduced, aiid . the
hemogl~o'bin
~turiis~;'~. y ',
a dark red color.
The oxygen, transportation functions of the body rely
., , ' essentially eriti.rely on:: the presence of hemoglobin. in ahe
~ _ ..
,
red cbrpuscles: - 'Red~'corpuscles ~ greatly~~: outnu~nber~~,other'.
~: _~
corpuscles, being about 7.o0ytimes greater than .the number. of
. .
white corpuscles in a healthy human subject:
Medical professionals routinely desire. to.knaw the
. hematocrit,of a patient. In.order to determine hematocrit
using any of the techniques available to date, it is
necessary to draw a sample of blood by.~uncturing a vein or
~~invading ~~a capillary. ~I'hen~; ~" iisiiig -~a~~"widely 'accepted
technique-, - the- .s.amp~.e :.of.. bla~od ,is... subj ected.
to. ~.~ "high speed ,._ : r
centrifuge treatment for several minutes (ela., 7 or more
minutes). The centrifuging process, if properly carried
out, separates the corpuscles into a packed mass. The
volume occupied by the packed corpuscles, expressed as a -
percentage of the total volume of the plasma/corpuscle
combination, is taken as the hematocrit.
- It will be appreciated that the centrifuge process
provides a hematocrit value which includes all corpuscles,
-- not just red corpuscles. Nevertheless, the vastly greater
numbers of red corpuscles in a healthy subject allows the
hematocrit value obtained by the centrifuge process to be
clinically usable'in such healthy subjects. Nevertheless,
in subjects with low hematocrit or dramatically high white
corpuscle content, it may be desirable to diminish the
effect of the non-red corpuscles when obtaining an
hematocrit value.
There have been various techniques and devices
introduced which have automated and increased the precision
of obtaining a hematocrit value. Nevertheless, all the
previously available techniques have one or more drawbacks.
Specifically, the previously available techniques all
require that a sample of blood be withdrawn from the
CA 02449621 2003-12-09
_ -3-
patient-.f, or - in-vitro. analysis.,.. Ariy ..invasion -of rthe' siibj ect
~to obtain , blood is - . accompanied ~ ~by the' 7problems of
inconvenience, stress, and discomfort imposed upon the
subject and- also v the risks which are" always present :when ~._ .. .
- v ~ - 5' Y the body ~is- .invaded. ~ - DraW'ing.,blood-ako
.createsL.certain~.
contamination ' risks -'. to the paramedical ~ .professional.
Moreover, even in a setting where obtaining a blood sample
does not impose any - ~addit~ional problems., e...a.. , .during .
surgery, the previ~usly available techniques reqwire a
delay between the time that the ,sample is drawn and the.
hematocrit value is obtained. Still .further, none of the
previously-avai~.abhe techri~iques'alToW coiltiriudus iaohittiririt~"
.. .of ..a.
subject'a.:.hemato:c.rit,...,as...Might;..,be:..desirable..~.du.,~irig.. .
some surgical procedures ar intensive care treatment, but
require the periodic withdrawal and processing of blood
samples.
In view of the drawbacks inherent in the available art
dealing with invasive hematocrit determinations, it would'
be an advance in the art to noninvasively and
quantitatively determine a subject's hematocrit value. It
would also be an advance in the art to provide a system and
- method for noninvasive hematocrit monitoring which can be
applied to a plurality-of body parts, and which utilizes
electromagnetic emissions as an hematocrit information
carrier. It would be another advance in the art to provide
a system and method which can provide both immediate and
continuous hematocrit information for a subject. It would
be yet another advance to provide repeatable and reliable
systems for noninvasive monitoring of a subject's
hematocrit. It wauld be still another advance in the art
to noninvasively and accurately determine a subject's blood
oxygen saturation while accounting for the pati'ent's low or
varying hematocrit and/or under conditions of low
perfusion. -
CA 02449621 2003-12-09
0
_ _4_
. . . . : ... .. ' ~. .. . . : .; .. _-.: BRIEF . ST ..TMMARY ~ AND OBJEC'rS~
OF~ 'THE~~~ ZNV~NTIOf~..'- ' ", ' ': ~ < . . -: ..
The present invention ~ misdirected ~ toapparatus and
methods for determining biologic; constituent values, such
. . as the w liematocrit: . va~hx~., :~ .. trans~u~aneous.ly . , :and -. . ...
~ ~ .noninvasivel~~.~ ~. ~ ~TYiis.~ isv achxevecl.: ~by ~ passing..; at .,
lease.. t~ao .. ~ ..~. '
wavelengths ~ -of ~ light onto-. or through ~ body : tissues ':such - asw :- _
the finger; earlobe; or.scal.p; lets. and then compensating
for the effects body' tissue and fluid.:.effects.. '. As: used. .
herein, the term biologic constituent includes proteins,
red cells , metabolites , drugs, c,ytochromes, hormones,.etc. .
In one embodiment within the scope of the present
iriventi~on',"t~iev"wavelengths of light 'are welected to~'be'nea~rw
.. .. . . .. or ...at.....:the.,. . iso..bestic.. .:points.. -c~~.
,,.reduced., _ hemoglobin, and' ,_ .
oxyhemoglobin to eliminate the effects of variable blood
oxygenation. At an isobestic wavelength, the extinction
coefficient, e, is the same for both reduced and oxygenated
hemoglobin. Thus, at isobestic~wavelengths, the amount of
light absorption is independent of the amount of-
oxygenated or reduced hemoglobin in the red cells.
Means are provided for delivering and detecting those
wavelengths of light and for analyzing the light
intensities. The sensing and radiation emitting elements
are preferably spatially arranged to allow ease of use and'
to be accessible to a,patient's exterior body parts. The
configuration of the sensing and emitting ele~uents is
'important to give optimum repeatability of the signals and
data derived therefrom.
Memory and calculation means are included which are
capable of storing, manipulating, and displaying the
detected signals i1~ a variety of ways. For instance, the
continuous pulse wave contour, the pulse rate value, the
hematocrit value and the continuous analog hematocrit curve
in real time, the hematocrit-independent oxygen saturation
value, and the oxygen content value of the blood, all as
CA 02449621 2003-12-09
m
_. _5_
.-- digital,-vahues".or as co~tinuous.analog curve~,:,'in real: ~t~mp'~
,. : .' ......~'..:':: . ~.' h.. ... ~.,..... ,.... ;. ._.,..~... ., .. ~~ ..
~ .... . ., . ,..~ . . ..
are capably of being displayed.
An important advantage of monitoring and analyzing
w _~ each v~ ind,ividiral--- ~ :.pulsatile~: rsyiial ~. ~is- - ~ that w
a~xer~i~g.irig ...
' -algorithms .inay~.~=be.'perfo'rmedv.for. identifying- -andvreject~.ng= '.
erroneous data . , . - In y addition, such , techniques also y impvove
repeatability. ' ~ v . - -.
Another significant~advantage of the present 'invention.
- is the capability. of monitoring multiple wavelengths
(including nonisobestic wavelengths) for the simultaneous
real time computation and display .~f the -hematocrit
i~ndependent~ oxygen -saturation va:Lue:'~ ' Techiiiqiies iii-"prior' '~'"
.. . .. .ai , .... . ox.imet-ry . ...,have'.. . :a~Z.l . .. .suffered.
:.inaccuracies. .. . dare .... . tp ,.., _. .
hematocrit sensitivities.
Rather than apply AC-DC cancellation techniques only,
it is also within the° scope of the present invention to
detect and analyze multiple wavelengths using a logarithmic "
DC analysis technique. In this embodiment, a pulse wave is-
not required. Hence, this embodiment may be utilized in
states of low blood pressure or low blood flow.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a perspective 'view of a first presently
preferred embodiment of the present invention.
Figure 1A is an enlarged cross sectional view of the
body part (finger) and system~components represented in
Figure 1 used in a transmission mode.
Figure 1B is an enlarged cross sectional view of the
body part (finger) and associated system components
represented in Figure 1 used in a reflective mode.
Figure 2 is a chart showing the optical absorption
coefficients of oxyhemoglobin (Hb02), reduced
hemoglobin (Hb), .and water (H2o) versus wavelength.
CA 02449621 2003-12-09
b
- -s-
. . . . Y.. . ~. Figure. _. 3 ~~ is. a chart showing .. the. relationshiP_
between
the' extinction coefficient ~of Tight. at~ three different .~
wavelengths versus hematocrit for whole blood.
' ., , ~ . ,-, y _ - ,~ , . ~Figure~ 4 ~ is a ~ chatty showing : ahe
relationship'.. betwe~eri
:, . ... , .
' S the ratio . o~ .the ~ extinction coeff ic~ients~ of twow rays having
differing - ra~vei~engtlis- 'versus,. ~hematocrit:; : . .
Figures SA-5E provide a flaw chart showing the steps
carried out during one presently preverred mEthod of the
present ,invention, using the pulsatile component of the
l0 subject's blood flow to provide accurate hematocrit and
blood oxygen saturation values: -
~Figure ~6 ~is~~ a~~~perspective view- of '~a- secoiid~ presently '
-. . - . - . pr~efe.rred~.. ~sys~tem of...t;he,-.,present : invention. wh.i.ch
is,. applied. .
to the ear and includes structures to squeeze out the blood
15 to blanch the ear tissues.
Figure 6A is ari enlarged cross sectional view of the
ear and system components represented in Figure 6.
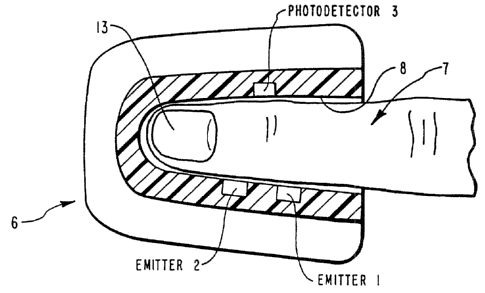
Figure ? provides a detailed schematic diagram of the-
low level sensor circuitry included in the presently
20 preferred system of the present invention.
Figures 8A-8C provide a detailed schematic diagram
digital section circuitry included in the presently
preferred system of the present invention.
Figures 9A-9D provide a detailed schematic diagram of
25 the analog section circuitry included in the presently
preferred system of the present invention.
Figures l0A-lOC provide a detailed schematic diagram
of the power supply and input/output (I/O) section included
in the presently preferred system of the present invention.
30 Figure 11 is a graph showing variation in oxygen
saturation as a function of hematocrit.
Figure 12 is a graph of E /E versus Hematocrit.
eaos mo
Figures 13A-13B are graphs of E versus Hematocrit at
two non-preferred Wavelengths and e~/EZ versus Hematocrit at
35 those non-preferred wavelengths.
CA 02449621 2003-12-09
. : . , : . . . .. : . . _Figures . 14.A~.1.48. ark :~.g~r.~~hs.._pf E
versus
. .. . . : Hematocr. it. . at ~ ..
two non-preferred wavelengths and E~/e2 versus Hematacrit at
those non-preferred wavelengths.
.. . . , Figure.. .1.5 ...iilustrat.e.. "verti.ca.7. :.em.itter..
alxgn~ent.
;a~izd .....~
. . . - .:.. the resu3aing non-identical dXb ~ regions ; .. . ... . . . _ .
. . 5 . ~
. . ~- ~..Figure ~ 16 .-illustrates. horizontal. emitter. alignment.
DETAILED~DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present-invention.. is. directed to apparatus and
methods for determining biologic~constituent values, such
as the hematocrit value, transcutaneously and
.
is achieved by passing at least two
noninvasively.This
~aavelsng~lis ~ of'~' ~:~ight "onto bf~ '~thxoiigh-~ bbdy -W~idsuesr
stitch ~~ as~ ' -
the finger, earlobe, or scalp, etc., and then compensating.
for the effects of body tissue and fluid by modifying the
Beer-Lambert Law. The_principles within the scope of the
present invention may also be utilized to provide a
hematocrit-independent oxygen saturation and oxygen content-
measurements as well as noninvasive measurement of blood
' constituents such as glucose, cholesterol, bilirubin,
creatinine, etc.
Although the present invention will describe in great,
detail the transillumination of various body parts, it will
be appreciated that reflectance spectrophotometry may
alternatively be employed where transillurnination is
difficult to accomplish. As used herein, the term "body
part" is intended to include skin, earlobe, finger, lip,
forehead, etc. Because the principles within the scope of
the present invention can be adapted by those skilled in
the art for in vitro measurement of hematocrit and other
blood constituents, the term "body part" is also intended
to include various in vitro blood containers such as tubes
and cuvettes.
CA 02449621 2003-12-09
.. : :.. . . : .1... -. ,,S,~~ectrophotome~ric Methods. , , ~ y ' ' y . . . .
.. ' . ' ' .
Spectrophotometric methods have been described in the
prior art which monitor various metabolites in bady fluids.
. , ~, . _ , . . . . : .Radiation,, ....'typica.l.ly.. ..in . the;. visible :.
or. , .near . infrared v
5'~~ ~'regioii; ~ is directedonto' aii " exterior body ~ part ' for
transcutaneous.penetration of.the.,radiation. The radiation.
is then monitored reflectively or transmissively by a
photodetector or similar sensor: Radiation spectra are
chosen at wavelengths where the metabolite or compound
sought for either absorbs highly or poorly. Some examples
of such spectrophotometric methods ark described in U.S.
Patent No. 4,653,498 for pulse oximetry, U.S. Patent
. . No . 4 , 655'; 225 ' ' foW .: 'bloo~d~ =~glwcosew...monitoring ~ ~ ~ and=
more ~ .. ,
recently U.S. Patent No. 4,805,623 for monitoring various
blood metabolites (glucose, cholesterol,-etc.).
A theoretical. basis for the spectrophotometric
techniques is the Beer-Lambert Law:
Ioe -Eud ( 1)
Equation (1) may also be written:
ln(IjIo) - ~-eXd (la)
wherein IQ is the incident intensity of the source
radiation, I is the transmitted intensity of the source
through the sample, E is the ext:i~ction coefficient of the
sought for component, X is the concentration of the sample
component in the tissue itself, and d is the optical path
length (distance).
The Beer-Lambert Law (1) permits in vitro solute
concentration determinations. However, quantitative
measurements have not been possible in the body since the
scattering of the incident photons passing into and through
the integument and subdermal regions is extensive and
CA 02449621 2003-12-09
_ . _9_
" v. highly 'variabl.e. ' . ~Thi.s, scattering .s~po,i~.s the BeerLambert . .
,
Law by adding a variable loss of radiation to the
measurement and also extends the path length of the
., .. , .... incident,,.photons. .b~ an' unknown amount as well.,
' ' S 'Even though opti~calw ~ pulse rate ~monitors~,
plethysmographs,.,and pulse oximeters are."known, their-
development has been accelerated by techniques which allow
for cancellation of the optical scattering effects to a
large extent. This development began with U.S. Patent
No. 2,706,927 and was further refined by Yoshiya, et. al.
(Med. and Biol. Eng. and Computing, 1980 Vol. 18,
Pages. 27-32 ) , Koneshi in U. S. Patent ~ No. 3, 998, 550, and
. . .-. . . . . . . . Ramagur~i iv wU.-S ~ -- N~.w-patent-:.;.4.,:2661, 554.,
..~whicb ut~i.lized.:..a_ .. . ..
technique of analyzing the resultant opto-electronic signal
by dividing it into its AC and DC components. The AC and
DC components are mani.'pulated with logarithmic amplifiers
in such a way as to eliminate the above-mentioned
transdermal optical effects (the variable amount of-
radiation loss due to scattering in the tissue and the
2~0 unknown and variable amounts of optical path length
increase).
Until now, the AC-DC cancellation techniques have not
been successfully adapted for the measurement of hematocrit
or hematocrit-independent blood oxygen saturation.
2. Noninvasive Differential-Ratiometric Spectro_pho.tometry
' It is assumed that incident radiation passing onto or
into a living tissue will pass through a combination of
blood, tissue, and interstitial fluid compartments. The
CA 02449621 2003-12-09
_ -10-
. , light ,attenuated .: b~~, such a , living , tissue. ~ can .be .expres.sed
':
by the modified Beer-Lambert equation:
. ... .. . . . . . . ~ z ._ . .Ipe <eb<xetx">+~txta.;xi )a+c _ . . , , . ( 2
). . . .
Equation .( 2 ) may also be written , . ,
ln(I/Ip) - -(Eb(Xe+XY)+etXt+Eixi)d+G (2a)
Where Eb, Et, and Ei represent the extinction coefficient in
the blood, tissue, and interstitial fluid compartments,
respectively; Xa and X~ represent the arterial and venous
blodd~ :~ _ .C.oncentrationw: (Xb-.Xe+X~j :m .: .. :Xt .,..,.represents -. the
:.
concentration of the tissue absorbers, and Xi represents the
relative concentration of water and dissolved components in
the interstitial fluid compartment; d represents the
intrasensor spacing; and G is a constant of the geometric -~
configuration.
As the blood layer pulsates, the concentration terms
change. The term d can be fixed by the geometric
configuration of the device., Taking the partial
derivatives of equation (2) with respect to time and
dividing by equation (2) gives:
aI/at =~E~(axa/at+ax~/at) + EtaXt/at + E;ax;/at~d +aG/at ( 3 )
which can be simplified at each compartment and wavelength
by letting X' =ax/at, and G' =aG/<'3t, and V~=-(c3IIc3t' to give
Jx
V~ _~Eb(Xa+Xv~ + EtX C + E iXild +G/ (,4 )
Assuming that Xt and G do not vary significantly over the
pulse time interval, then G'=0 and X't=0, and equation (4)
can be simplified to
, CA 02449621 2003-12-09
. _11-
. , . , . . .. ~.. ~Z-(.Eb~Xa+Xv~ *,E;~;~a: . . ,: . ..' . .. _ .. ~, ,,' :(
~:. .
. ~ Exami~ing....the vtranspovtwb~etween. X8... and....~~~. ~"~~ -~ca~i-
:..farm~.a..: - .
proportionality constant I~ such that, ~ X'.~ -~,X' a,
representing the reactionary nature of the venous
component, and further reduce the above equation to
Vz =(Eb~l_Kv~Xa + E iXi)d ( 6 )
Since X' a and X' i ' are not wavelength (~l) wdependent, V' ~
. . value. . :a~.. .. dif.f~.rent. wavelengths can , be....dif ferentially, .
subtracted to produce a hematocrit independent term which
contains only EiX'i information. Although the term
V~ sos/V~ ~sio Provides- useful information regarding relative
. changes in hematocrit, it should be recognized that the
simple V' ~s/V' ~3~o ratio is not sufficiently accurate for
hematocrit value determination unless the EiX'i term is
known or eliminated. For example, the EiX' i8os term can be
neglected since ei~s is extremely small, whereas the EiX' ;310
term is about 25%-50% of the Eb~3~~ value of blood itself and
cannot, therefore, be neglected without affecting accuracy:
Figures 3 and 12 suggest that a linear combination of
V° z at ~=805 nm and a=970 nm will have a near constant value
.for a range of Hct values. Since the extinction
coef f icients E iaos and E i97o are well known, or can be
empirically determined, a precise proportionality constant
R~ can be found to produce
i_ i i
Ei970Xi -V970~R1V80s ( )
This correction term can now be applied with a second
proportionality constant R2 (whe:re RZ is approximately equal
CA 02449621 2003-12-09
. -1.2-
. to . E i f3t~°~ i970)...: t~ -~~e y'~.~3~0 . termvto: exactly. remove
its.,. E.;y3lox~..;.. .., . .....
sensitivity, hence: '
r r _ r _ m g
. . ' ' y. , . ~ . Eb1310~f -K.r,Xa.' V13'10 R2(VSt70 R1~805~.; ...:. . . .. (
) '
... , , .; . .... . .. . .., . .. :,. . .. , . , : . : . , _ . . . .. ,. .,::
.. .
,. . .. . ,.,.. ' ..
This corrected' term 'can now "be 'used ' ratiometrically with
V' $0s to remove the ( 1-ICY) X' 8 ', and leave the pure extinction
coefficient ratio represented by Equation, (9) below and
shown graphically in Figure 4.
r
E aaos __ Vaos
~b1310 / _ ( ~~ _ I
V 1310 2 970 , . . . BOS
.. .... . : . . . . ....... . .... .,. ,. .. . .,. . . ... .. .. ., . .....,..
. .. . .. . . . ., . ... : . . . V .. .. . .. . . . . ...... .. . ~. ... . ...
. ., . . .
.,. R.'V R1
It should be noticed that the following assumptions
to and requirements are essential in hematocrit determinations
(but in the case of pulse oximetry these requirements may
not be of the same degree of significance).
A. Even though wavelengths ~1=805 nm and ~1=1310 nm
- are near isobestic, the actual function of E versus
Hematocrit at each given wavelength must hold hematocrit
information that is different in curvature, or offset, or
linearity, or sign from the other. See Figure 3. If the
functions Ea versus hematocr3.t are not sufficiently
different, then the ratio Eb~1/Eb~z will not hold hematocrit
~.nformation. See Figures 13A and 13B and Figures 14A
and 14B. Even though the foregoing discussion refers to
the isobestic wavelengths of Jl=805 nm and A=1310 nm, it
will be appreciated that other isobestic wavelengths, such
as ~=570 nm, .1=589 nm, and JL=155() nm, may also be utilized.
B. Further, the wavelengths should be selected close
enough to one another such that the optical path
lengths, d, are approximately the same. Longer wavelengths
are preferred since they exhibit less sensitivity to
scattering, s:
CA 02449621 2003-12-09
- -13-
s« 2 (10)
C. The, geometric. or , spat ial relationship of the.
. . . . . . . 'emitters ~ and ~ ~seiisorsv ~~'ls ... . im~or~.ai~t : " Fo"r
, .. . ' "iiistar~c~~; -'... vif..:~ . _ ..
vertically aligned vemitters axe used in an earlobe-
measuring device, then~the top=most emitter may illuminate
a different amount of blood filled tissue than the lower
emitter. If only one sensor is used, then there will be a
disparity between X'b at each wavelength. See Figure 15,
wherein Xb> > X~ > X~. Furthermore, the sensor-emitter
spatial separation distance is very important because the
, pressure _ ;applied ., to ..the, tissue between , the sensor
,. and . ,
,
.
arteriolar and capillary vessel
the
emitters affects
compliance. This changes the X' as the pressure {or
distance) changes. This change in X' then modulates the V' a
function. Therefore, the sensor-emitter separation
distance must be such that the pressure applied to the
earlobe, fingertip, or other body member, does not affect
function. This sensor separation distance is
the V'
~
empirically determined and should generate less than 40 mm
.- Hg applied transmural pressure.
A horizontal alignment (Figure 15) of the emitters
with respect to the single sensor can be arranged so that
the emitters and sensors illuminate and detect identical
regions of aX~~ and aX~2. It is important to note that the
term d, the sensor-emitter separation, will be different
between ~,~ and 7lz by the cosine of the angle between the
sensor and emitter. Therefore, if any misalignment from
normal occurs, the term d will not cancel to obtain
equation {9).
The preferred arrangement is wherein all the emitters
(660, 805, 950, and 1310 nm) are located on the same
substrate. This is~ preferred because the emitters will
then illuminate essentially the same Xb region.
CA 02449621 2003-12-09
D. In the case of reflectance spectropho-tometry, an,
ag~rture for the sensor and each emitter is required. See
Figure 1B. Also, a sensor-emitter separation is required
y y . so that ,he reflectance of the .first .layer. o~f wtissue, Rt, ~ y(a: '
..
non-blood layer of epithelium) does. not further exaggerate
a multiple scattering effect, i.e. the total reflectance,
R, measured would contain spurious information of the
epithelial layers' reflectance as well, where:
2~
R=Rt+ Tt Rb (11)
t1 _ Rb~ Rt)
1'0 c~there R "is the total retiectawce, Rt is the reflectancewdue . .
to the first tissue-epithelial layer, Rb is the reflectance
due to the blood layer, and Tt is the transmission through
the first tissue layer'.
The reflectance equations describing Rt or Rb must now
l~ sum all of the backscattered light that the sensor detects,
i.e.,:
Rb=~~~(source function)~(scattering function) (12)
While equation (9) describes the theory of the
noninvasive hematocrit device, the four' assumptions (A-D)
20 are important to the repeatability and accurate functioning
of the hematocrit device.
Assuming items A throutgh D are dealt with
appropriately, then (9) becomes:
Ebxt _ (st +'_~t~ ( 13 )
Ebd2 ' ~SZ ~~ k2,
CA 02449621 2003-12-09
-15-
where s is a scattering constant and k ~.s an absorption
constant, and where in whole blood:
~. , . . ~ ~ ~S - 'y ffSHct (~l-Hct) .., . ' ~ ;~ ~.;;: .. ,.~ ~~ ~f~14 ), ,.
k = vaHct ( at isobestic wavelengths ) ( 15
where vs is the scattering cross section and va is the
absorption cross section.
l0 From the foregoing, E, the extinction coefficient, is
not a simple function of the absorption coefficient, k,
normally determined in pure solutions. Rather, it contains
a diffusion or scattering term, s, which must be accounted
for in a non-pure solution media such as whole blood and
. tissue.
Finally, substituting (14) and (15) into (13):.
E~1 _ Qs1(1-Hct) +Qat (16) ._
E.12 Qs2 ( 1-HCt) + Qa2
Therefore, the ratio e~'/e~2 is a function of
hematocrit. From Figure 4, a look up table or polynomial
curve fit equation may be obtained and utilized in the
final displayed hematocrit results. Knowing the actual
hematocrit value, it is straightforward to see (Figure 2)
that a wavelength at 660 nanometers can be~ selected to
obtain an E ratio wherein the hematocrit-independent oxygen
saturation value is derived. For example, equation (16)
would become:
Ego _ Qs~o ( 1' Hct) + aabbo + Sa02 ( Qao~o - Qa~s~oj
E ~ Q (1-Hct +a +S 0 a -a (17)
bB05 s805 ) a805 a 2 ( ao805 as805~
Equation (17) shows both the hematocrit and oxygen
saturation dependence on each other.
CA 02449621 2003-12-09
-ls-
,Figure 11 graphically demonstrates the need for a
hematocrit-independent blood saturation device. As either
the hematocrit value or percent oxygen saturation
decreas~es,~ the ypercent . . saturation ~ ~y error ~~ .becomes.. ...
unacceptable for clinical usage. For examgle, it is not
uncommon to see patients with a low hematocrit (about 20%)
who.have respiratory embarrassment (low oxygen saturation)
as well. Hence, the clinician simply requires more
accurate oxygen saturation values.
to Knowing the hematocrit and oxygen saturation values,
the computation of the Oxygen Content ~s trivial and maybe
displayed directly (a value heretofore unavailable to the
clinician as a continuous, real-time, noninvasive result):
[ Oxygen Content ] - ;Hct ~ SaO~ ~ K ( 18 )
where K is an empirically determined constant.
Referring to the equations (16) and (9) a decision
must be made by the computer as to the suitability of
utilizing the Taylor expansion approximation to the
logarithm. This algorithm is maintained in the software as
a qualifying decision for the averaging and readout
algorithms. The Taylor approximation is only valid for
small aI/r~t values.
3. Nonpulsatile Applications
' a. Valsalva's Maneuver to Simulate Pulsatile Case
It is interesting to see the similarities between this
AC pulsatile derivation and an analogous DC technique. By
taking the logarithm of two intensity ratios, values of Eb
3o and e~ can be obtained from the modified Beer-Lambert
equation (equation (2a)). These same extinction
coefficients can be manipulated by the identical
proportionality constants R~ and Rz found previously to
exactly eliminate E;~3~oX; and yield
CA 02449621 2003-12-09
. '17-
E bao5 '_ UaoS ( 19 )
Eb1310 ~ U1310 R2 ~rJ9T0'~ R1U805~ .
I
Where the term ' ' ~ ~ Ua= In ~ 2 .
I1 ~k represents v the l'ogarithm' ~ of
intensity ratios at Xb values of X1 and Xz.
It should also be noted that the two derivations (AC
and DC) fold into' one another through the power series
expansion of the ln(1+Z) function:
Z2 .Z3
ln(1+z) =z- +s-... (20)
2 3
When the.value DI=Iz-It, it can be seen that
1nC Iyl = 1nC ~ 1I11 = 1n(1+ ~~ _ ~ + High Order Terms ( 21)
which means that for small changes in Xb, the AC (partial_
derivative) and DC (logarithmic) derivations are similar
_ and can each be precisely compensated through this
differential-ratiometric . technique to provide an
noninvasive e~os/eb~3lfl ratio which is independent of both the
constant and time-varying tissue and interstitial fluid'
terms.
One currently preferred method of obtaining the two
intensity ratios is to have the patient perform Valsalva's
maneuver. Valsalva's maneuver is an attempt to forcibly
exhale with the glottis, nose, and mouth closed. This
maneuver increases intrathoracic pressure, slows the pulse,
decreases return of blood to the heart, and increases
venous pressure. Obtaining intensity measurements before
and during Valsalva's maneuver provide sufficiently
different intensity ratios to utilize equatian (19). Even
a deep breath can be enough to obtain sufficiently
different intensity ratios.
CA 02449621 2003-12-09
_ . _18.
b. Stepper Motor Technicrue
Another technique to simulate pulsatile blood flow arid
to eliminate the skin's optical scattering effects, while
a-t~ the same time preserving -the blood~~borne .hematocrit and
oxygen saturation information, is described below. By
utilizing a stepper motor 9 in the earlobe clip assembly l0
on an earlobe 11 of a patient such as that illustrated in
Figures 5, 6A, 15, and 16, one can produce a variation of
Xb sufficient to utilize equation 19. The stepper motor 9
could even produce a bloodless (Xe 0) state, if required.
However, equation 19 shows that only a' difference between
Xb~ and Xb2 is needed.
The major advantage of this technique is that under
clinical conditions of poor blood flow, poor blood
pressure, or peripheral. vascular disease, where pulse wave
forms are of poor quality for the (c3I/c?t)/I technique, this
DC stepper motor technique could be utilized.
c.. Oxvcren Saturation Determination
The above techniques describe conditions and equations
wherein isobestic wavelengths are chosen such that the
hematocrit value abtained has no interference from oxygen
saturation, hence an independently determined hematocrit
value.
One, however, may choose k2 (the reference wavelength)
in equation (13) at 1550 nm as well. In the radiation
legion 900 to 2000 nm the blood absorption coefficients
depend on hematocrit and water, whereas at 805 nm the blood
absorption coefficient only depends on hematocrit.
Therefore, utilizing in combination, wavelengths of 660,
805, and 1550 will also give a technique to determine
hematocrit (~$os/E~sso) and oxygen saturation (~~o/e8os)'
These 3 wavelengths are particularly important since
660, 805, and 1550 nm (or 1310 nm) are readily available
LEDs, such-as, respectively, MLED76-Motorola, HLP30RGB
Hitachi, and ETX1550-EPITAXX (or NDL5300-NEC), with the
CA 02449621 2003-12-09
- -19-
benefits of low cost and low optical power (reducing any
question of possible eye damage).
The manufacturing of a multi-chip LED emitter becomes
reasonable, cost-wise, and provides increased accuracy
since the LED sources have practically no separation
distances and appear as a single point source,
This invention may be applied to th.e determination of
other components (included, but not limited to, glucose, or
cholesterol) in any range of the electromagnetic spectrum
in_which spectrophotometric techniques can be utilized.
4. Currently Preferred Apparatus -
An earlobe clip assembly 10 as in Figures 6, 6A, 15,
and 16 (with or without the stepper motor 9 shown in
Figure 6A) a finger clip assembly 6 used on a finger 7 as
shown in Figures 1, 1A, and 1B are two currently preferred
embodiments for practicing the present invention. The
photodiodes 3 and emitters 1 and 2 in each are placed in
accordance with appropriate alignment. -
Consider first the sensor technology in the
transmissive mode of operation. An earlobe or fingertip
housing can be provided with discreet emitters and two
photodiode chips (of different sensitivity ranges, 600-
1000 nm and 1000-1700 nm ranges] placed on one substrate,
such as a TO-5 can (Hamamatsu K1713-03j. The emitters
likewise can be two or more emitter chips (i.e., ~1 = 805,
'1310, 660, and 950 nm) placed on a common substrate and
illuminated through a TO-39 can.
Finally, a single substrate multi-wavelength emitter
and a multi-wavelength detector, assembled in one. small
physical housing for each, make alignment and detection
sensitivity more repeatable, and hence more accurate.
The preferred emitter chips would have wavelengths,
for hematocrit-only measurements, at 805 nm, 950 nm,. and
1310 nm (or 805 nm, 950 nm, and 1550 nm). Although in
theory, an emitter having a wavelength of 970 nanometers,
CA 02449621 2003-12-09
-20-
rat~:er than .950 nm, would provide more accurate
information, 970 nm emitters are not presently available
commercially. These wavelengths are currently preferred y
because of the different curvature and baseline offset of
the E versus Hematocrit at those wavelengths. See
Figure 3. Hence, the hematocrit information will exist in
the ratio e~~/E~2. See Figure 4.
Furthermore, the choice of 805 nm and 1310 nm (or
1550 nm) rather than 570 nm and 805 nm is because there is
no water absorption in the 570 nm (or 589 nm) and 805 nm
isobestic wavelengths. However, there is tremendous water
absorption at 1310 nm and 1550 :nm. Hence, the ratio of
570 nm to 805 nm, as a reference, would not yield
hematocrit information because.there would be no offset due
to water in the plasma. See Figures _13A and 13B and
Figures 14A and 14B. _
If hematocrit-independent oxygen saturation is desired
then the emitter chip wavelengths would be 660 nm, 805 nm,w
950 nm, and 1310 nm (or 1550 nra) (the 660 nm is MLED76,
Motorola or ~ TOLD 9200, Toshiba). Likewise, the
photodetector single substrate could house at least two
chips, such as a Hamamatsu K1713--03.
It will be appreciated that those skilled. in the art
would be able to add other chips to the single substrate at
wavelengths sensitive to other metabolites (glucose,
cholesterol, etc.). The above mentioned emitter and
detector connections can be seen in the analog schematic
diagram illustrated in Figures 7 and 9B-9D.
The sensor technology in the reflectance mode must
conform to two embodiment parameters. See Figure 1B. The
diameter and thickness of the aperture 8 in which figure 7
is received in combination with the sensor-emitter
separation distance are important to provide a detection
region within the subdermis 1:? at points a and b of
Figure 1B, where the radiation impinges on blood-tissue
CA 02449621 2003-12-09
- -21-
without the multiple scattering effects of th;e epithelial
layer, Rt. The determination of optimum sensor 3 separation
and aperture 8 sizeswis done empirically' from numerous
fingers 8 with varying callous and fingernails 13. Minimum
sensor separation and aperture diameters can be established
wherein Rt, of equation (14) is eliminated.
Figures 7, 8A-8C, 9A=9D, and l0A-lOB detail .the
electronics of one circuit suitable for use within the
scope of the present invention. The memory and computation
means (Figures 8A-8C) are connected via a 'bus" structure
between PROMS (U110,U111), microprocessor MC68HC000 (U106),
static RAMS (U112,U113), and isolation buffers to the low-
level analog circuitry (Figure 7). A crystal controlled
oscillator circuit (UlOIA,B) is divided by 2 to provides a
symmetric master clock to the microprocessors this clock is
further subdivided~and used to provide clocking for the
r analog-to-digital converter (U208) and timer (U109).-
Strobe lines are generated through a decoder arrangement to-
drive each of the subsystems of 'the device and also control
the isolation bus buffers (U201,U202).
Timer outputs are fed back into the microprocessor and
encoded (U104) to produce interrupts at specific intervals
for system functions. One timer is shared by subsystems
which control the liquid crystal display means, the
keyboard entry means, the audible indicator, and the
Cycling background system self-test. Another timer is
dedicated exclusively to provide a high priority interrupt
to the microprocessor; this interrupt drives software which
controls the basic sensor sampling mechanism. An expansion
connector (J101) is included to allow extended testing of
the device or connection to external data-logging equipment
such as a printer or computer interface.
The local bus isolates the sensitive analog circuitry
from the main digital circuitry. Th~,s prevents spurious
crosstalk from digital signals into the analog circuitry
CA 02449621 2003-12-09
-22-
and thereby reduces superimposed noise on the measured
signals. It is on this local bus that the Digital-to
Analog Converters (DAC) and Analog-to-Digital Convertors
(ADC) transmit and receive digital information while'
processing the low-level analog signals.
The Low Level Sensor electronic section, Figure 7,
combines subsystems to both measure and.modulate the
current produced from each optical sensor. Since the
pulsatile component of the optical energy transmitted
through or reflected off of tissue, comprises only a small
part of the overall optical energy incident on the sensor,.
means are provided to '°null out" in a carefully controlled
and accurately known way the non-pulsatile component of the
light-produced current in the sensing detector. The
remaining signal can then be dc-amplified and filtered in
a straightforward manner and prs~sented to the ADC (U208)
for conversion into a digital value representative of the
relative AC pulsatile component. Furthermore, because the.-
relationship between the pulling current and the average
value of this AC component is known; the DC component can
easily be caicuiated as a 'function of the sensing means'
sensitivities and the electronic stages' gains': The
functions determining these AC and DC values can (if~
necessary) be trimmed in software by calibration constants
which are stored in EEPROM (U307) and retrieved each time
the unit is powered on.
The current which modulates the optical sources (LEDs
or Laser Diodes) is also controlled (U203) and precisely
adjusted (U306) to optimize signal reception arid detection.
~ Through software control, the modulation current can be
adjusted on a pulse-by-pulse basis to minimize nvise-
induced inaccuracies. Furthermore, by sampling the sensors
with the modulation sources disabled appropriately,
background noise (such as 60 Hz) can be rejected digitally
~ 35 as common-mode noise. Thus, by controlling the optical
CA 02449621 2003-12-09
-2~3-
source energy and modulating the nulling current in the
photosensor circuitry, it is possible to effectively cancel
the effects of ambient radiation levels and accurately
measure both the static (DC) and time-varying (AC)
5, components of transmitted or reflected light.
Interrupt-driven software algorithms acquire the
. sensor data, provide a real-time pulse wave contour, and
determine pulse boundaries. Completed buffers (i.e. one
entire pulse per buffer) of sensor data are then passed to
the foreground software processes for computation. This
involves the determination of~the background-compensated AC
pulsatile and DC static values of intensities for each
wavelength. Through averaging and selective elimination of
abnormal values, results are then calculated using
equation (9) and displayed on the LCD. The modulating and
pulling currents are .(if necessary) also adjusted to
utilize the electronic hardware efficiently and optimally.
5. Summary
Although the foregoing discussion has related to
noninvasive analysis of blood hematocrit information, it
will be appreciated that the above-mentioned emitters,
sensors, and circuitry may be adapted for invasive in vitro
analysis of blood hematocrit values. The principles within
the scope of the present invention which,compensate for
spatial, geometric, and tissue variations may be used to
compensate for similar variations in an in vitro blood
container. Such a device would allow hematocrit values to
be determined-rapidly and accurately.
Those skilled in the art will also appreciate.that the
methods within the scope of the present invention for
determining blood hematocrit values may be adapted for
determining non-hematocrit biologic constituent values such
as glucose, cholesterol, etc. To determine biologic
constituent information, the effects of competing blood,
tissue, and interstitial fluid constituents must be
CA 02449621 2003-12-09
-24-
eliminated. It is believed that these. effects may be
eliminated by appropriate modification of the differential
ratiometric techniques described above.
It is important to recognize that the present
invention is not directed to determining the tissue
hematocrit value. The tissue hematocrit value, in contrast
with the blood hematocrit value, reflects the amount of red
blood cells in a given volume of tissue (blood,
interstitial fluids, fat, hair follicles, etc.). The
present invention is capable of determining actual
i:ntravascular blood hematocrit and hemog~5.obin values.
From the foregoing, it will be appreciated that the
present invention provides a system and method for
noninvasively and quantitatively determining a subject's
hematocrit or other blood constituent vale. The present
invention determines the hematocrit noninvasively by
utilizing electromagnetic radiation as the transcutaneous
information carrier. Importantly, the present inventionw-
may be used on various body parts to provide accurate
quantitative hematocrit values.
It will also be appreciated that the present invention
also provides a system and method which can provide
immediate and continuous hematocrit information for a
subject. The present invention further provides a system
and method for noninvasively determining a subjects's blood
oxygen saturation (S802) independent of the subject's
hematocrit. In addition, the present invention provides a
system and method for noninvasively determining a subject's
hematocrit and/or blood oxygen saturation even under
conditions of low blood perfusion.
The present invention may be embodied in other
specific forms without departing from its spirit or
essential characteristics. The described embodiments are
to be considered in all respects only as illustrative and
not restrictive. The scope of the invention is, therefore,
CA 02449621 2003-12-09
-25-
indicated by the appende3 ,claims rather than by the
foregoing description. All changes which come within the
meaning and range of equivalency of the claims are to be
embraced within their scope.